Abstract
Background
Approximately 80% of breast cancers amongst premenopausal women are hormone receptor‐positive. Adjuvant endocrine therapy is an integral component of care for hormone receptor‐positive breast cancer and in premenopausal women includes oestrogen receptor blockade with tamoxifen, temporary suppression of ovarian oestrogen synthesis by luteinising hormone releasing hormone (LHRH) agonists, and permanent interruption of ovarian oestrogen synthesis with oophorectomy or radiotherapy. Recent international consensus statements recommend single‐agent tamoxifen or aromatase inhibitors with ovarian function suppression (OFS) as the current standard adjuvant endocrine therapy for premenopausal women (often preceded by chemotherapy). This review examined the role of adding OFS to another treatment (i.e. chemotherapy, endocrine therapy, or both) or comparing OFS to no further adjuvant treatment.
Objectives
To assess effects of OFS for treatment of premenopausal women with hormone receptor‐positive early breast cancer.
Search methods
For this review update, we searched the Specialised Register of the Cochrane Breast Cancer Group, MEDLINE, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 8), the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), and ClinicalTrials.gov on 26 September 2019. We screened the reference lists of related articles, contacted trial authors, and applied no language restrictions.
Selection criteria
We included all randomised trials assessing any method of OFS, that is, oophorectomy, radiation‐induced ovarian ablation, or LHRH agonists, as adjuvant treatment for premenopausal women with early‐stage breast cancer. We included studies that compared (1) OFS versus observation, (2) OFS + chemotherapy versus chemotherapy, (3) OFS + tamoxifen versus tamoxifen, and (4) OFS + chemotherapy + tamoxifen versus chemotherapy + tamoxifen.
Data collection and analysis
Two review authors independently extracted data and assessed risk of bias and certainty of evidence using the GRADE approach. Hazard ratios (HRs) were derived for time‐to‐event outcomes, and meta‐analysis was performed using a fixed‐effect model. The primary outcome measures were overall survival (OS) and disease‐free survival (DFS). Toxicity, contralateral breast cancer, and second malignancy were represented as risk ratios (RRs), and quality of life data were extracted when provided.
Main results
This review update included 15 studies involving 11,538 premenopausal women with hormone receptor‐positive early breast cancer; these studies were conducted from 1978 to 2014. Some of these treatments are not current standard of care, and early studies did not assess HER2 receptor status. Studies tested OFS versus observation (one study), OFS plus chemotherapy versus chemotherapy (six studies), OFS plus tamoxifen versus tamoxifen (six studies), and OFS plus chemotherapy and tamoxifen versus chemotherapy and tamoxifen (two studies). Of those studies that reported the chemotherapy regimen, an estimated 72% of women received an anthracycline. The results described below relate to the overall comparison of OFS versus no OFS.
High‐certainty evidence shows that adding OFS to treatment resulted in a reduction in mortality (hazard ratio (HR) 0.86, 95% confidence interval (CI) 0.78 to 0.94; 11 studies; 10,374 women; 1933 reported events). This treatment effect was seen when OFS was added to observation, to tamoxifen, or to chemotherapy and tamoxifen. The effect on mortality was not observed when OFS was added to chemotherapy without tamoxifen therapy (HR 0.95, 95% CI 0.82 to 1.09; 5 studies; 3087 women; median follow‐up: range 7.7 to 12.1 years). The addition of OFS resulted in improved DFS (HR 0.83, 95% CI 0.77 to 0.90; 10 studies; 8899 women; 2757 reported events; high‐certainty evidence). The DFS treatment effect persisted when OFS was added to observation, to tamoxifen, and to chemotherapy and tamoxifen. The effect on DFS was reduced when OFS was added to chemotherapy without tamoxifen therapy (HR 0.90, 95% CI 0.79 to 1.01; 5 studies; 2450 women). Heterogeneity was low to moderate across studies for DFS and OS (respectively).
Evidence suggests that adding OFS slightly increases the incidence of hot flushes (grade 3/4 or any grade; risk ratio (RR) 1.60, 95% CI 1.41 to 1.82; 6 studies; 5581 women; low‐certainty evidence, as this may have been under‐reported in these studies). Two other studies that could not be included in the meta‐analysis reported a higher number of hot flushes in the OFS group than in the no‐OFS group. Seven studies involving 5354 women collected information related to mood; however this information was reported as grade 3 or 4 depression, anxiety, or neuropsychiatric symptoms, or symptoms were reported without the grade. Two studies reported an increase in depression, anxiety, and neuropsychiatric symptoms in the OFS group compared to the no‐OFS group, and five studies indicated an increase in anxiety in both treatment groups (but no difference between groups) or no difference overall in symptoms over time or between treatment groups. A single study reported bone health as osteoporosis (defined as T score < ‐2.5); this limited evidence suggests that OFS increases the risk of osteoporosis compared to no‐OFS at median follow‐up of 5.6 years (RR 1.16, 95% CI 1.10 to 28.82; 2011 women; low‐certainty evidence).
Adding OFS to treatment likely reduces the risk of contralateral breast cancer (HR 0.75, 95% CI 0.57 to 0.97; 9 studies; 9138 women; moderate‐certainty evidence).
Quality of life was assessed in five studies; four studies used validated tools, and the fifth study provided no information on how data were collected. Two studies reported worse quality of life indicators (i.e. vaginal dryness, day and night sweats) for women receiving OFS compared to those in the no‐OFS group. The other two studies indicated worsening of symptoms (e.g. vasomotor, gynaecological, vaginal dryness, decline in sexual interest, bone and joint pain, weight gain); however these side effects were reported in both OFS and no‐OFS groups. The study that did not use a validated quality of life tool described no considerable differences between groups.
Authors' conclusions
This review found evidence that supports adding OFS for premenopausal women with early, hormone receptor‐positive breast cancers. The benefit of OFS persisted when compared to observation, and when added to endocrine therapy (tamoxifen) or chemotherapy and endocrine therapy (tamoxifen). The decision to use OFS may depend on the overall risk assessment based on tumour and patient characteristics, and may follow consideration of all side effects that occur with the addition of OFS.
Plain language summary
Ovarian function suppression for treating premenopausal women with hormone receptor‐positive early breast cancer
What is the aim of this review?
The aim of this Cochrane Review was to find out whether adding ovarian function suppression to treatment for early breast cancer improves survival, reduces the risk of cancer coming back, and is safe for premenopausal women with hormone receptor‐positive early breast cancer. Cochrane Review authors collected and analysed all relevant studies to answer these questions and found 15 studies.
Key messages?
Adding ovarian suppression function to therapy improved survival (women lived longer) and reduced the chance of cancer returning in women with operable early breast cancer, but the use of ovarian function suppression appears to increase the risk of hot flushes and may affect bone health. The decision to use OFS needs to be personalised after the risk and benefit profile is considered.
What was studied in the review?
Around eight out of ten premenopausal women who develop breast cancer have a type of cancer that is sensitive to hormones, termed 'hormone receptor‐positive' disease. To slow the growth of any cancer cells that remain after surgery, hormonal therapy can be used to reduce the availability of natural hormone oestrogen to cancer cells. This can be done by blocking oestrogen receptors on the cells with drugs such as tamoxifen, by suppressing the production of oestrogen by drugs called luteinising hormone releasing hormone (LHRH) agonists, or by removing the ovaries with surgery or impairing their ability to produce hormones using radiotherapy.
This review examined the role of ovarian function suppression (i.e. LHRH agonists, removal of the ovaries, or radiation‐induced ovarian suppression) for premenopausal women with hormone receptor‐positive early‐stage breast cancer. The practice of suppressing ovarian function in addition to providing other treatments has been of interest over the last five years, as new data from clinical trials have become available. A review of these data is needed to find the benefits of adding ovarian function suppression to treatment, to identify side effects from ovarian function suppression, and to discover how treatment is affecting a woman's overall well‐being (quality of life).
The funding source for the conduct of these studies was government (four studies), government and pharmaceutical companies combined (three studies), government and not‐for‐profit organisations combined (two studies), not‐for‐profit organisations and pharmaceutical companies (two studies), and a pharmaceutical company (one study); three studies did not report a funding source.
What are the main results of the review?
Review authors found 15 relevant studies involving 11,538 women. To achieve ovarian function suppression, nine studies used LHRH agonists (most used goserelin), two studies induced ovarian function suppression through surgery, and four studies allowed any method (LHRH agonists, surgery, or radiotherapy). LHRH agonists were given to women for a minimum of one year.
The woman's health was monitored for at least two years from the start of the study. Some studies monitored women for over 12 years.
Review authors found that adding ovarian suppression function to treatment:
• improves survival and reduces the risk of cancer coming back compared to treatment without ovarian function suppression;
• appears to increase the chance of severe hot flushes compared to treatment without ovarian function suppression;
• probably reduces the risk of a second breast cancer in the other breast compared to treatment without ovarian function suppression;
• may or may not have an effect on mood (e.g. anxiety, depression) compared to treatment without ovarian function suppression;
• may increase the risk of osteoporosis compared to treatment without ovarian function suppression (however, this finding was based on one study); and
• may make little or no difference in quality of life for women compared to treatment without ovarian function suppression. Five of 15 studies provided some information on the quality of life of women.
How up‐to‐date is this review?
The review authors searched for studies that had been published up to September 2019.
Summary of findings
Summary of findings for the main comparison. OFS compared to no OFS for adjuvant treatment of early breast cancer.
| OFS compared to no OFS for adjuvant treatment of early breast cancer | ||||||
| Patient or population: women with early breast cancer Setting: outpatient Intervention: OFS (± other treatment) Comparison: no OFS (± other treatment) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no OFS | Risk with OFS | |||||
| Overall survival (OS) Median follow‐up: range 5.3 to 12.1 years (*baseline risk at 5 and 10 years estimated from the control arm in 8 and 7 studies, respectively) |
5‐year risk of death* | HR 0.86 (0.78 to 0.94) | 10,374 (11 RCTs) | ⊕⊕⊕⊕ HIGH | ||
| 110 per 1000 | 95 per 1000 (87 to 104) | |||||
| 10‐year risk of death* | ||||||
| 310 per 1000 | 273 per 1000 (251 to 294) | |||||
| Disease‐free survival (DFS)
Median follow‐up: range 5.3 to 12.1 years (*baseline risk at 5 and 10 years estimated from the control arm in 9 and 7 studies, respectively) |
5‐year risk of recurrence* | HR 0.83 (0.77 to 0.90) | 8899 (10 RCTs) | ⊕⊕⊕⊕ HIGH | ||
| 250 per 1000 | 212 per 1000 (199 to 228) | |||||
| 10‐year risk of recurrence* | ||||||
| 440 per 1000 | 382 per 1000 (360 to 407) | |||||
| Toxicity ‐ hot flushes (a combination of "grade 3/4" and "any grade" toxicity) Follow‐up: range 2 to 5 years |
97 per 1000 | 154 per 1000 (136 to 176) | RR 1.60 (1.41 to 1.82) | 5581 (6 RCTs) | ⊕⊕⊕⊝ LOWa | An additional 2 studies (studied population: 246 in ABCTCG, not detailed in IBCSG VIII) reported a higher number of hot flushes in the OFS group than in the no OFS group. These results are consistent with the overall effect estimate |
| Toxicity ‐ mood Follow‐up: range 1 year to 9.6 years |
7 out of 15 studies reported Grade 3 or 4 depression, anxiety, or neuropsychiatric symptoms or did not report the grade of these side effects. Two studies ‐ ABCTCG; E‐3193, INT‐0142 ‐ reported an increase in depression, anxiety, and neuropsychiatric symptoms in the OFS group compared to the non‐OFS group. Five studies ‐ ECOG 5188, INT‐0101; GABG IV‐B‐93; SOFT; Yi 2016; ZIPP ‐ indicated an increase in anxiety in both treatment groups (but no differences between groups) or no difference overall in symptoms over time or between treatment groups | ‐ | 5354 (7 RCTs) | ⊕⊕⊕⊝ MODERATEb | ||
| Toxicity ‐ bone health (osteoporosis defined by a T score < ‐2.5) Follow‐up: median 5.6 years |
35 per 1000 | 58 per 1000 (38 to 87) | RR 1.66 (1.10 to 2.50) | 2011 (1 RCT) | ⊕⊕⊝⊝c LOW | |
| Contralateral breast cancer Follow‐up: 4.75 to median 12.1 years |
31 per 1000 | 23 per 1000 (18 to 30) | RR 0.75 (0.57 to 0.97) | 7856 (8 RCTs) | ⊕⊕⊕⊝ MODERATEd | |
| Quality of life Follow‐up: 2 to 6 years | Four out of 15 studies collected data on quality of life using validated tools (ABCTCG; E‐3193, INT‐0142; IBCSG VIII; SOFT), and 1 study collected quality of life‐type information without describing a validated tool (ZBCSG Trial B). Two studies ‐ ABCTCG; E‐3193, INT‐0142 ‐ reported worse quality of life indicators (i.e. vaginal dryness, day and night sweats) in the OFS group than in the no OFS group. The other 2 studies ‐ IBCSG VIII; SOFT ‐ indicated worsening of symptoms (e.g. vasomotor, gynaecological, vaginal dryness, decline in sexual interest, bone and joint pain, weight gain); however these side effects were reported in both OFS and no OFS groups. The study that did not use a validated quality of life tool described no considerable differences between groups | ‐ | Estimated to be 2996 (5 RCTs) | ⊕⊕⊝⊝ LOWe | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; RCT: randomised controlled trial; RR: risk ratio; OFS: ovarian function suppression. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aThree studies reported Grade 3 or 4 hot flushes using a standardised toxicity symptom scale (E‐3193, INT‐0142; ECOG 5188, INT‐0101; SOFT), and the other three studies that contributed to the meta‐analysis reported any grade of hot flushes/sweats without reporting the scale used (Arriagada 2005; ZBCSG Trial B; ZIPP). This outcome was downgraded due to variability in reporting of hot flushes across studies and uncertainty as to whether unblinding of treatment allocation may have affected patient reporting and concerns about selective outcome reporting. Therefore downgraded by two points overall. bThis outcome was downgraded because measures appeared to be different across studies (ranging from 'neuropsychiatric' to anxiety) and were patient‐reported, with most studies not describing the toxicity symptom scale used. The direction of the treatment effect was also inconsistent across studies. Therefore we downgraded by one point overall for risk of bias and inconsistency. cThis outcome was reported by a single study, and the number of events did not meet the optimal information size. There are also concerns that the follow‐up period was relatively short for this type of outcome. We downgraded by two points. dThe number of events did not meet the optimal information size; therefore we downgraded by one point for imprecision. eThis outcome was downgraded because all measures were patient‐reported, taking place in open‐label studies, and therefore were at high risk of bias. Although most studies used validated questionnaires, the time frames when women were given the questionnaires was variable, the direction of effect was variable across studies, and the length of follow‐up was different (ranging from 2 years to 6 years). Few studies provided the number of participants who responded to the quality of life questionnaires over time.
Background
Description of the condition
Breast cancer is a major cause of morbidity and mortality. Approximately 20% of women who were diagnosed and treated for early breast cancer will eventually die of the disease (Jemal 2008). Based on data from the Surveillance, Epidemiology, and End Results (SEER) registries in the United States, approximately 80% of tumours in premenopausal women are hormone sensitive and may be suitable for hormonal treatment (Howlader 2014).
Description of the intervention
The aim of adjuvant therapy is to prevent recurrence and improve overall survival. In premenopausal women with hormone receptor‐positive early breast cancer, options for adjuvant therapy include cytotoxic chemotherapy and hormonal therapy (Gelber 1996). The goal of hormonal therapy is to reduce the availability of oestrogen to cancer cells. This can be achieved by blocking oestrogen receptors (e.g. using tamoxifen) or by using ovarian function suppression (OFS) to suppress oestrogen synthesis.
OFS can occur irreversibly with surgical oophorectomy or with radiation‐induced ovarian ablation. It can occur reversibly with luteinising hormone releasing hormone (LHRH) agonists. LHRH agonists act by binding to pituitary LHRH receptors, resulting in down‐regulation of receptors and subsequent suppression of luteinising hormone and oestradiol (Furr 1989). The most commonly prescribed LHRH agonist is goserelin. The major side effects of OFS are infertility, decreased libido, hot flushes, sweating, headache, blood pressure changes, loss of bone density, hypercalcaemia, and several other rare complications.
How the intervention might work
Chemotherapy has been shown to induce amenorrhoea in 60% to 80% of premenopausal women who receive adjuvant treatment (Bines 1996; Walshe 2006). Women who become amenorrhoeic following chemotherapy have better disease‐free survival than those who do not, particularly in the case of hormone‐sensitive disease (Pagani 1998; Davidson 2003; Walshe 2006). This suggests that at least some of the beneficial effect of chemotherapy in premenopausal women is mediated via its toxic effects on the ovaries and results in ovarian suppression. The value of ovarian ablation by oophorectomy or radiotherapy is clearly demonstrated by a meta‐analysis from the Early Breast Cancer Trialists' Collaborative Group (EBCTCG 1996; EBCTCG 2003; EBCTCG 2005), which focused on women under the age of 50 years, most of whom were likely to be premenopausal. For women who underwent ovarian ablation in the absence of chemotherapy, a 25% reduction in the annual odds of recurrence was reported, along with a 24% reduction in the annual odds of death. Benefit was seen in both node‐positive and node‐negative women. Among women randomised to ovarian ablation following chemotherapy, the benefit of ablation appeared smaller and was not statistically significant (reduction in the annual odds of recurrence was 10% with a standard error (SE) of 9%; reduction in the annual odds of death was 8% with an SE of 10%).
Early clinical trials compared outcomes of OFS versus chemotherapy (SCTBG and ICRF 1993; Ejlertsen 1999); researchers often did not select participants based on their hormone status. Current guidelines recommend hormone manipulation in all women with hormone receptor‐positive breast cancer (Cardoso 2019; NCCN 2019). One of the increasingly important questions on treatment of premenopausal women with early breast cancer has become the value of adding OFS to treatment.
Why it is important to do this review
The role of OFS has been extensively researched since 1896, including a comprehensive review using individual participant data and conducted by the Early Breast Cancer Trialists' Collaborative Group (Clarke 1998; EBCTCG, Clarke 1998; EBCTCG 2005). This early research was conducted before the era of treatment driven by hormone receptor status or assessment of HER2 receptor status. Recent international consensus statements recommend single‐agent tamoxifen or aromatase inhibitors with ovarian function suppression (OFS) as the current standard adjuvant endocrine therapy for very young premenopausal women or high‐risk premenopausal women receiving chemotherapy. This Cochrane Review examined the role of adding OFS to many different treatments (including various chemotherapy regimens, endocrine therapy, or observation) for women with hormone receptor‐positive early breast cancer. The findings of this review will assist consumers and clinicians, guideline developers, and funding bodies (e.g. NIHR UK).
The aim of this review was to clarify effects of OFS for adjuvant treatment of hormone receptor‐positive early breast cancer in premenopausal women by performing a systematic review of available randomised trials. An earlier version of this review focuses on effects of LHRH agonists in the adjuvant treatment of breast cancer with confounded comparisons (Goel 2009). This current systematic review addresses the modern question of OFS compared to no OFS in premenopausal, hormone receptor‐positive women given that a number of trials have been reported in full or new trials have been reported since the time that Goel 2009 was published, and that adjustments to eligibility criteria have resulted in the inclusion of additional trials.
Objectives
To assess effects of OFS for treatment of premenopausal women with hormone receptor‐positive early breast cancer.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled studies. Quasi‐randomised studies were not eligible.
We included studies published as full‐text articles or as conference abstracts.
Types of participants
We included premenopausal women with a histological diagnosis of hormone receptor‐positive early breast cancer. 'Early breast cancer' is defined as tumour‐node‐metastasis (TNM) stage I, II, and III. 'Premenopausal' is defined by the studies, usually as menses in the last 3 to 12 months and/or oestradiol levels in premenopausal ranges.
We excluded studies of women with metastatic disease.
Types of interventions
We defined an intervention as any form of OFS (i.e. oophorectomy, radiation‐induced ovarian ablation, or LHRH agonists). LHRH agonists could include buserelin, goserelin, leuprorelin, nafarelin, and triptorelin, and had to be used for at least 12 months.
We defined a comparator as any regimen that did not contain OFS. Endocrine therapy and chemotherapy were allowed if the same treatment was given to both groups.
Comparisons could include the following.
OFS versus observation.
OFS + chemotherapy versus chemotherapy.
OFS + tamoxifen versus tamoxifen.
OFS + chemotherapy + tamoxifen versus chemotherapy + tamoxifen.
Types of outcome measures
Primary outcomes
Overall survival (OS), defined as the time from date randomised to date of death due to any cause
Disease‐free survival (DFS), defined as the time from date randomised to first recurrence, contralateral breast cancer, second breast cancer, or death, or as defined by the study
Secondary outcomes
Contralateral breast cancer
Second malignancy
Adverse events including hot flushes, mood disorders, reduced bone density, arthralgias, altered sexual function, increased cardiovascular risk, deep vein thrombosis, pulmonary embolism, impaired cognitive function, treatment‐related death, and any other significant toxicities reported by the studies. Toxicities could be defined as per the World Health Organization (WHO)/National Cancer Institute of Canada (NCIC) toxicity criteria, or as per the study
Compliance with treatment
Quality of life, assessed by validated or trial‐specific instruments such as the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire
Search methods for identification of studies
Electronic searches
We searched the following databases on 17 September 2018 and performed a top‐up search on 26 September 2019.
Specialised Register of the Cochrane Breast Cancer Group. Details of the search strategy used by the Group for identification of studies and the procedure used to code references are outlined in the Group's module (www.mrw.interscience.wiley.com/cochrane/clabout/articles/BREASTCA/frame.html). We carried out a search for the following text words: 'buserelin', 'goserelin', 'gonadotropin‐releasing hormone', 'leuprorelin', 'triptorelin', 'nafarelin', 'LHRH', 'oophorectomy', 'ovariectomy', 'ablation', and 'ovarian function depression'.
Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 8) (see Appendix 1).
MEDLINE (via OvidSP) (see Appendix 2).
Embase (via OvidSP) (see Appendix 3).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal for all prospectively registered and ongoing trials (see Appendix 4).
ClinicalTrials.gov register (clinicaltrials.gov) for additional unpublished and ongoing studies (see Appendix 5).
Searching other resources
We contacted some of the investigators of potentially eligible studies for unpublished data or clarification of data analysis (i.e. whether or not analyses were adjusted) and checked PubMed to learn whether eligible conference abstracts had been published as full‐text articles. These approaches are recognised as appropriate methods (Young 2011; Scherer 2018).
For the previous versions of this review (Goel 2009), we handsearched the proceedings of annual meetings of the American Society of Clinical Oncology and the San Antonio Breast Cancer Symposium (2005 to 2008). For the 2019 review update, handsearching of these conference proceedings was not required because these are now imported and searched via the aforementioned Embase search (as outlined in Appendix 3).
Data collection and analysis
Selection of studies
We applied the eligibility criteria to each of the retrieved references. In the first instance, we used study publications to assess each study's eligibility. If a study had not been published, we attempted to find the necessary information from a study protocol or a clinical trial registry record.
For the original review and the review update, two review authors (review update: TB, AG) independently assessed each potentially eligible study. A third review author was not required as there were no disagreements regarding eligibility.
We recorded excluded studies in the Characteristics of excluded studies table.
We applied no language restrictions.
Data extraction and management
For the original review and the review update, two review authors (review update: TB, MW)) independently extracted data from the included studies. If required, a third review author (AG) was available to resolve any discrepancies regarding extraction of quantitative data. We collected information on study design, participants (including hormone receptor status and nodal involvement), settings, interventions, primary and secondary outcomes, follow‐up, and sources of funding. For studies with more than one publication, we extracted data from these publications, and we considered the final or updated version of each study as the primary reference. For one included study, four colleagues at the Japanese Cochrane Centre conducted data extraction and risk of bias assessments and double‐checked the translated material.
Assessment of risk of bias in included studies
For the review update, we used Cochrane's 'Risk of bias' assessment tool to assess potential sources of bias in the included studies (Higgins 2011). Two review authors (TB, MW) independently assessed the potential risk of bias for each study and resolved any differences in judgement through discussion. The domains assessed were random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. We assigned a rating of 'high', 'low', or 'unclear' risk of bias to each domain for each included study in keeping with the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Among phase 3 oncology studies, open‐label studies are common due to the difficulty involved in concealing different chemotherapy schedules and toxicities. We therefore grouped the blinding of outcome assessment domain with outcome measures from most unlikely to most likely to be influenced by lack of blinding. We segregated outcomes into (1) OS, (2) DFS, (3) toxicity, and (4) quality of life.
Measures of treatment effect
Two review authors extracted data from each trial.
The primary outcomes for this review were OS and DFS, with both considered as time‐to‐event outcomes. Hazard ratios (HRs) and variances were extracted from trial publications, when available. If not reported, statistics were extracted from publications via the methods described by Tierney et al using other summary statistics (Tierney 2007). These indirect methods were recorded in the Notes section in the Characteristics of included studies tables. All efficacy analyses used an intention‐to‐treat population when this was reported. Furthermore, data for OS and DFS were extracted for the hormone receptor‐positive population or if more than 50% of the study population had hormone receptor‐positive cancers.
Contralateral breast cancer and second malignancy were collected and reported as risk ratios (RRs) with 95% confidence intervals (CIs).
Toxicity data were extracted from each study; when possible, this was done for the treated population rather than the intention‐to‐treat population. As definitions of toxic events varied between trials, events were extracted and summarised to best reflect clinically important outcomes. Pooled RRs and 95% CIs were calculated for hot flushes; all other toxicities were presented as frequencies and proportions and were not included as part of a meta‐analysis due to the paucity of data. For this review update, if efficacy data were reported separately for hormone receptor‐positive cancers but not for toxicity outcomes, we extracted the toxicity data regardless of the proportion of the studied population with hormone receptor‐positive tumours because we expected toxicity to be the same regardless of hormone receptor status.
We collected quality of life data irrespective of the questionnaire used. We made no attempt to statistically synthesise quality of life data, which we summarised and reported qualitatively.
Unit of analysis issues
Three studies were three‐arm studies (ECOG 5188, INT‐0101; IBCSG VIII; ZBCSG Trial B). For all three studies, data from two of the three arms were used and were relevant for this review topic. The third arm contained a confounded comparator or intervention group.
One study was a 2 × 2 factorial study (ZIPP). For the analysis, there were two relevant intervention arms (goserelin ± elective tamoxifen, and goserelin + randomised tamoxifen) and two relevant comparator arms (observation ± elective tamoxifen, and tamoxifen). Data from the two intervention arms were combined and were compared to data from the two comparator arms.
Dealing with missing data
When data were missing, we contacted the original investigators (by written correspondence) to request missing data. For the review update, we contacted the following trialists for summary statistics, numbers of events for each treatment arm (for overall survival or disease‐free survival), and clarification on whether HRs were adjusted or unadjusted: ABCTCG; Arriagada 2005; ASTRRA; GABG IV‐B‐93; Uslu 2014; Yang 2013. We received additional data from the trialists for two studies: ASTRRA; GABG IV‐B‐93.
Assessment of heterogeneity
We assessed heterogeneity by using the Chi² test and the I² statistic and by visually inspecting forest plots. We inspected the graphical representation of data; if confidence intervals for the results of individual studies had poor overlap, this generally indicated the presence of statistical heterogeneity.
We interpreted the I² statistic as per guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011): 0% to 40% might not be important; 30% to 60% represented moderate heterogeneity; 50% to 90% represented substantial heterogeneity; and 75% to 100% represented considerable heterogeneity.
As there was minimal heterogeneity in the majority of the studies analysed in this review, we used the fixed‐effect model. When there was considerable heterogeneity (for hot flushes), we used the random‐effects model and explored sources of heterogeneity; however we ultimately used a fixed‐effect model for these given that the conclusions were the same based on fixed‐effect and random‐effects analyses.
