Abstract
Alzheimer’s disease (AD) is a devastating neurodegenerative disease and a major cause of dementia in elderly individuals world-wide. Increased deposition of insoluble amyloid β (Aβ) fibrils in the brain is thought be a key neuropathological hallmark of AD. Many recent studies show that natural products such as polyphenolic flavonoids inhibit the formation of insoluble Aβ fibrils and/or destabilize β-sheet-rich Aβ fibrils to form non-cytotoxic aggregates. In the present study, we explored the structure-activity relationship of naturally-occurring biflavonoids on Aβ amyloidogenesis utilizing an in vitro thioflavin T assay with Aβ1–42 peptide which is prone to aggregate more rapidly to fibrils than Aβ1–40 peptide. Among the biflavonoids we tested, we found amentoflavone revealed the most potent effects on inhibiting Aβ1–42 fibrillization (IC50: 0.26 μM), as well as on disassembling preformed Aβ1–42 fibrils (EC50: 0.59 μM). Our structure-activity relationship study suggests that the hydroxyl groups of biflavonoid compounds play an essential role in their molecular interaction with the dynamic process of Aβ1–42 fibrillization. Our atomic force microscopic imaging analysis demonstrates that amentoflavone directly disrupts the fibrillar structure of preformed Aβ1–42 fibrils, resulting in conversion of those fibrils to amorphous Aβ1–42 aggregates. These results indicate that amentoflavone affords the most potent anti-amyloidogenic effects on both inhibition of Aβ1–42 fibrillization and disaggregation of preformed mature Aβ1–42 fibrils.
Keywords: Alzheimer’s disease, Amyloid β (Aβ), Fibrillization, Disaggregation, Structure-activity relationship, Biflavonoids
INTRODUCTION
Alzheimer’s disease (AD) is a neurodegenerative disease that is a major cause of dementia, affecting more than 26 million individuals worldwide (Luchsinger et al., 2005; Weiner, 2008; Querfurth and LaFerla, 2010; Reitz et al., 2010, 2011; Ubhi and Masliah, 2013). The biggest risk factor for this disease is age – the occurrence of the disease doubles every 5 years after the age of 65 (Querfurth and LaFerla, 2010). Symptoms include memory lapses of recent events, decreases in the ability to judge situations and problems, decreases in the ability to perform familiar tasks, and changes in mood and personality, such as increased depression. The dominant hypothesis for the pathogenesis of AD is the amyloid β (Aβ) cascade hypothesis. Neuropathological features of AD include the accumulation of insoluble Aβ fibrils in brain parenchyma as neuritic plaques and in cerebrovasculature as cerebral amyloid angiopathy (CAA) (Glenner and Wong, 1984; Masters et al., 1985; Rensink et al., 2003). Aβ peptide (commonly Aβ1–40 and Aβ1–42) is produced via endoproteolytic cleavage of the amyloid precursor protein (APP), a single transmembrane protein, and soluble forms of the peptide are secreted into the brain extracellular space throughout life (Bredesen, 2009). This peptide is eliminated via multiple pathways, including uptake in neurovascular cells, proteolytic degradation by proteases, and transport across the blood-brain barrier or the perivascular drainage pathway (Bu et al., 2006; Bates et al., 2009; Deane et al., 2009; Mandrekar et al., 2009; Kanekiyo et al., 2011; Kurz and Perneczky, 2011; Kanekiyo et al., 2012). In patients with AD, the production of the neurototoxic Aβ1–42 is increased, leading to an imbalance in the ratio with the non-toxic form, Aβ40 (Bates et al., 2009). These misfolded Aβ species (oligomers and fibrils) are deposited in the brain parenchyma (mainly consisted of Aβ1–42) and cerebrovasculature (mainly consisted of Aβ1–40), and are responsible for the development of AD pathology (Herzig et al., 2004). The most cytotoxic Aβ species, soluble Aβ1–42 oligomers found in the brains of AD patients, is associated with impaired long-term potentiation and endoplasmic reticulum stress (Walsh et al., 2002; Resende et al., 2008). Deposition of insoluble Aβ fibrils has also been found to be involved in the tissue neuroinflammatory response, contributing to the pathogenesis of the disease (Cunningham, 2013; Han et al., 2015).
Aβ amyloidogenesis involves multiple steps consisting of protein misfolding, nucleation, and elongation (Sgarbossa, 2012; Eisele et al., 2015; Tycko, 2015). Soluble Aβ peptides are converted to misfolded Aβ oligomers with β-sheet structures. The addition of individual peptides or other preformed β-sheet rich oligomers to these Aβ oligomers cause them to exponentially grow to form Aβ protofibrils. The Aβ protofibrils in turn are converted into insoluble, mature Aβ fibrils by stacking the protofibrils so that their longitudinal axes are parallel to each other (Cohen et al., 2013; Tycko, 2015; Chen et al., 2017). The Aβ fibrillization is a reversible process, and there is a dynamic association-dissociation equilibrium between the different Aβ species (Sgarbossa, 2012). Some aromatic compounds, including thioflavin T/S and their derivatives, and Congo red, selectively bind to the β-sheet-rich fibrils and can be used as an experimental tool to detect Aβ fibrils in AD brain tissues and in vitro studies with synthetic Aβ peptide (Giorgetti et al., 2018). Interestingly, we have found that another aromatic compound, resorufin, preferentially binds amyloid plaques in the cerebral arteries, but not in the brain parenchyma of the AD-affected brain tissues of humans and APP transgenic mice (Han et al., 2011).
