Abstract
Background
Shock wave therapy has seen widespread use since the 1990s to treat various musculoskeletal disorders including rotator cuff disease, but evidence of its efficacy remains equivocal.
Objectives
To determine the benefits and harms of shock wave therapy for rotator cuff disease, with or without calcification, and to establish its usefulness in the context of other available treatment options.
Search methods
We searched Ovid MEDLINE, Ovid Embase, CENTRAL, ClinicalTrials.gov and the WHO ICTRP up to November 2019, with no restrictions on language. We reviewed the reference lists of retrieved trials to identify potentially relevant trials.
Selection criteria
We included randomised controlled trials (RCTs) and controlled clinical trials (CCTs) that used quasi‐randomised methods to allocate participants, investigating participants with rotator cuff disease with or without calcific deposits. We included trials of comparisons of extracorporeal or radial shock wave therapy versus any other intervention. Major outcomes were pain relief greater than 30%, mean pain score, function, patient‐reported global assessment of treatment success, quality of life, number of participants experiencing adverse events and number of withdrawals due to adverse events.
Data collection and analysis
Two review authors independently selected studies for inclusion, extracted data and assessed the certainty of evidence using GRADE. The primary comparison was shock wave therapy compared to placebo.
Main results
Thirty‐two trials (2281 participants) met our inclusion criteria. Most trials (25) included participants with rotator cuff disease and calcific deposits, five trials included participants with rotator cuff disease and no calcific deposits, and two trials included a mixed population of participants with and without calcific deposits.
Twelve trials compared shock wave therapy to placebo, 11 trials compared high‐dose shock wave therapy (0.2 mJ/mm² to 0.4 mJ/mm² and above) to low‐dose shock wave therapy. Single trials compared shock wave therapy to ultrasound‐guided glucocorticoid needling, ultrasound‐guided hyaluronic acid injection, transcutaneous electric nerve stimulation (TENS), no treatment or exercise; dual session shock wave therapy to single session therapy; and different delivery methods of shock wave therapy. Our main comparison was shock wave therapy versus placebo and results are reported for the 3 month follow up.
All trials were susceptible to bias; including selection (74%), performance (62%), detection (62%), and selective reporting (45%) biases.
No trial measured participant‐reported pain relief of 30%. However, in one trial (74 participants), at 3 months follow up, 14/34 participants reported pain relief of 50% or greater with shock wave therapy compared with 15/40 with placebo (risk ratio (RR) 1.10, 95% confidence interval (CI) 0.62 to 1.94); low‐quality evidence (downgraded for bias and imprecision). Mean pain (0 to 10 scale, higher scores indicate more pain) was 3.02 points in the placebo group and 0.78 points better (0.17 better to 1.4 better; clinically important change was 1.5 points) with shock wave therapy (9 trials, 608 participants), moderate‐quality evidence (downgraded for bias). Mean function (scale 0 to 100, higher scores indicate better function) was 66 points with placebo and 7.9 points better (1.6 better to 14 better, clinically important difference 10 points) with shock wave therapy (9 trials, 612 participants), moderate‐quality evidence (downgraded for bias). Participant‐reported success was reported by 58/150 people in shock wave therapy group compared with 35/137 people in placebo group (RR 1.59, 95% CI 0.87 to 2.91; 6 trials, 287 participants), low‐quality evidence (downgraded for bias and imprecision). None of the trials measured quality of life.
Withdrawal rate or adverse event rates may not differ between extracorporeal shock wave therapy and placebo, but we are uncertain due to the small number of events. There were 11/34 withdrawals in the extracorporeal shock wave therapy group compared with 13/40 withdrawals in the placebo group (RR 0.75, 95% CI 0.43 to 1.31; 7 trials, 581 participants) low‐quality evidence (downgraded for bias and imprecision); and 41/156 adverse events with extracorporeal shock wave therapy compared with 10/139 adverse events in the placebo group (RR 3.61, 95% CI 2.00 to 6.52; 5 trials, 295 participants) low‐quality evidence (downgraded for bias and imprecision).
Subgroup analyses indicated that there were no between‐group differences in pain and function outcomes in participants who did or did not have calcific deposits in the rotator cuff.
Authors' conclusions
Based upon the currently available low‐ to moderate‐certainty evidence, there were very few clinically important benefits of shock wave therapy, and uncertainty regarding its safety. Wide clinical diversity and varying treatment protocols means that we do not know whether or not some trials tested subtherapeutic doses, possibly underestimating any potential benefits.
Further trials of extracorporeal shock wave therapy for rotator cuff disease should be based upon a strong rationale and consideration of whether or not they would alter the conclusions of this review. A standard dose and treatment protocol should be decided upon before further research is conducted. Development of a core set of outcomes for trials of rotator cuff disease and other shoulder disorders would also facilitate our ability to synthesise the evidence.
Keywords: Humans, Middle Aged, Rotator Cuff, Calcinosis, Calcinosis/therapy, Exercise Therapy, Extracorporeal Shockwave Therapy, Extracorporeal Shockwave Therapy/adverse effects, Extracorporeal Shockwave Therapy/methods, Glucocorticoids, Glucocorticoids/administration & dosage, Hyaluronic Acid, Hyaluronic Acid/administration & dosage, Muscular Diseases, Muscular Diseases/therapy, Patient Dropouts, Patient Dropouts/statistics & numerical data, Randomized Controlled Trials as Topic, Shoulder Pain, Shoulder Pain/therapy, Transcutaneous Electric Nerve Stimulation, Viscosupplements, Viscosupplements/administration & dosage
Plain language summary
Shock wave therapy for rotator cuff disease with or without calcification
Background
Rotator cuff disease is the most common cause of shoulder pain, especially at night and when lifting the arm above the head. Calcium deposits may form on the tendons in the shoulder joint.
Shock wave therapy passes sound or shock waves through the skin to the affected area, and may break up calcium deposits. There is currently no standard dose or treatment regimen.
Review question
In people with rotator cuff disease with or without calcific deposits, what are the benefits and harms of shock wave therapy compared to placebo (pretend) or other available treatments?
Study characteristics
We included 32 trials (2281 participants), published up to November 2019.
Twelve trials compared shock wave therapy to placebo. Eleven trials compared high‐ and low‐dose shock wave therapy, although dosages varied across trials. Single trials compared shock wave therapy to other treatments including ultrasound‐guided glucocorticoid needling, transcutaneous electric nerve stimulation (TENS), exercise, or no treatment; or different regimens of shock wave therapy.
Overall, 61% of participants were women, the average age was 52 years, and the average duration of the condition was 33 months. Two trials were funded by manufacturers of shock wave machines.
Key results for the primary comparison, shock wave therapy versus placebo
Participant‐reported pain relief of 50% or greater (one trial):
• four more people out of 100 reported pain relief of 50% or more (ranging from 19 fewer to 26 more).
42 out of 100 people reported pain relief of 50% or greater with shock wave therapy compared with 38 out of 100 with placebo.
Pain (higher scores mean more pain) (nine trials):
• Improved pain by 8% (ranging from 2% better to 14% better) or 0.78 points better (ranging from 0.17 better to 1.4 better) on a 0‐ to 10‐point scale.
People who had shock wave therapy rated their pain as 2.2 points and people who had placebo rated their pain as 3 points.
Function (ability to use the shoulder; higher scores meanbetter function) (nine trials):
• Improved by 8% (ranging from 1.6% to 14%) or 8 points better (ranging from 1.6 better to 14 better) on a 0‐ to 100‐point scale.
People who had shock wave therapy rated their function as 74 points and people who had placebo rated their function as 66 points.
Participant‐reported success (six trials):
• 15% (ranging from 3% fewer to 49% more) more people reported their treatment a success.
41 out of 100 people reported treatment success with shock wave therapy and 26 out of 100 people reported treatment success with placebo.
Withdrawals due to side effects (seven trials):
• 3% fewer (ranging from 6% fewer to 3% more) people withdrew from treatment due to side effects.
8 out of 100 people withdrew from treatment with shock wave therapy and 10 out of 100 people withdrew from the placebo group.
Side effects (five trials):
• 19% more people reported side effects (ranging from 7% more to 40% more):
26 out of 100 people had a side effect with shock wave therapy and seven out of 100 people had a side effect with placebo.
Certainty of the evidence
In people with rotator cuff disease, moderate‐certainty evidence (downgraded due to bias) shows that shock wave therapy probably does not improve pain and function compared with placebo, and low‐certainty evidence (downgraded due to bias and lack of accuracy) shows there may be no improvement in those with a pain reduction of 50% or more and participant‐reported success. We are uncertain if withdrawals or side effects differed between groups due to small number of events. It did not appear to matter if participants had calcific deposits or not. We are uncertain if higher doses of shock wave therapy have benefits with more side effects compared with lower doses, as there was only low‐ or very low‐certainty evidence available, and we cannot recommend a particular treatment dose.
Side effects included treatment‐related pain, bruising and bleeding although these were generally minor and short‐lived. Rare and serious side effects, including loss of blood supply and bone death, while possible, were not reported.
Summary of findings
Summary of findings for the main comparison. Shock wave therapy versus placebo for rotator cuff disease with or without calcification.
| Shock wave therapy for rotator cuff disease with or without calcification at 3 months | ||||||
| Patient or population: rotator cuff disease with or without calcification Setting: outpatient clinic Intervention: shock wave therapy Comparison: placebo therapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with shock wave therapy | |||||
|
Pain relief > 50%a Follow‐up: 3 months |
375 per 1000 | 413 per 1000 (232 to 728) |
RR 1.10 (0.62 to 1.94) | 74 (1 study) |
⊕⊕⊝⊝ Lowb,c |
Shockwave therapy may provide no improvement in the number of participants with a pain reduction of 50% or more. Absolute change 4% more had relief (19% fewer to 26% more); relative change 10% more had relief (38% fewer to 94% more); NNTB: NAd |
|
Pain Multiple scalese translated to VAS 0–10 (10 was severe pain)f Follow‐up: 3 months |
Mean pain in the control group was 3.02 pointsg | Mean pain in the intervention group was 0.78 points better (0.17 better to 1.4 better) |
SMD –0.49 (95% CI –0.88 to –0.11) | 608 (9 studies) | ⊕⊕⊕⊝ Moderateh |
Shockwave therapy probably results in little or no clinically important improvement in pain. Mean pain did not appear to differ in participants with and without calcification: test for subgroup differences: Chi² = 0.25, df = 1 (P = 0.62), I² = 0% Absolute change 8% better (2% to 14% better); relative change 14% better (3% better to 25% better);i NNTB: 4 (95% CI 2 to 34)d |
|
Function Multiple scalese translated to Constant 0–100 scale (100 was best function)f Follow‐up: 3 months |
Mean function in the control group was 66 pointsg | Mean function in the intervention group was 7.9 points better (1.6 better to 14 better) | SMD 0.62 (95% CI 0.13 to 1.11) | 612 (9 studies) | ⊕⊕⊕⊝ Moderatej |
Shockwave therapy probably results in little or no clinically important improvement in function. Mean function did not appear to differ in participants with and without calcification: test for subgroup differences: Chi² = 1.00, df = 1 (P = 0.32), I² = 0.1% Absolute change: 8% better (1.6% to 14% better); relative change 12% better (3% to 22% better);i NNTB: 3 (95% CI 2 to 18)d |
|
Participant‐reported success Follow‐up: end of studies |
255 per 1000 | 406 per 1000 (222 to 743) | RR 1.59 (0.87 to 2.91) | 287 (6 studies) |
⊕⊕⊝⊝ Lowb,c |
Shockwave therapy may provide no improvement in the number of participants reporting treatment success. Absolute change 15% more had success (3% fewer to 49% more); relative change 59% more (13% fewer to 191% more); NNTB: NAd |
| Quality of life | — | — | — | — | — | Not measured |
| Number of participant withdrawals due to adverse events or treatment intolerance | 103 per 1000 | 77 per 1000 (44 to 135) | RR 0.75 (0.43 to 1.31) | 581 (7 studies) | ⊕⊕⊝⊝ Lowb,c |
We are uncertain if shockwave therapy increases withdrawal rates. Absolute change 3% less events (6% less to 3% more); relative change 25% less (57% less to 31% more); NNTH: NAd |
|
Number of participants experiencing any adverse event Follow‐up: 12 months |
72 per 1000 | 260 per 1000 (144 to 469) | RR 3.61 (2.00 to 6.52) | 295 (5 studies) | ⊕⊕⊝⊝ Lowb,c |
We are uncertain if shockwave therapy increases adverse events. Absolute difference: 19% more events (7% more to 40% more); relative change: 261% more (100% more to 552% more); NNTH: NAd |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NA: not applicable; NNTB: number needed to treat for an additional beneficial outcome; RR: risk ratio; SMD: standardised mean difference; VAS: Visual Analogue Scale. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aThe a priori outcome was pain relief 30% or greater, which was not reported in any studies; thus we reported pain relief 50% or greater. b Downgraded one level due to study limitations (including risk of selection, detection, attrition, and reporting bias).
cDowngraded one level for imprecision due to wide confidence intervals, or small number of participants or small number of events.
dNumber needed to treat for an additional beneficial outcome (NNTB), or an additional harmful outcome (NNTH) not applicable (n/a) when result is not statistically significant. NNTB or NNTH for dichotomous outcomes calculated using Cates NNT calculator (www.nntonline.net/visualrx/). NNTB or NNTH for continuous outcomes calculated using Wells Calculator (CMSG editorial office), with an assumed minimal clinical important difference for pain of 1.5 points on 0 to 10 VAS, and for function of 10 points on 0 to 100 Constant score. ePain scores: VAS 0 to 10, VAS 0 to 100, Constant Score 0 to 15 (also called Constant score); function scores: Constant‐Murley 0 to 100, Shoulder Pain And Disability Index 0 to 100. fTranslated from SMD and 95% CIs to 0 to 10 VAS for pain and 0 to 100 Constant scale for function by multiplying the SMD by the standard deviation (SD) at baseline in the placebo group from Gerdesmeyer 2003 (values were mean (SD) VAS pain 5.6 (1.6), and mean Constant score (SD) 64.2 (12.8). gControl group mean (SD) values at 3 months' follow‐up from Gerdesmeyer 2003: values were 3.8 (2.3) on 0 to 10 VAS pain; 74 (15.5) on 0 to 100 Constant function score. h Downgraded one level due to study limitations (including risk of selection, detection, and attrition bias). Although this outcome had a high I2 (80%), the outcome was not downgraded for inconsistency. This high I2 was due to one outlier,Hsu 2008 and removing this outlier removes the statistical heterogeneity (I2 = 0%) and does not change the direction of the effect
iRelative changes calculated as absolute change (mean difference) divided by mean at baseline in the control group from Gerdesmeyer 2003 (values were 5.6 on 0 to 10 point VAS pain; 64.2 on 0 to 100 Constant score).
jDowngraded one level due to study limitations (including risk of selection, detection, and attrition bias), and one level due to inconsistency (I² = 91%). Removing the potential extreme outlier reported in Hsu 2008 still left considerable heterogeneity (I² = 72%), additional removal of another, less extreme outlier (Cosentino 2003) resulted in I² = 38%. As we could explain the heterogeneity, we did not downgrade the certainty further.
Background
Description of the condition
Shoulder disorders are common, with a reported prevalence ranging from 7% to 26% in adults (Luime 2004). Shoulder problems account for 1.3% of all general practice encounters in Australia (Britt 2016), and up to 14% of all referrals to physiotherapists in the UK (May 2003). Shoulder pain persists or recurs in 40% of people within one year after their first visit to a primary care physician (van der Windt 1996), and has a substantial impact upon quality of life (MacDermid 2004; Taylor 2005).
Rotator cuff disease is the most common cause of shoulder pain seen by physicians (Ostor 2005), and is estimated to occur in up to 50% of people aged 75 years or over (Urwin 1998). The incidence is expected to rise with the ageing of the population (Gomoll 2004). A wide range of pathophysiological conditions are included under the umbrella term of 'rotator cuff disease', including rotator cuff tendonitis or tendinopathy, supraspinatus, infraspinatus or subscapularis tendonitis, subacromial bursitis, and partial and complete rotator cuff tears. There is no uniformity in how these conditions are labelled and defined (Green 1998; Lewis 2009). Among published controlled trials for rotator cuff disease, the definition most commonly used is based on clinical features and includes the presence of positive impingement signs including a painful arc and pain with resisted movements or normal passive range of movement (ROM) (Green 1998).
The pathophysiology of rotator cuff disease has traditionally been viewed as a continuum that ranges from impingement syndrome to partial‐ and full‐thickness rotator cuff tears (Neer 1983). While it is commonly believed that intrinsic degeneration of the rotator cuff tendons together with repetitive microtrauma contribute to its development (Ogata 1990), it is probably multifactorial, and many conflicting theories have been presented (Lewis 2007). Based on magnetic resonance imaging (MRI) scans, asymptomatic partial and full‐thickness rotator cuff tears have been demonstrated in 4% of people aged less than 40 years and in more than 50% of people aged more than 60 years (Sher 1995). It is currently not known how many asymptomatic rotator cuff tears will subsequently become symptomatic. For example, one study of people aged 50 to 80 years who presented with unilateral shoulder pain and had the contralateral shoulder examined by ultrasound suggested that 50% of asymptomatic rotator cuff tears become symptomatic within five years (Yamaguchi 2001). Another study in asymptomatic young elite athletes aged 18 to 38 years participating in sports involving the shoulder, none of the eight athletes with partial or full‐thickness tears found on MRI had developed symptoms five years later (Connor 2003).
The diagnosis of rotator cuff disease in primary care is predominantly made by history and physical examination. People may present with impingement‐type symptoms, pain at night and at rest, and painful movement, with or without features of a torn rotator cuff tendon such as painful weakness and atrophy. The diagnostic utility of various physical examination tests is limited (Hegedus 2008); however, rotator cuff disease is usually distinguishable from adhesive capsulitis by the lack of global restriction of movement. Imaging techniques are also limited in their usefulness for diagnosis. X‐rays may exclude other causes of shoulder pain such as glenohumeral osteoarthritis, calcific tendinitis indicated by the presence of calcific deposits situated just proximal to the rotator cuff insertion in the setting of acute onset of pain, or an acromial spur that might impinge on the rotator cuff. Elevation of the humeral head, together with narrowing of the subacromial space, might indicate the presence of a large rotator cuff tear (Weiner 1970). Imaging modalities such as ultrasound and MRI are able to detect full thickness rotator cuff tears but have less accuracy for detection of partial‐thickness tears (Dinnes 2003; Lewis 2007).
Description of the intervention
The objectives of treatment of symptomatic rotator cuff disease are to relieve pain and restore movement and function of the shoulder. Conservative treatments include corticosteroid injections (Buchbinder 2003), analgesics (Paoloni 2005), non‐steroidal anti‐inflammatories (NSAIDs) (Green 1999), and physical modalities including exercise (Page 2016a; Page 2016b). Topical glyceryl trinitrate has also been proposed as a treatment (Cumpston 2009). These treatments may be used in combination or sequentially. Surgery (decompression with or without rotator cuff repair) is usually reserved for people who do not respond to non‐operative treatment (Karjalainen 2019a; Karjalainen 2019b).
Shock wave therapy can be either extracorporeal or radial. Extracorporeal shock wave therapy (ESWT) is a non‐invasive treatment that involves passing sound waves (or shock waves) through the skin to the affected area, sometimes used with ultrasound‐guided positioning of the device. Shock waves are single pulsed acoustic or sonic waves, which dissipate mechanical energy at the interface of two substances with different acoustic impedance (Loew 1997). They are produced by generators of an electrical energy source and require an electroacoustic conversion mechanism and a focusing device (Ueberle 1997). Three types of systems can be distinguished based upon the sound source: electrohydraulic, electromagnetic and piezoelectric systems. Various doses appear to be used, with no apparent consensus on the minimum therapeutic dose. The definition that will be used throughout this review was defined by Cacchio 2006 as low‐energy shock waves: less than 0.1 mJ/mm² and high‐energy shock waves: 0.2 mJ/mm² to 0.4 mJ/mm²).
Radial shock wave therapy (RSWT) is generated through the acceleration of a projectile inside the handpiece of the treatment device and then transmitted radially from the tip of the applicator to the target zone. Radial shock waves show a lower peak pressure and a considerably longer rise time than extracorporeal shock waves. In RSWT, the focal point is not centred on a target zone, as occurs in ESWT, but on the tip of the applicator (Cacchio 2006).
ESWT has been used since the 1990s to treat various musculoskeletal disorders, but evidence of its efficacy remains equivocal, with trials and reviews reporting conflicting results and there is no known standard dose and treatment protocol. Evidence from one Cochrane systematic review indicated that ESWT did not improve pain and function in lateral elbow pain (Buchbinder 2005; Buchbinder 2006), while another Cochrane Review reported that the evidence for heel pain was equivocal (Crawford 2003). In terms of safety, adverse effects that have been described include local erythema and pain although these are generally minor and short‐lived and no serious adverse effects have been reported.
How the intervention might work
The mechanism of action of ESWT on damaged tendons is not understood. Possible mechanisms have been proposed including overstimulation of pain nerve fibre endings producing an analgesic effect (Melzack 1975; Rompe 1996), or disruption of the tendon tissue by the physical effects of the sound waves (or radial shock wave) resulting in induction of a healing process of the tendon (Loew 1997).
Why it is important to do this review
Despite widespread use of shock wave therapy, evidence of its effectiveness for rotator cuff disease is equivocal. Several systematic reviews have been published (Bannuru 2014; Ioppolo 2013; Vavken 2009; Verstraelen 2014). Three reviews only considered participants with calcific rotator cuff tendinitis (Ioppolo 2013; Vavken 2009; Verstraelen 2014). Vavken 2009 included 14 trials (995 participants) published up to 2008 and concluded that high‐dose ESWT was effective for calcific tendinitis but noted that the conclusions were susceptible to bias. They did not separate placebo from other treatments in their pooled comparative analyses. Ioppolo 2013 included six trials published between 1992 and 2011 and reported that ESWT increased shoulder function, reduced pain and was effective in dissolving calcifications. Verstraelen 2014 included five trials (359 participants) that compared low‐ to high‐energy shock wave therapy for calcific tendinitis and reported that high‐energy shock waves resulted in greater benefits with respect to function and resorption of the calcific deposits at three months compared with low‐energy shock waves. Bannuru 2014 included 28 trials (1745 participants) investigating different energy levels of ESWT for people with both calcific or non‐calcific rotator cuff tendinitis. They were unable to perform any meta‐analyses due to clinical heterogeneity but concluded that high‐energy ESWT was only of benefit for improving pain and function in chronic calcific shoulder tendinitis. An updated high‐quality systematic review is needed to synthesise all the available data up to the present day.
Objectives
To determine the benefits and harms of shock wave therapy for rotator cuff disease, with or without calcification, and to establish its usefulness in the context of other available treatment options.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and controlled clinical trials (CCTs) that used quasi‐randomised methods to allocate participants, for example by date of birth, hospital record number or alternation. We included trials published in any language.
Types of participants
We included trials with participants described as having rotator cuff disease (rotator cuff tendonitis or tendinopathy, supraspinatus, infraspinatus or subscapularis tendonitis, subacromial bursitis or rotator cuff tears) with or without calcific deposits. We also planned to include studies of multiple soft tissue diseases and pain due to tendonitis in different parts of the body provided that the rotator cuff disease results were presented separately, or greater than 90% of participants in the study had rotator cuff disease, but we did not identify any such studies. We excluded RCTs that included participants with a history of significant injury or systemic inflammatory conditions such as rheumatoid arthritis.
Types of interventions
We included all randomised controlled comparisons of shock wave therapy (ESWT or RSWT) versus placebo, or another treatment, or of varying types and dosages of ESWT. Trials that included co‐interventions were eligible for inclusion provided co‐interventions were given to both experimental and control groups.
Types of outcome measures
There is considerable variation in the outcome measures reported in clinical trials of interventions for pain. For the purpose of this systematic review, we aimed to include clinically important changes in pain, as recommended by the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT). Reductions in pain intensity of 30% or greater reflect moderate clinically important differences and 50% or greater reflect substantial clinically important differences, and it is recommended that the proportion of patients who respond with these degrees of pain relief be reported (Dworkin 2008).
Continuous outcome measures in pain trials (such as mean change on a 100 mm Visual Analogue Scale (VAS)) may not follow a Gaussian distribution. Often, a bimodal distribution is seen instead, where patients tend to report either very good or very poor pain relief (Moore 2010). This creates difficulty in interpreting mean changes in continuous pain measures. For this reason, a dichotomous outcome measure (the proportion of participants reporting 30% or greater pain relief) is likely to be more clinically relevant and was the main outcome measure of benefit in this review. However, it is recognised that it has been the practice in most trials of interventions for chronic pain to report continuous measures and, therefore, the mean pain score or mean change in pain score were also included as major outcomes.
The pain state at the end of a clinical trial of an analgesic intervention, in contrast to measures of pain improvement, has also been recommended as a clinically relevant dichotomous outcome measure and was included as a secondary efficacy measure in this review (Moore 2010). A global rating of treatment satisfaction, such as the Patient Global Impression of Change scale (PGIC), which provides an outcome measure that integrates pain relief, changes in function and adverse effects, into a single, interpretable measure, is also recommended by IMMPACT, and was included as a major outcome (Dworkin 2008).
Major outcomes
We presented the major outcomes below in the 'Summary of findings' tables.
Participant‐reported pain relief of 30% or greater.
Mean pain score, or mean change in pain score on VAS or Numerical Rating Scale (NRS) or categorical rating scale (in that order of preference).
-
Disability or function. Where trialists reported outcome data for more than one function scale, we extracted data on the scale that was highest on the following an a priori consensus‐based list:
Shoulder Pain And Disability Index (SPADI);
Shoulder Disability Questionnaire (SDQ);
Constant score;
Disabilities of the Arm, Shoulder and Hand (DASH);
Health Assessment Questionnaire (HAQ);
any other function scale.
Composite endpoints measuring 'success' of treatment such as participants feeling no further symptoms.
Quality of life.
Number of participant withdrawals, for example, due to adverse events or intolerance to treatment.
Number of participants experiencing any adverse event.
Minor outcomes
Proportion of participants achieving pain score below 30/100 mm on VAS.
ROM active preferred over passive measures: shoulder abduction, flexion, external rotation and internal rotation (measured in degrees or other; e.g. hand‐behind‐back distance in centimetres).
For participants with calcification, the effect of ESWT on the size of the calcification.
For participants with calcific deposits, the number of participants with complete or partial resolution (defined or not) of calcific deposits.
We extracted outcome measures assessing benefits of treatment (e.g. pain, function, success, quality of life) at the time points:
up to six weeks;
greater than six weeks to three months (this was the primary time point);
greater than three months to up to six months;
greater than six months to 12 months;
greater than 12 months.
If data were available in a trial at multiple time points within each of the above periods (e.g. at four, five and six weeks), we only extracted data at the latest possible time point of each period. We extracted adverse events, calcification resolution and treatment success at the end of the trial.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases, unrestricted by date or language, on 11 November 2019:
the Cochrane Central Register of Controlled Trials (CENTRAL, via the Cochrane Library);
MEDLINE (Ovid);
Embase (Ovid);
ClinicalTrials.gov;
World Health Organization (WHO) International Clinical Trials Registry Platform.
For the database searches, we combined search terms and text words describing rotator cuff disease and ESWT for the CENTRAL search (Appendix 1), and with validated methodological filters designed to identify CCTs for the MEDLINE database (Appendix 2) (Lefebvre 2011), and the Embase database (Appendix 3). We searched ClinicalTrials.gov (Appendix 4) and the WHO International Clinical Trials Registry Platform (www.who.int/trialsearch/Default.aspx) (Appendix 5) for ongoing trials.
Searching other resources
We checked reference lists of all included articles for additional references.
Data collection and analysis
Selection of studies
Two review authors (SJS, JD) independently selected the trials to be included in the review and retrieved all articles selected by at least one of the review authors for further examination. The review authors were not blinded to the journal or authors. A third review author (RJ) resolved disagreement about inclusion or exclusion of individual studies.
Data extraction and management
Two review authors (SJS, JD) independently extracted data using a standard data extraction form developed for this review. The authors resolved any discrepancies through discussion or adjudication by a third author (RJ or RB), until we reached consensus. We pilot tested the data extraction form and modified it accordingly before use. In addition to items for assessing risk of bias and numerical outcome data, we extracted the following data.
Trial characteristics, including type (e.g. parallel or cross‐over), country, source of funding and trial registration status (with registration number recorded if available).
Participant characteristics, including age, sex, duration of symptoms and inclusion/exclusion criteria.
Intervention characteristics, including description of modality used, dose of treatment, method of administration, frequency of administration and use of co‐interventions.
Outcomes reported, including measurement instrument used and timing of outcome assessment.
Two review authors (SJS, JD) each independently compiled half of the comparisons and entered outcome data into Review Manager 5 (Review Manager 2014). The two review authors (SJS, JD) then independently checked the other author's work to ensure all data were accurate.
For a particular systematic review outcome there may be a multiplicity of results available in the trial reports (e.g. multiple scales, time points and analyses). To prevent selective inclusion of data based on the results (Page 2015), we used the following a priori defined decision rules to select data from trials.
Where trialists reported both final values and change from baseline values for the same outcome, we extracted final values.
Where trialists reported both unadjusted and adjusted values for the same outcome, we extracted unadjusted values.
Where trialists reported data analysed based on the intention‐to‐treat (ITT) sample and another sample (e.g. per‐protocol, as‐treated), we extracted ITT‐analysed data.
For cross‐over RCTs, we extracted data from the first period only.
Where trials did not include a measure of overall pain but included one or more other measures of pain, for the purpose of combining data for the primary analysis of overall pain, we combined overall pain with other types of pain in the following hierarchy:
overall or unspecified pain;
pain at rest;
pain with activity;
daytime pain;
night‐time pain.
Where trials included more than one measure of disability or function, we extracted data from the one function scale that was highest on the following a priori defined list:
SPADI;
SDQ;
Constant score;
DASH;
HAQ;
any other function scale.
Where trials included more than one measure of treatment success, we extracted data from the one function scale that was highest on the following a priori defined list:
participant‐defined measures of success, such as asking participants if treatment was successful;
trialist‐defined measures of success, such as a 30‐point increase on the Constant Score.
For ROM, we only extracted active ROM (abduction or flexion) measured in number of degrees.
Assessment of risk of bias in included studies
Three review authors (SJS, JD, RJ) independently assessed the risk of bias of each included study. The authors resolved any discrepancies through discussion or adjudication by a fourth author (RB), until consensus was reached.
We assessed the following methodological domains, as recommended by Cochrane (Higgins 2011a):
sequence generation (to determine if the method of generating the randomisation sequence was adequate, such as random‐number tables, computer‐generated random numbers, minimisation, coin tossing, shuffling of cards and drawing of lots);
allocation sequence concealment (to determine if adequate methods were used to conceal allocation, such as central randomisation and sequentially numbered, sealed, opaque envelopes);
blinding of participants and personnel;
blinding of outcome assessors: we considered blinding of assessors of self‐reported subjective outcomes (pain, function, success, quality of life) separately from assessors of more objective outcomes (such as calcification and adverse events);
incomplete outcome data;
selective outcome reporting;
other potential threats to validity including baseline imbalance, unit of analysis issues, inappropriate or unequal application of co‐interventions across treatment groups.
Measures of treatment effect
When possible, we based analyses on ITT data (outcomes provided for every randomised participant) from the individual trials. For each trial, we presented outcome data as point estimates with mean and standard deviation (SD) for continuous outcomes and risk ratios (RRs) with corresponding 95% confidence interval (CI) for dichotomous outcomes. Where possible, for continuous outcomes, we extracted end of treatment scores, rather than change from baseline scores.
For continuous data, we presented results as mean differences (MD), if possible. When studies used different scales to measure the same conceptual outcome (e.g. disability), we calculated standardised mean differences (SMD), with corresponding 95% CI. SMD was back‐translated to a typical scale (e.g. 0 to 10 for pain) by multiplying the SMD by a typical among‐person SD (e.g. the SD of the control group at baseline from the most representative trial) (Schünemann 2011a). For ESWT versus placebo, we converted pain (Analysis 1.2) to a 0‐ to 10‐point VAS score using the SD reported at baseline in the placebo group from Gerdesmeyer 2003 (mean (SD): 5.1 (1.6)). For ESWT versus placebo, we converted function (Analysis 1.3) to a 0‐ to 100‐point Constant scale using the SD reported at baseline in the placebo group from Gerdesmeyer 2003 (mean (SD): 64.2 (12.8)). For high‐dose versus low‐dose ESWT, we converted pain (Analysis 8.1) to a 0‐ to 10‐point VAS score using the SD reported at baseline in the placebo group from Gerdesmeyer 2003 (mean (SD): 5.1 (1.6)). For high‐dose versus low‐dose ESWT, we converted function (Analysis 8.2) to a 0‐ to 100‐point Constant scale using the SD reported at baseline in the placebo group from Gerdesmeyer 2003 (mean (SD): 64.2 (12.8)).
1.2. Analysis.
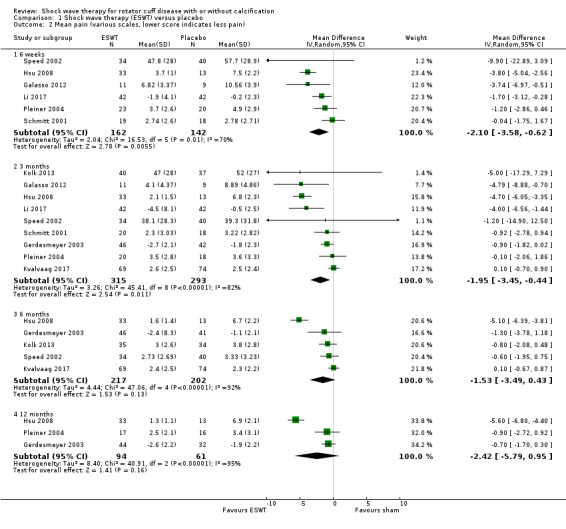
Comparison 1 Shock wave therapy (ESWT) versus placebo, Outcome 2 Mean pain (various scales, lower score indicates less pain).
1.3. Analysis.
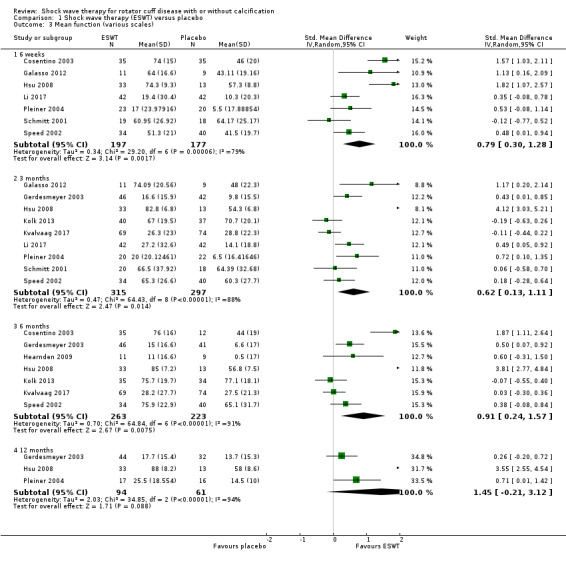
Comparison 1 Shock wave therapy (ESWT) versus placebo, Outcome 3 Mean function (various scales).
8.1. Analysis.
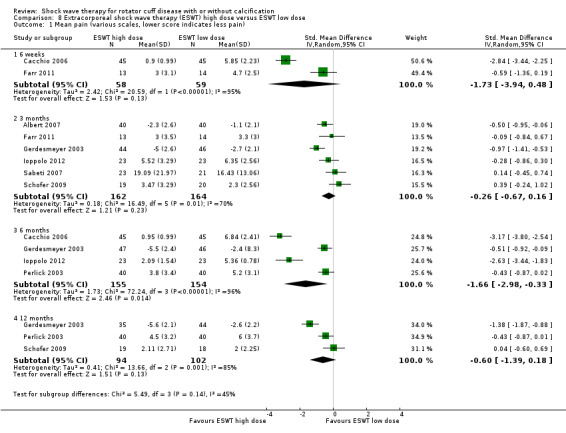
Comparison 8 Extracorporeal shock wave therapy (ESWT) high dose versus ESWT low dose, Outcome 1 Mean pain (various scales, lower score indicates less pain).
8.2. Analysis.
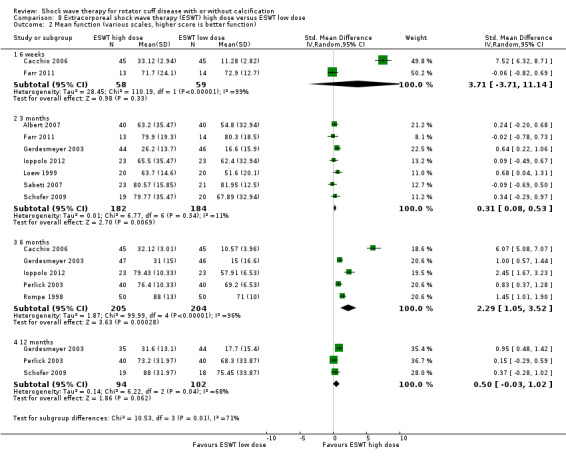
Comparison 8 Extracorporeal shock wave therapy (ESWT) high dose versus ESWT low dose, Outcome 2 Mean function (various scales, higher score is better function).
In the 'Comments' column of the 'Summary of findings' table, we reported the absolute percent difference and the relative percent change from baseline.
For dichotomous outcomes, we calculated the absolute risk difference using the risk difference statistic in Review Manager 5 (Review Manager 2014), and the result expressed as a percentage. For continuous outcomes, we calculated the absolute benefit as the improvement in the intervention group minus the improvement in the control group (MD), in the original units, and expressed as a percentage.
We calculated the relative percent change for dichotomous data as the RR – 1 and expressed as a percentage. For continuous outcomes, we calculated the relative difference as the absolute benefit divided by the baseline mean of the control group, expressed as a percentage.
Unit of analysis issues
Where a single trial reported multiple trial arms, we included only the relevant arms. For the comparison, ESWT versus placebo, if two different energy doses of shock wave therapy and a placebo or control arm were included in a three arm trial (Gerdesmeyer 2003; Peters 2004), we chose the lower dose shock wave therapy as the shock wave arm and compared this to placebo to avoid the data for that study population being over‐represented in the meta‐analysis. The rationale for choosing the lower dose was to reduce clinical heterogeneity within the meta‐analysis, as the lower dose seemed closer to the dose used in the active treatment group of the two arm trials, and there did not appear to be consensus for a definition of a clinical therapeutic dose.
Two trials treated two shoulders in a single participant without adjusting their analysis for the lack of independence (Pan 2003; Pleiner 2004). We reported this as a potential source of additional bias and assessed the impact of including these trials in a sensitivity analysis. When the data for these studies was extracted, the number of shoulders was taken as the population for the study.
If we had identified cross‐over trials, we planned to extract data from the first phase of the trial to avoid potential carry over effects. If we had identified cluster‐randomised trials that did not adjust for potential unit of analysis issues, we would note this and assess the effect of including studies with potential unit of analysis issues in a sensitivity analysis.
Dealing with missing data
Where data were missing or incomplete, we sought further information from the study authors.
In cases where participants were missing from the reported results, we assumed the missing values to have a poor outcome. For dichotomous outcomes that measured adverse events (e.g. number of withdrawals due to adverse events), we calculated the withdrawal rate using the number of participants who received treatment as the denominator (worst‐case analysis). For dichotomous outcomes that measured benefits (e.g. proportion of participants with 30% or more reduction in pain), we calculated the worst‐case analysis using the number of randomised participants as the denominator. For continuous outcomes (e.g. pain), we calculated the MD or SMD based on the number of participants analysed at the time point. If the number of participants analysed were not presented for each time point, we used the number of randomised participants in each group at baseline.
Where possible, we computed missing SDs from other statistics such as standard errors, CIs or P values, according to the methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). If SDs could not be calculated, they were imputed (e.g. from other studies in the meta‐analysis (Higgins 2011c).
Assessment of heterogeneity
We assessed clinical heterogeneity by determining whether the characteristics of participants, interventions, outcome measures and timing of outcome measurement were similar across trials. We assessed statistical heterogeneity using the Chi² statistic and the I² statistic (Higgins 2002). We interpreted the I² statistic using the following as an approximate guide.
0% to 40% may not be important heterogeneity.
30% to 60% may represent moderate heterogeneity.
50% to 90% may represent substantial heterogeneity.
75% to 100% may represent considerable heterogeneity (Deeks 2011).
Assessment of reporting biases
To determine whether reporting bias was present, we determined whether the protocol of the RCT was published before recruitment of participants of the study was started. For studies published after 1 July 2005, we screened the WHO International Clinical Trials Registry Platform (apps.who.int/trialssearch). We evaluated whether selective reporting of outcomes was present (outcome reporting bias).
We compared the fixed‐effect estimate against the random‐effects model to assess the possible presence of small‐sample bias in the published literature (i.e. in which the intervention effect is more beneficial in smaller studies). In the presence of small‐sample bias, the random‐effects estimate of the intervention is more beneficial than the fixed‐effect estimate (Sterne 2011).
The potential for small‐study effects in the main outcomes of the review were further explored using funnel plots if at least 10 studies were included in a meta‐analysis for the main efficacy outcome.
Data synthesis
For clinically similar studies that used a common comparator, we pooled outcomes in a meta‐analysis using the random‐effects model as a default, and performed a sensitivity analysis with the fixed‐effect model.
'Summary of findings' table
We created a 'Summary of findings' table using the following outcomes: pain relief greater than 50% (the a priori outcome was pain relief of 30% or greater, which none of the studies reported so we reported pain relief greater than 50%), mean pain score, function, participant‐reported success, quality of life, number of participant withdrawals due to adverse events or treatment intolerance, and number of participants experiencing any adverse event. We selected three months as the primary time point (for the outcomes assessing benefits of treatment) and placebo as the main comparator.