Assessment of reporting biases
We followed recommendations for testing for funnel plot asymmetry as described in Section 10.4.3.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Funnel plot asymmetry may be due to reporting bias; we addressed this possibility in the Results and Discussion sections of the review for the two primary outcomes. Supplementary to visual inspection of the funnel plot, we conducted Egger's test for the primary outcomes using R (metafor package; R).
Data synthesis
For time‐to‐event outcome data (i.e. OS and DFS), we used a fixed‐effect (inverse‐variance method) model.
For dichotomous outcome data (i.e. contralateral breast cancer and second malignancy), we used the fixed‐effect (inverse‐variance method) model. For the toxicity outcome ‐ hot flushes ‐ we used the fixed‐effect (Mantel‐Haenszel method) model. For all other outcomes (including most toxicity outcomes and compliance with treatment), we reported data when available and summarised the information narratively.
We performed all analyses using Review Manager software (RevMan).
Summary of findings
We used the GRADE approach to assess the certainty of evidence for the following seven main outcomes: overall survival, disease‐free survival, hot flushes (grade 3/4), mood, bone health, contralateral breast cancer, and quality of life. We used GRADEproGDT software to develop the 'Summary of findings' table and followed GRADE guidance (GRADEproGDT; Schünemann 2019). Two review authors (TB, MW) graded the certainty of evidence for this review update.
To calculate absolute risk of the comparator group for time‐to‐event outcomes, we estimated the event rate at two specific time points (i.e. five and ten years for overall survival and disease‐free survival) from the Kaplan‐Meier curves or reported event rates. We entered these estimated values into GRADEproGDT, and the corresponding absolute risks for the intervention group at five and ten years were automatically populated by GRADEproGDT.
Subgroup analysis and investigation of heterogeneity
We planned to perform the following post‐hoc subgroup analysis for OS and DFS: HER2 ISH status: positive or negative; age group: younger than 35 years of age versus 35 to 40 years of age versus over 40 years of age; molecular subtypes: luminal A versus luminal B; chemotherapy regimen: non‐anthracycline/taxane versus anthracycline/taxane versus dose‐dense anthracycline/taxane; breast cancer stage: locally advanced breast cancer that is inoperable at presentation (stage III) versus stage I/II breast cancer at presentation. However, data were not available for these analyses.
We performed the following post‐hoc subgroup analyses for OS and DFS.
Duration of OFS: fewer than three years versus three years or longer.
Age of studied population: younger than 40 years versus 40 years of age or older.
Chemotherapy use irrespective of treatment combinations (i.e. chemotherapy alone or with endocrine therapy): yes or no.
Method of OFS: surgery versus LHRH agonists versus radiation‐induced ovarian ablation.
Lymph node status: positive (defined as ≥ 50% of population with node‐positive disease) versus negative (defined as < 50% of the population with node‐positive disease).
Sensitivity analysis
We planned to perform sensitivity analysis in relation to studies that were at high risk of bias and publication status (fully published trials versus trials published in abstract form only). However, none of the included studies met these criteria; therefore sensitivity analyses were not conducted.
Results
Description of studies
Results of the search
For this review update, searching yielded 1987 records from the Specialised Register of the Cochrane Breast Cancer Group, MEDLINE, Embase, CENTRAL, WHO ICTRP, and ClinicalTrials.gov on 26 September 2019. Searching relevant review papers revealed one additional record. After removing duplicates, we screened the titles and abstracts of the 1846 remaining records and excluded 1697 of them based on information found in the abstract alone. We further assessed the full‐text articles or ongoing study records for 149 records. We excluded 86 records after full‐text review and provided reasons in the PRISMA flowchart (Figure 1). Further details for exclusion are provided for a subset of studies in the Characteristics of excluded studies table.
1.
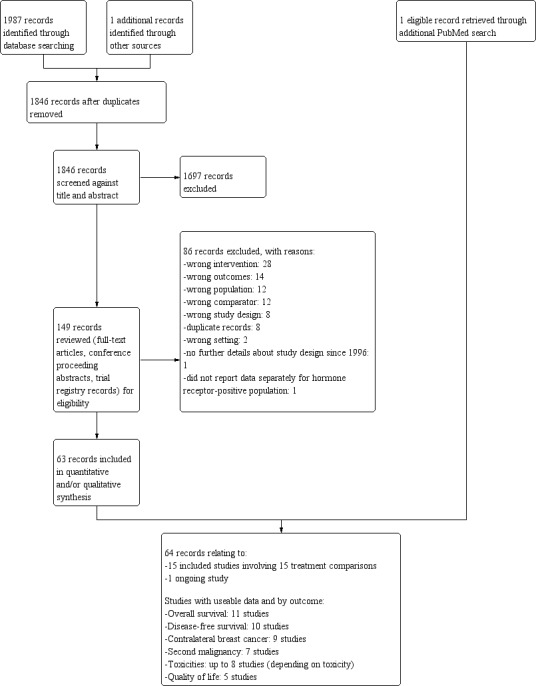
Study flow diagram: 2019 review update.
Of the 63 remaining records, 62 records related to 15 eligible studies (ABCTCG; Arriagada 2005; ASTRRA; E‐3193, INT‐0142; ECOG 5188, INT‐0101; GABG IV‐B‐93; IBCSG II; IBCSG VIII; SOFT; SWOG 1996; Uslu 2014; Yang 2013; Yi 2016; ZBCSG Trial B; ZIPP), and one record was classified as an 'ongoing' study (NCT02132390). An additional record relating to an already identified eligible study was noted in a supplementary PubMed search. In sum, upon applying eligibility criteria, we identified 15 eligible randomised trials (relating to 64 records) that addressed the role of OFS in the adjuvant treatment of premenopausal women with hormone receptor‐positive early breast cancer. One relevant ongoing trial was identified and described in the Characteristics of ongoing studies table.
We have outlined the search process in the PRISMA flowchart in Figure 1. Details of the PRISMA flowchart for the previous version of this review can be found in Goel 2009.
Included studies
See the Characteristics of included studies table.
The 15 included studies, involving 11,538 premenopausal women with hormone‐positive early breast cancers, contributed to the following treatment comparisons.
OFS versus observation: one study (ZIPP).
OFS + chemotherapy versus chemotherapy: six studies (Arriagada 2005; ECOG 5188, INT‐0101; GABG IV‐B‐93; IBCSG II; IBCSG VIII; SWOG 1996).
OFS + tamoxifen versus tamoxifen: six studies (ABCTCG; E‐3193, INT‐0142; SOFT; Yang 2013; Yi 2016; ZBCSG Trial B), although the ZIPP study would have been eligible if long‐term data had been reported separately for this comparison.
OFS + chemotherapy + tamoxifen versus chemotherapy + tamoxifen: two studies (ASTRRA; Uslu 2014).
Standard dosing of LHRH agonists was observed across studies (i.e. goserelin 3.6 mg subcutaneously, triptorelin 3.75 mg intramuscularly, leuprorelin 3.75 mg subcutaneously, every 4 weeks). When tamoxifen was used, the most common dose was 20 mg daily (ABCTCG; ASTRRA; E‐3193, INT‐0142; SOFT; Uslu 2014). One study allowed 20 mg or 40 mg daily (ZIPP), another study used tamoxifen 10 mg twice daily (Yang 2013), one study allowed tamoxifen 10 mg twice daily or 20 mg daily (ZBCSG Trial B), and another study did not specify the dosing regimen used (Yi 2016).
Ovarian function suppression versus observation
ZIPP was an international collaboration between four breast cancer research groups that adopted similar protocols with the intention of combining their results in a prospective meta‐analysis. The study used a 2 × 2 factorial design to randomise 2710 participants into four arms of goserelin and tamoxifen, goserelin, tamoxifen alone, and observation alone. Study medications were continued for two years. Elective tamoxifen was allowed in two of the four collaborative groups. Fifty‐one per cent of participants had cancers that were oestrogen‐positive, and 53% were node‐positive, and 43% of participants received (neo)adjuvant chemotherapy. Overall, 48% of participants electively received tamoxifen, which included 95% of participants in whom it was permissible to do so (Baum 2006). The median 12‐year follow‐up data from the ZIPP collaboration included complete efficacy outcomes only for the comparison of OFS or no OFS in the overall population, including both hormone receptor‐positive and hormone receptor‐negative cancers. Results that were stratified by hormone status were reported in subgroups by the receipt of tamoxifen (electively or by randomisation). For this reason, efficacy outcomes for the ZIPP collaboration have been reported under the OFS compared to observation analysis only; it is the only study that performed this comparison.
Ovarian function suppression + chemotherapy versus chemotherapy
These six studies randomised 4376 women to OFS and chemotherapy compared to chemotherapy alone (Arriagada 2005; ECOG 5188, INT‐0101; GABG IV‐B‐93; IBCSG II; IBCSG VIII; SWOG 1996). Four studies recruited participants with mostly hormone receptor‐positive cancers (100% in ECOG 5188, INT‐0101 and SWOG 1996; 90% in IBCSG VIII; 70% in Arriagada 2005), and only 33% and 40% of participants had hormone receptor‐positive cancers in IBCSG II and GABG IV‐B‐93, respectively. Most participants had node‐positive disease (100% in IBCSG II, ECOG 5188, INT‐0101, and SWOG 1996, and at least 90% in Arriagada 2005 and GABG IV‐B‐93) with the exception of one study, which recruited only participants with node‐negative disease (IBCSG VIII). Five studies mandated the type of chemotherapy provided, although the regimen varied between studies. Mandated chemotherapy regimens included cyclophosphamide, methotrexate, and fluorouracil (CMF); CMF and prednisone; CMF, vincristine, and prednisone; cyclophosphamide, doxorubicin, and fluorouracil; and epirubicin and cyclophosphamide followed by CMF. Delivery of OFS also varied. Three studies used goserelin: IBCSG VIII (18 months), GABG IV‐B‐93 (two years), and ECOG 5188, INT‐0101 (five years). Two studies used oophorectomy (IBCSG II; SWOG 1996), and one study allowed any method of OFS (oophorectomy, pelvic radiotherapy, or triptorelin for three years; Arriagada 2005).
Ovarian function suppression + tamoxifen versus tamoxifen
These six studies randomised 3504 women to receive ovarian function suppression and tamoxifen compared to tamoxifen alone (ABCTCG; E‐3193, INT‐0142; SOFT; Yang 2013; Yi 2016; ZBCSG Trial B). Five studies included only participants with hormone receptor‐positive cancers (E‐3193, INT‐0142; SOFT; Yang 2013; Yi 2016; ZBCSG Trial B), and only 39% of participants had hormone receptor‐positive cancers in ABCTCG. Node positivity varied from 0% to 61%. Chemotherapy was permitted in three studies (ABCTCG; SOFT; Yang 2013), with rates varying from 53% to 88%. Chemotherapy use was not allowed in one study (E‐3193, INT‐0142), and chemotherapy use was not specified in two studies (Yi 2016; ZBCSG Trial B). Delivery OFS varied. All studies allowed LHRH agonists to be used, although one study used triptorelin (SOFT), three studies used goserelin (Yang 2013; Yi 2016; ZBCSG Trial B), and two studies allowed either goserelin or leuprorelin (ABCTCG; E‐3193, INT‐0142). Two studies allowed other methods of OFS (oophorectomy, pelvic radiotherapy; ABCTCG; E‐3193, INT‐0142). The duration of LHRH agonists varied between 12 months (Yi 2016), 18 months (Yang 2013), 2 years (ABCTCG; ZBCSG Trial B), and 5 years (E‐3193, INT‐0142; SOFT). The duration of tamoxifen therapy varied between 12 months (Yi 2016), 2 years (ZBCSG Trial B), and 5 years (ABCTCG; E‐3193, INT‐0142; SOFT; Yang 2013).
Ovarian function suppression + chemotherapy + tamoxifen versus chemotherapy + tamoxifen
These two studies randomised 1390 women to receive OFS, chemotherapy, and tamoxifen compared to chemotherapy and tamoxifen (ASTRRA; Uslu 2014). All participants had hormone receptor‐positive cancers. All cancers were node positive in Uslu 2014, and 56% of cancers were node positive in ASTRRA. In both studies, an anthracycline‐based chemotherapy was predominantly used (Uslu 2014 100%; ASTRRA 94%), tamoxifen was continued for five years, and OFS was achieved with goserelin for two years. Uslu 2014 allowed a switch to an aromatase inhibitor if menopause occurred whilst on tamoxifen alone.
Excluded studies
We excluded 86 records from this review update and provided a list of notable excluded studies under Characteristics of excluded studies. The main reason for excluding these studies was use of an incorrect comparator where the comparator arm received additional or different treatment regimens from the intervention arm and therefore was confounded (ABCSG 5; ABCSG‐12; Baum 1996; FASG 06; GABG IV‐A‐93; Grocta 02; Li 2019; MAM 01 GOCSI; PERCHE; Ragaz 1997; Soreide 2002; TABLE; Yu 2019; ZEBRA). In addition, one study stratified participants by oophorectomy status and did not randomise to either OFS or no OFS (Manson 2019), one study did not report outcomes by hormone receptor status (Pretoria), one study used either LHRH or tamoxifen and did not report data separately for LHRH (HMFEC), and two studies were registered or published in the 1990s or early 2000s and no further details have been published since that time (Baum 1996; UKCCR).
Risk of bias in included studies
Refer to Figure 2 for a summary of risk of bias judgements for the included studies for each risk of bias domain.
2.
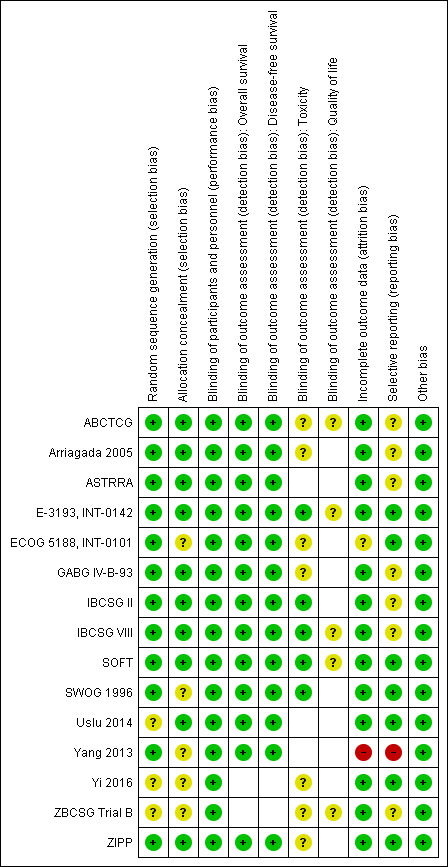
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The 15 studies were described as randomised. The method of random sequence generation was described adequately (i.e. with low risk of bias) in 12 studies (ABCTCG; Arriagada 2005; ASTRRA; E‐3193, INT‐0142; ECOG 5188, INT‐0101; GABG IV‐B‐93; IBCSG II; IBCSG VIII; SOFT; SWOG 1996; Yang 2013; ZIPP). These studies reportedly stratified randomisation or permuted block design and/or had no baseline imbalances. It was not possible to accurately assess the method of random sequence generation used in three studies owing to lack of information presented in the published article or the presence of imbalanced randomised arms (Uslu 2014; Yi 2016; ZBCSG Trial B). In particular, ZBCSG Trial B recruited 20 participants in the OFS group and 94 participants in the comparator group. These studies were classified as having unclear risk of bias.
Ten of the 15 studies were at low risk of bias for allocation concealment. These studies described central randomisation systems (internet‐based or co‐ordinating centre) (ABCTCG; Arriagada 2005; ASTRRA; E‐3193, INT‐0142; GABG IV‐B‐93; IBCSG II; IBCSG VIII; SOFT; Uslu 2014; ZIPP). Five studies did not describe methods of allocation concealment used or did not provide sufficient detail in the trial publication and were judged as having unclear risk of bias (ECOG 5188, INT‐0101; SWOG 1996; Yang 2013; Yi 2016; ZBCSG Trial B).
Blinding
All fifteen studies were described as 'open‐label' or most likely were open‐label studies, but this was not specifically mentioned in the trial publication. Performance bias was not considered to be a concern given that there was considerable equipoise at the time at which these studies were conducted such that knowing the treatment allocation was unlikely to affect the behaviour of clinicians and participants. Therefore we judged the 15 studies as having low risk of bias for this domain.
Detection bias was assessed by grouping outcomes with similar risks of bias: (1) OS, (2) DFS, (3) toxicity, and (4) quality of life. For OS and DFS, lack of blinding was perceived as unlikely to have an impact on this outcome assessment. Therefore all studies that reported these outcomes were perceived to be at low risk of bias. For outcome measures that were more likely to be influenced by lack of blinding (i.e. toxicity), we assessed whether outcome assessments were made using validated and standardised grading of symptom assessment tools and included biochemical tests. Of the 12 studies that reported toxicities, five used standardised grading symptoms, and therefore knowing the treatment allocation may have had an immaterial effect on the grading of symptoms by clinicians or participants. These five studies were rated as having low risk of bias on this domain (E‐3193, INT‐0142; IBCSG II; IBCSG VIII; SOFT; SWOG 1996). For the other seven studies, toxicities were self‐reported with no standardised tools; therefore reporting of this outcome may have been affected by lack of blinding. These seven studies were rated as having unclear risk of bias (ABCTCG; Arriagada 2005; ECOG 5188, INT‐0101; GABG IV‐B‐93; Yi 2016; ZBCSG Trial B; ZIPP). Quality of life measures were likely to be affected by lack of blinding to treatment. Five studies planned to collect and report quality of life (QoL) data using validated questionnaires (ABCTCG; E‐3193, INT‐0142; IBCSG VIII; SOFT; ZBCSG Trial B). Quality of life questionnaires were completed by participants who were unblinded to the treatment allocation; therefore it is uncertain whether this introduced risk of bias. We judged these five studies as having unclear risk of bias for this domain.
Incomplete outcome data
Thirteen studies described intention‐to‐treat analysis and minimal patient loss to follow‐up that was accounted for; therefore we judged them to be at low risk of bias: ABCTCG; Arriagada 2005; ASTRRA; E‐3193, INT‐0142; GABG IV‐B‐93; IBCSG II; IBCSG VIII; SOFT; SWOG 1996; Uslu 2014; Yi 2016; ZBCSG Trial B; ZIPP. One study was judged as having unclear risk of bias due to reporting that the data were analysed as intention‐to‐treat; however 34 participants were excluded from the analysis, and the division of excluded participants across treatment allocations was not provided in the trial publication (ECOG 5188, INT‐0101). The trial publication did state that results were similar between the modified intention‐to‐treat analysis and the full intention‐to‐treat analysis but did not report details. One study was judged as having high risk of bias because study authors did not analyse data by intention‐to‐treat and stated that 19 participants dropped out but provided no reasons or mention of the split across treatment groups (Yang 2013).
Selective reporting
Seven studies reported results for outcomes listed in the methods section of the trial publication (E‐3193, INT‐0142; ECOG 5188, INT‐0101; SOFT; Uslu 2014; Yi 2016; ZIPP) or provided a trial registration record with listed outcomes found in the methods and results sections of the trial publication (SOFT). In seven studies, it was assessed that there was partial or no reporting of toxicity outcomes when it was very likely that these outcomes were collected (ABCTCG; Arriagada 2005; ASTRRA; GABG IV‐B‐93; IBCSG II; ZBCSG Trial B), or that only partial numerical data were provided (IBCSG VIII). In the case of the ASTRRA study, previous publications had reported that data relating to tolerability of the medicines would be assessed. Therefore these seven studies were ranked as having unclear risk of bias. Yang 2013 was judged as having high risk of bias for this domain because not all outcomes measured (i.e. toxicities) were reported in the trial publication, and new outcomes were re‐assigned as primary outcomes in the final trial publication but were not reported in previous trial publications nor in the clinical trial registry record.
Other potential sources of bias
No other sources of bias were evident in 15 studies (ABCTCG; Arriagada 2005; ASTRRA; E‐3193, INT‐0142; ECOG 5188, INT‐0101; GABG IV‐B‐93; IBCSG II; IBCSG VIII; SOFT; SWOG 1996; Uslu 2014; Yang 2013; Yi 2016; ZBCSG Trial B; ZIPP).
Effects of interventions
See: Table 1
Refer to Table 1.
Overall survival
Thirteen of 15 studies collected overall survival data (all except Yi 2016 and ZBCSG Trial B); however, two studies did not provide sufficient information for analysis (IBCSG II; Uslu 2014). With median follow‐up across studies ranging from 5.3 to 12.1 years, the remaining 11 studies showed that adding OFS to treatment resulted in a reduction in mortality (HR 0.86, 95% CI 0.78 to 0.94; high‐certainty evidence; Figure 3). A total of 10,374 women were included in the OS analysis, with an estimated 1933 deaths reported from nine of the eleven studies (neither ABCTCG nor GABG IV‐B‐93 provided the number of events in each treatment group). A funnel plot and Egger's test did not support any publication bias for the studies reviewed (Figure 4; Egger's test: P = 0.12).
3.
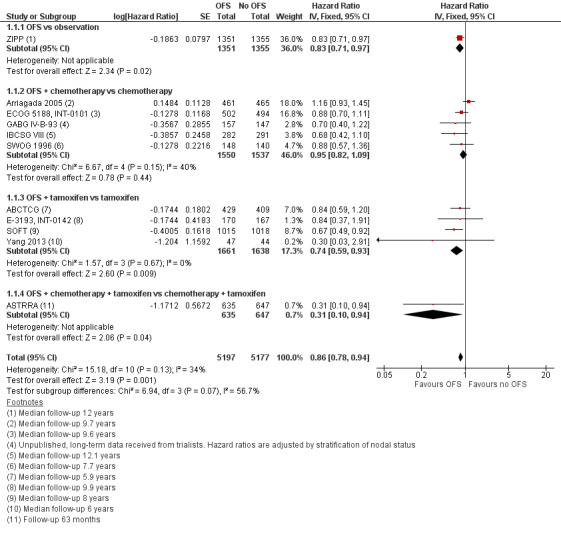
Forest plot of comparison: 1 OFS versus no OFS, outcome: 1.1 Overall survival.
4.
Funnel plot of comparison: 1 OFS versus no OFS, outcome: 1.1 Overall survival.
This treatment effect was present when OFS was added to observation (HR 0.83, 95% CI 0.71 to 0.97; 1 study; 2706 women), to tamoxifen (HR 0.74, 95% CI 0.59 to 0.93; 4 studies; 3299 women), or to chemotherapy and tamoxifen (HR 0.31, 95% CI 0.10 to 0.94; 1 study; 1282 women). The effect was reduced when ovarian function suppression was added to chemotherapy only (HR 0.95, 95% CI 0.82 to 1.09; 5 studies; 3087 women). Refer to Analysis 1.1.
1.1. Analysis.
Comparison 1 OFS versus no OFS, Outcome 1 Overall survival.
Subgroup analysis
Duration of OFS
The addition of OFS did not result in reduced mortality when OFS was used for three years or longer (HR 0.93, 95% CI 0.81 to 1.07; 5 studies; 4580 participants); however the studies contributing to this result had provided follow‐up for a period ranging from 7.7 to 9.89 years. The effect of OFS during adjuvant treatment resulted in a reduction in mortality when OFS was used for less than three years but for longer than one year (HR 0.79, 85% CI 0.69 to 0.91; 5 studies; 4956 women). For these studies, women had been monitored for a longer time period, with most studies providing a median follow‐up period of 12 years.
Age of studied population
Two studies reported overall survival by age and were underpowered (women younger than 40 years of age: HR 0.73, 95% CI 0.51 to 1.04; 394 women; women who were 40 years of age or older: HR 0.89, 95% CI 0.69 to 1.14; 1175 women) (ECOG 5188, INT‐0101; IBCSG VIII). Refer to Analysis 3.1
3.1. Analysis.
Comparison 3 Age of studied population: < 40 years vs ≥ 40 years, Outcome 1 Overall survival.
Chemotherapy use irrespective of treatment combinations
Two studies in which chemotherapy was not mandatory reported overall survival by receipt of chemotherapy (SOFT; ZIPP). In all studies that used chemotherapy, the addition of OFS resulted in a reduction in mortality (HR 0.86, 95% CI 0.76 to 0.97; 8 studies; 5453 women), and in those studies in which women did not receive chemotherapy, the addition of OFS did not reduce mortality (HR 0.89, 95% CI 0.62 to 1.28; 3 studies; 1286 women). Refer to Analysis 4.1.
4.1. Analysis.
Comparison 4 Chemotherapy use: yes or no, Outcome 1 Overall survival.
Method of OFS
Reduction in mortality with the addition of OFS was observed when LHRH agonists were used for OFS (HR 0.80, 95% CI 0.71 to 0.89; 8 studies; 8101 women). No mortality benefit was noted when OFS was provided via surgery (HR 0.86, 95% CI 0.57 to 1.28, 2 studies; 415 women) or radiotherapy (HR 1.75, 95% CI 0.50 to 6.16; 1 study; 77 women). Refer to Analysis 5.1.
5.1. Analysis.
Comparison 5 Method of OFS, Outcome 1 Overall survival.
Lymph node status
Reduction in mortality was observed with the addition of OFS in lymph node‐positive cancers (HR 0.89, 95% CI 0.81 to 0.99; 7 studies; 7340 women) and in lymph node‐negative cancers (HR 0.69, 95% CI 0.53 to 0.88; 3 studies; 2943 women). Refer to Analysis 6.1.
6.1. Analysis.
Comparison 6 Lymph node status: positive or negative, Outcome 1 Overall survival.
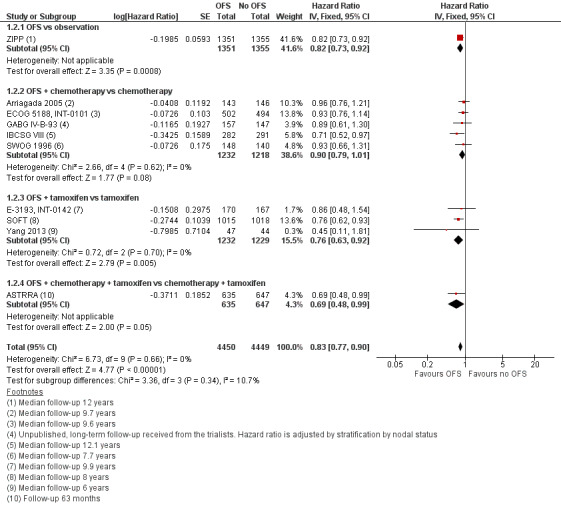
Disease‐free survival
Thirteen of the 15 studies collected DFS data (all except Yi 2016 and ZBCSG Trial B); however, three studies did not provide sufficient information for analysis (ABCTCG; IBCSG II; Uslu 2014). IBCSG II did not report outcomes by hormonal status in the follow‐up publication. Median follow‐up ranged from 4 to 12.1 years. The addition of OFS resulted in improvement in DFS (HR 0.83, 95% CI 0.77 to 0.90; 10 studies; high‐certainty evidence; Figure 5). A total of 8899 women were included in the DFS analysis, with an estimated 2757 DFS events reported from nine studies (Yang 2013 did not provide the number of events in each treatment group). A funnel plot did not support any publication bias for the studies reviewed (Figure 6; Egger's test: P = 0.6285).
5.
Forest plot of comparison: 1 OFS versus no OFS, outcome: 1.2 Disease‐free survival.
6.
Funnel plot of comparison: 1 OFS versus no OFS, outcome: 1.2 Disease‐free survival.
The effect persisted when OFS was added to observation (HR 0.82, 95% CI 0.73 to 0.93; 1 study; 2706 women), to tamoxifen (HR 0.76, 95% CI 0.76 to 0.92; 3 studies; 2461 women), and to chemotherapy and tamoxifen (HR 0.69, 95% CI 0.48 to 0.99; 1 study; 1282 women). The effect was reduced when OFS was added to chemotherapy only (HR 0.90, 95% CI 0.79 to 1.01; 5 studies; 2450 women). Refer to Analysis 1.2
1.2. Analysis.
Comparison 1 OFS versus no OFS, Outcome 2 Disease‐free survival.
