Abstract
In the last few years, single-cell profiling of taste cells and ganglion cells has advanced our understanding of transduction, encoding, and transmission of information from taste buds as relayed to the central nervous system. This review focuses on new knowledge from these molecular approaches and attempts to place this in the context of previous questions and findings in the field. The individual taste cells within a taste bud are molecularly specialized for detection of one of the primary taste qualities: salt, sour, sweet, umami, and bitter. Transduction and transmitter release mechanisms differ substantially for taste cells transducing sour (Type III cells) compared with those transducing the qualities of sweet, umami, or bitter (Type II cells), although ultimately all transmission of taste relies on activation of purinergic P2X receptors on the afferent nerves. The ganglion cells providing innervation to the taste buds also appear divisible into functional and molecular subtypes, and each ganglion cell is primarily but not exclusively responsive to one taste quality.
Keywords: taste bud, transduction, geniculate ganglion, purinergic transmission, serotonin, taste, signalling, nervous system
This review will focus on progress in the last few years in understanding transduction, coding, and specificity of the peripheral gustatory system starting with the cellular components of taste buds. Taste buds comprise 50 to 100 taste cells, which are specialized epithelial cells including signal transducing cells and glial-like supporting cells. The taste cells respond to taste substances in the saliva to generate a biological signal that then must be transmitted to the taste sensory nerves conveying the message to the nucleus of the solitary tract in the brain stem. This review will focus on recent discoveries in the processes of taste transduction and transmission, which significantly impact our understanding of this system.
In mammals, the sense of taste detects a wide variety of compounds which canonically fall into only five main sensory qualities (or modalities): sweet, umami (savory), bitter, sour, and salty. Each taste cell is most responsive to a single taste quality 1, and each taste bud contains one or more cells capable of responding to each of the taste qualities; that is, taste cells are “chemically tuned” whereas taste buds are not.
Methodological advances
Two relatively new methodologies have been put to good effect in analyzing taste buds and their associated ganglia. Functional imaging of ganglion cells expressing genetically encoded activity sensors has allowed direct analysis of the breadth of tuning of inputs to the central nervous system (CNS) 2, 3, whereas single-cell transcriptomics has permitted molecular and functional classification of both taste cells and ganglion cells 4– 8. The implications of these and other recent studies are described below and summarized in Table 1.
Table 1. Reported molecular characteristics of taste cells and ganglion cells.
| Taste
quality |
Sweet | Umami | Bitter | Sour | Salty |
|---|---|---|---|---|---|
| Ganglion
cells |
Spon1 | Cdh4? a | Cdh13 ? a | Penk, Htr3a | Egr2? a |
| Taste cells | Sema7A | Sema3A |
Pkd2L1,
OTOP1 |
Data are from 6, 8– 11. aThe meta-analysis by Anderson and Larson 11 does not support Cdh4, Cdh13, and Egr2 as marking unique clusters. Cdh13 marked two clusters that also express Olfm3. Olfm3 is associated with several ganglion cell clusters but is never associated with Penk and so may mark cells innervating Type II cells 4 but not a particular subset of Type II cells. Factors in question are indicated by “?”.
Transduction
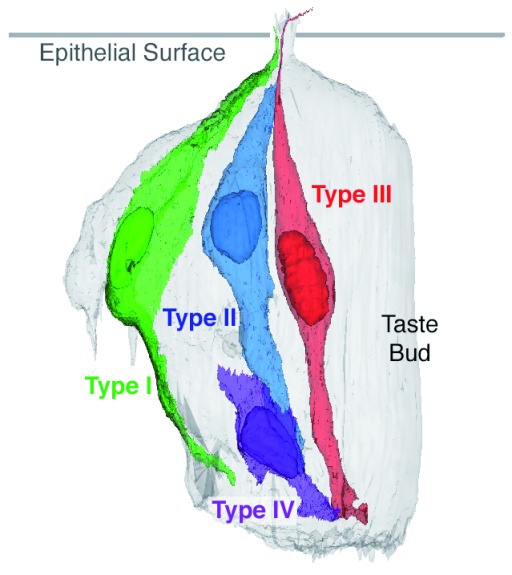
The taste cells are divisible into four types characterized by both morphological and molecular features and given the names Type I, Type II, Type III and Type IV ( Figure 1). Type I cells are similar in many ways to astrocytes and Type IV cells are immature cells, whereas Type II and Type III cells serve as the transducing elements for different taste qualities.