All review authors independently assessed the certainty of the evidence. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of the body of evidence as it related to the studies which contribute data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in Section 8.5, Section 8.7, Chapter 11 and Section 13.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a; Reeves 2011; Schünemann 2011b) using GRADEpro software (GRADEpro GDT 2015). We justified all decisions to downgrade the certainty of the studies using footnotes and made comments to aid the reader's understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses:
those with and without calcification.
We used the following outcomes in subgroup analyses, for the main comparison (ESWT versus placebo):
pain;
function.
Sensitivity analysis
We performed the following sensitivity analyses for the main comparator (ESWT vs placebo), for the outcomes pain and function:
adequate allocation concealment (selection bias);
participant blinding (detection bias).
We removed the trials that reported inadequate or unclear allocation concealment and lack of participant blinding from the meta‐analysis of pain and function for the main comparison (ESWT versus placebo), at the primary time point (three months) to assess the effect of potential selection and detection biases on the overall treatment effect.
Results
Description of studies
Results of the search
The database searches conducted up to 11 November 2019 resulted in retrieval of 461 records. After removal of duplicates, 285 unique records remained. After screening the abstracts, we retrieved 104 unique studies for full‐text screening, out of which we excluded 58 studies (see Characteristics of excluded studies table). We selected 32 trials for inclusion (Albert 2007; Cacchio 2006; Cosentino 2003; De Boer 2017; Del Castillo‐Gonzales 2016; Duymaz 2019; Engebretsen 2009; Farr 2011; Frizziero 2017; Galasso 2012; Gerdesmeyer 2003; Haake 2002; Hearnden 2009; Hsu 2008; Ioppolo 2012; Kim 2014; Kolk 2013; Kvalvaag 2017; Li 2017; Loew 1999; Melegati 2000; Pan 2003; Perlick 2003; Peters 2004; Pleiner 2004; Rompe 1998; Sabeti 2007; Sabeti‐Aschraf 2005; Schmitt 2001; Schofer 2009; Speed 2002; Tornese 2011; Characteristics of included studies table). Nine additional trials are awaiting classification, as they could not be translated (Berner 2004; Diehl 2011; Gross 2002; Loew 1995; Mao 2003; Paternostro‐Sluga 2004; Rompe 1997a; Rompe 1997b; Seil 1999; Characteristics of studies awaiting classification table). We identified five ongoing trials in clinical trials registries (ChiCTR1900022932; NCT02677103; NCT03779919; NTR7093; PACTR201910650013453; Characteristics of ongoing studies table). Figure 1 shows the flow diagram of the study selection process.
1.
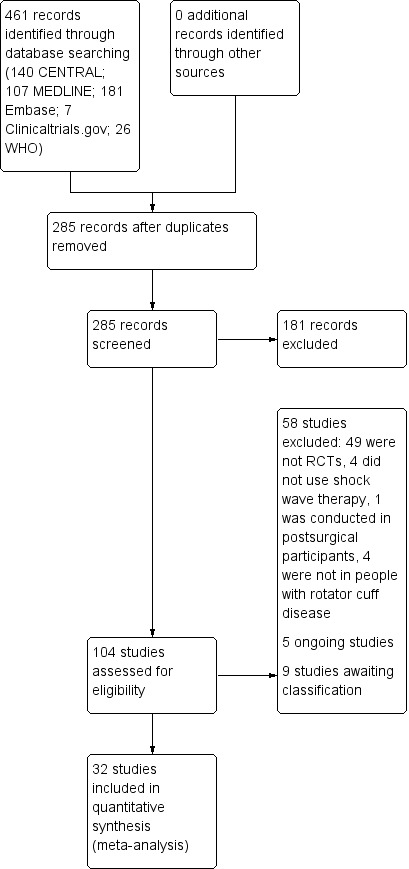
Study flow diagram.
Included studies
A full description of all included trials is provided in the Characteristics of included studies table. We contacted authors of 24 trials to request information about study design, participants, interventions and outcomes of the trial; information required to complete the risk of bias assessments; or missing data for unreported or partially reported outcomes (Albert 2007; Cacchio 2006; Cosentino 2003; Engebretsen 2009; Farr 2011; Frizziero 2017; Galasso 2012; Gerdesmeyer 2003; Haake 2002; Hearnden 2009; Hsu 2008; Ioppolo 2012; Kim 2014; Kolk 2013; Loew 1999; Melegati 2000; Pan 2003; Perlick 2003; Peters 2004; Pleiner 2004; Rompe 1998; Sabeti‐Aschraf 2005; Sabeti 2007; Tornese 2011). We received replies from five trialists (Engebretsen 2009; Frizziero 2017; Galasso 2012; Kolk 2013; Sabeti 2007).
Study design and setting
All studies were parallel‐group RCTs. Twenty‐eight trials included two intervention arms, three trials included three intervention arms (Peters 2004; Gerdesmeyer 2003; Melegati 2000), and one trial included four intervention arms (Loew 1999).
Trials were set in Italy (Cacchio 2006; Cosentino 2003; Frizziero 2017; Galasso 2012; Ioppolo 2012; Melegati 2000; Tornese 2011), Germany (Haake 2002; Loew 1999; Perlick 2003; Peters 2004; Rompe 1998; Schmitt 2001; Schofer 2009), Austria (Farr 2011; Pleiner 2004; Sabeti 2007; Sabeti‐Aschraf 2005), Germany and Austria (Gerdesmeyer 2003), Norway (Engebretsen 2009; Kvalvaag 2017), the Netherlands (De Boer 2017; Kolk 2013); UK (Hearnden 2009; Speed 2002), China (Hsu 2008; Li 2017), France (Albert 2007), Taiwan (Pan 2003), Spain (Del Castillo‐Gonzales 2016), Turkey (Duymaz 2019), and South Korea (Kim 2014).
Two studies were funded by manufacturers of shock wave machines (Galasso 2012; Kolk 2013), seven studies were funded by grants from research foundations or universities (Albert 2007; Del Castillo‐Gonzales 2016; Engebretsen 2009; Gerdesmeyer 2003; Ioppolo 2012; Kvalvaag 2017; Li 2017), three studies were provided with the shock wave machines (Albert 2007; Gerdesmeyer 2003; Pleiner 2004), nine studies explicitly reported they received no funding (Cacchio 2006; Duymaz 2019; Hearnden 2009; Kim 2014; Loew 1999; Pan 2003; Schmitt 2001; Speed 2002; Tornese 2011), while 13 studies did not report either way (Cosentino 2003; De Boer 2017; Farr 2011; Frizziero 2017; Haake 2002; Hsu 2008; Melegati 2000; Perlick 2003; Peters 2004; Rompe 1998; Sabeti 2007; Sabeti‐Aschraf 2005; Schofer 2009).
Participant characteristics
The 32 trials included 2281 participants, and the number of participants per trial ranged from 20 to 243. Of the 16 studies that reported mean age of the overall cohort, the mean age of participants ranged from 48 years to 56.2 years. Of the seven studies that reported the mean duration of symptoms of the overall cohort, the mean duration of symptoms ranged from 7.1 to 60 months. Of the 30 studies that reported population gender numbers, 61% of participants were female.
Inclusion criteria or definitions of the included conditions (or both) varied between trials. Ten trials specified calcific or calcifying tendonitis or tendinopathy without specifying the involved tendons (Albert 2007; Cacchio 2006; Duymaz 2019; Farr 2011; Gerdesmeyer 2003; Haake 2002; Hsu 2008; Pan 2003; Sabeti‐Aschraf 2005; Tornese 2011); 11 trials specified the presence of symptoms such as pain (De Boer 2017; Del Castillo‐Gonzales 2016; Duymaz 2019; Frizziero 2017; Haake 2002; Hsu 2008; Kvalvaag 2017; Peters 2004; Pleiner 2004; Rompe 1998; Sabeti‐Aschraf 2005), four trials specified supraspinatus or infraspinatus calcification (Cosentino 2003; Hearnden 2009; Kim 2014; Sabeti 2007), two trials specified non‐calcific tendonitis of the supraspinatus tendon (Schmitt 2001; Schofer 2009), two trials specified non‐calcific tendonitis of any part of the rotator cuff (Galasso 2012; Speed 2002), four trials specified calcific deposits without tendonitis (De Boer 2017; Del Castillo‐Gonzales 2016; Ioppolo 2012; Kim 2014), two trials specified subacromial shoulder pain (Engebretsen 2009; Kvalvaag 2017), two trials included shoulder pain without a specified location (Loew 1999; Perlick 2003), one trial specified subacromial impingement syndrome (Melegati 2000), and two trials specified chronic tendonitis (Kolk 2013; Li 2017). Twenty trials included radiographic imaging as part of their definition for the condition (Albert 2007; Cacchio 2006; Cosentino 2003; De Boer 2017; Frizziero 2017; Gerdesmeyer 2003; Haake 2002; Hearnden 2009; Ioppolo 2012; Kim 2014; Melegati 2000; Pan 2003; Perlick 2003; Peters 2004; Pleiner 2004; Rompe 1998; Sabeti 2007; Sabeti‐Aschraf 2005; Speed 2002; Tornese 2011).
Twenty‐three trials only included participants with calcific tendinitis (Albert 2007; Cacchio 2006; Cosentino 2003; De Boer 2017; Del Castillo‐Gonzales 2016; Duymaz 2019; Farr 2011; Gerdesmeyer 2003; Haake 2002; Hearnden 2009; Hsu 2008; Ioppolo 2012; Kim 2014; Kvalvaag 2017; Loew 1999; Pan 2003; Perlick 2003; Peters 2004; Pleiner 2004; Rompe 1998; Sabeti 2007; Sabeti‐Aschraf 2005; Tornese 2011), seven trials only included participants without calcific deposits (Frizziero 2017; Galasso 2012; Li 2017; Melegati 2000; Schmitt 2001; Schofer 2009; Speed 2002), and two trials included participants with or without calcific deposits (Engebretsen 2009; Kolk 2013). Only Kolk 2013 reported data for participants with and without calcific deposits separately.
Interventions
A detailed description of the interventions delivered in each trial is summarised in the Characteristics of included studies table and a summary of the shock wave technique and comparison tested in each trial is presented in Table 2. Shock wave treatments were very heterogeneous across trials and varied in the machines used to generate the shock waves, number and size of energy pulses, and the number of treatment sessions (one to six sessions varying from seven to 16 days apart).
1. Characteristics of interventions used in included trials.
| Study ID | Shock wave machine | Type of shock wave | Number, frequency and dose | Comparison | Use of anaesthesia | Number of treatments |
| Albert 2007 | Modulith SLK (Storz Medical AG, Tagerwilen, Switzerland) electromagnetic shock wave generator with fluoroscopic and sonographic guidance | ESWT | High‐dose shock wave: 2500 impulses, frequency 1 Hz for first 200 and 2 Hz thereafter. Goal intensity was maximum energy level tolerated by participant without exceeding 0.45 mJ/mm² per impulse |
Low dose: 2500 impulses, frequency 1 Hz for first 200 and 2Hz thereafter. The energy intensity gradually increased from 0.02 mJ/mm² to 0.06 mJ/mm² per shock |
None | 2 sessions 14 days apart |
| Cacchio 2006 | Physio Shock Wave Therapy device consisting of a control unit, a handpiece with 3 different head applicators and medical air compressor | rESWT | High dose: 2500 impulses per session (500 impulses with pressure 1.5 bar and frequency 10 Hz), EFD 0.10 mJ/mm² and fixed impulse time of 2 ms |
Low dose: 25 impulses per session (5 impulses with a pressure of 1.5 bar and frequency of 4.5 Hz and 20 impulses with pressure 2.5 bar and frequency 10 Hz), EFD 0.10 mJ/mm² and fixed impulse time of 2 ms |
None | 4 sessions 7 days apart |
| Cosentino 2003 | 'Orthima' by Direx Medical System Ltd | ESWT | Shock wave: 1200 shocks at 120 shocks/minute of 0.03 mJ/mm² |
Placebo: 1200 shocks at 120 shock/minute of 0 mJ/mm² |
None | 4 sessions 4–7 days apart |
| De Boer 2017 | Masterpuls MP 100 (Storz Medical, Tagerwilen, Switzerland) | rESWT | Shock wave: 500 pulses of 1.5 bar (150 kPa) with a frequency of 4.5 Hz, followed by 2000 pulses of 2.5 bar (250 kPa) with a frequency 10 Hz; EFD)0.10 mJ/mm², duration of pulses was 2 ms |
Ultrasound‐guided needling | None | 4 sessions, 1 week apart |
| Del Castillo‐Gonzales 2016 | Swiss DolorClast device | ESWT | Shock wave: Total of 2000 impacts (2 series of 1000 each) at frequency 8–10 Hz and EFD 0.20 J/mm² |
Ultrasound‐guided percutaneous lavage | None | Twice per week for 4 weeks |
| Duymaz 2019 | ShockMaster 500 device (GymnaUniphy NV, Bilzen, Belgium) | rESWT | Shock wave: 1500 shocks with a frequency of 150 shocks per minute. all participants were treated with a low‐energy density of 0.03 mJ/mm² for the first 5 minutes, which was then progressively increased to 0.28 mJ/mm². Duration of pulses was 10 minutes |
Physiotherapy: ultrasound (1.0 MHz, 5 minutes, continuous), TENS (conventional, 20 minutes), shoulder joint ROM and stretching exercises, and ice application |
None | 1 session weekly for 4 weeks |
| Engebretsen 2009 | Swiss Dolor Clast, EMS | rESWT | Shock wave: 8–12 Hz at 2000 impulses/second with a pressure of 2.5–4.0 bar |
Supervised exercises | None | 1 session weekly for 4–6 weeks for rESWT OR 2 × 45‐minute sessions weekly for up to 12 weeks for supervised exercises |
| Farr 2011 | Storz Modulith SLK lithotripter in combination with a fluoroscopy‐guided 3D computer‐assisted navigation device | ESWT | High dose: 3200 impulses at 0.3 mJ/mm²; twice |
Low dose: 1600 impulses at 0.02 mJ/mm²; once |
5 mL xylocaine subacromially | Once only for low dose OR 2 sessions 7 days apart for high dose |
| Frizziero 2017 | Modulith SLK (Storz Medical AG, Tagerwilen, Switzerland) | ESWT | Shock wave (low dose): 1600 impulses at a frequency of 4 Hz not exceeding 0.15 mJ/mm² |
Ultrasound‐guided injection with low molecular weight hyaluronic acid | None | Weekly shock wave sessions for 4 weeks OR 1 injection weekly for 3 weeks |
| Galasso 2012 | Modulith SLK system | ESWT | Shock wave: 3000 shocks of 0.068 mJ/mm² |
Placebo: Same protocol but with shock wave generator disconnected |
Subcutaneous injection of 2 mL of 2% lidocaine above the subacromial space of the affected shoulder prior to each treatment | 2 sessions 7 days apart |
| Gerdesmeyer 2003 | Not reported | ESWT | Shock wave (low dose): 6000 shocks at 120 impulses/minute of 0.08 mJ/mm² |
High dose: 6000 shocks at 120 impulses/minute of 0.32 mJ/mm² OR Placebo: 1500 shocks at 120 impulses/minute of 0.32 mJ/mm² with participant insulated from shock waves |
None | 2 sessions 12–16 days apart |
| Haake 2002 | Adapted shock wave generator Storz Minilith SL‐1 (Storz Medical AG, CH 8280 Kreuzlingen, Switzerland) | ESWT | At site of calcification: 2000 impulses of a positive EFD 0.35 mJ/mm² measured with a membrane hydrophone (equivalent to 0.78 mJ/mm² measured with a fibreoptic hydrophone) at 120 impulses/minute |
Supraspinatus site: 2000 impulses of a positive EFD 0.35 mJ/mm² measured with a membrane hydrophone (equivalent to 0.78 mJ/mm² measured with a fibreoptic hydrophone) at 120 impulses/minute |
15 mL mepivacaine 1% subacromially | 2 sessions 7 days apart |
| Hearnden 2009 | Not reported | ESWT | Shock wave: 2000 shocks of 0.28 mJ/mm² |
Placebo: 20 shocks of 0.03 mJ/mm² |
20 mL of 0.5% marcaine at site of calcific deposit | 1 session |
| Hsu 2008 | OrthoWave machine (MTS, Konstanz, Germany) | ESWT | Shock wave: 1000 shocks at 2 wave pulses/second of 0.55 mJ/mm² |
Placebo: dummy electrode |
10 mL of 2% lidocaine injected into affected area from a lateral approach with a 24‐gauge needle | 2 sessions 14 days apart |
| Ioppolo 2012 | ESWT (Modulith SLK system, Storz Medical, Tager‐wilen, Switzerland) equipped with an in‐line ultrasound positioning system on the target zone | ESWT | Low dose: 2400 impulses at 0.10 mJ/mm² |
High dose: 2400 impulses at 0.20 mJ/mm² |
None | 4 sessions 7 days apart |
| Kim 2014 | Not reported | ESWT | Shock wave: 1000 impulses, 0.32 mJ/mm² |
Glucocorticoid needling 1 mL Depo‐Medrol (glucocorticoid) ultrasound guidance |
2% lidocaine in the corticosteroid group | 3 sessions 1 week apart for ESWT OR 1 steroid injection |
| Kolk 2013 | Swiss DolorClast radial shock wave device (EMS Electro Medical Systems, Nyon, Switzerland) | rESWT | Shock wave: 2000 impulses of 0.11 mJ/mm² |
Placebo: 2000 impulses of 0.11 mJ/mm² with a sham probe |
None | 3 sessions 10–14 days apart |
| Kvalvaag 2017 | EMS Swiss DolorClast/Enimed | rESWT | Shock wave: 2000 impulses at 0.35 mJ/mm² pressure 1.5–3 bar, depending on what the participant tolerated |
Placebo: 2000 impulses at 0.35 mJ/mm² with a sham probe |
None | 1 session weekly for 4 weeks |
| Li 2017 | Pain Treatment System of Radial shock wave Device (Sonothera, Hanil Tm Co. Ltd, Korea) | ESWT | Shock wave: 3000 pulses of 0.11 mJ/mm² at frequency 15 Hz. Pressure 3 bar |
Placebo: identical‐looking placebo probe used |
None | 5 sessions, 3 days apart |
| Loew 1999 | Electrohydraulic lithotripter (MFL 5000; Philips, Hamburg, Germany) | ESWT | Group 1: 1 dose of 2000 impulses of 0.1 mJ/mm² Group 2: 1 dose of 2000 impulses of 0.3 mJ/mm² Group 3: 2 doses of 2000 impulses of 0.3 mJ/mm² 1 week apart |
No treatment | 15–20 mL bupivacaine hydrochloride | 1 session OR 2 sessions 1 week apart |
| Melegati 2000 | Epos Ultra electromagnetic apparatus fitted with a 7.5 MHz linear echographic sound | ESWT | 200 shots of 0.22 mJ/mm² reached in 400 shots | Kinesitherapy | None | 3 sessions 7 days apart for ESWT OR 6 × 40‐minute sessions 3 weeks apart for kinesitherapy |
| Pan 2003 | Orthospec (Medispec Ltd, Germantown, MD, USA) | ESWT | 2000 shock waves at 2 Hz of 0.26–0.32 mJ/mm² | TENS | None | 2 sessions 14 days apart for ESWT OR 3 times a week for 4 weeks for TENS |
| Perlick 2003 | Siemens Lithostar‐Lithotripter | ESWT | 2000 impulses of 0.23 mJ/mm² | 2000 impulses of 0.42 mJ/mm² | 10 mL bupivacaine hydrochloride 0.5% | 2 sessions 3 weeks apart |
| Peters 2004 | The miniaturised shock wave source Minilith (15 cm diameter, 15 cm length) (Stroz Medical, Switzerland) with an in‐line ultrasound device | ESWT | 1500 impulses of 0.15 mJ/mm² | 1500 impulses of 0.44 mJ/mm² OR system turned off |
None | 1–5 sessions at 6‐week intervals |
| Pleiner 2004 | Electrohydraulic system (Orthospec, Medispec Inc, Montgomery Village, MD, USA) | ESWT | High dose: 2 × 2000 impulses at frequency 2.5 Hz, dose 0.28 mJ/mm² |
Placebo 2 × 2000 impulses at frequency 2.5 Hz, dose < 0.07 mJ/mm² dampened with a foam membrane |
None | 2 sessions |
| Rompe 1998 | ESWT with an experimental device characterised by the integration of an electromagnetic shock wave generator and a mobile fluoroscopy unit (Siemens AG, 91052 Erlangen, Germany) | ESWT | 1500 impulses of 0.06 mJ/mm² | 1500 impulses of 0.28 mJ/mm² | None | 1 session |
| Sabeti 2007 | Lithotripter (Storz Modulith SLK, Storz Medical Products, Kreuzlingen, Switzerland) | ESWT | 1000 impulses of 0.08 mJ/mm² | 2000 impulses of 0.02 mJ/mm² | 5 mL Xyloneural subacromially | 3 sessions 7 days apart for low dose OR 2 sessions 7 days apart for higher dose |
| Sabeti‐Aschraf 2005 | Lithotripter (Modulith SLK, Storz Medical Products, Kreuzlingen, Switzerland) | ESWT | 1000 impulses of 0.08 mJ/mm² with frequency 4 Hz | 1000 impulses of 0.08 mJ/mm² with frequency 4 Hz | None | 3 sessions 7 days apart |
| Schmitt 2001 | Storz Minilith SL 1 (Storz Medical AG, Kreuzlingen, Switzerland) | ESWT | 2000 impulses at 120 impulses/minute of 0.11 mJ/mm² | 2000 impulses at 120 impulses/minute of 0.11 mJ/mm² with the participant insulated from the shock waves | 10 mL mepivacaine subacromially | 3 sessions 7 days apart |
| Schofer 2009 | Minilith SL 1 shock wave generator (Storz Medical, Switzerland) | ESWT | 2000 impulses at 120 impulses/second of 0.33 mJ/mm² | 2000 impulses at 120 impulses/second of 0.78 mJ/mm² | 10 mL mepivacaine 1% subacromially | 3 sessions 7 days apart |
| Speed 2002 | Sonocur Plus Unit (Siemens, Munich, Germany) | ESWT | 1500 impulses of 0.12 mJ/mm² | 1500 impulses of 0.04 mJ/mm² with the machine head deflated, no contact gel applied and standard skin contact avoided | None | 3 sessions 1 month apart |
| Tornese 2011 | Electromagnetic lithotriptor (Epos Ultra; Dornier MedTech Wessling, Germany) fitted with a linear ultrasonographic probe | ESWT | 1800 pulses of up to 0.22 mJ/mm² which was reached within 400 impulses | 1800 pulses of up to 0.22 mJ/mm² which was reached within 400 impulses | None | 3 sessions 7 days apart |
EFD: energy fluctuation density; ESWT: extracorporeal shock wave therapy; rESWT: radial extracorporeal shock wave therapy; ROM: range of movement; TENS: transcutaneous electrical nerve stimulation.
Twelve trials compared ESWT to a placebo control (Cosentino 2003; Galasso 2012; Gerdesmeyer 2003; Hearnden 2009; Hsu 2008; Kolk 2013; Kvalvaag 2017; Li 2017; Peters 2004; Pleiner 2004; Schmitt 2001; Speed 2002). The trials the placebo control variably. Six trials used negligible or 0 mJ/mm² energy density (Cosentino 2003; Hearnden 2009; Hsu 2008; Kolk 2013; Peters 2004; Speed 2002), four trials physically blocked or dampened the shock waves (Gerdesmeyer 2003; Li 2017; Pleiner 2004; Schmitt 2001), one trial disconnected the shock wave device in the placebo group (Galasso 2012), and one trial did not clearly describe the sham procedure (Kvalvaag 2017).
Ten trials compared high‐dose to low‐dose ESWT (Albert 2007; Farr 2011; Gerdesmeyer 2003; Ioppolo 2012; Loew 1999; Perlick 2003; Peters 2004; Rompe 1998; Sabeti 2007; Schofer 2009), and one trial compared high‐dose to low‐dose RSWT (Cacchio 2006). Trials differed in their definition of high and low dose (Table 2).
One trial compared ESWT directed to the calcific deposit versus directed to the origin of the supraspinatus tendon (Haake 2002); one trial compared ESWT with the arm hyperextended versus with the arm in a neutral position (Tornese 2011); one trial compared fluoroscopic‐guided ultrasound targeted to the calcific deposit versus the shock waves directed to the area of maximum tenderness (Sabeti‐Aschraf 2005); one trial compared shock wave therapy plus physiotherapy to physiotherapy alone (Duymaz 2019); and one trial compared two versus one session of ESWT (Loew 1999).
Four trials compared ESWT to ultrasound‐guided needling (De Boer 2017; Del Castillo‐Gonzales 2016; Frizziero 2017; Kim 2014); one trial compared shock wave therapy to TENS (Pan 2003); ESWT to no treatment (Loew 1999); and combination of ESWT and exercise to exercise alone or advice alone (Melegati 2000). One trial compared RSWT to supervised exercise (Engebretsen 2009).
Outcomes
Of the major outcomes, no trial measured participant‐reported pain relief of 30% or greater or quality of life. However, one study reported participant‐reported pain relief of 50% or greater (Speed 2002); thus, we report this outcome as a major outcome.
Twenty‐nine trials measured pain (mean or mean change), with most using a 0‐ to 10‐point VAS with 10 indicating the worst pain. Of these, five partially reported the pain outcome (Cosentino 2003; Frizziero 2017; Hearnden 2009; Kim 2014; Speed 2002). Three trials did not measure the pain outcome (Loew 1999; Melegati 2000; Rompe 1998).
Thirty trials measured function, with the Constant score being the most commonly used. Of these, four trials partially reported the function outcome (Hearnden 2009; Kim 2014; Perlick 2003; Rompe 1998). Two trials did not measure function (Del Castillo‐Gonzales 2016; Peters 2004).
Fourteen trials measured treatment success using a variety of methods (Albert 2007; Cacchio 2006; De Boer 2017; Del Castillo‐Gonzales 2016; Galasso 2012; Gerdesmeyer 2003; Haake 2002; Hearnden 2009; Hsu 2008; Loew 1999; Peters 2004; Sabeti 2007; Schmitt 2001; Speed 2002).
Eight trials measured withdrawals due to adverse events (Engebretsen 2009; Gerdesmeyer 2003; Kolk 2013; Kvalvaag 2017; Li 2017; Peters 2004; Pleiner 2004; Speed 2002). Twenty‐seven trials measured adverse events (Albert 2007; Cacchio 2006; Cosentino 2003; De Boer 2017; Del Castillo‐Gonzales 2016; Engebretsen 2009; Farr 2011; Galasso 2012; Gerdesmeyer 2003; Haake 2002; Hearnden 2009; Hsu 2008; Ioppolo 2012; Kolk 2013; Kvalvaag 2017; Li 2017; Loew 1999; Pan 2003; Perlick 2003; Peters 2004; Pleiner 2004; Rompe 1998; Sabeti 2007; Sabeti‐Aschraf 2005; Schmitt 2001; Schofer 2009; Speed 2005), and of these one partially reported the adverse event outcome (Hearnden 2009). Five trials did not measure adverse events (Duymaz 2019; Frizziero 2017; Kim 2014; Melegati 2000; Tornese 2011)
We contacted authors of all trials who did not fully report outcomes to request missing data, and received missing data from two authors (Engebretsen 2009; Frizziero 2017). In two studies, it was possible to use alternate scores or extrapolation to extract the data for review (Kolk 2013; Sabeti 2007).
Of the minor outcomes, one trial measured pain below 30/100 on a VAS (Haake 2002), three trials measured active ROM (Cacchio 2006 measured active flexion; Duymaz 2019 measured flexion, extension, abduction and external rotation; and Engebretsen 2009 measured active abduction). Twenty‐one trials measured calcification size (mean size, mean change in size or disappearance/resolution of calcification) (Albert 2007; Cacchio 2006; Cosentino 2003; De Boer 2017; Del Castillo‐Gonzales 2016; Farr 2011; Gerdesmeyer 2003; Haake 2002; Hearnden 2009; Hsu 2008; Ioppolo 2012; Kim 2014; Loew 1999; Pan 2003; Perlick 2003; Peters 2004; Pleiner 2004; Rompe 1998; Sabeti 2007; Sabeti‐Aschraf 2005; Tornese 2011).
Excluded studies
A full description of all excluded trials is provided in the Characteristics of excluded studies table. Of the 58 full‐text articles excluded, 49 were not RCTs (Adamietz 2003; Astore 2003; Avancini‐Dobrovic 2011; Barnsley 2001; Boxberg 1996; Buch 1999; Buselli 2010; Bytomski 2006; Charrin 2001; Cheing 2003; Cosentino 2004; Costa 2002; Cyteval 2003; Friedberg 2010; Garcia Marti 2004; Hayes 2005; Jakobeit 2002; Labek 1999; Lee 2011; Lippincott 2010; Loew 1995; Lorbach 2008; Magosch 2003; Maier 2000; Mangone 2010; Manske 2004; Meier 2000; Moretti 2005; Mundy 2004; Noel 1999; Notarnicola 2011; Pigozzi 2000; Rebuzzi 2008; Rees 2009; Rompe 1995; Rompe 2000; Rompe 2001; Rompe 2003; Sabeti‐Aschraf 2004; Sarrat 2004; Seil 2006; Sistermann 1998; Speed 2005; Spindler 1998; Steinacker 2001; Thigpen 2010; Wang 2001; Wang 2003; Wiley 2002), four studies did not investigate shock wave therapy (Bringmann 2001; Krasny 2005; Polimeni 2003; Saggini 2010), four studies investigated conditions other than rotator cuff disease (Ali 2016; Chow 2007; Liu 2012; Njawaya 2018), and one study included postsurgical participants (Kim 2012).
Studies awaiting classification
Nine trials are awaiting classification, subject to translation into English (Berner 2004; Diehl 2011; Gross 2002; Loew 1995; Mao 2003; Paternostro‐Sluga 2004; Rompe 1997a; Rompe 1997b; Seil 1999; Characteristics of studies awaiting classification table).
Ongoing studies
At the time of publication of this review, there were five ongoing studies that did not have study results available at the time of submission of this review (ChiCTR1900022932; NCT02677103; NCT03779919; NTR7093; PACTR201910650013453). A description of these trials is provided in the Characteristics of ongoing studies table.
Risk of bias in included studies
All trials were susceptible to bias. Overall, 24/32 (75%) trials were susceptible to selection bias, 20 (62%) trials at risk of performance bias, 20 (62%) trials at risk of detection bias and 14 (45%) trials at risk of selective reporting bias (Figure 2).
2.
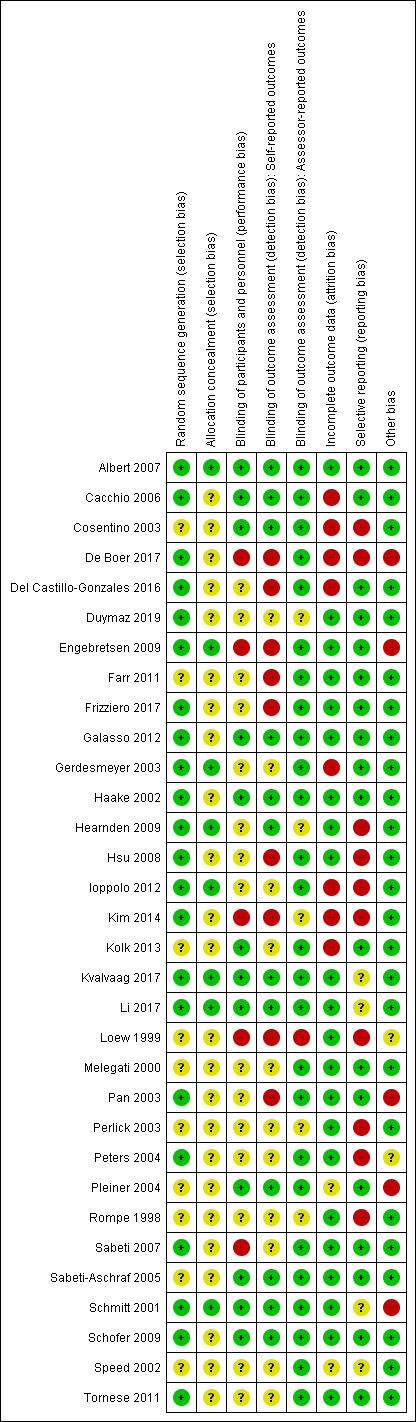
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Only eight (26%) trials used appropriate methods to both generate and conceal their allocation sequence, and so were rated at low risk of selection bias (Albert 2007; Engebretsen 2009; Gerdesmeyer 2003; Hearnden 2009; Ioppolo 2012; Kvalvaag 2017; Li 2017; Schmitt 2001).
Ten (32%) trials did not clearly report their method of sequence generation (Cosentino 2003; Farr 2011; Kolk 2013; Loew 1999; Melegati 2000; Perlick 2003; Pleiner 2004; Rompe 1998; Sabeti‐Aschraf 2005; Speed 2002), and 24 (75%) trials did not adequately report their method of allocation concealment (Cacchio 2006; Cosentino 2003; De Boer 2017; Del Castillo‐Gonzales 2016; Duymaz 2019; Farr 2011; Frizziero 2017; Galasso 2012; Haake 2002; Hsu 2008; Kim 2014; Kolk 2013; Loew 1999; Melegati 2000; Pan 2003; Perlick 2003; Peters 2004; Pleiner 2004; Rompe 1998; Sabeti 2007; Sabeti‐Aschraf 2005; Schofer 2009; Speed 2002; Tornese 2011). Therefore, the risk of selection bias in these trials was unclear.
Blinding
We judged 12 (38%) trials at low risk of performance bias because participants and personnel were likely successfully blinded (Albert 2007; Cacchio 2006; Cosentino 2003; Galasso 2012; Haake 2002; Kolk 2013; Kvalvaag 2017; Li 2017; Pleiner 2004; Sabeti‐Aschraf 2005; Schmitt 2001; Schofer 2009). We judged five (15%) trials at high risk of performance bias as participants or personnel were not successfully blinded to treatment groups (De Boer 2017; Engebretsen 2009; Kim 2014; Loew 1999; Sabeti 2007).
In the remaining 15 trials (50%) the risk of performance bias was unclear as it was not clearly reported if personnel or participants, or both, were blinded (Del Castillo‐Gonzales 2016; Duymaz 2019; Farr 2011; Frizziero 2017; Gerdesmeyer 2003; Hearnden 2009; Hsu 2008; Ioppolo 2012; Melegati 2000; Pan 2003; Perlick 2003; Peters 2004; Rompe 1998; Speed 2002; Tornese 2011).
Twelve (38%) trials were at low risk of detection bias in self‐reported outcomes because participants were probably successfully blinded to treatment (Albert 2007; Cacchio 2006; Cosentino 2003; Galasso 2012; Haake 2002; Hearnden 2009; Kvalvaag 2017; Li 2017; Pleiner 2004; Sabeti‐Aschraf 2005; Schmitt 2001; Schofer 2009).
We judged 11 (32%) trials at unclear risk of detection bias due to lack of reporting of blinding methods (Duymaz 2019; Gerdesmeyer 2003; Ioppolo 2012; Kolk 2013; Melegati 2000; Perlick 2003; Peters 2004; Rompe 1998; Sabeti 2007; Speed 2002; Tornese 2011). We judged nine (29%) trials at high risk of detection bias as participants were either not blinded or likely guessed their treatment group due to the differing nature of the treatment groups (De Boer 2017; Del Castillo‐Gonzales 2016; Engebretsen 2009; Farr 2011; Frizziero 2017; Hsu 2008; Kim 2014; Loew 1999; Pan 2003).
Twenty‐seven trials included assessor‐rated outcomes (calcification size, ROM). There was a low risk of detection bias for these outcomes in 26 (84%) trials, as assessors were adequately blinded (Albert 2007; Cacchio 2006; Cosentino 2003; De Boer 2017; Del Castillo‐Gonzales 2016; Engebretsen 2009; Farr 2011; Frizziero 2017; Galasso 2012; Gerdesmeyer 2003; Haake 2002; Hsu 2008; Ioppolo 2012; Kolk 2013; Kvalvaag 2017; Li 2017; Melegati 2000; Pan 2003; Peters 2004; Pleiner 2004; Sabeti 2007; Sabeti‐Aschraf 2005; Schmitt 2001; Schofer 2009; Speed 2002; Tornese 2011). Outcome assessors were not blinded in one (3%) study, which was judged at high risk of detection bias (Loew 1999). It was unclear if assessors were blinded in five (15%) trials (Duymaz 2019; Hearnden 2009; Kim 2014; Perlick 2003; Rompe 1998).
Incomplete outcome data
We rated 22 (68%) trials at low risk of attrition bias because they had no dropouts or the losses to follow‐up, exclusions or attrition was sufficiently small that it was unlikely to have biased the results (Albert 2007; Duymaz 2019; Engebretsen 2009; Farr 2011; Frizziero 2017; Galasso 2012; Haake 2002; Hearnden 2009; Hsu 2008; Kvalvaag 2017; Li 2017; Loew 1999; Melegati 2000; Pan 2003; Perlick 2003; Peters 2004; Rompe 1998; Sabeti 2007; Sabeti‐Aschraf 2005; Schmitt 2001; Schofer 2009; Tornese 2011). In eight (26%) trials there was differential dropout across groups or reasons for drop out were related to treatment (e.g. no effect in placebo group) and thus we rated these trials as high risk of attrition bias (Cacchio 2006; Cosentino 2003; De Boer 2017; Del Castillo‐Gonzales 2016; Gerdesmeyer 2003; Ioppolo 2012; Kim 2014; Kolk 2013). The remaining two (6.4%) trials did not clearly report the amount of incomplete outcome data or reasons for incomplete outcome data so the risk of attrition bias was unclear (Pleiner 2004; Speed 2002).
Selective reporting
We rated 18 (56%) trials at low risk of selective reporting bias (Albert 2007; Cacchio 2006; Del Castillo‐Gonzales 2016; Duymaz 2019; Engebretsen 2009; Farr 2011; Frizziero 2017; Galasso 2012; Gerdesmeyer 2003; Haake 2002; Kolk 2013; Melegati 2000; Pan 2003; Pleiner 2004; Sabeti 2007; Sabeti‐Aschraf 2005; Schofer 2009; Tornese 2011). One trial reported all outcomes listed in the study protocol (Galasso 2012). One trial measured several outcomes which were not specified in the ClinicalTrials.gov registry but were added to the publication (e.g. function, active ROM, work status) (Engebretsen 2009). For the other 15 trials, while there was no published study protocol, results were reported for all outcomes measured (as stated in the methods) and included all major outcomes (except for quality of life, which no trial measured) sufficient for us to judge these as having a probable low risk of selective reporting bias (Albert 2007; Cacchio 2006; Del Castillo‐Gonzales 2016; Farr 2011; Frizziero 2017; Gerdesmeyer 2003; Haake 2002; Kolk 2013; Melegati 2000; Pan 2003; Pleiner 2004; Sabeti 2007; Sabeti‐Aschraf 2005; Schmitt 2001; Schofer 2009; Tornese 2011).
We rated four (13%) trials at unclear risk of selective reporting bias due to incomplete reporting of outcomes (Kvalvaag 2017; Li 2017; Schmitt 2001; Speed 2005). One study reported changes from baseline at the follow‐up (Li 2017). The trial protocol for Kvalvaag 2017 stated that return to work and health‐related quality of life were measured as secondary outcomes, but these outcomes were not reported in the results paper. Another trial had a significant number of unexplained dropouts without clear reporting of the number of participants who completed outcome measurements (Speed 2002). In one trial an outcome (treatment success) was possibly added post‐hoc (Schmitt 2001).
We rated 10 (32%) trials at high risk of selective reporting bias, as data were missing for one or more outcomes listed as measured in the methods (Cosentino 2003; De Boer 2017; Hearnden 2009; Loew 1999), or measures of variance were not reported for one or more outcomes (Hsu 2008; Ioppolo 2012; Kim 2014; Perlick 2003; Peters 2004; Rompe 1998).
Other potential sources of bias
Five (16%) trials were at high risk of other bias (De Boer 2017; Engebretsen 2009; Pan 2003; Pleiner 2004; Schmitt 2001). Two trials were at high risk of unit of analysis bias as trialists in both cases did not adjust for the non‐independence between groups due to bilateral treatment (Pan 2003; Pleiner 2004). One trial showed a high risk of bias as it was terminated prematurely because of higher pain in the shock wave group (De Boer 2017). Another trial was at high risk of bias because of imbalance between groups in the number of additional treatments received outside of the trial setting, which may have biased the results in favour of the radial extracorporeal shock wave therapy group (rESWT) (Engebretsen 2009). In another trial, 40% of participants were not satisfied with the allocated treatment and were unmasked and informed of their treatment group, and participants in the placebo group were offered shock wave therapy (Schmitt 2001). The remaining 26 (84%) trials were rated as being free from other potential sources of bias.
Effects of interventions
See: Table 1
Shock wave therapy versus placebo
Twelve studies assessed shock wave therapy (using ESWT) compared to placebo (Cosentino 2003; Galasso 2012; Gerdesmeyer 2003; Hearnden 2009; Hsu 2008; Kolk 2013; Kvalvaag 2017; Li 2017; Peters 2004; Pleiner 2004; Schmitt 2001; Speed 2002).
Major outcomes
Participant‐reported pain relief of 30% or greater
The studies did not report pain relief of 30% or greater but did report pain relief of 50% or greater, which we report below.
Participant‐reported pain relief of 50% or greater
One study reported participant‐reported pain relief of 50% or greater at three months' follow‐up (Speed 2002). Speed 2002 reported that 14/34 participants in the ESWT group and 15/40 participants in the placebo group reported 50% or greater improvement in pain relief, a difference that was not statistically different, but of some uncertainty as it is based on low‐certainty evidence (RR 1.10, 95% CI 0.62 to 1.94; 74 participants), or in absolute terms, 4% more had pain relief (19% fewer to 26% more), and a relative change of 10% (38% fewer to 94% more) (Table 1).
