Abstract
Introduction
Chronic kidney disease of unknown origin (CKDu) is an epidemic concentrated in agricultural communities in Central and South America, including young, male sugarcane harvesters. The purpose of this analysis is to understand early changes in kidney function among a cohort of first-time sugarcane harvesters and to determine risk factors for kidney function decline.
Methods
Joint latent class mixed models were used to model sub-population kidney function trajectory over the course of 4 years (2012–2016). Probability weighted logistic regression was used to determine personal health, community, and individual behavior risk factors associated with sub-population assignment. Data analysis occurred in 2019.
Results
Of 181 new workers median age 19 years old (IQR: 4), 39 (22%) were identified as having non-stable kidney function with an annual age-adjusted decline of estimated glomerular filtration rate (eGFR) of -1.0 ml/min per 1.73 m2 (95% CI: -3.4, 1.3). Kidney function (OR: 0.96; 95% CI: 0.93, 0.98), mild hypertension (OR: 5.21; 95% CI: 2.14, 13.94), and having a local home of residence (OR: 7.12; 95% CI: 2.41, 26.02) prior to employment in sugarcane were associated with non-stable eGFR sub-population assignment.
Conclusions
Mild hypertension may be an early indicator of the development of CKDu. A better understanding of preexisting risk factors is needed to determine why individuals are entering the workforce with reduced kidney function and elevated blood pressure and increased risk of renal function decline.
Introduction
Chronic kidney disease of unknown origin (CKDu) is an emerging epidemic concentrated in agricultural communities in Central and South America [1]. Unlike other forms of chronic kidney disease, CKDu is defined in the absence of traditional risk factors such as diabetes and hypertension [2, 3]. Characterized as a form of tubulointerstitial nephritis with varying degrees of fibrosis [4], clinical and epidemiologic studies have shown that young men are at the greatest risk for developing the disease with the age at diagnosis most often between 30 and 50 years [2].
Because the epidemic is concentrated in areas where a high percentage of the labor force conducts physically demanding manual labor [5] one of the leading hypotheses is that CKDu is caused, at least in part, by carrying out heavy work in hot climates while under a state of dehydration [1, 6–8]. Much of the research has been focused in either agricultural worker cohorts or community cohorts where a large percentage of the population is composed of current or former agricultural workers [2, 9–13]. These cross-sectional studies have implicated sugarcane harvesting and the number of years of work in agriculture as predictors of development of disease [10, 11]. However, some recent lines of evidence suggest that CKDu may also be occurring in communities among individuals who are not agricultural workers [14–17]. Recent studies suggest that there may be early indicators of renal damage among adolescents [18, 19], suggesting the possibility that there may be non-occupational contributors to the development of disease.
In order to better understand the possible combined contributions of individual health, community, and work-related risk factors to renal function decline, we conducted a four-year longitudinal analysis of apparently healthy, asymptomatic young men seeking their first job as sugarcane harvesters in southwest Guatemala. We hypothesized that there are multifactorial community, personal health, and lifestyle risk factors present prior to employment that contribute to observed changes in renal function over the course of their time employed in sugarcane.
Methods
Cohort
Workers from the region surrounding a sugarcane mill in southwest Guatemala (local workers), as well as migrant workers from other parts of Guatemala, are recruited annually by a large agribusiness. Each year, prior to the start of the 6-month sugarcane harvest, the agribusiness conducts a pre-employment screening which includes a medical exam (blood pressure, heart rate, height, and weight), questionnaire (demographic, lifestyle, medical, and occupational history questions), and venipuncture for measurement of serum creatinine of each individual seeking employment as a sugarcane harvester. Full details on recruitment and pre-employment screening are published elsewhere [20].
Subjects for our analysis were retrospectively selected from workers seeking employment as sugarcane harvesters between November 2012 and November 2015 at the agribusiness. To be included in this analysis, workers must have 1) reported never working a previous sugarcane harvest, whether at this company or another, 2) been hired and worked at least one week of the harvest after hiring date, and 3) have returned for pre-employment screening the subsequent year. Once this cohort was identified, the agribusiness used employment records to verify that these individuals had not previously worked for the company.
Data from the pre-employment screenings and information on the productivity of workers (to verify employment and attrition) were provided by the agribusiness. Data analysis occurred in 2019. As this was a secondary evaluation of de-identified data collected for business purposes, informed consent was not obtained. Ethics review and approval for our evaluation of these data was received from Colorado Multiple Institutional Review Board (COMIRB).
