Introduction:
Eosinophilic esophagitis (EoE), is an allergic disease of the esophagus that affects both children and adults and is defined by clinical, endoscopic, and histologic characteristics. Diagnostic criteria include eosinophilic infiltration of the esophagus with ≥ 15 eosinophils/high-powered field (eos/hpf), in the setting of esophageal dysfunction1. EoE is considered to be active based on histopathology - if the peak eosinophil count is ≥15 eos/hpf, the disease is considered active. EoE is in remission if the eosinophil count decreases to <15 eos/hpf in a patient with previously diagnosed EoE1, 2.
Esophageal dysfunction in EoE may present differently in children and adults. Pediatric patients typically present with feeding difficulty, abdominal pain, and vomiting, and adolescents and adults present with dysphagia and food impaction3. The current theory is that eosinophilic inflammation in the esophagus over time leads to fibrosis and narrowing of the esophagus, thus it is hypothesized that many, though likely not all, older patients with increased duration of disease have more sequela of fibrostenosis4. While EoE is a life-long disease5, therapy has been shown to reduce side effects, improve symptomatic burden, and decrease necessity of esophageal dilation6, 7, highlighting the importance of understanding disease activity even in the asymptomatic patient8–10.
In order to perform this histologic investigation to determine disease activity in EoE, an endoscopy is required to obtain a biopsy of the esophageal mucosa. After each therapeutic change an endoscopy is performed to determine response. Thus, the burden of endoscopy on the EoE patient is great. In most cases, this involves a diagnostic endoscopy, followed by a few attempted therapies (drug or diet interventions). Patients who do not respond to the first therapy or who choose diet therapy, often have many endoscopies in rapid succession3, 11. The importance of this monitoring must be balanced with cost-effectiveness 12 as well as risk to the patient - although adverse events during endoscopic procedures under anesthesia are rare13, they do occur and must be taken into account when discussing risks and benefits of monitoring with patients and their families. Additionally, in 2016, the US Food and Drug Administration “Drug Safety Communication” issued a black box warning related to the use of anesthetics in children under the age of 3, suggesting that it “may affect the development of children’s brains” (https://www-fda-gov.proxy.library.upenn.edu/Drugs/DrugSafety/ucm532356.htm). These recommendations were based in large part on studies in which animals received multiple rounds of anesthesia or prolonged anesthesia during early-life. Animals exposed to anesthesia were more likely to demonstrate brain/neuron development and cognition issues than unexposed animals14–16. Understanding the risks and benefits of these procedures and the information that that will be gained from each endoscopy is crucial for the physicians caring for EoE patients.
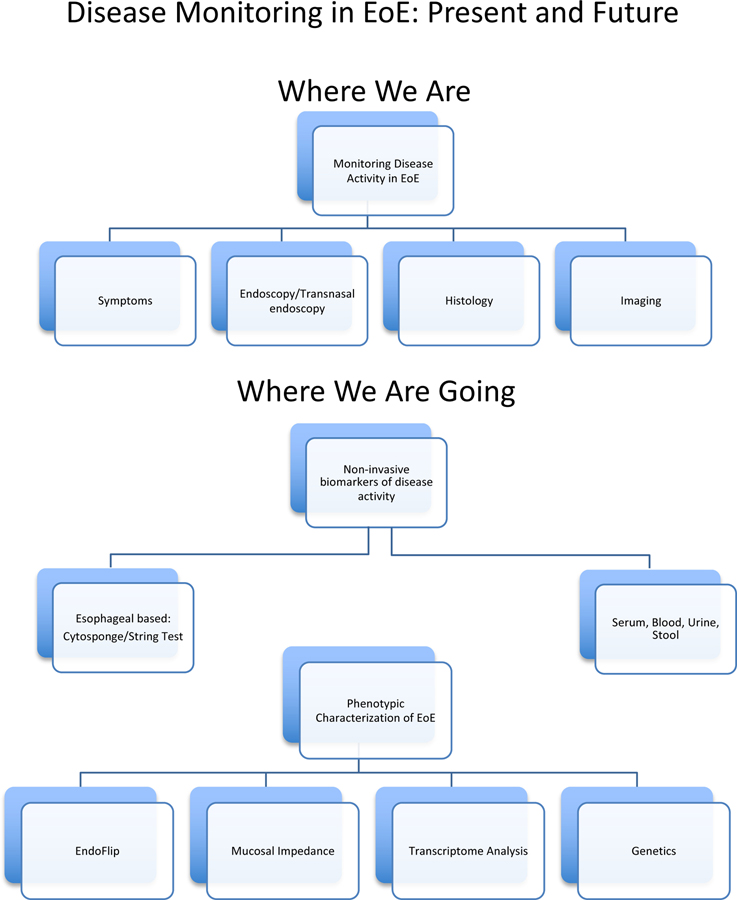
Additional tools, both invasive and non-invasive, have been researched for monitoring and understanding eosinophilic esophagitis A literature search was performed using PubMed with keyword combinations of EoE and monitoring as well as various techniques used for monitoring, including, but not limited to, symptoms, endoscopy, histology, fluoroscopy, EndoFLIP, non-invasive monitoring and biomarkers. Case-control studies, observational studies, peer-reviewed reviews and guidelines, and systematic reviews were selected, reviewed, and summarized here. The first portion of this article aims to summarize current disease monitoring practice as well as potential candidates for future less-invasive monitoring in EoE. We will then describe new research applications that serve to better characterize EoE disease activity through personalized medicine approaches including both molecular and functional analysis (Fig. 1).