Mean pain
Six trials reported pain at zero to six weeks measured on two scales, a VAS score (Hsu 2008; Li 2017; Pleiner 2004; Schmitt 2001; Speed 2002) and Constant score (Galasso 2012). There was a small statistically significant but clinically uncertain reduction in pain with ESWT compared to placebo at six weeks' follow‐up (SMD –0.75, 95% CI –1.33 to –0.17; I² = 81%; 304 participants; Analysis 1.2). Based on an SD of 1.6 (Gerdesmeyer 2003), this was equivalent to a mean reduction of 1.2 points (95% CI –2.13 to –0.27) on a 0‐ to 10‐point VAS score, where 1.5 points is considered a clinically important difference in pain. Hsu 2008 found a large benefit in favour of shock wave therapy, which appears to be the main contributor to the large heterogeneity; removing data from Hsu 2008 removes the statistical heterogeneity (I² = 0%) without changing the direction of the effect (SMD –0.41, 95% CI –0.66 to –0.16; I² = 0%); this was equivalent to a pain reduction of 0.66 (95% CI –1.06 to –0.26 on a 0‐ to 10‐point scale).
Nine trials reported pain at six weeks to three months on two scales, a VAS score (Gerdesmeyer 2003; Hsu 2008; Kolk 2013; Kvalvaag 2017; Li 2017; Pleiner 2004; Schmitt 2001; Speed 2002) and Constant score (Galasso 2012). Low‐certainty evidence indicated a clinically unimportant reduction in pain with shock wave therapy compared to placebo (SMD –0.49, 95% CI –0.88 to –0.11; I² = 80%; 608 participants; Analysis 1.2). Based on an SD of 1.6 (Gerdesmeyer 2003), this translated to a mean improvement of 0.78 points (95% CI –1.4 to –0.17) on a 0‐ to 10‐point scale; or 7.8% improvement in pain (95% CI 2% to 14%), relative improvement of 14% (95% CI 3% to 25%) and number need to treat for an additional beneficial outcome (NNTB) of 4 (95% CI 2 to 34) (Table 1).
The high heterogeneity was due to the large outlier reported in Hsu 2008; removing these data removed most heterogeneity (SMD –0.36, 95% CI –0.59 to –0.13; I² = 18%) (equivalent to a mean reduction of 0.58 points, 95% CI –0.94 to –0.21 on a 0 to 10 scale).
Five trials reported pain at three to six months using a 0‐ to 10‐point VAS score (higher score indicating more pain) (Gerdesmeyer 2003; Hsu 2008; Kolk 2013; Kvalvaag 2017; Speed 2002). There was no evidence of a between‐group difference in pain (MD ‐1.53, 95% CI ‐3.49 to 0.43) I2 = 90%; 419 participants; Analysis 1.2).
Three trials reported pain at six to 12 months using a 0‐ to 10‐point VAS score (higher score indicating more pain) (Gerdesmeyer 2003; Hsu 2008; Pleiner 2004). There was no evidence of a between‐group difference in pain (MD –2.42, 95% CI –5.79 to 0.95; I² = 95%; 155 participants; Analysis 1.2). The high heterogeneity was due to the large outlier reported in Hsu 2008; removing these data removed all heterogeneity (MD –0.75, 95% CI –1.62 to 0.13; I² = 0%).
For the subgroup analysis comparing outcomes for participants with and without calcification, we pooled six‐week to three‐month data from five studies of people with calcific deposits (Gerdesmeyer 2003; Hsu 2008; Kolk 2013; Kvalvaag 2017; Pleiner 2004; 256 participants) and five studies of people without calcific deposits (Galasso 2012; Kolk 2013; Li 2017; Schmitt 2001; Speed 2002; 253 participants). Subgroups did not appear to differ with respect to mean pain (with calcific deposits: SMD –0.59, 95% CI –1.33 to 0.14; 256 participants; without calcific deposits: SMD –0.39, 95% CI –0.70 to –0.09; 253 participants; test for subgroup differences: Chi² = 0.25, df = 1 (P = 0.62), I² = 0% despite the 'without calcification' group achieving statistical significance; Analysis 1.11).
1.11. Analysis.
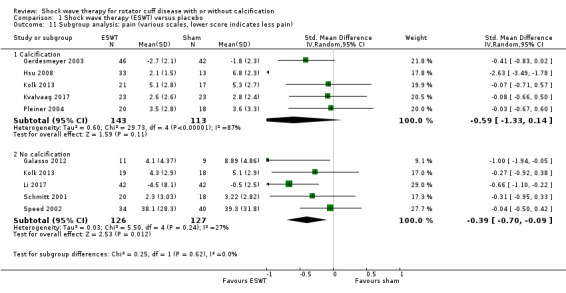
Comparison 1 Shock wave therapy (ESWT) versus placebo, Outcome 11 Subgroup analysis: pain (various scales, lower score indicates less pain).
In the sensitivity analyses for pain at six weeks to three months, removing studies at risk of selection bias or detection bias did not alter the findings substantially.
Removal of five studies with possible selection bias (Galasso 2012; Hsu 2008; Kolk 2013; Pleiner 2004; Speed 2002) changed the effect size from SMD –0.66 (95% CI –1.14 to –0.18; I² = 81%; 8 studies, 465 participants) to SMD –0.49 (95% CI –0.76 to –0.21; I² = 0%; 2 studies, 210 participants).
Removal of five studies with possible detection bias (Gerdesmeyer 2003; Hsu 2008; Kolk 2013; Pleiner 2004; Speed 2002) changed the effect size from SMD –0.66 (95% CI –1.14 to –0.18; I² = 81%; 8 studies, 465 participants) to SMD –0.61 (95% CI –0.94 to –0.27; I² = 0%; 3 studies, 142 participants).
Function
Ten trials reported mean function using the Constant score (lower score is worse) (Cosentino 2003; Galasso 2012; Gerdesmeyer 2003; Hearnden 2009; Hsu 2008; Kolk 2013; Kvalvaag 2017; Li 2017; Pleiner 2004; Schmitt 2001), and one trial used the SPADI score (lower score is better) (Speed 2002). We changed the direction of the SPADI scores to 0 to 100 score with a higher score indicating better function.
Seven trials reported function at zero to six weeks (Cosentino 2003; Galasso 2012; Hsu 2008; Li 2017; Pleiner 2004; Schmitt 2001; Speed 2002). There was a statistically significant improvement in function when comparing ESWT to placebo at six weeks' follow‐up (SMD 0.79, 95% CI 0.30 to 1.28; I² = 79%; 374 participants; Analysis 1.3). Using the SD of 12.8 from Gerdesmeyer 2003, this is equivalent to a mean increase of 10.11 points (95% CI 3.84 to 16.38) on a 0‐ to 100‐point scale. The clinical importance of this improvement was uncertain as the 95% CIs included both a clinically important (greater than 10‐point) increase and clinically unimportant (less than 10 points) change.
Nine trials reported function at six weeks to three months (Galasso 2012; Gerdesmeyer 2003; Hsu 2008; Kolk 2013; Kvalvaag 2017; Li 2017; Pleiner 2004; Schmitt 2001; Speed 2002). Based on low‐certainty evidence, there was a statistically significant improvement of uncertain clinical importance in function when comparing ESWT to placebo at three months' follow‐up (SMD 0.62, 95% CI 0.13 to 1.11; I² = 88%; 612 participants; Analysis 1.3). Using the SD of 12.8 from Gerdesmeyer 2003, this translated to a mean increase of 7.93 points (95% CI 1.66 to 14.2) on a 0‐ to 100‐point scale, an absolute improvement of 8% (95% CI 1.6% to 14%), relative improvement of 12% (95% CI 3% to 22%), or NNTB of 3 (95% CI 2 to 18) (Table 1). Removal of the extreme outlier reported in Hsu 2008 reduced heterogeneity to a moderate level (I² = 47%), and removed any clinical significance from the results (SMD 0.26, 95% CI –0.00 to 0.52; I² = 56%), translating to a mean improvement of 3.33 points on a 0‐ to 100‐point scale (95% CI 0.00 to 6.65).
Seven trials reported function at three to six months (Cosentino 2003; Gerdesmeyer 2003; Hearnden 2009; Hsu 2008; Kolk 2013; Kvalvaag 2017; Speed 2002). There was a statistically significant but clinically unimportant improvement in function favouring the ESWT group (SMD 0.91, 95% CI 0.24 to 1.57; I² = 91%; 471 participants; Analysis 1.3). Using the SD of 12.8 from Gerdesmeyer 2003, this translated to a mean increase on the Constant scale of 11.65 points (95% CI 3.07 to 20.1).
Three trials reported function at six to 12 months (Gerdesmeyer 2003; Hsu 2008; Pleiner 2004). There was no evidence of a between‐group difference in function measured using the Constant score (MD 15.18, 95% CI –2.55 to 32.91; I² = 94%; 155 participants). The significant heterogeneity was largely due to Hsu 2008; removal of these more extreme data reduced the heterogeneity to a likely unimportant level, without changing the direction of the effect (MD 6.51, 95% CI –0.07 to 13.10; I² = 20%).
For the subgroup analysis comparing outcomes for participants with and without calcification, we pooled six week to three month data from five trials that included people with calcific deposits (Gerdesmeyer 2003; Hsu 2008; Kolk 2013; Kvalvaag 2017; Pleiner 2004) and five trials including people without calcific deposits (Galasso 2012; Kolk 2013; Li 2017; Schmitt 2001; Speed 2002). Subgroups did not appear to differ with respect to mean function (with calcific deposits: SMD 0.84, 95% CI –0.20 to 1.89; 260 participants; without calcific deposits: SMD 0.29, 95% CI –0.04 to 0.61; 253 participants; test for subgroup differences: Chi² = 1.00, df = 1 (P = 0.32), I² = 0.1%; Analysis 1.12).
1.12. Analysis.
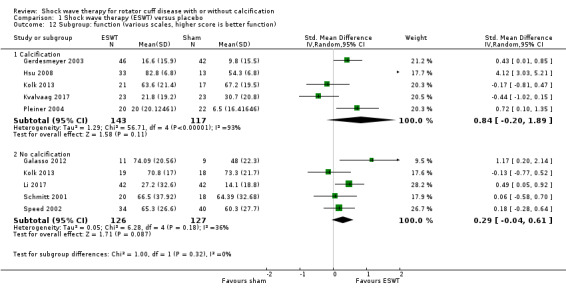
Comparison 1 Shock wave therapy (ESWT) versus placebo, Outcome 12 Subgroup: function (various scales, higher score is better function).
In the sensitivity analyses for function at six weeks to three months, removing studies at risk of selection bias or detection bias did not alter the effect size dramatically.
Removal of five studies with possible selection bias (Galasso 2012; Hsu 2008; Kolk 2013; Pleiner 2004; Speed 2002) changed the effect size and eliminated the slight between‐group statistical difference from SMD 0.74 (95% CI 0.18 to 1.31; I² = 88%; 8 studies, 469 participants) to SMD 0.38 (95% CI 0.11 to 0.66; I² = 0%; 3 studies, 210 participants) at six weeks to three months.
Removal of five studies with possible detection bias (Gerdesmeyer 2003; Hsu 2008; Kolk 2013; Pleiner 2004; Speed 2002) changed the effect size and eliminated the slight between‐group statistical difference from SMD 0.74 (95% CI 0.18 to 1.31; I² = 88%; 8 studies, 469 participants) to SMD 0.48 (95% CI –0.02 to 0.97; I² = 45%; 3 studies, 142 participants) at six weeks to three months.
Participant‐reported success
Six trials reported treatment success (Galasso 2012; Gerdesmeyer 2003; Hearnden 2009; Peters 2004; Schmitt 2001; Speed 2002). Low‐certainty evidence indicated there may be no statistical difference in the number reporting success: 255 per 1000 participants reported success with placebo and 405 per 1000 reported success with shock wave therapy (RR 1.59, 95% CI 0.87 to 2.91; I² = 53%; 287 participants; Analysis 1.4), or 15% more (3% fewer to 49% more) participants had success with shock wave therapy, a relative increase of 59% (13% fewer to 191% more) (Table 1).
1.4. Analysis.
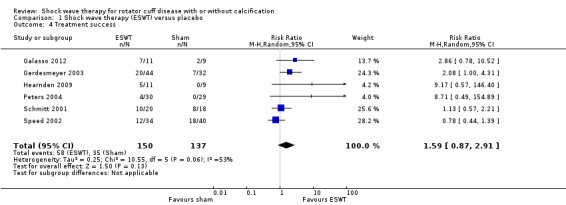
Comparison 1 Shock wave therapy (ESWT) versus placebo, Outcome 4 Treatment success.
Quality of life
None of the trials reported quality of life.
Number of participant withdrawals
Withdrawals specifically due to adverse events were not well reported across studies. Three trials reported that there were no withdrawals for any reasons (Galasso 2012; Peters 2004; Schmitt 2001; 167 participants), while only one study explicitly reported a withdrawal due to an adverse event, namely, a single participant withdrew due to intolerance of the shock wave therapy (Speed 2002). Kvalvaag 2017 reported that four participants withdrew from each group with two discontinuing intervention in the shock wave group and three discontinuing intervention in the placebo group due to an adverse event. Cosentino 2003 reported that 23/35 participants dropped out from the placebo group at six months' follow‐up, without reporting the reasons, and also did not explicitly report if any participants dropped out from the shock wave group. Therefore, we could not include data from this study in the analysis.
For withdrawals due to adverse events or treatment intolerance, seven trials provided low‐certainty evidence (Gerdesmeyer 2003;Kolk 2013; Kvalvaag 2017; Li 2017; Peters 2004; Pleiner 2004; Speed 2002). There was no between‐group difference in withdrawals, 103 per 1000 withdrawals in the placebo group compared with 77 per 1000 in the shock wave therapy group (RR 0.75, 95% CI 0.43 to 1.31; I² =0%; 581 participants; Analysis 1.5), an absolute difference of 3% less events (6% less to 3% more), or a relative change of 25% less (57% less to 31% more) (Table 1). One participant in the shock wave group withdrew due to intolerance of the therapy, while other 10 participants who withdrew from active treatment offered no reason. From the placebo group, one participant withdrew due to deteriorating symptoms and a further 12 did not complete treatment but offered no reason for withdrawing.
1.5. Analysis.
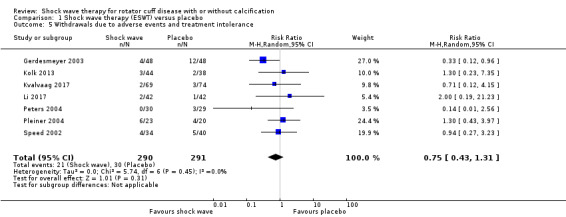
Comparison 1 Shock wave therapy (ESWT) versus placebo, Outcome 5 Withdrawals due to adverse events and treatment intolerance.
Number of participants experiencing any adverse event
Several trials reported adverse events incompletely. Cosentino 2003 and Pleiner 2004 explicitly reported that there were zero adverse events in either treatment group, although Cosentino 2003 also reported that there was transient treatment pain associated with shock wave therapy without reporting the number of people who had the event. Hsu 2008 reported transient treatment‐associated pain treated with ice and paracetamol, but did not report the number of participants with the event. Hearnden 2009 reported bruising in 7/11 (62%) participants in the shock wave group, but did not report if participants in the placebo group had any adverse events. Thus treatment‐related pain from these three studies could not be included in the meta‐analysis.
Five trials provided data on the number of participants per treatment group with adverse events for a meta‐analysis (Galasso 2012; Gerdesmeyer 2003; Hsu 2008; Peters 2004; Speed 2002). Low‐certainty evidence indicated no between‐group difference in the proportion of people with adverse events, 72 per 1000 in the placebo group compared with 260 per 1000 in the shock wave therapy group (RR 3.61, 95% CI 2.00 to 6.52; 295 participants; Analysis 1.7), an absolute change of 19% more adverse events with shock wave therapy (7% more to 40% more), or a relative change of 261% more (100% more to 552% more) (Table 1).
1.7. Analysis.
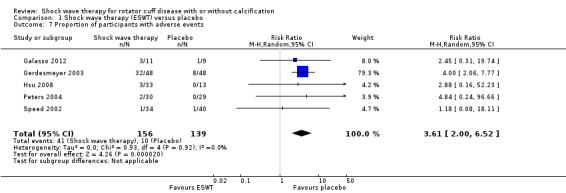
Comparison 1 Shock wave therapy (ESWT) versus placebo, Outcome 7 Proportion of participants with adverse events.
The type of adverse events included: pain associated with shock wave therapy or placebo treatment (Cosentino 2003; Galasso 2012; Gerdesmeyer 2003; Hsu 2008; Peters 2004; Speed 2002); localised redness, bleeding or bruising (Gerdesmeyer 2003; Hearnden 2009; Hsu 2008; Peters 2004); and increased shoulder pain following treatment (Peters 2004).
Minor outcomes
Proportion of participants achieving pain score below 30/100 mm on Visual Analogue Scale
None of the trials reported proportion of participants achieving pain score below 30/100 mm on VAS.
Range of movement
None of the trials reported ROM.
Calcification size: number with complete resolution
Four trials reported number of participants with complete resolution of calcium deposits (Cosentino 2003; Hsu 2008; Peters 2004; Pleiner 2004; 218 participants). Peters 2004 (59 participants) reported that no participants in either treatment group had complete resolution of deposits and was not included in the analysis. Based on the other three trials, there was a statistically significant increase in the number of calcium deposits which completely resolved with ESWT compared to placebo although this is of uncertain clinical importance (RR 4.78, 95% CI 1.31 to 17.39; 159 participants; Table 3; Analysis 1.8).
2. Shock wave therapy versus placebo secondary outcomes.
| Outcome | Number of studies |
Number of participants: shock wave |
Number of participants: placebo |
Statistic random‐effects Mantel‐Haenszel |
Effect estimate (95% CI) |
| Proportion achieving pain score below 30/100 mm on VAS | 0 | Not reported | Not reported | Not reported | Not reported |
| Range of movement | 0 | Not reported | Not reported | Not reported | Not reported |
| Mean change in calcification width (mm) at 3 months | 1 | 46 | 42 | Mean difference (95% CI) |
–26.00 (–85.77 to 33.77) |
| Proportion with complete calcification resolution | 3 | 91 | 68 | Risk ratio (95% CI) |
4.78 (1.31 to 17.39) |
| Proportion with partial calcification partial resolution | 3 | 91 | 68 | Risk ratio (95% CI) |
3.41 (0.95 to 12.23) |
CI: confidence interval; VAS: Visual Analogue Scale.
1.8. Analysis.
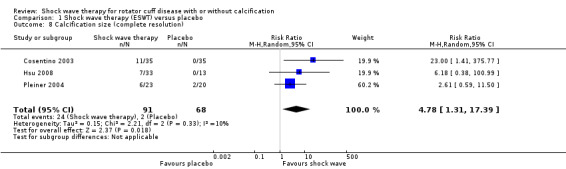
Comparison 1 Shock wave therapy (ESWT) versus placebo, Outcome 8 Calcification size (complete resolution).
Calcification size: number with partial resolution
Four trials reported the number of participants with partial resolution of calcium deposits (Cosentino 2003; Hsu 2008; Peters 2004; Pleiner 2004: 218 participants). Peters 2004 (59 participants) reported that no participants in either treatment group had partial resolution of deposits and was not included in the analysis. Based upon the other three trials, there was no statistically significant difference in the number of calcium deposits which partially resolved in the ESWT group compared to the placebo group (RR 3.41, 95% CI 0.95 to 12.23; 159 participants; Table 3; Analysis 1.9).
1.9. Analysis.
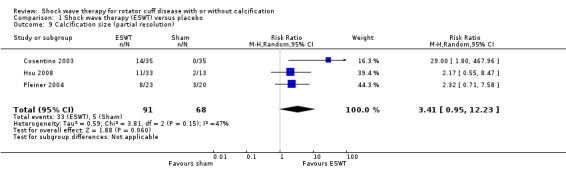
Comparison 1 Shock wave therapy (ESWT) versus placebo, Outcome 9 Calcification size (partial resolution).
Calcification size: mean or change in mean calcification size
One trial reported mean calcification width at six weeks to three months (Gerdesmeyer 2003). Mean change in size was 56.3 mm in the treatment group compared with 30.3 mm in the placebo group, which was not statistically different (MD –26.00, 95% CI –85.77 to 33.77; 88 participants; Table 3; Analysis 1.10).
1.10. Analysis.
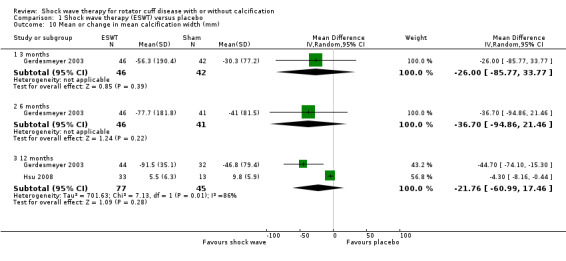
Comparison 1 Shock wave therapy (ESWT) versus placebo, Outcome 10 Mean or change in mean calcification width (mm).
One trial reported mean change in calcification size at three to six months (Gerdesmeyer 2003; 46 participants). Mean change was –77.7 mm in the treatment group and –41 mm in the placebo group, which was not statistically different (MD –36.70, 95% CI –94.86 to 21.46; 87 participants; Table 3; Analysis 1.10).
Two trials reported mean calcification width at six to 12 months (Gerdesmeyer 2003; Hsu 2008). Mean change was 5.5 mm in the ESWT group and 9.8 mm in the placebo group, which was not statistically significantly different (MD –21.76, 95% CI –60.99 to 17.46; I² = 86%; 122 participants; Table 3; Analysis 1.10).
Shock wave therapy versus no treatment
One study compared shock wave therapy versus no treatment (Loew 1999).
Major outcomes
Function
There was no between‐group difference in function (Constant score) at three months (mean function: 51.6 in the shock wave group and 47.8 in the no treatment group; MD 3.80, 95% CI –6.33 to 13.93; 40 participants; Analysis 2.1).
2.1. Analysis.
Comparison 2 Shock wave therapy (ESWT) versus no treatment, Outcome 1 Mean function (Constant score 0–100, 100 indicating best).
Participant‐reported success
At the end of the trial, there was no between‐group difference in the number of participants who reported that the treatment was successful (6/20 participants in the shock wave group versus 1/20 participants in the no treatment group; RR 6.00, 95% CI 0.79 to 45.42; Analysis 2.2).
2.2. Analysis.
Comparison 2 Shock wave therapy (ESWT) versus no treatment, Outcome 2 Treatment success as determined by participant.
Other major outcomes
The study did not report participant‐reported pain relief of 30% or greater, mean pain, participant‐reported success, quality of life, number of participant withdrawals and number of participants experiencing any adverse event.
Minor outcomes
Number of participants with complete resolution of calcific deposits
At the end of the trial, there were no between‐group differences in the number of participants who had achieved complete resolution of calcific deposits (4/20 participants in the shock wave group versus 2/20 participants in the no treatment group; RR 2.00, 95% CI 0.41 to 9.71; Analysis 2.3).
2.3. Analysis.
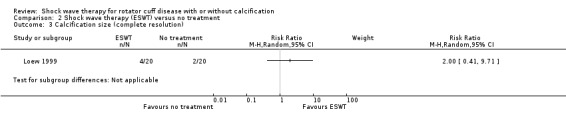
Comparison 2 Shock wave therapy (ESWT) versus no treatment, Outcome 3 Calcification size (complete resolution).
Other minor outcomes
The study did not report proportion of participants achieving pain score below 30/100 mm on VAS, ROM or effect of ESWT on the size of the calcification.
Shock wave therapy versus ultrasound‐guided needling with glucocorticoid
One study assessed ESWT versus ultrasound‐guided needling with glucocorticoid (Kim 2014).
Major outcomes
Mean pain
The study incompletely reported mean pain (no measures of variance), therefore, we could not extract or substantiate these data. The authors reported a greater improvement in pain and function with ultrasound‐guided needling than with shock wave therapy.
Function
The study incompletely reported function (no measures of variance), therefore, we could not extract or substantiate these data. The authors reported a greater improvement in pain and function with ultrasound‐guided needling than with shock wave therapy.
Other major outcomes
The study did not report participant reported pain relief of 30% or greater, participant‐reported success, quality of life, number of withdrawals due to adverse events and number of participants experiencing any adverse event.
Minor outcomes
Calcification size: mean calcification width
Mean calcification width decreased in both groups but the difference favoured glucocorticoid needling (mean calcification size was 5.6 mm in the shock wave group versus 0.45 mm in the glucocorticoid needling group; MD 5.15, 95% CI 4.84 to 5.46; 54 participants; Analysis 3.1). This difference is of uncertain clinical importance.
3.1. Analysis.
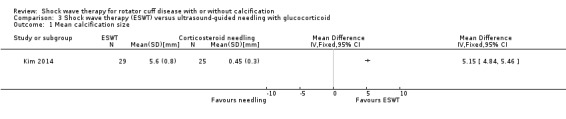
Comparison 3 Shock wave therapy (ESWT) versus ultrasound‐guided needling with glucocorticoid, Outcome 1 Mean calcification size.
Calcification size: number with complete resolution
Complete resolution of calcific deposits occurred less frequently in the shock wave therapy group (12/29 participants in the shock wave group versus 18/25 participants in the glucocorticoid needling group; RR 0.57, 95% CI 0.35 to 0.95; Analysis 3.2). This difference is of uncertain clinical importance.
3.2. Analysis.
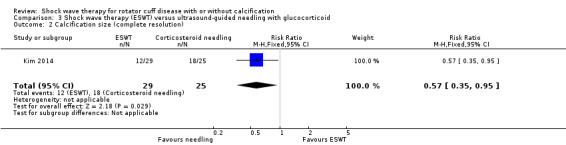
Comparison 3 Shock wave therapy (ESWT) versus ultrasound‐guided needling with glucocorticoid, Outcome 2 Calcification size (complete resolution).
Calcification size: number with partial resolution
There was no between‐group difference in the number of participants who had partial resolution of calcific deposits (5/29 participants in the shock wave group versus 3/25 participants in the needling group; RR 1.44, 95% CI 0.38 to 5.42; Analysis 3.3).
3.3. Analysis.
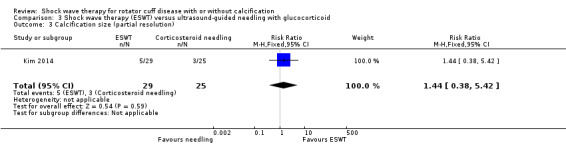
Comparison 3 Shock wave therapy (ESWT) versus ultrasound‐guided needling with glucocorticoid, Outcome 3 Calcification size (partial resolution).
Other minor outcomes
The study did not report proportion of participants achieving pain score below 30/100 mm on VAS and ROM.
Radial shock wave therapy versus ultrasound‐guided needling with glucocorticoids
One study assessed RSWT versus ultrasound‐guided needling with glucocorticoids (De Boer 2017).
Major outcomes
Pain
At six weeks to three months, there was a statistically significant and clinically important increase in mean pain (NRS 0 to 10, higher score indicating greater pain) in participants who received RSWT compared to participants who underwent ultrasound‐guided needling with glucocorticoids (MD 1.60, 95% CI 0.13 to 3.07; 25 participants; Analysis 4.1).
4.1. Analysis.
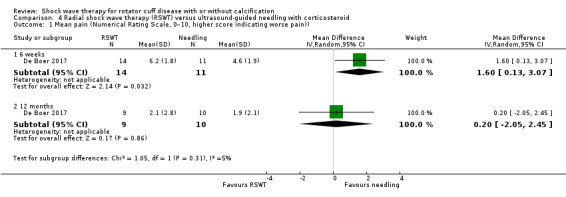
Comparison 4 Radial shock wave therapy (RSWT) versus ultrasound‐guided needling with corticosteroid, Outcome 1 Mean pain (Numerical Rating Scale, 0–10, higher score indicating worse pain)).
At 12 months and greater, there was no statistically significant or clinically important change in mean pain (NRS 0 to 10, higher score indicating greater pain) in participants who received RSWT compared to participants who underwent ultrasound‐guided needling with glucocorticoids (MD 0.20, 95% CI –2.05 to 2.45; 19 participants; Analysis 4.1).
Function
At six weeks to three months, there was no statistically significant or clinically important change in mean function (Constant score 0 to 100, higher score indicating better function or Oxford score, 12 to 60 with a higher score indicating better function) in participants who received RSWT compared to participants who underwent ultrasound‐guided needling with glucocorticoids (Constant score: MD –11.70, 95% CI –24.79 to 1.39; 25 participants; Analysis 4.2; Oxford score: MD –2.30; 95% CI –9.30 to 4.70; 25 participants; Analysis 4.3).
4.2. Analysis.
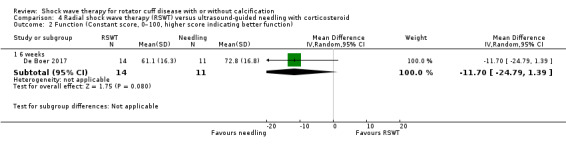
Comparison 4 Radial shock wave therapy (RSWT) versus ultrasound‐guided needling with corticosteroid, Outcome 2 Function (Constant score, 0–100, higher score indicating better function).
4.3. Analysis.
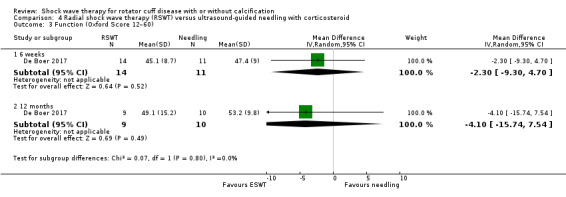
Comparison 4 Radial shock wave therapy (RSWT) versus ultrasound‐guided needling with corticosteroid, Outcome 3 Function (Oxford Score 12–60).
At 12 months and greater, there was no statistically significant or clinically important change in mean function (Oxford score, 12 to 60, higher score indicating better function) in participants who received RSWT compared to participants who underwent ultrasound‐guided needling with glucocorticoids (MD –4.10, 95% CI –15.74 to 7.54; 19 participants; Analysis 4.3).
Participant‐reported success
At the end of the trial, there was no difference in treatment success (proportion of participants with no complaints) in participants who received RSWT compared to participants who underwent ultrasound‐guided needling with glucocorticoids (4/9 participants with RSWT versus 4/10 participants with ultrasound‐guided needling with glucocorticoids; RR 1.11, 95% CI 0.39 to 3.19; Analysis 4.4).
4.4. Analysis.

Comparison 4 Radial shock wave therapy (RSWT) versus ultrasound‐guided needling with corticosteroid, Outcome 4 Treatment success (proportion of participants with no complaints).
Number of participants experiencing any adverse event
At the end of the trial, there was no difference in the proportion of participants with adverse events in participants who received RSWT compared to participants who underwent ultrasound‐guided needling with glucocorticoids (5/14 participants with RSWT versus 1/11 participants with ultrasound‐guided needling with glucocorticoids; RR 3.93, 95% CI 0.53 to 28.93; Analysis 4.5).
4.5. Analysis.
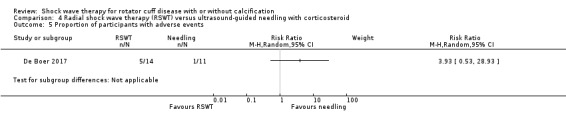
Comparison 4 Radial shock wave therapy (RSWT) versus ultrasound‐guided needling with corticosteroid, Outcome 5 Proportion of participants with adverse events.
Other major outcomes
The trial did not report participant‐reported pain relief of 30% or greater and quality of life. There were no withdrawals listed due to adverse events.
Minor outcomes
Calcification size
At the end of the trial, there was no difference in the calcification size (number with complete resolution) in participants who received RSWT compared to participants who underwent ultrasound‐guided needling with glucocorticoids (1/14 participants with RSWT versus 5/11 participants with ultrasound‐guided needling with glucocorticoids; RR 0.16, 95% CI 0.02 to 1.16; Analysis 4.6).
4.6. Analysis.
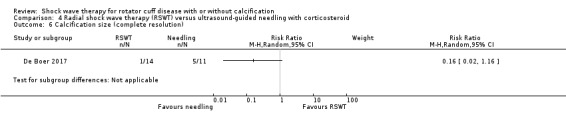
Comparison 4 Radial shock wave therapy (RSWT) versus ultrasound‐guided needling with corticosteroid, Outcome 6 Calcification size (complete resolution).
Radial shock wave therapy versus supervised exercise
One study assessed rESWT versus supervised exercises (Engebretsen 2009).
Major outcomes
Pain
There was no between‐group differences in mean pain (Likert 0 to 9, 9 indicating severe pain) at any time point (six weeks: 2.9 with shock wave versus 2.6 with supervised exercises; MD 0.30, 95% CI –0.53 to 1.13; 90 participants; six weeks to three months: 2.9 with shock wave versus 2.5 with supervised exercises; MD 0.40, 95% CI –0.36 to 1.16; 102 participants; three to six months: 2.7 with shock wave versus 2.5 with supervised exercises; MD 0.20, 95% CI –0.56 to 0.96; 100 participants; one year: 2.6 with shock wave versus 2.1 with supervised exercises; MD 0.50, 95% CI –0.20 to 1.2; 97 participants; Analysis 5.1).
5.1. Analysis.
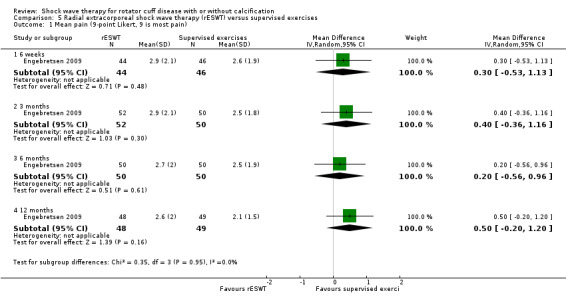
Comparison 5 Radial extracorporeal shock wave therapy (rESWT) versus supervised exercises, Outcome 1 Mean pain (9‐point Likert, 9 is most pain).
Function
There was no between‐group differences in mean function (SPADI 0 to 100, 100 indicating worst function) at any time point (six weeks: 33.5 with shock wave versus 25.8 with supervised exercises; MD 7.70, 95% CI –1.57 to 16.97; 90 participants; six weeks to three months: 36.1 with shock wave versus 27.0 with supervised exercises; MD 9.10, 95% CI –1.13 to 19.33; 102 participants; three to six months: 29.2 with shock wave versus 24.5 with supervised exercises; MD 4.70, 95% CI –5.39 to 14.79; 100 participants; 12 months: 27.9 with shock wave versus 24.0 with supervised exercises; MD 3.90, 95% CI –6.08 to 13.88; 97 participants; Analysis 5.2).
5.2. Analysis.
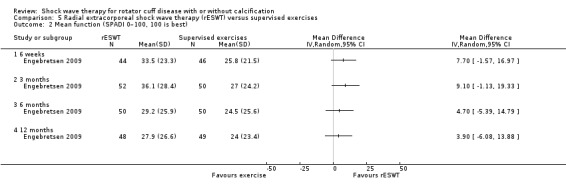
Comparison 5 Radial extracorporeal shock wave therapy (rESWT) versus supervised exercises, Outcome 2 Mean function (SPADI 0–100, 100 is best).
Number of participant withdrawals
There was no between‐group difference in withdrawals due to adverse events, but the event rates were too low to be certain (2/52 participants with shock wave versus 1/50 participants with supervised exercise; (RR 3.00, 95% CI 0.32 to 27.91; 104 participants; one study; Analysis 5.3). Withdrawal of one participant from the supervised exercise group was due to increased pain and stiffness consistent with adhesive capsulitis and the two withdrawals from the shock wave group were due to aggravation of pain.
5.3. Analysis.
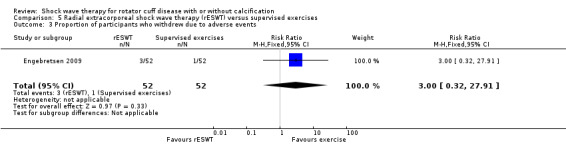
Comparison 5 Radial extracorporeal shock wave therapy (rESWT) versus supervised exercises, Outcome 3 Proportion of participants who withdrew due to adverse events.
Number of participants experiencing any adverse event
Adverse events included frozen shoulder (two in the exercise group, one in the shock wave group); polymyalgia rheumatica (one in the exercise group); depression (one in the shock wave group); aggravation of pain (two in the shock wave group, crossed over to exercise), and one participant from shock wave group had surgery (unreported if this was due to an adverse event or inefficacy; we have included this as an adverse event). Total adverse events did not differ statistically between groups (5/52 participants with shock wave versus 3/50 participants with supervised exercise (RR 1.60, 95% CI 0.40 to 6.36; Analysis 5.4).
5.4. Analysis.
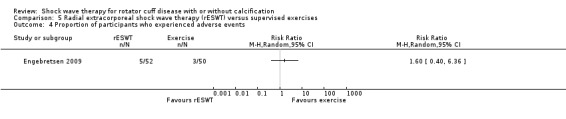
Comparison 5 Radial extracorporeal shock wave therapy (rESWT) versus supervised exercises, Outcome 4 Proportion of participants who experienced adverse events.
Other major outcomes
The study did not report participant‐reported pain relief of 30% or greater, participant‐reported success and quality of life.
Minor outcomes
Range of movement
There was no between‐group difference in mean active abduction (measured in degrees, data supplied by the trial authors) at any time point (six weeks to three months: 167.65 degrees with shock wave versus 169.6 degrees with supervised exercise group; MD –1.95 degrees, 95% CI –10.50 to 6.60; three to six months: 154.78 degrees with shock wave versus 166.6 degrees with supervised exercise; MD –11.82 degrees, 95% CI –25.37 to 1.73; Analysis 5.5). Data were not reported at one year.
5.5. Analysis.
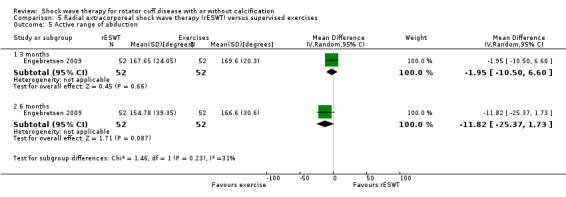
Comparison 5 Radial extracorporeal shock wave therapy (rESWT) versus supervised exercises, Outcome 5 Active range of abduction.
Other minor outcomes
The trial did not report proportion of participants achieving pain score below 30/100 mm on a VAS, size of the calcification and number of participants with complete or partial resolution.
Shock wave therapy plus exercise and advice versus exercise and advice alone
One study assessed ESWT plus a supervised exercise programme (called kinesitherapy) and advice versus kinesitherapy and advice alone (Melegati 2000).
Major outcomes
Function
At six to 12 months, there was a statistically significant but clinically unimportant improvement in function in the shock wave plus exercise and advice group compared to the exercise and advice control group (Constant score: 74.5 with shock wave plus exercise and advice versus 65.15 with exercise and advice control; MD 9.35, 95% CI 4.98 to 13.72; 60 participants; Analysis 13.1).
13.1. Analysis.
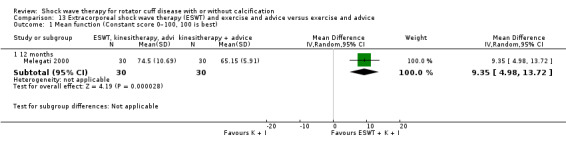
Comparison 13 Extracorporeal shock wave therapy (ESWT) and exercise and advice versus exercise and advice, Outcome 1 Mean function (Constant score 0–100, 100 is best).
Other major outcomes
The study did not report participant‐reported pain relief of 30% or greater, mean pain, participant‐reported success of treatment, quality of life, number of participant withdrawals and number of participants experiencing any adverse event.
Minor outcomes
The study did not report proportion of participants achieving pain score below 30/100 mm on VAS, ROM, effect of ESWT on the size of the calcification and number of participants with complete or partial resolution.
Shock wave therapy versus transcutaneous electrical nerve stimulation
One study compared ESWT to TENS (Pan 2003).
Major outcomes
Pain
At six weeks, the MD in pain (measured by 0‐ to 10‐point VAS, higher score indicating more pain) favoured shock wave therapy but the CIs indicated that this may or may not be of clinical importance (pain improvement: 3 points with shock wave therapy versus 1.1 points with TENS; MD –1.90, 95% CI –2.98 to –0.82; 62 participants; Analysis 7.1). At three months, there was a clinically important difference in pain in favour of shock wave therapy (–4.08 points with ESWT versus –1.74 points with TENS; MD –2.34, 95% CI –3.53 to –1.15; 62 participants; Analysis 7.1).
7.1. Analysis.
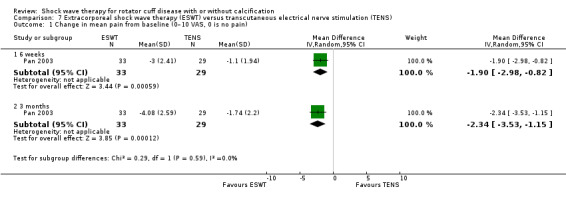
Comparison 7 Extracorporeal shock wave therapy (ESWT) versus transcutaneous electrical nerve stimulation (TENS), Outcome 1 Change in mean pain from baseline (0–10 VAS, 0 is no pain).
Function
At six weeks, the MD in function (measured by Constant score) favoured shock wave therapy but the CIs indicated that this may or may not be of clinical importance (mean function improvement: 24.12 points with shock wave versus 9.59 points with TENS; MD 14.53, 95% CI 8.70 to 20.36; 62 participants; Analysis 7.2). At three months, there was a clinically important difference in function favouring shock wave therapy (mean function improvement: 28.31 points with shock wave versus 11.86 points with TENS; MD 16.45, 95% CI 9.86 to 23.04; 62 participants; Analysis 7.2).
7.2. Analysis.
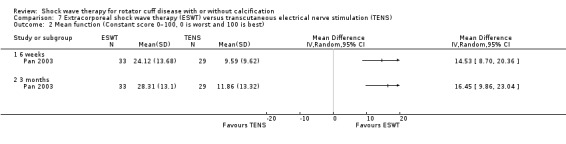
Comparison 7 Extracorporeal shock wave therapy (ESWT) versus transcutaneous electrical nerve stimulation (TENS), Outcome 2 Mean function (Constant score 0–100, 0 is worst and 100 is best).
