Abstract
Background
In spite of demonstrating prognostic and possibly predictive benefit in retrospective cohorts and meta-analyses of cancer populations, including colorectal cancer (CRC), prospective evaluation of the relationship between neutrophil to lymphocyte ratio (NLR) and treatment outcomes in previously untreated mCRC patients receiving bevacizumab-based therapy has not yet been performed.
Methods
An open-label, single arm, multi-centre study. Patients received first-line bevacizumab plus XELOX or mFOLFOX6 (Phase-A) and continued bevacizumab plus FOLFIRI beyond first progression (Phase-B). Analyses included the association of NLR with phase A progression free survival (PFS) and overall survival (OS). A sub-study investigated the safety in patients with the primary in situ tumor. An exploratory sub-study examined relationships of circulating proteomic markers with PFS.
Results
Phase-A enrolled 128 patients; median age was 64 years (range: 26–84), 70 (55%) were female, 71 (56%) were PS-0 and 51 (40%) had primary in situ tumor. Fifty-three (41%) patients entered Phase-B. The median baseline (b) NLR was 3.2 (range: 1.5–20.4) with 32 (25%) patients having bNLR > 5. The PFS hazard ratio (HR) by bNLR > 5 versus ≤ 5 was 1.4 (95% CI: 0.9–2.2; p = 0.101). The median PFS was 9.2 months (95% CI: 7.9–10.8) for Phase-A and 6.7 months (95% CI: 3.0–8.2) for Phase-B. The HR for OS based on bNLR > 5 versus ≤ 5 was 1.6 (95% CI: 1.0–2.7; p = 0.052). The median OS was 25 months (95% CI: 19.2–29.7) for the full analysis set and 14.9 months for Phase-B. Baseline levels of nine proteomic markers showed a relationship with PFS. Treatment related toxicities were consistent with what has previously been published. There were 4 (3%) instances of GI perforation, of which, 3 (6%) occurred in the primary in situ tumor group.
Conclusions
Results from this study are aligned with the previously reported trend towards worse PFS and OS in patients with higher bNLR.
Trial registration
ClinicalTrials.gov: NCT01588990; posted May 1, 2012.
Background
The efficacy of bevacizumab in combination with fluoropyrimidine-containing chemotherapy has been demonstrated in metastatic colorectal cancer (mCRC).[1–7] However, to date, there are no reproducible, validated, simple and inexpensive prognostic biomarkers to aid treatment selection. The optimal treatment duration and the role of bevacizumab in certain patient subgroups, specifically those considered at particular risk of bevacizumab-mediated toxicity, also require further investigation.
The microenvironment of the tumor and the inflammatory response are considered important effectors of cancer biology and tumorigenesis.[8] Tumor development and progression induced by an inflammatory response are mediated by interactions between pro-inflammatory cytokines and cellular pathways, including the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-ΚB) and the signal transducer and activator of transcription-3 (STAT-3).[9] The role of inflammatory markers as predictive or prognostic tools in the setting of bevacizumab has been investigated retrospectively.[10, 11] The use of blood-based markers such as neutrophil/lymphocyte ratio (NLR) as prognostic/predictive biomarkers in patients receiving bevacizumab-based chemotherapy had not been previously evaluated in this setting.
This study[12] aimed to evaluate the relationship between the host inflammatory response, measured by NLR, selected proteomic plasma markers and treatment outcomes, in patients with previously untreated mCRC receiving bevacizumab-based first- and second-line treatments.
Methods
Study design
An open-label, prospective, single arm, phase-IV, multi-center study (NCT01588990) evaluating the relationship between NLR and treatment outcomes in patients with histologically confirmed, previously untreated mCRC; had World Health Organization (WHO) performance status (PS) of 0–1 and life expectancy of ≥ 3 months; and were eligible to received bevacizumab-based, first- and second-line, treatment. The study protocol has previously been published.[12] In summary, there were two phases of treatment; in Phase-A bevacizumab (7.5 mg/kg every 3 weeks) plus XELOX or bevacizumab (5mg/kg every 2 weeks) plus mFOLFOX6 were administered from study start until first disease progression. In Phase-B bevacizumab (5.0 mg/kg every 2 weeks) plus FOLFIRI were administered from first disease progression until second disease progression. The study planned to recruit a total of 150 patients; however, due to competing recruitment only 144 patients were enrolled. The study was conducted in accordance with local guidelines and in line with the principles of the Declaration of Helsinki and Good Clinical Practice Guidelines. Ethics approval was obtained from the following Human Research Ethics committees (HREC): Australian Capital Territory Health HREC (ETH.7.12.168; August 2012); Calvary Health Care Adelaide HREC (Reference number: 12-CHREC-F002; April 2012); Cancer Institute NSW Clinical Research Ethics Committee (HREC/12/CIC/3; May 2012); Central Northern Adelaide Health Service Ethics of Human Research Committee (HREC/12/TQEHLMH/63; October 2012); Melbourne Health HREC (HREC/11/MH/383; March 2012); St John of God Health Care Ethics Committee (HREC#573; October 2012); St Vincent's Hospital HREC (Reference number: 12/109; June 2012); Sydney Adventist Hospital Group HREC (HREC-2012-020; August 2012); Sydney Local Health District Ethics Review Committee (Royal Prince Alfred Hospital Zone; X12-0243; August 2012); and Tasmanian Health and Medical HREC (H12421; May 2012). All participating patients provided written informed consent prior to participation in the study.
