Abstract
Changing trends in anticancer research have altered the treatment paradigm to the extent that it is difficult to investigate any anticancer drugs without mentioning immunotherapy. Thus, we are finally contemplating tumour regression using magic bullets known as immunotherapy drugs. This review explores the possible options and pitfalls in tumour regression by first elucidating the features of cancer and the importance of tumour microenvironments. Next, we evaluated the trends of anticancer therapeutics regulating tumour microenvironment. Finally, we introduced the concept of tumour regression and various targets of tumour microenvironment, which can be used in combination with current immunotherapy for tumour regression. In particular, we emphasize the importance of regulating the neurological manifestations of tumour microenvironment (N) in addition to inflammation (I) and hypoxia (H) in cancer.
Keywords: Immunotherapy, Tumour regression, Tumour microenvironment, Neurological, Inflammation, Hypoxia
INTRODUCTION
Cancer is the leading cause of death in the world (Bray et al., 2018). In particular, 90% of cancer deaths are caused by cancer metastasis (Bray et al., 2018). In order to overcome this fatal disease, we need to understand what cancer is. Based on several studies, cancer is now considered a disease of organs rather than a genetic diseases. Thus, cancer should be understood as an abnormal organs containing various cellular aggregates resulting in a whole-body tumour microenvironment (Egeblad et al., 2010).
The first purpose of this review is to share with researchers the concept that immunotherapeutic agents against cancer can be combined with other anticancer drugs leading to tumour regression. The next step might be to determine which target-controlling drug would be appropriate in combination with immunotherapy. We wanted to find clues that could help determine an answer to this question based on the experiences of healers (those who overcome cancer) and based on spontaneous regression (a phenomenon of natural healing of cancer) (Sengupta et al., 2010; Turner, 2014). From this, we investigate substances that regulates neurological, inflammatory, and hypoxic tumour microenvironments (NIH), which is regarded as a promising target for combination with immunotherapy.
Of course, while developing compounds that control these NIHs, we believed that there were many ways to attain tumour regression. In future, combination studies involving compounds that regulate promising new targets that we have overlooked are also likely to be pursued.
WHAT IS CANCER?
Hallmarks of cancer
The hallmarks of cancer are summarized in 10 categories (Fig. 1) (Hanahan and Weinberg, 2011). They include maintenance of proliferation signals, avoidance of growth inhibition, avoidance of immune destruction, possible replication immortality, inflammation promoting cancer, invasion and metastasis, induction of angiogenesis, genetic instability and mutation, resistance to apoptosis, and de-regulation of cellular metabolism (Hanahan and Weinberg, 2011). It is believed that it is possible to inhibit cancer by blocking these features (Fig. 1) (Hanahan and Weinberg, 2011).
Fig. 1.
Hallmarks of cancer. Hallmarks of cancer include maintenance of proliferation signals, avoidance of growth inhibition, avoidance of immune destruction, possible replication immortality, inflammation promoting cancer, invasion and metastasis, induction of angiogenesis, genetic instability and mutation, resistance to apoptosis, and de-regulation of cellular metabolism (Hanahan and Weinberg, 2011). Seven of cancer hallmarks are originated from cancer cells, while the remaining are related to tumour microenvironments.
Clonal evolution of cancer, plasticity and tumour heterogeneity
Cancer has evolved via an iterative process of clonal expansion, genetic diversity, and clonal selection (McGranahan and Swanton, 2017). In particular, it is selected by resistance to anticancer drugs. In addition, treatment with anticancer drugs inhibits cancer cells heterogeneity, and facilitates the survival of resistant cancer cells (Jamal-Hanjani et al., 2015). Cancer cells also exhibit plasticity, which is altered by tumour microenvironment (da Silva-Diz et al., 2018). Accordingly, cancer cells initiated by a single clone exhibit heterogeneity. Genetic mutations, epigenetics, and changes in tumour microenvironment are major contributors to tumour heterogeneity. Blocking the action of tumour microenvironment is a good strategy to overcome cancer heterogeneity for preventing cancer growth (Roma-Rodrigues et al., 2019).
TUMOUR MICROENVIRONMENT
Importance of tumour microenvironment
Regulation of tumour microenvironment is emerging as an important strategy in overcoming cancer heterogeneity since tumour microenvironment itself contributes to heterogeneity of cancer (Junttila and de Sauvage, 2013; Park and Lee, 2019). Noncancerous cells constituting tumour microenvironments include macrophages, dermal cells, vascular endothelial cells, and neutrophils, which promote or inhibit cancer (Bhome et al., 2015; Park and Lee, 2019). It is therefore necessary to retrain the microenvironment that promotes cancer to a microenvironment that inhibits cancer (Quail and Joyce, 2013; Kowal et al., 2019; Park and Lee, 2019). In particular, the ability of immune cells to destroy cancer cells is low in tumour microenvironment. Immune checkpoint inhibitors (ICIs) can retrain immune cells to attack cancer cells (Egeblad et al., 2010).
Emergence of resistance to therapies for tumour microenvironment and the need for combination therapy
In the case of glioblastoma multiforme (GBM), the major cells in tumour microenvironment include macrophages and microglia, constituting 30% of the total cancer cells. Inhibition of the colony-stimulating factor-1 receptor (CSF-1R) has been demonstrated in animal models, however, 50% of the mice show recurrent cancer, which may result in mutations in macrophages. Animal models have suggested that increased production of IGF-1 by IL-4 treatment of these macrophages induces activation of the PI3K pathway in cancer cells, thereby promoting survival and invasion of cancer cells (Pyonteck et al., 2013; Quail et al., 2016). In this study, the recurrence of cancers in mice was inhibited by a combination of anti-CSF-1R therapy and IGF pathway inhibitor, which suppressed the cancer cell resistance induced by macrophages. This finding suggests that even in the case of therapeutic agents regulating tumour microenvironment, the risk of mutations was lower than in cancer cells; however, a combination approach may be required to overcome the possible resistance.
TRENDS IN ANTICANCER THERAPY: EMERGENCE AND LIMITATION OF IMMUNOTHERAPY
Recent key results in cancer therapeutics
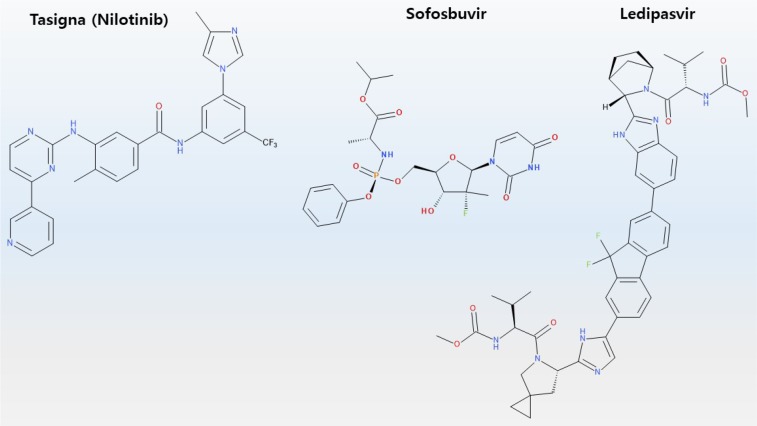
The development of antiviral drug against hepatitis C encountered new experiences. Harvoni, a combination drug with various mechanisms (including Sovaldi) to treat patients with hepatitis C, which accounts for 10% of the causes of hepatocellular carcinoma, has resulted in 94–99% cure rate, and not only improved symptoms, but also obviated the need to changing medications in cases associated with severe mutations (Fig. 2) (Keating, 2015). Ironically, the number of patients decreased and sales declined (Lindsley, 2017).
Fig. 2.
Chemical structure of Harvoni and Tasigna. Tasigna is a tradename of nilotinib which is a selective Bcr-Abl tyrosine kinase inhibitor. Harvoni is a combination of ledipasvir and sofosbuvir.
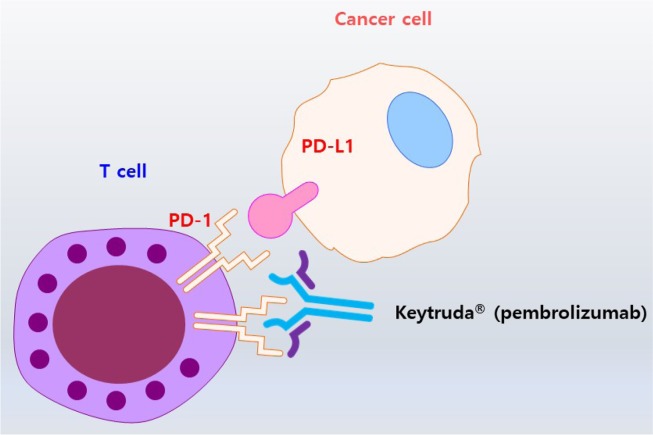
An unexpected surprise occurred in the development of anticancer drugs. For example, Tasigna, an anticancer drug for chronic myelogenous leukemia, acquired a treatment free remission label (Fig. 2) (Hochhaus et al., 2017; Mahon et al., 2018). Treatment-free remission refers to molecular response even after discontinuing drug therapy, especially in patients with Philadelphia chromosome-positive chronic myelogenous leukemia (Hochhaus et al., 2017). Another striking example is related to former US President Jimmy Carter who suffered melanoma, which had spread to the brain. Pembrolizumab (Keytruda®), an antibody drug binding to the PD-1 receptor, has been used to successfully treat metastatic melanoma (Fig. 3) (Choi and Yang, 2018). ICI attracted the attention of general public, after MRI scans of Jimmy Carter’s brain showed the disappearance of metastatic melanoma after treatment with pembrolizumab (Keytruda®). Thus, immunotherapy is a key factor to induce tumour regression.