Figure 1. Cell types in taste buds.
Four different morphological and molecularly distinct types of cells populate taste buds. Types II and III transduce different classes of tastes, whereas Type I cells are more glial-like. Type IV cells are the immature population, which develop into the other cell types over the span of a few days. Figure generated from data in 12.
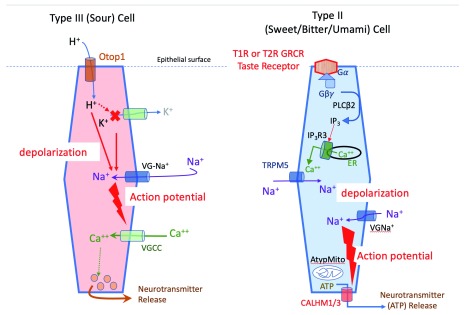
Type II cells use G protein–coupled receptors for sweet (T1R2 + T1R3), umami (T1R1 + T1R3), or bitter (T2Rs) to initiate a transduction cascade, whereas Type III cells rely on ion channels for transduction of the ionic tastes of salty and sour. The receptors and downstream signaling cascade for the Type II cells (sweet, umami, or bitter) have been well described since the early part of this century 13 and involve a phospholipase C (PLC)-mediated cascade culminating in the activation of the Ca ++-responsive channels TRPM5 and TRPM4 14 to depolarize the cell sufficiently to generate an action potential via voltage-gated Na + channels (SCN2A, SCN3A, and SCN9A 15). Why axonless receptor cells should generate action potentials is of interest and is likely related to the release mechanism for neurotransmitter from Type II taste cells as described below.
Whereas early studies suggested that a single sweet taste receptor (T1R2 + T2R3) mediates all responses to sugars and sweeteners 16, recent studies suggest that other mechanisms also play a role for glucose-containing sugars but not for artificial sweeteners. Glucose transporters and the K ATP channel, which are expressed in sweet-responsive (T1R3-expressing) taste cells 17, are involved in cephalic phase insulin release independent of the neural signal for sweet transmitted to the nervous system 18. The exact mechanism by which activation of the taste cells evokes insulin release is unclear but may involve humoral rather than neural signals.
Sour
In 2006, Huang et al. 19 showed that sour detection depended on cells expressing PKD2L1—cells subsequently identified as a subset of Type III cells 20. In 2011, Horio et al. showed that PKD2L1 itself was not necessary for transduction of protons 21. Rather, transduction of sour involves permeation of H + through an apical ion channel 22 subsequently identified as OTOP1 6. Using PKD2L1 as a molecular identifier for sour-responsive taste cells, Liman and co-workers 7 and Zhang et al. 8 went on to confirm OTOP1 as the necessary transduction channel underlying sour taste. The entering H + ions not only directly depolarize the taste cells but also block Kir2.1 K + channels 23, thereby amplifying the depolarization of the entering H + ions. The resulting depolarization triggers voltage-gated Na + channels (SCN2A 15) to generate action potentials that activate voltage-gated Ca ++ channels triggering the release of synaptic vesicles 24.
In keeping with the PKD2L1 cells being the sour-transducing cells, optogenetic driving of these cells evokes an aversive response 25. Curiously, another study 26 reported that optogenetic driving of the PKD2L1 population drives drinking behavior in thirsty mice. Why the mice should respond with drinking to a sensation of sour is still unresolved, although Zocchi et al. 26 suggest that a subset of PKD2L1-expressing cells may convey a specific “water taste” as is known for insects 27– 29.
Salt
Historically, responsiveness to salt has been separated into amiloride-sensitive (AS) and amiloride-insensitive (AI) modalities 30. Confounding our understanding of salt taste is that low concentrations of salt are appetitive whereas high concentrations are aversive. Furthermore, while Na + is important perceptually for salt, other substances, not containing Na +, also are salty-tasting. The multiple perceptual and chemical properties suggest that more than one transduction mechanism may be involved. Supporting this concept are the results from 31, 32. These studies argue that the molecular correlate of AS salt, the epithelial sodium channel (ENaC), underlies the appetitive qualities of Na + but that AI salt detection of high concentrations of Na + relies on a subpopulation of the bitter-responsive Type II cells and a subset of the sour-responsive Type III cells 5, 32, 33. Furthermore, a recent study 34 suggests that the AI salt transduction mechanism may directly involve Cl −, but the actual mechanism remains elusive since known Cl − channel and transporter blockers have no effect on salt taste. Two recent works confirm a previous study 35 suggesting that AS-responsive taste cells fall into a unique class of taste cells not identified by the canonical reporters (TRPM5 for Type II and CAR4 for Type III 32 or PIRT for Types II and III 34). Further confounding the interpretation of these studies on Na + transduction is the finding that, compared with rodents, humans—who enjoy low salt and avoid high salt—do not have a large AS component of salt taste 36, although chimpanzees do have a minor AS component 37.
