Abstract
Background
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews (2009, Issue 3).Tea is one of the most commonly consumed beverages worldwide. Teas from the plant Camellia sinensis can be grouped into green, black and oolong tea, and drinking habits vary cross‐culturally. C sinensis contains polyphenols, one subgroup being catechins. Catechins are powerful antioxidants, and laboratory studies have suggested that these compounds may inhibit cancer cell proliferation. Some experimental and nonexperimental epidemiological studies have suggested that green tea may have cancer‐preventative effects.
Objectives
To assess possible associations between green tea consumption and the risk of cancer incidence and mortality as primary outcomes, and safety data and quality of life as secondary outcomes.
Search methods
We searched eligible studies up to January 2019 in CENTRAL, MEDLINE, Embase, ClinicalTrials.gov, and reference lists of previous reviews and included studies.
Selection criteria
We included all epidemiological studies, experimental (i.e. randomised controlled trials (RCTs)) and nonexperimental (non‐randomised studies, i.e. observational studies with both cohort and case‐control design) that investigated the association of green tea consumption with cancer risk or quality of life, or both.
Data collection and analysis
Two or more review authors independently applied the study criteria, extracted data and assessed methodological quality of studies. We summarised the results according to diagnosis of cancer type.
Main results
In this review update, we included in total 142 completed studies (11 experimental and 131 nonexperimental) and two ongoing studies. This is an additional 10 experimental and 85 nonexperimental studies from those included in the previous version of the review.
Eleven experimental studies allocated a total of 1795 participants to either green tea extract or placebo, all demonstrating an overall high methodological quality based on 'Risk of bias' assessment. For incident prostate cancer, the summary risk ratio (RR) in the green tea‐supplemented participants was 0.50 (95% confidence interval (CI) 0.18 to 1.36), based on three studies and involving 201 participants (low‐certainty evidence). The summary RR for gynaecological cancer was 1.50 (95% CI 0.41 to 5.48; 2 studies, 1157 participants; low‐certainty evidence). No evidence of effect of non‐melanoma skin cancer emerged (summary RR 1.00, 95% CI 0.06 to 15.92; 1 study, 1075 participants; low‐certainty evidence). In addition, adverse effects of green tea extract intake were reported, including gastrointestinal disorders, elevation of liver enzymes, and, more rarely, insomnia, raised blood pressure and skin/subcutaneous reactions. Consumption of green tea extracts induced a slight improvement in quality of life, compared with placebo, based on three experimental studies.
In nonexperimental studies, we included over 1,100,000 participants from 46 cohort studies and 85 case‐control studies, which were on average of intermediate to high methodological quality based on Newcastle‐Ottawa Scale 'Risk of bias' assessment. When comparing the highest intake of green tea with the lowest, we found a lower overall cancer incidence (summary RR 0.83, 95% CI 0.65 to 1.07), based on three studies, involving 52,479 participants (low‐certainty evidence). Conversely, we found no association between green tea consumption and cancer‐related mortality (summary RR 0.99, 95% CI 0.91 to 1.07), based on eight studies and 504,366 participants (low‐certainty evidence).
For most of the site‐specific cancers we observed a decreased RR in the highest category of green tea consumption compared with the lowest one. After stratifying the analysis according to study design, we found strongly conflicting results for some cancer sites: oesophageal, prostate and urinary tract cancer, and leukaemia showed an increased RR in cohort studies and a decreased RR or no difference in case‐control studies.
Authors' conclusions
Overall, findings from experimental and nonexperimental epidemiological studies yielded inconsistent results, thus providing limited evidence for the beneficial effect of green tea consumption on the overall risk of cancer or on specific cancer sites.
Some evidence of a beneficial effect of green tea at some cancer sites emerged from the RCTs and from case‐control studies, but their methodological limitations, such as the low number and size of the studies, and the inconsistencies with the results of cohort studies, limit the interpretability of the RR estimates. The studies also indicated the occurrence of several side effects associated with high intakes of green tea. In addition, the majority of included studies were carried out in Asian populations characterised by a high intake of green tea, thus limiting the generalisability of the findings to other populations. Well conducted and adequately powered RCTs would be needed to draw conclusions on the possible beneficial effects of green tea consumption on cancer risk.
Plain language summary
Green tea for the prevention of cancer
Background There is a high consumption worldwide of green tea (Camellia sinensis), that contains polyphenols which have a powerful antioxidant activity that can prevent the formation of free radicals that may cause damage and cell death. Therefore it has been suggested that green tea might reduce cancer risk, a theory that has been tested through a number of studies on human populations, which examined the link between green tea consumption and cancer.
The aim of the review We assessed the association between green tea consumption and the risk of developing cancer in epidemiologic studies.
Main findings In this review we included 142 studies with more than 1.1 million participants looking for an association between green tea consumption and cancers of the digestive tract and the female reproductive system, breast, prostate, kidney and urinary tract, nasopharynx, lung, blood, skin, thyroid and brain. The majority of the studies were of medium to high quality in terms of how they were conducted. Overall, the evidence from the studies showed that the consumption of green tea to reduce the risk of cancer was inconsistent.
Some studies suggested a beneficial effect on cancer risk, while others indicated no effect, and even suggested a slightly increased cancer risk. In particular, results from experimental studies suggested that green tea extract supplementation yielded a decreased risk for prostate cancer, but increased risk for gynaecological cancers. For non‐melanoma skin cancer no difference in cancer cases emerged. Green tea supplementation seemed to slightly improve quality of life compared with placebo, although it was associated with some adverse effects including gastrointestinal disorders, higher levels of liver enzymes, and, more rarely, insomnia, raised blood pressure and skin reactions.
In nonexperimental studies, comparing people consuming the highest amount of green tea to those in the lowest category of consumption, we found an indication of a lower occurrence of new cases of overall types of cancer, while no difference emerged for lethal cases. However, results according to the type of cancer and study design were inconsistent.
What are the conclusions? A beneficial effect of green tea consumption on cancer prevention remains unproven so far. Caution is advised regarding supplementation with high‐dose green tea extracts due to the possible adverse effects.
Summary of findings
Background
This review is an update of a previously published Cochrane review (Boehm 2009).
Description of the intervention
Tea (Camellia sinensis) is the most highly consumed manufactured drink in the world (FAO 2018). Between 2007 and 2016, world tea production grew by an average annual rate of 4.4%. Global tea consumption was 5.53 million tonnes in 2016 with an annual growth rate of 4.5% between 2007 and 2016. Three‐quarters of global production is consumed locally, driven particularly by China, India and other emerging economies (FAO 2018). In high‐income countries, consumption is much lower, being generally one‐fifth of that found in low‐ and middle‐income countries. Tea consumption has stabilised in recent years, with a few exceptions (FAO 2015), for example between 1990 and 2014, total tea consumption increased in the USA by about 38% (USDA 2018).
Brewed tea is obtained from the infusion of leaves and buds of Camellia sinensis. The most commonly consumed types of tea are green and black tea. Approximately 20% of the world's Camellia sinensis consumption is in the form of green tea; the other 80% is black (FAO 2015). Tea is characterised by the manufacturing process that the leaves undergo after harvesting. Green tea is made by processing fresh leaves using heat or hot steam immediately after collection, thus minimising any oxidation processes. Conversely, in black tea, the leaves undergo several treatments, including withering by blowing air, preconditioning, 'cut‐tear‐curl', fermentation and final drying, which result in an oxidised tea (Preedy 2014). Depending on these processes, the degree of oxidation may vary greatly, thus influencing the content of antioxidant compounds (Preedy 2014).
Due to the high content of antioxidant compounds, a great deal of attention has been given to green tea with regard to the possible prevention of chronic diseases and cancer (Eisenstein 2019; Yang 2019), as well as possible beneficial effects on cardiovascular disease, insulin sensitivity and lipid profiles (Liu 2013b; Yang 2019; Yu 2017).
How the intervention might work
Pharmacology of Camellia sinensis
The active ingredients of green tea include polyphenols most of which are flavonols, commonly known as catechins. These account for 30% to 40% of the extractable solids of dried green tea leaves. Other active ingredients are alkaloids, such as caffeine and theobromine, carbohydrates, and minerals and other trace elements, such as fluoride and aluminium (Coppock 2016; Filippini 2019; Milani 2019; Yang 2019). Green tea contains higher amounts of catechins than black tea (Peluso 2017), and green tea processing prevents oxidation (Chen 2007). After fermentation from green to black tea, about 15% of catechins remain unchanged while the rest of the catechins are converted to theaflavins, which are polyphenol pigments and thearubigins (Blumenthal 2003). Brewing conditions, including water temperature and infusion time, influence the antioxidant capacity of green tea (Sharpe 2016).
The catechins found in green tea include epigallocatechin‐3‐gallate (EGCG), epigallocatechin, epicatechin‐3‐gallate and epicatechin, gallocatechins and gallocatechin gallate. EGCG is the predominant and most studied catechin in green tea (Peluso 2017; Yang 2019), as it is a powerful antioxidant believed to be an important determinant of the therapeutic qualities of green tea (Chen 2019; Gao 2016; Peluso 2017). It is suggested that EGCG works by suppressing the formation of new blood vessels (angiogenesis) and regulating their permeability, thereby cutting off the blood supply to cancerous cells (Demeule 2002; Diniz 2017; Maiti 2003; Rashidi 2017; Yang 2019). In vitro studies and in vivo animal models have shown EGCG to be a potent chemo‐preventative agent (Liao 2001; Shirakami 2018; Xu 2019).
Green tea catechins have also been shown to decrease plasma lipid peroxide and malondialdehyde concentrations, to increase plasma ascorbate concentrations, to decrease non‐haem iron absorption, and increase the resistance of low‐density lipoproteins to oxidation (Williamson 2005). It is recognised that most classes of catechins are sufficiently well absorbed to have the potential to induce biological effects, since they cross the intestinal barrier and reach concentrations in the blood stream that have been shown in vitro to exert effects (Liao 2001; Manach 2005; Scalbert 2000). They are reported to be rapidly absorbed and eliminated in humans. Peak plasma concentrations were observed between one to three hours after oral administration and reached total catechin concentrations in the sub‐ or low‐μM range, and with a half‐life of two to four hours. Parent flavonoids are deglycosylated during digestion, are absorbed in the small intestine, and appear in the blood as phase II metabolites (Williamson 2018). Pharmacokinetic studies show that the flavonoid epicatechin is absorbed in the small intestine with a number of structural‐related epicatechin metabolites (SREM) attaining sub‐μmol/L peak plasma concentrations (Cmax) approximately one hour after ingestion (Borges 2018). The SREMs are excreted in urine over a 24‐hour period in amounts corresponding to 20% of epicatechin intake. If unabsorbed along the small intestine epicatechin undergoes microbiota‐mediated conversions in the colon, which, being absorbed, appear in plasma as phase II metabolites with a Cmax of 5.8 hours after consumption, and they are excreted in quantities equivalent to 42% of the ingested epicatechin (Borges 2018).
Possible mechanisms of action of Camellia sinensis polyphenols
Green tea polyphenols inhibit cell proliferation and viability, and have been shown (primarily in in vitro and ex vivo studies) to exert a powerful antioxidant activity (Ahmad 1999; Romano 2013; Schröder 2019; Yang 1993; Yang 1997). Several mechanisms have been proposed for the potential anticancer activity of green tea catechins (Yang 2019). Their polyphenolic structure allows electron delocalisation, conferring the ability to quench free radicals. EGCG, has been shown to reduce reactive oxygen species, such as superoxide radical, singlet oxygen, hydroxyl radical, peroxyl radical, nitric oxide, nitrogen dioxide and peroxynitrite (Sang 2011). Tea polyphenols are also strong chelators of metal ions, thus hampering the formation of reactive oxygen species. Several hypotheses have been put forward to explain a possible cancer‐preventive activity of catechins (Fujiki 1999; Yang 2019), including counteraction of tumour growth, invasion, metastasis and cell transformation, as well as inhibiting the interaction of tumour promoters, hormones and various growth factors with their receptors (Beltz 2006; Peluso 2017; Rahmani 2015; Rashidi 2017; Yang 2019). However, although in vitro and animal mechanistic studies indicate that flavonoids have anticancer properties, much of the evidence is derived from culture studies using unmetabolised flavonoids, and the simple antioxidant hypothesis is no longer an acceptable explanation (Kerimi 2018). In animal models, where high doses of green tea extracts and constituents have been used, strong evidence for the cancer‐preventive activity of tea constituents has been noted (Yang 2009; Yang 2011b). However, because of differences in endogenous metabolism and gut microflora, animal studies produce data that may not be necessarily be extrapolated to humans (Borges 2016).
Why it is important to do this review
Many reviews have been undertaken in recent years to examine the association between green tea consumption and cancer risk. Examples include a meta‐analysis that concluded that there is a dose‐response relationship between green tea consumption and prevention of prostate cancer when more than seven cups of tea are consumed per day (Guo 2017). Another recent dose‐response meta‐analysis reported a relationship between green tea drinking and prevention of liver cancer, with the downward trend being most obvious when the consumption was increased to four cups per day (Ni 2017). Similarly, dose‐response analysis of green tea consumption and biliary tract cancer suggested that the risk decreased by 4% with each additional cup of tea per day, especially in women (Xiong 2017). A systematic review examining the effect of green tea on risk of breast cancer suggested a protective effect (Gianfredi 2018), whereas another was inconclusive (Najaf 2018). With regard to gastric cancer, the effect of green tea may be temperature‐dependent, with high‐dose, long‐term consumption reducing the risk, whereas very high‐temperature green tea may possibly increase the risk of gastric cancer (Huang 2017).
Since several additional experimental and nonexperimental epidemiological studies have become available since the previous version of this Cochrane Review, we undertook an update to assess the relationship between peoples' green tea or EGCG consumption and cancer risk.
Objectives
To assess possible associations between green tea consumption and the risk of cancer incidence and mortality as primary outcomes, and safety data and quality of life as secondary outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We included studies in which participants consumed green tea orally, either as drinkable tea or as extracts. Studies used one of the following designs.
Experimental studies: randomised controlled trials (RCTs)
Nonexperimental studies: both cohort and case‐control observational studies
We did not consider case‐series, case reports and other studies without a comparator, editorials, reviews, animal studies and in vitro studies for this review.
Types of participants
Adult participants (18 years of age and older).
Types of interventions
We were interested in studies that focused on the consumption of green tea, either as part of an intervention (experimental) study or measured in a nonexperimental study. The exposure variable was the consumption of green tea or green tea extract (only monotherapy preparations for oral consumption in liquid, powder or tablet form). We excluded studies that used green tea extract supplementation as part of a multi‐component preparation if they did not include a study arm using green tea extracts in monotherapy.
We defined green tea as non‐fermented tea leaves, and studies had to mention that green tea, non‐fermented tea or 'matsu‐cha', as it is called in Asia, had been consumed. We considered any method of quantifying this variable (e.g. direct recording, recall questionnaire) as potentially valid. We excluded studies that did not distinguish the type of tea (e.g. black tea versus green tea) or did not report quantitative data of at least two different amounts or frequencies of green tea consumption.
We excluded pharmacokinetic‐type studies because they were unlikely to contribute useful data on long‐term effects of green tea.
Only studies reporting the duration of green tea consumption in their summary were included.
Types of outcome measures
Primary outcomes
The primary outcome measures were:
the number of participants developing cancer (incidence);
the number of participants dying from cancer (mortality).
Results from nonexperimental epidemiological studies had to include an estimate of the risk ratio (RR), or sufficient data for us to calculate it.
We used the following categories to combine and analyse different types of cancer.
Gastrointestinal cancers: including oral cancer, pharyngeal cancer, laryngeal cancer, oesophageal cancer, stomach cancer, liver cancer, pancreatic cancer, biliary tract cancer, and colorectal cancer
Respiratory tract cancer: including nasopharyngeal cancer, lung cancer, and mesothelioma
Breast cancer
Urogenital tract cancers: including prostate cancer, endometrial cancer, ovarian cancer, renal cancer, and urinary tract cancer
Haematological cancers: including haematopoietic cancer, leukaemia, lymphoma, and multiple myeloma
All other types of cancer
Secondary outcomes
Safety data and data on quality of life
Search methods for identification of studies
Electronic searches
For the original review we searched the following electronic databases from inception to January 2009 to retrieve studies for potential inclusion: the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (Ovid), Embase (Ovid), Amed, CancerLit, PsychInfo and Phytobase.
For this update we searched the following electronic databases up to January 2019:
the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 1) in the Cochrane Library (Appendix 1);
MEDLINE via Ovid (January 2009 to January week 1, 2019) (Appendix 2);
Embase via Ovid (January 2009 to 2019 Week 1) (Appendix 3).
Searching other resources
We systematically checked references from published studies for further studies. We specifically screened the reference list from studies retrieved in full text, and also from previous systematic reviews and meta‐analysis on the topic, including non‐English papers, though we assumed that some of the articles from Asian countries would not be retrievable via Western medical databases. We obtained all relevant non‐English articles and a Japanese/Chinese Cochrane collaborator acted as a filter for study selection. Publications in languages other than English were translated in‐house or by using relevant services. Finally, we also checked for other relevant studies in the clinical trials registry ClinicalTrials.gov up to January 2019.
Data collection and analysis
Selection of studies
To be included, studies had to report on the consumption of green tea, non‐fermented tea or 'matsu‐cha'. Two review authors checked studies identified by the searches and included articles on initial screen only if they could determine from the abstract that the article was a report of either an experimental intervention or a nonexperimental study. When we could not reject with certainty a title or abstract, we assessed the full text.
Two review authors independently analysed the full text of all potentially relevant eligible studies. Reasons for excluding any study are stated in Criteria for considering studies for this review. All disagreements were resolved by discussion between the two review authors. If any data were missing from the study reports, we attempted to obtain the data by contacting the study authors.
Data extraction and management
Two review authors independently performed data extraction using pre‐defined and pre‐tested data extraction forms. We resolved discrepancies by discussion. We categorised studies into experimental (RCTs) and nonexperimental studies (i.e. cohort studies, including cohort‐nested studies, and case‐control studies). We also grouped data according to study design and cancer type. We entered the extracted data into Review Manager 5 (Review Manager 2014), and two review authors double‐checked the entries.
Assessment of risk of bias in included studies
Four review authors independently assessed the risk of bias of the included studies.
Experimental studies
We used the Cochrane 'Risk of bias' tool to assess risk of bias in the included RCTs (Higgins 2017). The criteria relate to the following domains:
Selection bias: random sequence generation and allocation concealment
Performance bias: blinding of participants and personnel (i.e. treatment providers)
Detection bias: blinding of outcome assessment
Attrition bias: incomplete outcome data
Reporting bias: selective reporting of outcomes
other possible sources of bias (please specify)
We considered studies that we assessed as 'adequate' in all main domains to be at low risk of bias. Studies in which there was no clear judgement concerning the procedures in one or more key domains we considered to be at least of medium risk of bias. Studies with clearly inadequate procedures in one or more of the key domains we considered to be at high risk of bias.
Nonexperimental studies
We used the Newcastle‐Ottawa Scale (NOS) to assess the methodological quality of epidemiologic studies (Wells 2001). The NOS is based on a 'star' system in which a study is assessed on three broad perspectives:
selection of study groups;
comparability of the groups;
ascertainment of outcome or exposure of interest for cohort or case‐control studies, respectively.
High‐quality answers to each NOS question are identified with a star/asterisk. Details used during the evaluation are reported in two templates, one for cohort (Appendix 4), and one for case‐control studies (Appendix 5). Both cohort and case‐control studies can receive a maximum of nine stars or points. We considered studies with six or fewer points as low quality, with seven to eight points as medium quality, and with nine points as high quality.
Measures of treatment effect
We used the following measures of the effect of treatment or exposure.
For dichotomous outcomes (i.e. cancer risk), we used the risk ratio (RR) for both experimental and nonexperimental studies.
For continuous outcomes (evaluation of scores for quality of life), we used the mean difference between treatment arms in experimental studies.
Unit of analysis issues
We did not note any unit of analysis issues.
Dealing with missing data
When a study had missing data in the level of exposure assessment, risk estimates or confidence intervals, we attempted to obtain the data by contacting the study authors. Nevertheless, we reported the available data in Characteristics of included studies. We did not impute missing data for any of the outcomes for data analysis.
Assessment of heterogeneity
We used the Chi2 test for heterogeneity and the I2 statistic (Higgins 2003), to quantify heterogeneity of study results. We interpreted the I2 statistic as per guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017): 0% to 40% might not be important; 30% to 60% represented moderate heterogeneity; 50% to 90% represented substantial heterogeneity; and 75% to 100% represented considerable heterogeneity.
Assessment of reporting biases
We followed the recommendations for testing for funnel plot asymmetry as described in the Cochrane Handbook for Systematic Reviews of Interventions (Sterne 2017). Funnel plot asymmetry may be due to reporting bias. We produced funnel plots to assess the potential for small‐study effects when at least five studies reported results for the same type of cancer (Egger 1997).
Data synthesis
We carried out a meta‐analysis of the included studies when the study results reported estimate for cancer risk or we could compute it from raw data. When studies reported more than one estimated risk, we used the results generated by the most adjusted model. We used a random‐effects model for all analyses. We carried out and reported overall analyses and analyses stratified by study design.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analysis for any cancer incidence and mortality and, whenever possible, for all different types of cancer site according to categories identified in Primary outcomes. In order to investigate possible sources of heterogeneity, we also performed stratified analyses according to study design of nonexperimental studies (hospital‐based case‐control design, population‐based case‐control design, cohort design).
Sensitivity analysis
We performed the following sensitivity analyses:
study design of nonexperimental studies (hospital‐based case‐control design, population‐based case‐control design, cohort design).
Summary of findings and assessment of the certainty of the evidence
We presented the overall certainty of evidence of all outcomes investigated in the experimental studies, namely prostate cancer, gynaecological cancer and non‐melanoma skin cancer. We also presented the certainty of evidence for the primary outcomes of nonexperimental studies and for which it was possible to evaluate publication bias, that is, when at least five studies reported results for the same type of cancer (Egger 1997).
We evaluated the overall certainty of evidence according to the GRADE approach (Atkins 2004), which takes into account issues related not only to internal validity (risk of bias, inconsistency, imprecision, publication bias) but also to external validity, such as directness of results (Langendam 2013). We created three 'Summary of findings' tables (Table 1; Table 2; Table 3), adhering to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2017), and using GRADEpro GDT. We used the GRADE checklist and GRADE Working Group certainty of evidence definitions (Meader 2014), as follows.
Summary of findings 1. Green tea extract supplementation compared with placebo for preventing cancer: experimental studies.
| Green tea extract supplementation compared with placebo for cancer prevention: experimental studies | |||||||
|
Patient or population: adults (aged at least 18 years) Settings: outpatients Intervention: green tea extract supplementation Comparison: placebo | |||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) (studies) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments/explanations | ||
| Placebo | Green tea extract supplementation | Difference | |||||
| Prostate cancer incidence | RR 0.50 (0.18 to 1.36) | 22.0% | 11.7% (4.4 to 28.7) |
10.3% fewer (17.6 fewer to 6.7 more) |
201 (3 RCTs) |
⊕⊕⊝⊝ Low | Very large effects, but all participants were at high risk of prostate cancer at the time of recruitment, with high‐grade prostatic intraepithelial neoplasia and/or atypical small acinar proliferation less than 3 months before, thus the indirectness in transferring the results to the general population, high imprecision of the summary estimates based on only 201 participants and 32 cases, and high inconsistency between study results |
| Gynaecological cancer incidence | RR 1.50 (0.41 to 5.48) | 0.9% | 1.3% (0.4 to 4.6) |
0.4% more (0.5 fewer to 3.7 more) |
1157 (2 RCTs) |
⊕⊕⊝⊝ Low | Large effects, but high imprecision of the summary RR and high inconsistency of results due to contradictory findings from two available studies. |
| Non‐melanoma skin cancer incidence | RR 1.00 (0.06 to 15.92) | 0.2% | 0.2% (0.0 to 2.9) |
0.0% fewer (0.0 fewer to 2.7 more) |
1075 (1 RCT) |
⊕⊕⊝⊝ Low | Very high imprecision based on only one study and no large effect detected. |
| Quality of life | Not estimable | ‐ | ‐ | ‐ | ‐ | Not assessed | Due to the large number of different scales used it was not possible to the overall certainty of evidence. |
| The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||||
Summary of findings 2. Highest compared with lowest green tea exposure for preventing cancer: primary outcomes in nonexperimental studies.
| Highest compared with lowest green tea exposure for preventing cancer in nonexperimental studies | ||||
|
Patient or population: adults (aged at least 18 years) Setting: outpatient Intervention: highest green tea exposure Comparison: lowest green tea exposure | ||||
| Outcomes (number of studies) | Relative effect (95% CI) | Number of participants (number of cases) | Certainty of the evidence (GRADE) | Comments |
|
Any cancer incidence (3 studies) |
RR 0.83 (0.65 to 1.07) | 52,479 (4962 cases) | ⊕⊕⊝⊝ Low | Largea but imprecise effect. Similar but imprecise effect from the 2 cohort studies (RR 0.81, 95% CI 0.50 to 1.32) |
|
Any cancer mortality (8 studies) |
RR 0.99 (0.91 to 1.07) | 504,366 (21,439 cases) | ⊕⊕⊝⊝ Low | Not a large effect. All cohort studies |
| The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||
aUpgrading criteria for nonexperimental studies considered are: large effect estimates.
Summary of findings 3. Highest compared with lowest green tea exposure for preventing cancer: individual cancer types in nonexperimental studies.
| Highest compared with lowest green tea exposure for preventing cancer in nonexperimental studies | ||||
|
Patient or population: adults (aged at least 18 years) Setting: outpatient Intervention: highest green tea exposure Comparison: lowest green tea exposure | ||||
| Outcomes (Number of studies) | Relative effect (95% CI) | Number of participants (number of cases) | Certainty of the evidence (GRADE) | Comments |
|
Oral cancer risk (5 studies) |
RR 0.71 (0.62 to 0.82 | 55,977 (2343 cases) | ⊕⊕⊝⊝ Low | Large effecta, but possible serious risk of bias due to case‐control studies. Smaller but more imprecise effect from the single cohort study (RR 0.44, 95% CI 0.19 to 1.04) |
|
Any gut cancer risk (7 studies) |
RR 0.78 (0.59 to 1.02) | 70,299 (3191 cases) | ⊕⊕⊝⊝ Low | Large effecta, but possible serious risk of bias due to case‐control studies. Smaller but more imprecise decreased risk for cohort studies alone (RR 0.86, 95% CI 0.27 to 2.79; 2 studies) |
|
Oesophageal cancer risk (13 studies) |
RR 0.81 (0.64 to 1.04) | 74,895 (4595 cases) | ⊕⊝⊝⊝ Very low | Large effecta, but possible serious risk of bias due to case‐control studies. Possible publication bias. Increased though highly imprecise risk from the single cohort study (RR 1.67, 95% CI 0.88 to 3.16) |
|
Stomach cancer risk (18 studies) |
RR 0.86 (0.74 to 1.01) | 438,595 (10,183 cases) | ⊕⊝⊝⊝ Very low | Large effecta, but possible serious risk of bias due to case‐control studies. Null risk from cohort studies alone (RR 0.99, 95% CI 0.85 to 1.14; 7 studies) |
|
Liver cancer risk (6 studies) |
RR 0.88 (0.68 to 1.14) | 198,885 (1284 cases) | ⊕⊕⊝⊝ Low | Small but imprecise effect. Mostly cohort studies showing similar but smallest risk (RR 0.93, 0.71 to 1.20; 5 studies) |
|
Pancreatic cancer risk (9 studies) |
RR 0.88 (0.70 to 1.10) | 326,564 (2386 cases) | ⊕⊕⊝⊝ Low | Small but imprecise effect. Possible serious risk of bias due to case‐control studies. Null risk for only cohort studies (RR 1.04, 95% CI 0.84 to 1.30; 6 studies) |
|
Colorectal cancer risk (16 studies) |
RR 0.84 (0.74 to 0.96) | 610,295 (8601 cases) | ⊕⊕⊝⊝ Low | Large effecta, but possible serious risk of bias due to case‐control studies. Null risk for cohort studies alone (RR 1.00, 95% CI 0.92 to 1.08; 9 studies) |
|
Colon cancer risk (10 studies) |
RR 0.89 (0.80 to 0.98) | 389,974 (4118 cases) | ⊕⊕⊝⊝ Low | Large effecta, but possible serious risk of bias due to case‐control studies. Smaller but more imprecise decreased risk for cohort studies alone (RR 0.93, 95% CI 0.82 to 1.05; 6 studies) |
|
Rectal cancer risk (9 studies) |
RR 0.89 (0.75 to 1.05) | 356,851 (2679 cases) | ⊕⊕⊝⊝ Low | Small effect. Smaller but more imprecise effect from only cohort studies (RR 0.92, 95% CI 0.77 to 1.09, 5 studies) |
|
Lung cancer risk (17 studies) |
RR 0.88 (0.76 to 1.02) | 269,565 (9180 cases) | ⊕⊝⊝⊝ Very low | Small effect, but possible serious risk of bias due to case‐control studies. Null risk for cohort studies alone (RR 1.02, 95% CI 0.79 to 1.31; 6 studies) |
|
Breast cancer risk (14 studies) |
RR 0.88 (0.75 to 1.02) | 250,822 (9378 cases) | ⊕⊝⊝⊝ Very low | Small effect, but possible serious risk of bias due to case‐control studies. Null risk for cohort studies alone (RR 1.01, 95% CI 0.86 to 1.19; 5 studies) |
|
Gynaecological cancer risk (10 studies) |
RR 0.69 (0.57 to 0.83) | 66,738 (5506 cases) | ⊕⊕⊝⊝ Low | Large effecta, but possible serious risk of bias due to case‐control studies. Similar but more imprecise effect from the single cohort study (RR 0.75, 95% CI 0.43 to 1.30) |
|
Endometrial cancer risk (5 studies) |
RR 0.77 (0.65 to 0.91) | 60,416 (2835 cases) | ⊕⊕⊝⊝ Low | Large effect, but possible serious risk of bias due to case‐control studies. Smaller but imprecise effect from the single cohort study (RR 0.75, 95% CI 0.43 to 1.30) |
|
Ovarian cancer risk (5 studies) |
RR 0.64 (0.45 to 0.90) | 6,322 (2671 cases) | ⊕⊕⊝⊝ Low | Large effecta, but possible serious risk of bias due to all case‐control studies |
|
Prostate cancer risk (13 studies) |
RR 0.73 (0.56 to 0.94) | 127,239 (2926 cases) | ⊕⊝⊝⊝ Very low | Large effecta, but possible serious risk of bias due to case‐control studies. Increased though imprecise risk for cohort studies alone (RR 1.09, 95% CI 0.89 to 1.32; 5 studies). Possible publication bias |
|
Urinary tract cancer risk (7 studies) |
RR 1.04 (0.79 to 1.37) | 156,039 (2235 cases) | ⊕⊝⊝⊝ Very low | Small and imprecise effect. Increased but imprecise effect from cohort studies alone (RR 1.24, 95% CI 0.87 to 1.76; 3 studies) |
| The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||
aUpgrading criteria for nonexperimental studies considered are: large effect estimates.
High‐certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate‐certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low‐certainty: our confidence in the effect estimate is limited, and the true effect may be substantially different from the estimate of the effect.
Very low‐certainty: we have very little confidence in the effect estimate, and the true effect is likely to be substantially different from the estimate of effect.
Results
Description of studies
Results of the search
For the initial version of this review (Boehm 2009), we identified a total of 675 hits from the literature searches from database inception to January 2009. However, 586 clearly did not match inclusion criteria and were excluded by title and abstract screening. The main reasons for exclusion were that the paper did not investigate people or cancer. Of the remaining 89 papers, we retrieved the full‐text articles and assessed them according to the inclusion criteria provided in the protocol. Thirty‐eight of them did not fulfil the inclusion criteria. The main reasons for exclusion were as follows: no distinction between green and black tea, endpoints other than cancer, frequency of green tea consumption was not specified, or they were duplicate publications. Of the remaining records we identified 51 studies for inclusion (1 RCT, 23 prospective cohort studies and 27 retrospective case‐control studies).
In this update of the original review, we conducted the literature search from January 2009 to January 2019, and we retrieved an additional 2399 hits from database searching. We included a further 21 articles of potential relevance from trials registries and citation chasing (Booth 2008; EUnetHTA 2017). After de‐duplication, we screened a total of 1932 references. Of these, we excluded 1742 references as clearly irrelevant on the basis of the title and abstract review. We then retrieved the full‐text articles for the remaining 190 publications and assessed them for possible relevance. We considered 130 of these publication as eligible for inclusion. For the 60 studies that we excluded, 30 studies reported exposure not including green tea separately, 13 had an ineligible study design (e.g. cross‐sectional studies or pharmacokinetic studies), 11 did not include cancer among the outcomes, four were undertaken in non‐healthy individuals all with cancer, and two studies were from paediatric populations (Figure 1).
1.
PRISMA flow‐chart
In total we identified 181 references (51 from the original review and 130 from the update searches) referring to 144 studies (22 references for 11 experimental studies, 157 references for 131 nonexperimental studies and two ongoing studies) for inclusion in this review (Figure 1; Characteristics of included studies; Characteristics of ongoing studies). Only the number of studies, not the number of references, was subsequently mentioned in the review.
Included studies
Overall, the 142 epidemiological studies of experimental and nonexperimental design considered in this review included over 1,100,000 participants. A total number of 1795 participants were included in experimental studies (Table 4), over 957,000 participants in cohort studies (Table 5), and 47,973 cases and 130,306 referents in case‐control studies (Table 6). Studies were carried out in 10 different countries.
1. Summary characteristics of experimental studies.
| Study | Country | Target cancer | Outcomes | Participants | Intervention | Duration |
| Bettuzzi 2006 | Italy | Prostate cancer | Prostate cancer incidence LUTS PSA values QoL Safety data |
60 men | Green tea total catechins 600 mg/day = EGCG: ~300 mg/day |
12 months |
| Dostal 2015 | USA | Breast cancer | Breast cancer biomarkers Circulating F2‐isoprostane levels Oestrogen metabolite levels Non‐melanoma skin cancer Uterine cancer Safety data |
1075 women | Green tea total catechins: 1315 (± 116) mg/day = EGCG: 843 (± 44) mg/day |
12 months |
| Dryden 2013 | USA | ‐ | Ulcerative disease activity QoL Safety data |
20 men and women | Green tea extracts: Polyphenon E = EGCG: 200 mg or 400 mg/day |
56 days |
| Garcia 2014 | USA | Cervical cancer | Oncogenic HPV clearance CIN1 clearance Safety data |
98 women | Green tea extracts: Polyphenon E = EGCG: 800 mg/day | 4 months |
| Garland 2006 | USA | Lung cancer | Biomarkers of oxidative stress Safety data |
178 (89 men and 89 women) | Green tea extracts: Polyphenon E = ECGC 800 mg/day | 6 months |
| Kumar 2015 | USA | Prostate cancer | Prostate cancer incidence Safety data |
97 men | Green tea extracts: Polyphenon E = EGCG: 400 mg/day | 12 months |
| Lane 2018 | UK | Prostate cancer | PSA levels Clinical outcome (weight and blood pressure) Safety data |
88 men | Green tea extracts = EGCG: 600 mg/day | 6 months |
| Micali 2017 | Italy | Prostate cancer | Prostate cancer incidence PSA levels QoL Safety data |
60 men | Green tea extracts 600 mg = EGCG: 300 mg/day | 12 months |
| Roshdy 2013 | Egypt | Uterine fibroids | Severity of symptoms QoL Safety data |
39 women | Green tea extracts = EGCG: ~400 mg/day | 4 months |
| Sinicrope 2017 | USA | Colon cancer | Change in rectal aberrant crypt foci Safety data |
39 (14 men and 25 women) | Green tea extracts = EGCG: 400 mg/day | 6 months |
| Tsao 2009 | Japan | Oral cancer | Histological response Safety data |
41 (19 men and 22 women) | Green tea extracts 500, 750 or 1000 mg/day | 12 weeks |
| Shannon 2010 | USA | Prostate cancer | Prostate cancer incidence Immunoistochemical response |
67 men out of 120 planned | Green tea polyphenols = EGCG ~600 mg/day) | 12 weeks |
| NCT01496521 | China | Oesophageal cancer | Oesophageal cancer incidence Occurrence of high grade dysplasia Invasive oesophageal squamous cell carcinoma |
Not reported | Tea polyphenols 600 mg/day | 12 months |
| EGCG: (–)‐epigallocatechin‐3‐gallate; CIN: cervical intraepithelial neoplasia; HPV: human papillomavirus LUTS: lower urinary tract symptoms; PSA: prostate‐specific antigens; QoL: quality of life | ||||||
2. Summary characteristics of cohort studies.
| Study | Country | Cohort | Cancer | Outcome | Participants | Cases |
| Allen 2004 | Japan | Life Span Study | Prostate | Incidence | 18,115 men | 193 |
| Chyou 1993 | USA | Honolulu Hearth Program | Bladder | Incidence | 7991 men | 96 |
| Dai 2010 | China | Shangai Women's Health Study | Breast | Incidence | 72,861 | 614 |
| Fujino 2002 in: Inoue 2009a | Japan | JACC Study | Stomach | Incidence | 44,930 | 379 |
| Galanis 1998 | USA | Hawaii Health Surveillance Program | Stomach | Incidence | 11,907 | 108 |
| Hoshiyama 2002 in: Inoue 2009a | Japan | JACC Study | Stomach | Mortality | 44,930 | 359 |
| Ide 2007 | Japan | JACC Study | Oral | Incidence | 50,221 | 37 |
| Inoue 2009a | Japan | JACC, JPHC‐I, JPHC‐II, MIYAGI, 3‐pref MIYAGI, and 3‐pref AICHI Studies | Stomach | Incidence | 219,080 | 3577 |
| Inoue 2009b | Japan | JPHC‐II Study | Liver | Incidence | 18,815 | 110 |
| Ishikawa 2006 | Japan | MIYAGI and 3‐pref MIYAGI Studies | Oesophageal | Incidence | 26,723 | 78 |
| Iwai 2002 | Japan | JACC Study | Any cancer | Mortality | 2855 | 31 |
| Iwasaki 2010a | Japan | JPHC‐I and JPHC‐II Studies | Breast | Incidence | 67,422 | 581 |
| Iwasaki 2010b in: Iwasaki 2010a | Japan | JPHC‐I and JPHC‐II Studies | Breast | Incidence | 67,422 | 144 |
| Key 1999 | Japan | Life Span Study | Breast | Incidence | 34,765 | 405 |
| Khan 2004 | Japan | Public Health Centers in Hokkaido Prefecture | Any cancer Lung Stomach Colorectal Pancreatic |
Mortality | 3158 | 243 51 51 29 25 |
| Kikuchi 2006 | Japan | Ohsaki Cohort Study | Prostate | Incidence | 18,961 | 110 |
| Koizumi 2003 in: Inoue 2009a | Japan | MIYAGI and 3‐pref MIYAGI Studies | Stomach | Incidence | 65,915 | 733 |
| Kurahashi 2007 | Japan | JPHC‐I and JPHC‐II Studies | Prostate | Incidence | 49,920 | 404 |
| Kurahashi 2009 | Japan | JPHC‐I and JPHC‐II Studies | Bladder | Incidence | 104,440 | 206 |
| Kuriyama 2006 | Japan | Ohsaki Cohort Study | Any cancer Lung Stomach Colorectal |
Mortality | 40,530 | 1134 218 193 132 |
| Lee 2007 | Japan | JPHC‐I and JPHC‐II Studies | Colorectal | Incidence | 96,162 | 1158 |
| Li 2008 | Japan | Ohsaki Cohort Study | Lung | Incidence | 41,440 | 302 |
| Li 2018 | China | Kailuan Cohort | Lung | Incidence | 103,010 | 964 |
| Lin 2008 | Japan | JACC Study | Pancreatic | Mortality | 77,850 | 292 |
| Liu 2016 | China | Chinese Prospective Smoking Study | Any cancer | Mortality | 164,681 | 7002 |
| Luo 2007 | Japan | JPHC‐I and JPHC‐II Studies | Pancreatic | Incidence | 102,137 | 233 |
| Makiuchi 2016 | Japan | JPHC‐I and JPHC‐II Studies | Biliary tract | Incidence | 140,420 | 271 |
| Michikawa 2011 | Japan | JPHC‐I and JPHC‐II Studies | Thyroid | Incidence | 100,507 | 159 |
| Montague 2012 | China | Singapore Chinese Health Study | Prostate | Incidence | 27,293 | 298 |
| Nagano 2001 | Japan | Life Span Study | Any cancer and several specific types | Incidence | 38,540 | 4049 |
| Naganuma 2009 | Japan | Ohsaki Cohort Study | Hematopoitic | Incidence | 41,761 | 157 |
| Nakachi 2000 | Japan | Saitama Prefecture | Any cancer | Incidence | 8552 | 488 |
| Nakamura 2011 | Japan | Takayama and Gigu Prefectures | Pancreatic | Mortality | 30,826 | 52 |
| Nechuta 2012 | China | Shangai Women's Health Study | Digestive system | Incidence | 67,230 | 1239 |
| Oba 2006 | Japan | Takayama and Gigu Prefectures | Colon | Incidence | 30,836 | 213 |
| Odegaard 2015 | China | Singapore Chinese Health Study | Any cancer | Mortality | 52,584 | 4092 |
| Ogawa 2016 | Japan | JPHC‐I and JPHC‐II Studies | Brain | Incidence | 106,324 | 155 |
| Saito 2015 | Japan | JPHC‐I and JPHC‐II Studies | Any cancer | Mortality | 90,914 | 5327 |
| Sasazuki 2004 in: Inoue 2009a | Japan | JPHC‐I and JPHC‐II Studies | Stomach | Incidence | 72,943 | 892 |
| Sauvaget 2005 in: Nagano 2001 | Japan | Life Span Study | Stomach | Incidence | 38,576 | 1270 |
| Severson 1989 | USA | Honolulu Hearth Program | Prostate | Incidence | 7821 | 174 |
| Shimazu 2008 | Japan | JPHC‐I and JPHC‐II Studies | Endometrial | Incidence | 53,724 | 117 |
| Sun 2007 | China | Singapore Chinese Health Study | Colorectal | Incidence | 61,320 | 845 |
| Suzuki 2004 | Japan | MIYAGI and 3‐pref MIYAGI Studies | Breast | Incidence | 35,004 | 222 |
| Suzuki 2005 | Japan | MIYAGI and 3‐pref MIYAGI Studies | Colorectal | Incidence | 65,915 | 516 |
| Suzuki 2009 | Japan | Prospective Shizuoka Elderly Cohort | Any cancer Stomach Lung Colorectal |
Mortality | 12,251 | 400 68 88 43 |
| Tamura 2018 | Japan | Takayama and Gigu Prefectures | Liver | Incidence | 30,824 | 172 |
| Tsubono 2001 in: Inoue 2009a | Japan | 3‐pref MIYAGI Study | Stomach | Incidence | 26,311 | 419 |
| Ugai 2017 | Japan | JPHC‐I and JPHC‐II Studies | Lymphoma Multiple myeloma |
Incidence | 95,807 | 411 138 |
| Ugai 2018 | Japan | JPHC‐I and JPHC‐II Studies | Acute myeloid leukaemia | Incidence | 95,807 | 85 |
| Ui 2009 | Japan | Ohsaki Cohort Study | Liver | Incidence | 41,761 | 247 |
| Yang 2007 in: Nechuta 2012 | China | Shangai Women's Health Study | Colorectal | Incidence | 69,710 | 256 |
| Yang 2011a | China | Shangai Men's Health Study | Colorectal | Incidence | 60,567 | 243 |
| Zhao 2017 | China | Shangai Women's Health Study and Shangai Men's Health Study |
Any cancer | Incidence | 115,954 | 3210 |
3. Summary characteristics of case‐control studies.
| Study | Country | Study type | Cancer |
Cases/ Controls |
Sex |
| Bandera 2010 | USA | PCC | Endometrial | 397/373 | Women |
| Berroukche 2012 | Algeria | HCC | Prostate | 160/160 | Men |
| Bonner 2005 | China | PCC | Lung | 122/121 | Both |
| Chen 2011 | China | HCC | Oesophageal | 150/300 | Both |
| Chen 2015 in: Chen 2017a | China | HCC | Oral | 203/572 | Both |
| Chen 2016 in: Chen 2017a | China | HCC | Oral | 207/480 | Women |
| Chen 2017a | China | HCC | Oral | 586/1024 | Both |
| Fu 2013 | China | HCC | Oral | 723/857 | Both |
| Gao 1994 | China | PCC | Oesophageal | 902/1312 | Both |
| Gao 2005 | China | PCC | Endometrial | 955/1087 | Women |
| Gavrilas 2018 | Romania | HCC | Colorectal | 151/151 | Both |
| Goodman 2003 | USA | PCC | Ovarian | 164/194 | Women |
| Goto 1990 | Japan | PCC | Pancreatic | 71/142 | Both |
| Green 2014 | Australia | PCC | Colorectal | 854/948 | Both |
| Hakim 2000 | USA | PCC | Skin | 243/216 | Both |
| Han 2008 | China | PCC | Lung | 523/1924 | Both |
| Hemelt 2010 | China | HCC | Bladder | 419/392 | Both |
| Hoshiyama 1992 | Japan | PCC | Stomach | 251/483 | Men |
| Hoshiyama 2004 in: Inoue 2009a | Japan | Nested case‐cohort | Stomach | 151/256 | Both |
| Hsu 2012 | China | PCC | Nasopharyngeal | 368/317 | Both |
| Huang 1999 | Japan | HCC | Stomach | 887/28,619 | Both |
| Inoue 1994 in: Huang 1999 | Japan | HCC | Stomach | 668/668 | Both |
| Inoue 1998 | Japan | HCC | Oesophageal Stomach Colorectal |
1706/21,128 | Both |
| Inoue 2008 | China | PCC | Breast | 380/662 | Women |
| Islami 2009 | Iran | PCC | Oesophageal | 266/571 | Both |
| Iwasaki 2014 | Japan | HCC | Breast | 369/405 | Both |
| Ji 1996 | China | PCC | Stomach | 1029/1347 | Both |
| Ji 1997 | China | PCC | Colorectal Pancreatic |
2156/1552 | Both |
| Jia 2016 | China | CC | Lung Mesothelioma |
53/106 | Both |
| Jian 2004 | China | HCC | Prostate | 130/274 | Men |
| Jin 2013 | China | PCC | Lung | 799/2020 | Both |
| Kakuta 2009 | Japan | PCC | Endometrial | 152/285 | Women |
| Kato 1990 | Japan | HCC | Colorectal | 221/578 | Both |
| Kato 1990a in: Huang 1999 | Japan | HCC | Stomach | 427/3014 | Both |
| Kono 1988 | Japan | PCC/HCC | Stomach | 139/278 139/2575 |
Both |
| Kubik 2004 in: Kubik 2008 | Czech Republic | HCC | Lung | 435/1710 | Women |
| Kubik 2008 | Czech Republic | HCC | Lung | 1096/2966 | Both |
| Kuo 2009 | China | PCC | Leukaemia | 93/223 | Both |
| Lassed 2016 | Algeria | HCC | Prostate | 90/190 | Men |
| Lee 2017 | China | HCC | Prostate | 404/395 | Men |
| Lei 1994 | China | HCC | Lung | 792/792 | Both |
| Le Marchand 2000 | USA | PCC | Lung | 582/582 | Both |
| Leung 2016 | China | PCC | Ovarian | 104/471 | Women |
| Li 2011a | China | PCC/HCC | Any cancer Breast Colorectal Leukaemia |
425/540 425/540 |
Both |
| Li 2011b in: Mu 2003 | China | PCC | Liver | 204/415 | Both |
| Li 2014 | China | PCC | Prostate | 250/500 | Men |
| Li 2016 | China | HCC | Breast | 756/789 | Women |
| Lin 2012 | China | HCC | Lung | 170/340 | Both |
| Liu 2010 | China | PCC | Stomach | 641/1847 | Both |
| Liu 2017 | China | HCC | Leukaemia | 442/442 | Both |
| Mao 2011 | China | HCC | Stomach | 200/200 | Both |
| Mizoo 2013 | Japan | HCC | Breast | 472/464 | Women |
| Mizuno 1992 | Japan | HCC | Pancreatic | 124/124 | Both |
| Mu 2003 | China | HCC | Stomach Liver Oespphagus |
628/415 | Both |
| Mu 2005 in: Mu 2003 | China | PCC | Stomach | 206/415 | Both |
| Nagle 2010 | Australia | PCC | Ovarian | 1368/1462 | Women |
| Oze 2014 | Japan | PCC | Upper digestive system | 922/2883 | Both |
| Peng 2013 | China | PCC | Colorectal | 672/672 | Both |
| Peng 2015 | China | PCC | Oesphageal | 285/570 | Both |
| Ruan 2010 | China | HCC | Nasopharyngeal | 1355/1459 | Both |
| Setiawan 2001 | China | PCC | Stomach | 133/433 | Both |
| Shrubsole 2009 | China | PCC | Breast | 3371/3380 | Women |
| Song 2008 | USA | PCC | Ovarian | 781/1263 | Women |
| Sonoda 2004 | Japan | HCC | Prostate | 140/140 | Men |
| Tajima 1985 | Japan | HCC | Stomach | 93/93 | Both |
| Takezaki 2000 | Japan | HCC | Pharynx Oesophageal |
346/11,936 | Both |
| Takezaki 2001 | Japan | HCC | Lung | 945/4153 | Both |
| Tewes 1990 | China | HCC | Lung | 200/200 | Women |
| Tse 2017 | China | HCC | Prostate | 431/402 | Men |
| Wakai 2004 | Japan | HCC | Bladder | 124/620 | Both |
| Wang 1999 | China | PCC | Oesophageal and stomach | 209/209 | Both |
| Wang 2006 | China | PCC | Oesophageal | 107/107 | Both |
| Wang 2007 | China | PCC | Oesophageal | 355/209 | Both |
| Wang 2012a | China | HCC | Renal | 250/299 | Both |
| Wang 2012b | China | HCC | Multiple myeloma | 220/220 | Both |
| Wang 2012c | China | PCC | Pancreatic | 908/1067 | Both |
| Wang 2013a | China | HCC | Breast | 157/314 | Women |
| Wang 2013b | USA | HCC | Bladder | 1007/1299 | Both |
| Wang 2015 | China | HCC | Stomach | 160/320 | Both |
| Wilkens 1996 | USA | PCC | Bladder | 261/522 | Both |
| Wu 2003 | USA | PCC | Breast | 501/594 | Women |
| Wu 2009a | China | HCC | Prostate | 142/142 | Men |
| Wu 2009b | China | PCC | Oesophageal | 1,502/3,879 | Both |
| Xu 2007 | China | PCC | Endometrial | 1204/1212 | Women |
| Xu 2013 | China | PCC | Lung | 1225/1234 | Both |
| Yan 2016 | China | PCC | Oral | 593/1128 | Both |
| Ye 1998 | China | PCC | Stomach | 272/544 | Both |
| Yu 1995 | China | PCC | Stomach | 711/711 | Both |
| Zhang 2002 | China | PCC/HCC | Ovarian | 254/261 254/340 |
Women |
| Zhang 2007 | China | HCC | Breast | 1009/1009 | Women |
| Zhang 2008 | China | HCC | Leukaemia | 107/110 | Both |
| Zheng 1993 | China | HCC | Oral | 404/404 | Both |
| Zhong 2001 | China | PCC | Lung | 649/675 | Both |
| HCC: hospital‐based case‐control study; PCC: population‐based case‐control study | |||||
In the experimental group, six studies were carried out in the USA, two in Italy, and one each in the UK, Egypt, and Japan. The ongoing experimental studies are being carried out in the USA and China.
In the nonexperimental group, 63 studies (9 cohort and 54 case‐control) were carried out in China, 50 (34 cohort and 16 case‐control) in Japan, 11 (3 cohort and 8 case‐control) in the USA, two (case‐control) each in Algeria and Australia, and one (case‐control) each in Czech Republic, Iran and Romania. The studies were published between 1985 and 2018. The majority of references (N = 165) were published in English, while 15 were published in Chinese and one in Japanese (Characteristics of included studies).
Outcomes
Of the 46 cohort studies, 37 measured cancer incidence and 9 measured cancer mortality (Iwai 2002; Khan 2004; Kuriyama 2006; Lin 2008; Liu 2016; Naganuma 2009; Odegaard 2015; Saito 2015; Suzuki 2009). All of the 85 case‐control studies assessed any association between green tea consumption and cancer risk. Details of individual study results are reported in Table 7; Table 8. The 11 included RCTs (Bettuzzi 2006; Dostal 2015; Dryden 2013; Garcia 2014; Garland 2006; Kumar 2015; Lane 2018; Micali 2017; Roshdy 2013; Sinicrope 2017; Tsao 2009), and two ongoing studies (Shannon 2010; NCT01496521), investigated, amongst other outcomes, cancer incidence (namely prostate cancer, gynaecological cancers and non‐melanoma skin cancer), quality of life (Bettuzzi 2006; Dryden 2013; Micali 2017; Roshdy 2013), and safety data (Bettuzzi 2006; Dostal 2015; Dryden 2013; Garcia 2014; Garland 2006; Kumar 2015; Lane 2018; Micali 2017; Roshdy 2013; Sinicrope 2017; Tsao 2009). Details of individual study results are reported in Table 9.
4. Detailed summary results of included experimental studies.
| References | Detailed results |
| Bettuzzi 2006 | Prostate cancer incidence Bettuzzi 2006: one prostate cancer case in the treatment group and 9 cases in the placebo group Brausi 2008 reported a longer follow‐up on a subset of participants: 13 cases in the intervention group and 9 in the placebo group, all after the suspension of the treatment. Further 3 cases of prostate cancer were diagnosed, 1 in the treatment group and 2 cases in the placebo group LUTS: a slightly higher decrease in IPSS was found in the treatment group from 11.12 to 9.12, than in the placebo group, from 8.27 to 7.00 PSA levels: no substantial difference in the PSA levels between groups QoL: QoL score assessed after 3 months decreased in the intervention group (from 2.06 to 1.76), while slightly increased in the placebo group (from 1.30 to 1.47) Safety data: 2 cases of diarrhoea in each arm, reported as very mild disorders |
| Dostal 2015 |
Dostal 2015 Follow‐up: 59 participants (39 in the intervention and 20 in the control group) stopped taking study product but remained in the study. Participants stopped mainly due to adverse symptoms (N = 50). Dropout: 138 participants withdrew from the study due to request (N = 93), adverse event (N = 22), protocol violation (N = 10), lost to follow‐up (N = 10), investigator judgment (N = 3) and death (N = 1). 18 of 22 (82%) who withdrew to adverse events were in the intervention group. Cancer incidence: 2 participants in the placebo group were diagnosed with uterine cancer after randomisation, 1 woman 1 day after beginning study product (for this reason not included in the risk analysis) and the other during the last month of participation. 2 women (1 in the treatment and 1 in the placebo group) reported a diagnosis of non‐melanoma skin cancer during the study. Safety data: total 1141 adverse events documented in the intervention group and 1031 in the placebo group. The most common events were infections; gastrointestinal disorders (nausea, indigestion, diarrhoea, constipation, vomiting, increased gassiness, abdominal pain, increased acid reflux); vascular disorders; respiratory, thoracic and mediastinal disorders; general disorders and administration site condition; musculoskeletal and connective tissue disorders, mainly of grade 1 and 2 of severity. Higher adverse effects in intervention groups were skin and subcutaneous tissue disorders (mainly rash or allergic skin reaction), ALT elevations and nausea and partially indigestion and constipation. Groups did not differ in severity of adverse effects. Yu 2017 in: Dostal 2015 Follow‐up: in the present report data on 513 men in the intervention group and 508 women in the control group are reported, due to missing data of ALT at baseline (N = 3) or during follow‐up (N = 51). Treatment increased both ALT and AST, whereas no increase was reported in the control group. AKP and total bilirubin did not increase in both treatment and control group. Webster 2018 in: Dostal 2015 QoL: data on overall QoL of recruited women. QoL assessed using MENQOL scale showed overall scores higher in women aged 50‐54.9 years |
| Dryden 2013 | Follow‐up: 10 participants randomised in cohort 1:8 to treatment group (low‐dose) and 2 to placebo: 10 participants randomised in cohort 2: 8 to treatment group (high‐dose) and 2 in placebo Safety data: no participants required termination due to serious adverse event. 1 participant in the treatment group required hospitalisation due to disease progression. Higher incidence of heartburn, increased thirst and increased diarrhoea were found in treatment group. QoL: measured with the Inflammatory Bowel Disease Questionnaire, QoL generally inversely correlated with the activity of the disease. |
| Garcia 2014 | Follow‐up: 82 participants (41 in the intervention and 41 in the control group) analysed for primary outcome Primary outcome Complete response (negative high‐risk HPV and normal histopathology) was noted in 7 (17.1%) and 6 (14.6%) for the treatment and control group, respectively Partial response (negative high‐risk HPV and evidence of low‐grade CIN) occurred more frequently in the control group, 1 (2.4%) vs. 614.6%) Progression/negative response (persistence of high‐risk HPV+ and worsening of CIN or invasive cancer) was more common in the treatment group, 6 (14.6%) vs 3 (7.3%) No response (persistence of high‐risk HPV+ with no progression similar to intervention and control group, 27 (65.9%) vs. 26 (63.4%) Secondary outcome Safety data: 163 and 136 adverse events in the control and in the intervention group. 2 serious events occurred in 1 participant in the placebo group. 1 and 2 participants in the control and in the intervention group respectively discontinued the study due to adverse events Adverse events were all Grade 1 and Grade 2, except 1 Grade 3 ALT elevation and one Grade 3 back pain in the Polyphenon E arm and one Grade 3 ALT and AST elevation in the placebo arm. Nausea was reported more frequently by participants receiving Polyphenon E compared to those receiving placebo, 32.0% vs 18.8%, respectively, as well as elevated ALT/AST, 10.0%/8.0% and 2.1%/2.1%, respectively, as well as was incidence of dizziness (14.0% vs 6.3%) |
| Garland 2006 | Primary outcome Urinary 8‐hydroxydeoxyguanosine levels increased in Group A (+2.36) and B (+5.20), while decreased in Group C (−1.08) Urinary 8‐F2‐isoprostanes levels decreased in both Group A (−39.87) and B (−35.80), with no change in Group C (0.71) Secondary outcome Safety data: no significant adverse events were reported, including no liver toxicity, but higher frequency of nausea, constipation and gastrointestinal reflux disease detected |
| Kumar 2015 | Prostate cancer incidence: total 14 cases at the end of the study (1 year): 5 prostate cancer cases in the treatment group and 9 cases in the control group. Prostate cancer + ASAP: total 13 (8 + 5) cases at the end of the study (1 year): 3 (3 + 0) prostate cancer cases in the treatment group and 10 (5 + 5) cases in the control group. No significant differences between the treatment and placebo arms were observed in LUTS and QoL scores from baseline to end of study. Safety data: 26 adverse effects (14 in the treatment group and 12 in control group censored at 6 months due to adverse effects), 2 (1 + 1) between 6 and 12 months and 55 (29 + 26) adverse effects at 12 months. Higher possible and probable events in the treatment arm, all but 1 of grade 1 and 2. Total 381 adverse effects, 212 in the treatment group and 169 in the control group. 11 and 7 in treatment and control groups respectively met off‐study criteria due to adverse effects. Higher incidence of coagulation toxicity, gastrointestinal and pain reported in the treatment group as well as toxicity to skin and musculoskeletal/soft tissue. Data on adverse effects also reported in Kumar 2016. Data on body weight or abdominal obesity in Kumar 2017 |
| Lane 2018 | Primary outcome Risk for prostate cancer NR Secondary outcomes PSA levels did not differ between green tea groups after 6 months of treatment Safety data: most incident adverse events were nocturia and insomnia that were higher in green tea drink (46% and 22%) and green tea capsules (50% and 21%) compared to placebo (33% and 8%). Hypertension was similar across groups, while fatigue was higher in green tea capsules (24%) and placebo (18%) than green tea drink (5%). Less frequent adverse events were cramp, shortness of breath, heartburn, headache and diarrhoea. |
| Micali 2017 | Follow‐up: 44 (22 in the intervention and 22 in the control group) completed the study. 16 participants (8 in the intervention and 8 in the control group) chose to withdraw from the study. Primary outcome Prostate cancer incidence After 6 months, 2/22 (9%) and 4/22 (18%) prostate cancer occurred in the treatment and control group, respectively. After 1 year total prostate cancer cases were 4/22 (18%) in both groups Secondary outcomes Safety data: only Grade 1 and 2 side effects occurred, including nausea, emesis, abdominal pain, insomnia, fatigue and diarrhoea Mean (SD) PSA levels decreased from 5.9 (2.3) ng/mL to 3.8 (1.8) in treatment group and increased from 4.7 (2.5) to 5.8 (2.6) in control group LUTS and QoL scores reported to be improved after 1‐year study but results were NR. |
| Roshdy 2013 | Primary outcome Total fibroid volume decreased by an average of 32.6% in the treatment group and increased by an average of 24.2% in the placebo group Secondary outcomes HRQoL Scale 1: mean change in score for the intervention group of −25.28 (SD ± 17.38) mean change of 7.1 (SD ± 15.5) in placebo group Scale 2: overall increase of 20.7 (SD ± 21) in the percentile scores for HRQoL in the treatment group and 2.19 (SD ± 17.4) in the placebo group Safety data: none of the participants reported any adverse events of any grade throughout the whole study period. |
| Sinicrope 2017 | Percent change in rectal ACF did not differ between the study arms after 6 months of treatment Safety data: similar adverse events between the study arms. 1 participant in placebo arm reported grade 3 adverse event and 2 participants (1 in each arm) reported grade 2+ adverse events, namely elevated AST, ALT in the treatment group |
| Tsao 2009 | Histological response Higher response rate in the 3 combined GTE arms (50%) versus placebo (18.2%), with dose‐dependent effect: 58% in the combined higher‐dose GTE arms (750 and 1000 mg/m2) versus 36.4% (GTE at 500 mg/m2) and 18.2% (placebo) Higher histological response rate in the 3 combined GTE arms (21.4%) vs placebo (9.1%), not dose‐dependent Safety data: adverse events reported by 28 of the 30 (93.3%) participants in treatment groups and 9 of the 11 (81.8%) participants of control group The most frequently reported adverse effects are grade 1 to 2, including insomnia, headache, nausea and nervousness. The grade 3 toxicity was reported by 2 participants in Group B, namely insomnia, diarrhoea and oral/neck pain. Insomnia was the most frequent adverse event. |
5. Detailed summary results of included nonexperimental cohort studies.
| References | Detailed summary results | The Newcastle‐Ottawa Scale (NOS) |
| Allen 2004 | Reference category: lowest exposure Prostate cancer Intermediate exposure: RR 1.03 (95% CI 0.69‐1.55) Highest exposure: RR 1.29 (95% CI 0.84‐1.98) |
Low risk: Selection: 3/4 stars since the exposed population is a highly selected group (survivors of the atomic bomb). Comparability: 1/2 stars, since the study did not control for smoking habits. Outcome: 3/3 stars, low risk Total score: 7/9 stars, moderate quality. |
| Chyou 1993 | Urinary tract cancer Reference category: lowest exposure Highest exposure: RR 1.34 (95% CI 0.79‐2.27) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Dai 2010 | Reference category: lowest exposure Breast cancer Exposure assessment A: intake of green tea Highest exposure: HR 1.04 (95% CI 0.88‐1.26) Exposure assessment B: dosage of green tea: Intermediate exposure 1: HR 1.07 (95% CI 0.81‐1.42) Intermediate exposure 2: HR 0.98 (95% CI 0.75‐1.29) Intermediate exposure 3: HR 1.00 (95% CI 0.68‐1.48) Highest exposure: HR 1.18 (95% CI 0.86‐1.61) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 2/3 stars, follow‐up < 5 years Total score: 8/9 stars, moderate quality. |
| Galanis 1998 | Stomach cancer Reference category: lowest exposure All Intermediate exposure: HR 1.3 (95% CI 0.7‐2.1) Highest exposure: HR 1.5 (95% CI 0.9‐2.3) Men Intermediate exposure: HR 1.2 (95% CI 0.6‐2.5) Highest exposure: HR 1.6 (95% CI 0.9‐2.9) Women Intermediate exposure: HR 1.3 (95% CI 0.6‐2.9) Highest exposure: HR 1.3 (95% CI 0.6‐2.6) |
Low risk: Selection: 4/4 stars, low risk Comparability: 1/2 stars, the study did not control for smoking in the overall analysis, only in men Outcome: 2/3 stars, follow‐up < 5 years, low risk Total score: 7/9 stars, moderate quality. |
| Ide 2007 | Reference category: lowest exposure Oral cancer All participants Intermediate exposure 1: HR 0.65 (95% CI 0.22‐1.94) Intermediate exposure 2: HR 0.69 (95% CI 0.28‐1.71) Highest exposure: HR 0.44 (95% CI 0.19‐1.04) Men Intermediate exposure 1: HR 0.79 (95% CI 0.18‐3.57) Intermediate exposure 2: HR 0.81 (95% CI 0.22‐3.03) Highest exposure HR 0.61 (95% CI 0.18‐2.06) Women Intermediate exposure 1: HR 0.51 (95% CI 0.10‐2.68) Intermediate exposure 2: HR 0.60 (95% CI 0.17‐2.10) Highest exposure: HR 0.31 (95% CI 0.09‐1.07) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Inoue 2009a | Reference category: lowest exposure Stomach cancer Men All cohorts Intermediate exposure 1: HR 0.97 (95% CI 0.83‐1.12) Intermediate exposure 2: HR 0.93 (95% CI 0.81‐1.08) Highest exposure: HR 1.06 (95% CI 0.86‐1.30) JPHC‐I Intermediate exposure 1: HR 0.85 (95% CI 0.62‐1.17) Intermediate exposure 2: HR 0.87 (95% CI 0.65‐1.16) Highest exposure: HR 0.97 (95% CI 0.73‐1.28) JPHC‐II Intermediate exposure 1: HR 1.11 (95% CI 0.82‐1.52) Intermediate exposure 2: HR 1.08 (95% CI 0.80‐1.45) Highest exposure: HR 1.06 (95% CI 0.78‐1.43) JACC Intermediate exposure 1: HR 0.80 (95% CI 0.59‐1.08) Intermediate exposure 2: HR 0.75 (95% CI 0.57‐1.00) Highest exposure: HR 0.81 (95% CI 0.63‐1.05) MIYAGI Intermediate exposure 1: HR 0.90 (95% CI 0.67‐1.20) Intermediate exposure 2: HR 0.87 (95% CI 0.65‐1.17) Highest exposure: HR 0.88 (95% CI 0.67‐1.15) 3‐pref MIYAGI Intermediate exposure 1: HR 1.28 (95% CI 0.84‐1.94) Intermediate exposure 2: HR 1.20 (95% CI 0.79‐1.80) Highest exposure: HR 1.55 (95% CI 1.09‐2.20) 3‐pref AICHI Intermediate exposure 1: HR 1.27 (95% CI 0.74‐2.21) Intermediate exposure 2: HR 1.22 (95% CI 0.73‐2.03) Highest exposure: HR 1.60 (95% CI 0.97‐2.63) Women: All cohorts: Intermediate exposure 1: HR 0.90 (95% CI 0.73‐1.10) Intermediate exposure 2: HR 0.92 (95% CI 0.76‐1.11) Highest exposure: HR 0.79 (95% CI 0.65‐0.96) JPHC‐I Intermediate exposure 1: HR 0.75 (95% CI 0.45‐1.25) Intermediate exposure 2: HR 0.90 (95% CI 0.58‐1.42) Highest exposure: HR 0.58 (95% CI 0.36‐0.95) JPHC‐II Intermediate exposure 1: HR 0.93 (95% CI 0.56‐1.56) Intermediate exposure 2: HR 1.18 (95% CI 0.74‐1.86) Highest exposure: HR 0.74 (95% CI 0.45‐1.20) JACC Intermediate exposure 1: HR 1.04 (95% CI 0.71‐1.53) Intermediate exposure 2: HR 0.85 (95% CI 0.60‐1.19) Highest exposure: HR 0.88 (95% CI 0.64‐1.21) MIYAGI Intermediate exposure 1: HR 0.81 (95% CI 0.53‐1.26) Intermediate exposure 2: HR 0.89 (95% CI 0.59‐1.35) Highest exposure: HR 0.67 (95% CI 0.44‐1.02) 3‐pref MIYAGI Intermediate exposure 1: HR 0.82 (95% CI 0.45‐1.49) Intermediate exposure 2: HR 0.72 (95% CI 0.41‐1.27) Highest exposure: HR 0.83 (95% CI 0.51‐1.35) 3‐pref AICHI Intermediate exposure 1: HR 1.20 (95% CI 0.49‐2.95) Intermediate exposure 2: HR 1.29 (95% CI 0.59‐2.80) Highest exposure: HR 1.54 (95% CI 0.72‐3.28) Stratified data available by sex and further stratified by smoking status (never smokers and current smokers) Stratified data available by subsite (proximal and distal stomach cancer) Previous reports Fujino 2002 Stomach cancer mortality Reference category: lowest exposure Men Intermediate exposure: RR 0.82 (95% CI 0.41‐1.64) Highest exposure: RR 1.11 (95% CI 0.75‐1.63) Women Intermediate exposure: RR 1.74 (95% CI 0.71‐4.26) Highest exposure: RR 1.43 (95% CI 0.78‐2.62) Hoshiyama 2002 Reference category: lowest exposure Stomach cancer mortality Men Intermediate exposure 1: RR 1.6 (95% CI 0.9‐2.9) Intermediate exposure 2: RR 1.1 (95% CI 0.6‐1.9) Intermediate exposure 3: RR 1.0 (95% CI 0.5‐2.5) Highest exposure: RR 1.0 (95% CI 0.5‐2.0) Women Intermediate exposure 1: RR 1.1 (95% CI 0.5‐2.5) Intermediate exposure 2: RR 1.0 (95% CI 0.5‐2.5) Intermediate exposure 3: RR 0.8 (95% CI 0.4‐1.6) Highest exposure: RR 0.7 (95% CI 0.3‐2.1) Hoshiyama 2004 Reference category: lowest exposure Stomach cancer Intermediate exposure 1: RR 1.3 (95% CI 0.6‐2.8) Intermediate exposure 2: RR 1.0 (95% CI 0.5‐1.9) Intermediate exposure 3: RR 0.8 (95% CI 0.4‐1.6) Highest exposure: RR 1.2 (95% CI 0.6‐2.5) Koizumi 2003 Stomach cancer Intermediate exposure 1: HR 1.01 (95% CI 0.80‐1.27) Intermediate exposure 2: HR 0.89 (95% CI 0.70–1.13) Highest exposure: HR 1.06 (95% CI 0.86–1.30) Stratified data available by histological subtype (differentiated and nondifferentiated) Stratified data available by subsite (cardia, body and antrum) Tsubono 2001 Stomach cancer All participants Intermediate exposure 1: RR 1.2 (95% CI 0.8‐1.8) Intermediate exposure 2: RR 1.0 (95% CI 0.7–1.5) Highest exposure: RR 1.4 (95% CI 1.0‐1.9) Men Intermediate exposure 1: RR 1.2 (95% CI 0.7‐1.9) Intermediate exposure 2: RR 1.2 (95% CI 0.7‐1.9) Highest exposure: RR 1.5 (95% CI 1.0‐2.3) Women Intermediate exposure 1: RR 1.2 (95% CI 0.6‐2.3) Intermediate exposure 2: RR 0.7 (95% CI 0.4‐1.5) Highest exposure: RR 1.1 (95% CI 0.6‐2.0) Sasazuki 2004 Reference category: lowest exposure Men Stomach cancer Intermediate exposure 1: RR 0.95 (95% CI 0.74–1.21) Intermediate exposure 2: RR 0.89 (95% CI 0.71–1.13) Highest exposure: RR 0.97 (95% CI 0.77–1.22) Subsite Upper‐third including cardia cancer Intermediate exposure 1: RR 1.07 (95% CI 0.53–2.17) Intermediate exposure 2: RR 0.88 (95% CI 0.44–1.75) Highest exposure: RR 1.24 (95% CI 0.65–2.35) Distal cancer Intermediate exposure 1: RR 0.88 (95% CI 0.65–1.17) Intermediate exposure 2: RR 0.85 (95% CI 0.64–1.12) Highest exposure: RR 0.88 (95% CI 0.67–1.16) Women Stomach cancer Intermediate exposure 1: RR 0.93 (95% CI 0.61–1.41) Intermediate exposure 2: RR 1.10 (95% CI 0.75–1.60) Highest exposure: RR 0.70 (95% CI 0.47–1.05) Subsite: Upper‐third including cardia cancer Intermediate exposure 1: RR 2.28 (95% CI 0.56–9.33) Intermediate exposure 2: RR 0.70 (95% CI 0.13–3.62) Highest exposure: RR 1.74 (95% CI 0.44–6.86) Distal cancer Intermediate exposure 1: RR 0.92 (95% CI 0.58–1.47) Intermediate exposure 2: RR 1.05 (95% CI 0.69–1.60) Highest exposure: RR 0.53 (95% CI 0.33–0.85) |
Low risk: Inoue 2009a Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. Fujino 2002 Selection: 4/4 stars, low risk Comparability: 1/2 stars, the study did not control for smoking Outcome: 2/3 stars, follow‐up < 90%. Total score: 8/9 stars, moderate quality. Hoshiyama 2002 Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 2/3 stars, follow‐up < 90% Total score: 8/9 stars, moderate quality. Hoshiyama 2004 Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 2/3 stars, follow‐up < 90%. Total score: 8/9 stars, moderate quality. Sasazuki 2004 Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. Tsubono 2001 Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Inoue 2009b | Reference category: lowest exposure Liver cancer Men Intermediate exposure: HR 1.20 (95% CI 0.64‐2.23) Highest exposure: HR 1.18 (95% CI 0.63‐2.20) Women Intermediate exposure: HR 2.58 (95% CI 1.01‐6.59) Highest exposure: HR 1.48 (95% CI 0.54‐4.08) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Ishikawa 2006 | Reference category: lowest exposure Oesophageal cancer Cohort 1 + cohort 2 Intermediate exposure 1: HR 1.03 (95% CI 0.46‐2.28) Intermediate exposure 2: HR 1.13 (95% CI 0.53‐2.42) Highest exposure: HR 1.67 (95% CI 0.89‐3.16) Cohort 1 Intermediate exposure 1: HR 0.69 (95% CI 0.17‐2.91) Intermediate exposure 2: HR 1.58 (95% CI 0.52‐4.76) Highest exposure: HR 1.78 (95% CI 0.66‐4.82) Cohort 2 Intermediate exposure 1: HR 1.22 (95% CI 0.47‐3.19) Intermediate exposure 2: HR 0.85 (95% CI 0.30‐2.40) Highest exposure: HR 1.61 (95% CI 0.71‐3.66) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 2/3 stars, follow‐up rate < 90% Total score: 8/9 stars, moderate quality. |
| Iwai 2002 | Reference category: lowest exposure Total cancer mortality Intermediate exposure: HR 0.93 (95% CI 0.51‐1.70) Highest exposure: HR 0.92 (95% CI 0.49‐1.73) |
Low risk: Selection: 4/4 stars, low risk Comparability: 1/2 stars, the study did not control for smoking Outcome: 3/3 stars, low risk Total score: 8/9 stars, moderate quality. |
| Iwasaki 2010a | Reference category: lowest exposure Breast cancer Iwasaki 2010a Intermediate exposure: HR 1.19 (95% CI 0.80‐1.76) Intermediate exposure 2: HR 1.13 (95% CI 0.72‐1.75) Intermediate exposure 3: HR 1.13 (95% CI 0.81‐1.58) Intermediate exposure 4: HR 1.17 (95% CI 0.85‐1.62) Highest exposure: HR 1.12 (95% CI 0.81‐1.56) Iwasaki 2010b Highest exposure: OR 1.02 (95% CI 0.62‐1.65) |
Low risk: Iwasaki 2010a: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. Iwasaki 2010b Selection: 4/4 stars, low risk Comparability: 1/2 stars, the study did not control for smoking Outcome: 3/3 stars, low risk Total score: 8/9 stars, moderate quality. |
| Key 1999 | Reference category: lowest exposure Breast cancer Intermediate exposure: RR 1.02 (95% CI 0.76‐1.36) Highest exposure: RR 0.86 (95% CI 0.62‐1.21) |
Low risk: Selection: 3/4 stars, exposure based on self‐report not within a structured interview or questionnaire. Comparability: 1/2 stars, the study did not control for smoking Outcome: 3/3 stars, low risk Total score: 7/9 stars, moderate quality. |
| Khan 2004 | Reference category: lowest exposure Men Total cancer mortality Highest exposure: RR 1.0 (955 CI 0.7‐1.6) Lung cancer mortality Highest exposure: RR 0.6 (95% CI 0.3‐1.2) Stomach cancer mortality Highest exposure: RR 1.1 (95% CI 0.4‐2.5) Colorectal cancer mortality Highest exposure: RR 1.3 (95% CI 0.3‐5.9) Pancreatic cancer mortality Highest exposure: RR not estimated Women Total cancer mortality Highest exposure: RR 1.0 (955 CI 0.6‐1.6) Lung cancer mortality Highest exposure: RR 0.7 (95% CI 0.2‐2.9) Stomach cancer mortality Highest exposure: RR 0.7 (95% CI 0.2‐1.9) Colorectal cancer mortality Highest exposure: RR 1.2 (95% CI 0.3‐4.4) Pancreatic cancer mortality Highest exposure: RR 0.5 (95% CI 0.2‐1.6) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Kikuchi 2006 | Reference category: lowest exposure Prostate cancer Intermediate exposure 1: HR 0.77 (95% CI 0.42‐1.40) Intermediate exposure 2: HR 1.15 (95% CI 0.69‐1.94) Highest exposure: HR 0.85 (95% CI 0.50‐1.43) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Kurahashi 2007 | Reference category: lowest exposure Prostate cancer Intermediate exposure 1: RR 0.96 (95% CI 0.68‐1.35) Intermediate exposure 2: RR 0.94 (95% CI 0.68‐1.30) Highest exposure: RR 0.89 (95% CI 0.65‐1.21) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Kurahashi 2009 | Reference category: lowest exposure Bladder cancer Men Intermediate exposure 1: HR 1.18 (95% CI 0.73‐1.91) Intermediate exposure 2: HR 0.71 (95% CI 0.43‐1.18) Highest exposure: HR 0.90 (95% CI 0.56‐1.45) Women Intermediate exposure: HR 1.22 (95% CI 0.49‐3.00) Highest exposure: HR 2.29 (95% CI 1.06‐4.92) Analysis also available stratified by smoking status |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Kuriyama 2006 | Reference category: lowest exposure All participants Total cancer mortality Intermediate exposure 1: HR 1.11 (95% CI 0.93‐1.34) Intermediate exposure 2: HR 1.16 (95% CI 0.97‐1.38) Highest exposure: HR 1.11 (95% CI 0.94‐1.31) Stomach cancer mortality Intermediate exposure 1: HR 1.33 (95% CI 0.86‐2.04) Intermediate exposure 2: HR 1.00 (95% CI 0.64‐1.58) Highest exposure: HR 1.17 (95% CI 0.78‐1.76) Lung cancer mortality Intermediate exposure 1: HR 1.03 (95% CI 0.67‐1.58) Intermediate exposure 2: HR 1.05 (95% CI 0.69‐1.59) Highest exposure: HR 1.18 (95% CI 0.81‐1.72) Colorectal cancer mortality Intermediate exposure 1: HR 1.04 (95% CI 0.59‐1.82) Intermediate exposure 2: HR 1.45 (95% CI 0.87‐2.42) Highest exposure: HR 1.10 (95% CI 0.67‐1.82) Men Total cancer mortality Intermediate exposure 1: HR 1.02 (95% CI 0.82‐1.28) Intermediate exposure 2: HR 1.18 (95% CI 0.95‐1.46) Highest exposure: HR 1.11 (95% CI 0.90‐1.36) Stomach cancer mortality Intermediate exposure 1: HR 1.29 (95% CI 0.78‐2.16) Intermediate exposure 2: HR 1.19 (95% CI 0.71‐2.00) Highest exposure: HR 1.20 (95% CI 0.74‐1.95) Lung cancer mortality Intermediate exposure 1: HR 0.88 (95% CI 0.54‐1.42) Intermediate exposure 2: HR 0.97 (95% CI 0.61‐1.54) Highest exposure: HR 1.14 (95% CI 0.75‐1.73) Colorectal cancer mortality Intermediate exposure 1: HR 1.09 (95% CI 0.57‐2.09) Intermediate exposure 2: HR 1.23 (95% CI 0.66‐2.29) Highest exposure: HR 0.88 (95% CI 0.47‐1.63) Women Total cancer mortality Intermediate exposure 1: HR 1.27 (95% CI 0.93‐1.74) Intermediate exposure 2: HR 1.09 (95% CI 0.79‐1.49) Highest exposure: HR 1.07 (95% CI 0.80‐1.44) Stomach cancer mortality Intermediate exposure 1: HR 1.32 (95% CI 0.59‐2.94) Intermediate exposure 2: HR 0.64 (95% CI 0.26‐1.63) Highest exposure: HR 1.08 (95% CI 0.50‐2.33) Lung cancer mortality Intermediate exposure 1: HR 1.83 (95% CI 0.68‐4.96) Intermediate exposure 2: HR 1.46 (95% CI 0.54‐3.95) Highest exposure: HR 1.59 (95% CI 0.63‐4.05) Colorectal cancer mortality Intermediate exposure 1: HR 0.98 (95% CI 0.32‐2.97) Intermediate exposure 2: HR 1.96 (95% CI 0.78‐4.95) Highest exposure: HR 1.49 (95% CI 0.60‐3.71) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Lee 2007 | Reference category: lowest exposure Men Colorectal cancer Intermediate exposure 1: RR 0.80 (95% CI 0.57‐1.06) Intermediate exposure 2: RR 1.00 (95% CI 0.73‐1.35) Intermediate exposure 3: RR 1.04 (95% CI 0.78‐1.40) Highest exposure: RR 0.96 (95% CI 0.71‐1.29) Colon cancer Intermediate exposure 1: RR 0.73 (95% CI 0.47‐1.14) Intermediate exposure 2: RR 1.09 (95% CI 0.75‐1.59) Intermediate exposure 3: RR 1.06 (95% CI 0.74‐1.53) Highest exposure: RR 0.92 (95% CI 0.63‐1.33) Rectal cancer Intermediate exposure 1: RR 0.93 (95% CI 0.53‐1.67) Intermediate exposure 2: RR 0.81 (95% CI 0.47‐1.39) Intermediate exposure 3: RR 1.01 (95% CI 0.60‐1.67) Highest exposure: RR 1.04 (95% CI 0.63‐1.72) Women Colorectal cancer Intermediate exposure 1: RR 1.01 (95% CI 0.67‐1.52) Intermediate exposure 2: RR 0.81 (95% CI 0.55‐1.22) Intermediate exposure 3: RR 0.96 (95% CI 0.66‐1.40) Highest exposure: RR 1.02 (95% CI 0.70‐1.47) Colon cancer Intermediate exposure 1: RR 0.97 (95% CI 0.57‐1.63) Intermediate exposure 2: RR 0.81 (95% CI 0.49‐1.35) Intermediate exposure 3: RR 0.99 (95% CI 0.62‐1.57) Highest exposure: RR 1.10 (95% CI 0.70‐1.73) Rectal cancer Intermediate exposure 1: RR 1.09 (95% CI 0.56‐2.13) Intermediate exposure 2: RR 0.81 (95% CI 0.42‐1.57) Intermediate exposure 3: RR 0.92 (95% CI 0.49‐1.71) Highest exposure: RR 0.85 (95% CI 0.45‐1.61) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Li 2008 | Reference category: lowest exposure Lung cancer All participants Intermediate exposure 1: HR 1.14 (95% CI 0.80‐1.62) Intermediate exposure 2: HR 1.18 (95% CI 0.83‐1.66) Highest exposure: HR 1.17 (95% CI 0.85‐1.61) Men Intermediate exposure 1: HR 1.05 (95% CI 0.70‐1.57) Intermediate exposure 2: HR 1.21 (95% CI 0.82‐1.79) Highest exposure: HR 1.17 (95% CI 0.82‐1.68) Women Intermediate exposure 1: HR 1.48 (95% CI 0.71‐3.10) Intermediate exposure 2: HR 1.11 (95% CI 0.52‐2.37) Highest exposure: HR 1.30 (95% CI 0.65‐2.60) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Li 2018 | Reference category: lowest exposure Lung cancer Highest exposure: HR 1.88 (95% CI 0.93‐3.77) |
Low risk: Selection: 3/4 stars, exposure based on self‐report not within a structured interview or questionnaire. Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 8/9 stars, moderate quality. |
| Lin 2008 | Reference category: lowest exposure Pancreatic cancer mortality All participants Intermediate exposure 1: RR 1.04 (95% CI 0.67‐1.60) Intermediate exposure 2: RR 1.14 (95% CI 0.80‐1.63) Intermediate exposure 3: RR 0.99 (95% CI 0.69‐1.42) Highest exposure: RR 1.23 (95% CI 0.84‐1.80) Men Intermediate exposure 1: RR 0.79 (95% CI 0.42‐1.51) Intermediate exposure 2: RR 1.09 (95% CI 0.65‐1.83) Intermediate exposure 3: RR 0.88 (95% CI 0.53‐1.48) Highest exposure: RR 0.95 (95% CI 0.5‐1.65) Women Intermediate exposure 1: RR 1.32 (95% CI 0.73‐2.38) Intermediate exposure 2: RR 1.20 (95% CI 0.73‐1.97) Intermediate exposure 3: RR 1.08 (95% CI 0.66‐1.78) Highest exposure: RR 1.54 (95% CI 0.91‐2.60) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Liu 2016 | Reference category: highest exposure Total cancer mortality Intermediate exposure 1: HR 0.93 (95% CI 0.85‐1.01) Intermediate exposure 2: HR 0.91 (95% CI 0.85‐0.98) Lowest exposure: HR 0.86 (95%CI 0.79‐0.93) Available also stratified analysis by smoking status, alcohol drinking, rural and urban locality |
Low risk: Selection: 4/4 stars, low risk. Comparability: 2/2 stars, low risk. Outcome: 3/3 stars, low risk. Total score: 9/9 stars, high quality. |
| Luo 2007 | Reference category: lowest exposure Pancreatic cancer All participants Intermediate exposure 1: HR 1.1 (95% CI 0.6‐1.9) Intermediate exposure 2: HR 1.1 (95% CI 0.7‐1.9) Intermediate exposure 3: HR 1.2 (95% CI 0.7‐2.0) Highest exposure: HR 1.2 (95% CI 0.7‐1.9) Men Intermediate exposure 1: HR 1.3 (95% CI 0.6‐2.9) Intermediate exposure 2: HR 1.4 (95% CI 0.7‐2.9) Intermediate exposure 3: HR 1.5 (95% CI 0.7‐3.1) Highest exposure: HR 1.4 (95% CI 0.7‐2.8) Women Intermediate exposure 1: HR 0.9 (95% CI 0.4‐2.1) Intermediate exposure 2: HR 0.9 (95% CI 0.4‐1.8) Intermediate exposure 3: HR 0.9 (95% CI 0.5‐1.9) Highest exposure: HR 1.0 (95% CI 0.5‐2.0) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Makiuchi 2016 | Reference category: lowest exposure Biliary tract cancer All participants Intermediate exposure 1: HR 0.74 (95% CI 0.52‐1.04) Intermediate exposure 2: HR 0.86 (95% CI 0.62‐1.21) Highest exposure: HR 0.67 (95% CI 0.46‐0.97) Men Intermediate exposure 1: HR 0.74 (95% CI 0.48‐1.15) Intermediate exposure 2: HR 0.89 (95% CI 0.58‐1.37) Highest exposure: HR 0.66 (95% CI 0.40‐1.08) Women Intermediate exposure 1: HR 0.74 (95% CI 0.42‐1.29) Intermediate exposure 2: HR 0.84 (95% CI 0.49‐1.44) Highest exposure: HR 0.66 (95% CI 0.37‐1.20) Gallbladder Intermediate exposure 1: HR 0.56 (95% CI 0.32‐0.97) Intermediate exposure 2: HR 0.88 (95% CI 0.54‐1.45) Highest exposure: HR 0.57 (95% CI 0.32‐1.01) Extrahepatic bile duct cancer Intermediate exposure 1: HR 0.83 (95% CI 0.53‐1.31) Intermediate exposure 2: HR 0.79 (95% CI 0.50‐1.26) Highest exposure: HR 0.69 (95% CI 0.41‐1.15) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Michikawa 2011 | Reference category: lowest exposure Thyroid cancer Men Intermediate exposure 1: HR 1.13 (95% CI 0.39‐3.27) Intermediate exposure 2: HR 0.95 (95% CI 0.33‐2.78) Highest exposure: HR 0.71 (95% CI 0.22‐2.28) Women Intermediate exposure 1: HR 0.64 (95% CI 0.37‐1.14) Intermediate exposure 2: HR 1.10 (95% CI 0.70‐1.75) Highest exposure: HR 0.91 (95% CI 0.56‐1.48) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality |
| Montague 2012 | Reference category: lowest exposure Prostate cancer Intermediate exposure 1: HR 1.07 (95% CI 0.73‐1.56) Intermediate exposure 2: HR 1.09 (95% CI 0.80‐1.48) Highest exposure: HR 1.08 (95% CI 0.79‐1.48) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Nagano 2001 | Reference category: lowest exposure Total cancer Intermediate exposure: RR 1.0 (95% CI 0.93‐1.1) Highest exposure: RR 0.98 (95% CI 0.89‐1.1) Total solid cancer Intermediate exposure: RR 1.0 (95% CI 0.92‐1.1) Highest exposure: RR 0.98 (95% CI 0.88‐1.1) Stomach cancer (also reported in Sauvaget 2005) Intermediate exposure: RR 1.0 (95% CI 0.82‐1.2) Highest exposure: RR 0.95 (95% CI 0.76‐1.2) Colon cancer Intermediate exposure: RR 1.0 (95% CI 0.76‐1.4) Highest exposure: RR 1.0 (95% CI 0.76‐1.4) Rectal cancer Intermediate exposure: RR 1.3 (95% CI 0.80‐2.0) Highest exposure: RR 1.3 (95% CI 0.77‐2.1) Liver cancer Intermediate exposure: RR 1.1 (95% CI 0.80‐1.4) Highest exposure: RR 0.95 (95% CI 0.69‐1.3) Gallbladder cancer Intermediate exposure: RR 0.9 (95% CI 0.57‐1.7) Highest exposure: RR 1.2 (95% CI 0.66‐2.2) Pancreatic cancer Intermediate exposure: RR 0.8 (95% CI 0.51‐1.4) Highest exposure: RR 0.79 (95% CI 0.45‐1.4) Lung cancer Intermediate exposure: RR 0.78 (95% CI 0.60‐1.0) Highest exposure: RR 0.79 (95% CI 0.59‐1.1) Breast cancer Intermediate exposure: RR 1.2 (95% CI 0.86‐1.8) Highest exposure: RR 1.0 (95% CI 0.67‐1.6) Bladder cancer Intermediate exposure: RR 1.1 (95% CI 0.62‐2.0) Highest exposure: RR 1.1 (95% CI 0.6‐2.1) Haematopoietic cancer Intermediate exposure: RR 1.2 (95% CI 0.75‐1.8) Highest exposure: RR 0.99 (95% CI 0.61‐1.7) Sauvaget 2005: Reference category: lowest exposure Stomach cancer: Intermediate exposure: IRR 1.03 (95% C 0.89–1.19) Highest exposure: IRR 1.06 (95% CI 0.89–1.25) |
Low risk: Nagano 2001 Selection: 3/4 stars, exposure based on self‐report not within a structured interview or questionnaire. Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 8/9 stars, moderate quality. Sauvaget 2005 Selection: 3/4 stars, exposure based on self report not within a structured interview or questionnaire. Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 8/9 stars, moderate quality. |