Fig. 3.
Immue checkpoint inhibitor: Keytruda®. PD-L1 in cancer cells binds to its receptor PD-1, which is expressed on the surface of T cells, to escape the destruction initiated by T cells (Nam et al., 2019). Keytruda® (Pembrolizumab) is a therapeutic antibody that binds to and blocks PD-1 present on T cells, thereby resulting in the killing of cancer cells.
Emergence of immunotherapy
It is impossible to treat the heterogeneity and plasticity of cancer cells with targeted therapy. Immunotherapy including monoclonal antibodies, ICIs, cancer vaccines, and cell-based therapies, has been used to control the heterogeneity of cancer cells. ICIs induced a marked response in melanoma and non-small cell lung cancer, and CAR-T therapy evoked a similar response in B-cell acute lymphoblastic leukaemia (Khalil et al., 2016; Park et al., 2018). In addition, a variety of clinical trials involving cancer immunotherapies (more than 1,700) are listed at ClinicalTrials.gov (Ventola, 2017a).
However, immunotherapy is not a panacea for all cancers. Evidence supporting clinical response with ICIs is accumulating in many different types of cancer, leading to expanded treatment indications for these agents (Helissey et al., 2016). Indeed, clinical trial data have shown that approximately 15% to 25% (or higher) of patients with various types of cancer respond to ICIs (Grady, 2016). Positive objective response rates (ORR) following ICI treatment have been reported in many malignancies, including gastric cancer (20%), HNSCC (12% to 25%), hepatocellular carcinoma (20%), ovarian cancer (15%), small-cell lung cancer (15%), triple-negative breast cancer (20%), urothelial cancer (25%), mismatch repair deficient CRC (60%), and Hodgkin’s lymphoma (65% to 85%) (Helissey et al., 2016). Patients with bladder cancer who manifest a high expression of PD-L1 have demonstrated ORRs as high as 40% (Ventola, 2017b).
Two antibodies targeting CTLA-4 including ipilimumab and tremelimumab induced relatively rare regression rates and in a few patients with advanced metastastic melanoma, the 15% rate of objective radiographic response has lasted more than 10 years after termination cessation (Eroglu et al., 2015; Schadendorf et al., 2015; Xia et al., 2019).
Currently, the response observed with ICIs is most often a partial response (PR), comparable to other targeted agents or chemotherapy. However, treatment with ICIs is durable and can result in 80% to 90% tumour shrinkage (Grady, 2016). Until recently, surgeons have been reluctant to operate on a patient diagnosed with advanced metastatic cancer because of perceived lack of benefit in extending patient’s life (Grady, 2016). However, in a few such patients, ICIs have been used to eliminate or shrink the tumours to sizes and locations amenable to surgical removal (Grady, 2016). Furthermore, even a PR to ICI treatment is durable compared with chemotherapy or other targeted therapies (Ventola, 2017b). In some cases the prolonged benefit observed with ICI treatment has been considered a functional cure (Ventola, 2017b). However, because CTLA-4 agents stimulating the circulating anticancer T cells, the activation of T cells to elicit a significant response may require months (Grady, 2016; Ventola, 2017b). In contrast, with anti-PD-1/PD-L1 therapies, a more rapid response has often been observed because these drugs act on primed T cells that are already located in the tumour (Ventola, 2017b). Nevertheless, in most types of cancer, only a minority of patients respond to currently available ICIs. Therefore, the focus of investigation ought to involve development of other agents that target additional immune checkpoints in these cancers, as well as identifying combination therapies that use several targeted agents (Ventola, 2017b).
Side effects of immunotherapy
Immunological relevant side effects of ipilimumab treatment in clinical settings include toxicity due to disinhibited immune response. They include enterocolitis, pneumonitis, hepatitis, dermatitis, neuropathy, endocrinopathy, arthritis, nephritis, meningitis, pericarditis, and uveitis. Additional, side effects may include iritis, anaemia, and neutropenia (Khalil et al., 2016).
The toxicities observed with the currently used anti-CTLA-4 and anti-PD-1/PD-L1 ICI agents are similar, but show varying frequency of occurrence (Helissey et al., 2016). The toxicity of anti-CTLA-4 agents has been observed in more than 10% of patients, and symptoms include anorexia, abdominal pain, diarrhea, fatigue, nausea, pruritus, rash, and vomiting (Helissey et al., 2016). The toxicities associated with anti-PD-1/PD-L1 agents has been observed in more than 10% of patients, and include arthralgia, diarrhea, fatigue, nausea, pruritus, and rash (Helissey et al., 2016). In addition, the adverse effects of anti-PD-1/PD-L1 agents have been considered milder than those of anti-CTLA-4 ICIs; with the rate of grade 3 or 4 toxicities associated with anti-CTLA-4 agents ranging between 20% and 30% versus 10% and 15% with anti-PD-1 agents (Helissey et al., 2016). Importantly, although severe immune related adverse events occur in a small minority of patients receiving ICI treatment, they can become life-threatening if not detected early and managed appropriately (Helissey et al., 2016). The main life-threatening toxicities associated with anti-CTLA-4 treatment and PD-1/PD-L1 agents include dysimmune colitis and interstitial pneumonitis, respectively (Haanen et al., 2018). However, other severe toxicities associated with ICI treatment have been reported, including autoimmune anemia, infusion reactions, type-1 diabetes with ketoacidosis, Guillain–Barré syndrome, Stevens–Johnson syndrome, and thrombocytopenia with bleeding complications (Helissey et al., 2016).
Recent studies have reported additional interesting observations such as pseudoprogression. For example, a few patients display unconventional responses to treatment such as mixed responses or pseudoprogression, which is defined as an initial surge in tumour burden, typically detected on imaging, followed by tumour shrinkage (Wolchok et al., 2009). This phenomenon is a clinical challenge because differentiation discrimination of pseudoprogression from disease progression is not facilitated by the initial disease assessment. Additional imaging assessment is required (usually at 4 weeks) to establish or invalidate possible disease progression (Wolchok et al., 2009). The detection of pseudoprogression has prompted the development of immunerelated response criteria such as irRC (Wolchok et al., 2009), irRECIST (Bohnsack et al., 2014) and iRECIST (Seymour et al., 2017). Pseudoprogression was initially described in ∼10% of patients diagnosed with melanoma receiving anti-CTLA-4 antibodies (Hodi et al., 2016) and is usually associated with a survival benefit. A report published in 2018 suggests, however, that pseudoprogression might be less frequent (∼5% of patients with NSCLC treated with anti-PD-1/PD-L1 antibodies) (Tazdait et al., 2018). Finally, evidence from several studies indicates that a subset of patients might present with accelerated disease progression upon treatment with anti-PD-1/PD-L1 antibodies, often resulting in their disease deterioration (Champiat et al., 2017; Kato et al., 2017; Saâda-Bouzid et al., 2017; Ferrara et al., 2018; Zuazo-Ibarra et al., 2018). This phenomenon is referred to as hyperprogressive disease, a phenomenon that is currently not fully understood.
Limitation of immunotherapy
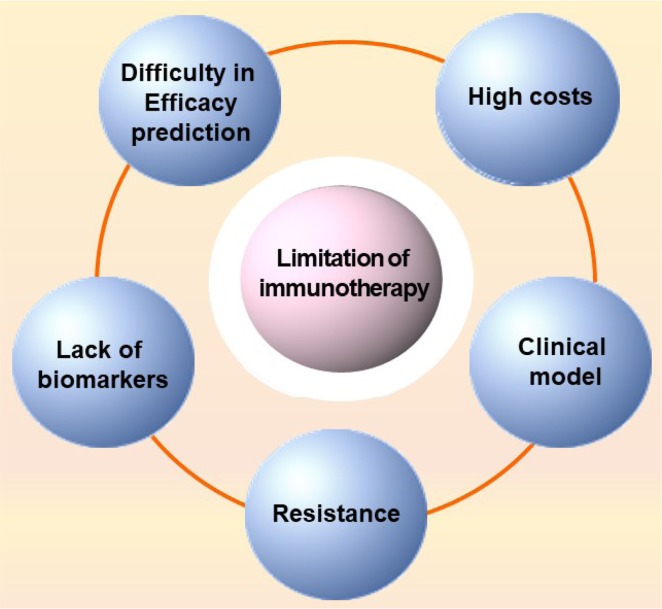
The limitations of immunotherapy are listed based on the report of Ventola as follows (Fig. 4) (Ventola, 2017c).