Peripheral neurotransmission
Whatever means are used for transduction, the Type II and Type III cells ultimately must release one or more neurotransmitters to activate the afferent nerves. Activity-dependent release of ATP from Type II cells 38, 39 and 5-HT from Type III cells 40 was clearly demonstrated by several investigators, but whether other transmitters (for example, glutamate or acetylcholine) also may be involved remains unclear. Although ATP acting on neural P2X receptors was identified as a crucial transmitter for all tastes over a decade ago 41, only recently has the contribution of 5-HT acting on neural 5-HT 3 receptors been elucidated in terms of transmission of sour taste 42. In addition, taste cells may directly release peptides such as glucagon-like peptide 1 (GLP-1) 43, 44. These additional transmitters may act to modulate adjacent taste cells 45 and activate afferent nerve fibers.
Although both Type II and Type III cells require action potentials for transmitter release, the mechanisms of release for these two cell types are quite different ( Figure 2). Type III cells use a conventional synapse involving voltage-gated Ca ++ channels and SNARE mechanisms to effect release of synaptic vesicles 24, 46, 47. In contrast, Type II taste cells (transducing bitter, sweet, or umami) rely on action potentials to trigger the voltage-gated large-pore channel CALHM1 to release ATP 48– 50. The pore size of this channel is sufficient to permit passage of ATP which serves as an obligatory transmitter in this system 41, 51, 52. The biophysical properties of CALHM1 as described in the seminal article on this channel 48 did not exactly match the properties of the release channel in taste buds. A more recent report 49 showed that the channel in taste buds consists of two subunits, CALHM1 and CALHM3, which together form a channel matching the properties of the Type II cell release channel.
Figure 2. Taste transduction cascades.
Transduction pathways for the two different types of taste-transducing cells: Type III for sour and Type II for sweet, bitter, or umami. The different responsiveness of Type II cells is dictated by the type of receptor each cell expresses, not by downstream members of the transduction cascade. AtypMito, atypical mitochondria; ER, endoplasmic reticulum; Gα, alpha subunit of G protein; Gβγ, beta-gamma subunits of G protein; IP 3, inositol trisphosphate; IP 3R3, inositol trisphosphate receptor isoform 3; PLCβ2, phospholipase C isoform β2; TRPM5, transient receptor potential cation channel subfamily M member 5; VGCC, voltage-gated calcium channel; VG-Na +, voltage-gated sodium channel.
Whereas the mechanism of release for ATP is well established for Type II cells, the source of ATP for transmission of sour information from Type III cells remains enigmatic. No one has yet demonstrated direct release of ATP from Type III cells, yet transmission of Type III cell taste qualities (for example, sour) is dependent on intact purinergic signaling to P2X receptors 41. Type III cells do not express CALHM1 48 and so do not use CALHM1/3-mediated release. Another possible means of release of ATP is via synaptic vesicles, but Type III cells are reported to lack the vesicular ATP-transporter, VNUT 53, presumed to be necessary for loading of synaptic vesicles with ATP. So, what other possible sources exist for release of ATP? One suggestion is that Type III cells may trigger ATP release via interaction with other taste cell types. If so, this interaction does not require participation of Type II cells since mice lacking Type II cells (Skn1A-KO) 54 and CALHM1 KO 48 mice show nearly normal responses to sour. Hence, the transmission of sour information to taste nerves does not require the presence of Type II taste cells nor the function of CALHM1 to release ATP. Furthermore, a recent study showing high-resolution reconstructions of taste buds shows that Type III cells seldom directly contact Type II cells since processes from Type I cells intervene 12, suggesting that any interactions between Type II and Type III cells may be indirect.
