Abstract
Silibinin exhibits antidiabetic potential by preserving the mass and function of pancreatic β-cells through up-regulation of estrogen receptor-α (ERα) expression. However, the underlying protective mechanism of silibinin in pancreatic β-cells is still unclear. In the current study, we sought to determine whether ERα acts as the target of silibinin for the modulation of antioxidative response in pancreatic β-cells under high glucose and high fat conditions. Our in vivo study revealed that a 4-week oral administration of silibinin (100 mg/kg/day) decreased fasting blood glucose with a concurrent increase in levels of serum insulin in high-fat diet/streptozotocin-induced type 2 diabetic rats. Moreover, expression of ERα, NF-E2-related factor 2 (Nrf2), and heme oxygenase-1 (HO-1) in pancreatic β-cells in pancreatic islets was increased by silibinin treatment. Accordingly, silibinin (10 μM) elevated viability, insulin biosynthesis, and insulin secretion of high glucose/palmitate-treated INS-1 cells accompanied by increased expression of ERα, Nrf2, and HO-1 as well as decreased reactive oxygen species production in vitro. Treatment using an ERα antagonist (MPP) in INS-1 cells or silencing ERα expression in INS-1 and NIT-1 cells with siRNA abolished the protective effects of silibinin. Our study suggests that silibinin activates the Nrf2-antioxidative pathways in pancreatic β-cells through regulation of ERα expression.
Keywords: Diabetes mellitus, Silibinin, Pancreatic β-cell, Estrogen receptor-α, Antioxidative response
INTRODUCTION
Diabetes mellitus, diagnosed by observing elevated levels of blood glucose, is globally approaching epidemic proportions (Mackenbach et al., 2018; Yang and Kang, 2018). Inadequate production of insulin caused by loss of pancreatic β-cell mass and function is central to the development of diabetes (Kawamori, 2017; Chu et al., 2018). As the predominant form of diabetes, type 2 diabetes mellitus (T2DM) accounts for around 90% of diabetic patients globally (International Diabetes Federation, 2017). The dysregulation of glucose homeostasis in T2DM is associated with body fat accumulation (Shulman, 2014). Increased intracellular reactive oxygen species (ROS) have been reported to be involved in glucose-induced glucotoxicity and fatty acid-induced lipotoxicity in insulin-producing pancreatic β-cells (Robertson, 2006; Lee et al., 2013). The ROS produced could be scavenged by endogenous antioxidative enzymes such as heme oxygenase-1 (HO-1) and superoxide dismutase 2 (SOD2), while the imbalance between antioxidative defense and ROS production leads to oxidative stress. The transcription of HO-1 and SOD2 genes are regulated by the transcription factor NF-E2-related factor 2 (Nrf2), which acts as a master regulator of the protective response to oxidative stress (Rochette et al., 2014; Gerber and Rutter, 2017; Khan et al., 2017).
Polyphenols are widely distributed in plants and are commonly referred to as antioxidants (Morillas-Ruiz et al., 2006). A variety of polyphenols such as curcumin, resveratrol, quercetin, epigallocatechin-3-gallate, caffeic acid, hydroxytyrosol, genistein, lycopene, and ellagic acid exert antioxidative activities through targeting Nrf2 and consequently activating the antioxidant response element-related cytoprotective genes (Nabavi et al., 2016). Silibinin, a major polyphenolic compound extracted from milk thistle (Silibum marianum), has been widely used for the treatment of gallbladder and hepatic diseases via its antioxidative and hepatoprotective properties (Zou et al., 2017). Further studies revealed that silibinin exerted strong antidiabetic effects by increasing the viability and improving the function of pancreatic β-cells (Wang et al., 2012; Chen et al., 2014). Silibinin was shown to increase the functional insulin-producing β-cell mass through the activation of estrogen receptor-α (ERα) in our recent study (Yang et al., 2018). ROS production in pancreatic β-cells can be induced by hyperglycemia during the pathogenesis of type 2 diabetes, and the excessive concentrations of ROS cause pancreatic β-cell dysfunction and impair insulin action (Sone and Kagawa, 2005; Robertson, 2006). The activation of ERα has been shown to regulate the antioxidative response in breast cancer cells by activating Nrf2 (Wu et al., 2014), but the relationship between ERα and Nrf2 in pancreatic β-cells is still unclear. In the current study, whether silibinin protects pancreatic β-cells against oxidative damage under high-glucose and high-fat conditions was determined, and the underlying mechanisms involving the regulation of ERα and the Nrf2-antioxidative pathway were explored.
MATERIALS AND METHODS
Chemicals and reagents
Silibinin (purity ≥99%) was purchased from Jurong Best Medicine Material (Zhenjiang, Jiangsu, China). Streptozotocin (STZ, purity≥98%), Metformin (purity≥98%), 3-(4,5-dimethyl-2-thiazolyl)-2, 5-diphenyltetrazolium bromide (MTT), ERα-specific agonist 1,3,5-tris (4-hydroxyphenyl)-4-propyl-1H-pyrazole (PPT), and ERα-specific antagonist 1,3-bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole-dihydro-chloride (MPP) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Mouse anti-insulin and rabbit anti-ERα primary antibodies as well as Rhodamine-, FITC-, and HRP-conjugated secondary antibodies were purchased from Proteintech Group (Chicago, IL, USA). Rabbit antibodies against Nrf2, HO-1, SOD2 and β-actin were obtained from Wanlei Life Science (Shenyang, China). Mouse anti-8-OHdG antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The enzyme-linked immunosorbent assay (ELISA) kit for detecting rat insulin was purchased from Shanghai Huayi Biological Technology (Shanghai, China). CMC-Na, palmitate, glucose, and DAPI-containing mounting media were provided by Meilun Biotech (Dalian, China).