Fig. 4.
Limitations of immunotherapy. Limitations of immunotherapy include difficulty in efficacy prediction, high costs of drugs, inappropriate clinical model, drug resistance, and lack of an appropriate biomarker (Ventola, 2017b).
Difficulty in efficacy prediction: Patients with lung cancer carrying KRAS mutations do not respond well to EGFR tyrosine kinase inhibitors (Massarelli et al., 2007). Similarly, the efficacy of immunotherapy has been predicted by the level of PD-L1 in tumours given that cancer patients with higher levels of PD-L1 expression are more responsive to treatment (Pardoll, 2015; Sambi et al., 2019).
Clinical benefits of ICIs are only observed in certain specific cancers, such as lung cancer, and in particular in a small number of patients (Chiriva-Internati and Bot, 2015). Clinical trial data have shown that approximately 15% to 25% or higher proportion of patients diagnosed with various types of cancer respond to cytotoxic T-lymphocyte–associated protein 4 (CTLA-4) receptor or programmed death-1 (PD-1)/programmed death ligand-1 (PD-L1) ICIs (Alatrash et al., 2013). The reason for the difference in response to ICIs among cancer patients is thought to be owing to the existence of different kinds of additional immune checkpoints that suppress anticancer immune defenses. Thus, it is necessary to identify the additional checkpoints (Grady, 2016). Additional genetic mutations, cancer pathways, and immune checkpoints have been under investigation to develop new targeted drug therapies (Yang, 2015; Grady, 2016). Although challenging, these efforts will likely lead to more promising targeted cancer treatments (Zugazagoitia et al., 2016). The efficacy of cancer immunotherapies has been limited by the longstanding use of conventional chemotherapy as first-line cancer treatment (West, 2014). Consequently, because cancer immunotherapies have yet to be widely used as first-line treatments, they are typically administered to patients with compromised immune systems due to advanced disease and/or previous therapies. The role of cancer immunotherapies in restoring antitumor immune function under these conditions has been a challenge. Therefore, early intervention using personalized cancer immunotherapies may lead to higher efficacy rates in a greater percentage of patients and elicit a robust antitumor response and restore the immune system. HLA class-I is also a predictive marker to distinguish responders from non-responders. Patient’s tumours lacking HLA-class-I molecular diversity have been associated with poor survival (Chowell et al., 2018).
Lack of appropriate biomarkers: Clinical biomarkers can have diagnostic, predictive, prognostic, and pharmacogenomic values. Particularly predictive biomarkers are most commonly used routine clinical setting. There is still a lack of biomarkers for predicting whether a patient can benefit from the use of immunotherapeutic agents (Zugazagoitia et al., 2016). Progress has been observed in the biomarker field. For example, human epidermal growth factor receptor 2 (HER2) amplification has been found in 20% of patients with gastric cancer. Such patients have been found to exhibit a response rate of 40% to 50% when treated with the monoclonal antibody trastuzumab (Zugazagoitia et al., 2016).
Although PD-L1 is the most studied predictive biomarker, nivolumab has a high ORR of 50% among patients with high PD-L1 expression and a response rate of 20–30% in patients with a low PD-L1 expression in non-small-cell lung cancer (Meyers et al., 2018). These findings indicate the presence of other types of biomarkers.
PD-L1 is also an inducible marker with a varying degree of expression depending on the cancer (Robert et al., 2015; Weber et al., 2015; Gibney et al., 2016; Zugazagoitia et al., 2016; Ventola, 2017c). In the presence of conserved biomarkers on the surface of cancer cells, immunotherapy may be applicable to a wider range of patients (Ventola, 2017c).
For example, the FDA approved pembrolizumab for pediatric and adult patients with microsatellite instability-high (MSI-H) or mismatch repair-deficient solid tumours. This FDA approaval is the first in cancer treatment based on a common biomarker rather than long-term cancer pathology, especially in the subset of colorectal and noncolorectal carcinomas (Chang et al., 2018).
Recently, the importance of intestinal microbiome as a biomarker for ICI has been reported. Patients with altered intestinal microflora in response to antibiotic treatment one to two months prior to treatment with the ICI anticancer drug showed shorter progression free survival (PFS) and OS (Routy et al., 2018; Otoshi et al., 2019). In particular, it has been reported that Bifidobacterium increases antitumor immunity and promotes anti-PD-L1 efficacy (Sivan et al., 2015).
Resistance to immunotherapy: The effects of pembrolizumab therapy on advanced metastatic melanoma have been evaluated (Ribas et al., 2016; Ventola, 2017c). The 12 month median PFS was 35% and the median OS was 23 months (Ribas et al., 2016). At 21 months, cancer recurred and resistance developed (Ribas et al., 2016). These results suggested that the therapeutic effects of ICIs are unreliable (Milano, 2017). The heterogeneity of cancer and the emergence of resistant cancer clones during immune therapy are related to each other (Ventola, 2017c). Mutations in the JAK1 and JAK2 genes were observed in two patients, leading to abnormal IFN-gamma signaling and a decrease in the genes associated with the recognition and destruction of cancer cells by T cells (Zaretsky et al., 2016). Mutations of β-2-microglobulin (B2M) gene have been identified in other patients, which encode proteins on the surface of immune cells that recognize and kill cancer cells (Zaretsky et al., 2016).
In addition, although the proposed mechanism has not been fully identified in clinical studies, various resistance mechanisms have been reported by Jiang et al. (2019). For example, the activation of AXL by eIF2B and the induction of MITF inhibition induce phenotypes resistant to chemotherapy and tolerance to adoptive T-cell and anti-PD-1 immunotherapy (Falletta et al., 2017). Treatment with anti-CTLA-4 mAbs stimulates the accumulation of TNF-α and T cells in the cells, promoting enhancer of zeste homolog 2 (Ezh2) expression, resulting in loss of cancer immunity, reduction of antigen expression, and resistance to immunotherapy (Zingg et al., 2017). These results suggest that Ezh2 mediates the resistance to immunotherapy (Zingg et al., 2017). Cbl-b is one of the E3 ubiqutin ligases. The antibody against PD-L1 had no effect in mice lacking cbl-b (Fujiwara et al., 2017).
A correlation between activation of Wnt/β-catenin and absence of T cell gene expression has been reported in metastatic melanoma (Spranger et al., 2015). Spranger et al. (2015) reported that the activation of Wnt/β-catenin by immune exclusion in melanoma resulted in resistance to immunotherapy of anti-CTLA-4 and anti-PD-L1 mAbs due to defective recruitment of CD103 + dendritic cells.
A strong correlation between loss of PTEN and pembrolizumab resistance has been reported. PTEN loss activates the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) pathway (George et al., 2017). In addition, clinical studies of anti-PD1 therapy have shown an increase in the expression of TIM3, another immune checkpoint (Koyama et al., 2016).
Inappropriate clinical model: The criteria for evaluation of cancer immunotherapies should be distinguished from those evaluating response to chemotherapy or other cytotoxic agents (Wayteck et al., 2014). Immunotherapies do not directly attack cancer cells but activate the immune system, resulting in delayed anticancer effects and variation in response kinetics. In addition, immunotherapy may delay the side effects (Anagnostou et al., 2017). In the case of traditional cytotoxic chemotherapy, it is important to determine the maximum tolerated dose in phase I, whereas immunotherapy, especially antibody drugs, the minimal effective dose is more appropriate (Anagnostou et al., 2017). Therefore, the endpoints used in clinical trials of cytotoxic chemotherapy are not appropriate, based on the clinical trial results of anti-CTLA-4 ICI. In the case of immunotherapy clinical trials, the investigation of iplimumab was prolonged and the FDA approved the drug for melanoma treatment based on clinical data (Hoos and Britten, 2012). Other immune-related criteria have been proposed such as the expression and function of immune cells including cancer-specific cytotoxic T lymphocytes and the evaluation of immune memory (Alatrash et al., 2013; Ventola, 2017c). Although immune-related response criteria (irRC) have been proposed to characterize the standard response to immunotherapy in a clinical trial based on the characteristics of immunotherapy, such data need to be validated for various cancers (Wolchok et al., 2009).
High costs of immunotherapy: Immunotherapeutic approaches and molecular targeted therapies are becoming game changers in the field of cancer therapy, albeit at an exorbitant price. In particular, pembrolizumab has been estimated to cost $145,010 and $130,511 a year for treatment of melanoma and non-small cell lung cancer, respectively (Tartari et al., 2016). This treatment cost has resulted in a PFS of 6.3 months in each cancer. Similarly, the costs for the treatment of melanoma and non-small cell lung cancer using nivolumab were an estimated $64,680 and $44,100, respectively, resulting in a PFS of 5.1 months and 3.5 months, respectively (Tartari et al., 2016). Finally, the cost of treatment is likely to be manageable with appropriate doses tailored to patients need (Vergnenègre and Chouaïd, 2018). Accordingly, nivolumab has not been cost-effective in a patient cohort with general lung cancer but has been cost-effective in patients with high PD-L1 expression whereas pembrolizumab is cost-effective in patients diagnosed with previously treated or newly diagnosed metastatic non-small cell lung cancer (Verma et al., 2018).
