Abstract
Toxoplasma gondii, a member of the Apicomplexa, is known for its ability to infect an impressive range of host species. It is a common human infection that causes significant morbidity in congenitally infected children and immunocompromised patients. This parasite can be transmitted by bradyzoites, a slowly-replicating life stage found within intracellular tissue cysts, and oocysts, the sexual life cycle stage that develops in domestic cats and other Felidae. T. gondii bradyzoites retain the capacity to revert back to the quickly-replicating tachyzoite life stage, and when the host is immune compromised unrestricted replication can lead to significant tissue destruction. Bradyzoites are refractory to currently available Toxoplasma treatments. Improving our understanding of bradyzoite biology is critical for the development of therapeutic strategies to eliminate latent infection. This chapter describes a commonly used protocol for the differentiation of T. gondii tachyzoites into bradyzoites using human foreskin fibroblast cultures and a CO2-limited alkaline cell media, which results in a high proportion of differentiated bradyzoites for further study. Also described are methods for purifying tissue cysts from chronically infected mouse brain using isopycnic centrifugation and a recently developed approach for measuring bradyzoite viability.
Keywords: Toxoplasma, Bradyzoite, Tissue cyst, Differentiation, Stress, in vitro, in vivo
1. Introduction
The term bradyzoite is classically used to describe a slow-growing life stage of certain organisms belonging to a subclass of apicomplexans known as the Coccidia [1]. In the case of Toxoplasma gondii, bradyzoites are 7 × 1.5μm crescent-shaped organisms found within intracellular tissue cysts most commonly in neural and muscular tissue [2]. Tissue cysts vary in size and can contain from two to several thousand individual bradyzoites [2]. Within an intermediate host, when infected tissue containing tissue cysts is ingested, the released bradyzoites differentiate into tachyzoites, a quickly-replicating life stage that disseminates to various organs, causing an acute infection [3]. Acute infection may also occur from the ingestion of oocysts containing sporozoites, which differentiate into tachyzoites shortly after invasion of the host [3]. During the course of acute infection in mice, tachyzoites differentiate into bradyzoites in various tissues, with most bradyzoites appearing in the brain [4]. Chronic infection results when a competent immune response controls and clears the tachyzoite population within the host, leaving predominately the bradyzoite population intact [3]. It is thought that bradyzoites persist within their host during chronic infection by repeated cycles of tissue cyst rupture, infection of new host cells, and the formation of new tissue cysts [5]. The likelihood of an individual parasite behaving as a tachyzoite or bradyzoite can be thought of as a continuum, where a bradyzoite or a tachyzoite can be identified on the basis of specific markers, but an intermediate state cannot be easily identified. The decision to form a bradyzoite vacuole (also known as a tissue cyst) or a tachyzoite vacuole is likely an early event during the course of host cell invasion [2]. Support for this notion is provided by the finding that tissue cyst markers can be identified within vacuoles containing an individual parasite shortly after invasion [2,6]. Although it is classically thought that bradyzoites are a quiescent life stage that replicate asynchronously, recent work has demonstrated that bradyzoites replicate in dynamic fashion during the course of chronic infection in mice [7].
In their host cells, both tachyzoites and bradyzoites reside within a specialized compartment referred to as the parasitophorous vacuole, within which the parasite scavenges nutrients and replicates [8,9]. When imaged with transmission electron microscopy (TEM), the parasitophorous vacuoles and the morphology of bradyzoites and tachyzoites differ dramatically. Bradyzoites typically contain a posteriorly located nucleus, electron dense rhoptry organelles, and an abundance of amylopectin storage granules that stain positive with Periodic Acid-Schiff Base (PAS) [2,10]. Within tissue cysts, bradyzoites typically reside in an electron dense matrix or ground substance [10]. Bradyzoite tissue cysts also contain an amorphous collection of proteinaceous material just beneath the limiting membrane of the parasitophorous vacuole, known as the cyst wall [11]. Cyst wall thickness varies between 50 to 500nm depending on the age of the cyst, which is generally inferred by the size of the tissue cyst and the development of the cyst wall [12]. In contrast to bradyzoite vacuoles, tachyzoite vacuoles lack the ground substance and cyst wall that typify tissue cysts, instead residing in relatively clear vacuoles containing an intertubular (or intravacuolar) network (IVN) between individual tachyzoites [13].