Number of participant withdrawals
There was only one withdrawal due to severe pain from the TENS group. It was not clearly reported if the pain was due to the TENS treatment (or due to the shoulder disorder). The difference between groups was not statistically significant, but there were too few events to be conclusive (0/33 participants with shock wave versus 1/29 participants with TENS; RR 0.29, 95% CI 0.01 to 6.95; Analysis 7.3).
7.3. Analysis.
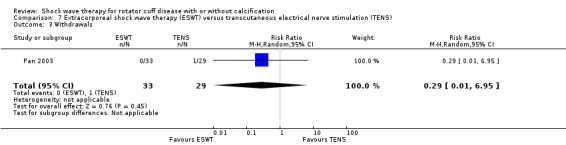
Comparison 7 Extracorporeal shock wave therapy (ESWT) versus transcutaneous electrical nerve stimulation (TENS), Outcome 3 Withdrawals.
Number of participants experiencing any adverse event
Reported adverse events included soreness due to the shock wave therapy (five participants) or pain, possibly due to TENS (one participant), anxiety resulting in heart palpitations in the shock wave group (one participant). No haematomas or paraesthesia were reported. There were no statistical differences between the number of participants who experienced an adverse event, but there were too few events to be certain (6/33 participants with shock wave versus 1/29 with TENS; RR 5.27, 95% CI 0.67 to 41.00; Analysis 7.4).
7.4. Analysis.
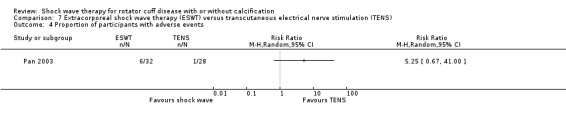
Comparison 7 Extracorporeal shock wave therapy (ESWT) versus transcutaneous electrical nerve stimulation (TENS), Outcome 4 Proportion of participants with adverse events.
Other major outcomes
The study did not report participant‐reported pain relief of 30% or greater, participant‐reported success and quality of life were not reported.
Minor outcomes
Calcification size: mean calcification width
At six weeks, there was a greater reduction in mean width of calcific deposits in the shock wave therapy group (mean change: –3.16 mm with shock wave versus –0.75 mm with TENS; MD –2.41, 95% CI –3.94 to –0.88; 62 participants; Analysis 7.5). This is of unknown clinical relevance.
7.5. Analysis.
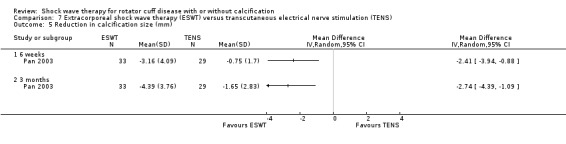
Comparison 7 Extracorporeal shock wave therapy (ESWT) versus transcutaneous electrical nerve stimulation (TENS), Outcome 5 Reduction in calcification size (mm).
At six weeks to three months, there was a greater reduction in mean width of calcific deposits in the shock wave therapy group (mean change: –4.39 mm with shock wave versus –1.65 mm with TENS; MD –2.74, 95% CI –4.39 to –1.09; 62 participants; Analysis 7.5). This is of unknown clinical relevance.
Other minor outcomes
The study did not report proportion of participants achieving pain score below 30/100 mm on VAS, ROM and complete or partial resolution of calcification.
Comparisons of different parameters of shock wave therapy
High‐dose versus low‐dose shock wave therapy
Eleven studies compared high‐dose to low‐dose shock wave therapy (Albert 2007; Cacchio 2006; Farr 2011; Gerdesmeyer 2003; Ioppolo 2012; Loew 1999; Perlick 2003; Peters 2004; Rompe 1998; Sabeti 2007; Schofer 2009).
Major outcomes
Participant reported pain relief of 30% or greater
None of the trials reported participant reported pain relief of 30% of greater.
Pain
Two trials reported pain at six weeks (Cacchio 2006; Farr 2011). There was a slight improvement in pain that favoured high‐dose shock wave therapy (mean pain on a 0‐ to 10‐point VAS, 10 indicating most pain: 2 points with high‐dose versus 5 points with low‐dose; SMD ‐1.73, 95% CI ‐3.94 to 0.48; 117 participants; I2 = 95%; Analysis 8.1). Although the 95% CIs included both a clinically important and a clinically unimportant pain reduction (assuming a clinically important difference is 1.5 points), the clinical significance of this improvement may be unimportant. The high heterogeneity was largely driven by Cacchio 2006, who reported a large improvement with high‐dose therapy.
Six trials reported pain at three months (Albert 2007; Farr 2011; Gerdesmeyer 2003; Ioppolo 2012; Sabeti 2007; Schofer 2009). There was no statistical between‐group difference in pain (SMD –0.26, 95% CI –0.67 to 0.16; I² = 70%; 326 participants; Analysis 8.1). Based on an SD of 1.9 (Gerdesmeyer 2003), this translates to a mean reduction in pain of 0.49 points (95% CI –1.27 to 0.31) on a 0‐ to 10‐point scale.
Four trials reported pain at three to six months (Cacchio 2006; Gerdesmeyer 2003; Ioppolo 2012; Perlick 2003). There was a slight, possibly clinically unimportant, improvement in pain favouring the high‐dose group (SMD –1.66, 95% CI –2.98 to –0.33; I² = 96%; 326 participants; Analysis 8.1). Based on an SD of 1.9 (Gerdesmeyer 2003), this translates to a mean reduction of 3.15 points (95% CI –5.66 to –0.63) on a 0‐ to 10‐point scale, the 95% CIs include both a clinically important and a clinically unimportant pain reduction. The heterogeneity was driven by the more extreme improvements reported in Cacchio 2006 and Ioppolo 2012; removing their data reduced heterogeneity to zero (SMD –0.47, 95% CI –0.77 to –0.17).
Three trials reported pain at six to 12 months (Gerdesmeyer 2003; Perlick 2003; Schofer 2009). There was no between‐group difference in pain (SMD –0.60, 95% CI –1.39 to 0.18, I² = 85%; 196 participants; Analysis 8.1). Based on a SD of 1.9 (Gerdesmeyer 2003), this translated to a mean reduction of 1.14 points (95% CI –2.64 to 0.34) on a 0‐ to 10‐point scale.
Function
Two trials reported function at six weeks (Cacchio 2006; Farr 2011). While there were no between‐group differences (SMD 3.71, 95% CI –3.71 to 11.14; I² = 99%; 117 participants; Analysis 8.2), the heterogeneity meant the pooled effect size was uninterpretable. Cacchio 2006 found a large benefit favouring high‐dose therapy while Farr 2011 found no between‐group difference.
Seven trials reported function at three months (Albert 2007; Farr 2011; Gerdesmeyer 2003; Ioppolo 2012; Loew 1999; Sabeti 2007; Schofer 2009). There was a clinically unimportant benefit favouring high‐dose therapy (SMD 0.31, 95% CI 0.08 to 0.53; I² = 11%; 366 participants; Analysis 8.2). Based on an SD of 12.8 (Gerdesmeyer 2003), this translated to a mean increase of 4.0 points (95% CI 1.02 to 6.78) on a 0‐ to 100‐point scale. Assuming an minimal clinically important difference of 10 points, this benefit was not clinically significant.
Five trials reported function at six months (Cacchio 2006; Gerdesmeyer 2003; Ioppolo 2012; Perlick 2003; Rompe 1998). The analysis favoured the high‐dose ESWT group, although there was significant heterogeneity (SMD 2.29, 95% CI 1.05 to 3.52; I² = 96%; 409 participants; Analysis 8.2). Based on an SD of 12.8 (Gerdesmeyer 2003), this translated to a mean increase of 29.31 points (95% CI 13.44 to 45.06) on a 0‐ to 100‐point Constant scale. Heterogeneity was reduced but still substantial with removal of the more outlying study (I² = 79%; Cacchio 2006) (SMD 1.36, 95% CI 0.81 to 1.91; equivalent to a mean increase of 17.4 points, 95% CI 10.4 to 24.4, on a 0‐ to 100‐point function scale).
Three trials reported function at 12 months using the Constant score (Gerdesmeyer 2003; Perlick 2003; Schofer 2009). The MD favoured the high‐dose group but the CIs indicated that this may or may not be of clinical importance (MD 12.47, 95% CI 6.91 to 18.03; I² = 0%; 196 participants); Analysis 8.2).
Participant‐reported success
Six trials reported participant‐reported success at the end of the trial (Albert 2007; Cacchio 2006; Gerdesmeyer 2003; Loew 1999; Peters 2004; Rompe 1998). There was a clinically important increase in the proportion of successful treatments in the high‐dose compared with the low‐dose ESWT group (174/221 participants with high dose versus 61/229 participants with low dose; RR 2.74, 95% CI 1.58 to 4.77; I² = 80%; 450 participants; Analysis 8.3). However, the large effect and the high heterogeneity was driven largely by Cacchio 2006 who reported no success with low‐dose therapy, and Peters 2004 who reported that 31/31 (100%) participants had success in the high‐dose group compared to only 4/30 (13%) in the low‐dose group. Removal of these two studies with outlying results modified the effect size to a more moderate increase in success rate and eliminated statistical heterogeneity (RR 1.96, 95% CI 1.57 to 2.45; I² = 0%).
8.3. Analysis.
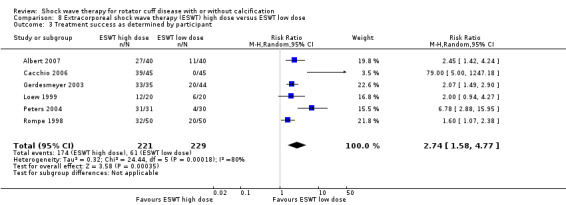
Comparison 8 Extracorporeal shock wave therapy (ESWT) high dose versus ESWT low dose, Outcome 3 Treatment success as determined by participant.
Number of participant withdrawals
Cacchio 2006 reported that no participants withdrew from the study due to adverse events. No other studies reported if there were any withdrawals.
Number of participants experiencing any adverse event
Five trials reported adverse events (Albert 2007; Cacchio 2006; Perlick 2003; Peters 2004; Schofer 2009). A sixth trial reported that haematomas occurred in participants in the high‐dose group, without reporting the number of participants who had the event, so data from this study could not be included in the analysis (Loew 1999). More participants reported adverse events in the high‐dose shock wave group (89/175 participants with high dose versus 23/173 participants with low dose; (RR 3.51, 95% CI 1.53 to 8.03; I2 = 17%; 351 participants; Analysis 8.5).
8.5. Analysis.
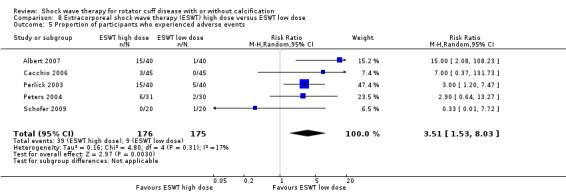
Comparison 8 Extracorporeal shock wave therapy (ESWT) high dose versus ESWT low dose, Outcome 5 Proportion of participants who experienced adverse events.
Adverse events included bruising or skin lesions with high‐dose treatment (Albert 2007; Cacchio 2006; Loew 1999; Perlick 2003; Peters 2004); increased shoulder pain following treatment (Perlick 2003; Peters 2004; Schofer 2009); and acute subacromial bursitis possibly associated with shock wave penetration (Perlick 2003).
One participant in the low‐dose group of one trial reported a panic attack (Albert 2007).
Minor outcomes
Proportion of participants achieving pain score below 30/100 mm on Visual Analogue Scale
None of the trials reported the proportion of participants achieving pain score below 30/100 mm on VAS.
Range of movement
One trial reported ROM (Cacchio 2006).
At six weeks, active flexion was much greater in the high‐dose shock wave group (134 degrees with high dose versus 85.00 degrees with low dose; MD 49.35, 95% CI 37.39 to 61.31; 90 participants; Analysis 8.6).
8.6. Analysis.
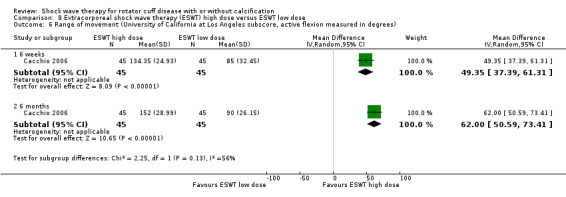
Comparison 8 Extracorporeal shock wave therapy (ESWT) high dose versus ESWT low dose, Outcome 6 Range of movement (University of California at Los Angeles subscore, active flexion measured in degrees).
At six months, active flexion favoured the high‐dose group (152.00 degrees with high dose versus 90 degrees with low dose; MD 62.00, 95% CI 50.59 to 73.41; 90 participants; Analysis 8.6).
Calcification size: number with complete resolution
Five trials reported the number of participants with complete resolution of calcium deposits at the end of the trial (Loew 1999; Perlick 2003; Peters 2004; Pleiner 2004; Rompe 1998). More participants in the high‐dose shock wave therapy group had complete resolution (73/172 (42%) participants with high dose versus 20/166 (12%) participants with low dose; (RR 2.91, 95% CI 1.04 to 8.15; I2 = 72%; 281 participants; Analysis 8.7).
8.7. Analysis.
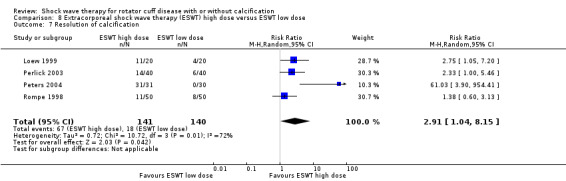
Comparison 8 Extracorporeal shock wave therapy (ESWT) high dose versus ESWT low dose, Outcome 7 Resolution of calcification.
Calcification size: number with partial resolution
Two trials reported number of participants with partial resolution of calcium deposits at the end of the trial (Perlick 2003; Rompe 1998). There was no between‐group differences in partial resolution (29/90 participants with high dose versus 26/90 participants with low dose; RR 1.13, 95% CI 0.73 to 1.75; I² = 0%; Analysis 8.8).
8.8. Analysis.
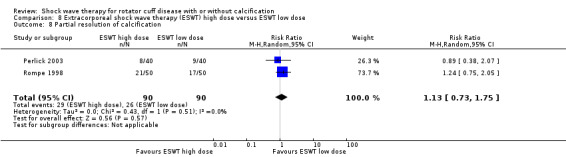
Comparison 8 Extracorporeal shock wave therapy (ESWT) high dose versus ESWT low dose, Outcome 8 Partial resolution of calcification.
Calcification size: mean calcification width
Three trials reported mean change in calcification size at six months (Cacchio 2006; Gerdesmeyer 2003; Ioppolo 2012). There was a greater reduction in the high‐dose therapy group (MD –24.19, 95% CI –44.83 to –3.55; I² = 31%; 229 participants; Analysis 8.9).
8.9. Analysis.
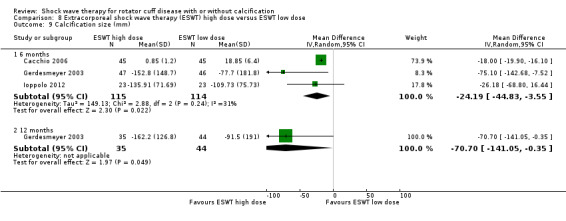
Comparison 8 Extracorporeal shock wave therapy (ESWT) high dose versus ESWT low dose, Outcome 9 Calcification size (mm).
One trial reported mean change in calcification size at 12 months (Gerdesmeyer 2003). There was a greater reduction in the high‐dose group (MD –70.70, 95% CI –141.05 to –0.35; 79 participants; Analysis 8.9).
Calcification size: greater than 80% reduction of calcified surface on anteroposterior view
One trial reported proportion of participants with greater than 80% reduction of calcified surface on anteroposterior view at the end of the trial (Albert 2007). There was no evidence of a difference (6/40 participants with high dose versus 2/40 participants with low dose; RR 3.00, 95% CI 0.64 to 13.98; 80 participants; Analysis 8.10).
8.10. Analysis.
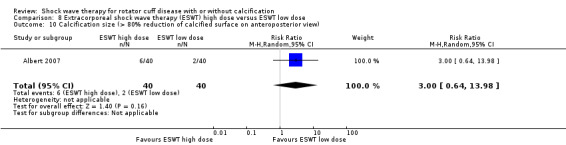
Comparison 8 Extracorporeal shock wave therapy (ESWT) high dose versus ESWT low dose, Outcome 10 Calcification size (> 80% reduction of calcified surface on anteroposterior view).
Two versus one treatment session of shock wave therapy
One small trial (40 participants) compared one versus two treatment sessions of ESWT (Loew 1999), and reported only function, treatment success and adverse events. Findings were uncertain given that the evidence was very‐low certainty due to the small number of participants and potential for selection, performance, detection and selective reporting bias.
Major outcomes
Function
There was no evidence of a difference in function at three months (mean function using Constant score 0 to 100, 0 indicating worst function: 68.5 (SD 13.1) with two sessions versus 63.7 (SD 14.6) with one session (MD 4.80, 95% CI ‐3.80 to 13.40; one study, 40 participants; Analysis 9.1).
9.1. Analysis.
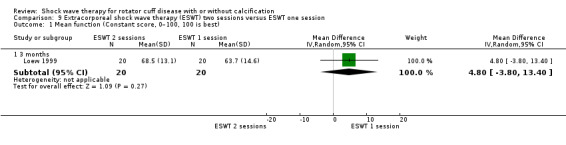
Comparison 9 Extracorporeal shock wave therapy (ESWT) two sessions versus ESWT one session, Outcome 1 Mean function (Constant score, 0–100, 100 is best).
Participant‐reported success
There was no evidence of a difference in treatment success (proportion of participants satisfied with the treatment) (14/20 participants with two sessions versus 12/20 participants with one session (RR 1.17, 95% CI 0.74 to 1.85; one study, 40 participants; Analysis 9.2).
9.2. Analysis.
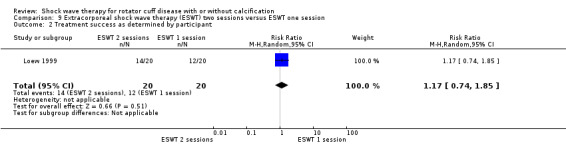
Comparison 9 Extracorporeal shock wave therapy (ESWT) two sessions versus ESWT one session, Outcome 2 Treatment success as determined by participant.
Number of participants experiencing any adverse event
Loew 1999 reported that haematomas occurred in participants in the two‐session group, without reporting the number of participants who had the event, so these data could not be included in an analysis.
Other major outcomes
The study did not report participant‐reported pain relief of 30% or greater, pain, quality of life, withdrawals due to adverse events and the number of people with adverse events.
Minor outcomes
Proportion with resolution of calcification
There was no evidence of a difference in the number of participants with resolution of calcifications (12/20 participants with two sessions versus 11/20 participants with one session (RR 1.09, 95% CI 0.64 to 1.86; 40 participants one study; Analysis 9.3).
9.3. Analysis.
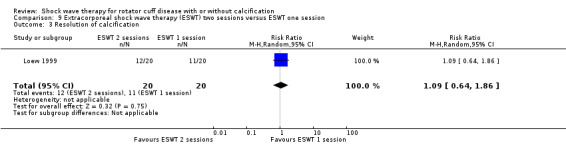
Comparison 9 Extracorporeal shock wave therapy (ESWT) two sessions versus ESWT one session, Outcome 3 Resolution of calcification.
Other minor outcomes
The study did not report proportion of participants achieving pain score below 30/100 mm on VAS, ROM and size of the calcification.
Shock wave therapy directed to the calcific deposits or to the supraspinatus origin
One study compared calcification‐focused ESWT with supraspinatus origin‐focused ESWT (Haake 2002).
Major outcomes
Participant reported pain relief of 30% or greater
The trial did not report participant‐reported pain relief of 30% or greater.
Pain
There was no statistically significant difference in pain when comparing calcification‐focused ESWT with supraspinatus origin‐focused ESWT at three months' follow‐up (mean pain at rest, visual NRS 0 to 11, 11 indicating worst pain: 3.21 with calcification‐focused ESWT versus 4.74 with supraspinatus origin‐focused ESWT; MD –1.53, 95% CI –3.24 to 0.18; 47 participants; Analysis 10.1).
10.1. Analysis.
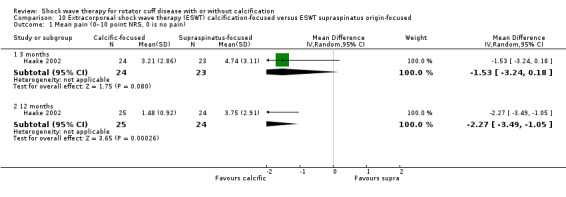
Comparison 10 Extracorporeal shock wave therapy (ESWT) calcification‐focused versus ESWT supraspinatus origin‐focused, Outcome 1 Mean pain (0–10 point NRS, 0 is no pain).
There was a statistically significant but clinically unimportant decrease in pain when comparing calcification‐focused ESWT with supraspinatus origin‐focused ESWT at 12 months' follow‐up (mean pain at rest, visual NRS 0 to 11, 11 indicating worst pain: 1.48 with calcification‐focused ESWT versus 3.75 with supraspinatus origin‐focused ESWT; MD –2.27, 95% CI –3.49 to –1.05; 49 participants; Analysis 10.1).
Function
There was a statistically significant and clinically important increase in function when comparing calcification‐focused ESWT with supraspinatus origin‐focused ESWT at six weeks to three months' follow‐up (mean function using Constant score 0 to 100, 1000 indicating best function: 104.59 with calcification‐focused ESWT versus 73.08 with supraspinatus origin‐focused ESWT; MD 31.51, 95% CI 16.33 to 46.69; 47 participants; Analysis 10.2).
10.2. Analysis.
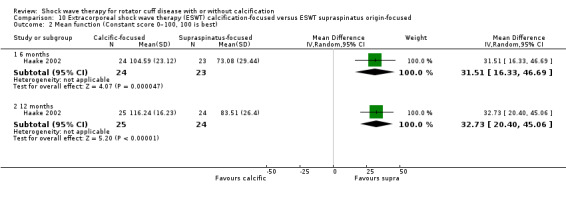
Comparison 10 Extracorporeal shock wave therapy (ESWT) calcification‐focused versus ESWT supraspinatus origin‐focused, Outcome 2 Mean function (Constant score 0–100, 100 is best).
There was a statistically significant and clinically important increase in function when comparing calcification‐focused ESWT with supraspinatus origin‐focused ESWT at 12 months' follow‐up (mean function using Constant score 0 to 100, 100 indicating best function: 116.24 with calcification‐focused ESWT versus 83.51 with supraspinatus origin‐focused ESWT; MD 32.73, 95% CI 20.40 to 45.06; 49 participants; Analysis 10.2).
Participant‐reported success
Haake 2002 measured treatment success by the proportion of participants satisfied with the treatment. There was a statistically significant but clinically unimportant increase in success rate in the calcification‐focused ESWT group compared with the supraspinatus origin‐focused ESWT group (25/25 participants with calcification‐focused ESWT versus 10/24 participants with supraspinatus origin‐focused ESWT; RR 2.34, 95% CI 1.47 to 3.71; Analysis 10.3).
10.3. Analysis.
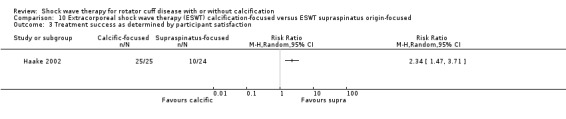
Comparison 10 Extracorporeal shock wave therapy (ESWT) calcification‐focused versus ESWT supraspinatus origin‐focused, Outcome 3 Treatment success as determined by participant satisfaction.
Quality of life
The trial did not report quality of life.
Number of participant withdrawals
The trial did not report number of participant withdrawals.
Number of participants experiencing any adverse event
Haake 2002 reported that no participants experienced adverse events during the study. These data could not be analysed in this review.
Minor outcomes
Calcification size: number with complete resolution
There was a statistically significant increase of uncertain clinical significance in the number with complete resolution in the calcification‐focused ESWT group compared with supraspinatus origin‐focused ESWT group at the end of the trial (14/24 participants with calcification‐focused ESWT group versus 8/22 participants with supraspinatus origin‐focused ESWT (RR 1.60, 95% CI 0.84 to 3.07; 46 participants; one study; Analysis 10.4).
10.4. Analysis.
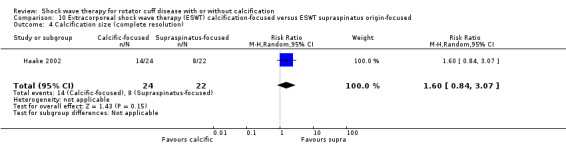
Comparison 10 Extracorporeal shock wave therapy (ESWT) calcification‐focused versus ESWT supraspinatus origin‐focused, Outcome 4 Calcification size (complete resolution).
Other minor outcomes
The trial did not report proportion of participants achieving pain score below 30/100 mm on VAS, ROM and calcification width.
Palpation‐guided versus image‐guided shock wave therapy
One study compared palpation‐guided ESWT to image‐guided ESWT (Sabeti‐Aschraf 2005).
Major outcomes
Pain
There was a statistically significant and clinically important difference in improvement in pain favouring the image‐guided ESWT at three months (mean pain using a 0‐ to 100‐point VAS, 100 indicating most pain: 18.21 with image‐guided ESWT versus 33.36 with palpation‐guided ESWT; MD –15.15, 95% CI –26.62 to –3.68; 50 participants; Analysis 11.1).
11.1. Analysis.
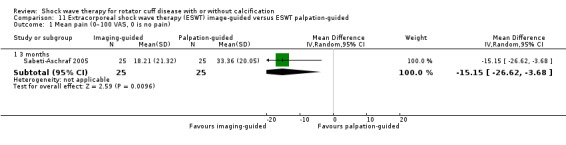
Comparison 11 Extracorporeal shock wave therapy (ESWT) image‐guided versus ESWT palpation‐guided, Outcome 1 Mean pain (0–100 VAS, 0 is no pain).
Function
There was no between‐group difference in function at three months (mean Constant score: 79.48 with image‐guided ESWT versus 73.00 with palpation‐guided ESWT; MD 6.48, 95% CI –2.22 to 15.18; 50 participants; Analysis 11.2).
11.2. Analysis.
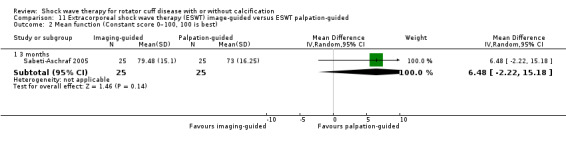
Comparison 11 Extracorporeal shock wave therapy (ESWT) image‐guided versus ESWT palpation‐guided, Outcome 2 Mean function (Constant score 0–100, 100 is best).
Number of participants experiencing any adverse event
There were no adverse events reported.
Other major outcomes
The trial did not report participant‐reported pain relief of 30% or greater, participant‐reported success, quality of life and withdrawals due to adverse events.
Minor outcomes
Calcific deposits: number with complete resolution
There was no difference in the number of participants who had complete resolution of calcific deposits at the end of the trial (6/25 participants with image‐guided ESWT versus 1/25 participants with palpation‐guided ESWT; RR 6.00, 95% CI 0.78 to 46.29; Analysis 11.3).
11.3. Analysis.
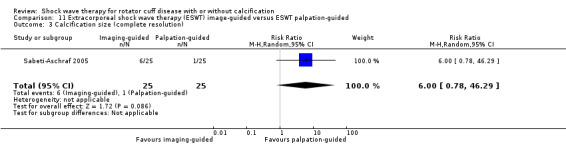
Comparison 11 Extracorporeal shock wave therapy (ESWT) image‐guided versus ESWT palpation‐guided, Outcome 3 Calcification size (complete resolution).
Calcification size: number with partial resolution
There was no difference in the number of participants who had partial resolution of calcific deposits at the end of the trial (7/25 participants with image‐guided ESWT versus 5/25 participants with palpation‐guided ESWT; RR 1.40, 95% CI 0.51 to 3.82; Analysis 11.4).
11.4. Analysis.
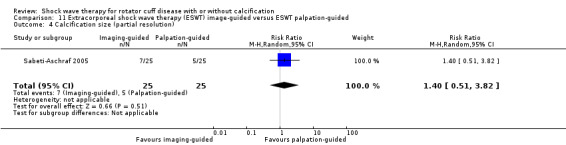
Comparison 11 Extracorporeal shock wave therapy (ESWT) image‐guided versus ESWT palpation‐guided, Outcome 4 Calcification size (partial resolution).
Other minor outcomes
The trial did not report proportion of participants achieving pain score below 30/100 mm on VAS, ROM and mean calcification width.
ESWT with hyperextended arm position versus ESWT with neutral arm position
One trial compared ESWT treatment given in a neutral arm position compared with a hyperextended arm position (Tornese 2011).
Major outcomes
Pain
There was no statistically significant difference in pain when comparing hyperextended arm position ESWT with neutral arm position ESWT at 3 months' follow‐up (mean pain using 0‐ to 15‐point VAS, 15 indicating worst pain: 10.9 with hyperextended arm position ESWT versus 9.2 with neutral arm position ESWT; MD 1.70, 95% CI –0.55 to 3.95; 35 participants; Analysis 12.1).
12.1. Analysis.
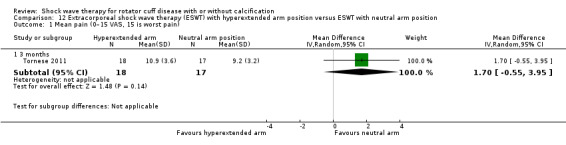
Comparison 12 Extracorporeal shock wave therapy (ESWT) with hyperextended arm position versus ESWT with neutral arm position, Outcome 1 Mean pain (0–15 VAS, 15 is worst pain).
Function
There was a statistically significant but clinically unimportant increase in function when comparing hyperextended arm position ESWT with neutral arm position ESWT at three months' follow‐up (mean function using Constant score 0 to 100, 100 indicating best function: 76.9 with hyperextended arm position ESWT versus 67.9 with neutral arm position ESWT; MD 9.00, 95% CI 0.72 to 17.28; 35 participants; Analysis 12.2).
12.2. Analysis.
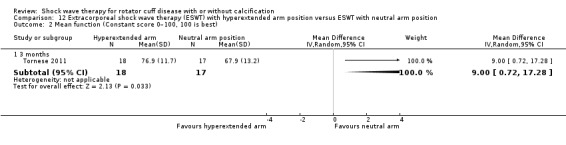
Comparison 12 Extracorporeal shock wave therapy (ESWT) with hyperextended arm position versus ESWT with neutral arm position, Outcome 2 Mean function (Constant score 0–100, 100 is best).
Other major outcomes
The study did not report participant‐reported pain relief of 30% or greater, treatment success, quality of life, withdrawals due to adverse events and number of participants experiencing any adverse event.
Minor outcomes
Calcification size: greater than 80% reduction of calcified surface on anteroposterior view
There was no difference in number of participants who achieved greater than 80% reduction of calcified surface on anteroposterior view at the end of the trial (12/18 participants with hyperextended arm position ESWT versus 6/17 with neutral arm position ESWT; RR 1.89, 95% CI 0.92 to 3.89; Analysis 12.3).
12.3. Analysis.
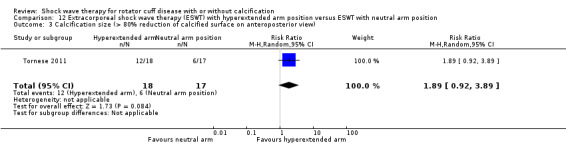
Comparison 12 Extracorporeal shock wave therapy (ESWT) with hyperextended arm position versus ESWT with neutral arm position, Outcome 3 Calcification size (> 80% reduction of calcified surface on anteroposterior view).
Other minor outcomes
The study did not report proportion of participants achieving pain score below 30/100 mm on VAS, ROM and mean calcific deposit width.
ESWT versus ultrasound‐guided percutaneous lavage
One study investigated ESWT versus ultrasound‐guided percutaneous lavage (Del Castillo‐Gonzales 2016).
Major outcomes
Pain
There was no evidence of a difference in mean pain (0‐ to 10‐point VAS, 10 indicating worst pain) when comparing ESWT to ultrasound‐guided percutaneous lavage at zero to six weeks (MD –0.10, 95% CI –0.26 to 0.06; 201 participants; Analysis 6.1).
6.1. Analysis.
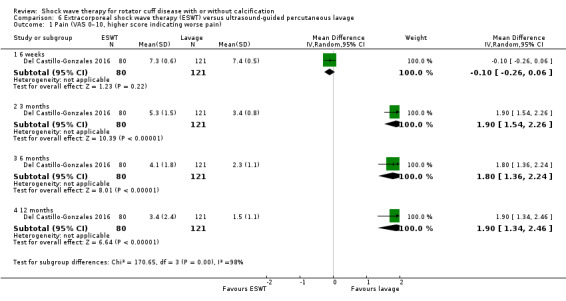
Comparison 6 Extracorporeal shock wave therapy (ESWT) versus ultrasound‐guided percutaneous lavage, Outcome 1 Pain (VAS 0–10, higher score indicating worse pain).
There was a statistically and clinically significant increase in mean pain (0‐ to 10‐point VAS, 10 indicating worst pain) when comparing ESWT to ultrasound‐guided percutaneous lavage at six weeks to three months (MD 1.90, 95% CI 1.54 to 2.26; 201 participants; Analysis 6.1).
There was a statistically significant increase in mean pain of uncertain clinical significance (0‐ to 10‐point VAS, 10 indicating worst pain) when comparing ESWT to ultrasound‐guided percutaneous lavage at three to six months (MD 1.80, 95% CI 1.36 to 2.24; 201 participants; Analysis 6.1).
There was a statistically significant increase in mean pain of uncertain clinical significance (0‐ to 10‐point VAS, 10 indicating worst pain) when comparing ESWT to ultrasound‐guided percutaneous lavage (MD 1.90, 95% CI 1.34 to 2.46; 201 participants; Analysis 6.1).
Participant‐reported success
There was no statistically significant difference in treatment success (proportion of participants who were pain‐free) when comparing ESWT to ultrasound‐guided percutaneous lavage at the end of the trial (RR 0.91, 95% CI 0.81 to 1.03; 201 participants; Analysis 6.2).
6.2. Analysis.
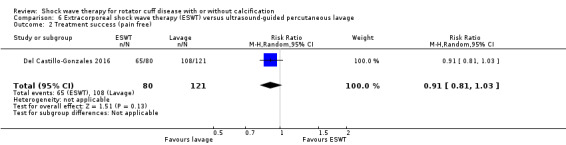
Comparison 6 Extracorporeal shock wave therapy (ESWT) versus ultrasound‐guided percutaneous lavage, Outcome 2 Treatment success (pain free).
Number of participants experiencing any adverse event
There was a statistically significant increase in the risk of experiencing an adverse event when comparing ESWT to ultrasound‐guided percutaneous lavage at the end of the trial (RR 0.08, 95% CI 0.00 to 1.36; 243 participants; Analysis 6.3).
6.3. Analysis.
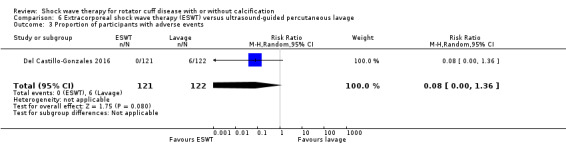
Comparison 6 Extracorporeal shock wave therapy (ESWT) versus ultrasound‐guided percutaneous lavage, Outcome 3 Proportion of participants with adverse events.
Minor outcomes
Calcification size
There was a statistically significant decrease in calcification size when comparing ESWT to ultrasound‐guided percutaneous lavage at zero to six weeks (MD –2.00 mm, 95% CI –2.94 to –1.06; 201 participants; Analysis 6.4).
6.4. Analysis.
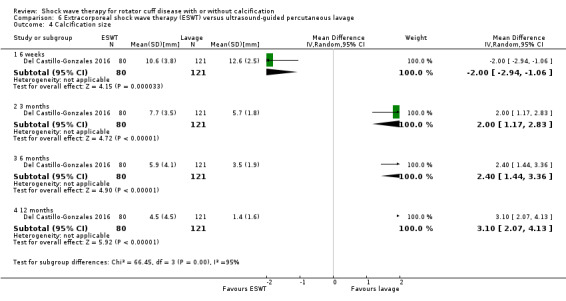
Comparison 6 Extracorporeal shock wave therapy (ESWT) versus ultrasound‐guided percutaneous lavage, Outcome 4 Calcification size.
There was a statistically significant increase in calcification size when comparing ESWT to ultrasound‐guided percutaneous lavage at six weeks to three months (MD 2.00 mm, 95% CI 1.17 to 2.83; 201 participants; Analysis 6.4).
There was a statistically significant increase in calcification size when comparing ESWT to ultrasound‐guided percutaneous lavage at three to six months (MD 2.40 mm, 95% CI 1.44 to 3.36; 201 participants; Analysis 6.4).
There was a statistically significant increase in calcification size when comparing ESWT to ultrasound‐guided percutaneous lavage at six to twelve months (MD 3.10 mm, 95% CI 2.07 to 4.13; 201 participants; Analysis 6.4).
Calcification size (complete resolution)
There was a statistically significant increase in the chance of complete resolution of calcification when comparing ESWT to ultrasound‐guided percutaneous lavage at the end of the trial (RR 0.65, 95% CI 0.53 to 0.80; 201 participants; Analysis 6.5).
6.5. Analysis.
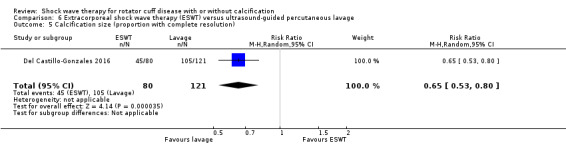
Comparison 6 Extracorporeal shock wave therapy (ESWT) versus ultrasound‐guided percutaneous lavage, Outcome 5 Calcification size (proportion with complete resolution).
ESWT versus ultrasound‐guided hyaluronic acid injection
One study compared shock wave therapy to ultrasound‐guided hyaluronic acid injection (Frizziero 2017).
Major outcomes
Pain
The study measured pain on the DASH scale postintervention and at three months' follow‐up. We did not use these data in our review.
Function
There was evidence of a difference in function when comparing the ESWT group with the ultrasound‐guided hyaluronic acid injection group at three months' follow‐up (mean function using Constant score 0 to 100, 100 indicating best function: 76.5 (SD 20.6) with ESWT versus 81.8 with ultrasound‐guided hyaluronic acid injection; SMD –0.26, 95% CI –0.94 to 0.41; 34 participants; Analysis 14.1).
14.1. Analysis.
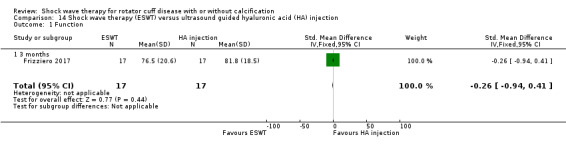
Comparison 14 Shock wave therapy (ESWT) versus ultrasound guided hyaluronic acid (HA) injection, Outcome 1 Function.
Other major outcomes
The study did not report participant‐reported pain relief of 30% or greater, participant‐reported success, quality of life, proportion of participants with adverse events and withdrawals.
Minor outcomes
The study did not report proportion of participants achieving pain score below 30/100 mm on VAS, ROM, size of the calcification and number of participants with complete or partial resolution.
rESWT plus physiotherapy versus physiotherapy
One study compared rESWT plus physiotherapy with physiotherapy alone (Duymaz 2019).
Major outcomes
Pain
There was a statistically significant but clinically unimportant improvement in pain in the rESWT plus physiotherapy group compared to the physiotherapy group alone postintervention (mean pain measured on 0‐ to 10‐point VAS, 10 indicating most pain: 1.3 with rESWT plus physiotherapy versus 2.5 with physiotherapy alone; MD –1.20, 95% CI –1.58 to –0.82; 80 participants; Analysis 15.1).
15.1. Analysis.
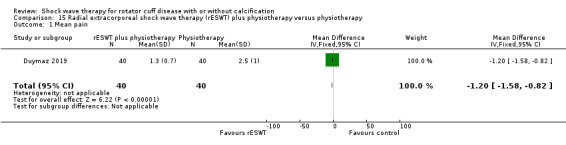
Comparison 15 Radial extracorporeal shock wave therapy (rESWT) plus physiotherapy versus physiotherapy, Outcome 1 Mean pain.
Function
There was a statistically significant and clinically important improvement in function in the rESWT plus physiotherapy group compared to the physiotherapy group alone (mean function measured on quickDASH scale of 0 to 100, 100 indicating most disability: 1.3 with rESWT plus physiotherapy versus 12.6 with physiotherapy alone; MD –11.30, 95% CI –14.75 to –7.85; 80 participants; Analysis 15.2).
15.2. Analysis.
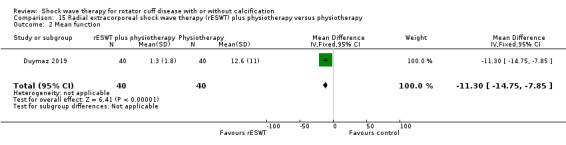
Comparison 15 Radial extracorporeal shock wave therapy (rESWT) plus physiotherapy versus physiotherapy, Outcome 2 Mean function.
Other major outcomes
The study did not report participant‐reported pain relief of 30% or greater, quality of life, number of participant withdrawals and number of participants experiencing any adverse event.
