Abstract
Background
Treatment strategies for childhood cancer are improving, resulting in higher survival rates. However, the consequences of childhood cancer do not end with the successful completion of cancer treatment. Most patients will develop late effects after cessation of treatment. Severe fatigue is seen as a common and debilitating late effect in cancer survivors. Although most research on fatigue has been performed in patients after adult‐onset cancer, our review focuses on fatigue after childhood cancer.
Objectives
To estimate the prevalence of severe fatigue after treatment for childhood cancer. Secondary objectives are to describe the course of severe fatigue following cancer treatment and to examine risk factors for fatigue, or factors associated with it.
Search methods
We searched the Cochrane Central Register of Controlled Trials (the Cochrane Library 2019; issue 8 March 2019), MEDLINE/PubMed (from 1945 to 8 March 2019), Embase/Ovid (from 1947 to 8 March 2019), reference lists of included articles and several conference proceedings from 2011 to 2018.
Selection criteria
Observational studies, randomised controlled trials and controlled clinical trials reporting on fatigue in participants after treatment for childhood cancer. Case series and case reports were not eligible for inclusion.
Data collection and analysis
Two review authors independently extracted data and assessed risks of bias. If the publication did not present the prevalence of severe fatigue, we contacted study authors for additional information.
Main results
We included 30 studies (18,682 participants in total). Eighteen studies contributed to the main objective and 22 studies contributed to the secondary objectives. We found substantial differences between studies in cancer diagnosis, cancer treatment, age of participants, questionnaires used to assess fatigue, and sample size. All included studies scored at least one 'Risk of bias' item as unclear or high risk.
We identified both clinical and statistical heterogeneity and therefore could not pool results, so we present them descriptively. Eighteen studies (describing 14,573 survivors) reported the prevalence of severe fatigue, which ranged from 0% to 61.7%. In a subgroup of three studies including children aged up to 18 years at fatigue assessment (268 survivors), prevalence rates ranged from 6.7% to 12.5%. In comparison, in a subgroup of 12 studies including participants aged 16 and over (13,952 survivors), prevalence rates ranged from 4.4% to 61.7%. The prevalence of severe fatigue in a subgroup of survivors of haematological cancer was presented in seven studies and ranged from 1.8% to 35.9% (1907 survivors). Prevalence of severe fatigue in brain cancer survivors was presented in two studies (252 survivors) and was 14.6% and 21.1% respectively. One study presented a prevalence for bone cancer survivors of 0.0% (17 survivors). Four studies provided prevalence rates of severe fatigue in control groups of siblings or population‐based controls, which ranged from 3.1% to 10.3%. In these four studies, survivors were more often fatigued than controls, but this difference was statistically significant in only two studies.
Studies assessing risk and associated factors for fatigue were heterogeneous, and definitions of the factors under study were often inconsistent, with results therefore presented descriptively. They found that depression might be associated with fatigue. In contrast, age at diagnosis and education level did not seem to be associated with fatigue. We were unable to calculate any overall risk estimate for any of the reported risks and associated factors, because we could not conduct meta‐analysis.
One study provided information about the course of fatigue over time, and found that over the course of 2.7 years, 32 of the 102 participants (31.4%) reported persistent severe fatigue.
Authors' conclusions
It is unclear how many childhood cancer survivors suffer from severe fatigue. This review encountered several difficulties. We found statistical and clinical heterogeneity and great variation in the reporting of possible risk and associated factors. The evidence in this review is therefore weak, and the exact prevalence of severe fatigue after treatment for childhood cancer remains to be determined. This is also the case for the course of severe fatigue following treatment and the strength of the relationship between fatigue and associated and risk factors. Despite these limitations, our review does provide a comprehensive overview of the existing literature about severe fatigue after treatment for childhood cancer.
Keywords: Adolescent, Adult, Child, Humans, Young Adult, Cancer Survivors, Antineoplastic Agents, Antineoplastic Agents/adverse effects, Antineoplastic Agents/therapeutic use, Fatigue, Fatigue/etiology, Neoplasms, Neoplasms/drug therapy, Randomized Controlled Trials as Topic, Risk Factors
Plain language summary
Severe fatigue after treatment for childhood cancer
Review question
We reviewed the literature to determine how common (prevalence) severe fatigue is in patients after treatment for childhood cancer. We also wanted to describe the course of severe fatigue after completion of cancer treatment, and to identify possible risk factors for the development of fatigue in this population.
Background
Treatments for childhood cancer are improving and becoming more effective in curing cancer. The impact of having had cancer at a young age, together with often intensive cancer therapy, can affect physical and mental well‐being later in life. Most survivors will develop one or more of these so‐called late effects. Severe fatigue is a common late effect in people with adult‐onset cancer and can affect a person's daily life in many ways. We do not currently know how often severe fatigue occurs after treatment for childhood cancer, nor which risk factors might be responsible for developing fatigue.
Study characteristics
The evidence is up to date to March 2019.
We include 30 studies, describing 18,682 participants after treatment for childhood cancer. We found a lot of variation between studies in cancer diagnosis, cancer treatment, age of participants, the questionnaires used to assess fatigue, and the size of the study.
Key results
Eighteen studies reported a prevalence of severe fatigue, which ranged from 0% to 61.7%. Four studies reported a prevalence of severe fatigue in the patient's brothers and sisters or in population‐based controls. Prevalence rates in these control groups ranged from 3.1% to 10.3%. In these four studies, survivors were more often fatigued than controls. This difference was only significant in two studies.
When we looked at the prevalence of severe fatigue in survivors of lymphoma and leukaemia (types of blood cancers), we found that they ranged from 1.8% to 35.9%. Two studies reported on severe fatigue in brain cancer survivors, with rates of 21.13% and 14.6%. One study in bone cancer survivors reported no cases of severe fatigue. For survivors aged 18 and younger, prevalence rates ranged from 6.7% to 12.5%. By contrast, in studies including participants aged 16 years and over (but mostly over 18), prevalence rates ranged from 4.4% to 61.7%.
Twenty‐two studies assessed one or more possible risk factors for fatigue. Our review shows that depression might increase fatigue. The age at cancer diagnosis and the education level of the survivor did not seem to influence fatigue.
Only one study provided information about the course of fatigue over time, and found that over the course of 2.7 years 32 of the 102 participants (31.4%) reported persistent severe fatigue.
Quality of the evidence
All included studies had problems with the quality of the evidence, and we found many differences between studies for several characteristics. The evidence to address our review question is therefore weak. The occurrence of severe fatigue after treatment for childhood cancer remains uncertain. This is also the case for the course of severe fatigue after completion of cancer treatment and the risk factors that might be responsible for developing fatigue.
Background
Description of the condition
With current treatment regimens, about 80% of children with cancer are expected to survive at least five years post‐diagnosis (American Cancer Society 2014; Gatta 2014). Unfortunately, the consequences of childhood cancer do not end with the successful completion of cancer treatment. Most childhood cancer survivors (CCS) will develop late effects during their life (Armstrong 2014; Geenen 2007; Hudson 2013). Late effects are defined as adverse long‐term health problems which are related to childhood cancer and its treatment, for example cardiac dysfunction, renal insufficiency and hepatic complications (Kooijmans 2019; Mulder 2019; Nathan 2016). They can occur years after the completion of treatment and cause substantial excess morbidity and mortality (Armstrong 2014; Diller 2009; Hudson 2013). Research groups in the USA (Oeffinger 2006) and the Netherlands (Geenen 2007) estimate that the cumulative incidence of severe, disabling, and/or life‐threatening late effects is about 40% at 25 to 30 years after childhood cancer diagnosis. The need for long‐term follow‐up is therefore uniformly recognised (Skinner 2006).
Cancer‐related fatigue (CRF) is one of the most common and debilitating symptoms in cancer survivors (Mulrooney 2008; Servaes 2002). CRF is defined by the National Comprehensive Cancer Network (NCCN) of the USA as "a distressing, persistent, subjective sense of physical, emotional and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning" (Mock 2000). It can impair performance so severely that the person is unable to work or attend school. As such, CRF has a negative effect on quality of life (QoL) (Hjermstad 2006; Kanellopoulos 2013; Meeske 2007; Zeltzer 2009).
The aetiology of CRF is poorly understood and is likely to be the result of a complex interaction of multiple factors, involving the dysregulation of inter‐related physiological, biochemical (e.g. inflammation) and psychological systems (Barsevick 2010; Reyes‐Gibby 2008; Ryan 2007). Possible risk factors for CRF can be classified into predisposing (genetic disposition), triggering (disease‐ and treatment‐related factors), maintaining (current health, demographic and life‐style factors) and modulating (age at diagnosis and gender) factors. Given the multiplicity of factors contributing to CRF, interventions should be tailored to each of the contributing factors and to the specific needs of the individual survivor.
Many different instruments have been developed for the assessment of fatigue. These vary from a single‐item question about the presence of fatigue to fatigue severity scales and multidimensional assessment tools measuring different dimensions of fatigue (e.g. cognitive, emotional or physical fatigue, or combinations of these) (Bower 2014b; Jacobsen 2004; Minton 2008). Besides the fatigue questionnaires, there are also questionnaires that assess different symptoms or quality of life, aside from fatigue dimensions (e.g. EORTC QLQ‐C30; Aaronson 1993). To date, there is no consensus on how fatigue should be assessed. Furthermore, the presence of fatigue on a fatigue assessment tool does not by definition mean that a cancer survivor suffers from CRF, as fatigue, according to the definition of CRF, must be persistent, severe, not related to recent activity or comorbidity, and it must interfere with daily functioning (Bower 2014b). Current research has mostly focused on severe fatigue as an aspect of CRF. The focus of this review is therefore on severe fatigue, with other aspects of CRF not taken into account, for example its relation to activity and its impact on daily functioning.
Previous reviews of severe fatigue in cancer survivors have focused mainly on the prevalence, duration and factors associated with severe fatigue in adult cancer survivors (ACS). A review of adult Hodgkin lymphoma survivors, for instance, estimated the prevalence of severe fatigue at between 11% and 76% (Daniels 2013). The mean prevalence of severe fatigue in breast cancer survivors has been reported to be 27% (range 7% to 52%) in a recent meta‐analysis (Abrahams 2016). These studies show severe fatigue to be a frequently‐occurring problem in adult cancer survivors. However, the reported prevalence rates vary substantially between studies. Persistence of fatigue in ACS long after completion of cancer therapy has been demonstrated in many longitudinal studies (Bower 2006; Prue 2006; Reinertsen 2010; Servaes 2007). Factors associated with severe fatigue, reported in ACS, are: higher stage of cancer, intensive cancer treatment, sleep disturbance, lower levels of physical activity, elevated body mass index (BMI) and psychosocial problems (Abrahams 2016; Abrahams 2018; Bower 2014a; Gielissen 2007; Spathis 2015). It is unknown, however, whether these findings from ACS studies can be generalised to CCS.