Animals
Seven-week-old male specific pathogen free Sprague-Dawley (SD) rats were obtained from the Experimental Animal Center of Shenyang Pharmaceutical University (Shenyang, China). Animal experiments were conducted in accordance with the Guideline of Animal Experimentation and the protocol was subject to approval by the Animal Ethics Committee of Shenyang Pharmaceutical University. All rats were housed under conventional conditions with appropriate temperature (22 ± 0.5°C), humidity (50-60%) control and a 12/12 h light/dark cycle with free access to food and water. T2DM was induced by feeding rats with high-fat diet (HFD, H10045; 4.73 kcal/g, 45 kcal% fat, 35 kcal% carbohydrates, and 20 kcal% protein; Beijing HFK Bioscience, Beijing, China) for 5 weeks followed by intraperitoneal (i.p.) injection of 35 mg/kg STZ. Rats were considered to be hyperglycemic when blood glucose levels were >11.1 mmol/L after a 1 week waiting period (Xu et al., 2018). Silibinin (100 mg/kg) and metformin (100 mg/kg) suspensions prepared in 0.5% CMC-Na were administered to rats once daily by oral gavage for 4 consecutive weeks, and the untreated rats in diabetic control group were administrated with 0.5% CMC-Na only (n=6 in each group). Fasting blood glucose was measured by glucose oxidase/peroxidase method (Wako Diagnostics, Tokyo, Japan) at the indicated time points. By the end of the study, overnight-fasted animals were anesthetized with intraperitoneal injection of chloral hydrate (350 mg kg−1), and serum was collected for assessment of IL-1β, TNF-α (Nanjing Jiancheng Bio engineering Institute, Nanjing, China) and insulin (Shanghai Huayi Biological Technology). Pancreas tissues were harvested and fixed with 4% paraformaldehyde for immunofluorescence analysis.
Immunofluorescence
Paraformaldehyde-fixed pancreas tissues were embedded with paraffin and sectioned into 4 μm slices. The sections were permeabilized with 0.15% Triton X-100, and then blocked with blocking buffer containing 5% normal goat serum before incubation with anti-insulin antibody and antibodies targeting ERα, Nrf2 or HO-1. The primary antibodies were located with FITC- or Rhodamine-conjugated secondary antibody. Slides were then mounted with DAPI-containing mounting medium, and the images were taken using a fluorescence microscope (Eclipse 90i; Nikon Instruments, Tokyo, Japan) at 200x magnification.
Cell culture
Rat pancreatic β-cell line INS-1 was obtained from National Platform of Experimental Cell Resources for Sci-Tech (Beijing, China), and the cells were cultured in RPMI 1640 medium (Gibico, Grand Island, NY, USA) containing 11.1 mmol/L glucose supplemented with 10% FBS, 10 mmol/L HEPES, 2 mmol/L L-glutamine, 1 mmol/L sodium pyruvate, 100 U/mL penicillin G sodium, 100 μg/mL streptomycin sulfate, and 50 μmol/L β-mercaptoethanol. Mouse pancreatic β-cell line NIT-1 was obtained from Newgainbio (Wuxi, China), the cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 11.1 mmol/L glucose supplemented with 10% FBS, 100 U/mL penicillin G sodium, and 100 μg/mL streptomycin sulfate. The stock solution of palmitate (5 mM) bound to 3.75% bovine serum albumin (BSA) was prepared by following the published protocol (Lee et al., 2013), and the stock solution was diluted in culture medium to obtain 0.4 mM palmitate conjugated with 0.3% BSA. Glucolipotoxcity-mediated cell damage was induced by incubating INS-1 or NIT-1 cells with high glucose (HG, 25 mM) and 0.4 mM palmitate/BSA (PA) for 24 h. To determine the protective effects of silibinin against glucolipotoxicity through the regulation of ERα expression, cells were pre-incubated with silibinin (2.5, 5, and 10 μM), PPT (10 μM) or MPP (15 μM) for 2 h before incubation with HG/PA. Moreover, small interfering RNA (siRNA) against ERα and control scrambled siRNA were transfected into INS-1 or NIT-1 cells with siRNA-Mate (Shanghai Gene Pharma, Shanghai, China) to confirm the study using ERα antagonist, and the pre-incubation of cells with silibinin was then performed 24 h after transfection. Insulin in cells and conditioned medium was determined using the commercial ELISA kit (Shanghai Huayi Biological Technology, Shanghai, China). The cells were incubated at 37°C with 5% CO2 in a humidified atmosphere during the study.
MTT assay
INS-1 cells were plated into 96-well cell culture plates (Costar, Corning, NY, USA) at a density of 1.0×104 cells/well. After cultured for 24 h, the cells were subjected to the indicated treatments followed by incubation with 100 μL of 0.5 mg/mL MTT solution at 37°C for additional 3 h. The formazan dye product of residual cell layer was dissolved with 150 μL of DMSO. The absorbance of each sample was measured at 490 nm wavelength using a microplate reader (SpectraMax M2, Molecular Devices, Sunnyvale, CA, USA). Data were expressed as the cell viability versus that of control.
Western blot assay
INS-1 or NIT-1 cells were lysed in RIPA buffer containing phosphatase inhibitors and protease inhibitors (Roche Diagnostics, Indianapolis, IN, USA). The soluble protein was isolated by centrifugation at 12,000 g for 10 min. Equal amounts of total protein of each sample were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to PVDF membrane. After blocking with 5% skimmed milk in PBS containing 0.1% Tween-20, the membranes were blotted with primary antibody against ERα, Nrf2, HO-1, SOD2 or β-actin. The primary antibodies were then located with HRP-conjugated secondary antibody, and the protein bands were finally visualized by using ECL kit (Amersham, Buckinghamshire, UK). The band density was analyzed using Image J software (National Institutes of Health, Bethesda, MD, USA).
ROS production
Intracellular ROS of INS-1 or NIT-1 cells was detected by a fluorescent probe 2′,7′-dichlorofluorescein diacetate (DCFH-DA) (Meilun Biotech). DCFH-DA is converted by intracellular esterases, which is oxidized into the highly fluorescent dichlorofluorescein (DCF) in the presence of oxidants. The fluorescence was measured by using the microplate reader (SpectraMax M2, Molecular Devices) with excitation at 485 nm and emission at 530 nm.
Statistics
In experiments with multiple treatments, the statistical comparisons were analyzed by ANOVA with Tukey’s test. Results were presented as the mean ± SEM. p<0.05 was considered statistically significant.