Recent studies have shown that naturally-occurring polyphenols, including epigallocatechin-3-gallate (EGCG), curcumin, and resveratrol, have anti-amyloidogenic activity (Dasilva et al., 2010; Velander et al., 2017). For instance, in vitro studies reveal that EGCG inhibits the formation of toxic pre-fibrillar oligomers and amyloid fibrils, and converts previously existing amyloid fibrils into less toxic insoluble aggregates (Wang et al., 2010; Xu et al., 2017). In addition, a recent study has shown that naturally-occurring biflavonoids, such as taiwaniaflavone and amentoflavone, inhibit Aβ1–42 polymerization and elongation, and disaggregate preformed Aβ1–42 fibrils (Thapa et al., 2011). Interestingly, the authors found that biflavonoids increase the dose-dependent formation of Aβ1–42 oligomers that do not cause neurotoxicity. In line with these findings, we have previously reported the biflavonoid amentoflavone and its derivatives attenuate neuronal cell death induced by various cytotoxic insults, including the effects of the Aβ peptide in human neuroblastoma SH-SY5Y cells (Kang et al., 2005). These results suggest that biflavonoids attenuate Aβ-induced cytotoxicity by directly inhibiting the cell-death signaling cascade and/or by promoting the formation of non-toxic Aβ species. Chen et al. (2018) reported that amentoflavone significantly attenuates Aβ1–42-induced cognitive deficits and hippocampal neuronal cell death in the rat model of AD. The authors, however, have not addressed the effects of amentoflavone on aggregation and/or disaggregation of Aβ1–42 as its possible mechanism of action against Aβ1–42 neurotoxicity. More recently, Sirimangkalakitti et al. (2019) reported that amentoflavone and its related biflavonoids attenuate the fibrillization of Aβ1–40 in an in vitro assay system. It, however, still needs to investigate the effects of those biflavonoids on the dynamic process of aggregation and disaggregation of Aβ1–42 which the major neurotoxic species found in amyloid plaque in patients with AD. We sought to further explore the structure-activity relationship of amentoflavone-like biflavonoids on Aβ1–42 fibril formation and destabilization of preformed Aβ1–42 fibrils.
MATERIALS AND METHODS
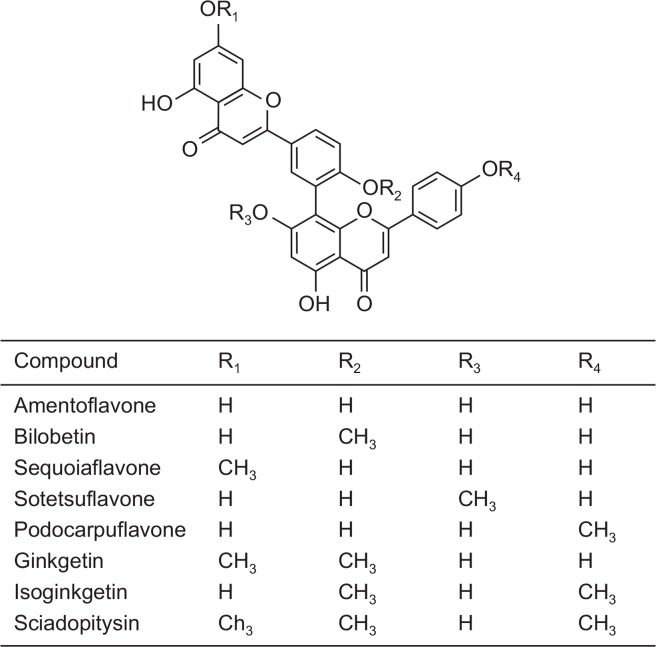
In the present study, we used 8 amentoflavone-like biflavonoids consisting of monoflavonoid dimers linked by C-C covalent bonds (Fig. 1). Biflavonoids compounds were prepared as we previously reported (Kang et al., 2005; Sasaki et al., 2010). Briefly, bilobetin, ginkgetin, isoginkgetin, and sciadopitysin were purified from Gingko biloba (Kang et al., 1990; Lee et al., 1995). Amentoflavone was purified from Selaginella tamariscina (Kang et al., 2005). Sequoiaflavone, sotetsuflavone, and podocarpuflavone were kindly gifted from Dr. Kiyotaka Koyama at Meiji Pharmaceutical University (Tokyo, Japan) (Sasaki et al., 2010). The purity of the flavonoids used in the study was greater than 98% (Chang et al., 1993). Recombinant human Aβ1–42 peptide purified with 1,1,1,3,3,3-hexafluoro-2-propanol were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Fig. 1.
Chemical structures of biflavonoids used in the present study.
Aβ1–42 aggregation assay
To test the inhibitory effects of biflavonoids on Aβ1–42 aggregation, we performed the thioflavin T (ThT) fluorescent assay per the published methods with modification (McKoy et al., 2012; Ryan et al., 2012). Recombinant human Aβ1–42 peptide (Sigma-Aldrich) purified with 1,1,1,3,3,3-hexafluoro-2-propanol was dissolved in dimethyl sulfoxide (DMSO) and kept at −20°C until use. Aβ1–42 was then diluted in PBS buffer (50 mM NaH2PO4 and 100 mM NaCl, pH 7.40) and added to a 96-well plate containing final concentrations of 20 μM Aβ1–42, 5 μM ThT, and with or without various concentrations of test compounds. Test compounds were first dissolved in DMSO at a concentration of 10 mM, and further diluted in the reaction buffer at final concentrations of 10, 2, 0.4, and 0.08 μM. The reaction samples were incubated at 37°C for 24 h and the ThT fluorescence was measured every 10 minutes at 508 nm (excitation at 460 nm) on a Bio-Tek plate reader (Winooski, VT, USA).
Aβ1–42 disaggregation assay
To measure the effects of the test compounds on destabilizing the preformed Aβ1–42 fibrils, 20 μM of recombinant human Aβ1–42 peptide in the assay buffer was co-incubated with 5 μM ThT at 37°C for 24 h in order to make Aβ1–42 fibrils. The preformed Aβ1–42 fibrils were treated with test compounds at final concentrations of 10, 2, 0.4, and 0.08 μM or control (DMSO), followed by incubation at 37°C for 7 h. The ThT fluorescent intensity was measured every 2.5 minutes as described above. Four independent experiments with duplicate samples were performed and plotted using the GraphPad Prism 8 software program (GraphPad, San Diego, CA, USA).
