Abstract
Background
CNS tumors, including medulloblastoma and pediatric glioblastoma (pGBM) account for the majority of solid pediatric malignancies. There remains an unmet need to identify novel treatment approaches in poor prognosis and relapsed pediatric brain tumors, where therapeutic options are limited. Small-molecule B-cell lymphoma 2 (BCL-2) family inhibitors may enhance tumor cell killing when combined with conventional and targeted chemotherapeutic agents. We investigated the effect of disrupting BCL-2 and B cell lymphoma-extra large (BCL-XL) protein function using ABT-263, ABT-199 and WEHI-539 in medulloblastoma and pGBM cells following treatment with MLN8237, an Aurora kinase inhibitor under investigation as a novel agent for the treatment of malignant brain tumors.
Methods
Tumor cell growth and viability were determined by MTT/WST-1 assays and flow cytometry. Effects on cell phenotype, cell cycle progression, and ploidy were determined by live cell imaging and DNA content analysis. Apoptosis was determined by annexin V/propidium iodide staining and time-lapse microscopy and confirmed by measuring caspase-3/7 activity and western blotting and by short interfering RNA (siRNA) knockdown of BCL-2 associated X protein/BCL-2 antagonist killer (BAX/BAK).
Results
ABT-263, in combination with MLN8237, reduced mitotic slippage and polyploidy and promoted the elimination of mitotically defective cells via a BAX/BAK-dependent, caspase-mediated apoptotic pathway. The BCL-XL antagonist, WEHI-539, significantly augmented tumor cell killing when used in combination with MLN8237, as well as sensitized resistant brain tumor cells to a novel BAX activator, SMBA1. In addition, siRNA-mediated knockdown of BCL-XL sensitized pGBM and medulloblastoma cells to MLN8237 and mimicked the effect of combination drug treatment.
Conclusion
Selective small-molecule inhibitors of BCL-XL may enhance the efficacy of MLN8237 and other targeted chemotherapeutic agents.
Keywords: ABT-263, apoptosis, brain tumors, MLN8237, WEHI-539
Importance of the study
Medulloblastoma and pGBM account for the majority of brain tumor–related deaths in children and present a significant challenge for conventional multimodal therapy. Although targeted therapies represent an important approach to improving outcomes for children suffering from these aggressive brain tumors, drug resistance remains a significant problem and contributes to tumor recurrence after initial treatment. Using ABT-263, ABT-199, and WEHI-539, we identify BCL-XL as a candidate drug target. Ours is the first study in pediatric brain tumors to demonstrate that the BCL-XL antagonist, WEHI-539, can promote apoptosis when used in combination with other chemotherapeutics, including MLN8237, an Aurora kinase inhibitor currently being tested in clinical studies for the treatment of CNS tumors, and a novel BAX agonist, previously untested in brain tumor models. Such therapeutic combinations represent novel, targeted treatments that could improve the clinical management of these aggressive, and therapeutically challenging, brain tumors.
Medulloblastoma is the most prevalent (type of) brain tumor in the pediatric population,1 and can be classified into at least 4 subtypes (wingless [WNT], sonic hedgehog [SHH], Group 3, and Group 4) based on their distinct molecular biology.2 In children over 3 years old, effective treatment consists of surgical resection, irradiation, and chemotherapy.3 Survival rates are greater than 75% for patients classified as having average-risk disease (total surgical resection, nonmetastatic, non-MYC/MYCN amplified tumors; TP53-wild type SHH, Group 3, and nonmetastatic Group 4; without chromosome 11 loss).4 However, effective treatment options for high-risk patients with poor prognosis tumors are limited, and current therapies lack target specificity and are associated with significant neurocognitive sequelae and psychosocial deficits.5,6 Tumor recurrence following conventional treatment remains a significant problem, and the majority of patients with relapsed medulloblastoma succumb to the disease.7 Other high-grade tumors, including glioblastoma (GBM), are rarer in children and adolescents (<3.0% of CNS tumors).8 However, the outlook for these patients remains poor (5-y survival: ~18%)9 and is partly due to the intrinsic resistance of these tumors to radiation and chemotherapy. The clinical management of aggressive brain tumors in children remains a significant challenge and there is a need for novel, efficacious therapies directed against defined molecular targets.
B-cell lymphoma 2 (BCL-2) family proteins containing only the BCL-2 homology 3 (BH3) domain are inducers of apoptosis and include BCL-2 associated agonist of cell death (BAD), which promotes apoptosis via binding of anti-apoptotic BCL-2, B-cell lymphoma extra large (BCL-XL), and BCL-2 like protein 2 (BCL-w).10 Navitoclax/ABT-263 is a BAD-like BH3-mimetic and inhibits BCL-2, BCL-XL, and BCL-w, but not myeloid cell leukemia 1 (MCL-1).11 ABT-199/Venetoclax and WEHI-539 are selective small-molecule inhibitors of BCL-2 and BCL-XL, respectively.12,13 WEHI-539 has been shown to sensitize tumor-initiating, colon cancer stem-cell like cells to a variety of chemotherapeutic agents.14 The combination of BCL-2/BCL-XL inhibitors with other targeted therapeutics may represent an efficient way to selectively kill brain tumor cells and could lead to a more durable response than single agent treatment. Aurora kinase A and B regulate cell cycle events from G2 phase to cytokinesis, and have emerged as attractive drug targets in medulloblastoma and pediatric GBM (pGBM).15–17 MLN8237 (alisertib), an Aurora kinase inhibitor,18 has shown in vitro and in vivo activity in preclinical pediatric cancer models.19 Orally administered MLN8237 was tolerated by children with relapsed/refractory solid tumors in a phase I pediatric study20 and has been evaluated as a single agent in pediatric patients with recurrent atypical teratoid/rhabdoid tumors (AT/RT).21 However, the factors contributing to drug response and the mechanisms involved in tumor cell elimination following disruption of mitosis are not fully understood.
In medulloblastoma and pGBM cells, we demonstrate that ABT-263 significantly decreased cell survival, increased caspase-mediated apoptosis, and decreased mitotic exit following mitotic disruption by MLN8237. Combined WEHI-539/MLN8237 treatment revealed that ABT-263 increases sensitivity to MLN8237 via a mechanism of BCL-XL inhibition. In addition, we show that WEHI-539 augments tumor cell killing in resistant pGBM cells when combined with a newly developed BAX agonist.22 Our findings confirm BCL-XL as a relevant anti-apoptotic target in pediatric brain tumor cells, which may have implications in the context of targeted combination therapy for these aggressive tumors.