Outcome
Creatinine measures were collected at the time of pre-employment screenings. Blood samples were sent to an independent, licensed clinical laboratory (Herrera Llerandi laboratory, Guatemala City, Guatemala). The Creatinine Jaffe Generation 2 method was used to determine serum creatinine. Further detail on the laboratory methods has been published elsewhere [21]. The primary outcome was the pattern of change in estimated glomerular filtration rate (eGFR) over a period of up to 4 years (2012–2016). Data on eGFR from 2016 were used for follow-up eGFR measurements only. To calculate the eGFR we used the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula [22]. Race was considered “non-black” and all workers were male. From 2009 to 2015 it was the policy of the company to only hire workers with a serum creatinine of 1.45 mg/dL or less. The policy has since been updated (2017) to only hire cane cutters with an eGFR above 90 ml/min per 1.73 m2.
Covariates and potential risk factors
Baseline covariates were defined based on the first pre-employment screening of an individual. These included eGFR, age, body mass index (BMI), blood pressure, location of home residence, home drinking water source, alcohol consumption, and smoking status.
Pre-employment blood pressure measurements were taken by a licensed nurse following standard protocol. If there was doubt regarding the reading, a medical doctor in charge of pre-employment evaluation obtained a second measurement. The worker was notified of his result. We used the cut-off of systolic blood pressure ≥130 or diastolic blood pressure ≥ 80 to define mild hypertension in accordance with the 2017 American Academy of Cardiology guidelines [23].
Statistical methods
Because kidney function at time of hire is related to attrition from the workforce [24] we used joint latent class mixed models to model the shape of longitudinal kidney function change while simultaneously modeling loss to follow-up [25]. In our analysis, time was treated as season (1–5 harvest seasons) in the linear mixed model. The longitudinal change in eGFR was modelled with a quadratic time trend with random terms for intercept and 2-degree polynomial time. Unconditional models were used to determine class-membership. Class-specific baseline risk functions were adjusted for continuous age. A full discussion of our modelling approach and final model specifications can be found in the Supporting Information. The R package “lcmm” was used [26].
Sub-population assignment
Each individual was assigned a probability for sub-population assignment from the joint latent class mixed model. Annual change in eGFR for each sub-population was calculated using an age-adjusted Bayesian generalized linear multilevel model with random intercept for individual [27]. Age-adjusted associations between baseline covariates, as measured by their first pre-employment survey as described above, and probability of sub-population assignment were assessed using probability weighted logistic regression. Models were then further adjusted for baseline eGFR. For descriptive tables, individuals were assigned to the sub-population with which they had the highest probability. Comparisons between groups were done using Chi-squared, Fisher’s Exact, or T-tests as appropriate. All analyses were conducted in R version 3.4.3 [28].
Sensitivity analysis
To address the potential misclassification of individuals with mild hypertension we conducted a sensitivity analysis. We took random samples of 20% and 40% of the workers with mild hypertension and re-classified them as non-hypertensive. We re-ran the sub-population assignment analysis as described above with the updated datasets to assess the stability of the hypertension estimates.
Results
Cohort and follow-up
We identified 534 male individuals who were hired and worked at least one week as first time sugarcane harvester between 2012 and 2015. Of these, 181 (34%) returned the subsequent year for the next pre-employment screening, making up the analysis cohort (S1 Fig). Of the 181 workers with longitudinal measurements of eGFR, subsequent measures of eGFR were available for 100 workers for one year, 52 workers for two years, 26 workers for three years, and 3 workers for four years. A comparison between those selected and not selected for the cohort is provided in the Supporting Information (S1 Table).