Minor outcomes
Range of movement
There was a statistically significant improvement in flexion with rESWT plus physiotherapy compared to physiotherapy alone postintervention (measured using a goniometer: 171.1 with rESWT plus physiotherapy versus 139.5 with physiotherapy alone; MD 31.60, 95% CI 24.04 to 39.16; 80 participants; Analysis 15.3). There was a statistically significant improvement in extension with rESWT plus physiotherapy group compared to physiotherapy alone postintervention (measured using a goniometer: 33.8 with rESWT plus physiotherapy versus 16.8 with physiotherapy alone; MD 17.00, 95% CI 14.10 to 19.90; 80 participants; Analysis 15.4). There was a statistically significant improvement in abduction with rESWT plus physiotherapy group compared to physiotherapy alone postintervention (measured using a goniometer: 167 with rESWT plus physiotherapy versus 125.2 with physiotherapy alone; MD 41.80, 95% CI 32.79 to 50.81; 80 participants; Analysis 15.5). There was a statistically significant improvement in external rotation with rESWT plus physiotherapy compared to physiotherapy alone postintervention (measured using a goniometer: 49 with rESWT plus physiotherapy versus 25.8 with physiotherapy alone; MD 23.20, 95% CI 16.98 to 29.42; 80 participants; Analysis 15.6).
15.3. Analysis.
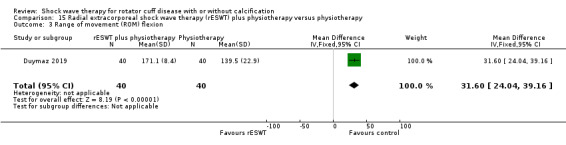
Comparison 15 Radial extracorporeal shock wave therapy (rESWT) plus physiotherapy versus physiotherapy, Outcome 3 Range of movement (ROM) flexion.
15.4. Analysis.
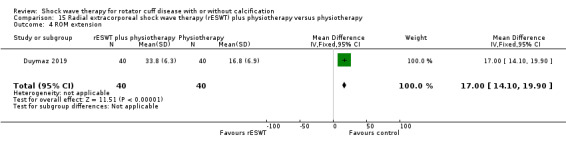
Comparison 15 Radial extracorporeal shock wave therapy (rESWT) plus physiotherapy versus physiotherapy, Outcome 4 ROM extension.
15.5. Analysis.
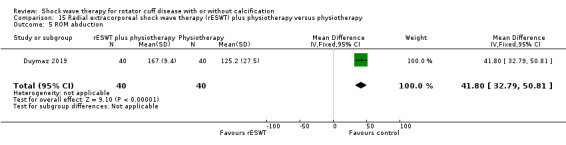
Comparison 15 Radial extracorporeal shock wave therapy (rESWT) plus physiotherapy versus physiotherapy, Outcome 5 ROM abduction.
15.6. Analysis.
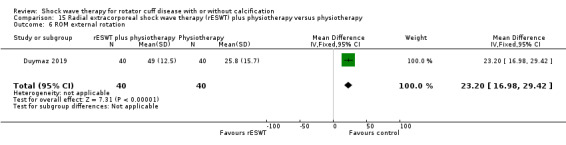
Comparison 15 Radial extracorporeal shock wave therapy (rESWT) plus physiotherapy versus physiotherapy, Outcome 6 ROM external rotation.
Other minor outcomes
The study did not report proportion of participants achieving pain score below 30/100 mm on VAS, size of the calcification and number of participants with complete or partial resolution.
Discussion
Summary of main results
Compared to placebo, there was moderate‐certainty evidence that shockwave therapy provides no clinically important improvement in pain and function at three months following treatment, and low‐certainty evidence indicating there may also be no improvement in the number of participants with a pain reduction of 50% or more and the number with participant‐reported treatment success. It is uncertain if therapy increases withdrawal rate and adverse events, due to the small number of events (Table 1). None of the studies measured quality of life. There were also no clinically important differences between shock wave therapy and placebo at any other time points.
Subgroup analyses indicated that pain and function outcomes did not differ between those participants who did or did not have calcific deposits.
Shock wave therapy was associated with an increased rate of complete resolution of calcium deposits by the end of the trial, but this was of uncertain clinical significance. The studies did not measure the proportion of participants achieving a pain score below 30/100 mm on VAS, ROM and number of participants with partial resolution of calcific deposits.
Evidence was downgraded due to the risk of selection, detection or reporting bias, or a combination of these biases, as well as imprecision or heterogeneity.
We are uncertain if shock wave therapy has any benefits over ultrasound‐guided needling, TENS, supervised exercises, no treatment, percutaneous lavage or multiple versus single treatments, as there was only low‐ to very low‐certainty evidence from single or few small studies.
There was very low‐certainty evidence that high‐dose shockwave therapy may provide a clinically important benefit compared with low‐dose shock wave therapy at the end of the trial with respect to treatment success and function. Higher doses also had a benefit of uncertain clinical significance with respect to ROM and reduction of calcific deposits. High‐dose therapy had a higher risk of adverse events but not withdrawals. There were no clinically important differences between high‐dose and low‐dose shock wave therapy at any other time points. Evidence was downgraded due to the risk of multiple biases, imprecision, heterogeneity and indirectness for pain and function.
Adverse events of shock wave therapy reported in the trials included treatment‐related pain, bruising and bleeding, although these were self‐limiting.
Rare and potential serious adverse events, such as osteonecrosis of the bone of the upper arm (loss of blood supply and bone death) while theoretically possible, were not reported in the studies.
Overall completeness and applicability of evidence
There was inconsistent reporting of major outcomes across trials (Table 4). Overall pain and function were reported commonly (96% of trials for both), but were often not reported fully, for example without measures of variance, or in some studies only reported in the treatment group, precluding their inclusion in the analyses. A lower proportion of trials measured the other major outcomes. No trial reported participant‐reported pain relief of 30% or greater, although one trial reported pain relief of 50% or greater, which we reported. Fifty percent of trials reported treatment success, no trial included quality of life, 25% reported withdrawals and 64% reported withdrawals due to adverse events.
3. Outcome Reporting Bias In Trials (ORBIT) matrix.
| Study ID | Major outcomes | ||||||
|
Participant‐reported pain relief ≥ 50% |
Pain |
Function or disability |
Treatment success |
Quality of life |
Withdrawal due to adverse events |
Adverse events | |
| Albert 2007 | ? | Full | Full | Full | ? | ? | Full |
| Cacchio 2006 | ? | Full | Full | Full | ? | Full | Full |
| Cosentino 2003 | ? | Partial | Full | ? | ? | ? | Full |
| De Boer 2017 | ? | Full | Full | Full | ? | ? | Full |
| Del Castillo‐Gonzales 2016 | ? | Full | ? | Full | ? | ? | Full |
| Duymaz 2019 | ? | Full | Full | ? | ? | ? | ? |
| Engebretsen 2009 | ? | Full | Full | ? | ? | Full | Full |
| Farr 2011 | ? | Full | Full | ? | ? | ? | Full |
| Frizziero 2017 | ? | Partial | Full | ? | ? | ? | ? |
| Galasso 2012 | ? | Full | Full | Full | ? | Full | Full |
| Gerdesmeyer 2003 | ? | Full | Full | Full | ? | ? | Full |
| Haake 2002 | ? | Full | Full | Full | ? | ? | Full |
| Hearnden 2009 | ? | Partial | Partial | Full | ? | ? | Partial |
| Hsu 2008 | ? | Full | Full | Full | ? | ? | Full |
| Ioppolo 2012 | ? | Full | Full | ? | ? | ? | ? |
| Kim 2014 | ? | Partial | Partial | ? | ? | ? | ? |
| Kolk 2013 | ? | Full | Full | ? | ? | ? | ? |
| Kvalvaag 2017 | ? | Full | Full | ? | ? | Full | Partial |
| Li 2017 | ? | Full | Full | ? | ? | ? | Full |
| Loew 1999 | ? | Not measured | Full | Full | ? | ? | ? |
| Melegati 2000 | ? | Not measured | Full | ? | ? | ? | ? |
| Pan 2003 | ? | Full | Full | ? | ? | Full | Full |
| Perlick 2003 | ? | Full | Partial | ? | ? | ? | Full |
| Peters 2004 | ? | ? | ? | Full | ? | Full | Full |
| Pleiner 2004 | ? | Full | Measured | ? | ? | ? | ? |
| Rompe 1998 | ? | Not measured | Partial | Full | ? | ? | ? |
| Sabeti 2007 | ? | Full | Full | Full | ? | ? | ? |
| Sabeti‐Aschraf 2005 | ? | Full | Full | ? | ? | ? | Full |
| Schmitt 2001 | ? | Full | Full | Full | ? | Full | Full |
| Schofer 2009 | ? | Full | Full | ? | ? | ? | Full |
| Speed 2002 | Full | Partial | Full | Full | ? | Full | Full |
| Tornese 2011 | ? | Full | Full | ? | ? | ? | ? |
'Full': sufficient data for inclusion in a meta‐analysis was reported (e.g. mean, standard deviation and sample size per group for continuous outcomes). 'Partial': insufficient data for inclusion in a meta‐analysis was reported (e.g. means only, with no measures of variance). 'Measured': outcome was measured but no outcome data was reported. 'Not measured': outcome was not measured by the trialists. '?': unclear whether the outcome was measured or not (as a trial protocol was unavailable).
Inclusion of a core outcome measures in future trials would facilitate the ability to synthesise the evidence, compare results between trials and increase the certainty of our conclusions (Buchbinder 2017; Page 2015; Page 2016c; Page 2018).
Additionally, there was no standard approach to shock wave therapy in terms of type of shock wave, dose and frequency of treatment, and the placebo controls varied across trials. This resulted in marked clinical heterogeneity across studies leading to uncertainty in interpreting the pooled analyses.
While we did find that shock wave therapy, particularly in high doses, resulted in a greater number of people with complete resolution of calcific deposits when present, this did not appear to translate into improved patient‐relevant outcomes of pain, function or treatment success.
Two RCTs that were potentially eligible for inclusion in this review did not have available results. However, we believe it is unlikely that inclusion of these studies in our review would change our conclusions.
Quality of the evidence
We used the GRADE approach to assess the certainty of the evidence (Schünemann 2011a). Moderate‐certainty evidence suggests that compared to placebo, shock wave therapy results in a small but clinically uncertain improvement in mean pain and function. It did not appear to matter if participants had calcific deposits or not. Evidence was downgraded due to the potential for selection, performance, detection and reporting biases, There was also considerable heterogeneity, but, as it was largely driven by a pseudo‐randomised trial with outlier results, we did not downgrade the evidence further.
Shock wave therapy may not have an effect on participant‐reported pain relief of 50% or greater and treatment success, but as this is based on low‐certainty evidence, we could not be certain. Evidence was downgraded due potential bias arising from inadequate study design and imprecision: only a single poorly designed study reported pain relief of 50% or more, and although 287 participants from six poorly reported studies reported treatment success, CIs around the effect estimate were wide, due to the small number of events in most studies. Low‐certainty evidence was also available from seven studies for withdrawals and five studies for adverse events. Evidence was downgraded due to potential for bias and imprecision. We are uncertain if withdrawals or adverse events differed between groups due to the small number of events. Shock wave therapy did result in more people with complete resolution of calcium deposits compared to placebo. Quality of life was not measured.
We are uncertain if shock wave therapy has any benefits over ultrasound‐guided glucocorticoid needling, TENS, exercise or no treatment, or different regimens of shock wave therapy as there was only low‐certainty evidence from single or few small studies, subject to bias and imprecision.
We are uncertain if higher doses of shock wave therapy has any benefit and more adverse events over lower doses, due to very low‐certainty evidence. Evidence was downgraded due to imprecision, bias, heterogeneity and indirectness due to variability and lack of consensus in recommended treatment dose.
Potential biases in the review process
We performed a thorough search of CENTRAL, MEDLINE, Embase, ClinicalTrials.gov and WHO International Clinical Trials Registry Platform databases using a sensitive search strategy without restricting by date or language to identify published and unpublished studies, so it is unlikely that we missed any relevant studies. We could not fully assess publication bias because we did not have enough trials. However, unpublished trials may be more likely to show no benefit of shock wave therapy and are, therefore, unlikely to change our conclusions.
We identified five ongoing studies, one comparing needle aspiration of calcific deposits versus ESWT (NTR7093), one comparing rESWT to ultrasound‐guided needle puncture or to a combination of both interventions (NCT02677103), one comparing focussed ESWT to rESWT (ChiCTR1900022932), another comparing high energy ESWT to low energy ESWT to sham (NCT03779919), and one comparing ESWT to steroid injection (PACTR201910650013453). As these studies have varied comparators and would be presented as single studies in stand‐alone comparisons, it appears that inclusion of the results when available are unlikely to impact on the conclusions of this review.
Two review authors independently assessed the trials for inclusion, extracted data and assessed the risk of bias, and a third review author adjudicated when any discrepancy arose. Review questions of interest were defined with full knowledge of the possible comparisons that could be undertaken, but no knowledge of the results of any comparisons. To prevent selective inclusion of results we used predefined decision rules to select data from trials when multiple measurement scales, time points and analyses were reported.
A limitation of the review was that many trials did not report major outcomes or presented outcome data incompletely and attempts to obtain unpublished data from trialists were largely unsuccessful.
We identified nine studies published in languages other than English that we could not translate at the time of submission of the review, and thus these studies are still awaiting classification. We do not consider that the results of these studies are likely to alter the conclusions of the review substantially.
Agreements and disagreements with other studies or reviews
Two other systematic reviews comparing shock wave therapy to placebo have been published (Bannuru 2014; Ioppolo 2013). However, Bannuru 2014 did not identify the time points at which it was extracting data and stratified its included trials based on higher doses versus sham to lower dose versus sham, as well as for studies with higher doses versus calcification and lower doses versus calcifications. Ioppolo 2013 only synthesised the data for calcific deposit resolution for meta‐analysis. We identified two other meta‐analysis of ESWT; however, one only compared high‐dose to low‐dose therapy (Verstraelen 2014) and one pooled data for ESWT versus any other treatment (Vavken 2009). Therefore, to our knowledge ours is the most comprehensive review of shock wave therapy for rotator cuff disease.
Our conclusions about the benefits and harms of ESWT are consistent with other reviews in that it is likely to help resolve calcification deposition, but that this is of uncertain clinical significance. Our review also suggests that higher‐dose therapy may be more beneficial than lower‐dose therapy. Where our review differs, is that Bannuru 2014 and Ioppolo 2013 both recommend ESWT as an effective treatment over sham. These discrepancies appear to derive from how these reviews handled their data. Due to the high heterogeneity of studies, Bannuru 2014 did not synthesise the data from its included studies for meta‐analysis. Instead it appears to have based its recommendations on visualisations of the mean and 95% CIs for studies, and further narrowed its recommendations to "high‐dose" studies including only people with calcifications. These results should be interpreted cautiously, however, as even within these high‐dose trials treatment regimens varied greatly. Furthermore, from the published information, it was not possible to determine which time points Bannuru 2014 was referring to, which groups it extracted its data for (as one included high‐dose trial had three arms) and it should be noted that they considered a trial which compared ESWT to no treatment as sham, where our review considered no treatment at all as not equitable to a sham treatment. Finally, due to the great differences between treatment regimens, our review did not pool all trials with calcifications, but only used trials which reported data separately for people with and without calcifications to consider the potential different effectiveness of ESWT on these groups. As for Ioppolo 2013, the study authors did not synthesise data for outcomes other than calcification resorption for meta‐analysis. Their recommendations about the effectiveness of ESWT over sham for pain and function outcomes are based on the mean change in mean for the treatment groups in their included studies' outcome scores (such as VAS or Constant score), but did not include considerations of CIs or MDs. Meanwhile, our review based its recommendations on how ESWT performed when compared to sham on the MD between treatment and control groups, and considered that for a change to be clinically important its 95% CIs must not have left the range of clinical importance.
Finally, discrepancies over the recommendations that can be made from the results of these meta‐analyses appear to be driven by less frequent consideration of the overall certainty of evidence in these reviews (i.e. while study risk of bias was assessed, other domains of the GRADE approach (imprecision, inconsistency, indirectness and publication bias) were not).
Authors' conclusions
Implications for practice.
Based upon the currently available low‐ to moderate‐certainty evidence, our review indicates few clinically important benefits of shock wave therapy compared with placebo, ultrasound‐guided needling, transcutaneous electrical nerve stimulation, supervised exercises or percutaneous lavage for the treatment of rotator cuff disease with or without calcific deposits. There is also uncertainty regarding its safety. Wide clinical heterogeneity and varying treatment protocols means that we do not know whether 'subtherapeutic' doses were tested in some trials underestimating any potential benefits.
Implications for research.
Further trials of shock wave therapy for rotator cuff disease should be based upon a strong rationale, be of high quality, include a core set of outcomes and be adequately powered to test for important patient‐relevant benefits. To reduce research wastage, further trials should only be conducted with explicit consideration of whether or not they would alter the conclusions of this review. A standard dose and treatment protocol should be defined and evaluated in a consistent and comparable manner. Updates of this review will only be performed if new trials that may change the conclusions of this review become available.
What's new
| Date | Event | Description |
|---|---|---|
| 30 July 2014 | Amended | CMSG ID C173‐R |
History
Protocol first published: Issue 1, 2011 Review first published: Issue 3, 2020
| Date | Event | Description |
|---|---|---|
| 14 November 2008 | Amended | Converted to new review format. |
Acknowledgements
We are grateful to Louise Falzon, Trial Search Co‐ordinator, Cochrane Musculoskeletal Group for designing the original search strategy and Tamara Rader, Trial Search Co‐ordinator, Cochrane Musculoskeletal Group for updating and executing the searches. We would also like to thank Juliana Roos for her substantial contribution to the protocol.
We would like to acknowledge the peer‐referees:
Hans Lund, Western Norway University of Applied Sciences (Cochrane Musculoskeletal Editor);
Professor Susanna Proudman, Rheumatology Unit, Royal Adelaide Hospital and Discipline of Medicine, University of Adelaide, South Australia;
Professor G David Baxter, Centre for Health, Activity and Rehabilitation Research, University of Otago.
Appendices
Appendix 1. Cochrane Central Register of Controlled Trials (CENTRAL, via the Cochrane library) search strategy
#1 shoulder (7054)
#2 MeSH descriptor: [Rotator Cuff] explode all trees (382)
#3 #1 or #2 (7127)
#4 MeSH descriptor: [Calcium] this term only (3250)
#5 MeSH descriptor: [Bursitis] explode all trees (248)
#6 #4 or #5 (3498)
#7 #3 and #6 (185)
#8 MeSH descriptor: [Shoulder Pain] this term only (637)
#9 MeSH descriptor: [Shoulder Impingement Syndrome] this term only (232)
#10 MeSH descriptor: [Rotator Cuff Injuries] this term only (201)
#11 rotator cuff:ti,ab or supraspinatus:ti,ab or infraspinatus:ti,ab or subscapular*:ti,ab or teres:ti,ab (1131)
#12 ((shoulder*:ti,ab or subacromial:ti,ab or rotator cuff:ti,ab) near/5 (tendon*:ti,ab or tendin*:ti,ab or bursitis:ti,ab or calcium:ti,ab or calcif*:ti,ab or impinge*:ti,ab or tear*:ti,ab or pain:ti,ab)) (2488)
#13 #7 or #8 or #9 or #10 or #11 or #12 (3184)
#14 shockwave or shock wave (1587)
#15 MeSH descriptor: [Radiation, Nonionizing] explode all trees (2866)
#16 hesw (4)
#17 extracorporeal shock (1135)
#18 radial shock (133)
#19 #14 or #15 or #16 or #17 or #18 (4451)
#20 #13 and #19 (114)
Appendix 2. MEDLINE (Ovid) search strategy
1 Shoulder/ (11707)
2 rotator cuff/ (5569)
3 1 or 2 (16726)
4 calcium/ (257945)
5 3 and 4 (21)
6 shoulder pain/ (4123)
7 Shoulder Impingement Syndrome/ (1590)
8 rotator cuff injuries/ (4607)
9 (rotator cuff or supraspinatus or infraspinatus or subscapular$ or teres).tw. (13379)
10 ((shoulder$ or subacromial or rotator cuff) adj5 (tendon$ or tendin$ or calcium or calcif$ or impinge$ or tear$ or pain)).tw. (12875)
11 or/5‐10 (22171)
12 exp Radiation, Nonionizing/ (258404)
13 (shockwave$ or shock wave$).tw. (8449)
14 hesw.tw. (69)
15 ((extracorporeal or radial) adj shock$).tw. (5340)
16 or/12‐15 (265915)
17 11 and 16 (203)
18 randomized controlled trial.pt. (461808)
19 controlled clinical trial.pt. (92422)
20 randomized.ab. (361584)
21 placebo.ab. (173004)
22 drug therapy.fs. (2023031)
23 randomly.ab. (250488)
24 trial.ab. (374968)
25 groups.ab. (1564818)
26 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 (3900446)
27 exp animals/ not humans.sh. (4463900)
28 26 not 27 (3328315)
29 17 and 28 (96)
30 limit 29 to ed=19740101‐20180503 (96)
Appendix 3. Embase (Ovid) search strategy
1 shoulder/ (30686)
2 rotator cuff/ (5460)
3 1 or 2 (34156)
4 calcium/ (275714)
5 3 and 4 (171)
6 shoulder pain/ (13982)
7 shoulder impingement syndrome/ (2452)
8 rotator cuff injury/ (1836)
9 rotator cuff rupture/ (5831)
10 (rotator cuff or supraspinatus or infraspinatus or subscapular$ or teres).tw. (18794)
11 ((shoulder$ or subacromial or rotator cuff) adj5 (tendon$ or tendin$ or calcium or calcif$ or impinge$ or tear$ or pain)).tw. (19790)
12 or/5‐11 (37690)
13 exp radiation/ (556038)
14 (shockwave$ or shock wave$).tw. (13099)
15 hesw.tw. (73)
16 ((extracorporeal or radial) adj shock$).tw. (7611)
17 or/13‐16 (567797)
18 12 and 17 (849)
19 random$.tw. (1312577)
20 factorial$.tw. (33026)
21 crossover$.tw. (66509)
22 cross over.tw. (29264)
23 cross‐over.tw. (29264)
24 placebo$.tw. (275824)
25 (doubl$ adj blind$).tw. (190416)
26 (singl$ adj blind$).tw. (21300)
27 assign$.tw. (340342)
28 allocat$.tw. (128622)
29 volunteer$.tw. (233888)
30 crossover procedure/ (55878)
31 double blind procedure/ (151040)
32 randomized controlled trial/ (506798)
33 single blind procedure/ (31640)
34 or/19‐23 (1378960)
35 18 and 34 (155)
36 limit 35 to em=197401‐201818 (154)
Appendix 4. ClinicalTrials.gov search strategy
Database: clinicaltrials.gov/ 3 May 2018
Search strategy: Shock wave AND shoulder (6)
Appendix 5. WHO International Clinical Trials Registry Platform (ICTRP)
Database: apps.who.int/trialsearch/default.aspx 3 May 2018
Search strategy: Shock wave AND shoulder (16)
Data and analyses
Comparison 1. Shock wave therapy (ESWT) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants with ≥ 50% improvement in pain | 1 | 74 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.62, 1.94] |
| 2 Mean pain (various scales, lower score indicates less pain) | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 6 weeks | 6 | 304 | Mean Difference (IV, Random, 95% CI) | ‐2.10 [‐3.58, ‐0.62] |
| 2.2 3 months | 9 | 608 | Mean Difference (IV, Random, 95% CI) | ‐1.95 [‐3.45, ‐0.44] |
| 2.3 6 months | 5 | 419 | Mean Difference (IV, Random, 95% CI) | ‐1.53 [‐3.49, 0.43] |
| 2.4 12 months | 3 | 155 | Mean Difference (IV, Random, 95% CI) | ‐2.42 [‐5.79, 0.95] |
| 3 Mean function (various scales) | 11 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 6 weeks | 7 | 374 | Std. Mean Difference (IV, Random, 95% CI) | 0.79 [0.30, 1.28] |
| 3.2 3 months | 9 | 612 | Std. Mean Difference (IV, Random, 95% CI) | 0.62 [0.13, 1.11] |
| 3.3 6 months | 7 | 486 | Std. Mean Difference (IV, Random, 95% CI) | 0.91 [0.24, 1.57] |
| 3.4 12 months | 3 | 155 | Std. Mean Difference (IV, Random, 95% CI) | 1.45 [‐0.21, 3.12] |
| 4 Treatment success | 6 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.87, 2.91] |
| 5 Withdrawals due to adverse events and treatment intolerance | 7 | 581 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.43, 1.31] |
| 6 Total withdrawals | 8 | 621 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.52, 1.07] |
| 7 Proportion of participants with adverse events | 5 | 295 | Risk Ratio (M‐H, Random, 95% CI) | 3.61 [2.00, 6.52] |
| 8 Calcification size (complete resolution) | 3 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 4.78 [1.31, 17.39] |
| 9 Calcification size (partial resolution) | 3 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 3.41 [0.95, 12.23] |
| 10 Mean or change in mean calcification width (mm) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 3 months | 1 | 88 | Mean Difference (IV, Random, 95% CI) | ‐24.00 [‐85.77, 33.77] |
| 10.2 6 months | 1 | 87 | Mean Difference (IV, Random, 95% CI) | ‐36.7 [‐94.86, 21.46] |
| 10.3 12 months | 2 | 122 | Mean Difference (IV, Random, 95% CI) | ‐21.76 [‐60.99, 17.46] |
| 11 Subgroup analysis: pain (various scales, lower score indicates less pain) | 9 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 Calcification | 5 | 256 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐1.33, 0.14] |
| 11.2 No calcification | 5 | 253 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.70, ‐0.09] |
| 12 Subgroup: function (various scales, higher score is better function) | 9 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 Calcification | 5 | 260 | Std. Mean Difference (IV, Random, 95% CI) | 0.84 [‐0.20, 1.89] |
| 12.2 No calcification | 5 | 253 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.04, 0.61] |
1.1. Analysis.
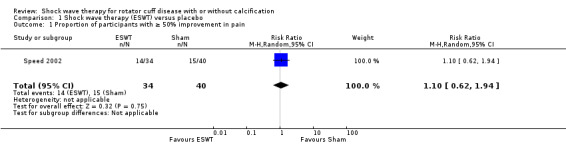
Comparison 1 Shock wave therapy (ESWT) versus placebo, Outcome 1 Proportion of participants with ≥ 50% improvement in pain.
1.6. Analysis.
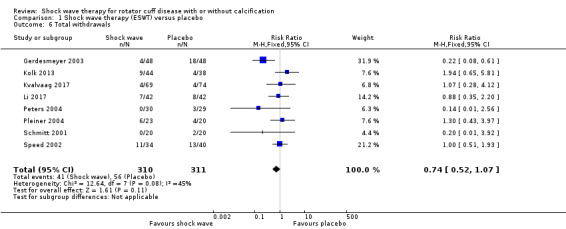
Comparison 1 Shock wave therapy (ESWT) versus placebo, Outcome 6 Total withdrawals.
Comparison 2. Shock wave therapy (ESWT) versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean function (Constant score 0–100, 100 indicating best) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 3 months | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 3.80 [‐6.33, 13.93] |
| 2 Treatment success as determined by participant | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3 Calcification size (complete resolution) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
Comparison 3. Shock wave therapy (ESWT) versus ultrasound‐guided needling with glucocorticoid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean calcification size | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Calcification size (complete resolution) | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.35, 0.95] |
| 3 Calcification size (partial resolution) | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.38, 5.42] |
Comparison 4. Radial shock wave therapy (RSWT) versus ultrasound‐guided needling with corticosteroid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean pain (Numerical Rating Scale, 0–10, higher score indicating worse pain)) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 6 weeks | 1 | 25 | Mean Difference (IV, Random, 95% CI) | 1.60 [0.13, 3.07] |
| 1.2 12 months | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐2.05, 2.45] |
| 2 Function (Constant score, 0–100, higher score indicating better function) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 6 weeks | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐11.70 [‐24.79, 1.39] |
| 3 Function (Oxford Score 12–60) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 6 weeks | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐2.30 [‐9.30, 4.70] |
| 3.2 12 months | 1 | 19 | Mean Difference (IV, Random, 95% CI) | ‐4.10 [‐15.74, 7.54] |
| 4 Treatment success (proportion of participants with no complaints) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5 Proportion of participants with adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6 Calcification size (complete resolution) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
Comparison 5. Radial extracorporeal shock wave therapy (rESWT) versus supervised exercises.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean pain (9‐point Likert, 9 is most pain) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 6 weeks | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.53, 1.13] |
| 1.2 3 months | 1 | 102 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.36, 1.16] |
| 1.3 6 months | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.56, 0.96] |
| 1.4 12 months | 1 | 97 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐0.20, 1.20] |
| 2 Mean function (SPADI 0–100, 100 is best) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 6 weeks | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 3 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 12 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Proportion of participants who withdrew due to adverse events | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.32, 27.91] |
| 4 Proportion of participants who experienced adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Active range of abduction | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 3 months | 1 | 104 | Mean Difference (IV, Random, 95% CI) | ‐1.95 [‐10.50, 6.60] |
| 5.2 6 months | 1 | 104 | Mean Difference (IV, Random, 95% CI) | ‐11.82 [‐25.37, 1.73] |
Comparison 6. Extracorporeal shock wave therapy (ESWT) versus ultrasound‐guided percutaneous lavage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (VAS 0–10, higher score indicating worse pain) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 6 weeks | 1 | 201 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.26, 0.06] |
| 1.2 3 months | 1 | 201 | Mean Difference (IV, Random, 95% CI) | 1.9 [1.54, 2.26] |
| 1.3 6 months | 1 | 201 | Mean Difference (IV, Random, 95% CI) | 1.80 [1.36, 2.24] |
| 1.4 12 months | 1 | 201 | Mean Difference (IV, Random, 95% CI) | 1.90 [1.34, 2.46] |
| 2 Treatment success (pain free) | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.81, 1.03] |
| 3 Proportion of participants with adverse events | 1 | 243 | Risk Ratio (M‐H, Random, 95% CI) | 0.08 [0.00, 1.36] |
| 4 Calcification size | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 6 weeks | 1 | 201 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐2.94, ‐1.06] |
| 4.2 3 months | 1 | 201 | Mean Difference (IV, Random, 95% CI) | 2.0 [1.17, 2.83] |
| 4.3 6 months | 1 | 201 | Mean Difference (IV, Random, 95% CI) | 2.40 [1.44, 3.36] |
| 4.4 12 months | 1 | 201 | Mean Difference (IV, Random, 95% CI) | 3.1 [2.07, 4.13] |
| 5 Calcification size (proportion with complete resolution) | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.53, 0.80] |
Comparison 7. Extracorporeal shock wave therapy (ESWT) versus transcutaneous electrical nerve stimulation (TENS).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in mean pain from baseline (0–10 VAS, 0 is no pain) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 6 weeks | 1 | 62 | Mean Difference (IV, Random, 95% CI) | ‐1.9 [‐2.98, ‐0.82] |
| 1.2 3 months | 1 | 62 | Mean Difference (IV, Random, 95% CI) | ‐2.34 [‐3.53, ‐1.15] |
| 2 Mean function (Constant score 0–100, 0 is worst and 100 is best) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 6 weeks | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 3 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Withdrawals | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.01, 6.95] |
| 4 Proportion of participants with adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Reduction in calcification size (mm) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 6 weeks | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 3 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 8. Extracorporeal shock wave therapy (ESWT) high dose versus ESWT low dose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean pain (various scales, lower score indicates less pain) | 8 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 6 weeks | 2 | 117 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.73 [‐3.94, 0.48] |
| 1.2 3 months | 6 | 326 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.67, 0.16] |
| 1.3 6 months | 4 | 309 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.66 [‐2.98, ‐0.33] |
| 1.4 12 months | 3 | 196 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.39, 0.18] |
| 2 Mean function (various scales, higher score is better function) | 10 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 6 weeks | 2 | 117 | Std. Mean Difference (IV, Random, 95% CI) | 3.71 [‐3.71, 11.14] |
| 2.2 3 months | 7 | 366 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [0.08, 0.53] |
| 2.3 6 months | 5 | 409 | Std. Mean Difference (IV, Random, 95% CI) | 2.29 [1.05, 3.52] |
| 2.4 12 months | 3 | 196 | Std. Mean Difference (IV, Random, 95% CI) | 0.50 [‐0.03, 1.02] |
| 3 Treatment success as determined by participant | 6 | 450 | Risk Ratio (M‐H, Random, 95% CI) | 2.74 [1.58, 4.77] |
| 4 Withdrawals | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5 Proportion of participants who experienced adverse events | 5 | 351 | Risk Ratio (M‐H, Random, 95% CI) | 3.51 [1.53, 8.03] |
| 6 Range of movement (University of California at Los Angeles subscore, active flexion measured in degrees) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 6 weeks | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 49.35 [37.39, 61.31] |
| 6.2 6 months | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 62.0 [50.59, 73.41] |
| 7 Resolution of calcification | 4 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 2.91 [1.04, 8.15] |
| 8 Partial resolution of calcification | 2 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.73, 1.75] |
| 9 Calcification size (mm) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 6 months | 3 | 229 | Mean Difference (IV, Random, 95% CI) | ‐24.19 [‐44.83, ‐3.55] |
| 9.2 12 months | 1 | 79 | Mean Difference (IV, Random, 95% CI) | ‐70.70 [‐141.05, ‐0.35] |
| 10 Calcification size (> 80% reduction of calcified surface on anteroposterior view) | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.64, 13.98] |
8.4. Analysis.
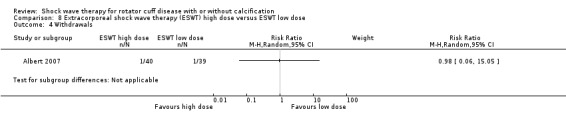
Comparison 8 Extracorporeal shock wave therapy (ESWT) high dose versus ESWT low dose, Outcome 4 Withdrawals.
Comparison 9. Extracorporeal shock wave therapy (ESWT) two sessions versus ESWT one session.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean function (Constant score, 0–100, 100 is best) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 3 months | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 4.80 [‐3.80, 13.40] |
| 2 Treatment success as determined by participant | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.74, 1.85] |
| 3 Resolution of calcification | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.64, 1.86] |
Comparison 10. Extracorporeal shock wave therapy (ESWT) calcification‐focused versus ESWT supraspinatus origin‐focused.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean pain (0–10 point NRS, 0 is no pain) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 3 months | 1 | 47 | Mean Difference (IV, Random, 95% CI) | ‐1.53 [‐3.24, 0.18] |
| 1.2 12 months | 1 | 49 | Mean Difference (IV, Random, 95% CI) | ‐2.27 [‐3.49, ‐1.05] |
| 2 Mean function (Constant score 0–100, 100 is best) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 6 months | 1 | 47 | Mean Difference (IV, Random, 95% CI) | 31.51 [16.33, 46.69] |
| 2.2 12 months | 1 | 49 | Mean Difference (IV, Random, 95% CI) | 32.73 [20.40, 45.06] |
| 3 Treatment success as determined by participant satisfaction | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Calcification size (complete resolution) | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [0.84, 3.07] |
Comparison 11. Extracorporeal shock wave therapy (ESWT) image‐guided versus ESWT palpation‐guided.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean pain (0–100 VAS, 0 is no pain) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 3 months | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐15.15 [‐26.62, ‐3.68] |
| 2 Mean function (Constant score 0–100, 100 is best) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 3 months | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 6.48 [‐2.22, 15.18] |
| 3 Calcification size (complete resolution) | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 6.0 [0.78, 46.29] |
| 4 Calcification size (partial resolution) | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.4 [0.51, 3.82] |
Comparison 12. Extracorporeal shock wave therapy (ESWT) with hyperextended arm position versus ESWT with neutral arm position.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean pain (0–15 VAS, 15 is worst pain) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 3 months | 1 | 35 | Mean Difference (IV, Random, 95% CI) | 1.70 [‐0.55, 3.95] |
| 2 Mean function (Constant score 0–100, 100 is best) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 3 months | 1 | 35 | Mean Difference (IV, Random, 95% CI) | 9.0 [0.72, 17.28] |
| 3 Calcification size (> 80% reduction of calcified surface on anteroposterior view) | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 1.89 [0.92, 3.89] |
Comparison 13. Extracorporeal shock wave therapy (ESWT) and exercise and advice versus exercise and advice.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean function (Constant score 0–100, 100 is best) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 12 months | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 9.35 [4.98, 13.72] |
Comparison 14. Shock wave therapy (ESWT) versus ultrasound guided hyaluronic acid (HA) injection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Function | 1 | 34 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.94, 0.41] |
| 1.1 3 months | 1 | 34 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.94, 0.41] |
Comparison 15. Radial extracorporeal shock wave therapy (rESWT) plus physiotherapy versus physiotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean pain | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐1.58, ‐0.82] |
| 2 Mean function | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐11.30 [‐14.75, ‐7.85] |
| 3 Range of movement (ROM) flexion | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 31.60 [24.04, 39.16] |
| 4 ROM extension | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 17.00 [14.10, 19.90] |
| 5 ROM abduction | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 41.8 [32.79, 50.81] |
| 6 ROM external rotation | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 23.2 [16.98, 29.42] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Albert 2007.
| Methods |
Study design: single‐centre, parallel group, two‐arm RCT Setting: outpatient setting, Rennes University Hospital, France Trial time period: December 2002 to August 2004 Interventions: high‐dose ESWT vs low‐dose ESWT Sample size calculation: 40 people per treatment group required to achieve 95% power to detect a difference of ≥ 15% in the change in CMS between the active treatment and control groups at 3 months' follow‐up Analysis: ITT |
|
| Participants |
Number of participants:
Inclusion criteria:
Exclusion criteria:
Baseline characteristics: High‐dose ESWT (40 participants):
Low‐dose ESWT(40 participants):
Pretreatment group differences: participants in high‐dose ESWT group had a longer duration of symptoms compared with low‐dose group. The low‐dose participants had more subacromial injections in past than those in high‐dose group. |
|
| Interventions |
High‐energy ESWT:
Low‐energy ESWT:
|
|
| Outcomes | Measured at baseline and 3 months Outcomes included in review:
Other outcomes in trial, excluded from review:
|
|
| Source of funding | Funding by Clinical Research Commission of Rennes University Hospital. The electronic dynamometer was provided by Smith‐Nephew France (ZITournes‐Cliron BP 1109, Tournes, France). | |
| Notes |
Trial registration: not registered Time points included in review: 3 months Data analysis: range was the only measure of variance reported for pain and function; we used SD for change in pain from Gerdesmeyer 2003, and SD for mean function score from Ioppolo 2012 Withdrawals: 1/40 in high‐dose group due to resolution of symptoms and 1/40 in low‐dose group due to a panic attack Adverse events: High‐dose ESWT:
Low‐dose ESWT:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised randomisation list using block sizes of 4 used. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment centralised; thus, risk of selection bias was probably low. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were reported as blinded, the physician measuring outcomes at the follow‐up was not blinded; however, authors stated that "the assignment chart was not consulted before the assessment was completed." |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Participants were blinded to their treatment group, and self‐reported outcomes of pain, function and treatment success were unlikely to be affected by bias. |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes | Low risk | Radiological assessment performed by an assessor unaware of treatment allocation. The other 'assessing physician' although unblinded did not have access to the assignment chart until measurements were completed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/80 withdrew (1/40 in each group); follow‐up data was collected from both participants but the low‐dose group participant refused to attend the clinic visit so data collection was done via telephone. |
| Selective reporting (reporting bias) | Low risk | No published study protocol, but results included all major outcomes, thus the risk of reporting bias was probably low. |
| Other bias | Low risk | No other biases apparent. |
Cacchio 2006.
| Methods |
Study design: single‐centre, parallel‐group, two‐arm, single‐blind, RCT Setting: San Salvatore Hospital of L'Aquila, Coppito‐L Aquila, Italy Trial time period: November 2002 to December 2003 Interventions: high‐dose RSWT vs low‐dose RSWT Sample size calculation: not performed Analysis: ITT |
|
| Participants |
Number of participants
Inclusion criteria:
Exclusion criteria:
Baseline characteristics: High‐dose RSWT (45 participants):
Low‐dose shock wave therapy (45 participants):
Pretreatment group differences: none |
|
| Interventions |
High‐dose RSWT:
Low‐dose RSWT group:
|
|
| Outcomes | Measured at 1 week and 6 months after treatment Outcomes included in review:
Outcomes excluded from review:
|
|
| Source of funding | Not reported | |
| Notes |
Trial registration: not registered Time points included in review: 1 and 6 months Data analysis: we used the enrolled population (45 participants per group) as the denominator to measure treatment success. We extracted data that included transformed data for 6 participants in low‐dose group who had received additional treatment for pain and function at 6 months. Withdrawals: 0/45 in high‐dose group; 6/45 in low‐dose group as they received local steroid injections Adverse events: High‐dose RSWT:
Low‐dose RSWT:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated 1:1 randomisation scheme. |
| Allocation concealment (selection bias) | Unclear risk | Used 'sealed envelopes'; insufficient information to determine if this was adequate to conceal the random sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel unaware of treatment group. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Participants blinded. Low risk of bias in self‐reported outcomes. |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes | Low risk | Outcome assessors and the radiologist blinded to treatment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6/90; 0/45 in high‐dose group, 6/45 (13%) in low‐dose group as they received local steroid injections; however, their data were included in final analysis. The trialists used the final mean change recorded in per‐protocol completer population instead, possibly overestimating the benefits of high‐dose shock wave. |
| Selective reporting (reporting bias) | Low risk | Although there was no trial protocol, data reported for all outcomes measured (as reported in methods). |
| Other bias | Low risk | No other biases apparent |
Cosentino 2003.