Subgroup analysis
Duration of OFS
The addition of OFS improved DFS among participants regardless of whether OFS was continued for three years or longer (HR 0.88, 95% CI 0.78 to 0.98; 5 studies; 3943 women) or for less than three years (HR 0.80, 95% CI 0.72 to 0.88; 5 studies; 4956 women; Analysis 2.2). In the three studies that reported DFS by age, a large improvement in disease‐free survival was seen with the addition of OFS in women younger than 40 years of age (HR 0.65, 95% CI 0.50 to 0.83; 3 studies; 1764 women), and no difference was seen among women 40 years of age or older (HR 0.95, 95% CI 0.78 to 1.15; 3 studies; 1504 women). Refer to Analysis 3.2.
2.2. Analysis.
Comparison 2 Duration of OFS: < 3 years vs ≥ 3 years, Outcome 2 Disease‐free survival.
3.2. Analysis.
Comparison 3 Age of studied population: < 40 years vs ≥ 40 years, Outcome 2 Disease‐free survival.
Chemotherapy use irrespective of treatment combinations
In studies in which chemotherapy was not mandatory, two studies reported DFS by receipt of chemotherapy (SOFTZIPP). In all studies that used chemotherapy, the addition of OFS resulted in improvement in disease‐free survival among women who received chemotherapy (HR 0.86; 95% CI 0.76 to 0.97; 8 studies; 5453 women) but not among women who did not receive chemotherapy (HR 0.87, 95% CI 0.62 to 1.28; 3 studies). Refer to Analysis 4.2.
4.2. Analysis.
Comparison 4 Chemotherapy use: yes or no, Outcome 2 Disease‐free survival.
Method of OFS
Improvement in DFS was seen with the addition of OFS when LHRH agonists were used as the OFS method (HR 0.81, 95% CI 0.75 to 0.88; 8 studies; 8101 women), but not when the OFS method was surgery (HR 0.96, 95% CI 0.70 to 1.30; 2 studies; 415 women) or radiotherapy (HR 0.94, 95% CI 0.28 to 3.13; 1 study; 77 women), although the certainty of evidence for this is considered to be low due to small sample size and wide confidence intervals. Refer to Analysis 5.2.
5.2. Analysis.
Comparison 5 Method of OFS, Outcome 2 Disease‐free survival.
Lymph node status
The addition of OFS resulted in improvement in DFS in lymph node‐positive cancers (HR 0.86, 95% CI 0.79 to 0.93; 6 studies; 5865 women) and in lymph node‐negative cancers (HR 0.75, 95% CI 0.64 to 0.89; 2943 women). Refer to Analysis 6.2.
6.2. Analysis.
Comparison 6 Lymph node status: positive or negative, Outcome 2 Disease‐free survival.
Contralateral breast cancer
Nine of 15 studies reported outcomes of contralateral breast cancer (Arriagada 2005; ASTRRA; ECOG 5188, INT‐0101; GABG IV‐B‐93; IBCSG VIII; SOFT; SWOG 1996; Uslu 2014; ZIPP). The addition of OFS likely reduces the risk of contralateral breast cancer (HR 0.75, 95% CI 0.57 to 0.97; moderate‐certainty evidence; Analysis 1.3). A total of 9138 women were included in this analysis, with an estimated 196 contralateral breast cancers reported during the follow‐up period (range 4 to 10 years). Data were insufficient to meaningfully report on effects of OFS on contralateral breast cancer outcomes by duration of OFS, age, type of OFS, receipt of chemotherapy, or lymph node status.
1.3. Analysis.
Comparison 1 OFS versus no OFS, Outcome 3 Contralateral breast cancer.
Second malignancy
Seven of 15 studies reported outcomes of second malignancy (Arriagada 2005; ASTRRA; ECOG 5188, INT‐0101; GABG IV‐B‐93; IBCSG VIII; SOFT; SWOG 1996). The addition of OFS likely does not reduce the risk of second malignancy (HR 0.89, 95% CI 0.64 to 1.25; moderate‐certainty evidence; 6327 women). Data were insufficient to meaningfully report on effects of OFS on contralateral breast cancer outcomes by duration of OFS, age, type of OFS, receipt of chemotherapy, or lymph node status.
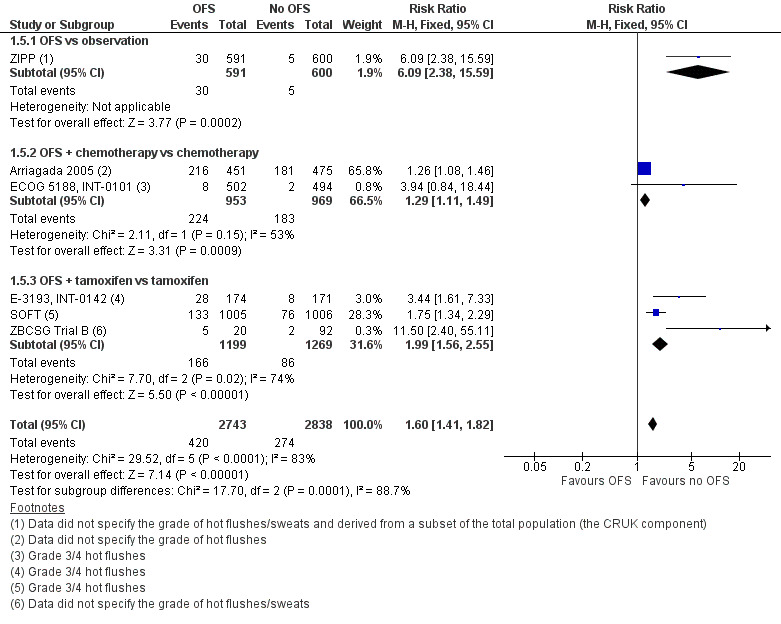
Hot flushes
Eight of 15 studies reported on hot flushes/sweats, with six of these studies contributing to a pooled analysis. Evidence suggests that the addition of OFS slightly increases the incidence of hot flushes (grade 3/4 or any grade; risk ratio (RR) 1.60, 95% CI 1.41 to 1.82; low‐certainty evidence; Analysis 1.5; Figure 7). Two additional studies reported a higher number of hot flushes in the OFS group compared to the not OFS group (ABCTCG; IBCSG VIII). Refer to Table 2.
1.5. Analysis.
Comparison 1 OFS versus no OFS, Outcome 5 Hot flushes.
7.
Forest plot of comparison: 1 OFS versus no OFS, outcome: 1.5 Hot flushes.
1. Toxicity: hot flushes, bone density, arthralgias, and mood.
| Study | Hot flushes/menopausal symptoms# | Bone health: bone density/fractures | Arthralgias (joint pain) | Mood (anxiety, depression, other as indicated in footnotes) | ||||
| OFS (n/N) | Comparator (n/N) | OFS (n/N) | Comparator (n/N) | OFS (n/N) | Comparator (n/N) | OFS (n/N) | Comparator (n/N) | |
| OFS vs observation | ||||||||
| ZIPP* | Reported as: Sweating: 30a/591 Vasodilation: 235a/591 | Reported as: Sweating: 5a/600 Vasodilation: 78a/600 | NR | NR | 17/591 | 6/600 | 34/591 | 11/600 Reported as anxiety/depression/irritability |
| OFS + tamoxifen vs tamoxifen | ||||||||
| ABCTCG | Participants in the OFS group (n = 118) experienced more menopausal symptoms (night sweats, P = 0.005) and day sweats (P < 0.001) than those in the no OFS group (n = 128) | NR | NR | NR | NR | Participants in the OFS group had increased depression (P = 0.05) and anxiety (P = 0.04) over 30 months compared to those in the no OFS group (total number of participants = 436, data not reported separately by treatment group) | ||
| E‐3193, INT‐0142 | 28/174 | 8/171 Difference between groups was observed at 1, 2, and 3 years | NR | NR | NR | NR | 4b/174 | 4b/171 |
| SOFT | 133/1005 | 76/1006 | 3/1005 | 1/1006 Grade 3/4 osteoporosis | 55/1005 | 63/1006 Grade 3/4 musculoskeletal symptoms | 44/1005 | 38/1006 Grade 3/4 depression |
| Yang 2013 | NR | NR | NR | NR | NR | NR | NR | NR |
| Yi 2016 | NR | NR | NR | NR | NR | NR | (a) No differences were observed in HAM‐D, HAM‐A, MDQ, HCL‐32, ASI at baseline between groups, and (b) no significant time, group, or time × group differences were observed in HAM‐D and BDI score, or in HAM‐A score, between treatment groups Moderate to severe anxiety was noted in 41.9% (13/32) in the OFS group and 44.8% (14/32) in the tamoxifen group. Both treatment groups had an increase in anxiety levels over time, but there were no significant differences between treatment groups |
|
| ZBCSG Trial B | 5a/20 | 2a/92 | NR | NR | NR | NR | NR | NR |
| OFS + chemotherapy vs chemotherapy | ||||||||
| Arriagada 2005 | 216a/451^ | 181a/475^ | NR | NR | NR | NR | NR | NR |
| ECOG 5188, INT‐0101 | 8/502^ | 2/494^ | NR | NR | NR | NR | 14c/502 | 6c/494 |
| GABG IV‐B‐93 | NR | NR | NR | NR | NR | NR | 3d/160 | 0d/151 |
| IBCSG II | NR | NR | NR | NR | NR | NR | NR | NR |
| IBCSG VIII | The median hot flushes score appeared to be worse in the OFS group (goserelin plus chemotherapy) compared to the chemotherapy alone group from approximately 7 months onwards (Figure 1Bernhard 2007). The median hot flushes score seemed to improve from 9 months; however the OFS group still had slightly worse scores than the chemotherapy alone group up until around 30 months (no statistical analyses of treatment comparisons provided in the paper) | NR | NR | NR | NR | NR | NR | |
| SWOG 1996 | NR | NR | NR | NR | NR | NR | NR | NR |
| OFS + chemotherapy + tamoxifen vs chemotherapy + tamoxifen | ||||||||
| ASTRRA | NR | NR | NR | NR | NR | NR | NR | NR |
| Uslu 2014 | NR | NR | NR | NR | NR | NR | NR | NR |
n: number of events; N: number of women studied in each group; NR: not reported.
#Grade 3 or 4 toxicities unless otherwise stated. *Toxicity data reported on a subset of women in the study (i.e. those from the CRUK enrolment phase). ^Denominator is the randomised number rather than the number of participants who received allocated treatment and assessed while on treatment. aThis study did not report whether toxicity was assessed using a standardised tool and did not specify the grade of the hot flushes or sweats. ZIPP did not report the grade of vasodilation. bGrade 3 or 4 neuropsychiatric adverse effects on the NCI CTC; these included outcomes such as anxiety, depression, somnolence, and confusion. cGrade 3 or 4 neuropsychiatric adverse effects but type of event not specified. dReported as neuropsychiatric disorders but no further details of how assessed or on severity of the disorders.
In relation to each treatment comparison, ZIPP reported sweating separately for each treatment group as 0% (observation), 1% (tamoxifen), 5% (goserelin), and 5% (goserelin and tamoxifen; Table 2 presents the combined data). ZIPP also reported the incidence of “vasodilation”, which was reported as 0% (observation), 17% (tamoxifen alone), 26% (goserelin alone), and 44% (goserelin and tamoxifen).
Two studies compared OFS and chemotherapy to chemotherapy alone, with any‐grade hot flushes reported in the OFS arm as 48% ‐ Arriagada 2005 ‐ and 79.5% ‐ ECOG 5188, INT‐0101 ‐ and in the control arm as 38% ‐ Arriagada 2005 ‐ and 59.4% ‐ ECOG 5188, INT‐0101. Grade 3 hot flushes were reported in 1.6% in the OFS arm and in 0.4% in the control arm (ECOG 5188, INT‐0101; Table 2).
Four studies reported outcome data for the comparison of OFS and tamoxifen to tamoxifen alone (ABCTCG; E‐3193, INT‐0142; SOFT; ZBCSG Trial B). Two studies reported grade 3 hot flushes in the OFS arm as 13.2% ‐ SOFT ‐ and 16.1% ‐ E‐3193, INT‐0142 ‐ and in the control arm as 7.6% ‐ SOFT ‐ and 4.7% ‐ E‐3193, INT‐0142. One of these studies also reported any‐grade hot flushes as 93.4% in the OFS arm and 79.8% in the control arm (SOFT). ABCTCG reported a higher incidence of night sweats and day sweats in the OFS arm compared to the control arm. Any‐grade sweating was reported in the SOFT study in the OFS arm as 61.8% and in the control arm as 48.3%. No grade 3 or higher events of sweating were reported. ZBCSG Trial B did not state the grade nor any other details for assessing hot flushes; translated information indicated that 25% in the OFS arm and 2.2% in the control arm experienced hot flushes.
Mood
Seven of the 15 studies reported outcomes related to mood, although they were reported in different ways that did not permit a meta‐analysis (refer to Table 2). Two studies reported the incidence of (neuro)psychiatric symptoms. Neuropsychiatric symptoms of grade 3 or higher were reported in the OFS arm as 2.8% ‐ ECOG 5188, INT‐0101 ‐ and 2.3% ‐ E‐3193, INT‐0142 ‐ and in the control arm as 1.2% ‐ ECOG 5188, INT‐0101 ‐ and 2.3% ‐ E‐3193, INT‐0142. ECOG 5188, INT‐0101 also reported any‐grade neuropsychiatric symptoms (including anxiety and depression) in the OFS arm of 32.2% and in the control arm of 16.4%. Psychiatric symptoms of any grade were reported in the OFS arm as 1.9% and in the control arm as 0% (GABG IV‐B‐93). ZIPP reported anxiety, depression, and irritability as a combined measure, with incidence reported as 0% (observation), 2% (tamoxifen), 6% (goserelin), and 6% (goserelin and tamoxifen; see Table 2 for combined data). SOFT reported grade 3 or higher depression in the OFS arm as 4.4% and in the control arm as 3.8%, and the incidence of any‐grade depression in the OFS arm as 51.9% and in the control arm as 46.6%. Yi 2016 reported the incidence of moderate to severe anxiety as 41.9% in the OFS arm and 44.8% in the control arm. ABCTCG reported the OFS arm had a greater incidence of depression and anxiety. See Table 2.
Bone health
Bone health outcomes were reported in one study (SOFT). At a median follow up of 5.6 years, osteoporosis (based on T score < ‐2.5) was reported in the OFS arm as 5.8% and in the control arm as 3.5%. Osteoporosis appears to be worse in the OFS arm than in the control arm (RR 1.16, 95% CI 1.10 to 2.50; 2011 participants; 1 study; low‐certainty evidence). Grade 3 osteoporosis was reported in three participants in the OFS arm and in one participant in the control arm with certainty of evidence considered to be low due to the small number of events and the wide confidence intervals (RR 3.00, 95% CI 0.31 to 28.82; 1 study; 2011 women; Table 2). Any‐grade osteoporosis was reported in the OFS arm as 20%, and in the control arm as 12.3%. In addition, fractures (defined as 'any event') were reported in the OFS arm as 5.4% (54/1005) and 4.9% (49/100 6) at a median follow‐up of 5.6 years (SOFT).
Arthralgia (joint pain)
Arthralgia was specifically reported in ZIPP. Any‐grade arthralgia was reported in 0% (observation), 1% (tamoxifen only), 5% (goserelin only), and 2% (goserelin and tamoxifen; see Table 2 for combined data). SOFT reported on the incidence of musculoskeletal symptoms as any‐grade symptoms in the OFS arm of 75.1% and in the control arm 69.0%, and as grade 3 or higher in the OFS arm of 5.5% and in the control arm 6.3%.
Sexual function
Four of the 15 studies reported outcomes related to sexual function. Any‐grade vaginal dryness was reported in SOFT in the OFS arm as 49.8% and in the control arm as 41.8%, with no grade 3 or higher events. Vaginal dryness was reported in E‐3193, INT‐0142 as grade 3 or higher toxicity in 0.6% (1 of 174 women) of the OFS arm, and no events were reported in the control arm. This was reported descriptively in ZIPP as greatest in the goserelin arm over time compared to each other arm, with the tamoxifen only arm and the goserelin and tamoxifen arm reporting more vaginal dryness than the observation arm. Any‐grade decreases in libido were reported in SOFT in the OFS arm as 47.5% and in the control arm as 42.4%. In ABCTCG, the OFS arm was reported to have more vaginal dryness than the control arm, although there were no differences in sexual function. No numerical data were provided in ABCTCG. See Table 3.
2. Toxicity: sexual function, cardiovascular symptoms, cognitive function, and treatment‐related death.
| Study | Sexual function/vaginal dryness | Cardiovascular risk, DVT/PE | Cognitive function | Treatment‐related death | Other toxicities | ||||
| OFS (n/N) | Comparator (n/N) | OFS (n/N) | Comparator (n/N) | OFS (n/N) | Comparator (n/N) | OFS (n/N) | Comparator (n/N) | ||
| OFS vs observation | |||||||||
| ZIPP | NR | NR | NR | NR | NR | NR | NR | NR | Weight gain: 55/591 in OFS group, 32/600 in no OFS group |
| OFS + tamoxifen vs tamoxifen | |||||||||
| ABCTCG | Participants in the OFS group (n = 118) experienced more vaginal dryness (P < 0.001) than those in the no OFS group (n = 128) | NR | NR | NR | NR | 0/1063 | 2/1081 | ||
| E‐3193, INT‐0142 | 1a/174 | 0a/170 | NR | NR | NR | NR | 0/174 | 0/171 | |
| SOFT | Decreased libido (any event): 477/1005 Vaginal dryness (any event): 500/1005 | Decreased libido (any event): 427/1006 Vaginal dryness (any event); 421/1006 |
Glucose intolerance (Grade 3/4): 14/1005 Hypertension (Grade 3/4): 75/1005 | Glucose intolerance (Grade 3/4): 3/1006 Hypertension (Grade 3/4): 54/1006 | NR | NR | NR | NR | Supplementary data in the 2015 trial publication provide incidence of events for a number of toxicities including insomnia, fatigue, and nausea p.27 |
| Yang 2013 | NR | NR | NR | NR | NR | NR | NR | NR | In the protocol publication where baseline characteristics were reported, it was reported that "serious adverse events were not observed during the period of intervention or follow‐up" p.585 |
| Yi 2016 | NR | NR | NR | NR | NR | NR | NR | NR | |
| ZBCSG Trial B | NR | NR | NR | NR | NR | NR | NR | NR | Weight gain noted in 2/20 in the OFS group, 5/92 in the no OFS group |
| OFS + chemotherapy vs chemotherapy | |||||||||
| Arriagada 2005 | NR | NR | NR | NR | NR | NR | NR | NR | "No severe adverse effects were documented" p.395. Body weight was recorded with no difference between OFS and no OFS treatment groups |
| ECOG 5188, INT‐0101 | NR | NR | 14/502 | 16/494 Reported as Grade 3/4 diabetes | NR | NR | 1/502 lethal event (cardiomyopathy) during maintenance phase | 6/494 lethal adverse events were recorded during chemotherapy (4 events ‐ 2 sepsis, 1 myocardial infarction, and 1 cardiomyopathy and pneumonia) and in maintenance phase (2 events ‐ suicide, unspecified pulmonary disease) |
An increase in weight and hypertension was noted in the OFS group |
| GABG IV‐B‐93 | NR | NR | NR | NR | NR | NR | 0/160 | 0/151 | Leukopenia: 1/160 (OFS), 5/151 (no OFS); emesis/nausea: 1/160 (OFS), 2/151 (no OFS); paravasation: 1/160 (OFS), 0/151 (no OFS). In the OFS group, 2 participants each had the following: wound healing and erysipelas; in the no OFS group, there were 2 participants each with seroma and abscess. One participant in each group had the following: infection, wound pain, endometrial hyperproliferation,, mastopathy, thrombophlebitis, hyponatraemia, stomatitis, vertigo, infection, and fever |
| IBCSG II | NR | NR | NR | NR | NR | NR | NR | NR | |
| IBCSG VIII | NR | NR | NR | NR | NR | NR | 0/357^ | 0/360^ | In the OFS group: 1/360 life‐threatening (suicidal) depression reported after 6 months of chemotherapy and 4 goserelin implants. Alopecia reported but only in those participants who had chemotherapy; information was not presented separately for each group. Weight gain was mentioned only in the OFS group and further details were provided in the trial publication |
| SWOG 1996 | NR | NR | NR | NR | NR | NR | 0/148^ | 0/140^ | Leukopenia: 27/148 (OFS), 22/140 (no OFS); neuropathy: 4/148 (OFS), 9/140 (no OFS); mucositis: 20/148 (OFS), 19/148 (no OFS); fatigue: 29/148 (OFS), 29/140 (no OFS) |
| OFS + chemotherapy + tamoxifen vs chemotherapy + tamoxifen | |||||||||
| ASTRRA | NR | NR | NR | NR | NR | NR | NR | NR | |
| Uslu 2014 | NR | NR | NR | NR | NR | NR | NR | NR | |
n: number of events; N: number of women studied in each group; NR: not reported.
aGrade 3 or 4 vaginal dryness reported using the NCI CTC. ^Denominator is the randomised number rather than the number of participants who received the allocated treatment and assessed while on treatment.
Cardiovascular risk/DVT/PE
Two of the 15 studies reported outcomes related to cardiovascular conditions. Any‐grade glucose intolerance was reported in the OFS arm as 44.2% ‐ ECOG 5188, INT‐0101 ‐ and 3.5% ‐ SOFT ‐ and in the control arm as 36.9% ‐ ECOG 5188, INT‐0101 ‐ and 1.8% ‐ SOFT. Grade 3 or higher glucose intolerance was reported in the OFS arm as 2.8% ‐ ECOG 5188, INT‐0101 ‐ and 1.4% ‐ SOFT ‐ and in the control arm as 3.2% ‐ ECOG 5188, INT‐0101 ‐ and 0.3% ‐ SOFT. Any‐grade hypertension was reported in the OFS arm as 22.2% and in the control arm as 17.2% (SOFT). Grade 3 or higher hypertension was reported in the OFS arm as 7.5% and in the control arm as 5.4% (SOFT). Thrombosis or embolism of any grade was reported in the OFS arm as 2.3% and in the control arm as 2.2% (SOFT). See Table 3.
Cognitive function
Two of 15 studies reported outcomes related to cognitive function. One study within the ZIPP collaboration reported that memory and concentration problems were not affected by either treatment. SOFT reported no difference in the objective measurement of cognitive function between arms, although a decline in self‐reported cognitive function was noted in the OFS arm compared to the control arm. See Table 3.
Treatment‐related death
Six of 15 studies reported outcomes for treatment‐related deaths (ABCTCG; ECOG 5188, INT‐0101; E‐3193, INT‐0142; GABG IV‐B‐93; IBCSG VIII; SWOG 1996). ABCTCG reported two deaths due to chemotherapy toxicity and no treatment‐related deaths in the OFS treatment group. ECOG 5188, INT‐0101 reported four lethal adverse events during chemotherapy from sepsis (two participants), myocardial infarction (one participant), and cardiomyopathy and pneumonitis (one participant). Four lethal adverse events were reported during the maintenance phase ‐ one in the OFS arm (cardiomyopathy) and two in the control arm (suicide and unspecified pulmonary disease, respectively). E‐3193, INT‐0142, GABG IV‐B‐93, IBCSG VIII, and SWOG 1996 reported no treatment‐related deaths. See Table 3.
Other toxicities
Seven of the 15 studies also reported other toxicities not otherwise mentioned above (see Table 3). GABG IV‐B‐93, IBCSG II, and SWOG 1996 reported on toxicities that appeared to be chemotherapy related, including myelosuppression, gastrointestinal symptoms, peripheral neuropathy, rash, and lung problems. ZIPP reported weight gain as 0% (observation), 7% (tamoxifen), 4% (goserelin), and 11% (goserelin and tamoxifen). Goserelin and goserelin and tamoxifen arms had a higher incidence of problems with body image than the tamoxifen alone arm. There was no difference in sleep or fatigue between arms. ZBCSG Trial B reported weight gain of 10% in the OFS group and 5.4% in the control group, and so too did IBCSG VIII; Arriagada 2005 recorded no differences between treatment groups in relation to weight gain. ECOG 5188, INT‐0101 reported an increase in hypertension and weight in the OFS group. In SOFT, there were higher rates of any‐grade insomnia in the OFS arm (57.2%) than in the control arm (46.3%) and of grade 3 or higher events of insomnia (4.6% versus 2.9%, respectively).
Quality of life
Four out of 15 studies collected data on quality of life using validated tools (ABCTCG; E‐3193, INT‐0142; IBCSG VIII; SOFT), and one study collected quality of life‐type information without describing a validated tool (ZBCSG Trial B). Two studies reported worse quality of life indicators (i.e. vaginal dryness, day and night sweats) in the OFS group than in the no OFS group (ABCTCG; E‐3193, INT‐0142). The other two studies indicated worsening of symptoms (e.g. vasomotor, gynaecological, vaginal dryness, decline in sexual interest, bone and joint pain, and weight gain); however these side effects were reported in both OFS and no OFS groups (IBCSG VIII; SOFT). The study that did not use a validated quality of life tool described no considerable differences between groups (ZBCSG Trial B).
Compliance with treatment
In this review, compliance with treatment referred to participants who received their allocated treatment and did not stop treatment early due to toxicities. Six of 15 studies reported the number of women who received LHRH agonists and discontinued due to toxicity (Arriagada 2005; E‐3193, INT‐0142; ECOG 5188, INT‐0101; GABG IV‐B‐93; IBCSG VIII; SOFT). Over 78% of women completed the intended course of OFS in these studies: Arriagada 2005: 85% (151/177), E‐3193, INT‐0142: 90% (153/170), GABG IV‐B‐93: 92.5% (296/320), ECOG 5188, INT‐0101: 84.3% (348/413), IBCSG VIII: 93% (332/357), and SOFT: 78% (792/1015). Two of the six studies provided hormonal treatment in the control arm, with treatment completion rates of 93.8% (155/167) ‐ E‐3193, INT‐0142 ‐and 77.5% (789/1018) ‐ SOFT.
Discussion
Summary of main results
In premenopausal women with hormone‐positive early breast cancer, the addition of ovarian function suppression (OFS) resulted in a modest improvement in overall survival and disease‐free survival, as well as an overall reduction in contralateral breast cancers. This benefit seen in overall survival and disease‐free survival persisted among participants who were randomised to OFS, OFS and endocrine therapy, and OFS combined with chemotherapy and endocrine therapy. Chemotherapy regimens included cyclophosphamide, methotrexate, and fluorouracil (CMF) and anthracycline regimens, and endocrine therapy was tamoxifen in the included studies. No studies tested OFS plus aromatase inhibitors versus no OFS plus aromatase inhibitors as expected in women who are premenopausal. In some subgroups, the addition of OFS resulted in improvement in disease‐free survival without improvement in overall survival. These included participants who were younger than 40 years of age, participants who had less than three years of OFS, and participants who did not receive chemotherapy at all. The results may be due to smaller numbers in these subgroups, especially as not all studies stratified their results by these factors, and because duration of follow‐up was shorter in these groups.
Using OFS probably resulted in a higher incidence of any‐grade and severe hot flushes, and may increase the risk of osteoporosis, although longer follow‐up may be required to assess bone health. For most studies, there were minimal differences in arthralgia, mood, sexual function, cardiovascular outcomes, and cognitive function, although few studies reported toxicity data well. The addition of OFS did not appear to increase treatment‐related deaths nor second malignancy.