Figure 1:
Current and future modalities for monitoring in Eosinophilic Esophagitis
Monitoring of Disease Activity in EoE –Where We Are
As we look towards the future of monitoring for EoE in less invasive ways, it is important to define what measure we have at our fingertips for use today and how we use them in an evidence-based and clinically relevant way. The current modalities being used and studied focus on defining disease state and considering whether further therapeutic changes are necessary based on findings detected at a specific moment in time, on a particular diet or medication. The following section describes the tools that have been investigated, and in many cases are currently being used, to make this determination.
Symptoms
Endoscopy and biopsy provide the gold standard for diagnosis and disease activity monitoring, because symptoms have been shown to be less reliable modality17. One reason for this finding is coping mechanisms. Patients adapt their diet to exclude foods that cause dysphagia, increase lubrication with extra mealtime beverages, and increase chewing time. It can be challenging to assess symptoms in patients who have had undiagnosed inflammation for years4 who have adapted these subtle behaviors.
There are three validated patient reported outcome (PRO) tools: Pediatric EoE Symptom Score (PEESS v2.0)18, the dysphagia symptom questionnaire (DSQ)19, and the adult eosinophilic esophagitis activity index (EEsAI)20. Unfortunately, these tools have not shown adequate sensitivity for detecting esophageal eosinophilia based on symptoms17, 21.
In order to capture the myriad of pediatric complaints in the EoE population, validation of the PEESS 2.0 evaluated 4 domains: pain, reflux, nausea/vomiting, and dysphagia. Each of these four domains was compared to histologic factors including eosinophil count and eosinophil/mast cell derived proteins, as well as gene expression of the top 96 dysregulated genes in EoE. Despite the broad approach, mast cell products (typtase/chymase) and eosinophil peroxidase correlated significantly with only the dysphagia domain.
Symptom scoring in adults puts more weight on dysphagia. The DSQ evaluates dysphagia daily for 30 days using a simple 3 question survey, and the EEsAI follows patients for 7 days and additionally asks about behavioral adaptions and food avoidance that may occur as a result of dysphagia. EEsAI was found to have an accuracy of 0.6–0.7 for detecting disease activity in EoE. Specifically the mild/modest disease activity, without fibrosis, seemed more difficult to detect via symptomatology alone.
The inability to follow symptoms has been a major obstacle to drug development in EoE. In order for drugs to be approved by the FDA, the trial must show an improvement in symptoms greater than placebo. As discussed symptoms vary greatly by eating behavior and do not always correlate with histologic endpoints, especially in the setting of post-inflammatory esophageal remodeling in which symptoms can persist despite resolution of disease. In order to try to achieve the FDA mandates, many EoE clinical trials have restricted entry to include only those with severe dysphagia. This provides two possibly unintended side-effects: 1) the majority of pediatric patients are excluded and 2) there is a bias toward more severe disease.
The development and validation of symptom scores are crucial to our understanding of the symptomatology of EoE, however, they are not currently adequate to detect disease activity. And our inability to utilize symptom as a clinical outcome measure has had unforeseen effects on clinical trial development.
Endoscopy and Histology
The current gold standard for monitoring activity in EoE is endoscopy with biopsy. This is typically done 8 to 12 weeks after a change in therapy. While there has not been a study evaluating timing for endoscopy after therapeutic changes, there is evidence that endoscopy beyond 3 months in steroid non-responders did not yield additional information22. The eosinophilic esophagitis endoscopic reference score (EREFS) is a classification system used to score endoscopic findings23. The EREFS score accounts for the presence and severity of findings that are classic in appearance for EoE, including esophageal rings, exudates, furrowing, mucosal fragility, edema and associated decreased vascular markings, and esophageal stricture. This scoring system has been showed to correspond to histologic improvement in EoE, supporting its validity24.