RESULTS
Silibinin decreases blood glucose and increases serum insulin of diabetic rats
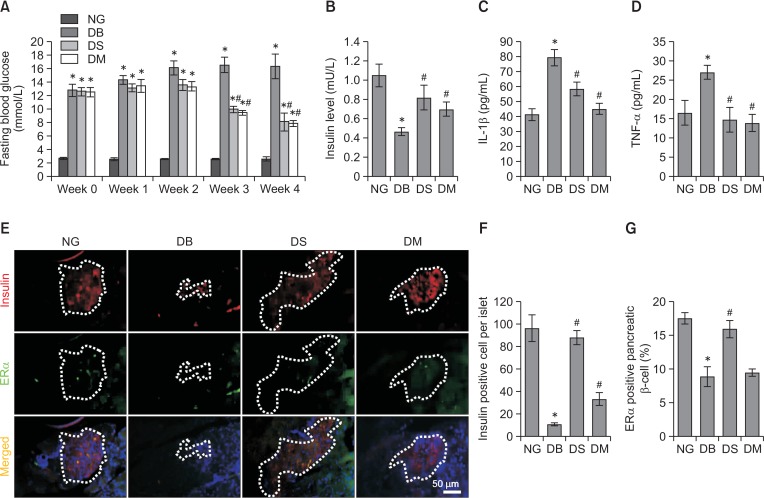
Fasting blood glucose in HFD/STZ-induced type 2 diabetic Sprague-Dawley (SD) rats was significantly higher than in normoglycemic control rats. However, silibinin (100 mg/kg) and the positive control metformin (100 mg/kg) significantly decreased the fasting blood glucose of diabetic rats 3 weeks after oral administration (p<0.05) (Fig. 1A). Insulin secretion was impaired in diabetic SD rats as evidenced by the lower insulin level in diabetic rats compared with normoglycemic rats (p<0.05), while the administration of silibinin or metformin significantly reversed insulin secretion (p<0.05) (Fig. 1B). Since TNF-α and IL-1β are the crucial mediators leading to β-cell destruction (Yang et al., 2018), we further measured the level of TNF-α and IL-1β in serum of rats from different groups. Diabetic rats showed higher levels of TNF-α and IL-1β relative to normoglycemic rats, and these levels were significantly attenuated by silibinin or metformin administration (p<0.05) (Fig 1C, 1D).
Fig. 1.
Effects of silibinin on fasting blood glucose, serum insulin and pro-inflammatory cytokines as well as expression of ERα in pancreatic β-cells in type 2 diabetic SD rats. (A) Fasting blood glucose of SD rats in different groups was monitored during the study. (B) Rats were euthanized after 4 weeks of silibinin or metformin administration, and the serum was collected to evaluate the insulin secretion of rats with different treatments. (C, D) Levels of IL-1β and TNF-α in serum of rats from different groups were determined. (E) Representative immunofluorescence images showed insulin- and ERα-positive cells in pancreatic islet of normoglycemic and diabetic SD rats with different treatments (magnification, ×200; scale bar, 50 μm). (F, G) Quantification of the insulin-positive cell number and ERα-positive pancreatic β-cell percentage in islets. NG, normoglycemia; DB, diabetes; DS, diabetes+silibinin; DM, diabetes+metformin. Data are mean ± SEM (n=6 in each group). *p<0.05 vs. NG group; #p<0.05 vs. DB group.
Silibinin elevates the expression of ERα in pancreatic β-cells of diabetic rats
The pancreatic β-cells in pancreatic islets were located by immunofluorescence using anti-insulin antibody (Fig. 1E). Consistent with the data shown in Fig. 1B, the quantitative result of insulin immunofluorescence revealed that the number of pancreatic β-cells in pancreatic islets of diabetic rats decreased significantly compared with the normoglycemic control group (p<0.05), while the administration of silibinin or metformin increased the number of pancreatic β-cells in diabetic rats (p<0.05) (Fig. 1F). The expression of ERα in pancreatic β-cells was shown by colocalization of ERα and insulin (Fig. 1E). ERα-positive pancreatic β-cell percentages in diabetic pancreatic islets were lower than in the normoglycemic control group (p<0.05), while silibinin (p<0.05) rather than metformin (p>0.05) significantly increased the expression of ERα in pancreatic β-cells (Fig. 1G).
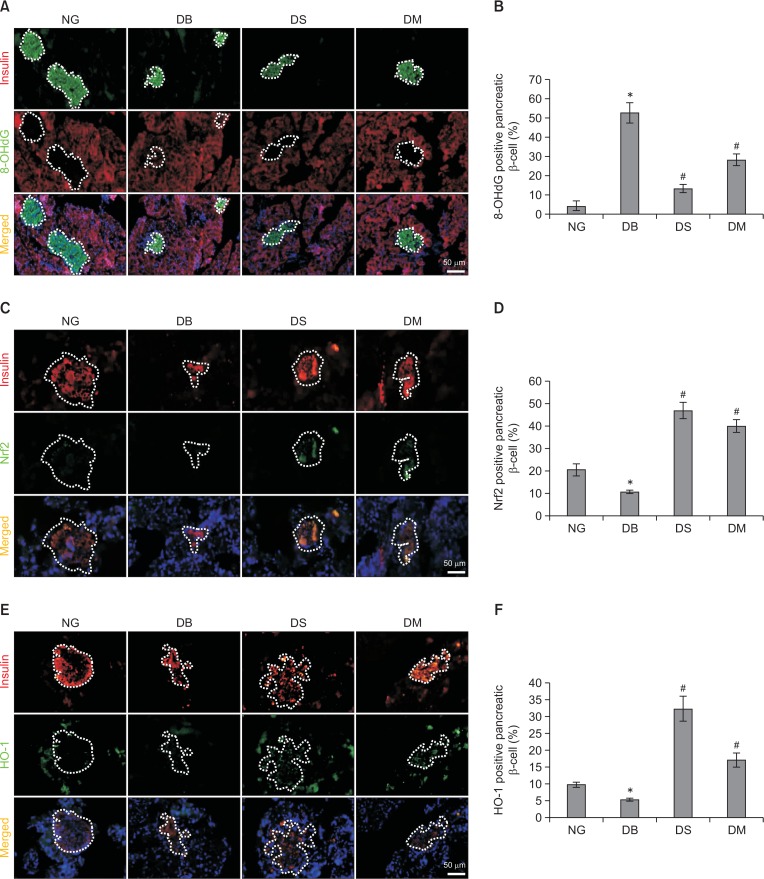
Silibinin increases the expression of Nrf2 and HO-1 in pancreatic β-cells of diabetic rats
To determine whether the antioxidative activity of silibinin contributed to the protective effects in pancreatic β-cells, the expression of 8-OHdG, a marker of oxidative stress to DNA, was determined by double immunofluorescence labeling of insulin and 8-OHdG. As shown in Fig. 2A and 2B, the percentage of 8-OHdG-positive pancreatic β-cells in diabetic control rats was significantly higher than in the normoglycemic control group. However, the administration of silibinin or metformin in diabetic rats decreased 8-OHdG-positive cell percentages. In addition, the expression of oxidative sensor Nrf2 and its downstream factor HO-1 was also evaluated by double immunofluorescence with antibodies targeting insulin and Nrf2/HO-1. The results showed that the expression of Nrf2 decreased significantly in pancreatic β-cells of diabetic rats, while silibinin caused a 3.30-fold increase of Nrf2 expression (p<0.05). Metformin also increased the level of Nrf2 by 2.66-fold (p<0.05) (Fig. 2C, 2D). Accordingly, silibinin and metformin significantly increased the expression of the antioxidative enzyme HO-1 by 5.04- and 2.19-fold, respectively (p<0.05) (Fig. 2E, 2F).