| Naganuma 2009 | Reference category: lowest exposure All participants Haematopoietic cancer Intermediate exposure 1: HR 0.88 (95% CI 0.57‐1.38) Intermediate exposure 2: HR 0.90 (95% CI 0.59‐1.39) Highest exposure: HR 0.58 (95% CI 0.37‐0.89) Lymphoid cancer Intermediate exposure 1: HR 1.00 (95% CI 0.61‐1.65) Intermediate exposure 2: HR 0.92 (95% CI 0.56‐1.52) Highest exposure: HR 0.52 (95% CI 0.31‐1.87) Myeloid cancer Intermediate exposure 1: HR 0.57 (95% CI 0.20‐1.64) Intermediate exposure 2: HR 0.91 (95% CI 0.37‐2.23) Highest exposure: HR 0.76 (95% CI 0.32‐1.78) Men Hematopoietic cancer Intermediate exposure 1: HR 0.75 (95% CI 0.41‐1.35) Intermediate exposure 2: HR 0.82 (95% CI 0.47‐1.46) Highest exposure: HR 0.57 (95% CI 0.32‐1.00) Women Hematopoietic cancer Intermediate exposure 1: HR 1.09 (95% CI 0.55‐2.16) Intermediate exposure 2: HR 1.01 (95% CI 0.52‐1.99) Highest exposure: HR 0.58 (95% CI 0.29‐1.16) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Nakachi 2000 | Reference category: lowest exposure Total cancer All participants Intermediate exposure: RR 0.81 (95% CI 0.52‐1.27) Highest exposure: RR 0.59 (95% CI 0.35‐0.98) Men Intermediate exposure: RR 1.00 (95% CI 0.50‐2.04) Highest exposure: RR 0.54 (95% CI 0.22‐1.34) Women Intermediate exposure: RR 0.92 (95% CI 0.64‐1.31) Highest exposure: RR 0.57 (95% CI 0.34‐0.98) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Nakamura 2011 | Pancreatic cancer mortality Reference category: lowest exposure Men Intermediate exposure: HR 2.02 (95% CI 0.61‐6.63) Highest exposure: HR 1.77 (95% CI 0.78‐4.04) Women Intermediate exposure: HR 0.31 (95% CI 0.04‐2.59) Highest exposure: HR 0.59 (95% CI 0.21‐1.61) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Nechuta 2012 | Reference category: lowest exposure Digestive system cancer Highest exposure: HR 0.86 (95% CI 0.75‐1.00) Stomach cancer Highest exposure: HR 0.79 (95% CI 0.58‐1.07) Stomach and oesophageal cancer Highest exposure: HR 0.77 (95% CI 0.57‐1.03) Colorectal cancer Highest exposure: HR 0.91 (95% CI 0.74‐1.12) Colon cancer Highest exposure: HR 0.96 (95% CI 0.74‐1.24) Rectal cancer Highest exposure: HR 0.84 (95% CI 0.60‐1.17) Liver cancer Highest exposure: HR 0.89 (95% CI 0.58‐1.38) Pancreatic cancer Highest exposure: HR 0.96 (95% CI 0.62‐1.49) Gallbladder and bile duct cancer Highest exposure: HR 0.73 (95% CI 0.40‐1.35) Yang 2007: Colorectal cancer Highest exposure: RR 0.63 (95% CI 0.45‐0.88) Further divided in: Highest exposure A: RR 0.70 (95% CI 0.47‐1.02) Highest exposure B: RR 0.56 (95% CI 0.32‐0.98) |
Low risk: Nechuta 2012 Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. Yang 2007 Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Oba 2006 | Reference category: lowest exposure Colon cancer Men Intermediate exposure: RR 0.99 (95% CI 0.61–1.63) Highest exposure: RR 0.75 (95% CI 0.49–1.16) Women Intermediate exposure: RR 1.00 (95% CI 0.53–1.86) Highest exposure: RR 1.08 (95% CI 0.67–1.76) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Odegaard 2015 | Reference category: lowest exposure Total cancer mortality Intermediate exposure 1: HR 1.00 (95% CI 0.92‐1.08) Intermediate exposure 2: HR 0.95 (95% CI 0.83‐1.09) Highest exposure: HR 1.10 (95% CI 0.97‐1.25) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Ogawa 2016 | Reference category: lowest exposure Brain cancer All participants Intermediate exposure: HR 0.96 (95% CI 0.58‐1.59) Highest exposure: HR 1.07 (95% CI 0.70‐1.62) Men Intermediate exposure: HR 1.19 (95% CI 0.59–2.40) Highest exposure: HR 0.96 (95% CI 0.51–1.81) Women Intermediate exposure: HR 0.74 (95% CI 0.35–1.55) Highest exposure: HR 1.17 (95% CI 0.66–2.06) Glioma Intermediate exposure: HR 1.12 (95% CI 0.51–2.43) Highest exposure: HR 1.05 (95% CI 0.54–2.05) Meningioma Intermediate exposure: HR 0.89 (95% CI 0.38–2.12) Highest exposure: HR 1.01 (95% CI 0.49–2.08) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Saito 2015 | Reference category: lowest exposure Total cancer mortality Men Intermediate exposure 1: HR 1.09 (95% CI 0.97‐1.22) Intermediate exposure 2: HR 1.03 (95% CI 0.92‐1.16) Highest exposure: HR 1.04 (95% CI 0.93‐1.17) Women Intermediate exposure 1: HR 0.86 (95% CI 0.73‐1.01) Intermediate exposure 2: HR 0.93 (95% CI 0.80‐1.07) Highest exposure: HR 0.87 (95% CI 0.75‐1.01) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Severson 1989 | Prostate cancer Reference category: lowest exposure Highest exposure: RR 1.47 (95% CI 0.99‐2.19) |
Low risk: Selection: 4/4 stars, low risk Comparability: 1/2 stars, the study did not control for smoking Outcome: 3/3 stars, low risk Total score: 8/9 stars, moderate quality. |
| Shimazu 2008 | Reference category: lowest exposure Endometrial cancer Intermediate exposure 1: HR 1.04 (95% CI 0.62‐1.74) Intermediate exposure 2: HR 0.79 (95% CI 0.47‐1.35) Higest exposure: HR 0.75 (95% CI 0.44‐1.30) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Sun 2007 | Reference category: lowest exposure Colorectal cancer All participants Exposure assessment A: green tea intake Highest exposure: RR 1.12 (95% CI 0.97–1.29) Exposure assessment B: green tea intake Intermediate exposure 1: RR 1.05 (95% CI 0.84–1.31) Intermediate exposure 2: RR 1.11 (95% CI 0.92–1.35) Highest exposure: RR 1.18 (95% CI 0.97–1.45) Men Exposure assessment A: green tea intake Highest exposure: RR 1.31 (95% CI 1.08–1.58) Exposure assessment B: green tea intake Intermediate exposure 1: RR 1.32 (95% CI 0.98–1.78) Intermediate exposure 2: RR 1.25 (95% CI 0.98–1.61) Highest exposure: RR 1.36 (95% CI 1.06–1.74) Women Exposure assessment A: green tea intake Highest exposure: RR 0.89 (95% CI 0.71–1.12) Exposure assessment B: green tea intake Intermediate exposure 1: RR 0.79 (95% CI 0.56–1.13) Intermediate exposure 2: RR 0.96 (95% CI 0.71–1.31) Highest exposure: RR 0.91 (95% CI 0.63–1.32) Reported stratified analysis in colon cancer and rectal cancer, only in men. Reported stratified analysi by localised and advanced disease. Reported nondrinkers vs. drinkers: Men with colon cancer Localised Highest exposure: RR 1.23 (95% CI 0.81–1.87) Advanced Highest exposure: RR 1.75 (95% CI 1.24–2.46) Men with rectal cancer: Localised Highest exposure: RR 1.17 (95% CI 0.75–1.81) Advanced Highest exposure: RR 1.32 (95% CI 0.90–1.91) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Suzuki 2004 | Reference category: lowest exposure Breast cancer Intermediate exposure 1: RR 0.87 (95% CI 0.57‐1.32) Intermediate exposure 2: RR 1.07 (95% CI 0.73‐1.57) Highest exposure: RR 0.84 (95% CI 0.57‐1.24) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Suzuki 2005 | Reference category: lowest exposure All participants Colon cancer Intermediate exposure 1: RR 1.06 (95% CI 0.74‐1.52) Intermediate exposure 2: RR 1.10 (95% CI 0.78‐1.55) Highest exposure: RR 0.97 (95% CI 0.70‐1.35) Rectal cancer Intermediate exposure 1: RR 0.85 (95% CI 0.56‐1.29) Intermediate exposure 2: RR 0.70 (95% CI 0.45‐1.08) Highest exposure: RR 0.85 (95% CI 0.58‐1.23) Men Colon cancer Intermediate exposure 1: RR 1.32 (95% CI 0.83‐2.10) Intermediate exposure 2: RR 1.35 (95% CI 0.86‐2.12) Highest exposure: RR 1.12 (95% CI 0.72‐1.74) Rectal cancer Intermediate exposure 1: RR 0.85 (95% CI 0.50‐1.45) Intermediate exposure 2: RR 0.58 (95% CI 0.32‐1.04) Highest exposure: RR 0.62 (95% CI 0.38‐1.02) Women Colon cancer Intermediate exposure 1: RR 0.78 (95% CI 0.43‐1.40) Intermediate exposure 2: RR 0.78 (95% CI 0.45‐1.35) Highest exposure: RR 0.79 (95% CI 0.49‐1.29) Rectal cancer Intermediate exposure 1: RR 0.81 (95% CI 0.40‐1.66) Intermediate exposure 2: RR 0.95 (95% CI 0.48‐1.89) Highest exposure: RR 1.30 (95% CI 0.70‐2.42) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Suzuki 2009 | Reference category: lowest exposure All participants Total cancer mortality Intermediate exposure 1: RR 0.63 (95% CI 0.34–1.16) Intermediate exposure 2: RR 0.76 (95% CI 0.42–1.37) Highest exposure: RR 0.82 (95% CI 0.45–1.50) Stomach cancer mortality Intermediate exposure 1: RR 0.49 (95% CI 0.11–2.28) Intermediate exposure 2: RR 0.78 (95% CI 0.19–3.30) Highest exposure: RR 0.81 (95% CI 0.18–3.54) Lung cancer mortality Intermediate exposure 1: RR 0.85 (95% CI 0.19–3.74) Intermediate exposure 2: RR 1.13 (95% CI 0.27–4.68) Highest exposure: RR 1.24 (95% CI 0.29–5.25) Colorectal cancer mortality Intermediate exposure 1: RR 0.47 (95% CI 0.10–2.18) Intermediate exposure 2: RR 0.35 (95% CI 0.08–1.55) Highest exposure: RR 0.36 (95% CI 0.07–1.74) Men Total cancer mortality Intermediate exposure 1: RR 0.61 (95% CI 0.32–1.17) Intermediate exposure 2: RR 0.69 (95% CI 0.37–1.27) Highest exposure: RR 0.82 (95% CI 0.44–1.55) Women Total cancer mortality Intermediate exposure 1: RR 1.14 (95% CI 0.15–8.82) Intermediate exposure 2: RR 1.85 (95% CI 0.25–13.57) Highest exposure: RR 1.31 (95% CI 0.17–10.01) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Tamura 2018 | Reference category: lowest quartile Liver cancer Intermediate exposure 1: HR 1.36 (95% CI 0.86‐2.16) Intermediate exposure 2: HR 1.08 (95% CI 0.60‐1.94) Intermediate exposure 3: HR 0.75 (95% CI 0.51‐1.11) Highest exposure: HR 1.25 (95% CI 0.77‐2.04) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Ugai 2017 | Reference category: lowest exposure Malignant lymphoma All participants Intermediate exposure 1: HR 0.84 (95% CI 0.53–1.31) Intermediate exposure 2: HR 1.38 (95% CI 0.92–1.94) Intermediate exposure 3: HR 1.10 (95% CI 0.76–1.59) Highest exposure: HR 0.89 (95% CI 0.61–1.29) Men Intermediate exposure 1: HR 0.81 (95% CI 0.44–1.50) Intermediate exposure 2: HR 1.45 (95% CI 0.87–2.39) Intermediate exposure 3: HR 1.21 (95% CI 0.73–2.00) Highest exposure: HR 1.08 (95% CI 0.65–1.79) Women Intermediate exposure 1: HR 0.87 (95% CI 0.45–1.69) Intermediate exposure 2: HR 1.21 (95% CI 0.70–2.11) Intermediate exposure 3: HR 0.96 (95% CI 0.56–1.67) Highest exposure: HR 0.67 (95% CI 0.38–1.19) Multiple myeloma All participants Intermediate exposure 1: HR 0.98 (95% CI 0.47–2.03) Intermediate exposure 2: HR 0.84 (95% CI 0.43–1.66) Intermediate exposure 3: HR 1.18 (95% CI 0.64–2.20) Highest exposure: HR 0.74 (95% CI 0.38–1.41) Men Intermediate exposure 1: HR 0.81 (95% CI 0.29–2.28) Intermediate exposure 2: HR 0.77 (95% CI 0.31–1.94) Intermediate exposure 3: HR 1.13 (95% CI 0.49–2.61) Highest exposure: HR 0.55 (95% CI 0.22–1.37) Women Intermediate exposure 1: HR 1.18 (95% CI 0.41–3.44) Intermediate exposure 2: HR 0.93 (95% CI 0.34–2.53) Intermediate exposure 3: HR 1.25 (95% CI 0.50–3.15) Highest exposure: HR 0.92 (95% CI 0.36–2.38) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Ugai 2018 | Reference category: lowest exposure Acute myeloid leukaemia All participants Intermediate exposure 1: HR 0.91 (95% CI 0.44–1.90) Intermediate exposure 2: HR 1.19 (95% CI 0.62–2.31) Highest exposure: HR 1.20 (95% CI 0.62–2.32) Men Intermediate exposure 1: HR 0.66 (95% CI 0.24–1.76) Intermediate exposure 2: HR 1.26 (95% CI 0.56–2.84) Highest exposure: HR 0.86 (95% CI 0.36–2.06) Women Intermediate exposure 1: HR 1.53 (95% CI 0.50–4.69) Intermediate exposure 2: HR 1.08 (95% CI 0.34–3.44) Highest exposure: HR 1.96 (95% CI 0.68–5.67) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Ui 2009 | Reference category: lowest exposure Liver cancer All participants Intermediate exposure 1: HR 0.78 (95% CI 0.54–1.12) Intermediate exposure 2: HR 0.98 (95% CI 0.69–1.37) Highest exposure: HR 0.58 (95% CI 0.41–0.83) Men Intermediate exposure 1: HR 0.83 (95% CI 0.53–1.30) Intermediate exposure 2: HR 1.11 (95% CI 0.73–1.68) Highest exposure: HR 0.63 (95% CI 0.41–0.98) Women Intermediate exposure 1: HR 0.68 (95% CI 0.35–1.31) Intermediate exposure 2: HR 0.79 (95% CI 0.44–1.44) Highest exposure: HR 0.50 (95% CI 0.27–0.90) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Yang 2011a | Reference category: lowest exposure Exposure assessment A: intake of green tea Colorectal cancer Highest exposure: HR 0.77 (95% CI 0.59‐1.01) Colon cancer Highest exposure: HR 0.69 (95% CI 0.48‐0.98) Rectal cancer Highest exposure: HR 0.89 (95% CI 0.59‐1.34) Exposure assessment B: consumption of green tea Intermediate exposure: HR 0.66 (95% CI 0.46‐0.93) Highest exposure: HR 0.85 (95% CI 0.62‐1.15) Data also available stratified by smoking status (non‐smokers and smokers) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Zhao 2017 | Reference category: lowest exposure Total cancer mortality All participants Exposure assessment A: intake of green tea Highest exposure: HR 1.01 (95% CI 0.93–1.10) Exposure assessment B: consumption of green tea Intermediate exposure: HR 0.98 (95% CI 0.87‐1.10) Highest exposure: HR 1.04 (95% CI 0.95‐1.14) Men Exposure assessment A: intake of green tea Highest exposure: HR 1.06 (95% CI 0.94‐1.19) Exposure assessment B: consumption of green tea Intermediate exposure: HR 1.06 (95% CI 0.92‐1.22) Highest exposure: HR 1.06 (95% CI 0.93‐1.21) Women Exposure assessment A: intake of green tea Highest exposure: HR 0.97 (95% CI 0.86–1.08) Exposure assessment B: consumption of green tea Intermediate exposure: HR 0.82 (95% CI 0.67‐1.01) Highest exposure: HR 1.03 (95% CI 0.91‐1.17) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Outcome: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
6. Detailed summary results of included nonexperimental case‐control studies.
| References | Detailed summary results | The Newcastle‐Ottawa Scale (NOS) |
| Bandera 2010 | Reference category: lowest exposure Endometrial cancer Intermediate exposure: OR 1.04 (95% CI 0.72‐1.50) Highest exposure: OR 0.76 (95% CI 0.48‐1.21) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Exposure: 2/3 stars, difference in response rate between cases and controls or no designation Total score: 8/9 stars, moderate quality. |
| Berroukche 2012 | Reference category: lowest exposure Prostate cancer Intermediate exposure 1: OR 1.4 (95% CI 0.8‐2.2) Intermediate exposure 2: OR 0.9 (95% CI 0.5‐1.4) Highest exposure: OR 0.6 (95% CI 0.3‐1.1) |
Low risk: Selection: 3/4 stars, hospital controls Comparability: 2/2 stars, low risk Exposure: 3/3 stars, low risk Total score: 8/9 stars, moderate quality. |
| Bonner 2005 | Reference category: lowest exposure Lung cancer Intermediate exposure: OR 0.84 (95% CI 0.38‐1.85) Highest exposure: OR 0.59 (95% CI 0.26‐1.37) |
Low risk: Selection: 3/4 stars, no description of source of controls Comparability: 2/2 stars, low risk Exposure: 2/3 stars, low risk Total score: 7/9 stars, moderate quality. |
| Chen 2011 | Oesophageal cancer Reference category: lowest exposure Intermediate exposure 1: OR 1.27 (95% CI 0.72‐1.89) Intermediate exposure 2: OR 0.97 (95% CI 0.59‐2.56) Highest exposure: OR 0.92 (95% CI 0.49‐2.32) |
Low risk: Selection: 3/4 stars, hospital controls Comparability: 2/2 stars, low risk Exposure: 3/3 stars, low risk Total score: 8/9 stars, moderate quality. |
| Chen 2017a | Oral cancer Reference category: lowest exposure Chen 2015 Highest exposure: OR 0.48 (95% CI 0.28‐0.82) Chen 2016 Highest exposure: OR 0.501 (95% CI 0.284‐0.883) Chen 2017a non‐smokers Highest exposure: OR 0.515 (95% CI 0.323‐0.821) Former/current smokers Highest exposure: OR 0.849 (95% CI 0.556‐1.298) Non‐alcohol drinkers Highest exposure: OR 0.551 (95% CI 0.372‐0.817) Former/current alcohol drinkers Highest exposure: OR 0.934 (95% CI 0.934‐1.563) |
Low risk: Chen 2015 Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Exposure: 3/3 stars, low risk Total score: 9/9 stars, high quality. Chen 2016 Selection: 3/4 stars, hospital controls Comparability: 2/2 stars, low risk Exposure: 3/3 stars, low risk Total score: 8/9 stars, moderate quality. Chen 2017a Selection: 3/4 stars, hospital controls Comparability: 2/2 stars, low risk Exposure: 3/3 stars, low risk Total score: 8/9 stars, moderate quality. |
| Fu 2013 | Oral cancer Reference category: lowest exposure Men Intermediate exposure: OR 0.82 (95% CI 0.69‐1.07) Highest exposure: OR 0.72 (95% CI 0.54‐0.93) Women Intermediate exposure: OR 1.00 (95% CI 0.80‐1.25) Highest exposure: OR 0.93 (95% CI 0.74‐1.26) |
Low risk: Selection: 3/4 stars, hospital controls Comparability: 2/2 stars, low risk Exposure: 3/3 stars, low risk Total score: 8/9 stars, moderate quality. |
| Gao 1994 | Oesophageal cancer Reference category: lowest exposure Exposure assessment A: intake of green tea Men Highest exposure: OR 0.80 (95% CI 0.58‐1.09) Women Highest exposure: OR 0.50 (95% CI 0.30‐0.83) Exposure assessment B: green tea consumption Men Intermediate exposure: OR 0.79 (95% CI 0.53‐1.17) Highest exposure: OR 0.79 (95% CI 0.56‐1.13) Women Intermediate exposure: OR 0.77 (95% CI 0.39‐1.53) Highest exposure: OR 0.34 (95% CI 0.17‐0.69) |
Low risk: Selection: 3/4 stars, hospital controls Comparability: 2/2 stars, low risk Exposure: 2/3 stars, non‐response rate difference between cases and controls or NR Total score: 7/9 stars, moderate quality. |
| Gao 2005 | Reference category: lowest exposure Endometrial cancer Highest exposure: OR 0.81 (95% CI 0.65‐1.00) |
Low risk: Selection: 4/4 stars, low risk Comparability: 1/2 stars, the study did not control for smoking. Exposure: 2/3 stars, nonresponse rate different between cases and controls or NR Total score: 7/9 stars, moderate quality. |
| Gavrilas 2018 | Colorectal cancer Reference category: lowest exposure Intermedaite exposure 1: OR 0.34 (95% CI 0.15‐0.76) Intermediate exposure 2: OR 0.03 (95% CI 0.01‐0.10) Highest exposure: OR 0.14 (95% CI 0.05‐0.34) |
Low risk: Selection: 3/4 stars, no description of controls Comparability: 2/2 stars, low risk Exposure: 2/3 stars, non‐response rate NR Total score: 7/9 stars, moderate quality. |
| Goodman 2003 | Ovarian cancer Reference category: lowest exposure Highest exposure: OR 0.9 (95% CI 0.6‐1.5) Highest exposure A: OR 1.0 (95% CI 0.6‐1.9) Highest exposure B: OR 0.9 (95% CI 0.5‐1.6) |
Low risk: Selection: 4/4 stars, low risk Comparability: 1/2 stars, the study did not control for smoking Exposure: 3/3 stars, low risk Total score: 8/9 stars, moderate quality. |
| Goto 1990 | Pancreatic cancer Reference category: lowest exposure Highest exposure: OR 0.34 (95% CI 0.17‐0.67) |
Low risk: Selection: 3/4 stars, no description of source of controls Comparability: 2/2 stars, low risk Exposure: 2/3 stars, non‐response rate different between cases and controls or NR Total score: 7/9 stars, moderate quality. |
| Green 2014 | Reference category: lowest exposure Colorectal cancer Intermediate exposure 1: OR 0.99 (95% CI 0.64‐1.52) Intermediate exposure 2: OR 1.15 (95% CI 0.62‐2.13) Highest exposure: OR 0.99 (95% CI 0.62‐1.58) Proximal colon cancer Intermediate exposure 1: OR 1.36 (95% CI 0.76‐2.41) Intermediate exposure 2: OR 0.61 (95% CI 0.20‐1.83) Highest exposure: OR 0.95 (95% CI 0.50‐1.78) Distal colon cancer Intermediate exposure 1: OR 0.75 (95% CI 0.38‐1.46) Intermediate exposure 2: OR 1.31 (95% CI 0.55‐3.10) Highest exposure: OR 0.97 (95% CI 0.47‐2.03) Rectal cancer Intermediate exposure 1: OR 0.91 (95% CI 0.48‐1.71) Intermediate exposure 2: OR 1.49 (95% CI 0.67‐3.32) Highest exposure: OR 1.05 (95% CI 0.53‐2.10) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Exposure: 2/3 stars, non‐response rate different between cases and controls or NR Total score: 8/9 stars, moderate quality. |
| Hakim 2000 | Skin cancer Reference category: lowest exposure Highest exposure: OR 0.82 (95% CI 0.35‐1.90) |
Low risk: Selection: 4/4 stars, low risk Comparability: 1/2 stars, the study did not control for smoking. Exposure: 3/3 stars, low risk Total score: 8/9 stars, moderate quality. |
| Han 2008 | Reference category: lowest exposure Lung cancer Exposure assessment 1: Highest exposure: OR 0.520 (95% CI 0.392‐0.691) Exposure assessment 2: Intermediate exposure 1: OR 0.222 (95% CI 0.127‐0.390) Intermediate exposure 2: OR 0.687 (95% CI 0.448‐1.052) Highest exposure: OR 0.734 (95% CI 0.476‐1.132) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Exposure: 2/3 stars, non‐response rate different between cases and controls or NR Total score: 8/9 stars, moderate quality. |
| Hemelt 2010 | Bladder cancer Reference category: lowest exposure Exposure assessment 1: Highest exposure: OR 0.95 (95% CI 0.68‐1.32) Exposure assessment 2: Intermediate exposure: OR 0.83 (95% CI 0.54‐1.27) Highest exposure: OR 1.02 (95% CI 0.71‐1.48) further divided by cups/d: < 4 cups/d OR 1.23 (95% CI 0.76‐1.97); > 4 cups/d OR 0.83 (95% CI 0.53‐1.28) |
Low risk: Selection: 3/4 stars, hospital controls Comparability: 2/2 stars, low risk Exposure: 2/3 stars, non‐response rate different between cases and controls or NR Total score: 7/9 stars, moderate quality. |
| Hoshiyama 1992 | Reference category: lowest exposure Single stomach cancer Intermediate exposure: OR 1.2 (95% CI 0.8‐1.7) Highest exposure: OR 0.9 (95% CI 0.6‐1.3) Multiple stomach cancer Intermediate exposure: OR 1.5 (95% CI 0.6‐3.5) Highest exposure: OR 1.6 (95% CI 0.7‐3.9) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Exposure: 3/3 stars, non‐response rate different between cases and controls or NR Total score: 8/9 stars, moderate quality. |
| Hsu 2012 | Reference category: lowest exposure Nasopharyngeal carcinoma Intermediate exposure: OR 0.58 (95% CI 0.35‐0.98) Highest exposure: OR 0.61 (95% CI 0.40‐0.91) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Exposure: 2/3 stars, non‐response rate different between cases and controls or NR Total score: 8/9 stars, moderate quality. |
| Huang 1999 |
Huang 1999 Reference category: lowest exposure Stomach cancer Intermediate exposure 1: OR 0.88 (95% CI 0.73‐1.05) Intermediate exposure 2: OR 1.08 (95% CI 0.90‐1.24) Highest exposure: OR 0.90 (95% CI 0.73‐1.11) Inoue 1994 Reference category: lowest exposure Stomach cancer (total) Highest exposure: OR 1.09 (95% CI 0.83‐1.43) Subsite Cardia Highest exposure: OR 1.12 (95% CI 0.70‐1.79) Middle Highest exposure: OR 1.06 (95% CI 0.73‐1.54) Antrum Highest exposure: OR 1.10 (95% CI 0.78‐1.57) Kato 1990a Reference category: lowest exposure Stomach cancer Men Intermediate exposure: RR 1.14 (95% CI 0.82‐1.60) Highest exposure: RR 1.01 (95% CI 0.70‐1.47) Women Intermediate exposure: RR 0.71 (95% CI 0.45‐1.14) Highest exposure: RR 0.81 (95% CI 0.51‐1.27) |
High risk: Huang 1999 Selection: 3/4 stars, hospital controls Comparability: 2/2 stars, low risk Exposure: 2/3 stars, non‐respondent described Total score: 7/9 stars, moderate quality. Kato 1990a Selection: 2/4 stars, potential bias in selection of cases and hospital controls Comparability: 1/2 stars, the study did not control for smoking. Exposure: 2/3 stars, self‐reported exposure not within a structured interview or questionnaire. Total score: 5/9 stars, low quality. Inoue 1994 Selection: 3/4 stars, hospital controls Comparability: 1/2 stars, the study did not control for smoking. Exposure: 2/3 stars, self‐reported exposure not within a structured interview or questionnaire. Total score: 6/9 stars, low quality. |
| Inoue 1998 | Reference category: lowest exposure Oesophageal cancer Intermediate exposure 1: OR 1.02 (95% CI 0.50‐2.10) Intermediate exposure 2: OR 1.07 (95% CI 0.58‐2.00) Intermediate exposure 3: OR 0.96 (95% CI 0.50‐1.83) Highest exposure: OR 1.14 (95% CI 0.55‐2.34) Stomach cancer (also reported in Inoue 1994 in: Huang 1999 and Inoue 2009a) Intermediate exposure 1: OR 1.00 (95% CI 0.77‐1.44) Intermediate exposure 2: OR 0.96 (95% CI 0.70‐1.32) Intermediate exposure 3: OR 1.01 (95% CI 0.74‐1.39) Highest exposure: OR 0.69 (95% CI 0.48‐1.00) Colon cancer Intermediate exposure 1: OR 0.62 (95% CI 0.36‐1.05) Intermediate exposure 2: OR 0.64 (95% CI 0.42‐1.00) Intermediate exposure 3: OR 0.76 (95% CI 0.49‐1.17) Highest exposure: OR 0.77 (95% CI 0.47‐1.26) Rectal cancer Intermediate exposure 1: OR 1.41 (95% CI 0.70‐2.83) Intermediate exposure 2: OR 1.04 (95% CI 0.55‐1.98) Intermediate exposure 3: OR 1.42 (95% CI 0.75‐2.69) Highest exposure: OR 1.25 (95% CI 0.62‐2.51) |
Low risk: Selection: 3/4 stars, hospital controls Comparability: 2/2 stars, low risk Exposure: 2/3 stars, self‐reported exposure not within a structured interview or questionnaire. Total score: 7/9 stars, moderate quality. |
| Inoue 2008 | Reference category: lowest exposure Breast cancer Intermediate exposure: OR 0.65 (95% CI 0.45‐0.94) Highest exposure: OR 1.00 (95% CI 0.82‐1.22) |
Low risk: Selection: 4/4 stars, low risk Comparability: 1/2 stars, the study did not control for smoking Exposure: 2/3 stars, non‐response rate different between cases and controls Total score: 7/9 stars, moderate quality. |
| Islami 2009 | Reference category: lowest exposure Oesophageal cancer Highest exposure: OR 0.89 (95% CI 0.38‐2.09) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Exposure: 2/3 stars, non‐respondents described Total score: 8/9 stars, moderate quality. |
| Iwasaki 2014 | Reference category: lowest exposure Breast cancer Intermediate exposure: OR 0.86 (95% CI 0.53‐1.41) Highest exposure: OR 1.27 (95% CI 0.75‐2.14) |
Low risk: Selection: 3/4 stars, hospital controls Comparability: 2/2 stars, low risk Exposure: 2/3 stars, non‐respondents described Total score: 7/9 stars, moderate quality. |
| Ji 1996 | Reference category: lowest exposure Stomach cancer Men Exposure assessment A: green tea drinking status Highest exposure: OR 0.96 (95% CI 0.77‐1.21) Exposure assessment B: consumption of green tea leaves Intermediate exposure 1: OR 1.06 (95% CI 0.76‐1.49) Intermediate exposure 2: OR 1.15 (95% CI 0.82‐1.61) Intermediate exposure 3: OR 0.88 (95% CI 0.55‐1.24) Highest exposure: OR 0.76 (95% CI 0.55‐1.27) Women Exposure assessment A: green tea drinking status Highest exposure: OR 0.77 (95% CI 0.52‐1.13) Exposure assessment B: consumption of green tea leaves Intermediate exposure: OR 0.74 (95% CI 0.45‐1.21) Highest exposure: OR 0.81 (95% CI 0.46‐1.43) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Exposure: 2/3 stars, non‐response rate different between cases and controls or NR Total score: 8/9 stars, moderate quality. |
| Ji 1997 | Reference category: lowest exposure Men Colon cancer Exposure assessment A: green tea drinking habit Highest exposure: OR 0.99 (95% CI 0.74‐1.33) Exposure assessment B: green tea consumption Intermediate exposure 1: OR 1.13 (95% CI 0.80‐1.61) Intermediate exposure 2: OR 0.92 (95% CI 0.62‐1.37) Highest exposure: OR 0.82 (95% CI 0.52‐1.28) Exposure assessment C: lifetime green tea consumption Intermediate exposure 1: OR 0.93 (95% CI 0.63‐1.35) Intermediate exposure 2: OR 1.06 (95% CI 0.72‐1.56) Highest exposure: OR 1.01 (95% CI 0.67‐1.51) Rectal cancer Exposure assessment A: green tea drinking habit Highest exposure: OR 0.82 (95% CI 0.61‐1.10) Exposure assessment B: green tea consumption Intermediate exposure 1: OR 0.99 (95% CI 0.69‐1.41) Intermediate exposure 2: OR 0.66 (95% CI 0.43‐0.99) Highest exposure: OR 0.72 (95% CI 0.46‐1.13) Exposure assessment C: lifetime green tea consumption Intermediate exposure 1: OR 0.88 (95% CI 0.60‐1.27) Intermediate exposure 2: OR 0.75 (95% CI 0.50‐1.13) Highest exposure: OR 0.80 (95% CI 0.53‐1.23) Pancreatic cancer Exposure assessment A: green tea drinking habit Highest exposure: OR 0.88 (95% CI 0.60‐1.31) Exposure assessment B: green tea consumption Intermediate exposure 1: OR 1.23 (95% CI 0.79‐1.92) Intermediate exposure 2: OR 0.57 (95% CI 0.32‐1.03) Highest exposure: OR 0.63 (95% CI 0.34‐1.17) Exposure assessment C: lifetime green tea consumption Intermediate exposure 1: OR 1.11 (95% CI 0.69‐1.79) Intermediate exposure 2: OR 0.86 (95% CI 0.51‐1.45) Highest exposure: OR 0.59 (95% CI 0.32‐1.07) Women Colon cancer Exposure assessment A: green tea drinking habit Highest exposure: OR 0.77 (95% CI 0.56‐1.06) Exposure assessment B: green tea consumption Intermediate exposure: OR 0.83 (95% CI 0.57‐1.21) Highest exposure: OR 0.67 (95% CI 0.41‐1.10) Exposure assessment C: lifetime green tea consumption Intermediate exposure: OR 0.87 (95% CI 0.60‐1.27) Highest exposure: OR 0.62 (95% CI 0.38‐1.02) Rectal cancer Exposure assessment A: green tea drinking habit Highest exposure: OR 0.51 (95% CI 0.36‐0.73) Exposure assessment B: green tea consumption Intermediate exposure: OR 0.51 (95% CI 0.33‐0.79) Highest exposure: OR 0.57 (95% CI 0.34‐0.97) Exposure assessment C: lifetime green tea consumption Intermediate exposure: OR 0.54 (95% CI 0.35‐0.84) Highest exposure: OR 0.52 (95% CI 0.30‐0.89) Pancreatic cancer: Exposure assessment A: green tea drinking habit Highest exposure: OR 0.47 (95% CI 0.29‐0.77) Exposure assessment B: green tea consumption Intermediate exposure: OR 0.47 (95% CI 0.25‐0.89) Highest exposure: OR 0.53 (95% CI 0.25‐1.09) Exposure assessment C: lifetime green tea consumption Intermediate exposure: OR 0.59 (95% CI 0.32‐1.10) Highest exposure: OR 0.38 (95% CI 0.18‐0.82) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Exposure: 2/3 stars, non‐respondents described Total score: 8/9 stars, moderate quality. |
| Jia 2016 | Reference category: lowest exposure Lung cancer + mesothelioma mortality Intermediate exposure 1: OR 0.88 (95% CI 0.66‐0.87) Intermediate exposure 2: OR 2.18 (95% CI 0.96‐3.50) Highest exposure: OR 0.70 (95% CI 0.57‐0.86) |
Low risk: Selection: 4/4 stars, low risk Comparability: 1/2 stars, the study did not control for smoking Exposure: 2/3 stars, interview not blinded to case/control status Total score: 7/9 stars, moderate quality. |
| Jian 2004 | Prostate cancer Reference category: lowest exposure Exposure assessment A: green tea drinking habits Highest exposure: OR 0.28 (95% CI 0.17‐0.47) Exposure assessment B: intake of green tea Intermediate exposure: OR 0.53 (95% CI 0.30‐0.94) Highest exposure: OR 0.27 (95% CI 0.15‐0.48) |
Low risk: Selection: 3/4 stars, hospital controls Comparability: 2/2 stars, low risk Exposure: 3/3 stars, low risk Total score: 8/9 stars, moderate quality. |
| Jin 2013 | Reference category: lowest exposure Lung cancer Exposure assessment A: green tea drinking habits Highest exposure: OR 0.78 (95% CI 0.65‐0.95) Exposure assessment B: consumption of green tea Intermediate exposure 1: OR 0.76 (95% CI 0.54‐1.07) Intermediate exposure 2: OR 0.84 (95% CI 0.66‐1.08) Highest exposure: OR 0.74 (95% CI 0.56‐0.99) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Exposure: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Kakuta 2009 | Reference category: lowest exposure Endometrial cancer Intermediate exposure 1: OR 0.77 (95% CI 0.37‐1.58) Intermediate exposure 2: OR 0.61 (95% CI 0.30‐1.23) Highest exposure: OR 0.33 (95% CI 0.15‐0.75) |
Low risk: Selection: 3/4 stars, hospital controls Comparability: 2/2 stars, low risk Exposure: 3/3 stars, low risk Total score: 8/9 stars, moderate quality. |
| Kato 1990 | Reference category: lowest exposure Colon cancer Highest exposure: RR 0.61 (95% CI 0.41‐0.91) Rectal cancer Highest exposure: RR 1.32 (95% CI 0.78‐2.23) |
High risk: Selection: 3/4 stars, potential for selection bias in cases Comparability: 1/2 stars, the study did not control for smoking Exposure: 2/3 stars, self‐reported exposure not within a structured interview or questionnaire. Total score: 6/9 stars, low quality. |
| Kono 1988 | Stomach cancer Reference category: lowest exposure Population controls Highest exposure: OR 0.3 (95% CI 0.1‐0.7) Hospital controls Highest exposure: OR 0.5 (95% CI 0.3‐1.1) |
High risk: Selection: 4/4 stars when using population controls and 3/4 when using hospital controls Comparability: 2/2 stars, low risk Exposure: 1/3 stars, different methods of exposure ascertainment in cases and controls and self‐reported exposure not within a structured interview or questionnaire. Total score: 7/9 stars, moderate quality and 6/9 stars low quality. |
| Kubik 2008 |
Kubik 2008 Reference category: lowest exposure (OR for trend only reported) Lung cancer Men nonsmokers Highest exposure: OR 1.08 (95% CI 0.56‐2.08) Men smokers Highest exposure: OR 0.93 (95% CI 0.63‐1.38) Women nonsmokers Highest exposure: OR 0.88 (95% CI 0.61‐1.27) Women smokers Highest exposure: OR 1.09 (95% CI 0.80‐1.49) Data on former smokers who quit from 10 to < 20 years were excluded from the reported analysis. Previous report on fewer female cases reported in Kubik 2004. Kubik 2004 Reference category: lowest exposure Lung cancer Intermediate exposure: OR 0.97 (95% CI 0.69‐1.38) Highest exposure: OR 1.02 (95% CI 0.74‐1.40) Also analysis available stratified by smoking habits Subsequent report in Kubik 2008 with longer recruitment of women from the same area |
Low risk: Kubik 2004 Selection: 3/4 stars, hospital controls Comparability: 2/2 stars, low risk Exposure: 2/3 stars, non‐response rate different between cases and controls or NR Total score: 7/9 stars, moderate quality. Kubik 2008 Selection: 3/4 stars, hospital controls Comparability: 2/2 stars, low risk Exposure: 2/3 stars, non‐response rate different between cases and controls or NR Total score: 7/9 stars, moderate quality. |
| Kuo 2009 | Leukaemia Green‐tea‐only consumption compared to no‐tea consumption Cases exposed = 9, controls exposed = 24, cases not exposed = 63, controls not exposed = 123 OR 0.73 (95% CI 0.32‐1.67) Consumption of green tea with other types of tea compared to no‐tea consumption Cases exposed = 17, controls exposed = 61, cases not exposued = 63, controls not exposed = 123 OR 0.54 (95% CI 0.29‐1.01) |
High risk: Selection: 4/4 stars, low risk Comparability: 0/2 stars, only crude analysis without adjustment for confounding factors Exposure: 2/3 stars, non‐response rate different between cases and controls or NR Total score: 6/9 stars, low quality. |
| Lassed 2016 | Reference category: lowest exposure Postate cancer Intermediate exposure: OR 0.64 (95% CI 0.36‐1.15) Highest exposure: OR 0.40 (95% CI 0.08‐1.92) |
High risk: Selection: 3/4 stars, hospital controls Comparability: 0/2 stars, crude analysis with no adjustment for confounding factors Exposure: 3/3 stars, low risk Total score: 6/9 stars, low quality. |
| Le Marchand 2000 | Reference category: lowest exposure Lung cancer Intermediate exposure 1: OR 1.0 (95% CI 0.6‐1.7) Intermediate exposure 2: OR 0.7 (95% CI 0.4‐1.3) Highest exposure: OR 0.9 (95% CI 0.5‐1.6) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Exposure: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Lee 2017 | Reference category: lowest exposure Prostate cancer Exposure assessment A Highest exposure: OR 0.60 (95% CI 0.37‐0.98) Exposure assessment B Intermediate exposure 1: OR 1.53 (95% CI 0.39‐5.93) Intermediate exposure 2: OR 0.16 (95% CI 0.01‐2.22) Highest exposure: OR 0.34 (95% CI 0.02‐6.05) |
Low risk: Selection: 3/4 stars, hospital controls Comparability: 2/2 stars, low risk Exposure: 2/3 stars, non‐response rate different between cases and controls or NR Total score: 7/9 stars, moderate quality. |
| Lei 1994 | Reference category: lowest exposure Lung cancer Highest exposure: OR 0.71 (95% CI 0.43‐1.18) |
High risk: Selection: 1/4 stars, no description of identification of cases with potential selection bias and hospital controls Comparability: 1/2 stars, the study did not control for smoking. Exposure: 1/3 star, no description of exposure ascertainment and non‐response rate between cases and controls NR Total score: 3/9 stars, low quality. |
| Leung 2016 | Reference category: lowest exposure Ovarian cancer Exposure assessment A Highest exposure: OR 0.84 (95% CI 0.54‐1.30) Exposure assessment B Intermediate exposure 1: OR 1.03 (95% CI 0.50‐2.00) Intermediate exposure 2: OR 0.78 (95% CI 0.37‐1.55) Highest exposure: OR 0.77 (95% CI 0.39‐1.46) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Exposure: 3/3 stars, low risk Total score: 9/9 stars, high quality |
| Li 2011a | Reference category: lowest exposure Results using population controls All cancer Exposure assessment A Highest exposure: OR 0.78 (95% CI 0.60‐1.01) Exposure assessment B Intermediate exposure: OR 0.71 (95% CI 0.47‐1.08) Highest exposure: OR 0.51 (95% CI 0.27‐0.74) Breast cancer Exposure assessment A Highest exposure: OR 0.61 (95% CI 0.32‐1.18) Exposure assessment B Intermediate exposure: OR 0.60 (95% CI 0.24‐1.50) Highest exposure: OR 0.07 (95% CI 0.01‐0.47) Colorectal cancer Exposure assessment A Highest exposure: OR 0.62 (95% CI 0.42‐0.92) Exposure assessment B Intermediate exposure: OR 0.55 (95% CI 0.28‐1.06) Highest exposure: OR 0.45 (95% CI 0.25‐0.82) Leukaemia Exposure assessment A Highest exposure: OR 1.55 (95% CI 0.61‐3.96) Exposure assessment B Intermediate exposure: OR not estimable Highest exposure: OR 0.57 (95% CI 0.07‐4.79) Results using hospital controls All cancer Exposure assessment A Highest exposure: OR 0.77 (95% CI 0.59‐1.00) Exposure assessment B: Intermediate exposure: OR 0.60 (95% CI 0.40‐0.92) Highest exposure: OR 0.51 (95% CI 0.31‐0.83) Breast cancer Exposure assessment A Highest exposure: OR 0.55 (95% CI 0.28‐1.06) Exposure assessment B Intermediate exposure: OR 0.54 (95% CI 0.22‐1.33) Highest exposure: OR 0.06 (95% CI 0.01‐0.61) Colorectal cancer Exposure assessment A Highest exposure: OR 0.67 (95% CI 0.45‐0.99) Exposure assessment B Intermediate exposure: OR 0.51 (95% CI 0.26‐0.99) Highest exposure: OR 0.52 (95% CI 0.29‐0.94) Leukaemia Exposure assessment A Highest exposure: OR 1.46 (95% CI 0.55‐3.84) Exposure assessment B Intermediate exposure: OR not estimable Highest exposure: OR 0.65 (95% CI 0.08‐5.63) |
Low risk: Selection: 4/4 stars when using population controls and 3/4 when using hospital controls, low risk Comparability: 2/2 stars, low risk Exposure: 2/3 stars, non‐response rate different between cases and controls or NR Total score: 8/9 stars and 7/9 stars, moderate quality. |
| Li 2014 | Reference category: lowest exposure Prostate cancer Highest exposure: OR 0.59 (95% CI 0.40‐0.87) |
Low risk: Selection: 4/4 stars, low risk Comparability: 1/2 stars, the study did not control for smoking Exposure: 2/3 stars, self‐reported exposure not within a structured interview or questionnaire. Total score: 7/9 stars, moderate quality. |
| Li 2016 | Reference category: lowest exposure Breast cancer Highest exposure: OR 1.20 (95% CI 0.80‐1.78) |
Low risk: Selection: 3/4 stars, hospital controls Comparability: 1/2 stars, the study did not control for smoking Exposure: 3/3 stars, low risk Total score: 7/9 stars, moderate quality. |
| Lin 2012 | Reference category: highest exposure Lung cancer Intermediate exposure: RR 3.01 (95% CI 1.13‐8.05) Lowest exposure: RR 6.34 (95% CI 2.69‐14.91) |
High risk: Selection: 3/4 stars, hospital controls Comparability: 1/2 stars, the study did not control for smoking. Exposure: 2/3 stars, self‐reported exposure not within a structured interview or questionnaire. Total score: 6/9 stars, low quality. |
| Liu 2010 | Reference category: lowest exposure Stomach cancer: Highest exposure: OR 0.42 (95%CI 0.32‐0.55) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Exposure: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Liu 2017 | Reference category: highest exposure Leukaemia Lowest exposure: OR 0.71 (95% CI 0.46‐1.07) |
Low risk: Selection: 3/4 stars, hospital controls Comparability: 2/2 stars, low risk Exposure: 3/3 stars, low risk Total score: 8/9 stars, moderate quality. |
| Mao 2011 | Reference category: lowest exposure Stomach cancer Exposure assessment A: green‐tea drinking status Intermediate exposure: OR 0.58 (95% CI 0.46‐5.03) Highest exposure: OR 1.02 (95% CI 0.70‐1.64) Exposure assessment B: green tea consumption Intermediate exposure 1: OR 0.88 (95% CI 0.57‐1.36) Intermediate exposure 2: OR 1.15 (95% CI 0.76‐2.35) Highest exposure: OR 1.13 (95% CI 0.52‐2.67) |
High risk: Selection: 2/4 stars, hospital controls with no description Comparability: 2/2 stars, low risk Exposure: 2/3 stars, self‐reported exposure not within a structured interview or questionnaire. Total score: 6/9 stars, low quality. |
| Mizoo 2013 | Reference category: lowest exposure Breast cancer Intermediate exposure 1: OR 0.97 (95% CI 0.71‐1.33) Intermediate exposure 2: OR 0.63 (95% CI 0.43‐0.93) Highest exposure: OR 0.72 (95% CI 0.46‐1.12) |
Low risk: Selection: 3/4 stars, hospital controls Comparability: 1/2 stars, the study did not control for smoking Exposure: 3/3 stars, low risk Total score: 7/9 stars, moderate quality. |
| Mizuno 1992 | Reference category: lowest exposure Pancreatic cancer Highest exposure: OR 1.94 (95% CI 1.06‐3.55) |
High risk: Selection: 3/4 stars, hospital controls Comparability: 1/2 stars, the study did not control for smoking Exposure: 2/3 stars, self‐reported exposure not within a structured interview or questionnaire. Total score: 6/9 stars, low quality. |
| Mu 2003 | Reference category: lowest exposure Stomach cancer (Mu 2005) Intermediate exposure 1: OR 1.09 (95% CI 0.53‐2.23) Intermediate exposure 2: OR 0.44 (95% CI 0.19‐1.01) Highest exposure: OR 0.40 (95% CI 0.14‐1.09) Exposure assessment B: green tea intake Highest exposure: OR 0.59 (95% CI 0.34‐1.01) Liver cancer (Li 2011b) Intermediate exposure 1: OR 1.21 (95% CI 0.62‐2.36) Intermediate exposure 2: OR 0.76 (95% CI 0.38‐1.51) Highest exposure: OR 0.55 (95% CI 0.28‐1.09) Oesophageal cancer Intermediate exposure 1: OR 1.13 (95% CI 0.67‐1.92) Intermediate exposure 2: OR 0.78 (95% CI 0.46‐1.34) Highest exposure: OR 0.58 (95% CI 0.35‐0.97) |
Low risk: Li 2011b Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Exposure: 2/3 stars, non‐response rate different between cases and controls or NR Total score: 8/9 stars, moderate quality. Mu 2003 Selection: 3/4 stars, potential selection bias for cases Comparability: 2/2 stars, low risk Exposure: 2/3 stars, non‐response rate different between cases and controls or NR Total score: 7/9 stars, moderate quality. Mu 2005 Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Exposure: 2/3 stars, non‐response rate different between cases and controls or NR Total score: 8/9 stars, moderate quality. |
| Nagle 2010 | Reference category: lowest exposure Ovarian cancer Intermediate exposure 1: OR 0.77 (95% CI 0.61‐0.98) Intermediate exposure 2: OR 0.85 (95% CI 0.63‐1.15) Intermediate exposure 3: OR 0.80 (95% CI 0.54‐1.19) Intermediate exposure 4: OR 0.92 (95% CI 0.57‐1.49) Highest exposure: OR 0.82 (95% CI 0.38‐1.79) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Exposure: 2/3 stars, non‐response rate different between cases and controls or NR Total score: 8/9 stars, moderate quality |
| Oze 2014 | Reference category: lowest exposure Upper aerodigestive tract cancer Intermediate exposure 1: OR 1.25 (95% CI 0.98–1.60) Intermediate exposure 2: OR 1.02 (95% CI 0.77–1.34) Highest exposure: OR 1.39 (95% CI 1.13–1.70) Subsite Oesophageal cancer Intermediate exposure 1: OR 1.20 (95% CI 0.82–1.77) Intermediate exposure 2: OR 1.00 (95% CI 0.65–1.55) Highest exposure: OR 1.31 (95% CI 0.95–1.81) Oral, pharyngeal and laryngeal cancer Intermediate exposure 1: OR 1.34 (95% CI 0.97–1.86) Intermediate exposure 2: OR 1.03 (95% CI 0.71–1.50) Highest exposure: OR 1.47 (95% CI 1.12–1.93) Available also stratified analysis by smoking status (never, ever) and alcoholic drinker |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Exposure: 2/3 stars, self‐reported exposure not within a structured interview or questionnaire. Total score: 8/9 stars, moderate quality. |
| Peng 2013 | Reference category: lowest exposure Colorectal cancer Highest exposure: OR 0.54 (95% CI 0.41‐0.72) |
Low risk: Selection: 4/4 stars, low risk Comparability: 1/2 stars, the study did not control for smoking. Exposure: 2/3 stars, self‐reported exposure not within a structured interview or questionnaire. Total score: 7/9 stars, moderate quality |
| Peng 2015 | Reference category: lowest exposure Oesophageal cancer Highest exposure: OR 0.63 (95% CI 0.45‐0.91) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Exposure: 2/3 stars, self‐reported exposure not within a structured interview or questionnaire. Total score: 8/9 stars, moderate quality. |
| Ruan 2010 | Reference category: lowest exposure Nasopharyngeal cancer Highest exposure: OR 0.44 (95% CI 0.36–0.54) |
Low risk: Selection: 3/4 stars, hospital controls Comparability: 1/2 stars, the study did not control for smoking Exposure: 3/3 stars, low risk Total score: 7/9 stars, moderate quality. |
| Setiawan 2001 | Reference category: lowest exposure Stomach cancer Exposure assessment A Highest exposure: OR 0.52 (95% CI 0.29–0.94) Exposure assessment B Intermediate exposure: OR 0.70 (95% CI 0.36–1.36) Highest exposure: OR 0.39 (95% CI 0.15–1.01) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Exposure: 2/3 stars, non‐response rate different between cases and controls or NR Total score: 8/9 stars, moderate quality. |
| Shrubsole 2009 | Reference category: lowest exposure Breast cancer Exposure assessment A Highest exposure: OR 0.88 (95% CI 0.79–0.98) Exposure assessment B Intermediate exposure 1: OR 0.89 (95% CI 0.73–1.08) Intermediate exposure 2: OR 0.93 (95% CI 0.77–1.12) Intermediate exposure 3: OR 0.72 (95% CI 0.59–0.88) Highest exposure: OR 0.97 (95% CI 0.80–1.16) Stratified analysis also available in pre‐ and post‐menopausal women |
Low risk: Selection: 4/4 stars, low risk Comparability: 1/2 stars, the study did not control for smoking. Exposure: 2/3 stars, self‐reported exposure not within a structured interview or questionnaire. Total score: 7/9 stars, moderate quality. |
| Song 2008 | Reference category: lowest exposure Ovarian cancer Intermediate category: OR 0.82 (95% CI 0.66‐1.04) Highest exposure: OR 0.46 (95% CI 0.26‐0.84) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Exposure: 2/3 stars, self‐reported exposure not within a structured interview or questionnaire. Total score: 8/9 stars, moderate quality |
| Sonoda 2004 | Reference category: lowest exposure Prostate cancer Intermediate exposure 1: OR 0.99 (95% CI 0.48‐2.03) Intermediate exposure 2: OR 0.79 (95% CI 0.38‐1.63) Highest exposure: OR 0.67 (95% CI 0.27‐1.64) |
Low risk: Selection: 3/4 stars, hospital controls Comparability: 2/2 stars, low risk Exposure: 2/3 stars, self‐reported exposure not within a structured interview or questionnaire. Total score: 7/9 stars, moderate quality. |
| Tajima 1985 | Reference category: lowest exposure Stomach cancer Highest exposure: OR 0.64, CIs NR Colon cancer Highest exposure: OR 0.97, CIs NR Rectal cancer Highest exposure: OR 0.91, CIs NR |
High risk: Selection: 3/4 stars, hospital controls Comparability: 1/2 stars, the study did not control for smoking Exposure: 2/3 stars, self‐reported exposure not within a structured interview or questionnaire. Total score: 6/9 stars, low quality. |
| Takezaki 2000 | Reference category: lowest exposure Pharynx (hypopharynx) cancer Intermediate exposure: OR 0.8 (95% CI 0.4‐1.5) Highest exposure: OR 0.8 (95% CI 0.3‐2.3) Oesophageal cancer Intermediate exposure: OR 0.8 (95% CI 0.6‐1.1) Highest exposure: OR 0.7 (95% CI 0.4‐1.2) Analysis also reported for upper, middle and lower third of the oesophagus |
Low risk: Selection: 3/4 stars, hospital controls Comparability: 2/2 stars, low risk Exposure: 3/3 stars, low risk Total score: 8/9 stars, moderate quality. |
| Takezaki 2001 | Reference category: lowest quartile Adenocarcinoma Men Intermediate exposure 1: OR 1.06 (95% CI 0.72‐1.57) Intermediate exposure 2: OR 1.11 (95% CI 0.74‐1.66) Highest exposure: OR 1.33 (95% CI 0.83‐2.15) Women Intermediate exposure 1: OR 0.98 (95% CI 0.58‐1.66) Intermediate exposure 2: OR 1.14 (95% CI 0.68‐1.93) Highest exposure: OR 1.14 (95% CI 0.61‐2.12) Squamous cell and small‐cell carcinoma Men Intermediate exposure 1: OR 0.99 (95% CI 0.67‐1.47) Intermediate exposure 2: OR 1.17 (95% CI 0.78‐1.73) Highest exposure: OR 1.08 (95% CI 0.66‐1.75) Women Intermediate exposure 1: OR 0.36 (95% CI 0.14‐0.93) Intermediate exposure 2: OR 0.41 (95% CI 0.16‐1.04) Highest exposure: OR 0.49 (95% CI 0.17‐1.46) |
Low risk: Selection: 3/4 stars, hospital controls Comparability: 2/2 stars, low risk Exposure: 3/3 stars, low risk Total score: 8/9 stars, moderate quality. |
| Tewes 1990 | Reference category: lowest exposure Lung cancer Highest exposure: OR 2.74 (95% CI 1.10‐6.80) |
Low risk: Selection: 3/4 stars, case identification with no independent validation Comparability: 2/2 stars, low risk Exposure: 2/3 stars, self‐reported exposure not within a structured interview or questionnaire. Total score: 7/9 stars, moderate quality. |
| Tse 2017 | Reference category: lowest exposure Prostate cancer Highest exposure: OR 0.56 (95% CI 0.34‐0.91) |
High risk: Selection: 3/4 stars, hospital controls Comparability: 1/2 stars, the study did not control for smoking. Exposure: 2/3 stars, self‐reported exposure not within a structured interview or questionnaire. Total score: 6/9 stars, low quality. |
| Wakai 2004 | Reference category: lowest exposure Bladder cancer Intermediate exposure 1: OR 1.49 (95% CI 0.78–2.84) Intermediate exposure 2: OR 2.79 (95% CI 1.49–5.23) Highest exposure: OR 1.24 (95% CI 0.51–2.99) |
Low risk: Selection: 3/4 stars, hospital controls Comparability: 2/2 stars, low risk Exposure: 3/3 stars, low risk Total score: 8/9 stars, moderate quality. |
| Wang 1999 | Reference category: lowest exposure Oesophageal cancer Highest exposure: OR 0.20 (95% CI 0.06‐0.67) Other stomach cancer Highest exposure: OR 0.28 (CIs NR) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Exposure: 1/3 stars, no description of exposure ascertainment and non‐response rate different between cases and controls or NR Total score: 7/9 stars, moderate quality. |
| Wang 2006 | Reference category: lowest quartile Oesophageal cancer Highest exposure: OR 0.13 (95% CI 0.03‐0.62) |
High risk: Selection: 4/4 stars, low risk Comparability: 0/2 stars, no information on confounders as smoking Exposure: 2/3 stars, self‐reported exposure not within a structured interview or questionnaire. Total score: 6/9 stars, low quality. |
| Wang 2007 | Reference category: lowest exposure Oesophageal cancer Men Highest exposure: OR 1.368 (95% CI 0.948‐1.975) Women Highest exposure: OR 0.257 (95% CI 0.070‐0.941) Available analysis according green tea drinking duration (years) |
Low risk: Selection: 4/4 stars, low risk Comparability: 1/2 stars, the study did not control for smoking. Exposure: 2/3 stars, self‐reported exposure not within a structured interview or questionnaire. Total score: 7/9 stars, moderate quality. |
| Wang 2012a | Reference category: lowest exposure Renal cancer Highest exposure: OR 0.34 (95% CI 0.21–0.55) |
High risk: Selection: 3/4 stars, hospital controls Comparability: 1/2 stars, the study did not control for smoking. Exposure: 2/3 stars, non‐response rate different between cases and controls or NR Total score: 6/9 stars, low quality. |
| Wang 2012b | Reference category: lowest exposure Multiple myeloma Intermediate exposure 1: OR 0.94 (95% CI 0.52–1.72) Intermediate exposure 2: OR 0.51 (95% CI 0.35‐0.73) Highest exposure: OR 0.27 (95% CI 0.13–0.58) Overall exposure: OR 0.38 (95% CI 0.27‐0.53) |
Low risk: Selection: 3/4 stars, hospital controls Comparability: 1/2 stars, the study did not control for smoking. Exposure: 3/3 stars, low risk Total score: 7/9 stars, moderate quality. |
| Wang 2012c | Reference category: lowest exposure Pancreatic cancer Men Exposure assessment A Highest exposure: OR 1.02 (95% CI 0.78‐1.35) Exposure assessment B Intermediate exposure 1: OR 0.99 (95% CI 0.71‐1.40) Intermediate exposure 2: OR 1.38 (95% CI 0.91‐2.11) Highest exposure: OR 0.91 (95% CI 0.65‐1.27) Women Exposure assessment A Highest exposure: OR 0.68 (95% CI 0.48‐0.96) Exposure assessment B Intermediate exposure 1: OR 0.85 (95% CI 0.49–1.46) Intermediate exposure 2: OR 0.64 (95% CI 0.37‐1.11) Highest exposure: OR 0.56 (95% CI 0.32‐0.98) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Exposure: 2/3 stars, non‐response rate different between cases and controls or NR Total score: 8/9 stars, moderate quality. |
| Wang 2013a | Reference category: lowest exposure Breast cancer Highest exposure: OR 0.65 (95% CI 0.44–0.97) |
Low risk: Selection: 3/4 stars, hospital controls Comparability: 1/2 stars, the study did not control for smoking. Exposure: 3/3 stars, low risk Total score: 7/9 stars, moderate quality. |
| Wang 2013b | Reference category: lowest exposure Bladder cancer Intermediate exposure: OR 0.82 (95% CI 0.61–1.11) Highest exposure: OR 0.60 (95% CI 0.45–0.79) |
Low risk: Selection: 3/4 stars, hospital controls Comparability: 2/2 stars, low risk Exposure: 2/3 stars, non‐response rate different between cases and controls or NR Total score: 7/9 stars, moderate quality. |
| Wang 2015 | Reference category: lowest exposure Stomach cancer Exposure assessment A: green tea intake Highest exposure: OR 0.72 (95% CI 0.32‐0.98) Exposure assessment B: green tea consumption Intermediate exposure: OR 0.87 (95% CI 0.43‐1.81) Intermediate exposure: OR 0.66 (95% CI 0.36‐1.17) Highest exposure: OR 0.53 (95% CI 0.23‐0.97) |
Low risk: Selection: 3/4 stars, hospital controls Comparability: 2/2 stars, low risk Exposure: 2/3 stars, non‐response rate different between cases and controls or not reported Total score: 7/9 stars, moderate quality. |
| Wilkens 1996 | Reference category: lowest exposure Urinary tract cancer Men Intermediate exposure: OR 1.1 (95% CI 0.6‐1.9) Highest exposure: OR 1.1 (95% CI 0.6‐2.3) Women Intermediate exposure: OR 0.8 (95% CI 0.3‐2.1) Highest exposure: OR 0.9 (95% CI 0.3‐2.6) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Exposure: 2/3 stars, non‐response rate different between cases and controls or NR Total score: 8/9 stars, moderate quality. |
| Wu 2003 | Reference category: lowest exposure Breast cancer Intermediate exposure: OR 0.74 (95% CI 0.52‐1.04) Highest exposure: OR 0.61 (95% CI 0.40‐0.93) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Exposure: 3/3 stars, low risk Total score: 9/9 stars, high quality. |
| Wu 2009a | Reference category: lowest exposure Prostate cancer Highest exposure: OR 0.52 (95% CI 0.28‐0.96) Intermediate categories NR |
High risk: Selection: 2/4 stars, potential selection bias for cases and hospital controls Comparability: 1/2 stars, the study did not control for smoking. Exposure: 2/3 stars, non‐response rate different between cases and controls or NR Total score: 5/9 stars, low quality. |
| Wu 2009b | Reference category: lowest exposure Oesophageal cancer Dafeng (high risk area) Highest exposure: OR 1.0 (95% CI 0.7‐1.3) Ganyu (low risk area) Highest exposure: OR 1.3 (95% CI 0.9‐1.7) |
Low risk: Selection: 3/4 stars, potential for selection bias in cases Comparability: 2/2 stars, low risk Exposure: 3/3 stars, low risk Total score: 8/9 stars, moderate quality. |
| Xu 2007 | Reference category: lowest exposure Endometrial cancer Highest exposure: OR 0.8 (95% CI 0.6‐0.9) |
Low risk: Selection: 4/4 stars, low risk Comparability: 1/2 stars, the study did not control for smoking. Exposure: 3/3 stars, low risk Total score: 8/9 stars, moderate quality. |
| Xu 2013 | Reference category: lowest exposure Lung cancer Highest exposure: OR 0.333 (95% CI 0.154 ‐0.720) |
High risk: Selection: 1/4 star, no description of cases, potential selection bias in cases, no description of controls Comparability: 1/2 stars, the study did not control for smoking. Exposure: 2/3 stars, non‐response rate different between cases and controls or NR Total score: 4/9 stars, low quality. |
| Yan 2016 | Reference category: lowest exposure Oral cancer Highest exposure: OR 0.58 (95% CI 0.42‐0.79) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Exposure: 2/3 stars, non‐response rate different between cases and controls or NR Total score: 8/9 stars, moderate quality. |
| Ye 1998 | Reference category: lowest exposure Stomach cancer Highest exposure: OR 1.72 (95% CI 1.26‐2.36) |
Low risk: Selection: 4/4 stars, low risk Comparability: 1/2 stars, the study did not control for smoking. Exposure: 2/3 stars, self‐reported exposure not within a structured interview or questionnaire. Total score: 7/9 stars, moderate quality. |
| Yu 1995 | Reference category: lowest exposure Stomach cancer Highest exposure: OR 0.71 (95% CI 0.54‐0.93) Highest exposure A: OR 0.76 (95% CI 0.57‐1.03) Highest exposure B: OR 0.54 (95% CI 0.33‐0.88) Subsite Cardia Highest exposure: OR 0.95 (95% CI 0.51‐1.77) Highest exposure A: OR 0.94 (95% CI 0.47‐1.87) Highest exposure B: OR 0.98 (95% CI 0.34‐2.89) Pylori Highest exposure: OR 0.29 (95% CI 0.13‐0.68) Highest exposure A: OR 0.30 (95% CI 0.13‐0.73) Highest exposure B: OR 0.24 (95% CI 0.05‐1.17) Antrum Highest exposure: OR 0.67 (95% CI 0.41‐1.08) Highest exposure A: OR 0.79 (95% CI 0.48‐1.31) Highest exposure B: OR 0.29 (95% CI 0.12‐0.71) Other sites Highest exposure: OR 0.82 (95% CI 0.41‐1.65) Highest exposure A: OR 0.83 (95% CI 0.40‐1.73) Highest exposure B: OR 0.76 (95% CI 0.15‐3.87) Site unknown Highest exposure: OR 0.69 (95% CI 0.23‐2.06) Highest exposure A: OR 0.62 (95% CI 0.17‐2.18) Highest exposure B: OR 0.87 (95% CI 0.15‐5.00) |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Exposure: 2/3 stars, non‐response rate different between cases and controls or NR Total score: 8/9 stars, moderate quality. |
| Zhang 2002 | Reference category: lowest exposure Ovarian cancer Intermediate exposure 1: OR 0.42 (95% CI 0.24‐0.73) Intermediate exposure 2: OR 0.40 (95% CI 0.23‐0.70) Highest exposure: OR= 0.43 (95% CI 0.30‐0.63) |
Low risk: Selection: 3/4 stars, hospital controls Comparability: 2/2 stars, low risk Exposure: 2/3 stars, self‐reported exposure not within a structured interview or questionnaire. Total score: 7/9 stars, moderate quality. |
| Zhang 2007 | Reference category: lowest exposure Breast cancer Intermediate exposure 1: 0.85 (95% CI 0.68–1.05) Intermediate exposure 2: 0.92 (95% CI 0.75–1.12) Intermediate exposure 3: 0.57 (95% CI 0.38–0.85) Highest exposure: 0.57 (95% CI 0.47–0.69) |
Low risk: Selection: 3/4 stars, hospital controls Comparability: 2/2 stars, low risk Exposure: 3/3 stars, low risk Total score: 8/9 stars, moderate quality. |
| Zhang 2008 | Reference category: lowest exposure Leukaemia Exposure assessment A: intake of green tea Highest exposure: OR 0.51 (95% CI 0.27–0.96) Exposure assessment B: intake of green tea Intermediate exposure: OR 0.40 (95% CI 0.14–1.14) Highest exposure: OR 0.40 (95% CI 0.19–0.82) |
Low risk: Selection: 3/4 stars, hospital controls Comparability: 2/2 stars, low risk Exposure: 2/3 stars, self‐reported exposure not within a structured interview or questionnaire. Total score: 7/9 stars, moderate quality. |
| Zheng 1993 | Reference category: lowest exposure Oral cancer Highest exposure: HR 0.85 (95% CI 0.32–2.31) |
Low risk: Selection: 3/4 stars, hospital controls Comparability: 2/2 stars, low risk Exposure: 2/3 stars, self‐reported exposure not within a structured interview or questionnaire Total score: 7/9 stars, moderate quality. |
| Zhong 2001 | Reference category: lowest exposure Lung cancer Highest exposure: OR 0.65 (95% CI 0.45‐0.93) in nonsmokers Highest exposure: OR 0.94 (95% CI 0.40‐2.22) in smokers |
Low risk: Selection: 4/4 stars, low risk Comparability: 2/2 stars, low risk Exposure: 2/3 stars, non‐response rate different between cases and controls or NR Total score: 8/9 stars, moderate quality. |
Diagnoses
Any cancer type
Two cohort studies (Nagano 2001; Nakachi 2000), and one case‐control study (Li 2011a), reported incidence of any cancer. Eight cohort studies (Iwai 2002; Khan 2004; Kuriyama 2006; Liu 2016; Odegaard 2015; Saito 2015; Suzuki 2009; Zhao 2017) reported any cancer mortality.
Gastrointestinal tract cancer
Two experimental studies reported data on gastrointestinal cancer: one study assessed the clinical and histologic response of high‐risk oral lesions after green tea as ministration (Tsao 2009), and one study assessed the histological presence of rectal aberrant crypt foci of colon cancer (Sinicrope 2017). One experimental study assessing incidence of oesophageal cancer is ongoing (NCT01496521).
Seventy‐one nonexperimental studies reported data on the risk of cancer of the gastrointestinal tract and provided data on nine different types of cancer.
Oral cancer: one cohort study (Ide 2007), four case‐control studies (Chen 2017a; Fu 2013; Yan 2016; Zheng 1993)
Overall oral, pharyngeal and laryngeal cancer: one cohort study (Oze 2014)
Pharyngeal cancer: one case‐control study (Takezaki 2000)
Oesophageal cancer: three cohort studies (Ishikawa 2006, Nagano 2001; Nechuta 2012), 12 case‐control studies (Chen 2011; Gao 1994; Inoue 1998; Islami 2009; Mu 2003; Oze 2014; Peng 2015; Takezaki 2000Wang 1999; Wang 2006; Wang 2007; Wu 2009b)
Stomach cancer: seven cohort studies (Galanis 1998; Inoue 2009a; Khan 2004; Kuriyama 2006; Nagano 2001; Nechuta 2012; Suzuki 2009), 13 case‐control studies (Hoshiyama 1992; Huang 1999; Ji 1996; Kono 1988; Liu 2010; Mao 2011; Mu 2003; Setiawan 2001; Tajima 1985; Wang 1999; Wang 2015; Ye 1998; Yu 1995).
Liver cancer: five cohort studies (Inoue 2009b; Nagano 2001; Nechuta 2012; Tamura 2018; Ui 2009), one case‐control study (Mu 2003)
Pancreatic cancer: six cohort studies (Khan 2004; Lin 2008; Luo 2007; Nagano 2001; Nakamura 2011; Nechuta 2012), four case‐control studies (Goto 1990; Ji 1997; Mizuno 1992; Wang 2012c)
Biliary tract cancer: three cohort studies (Makiuchi 2016; Nagano 2001; Nechuta 2012)
Colorectal cancer: nine cohort studies (Khan 2004; Kuriyama 2006; Lee 2007; Nagano 2001; Nechuta 2012; Sun 2007; Suzuki 2005; Suzuki 2009; Yang 2011a), eight case‐control studies (Gavrilas 2018; Green 2014; Inoue 1998; Ji 1997; Kato 1990; Li 2011a; Peng 2013; Tajima 1985)
Colon cancer only: six cohort studies (Lee 2007; Nagano 2001; Nechuta 2012; Oba 2006; Suzuki 2005; Yang 2011a), four case‐control studies (Green 2014; Inoue 1998; Ji 1997; Kato 1990)
Rectal cancer only: six cohort studies (Lee 2007; Nagano 2001; Nechuta 2012; Oba 2006; Suzuki 2005; Yang 2011a), four case‐control studies (Green 2014; Inoue 1998; Ji 1997; Kato 1990).
Respiratory tract cancer
One experimental study assessing lung cancer risk reported data on quality of life only (Garland 2006).
Twenty‐one nonexperimental studies reported data on the risk of cancer of the respiratory tract and provided data on three different types of cancer.
Nasopharyngeal cancer: two case‐control studies (Hsu 2012; Ruan 2010)
Lung cancer: six cohort studies (Khan 2004; Kuriyama 2006; Li 2008; Li 2018; Nagano 2001; Suzuki 2009), and 11 case‐control studies (Bonner 2005; Han 2008; Jin 2013; Kubik 2008; Lei 1994; Le Marchand 2000; Lin 2012; Takezaki 2001; Tewes 1990; Xu 2013; Zhong 2001)
Lung cancer and mesothelioma: one case‐control study (Jia 2016).
Breast cancer
One experimental study carried out for prevention of breast cancer (Dostal 2015) did not report results on this primary outcome, but data on secondary outcomes (other types of cancer, quality of life) are available.
Fifteen nonexperimental studies reported data on the risk of breast cancer: five cohort studies (Dai 2010; Iwasaki 2010a; Key 1999; Nagano 2001; Suzuki 2004), and nine case‐control studies (Inoue 2008; Iwasaki 2014; Li 2011a; Li 2016; Mizoo 2013; Shrubsole 2009; Wang 2013a; Wu 2003; Zhang 2007)
Urogenital tract cancer
Eight experimental studies reported data on cancer of the urogenital tract and provided data on three different types of cancer.
Cervical cancer: one study reported data on cervical cancer incidence (Garcia 2014)
Endometrial cancer: one study reported data on endometrial cancer incidence (Dostal 2015) and one study carried out in women with uterine fibroids reported data on quality of life only (Roshdy 2013)
Prostate cancer: three studies reported data on incidence of prostate cancer (Bettuzzi 2006; Kumar 2015; Micali 2017), one study assessing prostate cancer incidence is ongoing (Shannon 2010), and one study assessing prostate cancer incidence reported data on PSA levels and clinical outcomes only (Lane 2018)
Thirty‐one nonexperimental studies reported data on the risk of cancer of the urogenital tract and provided data on five different types of cancer.
Prostate cancer: five cohort studies (Allen 2004; Kikuchi 2006; Kurahashi 2007; Montague 2012; Severson 1989), eight case‐control studies (Berroukche 2012; Jian 2004; Lassed 2016; Lee 2017; Li 2014; Sonoda 2004; Tse 2017; Wu 2009a)
Endometrial cancer: one cohort study (Shimazu 2008), and four case‐control studies (Bandera 2010: Gao 2005; Kakuta 2009; Xu 2007)
Ovarian cancer: five case‐control studies (Goodman 2003; Leung 2016; Nagle 2010; Song 2008; Zhang 2002)
Renal cancer: one case‐control study (Wang 2012a)
Urinary tract cancer: three cohort studies (Chyou 1993; Kurahashi 2009; Nagano 2001), four case‐control studies (Hemelt 2010; Wakai 2004; Wang 2013b; Wilkens 1996)
Haematopoietic cancer
Nine nonexperimental studies reported data on the risk of cancer of the haematopoietic system and reported data on four different types of cancer.
Hematopoetic cancer: two cohort studies (Nagano 2001; Naganuma 2009).
Leukaemia: one cohort study (Ugai 2018) assessed acute myeloid leukaemia, and four case‐control studies (Kuo 2009; Li 2011a; Liu 2017; Zhang 2008) assessed overall leukaemia.
Lymphoma: one cohort study (Ugai 2017).
Multiple myeloma: one cohort study (Ugai 2017) and one case‐control study (Wang 2012b).
Other types of cancer
One experimental study reported also data on incidence of non‐melanoma skin cancer (Dostal 2015). One case‐control study assessed the association between green tea consumption and non‐melanoma skin cancer (Hakim 2000), while two cohort studies investigated the risk of thyroid cancer (Michikawa 2011) and brain cancer (Ogawa 2016).
Exposure
In experimental studies, amount of supplemented green tea extracts or total green tea polyphenols ranged from 400 mg/day up to 1315 mg/day, corresponding to EGCG intakes ranging from 200 up to 843 mg/day (Table 4).
Nonexperimental studies assessed green tea exposure through administration of either food‐frequency questionnaires, structured interviews by trained personnel, or self‐administrated surveys in which participants had to declare the frequency and amount of certain food and beverage intakes.
Amounts of green tea consumption were rated either per day, per week, per month or per year and ranged from 0 cups to 10 cups or more per day or week. Some studies specified the amount in grams of green tea leaves consumed over a defined period of time (e.g. month or year). Drinking green tea has often been defined as consumption of one or more cups per week for at least six months. Finally, some studies estimated lifetime consumption of green tea in grams per month per year of drinking.
Sponsorship
All but two experimental studies reported funding sources, mainly grants of National Institutes of Health or National Cancer Institute for studies in the USA, and a Cancer Research UK grant in one Italian study. Three studies reported funding from pharmaceutical companies, two in Italy (Bettuzzi 2006; Micali 2017), and one in Japan (Tsao 2009), while one ongoing trial carried out in the USA mentioned that no significant financial relationships to disclose were present (Shannon 2010), and conversely, the other ongoing RCT did not report any financial source (NCT01496521). Of the 131 nonexperimental studies, only 27 did not declare any type of sponsorship or founding source. For the remaining studies, National Institutes of Health or National Cancer Institute generally sponsored the USA studies. In Japan, mainly the Ministry of Health, Labour and Welfare or the Ministry of Education, Science and Culture sponsored the investigations. In China, the Natural Science Foundation sponsored some of the studies. Finally, the 'Ministry of Health' and 'National Health and Medical Research Council' supported with grants the studies from Czech Republic and Australia, respectively.
Excluded studies
For the 60 studies that we excluded, 30 studies reported exposure not including green tea separately, 13 had an ineligible study design (e.g. cross‐sectional studies or pharmacokinetic studies), 11 did not include cancer among the outcomes, four were undertaken in non‐healthy individuals all with cancer, and two studies were from paediatric populations. See Characteristics of excluded studies.
Risk of bias in included studies
Experimental studies
We used the Cochrane tool for assessing risk of bias for the experimental studies (Deeks 2017). Detailed 'Risk of bias' assessments of included experimental studies are reported in study‐specific tables (Characteristics of included studies) and are summarised in Table 10.
7. Methodological quality of experimental studies.
| Study | Random sequence generation | Allocationconcealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
| Bettuzzi 2006 | Low risk | Unclear risk | Low risk (all outcomes) | Low risk: PSA levels Unclear risk: prostate cancer and LUTS |
Low risk: prostate cancer and PSA levels Unclear risk: LUTS |
Unclear risk | Low risk |
| Dostal 2015 | Low risk | Low risk | Low risk (all outcomes) | Low risk (all outcomes) | Low risk (all outcomes) | Low risk | Unclear risk |
| Dryden 2013 | Low risk | Low risk | Low risk (all outcomes) | Low risk (all outcomes) | Low risk (all outcomes) | Low risk | High risk |
| Garcia 2014 | Low risk | Low risk | Low risk (all outcomes) | Low risk (all outcomes) | Low risk (all outcomes) | Low risk | Low risk |
| Garland 2006 | Low risk | Unclear risk | Low risk (all outcomes) | Low risk (all outcomes) | High risk | Low risk | Unclear risk |
| Kumar 2015 | Low risk | Low risk | Low risk (all outcomes) | Low risk (all outcomes) | Low risk: prostate cancer and PSA levels Unclear risk: LUTS |
Low risk | Unclear risk |
| Lane 2018 | Low risk | Low risk | Low risk (all outcomes) | Low risk (all outcomes) | Unclear risk (all outcomes) | High risk | Unclear risk |
| Micali 2017 | Low risk | Unclear risk | Low risk (all outcomes) | Low risk (all outcomes) | Low risk (all outcomes) | Unclear risk | High risk |
| Roshdy 2013 | Low risk | Low risk | Low risk (all outcomes) | Low risk (all outcomes) | Unclear risk (all outcomes) | Low risk | Unclear risk |
| Sinicrope 2017 | Low risk | Unclear risk | Low risk (al outcomes) | Unclear risk (all outcomes) | Low risk (all outcomes) | Low risk | Low risk |
| Tsao 2009 | Low risk | Low risk | Low risk (al outcomes) | Low risk: other outcomes Unclear risk: oral lesions |
Unclear risk (all outcomes) | Unclear risk | Low risk |
| LUTS: lower urinary tract symptoms; PSA: prostate‐specific antigens | |||||||
Allocation
All studies were randomised. Four studies did not clearly specify their method of allocation concealment (Bettuzzi 2006; Garland 2006; Micali 2017; Sinicrope 2017) so we judged them to be at unclear risk of bias for this domain. We judged the remaining studies at a low risk of bias.
Blinding
All studies were double‐blinded and, specifically, we judged all studies at low risk of bias regarding blinding of participants and personnel involved in the recruitment. Conversely, we judged blinding during outcome assessment at unclear risk for some but not all outcomes in three studies (Bettuzzi 2006; Sinicrope 2017; Tsao 2009).
Incomplete outcome data
All but two studies (Garland 2006; Lane 2018), reported summary results for all outcomes. However, in three studies incomplete reporting for some outcomes can be noted. In particular, two studies reported results for lower urinary tract symptoms (Bettuzzi 2006; Kumar 2015), and one study assessed uterine leiomyoma burden (Roshdy 2013) but none of these studies stated the number of participants included in the analysis.
Selective reporting
The study protocol was not available for four studies so we judged them at unclear (Bettuzzi 2006; Micali 2017; Tsao 2009), or high (Lane 2018), risk of selective reporting bias.
Other potential sources of bias
Four RCTs reported a high number of withdrawals (Dostal 2015; Kumar 2015; Micali 2017; Roshdy 2013). In two RCTs other concomitant interventions were reported. In particular, some participants were taken lycopene capsules during the study (Lane 2018), while the other RCTs some participants in the treatment group, but not in the placebo arm, took immunomodulatory drugs, possible affecting response rates (Dryden 2013).
Nonexperimental studies
We assessed the methodological quality of the nonexperimental studies by using the Newcastle Ottawa Scale (NOS), for both cohort (Appendix 4) and case‐control studies (Appendix 5).
Cohort studies
All but one of the cohort studies were of high methodological quality and reached 8 or 9 stars on the NOS (Wells 2001), while one study was of medium methodological quality reaching 7 stars (Key 1999). The median score was 9 (out of 9) for the 46 cohort studies with a range of 7 to 9 stars (Table 8; Figure 2). Detailed results of single NOS items are shown in Table 11. Regarding 'selection' items, we judged study participants as being truly representative of the average general population in almost all studies, while two studies restricted participants to those of Japanese ancestry (Chyou 1993; Galanis 1998), some participants in three studies were survivors from the atomic bomb in Hiroshima and Nagasaki (Allen 2004; Key 1999; Nagano 2001), and one study was carried out in working people (Li 2018). All studies described the modality of exposure assessment of green tea consumption, generally relying on the use of structured interviews or food‐frequency questionnaires, and all studies excluded participants with previous history of cancer. For 'comparability' items, all studies controlled the analysis for age, and all but six adjusted for smoking habit in the multivariate model (Allen 2004; Galanis 1998; Inoue 2008; Iwai 2002; Key 1999; Severson 1989). Concerning 'outcome' items, all studies used a record linkage based on a cancer registry for outcome assessment, with a follow‐up at least of five years in all but two studies (Dai 2010; Galanis 1998). Finally, while we considered the follow‐up rate inadequate (i.e. less than 90% of participants and no description of those lost) in three studies studies (Hoshiyama 2002 and Hoshiyama 2004 in: Inoue 2009a; Ishikawa 2006).
2.
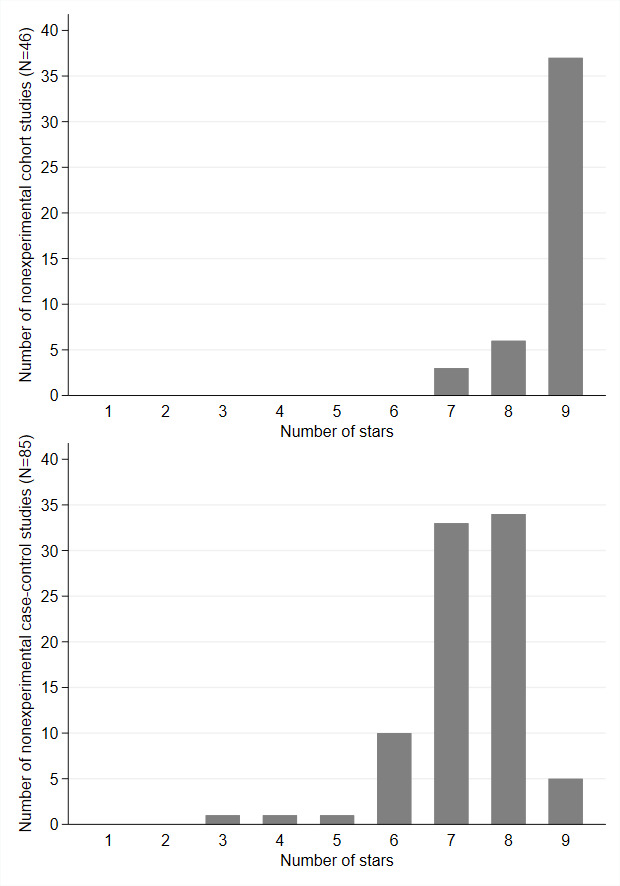
Newcastle‐Ottawa scale for nonexperimental studies
8. Methodological quality of nonexperimental cohort studies.
| Study | Selection | Comparability | Outcome | Total (out of 9) | ||||||
| S1 | S2 | S3 | S4 | C1 | C2 | O1 | O2 | O3 | ||
| Allen 2004 | c(0) | a(1) | b(1) | a(1) | 1 | 0 | b(1) | a(1) | a(1) | 7 |
| Chyou 1993 | b(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | a(1) | 9 |
| Dai 2010 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | b(0) | b(1) | 8 |
| Fujino 2002 in: Inoue 2009a | a(1) | a(1) | b(1) | a(1) | 1 | 0 | b(1) | a(1) | a(1) | 8 |
| Galanis 1998 | b(1) | a(1) | b(1) | a(1) | 1 | 0 | b(1) | b(0) | b(1) | 7 |
| Hoshiyama 2002 in: Inoue 2009a | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | c(0) | 8 |
| Hoshiyama 2004a in: Inoue 2009a | a(1) | a(1) | a(1) | a(1) | 1 | 1 | b(1) | a(1) | c(0) | 8 |
| Ide 2007 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | a(1) | 9 |
| Inoue 2009a | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | b(1) | 9 |
| Inoue 2009b | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | a(1) | 9 |
| Ishikawa 2006 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | c(0) | 8 |
| Iwai 2002 | a(1) | a(1) | b(1) | a(1) | 1 | 0 | b(1) | a(1) | b(1) | 8 |
| Iwasaki 2010a | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | b(1) | 9 |
| Iwasaki 2010b in: Iwasaki 2010a | a(1) | a(1) | b(1) | a(1) | 1 | 0 | b(1) | a(1) | b(1) | 8 |
| Key 1999 | c(0) | a(1) | b(1) | a(1) | 1 | 0 | b(1) | a(1) | a(1) | 7 |
| Khan 2004 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | a(1) | 9 |
| Kikuchi 2006 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | b(1) | 9 |
| Kurahashi 2007 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | b(1) | 9 |
| Kurahashi 2009 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | b(1) | 9 |
| Kuriyama 2006 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | a(1) | 9 |
| Lee 2007 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | b(1) | 9 |
| Li 2008 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | b(1) | 9 |
| Li 2018 | c(0) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | b(1) | 8 |
| Lin 2008 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | a(1) | 9 |
| Liu 2016 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | a(1) | 9 |
| Luo 2007 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | b(1) | 9 |
| Makiuchi 2016 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | b(1) | 9 |
| Michikawa 2011 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | b(1) | 9 |
| Montague 2012 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | b(1) | 9 |
| Nagano 2001 | c(0) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | a(1) | 8 |
| Naganuma 2009 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | a(1) | 9 |
| Nakachi 2000 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | a(1) | 9 |
| Nakamura 2011 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | b(1) | 9 |
| Nechuta 2012 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | a(1) | 9 |
| Oba 2006 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | b(1) | 9 |
| Odegaard 2015 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | b(1) | 9 |
| Ogawa 2016 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | b(1) | 9 |
| Saito 2015 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | b(1) | 9 |
| Sasazuki 2004 in: Inoue 2009a | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | b(1) | 9 |
| Sauvaget 2005 in: Nagano 2001 | c(0) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | a(1) | 8 |
| Severson 1989 | a(1) | a(1) | b(1) | a(1) | 1 | 0 | b(1) | a(1) | b(1) | 8 |
| Shimazu 2008 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | b(1) | 9 |
| Sun 2007 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | b(1) | 9 |
| Suzuki 2004 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | b(1) | 9 |
| Suzuki 2005 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | b(1) | 9 |
| Suzuki 2009 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | b(1) | 9 |
| Tamura 2018 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | b(1) | 9 |
| Tsubono 2001 in: Inoue 2009a | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | b(1) | 9 |
| Ugai 2017 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | b(1) | 9 |
| Ugai 2018 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | b(1) | 9 |
| Ui 2009 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | b(1) | 9 |
| Yang 2007 in: Nechuta 2012 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | b(1) | 9 |
| Yang 2011a | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | b(1) | 9 |
| Zhao 2017 | a(1) | a(1) | b(1) | a(1) | 1 | 1 | b(1) | a(1) | b(1) | 9 |
aCase‐cohort study
Case‐control studies
The median score was 7 (out of 9) for the 85 case‐control studies with an overall range from 3 to 9 stars (Table 9; Figure 2). Detailed results of single NOS items (see Table 12) showed that, regarding 'selection' items almost all studies adequately identified cases by accessing medical records in hospitals or cancer registries, or both. Two studies (Lei 1994; Xu 2013), did not describe the source of cases, and only one study (Tewes 1990), was based on self‐reports. All but six studies (Kato 1990; Lei 1994; Mu 2003; Wu 2009a; Wu 2009b; Xu 2013), selected consecutive or obviously representative series of cases during a clear, identified period. Regarding selection of controls, they were recruited from the same community of the corresponding paired case in 42 studies, while they were recruited from hospital attenders in 39 studies. Three studies recruited and presented results using two sets of controls in the analyses: population or hospital controls (Kono 1988; Li 2011a; Zhang 2002). Two studies (Gavrilas 2018; Xu 2013) did not provide a clear description of selection of controls. Regarding 'comparability' items, all but three studies (Kuo 2009; Lassed 2016; Wang 2006), controlled for age and approximately one‐third (N = 28) of the studies did not include smoking habits in the statistical model. Regarding exposure‐related items, four studies implemented a food‐frequency questionnaire or a structured interview to assess green tea exposure (Bonner 2005; Inoue 1998; Jia 2016; Kato 1990), while two studies did not provide a description (Lei 1994; Wang 1999). The same method of exposure assessment for both cases and controls was clearly used in all but one study (Kono 1988). Finally, in relation to the response rate, it was largely comparable for both cases and controls in 30 studies or, if rates were different, non‐respondents were described in 24 studies, while 31 studies did not provide a description of non‐respondents.
9. Methodological quality of nonexperimental case‐control studies.
| Study | Selection | Comparability | Exposure | Total (out of 9) | ||||||
| S1 | S2 | S3 | S4 | C1 | C2 | E1 | E2 | E3 | ||
| Bandera 2010 | a(1) | a(1) | a(1) | a(1) | 1 | 1 | b(1) | a(1) | c(0) | 8 |
| Berroukche 2012 | a(1) | a(1) | b(0) | a(1) | 1 | 1 | b(1) | a(1) | a(1) | 8 |
| Bonner 2005 | a(1) | a(1) | a(1) | b(0) | 1 | 1 | c(0) | a(1) | a(1) | 7 |
| Chen 2011 | a(1) | a(1) | b(0) | a(1) | 1 | 1 | b(1) | a(1) | a(1) | 8 |
| Chen 2015 in: Chen 2017a | a(1) | a(1) | a(1) | a(1) | 1 | 1 | b(1) | a(1) | a(1) | 9 |
| Chen 2016 in: Chen 2017a | a(1) | a(1) | b(0) | a(1) | 1 | 1 | b(1) | a(1) | a(1) | 8 |
| Chen 2017a | a(1) | a(1) | b(0) | a(1) | 1 | 1 | b(1) | a(1) | a(1) | 8 |
| Fu 2013 | a(1) | a(1) | b(0) | a(1) | 1 | 1 | b(1) | a(1) | a(1) | 8 |
| Gao 1994 | a(1) | a(1) | b(0) | a(1) | 1 | 1 | b(1) | a(1) | c(0) | 7 |
| Gao 2005 | a(1) | a(1) | a(1) | a(1) | 1 | 0 | b(1) | a(1) | c(0) | 7 |
| Gavrilas 2018 | a(1) | a(1) | c(0) | a(1) | 1 | 1 | b(1) | a(1) | c(0) | 7 |
| Goodman 2003 | a(1) | a(1) | a(1) | a(1) | 1 | 0 | b(1) | a(1) | a(1) | 8 |
| Goto 1990 | a(1) | a(1) | a(1) | b(0) | 1 | 1 | b(1) | a(1) | c(0) | 7 |
| Green 2014 | a(1) | a(1) | a(1) | a(1) | 1 | 1 | b(1) | a(1) | c(0) | 8 |
| Hakim 2000 | a(1) | a(1) | a(1) | a(1) | 1 | 0 | b(1) | a(1) | a(1) | 8 |
| Han 2008 | a(1) | a(1) | a(1) | a(1) | 1 | 1 | b(1) | a(1) | c(0) | 8 |
| Hemelt 2010 | a(1) | a(1) | b(0) | a(1) | 1 | 1 | b(1) | a(1) | c(0) | 7 |
| Hoshiyama 1992 | a(1) | a(1) | a(1) | a(1) | 1 | 1 | b(1) | a(1) | c(0) | 8 |
| Hsu 2012 | a(1) | a(1) | a(1) | a(1) | 1 | 1 | b(1) | a(1) | c(0) | 8 |
| Huang 1999 | a(1) | a(1) | b(0) | a(1) | 1 | 1 | b(1) | a(1) | b(0) | 7 |
| Inoue 1994 in: Huang 1999 | a(1) | a(1) | b(0) | a(1) | 1 | 0 | d(0) | a(1) | a(1) | 6 |
| Inoue 1998 | a(1) | a(1) | b(0) | a(1) | 1 | 1 | d(0) | a(1) | a(1) | 7 |
| Inoue 2008 | a(1) | a(1) | a(1) | a(1) | 1 | 0 | b(1) | a(1) | c(0) | 7 |
| Islami 2009 | a(1) | a(1) | a(1) | a(1) | 1 | 1 | b(1) | a(1) | b(0) | 8 |
| Iwasaki 2014 | a(1) | a(1) | b(0) | a(1) | 1 | 1 | b(1) | a(1) | b(0) | 7 |
| Ji 1996 | a(1) | a(1) | a(1) | a(1) | 1 | 1 | b(1) | a(1) | c(0) | 8 |
| Ji 1997 | a(1) | a(1) | a(1) | a(1) | 1 | 1 | b(1) | a(1) | b(0) | 8 |
| Jia 2016 | a(1) | a(1) | a(1) | a(1) | 1 | 0 | c(0) | a(1) | a(1) | 7 |
| Jian 2004 | a(1) | a(1) | b(0) | a(1) | 1 | 1 | b(1) | a(1) | a(1) | 8 |
| Jin 2013 | a(1) | a(1) | a(1) | a(1) | 1 | 1 | b(1) | a(1) | a(1) | 9 |
| Kakuta 2009 | a(1) | a(1) | b(0) | a(1) | 1 | 1 | b(1) | a(1) | a(1) | 8 |
| Kato 1990 | a(1) | b(0) | a(1) | a(1) | 1 | 0 | d(0) | a(1) | a(1) | 6 |
| Kato 1990a in: Huang 1999 | a(1) | b(0) | b(0) | a(1) | 1 | 0 | d(0) | a(1) | a(1) | 5 |
| Kono 1988a | a(1) | a(1) | a(1) | a(1) | 1 | 1 | b(1) | b(0) | b(0) | 7 |
| Kono 1988b | a(1) | a(1) | b(0) | a(1) | 1 | 1 | b(1) | b(0) | b(0) | 6 |
| Kubik 2004 in: Kubik 2008 | a(1) | a(1) | b(0) | a(1) | 1 | 1 | b(1) | a(1) | c(0) | 7 |
| Kubik 2008 | a(1) | a(1) | b(0) | a(1) | 1 | 1 | b(1) | a(1) | c(0) | 7 |
| Kuo 2009 | a(1) | a(1) | a(1) | a(1) | 0 | 0 | b(1) | a(1) | c(0) | 6 |
| Lassed 2016 | a(1) | a(1) | b(0) | a(1) | 0 | 0 | b(1) | a(1) | a(1) | 6 |
| Le Marchand 2000 | a(1) | a(1) | a(1) | a(1) | 1 | 1 | b(1) | a(1) | a(1) | 9 |
| Lee 2017 | a(1) | a(1) | b(0) | a(1) | 1 | 1 | b(1) | a(1) | c(0) | 7 |
| Lei 1994 | c(0) | b(0) | b(0) | a(1) | 1 | 0 | e(0) | a(1) | c(0) | 3 |
| Leung 2016 | a(1) | a(1) | a(1) | a(1) | 1 | 1 | b(1) | a(1) | a(1) | 9 |
| Li 2011aa | a(1) | a(1) | a(1) | a(1) | 1 | 1 | b(1) | a(1) | c(0) | 8 |
| Li 2011ab | a(1) | a(1) | b(0) | a(1) | 1 | 1 | b(1) | a(1) | c(0) | 7 |
| Li 2011b in: Mu 2003 | a(1) | a(1) | a(1) | a(1) | 1 | 1 | b(1) | a(1) | c(0) | 8 |
| Li 2014 | a(1) | a(1) | a(1) | a(1) | 1 | 0 | b(1) | a(1) | b(0) | 7 |
| Li 2016 | a(1) | a(1) | b(0) | a(1) | 1 | 0 | b(1) | a(1) | a(1) | 7 |
| Lin 2012 | a(1) | a(1) | b(0) | a(1) | 1 | 0 | b(1) | a(1) | b(0) | 6 |
| Liu 2010 | a(1) | a(1) | a(1) | a(1) | 1 | 1 | b(1) | a(1) | a(1) | 9 |
| Liu 2017 | a(1) | a(1) | b(0) | a(1) | 1 | 1 | b(1) | a(1) | a(1) | 8 |
| Mao 2011 | a(1) | a(1) | b(0) | b(0) | 1 | 1 | b(1) | a(1) | b(0) | 6 |
| Mizoo 2013 | a(1) | a(1) | b(0) | a(1) | 1 | 0 | b(1) | a(1) | a(1) | 7 |
| Mizuno 1992 | a(1) | a(1) | b(0) | a(1) | 1 | 0 | b(1) | a(1) | b(0) | 6 |
| Mu 2003 | a(1) | b(0) | a(1) | a(1) | 1 | 1 | b(1) | a(1) | c(0) | 7 |
| Mu 2005 in: Mu 2003 | a(1) | a(1) | a(1) | a(1) | 1 | 1 | b(1) | a(1) | c(0) | 8 |
| Nagle 2010 | a(1) | a(1) | a(1) | a(1) | 1 | 1 | b(1) | a(1) | c(0) | 8 |
| Oze 2014 | a(1) | a(1) | a(1) | a(1) | 1 | 1 | b(1) | a(1) | b(0) | 8 |
| Peng 2013 | a(1) | a(1) | a(1) | a(1) | 1 | 0 | b(1) | a(1) | b(0) | 7 |
| Peng 2015 | a(1) | a(1) | a(1) | a(1) | 1 | 1 | b(1) | a(1) | b(0) | 8 |
| Ruan 2010 | a(1) | a(1) | b(0) | a(1) | 1 | 0 | b(1) | a(1) | a(1) | 7 |
| Setiawan 2001 | a(1) | a(1) | a(1) | a(1) | 1 | 1 | b(1) | a(1) | c(0) | 8 |
| Shrubsole 2009 | a(1) | a(1) | a(1) | a(1) | 1 | 0 | b(1) | a(1) | b(0) | 7 |
| Song 2008 | a(1) | a(1) | a(1) | a(1) | 1 | 1 | b(1) | a(1) | b(0) | 8 |
| Sonoda 2004 | a(1) | a(1) | b(0) | a(1) | 1 | 1 | b(1) | a(1) | b(0) | 7 |
| Tajima 1985 | a(1) | a(1) | b(0) | a(1) | 1 | 0 | b(1) | a(1) | b(0) | 6 |
| Takezaki 2000 | a(1) | a(1) | b(0) | a(1) | 1 | 1 | b(1) | a(1) | a(1) | 8 |
| Takezaki 2001 | a(1) | a(1) | b(0) | a(1) | 1 | 1 | b(1) | a(1) | a(1) | 8 |
| Tewes 1990 | b(0) | a(1) | a(1) | a(1) | 1 | 1 | b(1) | a(1) | b(0) | 7 |
| Tse 2017 | a(1) | a(1) | b(0) | a(1) | 1 | 0 | b(1) | a(1) | b(0) | 6 |
| Wakai 2004 | a(1) | a(1) | b(0) | a(1) | 1 | 1 | b(1) | a(1) | a(1) | 8 |
| Wang 1999 | a(1) | a(1) | a(1) | a(1) | 1 | 1 | e(0) | a(1) | c(0) | 7 |
| Wang 2006 | a(1) | a(1) | a(1) | a(1) | 0 | 0 | b(1) | a(1) | b(0) | 6 |
| Wang 2007 | a(1) | a(1) | a(1) | a(1) | 1 | 0 | b(1) | a(1) | b(0) | 7 |
| Wang 2012a | a(1) | a(1) | b(0) | a(1) | 1 | 0 | b(1) | a(1) | c(0) | 6 |
| Wang 2012b | a(1) | a(1) | b(0) | a(1) | 1 | 0 | b(1) | a(1) | a(1) | 7 |
| Wang 2012c | a(1) | a(1) | a(1) | a(1) | 1 | 1 | b(1) | a(1) | c(0) | 8 |
| Wang 2013a | a(1) | a(1) | b(0) | a(1) | 1 | 0 | b(1) | a(1) | a(1) | 7 |
| Wang 2013b | a(1) | a(1) | b(0) | a(1) | 1 | 1 | b(1) | a(1) | c(0) | 7 |
| Wang 2015 | a(1) | a(1) | b(0) | a(1) | 1 | 1 | b(1) | a(1) | c(0) | 7 |
| Wilkens 1996 | a(1) | a(1) | a(1) | a(1) | 1 | 1 | b(1) | a(1) | c(0) | 8 |
| Wu 2003 | a(1) | a(1) | a(1) | a(1) | 1 | 1 | b(1) | a(1) | a(1) | 9 |
| Wu 2009a | a(1) | b(0) | b(0) | a(1) | 1 | 0 | b(1) | a(1) | c(0) | 5 |
| Wu 2009b | a(1) | b(0) | a(1) | a(1) | 1 | 1 | b(1) | a(1) | a(1) | 8 |
| Xu 2007 | a(1) | a(1) | a(1) | a(1) | 1 | 0 | b(1) | a(1) | a(1) | 8 |
| Xu 2013 | c(0) | b(0) | c(0) | a(1) | 1 | 0 | b(1) | a(1) | c(0) | 4 |
| Yan 2016 | a(1) | a(1) | a(1) | a(1) | 1 | 1 | b(1) | a(1) | c(0) | 8 |
| Ye 1998 | a(1) | a(1) | a(1) | a(1) | 1 | 0 | b(1) | a(1) | b(0) | 7 |
| Yu 1995 | a(1) | a(1) | a(1) | a(1) | 1 | 1 | b(1) | a(1) | c(0) | 8 |
| Zhang 2002 | a(1) | a(1) | b(0) | a(1) | 1 | 1 | b(1) | a(1) | b(0) | 7 |
| Zhang 2007 | a(1) | a(1) | b(0) | a(1) | 1 | 1 | b(1) | a(1) | a(1) | 8 |
| Zhang 2008 | a(1) | a(1) | b(0) | a(1) | 1 | 1 | b(1) | a(1) | b(0) | 7 |
| Zheng 1993 | a(1) | a(1) | b(0) | a(1) | 1 | 1 | b(1) | a(1) | b(0) | 7 |
| Zhong 2001 | a(1) | a(1) | a(1) | a(1) | 1 | 1 | b(1) | a(1) | c(0) | 8 |
aPopulation controls considered; bHospital controls considered.
Publication bias
There were too few studies to yield reliable funnel plots for experimental studies, but we were able to assess the reporting bias for most outcomes from the nonexperimental studies. For the latter studies, the funnel plot for any cancer mortality (Figure 3), showed a symmetrical distribution, as did the funnel plots for oral cancer (Figure 4) and any gut cancer (Figure 5). Conversely, analysis for oesophageal cancer showed an asymmetrical distribution (Figure 6), mainly from results of case‐control studies. In other gastrointestinal cancers we did not detect evidence of publication bias (Figure 7; Figure 8; Figure 9; Figure 10; Figure 11; Figure 12), as was the case for lung cancer (Figure 13), breast cancer (Figure 14), and other gynaecological cancer (Figure 15; Figure 16; Figure 17). For prostate cancer, an indication of publication bias toward a decreased risk of cancer emerged (Figure 18), while we found symmetry from the studies assessing urinary tract cancer (Figure 19).
3.

