Abstract
Nutritional Programming (NP) has been shown to counteract the negative effects of dietary plant protein (PP) by introducing PP at an early age towards enhancement of PP utilization during later life stages. This study explored the effect of NP and its induction time on growth, expression of appetite-stimulating hormones, and any morphological changes in the gut possibly responsible for improved dietary PP utilization. At 3 days post-hatch (dph) zebrafish were distributed into 12 (3 L) tanks, 100 larvae per tank. This study included four groups: 1) The control (NP-FM) group received fishmeal (FM)-based diet from 13–36 dph and was challenged with PP-based diet during 36–66 dph; 2) The NP-PP group received NP with dietary PP in larval stage via live food enrichment during 3–13 dph followed by FM diet during 13–36 dph and PP diet during 36–66 dph; 3) The T-NP group received NP between 13–23 dph through PP diet followed by FM diet during 23–36 dph and PP diet during 36–66 dph; and 4) The PP group received PP diet from 13–66 dph. During the PP challenge the T-NP group achieved the highest weight gain compared to control and PP. Ghrelin expression in the brain was higher in T-NP compared to NP-FM and NP-PP, while in the gut it was reduced in both NP-PP and T-NP groups. Cholecystokinin expression showed an opposite trend to ghrelin. The brain neuropeptide Y expression was lower in NP-PP compared to PP but not different with NP-FM and T-NP groups. The highest villus length to width ratio in the middle intestine was found in T-NP compared to all other groups. The study suggests that NP induced during juvenile stages improves zebrafish growth and affects digestive hormone regulation and morphology of the intestinal lining–possible mechanisms behind the improved PP utilization in pre-adult zebrafish stages.
Introduction
Replacement of fishmeal (FM) in fish diets with plant protein (PP) has been an ongoing challenge in the aquaculture industry. High-quality PP sources such as soy or pea protein concentrates and wheat or corn gluten have been widely used by the feed industry since their digestibility in some species is comparable to FM. However, their price can often exceed the cost of marine raw materials. Although some progress with utilization of lower-quality PP, such as soybean meal, has been made, a number of concerns must still be overcome including a presence of anti-nutritional factors responsible for inducing intestinal inflammation, to maintain acceptable growth rates and feed efficiency values at high FM substitution levels.
Nutritional programming (NP) has been found to be a promising approach to counteract the negative effects of PP in feeds. NP in fish only recently has received more attention and some studies already indicate it is possible to “imprint” fish during their young age with alternative raw materials or nutrient levels to allow them to utilize different dietary compounds more efficiently later in life. Geurden et al. [1] first found that juvenile rainbow trout Oncorhynchus mykiss subjected to PP-based diet during the first three weeks of life showed improved acceptance and utilization of the same dietary PP-based diet when given during later life stages. Furthermore, two other studies reported that early exposure of young European seabass Dicentrarchus labrax to diets deficient in long-chain polyunsaturated fatty acids induced higher expression of delta-6 desaturase mRNA levels in juveniles–the rate-limiting enzyme involved in biosynthesis of highly unsaturated fatty acids [2,3]. Most of the NP investigations on fish focus, however, on NP with PP induced during fish juvenile stages. Perera and Yufera [4] attempted to nutritionally program zebrafish Danio rerio in its larval stage with soybean meal-based feeds at first feeding but without success. It is therefore critical to understand if an optimal timing exists for NP to take effect and improve the capacity of the fish to utilize PP better for growth later in life.
Although NP has a great potential to improve fish growth and health performance during the grow-out phase, the mechanism behind the NP phenomenon remains elusive. Balasubramanian et al. [5] indicated that early PP diet exposure in rainbow trout might mediate feed acceptance of the same diet at a later life stage by affecting pathways regulating the sensory perception of taste, odor, and vision. If improved dietary PP utilization induced by NP is a result of improved palatability towards dietary PP, can the negative effects of anti-nutritional factors present in PP still induce morphological changes in fish digestive tract as seen in other studies [6–9] Dietary PP have been associated with many cases of intestinal inflammation in several fish species limiting their dietary inclusion rates [4,6,10–14]. Some of the typical signs of dietary PP-induced inflammation in the intestinal mucosa include shortening of the mucosal folds which decrease the capacity of the digestive tract to digest, absorb, and utilize nutrients consequently affecting fish growth and health by diminishing their response to pathogens [15]. Whether the process of NP is driven solely by increased palatability and therefore improved feed intake, or by specific intestinal responses to dietary PP that lead to morphological adaptations remains unknown. We believe that NP with dietary PP alters the gut epithelial lining and consequently increases fish resistance to negative side-effects of PP, thereby improving fish ability to cope with those alternative raw materials. Furthermore, we also believe that if improved taste is in fact the key driver for improved growth performance in previously “programmed” fish, we would expect to observe differential expression of digestive hormones responsible for appetite control between programmed and non-programmed individuals. Therefore, the objective of our study was to explore the effect of NP and its induction time on growth, expression of appetite-stimulating hormones, and any morphological changes in the gut possibly responsible for improved dietary PP utilization in zebrafish. This study found that NP with PP induced during juvenile stages improves zebrafish growth and affects digestive hormone regulation and morphology of the intestinal lining—possible mechanisms behind the improved PP utilization in pre-adult zebrafish stages.
Materials and methods
The feeding trial was conducted in the Center for Fisheries, Aquaculture, and Aquatic Sciences at Southern Illinois University-Carbondale (SIUC), IL. All experiments were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of SIUC. The SIUC Institutional Animal Care and Use approved all of the protocols performed (protocol# 18–007). During fish handling anesthesia was performed using water bath immersion in tricaine methanesulfonate (MS222) at a recommended concentration, and all efforts were made to minimize pain, stress, and discomfort in the animals.
Experimental fish
The experiment was conducted with zebrafish since it is considered an established model species for various research areas including genetics, nutrition, biomedicine, and toxicology; additionally, its popularity is now growing in the aquaculture research field [16]. It is an omnivorous teleost species that can utilize both plant and animal protein sources efficiently for growth serving as a great model for assessment of nutritional pathways in carnivorous and herbivorous aquaculture species [16].
All experiments were carried using recirculated aquaculture system (Pentair Aquatic Eco-systems, Cary, NC) consisting of biofilter, carbon filter, UV light, and pH/conductivity automatic adjustment feature. The experimental culture system used reversed osmosis as the main water source where pH and conductivity were maintained at optimal levels of 6.9±0.2 and 1584±27 μS, respectively. The average water temperature throughout the feeding trial was 27.1 ± 0.2 °C. The photoperiod consisted of 14 hours of darkness and 10 hours of light, with the overhead lights on from 8:00–18:00.
Zebrafish broodstock was obtained from a local pet store (Petco, Carbondale, IL). Males and females were kept in separate tanks and fed 2–3 times a day with commercial feed (Otohime, Japan) and Artemia nauplii for two weeks before breeding. Fish were combined for breeding in a 2:1 ratio of females to males. A wire net with a 1.5 mm mesh was placed in breeding tank with artificial plants to induce spawning. The fish were left to breed for 24 hours. The broodstock was then removed after spawning on the next day. The wire mesh was taken out, and eggs hatched approximately 48 hours at 27 °C. At 3 days post-hatch (dph) after mouth opening and when the larvae started actively swimming, they were randomly distributed into experimental tanks.
Zebrafish were fed with rotifers Brachionus plicatilis only for the first two days after the swim up stage (3–4 dph). The rotifers were obtained from cysts purchased from a commercial vendor (Brine Shrimp Direct, Ogden, Utah). Starting at 5 dph all fish received Artemia nauplii obtained from hatching of cysts from a commercial source (GSL Brine Shrimp, Ogden, Utah) together with rotifers (5–7 dph) and then Artemia nauplii only from 8–13 dph. All groups were fed ad libitum throughout the study.
Experimental diets
All the experimental feeds were formulated and produced at SIUC. Two experimental diets were tested: a diet based on soybean meal and soy protein concentrate as main protein sources replacing 80% of marine animal protein (PP diet; the soy concentrate was included to adjust dietary crude protein while leaving room for other ingredients in the formulation, including a minimum level of starch to allow expansion and floatability of the experimental diets); and a diet based on fishmeal as a main protein source (FM diet). Both diets were formulated to be isonitrogenous (49% crude protein) and isolipidic (10% lipid) (Table 1).