Tuning specificity of taste cells and nerve fibers
One of the classic discussions in taste research concerns the specificity of chemical tuning in the peripheral taste system, that is, whether individual taste cells or nerve fibers respond to single classes of taste stimuli or respond more broadly across several modalities. The former, known as “labeled line” coding, suggests that taste information (for example, for sweet taste) is transduced by a unique subset of taste cells and transmitted over dedicated nerve fibers that signal that particular quality (sweet) to the brain. Conversely, a broadly tuned peripheral system, utilizing “cross-fiber pattern” coding, relies on nerve fibers that are more broadly responsive but that respond maximally to particular qualities, necessitating comparison of activity across the fiber population in order for the brain to extract quality information. Receptor expression data strongly indicate that taste receptor cells largely express receptors for a single taste quality and this suggests that labeled line coding is plausible 55.
So, the question then is how specifically the taste cells activate particular nerve fibers. Two articles published in 2015 address this question but arrive at somewhat different conclusions, although detailed comparison of the data in the two studies shows similar results. In both works, the investigators used optical recording methods to assess activity in geniculate ganglion cells that innervate taste buds in fungiform papillae on the front of the tongue. Barretto et al. 2, using relatively low concentrations of taste stimuli, report that taste ganglion cells largely respond to a single stimulus class, although many multiply responsive cells are evident in the data. Conversely, Wu et al. 3 found that ganglion cell specificity is dominant only for low concentrations of tastants. As concentrations increase, more and more ganglion cells are recruited so that the majority of cells will respond to more than one class of tastant at moderate - high stimulus levels. This finding tends to support a cross-fiber model of decoding afferent input, particularly at higher stimulus concentrations. It should be noted that loss of specificity at high stimulus levels is similar to encoding in other sensory modalities; for example, a dim blue light of 505 nm will selectively activate the blue-sensitive, short-wavelength cones in the retina. But when the same wavelength of light is more intense, it will also activate the green, medium-wavelength cones and ultimately the red, long-wavelength cones. So absolute color cannot be determined by reading the output of only one class of cones but rather requires comparisons of activity levels across the different types of cones. Similarly, taste quality identification may require comparison of activity levels across incoming lines of information which preferentially, but not absolutely, encode particular qualities. In other words, while the afferent input is “tuned” as would be necessary for a labeled line system, absolute quality information can be extracted only by comparing the pattern and level of activity across fiber classes.
Another requirement for labeled line coding is connectional specificity between taste cells and nerve fibers; that is, sweet receptor-expressing taste cells should connect to nerve fibers receiving synaptic input only from other sweet receptor cells and so forth. Such specificity has been suggested recently for both Type III cells and Type II cells. Taste nerve fibers highly expressing the serotonin receptor 5-HT 3 show preferential connectivity with Type III taste cells (sour) that accumulate and release serotonin 9. Argument for Type II cell connectional specification is based on transcriptome profiling of geniculate ganglion cells. Like dorsal root ganglion cells 56, geniculate ganglion cells fall into distinct molecular classes 4. Those ganglion cells that innervate taste buds express Phox2b, whereas those providing somatosensory innervation to the ear do not 57, so this transcription factor can be used to identify ganglion cells that innervate taste buds. Lee et al. 10 showed that connectional specificity for Type II cells is maintained by molecular encoding of receptor cell and nerve fiber identities by SEMA family guidance molecules. Likewise, Zhang et al. 8 found that genetic deletion of particular gene products correlates with loss of behavioral response to particular tastants— Cdh4 for umami, Cdh13 for bitter, and Egr2 for salty—suggesting that these factors may serve to identify particular classes of gustatory ganglion cells (see Table 1). Since these proteins are expressed widely in the CNS (including in taste-processing areas), it is unclear whether the reported behavioral changes are attributable to changes in ganglion cell functionality or changes higher in the neuraxis. Furthermore, a more recent meta-analysis of these and other transcriptome data on ganglion cell subclasses fails to support the segregation of geniculate ganglion cell subtypes according to expression of these cadherins 11. Whether this all equates to absolute functional specificity of the taste neurons remains open to question. Substantial evidence exists for the possibility of cell-to-cell communication in taste buds 1, 58, and side-band activity in the taste nerves might result from such interactions between taste cells rather than direct convergence of input onto single fibers.