Funnel plot of comparison 2. Nonexperimental studies: highest versus lowest green tea exposure, outcome 2.2, any cancer mortality
4.

Funnel plot of comparison 2. Nonexperimental studies: highest versus lowest green tea exposure, outcome 2.3, oral cancer
5.

Funnel plot of comparison 2. Nonexperimental studies: highest versus lowest green tea exposure, outcome 2.6, any gut cancer
6.

Funnel plot of comparison 2. Nonexperimental studies: highest versus lowest green tea exposure, outcome 2.7, oesophageal cancer
7.

Funnel plot of comparison 2. Nonexperimental studies: highest versus lowest green tea exposure, outcome 2.21, prostate cancer
8.

Funnel plot of comparison 2. Nonexperimental studies: highest versus lowest green tea exposure, outcome 2.23, urinary tract cancer
9.

Funnel plot of comparison 2. Nonexperimental studies: highest versus lowest green tea exposure, outcome 2.8, stomach cancer
10.

Funnel plot of comparison 2. Nonexperimental studies: highest versus lowest green tea exposure, outcome 2.9, liver cancer
11.

Funnel plot of comparison 2. Nonexperimental studies: highest versus lowest green tea exposure, outcome 2.10, pancreatic cancer
12.

Funnel plot of comparison 2. Nonexperimental studies: highest versus lowest green tea exposure, outcome 2.12, colorectal cancer
13.

Funnel plot of comparison 2. Nonexperimental studies: highest versus lowest green tea exposure, outcome 2.13, colon cancer
14.

Funnel plot of comparison 2. Nonexperimental studies: highest versus lowest green tea exposure, outcome 2.14, rectal cancer
15.

Funnel plot of comparison 2. Nonexperimental studies: highest versus lowest green tea exposure, outcome 2.16, lung cancer
16.

Funnel plot of comparison 2. Nonexperimental studies: highest versus lowest green tea exposure, outcome 2.17, breast cancer
17.

Funnel plot of comparison 2. Nonexperimental studies: highest versus lowest green tea exposure, outcome 2.18, gynaecological cancer
18.

Funnel plot of comparison 2. Nonexperimental studies: highest versus lowest green tea exposure, outcome 2.19, endometrial cancer
19.

Funnel plot of comparison 2. Nonexperimental studies: highest versus lowest green tea exposure, outcome 2.20, ovarian cancer
Effects of interventions
See: Table 1; Table 2; Table 3
Experimental studies
Primary outcome
We included 11 RCTs with administration of green tea extracts (Table 4; Table 1).
Prostate cancer
Low‐certainty evidence from three studies reporting data on prostate cancer incidence in 201 men (101 in the intervention group and 100 in the control group) with high‐grade prostatic intraepithelial neoplasia, thus at high risk of prostate cancer, yielded a summary RR of 0.50 (95% CI 0.18 to 1.36; Analysis 1.1; Bettuzzi 2006; Kumar 2015; Micali 2017) in the intervention arms.
1.1. Analysis.

Comparison 1: Experimental studies: highest versus lowest green tea exposure, Outcome 1: Prostate cancer incidence
Gynaecological cancer
Low‐certainty evidence from two studies reporting data on gynaecological cancer, showed that green tea moderately increased the incidence of gynaecological cancer (summary RR 1.50, 95% CI 0.41 to 5.48), but findings for the two studies individually yielded contradictory results (Analysis 1.2). One study favoured experimental group and reported a decreased risk of endometrial cancer (RR 0.33, 95% CI 0.01 to 8.15) (Dostal 2015). Conversely, the other study favoured control group and reported increased risk of cervical cancer (RR 2.00, 95% CI 0.54 to 7.46) (Garcia 2014). Overall, all the RRs generated by these RCTs were statistically imprecise.
1.2. Analysis.

Comparison 1: Experimental studies: highest versus lowest green tea exposure, Outcome 2: Gynaecological cancer incidence
Non‐melanoma skin cancer
Low‐certainty evidence from one study assessing non‐melanoma skin cancer showed no difference in effect due to green tea extract supplementation (RR 1.00, 95% CI 0.06 to 15.92; Analysis 1.3; Dostal 2015).
1.3. Analysis.

Comparison 1: Experimental studies: highest versus lowest green tea exposure, Outcome 3: Non‐melanoma skin cancer incidence
Secondary outcomes
Quality of life
Three studies assessed quality of life in relation to administration of green tea extracts, where quality of life was slightly improved in the intervention group compared to the placebo group (Bettuzzi 2006; Micali 2017; Roshdy 2013). Bettuzzi 2006 assessed quality of life (Grumann 2001), in relation to lower urinary tract symptoms after three months of treatment and found that it decreased in the intervention group (from 2.06 to 1.76), while it slightly increased in the placebo group (from 1.30 to 1.47). Similarly, Micali 2017 reported lower urinary tract symptoms (Denis 1994), and quality‐of‐life scores (Grumann 2001), to be improved after one year but did not present detailed results. Roshdy 2013 used two different questionnaires to evaluate severity of fibroid‐specific symptoms and health‐related quality of life (Spies 2002; Wyatt 2001), and reported that green tea extract administration improved quality of life. The first scale showed a decrease in symptom severity with a mean change in the intervention group of −25.28 (SD ± 17.38) compared to a mean change of +7.1 (SD ± 15.5) in the placebo group (Spies 2002). Similarly, in the percentile scores for health‐related quality of life there was an overall increase of 20.7 (SD ± 21.0) in the treatment group and 2.19 (SD ± 17.4) in the placebo group (Wyatt 2001).
Conversely, Kumar 2015 observed no significant differences between the treatment and placebo arms in lower urinary tract symptoms (Marberger 2013), and quality‐of‐life scores (McHorney 1993), from baseline to the end of the study.
Finally, two studies reported only baseline evaluation of quality of life: Dostal 2015 used the Menopause‐Specific Quality of Life questionnaire (Lewis 2005), and Dryden 2013 used the Inflammatory Bowel Disease Questionnaire (Guyatt 1989).
Safety data
All the experimental studies assessed the safety of green tea supplementation (see Characteristics of included studies), and only two studies reported no difference in adverse effects between groups (Bettuzzi 2006; Roshdy 2013). Conversely, the most common adverse effects related to green tea extracts were gastrointestinal disorders, including generally mild‐to‐moderate or grade 1 to 2 disorders, particularly nausea, but also diarrhoea and other mild gastrointestinal disorders, for example, indigestion, constipation or gastroesophageal reflux (green tea versus placebo: 28.6% versus 24.6% (Dostal 2015); 32.0% versus 18.8% (Garcia 2014); 6.35% versus 5.48% (Garland 2006); 35% versus 25% (Kumar 2015); percentage not reported in Micali 2017; 30.0% versus 18.2% (Tsao 2009)). Three studies reported the elevation of liver enzymes (ALT and/or AST), generally of grade 2+ or 3, more frequently in the treatment group compared with placebo (green tea versus placebo: 6.7% versus 0.7% (Dostal 2015); 10% versus 2.1% (Garcia 2014); 5.26% versus 0% (Sinicrope 2017)). Similarly, three studies reported insomnia more frequently in the treatment group (green tea versus placebo: 21% versus 8% (Lane 2018); percentage not reported in Micali 2017; 36.7% versus 18.2% (Tsao 2009)), although it was generally of low grade, except for Tsao 2009. Two studies reported hypertension to be slightly more frequent in the treatment arm (green tea versus placebo: 21.8% versus 19.7% (Dostal 2015); 21% versus 15% (Lane 2018)). Also, two studies reported slightly higher frequencies of skin and subcutaneous tissue disorders (mainly rash or allergic skin reactions) in the treatment group (green tea versus placebo: 3.4% versus 1.5% (Dostal 2015); 14% versus 6% (Kumar 2015)), and two studies reported higher incidence of dizziness (green tea versus placebo: 14.0% versus 6.3% (Garcia 2014); 6.7% versus 0% (Tsao 2009)). Finally, Dryden 2013 reported higher incidence of heartburn and increased thirst (27% versus 0%).
Nonexperimental studies
We compared the risk of cancer in the highest category of green tea intake with the bottom category of exposure, by computing a summary risk ratio (RR) along with its 95% CI (Table 5; Table 6; Table 2).
Any cancer
We meta‐analysed results of two cohort studies on any cancer incidence (Nagano 2001; Nakachi 2000), and one population‐based case‐control study (Li 2011a), along with data from eight cohort studies evaluating any cancer mortality (Iwai 2002; Khan 2004; Kuriyama 2006; Liu 2016; Odegaard 2015; Saito 2015; Suzuki 2009; Zhao 2017), and a total of over 530,000 participants. For participants in the highest category of green tea intake compared with those in the lowest exposure category, the summary RR for any cancer incidence was 0.83 (95% CI 0.65 to 1.07; 3 studies, 52,479 participants; low‐certainty evidence; (Analysis 2.1; Table 2), and for any cancer mortality 0.99 (95% CI 0.91 to 1.07; low‐certainty evidence; Analysis 2.2). We observed moderate heterogeneity for both incidence (I2 = 66%) and mortality (I2 = 58%) studies.
2.1. Analysis.

Comparison 2: Nonexperimental studies: highest versus lowest green tea exposure, Outcome 1: Any cancer incidence
2.2. Analysis.

Comparison 2: Nonexperimental studies: highest versus lowest green tea exposure, Outcome 2: Any cancer mortality
Gastrointestinal cancers
Oral, laryngeal and pharyngeal cancer
One cohort study (Ide 2007), found that increased green tea consumption was associated with a lower risk of oral cancer (RR 0.44, 95% CI 0.19 to 1.04). Results from case‐control studies generally showed a decreased RR of oral cancer associated with the highest green tea consumption in either the one population‐based study (RR 0.58, 95% CI 0.42 to 0.79; Yan 2016), and the three hospital‐based studies with summary RR of 0.77 (95% CI 0.65 to 0.90; Analysis 2.3; Chen 2017a; Fu 2013; Zheng 1993). Conversely, one cohort study evaluating risk of oral, pharyngeal and laryngeal cancer found an increased risk in the highest category of green tea intake, with RR of 1.47 (95% CI 1.12 to 1.93; Analysis 2.4; Oze 2014). The one hospital‐based case‐control study showed a decreased but statistically imprecise risk of pharyngeal cancer (OR 0.83, 95% CI 0.30 to 2.30; Analysis 2.5; Takezaki 2000). The analysis of any gut cancer by combining all these nonexperimental studies showed a decreased risk in the highest category of green tea exposure, with a summary RR of 0.78 (95% CI 0.59 to 1.02),high heterogeneity (I2 = 73%) and low‐certainty evidence between study results (Analysis 2.6).
2.3. Analysis.

Comparison 2: Nonexperimental studies: highest versus lowest green tea exposure, Outcome 3: Oral cancer
2.4. Analysis.

Comparison 2: Nonexperimental studies: highest versus lowest green tea exposure, Outcome 4: Oral, pharyngeal and laryngeal cancer
2.5. Analysis.

Comparison 2: Nonexperimental studies: highest versus lowest green tea exposure, Outcome 5: Pharyngeal cancer
2.6. Analysis.

Comparison 2: Nonexperimental studies: highest versus lowest green tea exposure, Outcome 6: Any gut cancer
Oesophageal cancer
Overall results showed that the highest green tea intake category was associated with lower oesophageal cancer risk (summary RR 0.81, 95% CI 0.64 to 1.04), with high heterogeneity (I2 = 69%) and very low‐certainty evidence between study results (Analysis 2.7; Table 3). Two cohort studies reported only the total number of cases, thus we could not include them in the meta‐analysis (Nagano 2001; Nechuta 2012), while Ishikawa 2006 found an increased risk of oesophageal cancer in participants consuming the highest amounts of green tea (summary RR 1.67, 95% CI 0.88 to 3.16; Analysis 2.7). Conversely, overall risk estimate of the 12 case‐control studies found a decreased risk of oesophageal cancer in participants in the highest category of green tea intake, with summary RR of 0.74 (95% CI 0.55 to 1.00) for the nine population‐based case‐control studies, and 0.86 (95% CI 0.57 to 1.27) for the three hospital‐based case‐control studies (Analysis 2.7; Table 3; Chen 2011; Gao 1994; Inoue 1998; Islami 2009; Mu 2003; Oze 2014; Peng 2015; Takezaki 2000; Wang 1999; Wang 2006; Wang 2007; Wu 2009b).
2.7. Analysis.

Comparison 2: Nonexperimental studies: highest versus lowest green tea exposure, Outcome 7: Oesophageal cancer
Stomach cancer
Of the 20 nonexperimental studies assessing stomach cancer risk, two did not report confidence intervals of risk estimates, thus we could not include them in the meta‐analysis (Tajima 1985; Wang 1999). Overall results from available studies suggest an association between green tea intake and decreased stomach cancer risk, with summary RR of 0.86 (95% CI 0.74 to 1.01) and high heterogeneity (I2 = 75%) and very‐low certainty evidence between study results (Analysis 2.8; Table 3). In the overall estimate from seven cohort studies (Galanis 1998; Inoue 2009a; Khan 2004; Kuriyama 2006; Nagano 2001; Nechuta 2012; Suzuki 2009), there was no association between green tea consumption and decreased risk of stomach cancer (summary RR 0.99, 95% CI 0.85 to 1.14; Analysis 2.8). Conversely, summary findings from case‐control studies showed a lower stomach cancer risk in participants in the highest category of green tea intake with summary RR of 0.74 (95% CI 0.53 to 1.02) from eight population‐based case‐control studies (Hoshiyama 1992; Ji 1996; Kono 1988; Liu 2010; Mu 2003; Setiawan 2001; Ye 1998; Yu 1995), and summary RR of 0.90 (95% CI 0.74 to 1.09) from the three hospital‐based case‐control studies (Huang 1999; Mao 2011; Wang 2015) see Analysis 2.8 and Table 3. We observed moderate heterogeneity (I2 = 39%) between results of cohort studies, and high (I2 = 85%) and no heterogeneity (I2 = 0%) between population‐based and hospital‐based case‐control studies, respectively.
2.8. Analysis.

Comparison 2: Nonexperimental studies: highest versus lowest green tea exposure, Outcome 8: Stomach cancer
Liver cancer
Overall study results showed a slightly but imprecise decreased risk with a higher intake of green tea (summary RR 0.88, 95% CI 0.68 to 1.14), with moderate heterogeneity (I2 = 46%) and low‐certainty evidence (Analysis 2.9; Table 3). Summary findings from five cohort studies found a slightly lower liver cancer risk in association with the highest green tea intake (summary RR 0.93, 95% 0.71 to 1.20; Analysis 2.9; Table 3; Inoue 2009b; Nagano 2001; Nechuta 2012; Tamura 2018; Ui 2009). In the only population‐based case‐control study, an indication of decreased liver cancer risk with increasing green tea intake emerged (RR 0.55, 95% 0.28 to 1.09; Analysis 2.9; Mu 2003).
2.9. Analysis.

Comparison 2: Nonexperimental studies: highest versus lowest green tea exposure, Outcome 9: Liver cancer
Pancreatic cancer
Overall, an inverse but imprecise association emerged for pancreatic cancer (summary RR of 0.88, 95% CI 0.70 to 1.10) in subjects with the highest green tea intake, high heterogeneity (I2 = 63%) and low‐certainty evidence (Analysis 2.10; Table 3). Null association was found from six cohort studies (Khan 2004; Lin 2008; Luo 2007; Nagano 2001; Nakamura 2011; Nechuta 2012), assessing pancreatic cancer risk (summary RR 1.04, 95% CI 0.84 to 1.30; Analysis 2.10). Conversely, overall findings from three population‐based case‐control studies (Goto 1990; Ji 1997; Wang 2012c), and one hospital‐based case‐control study (Mizuno 1992), showed contradictory findings with summary RR of 0.67 (95% CI 0.48 to 0.96) and 1.94 (95% CI 1.06 to 3.55), respectively (Analysis 2.10). We observed low heterogeneity in cohort studies (I2 = 8%), whereas there was high heterogeneity in population‐based case‐control studies (I2 = 73%).
2.10. Analysis.

Comparison 2: Nonexperimental studies: highest versus lowest green tea exposure, Outcome 10: Pancreatic cancer
Biliary tract cancer
Summary results from the three cohort studies (Makiuchi 2016; Nagano 2001; Nechuta 2012), assessing biliary tract cancer risk and consumption of green tea showed an indication of lower risk with higher green tea intake (summary RR 0.79, 95% CI 0.57 to 1.11; Analysis 2.11), with low heterogeneity (I2 = 25%).
2.11. Analysis.

Comparison 2: Nonexperimental studies: highest versus lowest green tea exposure, Outcome 11: Biliary tract cancer
Colorectal cancer
One study did not report confidence intervals of risk estimates, thus we could not include it in the meta‐analysis (Tajima 1985). Findings of 16 nonexperimental studies investigating the association between green tea intake and risk of colorectal cancer are contrasting. Overall results suggested a decreased risk of colorectal cancer in subjects with the highest green tea intake, with a summary RR of 0.84 (95% CI 0.74 to 0.96), with high heterogeneity (I2 = 65%) and low‐certainty evidence (Analysis 2.12; Table 3). However, nine cohort studies (Khan 2004; Kuriyama 2006; Lee 2007; Nagano 2001; Nechuta 2012; Sun 2007; Suzuki 2005; Suzuki 2009; Yang 2011a), found no association, with a summary RR of 1.00 (95% CI 0.92 to 1.08) and no heterogeneity. Conversely, case control studies reported an inverse association: in population‐based case‐control studies we found a summary RR of 0.74 (95% CI 0.61 to 0.90; Green 2014; Ji 1997; Kato 1990; Li 2011a; Peng 2013), and in hospital‐based case‐control studies a summary RR of 0.53 (95% CI 0.17 to 1.60; Gavrilas 2018; Inoue 1998), with high heterogeneity (I2 = 62% and I2 = 88%, respectively; Analysis 2.12). Stratified analysis by dividing colon and rectal cancer showed comparable results (Analysis 2.13; Analysis 2.14; Table 3).
2.12. Analysis.