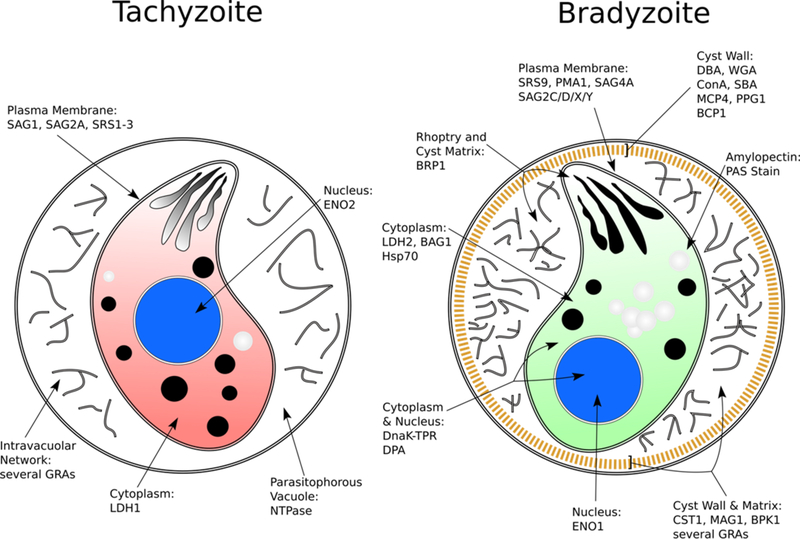
Decades of research has led to the identification of several stage-specific cytoplasmic and secreted proteins in bradyzoites that can be identified during the course of differentiation in vitro (illustrated in Figure 1) (see ref [14]). The small heat shock protein BAG1 (formerly known as BAG5) was one of the earliest identified bradyzoite markers, shown to localize to the cytoplasm of bradyzoites as early as 1 day post-differentiation by alkaline media induction [15,16]. A few cyst wall proteins that play an important role in cyst wall rigidity and bradyzoite persistence have been described, such as BPK1 and CST1 [17,18]. It is well known that the bradyzoite-derived cyst wall is glycosylated, as various lectins have been shown to react with the cyst wall and are commonly used as markers for bradyzoite differentiation [19–21]. The use of transgenic reporter strains also allows bradyzoites to be identified in vitro and in vivo through the use of fluorescent proteins and enzymes expressed under the control of stage-specific gene promoters [22,23]. It is important to note that not every bradyzoite protein marker is expressed at the same time during the course of tachyzoite to bradyzoite conversion. The bradyzoite proteins BAG1 and CST1 appear to be expressed early following in vitro induction, whereas the proteins SRS9 and LDH2 tend to be expressed at later time points following in vitro induction [16,18,22,24].
Figure 1.
Markers used to distinguish tachyzoites and bradyzoites. Note that certain markers appear earlier than others during the course of tachyzoite to bradyzoite differentiation. Modified from ref [14] with permission from Elsevier.
In vitro derived cysts and in vivo derived cysts have shown some differences in protein localization. One particular cyst wall protein, BCP1, was shown to exhibit punctate localization within in vitro derived tissue cysts [25]. In contrast, this same protein was shown to localize to the cyst wall and was translated into a protein of larger size within in vivo derived cysts [25]. While this suggests that not every observation made using the in vitro model of bradyzoite differentiation is true of bradyzoites that differentiate in vivo, the in vitro model has proven to be a useful tool in the field, identifying several bradyzoite antigens whose size and localization has been confirmed using tissue cysts isolated from in vivo sources. Hence, in vitro models are powerful, but need to be interpreted with caution and should be confirmed with additional in vivo assays.
Although the markers described above have proven useful in characterizing bradyzoite differentiation, it is worth noting that the most sensitive marker in identifying mature bradyzoite tissue cysts is determination of the pre-patent period in felines; that is, the time to oocyst release by a cat following ingestion of parasites (3 to 10 days in the case of bradyzoite tissue cyst ingestion) [26]. Most laboratories do not have access to felines for bioassays or are not well equipped to work with oocysts; therefore, such assays are not commonly used.
The factors governing tachyzoite to bradyzoite differentiation (and vice-versa) have been the subject of intense research, and several reviews exist addressing this topic [27–29]. It is clear from collective observations that bradyzoite differentiation is in response to stress, either perceived through the infected host cell or directly on the parasite itself. Examples of stressful stimuli, which have all been demonstrated as feasible in vitro induction methods, include gamma-interferon treatment, induction of host cell nitric oxide, heat-shock, nutrient deprivation, and alterations in pH [29]. Several chemical compounds have also been shown to induce bradyzoite differentiation through various mechanisms, reviewed in [30]. The cell type that is infected can also influence bradyzoite differentiation, as post-mitotic cells such as neurons or skeletal muscle cells have been shown to facilitate bradyzoite differentiation more so than tachyzoite replication [31,32]. Indeed, neurons have been shown to be the most common cell type to harbor tissue cysts within the brain [11, 33, 41].