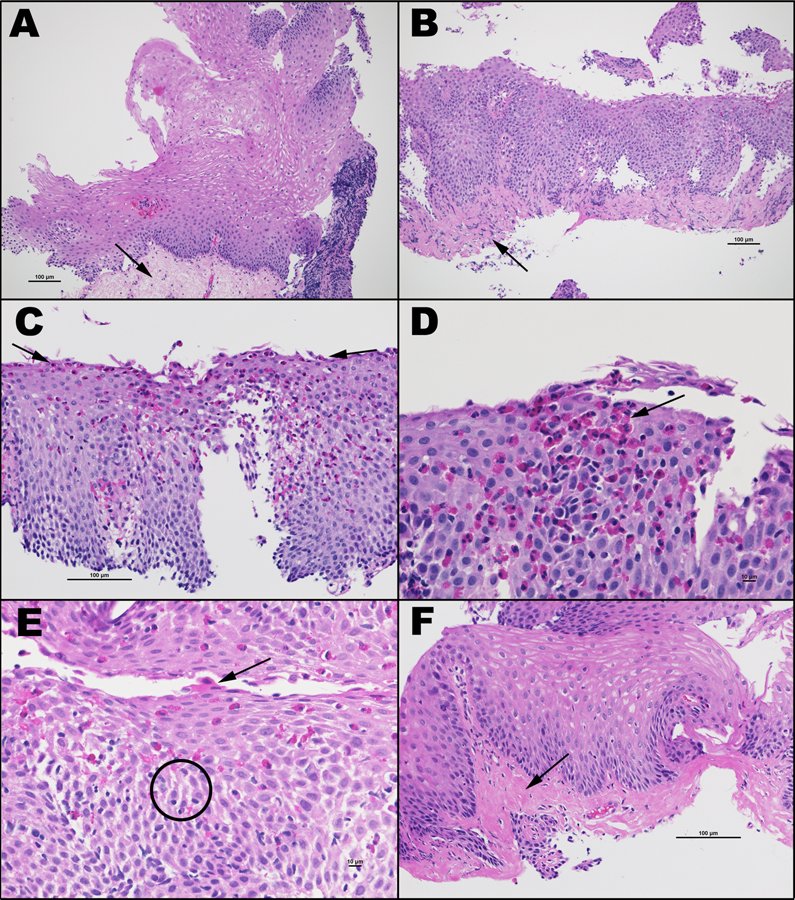
Beyond visual inspection, histologic evaluation of the esophagus is essential, with the guideline of 15 or more eos/hpf in the esophagus determining whether a person is thought to have active or inactive EoE. There is current work looking into an advanced histologic scoring system (EoEHSS) to evaluate a wider range of histologic findings, including eosinophil density, basal zone hyperplasia, eosinophil abscesses, eosinophil surface layering, dilated intercellular spaces, surface epithelial alteration, dyskeratotic epithelial cells, and lamina propria fibrosis25, 26 (Figure 2). Some of these characteristics were found to be quite rare with only a small proportion of patients with EoE demonstrating dyskeratosis and superficial epithelial alteration, whereas others such as basal cell hyperplasia and dillated intracellular spaces were more common.
Figure 2:
Histologic changes in active Eosinophilic Esophagitis: in addition to eosinophilia, other mucosal and submucosal abnormalities include basal cell hyperplasia, dilated intercellular spaces, and lamina propria fibrosis.
The importance of an in depth histologic epithelial evaluation in addition to simply eosinophil count was highlighted in a recent paper describing persistent symptoms in inactive EoE patients. Symptomatology and endoscopic findings were found to be more common in those patients with persistent basal cell hyperplasia seen on pathology 7. Similarly, the presence of dilated intercellular spaces (DIS) has also shown clinical relevance, with decrease in DIS in patients on therapy 27. These studies highlight the importance of histologic evaluation that goes beyond eosinophils alone in clinical decision making, and may help guide the clinician when there are <15 eosinophils per high powered field but ongoing symptoms.
In addition to discussion regarding expanding histologic criterion used in diagnosis and monitoring in eosinophilic esophagitis, there is also discussion in the field regarding how many biopsies are necessary for diagnosis and monitoring in EoE, and significance of the location of those biopsies. EoE is thought to be a patchy disease in the esophagus, leading to the possibility of a missed diagnosis or inaccurate determination of remission status if biopsies are not taken in an area with active esophageal inflammation. Three biopsy specimens in the esophagus yields a 97% sensitivity, and that sensitivity increases further if biopsies are taken in a variety of locations in the esophagus, including proximal and distal esophagus28.
It is important to note that clinical trials often use a different, sometimes more stringent, set of criteria to define remission. Histologic endpoints in clinical trials can vary, including definitions of endpoints with as low as < 5 eos/hpf in the esophagus despite the fact that there may be markedly improved eosinophil density. Percentage reduction in eosinophils in the esophagus has also been used as an endpoint in clinical trials. Recent studies also report an expanded list of endpoints including EoE HSS, endoscopic findings via EREFS, and esophageal distensibility. Again, the importance of discussing these endpoints lays in the difficulty of obtaining FDA approval for many drugs being studies for EoE, and how our monitoring for remission when caring for a patient on a medication may differ from that being used when defining efficacy of a particular drug.
Transnasal Endoscopy
A recent advance in endoscopic monitoring of EoE has been transnasal esophagoscopy or endoscopy29. This is an endoscopy that is done in an awake patient that has shown to generate biopsy samples that are not statistically different from biopsies obtained during standard endoscopy, therefore creating a way to monitor EoE activity without sedation and anesthesia. It has been reported recently that virtual reality video goggles have been used to aid children in undergoing this procedure30. Transnasal endoscopy will allow outpatient endoscopy, done in the office, decreasing time and financial cost both to the patient and the healthcare provider.
The monitoring tools described above generally evaluate for what we in practice call clinical remission. Clinical remission takes symptoms and endoscopic findings into account while also seriously considering esophageal eosinophil density with a goal of < 15 eos/hpf to be defined. Ideally, the histologic criteria is used only as a piece of the puzzle – the patient’s quality of life, eating behaviors, EREFS score, esophageal disensibility and expanded histologic criteria should all be considered when making decisions regarding whether further steps in treatment should be taken or not.
Imaging
While fluoroscopic imaging such as esophagram cannot be used to quantify eosinophils in the esophageal mucosa, it is an important tool when monitoring disease activity in EoE. As discussed previously, over time EoE can lead to formation of clinically significant strictures, which may be too subtle to be noted at time of endoscopy31. Esophagram may be able to point to disease activity by identifying rings or narrow caliber esophagus, and may also guide preparation for endoscopy with dilation32, as well as prevent complication such as perforation or mucosal tear which may occur when endoscopy is performed without prior knowledge of a stricture. While esophagram will not provide definitive information on disease activity, it does serve to better characterize the esophagus and any potential narrowing more accurately than endoscopy.