Here we present results for the primary endpoint which investigated the prognostic value of the host inflammatory response as assessed by the NLR (≤ 5 versus > 5) on Progression Free Survival (PFS); and the secondary endpoints which investigated the safety profile of bevacizumab, efficacy by treatment phase, the role of NLR as predictor of Overall Survival (OS); the association between post-baseline changes in NLR, PFS and OS. The incidence of serious adverse events related to the primary tumor in the primary in situ tumor patient cohort is also presented.
Proteomic analysis
Pre-treatment, baseline plasma samples were independently prepared in duplicate by reduction and alkylation and then digested overnight at 37°C with trypsin. For each preparation, 1 μg of peptide was analyzed as technical duplicates by nano liquid chromatography-selected reaction monitoring mass spectrometry assays of 66 peptides representing 32 acute phase and inflammation related plasma proteins as previously described.[13, 14] Individual peptide peak areas were obtained following normalization to total peak area, and the means and variances reported.
Statistical methodologies
The statistical and analytical plan has been previously published.[12] The analysis populations included the Full Analysis Set (FAS), defined as subjects who received at least one dose of bevacizumab; the “primary in situ population” was defined as all subjects in the FAS with a primary in situ tumor; and the “resected primary tumor population” was defined as all subjects in the FAS without a primary in situ tumor. PFS was defined as the time from the start of initial treatment to documentation of first disease progression or death from any cause, whichever occurred first. PFS in Phase-B was defined as the time from the start of Phase-B treatment to documentation of second disease progression or death from any cause, whichever occurred first. OS was defined as the time from the start of initial treatment to the date of death, regardless of the cause of death. OS in Phase-B was defined as the time from the start of treatment in Phase-B to death of any cause.
All analyses were conducted (using the Statistical Analysis System, SAS v9.3) on the FAS unless otherwise stated. Testing of statistical hypotheses was conducted at two-sided alpha of 0.05.
The NLR at the start of treatment was dichotomized between ≤ 5 and > 5 and tested in a standard Proportional Hazards Cox regression model [15] for an association with PFS (primary analysis) and OS (secondary analysis), in a model adjusted for the default covariates, which included the WHO PS (0 versus. 1), metastatic disease in the liver (Yes/No), number of different sites of metastatic disease (≤ 3 versus. > 3), and presence of metastatic disease in the liver with no other sites involved (Yes/No). The association between longitudinal NLR measurements and PFS and OS was assessed by including NLR (≤ 5 versus > 5) as a time-varying covariate in the primary model, adjusted for all the covariates that were defined as covariates in the primary model.
Results
Patients
A total of 144 patients were screened (signed the informed consent) from 17 sites; 16 patients were excluded (Fig 1). A total of 128 patients were enrolled in Phase-A (June-2012 to September-2016) and received treatment. Fifty-eight patients ended Phase-A due to disease progression; of these 53 patients continued into Phase-B (Fig 1). The median age of the overall population was 64 years (range: 26–84). There were more female (n = 70; 55%; Table 1). The majority (n = 100; 78%) had a Charlson comorbidity index ≤ 1. Seventy-one patients (56%) had WHO PS 0 and 56 (44%) patients had PS of 1. Overall, 96 (75%) patients had baseline (b) NLR ≤ 5 while 32 (25%) patients had bNRL > 5, with median bNLR of 3 (range: 1.2–20.0). Fifty-one patients had primary in situ tumor and 77 had resected primary tumor.
Fig 1. Patient disposition.