Another interesting outcome of the molecular profiling of the geniculate ganglion neurons innervating taste buds is the identification of a population expressing Piezo2, a marker of touch-sensitive ganglion cells 4. Such touch sensitivity of a subpopulation of gustatory ganglion cells would explain the residual tactile responsiveness of the gustatory nerves following genetic deletion of P2X receptors necessary for transmission from taste cells to nerve fibers 41. Thus, the nerve fibers themselves may be touch-sensitive and require no activation from the taste buds for tactile activation.
Is “fatty” a taste?
A classic question in the field of taste research is “How many primary tastes are there?” In the 20th century, the debate focused on whether umami (savory) was a primary taste distinct from the classic qualities of salty, sour, sweet, and bitter. The discovery of a distinct molecular receptor for glutamate, coupled with the identification of glutamate (umami)-specific fibers in gustatory nerves and behavioral experiments showing the discriminability of glutamate from other tastes, clinched the case that umami is indeed a distinct primary taste. In the last few decades, the question of whether the taste of fat is a primary taste quality has been debated 59. Potential fat receptors GPR120 and CD36 were identified molecularly in taste buds about a decade ago 60, 61. But the mere presence of a receptor does not necessarily equate to the existence of a separate coding channel as would be expected of a primary taste quality. This year, Ninomiya et al. 62 identified, for the first time, a population of nerve fibers (F-fibers) in the chorda tympani nerve that respond uniquely to a fatty acid, linoleic acid, lending further credence to the idea that fat is a unique taste quality. Complicating the situation is that some of the F-fibers also show responses to glutamate, and although mice can be trained to recognize the taste of linoleic acid, they confound it with glutamate (see also 63), suggesting that fatty taste sensations may be intermingled with those for umami. Thus, the question remains as to whether fatty acid taste is a distinct primary taste or more a modulator of other taste qualities.
Remaining unanswered questions
What mechanisms and cells underlie transduction of AI salt taste, and what cells are responsible for AS salt taste?
What is the source of ATP required for transmission of information from Type III (sour) cells to the afferent nerves?
How much intercellular communication occurs within taste buds, and how does this affect the nature of the signal transmitted to the nerve fibers?
Is fatty a primary taste quality? And how many other unrecognized primary taste qualities may exist?
Acknowledgments
The authors thank Brigit High and Eric Larson (Univ. Colorado Sch. Medicine) for comments on this work, and Yannick K. Dzowo (Univ. Colorado Sch. Medicine) for assistance with preparing Figure 1.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Stephen Roper, Department of Physiology & Biophysics and Department of Otolaryngology, University of Miami Miller School of Medicine, Miami, FL, 33136, USA
Susan Travers, Division of Biosciences, College of Dentistry, Ohio State University, Columbus, OH, USA
Funding Statement
This work was supported in part by grants from the National Institute on Deafness and Other Communication Disorders: 1R01DC012931 and 1R01DC014728 (TEF), 1R01DC017679 and 1RO1DC012555 (SCK).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 2 approved]
References
- 1. Roper SD: Signal transduction and information processing in mammalian taste buds. Pflugers Arch. 2007;454(5):759–76. 10.1007/s00424-007-0247-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barretto RPJ, Gillis-Smith S, Chandrashekar J, et al. : The neural representation of taste quality at the periphery. Nature. 2015;517(7534):373–6. 10.1038/nature13873 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 3. Wu A, Dvoryanchikov G, Pereira E, et al. : Breadth of tuning in taste afferent neurons varies with stimulus strength. Nat Commun. 2015;6:8171. 10.1038/ncomms9171 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 4. Dvoryanchikov G, Hernandez D, Roebber JK, et al. : Transcriptomes and neurotransmitter profiles of classes of gustatory and somatosensory neurons in the geniculate ganglion. Nat Commun. 