Work on the cellular mechanisms within the parasite responsible for stage conversion between tachyzoites and bradyzoites is still ongoing. Apicomplexan AP2 proteins (ApiAP2), as well as the phosphorylation status of the parasite eukaryotic initiation factor-2 (eIF2), have been shown to play important roles in facilitating or repressing tachyzoite to bradyzoite differentiation [34,35].
Excellent protocols on the isolation of in vivo bradyzoites from mouse brain and the differentiation of bradyzoites in vitro are available elsewhere [36,37]. The focus of this chapter is to describe three separate methods. The first is a simple approach towards obtaining differentiated bradyzoites in vitro through the use of human foreskin fibroblasts cultures and an alkaline, CO2-depleted media in the absence of gaseous CO2, which appears to be the most common approach for in vitro bradyzoite induction in modern Toxoplasma research. This method is reproducible and results in a large proportion of differentiated bradyzoites, which can be confirmed through the use of reporter strains or via immunofluorescence of bradyzoite specific markers. The second method describes isopycnic separation of T. gondii tissue cysts from chronically infected mouse brains using a Percoll buffer, originally described by Cornelissen et al. [38]. This approach ultimately results in a pellet containing tissue cysts that can be used for further experimentation and analysis. Finally, we describe an approach for assessing viability of bradyzoites based on quantitative PCR (qPCR) combined with a plaque assay.
2. Materials
2.1. Cell Culture
Human Foreskin Fibroblasts, HFF-1 (ATCC®SCRC-1041™)
Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 2mM L-glutamine. A 1:200 dilution of a 10,000 U/mL penicillin and streptomycin stock can be added if desired (these help prevent bacterial contamination of the media).
T. gondii strain/transgenic line of choice (see Note 1)
Tissue culture vessels of choice.
Tissue culture CO2 incubators.
5μm filters and Luer-Lock syringes
2.2. Bradyzoite Differentiation
Differentiation Media: Gibco™ High glucose DMEM powder, 50mM HEPES, 1% FBS. Media adjusted to pH 8.2. A 1:200 dilution of 10,000 U/mL penicillin and streptomycin stock can be added if desired (these help prevent bacterial contamination of the media). Prepare 1L of differentiation media by dissolving 1 packet of DMEM powder with 11.91g HEPES powder in 800mL ultra-pure water. Adjust the pH to 8.2 by measuring with a pH probe and adding freshly made 1M NaOH. Adjust final volume accordingly with ultra-pure water. Filter solution through a 0.45μm or 0.22μm bottle-top filter. Add FBS after filtration, as well as penicillin/streptomycin if desired. Store differentiation media at 4oC until needed (see Note 2). Warm the media to 37 oC before use.
1M NaOH.
Ultra pure water (e.g. Milli-Q® filtered water).
pH probe.
Bottle-top filters (0.45μm or 0.22 μm).
2.3. In vivo Tissue Cyst Purification
Mouse strain of choice chronically infected with T. gondii strain/transgenic line of choice (see Notes 7 and 8).
Sterile dissection instruments (e.g. scissors, forceps, and spatula).
Equipment for euthanasia (e.g. CO2 chamber).
Luer-Lock syringe (10mL or larger).
Petri dishes (large enough to hold more than 10mL).
Thomas® Pestle Tissue Grinder Assembly, using either a Teflon® pestle or a size B glass pestle. Items should be autoclaved before use (see Note 9).
Percoll® (P1644 Sigma).
1X Phosphate Buffered Saline (PBS).
70% ethanol.
Centrifuge capable of speeds up to 26,600 x g.
Centrifuge tubes (resistant to breaking at 26,600 x g).
50mL conical tubes.
2.4. Bradyzoite Viability assay
Tissue culture 6-well plates.
Hank’s Balanced Salt Solution (HBSS) (without Ca2+ and Mg2+).
Cell scrapers.
10mL Luer-Lock syringe.
25 or 27 gauge needles.