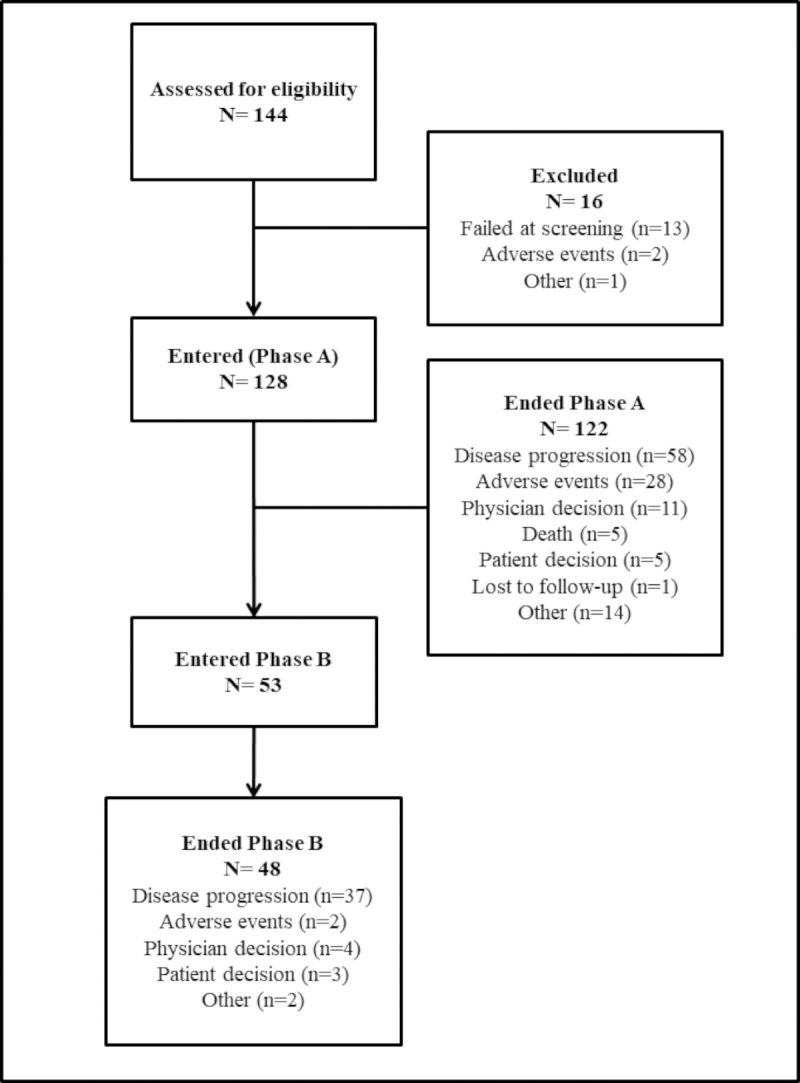
Table 1. Summary of demographics and patient characteristics at baseline (full analysis set population).
| NLR ≤ 5 (N = 96) | NLR > 5 (N = 32) | Total (N = 128) | |
|---|---|---|---|
| Age (years) | |||
| n | 96 | 32 | 128 |
| median (min; max) | 64 (31; 82) | 63 (26; 84) | 64 (26;84) |
| Gender, (n, %) | |||
| Male | 44 (46) | 14 (44) | 58 (45) |
| Female | 52 (54) | 18 (56) | 70 (55) |
| Tumor Status (n, %) | |||
| Primary in situ (PIS) | 33 (34) | 18 (56) | 51 (40) |
| Resected primary tumor (RPT) | 63 (66) | 14 (44) | 77 (60) |
| WHO Performance Status (n, %) | |||
| Missing | 1 (1) | 0 | 1 (1) |
| 0 | 56 (58) | 15 (47) | 71 (56) |
| ≥ 1 | 39 (41) | 17 (53) | 56 (44) |
| Charlson Comorbidity Index (n, %) | |||
| Missing | 3 (3) | 2 (6) | 5 (4) |
| ≤ 1 | 73 (76) | 27 (84) | 100 (78) |
| > 1 | 20 (20) | 3 (9) | 23 (18) |
| NLR | |||
| Mean (SD) | 3 (1) | 9 (3) | 4 (3) |
| Median (range | 3 (1–5) | 8 (5–20) | 3 (1–20) |
| PLR (n, %) | |||
| ≤ 150 | 39 (41) | 2 (6) | 41 (32) |
| > 150 and ≤ 300 | 52 (54) | 10 (31) | 62 (48) |
| > 300 | 5 (5) | 20 (63) | 25 (20) |
| Glasgow Prognostic Index (n, %) | |||
| 0 | 29 (30) | 0 | 29 (23) |
| 1 | 37 (39) | 0 | 37 (29) |
| 2 | 13 (14) | 2 (6) | 15 (12) |
| 3 | 16 (17) | 11 (34) | 27 (21) |
| 4 | 1 (1) | 12 (38) | 13 (10) |
| 5 | 0 | 7 (22) | 7 (6) |
| Site of Metastatic Disease (n (%)) | |||
| Liver | 65 (68) | 24 (75) | 89 (70) |
| Lung | 37 (39) | 16 (50) | 53 (41) |
| Bone | 8 (8) | 8 (25) | 16 (13) |
| Lymph nodes | 40 (42) | 14 (44) | 54 (42) |
| Peritoneal/omental fat | 15 (16) | 6 (19) | 21 (16) |
Abbreviations: max = maximum; min = minimum; NLR = neutrophil/lymphocyte ratio, PLR = platelet/lymphocyte ratio; SD = standard deviation.
