Abstract
Background
Intra‐uterine insemination (IUI) is a widely‐used fertility treatment for couples with unexplained subfertility. Although IUI is less invasive and less expensive than in vitro fertilisation (IVF), the safety of IUI in combination with ovarian hyperstimulation (OH) is debated. The main concern about IUI treatment with OH is the increase in multiple pregnancy rates.
Objectives
To determine whether, for couples with unexplained subfertility, the live birth rate is improved following IUI treatment with or without OH compared to timed intercourse (TI) or expectant management with or without OH, or following IUI treatment with OH compared to IUI in a natural cycle.
Search methods
We searched the Cochrane Gynaecology and Fertility (CGF) Group trials register, CENTRAL, MEDLINE, Embase, PsycINFO, CINAHL and two trials registers up to 17 October 2019, together with reference checking and contact with study authors for missing or unpublished data.
Selection criteria
Randomised controlled trials (RCTs) comparing IUI with TI or expectant management, both in stimulated or natural cycles, or IUI in stimulated cycles with IUI in natural cycles in couples with unexplained subfertility.
Data collection and analysis
Two review authors independently performed study selection, quality assessment and data extraction. Primary review outcomes were live birth rate and multiple pregnancy rate.
Main results
We include 15 trials with 2068 women. The evidence was of very low to moderate quality. The main limitation was very serious imprecision.
IUI in a natural cycle versus timed intercourse or expectant management in a natural cycle
It is uncertain whether treatment with IUI in a natural cycle improves live birth rate compared to treatment with expectant management in a natural cycle (odds ratio (OR) 1.60, 95% confidence interval (CI) 0.92 to 2.78; 1 RCT, 334 women; low‐quality evidence). If we assume the chance of a live birth with expectant management in a natural cycle to be 16%, that of IUI in a natural cycle would be between 15% and 34%. It is uncertain whether treatment with IUI in a natural cycle reduces multiple pregnancy rates compared to control (OR 0.50, 95% CI 0.04 to 5.53; 1 RCT, 334 women; low‐quality evidence).
IUI in a stimulated cycle versus timed intercourse or expectant management in a stimulated cycle
It is uncertain whether treatment with IUI in a stimulated cycle improves live birth rates compared to treatment with TI in a stimulated cycle (OR 1.59, 95% CI 0.88 to 2.88; 2 RCTs, 208 women; I2 = 72%; low‐quality evidence). If we assume the chance of achieving a live birth with TI in a stimulated cycle was 26%, the chance with IUI in a stimulated cycle would be between 23% and 50%. It is uncertain whether treatment with IUI in a stimulated cycle reduces multiple pregnancy rates compared to control (OR 1.46, 95% CI 0.55 to 3.87; 4 RCTs, 316 women; I2 = 0%; low‐quality evidence).
IUI in a stimulated cycle versus timed intercourse or expectant management in a natural cycle
In couples with a low prediction score of natural conception, treatment with IUI combined with clomiphene citrate or letrozole probably results in a higher live birth rate compared to treatment with expectant management in a natural cycle (OR 4.48, 95% CI 2.00 to 10.01; 1 RCT; 201 women; moderate‐quality evidence). If we assume the chance of a live birth with expectant management in a natural cycle was 9%, the chance of a live birth with IUI in a stimulated cycle would be between 17% and 50%. It is uncertain whether treatment with IUI in a stimulated cycle results in a lower multiple pregnancy rate compared to control (OR 3.01, 95% CI 0.47 to 19.28; 2 RCTs, 454 women; I2 = 0%; low‐quality evidence).
IUI in a natural cycle versus timed intercourse or expectant management in a stimulated cycle
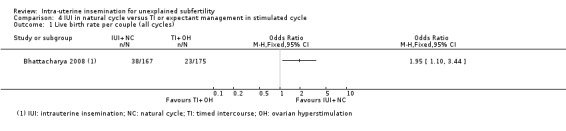
Treatment with IUI in a natural cycle probably results in a higher cumulative live birth rate compared to treatment with expectant management in a stimulated cycle (OR 1.95, 95% CI 1.10 to 3.44; 1 RCT, 342 women: moderate‐quality evidence). If we assume the chance of a live birth with expectant management in a stimulated cycle was 13%, the chance of a live birth with IUI in a natural cycle would be between 14% and 34%. It is uncertain whether treatment with IUI in a natural cycle results in a lower multiple pregnancy rate compared to control (OR 1.05, 95% CI 0.07 to 16.90; 1 RCT, 342 women; low‐quality evidence).
IUI in a stimulated cycle versus IUI in a natural cycle
Treatment with IUI in a stimulated cycle may result in a higher cumulative live birth rate compared to treatment with IUI in a natural cycle (OR 2.07, 95% CI 1.22 to 3.50; 4 RCTs, 396 women; I2 = 0%; low‐quality evidence). If we assume the chance of a live birth with IUI in a natural cycle was 14%, the chance of a live birth with IUI in a stimulated cycle would be between 17% and 36%. It is uncertain whether treatment with IUI in a stimulated cycle results in a higher multiple pregnancy rate compared to control (OR 3.00, 95% CI 0.11 to 78.27; 2 RCTs, 65 women; low‐quality evidence).
Authors' conclusions
Due to insufficient data, it is uncertain whether treatment with IUI with or without OH compared to timed intercourse or expectant management with or without OH improves cumulative live birth rates with acceptable multiple pregnancy rates in couples with unexplained subfertility. However, treatment with IUI with OH probably results in a higher cumulative live birth rate compared to expectant management without OH in couples with a low prediction score of natural conception. Similarly, treatment with IUI in a natural cycle probably results in a higher cumulative live birth rate compared to treatment with timed intercourse with OH. Treatment with IUI in a stimulated cycle may result in a higher cumulative live birth rate compared to treatment with IUI in a natural cycle.
Plain language summary
Intra‐uterine insemination for unexplained subfertility
Review question
Does treatment with intra‐uterine insemination (IUI), with or without fertility drugs, compared to timed intercourse or expectant management (watchful waiting) with or without fertility drugs, or treatment with IUI with fertility drugs compared to IUI without fertility drugs improve live birth rates in couples with unexplained subfertility?
Background
IUI is a treatment often used for couples with unexplained subfertility. In an IUI cycle, the male partner's sperm is prepared and placed directly in the womb around the time of ovulation. IUI cycles can be used in combination with fertility drugs to increase the number of available eggs. However, these drugs can have side effects, and also increase the risk of multiple pregnancies. Expectant management and timed intercourse have also been shown to increase pregnancy rates, resulting in live births. With this review, we would like to enhance decision‐making for couples starting treatment for unexplained subfertility.
Study characteristics
Cochrane authors identified 15 randomised controlled trials which included 2068 women. Women who were treated with IUI, with or without fertility drugs, were compared to those who were assigned timed intercourse or expectant management with or without fertility drugs, or women who received IUI with fertility drugs were compared to those who were treated with IUI without fertility drugs. The main outcomes of interest were live birth rates and multiple pregnancy rates. Other outcomes included pregnancy rate, miscarriage rate and other adverse events. The evidence is current to October 2019.
Key results
For most treatment groups, it is uncertain whether treatment with one IUI treatment type improves cumulative live birth rates (i.e. rates at the end of a course of treatment) with acceptable multiple pregnancy rates when compared to another treatment type. However, there is moderate‐quality evidence that, in couples with a low chance of natural conception, treatment with IUI combined with fertility drugs probably improves cumulative live birth rates compared to treatment with expectant management. Similarly, there is moderate‐quality evidence that treatment with IUI alone probably improves cumulative live birth rates compared to treatment with expectant management combined with fertility drugs.
Quality of the evidence
The evidence was of low to moderate quality for cumulative live birth and of low quality for multiple pregnancy. The main limitation of the evidence was a lack of precision in the findings, due to the inclusion of few studies with small sample sizes.
Summary of findings
Background
Description of the condition
Of all couples presenting with fertility problems, about 25% have no cause that can be identified (NICE 2013). Couples are classified as having unexplained subfertility when they have tried to conceive for at least one year and the fertility work‐up has shown patent fallopian tubes, an ovulatory menstrual cycle and a normal semen analysis.
Description of the intervention
Intra‐uterine insemination (IUI) is a commonly‐used treatment in couples with unexplained subfertility. IUI is a relatively simple procedure in which semen is 'washed' in the laboratory and inserted in the uterine cavity using a small catheter at the time of ovulation. IUI can be performed with or without drugs for ovarian hyperstimulation (OH). For correct timing of the insemination, cycle monitoring is performed. This is usually done by ultrasound assessment of follicle growth or by monitoring the pre‐ovulatory luteinising hormone rise in blood or urine. In hyperstimulated cycles, ovulation is often induced by an injection of human chorionic gonadotropin (hCG), which improves timing possibilities. In contrast to IUI procedure, with expectant management couples either receive cycle monitoring for correct timing of sexual intercourse, i.e. timed intercourse (TI), or no intervention at all.
How the intervention might work
The rationale for performing IUI is that the motile spermatozoa, which are morphologically normal, can be concentrated in a small volume and placed directly into the uterus close to the released oocyte. In this way the cervix, which also acts as a reservoir for sperm, is bypassed. Accurate timing of the insemination is therefore of great importance. IUI can be performed with or without ovarian hyperstimulation (OH). The role of IUI in fertility treatment is often debated, in particular whether or not it is superior to TI, and whether or not OH should be used at the same time (Cohlen 2005; Hughes 2003; Stewart 2003). Commonly‐used drugs for ovarian hyperstimulation are clomiphene citrate (CC) and aromatase inhibitors, such as letrozole, both of which are oral treatments, and gonadotropins which are administered by subcutaneous injection. The aim of OH is to increase the number of oocytes available for fertilisation and to enhance accurate timing.
The use of OH in fertility treatment for unexplained subfertility is associated with benefits as well as increased risk of multiple pregnancies. When Hughes 1997 published a meta‐analysis indicating that the average fecundability is approximately five‐fold higher for treatment with IUI and OH, the Royal College of Obstetricians and Gynaecologists (RCOG 1998) concluded accordingly that "OH with IUI is an effective treatment for couples with unexplained infertility". However, major concerns were raised about the incidence of multiple pregnancies, and OH became less popular. These concerns have resulted in an adjustment of the advice for treatment of couples with unexplained subfertility. The NICE fertility guideline states that "ovarian hyperstimulation should not be offered to women with unexplained subfertility" (NICE 2013).
The increase in multiple pregnancies is a logical consequence of stimulated growth of multiple follicles. The incidence of multiple pregnancies after treatment with OH and IUI varies between 10% and 40%, and the overall contribution of this treatment to multiple births is estimated to be around 30% (Fauser 2005). However, recent studies have reported lower incidence of multiple pregnancies with OH. For example, a recent randomised controlled trial has reported multiple pregnancy rates of between 1.4% and 2.2% with OH (Danhof 2018); rates of between 3% and 10% have also been reported in another trial (Diamond 2015). The question is whether this multiple pregnancy rate is acceptable or whether it can be reduced to acceptable numbers. Recently, more evidence has been collected showing that mild ovarian hyperstimulation with strict cancellation criteria reduces the risk of achieving multiple pregnancies to approximately 10%, without compromising pregnancy rates (ESHRE 2006; Ragni 2006; Van Rumste 2006). Because maternal and neonatal morbidity and mortality rates are significantly increased in multiple pregnancies (Fauser 2005; Ombelet 2005), caregivers should take extra care to keep the multiple pregnancy rate to a minimum. Couples should be well‐informed by their physicians, especially as many couples wish to conceive twins (Ryan 2004) and prefer a higher pregnancy chance over safety.
Some authors have stated that treatment with OH results in an unacceptably high incidence of high‐order multiple pregnancies (Gleicher 2000; Nan 1994) and treatment with IUI in natural cycles should be preferred (Fauser 2005; Goverde 2005). Others have reported that the risk of multiple pregnancies could be reduced, with strict monitoring of the people undergoing treatment (Dickey 2005; Tur 2005). Te Velde 1999 concluded that IUI with OH is an appropriate treatment option if done with a mild stimulation protocol, careful cycle monitoring and with strict cancellation criteria. It is, however, still not known to what extent multiple pregnancies can be avoided if these criteria are met. Besides, the use of strict cycle cancellation criteria might result in a reduced overall pregnancy rate. Several trials using mild stimulation protocols for IUI have been published, showing promising results of acceptable pregnancy rates with very low multiple pregnancy rates (Balasch 2004). As IVF allows better control over reducing the risk of a multiple pregnancy (Gleicher 2000), and IVF with single‐embryo transfer is becoming more accepted, it has been argued that IVF is a safer treatment option than IUI with OH.
Why it is important to do this review
Although ovarian hyperstimulation seems to result in higher pregnancy rates, it also increases the risk of multiple pregnancies and ovarian hyperstimulation syndrome (OHSS). High risk of multiple pregnancies, for instance, increases the risk of neonatal morbidity and mortality and maternal morbidity (Guzick 1999; Ombelet 2006). Fertility guidelines recommend IUI without OH for couples with unexplained subfertility because of the increased risk of multiple pregnancies and OHSS associated with OH (NICE 2013). This systematic review, originally published in 2006, and updated in 2012 (Veltman‐Verhulst 2012) and 2016 (Veltman‐Verhulst 2016), was therefore undertaken to assess the evidence on the benefits and adverse events associated with IUI with or without OH, compared to timed intercourse or expectant management with or without OH, or with IUI with OH compared to IUI without OH, for couples with unexplained subfertility.
Objectives
To determine whether, for couples with unexplained subfertility, the live birth rate is improved following IUI treatment with or without OH compared to timed intercourse (TI) or expectant management with or without OH, or following IUI treatment with OH compared to IUI in a natural cycle.
Methods
Criteria for considering studies for this review
Types of studies
Published and unpublished randomised controlled trials (RCTs) were eligible for inclusion. We excluded non‐randomised studies (e.g. studies with evidence of inadequate sequence generation such as alternate days, patient numbers) as they are associated with a high risk of bias. We attempted to contact the author of the study if the randomisation or allocation method was unclear.
We excluded trials that did not report separate data for women with unexplained subfertility or where such data were not obtainable from the authors. We assessed the trial design (cross‐over or parallel) and included cross‐over trials if we could extract pre‐cross‐over data.
Types of participants
I. Couples with unexplained subfertility, defined as follows.
Normal ovulatory status (determined by either biphasic basal body temperature chart, normal luteal progesterone, in‐phase endometrial biopsy or ovulation detected with ultrasound).
Tubal patency (determined by hysterosalpingography or laparoscopy, or both).
A normal semen sample according to World Health Organization (WHO) criteria current at the time of the trial.
II. Couples who had tried to conceive for at least one year.
Participants excluded were: couples with a known cause of infertility including a moderate male factor, moderate to severe endometriosis (according to the American Society for Reproductive Medicine (ASRM) classification), tubal disease, or a cervical factor.
We contacted study authors to obtain data of couples with unexplained infertility if groups with mixed infertility causes were studied. If we could not extract relevant data separately for included participants, we excluded the study.
We excluded trials of participants with mild to moderate endometriosis only.
Types of interventions
Trials with at least one of the following comparisons:
Intra‐uterine insemination (IUI) in a natural cycle versus timed intercourse (TI) or expectant management in a natural cycle;
IUI in a stimulated cycle versus TI or expectant management in a stimulated cycle;
IUI in a stimulated cycle versus TI or expectant management in a natural cycle;
IUI in a natural cycle versus TI or expectant management in a stimulated cycle;
IUI in a stimulated cycle versus IUI in a natural cycle.
Ovarian hyperstimulation (OH) was achieved with either clomiphene citrate, aromatase inhibitors (letrozole) or gonadotropins. We included expectant management as a variant of timed intercourse.
We excluded the following interventions:
intra‐cervical insemination, because we consider this to be a different treatment modality (Ripps 1994) and it is the topic of another Cochrane Review (Kop 2018);
donor insemination.
Types of outcome measures
Primary outcomes
Live birth rate per couple: all cycles. Live birth is defined as delivery of a live foetus after 20 completed weeks of gestational age;
Multiple pregnancy rate per couple. Multiple pregnancies confirmed by ultrasound, with or without selective reduction, were recorded.
Secondary outcomes
Pregnancy rate per couple: all cycles. Pregnancy includes clinical pregnancy, defined by the presence of an intra‐uterine gestational sac or foetal heartbeat visualised by an ultrasound scan before 12 weeks, and/or ongoing pregnancy, defined as a pregnancy extending beyond 12 weeks of gestation, confirmed by ultrasound or delivery.
Other adverse events:
Moderate or severe ovarian hyperstimulation syndrome (OHSS), rate per woman;
Miscarriage rate per couple;
Ectopic pregnancy rate per couple.
We excluded pregnancies confirmed only by detection of hCG in serum or urine (biochemical pregnancies). When pregnancy was not further defined, and remained unclear even after contacting the authors, we assumed the pregnancy to be clinical.
Search methods for identification of studies
We searched for all reports which describe (or might describe) randomised controlled trials of IUI with or without OH, in couples with unexplained infertility. The original search was performed in 2005 and was last updated in October 2019, in consultation with the Gynaecology and Fertility Group (CGF) Information Specialist.
Electronic searches
We searched:
Cochrane Gynaecology and Fertility Specialised Register; PROCITE platform (searched 17 October 2019) (Appendix 1);
CENTRAL; via the Cochrane Register of Studies Online (CRSO); web platform (searched 17 October 2019) (Appendix 2);
MEDLINE; OVID platform (searched from 1946 to 17 October 2019) (Appendix 3);
Embase; OVID platform (searched from 1980 to 17 October 2019) (Appendix 4);
PsycINFO; OVID platform (searched from 1806 to 17 October 2019) (Appendix 5);
CINAHL; EBSCO platform (searched from 1961 to October 2019) (Appendix 6).
We combined the MEDLINE search with the Cochrane highly sensitive search strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision; Lefebvre 2011). We combined the Embase and PsycINFO searches with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) ( www.sign.ac.uk/methodology/filters.html#random).
Other electronic sources of trials included:
-
Trial registers for ongoing and registered trials (up to October 2019):
www.clinicaltrials.gov (a service of the US National Institutes of Health);
www.who.int/trialsearch/Default.aspx (The World Health Organization International Trials Registry Platform search portal) Note: it is now mandatory for Cochrane Reviews to include searches of trial registers;
Database of Abstracts of Reviews of Effects (DARE) in the Cochrane Library at onlinelibrary.wiley.com/o/cochrane/cochrane_cldare_articles_fs.html (for reference lists from relevant non‐Cochrane reviews) (up to October 2019);
Web of Knowledge at wokinfo.com/ (another source of trials and conference abstracts) (up to October 2019);
OpenGrey at www.opengrey.eu/ for unpublished literature from Europe (up to October 2019);
LILACS database at regional.bvsalud.org/php/index.php?lang=en (for trials from the Portuguese‐ and Spanish‐speaking world) (up to October 2019);
PubMed and Google Scholar (for recent trials not yet indexed in MEDLINE) (up to October 2019);
Epistemonikos database at www.epistemonikos.org/en for systematic reviews that are useful for reference‐checking for trials (up to October 2019);
European Society of Human Reproduction and Embryology (ESHRE) and American Society for Reproductive Medicine (ASRM) 2019 conference abstracts.
Searching other resources
We handsearched reference lists of articles retrieved by the search and contacted experts in the field to obtain additional data. We also handsearched relevant journals and conference abstracts that were not covered in the Cochrane Gynaecology and Fertility register, in liaison with the Information Specialist.
Data collection and analysis
Selection of studies
After an initial screening of titles and abstracts produced by the search, we retrieved the full texts of all potentially eligible studies. Two review authors (ROA and JDA) independently examined these full‐text articles for compliance with the inclusion criteria and selected studies eligible for inclusion in the 2020 update. We contacted study investigators as required, to clarify study eligibility. We resolved any disagreements about study eligibility by discussion. We documented the selection process with a 'PRISMA' flow chart (Liberati 2009).
Data extraction and management
Two review authors (ROA and JDA) independently extracted data from eligible studies using a data extraction form designed and pilot‐tested by the review authors, resolving any disagreements by discussion. Data extracted included study characteristics and outcome data. Where studies had multiple publications the review authors collated multiple reports of the same study, so that each study, rather than each report, was the unit of interest in the review, and such studies had a single study ID with multiple references. We contacted study investigators for further data on methods or results, or both, as required.
Assessment of risk of bias in included studies
Two review authors (ROA and JDA) independently assessed the included studies for risks of bias using the Cochrane 'Risk of bias' assessment tool (Higgins 2017) to assess: selection bias (random sequence generation and allocation concealment); performance bias (blinding of participants and personnel); detection bias (blinding of outcome assessors); attrition bias (incomplete outcome data); reporting bias (selective reporting); and other potential bias. We resolved disagreements by discussion or by involving a third review author. We described all judgements fully in the 'Risk of bias' table for each included study and incorporated our judgements into the interpretation of the review findings.
Measures of treatment effect
Only dichotomous data were reported in this review and we used the numbers of events in the control and intervention groups of each study to calculate Mantel‐Haenszel odds ratios (ORs). We reversed the direction of effect of individual studies, where required, to ensure consistency across trials. We presented 95% confidence intervals (CIs) for all outcomes. We assessed whether the estimates calculated in the review for individual studies were compatible in each case with the estimates reported in the study publications.
Unit of analysis issues
We analysed data per randomised couple or woman, because per‐treatment‐cycle data may lead to biased results (Dias 2008). In the case of a cross‐over trial, we only analysed data prior to cross‐over. For studies where data did not allow analysis (e.g. per‐cycle data) we contacted study authors for per‐woman data. Where we could not obtain appropriate data after contact with authors, we excluded such data (per‐cycle) from meta‐analyses. We counted multiple live births (e.g. twins or triplets) as one live birth event.