SDS-PAGE electrophoresis and immunoblotting
We first made Aβ1-42 fibrils by incubating recombinant human Aβ1–42 peptide at 1 mM in PBS buffer at 37°C for 48 h. Aβ1–42 fibrils at a final concentration of 25 μM were incubated for 1 h with 25 μM amentoflavone or vehicle (PBS), and SDS protein sample loading buffer was added. In a separate preparation, the protein samples were treated with a crosslinking agent, 0.01% glutaraldehyde for 5 min. Samples were separated on a 4–20% gradient tris-tricine gel (Bio-Rad, Hercules, CA, USA) per our published protocol (Shin et al., 2006; Han et al., 2008). The gel was transferred to a nitrocellulose membrane (Bio-Rad) at 4°C for 3 h. Blots were blocked with PBS containing 5% dry milk for 1 h and incubated in PBS solution containing an anti-Aβ antibody 6E10 (1:2000 dilution; Covance, San Diego, CA, USA). After wash, blots were incubated with goat anti-mouse IgG conjugated with horseradish peroxidase (Bio-Rad). The signal was visualized using the HRP chemiluminescent kit (ThermoFisher Scientific, Waltham, MA, USA), and photographic images were taken using a Syngene gel imaging system (Frederick, MD, USA).
Atomic force microscopy (AFM) imaging
AFM imaging was performed using an Asylum Research MFP-3D-BIO atomic force microscope mounted on an Olympus X711 microscope according to the manufacturer’s protocol (Olympus, Goleta, CA, USA). Aβ1–42 peptide samples prepared as described above were loaded on AFM standard cantilevers (MikroMasch, Lady’s Island, SC, USA) and scanned images were captured.
RESULTS
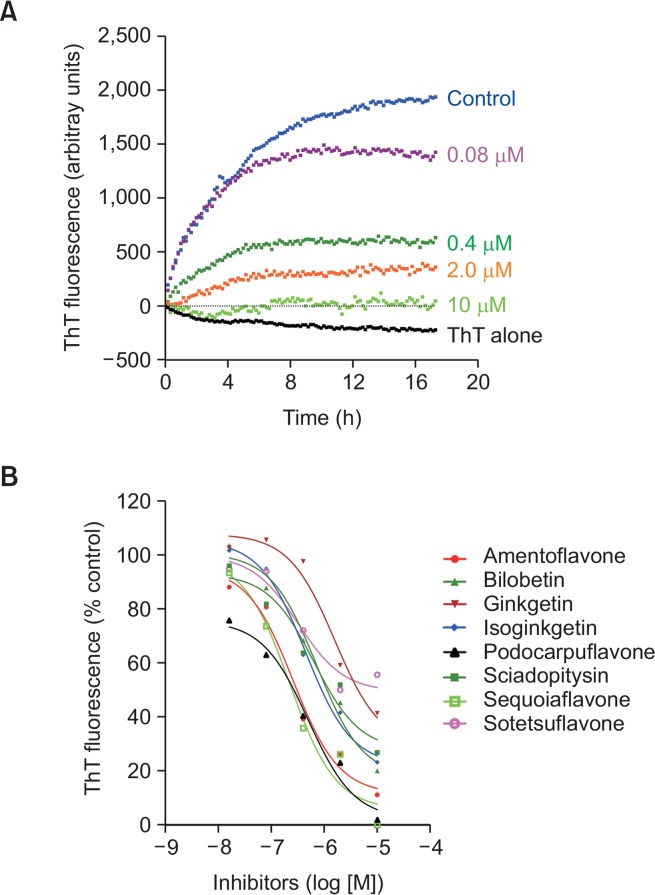
In the present study, we used 8 amentoflavone-like biflavonoids consisting of monoflavonoids dimers linked by C-C covalent bonds (Fig. 1). First, to determine the inhibitory effects of the test compounds on the formation of Aβ1–42 fibrils, soluble Aβ1–42 peptide was incubated with thioflavin T (ThT) in the presence of various concentrations of test compounds in phosphate-buffered saline at 37°C. ThT is known to bind only to the fibrillar forms of Aβ species (McKoy et al., 2012; Ryan et al., 2012). We found there was an increase in the ThT fluorescent intensity, reaching the plateau by 12 h after the start of incubation without the test compound amentoflavone (Fig. 2A). However, there was no increase in the ThT fluorescent intensity in the absence of Aβ peptide (ThT alone; Fig. 2A), indicating the specificity of the signals for Aβ fibrils. We found that amentoflavone inhibited Aβ1–42 fibrillization in a concentration-dependent fashion (Fig. 2A). Inhibitory effects (IC50 values) of the test compounds on Aβ1–42 fibrillization were calculated and presented in Table 1. All the biflavonoid molecules we tested possessed the inhibitory effects on Aβ1–42 fibrillization with IC50 values lower than 10 μM (Table 1). Among the biflavonoids, we found amentoflavone (IC50: 0.26 ± 0.03 μM), sequoiaflavone (IC50: 0.29 ± 0.01 μM), and podocarpuflavone (IC50: 0.19 ± 0.01 μM) revealed more potent inhibitory actions against Aβ1–42 fibrillization when compared to the other compounds.
Fig. 2.
Inhibitory effects of flavonoids on the formation of Aβ1–42 fibrils. Monomeric human Aβ1–42 peptide (20 μM) was incubated with various concentrations of test compounds and 5 μM thioflavin T (ThT) at 37°C. The fluorescent intensity of ThT-Aβ fibrils were measured every 10 min using a plate reader. Note that the fluorescent intensity in the samples of ThT alone declined over time due to photobleaching after repetitive measurements. (A) Time-course of Aβ1–42 fibrillization in the presence of various concentrations of amentoflavone. (B) Concentration-dependent inhibitory effects of the test compounds on Aβ1–42 fibrillization were plotted.
Table 1.
Inhibitory effects of test compounds on the formation of Aβ fibrils
| Compound | IC50 (μM) | Compound | IC50 (μM) |
|---|---|---|---|
| Amentoflavone | 0.26 ± 0.03 | Podocarpuflavone | 0.19 ± 0.01 |
| Bilobetin | 1.53 ± 0.20 | Ginkgetin | 4.92 ± 1.31 |
| Sequoiaflavone | 0.29 ± 0.01 | Isoginkgetin | 1.80 ± 0.38 |
| Sotetsuflavone | 8.70 ± 1.21 | Sciadopitysin | 2.28 ± 0.23 |
Data represent mean ± SEM from 4 independent experiments.