Overall completeness and applicability of evidence
Generalisability of these results may be affected by the use of outdated chemotherapy regimens, as some included studies were old, and women with hormone‐positive and/or HER2‐positive cancers may not have received any form of hormonal manipulation or HER2‐targeted therapy as part of the standard of care. Among studies that reported the chemotherapy regimen used, it was estimated that 72% (3091/4296) of women received an anthracycline; in one case, anthracyclines were used solely for women at high risk (with four to nine lymph nodes involved). Inclusion of the SOFT study as a more recent study where HER2 receptor was assessed does support the addition of OFS in the context of modern treatment paradigms.
Most studies reported on overall survival and disease‐free survival outcomes. Most studies did not report the incidence of contralateral breast cancer or second malignancy. Most studies did not report on toxicity outcomes. There was variation in which side effects were collected and how they were reported. Toxicities were reported as the incidence of any‐grade toxicity, the incidence of high‐grade toxicity, or a description of toxicities without a numerical comparison provided. The intensity of side effects over time was not captured. Notably, only one study reported bone health outcomes, which were defined by bone mineral density thresholds. Although the incidence of fractures was also reported, rates of bisphosphonate use were not. Bone health is more likely to be affected in the long term than in the short to medium term; a longer duration of follow‐up is therefore required. Most studies did not measure quality of life outcomes. When quality of life was measured, each of the five studies used a different tool or did not report whether a validated questionnaire was used.
Quality of the evidence
This systematic review provides evidence from 15 studies involving 11,538 premenopausal women with hormone‐positive early breast cancer. High‐certainty evidence supports the addition of OFS to standard treatment in the adjuvant setting with an increase in overall survival and disease‐free survival. These were open‐label studies, which may increase the risk of bias, particularly for toxicity and quality of life assessments. Clinical heterogeneity was evident in the duration of OFS, the method of OFS, the node positivity of cancers, and the type of chemotherapy administered (where applicable).
Potential biases in the review process
We did not include studies that used OFS for 12 months or less and reported on primary endpoints focused on fertility, as these were outside the scope of this review. These may have provided evidence to indicate the presence of a dose‐dependent relationship between the addition of OFS and efficacy outcomes.
When the total population of the studies included both hormone‐positive and hormone‐negative cancers, and when results were not stratified by hormone status, we used the data for the total population (as long as the incidence of hormone‐positive cancers was > 50%). This may have underestimated the efficacy of the addition of OFS, which is considered to impact outcomes only in hormone‐positive cancers.
Agreements and disagreements with other studies or reviews
Our results are consistent with the EBCTCG meta‐analysis (EBCTCG 2005), which examined individual participant data of 8000 women and found that OFS resulted in a reduction in recurrence and breast cancer mortality, although the effect was smaller when OFS was used in conjunction with other treatments. The population included 47% of participants with untested hormonal status who were randomised to a clinical study before the year 2000 in studies that began before 1995. This current review includes women who had hormone‐positive cancers and had received more modern treatments such as anthracyclines. Some of the studies included in the individual participant data review were not included in this Cochrane Review because they did not stratify by hormone receptor status, or they provided insufficient details about the duration of OFS. In this Cochrane Review, we included additional studies in our meta‐analysis that reflected aggregate data and new studies published since 2005.
Other reviews that examined the role of OFS in adjuvant treatment for early breast cancer include Cheer 2005 and Chlebowski 2017.
Cheer 2005 examined the effects of goserelin in treatment of early breast cancer, and included three of the studies that we had identified (ECOG 5188, INT‐0101; IBCSG VIII; ZIPP). Our review includes longer‐follow‐up for two of these studies and examines the role of OFS rather than goserelin only. The remaining four studies included in the Cheer 2005 review did not fulfil our eligibility criteria and included comparisons of goserelin against chemotherapy or added tamoxifen as well as goserelin to the intervention arm.
Chlebowski 2017 examined the effects of OFS in combination endocrine adjuvant therapy and included two of the studies identified in our search (E‐3193, INT‐0142; SOFT). Our review includes longer follow‐up for SOFT and examines the broader effect of OFS by considering the addition of OFS to observation, chemotherapy, and/or tamoxifen rather than just to other hormonal agents. The remaining two studies included in the Chlebowski 2017 review did not fulfil our eligibility criteria and included the comparison of OFS and aromatase inhibitors to OFS and tamoxifen.
Authors' conclusions
Implications for practice.
This review provides evidence that supports the addition of OFS to standard therapy in premenopausal women with early, hormone‐positive breast cancers. The decision to use OFS may depend on the overall risk assessment based on tumour and patient characteristics, and consideration of the toxicities, particularly of hot flushes, that occur with OFS.
Implications for research.
Further research is required to identify women who will derive the most benefit from OFS. The role of aromatase inhibitors and OFS in the adjuvant setting also needs to be clarified, given that there has been improvement in disease‐free survival of this combination compared with tamoxifen and OFS (Francis 2018). No reduction in mortality has been observed with this combination. This review has highlighted the need for improved safety and quality of life data collection.
Acknowledgements
We are very grateful for data extraction and risk of bias assessments conducted by Anna Kono and Rina Shoki, and for quality checks undertaken by Noyuri Yamaji and Erika Ota at the Japanese Cochrane Centre. We also thank Ava Grace Tan‐Koay for conducting searches of databases and registries for the 2019 review update. Mike Clarke assisted with completion of the original review including modification of what had been published in the original review (Sharma 2005).
We also wish to thank the following people for their helpful peer review: Mike Clarke (clinical and methodological editor), who has been a member of the EBCTCG Secretariat since 1989 and was responsible for the overview of ovarian ablation and suppression; Matteo Lambertini (external clinical reviewer), MD, PhD, IRCC, Ospedale Policlino San Martino, University of Genova; the statistical editor, who wishes to remain anonymous; Sandra Finestone (consumer reviewer), PhD; and Bonner Cutting (consumer reviewer), who is also a member of the Alamo Breast Cancer Foundation and the Alamo Advocate Program Committee for the SABCS.
Appendices
Appendix 1. CENTRAL
#1 MeSH descriptor: [Breast Neoplasms] explode all trees #2 breast near cancer #3 breast near neoplasm* #4 breast near carcinoma* #5 breast near tumour* #6 breast near tumor* #7 breast near malignan* #8 #1 or #2 or #3 or #4 or #5 or #6 or #7 #9 MeSH descriptor: [Gonadotropin‐Releasing Hormone] explode all trees #10 MeSH descriptor: [Buserelin] explode all trees #11 MeSH descriptor: [Goserelin] explode all trees #12 MeSH descriptor: [Leuprolide] explode all trees #13 MeSH descriptor: [Nafarelin] explode all trees #14 MeSH descriptor: [Triptorelin Pamoate] explode all trees #15 (buserelin or goserelin or gonadorelin or leuprolide or nafarelin or triptorelin or leuprorelin or leuproprelin) #16 LHRH #17 (luteinising hormone releasing hormone) #18 MeSH descriptor: [Ovariectomy] explode all trees #19 oophorectom* #20 ovar* near ablat* #21 ovarian suppress* #22 ovar* near function near suppress* #23 ovarian function suppression #24 #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 #25 #8 AND #24
Appendix 2. MEDLINE
| 1 | randomized controlled trial.pt. |
| 2 | controlled clinical trial.pt. |
| 3 | randomized.ab. |
| 4 | placebo.ab. |
| 5 | Clinical Trials as Topic/ |
| 6 | randomly.ab. |
| 7 | trial.ti. |
| 8 | (crossover or cross‐over).tw. |
| 9 | Pragmatic Clinical Trials as Topic/ |
| 10 | pragmatic clinical trial.pt. |
| 11 | Randomized Controlled Trials as Topic/ |
| 12 | or/1‐11 |
| 13 | exp Breast Neoplasms/ |
| 14 | (breast adj6 cancer$).tw. |
| 15 | (breast adj6 neoplasm$).tw. |
| 16 | (breast adj6 carcinoma$).tw. |
| 17 | (breast adj6 tumo?r$).tw. |
| 18 | or/13‐17 |
| 19 | exp Gonadotropin‐Releasing Hormone/ |
| 20 | exp BUSERELIN/ |
| 21 | exp GOSERELIN/ |
| 22 | exp LEUPROLIDE/ |
| 23 | exp NAFARELIN/ |
| 24 | exp Triptorelin Pamoate/ |
| 25 | (buserelin or goserelin or gonadorelin or leuprolide or nafarelin or triptorelin or leuprorelin or leuproprelin).mp. |
| 26 | LHRH.mp. |
| 27 | luteinising hormone releasing hormone.tw. |
| 28 | exp Ovariectomy/ |
| 29 | oophorectom*.mp. |
| 30 | (ovar* adj6 ablat*).tw. |
| 31 | ovarian suppress*.tw. |
| 32 | (ovar* adj6 function adj6 suppress*).tw. |
| 33 | ovarian function suppression.mp. |
| 34 | or/19‐33 |
| 35 | 12 and 18 and 34 |
| 36 | animals/ not humans/ |
| 37 | 35 not 36 |
Appendix 3. Embase
| 1 | Randomized controlled trial/ |
| 2 | Controlled clinical study/ |
| 3 | Random$.ti,ab. |
| 4 | randomization/ |
| 5 | intermethod comparison/ |
| 6 | placebo.ti,ab. |
| 7 | (compare or compared or comparison).ti. |
| 8 | (open adj label).ti,ab. |
| 9 | ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. |
| 10 | double blind procedure/ |
| 11 | parallel group$1.ti,ab. |
| 12 | (crossover or cross over).ti,ab. |
| 13 | ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. |
| 14 | (assigned or allocated).ti,ab. |
| 15 | (controlled adj7 (study or design or trial)).ti,ab. |
| 16 | (volunteer or volunteers).ti,ab. |
| 17 | trial.ti. |
| 18 | or/1‐17 |
| 19 | exp breast/ |
| 20 | exp breast disease/ |
| 21 | (19 or 20) and exp neoplasm/ |
| 22 | exp breast tumor/ |
| 23 | exp breast cancer/ |
| 24 | exp breast carcinoma/ |
| 25 | (breast$ adj5 (neoplas$ or cancer$ or carcin$ or tumo$ or metasta$ or malig$)).ti,ab. |
| 26 | or/21‐25 |
| 27 | exp gonadorelin/ |
| 28 | exp buserelin/ |
| 29 | exp goserelin/ |
| 30 | exp leuprorelin/ |
| 31 | exp nafarelin/ |
| 32 | exp triptorelin/ |
| 33 | (gonadorelin or buserelin or goserelin or leuprolide or nafarelin or triptorelin or leuprorelin or leuproprelin).mp. |
| 34 | LHRH.mp. |
| 35 | luteinising hormone releasing hormone.mp. |
| 36 | Gonadotropin Releasing Hormone.mp. |
| 37 | exp ovariectomy/ |
| 38 | oophorectom*.mp. |
| 39 | (ovar* adj6 ablat*).tw. |
| 40 | ovarian suppress*.tw. |
| 41 | (ovar* adj6 function adj6 suppress*).tw. |
| 42 | ovarian function suppression.mp. |
| 43 | or/27‐42 |
| 44 | 18 and 26 and 43 |
| 45 | limit 44 to (human and (conference abstracts or embase)) |
Appendix 4. WHO ICTRP
Basic search 1:
breast cancer AND luteinising hormone releasing hormone
Basic search 2:
breast cancer AND ovarian suppression
Advanced search 1: Condition: Breast cancer OR Breast neoplasm
Intervention: buserelin OR goserelin OR gonadorelin OR leuprolide OR nafarelin OR triptorelin OR leuprorelin OR leuproprelin
Recruitment Status: ALL
Advanced search 2: Condition: Breast cancer OR Breast neoplasm
Intervention: ovarian ablation OR ovarian function OR ovarian suppression
Recruitment Status: ALL
Advanced search 3: Condition: Breast cancer OR Breast neoplasm
Intervention: oophorectomy or ovariectomy
Recruitment Status: ALL
Appendix 5. ClinicalTrials.gov
Basic search 1:
Condition or disease: Breast Cancer
Other terms: luteinising hormone releasing hormone
Basic search 2:
Condition or disease: Breast Cancer
Other terms: Ovarian suppression
Advanced search 1:
Condition or disease: Breast cancer OR Breast neoplasm
Intervention/treatment: buserelin OR goserelin OR gonadorelin OR leuprolide OR nafarelin OR triptorelin OR leuprorelin OR leuproprelin
Study type: All studies
Study Results: All studies
Advanced search 2:
Condition or disease: Breast cancer OR Breast neoplasm
Intervention/treatment: ovarian (ablation OR function OR suppression)
Study type: All studies
Study Results: All studies
Advanced search 3:
Condition or disease: Breast cancer OR Breast neoplasm
Intervention/treatment: oophorectomy or ovariectomy
Study type: All studies
Study Results: All studies
Data and analyses
Comparison 1. OFS versus no OFS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 11 | 10374 | Hazard Ratio (Fixed, 95% CI) | 0.86 [0.78, 0.94] |
| 1.1 OFS vs observation | 1 | 2706 | Hazard Ratio (Fixed, 95% CI) | 0.83 [0.71, 0.97] |
| 1.2 OFS + chemotherapy vs chemotherapy | 5 | 3087 | Hazard Ratio (Fixed, 95% CI) | 0.95 [0.82, 1.09] |
| 1.3 OFS + tamoxifen vs tamoxifen | 4 | 3299 | Hazard Ratio (Fixed, 95% CI) | 0.74 [0.59, 0.93] |
| 1.4 OFS + chemotherapy + tamoxifen vs chemotherapy + tamoxifen | 1 | 1282 | Hazard Ratio (Fixed, 95% CI) | 0.31 [0.10, 0.94] |
| 2 Disease‐free survival | 10 | 8899 | Hazard Ratio (Fixed, 95% CI) | 0.83 [0.77, 0.90] |
| 2.1 OFS vs observation | 1 | 2706 | Hazard Ratio (Fixed, 95% CI) | 0.82 [0.73, 0.92] |
| 2.2 OFS + chemotherapy vs chemotherapy | 5 | 2450 | Hazard Ratio (Fixed, 95% CI) | 0.90 [0.79, 1.01] |
| 2.3 OFS + tamoxifen vs tamoxifen | 3 | 2461 | Hazard Ratio (Fixed, 95% CI) | 0.76 [0.63, 0.92] |
| 2.4 OFS + chemotherapy + tamoxifen vs chemotherapy + tamoxifen | 1 | 1282 | Hazard Ratio (Fixed, 95% CI) | 0.69 [0.48, 0.99] |
| 3 Contralateral breast cancer | 9 | 9138 | Risk Ratio (Fixed, 95% CI) | 0.75 [0.58, 0.98] |
| 3.1 OFS vs observation | 1 | 2710 | Risk Ratio (Fixed, 95% CI) | 0.75 [0.47, 1.17] |
| 3.2 OFS + chemotherapy vs chemotherapy | 5 | 3012 | Risk Ratio (Fixed, 95% CI) | 0.82 [0.56, 1.20] |
| 3.3 OFS + tamoxifen vs tamoxifen | 1 | 2033 | Risk Ratio (Fixed, 95% CI) | 0.56 [0.29, 1.07] |
| 3.4 OFS + chemotherapy + tamoxifen vs chemotherapy + tamoxifen | 2 | 1383 | Risk Ratio (Fixed, 95% CI) | 1.02 [0.26, 4.06] |
| 4 Second malignancy | 7 | 6327 | Risk Ratio (Fixed, 95% CI) | 0.89 [0.64, 1.25] |
| 4.1 OFS + chemotherapy vs chemotherapy | 5 | 3012 | Risk Ratio (Fixed, 95% CI) | 0.94 [0.60, 1.45] |
| 4.2 OFS + tamoxifen vs tamoxifen | 1 | 2033 | Risk Ratio (Fixed, 95% CI) | 0.96 [0.55, 1.67] |
| 4.3 OFS + chemotherapy + tamoxifen vs chemotherapy + tamoxifen | 1 | 1282 | Risk Ratio (Fixed, 95% CI) | 0.38 [0.10, 1.43] |
| 5 Hot flushes | 6 | 5581 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.41, 1.82] |
| 5.1 OFS vs observation | 1 | 1191 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.09 [2.38, 15.59] |
| 5.2 OFS + chemotherapy vs chemotherapy | 2 | 1922 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.11, 1.49] |
| 5.3 OFS + tamoxifen vs tamoxifen | 3 | 2468 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.56, 2.55] |
1.4. Analysis.
Comparison 1 OFS versus no OFS, Outcome 4 Second malignancy.
Comparison 2. Duration of OFS: < 3 years vs ≥ 3 years.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 10 | 9536 | Hazard Ratio (Fixed, 95% CI) | 0.86 [0.78, 0.95] |
| 1.1 < 3 years of OFS | 5 | 4956 | Hazard Ratio (Fixed, 95% CI) | 0.79 [0.69, 0.91] |
| 1.2 ≥ 3 years of OFS | 5 | 4580 | Hazard Ratio (Fixed, 95% CI) | 0.93 [0.81, 1.07] |
| 2 Disease‐free survival | 10 | 8899 | Hazard Ratio (Fixed, 95% CI) | 0.83 [0.77, 0.90] |
| 2.1 < 3 years of OFS | 5 | 4956 | Hazard Ratio (Fixed, 95% CI) | 0.80 [0.72, 0.88] |
| 2.2 ≥ 3 years of OFS | 5 | 3943 | Hazard Ratio (Fixed, 95% CI) | 0.88 [0.78, 0.98] |
2.1. Analysis.
Comparison 2 Duration of OFS: < 3 years vs ≥ 3 years, Outcome 1 Overall survival.
Comparison 3. Age of studied population: < 40 years vs ≥ 40 years.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 2 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 1.1 < 40 years | 2 | 394 | Hazard Ratio (Fixed, 95% CI) | 0.73 [0.51, 1.04] |
| 1.2 ≥ 40 years | 2 | 1175 | Hazard Ratio (Fixed, 95% CI) | 0.89 [0.69, 1.14] |
| 2 Disease‐free survival | 3 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 2.1 < 40 years | 3 | 1764 | Hazard Ratio (Fixed, 95% CI) | 0.65 [0.50, 0.83] |
| 2.2 ≥ 40 years | 3 | 1504 | Hazard Ratio (Fixed, 95% CI) | 0.95 [0.78, 1.15] |
Comparison 4. Chemotherapy use: yes or no.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 9 | 6739 | Hazard Ratio (Fixed, 95% CI) | 0.87 [0.77, 0.97] |
| 1.1 Chemotherapy | 8 | 5453 | Hazard Ratio (Fixed, 95% CI) | 0.86 [0.76, 0.97] |
| 1.2 No chemotherapy | 3 | 1286 | Hazard Ratio (Fixed, 95% CI) | 0.89 [0.62, 1.28] |
| 2 Disease‐free survival | 9 | 6102 | Hazard Ratio (Fixed, 95% CI) | 0.83 [0.76, 0.90] |
| 2.1 Chemotherapy | 8 | 4816 | Hazard Ratio (Fixed, 95% CI) | 0.85 [0.77, 0.93] |
| 2.2 No chemotherapy | 3 | 1286 | Hazard Ratio (Fixed, 95% CI) | 0.73 [0.59, 0.90] |
Comparison 5. Method of OFS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 9 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 1.1 OFS via surgery vs no OFS | 2 | 415 | Hazard Ratio (Fixed, 95% CI) | 0.86 [0.57, 1.28] |
| 1.2 OFS via LHRH agonists vs no OFS | 8 | 8101 | Hazard Ratio (Fixed, 95% CI) | 0.80 [0.71, 0.89] |
| 1.3 OFS via RT vs no OFS | 1 | 77 | Hazard Ratio (Fixed, 95% CI) | 1.75 [0.50, 6.16] |
| 2 Disease‐free survival | 9 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 2.1 OFS via surgery vs no OFS | 2 | 415 | Hazard Ratio (Fixed, 95% CI) | 0.96 [0.70, 1.30] |
| 2.2 OFS via LHRH agonists vs no OFS | 8 | 8101 | Hazard Ratio (Fixed, 95% CI) | 0.81 [0.75, 0.88] |
| 2.3 OFS via RT vs no OFS | 1 | 77 | Hazard Ratio (Fixed, 95% CI) | 0.94 [0.28, 3.13] |
Comparison 6. Lymph node status: positive or negative.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 10 | 10283 | Hazard Ratio (Fixed, 95% CI) | 0.86 [0.78, 0.94] |
| 1.1 Positive | 7 | 7340 | Hazard Ratio (Fixed, 95% CI) | 0.89 [0.81, 0.99] |
| 1.2 Negative | 3 | 2943 | Hazard Ratio (Fixed, 95% CI) | 0.69 [0.53, 0.88] |
| 2 Disease‐free survival | 9 | 8808 | Hazard Ratio (Fixed, 95% CI) | 0.83 [0.77, 0.90] |
| 2.1 Positive | 6 | 5865 | Hazard Ratio (Fixed, 95% CI) | 0.86 [0.79, 0.93] |
| 2.2 Negative | 3 | 2943 | Hazard Ratio (Fixed, 95% CI) | 0.75 [0.64, 0.89] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ABCTCG.
| Methods | Accrual: January 7, 1993, until September 27, 2000 Multi‐centre: 106 sites in UK, 16 non‐UK sites Phase of trial: III Study design: RCT Country or countries where the trial was conducted: UK, India, Egypt, Malta, New Zealand, Saudi Arabia, Sri Lanka, Iran, Pakistan, Tehran, Singapore Median follow‐up: 5.9 years |
|
| Participants | 2144 participants randomised
Hormone receptor status: ER+ in 39.1% (838/2144); 66.8% to 69.5% of patients with known receptor status
Mean age: 43 years, SD 5.7 years, 24% < 40 years Pre‐ or perimenopausal defined as last menstrual period occurring within 12 months preceding breast diagnostic surgery Tumour size: < 2 cm: 37.2%, 2 to 5 cm: 47.8%, > 5 cm: 9.5% Nodal status: positive 61%; 1 to 3 nodes: 37.5% (805 patients); ≥ 4 nodes: 21.4% (458 patients); positive unknown: 1.6% (35 patients) First line Notable exclusion criteria: nil |
|
| Interventions | ARM 1
Intervention: ovarian ablation or suppression and tamoxifen 20 mg daily (for 5 years) Ovarian function suppression with: ‐ Radiation induced 1600 Gy in 4 fractions to midplane by the anteroposterior fields of the pelvis after ultrasound localisation of ovaries, or ‐ Goserelin 3.6 mg SC q28d or leuprorelin 3.75 mg SC q28d for at least 2 years, or ‐ Surgical ablation 68.8% had radiation‐induced menopause, 22.8% had surgical ablation, and 8.4% had leuprorelin ARM 2 Comparator: tamoxifen 20 mg (5 years, but could participate in different trial assessing duration) Mastectomy 63%, local excision 35%, other surgery 2% Radiotherapy 73% Chemotherapy 80% |
|
| Outcomes | Primary outcomes: ‐ Overall survival: time from date of random assignment to date of death Secondary outcomes: ‐ Disease (relapse)‐free survival: time from date of random assignment to date of first recurrence or death from breast cancer with no known date of relapse ‐ Breast cancer‐specific mortality ‐ Quality of life using EORTC QLQ‐C30, BR23 breast cancer module, Hospital Anxiety and Depression Scale (HAS), and Menopausal Symptom Scale (MSS) (reported in 2004 abstract) |
|
| Notes | Trial registration links: https://clinicaltrials.gov/ct2/show/nct00002582 https://clinicaltrials.gov/ct2/show/nct00002580 (Scottish) Contacted trial authors 7 August 2019 to request information related to ER+ tumours (i.e. numbers of events and participants in each treatment group for RFS and OS, hazard ratio for RFS) and quality of life information All randomised patients analysed in intention to treat No estimations undertaken Funding considerations: trial funding not reported; funding for Open Access publication provided by Cancer Research UK |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was performed by randomly permutated blocks stratified by hospital and elective chemotherapy treatment" p.518 |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization and data management were carried out at four academic trials units in the United Kingdom… and at the Ministrry of Health Clinical Trials and Epidemiology Research Unit, Singapore" p.518 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label study. Participants were unable to be blinded to ovarian function suppression due to nature of treatment (radiotherapy, injection, or surgery). Reasonable clinical equipoise such that knowing treatment allocation was unlikely to affect the behaviour of clinicians and patients |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Lack of blinding unlikely to influence this outcome |
| Blinding of outcome assessment (detection bias) Disease‐free survival | Low risk | Lack of blinding unlikely to influence this outcome. Patients were followed up annually. Lack of blinding is unlikely to lead to a material bias when DFS events are assessed |
| Blinding of outcome assessment (detection bias) Toxicity | Unclear risk | Toxicity outcomes (e.g. day sweats, night sweats) are self‐reported; reporting may be influenced by knowing the treatment received |
| Blinding of outcome assessment (detection bias) Quality of life | Unclear risk | Assessed with EORTC QLQ‐C30, BR23 breast cancer module. Outcome is self‐reported, and reporting may be influenced by knowing the treatment received |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants included in intention‐to‐treat analysis for OS and other efficacy outcomes |
| Selective reporting (reporting bias) | Unclear risk | Outcomes mentioned in the methods section are reported on in the results section. There was minimal reporting of toxicity outcomes, and these outcomes were likely to be collected |
| Other bias | Low risk | None identified |
Arriagada 2005.
| Methods | Accrual: January 1989 to February 1998 Multi‐centre: France, Chile (total 12 sites) Phase of trial: III (presumed, not explicitly stated) Study design: RCT Country or countries where the trial was conducted: France, Chile Median follow‐up: 9.7 years in OFS arm, 9.8 years in control arm |
|
| Participants | 926 participants randomised
Hormonal receptor status: ER+ PR+: 69.7% (645/926) ‐ER+/PR+: 55% (509 patients), ER+/PR‐ or ER‐/ PR+: 14.7% (136 patients), ER‐/PR‐: 17.2% (159 patients); unknown: 15.3% (122 patients) Mean age: 43 years Premenopausal defined as menstruation for 3 months preceding diagnosis, or if oestradiol ≥ 60 pmol/mL or FSH ≤ 30 mU/mL Tumour size: T0 to 1: 37% (343), T2: 50.9% (472), T3 to 4: 9.3% (86) Nodal status: positive 90% (N0: 9.6% (89), N1 to 3: 56.6% (524), N4+: 33% (306)); no lymph node dissection in 7 patients First line Notable exclusion criteria: nil |
|
| Interventions | ARM 1
Intervention: chemotherapy (physician choice) followed by ovarian function suppression
Ovarian function suppression with: ‐ Radiotherapy: 4#, delivered to mid‐plane of pelvis at 12 Gy in 4 fractures on 4 consecutive days, field 14 × 10 cm (ultrasound localisation of ovaries not mandatory), or ‐ Triptorelin 3.7 mg IM monthly for 3 years, or ‐ Surgical oophorectomy ARM 2 Comparator: chemotherapy (physician choice) alone Mastectomy 43%, lumpectomy 56%, no surgery 1% Axillary clearance: 90% Radiotherapy: to breast or chest wall 90%, to lymph nodes 82% Chemotherapy: postoperative 83%, neoadjuvant chemotherapy 6%, both adjuvant and neoadjuvant 11% Tamoxifen (violating study protocol): 5% |
|
| Outcomes | Primary outcomes:
‐ Overall survival: time from date of randomisation and date of last follow‐up or death ‐ Disease‐free survival: date of randomisation and date of last follow‐up or date of best available evidence concerning the first unfavourable event (i.e. locoregional recurrence, distant metastasis, contralateral breast cancer, or death) Secondary outcomes: ‐ Hot flushes ‐ Serious adverse events (SAEs) mentioned in Discussion |
|
| Notes | Trial registration link: could not be found
Contacted authors 7 August 2019 for data related to ER+ tumours (i.e. number of events in both treatment groups, unadjusted hazard ratio for overall survival)
All randomised patients included in intention‐to‐treat analysis
Hazard ratio estimated for OS and DFS using Tierney method 7 Funding considerations: Ipsen‐Biotech, France – provided drug for free and partially funded updating of trial data. Data analysis performed independently by Biostatistics Department, Institut Gustave‐Roussy |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was stratified by centre and hormone receptor status. Baseline characteristics across groups were balanced (as reported in Table 2). Random sequence generation: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization was centralized at the Institut Gustave‐Roussy by telephone or fax …" p.390 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label study. Participants were unable to be blinded to ovarian function suppression due to nature of treatment (radiotherapy, injection, or surgery). Reasonable clinical equipoise such that knowing treatment allocation was unlikely to affect the behaviour of clinicians and patients |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Lack of blinding unlikely to influence this outcome |
| Blinding of outcome assessment (detection bias) Disease‐free survival | Low risk | Quote: "patients were seen every 6 months for the first 2.5 years and yearly thereafter with a yearly mammogram and a clinical visit at each visit" p.390. Lack of blinding is unlikely to lead to a material bias when DFS events are assessed |
| Blinding of outcome assessment (detection bias) Toxicity | Unclear risk | No details provided in trial publication aside from the incidence of hot flushes p393. Outcome is self‐reported, and reporting may be influenced by knowing treatment received |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants included in intention‐to‐treat analysis for OS and other efficacy outcomes |
| Selective reporting (reporting bias) | Unclear risk | Outcomes mentioned in the methods section are reported on in the results section. There was minimal reporting of toxicity outcomes, and these outcomes were likely to be collected |
| Other bias | Low risk | None identified |
ASTRRA.