Dealing with missing data
We analysed the data on an intention‐to‐treat basis as far as possible. In the case of missing data we contacted authors of the published trials and included the newly‐obtained data in the analysis. However, where the study authors did not provide additional data, we assumed that no live births occurred in participants without a reported outcome. We assumed that women who dropped out or were excluded after randomisation were not pregnant. Women who were excluded because they conceived before receiving treatment were included as a success in the allocated group in the ITT analysis. For other outcomes, we analysed only the available data.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity using the I2 statistic. We took an I2 statistic measurement greater than 50% to indicate substantial heterogeneity (Deeks 2017).
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, we minimised their potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data. Where there were 10 or more studies in an analysis, we had intended to use a funnel plot to explore the possibility of small‐study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies) (Sterne 2017).
Data synthesis
Where the studies were sufficiently similar, we combined the data using a fixed‐effect model in the following comparisons:
Intra‐uterine insemination (IUI) in a natural cycle versus timed intercourse (TI) or expectant management in a natural cycle;
IUI in a stimulated cycle versus TI or expectant management in a stimulated cycle;
IUI in a stimulated cycle versus TI or expectant management in a natural cycle;
IUI in a natural cycle versus TI or expectant management in a stimulated cycle;
IUI in a stimulated cycle versus IUI in a natural cycle.
An increase in the odds of a particular outcome, which may be beneficial (e.g. live birth) or detrimental (e.g. adverse events), was displayed graphically in the meta‐analyses to the right of the centre‐line and a decrease in the odds of an outcome to the left of the centre‐line. When pre‐cross‐over data were available, we included cross‐over trials in the analysis and pooled them with parallel trials.
Subgroup analysis and investigation of heterogeneity
Where data were available, we planned to conduct subgroup analyses to determine the separate evidence on the number of treatment cycles for live birth, and methods of ovarian hyperstimulation. However, there were insufficient data to perform subgroup analyses for the number of treatment cycles in any the comparisons, and for methods of ovarian hyperstimulation in some of the comparisons. We therefore did not report the findings by subgroups for any of the comparisons, except where data could not be pooled in meta‐analyses due to the presence of high heterogeneity. Where a visual scan of the forest plots or the results of statistical tests indicated substantial heterogeneity, we explored possible explanations in the text or in subgroup analyses.
Sensitivity analysis
Where appropriate, we performed sensitivity analyses for the review's primary outcomes (live birth and multiple pregnancy) to determine whether the results were robust to decisions made during the review process. These analyses considered the effects of excluding the following studies:
Studies that did not clearly describe adequate procedures for random sequence generation and allocation concealment;
Studies with a unit‐of‐analysis error (such as those in which cross‐over data were analysed as if they derived from parallel studies).
Overall quality of the body of evidence: 'Summary of findings' tables
We prepared 'Summary of findings' tables using GRADEpro software (GRADEpro GDT 2015) and Cochrane methods. These tables evaluate the overall quality of the body of evidence for the review outcomes (live birth, multiple pregnancy, pregnancy, OHSS, miscarriage, ectopic pregnancy) for the main review comparisons:
IUI versus TI or expectant management both in a natural cycle;
IUI in a stimulated cycle versus TI or expectant management in a stimulated cycle;
IUI in a stimulated cycle versus TI or expectant management in a natural cycle;
IUI in a natural cycle versus TI or expectant management in a stimulated cycle; and
IUI in a stimulated cycle versus IUI in a natural cycle).
We assessed the quality of the evidence using GRADE criteria: risk of bias, consistency of effect, indirectness, imprecision and publication bias (Schünemann 2017). Two review authors working independently made judgements about evidence quality (high, moderate, low or very low), with disagreements resolved by discussion. We justified, documented, and incorporated our judgements into the reporting of results for each outcome.
Results
Description of studies
Results of the search
For the 2020 update, the searches identified 925 records (most of which were also identified during the previous updates) after removal of duplicates. We retrieved three full‐text articles, one of which was eligible for inclusion (Farquhar 2018). Fourteen studies were identified from the previous updates; we therefore include a total of 15 studies (21 references) in the review. See Figure 1 for details of the screening and selection process and Characteristics of included studies for studies included in the review.
1.
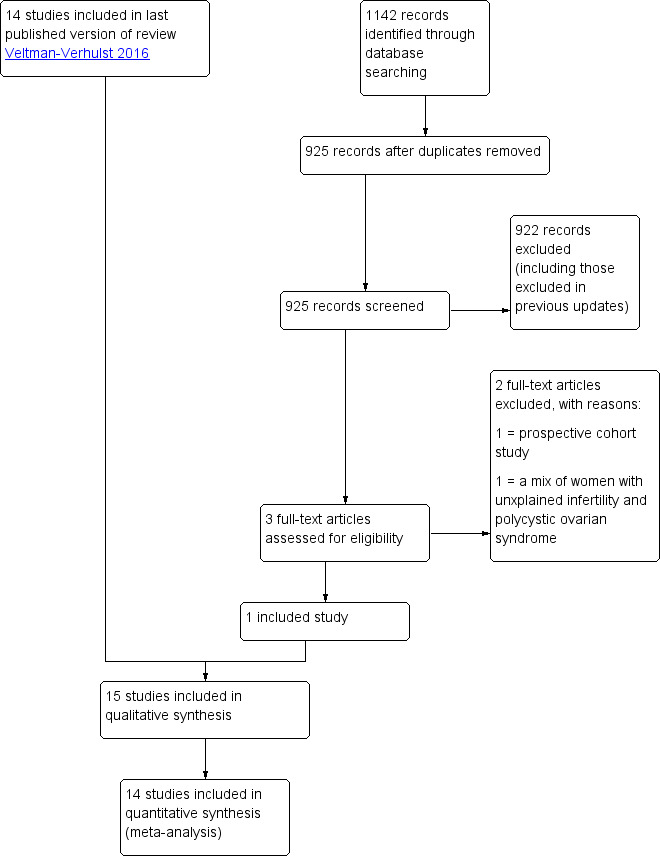
Study flow diagram.
We tried to contact all study authors to retrieve unpublished details. Responses from three study authors resulted in the exclusion of the following trials: Nulsen 1993; Prentice 1995; Serhal 1988. Seven study authors (Agarwal 2004; Arici 1994; Bhattacharya 2008; Guzick 1999; Melis 1995; Murdoch 1991; Steures 2006a) provided unpublished information or data, which we used in this analysis. Some authors could not provide us with the requested data, while others did not respond to our requests.
Included studies
Design
We included 15 RCTs. All trials were published in journals (Janko 1998 was published as an abstract only) and were available in English. The trials were carried out in different countries: UK (Bhattacharya 2008; Chung 1995; Murdoch 1991), USA (Arici 1994; Deaton 1990; Guzick 1999), Italy (Arcaini 1996; Melis 1995), the Netherlands (Goverde 2000;Steures 2006a), India (Agarwal 2004), New Zealand (Farquhar 2018), Slovakia (Janko 1998), Sweden (Karlstrom 1993), and multiple European countries (Crosignani 1991).
Twelve were parallel‐group studies (Agarwal 2004; Arcaini 1996; Bhattacharya 2008; Chung 1995; Farquhar 2018; Goverde 2000;Guzick 1999; Janko 1998; Karlstrom 1993; Melis 1995; Murdoch 1991; Steures 2006a), and three were cross‐over studies (Arici 1994; Crosignani 1991; Deaton 1990).
Power calculation
Bhattacharya 2008, Farquhar 2018, Goverde 2000 and Steures 2006a were the only studies which performed a power calculation. All four studies reached the targeted inclusion number to obtain enough power (80% to 90% with 5% level of significance). Further details about the included trials are provided in the Characteristics of included studies table.
Participants
The 15 trials comprised a total of 2068 women with unexplained subfertility.
The ages of the women were mentioned in most trials as either a mean ± standard deviation (SD) or a median and range. One trial (Janko 1998) did not report the women's ages. The overall age reported in the studies was similar. The mean or median age was between 30 years and 34 years (with comparable SDs). The maximum age of the participants was stated in six studies, only one of whom was above the age of 40 years (Arici 1994).
The duration of subfertility was given in 11 trials and reported as a mean duration or range. Five studies only included couples with subfertility for more than at least three years. The overall subfertility duration ranged from one year to 15 years.
Two studies used a prediction score of validated Hunault model (Hunault 2004), including women with a prediction score of natural conception of less than 30% (Farquhar 2018) or between 30% and 40% (Steures 2006a).
Types of subfertility
The definition of unexplained subfertility was similar between studies. Six trials enrolled participants with unexplained subfertility only. Five trials also included participants with male factor subfertility. In those studies the data for unexplained subfertility were either reported separately or obtained from the authors. One study selected couples with unexplained subfertility and an intermediate prognosis (Steures 2006a). Five studies reported the inclusion of women with either surgically‐corrected endometriosis (Deaton 1990), mild or stage II treated endometriosis (Guzick 1999) or minimal/mild endometriosis( Bhattacharya 2008; Farquhar 2018; Karlstrom 1993), which we considered to be unexplained subfertility. Melis 1995 specifically excluded participants if minor disorders such as minimal endometriosis were found in the investigation. Although our protocol stipulated only women with minimal and mild endometriosis, we decided to include Deaton 1990, despite the inclusion of three participants (out of 51 in total) with moderate endometriosis.
All studies reported a thorough fertility investigation, including a laparoscopy. A semen analysis was performed at least once in all studies. In nine studies the semen quality was reported according to the WHO criteria. Two studies (Arcaini 1996; Janko 1998) did not specify the criteria for a normal semen analysis. Chung 1995 used a sperm count per ejaculate instead of per ml. The data in Guzick 1999 were based only on a normal sperm count and a normal motility according to Kruger criteria.
Primary or secondary subfertility
Nine trials contained a mixed population of couples who had never achieved a pregnancy (primary subfertility) and those who had previously been pregnant (secondary subfertility). The remaining trials did not give any description for inclusion of people with secondary subfertility.
Previous treatment
Couples who have previously had failed fertility treatment have a lower probability of conception in subsequent treatment attempts. It is therefore important in fertility trials to report if couples have undergone previous treatment. Of the 15 included studies only two trials included couples who had previously had unsuccessful fertility treatment (Farquhar 2018; Melis 1995). Five trials did not include previously‐treated participants (Agarwal 2004; Arici 1994; Guzick 1999; Karlstrom 1993; Murdoch 1991) and the remaining trials did not provide information about previous treatment.
Interventions
Number of trials included per comparison
IUI in a natural cycle versus expectant management in a natural cycle: one trial (Bhattacharya 2008)..
IUI in a stimulated cycle versus TI in a stimulated cycle: seven trials (Agarwal 2004; Arcaini 1996; Chung 1995; Crosignani 1991; Janko 1998; Karlstrom 1993; Melis 199
IUI in a stimulated cycle versus TI or expectant management in a natural cycle: three trials (Deaton 1990; Farquhar 2018; Steures 2006a).
IUI in a natural cycle versus expectant management in a stimulated cycle: one trial (Bhattacharya 2008).
IUI in a stimulated cycle versus IUI in natural cycle: four trials (Arici 1994; Goverde 2000; Guzick 1999; Murdoch 1991)
Agarwal 2004, although included in the review, was excluded from the primary analysis. This Indian study had a high dropout percentage (37%) in the treatment group which caused severely unbalanced groups. The main reason for dropout was financial constraints, so this introduces a considerable bias. In three of the included studies (Bhattacharya 2008; Farquhar 2018; Steures 2006a) expectant management was performed instead of TI.
Treatment
The treatment methods varied substantially between studies. Seven studies used gonadotropins for ovarian hyperstimulation. Arcaini 1996 offered both gonadotropins and clomiphene citrate, which resulted in a high‐dose hyperstimulation. Five studies used clomiphene only, one study used clomiphene and letrozole (Farquhar 2018) and Crosignani 1991 did not report the method of ovarian hyperstimulation. The different fertility centres in this multicentre trial used different treatments. More details on drug dose and method can be found in the prognostic factor table (Appendix 7) and the Characteristics of included studies table. Additional gonadotropin‐releasing hormone agonist (GnRHa) was used by Chung 1995 and Murdoch 1991. All studies used human chorionic gonadotropin (hCG) (5000 to 10,000 IU) for triggering ovulation. Chung 1995 also provided hCG in the post‐ovulatory phase.
The timing of IUI was similar among the studies. Follicle development was usually monitored by ultrasound scan (USS) and serum estradiol levels (serum‐E2). The hCG was given when the dominant follicles reached a mean diameter of 16 mm to 18 mm. Insemination was performed 30 hours to 48 hours after hCG administration. Arcaini 1996 performed a double insemination at 24 and 48 hours, and in Murdoch 1991 insemination took place on alternate days until ovulation was confirmed. Follicular development in natural cycles was monitored by ultrasound or luteinising hormone (LH) urine tests, and intercourse was advised at 12 hours to 40 hours after the hCG or LH surge. Couples were mostly advised to have intercourse more than once.
In the studies with expectant management instead of TI (Bhattacharya 2008; Farquhar 2018; Steures 2006a), couples were given general advice about the need for regular intercourse.
The number of cycles in included studies ranged from one to eight.
Cancellation criteria
The most serious adverse effects of ovarian hyperstimulation are multiple pregnancies and ovarian hyperstimulation syndrome (OHSS). These risks can both be reduced by the cancellation of the treatment cycle if excessive follicle stimulation occurs. It is important that fertility trials report the cancellation criteria they applied. Firstly, this ensures that participants were not exposed to a higher risk of multiple pregnancy or OHSS to increase the pregnancy rate, and secondly, it reduces the bias introduced by cancellation of treatment in initially randomised groups.
Eleven studies described criteria for cancellation of the treatment cycle. Insemination or hCG administration did not take place if the cancellation criteria were met. Six studies used serum‐E2 levels to determine over‐ or under‐stimulation as well as a maximum of dominant follicles (four follicles of a maximum 16 mm diameter). Arcaini 1996 accepted a maximum of six dominant follicles. Four studies did not describe any cancellation criteria.
Outcomes
Ten trials reported live birth, our primary outcome of interest. The other studies reported pregnancy as the main outcome. Pregnancy was confirmed by ultrasound in nine trials. In Guzick 1999 pregnancy was confirmed by two hCG measurements or live birth. Others did not report the method of pregnancy confirmation. The reported pregnancies were mostly clinical. The multiple pregnancy rate was mentioned in 13 trials, miscarriage in 11, ectopic pregnancy in 11, and OHSS in 10 trials. These events were often reported as total numbers or as post‐cross‐over data and therefore often could not be used in the meta‐analysis.
Excluded studies
For the 2020 update, we excluded two studies in addition to those previously excluded. One was excluded because it was a prospective cohort study (Van Eekelen 2019) while the other study (Zolghadri 2012) was excluded because it included a mix of women with unexplained infertility and polycystic ovarian syndrome, with no separate data for the two groups. We contacted the study authors for data on women with unexplained infertility but we did not receive any response. We had excluded 23 studies during the previous updates (see details below); we therefore excluded a total of 25 studies from this review update. See Characteristics of excluded studies.
For the 2016 update, 10 studies were excluded (Aanesen 2014; Check 2013; Barros Delgadillo 2008; Barros‐Delgadillo 2010; Kabouk 2010; Leanza 2014a; Leanza 2014b; Peeraer 2013; Wadhwa 2013; Xu 2014). Four studies were not RCTs (Check 2013; Barros Delgadillo 2008; Leanza 2014a; Leanza 2014b), four studies did not include a comparison of interest to this review (Barros‐Delgadillo 2010; Kabouk 2010; Peeraer 2013; Wadhwa 2013), one study was a cohort study (Aanesen 2014) and another study was ineligible as it investigated donor sperm (Xu 2014).
For the 2012 update, 13 studies were excluded (Aboulghar 1993; Doyle 1991; Evans 1991; Gregoriou 1995; Ho 1998; Kirby 1991; Martinez 1990; Martinez 1991; Nulsen 1993; Prentice 1995; Serhal 1988; Tummon 1997; Zikopoulos 1993). Two studies were found not to be randomised studies (Aboulghar 1993; Serhal 1988); inadequate method of randomisation was the reason for exclusion of another two trials (Nulsen 1993; Prentice 1995); one study (Tummon 1997) included women with endometriosis only and thus did not focus on unexplained subfertility; Martinez 1990 reported biochemically‐confirmed pregnancies only and was therefore excluded; Ho 1998 did not report separate data for couples with unexplained subfertility; six studies (Doyle 1991; Evans 1991; Gregoriou 1995; Kirby 1991; Martinez 1991; Zikopoulos 1993) reported pre‐ or post‐cross‐over 'per‐cycle' only, instead of 'per randomised woman', and therefore could not be included. We contacted authors of these studies to obtain relevant data such as pre‐cross‐over 'per randomised woman' data but we did not receive any response from the authors.
Studies awaiting classification
There are no studies awaiting classification. However if pre‐cross‐over data of the excluded studies become available we will reconsider inclusion and report the studies in an update of this review.
Ongoing studies
We identified one study which was ongoing and appeared to meet the inclusion criteria (NCT03455426).
Risk of bias in included studies
See the ’Risk of bias’ graph (Figure 2) and ’Risk of bias’ summary (Figure 3).
2.
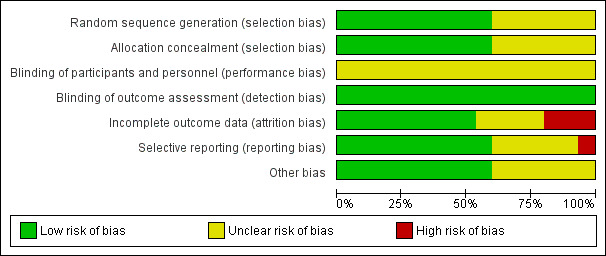
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
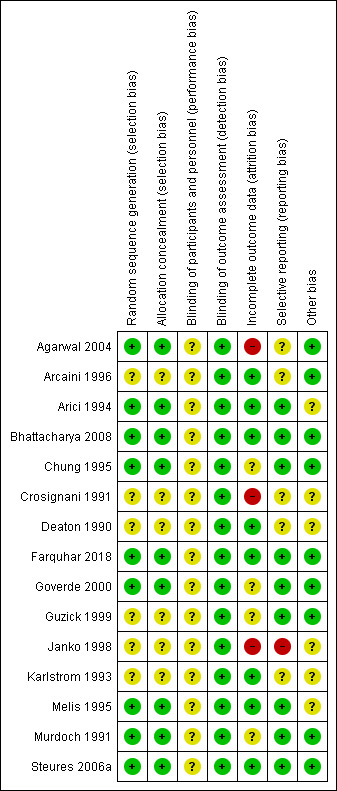
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In nine of the included studies (Agarwal 2004; Arici 1994; Bhattacharya 2008; Chung 1995; Farquhar 2018; Goverde 2000; Melis 1995; Murdoch 1991; Steures 2006a), we considered the methods used for sequence generation and allocation concealment to be adequate and we therefore rated them as being at low risk of bias. In the remaining six trials (Arcaini 1996; Crosignani 1991; Deaton 1990; Guzick 1999; Janko 1998; Karlstrom 1993), the methods used for random sequence generation and allocation concealment were not sufficiently described to draw a conclusive judgement, so we rated the risk of bias as unclear.
Blinding
We assessed all the included studies as being at unclear risk for performance bias, as none of them reported blinding due to the nature of the interventions. For detection bias, we rated all the included studies as being at low risk; we considered that non‐blinding was unlikely to affect the outcomes of interest, as they were objectively assessed.
Incomplete outcome data
We used an intention‐to‐treat (ITT) analysis when possible. In three trials an ITT analysis was not possible (Crosignani 1991; Deaton 1990; Karlstrom 1993), as the trials only reported the number of participants analysed.
In Murdoch 1991 one woman became pregnant spontaneously between treatment cycles. This pregnancy resulted in a live birth and was entered as such in the analysis. Goverde 2000 also reported spontaneous pregnancies that occurred between treatment cycles. Because it was unclear in which group these pregnancies occurred, we could not use them in the ITT analysis.
Seven of the 15 included trials clearly mentioned the number of dropouts and the reasons for dropping out (Arici 1994; Deaton 1990; Farquhar 2018; Goverde 2000; Guzick 1999; Melis 1995; Steures 2006a). Murdoch 1991 reported the number of dropouts but did not give any information on reasons for dropping out. Bhattacharya 2008 had a dropout rate of less than 1%. The studies with the highest dropout rates were Arcaini 1996 (dropout of 20.6%) and Agarwal 2004 (19%). In Agarwal the couples mainly left the study for financial reasons, which resulted in an unevenly‐distributed dropout rate of 37% in the treatment group as compared to 1% in the control group. The dropout rate usually increased in studies with a longer follow‐up period. Because this review included trials with different durations, it was difficult to compare the dropout rates. We rated nine of the included studies as being at low risk of bias, three as unclear and another three as high risk of bias.
Selective reporting
There was a risk of selective reporting in some of the studies included in this review. Live birth data were not reported in five studies (Arcaini 1996; Crosignani 1991; Deaton 1990; Janko 1998; Karlstrom 1993). Adverse events were often not reported by group but as a study total, which could not be included in the analysis. Multiple pregnancy rates were not reported in two trials (Crosignani 1991; Janko 1998). We rated nine of the included studies as being at low risk of bias, five as being at unclear risk and one as being at high risk. We did not construct a funnel plot for any of the analyses, as none of them included up to 10 studies.
Other potential sources of bias
To reduce bias introduced by a cross‐over study design, we included pre‐cross‐over data only. Three studies used a cross‐over design (Arici 1994; Crosignani 1991; Deaton 1990). In this design participants were initially randomised to the treatment or control group but then crossed over to the other group after a certain number of treatment cycles. The duration of these studies varied from two to eight treatment cycles per couple. In two studies (Arici 1994; Crosignani 1991) the participants crossed over after one treatment cycle. In Deaton 1990 participants crossed over after four cycles.