Table 1. Dietary formulation (g/100 g) of experimental diet.
| Ingredients | FM-diet | PP-diet |
|---|---|---|
| Fish meal1 | 63.7 | 0 |
| Soybean meal2 | 0.0 | 45.5 |
| Soy protein concentrate3 | 0.0 | 16.0 |
| Krill Meal4 | 10.0 | 10.0 |
| CPSP5 | 5.0 | 5.0 |
| Dextrin3 | 5.4 | 0.0 |
| Fish Oil6 | 4.2 | 7.8 |
| Soy Lecithin6 | 5.0 | 5.0 |
| Mineral mix3 | 2.5 | 2.5 |
| CaHPO47 | 0.0 | 1.5 |
| Vitamin mix3 | 2.0 | 2.0 |
| Vitamin C8 | 0.1 | 0.1 |
| Choline chloride3 | 0.1 | 0.1 |
| Methionine3 | 0.0 | 0.5 |
| Lysine3 | 0.0 | 2.0 |
| Threonine3 | 0.0 | 0.1 |
| Taurine3 | 1.0 | 1.0 |
| Guar Gum3 | 1.0 | 1.0 |
| Sum | 100 | 100 |
1 Omega Protein, Reedville, VA, USA
2 Premium Feeds, Perryville, MO, USA
3 Dyets Inc, Bethlehem, PA, USA
4 Florida Aqua Farms, Dade City, FL
5 Soluble fish protein concentrate, Sopropeche, France
6 MP Biomedicals, Solon, OH, USA
7 Acros Organics, NJ, USA
8 Argent Aquaculture, Redmond, WA, USA
All dry protein ingredients (fishmeal, krill meal, and soybean meal) were added to a centrifugal mill (Retsch Haan, Germany) and ground to 0.5 micrometers. After the grinding process, all ingredients were manually sieved through a 0.25-micrometer sieve to ensure all particles were of the appropriate and uniform size. All the dry ingredients (excluding soy lecithin and choline chloride) were added together and mixed for 15 minutes and the fish oil was then added with the soy lecithin dissolved in the oil. The oil and dry ingredients were mixed again for 15 minutes. Finally water (~10–15% of total mass of feed) was added with dissolved choline chloride. Feeds were then slowly added to the extruder (Caleva Extruder 20, Sturminster Newton Dorset, England) at levels between 20–24 rpm to obtain a proper extrudate size and firmness. Extrudates were then processed using a spheronizer (Caleva, Sturminster Newton Dorset, England) at 600 rpm for 3 min, 1800 rpm for 30 seconds, and then 600 rpm for 2–5 minutes to finish the process. Finally, the extrudates were dried using a freeze dryer (Labconco, Kansas City, MO). All dried pellets were sieved to appropriate sizes using a vibratory sieve shaker (Retsch Hann, Germany). All finished feeds were stored in bags at -20°C. While the feeds were being used in experimentation, they were kept at 4°C.
Experimental design
At 3 dph zebrafish larvae were randomly distributed into 12 (3 L) tanks, 100 larvae per tank. The study lasted until fish reached 66 dph. Four different feeding regimes were investigated: 1) The first group received FM-based diet after the live food period from 13–36 dph and was challenged with PP-based diet between 36–66 dph (control, NP-FM); 2) The second group received NP with dietary PP in the early larval stage via live food enrichment during 3–13 dph followed by fishmeal (FM)-based diet during 13–36 dph and PP-based diet during 36–66 dph (NP-PP); 3) The third group received NP with dietary PP between 13–23 dph through formulated diet after the live food period followed by FM-based diet during 23–36 dph and PP-based diet during 36–66 dph (T-NP); and 4) The fourth group received PP-based diet after the live food period from 13–66 dph (negative control; PP) (Fig 1).
Fig 1. Dietary treatment regimens tested in the study.
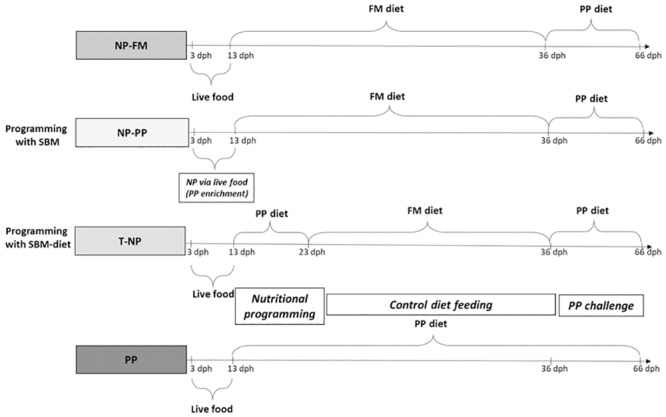
NP–nutritional programming, FM–fishmeal, PP–plant protein, SBM–soybean meal.
For the NP of the NP-PP group an enrichment of the live food was prepared with soybean meal. Both rotifers and Artemia nauplii were enriched by adding previously sieved finely ground soybean meal (<0.15mm) into the water of the rotifer or Artemia culture for a minimum of 3 hours prior to fish feeding (Fig 2). All the other groups (NP-FM, T-NP, and negative control) received live food enriched with Spirulina algae (Earthrise, Irvine, CA) using the same enrichment procedure.
Fig 2. Rotifers after 2 hours of enrichment with Spirulina or ground, blended, and sieved soybean meal.
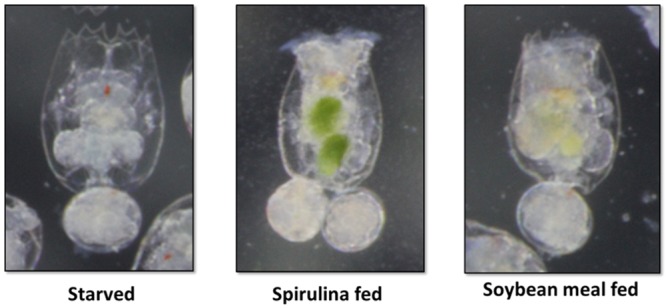
Measured responses
At the end of the feeding trial, fish in each tank were counted and weighed. The following quantified growth performance parameters were assessed:
Survival (%) = 100 × (final number of fish/initial number of fish)
Final Weight (g) = Final body weight–initial body weight
Weight gain (% of initial weight) = 100 × (final body weight–initial body weight)/initial body weight.
At the beginning (NP phase), in the middle (control phase), and at the end of the trial (PP challenge) feed intake was assessed by measuring the amount of feed that was consumed in one meal in each tank (feeding was ceased when fish showed signs of feed rejection).
At the end of the study, samples of fish were taken 3 hours (postprandial levels) and 24 hours (physiological baseline) after feeding from each group and preserved in RNALater® (Sigma-Aldrich, St Louis, MO) to assess the expression of digestive hormones. In addition, three fish from each tank were sampled, their digestive tracts were dissected and preserved in 10% buffered formalin for histological evaluation of the intestinal villi.
Digestive hormone analysis
The digestive hormones gene expression analysis was conducted in the laboratory of Animal Biotechnology and Aquaculture of the Department of Biotechnologies and Life Sciences at University of Insubria, Varese, Italy.
Total RNA extraction and cDNA synthesis
Total RNA was extracted from zebrafish brain and intestine samples using an automatic system (Maxwell® 16 Instrument, Promega). The extracted RNA was quantified by using NanoDrop™ 2000c spectrophotometer (Thermo Scientific) and reverse transcribed into cDNA following the protocol described in the SuperScript III Reverse Transcriptase kit (Invitrogen, Milan, Italy).
Primer design and amplification, and molecular cloning and sequencing of five target genes
The primers for the amplification of ghrelin, leptin, cholecystokinin (CCK), orexin, and neuropeptide Y (NPY) genes were designed based on the cDNA sequences of these genes in Danio rerio available in GenBank database. The accession numbers of the sequences are AM055940.1 for ghrelin, BN000830.1 for leptin, XM_001346104.6 for CCK, NM_001077392 for orexin, and BC162071.1 for NPY gene. The primer sequences are listed in Table 2.