Treatment exposure
Patients received median 15 cycles (range: 1–91) of bevacizumab treatment; 11 cycles (range: 1–91) in Phase-A and 8 cycles (range: 1–79) in Phase-B. At study end, 6 patients remained on treatment in Phase-A and 5 patients in Phase-B. Bevacizumab dose interruption due to adverse events (AEs) were recorded in 78 (61%) patients overall; 70 (55%) patients in Phase-A and 20 (38%) patients in Phase-B. The major reasons for treatment discontinuation were disease progression (n = 58; 45%) and AEs (n = 28; 22%) in Phase-A and disease progression (n = 37; 70%) in Phase-B (Fig 1).
XELOX was administered in 38 patients in Phase-A, with median 32 weeks (range: 3–189). Eighty patients received mFOLFOX6; median number of weeks was 23 (range: 2–189). In Phase-B, 53 patients received a median of 18 weeks of FOLFIRI (range: 0–198).
Efficacy analyses
Using the Cox proportional hazards model (Table 2) for the primary analyses, NLR was not shown to be prognostic of PFS; the Hazard Ratio (HR) [NLR > 5 versus NLR ≤ 5] was 1.4 (95% CI: 0.9–2.2; p = 0.101). An HR of 1.4 equates to a predicted difference in 12-month PFS from 56% for patient with bNLR ≤ 5 vs 44%, had that same patient had bNLR >5 (S1 Table).
Table 2. Multivariate cox proportional hazards analysis of the influence of baseline characteristics on PFS and OS (full analysis set).
| Progression Free Survival | Overall Survival | |||||
|---|---|---|---|---|---|---|
| Covariate | HR | 95% CI | p | HR | 95% CI | p |
| NLR (> 5 versus ≤ 5) | 1.4 | 0.9,2.2 | 0.101 | 1.6 | 1.0, 2.7 | 0.052 |
| WHO PS (≥ 1 versus 0) | 1.6 | 1.1, 2.4 | 0.013 | 1.8 | 1.1, 2.8 | 0.011 |
| Metastatic liver disease (yes versus no) | 1.5 | 1.0, 2.4 | 0.079 | 1.4 | 0.8, 2.4 | 0.206 |
| Number of metastatic sites (> 3 versus ≤ 3) | 1.0 | 0.5, 2.0 | 0.886 | 1.5 | 0.8, 3.1 | 0.219 |
| Metastatic liver disease with no other sites (yes versus no) | 1.0 | 0.6, 1.7 | 0.916 | 0.8 | 0.4, 1.5 | 0.440 |
The HR for OS (bNLR > 5 versus ≤ 5) was 1.6 (95% CI: 1.0–2.7; p = 0.052). The predicted OS probability at 12 months from the primary model for subjects with WHO PS 0, no metastatic liver disease and ≤3 sites of metastatic disease, was 87% for subjects with bNLR ≤ 5 and 79% for subjects with bNLR > 5 (S2 Table).
To determine whether post baseline normalization of NLR was a determinant of response to treatment, the longitudinal NLR was added as a time-varying covariate to the primary model. The model generated a PFS HR = 1.3 (95% CI: 0.9–1.9; p = 0.188) and an OS HR = 2.2 (95% CI: 1.2–4.0; p = 0.016). While these results do not support the hypothesis that normalization of NLR is a predictor of response, they suggest that NLR status of the patient at any time is associated with an increased rate of mortality.