Materials and Methods
Cell Culture
Pediatric GBM cell lines SF188 (H3F3A wild-type) and KNS42 (H3F3A G34V-mutant) were donated by C. Jones (Institute of Cancer Research, London) and cultured in Dulbecco’s modified Eagle’s medium (Sigma-Aldrich) containing 10% (v/v) fetal bovine serum. DAOY (SHH MB)23 and D283 (Group 3 MB)24 were purchased from American Type Culture Collection and cultured in Improved MEM Zinc Option (Gibco, Life Technologies) supplemented with 10% (v/v) fetal bovine serum, 1% (v/v) nonessential amino acid (Sigma-Aldrich), and 0.002% Phenol Red (Sigma-Aldrich). The identity of cell lines was confirmed by short tandem repeat profiling and all were cultured at 37°C in 5% CO2.
Chemicals
All chemicals were purchased from Sigma-Aldrich unless otherwise stated. Stock solutions of MLN8237, ABT-263, ABT-199 (Selleckchem), WEHI-539 (Bioquote), and zVAD-fmk (Becton Dickinson) were prepared in dimethyl sulfoxide. SMBA122 was donated by J. Zhou (University of Texas Medical Branch, Galveston, Texas).
Cell Viability Assays
Cells were seeded in triplicate prior to treatment. Viability was determined by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) for adherent cell lines (SF188, DAOY, KNS42) and by water-soluble tetrazolium salt 1 (WST-1) assays for the semi-adherent D283 cell line, as per the manufacturers’ instructions.
Analysis of Cell Death
Membrane integrity was determined by flow cytometric quantification of propidium iodide (PI) uptake. Cells were seeded in triplicate and after treatment were harvested and washed with ice-cold phosphate buffered saline (PBS) containing sodium azide (0.1% v/v). Cell pellets were resuspended in PBS/sodium azide solution containing PI (0.83 mg/mL). Apoptosis was quantified using the FITC Annexin V apoptosis detection kit (BD Pharmingen) according to the manufacturer’s instructions. Cell death was analyzed using an Attune Acoustic focusing cytometer (Applied Biosystems) and Attune Cytometric Software.
Cell Cycle Analysis
Treated cells and supernatants were harvested and pelleted before fixation with ice-cold 70% (v/v) ethanol/PBS. Cells were stained in fluorescence activated cell sorting buffer (PBS + 0.1% [w/v] bovine serum albumin and 0.1% [v/v] Tween-20) containing 20 µg/mL PI and 200 µg RNase A. Cells were analyzed using an Attune Acoustic focusing cytometer (Applied Biosystems) and ModFit LT software (Verity Software House).
Live Cell Imaging
Cells were seeded at subconfluent densities in imaging dishes (Ibidi) and placed in a Nikon BioStation IM CELL-S1 microscopy system. Phase contrast images (x20) were captured every 3 minutes for 72 h. At least 50 mitotic cells were analyzed per condition and data were processed for the following parameters: total time in mitosis, completed/uncompleted mitosis, mitotic slippage, and cell death during mitosis.
Apoptosis was monitored in real time by time-lapse microscopy, using a reversible, polarity-sensitive annexin-V probe (pSIVA-IANBD) according to the manufacturer’s instructions (Abcam). Live cell imaging was performed using the IncuCyte zoom live cell imaging system (Essen Bioscience). Whole well scans (x4) and higher resolution scans (x20) over multiple fields of view were performed every 30 minutes (for 72 h) and pSIVA-IANBD fluorescence was measured using conventional green filter sets (excitation maximum 488 nm and emission maximum 530 nm).
Quantification of Caspase-3/7 Activity
Caspase-3/7 activity was assayed using the Caspase-Glo assay (Promega) according to the manufacturer’s instructions. Luminescence was measured using a Mithras LB 940 multimode plate reader.
Western Blotting
Cell lysates were prepared and separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and probed with antibodies against poly(ADP-ribose) polymerase (PARP), Lamin A/C, BCL-XL, BCL-2, BCL-w, BAX, BAK, Bid, Puma, Bad, p-Bad (Ser112), Bik, Bim, phospho–Aurora A (Thr288)/phospho–Aurora B (Thr232)/phospho–Aurora C (Thr198), and total Aurora A and B (Cell Signaling Technologies). Antibody binding was detected using the Odyssey imaging system (LI-COR Biosciences) or the Supersignal West Pico Mouse and Rabbit IgG detection kits (Life Technologies). Equal loading was confirmed using a β-actin antibody (Sigma-Aldrich).
RNA Interference Studies
On-Target plus Smartpool short interfering RNAs (siRNAs) were purchased from Thermo Scientific. Cells were transfected with 75 nM BCL-XL, BCL-2, and BAX or BAK siRNA alone or in combination. A control siRNA was included. Transfections were carried out in Optimem (Gibco, Life Technologies) using Lipofectamine RNAiMax (Invitrogen) for 6–7 h. Media were replenished with serum and supplements and cells cultured for 48 h. Cells were treated with either MLN8237/ABT-263/ABT-199/WEHI-539 alone or in combination. Lysates were prepared in parallel and subjected to SDS-PAGE and membranes probed to confirm successful target knockdown.
Statistical Analysis
SPSS (IBM) was used to analyze quantitative data from independent experiments. Statistical significance between multiple groups was determined by Kruskal–Wallis ANOVA tests, which were followed by Bonferroni-corrected Mann–Whitney U tests for paired comparisons.