Pepsin solution 2X: 340mM NaCl, 120mM HCl, 0.52mg/mL pepsin (porcine gastric mucosa ≥250 units/mg solid). To prepare 50mL of pepsin solution 2X, dissolve 26mg pepsin and 0.993g of NaCl in 45mL ultra-pure water, adjust pH to 2.0, and bring the solution to 50mL with ddH2O. Filter the solution using a 0.2μm filter and store at 4°C. Prepare fresh solution for each use.
Neutralization buffer 2x: 188 mM Na2CO3. To prepare 50mL of neutralization buffer 2X, dissolve 1g Na2CO3 in 50mL ultra-pure water. Filter the solution using a 0.2μm filter and store at 4°C.
Genomic extraction kit.
qPCR machine.
qPCR reagents.
Primers for qPCR. TUB-for 5’-GCG TCT TCT TGG ATT TGG AG-3’ and TUB-rev 5’-TGG AGA CCA GTG CAG TTG TC-3’
15mL conical polystyrene tubes.
3. Methods
3.1. Cell Culture
T. gondii tachyzoites can be maintained in confluent HFF monolayers grown in supplemented DMEM media within 37oC incubators at 5% CO2. When the majority of host cells have lysed and freshly egressed parasites are seen, the tachyzoites can be passaged by inoculating a fresh confluent HFF monolayer.
3.2. Bradyzoite Differentiation
Collect freshly egressed tachyzoites via filtration through a 5μm filter. If the culture has not fully lysed, the culture can be scraped with a cell scraper and passed through needles of increasingly smaller diameter (up to 27.5 gauge) with a syringe before filtration. Freshly egressed tachyzoites and lysed intracellular parasites have similar rates of differentiation.
Count the filtrate in a hemocytometer. Calculate the amount of parasites needed to achieve the desired multiplicity of infection (MOI) (see Note 3).
Infect an HFF monolayer plated in a tissue culture vessel of choice with the appropriate amount of filtered parasites. Place the infected culture in a 37 oC incubator at 5% CO2.
Two hours post-infection (see Note 4), replace the media in the culture with differentiation media (see section 2.2) and transfer the culture to a 37 oC incubator set at 0% CO2 (ambient air, see Note 5).
Replace media every 1 to 2 days with fresh differentiation media. This reduces the likelihood of bradyzoites reverting to tachyzoites.
Visualize or collect bradyzoites for desired assay as needed at a given time point post-differentiation (see Notes 3 and 6). After 3 days in culture, the ME49 or Prugniaud T. gondii strains have over 90% of vacuoles expressing bradyzoite markers. The proportion of differentiated bradyzoites can be confirmed with reporter strains and/or immunofluorescence assays using antibodies to bradyzoite specific markers (see Figure 1).
3.3. In vivo Tissue Cyst Purification
Collect mice chronically infected with T. gondii (see Notes 7 and 8), and sacrifice following an approved animal euthanasia protocol.
After mice have been sacrificed, transfer mice to a biosafety cabinet and spray down the skin of the head with 70% ethanol.
Remove the skin of the head by cutting from the base of the neck down to the nose, further removing skin with forceps if necessary.
Make a single incision at the base of the skull, cut along the base of the skull on both the right and left side, starting at the initial incision. The skull can usually be peeled away with forceps after these cuts are made. Collect the brain with a spatula, severing the connection to the olfactory bulbs in the process. If desired, the sacrificed mouse can be decapitated prior to skull dissection. Repeat for other mice.
Transfer the brains into a 50mL conical tube, and rinse the brains several times with pre-chilled PBS to remove erythrocytes
Transfer brains onto a petri dish, and homogenize the brains using a Luer-Lock syringe without an attached needle in 10mL of PBS (no more than 5 brains should be homogenized in 10mL). After homogenization and if desired, check the Petri dish for intact tissue cysts under a microscope.
Collect the homogenate with a syringe and transfer into the Thomas® Pestle Tissue Grinder Assembly. Homogenize further by grinding with at least 10 full passes. Keep the homogenate on ice.
Make 50mL of a 45% Percoll solution (22.5mL Percoll in 27.5mL PBS).
Divide 25mL of the 45% Percoll solution into two centrifuge tubes. Add 5mL of the homogenized cyst solution into each tube.
Centrifuge the two solutions at 26,600 x g for 20 minutes at 4oC
Carefully remove 3mL from the bottom of each tube and discard (Note 10).
Carefully remove the next 20mL from the bottom of each tube, and place into new 50mL conical tubes. Fill up each tube to 50mL with PBS.
Centrifuge the solutions at 130 x g for 10 minutes at 4oC.