To determine whether any of the baseline characteristics or other laboratory values were confounding for the effect of NLR, the demographic and laboratory values were each individually included in the primary model (S3 Table). None of these changed the association to being statistically significant. Only the inclusion of the Glasgow Prognostic Index as a linear variable changed the direction of the association, but the effect was still not statistically significant.
Time to event measures for each of the following secondary outcome variables, PFS in Phase-A and Phase-B, OS in the FAS population and Phase-B OS are summarised in Table 3.
Table 3. Secondary efficacy analyses.
| Outcome (population) | N | Median (mo) (95% CI) | Range (min, max) | 12-mo predicted Proportion (95% CI) |
|---|---|---|---|---|
| PFS until 1st Progression (FAS) | 128 | 9.2 (7.9, 10.8) | 1.0, 42.5 | 35.8% (27.4, 44.3) |
| OS (FAS) | 128 | 25.0 (19.2, 29.7) | 1.0, 47.5 | 75.6% (67.0, 82.3) |
| Survival beyond first progression (Progressed in Phase-A) | 101 | 12.6 (8.8, 15.9) | 0.2, 45.7 | 52.0% (41.4, 61.6) |
| PFS (Phase B) | 53 | 6.7 (3.0, 8.2) | 0.0, 44.1 | 15.9% (7.1, 28.1) |
| OS (Phase B) | 53 | 14.9 (8.2, 17.5) | 0.0, 44.8 | 59.4% (44.0, 71.9) |
Abbreviations: FAS = full analysis set; mo = Months; PFS = Progression Free Survival; OS = Overall Survival
Safety analyses
All 128 patients experienced at least one AE. Most patients experienced mild (n = 125; 98%) or moderate (n = 119; 93%) AEs. Grade 3–5 AEs were reported in 102 (79.7%) patients overall; 97 (75.8%) patients in Phase-A and 24 (45.3%) patients in Phase-B. The incidence of the most frequent AEs by primary tumor status is presented in Table 4.
Table 4. Summary of non-serious adverse events reported by ≥ 20% of patients (full analysis set).
| Preferred Term | PIS (N = 51) n (%) | RPT (N = 77) n (%) | Total (N = 128) n (%) |
|---|---|---|---|
| Nausea | 37 (72.5) | 50 (64.9) | 87 (68.0) |
| Fatigue | 33 (64.7) | 47 (61.0) | 80 (62.5) |
| Neuropathy peripheral | 28 (54.9) | 52 (67.5) | 80 (62.5) |
| Diarrhea | 28 (54.9) | 44 (57.1) | 72 (56.3) |
| Constipation | 26 (51.0) | 26 (33.8) | 52 (40.6) |
| Abdominal pain | 14 (27.5) | 30 (39.0) | 44 (34.4) |
| Palmar-plantar erythrodysaesthesia syndrome | 14 (27.5) | 24 (31.2) | 38 (29.7) |
| Vomiting | 14 (27.5) | 26 (33.8) | 40 (31.3) |
| Neutropenia | 15 (29.4) | 18 (23.4) | 33 (25.8) |
| Mucosal inflammation | 14 (27.5) | 17 (22.1) | 31 (24.2) |
| Epistaxis | 9 (17.6) | 21 (27.3) | 30 (23.4) |
| Decreased appetite | 16 (31.4) | 12 (15.6) | 28 (21.9) |
| Gastroesophageal reflux disease | 15 (29.4) | 12 (15.6) | 27 (21.1) |
| Paraesthesia | 10 (19.6) | 16 (20.8) | 26 (20.3) |
| Alopecia | 7 (13.7) | 19 (24.7) | 26 (20.3) |
Abbreviations: NLR = neutrophil/lymphocyte ratio; PIS = primary in situ tumor; RPT = resected primary tumor. The denominator for percentages is the number of patients in the FAS for each Primary Intact or Resected group. Sorted in descending order of frequency based on the total column. Note: This table contains counts of subjects. If a subject experienced more than one episode of an adverse event, the subject is counted only once within a preferred term.
Of particular interest within the study context, any grade anal abscess and enterovesical fistula were reported in two patients each; and anal fistula, gastrointestinal perforation, intestinal perforation, large intestine perforation and rectal perforation in one patient each. Anal abscess, enterovesical fistula, and anal fistula were mostly reported in primary in situ tumor patients (4 of 5 cases) and in patients with baseline NLR ≤ 5 (4 of 5 cases). Similarly, gastro-intestinal perforations were mostly observed in patients with primary in situ tumor (3 of 4 cases) and in patients with baseline NLR ≤ 5 (3 of 4 cases).
