Abstract
Background/Aim
The incidence of hepatocellular carcinoma (HCC) in patients with transfusion dependent thalassemia (TDT) has been increasing, where viral hepatitis and iron overload are the two established HCC risk factors. The aim of this study was to investigate the etiological factors of HCC development and to evaluate the possible factors associated with survival in our cohort of TDT patients with HCC.
Methods
Records of patients with TDT diagnosed with HCC from 2008 to 2018 were reviewed. Liver iron concentration (LIC) has been assessed by the signal-intensity-ratio MRI. The diagnosis of HCC was made by a 3-phase contrast magnetic resonance imaging (MRI) and patients were staged and treated for HCC according to Barcelona Clinic Liver Cancer (BCLC) grading system.
Results
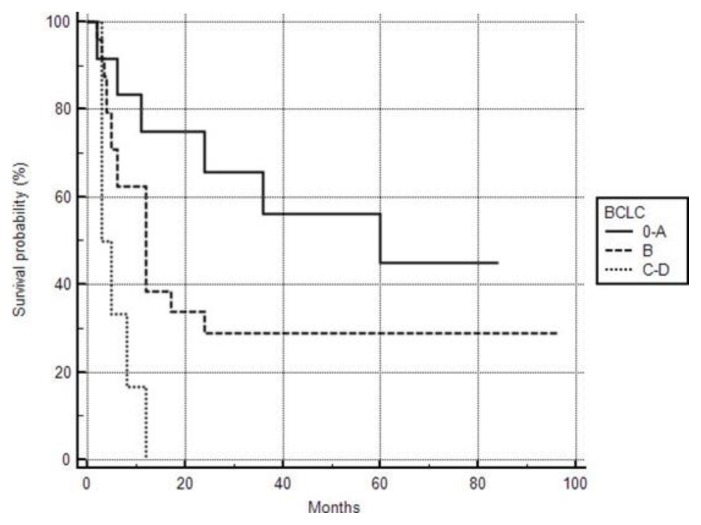
Forty-two TDT patients with HCC have been included. Most of them (78.5%) were anti-HCV positive, 59.5% HCV-RNA positive, and 16.5% had serological markers of resolved HBV infection. Patients with HCV infection have been treated successfully with either Peg-IFNa±Ribavirin or with the new direct antivirals (DAAs). At the time of HCC diagnosis, all patients with chronic HCV infection were HCV-RNA negative, 78.5% had underlying cirrhosis, and the vast majority (98%) had average or mild elevated LIC values. According to the BCLC system, patients were classified as 0-A: 28.5%, B: 57% and C–D: 14.5%. HCC has been treated with loco-regional treatment in 78.5% of our patients, while the rest have received sorafenib. Twenty-eight patients (66.5%) died due to HCC with a median survival time of 6 months (range: 2–60). Using the Cox proportional hazard model, the only factors associated with poor survival were BCLC stages C and D.
Conclusions
In conclusion, BCLC staging is the main prognostic factor of survival in patients with TDT who develop HCC.
Keywords: Hepatocellular carcinoma (HCC), Thalassemia major (TM), Viral hepatitis, Cirrhosis, Hemosiderosis
Introduction
The incidence of hepatocellular carcinoma (HCC) in patients with thalassemia has been increasing in recent years, where chronic viral hepatitis and iron overload are the two established HCC risk factors in this particular patient population.1 Importantly all these factors are both preventable and treatable.