| Methods |
Study design: single‐centre, parallel‐group, two‐arm, single‐blind, RCT Setting: Italy Trial time period: not reported Interventions: ESWT vs sham procedure Sample size calculation: not performed Analysis: method was not described |
|
| Participants |
Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics: ESWT (35 participants):
Sham procedure (35 participants):
Pretreatment group differences: not reported |
|
| Interventions |
ESWT:
Sham procedure:
|
|
| Outcomes | Pain and function measured at baseline, end of treatment, 1 month and 6 months; calcifications measured at 1 month Outcomes included in review:
Outcomes excluded from review:
|
|
| Source of funding | No source of funding reported | |
| Notes |
Trial registration: not registered Time points included in review: 1 and 6 months Data analysis: trialists did not report pain for the sham group, thus we excluded this study from Analysis 1.2. Trialists did not report the number of withdrawals from the shock wave group; we excluded this study from Analysis 1.5. The mean Constant score was extracted using the WebPlotDigitier program found at arohatgi.info/WebPlotDigitizer/app. It was unclear whether the graph also displayed SE or SD; we assumed SD. Where extracted numbers differed from a reported figure, the reported figure was used. Proportion of participants with adverse events was reported as 0 for both groups (apart from initial transient treatment pain, although the number of participants with the event in each group was not explicitly reported) Withdrawals: 23/35 participants from the sham group and 0/35 from the shock wave group at 6 months' follow‐up. No reasons for withdrawal were given. Adverse events: ESWT:
Sham procedure:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study personnel not blinded; however, the radiologist assessing calcifications was blinded. Participants blinded to group allocations. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Due to blinding of participants, there was low risk of bias in reporting of pain and function. |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes | Low risk | Radiologist who measured calcification size blinded to the group allocations, objective outcomes in Constant score were not used in this review. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 23/35 (66%) participants were lost from the sham group at 6 months, with no reason given; 0/35 lost from the shock wave group. |
| Selective reporting (reporting bias) | High risk | Study outcomes not reported clearly, means given but ranges were missing for baseline values. Pain scores given only for the treatment group and not for the sham group. |
| Other bias | Low risk | No other biases apparent |
De Boer 2017.
| Methods |
Study design: single‐centre, parallel‐group, two‐arm, RCT Setting: outpatient clinic, Department of Orthopaedic Surgery, Hospital in the Netherlands Trial time period: May 2010 and March 2011 Interventions: RSWT vs US‐guided needling Sample size calculation: in initial power calculation of the medical ethical committee, 40 participants were needed for inclusion (20 in each group) Analysis: as‐treated |
|
| Participants |
Number of participants:
Inclusion criteria:
Exclusion criteria:
Baseline characteristics: RSWT (14 participants):
US‐guided needling (11 participants):
|
|
| Interventions |
RSWT:
US‐guided needling:
|
|
| Outcomes | Measured at baseline, 6 weeks and 12 months Outcomes included in review:
Note that both function scores were extracted as the Constant score was not reported at 1 year Outcomes excluded from review:
|
|
| Source of funding | None reported | |
| Notes |
Trial registration: not registered Time points included in review: 6 weeks and 12 months Data analysis: outcome data extracted at 6 weeks and 12 months Withdrawals: 5/14 in shock wave group (severe pain); 1/11 in US needling group (severe pain) Adverse events: RSWT:
US‐guided needling:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done by allowing the patient to choose an unmarked envelope containing the treatment protocol for either UN or RSWT from a box. The envelopes were randomized in blocks (6 envelopes, 3 of each treatment). When a block was finished, the next block was started." |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither participants nor personnel were blinded. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Participants were not blinded to their treatment group due to the nature of their intervention, and this was likely to affect the measurement of pain and function. |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes | Low risk | The X‐ray results were collected by a nurse practitioner and though it was not clear if they were blinded, this was unlikely to affect the data. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6/25; 5/14 (35.7%) in shock wave group (severe pain) and 1/11 (9%) in US needling group. The trialists did not use an ITT analysis and excluded these 6 participants from the follow‐up analysis. |
| Selective reporting (reporting bias) | High risk | No access to the study protocol and trial was not registered. In outcome data, 12‐month Constant scores not reported. |
| Other bias | High risk | Data Safety Monitoring Board prematurely terminated inclusion because of the higher pain score in shock wave group. |
Del Castillo‐Gonzales 2016.
| Methods |
Study design: single‐centre, parallel‐group, two‐arm, single‐blind, RCT Setting: Centro Medico Deyre, Madrid, Spain Trial time period: January 2007 to December 2013. Interventions: ESWT vs US‐guided percutaneous lavage Sample size calculation: not described Analysis: only performed in participants who completed study |
|
| Participants |
Number of participants:
Inclusion criteria:
Exclusion criteria:
Baseline characteristics: not provided |
|
| Interventions |
ESWT:
US‐guided percutaneous lavage:
|
|
| Outcomes | Measured at baseline, 3, 6 and 12 months Outcomes included in review:
Outcomes excluded from review:
|
|
| Source of funding | Partly funded by grant awarded by the Santander Group to the Foundation Alfonso X el Sabio University | |
| Notes |
Trial registration: not registered Time points included in review: 3, 6 and 12 months Data analysis: review data extracted at 3, 6 and 12 months. Mild discomfort during shock wave was not counted as an adverse event in this review. Withdrawals: 41/121 in ESWT group (38 did not complete intervention, 3 did not attend examinations) vs 1/122 in lavage group (lost to follow‐up) Adverse events: ESWT:
US‐guided percutaneous lavage:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using tables of randomized numbers (www.randomized.org), a collaborator who took no further part in the study randomly assigned patients to undergo either ESWT (N=121) or UGPL [ultrasound‐guided percutaneous lavage] (N=122)." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants not blinded but study staff were blinded to the group allocation. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | As participants were not blinded it is likely that the self‐reported outcome (pain) was subject to bias. |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes | Low risk | Outcome assessors blinded to the group allocation so low risk of bias in measurement of calcifications. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 41/121 (34%) in ESWT group (38 not completing intervention, 3 not attending examinations) vs 1/122 (0.8%) in lavage group. As‐treated analysis performed. |
| Selective reporting (reporting bias) | Low risk | No access to a protocol, but results were reported for all outcomes listed as measured in methods. |
| Other bias | Low risk | No other biases apparent |
Duymaz 2019.
| Methods |
Study design: single‐centre, parallel‐group, two‐arm, RCT Setting: Istanbul Bilgi University Faculty of Health Sciences, Turkey Trial time period: August 2017 to April 2018 Interventions: rESWT plus conventional physiotherapy vs conventional physiotherapy Sample size calculation: standard effect size of 0.72 and ≥ 80 cases with a 95% CI and a power of 80% so 40 participants were recruited for each arm. Analysis: ITT |
|
| Participants |
Number of participants:
Inclusion criteria:
Exclusion criteria:
Baseline characteristics: rESWT plus physiotherapy group:
Physiotherapy group:
Pretreatment group differences: function was worse in rESWT group compared with the control group at baseline. |
|
| Interventions |
rESWT plus conventional physiotherapy:
Conventional physiotherapy:
|
|
| Outcomes | Measured at end of treatment Study outcomes:
Outcomes used in review:
|
|
| Source of funding | Authors reported that they did not receive any funding for this study | |
| Notes |
Trial registration: not registered Withdrawals: none Adverse events: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence used to generate the random schedule. |
| Allocation concealment (selection bias) | Unclear risk | Unclear how allocation to groups was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and study personnel not reported. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Unclear risk | Unclear whether participants were blinded; unclear risk of bias in measurement of pain and function. |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes | Unclear risk | Unclear whether outcome assessors were blinded; unclear risk of bias in measurement of ROM. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals reported. |
| Selective reporting (reporting bias) | Low risk | Although the trial was not registered and there was no study protocol, results for all prespecified outcomes were clearly reported. |
| Other bias | Low risk | Function scores were lower in control group; however, this did not affect the study findings. |
Engebretsen 2009.
| Methods |
Study design: parallel‐group, two‐arm, single‐blind, RCT Setting: outpatient clinic of the Physical Medicine and Rehabilitation Department at Ullevaal University Hospital, Oslo, Norway Trial time period: July 2006 to August 2007 Interventions: rESWT vs supervised exercises Sample size calculation: study designed to detect a difference of 10 points in SPADI score between groups with α = 0.05 (type I error) and β = 0.2 (type II error). 48 participants were required per group to detect a 10‐point change in SPADI score with a 20‐point SD. Analysis: ITT |
|
| Participants |
Number of participants:
Inclusion criteria
Exclusion criteria
Baseline characteristics: Radial ESWT (52 participants):
Supervised exercises (52 participants):
Pretreatment group differences: groups similar at baseline with regard to demographic and outcome variables. |
|
| Interventions |
rESWT:
Supervised exercises:
|
|
| Outcomes | Outcomes measured at baseline, 6 weeks, 12 weeks, 18 weeks and 1 year Outcomes included in review
Outcomes excluded from review
|
|
| Source of funding | Study supported by Health Region East, Norway | |
| Notes |
Trial registration: ClinicalTrials.gov identifier NCT00653081 Time points included in review: 6 weeks and 12 months Data analysis: author provided unpublished information: mean age of cohort participants and duration of symptoms, methods of randomisation and allocation concealment, attrition rates, active range of abduction (mean and SD). 13/52 in shock wave group and 3/52 in exercise group received additional treatment (cortisone injections, chiropractic treatment, physical therapy or supervised exercises) between 12 and 18 weeks. Withdrawals: 4/52 in shock wave group (1 death, 1 loss to follow‐up, 2 incomplete questionnaires) and 3/52 in exercise group (2 loss to follow‐up, 1 incomplete questionnaire) Adverse events: RSWT:
Supervised exercises:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A statistician not involved in data collection or analysis randomly allocated patients to treatment groups in blocks of four to six. Randomisation was stratified by sex." Comment: adequate method used to generate the allocation sequence. |
| Allocation concealment (selection bias) | Low risk | Quote: "A person not involved in the treatments opened the sealed envelopes and assigned appointments according to treatment group." Comment: adequate method likely used to conceal the allocation sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Participants and personnel could not be blinded for this trial." Comment: given the nature of the interventions, participants were not blind to treatment, and may have had different expectations about the benefits of each intervention. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: unblinded participants, who may have had different expectations about the benefits of the intervention they received. |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes | Low risk | Quote: "A blinded physiotherapist made the baseline and follow‐up measurements. The patients were instructed not to discuss their treatment with the blinded physiotherapist." Comment: assessor of objective outcomes was likely blinded to the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 10 participants per group did not return for all follow‐up measures, and while reasons for loss to follow‐up were not reported, an ITT analysis was performed; 7/104; 4/52 in shock wave group (1 death, 1 loss to follow‐up, 2 incomplete questionnaires) and 3/52 in exercise group (2 loss to follow‐up, 1 incomplete questionnaire). |
| Selective reporting (reporting bias) | Low risk | Active ROM data were not reported; the authors supplied the unpublished data upon request. Function, work status were not listed in trial protocol but were in results paper. |
| Other bias | High risk | More people in shock wave group received additional treatments outside of the trial setting, including injection, physiotherapy or chiropractice (13 from shock wave vs 3 from exercise), which may have biased the results in their favour. |
Farr 2011.
| Methods |
Study design: prospective, parallel‐group, two‐arm, single‐blind, RCT Setting: orthopaedic department's outpatient clinic, Austria Trial time period: not reported Interventions: single high‐dose ESWT vs 2 treatments of low‐dose ESWT Sample size calculations: not performed for this study, the authors reported that 200 participants per group would be required to detect differences between groups for the VAS (at rest) and Constant score Analysis: study did not report using an ITT analysis |
|
| Participants |
Number of participants:
Inclusion criteria:
Exclusion criteria: not reported Baseline characteristics: High‐dose ESWT(15 participants):
Low‐dose ESWT(15 participants):
Pretreatment group differences: no significant differences in age, sex, weight, VAS (rest), Constant score at baseline. Duration of symptoms and treatment history not reported. |
|
| Interventions |
High‐dose ESWT:
Low‐dose ESWT:
|
|
| Outcomes | Measured at 6 and 12 weeks Outcomes included in review:
Outcomes excluded from review:
|
|
| Source of funding | Not reported | |
| Notes |
Trial registration: not reported Time points included in review: 6 and 12 weeks Data analysis: function and pain at rest extracted at 6 and 12 weeks. Radiological changes extracted at study conclusion (12 weeks) Withdrawals: 2/15 in high‐dose group and 1/15 in low‐dose group were lost to follow‐up Adverse events: High‐dose shock wave therapy:
Low‐dose shock wave therapy
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of randomisation method were not reported. |
| Allocation concealment (selection bias) | Unclear risk | Methods of allocation concealment were not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | 'Observers' were blinded and participants were not blinded. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Due to the nature of the intervention participants were unable to be blinded; thus, there was a potential risk of bias in the self‐reported outcomes of pain and function. |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes | Low risk | Outcome assessors were blinded so there was low risk of bias in the assessment of radiographic outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/15 in high‐dose group and 1/15 in low‐dose group were lost to follow‐up, data were not collected from them for the final analysis. |
| Selective reporting (reporting bias) | Low risk | There was no published study protocol, but results were reported for all outcomes as mentioned in methods, and included major outcomes. |
| Other bias | Low risk | No other biases were apparent in study. |
Frizziero 2017.
| Methods |
Study design: single‐centre, parallel‐group, two‐arm, single‐blind, RCT Setting: Department of Physical Medicine and Rehabilitation, University of Padua, Italy Trial time period: not reported Interventions: LMW‐HA injection vs low‐energy ESWT Sample size calculation: not reported Analysis: ITT |
|
| Participants |
Number of participants:
Inclusion criteria:
Exclusion criteria:
Baseline characteristics: Low‐energy ESWT (17 participants):
LMW‐HA (17 participants):
Pretreatment group differences: mean Constant scores were higher in low‐energy ESWT group, while the DASH scores were slightly higher in LMW‐HA group. |
|
| Interventions |
LMW‐HA:
Low‐energy ESWT:
|
|
| Outcomes | Measured at baseline, postintervention and 3 months Outcomes used in review:
Outcomes excluded from review:
|
|
| Source of funding | Not reported | |
| Notes |
Trial registration: not reported Time points included in review: 3 months Data analysis: author provided SDs and SEs for DASH and Constant scores which were not published in paper. Withdrawals: none Adverse events: not measured |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation performed using a computer‐generated schedule. |
| Allocation concealment (selection bias) | Unclear risk | Unclear whether concealment of the group allocation was done. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were unable to be blinded, study personnel were blinded to the treatment allocation. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Due to the nature of the interventions, participants could not be blinded; there was risk of bias in measurement of self‐reported outcomes of pain and function. |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes | Low risk | Study personnel were blinded so there was low risk of bias in measurement of objective outcomes of function using the CMS. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals. |
| Selective reporting (reporting bias) | Low risk | Author provided SDs and SEs, as well as information on method of randomisation upon request. |
| Other bias | Low risk | No other biases apparent. |
Galasso 2012.
| Methods |
Study design: parallel‐group, two‐arm, double‐blind, randomised placebo‐controlled trial Setting: outpatients, Italy Trial time period: not reported Interventions: ESWT vs sham therapy Sample size calculation: not performed Analysis: as‐treated |
|
| Participants |
Number of participants:
Inclusion criteria
Exclusion criteria
Baseline characteristics: ESWT (11 participants):
Sham group (9 participants)
Pretreatment group differences: no differences in baseline, except BMI |
|
| Interventions |
ESWT:
Sham shock wave therapy:
|
|
| Outcomes | Measured at baseline, 6 and 12 weeks Outcomes included in review:
Outcomes excluded from review:
|
|
| Source of funding | Study supported, in part, by Storz Medical AG, Tagerwilen, Switzerland | |
| Notes |
Trial registration: ISRCTN registry ISRCTN41236511 Time points included in review: 6 and 12 weeks Data analysis: the Constant pain subscore was subtracted from 15 to reverse the direction of the scale (lower score would indicate less pain). We e‐mailed the study contact to ask for clarification of methods of allocation concealment (no response at time of publication of this review) Withdrawals: 0/11 in ESWT group; 1/9 in sham group were lost to follow‐up Adverse events: ESWT:
Sham treatment:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified random permuted blocks with an allocation ratio of 1:1. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and outcome assessors were blinded; local anaesthetic was used in all participants to mask active or placebo treatment. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Participants were blinded so low risk of bias in measurement of pain and function. |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes | Low risk | Outcome assessors who measured radiographic outcomes were blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 0/11 in ESWT group and 1/10 in sham group (lost to follow‐up). |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in study protocol were reported in results paper. |
| Other bias | Low risk | No other biases apparent. |
Gerdesmeyer 2003.
| Methods |
Study design: multicentre, parallel‐group, three‐arm, double‐blind, randomised, placebo‐controlled trial Setting: 7 orthopaedic departments in Germany and Austria Trial time period: February 1997 to March 2001 Interventions: high‐energy ESWT vs low‐energy ESWT vs sham therapy Sample size calculation: 144 participants would have 90% power to find a 15% difference in Constant score between therapy and placebo, given α = 0.025. Analysis: ITT analysis was used for primary outcomes, with missing data imputed using last observation carried forward |
|
| Participants |
Number of participants:
Inclusion criteria:
Exclusion criteria:
Baseline characteristics: High‐dose ESWT (48 participants):
Low‐dose ESWT (48 participants)
Sham treatment (48 participants):
Pretreatment group differences: no group differences were found at baseline with the exception of the calcific deposit which was smaller in sham group compared to the ESWT group |
|
| Interventions |
High‐energy ESWT:
Low‐energy ESWT:
Sham treatment:
|
|
| Outcomes | Measured at baseline, 3, 6 and 12 months Outcomes included in review:
Outcomes excluded from review:
|
|
| Source of funding | Supported by the German Association for Orthopedics and Orthopedic Surgery (DGOCC). Shock wave equipment supplied by Domier Medlizintechnilk, Wessling, Germany. | |
| Notes |
Trial registration: not reported Time points included in review: 3, 6 and 12 months Data analysis: as there were 2 intervention groups, the low‐energy group data were used for the comparison ESWT vs sham as it was more consistent with the energy levels used in other trials. The high‐energy group and low‐energy group were used for the comparison high vs low dose. The 95% CI was converted to a SD using the following equation in excel: (('upper CI' – 'lower CI')/3.92) × √'population'). Results were then rounded to 1 decimal place. Proportion of participants with ≥ 30% increase in CMS, noted in trial as a clinically relevant improvement, was taken as the measure of treatment success. As proportion of participants who experienced adverse events was reported by category of adverse event, the largest number from any category was used in data extraction as a best estimate. Withdrawals: 13/48 in high‐dose ESWT group (7 refused follow‐up visit, 6 reported no reason): 4/48 in low‐dose ESWT group (2 had drug therapy and 2 had surgery) and 18/48 in placebo group (7 had drug therapy, 5 had surgery, 5 refused follow‐up visits, 1 moved). We assumed 4/48 in ESWT and 12/48 in placebo were intolerant to treatment (Analysis 1.5). Adverse events: High‐dose ESWT:
Low‐dose ESWT:
Sham treatment:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation using a computer‐generated sequence at a central location. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes stored at a central location, allocation by telephone. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and evaluators were reported as blinded to treatment; however as intravenous analgesics and sedation were offered 'as needed', and local anaesthetic was not allowed, it was unclear if participants could guess their assignment. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Unclear risk | Unclear if participants may have guessed their treatment group, thus reporting of pain, function and treatment success could have been subject to bias. |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes | Low risk | Radiologists who assessed calcification were blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 33/144; 13/48 (27%) in high‐dose ESWT group (7 refused follow‐up visit, 6 withdrawn), 4/48 (8.3%) in low‐dose ESWT group (no reasons given) and 16/48 (33%) in sham group (5 refused follow‐up visits, 11 withdrawn). |
| Selective reporting (reporting bias) | Low risk | No published study protocol, but results were reported for all outcomes as mentioned in the methods, and included major outcomes. Low risk of reporting bias. |
| Other bias | Low risk | No other biases apparent in the study. |
Haake 2002.
| Methods |
Study design: parallel‐group, two‐arm, single‐blind, RCT Setting: Germany Trial time period: participant enrolment from September 1998 to December 1999 Interventions: ESWT focused on the origin of supraspinatus tendon vs ESWT focused on the calcific deposit Sample size calculation: sample size calculation not performed Analysis: ITT |
|
| Participants |
Number of participants:
Inclusion criteria
Exclusion criteria
Baseline characteristics: ESWT to supraspinatus tendon (25 participants):
ESWT to calcific deposit (25 participants):
Pretreatment group differences: none |
|
| Interventions |
ESWT to supraspinatus tendon
ESWT to calcific deposit
|
|
| Outcomes | Measured at 12 weeks and 1 year Outcomes included in review:
Outcomes excluded from review:
|
|
| Source of funding | Not reported | |
| Notes |
Trial registration: not reported Time points included in review: 12 weeks and 12 months Data analysis: function and pain at rest extracted at 12 weeks and 1 year; resorption of calcific deposit extracted at 1 year; participant satisfaction extracted at end of study period. Participant satisfaction was extracted as the measure to represent treatment success, over the number achieving 80% of the normal value for the age‐standardised Constant score Withdrawals: 1/25 in ESWT to supraspinatus tendon group (withdrew consent after randomisation); 0/25 in ESWT to calcific deposit group Adverse events: ESWT to supraspinatus tendon:
ESWT to calcific deposit:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation in permutated blocks. |
| Allocation concealment (selection bias) | Unclear risk | Methods not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and outcome assessors blinded to treatment assignments. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Participants blinded to treatment allocation, thus there was a low risk of detection bias in reporting of self‐reported outcomes (including pain, function and patient satisfaction). |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes | Low risk | Blinded independent observers assessed other outcomes, such as radiographic assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/25 in ESWT to supraspinatus tendon group (withdrew consent after randomisation) and 0/25 in ESWT to calcific deposit group. |
| Selective reporting (reporting bias) | Low risk | No published study protocol, but results reported for all outcomes as mentioned in methods, and included major outcomes. |
| Other bias | Low risk | No other biases apparent. |
Hearnden 2009.
| Methods |
Study design: single‐centre, parallel‐group, two‐arm, single‐blind randomised placebo‐controlled trial Setting: orthopaedics referrals from general practice, Wrightington Hospital, UK Trial time period: not reported Interventions: ESWT vs placebo Sample size calculation: not performed Analysis: ITT |
|
| Participants |
Number of participants:
Inclusion criteria:
Exclusion criteria:
Baseline characteristics: numerical data were not reported Pretreatment group differences: authors reported that participants in both groups were well matched in demographics, symptoms and calcific deposits (no numerical data reported) |
|
| Interventions |
ESWT:
Placebo
|
|
| Outcomes | Measured at 6 months Outcomes included in review:
Outcomes excluded from review:
|
|
| Source of funding | Not reported | |
| Notes |
Trial registration: not reported Time points included in review: 6 months Data analysis: function was extracted at 6 months, treatment success was extracted at the study's conclusion (6 months). Pain, calcification size and adverse events could not be extracted as the data were only reported for the intervention group. As no SDs were reported for function and there were none available in study, the SD at 6 months for active and sham groups were taken from Gerdesmeyer 2003. Withdrawals: none Adverse events: ESWT:
Placebo:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation 'using a centralised list in blocks to get two equal groups'. |
| Allocation concealment (selection bias) | Low risk | Treatment allocations were kept in sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants blinded to treatment allocation, not reported if outcome assessors were blinded. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Participants unaware of treatment received, thus the risk of bias was low for self‐reported outcomes (pain, function and treatment success). |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes | Unclear risk | Not reported if the radiographer measuring calcification was blinded to the group allocations. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals. |
| Selective reporting (reporting bias) | High risk | There was no published study protocol. The summary data for both groups were not reported. VAS pain scores and calcification changes in placebo group were not reported. |
| Other bias | Low risk | No other biases apparent. |
Hsu 2008.
| Methods |
Study design: single‐centre, parallel‐group, two‐arm, single‐blind, RCT Setting: hospital outpatient clinic, China Trial time period: enrolment July 2002 to February 2004 Interventions: ESWT vs sham treatment Sample size calculations: not performed Analysis: ITT |
|
| Participants |
Number of participants:
Inclusion criteria:
Exclusion criteria:
Baseline characteristics ESWT (33 participants):
Sham treatment (13 participants):
Pretreatment group differences: no statistically significant differences between groups at baseline |
|
| Interventions |
ESWT:
Sham treatment:
|
|
| Outcomes | Measured at 6 weeks, 12 weeks, 6 months and 12 months Outcomes included in review:
Outcomes excluded from review:
|
|
| Source of funding | Not reported | |
| Notes |
Trial registration: not reported Time points included in review: 6 weeks, 6 months and 12 months Data analysis: the mean Constant score was extracted using the WebPlotDigitier program found at arohatgi.info/WebPlotDigitizer/app. It was not reported whether the graphs were reporting SD or SE; we assumed SDs were reported. The numbers were extracted and rounded to 1 decimal place. Where measured numbers differed from a reported figure, the reported figure was used. Withdrawals: none reported Adverse events: ESWT:
Sham treatment:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Systematic random sampling in multiples of 3, allocation ratio set to 2:1 for intervention:placebo. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although local anaesthetic was used, the study did not report if participants were blinded, but outcome assessors were blinded. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Blinding of participants was not reported so there was a risk of bias in self‐reported outcomes of pain, function and treatment success. |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes | Low risk | Radiologist who assessed calcification was reported as blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals. |
| Selective reporting (reporting bias) | High risk | There was no published study protocol, and participant satisfaction was measured but results were not reported. SDs for all outcomes were not reported. All study outcomes presented only as graphs without numerical tables. |
| Other bias | Low risk | No other biases apparent. |
Ioppolo 2012.
| Methods |
Study design: single‐centre, parallel‐group, two‐arm, single‐blind RCT Setting: outpatients at university hospital, Italy Trial time period: enrolment November 2008 to June 2010 Interventions: high‐energy ESWT vs low‐energy ESWT Sample size calculation: a sample size of 46 participants achieved a power over 80% to detect a 15% difference in Constant score. The statistical level of significance was set at α = 0.05, and the assumed SD was set at 17.7 points Analysis: ITT analysis used, with missing data imputed with the last observation carried forward |
|
| Participants |
Number of participants:
Inclusion criteria:
Exclusion criteria:
Baseline characteristics: High‐energy ESWT (23 participants):
Low‐dose ESWT(23 participants):
Pretreatment group differences: none |
|
| Interventions |
High‐energy ESWT:
Low‐dose ESWT:
|
|
| Outcomes | Measured at 3, 6 and 12 months Outcomes included in review:
Outcomes excluded from review:
|
|
| Source of funding | Study supported by a grant from 'La Sapienza' University of Rome. | |
| Notes |
Trial registration: ClinicalTrials.gov identifier NCT01602653 Time points included in review: 3, 6 and 12 months Data analysis: mean pain and function extracted at 3 and 6 months; mean change in calcific size extracted at 6 months. As no measure of variance was reported for pain or function, the SD was taken from Schofer 2009 Withdrawals: 7/23 in high‐dose ESWT group and 3/23 in low‐dose ESWT group, no reasons given in either group Adverse events: High‐dose ESWT:
Low‐dose ESWT:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated 1:1 randomisation scheme used. |
| Allocation concealment (selection bias) | Low risk | An adequate method of numbered, opaque envelopes used to conceal the randomisation scheme. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Protocol explicitly reported that investigators were blinded to treatment but no information on whether participants were blinded or not. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Unclear risk | Unclear if participants were aware of their treatment group, so reporting of pain and function may have been affected by bias. |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes | Low risk | Radiographer who assessed calcific deposits was reported as blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10/46; 7/23 (30%) in high‐dose ESWT group and 3/23 (13%) in low‐dose ESWT group no reasons for withdrawal given. |
| Selective reporting (reporting bias) | High risk | VAS and Constant score outcomes were only reported in exact figures for 6 months' follow‐up and only graphically with no measures of variance at other time points, and 12 months' follow‐up date were not reported at all. |
| Other bias | Low risk | No other biases apparent. |
Kim 2014.
| Methods |
Study design: single‐centre, parallel‐group, two‐arm, RCT Setting: orthopaedic surgery outpatient department, St Mary's Hospital, the Catholic University of Korea, Seoul, South Korea Trial time period: November 2005 to March 2011 Interventions: ESWT vs US‐guided needling Sample size calculation: 30 participants per group were needed to detect a significant difference (mean difference 8 points; SD 12 points) between groups in ASES scores, with power of 80%, at a type I error level of 0.05 Analysis: not ITT |
|
| Participants |
Number of participants:
Inclusion criteria:
Exclusion criteria:
Baseline characteristics: ESWT (32 participants):
US‐guided glucocorticoid needling (30 participants):
Pretreatment group differences: none |
|
| Interventions |
ESWT:
US‐guided needling:
|
|
| Outcomes | Measured at 6 weeks, 12 weeks, 6 months, 12 months, and last follow‐up visit. Mean follow‐up: 23.0 (range 12.1–28.5) months after treatment Outcomes included in review:
Other outcomes in trial, excluded from review
|
|
| Source of funding | The authors, their immediate families, and any research foundation with which they were affiliated received no financial payments or other benefits from any commercial entity related to the subject of the article | |
| Notes |
Trial registration: not reported Time points included in review: 3, 6 and 12 months Data analysis: no SDs or any other measures of variance were reported or could be calculated for the pain scores or the function scores at follow‐up, thus we could not analyse these outcomes. The size of calcific deposits was extracted at last follow‐up (12 months) Withdrawals: 3/32 in ESWT group, 5/30 in needling group were lost to follow‐up Adverse events: not measured |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization was conducted by an independent statistician who provided us with a computer‐generated randomization list." Comment: adequate. |
| Allocation concealment (selection bias) | Unclear risk | Not reported if the randomisation list was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither participants nor personnel were blinded to treatment allocation. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Participants were not blinded to treatment allocation, thus there was risk of detection bias in reporting of pain and function. |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes | Unclear risk | It was not reported if the radiographer assessing calcification size was blinded and the effect on measurement of this outcome was unclear. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3/32 (9%) in ESWT group and 5/30 (16.6%) in needling group were lost to follow‐up. |
| Selective reporting (reporting bias) | High risk | There was no published study protocol, and measures of variance were not reported at follow‐up for most data. |
| Other bias | Low risk | No other biases apparent. |
Kolk 2013.
| Methods |
Study design: multicentre, parallel‐group, two‐arm, double‐blind, randomised placebo‐controlled trial Setting: outpatient clinics of 5 Dutch hospitals Trial time period: enrolment 2001–2003 Interventions: rESWT vs placebo Sample size calculation: sample size calculation was based on an unpublished pilot study, estimated that 35 participants per group were needed to detect a 50% difference in pain (VAS) between groups Analysis: ITT. Missing values were included in analyses using the 'last case carried forward' principle |
|
| Participants |
Number of participants:
Inclusion criteria:
Exclusion criteria:
Baseline characteristics: rESWT (44 participants):
Placebo (38 participants)
Pretreatment group differences: none |
|
| Interventions |
rESWT:
Placebo:
|
|
| Outcomes | Measured at 3 and 6 months Outcomes included in review:
Outcomes excluded from review:
|
|
| Source of funding | Study supported by EMS. Although none of the authors received benefits for personal or professional use from a commercial party related directly or indirectly to the subject of the article. | |
| Notes |
Trial registration: not reported Time points included in review: 3 and 6 months Data analysis: the VAS and CMS were extracted at 6 months. The study contact was e‐mailed to gain further information on the mean duration of symptoms of the overall cohort of study participants and for the methods of allocation concealment Withdrawals: 9/44 in rESWT group (2 lack of treatment effect, 1 lack of confidence in physiotherapist, 6 unspecified) and 4/38 in placebo group (2 failure of clinician instructions, 2 unspecified). We assumed withdrawals due to adverse events or intolerance to treatment was 3/44 in rESWT group and 2/38 in placebo group Adverse events: rESWT:
Placebo:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "An independent coordinator, who was not involved in the treatment or evaluation of the patients performed the randomisation by a closed envelope system." |
| Allocation concealment (selection bias) | Unclear risk | A closed envelope system was used; however, it was not reported if the envelopes were opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and surgeon were blinded to treatment allocation. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Unclear risk | Local anaesthesia was not used so participants may have been able to guess their allocation. |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes | Low risk | Low risk of bias in assessor‐reported outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 13/82; 9/44 (20%) in rESWT group (2 lack of treatment effect, 1 lack of confidence in physiotherapist, 6 unspecified) and 4/38 (10%) in placebo group (2 failure of clinician instructions, 2 unspecified) were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | No published study protocol and trial was not registered; however, all specified outcomes were measured and reported. |
| Other bias | Low risk | No other biases apparent. |
Kvalvaag 2017.
| Methods |
Study design: single‐centre, parallel‐group, two‐arm, double‐blind, randomised sham‐controlled trial Setting: outpatient shoulder clinic, Department of Physical Medicine and Rehabilitation, Oslo University hospital, Norway Trial time period: enrolment 1 January 2012 to 15 April 2014 Interventions: supervised exercises plus rESWT vs supervised exercises plus sham rESWT Sample size calculation: study was designed to detect a clinically relevant difference of 10 points (SD 20 points) between the groups with significance level (a) of 0.05 and power (b) of 80%. The sample size was calculated as 50 in each group. We included 143 participants to account for dropouts. Analysis: ITT |
|
| Participants |
Number of participants:
Inclusion criteria:
Exclusion criteria:
Baseline characteristics: Supervised exercises plus rESWT (69 participants)
Supervised exercises plus sham rESWT (74 participants)
Pretreatment group differences: none. |
|
| Interventions |
Supervised exercises plus rESWT: Supervised exercises:
rESWT:
Supervised exercises plus sham rESWT: Supervised exercises:
Sham rESWT:
|
|
| Outcomes | Measured at baseline, 12 and 24 weeks Outcomes included in review:
Outcomes excluded from review:
|
|
| Source of funding | Sophies Minde Ortopedi, Norway | |
| Notes |
Trial registration: ClinicalTrials.gov identifier NCT01441830 Time points included in review: 12 and 24 weeks Data analysis: function measured on SPADI and pain on the 11‐point Likert‐type scale were extracted at 12 and 24 weeks Withdrawals: 4/69 in shock wave group (2 loss to follow‐up, 2 discontinued intervention (1 developed adhesive capsulitis and 1 developed synovial chondromatosis)) and 4/74 in sham group (1 loss to follow‐up, 3 discontinued intervention, (1 developed adhesive capsulitis, 1 developed increased pain, 1 developed other serious disorder)) Adverse events: Supervised exercise plus rESWT:
Supervised exercise plus sham rESWT:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation in blocks of 20 in a 1:1 ratio used. |
| Allocation concealment (selection bias) | Low risk | Allocation concealed using sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and study personnel was done. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Blinding of participants resulted in low risk of bias in self‐reported outcomes of pain, disability and function. |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes | Low risk | Blinding of outcome assessors was done; however, not applicable in the measurement of study outcomes which were all self‐reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 withdrawals from this study, 4/69 in supervised exercise plus rESWT group and 4/74 in supervised exercise plus sham group, however none were excluded from the analysis in both groups. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registration accessible to review authors. All measured outcomes were reported; however, the protocol stated return to work and health‐related quality of life as secondary outcomes, which were not measured in study. |
| Other bias | Low risk | No other biases apparent. |
Li 2017.
| Methods |
Study design: single‐centre, parallel‐group, two‐arm, double‐blind, randomised, placebo‐controlled trial Setting: First Hospital of Harbin City, China Trial time period: February 2015 to January 2017 Interventions: ESWT vs placebo Sample size calculation: sample size calculation was based on the 50% difference in NRS score with α = 0.5, β = 0.8, and assuming a 20% dropout rate. Therefore, the required sample size of the present study was estimated to be 84 participants, with 42 assigned to each group. Analysis: all outcome data by ITT |
|
| Participants |
Number of participants:
Inclusion criteria:
Exclusion criteria:
Baseline characteristics: ESWT (42 participants):
Placebo (42 participants):
Pretreatment group differences: none |
|
| Interventions |
ESWT:
Placebo:
|
|
| Outcomes | Measured at 4 and 8 weeks Outcomes included in review:
Outcomes excluded from review:
|
|
| Source of funding | Study funded by grants from the Science and Technology Talents Program of Harbin (2014RFXGJ041, 2014RFQGJ094), Harbin First Hospital postdoctoral fellowship program (HRBSDYYYBSH‐1); Postdoctoral Fund (160780); Harbin high level talent fund (HRBGCCRCJJ‐6, 2013SYYRCYJ01–1); China Postdoctoral Science Foundation, Heilongjiang Natural Science Foundation (QC2016102, H2016002) | |
| Notes |
Trial registration: not registered Time points included in review: 4 and 8 weeks Data analysis: only changes from baseline values were reported for all study outcomes at both time points. Withdrawals: 7/42 in ESWT group (2 withdrawal of consent, 5 lost to follow‐up) and 8/42 in placebo group (1 withdrawal of consent, 7 lost to follow‐up). As reasons for withdrawal of consent were not reported, we assumed it may have been due to treatment intolerance, and included these data in Analysis 1.5: 2/42 in ESWT group and 1/42 in placebo group. Adverse events: rESWT:
Placebo:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization schedule was operated by a computerized number generated using SAS package (Version 8.2; SAS Institute Inc. Cary, NC) at a 1:1 ratio." |
| Allocation concealment (selection bias) | Low risk | Quote: "All information of assignments and allocation were concealed in sequentially numbered, opaque, and sealed envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both participants and study personnel were blinded to treatment allocation. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | As participants were blinded to group allocation, there was unlikely to have been any effect on subject outcome data. |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes | Low risk | Quote: "the outcome assessors and data analysts were also blinded in this study." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7/42 in ESWT group (2 withdrawal of consent, 5 lost to follow‐up) and 8/42 in placebo group (1 withdrawal of consent, 7 lost to follow‐up). |
| Selective reporting (reporting bias) | Unclear risk | The review author did not have access to a protocol, the results were reported as change from baseline and no summary data were given for each group for all study outcomes. |
| Other bias | Low risk | No other biases apparent. |
Loew 1999.
| Methods |
Study design: single‐centre, parallel‐group, four‐arm randomised trial Setting: outpatient clinic Trial time period: July 1993 to December 1994 Interventions: no treatment vs single session low‐dose ESWT vs single session high‐dose ESWT vs dual session high‐dose ESWT Sample size calculation: not reported Analysis: not reported if ITT analysis was used, but seemed that all allocated to treatments were followed up |
|
| Participants |
Number of participants:
Inclusion criteria:
Exclusion criteria:
Baseline characteristics: No treatment (20 participants):
Low‐dose ESWT (20 participants):
Single session high‐dose ESWT (20 participants):
Dual session high‐dose ESWT (20 participants):
Pretreatment group differences: no differences in Constant scores but data on demographic variables were not reported. |
|
| Interventions |
PART A: Low‐dose ESWT:
Single session high‐dose ESWT:
Dual session high‐dose ESWT:
No treatment control:
PART B: Single session high‐dose ESWT:
Dual session high‐dose ESWT:
|
|
| Outcomes | Measured at baseline and 3 months (Part A) and 6 months (Part B). Outcomes included in review (Part A only):
|
|
| Source of funding | Authors reported that they did not receive any funding | |
| Notes |
Trial registration: not registered Time points included in review: 3 months Data analysis: data from Part A were included in this review. As Part B was probably not a randomised study, it was excluded from this review Withdrawals: 0 in Part A. Part B data not included in this review Adverse events: Low‐dose ESWT:
Single session high‐dose ESWT:
Small haematomas in high‐dose group, the exact number of participants was not reported Dual session high‐dose ESWT:
Small haematomas in high‐dose group, the exact number of participants was not reported No treatment:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "In order of their entry to the trial, 80 patients were divided into groups of 20." Comment: method of randomisation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Concealment of allocation not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither participants nor personnel were blinded to treatment group. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | As participants were aware of their treatment group, this may have biased self‐reported outcomes of pain and function. |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes | High risk | As assessors were not blinded, there was risk of bias in radiographic assessment of calcific deposits |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | High risk | There was no published study protocol, important outcomes, such as pain were not reported. Adverse events were measured, but incompletely reported. |
| Other bias | Unclear risk | Unclear how participants were enrolled in study, baseline characteristics of each of the 4 groups were not provided. |
Melegati 2000.
| Methods |
Study design: single‐centre, parallel‐group, three‐arm, RCT Setting: Department of Physical Therapy and Rehabilitation, Istituto Ortopedico G. Pini, Milan, Italy Trial time period: December 1998 to May 1999 Interventions: ESWT plus kinesitherapy vs kinesitherapy alone vs control (postural advice only) Sample size calculation: not reported Analysis: not reported |
|
| Participants |
Number of participants:
Inclusion criteria:
Exclusion criteria:
Baseline characteristics: Kinesitherapy (30 participants):
ESWT plus kinesitherapy (30 participants):
Control (postural hygiene) (30 participants):
Pretreatment group differences: none |
|
| Interventions |
Kinesitherapy:
ESWT plus exercises:
Control (advice only):
|
|
| Outcomes | Measured at 8 months Outcomes included in review:
Outcomes excluded from review: none |
|
| Source of funding | Not reported | |
| Notes |
Trial registration: not registered Time points included in review: 8 months Data analysis: 2 treatment groups were included in this review for the comparison: ESWT plus kinesitherapy vs kinesitherapy alone. Function measured by Constant score 8 months after last intervention. An e‐mail requesting information (population in follow‐up outcomes, Constant subscore of pain) was not able to be sent to the study author because an e‐mail address was not reported in published study. Withdrawals: none Adverse events: not measured |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment methods not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding methods not reported. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Unclear risk | Unknown whether the participants were blinded, hence there was a risk of bias in self‐reported outcomes of pain and function. |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes | Low risk | No assessor‐reported outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Low risk | No published study protocol, but the study outcome was reported (function). |
| Other bias | Low risk | No other biases apparent. |
Pan 2003.