To our knowledge, there is no systematic review describing the prevalence and course of severe fatigue in CCS or its risk and associated factors. Cross‐sectional studies assessing the prevalence of severe fatigue in subgroups of CCS, with different time intervals since diagnosis, different fatigue assessment tools and using different comparison groups are available (e.g. Johannsdottir 2012; Langeveld 2003; Meeske 2005; Mulrooney 2008). Unfortunately, the results are contradictory, from no excess fatigue to most of the CCS group being severely fatigued. Zeller 2014a conducted a longitudinal study in 102 long‐term survivors of childhood lymphoma and acute lymphoblastic leukaemia. At the first fatigue measurement 27.2% had severe fatigue and after a median interval of 2.7 years 60% of this group was still severely fatigued. Important to note is that this CCS group included survivors with major somatic comorbidities, which could also explain the severe fatigue. Kenney 2010 studied the health status of adult CCS with the longest follow‐up period so far (age more than 50 years, treated for cancer between 1947 and 1968). CCS reported significantly higher scores for fatigue compared to their sibling controls, indicating that problems due to fatigue could persist decades after cancer treatment. Persistent fatigue may also be a problem in CCS subgroups.
Abrahams 2016 reported a relatively large decrease in the prevalence of severe fatigue in the first six months after completion of breast cancer treatment in ACS. Interventions during this period would therefore most probably not be cost‐effective and could put undue strain on cancer survivors, since severe fatigue may still resolve spontaneously. No longitudinal studies have been published on the natural course of fatigue directly from completion of cancer treatment in CCS. This makes it difficult to determine at which time point an intervention for CRF can best be offered to CCS.
Why it is important to do this review
As far as we know, no meta‐analysis has been conducted to estimate the prevalence of severe fatigue, to assess its course since end of cancer treatment, and to identify possible risk factors for the development of severe fatigue following treatment for childhood cancer. As the number of CCS increases due to better treatment options, more survivors will be at risk for the development of severe fatigue as a late effect of childhood cancer and its treatment. It is unknown if the prevalence rates and risk factors for severe fatigue found in ACS can be generalised to the CCS population, as the group of CCS differs from ACS and is very heterogeneous, with different cancer diagnoses, treatment modalities, late effects, and age at the start of cancer treatment. It is crucial to identify which CCS are more likely to develop severe fatigue following cancer treatment, in order to develop guidelines for follow‐up and management of severe fatigue in CCS (Berger 2015). Knowledge about the natural course of severe fatigue in CCS will help to initiate timely and adequate interventions to alleviate severe fatigue and to improve the associated quality of life in CCS.
This review focuses on severe fatigue as an aspect of CRF, because severe fatigue has frequently been shown to have a negative effect on a person's daily life, school performance and/or work ability, and on their quality of life. We have attempted, through analysis of the published data on severe fatigue in CCS, to increase our knowledge of the prevalence of severe fatigue, of its course, and of factors associated with severe fatigue or increasing the risk of developing it in CCS.
Objectives
The primary objective is to estimate the prevalence of severe fatigue after treatment for childhood cancer.
The secondary objective is to describe the course of severe fatigue following cancer treatment and examine risk factors for fatigue, or factors associated with it (e.g. demographic, life‐style, cancer‐ and cancer treatment‐related factors, and comorbidity).
Methods
Criteria for considering studies for this review
Types of studies
We have included studies with a cohort, case‐control or cross‐sectional design and longitudinal studies. If we had included randomised controlled trials (RCTs) and controlled clinical trials (CCTs) in CCS, they should not have preselected participants based on the presence or severity of fatigue, and baseline characteristics should have included data on fatigue. Finally, we would have included RCTs and CCTs in participants with childhood cancer, testing the efficacy of cancer treatments, if they reported data on fatigue assessment after cessation of cancer treatment. We excluded case reports and case series (i.e. a description of non‐consecutive patients).
Types of participants
Studies that involved childhood cancer survivors of any age, who were diagnosed and treated for any type of cancer before the age of 18 years, were eligible for inclusion. For this review we considered a participant a CCS from end of treatment onwards, in order to be able to report the course of severe fatigue after completion of cancer treatment. In addition, the survivor should be in persistent complete remission at the time of fatigue assessment. We interpreted 'complete remission' as participants being off treatment; or having no active disease of a recurrence or second malignancy; or had no evidence of the disease at the time of the study; or visited a long‐term follow‐up clinic or had a mean time since diagnosis of at least five years. Studies that include both CCS and adult cancer survivors (ACS) were only eligible for inclusion if more than 90% of the survivors were under the age of 18 years at cancer diagnosis, or when the study presented separate results for survivors who were under the age of 18 years at cancer diagnosis.
Types of interventions
We included all studies that reported on CCS treated with one or a combination of cancer treatment modalities. Treatments included: chemotherapy, targeted therapy, immunotherapy, stem cell transplantation/bone marrow transplantation, radiotherapy or surgery or both for childhood cancer.
Types of outcome measures
Primary outcomes
The primary outcome is the prevalence of severe fatigue in CCS. We anticipated that studies would use a variety of tools and outcome measures to evaluate severe fatigue. We took severe fatigue as the main outcome measure, rather than cancer‐related fatigue (CRF), which requires that several other criteria be met by participants (Mock 2000). We defined severe fatigue as scoring above a published cut‐off score on a validated or non‐validated fatigue questionnaire. We included all studies that measured severe fatigue with any questionnaire (e.g. fatigue questionnaire, fatigue items as part of a quality‐of‐life questionnaire, or a criterion in an interview), with the exception of studies that assessed (severe) fatigue with a dichotomous outcome, which we excluded.
We also included studies assessing fatigue with a questionnaire lacking a published cut‐off score but with published normative data from a healthy reference group. For these studies we based the criterion for severe fatigue on normative data, and defined it as scoring below or above two standard deviations (SDs) of the normative mean (depending on the direction of the score on the questionnaire).
Secondary outcomes
The course of severe fatigue over time. We assessed the course of severe fatigue in longitudinal studies with more than one fatigue assessment point.
Risk factors for fatigue or factors associated with fatigue. We included longitudinal studies with more than one consecutive fatigue assessment point, together with studies reporting on gender, ethnicity and disease‐ and treatment‐related variables in relation to fatigue, to assess risk factors. These variables are most likely present before the onset of fatigue and are therefore interpreted as possible risk factors. All other variables that were not assessed in a longitudinal study were included in the analysis as factors associated with fatigue. We used data on demography, life style (e.g. BMI and physical activity), and current health status (e.g. comorbidity, late effects of cancer treatment, sleep disturbance or psychosocial problems or both) to identify risk and associated factors. The presence of data about the course of severe fatigue and its associated/risk factors was not an inclusion criterion.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases:
Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library; issue 8 March 2019);
MEDLINE/PubMed (from 1945 to 8 March 2019);
Embase/Ovid (from 1947 to 8 March 2019).
All electronic searches have been developed in co‐operation with Cochrane Childhood Cancer. The search strategies for the different electronic databases (using a combination of controlled vocabulary and text‐word terms) are shown in the appendices (Appendix 1; Appendix 2; Appendix 3).
Cochrane Childhood Cancer ran the searches in CENTRAL, MEDLINE/PubMed, and Embase/Ovid, with all other searches conducted by the review authors. We imposed no language restrictions.
Searching other resources
We located information about studies not registered in CENTRAL, MEDLINE/PubMed or Embase/Ovid, either published or unpublished, by searching the reference lists of relevant articles and review articles. We also scanned the proceedings abstracts from 2011 to 2018 electronically of:
the International Society of Paediatric Oncology (SIOP);
American Society of Clinical Oncology (ASCO);
American Society of Pediatric Hematology/Oncology (ASPHO);
International Conference on Long‐Term Complications of Treatment of Children and Adolescents for Cancer;
European Symposium on Late Complications after Childhood Cancer (ESLCCC).
Appendix 4 describes how we conducted the search. Experts in the field provided information on additional studies.
Data collection and analysis
Selection of studies
After performing the searches described above, two review authors independently determined the eligibility of studies by reading the abstract of each study, and independently eliminating studies that did not meet the inclusion criteria. One review author performed a search of the reference lists of relevant articles and review articles, as well as the search within the conference proceedings. Two review authors read the full‐text versions of the potentially eligible studies, to determine whether they met the inclusion criteria, and resolving discrepancies between them by consensus. In case of no consensus, a third review author acted as arbiter for a final decision. When there were multiple publications of the same study population, we included a single report, if possible the publication with the most participants or the most recent data. We recorded reasons for the exclusion of studies that we had considered for inclusion on the basis of title or abstract. We include a PRISMA flow chart of the selection of studies (see Figure 1).
Data extraction and management
Two review authors independently performed data extraction using standardised forms. In cases of disagreement, we re‐examined publications and discussed the data extraction items until we reached a consensus. If this was not possible, a third review author made a final decision. We extracted the following data.
Study characteristics, including:
study design;
number of CCS in the study;
inclusion/exclusion criteria for participation in the study;
'Risk of bias' items;
funding sources;
declarations of interest.
Outcome measures, including:
instruments used to assess fatigue;
cut‐off score or criterion for severe fatigue;
time point(s) at which outcome data were collected;
number (percentage) of survivors with severe fatigue.
Demographic and disease‐ and treatment‐related risk factors for fatigue:
gender;
ethnicity;
age at cancer diagnosis;
tumour type and stage;
type of cancer treatment: number of patients who received chemotherapy, or targeted therapy, or immunotherapy, or stem cell transplantation/bone marrow transplantation (SCT/BMT), or radiotherapy or surgery for primary cancer, or combinations of cancer treatment;
received chemotherapeutic agent;
duration of follow‐up since cancer diagnosis.