Both alpha- and beta-thalassemia are more prevalent in tropical and subtropical regions of the world. The southern regions of Europe, such as Italy and Greece, are the most likely areas to be affected in Europe. Greece, a country of approximately 11 million people, has a mean frequency of thalassaemia carriers at 7%.2
The role of viral hepatitis as a risk factor for hepatocarcinoma is essential in thalassemia, mainly in older patients, since the risk of viral transmission through blood transfusion was significantly reduced after the 1990s with the identification of the HCV virus and the universal screening of blood donors.3 The estimation of the current prevalence of Hepatitis C in Greece ranges from 0.5% to 2% according to the population studied, while the prevalence of HBsAg was 0.84% in a 6-year blood donor based study in Athens.4,5 HBV-induced hepatocarcinogenesis is a multifactorial process that involves the presence of chronic hepatitis and cirrhosis and also the direct role of hepatitis B virus (HBV), development of HCC in the absence of cirrhosis, mainly through HBV-DNA integration into the host DNA which alters the function of endogenous genes or induces chromosomal instability.6 On the other hand, HCV-induced hepatocarcinogenesis of HCC is a gradual process that relates to chronic hepatitis and the duration of disease leading to liver cirrhosis.7–10 Furthermore, the risk of HCC is greatly reduced in HCV cirrhotic patients who obtained a sustained virological response (SVR) after HCV treatment but is not eliminated.11,12
Both the incidence and the etiology of HCC changed over the last 25 years. In a recently published study performed in Crete island, authors indicate a rose from 6.0 new cases per 100,000 inhabitants in the first five-year period (1990–1994) to 16.8 in the last five years (2010–2014). The change was mostly attributable to a gradual increase in the incidence of HB, alcohol and non-alcoholic fatty liver disease (NAFLD) related cases, especially during the last decade.13
The hepatocarcinogenicity of iron seems to be a very complex phenomenon. It is well established that patients with hereditary hemochromatosis have a risk of developing HCC even in the absence of cirrhosis.14 Even more, several reports have described the development of HCC in non-cirrhotic patients with thalassemia syndromes who were negative for HBV and HCV but had a hepatic iron overload. The primary hypothesis is that free iron, even in the absence of cirrhosis, is hepatocarcinogenic due to the generation of reactive oxygen species (ROS), which causes peroxidation of membrane fatty acids that impair protein synthesis and disrupt DNA synthesis.15
Data concerning etiological factors and treatment outcomes of HCC appear to be lacking in patients with transfusion dependent thalassemia (TDT). The aim of this study was to investigate the etiological factors of HCC development and to evaluate the possible factors associated with survival among Greek patients with TDT and HCC.
Patients and Methods
We review the records of all patients with TDT who developed HCC from a referral tertiary liver center of Athens from 2008–2018. The database included patient demographic and epidemiological characteristics, medical history data, as well as clinical and laboratory data. All patients were under systemic transfusions at 2–4-wk intervals (age of initial treatment: 3 months to 5 years) and iron chelation therapy either with deferoxamine (DFO) or deferiprone (DFP) or deferasirox (DFX) (age of initial treatment: 2–15 years). Hematological/biochemical parameters including ferritin levels, serological markers of hepatitis B (hepatitis B surface antigen, antibodies to hepatitis B surface and core antigens) and hepatitis C (antibodies to hepatitis C virus) have been determined by commercially available assays. Serum α-fetoprotein (αFP) levels have been determined by a commercially available assay (R&D) with a lower cut-off value of 20 ng/mL. All anti-HCV positive patients were further evaluated with HCV-RNA by commercially available quantitative PCR assays (COBAS TaqMan HCV v1.0 or v2.0; Roche Diagnostics) with a lower limit of detection of 43 and 15 IU/ml respectively.
The average of the last four measurements before the diagnosis of HCC has been used to represent the levels of ferritin and hemoglobin for the purpose of our analysis. All included patients in the study had serum ferritin levels less than 1,000 ng/ml. Hepatic magnetic resonance imaging (MRI) is now considered the gold standard method for estimating and monitoring liver iron concentration (LIC) in these patients. Thus, liver iron overload has been assessed by the signal-intensity-ratio MRI (Rennes University algorithm) at the time of HCC diagnosis.16,17 Patients with LIC values ≤40μmolFe/g were considered as normal, those with LIC values between 40 and ≤100 μmolFe/g as having mild hemosiderosis, those with LIC values between 100 and ≤200 μmolFe/g as having moderate hemosiderosis and those with LIC values above 200 μmolFe/g as having severe iron overload.16 Liver cirrhosis has been diagnosed using imaging methods (computed tomography scan or liver ultrasound), while in four of them (9.5%) has been documented by liver biopsy. The diagnosis of HCC was done by magnetic resonance imaging (MRI) with contrast (3 phase) and was confirmed by guided liver biopsy in uncertain by MRI cases. All patients were assessed using Barcelona Clinic Liver Cancer (BCLC) grading system and were classified as very early or early stage (0-A), intermediate stage (B), and as advanced or terminal stage (C–D).18 The very early stage (BCLC 0) is defined as the presence of a single nodule < 2 cm in diameter in patients with well-preserved liver function (Child-Pugh A). The early stage (BCLC A) corresponds to patients with one nodule < 5 cm or up to three nodules each < 3 cm. Patients with BCLC stages 0 and A are candidates for potentially curative treatment options such as surgical resection, liver transplantation, or local radiofrequency ablation (RFA). The intermediate stage (BCLC B) includes asymptomatic patients with large or multifocal tumors. The advanced stage (BCLC C) concerns patients with cancer-related symptoms, macrovascular invasion, or extrahepatic spread. Patients with BCLC B and C are treated with palliative approaches, such as transarterial chemoembolization (TACE) or systemic therapy with sorafenib. Patients in the terminal stage (BCLC D) receive best supportive care.19 The study has been performed according to the World Medical Association Declaration of Helsinki and has been approved by the hospital ethics committee. All patients were informed and consent to access their confidential data from the hospital’s medical records.