Comparison 2: Nonexperimental studies: highest versus lowest green tea exposure, Outcome 12: Colorectal cancer
2.13. Analysis.

Comparison 2: Nonexperimental studies: highest versus lowest green tea exposure, Outcome 13: Colon cancer
2.14. Analysis.

Comparison 2: Nonexperimental studies: highest versus lowest green tea exposure, Outcome 14: Rectal cancer
Respiratory tract cancers
Nasopharyngeal cancer
The two case‐control studies on nasopharyngeal cancer, one population‐based (Hsu 2012), and one hospital‐based (Ruan 2010), reported a negative association between green tea intake and risk, with a summary RR of 0.49 (95% CI 0.36 to 0.67; Analysis 2.15). We observed moderate heterogeneity (I2 = 51%) between studies.
2.15. Analysis.

Comparison 2: Nonexperimental studies: highest versus lowest green tea exposure, Outcome 15: Nasopharyngeal carcinoma
Lung cancer
Overall, a negative association was found between green tea consumption and lung cancer risk, with a summary RR of 0.88 (95% CI 0.76 to 1.02) and moderate heterogeneity (I2 = 55%), with very low‐certainty evidence (Analysis 2.16; Table 3). However, the five cohort studies found no association between green tea intake and lung cancer risk (summary RR 1.02, 95% CI 0.79 to 1.31), with low‐to‐moderate heterogeneity (I2 = 38%) (Analysis 2.16; Table 3Khan 2004; Kuriyama 2006; Li 2008; Li 2018; Nagano 2001; Suzuki 2009). Conversely, results from the five population‐based case‐control studies (Han 2008; Jin 2013; Le Marchand 2000; Xu 2013; Zhong 2001), and the six hospital‐based case‐control studies (Bonner 2005; Kubik 2008; Lei 1994; Lin 2012; Takezaki 2001; Tewes 1990), suggested a lower risk in association with higher green tea intake, with summary RR of 0.73 (95% CI 0.61 to 0.87) and 0.90 (95% CI 0.69 to 1.17), respectively. Heterogeneity for these study categories was low (I2 = 13%) and high (I2 = 63%), respectively (Analysis 2.16).
2.16. Analysis.

Comparison 2: Nonexperimental studies: highest versus lowest green tea exposure, Outcome 16: Lung cancer
Breast cancer
Summary findings from nonexperimental studies on breast cancer risk suggested a lower risk following higher green tea intake, with summary RR of 0.88 (95% CI 0.75 to 1.02), with high heterogeneity (I2 = 67%) and very low‐certainty evidence (Analysis 2.17; Table 3). The five cohort studies investigating the association between green tea intake and risk of breast cancer in women found no association (summary RR 1.01, 95% CI 0.86 to 1.19; Analysis 2.17Dai 2010; Iwasaki 2010a; Key 1999; Nagano 2001; Suzuki 2004). However, four population‐based (Inoue 2008; Li 2011a; Shrubsole 2009; Wu 2003), and five hospital‐based (Iwasaki 2014; Li 2016; Mizoo 2013; Wang 2013a; Zhang 2007), case‐control studies, showed a slight inverse association (summary RR 0.87, 95% 0.70 to 1.08, and 0.81, 95% CI 0.58 to 1.13, respectively). We observed no heterogeneity in the cohort studies, while it was moderate (I2 = 51%) and high (I2 = 76%) in the population‐based and hospital‐based control studies, respectively (Analysis 2.17).
2.17. Analysis.

Comparison 2: Nonexperimental studies: highest versus lowest green tea exposure, Outcome 17: Breast cancer
Gynaecological cancer
In general, the nonexperimental studies evaluating the association between green tea intake and gynaecological cancer showed a negative association for both endometrial and ovarian cancer risk, with a summary RR of 0.69 (95% CI 0.57 to 0.83), with moderate heterogeneity (I2 = 42%) and low‐certainty evidence (Analysis 2.18; Table 3). In particular, studies assessing endometrial cancer risk showed a summary RR of 0.77 (95% CI 0.65 to 0.91), based on RR of 0.75 (95% CI 0.43 to 1.30) of one cohort study (Shimazu 2008), and on summary RR of 0.75 (95% CI 0.61 to 0.94) from the four population‐based case‐control studies (Analysis 2.19; Bandera 2010; Gao 2005; Kakuta 2009; Xu 2007), with low heterogeneity between study results. Regarding ovarian cancer, the RR from the five population‐based case‐control studies suggested an inverse association for participants in the highest category of green tea intake with a summary RR of 0.64 (95% CI 0.45 to 0.90) and moderate (I2 = 52%) heterogeneity (Analysis 2.20; Goodman 2003; Leung 2016; Nagle 2010; Song 2008; Zhang 2002).
2.18. Analysis.

Comparison 2: Nonexperimental studies: highest versus lowest green tea exposure, Outcome 18: Gynaecological cancer
2.19. Analysis.

Comparison 2: Nonexperimental studies: highest versus lowest green tea exposure, Outcome 19: Endometrial cancer
2.20. Analysis.

Comparison 2: Nonexperimental studies: highest versus lowest green tea exposure, Outcome 20: Ovarian cancer
Urogenital tract cancer
Prostate cancer
Results from nonexperimental studies comparing highest versus lowest intake of green tea and prostate cancer risk showed a lower risk in overall analysis (summary RR 0.73, 95% CI 0.56 to 0.94; I2 = 72%; very low‐certainty evidence; Analysis 2.21; Table 3), but there were conflicting results in stratified analysis according to the study design. Indeed, a slightly increased risk was found overall in five cohort studies with a summary RR of 1.09 (95% CI 0.89 to 1.32), with low heterogeneity (I2 = 25%; Analysis 2.21; Allen 2004; Kikuchi 2006; Kurahashi 2007; Montague 2012; Severson 1989), and a decreased for case‐control studies. In the latter eight studies, a negative association for participants in the highest category of green intake emerged for population‐based studies (summary RR 0.59, 95% CI 0.40 to 0.87) and for hospital‐based studies (summary RR 0.50, 95% CI 0.39 to 0.63; Analysis 2.21; Berroukche 2012; Jian 2004; Lassed 2016; Lee 2017; Li 2014; Sonoda 2004; Tse 2017; Wu 2009a).
2.21. Analysis.

Comparison 2: Nonexperimental studies: highest versus lowest green tea exposure, Outcome 21: Prostate cancer
Renal cancer
The one hospital‐based case‐control study investigating kidney cancer found a strong negative association between green tea consumption and risk (OR 0.34, 95% CI 0.21 to 0.55; Analysis 2.22; Wang 2012a).
2.22. Analysis.

Comparison 2: Nonexperimental studies: highest versus lowest green tea exposure, Outcome 22: Renal cancer
Urinary tract cancer
Nonexperimental studies investigating green tea consumption and risk of urinary tract (mainly urinary bladder) cancer showed no association (summary RR 1.04, 95% CI 0.79 to 1.37), with moderate heterogeneity (I2 = 56%) and very low‐certainty evidence (Analysis 2.23; Table 3). However, the summary estimate from the three cohort studies showed a positive association with a summary RR of 1.24 (95% CI 0.87 to 1.76) and low heterogeneity (I2 = 31%; Analysis 2.23; Chyou 1993; Kurahashi 2009; Nagano 2001). Conversely, one population‐based case‐control study (Wilkens 1996), and three hospital‐based case‐control studies (Hemelt 2010; Wakai 2004; Wang 2013b), found little to no association between green tea consumption and urinary tract cancer risk, with summary RR of 1.08 (95% CI 0.61 to 1.92) and 0.84 (95% CI 0.53 to 1.32), respectively.
2.23. Analysis.

Comparison 2: Nonexperimental studies: highest versus lowest green tea exposure, Outcome 23: Urinary tract cancer
Haematological cancer
Findings from overall haematological cancers showed a lower, though imprecise risk in the highest category of green tea consumption (summary RR 0.75, 95% CI 0.45 to 1.27), with moderate heterogeneity (I2 = 60%; Analysis 2.24). Indeed, results of studies assessing leukaemia risk were highly variable (Analysis 2.25), with high risk in one cohort study (Ugai 2018), assessing acute myeloid leukaemia risk (RR 1.20, 95% CI 0.62 to 2.32), null risk from the two population‐based case‐control studies assessing all leukaemia (summary RR 1.03, 95% CI 0.50 to 2.14; Kuo 2009; Li 2011a), and lower risk from the two hospital‐based case‐control studies assessing all leukaemias (summary RR 0.64, 95% 0.45 to 0.91; Analysis 2.25; Liu 2017; Zhang 2008). Similarly, a slightly lower risk (RR 0.89, 95% CI 0.61 to 1.30) was reported in one cohort study evaluating risk of lymphoma (Analysis 2.26; Ugai 2017), as well as from studies assessing risk of multiple myeloma (summary RR 0.50, 95% CI 0.26 to 0.95; Analysis 2.27).
2.24. Analysis.

Comparison 2: Nonexperimental studies: highest versus lowest green tea exposure, Outcome 24: Hematopoietic cancer
2.25. Analysis.

Comparison 2: Nonexperimental studies: highest versus lowest green tea exposure, Outcome 25: Leukaemia
2.26. Analysis.

Comparison 2: Nonexperimental studies: highest versus lowest green tea exposure, Outcome 26: Lymphoma
2.27. Analysis.

Comparison 2: Nonexperimental studies: highest versus lowest green tea exposure, Outcome 27: Multiple myeloma
Other types of cancers
The one case‐control study assessing non‐melanoma skin cancer risk showed an indication of lower risk in the highest category of green tea consumption (OR 0.82, 95% CI 0.35 to 1.90; Analysis 2.28; Hakim 2000). Similarly, a lower risk was reported for thyroid cancer (RR 0.88, 95% CI 0.56 to 1.37) by one cohort study (Analysis 2.29; Michikawa 2011). Conversely, slightly increased and highly imprecise risk was reported by one cohort study (RR 1.07, 95% CI 0.71 to 1.62) assessing brain cancer risk (Analysis 2.30; Ogawa 2016).
2.28. Analysis.

Comparison 2: Nonexperimental studies: highest versus lowest green tea exposure, Outcome 28: Non‐melanoma skin cancer
2.29. Analysis.

Comparison 2: Nonexperimental studies: highest versus lowest green tea exposure, Outcome 29: Thyroid cancer
2.30. Analysis.