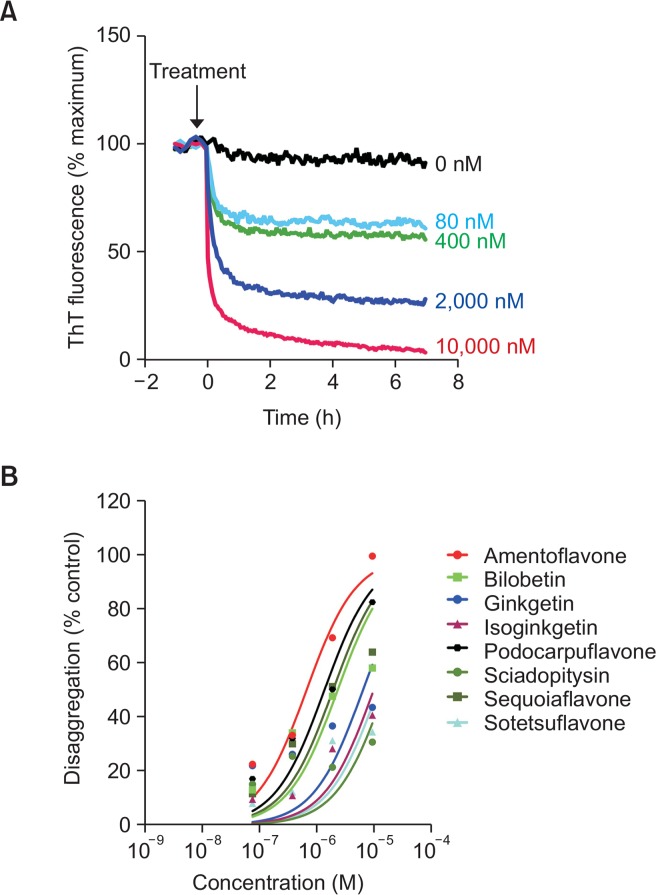
Next, to test whether the test compounds could disassemble Aβ1–42 fibrils, we carried out a time-course measurement of the fluorescent intensity of ThT bound to preformed Aβ1–42 fibrils in the presence or absence of the test compounds. During the 7-hour-course of the experiments, preformed Aβ1–42 fibrils remained at a steady state in the absence of amentoflavone, indicating the stability of the Aβ1–42 fibrils in the assay condition (Fig. 3A). However, there was a concentration-dependent, stiff decline of the ThT fluorescent intensity within 30 minutes of the addition of amentoflavone. Percent change in the ThT intensity vs. the concentrations of the competitors were plotted (Fig. 3A), and the EC50 values were calculated from the concentration-response curves and presented in Table 2. We found that amentoflavone (EC50: 0.59 ± 0.19 μM) most potently disassembled Aβ1–42 fibrils into ThT-negative Aβ1–42 species when compared with the other biflavonoids we tested.
Fig. 3.
Effects of flavonoids on disaggregation of preformed Aβ1–42 fibrils. Preformed Aβ1–42 fibrils (20 μM) were incubated with ThT (5 μM) various concentrations of test compounds. The fluorescent intensity of ThT-Aβ fibrils were measured every 2.5 min using a plate reader. (A) Time-course of disaggregation of Aβ1–42 fibrils in the presence of various concentrations of amentoflavone. (B) Concentration-dependent disaggregating effects of the test compounds on Aβ1–42 fibrils were plotted.
Table 2.
Effect of test compounds on disaggregation of Aβ fibrils
| Compound | EC50 (μM) | Compound | EC50 (μM) |
|---|---|---|---|
| Amentoflavone | 0.59 ± 0.19 | Podocarpuflavone | 1.45 ± 0.40 |
| Bilobetin | 2.45 ± 1.28 | Ginkgetin | 6.81 ± 4.08 |
| Sequoiaflavone | 2.04 ± 0.79 | Isoginkgetin | >10 |
| Sotetsuflavone | >10 | Sciadopitysin | >10 |
Data represent mean ± SEM from 4 independent experiments.
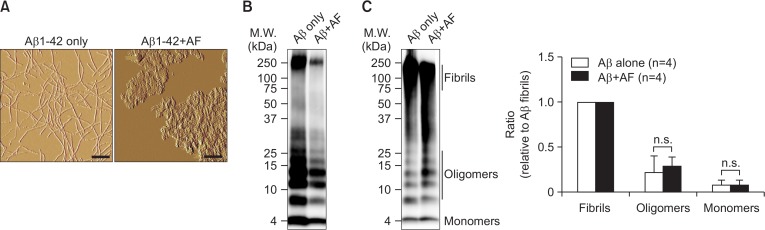
We next sought to confirm that amentoflavone directly interacts with Aβ1–42 fibrils and disassembles β-sheet-rich Aβ1–42 fibrils. Atomic force microscopic images showed that in our assay condition monomeric Aβ1–42 peptide was converted to Aβ1–42 fibrils (Fig. 4A, left panel). Co-incubation of preformed Aβ1–42 fibrils with amentoflavone led to conformational changes of the fibrils into amorphous Aβ1–42 aggregates where the extended fibrillar structure of Aβ1–42 fibrils no longer existed (Fig. 4A, right panel). To further determine the structural change of the Aβ1–42 fibrils in the presence of amentoflavone, the protein samples were subjected to SDS-PAGE electrophoresis and immunoblot analysis with the anti-Aβ antibody 6E10 (Fig. 4B, 4C). Under the denatured condition in the presence of the denaturing agent sodium dodecyl sulfate (SDS), the majority of Aβ1–42 fibrils (a molecular mass of ∼250 kDa) was converted to Aβ1–42 oligomers (a molecular mass of 8–30 kDa) and monomers (a molecular mass of 4 kDa) similarly in both samples containing Aβ1–42 fibrils only and Aβ1–42 fibrils plus amentoflavone (Fig. 4B). To preserve the integrity of Aβ1–42, the protein samples were treated with the cross-linking agent glutaraldehyde, followed by SDS-PAGE electrophoresis and immunoblot with the anti-Aβ antibody 6E10 (Fig. 4C). We found that both reaction samples contained predominantly Aβ1–42 fibrils, while amentoflavone did not increase the conversion of Aβ1–42 fibrils to oligomers and monomers (Fig. 4C). Taken together, these data indicate that amentoflavone rapidly disrupts the fibrillar structure of Aβ1–42 into amorphous Aβ1–42 aggregates without generation of cytotoxic Aβ1–42 oligomers.
