Abstract
Histone deacetylase (HDAC) inhibitors represent a novel class of anticancer agents, which can be used to inhibit cell proliferation and induce apoptosis in several types of cancer cells. In this study, we investigated the anticancer activity of MHY4381, a newly synthesized HDAC inhibitor, against human prostate cancer cell lines and compared its efficacy with that of suberoylanilide hydroxamic acid (SAHA), a well-known HDAC inhibitor. We assessed cell viability, apoptosis, cell cycle regulation, and other biological effects in the prostate cancer cells. We also evaluated a possible mechanism of MHY4381 on the apoptotic cell death pathway. The IC50 value of MHY4381 was lower in DU145 cells (IC50=0.31 μM) than in LNCaP (IC50=0.85 μM) and PC-3 cells (IC50=5.23 μM). In addition, the IC50 values of MHY4381 measured in this assay were significantly lower than those of SAHA against prostate cancer cell lines. MHY4381 increased the levels of acetylated histones H3 and H4 and reduced the expression of HDAC proteins in the prostate cancer cell lines. MHY4381 increased G2/M phase arrest in DU145 cells, and G1 arrest in LNCaP cells. It also activated reactive oxygen species (ROS) generation, which induced apoptosis in the DU145 and LNCaP cells by increasing the ratio of Bax/Bcl-2 and releasing cytochrome c into the cytoplasm. Our results indicated that MHY4381 preferentially results in antitumor effects in DU145 and LNCaP cells via mitochondria-mediated apoptosis and ROS-facilitated cell death pathway, and therefore can be used as a promising prostate cancer therapeutic.
Keywords: HDAC inhibitor, MHY4381, Prostate cancer, Apoptosis, Reactive oxygen species
INTRODUCTION
Prostate cancer is the most common type of cancer diagnosed in men. In 2018, prostate cancer represented 19% of all cancer diagnoses in men in the United States, which is the highest in the entire world (Siegel et al., 2018). The current front-line therapies for prostate cancer are either surgical removal of the tumor or radiation therapy, regardless of androgen sensitivity (Balk and Knudsen, 2008; Schröder et al., 2012). The androgen receptor (AR) is a transcription factor that plays a pivotal role in the regulation of androgen levels in tumors, and in the development of advanced prostate cancer (Balk, 2009). However, the mechanisms underlying the initiation and progression of prostate cancer are still not fully understood. In the early stages of prostate cancer, patients usually receive anti-androgen therapy; however, relapses occur frequently within 1 to 3 years, and patients then require treatment with a continuous conventional therapy. The effectiveness of chemotherapy remains limited, and is associated with serious adverse effects and a compromised quality of life (Keizman and Eisenberger, 2010; Kuban et al., 2013). Owing to increased mortality rates and failure of conventional chemotherapy in advanced prostate cancer patients, there is an urgent need for new alternative therapies (Bilusic et al., 2017; Litwin and Tan, 2017).
Several studies have indicated that epigenetic aberrations can contribute to cancer development (Ducasse and Brown, 2006; Dokmanovic et al., 2007; Park and Han, 2019). Histone protein acetylation is controlled by the balance of histone acetyltransferases (HATs) and histone deacetylases (HDACs). Altered histone protein acetylation regulates the transcription of genes, particularly different types of oncogenes, tumor suppressor genes, and DNA repair genes (Dokmanovic and Marks, 2005; Yoon and Eom, 2016). Hence, abnormal expression of HDACs is coupled with the development of cancer (Mottet and Castronovo, 2008). Currently, the study of HDAC inhibitors is focused on their potential as emerging drugs in cancer therapy. However, the exact mechanisms by which individual HDACs regulate tumorigenesis are quite diverse. Four HDAC classes have been identified. Class I HDACs (HDAC1, 2, 3, and 8) are principally located in the nucleus and have uniform expression in all tissues, including prostate tissue (Weichert et al., 2008). Class II HDACs (HDAC4, 5, 6, 7, 9, and 10) are expressed in both the nucleus and cytoplasm of tissues. Class IV consists of HDAC11 only, which exhibits the properties of both class I and II HDACs (Perry et al., 2010). Overexpression of HDAC1, 2 and 3 have been reported in prostate cancer (Waltregny et al., 2004; Lin et al., 2013). Nevertheless, there are relatively few highly selective HDAC inhibitors, but a few drugs with proposed selectivity for several class I and class II HDACs have been developed. Pracinostat belongs to a class of hydroxamic acids that specifically target HDAC class I, II, and IV, and is currently in phase II of clinical trials for the treatment of prostate cancer patients (Ganai, 2016; Eckschlager et al., 2017). The relative abilities of pan- and selective-Class I HDAC inhibitors to attenuate androgen receptor (AR)-mediated target gene expression and proliferation were assessed in several prostate cancer cell lines (Ruscetti et al., 2016; McLeod et al., 2018). Additionally, HDAC inhibitors disrupt cytoplasmic AR via HDAC6 inhibition, leading to AR degradation and growth suppression of prostate cancer cells. Therefore, HDAC inhibitors can also be important therapeutic agents in castration-resistant prostate cancer (CRPC). However, current HDAC inhibitors are not effective in clinical trials treating CRPC.
The aim of this study was to evaluate the differential anticancer efficacy of the new HDAC inhibitor MHY4381 in both androgen-sensitive and androgen-independent cancer prostate cells. Our results suggest that the MHY4381 preferentially results in antitumor effects in DU145 and androgen-dependent LNCaP cells via both mitochondria-mediated apoptosis and reactive oxygen species (ROS)-facilitated cytotoxicity.
MATERIALS AND METHODS
Reagents
Suberoylanilide hydroxamic acid (SAHA) and other chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA). MHY4381 (purity, 98.5%) was synthesized by Prof. Moon (College of Pharmacy, Pusan National University, Busan, Korea). Stock solutions (10 mM) of drugs were prepared in sterile dimethyl sulfoxide (DMSO) and stored at −20°C until use. Culture media and fetal bovine serum (FBS) were purchased from Gibco Invitrogen Corporation (Carlsbad, CA, USA). Primary antibodies against acetyl-H3, acetyl-H4, Bax, poly-ADP-ribose polymerase (PARP), Bcl-2, p53, cytochrome C, cyclin A, cyclin B, cyclin D, HDACs, caspase-3 and 9, and β-actin were purchased from Cell Signaling (Beverly, MA, USA). Antibodies against p27 and p21 and horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide) and DAPI (4′-6-diamidino-2-phenylindole) were purchased from Thermo Fisher Scientific, Inc (Invitrogen, MA, USA), while DCFH-DA (2′,7′-dichlo rofluorescein-diacetate) was obtained from Sigma-Aldrich.
Cell lines and culture conditions
Human prostate cancer cell lines (LNCaP, PC-3, and DU145) were obtained from the American Type Culture Collection (Manassas, VA, USA). DU145 and LNCaP cells were grown in RPMI 1640 (with 10% FBS), and PC-3 cells were maintained in F12 media supplemented with 4 mmol/L l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. All cells were maintained as monolayers in a humidified atmosphere containing 5% CO2 at 37°C, and the culture medium was replaced every two days.