RECIPE FOR A PERFECT INTERVENTION
In the tumour microenvironment, various factors besides immune cells are involved. Therefore, the activation of immune cells alone is insufficient to inhibit cancer. As mentioned earlier, a few cancers respond well to immunotherapy, while others do not. Immunotherapy is not indicated for cancers associated with inflammation.
Genomically targeted therapy improves median survival compared with systemic chemotherapy but does not show a long-term durable response. Immunotherapy improves median survival and long-term, durable response that increases the tail of the survival curve. Therefore, the combination of genomically targeted therapies and immune checkpoint therapies is expected to improve the median survival (Champiat et al., 2014; Sharma and Allison, 2015). Accordingly, pharmaceutical companies have been conducting a series of clinical trials with a combination of ICIs are now conducting a variety of combined clinical trials to create a perfect blend or therapeutic cocktail that can be used to treat cancer comprehensively (Ledford, 2016).
Problems to solve
The biggest challenge associated with cancer involves trials with multiple therapies that may not always be successful and existing chemotherapies, which are of limited value in the treatment of cancer. Although immunotherapeutic agents are effective in the early stages of cancer progression, their effects are limited in advanced and resistant cancers (Ventola, 2017c). The recent advances of immunotherapy and the emergence of curative medicines (e.g., Harvoni and Tasigna) represent a challenge in the area of cancer treatment.
Thus, drugs are needed to achieve the goal of remission beyond extension of life in cancer patients.
Tumour regression
Tumour regression refers to a phenomenon in which cancer does not worsen and is treated naturally or using drugs, alternative therapies, and the like. Spontaneous remission in cancer is considered a very rare phenomenon; however, melanoma, neuroblastoma, and lymphoma may show remission compared with carcinoma, with a reported frequency of 1/100,000, which is more than 1/700,000 likelihood of being hit by lightning (Hobohm, 2001). The probability of tumour remission may be overestimated or underestimated, and a carefully designed study suggests that 22% of patients with invasive breast cancer were expected to experience tumour regression (Zahl et al., 2008). One of the phenomena associated with spontaneous remission is heat emission due to infection, which may be related to the immune response or the vulnerability of cancer cells (Hobohm, 2005; Sengupta et al., 2010).
Induction of tumour remission: Encouraging clues from cancer survivors suggest ideas to overcome cancer: 1) do not try to beat cancer; 2) take a good night’s sleep; 3) enhance immunity; 4) relax your mind and get rid of stress; 5) breathe healthy air; 6) eat natural foods. We can interpret cancer survivors’ experience from a cancer biology perspective. ‘Do not try to beat cancer’ is interpreted as ‘Avoid harmful cancer treatments’. ‘Take a good night’s sleep’ has been interpreted as ‘care for the maintenance of the circadian rhythm’. ‘Enhance immunity’ can be interpreted as ‘immune activation’ which has been already achieved by immune checkpoint blockers. ‘Relax your mind and get rid of stress’ can be interpreted as ‘block activation of sympathetic nerves’. ‘Breath healthy air’ is interpreted as ‘supply of oxygen or avoidance of hypoxic conditions’. ‘Eat natural foods’ has been translated as ‘reduce intake of food containing harmful synthetic additives and manage gut microbes’. The challenge is to turn these clues into anticancer therapy.
The activation of immunity has become feasible by using agent such as ICIs (Turner, 2014). However, the ICIs have therapeutic effects on a limited number of cancer patients, indicating the need for new tools to induce disease remission.
We developed a hypothesis of NIH based on patients testimonies who have successfully overcome cancer targeting the three important targets. Inflammation and hypoxia have already been recognized as targets of cancer therapy and are important factors in tumour microenvironments. In addition, recent reports suggests that neurological factors play a major factor in tumour microenvironment (Mancino et al., 2011; Cole et al., 2015). However, studies have yet to develop a therapeutic agent targeting neurological factors, inflammation, and hypoxia to induce remission of cancer in an integrated manner.
Neurological aspects: The importance of neuronal effects on cancer progression and microenvironment has been emphasized. For example, activation of sympathetic nerves due to stress and the reduced density of tumour innervation resulted in higher recurrence-free survival (Cole et al., 2015; Makale et al., 2017). The increased chemotherapeutic response by β-blockers is mediated via anticancer and anti-angiogenic activities (Pasquier et al., 2013).
Melatonin (N-acetyl-5-methoxy-tryptamine), known as a biological clock regulator, has been implicated in the induction of apoptosis, cell cycle arrest, proliferation inhibition, and immune regulation (Vijayalaxmi et al., 2002). Defects in biological clock and activation of the sympathetic nerve are induced by various interactions between neurological factors and tumour microenvironment resulting in the progression of cancer (Tabebi et al., 2018; Verlande and Masri, 2019).
Studies investigating the regulation of tumour microenvironment have not considered the effect of nervous system on cancer cells and tumour microenvironment. Therefore, it is important to determine the role of neuronal interactions in biological clocks and tumour microenvironment, and retrain the tumour microenvironment to facilitate antitumour environment. In addition, several components of the nervous system may not target the correct molecule in different cancers.
Signaling substances released from neurons mediate cancer malignancy via various pathways that increase neovascularization, metabolic activity, immunosuppression, cancer cell proliferation and metastasis of cancer cells.
Inflammation: Inflammation plays an important role in cancer. Chronic, out-of-control, and persistent unresolved inflammation is associated with increased risk of cancer (Crusz and Balkwill, 2015). Infection and activation of oncogenes trigger inflammation and activate inflammatory transcription factors in cancer cells. Cancer cells secrete inflammatory cytokines and enzymes to recruit inflammatory cells, which lead to cancer-induced inflammation, survival and proliferation, inhibition of the immune response, angiogenesis, tissue infiltration and metastasis. In addition, inflammation induces DNA damage, thereby increasing the risk of mutations in cancer cells (Kiraly et al., 2015).
Recent studies have suggested that inflammation is terminated by substances that actively mediate the termination in contrast to inflammation that is terminated when the inflammatory triggers disappear (Lee, 2012, 2018).
The substances causing the resolution of inflammation include lipoxins, resolvins, protectins and maresins, annexinA1 and related peptides, gaseous substances such as carbon monoxide and hydrogen sulphide, adenosine, and neurotransmitters that regulate vagus nerve (Headland and Norling, 2015). Proresolving compounds induce: 1) neutrophil ceases penetration into the tissue, 2) counter regulation of chemokines and cytokines, 3) efferocytosis by macrophages, 4) conversion of macrophages from activated M1 to M2, 5) return of non-apoptotic cells to blood vessels and lymph nodes, 6) induction of the healing process and so on, resulting in homeostasis of the tissue (Serhan and Savill, 2005). Failure of various types of inflammation resolution results in many diseases including atherosclerosis, chronic obstructive pulmonary disease, obesity, cancer, multiple sclerosis, asthma, inflammatory bowel disease, and rheumatoid arthritis (Nathan and Ding, 2010).
The major issue related to resolution of inflammation in cancer is whether compounds such as resolvin and lipoxin promote or retard cancer progression. Treatment of macrophages with resolvin results in M2-type macrophages, which are similar to tumour-associated macrophages that constitute the tumour microenvironment. Therefore, it is a major issue whether resolution of inflammation is induced in order to overcome the unresolving cancer or convert to M2 TAMs in tumour microenvironment from M1.
Therefore, it is necessary to distinguish between pro-resolving and resoleotoxic cases of anti-inflammatory drugs and natural products, and investigating their effects on cancer and tumour microenvironment. An ideal inflammatory regulator is needed to suppress cancer and tumour microenvironment.
The expression of abnormal tissues such as cancer induces immune response, and the entire process such as recruitment, polarization/differentiation, and activation of macrophages plays an important role in the growth and metastasis of cancer. Macrophages present in cancer tissues produce signaling substances necessary for growth and survival of cancer, and creating barriers to the treatment of cancer (Ostuni et al., 2015).
Hypoxia: Cancer cells and various immune cells thrive in hypoxic- and nutrient-deficient and acidic microenvironment. Hypoxia-inducible factor-1α (HIF-1α) is a typical transcription factor that senses low O2 condition and induces cellular programs adaptive to hypoxic condition (Ryu et al., 2018). HIF-1α is stabilized in hypoxic tumour microenvironment, and cancer becomes aggressive by expressing gene involved in neovascularization, metastasis, growth and metabolism of cancer cells (Semenza, 2003). Hypoxia mediates the regulation of natural killer (NK) and natural killer T cells (NKT) and protects cancer cells from T lymphocyte mediated-cytotoxicity. In addition, inhibition of immune activity of tumour-associates macrophages (TAM) causes immune tolerance of cancer cells (Chouaib et al., 2017). HIF-1α induces adenosine-dependent immunosuppression by increasing the extracellular adenosine level via activation of CD39 and CD73 (Ohta, 2016). Hypoxia is a major factor in characterizing the tumour microenvironment. Hypoxia promotes growth and angiogenesis of primary cancer. It also promotes metastasis to organs such as lymph node, bone, and lung. It also increases the heterogeneity of cancer cells (Terry et al., 2017). Hypoxia also induces the termination of inflammation and immunosuppression.