| Methods |
Study design: single‐centre, parallel‐group, two‐arm, RCT Setting: outpatient clinics of the departments of Physical Medicine and Rehabilitation and of Orthopedics and Traumatology, Taipei Veterans General Hospital, Taiwan Trial time period: January 2001 to January 2002 Interventions: ESWT vs TENS Sample size calculation: not performed Analysis: unclear if ITT analysis was planned; dropouts did not contribute data |
|
| Participants |
Number of participants:
Inclusion criteria:
Exclusion criteria:
Baseline characteristics: ESWT (32 participants):
TENS (28 participants):
Pretreatment group differences: no baseline differences between the 2 groups |
|
| Interventions |
ESWT:
TENS:
|
|
| Outcomes | Measured at baseline, 2, 4 and 12 weeks Outcomes included in review:
Outcomes excluded from review:
|
|
| Source of funding | Not reported | |
| Notes |
Trial registration: not registered Time points included in review: 4 and 12 weeks Data analysis: Constant score, VAS scores and changes in calcification size were extracted at 4 and 12 weeks. Adverse events and withdrawal due to adverse events were extracted at the conclusion of the study (12 weeks). The number of shoulders rather than the number of participants was used in analysis of pain, function and calcification size. Withdrawals: 0/32 in ESWT group, 1/28 in TENS group due to severe pain. The adverse events in ESWT group were not included as they subsided without treatment and did not affect intervention. Adverse events: ESWT:
TENS:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All patients were randomly assigned to ESWT or TENS groups by draw." Comment: drawing lots was an adequate randomisation method. |
| Allocation concealment (selection bias) | Unclear risk | Allocation process not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not reported. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Participants were unblinded, there was a risk of bias in measurement of pain and function. |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes | Low risk | Quote: "The baseline and posttreatment sonographic assessments were performed by the same radiologist, who was blind to the assignment of the subjects." Comment: low risk of bias for the measurement of calcification size. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/28 from the TENS group due to severe pain leading to withdrawal, 0/32 in ESWT group |
| Selective reporting (reporting bias) | Low risk | Comment: no published study protocol, but results were reported for all outcomes as mentioned in methods. |
| Other bias | High risk | Unit of analysis bias: the trialist did not report if they adjusted for the non‐independence between shoulders for the participants who had bilateral treatment. This may underestimate any treatment differences. |
Perlick 2003.
| Methods |
Study design: parallel‐group, two‐arm, RCT Setting: outpatient setting Trial time period: participant enrolment 1995–1998 Interventions: low‐dose ESWT vs high‐dose ESWT Sample size calculation: sample size calculation not performed Analysis: the study did not state whether ITT analysis was used |
|
| Participants |
Number of participants:
Inclusion criteria:
Exclusion criteria:
Baseline characteristics Low‐dose ESWT (40 participants):
High‐dose ESWT (40 participants):
Pretreatment group differences: demographic characteristics for each group were not reported. |
|
| Interventions |
Low‐dose ESWT
High‐dose ESWT
|
|
| Outcomes | Measured at 3 and 12 months Outcomes included in review:
Outcomes excluded from review:
|
|
| Source of funding | Not reported | |
| Notes |
Trial registration: not registered Time points included in review: 3 and 12 months Data analysis: pain and function extracted at 3 and 12 months; calcification resorption and adverse events extracted at 12 months. Pain scores were reversed in direction by subtracting the score from 15 so that they could be compared with VAS scores of other studies (where VAS 0–10, 10 indicating most pain). SDs were not reported for function scores. The SD was imputed from Ioppolo 2012 at 6 months and Schofer 2009 at 12 months for analyses. No author contact details were provided, so we could not request missing data. We reported 5/40 adverse events in low‐dose group and 15/40 adverse events in high‐dose group. We did not include petechial bleeding as it was mild and local in both groups Withdrawals: none Adverse events: Low‐dose shock wave therapy:
High‐dose shock wave therapy
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly assigned in a blinded fashion to two groups." Comment: method of randomisation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Methods of allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of the personnel or study participants not described; however, local anaesthetic used in both groups. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Unclear risk | As the blinding of the participants was not adequately reported, there was an unclear risk of detection bias on the self‐reported outcomes of pain and function. |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes | Unclear risk | Blinding of assessors not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | High risk | No published study protocol, but results were reported for all outcomes as mentioned in methods. A measure of variance was not reported for function outcomes. |
| Other bias | Low risk | No other biases apparent. |
Peters 2004.
| Methods |
Study design: parallel‐group, three‐arm, double‐blind, RCT Setting: not reported Trial time period: not reported Interventions: low‐dose ESWT vs high‐dose ESWT vs sham ESWT Sample size calculation: not done Analysis: did not report using ITT analysis |
|
| Participants |
Number of participants:
Inclusion criteria:
Exclusion criteria:
Baseline characteristics: not reported |
|
| Interventions |
Low‐dose ESWT:
High‐dose ESWT:
Sham ESWT:
|
|
| Outcomes | Measured at 6 months Outcomes included in review:
Outcomes excluded from review:
|
|
| Source of funding | Not reported | |
| Notes |
Trial registration: not registered Time points included in review: 6 months Data analysis: as there were 2 active intervention groups, the low‐dose ESWT data were included for the comparison ESWT vs placebo as it was more consistent with that in given in other studies. The high‐dose group and low‐dose group were used for the comparison high‐dose vs low‐dose ESWT. Pain during treatment was not considered by the study to be adverse events, and were, therefore, not able to be extracted as adverse events in this review. The outcome of 'treatment success' was obtained by the equation of: proportion of successes = 100% – proportion of relapses; or number of successes = total population – number of relapsed participants. Withdrawals: 0/30 in low‐dose ESWT group, 0/31 in high‐dose ESWT group, 3/29 in sham group (unresolved pain after 3 sessions). We assumed withdrawals due to intolerance were 0/30 in shock wave and 3/29 in placebo (Analysis 1.5) Adverse events: Low‐dose ESWT:
4/30 had pain during ESWT 2/30 had haematomas High‐dose ESWT:
31/31 had pain during shock wave 6/31 had haematomas Sham shock wave therapy:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Spreadsheet used to generate a list of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Both participants and study staff were blinded to treatment allocation. But local anaesthesia was not used for both groups so participants may have guessed if they were in placebo group. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Unclear risk | Since no local anaesthetic was used, participants may have been biased in reporting treatment success. |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes | Low risk | Radiologists assessing the X‐rays were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/90; 0/30 in low‐dose ESWT group, 0/31 in high‐dose ESWT group, 3/29 in sham group (unresolved pain after 3 sessions) |
| Selective reporting (reporting bias) | High risk | No published study protocol; the study reported outcomes mentioned in methods but did not report SDs for pain |
| Other bias | Unclear risk | Authors reported that demographic data of the groups were comparable with regard to age, size and type of calcification. However, baseline data were not reported. |
Pleiner 2004.
| Methods |
Study design: single‐centre, parallel‐group, two‐arm, double‐blind, randomised placebo‐controlled trial Setting: not reported Trial time period: not reported Interventions: ESWT vs placebo Sample size calculations: a sample size based on priori assumption of α = 0.05 and β = 0.20, was performed, but the number of participants needed per group was not reported. Analysis: ITT analysis was used when assessing changes in Gärtner score for X‐rays |
|
| Participants |
Number of participants:
Inclusion criteria:
Exclusion criteria:
Baseline characteristics: ESWT (23 participants)
Placebo (20 participants)
Pretreatment group differences: none |
|
| Interventions |
ESWT:
Placebo:
|
|
| Outcomes | Measured at 1 week, 3 months and 7 months Outcomes included in review:
Outcomes excluded from review:
|
|
| Source of funding | Assistance and technical support from Werner Kostler and the Ad Rem Team and the Medispec team for providing Orthospeo ESWT system | |
| Notes |
Trial registration: not registered Time points included in review: 1 week, 3 months and 7 months Data analysis: unit of randomisation was the participant, so those with bilateral calcifications received the same intensity of shock wave therapy for both shoulders. Constant mean change in function and SE were only presented graphically; thus mean and SE were estimated from the graph and SD calculated from SE using the formula: SD = SE × N. We did not extract pain during shock wave as an adverse event Withdrawals: 6/23 in ESWT group had another treatment (US, surgery) and 4/20 in placebo group ('personal reasons'). We assumed the withdrawals in both groups were due to intolerance and included the data in Analysis 1.5. Adverse events: ESWT:
Placebo:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods not reported, therefore, there was an unclear risk of selection bias. |
| Allocation concealment (selection bias) | Unclear risk | Methods not reported, therefore, there was an unclear risk of selection bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and study staff were blinded to treatment allocation. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Due to participant blinding, low risk of bias in self‐reported outcomes of pain and function. |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes | Low risk | Radiologists were unaware of the treatment assignment, there was a low risk of bias in measurement of calcification size. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 10/43; 6/23 in ESWT group and 4/20 in placebo group; overall reasons included alternative treatment and loss to follow‐up but reasons per group were not given. |
| Selective reporting (reporting bias) | Low risk | No published study protocol and trial was not registered, but results were reported for all outcomes as mentioned in methods. |
| Other bias | High risk | Unit of analysis bias: there was a high risk of unit of analysis bias as trialist did not adjust for the non‐independence between groups due to bilateral treatment. Therefore, the true difference between the groups may have been smaller than reported. |
Rompe 1998.
| Methods |
Study design: parallel‐group, two‐arm, RCT Setting: not reported Trial time period: 2‐year trial exact time period not reported Interventions: low‐dose ESWT vs high‐dose ESWT Sample size calculation: sample size calculation not performed Analysis: study did not state if it used ITT analysis |
|
| Participants |
Number of participants:
Inclusion criteria:
Exclusion criteria:
Baseline characteristics: Low‐dose ESWT (50 participants)
High‐dose ESWT (50 participants)
Pretreatment group differences: none |
|
| Interventions |
Low‐dose ESWT:
High‐dose ESWT:
|
|
| Outcomes | Measured at 6 weeks and 6 months Outcomes included in review:
Outcomes excluded from review:
|
|
| Source of funding | Not reported | |
| Notes |
Trial registration: not registered Time points included in review: 6 weeks and 6 months Data analysis: function extracted at 6 weeks and 24 weeks. Complete and partial resorption of calcification and participant satisfaction extracted at 24 weeks. Pain was not able to be extracted as not reported in results section (this is subset of Constant score) as only ranges were reported by the study. The SD for the Constant score was extracted using the WebPlotDigitier program found at arohatgi.info/WebPlotDigitizer/app. As it was unclear whether the graph displayed SEs or SDs, it was agreed that the data would be treated as SDs. The data were extracted and rounded to the nearest whole number. Where measured numbers differed from a reported figure, the reported figure was used Withdrawals: none Adverse events: Low‐dose ESWT:
High‐dose ESWT:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly assigned in a blinded fashion to two groups." Method of randomisation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Methods were not reported, therefore, there was an unknown risk of selection bias due to unknown allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information on blinding of study personnel or participants |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Unclear risk | Methods of participant blinding were not reported, therefore, there was an unclear risk of detection bias regarding the self‐reported outcomes of Constant score and treatment success. |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes | Unclear risk | As blinding of assessors was not reported, there was risk of bias in the measurement of radiographic outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals in either group. |
| Selective reporting (reporting bias) | High risk | There was no published study protocol, but results were reported for all outcomes as mentioned in methods. The breakdown of the Constant score was not reported (including pain) and SDs were not provided for any outcome measure. |
| Other bias | Low risk | No other biases apparent. |
Sabeti 2007.
| Methods |
Study design: single‐centre, parallel‐group, two‐arm, single‐blind, RCT Setting: outpatient clinic in Department of Orthopedics, Vienna Medical School, Vienna, Austria Trial time period: not reported Interventions: low‐dose ESWT vs high‐dose ESWT Sample size calculation: not performed Analysis: not ITT |
|
| Participants |
Number of participants:
Inclusion criteria:
Exclusion criteria:
Baseline characteristics: Low‐dose ESWT (22 participants):
High‐dose ESWT (25 participants):
Pretreatment group differences: none |
|
| Interventions |
Low‐dose ESWT:
High‐dose ESWT:
|
|
| Outcomes | Measured at baseline and 12 weeks Outcomes included in review:
Outcomes excluded from review:
|
|
| Source of funding | Not reported | |
| Notes |
Trial registration: not registered Time points included in review: 12 weeks Data analysis: pain, function, calcification resorption and treatment success extracted at 12 weeks. The study contact was e‐mailed to request further information on the methods of allocation concealment, and their response was used to guide the risk of bias assessment Withdrawals: 4/22 in low‐dose group (2 excluded due to strong pain during therapy, 1 had urgent personal reasons, 1 was lost to follow‐up) and 2/25 in high‐dose group due to loss to follow‐up Adverse events: Low‐dose ESWT:
High‐dose ESWT
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization into two groups was performed after every ten consecutive patients were enrolled, thus a total of five randomization procedures were carried out. Patients' names were written on cards that were put into envelopes, mixed and randomised." |
| Allocation concealment (selection bias) | Unclear risk | Response from study team: "One of the nurses, working with us in the treatment rooms wrote the names on cards, which were put in envelopes and sealed and put in a cup. As noted in the paper, as soon as ten patients were collected the nurse chose randomly 5 envelopes which were assigned to Group one, and the remaining 5 to Group two. The patients were recruited by the outpatient clinics and were consecutively included, meaning: the first eligible patient´s name was put in the envelope, – put in the Cup, – Cup with ten names,‐ random Distribution 5 vs 5." Comment: no information was provided on whether the envelopes used were opaque and sealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study described itself as an "observer‐blinded" study and participants were not blinded to treatment allocation. Quote: "The treatment room was the same for both groups, but patients were scheduled at different times so that individuals within the groups would not contact each other." Comment: as the 2 study groups differed in session number, dose and presence of anaesthesia, it was difficult to assess how the treatment results would have been affected. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Unclear risk | As there was no report of participant blinding, there was a risk of bias in self‐reported outcomes of pain, function and treatment success. |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes | Low risk | Quote: "The clinical follow‐up examination was carried out by an independent observer who had no information about the treatment protocol. X‐rays were evaluated by an independent observer." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6/50; 4/22 (18%) in low‐dose group (2 excluded due to strong pain during therapy,1 urgent personal reason, 1 loss to follow‐up) and 2/25 (8%) in high‐dose group due to loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Comment: no published study protocol, but results were reported for all outcomes as mentioned in methods. There is, therefore, a low risk of reporting bias. |
| Other bias | Low risk | Comment: no other biases apparent. |
Sabeti‐Aschraf 2005.
| Methods |
Study design: single‐centre, parallel‐group, two‐arm, single‐blind, RCT Setting: outpatient clinic in Department of Orthopedics, Vienna Medical School, Vienna, Austria Trial time period: not reported Interventions: palpation‐guided ESWT vs imaging‐guided ESWT Sample size calculation: sample size calculation was not performed Analysis: the study did not report if ITT analysis was used, and did not report if any participants dropped out |
|
| Participants |
Number of participants:
Inclusion criteria:
Exclusion criteria:
Baseline characteristics: Palpation‐guided ESWT (25 participants)
Image‐guided ESWT (25 participants)
Pretreatment group differences: none |
|
| Interventions |
Palpation‐guided ESWT:
Image‐guided ESWT:
|
|
| Outcomes | Measured at baseline and 12 weeks Outcomes included in review:
Outcomes excluded from review:
|
|
| Source of funding | Not reported | |
| Notes |
Trial registration: not registered Time points included in review: 12 weeks Data analysis: pain, function and calcification deposit data were extracted at 3 months Withdrawals: none Adverse events: Palpation‐guided ESWT:
Image‐guided ESWT:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was performed by the Department of Medical Statistics, but the method of generating the sequence was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were reported as blinded, and study personnel were not blinded; however, calcification was measured by an independent observer. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Participants were adequately blinded so there was a low risk of detection bias in regards to self‐reported outcome assessment. |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes | Low risk | Radiographic outcomes (of calcification size) were analysed by an independent observer. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals in either group. |
| Selective reporting (reporting bias) | Low risk | There was no published study protocol, but results were reported for all outcomes as mentioned in methods. |
| Other bias | Low risk | No other biases apparent. |
Schmitt 2001.
| Methods |
Study design: parallel‐group, two‐arm, single‐blind, randomised, placebo‐controlled trial Setting: not reported Trial time period: enrolment from March 1999 to February 2000 Interventions: ESWT vs sham ESWT Sample size calculation: an a priori analysis gave a total sample size of 16,818 participants for a given power of 95% was needed to prove the study effect of ESWT Analysis: ITT |
|
| Participants |
Number of participants:
Inclusion criteria:
Exclusion criteria:
Baseline characteristics: ESWT group (20 participants):
Sham group (19 participants):
Pretreatment group differences: none |
|
| Interventions |
ESWT:
Sham ESWT:
|
|
| Outcomes | Measured at 6 and 12 weeks Outcomes included in review:
Outcomes excluded from review:
|
|
| Source of funding | Not reported | |
| Notes |
Trial registration: not registered Time points included in review: 6 and 12 weeks Data analysis: function and pain at rest extracted at 6 and 12 weeks and treatment success and adverse events was extracted at 12 weeks. Although 12‐months follow‐up data were reported, participants who reported no improvement at 12 weeks were told of their treatment group and allowed to cross‐over to the ESWT treatment if they had placebo previously; we considered this part of the trial no longer randomised and did not include the 12‐month data. It is possible that treatment success was possibly added post‐hoc Withdrawals: 0/20 in ESWT group and 2/20 in placebo group (1 loss to follow‐up, 1 withdrew consent just after randomisation). We assumed no withdrawals in either group due to adverse events or treatment intolerance Adverse events: ESWT:
Sham:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment allocation was done using random permutated blocks through telephone hotline. |
| Allocation concealment (selection bias) | Low risk | Participants were centrally allocated via a telephone hotline. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both participants and study personnel were blinded to group allocation. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Participants were likely unaware of treatment, thus there was low risk of detection bias in reporting of function and treatment success. |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes | Low risk | An 'independent observer' who was unaware of treatment measured other outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/40; 0/20 in ESWT group and 2/20 in sham group due to loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | There was no published study protocol, and all outcomes were reported; however, it is possible that treatment success was possibly added post‐hoc. |
| Other bias | High risk | At 12 weeks, 16 participants reported they were not satisfied with treatment so they were unmasked and informed of their treatment group, and participants in placebo group were offered ESWT, effectively ending the randomised part of the study. |
Schofer 2009.
| Methods |
Study design: parallel‐group, two‐arm, double‐blind, RCT Setting: outpatient clinic, Department of Orthopedics, University Hospital Marburg, Germany Trial time period: not reported Interventions: high‐dose ESWT vs low‐dose ESWT Sample size calculation: a priori analysis using GPower to find the sample size for a larger confirmatory study gave a total sample of 156 participants for the 12‐week Constant score (effect size d = 0.384 at α = 0.05 and power 80%) and total sample of 94 participants for 12‐month Constant score (effect size d = 0.518 at α = 0.05 and 80% power). Analysis: the study did not report using ITT analysis |
|
| Participants |
Number of participants:
Inclusion criteria:
Exclusion criteria:
Baseline characteristics: High‐dose ESWT (20 participants):
Low‐dose ESWT (20 participants):
Pretreatment group differences: prior to treatment there was no significant difference in primary outcome parameter. In 1 of the secondary outcome measurements (pain at rest) there was a statistically significant difference of 2 points on the VAS. |
|
| Interventions |
High‐dose ESWT
Low‐dose ESWT
|
|
| Outcomes | Measured at 3 and 12 months Outcomes included in review:
Outcomes excluded from review:
|
|
| Source of funding | No benefits or funds were received in support of this study | |
| Notes |
Trial registration: not registered Time points included in review: 3 and 12 months Data analysis: function and pain at rest extracted for review at 3 and 12 months. Adverse events extracted at study end Withdrawals: 2/20 in high‐dose group (1 loss to follow‐up, 1 underwent surgery) and 1/20 in low‐dose group (1 loss to follow‐up) Adverse events: High‐dose ESWT:
Low‐dose ESWT:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were randomised externally using random permuted blocks." Comment: low risk of selection bias. |
| Allocation concealment (selection bias) | Unclear risk | No information on how the allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and study personnel was done. All participants received local anaesthetics making it more likely participants were blinded to high‐dose vs low‐dose treatment; however, as personnel were not blinded, there was an unclear risk of performance bias. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | As participants were blinded to group allocation there was low risk of bias in measurement of subjective outcomes of pain and function. |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes | Low risk | Low risk of bias in Constant score measurements (assessed by blinded assessors). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/40; 2/20 in high‐dose group (1 loss to follow‐up, 1 underwent surgery) and 1/20 in low‐dose group (1 loss to follow‐up). |
| Selective reporting (reporting bias) | Low risk | No published study protocol, but results were reported for all outcomes as mentioned in methods. |
| Other bias | Low risk | No other bias apparent. |
Speed 2002.
| Methods |
Study design: single‐centre, parallel‐group, two‐arm, double‐blind randomised, placebo‐controlled trial Setting: not reported Trial time period: not reported Interventions: ESWT vs placebo Sample size calculation: sample size calculation not performed Analysis: ITT analysis reported but not performed |
|
| Participants |
Number of participants:
Inclusion criteria:
Exclusion criteria:
Baseline characteristics: ESWT:
Placebo:
Pretreatment group differences: none |
|
| Interventions |
ESWT:
Placebo:
|
|
| Outcomes | Measured at baseline, 1, 2, 3 and 6 months Outcomes included in review:
|
|
| Source of funding | Not reported | |
| Notes |
Trial registration: not registered Time points included in review: 1, 3 and 6 months Data analysis: we extracted pain and function at 1, 3 and 6 months. For SPADI function, we subtracted total from 100 so that higher score indicated better function. Treatment success and withdrawals were extracted at last follow‐up Withdrawals: 11/34 in ESWT group (4 did not complete treatment (1 did not tolerate treatment, 3 did not give a reason) and 7 completed treatment but did not attend follow‐up) and 13/40 in placebo group (5 did not complete treatment (1 did not tolerate treatment due to worsening symptoms, 4 did not give a reason) and 8 completed treatment but did not attend follow‐up). We assumed 4/34 in ESWT group and 5/40 in placebo group withdrew due to intolerance (Analysis 1.5) Adverse events: Shock wave therapy:
Sham therapy:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of generating the random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported if blinding was used. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Unclear risk | Unclear if participants were blinded. |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes | Low risk | There were no assessor‐reported outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 24/74; 11/34 (32%) in shock wave group (4 did not complete treatment, 7 did not attend follow‐up assessments) and 13/40 (32%) in sham group (5 did not complete treatment, 8 did not attend follow‐up assessments) reasons for non‐completion of treatment were not clearly reported. |
| Selective reporting (reporting bias) | Unclear risk | No published protocol for this study; but all measured outcomes were reported. The number of withdrawals was unclear, number of participants in final outcome measurement was not clearly reported. |
| Other bias | Low risk | No other biases apparent. |
Tornese 2011.
| Methods |
Study design: parallel‐group, two‐arm, RCT Setting: Outpatient Department of the Center for Sports Rehabilitation of the Galeazzi Orthopedics Institute in Milan, Italy Trial time period: participant enrolment January 2009 to September 2009 Interventions: ESWT neutral position technique vs ESWT with hyperextended internal rotation technique Sample size calculation: not performed Analysis: study did not report using ITT analysis |
|
| Participants |
Number of participants:
Inclusion criteria:
Exclusion criteria:
Baseline characteristics: ESWT neutral position technique (17 participants):
ESWT with hyperextended internal rotation technique (18 participants):
Pretreatment group differences: none |
|
| Interventions |
ESWT with neutral position technique:
ESWT with hyperextended internal rotation technique:
|
|
| Outcomes | Measured at 3 months Outcomes Included in review:
Outcomes excluded from review:
|
|
| Source of funding | Research received no specific grant from any funding agency in the public, commercial or not‐for‐profit sectors | |
| Notes |
Trial registration: not registered Time points included in review: 3 months Data analysis: pain, function and calcification size data were extracted at 3 months Withdrawals: none Adverse events: not measured |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomized assignment was by a casual number generation software into two groups." Comment: low risk of selection bias. |
| Allocation concealment (selection bias) | Unclear risk | Comment: methods not reported, therefore, there was an unclear risk of selection bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study reported assessors were blinded but it was not clear whether participants were blinded. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Unclear risk | As participants were not reported to be blinded there was risk of bias in self‐reported outcomes of pain and function. |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes | Low risk | Assessors were blinded so low risk of bias in radiographic assessments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants completed follow‐up assessments. |
| Selective reporting (reporting bias) | Low risk | There was no published study protocol, but results were reported for all outcomes as mentioned in methods. |
| Other bias | Low risk | No other biases apparent. |
ADL: activities of daily living; ASES: American Shoulder and Elbow Surgeons; BMI: body mass index; CI: confidence interval; CMS: Constant Score; DASH: Disabilities of the Arm, Shoulder and Hand; EFD: energy fluctuation density; EMS: Electro Medical Systems; ESWT: extracorporeal shock wave therapy; EQ‐VAS: EuroQol‐Visual Analogue Scale; EQ‐5D: EuroQol‐5D; IQR: interquartile range; ITT: intention to treat; LMW‐HA: low molecular weight hyaluronic acid; MRI: magnetic resonance imaging; NRS: Numerical Rating Scale; NSAID: non‐steroidal anti‐inflammatory drug; RCT: randomised controlled trial; rESWT: radial extracorporeal shock wave therapy; ROM: range of movement; RSWT: radial shock wave therapy; SD: standard deviation; SE: standard error; SPADI: Shoulder Pain And Disability Index; SST: Simple Shoulder Test; TENS: transcutaneous electric nerve stimulation; UCLA: University of California at Los Angeles; US: ultrasound; VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adamietz 2003 | Not an RCT |
| Ali 2016 | Examined treatment of myofascial trigger points of rotator cuff muscle dysfunction. |
| Astore 2003 | Not an RCT |
| Avancini‐Dobrovic 2011 | Not an RCT |
| Barnsley 2001 | Not an RCT |
| Boxberg 1996 | Not an RCT |
| Bringmann 2001 | Not investigating shock wave therapy |
| Buch 1999 | Not an RCT |
| Buselli 2010 | Not an RCT |
| Bytomski 2006 | Not an RCT |
| Charrin 2001 | Not an RCT |
| Cheing 2003 | Not an RCT |
| Chow 2007 | Studied ESWT therapy for heel pain |
| Cosentino 2004 | Not an RCT |
| Costa 2002 | Not an RCT |
| Cyteval 2003 | Not an RCT |
| Friedberg 2010 | Not an RCT |
| Garcia Marti 2004 | Not an RCT |
| Hayes 2005 | Not an RCT |
| Jakobeit 2002 | Not an RCT |
| Kim 2012 | Participants were postsurgical repair |
| Krasny 2005 | Studied ultrasound‐guided needling |
| Labek 1999 | Not an RCT |
| Lee 2011 | Not an RCT |
| Lippincott 2010 | Not an RCT |
| Liu 2012 | Study was on bicipital tenosynovitis |
| Loew 1995 | Not an RCT |
| Lorbach 2008 | Not an RCT |
| Magosch 2003 | Not an RCT |
| Maier 2000 | Not an RCT |
| Mangone 2010 | Not an RCT |
| Manske 2004 | Not an RCT |
| Meier 2000 | Not an RCT |
| Moretti 2005 | Not an RCT |
| Mundy 2004 | Not an RCT |
| Njawaya 2018 | Study had planned inclusion of participants in 3 arms – those with calcific supraspinatus tendinopathy, plantar fasciitis and Achilles tendinopathy. However, due to poor recruitment in first arm (2 participants), they abandoned this arm of the study and have excluded these 2 participants from the results. Hence, the study did not include participants with rotator cuff disease, |
| Noel 1999 | Not an RCT |
| Notarnicola 2011 | Not an RCT |
| Pigozzi 2000 | Not an RCT |
| Polimeni 2003 | Did not study ESWT |
| Rebuzzi 2008 | Not an RCT |
| Rees 2009 | Not an RCT |
| Rompe 1995 | Not an RCT |
| Rompe 2000 | Not an RCT |
| Rompe 2001 | Not an RCT |
| Rompe 2003 | Not an RCT |
| Sabeti‐Aschraf 2004 | Not an RCT |
| Saggini 2010 | Did not study ESWT |
| Sarrat 2004 | Not an RCT |
| Seil 2006 | Not an RCT |
| Sistermann 1998 | Not an RCT |
| Speed 2005 | Not an RCT |
| Spindler 1998 | Not an RCT |
| Steinacker 2001 | Not an RCT |
| Thigpen 2010 | Not an RCT |
| Wang 2001 | Not an RCT |
| Wang 2003 | Not an RCT |
| Wiley 2002 | Commentary, not an RCT |
ESWT: extracorporeal shock wave therapy; RCT: randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
Berner 2004.
| Methods | Requires translation |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Diehl 2011.
| Methods | Requires translation |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Gross 2002.
| Methods | Requires translation |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Loew 1995.
| Methods | Requires translation |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Mao 2003.
| Methods | Requires translation |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Paternostro‐Sluga 2004.
| Methods | Requires translation |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Rompe 1997a.
| Methods | Requires translation |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Rompe 1997b.
| Methods | Requires translation |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Seil 1999.
| Methods | Requires translation |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
ChiCTR1900022932.
| Trial name or title | Effect of focused versus radial extracorporeal shock‐wave therapy for tendonitis of rotator cuff |
| Methods |
Study design: parallel‐group, two‐arm, randomised controlled trial Setting: China Japan Friendship Hospital, China Intervention: focused ESWT vs rESWT Analysis: not reported |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention: focused ESWT Control: rESWT |
| Outcomes |
Outcomes: Visual Analogue Scale; Constant Score |
| Starting date | 4 May 2019 |
| Contact information | Sun Wei 2 Yinghua Street East, Chaoyang District, Beijing, China Tel: +86 17801203237 E‐mail: Sun887@126.com |
| Notes |
Estimated completion date: 23/04/2020 Trial registration: ChiCTR1900022932. Date of first enrolment: 30 April 2019. Retrospective registration. Status on 11 November 2019 recruitment continuing. |
NCT02677103.
| Trial name or title | Extracorporeal shock‐wave therapy for supraspinatus calcifying tendonitis: a randomized clinical trial comparing two different energy levels |
| Methods |
Study design: Parallel, three‐arm, randomised controlled trial Setting: Shin Kong Wu Ho‐Su Memorial Hospital, Tapei, Taiwan Interventions: rESWT vs US‐guided needle puncture vs rESWT plus US‐guided needle puncture Analysis: not reported |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
rESWT:
US‐guided needle puncture:
rESWT plus US‐guided needle puncture:
|
| Outcomes |
Outcomes included in review:
Outcomes excluded from review:
|
| Starting date | April 2013 |
| Contact information | Lin‐Fen Hsieh, MD, Shin Kong Wu Ho‐Su Memorial Hospital |
| Notes |
Estimated completion date: study completed, no results posted Trial registration: ClinicalTrials.gov identifier NCT02677103. Status on 9 May 2018: recruitment completed, 61 participants enrolled, no study results available. Last update posted on 25 March 2016 on the ClinicalTrials.gov website. |
NCT03779919.
| Trial name or title | The therapeutic effect of the extracorporeal shock wave therapy on shoulder calcific tendinitis |
| Methods |
Study design: parallel, three‐arm, triple‐blind, randomised controlled trial Setting: ChiMei Medical Center, Taiwan Interventions: high‐energy ESWT vs low‐energy ESWT vs sham therapy Analysis: not reported |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
High‐energy ESWT:
Low‐energy ESWT:
Sham:
|
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Starting date | 19 December 2018 |
| Contact information | Hsin‐Han Cheng, MD Tel: +886926722119 E‐mail: a11010147@gmail.com |
| Notes |
Estimated completion date: 31 May 2020 Trial registration: NCT03779919; status on 11 November 2019, recruiting participants |
NTR7093.
| Trial name or title | Needle aspiration of calcific deposits versus extracorporeal shock wave therapy for conservative therapy resistant calcifying tendinitis of the shoulder |
| Methods |
Study design: parallel, two‐arm, randomised controlled trial Setting: Maxima Medical Centre, Netherlands Interventions: needle aspiration of calcific deposits vs ESWT Analysis: not reported |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Needle aspiration of calcific deposits: sonographically guided removal of the calcific deposits will be performed ESWT: participants will receive a focused ESWT Both procedures will be conducted according to a standardised protocol |
| Outcomes |
Outcomes included in review:
Outcomes excluded from review:
|
| Starting date | 1 April 2018 |
| Contact information | Dr Max Reijman PhD, Maxima Medical Centre, Netherlands |
| Notes |
Estimated completion date: 1 January 2021 Trial registration: NTR7093. Status on 8 May 2018, recruitment not yet commenced. |
PACTR201910650013453.
| Trial name or title | Shock wave therapy versus local corticosteroid injection in shoulder impingement syndrome |
| Methods |
Study design: parallel, two‐arm, randomised controlled trial Setting: Cairo, Egypt Intervention: shock wave therapy vs local corticosteroid injection Analysis: not reported |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions | Shock wave Corticosteroid injection Strengthening exercise for rotator cuff muscles and scapular stabilisers and shoulder mobilisation |
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Starting date | 30 September 2019 |
| Contact information | Ahmed Elerian Elmaadi 0025 Cairo Egypt Tel: 002201116752333 E‐mail: dr_ahmed_elerian77@yahoo.com |
| Notes |
Estimated completion date: not reported Trial registration: PACTR201910650013453 prospectively registered on 16 September 2019. Status on 11 November 2019, recruitment commenced and ongoing |
CT: computer tomography; ESWT: extracorporeal shock wave therapy; MRI: magnetic resonance imaging; NA: not available; rESWT: radial extracorporeal shock wave therapy; ROM: range of movement; SF‐36: 36‐Item Short‐Form Health Survey; US: ultrasound; VAS: Visual Analogue Scale.
Differences between protocol and review
We changed the major outcomes to be included in the summary of findings tables after publication of the protocol, from six (participant‐reported pain relief of 30% or greater; mean pain score, or mean change in pain score on VAS or NRS; disability or function (various scales); composite endpoints measuring 'success' of treatment such as participants feeling no further symptoms; participant withdrawals due to adverse events; number of participants experiencing any adverse event) to seven, by the addition of quality of life. As no studies reported the outcome "pain relief of 30% or greater" we instead used the outcome "Pain relief of 50% or greater", as one study reported the latter.
We specified three months as our main time point as it was thought clinically likely to allow enough time for a treatment effect to occur.
We specified sham as our main comparator (i.e. presented in 'Summary of findings' table) as it was most likely to demonstrate a treatment benefit independent of a placebo effect (e.g. if comparing to a no‐treatment control).
We specified that we would perform a subgroup analysis comparing people aged older than 65 years with those aged 65 years or younger, but as there was no identifiable rationale for this analysis we did not perform this analysis.
We specified, post hoc, that we would perform sensitivity analyses on the presence of adequate allocation concealment and participant blinding to assess the possible effects of selection and detection biases on pain and function and assessed the effect of including trials with a unit of analysis issue due to bilateral treatment in some participants in a sensitivity analysis.
The text word "Radiofrequency" was removed from the search strategy, as it was made redundant by the broader search term "Radiation, non‐ionizing" and no relevant studies were lost upon its removal.
Contributions of authors
RB, RJ and a previous author, Juliana Roos (JR) drafted the protocol (Buchbinder 2011).
SJS and JD were responsible for performing the searches, selecting trials, performing risk of bias assessment, data extraction, analysing the data and interpreting the results of the review, and writing the first draft of the review.
RJ and RB were responsible for checking the quality of the review, performing the risk of bias assessment, performing the GRADE assessment and 'Summary of findings' tables, interpretation of results and editing of the final manuscript.
Sources of support
Internal sources
-
Department of Epidemiology and Preventive Medicine, School of Public Health and Preventive Medicine, Monash University, Australia.
In kind support
-
Cabrini Institute, Cabrini Hospital, Malvern, Victoria, Australia.
In kind support
External sources
No sources of support supplied
Declarations of interest
RB has authored two randomised controlled trials; one of ESWT for heel pain and one for lateral elbow pain (Buchbinder 2002; Staples 2008), as well as a Cochrane systematic review of ESWT for lateral elbow pain (Buchbinder 2005). RB has received royalties from Wolters Kluwer Health for writing a chapter on plantar fasciitis in UpToDate. She is also the Co‐ordinating Editor of Cochrane Musculoskeletal, but is not involved in editorial decisions regarding this review. She is a recipient of a National Health and Medical Research Council (NHMRC) Cochrane Collaboration Round 7 Funding Program Grant, which supports the activities of Cochrane Musculoskeletal ‐ Australia and Cochrane Australia, but the funders do not participate in the conduct of reviews.
JD has been employed by Alfred Health from January 2016 to present as a Hospital Medical Officer (HMO).
RJ is the Managing Editor of Cochrane Musculoskeletal, but is not involved in editorial decisions regarding this review. She is a recipient of an NHMRC (Australia) Cochrane Collaboration Round 7 Funding Program Grant, which supports the Cochrane Musculoskeletal Australian Editorial base, but the funders do not participate in the conduct of this review.
SJS: none known.
New
References
References to studies included in this review
Albert 2007 {published and unpublished data}
- Albert JD, Meadeb J, Guggenbuhl P, Marin F, Benkalfate T, Thomazeau H, et al. High‐energy extracorporeal shock‐wave therapy for calcifying tendinitis of the rotator cuff. Journal of Bone and Joint Surgery 2007;89(3):335‐41. [DOI] [PubMed] [Google Scholar]
Cacchio 2006 {published data only}
- Cacchio A, Paoloni M, Barile A, Don R, Paulis F, Calvisi V, et al. Effectiveness of radial shock‐wave therapy for calcific tendinitis of the shoulder: single‐blind, randomized clinical study. Physical Therapy 2006;86(5):672‐82. [PubMed] [Google Scholar]
Cosentino 2003 {published data only}
- Cosentino R, Stefano R, Selvi E, Frati E, Manca S, Frediani B, et al. Extracorporeal shock wave therapy for chronic calcific tendinitis of the shoulder: single blind study. Annals of the Rheumatic Diseases 2003;62(3):248‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Boer 2017 {published data only}
- Boer FA, Mocking F, Neilssen EM, Kampen PM, Huijsmans PE. Ultrasound guided needling vs radial shockwave therapy in calcific tendinitis of the shoulder: a prospective randomized trial. Journal of Orthopaedics 2017;14(4):466‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Del Castillo‐Gonzales 2016 {published data only}
- Castillo‐Gonzales F, Ramos‐Alvarez JJ, Rodriguez‐Fabian G, Gonzalez‐Perez J, Jimenez‐Herranz E, Varela E. Extracorporeal shockwaves versus ultrasound‐guided percutaneous lavage for the treatment of rotator cuff calcific tendinopathy: a randomized controlled trial. European Journal of Physical and Rehabilitation Medicine 2016;52(2):145‐51. [PubMed] [Google Scholar]
Duymaz 2019 {published data only}
- Duymaz T, Sindel D. Comparison of radial extracorporeal shock wave therapy and traditional physiotherapy in rotator cuff calcific tendinitis treatment. Archives of Rheumatology 2019;34(3):281‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Engebretsen 2009 {published data only}
- Engebretsen K, Grotle M, Bautz‐Holter E, Ekeberg OM, Brox JI. Predictors of shoulder pain and disability index (SPADI) and work status after 1 year in patients with subacromial shoulder pain. BMC Musculoskeletal Disorders 2010;17(2):218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engebretsen K, Grotle M, Bautz‐Holter E, Ekeberg OM, Juel NG, Brox JI. Supervised exercises compared with radial extracorporeal shock‐wave therapy for subacromial shoulder pain: 1‐year results of a single‐blind randomized controlled trial. Physical Therapy 2011;91:37‐47. [DOI] [PubMed] [Google Scholar]
- Engebretsen K, Grotle M, Bautz‐Holter E, Ekeburg OM, Juel NG, Brox JI. Supervised exercises compared with radial extracorporeal shock wave therapy (rESWT) in patients with SIS. clinicaltrials.gov/ct2/show/study/NCT00653081 (first received 4 April 2008).