Total HDAC enzyme activity assay
HDAC enzyme activity was assessed using an HDAC fluorogenic assay kit (BPS, Bioscience, San Diego, CA, USA) according to the manufacturer’s instructions. Briefly, HDAC enzymes were incubated with vehicle, various concentrations of MHY4381, or SAHA at 37°C for 30 min in the presence of an HDAC fluorometric substrate. Fluorescence was measured using VICTOR 3 (PerkinElmer, MA, USA) with excitation at 360 nm and emission at 460 nm. Data were analyzed using GraphPad Prism (GraphPad Software, San Diego, CA, USA).
Cytotoxicity assay
The cytotoxic effects of MHY4381 and SAHA were assessed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT, 5 mg/mL). Approximately, 3×103 cells per well were seeded into a 96 well plate. After 24 h, cells were treated with a range of drug concentrations (0.01–10 μM) for 24 and 48 h. One hundred microliters of MTT solution was added to each well at the end of the experiment and incubated for 4 h at 37°C in the dark. A VERSA Max Microplate Reader (Molecular Devices Corp., CA, USA) was then used to measure the optical density at 540 nm. The data obtained from three independent experiments were used to calculate the IC50 from sigmoidal dose-response curves using SigmaPlot 10 software (Jandel Scientific, San Rafael, CA, USA).
Colony formation assay
A total of 500 cells/well were seeded into 6 well plates and then incubated with MHY4381 (1 μM) or SAHA (1 μM) for 14 days. Viable colonies were fixed with methanol, stained with 0.05% crystal violet for 20 min, washed with phosphate buffered saline (PBS), and air dried. Colonies with more than 30 cells were counted and then normalized to the numbers in the control group (Franken et al., 2006).
Western blot analysis
After 48 h of treatment with MHY4381 (0.1, 0.5, or 1 μM) or SAHA (1 μM), cells were collected and washed with cold PBS. Total protein was isolated using a PRO-PREPTM protein extraction solution (Bio-Rad, Hercules, CA, USA). Equal amounts of protein (20 μg) were loaded onto 6–15% SDS polyacrylamide gels and then transferred to PVDF membranes. The membranes were then incubated with the desired primary antibodies at 4°C overnight, washed with TBS buffer, and then incubated with HRP-conjugated anti-mouse or anti-rabbit antibodies. The immunoreactive bands were visualized using Chemi DocTM (Bio-Rad, Hercules, CA, USA), and the blots were quantitatively analyzed using an image analyzer (LAS-4000; Fuji film, Tokyo, Japan).
Cell cycle analysis by flow cytometry
Cells were seeded and allowed to attach overnight, and were then treated with MHY4381 (0.1, 0.5 or 1 μM) or SAHA (1 μM) for 48 h. The total number of cells, including the ones in suspension and those adhered to the walls of the wells, were harvested separately to identify sub-G1 or other cell cycle stages, respectively, and were washed in 1% bovine serum albumin (BSA) before fixing in 95% ice-cold ethanol containing 0.5% Tween-20 for 1 h. Cells (1×106) were washed in a solution containing 1% BSA, stained with cold propidium iodide (PI) staining solution (10 μg/mL PI and 100 μg/mL RNase in PBS), and incubated in the dark for 30 min at room temperature. Data were analyzed using a flow cytometer (Guava EasyCyte flow cytometer, Millipore, Billerica, MA, USA).
AnnexinV-FITC binding assay
The AnnexinV-FITC binding assay was carried out using the AnnexinV-FITC detection kit (BD, Bioscience). The cells were treated with MHY4381 (0.1, 0.5, or 1 μM) or SAHA (1 μM) for 24 and 48 h. The samples were prepared according to the manufacturer’s instructions and analyzed by flow cytometry (Guava EasyCyte flow cytometer, Millipore).
4′-6-Diamidino-2-phenylindole (DAPI) staining
Morphological changes in the nuclear chromatin in apoptotic cells were assessed by DAPI staining. Cells (1×104) were seeded into a 6-well plate dish and treated with MHY4381 (0.1, 0.5, or 1 μM) or SAHA (1 μM) for 48 h, followed by fixation with methanol and staining with DAPI solution (1 μg/mL). The staining solution was discarded, and the cells were washed with cold PBS. Confocal microscopy was performed using an FV10i microscope (40x, Olympus, Tokyo, Japan) to visualize apoptotic cells.
Intracellular ROS assay
A non-fluorescent 2′,7′-dichlo rofluorescein-diacetate (DCFH-DA, 10 μM) intracellular probe was used to detect intracellular ROS formation. Approximately 1×103 cells/well were seeded into a 96-well plate. After 24 h, the cells were exposed to MHY4381 (1 μM) or SAHA (1 μM) for 12 h followed by incubation with 10 μM DCFH-DA in serum-free media for 30 min at 37°C. The cells were washed twice with PBS, and DCF fluorescence was detected using a fluorometric imaging plate reader at excitation and emission wavelengths of 488 nm and 520 nm, respectively. To observe the fluorescence image, the cells were seeded into a confocal dish and treated as described previously with the given compounds (MHY4381 or SAHA, each 1 μM) and DCFH-DA in serum-free media for 30 min at 37°C. After washing the cells with PBS, their images were captured using an FV10i microscope (Olympus).
Statistical analysis
All the data are presented as mean ± SD from at least three independent experiments. Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Bonferroni’s test. Analysis was performed using Graph Pad Prism Software Version 5.0 (GraphPad Software). A p-value of <0.05 was considered statistically significant.
RESULTS
MHY4381 inhibits HDAC enzyme activity and expression in prostate cancer cells
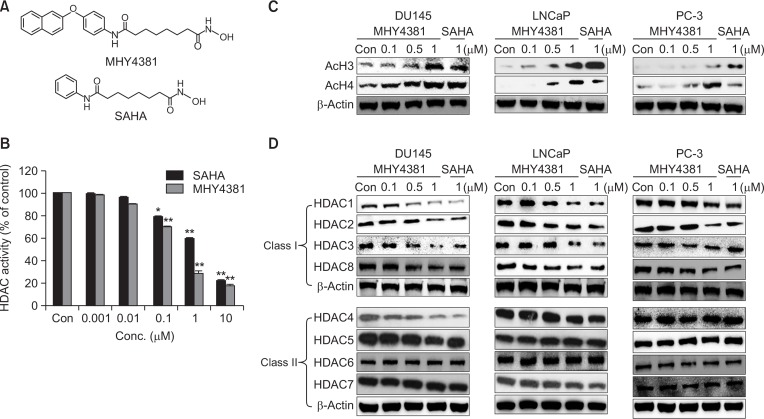
The chemical structures of MHY4381 and SAHA are shown in Fig. 1A. Both MHY4381 and SAHA treatment significantly inhibited HDAC enzyme activity in a concentration-dependent manner, and the IC50 value (0.31 μM) for MHY4381 was considerably lower than that of SAHA (1.21 μM) (Fig. 1B). The effect of MHY4381 on the expression levels of acetylated histone proteins and HDACs was measured in three prostate cancer cell lines (DU145, LNCaP, and PC-3) by western blotting. MHY4381 treatment markedly increased acetylated H3 and H4 protein levels in both DU145 and LNCaP cells at 0.5 μM and 1 μM (Fig. 1C). In PC-3 cells, hyperacetylation of H3 and H4 was observed at 1 μM MHY4381 (Fig. 1C). After 48 h of MHY4381 treatment, class I HDACs (1–3 and 8) were noticeably downregulated in the three prostate cancer cell lines. In contrast, the expression of HDAC4 (class II) was downregulated only in DU145 cells but was unchanged in both LNCaP and PC-3 cells by MHY4381 treatment. The expression of the other class II HDACs (5, 6, and 7) was unaffected by treatment with MHY4381 in all three prostate cancer cell lines (Fig. 1D).