eGFR sub-populations
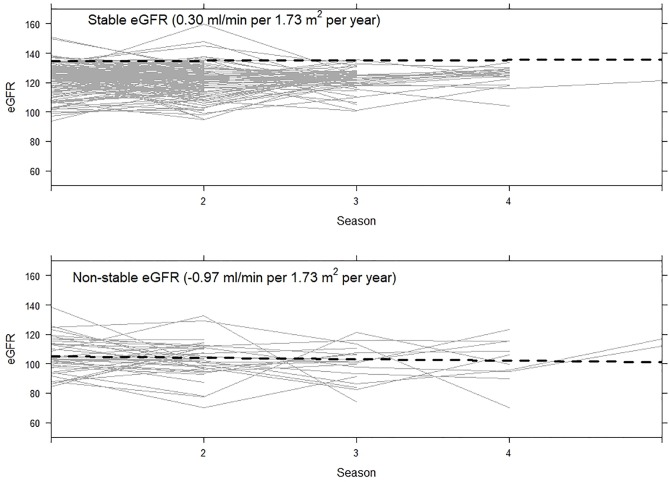
We identified two distinct sub-populations of first year sugarcane harvesters: those who slightly declined in kidney function over time (non-stable) and those who remained stable (Fig 1). Most workers fell into the stable category (78%; n = 142); however, almost a quarter (22%; n = 39) were identified in the non-stable category. The average annual change in eGFR for workers in the non-stable group was -1.0 ml/min per 1.73 m2 (95% CI: -3.4, 1.3) compared to 0.3 ml/min per 1.73 m2 (95% CI: -0.9, 1.5) for the stable group.
Fig 1. Individual longitudinal eGFR patterns stratified by assigned sub-population.
The two groups did significantly differ on baseline eGFR. While no workers in our analysis had baseline kidney dysfunction when hired (defined as eGFR < 60 ml/min per 1.73 m2) [29], five workers assigned to the non-stable group had eGFR < 90 ml/min per 1.73 m2 at the time of hire (Minimum: 84 ml/min per 1.73 m2). Those in the non-stable group had on average a baseline eGFR 17 ml/min per 1.73 m2 lower (95% CI: -21 ml/min per 1.73 m2, -12 ml/min per 1.73 m2) than those in the stable group (Table 1). They also tended to be older. The average baseline BMI for those in the non-stable group was higher than those in the stable group.
Table 1. Baseline covariates of new sugarcane workers by assigned sub-population in mean (SD) or n (%).
Data collected from workers seeking first time work as sugarcane harvesters in Guatemala from 2012–2015.
| Baseline Characteristic | Non-stable Kidney Function (n = 39) |
Stable Kidney Function (n = 142) |
p-value |
|---|---|---|---|
| Creatinine, mg/dL | 0.97 (0.12) | 0.86 (0.10) | <0.001 |
| eGFR, ml/min per 1.73 m2 | 105.24 (12.43) | 123.33 (10.11) | <0.001 |
| Age, years | 28 (7) | 20 (2) | <0.001 |
| BMI, kg/m2 | 23.75 (2.70) | 22.60 (2.15) | 0.005 |
| Systolic, mmHg | 111 (10) | 110 (12) | 0.686 |
| Diastolic, mmHg | 76 (7) | 73 (8) | 0.050 |
| Hypertensiona | 26 (67%) | 59 (42%) | 0.009 |
| Stage 1 | 25 (64%) | 49 (35%) | 0.002 |
| Stage 2 | 1 (3%) | 10 (7%) | 0.461 |
| Local home of residence (vs. migrant) | 16 (41%) | 46 (32%) | 0.314 |
| Well water source | 11 (28%) | 42 (30%) | 0.868 |
| Consumes alcohol | 2 (5%) | 7 (5%) | 0.868 |
| Current or former smoker | 0 (0%) | 12 (9%) | 0.072 |
| Weeks worked first year | 26 (1) | 26 (2) | 0.500 |
aStage 1 hypertension defined as systolic blood pressure ≥130 or diastolic blood pressure ≥ 80; Stage 2 hypertension defined as systolic blood pressure ≥140 or diastolic blood pressure ≥ 90.
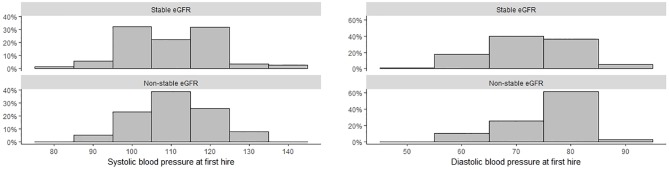
There were notable differences in the distribution of blood pressure between the non-stable and stable groups (Fig 2). A total of 85 workers (47%) in this analysis met the definition of mild hypertension. The proportion of individuals with mild hypertension differed significantly between groups, with 26 (67%) individuals in the non-stable eGFR group having mild hypertension compared to 59 (42%) in the stable eGFR group (p-value: 0.01).