Fig. 4.
Amentoflavone directly disrupts the fibrillar structure of preformed Aβ1–42 fibrils. (A) Representative images taken by atomic force microscopy (AFM). Preformed Aβ1–42 fibrils was incubated in the absence or in the presence of an equal concentration (25 μM) of amentoflavone (AF), and subjected to AFM imaging. Scale bar indicates 200 nm. (B, C) Representative images of Western immunoblotting. The reaction samples from the experiment A were separated by SDS-PAGE in denatured condition (B) or after cross-linking using glutaraldehyde (C), followed by immunoblotting with a monoclonal anti-Aβ antibody, 6E10. Representative images from three independent experiments are presented. The intensity of the signals corresponding to Aβ fibrils (M.W.: ≥75 kDa), oligomers (M.W.: 8–30 kDa) and monomers (M.W.: 4 kDa) were quantified and presented as relative ratio to the fibrils. Data indicate mean ± standard error of mean (n= 4). n.s.: not significant (p>0.05) analyzed by t-test.
DISCUSSION
In the present study, we explored the structure-activity relationship of amentoflavone-type biflavonoids on the inhibition of Aβ fibrillization as well as on disaggregation of preformed Aβ fibrils. Aβ peptide (39–43 amino acid residues) is produced in humans as soluble forms via enzymatic cleavage of amyloid precursor protein (APP) by secretases. The longer form (Aβ1–42 and Aβ1–43) is more amyloidogenic than the shorter form (Aβ1–40) and prone to aggregate to form β-sheet fibrils. These Aβ fibrils are essential for amyloid plaque formation, and deposit in the brain parenchyma as senile plaques (mainly comprised of Aβ1–42), and in the cerebral arteries as cerebral amyloid angiopathy (mainly comprised of Aβ1–40) in human patients with AD and in APP transgenic mouse models of AD (McGowan et al., 2005; Kim et al., 2007; Han et al., 2008; Ubhi and Masliah, 2013). Therefore, small molecules that can intervene the process of amyloidogenesis has provided therapeutic potential for the treatment of AD. A recent study reported the structure-activity relationship of the anti-amyloid aggregation activity of biflavonoids using synthetic Aβ1–40 in an in vitro assay system (Sirimangkalakitti et al., 2019). This study, however, lacks the antiamyloidogenic activity of those biflavonoids on the major neurotoxic species Aβ1–42. In the present study, we sought to further explore the structure activity relationship of biflavonoids on inhibition of the Aβ aggregation and promotion of the disaggregation of Aβ fibrils using recombinant human Aβ1–42.
In our study, we carried out an in vitro assay utilizing the fluorescent dye thioflavin T (ThT) which is well known to bind specifically to Aβ1–42 fibrils, but not to Aβ monomers or oligomers. In line with the previous reports (Sgarbossa et al., 2015; Thapa and Chi, 2015), we observed that Aβ1–42 fibrillization without biflavonoids increased gradually, to reach a plateau 15 hours after the start of incubation in our assay condition (Fig. 2A). We found that co-incubation of Aβ1–42 peptide with various concentrations of amentoflavone inhibited the formation of Aβ1–42 fibrils in a concentration-dependent manner (Fig. 2A). This result was not attributed by direct molecular interaction between ThT and amentoflavone, as amentoflavone did not quench or decrease the fluorescence intensity of ThT (data not shown). To compare the inhibitory activity of biflavonoids on inhibition of amyloidogenesis, we calculated the IC50 values from the concentration-effect curves (Fig. 2B, Table 2). We found that amentoflavone having 4 hydroxyl groups had the most potent inhibitory effect on Aβ fibrillization when compared to the other biflavonoids with at least one substitution with a methoxy group (Fig. 1). In addition, our data suggest that the hydroxyl groups at both R2 and R3 positions (Table 2) are critical for the anti-aggregation activity of the biflavonoids, whereas substitution with a methoxy group at either R1 (sequoiaflavone) or at R4 position (podocarpuflavone) did not change their inhibitory effects on Aβ1–42 fibrillization. In agreement of our findings, Sirimangkalakitti, et al. (2019) has recently reported that amentoflavone inhibits most potently the aggregation of Aβ1–40, while an increase in the number of methoxy substituents of biflavonoids diminishes their inhibitory effects on Aβ1–40 aggregation. However, amentoflavone requires a higher concentration (IC50: ∼5 μM) to inhibit fibrillization of Aβ1–40 (Sirimangkalakitti et al., 2019), whereas we found it is more potent to inhibit fibrillization of Aβ1–42 (IC50: 0.26 μM) in our experimental condition (Table 1). These results suggest that amentoflavone may have higher affinity for Aβ1–42 fibrils than Aβ1–40 fibrils. Each Aβ species have distinct aggregation kinetics and 3D structures. For example, the last two amino acid residues of human Aβ1–42 (i.e., Ile and Ala) are involved in hydrophobic interaction serving as an interface for Aβ1–42 aggregation, and it is shown that Aβ1–42 aggregates into β-sheet-rich fibrils at a higher rate than Aβ1–40 (Vandersteen et al., 2012; Zhang et al., 2013). It would be possible that amentoflavone disrupts the hydrophobic interaction through the C-terminal residues which is essential for Aβ1–42 aggregation. We are currently exploring the molecular interaction between the biflavonoids and Aβ1–42 fibrils at the atomic level utilizing computational modeling systems.