Fig. 1.
Effect of MHY4381 and SAHA on histone deacetylase (HDAC) expression and activity and acetylation of histones (H3 and H4). (A) The chemical structures of MHY4381 and SAHA. (B) HDAC enzyme activity was measured using a fluorogenic HDAC assay kit. HDAC enzyme activity is shown relative to that of the control. Three independent experiments were performed and results are expressed as mean ± SD. Statistical analysis was performed using one-way analysis of variance, followed by Bonferroni’s multiple comparison tests. *p<0.05 and **p<0.01 indicate significant differences between the control and treatment groups. (C) Effect of MHY4381 and SAHA on the levels of acetylated H3 and H4. Prostate cancer (DU145, LNCaP, and PC-3) cells were exposed to the indicated concentrations of MHY4381 and SAHA for 48 h. β-Actin was used as a loading control. (D) Effect of MHY4381 and SAHA on the protein expression levels of HDACs. Prostate cancer (DU145, LNCaP, and PC-3) cells were exposed to the indicated concentrations of MHY4381 and SAHA for 48 h and expression of HDACs (class I and II) was measured by western blot analysis. β-Actin was used as a loading control.
MHY4381 inhibits the proliferation of prostate cancer cells
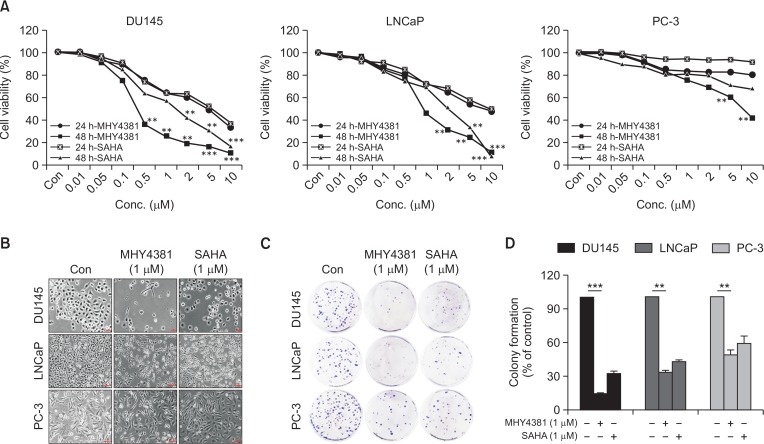
The cytotoxicity of MHY4381 in the human prostate cancer cell lines (DU145, LNCaP, and PC-3) was assessed by MTT assay. Both MHY4381 and SAHA prevented cancer cell proliferation in a concentration-and time-dependent manner. MHY4381 was more potent than SAHA (IC50, 5.48 μM) with the following IC50 values in the different cell lines after treatment: DU145 (IC50=0.31 μM), LNCaP (IC50=0.85 μM), and PC-3 (IC50=5.23 μM) (Fig. 2A). All three human prostate cancer cell lines showed different growth inhibition responses when exposed to MHY4381. Prominent morphological changes included cellular elongation induced by MHY4381 in both DU145 and LNCaP cells after 48 h of treatment (Fig. 2B).
Fig. 2.
Effect of MHY4381 and SAHA on viability and morphology of prostate cancer (DU145, LNCaP, or PC-3) cells. (A) The cells were treated with MHY4381 or SAHA at various concentrations (0.01-10 μM) for 24 h and 48 h. Cell viability was assessed using an MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay, and the data represent the mean ± SD of three independent experiments. (B) Morphological changes (cell body shrinkage and reduction of cell number) in the cells were documented by photography after MHY4381 (1 μM) and SAHA (1 μM) treatment for 48 h. Light microscopic images representative of three independent experiments are shown. Scale bar, 100 μm. (C) The effect of MHY4381 or SAHA on prostate cancer single cell proliferation assessed by colony formation assay. Prostate cancer cells were treated with MHY4381 (1 μM) or SAHA (1 μM), and allowed to form colonies in fresh medium for 14 days. (D) Representative histogram showing the plating efficiency percentage in prostate cancer cells. Data are expressed as mean ± SD (n=3). **p<0.01, ***p<0.001 versus control group.
MHY4381 inhibits colony formation in prostate cancer cells
To evaluate the anti-tumor effects of MHY4381, a colony formation assay was performed. The ability to form colonies is a fundamental characteristic of cancer cells (Nair et al., 2004; Franken et al., 2006). As shown in Fig. 2C and 2D, colony formation of DU145 cells (15% of control), LNCaP cells (34% of control), and PC-3 (49.17% of control) cells was significantly inhibited after treatment with MHY4381. The inhibitory potency of MHY4381 on colony formation was higher than that of SAHA.