Predisposing, demographic, life‐style and current health factors that might increase the risk or are associated with fatigue:
genetic factors/mutations;
marital status;
highest completed education level;
employment status;
age at fatigue assessment;
physical activity level;
BMI;
sleeping problems;
psychosocial problems;
comorbidity, including late effects.
Assessment of risk of bias in included studies
Two review authors independently assessed the risks of bias in the included studies, resolving discrepancies by consensus or, in case of doubt, by a third‐party arbiter. For the assessment of risk of bias in observational studies, we used a modified checklist based on previously‐published checklists for observational studies, according to evidence‐base medicine criteria (Grimes 2002; Von Elm 2007). We scored 'Risk of bias' assessments by the criteria mentioned in Table 1. If we had included RCTs and CCTs, we would have assessed them with the 'Risk of bias' items as described in the module of the Childhood Cancer Group (Kremer 2016), which are based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We took risks of bias into account when interpreting the review's results.
1. Risk of bias assessment for observational studies.
| Internal validity | External validity | |
| Study group |
Selection bias (representative: yes/no) ‐ if the described study group consisted of more than 90% of the original cohort of cancer survivors ‐ or if the study population was a random sample with respect to the cancer treatment of the original cohort of cancer survivors |
Reporting bias (well‐defined: yes/no) ‐ if the type of cancer and cancer treatment was mentioned (i.e. information about surgery, chemotherapeutic agents, radiotherapy fields and doses are provided) ‐ if the inclusion and exclusion criteria are described (i.e. provide enough information to describe how the study population was established) |
| Follow‐up |
Attrition bias (adequate: yes/no) ‐ if the outcome was assessed for more than 95% of the study group of interest (++) ‐ or if the outcome was assessed for 65% to 95% of the study group of interest (+) |
Reporting bias (well‐defined: yes/no) ‐ if the length of follow‐up (i.e. time since diagnosis or time since end of therapy) was mentioned |
| Outcome |
Detection bias (blind: yes/no) ‐ if the outcome assessors were blinded to the investigated determinant |
Reporting bias (well‐defined: yes/no) Outcome severe fatigue: ‐ if the authors reported what instruments they used to assess fatigue and what they considered to be severe fatigue Outcome fatigue: ‐ if the authors reported what instruments they used to assess fatigue and mentioned how fatigue was interpreted (e.g. continuous scale, moderate fatigue, etc.) |
| Risk estimation |
Confounding (adjustment for other factors: yes/no) ‐ if possibly important prognostic factors (i.e. age, sex, co‐treatment, comorbidity) and follow‐up were taken adequately into account (i.e. multivariable analyses) |
Analyses (well‐defined: yes/no) ‐ if one of the following items were calculated: prevalence, cumulative incidence, mean difference, correlation coefficient, regression coefficient, relative risk, risk ratio, or odds ratio (i.e. an item that provides information about the direction of effect) |
Measures of treatment effect
The focus of this review is on the estimation of the prevalence of severe fatigue, the course of severe fatigue and risk factors for fatigue or factors associated with it after treatment for childhood cancer. We combined the prevalence of severe fatigue and the course of severe fatigue with validated and non‐validated questionnaires in the analyses. If we had included studies with non‐validated questionnaires, we would have performed separate analyses of the data from the subgroup of studies that used validated questionnaires. We used the following data: prevalence, cumulative incidence, mean difference, absolute and relative risk, odds ratio, attributable risk, and other associated outcomes. We presented all measures with a 95% confidence interval.
Dealing with missing data
When possible, we contacted authors of individual studies for clarification of unspecified or unclear data, or to obtain missing data about study selection, data extraction and 'Risk of bias' assessment. We contacted study authors to request information when it was not clear if at least 90% of the survivors were under 18 years at diagnosis, or to request additional data when the prevalence of severe fatigue was not presented. We sent a reminder approximately four weeks after the initial request. If study authors did not respond to the information request about age at diagnosis, we designated the studies as 'Awaiting classification'.
Assessment of heterogeneity
We assessed heterogeneity both by visual inspection of the forest plots and by a formal statistical test for heterogeneity, i.e. the I2 statistic. We defined significant heterogeneity as I2 > 50% (Deeks 2017).
Assessment of reporting biases
If we had been able to pool the results, we would have produced a funnel plot to identify the possible presence of publication bias, provided there were a sufficient number of included studies (i.e. when at least 10 studies are available for meta‐analysis). If there are fewer than 10 studies, the power of the test is too low to distinguish chance variation from real asymmetry (Sterne 2017).
Data synthesis
We would have pooled results if (observational) studies were comparable, including the outcome definitions and study population (e.g. cancer type and cancer treatment). If we had been able to pool results, we would have plotted the pooled prevalence rates of longitudinal studies in a graph, to provide an overview of the course of severe fatigue over time. If it had been possible to pool the results, we would have used a random‐effects model. Since pooling of the studies was not feasible, we summarized studies descriptively. We conducted all analyses according to the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017). We calculated prevalence rates and 95% confidence intervals using the Wilson method. As this was not possible in Review Manager 5, we used the tool EpiTools epidemiological calculators (Epitools 2018). We used R Statistical Software 2016 to produce the forest plots, and we entered the data into Review Manager 5 software as provided by Cochrane (Review Manager 2014).
Subgroup analysis and investigation of heterogeneity
Where possible, we performed subgroup analysis based on cancer diagnosis (haematological cancer, bone cancer, brain cancer or other solid tumours), cancer treatment (chemotherapy, stem cell transplantation/bone marrow transplantation, surgery for primary cancer, radiotherapy and radiotherapy on CNS localisation versus non‐CNS localisation), gender (male/ female), age at cancer diagnosis (0 to 4 years / 4 to 12 years / over 12 years), age at fatigue assessment (child/adult) and follow‐up time since cancer diagnosis (less than 5 years / 5 to 15 years / more than 15 years). We defined these subgroups because we anticipated that prevalence rates of severe fatigue might differ between them. If we found significant heterogeneity (I2 > 50%) (Deeks 2017), we explored possible reasons based on clinical differences and made a decision on whether pooling of the data was justified, using a random‐effects model.
Sensitivity analysis
If we had been able to pool results, we would have conducted sensitivity analyses for every individual 'Risk of bias' item. We would have excluded those studies with a high risk of bias and studies for which the risk of bias was unclear from these analyses. We would have performed a sensitivity analysis for all outcomes for which pooling was possible if at least two studies were left in the analysis after excluding studies at high and unclear risk, and compared them to the results of all available studies.
Results
Description of studies
Results of the search
We identified 2218 records from the electronic database search, and retrieved 71 records through screening of conference proceedings. After removal of duplicates, we screened the remaining 1844 records for relevance, based on title and abstract. We retrieved 99 full‐text articles. We found eight full‐text articles through reference checking of included studies, two additional studies after screening the reference list of 20 review papers, and one study identified by experts in the field. We assessed these 110 full‐text articles for eligibility. See also the PRISMA study flow diagram (Figure 1). Thirty studies met the eligibility criteria and are included in this review, 12 studies were classified as awaiting classification (Characteristics of studies awaiting classification), one study as an ongoing study, and 67 studies were excluded.
1.
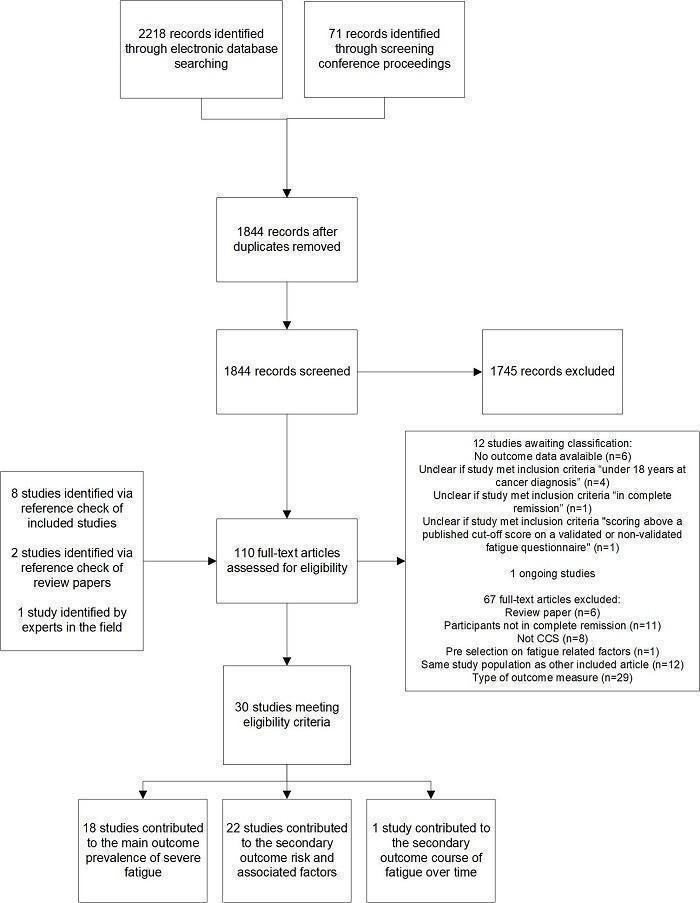
Study flow diagram.
Included studies
Characteristics of the 30 included studies can be found in the Characteristics of included studies at the end of this review. Twenty‐nine studies had a cross‐sectional design (Barrera 2012; Berbis 2013; Brand 2016; Calaminus 2014; Cheung 2017; Crom 1999; Daniel 2019; Gordijn 2013; Hamre 2013a; Harila 2010; Ho 2019; Johannsdottir 2012; Kenney 2010; Khan 2014; Langeveld 2003; Lopez 2011; Meeske 2005; Mört 2011; Mulrooney 2008; Pemberger 2005; Puhr 2019; Reulen 2007; Ruccione 2013; Rueegg 2013; Sundberg 2013; Tremolada 2018; Van Dijk 2008; Verberne 2012; Wright 2013), and we included one case‐control study that assessed severe fatigue at two time points (Zeller 2014a). This case‐control study was a follow‐up study of Hamre 2013a. Hamre 2013a was included to report on the prevalence of severe fatigue and associated factors, while Zeller 2014a was included to describe changes in severe fatigue over time and risk factors for fatigue. Daniel 2019 and Mulrooney 2008 report on the same study population; Daniel 2019 was included to describe the prevalence of severe fatigue, and Mulrooney 2008 was added to the description of risk and associated factors.