| Methods | Accrual: March 2009 to March 2014 Multi‐centre: 35 centres Phase of trial: III State study design: RCT Country or countries where trial was conducted: 35 institutions in South Korea Median follow‐up: 63 months |
|
| Participants | 1289 participants randomised Hormone receptive status: ER+ in 100% Median age: 40 years (range 24 to 45 years) Premenopausal status assessed 3 months after final dose of chemotherapy and at 6, 12, 18, and 24 months; defined as serum FSH levels < 30 mIU/mL or bleeding history within 6 months of each visit Nodal status: positive 55.0% Grade: Grade 1 16.1%, Grade 2 51.7%, Grade 3 23.8%, unidentified 8.4% Histology: invasive ductal carcinoma 88.5%, invasive lobular carcinoma 4.7%, others 6.4%, unidentified 0.5% HER2‐positive: 13.8% First line Notable exclusion criteria: nil |
|
| Interventions | ARM 1
Intervention: chemotherapy (physician choice) + goserelin 3.6 mg SC q28 days for 2 years + tamoxifen 20 mg daily for 5 years ARM 2 Comparator: chemotherapy (physician choice) + tamoxifen 20 mg daily for 5 years Mastectomy 40.0%, breast‐conserving surgery 57.9%, unidentified 2.7% Chemotherapy: anthracycline‐containing regimen 41.0%, anthracycline and taxane‐containing regimen 56.4%, other taxane regimen 1%, other non‐taxane regimen 0.7%, unidentified 0.9% Neoadjuvant or adjuvant chemotherapy allowed |
|
| Outcomes | Primary outcomes:
‐ Disease‐free survival defined as time from enrolment to detection of invasive local recurrence, regional recurrence, distant recurrence, contralateral breast cancer, secondary malignancy, or death by any cause Secondary outcomes: ‐ Overall survival ‐ 5‐year DFS between post‐ and pre‐menopausal women ‐ Tolerability of tamoxifen with or without goserelin Study explicitly stated that data on adverse events were not collected |
|
| Notes | Trial registration link: https://clinicaltrials.gov/ct2/show/NCT00912548
Contacted trialists on 7 August 2019 (prior to full‐text publication) for numbers of events (death and DFS‐related events) and number of participants in each treatment group, and any toxicity data. Study authors provided efficacy data and indicated that toxicity data were not included
Not all randomised patients were included in intention‐to‐treat analysis Funding considerations: supported by an investigator‐sponsored study programme at AstraZeneca. The study was also partially supported by the Korea Institute of Radiological and Medical Sciences, funded by the Ministry of Science and ICT, Republic of Korea |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization performed by means of an inter‐based system" p.3. Also randomisation was stratified according to lymph node status and by institution |
| Allocation concealment (selection bias) | Low risk | Quote: "Internet based system" p.3 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label study. Participants were unable to be blinded to ovarian function suppression due to nature of treatment (injections). Reasonable clinical equipoise such that knowing treatment allocation was unlikely to affect the behaviour of clinicians and patients |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Lack of blinding unlikely to influence this outcome |
| Blinding of outcome assessment (detection bias) Disease‐free survival | Low risk | Lack of blinding unlikely to influence this outcome. Patients were followed up every 6 months for 5 years, and at least yearly thereafter, according to the institute's routine practice |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of participants randomised was different from the number of participants included in the intention‐to‐treat analysis. Four participants in the goserelin + tamoxifen group were excluded (due to inadequate data or consent withdrawal), and 7 participants in the tamoxifen group were excluded for similar reasons |
| Selective reporting (reporting bias) | Unclear risk | Reported DFS and OS but other outcomes (such as tolerability) were not reported in the full‐text publication |
| Other bias | Low risk | None identified |
E‐3193, INT‐0142.
| Methods | Accrual: September 1994 to November 1997 Multi‐centre: no further details provided Phase of trial: III RCT Country or countries where the trial was conducted: United States of America Median follow‐up: 9.9 years (range 0.2 to 12.3 years) for recurrence and survival; 5.86 years for patient‐reported endpoints |
|
| Participants | 345 participants were randomised Hormone receptor status: ER+ and/or PR+ in 100% ‐ER+/PR+ 88%, ER+/PR‐ 8%, ER+/PR unknown 1%, ER‐/PR+ 2.4%, ER unknown/PR+ 1% Age: median 45 years (range 26 to 55) Premenopausal status defined as menstrual period within past 6 months without prior oophorectomy, or in the case of prior hysterectomy, as age 55 years or younger with 1 or both ovaries remaining and an oestradiol level in normal premenopausal range Tumour size: ≤ 1 cm 11.0%, 1.1 to 2 cm 72.1%, > 2 cm 16.9% Nodal status: positive 0% First line Notable exclusion criteria: no adjuvant systemic chemotherapy allowed |
|
| Interventions | ARM 1
Intervention: OFS (goserelin 3.6 mg SC q4weeks for 5 years OR leuprorelin 3.75 mg SC q4weeks for 5 years, surgical ablation or radiation 20 Gy in 10 fractions) + tamoxifen 20 mg daily for 5 years Choice of OFS by participants: 42% had an oophorectomy 36% decided on LHRH 13% decided on radiation 9% refused OFS ARM 2 Comparator: tamoxifen 20 mg daily for 5 years Mastectomy 40.0%, breast‐conserving surgery 62.0% |
|
| Outcomes | Primary outcomes: ‐ Overall survival, defined as time from random assignment to death as a result of any cause, with living patients censored at last evaluation date ‐ Disease‐free survival, defined as time from random assignment to earliest time of disease recurrence, new primary breast cancer, or death as a result of any cause, censoring patients without recurrence or death at the date of last disease assessment known to be disease free Secondary outcomes: ‐ Toxicity, assessed using the National Cancer Institute Common Toxicity Criteria version 1 ‐ Patient‐reported outcomes including: (a) menopausal symptoms, using the Postmenopausal Estrogen/Progestin Intervention checklist (b) sexual function, using the Sexual Activity Questionnaire (c) HRQoL, using FACT‐B (version 3) |
|
| Notes | Trial registration link could not be found Trial authors were not contacted Efficacy outcomes analyses according to modified intention‐to‐treat; toxicity outcomes analysed according to assigned treatment arm (as‐treated analysis) No estimations undertaken Funding considerations: funded in part by Public Health Service Grants from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, and Clinical and Translational Science Award programme through NIH National Center for Advancing Translational Sciences |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned 1:1 by using permuted blocks within strata with dynamic balancing across main institutions and their affiliated networks" p.3949 |
| Allocation concealment (selection bias) | Low risk | No description whether randomisation was centralised, although probably done as involved randomisation across multiple sites |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label study. Participants were unable to be blinded to ovarian function suppression due to nature of treatment (radiotherapy, injection, or surgery). Reasonable clinical equipoise such that knowing treatment allocation was unlikely to affect the behaviour of clinicians and patients |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Lack of blinding unlikely to influence this outcome |
| Blinding of outcome assessment (detection bias) Disease‐free survival | Low risk | Lack of blinding unlikely to influence this outcome. Outcomes assessed every 6 months for the first 5 years and annually thereafter |
| Blinding of outcome assessment (detection bias) Toxicity | Low risk | Toxicity outcomes were graded as per CTCAE p3449. Although the study is open‐label, grading symptoms using the CTCAE is standardised; therefore knowing treatment allocation may have an immaterial effect on the grading of outcomes |
| Blinding of outcome assessment (detection bias) Quality of life | Unclear risk | Assessed with FACT‐B; however this is self‐reported, and participant knowledge of treatment allocation may have influenced reporting of results |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | CONSORT diagram (p.3950), note 5 patients (OFS) and 4 patients (control) did not proceed with study treatment. The trial publication states that it is an intention‐to‐treat analysis although likely modified intention‐to‐treat (due to ineligible patients, listed above) |
| Selective reporting (reporting bias) | Low risk | Outcomes described in the methods section in the trial publication are reported. We did not identify a trial record to cross‐check outcome reporting |
| Other bias | Low risk | None identified |
ECOG 5188, INT‐0101.
| Methods | Accrual: July 1989 to February 1994
Multi‐centre
Phase of trial: III
RCT Country or countries where the trial was conducted: USA Median follow‐up: 9.6 years |
|
| Participants | 996 participants randomised Hormone receptor status: ER+ and/or PR+ 100% ‐ER+/PR+ 75.6%, ER+/PR‐ 11.0%, ER‐/PR+ 13.4% Age: < 40 years 29.0%, ≥ 40 years 71.0% Premenopausal status defined by: < 4 months since menses; 4 to 12 months since menses with premenopausal FSH level, or < 61 years of age with previous ovary‐sparing hysterectomy and premenopausal FSH Tumour size: ≤ 2 cm 36.8%, > 2 cm 58.8%; unknown 4.3% Nodal status: positive 100%; 1 to 3 LN 59.2%, 4 to 9 LN 31.3%, ≥ 10 LN 9.4% First line Notable exclusion criteria: nil |
|
| Interventions | Three‐arm trial; however only 1 comparison relevant for inclusion in this Cochrane Review ARM 1 Intervention: chemotherapy (CAF) followed by goserelin 3.6 mg SC q4weeks for 5 years, beginning on cycle 6, day 29 of chemotherapy Chemotherapy (CAF): 6 × cyclophosphamide 100 mg/m² PO on day 1 for 14 days, doxorubicin 30 mg/m² IV, and fluorouracil 500 mg/m² IV on days 1 and 8, every 28 days Goserelin dose was doubled if a patient was not amenorrhoeic, and if serum oestradiol level was not in the postmenopausal range after 8 weeks. ARM 2 Comparator: chemotherapy (CAF) alone Colony‐stimulating factor permitted only for treatment of febrile neutropenia Mastectomy 82.4%, breast conservation 17.6% Post‐mastectomy radiotherapy 11% |
|
| Outcomes | Primary outcomes: ‐ Time to recurrence (TTR), defined as time from random assignment to disease recurrence or new breast cancer primary, where death without recurrence censored ‐ Disease‐free survival (DFS), defined as time from random assignment to disease recurrence, new breast cancer primary, or death, whichever occurred first ‐ Overall survival (OS), defined as time from random assignment to death Secondary outcomes: ‐ Toxicity, grades 1 to 4 (no further information provided in trial publication) ‐ Hormone levels (luteinising hormone, follicle‐stimulating hormone, and 17β oestradiol) ‐ Amenorrhoea |
|
| Notes | Trial registration link could not be found
Trial authors were not contacted All patients analysed according to assigned treatment arm No estimations undertaken Funding considerations: supported by Eastern Cooperative Oncology Group (ECOG) and in part by Public Health Service Grants from National Cancer Institute, National Institutes of Health, and Department of Health and Human Services |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Publication states that "patients were randomly assigned to receive…" p.5974 with no baseline imbalances. Trial conducted by ECOG and likely to have used an adequate random sequence |
| Allocation concealment (selection bias) | Unclear risk | No description whether randomisation was centralised |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label study. Reasonable clinical equipoise such that knowing treatment allocation was unlikely to affect the behaviour of clinicians and patients |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Lack of blinding unlikely to influence this outcome |
| Blinding of outcome assessment (detection bias) Disease‐free survival | Low risk | Lack of blinding unlikely to influence this outcome. Patients reviewed every 3 months for first 6 months, every 4 months after chemotherapy for first 5 years, every 6 months for next 3 years, and annually thereafter |
| Blinding of outcome assessment (detection bias) Toxicity | Unclear risk | Very few details about how toxicity outcome data were collected, except that the maximum toxicity grade was collected; some toxicities listed would be blood tests (e.g. anaemia), and others were self‐reported (e.g. nausea, hot flashes). Therefore it is unclear whether lack of blinding had an effect on results reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Publication reports that data were analysed as intention‐to‐treat (ITT); however 34 patients were excluded and a modified intention‐to‐treat analysis appears to have been conducted. Reasons for patient exclusion are provided but no indication of the numbers in each group provided, and if they varied considerably across treatment groups. Results from full ITT are not reported, although the paper states that results were similar between ITT and mITT |
| Selective reporting (reporting bias) | Low risk | Outcomes described in the method section in the trial publication are reported on in the results section. We did not identify a trial record to cross‐check outcome reporting |
| Other bias | Low risk | None identified |
GABG IV‐B‐93.
| Methods | Accrual: February 1993 to December 2000 Multi‐centre (66 centres) Phase of trial: III RCT Country or countries where the trial was conducted: Germany Median follow‐up: 4.7 years (for entire cohort); 4 years for hormone receptor‐positive group |
|
| Participants | 776 participants randomised ‐ Initially patients were ER‐/PR‐ but inclusion criteria were extended to include women with ER+ and/or PR+ tumours in April 1997 Hormone receptor status: hormone receptor‐positive 40.1% (311/776) Age: ≤ 40 years 26.4%, > 40 years 73.6% Premenopausal status defined as regular menses in the last 6 months or FSH < 20 IU/L, LH > 50 pg/mL Tumour size: ≤ 2 cm 49.5%, > 2 cm 50.5% Nodal status: 0 LN 8.0%, 1 to 3 LN 57.9%. 4 to 9 LN 34.1% Grade: Grade 1 8.4%, Grade 2 54.7%, Grade 3 34.7%, unknown 2.3% First line Notable exclusion criteria: nil |
|
| Interventions | ARM 1
Intervention: chemotherapy (risk‐adjusted) + goserelin 3.6 mg SC every 4 weeks for 2 years Chemotherapy (risk‐adjusted): ‐ 0 to 3 positive lymph nodes: 3 × CMF (cyclophosphamide 500 mg/m², methotrexate 40 mg/m², and 5‐fluorouracil 600 mg/m² IV on days 1 and 8) ‐ 4 to 9 positive lymph nodes: 4 × EC (epirubicin 90 mg/m² and cyclophosphamide 600 mg/m² IV) every 3 weeks followed by 3 × CMF (as above) ARM 2 Comparator: risk‐adjusted chemotherapy (as above) only and no further treatment Mastectomy 41.5%, breast‐conserving surgery 58.5% Radiotherapy 65.6% |
|
| Outcomes | Primary outcomes: ‐ Event‐free survival, defined as time from definitive primary surgery to first event of failure (locoregional recurrent metastases, second primaries including contralateral breast cancer, or death) ‐ First event failure defined as isolated locoregional recurrence if locoregional recurrence occurred at least 4 weeks before an event at a distant site Secondary outcomes: ‐ Overall survival, defined as the interval from definitive primary surgery to death of any cause ‐ Toxicity outcomes |
|
| Notes | Trial registration link could not be found
Contacted study authors 7 August 2019 to ask for details about the unadjusted hazard ratio for overall survival. Trialists provided adjusted, long‐term (unspecified), unpublished hazard ratios for overall survival and event‐free survival on 5 September 2019 All patients analysed according to assigned treatment arm Unadjusted hazard ratio estimated for EFS using Tierney method 7 Funding considerations: funded by Deutsche Krebshilfe and in part by Pharmacia and AstraZeneca (Germany) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…centre block randomisation with randomly varying block size…" p.2352 |
| Allocation concealment (selection bias) | Low risk | Quote: "patients were centrally randomised, stratified by participating site" p.2352 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label study. Reasonable clinical equipoise such that knowing treatment allocation was unlikely to affect the behaviour of clinicians and patients |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Lack of blinding unlikely to influence this outcome |
| Blinding of outcome assessment (detection bias) Disease‐free survival | Low risk | Lack of blinding unlikely to influence this outcome. Follow‐up examinations were scheduled every 3 months for the first 2 years, every 6 months up to year 5, and annually thereafter |
| Blinding of outcome assessment (detection bias) Toxicity | Unclear risk | No details of how toxicity outcomes were collected; some toxicities noted would be based on blood tests (e.g. leucopenia), while others were self‐reported (e.g. nausea); therefore it is unclear whether lack of blinding had an effect on results reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis and data did not appear to be missing |
| Selective reporting (reporting bias) | Unclear risk | Outcomes described in the methods section in the trial publication are reported on in the results section. We did not identify a trial record to cross‐check outcome reporting. There was minimal reporting of toxicity outcomes, and these outcomes were likely to be collected |
| Other bias | Low risk | None identified |
IBCSG II.
| Methods | Accrual: 1 July 1978 to 31 August 1981 Multi‐centre: 18 sites Phase of trial: III (presumed, not explicitly stated) Study design: RCT Country or countries where the trial was conducted: Switzerland, United States of America, South Africa, Germany, Sweden, Yugoslavia, United Kingdom, Spain, Australia, New Zealand Median follow‐up: 13 years |
|
| Participants | 327 participants randomised Hormone receptor status: ER+ in 32.7% (107/327); approximately 78.9% hormone receptor positive (281/356) ‐ER+ ≥ 10 fmol: 33%, ER+ 0 to 9 fmol: 28%, ER unknown: 39% ‐PR+ ≥ 10 fmol: 26%; PR+ 0 to 9 fmol: 30%, PR unknown 44% ‐ER+/PR+: 20%, ER‐/PR‐: 21% Pre‐ and perimenopausal status defined as ‐ Normal menstruation ‐ Amenorrhoea for less than 1 year ‐ Biochemical evidence of ovarian function ‐ Amenorrhoea for 1 to 3 years in patients younger than 52 years ‐ Hysterectomy without bilateral oophorectomy for patients younger than 56 years Age: ≥ 40 years: 73%; ≤ 39 years: 27% All had ≥ 4 axillary lymph nodes (LN) involved: 4 to 6 LN 48%, 7 to 10 LN 24%, > 10 LN 28% Grade: Grade 1 17%, Grade 2 49%, Grade 3 32% First line Notable exclusion criteria: nil |
|
| Interventions | ARM 1
Intervention: surgical oophorectomy followed by chemotherapy Chemotherapy (CMFp) given q28 days: ‐ Cyclophosphamide 100 mg/m² po days 1 to 14 ‐ Methotrexate 40 mg/m² IV days 1 and 8 ‐ Fluorouracil 600 mg/m² IV days 1 and 8 ‐ Prednisone 5 mg mane, 2.5 mg nocte PO continuous ARM 2 Comparator: CMFp alone (as above) Adjuvant treatment commenced within 6 weeks of mastectomy All patients had primary treatment by mastectomy and axillary clearance |
|
| Outcomes | Primary outcomes:
‐ Disease‐free survival: failure was defined as any recurrence, appearance of a second primary malignancy (including contralateral breast cancer), or death, whichever occurred first, measured from the date of randomisation ‐ Overall survival Secondary outcome: ‐ Not stated although toxicity outcomes (related to CMFp) were described in the results section of trial publication |
|
| Notes | Trial registration link could not be found Trial authors were not contacted All randomised patients included in intention‐to‐treat analysis No estimations undertaken Funding considerations: central co‐ordination, data management, and statistics provided by The Swiss Cancer League, The Cancer League of Ticino, The Swedish Cancer League, The Australia‐New Zealand Breast Cancer Trials Group, The Australian Cancer Society, The Frontier Science and Technology Research Foundation, and The Swiss Institute for Applied Cancer Research |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization schedule was produced using pseudo‐random numbers generated by a congruence method executed on a DEC‐2060 computer" (1985, p.1060) |
| Allocation concealment (selection bias) | Low risk | "Randomization was conducted centrally by the Study Coordinaion Center (Bern, Switzerland)" (1985, p.1060) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label study. Participants were unable to be blinded to ovarian function suppression due to nature of treatment (surgery). Reasonable clinical equipoise such that knowing treatment allocation was unlikely to affect the behaviour of clinicians and patients |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Lack of blinding unlikely to influence this outcome |
| Blinding of outcome assessment (detection bias) Disease‐free survival | Low risk | Lack of blinding unlikely to influence this outcome. Patients were followed‐up through clinical, haematological, and biochemical assessment every 3 months for 2 years, and thereafter every 6 months until death. All records (i.e. toxicity and recurrence) were reviewed centrally by the study co‐ordinator |
| Blinding of outcome assessment (detection bias) Toxicity | Low risk | Patients were followed up through clinical, haematological, and biochemical assessment every 3 months for 2 years, and thereafter every 6 months until death. Brief toxicity outcomes provided for chemotherapy (1985, p.1062) but not for OFS, with most being 'objective' toxicities based on results from blood tests or clinical review. Therefore lack of blinding unlikely to influence these outcomes reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No CONSORT diagram, but Table 10 (1985, p.1061) reporting baseline data also includes randomised participants who did not undergo study treatment (intention‐to‐treat analysis) |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported in the methods section are reported on in the trial publication for main outcomes. There was minimal reporting of toxicity outcomes and these outcomes were likely to be collected |
| Other bias | Low risk | None identified |
3. Quality of life.
| Study | Questionnaire used | Summary of findings | Follow‐up period |
| OFS versus observation | |||
| ZIPP | NR | NR | NR |
| OFS + tamoxifen vs tamoxifen | |||
| ABCTCG | EORTC QLQ‐C30, BR23 breast cancer module | 246 out of 2144 participants agreed to take part in the Quality of Life substudy. A narrative synthesis of the key findings was provided in a conference abstract: there was no deterioration in role function, global QL, body image, or sexual function | 30 months |
| E‐3193, INT‐0142 | FACT‐General and FACT‐B | Health‐related QoL mean scores were worse in the OFS group than in the no OFS group, based on scores from the FACT‐General and FACT‐B cancer subscales at all time points (i.e. 6 months, 12 months, years 2, 3, 4, and 5). This was more pronounced over time and reached statistical and clinical significance for FACT‐G at 3 years. This statistical difference did not persist at 4 and 5 years, and decreased over time | 5.86 years |
| SOFT | International Breast Cancer Study Group (IBCSG) QoL core form and a symptom‐specific module | 1722 out of 2045 participants were tested at baseline, at 6, 12, 18, and 24 months, and then annually during 3 to 6 years. Baseline global QoL scores were similar in both arms and were similar between treatments over the whole treatment period The OFS group reported worse endocrine symptoms and sexual functioning during the first 2 years of treatment. In particular, a decline in sexual interest was significantly greater among participants in the OFS group at 6 months compared to the no OFS group, but not at 24 and 60 months. Participants in the OFS group also experienced worse vaginal dryness over the whole treatment period, while participants in the no OFS group experienced a significantly greater increase in vaginal discharge and itching in the short and intermediate term. Both groups had worsening of bone and joint pain and weight gain. Participants in the OFS arm had more sleep disturbance at 6 months than those receiving tamoxifen alone but not at later time points. No significant differences between groups were observed for all other symptoms. For example, changes in other symptoms (headache, irritable, feeling dizzy, appetite, feeling sick, tired) and global indicators for physical well‐being, mood, and health perception were small over time and were similar between groups The OFS group had slightly less improvement in coping effort and were more burdened by treatment at 6 and 24 months than those in the no OFS group |
6 years |
| Yang 2013 | NR | NR | NR |
| Yi 2016 | NR | NR | NR |
| ZBCSG Trial B | Assessed using 7 domains: daily activity, appetite, sleep, mental, economic, menopause symptoms and menstruation. No further details provided | None of the scores changed considerably before administration of and after treatments | 2 years |
| OFS + chemotherapy vs chemotherapy | |||
| Arriagada 2005 | NR | NR | NR |
| ECOG 5188, INT‐0101 | NR | NR | NR |
| GABG IV‐B‐93 | NR | NR | NR |
| IBCSG II | NR | NR | NR |
| IBCSG VIII | QoL IBCSG core questionnaire | QoL scores were similar across ER‐positive and ER‐negative cohorts (however data were not shown). There were no differences between groups except for hot flushes at 3 years. Quality of life measures were taken at baseline, 3 months, 6 months, and 36 months. The Discussion section of the trial publication stated that "CMF followed by goserelin showed the same effect on all QoL indicators as CMF alone" p.269 | 3 years after randomisation |
| SWOG 1996 | NR | NR | NR |
| OFS + chemotherapy + tamoxifen vs chemotherapy + tamoxifen | |||
| ASTRRA | NR | NR | NR |
| Uslu 2014 | NR | NR | NR |
NR: not reported (meaning not measured).