AEs possibly related to bevacizumab were reported in 88 (69%) patients; 38 (30%) patients experienced at least 1 Grade 3–5 AE possibly related to bevacizumab, the most frequently reported of which were pulmonary embolism (n = 13; 10%) and hypertension (n = 8; 6%), followed by neutropenia (n = 7; 6%), and proteinuria (n = 3; 2%).
Death on study
Eighty-two subjects died on study, the majority due to disease progression. Fatal AEs were reported in 7 (6%) patients and included acute renal failure, intestinal obstruction, pulmonary embolism, pneumonia, and sepsis in the primary in situ tumor population, and gangrene and aspiration pneumonia in the resected primary tumor population. The pulmonary embolism was considered related to bevacizumab.
Proteomic analysis
Fifty-one (40%) patients were included in the proteomic analyses. We examined whether the baseline abundances of 32 high-medium abundance plasma proteins had a relationship with PFS (S4 Table). Nine acute phase reactants were found to be significantly related with PFS (p < 0.05) after adjusting for the effect of NLR, and warrant further investigation in larger cohorts: A1AGLP [HR = 1.13 (95% CI: 1.00–1.28; p = 0.047)], A1MICG [HR = 0.09 (95% CI: 0.01–0.99; p = 0.049)] AACT [HR = 1.62 (95% CI: 1.04–2.53; p = 0.033)], APOC3 [HR = 0.77 (95% CI: 0.60–1.00; p = 0.049)], CRLPLSMN [HR = 14.65 (95% CI: 1.06–202.32; p = 0.045)], CRP (Logged and raw) [HR = 1.48 (95% CI: 1.09–2.02; p = 0.013], FIBB [HR = 0.80 (95% CI: 0.67–0.96; p = 0.014)], KNG1 [HR = 0 (95% CI: 0.00–0.49; p = 0.024)] and PREALB [HR = 0.01 (95% CI: 0.00–0.59; p = 0.027).
There were three Grade 3–5 AEs in the first cycle amongst patients in the proteomic sub-study population, therefore significance of the proteomic markers to predict Grade 3–5 AEs in the first cycle could not be assessed.
Discussion
This multi-center study was the first to prospectively evaluate the relationship between the host inflammatory response, measured by NLR, and outcomes in subjects with previously untreated mCRC who received bevacizumab-based first- and second-line treatment. Progression was based not on the RECIST criteria, but on investigators’ routine clinical assessment which enabled the primary endpoint to reflect the clinical course of disease under routine clinical practice. The observed median PFS and OS in both phases of our study is consistent with the published literature, reporting that patients continue to derive benefits from bevacizumab when used beyond progression.[16–18]
Although this study is significantly smaller than the retrospective, published, literature on NLR in other cancers, it did have enough power to detect a HR of 1.7 [10]. While our results did not prove the claim that NLR > 5 is prognostic for lower PFS [10, 19], the size of the association (HR of 1.4) is consistent with data published by Chua et al [10] and other subsequent data.[20, 21]
The observed association between longitudinal NLR measurements and OS [HR = 2.2 (95% CI: 1.2–4.0; p = 0.016)] indicates that NLR status of the patient at any time is associated with an increased rate of mortality and warrants further investigation. Results were after adjustment for the baseline disease characteristics included in the primary model. This is consistent with results from a recent meta-analysis [21] which analysed data from 9363 colorectal cancer patients and showed that elevated NLR was a negative predictor of outcome.[22]
The detected associations of PFS with the A1AGLP, A1MICG, AACT, APOC3, CRLPLSMN, CRP (Logged and raw), FIBB, KNG1 and PREALB proteomic markers were found through post-hoc exploratory analysis. Further validation of the associations is required, especially interesting for those proteins where large effects were observed (CRLPLSMN, KING1, PREALB).
The safety profile observed in the study was consistent with the known safety profile of bevacizumab, with no apparent differences between the primary in situ tumor and resected primary tumor populations. The numbers of reported perforations were not higher than the published data.
Conclusion
Although this study did not confirm the prognostic value of NLR in metastatic colorectal cancer patients, treated with bevacizumab, there was a trend towards worse PFS and OS with higher bNLR which is consistent with previous studies.[21]. Treatment related toxicities were consistent with prior experience with no apparent differences between the primary in situ tumor and resected primary tumor populations. No new safety signals were reported for this study.