All studies combined described 18,682 individual participants after treatment for childhood cancer, of whom 9156 were female and 9515 male. Hamre 2013a did not provide information on gender of all 290 study participants, but only for the 279 participants that were included in the risk factor analysis. Sample sizes of the included studies ranged from 17 to 10,189 and 13 studies described fewer than 100 study participants (Barrera 2012; Cheung 2017; Gordijn 2013; Harila 2010; Kenney 2010; Lopez 2011; Pemberger 2005; Ruccione 2013; Sundberg 2013; Tremolada 2018; Van Dijk 2008; Wright 2013; Zeller 2014a). Three studies included both childhood cancer survivors and adult cancer survivors, but they were eligible for inclusion because more than 90% of the study population was under the age of 18 years at cancer diagnosis (Brand 2016; Daniel 2019; Mulrooney 2008). Age at diagnosis of the included studies ranged from a minimum of 0 to 6 years to a maximum of 12 to 22 years. Years of follow‐up were reported as time since cancer diagnosis in 14 studies (Berbis 2013; Brand 2016; Calaminus 2014; Cheung 2017; Crom 1999; Hamre 2013a; Harila 2010; Johannsdottir 2012; Kenney 2010; Mört 2011; Mulrooney 2008; Rueegg 2013; Van Dijk 2008; Zeller 2014a), and as time since end of cancer therapy in 10 studies (Gordijn 2013; Ho 2019; Langeveld 2003; Lopez 2011; Meeske 2005; Puhr 2019; Sundberg 2013; Tremolada 2018; Verberne 2012; Wright 2013). Two studies reported both time since cancer diagnosis and time since end of cancer therapy (Khan 2014; Pemberger 2005), and four studies did not report mean time since diagnosis or time since end of therapy (Barrera 2012; Daniel 2019; Reulen 2007; Ruccione 2013). Time since cancer diagnosis ranged from two years to 65 years, and time since end of cancer therapy ranged from less than six months to a maximum of 33 years.
Age at assessment varied considerably between studies. Five studies included children up to 18 years of age at fatigue assessment, with the mean age at assessment of individual studies ranging from 9.7 years to 16 years (Gordijn 2013; Ho 2019; Mört 2011; Verberne 2012; Wright 2013). Seventeen studies included participants from 16 years and older, consisting of mainly adult survivors who were older than 18 years at fatigue assessment (range of mean or median age at assessment of individual studies 22.6 to 56 years) (Barrera 2012; Calaminus 2014; Crom 1999; Daniel 2019; Hamre 2013a; Harila 2010; Kenney 2010; Langeveld 2003; Meeske 2005; Mulrooney 2008; Pemberger 2005; Puhr 2019; Reulen 2007; Rueegg 2013; Sundberg 2013; Van Dijk 2008; Zeller 2014a). The remaining eight studies included a mix of children and adults, with the mean or median age of individual studies ranging from 14 to 20.2 years (Berbis 2013; Brand 2016; Cheung 2017; Johannsdottir 2012; Khan 2014; Lopez 2011; Ruccione 2013; Tremolada 2018).
There was substantial variation between studies in the occurrence of different cancer diagnoses. Five studies only included participants treated for acute lymphoblastic leukaemia (ALL) (Cheung 2017; Gordijn 2013; Harila 2010; Khan 2014; Meeske 2005), two studies only included participants treated for bone cancer (Barrera 2012; Lopez 2011), two studies only participants treated for brain cancer (Brand 2016; Puhr 2019) and one study only participants with Hodgkin lymphoma (HL) diagnosis (Calaminus 2014). The other 20 studies included more than one cancer type. Of these studies, 15 included more than three different cancer types, covering a broad range of childhood cancer diagnoses (Crom 1999; Daniel 2019; Ho 2019; Kenney 2010; Langeveld 2003; Mört 2011; Mulrooney 2008; Pemberger 2005; Reulen 2007; Ruccione 2013; Rueegg 2013; Tremolada 2018; Van Dijk 2008; Verberne 2012; Wright 2013). Of the remaining five studies, one included acute myeloid leukaemia (AML), infratentorial astrocytoma (IA) and Wilms tumour (WT) survivors (Johannsdottir 2012), one study included ALL and AML survivors (Berbis 2013), one study included ALL and lymphoblastic lymphoma survivors (Sundberg 2013), and two studies included ALL, non‐Hodgkin lymphoma (NHL) and HL survivors (Hamre 2013a; Zeller 2014a).
Reported treatment modalities were chemotherapy, radiotherapy, cranial irradiation, surgery and SCT/BMT, and were mostly reported as a combination of treatments. In two studies that included only ALL survivors, participants received chemotherapy only (Cheung 2017; Gordijn 2013). SCT/BMT was reported in 12 studies (Berbis 2013; Brand 2016; Harila 2010; Ho 2019; Johannsdottir 2012; Lopez 2011; Meeske 2005; Mört 2011; Ruccione 2013; Rueegg 2013; Sundberg 2013; Tremolada 2018), and cranial irradiation was explicitly reported in 10 studies (Berbis 2013; Daniel 2019; Hamre 2013a; Harila 2010; Khan 2014; Meeske 2005; Puhr 2019; Rueegg 2013; Sundberg 2013; Verberne 2012). Limited information was available about received chemotherapeutic agents, radiation fields and received cumulative doses.
Fatigue was assessed with 10 different questionnaires, which were all validated. The most frequently used instrument was the Short Form‐36 (SF‐36) vitality subscale (Berbis 2013; Harila 2010; Pemberger 2005; Reulen 2007; Rueegg 2013; Sundberg 2013; Tremolada 2018; Van Dijk 2008). The Pediatric Quality of Life Multidimensional Fatigue scale self‐reported and/or parent‐proxy form (PedsQL MFS) was used in six studies (Brand 2016; Cheung 2017; Gordijn 2013; Mört 2011; Ruccione 2013; Verberne 2012). The EORTC‐QLQ‐C30 symptom scale fatigue was used in four studies (Barrera 2012; Calaminus 2014; Crom 1999; Lopez 2011), and the Fatigue Questionnaire (FQ) was also used in four studies (Hamre 2013a; Johannsdottir 2012; Puhr 2019; Zeller 2014a), while the Functional Assessment of Chronic Illness therapy ‐ Fatigue scale (FACIT‐F) was used in three studies (Daniel 2019; Kenney 2010; Mulrooney 2008). Other questionnaires that were used included the Revised‐Piper Fatigue scale (R‐PFS) (Meeske 2005), the Brief Fatigue Inventory (BFI) (Khan 2014), the Multidimensional Fatigue Inventory (MFI) (Langeveld 2003), the Fatigue Scale – Adolescent (FS‐A) (Wright 2013) and the Chinese versions of the FS‐A and the Fatigue Scale ‐ Children (FS‐C) (Ho 2019).
Nineteen studies provided a prevalence of severe fatigue, either in the study report or provided by the study authors upon request, but two studies described the same study population and therefore the same prevalence (Hamre 2013a; Zeller 2014a). As a result, 18 prevalence rates of different samples were included to describe the main outcome. Twenty‐two studies provided information about risk and associated factors and one study provided information about the course of fatigue over time.
Excluded studies
We excluded 67 full‐text articles for the following reasons: study design was a literature review (n = 6), participants were not in complete remission (n = 11), participants were not childhood cancer survivors (e.g. survivors of adult‐onset cancer, other diseases) (n = 8), preselection on fatigue‐related factors (n = 1), the study population was the same as other included studies (n = 12) and type of outcome measure did not meet the inclusion criteria (n = 29). Specific reasons for exclusion are shown in the Characteristics of excluded studies table.
Risk of bias in included studies
Data for the 'Risk of bias' assessments are presented in the Characteristics of included studies tables and Figure 2. All studies scored at least one 'Risk of bias' item as unclear or high risk.
2.
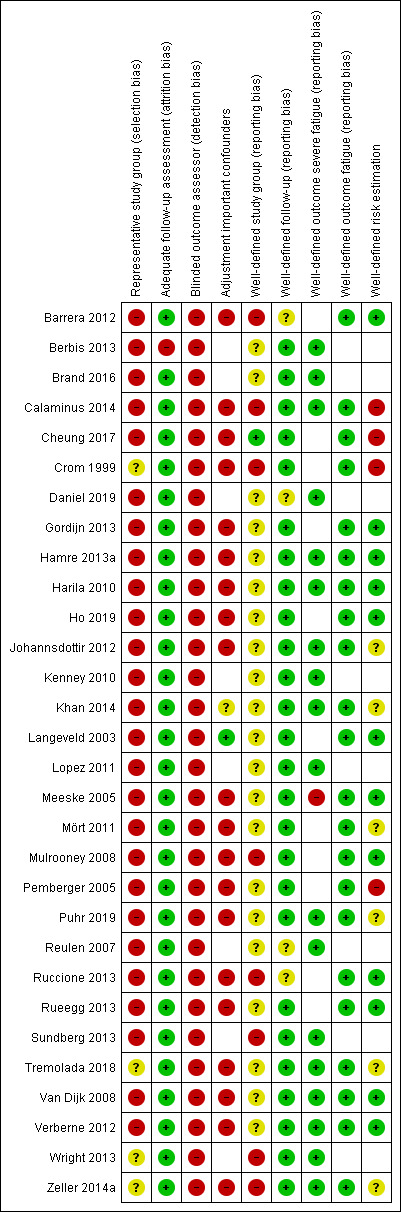
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Internal validity
Representative study group (selection bias)
When more than 90% of the original cohort of cancer survivors was described or if the study population was a random sample of the original cohort with respect to cancer treatment, then we judged the study to be representative (i.e. low risk of bias). The study population of one study was a random sample of the original cohort of cancer survivors with respect to treatment intensity, but it was unclear which specific treatments were received by the study participants (Crom 1999). We therefore rated this study as being at unclear risk of bias. Seventeen studies did not report the size of the original cohort, but based on the amount of eligible participants it was clear that the described study group did not consist of 90% or more of the original cohort and we judged them as being at high risk of bias (Barrera 2012; Brand 2016; Calaminus 2014; Gordijn 2013; Hamre 2013a; Harila 2010; Ho 2019; Johannsdottir 2012; Khan 2014; Langeveld 2003; Meeske 2005; Mört 2011; Pemberger 2005; Ruccione 2013; Rueegg 2013; Van Dijk 2008; Verberne 2012). In addition, nine studies reported the size of the original cohort but did not describe at least 90% of the original cohort and were also judged as being at high risk of bias (Berbis 2013; Cheung 2017; Daniel 2019; Kenney 2010; Lopez 2011; Mulrooney 2008; Puhr 2019; Reulen 2007; Sundberg 2013). The remaining three studies did not report the size of the original cohort nor the eligible sample size and were rated at unclear risk of bias for this domain (Tremolada 2018; Wright 2013; Zeller 2014a). In summary, four studies were at unclear risk of bias (Crom 1999; Tremolada 2018; Wright 2013; Zeller 2014a) and 26 studies were at high risk of bias (Barrera 2012; Berbis 2013; Brand 2016; Calaminus 2014; Cheung 2017; Daniel 2019; Gordijn 2013; Hamre 2013a; Harila 2010; Ho 2019; Johannsdottir 2012; Kenney 2010; Khan 2014; Langeveld 2003; Lopez 2011; Meeske 2005; Mört 2011; Mulrooney 2008; Pemberger 2005; Puhr 2019; Reulen 2007; Ruccione 2013; Rueegg 2013; Sundberg 2013; Van Dijk 2008; Verberne 2012).