Monitoring of Disease Activity in EoE –Where We Are Going
The diagnosis and treatment strategies in EoE rely heavily on endoscopic biopsy. Being able to non-invasively sample the esophagus or sample surrogate tissue (ie, blood/urine) would decrease anesthesia exposure and risk. It would also allow for patients to wean their medications or try a new diet without needing to undergo a procedure with each small modification.
String Test
An innovative development in monitoring EoE disease activity that is currently being validated is the esophageal string test (EST)33. The EST is a nylon string that has a gelatin capsule attached to the distal end. The patient swallows the capsule while the proximal string is wrapped around the finger and is then taped to the cheek. A small metal ball keeps the string in place in the patient’s stomach. When the string is removed after an hour34, it can be analyzed to detect eosinophil derived proteins. Current data suggests that the EST is a sensitive tool for detecting esophageal eosinophilia. Further research is underway to further support this data and drive clinical development of this exciting tool which could decrease costs and patient risk by limiting need for endoscopy with or without anesthesia.
Cytosponge
The Cytosponge is another minimally invasive tool that has showed promising results in the monitoring of eosinophilic esophagitis35. The Cytosponge is enclosed in a capsule that dissolves within 5 minutes of entering the stomach. Once the capsule dissolves, a 3cm mesh sponge emerges and can be withdrawn by pulling the string that is attached to it. Specimens from the sponge are embedded in paraffin and undergo standard hematoxylin and eosin (H&E) staining as well as analyzed with trefoil factor-3. Accuracy of detection of esophageal eosinophilia has been promising36 with a sensitivity of 75% and a specificity of 86%, although side effects such as esophageal abrasions and detachment of the sponge have been reported.
Blood, urine, salivary, and breath testing
Monitoring disease activity in eosinophilic esophagitis via blood tests is an active area of current research endeavors. A recent systematic review reports 41 studies evaluating disease activity with blood tests with a large proportion occurring in the last 5 years37. The authors note that major weaknesses of these studies involve 1) including proper controls (reflux or atopic controls) 2) timing the blood draw close to endoscopy 3) and retrospective design. Many approaches have been undertaken to find a clue to disease activity in the blood including cytokine levels, cell surface markers and granule proteins, as well as absolute eosinophil count (AEC). In fact, AEC has been evaluated in 16 different studies, unfortunately with varying and limited success.
While blood testing has been the most common approach taken for non-invasive biomarker development, urine, saliva, exhaled nitric oxide and stool have been evaluated as well38–42. None of these non-invasive biomarkers are currently being used beyond research, and many studies are moving beyond these bodily fluids and trying to capture the esophageal epithelium or the milieu using alternative modalities such as the string and the cytosponge (see below). At this moment modalities of assessing for esophageal inflammation that do not use actual esophageal tissue are not proven to be helpful and thus far should not be used in clinical practice.
Personalized Medicine and Phenotypic Characterization in EoE
In addition to the tests listed above, both invasive and non-invasive, a new realm of diagnostic modalities are being discovered and explored that will allow us to comment on future disease course and particular therapeutic options based on personalized medicine, expanding our ability to determine activity or inactivity at a moment in time and look ahead to effectively guide the disease course for each individual patient.
As highlighted in a recent review by Atkins et al43, age of presentation, number of food triggers, progression to fibrostenosis, response to proton-pump inhibitors and many more factors vary greatly from patient to patient. Understanding the molecular and functional underpinnings of these phenotypes could lead to better characterization of disease and personalization of therapy. The promise of the tests described above is one of precision medicine and the ability to predict disease course and optimal treatment for individual patients.
Functional assessment of the esophagus
A major weakness of esophageal biopsy specimens is that it only captures a small portion of the esophageal epithelium and contains very little submucosa (ie, lamina propria and muscularis). In fact only about 50% of esophageal biopsies contain evaluable lamina propria and the tissue that is obtained is often crushed by biopsy forceps, making the yield even less44. Thus, there is a critical need to detect subtle degrees of remodeling that may be occurring but are not evaluable with current diagnostic modalities, including endoscopy31.
The Endoluminal Functional Imaging System (FLIP) is a technology developed to measure the pressure-geometry relationship of hollow organs through the digestive track (Fig 3). This tool is now being utilized to evaluate esophageal diameter and pressure in the context of EoE44–47. The first studies were performed in adults and these showed that in EoE there is reduced esophageal distensibility in the esophagus and that decreased esophageal distensibility could predict future dilation or food impaction. In adults there was no difference between patients with active EoE compared to inactive EoE, but distensibility did improve with therapy48.