Statistical analysis
All data were analyzed using the statistical package MedCalc (version 18.11). Statistical analysis was performed using a t-test for comparisons of continuous variables between groups and corrected chi-squared test for comparisons of qualitative data. A two-tailed p value <0.05 was considered to be statistically significant. The survival time for each patient, in terms of months, was defined as the interval between the dates of HCC diagnosis and the date of death or the end of the study. Cumulative survival rates were estimated using the Kaplan-Meier method, while cox proportional hazards model was also used to identify risk factors relating to survival.
Results
In total, 42 patients were included. Their mean age was 45.5±5.8 years, whereas 27 of them (64.5%) were males. At the time of HCC diagnosis, most of the patients (78.5%) were diagnosed with compensated cirrhosis (Child-Pugh A).
Out of 42 patients, 33 (78.5%) were anti-HCV positive. Most of them 25/42 (59.5%) were HCV-RNA positive within the last 10 years. The rest of the anti-HCV positive patients (8/42) were always HCV-RNA negative indicative of spontaneous viral clearance after exposure to the virus. All viraemic patients have been treated either with Pegylated - interferon alpha (Peg-IFN) ± ribavirin (RBV) [8/25, (32%)] or with the new interferon-free, direct acting antivirals (DAAs) regimens [17/25, (68%)]. All patients had achieved sustained virological response (SVR) at least 12 months before HCC diagnosis.
Regarding HBV infection, none of our patients was HBsAg positive. Thus, no one had received anti-viral treatment. However, 7 patients (16.5%) had serological evidence of resolved HBV infection [HBsAg(−)/anti-HBc(+)/anti-HBs(+)] without prior anti-HBV treatment. All of these patients had a history of acute HBV infection, and finally, they have achieved HBsAg clearance.
The mean LIC values were 48.6±29.5 μmolFe/g. More than half of our patients (55%) had average LIC values, 18 patients (43%) were classified as having mild hemosiderosis and only one patient (2%) has been classified as having moderate hemosiderosis.
αFP levels have been assessed in all patients and were ≤100 ng/ml in 26 of them (62%).
The tumor has been diagnosed as multifocal (≥3) in 25 patients (59.5%), while those with maximum diameter ≥5cm have been diagnosed in 18 patients (43%). According to the BCLC grading system, patients were classified as 0-A: 12/42 (28.5%), B: 24/42 (57%) and as C–D: 6 patients (14.5%).
HCC has been treated with TACE or RFA in 33 patients (78.5%) while the rest 9 patients were treated with sorafenib. Two patients with very early stage and no other contraindication underwent surgical excision but eventually, they have been treated with loco-regional therapies due to HCC relapse in the first six months. These two patients have been included in TACE/RFA group.
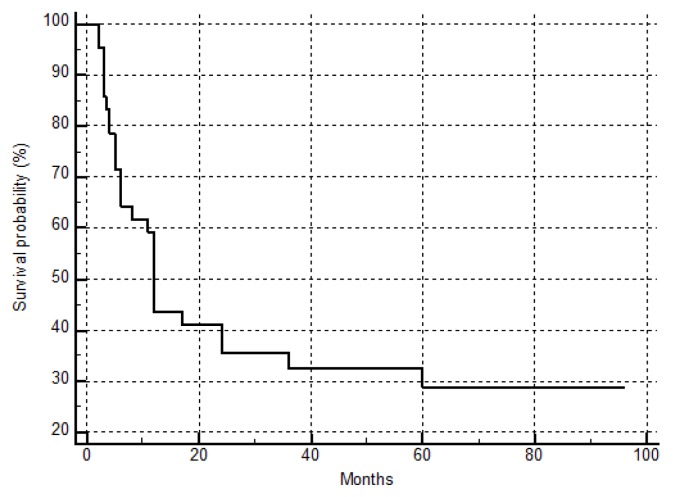
Overall, 28 patients (66.5) have eventually died due to HCC, with a median survival time of 6 months (2–60), while a median survival time of 60 months (6–96) has been observed in still living patients at the end of the study (Table 1).