HIF-1α, which is overexpressed in hypoxic conditions, affects not only cancer but also inflammation and neurological aggravation via regulation of cancer cells, immune cells and nerve cells. Therefore, the HIF-1α inhibitor can be used to treat cancer, inflammation and neurological diseases by regulating the expression of genes in cancer cells, immune cells and neurons, thereby reconstructing the tumour microenvironment. HIF-1α inhibitor selectively inhibits HIF-1α accumulation in cancer tissues and inhibits the expression of target genes, leading to inhibition of growth, neovascularization, and cancer metastasis, and induction of apoptosis, resulting in antitumor efficacy.
HIF-1α inhibitors alter the hypoxic microenvironment of tumours and regulate the activity of immune cells (Noman et al., 2015; Bhattarai et al., 2018). The limitations of immunotherapy can be overcome by, using a combination of drugs to control the immune evasion mechanisms of cancer cells by HIF-1α. HIF-1α inhibitors show a synergistic efficacy in overcoming resistance chemotherapy and radiotherapy, and are associated with multiple control mechanisms within the cell, which can lead to multi-targeting effects.
Nano-formulation to induce cancer remission: In case of anticancer drugs and antiviral drugs that show remission, the effects of single gene (bcr-abl) can be overcome by addressing the root cause. In addition, the management of cancers that are amenable to curative interventions using anticancer drugs, can be enhanced using nanotechnologies to maximize the exposure of cancerous tissues to therapeutic agents for remission of solid tumours.
Since the discovery that polymer formulations and nanodrugs accumulate in solid tumours, formulation studies based on enhanced permeability and retention (EPR) effects have become more active. FDA approved the first nano-formulations of anticancer drugs Doxil (doxorubicin liposome formulation), DaunoXome (daunorubicin), and Abraxane (albumin-bound paclitaxel). Among several published studies, only a few have been FDA approved. This suggests that nano-formulation development was not successful. Nano-formulation studies aim to exploit the effects of EPR in cancer, but many studies on nano-formulation have shown unsatisfactory results when implemented in clinical trials. The reason for this failure is that there are many differences in the EPR environment between individuals or within individuals (Danhier, 2016; Wilhelm et al., 2016; Maeda and Khatami, 2018). Therefore, the method of enhancing the effect of nano-formulation through patient selection has been proposed as a coping plan for the future (Natfji et al., 2017). In recent years, nano-formulation has been proposed to increase cancer immunity by relaying tumour infiltration and activation of immune cells (Shen et al., 2017).
When nano-drug is administered systemically, it is adsorbed to plasma protein systemically. Barriers to drug delivery include accumulation in off-target regions, abnormal blood vessels, dense extracellular matrix, stromal cells, dense cancer cells, difference in oxygen partial pressure, and interstitial fluid pressure. Development of nano-formulations that overcome these constraints is required.
Tumour regression strategy
We are told not to be stressed or to increase immunity, but from the people who overcome cancer, the so-called healers. Immune checkpoint inhibitors made us realize that these stories from healers are important factors that we should consider in treating cancer. Hence, we derive the factors necessary to induce tumour regression based on the stories of healer and from spontaneous tumour regression. In particular, we believe that immunotherapy needs to be combined with other cancer agents in order to cure cancer (Sharma and Allison, 2015). Of course, no clinical results have to date revealed whether such a combinational immunotherapy is effective.
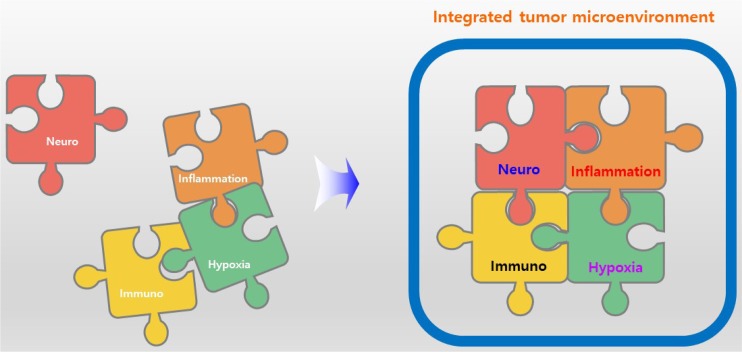
NIH is a new target to induce tumour remission, and substances that target NIH can be used to treat cancer either alone or as a combination therapy, or alleviate the side effects of immunotherapy. The combination of ICIs and antitumor compounds targeting NIH can enhance the efficacy of treatment and alleviate the potential side effects (Fig. 5).
Fig. 5.
Integrated view of tumour microenvironment including NIH. Integrative view of tumour microenvironment add ‘neuro’ to existing tumour microenvironment including immune, inflammation, and hypoxia. Sometimes, ‘inflammation’ includes ‘immuno’ but in here, ‘immuno’ is separated to emphasize ‘immuno’.
We plan to induce tumour regression by combination of immune checkpoint inhibitor with hypoxia blocker, pro-resolving compounds, and β-blockers because our data as well as data from other study shows that each class of compounds also possess anticancer activities. In addition, drug resistance is a barrier that causes difficulties in the treatment of cancer patients (Xia and Lee, 2010). Hypoxia can induce the expression of various drug resistance genes. Therefore, anti-hypoxia compounds can suppress drug resistance of cancer cells (Warfel and El-Deiry, 2014). Epithelial-mesenchymal transition is also closely associated with anticancer drug resistance. It is possible to overcome anticancer drug resistance by inhibiting EMT using resolvin, a pro-resolving lipids (Lee et al., 2013). Therefore, we aim to regulate drug resistance by suppressing hypoxia and pro-resolving lipids such as resolvins.
We also use nano-formulation that penetrate solid tumour and microenvironment. We expect that such a combination might alleviate the side effects of ICI and suppress tumour progression via stimulation from neuronal, inflammatory, and hypoxic tumour microenvironments. Whether the NIH controlling agent can restore immune response in combination with or without the ICI needs to be determined. The findings can be shed to determine the anticancer effects of immune activation of NIH controlling agent.
It is also necessary to determine whether the NIH control agent affects the prognosis of autoimmune disease and to test the possibility that the NIH control substance can control the autoimmune-related side effects caused by ICIs. The results can be used to determine the possibility of combining NIH regulatory substances with ICIs. In addition, it is important to evaluate whether the effects of NIH control substances vary between cancer that responds to ICI (hot cancer) and those that do not (cold cancer).
Therefore, a dual-disease mouse model capable of simultaneously inducing cancer and autoimmune diseases such as vitiligo and multiple sclerosis is needed. Using specific compounds and peptides, transgenic mice can be induced to develop cancers of lung, breast, and pancreas. It is interesting to investigate whether induction of tumour remission is possible with only three NIH and ICI combinations or whether regulation of new agent contributing tumour remission (for example T cell or macrophage metabolism) (Hope and Salmond, 2019; Vitale et al., 2019) is necessary.
CONCLUSION
The presumption that cancer is a disease that leads to death and cannot be cured has been a major obstacle to the treatment of cancer. However, our indomitable attempts to treat cancer eventually led to a glimmer of hope in targeted therapies such as Gleevec. A renewed attempt to treat the immune system could set goals for tumour regression. Subsequent studies using explosive combination therapies with immunosuppressive agents have attempted to induce tumour regression. We may be able to find an answer to tumour regression challenge among the clinically untested therapies that target cancer.
Especially, neuronal and hypoxic tumour environments are considered as promising areas to overcome the challenges associated with immune suppression. Fortunately, medicines that control the neuronal tumour microenvironment may already exist, although their effectiveness in cancer patients remains to be proven. Therefore, a new combination therapy that regulates NIH targets may be a new strategy to overcome tumour regression. However, appropriate drug combination strategies that can overcome the entry barriers and access the tumour microenvironment successfully, are also needed.
Acknowledgments
This study was supported by grants (NRF-2017R1A2A1A 05000878 and NRF-2018R1A5A2023127) of the Basic Science Research Program, through the National Research Foundation (NRF) of Korea.
Footnotes
CONFLICT OF INTEREST
None.