Fig. 2.
Silibinin treatment up-regulates the expression of Nrf2 and HO-1 in pancreatic β-cells of diabetic rats. (A, B) Representative images of immunofluorescence labeling of insulin and 8-OHdG, and quantitative results of 8-OHdG-positive pancreatic β-cell percentage in islets. (C, D) Representative images of insulin/Nrf2 double immunofluorescence staining and quantitative analysis of Nrf2-positive pancreatic β-cell percentage in islets. (E, F) Representative images and the quantitative results of insulin/HO-1 double immunofluorescence staining. Magnification, ×200; scale bar, 50 μm. NG, normoglycemia; DB, diabetes; DS, diabetes+silibinin; DM, diabetes+metformin. Data are mean ± SEM (n=6 in each group). *p<0.05 vs. NG group; #p<0.05 vs. DB group.
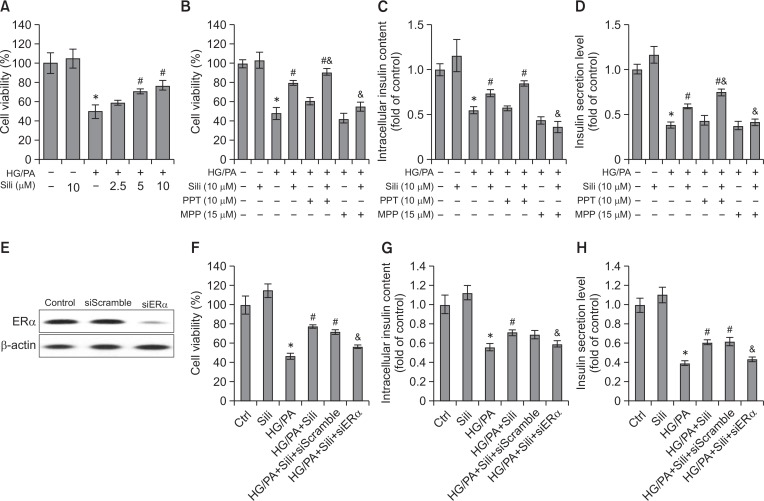
Silibinin increases viability and insulin synthesis and secretion of rat pancreatic INS-1 cells in an ERα-dependent manner
To investigate whether silibinin exerts antioxidative effects through regulation of ERα expression, the rat pancreatic β-cell line INS-1 was used in the in vitro study. HG/PA-induced glucolipotoxicity markedly reduced INS-1 cell viability, while preincubation with silibinin (5 or 10 μM) resulted in a significant increase of cell viability (p<0.05). In addition, 10 μM silibinin alone did not reduce the viability of INS-1 cells (p>0.05) (Fig. 3A). PPT and MPP were used as ERα-selective agonist and antagonist, respectively. Treatment with PPT further increased the viability of INS-1 cells induced by silibinin (p<0.05), while treatment with MPP significantly reduced cell viability (p<0.05) (Fig. 3B). The impact of ERα expression on insulin synthesis and secretion of INS-1 cells was further determined. In accordance with the in vivo results (Fig. 1B, 1F), silibinin significantly increased the insulin level in INS-1 cells and its culture medium (p<0.05), which was attenuated by cotreatment with MPP (p<0.05) (Fig. 3C, 3D). In addition, PPT augmented the level of insulin secretion induced by silibinin (p<0.05) (Fig. 3D). To confirm results using an ERα antagonist, ERα siRNA was transfected into INS-1 cells. ERα protein expression was shown to be effectively suppressed by ERα siRNA transfection (Fig. 3E). ERα siRNA transfection significantly attenuated silibinin-elevated cell viability and levels of intracellular and secreted insulin of INS-1 cells cultured with HG/PA (p<0.05), while the control siRNA failed to exert these effects (Fig. 3F–3H). Taken together, silibinin, through the regulation of ERα, protected INS-1 cells against cytotoxicity and tackled dysfunction induced by the effects of HG/PA.
Fig. 3.
Effects of silibinin on the viability and insulin secretion of INS-1 cells. (A) The effects of silibinin on the viability of INS-1 cells treated with HG/PA were determined by MTT assay. (B) The impacts of ERα selective agonist PPT and antagonist MPP on silibinin-enhanced INS-1 cell viability were determined by MTT assay. (C, D) Insulin in cells and medium was evaluated by ELISA assay. (E) INS-1 cells were transfected with ERα siRNA or control siRNA, and ERα protein expression was examined by Western blot assay 48 h after transfection. The cell viability (F) as well as intracellular (G) and secreted (H) insulin levels of cells with administration of silibinin and/or siRNA transfection were further evaluated. Serum albumin (0.3% BSA) was present under all conditions. HG/PA, high glucose and palmitate/BSA; Sili, silibinin. Data are mean ± SEM of 3 independent experiments. *p<0.05 vs. control group; #p<0.05 vs. HG/PA group; &p<0.05 vs. HG/PA+Sili group.