The tissue cysts should form a pellet at the bottom of the tubes by this step. Aspirate the supernatant and resuspend the pellet in 500μL PBS. Take a small aliquot (e.g. 10 μL) of these solutions and make a wet mount with a coverslip and microscope slide, counting the number of cysts present in the aliquot and determining the total number of cysts in the pellet. If cyst are to be used for murine infection the appropriate amount of cysts can be used directly from this suspension.
Concentrate the cysts again by centrifuging the samples at 1,400 x g for 4 minutes at room temperature.
Remove the supernatant. At this stage, the pellet can be stored in −80oC for future analysis or fixed for electron microscopy.
3.4. Bradyzoite Viability assay
-
1.
Differentiate bradyzoites as described in section 3.2
-
2.
After 7 days, parasites are largely converted into bradyzoites. If any treatment is to be tested on fully converted bradyzoites, it should start on day 7 post-infection. Change media every day or two with fresh differentiation media (supplemented with fresh drug if applying a treatment) until the selected time point(s) (Note 11).
-
4.
At the time point(s) selected to perform the viability assay (Note 12) remove media from the infected monolayers and then wash infected monolayers 3 times with HBSS (without Ca2+ and Mg2+).
-
5.
Add 2mL of HBSS, scrape the monolayer with a cell scraper and then syringe up and down 4 times with a 25 or 27 gauge needle.
-
6.
Recover the media containing scraped cells from each well, transfer to a 15mL conical tube, wash the well with 1.5mL of HBSS, and add to the same 15mL tube.
-
7.
Add 3.5 mL of pepsin solution 2X, mix and incubate samples in a water bath for 30 minutes at 37°C.
-
8.
Stop the reaction by adding 7mL of neutralization buffer 2X (final concentration 94mM Na2CO3).
-
9.
Centrifuge 1800 x g for 10 minutes at RT.
-
10.
Very carefully pour off the supernatant and then resuspend in 1mL of supplemented DMEM.
-
11.
Count parasites (add trypan blue, to avoid counting dead parasites killed by pepsin treatment) in the control (untreated) sample using a hemocytometer.
-
12.
Take 100, 1000 and 5000 parasites (based on the number from the control) and infect HFF monolayers grown in 6-well plates in triplicate for plaque assay (in 3mL of fresh supplemented DMEM).
-
13.
Incubate the 6-well plates undisturbed for 10 to 14 days (depending from the parasite strain) in a humidified 37°C incubator with 5% CO2.
-
14.
After the incubation period, check the 6-well plates on an inverted microscope with a low power objective for plaques. If present, stain with crystal violet, and count plaques (Note 13).
-
15.
To perform qPCR to normalize the total number of bradyzoites added to 6-well plates for plaque assay (Note 14), spin 800μL bradyzoites from step 5j and centrifuge the samples at 1800 xg for 10 minutes at RT.
-
16.
Carefully remove with a pipette all the supernatant without disturbing the pellet.
-
17.
Purify nucleic acids gDNA using NucleoSpin Tissue kit (Macherey-Nagel, cat. no. 740952) or any other commercial genomic extraction kit (the pellets can be frozen at −80°C for future purification).
-
18.
In parallel, prepare a reference gDNA from a known number of parasites (e.g., 107 or 108 tachyzoites). Dilute the reference gDNA to have a concentration of DNA corresponding to 105, 104, 103, 102 and 101 tachyzoites/μL to generate a standard curve.
-
19.
Perform qPCR using the TUB-for and TUB-rev primers for the tubulin gene (or primers to amplify any other genomic fragment). Use the highest volume suggested by the manufacturer of the qPCR machine being utilized. In addition, generate a standard curve with 6 reference points using gDNA and using sterile ultra-pure water as a negative control.
-
20.
Utilize a dual step cycle of 98°C/5 sec and 68°C/30 sec for 40 cycles. Set a melting curve from 58°C to 95°C, 5 sec/step, 0.5°C increments to assess that your primers produce only one amplicon.
-
21.
Use the reference curve generated to calculate how many parasite genomes went into the 6-well plates for the plaque assay to obtain the number of plaques formed from X number of parasite genomes.
Acknowledgement
This work was supported by 1F31AI136401 (J.M.), R01AI134753 (L.M.W.), and R21AI123495 (L.M.W.).
Footnotes
Type II and III strains differentiate into bradyzoites more readily compared to Type I. All strains display varying degrees of spontaneous differentiation in the absence of added stress (albeit at low rates). Type II strains are very amendable to in vitro differentiation protocols, as previously described [27].