REFERENCES
- Alatrash G, Jakher H, Stafford PD, Mittendorf EA. Cancer immunotherapies, their safety and toxicity. Expert Opin Drug Saf. 2013;12:631–645. doi: 10.1517/14740338.2013.795944. [DOI] [PubMed] [Google Scholar]
- Anagnostou V, Yarchoan M, Hansen AR, Wang H, Verde F, Sharon E, Collyar D, Chow LQ, Forde PM. Immuno-oncology trial endpoints: capturing clinically meaningful activity. Clin Cancer Res. 2017;23:4959–4969. doi: 10.1158/1078-0432.CCR-16-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattarai D, Xu X, Lee K. Hypoxia-inducible factor-1 (HIF-1) inhibitors from the last decade (2007 to 2016): a “structure-activity relationship” perspective. Med Res Rev. 2018;38:1404–1442. doi: 10.1002/med.21477. [DOI] [PubMed] [Google Scholar]
- Bhome R, Bullock MD, Al Saihati HA, Goh RW, Primrose JN, Sayan AE, Mirnezami AH. A top-down view of the tumor microenvironment: structure, cells and signaling. Front Cell Dev Biol. 2015;3:33. doi: 10.3389/fcell.2015.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnsack O, Hoos A, Ludajic K. Adaptation of the immune related response criteria: irRECIST [Internet] Lugano, OncologyPRO. 2014:c2014. [cited 2014 Sep 29]. Available from: https://oncologypro.esmo.org/Meeting-Resources/ESMO-2014/Adaptation-of-the-immune-related-response-criteria-irRECIST/.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S, Chaput N, Eggermont A, Marabelle A, Soria J.-C. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res. 2017;23:1920–1928. doi: 10.1158/1078-0432.CCR-16-1741. [DOI] [PubMed] [Google Scholar]
- Champiat S, Ileana E, Giaccone G, Besse B, Mountzios G, Eggermont A, Soria JC. Incorporating immune-checkpoint inhibitors into systemic therapy of NSCLC. J Thorac Oncol. 2014;9:144–153. doi: 10.1097/JTO.0000000000000074. [DOI] [PubMed] [Google Scholar]
- Chang L, Chang M, Chang HM, Chang F. Microsatellite instability: a predictive biomarker for cancer immunotherapy. Appl Immunohistochem Mol Morphol. 2018;26:e15–e21. doi: 10.1097/PAI.0000000000000575. [DOI] [PubMed] [Google Scholar]
- Chiriva-Internati M, Bot A. A new era in cancer immunotherapy: discovering novel targets and reprogramming the immune system. Int Rev Immunol. 2015;34:101–103. doi: 10.3109/08830185.2015.1015888. [DOI] [PubMed] [Google Scholar]
- Choi E, Yang JW. Updates to clinical information on anticancer immunotherapy. Korean J Clin Pharm. 2018;28:65–75. doi: 10.24304/kjcp.2017.28.1.65. [DOI] [Google Scholar]
- Chouaib S, Noman M, Kosmatopoulos K, Curran M. Hypoxic stress: obstacles and opportunities for innovative immunotherapy of cancer. Oncogene. 2017;36:439. doi: 10.1038/onc.2016.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowell D, Morris LG, Grigg CM, Weber JK, Samstein RM, Makarov V, Kuo F, Kendall SM, Requena D, Riaz N. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science. 2018;359:582–587. doi: 10.1126/science.aao4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, Sood AK. Sympathetic nervous system regulation of the tumour microenvironment. Nat. Rev. Cancer. 2015;15:563–572. doi: 10.1038/nrc3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol. 2015;12:584–596. doi: 10.1038/nrclinonc.2015.105. [DOI] [PubMed] [Google Scholar]
- da Silva-Diz V, Lorenzo-Sanz L, Bernat-Peguera A, Lopez-Cerda M, Munoz P. Cancer cell plasticity: impact on tumor progression and therapy response. Semin Cancer Biol. 2018;53:48–58. doi: 10.1016/j.semcancer.2018.08.009. [DOI] [PubMed] [Google Scholar]
- Danhier F. To exploit the tumor microenvironment: since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control. Release. 2016;244:108–121. doi: 10.1016/j.jconrel.2016.11.015. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Nakasone ES, Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Dev. Cell. 2010;18:884–901. doi: 10.1016/j.devcel.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu Z, Kim DW, Wang X, Camacho LH, Chmielowski B, Seja E, Villanueva A, Ruchalski K, Glaspy JA, Kim KB. Long term survival with cytotoxic T lymphocyte-associated antigen 4 blockade using tremelimumab. Eur. J. Cancer. 2015;51:2689–2697. doi: 10.1016/j.ejca.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falletta P, Sanchez-del-Campo L, Chauhan J, Effern M, Kenyon A, Kershaw CJ, Siddaway R, Lisle R, Freter R, Daniels MJ. Translation reprogramming is an evolutionarily conserved driver of phenotypic plasticity and therapeutic resistance in melanoma. Genes Dev. 2017;31:18–33. doi: 10.1101/gad.290940.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara R, Mezquita L, Texier M, Lahmar J, Audigier-Valette C, Tessonnier L, Mazieres J, Zalcman G, Brosseau S, Le Moulec S, Leroy L, Duchemann B, Lefebvre C, Veillon R, Westeel V, Koscielny S, Champiat S, Ferté C, Planchard D, Remon J, Boucher ME, Gazzah A, Adam J, Bria E, Tortora G, Soria JC, Besse B, Caramella C. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol. 2018;4:1543–1552. doi: 10.1001/jamaoncol.2018.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara M, Anstadt EJ, Clark RB. Cbl-b deficiency mediates resistance to programmed death-ligand 1/programmed death-1 regulation. Front Immunol. 2017;8:42. doi: 10.3389/fimmu.2017.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S, Miao D, Demetri GD, Adeegbe D, Rodig SJ, Shukla S, Lipschitz M, Amin-Mansour A, Raut CP, Carter SL, Hammerman P, Freeman GJ, Wu CJ, Ott PA, Wong KK, Van Allen EM. Loss of PTEN is associated with resistance to anti-PD-1 checkpoint blockade therapy in metastatic uterine leiomyosarcoma. Immunity. 2017;46:197–204. doi: 10.1016/j.immuni.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17:e542–e551. doi: 10.1016/S1470-2045(16)30406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady D. Harnessing the immune system to fight cancer. The New York Times. 2016 Jul 30; Available from: https://www.nytimes.com/2016/07/31/health/harnessing-the-immune-system-to-fight-cancer.html/.
- Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, Jordan K, ESMO Guidelines Committee Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv264–iv266. doi: 10.1093/annonc/mdy162. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Headland SE, Norling LV. In Seminars in immunology. Vol. 27. Elsevier; 2015. The resolution of inflammation: principles and challenges; pp. 149–160. [DOI] [PubMed] [Google Scholar]
- Helissey C, Vicier C, Champiat S. The development of immunotherapy in older adults: new treatments, new toxicities? J Geriatr Oncol. 2016;7:325–333. doi: 10.1016/j.jgo.2016.05.007. [DOI] [PubMed] [Google Scholar]
- Hobohm U. Fever and cancer in perspective. Cancer Immunol Immunother. 2001;50:391–396. doi: 10.1007/s002620100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobohm U. Fever therapy revisited. Br. J. Cancer. 2005;92:421–425. doi: 10.1038/sj.bjc.6602386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochhaus A, Masszi T, Giles FJ, Radich JP, Ross DM, Gomez Casares MT, Hellmann A, Stentoft J, Conneally E, Garcia-Gutierrez V, Gattermann N, Wiktor-Jedrzejczak W, le Coutre PD, Martino B, Saussele S, Menssen HD, Deng W, Krunic N, Bedoucha V, Saglio G. Treatment-free remission following frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the ENESTfreedom study. Leukemia. 2017;31:1525–1531. doi: 10.1038/leu.2017.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi FS, Hwu W.-J, Kefford R, Weber JS, Daud A, Hamid O, Patnaik A, Ribas A, Robert C, Gangadhar TC, Joshua AM, Hersey P, Dronca R, Joseph R, Hille D, Xue D, Li XN, Kang SP, Ebbinghaus S, Perrone A, Wolchok JD. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol. 2016;34:1510–1517. doi: 10.1200/JCO.2015.64.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoos A, Britten CM. The immuno-oncology framework: Enabling a new era of cancer therapy. Oncoimmunology. 2012;1:334–339. doi: 10.4161/onci.19268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope HC, Salmond RJ. Targeting the tumor microenvironment and T cell metabolism for effective cancer immunotherapy. Eur J Immunol. 2019;49:1147–1152. doi: 10.1002/eji.201848058. [DOI] [PubMed] [Google Scholar]