Table 1.
Baseline characteristics of 42 patients with thalassemia major and HCC.
| Total (N=42) | Dead (n=28) | Alive (n=14) | |
|---|---|---|---|
|
| |||
| Age, years | 45.5±5.8 | 46±5.8 | 44.5±6 |
|
| |||
| Gender (males), n (%) | 27 (64.5) | 19 (68) | 8 (57) |
|
| |||
| History of anti-HCV positivity, n(%) | 33 (78.5) | 21 (75) | 12 (86) |
| • History of HCV RNA positivity, n (%) | 25 (59.5) | 19 (90.5) | 6 (43) |
| • History of SVR, n (%) | 25 (100) | 19 (100) | 6 (100) |
|
| |||
| anti-HBc positivity, n (%) | 7 (16.5%) | 6 (21.5) | 1 (9) |
|
| |||
| Cirrhosis at diagnosis of HCC, n (%) | 33 (78.5%) | 24 (86) | 9 (64.5) |
| • C/P A | 33 (100) | 24 (100) | 9 (100) |
|
| |||
| LIC, μmolFe/g | 48.6±29.5 | 49.25±31.8 | 47.2±25.4 |
|
| |||
| Grade of siderosis at diagnosis of HCC, n (%) | |||
| • normal LIC (≤40 μmolFe/g) | 23 (55) | 15 (53.5) | 8 (57) |
| • mild (40<LIC≤100 μmolFe/g) | 18 (43) | 12 (43) | 6 (43) |
| • modereate (100<LIC≤200 μmolFe/g) | 1 (2) | 1 (3.5) | 0 |
| • severe (>200 μmolFe/g) | 0 | 0 | 0 |
|
| |||
| ALT, IU/L | 34±32.5 | 49.2±34.5 | 21±5 |
|
| |||
| AST, IU/L | 34±55.5 | 82.3±58 | 30±10 |
|
| |||
| Albumin levels, mg/dl | 3.6±0.6 | 3.4±0.6 | 4±0.6 |
|
| |||
| αFP, ng/ml (range) | 20 (1.7–215,100) | 28.35 (1.9–215,100) | 20 (1.7–20,300) |
|
| |||
| αFP classification (lower cut-off value of 20 ng/mL) | |||
| • ≤100 ng/ml, n (%) | 26 (62) | 16 (57) | 10 (71.5) |
| • >100 ng/ml, n (%) | 16 (38) | 12 (43) | 4 (28.5) |
|
| |||
| Number of HCC lesions, n (%) | |||
| • <3 | 17 (40.5) | 11 (39) | 6 (43) |
| • ≥3 | 25 (59.5) | 17 (61) | 8 (57) |
|
| |||
| Maximum diameter of HCC, n (%) | |||
| • <5 cm | 24 (57) | 14 (50) | 10 (71.5) |
| • ≥5 cm | 18 (43) | 14 (50) | 4 (28.5) |
|
| |||
| BCLC staging at diagnosis of HCC, n (%) | |||
| • 0-A | 12 (28.5) | 6 (21.5) | 6 (43) |
| • B | 24 (57) | 16 (57) | 8 (57) |
| • C–D | 6 (14.5) | 6 (21.5) | 0 |
|
| |||
| Treatment, n (%) | |||
| • TACE/RFA | 33 (78.5) | 21 (75) | 12 (85.5) |
| • Sorafenib | 9 (21.5) | 7 (25) | 2 (14.5) |
|
| |||
| Median Survival after HCC occurrence, months (range) | 12 (2–96) | 6 (2–60) | 60 (6–96) |
|
| |||
| Median Survival according to treatment, months (range) | |||
| • Sorafenib | 5 (3–12) | 3.5 (3–12) | 6 (6–6) |
| • TACE/RFA | 12 (2–96) | 11 (2–60) | 66 (12–96) |
HCC: Hepatocellular Carcinoma, HCV: Hepatitis C Virus, SVR: Sustained Virological Response, C/P: Child/Pugh, LIC: Liver iron overload, BCLC: Barcelona Clinic Liver Cancer, TACE: transarterial chemoembolization, RFA: radiofrequency ablation.