We rated nine of the included studies as being at low risk of bias because baseline demographic characteristics of participants between the two treatment groups were similar. We rated the remaining six studies at unclear risk for this domain because there was insufficient information to make a conclusive judgement on the baseline demographic characteristics of participants.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Summary of findings for the main comparison. IUI in a natural cycle compared to TI or expectant management in a natural cycle for unexplained subfertility.
| IUI compared to TI or expectant management both in natural cycle for unexplained subfertility | ||||||
| Patient or population: participants with unexplained subfertility Settings: fertility clinic Intervention: IUI Comparison: TI or expectant management both in natural cycle | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| TI or expectant management both in natural cycle | IUI | |||||
| Live birth rates per couple (all cycles) | 156 per 1000 | 228 per 1000 (145 to 339) | OR 1.60 (0.92 to 2.78) | 334 (1 study) | ⊕⊕⊝⊝ lowa | ‐ |
| Multiple pregnancy rate per couple | 12 per 1000 | 6 per 1000 (0 to 63) | OR 0.50 (0.04 to 5.53) | 334 (1 study) | ⊕⊕⊝⊝ lowa | ‐ |
| Pregnancy rate per couple (all cycles) | 162 per 1000 | 228 per 1000 (145 to 338) | OR 1.53 (0.88 to 2.64) | 334 (1 study) | ⊕⊕⊝⊝ lowa | ‐ |
| Ovarian hyperstimulation syndrome rate per woman ‐ not reported | See comment | See comment | ‐ | ‐ | See comment | No data reported on outcome |
| Miscarriage rate per couple | 54 per 1000 | 42 per 1000 (16 to 107) | OR 0.77 (0.28 to 2.11) | 334 (1 study) | ⊕⊕⊝⊝ lowa | ‐ |
| Ectopic pregnancy rate per couple | Not estimable (no events in control group) | ‐ | OR 05.06 (0.24 to 1106.21) | 334 (1 study) | ⊕⊕⊝⊝ lowa | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aDowngraded by two levels for very serious imprecision: small sample size with a low event rate and effect estimate with a wide confidence interval.
Summary of findings 2. IUI in a stimulated cycle compared to TI or expectant management in a stimulated cycle for unexplained subfertility.
| IUI compared to TI or expectant management both in stimulated cycle for unexplained subfertility | ||||||
| Patient or population: participants with unexplained subfertility Settings: fertility clinic Intervention: IUI Comparison: TI or expectant management both in stimulated cycles | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| TI or expectant management both in stimulated cycle | IUI | |||||
| Live birth rate per couple (all cycles) | 255 per 1000 | 352 per 1000 (231 to 496) | OR 1.59 (0.88 to 2.88) | 208 (2 studies) | ⊕⊕⊝⊝ lowa | ‐ |
| Multiple pregnancy rate per couple | 43 per 1000 | 62 per 1000 (24 to 148) | OR 1.46 (0.55 to 3.87) | 316 (4 studies) | ⊕⊕⊝⊝ lowa | ‐ |
| Pregnancy rate per couple (all cycles) | 234 per 1000 | 340 per 1000 (258 to 436) | OR 1.69 (1.14 to 2.53) | 517 (6 studies) | ⊕⊕⊝⊝ lowb,c | ‐ |
| Ovarian hyperstimulation syndrome rate per woman | Not estimable (no events in control group) | OR 2.75 (0.11 to 69.83) | 68 (1 study) | ⊕⊝⊝⊝ very lowa,b | ‐ | |
| Miscarriage rate per couple | 57 per 1000 | 91 per 1000 (33 to 228) | OR 1.66 (0.56 to 4.88) | 208 (2 studies) | ⊕⊕⊝⊝ lowa | ‐ |
| Ectopic pregnancy rate per couple | Not estimable (no events in control group) | ‐ | OR 3.06 (0.12 to 76.95) | 100 (1 study) | ⊕⊕⊝⊝ lowa | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aDowngraded by two levels for very serious imprecision: small sample size with a low event rate and effect estimate with a wide confidence interval. bDowngraded by one level for serious risk of bias: most domains of risk of bias were assessed as 'unclear'. cDowngraded by one level for serious imprecision: small sample size.
Summary of findings 3. IUI in a stimulated cycle compared to TI or expectant management in a natural cycle for unexplained subfertility.
| IUI in stimulated cycle compared to TI or expectant management in natural cycle for unexplained subfertility | ||||||
| Patient or population: participants with unexplained subfertility Settings: fertility clinic Intervention: IUI in stimulated cycle Comparison: TI or expectant management in natural cycle | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| TI or expectant management in a natural cycle | IUI in a stimulated cycle | |||||
| Live birth rate per couple (all cycles)‐ clomiphene citrate or letrozole | 90 per 1000 | 307 per 1000 (165 to 497) | OR 4.48 (2 to 10.01) | 201 (1 study) | ⊕⊕⊕⊝ moderatea | ‐ |
| Live birth rate per couple (all cycles)‐ clomiphene citrate or gonadotropins | 238 per 1000 | 204 per 1000 (123 to 318) | OR 0.82 (0.45 to 1.49) | 253 (1 study) | ⊕⊕⊝⊝ lowb | ‐ |
| Multiple pregnancy rate per couple | 4 per 1000 | 13 per 1000 (2 to 79) | OR 3.01 (0.47 to 19.28) | 454 (2 studies) | ⊕⊕⊝⊝ lowb | ‐ |
| Pregnancy rate per couple (all cycles)‐ clomiphene citrate or letrozole | 110 per 1000 | 366 per 1000 (215 to 549) | OR 4.68 (2.22 to 9.86) | 201 (1 study) | ⊕⊕⊕⊝ moderatea | ‐ |
| Ovarian hyperstimulation syndrome rate per woman | See comment | See comment | See comment | See comment | See comment | No events in intervention or control groups |
| Miscarriage rate per couple | 31 per 1000 | 84 per 1000 (36 to 183) | OR 2.87 (1.18 to 7.01) | 454 (2 studies) | ⊕⊕⊕⊝ moderatea | ‐ |
| Ectopic pregnancy rate per couple | Not estimable (no events in intervention group) | ‐ | OR 9.28 (0.49 to 174.6) | 201 (1 study) | ⊕⊕⊝⊝ lowb | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aDowngraded by one level for serious imprecision: small sample size with a low event rate. bDowngraded by two levels for very serious imprecision: small sample size with a low event rate and effect estimate with a wide confidence interval.
Summary of findings 4. IUI in a natural cycle compared to TI or expectant management in a stimulated cycle for unexplained subfertility.
| IUI in natural cycle compared to TI or expectant management in stimulated cycle for unexplained subfertility | ||||||
| Patient or population: participants with unexplained subfertility Settings: fertility clinic Intervention: IUI in natural cycle Comparison: TI or expectant management in stimulated cycle | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| TI or expectant management in stimulated cycle | IUI in natural cycle | |||||
| Live birth rate per couple (all cycles) | 131 per 1000 | 227 per 1000 (142 to 341) | OR 1.95 (1.10 to 3.44) | 342 (1 study) | ⊕⊕⊕⊝ moderatea | ‐ |
| Multiple pregnancy rate per couple | 6 per 1000 | 6 per 1000 (0 to 88) | OR 1.05 (0.07 to 16.90) | 342 (1 study) | ⊕⊕⊝⊝ lowb | ‐ |
| Pregnancy rate per couple (all cycles) | 143 per 1000 | 228 per 1000 (144 to 339) | OR 1.77 (1.01 to 3.08) | 342 (1 study) | ⊕⊕⊕⊝ moderatea | ‐ |
| Ovarian hyperstimulation syndrome rate per woman ‐ not reported | See comment | See comment | ‐ | ‐ | See comment | No data reported on outcome |
| Miscarriage rater per couple | 46 per 1000 | 42 per 1000 (15 to 111) | OR 0.91 (0.32 to 2.58) | 342 (1 study) | ⊕⊕⊝⊝ lowb | ‐ |
| Ectopic pregnancy rate per couple | Not estimable (no events in control group) | ‐ | OR 5.30 (0.25 to 111.26) | 342 (1 study) | ⊕⊕⊝⊝ lowb | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aDowngraded by one level for serious imprecision: small sample size with a low event rate. bDowngraded by two levels for very serious imprecision: small sample size with a low event rate and effect estimate with a wide confidence interval.
Summary of findings 5. IUI in a stimulated cycle compared to IUI in a natural cycle for unexplained subfertility.
| IUI in stimulated cycle compared to IUI in natural cycle for unexplained subfertility | ||||||
| Patient or population: participants with unexplained subfertility Settings: fertility clinic Intervention: IUI in a stimulated cycle Comparison: IUI in a natural cycle | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| IUI in a natural cycle | IUI in a stimulated cycle | |||||
| Live birth rare per couple (all cycles) | 139 per 1000 | 250 per 1000 (165 to 361) | OR 2.07 (1.22 to 3.50) | 396 (4 studies) | ⊕⊕⊝⊝ lowa,b | ‐ |
| Multiple pregnancy rate per couple | Not estimable (no events in control group) | ‐ | OR 3.00 (0.11 to 78.27) | 39 (1 study) | ⊕⊕⊝⊝ lowc | ‐ |
| Pregnancy rate per couple (all cycles) | 63 per 1000 | 302 per 1000 (36 to 831) | OR 6.43 (0.56 to 73.35) | 26 (1 study) | ⊕⊕⊝⊝ lowc | ‐ |
| Ovarian hyperstimulation syndrome rate per woman ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No events in intervention and control groups |
| Miscarriage rate per couple | Not estimable (no events in control group) | ‐ | OR 5.21 (0.19 to 141.08) | 26 (1 study) | ⊕⊕⊝⊝ lowc | ‐ |
| Ectopic pregnancy rate per couple | Not estimable (no events in control group) | ‐ | OR 6.48 (0.33 to 127.09) | 211 (1 study) | ⊕⊝⊝⊝ very lowb,c | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aDowngraded by one level for serious imprecision: small sample size with a low event rate. bDowngraded by one level for serious risk of bias: sequence generation and allocation concealment rated as 'unclear' for the largest/larger study. cDowngraded by two levels for very serious imprecision: small sample size with a low event rate and effect estimate with a wide confidence interval.
This section describes the results of the meta‐analyses and sensitivity analyses.
Comparison 1. IUI in a natural cycle versus timed intercourse (TI) or expectant management in a natural cycle
The results from this comparison were all obtained from Bhattacharya 2008. Data for the unexplained subfertility group only were provided by the trial author.
1.1 Live birth rate per couple (all cycles)
Analysis 1.1 It is uncertain whether treatment with IUI in a natural cycle results in a higher cumulative live birth rate compared to treatment with expectant management in a natural cycle (OR 1.60, 95% CI 0.92 to 2.78; 1 RCT, 334 women; low‐quality evidence). The evidence suggests that if we assume the chance of a live birth using expectant management to be 16%, that of IUI would be between 15% and 34%.
1.1. Analysis.
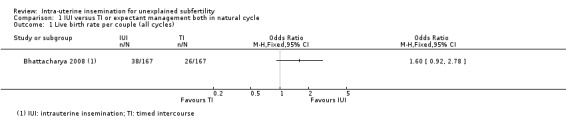
Comparison 1 IUI versus TI or expectant management both in natural cycle, Outcome 1 Live birth rate per couple (all cycles).
1.2 Multiple pregnancy rate per couple
1.2. Analysis.
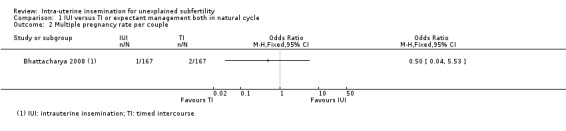
Comparison 1 IUI versus TI or expectant management both in natural cycle, Outcome 2 Multiple pregnancy rate per couple.
It is uncertain whether treatment with IUI in a natural cycle results in a lower multiple pregnancy rate compared to treatment with expectant management in a natural cycle (OR 0.50, 95% CI 0.04 to 5.53; 1 RCT, 334 women; low‐quality evidence). The evidence suggests that if we assume the risk of a multiple pregnancy using expectant management to be 1%, the risk using IUI would be between 0% and 6%.
We did not perform sensitivity analyses as only one trial with low risk of selection bas was included in the analysis for both primary outcomes.
1.3 Pregnancy rate per couple (all cycles)
Analysis 1.3 Compared to expectant management in a natural cycle, it is uncertain whether treatment with IUI in a natural cycle results in a higher cumulative pregnancy rate (OR 1.53, 95% CI 0.88 to 2.64; 1 RCT, 334 women; low‐quality evidence). The evidence suggests that if we assume the chance of a pregnancy using expectant management to be 16%, that of IUI would be between 15% and 34%.
1.3. Analysis.
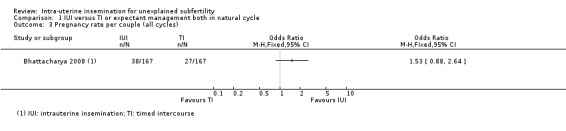
Comparison 1 IUI versus TI or expectant management both in natural cycle, Outcome 3 Pregnancy rate per couple (all cycles).
Other adverse events
Moderate or severe ovarian hyperstimulation syndrome rate per woman
Data on OHSS were not reported.
1.4 Miscarriage rate per couple
1.4. Analysis.

Comparison 1 IUI versus TI or expectant management both in natural cycle, Outcome 4 Miscarriage rate per couple.
It is uncertain whether treatment with IUI in a natural cycle results in a lower miscarriage rate compared to treatment with expectant management in a natural cycle. Sixteen miscarriages were reported in a total of 334 couples, seven in the IUI group and nine in the expectant management group (OR 0.77, 95% CI 0.28 to 2.11; 1 RCT, 334 women; low‐quality evidence). The evidence suggests that if we assume the risk of a miscarriage using expectant management to be 5%, that of IUI would be between 2% and 11%.
1.5 Ectopic pregnancy rate per couple
Analysis 1.5 Compared to expectant management in a natural cycle, it is uncertain whether treatment with IUI in a natural cycle results in a lower ectopic pregnancy rate. Two ectopic pregnancies were reported in a total of 334 couples, with both occurring in the IUI group (OR 5.06, 95% CI 0.24 to 106.21; 1 RCT, 334 women; low‐quality evidence).
1.5. Analysis.
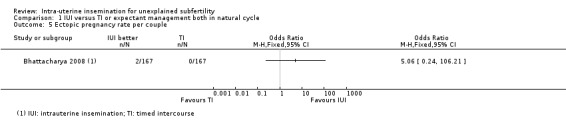
Comparison 1 IUI versus TI or expectant management both in natural cycle, Outcome 5 Ectopic pregnancy rate per couple.
Comparison 2. IUI in a stimulated cycle versus TI or expectant management in a stimulated cycle
2.1 Live birth rate per couple (all cycles)
Analysis 2.1 Only two of the six trials included in the analysis reported live birth rates (Chung 1995; Melis 1995). It is uncertain whether treatment with IUI in a stimulated cycle results in a higher cumulative live birth rate compared to treatment with TI in a stimulated cycle (OR 1.59, 95% CI 0.88 to 2.88; 2 RCTs, 208 women; I2 = 72%; low‐quality evidence). The evidence suggests that if we assume the chance of a live birth using TI to be 26%, that of IUI would be between 23% and 50%. Statistical heterogeneity was detected (P = 0.06, I2 = 72%) between the two studies, with inconsistency in the directions of effect estimates. This may be explained by the fact that all participants in Melis 1995 had previously received fertility treatment.
2.1. Analysis.
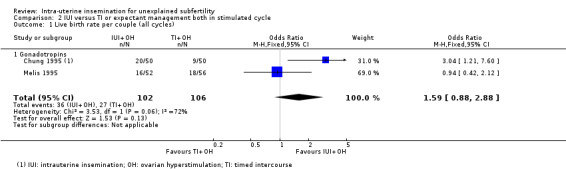
Comparison 2 IUI versus TI or expectant management both in stimulated cycle, Outcome 1 Live birth rate per couple (all cycles).
We did not perform sensitivity analysis excluding studies at unclear or high selection bias as both trials were at low risk of selection bias
2.2 Multiple pregnancy rate per couple
Analysis 2.2 Four studies reported their multiple pregnancies per treatment arm (Arcaini 1996; Chung 1995; Karlstrom 1993; Melis 1995), with a total of 17 multiple pregnancies in 316 couples. Arcaini 1996, Chung 1995 and Karlstrom 1993 reported one high‐order multiple pregnancy each. The studies reported 11 multiple pregnancies in the IUI group and six in the TI group (representing 13.5% of the total number of pregnancies in these studies). Pooling the data from these studies showed that it is uncertain whether treatment with IUI in a stimulated cycle results in a lower multiple pregnancy rate compared to treatment with TI in a stimulated cycle (OR 1.46, 95% CI 0.55 to 3.87; 4 RCTs, 316 women; I2 = 0%; low‐quality evidence). The evidence suggests that if we assume the risk of a multiple pregnancy using TI to be 4%, the risk using IUI would be between 2% and 15%.
2.2. Analysis.
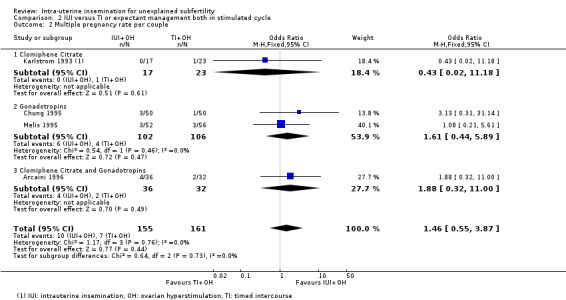
Comparison 2 IUI versus TI or expectant management both in stimulated cycle, Outcome 2 Multiple pregnancy rate per couple.
2.3 Pregnancy rate per couple (all cycles)
Analysis 2.3 Six trials reported pregnancy rates per couple. There were 517 women included in this analysis, reporting 149 cumulative pregnancies. The result showed that treatment with IUI in a stimulated cycle may result in a higher cumulative pregnancy rate compared to treatment with TI in a stimulated cycle (OR 1.69, 95% CI 1.14 to 2.53; 6 RCTs, 517 women; I2 = 8%; low‐quality evidence). This suggests that if we assume the chance of a pregnancy with TI to be 23%, the chance of a pregnancy in women using IUI would be between 26% and 44%.
2.3. Analysis.
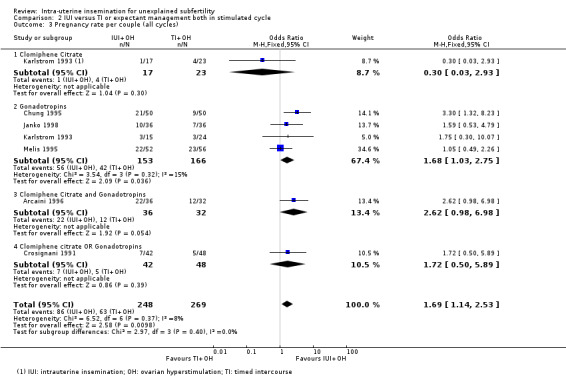
Comparison 2 IUI versus TI or expectant management both in stimulated cycle, Outcome 3 Pregnancy rate per couple (all cycles).
Other adverse events
2.4 Moderate to severe ovarian hyperstimulation syndrome rate per woman
Analysis 2.4 One case of OHSS was reported in 36 couples who were treated with IUI, while none was reported in 32 couples who received TI. It is, however, uncertain whether treatment with IUI in a stimulated cycle results in a lower OHSS rate compared to treatment with TI in a stimulated cycle (OR 2.75, 95% CI 0.11 to 69.83; 1 RCT, 68 women; very low‐quality evidence).
2.4. Analysis.
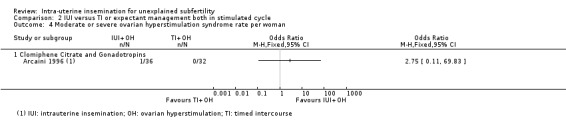
Comparison 2 IUI versus TI or expectant management both in stimulated cycle, Outcome 4 Moderate or severe ovarian hyperstimulation syndrome rate per woman.
2.5 Miscarriage rate per couple
Analysis 2.5 Twenty‐seven miscarriages were reported in total. Fifteen were reported by treatment arm, nine in the IUI group and six in the TI group. It is uncertain whether treatment with IUI in a stimulated cycle results in a lower miscarriage rate compared to treatment with TI in a stimulated cycle (OR 1.66, 95% CI 0.56 to 4.88; I2 = 0%; 2 RCTs, 208 women; low‐quality evidence). The evidence suggests that if we assume the risk of miscarriage using TI to be 6%, the risk using IUI would be between 3% and 23%.
2.5. Analysis.
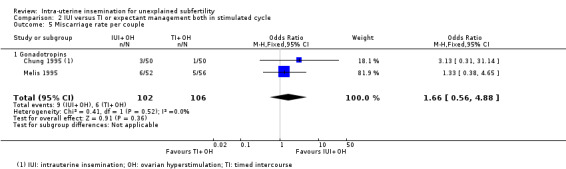
Comparison 2 IUI versus TI or expectant management both in stimulated cycle, Outcome 5 Miscarriage rate per couple.
2.6 Ectopic pregnancy rate per couple
Analysis 2.6 One case of ectopic pregnancy, occurring in the IUI group, was reported by one study of 100 couples. It is, however, uncertain whether treatment with IUI in a stimulated cycle results in a higher ectopic pregnancy rate compared to treatment with TI in a stimulated cycle (OR 3.06, 95% CI 0.12 to 76.95; 1 RCT, 100 women; low‐quality evidence).
2.6. Analysis.

Comparison 2 IUI versus TI or expectant management both in stimulated cycle, Outcome 6 Ectopic pregnancy rate per couple.