2017;8(1):760. 10.1038/s41467-017-01095-1 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 5. Sukumaran SK, Lewandowski BC, Qin Y, et al. : Whole transcriptome profiling of taste bud cells. Sci Rep. 2017;7(1):7595. 10.1038/s41598-017-07746-z [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 6. Tu YH, Cooper AJ, Teng B, et al. : An evolutionarily conserved gene family encodes proton-selective ion channels. Science. 2018;359(6379):1047–50. 10.1126/science.aao3264 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 7. Teng B, Wilson CE, Tu YH, et al. : Cellular and Neural Responses to Sour Stimuli Require the Proton Channel Otop1. Curr Biol. 2019;29(21):3647–3656.e5. 10.1016/j.cub.2019.08.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang J, Jin H, Zhang W, et al. : Sour Sensing from the Tongue to the Brain. Cell. 2019;179(2):392–402.e15. 10.1016/j.cell.2019.08.031 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 9. Stratford JM, Larson ED, Yang R, et al. : 5-HT 3A -driven green fluorescent protein delineates gustatory fibers innervating sour-responsive taste cells: A labeled line for sour taste? J Comp Neurol. 2017;525(10):2358–75. 10.1002/cne.24209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee H, Macpherson LJ, Parada CA, et al. : Rewiring the taste system. Nature. 2017;548(7667):330–3. 10.1038/nature23299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anderson CB, Larson ED: Single Cell Transcriptional Profiling of Phox2b-Expressing Geniculate Ganglion Neurons.bioRxiv,2019;812578 10.1101/812578 [DOI] [Google Scholar]
- 12. Yang R, Dzowo YK, Wilson CE, et al. : Three-dimensional reconstructions of mouse circumvallate taste buds using serial blockface scanning electron microscopy: I. Cell types and the apical region of the taste bud. J Comp Neurol. 2019;25:205. 10.1002/cne.24779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang Y, Hoon MA, Chandrashekar J, et al. : Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112(3):293–301. 10.1016/s0092-8674(03)00071-0 [DOI] [PubMed] [Google Scholar]
- 14. Dutta Banik D, Martin LE, Freichel M, et al. : TRPM4 and TRPM5 are both required for normal signaling in taste receptor cells. Proc Natl Acad Sci U S A. 2018;115(4):E772–E781. 10.1073/pnas.1718802115 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 15. Gao N, Lu M, Echeverri F, et al. : Voltage-gated sodium channels in taste bud cells. BMC Neurosci. 2009;10:20. 10.1186/1471-2202-10-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nelson G, Hoon MA, Chandrashekar J, et al. : Mammalian sweet taste receptors. Cell. 2001;106(3):381–390. 10.1016/s0092-8674(01)00451-2 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 17. Yee KK, Sukumaran SK, Kotha R, et al. : Glucose transporters and ATP-gated K+ (KATP) metabolic sensors are present in type 1 taste receptor 3 (T1r3)-expressing taste cells. Proc Natl Acad Sci U S A. 2011;108(13):5431–6. 10.1073/pnas.1100495108 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Glendinning JI, Frim YG, Hochman A, et al. : Glucose elicits cephalic-phase insulin release in mice by activating K ATP channels in taste cells. Am J Physiol Regul Integr Comp Physiol. 2017;312(4):R597–R610. 10.1152/ajpregu.00433.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 19. Huang AL, Chen X, Hoon MA, et al. : The cells and logic for mammalian sour taste detection. Nature. 2006;442(7105):934–8. 10.1038/nature05084 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Kataoka S, Yang R, Ishimaru Y, et al. : The candidate sour taste receptor, PKD2L1, is expressed by type III taste cells in the mouse. Chem Senses. 2008;33(3):243–54. 10.1093/chemse/bjm083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Horio N, Yoshida R, Yasumatsu K, et al. : Sour taste responses in mice lacking PKD channels. PLoS One. 2011;6(5):e20007. 10.1371/journal.pone.0020007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chang RB, Waters H, Liman ER: A proton current drives action potentials in genetically identified sour taste cells. Proc Natl Acad Sci U S A. 2010;107(51):22320–5. 10.1073/pnas.1013664107 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 23. Ye W, Chang RB, Bushman JD, et al. : The K + channel K IR2.1 functions in tandem with proton influx to mediate sour taste transduction. Proc Natl Acad Sci U S A. 2016;113(2):E229–E238. 