Effect of MHY4381 on cell cycle regulation in prostate cancer cells
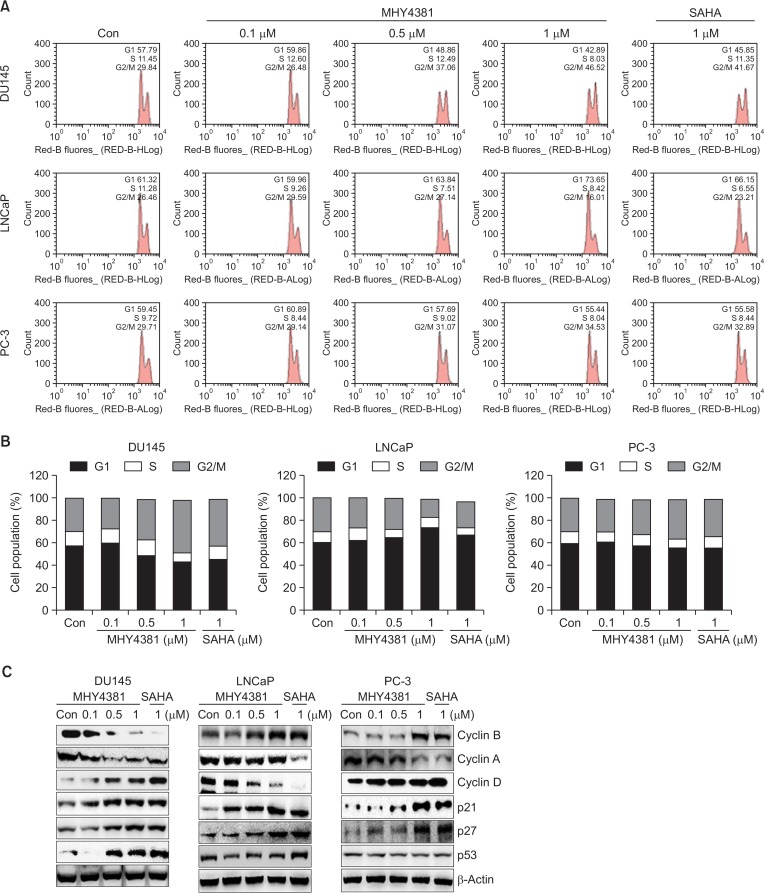
HDAC inhibitors reduce cellular growth by arresting the cell cycle at various checkpoints. The three prostate cancer cell lines were treated with MHY4381 (0.1, 0.5, or 1 μM) or SAHA (1 μM) for 48 h, and their DNA content was measured by flow cytometry. As shown in Fig. 3A, MHY4381 significantly induced G2/M accumulation in DU145 cells and simultaneously decreased the number of cells in S phase. In comparison, MHY4381 arrested cells at the G1 phase of the cell cycle in LNCaP cells. A similar effect was observed with SAHA treatment and occurred in a concentration-dependent manner. Both MHY4381 and SAHA arrested cells at the G2/M phase of the cell cycle in PC-3 cells, but this was not significant. The relative distribution of the effects on different phases of the cell cycle is shown in Fig. 3B. The effect of MHY4381 on the expression of cell cycle proteins was studied by western blotting. The expression levels of cyclin B and cyclin A were markedly decreased, whereas the expression of cyclin D was increased in DU145 cells after MHY4381 treatment. In LNCaP cells, the expression of cyclin D was reduced after MHY4381 treatment. MHY4381 considerably enhanced both p21WAF1/CIP1 and p27 protein levels in DU145 and LNCaP cells, and to a lesser extent in PC-3 cells, in a concentration-dependent manner. Furthermore, p53 was also upregulated in DU145 and LNCaP cells, whereas the expression levels of p53 did not noticeably change in PC-3 cells after MHY4381 treatment (Fig. 3C).
Fig. 3.
Effect of MHY4381 or SAHA on cell cycle distribution in prostate cancer cells (DU145, LNCaP, and PC-3). (A) Cells were treated with MHY4381 or SAHA at the indicated concentrations for 48 h, stained with propidium iodide (PI) and analyzed by flow cytometry to determine the cell cycle distribution. (B) Bar diagram showing the G1, S, and G2/M phase distributions of prostate cancer cells treated with vehicle control, MHY4381, or SAHA. Data are expressed as mean (n=3). (C) Effect of MHY4381 or SAHA on the protein expression levels of cell cycle regulatory proteins. DU145, LNCaP, and PC-3 cells were treated with MHY4381 (0.1, 0.5, or 1.0 μM) or SAHA (1 μM) for 48 h. The expression levels of cyclin B, cyclin A, cyclin D, p21, p27, and p53 were detected by western blot analysis. Data are representative of three independent experiments. β-Actin was used as a loading control.
MHY4381 induces apoptosis in prostate cancer cells
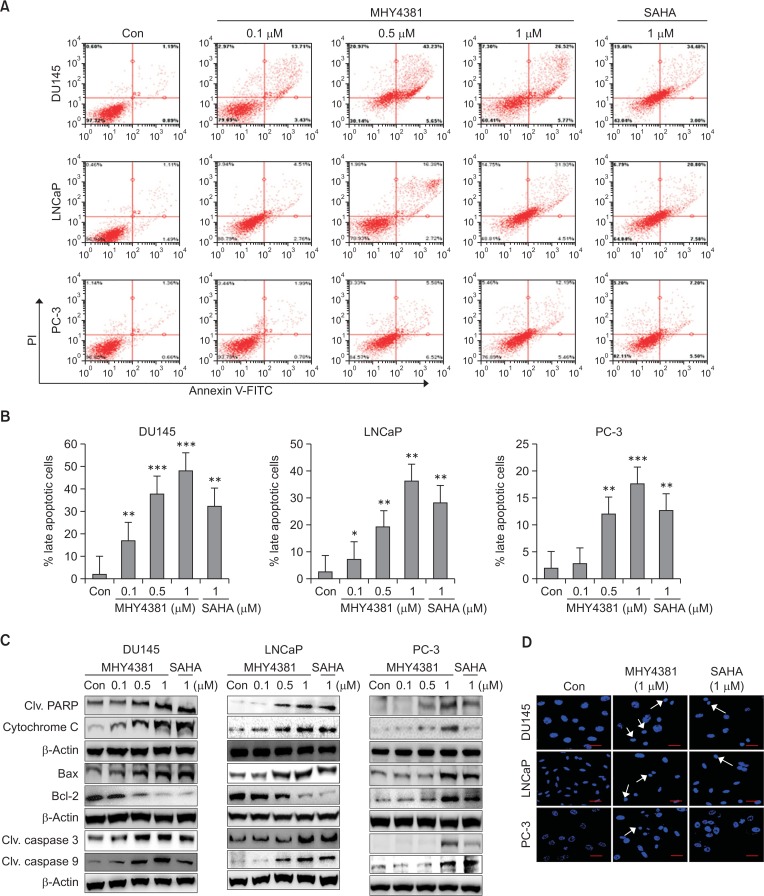
AnnexinV/PI double staining and western blot analysis were performed to understand the mechanism of prostate cancer cell growth inhibition after MHY4381 treatment. In both DU145 and LNCaP cells, the number of late stage apoptotic cells were increased in a concentration-dependent manner after MHY4381 treatment when compared to the control (Fig. 4A, 4B). The expression of apoptosis-related proteins was measured by western blotting. Treatment with MHY4381 increased the levels of cleaved PARP. MHY4381 also caused the cytoplasmic release of cytochrome c and decreased Bcl-2 expression levels in both DU145 and LNCaP cells in a concentration-dependent manner (Fig. 4C). Next, we used DAPI staining to analyze the apoptotic cells by confocal microscopy. The number of apoptotic cells with enhanced fluorescence increased after DAPI staining of MHY4381 treated cells (Fig. 4D). PC-3 cells did not show any significant increase in apoptotic cell death with FACS analysis.
Fig. 4.