Comparison 3. IUI in a stimulated cycle versus TI or expectant management in a natural cycle
3.1 Live birth rate per couple (all cycles)
Analysis 3.1; Figure 4 Data on this outcome were reported by two studies (Farquhar 2018; Steures 2006a). We did not pool data from the two studies, because of extreme statistical heterogeneity (I2 = 91%). The presence of extreme heterogeneity could be due to the use of different population groups and OH drugs by the two studies: Farquhar 2018 investigated women with a low chance of natural conception (prediction score of natural conception less than 30%) using clomiphene citrate or letrozole, while Steures 2006a included couples with an intermediate chance of spontaneous conception (prediction score of natural conception between 30% and 40%), using clomiphene citrate or gonadotropins.
3.1. Analysis.
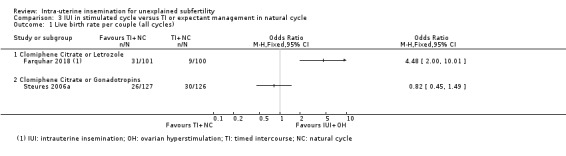
Comparison 3 IUI in stimulated cycle versus TI or expectant management in natural cycle, Outcome 1 Live birth rate per couple (all cycles).
4.
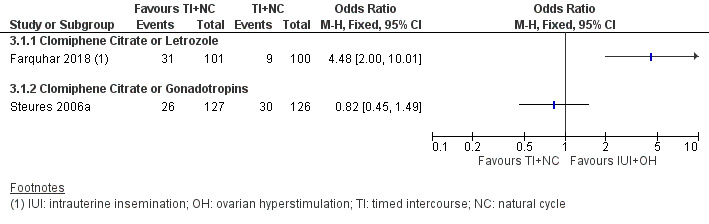
Forest plot of comparison 3: IUI in stimulated cycle versus TI or expectant management in natural cycle; outcome 3.1 Live birth rate per couple.
3.1.1 Clomiphene citrate or letrozole (Farquhar 2018)
In couples with a low prediction score of natural conception, treatment with IUI in a stimulated cycle probably results in a higher cumulative live birth rate compared to treatment with expectant management in a natural cycle (OR 4.48, 95% CI 2.00 to 10.01; 1 RCT; 201 women; moderate‐quality evidence). The evidence suggests that if we assume the chance of a live birth with expectant management in a natural cycle to be 9%, the chance of a live birth with IUI in a stimulated cycle would be between 17% and 50%.
3.1.2 Clomiphene citrate or gonadotropins (Steures 2006a)
It is uncertain whether treatment with IUI in a stimulated cycle results in a higher cumulative live birth rate compared to treatment with expectant management in a natural cycle (OR 0.82, 95% CI 0.45 to 1.49; 1 RCT, 253 women; low‐quality evidence). The evidence suggests that if we assume the chance of a live birth with expectant management in a natural cycle to be 24%, the chance of a live birth with IUI in a stimulated cycle would be between 12% and 32%.
3.2 Multiple pregnancy rate per couple
3.2. Analysis.
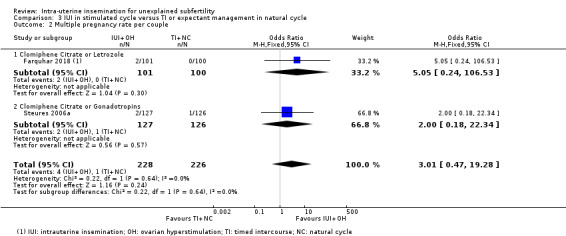
Comparison 3 IUI in stimulated cycle versus TI or expectant management in natural cycle, Outcome 2 Multiple pregnancy rate per couple.
Data on this outcome were reported by Farquhar 2018 and Steures 2006a. It is uncertain whether treatment with IUI in a stimulated cycle results in a lower multiple pregnancy rate compared to treatment with expectant management in a natural cycle (OR 3.01, 95% CI 0.47 to 19.28; 2 RCTs, 454 women; I2 = 0%; low‐quality evidence). The evidence suggests that if we assume the risk of a multiple pregnancy with expectant management in a natural cycle to be 0%, the risk of a multiple pregnancy with IUI in a stimulated cycle would be between 0% and 8%.
We did not perform sensitivity analyses excluding studies at unclear or high selection bias, as both trials were at low risk of selection bias for the primary outcomes.
3.3 Pregnancy rate per couple (all cycles)
3.3. Analysis.
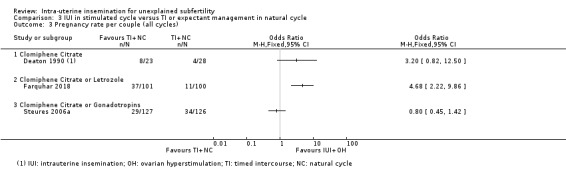
Comparison 3 IUI in stimulated cycle versus TI or expectant management in natural cycle, Outcome 3 Pregnancy rate per couple (all cycles).
Data on this outcome were reported by three studies (Deaton 1990; Farquhar 2018; Steures 2006a). We did not pool data from the three studies, because of extreme statistical heterogeneity (I2 = 86%). The presence of significant heterogeneity might be due to the use of different population groups and OH drugs by the three studies: Deaton 1990 investigated women with mild or moderate endometriosis, using clomiphene citrate only; Farquhar 2018 investigated women with a low chance of spontaneous conception (prediction score of natural conception less than 30%), using clomiphene citrate or letrozole; Steures 2006a included couples with an intermediate chance of spontaneous conception (prediction score of natural conception between 30% and 40%). using clomiphene citrate or gonadotropins.
3.3.1 Clomiphene citrate only (Deaton 1990)
It is uncertain whether treatment with IUI in a stimulated cycle results in a higher cumulative pregnancy rate compared to treatment with TI in a natural cycle (OR 3.20, 95% CI 0.82 to 12.50; 1 RCT, 51 women; low‐quality evidence).
3.3.2 Clomiphene citrate or letrozole (Farquhar 2018)
Treatment with IUI in a stimulated cycle probably results in a higher cumulative pregnancy rate compared to treatment with expectant management in a natural cycle (OR 4.68, 95% CI 2.22 to 9.86; 1 RCT, 201 women; moderate‐quality evidence). The evidence suggests that if we assume the chance of a pregnancy with expectant management in a natural cycle to be 11%, the chance of a pregnancy with IUI in a stimulated cycle would be between 22% and 55%.
3.3.3 Clomiphene citrate or gonadotropins (Steures 2006a)
It is uncertain whether treatment with IUI in a stimulated cycle results in a higher cumulative pregnancy rate compared to treatment with expectant management in a natural cycle (OR 0.80, 95% CI 0.45 to 1.42; 1 RCT, 253 women, low‐quality evidence).
Other adverse events
Moderate or severe ovarian hyperstimulation syndrome rate per woman
Two studies (Deaton 1990; Farquhar 2018) reported on OHSS in this comparison; there were no cases of OHSS in either study.
3.4 Miscarriage rate per couple
3.4. Analysis.

Comparison 3 IUI in stimulated cycle versus TI or expectant management in natural cycle, Outcome 4 Miscarriage rate per couple.
Data on this outcome were reported by Farquhar 2018 and Steures 2006a. Treatment with IUI in a stimulated cycle probably results in a higher miscarriage rate compared to treatment with expectant management in a natural cycle (OR 2.87, 95% CI 1.18 to 7.01; 2 RCTs, 454 women; I2 = 0%; moderate‐quality evidence). The evidence suggests that if we assume the risk of miscarriage with expectant management in a natural cycle to be 3%, the risk of miscarriage with IUI in a stimulated cycle would be between 4% and 18%.
3.5 Ectopic pregnancy rate per couple
3.5. Analysis.
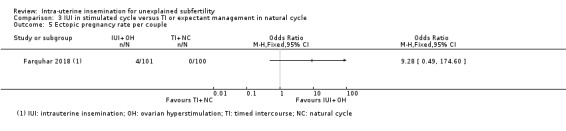
Comparison 3 IUI in stimulated cycle versus TI or expectant management in natural cycle, Outcome 5 Ectopic pregnancy rate per couple.
Data on this outcome were reported only by Farquhar 2018. It is uncertain whether treatment with IUI in a stimulated cycle results in a lower ectopic pregnancy rate compared to treatment with expectant management in a natural cycle (OR 9.28, 95% CI 0.49 to 174.60; 1 RCT, 201 women; low‐quality evidence)
Comparison 4. IUI in a natural cycle versus TI or expectant management in a stimulated cycle
Bhattacharya 2008 studied this comparison with IUI in a natural cycle compared to TI in a clomiphene citrate‐stimulated cycle.
4.1 Live birth rate per couple (all cycles)
4.1. Analysis.
Comparison 4 IUI in natural cycle versus TI or expectant management in stimulated cycle, Outcome 1 Live birth rate per couple (all cycles).
Treatment with IUI in a natural cycle probably results in a higher cumulative live birth rate compared to expectant management in a stimulated cycle (OR 1.95, 95% CI 1.10 to 3.44; 1 RCT, 342 women: moderate‐quality evidence). The evidence suggests that if we assume the chance of a live birth with expectant management in a stimulated cycle to be 13%, the chance of a live birth with IUI in a natural cycle would be between 14% and 34%.
4.2 Multiple pregnancy per couple
4.2. Analysis.
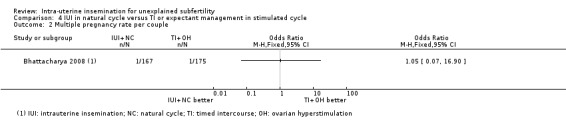
Comparison 4 IUI in natural cycle versus TI or expectant management in stimulated cycle, Outcome 2 Multiple pregnancy rate per couple.
It is uncertain whether treatment with IUI in a natural cycle results in a lower multiple pregnancy rate compared to treatment with expectant management in a stimulated cycle (OR 1.05, 95% CI 0.07 to 16.90; 1 RCT, 342 women; low‐quality evidence). The evidence suggests that if we assume the risk of a multiple pregnancy in expectant management in a stimulated cycle to be 1%, the risk of a multiple pregnancy with IUI in a natural cycle would be between 0% and 9%.
We did not perform sensitivity analysis for the main outcomes, as we included only one study with low risk of selection bias in this comparison.
4.3 Pregnancy rate per couple (all cycles)
Treatment with IUI in a natural cycle probably results in a higher cumulative pregnancy rate compared to treatment with expectant management in a stimulated cycle (OR 1.77, 95% CI 1.01 to 3.08; 1 RCT, 342 women; moderate‐quality evidence). The evidence suggests that if we assume the chance of a pregnancy with expectant management in a stimulated cycle to be 14%, the chance of a pregnancy with IUI in a natural cycle would be between 14% and 34%.
Other adverse events
Moderate or severe ovarian hyperstimulation syndrome rate per woman
OHSS was not reported.
4.4 Miscarriage rate per couple
Analysis 4.4 It is uncertain whether treatment with IUI in a natural cycle results in a lower miscarriage rate compared to treatment with expectant management in a stimulated cycle (OR 0.91, 95% CI 0.32 to 2.58; 1 RCT, 342 women; low‐quality evidence). The evidence suggests that if we assume the risk of a miscarriage with expectant management in a stimulated cycle to be 5%, the risk of a miscarriage with IUI in a natural cycle would be between 2% and 11%.
4.4. Analysis.
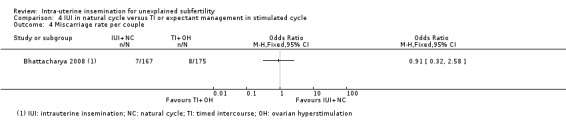
Comparison 4 IUI in natural cycle versus TI or expectant management in stimulated cycle, Outcome 4 Miscarriage rate per couple.
4.5 Ectopic pregnancy
Analysis 4.5 Although two ectopic pregnancies occurred in the IUI group, it is uncertain whether treatment with IUI in a natural cycle results in a lower ectopic pregnancy rate compared to treatment with expectant management in a stimulated cycle (OR 5.30, 95% CI 0.25 to 111.26; 1 RCT, 342 women; low‐quality evidence).
4.5. Analysis.
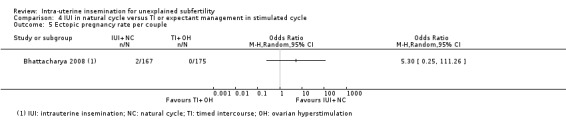
Comparison 4 IUI in natural cycle versus TI or expectant management in stimulated cycle, Outcome 5 Ectopic pregnancy rate per couple.
Comparison 5. IUI in a stimulated cycle versus IUI in a natural cycle
5.1 Live birth rate per couple (all cycles)
Analysis 5.1 Three trials reported the number of live births per treatment arm (Arici 1994; Goverde 2000; Murdoch 1991). We obtained the live birth data from Guzick 1999 after contacting the study authors. Treatement with IUI in a stimulated cycle may result in a higher cumulative live birth rate compared to treatment with IUI in a natural cycle (OR 2.07, 95% CI 1.22 to 3.50; I2 = 0%; 4 RCTs, 396 women; low‐quality evidence). The evidence suggests that if we assume the chance of a live birth with IUI in a natural cycle to be 14%, the chance of a live birth with IUI in a stimulated cycle would be between 17% and 36%. In a sensitivity analysis, we obtained a similar result by excluding Arici 1994, a cross‐over trial (OR 2.02, 95% CI 1.18 to 3.45; I2 = 0%; 3 RCTs, 370 women); however, it is uncertain whether treatment with IUI in a stimulated cycle results in a higher cumulative live birth rate compared to treatment with IUI in a natural cycle when we excluded Guzick 1999, a study with unclear risk of bias for random sequence generation and allocation concealment (OR 1.69, 95% CI 0.83 to 3.44; I2 = 0%; 3 RCTs, 185 women).
5.1. Analysis.
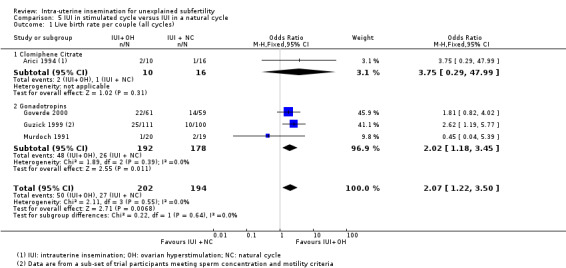
Comparison 5 IUI in stimulated cycle versus IUI in a natural cycle, Outcome 1 Live birth rate per couple (all cycles).
5.2 Multiple pregnancy rate per couple
Analysis 5.2 It is uncertain whether treatment with IUI in a stimulated cycle results in a higher multiple pregnancy rate compared to treatment with IUI in a natural cycle (OR 3.00, 95% CI 0.11 to 78.27; 1 RCT, 39; women; low‐quality evidence) (Figure 5).
5.2. Analysis.
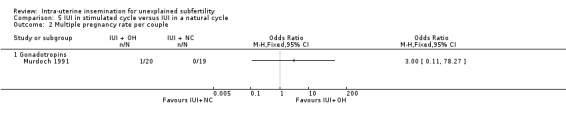
Comparison 5 IUI in stimulated cycle versus IUI in a natural cycle, Outcome 2 Multiple pregnancy rate per couple.
5.

Forest plot of comparison 5: IUI in stimulated cycle versus IUI in a natural cycle; outcome 5.2 Multiple pregnancy rate per couple.
5.3 Pregnancy rate per couple (all cycles)
Analysis 5.3 It is uncertain whether treatment with IUI in a stimulated cycle results in a higher cumulative pregnancy rate compared to treatment with IUI in a natural cycle (OR 6.43, 95% CI 0.56 to 73.35; 1 RCT, 26 women; low‐quality evidence). The evidence suggests that if we assume the chance of a pregnancy with IUI in a natural cycle to be 6%, the chance of a pregnancy with IUI in a stimulated cycle would be between 4% and 83%.
5.3. Analysis.
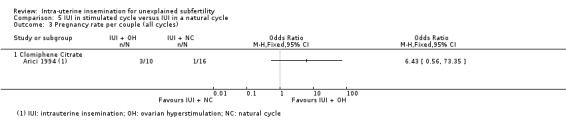
Comparison 5 IUI in stimulated cycle versus IUI in a natural cycle, Outcome 3 Pregnancy rate per couple (all cycles).
Other adverse events
Moderate or severe ovarian hyperstimulation syndrome rate per woman
There were no reported cases of OHSS.
5.4 Miscarriage rate per couple
5.4. Analysis.

Comparison 5 IUI in stimulated cycle versus IUI in a natural cycle, Outcome 4 Miscarriage rate per couple.
One miscarriage in the stimulated group was reported by Arici 1994. Guzick 1999 reported a total miscarriage rate of approximately 24% in all couples undergoing IUI treatment. It is uncertain whether treatment with IUI in a stimulated cycle results in a higher miscarriage rate compared to treatment with IUI in a natural cycle (OR 5.21, 95% CI 0.19 to 141.08; 1 RCT, 26 women; low‐quality evidence).
5.5 Ectopic pregnancy rate per couple
Analysis 5.5 It is uncertain whether treatment with IUI in a stimulated cycle results in a higher ectopic pregnancy rate compared to treatment with IUI in a natural cycle (OR 6.48, 95% CI 0.33 to 127.09; 1 RCTs, 211 women; very low‐quality evidence).
5.5. Analysis.
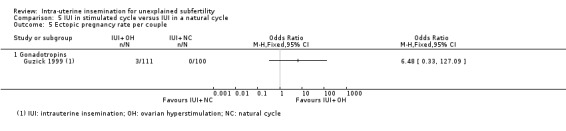
Comparison 5 IUI in stimulated cycle versus IUI in a natural cycle, Outcome 5 Ectopic pregnancy rate per couple.
Discussion
Summary of main results
This is an update of a Cochrane Review which assessed whether the live birth rate is improved with intrauterine insemination (IUI), with or without ovarian hyperstimulation (OH), compared to timed intercourse (TI) or expectant management with or without OH, or with IUI with OH compared to IUI in a natural cycle, for couples with unexplained subfertility. Fifteen studies met the eligibility criteria for inclusion; they investigated different IUI treatment modalities in five comparisons involving a total of 2068 women. Primary outcomes reported were cumulative live birth rates and multiple pregnancy rates. Secondary outcomes reported were cumulative pregnancy rates and other adverse events, including moderate to severe ovarian hyperstimulation syndrome (OHSS) rates, miscarriage rates, and ectopic pregnancy rates.The five comparisons identified in the included studies are classified into two broad groups as follows:
1. IUI with or without OH versus TI or expectant management with or without OH
We identified four comparisons in this group:
IUI in a natural cycle versus TI or expectant management in a natural cycle;
IUI in a stimulated cycle versus TI or expectant management in a stimulated cycle;
IUI in a stimulated cycle versus TI or expectant management in a natural cycle; and
IUI in a natural cycle versus TI or expectant management in a stimulated cycle.
For live birth, it is uncertain whether treatment with IUI in a natural cycle results in a higher cumulative live birth rate compared to treatment with expectant management in a natural cycle. We obtained a similar result with treatment with IUI in a stimulated cycle compared to TI in a stimulated cycle. However, there is moderate‐quality evidence that treatment with IUI in a stimulated cycle is likely to result in a higher cumulative live birth rate compared to treatment with expectant management in a natural cycle in couples with a low prediction score of natural conception. The evidence suggests that if we assume the chance of a live birth with expectant management in a natural cycle to be 9%, the chance of a live birth with IUI in a stimulated cycle would be between 17% and 50%. Similarly, there is moderate‐quality evidence that treatment with IUI in a natural cycle is likely to result in a higher cumulative live birth rate compared to expectant management in a stimulated cycle. The evidence suggests that if we assume the chance of a live birth with expectant management in a stimulated cycle to be 13%, the chance of a live birth with IUI in a natural cycle would be between 14% and 34%.
For multiple pregnancy, it is uncertain whether treatment with IUI with or without OH results in lower multiple pregnancy rates compared to treatment with TI or expectant management, with or without OH. For other adverse events such as OHSS rates, miscarriage rates and ectopic pregnancy rates, there were insufficient data to adequately assess the impact of these treatment modalities; data on these outcomes were either not reported or reported in single studies with small sample sizes.
2. IUI in a stimulated cycle versus IUI in a natural cycle
For live birth, there is low‐quality evidence that treatment with IUI in a stimulated cycle may result in a higher cumulative live birth rate compared to treatment with IUI in a natural cycle. The evidence suggests that if we assume the chance of a live birth with IUI in a natural cycle to be 14%, the chance of a live birth with IUI in a stimulated cycle would be between 17% and 36%. We tested the robustness of this finding in a sensitivity analysis by excluding a study with unclear risk of bias for random sequence generation and allocation concealment; the result showed that without this trial there was probably little or no difference in live birth rates between the two treatment groups.
For multiple pregnancy, it is uncertain whether treatment with IUI in a stimulated cycle results in a lower multiple pregnancy rate compared with treatment with IUI in a natural cycle. For other adverse events such as OHSS rates, miscarriage rates and ectopic pregnancy rates, there were insufficient data to adequately assess the impact of this treatment modality; data on these outcomes were mostly reported in single studies with small sample sizes.