Table 2. Primers used for the molecular cloning of target genes.
| Gene | Primer | Nucleotide sequence (5’- 3’) |
|---|---|---|
| Ghrelin | Forward | GCATGTTTCTGCTCCTGTGTG |
| Reverse | AGATTCTGAAGCACGGGACC | |
| Leptin | Forward | CGCTGACAAACCCATCCAAG |
| Reverse | CAGCTGGTCCAGATTATCGATCA | |
| CCK | Forward | CAGCTCTCTCTGCGTCTCTG |
| Reverse | TGCGGTATGAGCCTTTGGTT | |
| Orexin | Forward | TCCAGGTGCTCGTCTTCATG |
| Reverse | CTCCAGCCTCTTCCCCATTG | |
| NPY | Forward | AAGATGTGGATGAGCTGGGC |
| Reverse | TGGCATGGTGATCTCATCCAC |
An aliquot of cDNA obtained by reverse transcription was amplified by PCR using the designed primer sets. The plasmid was finally purified using the NucleoSpin® Plasmid kit (Macherey-Nagel, Milan, Italy) and then sequenced in both directions (T7 and SP6).
Quantitative real-time RT-PCR
Generation of in vitro-transcribed mRNAs for target genes
Based on the cDNA sequences of zebrafish ghrelin, leptin, CCK, orexin, and NPY genes, sequenced as aforementioned, forward and reverse primers were designed for each gene (Table 3). The forward primers were engineered to contain at the 5’ end the sequence of the T3 RNA polymerase promoter that is necessary for in vitro transcription of the mRNAs of each target gene. The T3 forward primers and their respective reverse primers were used in a conventional PCR reaction starting from plasmid. Then, the PCR product was checked on agarose gel, purified, and subsequently sequenced.
Table 3. Primers used for the synthesis of standard mRNAs.
| Gene | Primer | Nucleotide sequence (5’- 3’) |
|---|---|---|
| Ghrelin | T3 Forward | caattaaccctcactaaagggGCATGTTTCTGCTCCTGTGTG |
| Reverse | AGATTCTGAAGCACGGGACC | |
| Leptin | T3 Forward | caattaaccctcactaaagggCGCTGACAAACCCATCCAAG |
| Reverse | CAGCTGGTCCAGATTATCGATCA | |
| CCK | T3 Forward | caattaaccctcactaaagggCAGCTCTCTCTGCGTCTCTG |
| Reverse | TGCGGTATGAGCCTTTGGTT | |
| Orexin | T3 Forward | caattaaccctcactaaagggTCCAGGTGCTCGTCTTCATG |
| Reverse | CTCCAGCCTCTTCCCCATTG | |
| NPY | T3 Forward | caattaaccctcactaaagggAAGATGTGGATGAGCTGGGC |
| Reverse | TGGCATGGTGATCTCATCCAC |
Sequencing was necessary both to confirm the presence of the T7 promoter and to count the number of nucleotides present for the subsequent calculation of the molecular weight (MW—molecular weight) of each standard determined using the following formula: MW = [(n° of bases A x 329.2) + (n° of bases U x 306.2) + (n° of bases C x 305.2) + (n° of bases G x 345.2)] + 159.
In vitro transcription was performed using T3 RNA polymerase and other reagents supplied in the RiboProbe In Vitro Transcription System kit (Promega, Italy) according to the manufacturer’s protocol. The concentration of the mRNAs thus obtained was measured by reading the absorbance at 260 nm by means of NanoDrop ™ 2000c (Thermo Scientific). By knowing the molecular weight and the concentration of each mRNA it was possible to determine the number of molecules/μL for each target gene.
Generation of standard curves and real-time RT-PCR for quantification
The synthetic mRNAs of each gene were used as quantitative standards in the analysis of biological samples. For this, one hundred nanograms of total RNA extracted from fish samples were amplified via One-step SYBR® Green quantitative real-time RT-PCR, in the same plate with defined amounts of synthetic mRNAs at 10-fold dilutions, using iTaq™ Universal SYBR® Green One-Step kit (Bio-Rad, Italy). The sequences of primers used for target gene amplification are shown in Table 4. SYBR® Green PCR reactions were performed on a Bio-Rad® CFX96™ System.
Table 4. Primers and probes used for one-step SYBR® Green real-time RT-PCR.
| Gene | Primer | Nucleotide sequence (5’- 3’) |
|---|---|---|
| Ghrelin | Forward | GTGTCTCGAGTCTGTGAGCG |
| Reverse | CAGCTTCTCTTCTGCCCACT | |
| Leptin | Forward | TGTTGACCAGATACGCCGAG |
| Reverse | GTCCAGCGCTTTCCCATTTG | |
| CCK | Forward | GTTCAGTCTAATGTCGGCTCC |
| Reverse | TAGTTCGGTTAGGCTGCTGC | |
| Orexin | Forward | CTACGAGATGCTGTGCCGAG |
| Reverse | GAGTGAGAATCCCGACAGCG | |
| NPY | Forward | TGGGGACTCTCACAGAAGGG |
| Reverse | AATACTTGGCGAGCTCCTCC |
Data from the real time PCR runs were collected with CFX™ Software. The Ct values of the standard mRNAs amplification were used to create standard curves, which were then used for the calculation of the copies of mRNAs in the biologic samples.
Histological analyses
The intestines previously fixed in 10% neutral buffered formalin were processed to paraffin using a Sakura enclosed automated tissue processor (Netherlands). The three representative areas of zebrafish intestines were orientated for cross sections embedded together in the same block. Five micrometer serial sections were cut with a Leica manual microtome (Buffalo Grove, IL) and placed on water bath at 44°C. Sections were placed on positive charged slides. After drying, the slides were stained with hematoxylin and eosin and cover-slipped using acrylic mounting media. The histological analysis of the mid-gut portions of fish digestive tract focused on the tissue sections of medium diameter. Pictures of the samples were taken at 100x magnification using a microscope (Nikon SMZ1500, Japan) and a camera (Nikon Digital Sight, Japan).
To obtain villus length and width, pictures of each slide were obtained using a microscope (Leica DMI 300B) and camera (Leica DMC 290) combination, with the software LAS V4.4 (Leica Camera, Wetzler, Germany). From these pictures, individual lengths and widths were taken of intact villi using ImageJ (NIH, Betheseda, MD, USA). Length and width data were measured according to Karimi and Zhandi [17] in the three different segments of the intestine; proximal, middle, and distal. Villi length was measured from the tip of the villus to the luminal surface, and villi width was measured across the base of the villus at the luminal surface. The length-to-width ratio of each villus was determined by dividing the length by the width.
Statistical analyses
Results are presented as means ± standard deviation. One-way ANOVA was used followed by Tukey’s test using the software R. Differences with p values <.05 were considered significant.
Results
Growth performance
At the end of the study the lowest average weight was achieved by the PP group compared to all other groups. No differences were detected in average weight between NP-FM, NP-PP, or T-NP groups (Table 5).
Table 5. Dietary treatment effect on growth performance.
Values are presented as means (± std. dev). Superscript letters indicate statistical significance between groups. The significance was determined using a One-Way ANOVA and a Tukey Test with a p value <0.05.
| NP-FM | NP-PP | T-NP | PP | |
|---|---|---|---|---|
| Avg. Weight (g) | 0.37a (± 0.04) | 0.36a (± 0.05) | 0.42a (± 0.03) | 0.26b (± 0.03) |
| Avg. Weight Gain (g) | 0.29a (± 0.03) | 0.28a (±0.03) | 0.34a (±0.03) | 0.20b (±0.03) |
| Weight Gain (%) | 353.8ab (± 14.7) | 404.0ac (± 45.1) | 430.3c (±34.6) | 364.6b (± 29.9) |
During the PP challenge the T-NP group achieved the highest weight gain compared to both control groups NP-FM and PP. The NP-PP group achieved higher weight gain compared to the negative control; however, no differences were detected with NP-FM or T-NP groups. No differences in the feed intake were detected during NP phase, control feeding phase, or PP challenge. Similarly, no differences were found in the survival (~50% assessed for the trial duration from 3 until 66 dph) between the different dietary regimes.
Digestive hormones expression
At the end of the study expression of digestive hormones was measured in the gut and/or brain 3 and 24-hours after feeding representing postprandial and basal levels, respectively. Ghrelin expression in the brain was significantly higher in the T-NP group compared to NP-FM and NP-PP groups but not different with the PP group. Ghrelin expression in the gut was significantly reduced in both NP-PP and T-NP groups compared to the other groups. No differences were detected in the gut ghrelin expression between NP-PP and T-NP nor between NP-FM and PP groups (Fig 3).
Fig 3. Ghrelin expression in the brain and gut.