Apoptosis induced by MHY4381 and SAHA in DU145, LNCaP, and PC-3 prostate cancer cells. (A) Cells were treated with vehicle control, MHY4381 (0.1, 0.5, or 1 μM), or SAHA (1 μM) for 48 h, then stained with FITC-conjugated Annexin V and propidium iodide (PI) for flow cytometry analysis. The flow cytometry profile represents AnnexinV-FITC staining on the x axis and PI staining on the y axis. (B) Bar graph showing the percentage of late apoptotic cells. Data are expressed as mean ± SD. *p<0.05, **p<0.01, and ***p<0.001 versus the control group of three independent experiments. (C) Cells were treated with vehicle control, MHY4381 (0.1, 0.5, or 1 μM), or SAHA (1 μM) for 48 h and the expression of apoptosis-related proteins was analyzed by western blot. β-Actin was used as a loading control. (D) Effects of MHY4381 or SAHA on nuclear morphological changes assessed by DAPI staining. DU145, LNCaP, and PC-3 cells were treated with MHY4381 (1 μM) or SAHA (1 μM) for 48 h, followed by fixation and DAPI staining. Images were observed using an Olympus confocal microscope (FV10i. Magnification ×400, Olympus). Scale bar, 50 μm.
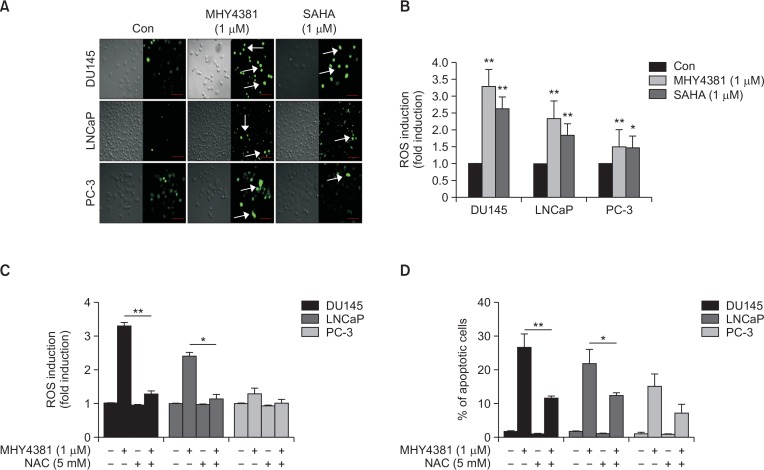
MHY4381 increases intracellular ROS formation in prostate cancer cells
Evidence has shown that intracellular ROS play an important role in cell death by inducing apoptosis (Robert and Rassool, 2012). Accordingly, we measured intracellular ROS formation in prostate cancer cell lines after MHY4381 treatment. As shown in Fig. 5A, the intracellular ROS levels increased about 3.4-fold compared to the control in DU145 cells after MHY4381 treatment, whereas in LNCaP cells, ROS levels increased by about 2.4-fold. In PC-3 cells, there was no significant generation of intracellular ROS. These data support the hypothesis that MHY4381 treatment increases ROS production, and that it could be involved in inducing apoptosis in prostate cancer cell lines (Fig. 5B). To further confirm the role of ROS in inducing apoptosis, an ROS scavenger (NAC) was used. Pretreatment with NAC completely erased the production of ROS induced by MHY4381 treatment in the prostate cancer cell lines. In parallel, NAC also significantly attenuated MHY4381-mediated apoptosis. These data support the notion that the generation of ROS by MHY4381 treatment may play a role in inducing apoptosis (Fig. 5C, 5D).
Fig. 5.
MHY4381 and SAHA induce generation of ROS in prostate cancer cells. (A) DU145, LNCaP, and PC-3 cells were treated with the indicated concentrations of MHY4381 and SAHA for 12 h and then stained with DCFH-DA (10 μM) for 30 min. Images were obtained using an Olympus confocal microscope (FV10i. Magnification ×400, Olympus). Scale bar, 50 μm. (B) Cells were treated with the indicated concentrations of MHY4381 and SAHA for 12 h and, stained with DCFH-DA (10 μM) and absorbance was read using a fluorometric imaging plate reader. Bar graph showing the ROS levels. Data are expressed as mean ± SD. *p<0.05, **p<0.01 versus control group for three independent experiments. (C) Bar graph showing the ROS level in cells after exposure to MHY4381 (1 μM), NAC (5 mM), or MHY4381 (1 μM) plus NAC (5 mM). *p<0.05, **p<0.01 versus MHY4381. (D) Apoptosis induced by ROS generation observed by flow cytometry. After exposure to MHY4381 (1 μM), NAC (5 mM), or MHY4381 (1 μM) plus NAC (5 mM) for 12 h, the apoptotic cells were observed by flow cytometry and depicted in a bar graph. The data are expressed as mean ± SD. *p<0.05, **p<0.01 versus MHY4381.
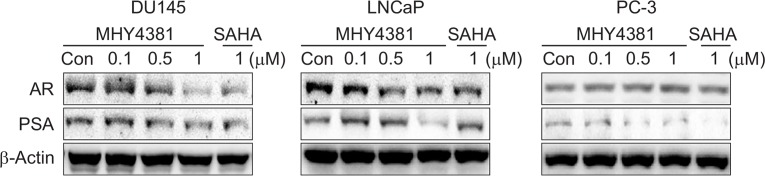
MHY4381 decreases AR and PSA expression in prostate cancer cells
In both androgen-dependent and independent prostate cancer cells, AR plays an important role in growth and proliferation. The effect of MHY4381 on AR expression levels and its target gene PSA was examined in cell lysates. As shown in Fig. 6, high concentrations of MHY4381 caused a significant reduction in both AR and PSA levels in DU145 and LNCaP cells, although not in PC-3 cells. The data show that there is an inhibitory effect of MHY4381 on the AR signaling pathway in prostate cancer cells which may affect the overall biological function of the cells.
Fig. 6.
Effects of MHY4381 and SAHA on the expression of the androgen receptor (AR) and prostate-specific antigen (PSA) in human prostate cancer cells. DU145, LNCaP, and PC-3 cells were treated with the indicated concentrations of MHY4381 and SAHA for 48 h. Western blotting using total protein lysate was used to assess the expression levels of AR and PSA. β-Actin was used as a loading control.
DISCUSSION
HDAC inhibitors induce cancer cell cycle arrest, apoptosis, and autophagy through the regulation of target gene expression by altering the acetylation status of chromatin and other non-histone proteins. Currently, there are a number of HDAC inhibitors in various stages of clinical trials, and some have been approved for use in cancer patients (Marks, 2010; Eckschlager et al., 2017). It has been indicated that HDACs may be essential for the maintenance of specific target genes required for survival and proliferation of cancer cells but not of normal cells (Ropero and Esteller, 2007). Taken together, expression of HDAC1, 2, and 3 is highly increased in the prostate carcinoma tissues compared with those of normal tissues (Weichert et al., 2008). Therefore, the development of highly potent and selective HDAC inhibitors is still necessary to delineate the associated biological effects for the complex signaling pathway of cancer therapy.