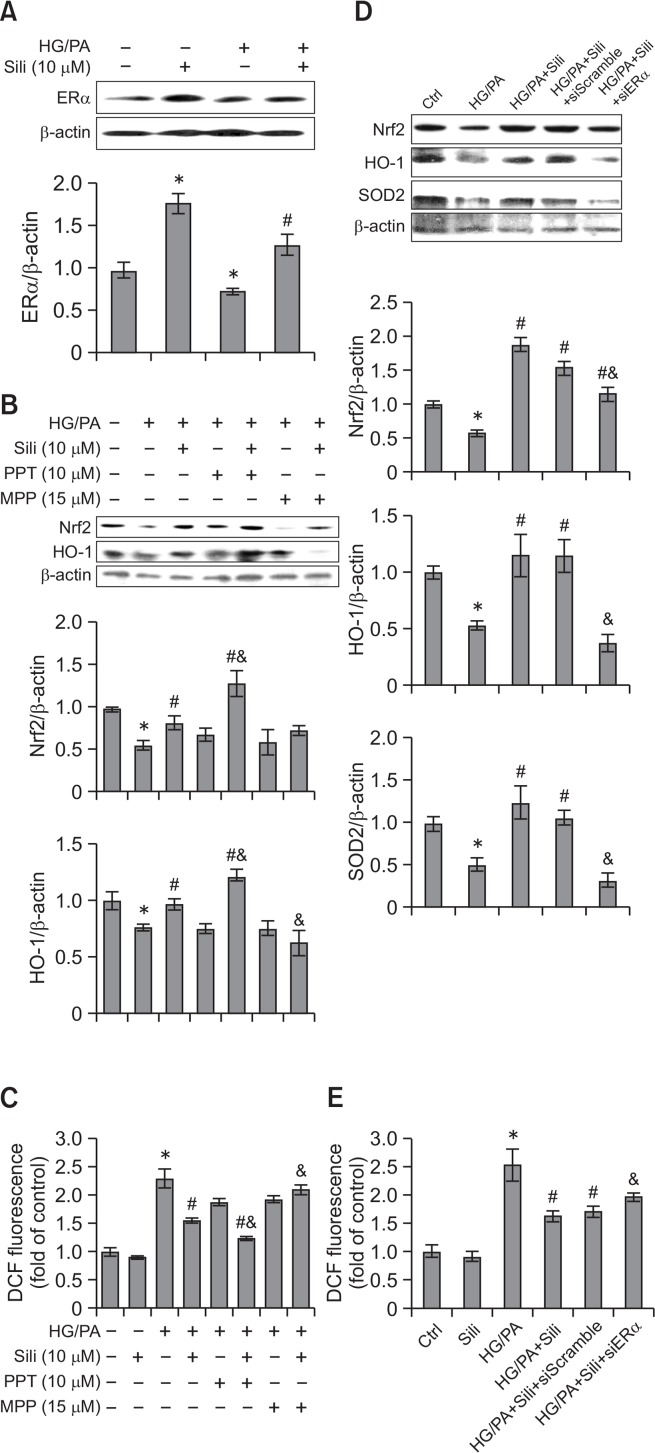
Silibinin decreases ROS production via the ERα/Nrf2/HO-1-SOD2 pathway in INS-1 and NIT-1 cells
As shown by the results of Western blot assay, silibinin (10 μM) up-regulated HG/PA-decreased ERα expression in INS-1 cells (p<0.05). Moreover, silibinin alone also significantly increased ERα expression compared with the control group (p<0.05) (Fig. 4A). The effects of silibinin on the expression of Nrf2 and HO-1 in INS-1 cells were further evaluated. As shown in Fig. 4B, the cells treated with HG/PA showed decreased expression of Nrf2 and HO-1 (p<0.05), while silibinin increased the expression of these proteins. To examine whether silibinin activated the Nrf2/HO-1 pathway through the regulation of ERα, PPT or MPP were added to the culture medium of INS-1 cells with silibinin. Cells cotreated with ERα agonist PPT and silibinin had significantly increased Nrf2 and HO-1 expression compared with the cells treated with silibinin alone (p<0.05), while silibinin-enhanced HO-1 expression was attenuated when cells were cultured with the ERα antagonist MPP (p<0.05). Moreover, ROS produced in INS-1 cells was detected by a DCFH-DA probe (Fig. 4C). HG/PA increased the level of ROS in INS-1 cells compared with the control group (p<0.05), while pre-incubation with silibinin significantly decreased ROS production (p<0.05). In addition, PPT further down-regulated silibinin-inhibited ROS production (p<0.05), and silibinin failed to exert antioxidative effects in the cells cotreated with MPP (p<0.05). To validate the involvement of ERα in the activation of the Nrf2-antioxidative signaling pathways in pancreatic β-cells, a loss-of-function study using ERα siRNA was performed in the rat pancreatic β-cell line INS-1 and a mouse pancreatic β-cell line NIT-1. Consistent with the results in Fig. 4A, silibinin (10 μM) up-regulated HG/PA-decreased ERα expression in NIT-1 cells (p<0.05) (Supplementary Fig. 1A). And ERα siRNA was shown to attenuate silibinin-increased Nrf2 and HO-1 expression in both INS-1 and NIT-1 cells cultured with HG/PA (p<0.05). In addition, the expression of SOD2, another antioxidative protein under the regulation of Nrf2, was also increased by silibinin administration in both cell lines, while ERα siRNA effectively attenuated silibinin-enhanced SOD2 expression (Fig. 4D, Supplementary Fig. 1B, 1C). Moreover, ERα siRNA up-regulated silibinin-decreased ROS production from both INS-1 and NIT-1 cells cultured with HG/PA (Fig. 4E, Supplementary Fig. 1D). These results indicated an inhibitory effect of silibinin on ROS production by activating ERα-dependent Nrf2-antioxidative signaling pathways.
Fig. 4.