IBCSG VIII.
| Methods | Accrual: March 1990 to October 1999
Multi‐centre, international Phase of trial: III RCT Country or countries where the trial was conducted: Canada, Hungary, Italy, Slovenia, South Africa, Spain, Sweden, Switzerland, Australia, New Zealand Median follow‐up: 12.1 years |
|
| Participants | 1063 participants randomised to 3‐arm treatment trial Hormone receptor status: ER+ 79.9% (573/717) Age in ER+ cohort: ≤ 34 years 5.6%, 35 to 39 years 12.7%, 40 to 44 years 27.4%, 45 to 49 years 36.6%, ≥ 50 years 17.6% Pre‐ or perimenopausal status defined by 1 of the following: > 52 years with last normal menstrual period within 1 year; ≤ 52 years with last normal menstrual period within 3 years; ≤ 55 years with hysterectomy but no bilateral oophorectomy or biochemical evidence of continuing ovarian function Tumour size: ≤ 1.0 cm 14.1%, 1.1 to 2.0 cm 51.8%, > 2 cm 32.8%, unknown 1.2% Nodal status: positive 0 Grade: Grade 1 21.5%, Grade 2 48.0%, Grade 3 29.1%, unknown 1.4% First line Notable exclusion criteria: nil |
|
| Interventions | Three‐arm trial; however only 1 comparison relevant for inclusion in this Cochrane Review ARM 1 Intervention: chemotherapy followed by goserelin 3.6 mg depot SC implants every 28 days for 18 months (beginning on day 28 of the sixth course of chemotherapy) Chemotherapy: CMF q28 days × 6 cycles ‐ Cyclophosphamide 100 mg/m² PO on D1 to 14 ‐ Methotrexate 40 mg/m² IV days 1 and 8 ‐ 5‐fluorouracil 600 mg/m² IV days 1 and 8 ARM 2 Comparator: chemotherapy (CMF) alone Mastectomy 43.1%, breast conservation with radiotherapy 51.8% |
|
| Outcomes | Primary outcomes: ‐ Disease‐free survival (DFS), defined as date of randomisation to any invasive breast cancer relapse (including ipsilateral or contralateral breast recurrence), appearance of second (non‐breast) malignancy, or death, whichever occurred first or was censored at date of last follow‐up ‐ Overall survival (OS), defined as date of randomisation to death from any cause ‐ Breast cancer‐free interval (BCFI), defined as date of randomisation to any invasive breast cancer relapse (including ipsilateral or contralateral breast recurrence) and censored at date of last follow‐up or date of death without relapse Secondary outcomes: ‐ Quality of life, assessed using the IBCSG QoL core questionnaire comprising self‐assessment on physical well‐being, mood, coping effort, appetite, tiredness, hot flashes, nausea/vomiting, perceived social support, restrictions in arm movement, and subjective health estimation in last 2 weeks ‐ Amenorrhoea ‐ Toxicity, as per modified World Health Organization toxicity grading criteria ‐ Treatment‐related death |
|
| Notes | Trial registration link could not be found
Trial authors were not contacted All patients analysed according to assigned treatment arm No estimations undertaken Funding considerations: supported by Ludwig Institute for Cancer Research, Cancer League of Ticino, Swedish Cancer Society, The Cancer Council Australia, Australian New Zealand Breast Cancer Trials Group (NHMRC grants), Frontier Science and Technology Research Foundation, Swiss Group for Clinical Cancer Research, Swiss Cancer League, and US National Institutes of Health |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the permuted blocks randomization schedule was produced by use of pseudorandom numbers generated by a congruence method" (p.1834, 2003 publication) |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization was conducted centrally (at the coordinating centers in Bern, Switzerland and Sydney, Australia" (p.1834, 2003 publication) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Most likely an open‐label study but not mentioned in trial publications. Reasonable clinical equipoise such as knowing treatment allocation was unlikely to affect the behaviour of clinicians and patients |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Lack of blinding unlikely to influence this outcome |
| Blinding of outcome assessment (detection bias) Disease‐free survival | Low risk | Lack of blinding unlikely to influence this outcome. Mammography was performed yearly |
| Blinding of outcome assessment (detection bias) Toxicity | Low risk | Toxicity outcomes collected according to WHO criteria; the study chair reviewed the records for all Grade 3 or worse toxicities. Clinical, haematological, and biochemical assessments were required every 3 months for the first year, every 6 months for the second year, and yearly thereafter |
| Blinding of outcome assessment (detection bias) Quality of life | Unclear risk | Measured through self‐reported/assessed outcomes using the IBSCG core questionnaire. It is unclear whether knowledge of treatment allocation would have influenced self‐assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis conducted |
| Selective reporting (reporting bias) | Unclear risk | Outcomes described in the methods section in the trial publication are reported on in the results section. We did not identify a trial record to cross‐check outcome reporting. For QoL outcomes, only means ± SEs for goserelin alone and chemotherapy alone provided; paper did not report numerical data (instead in graphs) for CMF followed by goserelin group (i.e. intervention of interest for this Review) |
| Other bias | Low risk | None identified |
SOFT.
| Methods | Accrual: December 2003 to January 2011 Multi‐centre Phase of trial: III RCT Country or countries where the trial was conducted: Australia, United States of America, Spain, Hungary, France, Italy, United Kingdom, Germany, Switzerland Median follow‐up: 8 years |
|
| Participants | 3066 participants were randomised, all premenopausal hormone‐positive women initially, as part of a 3‐arm randomisation Premenopausal was defined as oestradiol in the premenopausal range according to institutional parameters ‐ Unless no chemo AND regular menstruation in last 6 months before randomisation and not used any form of hormonal contraception or any other hormonal treatments during this time Age, range, median age: median 43 years ‐ < 35 years: 233 (11.5%), 35 to 39 years: 387 (19%), 40 to 49 years: 1224 (60.2%), ≥ 50 years: 189 (9.3%) Node negative: 1324 (61.5%), node positive 709 (34.9%) Tumour size ≤ 2 cm: 1332 (65.5%), > 2 cm 649 (31.9%) Grade 1 540 (26.6%), Grade 2 1006 (49.5%), Grade 3 439 (21.6%) HER2‐positive: 236 (11.6%) First line Notable exclusion criteria: Nil |
|
| Interventions | ARM 1
Intervention: OFS (triptorelin 3.75 mg q28 days for 5 years OR surgical oophorectomy OR ovarian irradiation) + tamoxifen 20 mg daily for 5 years ‐ 91% had GnRH analogue as initial method of OFS ARM 2 Comparator: tamoxifen 20 mg daily for 5 years Neo/adjuvant chemotherapy in 53% |
|
| Outcomes | Primary outcomes:
‐ Disease‐free survival: time from randomisation to first appearance of 1 of the following: invasive breast cancer recurrence and local, regional, or distant site, invasive contralateral breast cancer, second (non‐breast) invasive cancer, or death without cancer event, or censored at date of last follow‐up (prespecified analysis at 5 years) Secondary outcomes: ‐ Breast cancer‐free Interval: time from randomisation to invasive breast cancer recurrence at local, regional, or distant site, or invasive contralateral breast cancer; or censored at date of last follow‐up ‐ Distant recurrence‐free Interval: time from randomisation to invasive breast cancer recurrence at distant site, or invasive contralateral breast cancer; or censored at date of last follow‐up ‐ Overall survival: time from randomisation to death from any cause, or censored at date last known alive (prespecified analysis at 8 years) ‐ Toxicity ‐ Quality of life |
|
| Notes | Trial registration link: https://clinicaltrials.gov/ct2/show/NCT00066690 Trial authors were not contacted Most randomised patients included in intention‐to‐treat analysis – 2045 randomised, 12 withdrew consent, and 2033 were included in intention‐to‐treat population and analysis No estimations undertaken Funding considerations: study sponsor ‐ International Breast Cancer Study Group. Drugs donated by sponsors (Pfizer and Ipsen for exemestane and triptorelin) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation by (a) institution, (b) prior adjuvant/neoadjuvant chemotherapy, and (c) number of positive axillary and/or internal mammary lymph nodes (trial protocol provided in NEJM 2015) |
| Allocation concealment (selection bias) | Low risk | Internet system centralisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label study. Reasonable clinical equipoise such that knowing treatment allocation was unlikely to affect the behaviour of clinicians and patients |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Lack of blinding unlikely to influence this outcome |
| Blinding of outcome assessment (detection bias) Disease‐free survival | Low risk | Lack of blinding unlikely to influence this outcome. Patients were followed up every 3 months during 1 year, every 6 months during the next 5 years, and yearly thereafter |
| Blinding of outcome assessment (detection bias) Toxicity | Low risk | Toxicities measured using the standardised NCI Common Terminology Criteria for Adverse Events (CTCAE), version 3.0. It is possible that self‐reporting of some symptoms (e.g. fatigue) might be influenced by knowledge of treatment allocation but likely to have an immaterial effect on the grading of toxicity outcomes |
| Blinding of outcome assessment (detection bias) Quality of life | Unclear risk | Quality of life was measured using the International Breast Cancer Study Group (IBCSG) QoL core form and a symptom‐specific module at baseline, 6, 12, 18, and 24 months, then annually during 3 to 6 years. It is unclear whether knowledge of treatment allocation would have influenced self‐assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2045 were randomised, 12 withdrew consent, and 2033 were included in intention‐to‐treat population and analysis |
| Selective reporting (reporting bias) | Low risk | Outcomes reported in the clinical trial registry (NCT00066690) record have been reported on in multiple trial publications; results have also been uploaded into the clinical trial registry record |
| Other bias | Low risk | None identified |
SWOG 1996.
| Methods | Accrual: July 1979 to July 1989 Multi‐centre (trials group SWOG + ECOG group) Phase of trial: III (presumed, not explicitly stated) RCT Country or countries where the trial was conducted: United States of America (presumed) Median follow‐up: 7.7 years (maximum 13.2 years) |
|
| Participants | 288 participants randomised Hormone receptor status: ER+ in 100% Median age: 42 years (range 30 to 54) OFS; 44 years (24 to 59) control Premenopausal defined by clinical history, or based on FSH level when menopausal status in question Tumour size: ≤ 5 cm 89.9%; > 5 cm 10.1% Nodal status: positive 100%; 1 to 3 LN involved 43.8%, 4 to 6 LN involved 24.7%; ≥ 7 LN involved 31.6% First line Notable exclusion criteria: patients who had lumpectomy had to have lesions that were ≤ 5 cm, not diffuse, and not fixed to overlying skin |
|
| Interventions | ARM 1
Intervention: ovariectomy followed by CMFVP CMFVP ‐ Cyclophosphamide 60 mg/m² PO daily for 1 year ‐ Methotrexate 15 mg/m² IV weekly for 1 year ‐ 5‐FU 400 mg/m² IV weekly for 1 year ‐ Vincristine 0.625 mg/m² IV weekly for first 10 weeks ‐ Prednisone PO weeks 1 to 10 (decreasing dose 30 mg/m² to 2.5 mg/m²) ARM 2 Comparator: chemotherapy (CMFVP) Mastectomy 93.4%, lumpectomy 6.6% |
|
| Outcomes | Primary outcomes:
‐ Disease‐free survival: randomisation to first evidence of treatment failure, as defined by local, regional, or distant recurrence or death from any cause ‐ Overall survival: randomisation to time of death from any cause Secondary outcome: ‐ Toxicity: worst grade (SWOG criteria) experienced while on treatment |
|
| Notes | Trial registration link could not be found Trial authors were not contacted All patients analysed according to assigned treatment arm Unadjusted hazard ratio estimated for OS and DFS using Tierney et al’s method 7 Funding considerations: supported in part by Public Health Service Cooperative Agreements awarded by the National Cancer Institute, Department of Health and Human Services, USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Trial states patients were "randomized to receive 1 year of adjuvant CMFVP or ovariectomy followed by CMFVP for 1 year" p.47. Random sequence probably employed given trial conducted by ECOG |
| Allocation concealment (selection bias) | Unclear risk | No description whether randomisation was centralised |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label study: participants were unable to be blinded to ovarian function suppression due to nature of treatment (surgery). Reasonable clinical equipoise such that knowing treatment allocation was unlikely to affect the behaviour of clinicians and patients |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Lack of blinding unlikely to influence this outcome |
| Blinding of outcome assessment (detection bias) Disease‐free survival | Low risk | Lack of blinding unlikely to influence this outcome. Patients were followed up every 3 months, with chest X‐ray every 6 months, bone scan and mammogram annually, and after 5 years, patients were examined twice yearly |
| Blinding of outcome assessment (detection bias) Toxicity | Low risk | Toxicity outcomes reported appear to be chemotherapy‐related toxicity. During chemotherapy, patients were monitored weekly with blood tests. Some of the toxicity outcomes were more objective (i.e. severe lung problems, granulocytopenia) and others less so (e.g. fatigue). Unblinding may influence patient‐reported outcomes such as fatigue, but these outcomes were not viewed to be critical to studies on OFS |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No CONSORT diagram presented; however, for efficacy outcomes, no missing data are apparent. For toxicity outcomes, the number of patients who did not receive treatment or was not evaluated was provided (n = 19), but no details on the split of non‐evaluable patients in each group were provided |
| Selective reporting (reporting bias) | Low risk | Outcomes described in the methods section the trial publication are reported (i.e. DFS, OS, and toxicity as pre‐stated) |
| Other bias | Low risk | None identified |
Uslu 2014.
| Methods | Accrual: April 2003 to January 2011 Multi‐centre/single centre: NR Phase of trial: III (presumed, not explicitly stated) RCT Country or countries where the trial was conducted: Turkey Median follow‐up: 57 months (mean follow‐up 52.4 ± 2.8 months) |
|
| Participants | 101 participants randomised Hormone receptor status: ER+ and/or PR+ 100% ‐ER+/PR+ 80% (81/101); greater number of ER+ tumours in OFS group compared to control group (P = 0.01) Mean age: 47.4 years; participants younger in the OFS group compared to the control group (P = 0.04) Premenopausal status defined as cessation of cycles for at least 6 months with relevant luteinising hormone and oestradiol levels measured in the last 2 consecutive months Mean tumour size: 3.46 cm Node positive: 100%; mean no. of nodes involved: 7.5 LN Mean histological grade: Grade 2 tumours First line Notable exclusion criteria: neoadjuvant chemotherapy for present tumour |
|
| Interventions | ARM 1
Intervention: goserelin 3.6 mg SC q4 weeks for 2 years + chemotherapy + tamoxifen 20 mg daily for 5 years + chemotherapy options, given q3weekly × 6 cycles with G‐CSF D1‐5 ‐ Docetaxel 75 mg/m², doxorubicin 50 mg/m², cyclophosphamide 500 mg/m² (TAC) ‐ Docetaxel 75 mg/m², epirubicin 75 mg/m², cyclophosphamide 500 mg/m² (TEC) ARM 2 Comparator: chemotherapy + tamoxifen 20 mg daily for 5 years, or until menopause In the comparator arm, if women became menopausal while on treatment, their treatment was switched to anastrozole or letrozole to complete the remaining 5 years Radiotherapy 98% |
|
| Outcomes | Primary outcomes: ‐ Disease‐free survival, defined as the period of time from allocation to the occurrence of relapse locally, in the contralateral breast, or in a remote organ Secondary outcome: ‐ Overall survival (not explicitly stated as secondary outcome) |
|
| Notes | Trial registration link could not be found
Trial authors were contacted on 9 August 2019 to ask for details about the numbers of deaths in both treatment groups and the 95% confidence interval for overall survival and disease‐free survival, and for clarification on the median follow‐up
All patients analysed according to assigned treatment arm
No estimations undertaken Funding considerations: none listed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "parallel‑group method was used for randomization in which participants were arbitrarily allocated" (p.582); however no further details about the method of random sequence generation provided. Baseline characteristics were not balanced in particular for age (OFS group were younger than control group) and ER ‐positivity (higher number of participants with ER+ tumours in OFS group than in control group) |
| Allocation concealment (selection bias) | Low risk | Quote: "none of the investigators in this study participated in the assignment procedure. The allocation and randomization was performed by a third party and the participants did not obtain any information about the type of hormonal intervention until it was begun" (p.532) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label study. Reasonable clinical equipoise such that knowing treatment allocation was unlikely to affect the behaviour of clinicians and patients |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Lack of blinding unlikely to influence this outcome |
| Blinding of outcome assessment (detection bias) Disease‐free survival | Low risk | Lack of blinding unlikely to influence this outcome. No details of follow‐up assessments were reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "After randomization, no patient was lost to follow‑up or excluded due to severe side‑effects of treatment or non‑compliance" p.584 |
| Selective reporting (reporting bias) | Low risk | Reported all measures as identified (DFS main outcome) |
| Other bias | Low risk | None identified |
Yang 2013.
| Methods | Accrual: 22 June 2008 to 31 December 2009 Single‐centre Phase of trial: III (presumed, not explicitly stated) RCT Country or countries where the trial was conducted: China Median follow‐up: 72 months |
|
| Participants | 110 participants randomised Hormone receptor status: ER+ and/or PR+ 100% ‐ER+ 93%, PR+ 85% Median age: 42.4 years (OFS), 42.5 (control) Premenopausal defined as last menstruation < 6 months before trial entry; temporary chemotherapy‐induced amenorrhoea allowed if oestradiol level confirmed within 8 months before the final dose of chemotherapy Mean tumour size: 2.6 cm, tumours larger in OFS than in control (P = 0.017) Nodal status: mean number of involved nodes 1.8, more nodal involvement in OFS group than in control group (P = 0.04); percentage of participants who had node‐positive tumours was not reported Grade: Grade 1 10.9%, Grade 2 58.2%, Grade 3 8.2%, unknown 22.7% First line Notable exclusion criteria: nil |
|
| Interventions | ARM 1
Intervention: goserelin 3.6 mg q28days for 1.5 years plus tamoxifen 10 mg BD for 5 years ARM 2 Comparator: tamoxifen 10 mg BD for 5 years Modified radical surgery 91.8%, breast‐conserving surgery 6.4%, radical excision 1%, lumpectomy 1% Chemotherapy: 88.2% Radiotherapy: 42.7% |
|
| Outcomes | Primary outcomes: ‐ Mammographic breast density (as recorded in clinicaltrials.gov record) Secondary outcomes: ‐ Oestradiol level (as recorded in clinicaltrials.gov record) ‐ Ultrasonic endometrial thickness ‐ Blood lipid levels ‐ Disease‐free survival, referred to as a secondary endpoint in the conference abstract ‐ Overall survival; however later referred to as the primary endpoint in conference abstract ‐ Toxicity using criteria of the Eastern Cooperative Oncology Group (ECOG) |
|
| Notes | Trial registration link: https://clinicaltrials.gov/ct2/show/NCT00827307 Study authors contacted on 9 August 2019 for details about the numbers of deaths and DFS‐related events in both treatment groups, 95% confidence intervals for both overall survival and disease‐free survival, and toxicity data Confidence interval for overall survival and disease‐free survival estimated using hazard ratio and P value Not all patients analysed according to assigned treatment arm ‐ randomised 56 OFS, 54 control ‐ Analysed 51 OFS and 52 control for original paper (Yang 2013) ‐ Analysed 47 OFS and 44 control reported in abstracts 2016 due to dropout No estimations undertaken Funding considerations: AstraZeneca, the Chinese Anti‐Cancer Association, National Institutes of Health through MD Anderson’s Cancer Center Support Grant CA016672 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomised after enrolment into this study. Treatment allocation was based on the permuted block technique" p.583 |
| Allocation concealment (selection bias) | Unclear risk | No description whether randomisation was centralised |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label study. Reasonable clinical equipoise such that knowing treatment allocation was unlikely to affect the behaviour of clinicians and patients |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Lack of blinding unlikely to influence this outcome |
| Blinding of outcome assessment (detection bias) Disease‐free survival | Low risk | Patients were followed up at 3, 6, 12, and 18 months; underwent contralateral mammography at 12 and 18 months after endocrine treatment; routine haematological and clinical chemistry measurements conducted at each follow‐up visit. Lack of blinding unlikely to influence assessment of this outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Originally randomised 56 to OFS and 54 to control; "Because 19 participants dropped out, 47 patients in group A [OFS] and 44 patients in group B [control] were included in the final analysis" Intention‐to‐treat analysis not conducted |
| Selective reporting (reporting bias) | High risk | Not all outcomes collected (i.e. toxicities) reported in the publication. Added OS/DFS as outcomes (not in NCT record or original paper) in later publications and re‐assigned as primary or secondary endpoints |
| Other bias | Low risk | None identified |
Yi 2016.
| Methods | Accrual: January 2011 to December 2014 Single‐centre Phase of trial: not reported RCT Country or countries where trial was conducted: South Korea Median follow‐up: patients followed up for 12 months, no further details provided |
|
| Participants | 64 participants randomised Hormone receptor status: ER+ and/or PR+100% Premenopausal definition not reported Mean age: 44.86 years; 35 to 39 years: 6.25%, 40 to 49 years: 93.8% Mean tumour size: 1.06 cm Node status: not reported First line Notable exclusion criteria: nil |
|
| Interventions | ARM 1
Intervention: tamoxifen and goserelin for 12 months* ARM 2 Comparator: tamoxifen for 12 months* *No details of administered dose or co‐interventions were reported |
|
| Outcomes | Primary outcomes: ‐ Hamilton Depression Rating Scale (HAM‐D), from baseline to 12 months ‐ Hamilton Anxiety Rating Scale (HAM‐A), from baseline to 12 months Secondary outcomes: ‐ Beck Depression Inventory (BDI), 21‐item, self‐reported questionnaire to assess frequency of depressive symptoms over a 1‐week period; Korean version ‐ Albany Panic and Phobia Questionnaire (APPQ) ‐ Anxiety Sensitivity Index‐Revised (ASI‐R), Korean version ‐ Mood Disorder Questionnaire (MDQ) ‐ Hypomania Checklist (HCL‐32), measures severity of previous hypomanic episodes |
|
| Notes | Trial registration link could not be found Trial authors were not contacted All randomised patients included in intention‐to‐treat analysis No estimated undertaken Funding considerations: supported by the Institute for Information and communication Technology Promotion (IITP) grant funded by government (MSIP) and Samsung Medical Clinical Research Development Program, Samsung Medical Center Grant, Korea Health Technology R& D project (Ministry of Health and Welfare) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "eligible patients were randomized using a 1:1 allocation to one of the two treatment groups" (p.492); no further details on method of sequence generation reported. "No significant differences for age, tumor grade, body mass index, or family history were found at baseline between the two groups" |
| Allocation concealment (selection bias) | Unclear risk | No description whether randomisation was centralised |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Presumably an open‐label study. Reasonable clinical equipoise such that knowing treatment allocation was unlikely to affect the behaviour of clinicians and patients |
| Blinding of outcome assessment (detection bias) Toxicity | Unclear risk | Baseline psychological evaluation of participants was conducted by a trained psychologist blinded to psychiatrists' assessments. Depression, anxiety, mood states were evaluated using validated questionnaires (including Korean versions) and were self‐reported by participants. Therefore it is unclear whether knowing treatment allocation would have influenced the reporting of mood‐related levels by participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat likely and data did not appear to be missing |
| Selective reporting (reporting bias) | Low risk | All outcome measures related to baseline; 6‐ and 12‐month measures for anxiety, depression, and mood were reported. Outcomes specified in the methods section of the publication were reported on in the results section |
| Other bias | Low risk | None identified |
ZBCSG Trial B.
| Methods | Accrual: March 1994 to July 1998 Multi‐centre: 79 sites across Japan Phase of trial: not reported RCT Country or countries where the trial was conducted: Japan Median follow‐up: no details reported |
|
| Participants | 209 participants randomised
Oestrogen receptor‐positive: 100%
Characteristics of participants were provided for the 2 treatment arms and not for the treatment arm of interest in this Cochrane Review (i.e. goserelin + tamoxifen). In the 2 treatment arms, participants had the following characteristics ‐ Age: < 35 years: 5.8%, 36 to 40 years: 13.4%, 41 to 45 years: 35.8%, 46 to 50 years: 44.9% ‐ Premenopausal defined as women who have a regular menstrual cycle before menopause ‐ Participants were lymph node‐positive or had a tumour size > 3 cm and lymph node‐negative. No further details provided for OFS group (i.e. goserelin + tamoxifen); however details were provided for the tamoxifen alone group: node status: 74.5% positive (1 to 3 nodes: 60.6%, > 4: 13.8%); tumour size: 25.5% had tumours > 3 cm and no lymph node metastases Tumour stage: I 17.1%, II 73.3%, IIIa 9.6% First‐line Notable exclusion criteria: none |
|
| Interventions | Three‐arm trial; however only 1 comparison relevant for inclusion in this Cochrane Review ARM 1 Intervention: goserelin 3.6 mg depot, subcutaneous, every 4 weeks for 2 years + tamoxifen 10 mg 2 tablets per day or 20 mg 1 tablet per day by mouth, every day for 2 years ARM 2 Comparator: tamoxifen 10 mg 2 tablets per day or 20 mg 1 tablet per day by mouth, every day for 2 years Treatment arms received similar co‐interventions: no details reported There was no description of the types of surgery received |
|
| Outcomes | Primary outcomes (as listed in the trial publication): ‐ Disease‐free survival, definition not provided ‐ Relapse‐free survival, definition not provided ‐ Overall survival, definition not provided Secondary outcomes: ‐ Safety: side effects; hot flushes/menopausal symptoms (fatigue, sweating, weight gain, headache), sexual function/vaginal dryness (menstrual abnormalities and amenorrhoea), and other toxicity (increase in γ‐GTP, GOT, GPT, LDH, alkaline phosphatase, oestrogen, hypercholesterolaemia, hypertriglyceridaemia, anorexia) ‐ Quality of life: assessed by daily life, appetite, sleep, mental aspect, economic aspect, menopausal symptoms, physiological condition For the intervention (goserelin + tamoxifen) of interest in this review, the study did not report the primary outcomes. Side effects were reported |
|
| Notes | Trial registration link: http://www.pieronline.jp/content/article/0385‐0684/32130/2071 (Japanese)
209 randomised participants and 207 participants included in the analysis
Trial authors were not contacted Funding considerations: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were allocated to treatment groups using a dynamic allocation method (that took into account balancing randomisations between facilities; p.2072). The exact procedure of dynamic allocation method was not reported, and the number of participants in each treatment group did not match a clean 1:3:3 randomisation ratio |
| Allocation concealment (selection bias) | Unclear risk | No description whether randomisation was centralised |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Presumably an open‐label study. Reasonable clinical equipoise such that knowing treatment allocation was unlikely to affect the behaviour of clinicians and patients |
| Blinding of outcome assessment (detection bias) Toxicity | Unclear risk | Toxicities did not appear to be measured using a validated questionnaire. Some of the toxicities reported (i.e. sweats, headache) were patient reported. There may have been some influence of knowing treatment allocation on self‐reporting of side effects |
| Blinding of outcome assessment (detection bias) Quality of life | Unclear risk | Quality of life did not appear to be assessed using a validated questionnaire based on information provided by the translators. QoL was assessed through daily activities, appetite, sleep, mental, economic, climacteric symptoms, and physiological conditions; however no definition or further details were provided regarding these outcomes. There may have been some influence of knowing treatment allocation on self‐reporting of these quality of life measures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There did not appear to be any concern with missing outcome data. Two out of 109 participants dropped out |
| Selective reporting (reporting bias) | Unclear risk | There did not appear to be a study protocol to allow an assessment of whether all outcomes were reported as expected |
| Other bias | Low risk | None identified |
ZIPP.