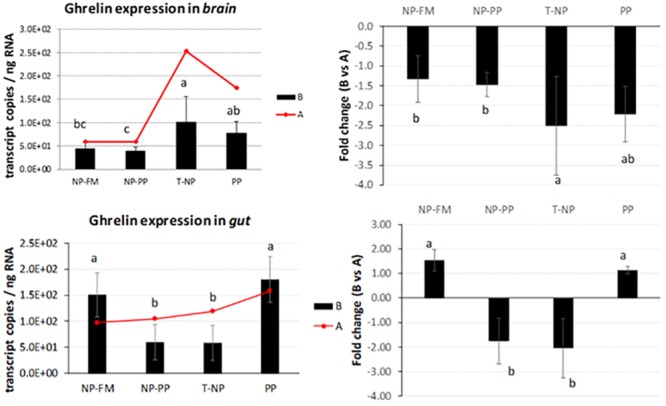
The expression of ghrelin presented as number of ghrelin transcript copies per ng of total RNA in zebrafish brain and gut at 3-hours (B) and 24-hours (A) after feeding. The fold change presents the difference in ghrelin transcript copies between 3 and 24-hour samples. Different letters indicate statistical difference at p<0.05.
CCK expression in the brain was significantly reduced in NP-PP and T-NP compared PP group but not different with NP-FM. CCK expression in the gut showed a similar trend, levels were lower in NP-PP and T-NP groups compared to PP but no difference was detected between T-NP and NP-FM (Fig 4).
Fig 4. CCK expression in the brain and gut.
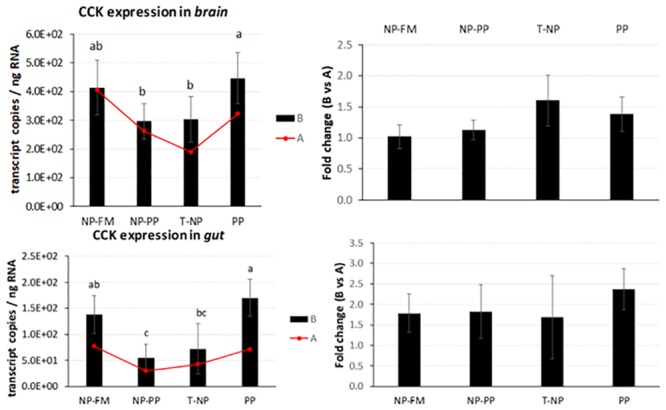
The expression of CCK presented as number of CCK transcript copies per ng of total RNA in zebrafish brain and gut at 3-hours (B) and 24-hours (A) after feeding. The fold change presents the difference in CCK transcript copies between 3 and 24-hour samples. Different letters indicate statistical difference at p <0.05.
Leptin expression in the brain and gut was not different between groups 3-hours after feeding, but the level of leptin mRNA in T-NP fish 3-hours after feeding in the gut was lower compared to 24-hour sampled fish (Fig 5).
Fig 5. Leptin expression in the brain and gut.
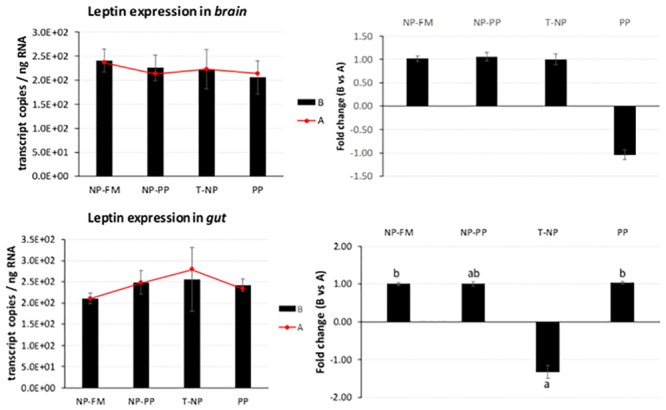
The expression of leptin presented as number of leptin transcript copies per ng of total RNA in zebrafish brain and gut at 3-hours (B) and 24-hours (A) after feeding. The fold change presents the difference in leptin transcript copies between 3 and 24-hour samples. Different letters indicate statistical difference at p <0.05.
No significance was detected in orexin expression in the brain nor the gut. However, an interesting trend was observed showing numerically higher expression level of brain orexin in T-NP and PP compared to the other groups (p>0.05). A similar trend was also seen in the gut but no significant differences were actually detected (Fig 6).
Fig 6. Orexin expression in the brain and gut.
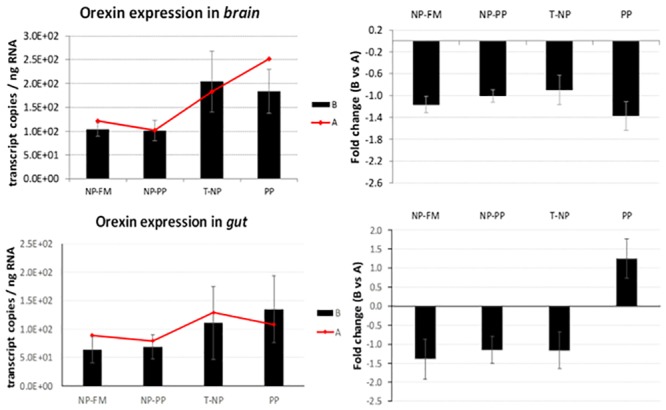
The expression of orexin presented as number of orexin transcript copies per ng of total RNA in zebrafish brain and gut at 3-hours (B) and 24-hours (A) after feeding. The fold change presents the difference in orexin transcript copies between 3 and 24-hour samples. Different letters indicate statistical difference at p <0.05.
NPY expression in the brain was significantly lower in the NP-PP compared to PP group but not different with NP-FM and T-NP groups (Fig 7).
Fig 7. NPY expression in the brain.
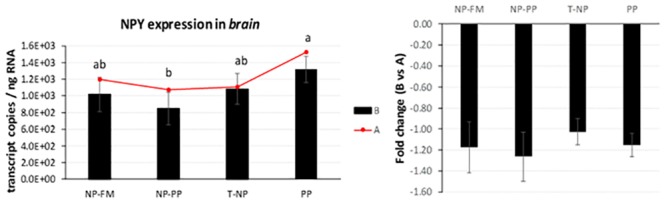
The expression of NPY presented as number of NPY transcript copies per ng RNA in zebrafish brain 3-hours (B) and 24-hours (A) after feeding. The fold change presents the difference in NPY transcript copies between 3 and 24-hour samples. Different letters indicate statistical difference at p <0.05.
Intestinal villi
The length of intestinal villi in the proximal intestine was the highest in NP-FM group compared to all other groups. The villi width was found higher in NP-FM group compared to PP and T-NP groups but not different with NP-PP. In the middle intestine, however, the T-NP fish displayed higher villi length compared to PP group and not different compared to NP-FM and NP-PP groups. Finally, in the distal portion the T-NP group had the highest villi length compared to NP-PP and PP groups and not different with NP-FM group. No differences were detected in villi width in the middle or distal intestine.
The highest villus length to villus width ratio in the proximal intestine was found in T-NP compared to both NP-PP and PP groups and not different with NP-FM group. In the middle intestine the T-NP group had the highest villus length to width ratio compared to all other groups and no differences were detected between NP-FM, NP-PP, and PP groups. No differences were detected among groups in the length to villus ratio in the distal intestine (Table 6).
Table 6. Treatment effect on intestinal villi length, width, and length to width ratio.