Fig 2. Blood pressure measurements at the time of first hire for first year sugarcane harvesters.
Adjusted associations with non-stable kidney function
In age-adjusted probability weighted models, baseline eGFR and baseline creatinine were associated with non-stable eGFR sub-population assignment (Table 2). Odds of assignment into the non-stable group decreased as baseline eGFR increased (OR: 0.96; 95% CI: 0.93, 0.98). In models that adjusted both for age and baseline eGFR, new workers with a local home of residence had 7.12 times the odds of being in the non-stable subgroup (95% CI: 2.41, 26.02) compared to those from other regions. Those with a well water source at home had 6.1 times the odds of being in the non-stable subgroup (95% CI: 1.80, 26.82) compared to all other sources of home water. Water source was highly correlated with region, with 60% of those with a local home of residence using well water compared to 13% from other regions (p-value: <0.001). Notably, the individuals with mild hypertension at the time of hire had 5.2 times the odds of assignment to the non-stable group (95% CI: 2.14, 13.94) compared to those with normal blood pressure. In a sensitivity analysis with 20% of the mild hypertensive workers randomly re-assigned to non-hypertensive, the results held (OR: 6.00; 95% CI: 2.24, 18.98; p-value: 0.002). Estimates were unstable in the extreme case of 40% of mild hypertensive workers randomly re-classified (OR: 0.54; 95%CI: 0.22, 1.33; p-value 0.17). A summary of all sensitivity analysis results is presented in S2 Table.
Table 2. Age-adjusted and age- and baseline eGFR-adjusted Odds Ratio (95% CI) of non-stable eGFR sub-population assignment.
Data collected from workers seeking first time work as sugarcane harvesters in Guatemala from 2012–2015.
| Age-adjusted OR for Non-stable | p-value | Age- and baseline eGFR adjusted OR for Non-stable |
p-value | |
|---|---|---|---|---|
| Baseline Creatinine (per 0.10 mg/dL) | 1.56 (1.15, 2.15) | 0.005 | ||
| Baseline eGFR, ml/min per 1.73 m2 | 0.96 (0.93, 0.98) | 0.004 | ||
| Baseline BMI, kg/m2 | 0.98 (0.81, 1.22) | 0.864 | 0.84 (0.67, 1.05) | 0.122 |
| Baseline Systolic (per 10 mmHg) | 0.96 (0.64, 1.44) | 0.850 | 0.95 (0.66, 1.36) | 0.769 |
| Baseline Diastolic (per 10 mmHg) | 1.45 (0.80, 2.64) | 0.225 | 1.72 (0.97, 3.10) | 0.066 |
| Baseline Hypertensiona | 2.80 (1.20, 6.74) | 0.020 | 5.21 (2.14, 13.94) | 0.001 |
| Local home of residence vs. migrant | 3.95 (1.40, 13.42) | 0.016 | 7.12 (2.41, 26.02) | 0.001 |
| Well water source | 3.48 (1.19, 12.94) | 0.038 | 6.07 (1.80, 26.82) | 0.009 |
| Weeks worked | 1.19 (0.70, 1.91) | 0.488 | 1.01 (0.57, 1.62) | 0.964 |
aHypertension defined as systolic blood pressure ≥130 or diastolic blood pressure ≥ 80.
Discussion
To our knowledge this is the first longitudinal analysis examining kidney function decline in a young, new worker population at a risk of developing CKDu. This analysis established that among newly hired sugarcane cutters, between 20% and 25% are expected to experience non-stable eGFR, with an average decline in eGFR at a rate of 1 ml/min per 1.73 m2 per year after adjusting for age. Workers who entered the workforce with lower levels of kidney function and mild hypertension were at a greater risk for experiencing declines in kidney function. Coupled with home of residence being an additional predictive risk factor for decline in kidney function suggests early exposures and community level factors play a role in the development of CKDu.