The overall Kaplan-Meier survival curve of the patients is shown in figure 1. In order to reveal possible risk factors relating to survival, we used the cox proportional hazard model adjusted for age (Table 2). BCLC stages C and D were associated with poor survival (HR: 5.4179, 95% CI: 1.4936–19.6531, p=0.0102) (Figure 2).
Figure 1.
Overall Kaplan-Meier survival curve.
Table 2.
Prognostic factors affecting survival using the Cox proportional hazard model.
| p value | HR | 95% CI | |
|---|---|---|---|
|
| |||
| Age, years | 0.5567 | 1.0233 | 0.9477–1.1050 |
|
| |||
| Gender | |||
| • Female | • 0.3088 | • 1.7627 | • 0.5917–5.2513 |
|
| |||
| Anti-HCV | |||
| • Negative | • 0.0960 | • 2.3158 | • 0.8615–6.2246 |
|
| |||
| Cirrhosis | |||
| • No | • 0.0851 | • 0.2708 | • 0.0612–1.1.1981 |
|
| |||
| Grade of siderosis | |||
| • mild/moderate | • 0.9444 | • 1.0331 | • 0.4139–2.5783 |
|
| |||
| αFP levels | |||
| • >100 ng/ml | • 0.5519 | • 0.7812 | • 0.1659–1.9732 |
|
| |||
| BCLC staging | |||
| • C–D | • 0.0102 | • 5.4179 | • 1.4936–19.6531 |
|
| |||
| Number of lesions | |||
| • <3 | • 0.6088 | • 1.3119 | • 0.4639–3.7099 |
|
| |||
| Maximum diameter | |||
| • ≥5 cm | • 0.2943 | • 2.0947 | • 0.5259–8.3432 |
HCV: Hepatitis C Virus, BCLC: Barcelona Clinic Liver Cancer.
Figure 2.
Kaplan-Meier survival curve according BCLC staging.
Discussion
In patients with TDT, the development of HCC represents evolving morbidity and mortality in the last years. The main risk factors for HCC are chronic iron accumulation, chronic HCV or HBV infection and cirrhosis. It is well established that patients with hereditary hemochromatosis have an increased risk of HCC irrespective of the presence of cirrhosis.14,15 Patients with TDT are exposed, at a very young age, to iron through transfusions, and most studies, including ours, denote that HCC develops below 50 years of age.20,21 Free iron induced oxidative stress and increased levels of ROS lead to mitochondrial injury, DNA damage and consequently, to hepatocarcinogenesis.22 In agreement with these reports, our patients had a mean age of 45.5±5.8 years old. However, all of them were under iron chelation treatment, had serum ferritin levels less than 1,000 ng/ml and the vast majority did not have (55%) or had only mild hemosiderosis (43%) at the time of HCC diagnosis. However, the amount and duration of exposure to excess iron are crucial to the development of liver injury. Moreover, the association between the iron overload and the development of HCC has been confirmed in studies in rats fed a high-iron diet. After 15 months, the iron-loaded liver developed HCC in the absence of cirrhosis.23 Furthermore, while TDT outcomes have been improving in recent years, particularly those related to heart disease due to iron chelation treatments, HCC has emerged as a new complication of liver disease.3,24
Both chronic viral hepatitis B and C and related cirrhosis remain important risk factors for the development of HCC.21 In our study 7 patients had serological markers of past HBV infection [HBsAg (−)/anti-HBc(+)/anti-HBs(+)] but all of them were cirrhotics. Theoretically, the hepatocarcinogenicity of HBV remains in patients with resolved HBV infection (spontaneous seroconversion of HBsAg) mainly through HBV-DNA integration into the host genome. However, the risk of HCC is significantly lower in HBsAg negative than in HBsAg positive patients and the presence of liver cirrhosis is the dominant risk factor for hepatocarcinogenesis.25 With respect to chronic HCV infection, most of our patients were anti-HCV positive (78.5%) of whom 76% had chronic HCV infection. All of them have eventually been treated and finally achieved SVR at least 12 months before HCC diagnosis. However, most of them were cirrhotics (79%) at that time. It is well known that cirrhosis is the main risk factor for HCC in CHC and all patients with cirrhosis should be closely monitored and followed even after successful antiviral therapy.26
Data concerning possible factors associated with the survival rate among patients with TDT who develop HCC remain unclear. It is well established that the development of HCC is the main factor affecting the survival of patients with chronic liver disease.27,28 The prognostic factors for survival in patients with HCC are related to tumor status (number and size of nodules, presence of vascular invasion, extrahepatic spread), liver function and general tumor-related health status.18 Previous population-based data from Italy suggested that in patients with TDT and HCC, the average survival was 3.5 months in 2004 and rose to 11.5 months in 2014.3,24 In our cohort, the analysis, after adjusting for age, has indicated that advanced BCLC stages were associated with poor survival. To our knowledge, this is the first real-life study evaluating prognostic factors affecting the survival of TDT patients with HCC.