To test the effects of amentoflavone on the destabilization of Aβ1–42, we incubated preformed Aβ1–42 fibrils along with ThT. We found that amentoflavone rapidly lowered the ThT fluorescent intensity within 1 h after the treatment in a concentration-dependent fashion (Fig. 3A). Our study using an atomic force microscope (AFM) further reveals that amentoflavone disrupts the fibrillar structure of Aβ1–42 fibrils, resulting in formation of disorderly aggregates (Fig. 4A). Similar to the previous report (Thapa et al., 2011), we found Aβ1–42 fibrils are unstable in the presence of a denaturing agent such as sodium dodecyl sulfate (SDS) when assessed by SDS-PAGE immunoblotting (Fig. 4B). We, therefore, pretreated the protein samples with a cross-linking agent, glutaraldehyde, and carried out SDS-PAGE immunoblotting with an anti-Aβ antibody. In this assay condition, Aβ fibrils as judged by the molecular size greater than 75 kDa was mostly preserved. Though amentoflavone disassembles the fibrillar structure of Aβ1–42 fibrils, it did not convert fibrils to smaller sizes of Aβ species such as oligomers (molecular size of 8–30 kDa) or monomers (4 kDa). It is important to note that Aβ oligomers are known to be the most neurotoxic species of Aβ peptide in the culture system as well as in the mouse models of AD (Resende et al., 2008; Sturchler et al., 2008; Broersen et al., 2010). Our data suggest that amentoflavone dismantles Aβ1–42 fibrils without causing increased generation of neurotoxic Aβ1–42 oligomers. We compared the effectiveness of biflavonoids on disaggregation of preformed Aβ1–42 fibrils (Fig. 3B, Table 2). Our results indicate a critical role of all hydroxyl groups at R1–4 positions in the ability of those molecules to access and disrupt Aβ1–42 fibrillar structure. Though both sequoiaflavone and podocarpuflavone also potently inhibited Aβ1–42 fibrillization (Fig. 1, Table 1), they had a weak effect on disassembling preformed Aβ1–42 fibrils (Fig. 3B, Table 2).
Our findings provide insights into the structure-activity relationship of amentoflavone-like biflavonoids on attenuating Aβ1–42 fibrillization, as well as on converting Aβ1–42 fibrils into thioflavin T-negative, Aβ1–42 aggregates without increased generation of the neurotoxic Aβ oligomers. Biflavonoids are small organic molecules that possess both hydrophilic and hydrophobic residues. Those functional residues allow them to bind Aβ peptides, oligomers, and fibrils through hydrogen bonds, and hydrophobic and aromatic interactions. These residues allow biflavonoids the ability to interact with the dynamic process of amyloidogenesis (Sgarbossa, 2012). Our data support this notion, as amentoflavone, which possesses four hydroxyl groups, most effectively inhibits Aβ fibrillization and promotes disaggregation of preformed Aβ fibrils when compared to the other biflavonoids. In addition, biflavonoids are known to bind various Aβ species through aromatic-aromatic interactions (Thapa et al., 2011) which can explain why biflavonoids (which have more aromatic rings) are more effective in inhibiting Aβ fibrillization than a monoflavonoid (Thapa et al., 2011). Interestingly, our data also suggest that hydrophilic groups are necessary for biflavonoids to interact with and destabilize β-sheet-rich Aβ fibrils (Table 2). Further study is warranted to understand the molecular mechanisms underlying the antiamyloidogenic effects of biflavonoids, which would provide insights into the design and discovery of small molecule drugs targeting the process of amyloidogenesis.
Acknowledgments
This work was financially supported by the Warner/Fermaturo and the A. T. Still University Board of Trustees Research Grant, and by the A. T. Still University.
Footnotes
CONFLICT OF INTEREST
None.
REFERENCES
- Bates KA, Verdile G, Li QX, Ames D, Hudson P, Masters CL, Martins RN. Clearance mechanisms of Alzheimer’s amyloid-beta peptide: implications for therapeutic design and diagnostic tests. Mol. Psychiatry. 2009;14:469–486. doi: 10.1038/mp.2008.96. [DOI] [PubMed] [Google Scholar]
- Bredesen DE. Neurodegeneration in Alzheimer’s disease: caspases and synaptic element interdependence. Mol Neurodegener. 2009;4:27. doi: 10.1186/1750-1326-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broersen K, Rousseau F, Schymkowitz J. The culprit behind amyloid beta peptide related neurotoxicity in Alzheimer’s disease: oligomer size or conformation? Alzheimers Res Ther. 2010;2:12. doi: 10.1186/alzrt36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu G, Cam J, Zerbinatti C. LRP in amyloid-beta production and metabolism. Ann N Y Acad Sci. 2006;1086:35–53. doi: 10.1196/annals.1377.005. [DOI] [PubMed] [Google Scholar]
- Chang SK, Youn JR, Kang SS. Seasonal variations of biflavone content from Ginkgo biloba leaves. Kor J Pharmacogn. 1993;24:54–57. [Google Scholar]
- Chen C, Li B, Cheng G, Yang X, Zhao N, Shi R. Amentoflavone ameliorates abeta1–42-induced memory deficits and oxidative stress in cellular and rat model. Neurochem Res. 2018;43:857–868. doi: 10.1007/s11064-018-2489-8. [DOI] [PubMed] [Google Scholar]
- Chen GF, Xu TH, Yan Y, Zhou YR, Jiang Y, Melcher K, Xu HE. Amyloid beta: structure, biology and structure-based therapeutic development. Acta Pharmacol Sin. 2017;38:1205–1235. doi: 10.1038/aps.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SI, Linse S, Luheshi LM, Hellstrand E, White DA, Rajah L, Otzen DE, Vendruscolo M, Dobson CM, Knowles TP. Proliferation of amyloid-beta42 aggregates occurs through a secondary nucleation mechanism. Proc Natl Acad Sci USA. 2013;110:9758–9763. doi: 10.1073/pnas.1218402110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C. Microglia and neurodegeneration: the role of systemic inflammation. Glia. 2013;61:71–90. doi: 10.1002/glia.22350. [DOI] [PubMed] [Google Scholar]