Results
ABT-263 Sensitizes Pediatric Brain Tumor Cells to MLN8237
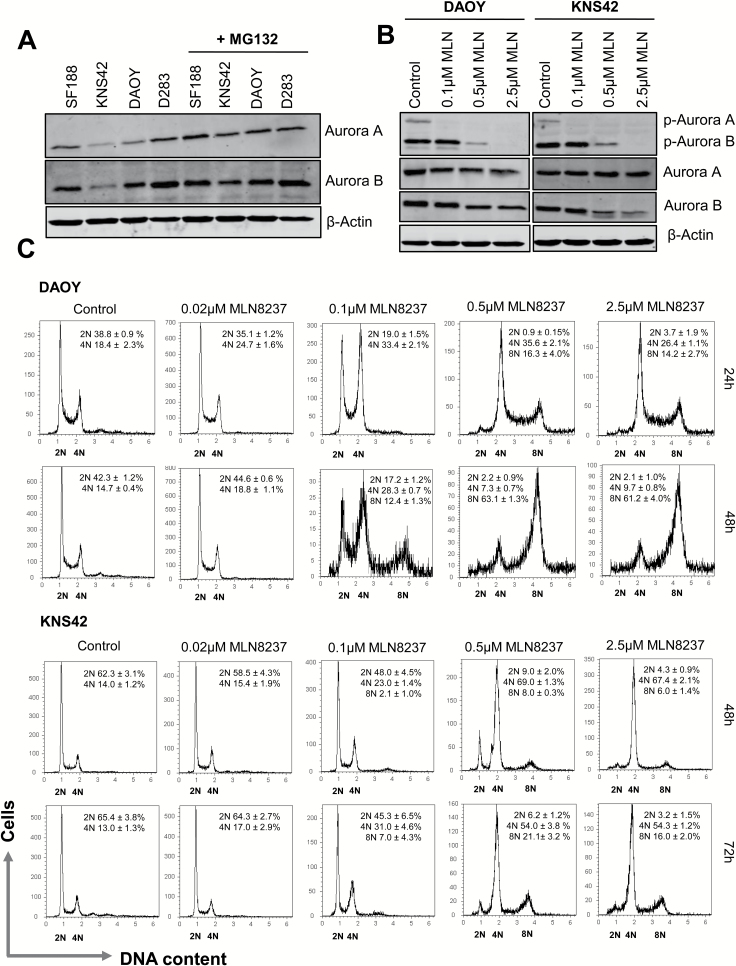
We observed detectable levels of Aurora A and B in two medulloblastoma (DAOY, D283) and pGBM (SF188, KNS42) cell lines under normal culture conditions, as well as in the presence of the proteasome inhibitor MG132, and confirmed the inhibitory effect of MLN8237 on autophosphorylation of Aurora A (Thr288) and Aurora B (Thr232)18 (Fig. 1A–B). DAOY has been shown to be representative of SHH MB,23 and the mitotic kinases have been identified as drug targets in this disease subtype.16H3F3A encodes histone variant H3.3 and is frequently mutated in pediatric high-grade gliomas (pHGGs).25 KNS42 cells carry the heterozygous H3F3A G34V mutation,17,26 which occurs in ~15% of cerebral hemispheric tumors in adolescents and young adults25,27 and has been associated with transcriptional upregulation of multiple genes, including MYCN.17 We used these cell lines as in vitro models of medulloblastoma and pGBM to assess the effect of MLN8237 on cell cycle progression (Fig. 1C). Exposure of DAOY cells to 0.1 µM MLN8237 for 24 h induced a significant G2/M accumulation (P ≤ 0.05, vehicle vs 0.1 µM MLN8237). We also observed a significant increase in the proportion of cells exhibiting 8N DNA content after treatment with 0.5–2.5 µM MLN8237 (P ≤ 0.05, 0.1 µM vs 0.5 µM MLN8237). By 48 h, the proportion of polyploid (>4N) cells had significantly increased to over 60%, suggesting that higher concentrations of MLN8237 induced mitotic slippage (P ≤ 0.01, 24 vs 48 h 0.5 µM MLN8237). Similar results were observed in the KNS42 cell line. However, while treatment with 0.5 µM MLN8237 induced a pronounced G2/M delay and a significant increase in cells with 4N DNA content (13.0% ± 1.3% to 54.0% ± 3.8% compared with vehicle, P ≤ 0.01) only 21% ± 3.2% were polyploid after 72 h, compared with DAOY cells.
Fig. 1.
MLN8237 induces G2/M accumulation and polyploidy in medulloblastoma and pGBM cell lines. (A) Expression of Aurora A and B was determined by immunoblotting in cells cultured +/− MG132 (20 µM) for 2 h (N ≥ 3). (B) DAOY and KNS42 cells were synchronized with 100 ng/mL nocodazole (16 h) and treated with MLN8237 + MG132 for 2 h. Equal loading was confirmed using a β-actin antibody. (C) Cells were treated with MLN8237 for 24–72 h and DNA content was quantified by PI uptake. Representative cell cycle profiles are shown; quantitative data are reported as mean ± SEM (N ≥ 3).
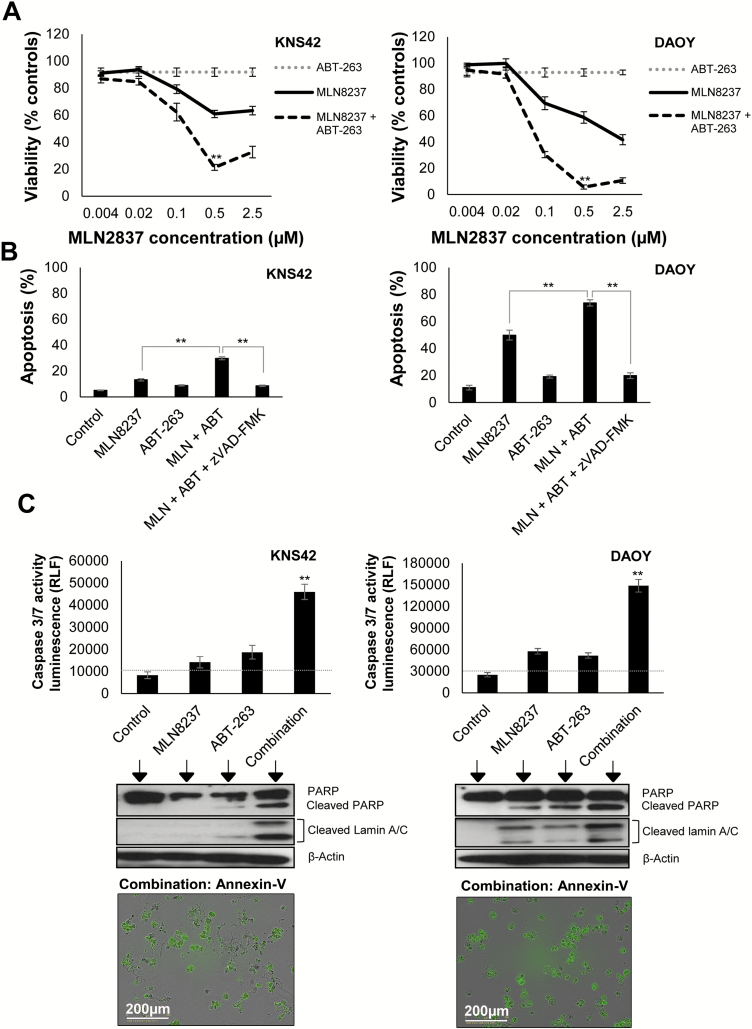
Sustained mitotic arrest and induction of polyploidy can increase the susceptibility of various cancer cell lines to the BCL-2/BCL-XL targeting drug, ABT-263.28–31 Based on our cell cycle data, we reasoned that the efficacy of MLN8237 may be improved by combination with ABT-263. Cell lines exhibited differential expression of BCL-2 family members, and when cultured with ABT-263 and the selective BCL-2 and BCL-XL antagonists, ABT-199 and WEHI-539, we found that ABT-263 was the most effective in reducing cell survival, with D283 cells (MCL-1 deficient) being most sensitive (Supplementary Figure S1). We found that high concentrations of MLN8237 (>0.1 μM) were required to attenuate tumor cell growth in vitro (Fig. 2A). We then examined whether the efficacy of MLN8237 could be enhanced by lowering the apoptotic threshold with ABT-263. We used a sublethal concentration of ABT-263 (1 µM) in combination with MLN8237, which reduced the half-maximal inhibitory concentration for MLN8237 after 72 h of treatment (KNS42: >2.5 µM vs 138 nM; DAOY: 1.1 µM vs 75.8 nM; Fig. 2A), with maximal inhibition of tumor cell growth observed with 0.5 µM MLN8237 and 1 µM ABT-263 (KNS42: 21.2% ± 2.1% viability; DAOY: 5.7% ± 1.5% viability, P ≤ 0.01 combination compared with MLN8237 alone). To determine whether the drug combination was cytotoxic, we quantified apoptosis by annexin V/PI staining (Fig. 2B and Supplementary Figure S2B) and