| Methods | Accrual: 27 August 1987 to 22 March 1999 Multi‐centre, planned as a prospective meta‐analysis (i.e. outcomes collected, study design, methods of analysis, etc were all pre‐planned) Phase of trial: III (presumed but not explicitly stated) RCT Country or countries where the trial was conducted: United Kingdom, Italy, Sweden, Belgium, Poland Median follow‐up: 12 years |
|
| Participants | 2710 participants were randomised
Hormone receptor status: ER+ in 51% Median age 44 years, range 22 to 56; ≤ 39 years 21%, > 40 years 79% Premenopausal criteria in CRUK/Stockholm trials only, no definition in CRUK provided. In Stockholm, defined as last menstruation < 6 months from start of the study. SE Sweden/GIVIO did not specify premenopausal status Tumour size ≤ 10 mm 11%, 11 to 20 mm 45%, 21 to 50 mm 33%, > 50 mm 2%, unknown 9% Nodal status: positive 53% First line Notable exclusion criteria: no bilateral breast tumours |
|
| Interventions | A 2 × 2 factorial trial ARM 1 Intervention: Two relevant arms ‐ Goserelin 3.6 mg q4weekly ± elective tamoxifen 20 mg or 40 mg for 2 years ‐ Goserelin 3.6 mg q4weekly + (randomised) tamoxifen 20 mg or 40 mg daily for 2 years ARM 2 Comparator: Two relevant arms ‐ Observation ± elective tamoxifen ‐ Tamoxifen 20 mg or 40 mg daily for 2 years Of total population: Mastectomy 49%, local excision 50%, unknown 1% Radiotherapy 60% Chemotherapy 43% |
|
| Outcomes | Primary outcomes: ‐ Event‐free survival, defined as the interval from randomisation to the date of first confirmed recurrence (local or distant), second primary cancer, or death. If none of these occurred, EFS was censored at the date of last follow‐up ‐ Overall survival, defined as the interval from randomisation to the date of death Secondary outcomes: ‐ Risk of recurrence ‐ Risk of dying from breast cancer ‐ Mood and sexual function |
|
| Notes | Trial registration link CRC‐PHASE‐III‐88002, UKM‐CRC‐BR‐UNDER‐50 No trial authors contacted All eligible, randomised patients included in analysis (n = 2706); number of patients randomised was 2710 but 4 ineligible patients were not included in intention‐to‐treat analysis No estimations undertaken Funding considerations: free drug supplied by ICI (now AstraZeneca) for CRUK BCTG and GIVIO trials, and payment for IHC testing for ER in CRUK. Grant from CRUK used in UK. AstraZeneca educational grant in Italy. King Gustaf V Jubilee Fund and unrestricted research grant from AstraZeneca in Stockholm |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were enrolled in a 2 × 2 factorial randomisation trial; randomisation was stratified in 3 groups based on nodal status (i.e. node negative, 1 to 3 positive nodes, and 4 or more) and use of other adjuvant therapies. Treatment allocation was based on balanced lists using the permuted block technique. Therefore considered to be at low risk |
| Allocation concealment (selection bias) | Low risk | Randomisation carried out at a central office where investigators would telephone the office and patient identifiers were recorded before allocation of treatment was revealed to the investigator. Therefore presumed that central allocation occurred |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label study. Reasonable clinical equipoise such that knowing treatment allocation was unlikely to affect the behaviour of clinicians and patients |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Lack of blinding unlikely to influence this outcome |
| Blinding of outcome assessment (detection bias) Disease‐free survival | Low risk | Lack of blinding unlikely to influence this outcome |
| Blinding of outcome assessment (detection bias) Toxicity | Unclear risk | Based on self‐reporting of hot flushes and weight gain. No further details provided in the trial publications. It is possible that self‐reported outcomes (such as hot flashes) may be influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A slightly modified intention‐to‐treat analysis likely based on eligible patients (2706); no. of patients randomised was 2710. Baseline characteristics of randomised and analysed patients were similar as reported in Table p.901 |
| Selective reporting (reporting bias) | Low risk | Outcomes described in the methods reported on in the results ‐ especially primary outcomes reported. Data related to HR+ as a subgroup provided less information |
| Other bias | Low risk | None identified |
BD: twice a day. CAF: cyclophosphamide, doxorubicin, fluorouracil. CMF: cyclophosphamide, methotrexate, fluorouracil. CMFVP: cyclophosphamide, methotrexate, fluorouracil, vincristine, prednisone. DFS: disease‐free survival. EFS: event‐free survival. EORTC QLQ‐C30: European Organization for Research and Treatment of Cancer core quality of life questionnaire. ER: oestrogen receptor. FSH: follicle‐stimulating hormone. GnRH: gonadotropin‐releasing hormone. GOT: aspartate aminotransferase. GPT: alanine aminotransferase. GTP: gamma‐glutamyl transferase. HRQoL: health‐related quality of life. IM: intramuscular. ITT: intention‐to‐treat. IV: intravenous. LDH: lactic acid dehydrogenase. LHRH: luteinising hormone releasing hormone. LN: lymph node. mITT: modified intention‐to‐treat. N: nodal status. OFS: ovarian function suppression. OS: overall survival. PR: progesterone receptor. RCT: randomised controlled trial. RFS: recurrence‐free survival. SAE: serious adverse event. SC: subcutaneous. SD: standard deviation. SWOG: Southwest Oncology Group. T: tumour size. TAC: docetaxel, doxorubicin, cyclophosphamide. TEC: docetaxel, epirubicin, cyclophosphamide. TTR: time to recurrence.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ABCSG 5 | Incorrect comparator. Comparator arm did not contain the same treatment as intervention arm but without OFS |
| ABCSG‐12 | Incorrect comparator. Goserelin was administered in all treatment groups |
| Baum 1996 | Unclear whether study meets the eligibility criteria for this review. The PDQ record was registered in 1996, and no further details about this potential study have been published since |
| FASG 06 | Incorrect comparator. Comparator arm did not provide the same treatment as the intervention arm but without OFS |
| GABG IV‐A‐93 | Incorrect comparator. Comparator arm did not provide the same treatment as the intervention arm but without OFS |
| Grocta 02 | Incorrect comparator. Comparator arm did not provide the same treatment as the intervention arm but without OFS |
| HMFEC | Hormone therapy could include LHRH or tamoxifen. Trial results were not reported separately for LHRH (in main publication or in appendices) |
| Li 2019 | Incorrect comparator. Comparator arm did not provide the same treatment as the intervention arm but without OFS |
| MAM 01 GOCSI | Incorrect comparator. Comparator arm did not provide the same treatment as the intervention arm but without OFS |
| Manson 2019 | Incorrect population. Patients were stratified by oophorectomy status and were not randomised to OFS or no OFS |
| PERCHE | OFS provided in both treatment arms |
| Pretoria | Does not measure hormone status and does not report outcomes by hormone status separately |
| Ragaz 1997 | Co‐interventions are not similar across treatment arms (i.e. 1 treatment arm received radiotherapy during randomisation, while the other treatment arm did not) |
| Soreide 2002 | Incorrect comparator. Comparator arm did not provide the same treatment as the intervention arm but without OFS |
| TABLE | Incorrect comparator. Comparator arm did not provide the same treatment as the intervention arm but without OFS |
| UKCCR | This reference was reported in the original Cochrane Review; no further details have been reported since 2005. Therefore we have excluded the record from this review update |
| Yu 2019 | Incorrect comparator. All treatment arms received leuprorelin |
| ZEBRA | Incorrect comparator. Comparator arm did not provide the same treatment as the intervention arm but without OFS |
LHRH: luteinising hormone releasing hormone. OFS: ovarian function suppression.
Characteristics of ongoing studies [ordered by study ID]
NCT02132390.
| Trial name or title | Adjuvant toremifene with or without goserelin in premenopausal women with stage I‐IIIA, hormonal receptor positive breast cancer accompanied with or without chemotherapy induced amenorrhoea |
| Methods | Accrual: not yet recruiting Accrual target: 300 participants Single‐centre Phase of trial: presumably phase III Country or countries where the trial is being conducted: China Any intended follow‐up details: for 5 years and annually thereafter State study design: RCT Blinding: participant, care provider, investigator, outcome assessor |
| Participants | Premenopausal women
Stage I to IIIA hormone receptor‐positive breast cancer With or without chemotherapy‐induced amenorrhoea |
| Interventions | ARM 1 Intervention: toremifene and goserelin (in participants with or without chemotherapy‐induced amenorrhoea) ARM 2 Comparator: toremifene (in participants with or without chemotherapy‐induced amenorrhoea) |
| Outcomes | Primary outcomes:
‐ Disease‐free survival
‐ Overall survival Secondary outcomes: ‐ Quality of life ‐ Bone mineral density loss ‐ Hormone levels ‐ Incidence of pregnancy |
| Starting date | Planned start date: May 2014 (although still not recruiting according to NCT record) Estimated completion date: May 2024 |
| Contact information | Contact: Yidong Zhou: wcj_sumy@126.com; |
| Notes | Trial registration link: clinicaltrials.gov/ct2/show/NCT02132390
Sponsor of the trial: Peking Union Medical College Hospital Funding considerations: not provided in trial record |
RCT: randomised controlled trial
Differences between protocol and review
The specific objectives outlined in this Review address different comparisons than those in previous versions of this Review (Goel 2009). The decision to include the comparisons listed in this version of the Review was made on the basis that the general objective was to assess the role of LHRH agonists as a method of ovarian function suppression in adjuvant therapy for premenopausal women with breast cancer in general, and not specifically in certain combinations or compared with certain therapies. No evidence suggests that any form of ovarian function suppression is clearly superior to another, which is why the intervention was broadened. Minimum criterion of 12 months' duration of ovarian function suppression was stipulated to exclude studies in which the primary outcomes were focused on fertility endpoints, which was outside the scope of this Review.
The specific differences and justification for changes between the previous review version ‐ Goel 2009 ‐ and the 2019 review update are listed below.
The background section has been modified to include information specifically related to each subheading and the breadth of the intervention being considered in this review update. The Background section now sets the scene for reviewing evidence on all methods of ovarian function suppression and not solely on the role of LHRH agonists
The objectives were broadened to reflect current clinical questions, which involved assessing the evidence on any method of ovarian suppression function as adjuvant therapy in hormone‐positive early breast cancer rather than one specific method of ovarian suppression function (i.e. LHRH agonists), as was the case in the previous review version (Goel 2009). Outcomes such as compliance with treatment were added to reflect current clinical questions
A number of treatment comparisons that were part of the previous review version ‐ Goel 2009 ‐ were removed from the current review because they have been extensively covered by an individual participant data (IPD) analysis (Cuzick 2007), and duplication of an IPD was not warranted. This meant that a number of eligible studies in the previous review version have been transferred to the excluded studies section due to the revised eligibility criteria of the current review
The methods section has been substantially updated to comply with Cochrane MECIR standards. This included, but was not limited to, removing the MERGE criteria for assessing the quality of studies; adding Cochrane's risk of bias tool and the GRADE approach for the most important outcomes; and splitting types of outcomes into primary and secondary
Contributions of authors
For previous versions of this review: RS designed the review and wrote the original protocol. All other review authors commented on the design and content of the protocol and the conduct of the review. SG wrote the current version of the review and approved the final review.
For the 2019 updated review:
Screening studies and retrieving papers: TB, AG. Conducting risk of bias assessments: TB, MW, AG. Extracting data: TB, MW. Entering data into Review Manager: TB. Analysing and interpreting data: TB, MW, AG. Providing clinical oversight: TB, AG.
Sources of support
Internal sources
No sources of support supplied
External sources
This project is funded by the National Institute for Health Research (NIHR) Cochrane Incentive Award 2018 (NIHR 128381). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care, UK.
Declarations of interest
Tam Bui: none known. Received funding to travel to the annual scientific meetings in 2019 (Australian and New Zealand Urogenital and Prostate Cancer Trials Group and Clinical Oncology Society of Australia) and in 2018 (Medical Oncology Group of Australia) to present PhD work on 'scanxiety: scan‐associated anxiety in people with cancer'. Payments did not support the design, conduct, or reporting of this research. Melina Willson: none known. Shom Goel: none related to this review. Shom has not received any payments from pharmaceutical companies directly related to this research project. Shom has received payments from Eli Lilly, Novartis, and G1 Therapeutics for serving on advisory boards for unrelated matters, and has received funding paid to his institution for laboratory and clinical research (in research areas unrelated to this Cochrane Review) from Eli Lilly and Novartis. Jane Beith: none related to this review. Jane received funding to travel to conferences from Roche and Novartis. Jane has served on advisory boards for Pfizer, Roche, Novartis, Lilly and Specialised Therapeutics for unrelated matters and payment has been made to her institution. Annabel Goodwin: none related to this review. There is no competing interest associated with funding of travel to attend a national and international educational meeting, or to provide expertise regarding Cancer Genetic counselling in Australia. Annabel has served on advisory boards for Pfizer in August 2019 and for AstraZeneca in 2018 for unrelated matters.
New
References
References to studies included in this review
ABCTCG {published data only}
- Adjuvant Breast Cancer Trials Collaborative Group. Ovarian ablation or suppression in premenopausal early breast cancer: results from the International Adjuvant Breast Cancer Ovarian Ablation or Suppression Randomized Trial. Journal of the National Cancer Institute 2007;99(7):516‐25. [DOI] [PubMed] [Google Scholar]
- Anonymous. Phase III randomised study of adjuvant therapy with tamoxifen vs endocrine ablation vs tamoxifen plus endocrine ablation vs neoadjuvant therapy in patients under 50 with operable breast cancer. UKCCCR National Register of Cancer Trials 1997.
- Brunt AM, Bliss JM, Benghiat A, Dawson C, Dewar J, Harnett AN, et al. The impact on quality of life of adding chemotherapy (CT) or ovarian suppression (OS) to adjuvant tamoxifen (TAM): outcomes from the UK NCRI Adjuvant Breast Cancer (ABC) trial. Journal of Clinical Oncology 2004;22(14 Suppl):8015. [Google Scholar]
- Brunt AM, Bliss JM, Johnson L, Lawrence D, Yarnold J. Results from the UK NCRI adjuvant breast cancer (ABC) international trial: polychemotherapy and ovarian ablation in women with early breast cancer prescribed 5 years tamoxifen. British Journal of Cancer 2004;91(Suppl 1):S1. [Google Scholar]
- George WD. Phase III randomised study of adjuvant tamoxifen with or without ovarian suppression and/or cyclophosphamide/methotrexate/fluorouracil (CMF) in premenopausal women with operable invasive breast cancer. Physician Data Query (PDQ) 1994.
- NCT00002582. Tamoxifen, ovarian ablation, and/or chemotherapy in treating women with stage 1, stage II or stage IIIA breast cancer. clinicaltrials.gov/show/NCT00002582, 8 July 2003.
- Yarnold JR. Phase III randomised study of adjuvant tamoxifen, ovarian suppression, and/or chemotherapy in women with T1‐3a, N0‐1, M0 breast cancer. Physician Data Query (PDQ) 1995.
- Yarnold JR, Bliss JM, Earl H, George D, Lawrence D, Mortazavi SH, et al. Ovarian ablation (OA) in pre‐menopausal women with early breast cancer prescribed 5 years tamoxifen (T) or T plus chemotherapy (CT) ‐ results from the UK NCRI Adjuvant Breast Cancer (ABC) international trial of 2,144 patients. American Society of Oncology 2004;22(14 Suppl):Abstract no. 535. [Google Scholar]
Arriagada 2005 {published data only}
- Arriagada R. Phase III randomised study of ovarian suppression in premenopausal women receiving adjuvant chemotherapy for poor‐prognosis nonmetastatic breast cancer. Physician Data Query (PDQ) 1995.
- Arriagada R, Le MG, Spielmann M, Mauriac L, Bonneterre J, Namer M, et al. Randomized trial of adjuvant ovarian suppression in 926 premenopausal patients with early breast cancer treated with adjuvant chemotherapy. American Society of Clinical Oncology 2003;22:no.4. [DOI] [PubMed] [Google Scholar]
- Arriagada R, Lê MG, Spielmann M, Mauriac L, Bonneterre J, Namer M, et al. Randomized trial of adjuvant ovarian suppression in 926 pre‐menopausal patients with early breast cancer treated with adjuvant chemotherapy. Annals of Oncology 2005;16(3):389‐96. [DOI] [PubMed] [Google Scholar]
ASTRRA {published data only}
- Kim HA, Ahn SH, Nam SJ, Park S, Ro J, Im SA, et al. The role of the addition of ovarian suppression to tamoxifen in young women with hormone‐sensitive breast cancer who remain premenopausal or regain menstruation after chemotherapy (ASTRRA): study protocol for a randomized controlled trial and progress. BMC Cancer 2016;16(319):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HA, Lee JW, Nam SJ, Park BW, Im SA, Lee ES, et al. Adding ovarian suppression to tamoxifen for premenopausal breast cancer: a randomized phase III trial. Journal of Clinical Oncology 2019;37:1‐10. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Ahn SH, Nam SJ, Park SH, Ro JS, Im SA, et al. Time course of changes in serum FSH, serum estradiol, and menstruation in premenopausal patients with breast cancer taking tamoxifen after completing chemotherapy: a report from the ASTRRA study. San Antonio Bresat Cancer Symposium 2018;79(4 Suppl 1):Abstract no. P4‐14‐04. [Google Scholar]
- NCT00912548. Evaluating the role of the addition of ovarian function suppression (OFS) to tamoxifen in young women (ASTRRA). clinicaltrials.gov/show/NCT00912548, 3 June 2009.
- Noh WC, Lee JW, Nam SJ, Park S, Im SA, Lee ES, et al. Role of adding ovarian function suppression to tamoxifen in young women with hormone‐sensitive breast cancer who remain premenopausal or resume menstruation after chemotherapy: the ASTRRA study. American Society of Oncology (ASCO) 2018;36(15 Suppl):Abstract no. 502. [Google Scholar]
E‐3193, INT‐0142 {published data only}
- Robert NJ, Wang M, Cella D, Martino S, Tripathy D, Ingle J, et al. Phase III comparison of tamoxifen versus tamoxifen and ovarian ablation in premenopausal women with axillary node‐negative receptor=positive breast cancer < 3cm. Proceedings of the American Society of Clinical Oncology 2003;22:5a. [Google Scholar]
- Tevaarwerk AJ, Wang M, Zhao F, Fetting JH, Cella D, Wagner LI, et al. Phase III comparison of tamoxifen versus tamoxifen plus ovarian function suppression in premenopausal women with node‐negative, hormone receptor‐positive breast cancer (E‐3193, INT‐0142): a trial of the Eastern Cooperative Oncology Group. Journal of Clinical Oncology 2014;32(35):3948‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
ECOG 5188, INT‐0101 {published data only}
- Davidson N, O’Neil A, Vukov A. Effect of chemohormonal therapy in pre‐menopausal, node (+), receptor (+) breast cancer: an Eastern Cooperative Oncology Group phase III Intergroup trial (E5188, INT‐0101). Proceedings of the American Society of Clinical Oncology. 2003; Vol. 5:Abstract 15.
- Davidson NE, O'Neill AM, Vukov AM, Osborne K, Martino S, White DR, et al. Chemoendocrine therapy for premenopausal women with axillary lymph node–positive, steroid hormone receptor–positive breast cancer: results from INT 0101 (E5188). Journal of Clinical Oncology 2005;23(25):5973‐82. [DOI] [PubMed] [Google Scholar]
GABG IV‐B‐93 {published data only}
- Kaufmann M, Graf E, Jonat W, Eiermann W, Vescia S, Geberthf M, et al. for the German Adjuvant Breast Cancer Study Group (GABG). A randomised trial of goserelin versus control after adjuvant, risk‐adapted chemotherapy in premenopausal patients with primary breast cancer – GABG‐IV B‐93. European Journal of Cancer 2007;43(16):2351‐8. [DOI] [PubMed] [Google Scholar]
- Kaufmann M, Graf E, Jonat W, Eiermann W, Zippel HH, Geberth M. Goserelin versus control after adjuvant, risk adapted chemotherapy in premenopausal women with breast cancer. GABG trial IV‐B‐93. Journal of Clinical Oncology 2004;22(14 Suppl):588. [Google Scholar]
IBCSG II {published data only}
- Castiglione‐Gertsch M, Johnsen C, Goldhirsch A, Gelber R D, Rudenstam CM, Collins J, et al. The International (Ludwig) Breast Cancer Study Group Trials I‐IV: 15 years follow‐up. Annals of Oncology 1994;5(8):717‐24. [DOI] [PubMed] [Google Scholar]
- International Breast Cancer Study Group. Late effects of adjuvant oophorectomy and chemotherapy upon premenopausal breast cancer patients. Annals of Oncology 1990;1(1):30‐5. [PubMed] [Google Scholar]
- Ludwig Breast Cancer Study Group. Chemotherapy with or without oophorectomy in high‐risk premenopausal patients with operable breast cancer. Journal of Clinical Oncology 1985;3(8):1059‐67. [DOI] [PubMed] [Google Scholar]
IBCSG VIII {published data only}
- Bernhard J, Zahrieh D, Castiglione‐Gertsch M, Hurny C, Gelber RD, Forbes JF, et al. Adjuvant chemotherapy followed by goserelin compared with either modality alone: the impact on amenorrhea, hot flashes, and quality of life in premenopausal patients ‐ the International Breast Cancer Study Group Trial VIII. Journal of Clinical Oncology 2007;25(3):263‐70. [DOI] [PubMed] [Google Scholar]
- Castiglione‐Gertsch M, O’Neill A, Gelber RD, et al. Is the addition of adjuvant chemotherapy always necessary in node negative (N‐) pre/perimenopausal breast cancer patients (pts) who receive goserelin? First results of IBCSG trial VIII. Proceedings of the American Society of Clinical Oncology. 2001; Vol. 38a:Abstract 149.
- International Breast Cancer Study Group. Adjuvant chemotherapy followed by goserelin versus either modality alone for premenopausal lymph node‐negative breast cancer: a randomized trial. Journal of the National Cancer Institute 2003;95(24):1833‐46. [DOI] [PubMed] [Google Scholar]
- Karlsson P, Sun Z, Braun D, Price KN, Castiglione‐Gertsch M, Rabaglio M, et al. Long‐term results of International Breast Cancer Study Group Trial VIII: adjuvant chemotherapy plus goserelin compared with either therapy alone for premenopausal patients with node‐negative breast cancer. Annals of Oncology 2011;22(10):2216‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
SOFT {published data only}
- Bellet M, Gray KP, Francis PA, Lang I, Ciruelos E, Lluch A, et al. Estrogen levels in premenopausal (prem) patients (pts) with hormone‐receptor positive (HR+) early breast cancer (BC) receiving adjuvant triptorelin (Trip) plus exemestane (E) or tamoxifen (T) in the SOFT trial: SOFT‐EST substudy. Journal of Clinical Oncology 2014;32(15 Suppl 1):Abstract no. 585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellet M, Gray KP, Francis PA, Lang I, Ciruelos E, Lluch A, et al. Twelve‐month estrogen levels in premenopausal women with hormone receptor‐positive breast cancer receiving adjuvant triptorelin plus exemestane or tamoxifen for the Suppression of Ovarian Function Trial (SOFT): the SOFT‐EST substudy. Journal of Clinical Oncology 2016;34(14):1584‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard J, Luo W, Ribi K, Colleoni M, Burstein HJ, Tondini C, et al. Patient‐reported endocrine symptoms, sexual functioning, and quality of life (QoL) in the IBCSG TEXT and SOFT trials: adjuvant treatment with exemestane (E) plus ovarian function suppression (OFS) versus tamoxifen (T) plus OFS in premenopausal women with hormone receptor‐positive (HR+) early breast cancer (BC). Journal of Clinical Oncology 2014;32(15 Suppl 1):Abstract no. 557. [Google Scholar]
- Bernhard J, Luo W, Ribi K, Colleoni M, Burstein HJ, Tondini C, et al. Patient‐reported outcomes with adjuvant exemestane versus tamoxifen in premenopausal women with early breast cancer undergoing ovarian suppression (TEXT and SOFT): a combined analysis of two phase 3 randomised trials. Lancet Oncology 2015;16(7):848‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figg WD II, Cook K, Clarke R. Aromatase inhibitor plus ovarian suppression as adjuvant therapy in premenopausal women with breast cancer. Cancer Biology & Therapy 2014;15(12):1586‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming G, Francis P. Phase III randomized study of ovarian function suppression in combination with tamoxifen versus ovarian function suppression in combination with exemestane versus tamoxifen alone in premenopausal women with endocrine‐responsive breast cancer. Physician Data Query (PDQ) 2003.
- Fleming G, Francis PA, Lang I, Ciruelos EM, Bellet M, Bonnefoi HR, et al. Randomized comparison of adjuvant tamoxifen (T) plus ovarian function suppression (OFS) versus tamoxifen in premenopausal women with hormone receptor‐positive (HR+) early breast cancer (BC): update of the SOFT trial. Cancer Research. Conference: San Antonio Breast Cancer Symposium, SABCS 2017;78(4 Suppl 1):GS4‐03. [Google Scholar]
- Francis PA, Fleming GF, Regan MM, Pagani O, Walley BA, Price KN, et al. Long‐term follow‐up of TEXT and SOFT trials of adjuvant endocrine therapies for premenopausal women with HR+ early breast cancer. Cancer Research. Conference: 39th Annual CTRC AACR San Antonio Breast Cancer Symposium 2017;77(4 Suppl 1):OT3‐02‐03. [Google Scholar]
- Francis PA, Pagani O, Fleming GF, Walley BA, Colleoni M, Lang I, et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. New England Journal of Medicine 2018;379(2):122‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis PA, Regan MM, Fleming GF, Lang I, Ciruelos E, Bellet M, et al. Adjuvant ovarian suppression in premenopausal breast cancer. New England Journal of Medicine 2015;372(5):436‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldhirsch A, Gelber RD, Francis PA, Regan MM, Fleming GF, Lang I, et al. Randomized comparison of adjuvant tamoxifen (T) plus ovarian function suppression (OFS) versus tamoxifen in premenopausal women with hormone receptor‐positive (HR+) early breast cancer (BC): analysis of the SOFT trial. Cancer Research. Conference: 37th Annual CTRC AACR San Antonio Breast Cancer Symposium 2015;75(9 Suppl 1):14‐S3‐08. [Google Scholar]
- Jancin B. SOFT trial endorses selective ovarian suppression in early breast cancer. Oncology Report 2015;11(1):5‐6. [Google Scholar]
- Johansson H, Gray KP, Pagani O, Regan MM, Viale G, Aristarco V, et al. CYP19A1 and ESR1 polymorphisms and selected early‐onset side effects during combined endocrine therapy in the IBCSG TEXT trial for premenopausal women with hormone receptor‐positive (HR+) early breast cancer. Cancer Research. Conference: 37th Annual CTRC AACR San Antonio Breast Cancer Symposium 2015;75(9 Suppl 1):14‐P1‐12‐01. [Google Scholar]
- Johansson H, Gray KP, Pagani O, Regan MM, Viale G, Aristarco V, et al. Impact of CYP19A1 and ESR1 variants on early‐onset side effects during combined endocrine therapy in the TEXT trial. Breast Cancer Research 2016;18(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00066690. Suppression of ovarian function with either tamoxifen or exemestane compared with tamoxifen alone in treating premenopausal women with hormone‐responsive breast cancer (SOFT). clinicaltrials.gov/ct2/show/NCT00066690 (date first received: 7 August 2003).