Adequate follow‐up assessment (attrition bias)
Follow‐up assessment was adequate when fatigue was assessed for at least 65% of the study group of interest. Twenty‐nine of the 30 included studies met this criterion and were therefore judged to be at low risk of bias. One study assessed fatigue for less than 65% of the study group of interest and was rated at high risk of bias (Berbis 2013). In this study only the survivors aged 18 years and older at the time of assessment were offered the fatigue questionnaire.
Blinding of outcome assessment (detection bias)
It was not possible to blind the outcome assessors in any of the 30 included studies. We therefore assessed all the included studies as being at high risk of bias for this domain.
Confounding
Assessment for risk of confounding was based on the adjustment for other factors in the risk factor analyses of the included studies. We rated one study (Langeveld 2003) at low risk of bias for this domain, because they adjusted for possibly important prognostic factors (i.e. age, sex, co‐treatment, comorbidity) and for time since completion of therapy in the multivariable analysis. Twenty studies performed only univariable analysis or did not include both possibly important prognostic factors and time since completion of therapy/time since diagnosis in the multivariable analysis, and were rated at high risk of bias (Barrera 2012; Calaminus 2014; Cheung 2017; Crom 1999; Gordijn 2013; Hamre 2013a; Harila 2010; Ho 2019; Johannsdottir 2012; Meeske 2005; Mört 2011; Mulrooney 2008; Pemberger 2005; Puhr 2019; Ruccione 2013; Rueegg 2013; Tremolada 2018; Van Dijk 2008; Verberne 2012; Zeller 2014a). For one study (Khan 2014) it was unclear which variables were included in the multivariable analysis and we judged it to be at unclear risk of bias.The remaining eight studies did not perform any risk factor analyses and we could therefore make no assessments for this domain (Berbis 2013; Brand 2016; Daniel 2019; Kenney 2010; Lopez 2011; Reulen 2007; Sundberg 2013; Wright 2013).
External validity
Reporting bias
Three 'Risk of bias' domains contributed to the assessment of possible reporting bias.
Reporting bias study group
The 'Risk of bias' domain for well‐defined study group was based on the description of inclusion and exclusion criteria and reporting of cancer diagnosis and cancer treatment of the study population. Inclusion and exclusion criteria were described and provided sufficient information for replication in all 30 included studies. Cancer diagnosis and cancer treatment were described in detail, including chemotherapeutic agents and cumulative doses, in one study, which we rated at low risk of bias (Cheung 2017). Cancer diagnosis and cancer treatment were described in the main categories of treatment (e.g. surgery, chemotherapy, radiotherapy), but detailed information about specific chemotherapeutic agents, radiotherapy fields and dose was partly reported or not available in 21 studies, which we therefore judged to be at unclear risk of bias (Berbis 2013; Brand 2016; Daniel 2019; Gordijn 2013; Hamre 2013a; Harila 2010; Ho 2019; Johannsdottir 2012; Kenney 2010; Khan 2014; Langeveld 2003; Lopez 2011; Meeske 2005; Mört 2011; Pemberger 2005; Puhr 2019; Reulen 2007; Rueegg 2013; Tremolada 2018; Van Dijk 2008; Verberne 2012). The remaining eight studies reported limited information about cancer treatment (e.g. fewer than the three main categories of treatment) and we rated them at high risk of bias (Barrera 2012; Calaminus 2014; Crom 1999; Mulrooney 2008; Ruccione 2013; Sundberg 2013; Wright 2013; Zeller 2014a).
Reporting bias follow‐up
We judged reporting of follow‐up as being at low risk of bias if time since cancer diagnosis or time since completion of therapy was reported. On this basis, we rated 26 studies at low risk of bias for this domain (Berbis 2013; Brand 2016; Calaminus 2014; Cheung 2017; Crom 1999; Gordijn 2013; Hamre 2013a; Harila 2010; Ho 2019; Johannsdottir 2012; Kenney 2010; Khan 2014; Langeveld 2003; Lopez 2011; Meeske 2005; Mört 2011; Mulrooney 2008; Pemberger 2005; Puhr 2019; Rueegg 2013; Sundberg 2013; Tremolada 2018; Van Dijk 2008; Verberne 2012; Wright 2013; Zeller 2014a). Four studies did not report the mean time since diagnosis or time since completion of therapy, but based on the inclusion criteria participants in these studies were at least five years since diagnosis (Barrera 2012; Daniel 2019; Reulen 2007) or completed therapy within the past six months (Ruccione 2013). We therefore judged these four studies to be at unclear risk of bias.
Reporting bias outcome
Reporting bias of the outcome was divided into two separate 'Risk of bias' domains: a well‐defined outcome of severe fatigue (outcome main objective) and a well‐defined outcome of fatigue (outcome secondary objective). We appraised both items only when the study contributed to the specific outcome.
The domain of well‐defined outcome: severe fatigue was available for 19 studies (Berbis 2013; Brand 2016; Calaminus 2014; Daniel 2019; Hamre 2013a; Harila 2010; Johannsdottir 2012; Kenney 2010; Khan 2014; Lopez 2011; Meeske 2005; Puhr 2019; Reulen 2007; Sundberg 2013; Tremolada 2018; Van Dijk 2008; Verberne 2012; Wright 2013; Zeller 2014a). If the authors of the included studies reported which instrument they used to assess severe fatigue and what they considered it to be, or provided additional information through a data query, we rated them at low risk of bias for this domain. We judged 18 studies to be at low risk of bias (Berbis 2013; Brand 2016; Calaminus 2014; Daniel 2019; Hamre 2013a; Harila 2010; Johannsdottir 2012; Kenney 2010; Khan 2014; Lopez 2011; Puhr 2019; Reulen 2007; Sundberg 2013; Tremolada 2018; Van Dijk 2008; Verberne 2012; Wright 2013; Zeller 2014a) and one study to be at high risk of bias (Meeske 2005).
The domain of well‐defined outcome: fatigue was available for 22 studies (Barrera 2012; Calaminus 2014; Cheung 2017; Crom 1999; Gordijn 2013; Hamre 2013a; Harila 2010; Ho 2019; Johannsdottir 2012; Khan 2014; Langeveld 2003; Meeske 2005; Mört 2011; Mulrooney 2008; Pemberger 2005; Puhr 2019; Ruccione 2013; Rueegg 2013; Tremolada 2018; Van Dijk 2008; Verberne 2012; Zeller 2014a). If the authors of the included studies reported which instrument they used to assess fatigue and they mentioned how fatigue was interpreted, we rated them at low risk of bias for this domain. We judged all 22 studies to be at low risk of bias for this domain.
Risk estimation
Risk estimation was well‐defined when one of the following items was calculated and presented in the study: prevalence, cumulative incidence, mean difference, correlation coefficient, regression coefficient, relative risk, risk ratio, or odds ratio. Twelve studies performed risk factor analyses and presented one of the items for all risk and associated factors in their report; we judged these studies to be at low risk of bias (Barrera 2012; Gordijn 2013; Hamre 2013a; Harila 2010; Ho 2019; Langeveld 2003; Meeske 2005; Mulrooney 2008; Ruccione 2013; Rueegg 2013; Van Dijk 2008; Verberne 2012). Six studies performed risk factor analyses but did not present one of the items for all risk and associated factors in the report, and were rated at unclear risk of bias (Johannsdottir 2012; Khan 2014; Mört 2011; Puhr 2019; Tremolada 2018; Zeller 2014a). Four studies did not present any effect estimate and were rated at high risk of bias (Calaminus 2014; Cheung 2017; Crom 1999; Pemberger 2005). The remaining eight studies did not perform any risk factor analyses and we therefore made no judgement on this domain (Berbis 2013; Brand 2016; Daniel 2019; Kenney 2010; Lopez 2011; Reulen 2007; Sundberg 2013; Wright 2013).
Effects of interventions
Prevalence of severe fatigue
Prevalence of severe fatigue was reported in 18 studies (Berbis 2013; Brand 2016; Calaminus 2014; Daniel 2019; Hamre 2013a; Harila 2010; Johannsdottir 2012; Kenney 2010; Khan 2014; Lopez 2011; Meeske 2005; Puhr 2019; Reulen 2007; Sundberg 2013; Tremolada 2018; Van Dijk 2008; Verberne 2012; Wright 2013). The studies were not comparable with respect to study population and outcome definition. For example, study populations differed greatly in reported cancer treatments and included cancer diagnoses, ranging from a study only including one specific cancer type to studies including a broad range of cancer diagnoses. In addition, fatigue was assessed with eight different questionnaires. Because the studies were so heterogeneous, both statistically and clinically, we did not pool prevalence rates and present the results descriptively. The prevalence rates of the 18 included studies, describing 14,573 survivors, ranged from 0% to 61.7% (Figure 3).
3.