10.1073/pnas.1514282112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vandenbeuch A, Zorec R, Kinnamon SC: Capacitance measurements of regulated exocytosis in mouse taste cells. J Neurosci. 2010;30(44):14695–701. 10.1523/JNEUROSCI.1570-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wilson CE, Vandenbeuch A, Kinnamon SC: Physiological and Behavioral Responses to Optogenetic Stimulation of PKD2L1 + Type III Taste Cells. eNeuro. 2019;6(2):pii: ENEURO.0107–19.2019. 10.1523/ENEURO.0107-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zocchi D, Wennemuth G, Oka Y: The cellular mechanism for water detection in the mammalian taste system. Nat Neurosci. 2017;20(7):927–33. 10.1038/nn.4575 [DOI] [PubMed] [Google Scholar]
- 27. Cameron P, Hiroi M, Ngai J, et al. : The molecular basis for water taste in Drosophila. Nature. 2010;465(7294):91–5. 10.1038/nature09011 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 28. Chen Z, Wang Q, Wang Z: The amiloride-sensitive epithelial Na + channel PPK28 is essential for drosophila gustatory water reception. J. Neurosci. 2010;30(18):6247–52. 10.1523/JNEUROSCI.0627-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matthews BJ, Younger MA, Vosshall LB: The ion channel ppk301 controls freshwater egg-laying in the mosquito Aedes aegypti. eLife. 2019;8:pii: e43963. 10.7554/eLife.43963 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Heck GL, Mierson S, DeSimone JA: Salt taste transduction occurs through an amiloride-sensitive sodium transport pathway. Science. 1984;223(4634):403–5. 10.1126/science.6691151 [DOI] [PubMed] [Google Scholar]
- 31. Chandrashekar J, Hoon MA, Ryba NJ, et al. : The receptors and cells for mammalian taste. Nature. 2006;444(7117):288–94. 10.1038/nature05401 [DOI] [PubMed] [Google Scholar]
- 32. Chandrashekar J, Kuhn C, Oka Y, et al. : The cells and peripheral representation of sodium taste in mice. Nature. 2010;464(7286):297–301. 10.1038/nature08783 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. Lewandowski BC, Sukumaran SK, Margolskee RF, et al. : Amiloride-Insensitive Salt Taste Is Mediated by Two Populations of Type III Taste Cells with Distinct Transduction Mechanisms. J Neurosci. 2016;36(6):1942–53. 10.1523/JNEUROSCI.2947-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Roebber JK, Roper SD, Chaudhari N: The Role of the Anion in Salt (NaCl) Detection by Mouse Taste Buds. J Neurosci. 2019;39(32):6224–32. 10.1523/JNEUROSCI.2367-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Vandenbeuch A, Clapp TR, Kinnamon SC: Amiloride-sensitive channels in type I fungiform taste cells in mouse. BMC Neurosci. 2008;9:1. 10.1186/1471-2202-9-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Desor JA, Finn J: Effects of amiloride on salt taste in humans. Chem Senses. 1989;14(6):793–803. 10.1093/chemse/14.6.793 [DOI] [Google Scholar]
- 37. Hellekant G, Ninomiya Y, Danilova V: Taste in chimpanzees II: single chorda tympani fibers. Physiol Behav. 1997;61(6):829–41. 10.1016/s0031-9384(96)00562-8 [DOI] [PubMed] [Google Scholar]
- 38. Huang YJ, Maruyama Y, Dvoryanchikov G, et al. : The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds Proc Natl Acad Sci U S A. 2007;104(15):6436–41. 10.1073/pnas.0611280104 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. Murata Y, Yasuo T, Yoshida R, et al. : Action potential-enhanced ATP release from taste cells through hemichannels. J Neurophysiol. 2010;104(2):896–901. 10.1152/jn.00414.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 40. Huang YJ, Maruyama Y, Lu KS, et al. : Mouse taste buds use serotonin as a neurotransmitter. J Neurosci. 2005;25(4):843–7. 10.1523/JNEUROSCI.4446-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 41. Finger TE, Danilova V, Barrows J, et al. : ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310(5753):1495–9. 10.1126/science.1118435 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Larson ED, Vandenbeuch A, Voigt A, et al. : The Role of 5-HT 3 Receptors in Signaling from Taste Buds to Nerves. J Neurosci. 2015;35(48):15984–95. 10.1523/JNEUROSCI.1868-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Shin YK, Martin B, Golden E, et al. : Modulation of taste sensitivity by GLP-1 signaling. J Neurochem. 2008;106(1):455–63. 10.1111/j.1471-4159.2008.05397.x [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 44. Takai S, Yasumatsu K, Inoue M, et al. : Glucagon-like peptide-1 is specifically involved in sweet taste transmission. FASEB J. 2015;29(6):2268–80. 10.1096/fj.14-265355 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Huang YA, Dando R, Roper SD: Autocrine and paracrine roles for ATP and serotonin in mouse taste buds. J Neurosci. 2009;29(44):13909–18. 10.1523/JNEUROSCI.2351-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yang R, Crowley HH, Rock ME, et al. : Taste cells with synapses in rat circumvallate papillae display SNAP-25-like immunoreactivity. J Comp Neurol. 2000;424(2):205–15. [DOI] [PubMed] [Google Scholar]