Mild hypertension at time of hire increased the odds of non-stable eGFR group assignment 5-fold. In addition, elevations of continuous diastolic blood pressure were also suggestive of non-stable eGFR in our age- and baseline eGFR-adjusted models. This is a notable finding because CKDu has been defined in absence of hypertension (systolic blood pressure ≥140 or diastolic blood pressure ≥ 90) but has not been examined in relationship to mild elevations of blood pressure. Our findings show that the non-stable group had both a higher prevalence of mild hypertension as well as lower baseline eGFR. Systemic arterial hypertension is almost always associated with, and likely driven by, subtle renal disease [30]. This, coupled with our findings presented here, suggest mild hypertension as an early indicator of the development of CKDu. Blood pressure screening in conjunction with renal function screenings should become a routine part of health monitoring for workers and community members at risk of CKDu, specifically those performing intense labor in hot environments. In addition, more research is warranted on the role of elevations of blood pressure, longitudinal changes in blood pressure, and the development of CKDu.
Our analysis showed that location of home of residence was associated with non-stable eGFR group assignment after adjusting for age and baseline eGFR. Workers living locally come from the communities surrounding the mill, which are at lower altitudes and closer to the coast than the seasonal migrant workers who generally are from the highlands, which are more mountainous, and at a higher altitude. Previous studies have shown that lower altitude and coastal home of residence are risk factors for CKDu [16, 31, 32] potentially due to differences in climatic factors and job history differences between these regions. While we are confident that the workers included in this analysis had never worked in sugarcane, we did not account for their job histories. There is the potential that the individuals evaluated here have had previous employment in other agricultural settings or participated in subsistence agriculture, which can differ by region, potentially explaining some of the differences we found between local and migrant workers. Limited research has been conducted to identify other differences in CKDu risk factors between local and migrant communities, but possible explanations could include differences in diets [33], airborne exposures [34], or water sources [35] which we showed to increase non-stable group assignment 6-fold and was highly associated with home of residence. Understanding differences in childhood and adolescent exposures may help determine why some young men enter the workforce with lower than expected eGFR and with elevated blood pressure.
This analysis carries several strengths. The models we employed help overcome common obstacles in most studies of CKDu, mainly the need for repeated measurements of eGFR, assumptions of linear trends in eGFR decline, and loss to follow-up. First, we had measurements on 181 first time sugarcane harvesters for up to four years, longer than any study we are aware of in the CKDu literature. Second, the joint linear mixed model allowed us to be flexible with the functional form of time when identifying sub-populations of renal function change. Third, informative loss to follow up in longitudinal worker cohorts is an issue, because workers with lower kidney function at the start of the harvest season are less likely to return to work [24]. Using joint linear mixed models allowed us to leverage this information.
Limitations
Despite these strengths, this analysis does face some limitations. We were limited in our ability to determine factors associated with a lower baseline eGFR, higher baseline blood pressure, and factors that differentiate local and migrant workers. Because these data were collected for hiring purposes, we were limited in our ability to examine additional hypothesized risk factors such as heat exposure [6, 11], use of pesticides [36, 37], and exposure to heavy metals [34, 38]. Due to the structure of our data, we were unable to assess the differences in recurrent dehydration [11], NSAID use [20], and other factors previously implicated in the development of the disease between the two identified groups. There is a possibility of nondifferential misclassification of survey variables including smoking status and alcohol intake. Due to the low number of respondents to questions regarding smoking and alcohol consumption we were unable to test the relationship with group assignment. We would be remiss not to mention that hypertension is clinically defined by elevated measurements at two time points, however we were limited in our classification to the single measurement taken at the time of hire. Finally, we lost many individuals to follow-up, limiting how many individuals for whom we had more than two time points. While we used a modeling approach that leveraged this information, it cannot be ignored that there are both observed and unobservable differences between those who choose to seek employment in subsequent years. Additionally, as loss to follow-up is associated with reduced kidney function [24], our analysis may underestimate the true rate of renal function decline as well as the association between renal function decline and mild-elevations in blood pressure.
Conclusions
To our knowledge this is the first cohort that examines the course of renal function change in newly hired, first time sugarcane harvesters who are at risk of CKDu. We identified nearly a quarter of the apparently healthy young men who experience on average a decline in eGFR of 1 ml/min per 1.73 m2 per year. Risk factors for this decline were decreased baseline eGFR, elevated baseline blood pressure, and residing locally in communities near the mill. A better understanding of non-occupational exposures and individual risk factors are needed to determine why these individuals are entering the workforce at higher risk. Improved surveillance practices of blood pressure and kidney function measurement are needed in the workplace in order to identify individuals at risk of kidney function decline. Workplaces should implement eGFR hiring cutoffs at 90 ml/min per 1.73 m2 as our study shows that all workers who had an eGFR < 90 ml/min per 1.73 m2 at the time of hire were assigned to the non-stable group. Studies of the possible mechanisms by which the combination of community, individual, and workplace risk factors contribute to renal function decline are needed.