Units for villi length and width are μm. Values are presented as means (± std. dev). Superscript letters indicate statistical significance between groups. The significance was determined using a One-Way ANOVA and a Tukey Test with a p value <0.05.
| Group | ||||
|---|---|---|---|---|
| Intestinal Segment | NP-FM | NP-PP | T-NP | PP |
| Proximal | ||||
| Length | 314.47b (± 58.14) | 213.12a (± 47.64) | 221.93a (± 72.21) | 188.24a (± 60.22) |
| Width | 112.18c (± 42.21) | 91.60bc (± 22.79) | 67.70a (± 13.40) | 79.43ab (± 17.80) |
| Ratio | 3.16ab (± 1.30) | 2.43a (± 0.66) | 3.37b (± 1.24) | 2.45a (± 0.91) |
| Middle | ||||
| Length | 130.19ab (± 13.87) | 129.84ab (± 31.74) | 156.64b (± 33.71) | 121.97a (± 22.25) |
| Width | 70.09 (± 23.91) | 68.89 (± 22.86) | 56.92 (± 11.73) | 69.89 (± 14.98) |
| Ratio | 2.07a (± 0.78) | 1.99a (± 0.59) | 2.84b (± 0.72) | 1.80a (± 0.40) |
| Distal | ||||
| Length | 160.92bc (± 21.99) | 106.41a (± 25.75) | 182.21c (± 43.33) | 130.62ab (± 52.30) |
| Width | 86.33 (± 20.76) | 63.63 (± 22.59) | 79.44 (± 20.18) | 81.86 (± 21.80) |
| Ratio | 1.98 (± 0.66) | 1.86 (± 0.82) | 2.40 (± 0.81) | 1.64 (± 0.71) |
Discussion
Nutritional programming has been shown to improve growth performance in different species such as rainbow trout [1], yellow perch Perca flavescens [18], and gilthead seabream Sparus aurata [19,20]. Other studies also point out the importance of maternal NP. For example, Izquierdo et al. [19] found that it is possible to achieve improved growth of 4-month old gilthead seabream juveniles fed low FM and low fish oil diets by previously exposing the broodstock of those fish to high vegetable-based oil feeds. Moreover, these authors later showed that the same fish at 16-month of age were still able to grow on low FM/fish oil diets better compared to control group suggesting positive long-term effect of NP on utilization of vegetable-based diet [20]. Perera and Yufera [4] induced NP during the first three days after mouth opening in zebrafish but did not observe any differences in the growth rate during later feeding with soybean meal-based diet. An induction of NP with dry feed as suggested by Perera and Yufera [4] in species that requires live food for proper growth, development, and survival, during the first feeding poses a risk of low or no food consumption defeating the actual NP outcome. We believe that programming with PP using a standard zebrafish rearing protocol utilizing live food as a vector for soybean meal could be a logical and straightforward approach in helping fish to adapt to this alternative protein source. Our experiment showed that dietary PP utilization and associated higher weight gain could potentially be improved in zebrafish with NP induced using live food as a PP vehicle and/or during zebrafish weaning period with dry PP-based feed. The experiment showed that after the PP challenge the T-NP group achieved the highest weight gain compared to both control groups suggesting that NP with dietary PP improves its utilization in zebrafish. The NP-PP group achieved higher weight gain compared to the negative control, however, no differences were detected with NP-FM or T-NP groups. It is possible that if the study was prolonged differences could also be detected between NP-PP and NP-FM groups. However, since the fish reach sexual maturation after approximately two months after hatching leading to size discrepancies within tanks and groups, the feeding trial had to be terminated at 66 dph before an obvious sexual dimorphism started to appear. Another possible scenario is that perhaps an optimal window exists for fish to respond to NP effectively. The live food used in the study, rotifers and Artemia nauplii, were both enriched with soybean meal and their enrichment status was assessed by change in body color (Fig 2) and therefore we believe there was an exposure to PP in larval zebrafish during the first days of feeding. It is possible, however, that fish must reach a certain developmental stage to be able to positively respond to the imposed nutritional trigger (such as soybean meal) to better adapt to it at a later age.
Flavor experiences during early life stages have been shown to be a driver for life-long flavor acceptance in adults’ life. For example, citric acid, which has a sour/bitter taste, is known to be an instinctively aversive substance for rats. However, rats exposed/programmed to citric acid during nursing showed increased voluntary ingestion of citric acid compared to rats that were not exposed to citric acid at all [21]. We hypothesized that since improved taste has been considered as one of the key drivers for improved growth performance in “programmed” fish [5], differential expression of digestive hormones responsible for appetite control between programmed and non-programmed fish would be observed. Fish gastrointestinal tract is characterized by certain amount of plasticity dependent on various signaling pathways which allow the gut to adapt to rapid shifts in environmental conditions including a diet [22]. Some of these signals are based on hormones that can be divided into two groups: those that induce (orexigenic), and those that inhibit (anorexigenic) appetite and food consumption. Peripheral signals originate in the gastrointestinal tract, liver, adipose tissue, and others, and reach the brain through both endocrine and neuroendocrine actions. The hypothalamus is considered the center that controls appetite and energy balance and integrates the different chemical (nutrient levels), mechanical (feed volume in the gut), and endocrine signals [23]. The available data on hormonal regulation of the gastrointestinal tract in teleosts suggest that some gastrointestinal hormones not only regulate digestion but also act as appetite/satiety modulating signals in the brain. When ghrelin was first discovered in rats it was demonstrated to be a powerful stimulator of food intake (the first known peripheral hormone with this effect) that stimulates body weight gain and adiposity in mammals. In the present study, fish brain and whole digestive tract samples were taken 3- and 24-hours after feeding. In T-NP fish 3-hours after feeding the level of ghrelin in the brain was higher compared to NP-PP and control fish. In the gut however, the level of ghrelin in both “programmed” groups was lower compared to control (NP-FM) and PP groups. Moreover, the level of ghrelin mRNA expression in NP-PP and T-NP groups 3-hours after feeding was lower compared to fish sampled 24-hours after feeding. Ghrelin in zebrafish is mainly expressed in endocrine pancreatic cells [24] and its higher mRNA levels have been associated with fasting [25]. The lower ghrelin expression level in the gut observed in our study might possibly indicate fish in “programmed” (NP-PP and T-NP) groups to be in a satiated (fed) state compared to both controls. In rainbow trout Oncorhynchus mykiss ghrelin was shown to have an anorexigenic effect that leads to suppression of food intake. However, in cyprinids such as goldfish Carassius auratus, ghrelin was reported to increase food intake through stimulation of NPY and orexin neurons in the brain and a potential feedback of orexin on ghrelin expression, which might help explain the elevated ghrelin levels in the T-NP fish in zebrafish brain in our study. Orexin has been found to stimulate appetite and food consumption after fasting in different fish species including zebrafish [26–29]. We found orexin expression levels in both brain and gut not significantly different among groups. However, there was a trend for higher levels of orexin in T-NP and PP groups compared to the other groups, particularly in the brain, possibly supporting the aforementioned assumption about the orexin-related increased ghrelin levels in T-NP group in the brain.
The role of NPY in feed regulation in fish has not been studied extensively but the available literature indicates that NPY in fish has orexigenic effects similar to those observed in mammals. More specifically, higher levels of NPY have been associated with increased food intake in zebrafish [30]. The NPY brain expression levels have been reported higher around feeding time and lower post-prandially in Chinook salmon Oncorhynchus tshawytscha, Coho salmon Oncorhynchus kisutchand [31], and in goldfish [32,33] further suggesting a role of NPY as an appetite stimulator consequently being widely considered as one of the most highly conserved neuropeptides in vertebrates [30]. Our study found NPY expression to be higher in the PP group compared to NP-PP and not different with T-NP and NP-FM groups, suggesting that perhaps fish in the PP group were in an “unfed” state 3-hours after feeding. Valen et al. [34] observed higher NPY mRNA levels in fed Atlantic salmon Salmo salar compared to unfed fish 1.5 and 9-hours after feeding. The authors argued, however, that the increased NPY expression was probably a result of digestive tract feedback since after 9 hours the feed had likely been evacuated from the digestive tract rendering the fish to be in “unfed” state again.