- Jamal-Hanjani M, Quezada SA, Larkin J, Swanton C. Translational implications of tumor heterogeneity. Clin Cancer Res. 2015;21:1258–1266. doi: 10.1158/1078-0432.CCR-14-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Li L, Li Y, Li Q. Molecular mechanisms and countermeasures of immunotherapy resistance in malignant tumor. J. Cancer. 2019;10:1764–1771. doi: 10.7150/jca.26481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junttila MR, de Sauvage FJ. Influence of tumour microenvironment heterogeneity on therapeutic response. Nature. 2013;501:346–354. doi: 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A, Kurzrock R. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res. 2017;23:4242–4250. doi: 10.1158/1078-0432.CCR-16-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating GM. Ledipasvir/Sofosbuvir: a review of its use in chronic hepatitis C. Drugs. 2015;75:675–685. doi: 10.1007/s40265-015-0381-2. [DOI] [PubMed] [Google Scholar]
- Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol. 2016;13:273–290. doi: 10.1038/nrclinonc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiraly O, Gong G, Olipitz W, Muthupalani S, Engelward BP. Inflammation-induced cell proliferation potentiates DNA damage-induced mutations in vivo. PLoS Genet. 2015;11:e1004901. doi: 10.1371/journal.pgen.1004901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal J, Kornete M, Joyce JA. Re-education of macrophages as a therapeutic strategy in cancer. Immunotherapy. 2019;11:677–689. doi: 10.2217/imt-2018-0156. [DOI] [PubMed] [Google Scholar]
- Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, Gandhi L, Redig AJ, Rodig SJ, Asahina H, Jones RE, Kulkarni MM, Kuraguchi M, Palakurthi S, Fecci PE, Johnson BE, Janne PA, Engelman JA, Gangadharan SP, Costa DB, Freeman GJ, Bueno R, Hodi FS, Dranoff G, Wong KK, Hammerman PS. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501. doi: 10.1038/ncomms10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledford H. Cocktails for cancer with a measure of immunotherapy. Nature. 2016;532:162–164. doi: 10.1038/532162a. [DOI] [PubMed] [Google Scholar]
- Lee CH. Resolvins as new fascinating drug candidates for inflammatory diseases. Arch Pharm Res. 2012;35:3–7. doi: 10.1007/s12272-012-0121-z. [DOI] [PubMed] [Google Scholar]
- Lee CH. Epithelial-mesenchymal transition: Initiation by cues from chronic inflammatory tumor microenvironment and termination by anti-inflammatory compounds and specialized pro-resolving lipids. Biochem Pharmacol. 2018;158:261–273. doi: 10.1016/j.bcp.2018.10.031. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Park MK, Lee EJ, Lee CH. Resolvin D1 inhibits TGF-β1-induced epithelial mesenchymal transition of A549 lung cancer cells via lipoxin A4 receptor/formyl peptide receptor 2 and GPR32. Int J Biochem Cell Biol. 2013;45:2801–2807. doi: 10.1016/j.biocel.2013.09.018. [DOI] [PubMed] [Google Scholar]
- Lindsley CW. New 2016 Data and Statistics for Global Pharmaceutical Products and Projections through 2017. ACS Publications. 2017 doi: 10.1021/acschemneuro.7b00253. [DOI] [PubMed] [Google Scholar]
- Maeda H, Khatami M. Analyses of repeated failures in cancer therapy for solid tumors: poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs. Clin Transl Med. 2018;7:11. doi: 10.1186/s40169-018-0185-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon FX, Boquimpani C, Kim DW, Benyamini N, Clementino NCD, Shuvaev V, Ailawadhi S, Lipton JH, Turkina AG, De Paz R, Moiraghi B, Nicolini FE, Dengler J, Sacha T, Takahashi N, Fellague-Chebra R, Acharya S, Wong S, Jin Y, Hughes TP. Treatment-free remission after secondline nilotinib treatment in patients with chronic myeloid leukemia in chronic phase: results from a single-group, phase 2, open-label study. Ann Intern Med. 2018;168:461–470. doi: 10.7326/M17-1094. [DOI] [PubMed] [Google Scholar]
- Makale MT, McDonald CR, Hattangadi-Gluth JA, Kesari S. Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nat Rev Neurol. 2017;13:52–64. doi: 10.1038/nrneurol.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancino M, Ametller E, Gascón P, Almendro V. The neuronal influence on tumor progression. Biochim. Biophys. Acta. 2011;1816:105–118. doi: 10.1016/j.bbcan.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Massarelli E, Varella-Garcia M, Tang X, Xavier AC, Ozburn NC, Liu DD, Bekele BN, Herbst RS, Wistuba II. KRAS mutation is an important predictor of resistance to therapy with epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. Clin Cancer Res. 2007;13:2890–2896. doi: 10.1158/1078-0432.CCR-06-3043. [DOI] [PubMed] [Google Scholar]
- McGranahan N, Swanton C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell. 2017;168:613–628. doi: 10.1016/j.cell.2017.01.018. [DOI] [PubMed] [Google Scholar]
- Meyers DE, Bryan PM, Banerji S, Morris DG. Targeting the PD-1/PD-L1 axis for the treatment of non-small-cell lung cancer. Curr Oncol. 2018;25:e324–e334. doi: 10.3747/co.25.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milano G. Resistance to immunotherapy: clouds in a bright sky. Invest New Drugs. 2017;35:649–654. doi: 10.1007/s10637-017-0456-x. [DOI] [PubMed] [Google Scholar]
- Nam S, Lee A, Lim J, Lim JS. Analysis of the expression and regulation of PD-1 protein on the surface of myeloid-derived suppressor cells (MDSCs) Biomol. Ther. (Seoul) 2019;27:63–70. doi: 10.4062/biomolther.2018.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natfji AA, Ravishankar D, Osborn HM, Greco F. Parameters affecting the enhanced permeability and retention effect: the need for patient selection. J Pharm Sci. 2017;106:3179–3187. doi: 10.1016/j.xphs.2017.06.019. [DOI] [PubMed] [Google Scholar]
- Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Noman MZ, Hasmim M, Messai Y, Terry S, Kieda C, Janji B, Chouaib S. Hypoxia: a key player in antitumor immune response. A review in the theme: cellular responses to hypoxia. Am J Physiol, Cell Physiol. 2015;309:C569–C579. doi: 10.1152/ajpcell.00207.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta A. A metabolic immune checkpoint: adenosine in tumor microenvironment. Front Immunol. 2016;7:109. doi: 10.3389/fimmu.2016.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostuni R, Kratochvill F, Murray PJ, Natoli G. Macrophages and cancer: from mechanisms to therapeutic implications. Trends Immunol. 2015;36:229–239. doi: 10.1016/j.it.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Otoshi T, Nagano T, Tachihara M, Nishimura Y. Possible Biomarkers for Cancer Immunotherapy. Cancers (Basel) 2019;11:E935. doi: 10.3390/cancers11070935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll D. In Seminars in Oncology. Vol. 42. Elsevier; 2015. Cancer and the immune system: basic concepts and targets for intervention; pp. 523–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, Sauter C, Wang Y, Santomasso B, Mead E. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378:449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MK, Lee CH. Role of sphingosylphosphorylcholine in tumor and tumor microenvironment. Cancers (Basel) 2019;11:E1696. doi: 10.3390/cancers11111696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquier E, Street J, Pouchy C, Carre M, Gifford A, Murray J, Norris M, Trahair T, Andre N, Kavallaris M. β-blockers increase response to chemotherapy via direct antitumour and anti-angiogenic mechanisms in neuroblastoma. Br. J. Cancer. 2013;108:2485–2494. doi: 10.1038/bjc.2013.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, Olson OC, Quick ML, Huse JT, Teijeiro V, Setty M, Leslie CS, Oei Y, Pedraza A, Zhang J, Brennan CW, Sutton JC, Holland EC, Daniel D, Joyce JA. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail DF, Bowman RL, Akkari L, Quick ML, Schuhmacher AJ, Huse JT, Holland EC, Sutton JC, Joyce JA. The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science. 2016;352:aad3018. doi: 10.1126/science.aad3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A, Hamid O, Daud A, Hodi FS, Wolchok JD, Kefford R, Joshua AM, Patnaik A, Hwu W.-J, Weber JS, Gangadhar TC, Hersey P, Dronca R, Joseph RW, Zarour H, Chmielowski B, Lawrence DP, Algazi A, Rizvi NA, Hoffner B, Mateus C, Gergich K, Lindia JA, Giannotti M, Li XN, Ebbinghaus S, Kang SP, Robert C. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315:1600–1609. doi: 10.1001/jama.2016.4059. [DOI] [PubMed] [Google Scholar]