The decision of the HCC treatment method was mainly based on the BCLC algorithm. However, we have to keep in mind that TDT has been considered as a relative contraindication for liver transplantation and this therapeutic approach was not available in our patients with early stage HCC. Moreover, major operations like surgical HCC resections are limited due to the problematic peri-operative management of these patients.29 Only two of our patients underwent resection of the tumor as initial treatment but both were relapsed within the next six months and were subsequently treated with loco-regional therapies. According to BCLC, systemic algorithm therapy is indicated in the advanced stage of the disease. In general, these patients bear a poor prognosis, with expected median survival times of 6–8 months.30 A large double-blinded placebo-controlled phase III study showed that the median overall survival of patients in the sorafenib group was 10.7 months compared to 7.9 months in the placebo group (HR, 0.69; 95% CI 0.55–0.87; p = 0.00058), representing a 31% decrease in the relative risk of death.31 In our study, these patients achieved a median survival period of 5 months (3–12). Patients with early stage HCC treated with RFA have an overall median survival of about 36 months which may extend to >5 years after successful treatment.30 Patients with intermediate stage HCC by BCLC treated with TACE have an overall survival of about 16 months which may extend to >40 months in well selected patients.32,33 Our patients who have been treated with TACE/RFA achieved an overall survival of 12 months (2–96) which is much lower than expected. A possible explanation is that mortality in these patients may be influenced by several other complications and also that the biological activity of HCC in these patients is more aggressive, as can be hypothesized by the very high αFP levels.
The absence of a comparable group of patients with TDT without HCC, which would further provide robustness on the estimation of surveillance of HCC, is the main limitation of our study. We did demonstrate, however, the characteristics and risk factors of HCC and the main prognostic factors of survival in this specific group of patients.
Conclusions
In conclusion, in patients with TDT, the development of HCC represents evolving morbidity and mortality in the last years. The main etiological factors for HCC are chronic iron accumulation in the liver, chronic HCV infection and cirrhosis. This population with HCC has discrete characteristics compared to the general population, such as young age at presentation of HCC and various comorbidities that limit the therapeutic options particularly transplantation and surgery. Since the HCC stage seems to be the major prognostic factor of survival, a personalized approach of surveillance is mandatory taking into consideration individual patient’s comorbidities. Once a surveillance test is positive, a more definitive imaging examination is recommended for noninvasive diagnosis and staging of HCC.
Footnotes
Competing interests: The authors declare no conflict of Interest.
References
- 1.Moukhadder HM, Halawi R, Cappellini MD, Taher AT. Hepatocellular carcinoma as an emerging morbidity in the thalassemia syndromes: a comprehensive review. Cancer. 2017;123:751–758. doi: 10.1002/cncr.30462. [DOI] [PubMed] [Google Scholar]
- 2.Loukopoulos D. Haemoglobinopathies in Greece: prevention programme over the past 35 years. Indian J Med Res. 2011;134:572–6. [PMC free article] [PubMed] [Google Scholar]
- 3.Borgna-Pignatti C, Garani MC, Forni GL, Cappellini MD, Cassinerio E, Fidone C, Spadola V, Maggio A, Restivo Pantalone G, Piga A, Longo F, Gamberini MR, Ricchi P, Costantini S, D’Ascola D, Cianciulli P, Lai ME, Carta MP, Ciancio A, Cavalli P, Putti MC, Barella S, Amendola G, Campisi S, Capra M, Caruso V, Colletta G, Volpato S. Hepatocellular carcinoma in thalassaemia: an update of the Italian Registry. Br J Haematol. 2014;167:121–126. doi: 10.1111/bjh.13009. [DOI] [PubMed] [Google Scholar]
- 4.Nikolopoulou G, Zisouli A. Viral hepatitis. Available from: http://www.keelpno.gr/en-us/home.aspx.