Leptin—an antagonist of ghrelin, is an anorexigenic hormone released mostly by adipose tissue in mammals. In fish, leptin has been found in liver, brain, muscle, gonads, kidneys, and other tissues [23,35–37] and its effects on energy metabolism in fish are still perplexing. An increased postprandial hepatic leptin expression have been observed in common carp Cyprinus carpio [35] and orange spotted grouper Epinephelus coioides [38] confirming its role as a satiety signal comparable to mammals. Murashita et al. [39] showed reduced growth performance of juvenile Atlantic salmon after intraperitoneal infusion of recombinant salmon specific leptin at a dose of 10 ng/g/h using implanted micro-osmotic pump over a three-week period. However, leptin response to feeding status varies between species and salmonids show an opposite trend to the mammalian model in which fasting or feed restriction actually leads to decreased leptin levels. Trombley et al. [36] reported 9-fold higher expression levels of hepatic leptin and 2.3 times higher plasma leptin levels in Atlantic salmon in the feed-restricted group (40% of the optimal feeding rate) compared to the control group (optimal feeding rate). Similar results were obtained by Kling et al. [40] who found plasma leptin levels to be higher in fasted rainbow trout compared to fed trout throughout 21 days of the experimental period. In our study, leptin expression in the brain was not significantly different among groups. Leptin expression in the gut was not different between groups 3-hours after feeding suggesting that either a longer time is required for the leptin signal to take effect or the link between diet and leptin regulation in zebrafish is not significant if all the fish receive similar food rations across groups as opposed to feeding versus starving. This first assumption can be consolidated by the observed rise in leptin mRNA levels in T-NP group 24-hours after feeding compared to 3-hour sampled fish potentially suggesting fish in T-NP group to be in a satiated state 24-hours after the last meal compared to all other groups. The second assumption is supported by results obtained by Oka et al. [41] who found no differences in leptin mRNA expression between overfed and control (optimal feeding rate) zebrafish. Huising et al. [35] reported that hepatic leptin expression levels corresponded to increase in plasma glucose levels 3-hours and 6-hours after feeding in common carp Cyprinus carpio. Yet, leptin expression was not affected by short-term fasting (six days) nor long-term-fasting (21 days) even though the latter fish lost 30% of their initial weight towards the end of the third week. Leptin’s role in satiety is supplemented by the actions of both ghrelin and CCK. Volkoff et al. [42] found that CCK might affect actions of leptin in goldfish. Interestingly, although in our study no differences were detected in leptin expression level among groups 3-hours after feeding, CCK expression level was significantly lower in both programmed groups compared to control and negative control in the brain. Similar trend was observed in the gut. CCK has been linked to stimulation of pancreatic enzyme secretion, gallbladder contraction, and intestinal peristalsis. In the brain CCK acts as a neurotransmitter involved in control of appetite as a satiation signal from the gut. In goldfish, CCK has been found to inhibit feed intake [43]. Consequently, decreased CCK expression in programmed groups potentially led to enhanced digestion and nutrient absorption and therefore better growth of fish in those two groups. However, CCK expression in the gut seemed to present a similar trend to ghrelin expression contradicting previous evidence on involvement of ghrelin in suppression of CCK anorexic effect in goldfish [44]. Although the majority of studies on several fish species indicate that fasting decreases CCK expression in the gut [45–50] the role of CCK response to fasting/feeding is species-, tissue-, and time-dependent [51]. For example, Macdonald and Volkoff [52] showed an increase in CCK expression in the gut after two weeks of fasting in winter skate Raja ocellata but no effect was detected in the brain. CCK has also been shown to differ between gastric and agastric fish species such as ballan wrasse Labrus bergylta [53]. The decreased expression of CCK in NP-PP and T-NP groups could also indicate these fish were actually in “starvation” compared to both control groups—an alternative scenario to the earlier statement on CCK status in our experiment. However, this speculation contradicts previous results presented for ghrelin, orexin, NPY, and leptin and therefore the results remain perplexing and suggest further investigation on the effects of nutritional status on the expression of CCK in zebrafish.
Soybean meal is the most commonly used PP source in aquaculture feeds, but its use has been limited due to its negative effects at higher dietary inclusion rates that lead to reduced growth and an intestinal inflammation [54]. Typical signs of dietary PP-induced inflammation in the intestinal mucosa include thickening of lamina propria and sub-epithelial mucosa; infiltration of inflammatory cells; and increased number of goblet cells in the epithelium; as well as shortening of the mucosal folds and hence, reduced absorptive capacity of the enterocytes lining the intestinal epithelium [15]. An increase in intestinal villi length has been associated with higher absorption of available nutrients due to increased surface area. However, assessment of villi length only does not consider variations in the surface area due to changes in intestinal villi width and consequently a ratio of intestinal villus length to width is commonly used. Our histological results revealed that the highest villus length to villus width ratio in the proximal intestine was found in T-NP and NP-FM groups compared to both NP-PP and PP groups. However, in the middle intestine the T-NP group had the highest villus length to villus width ratio compared to all other groups. Based on the differences in morphology and cell differentiation and function, Ng et al. [55] proposed that zebrafish intestinal tract should be divided into three distinct segments: the intestinal bulb (proximal intestine), middle intestine, and distal intestine. The digestive processes mostly occur in proximal and middle segments where absorptive enterocytes, digestive enzymes, and solute transporters are highly concentrated, while the distal region is likely involved in water and iron absorption [55,56]. Our results could therefore suggest that increased villus length to width ratio in T-NP group could be a result of morphological adaptation of the intestine allowing for more efficient dietary soybean meal digestion and nutrient absorption. Wang et al. [10] reported that 100% dietary fishmeal replacement with soybean meal led to inflammatory response manifested by significant reduction in intestinal villi length, width, and length to width ratio similar to the results obtained in our negative control group. The intestinal inflammation caused by presence of anti-nutritional factors in dietary soybean meal has been studied in both carnivorous and omnivorous species [10–12,57,58] where zebrafish has been suggested as a model species due to presence of similar inflammatory responses after ingestion of different plant-based ingredients [6].
Conclusions
Our study found that zebrafish is able to utilize PP-based diets more efficiently for growth when exposed to the same PP source early in life. This study also proposes that NP should probably be induced when fish are already in a juvenile stage when all the organs and systems are fully present to be able to respond to this early nutritional trigger more effectively. Furthermore, this study found that early exposure to PP affects gut responses on both molecular and histological level. The expression of three out of five genes coding for appetite-controlling hormones was significantly affected including ghrelin, cholecystokinin, and neuropeptide Y. The “programmed” fish group was also characterized by improved intestinal lining with the highest villus length to width ratio in the middle intestine. Taken together these results suggest that the mechanism behind NP might be associated with endocrine and morphological adaptation of the digestive system that leads to enhanced digestion and absorption capacity ultimately reflected by improved growth performance.
Supporting information
This file contains the raw data for the histological and gene expression analysis of the zebrafish intestines.
(XLSX)
Acknowledgments
We thank Saffron Scientific Histology Services (Carbondale, IL) for providing excellent histological services in a timely manner.
Federica Iannini is a PhD student of the “Dottorato in Scienze della Vita e Biotecnologie”, at the “Università degli Studi dell’Insubria”, in Varese, Italy.