Figure 3:
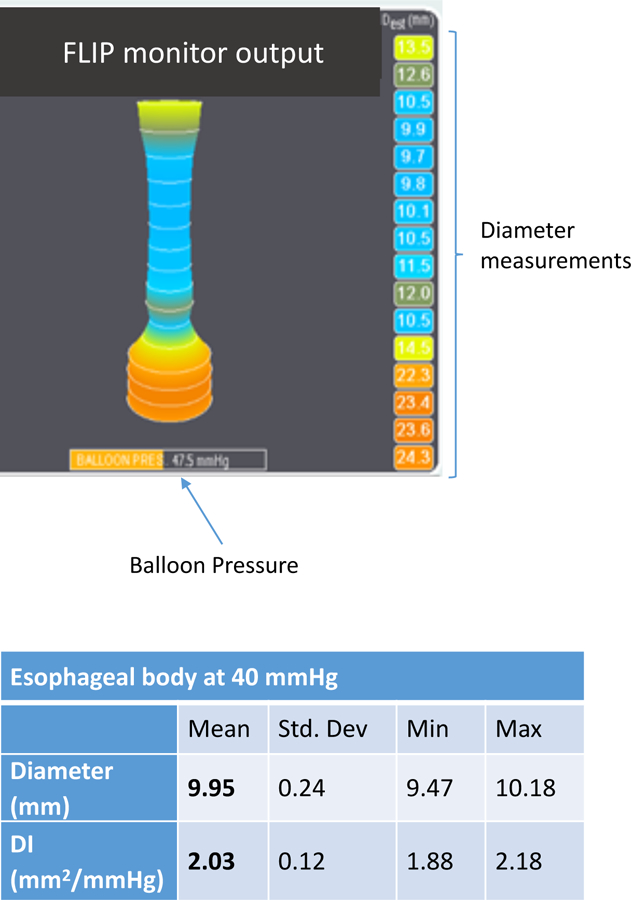
After the catheter is inserted and the balloon is inflated, a real time image of the esophagus appears on the FLIP monitor. The diameter is shown on the left and the balloon pressure at the bottom of the screen. Analysis is performed to determine the distensibility of the esophagus by taking the minimum diameter along the esophageal body at a pressure of 40mmHg accounting for peristalsis and respiration. This case represents a patient with EoE and lower esophageal narrowing not appreciated on endoscopy with a diameter of 9.95mm at a pressure of 40mmHg.
Pediatric studies have similarly found that there is decreased esophageal distensibility and compliance in the EoE esophagus compared to age/size matched control patients. However, in contrast to the studies performed in adults, Menard-Katcher et al showed that there are differences in distensibility between the active and inactive EoE population44. In fact, eosinophil count in the esophagus was negatively correlated with esophageal distensibility in the pediatric population. These findings suggest that in children, when there have been fewer years of active inflammation, there are reversible inflammatory changes rather than irreversible fibrogenesis and highlight the need for improved natural history studies to evaluate disease progression from childhood into adulthood.
Evaluation of the epithelial integrity
Mucosal impedance (MI) is a recently developed catheter designed to go through the endoscope to measure esophageal epithelial conductivity 49. This tool has been validated to detect changes in mucosal integrity that can help distinguish patients with GERD, EoE, or neither 50. Mucosal impedance measurements have been shown to correlate inversely with eosinophil counts and dilated intercellular spaces (DIS) in a pattern that differentiates EoE from GERD 51. This tool has been validated in pediatrics, with lower resistance in patients with active EoE compared to inactive EoE, NERD, or controls 52.
More recent analysis of mucosal impedance in EoE shows that the sub-upper esophageal sphincter (Sub-UES) region of the esophagus seems spared from these changes in mucosal integrity in the setting of EoE. In addition to normal MI values in the Sub-UES, Choski et al showed that this region was also spared in large part from histologic findings (intraepithelial eosinophils, dilated intracellular spaces and basal cell hyperplasia) found in the rest of the active EoE esophagus53. While this procedure is still used for research purposes only, understanding the variation in mucosal integrity along the esophageal body provides novel insight into the pathophysiology of EoE and may lead to more in depth characterization of patient phenotype.
Transcriptome Analysis
While EoE is generally considered a disease of atopic individuals who present with dysphagia, transcriptome analysis has revealed heterogeneity across the EoE population. The Eosinophilic Esophagitis Diagnostic Panel (EDP) utilizes quantitative PCR of the 95 most dysregulated genes in EoE54. This panel has been shown to accurately distinguish EoE biopsies from non-EoE biopsies. Additionally, this panel was used to correlate clinical, endoscopic, and histologic findings with transcriptome data revealing 3 distinct endotypes of EoE55. The first endotype, termed EoEe1, was found have a mild phenotype with decreased endoscopic and histologic findings. EoEe2 tended to patients who were refractory to treatment with topical steroids whereas EoEe3 type patients were more likely to have adult onset disease and narrow caliber esophagus. One downfall of using transcriptomes to define a patient’s individual disease is that there is evidence that the transcriptomes can change over time even within the same patient depending on disease activity.
Differentially dysregulated transcripts suggest that the pathophysiology of EoE may be heterogeneous as well. With these data, there is potential to provide prognostic information to patients (ie, possibility of dilation) and the potential to predict response to therapy.
Genetics
The first single nucleotide polymorphism associated with EoE was thymic stromal lymphopoietin (TSLP) gene, at 5q22. This gene polymorphism was identified by genome wide array studies (GWAS) by Rothenberg et al56. TSLP acts to trigger the inflammatory cascade in atopic conditions, inducing T-helper (Th)-2 type inflammation57. Studies have shown a gain of function effect in cases with the risk allele. Patients with one or two copies of the risk allele were more likely to have 3 or more EoE related food allergies58. Similarly, in vitro analysis showed that primary esophageal cultures from patients homozygous for the risk allele produced significantly more TSLP than those that were heterozygous.
TGFβ is the major effector cytokine in fibrosis59, 60. It activates fibroblasts to produce collagen and contract. It has been shown that patients with TT genotype at the C-509 SNP of the TGFβ promoter had more TGFβ content and epithelial remodeling than those with CC at this locus. These effects were enhanced in patients with food sensitization.