monitored the effect of drug treatment on annexin V exposure by time-lapse microscopy (videos S1–S2). Compared with MLN8237 treatment alone, the combination of ABT-263 with MLN8237 significantly increased apoptosis in KNS42 and DAOY cells (Fig. 2B). We obtained similar results in SF188 and D283 cells (Supplementary Figure S2A). Next, we investigated the mechanism of ABT-263 and MLN8237 cytotoxicity in KNS42 and DAOY cells using the general caspase inhibitor, zVAD-fmk, and by measuring caspase-3/7 activity and PARP and Lamin A/C cleavage (Fig. 2B–C). Inhibition of caspase activity with zVAD-fmk decreased apoptosis to levels observed in vehicle controls (Fig. 2B and Supplementary Figure S2B) and partially restored cell viability in KNS42 and DAOY cells (Supplementary Figure 6C). The combination of ABT-263 and MLN8237 led to a 2.5-fold increase in caspase-3/7 activity in KNS42 and DAOY cells, compared with MLN8237 or ABT-263 alone, and was accompanied by increased cleavage of PARP and Lamin A/C (Fig. 2C). Taken together, these results confirm that ABT-263 promotes caspase-dependent apoptosis following MLN8237 treatment.
Fig. 2.
ABT-263 enhances sensitivity of pediatric brain tumor cells to MLN8237 promoting caspase-mediated apoptosis. (A) Cells were treated with MLN8237 +/−ABT-263 (1 µM). Cell viability was assessed after 72 h by MTT assay. Data represent mean ± SEM (N ≥ 9): **P ≤ 0.01 MLN8237/ABT-263 versus MLN8237 alone. (B) Cells were treated with 0.5 µM MLN8237 (+/−1 µM ABT-263), and apoptosis was quantified by annexin V/PI staining 48 h (DAOY) or 72 h (KNS42) after treatment. Data (percentage annexin V–positive cells) represent the mean ± SEM (N ≥ 9): **P ≤ 0.01 MLN8237/ABT-263 combination versus MLN8237 alone, or combination + 50 µM zVAD-fmk. (C) Cells were treated with 0.5 µM MLN8237 (+/−1 µM ABT-263), and caspase-3/7 activity was quantified. Data represent the mean ± SEM (N ≥ 9): **P ≤ 0.01 MLN8237/ABT-263 versus MLN8237 or ABT-263 alone. Cleavages of PARP and Lamin A/C were detected by immunoblotting (N ≥ 3). Equal loading was confirmed using a β-actin antibody. Annexin V time-lapse microscopy was performed following addition of 0.5 µM MLN8237 and 1 µM ABT-263. Representative images are shown (72 h). Green fluorescence indicates annexin V–positive cells.
ABT-263 Promotes Caspase-Dependent Cell Death Following Disruption of Cell Division by MLN8237
In order to determine whether ABT-263 promoted caspase-dependent cell death during MLN8237-induced mitotic disruption, we examined the effect of drug treatment on cell cycle progression and on cell phenotype via time-lapse microscopy (Fig. 3A–C and Supplementary Figure S3). In KNS42 cells, DNA content analysis revealed that in comparison to MLN8237 treatment alone, combination of ABT-263 and 0.5 µM MLN8237 led to a small but significant increase in the proportion of cells with 4N DNA content (53.5% ± 3.8% to 71.0% ± 2.9%, P ≤ 0.05) and a decrease in the proportion of cells with 8N DNA content (21.1% ± 3.2% to 8.2% ± 1.3%, P ≤ 0.05). Similar effects were observed in the DAOY cell line, which was more prone to polyploidization. The ABT-263/MLN8237 combination significantly decreased the proportion of cells with 8N DNA content (63.1% ± 1.3% to 42.7% ± 5.7%) and increased the proportion of cells with 4N DNA content (7.3% ± 0.7% to 29.7% ± 4.9%) compared with MLN8237 alone (P ≤ 0.05, 0.5 µM MLN8237 vs 0.5 µM MLN8237/1 µM ABT-263). Time-lapse microscopy confirmed that vehicle or ABT-263 (1 µM) treated cultures underwent normal mitosis (Supplementary Figure S3 and videos S3–S4). KNS42 and DAOY cells, treated with 0.5 µM MLN8237 alone, showed clear evidence of protracted and defective mitoses, with the majority of cells exiting mitosis without division. However, ABT-263 addition decreased mitotic exit following cell division failure during MLN8237 treatment, increased cell elimination, and reduced polyploidization (Fig. 3B–C).
Fig. 3.