- 5.Kyriakis KP, Foudoulaki LE, Papoulia EI, Sofroniadou KE. Seroprevalence of hepatitis B surface antigen (HBsAg) among first-time and sporadic blood donors in Greece: 1991–1996. Transfusion Medicine. 2000;10:175–180. doi: 10.1046/j.1365-3148.2000.00257.x. [DOI] [PubMed] [Google Scholar]
- 6.Xu HZ, Liu YP, Guleng B, Ren JL. Hepatitis B Virus-Related Hepatocellular Carcinoma: Pathogenic Mechanisms and Novel Therapeutic Interventions. Gastrointest Tumors. 2014;1:135–45. doi: 10.1159/000365307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prati D, Zanella A, Farma E, De Mattei C, Bosoni P, Zappa M, Picone A, Mozzi F, Rebulla P, Cappellini MD, Allain JP, Sirchia G. A multicenter prospective study on the risk of acquiring liver disease in anti-hepatitis C virus negative patients affected from homozygous b-thalassemia. Blood. 1998;92:3460–3464. doi: 10.1182/blood.V92.9.3460. [DOI] [PubMed] [Google Scholar]
- 8.Mancuso A, Rigano P, Renda D, Di Salvo V, Pignatti CB, Guddo F, Buccellato A, Nicoli N, Maggio A. Hepatocellular carcinoma on cirrhosis-free liver in a HCV-infected thalassemic. Am J Hematol. 2005;78:158–159. doi: 10.1002/ajh.20289. [DOI] [PubMed] [Google Scholar]
- 9.Minola E, Prati D, Suter F, Maggiolo F, Caprioli F, Sonzogni A, Fraquelli M, Paggi S, Conte D. Age at infection affects the longterm outcome of transfusion-associated chronic hepatitis C. Blood. 2002;99:4588–4591. doi: 10.1182/blood-2001-12-0192. [DOI] [PubMed] [Google Scholar]
- 10.Papatheodoridis G, Dalekos G, Sypsa V, Yurdaydin C, Buti M, Goulis J, Calleja JL, Chi H, Manolakopoulos S, Mangia G, Gatselis N, Keskin O, Savvidou S, de la Revilla J, Hansen BE, Vlachogiannakos I, Galanis K, Idilman R, Colombo M, Esteban R, Janssen HL, Lampertico P. PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J Hepatol. 2016;64:800–806. doi: 10.1016/j.jhep.2015.11.035. [DOI] [PubMed] [Google Scholar]
- 11.Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158:329–337. doi: 10.7326/0003-4819-158-5-201303050-00005. [DOI] [PubMed] [Google Scholar]
- 12.Mancuso A. Hepatocellular carcinoma in thalassemia: A critical review. World J Hepatol. 2010;2:171–174. doi: 10.4254/wjh.v2.i5.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karageorgos SA, Stratakou S, Koulentaki M, Voumvouraki A, Mantaka A, Samonakis D, Notas G, Kouroumalis EA. Long-term change in incidence and risk factors of cirrhosis and hepatocellular carcinoma in Crete, Greece: a 25-year study. Ann Gastroenterol. 2017;30:357–363. doi: 10.20524/aog.2017.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kew MC. Hepatic iron overload and hepatocellular carcinoma. Liver Cancer. 2014;3:31–40. doi: 10.1159/000343856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kew MC. Hepatic iron overload and hepatocellular carcinoma. Cancer Lett. 2009;286:38–43. doi: 10.1016/j.canlet.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Gandon Y, Olivié D, Guyader D. Non-invasive assessment of hepatic iron stores by MRI. Lancet. 2004;363:357–362. doi: 10.1016/S0140-6736(04)15436-6. [DOI] [PubMed] [Google Scholar]
- 17.Hankins JS, McCarville MB, Loeffler RB, Smeltzer MP, Onciu M, Hoffer FA, Li CS, Wang WC, Ware RE, Hillenbrand CM. R2* magnetic resonance imaging of the liver in patients with iron overload. Blood. 2009;113:4853–4855. doi: 10.1182/blood-2008-12-191643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertuccio P, Turati F, Carioli G, Rodriguez T, La Vecchia C, Malvezzi M, Negri E. Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol. 2017;67:302–309. doi: 10.1016/j.jhep.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Richani M, Kolly P, Knoepfli M, Herrmann E, Zweifel M, von Tengg-Kobligk H, Candinas D, Dufour JF. Treatment allocation in hepatocellular carcinoma: Assessment of the BCLC algorithm. Ann Hepatol. 2016;15:82–90. doi: 10.5604/16652681.1184233. [DOI] [PubMed] [Google Scholar]