- Dasilva KA, Shaw JE, McLaurin J. Amyloid-beta fibrillogenesis: structural insight and therapeutic intervention. Exp Neurol. 2010;223:311–321. doi: 10.1016/j.expneurol.2009.08.032. [DOI] [PubMed] [Google Scholar]
- Deane R, Bell RD, Sagare A, Zlokovic BV. Clearance of amyloid-beta peptide across the blood-brain barrier: implication for therapies in Alzheimer’s disease. CNS Neurol. Disord. Drug Targets. 2009;8:16–30. doi: 10.2174/187152709787601867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele YS, Monteiro C, Fearns C, Encalada SE, Wiseman RL, Powers ET, Kelly JW. Targeting protein aggregation for the treatment of degenerative diseases. Nat Rev Drug Discov. 2015;14:759–780. doi: 10.1038/nrd4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgetti S, Greco C, Tortora P, Aprile FA. Targeting amyloid aggregation: an overview of strategies and mechanisms. Int J Mol Sci. 2018;19:E2677. doi: 10.3390/ijms19092677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenner GG, Wong CW. Alzheimer’s disease and Down’s syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984;122:1131–1135. doi: 10.1016/0006-291X(84)91209-9. [DOI] [PubMed] [Google Scholar]
- Han BH, Zhou ML, Abousaleh F, Brendza RP, Dietrich HH, Koenigsknecht-Talboo J, Cirrito JR, Milner E, Holtzman DM, Zipfel GJ. Cerebrovascular dysfunction in amyloid precursor protein transgenic mice: contribution of soluble and insoluble amyloid-beta peptide, partial restoration via gamma-secretase inhibition. J Neurosci. 2008;28:13542–13550. doi: 10.1523/JNEUROSCI.4686-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han BH, Zhou ML, Vellimana AK, Milner E, Kim DH, Greenberg JK, Chu W, Mach RH, Zipfel GJ. Resorufin analogs preferentially bind cerebrovascular amyloid: potential use as imaging ligands for cerebral amyloid angiopathy. Mol Neurodegener. 2011;6:86. doi: 10.1186/1750-1326-6-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han BH, Zhou ML, Johnson AW, Singh I, Liao F, Vellimana AK, Nelson JW, Milner E, Cirrito JR, Basak J, Yoo M, Dietrich HH, Holtzman DM, Zipfel GJ. Contribution of reactive oxygen species to cerebral amyloid angiopathy, vasomotor dysfunction, and microhemorrhage in aged Tg2576 mice. Proc Natl Acad Sci USA. 2015;112:E881–E890. doi: 10.1073/pnas.1414930112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig MC, Winkler DT, Burgermeister P, Pfeifer M, Kohler E, Schmidt SD, Danner S, Abramowski D, Sturchler-Pierrat C, Burki K, van Duinen SG, Maat-Schieman ML, Staufenbiel M, Mathews PM, Jucker M. Abeta is targeted to the vasculature in a mouse model of hereditary cerebral hemorrhage with amyloidosis. Nat Neurosci. 2004;7:954–960. doi: 10.1038/nn1302. [DOI] [PubMed] [Google Scholar]
- Kanekiyo T, Zhang J, Liu Q, Liu CC, Zhang L, Bu G. Heparan sulphate proteoglycan and the low-density lipoprotein receptor-related protein 1 constitute major pathways for neuronal amyloid-beta uptake. J Neurosci. 2011;31:1644–1651. doi: 10.1523/JNEUROSCI.5491-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekiyo T, Liu CC, Shinohara M, Li J, Bu G. LRP1 in brain vascular smooth muscle cells mediates local clearance of Alzheimer’s amyloid-beta. J Neurosci. 2012;32:16458–16465. doi: 10.1523/JNEUROSCI.3987-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SS, Kim JS, Kwak WJ, Kim KH. Flavonoids from the Leaves of Ginkgo biloba. Kor J Pharmacogn. 1990;21:111–120. [Google Scholar]
- Kang SS, Lee JY, Choi YK, Song SS, Kim JS, Jeon SJ, Han YN, Son KH, Han BH. Neuroprotective effects of naturally occurring biflavonoids. Bioorg Med Chem Lett. 2005;15:3588–3591. doi: 10.1016/j.bmcl.2005.05.078. [DOI] [PubMed] [Google Scholar]
- Kim J, Onstead L, Randle S, Price R, Smithson L, Zwizinski C, Dickson DW, Golde T, McGowan E. Abeta40 inhibits amyloid deposition in vivo. J Neurosci. 2007;27:627–633. doi: 10.1523/JNEUROSCI.4849-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz A, Perneczky R. Amyloid clearance as a treatment target against Alzheimer’s disease. J. Alzheimers Dis. 2011;24(Suppl 2):61–73. doi: 10.3233/JAD-2011-102139. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Choi JH, Son KH, Chang HW, Kang SS, Kim HP. Suppression of mouse lymphocyte proliferation in vitro by naturally-occurring biflavonoids. Life Sci. 1995;57:551–558. doi: 10.1016/0024-3205(95)00305-P. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65:545–551. doi: 10.1212/01.wnl.0000172914.08967.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar S, Jiang Q, Lee CY, Koenigsknecht-Talboo J, Holtzman DM, Landreth GE. Microglia mediate the clearance of soluble Abeta through fluid phase macropinocytosis. J Neurosci. 2009;29:4252–4262. doi: 10.1523/JNEUROSCI.5572-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan E, Pickford F, Kim J, Onstead L, Eriksen J, Yu C, Skipper L, Murphy MP, Beard J, Das P, Jansen K, Delucia M, Lin WL, Dolios G, Wang R, Eckman CB, Dickson DW, Hutton M, Hardy J, Golde T. Abeta42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron. 2005;47:191–199. doi: 10.1016/j.neuron.2005.