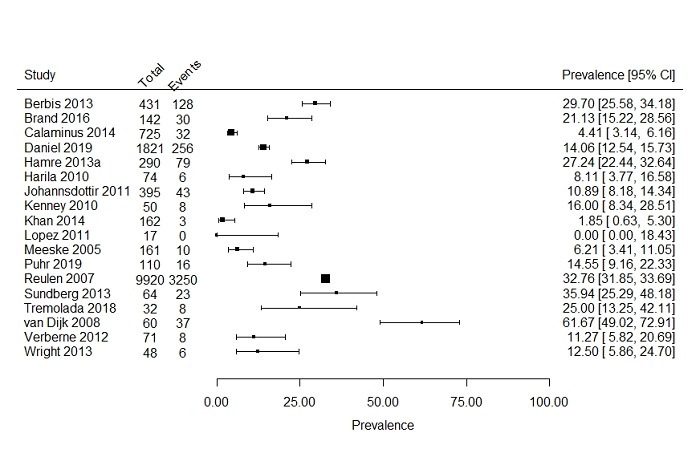
Forest plot: Prevalence and 95% confidence interval of severe fatigue including all studies
Three studies compared the prevalence of severe fatigue in survivors to either a control group of siblings or to population‐based controls (Hamre 2013a; Johannsdottir 2012; Kenney 2010) and one study provided data on the prevalence of severe fatigue in siblings through a data query (Daniel 2019). Prevalence of severe fatigue in the control groups was 3.1% for siblings aged between 48 and 70 years compared to 16.0% in survivors (Kenney 2010); 5.9% in population controls from Norway aged between 19 and 34 years compared to 10.9% in survivors (Johannsdottir 2012); 8.0% in population controls from Norway aged between 19 and 50 years compared to 27.2% in survivors (Hamre 2013a); and 10.3% in siblings aged 18 years and older compared to 14.1% in survivors (Daniel 2019). These four studies reported that survivors were more often fatigued than controls, but this difference was statistically significantly different in only two studies (Hamre 2013a; Johannsdottir 2012).
We formed subgroups based on cancer diagnosis and age at assessment. Seven studies included only survivors with a haematological cancer diagnosis, describing in total 1907 survivors (Berbis 2013; Calaminus 2014; Hamre 2013a; Harila 2010; Khan 2014; Meeske 2005; Sundberg 2013). Prevalence rates for severe fatigue after a haematological cancer reported in these studies ranged from 1.8% to 35.9% (Figure 4). Two studies reported a prevalence of severe fatigue in brain cancer survivors of 14.6% (110 survivors) and 21.1% (142 survivors) (Figure 5; Brand 2016; Puhr 2019). One study reported a prevalence for severe fatigue in bone cancer survivors of 0.0% (17 survivors) (Figure 6; Lopez 2011).
4.
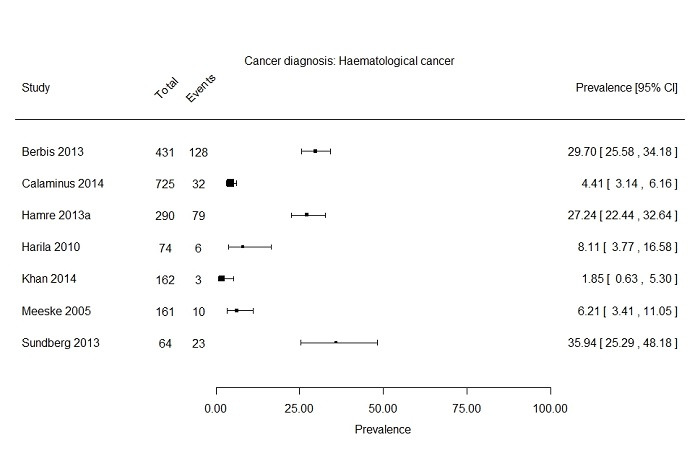
Forest plot: Prevalence and 95% confidence interval of severe fatigue in subgroup haemtological cancer diagnosis
5.
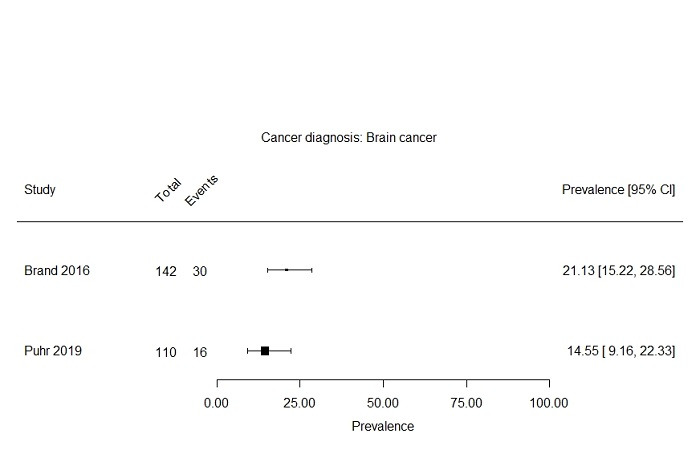
Forest plot: Prevalence and 95% confidence interval of severe fatigue in subgroup brain cancer diagnosis
6.
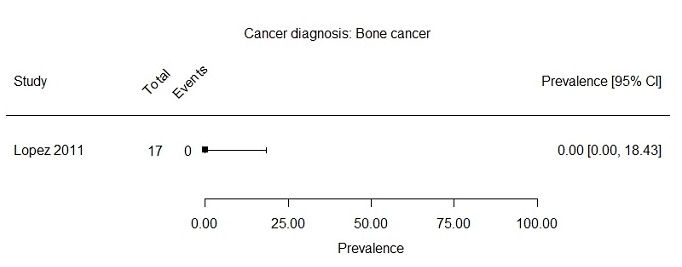
Forest plot: Prevalence and 95% confidence interval of severe fatigue in subgroup bone cancer diagnosis
Two subgroups could be formed based on age at assessment (i.e. child versus adult). Two studies included only children (Verberne 2012; Wright 2013), with a maximum age of 18 years at fatigue assessment, and 11 studies reported a prevalence rate of severe fatigue primarily in adults (age range 16 to 71) (Berbis 2013; Calaminus 2014; Daniel 2019; Hamre 2013a; Harila 2010; Kenney 2010; Meeske 2005; Puhr 2019; Reulen 2007; Sundberg 2013; Van Dijk 2008). Johannsdottir 2012 included both children and adults, but also presented the prevalence of severe fatigue separately for both age groups. This resulted in three studies for the subgroup of children (268 survivors) and 12 studies for the subgroup of adults (13,952 survivors). For the studies in children, prevalence rates of severe fatigue ranged from 6.7% to 12.5% (Figure 7). The 12 studies primarily including adults presented prevalence rates ranging from 4.4% to 61.7% (Figure 8).
7.
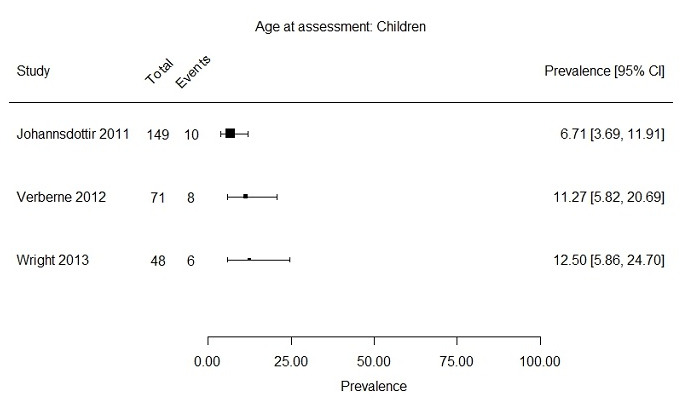
Forest plot: Prevalence and 95% confidence interval of severe fatigue in subgroup children (range 8 ‐ 18 years at assessment of fatigue)
8.
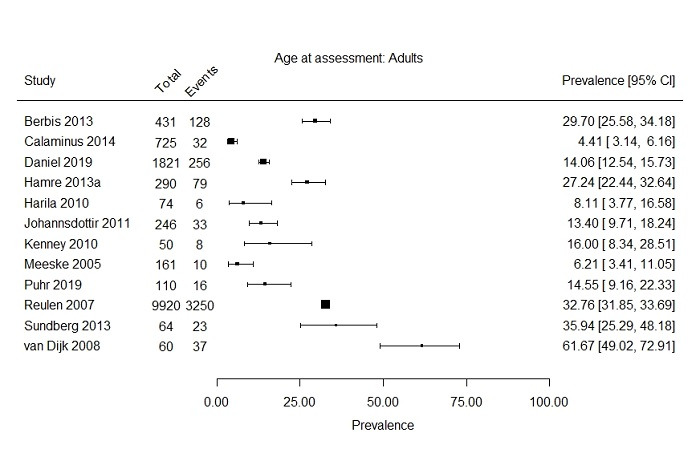
Forest plot: Prevalence and 95% confidence interval of severe fatigue in subgroup primarily adults (range 16 ‐ 71 years at assessment of fatigue)
It was not possible to form the prespecified subgroups based on gender, cancer treatment, age at diagnosis and follow‐up time since cancer diagnosis.
Course of severe fatigue over time
One study (Zeller 2014a) provided information on the course of severe fatigue over time. This study was a follow‐up to Hamre 2013a, in which all severely‐fatigued participants (n = 79) and 130 of the non‐severely‐fatigued participants at time point one were invited to participate. At a mean interval of 2.7 years (range 1 to 4.3 years) since the first fatigue assessment, 53 of the 79 severely‐fatigued survivors and 49 of the 130 non‐severely‐fatigued survivors participated in the second fatigue assessment. Forty‐one participants (40.2%) reported severe fatigue at the second fatigue assessment, of whom 32 participants (31.4%) reported severe fatigue at both time points and were defined as being persistently severely fatigued.
Risk and associated factors
Twenty‐two studies provided information on associated factors and risk factors for fatigue. Four studies performed analyses with severe fatigue as the dependent factor (i.e. outcome) (Hamre 2013a; Johannsdottir 2012; Puhr 2019; Zeller 2014a); one study performed analyses with moderate to severe fatigue as the dependent factor (Meeske 2005); one study performed analyses with mild to severe fatigue as the dependent factor (Khan 2014); two studies defined 'fatigued' as scoring below the 10th percentile of the siblings' scores (Mulrooney 2008; Rueegg 2013); and the remaining 14 studies performed analyses with a fatigue score on a continuous scale as the dependent factor (Barrera 2012; Calaminus 2014; Cheung 2017; Crom 1999; Gordijn 2013; Harila 2010; Ho 2019; Langeveld 2003; Mört 2011; Pemberger 2005; Ruccione 2013; Tremolada 2018; Van Dijk 2008; Verberne 2012). Since there was large variation in the methods used to analyse possible relations with fatigue (e.g. correlation, univariable regression, multivariable regression, dependent factor etc.), we could not conduct a meta‐analysis and we present the results descriptively. None of the included studies investigated genetics or tumour stage in relation to fatigue.