Supporting information
Study flow showing timeline along with the number of new workers screened and the number returning each subsequent year.
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Presented as Odds Ratio (95% Confidence Interval).
(DOCX)
Acknowledgments
We would like to thank the men and women employed by Pantaleon who worked tirelessly to ensure the accuracy of the data used in this evaluation. We would also like to thank Alicen Nelson, MD, MPH for her pilot work in assessing the pre-employment variables included in this analysis.
Data Availability
All files to reproduce the findings in this manuscript are housed on Open Science Framework (https://osf.io) DOI 10.17605/OSF.IO/4YGSB.
Funding Statement
This evaluation was supported in part by Pantaleon; the Chancellor, CU Anschutz; Centers for Disease Control and Prevention (CDC.gov) (U19 OH01127 to LSN) and National Institutes of Health (NIH.gov) (R21 ES028826 to LSN). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention, the National Institutes of Health, or the Department of Health and Human Services. The funders had no role in the evaluation design, data analysis, or data interpretation. Co-authors employed by the company (AC, CA, WDP) participated on the writing team. They provided details regarding work practices, but did not modify the results or conclusions of this report. The corresponding author had full access to all the raw data and had final responsibility for the decision to submit for publication. University of Colorado and Pantaleon are separate, independent organizations. University of Colorado employed appropriate research methods in keeping with academic freedom, based conclusions on critical analysis of the evidence and reported findings fully and objectively. The terms of this arrangement have been reviewed and approved by the University of Colorado in accordance with its conflict of interest policies.
References
- 1.Johnson RJ, Wesseling C, Newman LS. Chronic Kidney Disease of Unknown Cause in Agricultural Communities. New England Journal of Medicine. 2019;380(19):1843–52. 10.1056/NEJMra1813869 [DOI] [PubMed] [Google Scholar]
- 2.Correa-Rotter R, Wesseling C, Johnson RJ. CKD of unknown origin in Central America: the case for a Mesoamerican nephropathy. Am J Kidney Dis. 2014;63(3):506–20. Epub 2014/01/10. 10.1053/j.ajkd.2013.10.062 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trabanino RG RG AR, Silva CR, Mercado MO, Merino RL. End-stage renal disease among patients in a referral hospital in El Salvador. Rev Panam Salud Publica. 2002;12(3):202–6. 10.1590/s1020-49892002000900009 [DOI] [PubMed] [Google Scholar]
- 4.Wijkström J, González-Quiroz M, Hernandez M, Trujillo Z, Hultenby K, Ring A, et al. Renal Morphology, Clinical Findings, and Progression Rate in Mesoamerican Nephropathy. American Journal of Kidney Diseases. 2017;69(5):626–36. 10.1053/j.ajkd.2016.10.036 [DOI] [PubMed] [Google Scholar]
- 5.International Labour Organization. Statistics and databases [cited 2019 October 21]. https://www.ilo.org/global/statistics-and-databases/lang--en/index.htm.