Differentiation media remains effective for several months if stored at 4oC.
Depending on the MOI used, bradyzoites can be maintained in culture for several days, even weeks. We have observed greater amounts of parasite egress and reinvasion with MOI’s of 3:1 and greater. Therefore, depending on the nature of the experiment, the MOI should be chosen carefully to prevent premature HFF monolayer lysis. We have also observed less egress in the ME49 Type II strain compared to the Prugniaud Type II strain when equal MOIs are used.
Bradyzoite differentiation may be induced earlier than 2 hours post-infection if desired (e.g. at the time of invasion). The media of the HFF monolayer to be infected can be replaced with differentiation media, after which filtered tachyzoites can be pelleted and resuspended in differentiation media, followed by inoculation of the HFF monolayer with the resuspended parasites.
The absence of CO2 in differentiating cultures plays an important role in parasites lacking the UPRT gene, as it is thought these parasites exhibit increased sensitivity to CO2 starvation due to the need for CO2 in the de novo synthesis of pyrimidine, as described in [39]. Additionally, removing CO2 prevents its dissolution in the differentiation media, which could acidify the media and relieve alkaline stress.
If passaging of in vitro differentiated bradyzoites is desired, the culture should be scraped with a cell scraper and passed through needles of increasingly larger gauge (up to 27.5 gauge) with a syringe, followed by filtration through a 5μm filter. We have found that the liberation of individual bradyzoites from in vitro tissue cysts can be challenging depending on the age of the cysts. Several passes through syringe needles may be needed for effective cyst disruption.
The strain of mouse/parasite, life stage of parasite inoculum, route and amount of inoculum, as well as anti-parasite treatment during infection are all important considerations when generating in vivo tissue cysts, reviewed in [40]. Generally, Type II tissue cysts passaged between mice orally or intraperitoneally (or Type II tachyzoites taken from tissue culture in vitro and inoculated intraperitoneally) result in higher cyst yields in most strains of mice compared to other parasite types [36]. However, as noted by Watts et al., tissue cyst yield can be quite variable even between identical mouse strains infected with the same dose of parasites for the same length of time [36].
In mice, chronic infection with T. gondii begins after the immune response removes most replicating tachyzoites in the body, leaving predominately the bradyzoite population intact 3 weeks post-infection [10]. Therefore, bradyzoite tissue cysts from the mouse brain are usually harvested no earlier than 3 weeks post-infection.
Thomas® Pestle Tissue Grinder Assemblies are available in various sizes. We find those with a 30mL capacity work well for homogenizing 10mL volumes. Pestle clearance is important to minimize cyst breakage during tissue homogenization. We have found that the ‘B” size of glass pestles or Teflon pestles are useful for cyst purification.
For pipetting we use a 4 inch (or longer) 14 gauge blunt dispensing needle and a syringe, which facilitates selective aspiration from the bottom of the tube.
Converted parasites can be kept in culture as long as 5 weeks post-infection, depending on the strain used. PruΔku80 shows good viability 4 weeks post-conversion, whereas Prugniaud and ME49 lose most of their viability beyond 2 weeks post-conversion.
In principle, this viability assay can also be applied to cysts purified from the brains of chronically infected mice as described in section 3.3.
Some T. gondii strains do not form plaques that are visible with crystal violet. In this case count the number of infective areas using an optical microscope.
qPCR measures the total number of bradyzoites regardless of whether they are dead or alive. This is an important advantage of qPCR over counting parasites in a hemocytometer, since in our experience dead bradyzoites can be deformed and are not easily identified by microscopic methods.