- 20.Restivo Pantalone G, Renda D, Valenza F, D’Amato F, Vitrano A, Cassarà F, Rigano P, Di Salvo V, Giangreco A, Bevacqua E, Maggio A. Hepatocellular carcinoma in patients with thalassaemia syndromes: clinical characteristics and outcome in a long term single centre experience. Br J Haematol. 2010;150:245–247. doi: 10.1111/j.1365-2141.2010.08180.x. [DOI] [PubMed] [Google Scholar]
- 21.Balogh J, Victor D, Asham EH, Burroughs SG, Boktour M, Saharia A, Li X, Ghobrial RM, Monsour HP., Jr Hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2016;3:41–53. doi: 10.2147/JHC.S61146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sekine S, Ito K, Watanabe H, Nakano T, Moriya K, Shintani Y, Fujie H, Tsutsumi T, Miyoshi H, Fujinaga H, Shinzawa S, Koike K, Horie T. Mitochondrial iron accumulation exacerbates hepatic toxicity caused by hepatitis C virus core protein. Toxicol Appl Pharmacol. 2015;282:237–243. doi: 10.1016/j.taap.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Kew MC, Asare GA. Dietary iron overload in the African and hepatocellular carcinoma. Liver Int. 2007;27:735–741. doi: 10.1111/j.1478-3231.2007.01515.x. [DOI] [PubMed] [Google Scholar]
- 24.Borgna-Pignatti C, Rugolotto S, De Stefano P, Zhao H, Cappellini MD, Del Vecchio GC, Romeo MA, Forni GL, Gamberini MR, Ghilardi R, Piga A, Cnaan A. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. 2004;89:1187–1193. [PubMed] [Google Scholar]
- 25.Furuta M, Tanaka H, Shiraishi Y, Unida T, Imamura M, Fujimoto A, Fujita M, Sasaki-Oku A, Maejima K, Nakano K, Kawakami Y, Arihiro K, Aikata H, Ueno M, Hayami S, Ariizumi SI, Yamamoto M, Gotoh K, Ohdan H, Yamaue H, Miyano S, Chayama K, Nakagawa H. Characterization of HBV integration patterns and timing in liver cancer and HBV-infected livers. Oncotarget. 2018;9:25075–25088. doi: 10.18632/oncotarget.25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatol. 2018;69:461–511. doi: 10.1016/j.jhep.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 27.EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. European Association for the Study of the Liver. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 28.Cabibbo G, Enea M, Attanasio M, Bruix J, Craxi A, Camma C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010;51:1274–1283. doi: 10.1002/hep.23485. [DOI] [PubMed] [Google Scholar]
- 29.Staikou C, Stavroulakis E, Karmaniolou I. A narrative review of peri-operative management of patients with thalassaemia. Anaesthesia. 2014;69:494–510. doi: 10.1111/anae.12591. [DOI] [PubMed] [Google Scholar]
- 30.Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61–74. doi: 10.1055/s-0030-1247133. [DOI] [PubMed] [Google Scholar]
- 31.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc J-F, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 32.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 33.Burrel M, Reig M, Forner A, Barrufet M, de Lope CR, Tremosini S, Ayuso C, Llovet JM, Real MI, Bruix J. Survival of patients with hepatocellular carcinoma treated by transarterialchemoembolisation (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design. J Hepatol. 2012;56:1330–1335. doi: 10.1016/j.jhep.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]