Silibinin increases the expression of Nrf2, HO-1 and SOD2, and decreases ROS production in an ERα-dependent manner in INS-1 cells. INS-1 cells received indicated treatments, and the expression of ERα (A), Nrf2 and HO-1 (B) was examined by Western blot assay. (C) DCFH-DA probe was used to evaluate ROS production in INS-1 cells, and the fluorescence of oxidation product DCF was measured using a microplate reader with excitation at 485 nm and emission at 530 nm. (D, E) INS-1 cells were transfected with ERα siRNA or control siRNA, and then the expression of Nrf2, HO-1, and SOD2 as well as ROS production of cells with administration of silibinin and/or siRNA transfection were examined. Serum albumin (0.3% BSA) was present under all conditions. HG/PA, high glucose and palmitate/BSA; Sili, silibinin. Data are mean ± SEM of 3 independent experiments. *p<0.05 vs. control group; #p<0.05 vs. HG/PA group; &p<0.05 vs. HG/PA+Sili group.
DISCUSSION
According to ethnobotanical information, approximately 800 plants are used in the folk medicine to treat diabetes (Hung et al., 2012). However, only Aegle marmelos, Allium cepa, Gymnema sylvestre, Momordica charantia, Ocimum sanctum, Nigella sativa, Ocimum sanctum, Panax quinquefolius, Salacia reticulate, Trigonella foenum-graecum, and Silybum marianum showed antidiabetic effects in clinical studies (Ghorbani, 2013; Saad et al., 2017). Silybum marianum grows all through Europe and North America, South America, China, India, Africa, and Australia (Corchete, 2008; Voroneanu et al., 2016). Silibinin, a natural polyphenolic flavonoid, is a major bioactive component in the fruits and seeds of Silybum marianum (Jeong et al., 2011). In our previous study, silibinin increased the viability and insulin synthesis of pancreatic β-cells (INS-1 cells) through up-regulation of ERα (Yang et al., 2018). Our current study further revealed that the ERα-activated Nrf2-antioxidative signaling pathways are involved in the protective effects of silibinin in pancreatic β-cells.
Persistent hyperglycemia and elevated levels of free fatty acids contribute to the oxidative stress in pancreatic β-cells (Govindaraj and Sorimuthu, 2015). In the present in vivo study, the HFD/STZ-induced rat model of T2DM was used to evaluate the protective effects of silibinin against oxidative damage in pancreatic β-cells. Metformin, the most prescribed agent for T2DM worldwide (Tahrani et al., 2016), was used as the positive control in our study. Metformin could restore glucose homeostasis by activating glucose uptake in muscle and liver, suppressing hepatic glucose output, and preserving pancreatic β-cell function (Giannarelli et al., 2003). Our results showed that silibinin (100 mg/kg) down-regulated blood glucose and decreased levels of pro-inflammatory cytokines TNF-α and IL-1β similar to those in rats receiving the same dose of metformin treatment. Notably, as evidenced by higher levels of insulin-positive pancreatic β-cells per islet in diabetic animals, silibinin exerted stronger protective effect on pancreatic β-cell than did metformin. In addition, rat INS-1 pancreatic β-cells were treated with HG/PA to induce glucolipotoxcity-mediated cell damage in vitro. Accordant with the results of the in vivo study, the preincubation of silibinin (10 μM) protected INS-1 cells against HG/PA-induced injury as evidenced by elevated cell viability and insulin synthesis and secretion.
It has been reported that the activation of ERα enhanced insulin synthesis in pancreatic β-cells (Alonsomagdalena et al., 2008; Tiano and Mauvais-Jarvis, 2012). The role of ERα in the protective effects of silibinin in pancreatic β-cells was determined in the current study. We calculated the percentage of ERα-positive pancreatic β-cells in rat islets, and the data indicated that silibinin increased ERα expression in pancreatic β-cells of diabetic rats. In addition, HG/PA treatment decreased ERα expression in the rat INS-1 cell line and a mouse NIT-1 cell line, while the pre-incubation of silibinin up-regulated the expression of ERα in HG/PA-treated cells. Both in vivo and in vitro studies have revealed increased expression of ERα induced by silibinin. Additional research has determined the role of ERα in the protective effects of silibinin in INS-1 cells occurs via activating or inactivating ERα by PPT (agonist) and MPP (antagonist), respectively (Hidalgo-Lanussa et al., 2018). PPT was shown to enhance silibinin-improved INS-1 cell survival and insulin secretion, while MPP negated the beneficial effects of silibinin. To validate the study using an ERα antagonist, ERα siRNA was transfected into INS-1 cells. The loss-of-function study confirmed that silibinin exerts protective effects in INS-1 cells by increasing cell viability and insulin synthesis and secretion in an ERα-dependent manner. Consistent with our previous study (Yang et al., 2018), these results indicated that silibinin could regulate glucose homeostasis, at least partly, by preserving pancreatic β-cell mass and function through up-regulation of ERα. However, silibinin was reported to decrease the expression of ERα and finally induced autophagy and apoptosis in human breast cancer MCF-7 cells (Zheng et al., 2015). These contradictory results indicated that ERα might be differently regulated by silibinin in different cell types.
Nrf2-mediated antioxidative response plays an important role in protecting pancreatic β-cells against oxidative stress-induced impairment (Pi et al., 2010). It has been reported that ERα antagonist MPP blocked estrogen-induced Nrf2 activity in MCF-7 breast cancer cells, while the inhibition of ERβ, another classic ER, failed to reverse the activation of Nrf2 by estrogen (Wu et al., 2014). Conversely, the abrogation of ERβ attenuated the induction of Nrf2 activation by S-(-)equol, a metabolic product of phytoestrogen daidzein, but modulation of ERα had no effects on Nrf2 in human umbilical vein endothelial cells (Zhang et al., 2013). The relationship between ER and Nrf2 in pancreatic β-cells is still unclear. Our study revealed that the expression of Nrf2 and its downstream effector HO-1 was increased by silibinin in pancreatic β-cells of diabetic rats accompanied by up-regulated expression of ERα. However, metformin regulated the expression Nrf2 and HO-1 without affecting ERα expression, indicating that metformin might exert antioxidative activity through ERα-independent signaling pathways. In the in vitro study, treatment of INS-1 cells with the ERα agonist PPT enhanced silibinin-induced activation of the Nrf2/HO-1 pathway and inhibited the production of ROS, while the ERα antagonist MPP negated the effectiveness of silibinin. Accordantly, ERα siRNA transfection was shown to abolish silibinin-increased Nrf2, HO-1, and SOD2 expression, and up-regulate silibinin-decreased ROS production in both INS-1 and NIT-1 cells cultured with HG/PA. In our previous study, silibinin was shown to attenuate TNFα- or IL-1β-impaired PI3K/Akt pathway (Yang et al., 2018). ER-mediated PI3K/Akt activation is a well-documented pathway involved in protection against oxidative stress, and the inactivation of the PI3K/Akt pathway was reported to attenuate phytoestrogen Rb1-induced activation of Nrf2/HO-1 pathway (Hwang and Jeong, 2010). Thus, under the regulation of ER, the PI3K/Akt pathway might be involved in the antioxidative action of silibinin.