Comparison 2: Nonexperimental studies: highest versus lowest green tea exposure, Outcome 30: Brain cancer
Discussion
Summary of main results
The aims of this review were to examine the possible association between green tea consumption and the risk of cancer incidence and mortality, as well as quality of life. This updated review includes data from 11 experimental studies (all RCTs), 10 more than in the previous version of this review (Boehm 2009), and 160 (106 additional) nonexperimental studies, which were cohort and case‐control studies.
Experimental studies have reported contrasting results. For instance, a decreased risk was suggested for prostate cancer incidence, in men at high risk of prostate cancer, but with RRs ranging from highly beneficial effect to no effect in the three RCTs for this outcome, whilst an increased risk in the green tea‐supplemented participants was reported for gynaecological cancer incidence, and no effect emerged for non‐melanoma skin cancer. Conversely, results from most nonexperimental studies showed a decreased risk for any cancer incidence, but not for mortality. Also, either decreased or increased results were reported for site‐specific cancers. Though a general decreased risk emerged when we considered all studies, stratified analyses according to study design, that is, case‐control versus cohort studies, showed a null or even increased risk estimates in the upper category of green tea consumption for the latter, while results for the case‐control studies were generally contradictory. This was true for any gut cancer, stomach, liver, colorectal, lung cancer, and even more for prostate, pancreatic, urinary tract cancer, and leukaemia.
Overall completeness and applicability of evidence
We aimed to extend the previous assessment of the relationship between green tea exposure and cancer risk by including all experimental and nonexperimental studies in adult populations published up until January 2019, without any limitation of time and language. During the past few decades, a large number of epidemiological studies have examined the association between green tea consumption and risk of various cancers. We included 16 non‐English papers (15 in Chinese and 1 in Japanese).
We included all publications assessing the association between green tea intake and cancer risk independently from the source of exposure, that is, including supplementation with green extracts in experimental studies, and any green tea consumption in nonexperimental studies, in liquid and solid form. We excluded all studies in which green tea exposure could not be precisely or independently identified, including those assessing intake of tea without allowing the selective measurement of consumption of green tea. Besides the database search, we screened previous reviews on this topic published up to May 2019 (Booth 2008; EUnetHTA 2017). This allowed us to assess the most up‐to‐date evidence compared with several previous systematic reviews carried out on single specific outcomes (Borrelli 2004; Butler 2011; Chang 2014; Chen 2014; Chen 2017b; Fang 2015; Gao 2013; Gianfredi 2018; Guo 2017; Jacob 2017; Huang 2016; Huang 2017; Hou 2013; Lin 2014; Najaf 2018; Ni 2017; Qin 2012; Sang 2013; Tang 2015; Vieira 2017; Wang 2014b; Weng 2017Wu 2013b; Xiong 2017; Yang 2019; Yiannakopoulou 2014; Yu 2014; Zeng 2014; Zhang 2015b; Zheng 2011; Zheng 2012; Zheng 2013; Zhong 2014; Zhou 2016).
In contrast to the previous version of this review (Boehm 2009), we included a quantitative assessment of cancer risk related to green tea intake, adding a meta‐analysis of all cancer outcomes whenever there were sufficient data available to perform the analysis.
The inconsistency and high heterogeneity of results from epidemiological studies might have various possible explanations. One is exposure misclassification, since exposure to green tea polyphenols may vary greatly across study population in terms of either quantity (e.g. cups per day) of green tea consumption and amount and type of catechins, depending on the type of green tea, brewing time and temperature (Astill 2001; Sharpe 2016).
In addition, exposure to green tea catechins between experimental studies and nonexperimental studies may be very different. One capsule containing 200 mg of EGCG corresponds to two or three cups of brewed tea, therefore the tested doses up to 800 mg of EGCG or more per day in experimental studies are equivalent to the consumption of at least eight cups of green tea per day (Coppock 2016; Crew 2015), a high level of exposure. Such a high quantity is difficult to reach when consuming only brewed tea. Moreover some experimental studies have shown that green tea supplementation is not free from adverse effects, such as gastrointestinal adverse effects, elevation of liver enzymes, and insomnia, probably due to the caffeine residues during extraction of catechins or polyphenols (Coppock 2016), hypertension, and skin or subcutaneous tissue reactions.
Also, in nonexperimental studies, we cannot rule out residual, unmeasured confounding effects, due to smoking and alcohol consumption (Chen 2017a), but also to other possible beneficial and adverse factors of dietary and non‐dietary origin, possibly a major source of heterogeneity (Bhagwat 2014; Khan 2017; Malir 2014; Manach 2005; Podwika 2018; Rothwell 2017).
Quality of the evidence
Overall, both the experimental and nonexperimental studies that we included in this review were generally of high quality, based on the assessment of risk of bias. In the latter, a higher quality was seen in the cohort studies, with all NOS scores above 7 stars (Figure 1), thus indicating a medium and high methodological quality. Conversely, case‐control studies, particularly those with a hospital‐based design, showed lower scores, and those with an NOS score equal to or below 6 stars should be considered at high risk of bias (Figure 1). We found little evidence of publication bias for all outcomes except for oesophageal cancer and prostate cancer, thus for these latter outcomes the risk of low reporting/publication for studies with unfavourable or no cancer risk cannot be ruled out.
The certainty of the evidence presented in the Table 1, Table 2 and Table 3 showed low‐ or very low‐certainty evidence from both experimental and nonexperimental studies, independently from the detection of either decreased or increased cancer risk. In experimental studies, we downgraded the certainty mainly due to the high imprecision of the estimates, based on small sample sizes and few observed cases with the outcome of interest. Also, moderate to high inconsistency was generally detected across study results, such as the results being reversed for risk of gynaecological cancers. Similarly in non‐experimental studies, despite some generally decreased RR in the most exposed categories, we could not exclude a serious risk of bias due to the case‐control design of most studies, since null or increased risk was reported in cohort studies for several outcomes such as stomach, pancreatic, colorectal, lung, breast and prostate cancer.
Potential biases in the review process
We attempted to minimise bias at every step of the review process. We performed a comprehensive search of the literature by accessing leading electronic databases (e.g. CENTRAL, MEDLINE, Embase), indexing relevant research and by implementing citation‐chasing methods for identification of all other additional relevant research. Regarding experimental studies in particular, it is unlikely that we have not identified any RCTs. However, unpublished trials or ongoing trials not registered in clinical trials registries could have been missed. Should such trials be identified, we will include them in future updates of the review. For study selection and assessment, at least two review authors independently assessed studies, performed data extraction, assessed risk of bias and GRADE, with the additional contribution of a third review author in order to solve conflicts. Regarding publication bias, in experimental studies, we were unable to assess its presence due to the few studies available to generate reliable funnel plots. In nonexperimental studies, we found symmetrical distribution for most of the outcomes considered, except oesophageal and prostate cancer, mainly due to results from case‐control studies. However, we systematically performed stratified analysis according to study design.
Agreements and disagreements with other studies or reviews
A previous meta‐analysis showed that black tea but not green tea consumption was related to a lower risk of cancer mortality (Zhang 2015a), in line with the results of this review. Other reviews that stratified analyses according to study design, also reported results consistent with our findings; they generally showed inverse associations in case‐control studies and much weaker or no difference in cohort studies. For example, for prostate cancer, a recent meta‐analysis concluded that there is a dose‐response relationship between green tea consumption and prevention of prostate cancer (Guo 2017). However, when only cohort studies were considered, no difference in risk was found. Similarly, nonexperimental (observational) studies also reported an inverse association between green tea and risk of both endometrial and ovarian cancer when only case‐control studies were considered (Butler 2011). Conversely, in cohort studies no difference in risk emerged. Consistent with this finding, contradictory results according to study design were found for risk of lung cancer (Guo 2019). In addition, the preventive role of green tea on gastrointestinal cancer was not confirmed when tea temperature was also evaluated. For instance, with reference to oesophageal and gastric cancer, high‐doses and long‐term consumption seemed to reduce the risk, whereas very high‐temperature green tea appeared to increase the risk of cancer (Huang 2017; Yi 2019).
Authors' conclusions
Implications for practice.
Overall, findings from epidemiological studies yielded inconsistent results for the effect of green tea consumption on cancer risk, despite some indications of a beneficial effect of green tea on a few site‐specific cancers. In addition, the majority of included studies were carried out in Asian populations characterised by high intakes of green tea, thus limiting the generalisability of the findings to other populations. Therefore, the epidemiological evidence appears to be still inadequate to support a beneficial effect of green tea on cancer risk. In addition, the possibility that high consumption of green tea extracts may have adverse effects should be taken into careful consideration.
Implications for research.
Recommendations for future research arise from the observation that evidence for green tea preventing cancer risk is still highly inconsistent. Some evidence of a beneficial effect of green tea on prostate cancer risk emerged from the randomised controlled trials (RCTs), but their methodological limitations, such as the low number and size of the studies, and the inconsistencies of the results limit the interpretability of their results. The other cancer outcomes investigated in RCTs, gynaecological cancer and non‐melanoma skin cancer, were not clearly associated with either beneficial or adverse effects, and also suggested the possible occurrence of side effects associated with high intake of green tea extracts. Well conducted and adequately powered RCTs, together with nonexperimental cohort design studies, are therefore clearly needed to elucidate the possible effects of green tea consumption on cancer risk in humans. RCTs should be carried out using low to moderate doses of green tea to avoid side effects and to reflect more closely the exposure patterns in most populations. They should also have an adequate sample size and allow a long period of follow‐up in order to detect long‐term and even small decreases in cancer risk.
What's new
| Date | Event | Description |
|---|---|---|
| 17 November 2021 | Amended | Correction made to PLS. |
History
Protocol first published: Issue 4, 2004 Review first published: Issue 3, 2009
| Date | Event | Description |
|---|---|---|
| 18 July 2019 | New citation required but conclusions have not changed | We included an additional 130 references relating to 93 studies. However the conclusions overall remain unchanged. |
| 18 July 2019 | New search has been performed | An updated search from January 2009 to January 2019 was conducted and 93 new studies were added to 52 in the review. |
Acknowledgements
We would like to thank all members of Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers for their valuable support.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
The review authors and Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers team, are grateful to the following peer reviewers for their time and comments: Catherine Crespi, Kathie Godfrey and Duarte Torres, and also to one who wished to remain anonymous.
Appendices
Appendix 1. CENTRAL search strategy
#1. MeSH descriptor: [Tea] explode all trees #2. MeSH descriptor: [Camellia sinensis] this term only #3. (green or antiox* or anti‐ox* or matsu or mattsu* or gruner*) near/5 (tea* or tee* or thea* or cha*) #4. camellia sinensis #5. tea or thea or tee or matsu‐cha or mattsu‐cha #6. #1 or #2 or #3 or #4 or #5 #7. MeSH descriptor: [Neoplasms] explode all trees #8. cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or adenocarcinoma* or oncol* #9. #7 or #8 #10. #6 and #9
Appendix 2. Medline Ovid search strategy
1. exp Tea/ 2. Camellia sinensis/ 3. ((green or antiox* or anti‐ox* or matsu or mattsu* or gruner*) adj5 (tea* or tee* or thea* or cha*)).mp. 4. camellia sinensis.mp. 5. (tea or thea or tee or matsu‐cha or mattsu‐cha).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 6. 1 or 2 or 3 or 4 or 5 7. exp Neoplasms/ 8. (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or adenocarcinoma* or oncol*).mp. 9. 7 or 8 10. 6 and 9 11. randomized controlled trial.pt. 12. controlled clinical trial.pt. 13. randomized.ab. 14. placebo.ab. 15. clinical trials as topic.sh. 16. randomly.ab. 17. trial.ti. 18. exp case control studies/ 19. exp cohort studies/ 20. case control.tw. 21. (cohort adj (study or studies)).tw. 22. cohort analy*.tw. 23. (follow up adj (study or studies)).tw. 24. (observational adj (study or studies)).tw. 25. longitudinal.tw. 26. retrospective.tw. 27. cross sectional.tw. 28. cross‐sectional studies/ 29. 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 30. (animals not (humans and animals)).sh. 31. 29 not 30 32. 10 and 31
key: mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier pt = publication type ab = abstract fs = floating subheading
Appendix 3. Embase Ovid search strategy
1. exp tea/ 2. Camellia sinensis/ 3. ((green or antiox* or anti‐ox* or matsu or mattsu* or gruner*) adj5 (tea* or tee* or thea* or cha*)).mp. 4. camellia sinensis.mp. 5. (tea or thea or tee or matsu‐cha or mattsu‐cha).mp. 6. 1 or 2 or 3 or 4 or 5 7. exp neoplasm/ 8. (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or adenocarcinoma* or oncol*).mp. 9. 7 or 8 10. 6 and 9 11. exp controlled clinical trial/ 12. randomized.ab. 13. randomly.ab. 14. trial.ab. 15. groups.ab. 16. exp case control study/ 17. exp cohort analysis/ 18. case control.tw. 19. (cohort adj (study or studies)).tw. 20. cohort analy*.tw. 21. (follow up adj (study or studies)).tw. 22. (observational adj (study or studies)).tw. 23. longitudinal.tw. 24. retrospective.tw. 25. cross sectional.tw. 26. cross‐sectional studies/ 27. 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 28. (animals not (humans and animals)).sh. 29. 27 not 28 30. 10 and 29
key:
[mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
Appendix 4. Newcastle‐Ottawa Scale for nonexperimental cohort studies
Note: a study can be awarded a maximum of one star for each numbered item within the selection and outcome categories. A maximum of two stars can be given for comparability.
Selection
-
Representativeness of the exposed cohort
truly representative of the average general population in the community (*)
somewhat representative of the average general population in the community (*)
selected group of users eg nurses, volunteers
no description of the derivation of the cohort
-
Selection of the non exposed cohort
drawn from the same community as the exposed cohort (*)
drawn from a different source
no description of the derivation of the non exposed cohort
-
Ascertainment of exposure
secure record (eg surgical records) (*)
structured interview or use of food frequency questionnaire (*)
written self‐report
no description
-
Demonstration that outcome of interest was not present at start of study
yes (*)
no
Comparability
-
Comparability of cohorts on the basis of the design or analysis
study controls for age and sex (when appropriate) (*)
study controls for any additional factor (*) smoking habits
Outcome
-
Assessment of outcome
independent blind assessment (*)
record linkage (e.g. cancer registry) (*)
self‐report
no description
-
Was follow‐up long enough for outcomes to occur
yes, more than 5 years (*)
no, less than 5 years
-
Adequacy of follow‐up of cohorts
complete follow‐up ‐ all participants accounted for (*)
participants lost to follow‐up unlikely to introduce bias ‐ small number lost ‐ > 90% (select an adequate %) follow up, or description provided of those lost) (*)
follow‐up rate < 90 % (select an adequate %) and no description of those lost
no statement
Appendix 5. Newcastle‐Ottawa Scale for nonexperimental case‐control studies
Note: a study can be awarded a maximum of one star for each numbered item within the selection and exposure categories. A maximum of two stars can be given for comparability.
Selection
-
Is the case definition adequate?
yes, with independent validation (*)
yes, e.g. record linkage or based on self‐reports
no description
-
Representativeness of the cases
consecutive or obviously representative series of cases (*)
potential for selection biases or not stated
-
Selection of controls
community/population controls (*)
hospital controls
no description
-
4) Definition of controls
no history of disease (endpoint) (*)
no description of source
Comparability
Comparability of cases and controls on the basis of the design or analysis
study controls for age and sex (when appropriate) (*)
study controls for any additional factor (*) smoking status
Exposure
-
Ascertainment of exposure
secure record (eg surgical records) (*)
structured interview blinded to case/control status or use of food frequency quesionannaire (*)
interview not blinded to case/control status
written self‐report or medical record only
no description
-
Same method of ascertainment for cases and controls
yes (*)
no
-
Non‐response rate
same rate for both groups (*)
nonrespondents described
rate different and no designation
Data and analyses
Comparison 1. Experimental studies: highest versus lowest green tea exposure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Prostate cancer incidence | 3 | 201 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.18, 1.36] |
| 1.2 Gynaecological cancer incidence | 2 | 1157 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.41, 5.48] |
| 1.2.1 Endometrial cancer | 1 | 1075 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.15] |
| 1.2.2 Cervical cancer | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [0.54, 7.46] |
| 1.3 Non‐melanoma skin cancer incidence | 1 | 1075 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.06, 15.92] |
Comparison 2. Nonexperimental studies: highest versus lowest green tea exposure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Any cancer incidence | 3 | 52479 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.65, 1.07] |
| 2.1.1 Cohort studies | 2 | 51629 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.50, 1.32] |
| 2.1.2 Population‐based case‐control studies | 1 | 850 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.60, 1.01] |
| 2.2 Any cancer mortality | 8 | 504366 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.91, 1.07] |
| 2.3 Oral cancer | 5 | 55977 | Risk Ratio (IV, Fixed, 95% CI) | 0.71 [0.62, 0.82] |
| 2.3.1 Cohort studies | 1 | 50258 | Risk Ratio (IV, Fixed, 95% CI) | 0.44 [0.19, 1.04] |
| 2.3.2 Population‐based case‐control studies | 1 | 1721 | Risk Ratio (IV, Fixed, 95% CI) | 0.58 [0.42, 0.79] |
| 2.3.3 Hospital‐based case‐control studies | 3 | 3998 | Risk Ratio (IV, Fixed, 95% CI) | 0.77 [0.65, 0.90] |
| 2.4 Oral, pharyngeal and laryngeal cancer | 1 | 2040 | Risk Ratio (IV, Random, 95% CI) | 1.47 [1.12, 1.93] |
| 2.4.1 Cohort studies | 1 | 2040 | Risk Ratio (IV, Random, 95% CI) | 1.47 [1.12, 1.93] |
| 2.5 Pharyngeal cancer | 1 | 12282 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.30, 2.30] |
| 2.5.1 Hospital‐based case‐control study | 1 | 12282 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.30, 2.30] |
| 2.6 Any gut cancer | 7 | 70299 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.59, 1.02] |
| 2.6.1 Cohort studies | 2 | 52298 | Risk Ratio (IV, Random, 95% CI) | 0.86 [0.27, 2.79] |
| 2.6.2 Population‐based case‐control studies | 1 | 1721 | Risk Ratio (IV, Random, 95% CI) | 0.58 [0.42, 0.79] |
| 2.6.3 Hospital‐based case‐control studies | 4 | 16280 | Risk Ratio (IV, Random, 95% CI) | 0.77 [0.65, 0.90] |
| 2.7 Oesophageal cancer | 13 | 74895 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.64, 1.04] |
| 2.7.1 Cohort studies | 1 | 26801 | Risk Ratio (IV, Random, 95% CI) | 1.67 [0.88, 3.16] |
| 2.7.2 Population‐based case‐control studies | 9 | 14111 | Risk Ratio (IV, Random, 95% CI) | 0.74 [0.55, 1.00] |
| 2.7.3 Hospital‐based case‐control studies | 3 | 33983 | Risk Ratio (IV, Random, 95% CI) | 0.86 [0.57, 1.27] |
| 2.8 Stomach cancer | 18 | 438595 | Risk Ratio (IV, Random, 95% CI) | 0.86 [0.74, 1.01] |
| 2.8.1 Cohort studies | 7 | 398286 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.85, 1.14] |
| 2.8.2 Population‐based case‐control studies | 8 | 9923 | Risk Ratio (IV, Random, 95% CI) | 0.74 [0.53, 1.02] |
| 2.8.3 Hospital‐based case‐control studies | 3 | 30386 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.74, 1.09] |
| 2.9 Liver cancer | 6 | 198885 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.68, 1.14] |
| 2.9.1 Cohort studies | 5 | 198266 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.71, 1.20] |
| 2.9.2 Population‐based case‐control studies | 1 | 619 | Risk Ratio (IV, Random, 95% CI) | 0.55 [0.28, 1.09] |
| 2.10 Pancreatic cancer | 10 | 326564 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.70, 1.10] |
| 2.10.1 Cohort studies | 6 | 320596 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.84, 1.30] |
| 2.10.2 Population‐based case‐control studies | 3 | 5720 | Risk Ratio (IV, Random, 95% CI) | 0.67 [0.48, 0.96] |
| 2.10.3 Hospital‐based case‐control studies | 1 | 248 | Risk Ratio (IV, Random, 95% CI) | 1.94 [1.06, 3.55] |
| 2.11 Biliary tract cancer | 3 | 195800 | Risk Ratio (IV, Random, 95% CI) | 0.79 [0.57, 1.11] |
| 2.11.1 Cohort studies | 3 | 195800 | Risk Ratio (IV, Random, 95% CI) | 0.79 [0.57, 1.11] |
| 2.12 Colorectal cancer | 16 | 610295 | Risk Ratio (IV, Random, 95% CI) | 0.84 [0.74, 0.96] |
| 2.12.1 Cohort studies | 9 | 554298 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.92, 1.08] |
| 2.12.2 Population‐based case‐control studies | 5 | 12811 | Risk Ratio (IV, Random, 95% CI) | 0.74 [0.61, 0.90] |
| 2.12.3 Hospital‐based case‐control studies | 2 | 43186 | Risk Ratio (IV, Random, 95% CI) | 0.53 [0.17, 1.60] |
| 2.13 Colon cancer | 10 | 389974 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.80, 0.98] |
| 2.13.1 Cohort studies | 6 | 361348 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.82, 1.05] |
| 2.13.2 Population‐based case‐control studies | 3 | 7136 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.69, 1.00] |
| 2.13.3 Hospital‐based case‐control studies | 1 | 21490 | Risk Ratio (IV, Random, 95% CI) | 0.77 [0.47, 1.26] |
| 2.14 Rectal cancer | 9 | 356851 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.75, 1.05] |
| 2.14.1 Cohort studies | 5 | 329570 | Risk Ratio (IV, Random, 95% CI) | 0.92 [0.77, 1.09] |
| 2.14.2 Population‐based case‐control studies | 3 | 5887 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.56, 1.24] |
| 2.14.3 Hospital‐based case‐control studies | 1 | 21394 | Risk Ratio (IV, Random, 95% CI) | 1.25 [0.62, 2.51] |
| 2.15 Nasopharyngeal carcinoma | 2 | 2290 | Risk Ratio (IV, Random, 95% CI) | 0.49 [0.36, 0.67] |
| 2.15.1 Population‐based case‐control studies | 1 | 685 | Risk Ratio (IV, Random, 95% CI) | 0.61 [0.41, 0.91] |
| 2.15.2 Hospital‐based case‐control studiesCohort studies | 1 | 1605 | Risk Ratio (IV, Random, 95% CI) | 0.44 [0.36, 0.54] |
| 2.16 Lung cancer | 17 | 269565 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.76, 1.02] |
| 2.16.1 Cohort studies | 6 | 240987 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.79, 1.31] |
| 2.16.2 Population‐based case‐control studies | 5 | 9703 | Risk Ratio (IV, Random, 95% CI) | 0.73 [0.61, 0.87] |
| 2.16.3 Hospital‐based case‐control studies | 6 | 18875 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.69, 1.17] |
| 2.17 Breast cancer | 14 | 250822 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.75, 1.02] |
| 2.17.1 Cohort studies | 5 | 235706 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.86, 1.19] |
| 2.17.2 Population‐based case‐control studies | 4 | 9336 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.70, 1.08] |
| 2.17.3 Hospital‐based case‐control studies | 5 | 5780 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.58, 1.13] |
| 2.18 Gynaecological cancer | 10 | 66738 | Risk Ratio (IV, Random, 95% CI) | 0.69 [0.57, 0.83] |
| 2.18.1 Cohort studies | 1 | 53841 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.43, 1.30] |
| 2.18.2 Population‐based case‐control studies | 9 | 12897 | Risk Ratio (IV, Random, 95% CI) | 0.68 [0.56, 0.84] |
| 2.19 Endometrial cancer | 5 | 60416 | Risk Ratio (IV, Random, 95% CI) | 0.77 [0.65, 0.91] |
| 2.19.1 Cohort studies | 1 | 53841 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.43, 1.30] |
| 2.19.2 Population‐based case‐control studies | 4 | 6575 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.61, 0.94] |
| 2.20 Ovarian cancer | 5 | 6322 | Risk Ratio (IV, Random, 95% CI) | 0.64 [0.45, 0.90] |
| 2.20.1 Population‐based case‐control studies | 5 | 6322 | Risk Ratio (IV, Random, 95% CI) | 0.64 [0.45, 0.90] |
| 2.21 Prostate cancer | 13 | 127239 | Risk Ratio (IV, Random, 95% CI) | 0.73 [0.56, 0.94] |
| 2.21.1 Cohort studies | 5 | 123289 | Risk Ratio (IV, Random, 95% CI) | 1.09 [0.89, 1.32] |
| 2.21.2 Population‐based case‐control studies | 1 | 750 | Risk Ratio (IV, Random, 95% CI) | 0.59 [0.40, 0.87] |
| 2.21.3 Hospital‐based case‐control studies | 7 | 3200 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.39, 0.63] |
| 2.22 Renal cancer | 1 | 549 | Risk Ratio (IV, Random, 95% CI) | 0.34 [0.21, 0.55] |
| 2.22.1 Hospital‐based case‐control studies | 1 | 549 | Risk Ratio (IV, Random, 95% CI) | 0.34 [0.21, 0.55] |
| 2.23 Urinary tract cancer | 7 | 156039 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.79, 1.37] |
| 2.23.1 Cohort studies | 3 | 151395 | Risk Ratio (IV, Random, 95% CI) | 1.24 [0.87, 1.76] |
| 2.23.2 Population‐based case‐control studies | 1 | 783 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.61, 1.92] |
| 2.23.3 Hospital‐based case‐control studies | 3 | 3861 | Risk Ratio (IV, Random, 95% CI) | 0.84 [0.53, 1.32] |
| 2.24 Hematopoietic cancer | 2 | 80646 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.45, 1.27] |
| 2.24.1 Cohort studies | 2 | 80646 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.45, 1.27] |
| 2.25 Leukaemia | 5 | 97778 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.57, 1.15] |
| 2.25.1 Cohort studies ‐ acute myeloid leukaemia | 1 | 95892 | Risk Ratio (IV, Random, 95% CI) | 1.20 [0.62, 2.32] |
| 2.25.2 Population‐based case‐control studies ‐ all leukaemia | 2 | 785 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.50, 2.14] |
| 2.25.3 Hospital‐based case‐control studies ‐ all leukaemia | 2 | 1101 | Risk Ratio (IV, Random, 95% CI) | 0.64 [0.45, 0.91] |
| 2.26 Lymphoma | 1 | 96218 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.61, 1.30] |
| 2.26.1 Cohort studies | 1 | 96218 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.61, 1.30] |
| 2.27 Multiple myeloma | 2 | 96385 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.26, 0.95] |
| 2.27.1 Cohort studies | 1 | 95945 | Risk Ratio (IV, Random, 95% CI) | 0.74 [0.39, 1.41] |
| 2.27.2 Hospital‐based case‐control studies | 1 | 440 | Risk Ratio (IV, Random, 95% CI) | 0.38 [0.27, 0.53] |
| 2.28 Non‐melanoma skin cancer | 1 | 450 | Risk Ratio (IV, Random, 95% CI) | 0.82 [0.35, 1.90] |
| 2.28.1 Population‐based case‐control studies | 1 | 450 | Risk Ratio (IV, Random, 95% CI) | 0.82 [0.35, 1.90] |
| 2.29 Thyroid cancer | 1 | 100666 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.56, 1.37] |
| 2.29.1 Cohort studies | 1 | 100666 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.56, 1.37] |
| 2.30 Brain cancer | 1 | 106479 | Risk Ratio (IV, Random, 95% CI) | 1.07 [0.71, 1.62] |
| 2.30.1 Cohort studies | 1 | 106479 | Risk Ratio (IV, Random, 95% CI) | 1.07 [0.71, 1.62] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Allen 2004.
| Study characteristics | ||
| Methods | Cohort study in Japan | |
| Participants | Participants: more than 119,500 men from Hiroshima and Nagasaki. 18,115 men included in the present study Inclusion criteria: resident in Hiroshima and Nagasaki included in the Adult Health Study (a subcohort of the Life Span study cohort) carried out among atomic‐bomb survivors and in residents in the cities in either city in the period between 1950 and 1953 and who completed the questionnaire surveys (in 1963, 1965 and 1979 to 1981), free from prostate cancer before the survey Parent cohort: Adult Health Study, a subcohort of the Life Span Study Recruitment: from 1963‐1996 |
|
| Interventions | N/A | |
| Outcomes | Number of cases prostate cancer: 193 cases (out of total 196 identified) | |
| Green tea in exposure categories | Exposure assessment: intake of green tea Lowest exposure: < 1 time/d Intermediate exposure: 2‐4 times/d Highest exposure: > 5 times/d |
|
| Notes | Funding: RERF Research Protocols (RP) no. 26‐63 and 14‐78 Statistical methods: Poisson regression Variables controlled in analysis: age, calendar period, city of residence, radiation dose and education level Variables controlled by matching: ‐ |
|
Bandera 2010.
| Study characteristics | ||
| Methods | PCC in USA | |
| Participants | Participants: 397 cases and 373 controls (all women) Inclusion criteria: women aged ≥ 21 years, newly diagnosed and histologically confirmed from the Estrogen, Diet, Genetic and Endometrial Cancer study, from 6 counties, New Jersey, USA Recruitment: from 1 July 2001‐30 June 2005 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Endometrial cancer: 397 cases from the 469 eligible (all women) |
|
| Green tea in exposure categories | Exposure assessment: green tea intake Lowest exposure: 0 cup/week Intermediate exposure: < 1 cup/week Highest exposure: ≥ 1 cups/week |
|
| Notes | Funding: NIH‐K07 CA095666 and R01CA83918 Statistical methods: unconditional logistic regression Variables controlled in analysis: age, education, race, age at menarche, menopausal status and age at menopause for postmenopausal women, parity, oral contraceptive use, HRT use, BMI, smoking (pack‐years), smoking status, added sugar/honey, milk, cream, or nondairy creamer in tea Variables controlled by matching ‐ |
|
Berroukche 2012.
| Study characteristics | ||
| Methods | HCC in Algeria | |
| Participants | Participants: 160 cases and 160 controls (all men) Inclusion criteria: free from other prostatic diseases or malignant tumours, not being under dietary restrictions or patients in critical conditions from Department of Urology of Sidibel‐Abbes UHC and of Saida Hospital, Algeria Recruitment: from January 2007‐March 2011 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Prostate cancer: 160 cases (all men) |
|
| Green tea in exposure categories | Exposure assessment: intake of green tea Lowest exposure: ≤ 1 cup/d Intermediate exposure 1: 2‐3 cups/d Intermediate exposure 2: 4‐5 cups/d Highest exposure: > 6 cups/d |
|
| Notes | Funding: not declared Statistical methods: conditional logistic regression Variables controlled in analysis: tobacco smoking, total energy intake and family history of prostate cancer Variables controlled by matching: age (± 5 years) |
|
Bettuzzi 2006.
| Study characteristics | ||
| Methods | RCT, parallel, double‐blind in Italy | |
| Participants | Participants: 60 men with HG‐PIN, 30 in each group Inclusion criteria: age 45‐75 years, with HG‐PIN diagnosed needle biopsies collection, not consuming green tea or taking antioxidants, not vegetarians and not under antiandrogenic therapy Recruitment: NR |
|
| Interventions | Treatment group: 3 capsules of GTEs, containing green tea catechins (200 mg each) capsules/d = total 600 mg/d, corresponding to approximately 300 mg/d of EGCG Control group: placebo Duration: 1 year |
|
| Outcomes | Primary outcome Prostate cancer incidence Secondary outcomes LUTS using IPSS PSA levels QoL data Safety data |
|
| Green tea in exposure categories | N/A | |
| Notes | Grant support: PRIN 2004 (MIUR, Italy). Dr. Rizzi was supported by Genprofiler Srl (Bolzano, Italy). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Volunteers were randomly assessed to a placebo‐ or GTCs [green tea catechins]‐arm by simple randomisation" Comment: it is unclear how sequence was generated, however the baseline characteristics reported in Table 4 are mainly equally distributed. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "That same day [when they signed the informed consent, NDR], they were alternatively assigned to the placebo‐ or GTCs [green tea catechins]‐arm and given the appropriate treatment. To all subjects, capsules were given by the urologist according to the double blind method" Comment: insufficient information to answer |
| Blinding of participants and personnel (performance bias) Prostate cancer incidence | Low risk | Quote: "capsules were given by the urologist according to the double blind method" Comment: probably done |
| Blinding of participants and personnel (performance bias) Lower urinary tract symptoms | Low risk | Quote: "In the second arm, men received placebo (three identical capsules per day). To all subjects, capsules were given by the urologist according to the double blind method". Comment: probably done |
| Blinding of participants and personnel (performance bias) PSA levels | Low risk | Review authors do not believe this will introduce bias since this measurement is independent from individual evaluation |
| Blinding of outcome assessment (detection bias) Prostate cancer incidence | Unclear risk | No explicit statement on blinded outcome assessment |
| Blinding of outcome assessment (detection bias) Lower urinary tract symptoms | Unclear risk | No explicit statement on blinded outcome assessment |
| Blinding of outcome assessment (detection bias) PSA levels | Low risk | Review authors do not believe this will introduce bias since this measurement is independent from individual evaluation |
| Incomplete outcome data (attrition bias) Prostate cancer incidence | Low risk | All randomised participants were included in the analysis |
| Incomplete outcome data (attrition bias) Lower urinary tract symptoms | Unclear risk | Quote: "patients, diagnosed with prostate cancer at the 6 months biopsy check, left the study" Comment: number of participants included in analysis not stated |
| Incomplete outcome data (attrition bias) PSA levels | Low risk | All randomised participants were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available and it is not clear if the published reports include all expected outcomes. Insufficient information to answer |
| Other bias | Low risk | Study controlled for total serum PSA at the time of enrolment, prostate volume at the time of enrolment, prostate volume at the end of study, total number of HG‐PIN scores vs total scores taken at the time of enrolment, total number of HG‐PIN scores taken at the end of study; total number of mono‐focal or plurifocal HG‐PIN lesions by means of a multivariate analysis |
Bonner 2005.
| Study characteristics | ||
| Methods | PCC in China | |
| Participants | Participants: 122 (male/female: 79/43) cases and 121 (male/female: 78/43) controls Inclusion criteria: newly diagnosed lung cancer, mean age 54.71 (SD 11.45) in cases and 54.44 (SD 11.97) in controls from residents of Xuan Weu, China Recruitment: from March 1995‐March 1996 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Lung cancer: 122 (male/female: 79/43) cases |
|
| Green tea in exposure categories | Exposure assessment: intake of green tea Lowest exposure: never Intermediate exposure: 2‐3 times/week Highest exposure: ≥ 1 time/d |
|
| Notes | Funding: not declared Statistical methods: unconditional logistic regression Variables controlled in analysis: smoking (pack‐years) Variables controlled by matching: age, sex, village of residence, type of heating and cooking fuel |
|
Chen 2011.
| Study characteristics | ||
| Methods | HCC in China | |
| Participants | Participants: 150 (male/female: 102/48) cases and 300 (male/female: 204/96) controls Inclusion criteria: histologically confirmed squamous cell oesophageal carcinoma, mean age 54.5 (SD 6) in cases and 54.0 (SD 7) in control from First Affiliated Hospital of Ji'nan University, China Recruitment: from June 2004‐May 2010 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Oesophageal cancer: 150 (male/female: 102/48) cases |
|
| Green tea in exposure categories | Exposure assessment: green tea intake Lowest exposure: never Intermediate exposure 1: < 100 g/month Intermediate exposure 2: 100‐250 g/month Highest exposure: > 250 g/month |
|
| Notes | Funding: Medical Science fund of Guangong Province (B2008094) Statistical methods: conditional logistic regression Variables controlled in analysis: age, sex, education level, annual income, cancer family history, smoking status and alcohol drinking status Variables controlled by matching: age (± 3 years) and sex |
|
Chen 2017a.
| Study characteristics | ||
| Methods | HCC in China | |
| Participants | Chen 2015 Participants: 203 (male/female: 153/50) cases and 572 (male/female: 416/156) controls Inclusion criteria: newly diagnosed cases of oral cancer, living in Fujian, China for > 10 years, with no pathological diagnosis of oral inflammation, benign lesions, or secondary tumours and without critical illness Recruitment: from September 2010‐January 2015 Chen 2016 Participants: 207 cases and 480 controls (all women) Inclusion criteria: newly diagnosed cases of oral cancer, consecutively recruited from the First Affiliated Hospital of Fujian Medical University, China Recruitment: from September 2010‐January 2015 Chen 2017a and Chen 2017c with duplicate results) Participants: 586 (male/female: 379/207) cases and 1024 (male/female: 630/394) controls Inclusion criteria: newly diagnosed cases of oral cancer, consecutively recruited from the First Affiliated Hospital of Fujian Medical University, China Recruitment: from September 2010‐January 2015 |
|
| Interventions | N/A | |
| Outcomes | Number of cases In Chen 2015 Oral cancer: 188 cases out of 203 recruited (15 cases excluded because drinkers of other types of tea) In Chen 2016 Oral cancer: 196 cases out of 207 recruited (11 cases excluded because drinkers of other types of tea) In Chen 2017a Oral cancer: 586 (male/female: 379/207) cases, including squamous cell carcinoma (N = 507), adenocarcinoma (N = 59), others (N = 20) |
|
| Green tea in exposure categories | Exposure assessment: green tea drinking habit (same for Chen 2015, Chen 2016 and Chen 2017a) Lowest exposure: not drinking tea Highest exposure: drinking green tea (≥ 1 cup/week for ≥ 6 months) |
|
| Notes | Chen 2015 Article in Chinese Funding: Research Project of Science and Technology Department of Fujian Province (2015J01304); Research Project of Fujian Provincial Department of Education (JA13141); Research and Application of New Technology of Key Laboratory of Environment and Health, School of Public Health, Fujian Medical University (201201). Statistical methods: unconditional logistic regression Variables controlled in analysis: sex, age, occupation, ethnicity, marital status, education level, BMI and place of residence Variables controlled by matching: ‐ Chen 2016 Funding: grants from the Natural Science Foundation of China (Nos. 30771845 and 81172766), Natural Science Foundation of Fujian Province (No. 2015J01304) and the Scientific Research Program of Education Department of Fujian Province (No. JA13141) Statistical methods: unconditional logistic regression Variables controlled in analysis: age, family history of cancer, occupation, education, BMI, residence, marital status, tobacco smoking, alcohol drinking, cooking oil fumes and passive smoking Variables controlled by matching: ‐ Chen 2017a Funding: grants from Natural Science Foundation of Fujian Province (N. 2015J01304) and from University Development Foundation of National Financial Support (N. 1003‐03900130) Statistical methods: unconditional logistic regression Variables controlled in analysis: age, sex, occupation, education, BMI, marital status, residence, family cancer history, vegetables and fruits, alcohol drinking and tobacco smoking Variables controlled by matching: ‐ Data also available stratified according to milk consumption in non‐smoking and non‐drinking participants. Previous report on the same population also reported in Chen 2015 and Chen 2016 in: Chen 2017a. |
|
Chyou 1993.
| Study characteristics | ||
| Methods | Cohort study in USA | |
| Participants | Participants: 7991 male participants out of 8006 recruited Inclusion criteria: American men of Japanese ancestry, born from 1990‐1919 and residing on the Hawaiian island of Oahu, Hawaii, USA Parent cohort: Honolulu Hearth Program Recruitment: from 1965‐1968 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: May 1991 Urinary tract cancer: 96 cases, including urinary bladder (N = 83), pelvis (N = 8) and ureter (N = 5) |
|
| Green tea in exposure categories | Exposure assessment: green tea intake Lowest exposure: almost never Highest exposure: not specified, probably merging 4 categories: < 2 times/week, 2‐4 times/week, almost daily, ≥ 1 time/d |
|
| Notes | Funding: grants provided by National Cancer Institute (R01 CA33644) Statistical methods: proportional hazard regression Variables controlled in analysis: age and smoking Variables controlled by matching: ‐ |
|
Dai 2010.
| Study characteristics | ||
| Methods | Cohort study in China | |
| Participants | Participants: 72,861 Chinese women Inclusion criteria: women aged 40‐70 years, no history of cancer at baseline. 381 women regularly drinking only black or oolong tea excluded Parent cohort: Shangai Women's Health Study Recruitment: from March 1997‐May 2000 Data on the same cohort also reported in Nechuta 2012 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 31 December 2005 Breast cancer: 614 cases (all female) |
|
| Green tea in exposure categories | Exposure assessment: intake of green tea Lowest exposure: no Highest exposure: yes Exposure assessment: dosage of green tea Lowest exposure: no drinking Intermediate exposure 1: 0‐1.67 g/d Intermediate exposure 2: 1.68‐3.33 g/d Intermediate exposure 3: 3.34‐5.00 g/d Highest exposure: > 5.00 g/d |
|
| Notes | Funding: grants provided by National Institutes of Health (R01CA70867, R01CA106591, N02 CP1101066) Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age, educational achievement, income, family history of breast cancer, history of fibroadenoma, BMI, waist‐to‐hip ratio, physically active, smoking status, alcohol consumption status, passive smoking status, ginseng intake, age at menarche, age at first live birth, menopausal status, age at menopause, use of HRT and dietary intake of total energy, fruits, vegetables, red meat, fish and isoflavones Variables controlled by matching: ‐ |
|
Dostal 2015.
| Study characteristics | ||
| Methods | RCT, parallel, double‐blind in USA ‐ Minnesota Green Tea Trial (MGTT) | |
| Participants | Participants: 1075 post‐menopausal women (538 in the treatment group and 537 in the control group) Inclusion criteria: age 50‐70 years, classified as having high mammography density attending annual screening mammogram at 8 clinical centres in the Minneapolis‐St. Paul metropolitan area (Minnesota Green Tea Trial (MGTT), planning to reside in or near Minnesota for study duration. Exclusion criteria: tested positive for serological status of hepatitis B surface antigen or antibodies to hepatitis C virus; baseline ALT > 1.5 times the upper limit of 60 U/L; any history of cancer; any history of proliferative breast disease; history of breast augmentation; BMI < 18.5 or > 40 kg/m2; weight change > 4.6 kg during the previous 12 months; current or recent (within 6 months) use of HRT; current use of anti‐inflammatory agents including methotrexate or etanercept; current smoker; regular consumption of ≥ 7 alcoholic beverages/week; and regular consumption of ≥ 1 cups of green tea/week. Full details reported in Samavat et al. 2015. Recruitment: from August 2009‐April 2013 |
|
| Interventions | Treatment group: 4 oral GTE capsules, i.e. 1315 (± 116) mg of total catechins/d, including 843 (± 44) mg of EGCG Control group: placebo Duration: 1 year |
|
| Outcomes | Primary outcome Effects on biomarkers of breast cancer risk: mammographic density, circulating reproductive hormones and circulating insulin‐like growth factor axis proteins Secondary outcome Circulating F2‐isoprostanes, urinary oestrogen metabolites, anthropometric variables and obesity‐associated hormone concentrations QoL Safety data |
|
| Green tea in exposure categories | N/A | |
| Notes | Funding: from National Institutes of Health/National Cancer Institute grant R01 CA127236, Award Number T32CA132670 from the National Cancer Institute, the Department of Defense/US Army Medical Research and Materiel Command Award Number W81XWH‐11‐1‐0013, the University of Minnesota Agricultural Experiment Station Project Number MIN‐18‐103 and the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A detailed description of the Minnesota Green Tea Trial (MGTT) design, eligibility criteria, study conduct and patient flow through the trial will be published separately (Samavat et al., Cancer Causes and Control)." reporting that "Investigational Drug Services (IDS) pharmacy utilized a computer generated randomisation scheme using the permuted block method" Comment: probably low risk |
| Allocation concealment (selection bias) | Low risk | Quote: "A detailed description of the Minnesota Green Tea Trial (MGTT) design, eligibility criteria, study conduct and patient flow through the trial will be published separately (Samavat et al., Cancer Causes and Control)." reporting that "Randomization was performed by the Investigational Drug Services (IDS) pharmacy at University of Minnesota Medical Center ‐ Fairview". Central allocation. Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: from Samavat 2015 “In this double‐blinded study, study staff, participants, laboratory personnel and all parties involved with assessment of the study endpoints were blinded to treatment assignment. The treatment codes were only available to the IDS [Investigational Drug Services] pharmacy staff in charge of randomisation and a study biostatistician.” From Dostal 2015 : “Participants, investigators, laboratory staff and those monitoring clinical outcomes and adverse events were blinded to treatment assignment” and “Placebo capsules were identical in appearance to GTE". Study investigators were kept blinded to the assigned treatment of all participants experiencing an adverse effects Comment: probably done |
| Blinding of participants and personnel (performance bias) Non‐melanoma skin cancer incidence | Low risk | Quote: from Samavat 2015 “In this double‐blinded study, study staff, participants, laboratory personnel and all parties involved with assessment of the study endpoints were blinded to treatment assignment. The treatment codes were only available to the IDS [Investigational Drug Services] pharmacy staff in charge of randomisation and a study biostatistician.” From Dostal 2015 : “Participants, investigators, laboratory staff and those monitoring clinical outcomes and adverse events were blinded to treatment assignment” and “Placebo capsules were identical in appearance to GTE". Study investigators were kept blinded to the assigned treatment of all participants experiencing an adverse effects Comment: probably done |
| Blinding of participants and personnel (performance bias) Gynaecological cancer incidence | Low risk | Quote: from Samavat 2015 “In this double‐blinded study, study staff, participants, laboratory personnel and all parties involved with assessment of the study endpoints were blinded to treatment assignment. The treatment codes were only available to the IDS pharmacy staff in charge of randomisation and a study biostatistician.” From Dostal 2015 : “Participants, investigators, laboratory staff and those monitoring clinical outcomes and adverse events were blinded to treatment assignment” and “Placebo capsules were identical in appearance to GTE". Study investigators were kept blinded to the assigned treatment of all participants experiencing an adverse effects Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | From Dostal 2015 "Participants, investigators, laboratory staff and those monitoring clinical outcomes and adverse events were blinded to treatment assignment" and "Placebo capsules were identical in appearance to GTE". Study investigators were kept blinded to the assigned treatment of all participants experiencing an adverse effects Comment: probably done |
| Blinding of outcome assessment (detection bias) Non‐melanoma skin cancer incidence | Low risk | From Dostal 2015 "Participants, investigators, laboratory staff and those monitoring clinical outcomes and adverse events were blinded to treatment assignment" and "Placebo capsules were identical in appearance to GTE". Study investigators were kept blinded to the assigned treatment of all participants experiencing an adverse effects Comment: probably done |
| Blinding of outcome assessment (detection bias) Gynaecological cancer incidence | Low risk | From Dostal 2015 "Participants, investigators, laboratory staff and those monitoring clinical outcomes and adverse events were blinded to treatment assignment" and "Placebo capsules were identical in appearance to GTE". Study investigators were kept blinded to the assigned treatment of all participants experiencing an adverse effects Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number and reason of participants' withdrawal from the study reported. Study authors performed an ITT analysis. |
| Incomplete outcome data (attrition bias) Non‐melanoma skin cancer incidence | Low risk | Number and reason of participants' withdrawal from the study reported. Study authors performed an ITT analysis. |
| Incomplete outcome data (attrition bias) Gynaecological cancer incidence | Low risk | Number and reason of participants withdrawal from the study reported. Authors performed an ITT analysis. |
| Selective reporting (reporting bias) | Low risk | Results to the Minnesota Green Tea Trial RCT (ClinicalTrials.gov identifier (NCT number): NCT00917735) reported in several publications all included in this review. |
| Other bias | Unclear risk | Elevated number of withdrawals |
Dryden 2013.
| Study characteristics | ||
| Methods | RCT, parallel, double‐blind in USA | |
| Participants | Participants: 20 people with ulcerative colitis. Ratio 4:1 randomisation treatment/placebo Inclusion criteria: age ≥ 18 years, with mildly to moderately active disease Recruitment: NR |
|
| Interventions | Treatment groups: green tea catechins (using Polyphenon E, Mitsu‐Norin, Fujieda) in low dose of catechins containing 200 mg of EGCG and high dose of catechins containing 400 mg of EGCG Control group: placebo Duration: 56 d |
|
| Outcomes | Primary outcome Safety data Secondary outcome: QoL |
|
| Green tea in exposure categories | N/A | |
| Notes | Funding: National Institutes of Health grant (5K23DK073750), University of Louisville Research Foundation Project Initiation Grant, Polyphenon E supplied by Mitsui‐Norin (Fujieda, Japan) through the Chemoprevention Agent Development Group of the National Cancer Institute. Baseline characteristics were not equally distributed: both men and women included in the treatment groups, while only women were included in the placebo group. Treated participants were also slightly older and with higher mean weight and all used azathioprine. Tobacco use prevalence was 7% and 50% in treatment and control group, respectively. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients...were randomized in a double‐blinded fashion according to a random number generator compiled by a statistician not involved in the study" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients...were randomized in a double‐blinded fashion according to a random number generator compiled by a statistician not involved in the study" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients ….. were randomized in a double‐blinded fashion": Also UCDAI was assessed at day 0 and 56, while for other laboratory analyses at day 1, 14, 28, 56 and 70. Response: review authors do not believe this will introduce bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Patients ….. were randomized in a double‐blinded fashion": Also UCDAI was assessed at day 0 and 56, while for other laboratory analyses at day 1, 14, 28, 56 and 70. Response: review authors do not believe this will introduce bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number of participants included in analysis stated and reported |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (ClinicalTrials.gov identifier (NCT number): NCT00718094) and the published reports include all expected outcomes |
| Other bias | High risk | Results from Inflammatory Bowel Disease Questionnaire, a validated, IBD‐specific indicator of QoL, was significantly different between groups at baseline. Some participants in the treatment group, but not in the placebo arm, took immunomodulatory drugs |
Fu 2013.
| Study characteristics | ||
| Methods | HCC in China | |
| Participants | Participants: 723 (male/female: 485/238) cases and 857 (male/female: 576/281) controls Inclusion criteria: aged 30‐80 years, with incident and histologically confirmed oral squamous cell cancer from 8 cities in different parts of China Recruitment: from 2007‐2010 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Oral cancer: 723 (male/female: 485/238) cases of squamous cell cancer |
|
| Green tea in exposure categories | Exposure assessment: intake of green tea Lowest exposure: < 4 g/d Intermediate exposure: 4‐7 g/d Highest exposure: ≥ 8 g/d |
|
| Notes | Funding: Grant (N. 06dz22026) from the Science and Technology Commission of Shanghai Municipality. Statistical methods: unconditional logistic regression Variables controlled in analysis: age, sex, long‐term residency area, years of education, tobacco smoking and alcohol drinking Variables controlled by matching: sex, age (± 5 years) and long‐term residency area. |
|
Galanis 1998.
| Study characteristics | ||
| Methods | Cohort study of Japanese population living in Hawaii, USA | |
| Participants | Participants: 40,575 eligible participants (of whom 12,789 were Japanese). Final population 11,907 (male/female: 5610/6297), 882 excluded for missing information on covariates Inclusion criteria: Japanese participants, complete information on covariates Parent cohort: Hawaii Health Surveillance Program Cohort (HHSPC) Recruitment: from 1975‐1980 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Oucome assessment: 31 December 1994 Stomach cancer: 108 (male/female: 64/44) cases |
|
| Green tea in exposure categories | Exposure assessment: intake of green tea Lowest exposure: none Intermediate exposure: 1 cup/d Highest exposure: ≥ 2 cups/d |
|
| Notes | Funding: not declared Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age, years of education, Japanese place of birth and sex (in combined analyses). Analyses among men were also adjusted for cigarette smoking and alcohol intake status. Variables controlled by matching: ‐ Galanis 1997 is a letter reporting preliminary findings subsequently published in Galanis 1998. |
|
Gao 1994.
| Study characteristics | ||
| Methods | PCC in China | |
| Participants | Participants: 902 (66% male) cases and 1552 (55% male) controls. 1312 (male/female: 654/658) controls considered in the present analysis Inclusion criteria: aged 30‐74 years, permanent residents in Shangai, China Recruitment: from 1 October 1990‐31 January 1993 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Oesophageal cancer: 659 (male/female: 417/242) out of 902 eligible cases excluding participants drinking other types of tea, including squamous cell carcinoma (N = 605), adenocarcinoma (N = 51), other specified types (N = 25) and unspecified types (N = 53) |
|
| Green tea in exposure categories | Exposure assessment: intake of green tea Lowest exposure: nondrinker Highest exposure: drinkers (≥ 1 cup/week for ≥ 6 months) Exposure assessment: consumption of green tea Lowest exposure: nondrinker Intermediate exposure: 1‐199 g/month in men and 1‐149 g/month in women Highest exposure: ≥ 200 g/month in men and ≥ 150 g/month in women |
|
| Notes | Funding: Dr J Schneider funded by "Consejeria de Sanidad del Gobierno Vasco" and by "Fondo de Investigationed Sanitaria"; Dr MP Rubio funded by "Gobierno de Navarra". Statistical methods: unconditional logistic regression Variables controlled in analysis: age, education, birthplace, cigarette smoking and alcohol intake (only men) Variables controlled by matching: age (± 5 years) and sex |
|
Gao 2005.
| Study characteristics | ||
| Methods | PCC in China | |
| Participants | Participants: 995 cases and 1087 controls (all women) Inclusion criteria: aged 30‐69 years, living in Shangai, China Recruitment: from January 1997‐December 2002 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Endometrial cancer: 965 cases (out of total 995 identified) |
|
| Green tea in exposure categories | Exposure assessment: intake of green tea Lowest exposure: non drinkers Highest exposure: drinker (2 cups/week for ≥ 3 months) |
|
| Notes | Article in Chinese Funding: National Cancer Institute funded project (R01CA92585) Statistical methods: unconditional logistic regression Variables controlled in analysis: age, education level, age at menarche, number of pregnancies, whether to take oral contraceptives, first‐degree relatives, malignant tumours, history of the genus and BMI Variables controlled by matching: age |
|
Garcia 2014.
| Study characteristics | ||
| Methods | RCT, parallel, double‐blind in USA | |
| Participants | Participants: 98 women with persistent high‐risk HPV infection and low‐grade CIN (grade 1), 50 in the intervention group and 48 in the control group Inclusion criteria: aged ≥ 18 years, normal liver and kidney function and good performance status. Exclusion criteria: pregnant or breast feeding, consumed tea regularly within 1 month of enrolment, had a history of allergic reaction to tea or related dietary products, had been treated for genital condyloma within 30 days of enrolment, were receiving other investigational agents, had prior pelvic irradiation, were HIV‐positive, had uncontrolled inter‐current illness, had invasive or high‐grade intraepithelial neoplasia, or had a history of cancer except nonmelanoma skin cancer Recruitment: at the University of Arizona (Tucson, Arizona), with additional accrual at Maricopa Integrated Health System (Phoenix, Arizona) and Southern Pines Women's Health Center (Southern Pines, North Carolina), period NR. |
|
| Interventions | Treatment group: green tea catechins (using Polyphenon E capsules), corresponding to 800 mg of EGCG/d Control group: placebo Duration: 4 months |
|
| Outcomes | Primary outcome Oncogenic HPV clearance and clearance of CIN1 Secondary outcome: Safety data |
|
| Green tea in exposure categories | N/A | |
| Notes | Funding: Contract (N01‐CN35158) from the National Cancer Institute and the Arizona Cancer Center Support Grant (P30CA023074). The paper was partially written using funding provided by the National Cancer Institute of the National Institutes of Health under Award Number R25CA078447. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "participants were randomized to receive Polyphenon E or placebo. An adaptive allocation randomization procedure was implemented to balance the two groups on the basis of age." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "participants were randomized to receive Polyphenon E or placebo. An adaptive allocation randomization procedure was implemented to balance the two groups on the basis of age." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All histopathology specimens were reviewed in a blinded fashion by an experienced gynaecologic pathologist and were subjected to a second quality control review." Comment: done |
| Blinding of participants and personnel (performance bias) Gynaecological cancer incidence | Low risk | Quote: "All histopathology specimens were reviewed in a blinded fashion by an experienced gynaecologic pathologist and were subjected to a second quality control review." Comment: done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All histopathology specimens were reviewed in a blinded fashion by an experienced gynaecologic pathologist and were subjected to a second quality control review." Comment: done |
| Blinding of outcome assessment (detection bias) Gynaecological cancer incidence | Low risk | Quote: "All histopathology specimens were reviewed in a blinded fashion by an experienced gynaecologic pathologist and were subjected to a second quality control review." Comment: done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number of participants included in analysis stated. ITT analysis implemented |
| Incomplete outcome data (attrition bias) Gynaecological cancer incidence | Low risk | Number of participants included in analysis stated. ITT analysis implemented |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (ClinicalTrials.gov identifier (NCT number): NCT00303823) and the published report includes all expected outcomes |
| Other bias | Low risk | No reported withdrawals |
Garland 2006.
| Study characteristics | ||
| Methods | RCT, quadruple‐blind in USA | |
| Participants | 178 (male/female: 89/89) participants: 42 (male/female: 21/21) in group A, 63 (male/female: 31/32) in group B and 73 (male/female: 37/36) in group C, 89 men | |
| Interventions | Group A: green tea beverage and placebo capsules Group B: placebo beverage and Polyphenon E capsules (Mitsui‐Norin Co, Ltd, Shizuoka, Japan), corresponding to 800 mg/d of ECGC Group C: placebo beverage and placebo capsules Duration: 6 months |
|
| Outcomes | Primary outcome Change in levels of biomarkers of oxidative stress Secondary outcome Safety data |
|
| Green tea in exposure categories | N/A | |
| Notes | No results on lung cancer prevention published or reported on ClinicalTrials.gov (NCT00363805) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We are conducting a 6‐month randomized, controlled, double‐blind trial..." Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | No statement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Review authors do not believe this would introduce bias since all biological analyses were performed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Review authors do not believe this would introduce bias since all biological analyses were performed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data on lung cancer NR, only on biomarkers |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on ClinicalTrials.gov (NCT00363805) |
| Other bias | Unclear risk | The number of withdrawals is low, however since no full publication is available, only an abstract and report on ClinicalTrials.gov, it is difficult to judge if other biases are present. |
Gavrilas 2018.
| Study characteristics | ||
| Methods | HCC in Romania | |
| Participants | Participants: 151 (male/female: 92/59) cases and 151 (male/female: 90/61) controls Inclusion criteria: recently diagnosed cases undergoing conventional treatment recruited from MEDISPROF Oncology Hospital, Cluj‐Napoca, Romania Recruitment: from April 2015‐October 2017 |
|
| Interventions | N/A | |
| Outcomes | Colorectal cancer: 151 (male/female: 92/59) cases | |
| Green tea in exposure categories | Exposure assessment: drinking of green tea Lowest exposure: < 1 serving/week Intermediate exposure 1: 1‐2 servings/week Intermediate exposure 2: 3‐5 servings/week Highest exposure: > 5 servings/week |
|
| Notes | Funding: not declared Statistical methods: crude analysis with raw data Variables controlled in analysis: crude analysis Variables controlled by matching: age (± 5 years) Gavrilas 2018 (Bulletin UASVM Food Science and Technology) reports same results subsequently published in Gavrilas 2018 |
|
Goodman 2003.
| Study characteristics | ||
| Methods | PCC in USA | |
| Participants | Participants: 164 cases and 194 controls (all women) Inclusion criteria: aged 18‐84 years, diagnosed with epithelial ovarian cancer. Controls Oahu residents from Hawai Health Survey Program and from women aged ≥ 65 years, in the Health Care Financing Administration on Oahu, Hawaii, USA Recruitment: from 1 July 1993‐30 June 1999 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Ovarian cancer: 164 cases of epithelial ovarian cancer |
|
| Green tea in exposure categories | Exposure assessment: drinking of green tea (yes/no) Lowest exposure: no drinking Highest exposure: drinking green tea further divided in: Highest exposure 1: < 1 cup/week Highest exposure 2: ≥ 1 cups/week |
|
| Notes | Funding: by the Anneliese Lermann Fund for Cancer Research, US Public Health Service grants R01‐CA‐58598 and P30‐CA‐71789 and contracts N01‐CN‐55424 and N01‐PC‐67001 from the National Cancer Institute. Statistical methods: unconditional logistic regression Variables controlled in analysis: age, ethnicity, oral contraceptive pill use and tubal ligation. Variables controlled by matching: ‐ |
|
Goto 1990.
| Study characteristics | ||
| Methods | PCC in Japan | |
| Participants | Participants: 71 (male/female: NR) cases and 142 (male/female: NR) controls Inclusion criteria: permanent residents in Hokkaido Prefecture, Japan Recruitment: NR |
|
| Interventions | N/A | |
| Outcomes | Number of cases Pancreatic cancer: 71 (male/female: NR) cases |
|
| Green tea in exposure categories | Exposure assessment: drinking of green tea Lowest exposure: no drinking Highest exposure: drinking green tea almost every day |
|
| Notes | Article in Japanese Funding: not declared Statistical methods: unconditional logistic regression Variables controlled in analysis: sex, age and area of residence Variables controlled by matching: sex and age (± 3 years) |
|
Green 2014.
| Study characteristics | ||
| Methods | PCC in Australia | |
| Participants | Participants: 854 (male/female: 525/329) and 948 (male/female: 556/392) controls Inclusion criteria: histologically confirmed incident cases aged 40‐79 years, from the Western Australian Bowel Health Study (WABOHS) Recruitment: 1 June 2005‐31 August 2007 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Colorectal cancer: 854 (male/female: 525/329) cases Proximal colon cancer: 281 cases Distal colon cancer: 260 cases Rectal cancer: 323 cases |
|
| Green tea in exposure categories | Exposure assessment: intake of green tea Lowest exposure: no drinking Intermediate exposure 1: < 1 cup/month Intermediate exposure 2: < 1 cup/week Highest exposure: ≥ 1 cup/week |
|
| Notes | Funding: Australian National Health and Medical Research Council (Project Grant #353568 and Fellowship #37614900) and Dutch Cancer Society Statistical methods: conditional logistic regression Variables controlled in analysis: age group, sex, energy intake from food, alcohol intake, smoking status, use of multivitamins, diabetes, physical activity during the age period 19–34 years, BMI at age 40 years, socioeconomic status and country of birth Variables controlled by matching: sex and age |
|
Hakim 2000.
| Study characteristics | ||
| Methods | PCC in USA | |
| Participants | Participants: 234 (male/female: 138/96) cases and 216 (male/female: 125/91) controls
Inclusion criteria: aged ≥ 30 years, histopathologically confirmed squamous cell carcinoma of the skin diagnosed within 4 months before the 1st interview and had no prior history of a skin cancer, non‐Hispanic and Hispanic white cases' ethnicity from Southeastern Arizona Skin Cancer Study, Arizona, USA. Recruitment: from January 1993‐December 1996 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Skin cancer: 234 (138/96) cases of squamous cell carcinoma |
|
| Green tea in exposure categories | Exposure assessment: intake of hot green tea Lowest exposure: nondrinker Highest exposure: drinker, merging all categories (1–3 cups/month, 1–6 cups/week and ≥ 1 cup/d) |
|
| Notes | Funding: grant from Unilever Health Institute Vlaardingen, the Netherlands and by Public Health Service Grant P01 CA27502 Statistical methods: unconditional logistic regression Variables controlled in analysis: age, sex, energy intake, inability to tan after prolonged sun exposure and history of diagnosed and treated actinic keratosis Variables controlled by matching: sex and age (± 10 years) |
|
Han 2008.
| Study characteristics | ||
| Methods | PCC in China | |
| Participants | Participants: 523 cases (male/female: 365/158) and 1924 (male/female: 1367/557) controls
Inclusion criteria: newly diagnosed cases 35‐79 years of age; residence in Dafeng City, China Recruitment: from January 2003 from Dafeng City Center for Disease Control and Prevention |
|
| Interventions | N/A | |
| Outcomes | Number of cases Lung cancer: 523 (male/female: 365/158) cases |
|
| Green tea in exposure categories | Exposure assessment 1: green tea intake Lowest exposure: never drinking Highest exposure: drinking Exposure assessment 2: green tea consumption Lowest exposure: 0 g/month Intermediate exposure 1: 0‐49 g/month Intermediate exposure 2: 50‐99 g/month Highest exposure: ≥ 100 g/month |
|
| Notes | Article in Chinese Funding: Jiangsu Provincial Medical Engineering Key Talent Research Fund (RC2003090) Statistical methods: unconditional logistic regression Variables controlled in analysis: sex, age, education, income per capita, frequent exposure to cooking fumes, smoking habits, alcohol intake, family history of lung cancer, daily fruit and vegetable intake Variables controlled by matching: sex, age (± 2 years) and living in the same area. |
|
Hemelt 2010.
| Study characteristics | ||
| Methods | HCC in China | |
| Participants | Participants: 432 (male/female: 358/74) and 392 (male/female: 299/93) controls
Inclusion criteria: all incident bladder cancer cases (ICD‐10 C67), aged ≥ 40 years, admitted to 4 hospitals (First Affliated Hospital in Hangzhou, First Municipal Hospital in Guangzhou, Tongji Hospital in Wuhan and Second Xiangya Hospital in Changsha), China Recruitment: from October 2005‐June 2008 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Bladder cancer: 419 out of 432 eligible cases, mainly urothelial carcinoma (N = 323), followed by adenocarcinoma (N = 11) and squamous cell carcinoma (N = 7) |
|
| Green tea in exposure categories | Exposure assessment 1: green tea drinking Lowest exposure: no Highest exposure: yes Exposure assessment 2: green tea drinking Lowest exposure: no Intermediate exposure: < daily Highest exposure: daily, further divided in < 4 cups/d and ≥ 4 cups/d |
|
| Notes | Funding: not declared Statistical methods: unconditional logistic regression Variables controlled in analysis: age, sex, smoking status, smoking frequency and smoking duration. Hospital was modelled as a random effect Variables controlled by matching: sex and age (± 5 years) |
|
Hoshiyama 1992.
| Study characteristics | ||
| Methods | PCC in Japan | |
| Participants | Participants: 251 cases and 483 controls (all men)
Inclusion criteria: histologically confirmed single and multiple stomach cancer cases admitted to the Saitama Cancer Center Hospital, living in the Saitama Prefecture for at least 10 years, Japan Recruitment: from August 1984‐July 1990 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Stomach cancer: 419 out of 432 eligible cases: 216 single stomach cancer and 35 multiple stomach cancer (all men) |
|
| Green tea in exposure categories | Exposure assessment: green tea drinking Lowest exposure: ≤ 4 cups/d Intermediate exposure: 5‐7 cups/d Highest exposure: ≥ 8 cups/d |
|
| Notes | Funding: not declared Statistical methods: unconditional logistic regression Variables controlled in analysis: age and smoking status Variables controlled by matching: ‐ |
|
Hsu 2012.
| Study characteristics | ||
| Methods | PCC in China | |
| Participants | Participants: 371 (69.5% male) cases and 321 (69.2% male) controls. Only 317 of the 321 eligible controls) included in the analysis Inclusion criteria: aged ≤ 75 years, no previous diagnosis for nasopharyngeal carcinoma and residence in Taipei city/county, Taiwan (China) for > 6 months Recruitment: from 15 July 1991‐31 December 1994 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Nasopharyngeal carcinoma: 368 cases out of 371 eligible cases |
|
| Green tea in exposure categories | Exposure assessment: intake of green tea Lowest exposure: 0 times/week Intermediate exposure: < 1 time/week Highest exposure: ≥ 1 time/week |
|
| Notes | Funding: National Institutes of Health, USAA Statistical methods: unconditional logistic regression Variables controlled in analysis: age, sex, ethnicity, educational level, nasopharyngeal carcinoma family history, total calories intake, years of cigarette smoking and exposure to formaldehyde and wood dust Variables controlled by matching: ‐ |
|
Huang 1999.
| Study characteristics | ||
| Methods | HCCs in Japan | |
| Participants |
Huang 1999: Participants: 887 (male/female: 595/292) cases and 28,619 (male/female: 7892/20,727) controls Inclusion criteria: aged 20‐79 years, from the Aichi Cancer Center Hospital (ACCH) in Aichi Prefecture, Japan Recruitment: from 1988‐1995 Inoue 1994 Participants: 127,477 inhabitants of 45 areas of Japan, data retrieved from 26,426 participants: 668 (male/female: 420/248) cases and 668 (male/female: 420/248) controls Inclusion criteria: aged 20‐79 years, from the Aichi Cancer Center Hospital (ACCH) in Aichi Prefecture, Japan Recruitment: from January 1988‐June 1991 Kato 1990a Participants: 427 (male/female: 289/138) cases and 3014 (male/female: 1247/1767) controls Inclusion criteria: aged ≥ 18 years, who underwent endoscopic examination and with no other types of cancer attending at Aichi Cancer Center Hospital, Japan. Controls considered in the present analysis are those with normal gastric mucosa Recruitment: from April 1985‐March 1989 |
|
| Interventions | N/A | |
| Outcomes |
Huang 1999 Number of cases Stomach cancer: 887 (male/female: 595/292) cases Inoue 1994: Number of cases Stomach cancer: 668 cases, 123 of cardia, 218 of middle stomach and 256 of antrum Kato 1990a Number of cases Stomach cancer: 427 (male/female: 289/138) cases |
|
| Green tea in exposure categories | Exposure assessment: intake of green tea Lowest exposure: never Intermediate exposure 1: 1‐2 cups/d Intermediate exposure 2: 3‐5 cups/d Highest exposure: > 6 cups/d Inoue 1994 Exposure assessment: green tea drinking Lowest exposure: < every day Highest exposure: every day Kato 1990a Exposure assessment: consumption of green tea Lowest exposure: nondrinker Intermediate exposure: 1‐4 cups/d Highest exposure: ≥ 5 cups/d |
|
| Notes |
Huang 1999 Funding: National Institutes of Health, USAA Statistical methods: unconditional logistic regression Variables controlled in analysis: sex and age Variables controlled by matching: ‐ Inoue 1994 Funding: Grant‐in‐Aid for Cancer Research (4‐2) and for a comprehensive 10‐year Strategy for Cancer Control from the Ministry of Health and Welfare, Japan Statistical methods: conditional logistic regression Variables controlled in analysis: age and intake of fresh vegetables Variables controlled by matching: sex, age (± 2 years) and first time of hospital visit (± 2 months) Kato 1990a Funding: Grant‐in‐Aid for a Comprehensive 10‐year Strategy for Cancer Control. Japan, from the Ministry of Health and Welfare Statistical methods: Walker‐Duncan logistic regression Variables controlled in analysis: age and residence Variables controlled by matching: ‐ |
|
Ide 2007.
| Study characteristics | ||
| Methods | Cohort study in Japan | |
| Participants | Participants: 127,477 inhabitants of 45 areas of Japan, data retrieved from 20,550 men and 29,671 women Inclusion criteria: aged 40‐79 (≥ 18 years in 1949). Details reported in Ohno 2001. Parent cohort: Japan Collaborative Cohort Study of Evaluation of Cancer Risk (JACC Study) Recruitment: from 1988‐1990 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 2001 Oral cancer: 37 (male/female: 20/17) cases, including cancers of the tongue (N = 22, male/female: 13/9) and other oral cavity (N = 15, male/female: 7/8) |
|
| Green tea in exposure categories | Exposure assessment: intake of green tea Lowest exposure: < 1 cup/d Intermediate exposure 1: 1‐2 cups/d Intermediate exposure 2: 3‐4 cups/d Highest exposure: ≥ 5 cups/d |
|
| Notes | Funding: Grants‐in‐Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan (Monbusho) (N. 61010076, 62010074, 63010074, 1010068, 2151065, 3151064, 4151063, 5151069, 6279102, 11181101 and 12218237) Statistical methods: Cox hazard proportional regression Variables controlled in analysis: sex, age, smoking status, alcohol consumption, consumption of coffee, consumption of green/yellow vegetables, salty foods and fruits Variables controlled by matching: ‐ |
|
Inoue 1998.
| Study characteristics | ||
| Methods | HCC in Japan | |
| Participants | Participants: 127,477 inhabitants of 45 areas of Japan, data retrieved from 21,128 participants Inclusion criteria: aged 40‐79 years, from the Aichi Cancer Center Hospital (ACCH) in Aichi Prefecture, Japan Recruitment: from January 1988‐June 1991 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Oesophageal cancer: 185 (male/female: 161/24) cases Stomach cancer: 893 (male/female: 613/280) cases Colon cancer: 362 (male/female: 213/149) cases Rectal cancer: 266 (male/female: 173/93) cases |
|
| Green tea in exposure categories | Exposure assessment: intake of green tea Lowest exposure: rarely Intermediate exposure 1: occasional Intermediate exposure 2: 1‐2 cups/d Intermediate exposure 3: 4‐6 cups/d Highest exposure: ≥ 7 cups/d |
|
| Notes | Funding: Grant‐in‐Aid for Cancer Research (9‐4) from the Ministry of Health and Welfare, Japan and a grant from the Foundation of All Japan Coffee Association Statistical methods: unconditional logistic regression Variables controlled in analysis: coffee intake, black tea intake, sex, age, year and season at 1st hospital visit, habitual smoking, habitual alcohol drinking, regular physical exercise, fruit intake, rice intake and beef intake Variables controlled by matching: ‐ Data on Aichi also reported in Inoue 2009a for stomach cancer |
|
Inoue 2008.
| Study characteristics | ||
| Methods | PCC in China | |
| Participants | Participants: 63,257 (male/female: 27,959/35,298) participants, 380 cases and 662 controls (all female) Inclusion criteria: aged 40‐74 years, belonging to the Hokkien or Cantonese dialect group in Singapore, China Parent cohort: Singapore Chinese Health Study (SCHS) Recruitment: from April 1993‐December 1998 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 31 July 2007 Breast cancer: 380 female breast cancer out of 736 eligible cases |
|
| Green tea in exposure categories | Exposure assessment: green tea intake Lowest exposure: none or < weekly Intermediate exposure: weekly to < daily Highest exposure: daily |
|
| Notes | Funding: National Cancer Institute, Bethesda, MD (R01‐CA55069, R35‐CA53890 and R01‐CA80205) Statistical methods: unconditional logistic regression Variables controlled in analysis: age, year of enrolment, education, dialect, BMI, age when period became regular, number of live births and black tea intake Variables controlled by matching: ‐ |
|
Inoue 2009a.
| Study characteristics | ||
| Methods | Cohort studies in Japan | |
| Participants | Participants: 219,080 (male/female: 100,479/118,601) participants Inclusion criteria: all studies were carried out on Japanese populations starting from mid‐1980s to mid‐1990s with information on diet (including green tea) Parent cohorts Japan Public Health Center‐based Prospective Study (JPHC)‐I Japan Public Health Center‐based Prospective Study (JPHC)‐II Japan Collaborative Cohort Study (JACC) (only from 24 of the 45 investigated areas) Miyagi Cohort Study (MIYAGI) Three Prefecture Study ‐ Miyagi portion (3‐pref MIYAGI) Three Prefecture Study ‐ Aichi portion (3‐pref AICHI) Recruitment JPHC‐I: 1990 JPHC‐II: from 1993‐1994 JACC: from 1988‐1990 MIYAGI: 1990 3‐pref MIYAGI: 1984 3‐pref AICHI: 1985 Previous reports Fujino 2002 Participants: 127,477 inhabitants of 45 areas of Japan, data retrieved from 18,746 men and 26,184 women Inclusion criteria: aged 40‐79 (≥ 18 years in 1949). Details reported in Ohno 2001 Parent cohort: Japan Collaborative Cohort Study of Evaluation of Cancer Risk (JACC Study) Recruitment: from 1988‐1990 Hoshiyama 2002 Participants: 127,477 inhabitants of 45 areas of Japan, data retrieved from 18,746 men and 26,184 women Inclusion criteria: aged 40‐79 (≥ 18 years in 1949). Details reported in Ohno 2001 Parent cohort: Japan Collaborative Cohort Study of Evaluation of Cancer Risk (JACC Study) Recruitment: from 1988‐1990 Hoshiyama 2004: (nested case‐cohort study due to availability of serum samples) Participants: 127,477 inhabitants of 45 areas of Japan, data retrieved from 30,370 men and 42,281 women including 151 cases and 265 controls Inclusion criteria: aged 40‐79 (≥ 18 years in 1949). Details reported in Ohno 2001 Parent cohort: Japan Collaborative Cohort Study of Evaluation of Cancer Risk (JACC Study) Recruitment: from 1988‐1990 Koizumi 2003 Participants: 31,345 (male/female: 13,992/17,353) from cohort 1 and 47,605 (male/female: 22,836/24,769). Final included subjects 26,311 (male/female: 11,902/14,409) from cohort 1 and 39,604 (male/female: NR) from cohort 2 Inclusion criteria: aged ≥ 40 years, residents in three municipalities of the Miyagi Prefecture, Northern Japan (cohort 1); aged 40‐64 years (cohort 2) in residents of Miyagi Prefecture, Japan Parent cohorts Cohort 1: Three Prefecture Study ‐ Miyagi portion (3‐pref MIYAGI) Cohort 2: Miyagi Cohort Study (MIYAGI) Recruitment: from 1984 for cohort 2 and from 1990 for cohort 2 Tsubono 2001 Participants: 31,345 (male/female: 13,992/17,353) with final included participants 26,311 (male/female: 11,902/14,409) Inclusion criteria: aged ≥ 40 years, residents in 3 municipalities of the Miyagi Prefecture, Northern Japan Parent cohort: Three Prefecture Study ‐ Miyagi portion (3‐pref MIYAGI) Recruitment: from 1984 Sasazuki 2004 Participants: 140,420 (male/female: 68,722/71,698), with 61,595 from cohort 1 and 78,825 from cohort 2 and 72,943 (male/female: 34,832/38,111) participants with complete data Inclusion criteria: aged 40‐59 years, from 5 Public Health Centre areas (Iwate, Akita, Nagano, Okinawa and Tokyo) for cohort 1 and aged 40‐69 years for cohort 2, from 6 public health centre areas (Ibaraki, Niigata, Kochi, Nagasaki, Okinawa and Osaka), respondent at 5‐year follow‐up. Katsushika Public Health Center was excluded due to missing cancer data. Parent cohorts Cohort 1: Japan Public Health Center‐based Prospective Study (JPHC)‐I Cohort 2: Japan Public Health Center‐based Prospective Study (JPHC)‐II Recruitment: from 1990 for cohort 1 and 1993/1994 for cohort 2 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Inoue 2009b Outcome assessment JPHC‐I: 2001 JPHC‐II: 2003‐2004 JACC: 2001 MIYAGI: 2001 3‐pref MIYAGI: 1992 3‐pref AICHI: 2000 Stomach cancer: 3577 (male/female: 2495/1082) cases Previous reports Fujino 2002 Outcome assessment: 31 December 1997 Stomach cancer mortality: 379 (male/female: 261/118) cases Hoshiyama 2002 Outcome assessment: 31 December 1997 Stomach cancer mortality: 359 (male/female: 240/119) cases Hoshiyama 2004 Outcome assessment: 31 December 1997 Stomach cancer: 151 cases of the 804 eligible cases due to availability of serum samples Koizumi 2003 Outcome assessment: December 1992 for 3‐pref MIYAGI, NR for MIYAGI (probably 1997) Stomach cancer: 733 (male/female: NR) cases; 419 (male/female: 296/123) cases in 3‐pref‐MIYAGI I and 314 (male/female: NR) cases in MIYAGI‐ Tsubono 2001 Outcome assessment: 31 December 1992 for 3‐pref MIYAGI only Stomach cancer: 419 (male/female: 296/123) cases from 3‐pref MIYAGI only Sasazuki 2004 Outcome assessment: 31 December 2001 Stomach cancer: 892 (male/female: 665/227) cases, including 109 (male/female: 88/21) including upper‐third gastric cancers and 631 (male/female: 461/170) distal cancers and as histological categorisation, 471 (male/female: 386/85) cases of differentiated type and 312 (male/female: 197/115) cases of undifferentiated type |
|
| Green tea in exposure categories | Exposure assessment: green tea intake (same for Inoue 2009a, Koizumi 2003, Tsubono 2001 and Sasazuki 2004) Lowest exposure: < 1 cup/d Intermediate exposure 1: 1‐2 cups/d Intermediate exposure 2: 3‐4 cups/d Highest exposure: ≥ 5 cups/d Exposure assessment: intake of green tea (in Fujino 2002) Lowest exposure: ≤ 3 times/week Intermediate exposure: > 3 times/week Highest exposure: every day Exposure assessment: intake of green tea (In Hoshiyama 2002 and Hoshiyama 2004) Intermediate exposure 1: 1‐2 cups/d Intermediate exposure 2: 3‐4 cups/d Intermediate exposure 3: 5‐9 cups/d Highest exposure: ≥ 10 cups/d |
|
| Notes | Funding: in Tsubono: grants from the Japanese Ministry of Health and Welfare and the Japanese Ministry of Education, Science and Culture. In Koisumi 2003 not declared and this study was supported by a Grant for the Third Term Comprehensive Control Research for Cancer from the Ministry of Health, Labour and Welfare of Japan. Statistical methods: Cox proportional hazard regression Inoue 2009b Variables controlled in analysis: age, area (JPHC‐I, JPHC‐II and JACC only), smoking, ethanol intake, rice intake, soy bean paste soup, coffee intake, pickled vegetable intake and green–yellow vegetable intake Fujino 2002 Funding: Grants‐in‐Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan (Monbusho) (N. 61010076, 63010074, 1010068, 2151065, 3151064, 4151063, 5151069, 6279102, 11181101, 12218237 and 12218216). Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age Hoshiyama 2002 Funding: Ministry of Education, Science, Sports and Culture of Japan grants: 61010076, 62010074, 63010074, 1010068, 2151065, 3151064, 4151063, 5151069, 6279102, 11181101, 12218237 Statistical methods: Cox hazard proportional regression Variables controlled in analysis: age, smoking status, history of peptic ulcer, family history of stomach cancer, consumption of rice, miso soup, green‐yellow vegetables, white vegetables, fruits and preference for salty foods Hoshiyama 2004 Funding: Ministry of Education, Science, Sports and Culture of Japan grants: 61010076, 62010074, 63010074, 1010068, 2151065, 3151064, 4151063, 5151069, 6279102, 11181101, 12218237 Statistical methods: Cox hazard proportional regression Variables controlled in analysis: age, smoking status, HP infection, history of peptic ulcer, family history of stomach cancer, educational level, consumption of rice, miso soup, green‐yellow vegetables, white vegetables, fruits and preference for salty foods Koizumi 2003 Variables controlled in analysis: sex, age, type of health insurance, parental history of gastric cancer, history of peptic ulcer, cigarette smoking, alcohol consumption, consumption of rice, black tea, coffee, pickled vegetables, bean‐paste soup. Plus consumption of meat, green or yellow vegetables, other vegetables and fruits for cohort 1 and consumption of pork, ham, spinach, carrot, cabbage, Chinese cabbage, orange and other fruits for cohort 2 Tsubono 2001 Variables controlled in analysis: sex, age, type of health insurance, history of peptic ulcer, smoking status, alcohol consumption, daily consumption of rice, consumption of black tea and consumption of coffee, and consumption of meat, green or yellow vegetables, pickled vegetables, other vegetables, fruits and bean‐paste soup. Sasazuki 2004 Funding: Grant‐in‐Aid for Cancer Research and for the Second Term Comprehensive 10‐year Strategy for Cancer Control from the Ministry of Health, Labour and Welfare of Japan Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age, area and cigarette smoking (stratified by sex) Variables controlled by matching: ‐ Note: Ohno 2001 study report rationale for initiating the Japan Collaborative Cohort (JACC) Study |