Supporting information
(PDF)
(PDF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The authors would like to thank the clinical research team at Roche, the investigation sites and all the patients who participated in this study and their families. Medical writing assistance was provided by Dr Joseline Ojaimi from Roche Product, Pty. Limited.
List of abbreviations
- AACT
Acetoacetyl-coenzyme A thiolase
- AE
Adverse events
- A1AGLP
Alpha-1 acid glycoprotein
- A1MICG
Alpha-1 microglobulin
- APOC3
Apolipoprotein C-III
- CRLPLSMN
Ceruloplasmin
- CRP
C-reactive protein
- FAS
Full analysis set
- FIBB
Fibrinogen binding protein
- FOLFIRI
Folinic acid, fluorouracil, irinotecan regimen
- HR
Hazards ratio
- KNG1
Kininogen 1
- mCRC
metastatic colorectal cancer
- mFOLFOX6
Modified folinic acid, fluorouracil, oxaliplatin regimen
- NF-ΚB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NLR
Neutrophil/lymphocyte ratio
- OS
Overall survival
- PFS
Progression free survival
- PREALB
Prealbumin
- RECIST
Response evaluation criteria in solid tumors
- STAT-3
signal transducer and activator of transcription-3
- WHO
World health organization
- XELOX
Capecitabine plus oxaliplatin regimen
Data Availability
Availability of data and material: Qualified researchers may request access to individual patient level data through the clinical study data request platform (www.clinicalstudydatarequest.com). Further details on Roche's criteria for eligible studies are available here (https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Roche.aspx). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
Funding Statement
The study was sponsored by Roche Products, Pty. Limited. The funder provided support in the form of salaries for authors KJ as an employee of the funder and WR as an employee of the Clinical Research Organization contracted to perform the data management and statistical analyses of the study. The funder was involved in the study design, data collection and analysis, decision to publish, and preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.Welch S, Spithoff K, Rumble RB, Maroun J. Bevacizumab combined with chemotherapy for patients with advanced colorectal cancer: a systematic review. Ann Oncol. 2010;21(6):1152–62. 10.1093/annonc/mdp533 . [DOI] [PubMed] [Google Scholar]
- 2.Galfrascoli E, Piva S, Cinquini M, Rossi A, La Verde N, Bramati A, et al. Risk/benefit profile of bevacizumab in metastatic colon cancer: a systematic review and meta-analysis. Dig Liver Dis. 2011;43(4):286–94. 10.1016/j.dld.2010.10.010 . [DOI] [PubMed] [Google Scholar]
- 3.Wagner AD, Arnold D, Grothey AA, Haerting J, Unverzagt S. Anti-angiogenic therapies for metastatic colorectal cancer. Cochrane database of systematic reviews (Online). 2009;(3):CD005392. 10.1002/14651858.CD005392.pub3 . [DOI] [PubMed] [Google Scholar]
- 4.Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25(12):1539–44. 10.1200/JCO.2006.09.6305 . [DOI] [PubMed] [Google Scholar]
- 5.Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26(12):2013–9. 10.1200/JCO.2007.14.9930 . [DOI] [PubMed] [Google Scholar]
- 6.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. The New England journal of medicine. 2004;350(23):2335–42. 10.1056/NEJMoa032691 . [DOI] [PubMed] [Google Scholar]
- 7.Tebbutt NC, Wilson K, Gebski VJ, Cummins MM, Zannino D, van Hazel GA, et al. Capecitabine, bevacizumab, and mitomycin in first-line treatment of metastatic colorectal cancer: results of the Australasian Gastrointestinal Trials Group Randomized Phase III MAX Study. J Clin Oncol. 2010;28(19):3191–8. 10.1200/JCO.2009.27.7723 . [DOI] [PubMed] [Google Scholar]
- 8.Hanahan D, Weinberg Robert A. Hallmarks of Cancer: The Next Generation. Cell. 2011;144(5):646–74. 10.1016/j.cell.2011.02.013 S0092-8674(11)00127-9. [DOI] [PubMed] [Google Scholar]