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKoy AF, Chen J, Schupbach T, Hecht MH. A novel inhibitor of amyloid beta (Abeta) peptide aggregation: from high throughput screening to efficacy in an animal model of Alzheimer disease. J Biol Chem. 2012;287:38992–39000. doi: 10.1074/jbc.M112.348037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Reitz C, Tang MX, Schupf N, Manly JJ, Mayeux R, Luchsinger JA. A summary risk score for the prediction of Alzheimer disease in elderly persons. Arch Neurol. 2010;67:835–841. doi: 10.1001/archneurol.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7:137–152. doi: 10.1038/nrneurol.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensink AA, de Waal RM, Kremer B, Verbeek MM. Pathogenesis of cerebral amyloid angiopathy. Brain Res Brain Res Rev. 2003;43:207–223. doi: 10.1016/j.brainresrev.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Resende R, Ferreiro E, Pereira C, Resende de Oliveira C. Neurotoxic effect of oligomeric and fibrillar species of amyloid-beta peptide 1–42: involvement of endoplasmic reticulum calcium release in oligomer-induced cell death. Neuroscience. 2008;155:725–737. doi: 10.1016/j.neuroscience.2008.06.036. [DOI] [PubMed] [Google Scholar]
- Ryan TM, Friedhuber A, Lind M, Howlett GJ, Masters C, Roberts BR. Small amphipathic molecules modulate secondary structure and amyloid fibril-forming kinetics of Alzheimer disease peptide Abeta (1–42) J Biol Chem. 2012;287:16947–16954. doi: 10.1074/jbc.M111.321778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H, Miki K, Kinoshita K, Koyama K, Juliawaty LD, Achmad SA, Hakim EH, Kaneda M, Takahashi K. beta-Secretase (BACE-1) inhibitory effect of biflavonoids. Bioorg Med Chem Lett. 2010;20:4558–4560. doi: 10.1016/j.bmcl.2010.06.021. [DOI] [PubMed] [Google Scholar]
- Sgarbossa A. Natural biomolecules and protein aggregation: emerging strategies against amyloidogenesis. Int J Mol Sci. 2012;13:17121–17137. doi: 10.3390/ijms131217121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgarbossa A, Giacomazza D, di Carlo M. Ferulic acid: a hope for Alzheimer’s disease therapy from plants. Nutrients. 2015;7:5764–5782. doi: 10.3390/nu7075246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DH, Bae YC, Kim-Han JS, Lee JH, Choi IY, Son KH, Kang SS, Kim WK, Han BH. Polyphenol amentoflavone affords neuroprotection against neonatal hypoxic-ischemic brain damage via multiple mechanisms. J Neurochem. 2006;96:561–572. doi: 10.1111/j.1471-4159.2005.03582.x. [DOI] [PubMed] [Google Scholar]
- Sirimangkalakitti N, Juliawaty LD, Hakim EH, Waliana I, Saito A, Koyama K, Kinoshita K. Naturally occurring biflavonoids with amyloid β aggregation inhibitory activity for development of anti-Alzheimer agents. Bioorg Med Chem Lett. 2019;29:1994–1997. doi: 10.1016/j.bmcl.2019.05.020. [DOI] [PubMed] [Google Scholar]
- Sturchler E, Galichet A, Weibel M, Leclerc E, Heizmann CW. Site-specific blockade of RAGE-Vd prevents amyloid-beta oligomer neurotoxicity. J Neurosci. 2008;28:5149–5158. doi: 10.1523/JNEUROSCI.4878-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapa A, Woo ER, Chi EY, Sharoar MG, Jin HG, Shin SY, Park IS. Biflavonoids are superior to monoflavonoids in inhibiting amyloid-beta toxicity and fibrillogenesis via accumulation of nontoxic oligomer-like structures. Biochemistry. 2011;50:2445–2455. doi: 10.1021/bi101731d. [DOI] [PubMed] [Google Scholar]
- Thapa A, Chi EY. Biflavonoids as potential small molecule therapeutics for Alzheimer’s disease. Adv Exp Med Biol. 2015;863:55–77. doi: 10.1007/978-3-319-18365-7_3. [DOI] [PubMed] [Google Scholar]
- Tycko R. Amyloid polymorphism: structural basis and neurobiological relevance. Neuron. 2015;86:632–645. doi: 10.1016/j.neuron.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubhi K, Masliah E. Alzheimer’s disease: recent advances and future perspectives. J. Alzheimers Dis. 2013;33(Suppl 1):S185–S194. doi: 10.3233/JAD-2012-129028. [DOI] [PubMed] [Google Scholar]
- Vandersteen A, Hubin E, Sarroukh R, De Baets G, Schymkowitz J, Rousseau F, Subramaniam V, Raussens V, Wenschuh H, Wildemann D, Broersen K. A comparative analysis of the aggregation behavior of amyloid-beta peptide variants. FEBS Lett. 2012;586:4088–4093. doi: 10.1016/j.febslet.2012.10.022. [DOI] [PubMed] [Google Scholar]
- Velander P, Wu L, Henderson F, Zhang S, Bevan DR, Xu B. Natural product-based amyloid inhibitors. Biochem Pharmacol. 2017;139:40–55. doi: 10.1016/j.bcp.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Wang SH, Liu FF, Dong XY, Sun Y. Thermodynamic analysis of the molecular interactions between amyloid beta-peptide 42 and (-)-epigallocatechin-3-gallate. J. Phys. Chem. B. 2010;114:11576–11583. doi: 10.1021/jp1001435. [DOI] [PubMed] [Google Scholar]
- Weiner MF. Perspective on race and ethnicity in Alzheimer’s disease research. Alzheimers Dement. 2008;4:233–238. doi: 10.1016/j.jalz.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZX, Ma GL, Zhang Q, Chen CH, He YM, Xu LH, Zhou GR, Li ZH, Yang HJ, Zhou P. Inhibitory mechanism of epigallocatechin gallate on fibrillation and aggregation of amidated human islet amyloid polypeptide. Chemphyschem. 2017;18:1611–1619. doi: 10.1002/cphc.201700057. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Rempel DL, Zhang J, Sharma AK, Mirica LM, Gross ML. Pulsed hydrogen-deuterium exchange mass spectrometry probes conformational changes in amyloid beta (Abeta) peptide aggregation. Proc Natl Acad Sci USA. 2013;110:14604–14609. doi: 10.1073/pnas.1309175110. [DOI] [PMC free article] [PubMed] [Google Scholar]