ABT-263 reduces MLN8237-induced polyploidy and increases death in mitosis. (A) Cells were treated with 0.5 µM MLN8237 +/− ABT-263 (1 µM), and DNA content was quantified by PI uptake. Representative cell cycle profiles are displayed and quantitative data represent the mean ± SEM (N ≥ 3). (B) Alterations in cellular ploidy were quantified following cell cycle experiments, KNS42 (72 h) and DAOY (48 h) (N ≥ 3): *P ≤ 0.05 MLN8237/ABT-263 versus MLN8237 alone. (C) Cells were imaged for 72 h by time-lapse microscopy. A minimum of 50 cells per condition were analyzed.
Selective Inhibition of BCL-XL Increases Cell Death in Combination with Targeted Chemotherapeutic Agents
To assess the specific roles of BCL-2 and BCL-XL in promoting cell survival, we used ABT-199 and WEHI-539 in combination with MLN8237 (Fig. 4A and Supplementary Figure S4). In KNS42 and DAOY cells, WEHI-539 (BCL-XL selective) induced apoptosis as effectively as ABT-263 when used in combination with MLN8237. These results were reproducible in the SF188 and D283 cell lines, where combination of MLN8237 with WEHI-539, but not ABT-199, significantly increased cell death and mimicked the effect observed with ABT-263 and MLN8237 (Supplementary Figure S5). In order to address the functional role of BCL-XL in the response to MLN8237 treatment, we used siRNA to silence BCL-XL expression in KNS42 and DAOY cells (Fig. 4B). Knockdown of BCL-XL significantly increased cell death following treatment with MLN8237, and levels of cell death were comparable to those observed with MLN8237/ABT-263 or WEHI-539 (Fig. 4B). These data indicate that MLN8237 renders tumor cells increasingly dependent on BCL-XL for survival.
Fig. 4.
Selective inhibition of BCL-XL sensitizes pediatric brain tumor cells to MLN8237. (A) Cells were treated with 0.5 µM MLN8237 +/− BH3-mimetics (1 µM) and apoptosis was quantified by annexin V/PI staining 48 h (DAOY) or 72 h (KNS42). Data (percentage annexin V–positive cells) represent the mean ± SEM (N ≥ 9): **P ≤ 0.01 MLN8237/ABT-263 or MLN8237/WEHI-539 versus MLN8237 alone. (B) Cells were treated with BCL-XL or control siRNA, and lysates were immunoblotted using antibodies against BCL-XL and BCL-2 (N ≥ 3). Equal loading was confirmed using a β-actin antibody. Cell death was determined by PI uptake 48–72 h after drug treatment. Data represent the mean ± SEM (N ≥ 9): **P ≤ 0.01 MLN8237/control siRNA versus MLN8237/BCL-XL siRNA.
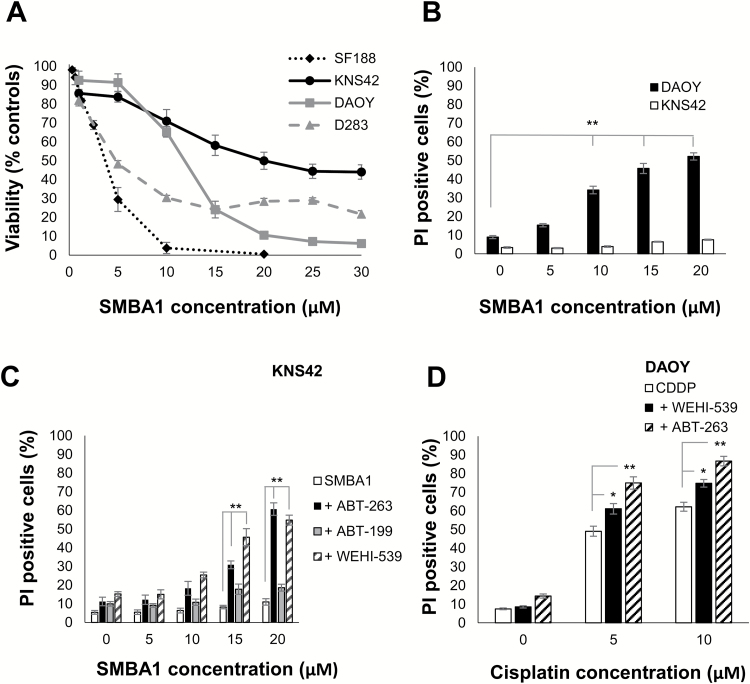
To further investigate whether BCL-XL contributes to chemotherapy resistance, we examined whether WEHI-539 could sensitize tumor cells to other chemotherapeutic agents and to a novel small-molecule BAX activator, SMBA122 (Fig. 5). All cell lines expressed comparable levels of BAX protein (Supplementary Figure S1A) but exhibited differential sensitivity to the BAX agonist (Fig. 5A). In DAOY cells, we observed a significant increase in cell death following treatment with SMBA1 compared with vehicle controls (34.1% ± 2.7% to 52.1% ± 2.6%, 10–20 µM of SMBA1, P ≤ 0.01). By contrast, KNS42 cells showed no significant increase in cell death, indicating that the observed decrease in viability was due to cytostatic rather than cytotoxic effects. Next, we examined the effect of combining ABT-263, ABT-199, and WEHI-539 with SMBA1 in KNS42 cells (Fig. 5C). Addition of 1 µM ABT-263 or WEHI-539, but not ABT-199, sensitized KNS42 cells to SMBA1 and significantly increased cell death, indicating that resistance to the BAX agonist was mediated by BCL-XL rather than BCL-2. We also investigated the use of these compounds in combination with the DNA damaging agent cisplatin (Fig. 5D). Our data show that WEHI-539 and ABT-263 potentiated cell death when used in combination with cisplatin in the DAOY cell line. Together, these data indicate that selective targeting of BCL-XL could increase the sensitivity of brain tumor cells to chemotherapeutic agents.
Fig. 5.
WEHI-539 increases cell death in combination with a BAX activator or cisplatin. (A) Cells were treated with SMBA1 for 72 h and cell viability determined by MTT (SF188, KNS42, DAOY) or WST-1 assay (D283). Data represent the mean ± SEM (N ≥ 9). (B) DAOY and KNS42 were treated with SMBA1 and cell death was determined by PI uptake after 72 h. The data represent the mean ± SEM (N ≥ 9): **P ≤ 0.01 10–20 µM SMBA1 versus controls. (C) KNS42 cells were treated with SMBA1 +/− BH3-mimetics (1 µM) for 72 h. Cell death was determined by PI uptake. Data represent the mean ± SEM (N ≥ 9): **P ≤ 0.01 15–20 µM SMBA1 + ABT-263/WEHI-539 versus SMBA1 alone. (D) DAOY cells were treated with cisplatin +/− ABT-263/WEHI-539 (1 µM) for 72 h. Cell death was determined by PI uptake. Data represent the mean ± SEM (N ≥ 9): *P ≤ 0.05, cisplatin/WEHI-539 versus cisplatin alone; **P ≤ 0.01 cisplatin/ABT-263 versus cisplatin alone.