- Engebretsen K, Grotle M, Bautz‐Holter E, Sandvik L, Juel NG, Ekeberg OM, et al. Radial extracorporeal shockwave treatment compared with supervised exercises in patients with subacromial pain syndrome: single blind randomised study. BMJ 2009;339:b3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farr 2011 {published data only}
- Farr S, Sevelda F, Mader P, Graf A, Petje G, Sabeti‐Aschraf M. Extracorporeal shockwave therapy in calcifying tendinitis of the shoulder. Knee Surgery, Sports Traumatology, Arthroscopy 2011;19(12):2085‐9. [DOI] [PubMed] [Google Scholar]
Frizziero 2017 {published data only}
- Frizziero A, Vittadini F, Barazzuol M, Gasparre G, Finotti P, Meneghini A, et al. Extracorporeal shockwaves therapy versus hyaluronic acid injection for the treatment of painful non‐calcific rotator cuff tendinopathies: preliminary results. Journal of Sports Medicine and Physical Fitness 2017;57(9):1162‐8. [DOI] [PubMed] [Google Scholar]
Galasso 2012 {published data only}
- Galasso O, Amelio E, Riccelli DA, Gasparini G. Short‐term outcomes of extracorporeal shock wave therapy for the treatment of chronic non‐calcific tendinopathy of the supraspinatus: a double‐blind, randomized, placebo‐controlled trial. BMC Musculoskeletal Disorders 2012;13(86):1471‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gerdesmeyer 2003 {published data only}
- Gerdesmeyer L, Wagenpfeil S, Haake M, Maier M, Loew M, Wörtler K, et al. Extracorporeal shock wave therapy for the treatment of chronic calcifying tendonitis of the rotator cuff: a randomized controlled trial. JAMA 2003;290(19):2573‐80. [DOI] [PubMed] [Google Scholar]
Haake 2002 {published data only}
- Haake M, Deike B, Thon A, Schmitt J. Exact focusing of extracorporeal shock wave therapy for calcifying tendinopathy. Clinical Orthopaedics and Related Research 2002;397:323‐31. [DOI] [PubMed] [Google Scholar]
- Haake M, Deike B, Thon A, Schmitt J. Importance of accurately focussing extracorporeal shock waves in the treatment of calcifying tendinitis [Bedeutung der exakten Fokussierung exktrakorporaler Stosswellen (ESWT) bei der therapie der tendinitis calcarea]. Biomedizinische Technik. Biomedical Engineering 2001;46:69‐74. [DOI] [PubMed] [Google Scholar]
Hearnden 2009 {published data only}
- Hearnden A, Desai A, Karmegam A, Flannery M. Extracorporeal shock wave therapy in chronic calcific tendonitis of the shoulder – is it effective?. Acta Orthopaedica Belgica 2009;75:25‐31. [PubMed] [Google Scholar]
Hsu 2008 {published data only}
- Hsu CJ, Wang DY, Tseng KF, Fong YC, Hsu HC, Jim YF. Extracorporeal shock wave therapy for calcifying tendinitis of the shoulder. Journal of Shoulder and Elbow Surgery 2008;17(1):55‐9. [DOI] [PubMed] [Google Scholar]
Ioppolo 2012 {published data only}
- Ioppolo F, Tattoli M, Sante L, Attanasi C, Venditto T, Servidio M, et al. Extracorporeal shock‐wave therapy for supraspinatus calcifying tendinitis: a randomized clinical trial comparing two different energy levels. Physical Therapy 2012;92(11):1376‐85. [DOI] [PubMed] [Google Scholar]
Kim 2014 {published data only}
- Kim SJ, Lee HJ, Kim YV, Kong CG. Which method is more effective in treatment of calcific tendinitis in the shoulder? Prospective randomized comparison between ultrasound‐guided needling and extracorporeal shock wave therapy. Journal of Shoulder and Elbow Surgery 2014;23:1640‐6. [DOI] [PubMed] [Google Scholar]
Kolk 2013 {published data only}
- Kolk A, Yang KG, Tamminga R, Hoeven H. Radial extracorporeal shock‐wave therapy in patients with chronic rotator cuff tendinitis. A prospective randomised double‐blind placebo‐controlled multicentre trial. Bone & Joint Journal 2013;95‐B(11):1521‐6. [DOI] [PubMed] [Google Scholar]
Kvalvaag 2017 {published and unpublished data}
- Kvalvaag E, Brox JI, Engebretsen KB, Soberg HL, Juel NG, Bautz‐Holter E, et al. Effectiveness of radial extracorporeal shock wave therapy (rESWT) when combined with supervised exercises in patients with subacromial shoulder pain: a double‐masked, randomized, sham‐controlled trial. American Journal of Sports Medicine 2017;45(11):2547‐54. [DOI] [PubMed] [Google Scholar]
- Kvalvaag E, Brox JI, Engebretsen KB, Søberg HL, Bautz‐Holter E, Røe C. Is radial extracorporeal shock wave therapy (rESWT) combined with supervised exercises (SE) more effective than sham rESWT and SE in patients with subacromial shoulder pain? Study protocol for a double‐blind randomised, sham‐controlled trial. BMC Musculoskeletal Disorders 2015;16:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2017 {published data only}
- Li W, Shou‐Xiang Z, Qi Y, Bao‐Lin L, Qing‐Gang M, Zheng‐Gui G. Effect of extracorporeal shock‐wave therapy for treating patients with chronic rotator cuff tendonitis. Medicine (Baltimore) 2017;96(35):e7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
Loew 1999 {published data only}
- Daecke W, Kusnierczak D, Loew M. Long‐term effects of extracorporeal shockwave therapy in chronic calcific tendinitis of the shoulder. Journal of Shoulder and Elbow Surgery 2002;11(5):476‐80. [DOI] [PubMed] [Google Scholar]
- Loew M, Daecke W, Kusnierczak D, Rahmanzadeh M, Ewerbeck V. Shock‐wave therapy is effective for chronic calcifying tendinitis of the shoulder. Journal of Bone and Joint Surgery. British Volume 1999;81(5):863‐7. [DOI] [PubMed] [Google Scholar]
Melegati 2000 {published and unpublished data}
- Melegati G, Tornese D, Bandi M. Effective of extracorporeal shock wave therapy associated with kinesitherapy in the treatment of subacromial impingement: a randomised, controlled study [Efficacia della terapia con onde d'urto extracorporee associata a chinesiterapia nel trattamento della sindrome da conflitto subacromiale: studio randomizzato controllato]. Journal of Sports Traumatology and Related Research 2000;22(2):58‐64. [Google Scholar]
Pan 2003 {published data only}
- Pan PJ, Chou CL, Chiou HJ, Ma HL, Lee HC, Chan RC. Extracorporeal shock wave therapy for chronic calcific tendinitis of the shoulders: a functional and sonographic study. Archives of Physical Medicine and Rehabilitation 2003;84:988‐93. [DOI] [PubMed] [Google Scholar]
Perlick 2003 {published data only}
- Perlick L, Luring C, Bathis H, Perlick C, Kraft C, Diedrich O. Efficacy of extracorporal shock‐wave treatment for calcific tendinitis of the shoulder: experimental and clinical results. Journal of Orthopaedic Science 2003;8:777‐83. [DOI] [PubMed] [Google Scholar]
Peters 2004 {published data only}
- Peters J, Luboldt W, Schwarz W, Jacobi V, Herzog C, Vogl TJ. Extracorporeal shock wave therapy in calcific tendinitis of the shoulder. Skeletal Radiology 2004;33:712‐8. [DOI] [PubMed] [Google Scholar]
- Peters J, Volkmar J, Thalhamer A, Vogl TJ. Extracorporeal shock wave lithotripsy in tendinitis calcarea of the shoulder: randomized comparison of different energy flux densities. Radiology 2001;221:560. [Google Scholar]
Pleiner 2004 {published data only}
- Pleiner J, Crevenna R, Langenberger H, Keilani M, Nuhr M, Kainberger F, et al. Extracorporeal shockwave treatment is effective in calcific tendonitis of the shoulder. A randomized controlled trial. Wiener Klinische Wochenschrift 2004;116(15‐16):536‐41. [DOI] [PubMed] [Google Scholar]
Rompe 1998 {published data only}
- Rompe JD, Buerer R, Hopf C, Eysel P. Shoulder function after extracorporal shock wave therapy for calcific tendinitis. Journal of Shoulder and Elbow Surgery 1998;7(5):505‐9. [DOI] [PubMed] [Google Scholar]
Sabeti 2007 {published data only}
- Sabeti M, Dorotka R, Goll A, Gruber M, Schatz KD. A comparison of two different treatments with navigated extracorporeal shock‐wave therapy for calcifying tendinitis – a randomized controlled trial. Wiener Klinische Wochenschrift 2007;119(3‐4):124‐8. [DOI] [PubMed] [Google Scholar]
Sabeti‐Aschraf 2005 {published data only}
- Sabeti‐Aschraf M, Dorotka R, Goll A, Trieb K. Extracorporeal shock wave therapy in the treatment of calcific tendinitis of the rotator cuff. American Journal of Sports Medicine 2005;33(9):1365‐8. [DOI] [PubMed] [Google Scholar]
Schmitt 2001 {published data only}
- Schmitt J, Haake M, Tosch A, Hildebrand R, Deike B, Griss P. Low‐energy extracorporeal shock‐wave treatment (ESWT) for tendinitis of the supraspinatus. A prospective, randomised study. Journal of Bone and Joint Surgery. British Volume 2001;83‐B(6):873‐6. [DOI] [PubMed] [Google Scholar]
- Schmitt J, Tosch A, Hunerkopf M, Haake M. Extracorporeal shockwave therapy (ESWT) as therapeutic option in supraspinatus tendon syndrome? One year results of a placebo controlled study [Die extrakorporale Stosswellentherapie (ESWT) als therapeutische Option beim Supraspinatussehnensyndrom? Ein‐Jahres‐Ergebnisse einer placebokontrollierten Studie]. Orthopade 2002;31:652‐7. [DOI] [PubMed] [Google Scholar]
Schofer 2009 {published data only}
- Schofer MD, Hinrichs F, Peterlein CD, Arendt M, Schmitt J. High‐ vs low‐energy extracorporeal shock wave therapy of rotator cuff tendinopathy: a prospective, randomised, controlled study. Acta Orthopaedica Belgica 2009;75(4):452‐8. [PubMed] [Google Scholar]
Speed 2002 {published data only}
- Speed CA, Richards C, Nichols D, Burnet S, Wies JT, Huphreys H, et al. Extracorporeal shock‐wave therapy for tendinitis of the rotator cuff: a double‐blind, randomised, controlled trial. Journal of Bone and Joint Surgery. British Volume 2002;84‐B(4):509‐12. [DOI] [PubMed] [Google Scholar]
Tornese 2011 {published data only}
- Tornese D, Mattei E, Bandi M, Zerbi A, Quaglia A, Melegati G. Arm position during extracorporeal shock wave therapy for calcifying tendinitis of the shoulder: a randomized study. Clinical Rehabilitation 2011;25(8):731‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adamietz 2003 {published data only}
- Adamietz B. Comment on the article by Gross MW, et al. The effectiveness of radiation treatment in comparison with extracorporeal shockwave therapy (ESWT) in supraspinatus tendon syndrome. (Strahlenther Onkol 2002;178:314‐20. No.6). Strahlentherapie und Onkologie 2003;179(2):129‐30; author reply 131‐2. [DOI] [PubMed] [Google Scholar]
Ali 2016 {published data only}
- Ali SA, Lasheen YR, Kamel RM, Genaidy AF. Efficacy of shockwave therapy in treatment of myofascial trigger points of rotator cuff muscle dysfunction. International Journal of PharmTech Research 2016;9(6):115‐26. [Google Scholar]
Astore 2003 {published data only}
- Astore F. Extracorporeal shock‐wave therapy for tendonitis of the rotator cuff. Journal of Bone and Joint Surgery. British Volume 2003;85(5):774; author reply 774. [PubMed] [Google Scholar]
Avancini‐Dobrovic 2011 {published data only}
- Avancini‐Dobrovic V, Frlan‐Vrgoc L, Stamenkovic D, Pavlovic I, Vrbanic TSL. Radial extracorporeal shock wave therapy in the treatment of shoulder calcific tendinitis. Collegium Antropologicum 2011;35 Suppl 2:221‐5. [PubMed] [Google Scholar]
Barnsley 2001 {published data only}
- Barnsley L, Martin J. New therapy for musculoskeletal conditions? Extracorporeal shockwave treatment. Medicine Today 2001;2:117‐8. [Google Scholar]
Boxberg 1996 {published data only}
- Boxberg W, Perlick L, Giebel G. Shockwave treatment of therapy refractory soft tissue pain. Chirurg 1996;67(11):1174‐8. [DOI] [PubMed] [Google Scholar]
Bringmann 2001 {published data only}
- Bringmann W. Chronic shoulder pain. Krankengymnastik 2001;53(2):244‐9. [Google Scholar]
Buch 1999 {published data only}
- Buch M, Klat J, Trager D, Siebert W. Prospective comparison of shock wave therapy and needling in calcareous tendinitis of the shoulder. Journal of Bone and Joint Surgery. British Volume 1999;81 Suppl 2:190. [Google Scholar]
Buselli 2010 {published data only}
- Buselli P, Coco V, Notarnicola A, Messina S, Saggini R, Tafuri S, et al. Shock waves in the treatment of post‐traumatic myositis ossificans. Ultrasound in Medicine & Biology 2010;36(3):397‐409. [DOI] [PubMed] [Google Scholar]
Bytomski 2006 {published data only}
- Bytomski JR, Black D. Conservative treatment of rotator cuff injuries. Journal of Surgical Orthopaedic Advances 2006;15(3):126‐31. [PubMed] [Google Scholar]
Charrin 2001 {published data only}
- Charrin JE, Noel ER. Shockwave therapy under ultrasonographic guidance in rotator cuff calcific tendinitis. Joint, Bone, Spine 2001;68(3):241‐4. [DOI] [PubMed] [Google Scholar]
Cheing 2003 {published data only}
- Cheing GL, Chang H. Extracorporeal shock wave therapy. Journal of Orthopaedic and Sports Physical Therapy 2003;33(6):337‐43. [DOI] [PubMed] [Google Scholar]
Chow 2007 {published data only}
- Chow IH, Cheing GL. Comparison of different energy densities of extracorporeal shock wave therapy (ESWT) for the management of chronic heel pain. Clinical Rehabilitation 2007;21(2):131‐41. [DOI] [PubMed] [Google Scholar]
Cosentino 2004 {published data only}
- Cosentino R, Selvi E, Stefano R, Frati E, Manca S, Hammoud M, et al. Extracorporeal shock wave therapy for chronic calcific tendinitis of the shoulder. Clinical Rheumatology 2004;23(5):475‐7. [DOI] [PubMed] [Google Scholar]
Costa 2002 {published data only}
- Costa M, Donell S, Schmitt J, Haake M. Low‐energy extracorporeal shock‐wave treatment (ESWT) for tendinitis of the supraspinatus (multiple letters). Journal of Bone and Joint Surgery. British Volume 2002;84(4):619‐20. [PubMed] [Google Scholar]
Cyteval 2003 {published data only}
- Cyteval C, Baron‐Sarrabere MP, Jorgensen C, Cottin A, Benis J, Sany J, et al. MRI study before and after extracorporal shock wave therapy in calcifying tendinitis of the shoulder. Journal de Radiologie 2003;84(6):681‐4. [PubMed] [Google Scholar]
Friedberg 2010 {published data only}
- Friedberg MW. Supervised exercise superior to radial extracorporeal shockwave treatment for subacromial pain syndrome. JCOM 2010;17(2):58‐9. [Google Scholar]
Garcia Marti 2004 {published data only}
- Garcia Marti S. Usefulness of extracorporeal shock waves in musculoskeletal disorders (Structured abstract). Health Technology Assessment Database 2004; Vol. 4.
Hayes 2005 {published data only}
- Hayes Inc. Extracorporeal shock wave therapy for tendonitis of the rotator cuff (Structured abstract). Health Technology Assessment Database 2005; Vol. 4.
Jakobeit 2002 {published data only}
- Jakobeit C, Winiarski B, Jakobeit S, Welp L, Spelsberg G. Ultrasound‐guided, high‐energy extracorporeal shock‐wave treatment of symptomatic calcareous tendinopathy of the shoulder. ANZ Journal of Surgery 2002;72(7):496‐500. [DOI] [PubMed] [Google Scholar]
Kim 2012 {published data only}
- Kim JY, Lee JS, Park CW. Extracorporeal shock wave therapy is not useful after arthroscopic rotator cuff repair. Knee Surgery, Sports Traumatology, Arthroscopy 2012;20(12):2567‐72. [DOI] [PubMed] [Google Scholar]
Krasny 2005 {published data only}
- Krasny C, Enenkel M, Aigner N, Wlk M, Landsiedl F. Ultrasound‐guided needling combined with shock‐wave therapy for the treatment of calcifying tendonitis of the shoulder. Journal of Bone and Joint Surgery. British Volume 2005;87(4):501‐7. [DOI] [PubMed] [Google Scholar]
Labek 1999 {published data only}
- Labek G, Auersperg V, Boehler N. Treatment of tendinosis calcarea and impingement by extracorporal shock wave therapy (ESWT). Journal of Bone and Joint Surgery. British Volume 1999;81‐B Supp 2:190. [Google Scholar]
Lee 2011 {published data only}
- Lee SY, Cheng B, Grimmer‐Somers K. The midterm effectiveness of extracorporeal shockwave therapy in the management of chronic calcific shoulder tendinitis. Journal of Shoulder and Elbow Surgery 2011;20(5):845‐54. [DOI] [PubMed] [Google Scholar]
Lippincott 2010 {published data only}
- Lippincott W, Lippincott W. Supervised exercise may be better than ESWT in treating shoulder pain. Lippincott's Bone and Joint Newsletter 2010;16(1):8. [Google Scholar]
Liu 2012 {published data only}
- Liu S, Zhai L, Shi Z, Jing R, Zhao B, Xing G. Radial extracorporeal pressure pulse therapy for the primary long bicipital tenosynovitis a prospective randomized controlled study. Ultrasound in Medicine & Biology 2012;38(5):727‐35. [DOI] [PubMed] [Google Scholar]
Loew 1995 {published data only}
- Loew M, Jurgowski W, Mau HC, Thomsen M. Treatment of calcifying tendinitis of rotator cuff by extracorporeal shock waves: a preliminary report. Journal of Shoulder and Elbow Surgery 1995;4(2):101‐6. [DOI] [PubMed] [Google Scholar]
Lorbach 2008 {published data only}
- Lorbach O, Kusma M, Pape D, Kohn D, Dienst M. Influence of deposit stage and failed ESWT on the surgical results of arthroscopic treatment of calcifying tendonitis of the shoulder. Knee Surgery, Sports Traumatology, Arthroscopy 2008;16(5):516‐21. [DOI] [PubMed] [Google Scholar]
Magosch 2003 {published data only}
- Magosch P, Lichtenberg S, Habermeyer P. Radial shock wave therapy in calcifying tendinitis of the rotator cuff – a prospective study. Zeitschrift fur Orthopadie und Ihre Grenzgebiete 2003;141(6):629‐36. [DOI] [PubMed] [Google Scholar]
Maier 2000 {published data only}
- Maier M, Stabler A, Lienemann A, Kohler S, Feitenhansl A, Durr HR, et al. Shockwave application in calcifying tendinitis of the shoulder – prediction of outcome by imaging. Archives of Orthopaedic and Trauma Surgery 2000;120(9):493‐8. [DOI] [PubMed] [Google Scholar]
Mangone 2010 {published data only}
- Mangone G, Veliaj A, Postiglione M, Viliani T, Pasquetti P. Radial extracorporeal shock‐wave therapy in rotator cuff calcific tendinosis. Clinical Cases in Mineral & Bone Metabolism 2010;7(2):91‐6. [PMC free article] [PubMed] [Google Scholar]
Manske 2004 {published data only}
- Manske RC, Reiman MP, Stovak ML. Nonoperative and operative management of snapping scapula. American Journal of Sports Medicine 2004;32(6):1554‐65. [DOI] [PubMed] [Google Scholar]
Meier 2000 {published data only}
- Meier M, Duerr HR, Koehler S, Staupendahl D, Pfahler M, Refior HJ. Analgetic effect of extracorporeal shockwaves used for tendinosis calcarea, epicondylitis humeri radialis and plantar fasciitis [Analgetische wirkung nieder‐energetischer extrakorporaler stosswellen bei tendinosis calcarea, epikondylitis humeri radialis und plantarfasziitis]. Zeitschrift fur Orthopadie und Unfallchirurgie 2000;138:34‐8. [DOI] [PubMed] [Google Scholar]
Moretti 2005 {published data only}
- Moretti B, Garofalo R, Genco S, Patella V, Mouhsine E. Medium‐energy shock wave therapy in the treatment of rotator cuff calcifying tendinitis. Knee Surgery, Sports Traumatology, Arthroscopy 2005;13(5):405‐10. [DOI] [PubMed] [Google Scholar]
Mundy 2004 {published data only}
- Mundy L, Merlin T, Hodgkinson B. Extracorporeal shock wave therapy for the treatment of chronic calcifying tendonitis of the rotator cuff. Horizon Scanning Prioritising Summary – Volume 3 (Structured abstract). Health Technology Assessment Database 2004; Vol. 4.
Njawaya 2018 {published and unpublished data}
- Njawaya MM, Moses B, Martens D, Orchard JJ, Driscoll T, Negrine J, et al. Ultrasound guidance does not improve the results of shock wave for plantar fasciitis or calcific achilles tendinopathy: a randomized control trial. Clinical Journal of Sport Medicine 2018;28(1):21‐7. [DOI] [PubMed] [Google Scholar]
Noel 1999 {published data only}
- Noel E, Charrin J. Extracorporeal shock wave therapy in calcific tendinitis of the shoulder. Revue du Rhumatisme 1999;66(12):691‐3. [PubMed] [Google Scholar]
Notarnicola 2011 {published data only}
- Notarnicola A, Moretti L, Tafuri S, Forcignano M, Pesce V, Moretti B. Reduced local perfusion after shock wave treatment of rotator cuff tendinopathy. World Federation for Ultrasound in Medicine & Biology 2011;37(3):417‐25. [DOI] [PubMed] [Google Scholar]
Pigozzi 2000 {published data only}
- Pigozzi F, Giombini A, Casciello G, Salvo V, Santori N, Mariani PP. The application of shock‐waves therapy in the treatment of resistant chronic painful shoulder: a clinical experience. Journal of Sports Medicine and Physical Fitness 2000;40(4):356‐61. [PubMed] [Google Scholar]
Polimeni 2003 {published data only}
- Polimeni V, Panuccio A, Furfari P, Crupi D, Barreca G, Forgione C, et al. Preliminary study on the efficacy of various rehabilitation therapies for shoulder pain. European Journal of Physical and Rehabilitation Medicine 2003;39(1):59‐63. [Google Scholar]
Rebuzzi 2008 {published data only}
- Rebuzzi E, Coletti N, Schiavetti S, Giusto F. Arthroscopy surgery versus shock wave therapy for chronic calcifying tendinitis of the shoulder. Journal of Orthopaedics and Traumatology 2008;9:179‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rees 2009 {published data only}
- Rees J, Maffulli N, Cook J. Management of tendinopathy. American Journal of Sports Medicine 2009;37(9):1855‐67. [DOI] [PubMed] [Google Scholar]
Rompe 1995 {published data only}
- Rompe JD, Rumler F, Hopf C, Nafe B, Heine J. Extracorporal shock wave therapy for calcifying tendinitis of the shoulder. Clinical Orthopaedics and Related Research 1995;321:196‐201. [PubMed] [Google Scholar]
Rompe 2000 {published data only}
- Rompe JD, Zollner J, Nafe B, Freitag C. Significance of the elimination of deposits in patients treated for calcifying tendinitis of the shoulder. Orthopadische Klinik der Johannes‐Gutenberg‐Universitat Mainz 2000;138:335‐9. [Google Scholar]
Rompe 2001 {published data only}
- Rompe JD, Zoellner J, Nafe B. Shock wave therapy versus conventional surgery in the treatment of calcifying tendinitis of the shoulder. Clinical Orthopaedics and Related Research 2001;387:72‐82. [DOI] [PubMed] [Google Scholar]
Rompe 2003 {published data only}
- Rompe JD. Letters to the editor. American Journal of Sports Medicine 2003;31(6):1049‐51. [PubMed] [Google Scholar]
Sabeti‐Aschraf 2004 {published data only}
- Sabeti‐Aschraf M, Dorotka R, Schatz KD, Schubert S, Ebenbichler G, Trieb K. Complication of extracorporal shockwave therapy in the treatment of calcifying tendinitis of the shoulder. Physikalische Medzin Rehabilitationsmedizin Kurotmedizin 2004;14:291‐4. [Google Scholar]
Saggini 2010 {published data only}
- Saggini R, Cavezza T, Pancrazio L, Pisciella V, Saladino G, Zuccaro MC, et al. Treatment of lesions of the rotator cuff. Journal of Biological Regulators and Homeostatic Agents 2010;24(4):453‐9. [PubMed] [Google Scholar]
Sarrat 2004 {published data only}
- Sarrat P, Cohen M, Carrasset S, Godde J, Franceschi JP, Aswad R. Focused lithotripsy in the treatment of tendinosis calcarea of the shoulder: results at 2 months and one year. Journal de Radiologie 2004;85:1721‐5. [DOI] [PubMed] [Google Scholar]
Seil 2006 {published data only}
- Seil R, Wilmes P, Nuhrenborger C. Extracorporeal shock wave therapy for tendinopathies. Expert Review of Medical Devices 2006;3(4):463‐70. [DOI] [PubMed] [Google Scholar]
Sistermann 1998 {published data only}
- Sistermann R, Katthagen BD. Complications, side effects and contraindications using middle and high energetic extracorporeal shock waves in orthopaedics. Zeitschrift fur Orthopadie und Ihre Grenzgebiete 1998;136:175‐81. [DOI] [PubMed] [Google Scholar]
Speed 2005 {published data only}
- Speed C. Shoulder pain. Clinical Evidence 2005;14:1543‐60. [PubMed] [Google Scholar]
Spindler 1998 {published data only}
- Spindler A, Berman A, Lucero E, Braier M. Extracorporeal shock wave treatment for chronic calcific tendinitis of the shoulder. Journal of Rheumatology 1998;25:1161‐3. [PubMed] [Google Scholar]
Steinacker 2001 {published data only}
- Steinacker T, Steuer M. Use of extracorporal shockwave therapy in sport orthopaedics. Sportverletzung Sportschaden 2001;15:45‐9. [DOI] [PubMed] [Google Scholar]
Thigpen 2010 {published data only}
- Thigpen C. Radial extracorporeal shockwave treatment or supervised exercises for subacromial pain syndrome?. Clinical Journal of Sport Medicine 2010;20:225‐6. [DOI] [PubMed] [Google Scholar]
Wang 2001 {published data only}
- Wang C, Ko J, Chen H. Treatment of calcifying tendinitis of the shoulder with shock wave therapy. Clinical Orthopaedics and Related Research 2001;387:83‐9. [DOI] [PubMed] [Google Scholar]
Wang 2003 {published data only}
- Wang C, Yang KD, Wang F, Chen H, Wang J. Shock wave therapy for calcific tendinitis of the shoulder. American Journal of Sports Medicine 2003;31(3):425‐30. [DOI] [PubMed] [Google Scholar]
Wiley 2002 {published data only}
- Wiley P. Low‐energy extracorporeal shock‐wave treatment for tendinitis of the supraspinatus. Clinical Journal of Sports Medicine 2002;12(4):262‐4. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Berner 2004 {published data only}
- Berner IC, Dudker J. Extracorporeal shock wave therapy for soft tissue rheumatisms: usefulness. [Les ondes de choc extracorporelles en pathologie abarticulaire: quelle utilite?]. Medecine et Hygiene 2004;62:549‐53. [Google Scholar]
Diehl 2011 {published data only}
- Diehl P, Gerdesmeyer L, Gollwitzer H, Sauer W, Tischer T. Calcific tendinitis of the shoulder [Die kalkschulter – tendinosis calcarea]. Der Orthopade 2011;40:733‐46. [DOI] [PubMed] [Google Scholar]
Gross 2002 {published data only}
- Gross MW, Sattler A, Haake M, Schmitt J, Hildebrandt R, Müller HH, et al. The value of radiotherapy in comparison with extracorporeal shockwave therapy for supraspinatus tendinitis [Die wertigkeit der strahlenbehandlung im vergleich zur extrakorporalen stoßwellentherapie (ESWT) beim supraspinatussehnensyndrom]. Strahlentherapie und Onkologie 2002;178:314‐20. [DOI] [PubMed] [Google Scholar]
Loew 1995 {published data only}
- Loew M, Jurgowski W, Thomsen M. Calcareous tendinitis of the shoulder – first experiences with a treatment by extracorporeal shock wave application (ESWA) [Die wirkung extrakorporaler atosswellen auf die tendinosis calcarea der schulter]. Urologe 1995;34:49‐53. [PubMed] [Google Scholar]
Mao 2003 {published data only}
- Mao YR, Huang DF, Ding JX, Xu GQ, Xu YL, Zhao M. Analysis of extracorporeal shock wave therapy in immediate treatment of musculoskeletal disorders. Chinese Journal of Clinical Rehabilitation 2003;7:3216‐7. [Google Scholar]
Paternostro‐Sluga 2004 {published data only}
- Paternostro‐Sluga T, Zoch C. Conservative treatment and rehabilitation of shoulder problems [Konservative therapie und rehabilitation von schulterbeschwerden]. Radiologe 2004;44:597‐603. [DOI] [PubMed] [Google Scholar]
Rompe 1997a {published data only}
- Rompe JD, Kullmer K, Vogel J, Eckardt A, Wahlmann U, Eysel P, et al. Extracorporeal shock‐wave therapy. Experimental basis, clinical application [Extrakorporale stoßwellentherapie. Experimentelle grundlagen, klinischer einsatz]. Orthopade 1997;26:215‐28. [DOI] [PubMed] [Google Scholar]
Rompe 1997b {published data only}
- Rompe JD. Extracorporeal shockwave therapy in orthopedics. Positive results in tennis elbow and tendinosis calcarea of the shoulder [Extrakorporale stosswellentherapie in der orthopadie. Positive ergebnisse beim tennisellenbogen und der tendinosis calcarea der schulter]. Fortschritte der Medizin 1997;115:29‐33. [PubMed] [Google Scholar]
Seil 1999 {published data only}
- Seil R, Rupp S, Hammer DS, Ensslin S, Gebhardt T, Kohn D. Extracorporeal shockwave therapy in tendionosis calcarea of the rotator cuff: comparison of different treatment protocols [Extrakorporale stosswellentherapie bei der tendionosis calcarea der rotatorenmanschette: vergleich verschiedener behandlungsprotokolle]. Zeitschrift fur Orthopadie und Ihre Grenzgebiete 1999;137:310‐5. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ChiCTR1900022932 {published data only}
- ChiCTR1900022932. http://www.chictr.org.cn/showproj.aspx?proj=38621.
NCT02677103 {published data only}
- NCT02677103. https://clinicaltrials.gov/show/NCT02677103.
NCT03779919 {published data only}
- NCT03779919. clinicaltrials.gov/ct2/show/NCT03779919 (first received 19 December 2018).
NTR7093 {published data only}
- NTR7093. https://www.trialregister.nl/trial/5527.
PACTR201910650013453 {published data only}
- PACTR201910650013453. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=PACTR201910650013453 (first received 16 September 2019).
Additional references
Bannuru 2014
- Bannuru RR, Flavin NE, Vaysbrot E, Harvey W, McAlindon T. High‐energy extracorporeal shock‐wave therapy for treating chronic calcific tendinitis of the shoulder: a systematic review. Annals of Internal Medicine 2014;160(8):542‐9. [DOI: 10.7326/M13-1982] [DOI] [PubMed] [Google Scholar]
Britt 2016
- Britt H, Miller GC, Henderson J, Bayram C, Harrison C, Valenti L, Pan Y, Charles J,Pollack AJ, Wong C, Gordon J.. General practice activity in Australia 2015–16. General practice series no. 40. Sydney: Sydney University Press, 2016.Available at <purl.library.usyd.edu.au/sup/9781743325131>.
Buchbinder 2002
- Buchbinder R, Ptasznik R, Gordon J, Buchanan J, Prabaharan V, Forbes A. Ultrasound‐guided extracorporeal shock wave therapy (ESWT) for plantar fasciitis (painful heel): a randomised controlled trial. JAMA 2002;288:1364‐72. [DOI] [PubMed] [Google Scholar]
Buchbinder 2003
- Buchbinder R, Green S, Youd JM. Corticosteroid injections for shoulder pain. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD004016] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buchbinder 2005
- Buchbinder R, Green S, Youd JM, Assendelft WJ, Barnsley L, Smidt N. Shock wave therapy for lateral elbow pain. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD003524.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buchbinder 2006
- Buchbinder R, Green S, Youd JM, Assendelft WJ, Barnsley L, Smidt N. Systematic review of the efficacy and safety of shock wave therapy for lateral elbow pain. Journal of Rheumatology 2006;33(7):1351‐63. [PubMed] [Google Scholar]
Buchbinder 2017
- Buchbinder R, Page MJ, Huang H, Verhagen AP, Beaton D, Kopkow C, et al. for the Shoulder Core Outcomes Set Special Interest Group. A preliminary core domain set for clinical trials of shoulder disorders: a report from the OMERACT 2016 Shoulder Core Outcome Set Special Interest Group. Journal of Rheumatology 2017;44:3. [DOI] [PubMed] [Google Scholar]
Connor 2003
- Connor PM, Banks DM, Tyson AB, Coumas JS, Alessandro DF. Magnetic resonance imaging of the asymptomatic shoulder of overhead athletes. American Journal of Sports Medicine 2003;31(5):724‐7. [DOI] [PubMed] [Google Scholar]
Crawford 2003
- Crawford F, Thomson CE. Interventions for treating plantar heel pain. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD000416] [DOI] [PubMed] [Google Scholar]
Cumpston 2009
- Cumpston M, Johnston RV, Wengier L, Buchbinder R. Topical glyceryl trinitrate for rotator cuff disease. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD006355.pub2] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Dinnes 2003
- Dinnes J, Loveman E, McIntyre L, Waugh N. The effectiveness of diagnostic tests for the assessment of shoulder pain due to soft tissue disorders: a systematic review. Health Technology Assessment 2003;7(29):1‐166. [DOI] [PubMed] [Google Scholar]
Dworkin 2008
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. Journal of Pain 2008;9(2):105‐21. [DOI] [PubMed] [Google Scholar]
Gomoll 2004
- Gomoll AH, Katz JN, Warner JJ, Millett PJ. Rotator cuff disorders: recognition and management among patients with shoulder pain. Arthritis and Rheumatism 2004;50(12):3751‐61. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Accessed on 20 November 2018.
Green 1998
- Green S, Buchbinder R, Glazier R, Forbes A. Systematic review of randomised controlled trials of interventions for painful shoulder: selection criteria, outcome assessment, and efficacy. BMJ 1998;316(7128):345‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Green 1999
- Green SE, Buchbinder R, Forbes A, Glazier R. Interventions for shoulder pain. Cochrane Database of Systematic Reviews 1999, Issue 2. [DOI: 10.1002/14651858.CD001156] [DOI] [PubMed] [Google Scholar]
Hegedus 2008
- Hegedus EJ, Goode A, Campbell S, Morin A, Tamaddoni M, Moorman CT III, et al. Physical examination tests of the shoulder: a systematic review with meta‐analysis of individual tests. British Journal of Sports Medicine 2008;42(2):80‐92. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;11:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Deeks JJ. Chapter 7: Selecting studies and collecting data. In: Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
- Higgins JP, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Ioppolo 2013
- Ioppolo F, Tattoli M, Sante L, Venditto T, Tognolo L, Delicata M, et al. Clinical improvement and resorption of calcifications in calcific tendinitis of the shoulder after shock wave therapy at 6 months' follow‐up: a systematic review and meta‐analysis. Archives of Physical Medicine and Rehabilitation 2013;94:1699‐706. [DOI] [PubMed] [Google Scholar]
Karjalainen 2019a
- Karjalainen TV, Jain NB, Page CM, Lähdeoja TA, Johnston RV, Salamh P, Kavaja L, Ardern CL, Agarwal A, Vandvik PO, Buchbinder R. Subacromial decompression surgery for rotator cuff disease. Cochrane Database of Systematic Reviews 2019, Issue 1. [DOI: 10.1002/14651858.CD005619.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karjalainen 2019b
- Karjalainen TV, Jain NB, Heikkinen J, Johnston RV, Page CM, Buchbinder R. Cochrane Database of Systematic Reviews 2019, Issue 12. Art. No.: CD013502. DOI: 10.1002/14651858.CD013502. Surgery for rotator cuff tears.. Cochrane Database of Systematic Reviews 2019, Issue 12. [DOI: 10.1002/14651858.CD013502] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lewis 2007
- Lewis J, Tennent D. How effective are diagnostic tests for the assessment of rotator cuff disease of the shoulder?. In: MacAuley D, Best TM editor(s). Evidence‐Based Sports Medicine. 2nd Edition. Malden (Netherlands): Blackwell Publishing, 2007:327‐60. [Google Scholar]
Lewis 2009
- Lewis JS. Rotator cuff tendinopathy/subacromial impingement syndrome: is it time for a new method of assessment?. British Journal of Sports Medicine 2009;43(4):259‐64. [DOI] [PubMed] [Google Scholar]
Loew 1997
- Loew M, Daecke W, Kusnierczak D. The effects of extracorporeal shock wave application (ESWA) in treatment of calcifying tendinitis of the shoulder. Journal of Bone and Joint Surgery. British Volume 1997;79B(Suppl 2):202‐3. [DOI] [PubMed] [Google Scholar]
Luime 2004
- Luime JJ, Koes BW, Hendriksen IJ, Burdof A, Verhagen AP, Miedema HS, et al. Prevalence and incidence of shoulder pain in the general population: a systematic review. Scandinavian Journal of Rheumatology 2004;33:73‐81. [DOI] [PubMed] [Google Scholar]
MacDermid 2004
- MacDermid JC, Raos J, Drosdowech D, Faber K, Patterson S. The impact of rotator cuff pathology on isometric and isokinetics strength, function, and quality of life. Journal of Shoulder and Elbow Surgery 2004;13:593‐8. [DOI] [PubMed] [Google Scholar]
May 2003
- Stephen May. An outcome audit for musculoskeletal patients in primary care. Physiotherapy Theory and Practice 2003;19(4):189‐198. [DOI: 10.1080/09593980390246724] [DOI] [Google Scholar]
Melzack 1975
- Melzack R. Prolonged relief of pain by brief, intense transcutaneous somatic stimulation. Pain 1975;1:357‐73. [DOI] [PubMed] [Google Scholar]
Moore 2010
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. "Evidence" in chronic pain – establishing best practice in the reporting of systematic reviews. Pain 2010;150(3):386‐9. [DOI] [PubMed] [Google Scholar]
Neer 1983
- Neer CS II. Impingement lesions. Clinical Orthopaedics and Related Research 1983;173:70‐7. [PubMed] [Google Scholar]
Ogata 1990
- Ogata S, Uhthoff HK. Acromial enthesopathy and rotator cuff tear: a radiologic and histologic postmortem investigation of the coracoacromial arch. Clinical Orthopaedics and Related Research 1990;254:39‐48. [PubMed] [Google Scholar]
Ostor 2005
- Ostor AJ, Richards CA, Prevost AT, Speed CA, Hazelman BL. Diagnosis and relation to general health of shoulder disorders presenting to primary care. Rheumatology 2005;44:800‐5. [DOI] [PubMed] [Google Scholar]
Page 2015
- Page MJ, McKenzie JE, Green SE, Beaton DE, Jain NB, Lenza M, et al. Core domain and outcome measurement sets for shoulder pain trials are needed: systematic review of physical therapy trials. Journal of Clinical Epidemiology 2015;68(11):1270‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2016a
- Page MJ, Green S, McBain B, Surace SJ, Deitch J, Lyttle N, et al. Manual therapy and exercise for rotator cuff disease. Cochrane Database of Systematic Reviews 2016, Issue 6. [DOI: 10.1002/14651858.CD012224] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2016b
- Page MJ, Green S, Mrocki MA, Surace SJ, Deitch J, McBain B, et al. Electrotherapy modalities for rotator cuff disease. Cochrane Database of Systematic Reviews 2016, Issue 6. [DOI: 10.1002/14651858.CD012225] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2016c
- Page MJ, Huang H, Verhagen AP, Buchbinder R, Gagnier JJ. Identifying a core set of outcome domains to measure in clinical trials for shoulder pain: a modified Delphi study. Rheumatic & Musculoskeletal Diseases 2016;2(2):e000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2018
- Page MJ, Huang H, Verhagen AP, Gagnier JJ, Buchbinder R. Outcome reporting in randomised trials for shoulder conditions: literature review to inform the development of a core outcome set. Arthritis Care & Research 2018;70(2):252‐9. [DOI] [PubMed] [Google Scholar]
Paoloni 2005
- Paoloni JA, Orchard JW. The use of therapeutic medications for soft‐tissue injuries in sports medicine. Medical Journal of Australia 2005;183(7):384‐8. [DOI] [PubMed] [Google Scholar]
Reeves 2011
- Reeves BC, Deeks JJ, Higgins JP, Wells GA. Chapter 13: Including non‐randomized studies. In: Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rompe 1996
- Rompe JD, Hopf C, Kullmer K, Heine J, Burger R. Analgesic effect of extracorporeal shock wave therapy on chronic tennis elbow. Journal of Bone and Joint Surgery 1996;78‐B(2):233‐7. [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Sher 1995
- Sher JS, Uribe JW, Posada A, Murphy BJ, Zlatkin MB. Abnormal findings on magnetic resonance imaging of asymptomatic shoulders. Journal of Bone and Joint Surgery 1995;77:10‐5. [DOI] [PubMed] [Google Scholar]
Staples 2008
- Staples M, Ptasznik R, Gordon J, Buchanan J, Forbes A, Buchbinder R. A randomized controlled trial of extracorporeal shock wave therapy for lateral epicondylitis (tennis elbow). Journal of Rheumatology 2008;35:1035‐46. [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Taylor 2005
- Taylor W. Musculoskeletal pain in the adult New Zealand population: prevalence and impact. New Zealand Medical Journal 2005;118(1221):U1629. [PubMed] [Google Scholar]
Ueberle 1997
- Ueberle F. Shock wave technology. In: Siebert W, Buch M editor(s). Extracorporeal Shock‐Waves in Orthopaedics. Berlin (Germany): Springer‐Verlag, 1997:59‐87. [Google Scholar]
Urwin 1998
- Urwin M, Symmons D, Allison T, Brammah T, Busby H, Roxby M, et al. Estimating the burden of musculoskeletal disorders in the community: the comparative prevalence of symptoms at different anatomical sites, and the relation to social deprivation. Annals of the Rheumatic Diseases 1998;57:649‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Windt 1996
- Windt DA, Koes BW, Boeke AJ, Deville W, Jong BA, Bouter LM. Shoulder disorders in general practice: prognostic indicators of outcome. British Journal of General Practice 1996;46(410):519‐23. [PMC free article] [PubMed] [Google Scholar]
Vavken 2009
- Vavken P, Holinka J, Rompe JD, Dorotka R. Focused extracorporeal shock wave therapy in calcifying tendinitis of the shoulder: a meta‐analysis. Sports Health 2009;1:137‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verstraelen 2014
- Verstraelen FU, In den Kleef NJ, Jansen L, Morrenhof JW. High‐energy versus low‐energy extracorporeal shock wave therapy for calcifying tendinitis of the shoulder: which is superior? A meta‐analysis. Clinical Orthopaedics and Related Research 2014;472(9):2816‐25. [DOI: 10.1007/s11999-014-3680-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiner 1970
- Weiner D, Macnab I. Superior migration of the humeral head: a radiological aid in the diagnosis of tears of the rotator cuff. Journal of Bone and Joint Surgery. British Volume 1970;52:523‐7. [PubMed] [Google Scholar]
Yamaguchi 2001
- Yamaguchi K, Tetro A, Blam O, Evanoff B, Teefey S, Middleton W. Natural history of asymptomatic rotator cuff tears: a longitudinal analysis of asymptomatic tears detected sonographically. Journal of Shoulder and Elbow Surgery 2001;10:199‐203. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Buchbinder 2011
- Buchbinder R, Johnston RV, Roos JF. Shock wave therapy for rotator cuff disease with or without calcification. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD008962] [DOI] [PMC free article] [PubMed] [Google Scholar]