References
- 1.Frenkel JK, Smith DD. (2003) Determination of the genera of cyst-forming coccidia. Parasitol Res. 91:384–389. [DOI] [PubMed] [Google Scholar]
- 2.Dubey JP, Lindsay DS, Speer CA. (1998) Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin Microbiol Rev. 11:267–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montoya JG, Liesenfeld O. (2004) Toxoplasmosis. Lancet. 363:1965–1976. [DOI] [PubMed] [Google Scholar]
- 4.Di Cristina M, Marocco D, Galizi R, Proietti C, Spaccapelo R, Crisanti A. (2008) Temporal and spatial distribution of Toxoplasma gondii differentiation into bradyzoites and tissue cyst formation in vivo. Infect Immun. 76:3491–3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rougier S, Montoya JG, Peyron F. (2017) Lifelong persistence of Toxoplasma cysts: A questionable dogma? Trends Parasitol. 33:93–101. [DOI] [PubMed] [Google Scholar]
- 6.Sahm M, Fischer HG, Gross U, Reiter-Owona I, Seitz HM. (1997) Cyst formation by Toxoplasma gondii in vivo and in brain-cell culture: a comparative morphology and immunocytochemistry study. Parasitol Res. 83:659–665. [DOI] [PubMed] [Google Scholar]
- 7.Watts E, Zhao Y, Dhara A, Eller B, Patwardhan A, Sinai AP. (2015) Novel approaches reveal that Toxoplasma gondii bradyzoites within tissue cysts are dynamic and replicating entities in vivo. MBio. 6:e01155–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dou Z, McGovern OL, Di Cristina M, Carruthers VB. (2014) Toxoplasma gondii ingests and digests host cytosolic proteins. MBio. 5:e01188–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caffaro CE, Boothroyd JC. (2011) Evidence for host cells as the major contributor of lipids in the intravacuolar network of Toxoplasma-infected cells. Eukaryot Cell. 10:1095–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferguson DJ, Hutchison WM. (1987) An ultrastructural study of the early development and tissue cyst formation of Toxoplasma gondii in the brains of mice. Parasitol Res. 73:483–491. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson DJ, Hutchison WM. (1987) The host-parasite relationship of Toxoplasma gondii in the brains of chronically infected mice. Virchows Archiv A, Pathological anatomy and histopathology. 411:39–43. [DOI] [PubMed] [Google Scholar]
- 12.Fortier B, Coignard-Chatain C, Soete M, Dubremetz JF. (1996) Structure and biology of Toxoplasma gondii bradyzoites. Comptes rendus des seances de la Societe de biologie et de ses filiales. 190:385–394. [PubMed] [Google Scholar]
- 13.Sibley LD, Niesman IR, Parmley SF, Cesbron-Delauw MF. (1995) Regulated secretion of multi-lamellar vesicles leads to formation of a tubulo-vesicular network in host-cell vacuoles occupied by Toxoplasma gondii. J. Cell Sci. 108:1669–1677. [DOI] [PubMed] [Google Scholar]
- 14.Tu V, Yakubu R, Weiss LM. (2017) Observations on bradyzoite biology. Microbes Infect. 20:466–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bohne W, Gross U, Ferguson DJ, Heesemann J. (1995) Cloning and characterization of a bradyzoite-specifically expressed gene (hsp30/bag1) of Toxoplasma gondii, related to genes encoding small heat-shock proteins of plants. Mol. Microbiol. 16:1221–1230. [DOI] [PubMed] [Google Scholar]
- 16.Parmley SF, Weiss LM, Yang S. (1995) Cloning of a bradyzoite-specific gene of Toxoplasma gondii encoding a cytoplasmic antigen. Mol. Biochem. Parasitol. 73:253–257. [DOI] [PubMed] [Google Scholar]
- 17.Buchholz KR, Bowyer PW, Boothroyd JC. Bradyzoite pseudokinase 1 is crucial for efficient oral infectivity of the Toxoplasma gondii tissue cyst. (2013) Eukaryot Cell. 12:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomita T, Bzik DJ, Ma YF, Fox BA, Markillie LM, Taylor RC, Kim K, Weiss LM. (2013) The Toxoplasma gondii cyst wall protein CST1 is critical for cyst wall integrity and promotes bradyzoite persistence. PLoS Pathog. 9):e1003823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sethi KK, Rahman A, Pelster B, Brandis H. (1977) Search for the presence of lectin-binding sites on Toxoplasma gondii. J. Parasitol. 63:1076–1080. [PubMed] [Google Scholar]
- 20.Caffaro CE, Koshy AA, Liu L, Zeiner GM, Hirschberg CB, Boothroyd JC. (2013) A nucleotide sugar transporter involved in glycosylation of the Toxoplasma tissue cyst wall is required for efficient persistence of bradyzoites. PLoS Pathog. 9:e1003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomita T, Sugi T, Yakubu R, Tu V, Ma Y, Weiss LM. (2017) Making Home Sweet and Sturdy: Toxoplasma gondii ppGalNAc-Ts Glycosylate in Hierarchical Order and Confer Cyst Wall Rigidity. MBio. 8:e02048–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang S, Parmley SF. (1997) Toxoplasma gondii expresses two distinct lactate dehydrogenase homologous genes during its life cycle in intermediate hosts. Gene. 184:1–12. [DOI] [PubMed] [Google Scholar]