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ, Hernberg MM, Lebbé C, Charles J, Mihalcioiu C, Chiarion-Sileni V, Mauch C, Cognetti F, Arance A, Schmidt H, Schadendorf D, Gogas H, Lundgren-Eriksson L, Horak C, Sharkey B, Waxman IM, Atkinson V, Ascierto PA. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- Roma-Rodrigues C, Mendes R, Baptista PV, Fernandes AR. Targeting tumor microenvironment for cancer therapy. Int J Mol Sci. 2019;20:E840. doi: 10.3390/ijms20040840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routy B, Le Chatelier E, Derosa L, Duong CP, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, Fidelle M, Flament C, Poirier-Colame V, Opolon P, Klein C, Iribarren K, Mondragón L, Jacquelot N, Qu B, Ferrere G, Clémenson C, Mezquita L, Masip JR, Naltet C, Brosseau S, Kaderbhai C, Richard C, Rizvi H, Levenez F, Galleron N, Quinquis B, Pons N, Ryffel B, Minard-Colin V, Gonin P, Soria JC, Deutsch E, Loriot Y, Ghiringhelli F, Zalcman G, Goldwasser F, Escudier B, Hellmann MD, Eggermont A, Raoult D, Albiges L, Kroemer G, Zitvogel L. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- Ryu D, Ryoo IG, Kwak MK. Overexpression of CD44 standard isoform upregulates HIF-1α signaling in hypoxic breast cancer cells. Biomol. Ther. (Seoul) 2018;26:487–493. doi: 10.4062/biomolther.2018.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saâda-Bouzid E, Defaucheux C, Karabajakian A, Coloma VP, Servois V, Paoletti X, Even C, Fayette J, Guigay J, Loirat D, Peyrade F, Alt M, Gal J, Le Tourneau C. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2017;28:1605–1611. doi: 10.1093/annonc/mdx178. [DOI] [PubMed] [Google Scholar]
- Sambi M, Bagheri L, Szewczuk MR. Current challenges in cancer immunotherapy: multimodal approaches to improve efficacy and patient response rates. J Oncol. 20192019:4508794. doi: 10.1155/2019/4508794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen T.-T, Berman DM, Wolchok JD. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33:1889–1894. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Sengupta N, MacFie TS, MacDonald TT, Pennington D, Silver AR. Cancer immunoediting and “spontaneous” tumor regression. Pathol Res Pract. 2010;206:1–8. doi: 10.1016/j.prp.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, Lin NU, Litière S, Dancey J, Chen A, Hodi FS, Therasse P, Hoekstra OS, Shankar LK, Wolchok JD, Ballinger M, Caramella C, de Vries EGE. RECIST working group (2017) iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 18:e143–e152. doi: 10.1016/S1470-2045(17)30074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Sun T, Hoang HH, Burchfield JS, Hamilton GF, Mittendorf EA, Ferrari M. In Seminars in Immunology. Vol. 34. Elsevier; 2017. Enhancing cancer immunotherapy through nanotechnology-mediated tumor infiltration and activation of immune cells; pp. 114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre M.-L, Chang EB, Gajewski TF. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231–235. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- Tabebi M, Soderkvist P, Jensen LD. Hypoxia Signaling and Circadian Disruption in and by Pheochromocytoma. Front. Endocrinol. (Lausanne) 2018;9:612. doi: 10.3389/fendo.2018.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartari F, Santoni M, Burattini L, Mazzanti P, Onofri A, Berardi R. Economic sustainability of anti-PD-1 agents nivolumab and pembrolizumab in cancer patients: recent insights and future challenges. Cancer Treat Rev. 2016;48:20–24. doi: 10.1016/j.ctrv.2016.06.002. [DOI] [PubMed] [Google Scholar]
- Tazdait M, Mezquita L, Lahmar J, Ferrara R, Bidault F, Ammari S, Balleyguier C, Planchard D, Gazzah A, Soria JC, Marabelle A, Besse B, Caramella C. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: Comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur. J. Cancer. 2018;88:38–47. doi: 10.1016/j.ejca.2017.10.017. [DOI] [PubMed] [Google Scholar]
- Terry S, Buart S, Chouaib S. Hypoxic stress-induced tumor and immune plasticity, suppression, and impact on tumor heterogeneity. Front Immunol. 2017;8:1625. doi: 10.3389/fimmu.2017.01625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner KA. Radical Remission : Surviving Cancer against All Odds. HarperOne; New York, NY: 2014. [Google Scholar]
- Ventola CL. Cancer immunotherapy, part 1: current strategies and agents. P T. 2017a;42:375–383. [PMC free article] [PubMed] [Google Scholar]
- Ventola CL. Cancer immunotherapy, part 2: efficacy, safety, and other clinical considerations. P T. 2017b;42:452–463. [PMC free article] [PubMed] [Google Scholar]
- Ventola CL. Cancer immunotherapy, part 3: challenges and future trends. P T. 2017c;42:514–521. [PMC free article] [PubMed] [Google Scholar]
- Vergnenègre A, Chouaïd C. Review of economic analyses of treatment for non-small-cell lung cancer (NSCLC) Expert Rev Pharmacoecon Outcomes Res. 2018;18:519–528. doi: 10.1080/14737167.2018.1485099. [DOI] [PubMed] [Google Scholar]
- Verlande A, Masri S. Circadian clocks and cancer: timekeeping governs cellular metabolism. Trends Endocrinol Metab. 2019;30:445–458. doi: 10.1016/j.tem.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma V, Sprave T, Haque W, Simone CB, 2nd, Chang JY, Welsh JW, Thomas CR., Jr A systematic review of the cost and cost-effectiveness studies of immune checkpoint inhibitors. J. Immunother. Cancer. 2018;6:128. doi: 10.1186/s40425-018-0442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayalaxmi, Thomas CR, Jr., Reiter RJ, Herman TS. Melatonin: from basic research to cancer treatment clinics. J Clin Oncol. 2002;20:2575–2601. doi: 10.1200/JCO.2002.11.004. [DOI] [PubMed] [Google Scholar]
- Vitale I, Manic G, Coussens LM, Kroemer G, Galluzzi L. Macrophages and Metabolism in the Tumor Microenvironment. Cell Metab. 2019;30:36–50. doi: 10.1016/j.cmet.2019.06.001. [DOI] [PubMed] [Google Scholar]
- Warfel NA, El-Deiry WS. HIF-1 signaling in drug resistance to chemotherapy. Curr Med Chem. 2014;21:3021–3028. doi: 10.2174/0929867321666140414101056. [DOI] [PubMed] [Google Scholar]
- Wayteck L, Breckpot K, Demeester J, De Smedt SC, Raemdonck K. A personalized view on cancer immunotherapy. Cancer Lett. 2014;352:113–125. doi: 10.1016/j.canlet.2013.09.016. [DOI] [PubMed] [Google Scholar]
- Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH, Jr., Lao CD, Linette GP, Thomas L, Lorigan P, Grossmann KF, Hassel JC, Maio M, Sznol M, Ascierto PA, Mohr P, Chmielowski B, Bryce A, Svane IM, Grob JJ, Krackhardt AM, Horak C, Lambert A, Yang AS, Larkin J. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- West H. Nivolumab as first line monotherapy for advanced non-small cell lung cancer: could we replace first line chemotherapy with immunotherapy? Transl Lung Cancer Res. 2014;3:400–402. doi: 10.3978/j.issn.2218-6751.2014.09.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WCW. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1:16014. doi: 10.1038/natrevmats.2016.14. [DOI] [Google Scholar]
- Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- Xia AL, Xu Y, Lu XJ. Cancer immunotherapy: challenges and clinical applications. J Med Genet. 2019;56:1–3. doi: 10.1136/jmedgenet-2018-105852. [DOI] [PubMed] [Google Scholar]
- Xia Y, Lee K. Targeting multidrug resistance with small molecules for cancer therapy. Biomol. Ther. (Seoul) 2010;18:375–385. doi: 10.4062/biomolther.2010.18.4.375. [DOI] [Google Scholar]
- Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest. 2015;125:3335–3337. doi: 10.1172/JCI83871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahl PH, Maehlen J, Welch HG. The natural history of invasive breast cancers detected by screening mammography. Arch Intern Med. 2008;168:2311–2316. doi: 10.1001/archinte.168.21.2311. [DOI] [PubMed] [Google Scholar]
- Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly L, Saco J, Homet Moreno B, Mezzadra R, Chmielowski B, Ruchalski K, Shintaku IP, Sanchez PJ, Puig-Saus C, Cherry G, Seja E, Kong X, Pang J, Berent-Maoz B, Comin-Anduix B, Graeber TG, Tumeh PC, Schumacher TN, Lo RS, Ribas A. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016;375:819–829. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingg D, Arenas-Ramirez N, Sahin D, Rosalia RA, Antunes AT, Haeusel J, Sommer L, Boyman O. The histone methyltransferase Ezh2 controls mechanisms of adaptive resistance to tumor immunotherapy. Cell Rep. 2017;20:854–867. doi: 10.1016/j.celrep.2017.07.007. [DOI] [PubMed] [Google Scholar]
- Zuazo-Ibarra M, Arasanz H, Fernandez-Hinojal G, Gato-Canas M, Hernandez-Marin B, Martinez-Aguillo M, Lecumberri MJ, Fernandez A, Teijeira L, Vera R, Kochan G, Escors D. Highly differentiated CD4 T cells unequivocally identify primary resistance and risk of hyperprogression to PD-L1/PD-1 immune checkpoint blockade in lung cancer. bioRxivorg. 2018 doi: 10.1101/320176. [DOI] [Google Scholar]
- Zugazagoitia J, Guedes C, Ponce S, Ferrer I, Molina-Pinelo S, Paz-Ares L. Current challenges in cancer treatment. Clin Ther. 2016;38:1551–1566. doi: 10.1016/j.clinthera.2016.03.026. [DOI] [PubMed] [Google Scholar]