- 47. DeFazio RA, Dvoryanchikov G, Maruyama Y, et al. : Separate populations of receptor cells and presynaptic cells in mouse taste buds. J Neurosci. 2006;26(15):3971–80. 10.1523/JNEUROSCI.0515-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Taruno A, Vingtdeux V, Ohmoto M, et al. : CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature. 2013;495(7440):223–6. 10.1038/nature11906 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Ma Z, Taruno A, Ohmoto M, et al. : CALHM3 Is Essential for Rapid Ion Channel-Mediated Purinergic Neurotransmission of GPCR-Mediated Tastes. Neuron. 2018;98(3):547–561.e10. 10.1016/j.neuron.2018.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 50. Romanov RA, Lasher RS, High B, et al. : Chemical synapses without synaptic vesicles: Purinergic neurotransmission through a CALHM1 channel-mitochondrial signaling complex. Sci Signal. 2018;11(529):pii: eaao1815. 10.1126/scisignal.aao1815 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 51. Kinnamon SC, Finger TE: A taste for ATP: neurotransmission in taste buds. Front Cell Neurosci. 2013;7:264. 10.3389/fncel.2013.00264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vandenbeuch A, Anderson CB, Ford AP, et al. : A selective P2X3, P2X2/3 receptor antagonist abolishes responses to all taste stimuli in mice. Chem Senses. 2013;38:86. [Google Scholar]
- 53. Iwatsuki K, Ichikawa R, Hiasa M, et al. : Identification of the vesicular nucleotide transporter (VNUT) in taste cells. Biochem Biophys Res Commun. 2009;388(1):1–5. 10.1016/j.bbrc.2009.07.069 [DOI] [PubMed] [Google Scholar]
- 54. Matsumoto I, Ohmoto M, Narukawa M, et al. : Skn-1a (Pou2f3) specifies taste receptor cell lineage. Nat Neurosci. 2011;14(6):685–7. 10.1038/nn.2820 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 55. Yarmolinsky DA, Zuker CS, Ryba NJ: Common sense about taste: from mammals to insects. Cell. 2009;139(2):234–44. 10.1016/j.cell.2009.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Usoskin D, Furlan A, Islam S, et al. : Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci. 2015;18(1):145–53. 10.1038/nn.3881 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. Ohman-Gault L, Huang T, Krimm R: The transcription factor Phox2b distinguishes between oral and non-oral sensory neurons in the geniculate ganglion. J Comp Neurol. 2017;525(18):3935–50. 10.1002/cne.24312 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. Roper SD: Taste buds as peripheral chemosensory processors. Semin Cell Dev Biol. 2013;24(1):71–9. 10.1016/j.semcdb.2012.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Besnard P, Passilly-Degrace P, Khan NA: Taste of Fat: A Sixth Taste Modality? Physiol Rev. 2016;96(1):151–76. 10.1152/physrev.00002.2015 [DOI] [PubMed] [Google Scholar]
- 60. Matsumura S, Mizushige T, Yoneda T, et al. : GPR expression in the rat taste bud relating to fatty acid sensing. Biomed Res. 2007;28(1):49–55. 10.2220/biomedres.28.49 [DOI] [PubMed] [Google Scholar]
- 61. Gaillard D, Laugerette F, Darcel N, et al. : The gustatory pathway is involved in CD36-mediated orosensory perception of long-chain fatty acids in the mouse. FASEB J. 2008;22(5):1458–68. 10.1096/fj.07-8415com [DOI] [PubMed] [Google Scholar]
- 62. Yasumatsu K, Iwata S, Inoue M, et al. : Fatty acid taste quality information via GPR120 in the anterior tongue of mice. Acta Physiol (Oxf). 2019;226(1):e13215. 10.1111/apha.13215 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 63. Stratford JM, Curtis KS, Contreras RJ: Linoleic acid increases chorda tympani nerve responses to and behavioral preferences for monosodium glutamate by male and female rats. Am J Physiol Regul Integr Comp Physiol. 2008;295(3):R764–R772. 10.1152/ajpregu.00916.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]