- 9.Jarnicki A, Putoczki T, Ernst M. Stat3: linking inflammation to epithelial cancer—more than a "gut" feeling? Cell Division. 2010;5(1):14 10.1186/1747-1028-5-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chua W, Charles KA, Baracos VE, Clarke SJ. Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br J Cancer. 2011;104(8):1288–95. Epub 2011/03/31. 10.1038/bjc.2011.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dirican A, Kucukzeybek Y, Alacacioglu A, Varol U, Aksun S, Bayoglu IV, et al. Impact of pre-angiogenic factors on the treatment effect of bevacizumab in patients with metastatic colorectal cancer. Med Oncol. 2014;31(4):905 Epub 2014/03/07. 10.1007/s12032-014-0905-8 . [DOI] [PubMed] [Google Scholar]
- 12.Clarke S, Burge M, Cordwell C, Gibbs P, Reece W, Tebbutt N. An Australian translational study to evaluate the prognostic role of inflammatory markers in patients with metastatic ColorEctal caNcer Treated with bevacizumab (Avastin) [ASCENT]. BMC Cancer. 2013;13:120 Epub 2013/03/19. 10.1186/1471-2407-13-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song X, Amirkhani A, Wu JX, Pascovici D, Zaw T, Xavier D, et al. Analytical performance of nano-LC-SRM using nondepleted human plasma over an 18-month period. Proteomics. 2016;16(15–16):2118–27. Epub 2016/05/29. 10.1002/pmic.201500507 . [DOI] [PubMed] [Google Scholar]
- 14.McKay MJ, Sherman J, Laver MT, Baker MS, Clarke SJ, Molloy MP. The development of multiple reaction monitoring assays for liver-derived plasma proteins. Proteomics Clin Appl. 2007;1(12):1570–81. Epub 2007/12/01. 10.1002/prca.200700305 . [DOI] [PubMed] [Google Scholar]
- 15.Cox DR. Regression models and life tables (with discussion). J R Statist Soc B. 1972;34:187–220. [Google Scholar]
- 16.Bennouna J, Sastre J, Arnold D, Osterlund P, Greil R, Van Cutsem E, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14(1):29–37. Epub 2012/11/22. 10.1016/S1470-2045(12)70477-1 . [DOI] [PubMed] [Google Scholar]
- 17.Iwamoto S, Takahashi T, Tamagawa H, Nakamura M, Munemoto Y, Kato T, et al. FOLFIRI plus bevacizumab as second-line therapy in patients with metastatic colorectal cancer after first-line bevacizumab plus oxaliplatin-based therapy: the randomized phase III EAGLE study. Annals of oncology: official journal of the European Society for Medical Oncology. 2015;26(7):1427–33. Epub 2015/04/25. 10.1093/annonc/mdv197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakayama G, Uehara K, Ishigure K, Yokoyama H, Ishiyama A, Eguchi T, et al. The efficacy and safety of bevacizumab beyond first progression in patients treated with first-line mFOLFOX6 followed by second-line FOLFIRI in advanced colorectal cancer: a multicenter, single-arm, phase II trial (CCOG-0801). Cancer Chemother Pharmacol. 2012;70(4):575–81. Epub 2012/08/14. 10.1007/s00280-012-1948-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dirican A, Varol U, Kucukzeybek Y, Alacacioglu A, Erten C, Somali I, et al. Treatment of metastatic colorectal cancer with or without bevacizumab: can the neutrophil/lymphocyte ratio predict the efficiency of bevacizumab? Asian Pac J Cancer Prev. 2014;15(12):4781–6. Epub 2014/07/08. 10.7314/apjcp.2014.15.12.4781 . [DOI] [PubMed] [Google Scholar]
- 20.Wei B, Yao M, Xing C, Wang W, Yao J, Hong Y, et al. The neutrophil lymphocyte ratio is associated with breast cancer prognosis: an updated systematic review and meta-analysis. Onco Targets Ther. 2016;9:5567–75. Epub 2016/09/24. 10.2147/OTT.S108419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng DC, Zheng C, Wu J, Ye H, Chen JJ, Zhou B, et al. Neutrophil-lymphocyte ratio predicts the prognosis of patients with colorectal cancer: a meta-analysis. Int J Clin Exp Med. 2016;9(1):78–90. ISI:000371356600009. [Google Scholar]
- 22.Kozloff MF, Berlin J, Flynn PJ, Kabbinavar F, Ashby M, Dong W, et al. Clinical outcomes in elderly patients with metastatic colorectal cancer receiving bevacizumab and chemotherapy: results from the BRiTE observational cohort study. Oncology. 2010;78(5–6):329–39. Epub 2010/08/25. 10.1159/000320222 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
Availability of data and material: Qualified researchers may request access to individual patient level data through the clinical study data request platform (www.clinicalstudydatarequest.com). Further details on Roche's criteria for eligible studies are available here (https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Roche.aspx). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).