Overall completeness and applicability of evidence
Most of the comparisons identified in this review were investigated in single trials, thereby making it difficult to combine data in meta‐analyses. Where we could pool data in meta‐analyses, the overall effect estimates usually had wide confidence intervals due to small sample sizes with low numbers of events. Furthermore, we were not able to assess all the stipulated outcomes for each comparison. There were not enough data available to adequately assess the effects of the various interventions on live birth, multiple pregnancy and other adverse events. Many studies were not included in the review as they reported per‐cycle data only. One of the areas of particular concern in fertility trials is what statisticians refer to as the 'unit of analysis' error (Vail 2003). It is methodologically incorrect to report data per cycle when it is women or couples who are randomised, because many of the women would have undergone more than one treatment cycle (Dias 2008; Johnson 2003; Vail 2003). Yet pregnancy rate per cycle is a commonly‐reported outcome in fertility trials and reviews. We could also not include many studies with a cross‐over design in the review because they did not report pre‐cross‐over data. We could not include post‐cross‐over data because couples would have received both treatment modalities. There have been many discussions about whether a cross‐over design is appropriate for fertility trials, mainly because participants drop out after treatment success, which results in a selected participant population post‐cross‐over. For this reason, it has been suggested that the cross‐over design has no place in infertility trials (Daya 1993). A cross‐over design could result in an overestimation of the treatment effect (Khan 1996; Norman 2000). Whether this overestimation could be statistically corrected for or whether it is clinically relevant remains under debate (Cohlen 1998; McDonnell 2004; Vail 2003).
Adverse events were often not mentioned, or mentioned by group instead of by treatment modality. We included studies mentioning 'no events' in the meta‐analyses, which might result in underestimation of the adverse events. On the other hand, these adverse events are mostly attributed to OH and not to IUI. It seems therefore unlikely that any significant difference in adverse events would be found when OH with TI is compared to OH with IUI. We could not determine the separate evidence for the number of treatment cycles and methods of ovarian hyperstimulation, due to insufficient studies. In addition, we did not examine the effects of important prognostic factors such as age, duration of subfertility and previous treatment in subgroup analyses, although there would have been insufficient data to do these analyses if we had decided to do so. The extent to which some of the findings which show evidence of a difference between the intervention groups are applicable to couples with different prognoses and baseline fecundity is therefore unclear.
The more recent studies adopted expectant management as a control treatment for IUI instead of timed intercourse (Bhattacharya 2008; Farquhar 2018; Steures 2006a). Because timing of intercourse (TI) interferes with the natural coital habits of a couple, expectant management has been proposed as the more appropriate comparison treatment for IUI (Wilcox 1995). A recent meta‐analysis, however, shows no significant difference in the pregnancy rate between studies comparing IUI versus TI and studies with IUI versus expectant management (Snick 2008). Whether it is appropriate to pool studies including TI and expectant management in a meta‐analysis remains unclear. We did not, however, pool the data from Bhattacharya 2008, Farquhar 2018 and Steures 2006a with data of trials applying TI because these trials comprised comparisons that were not subject to many other trials.
Quality of the evidence
Most of the studies included in this review were of moderately‐good quality in relation to risk of bias. We excluded studies with a cross‐over design which did not report pre‐cross‐over data. Most of the included studies clearly described their methods of sequence generation and allocation concealment. None of the included studies reported blinding, due to the nature of the interventions; however, lack of blinding may not have any effect on the outcomes of interest, as they were objectively assessed. For most comparisons, effect estimates were associated with imprecision due to small sample sizes and wide confidence intervals. We assessed the quality of the evidence for the review’s five comparisons; the quality of the evidence for our primary outcomes (cumulative live birth and multiple pregnancy) ranged from low to moderate. The main limitations in the body of evidence were very serious imprecision due to small sample sizes with low event rates and effect estimates with wide confidence intervals, and inadequate reporting of adverse events. We could not examine the presence of publication or reporting bias in funnel plots for any of the comparisons, as none of the analyses included 10 or more studies. We therefore did not downgrade the quality of the evidence for publication bias.
Potential biases in the review process
To prevent selection bias, the included studies were independently selected and data extracted by two review authors (ROA and SVV for the 2020 update). We resolved disagreements by discussion, keeping the protocol as our guideline to reduce bias. We consulted a statistician (Andy Vail) for the data extraction of complicated cross‐over trials. We have extracted and calculated the events per randomised couple for this meta‐analysis, which resulted in the exclusion of six studies reporting only post‐cross‐over or per‐cycle data. As a consequence, the analysis is statistically less biased, although a selection bias may have occurred. We could not construct a funnel plot for any of the outcomes to check for the presence of publication bias as none of the outcomes was reported by 10 or more studies. We have, however, minimised the effect of publication bias by undertaking a comprehensive search for all eligible studies.
Agreements and disagreements with other studies or reviews
Previous review articles and meta‐analyses (e.g. Aboulghar 2003; Balasch 2004; Cohlen 2005; Costello 2004; Hughes 1997; Zeyneloglu 1998) have all assessed the effectiveness of IUI and ovarian hyperstimulation, but they have all calculated pregnancy rates per cycle, which makes it difficult to compare their findings with those of this review which included per‐couple data only. Per‐cycle data produce biased results that may exaggerate treatment results (Dias 2008). A Cochrane Review and meta‐analysis published in 2019 (Wang 2019) evaluated the effectiveness and safety of different approaches to clinical management (expectant management, ovarian stimulation, intra‐uterine insemination, ovarian stimulation‐intra‐uterine insemination, and in vitro‐fertilisation/intracytoplasmic sperm injection) in couples with unexplained infertility. There was insufficient evidence of differences in live births between intra‐uterine insemination or ovarian stimulation‐intra‐uterine insemination compared to expectant management (OR 1.21, 95% CI 0.61 to 2.43; low‐certainty evidence; OR 1.61, 95% CI 0.88 to 2.94; low‐certainty evidence respectively). These findings are similar to our findings on live births in the corresponding comparisons in our review.
Authors' conclusions
Implications for practice.
Due to insufficient data, it is uncertain whether treatment with IUI with or without OH compared to timed intercourse or expectant management with or without OH, or treatment with IUI with OH compared to IUI in a natural cycle, improves cumulative live birth rates with acceptable multiple pregnancy rates in couples with unexplained subfertility. However, there is moderate‐quality evidence that treatment with IUI with OH probably results in a higher cumulative live birth rate compared to expectant management, without OH in couples with a low prediction score of natural conception. Similarly, there is moderate‐quality evidence that treatment with IUI in a natural cycle probably results in a higher cumulative live birth rate compared to treatment with timed intercourse with OH.
Implications for research.
None of the comparisons examined in this review were adequately addressed, due to insufficient data; questions still remain as to whether live birth rates can be improved while keeping the risk of multiple pregnancy and other adverse events to acceptable levels. There is therefore a need for better‐designed randomised controlled trials which evaluate the effects of different IUI modalities on important outcome measures. Furthermore, future trials should endeavour to report live birth and other outcome data in forms which enable appropriate analyses.
What's new
| Date | Event | Description |
|---|---|---|
| 17 October 2019 | New citation required but conclusions have not changed | The conclusions of the review have not changed. |
| 17 October 2019 | New search has been performed | Update of review and literature search; 1 new study added (Farquhar 2018) |
History
Protocol first published: Issue 3, 1999 Review first published: Issue 4, 2006
| Date | Event | Description |
|---|---|---|
| 16 August 2012 | New citation required but conclusions have not changed | Recognising that new citation warranted for update in 2011. |
| 22 June 2011 | New search has been performed | Two new RCTs added: Bhattacharya 2008 and Steures 2006a. |
| 13 June 2008 | Amended | Converted to new review format. |
| 5 August 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We wish to thank all colleagues of Cochrane Gynaecology and Fertility for their help. Special thanks to Elena Kostova, Marian Showell, Cindy Farquhar, Helen Nagels and statistician Andy Vail for all their advice and support. We also thank all authors for their responses and additional information on their trials.
We acknowledge the contribution of Professor Maas Jan Heineman and Professor Ed Hughes to previous versions of this review.
We would like to thank Abha Maheshwari, Rik van Eekelen, Astrid Cantineau, and Rui Wang for their valuable peer review comments.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Group specialised register search strategy
PROCITE platform
Searched 17 October 2019
Keywords CONTAINS "unexplained and endometriosis related infertility" or "unexplained infertility" or "unexplained subfertility" or "idiopathic infertility" or "idiopathic male infertility" or "idiopathic subfertility" or "idiopathic‐unexplained" or Title CONTAINS "unexplained and endometriosis related infertility" or "unexplained infertility" or "unexplained subfertility" or "idiopathic infertility" or "idiopathic male infertility" or "idiopathic subfertility" or "idiopathic‐unexplained"
AND
Keywords CONTAINS "Intrauterine Insemination" or "intrautero tuboperitoneal insemination" or "IUI" or "artificial insemination by donor" or "artificial insemination by partner" or "artificial insemination" or "superovulation" or "superovulation induction" or Title CONTAINS "Intrauterine Insemination" or "intrautero tuboperitoneal insemination" or "IUI" or "artificial insemination by donor" or "artificial insemination by partner" or "artificial insemination" or "superovulation" or "superovulation induction" (231 records)
Appendix 2. CENTRAL via the Cochrane Register of Studies Online (CRSO) search strategy
Web platform
Searched 17 October 2019
#1 MESH DESCRIPTOR Infertility EXPLODE ALL TREES 2963 #2 (unexplained or idiopathic):TI,AB,KY 11267 #3 #1 AND #2 404 #4 (unexplain* adj5 infertil*):TI,AB,KY 603 #5 (unexplain* adj5 subfertil*):TI,AB,KY 112 #6 (idiopathic adj5 subfertil*):TI,AB,KY 15 #7 (idiopathic adj5 infertil*):TI,AB,KY 129 #8 (unexplained adj3 steril*):TI,AB,KY 2 #9 (idiopathic adj3 steril*):TI,AB,KY 3 #10 (unknown adj3 steril*):TI,AB,KY 2 #11 #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 914 #12 MESH DESCRIPTOR Insemination, Artificial EXPLODE ALL TREES 359 #13 (intrauterine insemination*):TI,AB,KY 956 #14 (artificial insemination*):TI,AB,KY2 45 #15 IUI:TI,AB,KY 842 #16 superovulat*:TI,AB,KY 213 #17 (ovulation induc*):TI,AB,KY 2514 #18 (ovar* adj3 stimulat*):TI,AB,KY 2155 #19 (ovarian hyperstimulation):TI,AB,KY 1355 #20 COH:TI,AB,KY 386 #21 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 5324 #22 MESH DESCRIPTOR Clomiphene EXPLODE ALL TREES 603 #23 (Clomiphene or clomifene or Serophene):TI,AB,KY 1601 #24 clomid:TI,AB,KY 58 #25 MESH DESCRIPTOR Selective Estrogen Receptor Modulators EXPLODE ALL TREES 2838 #26 (selective estrogen receptor modulator*):TI,AB,KY 721 #27 (SERMs or SERM):TI,AB,KY 258 #28 (raloxifene or tamoxifen):TI,AB,KY 5461 #29 #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 7276 #30 MESH DESCRIPTOR Aromatase Inhibitors EXPLODE ALL TREES 700 #31 (Aromatase inhibitor*):TI,AB,KY 1945 #32 letrozole:TI,AB,KY 1792 #33 (femara or anastrozole):TI,AB,KY 1186 #34 (anti‐?estrogen* or anti?estrogen*):TI,AB,KY 610 #35 #30 OR #31 OR #32 OR #33 OR #34 4076 #36 MESH DESCRIPTOR Follicle Stimulating Hormone EXPLODE ALL TREES 1909 #37 MESH DESCRIPTOR Menotropins EXPLODE ALL TREES 425 #38 (Follicle Stimulating Hormone*):TI,AB,KY 3145 #39 (FSH or rFSH or recFSH):TI,AB,KY 4440 #40 (uFSH or rhFSH):TI,AB,KY 119 #41 (hpFSH or pFSH):TI,AB,KY 16 #42 (follitropin or Gonal F):TI,AB,KY 1557 #43 (menotropin* or menopur):TI,AB,KY 502 #44 corifollitropin:TI,AB,KY 114 #45 (urofollitropin or pergonal or bravelle* or follitrin):TI,AB,KY 126 #46 Follistim*:TI,AB,KY 19 #47 (Puregon or humegon or menogon):TI,AB,KY 128 #48 (human menopausal gonadotrop?in):TI,AB,KY 582 #49 (growth hormone):TI,AB,KY 5748 #50 HMG:TI,AB,KY 1948 #51 gonadotrop?in*:TI,AB,KY 5970 #52 HCG:TI,AB,KY 2801 #53 #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 17043 #54 #21 OR #29 OR #35 OR #53 26677 #55 #11 AND #54 652
Appendix 3. MEDLINE search strategy
OVID platform
Searched from 1946 to 17 October 2019
1 exp Infertility/ and unexplained.tw. (1979) 2 exp Infertility/ and idiopathic.tw. (1795) 3 (unexplain* adj5 infertil*).tw. (2199) 4 (unexplain* adj5 subfertil*).tw. (175) 5 (idiopathic adj5 subfertil*).tw. (75) 6 (idiopathic adj5 infertil*).tw. (1321) 7 (unknown adj3 infertil*).tw. (186) 8 (unknown adj3 subfertil*).tw. (13) 9 (unexplained adj3 steril*).tw. (56) 10 (idiopathic adj3 steril*).tw. (56) 11 (unknown adj3 steril*).tw. (48) 12 or/1‐11 (4784) 13 exp Insemination, Artificial/ (11506) 14 intrauterine insemination*.tw. (2428) 15 artificial insemination*.tw. (6501) 16 IUI.tw. (1698) 17 superovulat*.tw. (3348) 18 ovulation induc*.tw. (4066) 19 (ovar* adj2 stimulat*).tw. (6957) 20 ovarian hyperstimulation.tw. (4931) 21 or/13‐20 (30631) 22 exp Clomiphene/ (5216) 23 clomifene.tw. (134) 24 clomiphene.tw. (4986) 25 Serophene.tw. (4) 26 clomid.tw. (181) 27 selective estrogen receptor modulators/ (3934) 28 selective estrogen receptor modulator*.tw. (2917) 29 (SERMs or SERM).tw. (2084) 30 (raloxifene or tamoxifen).tw. (24483) 31 or/22‐30 (33648) 32 Aromatase Inhibitors/ (6001) 33 Aromatase inhibitor*.tw. (7054) 34 letrozole.tw. (2691) 35 (femara or anastrozole).tw. (1756) 36 (anti‐?estrogen* or anti?estrogen*).tw. (9127) 37 or/32‐36 (18678) 38 exp follicle stimulating hormone/ or exp follicle stimulating hormone, beta subunit/ or exp glycoprotein hormones, alpha subunit/ or exp menotropins/ or exp urofollitropin/ (39456) 39 Follicle Stimulating Hormone*.tw. (18990) 40 (FSH or rFSH or recFSH).tw. (34016) 41 (uFSH or rhFSH).tw. (244) 42 (hpFSH or pFSH).tw. (211) 43 (follitropin or Gonal F).tw. (724) 44 (menotropin* or menopur).tw. (210) 45 corifollitropin.tw. (108) 46 (urofollitropin or pergonal or bravelle* or follitrin).tw. (208) 47 Follistim*.tw. (12) 48 (Puregon or humegon or menogon).tw. (89) 49 human menopausal gonadotrop?in.tw. (1817) 50 growth hormone.tw. (54754) 51 HMG.tw. (14173) 52 gonadotrop?in*.tw. (62291) 53 HCG.tw. (24237) 54 or/38‐53 (169426) 55 21 or 31 or 37 or 54 (228388) 56 12 and 55 (1652) 57 randomized controlled trial.pt. (491546) 58 controlled clinical trial.pt. (93317) 59 randomized.ab. (457486) 60 randomised.ab. (91388) 61 placebo.tw. (207152) 62 clinical trials as topic.sh. (188724) 63 randomly.ab. (319887) 64 trial.ti. (206291) 65 (crossover or cross‐over or cross over).tw. (82021) 66 or/57‐65 (1307234) 67 exp animals/ not humans.sh. (4628394) 68 66 not 67 (1202791) 69 56 and 68 (441)
Appendix 4. Embase search strategy
OVID platform
Searched from 1980 to 17 October 2019
1 (exp infertility/ or exp infertility therapy/) and unexplained.tw. (4154) 2 (exp infertility/ or exp infertility therapy/) and idiopathic.tw. (3594) 3 (unexplain* adj5 infertil*).tw. (3387) 4 (unexplain* adj5 subfertil*).tw. (281) 5 (idiopathic adj5 subfertil*).tw. (91) 6 (idiopathic adj5 infertil*).tw. (1937) 7 (unknown adj3 infertil*).tw. (294) 8 (unknown adj3 subfertil*).tw. (17) 9 (unexplained adj3 steril*).tw. (61) 10 (idiopathic adj3 steril*).tw. (65) 11 (unknown adj3 steril*).tw. (58) 12 or/1‐11 (8148) 13 exp artificial insemination/ (16825) 14 intrauterine insemination*.tw. (3712) 15 artificial insemination*.tw. (5973) 16 (IUI or superovulation).tw. (5368) 17 exp ovulation induction/ (14132) 18 ovulation induc*.tw. (5543) 19 COH.tw. (2380) 20 (ovar* adj2 stimulat*).tw. (10979) 21 ovarian hyperstimulation.tw. (7354) 22 or/13‐21 (45645) 23 exp clomifene/ (4584) 24 clomifene.tw. (225) 25 clomiphene.tw. (5537) 26 Serophene.tw. (198) 27 clomid.tw. (950) 28 exp selective estrogen receptor modulator/ (7703) 29 exp raloxifene/ (11190) 30 exp tamoxifen citrate/ or exp tamoxifen/ (61475) 31 selective estrogen receptor modulator*.tw. (4005) 32 (SERMs or SERM).tw. (3169) 33 (raloxifene or tamoxifen).tw. (35757) 34 or/23‐33 (83442) 35 exp aromatase inhibitor/ (30464) 36 Aromatase inhibitor*.tw. (11332) 37 letrozole.tw. (5079) 38 (femara or anastrozole).tw. (3927) 39 (anti‐?estrogen* or anti?estrogen*).tw. (10783) 40 or/35‐39 (41113) 41 exp follitropin/ (51623) 42 exp human menopausal gonadotropin/ (8961) 43 exp urofollitropin/ (1693) 44 Follicle Stimulating Hormone*.tw. (19800) 45 (FSH or rFSH or recFSH).tw. (42009) 46 (uFSH or rhFSH).tw. (361) 47 (hpFSH or pFSH).tw. (225) 48 (follitropin or Gonal F).tw. (3170) 49 (menotropin* or menopur).tw. (852) 50 corifollitropin.tw. (226) 51 (urofollitropin or pergonal or bravelle* or follitrin).tw. (2064) 52 Follistim*.tw. (282) 53 (Puregon or humegon or menogon).tw. (2191) 54 human menopausal gonadotrop?in.tw. (1942) 55 growth hormone.tw. (57637) 56 HMG.tw. (18196) 57 gonadotrop?in*.tw. (64234) 58 or/41‐57 (184628) 59 22 or 34 or 40 or 58 (306922) 60 12 and 59 (3139) 61 Clinical Trial/ (954250) 62 Randomized Controlled Trial/ (572677) 63 exp randomization/ (84808) 64 Single Blind Procedure/ (36997) 65 Double Blind Procedure/ (164394) 66 Crossover Procedure/ (61141) 67 Placebo/ (330524) 68 Randomi?ed controlled trial$.tw. (213916) 69 Rct.tw. (34381) 70 random allocation.tw. (1919) 71 randomly.tw. (420773) 72 randomly allocated.tw. (33522) 73 allocated randomly.tw. (2487) 74 (allocated adj2 random).tw. (810) 75 Single blind$.tw. (23587) 76 Double blind$.tw. (197107) 77 ((treble or triple) adj blind$).tw. (1025) 78 placebo$.tw. (293366) 79 prospective study/ (557816) 80 or/61‐79 (2331046) 81 case study/ (65015) 82 case report.tw. (385105) 83 abstract report/ or letter/ (1076178) 84 or/81‐83 (1516287) 85 80 not 84 (2278531) 86 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5836378) 87 85 not 86 (2120418) 88 60 and 87 (920)
Appendix 5. PsycINFO search strategy
OVID platform
Searched from 1806 to 17 October 2019
1 exp reproductive technology/ (1768) 2 insemination.tw. (708) 3 iui.tw. (38) 4 or/1‐3 (2221) 5 subfertil$.tw. (92) 6 infertil$.tw. (3438) 7 superovulation.tw. (4) 8 (unexplained adj2 sterility).tw. (1) 9 ovulation induction.tw. (22) 10 clomiphene.tw. (48) 11 or/5‐10 (3535) 12 4 and 11 (680) 13 random.tw. (56396) 14 control.tw. (432002) 15 double‐blind.tw. (22399) 16 clinical trials/ (11457) 17 placebo/ (5379) 18 exp Treatment/ (1016626) 19 or/13‐18 (1402955) 20 12 and 19 (302)
Appendix 6. CINAHL search strategy
EBSCO platform
Searched from 1961 to 17 October 2019
S35 S22 AND S34 77 S34 S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 1,349,748 S33 TX allocat* random* 10,985 S32 (MH "Quantitative Studies") 23,433 S31 (MH "Placebos") 11,436 S30 TX placebo* 59,309 S29 TX random* allocat* 10,985 S28 (MH "Random Assignment") 55,536 S27 TX randomi* control* trial* 176,078 S26 TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) 1,029,085 S25 TX clinic* n1 trial* 251,722 S24 PT Clinical trial 86,284 S23 (MH "Clinical Trials+") 267,151 S22 S12 AND S21 161 S21 S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 3,652 S20 TX ovarian hyperstimulation* 826 S19 TX (ovar* N3 stimulat*) 989 S18 TX ovulation induc* 1,856 S17 TX superovulat* 84 S16 TX IUI 336 S15 TX artificial insemination* 776 S14 TX intrauterine insemination* 471 S13 (MM "Insemination, Artificial") 429 S12 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 592 S11 TX(unknown N5 steril*) 16 S10 TX(idiopathic N5 steril*) 7 S9 TX(unexplained N5 steril*) 1 S8 TX(unknown N5 subfertil*) 2 S7 TX(unknown N5 infertil*) 38 S6 TX(idiopathic N5 infertil*) 104 S5 TX(idiopathic N5 subfertil*) 5 S4 TX(unexplain* N5 subfertil*) 59 S3 TX(unexplain* N5 infertil*) 337 S2 (MM "Infertility") and TX idiopathic 114 S1 (MM "Infertility") and TX unexplained 250
Appendix 7. Prognostic factors in included studies
| Study ID | Age distribution | Subfertility years | Prim/sec infertility | Previous treatment | Stimulation Method | Single insemination |
| 1. IUI versus timed intercourse both in natural cycle | 1 study | |||||
| Bhattacharya 2008 | TI+NC: 32 (± 3.4) IUI+NC: 32 (± 3.7) (TI is expectant management) | TI+NC: 30 (25 ‐ 38) IUI+NC:30 (25 ‐ 40) months (Inter quartile range) | Mixed 117/386 (30%) Secondary |
Not stated | No stimulation | Single |
| 2. IUI versus timed intercourse both in stimulated cycle | 7 studies | |||||
| Agarwal 2004 | IUI+OH: 29.52 (± 3.65) TI +OH: 28,83 (± 4,76) | IUI+OH: 4.91(± 2.72) TI+OH: 4.93 (± 3.27) | Mixed 32/113 (28%) secondary | No | CC 50 ‐ 150 mg | Single |
| Arcaini 1996 | IUI+OH: 34.6 (± 4.9) TI+OH: 33.4 (± 4.7) | IUI+OH: 4.2 (± 1.6) TI+OH: 3.9 (± 2.3) | Mixed 7/68 (10%) secondary | Not stated | High dose: CC100mg and hMG 75 ‐ 225 IU | Double |
| Chung 1995 | IUI+OH: 31.8 (± 3.1) TI+OH: 32.1 (± 4.0) | IUI+OH: 4.7 (± 2.0) TI+OH: 5.3 (± 2.6) | Not clear | Not stated | hMG 150 IU starting dose and GnRHa | IUI: Single TI: Double |
| Crosignani 1991 | < 38 yrs | > 3 yrs | Not clear | Probably | Not stated | Not stated |