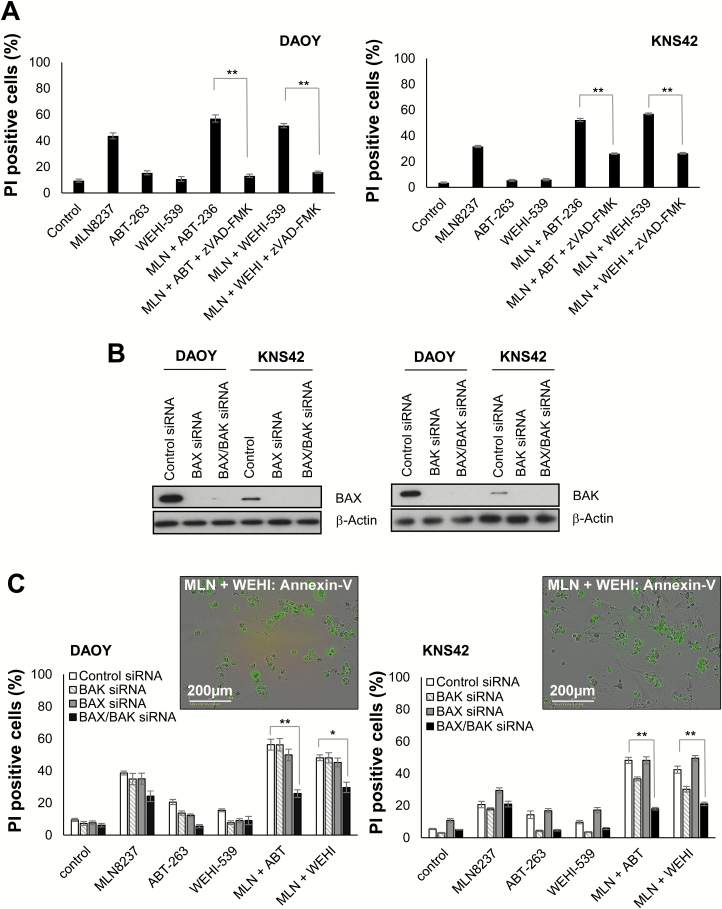
MLN8237 and WEHI-539 Cytotoxicity Is Attenuated by Caspase Inhibition and Requires BAX/BAK
Our data indicated that ABT-263 promoted caspase-dependent apoptosis following MLN8237 treatment. Similarly, we found that caspase inhibition significantly reduced cell death and increased viability following WEHI-539/MLN8237 treatment (Fig. 6A and Supplementary Figure S6A–C). Next, we examined the role of the mitochondrial death effectors, BAX and BAK (Fig. 6B–C). Immunoblotting confirmed knockdown of BAX and BAK (Fig. 6B and Supplementary Figure S6D). In DAOY cells, cytotoxicity induced by a combination of MLN8237/ABT-263 or WEHI-539 was only significantly reduced in cells with a double BAX/BAK knockdown (Fig. 6C). Cell death induced by MLN8237/ABT-263 was reduced by 30% following BAX/BAK knockdown (56.2% ± 3.4% to 26.7% ± 2.4%), and by 18% in cells treated with MLN8237/WEHI-539 (48.0% ± 1.8% to 29.6% ± 3.2%) compared with controls. Similarly, BAX/BAK knockdown reduced cell killing in KNS42 cells by 30% and 21% following treatment with MLN8237/ABT-263 and MLN8237/WEHI-539, respectively. In addition, specific knockdown of BAK significantly attenuated cell death following treatment with ABT-263 or WEHI-539 alone, and in combination with MLN8237 (P ≤ 0.01, BH3-mimetic/control siRNA vs BH3-mimetic/BAK siRNA). Overall, these data indicate that ABT-263 and WEHI-539 promote BAX/BAK-dependent, caspase-mediated apoptosis in pediatric brain tumor cells following MLN8237-induced mitotic disruption.
Fig. 6.
Combination of WEHI-539 with MLN8237 promotes caspase-mediated apoptosis and is BAX/BAK dependent. (A) Cells were pretreated with zVAD-fmk (50 µM) for 1 h before addition of WEHI-539 (1 µM), MLN8237 (0.5 µM), or both agents. Cells were treated with ABT-263 (1 µM) and MLN8237 (0.5 µM), alone or in combination as a control. Cell death was determined by PI uptake. Data represent the mean ± SEM (N ≥ 9): **P ≤ 0.01 MLN8237/ABT-263 versus MLN8237/ABT-263 + zVAD-fmk; MLN8237/WEHI-539 versus MLN8237/WEHI-539 + zVAD-fmk. (B) DAOY and KNS42 cells were treated with BAX and BAK or control siRNAs prior to treatment with MLN8237 (0.5 µM) and ABT-263 and WEHI-539 (1 µM) for 48–72 h. Expression levels of BAX and BAK are shown (N ≥ 3). Equal loading was confirmed using a β-actin antibody. (C) Cell death was determined by PI uptake. The data represent the mean ± SEM (N ≥ 9): *P ≤ 0.05, **P ≤ 0.01 MLN8237/BH3-mimetic + control siRNA versus MLN8237/BH3-mimetic + BAX/BAK siRNA.
Discussion
Pharmacological targeting of the apoptotic mitochondrial pathway represents a novel strategy for cancer therapy, and small-molecules targeting the BCL-2 family proteins, including ABT-263, can augment tumor cell killing when combined with other chemotherapeutic agents.28–32 There is considerable interest in Aurora kinase inhibitors as novel, targeted therapies for aggressive pediatric CNS tumors, including AT/RT, GBM, and medulloblastoma,15–17,21,33,34 and MLN8237 is a promising drug candidate, since it readily crosses the blood–brain barrier.35 MLN8237 significantly prolonged survival in models of relapsed medulloblastoma, via disruption of Aurora A/MYCN complexes and MYCN degradation.34 However, in this context, tumor regression appeared to be the consequence of cytostasis rather than induction of apoptosis. Here, we demonstrate that selective inhibition of BCL-XL significantly enhances the sensitivity of pediatric brain tumor cells to MLN8237 and augments tumor cell killing via activation of the mitochondrial apoptotic pathway. BAX is a key pro-apoptotic mitochondrial effector and, when deleted in ND2:SmoA1 transgenic mice, accelerates SHH-driven medulloblastoma formation.36 The discovery of new compounds which target BAX provides an additional and important route for apoptosis-based therapies.22 In this study, we demonstrated that a novel BAX agonist, SMBA1, reduced the growth of pediatric brain tumor cell lines in vitro and, when combined with WEHI-539, overcame resistance to apoptosis in the pGBM cell line, KNS42. Our study particularly identifies BCL-XL as a potential therapeutic target in pediatric brain tumor cells and is the first to demonstrate that selective inhibition of BCL-XL using WEHI-539 can sensitize medulloblastoma and pGBM cells to other targeted chemotherapeutic agents, including MLN8237 and SMBA1.