|
Inoue 2009b.
| Study characteristics | ||
| Methods | Cohort study in Japan | |
| Participants | Participants: 68,975 participants, results of 18,815 (male/female: 6414/12,401) participants Inclusion criteria: aged 40‐69 years, from 6 Public Health Centre areas across Japan Parent cohort: Japan Public Health Center‐based Prospective Study (JPHC)‐II Recruitment: from 1993‐1994 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Liver cancer: 110 (male/female: 73/37) cases Outcome assessment: 31 December 2006 |
|
| Green tea in exposure categories | Exposure assessment: green tea intake Lowest exposure: < 3 cups/d Intermediate exposure: 3‐4 cups/d Highest exposure: ≥ 5 cups/d |
|
| Notes | Funding: Grant‐in‐Aid for Cancer Research, for Research on Hepatitis and for the Third‐Term Comprehensive Control Research for Cancer from the Ministry of Health, Labour and Welfare of Japan Statistical methods: Cox proportional hazard regression Variables controlled in analysis: sex, age, area, smoking status, weekly ethanol intake, BMI, history of diabetes mellitus, coffee consumption, serum ALT level, hepatitis C infection status and hepatitis B infection status Variables controlled by matching: ‐ |
|
Ishikawa 2006.
| Study characteristics | ||
| Methods | Cohort study in Japan | |
| Participants | Participants: 31,345 (male/female: 13,991/17,354) participants in cohort 1 and 47,605 (male/female: 22,836/24,769) participants in cohort 2. Final participants are 26,723 (male/female: 9008/17,715) participants with covariate information Inclusion criteria: aged ≥ 40 years, in 3 municipalities of Miyagi Prefecture in cohort 1 and aged 40‐64 years, in the 14 municipalities of Miyagi Prefecture, Japan Parent cohorts Cohort 1: Three Prefecture Study ‐ Miyagi portion (3‐pref MIYAGI) Cohort 2: Miyagi Cohort Study (MIYAGI) Recruitment: from January 1994 (cohort 1) and from June‐August 1990 (cohort 2) |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 31 December 1992 (cohort 1) and 31 December 1997 (cohort 2) Oesophageal cancer: 38 + 40 in cohort 1 and cohort 2 respectively |
|
| Green tea in exposure categories | Exposure assessment: green tea consumption Lowest exposure: never or occasionally Intermediate exposure 1: 1‐2 cups/d Intermediate exposure 2: 3‐4 cups/d Highest exposure: ≥ 5 cups/d |
|
| Notes | Funding: not declared Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age, cigarette smoking, alcohol drinking and coffee consumption Variables controlled by matching: ‐ |
|
Islami 2009.
| Study characteristics | ||
| Methods | PCC in Iran | |
| Participants | Participants: 300 cases (male/female: 150/150) and 571 (male/female: 278/293) controls Inclusion criteria: newly diagnosed histologically confirmed oesophageal cancer in residents in the eastern part of Golestan Province, Iran, attending the Atrak Clinic. Recruitment: from December 2003 to March 2007 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Oesophageal cancer: 266/300 eligible cases |
|
| Green tea in exposure categories | Exposure assessment: frequency of green tea consumption Lowest exposure: never, < weekly Highest exposure: daily, weekly |
|
| Notes | Funding: not declared Statistical methods: conditional logistic regression Variables controlled in analysis: ethnicity, daily vegetable intake, alcohol consumption, tobacco or opium ever used, duration of residence in rural areas, education level, car ownership, tea temperature and black tea intake Variables controlled by matching: place of residence, age (± 2 years) and sex |
|
Iwai 2002.
| Study characteristics | ||
| Methods | Cohort study in Japan | |
| Participants | Participants: 4411 participants from the Tottori Prefecture, Japan. Data from 2855 (male/female: 1404/1451) participants, only men for cancer mortality Inclusion criteria: aged 40‐79 years Parent cohort: Japan Collaborative Cohort Study of Evaluation of Cancer Risk (JACC Study) Recruitment: from April‐May 1989 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 31 December 1999 Total cancer mortality: 31 cases (all men) |
|
| Green tea in exposure categories | Exposure assessment: frequency of green tea consumption Lowest exposure: < 0.5 cups/d Intermediate exposure: 0.5‐3 cups/d Highest exposure: ≥ 4 cups/d |
|
| Notes | Funding: Grant‐in‐Aid for Scientific Research from Monbusho, the Japanese Ministry of Education, Science and Culture (N.11181101) Statistical methods: Cox proportional hazard regression Variables controlled in analysis: coffee consumption, age, history of cancer and apoplexy, educational status and smoking status Variables controlled by matching: ‐ |
|
Iwasaki 2010a.
| Study characteristics | ||
| Methods | Cohort studies in Japan (Iwasaki 2010a) and case‐cohort nested study (Iwasaki 2010b) | |
| Participants | Participants: 140,420 (male/female: 68,722/71,698) from cohort 1 and cohort 2. From the available 67,422 women Inclusion criteria: incident breast cancer cases in women at baseline aged 40‐59 years in cohort 1 and 40‐69 years in cohort 2, with no history of breast cancer, respondent to baseline questionnaire, not moved out of study area In Iwasaki 2010b: included data with available plasma tea polyphenol levels. Final population of 144 cases and 288 controls Parent cohorts Japan Public Health Center‐based Prospective Study (JPHC)‐I Japan Public Health Center‐based Prospective Study (JPHC)‐II Recruitment JPHC‐I: 1990 JPHC‐II: from 1993‐1994 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 31 December 2006 in Iwasaki 2010a and 31 December 2002 in Iwasaki 2010b Breast cancer: 581 cases, based on baseline questionnaire data |
|
| Green tea in exposure categories | Exposure assessment: green tea intake at baseline Iwasaki 2010a Lowest exposure: < 1 cup/week Intermediate exposure 1: 1‐2 cups/week Intermediate exposure 2: 3‐4 cups/week Intermediate exposure 3: 1‐2 cups/d Intermediate exposure 4: 3‐4 cups/d Highest exposure: ≥ 5 cups/d Iwasaki 2010b Lowest exposure: < 1 cup/week Highest exposure: ≥ 5 cups/d |
|
| Notes |
Iwasaki 2010a Funding: Grant‐in‐Aid for Scientific Research from Monbusho, the Japanese Ministry of Education, Science and Culture (N.11181101) Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age, area (10 Public Health Centres), age at menarche, menopausal status at baseline, number of births, age at first birth, height, BMI, alcohol intake, smoking status, leisure time physical activity, exogenous hormone use, family history of breast cancer, oolong tea intake, black tea intake and coffee intake Variables controlled by matching: ‐ Iwasaki 2010b Funding: Grants‐in‐Aid for Cancer Research and for the Third Term Comprehensive Ten‐Year Strategy for Cancer Control from the Ministry of Health, Labour and Welfare of Japan and Grants‐in‐Aid for Scientific Research on Priority Areas (17015049) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. Statistical methods: crude data analysis Variables controlled in analysis: crude data Variables controlled by matching: ‐ Data of parent study Iwasaki 2010a were used for breast cancer analysis including a larger population. |
|
Iwasaki 2014.
| Study characteristics | ||
| Methods | HCC in Japan | |
| Participants | Participants: 405 cases and 405 controls (all women) Inclusion criteria: female, newly diagnosed and histologically confirmed breast cancer cases at 4 hospitals of Nagano prefecture, Japan Recruitment: from May 2001‐September 2005 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Breast cancer: 369/405 eligible cases due to missing information on green tea, SNP genotype information, or DNA sample. Major histologic types were invasive ductal carcinoma (85.6%), invasive lobular carcinoma (4.1%) and mucinous carcinoma (3.8%). |
|
| Green tea in exposure categories | Exposure assessment: green tea intake Lowest exposure: 1‐119 mL/d Intermediate exposure: 120‐599 mL/d Highest exposure: ≥ 600 mL/d |
|
| Notes | Funding: Grants‐in‐Aid for Research on Risk of Chemical Substances and the Third‐Term Comprehensive Ten‐Year Strategy for Cancer Control from the Ministry of Health, Labour and Welfare of Japan and Grants‐in‐Aid for Scientific Research on Innovative Areas (221S0001) and for Young Scientists (B) (22700934) from the Ministry of Education, Culture, Sports, Science and Technology of Japan and the Japan Society for the Promotion of Science and Foundation for Promotion of Cancer Research in Japan. Statistical methods: conditional logistic regression Variables controlled in analysis: menopausal status, number of births, family history of breast cancer, smoking status, moderate physical activity in the past 5 years, vitamin supplement use, oolong tea consumption, black tea consumption, coffee consumption and canned coffee consumption Variables controlled by matching: sex, age (± 3 years) and residential area |
|
Ji 1996.
| Study characteristics | ||
| Methods | PCC in China | |
| Participants | Participants: 1124 (male/female: 770/354) cases and 1451 (male/female: 819/632) controls. 1347 (male/female: 753/594) of the eligible controls included in the analysis. Inclusion criteria: aged 20‐69 years, permanent resident of the 10 urban districts of Shanghai, China Recruitment: from 1 December 1988‐30 November 1989 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Stomach cancer: 1029 (male/female: 684/345) of the eligible cases, including cancers of cardia (N = 145, 18.8% in men and N = 40, 11.3% in women), distal stomach (N = 530, 68.9% in men and N = 257, 72.6% in women) and unclassified (N = 95, 12.3% in men N = 57, 16.1% in women) |
|
| Green tea in exposure categories | Exposure assessment A: green tea drinking status Lowest exposure: nondrinker Highest exposure: regular drinker Exposure assessment B: consumption of green tea leaves Lowest exposure: nondrinker Men Intermediate exposure 1: ≤ 1200 g/year Intermediate exposure 2: 1200‐≤ 2000 g/year Intermediate exposure 3: 2000‐≤ 3000 g/year Highest exposure: > 3000 g/year Women Intermediate exposure: ≤ 1200 g/year Highest exposure: > 1200 g/year |
|
| Notes | Funding: not declared Statistical methods: logistic regression Variables controlled in analysis: age, income and educational level among women; further adjusted for smoking and alcohol drinking among men Variables controlled by matching: sex and age (± 5 years) |
|
Ji 1997.
| Study characteristics | ||
| Methods | PCC in China | |
| Participants | Participants: 2266 (male/female: NR) cases and 1552 (male/female: NR) controls Inclusion criteria: aged 30/74 years, permanent resident of the 10 urban districts of Shanghai, China Recruitment: from October 1990‐June 1993 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Colon cancer: 885 (male/female: 426/459) out of the 931 eligible cases Rectal cancer: 843 (male/female: 441/402) out of the 884 eligible cases Pancreatic cancer: 428 (male/female: 246/182) out of the 451 eligible cases |
|
| Green tea in exposure categories | Exposure assessment A: green tea drinking habit Lowest exposure: nondrinker Highest exposure: regular drinker Exposure assessment B: green tea consumption Men Lowest exposure: nondrinker Intermediate exposure 1: 1‐199 g/month Intermediate exposure 2: 200‐299 g/month Highest exposure: ≥ 300 g/month Women Lowest exposure: nondrinker Intermediate exposure: 1‐199 g/month Highest exposure: ≥ 200 g/month Exposure assessment C: lifetime green tea consumption Men Lowest exposure: nondrinker Intermediate exposure 1: 1‐3499 g/month per years of drinking Intermediate exposure 2: 3500‐8499 g/month per years of drinking Highest exposure: ≥ 8500 g/month per years of drinking Women Lowest exposure: nondrinker Intermediate exposure: 1‐3499 g/month per years of drinking Highest exposure: ≥ 3500 g/month per years of drinking |
|
| Notes | Funding: not declared Statistical methods: logistic regression Variables controlled in analysis: age, income, education and cigarette smoking Variables controlled by matching: sex and age (± 5 years) |
|
Jia 2016.
| Study characteristics | ||
| Methods | Case‐control study in China | |
| Participants | Participants: 53 (male/female: 19/34) cases and 106 (male/female: 48/58) controls Inclusion criteria: participants of the cohort of Dayao in Yunnan, a rural area with naturally occurring asbestos, China Recruitment: NR |
|
| Interventions | N/A | |
| Outcomes | Number of cases Lung cancer: 23 cases Pleural mesothelioma: 26 cases |
|
| Green tea in exposure categories | Exposure assessment: green tea intake Lowest exposure: never Intermediate exposure 1: 1‐3 times/week Intermediate exposure 2: 4‐6 times/week Highest exposure: ≥ 7 times/week |
|
| Notes | Article in Chinese Funding: National Natural Research Fundation of China (no. 41071064) Statistical methods: conditional logistic regression Variables controlled in analysis: sex, age, ethnicity, cultural level, BMI, alcohol consumption, history of lung cancer, family history of cancer Variables controlled by matching: sex and age (± 3 years) |
|
Jian 2004.
| Study characteristics | ||
| Methods | HCC in China | |
| Participants | Participants: 130 cases and 274 controls (all men) Inclusion criteria: aged ≥ 45 years, incident and histopathologically confirmed cases of adenocarcinoma of the prostate from 8 hospitals of Hangzhou, southeast China, residents in the Zhejiang Province, China for at least 10 years Recruitment: from July 2001‐June 2002 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Prostate cancer: 130 cases (all men) of prostatic adenocarcinoma |
|
| Green tea in exposure categories | Exposure assessment A: green tea drinking habits Lowest exposure: no Highest exposure: yes Exposure assessment B: intake of green tea Lowest exposure: < 1 cup/d Intermediate exposure: 1‐3 cups/d Highest exposure: > 3 cups/d |
|
| Notes | Funding: not declared Statistical methods: conditional logistic regression Variables controlled in analysis: age at interview, locality, education, family income, BMI, physical activity, alcohol consumption, tobacco smoking, total fat intake, marital status, age at marriage, number of children, vasectomy, family history of prostate cancer Variables controlled by matching: age and geographical area Jian 2007 Subsequent report on the exact same population, controlling for lycopene intake |
|
Jin 2013.
| Study characteristics | ||
| Methods | PCC in China | |
| Participants | Participants: 799 (male/female: 553/246) cases and 2010 (male/female: 1600/410) controls Inclusion criteria: aged 20‐90 years, residents in the Ganyu county, Jiangsu Province, China Recruitment: 2010 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Lung cancer: 799 (male/female:553/246) cases |
|
| Green tea in exposure categories | Exposure assessment A: intake of green tea Lowest exposure: nondrinker Highest exposure: drinker Exposure assessment B: consumption of green tea Lowest exposure: 0 cup/d Intermediate exposure 1: 1 cup/d Intermediate exposure 2: 2 cups/d Highest exposure: > 2 cups/d |
|
| Notes | Article in Chinese Funding: grant from Jiangsu Provincial Health Department (RC 2003090) Statistical methods: unconditional logistic regression Variables controlled in analysis: sex, age, education, income, BMI, family history of cancer, cigarette smoking, alcohol intake and garlic consumption Variables controlled by matching: sex and age (± 5 years) |
|
Kakuta 2009.
| Study characteristics | ||
| Methods | PCC in Japan | |
| Participants | Participants: 152 cases and 285 controls (all women) Inclusion criteria: aged < 80 years, having endometrial cancer and underwent total hysterectomy at either Tohoku University Hospital (centre 1) or at the Miyagi Cancer Center (centre 2), histologically confirmed and with no history of cancer at any other organ or site, Miyagi prefecture, Japan Recruitment: from November 2002‐March 2007 (centre 1) or from June 2005‐June 2006 (centre 2) |
|
| Interventions | N/A | |
| Outcomes | Number of cases Endometrial cancer: 152 cases of endometrioid adenocarcinoma |
|
| Green tea in exposure categories | Exposure assessment: consumption of green tea Lowest exposure: < 4 cups/week Intermediate exposure 1: 5‐6 cups/week‐1 cup/d Intermediate exposure 2: 2‐3 cups/d Highest exposure: > 4 cups/d |
|
| Notes | Funding: Grant‐in‐Aid for Scientific Research on Priority Areas, a Grant‐in‐Aid for Scientific Research, a Grant‐in‐Aid for Young Scientists, a Grant‐in‐Aid for Exploratory Research, from the Ministry of Education, Science, Sports and Culture, Japan; Grant‐in‐Aid from the Ministry of Health, Labour and Welfare, Japan; the 21st Century COE Program Special Research Grant (Tohoku University) from the Ministry of Education Science, Sports and Culture, Japan; Grant‐in‐aid from the Kurokawa Cancer Research Foundation, the Uehara Memorial Foundation, All Japan Coffee Association and the Third Term Comprehensive 10‐year Strategy for Cancer Control from the Ministry of Health, Labour and Welfare, Japan Statistical methods: conditional logistic regression Variables controlled in analysis: BMI, education, number of pregnancies, menopausal status, smoking status, diabetes mellitus, total calorie intake, miso soup consumption, tofu consumption and coffee consumption Variables controlled by matching: age (± 5 years) and area of residence |
|
Kato 1990.
| Study characteristics | ||
| Methods | PCC in Japan | |
| Participants | Participants: 221 (male/female: 138/83) cases and 578 (male/female: 377/201) controls Inclusion criteria: aged ≥ 18 years, receiving a coloscopic examination at Aichi Cancer Center Hospital, Japan Recruitment: from June 1986‐March 1990 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Colorectal cancer: 221 (male/female: 138/83) cases (2 cases have both colon and rectal cancer) Colon cancer: 132 (male/female: 79/53) cases Rectal cancer: 91 (male/female: 60/31) cases |
|
| Green tea in exposure categories | Exposure assessment: hot green tea Lowest exposure: < daily drinker Highest exposure: daily drinker |
|
| Notes | Funding: Grants‐in‐Aid for Cancer Research from the Ministry of Health and Welfare of Japan (61‐6 and 1‐6) Statistical methods: unconditional logistic regression Variables controlled in analysis: age, sex and residence Variables controlled by matching: age (± 5 years), sex and municipality |
|
Key 1999.
| Study characteristics | ||
| Methods | Cohort study in Japan | |
| Participants | Participants: 34,759 women from Hiroshima and Nagasaki Inclusion criteria: women (93,741) present in Hiroshima and Nagasaki at the time of the bombings and city residents on 1 October 1950 and residents (23,580) not present at the time of the bombings but present between 1950 and 1953. Data of women recruited in the 1st and 2nd mail surveys, alive on 1 September 1969 (survey 1) and alive in September 1979 (Hiroshima) and July 1979 (Nagasaki) Parental cohort: Radiation Effects Research Fundation's Life Span Study: final population of 34,765 women Recruitment: 1969‐1970 (survey 1) and 1979 (survey 2) |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 31 December 1993 Breast cancer: 405/427 eligible cases (22 cases excluded due to missing data on green tea intake) |
|
| Green tea in exposure categories | Exposure assessment: hot green tea intake Lowest exposure: ≤ 1 time/d Intermediate exposure: 2‐4 times/d Highest exposure: ≥ 5 times/d |
|
| Notes | Funding: research performed at the Radiation Effects Research Foundation (RERF), Hiroshima and Nagasaki, Japan. RERF is funded equally by the Japanese Ministry of Health and Welfare and the USA Department of Energy (DOE) Department of Energy through the National Academy of Sciences, Japan Statistical methods: Poisson regression Variables controlled in analysis: age, calendar period, city, age at the time of the bombing and radiation dose Variables controlled by matching: ‐ |
|
Khan 2004.
| Study characteristics | ||
| Methods | Cohort study in Japan | |
| Participants | Participants: 3158 (male/female: 1524/1634) participants Inclusion criteria: aged ≥ 40 years, attending the 45 Public Health Centres located in the Hokkaido Prefecture, Japan Recruitment: 2002 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 31 December 1993 Total cancer mortality: 243 (male/female: 154/89) cases Lung cancer mortality: 51 (male/female: 40/10) cases Stomach cancer mortality: 51 (male/female: 36/15) cases Colorectal cancer mortality: 29 (male/female: 15/14) cases Pancreatic cancer mortality: 25 (male/female: 12/13) cases Other cancers mortality: 88 (male/female: 51/37) cases |
|
| Green tea in exposure categories | Exposure assessment: green tea intake Lowest exposure: never drink or drink several times per year or per month Highest exposure: drink several times/week or every day |
|
| Notes | Funding: Department of Health and Welfare of Hokkaido Government, Japan Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age and smoking Variables controlled by matching: ‐ |
|
Kikuchi 2006.
| Study characteristics | ||
| Methods | Cohort study in Japan | |
| Participants | Participants: 26,481 participants (all men). Data on green tea consumption available for 18,961 men Inclusion criteria: aged 40‐79 years, living in 14 municipalities of Miyagi Prefecture in Japan Parent cohort: Ohsaki Cohort Study Recruitment: from 1994 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Otucome assessment: 2002 Prostate cancer: 110 cases |
|
| Green tea in exposure categories | Exposure assessment: green tea intake Lowest exposure: < 1 cup/d Intermediate exposure 1: 1‐2 cups/d Intermediate exposure 2: 3‐4 cups/d Highest exposure: ≥ 5 cups/d |
|
| Notes | Funding: Grant‐in‐aid of Third Term Comprehensive Control Research for Cancer from the Ministry of Health, Labour and Welfare, Japan (H16‐3ji‐gan‐010) Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age, BMI, alcohol consumption, smoking status, marital status, daily calorie intake, daily calcium intake, walking duration, consumption frequencies of black tea and coffee and consumption frequencies of meat and fish Variables controlled by matching: ‐ |
|
Kono 1988.
| Study characteristics | ||
| Methods | PCC and HCC in Japan | |
| Participants | Participants: 139 (male/female: 74/65) cases, 278 (male/female: 148/130) population controls and 2575 (male/female: 1171/1404) hospital controls Inclusion criteria: aged 20‐75 years, newly diagnosed cases from Karatsu city and 9 neighbouring villages in Saga Prefecture, Japan. Hospital controls were aged 25‐75 years, free from cancer at clinical/radiological examination. Population controls were residents of Saga Prefecture, Japan Recruitment: from 1979‐1982 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Stomach cancer: 139 (male/female: 74/65) cases |
|
| Green tea in exposure categories | Exposure assessment: green tea intake Lowest exposure: none or 1‐4 cups/d Intermediate exposure: 5‐9 cups/d Highest exposure: ≥ 10 cups/d |
|
| Notes | Funding: Grant‐in‐Aid, Ministry of Education, Science and Culture, Japan Statistical methods: unconditional logistic regression for hospital controls and conditional logistic regression for population controls Variables controlled in analysis: sex, age, class, smoking, consumption of mandarin oranges, fruits and others Variables controlled by matching: sex, age, class |
|
Kubik 2008.
| Study characteristics | ||
| Methods | HCC in Czech Republic | |
| Participants |
Kubik 2008 Participants: 1096 (male/female: 509/587) cases and 2966 (male/female: 788/2178) controls Inclusion criteria: aged 25‐89 years, with microscopically confirmed lung cancer attending Prague University Hospital Na Bulovce, Departments of pneumology, thoracic surgery and internal medicine Recruitment: from April 2002‐August 2006 (men) and from April 1998‐November 2006 (women) Kubik 2004 Participants: 435 cases and 1710 controls (all women) Inclusion criteria: women aged 25‐89 years, with microscopically confirmed lung cancer attending Prague University Hospital Na Bulovce, Departments of pneumology, thoracic surgery and internal medicine Recruitment: from April 1998‐November 2002 |
|
| Interventions | N/A | |
| Outcomes |
Kubik 2008 Number of cases Lung cancer: 1096 (male/female: 509/587) cases: 308 (male/female: 101/207) cases of adenocarcinoma, 398 (male/female: 249/149) squamous cell cancers and 213 (male/female: 81/132) small‐cell cancers Kubik 2004 Number of cases Lung cancer: 435 cases (all women) |
|
| Green tea in exposure categories |
Kubik 2008 Exposure assessment: green tea intake Lowest exposure: never Highest exposure: monthly or less, weekly or less, daily or several times/week Kubik 2004 Exposure assessment: green tea intake Lowest exposure: never Intermediate exposure: monthly (≤ 1 month ) or weekly (≤ 1 week but > 1 monthly) Highest exposure: daily (daily or several times/week) |
|
| Notes |
Kubik 2008 Funding: grant (N. NR/8411‐3) from the Internal Grant Agency of the Ministry of Health of the Czech Republic and by an institutional research project (N. MZO 00064211) from the Ministry of Health, Czech Republic Statistical methods: unconditional logistic regression Variables controlled in analysis: age, residence, education and pack‐years of smoking Variables controlled by matching: ‐ Kubik 2004 Funding: Internal Grant Agency of the Ministry of Health of the Czech Republic (NJ/6732–3) Statistical methods: unconditional logistic regression Variables controlled in analysis: age, residence, education and pack‐years of smoking Variables controlled by matching: ‐ |
|
Kumar 2015.
| Study characteristics | ||
| Methods | RCT, parallel, double‐blind in USA | |
| Participants | Participants: originally 97 men with HG‐PIN and/or ASAP (49 in the intervention group and 48 in the control group) Inclusion criteria: age 30‐80 years, with HG‐PIN and/or ASAP biopsy diagnosed < 3 months before randomisation, with no history of cancer, hepatic or renal disease, restricted from taking steroid or other supplements, or > 6‐12 cups of green tea/d Recruitment: from September 2008‐March 2013 (> 95%) at the Moffitt Cancer Center, James A. Haley VA Hospital, Tampa and University of Florida, Jacksonville, Florida |
|
| Interventions | Treatment group: Polyphenon E (Poly E) by Mitsui Norin Co (containing 200 mg of catechins/capsule), two capsules/d = total 400 mg/d of EGCG Control group: placebo Duration: 1 year |
|
| Outcomes | Primary outcome Prostate cancer incidence Secondary outcome Prostate cancer incidence + ASAP in men with HG‐PIN LUTS using the LUTS Symptoms Scale and QoL, using the Rand Short‐form (SF)‐36 Adverse effects (safety data) |
|
| Green tea in exposure categories | N/A | |
| Notes | Funding: NIH–National Cancer Institute R01 CA12060‐01A1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "participants were assigned to the intervention or placebo arm (1:1) using the SRAR system, a web‐delivered subject registration application." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "a web‐delivered subject registration application" Comment: central allocation, probably done |
| Blinding of participants and personnel (performance bias) Prostate cancer incidence | Low risk | Quote: "All study staff and participants, with the exception of the clinical pharmacist and biostatistician, were blinded to the assignments until the completion of the trial. Both PolyE and the matching placebo used in the trial were hard gelatin capsules with no difference in appearance, taste, or smell" Comment: probably done |
| Blinding of participants and personnel (performance bias) Lower urinary tract symptoms | Low risk | Quote: "All study staff and participants, with the exception of the clinical pharmacist and biostatistician, were blinded to the assignments until the completion of the trial. PolyE and matching placebo capsules were manufactured under contract to NCI" Comment: probably done |
| Blinding of participants and personnel (performance bias) PSA levels | Low risk | Review authors do not believe this would introduce bias |
| Blinding of outcome assessment (detection bias) Prostate cancer incidence | Low risk | Quote: "All study staff and participants, with the exception of the clinical pharmacist and biostatistician, were blinded to the assignments until the completion of the trial" Comment: probably done |
| Blinding of outcome assessment (detection bias) Lower urinary tract symptoms | Low risk | Quote: "All study staff and participants, with the exception of the clinical pharmacist and biostatistician, were blinded to the assignments until the completion of the trial. PolyE and matching placebo capsules were manufactured under contract to NCI" Comment: probably done |
| Blinding of outcome assessment (detection bias) PSA levels | Low risk | Review authors do not believe this would introduce bias |
| Incomplete outcome data (attrition bias) Prostate cancer incidence | Low risk | Comment: number of participants included in analysis stated |
| Incomplete outcome data (attrition bias) Lower urinary tract symptoms | Unclear risk | Comment: number of participants included in analysis NR |
| Incomplete outcome data (attrition bias) PSA levels | Low risk | Comment: number of participants included in analysis stated |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (ClinicalTrials.gov identifier (NCT number): NCT00596011) and the published reports include all expected outcomes |
| Other bias | Unclear risk | Elevated number of withdrawal |
Kuo 2009.
| Study characteristics | ||
| Methods | PCC in China | |
| Participants | Participants: 252 (male/female: NR) cases and 637 (male/female: NR) controls Inclusion criteria: aged > 30 years, and residents in Kaohsiung metropolitan area (Kaohsiung City and its suburbs including 4 complexes of Tsoying, Tasheh, Jenwu and Linyuan are in south‐western Taiwan) at the time of the diagnosis. Data on adults (16‐29 years) are included Recruitment: from November 1997‐December 2006 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Leukaemia: 93 (male/female: 42%/58%) adult cases and 223 (male/female: 39%/61%) controls |
|
| Green tea in exposure categories | Exposure assessment: green tea intake with the questionnaire and with catechin urinary levels. Only crude data of intake used. | |
| Notes | Funding: National Institutes of Health (ES09723, ES00002) Statistical methods: crude analysis Variables controlled in analysis: crude analysis Variables controlled by matching: ‐ |
|
Kurahashi 2007.
| Study characteristics | ||
| Methods | Cohort studies in Japan | |
| Participants | Participants: 65,659 men, with final data on 49,920 men with complete data Inclusion criteria: aged 40‐59 years, from 5 Public Health Centre areas (Iwate, Akita, Nagano, Okinawa and Tokyo) for cohort 1 and aged 40‐69 years for cohort 2, from 6 Public Health Centre areas (Ibaraki, Niigata, Kochi, Nagasaki, Okinawa and Osaka), Japan Parent cohorts Cohort 1: Japan Public Health Center‐based Prospective Study (JPHC)‐I Cohort 2: Japan Public Health Center‐based Prospective Study (JPHC)‐II Recruitment: from 1990 for cohort 1 and 1993 for cohort 2 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 31 December 2004 Prostate cancer: 404 cases, including 114 advanced, 271 localised and 19 of undetermined stage |
|
| Green tea in exposure categories | Exposure assessment: green tea consumption Lowest exposure: < 1 cup/d Intermediate exposure 1: 1‐2 cups/d Intermediate exposure 2: 3‐4 cups/d Highest exposure: ≥ 5 cups/d |
|
| Notes | Funding: Grants‐in‐Aid for cancer research from the Ministry of Health, Labour and Welfare of Japan for the Third Term Comprehensive 10‐Year Strategy for Cancer Control and by Grants‐in‐Aid for scientific research on priority areas from the Ministry of Education, Culture, Sports, Science and Technology for research on the risk of chemical substances Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age, area, smoking status, alcohol consumption, BMI, marital status and coffee, black tea, miso soup consumption, fruits, green or yellow vegetables, dairy food, soy food and genistein consumption Variables controlled by matching: ‐ |
|
Kurahashi 2009.
| Study characteristics | ||
| Methods | Cohort studies in Japan | |
| Participants | Participants: 133,084 (male/female: 65,660/67,424) and 104,440 (male/female: 49,566/54,874) people with complete data Inclusion criteria: aged 40‐59 years, from 5 Public Health Centre areas (Iwate, Akita, Nagano, Okinawa and Tokyo) for cohort 1 and aged 40‐69 years for cohort 2, from 6 Public Health Centre areas (Ibaraki, Niigata, Kochi, Nagasaki, Okinawa and Osaka), Japan Parent cohorts Cohort 1: Japan Public Health Center‐based Prospective Study (JPHC)‐I Cohort 2: Japan Public Health Center‐based Prospective Study (JPHC)‐II Recruitment: from 1990 for cohort 1 and 1993 for cohort 2 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 31 December 2005 Bladder cancer: 206 (male/female: 164/42) cases |
|
| Green tea in exposure categories | Exposure assessment: green tea consumption Men Lowest exposure: < 1 cup/d Intermediate exposure 1: 1‐2 cups/d Intermediate exposure 2: 3‐4 cups/d Highest exposure: ≥ 5 cups/d Women Lowest exposure: < 3 cups/d Intermediate exposure 1: 3‐4 cups/d Highest exposure: ≥ 5 cups/d |
|
| Notes | Funding: Grant‐in‐Aid for Cancer Research (19shi‐2) Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age, area, smoking status, alcohol drinking and coffee Variables controlled by matching: ‐ |
|
Kuriyama 2006.
| Study characteristics | ||
| Methods | Cohort study in Japan | |
| Participants | Participants: 52,029 participants. Final data on 40,530 participants included Inclusion criteria: aged 40‐79 years living in 14 municipalities of Miyagi Prefecture in the catchment area of the Ohsaki Public Health Centre, Miyagi, Japan. Parent cohort: Ohsaki Cohort Study Recruitment: from October‐December 1994 Data on the same cohort also reported in Naganuma 2009 and Ui 2009 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Total cancer mortality: 1134 (male/female: 734/395) cases Stomach cancer mortality: 193 (male/female: 138/55) cases Lung cancer mortality: 218 (male/female: 166/52) cases Colorectal cancer mortality: 132 (male/female: 84/48) cases Outcome assessment: 31 December 2001 |
|
| Green tea in exposure categories | Exposure assessment: green tea consumption Lowest exposure: < 1 cup/d Intermediate exposure 1: 1‐2 cups/d Intermediate exposure 2: 3‐4 cups/d Highest exposure: ≥ 5 cups/d |
|
| Notes | Funding: Health Sciences Research Grant for Health Services (H18‐Choju‐Ippan‐014, H16‐Seisaku‐Ippan‐023, H18‐ Junkankitou [Seisyu]‐Ippan‐012), Ministry of Health, Labour and Welfare, Japan Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age, sex, job status, years of education, BMI, engaging in sports or exercise, walking duration, history of hypertension, diabetes mellitus and gastric ulcer, smoking status, alcohol drinking, total energy intake/d, daily consumption of rice, daily consumption of miso (soybean paste) soup, daily consumption of soybean products, total meat, total fish, dairy products, total fruits and total vegetables and consumption of oolong tea, black tea and coffee Variables controlled by matching: ‐ For stomach cancer, data from Inoue 2009a were used |
|
Lane 2018.
| Study characteristics | ||
| Methods | RCT, parallel, double‐blind in UK | |
| Participants | Participants: 133 men at high risk of prostate cancer randomised Inclusion criteria: participants previously enrolled in the Prostate testing for cancer and Treatment (ProtecT) trial. Men aged 50‐69 years, with localised prostate cancer with no history of allergies to lycopene‐containing foods or green tea, current or prior prostate cancer, major co‐morbidities or 5‐ARI medication Recruitment: from December 2009‐May 2011 |
|
| Interventions | 3 lycopene (dietary advice, capsules, placebo) and 3 green tea (drink, capsules, placebo) interventions: 9 different interventions for 6 months, particularly regarding green tea: 45 participants in the green tea drink: at least 3 cups/d, around 600 mL/d of tea made with green tea bag, PG Tips, Unilever Ltd 45 participants in the green tea capsules: 2 capsules with 300 mg/d green tea leaf‐derived extracted = 600 mg/d EGCG (Frutarom Ltd.) 43 participants in the green tea placebo Duration: 6 months |
|
| Outcomes | Primary outcome Prostate cancer incidence Secondary outcomes PSA levels Safety data |
|
| Green tea in exposure categories | N/A | |
| Notes | Funding: Cancer Research UK (C11046/A10052) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly allocated to one of three lycopene interventions and to one of three green tea interventions using a blocked random allocation [1:1:1 ratio; generated by the trial statistician (C. Metcalfe) using the Stata uniform () "function]" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "opaque envelopes for allocation. The allocation was concealed from the study nurse recruiting individuals" Comment: probably done |
| Blinding of participants and personnel (performance bias) PSA levels | Low risk | Review authors do not believe this would introduce bias |
| Blinding of outcome assessment (detection bias) PSA levels | Low risk | Review authors do not believe this would introduce bias |
| Incomplete outcome data (attrition bias) PSA levels | Unclear risk | Missing outcome data without explanation |
| Selective reporting (reporting bias) | High risk | In the study protocol other outcomes are reported (ClinicalTrials.gov identifier (NCT number): NCT01105338) |
| Other bias | Unclear risk | Some men also took lycopene capsules |
Lassed 2016.
| Study characteristics | ||
| Methods | HCC in Algeria | |
| Participants | Participants: 90 cases and 190 controls (all men) Inclusion criteria: aged 50‐88 years, histologically confirmed prostate cancer followed at the service of urology and at the emergency department in Clinic of Urology‐Nephrology and Kidney Transplant Daski, Constantine, Algeria Recruitment: from 2011‐2013 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Prostate cancer: 90 cases (all men) |
|
| Green tea in exposure categories | Exposure assessment: green tea consumption Lowest exposure: < 1 cup/d Intermediate exposure: 1‐3 cups/d Highest exposure: > 3 cups/d |
|
| Notes | Funding: Individual Project (F0092012009) and research unit programmatic funding (VARENBIOMOL) at Constantine University, Algeria Statistical methods: Chi2 test (Woolf logit method) Variables controlled in analysis: ‐ Variables controlled by matching: ‐ |
|
Lee 2007.
| Study characteristics | ||
| Methods | Cohort study in Japan | |
| Participants | Participants: 96,162 (male/female: 46,023/50,139) people with complete data. Inclusion criteria: aged 40‐59 years, from 11 Prefectures, Japan (See also Kurahashi 2007) Parent cohort: Japan Public Health Centre‐based Prospective Study (JPHC) Recruitment: from 1990‐1994 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 31 December 2002 Colorectal cancer: 1158 (male/female: 724/434) cases out of 1163 eligible cases Colon cancer: 760 (male/female: 476/284) cases out of 763 eligible cases Rectal cancer: 398 (male/female: 248/150) cases out of 400 eligible cases |
|
| Green tea in exposure categories | Exposure assessment: green tea consumption Lowest exposure: almost never Intermediate exposure 1: < 1 cup/d Intermediate exposure 2: 1‐2 cups/d Intermediate exposure 3: 3‐4 cups/d Highest exposure: ≥ 5 cups/d |
|
| Notes | Funding: Cancer Research, Third‐Term Comprehensive Control Research for Cancer from the Ministry of Health, Labour and Welfare, Japan Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age, study area, BMI, smoking status, alcohol drinking, family history of colorectal cancer, physical activity and intake of green vegetables, beef, pork, coffee, Chinese tea and black tea Variables controlled by matching: ‐ |
|
Lee 2017.
| Study characteristics | ||
| Methods | HCC in China | |
| Participants | Participants: 404 cases and 395 controls (all men) Inclusion criteria: aged < 85 years, newly diagnosed primary prostate cancer cases referring at hospital of New Territories East Cluster of Hong Kong, China Recruitment: from August 2011‐June 2016 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Prostate cancer: 404 cases |
|
| Green tea in exposure categories | Exposure assessment A: green tea intake Lowest exposure: nondrinkers Highest exposure: green tea drinker Exposure assessment B: green tea concentration in 250 mL of tea Lowest exposure: < 2.5 g Intermediate exposure 1: 2.5‐5.0 g Intermediate exposure 2: 5.0‐7.5 g Highest exposure: ≥ 7.5 g |
|
| Notes | Funding: Grant from the Health and Medical Research Fund of the Hong Kong Special Administrative Region, China; Project N. 11121091 and 12131081 Statistical methods: unconditional logistic regression Variables controlled in analysis: age at interview, deep fried food consumption, green vegetable consumption, alcohol consumption, coffee consumption, tobacco consumption, education attainment and family prostate cancer history Variables controlled by matching: age (± 5 years) |
|
Lei 1994.
| Study characteristics | ||
| Methods | HCC in China | |
| Participants | Participants: 792 cases (male/female: NR) and 792 (male/female: NR) controls Inclusion criteria: people referred to hospitals of Guangzhou and residents in Guangzhou province, China Recruitment: 1986 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Lung cancer: 792 (male/female: NR) cases |
|
| Green tea in exposure categories | Exposure assessment: green tea drinking Lowest exposure: nondrinkers Highest exposure: drinkers |
|
| Notes | Article in Chinese Funding: not declared Statistical methods: conditional logistic regression Variables controlled in analysis: NR Variables controlled by matching: sex and age |
|
Le Marchand 2000.
| Study characteristics | ||
| Methods | PCC in USA | |
| Participants | Participants: 582 (male/female: 375/207) cases and 582 (male/female: 375/207) controls Inclusion criteria: aged 26‐79 years, histologically confirmed primary lung cancer, no history of lung cancer, appropriate ethnicity, Oahu residents, Hawaii State, USA Recruitment: from 1 January 1992‐31 March 1997 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Lung cancer: 582 (male/female: 375/27) cases |
|
| Green tea in exposure categories | Exposure assessment: green tea intake Lowest exposure: lowest quartile < 0.0 g/d Intermediate exposure 1: between 0.0 to median value g/d Intermediate exposure 2: between median value to 171.1 g/d Highest exposure: highest quartile > 171.1 g/d |
|
| Notes | Funding: Public Health Service R01CA55874 and contract N01CP67001 from National Cancer Institute and EDT‐78 grant from the American Cancer Society Statistical methods: Poisson regression Variables controlled in analysis: matching variables, smoking status, duration, duration^2, number of cigarettes smoked/d and beta‐carotene and saturated fat intake Variables controlled by matching: age, sex and ethnicity |
|
Leung 2016.
| Study characteristics | ||
| Methods | PCC in China | |
| Participants | Participants: 524 cases and 1587 controls (all women) from the Ovarian Cancer in Alberta and British Columbia (OVAL‐BC) Study Inclusion criteria: aged 20‐79 years, incident cases in residents in Alberta (AB) and aged 40‐79 years, incident cases in residents in British Columbia (BC), Canada Recruitment: from 2002‐2012 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Epithelian ovarian cancer: 104 cases out of 524 eligible cases with information on green tea and 471 out of 1587 eligible controls |
|
| Green tea in exposure categories | Exposure assessment A: green tea intake Lowest exposure: nondrinkers Highest exposure: green tea drinker Exposure assessment B: green tea consumption Lowest exposure: nondrinkers Intermediate exposure 1: ≤ 1 lifetime cups‐years Intermediate exposure 2: 1‐5 lifetime cups‐years Highest exposure: > 5 lifetime cups‐years |
|
| Notes | Funding: grants from the Canadian Institutes for Health Research and by a grant from WorkSafe BC (formerly the Workers’ Compensation Board of British Columbia) Statistical methods: unconditional logistic regression Variables controlled in analysis: study site, reference or diagnosis age, race, educational level, BMI, smoking, lifetime average alcohol drinking, first‐degree female relative history of ovarian/breast cancer, years of oral contraceptive use, parity, menopausal status and hormone therapy use Variables controlled by matching: age (± 5 years) |
|
Li 2008.
| Study characteristics | ||
| Methods | Cohort study in Japan | |
| Participants | Participants: 52,029 participants. Final data on 41,440 participants included Inclusion criteria: aged 40‐79 years, living in 14 municipalities of Miyagi Prefecture in the catchment area of the Ohsaki Public Health Centre, Miyagi, Japan Parent cohort: Ohsaki Cohort Study Recruitment: from October‐December 1994 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 31 December 2001 Lung cancer: 302 (male/female: 227/75) cases |
|
| Green tea in exposure categories | Exposure assessment: green tea consumption Lowest exposure: < 1 cup/d Intermediate exposure 1: 1‐2 cups/d Intermediate exposure 2: 3‐4 cups/d Highest exposure: ≥ 5 cups/d |
|
| Notes | Funding: Grant‐in‐Aid for Cancer Research and for the Third Term Comprehensive Ten‐Year Strategy for Cancer Control (H18‐3jigan‐ippan‐001), Ministry of Health, Labour and Welfare, Japan Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age, sex, education level, marital status, passive smoking, BMI, walking duration, family history of cancer, smoking status, number of cigarettes smoked/d, years of smoking, alcohol drinking, total energy intake/d and daily consumption of soybean products, total meat, total fish, dairy products, total fruits and total vegetables and consumption of coffee Variables controlled by matching: ‐ |
|
Li 2011a.
| Study characteristics | ||
| Methods | PCC and HCC in China | |
| Participants | Participants: 540 (male/female: NR) cases, 540 (male/female: NR) population controls and 540 (male/female: NR) hospital controls Inclusion criteria: aged 18‐85 years, participants with histopathology and haematology cancer diagnosed at the First Hospital of China Medical University from permanent residents of urban Shenyang, China Recruitment: from August 2009‐July 2010 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Total (any) cancer: 425 (male/female: NR) cases Breast cancer: 224 (male/female: NR) cases Colorectal cancer: 175 (male/female: NR) cases Leukaemia: 26 (male/female: NR) cases |
|
| Green tea in exposure categories | Exposure assessment A: green tea intake Lowest exposure: never or seldom Highest exposure: ≥ 1 time/d Exposure assessment B: dried green tea leaves Lowest exposure: 0 g/year Intermediate exposure: 500‐< 1000 g/year Highest exposure: ≥ 1000 g/year |
|
| Notes | Funding: National Health and Medical Research Council of Australia project grant (APP ID 572542) Statistical methods: (un)conditional logistic regression Variables controlled in analysis: education, BMI 5 years ago, smoking, passive smoking, alcohol consumption, physical activity, energy intake, cancer in first‐degree relative Variables controlled by matching: sex and age (± 5 years) |
|
Li 2014.
| Study characteristics | ||
| Methods | PCC in China | |
| Participants | Participants: 250 cases and 500 controls (all men) Inclusion criteria: newly diagnosed and histopathologically confirmed prostate cancer and with PSA value < 4.0 ng/mL from Changhai and Changzheng Hospitals of the Second Military Medical University and Zhongshan Hospital of the Fudan University located respectively in Yangpu, Huangpu and Xuhui District in Shangai city, China Recruitment: from 1 January 2007‐1 July 2013 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Prostate cancer: 250 cases (all men) |
|
| Green tea in exposure categories | Exposure assessment: green tea consumption Lowest exposure: never Highest exposure: consumption |
|
| Notes | Funding: National Natural Science Foundation of China (N. 81072377) Statistical methods: conditional logistic regression Variables controlled in analysis: multivariate model but not clear all factors included in the model Variables controlled by matching: race and age (± 5 years) |
|
Li 2016.
| Study characteristics | ||
| Methods | HCC in China | |
| Participants | Participants: 756 cases and 789 controls (all women) Inclusion criteria: aged 20‐84 years, Chinese women, newly diagnosed primary breast cancer in Hong Kong, China Recruitment: November 2011‐May 2014 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Breast cancer: 756 cases (all women) |
|
| Green tea in exposure categories | Exposure assessment: green tea consumption Lowest exposure: non‐tea drinkers Highest exposure: green tea drinker (any) |
|
| Notes | Funding: Research Grants Council of Hong Kong (N. 474811) Statistical methods: unconditional logistic regression Variables controlled in analysis: age at interview, age at menarche, age at first birth, parity, HRT, first‐degree family history and BMI Variables controlled by matching: age (± 5 years) |
|
Li 2018.
| Study characteristics | ||
| Methods | Cohort study in China | |
| Participants | Participants: 103,010 participants Inclusion criteria: aged ≥ 18 years, men, including employed and retired workers of Kailuan group, with no previous diagnosis of cancer, China Parent cohort: Kailuan Cohort Recruitment: from May 2006‐May 2014 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 31 December 2015 Lung cancer: 964 cases (all men) |
|
| Green tea in exposure categories | Exposure assessment: green tea consumption Lowest exposure: < 4 times/week Highest exposure: ≥ 4 times/week |
|
| Notes | Article in Chinese Funding: National Key R&D Plan (2016YFC0905400, 2016YFC1302500, 2017YFC0907900); Beijing Excellent Talent Cultivation Funding (2017000021223TD05), Central Health Special Fund (W2017BJ39), Concord Youth Fund (2017320013, 3332016131), National Natural Science Foundation of China (81673265). Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age, education, economic income, smoking status, drinking status, dust exposure, BMI, drinking tea type and family history of cancer Variables controlled by matching: ‐ |
|
Lin 2008.
| Study characteristics | ||
| Methods | Cohort study in Japan | |
| Participants | Participants: 110,792 inhabitants of 45 areas of Japan. Data retrieved for 77,850 (male/female: 32,774/45,076) Inclusion criteria: aged 40‐79 (≥ 18 years in 1949). Details reported in Ohno 2001 Parent cohort: Japan Collaborative Cohort Study of Evaluation of Cancer Risk (JACC Study) Recruitment: from 1988‐1990 Same population of Fujino 2002 and Hoshiyama 2002 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 31 December 2003 Pancreatic cancer mortality: 292 (male/female: 140/152) cases |
|
| Green tea in exposure categories | Exposure assessment: green tea drinking Lowest exposure: < 1 cup/d Intermediate exposure 1: 1‐2 cups/d Intermediate exposure 2: 3‐4 cups/d Intermediate exposure 3: 5‐6 cups/d Highest exposure: ≥ 7 cups/d |
|
| Notes | Funding: Grant‐in‐Aid for Scientific Research on Priority Areas 2 (No. 13220019) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. The JACC Study has also been supported by Grants‐in‐Aid for Scientific Research from the same ministry (Nos. 61010076, 62010074, 63010074, 1010068, 2151065, 3151064, 4151063, 5151069, 6279102 and 11181101). Statistical methods: Cox hazard proportional regression Variables controlled in analysis: age, sex, BMI, cigarette smoking, alcohol drinking, history of diabetes and history of gallbladder diseases Variables controlled by matching: ‐ |
|
Lin 2012.
| Study characteristics | ||
| Methods | HCC in China | |
| Participants | Participants: 170 (male/female: 102/68) cases and 340 (male/female: 204/136) controls Inclusion criteria: incident cancer aged < 80 years, diagnosed in Changhwa Christian Hospital (Changhwa County, Taiwan, China) Recruitment: from August 2004‐October 2008 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Lung cancer: 170 (male/female: 102/68) cases, including adenocarcinoma (N = 93), squamous cell carcinoma (N = 46) and others (N = 31, including small‐cell carcinoma, neuroendocrine carcinoma, mixed cell carcinoma and unspecific malignant cell) |
|
| Green tea in exposure categories | Exposure assessment: green tea drinking Lowest exposure: 0 cup/d Intermediate exposure: < 1 cup/d Highest exposure: ≥ 1 cup/d |
|
| Notes | Funding: National Science Council, Taiwan (NSC‐98‐2815‐C‐040‐028‐B; NSC 95‐2815‐C‐040‐019‐B; NSC 93‐2815‐C‐040‐008‐B) Statistical methods: conditional logistic regression Variables controlled in analysis: sex and age Variables controlled by matching: sex and age (± 5 years) |
|
Liu 2010.
| Study characteristics | ||
| Methods | PCC in China | |
| Participants | Participants: 641 (male/female: NR) cases and 1847 (male/female: NR) controls Inclusion criteria: mean age 63.5 years in both cases and controls, residents in Dafeng City, Jiangsu Province, China Recruitment: from January 2005‐December 2007 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Stomach cancer: 641 (male/female: NR) cases |
|
| Green tea in exposure categories | Exposure assessment: green tea drinking Lowest exposure: nondrinkers Highest exposure: > 21 cups/week |
|
| Notes | Article in Chinese Funding: not declared Statistical methods: unconditional logistic regression Variables controlled in analysis: age, sex, education level, income, smoking status, alcohol drinking and family history of stomach cancer Variables controlled by matching: ‐ |
|
Liu 2016.
| Study characteristics | ||
| Methods | Cohort study in China | |
| Participants | Participants: 222,279 men, including a total of 164,681 men who remained in the main analyses Inclusion criteria: aged > 40 years, men randomly selected from residential units within 45 nationally representative Disease Surveillance Points with no prior diagnosis of cancer, stroke, heart disease, chronic obstructive pulmonary disease, asthma, tuberculosis, peptic ulcer, diabetes, hypertension, kidney cirrhosis, chronic hepatitis Parent cohort: Chinese Prospective Smoking Study (CPSS) Recruitment: from 1990‐1991 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 31 December 2006 Total cancer mortality: 7002 cancer deaths (all men) |
|
| Green tea in exposure categories | Exposure assessment: green tea drinking Lowest exposure: nondrinkers Intermediate exposure 1: ≤ 5 g/month Intermediate exposure 2: 5‐10 g/month Highest exposure: ≥ 10 g/month |
|
| Notes | Funding: Chinese Ministry of Health, the UK Medical Research Council, British Heart Foundation and Cancer Research UK, the World Bank loan to China and the Canadian International Development Research Centre. Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age, BMI, marital status, urban locality, job status, smoking status, times of weekly fish consumption, times of weekly meat consumption, times of weekly poultry consumption, times of weekly egg consumption, times of weekly milk consumption, black tea drinker, jasmine tea drinker and other tea drinker Variables controlled by matching: ‐ |
|
Liu 2017.
| Study characteristics | ||
| Methods | HCC in China | |
| Participants | Participants: 442 (male/female: 256/186) cases and 442 (male/female: 256/186) controls Inclusion criteria: aged ≥ 16 years, with incident first‐time haematologically confirmed diagnosis of leukaemia residing in the respective provinces for at least 1 year and presenting as an inpatient to the participating hospitals, namely the First and the Second Affiliated Hospitals of Zhejiang University in Hangzhou, Zhejiang province and the First Hospital of China Medical University in Shenyang, Liaoning Province, China Recruitment: from August 2008‐August 2013 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Leukaemia: 442 (male/female: 256/186) cases |
|
| Green tea in exposure categories | Exposure assessment: green tea drinking Lowest exposure: nondrinker (< 1 time/month) Highest exposure: drinker |
|
| Notes | Funding: National Health and Medical Research Council (Australia) Project Grant (N. 572542) Statistical methods: conditional logistic regression Variables controlled in analysis: matching variable and resident locality, education, cigarette smoking and alcohol consumption Variables controlled by matching: sex, age (± 5 years) and study site |
|
Luo 2007.
| Study characteristics | ||
| Methods | Cohort study in Japan | |
| Participants | Participants: 133,084 (male/female: 65,660/67,424) and 102,137 (male/female: 48,783/53,354) participants with complete data Inclusion criteria: aged 40‐59 years, from 5 Public Health Centre areas (Iwate, Akita, Nagano, Okinawa and Tokyo) for cohort 1, and aged 40‐69 years cohort 2, from 6 Public Health Centre areas (Ibaraki, Niigata, Kochi, Nagasaki, Okinawa and Osaka) for cohort 2, Japan Parent cohorts: Cohort 1: Japan Public Health Centre‐based Prospective Study (JPHC)‐I Cohort 2: Japan Public Health Centre‐based Prospective Study (JPHC)‐II Recruitment: from 1990 for cohort 1 and 1993/1994 for cohort 2 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 31 December 2003 Pancreatic cancer: 233 (male/female: 135/98 ) cases |
|
| Green tea in exposure categories | Exposure assessment: green tea drinking Lowest exposure: rarely Intermediate exposure 1: < 1 cup/d Intermediate exposure 2: 1‐2 cups/d Intermediate exposure 3: 3‐4 cups/d Highest exposure: ≥ 5 cups/d |
|
| Notes | Funding: Grant‐in‐Aid from the Cancer Research and Third‐Term Comprehensive Control Research for Cancer from the Ministry of Labour, Health and Welfare of Japan. Statistical methods: Cox proportional hazard regression Variables controlled in analysis: sex, age, BMI, leisure‐time physical activity in terms of frequency of sports, smoking status, alcohol intake, history of diabetes, history of cholelithiasis, study area and coffee intake Variables controlled by matching: ‐ |
|
Makiuchi 2016.
| Study characteristics | ||
| Methods | Cohort study in Japan | |
| Participants | Participants: 140,420 (male/female: 68,722/71,698), with 61,595 from cohort 1 and 78,825 from cohort 2 and 89,555 (male 51.6%) participants with complete data Inclusion criteria: aged 40‐59 years, from 5 Public Health Centre areas (Iwate, Akita, Nagano, Okinawa and Tokyo) for cohort 1 and aged 40‐69 years for cohort 2, from 6 Public Health Centre areas (Ibaraki, Niigata, Kochi, Nagasaki, Okinawa and Osaka), respondent at 5‐year follow‐up Parent cohorts Cohort 1: Japan Public Health Centre‐based Prospective Study (JPHC)‐I Cohort 2: Japan Public Health Centre‐based Prospective Study (JPHC)‐II Recruitment: from 1990 for cohort 1 and 1993/1994 for cohort 2 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 31 December 2009 in Osaka Public Health Centre and 31 December 2010 in all other areas Biliary tract cancer: 271 (male/female: 160/111) cases out of 284 eligible cases, including cancers of gallbladder cancer (N = 116) and of extrahepatic bile duct (N =145) |
|
| Green tea in exposure categories | Exposure assessment: green tea consumption Lowest exposure: ≤ 120 mL/d Intermediate exposure 1: 120‐360 mL/d Intermediate exposure 2: 360‐720 mL/d Highest exposure: ≥ 720 mL/d |
|
| Notes | Funding: Grant‐in‐Aid from the Cancer Research and Third‐Term Comprehensive Control Research for Cancer from the Ministry of Labour, Health and Welfare of Japan Statistical methods: Cox proportional hazard regression Variables controlled in analysis: sex, age, BMI, leisure‐time physical activity in terms of frequency of sports, smoking status, alcohol intake, history of diabetes, history of cholelithiasis, study area and coffee intake Variables controlled by matching: ‐ |
|
Mao 2011.
| Study characteristics | ||
| Methods | HCC in China | |
| Participants | Participants: 200 (male/female: 139/61) cases and 200 (male/female: 139/61) controls Inclusion criteria: mean age 51.5 (SD = 7) years, with histologically confirmed cancer, referring at the Kunming General Hospital of Chinese PLA and the First People’s Hospital of Yunnan Province, China Recruitment: from May 2010‐February 2011 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Stomach cancer: 200 (male/female: 139/61) cases |
|
| Green tea in exposure categories | Exposure assessment A: green‐tea drinking status Lowest exposure: nondrinkers Intermediate exposure: former drinkers Highest exposure: current drinkers Exposure assessment B: green tea consumption Lowest exposure: never Intermediate exposure 1: < 150 g/month Intermediate exposure 2: 150‐250 g/month Highest exposure: > 250 g/month |
|
| Notes | Funding: not declared Statistical methods: conditional logistic regression Variables controlled in analysis: age, sex, education level, BMI, annual income, cancer family history, smoking and drinking status Variables controlled by matching: sex and age (± 5 years) |
|
Micali 2017.
| Study characteristics | ||
| Methods | RCT, parallel, double‐blind in Italy | |
| Participants | Participants: 60 men at high risk of prostate cancer (30 in the treatment group and 30 in the control group) Inclusion criteria: age 55‐65 years, with HG‐PIN assessed using prostate biopsy with no previous cancer, not anti‐androgenic or chemoprevention therapies, non‐obese, without diabetes or other endocrinological diseases Recruitment: from May 2007‐February 2011 |
|
| Interventions | Treatment group: 2 oral GTE capsules total 300 mg of Categ Plus, Sofar SPA, Milan, Italy = total 600 mg/d Control group: placebo Duration: 1 year |
|
| Outcomes | Primary outcome Prostate cancer incidence Secondaty outcomes Safety data: side effects PSA levels LUTS score QoL score |
|
| Green tea in exposure categories | N/A | |
| Notes | Funding: no specific funding reported. Sofar SPA, Milan provided free samples of Categ Plus employed in the trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The random allocation sequence was obtained by means of 'Easy Random Picker' software (TrustFm© 1998‐2016)" Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information |
| Blinding of participants and personnel (performance bias) Prostate cancer incidence | Low risk | Quote: "Both participants and care providers were blinded after assignment to interventions, in order to avoid any bias. Two capsules of Categ Plus® or placebo per day were given to all subjects by the clinical trial investigators, according to the double‐blind method" Comment: probably done |
| Blinding of participants and personnel (performance bias) Lower urinary tract symptoms | Low risk | Quote: " Both participants and care providers were blinded after assignment to interventions, in order to avoid any bias. Two capsules of Categ Plus® or placebo per day were given to all subjects by the clinical trial investigators, according to the double‐blind method.” Comment: Probably done |
| Blinding of participants and personnel (performance bias) PSA levels | Low risk | Review authors do not believe this would introduce bias |
| Blinding of outcome assessment (detection bias) Prostate cancer incidence | Low risk | Quote: "Both participants and care providers were blinded after assignment to interventions, in order to avoid any bias" Comment: probably done |
| Blinding of outcome assessment (detection bias) Lower urinary tract symptoms | Low risk | Quote: "Both participants and care providers were blinded after assignment to interventions, in order to avoid any bias" Comment: probably done |
| Blinding of outcome assessment (detection bias) PSA levels | Low risk | Review authors do not believe this would introduce bias |
| Incomplete outcome data (attrition bias) Prostate cancer incidence | Low risk | Comment: number of participants included in analysis stated |
| Incomplete outcome data (attrition bias) Lower urinary tract symptoms | Low risk | Comment: number of participants included in analysis not clearly stated but probably the same as prostate cancer risk |
| Incomplete outcome data (attrition bias) PSA levels | Low risk | Comment: number of participants included in analysis not clearly stated but probably the same as prostate cancer risk |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available and it is not clear if the published reports include all expected outcomes |
| Other bias | High risk | High dropout in both groups (26.7%) |
Michikawa 2011.
| Study characteristics | ||
| Methods | Cohort study in Japan | |
| Participants | Participants: 140,420 (male/female: 68,722/71,698), with 61,595 from cohort 1 and 78,825 from cohort 2 and 100,507 (male/female: 48,802/51,705) participants with complete data. Inclusion criteria: aged 40‐59 years, from 5 Public Health Centre areas (Iwate, Akita, Nagano, Okinawa and Tokyo) for cohort 1, and aged 40‐69 years for cohort 2 from 6 Public Health Centre areas (Ibaraki, Niigata, Kochi, Nagasaki, Okinawa and Osaka), respondent at 5‐year follow‐up. Katsushika Public Health Centre was excluded due to missing cancer data. Parent cohorts Cohort 1: Japan Public Health Centre‐based Prospective Study (JPHC)‐I Cohort 2: Japan Public Health Centre‐based Prospective Study (JPHC)‐II Recruitment: from 1990 for cohort 1 and 1993/1994 for cohort 2 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 31 December 2007 Thyroid cancer: 159 (male/female: 26/133) cases, including 133 cases of papillary carcinoma, 7 cases of follicular carcinoma, 1 case of anaplastic carcinoma and 18 cases of other or unknown histologic types |
|
| Green tea in exposure categories | Exposure assessment: green tea drinking Lowest exposure: < 1 cup/d Intermediate exposure 1: 1‐2 cups/d Intermediate exposure 2: 3‐4 cups/d Highest exposure: ≥ 5 cups/d |
|
| Notes | Funding: Grant‐in‐Aid for Cancer Research from the Ministry of Health, Labour and Welfare, Japan (until 2009) and by Management Expenses Grants from the Government to the National Cancer Center (since 2010). It has also supported by a Grant‐in‐Aid for the Third‐Term Comprehensive Control Research for Cancer from the Ministry of Health, Labour and Welfare, Japan and by a Grant‐in‐Aid from Keio Medical Association. Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age, area, smoking history, passive smoking in the workplace, alcohol consumption, BMI, consumption of green vegetable and seaweed, health screening in the previous year and coffee consumption. For women, additionally adjusted for menopausal status and use of exogenous female hormones. Variables controlled by matching: ‐ |
|
Mizoo 2013.
| Study characteristics | ||
| Methods | HCC in Japan | |
| Participants | Participants: 472 cases and 464 controls (all women) Inclusion criteria: aged ≥ 20 years, consecutive patients treated at the Okayama University Hospital, Okayama Rousai Hospital and Mizushima Kyodo Hospital in Okayama and at Kagawa Prefecture Central Hospital in Kagawa, Japan. Controls from women under breast cancer screening. Recruitment: from December 2010‐November 2011 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Breast cancer: 472 cases |
|
| Green tea in exposure categories | Exposure assessment: green tea consumption Lowest exposure: < 1 time/week Intermediate exposure 1: 1 time/week Intermediate exposure 2: 2‐3 times/week Highest exposure: ≥ 4 times/week |
|
| Notes | Funding: Grant‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan Statistical methods: unconditional logistic regression Variables controlled in analysis: age. Variables controlled by matching: ‐ |
|
Mizuno 1992.
| Study characteristics | ||
| Methods | HCC in Japan | |
| Participants | Participants: 124 (male/female: 68/56) cases, 124 (male/female: 68/56) controls Inclusion criteria: aged 40‐79 years, pathologically, radiographically and/or serodiagnostically confirmed diagnosis at the 7 co‐operating institutes, i.e. the National Cancer Center Hospital, Chiba University Hospital, Shinshu University Hospital, the Cancer Institute Hospital, Kobe University Hospital, Saitama Cancer Center Hospital and Nagasaki University Hospital, Japan Recruitment: from January 1989‐December 1990 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Pancreatic cancer: 124 (male/female: 68/57) cases |
|
| Green tea in exposure categories | Exposure assessment: green tea intake Lowest exposure: nondrinkers Intermediate exposure NR Highest exposure: ≥ 5 cups/d |
|
| Notes | Funding: Grant‐ in‐Aid for Cancer Research from the Ministry of Health and Welfare of Japan. Statistical methods: unconditional logistic regression Variables controlled in analysis: sex and age Variables controlled by matching: sex and age |
|
Montague 2012.
| Study characteristics | ||
| Methods | Cohort study in China | |
| Participants | Participants: 63,257 (male/female: 27,959/35,298) participants. Data on 27,293 men with no history of cancer Inclusion criteria: aged 45‐74 years, belonging to the Hokkien or Cantonese dialect group in Singapore, China Parent cohort: Singapore Chinese Health Study (SCHS) Recruitment: from April 1993 to December 1998 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 31 December 2007 Prostate cancer: 298 cases |
|
| Green tea in exposure categories | Exposure assessment: green tea intake Lowest exposure: none Intermediate exposure 1: monthly Intermediate exposure 2: weekly Highest exposure: daily, further divided in 1 cup/d and ≥ 2 cups/d |
|
| Notes | Funding: National Institute of Health grant R01CA144034. Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age, dialect group, interview year, education, BMI, smoking history and black tea intake. Variables controlled by matching: ‐ |
|
Mu 2003.
| Study characteristics | ||
| Methods | PCC in China | |
| Participants | Participants: 628 (male/female: 438/190) cases, 415 (male/female 287/128) controls Inclusion criteria: aged ≥ 20 years, newly diagnosed hospital‐based case‐control (HCC) cases included in the Taixing Tumor Registry and living for at least 10 years in Taixing, China Recruitment: from 1 June‐31 December 2000 for stomach cancer, from 1 January‐30 June 2000 for liver cancer and 2000 for oesophageal cancer In Mu 2005 Participants: 206 (male/female: 137/68) cases and 415 (male/female 287/128) controls Inclusion criteria: aged ≥ 20 years, newly diagnosed HCC cases included in the Taixing Tumor Registry and living for at least 10 years in Taixing, China. Recruitment: from 1 June‐31 December 2000 for stomach cancer In Li 2011b Participants: 204 (male/female: 159/45) cases and 415 (male/female: 287/128) controls Inclusion criteria: aged ≥ 20 years, newly diagnosed HCC cases included in the Taixing Tumor Registry and living for at least 10 years in Taixing, China. Recruitment: from 1 January‐30 June 2000 Data on liver cancer also reported in Mu 2003 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Mu 2003 Stomach cancer: 206 (male/female: 138/68) cases Liver cancer: 204 (male/female: 159/45) cases, HCC Oesophageal cancer: 218 (male/female: 141/77) cases Mu 2005 Stomach cancer: 206 (male/female: 137/68) cases, the majority (> 90%) with adenocarcinoma of the distal stomach Li 2011b Liver cancer: 204 (male/female: 159/45) cases of HCC |
|
| Green tea in exposure categories | Exposure assessment: green tea consumption Lowest exposure: never Intermediate exposure 1: < 125 g/month Intermediate exposure 2: 125‐250 g/month Highest exposure: ≥ 250 g/month Exposure assessment B: green tea intake (only for stomach cancer ‐ Mu 2005) Lowest exposure: never Highest exposure: ever |
|
| Notes |
Mu 2003: Article in Chinese Funding: not declared Statistical methods: unconditional logistic regression Variables controlled in analysis: age, education, smoking, alcohol drinking (for oesophageal cancer, other outcome reported in subsequent reports) Variables controlled by matching: ‐ Mu 2005 Funding: National Institute of Health, National Cancer Institute (ES06718, CA77954, CA09142, CA16042, CA42710, AT00151), UCLA Jonsson Comprehensive Cancer Center, UICC Technology Transfer Fellowship of the Foundation for the Author of National Excellent Doctoral Dissertation of P.R. China (200157) Statistical methods: unconditional logistic regression Variables controlled in analysis: age, sex, education, income, BMI, pack‐years of smoking, alcohol drinking, very hot food eating habit, H. pylori infection, stomach disease history and family history of stomach cancer Variables controlled by matching: ‐ Li 2011b Funding: International Union against Cancer Technology Transfer fellowship awarded to Dr. Li‐Na Mu and by the Foundation for the Author of National Excellent Doctoral Dissertation of PR China (N. 200157) awarded to Dr Lin Cai. The study was also partially supported by the NIH National Institute of Environmental Health Sciences, National Cancer Institute, Department of Health and Human Services, Grants CA09142, ES 011667 as well as the Alper Research Program for Environmental Genomics of the UCLA Jonsson Comprehensive Cancer Center. Statistical methods: unconditional logistic regression Variables controlled in analysis: age, sex, education, income, BMI, family history of cancer, smoking, alcohol drinking and HBSAg Variables controlled by matching: ‐ Mu 2003a, in reports same results as Mu 2003 |
|
Nagano 2001.
| Study characteristics | ||
| Methods | Cohort study in Japan | |
| Participants | Participants: 38,540 (male/female: 14,873/23,667) participants Inclusion criteria: aged 45‐74 years, carried out among atomic‐bomb survivors alive as of 1 September 1979, mean age at beginning of follow‐up of 52.8 years in men and 56.8 years in women Parent cohort: Life Span study cohort Recruitment: from 1979‐1981 Sauvaget 2005 Participants: people from Hiroshima and Kahasaki Inclusion criteria: participants (93,741) present in Hiroshima and Nagasaki at the time of the bombings and city residents on 1 October 1950 and residents (23,580) not present at the time of the bombings but present between 1950 and 1953. Data of 38,576 (male/female: 14,885/23,691) participants included are carried out in participants aged 34‐98 years, respondents to second mail surveys, alive on 1 September 1978. Parental cohort: Radiation Effects Research Fundation's Life Span Study Recruitment: 1979, completed on 1 January 1980 for men and 1 February 1981 for women |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 31 December 1994 Total cancer: 4049 (male/female: 1982/2087) cases Total solid cancer: 3881 (male/female: 1890/1991) cases Oesophageal cancer: 59 (male/female: 46/13) cases (risk not assessed) Stomach cancer: 901 (male/female: 518/383) cases Colon cancer: 432 (male/female: 221/211) cases Rectal cancer: 193 (male/female:100/93) cases Liver cancer: 418 (male/female: 260/158) cases Gallbladder cancer: 122 (male/female: 40/82) cases Pancreatic cancer: 122 (male/female: 43/79) cases Lung cancer: 436 (male/female: 265/171) cases Skin cancer: 89 (male/female: 36/53) cases (risk not assessed) Breast cancer: 281 (male/female: 276/5) cases Cervical cancer: 100 female cases (risk not assessed) Corpus uteri: 53 female cases (risk not assessed) Ovarian cancer: 49 female cases (risk not assessed) Prostate cancer: 92 male cases (risk not assessed) Bladder cancer: 122 (male/female: 88/34) cases Kidney cancer: 76 (male/female: 39/37) cases (risk not assessed) Thyroid cancer: 99 (male/female: 18/81) cases (risk not assessed) Other solid cancers: 237 (male/female: 119/118) cases (risk not assessed) Haematopoietic cancer: 188 (male/female: 92/96) cases Lymphoma: 94 (male/female: 45/ 51) cases (risk not assessed) Multiple myeloma: 40 (male/female: 20/20) cases (risk not assessed) Leukaemia: 52 (male/female: 27/25) cases (risk not assessed) In Sauvaget 2005 Outcome assessment: 31 December 1999 Stomach cancer: 1270 (male/female: 719/551) out of 1280 (male/female: NR) eligible cases, including 27% in the gastric body, 6% in the cardia, 5% in the lesser curve, 2% in the fundus and 27% were not specified. Regarding the histology type, 99% of the cases were classified as carcinoma and 1% as sarcoma (10 cases who were excluded from subsequent analyses) |
|
| Green tea in exposure categories |
Nagano 2001 Exposure assessment: green tea intake Lowest exposure: 0‐1 times/d Intermediate exposure: 2‐4 times/d Highest exposure: ≥ 5 times/d Sauvaget 2005 Exposure assessment: hot green tea Lowest exposure: < 2 times/d Intermediate exposure: 2‐4 times/d Highest exposure: ≥ 5 times/d |
|
| Notes |
Nagano 2001 Funding: RERF foundation, Hiroshima and Nagasaki, Japan equally funded by the Japanese Ministry of Health and Welfare and the US Department of Energy through the National Academy of Sciences Statistical methods: Poisson regression Variables controlled in analysis: city, age, sex, radiation dose, smoking status, alcohol drinking history, BMI, education level and calendar time Variables controlled by matching: ‐ Sauvaget 2005 Funding: Research performed at the Radiation Effects Research Foundation (RERF), Hiroshima and Nagasaki, Japan. RERF is funded equally by the Japanese Ministry of Health and Welfare and the US Department of Energy (DOE). RERF Research Protocols RP # 18–61 and 14–78 Statistical methods: Poisson regression Variables controlled in analysis: sex, sex‐specific age, city, radiation dose, sex‐specific smoking habits and education level |
|
Naganuma 2009.
| Study characteristics | ||
| Methods | Cohort study in Japan | |
| Participants | Participants: 52,029 participants. Final data on 41,761 (male/female: 19,749/22,012) participants included Inclusion criteria: aged 40‐79 years, living in 14 municipalities of Miyagi Prefecture in the catchment area of the Ohsaki Public Health Centre, Miyagi, Japan Parent cohort: Ohsaki Cohort Study Recruitment: from October‐December 1994 Data on the same cohort also reported in Kuriyama 2006 and Ui 2009. |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 31 December 2003 Haematopoietic cancer: 157 (male/female: 88/69) cases Lymphoid cancer: 119 (male/female: 66/53) cases Myeloid cancer: 36 (male/female: 20/16) cases |
|
| Green tea in exposure categories | Exposure assessment: green tea consumption Lowest exposure: < 1 cup/d Intermediate exposure 1: 1‐2 cups/d Intermediate exposure 2: 3‐4 cups/d Highest exposure: ≥ 5 cups/d |
|
| Notes | Funding: Health Sciences Research Grant for Health Services, Ministry of Health, Labour and Welfare of Japan (H19‐Seisaku‐Ippan‐026, H20‐Junkankitou(Seisyu)‐Ippan‐013, H21‐3jigan‐Ippan‐003). Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age, sex, educational level, cigarette smoking, alcohol drinking, fish consumption and soybean products consumption Variables controlled by matching: ‐ |
|
Nagle 2010.
| Study characteristics | ||
| Methods | PCC in Australia | |
| Participants | Participants: 1459 cases and 1462 controls with complete data on diet, but 24 and 113 omitted due to > 10% of missing items and with implausible total energy intake respectively. Final population of 1368 cases and 1416 controls (all women). Inclusion criteria: women aged 18‐79 years, from the Australian Ovarian Cancer Study diagnosed with epithelial ovarian cancer Recruitment: from January 2002‐June 2005 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Ovarian cancer: 1368 cases out of 1459 eligible cases of epithelial ovarian cancer |
|
| Green tea in exposure categories | Exposure assessment: green tea intake Lowest exposure: never Intermediate exposure 1: < 1 time/week Intermediate exposure 2: < 1 time/d Intermediate exposure 3: 1 time/d Intermediate exposure 4: 2‐3 times/d Highest exposure: ≥ 4 times/d |
|
| Notes | Funding: US Army Medical Research and Materiel Command under award DAMD17‐01‐1‐0729, the Cancer Council Tasmania and Cancer Foundation of Western Australia; the Australian Cancer Study was funded by the National Health and Medical Research Council of Australia (199600). Statistical methods: unconditional logistic regression Variables controlled in analysis: age, education, parity, hormonal contraceptive use, smoking status, fruit consumption, vegetable consumption, coffee consumption and other types of tea Variables controlled by matching: age (± 5 years) and state of residence |
|
Nakachi 2000.
| Study characteristics | ||
| Methods | Cohort study in Japan | |
| Participants | Participants: 8552 (male/female: NR) participants
Inclusion criteria: aged > 40 years, residents in a town in Saitama Prefecture, Japan Recruitment: from 1986 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 1997 Total cancer incidence: 488 (male/female: NR) cases, including stomach (N = 140), lung (N = 69), colorectal (N = 60) and liver (N = 35) cancer, not individually evaluated |
|
| Green tea in exposure categories | Exposure assessment: green tea drinking Lowest exposure: ≤ 3 cups/d Intermediate exposure: 4‐9 cups/d Highest exposure: ≥ 10 cups/d |
|
| Notes | Funding: Grants‐in‐Aid for Cancer Research from the Ministry of Education, Science, Sports and Culture of Japan, from the Ministry of Health and Welfare of Japan and from the Ministry of Health and Welfare for a 2nd‐Term Comprehensive 10‐Year Strategy for Cancer Control and by a grant from the Smoking Research Foundation of Japan Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age, cigarette smoking, alcohol consumption, intake of green and yellow vegetables and intake of rice Variables controlled by matching: ‐ Imai 1997 reports same results as Nakachi 2000 |
|
Nakamura 2011.
| Study characteristics | ||
| Methods | Cohort study in Japan | |
| Participants | Participants: 30,826 (male/female: 14,241/16,585) participants
Inclusion criteria: aged ≥ 35 years, non‐hospitalised inhabitants in Takayama, Gifu Prefecture, Japan Recruitment: from September 1992 Data on the same cohort also reported in Oba 2006. |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 31 December 1999 Pancreatic cancer mortality: 52 (male/female: 33/19) cases |
|
| Green tea in exposure categories | Exposure assessment: green tea drinking Lowest exposure: never Intermediate exposure: ≤ 1 cup/month to ≤ 4–6 cups/week Highest exposure: ≥ 1 cup/d |
|
| Notes | Funding: Grant from the Ministry of Education, Culture, Sports, Science and Technology, Japan. It was also funded by Grant‐in‐Aid for Cancer Research (21Shi‐11‐1) from the Ministry of Health, Labour and Welfare, Japan. Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age, smoking, BMI and history of diabetes mellitus Variables controlled by matching: ‐ |
|
Nechuta 2012.
| Study characteristics | ||
| Methods | Cohort study in China | |
| Participants | Participants: 74,941 Chinese women. Final data on 67,230 women witch complete and reliable data on dietary information and reporting consumption of green tea alone or in combination with other types of tea. In Yang 2007: final population of 69,710 participants Inclusion criteria: women aged 40‐70 years, no history of cancer at baseline recruited in seven urban areas in Shanghai, China Parent cohort: Shangai Women's Health Study Recruitment: from December 1996‐May 2000 Data on the same cohort also reported in Dai 2010 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Nechuta 2012 Outcome assessment: 31 December 2005 Digestive system cancer: 1239 cases Stomach cancer: 287 cases Stomach and oesophageal cancer: 314 cases Colorectal cancer: 579 cases Colon cancer: 355 cases Rectal cancer: 224 cases Liver cancer: 133 cases Pancreatic cancer: 131 cases Gallbladder and bile duct cancer: 82 cases Yang 2007 Outcome assessment: 2004 Colorectal cancer: 256 cases (all female) |
|
| Green tea in exposure categories | Exposure assessment: green tea consumption Lowest exposure: never drinking Highest exposure: ≥ 3 times/week for > 6 months In Yang 2007 Exposure assessment: intake of green tea Lowest exposure: nondrinker Highest exposure: drinker, further divided in amount of green tea consumption: Highest exposure A: 1‐4 g/d Highest exposure B: ≥ 5 g/d |
|
| Notes |
Nechuta 2012 Funding: National Cancer Institute (R37 CA70867) Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age, marital status, education, occupation, BMI, exercise, fruit and vegetable intake, meat intake, diabetes and family history of digestive system cancer Variables controlled by matching: ‐ Yang 2007 Funding: USPHS grant R01CA70867 and National Institue of Health intramural programme, Division of Cancer Epidemiology and Genetics (N02 CP1101066) Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age, education, household income, cigarette smoking, alcohol drinking, physical activity, BMI, menopausal status, nonsteroidal anti‐inflammatory drug use, vitamin supplement use, prior histories of colorectal polyps and chronic ulcerative colitis, family history of colorectal cancer and intakes of total energy, vegetables, fruits and red meat Variables controlled by matching: ‐ |
|
Oba 2006.
| Study characteristics | ||
| Methods | Cohort study in Japan | |
| Participants | Participants: 30,826 (male/female: 14,241/16,585) participants
Inclusion criteria: aged ≥ 35 years, non‐hospitalised inhabitants in Takayama, Gifu Prefecture, Japan Recruitment: from September 1992 Data on the same cohort also reported in Nakamura 2011 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 31 December 2000 Colon cancer: 213 (male/female: 111/102) cases |
|
| Green tea in exposure categories | Exposure assessment: green tea drinking Lowest exposure: never to < 1 cup/month Intermediate exposure: from 1 cup/month to < 1 cup/d Highest exposure: ≥ 1 cup/d |
|
| Notes | Funding: grants from the Ministry of Education, Culture, Science and Technology, the Ministry of Health, Labour and Welfare and the Japan Coffee Association Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age, height, BMI, total pack‐years of cigarette smoking, alcohol intake, physical activity, black tea intake and coffee intake Variables controlled by matching: ‐ |
|
Odegaard 2015.
| Study characteristics | ||
| Methods | Cohort study in China | |
| Participants | Participants: 63,257 (male/female: 27,959/35,298) participants. Data on 52,584, free from diabetes, cardiovascular disease and cancer at baseline Inclusion criteria: aged 45‐74 years, belonging to the Hokkien or Cantonese dialect group in Singapore, China Parent cohort: Singapore Chinese Health Study (SCHS) Recruitment: from April 1993‐December 1998 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 31 December 2011 Total cancer mortality: 4092 cases (deaths) |
|
| Green tea in exposure categories | Exposure assessment: green tea intake Lowest exposure: none Intermediate exposure 1: any to < 1 cup/d Intermediate exposure 2: 1 cup/d Highest exposure: ≥ 2 cups/d |
|
| Notes | Funding: NIH grants NCI RO1 CA055069, R35 CA053890, R01 CA080205, R01 CA098497, R01 CA144034 and R01 DK080720. Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age, sex, dialect, education, year of interview, smoking, moderate and vigorous activity, sleep, BMI, nonbeverage vegetable‐fruit‐soy–rich dietary pattern score, energy intake, intake of coffee, black tea, alcohol, soft drinks and juice Variables controlled by matching: ‐ |
|
Ogawa 2016.
| Study characteristics | ||
| Methods | Cohort study in Japan | |
| Participants | Participants: 140,420 (male/female: 68,722/71,698), with 61,595 from cohort 1 and 78,825 from cohort 2 and 106,324 (male/female: 50,438/55,886) participants with complete data in the present study Inclusion criteria: aged 40‐59 years, from 5 Public Health Centre areas (Iwate, Akita, Nagano, Okinawa and Tokyo) for cohort 1, and aged 40‐69 years for cohort 2 from 6 Public Health Centre areas (Ibaraki, Niigata, Kochi, Nagasaki, Okinawa and Osaka), respondent at 5‐year follow‐up Parent cohorts Cohort 1: Japan Public Health Centre‐based Prospective Study (JPHC)‐I cohort 2: Japan Public Health Centre‐based Prospective Study (JPHC)‐II Recruitment: from 1990 for cohort 1 and 1993/1994 for cohort 2 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 31 December 2012 Brain cancer: 155 (male/female: 70/85) cases out of 157 eligible, including glioma (N = 60), meningioma (N = 51), lymphoma (N = 9), schwannoma (N = 3), pituitary adenoma (N = 2) and unspecified brain tumour (N = 32) |
|
| Green tea in exposure categories | Exposure assessment: intake of green tea Lowest exposure: ≤ 4 d/week Intermediate exposure: 1‐2 cups/d Highest exposure: ≥ 3 cups/d |
|
| Notes | Funding: National Cancer Center Research and Development Fund (23‐A‐31[toku] and 26‐A‐2) (since 2011) and a Grant‐in‐Aid for Cancer Research from the Ministry of Health, Labour and Welfare of Japan (from 1989‐2010) Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age, sex, BMI, pack years of cigarettes, alcohol intake, coffee, past history of allergy and past history of diabetes mellitus Variables controlled by matching: ‐ |