Data Availability
All relevant data are within the manuscript and its supporting information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Geurden I, Borchert P, Balasubramanian MN, Schrama JW, Dupont-Nivet M, Quillet E, et al. The positive impact of the early-feeding of a plant-based diet on its future acceptance and utilisation in rainbow trout. PLoS One. 2013. December 27;8(12):e83162 10.1371/journal.pone.0083162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vagner M, Infante JZ, Robin JH, Person-Le Ruyet J. Is it possible to influence European sea bass (Dicentrarchus labrax) juvenile metabolism by a nutritional conditioning during larval stage? Aquaculture. 2007. July 3;267(1–4):165–74. [Google Scholar]
- 3.Vagner M, Robin JH, Zambonino-Infante JL, Tocher DR, Person-Le Ruyet J. Ontogenic effects of early feeding of sea bass (Dicentrarchus labrax) larvae with a range of dietary n-3 highly unsaturated fatty acid levels on the functioning of polyunsaturated fatty acid desaturation pathways. British journal of nutrition. 2009. May;101(10):1452–62. 10.1017/S0007114508088053 [DOI] [PubMed] [Google Scholar]
- 4.Perera E, Yúfera M. Soybean meal and soy protein concentrate in early diet elicit different nutritional programming effects on juvenile zebrafish. Zebrafish. 2016. February 1;13(1):61–9. 10.1089/zeb.2015.1131 [DOI] [PubMed] [Google Scholar]
- 5.Balasubramanian MN, Panserat S, Dupont-Nivet M, Quillet E, Montfort J, Le Cam A, et al. Molecular pathways associated with the nutritional programming of plant-based diet acceptance in rainbow trout following an early feeding exposure. BMC genomics. 2016. December;17(1):449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hedrera MI, Galdames JA, Jimenez-Reyes MF, Reyes AE, Avendaño-Herrera R, Romero J, et al. Soybean meal induces intestinal inflammation in zebrafish larvae. PLoS One. 2013. July 23;8(7):e69983 10.1371/journal.pone.0069983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baeverfjord G, Krogdahl Å. Development and regression of soybean meal induced enteritis in Atlantic salmon, Salmo salar L., distal intestine: a comparison with the intestines of fasted fish. Journal of Fish Diseases. 1996. September;19(5):375–87. [Google Scholar]
- 8.Urán PA, Gonçalves AA, Taverne-Thiele JJ, Schrama JW, Verreth JA, Rombout JH. Soybean meal induces intestinal inflammation in common carp (Cyprinus carpio L.). Fish & shellfish immunology. 2008. December 1;25(6):751–60. [DOI] [PubMed] [Google Scholar]
- 9.Urán PA, Schrama JW, Rombout JH, Obach A, Jensen L, Koppe W, et al. Soybean meal‐induced enteritis in Atlantic salmon (Salmo salar L.) at different temperatures. Aquaculture Nutrition. 2008. August;14(4):324–30. [Google Scholar]
- 10.Wang YR, Wang L, Zhang CX, Song K. Effects of substituting fishmeal with soybean meal on growth performance and intestinal morphology in orange-spotted grouper (Epinephelus coioides). Aquaculture Reports. 2017. February 1;5:52–7. [Google Scholar]
- 11.de Francesco M, Parisi G, Médale F, Lupi P, Kaushik SJ, Poli BM. Effect of long-term feeding with a plant protein mixture-based diet on growth and body/fillet quality traits of large rainbow trout (Oncorhynchus mykiss). Aquaculture. 2004. June 14;236(1–4):413–29. [Google Scholar]
- 12.Fontainhas-Fernandes A, Gomes E, Reis-Henriques MA, Coimbra J. Replacement of fish meal by plant proteins in the diet of Nile tilapia: digestibility and growth performance. Aquaculture International. 1999. January 1;7(1):57–67. [Google Scholar]
- 13.Kasper CS, Watkins BA, Brown PB. Evaluation of two soybean meals fed to yellow perch (Perca flavescens). Aquaculture Nutrition. 2007. December;13(6):431–8. [Google Scholar]
- 14.Marjara IS, Chikwati EM, Valen EC, Krogdahl Å, Bakke AM. Transcriptional regulation of IL-17A and other inflammatory markers during the development of soybean meal-induced enteropathy in the distal intestine of Atlantic salmon (Salmo salar L.). Cytokine. 2012. October 1;60(1):186–96. 10.1016/j.cyto.2012.05.027 [DOI] [PubMed] [Google Scholar]
- 15.Booman M, Forster I, Vederas JC, Groman DB, Jones SR. Soybean meal-induced enteritis in Atlantic salmon (Salmo salar) and Chinook salmon (Oncorhynchus tshawytscha) but not in pink salmon (O. gorbuscha). Aquaculture. 2018. January 20;483:238–43. [Google Scholar]
- 16.Ulloa PE, Iturra P, Neira R, Araneda C. Zebrafish as a model organism for nutrition and growth: towards comparative studies of nutritional genomics applied to aquacultured fishes. Reviews in Fish Biology and Fisheries. 2011. December 1;21(4):649–66. [Google Scholar]
- 17.Karimi K, Zhandi M. The effect of β-mannanase and β-glucanase on small intestine morphology in male broilers fed diets containing various levels of metabolizable energy. Journal of applied animal research. 2015. July 3;43(3):324–9. [Google Scholar]
- 18.Kemski M, Wick M, Dabrowski K. Nutritional programming effects on growth and reproduction of broodstock and embryonic development of progeny in yellow perch (Perca flavescens) fed soybean meal-based diets. Aquaculture. 2018. December 1;497:452–61. [Google Scholar]
- 19.Izquierdo MS, Turkmen S, Montero D, Zamorano MJ, Afonso JM, Karalazos V, et al. Nutritional programming through broodstock diets to improve utilization of very low fishmeal and fish oil diets in gilthead sea bream. Aquaculture. 2015. December 1;449:18–26. [Google Scholar]
- 20.Turkmen S, Zamorano MJ, Fernández-Palacios H, Hernández-Cruz CM, Montero D, Robaina L, et al. Parental nutritional programming and a reminder during juvenile stage affect growth, lipid metabolism and utilisation in later developmental stages of a marine teleost, the gilthead sea bream (Sparus aurata). British Journal of Nutrition. 2017. October;118(7):500–12. 10.1017/S0007114517002434 [DOI] [PubMed] [Google Scholar]
- 21.London RM, Snowdon CT, Smithana JM. Early experience with sour and bitter solutions increases subsequent ingestion. Physiology & behavior. 1979. June 1;22(6):1149–55. [DOI] [PubMed] [Google Scholar]
- 22.Buddington RK, Krogdahl Å. Hormonal regulation of the fish gastrointestinal tract. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2004. November 1;139(3):261–71. [DOI] [PubMed] [Google Scholar]
- 23.Rønnestad I, Gomes AS, Murashita K, Angotzi R, Jönsson E, Volkoff H. Appetite-controlling endocrine systems in teleosts. Frontiers in endocrinology. 2017. April 18;8:73 10.3389/fendo.2017.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eom J, Hong M, Cone RD, Song Y. Zebrafish ghrelin is expressed in pancreatic endocrine cells and regulated by metabolic state. Biochemical and biophysical research communications. 2013. September 13;439(1):115–20 10.1016/j.bbrc.2013.08.017 [DOI] [PubMed] [Google Scholar]
- 25.Amole N, Unniappan S. Fasting induces preproghrelin mRNA expression in the brain and gut of zebrafish, Danio rerio. General and comparative endocrinology. 2009. March 1;161(1):133–7. 10.1016/j.ygcen.2008.11.002 [DOI] [PubMed] [Google Scholar]
- 26.Novak CM, Jiang X, Wang C, Teske JA, Kotz CM, Levine JA. Caloric restriction and physical activity in zebrafish (Danio rerio). Neuroscience letters. 2005. July 22;383(1–2):99–104. 10.1016/j.neulet.2005.03.048 [DOI] [PubMed] [Google Scholar]
- 27.Nakamachi T, Matsuda K, Maruyama K, Miura T, Uchiyama M, Funahashi H, et al. Regulation by orexin of feeding behaviour and locomotor activity in the goldfish. Journal of neuroendocrinology. 2006. April;18(4):290–7. 10.1111/j.1365-2826.2006.01415.x [DOI] [PubMed] [Google Scholar]
- 28.Xu M, Volkoff H. Molecular characterization of prepro-orexin in Atlantic cod (Gadus morhua): cloning, localization, developmental profile and role in food intake regulation. Molecular and cellular endocrinology. 2007. June 15;271(1–2):28–37 10.1016/j.mce.2007.03.003 [DOI] [PubMed] [Google Scholar]
- 29.Buckley C, MacDonald EE, Tuziak SM, Volkoff H. Molecular cloning and characterization of two putative appetite regulators in winter flounder (Pleuronectes americanus): preprothyrotropin-releasing hormone (TRH) and preproorexin (OX). Peptides. 2010. September 1;31(9):1737–47. 10.1016/j.peptides.2010.05.017 [DOI] [PubMed] [Google Scholar]
- 30.Yokobori E, Azuma M, Nishiguchi R, Kang KS, Kamijo M, Uchiyama M, et al. Neuropeptide Y stimulates food intake in the zebrafish, Danio rerio. Journal of neuroendocrinology. 2012. May;24(5):766–73. 10.1111/j.1365-2826.2012.02281.x [DOI] [PubMed] [Google Scholar]