It has become clear that in antiandrogen-resistant cancers, the androgen receptor (AR) signaling axis is intact and is required for prostate cancer growth. Thus, there is a heightened interest in developing new drugs that function in part by down-regulating AR expression in prostate tumors. In the present study, we evaluated the anticancer effects of a novel HDAC inhibitor MHY4381 against three types of prostate cancer cell lines (DU145, LNCaP, and PC-3). Our data show that MHY4381 has potent anticancer activities at low doses, not only inhibiting HDAC activity, but also inducing apoptosis through cell cycle arrest and ROS generation in different types of prostate cancer cells. Histone protein acetylation was induced in DU145 and LNCaP cells by MHY4381 treatment at 0.5 and 1 μM, whereas in PC-3 cells, this effect was only observed at the highest concentration (1 μM). HDAC enzymes control the balance between the acetylation and deacetylation of histone and non-histone proteins, which normally regulate the expression of transcriptional factors. It has been reported that both class I and class II HDACs are present in prostate cancer cells at various levels (Waltregny et al., 2004). However, the exact functional role of individual HDAC subfamilies on anticancer activity in prostate cancer remains unclear. In this study, we ascertained that MHY4381 significantly reduced the expression of HDAC1, HDAC2, and HDAC3 in DU145 and LNCaP cells, whereas HDAC2 and HDAC4 expression was only reduced marginally in PC-3 cells. Therefore, more specific inhibitors of HDAC1 and HDAC2 could be therapeutically useful for patients with prostate cancer. However, the mechanism behind HDAC1/HDAC2 inhibition and the reasons why DU145 and LNCaP cells are more susceptible to HDAC1 and 2 inhibitors than PC-3 cells are unknown. One of the main reasons for different susceptibilities of MHY4381 against prostate cancer cells is that it closely associated with cell cycle arrest at a specific checkpoint.
Previous research has shown that different types of HDAC inhibitors induce cell growth arrest via different intracellular signaling pathways; they discontinue the cell cycle at the G0/G1 or G2/M phase (Park et al., 2004; Shankar and Srivastava, 2008; Feng et al., 2015). Cell cycle progression can be regulated at both the G0/G1 and G2/M phases by the tumor suppressor p53 to recover DNA damage (Kastan and Bartek, 2004). Taken together, the cell cycle is known to be regulated by cyclins, CDKs, p16, p21, and p27 (Kastan and Bartek, 2004; Telles and Seto, 2012). While p53 contributes to many cell processes, its primary function is as a transcription factor; improper functioning of p53 can therefore cause loss of control of the cell cycle, leading to aberrant cell growth. It has been reported that checkpoint kinase 1 (Chk1) has a unique role in enhancing the resistance of normal cells against HDAC inhibitors both in vitro as well as in vivo (Lee et al., 2011). In this study, MHY4381 treatment caused an accumulation of cell in the G2/M phase in DU145 cells, whereas the G1 phase arrest was markedly increased in LNCaP cells. These results indicate that MHY4381 could inhibit prostate cancer cells growth via inducing cell cycle arrest at the G1 and G2/M phase. These results were very similar to previously reported studies that SAHA can cause G1 or G2/M arrest in different cancer cell lines (Butler et al., 2000; Komatsu et al., 2006). MHY4381 reduced the expression of cyclin A/B and increased the expression of p21 and p27 in DU145 cells. In contrast, cyclin D, which is involved in G1 phase arrest was reduced in LNCaP cells after MHY4381 treatment. Several studies have reported that p21WAF1/CIP1 activation is mediated by HDAC inhibitors due to an enhancement of the acetylation of H3 and H4 histones associated with the p21WAF1/CIP1 promotor (Sambucetti et al., 1999; Richon et al., 2000). Typically 21WAF1/CIP1 accumulates at the G1 transition, although it may also have a role in the G2/M transition (Niculescu et al., 1998). We observed that MHY4381 dramatically increased p21 expression in three prostate cancer cell lines, leading to cell cycle arrest. Upregulation of p21 can occur via both p53-dependent and p53-independent pathways (Zhao et al., 2006; Wang et al., 2012). In this study, MHY4381 upregulated p53 expression in the DU145 and LNCaP cells, but not PC-3 cells. Among the three cell lines we used, LNCaP or DU145 is more sensitive than PC3 to MHY4381-induced cell growth inhibition. We also found that antiapoptotic protein Bcl-2 levels play an important role in PC3 resistance because Bcl-2 proteins increased in PC-3 cells after MHY4381 treatment.
MHY4381 possesses the ability to stimulate ROS production and cytochrome c release to activate cell death, similar to what is seen for SAHA (Ruefli et al., 2001). A reduction in the levels of anti-oxidant enzymes such as MnSOD and catalase can lead to ROS accumulation, which in turn creates oxidative stress in cancer cells (Rosato et al., 2008). The activation of p53 and the accumulation of ROS are concurrent processes that are responsible for mitochondrial membrane disruption. MHY4381 significantly induced ROS production in DU145 and LNCaP cells, which enhanced apoptotic cell death. MHY4381-induced ROS production and apoptosis levels were dramatically protected by a combination with NAC in DU145 and LNCaP cells. Mitochondrial membranes are depolarized due to the translocation of Bax, resulting in the formation of apoptosomes with Apaf-1 and other procaspase proteins such as caspase-3 and 9 (Bishayee et al., 2015). HDAC inhibitors induce apoptosis mostly through the mitochondrial pathway by upregulating apoptotic proteins Bax, cleaved caspase-3, or cleaved caspase-9 expression (Shankar and Srivastava, 2008; Bao et al., 2016). In particular, MHY4381 significantly induced PARP cleavage and cytochrome c release. These data suggest that alteration ratio of Bax/Bcl-2 proteins lead to the release of cytochrome c, supporting that MHY4381 may induce the intrinsic apoptotic pathway through mitochondria.
It has been reported that AR is involved in regulating the cell cycle, and that inhibiting AR expression results in cell cycle arrest in cancer cells (Balk and Knudsen, 2008; Koryakina et al., 2015). Although the majority of human prostate cancer cell lines are AR-negative, detectable levels of AR mRNA have been reported in both DU145 and PC-3 cells (Xu et al., 2006). Androgen-regulated G1 transition has been observed in prostate cancer cells, where androgen-deprived prostate cancer cells generally arrest in the G2 phase through CDK4 activation (Shrotriya et al., 2012). Activated AR stimulates cyclin D1 and promotes Rb phosphorylation, which in turn stimulates G1 phase entry (Alimirah et al., 2006). Therefore, hyperactivity of AR and the loss/absence of p21 are important in establishing the resistance of prostate cancer cells towards apoptosis (Wang et al., 2001). We observed a reduction in AR expression levels in LNCaP and DU145 cells at higher concentrations of MHY4381. This result was similar to a previous study showed that a reduction in AR expression levels and an increase in p21 levels has been reported following SAHA treatment (Marrocco et al., 2007). Further study is required to understand the mechanism by which MHY4381 decreases AR expression in androgen-dependent prostate cancer cells.
In summary, our data demonstrated that the MHY4381 could inhibit growth of human prostate cancer cells through mitochondria-mediated apoptosis and a reduction in AR expression in DU145 and LNCaP cells. These cellular events are accompanied by modifying the activity of cell regulatory proteins via ROS production. Therefore, we suggest that MHY4381 could be used as an anticancer agent for the treatment of prostate cancer.