There are other SNPS being investigated with regards to disease phenotype and characterization of disease; however, these examples represent direct relationships between genotype and patient phenotype. In the future, as we gain a better understanding of these polymorphisms in EoE, we may gain insight into prognosis and personalized therapies.
Conclusion
The diagnosis and monitoring of EoE continues to be an evolving process with a variety of emerging technologies being evaluated and investigated. Current monitoring practices being commonly used include patient reported outcomes, fluoroscopy, and endoscopy with histologic evaluation. A small number of sites around the country are routinely using new technology for monitoring of EoE, including FLIP and transnasal endoscopy. An incredible array of non-invasive biomarkers are under investigation for future use in monitoring of EoE, ranging from the string test and cytosponge to serum monitoring. The standard for monitoring of EoE will continue to change and develop in the upcoming years, allowing for techniques that undoubtedly have positive consequences for both patients and providers, allowing insight both into the patient’s current state of disease, as well as what their individual future may hold. The future of EoE is exciting from both a diagnostic and therapeutic standpoint with the existence of a clinical potential to tell patients the future course of their particular version of the disease along with the best treatment. As the world of personalized medicine continues to unfold, and unprecedented advancements continue to emerge, EoE is one of the fields in which these incredible discoveries will play a crucial role.
Key Messages.
The burden of endoscopy in EoE is great, with many patients undergoing multiple endoscopies in order to verify treatment success
The gold standard for monitoring disease activity EoE is histologic evaluation and eosinophil enumeration
While symptoms have been less reliable to track disease activity, there are multiple non-invasive methodologies being investigated to sample the esophageal tissue
A better understanding of disease phenotype, genotype and transcriptome may lead to more personalized approaches to diagnostics and therapeutics in EoE
Acknowledgments
Grant Support
This study was supported by the following NIH Grants: K08DK106444, R21 TR003039 (ABM), CEGIR (U54 AI117804) (ABM) is part of the Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), NCATS, and is funded through collaboration between NIAID, NIDDK, and NCATS. CEGIR is also supported by patient advocacy groups including APFED, CURED, and EFC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration: Not Applicable
Conflicts of interest: None
References
- 1.Dellon ES, Liacouras CA, Molina-Infante J, et al. Updated International Consensus Diagnostic Criteria for Eosinophilic Esophagitis: Proceedings of the AGREE Conference. Gastroenterology 2018;155:1022–1033 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reed CC, Wolf WA, Cotton CC, et al. Optimal Histologic Cutpoints for Treatment Response in Patients With Eosinophilic Esophagitis: Analysis of Data From a Prospective Cohort Study. Clin Gastroenterol Hepatol 2018;16:226–233 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonsalves N Clinical Presentation and Approach to Dietary Management of Eosinophilic Esophagitis. Gastroenterol Hepatol (N Y) 2018;14:706–712. [PMC free article] [PubMed] [Google Scholar]
- 4.Schoepfer AM, Safroneeva E, Bussmann C, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology 2013;145:1230–6 e1–2. [DOI] [PubMed] [Google Scholar]
- 5.Dellon ES, Kim HP, Sperry SL, et al. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc 2014;79:577–85 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Runge TM, Eluri S, Woosley JT, et al. Control of inflammation decreases the need for subsequent esophageal dilation in patients with eosinophilic esophagitis. Dis Esophagus 2017;30:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whelan KA, Godwin BC, Wilkins B, et al. Persistent Basal Cell Hyperplasia is Associated with Clinical and Endoscopic Findings in Patients With Histologically Inactive Eosinophilic Esophagitis. Clin Gastroenterol Hepatol 2019. [DOI] [PMC free article] [PubMed]
- 8.Chehade M, Nowak-Wegrzyn A. The asymptomatic patient with eosinophilic esophagitis: To treat or not to treat? Ann Allergy Asthma Immunol 2019;122:550–551. [DOI] [PubMed] [Google Scholar]
- 9.Muir A, Moore H, Spergel JM. Minimally symptomatic patients with eosinophilic esophagitis should still be actively treated-PRO. Ann Allergy Asthma Immunol 2019;122:572–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lieberman JA. Minimally symptomatic patients with eosinophilic esophagitis should still be actively treated-CON. Ann Allergy Asthma Immunol 2019;122:574–575. [DOI] [PubMed] [Google Scholar]
- 11.Molina-Infante J, Arias A, Alcedo J, et al. Step-up empiric elimination diet for pediatric and adult eosinophilic esophagitis: The 2–4-6 study. J Allergy Clin Immunol 2018;141:1365–1372. [DOI] [PubMed] [Google Scholar]
- 12.Dellon ES. Cost-effective care in eosinophilic esophagitis. Ann Allergy Asthma Immunol 2019;123:166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Najafi N, Veyckemans F, Vanhonacker D, et al. Incidence and risk factors for adverse events during monitored anaesthesia care for gastrointestinal endoscopy in children: A prospective observational study. Eur J Anaesthesiol 2019;36:390–399. [DOI] [PubMed] [Google Scholar]