| Janko 1998 | Not stated | > 3 yrs | Not clear | Not stated | hMG (10 amp per cycle) | Not stated |
| Karlstrom 1993 | 32 (range 21 ‐ 38) | 5 (range 2 ‐ 14) | Mixed 49/148 (33%) secondary (incl Pt in DIPI groups) | No | hMG (low‐dose step‐up) 75 IU starting dose OR CC 100 mg | IUI: Single TI: Double |
| Melis 1995 | 33.1 (± 5.2) | 4.3 (± 1.4) | Not clear | Yes, all participants | High dose: FSH 225 IU | Single |
| 3. IUI in natural cycle versus IUI in stimulated cycle | 4 studies | |||||
| Arici 1994 | 33 (range 24 ‐ 41) | 3.5 (range 1 ‐ 15) | Not clear | No | CC 50 mg | IUI+NC: Double IUI+OH: Single |
| Goverde 2000 | IUI+NC: 31.6 (± 3.7) IUI+OH: 31.7 (± 3.9) | IUI+NC: 3.9 (± 1.7) IUI+OH: 4.2 (± 1.9) | Mixed 13.5% secondary | Not stated | hMG 75 IU starting dose | Single |
| Guzick 1999 | IUI+NC: 32 (± 4) IUI+OH: 32 (± 4) < 40 yrs | IUI+NC: 3.8 (± 2.6) IUI+OH: 3.5 (± 2.2) | Mixed 40% secondary | No | FSH 150 IU | Single |
| Murdoch 1991 | IUI+NC: 30.5 (± 3.1) IUI+OH: 30.1 (± 2.9) | IUI+NC: 5.7 (± 2.4) IUI+OH: 5.1 (± 1.9) | Mixed 5/34 (15%) secondary | No | hMG (low dose) 75 IU + GnRHa | IUI+OH: Single IUI+NC: til USS evidence of ovulation |
| 4. IUI with OH versus TI in natural cycle | 3 studies | |||||
| Deaton 1990 | 33 (± 4.0) | 3.5 (± 1.7) | Mixed 21/51 (41%) secondary | Not stated | CC 50 mg | Single |
| Farquhar 2018 | IUI+OH: 34.4 (± 3.5) TI+NC: 33.6 (± 3.7) (TI is expectant management) |
Months (range) IUI+OH:41.0 (31.0 – 60·0) TI+NC: 46.0 (27.8–60·0) |
Mixed 176/201 (87.6%) secondary |
33/201 (16.4%) | CC; 50 – 150 mg, or letrozole; 2.5 – 7.5 mg | Single |
| Steures 2006 | IUI+OH: 33 (± 3.5) TI+NC: 33 (± 3.1) (TI is expectant management) | IUI+OH: 2.0 (± 0.5) TI+NC: 1.9 (± 0.5) | Mixed 58/253 (23%) secondary | Not stated | FSH 37 ‐ 150 IU or CC 50 ‐ 150 mg | Not stated |
| 5. IUI in natural cycle versus TI with OH | 1 study | |||||
| Bhattacharya 2008 | TI+OH: 32 (± 3.5) IUI+NC: 32 (± 3.7) | TI+OH: 30 (24 ‐ 38) IUI+NC: 30 (25 ‐ 40) months (Inter quartile range) | Mixed 109/387 (28%) | Not stated | CC 25 ‐ 50 mg | Single |
| * Mean age in years (± SD) or range | * Mean duration in years (± SD) or range | * Daily dose | ||||
Data and analyses
Comparison 1. IUI versus TI or expectant management both in natural cycle.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate per couple (all cycles) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Multiple pregnancy rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Pregnancy rate per couple (all cycles) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Miscarriage rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Ectopic pregnancy rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 2. IUI versus TI or expectant management both in stimulated cycle.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate per couple (all cycles) | 2 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.88, 2.88] |
| 1.1 Gonadotropins | 2 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.88, 2.88] |
| 2 Multiple pregnancy rate per couple | 4 | 316 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.55, 3.87] |
| 2.1 Clomiphene Citrate | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.02, 11.18] |
| 2.2 Gonadotropins | 2 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.61 [0.44, 5.89] |
| 2.3 Clomiphene Citrate and Gonadotropins | 1 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.32, 11.00] |
| 3 Pregnancy rate per couple (all cycles) | 6 | 517 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.14, 2.53] |
| 3.1 Clomiphene Citrate | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.03, 2.93] |
| 3.2 Gonadotropins | 4 | 319 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.03, 2.75] |
| 3.3 Clomiphene Citrate and Gonadotropins | 1 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.62 [0.98, 6.98] |
| 3.4 Clomiphene citrate OR Gonadotropins | 1 | 90 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.50, 5.89] |
| 4 Moderate or severe ovarian hyperstimulation syndrome rate per woman | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Clomiphene Citrate and Gonadotropins | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Miscarriage rate per couple | 2 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.56, 4.88] |
| 5.1 Gonadotropins | 2 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.56, 4.88] |
| 6 Ectopic pregnancy rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Gonadotropins | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. IUI in stimulated cycle versus TI or expectant management in natural cycle.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate per couple (all cycles) | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Clomiphene Citrate or Letrozole | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Clomiphene Citrate or Gonadotropins | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Multiple pregnancy rate per couple | 2 | 454 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.01 [0.47, 19.28] |
| 2.1 Clomiphene Citrate or Letrozole | 1 | 201 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.05 [0.24, 106.53] |
| 2.2 Clomiphene Citrate or Gonadotropins | 1 | 253 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 22.34] |
| 3 Pregnancy rate per couple (all cycles) | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Clomiphene Citrate | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Clomiphene Citrate or Letrozole | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Clomiphene Citrate or Gonadotropins | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Miscarriage rate per couple | 2 | 454 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.87 [1.18, 7.01] |
| 4.1 Clomiphene Citrate or Letrozole | 1 | 201 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.25 [0.74, 52.91] |
| 4.2 Clomiphene Citrate or Gonadotropins | 1 | 253 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.28 [0.84, 6.20] |
| 5 Ectopic pregnancy rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 4. IUI in natural cycle versus TI or expectant management in stimulated cycle.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate per couple (all cycles) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Multiple pregnancy rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Pregnancy rate per couple (all cycles) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Miscarriage rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Ectopic pregnancy rate per couple | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected |
4.3. Analysis.

Comparison 4 IUI in natural cycle versus TI or expectant management in stimulated cycle, Outcome 3 Pregnancy rate per couple (all cycles).
Comparison 5. IUI in stimulated cycle versus IUI in a natural cycle.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate per couple (all cycles) | 4 | 396 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.07 [1.22, 3.50] |
| 1.1 Clomiphene Citrate | 1 | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.75 [0.29, 47.99] |
| 1.2 Gonadotropins | 3 | 370 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.02 [1.18, 3.45] |
| 2 Multiple pregnancy rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Gonadotropins | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Pregnancy rate per couple (all cycles) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Clomiphene Citrate | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Miscarriage rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Clomiphene Citrate | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Gonadotropins | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Ectopic pregnancy rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Gonadotropins | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agarwal 2004.
| Methods | Trial design: parallel Single centre, India | |
| Participants | Couples with unexplained subfertility N randomised: IUI + OH 70; TI + OH 70 Age: IUI + OH 29.52 years (± 3.65); TI + OH 28.83 years (± 4.76) Duration of subfertility: IUI + OH 4.91 years (± 2.72); TI + OH 4.93 years (± 3.27) Basic fertility work‐up normal, semen normal according to WHO 1987 Previous treatment: no |
|
| Interventions | Comparison: IUI + OH versus TI + OH Stimulation method: 50 mg to 150 mg CC/day, day 3 to day 7 Ovulation: 10,000 IU hCG when not more than 4 follicles of > 16 mm were present Timing of IUI and TI: 36 hr to 40 hr after hCG Duration of treatment: 6 cycles max | |
| Outcomes | Live birth and PR per couple and per cycle
Miscarriage rate
Ectopic PR
Multiple pregnancies Pregnancy confirmed by USS showing gestational sac |
|
| Notes | ITT analysis: possible Author provided additional information Unbalanced groups: dropouts 37% in IUI group, 1% in TI group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table |
| Allocation concealment (selection bias) | Low risk | Adequate; sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not possible because of the nature of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was not possible because of the nature of the interventions and non‐blinding was not likely to affect the outcomes of interest |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was an unequal dropout in the treatment group due to financial reasons; Nr of withdrawals: IUI + OH 26 (37%); TI + OH 1 (total 19%) |
| Selective reporting (reporting bias) | Unclear risk | Reported on live birth, but authors provided additional information on ongoing pregnancies and twin pregnancies resulting in different data used for meta‐analysis |
| Other bias | Low risk | Baseline demographic characteristics similar between the 2 groups |
Arcaini 1996.
| Methods | Trial design: parallel Single centre, Italy | |
| Participants | Couples with unexplained subfertility N randomised: IUI + OH 36; TI + OH 32 Age: IUI + OH 34.6 years (± 4.9); TI + OH 33.4 years (± 4.7) Duration of subfertility: IUI + OH 4.2 years (± 1.6); TI + OH 3.9 years (± 2.3) Basic fertility work‐up normal, semen normal, not further specified Previous treatment: not stated |
|
| Interventions | Comparison: IUI + OH versus TI + OH Stimulation method: 100 mg CC/day, day 3 to day 7 and 1 to 3 ampoule hMG/day Ovulation: 10,000 IU hCG when 2 to 6 follicles of > 17 mm were present Timing IUI or TI: 24 hr and 48 hr after hCG Duration of treatment: 5 cycles max | |
| Outcomes | PR per couple
Miscarriage rate
Ectopic PR
Multiple pregnancies
OHSS Pregnancy confirmed by USS |
|
| Notes | ITT analysis: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not possible because of the nature of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was not possible because of the nature of the interventions and non‐blinding was not likely to affect the outcomes of interest |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 16 cancelled treatment cycles is described and analysed according to ITT. People who dropped out are clearly stated in a table |
| Selective reporting (reporting bias) | Unclear risk | Did not report on live birth, but did not intend to report on live birth |
| Other bias | Low risk | Nothing detected |
Arici 1994.
| Methods | Trial design: cross‐over (after 1 cycle) Single centre, USA | |
| Participants | Couples with unexplained subfertility and couples with male‐factor subfertility N randomised: 26 Age: mean 33 yrs (range 24 yrs to 41 yrs) Duration of subfertility: mean 3.5 yrs (range 1 yr to 15 yrs) Unexplained subfertility: basic fertility work‐up normal, semen normal according to WHO 1987 criteria Previous treatment: no |
|
| Interventions | Comparison: IUI + NC versus IUI + OH
Stimulation method: 50 mg CC/day, day 5 to day 9 Timing: Natural cycle: urinary LH test, IUI on day of LH peak and the next day Stimulated cycle: 10,000 IU hCG when at least 1 follicle of 18 mm was present; IUI 32 hr after hCG No cancellation criteria were given Duration of treatment: 4 cycles max |
|
| Outcomes | Live birth and PR per couple
PR per 1st cycle
PR per cycle
Miscarriage rate
Ectopic PR
Multiple pregnancies Pregnancy confirmed by USS showing gestational sac |
|
| Notes | ITT analysis: yes Author provided additional information 5 women with treated minimal endometriosis were included as unexplained subfertility | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random‐number table |
| Allocation concealment (selection bias) | Low risk | Adequate; computer system using locked files |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not possible because of the nature of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was not possible because of the nature of the interventions and non‐blinding was not likely to affect the outcomes of interest |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Author gave additional information on dropout rates of the couples with unexplained subfertility. Of the 26 women with unexplained subfertility, dropout occurred after 1 treatment cycle. Post‐cross‐over data are not included in the meta‐analysis |
| Selective reporting (reporting bias) | Low risk | Live‐birth data were obtained from the author |
| Other bias | Unclear risk | Insufficient information reported on baseline demographic characteristics |
Bhattacharya 2008.
| Methods | Trial design: parallel Multicentre (4 teaching hospitals, 1 general hospital), UK | |
| Participants | Couples with unexplained subfertility, (mild male factor infertility and minimal endometriosis) N randomised: 509 with unexplained subfertility only (total 580) Age: TI + NC 32 years (± 3.4); TI + OH 32 years (± 3.5); IUI + NC 32 (± 3.7) Duration of subfertility: minimum 2 years, median 30 months all groups Basic fertility work‐up normal, semen normal according to WHO (sperm motility < 20% included) Previous treatment: not stated |
|
| Interventions | Comparison: TI (expectant management) + NC versus TI + OH versus IUI + NC Stimulation method: 50 mg CC/day (starting dose), day 2 to day 6 Ovulation: confirmed by progesterone measure in TI + OH group, and urinary LH surge in IUI + NC group Timing of IUI and TI: IUI 20 hr to 30 hr after LH surge, timing intercourse advised on cycle day 12 to 18 Duration of treatment: 6 cycles max | |
| Outcomes | Live birth and PR per couple
Miscarriage rate
Ectopic PR
Multiple pregnancies Pregnancy confirmed by USS showing gestational sac and foetal heart beat |
|
| Notes | The author provided additional data on the couples with unexplained subfertility only The baseline characteristics of the participants reported are from the group total. ITT analysis was therefore possible and performed Author provided additional information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generated by independent statistician |
| Allocation concealment (selection bias) | Low risk | Adequate; central telephone randomisation system |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not possible because of the nature of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was not possible because of the nature of the interventions and non‐blinding was not likely to affect the outcomes of interest |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up and participants who received alternative treatment are presented in a flow‐chart; Nr of withdrawals: 4 |
| Selective reporting (reporting bias) | Low risk | Live birth data and adverse events are published |
| Other bias | Low risk | Nothing detected |
Chung 1995.
| Methods | Trial design: parallel Single centre, UK | |
| Participants | Couples with unexplained subfertility N randomised: 100 Age: IUI + OH 31.8 years (± 3.1); TI + OH 32.1 years (± 4.0) Duration of subfertility: IUI + OH 4.7 years (± 2.0); TI + OH 5.3 years (± 2.6) Basic fertility work‐up normal and semen 15 million motile per ejaculate Previous treatment: not stated |
|
| Interventions | Comparison: IUI + OH versus TI + OH Stimulation method: FSH 150 IU/day and GnRH nasal spray from day 21 on Ovulation: 5000 IU hCG when < 4 follicles > 16mm hCG post‐ovulatory for luteal support Timing TI: 24 hr + 48 hr after hCG Timing IUI: 36 hr to 48 hr after hCG Duration of treatment: 3 cycles max | |
| Outcomes | PR per couple and per cycle Total delivered Multiple pregnancies Ectopic Miscarriage rate | |
| Notes | ITT analysis: possible IUI was not possible on Sundays | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomisation scheme |
| Allocation concealment (selection bias) | Low risk | Adequate; numbered sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not possible because of the nature of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was not possible because of the nature of the interventions and non‐blinding was not likely to affect the outcomes of interest |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 8/50 withdrawn and 6 treatment cycles cancelled in TI group, 4/50 withdrawn and 11 treatment cycles cancelled in IUI group. Reason for cycle cancellation was excessive response. Reason for withdrawal was not stated |
| Selective reporting (reporting bias) | Low risk | Live birth data and complication numbers were reported |
| Other bias | Low risk | Nothing detected |
Crosignani 1991.
| Methods | Data from centre 10: Hedon, Montpellier, France Trial design: cross‐over (after 1 cycle) Multi centre (19 European fertility centres, 4 centres comparing IUI versus TI), Multiple European countries |
|
| Participants | Couples with unexplained subfertility N randomised: unclear; N analysed: total 90 (centre 10; 18 participants) Age: < 38 yrs Duration of subfertility: > 3 yrs Basic fertility work‐up normal, semen normal according to WHO 1987 Previous treatment: not stated |
|
| Interventions | Comparison: IUI + OH versus TI + OH Stimulation method: not stated Ovulation: not described Timing: not described No cancellation criteria were given Duration of treatment: 2 cycles max | |
| Outcomes | PR per 1st cycle PR per cycle | |
| Notes | ITT analysis: not possible Author replied; could not provide additional information Multicentre ESHRE trial Only 4 infertility centres compared IUI with superovulation alone. These centres were included in the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Each centre used own randomisation method. We could not obtain the by‐centre method |
| Allocation concealment (selection bias) | Unclear risk | Unclear; each centre used its own treatment allocation method. We could not obtain the by‐centre method |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not possible because of the nature of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was not possible because of the nature of the interventions and non‐blinding was not likely to affect the outcomes of interest |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Details on participant withdrawal or loss to follow‐up were not stated |
| Selective reporting (reporting bias) | Unclear risk | Live birth data were not reported |
| Other bias | Unclear risk | Insufficient information available to evaluate this risk domain |
Deaton 1990.
| Methods | Trial design: cross‐over (after 4 cycles) Single centre, USA | |
| Participants | Couples with unexplained subfertility and couples with surgically treated endometriosis N randomised: 67; N analysed: 51 total, unexplained: 24 Age: 33 years (± 4.0) Duration of subfertility: 3.5 years (±1.7) Basic fertility work‐up normal, semen normal according to WHO criteria 1987 Previous treatment: not stated |
|
| Interventions | Comparison: IUI + OH versus TI + NC
Stimulation method: 50 mg CC/day, day 5 to day 9 Timing: Natural cycle: urinary LH and BBT timed intercourse Stimulated cycle: 10,000 IU hCG when lead follicle was estimated to be at least 18 mm. IUI 36 hr after hCG injection No cancellation criteria were given Duration of treatment: 8 cycles max |
|
| Outcomes | Ongoing pregnancy rate
Multiple pregnancies
Ectopic pregnancies
Miscarriage rate
OHSS Pregnancy: not further defined |
|
| Notes | ITT analysis: not possible Participants with unexplained subfertility and endometriosis were included in this study; 3 participants with moderate and no participants with severe endometriosis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not possible because of the nature of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was not possible because of the nature of the interventions and non‐blinding was not likely to affect the outcomes of interest |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16/67 participants excluded from analysis due to anovulation, poor semen quality or inability to follow the treatment protocol. Of the remaining 51 participants, 6 couples did not complete treatment because of illness or relocation. 4/51 dropped out before cross‐over |
| Selective reporting (reporting bias) | Unclear risk | Live birth rate was not reported |
| Other bias | Unclear risk | Insufficient information available to evaluate this risk domain |
Farquhar 2018.
| Methods | Trial design: 2‐arm parallel design 2 centres, New Zealand |
|
| Participants | Women with unexplained infertility and those with mild endometriosis and polycystic ovarian syndrome N randomised: 201 Age: IUI + OH 34.4 years (± 3.5); EM 33.6 years (± 3.7) Duration of infertility (months): IUI + OH 41.0 (31.0 – 60.0); EM 46.0 (27.8 – 60.0) Basic fertility work‐up normal, semen normal Previous treatment: yes |
|
| Interventions | Comparison: IUI + OH versus EM
Stimulation method: oral clomiphene citrate; (50 – 150 mg, days 2 – 6) or oral letrozole; (2.5 – 7.5 mg,days 2 – 6) Timing:Stimulated cycle: IUI 24 hour after luteinising hormone surge or 36 hr after hCG injection Cancellation criteria: no rise in oestradiol or development of follicles, presence of > 3 follicles Duration of treatment: 3 cycles max |
|
| Outcomes | Live birth Clinical pregnancy Miscarriage Multiple pregnancy Ectopic pregnancy OHSS |
|
| Notes | This study investigated women with unexplained infertility and those with mild endometriosis and polycystic ovarian syndrome and a prediction score of natural conception leading to livebirth in the next year of < 30% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Allocations were concealed in sequentially‐numbered, sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not possible because of the nature of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was not possible because of the nature of the interventions and non‐blinding was not likely to affect the outcomes of interest |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Used ITT as part of the analyses |
| Selective reporting (reporting bias) | Low risk | All outcomes reported were prespecified in the Methods section |
| Other bias | Low risk | Not detected |
Goverde 2000.