- 6.Glaser J, Lemery J, Rajagopalan B, Diaz HF, García-Trabanino R, Taduri G, et al. Climate Change and the Emergent Epidemic of CKD from Heat Stress in Rural Communities: The Case for Heat Stress Nephropathy. Clin J Am Soc Nephrol. 2016;11(8):1472–83. Epub 2016/05/05. 10.2215/CJN.13841215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorensen C, Garcia-Trabanino R. A New Era of Climate Medicine—Addressing Heat-Triggered Renal Disease. New England Journal of Medicine. 2019;381(8):693–6. 10.1056/NEJMp1907859 [DOI] [PubMed] [Google Scholar]
- 8.Sorensen CJ, Butler-Dawson J, Dally M, Krisher L, Griffin BR, Johnson RJ, et al. Risk Factors and Mechanisms Underlying Cross-Shift Decline in Kidney Function in Guatemalan Sugarcane Workers. Journal of Occupational and Environmental Medicine. 2019;61(3):239–50. 10.1097/JOM.0000000000001529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kupferman J, Amador JJ, Lynch KE, Laws RL, López-Pilarte D, Ramírez-Rubio O, et al. Characterization of Mesoamerican Nephropathy in a Kidney Failure Hotspot in Nicaragua. American Journal of Kidney Diseases. 2016;68(5):716–25. 10.1053/j.ajkd.2016.06.012 [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Quiroz M, Smpokou E-T, Silverwood RJ, Camacho A, Faber D, Garcia BLR, et al. Decline in Kidney Function among Apparently Healthy Young Adults at Risk of Mesoamerican Nephropathy. J Am Soc Nephrol. 2018;29(8):2200–12. Epub 2018/06/15. 10.1681/ASN.2018020151 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wesseling C, Aragón A, González M, Weiss I, Glaser J, Rivard CJ, et al. Heat stress, hydration and uric acid: a cross-sectional study in workers of three occupations in a hotspot of Mesoamerican nephropathy in Nicaragua. BMJ Open. 2016;6(12):e011034–e. 10.1136/bmjopen-2016-011034 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raines NGM, Wyatt C, Kurzrok M, Pool C, Lemma T, Weiss I, Marín C, Prado V, Marcas E, Mayorga K, Morales JF, Aragón A, Sheffield P. Risk factors for reduced glomerular filtration rate in a Nicaraguan community affected by Mesoamerican nephropathy. MEDICC Rev. 2014;16(2):16–22. [DOI] [PubMed] [Google Scholar]
- 13.Laws RL, Brooks DR, Amador JJ, Weiner DE, Kaufman JS, Ramírez-Rubio O, et al. Changes in kidney function among Nicaraguan sugarcane workers. Int J Occup Environ Health. 2015;21(3):241–50. Epub 2015/01/28. 10.1179/2049396714Y.0000000102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrera Valdés R, Orantes CM, Almaguer López M, López Marín L, Arévalo PA, Smith González MJ, Morales FE, Bacallao R, Bayarre HD, Vela Parada XF. Clinical characteristics of chronic kidney disease of non-traditional causes in women of agricultural communities in El Salvador. Clinical Nephrology. 2015;83:56–83. 10.5414/cnp83s056 [DOI] [PubMed] [Google Scholar]
- 15.Orantes Navarro CM HVR, López MA, Calero DJ, Fuentes de Morales J, Alvarado Ascencio NP, Vela Parada XF, Zelaya Quezada SM, Granados Castro DV, Orellana de Figueroa P. Epidemiological characteristics of chronic kidney disease of non-traditional causes in women of agricultural communities of El Salvador. Clin Nephrol. 2015;83:24–31. 10.5414/cnp83s024 [DOI] [PubMed] [Google Scholar]
- 16.Torres C, Aragón A, González M, López I, Jakobsson K, Elinder C-G, et al. Decreased Kidney Function of Unknown Cause in Nicaragua: A Community-Based Survey. American Journal of Kidney Diseases. 2010;55(3):485–96. 10.1053/j.ajkd.2009.12.012 [DOI] [PubMed] [Google Scholar]
- 17.Lebov JF, Valladares E, Peña R, Peña EM, Sanoff SL, Cisneros EC, et al. A population-based study of prevalence and risk factors of chronic kidney disease in León, Nicaragua. Can J Kidney Health Dis. 2015;2:6-. 10.1186/s40697-015-0041-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agampodi SB, Amarasinghe GS, Naotunna PGCR, Jayasumana CS, Siribaddana SH. Early renal damage among children living in the region of highest burden of chronic kidney disease of unknown etiology (CKDu) in Sri Lanka. BMC nephrology. 2018;19(1):115-. 10.1186/s12882-018-0911-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramírez-Rubio O, Amador JJ, Kaufman JS, Weiner DE, Parikh CR, Khan U, et al. Urine biomarkers of kidney injury among adolescents in Nicaragua, a region affected by an epidemic of chronic kidney disease of unknown aetiology. Nephrol Dial Transplant. 2016;31(3):424–32. Epub 2015/08/25. 