Acknowledgments
This work was supported by grants from the Natio nal Research Foundation (NRF) of Korea (NRF-2016R1A2B 2011071, NRF-2018M3A9C8021792, and NRF2018R1D1A 11307048591), which is funded by the Korean Government.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- Alimirah F, Chen J, Basrawala Z, Xin H, Choubey D. DU-145 and PC-3 human prostate cancer cell lines express androgen receptor: Implications for the androgen receptor functions and regulation. FEBS Lett. 2006;580:2294–2300. doi: 10.1016/j.febslet.2006.03.041. [DOI] [PubMed] [Google Scholar]
- Balk SP. Increased expression of genes converting adrenal androgens to testosterone in castration-recurrent prostate cancer. In: Mohler J, Tindall D, editors. Androgen Action in Prostate Cancer. Springer; New York: 2009. pp. 123–139. [DOI] [Google Scholar]
- Balk SP, Knudsen KE. AR, the cell cycle, and prostate cancer. Nucl Recept Signal. 2008;6:e001. doi: 10.1621/nrs.06001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L, Diao H, Dong N, Su X, Wang B, Mo Q, Yu H, Wang X, Chen C. Histone deacetylase inhibitor induces cell apoptosis and cycle arrest in lung cancer cells via mitochondrial injury and p53 up-acetylation. Cell Biol Toxicol. 2016;32:469–482. doi: 10.1007/s10565-016-9347-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilusic M, Madan RA, Gulley JL. Immunotherapy of prostate cancer: facts and hopes. Clin Cancer Res. 2017;23:6764–6770. doi: 10.1158/1078-0432.CCR-17-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishayee K, Khuda-Bukhsh AR, Huh SO. PLGA-loaded gold-nanoparticles precipitated with quercetin downregulate HDAC-Akt activities controlling proliferation and activate p53-ROS crosstalk to induce apoptosis in hepatocarcinoma cells. Mol. Cells. 2015;38:518–527. doi: 10.14348/molcells.2015.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler LM, Agus DB, Scher HI, Higgins B, Rose A, Cordon-Cardo C, Thaler HT, Rifkind RA, Marks PA, Richon VM. Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Res. 2000;60:5165–5170. [PubMed] [Google Scholar]
- Dokmanovic M, Clarke C, Marks PA. Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res. 2007;5:981–989. doi: 10.1158/1541-7786.MCR-07-0324. [DOI] [PubMed] [Google Scholar]
- Dokmanovic M, Marks PA. Prospects: histone deacetylase inhibitors. J Cell Biochem. 2005;96:293–304. doi: 10.1002/jcb.20532. [DOI] [PubMed] [Google Scholar]
- Ducasse M, Brown MA. Epigenetic aberrations and cancer. Mol Cancer. 2006;5:60. doi: 10.1186/1476-4598-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckschlager T, Plch J, Stiborova M, Hrabeta J. Histone deacetylase inhibitors as anticancer drugs. Int J Mol Sci. 2017;18:E1414. doi: 10.3390/ijms18071414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Cai D, Zhang B, Lou G, Zou X. Combination of HDAC inhibitor TSA and silibinin induces cell cycle arrest and apoptosis by targeting survivin and cyclinB1/Cdk1 in pancreatic cancer cells. Biomed Pharmacother. 2015;74:257–264. doi: 10.1016/j.biopha.2015.08.017. [DOI] [PubMed] [Google Scholar]
- Franken NA, Rodermond HM, Stap J, Haveman J, Van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- Ganai SA. Histone deacetylase inhibitor pracinostat in doublet therapy: a unique strategy to improve therapeutic efficacy and to tackle herculean cancer chemoresistance. Pharm Biol. 2016;54:1926–1935. doi: 10.3109/13880209.2015.1135966. [DOI] [PubMed] [Google Scholar]
- Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- Keizman D, Eisenberger M. Is there a role for chemotherapy in nonmetastatic prostate cancer? Curr. Opin. Support Palliat. Care. 2010;4:141–146. doi: 10.1097/SPC.0b013e32833c6cfe. [DOI] [PubMed] [Google Scholar]
- Komatsu N, Kawamata N, Takeuchi S, Yin D, Chien W, Miller CW, Koeffler HP. SAHA, a HDAC inhibitor, has profound anti-growth activity against non-small cell lung cancer cells. Oncol Rep. 2006;15:187–191. [PubMed] [Google Scholar]
- Koryakina Y, Knudsen KE, Gioeli D. Cell-cycle-dependent regulation of androgen receptor function. Endocr. Relat. Cancer. 2015;22:249–264. doi: 10.1530/ERC-14-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuban DA, Hoffman KE, Corn P, Pettaway C. Prostate cancer. In: Rodriguez MA, Walters RS, Burke TW, editors. 60 Years of Survival Outcomes at the University of Texas MD Anderson Cancer Center. Springer; New York: 2013. pp. 35–43. [DOI] [Google Scholar]
- Lee JH, Choy ML, Ngo L, Venta-Perez G, Marks PA. Role of checkpoint kinase 1 (Chk1) in the mechanisms of resistance to histone deacetylase inhibitors. Proc Natl Acad Sci USA. 2011;108:19629–19634. doi: 10.1073/pnas.1117544108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Wang C, Kelly WK. Targeting epigenetics for the treatment of prostate cancer: recent progress and future directions. Semin Oncol. 2013;40:393–401. doi: 10.1053/j.seminoncol.2013.04.010. [DOI] [PubMed] [Google Scholar]
- Litwin MS, Tan HJ. The diagnosis and treatment of prostate cancer: a review. JAMA. 2017;317:2532–2542. doi: 10.1001/jama.2017.7248. [DOI] [PubMed] [Google Scholar]
- Marks PA. The clinical development of histone deacetylase inhibitors as targeted anticancer drugs. Expert Opin. Investig. Drugs. 2010;19:1049–1066. doi: 10.1517/13543784.2010.510514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrocco DL, Tilley WD, Bianco-Miotto T, Evdokiou A, Scher HI, Rifkind RA, Marks PA, Richon VM, Butler LM. Suberoylanilide hydroxamic acid (vorinostat) represses androgen receptor expression and acts synergistically with an androgen receptor antagonist to inhibit prostate cancer cell proliferation. Mol Cancer Ther. 2007;6:51–60. doi: 10.1158/1535-7163.MCT-06-0144. [DOI] [PubMed] [Google Scholar]
- McLeod AB, Stice JP, Wardell SE, Alley HM, Chang CY, McDonnell DP. Validation of histone deacetylase 3 as a therapeutic target in castration-resistant prostate cancer. Prostate. 2018;78:266–277. doi: 10.1002/pros.23467. [DOI] [PubMed] [Google Scholar]
- Mottet D, Castronovo V. Histone deacetylases: target enzymes for cancer therapy. Clin. Exp. Metastasis. 2008;25:183–189. doi: 10.1007/s10585-007-9131-5. [DOI] [PubMed] [Google Scholar]