| Methods | Trial design: parallel
Single centre, the Netherlands A power calculation was performed |
|
| Participants | Couples with unexplained subfertility and couples with male factor subfertility N randomised: 120 (unexplained IUI + NC and IUI + TI), 258 total Age: IUI + NC 31.6 years (± 3.7); IUI + OH 31.7 years (± 3.9) Duration of subfertility: IUI + NC 3.9 years (± 1.7); IUI + OH 4.2 years (± 1.9) Basic fertility work‐up normal, semen normal when > 20 million progressive motile in ejaculate Previous treatment: not stated |
|
| Interventions | Comparison: IUI + NC versus IUI + OH (versus IVF) Stimulation method: 75 IU FSH (starting dose) until 1 to 3 follicles of 18 mm were seen on USS hCG was withheld if > 3 follicles of 18 mm or > 6 of 14 mm were present Timing: Stimulated cycle: 10,000 IU hCG, IUI 40 hr to 42 hr after hCG; Natural cycle: IUI 20 hr to 30 hr after detection of urinary LH‐surge Cycles were cancelled when > 3 follicles of 18 mm or > 6 follicles of 14 mm were present Duration of treatment: 6 cycles max | |
| Outcomes | Live birth per couple OHSS | |
| Notes | ITT analysis: yes Some dropouts because of spontaneous pregnancy |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Adequate; numbered, masked and sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not possible because of the nature of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was not possible because of the nature of the interventions and non‐blinding was not likely to affect the outcomes of interest |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 7/120 withdrew before 1st treatment cycle. Details on dropout not separately available for unexplained subfertility. Some participants dropped out because of spontaneous pregnancy. It is not known whether these participants are included in the IUI unexplained subfertility group |
| Selective reporting (reporting bias) | Low risk | Live birth and complication data were reported |
| Other bias | Low risk | Nothing detected; baseline demographic characteristics similar between the 2 groups |
Guzick 1999.
| Methods | Trial design: Parallel Multicentre (10 clinical sites), USA | |
| Participants | Couples with unexplained subfertility and couples with stage I or II treated endometriosis or male factor subfertility N randomised: 932 (465 treated with IUI); N with unexplained subfertility: 211 Age: IUI + NC 32 years (± 4) IUI + OH 32 years (± 4) Duration of subfertility: IUI + NC 3.8 years (± 2.6); IUI + OH 3.5 years (± 2.2) Basic fertility work‐up normal, semen normal (according to WHO 1992) Previous treatment: no previous treatment. (Pt excluded if previous ART) |
|
| Interventions | Comparison: IUI + NC versus IUI + OH
Stimulation method: 150 IU FSH/day, day 3 to day 7
Ovulation: IUI + OH: 10,000 IU hCG when 2 follicles of > 18 mm were present
IUI + NC: urine LH testing
Timing: IUI + OH: 36 hr to 40 hr after hCG
IUI + NC: IUI the day after urinary LH surge Cycles were cancelled if serum E2 concentration > 3000 pg/ml Duration of treatment: 4 cycles max |
|
| Outcomes | Live birth per couple
PR per couple
Ectopic PR Pregnancy defined by 2 positive hCG tests. This is biochemical pregnancy, therefore not included in analysis |
|
| Notes | ITT analysis: not possible Author replied; provided additional information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Author could not guarantee whether or not participants were truly randomised |
| Allocation concealment (selection bias) | Unclear risk | Author could not guarantee whether or not participants were truly randomised |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not possible because of the nature of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was not possible because of the nature of the interventions and non‐blinding was not likely to affect the outcomes of interest |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal rates of the total group were presented: 4/465 treatment‐related withdrawal, 27/465 not treatment‐related. Numbers for unexplained subfertility group are not known |
| Selective reporting (reporting bias) | Low risk | Live birth and complication data were reported |
| Other bias | Low risk | Nothing detected |
Janko 1998.
| Methods | Trial design: parallel Single centre, Slovakia | |
| Participants | Couples with unexplained subfertility N randomised: 72 Age: not stated Duration of subfertility: > 3 yrs Basic fertility work‐up normal, semen normal not further specified Previous treatment: not stated |
|
| Interventions | Comparison: IUI + OH versus TI + OH Stimulation method: hMG (10 amp per cycle) Ovulation: 10,000 IU hCG Timing: not specified No cancellation criteria were given Duration of treatment: 3 cycles max | |
| Outcomes | PR per cycle Pregnancy not further defined |
|
| Notes | ITT analysis: possible Abstract only Data calculated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Unclear; not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not possible because of the nature of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was not possible because of the nature of the interventions and non‐blinding was not likely to affect the outcomes of interest |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not available |
| Selective reporting (reporting bias) | High risk | In this abstract the reported outcome data are minimal |
| Other bias | Unclear risk | Insufficient information available to evaluate this risk domain |
Karlstrom 1993.
| Methods | Trial design: parallel Single centre, Sweden | |
| Participants | Couples with unexplained subfertility and minimal or mild endometriosis N randomised: not clear; N analysed: 79 Age: 32 years (range 21 years to 38 years) Duration of subfertility: 5 years (range 2 years to 14 years) Basic fertility work‐up normal, semen normal according to WHO 1987 Previous treatment: no |
|
| Interventions | Comparison: IUI + OH versus TI + OH (vs DIPI + OH vs IUI and DIPI + OH)
Stimulation method 1: 150 IU hMG starting dose, til 1 follicle of at least 17 mm was present or the detection of a LH surge in serum or urine
Monitoring: USS and serum E2
Ovulation: 10,000 IU hCG
Timing: IUI 36 hr to 41 hr after hCG or 24 hr after detection of LH surge. TI the 2 following nights after hCG injection Stimulation method 2: 100 mg CC/day for 5 days Monitoring + ovulation: urinary LH timed Timing: IUI 20 hr to 28 hr after LH surge, TI day of LH surge and day after Cycles were cancelled according to serum E2 rise Duration of treatment: 1 cycle max |
|
| Outcomes | PR per cycle Ectopic PR Pregnancy not further defined | |
| Notes | ITT analysis: not possible When ovulation occurred during the weekend, participants were transferred to TI group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not possible because of the nature of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was not possible because of the nature of the interventions and non‐blinding was not likely to affect the outcomes of interest |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 withdrawals in clomiphene group due to absent LH surge, 5 withdrawals in hMG group due to absent LH surge, fast oestrogen rise or personal reasons |
| Selective reporting (reporting bias) | Unclear risk | Live birth data were not reported |
| Other bias | Unclear risk | Insufficient information was reported on demographic characteristics to make a conclusive judgement |
Melis 1995.
| Methods | Trial design: parallel Italy |
|
| Participants | Couples with unexplained subfertility and couples with mild male factor subfertility N randomised: 108; N analysed: 103 Age: 33.1 years (± 5.2) Duration of subfertility: 4.3 years (± 1.4) Basic fertility work‐up normal, semen normal according to WHO 1987 criteria Previous treatment: yes, all couples |
|
| Interventions | Comparison: IUI + OH versus TI + OH
Stimulation method: 3 amp FSH/day
Monitoring: USS and plasma E2
Ovulation: 10,000 IU hCG when at least 2 follicles of 16 mm were present
Timing: TI 12 hr after HCG, IUI 30 hr to 36 hr after HCG
Cycles cancelled when plasma E2 level > 1500 pg/ml Duration of treatment: 3 cycles max |
|
| Outcomes | Live birth per couple
PR/couple
Miscarriage
Multiple pregnancies
OHSS Pregnancy confirmed by USS showing foetal heart activity |
|
| Notes | ITT analysis: possible Author provided additional information All participants had previous fertility treatment Pt with minor abnormalities were excluded from the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random‐number list |
| Allocation concealment (selection bias) | Low risk | Adequate; numbered opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not possible because of the nature of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was not possible because of the nature of the interventions and non‐blinding was not likely to affect the outcomes of interest |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusion numbers were published for the overall group.The author provided additional information: 1/52 (IUI + OH group) withdrew, 4/56 (TI + OH group) withdrew. Reasons for dropout were family problems, poor response or exaggerated response |
| Selective reporting (reporting bias) | Low risk | Live birth data and complication numbers were available for analysis |
| Other bias | Unclear risk | Insufficient information was reported to make a conclusive judgement on participant demographic characteristics |
Murdoch 1991.
| Methods | Trial design: parallel UK |
|
| Participants | Couples with unexplained subfertility N randomised: 39; IUI + NC 19; IUI + OH 20 Age: IUI + NC 30.5 years (± 3.1); IUI + OH 30.1 years (± 2.9) Duration of subfertility: IUI + NC 5.7 years (± 2.4); IUI + OH 5.1 years (± 1.9) Basic fertility work‐up done, semen normal (according to WHO 1987) Previous treatment: no |
|
| Interventions | Comparison: IUI + NC versus IUI + OH (vs GIFT)
Stimulation method: 75 IU hMG/day and 200 micro gram buserelin 4 times daily intranasal
Ovulation: 5000 IU hCG, when < 4 follicles of > 16mm were seen.
Timing: 30 hr to 36 hr after hCG
Natural cycle: IUI on alternate days until ovulation confirmed on USS
Cycles were cancelled if > 4 dominant follicles were present Duration of treatment: 3 cycles max |
|
| Outcomes | PR per couple and per cycle
Live birth
Multiple pregnancies Clinical pregnancy defined by USS showing foetal heart activity |
|
| Notes | ITT analysis: yes
Author provided additional information 1 pregnancy between treatment cycles 10 cycles were abandoned because no treatment available at the weekend |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random‐number sequence |
| Allocation concealment (selection bias) | Low risk | Adequate; sequentially numbered opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not possible because of the nature of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was not possible because of the nature of the interventions and non‐blinding was not likely to affect the outcomes of interest |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop‐out rate 3/19 (IUI + NC), and 2/20 (IUI + OH) |
| Selective reporting (reporting bias) | Low risk | Live birth data were provided |
| Other bias | Low risk | Nothing detected |
Steures 2006a.
| Methods | Trial design: parallel Multicentre: 26 fertility centres in the Netherlands | |
| Participants | Couples with unexplained subfertility and an intermediate prognosis of conceiving within the next 12 months (Hunault 30% to 40%) N randomised: 253; IUI + OH 127; TI (expectant management) + NC 126 Age: IUI + OH 33 years (± 3.4); TI + NC 33 years (± 3.19) Duration of subfertility: IUI + OH 2.0 years (± 0.5);TI + NC 1.91 years (± 0.5) Basic fertility work‐up done, semen analysis according to WHO 1987, normal postcoital test Previous treatment: not stated |
|
| Interventions | Comparison: IUI + OH versus TI (expectant management) + NC
Stimulation method: FSH 37 to 150 IU/day or 50 mg to 150 mg CC/day
Monitoring: USS
Ovulation: 5000 or 10,000 IU hCG
Timing: IUI 36 hr to 40 hr after hCG Cycles were cancelled when > 3 follicles of 16 mm or > 5 follicles of 12 mm were present Duration of treatment: 6 months |
|
| Outcomes | Live birth/couple PR/couple Miscarriage rate Multiple pregnancies | |
| Notes | ITT analysis: yes
Author provided additional information Only couples with an intermediate prognosis of conceiving were included, so this influences the possible treatment effect |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence in balanced blocks |
| Allocation concealment (selection bias) | Low risk | Adequate; via opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not possible because of the nature of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was not possible because of the nature of the interventions and non‐blinding was not likely to affect the outcomes of interest |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | IUI + OH group, 3 participants lost to follow‐up, TI + NC group, 2 lost to follow‐up, 2 still pregnant |
| Selective reporting (reporting bias) | Low risk | Live birth and complications reported |
| Other bias | Low risk | Nothing detected |
ART: assisted reproductive technology CC: clomiphene citrate DIPI: direct intraperitoneal insemination EM: expectant management FSH: follicle stimulating hormone hCG: human chorionic gonadotropin hMG: human menopausal gonadotropin hr: hour(s) ITT: intention‐to‐treat IUI: intra‐uterine insemination OH: ovarian hyperstimulation PR: pregnancy rate USS: ultrasound scan
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aanesen 2014 | Cohort study |
| Aboulghar 1993 | The trial was not randomised |
| Barros Delgadillo 2008 | Not RCT |
| Barros‐Delgadillo 2010 | Did not include comparison of interest to this review |
| Check 2013 | Not RCT |
| Doyle 1991 | No pre‐cross‐over data available |
| Evans 1991 | No pre‐cross‐over data available |
| Gregoriou 1995 | No pre‐cross‐over data available |
| Ho 1998 | Abstract, full article not available. No separate data for couples with unexplained subfertility |
| Kabouk 2010 | Did not include comparison of interest to this review |
| Kirby 1991 | No pre‐cross‐over data available |
| Leanza 2014a | Did not include comparison of interest to this review |
| Leanza 2014b | Did not include comparison of interest to this review |
| Martinez 1990 | No per‐woman data. Biochemical pregnancies only reported |
| Martinez 1991 | No pre‐cross‐over data available |
| Nulsen 1993 | The trial (also published as an abstract in 1990) was not randomised |
| Peeraer 2013 | Did not include comparison of interest to this review |
| Prentice 1995 | This trial was quasi‐randomised, on the basis of hospital case record number |
| Serhal 1988 | The trial was not randomised |
| Tummon 1997 | The participants in this trial were all diagnosed with endometriosis |
| Van Eekelen 2019 | Cohort study |
| Wadhwa 2013 | Did not include comparison of interest to this review |
| Xu 2014 | Study involved donor semen |
| Zikopoulos 1993 | No pre‐cross‐over data available |
| Zolghadri 2012 | Included a mix of women with unexplained infertility and polycystic ovarian syndrome with no separate data for the 2 groups of women |
Characteristics of ongoing studies [ordered by study ID]
NCT03455426.
| Trial name or title | NCT03455426 |
| Methods | Randomised clinical trial |
| Participants | Women diagnosed with unexplained or mild male factor infertility scheduled for treatment with IUI |
| Interventions | IUI with letrozole versus IUI in natural cycle. In the group allocated to ovarian stimulation, women will receive oral tablets letrozole 5 mg daily from cycle day 3 ‐ 5 for 5 days. Investigators will treat the couples for 3 cycles, with a time horizon of 4 months |
| Outcomes | Primary outcome: ongoing pregnancy leading to live birth Secondary outcome: clinical pregnancy, multiple pregnancy, miscarriage rates, pregnancy complications and participants' costs |
| Starting date | March 15, 2018 |
| Contact information | Shuo Huang, PhD; 86‐13601203410; homelyleaf@aliyun.com |
| Notes |
Differences between protocol and review
We reclassified multiple pregnancy rate to be a primary outcome in the 2016 and 2020 updates.
We included aromatase inhibitors such as letrozole as agents for ovarian hyperstimulation in the 2020 update and this was reflected in the search strategies.
We conducted sensitivity analyses for the primary outcomes (live birth and multiple pregnancy) for studies with 'Risk of bias' or 'unit of analysis' errors in the 2020 update.
Contributions of authors
Reuben Olugbenga Ayeleke performed the updated search, selection of trials, data extraction and analyses; created the 'Summary of findings' tables and took the lead in writing and updating of the review.
Joyce Danielle Asseler performed selection of trials and contributed to the writing of the review.
Ben Cohlen performed selection of trial and contributed to the writing of the review
Susanne Veltman‐Verhulst performed selection of trials, data extraction and analyses and contributed to the writing of the review
Sources of support
Internal sources
No sources of support supplied
External sources
Van Harreveld Stichting, Netherlands.
Marco Polo fonds, Netherlands.
Stichting de Korinthiers, Netherlands.
Jan Kornelis de Kock Stichting, Netherlands.