In summary, silibinin up-regulated the viability and improved the function of β-cells in pancreatic islets of type 2 diabetic rats and HG/PA-treated β-cells in vitro. The protective effects of silibinin in pancreatic β-cells were established through the regulation of oxidative stress by activating ERα-dependent Nrf2-antioxidative signaling pathways. These findings suggest that silibinin may represent a potential therapeutic agent to improve glucose homeostasis in patients with diabetes. In addition, although ERα contributes to the maintenance of glucose homeostasis by increasing insulin synthesis in pancreatic β-cells, adverse effects such as insulin resistance might be caused if estrogenic action is not within physiological levels (Nadal et al., 2009). Therefore, further investigation of the safety of silibinin in diabetes control is necessary.
Acknowledgments
This work was funded by National Natural Science Foundation of China (81803603), China Postdoctoral Science Foundation (2017M621161; 2018T110462), Jiangsu Province “Innovative Entrepreneurship” Program, and Doctoral Starting-up Foundation of Liaoning Science and Technology Department (201601139).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- Alonsomagdalena P, Ropero AB, Carrera MP, Cederroth CR, Baquié M, Gauthier BR, Nef S, Stefani E, Nadal A. Pancreatic insulin content regulation by the estrogen receptor ERα. PLoS ONE. 2008;3:e2069. doi: 10.1371/journal.pone.0002069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Zhao L, He H, Wan X, Wang F, Mo Z. Silibinin protects beta cells from glucotoxicity through regulation of the insig-1/srebp-1c pathway. Int J Mol Med. 2014;34:1073–1080. doi: 10.3892/ijmm.2014.1883. [DOI] [PubMed] [Google Scholar]
- Chu C, Li D, Zhang S, Ikejima T, Jia Y, Wang D, Xu F. Role of silibinin in the management of diabetes mellitus and its complications. Arch Pharm Res. 2018;41:785–796. doi: 10.1007/s12272-018-1047-x. [DOI] [PubMed] [Google Scholar]
- Corchete P. Silybum marianum (L.) Gaertn: the source of silymarin. In Bioactive Molecules and Medicinal Plants. Springer; 2008. pp. 123–148. [Google Scholar]
- Gerber PA, Rutter GA. The role of oxidative stress and hypoxia in pancreatic beta-cell dysfunction in diabetes mellitus. Antioxid Redox Signal. 2017;26:501–518. doi: 10.1089/ars.2016.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorbani A. Best herbs for managing diabetes: a review of clinical studies. Braz J Pharm Sci. 2013;49:413–422. doi: 10.1590/S1984-82502013000300003. [DOI] [Google Scholar]
- Giannarelli R, Aragona M, Coppelli A, Del Prato S. Reducing insulin resistance with metformin: the evidence today. Diabetes Metab. 2003;29:6S28–6S35. doi: 10.1016/S1262-3636(03)72785-2. [DOI] [PubMed] [Google Scholar]
- Govindaraj J, Sorimuthu Pillai S. Rosmarinic acid modulates the antioxidant status and protects pancreatic tissues from glucolipotoxicity mediated oxidative stress in high-fat diet: Streptozotocin-induced diabetic rats. Mol Cell Biochem. 2015;404:143–159. doi: 10.1007/s11010-015-2374-6. [DOI] [PubMed] [Google Scholar]
- Hidalgo-Lanussa O, Avila-Rodriguez M, Baez-Jurado E, Zamudio J, Echeverria V, Garcia-Segura LM, Barreto GE. Tibolone reduces oxidative damage and inflammation in microglia stimulated with palmitic acid through mechanisms involving estrogen receptor beta. Mol Neurobiol. 2018;55:5462–5477. doi: 10.1007/s12035-017-0777-y. [DOI] [PubMed] [Google Scholar]
- Hung H-Y, Qian K, Morris-Natschke SL, Hsu C-S, Lee K-H. Recent discovery of plant-derived anti-diabetic natural products. Nat Prod Rep. 2012;29:580–606. doi: 10.1039/c2np00074a. [DOI] [PubMed] [Google Scholar]
- Hwang YP, Jeong HG. Ginsenoside Rb1 protects against 6-hydroxydopamine-induced oxidative stress by increasing heme oxygenase-1 expression through an estrogen receptor-related PI3K/Akt/Nrf2-dependent pathway in human dopaminergic cells. Toxicol Appl Pharm. 2010;242:18–28. doi: 10.1016/j.taap.2009.09.009. [DOI] [PubMed] [Google Scholar]
- International Diabetes Federation . IDF Diabetes Atlas. 8th ed. IDF; Brussels: 2017. [Google Scholar]
- Jeong JC, Shin WY, Kim TH, Kwon CH, Kim JH, Kim YK, Kim KH. Silibinin induces apoptosis via calpain-dependent aif nuclear translocation in U87MG human glioma cell death. J Exp Clin Cancer Res. 2011;30:44. doi: 10.1186/1756-9966-30-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamori D. Exploring the molecular mechanisms underlying α- and β-cell dysfunction in diabetes. Diabetol Int. 2017;8:248–256. doi: 10.1007/s13340-017-0327-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan NM, Haseeb A, Ansari MY, Devarapalli P, Haynie S, Haqqi TM. Wogonin, a plant derived small molecule, exerts potent anti-inflammatory and chondroprotective effects through the activation of ROS/ERK/Nrf2 signaling pathways in human osteoarthritis chondrocytes. Free Radic Biol Med. 2017;106:288–301. doi: 10.1016/j.freeradbiomed.2017.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Choi SE, Jung IR, Lee KW, Kang Y. Protective effect of nicotinamide on high glucose/palmitate-induced glucolipotoxicity to INS-1 beta cells is attributed to its inhibitory activity to sirtuins. Arch Biochem Biophys. 2013;535:187–196. doi: 10.1016/j.abb.