- Pagani O, Regan MM, Fleming GF, Walley BA, Colleoni M, Lang I, et al. Randomized comparison of adjuvant aromatase inhibitor exemestane (E) plus ovarian function suppression (OFS) vs tamoxifen (T) plus OFS in premenopausal women with hormone receptor positive (HR+) early breast cancer (BC): update of the combined TEXT and SOFT trials. Cancer Research. Conference: San Antonio Breast Cancer Symposium, SABCS 2017 2018;78(4 Suppl 1):GS4‐02. [Google Scholar]
- Pagani O, Regan MM, Francis PA. Are SOFT and TEXT results practice changing and how?. The Breast 2016;27:122‐5. [DOI] [PubMed] [Google Scholar]
- Pagani O, Regan MM, Walley B, Fleming GF, Colleoni M, Lang I, et al. Randomized comparison of adjuvant aromatase inhibitor (AI) exemestane (E) plus ovarian function suppression (OFS) vs tamoxifen (T) plus OFS in premenopausal women with hormone receptor‐positive (HR+) early breast cancer (BC): Joint analysis of IBCSG TEXT and SOFT trials. Cancer Research 2014;32(15 Suppl 1):GS4‐02. [Google Scholar]
- Pagani O, Regan MM, Walley BA, Fleming GF, Colleoni M, Lang I, et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. New England Journal of Medicine 2014;371(2):107‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Feng Y, Ribi K, Bernhard J, Puglisi F, Bellet M, et al. Co‐SOFT: the cognitive function substudy of the suppression of ovarian function trial (SOFT). Cancer Research. Conference: 37th Annual CTRC AACR San Antonio Breast Cancer Symposium 2015;75(9 Suppl 1):P1‐12‐06. [Google Scholar]
- Phillips KA, Regan MM, Ribi K, Francis PA, Puglisi F, Bellet M, et al. Adjuvant ovarian function suppression and cognitive function in women with breast cancer. British Journal of Cancer 2016;114(9):956‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan MM, Francis PA, Pagani O, Fleming GF, Walley BA, Viale G, et al. Absolute benefit of adjuvant endocrine therapies for premenopausal women with hormone receptor‐positive, human epidermal growth factor receptor 2‐negative early breast cancer: TEXT and SOFT trials. Journal of Clinical Oncology 2016;34(19):2221‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan MM, Pagani O, Fleming GF, Walley BA, Price KN, Rabaglio M, et al. Adjuvant treatment of premenopausal women with endocrine‐responsive early breast cancer: design of the TEXT and SOFT trials. Breast 2013;22(6):1094‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan MM, Pagani O, Francis PA, Fleming GF, Walley BA, Kammler R, et al. Predictive value and clinical utility of centrally assessed ER, PgR, and Ki‐67 to select adjuvant endocrine therapy for premenopausal women with hormone receptor‐positive, HER2‐negative early breast cancer: TEXT and SOFT trials. Breast Cancer Treatment and Treatment 2015;154(2):275‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan MM, Walley BA, Francis PA, Fleming GF, Lang I, Gomez HL, et al. Concurrent and sequential initiation of ovarian function suppression with chemotherapy in premenopausal women with endocrine‐responsive early breast cancer: an exploratory analysis of TEXT and SOFT. Annals of Oncology 2017;28(9):2225‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribi K, Luo W, Bernhard J, Francis PA, Bellet M, Burstein HJ, et al. Patient‐reported endocrine symptoms, sexual functioning and quality of life (QoL) in the IBCSG SOFT trial: adjuvant treatment with tamoxifen (T) alone versus tamoxifen plus ovarian function suppression (OFS) in premenopausal women with hormone receptor‐po. Cancer Research. Conference: 37th Annual CTRC AACR San Antonio Breast Cancer Symposium 2015;75(9 Suppl 1):14‐S3‐09. [Google Scholar]
- Ribi K, Luo W, Bernhard J, Francis PA, Burstein HJ, Ciruelos E, et al. Adjuvant tamoxifen plus ovarian function suppression versus tamoxifen alone in premenopausal women with early breast cancer: patient‐reported outcomes in the Suppression of Ovarian Function Trial. Journal of Clinical Oncology 2016;34(14):1601‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha P, Regan MM, Pagani O, Francis PA, Walley BA, Ribi K, et al. Treatment efficacy, adherence, and quality of life among women younger than 35 years in the International Breast Cancer Study Group TEXT and SOFT Adjuvant Endocrine Therapy Trials. Journal of Clinical Oncology 2017;35(27):3113‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zickl L, Francis P, Fleming G, Pagani O, Walley B, Price KN, et al. SOFT and TEXT: Trials of tamoxifen and exemestane with and without ovarian function suppression for premenopausal women with hormone receptor‐positive early breast cancer. Cancer Research. Conference: 35th Annual CTRC AACR San Antonio Breast Cancer Symposium 2012;72(24 Suppl 3):OT2‐2‐01. [Google Scholar]
SWOG 1996 {published data only}
- Rivkin S, Green S, Melch B, Cruz A, Tesh D, Glick J, et al. Adjuvant combination chemotherapy (CMFVP) versus oophorectomy followed by CMFVP (OCMFVP) for premenopausal women with ER+ operable breast cancer with positive axillary lymph nodes: an intergroup study. Breast Cancer Research and Treatment 1991;19(2):161. [Google Scholar]
- Rivkin SE, Green S, O'Sullivan J, Cruz AB, Abeloff MD, Jewell WR, et al. Adjuvant CMFVP versus adjuvant CMFVP plus ovariectomy for premenopausal, node‐positive, and estrogen receptor‐positive breast cancer patients: a Southwest Oncology Group study. Journal of Clinical Oncology 1996;14(1):46‐51. [DOI] [PubMed] [Google Scholar]
Uslu 2014 {published data only}
- Uslu A, Zengel B, Akpinar G, Postaci H, Yetis H, Corumlu B, et al. The outcome effect of double‐hormonal therapy in premenopausal breast cancer patients with high nodal‐status: result of a prospective randomized trial. Indian Journal of Cancer 2014;51(4):582‐6. [DOI] [PubMed] [Google Scholar]
Yang 2013 {published data only}
- Yang H, Yu X, Zong X, Chen D, Ding X, Yu Y, et al. Goserelin plus tamoxifen versus tamoxifen alone in pre‐or peri‐menopausal patients with hormone receptor‐positive early‐stage breast cancer: a randomized, controlled clinical trial in China. Journal of Clinical Oncology 2016;34(Suppl 15):Abstract no. 562. [Google Scholar]
- Yang H, Zong X, Yu Y, Shao G, Zhang L, Qian C, et al. Combined effects of goserelin and tamoxifen on estradiol level, breast density, and endometrial thickness in premenopausal and perimenopausal women with early‐stage hormone receptor‐positive breast cancer: a randomised controlled clinical trial. British Journal of Cancer 2013;109(3):582‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HJ, Yu XF, Zong XY, Chen DB, Ding XW, Yu Y, et al. Goserelin plus tamoxifen versus tamoxifen alone, in pre‐menopausal or peri‐menopausal patients with hormone receptor‐positive early stage breast cancer: a randomised controlled trial in China. European Journal of Cancer 2016;60(Suppl 1):e7‐8. [Google Scholar]
Yi 2016 {published data only}
- Heo JY, Yi H, Fava M, Mischoulon D, Kim K, Yoon S, et al. Agoraphobia and follicle‐stimulating hormone levels between tamoxifen and goserelin versus tamoxifen alone in premenopausal hormone receptor‐positive breast cancer: a 12‐month prospective randomized study. Psychiatry Investigation 2017;14(4):491‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi HW. Comparisons of anxiety and depression between premenopausal women who received tamoxifen and goserelin versus tamoxifen alone to manage breast cancer: a 12‐month prospective randomized study. European Journal of Cancer 2016;57(Suppl 2):S138. [Google Scholar]
- Yi HW, Nam SJ, Kim SW, Lee JE, Lee SK, Bae SY, et al. Depression and anxiety after adjuvant ovarian function suppression in premenopausal breast cancer patients. Cancer Research 2016;76(4 Suppl 1):Abstract no. P1‐11‐01. [Google Scholar]
ZBCSG Trial B {published data only}
- Mitsuyama S, Nomura Y, Ohno S, Miyauchi M, Yamamoto N, Kimura T, et al. Assessment of goserelin treatment in adjuvant therapy for premenopausal patients with breast cancer in Japan ‐ Zoladex Breast Cancer Study Group Trial‐B [Japanese]. Japanese Journal of Cancer Chemotherapy [Gan To Kagaku Ryoho] 2005;32(13):2071‐7. [PubMed] [Google Scholar]
ZIPP {published data only}
- Baum M, Hackshaw A, Houghton J, Rutqvist LE, Fornander T, Nordenskjold B, et al. Adjuvant goserelin in pre‐menopausal patients with early breast cancer: results from the ZIPP study. European Journal of Cancer 2006;42(7):895‐904. [DOI] [PubMed] [Google Scholar]
- Baum M, Houghton J, Riley DL, Melhuish PB. Cancer Research Campaign Adjuvant Breast Trial for patients under 50. Hormone Research 1989;32(Suppl 1):226‐30. [DOI] [PubMed] [Google Scholar]
- Baum M, Houghton J, Sawyer W, et al. Management of pre‐menopausal women with early breast cancer: is there a role for goserelin?. Proceedings of the American Society of Clinical Oncology. 2001; Vol. 38a:Abstract no. 103.
- Hackshaw A, Baum M, Fornander T, Nordenskjold B, Nicolucci A, Monson K, et al. Long‐term effectiveness of adjuvant goserelin in premenopausal women with early breast cancer. Journal of the National Cancer Institute 2009;101(5):341‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linderholm BK, Fornander T, Johansson H, Andersson J, Skoog L, Rutqvist LE. Expression of c‐erbB‐2 is correlated with expression of vascular endothelial growth factor receptor 2 (VEGFR2/KDR), VEGF and VERFR1 in premenopausal patients with primary human breast cancer receiving adjuvant endocrine therapy within a randomized phase III trial (ZIPP trial). Proceedings of the American Society of Clinical Oncology 2003;861:Abstract no. 3458. [Google Scholar]
- NCT00002460. Adjuvant hormone therapy in treating women with operable breast cancer. clinicaltrials.gov/ct2/show/NCT00002460 Date first received: 9 June 2004.
- Nordenskjold B. Adjuvant treatment of premenopausal breast cancer with zoladex and tamoxifen: results from randomised trials by the Cancer Research Campaign (CRC) Breast Cancer Trials Group, The Stockholm Breast Cancer Study Group, the South East Sweden Breast Cancer Group and Gruppo Interdisciplinare Valutazione Intervention Oncologia (GIVIO). European Journal of Cancer Supplement 1999;35(Suppl 4):S83. [Google Scholar]
- Nystedt M, Berglund G, Bolund C, et al. Side effects of adjuvant endocrine treatment in premenopausal breast cancer patients: a prospective randomised study. Journal of Clinical Oncology 2003;21:1836‐44. [DOI] [PubMed] [Google Scholar]
- Sverrisdottir A, Johansson H, Johansson U, Bergh J, Rotstein S, Rutqvist L, et al. Interaction between goserelin and tamoxifen in a prospective randomised clinical trial of adjuvant endocrine therapy in premenopausal breast cancer. Breast Cancer Research and Treatment 2011;128(3):755‐63. [DOI] [PubMed] [Google Scholar]
- Sverrisdottir A, Johansson H, Johansson U, Bergh J, Rotstein S, Rutqvist LE, et al. Abstract S1‐5: interaction between goserelin and tamoxifen in a controlled clinical trial of adjuvant endocrine therapy in premenopausal breast cancer. Cancer Research 2010;70(24 Suppl):S1‐5. [DOI] [PubMed] [Google Scholar]
- Sverrisdottir A, Nystedt M, Johansson H, Fornander T. Adjuvant goserelin and ovarian preservation in chemotherapy treated patients with early breast cancer: results from a randomized trial. Breast Cancer Research and Treatment 2009;117(3):561‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ABCSG‐12 {published data only}
- Gnant M, Mlineritsch B, Schippinger W, Luschin‐Ebengreuth G, Postlberger S, Menzel C, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. New England Journal of Medicine 2009;360(7):679‐91. [DOI] [PubMed] [Google Scholar]
ABCSG 5 {published data only}
- Gnant M, Greil R, Kubista E, Menzel C, Schippinger W, Seifert M, et al. The impact of treatment‐induced amenorrhea on survival of premenopausal patients with endocrine‐responsive breast cancer: 10‐year results of ABCSG‐05 (CMF vs. goserelin+tamoxifen). Breast Cancer Research and Treatment 2006;100 Suppl 1:10‐1 (abstract 17). [Google Scholar]
- Jakesz R, Hausmaninger H, Kubista E, Gnant M, Menzel C, Bauernhofer T, et al. Randomised adjuvant trial of tamoxifen and goserelin versus cyclophosphamide, methotrexate, and fluorouracil: evidence for the superiority of treatment with endocrine blockade in pre‐menopausal patients with hormone responsive breast cancer – Austrian Breast and Colorectal Cancer Study Group trial 5. Journal of Clinical Oncology 2002;20(24):4621‐7. [DOI] [PubMed] [Google Scholar]
Baum 1996 {published data only}
- Baum M. Phase III randomised study of adjuvant therapy with camomile vs endocrine ablation vs camomile plus endocrine ablation vs no adjuvant therapy in patients under age 50 with operable breast cancer. PDQ 1996.
FASG 06 {published data only}
- Roche H, Kerbrat P, Bonneterre J, Fargeot P, Fumoleau P, Monnier A, et al. Complete hormonal blockade versus epirubicin‐based chemotherapy in premenopausal, one to three node‐ positive, and hormone‐receptor positive, early breast cancer patients: 7‐year follow‐up results of French Adjuvant Study Group 06 randomised trial. Annals of Oncology 2006;17(8):1221‐7. [DOI] [PubMed] [Google Scholar]
- Roche H, Kerbrat P, Bonneterre J, et al. Complete hormonal blockade versus chemotherapy in pre‐menopausal early‐stage breast cancer patients (pts) with positive hormone‐receptor (HR+) and 1–3 node‐positive (N+) tumor: results of the FASG 06 trial. Proceedings of the American Society of Clinical Oncology. 2000:72a (abstract 279).
GABG IV‐A‐93 {published data only}
- Minckwitz G, Graf E, Geberth M, Eiermann W, Jonat W, Conrad B, et al. Goserelin versus CMF as adjuvant therapy for node‐negative, hormone receptor‐positive breast cancer in premenopausal women. The GABG trial IV‐A‐93 Trial. Proceedings of the American Society of Clinical Oncology. 2004:11s (abstract 534).
- Minckwitz G, Graf E, Geberth M, Eiermannd W, Jonate W, Conrad B, et al. CMF versus goserelin as adjuvant therapy for node‐negative, hormone‐receptor‐positive breast cancer in premenopausal patients: a randomised trial (GABG trial IV‐A‐93). European Journal of Cancer 2006;42:1780‐8. [DOI] [PubMed] [Google Scholar]
- Minckwitz G, de Assesn A, Conrad B, et al. Medical ovarian ablation versus polychemotherapy in premenopausal women with node‐negative, receptor positive breast cancer. The ongoing trial 'A‐93' of the German Adjuvant Greast Cancer Group (GABG). Proceedings of the American Society of Clinical Oncology. 1999:(abstract 395).
Grocta 02 {published data only}
- Boccardo F, Rubagotti A, Amoroso D, Mesiti M, Romeo D, Sismondi P, et al. Cyclophosphamide, methotrexate, and fluorouracil versus tamoxifen plus ovarian suppression as adjuvant treatment of oestrogen receptor‐positive pre‐/perimenopausal breast cancer patients: results of the Italian Breast Cancer Adjuvant Study Group 02 randomised trial. Journal of Clinical Oncology 2000;18(14):2718‐27. [DOI] [PubMed] [Google Scholar]
- Boccardo F, Rubagotti A, Amoroso D, et al. CMF versus tamoxifen (TAM) plus goserelin (GOS) as adjuvant treatment of ER positive (ER+) pre‐perimenopausal breast cancer (CA) patients (PTS). Preliminary results of the GROCTA 02 study. Proceedings of the American Sociey of Clinical Oncology. 1998:abstract 382.
- Boccardo F, Rubagotti A, Amoroso D, et al. CMF versus tamoxifen plus goserelin as adjuvant treatment of ER‐positive pre‐perimenopausal breast cancer patients: preliminary results of an ongoing Italian Breast Cancer Adjuvant Study Group (GROCTA) trial. Adjuvant Therapy of Cancer VIII. Philadelphia: Lippincott‐Raven, 1997:101‐8. [Google Scholar]
HMFEC {published data only}
- Coombes RC, Kilburn LS, Tubiana‐Mathieu N, Olmos T, Bochove A, Perez‐Lopez FR, et al. Epirubicin dose and sequential hormonal therapy ‐ Mature results of the HMFEC randomised phase III trial in premenopausal patients with node positive early breast cancer. European Journal of Cancer 2016;60:146‐53. [DOI] [PubMed] [Google Scholar]
Li 2019 {published data only}
- Li JW, Liu GY, Ji YJ, Yan X, Pang D, Jiang ZF, et al. Switching to anastrozole plus goserelin vs continued tamoxifen for adjuvant therapy of premenopausal early‐stage breast cancer: preliminary results from a randomized trial. Cancer Management and Research 2019;11:299‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
MAM 01 GOCSI {published data only}
- Bianco AR, Costanzo R, Lorenzo G, et al. The MAM‐1 GOCSI trial: a randomised trial with factorial design of chemo‐endocrine adjuvant treatment in node positive (N+) early breast cancer (ebc). Proceedings of the American Society of Clinical Oncology. 2001:27a (abstract 104).
- Placido S, Laurentiis M, Lena M, Lorusso V, Paradiso A, et al. A randomised factorial trial of sequential doxorubicin and CMF vs CMF and chemotherapy alone vs chemotherapy followed by goserelin plus tamoxifen as adjuvant treatment of node‐positive breast cancer. British Journal of Cancer 2005;92(3):467‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Manson 2019 {published data only}
- Manson JE, Aragaki AK, Bassuk SS, Chlebowski RT, Anderson GL, Rossouw JE, et al. Menopausal estrogen‐alone therapy and health outcomes in women with and without bilateral oophorectomy: a randomized trial. Annals of Internal Medicine 2019;171:406‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
PERCHE {published data only}
- Premenopausal endocrine responsive chemotherapy trial (PERCHE). clinicaltrials.gov/show/NCT00066807 (date first received 7 August 2003).
Pretoria {published data only}
- Falkson CI, Falkson HC, Falkson G. The effect of chemotherapy with or without GnHRA on serum hormone levels in premenopausal women with breast cancer. Proceedings of the American Society of Clinical Oncology. 1990:(abstract 90).
- Falkson CI, Jooste R, Falkson HC, Falkson G, Rens D, Aarde R, et al. Cyclophosphamide, methotrexate and fluorouracil (CMF) plus or minus depo‐buserelin in premenopausal women with lymph node positive breast cancer. Proceedings of American Society of Clincial Oncology 2001;20(Pt 2):Abstract no.1777. [Google Scholar]
- Falkson CI, Jooste R, Falkson HC, et al. Cyclophosphamide, methotrexate and fluorouracil (CMF) plus or minus depo‐buserelin in pre‐menopausal women with lymph node positive breast cancer. Proceedings of the American Society of Clinical Oncology. 2001:7b (abstract 1777).
Ragaz 1997 {published data only}
- Ragaz J, Jackson S, Wilson K, Plenderleith IH, Knowling M, Basco V. Randomized study of locoregional radiotherapy and ovarian ablation in premenopausal patients with breast cancer treated with adjuvant chemotherapy. Proceedings of the American Society of Clinical Oncology 1988;7(12):Abstract 45. [Google Scholar]
- Ragaz J, Jackson SM, Le N, Plenderleith IH, Wilson K, Knowling M, et al. Can ovarian ablation improve outcome of stage I‐II premenopausal breast cancer patients with estrogen receptor positive tumors treated with adjuvant chemotherapy? Long‐term analysis of British Columbia randomized trial. Proceedings of the American Society of Clinical Oncology 1997;16(142a):Abstract 501. [Google Scholar]
Soreide 2002 {published data only}
- Söreide JA, Varhaug JE, Fjösne HE, Erikstein B, Jacobsen AB, Skovlund E, et al. Adjuvant endocrine treatment (goserelin vs tamoxifen) in pre‐menopausal patients with operable node positive stage II breast cancer. A prospective randomised national multicenter study. European Journal of Surgical Oncology 2002;28(5):505‐10. [DOI] [PubMed] [Google Scholar]
TABLE {published data only}
- Schmid P, Untch M, Kosse V, Bondar G, Vassiljev L, Tarutinov V, et al. Leuprorelin acetate every‐3‐months depot versus cyclophosphamide, methotrexate, and fluorouracil as adjuvant treatment in premenopausal patients with node‐positive breast cancer: the TABLE study. Journal of Clinical Oncology 2007;25(18):2509‐15. [DOI] [PubMed] [Google Scholar]
- Schmid P, Untch M, Wallwiener D, Kossé V, Bondar G, Vassiljev L, et al. Cyclophosphamide, methotrexate and fluorouracil (CMF) versus hormonal ablation with leuprorelin acetate as adjuvant treatment of node positive, premenopausal breast cancer patients: preliminary results of the TABLE‐study (Takeda Adjuvant Breast Cancer Study with Leuprorelin acetate). Anticancer Research 2002;22(4):2325‐32. [PubMed] [Google Scholar]
- Untch M, Kahlert S, Kosse V, et al. LHRH‐analogue therapy with leuprorelin‐acetate three‐month depot offers similar efficacy to conventional adjuvant CMF chemotherapy in receptor positive, node positive breast cancer patients. San Antonio Breast Cancer Symposium. 2003:abstract 40.
UKCCR {published data only}
Yu 2019 {published data only}
- Yu KD, Wu SY, Liu GY, Wu J, Di GH, Hu Z, et al. Concurrent neoadjuvant chemotherapy and estrogen deprivation in patients with estrogen receptor‐positive, human epidermal growth factor receptor 2‐negative breast cancer (CBCSG‐036): a randomized, controlled, multicenter trial. Cancer 2019;125:2185‐93. [DOI] [PubMed] [Google Scholar]
ZEBRA {published data only}
- Jonat W, Kaufman M, Sauerbrei W, et al. Goserelin versus cyclophosphamide, methotrexate, and fluorouracil as adjuvant therapy in pre‐menopausal patients with node‐positive breast cancer: the Zoladex Early Breast Cancer Research Association study. Journal of Clinical Oncology 2002;20:4628‐35. [DOI] [PubMed] [Google Scholar]
- Kaufmann Ma, Jonat W, Blameyc R, Cuzick J, Namere M, Fogelman I, et al. on behalf of the Zoladex Early Breast Cancer Research Association (ZEBRA) Trialists’ Group. Survival analyses from the ZEBRA study: goserelin (ZoladexTM) versus CMF in premenopausal women with node‐positive breast cancer. European Journal of Cancer 2003;39:1711‐7. [DOI] [PubMed] [Google Scholar]
- Haes H, Olschewski M, Kaufmann M, Schumacher M, Jonat W, Sauerbrei W, Zoladex Early Breast Cancer Research Association Trialists Group. Quality of life in goserelin‐treated versus cyclophosphamide + methotrexate + fluorouracil‐treated premenopausal and perimenopausal patients with node‐positive, early breast cancer: the Zoladex Early Breast Cancer Research Association Trialists Group. Journal of Clinical Oncology 2003;21(24):4510‐6. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT02132390 {published data only}
- NCT02132390. Adjuvant toremifene with or without goserelin in premenopausal women with stage I‐IIIA, hormonal receptor positive breast cancer accompanied with or without chemotherapy induced amenorrhoea. clinicaltrials.gov/show/NCT02132390 (date first received: 7 May 2014).
Additional references
Baum 2006
- Baum M, Hackshaw A, Houghton J, et al. Adjuvant goserelin in pre‐menopausal patients with early breast cancer: results from the ZIPP study. European Journal of Cancer 2006;42:895‐904. [DOI] [PubMed] [Google Scholar]
Bernhard 2007
- Bernhard J, Zahrieh D, Castiglione‐Gertsch M, et al. Adjuvant chemotherapy followed by goserelin compared with either modality alone: the impact on amenorrhea, hot flashes, and quality of life in premenopausal patients: the International Breast Cancer Study Group Trial VIII. Journal of Clinical Oncology 2007;25:263‐70. [DOI] [PubMed] [Google Scholar]
Bines 1996
- Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. Journal of Clinical Oncology 1998;14:1718‐29. [DOI] [PubMed] [Google Scholar]
Cardoso 2019
- Cardoso F, Kyriakides S, Ohno S, Penault‐Llorca F, Poortmans P, Rubio IT, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Annals of Oncology 2019;30:1194‐1220. [DOI] [PubMed] [Google Scholar]
Cheer 2005
- Cheer SM, Plosker GL, Simpson D, Wagstaff AJ. Goserelin: a review of its use in the treatment of early breast cancer in premenopausal and perimenopausal women. Drugs 2005;65(18):2639‐55. [DOI] [PubMed] [Google Scholar]
Chlebowski 2017
- Chlebowski RT, Pan K, Col NF. Ovarian suppression in combination endocrine adjuvant therapy in premenopausal women with early breast cancer. Breast Cancer Research and Treatment 2017;161:185‐90. [DOI] [PubMed] [Google Scholar]
Clarke 1998
- Clarke MJ. Ovarian ablation in breast cancer, 1986 to 1998: milestones along hierarchy of evidence from case report to Cochrane review. BMJ 1998;317:1246‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cuzick 2007
- LHRH‐agonists in Early Breast Cancer Overview group, Cuzick J, Ambroisine L, Davidson N, Jakesz R, Kaufmann M, et al. Use of luteinising‐hormone‐releasing hormone agonists as adjuvant treatment in premenopausal patients with hormone‐receptor‐positive breast cancer: a meta‐analysis of individual patient data from randomised adjuvant trials. Lancet 2007;369(9574):1711‐23. [DOI] [PubMed] [Google Scholar]
Davidson 2003
- Davidson NE, O'Neill A, Vukov A, Osborne CK, Martino S, White D, et al. Chemohormonal therapy in premenopausal node‐positive, receptor positive breast cancer: an Eastern Cooperative Oncology Group phase III intergroup trial (E5188, INT‐0101). Proceedings of the American Society of Clinical Oncology. 2003. [DOI] [PubMed]
EBCTCG 1996
- Early Breast Cancer Trialists' Collaborative Group. Ovarian oblation in early breast cancer: overview of the randomised trials. Lancet 1998;348:1189‐96. [PubMed] [Google Scholar]
EBCTCG 2003
- Early Breast Cancer Trialists' Collaborative Group. Ovarian ablation for early breast cancer (Cochrane Review). Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD000485] [DOI] [Google Scholar]
EBCTCG 2005
- Early Breast Cancer Trialists' Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15‐year survival: an overview of the randomised trials. Lancet 2005;365:1687‐717. [DOI] [PubMed] [Google Scholar]
EBCTCG, Clarke 1998
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG), Clarke MJ. Ovarian ablation of early breast cancer. Cochrane Database of Systematic Reviews 1998, Issue 2. [DOI: 10.1002/14651858.CD000485] [DOI] [Google Scholar]
Ejlertsen 1999
- Ejlertsen B, Dombernowsky P, Mouridsen HT, Kamby C, Kjaer M, Pose C, et al. Comparable effect of ovarian ablation (OA) and CMF chemotherapy in premenopausal hormone receptor positive breast cancer patients. Proceedings of the American Society of Clinical Oncology 1999;18:66a. [Google Scholar]
Francis 2018
- Francis PA, Pagani O, Fleming GF, Walley BA, Colleoni M, Lang I, et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. New England Journal of Medicine 2018;379(2):122‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Furr 1989
- Furr BJA. Pharmacology of the luteinising hormone releasing hormone (LHRH) analog "Zoladex". Hormonal Research 1989;32 Suppl 1:86‐92. [DOI] [PubMed] [Google Scholar]
Gelber 1996
- Gelber RD, Cole BF, Goldhirsch A, Rose C, Fisher B, et al. Adjuvant chemotherapy plus tamoxifen compared with tamoxifen alone for postmenopausal breast cancer: meta‐analysis of quality adjusted survival. Lancet 1996;347:1066‐71. [DOI] [PubMed] [Google Scholar]
Goel 2009
- Goel S, Sharma R, Hamilton A, Beith J. LHRH agonists for adjuvant therapy of early breast cancer in premenopausal women. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD004562.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEproGDT
- GRADEproGDT: GRADEpro Guideline Development Tool [software]. Hamilton, ON: McMaster University, 2015 (developed by Evidence Prime, Inc). Available from www.gradepro.org.
Howlader 2014
- Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LAG, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. Journal of the National Cancer Institute 2014;106(5):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jemal 2008
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA: A Cancer Journal for Clinicians 2008;58(2):71‐96. [DOI] [PubMed] [Google Scholar]
NCCN 2019
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines), Breast Cancer, version 3.2019, September 6, 2019. NNCN.org (accessed 10 October 2019).
Pagani 1998
- Pagani O, O'Neil A, Castiglione M, et al. Prognostic impact of amenorrhoea after adjuvant chemotherapy in premenopausal breast cancer patients with axillary node involvement: results of the International Breast Cancer Study Group (IBCSG) Trial VI. European Journal of Cancer 1998;34:632‐40. [DOI] [PubMed] [Google Scholar]
R [Computer program]
- R Foundation for Statistical Computing. Vienna, Austria. ISBN 3‐900051‐07‐0, 2019. Available at http://www.R‐project.org/. R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. ISBN 3‐900051‐07‐0, 2019. Available at http://www.R‐project.org/, 2019.
Scherer 2018
- Scherer RW, Meerpohl JJ, Pfeifer N, Schmucker C, Schwarzer G, Elm E. Full publication of results initially presented in abstracts. Cochrane Database of Systematic Reviews 2018, Issue 11. [DOI: 10.1002/14651858.MR000005.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2019
- Schünemann HJ, Vist GE, Higgins JPT, Santesso N, Deeks JJ, Glaziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions vesrion 6.0 (updated July 2019). Cochrane, 2019 Available from www.training.cochrane.org/handbook.
SCTBG and ICRF 1993
- Scottish Cancer Trials Breast Group (SCTBG) and ICRF Breast Unit Guys Hospital London. Adjuvant ovarian ablation versus CMF in premenopausal women with pathological stage II breast carcinoma: the Scottish trial. Lancet 1993;341:1293‐9. [PubMed] [Google Scholar]
Walshe 2006
- Walshe JM, Denduluri N, Swain SM. Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. Journal of Clinical Oncology 2006;24:5769‐79. [DOI] [PubMed] [Google Scholar]
Young 2011
- Young T, Hopewell S. Methods for obtaining unpublished data. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.MR000027.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Sharma 2005
- Sharma R, Beith J, Hamilton A. Systematic review of LHRH agonists for the adjuvant treatment of early breast cancer. The Breast 2005;14:181‐91. [DOI] [PubMed] [Google Scholar]