- 23.Unno A, Suzuki K, Batanova T, Cha SY, Jang HK, Kitoh K, Takashima Y. (2009) Visualization of Toxoplasma gondii stage conversion by expression of stage-specific dual fluorescent proteins. Parasitology. 136:579–588. [DOI] [PubMed] [Google Scholar]
- 24.Kim SK, Boothroyd JC. (2005) Stage-specific expression of surface antigens by Toxoplasma gondii as a mechanism to facilitate parasite persistence. J. Immunol. 174:8038–8048. [DOI] [PubMed] [Google Scholar]
- 25.Milligan-Myhre K, Wilson SK, Knoll LJ. (2016) Developmental change in translation initiation alters the localization of a common microbial protein necessary for Toxoplasma chronic infection. Molec. Microbiol. 102:1086–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubey JP, Speer CA, Shen SK, Kwok OC, Blixt JA. (1997) Oocyst-induced murine toxoplasmosis: life cycle, pathogenicity, and stage conversion in mice fed Toxoplasma gondii oocysts. J. Parasitol. 83:870–82. [PubMed] [Google Scholar]
- 27.Weiss LM, Kim K. (2000) The development and biology of bradyzoites of Toxoplasma gondii. Front Biosci. 5:D391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lyons RE, McLeod R, Roberts CW. (2002) Toxoplasma gondii tachyzoite-bradyzoite interconversion. Trends Parasitol. 18:198–201. [DOI] [PubMed] [Google Scholar]
- 29.Luder CGK, Rahman T. (2017) Impact of the host on Toxoplasma stage differentiation. Microb. Cell. 4:203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sullivan WJ Jr., Jeffers V (2012) Mechanisms of Toxoplasma gondii persistence and latency. FEMS microbiology reviews. 36:717–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luder CG, Giraldo-Velasquez M, Sendtner M, Gross U. (1999) Toxoplasma gondii in primary rat CNS cells: differential contribution of neurons, astrocytes, and microglial cells for the intracerebral development and stage differentiation. Exp. Parasitol. 93:23–32. [DOI] [PubMed] [Google Scholar]
- 32.Swierzy IJ, Luder CG. (2015) Withdrawal of skeletal muscle cells from cell cycle progression triggers differentiation of Toxoplasma gondii towards the bradyzoite stage. Cell Microbiol. 17:2–17. [DOI] [PubMed] [Google Scholar]
- 33.Cabral CM, Tuladhar S, Dietrich HK, Nguyen E, MacDonald WR, Trivedi T, Devineni A, Koshy AA. (2016) Neurons are the Primary Target Cell for the Brain-Tropic Intracellular Parasite Toxoplasma gondii. PLoS Pathog. 12:e1005447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong DP, Radke JB, White MW. (2017) Opposing Transcriptional Mechanisms Regulate Toxoplasma Development. mSphere. 2 e00347–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holmes MJ, Augusto LDS, Zhang M, Wek RC, Sullivan WJ Jr. (2017) Translational Control in the Latency of Apicomplexan Parasites. Trends Parasitol. 33:947–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watts EA, Dhara A, Sinai AP. (2017) Purification Toxoplasma gondii Tissue Cysts Using Percoll Gradients. Curr. Prot. Microbiol. 45:20c.2.1–c.2.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tobin C, Pollard A, Knoll L. (2010) Toxoplasma gondii cyst wall formation in activated bone marrow-derived macrophages and bradyzoite conditions. J. Vis. Exp. Aug 12(42). pii: 2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cornelissen AW, Overdulve JP, Hoenderboom JM. (1981) Separation of Isospora (Toxoplasma) gondii cysts and cystozoites from mouse brain tissue by continuous density-gradient centrifugation. Parasitology. 83:103–108. [DOI] [PubMed] [Google Scholar]
- 39.Bohne W, Roos DS. (1997) Stage-specific expression of a selectable marker in Toxoplasma gondii permits selective inhibition of either tachyzoites or bradyzoites. Mol. Biochem. Parasitol. 88:115–126. [DOI] [PubMed] [Google Scholar]
- 40.Szabo EK, Finney CA. (2017) Toxoplasma gondii: One Organism, Multiple Models. Trends Parasitol. 33:113–127. [DOI] [PubMed] [Google Scholar]
- 41.Melzer TC, Cranston HJ, Weiss LM, Halonen SK. (2010) Host Cell Preference of Toxoplasma gondii Cysts in Murine Brain: A Confocal Study. J Neuroparasitology. 1 pii: N100505. [DOI] [PMC free article] [PubMed] [Google Scholar]