10.1093/ndt/gfv292 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butler-Dawson J, Krisher L, Asensio C, Cruz A, Tenney L, Weitzenkamp D, et al. Risk Factors for Declines in Kidney Function in Sugarcane Workers in Guatemala. Journal of occupational and environmental medicine. 2018;60(6):548–58. 10.1097/JOM.0000000000001284 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffin BR, Butler-Dawson J, Dally M, Krisher L, Cruz A, Weitzenkamp D, et al. Unadjusted point of care creatinine results overestimate acute kidney injury incidence during field testing in Guatemala. PLoS One. 2018;13(9):e0204614–e. 10.1371/journal.pone.0204614 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. Journal of the American College of Cardiology. 2018;71(19):e127 10.1016/j.jacc.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 24.Dally M, Butler-Dawson J, Krisher L, Monaghan A, Weitzenkamp D, Sorensen C, et al. The impact of heat and impaired kidney function on productivity of Guatemalan sugarcane workers. PLoS One. 2018;13(10):e0205181–e. 10.1371/journal.pone.0205181 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Proust-Lima C, Séne M, Taylor JMG, Jacqmin-Gadda H. Joint latent class models for longitudinal and time-to-event data: a review. Stat Methods Med Res. 2014;23(1):74–90. Epub 2012/04/19. 10.1177/0962280212445839 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Proust-Lima C, Philipps V, Liquet B. Estimation of Extended Mixed Models Using Latent Classes and Latent Processes: The R Package lcmm. 2017. 2017;78(2):56 Epub 2017-06-01. 10.18637/jss.v078.i02 [DOI] [Google Scholar]
- 27.Bürkner P-C. brms: An R Package for Bayesian Multilevel Models Using Stan. 2017. 2017;80(1):28 Epub 2017-08-29. 10.18637/jss.v080.i01 [DOI] [Google Scholar]
- 28.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 29.The Renal Association [Internet]. [cited Dec 20, 2017]. https://renal.org/information-resources/the-uk-eckd-guide/ckd-stages/.
- 30.Johnson RJ, Herrera-Acosta J, Schreiner GF, Rodríguez-Iturbe B. Subtle Acquired Renal Injury as a Mechanism of Salt-Sensitive Hypertension. New England Journal of Medicine. 2002;346(12):913–23. 10.1056/NEJMra011078 [DOI] [PubMed] [Google Scholar]
- 31.O’Donnell JK, Tobey M, Weiner DE, Stevens LA, Johnson S, Stringham P, et al. Prevalence of and risk factors for chronic kidney disease in rural Nicaragua. Nephrol Dial Transplant. 2011;26(9):2798–805. Epub 2010/07/08. 10.1093/ndt/gfq385 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.González-Quiroz M, Pearce N, Caplin B, Nitsch D. What do epidemiological studies tell us about chronic kidney disease of undetermined cause in Meso-America? A systematic review and meta-analysis. Clin Kidney J. 2018;11(4):496–506. Epub 2017/12/08. 10.1093/ckj/sfx136 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ananda Jayalal TB, Jayaruwan Bandara TWMA, Mahawithanage STC, Wansapala MAJ, Galappaththi SPL. A quantitative analysis of chronic exposure of selected heavy metals in a model diet in a CKD hotspot in Sri Lanka. BMC nephrology. 2019;20(1):208-. 10.1186/s12882-019-1371-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Blond JS, Woskie S, Horwell CJ, Williamson BJ. Particulate matter produced during commercial sugarcane harvesting and processing: A respiratory health hazard? Atmospheric Environment. 2017;149:34–46. 10.1016/j.atmosenv.2016.11.012. [DOI] [Google Scholar]
- 35.Jayasumana C, Paranagama P, Agampodi S, Wijewardane C, Gunatilake S, Siribaddana S. Drinking well water and occupational exposure to Herbicides is associated with chronic kidney disease, in Padavi-Sripura, Sri Lanka. Environ Health. 2015;14:6-. 10.1186/1476-069X-14-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valcke M, Levasseur M-E, Soares da Silva A, Wesseling C. Pesticide exposures and chronic kidney disease of unknown etiology: an epidemiologic review. Environ Health. 2017;16(1):49-. 10.1186/s12940-017-0254-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jayasumana C, Gajanayake R, Siribaddana S. Importance of Arsenic and pesticides in epidemic chronic kidney disease in Sri Lanka. BMC nephrology. 2014;15:124-. 10.1186/1471-2369-15-124 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scammell MK, Sennett CM, Petropoulos ZE, Kamal J, Kaufman JS. Environmental and Occupational Exposures in Kidney Disease. Seminars in Nephrology. 2019;39(3):230–43. 10.1016/j.semnephrol.2019.02.001 [DOI] [PubMed] [Google Scholar]