|
Oze 2014.
| Study characteristics | ||
| Methods | PCC in Japan | |
| Participants | Participants: 961 (male/female: 775/186) cases and 2883 (male/female: 2325/558) controls Inclusion criteria: aged 20‐79 years, from the Aichi Cancer Center Hospital (ACCH) in Aichi Prefecture, Japan Recruitment: from January 2001‐December 2005 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Upper aerodigestive tract cancer: 922 (male/female: NR) cases of the 961 (male/female: 775/186) eligible cases, including 420 (male/female: NR) cases of oesophageal cancer and 502 (male/female: NR) cases of oral, pharyngeal and laryngeal cancer |
|
| Green tea in exposure categories | Exposure assessment: green tea drinking Lowest exposure: < daily Intermediate exposure 1: 1 cup/d Intermediate exposure 2: 2 cups/d Highest exposure: ≥ 3 cups/d |
|
| Notes | Funding: Ministry of Education, Science, Sports, Culture and Technology of Japan (Grants‐in‐Aid for Scientific Research); Ministry of Health, Labour and Welfare of Japan [Third‐Term Comprehensive 10‐Year Strategy for Cancer Control and Health and Labor Sciences Research Grant for Clinical Cancer Research (H24‐Gannorinshou‐Ippan‐006)]; National Cancer Center Research and Development Fund (24‐ A‐3); Foundation for Promotion of Cancer Research in Japan and Japan Society for the promotion of Science A3 Foresight Program Statistical methods: conditional logistic regression Variables controlled in analysis: age, sex, coffee and green tea intake, alcohol consumption, cumulative smoking, fruit and vegetable intake, BMI, occupation and frequency of rice intake Variables controlled by matching: sex and age (10‐year categories) |
|
Peng 2013.
| Study characteristics | ||
| Methods | PCC in China | |
| Participants | Participants: 672 (male/female: 446/226) cases and 672 (male/female: 446/226) controls of unrelated ethnic Han Chinese from Fuzhou in Fujian Province and surrounding regions Inclusion criteria: histopathologically confirmed colorectal cancer cases from 3 hospitals (the Union Hospital of Medical University, the First Affiliated Hospital of Fujian Medical University and the National Fujian Hospital) in Fuzhou, in the Fujian Province of China. Controls recruited from local residents who underwent a routine health check and were free from any known major diseases Recruitment: from June 2006‐May 2010 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Colorectal cancer: 672 (male/female: 446/226) cases |
|
| Green tea in exposure categories | Exposure assessment: green tea drinking Lowest exposure: no Highest exposure: yes |
|
| Notes | Funding: National Natural Science Foundation of China (N. 81001279), the Fujian Science and Technology Innovation Foundation for Young Scientists (N. 2010J05067), the Key Program of Scientific Research of Fujian Medical University (N. 09ZD004) and the Foundation of Fujian Educational Committee (N. JA10138 and JA11106). Statistical methods: conditional logistic regression Variables controlled in analysis: age, sex, education, income, marriage, job, a family history of cancer in first‐degree relatives and intake of fruits, vegetables and meat, smoking status and alcohol drinking Variables controlled by matching: sex and age (± 5 years), ethnicity and area of residence |
|
Peng 2015.
| Study characteristics | ||
| Methods | PCC in China | |
| Participants | Participants: 285 (male/female: 168/117) cases and 570 (male/female: 336/234) controls of unrelated ethnic Han Chinese from AnXi or the surrounding regions Inclusion criteria: histologically confirmed oesophageal squamous cell carcinoma, at AnXi Hospital in the Fujian Province of China Recruitment: from June 2010‐May 2011 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Oesophageal cancer: 285 (male/female: 168/117) cases of oesophageal squamous cell carcinoma |
|
| Green tea in exposure categories | Exposure assessment: green tea drinking Lowest exposure: no Highest exposure: yes, defined as drinking at least 1 cup of green tea/week for > 6 months |
|
| Notes | Funding: grants from the Program for Outstanding Young Talents of Scientific Research in University of Fujian Province, China (No. JA11106), the National Natural Science Foundation of China (No. 81473047), the Key Program of Scientific Research of Fujian Medical University (09ZD004) and the Foundation of Fujian Province Key Laboratory of Environment and Health (201405) Statistical methods: conditional logistic regression Variables controlled in analysis: age, sex, education, income, marital status, alcohol drinking, smoking, pickled vegetables, fresh vegetables and fruits, meat, family history of oesophageal squamous cell carcinoma, history of reflux oesophagitis and hot beverage/food intake Variables controlled by matching: sex and age (± 5 years), ethnicity and area of residence |
|
Roshdy 2013.
| Study characteristics | ||
| Methods | RCT, parallel, double‐blind in Egypt | |
| Participants | Participants: 39 Egyptian women, 22 in the intervention group and 17 in the control group Inclusion criteria: age ≥18 years, women in pre‐menopause with follicle‐stimulating hormone level < 10 mIU/L, had reported at least moderately severe leiomyoma‐related symptoms (a score of ≥ 25 on the UF quality‐of‐life symptom severity subscale), had a total uterine volume of ≥ 160 mL by vaginal and abdominal ultrasound and at least 1 UF/leiomyoma that was ≥ 2 cm3, not pregnant or breastfeeding, with untreated abnormal pap smear, with no major morbidity of severe anaemia, elevated liver enzymes > 1.5 times the upper limit of normal, or active substance abuse and no use of such medication (oral or systemic corticosteroids, hormones i.e. oestrogen, progestin, oral contraceptives, herbal or botanical supplements with possible hormonal or GTE effects, or GnRH analogues or Depo‐Provera) in the previous 6 months Recruitment: from November 2010‐August 2011 |
|
| Interventions | Treatment group: 2 capsules (400 mg each)/d with 95% polyphenols and 45% GTE = total 800 mg/d Control group: placebo (brown rice) Duration: 4 months |
|
| Outcomes | Primary outcome Mean change in uterine leiomyoma burden Secondary outcomes Health‐related QoL assessed with 2 different questionnaires. Scale 1: the fibroid‐specific symptom severity (SS) scale ranges from 5‐40, where high values are indicative of greater symptom severity. Scale 2: the HRQoL questionnaire, which measures perceived impact of leiomyoma on activities of daily living, general concern and worry, energy, mood, sense of self‐control, self‐consciousness and sexual functioning of the participants. The scale ranges from 29‐145 where higher scores indicate better QoL. Safety monitoring: monthly haemoglobin levels, liver‐ and kidney‐function tests and pregnancy testing |
|
| Green tea in exposure categories | N/A | |
| Notes | Funding: Grant support: grant 1 ‐ R01 HD04 228‐01 from the National Institute of Child Health and Human Development, National Institutes of Health; RCMI grant 2 ‐ G12 RR003032 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The study pharmacist at Sohag Faculty of Medicine carried out the randomization process (by sequential digital assignment coding) and dispensed green tea extract or placebo capsules to participants, based on the assignment code randomly picked by each participant prior to treatment." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "based on the assignment code randomly picked by each participant prior to treatment" Comment: probably done |
| Blinding of participants and personnel (performance bias) Uterine leiomyoma burden | Low risk | Quote: "The study staff and the participants were blinded to the treatment assignment throughout the whole study. The placebo capsules were identical to the EGCG capsules in appearance and weight" Comment: probably done |
| Blinding of outcome assessment (detection bias) Uterine leiomyoma burden | Low risk | Quote: "The study staff and the participants were blinded to the treatment assignment throughout the whole study. The placebo capsules were identical to the EGCG capsules in appearance and weight" Comment: probably done |
| Incomplete outcome data (attrition bias) Uterine leiomyoma burden | Unclear risk | Comment: number of participants included in analysis not stated |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (ClinicalTrials.gov identifier (NCT number): NCT01311869) and the published reports include all expected outcomes |
| Other bias | Unclear risk | High rate of dropout in the placebo group (35%). In the protocol, treatment duration was reported to be 6 months. In the study, treatment duration was 4 months. |
Ruan 2010.
| Study characteristics | ||
| Methods | HCC in China | |
| Participants | Participants: 1387 (male/female: 1025/362) cases and 1459 (male/female: 1038/421) controls Inclusion criteria: aged < 80 years, histologically confirmed cases with no previous diagnosis of or treatment for nasopharyngeal cancer, residents in Guangdong province and referring at the Sun Yat‐sen University Cancer Center in Guangzhou, Guangdong province, southern China Recruitment: from October 2005‐October 2007 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Nasopharyngeal cancer: 1355 (male/female: NR) cases out of 1387 (male/female: 1025/362) eligible cases (green tea drinkers and non tea drinkers) |
|
| Green tea in exposure categories | Exposure assessment: intake of green tea Lowest exposure: nondrinker Highest exposure: drinkers (≥ 1 cup/week for ≥ 6 months) |
|
| Notes | Funding: National Natural Science Foundation of China (grant nos. 30671798 and 30471487), the National Science and Technology Support Program of China (N. 2006BAI02A11) and the National Major Basic Research Program of China (863 Program) (N. 2006AA02A404). Statistical methods: unconditional logistic regression Variables controlled in analysis: sex, age, education, dialect, household type and family history of nasopharyngeal cancer Variables controlled by matching: sex and age (± 5 years), education, dialect and household type (rural or urban) |
|
Saito 2015.
| Study characteristics | ||
| Methods | Cohort study in Japan | |
| Participants | Participants: 140,420 (male/female: 68,722/71,698), with 61,595 from cohort 1 and 78,825 from cohort 2 and 90,914 (male/female: 42,836/48,078) participants with complete data Inclusion criteria: aged 40‐59 years, from 5 Public Health Centre areas (Iwate, Akita, Nagano, Okinawa and Tokyo) for cohort 1, and aged 40‐69 years for cohort 2 from 6 Public Health Centre areas (Ibaraki, Niigata, Kochi, Nagasaki, Okinawa and Osaka), respondent at 5‐year follow‐up Parent cohorts Cohort 1: Japan Public Health Centre‐based Prospective Study (JPHC)‐I cohort 2: Japan Public Health Centre‐based Prospective Study (JPHC)‐II Recruitment: from 1990 for cohort 1 and 1993/1994 for cohort 2 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 31 December 2011 Total cancer mortality: 5327 (male/female: 3468/1859) cases |
|
| Green tea in exposure categories | Exposure assessment: green tea drinking Lowest exposure: < 1 cup/d Intermediate exposure 1: 1‐2 cups/d Intermediate exposure 2: 3‐4 cups/d Highest exposure: ≥ 5 cups/d |
|
| Notes | Funding: by the National Cancer Center Research and Development Fund (23‐A‐31[toku] and 26‐A‐2; since 2011), by a Grant‐in‐Aid for Cancer Research from the Ministry of Health, Labour and Welfare of Japan (from 1989 to 2010) and by Health and Labour Sciences Research Expenses for Commission (Comprehensive Research on Life‐Style Related Diseases including Cardiovascular Diseases and Diabetes Mellitus H26‐005) Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age, Public Health Centre area, BMI, history of hypertension, history of diabetes, leisure‐time sports or physical exercise, intake of coffee, Chinese tea, black tea and soda or juice, energy intake and intakes of fruits, vegetables, fish, meat, dairy products, rice and miso soup and job status (stratified by sex) Variables controlled by matching: ‐ |
|
Setiawan 2001.
| Study characteristics | ||
| Methods | PCC in China | |
| Participants | Participants: 133 (male/female: 93/40) cases and 433 (male/female: 214/219) controls Inclusion criteria: randomly selected patients, newly‐diagnosed at Yangzhong Central Hospital Endoscopy Unit, who had lived for at least 1 year in Yangzhong, China Recruitment: from 1 January 1995‐30 June 1995 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Stomach cancer: 133 (male/female: 93/40) cases |
|
| Green tea in exposure categories | Exposure assessment A: green tea intake Lowest exposure: no Highest exposure: yes Exposure assessment B: green tea consumption Lowest exposure: nondrinkers Intermediate exposure: 1‐21 cups/week Highest exposure: ≥ 21 cups/week |
|
| Notes | Funding: National Institute of Health National Cancer Institute, Department of Health and Human Services (CA77954, CA09142 and CA16042) and grants from the University of California‐Los Angeles Jonsson Comprehensive Cancer Center Foundation and the Weissman Fund. Statistical methods: unconditional logistic regression Variables controlled in analysis: age, sex, education, BMI, pack‐years of smoking and alcohol drinking. Variables controlled by matching: ‐ |
|
Severson 1989.
| Study characteristics | ||
| Methods | Cohort study in USA | |
| Participants | Participants: 7821 male participants out of 8006 recruited Inclusion criteria: American men of Japanese ancestry, born from 1990‐1919 and residing on the Hawaiian island of Oahu, Hawaii, USA Parent cohort: Honolulu Hearth Program Recruitment: from 1965‐1968 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 30 September 1986 Prostate cancer: 174 cases (all men) |
|
| Green tea in exposure categories | Exposure assessment: green tea drinking habit Lowest exposure: never Highest exposure: ever |
|
| Notes | Funding: USPHS Grant ROI CA 33644, awarded by the National Cancer Institute, Mil. Bethesda, MD Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age at examination Variables controlled by matching: ‐ |
|
Shimazu 2008.
| Study characteristics | ||
| Methods | Cohort study in Japan | |
| Participants | Participants: 53,724 women from the Japan Public Health Centre study Inclusion criteria: aged 40‐59 years, from 5 Public Health Centre areas (Iwate, Akita, Nagano, Okinawa and Tokyo) for cohort 1, and aged 40‐69 years for cohort 2 from 6 Public Health Centre areas (Ibaraki, Niigata, Kochi, Nagasaki, Okinawa and Osaka), respondent at 5‐year follow‐up Parent cohorts Cohort 1: Japan Public Health Centre‐based Prospective Study (JPHC)‐I Cohort 2: Japan Public Health Centre‐based Prospective Study (JPHC)‐II Recruitment: 1990 (cohort 1) and 1993 (cohort 2) |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 31 December 2005 Endometrial cancer: 117 cases |
|
| Green tea in exposure categories | Exposure assessment: green tea drinking habit Lowest exposure: ≤ 4 cups/week Intermediate exposure 1: 1‐2 cups/d Intermediate exposure 2: 3‐4 cups/d Highest exposure: ≥ 5 cups/d |
|
| Notes | Funding: not declared Statistical methods: Cox proportional hazard regression model Variables controlled in analysis: age, study area, BMI, menopausal status, age at menopause for menopausal women, parity, use of exogenous female hormones, smoking status, green vegetable consumption, beef consumption and pork consumption Variables controlled by matching: ‐ |
|
Shrubsole 2009.
| Study characteristics | ||
| Methods | PCC in China | |
| Participants | Participants: 3371 cases and 3380 controls (all female) Inclusion criteria: Shanghai Breast Cancer Study, with newly diagnosed cases aged 25–70 years, resident of urban Shanghai and with no previous history of any cancer Recruitment: from August 1996‐March 1998 (phase 1) and from April 2002 to February 2005 (phase 2) |
|
| Interventions | N/A | |
| Outcomes | Number of cases Breast cancer: 3371 cases |
|
| Green tea in exposure categories | Exposure assessment A: green tea intake Lowest exposure: never Highest exposure: ever Exposure assessment B: green tea consumption in tea leaves/month Lowest exposure: never regular Intermediate exposure 1: ≤ 50 g/month Intermediate exposure 2: 50‐< 100 g/month Intermediate exposure 3: 100‐< 225 g/month Highest exposure: ≥ 225 g/month |
|
| Notes | Funding: National Institute of Health National Cancer Institute (R01CA64277) Statistical methods: unconditional logistic regression Variables controlled in analysis: age, study phase, education, family history of breast cancer, personal history of fibroadenoma, age at menarche, parity, age at first live birth, age at menopause, physical activity, waist:hip ratio, total energy intake, total fruit and vegetable intake and fat intake Variables controlled by matching: age (± 5 years) |
|
Sinicrope 2017.
| Study characteristics | ||
| Methods | RCT, parallel, double‐blind in USA | |
| Participants | 39 (male/female: 14/25) participants: participants with prior advanced adenoma (N = 37), or colon cancer (N = 2) Inclusion criteria: at least 5 rectal ACF at baseline. 19 (male/female: 6/13) participants in the treatment group and 20 (male/female: 8/12) in the control group |
|
| Interventions | Group A: GTE (Polyphenon E, 2 capsules of 200 mg twice/d = 800 mg) containing 400 mg of EGCG Group B: placebo Duration: 6 months |
|
| Outcomes | Change in rectal ACF Safety data |
|
| Green tea in exposure categories | N/A | |
| Notes | ClinicalTrial.gov identifier: NCT01606124 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described as randomised in the abstract and in ClinicalTrials.gov |
| Allocation concealment (selection bias) | Unclear risk | No information about concealment process |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Probably done as it is described as double‐blind and placebo capsules were implemented |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Probably done as it is described as double‐blind and placebo capsules were implemented, but no explicit statement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All results reported for all included participants |
| Selective reporting (reporting bias) | Low risk | Results for the trial reported on (ClinicalTrials.gov identifier (NCT number): NCT01606124) |
| Other bias | Low risk | No withdrawals reported |
Song 2008.
| Study characteristics | ||
| Methods | PCC in USA | |
| Participants | Participants: 781 cases and 1263 controls (all women) Inclusion criteria: aged 35‐74 years, diagnosed with a primary invasive or borderline epithelial ovarian cancer in English‐speaking women who had residential telephones at the time of cancer diagnosis and were residents of a 13‐county area of western Washington State, USA Recruitment: from 2002‐2005 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Ovarian cancer: 781 cases |
|
| Green tea in exposure categories | Exposure assessment: green tea intake Lowest exposure: nondrinker Intermediate exposure: < 1 cup/d Highest exposure: ≥ 2 cups/d |
|
| Notes | Funding: National Institute of Health grant RO1 CA87538 Statistical methods: unconditional logistic regression Variables controlled in analysis: age, county, year of diagnosis/reference date, race/ethnicity, number of full‐term pregnancies, duration of hormonal contraception, education, BMI, smoking, tubal ligation/hysterectomy and family history of breast/ovarian cancer Variables controlled by matching: age, county of residence and year of diagnosis/reference date |
|
Sonoda 2004.
| Study characteristics | ||
| Methods | HCC in Japan | |
| Participants | Participants: 140 cases and 140 controls (all male) Inclusion criteria: aged 59‐73 years, cases with confirmed histological diagnosis of prostatic adenocarcinoma from the Department of Urology of Tsukuba University Hospital in Ibaraki and from the Department of Urology of Sapporo Medical University Hospital in Hokkaido, Japan. Controls were recruited from Department of Oral Surgery, Ophthalmology, or Dermatology of the same hospitals Recruitment: from January 1996‐September 2002 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Prostate cancer: 140 cases, including 2 cases of stage I, 86 cases of stage II, 36 cases of stage III and 16 cases of stage IV of adenocarcinoma |
|
| Green tea in exposure categories | Exposure assessment: green tea intake Lowest exposure: ≤ 1 cup/d Intermediate exposure 1: 2‐4 cups/d Intermediate exposure 2: 5‐9 cups/d Highest exposure: ≥ 10 cups/d |
|
| Notes | Funding: not declared Statistical methods: conditional logistic regression Variables controlled in analysis: age, cigarette smoking and energy intake Variables controlled by matching: age (± 5 years) |
|
Sun 2007.
| Study characteristics | ||
| Methods | Cohort study in China | |
| Participants | Participants: 63,257 (male/female: 27,959/35,298) participants. Data on 61,320 (male/female: NR) with no history of invasive cancer other than non‐melanoma skin cancer. Inclusion criteria: aged 45‐74 years, belonging to the Hokkien or Cantonese dialect group in Singapore, China Parent cohort: Singapore Chinese Health Study (SCHS) Recruitment: from April 1993‐December 1998 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 31 December 2004 Colorectal cancer: 845 (male/female: 470/375) cases, mainly adenocarcinoma Colon cancer: 516 (male/female: 241/275) cases Rectal cancer: 329 (male/female: 204/125) cases |
|
| Green tea in exposure categories | Exposure assessment A: green tea intake Lowest exposure: nondrinker Highest exposure: drinker Exposure assessment B: green tea intake Lowest exposure: nondrinker Intermediate exposure 1: monthly drinker Intermediate exposure 2: weekly drinker Highest exposure: daily drinker |
|
| Notes | Funding: National Cancer Institute, Bethesda, MD (R01 CA55069, R35 CA53890, R01 CA80205 and R01 CA98497) Statistical methods: Cox proportional hazard regression Variables controlled in analysis: sex, age at baseline interview, year of interview, dialect, education, family history of colorectal cancer, history of diabetes, cigarette smoking, alcohol drinking, coffee drinking, weekly moderate physical activity, BMI, total energy, total fat, dietary fibre, calcium, vitamin C and black tea intake Variables controlled by matching: ‐ |
|
Suzuki 2004.
| Study characteristics | ||
| Methods | Cohort study in Japan | |
| Participants | Participants: 31,345 (male/female: 13,992/17,353) from cohort 1 and 47,605 (male/female: 22,836/24,769) from cohort 2. Final included participants were 35,004 women, 14,409 from cohort 1 and 20,595 from cohort 2. Inclusion criteria: aged ≥ 40 years, residents in 3 municipalities of the Miyagi Prefecture, Northern Japan (cohort 1); aged 40‐64 years, (cohort 2) Parent cohorts Cohort 1: Three Prefecture Study ‐ Miyagi portion (3‐pref MIYAGI) Cohort 2: Miyagi Cohort Study (MIYAGI) Recruitment: from 1984 for cohort 1 and from 1990 for cohort 2 Same population and outcome of Tsubono 2001 in: Inoue 2009a for cohort 1 Characteristics of cohort 2 reported in Fukao 1995 in: Suzuki 2004. Same population also reported in Koizumi 2003 in: Inoue 2009a and Suzuki 2005 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: not clearly stated, 9 and 7 years of follow‐up for cohort 1 and cohort 2 respectively (probably 1992 and 1997) Breast cancer: 222 cases (all women), 103 in cohort 1 and 119 in cohort 2 |
|
| Green tea in exposure categories | Exposure assessment: green tea intake Lowest exposure: < 1 cup/d Intermediate exposure 1: 1‐2 cups/d Intermediate exposure 2: 3‐4 cups/d Highest exposure: ≥ 5 cups/d |
|
| Notes | Funding: not declared Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age, types of health insurance, age at menarche, menopausal status, age at first birth, parity, mother’s history of breast cancer, smoking, alcohol drinking, BMI, and consumption frequencies of black tea and coffee. Variables controlled by matching: ‐ |
|
Suzuki 2005.
| Study characteristics | ||
| Methods | Cohort study in Japan | |
| Participants | Participants: 31,345 (male/female: 13,992/17,353) from cohort 1 and 47,605 (male/female: 22,836/24,769) from cohort 2. Final included participants were 26,311 (male/female: NR) from cohort 1 and 39,604 (male/female: NR) from cohort 2 Inclusion criteria: aged ≥ 40 years, residents in 3 municipalities of the Miyagi Prefecture, Northern Japan (cohort 1); aged 40‐64 years (cohort 2) Parent cohorts Cohort 1: Three Prefecture Study ‐ Miyagi portion (3‐pref MIYAGI) Cohort 2: Miyagi Cohort Study (MIYAGI) Recruitment: from 1984 for cohort 1 and from 1990 for cohort 2 Same population and outcome of Tsubono 2001 in: Inoue 2009a for cohort 1 Characteristics of cohort 2 reported in Fukao 1995 in: Suzuki 2004. Same population also reported in Koizumi 2003 in:Koizumi 2003Inoue 2009a and Suzuki 2004 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 31 December 1992 for cohort 1 and 31 March 1997 for cohort 2 Colon cancer: 305 (male/female: 185/120) cases, 158 (male/female: NR) in cohort 1 and 147 (male/female: NR) in cohort 2 Rectal cancer: 211 (male/female: 119/92) cases 111 (male/female: NR) in cohort 1 and 100 (male/female: NR) in cohort 2 |
|
| Green tea in exposure categories | Exposure assessment: green tea intake Lowest exposure: < 1 cup/d Intermediate exposure 1: 1‐2 cups/d Intermediate exposure 2: 3‐4 cups/d Highest exposure: ≥ 5 cups/d |
|
| Notes | Funding: not declared Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age, family history of colorectal cancer, cigarette smoking, alcohol consumption, BMI and consumption of black tea and coffee Variables controlled by matching: ‐ Subsequent report on stomach cancer in Inoue 2009a, from which we used data in the analysis. |
|
Suzuki 2009.
| Study characteristics | ||
| Methods | Cohort study in Japan | |
| Participants | Participants: 13,636 (male/female: 6,916/6,720) eligible participants and 12,251 (male/female: 6231/6020) participants included in the present analysis Inclusion criteria: aged 65‐84 years, residents from 74 municipalities in Shizuoka, Japan, with reported information on green tea intake. Parent cohort: Prospective Shizuoka Elderly Cohort Recruitment: from December 1999 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: March 2006 Total cancer mortality: 400 (male/female: 304/96) cases (deaths) Stomach cancer mortality: 68 (male/female: NR) cases (deaths) Lung cancer mortality: 88 (male/female: NR) cases (deaths) Colorectal cancer mortality: 43 (male/female: NR) cases (deaths), including 28 colon cancer deaths and 15 rectal cancer deaths |
|
| Green tea in exposure categories | Exposure assessment: green tea intake Lowest exposure: < 1 cup/d Intermediate exposure 1: 1‐3 cups/d Intermediate exposure 2: 4‐6 cups/d Highest exposure: ≥ 7 cups/d |
|
| Notes | Funding: grant from Health and Labour Sciences Research Grants, Comprehensive Research on Aging and Health Statistical methods: Cox proportional hazard regression Variables controlled in analysis: smoking status, alcohol consumption, BMI and the frequency of physical activity Variables controlled by matching: ‐ |
|
Tajima 1985.
| Study characteristics | ||
| Methods | HCC in Japan | |
| Participants | Participants: 186 (male/female: NR) cases and 186 controls (male/female: NR) controls Inclusion criteria: aged 40‐70 years, newly diagnosed cases from the Aichi Cancer Center Hospital (ACCH) in Aichi Prefecture, Japan Recruitment: from April 1981‐March 1983 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Stomach cancer: 93 (male/female: NR) cases Colorectal cancer: 93 (male/female: NR) cases, including 42 colon cancer and 51 rectal cancer |
|
| Green tea in exposure categories | Exposure assessment: green tea drinking Lowest exposure: < 4 cups/d Highest exposure: ≥ 4 cups/d |
|
| Notes | Funding: Grant‐in‐Aid for Cancer Research from the Ministry of Health and Welfare Statistical methods: Mantel‐Haenszel method Variables controlled in analysis: sex and age Variables controlled by matching: ‐ The estimates cannot be included due to missing CIs |
|
Takezaki 2000.
| Study characteristics | ||
| Methods | HCC in Japan | |
| Participants | Participants: 127,477 inhabitants of 45 areas of Japan, data retrieved from 66,885 participants: 346 cases and 11,936 controls (all male) included in the present study Inclusion criteria: aged 40‐79 years, from the Aichi Cancer Center Hospital (ACCH) in Aichi Prefecture, Japan. Recruitment: from January 1988‐1997 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Pharynx (hypopharynx) cancer: 62 (male/female: NR) cases Oesophageal cancer: 284 cases, including 53, 159 and 72 cases in upper, middle and lower third of the oesophagus |
|
| Green tea in exposure categories | Exposure assessment: green tea drinking Lowest exposure: occasionally or less Intermediate exposure: 1‐6 cups/d Highest exposure: ≥ 7 cups/d |
|
| Notes | Funding: Grant‐in‐Aid for Cancer Research and the Comprehensive 10‐year Strategy for Cancer Control from the Ministry of Health and Welfare of Japan Statistical methods: unconditional logistic regression Variables controlled in analysis: age, year and season of visit, smoking and alcohol drinking Variables controlled by matching: ‐ |
|
Takezaki 2001.
| Study characteristics | ||
| Methods | HCC in Japan | |
| Participants | Participants: 945 cases (male/female: 748/297) and 4153 (male/female: 2964/1189) controls
Inclusion criteria: newly diagnosed cases, 40‐79 years of age from the Aichi Cancer Center Hospital (ACCH) in Aichi Prefecture, Japan Recruitment: from 1988‐1997 referring to Aichi Cancer Center Hospital |
|
| Interventions | N/A | |
| Outcomes | Number of lung cases: Lung cancer: Adenocarcinoma: 507 (male/female: 367/240) cases Squamous cell and small‐cell carcinomas 438 (male/female: 381/57) cases |
|
| Green tea in exposure categories | Exposure assessment: green tea intake Lowest exposure: < 1 cup/d Intermediate exposure 1: 1 cup/d Intermediate exposure 2: 2 cups/d Highest exposure: ≥ 3 cups/d |
|
| Notes | Funding: Grant‐in‐Aid for Cancer Research and the Comprehensive 10‐year Strategy for Cancer Control from the Ministry of Health and Welfare of Japan Statistical methods: unconditional logistic regression Variables controlled in analysis: age, season and year of visit, occupation, prior lung diseases, smoking and consumption of green vegetables and meat Variables controlled by matching: sex and age (± 5 years) |
|
Tamura 2018.
| Study characteristics | ||
| Methods | Cohort study in Japan | |
| Participants | Participants: 30,824 (14,240/16,584) participants
Inclusion criteria: aged ≥ 35 years, in residents in Takayama, Gifu Prefecture, Japan Recruitment: from 1 September 1992 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 31 March 2008 Liver cancer: 172 (male/female: 106/66) cases |
|
| Green tea in exposure categories | Exposure assessment: intake of green tea Lowest exposure: nondrinkers Intermediate exposure 1: < 1 time/d Intermediate exposure 2: 1 time/d Intermediate exposure 3: 2‐3 times/d Highest exposure: ≥ 4 times/d |
|
| Notes | Funding: grants from the Ministry of Education, Culture, Sports, Science and Technology and the Minister of Health, Labour and Welfare of Japan Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age, sex, ethanol intake, smoking status, BMI, education, total energy intake, physical activity and medical history of diabetes mellitus. Variables controlled by matching: ‐ |
|
Tewes 1990.
| Study characteristics | ||
| Methods | HCC in China | |
| Participants | Participants: 200 cases and 200 controls (all women)
Inclusion criteria: female Chinese participants living in Hong Kong, mean age 61.8 (SD 10.0) for cases and 60.6 (SD 9.6) for controls. Recruitment: from 1981‐1983 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Lung cancer: 200 cases (all women) |
|
| Green tea in exposure categories | Exposure assessment: drinking habit Lowest exposure: no drinking Highest exposure: usually drinking |
|
| Notes | Funding: not declared Statistical methods: conditional logistic regression Variables controlled in analysis: age, number of live births, schooling, smoking habits, alcohol drinking, frequency of consumption/month of fresh vegetables and fruits. Variables controlled by matching: age and district of residence. |
|
Tsao 2009.
| Study characteristics | ||
| Methods | RCT, parallel, double‐blind in Japan | |
| Participants | Participants: 41 (male/female: 19/22) participants aged 18‐75 years, with ≥ 1 histologically confirmed, bidimensionally measurable oral premalignant lesions, with Zubrod performance status < 2, adequate hematologic, liver, renal and cardiac function, with one of the following: harbouring at least mild dysplasia, located in a high‐risk area (i.e. floor of mouth, ventrolateral tongue and soft palate), significant extent of lesion tissue involvement and presence of symptoms. 11, 9 and 10 participants were randomised in intervention group A, B and C respectively and 11 participants in control group Recruitment: from August 2002‐March 2008 |
|
| Interventions | Treatment groups: GTE contains high amounts of polyphenols, including EGCG: Group A: 500 mg/m2 GTE, N = 11 (male/female: 5/6) Group B: 750 mg/m2 GTE, N = 9 (male/female: 4/5) Group C: 1,000 mg/m2 GTE, N = 10 (male/female: 4/6) Control group: placebo, N = 11 (male/female: 6/5) Duration: 12 weeks |
|
| Outcomes | Primary outcome Clinical and histologic response of high‐risk oral lesions Secondary outcome Safety data: qualitative and quantitative toxicities of GTE |
|
| Green tea in exposure categories | NA | |
| Notes | Funding: support to En Ltd., including YM Sagesaka as employee of Ito En Ltd | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization was done with the Pocock‐Simon dynamic allocation scheme" Comment: done |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization was done with the Pocock‐Simon dynamic allocation scheme" Comment: probably concealed |
| Blinding of participants and personnel (performance bias) Oral premalignant‐lesions | Low risk | Quote: "GTE and placebo capsules were supplied to the pharmacy in blister packs containing 10 capsules each" Comment: participants and personnel probably blinded |
| Blinding of outcome assessment (detection bias) Oral premalignant‐lesions | Unclear risk | No explicit statement on blinded outcome assessment, only for immunohistochemical staining |
| Incomplete outcome data (attrition bias) Oral premalignant‐lesions | Low risk | Data reported for all participants who completed the study. ITT analysis implemented |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available and it is not clear if the published reports include all expected outcomes |
| Other bias | Low risk | No other bias |
Tse 2017.
| Study characteristics | ||
| Methods | HCC in China | |
| Participants | Participants: 431 cases and 402 controls (all male) Inclusion criteria: aged 35‐84 years, newly diagnosed at Department of Surgery and Clinical Oncology from the Prince of Wales Hospital of New Territories East Cluster in Hong Kong, China Recruitment: from August 2011‐November 2016 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Prostate cancer: 431 cases (all male) |
|
| Green tea in exposure categories | Exposure assessment: drinking habit Lowest exposure: non habitual users Highest exposure: habitual users |
|
| Notes | Funding: grant from the Health and Medical Research Fund (N. 11121091), Hong Kong Special Administrative Region, China Statistical methods: unconditional logistic regression Variables controlled in analysis: age at interview, marital status, unemployment status, family prostate cancer history, consumption of deep fried food, consumption of pickled vegetables, nightshift work and cumulative bisphenol A exposure index Variables controlled by matching: age (± 5 years) |
|
Ugai 2017.
| Study characteristics | ||
| Methods | Cohort study in Japan | |
| Participants | Participants: 140,420 (male/female: 68,722/71,698), with 61,595 from cohort 1 and 78,825 from cohort 2 and 95,807 (male/female: 45,937/49,870) participants with complete data in the present study. Inclusion criteria: aged 40‐59 years, from 5 Public Health Centre areas (Iwate, Akita, Nagano, Okinawa and Tokyo) for cohort 1, and aged 40‐69 years for cohort 2 from 6 Public Health Centre areas (Ibaraki, Niigata, Kochi, Nagasaki, Okinawa and Osaka), respondent at 5‐year follow‐up. Parent cohorts Cohort 1: Japan Public Health Centre‐based Prospective Study (JPHC)‐I cohort 2: Japan Public Health Centre‐based Prospective Study (JPHC)‐II Recruitment: from 1990 for cohort 1 and 1993/1994 for cohort 2 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 31 December 2012 Malignant lymphoma: 411 (male/female: 237/174) cases Multiple myeloma: 138 (male/female: 66/72) cases |
|
| Green tea in exposure categories | Exposure assessment: green tea drinking Lowest exposure: almost none Intermediate exposure 1: 1‐4 times/week Intermediate exposure 2: 1‐2 cups/d Intermediate exposure 3: 3‐4 cups/d Highest exposure: ≥ 5 cups/d |
|
| Notes | Funding: National Cancer Center Research and Development Fund (23‐A‐31(toku) and 26‐A‐2; since 2011) and a Grant‐in‐Aid for Cancer Research from the Ministry of Health, Labour and Welfare of Japan (from 1989‐2010). Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age at baseline, sex, smoking status, alcohol consumption, BMI, occupation and study area Variables controlled by matching: ‐ |
|
Ugai 2018.
| Study characteristics | ||
| Methods | Cohort study in Japan | |
| Participants | Participants: 140,420 (male/female: 68,722/71,698), with 61,595 from cohort 1 and 78,825 from cohort 2 and 95,807 (male/female: 45,937/49,870) participants with complete data in the present study. Inclusion criteria: aged 40‐59 years, from 5 Public Health Centre areas (Iwate, Akita, Nagano, Okinawa and Tokyo) for cohort 1 and aged 40‐69 years in cohort 2, from 6 Public Health Centre areas (Ibaraki, Niigata, Kochi, Nagasaki, Okinawa and Osaka), respondent at 5‐year follow‐up Parent cohorts Cohort 1: Japan Public Health Centre‐based Prospective Study (JPHC)‐I Cohort 2: Japan Public Health Centre‐based Prospective Study (JPHC)‐II Recruitment: from 1990 for cohort 1 and 1993/1994 for cohort 2 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 31 December 2012 Acute myeloid leukaemia: 85 (male/female: 50/85) cases (Also reported 70 (male/female: 50/20) cases of myelodysplastic syndromes, not cancer) |
|
| Green tea in exposure categories | Exposure assessment: green tea drinking Lowest exposure: < 1 cup/d Intermediate exposure 1: 1‐2 cups/d Intermediate exposure 2: 3‐4 cups/d Highest exposure: ≥ 5 cups/d |
|
| Notes | Funding: National Cancer Center Research and Development Fund (23‐A‐31(toku) and 26‐A‐2; since 2011) and Ministry of Health, Labour and Welfare of Japan (from 1989‐2010; a Grant‐in‐Aid for Cancer Research) Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age at baseline, sex, smoking status, alcohol consumption, BMI, occupation and study area Variables controlled by matching: ‐ |
|
Ui 2009.
| Study characteristics | ||
| Methods | Cohort study in Japan | |
| Participants | Participants: 52,029 participants. Final data on 41,761 (male/female: 19,749/22,012) participants included Inclusion criteria: aged 40‐79 years, living in 14 municipalities of Miyagi Prefecture in the catchment area of the Ohsaki Public Health Centre, Miyagi, Japan Parent cohort: Ohsaki Cohort Study Recruitment: from October‐December 1994 Data on the same cohort also reported in Kuriyama 2006 and Naganuma 2009. |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 31 December 2002 Liver cancer: 247 (male/female: 164/83) cases |
|
| Green tea in exposure categories | Exposure assessment: green tea consumption Lowest exposure: < 1 cup/d Intermediate exposure 1: 1‐2 cups/d Intermediate exposure 2: 3‐4 cups/d Highest exposure: ≥ 5 cups/d |
|
| Notes | Funding: Grant‐in‐Aid for Cancer Research and for the Third Term Comprehensive Ten‐Year Strategy for Cancer Control (H18‐3jigan‐ippan‐001), Ministry of Health, Labour and Welfare, in Japan Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age, sex, alcohol consumption, smoking status, coffee consumption, vegetable consumption, dairy products consumption, fruit consumption, fish consumption and soybean consumption Variables controlled by matching: ‐ |
|
Wakai 2004.
| Study characteristics | ||
| Methods | HCC in Japan | |
| Participants | Participants: 124 (male/female: 100/24) cases and 620 (male/female: 500/120) controls Inclusion criteria: aged 40‐79 years, from the Aichi Cancer Center Hospital (ACCH) in Aichi Prefecture, Japan Recruitment: from January 1988‐December 2000 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Bladder cancer: 124 (male/female: 100/24) cases, including cancers of renal pelvis (N = 5), ureter (N = 6) or bladder (N = 113) |
|
| Green tea in exposure categories | Exposure assessment: green tea drinking Lowest exposure: < 1 cup/d Intermediate exposure 1: 1‐4 cups/d Intermediate exposure 2: 5‐9 cups/d Highest exposure: ≥ 10 cups/d |
|
| Notes | Funding: Grant‐in‐Aid for Cancer Research and the Comprehensive 10‐year Strategy for Cancer Control from the Ministry of Health, Labour and Welfare of Japan Statistical methods: unconditional logistic regression Variables controlled in analysis: age, sex, year of first visit, cumulative consumption of cigarettes, intake of green vegetables and intake frequency of eggs Variables controlled by matching: age (5 years, strata), sex and year of first visit |
|
Wang 1999.
| Study characteristics | ||
| Methods | PCC in China | |
| Participants | Participants: 209 cases (male/female: 129/80) and 209 (male/female: 129/80) controls
Inclusion criteria: 35‐79 years of age; residence in Yangzhong, Jiangsu Province, China Recruitment: 2000 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Oesophageal cancer: 68 cases Cardia (stomach) cancer: 69 cases Other stomach cancer: 72 cases |
|
| Green tea in exposure categories | Exposure assessment: drinking habit Lowest exposure: never drinking Highest exposure: drinking |
|
| Notes | Article in Chinese Sponsor: not declared Statistical methods: logistic regression Variables controlled in analysis: age, education, cigarette smoking and alcohol intake Variables controlled by matching: sex |
|
Wang 2006.
| Study characteristics | ||
| Methods | PCC in China | |
| Participants | Participants: 107 cases (male/female: 60/47) and 107 (male/female: 60/47) controls
Inclusion criteria: newly diagnosed unrelated ethnic Han Chinese and residents in 5 townships of Chuzhou District, which were located at the north side of the General Irrigation Canal, Huaian City, Jiangsu Province, China Recruitment: from 2002‐2003 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Oesophageal cancer: 107 (male/female: 60/47) cases of oesophageal squamous cell carcinoma |
|
| Green tea in exposure categories | Exposure assessment: green tea intake Lowest exposure: nondrinkers Highest exposure: drinking at least 1 cup/d for at least 6 months |
|
| Notes | Funding: grants CA94683 and CA90997 from NCI/NIH Statistical methods: conditional logistic regression Variables controlled in analysis: sex, age (± 5 years), residence, oesophageal lesion, eating fast, a family history of cancer, HP infection, clean up of storage utensils Variables controlled by matching: sex, age (± 5 years) and residence |
|
Wang 2007.
| Study characteristics | ||
| Methods | PCC in China | |
| Participants | Participants: 355 cases (male/female: 223/132) and 209 (male/female: 252/156) controls
Inclusion criteria: aged ≥ 30 years, referring to Yangzhong Cancer Research Institute and Yangzhong People's Hospital and living in Yangzhong, China Recruitment: from 1 January 2004‐28 February 2006 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Oesophageal cancer: 355 (male/female: 223/132) cases of squamous cell carcinoma |
|
| Green tea in exposure categories | Exposure assessment: drinking habit Lowest exposure: no drinking Highest exposure: drinking |
|
| Notes | Funding: National Nature Science Foundation of China Statistical methods: unconditional logistic regression Variables controlled in analysis: age, marital status and education years Variables controlled by matching: sex and age (± 5 years) |
|
Wang 2012a.
| Study characteristics | ||
| Methods | HCC in China | |
| Participants | Participants: 250 cases (male/female: 150/100) and 299 (male/female: 178/121) controls
Inclusion criteria: participants of Han Chinese ancestry newly diagnosed, pathologically confirmed sporadic cases of clear cell renal cell carcinoma at the Departments of Urology, the 1st and 2nd Affiliated Hospitals of Second Military Medical University, Shanghai, China Recruitment: from May 2007‐December 2009 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Renal cancer: 250 (male/female: 150/100) cases of clear cell renal cell carcinoma |
|
| Green tea in exposure categories | Exposure assessment: drinking habit Lowest exposure: < 500 mL/d Highest exposure: ≥ 500 mL/d |
|
| Notes | Sponsor: National Natural Science Foundation of China (30873041, 81025015) Statistical methods: unconditional logistic regression Variables controlled in analysis: age, BMI, hypertension, diabetes, urolithiasis, smoking, alcohol consumption and the polymorphisms Variables controlled by matching: sex and age (± 3 years) |
|
Wang 2012b.
| Study characteristics | ||
| Methods | HCC in China | |
| Participants | Participants: 220 cases (male/female: 135/85) and 220 (male/female: 135/85) controls
Inclusion criteria: participants with pathologically confirmed diagnosis and interviewed within 6 months of diagnosis referring at 5 hospitals (Xijing Hospital, Tangdu Hospital, Northwest Hospital, Xi'an Centre Hospital and Shaanxi Province People’s Hospital) in Xi'an, China. Recruitment: from August 2009‐December 2011 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Multiple myeloma: 220 (male/female: 135/85) cases |
|
| Green tea in exposure categories | Exposure assessment: drinking habit Lowest exposure: never Intermediate exposure 1: 1‐3 times/month Intermediate exposure 2: 1‐2 times/ week Highest exposure: ≥ 3 times/week |
|
| Notes | Sponsor: China Special Grant for the Prevention and Control of Infectious Diseases (2009ZX10002‐027). Statistical methods: conditional logistic regression Variables controlled in analysis: age, sex, education, family history of cancer in first‐degree relatives and dietary risk factors (shallot and garlic, soy food, fried food, cured/smoked food, black tea, fish and brined vegetables, pickle or sauerkraut) Variables controlled by matching: sex, age (± 5 years) and inpatient hospital |
|
Wang 2012c.
| Study characteristics | ||
| Methods | PCC in China | |
| Participants | Participants: 908 cases (male/female: 526/382) and 1067 (male/female: 605/462) controls
Inclusion criteria: aged 35–79 years, residents in urban Shanghai through an 'instant case reporting' system in 37 major hospitals newly diagnosed with pancreatic cancer and living in Shanghai, China. Participants reported to drink other type of tea than green tea were excluded. Recruitment: from December 2006‐January 2011 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Pancreatic cancer: 908 (male/female: 526/382) cases |
|
| Green tea in exposure categories | Exposure assessment A: green tea intake Lowest exposure: never Highest exposure: ever Exposure assessment B: green tea consumption Lowest exposure: 0 g/month Intermediate exposure 1: 1‐99 g/month Intermediate exposure 2: 100‐149 g/month Highest exposure: ≥ 150 g/month |
|
| Notes | Sponsor: grant of US National Cancer Institute (5R01CA114421), by the Science and Technology Commission of the Shanghai Municipality (08411954100), by the Shanghai Municipal Health Bureau (20114080) and by the Shanghai Cancer Institute (SB10‐06). Statistical methods: conditional logistic regression Variables controlled in analysis: age, BMI, education level, family history of cancer, smoking, history of type 2 diabetes, menopausal status, oral contraceptive use and menopausal hormone therapy. Variables controlled by matching: sex, age (± 5 years) and inpatient hospital |
|
Wang 2013a.
| Study characteristics | ||
| Methods | HCC in China | |
| Participants | Participants: 157 cases and 314 controls (all women)
Inclusion criteria: newly diagnosed and histologically confirmed cases in participants referring to Chung‐Shan Medical University Hospital Recruitment: from June 2009‐June 2011 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Breast cancer: 157 cases (all women) |
|
| Green tea in exposure categories | Exposure assessment: green tea intake Lowest exposure: no (< 1 cup/d) Highest exposure: yes (≥ 1 cup/d) |
|
| Notes | Sponsor: grant CSH‐2010‐A‐03 from Chung‐Shan Medical University Hospital, Taiwan Statistical methods: conditional logistic regression Variables controlled in analysis: education level, age at menarche and past hormone therapy Variables controlled by matching: age (± 2 years) |
|
Wang 2013b.
| Study characteristics | ||
| Methods | HCC in USA | |
| Participants | Participants: 1007 (male/female: 784/223) cases and 1299 (male/female: 1013/286) controls
Inclusion criteria: histologically confirmed bladder cancer with no prior treatment of chemotherapy or radiotherapy at the time of recruitment at the University of Texas MD Anderson Cancer Center and Baylor College of Medicine, Texas, USA. Recruitment: from 1999, still ongoing in 2013 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Bladder cancer: 1007 (male/female: 784/223) cases, all types, but generally transitional cell carcinoma |
|
| Green tea in exposure categories | Exposure assessment: green tea intake Lowest exposure: never Intermediate exposure: 0.1‐0.13 serving/d Highest exposure: ≥ 0.14 serving/d (one serving = cup 8 fl oz/240 mL) |
|
| Notes |
Sponsor: National Cancer Institute grants K07 CA134831 and R01 CA74880 Statistical methods: unconditional logistic regression Variables controlled in analysis: age, sex, ethnicity, energy intake and smoking Variables controlled by matching: sex, age (± 5 years) and ethnicity |
|
Wang 2015.
| Study characteristics | ||
| Methods | HCC in China | |
| Participants | Participants: 160 (male/female: 74/86) cases and 320 (male/female: 154/166) controls
Inclusion criteria: histological or cytological confirmed cases in participants referring to Zhengzhou University and Liaoning Cancer Hospital, Zhengzhou, China Recruitment: from February 2005‐February 2010 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Stomach cancer: 160 (male/female: 74/86) cases |
|
| Green tea in exposure categories | Exposure assessment A: green tea intake Lowest exposure: never Highest exposure: current Exposure assessment B: green tea consumption Lowest exposure: never Intermediate exposure 1: 1‐24 g/week Intermediate exposure 2: 25‐35 g/week Highest exposure: ≥ 35 g/week |
|
| Notes | Funding: not declared Statistical methods: conditional logistic regression Variables controlled in analysis: not clearly reported Variables controlled by matching: sex and age (± 3 years) |
|
Wilkens 1996.
| Study characteristics | ||
| Methods | PCC in USA | |
| Participants | Participants: 261 (male/female: 195/66) cases and 522 (male/female: 390/132) controls Inclusion criteria: white or Japanese ancestry with newly diagnosed cancer referring to the 7 largest civilian hospitals on the island of Oahu, Hawaii, USA Recruitment: from 1977‐1986 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Urinary tract cancer: 261 (male/female: 195/66) cases, mainly transitional cell cancer (95%), including urinary bladder (90%), renal pelvis (7%) and ureter (3%) |
|
| Green tea in exposure categories | Exposure assessment: green tea drinking habit Lowest exposure: first tertile Intermediate exposure: second tertile Highest exposure: third tertile Values of tertiles NR |
|
| Notes | Funding: National Cancer Institute grants R26 CA 25903 and NOl CA 15655 Statistical methods: conditional logistic regression Variables controlled in analysis: age, smoking status, pack‐years, employment in a high‐risk occupation, consumption of dark green vegetables in men and total vitamin C consumption in women. Variables controlled by matching: sex, age (± 5 years) and ethnic group |
|
Wu 2003.
| Study characteristics | ||
| Methods | PCC in USA | |
| Participants | Participants: 501 cases and 594 controls (all women) Inclusion criteria: aged 25‐74 years, in Asian Americans (Chinese, Japanese or Filipino), newly diagnosed cases identified through the Los Angeles County Cancer Surveillance Program, Los Angeles, USA Recruitment: from 1 January 1995‐31 December 1998 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Breast cancer: 501 cases (all women) |
|
| Green tea in exposure categories | Exposure assessment: green tea drinking habit Lowest exposure: nondrinkers Intermediate exposure: > 0‐85.7 mL/d Highest exposure: ≥ 85.7 mL/d |
|
| Notes | Funding: grants of California Breast Cancer Research Program (1RB‐0287, 3PB‐0102) and of USC/Norris Comprehensive Cancer Center (2 P30 CA14089‐26) Statistical methods: conditional logistic regression Variables controlled in analysis: age, Asian ethnicity, birthplace, education, age at menarche, pregnancy, current BMI, total caloric intake, menopausal status, use of menopausal hormones, intake of soy, dark green vegetables, smoking history, alcohol intake, physical activity and family history of breast cancer, coffee intake and black tea intake Variables controlled by matching: Asian ethnicity, age (5‐year groups) and birthplace |
|
Wu 2009a.
| Study characteristics | ||
| Methods | HCC in China | |
| Participants | Participants: 142 cases and 142 control (all men) Inclusion criteria: aged ≥ 18 years, referring to the First, Second and Third affiliated hospitals of Shanshan University and the Sun Yat‐Sen University, Department of Urology, Cancer Center in Guangdong, China Recruitment: from May 2005‐March 2008 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Prostate cancer: 142 cases (all men) |
|
| Green tea in exposure categories | Exposure assessment: green tea drinking habit Lowest exposure: occasionally or never Intermediate exposure 1: 3‐4 time/month Intermediate exposure 2: 1‐6 times/week Highest exposure: every d |
|
| Notes | Article in Chinese Funding: not declared Statistical methods: conditional logistic regression Variables controlled in analysis: age group (± 4‐5 years), ethnic group and type of residence Variables controlled by matching: age group (± 4‐5 years), ethnic group and type of residence |
|
Wu 2009b.
| Study characteristics | ||
| Methods | PCC in China | |
| Participants | Participants: 1502 (male/female: 1191/329) cases and 3879 (male/female: 2916/963) controls, including 637 (male/female: 426/211) cases and 1938 (male/female: 1368/570) controls in the Dafeng area and 883 (male/female: 765/118) cases and 1941 (male/female: 1548/393) controls in Ganyu. Inclusion criteria: participants resident in 2 counties, Dafeng and Ganyu, with high and low mortality for oesophageal cancer in Jiangsu province, China Recruitment: from 2003‐2007 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Oesophageal cancer: 1502 (male/female: 1191/329) cases |
|
| Green tea in exposure categories | Exposure assessment: green‐tea drinking habit Lowest exposure: never drinking Highest exposure: has ever drunk (≥ 1 cup/week for ≥ 6 months), further divided into former and current drinkers |
|
| Notes | Funding: Jiangsu Provincial Health Department (RC 2003090) Statistical methods: unconditional logistic regression Variables controlled in analysis: sex, age, education level, 10 years' income, cancer family history, BMI, pack‐year of smoking, alcohol drinking and tea temperature Variables controlled by matching: sex and age (± 5 years) |
|
Xu 2007.
| Study characteristics | ||
| Methods | PCC in China | |
| Participants | Participants: 1204 cases and 1212 controls (all women) Inclusion criteria: aged 30‐69 years, medical confirmed cases from Shanghai Cancer Registry with no history of cancer or hysterectomy, within the Shanghai Endometrial Cancer Study Recruitment: from 1997‐2003 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Endometrial cancer: 1204 cases (all women) |
|
| Green tea in exposure categories | Exposure assessment: green‐tea drinking habit Lowest exposure: never Highest exposure: primarily green tea drinking (≥ 3 times/week for ≥ 6 months) |
|
| Notes | Funding: USA Public Health Service grant R01CA92585 from the National Cancer Institute Statistical methods: unconditional logistic regression Variables controlled in analysis: age, education, menopausal status, years of menstruation, number of pregnancies, diagnosis of diabetes, alcohol consumption, BMI, physical activity, energy intake and total fruit and vegetable intake and soy protein intake Variables controlled by matching: age (± 5 years) |
|
Xu 2013.
| Study characteristics | ||
| Methods | PCC in China | |
| Participants | Participants: 1225 cases (male/female: NR) and 1234 (male/female: NR) controls
Inclusion criteria: newly diagnosed cases in China Recruitment: from 2006‐2012 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Lung cancer: 1225 cases (male/female: NR) |
|
| Green tea in exposure categories | Exposure assessment: drinking habit Lowest exposure: no drinking Highest exposure: drinking |
|
| Notes | Article in Chinese Sponsor: not declared Statistical methods: unconditional logistic regression Variables controlled in analysis: NR Variables controlled by matching: sex and age (± 3 years) |
|
Yan 2016.
| Study characteristics | ||
| Methods | PCC in China | |
| Participants | Participants: 593 (male/female: 392/201) cases and 1128 (male/female: 695/433) controls Inclusion criteria: aged 20‐80 years, newly diagnosed at the First Hospital of the University of Medical Sciences in residents for at least 10 years in Fujian Province, China Recruitment: from September 2010‐March 2006 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Oral cancer: 593 (male/female: 392/201) cases of oral squamous cell carcinoma |
|
| Green tea in exposure categories | Exposure assessment: green tea drinking habit Lowest exposure: nondrinkers Highest exposure: green tea drinker (≥ 1 cup/week for ≥ 6 consecutive months) |
|
| Notes | Article in Chinese Funding: grants of California Breast Cancer Research Program (1RB‐0287, 3PB‐0102) and of USC/Norris Comprehensive Cancer Center (2 P30 CA14089‐26) Statistical methods: unconditional logistic regression Variables controlled in analysis: sex, age, residence, smoking, drinking and eating vegetables and fruits Variables controlled by matching: sex and age |
|
Yang 2011a.
| Study characteristics | ||
| Methods | Cohort study in China | |
| Participants | Participants: 61,500 Chinese men, included in the present study 60,567 participants Inclusion criteria: men aged 40‐74 years, no history of cancer at baseline from 8 communities of Shanghai, China Parent cohort: Shangai Men's Health Study Recruitment: from 2002‐2006 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 31 December 2008 Colorectal cancer: 243 cases (all male), including colon cancer (N = 133) and rectal cancer (N = 130) |
|
| Green tea in exposure categories | Exposure assessment A: intake of green tea Lowest exposure: nondrinker Highest exposure: drinker Exposure assessment B: consumption of green tea Lowest exposure: never Intermediate exposure: < 250 g/month Highest exposure: ≥ 250 g/month |
|
| Notes | Funding: US Public Health Service grants (R01 CA082729, in part by R01 CA122364). Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age, education, cigarette smoking, pack‐years of cigarette smoking, alcohol consumption, regular exercise, BMI, history of diabetes, family history of colorectal cancer and intakes of vegetables, fruits and red meat. Variables controlled by matching: ‐ |
|
Ye 1998.
| Study characteristics | ||
| Methods | PCC in China | |
| Participants | Participants: 272 (male/female: 233/39) cases, 544 (male/female: 466/78) controls Inclusion criteria: age 30‐78 years, histologically confirmed or diagnosed by operation cases in residents in Changle City and Fuqing City for at least 20 years, Fujian Province, China Recruitment: from January 1993‐July 1995 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Stomach cancer: 272 (male/female: 233/39) cases |
|
| Green tea in exposure categories | Exposure assessment: intake of green tea Lowest exposure: < 0.75 kg/year Highest exposure: ≥ 75 kg/year |
|
| Notes | Funding: 8.5 National Major Project, No. 95‐914‐01‐10, China Statistical methods: conditional logistic regression Variables controlled in analysis: matching variables Variables controlled by matching: sex, age (± 3 years) and village |
|
Yu 1995.
| Study characteristics | ||
| Methods | PCC in China | |
| Participants | Participants: 711 (male/female: 453/258) cases and 711 (male/female: 453/258) controls Inclusion criteria: aged < 80 years, newly diagnosed cases among residents of Hongkou district of Shnaghai and Nanhui county in suburb area of Shangai, China Recruitment: from October 1991‐December 1993 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Stomach cancer: 711 (male/female: 453/258) cases, including cancers of cardia (N = 128), pylori (N = 216), antrum (N = 153), other sites (N = 124) and site unknown (N = 90) |
|
| Green tea in exposure categories | Exposure assessment: intake of green tea Lowest exposure: nondrinkers Highest exposure: drinkers further divided in: Highest exposure A: 1‐3 new batches Highest exposure B: ≥ 4 new batches |
|
| Notes | Funding: USA Public Health Service grant CA52560 from the National Cancer Institute, National Institutes of Health, Department of Health and Human Service Statistical methods: conditional logistic regression Variables controlled in analysis: sex, age education, birthplace, alcohol drinking and cigarette smoking Variables controlled by matching: sex and age (± 3 years) |
|
Zhang 2002.
| Study characteristics | ||
| Methods | PCC and HCC in China | |
| Participants | Participants: 254 cases and 652 controls, including 340 hospital and 261 population controls (all women) Inclusion criteria: aged < 75 years, newly diagnosed cases at Women’s Hospital, School of Medicine, Zhejiang University, Zhejiang Cancer Hospital and other general hospitals in residents for at least 10 years living in Zhejiang province, China Recruitment: from July 1999‐June 2000 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Ovarian cancer: 254 cases of epithelial ovarian cancer |
|
| Green tea in exposure categories | Exposure assessment: intake of green tea Lowest exposure: never or seldom Intermediate exposure 1: ≤1 time/week Intermediate exposure 2: 2‐6 times/week Highest exposure: ≥ 1 time/d |
|
| Notes | Funding: US Public Health Service grant CA52560 from the National Cancer Institute, National Institutes of Health, Department of Health and Human Service Statistical methods: conditional logistic regression Variables controlled in analysis: age at interview, education, living area, BMI, tobacco smoking, alcohol consumption, coffee drinking, family income, marital status, menopause status, parity, tubal ligation, oral contraceptive use, physical activity and family history of ovarian cancer Variables controlled by matching: age and geographical area |
|
Zhang 2007.
| Study characteristics | ||
| Methods | HCC in China | |
| Participants | Participants: 1009 cases and 1009 controls (all women) Inclusion criteria: aged 20‐87 years, newly diagnosed with invasive ductal carcinomas or in situ carcinoma of the breast, residents in Hangzhou, Zhejiang Province, China Recruitment: from July 2004‐September 2005 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Breast cancer: 1009 cases (all female) |
|
| Green tea in exposure categories | Exposure assessment: intake of green tea Lowest exposure: never or seldom Intermediate exposure 1: ≤ 1 cup/week Intermediate exposure 2: 2–6 times/week Intermediate exposure 3: 1 cup/d Highest exposure: ≥ 2 cups/d |
|
| Notes | Funding: not declared Statistical methods: conditional logistic regression Variables controlled in analysis: resident area, education, BMI, age at menarche, number of children breastfed, menopausal status, oral contraceptive use, HRT, biopsy‐confirmed benign breast diseases, breast cancer in first‐degree relatives, total energy intake, passive smoking, alcohol consumption, coffee consumption, physical activity, soy intake, vegetable intake and fruit intake Variables controlled by matching: age (± 5 years) Zhang 2009 reports same data as Zhang 2007 but stratified by intake of mushrooms. |
|
Zhang 2008.
| Study characteristics | ||
| Methods | HCC in China | |
| Participants | Participants: 107 (male/female: 66/41) cases and 110 (male/female: 70/40) controls Inclusion criteria: aged 16/81 years, histopathologically confirmed cases Zhejiang University residents in Hangzhou, Zhejiang Province, China Recruitment: from 2005‐2006 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Leukaemia: 107 (male/female: 66/41) cases, including acute myeloid leukaemia (N = 72), acute lymphocytic leukaemia (N = 22), chronic myeloid leukaemia (N = 10), chronic lymphocytic leukaemia (N = 3) |
|
| Green tea in exposure categories | Exposure assessment A: intake of green tea Lowest exposure: no Highest exposure: yes Exposure assessment B: consumption of green tea Lowest exposure: nondrinkers or ≤ 1 time/week Intermediate exposure: 2‐6 times/week Highest exposure: ≥ 1 time/d |
|
| Notes | Funding: not declared Statistical methods: unconditional logistic regression Variables controlled in analysis: age, sex, residence, education, smoking, medication use of chloromycetin, occupational exposure to benzene and organophosphorous Variables controlled by matching: age and hospital Zhang 2008b reports same data as Zhang 2008 but paper in Chinese |
|
Zhao 2017.
| Study characteristics | ||
| Methods | Cohort studies in China | |
| Participants | Participants: 61,491 men in Shanghai Men's Health Study and 74,941 women in Shanghai Women's Health Study. Total of 115,954 (male/female: 51,920/64,034) included in the present study Inclusion criteria: aged 40‐74 years, in men's study and 40‐70 years, in women's study with no prevalent cancer, coronary heart disease, stroke, or diabetes at the baseline survey and living in Shnaghai, China Parent cohorts: Shanghai Men's Health Study and Shanghai Women's Health Study Recruitment: from 2002‐2006 (for men's study) and from 1997‐2000 (for women's study) |
|
| Interventions | N/A | |
| Outcomes | Number of cases Outcome assessment: 2006 for men's study and 2000 for women's study Total cancer mortality: 3210 (male/female: 1378/1832) deaths |
|
| Green tea in exposure categories | Exposure assessment A: intake of green tea Lowest exposure: nondrinker Highest exposure: drinker Exposure assessment B: consumption of green tea Lowest exposure: 0 g/d Intermediate exposure: 0‐< median g/d Highest exposure: ≥ median g/d Median value = 8.22 g/d in men's study and 3.29 g/d in women's study |
|
| Notes | Funding: funds of State Key Laboratory of Oncogene and Related Genes (No. 91‐15‐10) and Shanghai Health Bureau Key Disciplines and Specialties Foundation and grants from the US National Institutes of Health (R37 CA070867 and UM1 CA182910, R01 CA082729 and UM1 CA173640) Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age, education, income, smoking status, alcohol intake, energy intake, BMI, physical activity, history of hypertension, gastritis, menopause status for women Variables controlled by matching: ‐ |
|
Zheng 1993.
| Study characteristics | ||
| Methods | HCC in China | |
| Participants | Participants: 404 (male/female: NR) cases and 404 (male/female: NR) controls Inclusion criteria: aged 18‐80 years, newly diagnosed oral cancer in residents in Beijing, China. Recruitment: from 1 May 1989‐24 December 1989 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Oral cancer: 404 (male/female: NR) cases |
|
| Green tea in exposure categories | Exposure assessment: intake of green tea Lowest exposure: < 1 cup/month Highest exposure: ≥ 1 cup/month |
|
| Notes | Funding: not declared Statistical methods: conditional logistic regression Variables controlled in analysis: sex, age, tobacco smoking, alcohol drinking, inadequate dentition, years of education and Quetelet Index Variables controlled by matching: sex and age (± 5 years) |
|
Zhong 2001.
| Study characteristics | ||
| Methods | PCC in China | |
| Participants | Participants: 649 cases and 675 controls (all women) Inclusion criteria: aged 35–69 years, newly diagnosed primary lung carcinoma through Shanghai Cancer Registry in residents in Shanghai, China Recruitment: from 1 February 1992‐31 January 1994 |
|
| Interventions | N/A | |
| Outcomes | Number of cases Lung cancer: 649 cases (all women), including 473 histologically confirmed: adenocarcinoma (N = 331, 70.0%), squamous cell carcinomas (N = 83, 17.5%), small‐cell carcinomas (N = 13, 2.7%), large‐cell carcinoma (N = 1, 0.2%) and mixed‐cell carcinomas (N = 45, 9.5%) |
|
| Green tea in exposure categories | Exposure assessment: intake of green tea Lowest exposure: on regular drinkers Highest exposure: regular drinkers |
|
| Notes | Funding: not declared Statistical methods: conditional logistic regression Variables controlled in analysis: age, income, number of years of exposure to environmental tobacco smoke at work, high‐risk occupation, family history of lung cancer, Vitamin C intake, cooking food at high temperature, and respondent status Variables controlled by matching: age (± 5 years) |
|
ACF: aberrant crypt foci; AKP: alkaline phosphatase; ALT: alanine aminotransferase; ASAP: atypical small acinar proliferation; AST: aspartate aminotransferase; BMI: body mass index; CIN: cervical intraepithelial neoplasia; CI: confidence interval; EGCG: (‐)‐epigallocatechin‐3‐gallate; GnRH: gonadotropin releasing hormone; GTE: green tea extract; HBSAg: hepatitis B surface antigen; HCC: hospital‐based case‐control study; HG‐PIN: high‐grade prostate intraepithelial neoplasia; HP:Helicobacter pylori; HPV: human papillomavirus; HR: hazard ratio; HRQoL: health‐related quality of life; HRT: hormone replacement therapy; IBD: inflammatory bowel disease; IPSS: International Prostate Symptom Score; ITT: intention‐to‐treat; LUTS: lower urinary tract symptoms; MENQOL: Menopause‐specific Quality of life; N/A: not applicable; NR: not reported; OR: odds ratio; PCC: population‐based case‐control study; PSA: prostate‐specific antigen; QoL: quality of life; RERF: Radiation Effects Research Foundation; RCT: randomised controlled trial; RR: risk ratio; SD: standard deviation; SNP: single nucleotide polymorphisms; UF: uterine fibroid
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahmad 2012 | Wrong exposure (not green tea) |
| Allen 2011 | Wrong exposure (not green tea) |
| Alsanad 2016 | Wrong patient population |
| Amarasinghe 2013 | Wrong outcomes |
| Arts 2001 | No distinction between green and black tea |
| Asgari 2011 | Wrong exposure (not green tea) |
| Askari 2014 | Wrong exposure (not green tea) |
| Azeem 2013 | Wrong exposure (not green tea) |
| Bailey 2017 | Paediatric population |
| Bamia 2015 | Wrong exposure (not green tea) |
| Bao 2015 | Wrong patient population |
| Baroudi 2014 | Wrong exposure (not green tea) |
| Bates 2007 | Wrong exposure (not green tea) |
| Bianchi 2000 | No distinction between green and black tea |
| Bonaventure 2013 | Paediatric population |
| Butler 2015 | Wrong exposure (not green tea) |
| Chen 2009 | Wrong exposure (not green tea) |
| Chyou 1995 | No green tea |
| DArena 2013 | Wrong patient population |
| Deandrea 2010 | Wrong exposure (not only green tea) |
| Emami 2014 | Wrong study design |
| Ettrich 2012 | Wrong outcomes |
| Ferrucci 2014 | Wrong exposure (not green tea) |
| Gao 2002 | Wrong exposure (not green tea) |
| Gao 2009 | Wrong exposure (not green tea) |
| Hara 1984 | Participants all people with cancer |
| He 2017 | Wrong exposure (not green tea) |
| Henning 2012 | Wrong outcomes |
| Ide 2008 | Wrong exposure (not green tea) |
| Il'yasova 2003 | No distinction between green and black tea |
| Inoue 1997 | Participants all people with cancer |
| Inoue 2001 | Study does not address cancer |
| Ishizuka 2003 | Measured gallstones |
| Jatoi 2003 | Participants all people with cancer |
| Jia 2012 | Cases not only people with cancer but included also participants with pre‐cancerous lesions |
| Johnson 2011 | Wrong exposure (not green tea) |
| Kono 1991 | Measured polyps of the colon |
| Kuwahara 2000 | Measured atrophic gastritis |
| Lee 1990 | Mixed reporting of results for oolong, black and green tea No distinction between at least 2 amounts of frequency of green tea consumption |
| Lee 2013 | Wrong exposure (not green tea) |
| Liu 2013a | Wrong exposure (not green tea) |
| Liu 2014 | Wrong exposure (not green tea) |
| Liu 2015 | Wrong outcomes |
| Luo 2010 | Wrong exposure (not green tea) |
| Menzler 2015 | Wrong outcomes |
| Mineharu 2011 | Wrong outcomes |
| Montella 2007 | No distinction between green and black tea |
| Montella 2009 | No distinction between green and black tea |
| Nagano 2000 | Summarised and added new data in Nagano 2001 |
| Nakachi 1998 | Participants all people with cancer |
| Nakachi 2003 | Paper reviews Nakachi and colleague's 1998 study, participants all people with cancer |
| Oguni 1992 | Abstract only, insufficient data |
| Ohno 1985 | No amount of frequency of green tea consumption specified |
| Ohno 1995 | "Okinawa tea" consumption, which is half‐fermented oolong tea |
| Parodi 2017 | Wrong exposure (not green tea) |
| Pisters 2001 | Participants all people with cancer |
| Ren 1991 | Type of tea not specified |
| Sasazuki 2008 | Wrong exposure (not green tea) |
| Sasazuki 2012 | Wrong study design |
| Sawada 2017 | Wrong study design |
| Seo 2013 | Wrong outcomes |
| Shibata 2000 | Measured atrophic gastritis |
| Shim 1995 | Study does not address cancer |
| Shimizu 2008 | Wrong outcomes |
| Shin 2018 | Wrong outcomes |
| Stingl 2011 | Wrong outcomes |
| Suganuma 1999 | Wrong study design |
| Sun 2002 | Not clear green tea exposure, but urinary oesophagogastroduodenoscopy |
| Tong 2014 | Wrong study design |
| Tsubono 1997 | Not related to cancer risk factors |
| Tsugane 2014 | Wrong study design |
| Wakai 1993 | Participants all people with cancer |
| Wang 2002 | No cancer (precancerous lesions) |
| Wang 2008 | Wrong exposure (not green tea) |
| Wang 2010 | Wrong study design |
| Wang 2012d | Wrong patient population |
| Wang 2012e | Wrong study design |
| Wang 2014a | Wrong study design |
| Wu 2003a | Amount of frequency of green tea consumption not specified |
| Wu 2013a | Wrong study design |
| Yu 1991 | Amount of frequency of green tea consumption not specified, not green tea only |
| YuanJ M 2007 | Wrong exposure (not green tea) |
| Zeegers 2001a | Wrong exposure (not green tea) |
| Zeegers 2001b | Wrong study design |
| Zhang 2004 | Follow‐up study to Zhang 2002, participants all people with cancer |
| Zhang 2006 | Results did not differentiate between black and green tea drinkers |
| Zhang 2009 | Wrong exposure (not green tea) |
| Zhang 2013 | Wrong exposure (not green tea) |
| Zhang 2016 | Wrong outcomes |
| Zhu 2016 | Wrong exposure (not green tea) |
Characteristics of ongoing studies [ordered by study ID]
NCT01496521.
| Study name | Chemoprevention of esophageal squamous cell carcinoma (ESCC) with aspirin and tea polyphenols (CREAT) |
| Methods | RCT, quadruple‐blind |
| Participants | Adults aged 40‐60 years |
| Interventions | Intervention group A: aspirin 100 mg/d Intervention group B: tea polyphenols 100 mg/d Control group: placebo |
| Outcomes | Primary outcomes Occurrence of high‐grade dysplasia and invasive ESCC (at six months) Secondary outcomes Mortality of the participants (at 6 months and at 3 or 5 years later) Number of participants with adverse events (at 6 months and at 3 or 5 years later) Occurrence of high‐grade dysplasia and invasive ESCC (at 3 or 5 years later) |
| Starting date | January 2012 |
| Contact information | Shu‐Tian Zhang, MD, Beijing Friendship Hospital Capital Medical University |
| Notes | Estimated study completion date: January 2013. No information on ClinicalTrials.gov |
Shannon 2010.
| Study name | Fish oil and green tea extract in preventing prostate cancer in patients who are at risk for developing prostate cancer ClinicalTrial ID: Shannon 2010 |
| Methods | Randomised, double‐blind, placebo‐controlled study |
| Participants | Men at high risk of prostate cancer Participants are stratified according to age (< 65 vs ≥ 65) |
| Interventions | Intervention group A: GTE polyphenols (75%) and EGCG (at least 30% = 300 mg) per 1000 mg capsule (2 capsules/d) Intervention group B: fish oil capsule per 1000 mg with ethyl esters of eicosapentaenoic acid (20:5n‐3) and docosahexanoic acid (3 capsules/d) Intervention group C: A+B Control group: placebo, olive oil capsule 2/3 times/d Duration: 12 weeks |
| Outcomes | Primary outcomes Fatty acid synthase expression by immunohistochemistry at pre‐ and post‐intervention (FAS Summary Score) Cell proliferation by Ki67‐immunohistochemistry at pre‐ and post‐intervention Incidence of prostate cancer |
| Starting date | July 2005 |
| Contact information | Jackie Shannon, Principal Investigator, OHSU Knight Cancer Institute |
| Notes | According to 2010 abstract 67 men completed the study, 4 were enrolled. Anticipated sample size 120 men, or 30 men/group |
EGCG: epigallocatechin‐3‐gallate; ESCC: (o)esophageal squamous cell carcinoma; FAS: fatty acid synthase; GTE: green tea extract; RCT: randomised controlled trial
Differences between protocol and review
In contrast to the previous version of this review (Boehm 2009), we included a quantitative assessment of cancer risk related to green tea intake, adding a meta‐analysis of all cancer outcomes whenever there were sufficient data available to perform the analysis.
Contributions of authors
Link with editorial base and co‐ordination of contributions from co‐reviewers (FB, MM, MV, TF) Draft protocol (AAI, FB; with contributions from all) Identify relevant titles (FB, MM, TF) Selection of included studies (MM, MV, TF) Extraction of data from included studies (MM, TF) Methodological quality assessment (AAI, FB, MM, MV, TF) Interpretation of analysis (all authors) Drafting final review (MV and TF, with contributions from all)
Sources of support
Internal sources
Pilkington Family Trusts, UK
External sources
AG Biologische Krebstherapie, Deutsche Krebshilfe, Bonn, Germany
Cochrane Gyneacological Cancer Review Group, UK
Nordic Cochrane Centre / ViFab, Denmark
Declarations of interest
TF: none known MM: none known FB: none known AAI: none known SF: none known MH: none known MV: none known
Edited (no change to conclusions)
References
References to studies included in this review
Allen 2004 {published data only}
- Allen NE, Sauvaget C, Roddam AW, Appleby P, Nagano J, Suzuki G, et al. A prospective study of diet and prostate cancer in Japanese men. Cancer Causes & Control 2004;15(9):911-20. [DOI] [PubMed] [Google Scholar]
Bandera 2010 {published data only}
- Bandera EV, Williams-King MG, Sima C, Bayuga-Miller S, Pulick K, Wilcox H, et al. Coffee and tea consumption and endometrial cancer risk in a population-based study in New Jersey. Cancer Causes & Control 2010;21(9):1467-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berroukche 2012 {published data only}
- Berroukche A, Bendahmane M, Kandouci BA. Association of diet with the risk of prostate cancer in Western Algeria. Oncologie 2012;14(12):674-8. [Google Scholar]
Bettuzzi 2006 {published data only}
- Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Research 2006;66(2):1234. [DOI] [PubMed] [Google Scholar]
- Brausi M, Rizzi F, Bettuzzi S. Chemoprevention of human prostate cancer by green tea catechins: two years later. A follow-up update. European Urology 2008;54(2):472-3. [DOI] [PubMed] [Google Scholar]
Bonner 2005 {published data only}
- Bonner MR, Rothman N, Mumford JL, He X, Shen M, Welch R, et al. Green tea consumption, genetic susceptibility, PAH-rich smoky coal, and the risk of lung cancer. Mutation Research 2005;582(1-2):53-60. [DOI] [PubMed] [Google Scholar]
Chen 2011 {published data only}
- Chen Z, Chen Q, Xia H, Lin J. Green tea drinking habits and esophageal cancer in southern China: a case-control study. Asian Pacific Journal of Cancer Prevention 2011;12(1):229-33. [PubMed] [Google Scholar]
Chen 2017a {published data only}
- Chen F, He B, Huang J, Liu F, Yan L, Hu Z, et al. Effect of tea on oral cancer in nonsmokers and nondrinkers: a case-control study. Zhonghua Yu Fang Yi Xue za Zhi [Chinese Journal of Preventive Medicine] 2015;49(8):683-7. [PubMed] [Google Scholar]
- Chen F, He B, Hu Z, Huang J, Liu F, Yan L, et al. Passive smoking and cooking oil fumes (COF) may modify the association between tea consumption and oral cancer in Chinese women. Journal of Cancer Research and Clinical Oncology 2016;142(5):995-1001. [DOI] [PubMed] [Google Scholar]
- Chen F, He B-C, Yan L-J, Liu F-P, Huang J-F, Hu Z-J, et al. Tea consumption and its interactions with tobacco smoking and alcohol drinking on oral cancer in southeast China. European Journal of Clinical Nutrition 2017;71(4):481-5. [DOI] [PubMed] [Google Scholar]
- Chen F, Yan L, Lin L, Qiu Y, Liu F, Huang J, et al. Independent and joint effects of tea and milk consumption on oral cancer among non-smokers and non-drinkers: a case-control study in China. Oncotarget 2017;8(30):50091-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chyou 1993 {published data only}
- Chyou PH, Nomura AM, Stemmermann GN. A prospective study of diet, smoking, and lower urinary tract cancer. Annals of Epidemiology 1993;3(3):211-6. [DOI] [PubMed] [Google Scholar]
Dai 2010 {published data only}
- Dai Q, Shu XO, Li H, Yang G, Shrubsole MJ, Cai H, et al. Is green tea drinking associated with a later onset of breast cancer? Annals of Epidemiology 2010;20(1):74-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dostal 2015 {published data only}
- Dostal A, Samavat H, Bedell S, Torkelson C, Wang R, Swenson K, et al. The safety of green tea extract supplementation in postmenopausal women at risk for breast cancer: results of the Minnesota Green Tea Trial. Food and Chemical Toxicology 2015;83:26-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samavat H, Dostal AM, Wang R, Bedell S, Emory TH, Ursin G, et al. The Minnesota Green Tea Trial (MGTT), a randomized controlled trial of the efficacy of green tea extract on biomarkers of breast cancer risk: study rationale, design, methods, and participant characteristics. Cancer Causes & Control 2015;26(10):1405-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samavat H, Ursin G, Emory TH, Lee E, Wang R, Torkelson CJ, et al. A randomized controlled trial of green tea extract supplementation and mammographic density in postmenopausal women at increased risk of breast cancer. Cancer Prevention Research (Philadelphia, Pa.) 2017;10(12):710-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster AD, Finstad DA, Kurzer MS, Torkelson CJ. Quality of life among postmenopausal women enrolled in the Minnesota Green Tea Trial. Maturitas 2018;108:1-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Samavat H, Dostal AM, Wang R, Torkelson CJ, Yang CS, et al. Effect of green tea supplements on liver enzyme elevation: results from a randomized intervention study in the United States. Cancer prevention research (Philadelphia, Pa.) 2017;10(10):571-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dryden 2013 {published data only}
- Dryden GW, Lam A, Beatty K, Qazzaz HH, McClain CJ. A pilot study to evaluate the safety and efficacy of an oral dose of (-)-epigallocatechin-3-gallate-rich polyphenon E in patients with mild to moderate ulcerative colitis. Inflammatory Bowel Diseases 2013;19(9):1904-12. [DOI] [PubMed] [Google Scholar]
Fu 2013 {published data only}
- Fu JY, Gao J, Zhang ZY, Zheng JW, Luo JF, Zhong LP, et al. Tea consumption and the risk of oral cancer incidence: a case-control study from China. Oral Oncology 2013;49(9):918-22. [DOI] [PubMed] [Google Scholar]
Galanis 1998 {published data only}
- Galanis DJ, Kolonel LN, Lee J, Nomura A. Intakes of selected foods and beverages and the incidence of gastric cancer among the Japanese residents of Hawaii: a prospective study. International Journal of Epidemiology 1998;27(2):173-80. [DOI] [PubMed] [Google Scholar]
- Galanis DJ, Lee J, Kolonel LN. The influence of cigarette smoking, alcohol, and green tea consumption on the risk of carcinoma of the cardia and distal stomach in Shanghai, China. Cancer 1997;79(9):1840-1. [PMID: ] [DOI] [PubMed]
Gao 1994 {published data only}
- Gao YT, McLaughlin JK, Blot WJ, Ji BT, Dai Q, Fraumeni JF Jr. Reduced risk of esophageal cancer associated with green tea consumption. Journal of the National Cancer Institute 1994;86(11):855-8. [DOI] [PubMed] [Google Scholar]
Gao 2005 {published data only}
- Gao J, Xiang YB, Xu WH, Shao CX, Ruan ZX, Cheng JR, et al. Green tea consumption and the risk of endometrial cancer: a population-based case-control study in urban Shanghai. Zhonghua Liu Xing Bing Xue za Zhi 2005;26(5):323-7. [PMID: ] [PubMed] [Google Scholar]
Garcia 2014 {published data only}
- Garcia FA, Cornelison T, Nuño T, Greenspan DL, Byron JW, Hsu CH, et al. Results of a phase II randomized, double-blind, placebo-controlled trial of Polyphenon E in women with persistent high-risk HPV infection and low-grade cervical intraepithelial neoplasia. Gynecologic Oncology 2014;132(2):377-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuno T, Garcia FA, Cornelison T, Mitchell AL, Greenspan DL, Byron JW, et al. Results of a phase II randomized, double-blind, placebo controlled trial of Polyphenon E in women with persistent high-risk HPV infection and low-grade cervical intraepithelial neoplasia. Cancer Prevention Research (Philadelphia, Pa.) 2013;6(11):Suppl. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garland 2006 {published data only}
- Garland LL, Chow HS, Einspahr J, Harris RB, Buckmeier J, Tobar M, et al. Phase III trial of chemoprevention of lung carcinogenesis using green tea beverage and tea polyphenols. Journal of Clinical Oncology 2006;24(18S):10262006. [Google Scholar]
- NCT00363805. Green tea or Polyphenon E in preventing lung cancer in former smokers with chronic obstructive pulmonary disease. ClinicalTrials.gov 2006: NCT00363805.
Gavrilas 2018 {published data only}
- Gavrilas L, Ciobarc AD, Revnic C, Ionescu C, Miere D. Importance of food groups rich in bioactive dietary components in colorectal cancer prevention. Clujul Medical 2017;90(Supplement 6):S135. [Google Scholar]
- Gavrilas LI, Ionescu C, Balacescu O, Muresan D, Revnic C, Lorena Filip L, et al. Intake of plant based foods and colorectal cancer. A case-control study in Romania. Bulletin UASVM Food Science and Technology 2018;75(2):1-8. [DOI: 10.15835/buasvmcn-fst: 2018.0005] [Google Scholar]
Goodman 2003 {published data only}
- Goodman MT, Tung KH, McDuffie K, Wilkens LR, Donlon TA. Association of caffeine intake and CYP1A2 genotype with ovarian cancer. Nutrition and Cancer 2003;46(1):23-9. [DOI] [PubMed] [Google Scholar]
Goto 1990 {published data only}
- Goto R, Masuoka H, Yoshida K, Mori M, Miyake H. A case control study of cancer of the pancreas. Gan No Rinsho 1990;Spec No:344-50. [PubMed] [Google Scholar]
Green 2014 {published data only}
- Green CJ, Dauwe P, Boyle T, Tabatabaei SM, Fritschi L, Heyworth JS. Tea, coffee, and milk consumption and colorectal cancer risk. Journal of Epidemiology / Japan Epidemiological Association 2014;24(2):146-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hakim 2000 {published data only}
- Hakim IA, Harris RB, Weisgerber UM. Tea intake and squamous cell carcinoma of the skin: influence of type of tea beverages. Cancer Epidemiology, Biomarkers & Prevention 2000;9(7):727-31. [PubMed] [Google Scholar]
Han 2008 {published data only}
- Han R-Q, Zhao J-K, Liu A-M, Wu M, Wamng P-H. The effect of green tea and its possible interactions with relevant factors on lung cancer in Dafeng county, Jiangsu province, China. Acta-Universitatis Medicinalis Nanjing 2008;28(3):354. [Google Scholar]
Hemelt 2010 {published data only}
- Hemelt M, Hu Z, Zhong Z, Xie LP, Wong YC, Tam PC, et al. Fluid intake and the risk of bladder cancer: results from the South and East China case-control study on bladder cancer. International Journal of Cancer 2010;127(3):638-45. [DOI] [PubMed] [Google Scholar]
Hoshiyama 1992 {published data only}
- Hoshiyama Y, Sasaba T. A case-control study of single and multiple stomach cancers in Saitama Prefecture, Japan. Japanese Journal of Cancer Research 1992;83(9):937-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hsu 2012 {published data only}
- Hsu WL, Pan WH, Chien YC, Yu KJ, Cheng YJ, Chen JY, et al. Lowered risk of nasopharyngeal carcinoma and intake of plant vitamin, fresh fish, green tea and coffee: a case-control study in Taiwan. PloS One 2012;7(7):e41779. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 1999 {published data only}