In a range of solid tumors, ABT-263 has been shown to potentiate tumor cell killing when used in combination with chemotherapeutics, predominantly via a mechanism of BCL-XL inhibition.13,28,29,31,37 Ham et al recently reported that MYCN-amplified neuroblastomas, characterized by low BCL-XL and high BCL-2 expression, retain sensitivity to ABT-199 partly due to the MYCN-driven upregulation of the MCL-1 antagonist, NOXA.38 In addition, combination of MLN8237 with ABT-199 was shown to increase apoptosis in vitro and improve tumor control in vivo. Differing expression levels and protein-protein interactions among individual BCL-2 family members are likely to contribute to differences observed in other tumor models where drug-induced mitotic arrest and polyploidization have been shown to increase BCL-XL dependency.28,29,31 Similarly, in our investigations in pediatric brain tumor cells, BCL-XL was revealed as the key target, since ABT-263/WEHI-539 treatment or siRNA-mediated knockdown of BCL-XL sensitized tumor cells to MLN8237 and potentiated cell death, whereas ABT-199 treatment did not. Our new findings have potentially important implications for brain tumor therapy, since addition of sublethal concentrations of ABT-263 significantly reduced cell viability, lowered the half-maximal inhibitory concentration of MLN8237, and augmented tumor cell killing. Post-chemotherapy survival of giant polyploid cells may contribute to tumor progression, relapse, and increased drug resistance via the generation of diploid escape cells.39 Thus, therapeutic combinations incorporating MLN8237 with BCL-XL targeting drugs may reduce the risk of tumor progression and relapse by decreasing mitotic slippage, suppressing polyploidization,28,31 or by eliminating polyploid cells.29 In a pediatric phase I trial of MLN8237, when patients were treated with the recommended maximum tolerated dose of 80 mg/m2/day, the peak plasma drug concentration was 7.5 µM (2 h post administration), and declined below 1 µM after 24 h.20 Our in vitro studies indicated that ABT-263 and WEHI-539 could enhance sensitivity to MLN8237 below this physiological range, indicating that the efficacy of MLN8237, at clinically relevant doses, could be enhanced via combination with BCL-XL inhibitors, such as A-1155463.37 However, on-target thrombocytopenia associated with BCL-XL targeting drugs12 and adequate CNS penetration are significant challenges affecting their use in the clinical setting.
In this study, we identify BCL-XL as a candidate drug target for sensitization to chemotherapy and present new evidence demonstrating how specific targeting of BCL-XL can promote apoptosis in pediatric brain tumor cell lines, in response to either MLN8237 or a small-molecule therapeutic designed to target pro-apoptotic BAX. Overall, our data indicate that combinatorial therapy with selective BCL-XL inhibitors and other targeted agents may be more effective against pediatric brain tumor cells than single agent treatment. Here, we utilized KNS42 (H3F3A G34V-mutant)17,26 pGBM cells and DAOY, an established medulloblastoma cell line (previously subtyped as SHH-MB23, TP53-mutant40), as in vitro models. TP53-mutant, SHH medulloblastomas are associated with a poor prognosis (<50% survival),4 and H3F3A G34V mutation occurs in up to 15% of cerebral hemispheric high-grade gliomas in children and adolescents.27 Follow-up preclinical studies focusing on pHGGs and specific medulloblastoma subtypes should be undertaken and include patient-derived cell lines and xenograft models to further determine treatment efficacy and durability of response in these aggressive pediatric brain tumors.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
This work was jointly funded by Candlelighters Childhood Cancer Charity in Yorkshire and Brain Tumour Research Across Yorkshire (BTRS).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary Material
References
- 1. Pfister S, Hartmann C, Korshunov A. Histology and molecular pathology of pediatric brain tumors. J Child Neurol. 2009;24(11):1375–1386. [DOI] [PubMed] [Google Scholar]
- 2. Northcott PA, Jones DT, Kool M et al. Medulloblastomics: the end of the beginning. Nat Rev Cancer. 2012;12(12):818–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gajjar A, Packer RJ, Foreman NK, Cohen K, Haas-Kogan D, Merchant TE; COG Brain Tumor Committee Children’s Oncology Group’s 2013 blueprint for research: central nervous system tumors. Pediatr Blood Cancer. 2013;60(6):1022–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ramaswamy V, Remke M, Bouffet E et al. Risk stratification of childhood medulloblastoma in the molecular era: the current consensus. Acta Neuropathol. 2016;131(6):821–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pizer B, Donachie PH, Robinson K et al. Treatment of recurrent central nervous system primitive neuroectodermal tumours in children and adolescents: results of a Children’s Cancer and Leukaemia Group study. Eur J Cancer. 2011;47(9):1389–1397. [DOI] [PubMed] [Google Scholar]
- 6. Frange P, Alapetite C, Gaboriaud G et al. From childhood to adulthood: long-term outcome of medulloblastoma patients. The Institut Curie experience (1980–2000). J Neurooncol. 2009;95(2):271–279. [DOI] [PubMed] [Google Scholar]
- 7. Packer RJ, Vezina G. Management of and prognosis with medulloblastoma: therapy at a crossroads. Arch Neurol. 2008;65(11):1419–1424. [DOI] [PubMed] [Google Scholar]
- 8. MacDonald TJ, Aguilera D, Kramm CM. Treatment of high-grade glioma in children and adolescents. Neuro Oncol. 2011;13(10):1049–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ostrom QT, Gittleman H, Liao P et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;16(Suppl 4):iv1–iv63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vela L, Marzo I. Bcl-2 family of proteins as drug targets for cancer chemotherapy: the long way of BH3 mimetics from bench to bedside. Curr Opin Pharmacol. 2015;23:74–81. [DOI] [PubMed] [Google Scholar]
- 11. Tse C, Shoemaker AR, Adickes J et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68(9):3421–3428. [DOI] [PubMed] [Google Scholar]
- 12. Souers AJ, Leverson JD, Boghaert ER et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19(2):202–208. [DOI] [PubMed] [Google Scholar]