Disease‐ and treatment‐related factors
Sixteen studies investigated the relationship between disease‐ and treatment‐related risk factors with fatigue (Barrera 2012; Calaminus 2014; Crom 1999; Hamre 2013a; Harila 2010; Ho 2019; Johannsdottir 2012; Khan 2014; Langeveld 2003; Meeske 2005; Mört 2011; Mulrooney 2008; Puhr 2019; Tremolada 2018; Van Dijk 2008; Zeller 2014a). A detailed overview of these variables can be found in Table 2. Eight studies investigated age at diagnosis as a risk factor for fatigue (Calaminus 2014; Langeveld 2003; Meeske 2005; Mört 2011; Mulrooney 2008; Puhr 2019; Tremolada 2018; Van Dijk 2008). Nine studies explored years of follow‐up, defined as time since diagnosis or time since end of therapy (Calaminus 2014; Ho 2019; Johannsdottir 2012; Langeveld 2003; Meeske 2005; Mört 2011; Puhr 2019; Tremolada 2018; Zeller 2014a). Four studies assessed having had a relapse as a risk factor for fatigue (Khan 2014; Meeske 2005; Mört 2011; Tremolada 2018). Reference and comparisons groups for cancer diagnosis varied between the nine studies investigating it (Crom 1999; Hamre 2013a; Ho 2019; Johannsdottir 2012; Langeveld 2003; Mört 2011; Mulrooney 2008; Puhr 2019; Tremolada 2018). Studies exploring the relationship between cancer treatment and fatigue were heterogeneous, with 10 studies including one or more treatment modalities in their analysis (Barrera 2012; Harila 2010; Ho 2019; Johannsdottir 2012; Langeveld 2003; Meeske 2005; Mört 2011; Mulrooney 2008; Puhr 2019; Zeller 2014a).
2. Disease and treatment‐related variables.
| Characteristics of study | Risk factors | ||||||
| Study ID | Tumour type | Dependent factor | Age at diagnosis | Years of follow‐up | Relapse | Diagnosis | Treatment |
| Barrera 2012 | Bone tumour | Fatigue (continuous) | ‐ | ‐ | ‐ | ‐ | S (type of surgery; LS mean 22.81 vs AMP mean 9.88) |
| Calaminus 2014 | HL | Fatigue (continuous) | NS (no further information) | NS (time since diagnosis, no further information) | ‐ | ‐ | ‐ |
| Crom 1999 | Mix | Fatigue (continuous) | ‐ | ‐ | ‐ | S (HL with mantle radiation, no further information) | ‐ |
| Hamre 2013a | ALL, HL, NHL | Severe fatigue | ‐ | ‐ | ‐ | NSa (ALL = ref; HL OR = 1.7; NHL OR = 1.5) | ‐ |
| Harila 2010 | ALL | Fatigue (continuous) | ‐ | ‐ | ‐ | ‐ | NS (irradiated mean 77 vs non‐irradiated mean 73) |
| Ho 2019b | Mix | Fatigue (continuous) | ‐ | Sa (time since end of therapy; 7 ‐ 12 yrs; b = −0.14) NSa (time since end of therapy; 13 ‐ 18 yrs; b = −0.13) |
‐ | NSa (7 ‐ 12 yrs; b = −0.01; 13 ‐ 18 yrs; b = −0.05) | NSa (7 ‐ 12 yrs; b = −0.11; 13 ‐ 18 yrs; b = 0.08) |
| Johannsdottir 2012 | AML, IA, WT | Severe fatigue | ‐ | NS (time since diagnosis, no further information) | ‐ | S (ref = GP; AML OR = 1.63; IA OR = 2.56; WT OR = 2.98) | NS (treatment modalities, not further specified) |
| Khan 2014 | ALL | Mild to severe fatigue | ‐ | ‐ | Sa,c (OR = 8.35) | ‐ | ‐ |
| Langeveld 2003 | Mix | Fatigue (continuous) | NSa (b = 0.06) | NSa (time since end of therapy; b = 0.02) | ‐ | NSa,d (solid tumour b = 0.02; CNS tumour b = −0.08) | NSa (ref = CT; RT b = 0.01; RT and CT b = 0.04) Sa,d (CRT, b = −0.16) |
| Meeske 2005 | ALL | Moderate to severe fatigue | NS (≤ 3 yrs = ref; 4 ‐ 6 yrs OR = 0.62; 7 ‐ 9 yrs OR = 1.26; ≥ 10 yr OR = 1.04) | NS (time since end of therapy; ref = ≤ 10 yrs; 11 ‐ 15 yrs OR = 0.64; ≥ 16 yrs OR = 0.87 | S (OR = 2.68) | ‐ | NS CRT (ref = 0 Gy; 18 Gy OR = 1.93; ≥ 24 Gy OR = 2.14) Anthracycline (ref = 0mg/m2; 75 ‐ 349 mg/m2 OR = 0.82; ≥ 350 mg/m2 OR = 1.96) BMT (OR = 0.76) |
| Mört 2011 | Mix | Fatigue (continuous) | NS (0 ‐ 4 yrs median 81.94 vs 5 ‐ 9 yrs median 83.33 vs 10 ‐ 12 yrs median 84.72) | NSa (time since diagnosis; ref = ≤ 10 yrs; > 10 yrs b = −3.6) | NS (yes median 86.81 vs no median 81.94) | Sa(ref = leukaemia; NHL b = −2.49; sarcoma b = −14.28; NBL b = −2.3; other b = −0.85) | NSa (ref = surgery alone; CT b = −4.2; RT b = −8.73; SCT b = −3.17; other b = −5.09) |
| Mulrooney 2008 | Mix | Fatigued | NSa (ref = 15+; 10 ‐ 14 yrs OR = 0.8; 5 ‐ 9 yrs OR = 0.9; 0 ‐ 4 yrs OR = 0.7) | ‐ | ‐ | NSa (ref = ALL; CNS OR = 1.3; HL OR = 1.2; STS OR = 1.0; Bone OR = 1.3) | Sa (RT: OR = 1.7) NSa (CT: OR = 1.0) |
| Puhr 2019 | CNS | Severe fatigue | NS (no further information) | S (time since end of therapy; fatigued mean 10.95 yrs vs non‐fatigued 14.32 yrs) | ‐ | NS (type of brain tumour; no further information) | S (surgery, CRT and chemotherapy; fatigued 26.9% vs non‐fatigued 8.3%) |
| Tremolada 2018 | Mix | Fatigue (continuous) | NS (r = 0.218) | NS (time since end of therapy; r = −0.012) | NS (r = −0.200) | NS (r = −0.042) | ‐ |
| Van Dijk 2008 | Mix | Fatigue (continuous) | NS (< 12 yrs mean 53.7 vs ≥ 12 yrs mean 49.8) | ‐ | ‐ | ‐ | ‐ |
| Zeller 2014a | ALL, HL, NHL | Persistent severe fatigue | ‐ | NS (time since diagnosis; cases mean 23 yrs vs controls mean 24 yrs) | ‐ | ‐ | NS (RT; cases n = 13 vs controls n = 14) |
Disease and treatment‐related risk factors for fatigue. Presented results are from univariable analyses, unless multivariable analyses were available. In that case, we present only the results of the multivariable analyses in this table. More information about the effect estimates can be found in the Characteristics of included studies tables.
ALL: acute lymphoblastoma leukaemia; AML: acute myeloid leukaemia; AMP: amputation; b: beta coefficient regression; BMT: bone marrow transplant; CNS: central nervous system tumour; CT: chemotherapy; GP: general population; HL: Hodgkins lymphoma; IA: infratentorial astrocytoma; NA: not applicable; NHL: non‐Hodgkins lymphoma; NS: non‐significant; LS: limb salvage; MD: mean difference; Mix: covering a broad range of childhood cancer diagnoses; NBL: neuroblastoma; OR: odds ratio; r: correlation coefficient; RT: radiotherapy; CRT: cranial irradiation; S: significant; STS: soft tissue sarcoma; WT: Wilms tumour
aresults of multivariable analyses. bHo 2019 performed separate analysis for survivors aged 7‐12 years and 13‐18 years at the time of fatigue assessment. cfor Khan 2014, it was unclear which other variables were included in the multivariable model. dLangeveld 2003 made the variable diagnosis with Leukaemia/non‐Hodgkins lymphoma without CRT as reference group and leukaemia/non‐Hodgkins lymphoma with CRT, solid tumour and brain/CNS tumour as comparison groups. In the analysis they found a significant reduction in fatigue between leukaemia/non‐Hodgkins lymphoma with CRT and the reference group and no significant effect for the other diagnosis groups.
Multivariable analyses
Six studies performed multivariable analysis that included disease‐ and treatment‐related factors (Hamre 2013a; Ho 2019; Khan 2014; Langeveld 2003; Mört 2011; Mulrooney 2008). Khan 2014 identified a significant effect for having relapsed. However, it was unclear which other variables, next to the variable relapse, were included in the multivariable regression model of this study. Mulrooney 2008 conducted a multivariable analysis including the disease‐ and treatment‐related factors chemotherapy, radiotherapy, age at diagnosis and diagnosis. Radiotherapy was the only significant factor in this multivariable model. The four remaining studies investigated disease‐ and treatment‐related factors in a multivariable model containing other factors (Hamre 2013a; Ho 2019; Langeveld 2003; Mört 2011). Hamre 2013a did not identify a significant effect for the variable of cancer diagnosis. Ho 2019 performed separate analyses for survivors aged seven to 12 years and 13 to 18 years at the time of fatigue assessment. They found a significant effect for time since the end of therapy for survivors aged seven to 12 years. No significant effects were identified for time since end of therapy in survivors aged 13 to 18 years, nor for diagnosis and treatment in either age group (Ho 2019). Mört 2011 found that survivors of a sarcoma were more at risk for fatigue compared to leukaemia survivors, and found no significant effect for cancer treatment and time since diagnosis. Finally, Langeveld 2003 investigated the variables of age at diagnosis, time since end of therapy, diagnosis and cancer treatment. They found that survivors of leukaemia and NHL with cranial irradiation were significantly less fatigued compared to leukaemia and NHL survivors without cranial irradiation (Langeveld 2003).