- Huang X, Tajima K, Hamajima N, Inoue M, Takezaki T, Kuroishi T, et al. Effect of life styles on the risk of subsite-specific gastric cancer in those with and without family history. Journal of Epidemiology 1999;9(1):40-5. [DOI] [PubMed] [Google Scholar]
- Inoue M, Tajima K, Hirose K, Kuroishi T, Gao CM, Kitoh T. Life-style and subsite of gastric cancer--joint effect of smoking and drinking habits. International Journal of Cancer 1994;56(4):494-9. [DOI] [PubMed] [Google Scholar]
- Kato I, Tominaga S, Ito Y, Kobayashi S, Yoshii Y, Matsuura A, et al. A comparative case-control analysis of stomach cancer and atrophic gastritis. Cancer Research 1990;50(20):6559-64. [PubMed] [Google Scholar]
Ide 2007 {published data only}
- Ide R, Fujino Y, Hoshiyama Y, Mizoue T, Kubo T, Pham TM, et al. A prospective study of green tea consumption and oral cancer incidence in Japan. Annals of Epidemiology 2007;17:821-6. [DOI] [PubMed] [Google Scholar]
Inoue 1998 {published data only}
- Inoue M, Tajima K, Hirose K, Hamajima N, Takezaki T, Kuroishi T, et al. Tea and coffee consumption and the risk of digestive tract cancers: data from a comparative case-referent study in Japan. Cancer Causes & Control 1998;9(2):209-16. [DOI] [PubMed] [Google Scholar]
Inoue 2008 {published data only}
- Inoue M, Robien K, Wang R, Van den Berg J, Koh WP, Yu MC. Green tea intake, MTHFR/TYMS genotype and breast cancer risk: the Singapore Chinese Health Study. Carcinogenesis 2008;29(10):1967-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Inoue 2009a {published data only}
- Fujino Y, Tamakoshi A, Ohno Y, Mizoue T, Tokui N, Yoshimura T. Prospective study of educational background and stomach cancer in Japan. Preventive Medicine 2002;35(2):121-7. [DOI] [PubMed] [Google Scholar]
- Hoshiyama Y, Kawaguchi T, Miura Y, Mizoue T, Tokui N, Yatsuya H, et al. A nested case-control study of stomach cancer in relation to green tea consumption in Japan. British Journal of Cancer 2004;90(1):135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshiyama Y, Kawaguchi T, Miura Y, Mizoue T, Tokui N, Yatsuya H, et al. A prospective study of stomach cancer death in relation to green tea consumption in Japan. British Journal of Cancer 2002;87(3):309-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Sasazuki S, Wakai K, Suzuki T, Matsuo K, Shimazu T, et al. Green tea consumption and gastric cancer in Japanese: a pooled analysis of six cohort studies. Gut 2009;58(10):1323-32. [DOI] [PubMed] [Google Scholar]
- Koizumi Y, Tsubono Y, Nakaya N, Nishino Y, Shibuya D, Matsuoka H, et al. No association between green tea and the risk of gastric cancer: pooled analysis of two prospective studies in Japan. Cancer Epidemiology, Biomarkers & Prevention 2003;12(5):472-3. [PubMed] [Google Scholar]
- Ohno Y, Tamakoshi A, JACC Study Group. Japan collaborative cohort study for evaluation of cancer risk sponsored by Monbusho (JACC study). Journal of Epidemiology 2001;11(4):144-50. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Sasazuki S, Inoue M, Hanaoka T, Yamamoto S, Sobue T, Tsugane S. Green tea consumption and subsequent risk of gastric cancer by subsite: the JPHC Study. Cancer Causes & Control 2004;15(5):483-91. [DOI] [PubMed] [Google Scholar]
- Tsubono Y, Nishino Y, Komatsu S, Hsieh CC, Kanemura S, Tsuji I, et al. Green tea and the risk of gastric cancer in Japan. New England Journal of Medicine 2001;344(9):632-6. [DOI] [PubMed] [Google Scholar]
Inoue 2009b {published data only}
- Inoue M, Kurahashi N, Iwasaki M, Shimazu T, Tanaka Y, Mizokami M, et al. Effect of coffee and green tea consumption on the risk of liver cancer: cohort analysis by hepatitis virus infection status. Cancer Epidemiology, Biomarkers & Prevention 2009;18(6):1746-53. [DOI] [PubMed] [Google Scholar]
Ishikawa 2006 {published data only}
- Ishikawa A, Kuriyama S, Tsubono Y, Fukao A, Takahashi H, Tachiya H, et al. Smoking, alcohol drinking, green tea consumption and the risk of esophageal cancer in Japanese men. Journal of Epidemiology 2006;16(5):185-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Islami 2009 {published data only}
- Islami F, Pourshams A, Nasrollahzadeh D, Kamangar F, Fahimi S, Shakeri R, et al. Tea drinking habits and oesophageal cancer in a high risk area in northern Iran: population based case-control study. BMJ 2009;338:b929. [DOI] [PMC free article] [PubMed] [Google Scholar]
Iwai 2002 {published data only}
- Iwai N, Ohshiro H, Kurozawa Y, Hosoda T, Morita H, Funakawa K, et al. Relationship between coffee and green tea consumption and all-cause mortality in a cohort of a rural Japanese population. Journal of Epidemiology 2002;12(3):191-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Iwasaki 2010a {published data only}
- Iwasaki M, Inoue M, Sasazuki S, Miura T, Sawada N, Yamaji T, et al. Plasma tea polyphenol levels and subsequent risk of breast cancer among Japanese women: a nested case-control study. Breast Cancer Research and Treatment 2010;124(3):827-34. [DOI] [PubMed] [Google Scholar]
- Iwasaki M, Inoue M, Sasazuki S, Sawada N, Yamaji T, Shimazu T, et al. Green tea drinking and subsequent risk of breast cancer in a population-based cohort of Japanese women. Breast Cancer Research 2010;12(5):R88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Iwasaki 2014 {published data only}
- Iwasaki M, Mizusawa J, Kasuga Y, Yokoyama S, Onuma H, Nishimura H, et al. Green tea consumption and breast cancer risk in Japanese women: a case-control study. Nutrition and Cancer 2014;66(1):57-67. [DOI] [PubMed] [Google Scholar]
Ji 1996 {published data only}
- Ji BT, Chow WH, Yang G, McLaughlin JK, Gao RN, Zheng W, et al. The influence of cigarette smoking, alcohol, and green tea consumption on the risk of carcinoma of the cardia and distal stomach in Shanghai, China. Cancer 1996;77(12):2449-57. [DOI] [PubMed] [Google Scholar]
Ji 1997 {published data only}
- Ji BT, Chow WH, Hsing AW, McLaughlin JK, Dai Q, Gao YT, et al. Green tea consumption and the risk of pancreatic and colorectal cancers. International Journal of Cancer 1997;70(3):255-8. [DOI] [PubMed] [Google Scholar]
Jia 2016 {published data only}
- Jia X, Mi J, Yang L, Wei B, Cao S, Hu L, et al. A case-control study on the relationship between food preference and lung cancer and mesothelioma in a rural area with naturally occurring asbestos. Wei Sheng Yan Jiu [Journal of Hygiene Research] 2016;45(5):771-6. [PMID: ] [PubMed] [Google Scholar]
Jian 2004 {published data only}
- Jian L, Lee AH, Binns CW. Tea and lycopene protect against prostate cancer. Asia Pacific Journal of Clinical Nutrition 2007;16(Suppl 1):453-7. [PubMed] [Google Scholar]
- Jian L, Xie LP, Lee AH, Binns CW. Protective effect of green tea against prostate cancer: a case-control study in southeast China. International Journal of Cancer 2004;108(1):130-5. [DOI] [PubMed] [Google Scholar]
Jin 2013 {published data only}
- Jin ZY, Han RQ, Zhang XF, Wang XS, Wu M, Zhang ZF, et al. The protective effects of green tea drinking and garlic intake on lung cancer, in a low cancer risk area of Jiangsu province, China. Zhonghua Liu Xing Bing Xue za Zhi 2013;34(2):114-9. [PubMed] [Google Scholar]
Kakuta 2009 {published data only}
- Kakuta Y, Nakaya N, Nagase S, Fujita M, Koizumi T, Okamura C, et al. Case-control study of green tea consumption and the risk of endometrial endometrioid adenocarcinoma. Cancer Causes & Control 2009;20(5):617-24. [DOI] [PubMed] [Google Scholar]
Kato 1990 {published data only}
- Kato I, Tominaga S, Matsuura A, Yoshii Y, Shirai M, Kobayashi S. A comparative case-control study of colorectal cancer and adenoma. Japanese Journal of Cancer Research 1990;81(11):1101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Key 1999 {published data only}
- Key TJ, Sharp GB, Appleby PN, Beral V, Goodman MT, Soda M, et al. Soya foods and breast cancer risk: a prospective study in Hiroshima and Nagasaki, Japan. British Journal of Cancer 1999;81(7):1248-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2004 {published data only}
- Khan MM, Goto R, Kobayashi K, Suzumura S, Nagata Y, Sonoda T, et al. Dietary habits and cancer mortality among middle aged and older Japanese living in Hokkaido, Japan by cancer site and sex. Asian Pacific Journal of Cancer Prevention 2004;5(1):58-65. [PubMed] [Google Scholar]
Kikuchi 2006 {published data only}
- Kikuchi N, Ohmori K, Shimazu T, Nakaya N, Kuriyama S, Nishino Y, et al. No association between green tea and prostate cancer risk in Japanese men: the Ohsaki Cohort Study. British Journal of Cancer 2006;95(3):371-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kono 1988 {published data only}
- Kono S, Ikeda M, Tokudome S, Kuratsune M. A case-control study of gastric cancer and diet in northern Kyushu, Japan. Japanese Journal of Cancer Research 1988;79(10):1067-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kubik 2008 {published data only}
- Kubik A, Zatloukal P, Tomasek L, Dolezal J, Syllabova L, Kara J, et al. A case-control study of lifestyle and lung cancer associations by histological types. Neoplasma 2008;55(3):192-9. [PubMed] [Google Scholar]
- Kubik AK, Zatloukal P, Tomasek L, Pauk N, Havel L, Krepela E, et al. Dietary habits and lung cancer risk among non-smoking women. European Journal of Cancer Prevention 2004;13(6):471-80. [DOI] [PubMed] [Google Scholar]
Kumar 2015 {published data only}
- Kumar NB, Patel R, Pow-Sang J, Spiess PE, Salup R, Williams CR, et al. Long-term supplementation of decaffeinated green tea extract does not modify body weight or abdominal obesity in a randomized trial of men at high risk for prostate cancer. Oncotarget 2017;8(58):99093-103. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar NB, Pow-Sang J, Egan KM, Spiess PE, Dickinson S, Salup R, et al. Randomized, placebo-controlled trial of green tea catechins for prostate cancer prevention. Cancer Prevention Research (Philadelphia, Pa.) 2015;8(10):879-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar NB, Pow-Sang J, Spiess PE, Park J, Salup R, Williams CR, et al. Randomized, placebo-controlled trial evaluating the safety of one-year administration of green tea catechins. Oncotarget 2016;7(43):70794-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuo 2009 {published data only}
- Kuo YC, Yu CL, Liu CY, Wang SF, Pan PC, Wu MT, et al. A population-based, case-control study of green tea consumption and leukemia risk in southwestern Taiwan. Cancer Causes & Control 2009;20(1):57-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kurahashi 2007 {published data only}
- Kurahashi N, Sasazuki S, Iwasaki M, Inoue M, Tsugane S. Green tea consumption and prostate cancer risk in Japanese men: a prospective study. American Journal of Epidemiology 2007;167(1):71-7. [DOI] [PubMed] [Google Scholar]
Kurahashi 2009 {published data only}
- Kurahashi N, Inoue M, Iwasaki M, Sasazuki S, Tsugane S, Japan Public Health Center (JPHC) Study Group. Coffee, green tea, and caffeine consumption and subsequent risk of bladder cancer in relation to smoking status: a prospective study in Japan. Cancer Science 2009;100(2):294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuriyama 2006 {published data only}
- Kuriyama S, Shimazu T, Ohmori K, Kikuchi N, Nakaya N, Nishino Y, et al. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan. Journal of the American Medical Association 2006;296:1255-65. [DOI] [PubMed] [Google Scholar]
Lane 2018 {published data only}
- Lane JA, Er V, Avery KN, Horwood J, Cantwell M, Caro GP, et al. ProDiet: a phase II randomized placebo-controlled trial of green tea catechins and lycopene in men at increased risk of prostate cancer. Cancer Prevention Research (Philadelphia, Pa.) 2018;11(11):687-96. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Lane JA, Er V, Horwood J, Avery K, Holly J, Martin R, et al. A randomized controlled feasibility trial of green tea and lycopene interventions in men at elevated risk of prostate cancer (ProDiet). European Journal of Surgical Oncology 2017;43(11):2236. [Google Scholar]
Lassed 2016 {published data only}
- Lassed S, Deus CM, Lourenco N, Dahdouh A, Rizvanov AA, Oliveira PJ, et al. Diet, lifestyles, family history, and prostate cancer incidence in an East Algerian patient group. BioMed Research International 2016;2016:5730569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2007 {published data only}
- Lee K-J, Inoue M, Otani T, Iwasaki M, Sasazuki S, Tsugane S. Coffee consumption and risk of colorectal cancer in a population-based prospective cohort of Japanese men and women. International Journal of Cancer 2007;121(6):1312-8. [DOI] [PubMed] [Google Scholar]
Lee 2017 {published data only}
- Lee PM, Ng CF, Liu ZM, Ho WM, Lee MK, Wang F, et al. Reduced prostate cancer risk with green tea and epigallocatechin 3-gallate intake among Hong Kong Chinese men. Prostate Cancer and Prostatic Diseases 2017;20(3):318-22. [DOI] [PubMed] [Google Scholar]
Lei 1994 {published data only}
- Lei Y, Cai W, Chen Y, Du Y. The analysis of non-smoking risk factors and primary lung cancer with the model of conditionallogistic regression. Academic Journal of Guangzhou Medical College 1994;22(5):5-10. [Google Scholar]
Le Marchand 2000 {published data only}
- Le Marchand L, Murphy SP, Hankin JH, Wilkens LR, Kolonel LN. Intake of flavonoids and lung cancer. Journal of the National Cancer Institute 2000;92(2):154-60. [PMID: ] [DOI] [PubMed] [Google Scholar]
Leung 2016 {published data only}
- Leung AC, Cook LS, Swenerton K, Gilks B, Gallagher RP, Magliocco A, et al. Tea, coffee, and caffeinated beverage consumption and risk of epithelial ovarian cancers. Cancer Epidemiology 2016;45:119-25. [DOI] [PubMed] [Google Scholar]
Li 2008 {published data only}
- Li Q, Kakizaki M, Kuriyama S, Sone T, Yan H, Nakaya N, et al. Green tea consumption and lung cancer risk: the Ohsaki study. British Journal of Cancer 2008;99(7):1179-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2011a {published data only}
- Li L, Zhang M, Holman D. Population versus hospital controls for case-control studies on cancers in Chinese hospitals. BMC Medical Research Methodology 2011;11:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2014 {published data only}
- Li ML, Lin J, Hou JG, Xu L, Cui XG, Xu XX, et al. Environmental and psycho-social factors related to prostate cancer risk in the Chinese population: a case-control study. Biomedical and Environmental Sciences 2014;27(9):707-17. [DOI] [PubMed] [Google Scholar]
Li 2016 {published data only}
- Li M, Tse LA, Chan WC, Kwok CH, Leung SL, Wu C, et al. Evaluation of breast cancer risk associated with tea consumption by menopausal and estrogen receptor status among Chinese women in Hong Kong. Cancer Epidemiology 2016;40:73-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2018 {published data only}
- Li X, Li N, Wang G, Su K, Li F, Chang S, et al. Tea consumption and the risk of lung cancer in Chinese males: a prospective cohort study. Zhonghua Yu Fang Yi Xue za Zhi [Chinese Journal of Preventive Medicine] 2018;52(5):511-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lin 2008 {published data only}
- Lin Y, Kikuchi S, Tamakoshi A, Yagyu K, Obata Y, Kurosawa M, et al. Green tea consumption and the risk of pancreatic cancer in Japanese adults. Pancreas 2008;37(1):25-30. [DOI] [PubMed] [Google Scholar]
Lin 2012 {published data only}
- Lin IH, Ho ML, Chen HY, Lee HS, Huang CC, Chu YH, et al. Smoking, green tea consumption, genetic polymorphisms in the insulin-like growth factors and lung cancer risk. PLos One 2012;7(2):e30951. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2010 {published data only}
- Liu A, Zhao JK, Zhang ZF. Case-control study of the effect of drinking green tea on the incidence of stomach cancer in residents in Dafeng City, Jiangsu Province, China. China Cancer 2010;19:585-8. [Google Scholar]
Liu 2016 {published data only}
- Liu J, Liu S, Zhou H, Hanson T, Yang L, Chen Z, et al. Association of green tea consumption with mortality from all-cause, cardiovascular disease and cancer in a Chinese cohort of 165,000 adult men. European Journal of Epidemiology 2016;31(9):853-65. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2017 {published data only}
- Liu P, Zhang M, Xie X, Jin J, Holman CD. Green tea consumption and glutathione S-transferases genetic polymorphisms on the risk of adult leukemia. European Journal of Nutrition 2017;56(2):603-12. [DOI] [PubMed] [Google Scholar]
Luo 2007 {published data only}
- Luo J, Inoue M, Iwasaki M, Sasazuki S, Otani T, Ye W, et al. Green tea and coffee intake and risk of pancreatic cancer in a large-scale, population-based cohort study in Japan (JPHC study). European Journal of Cancer Prevention 2007;16(6):542-8. [DOI] [PubMed] [Google Scholar]
Makiuchi 2016 {published data only}
- Makiuchi T, Sobue T, Kitamura T, Ishihara J, Sawada N, Iwasaki M, et al. Association between green tea/coffee consumption and biliary tract cancer: a population-based cohort study in Japan. Cancer Science 2016;107(1):76-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mao 2011 {published data only}
- Mao XQ, Jia XF, Zhou G, Li L, Niu H, Li FL, et al. Green tea drinking habits and gastric cancer in southwest China. Asian Pacific Journal of Cancer Prevention 2011;12(9):2179-82. [PubMed] [Google Scholar]
Micali 2017 {published data only}
- Micali S, Territo A, Pirola GM, Ferrari N, Sighinolfi MC, Martorana E, et al. Effect of green tea catechins in patients with high-grade prostatic intraepithelial neoplasia: results of a short-term double-blind placebo controlled phase II clinical trial. Archivio Italiano di Urologia, Andrologia : Organo Ufficiale [di] Societa Italiana di Ecografia Urologica e Nefrologica 2017;89(3):197-202. [PMID: ] [DOI] [PubMed] [Google Scholar]
Michikawa 2011 {published data only}
- Michikawa T, Inoue M, Shimazu T, Sasazuki S, Iwasaki M, Sawada N, et al. Green tea and coffee consumption and its association with thyroid cancer risk: a population-based cohort study in Japan. Cancer Causes & Control 2011;22(7):985-93. [DOI] [PubMed] [Google Scholar]
Mizoo 2013 {published data only}
- Mizoo T, Taira N, Nishiyama K, Nogami T, Iwamoto T, Motoki T, et al. Effects of lifestyle and single nucleotide polymorphisms on breast cancer risk: a case-control study in Japanese women. BioMed Central Cancer 2013;13:565. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mizuno 1992 {published data only}
- Mizuno S, Watanabe S, Nakamura K, Omata M, Oguchi H, Ohashi K, et al. A multi-institute case-control study on the risk factors of developing pancreatic cancer. Japanese Journal of Clinical Oncology 1992;22(4):286-91. [PubMed] [Google Scholar]
Montague 2012 {published data only}
- Montague JA, Butler LM, Wu AH, Genkinger JM, Koh WP, Wong AS, et al. Green and black tea intake in relation to prostate cancer risk among Singapore Chinese. Cancer Causes & Control 2012;23(10):1635-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mu 2003 {published data only}
- Li Y, Chang SC, Goldstein BY, Scheider WL, Cai L, You NC, et al. Green tea consumption, inflammation and the risk of primary hepatocellular carcinoma in a Chinese population. Cancer Epidemiology 2011;35(4):362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu L-N, Lu Q-Y, Yu S-Z, Jiang Q-W, Cao W, You N-C, et al. Green tea drinking and multigenetic index on the risk of stomach cancer in a Chinese population. International Journal of Cancer 2005;116(6):972-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu LN, Zhou XF, Ding BG, Wang RH, Zhang ZF, Chen CW, et al. A case-control study on drinking green tea and decreasing risk of cancers in the alimentary canal among cigarette smokers and alcohol drinkers. Zhonghua Liu Xing Bing Xue za Zhi 2003;24(3):192-5. [PubMed] [Google Scholar]
- Mu LN, Zhou XF, Ding BG, Wang RH, Zhang ZF, Jiang QW, et al. Study on the protective effect of green tea on gastric, liver and esophageal cancers. Zhonghua Yu Fang Yi Xue za Zhi [Chinese Journal of Preventive Medicine] 2003;37(3):171-3. [PubMed] [Google Scholar]
Nagano 2001 {published data only}
- Nagano J, Kono S, Preston DL, Mabuchi K. A prospective study of green tea consumption and cancer incidence, Hiroshima and Nagasaki (Japan). Cancer Causes & Control 2001;12(6):501-8. [DOI] [PubMed] [Google Scholar]
- Sauvaget C, Lagarde F, Nagano J, Soda M, Koyama K, Kodama K. Lifestyle factors, radiation and gastric cancer in atomic-bomb survivors (Japan). Cancer Causes & Control 2005;16(7):773-80. [DOI] [PubMed] [Google Scholar]
Naganuma 2009 {published data only}
- Naganuma T, Kuriyama S, Kakizaki M, Sone T, Nakaya N, Ohmori-Matsuda K, et al. Green tea consumption and hematologic malignancies in Japan: the Ohsaki study. American Journal of Epidemiology 2009;170(6):730-8. [DOI] [PubMed] [Google Scholar]
Nagle 2010 {published data only}
- Nagle CM, Olsen CM, Bain CJ, Whiteman DC, Green AC, Webb PM. Tea consumption and risk of ovarian cancer. Cancer Causes & Control 2010;21(9):1485-91. [DOI] [PubMed] [Google Scholar]
Nakachi 2000 {published data only}
- Imai K, Suga K, Nakachi K. Cancer-preventive effects of drinking green tea among a Japanese population. Preventive Medicine 1997;26:769-75. [DOI] [PubMed] [Google Scholar]
- Nakachi K, Matsuyama S, Miyake S, Suganuma M, Imai K. Preventive effects of drinking green tea on cancer and cardiovascular disease: epidemiological evidence for multiple targeting prevention. Biofactors 2000;13(1-4):49-54. [DOI] [PubMed] [Google Scholar]
Nakamura 2011 {published data only}
- Nakamura K, Nagata C, Wada K, Tamai Y, Tsuji M, Takatsuka N, et al. Cigarette smoking and other lifestyle factors in relation to the risk of pancreatic cancer death: a prospective cohort study in Japan. Japanese Journal of Clinical Oncology 2011;41(2):225-31. [DOI] [PubMed] [Google Scholar]
Nechuta 2012 {published data only}
- Nechuta S, Shu XO, Li HL, Yang G, Ji BT, Xiang YB, et al. Prospective cohort study of tea consumption and risk of digestive system cancers: results from the Shanghai Women's Health Study. American Journal of Clinical Nutrition 2012;96(5):1056-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Shu XO, Li H, Chow WH, Ji BT, Zhang X, et al. Prospective cohort study of green tea consumption and colorectal cancer risk in women. Cancer Epidemiology, Biomarkers & Prevention 2007;16(6):1219-23. [DOI] [PubMed] [Google Scholar]
Oba 2006 {published data only}
- Oba S, Shimizu N, Nagata C, Shimizu H, Kametani M, Takeyama N, et al. The relationship between the consumption of meat, fat, and coffee and the risk of colon cancer: a prospective study in Japan. Cancer Letters 2006;244(2):260-7. [DOI] [PubMed] [Google Scholar]
Odegaard 2015 {published data only}
- Odegaard AO, Koh WP, Yuan JM, Pereira MA. Beverage habits and mortality in Chinese adults. Journal of Nutrition 2015;145(3):595-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ogawa 2016 {published data only}
- Ogawa T, Sawada N, Iwasaki M, Budhathoki S, Hidaka A, Yamaji T, et al. Coffee and green tea consumption in relation to brain tumor risk in a Japanese population. International Journal of Cancer 2016;139(12):2714-21. [DOI] [PubMed] [Google Scholar]
Oze 2014 {published data only}
- Oze I, Matsuo K, Kawakita D, Hosono S, Ito H, Watanabe M, et al. Coffee and green tea consumption is associated with upper aerodigestive tract cancer in Japan. International Journal of Cancer 2014;135(2):391-400. [DOI] [PubMed] [Google Scholar]
Peng 2013 {published data only}
- Peng XE, Jiang YY, Shi XS, Hu ZJ. NQO1 609C>T polymorphism interaction with tobacco smoking and alcohol drinking increases colorectal cancer risk in a Chinese population. Gene 2013;521(1):105-10. [DOI] [PubMed] [Google Scholar]
Peng 2015 {published data only}
- Peng XE, Chen HF, Hu ZJ, Shi XS. Independent and combined effects of environmental factors and CYP2C19 polymorphisms on the risk of esophageal squamous cell carcinoma in Fujian Province of China. BioMed Central Medical Genetics 2015;16:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roshdy 2013 {published data only}
- Roshdy E, Rajaratnam V, Maitra S, Sabry M, Ait Allah AS, Al-Hendy A. Treatment of symptomatic uterine fibroids with green tea extract: a pilot randomized controlled clinical study. International Journal of Women's Health 2013;5(1):477-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruan 2010 {published data only}
- Ruan HL, Xu FH, Liu WS, Feng QS, Chen LZ, Zeng YX, et al. Alcohol and tea consumption in relation to the risk of nasopharyngeal carcinoma in Guangdong, China. Frontiers of Medicine in China 2010;4(4):448-56. [DOI] [PubMed] [Google Scholar]
Saito 2015 {published data only}
- Saito E, Inoue M, Sawada N, Shimazu T, Yamaji T, Iwasaki M, et al. Association of green tea consumption with mortality due to all causes and major causes of death in a Japanese population: the Japan Public Health Center-based Prospective Study (JPHC Study). Annals of Epidemiology 2015;25(7):512-518.e3. [DOI] [PubMed] [Google Scholar]
Setiawan 2001 {published data only}
- Setiawan VW, Zhang ZF, Yu GP, Lu QY, Li YL, Lu ML, et al. Protective effect of green tea on the risks of chronic gastritis and stomach cancer. International Journal of Cancer 2001;92(4):600-4. [DOI] [PubMed] [Google Scholar]
Severson 1989 {published data only}
- Severson RK, Nomura AM, Grove JS, Stemmermann GN. A prospective study of demographics, diet, and prostate cancer among men of Japanese ancestry in Hawaii. Cancer Research 1989;49(7):1857-60. [PubMed] [Google Scholar]
Shimazu 2008 {published data only}
- Shimazu T, Inoue M, Sasazuki S, Iwasaki M, Kurahashi N, Yamaji T, et al. Coffee consumption and risk of endometrial cancer: a prospective study in Japan. International Journal of Cancer 2008;123(10):2406-10. [DOI] [PubMed] [Google Scholar]
Shrubsole 2009 {published data only}
- Shrubsole MJ, Lu W, Chen Z, Shu XO, Zheng Y, Dai Q, et al. Drinking green tea modestly reduces breast cancer risk. Journal of Nutrition 2009;139(2):310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sinicrope 2017 {published data only}
- NCT01606124. Polyphenon E in treating patients with high-risk of colorectal cancer. ClinicalTrials.gov 2012: NCT01606124.
- Sinicrope FA, Viggiano TR, Buttar NS, Song LM, Schroeder KW, Kraichely RE, et al. Phase II randomized, double-blinded, placebo-controlled trial of Polyphenon E in patients with prior advanced adenomas or colon cancer. Gastroenterology 2017;152(5 Suppl 1):S839. [DOI: 10.1016/S0016-5085(17)32897-4] [DOI] [Google Scholar]
Song 2008 {published data only}
- Song YJ, Kristal AR, Wicklund KG, Cushing-Haugen KL, Rossing MA. Coffee, tea, colas, and risk of epithelial ovarian cancer. Cancer Epidemiology, Biomarkers & Prevention 2008;17(3):712-6. [DOI] [PubMed] [Google Scholar]
Sonoda 2004 {published data only}
- Sonoda T, Nagata Y, Mori M, Miyanaga N, Takashima N, Okumura K, et al. A case-control study of diet and prostate cancer in Japan: possible protective effect of traditional Japanese diet. Cancer Science 2004;95(3):238-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sun 2007 {published data only}
- Sun CL, Yuan JM, Koh WP, Lee HP, Yu MC. Green tea and black tea consumption in relation to colorectal cancer risk: the Singapore Chinese Health Study. Carcinogenesis 2007;28(10):2143-8. [DOI] [PubMed] [Google Scholar]
Suzuki 2004 {published data only}
- Fukao A, Tsubono Y, Komatsu S, Tsuji I, Minami Y, Hisamichi S, et al. A cohort study on the relation of lifestyle, personality and biologic markers to cancer in Miyagi, Japan: study design, response rate and profiles of the cohort subjects. Journal of Epidemiology 1995;5(3):153-7. [Google Scholar]
- Suzuki Y, Tsubono Y, Nakaya N, Suzuki Y, Koizumi Y, Tsuji I. Green tea and the risk of breast cancer: pooled analysis of two prospective studies in Japan. British Journal of Cancer 2004;90(7):1361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Suzuki 2005 {published data only}
- Suzuki Y, Tsubono Y, Nakaya N, Koizumi Y, Suzuki Y, Shibuya D, et al. Green tea and the risk of colorectal cancer: pooled analysis of two prospective studies in Japan. Journal of Epidemiology 2005;15(4):118-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Suzuki 2009 {published data only}
- Suzuki E, Yorifuji T, Takao S, Komatsu H, Sugiyama M, Ohta T, et al. Green tea consumption and mortality among Japanese elderly people: the prospective Shizuoka elderly cohort. Annals of Epidemiology 2009;19(10):732-9. [DOI] [PubMed] [Google Scholar]
Tajima 1985 {published data only}
- Tajima K, Tominaga S. Dietary habits and gastro-intestinal cancers: a comparative case-control study of stomach and large intestinal cancers in Nagoya, Japan. Japanese Journal of Cancer Research 1985;76(8):705-16. [PubMed] [Google Scholar]
Takezaki 2000 {published data only}
- Takezaki T, Shinoda M, Hatooka S, Hasegawa Y, Nakamura S, Hirose K, et al. Subsite-specific risk factors for hypopharyngeal and esophageal cancer (Japan). Cancer Causes & Control 2000;11(7):597-608. [DOI] [PubMed] [Google Scholar]
Takezaki 2001 {published data only}
- Takezaki T, Hirose K, Inoue M, Hamajima N, Yatabe Y, Mitsudomi T, et al. Dietary factors and lung cancer risk in Japanese: with special reference to fish consumption and adenocarcinomas. British Journal of Cancer 2001;84(9):1199-206. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tamura 2018 {published data only}
- Tamura T, Wada K, Konishi K, Goto Y, Mizuta F, Koda S, et al. Coffee, green tea, and caffeine intake and liver cancer risk: a prospective cohort study. Nutrition and Cancer 2018;70(8):1210-16. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tewes 1990 {published data only}
- Tewes FJ, Koo LC, Meisgen TJ, Rylander R. Lung cancer risk and mutagenicity of tea. Environmental Research 1990;52(1):23-33. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tsao 2009 {published data only}
- Tsao AS, Liu D, Martin J, Tang XM, Lee JJ, El-Naggar AK, et al. Phase II randomized, placebo-controlled trial of green tea extract in patients with high-risk oral premalignant lesions. Cancer Prevention Research (Philadelphia, Pa.) 2009;2(11):931-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tse 2017 {published data only}
- Tse LA, Lee PM, Ho WM, Lam AT, Lee MK, Ng SS, et al. Bisphenol A and other environmental risk factors for prostate cancer in Hong Kong. Environment International 2017;107:1-7. [DOI] [PubMed] [Google Scholar]
Ugai 2017 {published data only}
- Ugai T, Matsuo K, Kanda Y, Tsugane S, Sawada N, Iwasaki M, et al. Coffee and green tea consumption and subsequent risk of malignant lymphoma and multiple myeloma in Japan: the Japan Public Health Center-based Prospective Study. Cancer Epidemiology, Biomarkers & Prevention 2017;26(8):1352-6. [DOI] [PubMed] [Google Scholar]
Ugai 2018 {published data only}
- Ugai T, Matsuo K, Sawada N, Iwasaki M, Yamaji T, Shimazu T, et al. Coffee and green tea consumption and subsequent risk of acute myeloid leukemia and myelodysplastic syndromes in Japan. International Journal of Cancer 2018;142(6):1130-8. [DOI] [PubMed] [Google Scholar]
Ui 2009 {published data only}
- Ui A, Kuriyama S, Kakizaki M, Sone T, Nakaya N, Ohmori-Matsuda K, et al. Green tea consumption and the risk of liver cancer in Japan: the Ohsaki Cohort study. Cancer Causes & Control 2009;20(10):1939-45. [DOI] [PubMed] [Google Scholar]
Wakai 2004 {published data only}
- Wakai K, Hirose K, Takezaki T, Hamajima N, Ogura Y, Nakamura S, et al. Foods and beverages in relation to urothelial cancer: case-control study in Japan. International Journal of Urology 2004;11(1):11-9. [DOI] [PubMed] [Google Scholar]
Wang 1999 {published data only}
- Wang M, Guo C, Li M. A case-control study on the dietary risk factors of upper digestive tract cancer. Zhonghua Liu Xing Bing Xue za Zhi 1999;20(2):95-7. [PubMed] [Google Scholar]
Wang 2006 {published data only}
- Wang Z, Tang L, Sun G, Tang Y, Xie Y, Wang S, et al. Etiological study of esophageal squamous cell carcinoma in an endemic region: a population-based case control study in Huaian, China. BioMed Central Cancer 2006;6:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2007 {published data only}
- Wang JM, Xu B, Rao JY, Shen HB, Xue HC, Jiang QW. Diet habits, alcohol drinking, tobacco smoking, green tea drinking, and the risk of esophageal squamous cell carcinoma in the Chinese population. European Journal of Gastroenterology and Hepatology 2007;19(2):171-6. [DOI] [PubMed] [Google Scholar]
Wang 2012a {published data only}
- Wang G, Hou J, Ma L, Xie J, Yin J, Xu D, et al. Risk factor for clear cell renal cell carcinoma in Chinese population: a case-control study. Cancer Epidemiology 2012;36(2):177-82. [DOI] [PubMed] [Google Scholar]
Wang 2012b {published data only}
- Wang Q, Wang Y, Ji Z, Chen X, Pan Y, Gao G, et al. Risk factors for multiple myeloma: a hospital-based case-control study in Northwest China. Cancer Epidemiology 2012;36(5):439-44. [DOI] [PubMed] [Google Scholar]
Wang 2012c {published data only}
- Wang J, Zhang W, Sun L, Yu H, Ni QX, Risch HA, et al. Green tea drinking and risk of pancreatic cancer: a large-scale, population-based case-control study in urban Shanghai. Cancer Epidemiology 2012;36(6):e354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2013a {published data only}
- Wang L, Liao WC, Tsai CJ, Wang LR, Mao IF, Chen CC, et al. The effects of perceived stress and life style leading to breast cancer. Women Health 2013;53(1):20-40. [DOI] [PubMed] [Google Scholar]
Wang 2013b {published data only}
- Wang J, Wu X, Kamat A, Barton Grossman H, Dinney CP, Lin J. Fluid intake, genetic variants of UDP-glucuronosyltransferases, and bladder cancer risk. British Journal of Cancer 2013;108(11):2372-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2015 {published data only}
- Wang Y, Duan H, Yang H. A case-control study of stomach cancer in relation to Camellia sinensis in China. Surgical Oncology 2015;24(2):67-70. [DOI] [PubMed] [Google Scholar]
Wilkens 1996 {published data only}
- Wilkens LR, Kadir MM, Kolonel LN, Nomura AM, Hankin JH. Risk factors for lower urinary tract cancer: the role of total fluid consumption, nitrites and nitrosamines, and selected foods. Cancer Epidemiology, Biomarkers & Prevention 1996;5(3):161-6. [PubMed] [Google Scholar]
Wu 2003 {published data only}
- Wu AH, Yu MC, Tseng CC, Hankin J, Pike MC. Green tea and risk of breast cancer in Asian Americans. International Journal of Cancer 2003;106(4):574-9. [DOI] [PubMed] [Google Scholar]
Wu 2009a {published data only}
- Wu YJ, Liang CH, Zhou FJ, Gao X, Chen LW, Liu Q. A case-control study of environmental and genetic factors and prostate cancer in Guangdong. Zhonghua Yu Fang Yi Xue za Zhi [Chinese Journal of Preventive Medicine] 2009;43(7):581-5. [PubMed] [Google Scholar]
Wu 2009b {published data only}
- Wu M, Liu AM, Kampman E, Zhang ZF, Van't Veer P, Wu DL, et al. Green tea drinking, high tea temperature and esophageal cancer in high- and low-risk areas of Jiangsu Province, China: a population-based case-control study. International Journal of Cancer 2009;124(8):1907-13. [DOI] [PubMed] [Google Scholar]
Xu 2007 {published data only}
- Xu WH, Dai Q, Xiang YB, Long JR, Ruan ZX, Cheng JR, et al. Interaction of soy food and tea consumption with CYP19A1 genetic polymorphisms in the development of endometrial cancer. American Journal of Epidemiology 2007;166(12):1420-30. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Xu 2013 {published data only}
- Xu X, Cai L. A case-control study on tea consumption and the risk of lung cancer. Wei Sheng Yan Jiu [Journal of Hygiene Research] 2013;42(2):211-6. [PubMed] [Google Scholar]
Yan 2016 {published data only}
- Yan LJ, Chen F, Liu DM, Huang JF, Liu FP, Wu JF, et al. Tea, coffee intakes and risk of oral squamous cell carcinoma: a case-control study. Zhonghua Liu Xing Bing Xue za Zhi 2016;37(11):1531-5. [DOI] [PubMed] [Google Scholar]
Yang 2011a {published data only}
- Yang G, Zheng W, Xiang YB, Gao J, Li HL, Zhang X, et al. Green tea consumption and colorectal cancer risk: a report from the Shanghai Men's Health Study. Carcinogenesis 2011;32(11):1684-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ye 1998 {published data only}
- Ye WM, Yi YN, Luo RX, Zhou TS, Lin RT, Chen GD. Diet and gastric cancer: a case-control study in Fujian Province, China. World Journal of Gastroenterology 1998;4(6):516-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yu 1995 {published data only}
- Yu GP, Hsieh CC, Wang LY, Yu SZ, Li XL, Jin TH. Green-tea consumption and risk of stomach cancer: a population-based case-control study in Shanghai, China. Cancer Causes & Control 1995;6(6):532-8. [DOI] [PubMed] [Google Scholar]
Zhang 2002 {published data only}
- Zhang M, Binns CW, Lee AH. Tea consumption and ovarian cancer risk: a case-control study in China. Cancer Epidemiology, Biomarkers & Prevention 2002;11(8):713-8. [PubMed] [Google Scholar]
Zhang 2007 {published data only}
- Zhang M, Holman CD, Huang JP, Xie X. Green tea and the prevention of breast cancer: a case-control study in Southeast China. Carcinogenesis 2007;28(5):1074-8. [DOI] [PubMed] [Google Scholar]
- Zhang M, Huang J, Xie X, Holman CD. Dietary intakes of mushrooms and green tea combine to reduce the risk of breast cancer in Chinese women. International Journal of Cancer 2009;124(6):1404-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zhang 2008 {published data only}
- Zhang M, Zhao X, Zhang X, Holman CD. Possible protective effect of green tea intake on risk of adult leukaemia. British Journal of Cancer 2008;98(1):168-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XD, Zhao XY, Zhang M, Liang Y, Xu XH, D'Arcy C, et al. A case-control study on green tea consumption and the risk of adult leukemia. Zhonghua Liu Xing Bing Xue za Zhi 2008;29(3):290-3. [PubMed] [Google Scholar]
Zhao 2017 {published data only}
- Zhao LG, Li HL, Sun JW, Yang Y, Ma X, Shu XO, et al. Green tea consumption and cause-specific mortality: results from two prospective cohort studies in China. Journal of Epidemiology 2017;27(1):36-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zheng 1993 {published data only}
- Zheng T, Boyle P, Willett WC, Hu H, Dan J, Evstifeeva TV, et al. A case-control study of oral cancer in Beijing, People's Republic of China. Associations with nutrient intakes, foods and food groups. European Journal of Cancer. Part B, Oral Oncology 1993;29(1):45-55. [DOI] [PubMed] [Google Scholar]
Zhong 2001 {published data only}
- Zhong L, Goldberg MS, Gao YT, Hanley JA, Parent ME, Jin F. A population-based case-control study of lung cancer and green tea consumption among women living in Shanghai, China. Epidemiology 2001;12(6):695-700. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ahmad 2012 {published data only}
- Ahmad MR, Pervaiz MK, Pervaiz G. Non-occupational risk factors of urinary bladder cancer in Faisalabad and Lahore, Pakistan. Journal of the Pakistan Medical Association 2012;62(3):236-9. [PubMed] [Google Scholar]
Allen 2011 {published data only}
- Allen NE, Balkwill A, Beral V, Green J, Reeves G, Million Women Study Collaborators. Fluid intake and incidence of renal cell carcinoma in UK women. British Journal of Cancer 2011;104(9):1487-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alsanad 2016 {published data only}
- Alsanad SM, Howard RL, Williamson EM. An assessment of the impact of herb-drug combinations used by cancer patients. BMC Complementary and Alternative Medicine 2016;16(1):393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Amarasinghe 2013 {published data only}
- Amarasinghe HK, Usgodaarachchi U, Kumaraarachchi M, Johnson NW, Warnakulasuriya S. Diet and risk of oral potentially malignant disorders in rural Sri Lanka. Journal of Oral Pathology & Medicine 2013;42(9):656-62. [DOI] [PubMed] [Google Scholar]
Arts 2001 {published data only}
- Arts IC, Hollman PC, Bueno De Mesquita HB, Feskens EJ, Kromhout D. Dietary catechins and epithelial cancer incidence: the Zutphen elderly study. International Journal of Cancer 2001;92(2):298-302. [DOI] [PubMed] [Google Scholar]
Asgari 2011 {published data only}
- Asgari MM, White E, Warton EM, Hararah MK, Friedman GD, Chren MM. Association of tea consumption and cutaneous squamous cell carcinoma. Nutrition and Cancer 2011;63(2):314-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Askari 2014 {published data only}
- Askari F, Parizi MK, Jessri M, Rashidkhani B. Dietary patterns in relation to prostate cancer in Iranian men: a case-control study. Asian Pacific Journal of Cancer Prevention 2014;15(5):2159-63. [DOI] [PubMed] [Google Scholar]
Azeem 2013 {published data only}
- Azeem K, Sevcikova J, Tomaskova H, Horakova D, Prochazka V, Martinek A, et al. Pancreatic cancer and lifestyle factors. Klinicka Onkologie 2013;26(4):257-62. [DOI] [PubMed] [Google Scholar]
Bailey 2017 {published data only}
- Bailey HD, Lacour B, Guerrini-Rousseau L, Bertozzi A-I, Leblond P, Faure-Conter C, et al. Parental smoking, maternal alcohol, coffee and tea consumption and the risk of childhood brain tumours: the ESTELLE and ESCALE studies (SFCE, France). Cancer Causes & Control 2017;28(7):719-32. [DOI] [PubMed] [Google Scholar]
Bamia 2015 {published data only}
- Bamia C, Lagiou P, Jenab M, Trichopoulou A, Fedirko V, Aleksandrova K, et al. Coffee, tea and decaffeinated coffee in relation to hepatocellular carcinoma in a European population: multicentre, prospective cohort study. International Journal of Cancer 2015;136(8):1899-908. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bao 2015 {published data only}
- Bao PP, Zhao GM, Shu XO, Peng P, Cai H, Lu W, et al. Modifiable lifestyle factors and triple-negative breast cancer survival: a population-based prospective study. Epidemiology (Cambridge, Mass.) 2015;26(6):909-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baroudi 2014 {published data only}
- Baroudi O, Chaaben AB, Mezlini A, Moussa A, Omrane I, Jilson I, et al. Impact of lifestyle factors and nutrients intake on occurrence of gastrointestinal cancer in Tunisian population. Tumour Biology 2014;35(6):5815-22. [DOI] [PubMed] [Google Scholar]
Bates 2007 {published data only}
- Bates MN, Hopenhayn C, Rey OA, Moore LE. Bladder cancer and mate consumption in Argentina: a case-control study. Cancer Letters 2007;246(1-2):268-73. [DOI] [PubMed] [Google Scholar]
Bianchi 2000 {published data only}
- Bianchi GD, Cerhan JR, Parker AS, Putnam SD, See WA, Lynch CF, et al. Tea consumption and risk of bladder and kidney cancers in a population-based case-control study. American Journal of Epidemiology 2000;151(4):377-83. [DOI] [PubMed] [Google Scholar]
Bonaventure 2013 {published data only}
- Bonaventure A, Rudant J, Goujon-Bellec S, Orsi L, Leverger G, Baruchel A, et al. Childhood acute leukemia, maternal beverage intake during pregnancy, and metabolic polymorphisms [Erratum appears in Cancer Causes Control. 2014 Aug;25(8):1081]. Cancer Causes & Control 2013;24(4):783-93. [DOI] [PubMed] [Google Scholar]
Butler 2015 {published data only}
- Butler LM, Huang JY, Wang R, Lee MJ, Yang CS, Gao YT, et al. Urinary biomarkers of catechins and risk of hepatocellular carcinoma in the Shanghai Cohort Study. American Journal of Epidemiology 2015;181(6):397-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2009 {published data only}
- Chen YK, Lee CH, Wu IC, Liu JS, Wu DC, Lee JM, et al. Food intake and the occurrence of squamous cell carcinoma in different sections of the esophagus in Taiwanese men. Nutrition (Burbank, Los Angeles County, Calif.) 2009;25(7-8):753-61. [DOI] [PubMed] [Google Scholar]
Chyou 1995 {published data only}
- Chyou PH, Nomura AM, Stemmermann GN. Diet, alcohol, smoking and cancer of the upper aerodigestive tract: a prospective study among Hawaii Japanese men. International Journal of Cancer 1995;60(5):616-21. [DOI] [PubMed] [Google Scholar]
DArena 2013 {published data only}
- D'Arena G, Simeon V, De Martino L, Statuto T, D'Auria F, Volpe S, et al. Regulatory T-cell modulation by green tea in chronic lymphocytic leukemia. International Journal of Immunopathology and Pharmacology 2013;26(1):117-25. [DOI: 10.1177/039463201302600111] [DOI] [PubMed] [Google Scholar]
Deandrea 2010 {published data only}
- Deandrea S, Foschi R, Galeone C, La Vecchia C, Negri E, Hu J. Is temperature an effect modifier of the association between green tea intake and gastric cancer risk? European Journal of Cancer Prevention 2010;19(1):18-22. [DOI] [PubMed] [Google Scholar]
Emami 2014 {published data only}
- Emami H, Nikoobin F, Roayaei M, Ziya HR. Double-blinded, randomized, placebo-controlled study to evaluate the effectiveness of green tea in preventing acute gastrointestinal complications due to radiotherapy. Journal of Research in Medical Sciences 2014;19(5):445-50. [PMC free article] [PubMed] [Google Scholar]
Ettrich 2012 {published data only}
- Ettrich T, Stingl J, Muche R, Berger A, Seufferlein T. The MIRACLE trial: a randomized, controlled trial comparing green tea extract versus placebo for the prevention of metachronous colon adenomas in a screening population. Journal of Clinical Oncology 2015;33(3 Suppl):TPS786. [Google Scholar]
Ferrucci 2014 {published data only}
- Ferrucci LM, Cartmel B, Molinaro AM, Leffell DJ, Bale AE, Mayne ST. Tea, coffee, and caffeine and early-onset basal cell carcinoma in a case-control study. European Journal of Cancer Prevention 2014;23(4):296-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gao 2002 {published data only}
- Gao C-M, Takezaki T, Wu J-Z, Li Z-Y, Liu Y-T, Li S-P, et al. Glutathione-S-transferases M1 (GSTM1) and GSTT1 genotype, smoking, consumption of alcohol and tea and risk of esophageal and stomach cancers: a case-control study of a high-incidence area in Jiangsu Province, China. Cancer Letters 2002;188(1-2):95-102. [DOI] [PubMed] [Google Scholar]
Gao 2009 {published data only}
- Gao Y, Hu N, Han X, Giffen C, Ding T, Goldstein AM, et al. Jasmine tea consumption and upper gastrointestinal cancer in China. Cancer Causes & Control 2009;20(10):1997-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hara 1984 {published data only}
- Hara N, Sakata K, Nagai M, Fujita Y, Hashimoto T, Yanagawa H. Statistical analyses on the pattern of food consumption and digestive-tract cancers in Japan. Nutrition and Cancer 1984;6(4):220-8. [DOI] [PubMed] [Google Scholar]
He 2017 {published data only}
- He F, Xie JX, Liu CL, Xiong WM, Xu QP, Liu ZQ, et al. The relationship of lung cancer with menstrual and reproductive factors may be influenced by passive smoking, cooking oil fumes, and tea intake: a case-control study in Chinese women. Medicine 2017;96(46):e8816. [DOI] [PMC free article] [PubMed] [Google Scholar]
Henning 2012 {published data only}
- Henning SM, Wang P, Aronson WJ, Carpenter CL, Heber D. Inhibition of NFkappaB and proapoptotic effect of green tea in prostate tumor tissue: a phase II clinical trial in men with prostate cancer. Cancer Prevention Research (Philadelphia, Pa.) 2012;5(11 Suppl 1):B67. [DOI: ] [Google Scholar]
Ide 2008 {published data only}
- Ide R, Mizoue T, Fujino Y, Hoshiyama Y, Sakata K, Tamakoshi A, et al. Cigarette smoking, alcohol drinking, and oral and pharyngeal cancer mortality in Japan. Oral diseases 2008;14(4):314-19. [DOI] [PubMed] [Google Scholar]
Il'yasova 2003 {published data only}
- Il'yasova D, Martin C, Sandler RS. Tea intake and risk of colon cancer in African-Americans and whites: North Carolina colon cancer study. Cancer Causes & Control 2003;14(8):767-72. [DOI] [PubMed] [Google Scholar]
Inoue 1997 {published data only}
- Inoue M, Tajima K, Hirose K, Hamajima N, Takezaki T, Kuroishi T, et al. Epidemiological features of first-visit outpatients in Japan: comparison with general population and variation by sex, age, and season. Journal of Clinical Epidemiology 1997;50(1):69-77. [DOI] [PubMed] [Google Scholar]
Inoue 2001 {published data only}
- Inoue M, Tajima K, Mizutani M, Iwata H, Iwase T, Miura S, et al. Regular consumption of green tea and the risk of breast cancer recurrence: follow-up study from the Hospital-based Epidemiologic Research Program at Aichi Cancer Center (HERPACC), Japan. Cancer Letters 2001;167(2):175-82. [DOI] [PubMed] [Google Scholar]
Ishizuka 2003 {published data only}
- Ishizuka H, Eguchi H, Oda T, Ogawa S, Nakagawa K, Honjo S, et al. Relation of coffee, green tea, and caffeine intake to gallstone disease in middle-aged Japanese men. European Journal of Epidemiology 2003;18(5):401-5. [DOI] [PubMed] [Google Scholar]
Jatoi 2003 {published data only}
- Jatoi A, Ellison N, Burch PA, Sloan JA, Dakhil SR, Novotny P, et al. A phase II trial of green tea in the treatment of patients with androgen independent metastatic prostate carcinoma. Cancer 2003;97(6):1442-6. [DOI] [PubMed] [Google Scholar]
Jia 2012 {published data only}
- Jia Y, Hu T, Hang CY, Yang R, Li X, Chen ZL, et al. Case-control study of diet in patients with cervical cancer or precancerosis in Wufeng, a high incidence region in China. Asian Pacific Journal of Cancer Prevention 2012;13(10):5299-302. [DOI] [PubMed] [Google Scholar]
Johnson 2011 {published data only}
- Johnson S, Koh WP, Wang R, Govindarajan S, Yu MC, Yuan JM. Coffee consumption and reduced risk of hepatocellular carcinoma: findings from the Singapore Chinese Health Study. Cancer Causes & Control 2011;22(3):503-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kono 1991 {published data only}
- Kono S, Shinchi K, Ikeda N, Yanai F, Imanishi K. Physical activity, dietary habits and adenomatous polyps of the sigmoid colon: a study of self-defense officials in Japan. Journal of Clinical Epidemiology 1991;44(11):1255-61. [DOI] [PubMed] [Google Scholar]
Kuwahara 2000 {published data only}
- Kuwahara Y, Kono S, Eguchi H, Hamada H, Shinchi K, Imanishi K. Relationship between serologically diagnosed chronic atrophic gastritis, Helicobacter pylori, and environmental factors in Japanese men. Scandinavian Journal of Gastroenterology 2000;35(5):476-81. [DOI] [PubMed] [Google Scholar]
Lee 1990 {published data only}
- Lee HH, Wu HY, Chuang YC, Chang AS, Chao HH, Chen KY, et al. Epidemiologic characteristics and multiple risk factors of stomach cancer in Taiwan. Anticancer Research 1990;10(4):875-81. [PubMed] [Google Scholar]
Lee 2013 {published data only}
- Lee AH, Su D, Pasalich M, Binns CW. Tea consumption reduces ovarian cancer risk. Cancer Epidemiology 2013;37(1):54-9. [DOI] [PubMed] [Google Scholar]
Liu 2013a {published data only}
- Liu H, Jiang X, Zhang MW, Pan YF, Yu YX, Zhang SC, et al. Association of CASP9, CASP10 gene polymorphisms and tea drinking with colorectal cancer risk in the Han Chinese population. Journal of Zhejiang University. Science 2013;14(1):47-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2014 {published data only}
- Liu SZ, Chen WQ, Wang N, Yin MM, Sun XB, He YT. Dietary factors and risk of pancreatic cancer: a multi-centre case-control study in China. Asian Pacific Journal of Cancer Prevention 2014;15(18):7947-50. [DOI] [PubMed] [Google Scholar]
Liu 2015 {published data only}
- Liu P, Zhang M, Jin J, Holman CD. Tea consumption reduces the risk of de novo myelodysplastic syndromes. Leukemia Research 2015;39(2):164-9. [DOI] [PubMed] [Google Scholar]
Luo 2010 {published data only}
- Luo J, Gao YT, Chow WH, Shu XO, Li H, Yang G, et al. Urinary polyphenols and breast cancer risk: results from the Shanghai Women's Health Study [Erratum appears in Breast Cancer Res Treat. 2010 Apr;120(3):703]. Breast Cancer Research and Treatment 2010;120(3):693-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Menzler 2015 {published data only}
- Menzler S, Weinel R, Seufferlein TT. Does green tea prevent colorectal adenomas. Presentation of the design of a prospective, multicenter double-blind study. European Surgical Research. Europaische Chirurgische Forschung. Recherches Chirurgicales Europeennes 2015;55(1-2):107-8. [DOI: 10.1159/000381514] [DOI] [Google Scholar]
Mineharu 2011 {published data only}
- Mineharu Y, Koizumi A, Wada Y, Iso H, Watanabe Y, Date C, et al, JACC study Group. Coffee, green tea, black tea and oolong tea consumption and risk of mortality from cardiovascular disease in Japanese men and women. Journal of Epidemiology and Community Health 2011;65(3):230-40. [DOI] [PubMed] [Google Scholar]
Montella 2007 {published data only}
- Montella M, Polesel J, La Vecchia C, Dal Maso L, Crispo A, Crovatto M, et al. Coffee and tea consumption and risk of hepatocellular carcinoma in Italy. International Journal of Cancer 2007;120:1555-9. [DOI] [PubMed] [Google Scholar]
Montella 2009 {published data only}
- Montella M, Tramacere I, Tavani A, Gallus S, Crispo A, Talamini R, et al. Coffee, decaffeinated coffee, tea intake and risk of renal cell cancer. Nutrition and Cancer 2009;61(1):76-80. [DOI] [PubMed] [Google Scholar]
Nagano 2000 {published data only}
- Nagano J, Kono S, Preston DL, Moriwaki H, Sharp GB, Koyama K, et al. Bladder-cancer incidence in relation to vegetable and fruit consumption: a prospective study of atomic-bomb survivors. International Journal of Cancer 2000;86(1):132-8. [DOI] [PubMed] [Google Scholar]
Nakachi 1998 {published data only}
- Nakachi K, Suemasu K, Suga K, Takeo T, Imai K, Higashi Y. Influence of drinking green tea on breast cancer malignancy among Japanese patients. Japanese Journal of Cancer Research 1998;89(3):254-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nakachi 2003 {published data only}
- Nakachi K, Eguchi H, Imai K. Can teatime increase one's lifetime? Ageing Research Reviews 2003;2(1):1-10. [DOI] [PubMed] [Google Scholar]
Oguni 1992 {published data only}
- Oguni I, Chen SJ, Lin PZ. Protection against cancer risk by Japanese green tea. Preventive Medicine 1992;21:332. [Google Scholar]
Ohno 1985 {published data only}
- Ohno Y, Aoki K, Obata K, Morrison AS. Case-control study of urinary bladder cancer in metropolitan Nagoya. National Cancer Institute Monographs 1985;69:229-34. [PubMed] [Google Scholar]
Ohno 1995 {published data only}
- Ohno Y, Wakai K, Genka K, Ohmine K, Kawamura T, Tamakoshi A et al. Tea consumption and lung cancer risk: a case-control study in Okinawa, Japan. Japanese Journal of Cancer Research 1995;86(11):1027-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parodi 2017 {published data only}
- Parodi S, Merlo DF, Stagnaro E. Coffee and tea consumption and risk of leukaemia in an adult population: a reanalysis of the Italian multicentre case-control study. Cancer Epidemiology 2017;47:81-7. [DOI: 10.1016/j.canep.2017.01.005] [DOI] [PubMed] [Google Scholar]
Pisters 2001 {published data only}
- Pisters KM, Newman RA, Coldman B, Shin DM, Khuri FR, Hong WK, et al. Phase I trial of oral green tea extract in adult patients with solid tumors. Journal of Clinical Oncology 2001;19(6):1830-8. [DOI] [PubMed] [Google Scholar]
Ren 1991 {published data only}
- Ren A, Han X. Dietary factors and esophageal cancer: a case-control study. Zhonghua Liu Xing Bing Xue za Zhi 1991;12(4):200-4. [PubMed] [Google Scholar]
Sasazuki 2008 {published data only}
- Sasazuki S, Inoue M, Miura T, Iwasaki M, Tsugane S. Plasma tea polyphenols and gastric cancer risk: a case-control study nested in a large population-based prospective study in Japan. Cancer Epidemiology, Biomarkers & Prevention 2008;17(2):343-51. [DOI] [PubMed] [Google Scholar]
Sasazuki 2012 {published data only}
- Sasazuki S, Tamakoshi A, Matsuo K, Ito H, Wakai K, Nagata C, et al, Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan. Green tea consumption and gastric cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Japanese Journal of Clinical Oncology 2012;42(4):335-46. [DOI] [PubMed] [Google Scholar]
Sawada 2017 {published data only}
- Sawada N. Risk and preventive factors for prostate cancer in Japan: the Japan Public Health Center-based prospective (JPHC) study. Journal of Epidemiology 2017;27(1):2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seo 2013 {published data only}
- Seo AY, Lee DH, Shin CM, Kim SB, Son W-C, Kim N, et al. Green tea extracts for the prevention of colorectal adenomas and cancer: a preliminary report. Journal of Gastroenterology and Hepatology 2013;28:121. [DOI: ] [Google Scholar]
Shibata 2000 {published data only}
- Shibata K, Moriyama M, Fukushima T, Kaetsu A, Miyazaki M, Une H. Green tea consumption and chronic atrophic gastritis: a cross-sectional study in a green tea production village. Journal of Epidemiology 2000;10(5):310-6. [DOI] [PubMed] [Google Scholar]
Shim 1995 {published data only}
- Shim JS, Kang MH, Kim YH, Roh JK, Roberts C, Lee IP. Chemopreventive effect of green tea (Camellia sinensis) among cigarette smokers. Cancer Epidemiology, Biomarkers & Prevention 1995;4(4):387-91. [PubMed] [Google Scholar]
Shimizu 2008 {published data only}
- Shimizu M, Fukutomi Y, Ninomiya M, Nagura K, Kato T, Araki H, et al. Green tea extracts for the prevention of metachronous colorectal adenomas: a pilot study. Cancer Epidemiology, Biomarkers & Prevention 2008;17(11):3020-5. [DOI: 10.1158/1055-9965.EPI-08-0528] [DOI] [PubMed] [Google Scholar]
Shin 2018 {published data only}
- Shin CM, Lee DH, Seo AY, Lee HJ, Kim SB, Son W-C, et al. Green tea extracts for the prevention of metachronous colorectal polyps among patients who underwent endoscopic removal of colorectal adenomas: a randomized clinical trial. Clinical Nutrition 2018;37(2):452-8. [DOI: 10.1016/j.clnu.2017.01.014] [DOI] [PubMed] [Google Scholar]
Stingl 2011 {published data only}
- Stingl JC, Ettrich T, Muche R, Wiedom M, Brockmoller J, Seeringer A, et al. Protocol for minimizing the risk of metachronous adenomas of the colorectum with green tea extract (MIRACLE): a randomised controlled trial of green tea extract versus placebo for nutriprevention of metachronous colon adenomas in the elderly population. BMC Cancer 2011;11:360. [DOI: 10.1186/1471-2407-11-360] [DOI] [PMC free article] [PubMed] [Google Scholar]
Suganuma 1999 {published data only}
- Suganuma M, Okabe S, Sueoka N, Sueoka E, Matsuyama S, Imai K, et al. Green tea and cancer chemoprevention. Mutation Research 1999;428(1-2):339-44. [DOI] [PubMed] [Google Scholar]
Sun 2002 {published data only}
- Sun CL, Yuan JM, Lee MJ, Yang CS, Gao YT, Ross RK, et al. Urinary tea polyphenols in relation to gastric and esophageal cancers: a prospective study of men in Shanghai, China. Carcinogenesis 2002;23(9):1497-1503. [DOI] [PubMed] [Google Scholar]
Tong 2014 {published data only}
- Tong GX, Liang H, Chai J, Cheng J, Feng R, Chen PL, et al. Association of risk of gastric cancer and consumption of tobacco, alcohol and tea in the Chinese population. Asian Pacific Journal of Cancer Prevention 2014;15(20):8765-74. [DOI] [PubMed] [Google Scholar]
Tsubono 1997 {published data only}
- Tsubono Y, Takahashi T, Iwase Y, Iitoi Y, Akabane M, Tsugane S. Dietary differences with green tea intake among middle-aged Japanese men and women. Preventive Medicine 1997;26(5 Pt 1):704-10. [DOI] [PubMed] [Google Scholar]
Tsugane 2014 {published data only}
- Tsugane S, Sawada N. The JPHC study: design and some findings on the typical Japanese diet. Japanese Journal of Clinical Oncology 2014;44(9):777-82. [DOI] [PubMed] [Google Scholar]
Wakai 1993 {published data only}
- Wakai K, Ohno Y, Obata K, Aoki K. Prognostic significance of selected lifestyle factors in urinary bladder cancer. Japanese Journal of Cancer Research 1993;84(12):1223-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2002 {published data only}
- Wang LD, Zhou Q, Feng CW, Liu B, Qi YJ, Zhang YR, et al. Intervention and follow-up on human esophageal precancerous lesions in Henan, northern China, a high-incidence area for esophageal cancer. Gan to Kagaku Ryoho. Cancer & Chemotherapy 2002;29 Suppl 1:159-72. [PubMed] [Google Scholar]
Wang 2008 {published data only}
- Wang N, Zheng Y, Jiang Q, Yu X, Chen Y. Tea and reduced liver cancer mortality. Epidemiology (Cambridge, Mass.) 2008;19(5):761. [DOI] [PubMed] [Google Scholar]
Wang 2010 {published data only}
- Wang P, Aronson WJ, Huang M, Zhang Y, Lee RP, Heber D, et al. Green tea polyphenols and metabolites in prostatectomy tissue: implications for cancer prevention. Cancer Prevention Research (Philadelphia, Pa.) 2010;3(8):985-93. [DOI: 10.1158/1940-6207.CAPR-09-0210] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2012d {published data only}
- Wang P, Aronson W, Abgaryan N, Carpenter CL, Vadgama JV, Heber D, et al. Antioxidant activity of green tea: a phase II clinical trial in men with prostate cancer. Cancer Prevention Research (Philadelphia, Pa.) 2012;5 Suppl 1(11):B68. [DOI: ] [Google Scholar]
Wang 2012e {published data only}
- Wang ZH, Gao QY, Fang JY. Green tea and incidence of colorectal cancer: evidence from prospective cohort studies. Nutrition and Cancer 2012;64(8):1143-52. [DOI] [PubMed] [Google Scholar]
Wang 2014a {published data only}
- Wang L, Zhang X, Liu J, Shen L, Li Z. Tea consumption and lung cancer risk: a meta-analysis of case-control and cohort studies. Nutrition (Burbank, Los Angeles County, Calif.) 2014;30(10):1122-7. [DOI] [PubMed] [Google Scholar]
Wu 2003a {published data only}
- Wu AH, Tseng CC, Van den Berg D, Yu MC. Tea intake, COMT genotype, and breast cancer in Asian-American women. Cancer Research 2003;63(21):7526-9. [PubMed] [Google Scholar]
Wu 2013a {published data only}
- Wu S, Li F, Huang X, Hua Q, Huang T, Liu Z, et al. The association of tea consumption with bladder cancer risk: a meta-analysis. Asia Pacific Journal of Clinical Nutrition 2013;22(1):128-37. [DOI] [PubMed] [Google Scholar]
Yu 1991 {published data only}
- Yu GP, Hsieh CC. Risk factors for stomach cancer: a population-based case-control study in Shanghai. Cancer Causes & Control 1991;2(3):169-74. [DOI] [PubMed] [Google Scholar]
YuanJ M 2007 {published data only}
- Yuan J-M, Gao Y-T, Yang CS, Yu MC. Urinary biomarkers of tea polyphenols and risk of colorectal cancer in the Shanghai Cohort Study. International Journal of Cancer 2007;120(6):1344-50. [DOI] [PubMed] [Google Scholar]
Zeegers 2001a {published data only}
- Zeegers MP, Dorant E, Goldbohm RA, Van den Brandt PA. Are coffee, tea, and total fluid consumption associated with bladder cancer risk? Results from the Netherlands cohort study. Cancer Causes & Control 2001;12(3):231-8. [DOI] [PubMed] [Google Scholar]
Zeegers 2001b {published data only}
- Zeegers MP, Tan FE, Goldbohm RA, Van den Brandt PA. Are coffee and tea consumption associated with urinary tract cancer risk? A systematic review and meta-analysis. International Journal of Epidemiology 2001;30(2):353-62. [DOI] [PubMed] [Google Scholar]
Zhang 2004 {published data only}
- Zhang M, Lee AH, Binns CW, Xie X. Green tea consumption enhances survival of epithelial ovarian cancer. International Journal of Cancer 2004;112(3):465-9. [DOI] [PubMed] [Google Scholar]
Zhang 2006 {published data only}
- Zhang XH, Andreotti G, Gao YT, Deng J, Liu E, Rashid A, et al. Tea drinking and the risk of biliary tract cancers and biliary stones: a population-based case-control study in Shanghai, China. International Journal of Cancer 2006;118(12):3089-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2009 {published data only}
- Zhang M, Huang J, Xie X, Holman CD. Dietary intakes of mushrooms and green tea combine to reduce the risk of breast cancer in Chinese women. International Journal of Cancer 2009;124(6):1404-8. [DOI] [PubMed] [Google Scholar]
Zhang 2013 {published data only}
- Zhang SC, Jin W, Liu H, Jin MJ, Chen ZX, Ding ZY, et al. RPSA gene mutants associated with risk of colorectal cancer among the Chinese population. Asian Pacific Journal of Cancer Prevention 2013;14(12):7127-31. [DOI] [PubMed] [Google Scholar]
Zhang 2016 {published data only}
- Zhang Z, Garzotto M, Beer TM, Thuillier P, Lieberman S, Mori M, et al. Effects of omega-3 fatty acids and catechins on fatty acid synthase in the prostate: a randomized controlled trial. Nutrition and Cancer 2016;68(8):1309-19. [DOI: 10.1080/01635581.2016.1224365] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhu 2016 {published data only}
- Zhu H, Li X, Zhang X, Chen D, Li D, Ren J, et al. Polymorphisms in mismatch repair genes are associated with risk and microsatellite instability of gastric cancer, and interact with life exposures. Gene 2016;579(1):52-7. [DOI: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01496521 {published data only}
- NCT01496521. Chemoprevention of esophageal squamous cell carcinoma (ESCC) with aspirin and tea polyphenols. clinicaltrials.gov/ct2/show/NCT01496521.
Shannon 2010 {published data only}
- Shannon J, Lieberman S, Maxcy C, Thuillier P, Peters L, Garzotto M. Fish oil, green tea, and prostate cancer prevention. Journal of Clinical Oncology 2010;28 Suppl 15:17. [Google Scholar]
Additional references
Ahmad 1999
- Ahmad N, Hasan M. Green tea polyphenols and cancer: biological mechanisms and practical implications. Nutrition Review 1999;3:78-83. [DOI] [PubMed] [Google Scholar]
Astill 2001
- Astill C, Birch MR, Dacombe C, Humphrey PG, Martin PT. Factors affecting the caffeine and polyphenol contents of black and green tea infusions. Journal of Agricultural and Food Chemistry 2001;49(11):5340-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ (Clinical research ed.) 2004;328(7454):1490. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beltz 2006
- Beltz LA, Bayer DK, Moss AL, Simet IM. Mechanisms of cancer prevention by green and black tea polyphenols. Anti-cancer Agents in Medicinal Chemistry 2006;6(5):389-406. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bhagwat 2014
- Bagwat S, Haytowitz DB, Holden JM. USDA database for flavonoid content in selected foods. Beltsville, Maryland: US Department of Agriculture, 2014. [Google Scholar]
Blumenthal 2003
- Blumenthal M. The ABC Clinical Guide to Herbs. Published by the American Botanical Council, 2003. [<html><body id="body">ISBN: 9781588901576 / 9783131323910</body></html>] [Google Scholar]
Booth 2008
- Booth A. Unpacking your literature search toolbox: on search styles and tactics. Health Information and Libraries Journal 2008;25(4):313-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Borges 2016
- Borges G, Van der Hooft JJ, Crozier A. A comprehensive evaluation of the [2-(14)C](-)-epicatechin metabolome in rats. Free Radical Biology & Medicine 2016;99:128-38. [PMID: ] [DOI] [PubMed] [Google Scholar]
Borges 2018
- Borges G, Ottaviani JI, Van der Hooft JJ, Schroeter H, Crozier A. Absorption, metabolism, distribution and excretion of (-)-epicatechin: a review of recent findings. Molecular Aspects of Medicine 2018;61:18-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
Borrelli 2004
- Borrelli F, Capasso R, Russo A, Ernst E. Systematic review: green tea and gastrointestinal cancer risk. Alimentary Pharmacology & Therapeutics 2004;19(5):497-510. [DOI] [PubMed] [Google Scholar]
Butler 2011
- Butler LM, Wu AH. Green and black tea in relation to gynecologic cancers. Molecular Nutrition & Food Research 2011;55(6):931-40. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2014
- Chang B, Sang L, Wang Y, Tong J, Wang BY. Consumption of tea and risk for pancreatic cancer: a meta-analysis of published epidemiological studies. Nutrition and Cancer 2014;66(7):1109-23. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chen 2007
- Chen PC, Chang FS, Chen IZ, Lu FM, Cheng TJ, Chen RL. Redox potential of tea infusion as an index for the degree of fermentation. Analytica Chimica Acta 2007;594(1):32-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chen 2014
- Chen K, Zhang Q, Peng M, Shen Y, Wan P, Xie G. Relationship between tea consumption and pancreatic cancer risk: a meta-analysis based on prospective cohort studies and case-control studies. European Journal of Cancer Prevention 2014;23(5):353-60. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chen 2017b
- Chen Y, Wu Y, Du M, Chu H, Zhu L, Tong N, et al. An inverse association between tea consumption and colorectal cancer risk. Oncotarget 2017;8(23):37367-76. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2019
- Chen M, Wang F, Cao JJ, Han X, Lu WW, Ji X, et al. (-)-Epigallocatechin-3-gallate attenuates the toxicity of methylmercury in Caenorhabditis elegans by activating SKN-1. Chemico-biological Interactions 2019;307:125-35. [PMID: ] [DOI] [PubMed] [Google Scholar]
Coppock 2016
- Coppock RW, Dziwenka M. Chapter 46 - Green Tea Extract. In: Gupta Ramesh C, editors(s). Nutraceuticals, Efficacy, Safety and Toxicity. Boston: Academic Press, 2016:633-52. [Google Scholar]
Crew 2015
- Crew KD, Ho KA, Brown P, Greenlee H, Bevers TB, Arun B, et al. Effects of a green tea extract, Polyphenon E, on systemic biomarkers of growth factor signalling in women with hormone receptor-negative breast cancer. Journal of Human Nutrition and Dietetics 2015;28(3):272-82. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG (editors) on behalf ofthe Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking metaanalyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook 2011.
Demeule 2002
- Demeule M, Michaud-Levesque J, Annabi B, Gingras D, Boivin D, Jodoin J, et al. Green tea catechins as novel antitumor and antiangiogenic compound. Current Medicinal Chemistry & Anti-cancer Agents 2002;2(4):441-63. [DOI] [PubMed] [Google Scholar]
Denis 1994
- Denis LJ. Future implications for the management of benign prostatic hyperplasia. European Urology 1994;25 Suppl 1:29-34. [PMID: ] [DOI] [PubMed] [Google Scholar]
Diniz 2017
- Diniz C, Suliburska J, Ferreira IM. New insights into the antiangiogenic and proangiogenic properties of dietary polyphenols. Molecular Nutrition & Food Research 2017;61(6):1600912. [PMID: ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629-34. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eisenstein 2019
- Eisenstein M. Tea's value as a cancer therapy is steeped in uncertainty. Nature 2019;566(7742):S6-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
EUnetHTA 2017
- EunetHTA (European network for Health Technology Assessment). Process of Information Retrieval for Systematic Reviews and Health Technology Assessments on Clinical Effectiveness. Version 1.2 edition. Diemen, The Netherlands: EUnetHTA, December 2017. [Google Scholar]
Fang 2015
- Fang X, Wei J, He X, An P, Wang H, Jiang L, et al. Landscape of dietary factors associated with risk of gastric cancer: a systematic review and dose-response meta-analysis of prospective cohort studies. European Journal of Cancer (Oxford, England : 1990) 2015;51(18):2820-32. [PMID: ] [DOI] [PubMed] [Google Scholar]
FAO 2015
- FAO Intergovernmental Group on Tea: A Subsidiary Body of the FAO Committee on Commodity Problems (CCP). World tea production and trade current and future development. Rome: Food and Agriculture Organization of the United Nations, Rome, 2015. [Google Scholar]
FAO 2018
- FAO Intergovernmental Group on Tea. Emerging trends in tea consumption: informing a generic promotion process; 23rd Session of the Intergovernmental Group on Tea. www.fao.org/ccp/tea23/en/ 2018 (accessed 4 June 2019).
Filippini 2019
- Filippini T, Tancredi S, Malagoli C, Cilloni S, Malavolti M, Violi F, et al. Aluminum and tin: food contamination and dietary intake in an Italian population. Journal of trace elements in medicine and biology : organ of the Society for Minerals and Trace Elements (GMS) 2019;52:293-301. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fujiki 1999
- Fujiki H. Two stages of cancer prevention with green tea. Journal of Cancer Research and Clinical Oncology 1999;125(11):589-97. [DOI] [PubMed] [Google Scholar]
Gao 2013
- Gao M, Ma W, Chen XB, Chang ZW, Zhang XD, Zhang MZ. Meta-analysis of green tea drinking and the prevalence of gynecological tumors in women. Asia-Pacific Journal of Public Health / Asia-Pacific Academic Consortium for Public Health 2013;25(4 Suppl):43S-8S. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gao 2016
- Gao Z, Han Y, Hu Y, Wu X, Wang Y, Zhang X, et al. Targeting HO-1 by epigallocatechin-3-gallate reduces contrast-induced renal injury via anti-oxidative stress and anti-inflammation pathways. PloS One 2016;11(2):e0149032. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gianfredi 2018
- Gianfredi V, Nucci D, Abalsamo A, Acito M, Villarini M, Moretti M, et al. Green tea consumption and risk of breast cancer and recurrence-a systematic review and meta-analysis of observational studies. Nutrients 2018;10(12):1886. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 10 January 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Grumann 2001
- Grumann M, Schlag PM. Assessment of quality of life in cancer patients: complexity, criticism, challenges. Onkologie 2001;24(1):10-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guo 2017
- Guo Y, Zhi F, Chen P, Zhao K, Xiang H, Mao Q, et al. Green tea and the risk of prostate cancer: a systematic review and meta-analysis. Medicine 2017;96(13):e6426. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guo 2019
- Guo Z, Jiang M, Luo W, Zheng P, Huang H, Sun B. Association of lung cancer and tea-drinking habits of different subgroup populations: meta-analysis of case-control studies and cohort studies. Iranian Journal of Public Health 2019;48(9):1566-76. [PMC free article] [PubMed] [Google Scholar]
Guyatt 1989
- Guyatt G, Mitchell A, Irvine EJ, Singer J, Williams N, Goodacre R, et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology 1989;96(3):804-10. [PMID: ] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical Research Ed.) 2003;327(7414):557-60. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available fromwww.training.cochrane.org/handbook.
Hou 2013
- Hou IC, Amarnani S, Chong MT, Bishayee A. Green tea and the risk of gastric cancer: epidemiological evidence. World Journal of Gastroenterology 2013;19(24):3713-22. [PMID: ] [DOI] [PMC free article] [PubMed]
Huang 2016
- Huang YQ, Lu X, Min H, Wu QQ, Shi XT, Bian KQ, et al. Green tea and liver cancer risk: a meta-analysis of prospective cohort studies in Asian populations. Nutrition (Burbank, Los Angeles County, Calif.) 2016;32(1):3-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Huang 2017
- Huang Y, Chen H, Zhou L, Li G, Yi D, Zhang Y, et al. Association between green tea intake and risk of gastric cancer: a systematic review and dose-response meta-analysis of observational studies. Public Health Nutrition 2017;20(17):3183-92. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacob 2017
- Jacob SA, Khan TM, Lee LH. The effect of green tea consumption on prostate cancer risk and progression: a systematic review. Nutrition and Cancer 2017;69(3):353-64. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kerimi 2018
- Kerimi A, Williamson G. Differential impact of flavonoids on redox modulation, bioenergetics, and cell signaling in normal and tumor cells: a comprehensive review. Antioxidants & Redox Signaling 2018;29(16):1633-59. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2017
- Khan MR, Alothman ZA, Naushad M, Alomary AK, Alfadul SM, Alsohaimi IH, et al. Occurrence of acrylamide carcinogen in Arabic coffee Qahwa, coffee and tea from Saudi Arabian market. Scientific Reports 2017;7:41995. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Langendam 2013
- Langendam MW, Akl EA, Dahm P, Glasziou P, Guyatt G, Schunemann HJ. Assessing and presenting summaries of evidence in Cochrane Reviews. Systematic Reviews 2013;2:81. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 2005
- Lewis JE, Hilditch JR, Wong CJ. Further psychometric property development of the Menopause-Specific Quality of Life questionnaire and development of a modified version, MENQOL-Intervention questionnaire. Maturitas 2005;50(3):209-21. [PMID: ] [DOI] [PubMed] [Google Scholar]
Liao 2001
- Liao S, Kao YH, Hiipakka RA. Green tea: biochemical and biological basis for health benefits. Vitamins & Hormones 2001;62:1-94. [DOI] [PubMed] [Google Scholar]
Lin 2014
- Lin YW, Hu ZH, Wang X, Mao QQ, Qin J, Zheng XY, et al. Tea consumption and prostate cancer: an updated meta-analysis. World Journal of Surgical Oncology 2014;12:38. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2013b
- Liu K, Zhou R, Wang B, Chen K, Shi LY, Zhu JD, et al. Effect of green tea on glucose control and insulin sensitivity: a meta-analysis of 17 randomized controlled trials. American Journal of Clinical Nutrition 2013;98(2):340-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Maiti 2003
- Maiti FK, Chatterjee J, Dasgupta S. Effect of green tea polyphenols on angiogenesis induced by angiogenin-like protein. Biochemical and Biophysical Research Communications 2003;308(1):64-7. [DOI] [PubMed] [Google Scholar]
Malir 2014
- Malir F, Ostry V, Pfohl-Leszkowicz A, Toman J, Bazin I, Roubal T. Transfer of ochratoxin A into tea and coffee beverages. Toxins 2014;6(12):3438-53. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Manach 2005
- Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. American Journal of Clinical Nutrition 2005;81(1 Suppl):230S-42S. [DOI] [PubMed] [Google Scholar]
Marberger 2013
- Marberger M. Medical management of lower urinary tract symptoms in men with benign prostatic enlargement. Advances in Therapy 2013;30(4):309-19. [PMID: ] [DOI] [PubMed] [Google Scholar]
McHorney 1993
- McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical Care 1993;31(3):247-63. [PMID: ] [DOI] [PubMed] [Google Scholar]
Meader 2014
- Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Systematic Reviews 2014;3:82. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Milani 2019
- Milani RF, Silvestre LK, Morgano MA, Cadore S. Investigation of twelve trace elements in herbal tea commercialized in Brazil. Journal of Trace Elements in Medicine and Biology 2019;52:111-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Najaf 2018
- Najaf Najafi M, Salehi M, Ghazanfarpour M, Hoseini ZS, Khadem-Rezaiyan M. The association between green tea consumption and breast cancer risk: a systematic review and meta-analysis. Phytotherapy Research : PTR 2018;32(10):1855-64. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ni 2017
- Ni CX, Gong H, Liu Y, Qi Y, Jiang CL, Zhang JP. Green tea consumption and the risk of liver cancer: a meta-analysis. Nutrition and Cancer 2017;69(2):211-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
Peluso 2017
- Peluso I, Serafini M. Antioxidants from black and green tea: from dietary modulation of oxidative stress to pharmacological mechanisms. British Journal of Pharmacology 2017;174(11):1195-208. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Podwika 2018
- Podwika W, Kleszcz K, Krosniak M, Zagrodzki P. Copper, manganese, zinc, and cadmium in tea leaves of different types and origin. Biological Trace Element Research 2018;183(2):389-95. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Preedy 2014
- Preedy VR. Processing and Impact on Antioxidants in Beverages. Elsevier, 2014. [DOI: 10.1016/C2012-0-02151-5] [DOI] [Google Scholar]
Qin 2012
- Qin J, Xie B, Mao Q, Kong D, Lin Y, Zheng X. Tea consumption and risk of bladder cancer: a meta-analysis. World Journal of Surgical Oncology 2012;10:172. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rahmani 2015
- Rahmani AH, Al Shabrmi FM, Allemailem KS, Aly SM, Khan MA. Implications of green tea and its constituents in the prevention of cancer via the modulation of cell signalling pathway. BioMed Research International 2015;2015:925640. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rashidi 2017
- Rashidi B, Malekzadeh M, Goodarzi M, Masoudifar A, Mirzaei H. Green tea and its anti-angiogenesis effects. Biomedecine & Pharmacotherapie [Biomedicine & Pharmacotherapy] 2017;89:949-56. [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Romano 2013
- Romano B, Pagano E, Montanaro V, Fortunato AL, Milic N, Borrelli F. Novel insights into the pharmacology of flavonoids. Phytotherapy Research : PTR 2013;27(11):1588-96. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rothwell 2017
- Rothwell JA, Knaze V, Zamora-Ros R. Polyphenols: dietary assessment and role in the prevention of cancers. Current Opinion in Clinical Nutrition and Metabolic Care 2017;20(6):512-21. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sang 2011
- Sang S, Lambert JD, Ho CT, Yang CS. The chemistry and biotransformation of tea constituents. Pharmacological Research 2011;64(2):87-99. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sang 2013
- Sang LX, Chang B, Li XH, Jiang M. Green tea consumption and risk of esophageal cancer: a meta-analysis of published epidemiological studies. Nutrition and Cancer 2013;65(6):802-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Scalbert 2000
- Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. Jounal of Nutrition 2000;130(8S Suppl):2073S-85S. [DOI] [PubMed] [Google Scholar]
Schröder 2019
- Schröder L, Marahrens P, Koch JG, Heidegger H, Vilsmeier T, Phan-Brehm T, et al. Effects of green tea, matcha tea and their components epigallocatechin gallate and quercetin on MCF‑7 and MDA-MB-231 breast carcinoma cells. Oncology Reports 2019;41(1):387-96. [DOI] [PubMed] [Google Scholar]
Schünemann 2017
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Akl E, et al, on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Sharpe 2016
- Sharpe E, Hua F, Schuckers S, Andreescu S, Bradley R. Effects of brewing conditions on the antioxidant capacity of twenty-four commercial green tea varieties. Food Chemistry 2016;192:380-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shirakami 2018
- Shirakami Y, Shimizu M. Possible mechanisms of green tea and its constituents against cancer. Molecules (Basel, Switzerland) 2018;23(9):2284. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spies 2002
- Spies JB, Coyne K, Guaou Guaou N, Boyle D, Skyrnarz-Murphy K, Gonzalves SM. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstetrics and Gynecology 2002;99(2):290-300. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sterne 2017
- Sterne JA, Egger M, Moher D, Boutron I (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Tang 2015
- Tang J, Zheng JS, Fang L, Jin Y, Cai W, Li D. Tea consumption and mortality of all cancers, CVD and all causes: a meta-analysis of eighteen prospective cohort studies. British Journal of Nutrition 2015;114(5):673-83. [PMID: ] [DOI] [PubMed] [Google Scholar]
USDA 2018
- United States Department of Agriculture (USDA). The ERS Food Availability (Per Capita) Data System (FADS). United States Department of Agriculture’s Economic Research Service 2018.
Vieira 2017
- Vieira AR, Abar L, Chan DS, Vingeliene S, Polemiti E, Stevens C, et al. Foods and beverages and colorectal cancer risk: a systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR Continuous Update Project. Annals of Oncology 2017;28(8):1788-802. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wang 2014b
- Wang L, Zhang X, Liu J, Shen L, Li Z. Tea consumption and lung cancer risk: a meta-analysis of case-control and cohort studies. Nutrition (Burbank, Los Angeles County, Calif.) 2014;30(10):1122-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wells 2001
- Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. University of Ottawa, www.lri.ca/programs/ceu/oxford.htm (accessed 29 February 2009).
Weng 2017
- Weng H, Zeng XT, Li S, Kwong JS, Liu TZ, Wang XH. Tea consumption and risk of bladder cancer: a dose-response meta-analysis. Frontiers in Physiology 2017;7:693. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Williamson 2005
- Williamson G, Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. American Journal of Clinical Nutrition 2005;81(1 Suppl):243S-55S. [DOI] [PubMed] [Google Scholar]
Williamson 2018
- Williamson G, Kay CD, Crozier A. The bioavailability, transport, and bioactivity of dietary flavonoids: a review from a historical perspective. Comprehensive Reviews in Food Science and Food Safety 2018;17(5):1054-112. [DOI] [PubMed] [Google Scholar]
Wu 2013b
- Wu S, Li F, Huang X, Hua Q, Huang T, Liu Z, et al. The association of tea consumption with bladder cancer risk: a meta-analysis. Asia Pacific Journal of Clinical Nutrition 2013;22(1):128-37. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wyatt 2001
- Wyatt KM, Dimmock PW, Walker TJ, O'Brien PM. Determination of total menstrual blood loss. Fertility and Sterility 2001;76(1):125-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
Xiong 2017
- Xiong J, Lin J, Wang A, Wang Y, Zheng Y, Sang X, et al. Tea consumption and the risk of biliary tract cancer: a systematic review and dose-response meta-analysis of observational studies. Oncotarget 2017;8(24):39649-57. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Xu 2019
- Xu XY, Zhao CN, Cao SY, Tang GY, Gan RY, Li HB. Effects and mechanisms of tea for the prevention and management of cancers: an updated review. Critical Reviews in Food Science and Nutrition 2019;14:1-13. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yang 1993
- Yang CS, Wang ZY. Tea and cancer. Journal of the National Cancer Institute 1993;85:1038-49. [DOI] [PubMed] [Google Scholar]
Yang 1997
- Yang CS, Lee MJ, Chen L, Yang GY. Polyphenols as inhibitors of carcinogenesis. Environmental Health Perspectives 1997;105 Suppl 4:S971-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2009
- Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nature Reviews. Cancer 2009;9(6):429-39. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2011b
- Yang CS, Wang H, Li GX, Yang Z, Guan F, Jin H. Cancer prevention by tea: evidence from laboratory studies. Pharmacological Research 2011;64(2):113-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yang 2019
- Yang CS, Zhang J. Studies on the prevention of cancer and cardiometabolic diseases by tea: issues on mechanisms, effective doses, and toxicities. Journal of Agricultural and Food Chemistry 2019;67(19):5446-56. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yi 2019
- Yi M, Wu X, Zhuang W, Xia L, Chen Y, Zhao R, et al. Tea consumption and health outcomes: umbrella review of meta-analyses of observational studies in humans. Molecular Nutrition & Food Research 2019;63(16):e1900389. [DOI] [PubMed] [Google Scholar]
Yiannakopoulou 2014
- Yiannakopoulou EC. Interaction of green tea catechins with breast cancer endocrine treatment: a systematic review. Pharmacology 2014;94(5-6):245-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yu 2014
- Yu F, Jin Z, Jiang H, Xiang C, Tang J, Li T, et al. Tea consumption and the risk of five major cancers: a dose-response meta-analysis of prospective studies. BMC Cancer 2014;14:197. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yu 2017
- Yu J, Song P, Perry R, Penfold C, Cooper AR. The effectiveness of green tea or green tea extract on insulin resistance and glycemic control in type 2 diabetes mellitus: a meta-analysis. Diabetes & Metabolism Journal 2017;41(4):251-62. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zeng 2014
- Zeng JL, Li ZH, Wang ZC, Zhang HL. Green tea consumption and risk of pancreatic cancer: a meta-analysis. Nutrients 2014;6(11):4640-50. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2015a
- Zhang C, Qin Y-Y, Wei X, Yu F-F, Zhou Y-H, He J. Tea consumption and risk of cardiovascular outcomes and total mortality: a systematic review and meta-analysis of prospective observational studies. European Journal of Epidemiology 2015;30(2):103-13. [DOI] [PubMed] [Google Scholar]
Zhang 2015b
- Zhang YF, Xu Q, Lu J, Wang P, Zhang HW, Zhou L, et al. Tea consumption and the incidence of cancer: a systematic review and meta-analysis of prospective observational studies. European Journal of Cancer Prevention 2015;24(4):353-62. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zheng 2011
- Zheng J, Yang B, Huang T, Yu Y, Yang J, Li D. Green tea and black tea consumption and prostate cancer risk: an exploratory meta-analysis of observational studies. Nutrition and Cancer 2011;63(5):663-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zheng 2012
- Zheng P, Zheng HM, Deng XM, Zhang YD. Green tea consumption and risk of esophageal cancer: a meta-analysis of epidemiologic studies. BMC Gastroenterology 2012;12:165. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zheng 2013
- Zheng JS, Yang J, Fu YQ, Huang T, Huang YJ, Li D. Effects of green tea, black tea, and coffee consumption on the risk of esophageal cancer: a systematic review and meta-analysis of observational studies. Nutrition and Cancer 2013;65(1):1-16. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zhong 2014
- Zhong S, Chen Z, Yu X, Chen W, Lv M, Ma T, et al. Tea consumption and leukemia risk: a meta-analysis. Tumour Biology 2014;35(6):5205-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zhou 2016
- Zhou Q, Li H, Zhou JG, Ma Y, Wu T, Ma H. Green tea, black tea consumption and risk of endometrial cancer: a systematic review and meta-analysis. Archives of Gynecology and Obstetrics 2016;293(1):143-55. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Boehm 2009
- Boehm K, Borrelli F, Ernst E, Habacher G, Hung SK, Milazzo S, et al. Green tea (Camellia sinensis) for the prevention of cancer(Review). Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No: CD005004. [DOI: 10.1002/14651858.CD005004.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