- 14.Derderian CA, Szmuk P, Derderian CK. Behind the Black Box: The Evidence for the U.S. Food and Drug Administration Warning about the Risk of General Anesthesia in Children Younger than 3 Years. Plast Reconstr Surg 2017;140:787–792. [DOI] [PubMed] [Google Scholar]
- 15.Brambrink AM, Evers AS, Avidan MS, et al. Ketamine-induced neuroapoptosis in the fetal and neonatal rhesus macaque brain. Anesthesiology 2012;116:372–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Creeley C, Dikranian K, Dissen G, et al. Propofol-induced apoptosis of neurones and oligodendrocytes in fetal and neonatal rhesus macaque brain. Br J Anaesth 2013;110 Suppl 1:i29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Safroneeva E, Straumann A, Coslovsky M, et al. Symptoms Have Modest Accuracy in Detecting Endoscopic and Histologic Remission in Adults With Eosinophilic Esophagitis. Gastroenterology 2016;150:581–590 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franciosi JP, Hommel KA, DeBrosse CW, et al. Development of a validated patient-reported symptom metric for pediatric eosinophilic esophagitis: qualitative methods. BMC Gastroenterol 2011;11:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dellon ES, Irani AM, Hill MR, et al. Development and field testing of a novel patient-reported outcome measure of dysphagia in patients with eosinophilic esophagitis. Aliment Pharmacol Ther 2013;38:634–42. [DOI] [PubMed] [Google Scholar]
- 20.Schoepfer AM, Straumann A, Panczak R, et al. Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis. Gastroenterology 2014;147:1255–66 e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Safroneeva E, Schoepfer AM. Symptom-based patient-reported outcomes in adults with eosinophilic esophagitis: value for treatment monitoring and randomized controlled trial design. Curr Opin Allergy Clin Immunol 2019;19:169–174. [DOI] [PubMed] [Google Scholar]
- 22.Alexander JA, Jung KW, Arora AS, et al. Swallowed fluticasone improves histologic but not symptomatic response of adults with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2012;10:742–749 e1. [DOI] [PubMed] [Google Scholar]
- 23.Hirano I, Moy N, Heckman MG, et al. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut 2013;62:489–95. [DOI] [PubMed] [Google Scholar]
- 24.Dellon ES, Cotton CC, Gebhart JH, et al. Accuracy of the Eosinophilic Esophagitis Endoscopic Reference Score in Diagnosis and Determining Response to Treatment. Clin Gastroenterol Hepatol 2016;14:31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins MH, Martin LJ, Alexander ES, et al. Newly developed and validated eosinophilic esophagitis histology scoring system and evidence that it outperforms peak eosinophil count for disease diagnosis and monitoring. Dis Esophagus 2017;30:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warners MJ, Ambarus CA, Bredenoord AJ, et al. Reliability of histologic assessment in patients with eosinophilic oesophagitis. Aliment Pharmacol Ther 2018;47:940–950. [DOI] [PubMed] [Google Scholar]
- 27.Ravelli A, Villanacci V, Cadei M, et al. Dilated intercellular spaces in eosinophilic esophagitis. J Pediatr Gastroenterol Nutr 2014;59:589–93. [DOI] [PubMed] [Google Scholar]
- 28.Shah A, Kagalwalla AF, Gonsalves N, et al. Histopathologic variability in children with eosinophilic esophagitis. Am J Gastroenterol 2009;104:716–21. [DOI] [PubMed] [Google Scholar]
- 29.Friedlander JA, DeBoer EM, Soden JS, et al. Unsedated transnasal esophagoscopy for monitoring therapy in pediatric eosinophilic esophagitis. Gastrointest Endosc 2016;83:299–306 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen N, Lavery WJ, Capocelli KE, et al. Transnasal Endoscopy in Unsedated Children With Eosinophilic Esophagitis Using Virtual Reality Video Goggles. Clin Gastroenterol Hepatol 2019. [DOI] [PMC free article] [PubMed]
- 31.Menard-Katcher C, Swerdlow MP, Mehta P, et al. Contribution of Esophagram to the Evaluation of Complicated Pediatric Eosinophilic Esophagitis. J Pediatr Gastroenterol Nutr 2015;61:541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alexander JA. Endoscopic and Radiologic Findings in Eosinophilic Esophagitis. Gastrointest Endosc Clin N Am 2018;28:47–57. [DOI] [PubMed] [Google Scholar]
- 33.Furuta GT, Kagalwalla AF, Lee JJ, et al. The oesophageal string test: a novel, minimally invasive method measures mucosal inflammation in eosinophilic oesophagitis. Gut 2013;62:1395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ackerman SJ, Kagalwalla AF, Hirano I, et al. One-Hour Esophageal String Test: A Nonendoscopic Minimally Invasive Test That Accurately Detects Disease Activity in Eosinophilic Esophagitis. Am J Gastroenterol 2019;114:1614–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Januszewicz W, Tan WK, Lehovsky K, et al. Safety and Acceptability of Esophageal Cytosponge Cell Collection Device in a Pooled Analysis of Data From Individual Patients. Clin Gastroenterol Hepatol 2019;17:647–656 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katzka DA, Smyrk TC, Alexander JA, et al. Accuracy and Safety of the Cytosponge for Assessing Histologic Activity in Eosinophilic Esophagitis: A Two-Center Study. Am J Gastroenterol 2017;112:1538–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hines BT, Rank MA, Wright BL, et al. Minimally invasive