Declarations of interest
Reuben Ayeleke: none known. Joyce Danielle Asseler: none known. Ben Cohlen: none known. Susanne Veltman‐Verhulst: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Agarwal 2004 {published and unpublished data}
- Agarwal S, Mittal S. A randomised prospective trial of intrauterine insemination versus timed intercourse in superovulated cycles with clomiphene. Indian Journal of Medical Research 2004;120(6):519‐22. [PubMed] [Google Scholar]
Arcaini 1996 {published data only}
- Arcaini L, Bianchi S, Baglioni A, Marchini M, Tozzi L, Fedele L. Superovulation and intrauterine insemination vs. superovulation alone in the treatment of unexplained infertility. A randomized study. Journal of Reproductive Medicine 1996;41(8):614‐8. [PubMed] [Google Scholar]
Arici 1994 {published and unpublished data}
- Arici A, Byrd W, Bradshaw K, Kutteh WH, Marshburn P, Carr BR. Evaluation of clomiphene citrate and human chorionic gonadotropin treatment: A prospective, randomized, crossover study during intrauterine insemination cycles. Fertility and Sterility 1994;61(2):314‐8. [DOI] [PubMed] [Google Scholar]
Bhattacharya 2008 {published data only}
- Bhattacharya S, Harrild K, Mollison J, Wordsworth S, Tay C, Harrold A, et al. Clomifene citrate or unstimulated intrauterine insemination compared with expectant management for unexplained infertility: pragmatic randomised controlled trial. BMJ 2008;7(337):a716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wordsworth SA, Buchanan JA, Mollison JA, Harrild KA, Robertson LA, Tay C, et al. Clomifene citrate and intrauterine insemination as first‐line treatments for unexplained infertility: are they cost‐effective?. Human Reproduction 2011;26(2):369‐75. [DOI] [PubMed] [Google Scholar]
Chung 1995 {published data only}
- Chung CC, Fleming R, Jamieson ME, Yates RW, Coutts JR. Randomized comparison of ovulation induction with and without intrauterine insemination in the treatment of unexplained infertility. Human Reproduction 1995;10(12):3139‐41. [DOI] [PubMed] [Google Scholar]
Crosignani 1991 {published data only}
- Crosignani PG, Walters DE, Soliani A. The ESHRE multicentre trial on the treatment of unexplained infertility: a preliminary report (Centre 10: Hedon, Montpellier). Human Reproduction 1991;6(7):953‐8. [DOI] [PubMed] [Google Scholar]
- Crosignani PG, Walters DE, Soliani A. The ESHRE multicentre trial on the treatment of unexplained infertility: a preliminary report (Centre 13: Willemsen, Nijmegen). Human Reproduction 1991;6(7):953‐8. [DOI] [PubMed] [Google Scholar]
- Crosignani PG, Walters DE, Soliani A. The ESHRE multicentre trial on the treatment of unexplained infertility: a preliminary report (Centre 16: Pellicer, Valencia). Human Reproduction 1991;6(7):953‐8. [DOI] [PubMed] [Google Scholar]
- Crosignani PG, Walters DE, Soliani A. The ESHRE multicentre trial on the treatment of unexplained infertility: a preliminary report (Centre 19: Martinez, Amsterdam). Human Reproduction 1991;6(7):953‐8. [DOI] [PubMed] [Google Scholar]
Deaton 1990 {published data only}
- Deaton J, Gibson M, Blackmer K, Nakajima S, Badger G, Brumsted J. A randomized controlled trial of clomiphene citrate and intrauterine insemination in couples with unexplained infertility on surgically corrected endometriosis. Fertility and Sterility 1990;54(6):1083‐8. [PubMed] [Google Scholar]
Farquhar 2018 {published data only}
- Farquhar CM, Liu E, Armstrong S, Arroll N, Lensen S, Brown J. Intrauterine insemination with ovarian stimulation versus expectant management for unexplained infertility (TUI): a pragmatic, open‐label, randomised, controlled,two‐centre trial. Lancet 2018;391(10119):441‐50. [DOI] [PubMed] [Google Scholar]
- Liu E, Farquhar C, Armstrong S, Arroll N, Lensen S, Brown J. Randomised controlled trial of intrauterine insemination with ovarian stimulation for unexplained infertility. Australian & New Zealand Journal of Obstetrics & Gynaecology 2017;57(S1):11. [Google Scholar]
Goverde 2000 {published data only}
- Goverde AJ, McDonnell J, Vermeiden JP, Schats R, Rutten FF, Schoemaker J. Intrauterine insemination or in‐vitro fertilisation in idiopathic subfertility and male subfertility: a randomised trial and cost‐effectiveness analysis. Lancet 2000;355(9197):13‐8. [DOI] [PubMed] [Google Scholar]
Guzick 1999 {published data only}
- Guzick D, Carson S, Coutifaris C, Overstreet J, Factor‐Litvak P, Steinkampf MP, et al. Efficacy of superovulation and intrauterine insemination in the treatment of infertility. New England Journal of Medicine 1999;340(3):177‐83. [DOI] [PubMed] [Google Scholar]
Janko 1998 {published data only}
- Janko P, Hruzik P, Pruzinec J, Saliba H, Zidzik J. Induction of ovulation with or without intrauterine insemination in cases of unexplained sterility. Fertility and Sterility 1998;70(3):S442. [Google Scholar]
Karlstrom 1993 {published data only}
- Karlstrom P, Bergh T, Lundkvist O. A prospective randomized trial of artificial insemination versus intercourse in cycles stimulated with human menopausal gonadotropin or clomiphene citrate. Fertility and Sterility 1993;59(3):554‐9. [PubMed] [Google Scholar]
Melis 1995 {published and unpublished data}
- Melis GB, Paoletti AM, Ajossa S, Guerriero S, Depau GF, Mais V. Ovulation induction with gonadotropins as sole treatment in infertile couples with open tubes: a randomized prospective comparison between intrauterine insemination and timed vaginal intercourse. Fertility and Sterility 1995;64(6):1088‐93. [DOI] [PubMed] [Google Scholar]
Murdoch 1991 {published and unpublished data}
- Murdoch AP, Harris M, Mahroo M, Williams M, Dunlop W. Gamete intrafallopian transfer (GIFT) compared with intrauterine insemination in the treatment of unexplained infertility. British Journal of Obstetrics and Gynaecology 1991;98(11):1107‐11. [DOI] [PubMed] [Google Scholar]
Steures 2006a {published data only}
- Custers IM, Rumste MM, Steeg JW, Wely MA, Hompes PG, Bossuyt P, et al. Long‐term outcome in couples with unexplained subfertility and an intermediate prognosis initially randomized between expectant management and immediate treatment. Human Reproduction 2012;27(2):444‐50. [DOI] [PubMed] [Google Scholar]
- Steures P, Van der Steeg JW, Hompes PG, Habbema JD, Eijkemans MJ, Broekmans FJ, et al. Intrauterine insemination with controlled ovarian hyperstimulation versus expectant management for couples with unexplained subfertility and an intermediate prognosis: a randomised clinical trial. Lancet 2006;368(9531):216‐2. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aanesen 2014 {published data only}
- Aanesen A, Westerbotn M. Prospective study of a Swedish infertile cohort 2005‐08: Population characteristics, treatments and pregnancy rates. Family Practice. 2014;31(3):290‐7. [DOI] [PubMed] [Google Scholar]
Aboulghar 1993 {published data only}
- Aboulghar MA, Mansour RT, Serour GI, Amin Y, Abbas AM, Salah IM. Ovarian superstimulation and intrauterine insemination for the treatment of unexplained infertility. Fertility and Sterility 1993;60(2):303‐6. [DOI] [PubMed] [Google Scholar]
Barros Delgadillo 2008 {published data only}
- Barros Delgadillo JC, Martinez Barrios E, Moreno Aburto C, Godines Enriquez MS, Manzur Navarrete F, Sanchez Solis V, et al. Intrauterine insemination versus programmed intercourse in cycles of controlled ovaric hyperstimulation. Ginecologia y Obstetricia de Mexico 2008;76(1):18‐31. [PubMed] [Google Scholar]
Barros‐Delgadillo 2010 {published data only}
- Barros‐Delgadillo JC, Trejo‐Castaneda H, E‐Ormsby C, Gavino‐Gavino F. Differing response to GnRH antagonists in cycles of ovarian hyperstimulation plus intrauterine insemination. Ginecologia y Obstetricia de Mexico 2010;78(1):15‐28. [PubMed] [Google Scholar]
Check 2013 {published data only}
- Check JH, Liss J, Bollendorf A. Intrauterine insemination (IUI) does not improve pregnancy rates in infertile couples where semen parameters are normal and postcoital tests are adequate. Clinical and Experimental Obstetrics and Gynecology 2013;40(1):33‐4. [PubMed] [Google Scholar]
Doyle 1991 {published data only}
- Doyle M, DeCherney A. The value of empiric intrauterine insemination (IUI) with superovulation: a prospective‐ randomised clinical trial. Fertility and Sterility 1991;56:S34. [Google Scholar]
Evans 1991 {published data only}
- Evans J, Wells C, Gregory L, Walker S. A comparison of intrauterine insemination‐intraperitoneal insemination and natural intercourse in superovulated women. Fertility and Sterility 1991;56(6):1183‐7. [PubMed] [Google Scholar]
Gregoriou 1995 {published data only}
- Gregoriou O, Vitoratos N, Papadias C, Konidaris S, Gargaropoulos A, Louridas C. Controlled ovarian hyperstimulation with or without intrauterine insemination for the treatment of unexplained infertility. International Journal of Gynaecology and Obstetrics 1995;48:55‐9. [DOI] [PubMed] [Google Scholar]
Ho 1998 {published data only}
- Ho P, Yeung W, So W, Lau E. A randomised trial comparing the efficacy of ovarian stimulation and intrauterine insemination versus ovarian stimulation alone in the treatment of male infertility and unexplained infertility. British Journal of Obstetrics and Gynaecology 1998;105(suppl 17):43. [Google Scholar]
Kabouk 2010 {published data only}
- Kabouk GB, Donadio NF, Dzik A, Freitas GC, Justen R, Cavagna M. A prospective randomized study comparing clomiphene citrate supplemented with recombinant FSH or low‐dose hCG in ovarian stimulation for intrauterine insemination. Fertility and Sterility 2010;94 suppl 1(4):S160 Abstract no. P‐231. [Google Scholar]
Kirby 1991 {published data only}
- Kirby C, Flaherty S, Godfrey B, Warnes G, Matthews C. A prospective trial of intrauterine insemination of motile spermatozoa versus timed intercourse. Fertility and Sterility 1991;56(1):102‐7. [PubMed] [Google Scholar]
Leanza 2014a {published data only}
- Leanza V, Coco L, Grasso F, Leanza G, Zarbo G, Palumbo M, et al. Ovulation induction with clomiphene citrate for infertile couple. Minerva Ginecologica 2014;66(3):309‐12. [PubMed] [Google Scholar]
Leanza 2014b {published data only}
- Leanza V, Coco L, Grasso F, Leanza G, Zarbo G, Palumbo M, et al. Unexplained infertility and ovulatory induction with menopausal gonadotropins. Minerva Ginecologica 2014;66(3):303‐7. [PubMed] [Google Scholar]
Martinez 1990 {published data only}
- Martinez AR, Bernardus RE, Voorhorst FJ, Vermeiden JP, Schoemaker J. Intrauterine insemination does and clomiphene citrate does not improve fecundity in couples with infertility due to male or idiopathic factors: a prospective, randomized, controlled study. Fertility and Sterility 1990;53(5):847‐53. [DOI] [PubMed] [Google Scholar]
Martinez 1991 {published data only}
- Martinez AR, Bernardus RE, Voorhorst FJ, Vermeiden JP, Schoemaker J. Pregnancy rates after timed intercourse or intrauterine insemination after human menopausal gonadotropin stimulation of normal ovulatory cycles: a controlled study. Fertility and Sterility 1991;55(2):258‐65. [DOI] [PubMed] [Google Scholar]
Nulsen 1993 {published data only (unpublished sought but not used)}
- Nulsen JC, Walsh S, Dumez S, Metzger DA. A randomized and longitudinal study of human menopausal gonadotropin with intrauterine insemination in the treatment of infertility. Obstetrics and Gynecology 1993;82(5):780‐6. [PubMed] [Google Scholar]
Peeraer 2013 {published data only}
- Peeraer KA, Debrock S, Loecker P, Laenen A, Welkenhuyzen M, Spiessens C, et al. Effect of controlled ovarian stimulation with low dose human menopausal gonadotrophin or clomiphene on reproductive outcome after intrauterine insemination: a prospective, multicenter randomized trial. Human Reproduction 2013;28 suppl 1:i71‐i73 O‐172. [Google Scholar]
Prentice 1995 {published data only}
- Prentice A, Sacks GP, Morton NC, Deary AJ, Smith SK. Controlled ovarian stimulation (superovulation) and intrauterine insemination for the treatment of unexplained and minor male factor infertility. Human Reproduction 1995;10:112. [Google Scholar]
Serhal 1988 {published data only (unpublished sought but not used)}
- Serhal PF, Katz M, Little V, Woronowski H. Unexplained infertility ‐ the value of Pergonal superovulation combined with intrauterine insemination. Fertility and Sterility 1988;49(4):602‐6. [DOI] [PubMed] [Google Scholar]
Tummon 1997 {published data only}
- Tummon IS, Asher LJ, Martin JS, Tulandi T. Randomized controlled trial of superovulation and insemination for infertility associated with minimal or mild endometriosis. Fertility and Sterility 1997;68(1):8‐12. [DOI] [PubMed] [Google Scholar]
Van Eekelen 2019 {published data only}
- Eekelen R, Geloven N, Wely M, McLernon DJ, Mol F, Custers IM, et al. Is IUI with ovarian stimulation effective in couples with unexplained subfertility?. Human Reproduction 2019;34(1):84‐91. [DOI] [PubMed] [Google Scholar]
Wadhwa 2013 {published data only}
- Wadhwa LA, Khanna RA, Gupta TA, Gupta SA, Arora SA, Nandwani S. Evaluation of role of GnRH antagonist in intrauterine insemination (IUI) cycles with mild ovarian hyperstimulation (MOH). Fertility and Sterility 2013;100 Suppl(3):S17‐S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xu 2014 {published data only}
- Xu BF, Wang GY, Fan WM, Chen Q, Zhang AJ. Which is the best protocol of ovarian stimulation prior to artificial insemination by donor. Journal of Reproduction and Contraception 2014;25(1):41‐8. [Google Scholar]
Zikopoulos 1993 {published data only}
- Zikopoulos K, West C, Thong P, Kacser E, Morrison J, Wu F. Homologous intra‐uterine insemination has no advantage over timed natural intercourse when used in combination with ovulation induction for the treatment of unexplained infertility. Human Reproduction 1993;8(4):563‐7. [DOI] [PubMed] [Google Scholar]
Zolghadri 2012 {published data only}
- Zolghadri J, Younesi M, Tabibi A, Khosravi D, Behdin S, Vafayee H. Comparison of intrauterine insemination with timed intercourse method in controlled ovarian hyperstimulation. Iranian Journal of Reproductive Medicine 2012;10(Suppl 1):42. [Google Scholar]
References to ongoing studies
NCT03455426 {published data only}
- Huang S, Wang R, Li R, Yan H, Li N, Wang H, et al. Intrauterine insemination (IUI) with or without letrozole for unexplained or mild male factor infertility: a randomized pilot study. Human Reproduction 2019;34(Suppl 1):i149. [DOI] [PubMed] [Google Scholar]
Additional references
Aboulghar 2003
- Aboulghar MA, Mansour RT, Serour GI, Al‐Inany HG. Diagnosis and management of unexplained infertility: an update. Archives of Gynecology and Obstetrics 2003;267(4):177‐88. [DOI] [PubMed] [Google Scholar]
Balasch 2004
- Balasch J. Gonadotrophin ovarian stimulation and intrauterine insemination for unexplained infertility. Reproductive Biomedicine Online 2004;9(6):664‐72. [DOI] [PubMed] [Google Scholar]
Cohlen 1998
- Cohlen BJ, Velde ER, Looman CW, Eijckemans R, Habbema JD. Crossover or parallel design in infertility trials? The discussion continues. Fertility and Sterility 1998;70(1):40‐5. [DOI] [PubMed] [Google Scholar]
Cohlen 2005
- Cohlen B, Cantineau A, D'Hooghe T, Velde E. Multiple pregnancy after assisted reproduction. Lancet 2005;366(9484):452‐3. [DOI] [PubMed] [Google Scholar]
Costello 2004
- Costello MF. Systematic review of the treatment of ovulatory infertility with clomiphene citrate and intrauterine insemination. Australian & New Zealand Journal of Obstetrics & Gynaecology 2004;44(2):93‐102. [DOI] [PubMed] [Google Scholar]
Danhof 2018
- Danhof NA, Wely M, Repping S, Koks C, Verhoeve HR, Bruin JP, et al. Follicle stimulating hormone versus clomiphene citrate in intrauterine insemination for unexplained subfertility: a randomized controlled trial. Human Reproduction 2018;33(10):1866‐74. [DOI: 10.1093/humrep/dey268] [DOI] [PubMed] [Google Scholar]
Daya 1993
- Daya S. Is there a place for the crossover design in infertility trials?. Fertility and Sterility 1993;59(1):6‐7. [DOI] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG, editor(s) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking metaanalyses. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Diamond 2015
- Diamond MP, Richar SL, Coutifaris C, Alvero R, Robinson R, Casson P, et al. Letrozole, gonadotropin,or clomiphene for unexplained infertility. New England Journal of Medicine 2015;373(13):1230‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dias 2008
- Dias S, McNamee R, Vail A. Bias in frequently reported analyses of subfertility trials. Statistics in Medicine 2008;27(27):5605‐19. [DOI] [PubMed] [Google Scholar]
Dickey 2005
- Dickey RP. Risk factors for high‐order multiple pregnancy and multiple birth after controlled ovarian hyperstimulation: results of 4,062 intrauterine insemination cycles. Fertility and Sterility 2005;83(3):671‐83. [DOI] [PubMed] [Google Scholar]
ESHRE 2006
- Andersen AN, Gianaroli L, Felberbaum R, Mouzon J, Nygren KG. Assisted reproductive technology in Europe, 2002. Results generated from European registers by ESHRE. Human Reproduction 2006;21(7):1680‐97. [DOI] [PubMed] [Google Scholar]
Fauser 2005
- Fauser BC, Devroey P, Macklon NS. Multiple birth resulting from ovarian stimulation for subfertility treatment. Lancet 2005;365(9473):1807‐16. [DOI] [PubMed] [Google Scholar]
Gleicher 2000
- Gleicher N, Oleske DM, Tur‐Kaspa I, Vidali A, Karande V. Reducing the risk of high‐order multiple pregnancy after ovarian stimulation with gonadotropins. New England Journal of Medicine 2000;343(1):2‐7. [DOI] [PubMed] [Google Scholar]
Goverde 2005
- Goverde AJ, Lambalk CB, McDonnell J, Schats R, Homburg R, Vermeiden JP. Further considerations on natural or mild hyperstimulation cycles for intrauterine insemination treatment: effects on pregnancy and multiple pregnancy rates. Human Reproduction 2005;20(11):3141‐6. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc) available from www.gradepro.org. GRADEpro Guideline Development Tool. McMaster University (developed by Evidence Prime, Inc) available from www.gradepro.org, 2015.
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Hughes 1997
- Hughes EG. The effectiveness of ovulation induction and intrauterine insemination in the treatment of persistent infertility: a meta‐analysis. Human Reproduction 1997;12(9):1865‐72. [DOI] [PubMed] [Google Scholar]
Hughes 2003
- Hughes EG. Stimulated intra‐uterine insemination is not a natural choice for the treatment of unexplained subfertility.'Effective treatment' or 'not a natural choice'?. Human Reproduction 2003;18(5):912‐14. [DOI] [PubMed] [Google Scholar]
Hunault 2004
- Hunault CC, Habbema JD, Eijkemans MJ, Collins JA, Evers JL, Velde ER. Two new prediction rules for spontaneous pregnancy leading to live birth among subfertile couples, based on the synthesis of three previous models. Human Reproduction 2004;19(9):2019‐26. [DOI] [PubMed] [Google Scholar]
Johnson 2003
- Johnson NP, Proctor M, Farquhar CM. Gaps in the evidence for fertility treatment ‐ an analysis of the Cochrane Menstrual Disorders and Subfertility Group database. Human Reproduction 2003;18(5):947‐54. [PubMed] [Google Scholar]
Khan 1996
- Khan KS, Daya S, Collins JA, Walter SD. Empirical evidence of bias in infertility research: overestimation of treatment effect in crossover trials using pregnancy as the outcome measure. Fertility and Sterility 1996;65(5):939‐45. [DOI] [PubMed] [Google Scholar]
Kop 2018
- Kop PAL, Mochtar MH, O’Brien PA, Veen F, Wely M. Intrauterine insemination versus intracervical insemination in donor sperm treatment. Cochrane Database of Systematic Reviews 2018, Issue 1. [DOI: 10.1002/14651858.CD000317.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonnell 2004
- McDonnell J, Goverde AJ, Vermeiden JP. The place of the crossover design in infertility trials: a maximum likelihood approach. Human Reproduction 2004;19(11):2537‐44. [DOI] [PubMed] [Google Scholar]
Nan 1994
- Nan PM, Cohlen BJ, Velde ER, Kooij RJ, Eimers J, Zonneveld O, et al. Intra‐uterine insemination or timed intercourse after ovarian stimulation for male subfertility? A controlled study. Human Reproduction 1994;9(11):2022‐6. [DOI] [PubMed] [Google Scholar]
NICE 2013
- NICE (National Institute for Health and Care Excellence). Assessment and treatment for people with fertility problems. www.nice.org.uk/guidance/cg156 February 2013, issue 156:5. [guidance.nice.org.uk/cg156]
Norman 2000
- Norman GR, Daya S. The alternating‐sequence design (or multiple‐period crossover) trial for evaluating treatment efficacy in infertility. Fertility and Sterility 2000;74(2):319‐24. [DOI] [PubMed] [Google Scholar]
Ombelet 2005
- Ombelet W, Sutter P, Elst J, Martens G. Multiple gestation and infertility treatment: registration, reflection and reaction ‐ the Belgian project. Human Reproduction Update 2005;11(1):3‐14. [DOI] [PubMed] [Google Scholar]
Ombelet 2006
- Ombelet W, Martens G, Sutter P, Gerris J, Bosmans E, Ruyssinck G, et al. Perinatal outcome of 12,021 singleton and 3108 twin births after non‐IVF‐assisted reproduction: acohort study. Human Reproduction 2006;21(4):1025‐32. [DOI] [PubMed] [Google Scholar]
Ragni 2006
- Ragni G, Caliari I, Nicolosi AE, Arnoldi M, Somigliana E, Crosignani PG. Preventing high‐order multiple pregnancies during controlled ovarian hyperstimulation and intrauterine insemination: 3 years' experience using low‐dose recombinant follicle‐stimulating hormone and gonadotropin‐releasing hormone antagonists. Fertility and Sterility 2006;85(3):619‐24. [DOI] [PubMed] [Google Scholar]
RCOG 1998
- Royal College of Obsterricians and Gynaecologists. The management of infertility in secondary care ‐ evidence based guidelines No. 3. RCOG Press, London. London.
Ripps 1994
- Ripps BA, Minhas BS, Carson SA, Buster JE. Intrauterine insemination in fertile women delivers larger numbers of sperm to the peritoneal fluid than intracervical insemination. Fertility and Sterility 1994;61(2):398‐400. [DOI] [PubMed] [Google Scholar]
Ryan 2004
- Ryan GL, Zhang SH, Dokras A, Syrop CH, Voorhis BJ. The desire of infertile patients for multiple births. Fertility and Sterility 2004;81(3):500‐4. [DOI] [PubMed] [Google Scholar]
Schünemann 2017
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. on behalf of the Cochrane Applicability and Recommendations Methods Group. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Snick 2008
- Snick HK, Collins JA, Evers JL. What is the most valid comparison treatment in trials of intrauterine insemination, timed or uninfluenced intercourse? A systematic review and meta‐analysis of indirect evidence. Human Reproduction 2008;23(10):2239‐45. [DOI] [PubMed] [Google Scholar]
Sterne 2017
- Sterne JA, Egger M, Moher D, Boutron I, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Stewart 2003
- Stewart JA. Stimulated intra‐uterine insemination is not a natural choice for the treatment of unexplained subfertility. Should the guidelines be changed?. Human Reproduction 2003;18(5):903‐7. [DOI] [PubMed] [Google Scholar]
Te Velde 1999
- Velde ER, Cohlen BJ. The management of infertility. New England Journal of Medicine 1999;340(3):224‐6. [DOI] [PubMed] [Google Scholar]
Tur 2005
- Tur R, Barri PN, Coroleu B, Buxaderas R, Parera N, Balasch J. Use of a prediction model for high‐order multiple implantation after ovarian stimulation with gonadotropins. Fertility and Sterility 2005;83(1):116‐21. [DOI] [PubMed] [Google Scholar]
Vail 2003
- Vail A, Gardener E. Common statistical errors in the design and analysis of subfertility trials. Human Reproduction 2003;18(5):1000‐4. [DOI] [PubMed] [Google Scholar]
Van Rumste 2006
- Rumste MM, Hartog JE, Dumoulin JC, Evers JL, Land JA. Is controlled ovarian stimulation in intrauterine insemination an acceptable therapy in couples with unexplained non‐conception in the perspective of multiple pregnancies?. Human Reproduction 2006;21(3):701‐4. [DOI] [PubMed] [Google Scholar]
Wang 2019
- Wang R, Danhof NA, Tjon‐Kon‐Fat RI, Eijkemans MJ, Bossuyt PM, Mochtar MH, et al. Interventions for unexplained infertility: a systematic review and network meta‐analysis. Cochrane Database of Systematic Reviews 2019, Issue 9. [DOI: 10.1002/14651858.CD012692.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilcox 1995
- Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation. Effects on the probability of conception, survival of the pregnancy, and sex of the baby. New England Journal of Medicine 1995;333(23):1517‐21. [DOI] [PubMed] [Google Scholar]
Zeyneloglu 1998
- Zeyneloglu HB, Arici A, Olive DL, Duleba AJ. Comparison of intrauterine insemination with timed intercourse in superovulated cycles with gonadotropins: a meta‐analysis. Fertility and Sterility 1998;69(3):486‐91. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Cohlen 1999
- Cohlen BJ, Hughes E, Velde ER, Velde E. Intra‐uterine insemination for unexplained subfertility (Protocol). Cochrane Database of Systematic Reviews 1999, Issue 4. [DOI: 10.1002/14651858.CD001838] [DOI] [PubMed] [Google Scholar]
Veltman‐Verhulst 2006
- Veltman‐Verhulst SM, Cohlen BJ, Hughes E, Heineman MJ. Intra‐uterine insemination for unexplained subfertility. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD001838.pub3] [DOI] [PubMed] [Google Scholar]
Veltman‐Verhulst 2012
- Veltman‐Verhulst SM, Cohlen BJ, Hughes E, Heineman MJ. Intra‐uterine insemination for unexplained subfertility. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD001838.pub4] [DOI] [PubMed] [Google Scholar]
Veltman‐Verhulst 2016
- Veltman‐Verhulst SM, Hughes E, Ayeleke RO, Cohlen BJ. Intra‐uterine insemination for unexplained subfertility. Cochrane Database of Systematic Reviews 2016, Issue 2. [DOI: 10.1002/14651858.CD001838.pub5] [DOI] [PubMed] [Google Scholar]
Verhulst 2005
- Verhulst SM, Cohlen BBJ, Hughes E, Heineman MJ, Velde E. Intra‐uterine insemination for unexplained subfertility (Protocol). Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD001838.pub2] [DOI] [PubMed] [Google Scholar]