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Mackenbach JD, den Braver NR, Beulens JWJ. Spouses, social networks and other upstream determinants of type 2 diabetes mellitus. Diabetologia. 2018;61:1517–1521. doi: 10.1007/s00125-018-4607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morillas-Ruiz JM, García JAV, López FJ, Vidal-Guevara ML, Zafrilla P. Effects of polyphenolic antioxidants on exercise-induced oxidative stress. Clin Nutr. 2006;25:444–453. doi: 10.1016/j.clnu.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Nabavi SF, Barber AJ, Spagnuolo C, Russo GL, Daglia M, Nabavi SM, Sobarzo-Sanchez E. Nrf2 as molecular target for polyphenols: a novel therapeutic strategy in diabetic retinopathy. Crit Rev Clin Lab Sci. 2016;53:293–312. doi: 10.3109/10408363.2015.1129530. [DOI] [PubMed] [Google Scholar]
- Nadal A, Alonso-Magdalena P, Soriano S, Quesada I, Ropero AB. The pancreatic β-cell as a target of estrogens and xenoestrogens: implications for blood glucose homeostasis and diabetes. Mol Cell Endocrinol. 2009;304:63–68. doi: 10.1016/j.mce.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Pi J, Zhang Q, Fu J, Woods CG, Hou Y, Corkey BE, Collins S, Andersen ME. ROS signaling, oxidative stress and Nrf2 in pancreatic beta-cell function. Toxicol Appl Pharmacol. 2010;244:77–83. doi: 10.1016/j.taap.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson R. Oxidative stress and impaired insulin secretion in type 2 diabetes. Curr Opin Pharmacol. 2006;6:615–619. doi: 10.1016/j.coph.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Rochette L, Zeller M, Cottin Y, Vergely C. Diabetes, oxidative stress and therapeutic strategies. Biochim. Biophys. Acta. 2014;1840:2709–2729. doi: 10.1016/j.bbagen.2014.05.017. [DOI] [PubMed] [Google Scholar]
- Saad B, Zaid H, Shanak S, Kadan S. Anti-Diabetes and Anti-Obesity Medicinal Plants and Phytochemicals. Springer International Publishing; 2017. [Google Scholar]
- Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371:1131–1141. doi: 10.1056/NEJMra1011035. [DOI] [PubMed] [Google Scholar]
- Sone H, Kagawa Y. Pancreatic beta cell senescence contributes to the pathogenesis of type 2 diabetes in high-fat diet-induced diabetic mice. Diabetologia. 2005;48:58–67. doi: 10.1007/s00125-004-1605-2. [DOI] [PubMed] [Google Scholar]
- Tahrani AA, Barnett AH, Bailey CJ. Pharmacology and therapeutic implications of current drugs for type 2 diabetes mellitus. Nat Rev Endocrinol. 2016;12:566–592. doi: 10.1038/nrendo.2016.86. [DOI] [PubMed] [Google Scholar]
- Tiano JP, Mauvais-Jarvis F. Importance of oestrogen receptors to preserve functional β-cell mass in diabetes. Nat Rev Endocrinol. 2012;8:342–351. doi: 10.1038/nrendo.2011.242. [DOI] [PubMed] [Google Scholar]
- Voroneanu L, Nistor I, Dumea R, Apetrii M, Covic A. Silymarin in type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. J Diabetes Res. 2016;2016:5147468. doi: 10.1155/2016/5147468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Liu M, Liu WW, Hao WB, Tashiro S, Onodera S, Ikejima T. In vivo recovery effect of silibinin treatment on streptozotocin-induced diabetic mice is associated with the modulations of sirt-1 expression and autophagy in pancreatic beta-cell. J Asian Nat Prod Res. 2012;14:413–423. doi: 10.1080/10286020.2012.657180. [DOI] [PubMed] [Google Scholar]
- Wu J, Williams D, Walter GA, Thompson WE, Sidell N. Estrogen increases Nrf2 activity through activation of the PI3K pathway in MCF-7 breast cancer cells. Exp Cell Res. 2014;328:351–360. doi: 10.1016/j.yexcr.2014.08.030. [DOI] [PubMed] [Google Scholar]
- Xu F, Yang J, Negishi H, Sun Y, Li D, Zhang X, Hayashi T, Gao M, Ikeda K, Ikejima T. Silibinin decreases hepatic glucose production through the activation of gut-brain-liver axis in diabetic rats. Food Funct. 2018;9:4926–4935. doi: 10.1039/C8FO00565F. [DOI] [PubMed] [Google Scholar]
- Yang DK, Kang HS. Anti-diabetic effect of cotreatment with quercetin and resveratrol in streptozotocin-induced diabetic rats. Biomol. Ther. (Seoul) 2018;26:130–138. doi: 10.4062/biomolther.2017.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Sun Y, Xu F, Liu W, Hayashi T, Onodera S, Tashiro SI, Ikejima T. Involvement of estrogen receptors in silibinin protection of pancreatic β-cells from TNFα- or IL-1β-induced cytotoxicity. Biomed Pharmacother. 2018;102:344–353. doi: 10.1016/j.biopha.2018.01.128. [DOI] [PubMed] [Google Scholar]
- Zhang T, Liang X, Shi L, Wang L, Chen J, Kang C, Zhu J, Mi M. Estrogen receptor and PI3K/Akt signaling pathway involvement in S-(-)equol-induced activation of Nrf2/ARE in endothelial cells. PLoS ONE. 2013;8:e79075. doi: 10.1371/journal.pone.0079075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng N, Zhang P, Huang H, Liu W, Hayashi T, Zang L, Zhang Y, Liu L, Xia M, Tashiro S-I, Onodera S, Ikejima T. ERα down-regulation plays a key role in silibinin-induced autophagy and apoptosis in human breast cancer MCF-7 cells. J Pharmacol Sci. 2015;128:97–107. doi: 10.1016/j.jphs.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Zou H, Zhu XX, Zhang GB, Ma Y, Wu Y, Huang DS. Silibinin: an old drug for hematological disorders. Oncotarget. 2017;8:89307–89314. doi: 10.18632/oncotarget.19153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.