- Nair HK, Rao KV, Aalinkeel R, Mahajan S, Chawda R, Schwartz SA. Inhibition of prostate cancer cell colony formation by the flavonoid quercetin correlates with modulation of specific regulatory genes. Clin Diagn Lab Immunol. 2004;11:63–69. doi: 10.1128/CDLI.11.1.63-69.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niculescu AB, Chen X, Smeets M, Hengst L, Prives C, Reed SI. Effects of p21Cip1/Waf1 at both the G1/S and the G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication. Mol Cell Biol. 1998;18:629–643. doi: 10.1128/MCB.18.1.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JW, Han JW. Targeting epigenetics for cancer therapy. Arch Pharm Res. 2019;42:159–170. doi: 10.1007/s12272-019-01126-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Jung Y, Kim TY, Kim SG, Jong HS, Lee JW, Kim DK, Lee JS, Kim NK, Kim TY, Bang YJ. Class I histone deacetylase-selective novel synthetic inhibitors potently inhibit human tumor proliferation. Clin Cancer Res. 2004;10:5271–5281. doi: 10.1158/1078-0432.CCR-03-0709. [DOI] [PubMed] [Google Scholar]
- Perry AS, Watson RW, Lawler M, Hollywood D. The epigenome as a therapeutic target in prostate cancer. Nat Rev Urol. 2010;7:668–680. doi: 10.1038/nrurol.2010.185. [DOI] [PubMed] [Google Scholar]
- Richon VM, Sandhoff TW, Rifkind RA, Marks PA. Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc Natl Acad Sci USA. 2000;97:10014–10019. doi: 10.1073/pnas.180316197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C, Rassool FV. HDAC inhibitors: roles of DNA damage and repair. Adv Cancer Res. 2012;116:87–129. doi: 10.1016/B978-0-12-394387-3.00003-3. [DOI] [PubMed] [Google Scholar]
- Ropero S, Esteller M. The role of histone deacetylases (HDACs) in human cancer. Mol Oncol. 2007;1:19–25. doi: 10.1016/j.molonc.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosato RR, Almenara JA, Maggio SC, Coe S, Atadja P, Dent P, Grant S. Role of histone deacetylase inhibitor-induced reactive oxygen species and DNA damage in LAQ-824/fludarabine antileukemic interactions. Mol Cancer Ther. 2008;7:3285–3297. doi: 10.1158/1535-7163.MCT-08-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruefli AA, Ausserlechner MJ, Bernhard D, Sutton VR, Tainton KM, Kofler R, Smyth MJ, Johnstone RW. The histone deacetylase inhibitor and chemotherapeutic agent suberoylanilide hydroxamic acid (SAHA) induces a cell-death pathway characterized by cleavage of Bid and production of reactive oxygen species. Proc Natl Acad Sci USA. 2001;98:10833–10838. doi: 10.1073/pnas.191208598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti M, Dadashian EL, Guo W, Quach B, Mulholland DJ, Park JW, Tran LM, Kobayashi N, Bianchi-Frias D, Xing Y, Nelson PS, Wu H. HDAC inhibition impedes epithelial-mesenchymal plasticity and suppresses metastatic, castration-resistant prostate cancer. Oncogene. 2016;35:3781–3795. doi: 10.1038/onc.2015.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambucetti LC, Fischer DD, Zabludoff S, Kwon PO, Chamberlin H, Trogani N, Xu H, Cohen D. Histone deacetylase inhibition selectively alters the activity and expression of cell cycle proteins leading to specific chromatin acetylation and antiproliferative effects. J Biol Chem. 1999;274:34940–34947. doi: 10.1074/jbc.274.49.34940. [DOI] [PubMed] [Google Scholar]
- Schröder F, Crawford ED, Axcrona K, Payne H, Keane TE. Androgen deprivation therapy: past, present and future. BJU Int. 2012;109(Suppl 6):1–12. doi: 10.1111/j.1464-410X.2012.11215.x. [DOI] [PubMed] [Google Scholar]
- Shankar S, Srivastava RK. Histone deacetylase inhibitors: mechanisms and clinical significance in cancer: HDAC inhibitor-induced apoptosis. Adv Exp Med Biol. 2008;615:261–298. doi: 10.1007/978-1-4020-6554-5_13. [DOI] [PubMed] [Google Scholar]
- Shrotriya S, Gagan D, Ramasamy K, Raina K, Barbakadze V, Merlani M, Gogilashvili L, Amiranashvili L, Mulkijanyan K, Papadopoulos K, Agarwal C, Agarwal R. Poly[3-(3, 4-dihydroxyphenyl) glyceric acid] from Comfrey exerts anti-cancer efficacy against human prostate cancer via targeting androgen receptor, cell cycle arrest and apoptosis. Carcinogenesis. 2012;33:1572–1580. doi: 10.1093/carcin/bgs202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- Telles E, Seto E. Modulation of cell cycle regulators by HDACs. Front. Biosci Schol. Ed.) 2012;4:831–839. doi: 10.2741/s303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltregny D, North B, Van Mellaert F, De Leval J, Verdin E, Castronovo V. Screening of histone deacetylases (HDAC) expression in human prostate cancer reveals distinct class I HDAC profiles between epithelial and stromal cells. Eur J Histochem. 2004;48:273–290. [PubMed] [Google Scholar]
- Wang H, Zhou W, Zheng Z, Zhang P, Tu B, He Q, Zhu WG. The HDAC inhibitor depsipeptide transactivates the p53/p21 pathway by inducing DNA damage. DNA Repair (Amst.) 2012;11:146–156. doi: 10.1016/j.dnarep.2011.10.014. [DOI] [PubMed] [Google Scholar]
- Wang LG, Ossowski L, Ferrari AC. Overexpressed androgen receptor linked to p21WAF1 silencing may be responsible for androgen independence and resistance to apoptosis of a prostate cancer cell line. Cancer Res. 2001;61:7544–7551. [PubMed] [Google Scholar]
- Weichert W, Röske A, Gekeler V, Beckers T, Stephan C, Jung K, Fritzsche FR, Niesporek S, Denkert C, Dietel M, Kristiansen G. Histone deacetylases 1, 2 and 3 are highly expressed in prostate cancer and HDAC2 expression is associated with shorter PSA relapse time after radical prostatectomy. Br J Cancer. 2008;98:604–610. doi: 10.1038/sj.bjc.6604199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Chen SY, Ross KN, Balk SP. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res. 2006;66:7783–7792. doi: 10.1158/0008-5472.CAN-05-4472. [DOI] [PubMed] [Google Scholar]
- Yoon S, Eom GH. HDAC and HDAC inhibitor: from cancer to cardiovascular diseases. Chonnam Med J. 2016;52:1–11. doi: 10.4068/cmj.2016.52.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Lu S, Wu L, Chai G, Wang H, Chen Y, Sun J, Yu Y, Zhou W, Zheng Q, Wu M, Otterson GA, Zhu WG. Acetylation of p53 at lysine 373/382 by the histone deacetylase inhibitor depsipeptide induces expression of p21Waf1/Cip1. Mol Cell Biol. 2006;26:2782–2790. doi: 10.1128/MCB.26.7.2782-2790.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]