- 13. Lessene G, Czabotar PE, Sleebs BE et al. Structure-guided design of a selective BCL-X(L) inhibitor. Nat Chem Biol. 2013;9(6):390–397. [DOI] [PubMed] [Google Scholar]
- 14. Colak S, Zimberlin CD, Fessler E et al. Decreased mitochondrial priming determines chemoresistance of colon cancer stem cells. Cell Death Differ. 2014;21(7):1170–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Neben K, Korshunov A, Benner A et al. Microarray-based screening for molecular markers in medulloblastoma revealed STK15 as independent predictor for survival. Cancer Res. 2004;64(9):3103–3111. [DOI] [PubMed] [Google Scholar]
- 16. Markant SL, Esparza LA, Sun J et al. Targeting sonic hedgehog-associated medulloblastoma through inhibition of Aurora and Polo-like kinases. Cancer Res. 2013;73(20):6310–6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bjerke L, Mackay A, Nandhabalan M et al. Histone H3.3. mutations drive pediatric glioblastoma through upregulation of MYCN. Cancer Discov. 2013;3(5):512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Manfredi MG, Ecsedy JA, Chakravarty A et al. Characterization of alisertib (MLN8237), an investigational small-molecule inhibitor of aurora A kinase using novel in vivo pharmacodynamic assays. Clin Cancer Res. 2011;17(24):7614–7624. [DOI] [PubMed] [Google Scholar]
- 19. Maris JM, Morton CL, Gorlick R et al. Initial testing of the aurora kinase A inhibitor MLN8237 by the Pediatric Preclinical Testing Program (PPTP). Pediatr Blood Cancer. 2010;55(1):26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mossé YP, Lipsitz E, Fox E et al. Pediatric phase I trial and pharmacokinetic study of MLN8237, an investigational oral selective small-molecule inhibitor of Aurora kinase A: a Children’s Oncology Group Phase I Consortium study. Clin Cancer Res. 2012;18(21):6058–6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wetmore C, Boyett J, Li S et al. Alisertib is active as single agent in recurrent atypical teratoid rhabdoid tumors in 4 children. Neuro Oncol. 2015;17(6):882–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xin M, Li R, Xie M et al. Small-molecule Bax agonists for cancer therapy. Nat Commun. 2014;5:4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Triscott J, Lee C, Foster C et al. Personalizing the treatment of pediatric medulloblastoma: polo-like kinase 1 as a molecular target in high-risk children. Cancer Res. 2013;73(22):6734–6744. [DOI] [PubMed] [Google Scholar]
- 24. Snuderl M, Batista A, Kirkpatrick ND et al. Targeting placental growth factor/neuropilin 1 pathway inhibits growth and spread of medulloblastoma. Cell. 2013;152(5):1065–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sturm D, Bender S, Jones DT et al. Paediatric and adult glioblastoma: multiform (epi)genomic culprits emerge. Nat Rev Cancer. 2014;14(2):92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Levesley J, Steele L, Taylor C, Sinha P, Lawler SE. ABT-263 enhances sensitivity to metformin and 2-deoxyglucose in pediatric glioma by promoting apoptotic cell death. PLoS One. 2013;8(5):e64051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jones C, Karajannis MA, Jones DTW et al. Pediatric high-grade glioma: biologically and clinically in need of new thinking. Neuro Oncol. 2017;19(2):153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shi J, Zhou Y, Huang HC, Mitchison TJ. Navitoclax (ABT-263) accelerates apoptosis during drug-induced mitotic arrest by antagonizing Bcl-xL. Cancer Res. 2011;71(13):4518–4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shah OJ, Lin X, Li L et al. Bcl-XL represents a druggable molecular vulnerability during aurora B inhibitor-mediated polyploidization. Proc Natl Acad Sci U S A. 2010;107(28):12634–12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tan N, Malek M, Zha J et al. Navitoclax enhances the efficacy of taxanes in non-small cell lung cancer models. Clin Cancer Res. 2011;17(6):1394–1404. [DOI] [PubMed] [Google Scholar]
- 31. Bah N, Maillet L, Ryan J et al. Bcl-xL controls a switch between cell death modes during mitotic arrest. Cell Death Dis. 2014;5:e1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sun J, Knickelbein K, He K et al. Aurora kinase inhibition induces PUMA via NF-κB to kill colon cancer cells. Mol Cancer Ther. 2014;13(5):1298–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Venkataraman S, Alimova I, Tello T et al. Targeting aurora kinase A enhances radiation sensitivity of atypical teratoid rhabdoid tumor cells. J Neurooncol. 2012;107(3):517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hill RM, Kuijper S, Lindsey JC et al. Combined MYC and P53 defects emerge at medulloblastoma relapse and define rapidly progressive, therapeutically targetable disease. Cancer Cell. 2015;27(1):72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lehman NL, O’Donnell JP, Whiteley LJ et al. Aurora A is differentially expressed in gliomas, is associated with patient survival in glioblastoma and is a potential chemotherapeutic target in gliomas. Cell Cycle. 2012;11(3):489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Garcia I, Crowther AJ, Gama V et al. Bax deficiency prolongs cerebellar neurogenesis, accelerates medulloblastoma formation and paradoxically increases both malignancy and differentiation. Oncogene. 2013;32(18):2304–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Leverson JD, Phillips DC, Mitten MJ et al. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Science Trans Med. 2015;7(279):279ra240. [DOI] [PubMed] [Google Scholar]
- 38. Ham J, Costa C, Sano R et al. Exploitation of the apoptosis-primed state of MYCN-amplified neuroblastoma to develop a potent and specific targeted therapy combination. Cancer Cell. 2016;29(2):159–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Puig PE, Guilly MN, Bouchot A et al. Tumor cells can escape DNA-damaging cisplatin through DNA endoreduplication and reversible polyploidy. Cell Biol Int. 2008;32(9):1031–1043. [DOI] [PubMed] [Google Scholar]
- 40. Saylors RL 3rd, Sidransky D, Friedman HS et al. Infrequent p53 gene mutations in medulloblastomas. Cancer Res. 1991;51(17):4721–4723. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.