- 31.Silverstein JT, Breininger J, Baskin DG, Plisetskaya EM. Neuropeptide Y-like gene expression in the salmon brain increases with fasting. General and comparative endocrinology. 1998. May 1;110(2):157–65. 10.1006/gcen.1998.7058 [DOI] [PubMed] [Google Scholar]
- 32.Narnaware YK, Peyon PP, Lin X, Peter RE. Regulation of food intake by neuropeptide Y in goldfish. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2000. September 1;279(3):R1025–34. 10.1152/ajpregu.2000.279.3.R1025 [DOI] [PubMed] [Google Scholar]
- 33.Narnaware YK, Peter RE. Neuropeptide Y stimulates food consumption through multiple receptors in goldfish. Physiology & behavior. 2001. September 1;74(1–2):185–90. [DOI] [PubMed] [Google Scholar]
- 34.Valen R, Jordal AE, Murashita K, Rønnestad I. Postprandial effects on appetite-related neuropeptide expression in the brain of Atlantic salmon, Salmo salar. General and comparative endocrinology. 2011. May 1;171(3):359–66. 10.1016/j.ygcen.2011.02.027 [DOI] [PubMed] [Google Scholar]
- 35.Huising MO, Geven EJ, Kruiswijk CP, Nabuurs SB, Stolte EH, Spanings FT, et al. Increased leptin expression in common carp (Cyprinus carpio) after food intake but not after fasting or feeding to satiation. Endocrinology. 2006. December 1;147(12):5786–97. 10.1210/en.2006-0824 [DOI] [PubMed] [Google Scholar]
- 36.Trombley S, Maugars G, Kling P, Björnsson BT, Schmitz M. Effects of long-term restricted feeding on plasma leptin, hepatic leptin expression and leptin receptor expression in juvenile Atlantic salmon (Salmo salar L.). General and comparative endocrinology. 2012. January 1;175(1):92–9. 10.1016/j.ygcen.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 37.Kurokawa T, Murashita K, Suzuki T, Uji S. Genomic characterization and tissue distribution of leptin receptor and leptin receptor overlapping transcript genes in the pufferfish, Takifugu rubripes. General and comparative endocrinology. 2008. August 1;158(1):108–14. 10.1016/j.ygcen.2008.06.003 [DOI] [PubMed] [Google Scholar]
- 38.Zhang H, Chen H, Zhang Y, Li S, Lu D, Zhang H, et al. Molecular cloning, characterization and expression profiles of multiple leptin genes and a leptin receptor gene in orange-spotted grouper (Epinephelus coioides). General and comparative endocrinology. 2013. January 15;181:295–305. 10.1016/j.ygcen.2012.09.008 [DOI] [PubMed] [Google Scholar]
- 39.Murashita K, Jordal AE, Nilsen TO, Stefansson SO, Kurokawa T, Björnsson BT, et al. Leptin reduces Atlantic salmon growth through the central pro-opiomelanocortin pathway. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2011. January 1;158(1):79–86. [DOI] [PubMed] [Google Scholar]
- 40.Kling P, Rønnestad I, Stefansson SO, Murashita K, Kurokawa T, Björnsson BT. A homologous salmonid leptin radioimmunoassay indicates elevated plasma leptin levels during fasting of rainbow trout. General and comparative endocrinology. 2009. July 1;162(3):307–12. 10.1016/j.ygcen.2009.04.003 [DOI] [PubMed] [Google Scholar]
- 41.Oka T, Nishimura Y, Zang L, Hirano M, Shimada Y, Wang Z, et al. Diet-induced obesity in zebrafish shares common pathophysiological pathways with mammalian obesity. BMC physiology. 2010. December;10(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Volkoff H, Eykelbosh AJ, Peter RE. Role of leptin in the control of feeding of goldfish Carassius auratus: interactions with cholecystokinin, neuropeptide Y and orexin A, and modulation by fasting. Brain research. 2003. May 16;972(1–2):90–109. 10.1016/s0006-8993(03)02507-1 [DOI] [PubMed] [Google Scholar]
- 43.Himick BA, Peter RE. CCK/gastrin-like immunoreactivity in brain and gut, and CCK suppression of feeding in goldfish. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 1994. September 1;267(3):R841–51. [DOI] [PubMed] [Google Scholar]
- 44.Blanco AM, Bertucci JI, Valenciano AI, Delgado MJ, Unniappan S. Ghrelin suppresses cholecystokinin (CCK), peptide YY (PYY) and glucagon-like peptide-1 (GLP-1) in the intestine, and attenuates the anorectic effects of CCK, PYY and GLP-1 in goldfish (Carassius auratus). Hormones and behavior. 2017. July 1;93:62–71. 10.1016/j.yhbeh.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 45.Koven W, Schulte P. The effect of fasting and refeeding on mRNA expression of PepT1 and gastrointestinal hormones regulating digestion and food intake in zebrafish (Danio rerio). Fish physiology and biochemistry. 2012. December 1;38(6):1565–75. 10.1007/s10695-012-9649-6 [DOI] [PubMed] [Google Scholar]
- 46.MacDonald E, Volkoff H. Cloning, distribution and effects of season and nutritional status on the expression of neuropeptide Y (NPY), cocaine and amphetamine regulated transcript (CART) and cholecystokinin (CCK) in winter flounder (Pseudopleuronectes americanus). Hormones and behavior. 2009. June 1;56(1):58–65. 10.1016/j.yhbeh.2009.03.002 [DOI] [PubMed] [Google Scholar]
- 47.Murashita K, Fukada H, Hosokawa H, Masumoto T. Cholecystokinin and peptide Y in yellowtail (Seriola quinqueradiata): molecular cloning, real-time quantitative RT-PCR, and response to feeding and fasting. General and comparative endocrinology. 2006. February 1;145(3):287–97. 10.1016/j.ygcen.2005.09.008 [DOI] [PubMed] [Google Scholar]
- 48.Yuan D, Wang T, Zhou C, Lin F, Chen H, Wu H, et al. Leptin and cholecystokinin in Schizothorax prenanti: molecular cloning, tissue expression, and mRNA expression responses to periprandial changes and fasting. General and comparative endocrinology. 2014. August 1;204:13–24. 10.1016/j.ygcen.2014.05.013 [DOI] [PubMed] [Google Scholar]
- 49.Ji W, Ping HC, Wei KJ, Zhang GR, Shi ZC, Yang RB, et al. Ghrelin, neuropeptide Y (NPY) and cholecystokinin (CCK) in blunt snout bream (Megalobrama amblycephala): cDNA cloning, tissue distribution and mRNA expression changes responding to fasting and refeeding. General and comparative endocrinology. 2015. November 1;223:108–19. 10.1016/j.ygcen.2015.08.009 [DOI] [PubMed] [Google Scholar]
- 50.Babaei S, Sáez A, Caballero-Solares A, Fernández F, Baanante IV, Metón I. Effect of dietary macronutrients on the expression of cholecystokinin, leptin, ghrelin and neuropeptide Y in gilthead sea bream (Sparus aurata). General and comparative endocrinology. 2017. January 1;240:121–8. 10.1016/j.ygcen.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 51.Dalmolin C, Almeida DV, Figueiredo MA, Marins LF. Food intake and appetite control in a GH-transgenic zebrafish. Fish physiology and biochemistry. 2015. October 1;41(5):1131–41. 10.1007/s10695-015-0074-5 [DOI] [PubMed] [Google Scholar]
- 52.MacDonald E, Volkoff H. Neuropeptide Y (NPY), cocaine-and amphetamine-regulated transcript (CART) and cholecystokinin (CCK) in winter skate (Raja ocellata): cDNA cloning, tissue distribution and mRNA expression responses to fasting. General and comparative endocrinology. 2009. April 1;161(2):252–61. 10.1016/j.ygcen.2009.01.021 [DOI] [PubMed] [Google Scholar]
- 53.Le HT, Lie KK, Giroud-Argoud J, Rønnestad I, Sæle Ø. Effects of Cholecystokinin (CCK) on Gut Motility in the Stomachless Fish Ballan Wrasse (Labrus bergylta). Frontiers in neuroscience. 2019;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krogdahl Å, Gajardo K, Kortner TM, Penn M, Gu M, Berge GM, et al. Soya saponins induce enteritis in Atlantic salmon (Salmo salar L.). Journal of agricultural and food chemistry. 2015. April 10;63(15):3887–902. 10.1021/jf506242t [DOI] [PubMed] [Google Scholar]
- 55.Ng AN, de Jong-Curtain TA, Mawdsley DJ, White SJ, Shin J, Appel B, et al. Formation of the digestive system in zebrafish: III. Intestinal epithelium morphogenesis. Developmental biology. 2005. October 1;286(1):114–35. 10.1016/j.ydbio.2005.07.013 [DOI] [PubMed] [Google Scholar]
- 56.Wallace KN, Pack M. Unique and conserved aspects of gut development in zebrafish. Developmental biology. 2003. March 1;255(1):12–29. 10.1016/s0012-1606(02)00034-9 [DOI] [PubMed] [Google Scholar]
- 57.Bakke‐McKellep AM, Press CM, Baeverfjord G, Krogdahl Å, Landsverk T. Changes in immune and enzyme histochemical phenotypes of cells in the intestinal mucosa of Atlantic salmon, Salmo salar L., with soybean meal‐induced enteritis. Journal of Fish Diseases. 2000. March;23(2):115–27. [Google Scholar]
- 58.Krogdahl A, Bakke-McKellep AM, Roed KH, Baeverfjord G. Feeding Atlantic salmon Salmo salar L. soybean products: effects on disease resistance (furunculosis), and lysozyme and IgM levels in the intestinal mucosa. Aquaculture Nutrition. 2000. June 1;6(2):77–84. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file contains the raw data for the histological and gene expression analysis of the zebrafish intestines.
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its supporting information files.