biomarker studies in eosinophilic esophagitis: A systematic review. Ann Allergy Asthma Immunol 2018;121:218–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hiremath G, Shilts MH, Boone HH, et al. The Salivary Microbiome Is Altered in Children With Eosinophilic Esophagitis and Correlates With Disease Activity. Clin Transl Gastroenterol 2019;10:e00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benitez AJ, Hoffmann C, Muir AB, et al. Inflammation-associated microbiota in pediatric eosinophilic esophagitis. Microbiome 2015;3:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Konikoff MR, Blanchard C, Kirby C, et al. Potential of blood eosinophils, eosinophil-derived neurotoxin, and eotaxin-3 as biomarkers of eosinophilic esophagitis. Clin Gastroenterol Hepatol 2006;4:1328–36. [DOI] [PubMed] [Google Scholar]
- 41.Cunnion KM, Willis LK, Minto HB, et al. Eosinophil Quantitated Urine Kinetic: A novel assay for assessment of eosinophilic esophagitis. Ann Allergy Asthma Immunol 2016;116:435–9. [DOI] [PubMed] [Google Scholar]
- 42.Johnson K, Iyer V, Katzka D, et al. Poor Relationship Between Fractionated Exhaled Nitric Oxide and Disease Activity in Eosinophilic Esophagitis. Dysphagia 2019;34:138–144. [DOI] [PubMed] [Google Scholar]
- 43.Atkins D, Furuta GT, Liacouras CA, et al. Eosinophilic esophagitis phenotypes: Ready for prime time? Pediatr Allergy Immunol 2017;28:312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menard-Katcher C, Benitez AJ, Pan Z, et al. Influence of Age and Eosinophilic Esophagitis on Esophageal Distensibility in a Pediatric Cohort. Am J Gastroenterol 2017;112:1466–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carlson DA, Lin Z, Hirano I, et al. Evaluation of esophageal distensibility in eosinophilic esophagitis: an update and comparison of functional lumen imaging probe analytic methods. Neurogastroenterol Motil 2016;28:1844–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nicodeme F, Hirano I, Chen J, et al. Esophageal distensibility as a measure of disease severity in patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2013;11:1101–1107 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hassan M, Aceves S, Dohil R, et al. Esophageal Compliance Quantifies Epithelial Remodeling in Pediatric Patients With Eosinophilic Esophagitis. J Pediatr Gastroenterol Nutr 2019;68:559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carlson DA, Hirano I, Zalewski A, et al. Improvement in Esophageal Distensibility in Response to Medical and Diet Therapy in Eosinophilic Esophagitis. Clin Transl Gastroenterol 2017;8:e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lei WY, Vaezi MF, Naik RD, et al. Mucosal impedance testing: A new diagnostic testing in gastroesophageal reflux disease. J Formos Med Assoc 2019. [DOI] [PubMed]
- 50.Patel DA, Higginbotham T, Slaughter JC, et al. Development and Validation of a Mucosal Impedance Contour Analysis System to Distinguish Esophageal Disorders. Gastroenterology 2019;156:1617–1626 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katzka DA, Ravi K, Geno DM, et al. Endoscopic Mucosal Impedance Measurements Correlate With Eosinophilia and Dilation of Intercellular Spaces in Patients With Eosinophilic Esophagitis. Clin Gastroenterol Hepatol 2015;13:1242–1248 e1. [DOI] [PubMed] [Google Scholar]
- 52.Lowry MA, Vaezi MF, Correa H, et al. Mucosal Impedance Measurements Differentiate Pediatric Patients With Active Versus Inactive Eosinophilic Esophagitis. J Pediatr Gastroenterol Nutr 2018;67:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choksi Y, Chaparro J, Blanco M, et al. Impedance and Histologic Characteristics of the Sub-laryngeal Esophagus Distinguish Eosinophilic Esophagitis From Other Esophageal Disorders. Clin Gastroenterol Hepatol 2019. [DOI] [PMC free article] [PubMed]
- 54.Wen T, Stucke EM, Grotjan TM, et al. Molecular diagnosis of eosinophilic esophagitis by gene expression profiling. Gastroenterology 2013;145:1289–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shoda T, Wen T, Aceves SS, et al. Eosinophilic oesophagitis endotype classification by molecular, clinical, and histopathological analyses: a cross-sectional study. Lancet Gastroenterol Hepatol 2018;3:477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sherrill JD, Gao PS, Stucke EM, et al. Variants of thymic stromal lymphopoietin and its receptor associate with eosinophilic esophagitis. J Allergy Clin Immunol 2010;126:160–5 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muir AB, Wang JX, Nakagawa H. Epithelial-stromal crosstalk and fibrosis in eosinophilic esophagitis. J Gastroenterol 2019;54:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fahey LM, Chandramouleeswaran PM, Guan S, et al. Food allergen triggers are increased in children with the TSLP risk allele and eosinophilic esophagitis. Clin Transl Gastroenterol 2018;9:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cho JY, Doshi A, Rosenthal P, et al. Smad3-deficient mice have reduced esophageal fibrosis and angiogenesis in a model of egg-induced eosinophilic esophagitis. J Pediatr Gastroenterol Nutr 2014;59:10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muir AB, Dods K, Henry SJ, et al. Eosinophilic Esophagitis-Associated Chemical and Mechanical Microenvironment Shapes Esophageal Fibroblast Behavior. J Pediatr Gastroenterol Nutr 2016;63:200–9. [DOI] [PMC free article] [PubMed] [Google Scholar]