Demographic characteristics
Thirteen studies investigated the relationship between demographic characteristics and fatigue (Barrera 2012; Cheung 2017; Hamre 2013a; Ho 2019; Johannsdottir 2012; Langeveld 2003; Meeske 2005; Mört 2011; Mulrooney 2008; Pemberger 2005; Puhr 2019; Tremolada 2018; Zeller 2014a). Gender and ethnicity were assessed as possible risk factors for fatigue in 12 studies (Barrera 2012; Cheung 2017; Hamre 2013a; Ho 2019; Johannsdottir 2012; Langeveld 2003; Meeske 2005; Mört 2011; Mulrooney 2008; Pemberger 2005; Puhr 2019; Tremolada 2018) and one study (Meeske 2005) respectively (Table 3). Eleven studies included one or more demographic characteristics as possible associated factors in their analyses (Barrera 2012; Hamre 2013a; Ho 2019; Johannsdottir 2012; Langeveld 2003; Meeske 2005; Mört 2011; Mulrooney 2008; Puhr 2019; Tremolada 2018; Zeller 2014a). An overview of these variables can be found in Table 3. Age at assessment was assessed in nine studies (Barrera 2012; Hamre 2013a; Ho 2019; Johannsdottir 2012; Langeveld 2003; Meeske 2005; Mört 2011; Puhr 2019; Tremolada 2018). Education was assessed in six studies (Hamre 2013a; Johannsdottir 2012; Langeveld 2003; Meeske 2005; Tremolada 2018; Zeller 2014a). Employment was assessed in five studies (Johannsdottir 2012; Langeveld 2003; Meeske 2005; Mulrooney 2008; Zeller 2014a) and marital status was also assessed in five studies (Hamre 2013a; Johannsdottir 2012; Langeveld 2003; Meeske 2005; Mulrooney 2008). Definitions of education, employment and marital status differed considerably between studies.
3. Demographic characteristics.
| Characteristics of study | Risk factors | Associated factors | ||||||
| Study ID | Tumour type | Dependent factor | Gender | Ethnicity | Age at assessment | Education | Marital status | Employment |
| Barrera 2012 | Bone tumour | Fatigue (continuous) | S (female mean 26.19 vs male mean 11.11) | ‐ | NS (age ≤ 25 yrs mean 14.07 vs age ≥ 26 yrs mean 23.93) | ‐ | ‐ | ‐ |
| Cheung 2017 | ALL | Fatigue (continuous) | NS (no further information) | ‐ | ‐ | ‐ | ‐ | ‐ |
| Hamre 2013a | ALL, NHL, HL | Severe fatigue | NSa (female; OR = 0.8) | ‐ | NSa (OR = 1.05) | NS (≥ 11 yrs; OR = 1.6) | NS (not in a partnership; OR = 0.7) | ‐ |
| Ho 2019b | Mix | Fatigue (continuous) | NSa (7 ‐ 12 yrs b = −0.03; 13 ‐ 18 yrs; b = 0.06) | ‐ | NSa (7 ‐ 12 yrs b = −0.09; 13 ‐ 18 yrs b = 0.01) | ‐ | ‐ | ‐ |
| Johannsdottir 2012 | AML, IA, WT | Severe fatigue | NSa (female; OR = 1.54) | ‐ | Sa (OR = 1.08) | NSa (academic education; OR = 0.63) | NSa (married/cohabiting; OR = 1.09) | NSa (gainfully employed; OR = 1.18) |
| Langeveld 2003 | Mix | Fatigue (continuous) | Sa (female; b = 0.19) | ‐ | NSa (b = 0.01) | NSa (higher level; b = 0.03) | NSa (married; b = 0.04) | Sa (ref = unemployed; student/homemaker b = −0.12; employed b = −0.20) |
| Meeske 2005 | ALL | Moderate to severe fatigue | S (female; OR = 2.11) | S (ref = white; Hispanic OR = 2.56; other OR = 1.30) | NS (ref = 18 ‐ 19 yrs; 20 ‐ 24 yrs OR = 0.74; 25 ‐ 29 yrs OR = 1.93; 30 ‐ 41 yrs OR = 1.53) | NS (ref ≤ high school graduate; some college OR = 0.84; college graduate OR = 0.84) | Sa (married; OR = 0.11) | S (ref = work full‐time; work part‐time OR = 1.88; student OR = 1.25; student and working OR = 0.64; unemployed OR = 6.00) |
| Mört 2011 | Mix | Fatigue (continuous) | NSa (female; b = 2.99) | ‐ | Sa (b = −1.87) | ‐ | ‐ | ‐ |
| Mulrooney 2008 | Mix | Fatigued | Sa (female; OR = 1.9) | ‐ | ‐ | ‐ | Sa (not married; OR = 2.7) | NSa (not working full‐time; OR = 1.2) |
| Pemberger 2005 | Mix | Fatigue (continuous) | S (female, no further information) | ‐ | ‐ | ‐ | ‐ | ‐ |
| Puhr 2019 | CNS | Severe fatigue | NS (no further information) | ‐ | NS (no further information) | ‐ | ‐ | ‐ |
| Tremolada 2018 | Mix | Fatigue (continuous) | NS (r = −0.150) | ‐ | NS (r = 0.081) | NS (no further information) | ‐ | ‐ |
| Zeller 2014a# | ALL, NHL, HL | Persistent severe fatigue | ‐ | ‐ | ‐ | NS (higher level education ≥ 12 yrs; cases n = 11 vs controls n = 21) | ‐ | NS (at present in paid work; cases n = 17 vs controls n = 21) |
Demographic risk and associated factors for fatigue. Presented results are from univariable analyses, unless multivariable analyses were available. In that case, we present only the results of the multivariable analyses in this table. For Pemberger 2005, it was unclear if analysis was univariable or multivariable. More detailed information about the effect estimates can be found in the Characteristics of included studies tables.
ALL: acute lymphoblastoma leukaemia; AML: acute myeloid leukaemia; b: beta coefficient regression; CNS: central nervous system tumour; HL: Hodgkins lymphoma; IA: infratentorial astrocytoma; MD: mean difference; Mix: covering a broad range of childhood cancer diagnoses; NHL: non‐Hodgkins lymphoma; NS: non‐significant; OR: odds ratio; r: correlation coefficient; S: Significant; WT: Wilms tumour
aresults of multivariable analyses. bHo 2019 performed separate analysis for survivors aged 7 ‐ 12 years and 13 ‐ 18 years at the time of fatigue assessment. cZeller 2014a is a longitudinal study. The investigated factors can therefore be interpreted as risk factors instead of associated factors.
Multivariable analyses
For Pemberger 2005 it was unclear if the analysis was univariable or multivariable. Seven studies performed multivariable analysis that included demographic characteristics (Hamre 2013a; Ho 2019; Johannsdottir 2012; Langeveld 2003; Meeske 2005; Mört 2011; Mulrooney 2008). The multivariable regression models of these studies differed greatly in their risk and associated factors. Hamre 2013a and Ho 2019 did not identify a significant effect for the demographic characteristics of gender and age at assessment. Johannsdottir 2012 only found a significant association between fatigue and older age at assessment. Langeveld 2003 found a significantly increased risk for female gender, and being employed was associated with less fatigue. Meeske 2005 investigated marital status and identified that being married was associated with less fatigue. Mört 2011 investigated gender and age at assessment and identified a significant association between fatigue and older age at assessment. Mulrooney 2008 found a significantly increased risk for fatigue in female survivors and a significant association between not being married and fatigue. No significant effect was found for employment status (Mulrooney 2008).
Clinical and psychological variables
Several clinical and psychological variables were investigated in relation to fatigue in 11 studies (Gordijn 2013; Hamre 2013a; Ho 2019; Langeveld 2003; Meeske 2005; Mulrooney 2008; Puhr 2019; Ruccione 2013; Rueegg 2013; Verberne 2012; Zeller 2014a). The definitions that were used to describe the investigated clinical and psychological variables were inconsistent. A detailed overview of the variables can be found in Tables 4 to 7 (Table 4; Table 5; Table 6; Table 7). Depression was assessed in relation to fatigue in eight studies (Gordijn 2013; Hamre 2013a; Ho 2019; Langeveld 2003; Meeske 2005; Mulrooney 2008; Ruccione 2013; Zeller 2014a). The association between sleep problems and fatigue was investigated in four studies (Gordijn 2013; Meeske 2005; Verberne 2012; Zeller 2014a). Pain was assessed in three studies (Meeske 2005; Ruccione 2013; Zeller 2014a). Post‐traumatic stress was included as a possible associated factor in one study (Ruccione 2013). Body mass index (BMI) was assessed in five studies (Hamre 2013a; Meeske 2005; Mulrooney 2008; Rueegg 2013; Zeller 2014a). Physical activity was assessed in two studies (Ho 2019; Zeller 2014a). Two studies included a combined variable for late effects in their risk factor analysis (Langeveld 2003; Meeske 2005). The relationship between thyroid problems and fatigue was assessed in four studies (Hamre 2013a; Meeske 2005; Mulrooney 2008; Rueegg 2013). Cardiac problems, neurocognitive impairment, hearing problems and vision impairments were all assessed in two studies (Meeske 2005; Rueegg 2013). Lung fibrosis (Mulrooney 2008), digestive problems (Rueegg 2013), musculoskeletal/neurological problems (Rueegg 2013) and psychiatric comorbidity (Puhr 2019) were assessed in one study each. Finally, Meeske 2005 further investigated associations with fatigue for the clinical variables of second malignancy, chronic headaches or migraines, seizures, exercise‐induced symptoms, surgical procedure following therapy, menopausal symptoms, gonadal failure, growth hormone deficiency, hepatitis C and anaemia in the past 12 months.
4. Clinical and psychological variables part 1.
| Characteristics of study | Associated factors | ||||||||
| Study ID | Tumour type | Dependent factor | Depression | Sleep problems | Pain | Post‐traumatic stress | BMI | Physical activity | Late effects |
| Gordijn 2013 | ALL | Fatigue (continuous) | S (r = −0.45) | S (CSHQ parent, r = −0.60 ; ASHQ, parent form r = −0.74) NS (CSHQ child form, r = −0.44; ASHQ child form, r = −0.47) |
‐ | ‐ | ‐ | ‐ | ‐ |
| Hamre 2013a | ALL, NHL, HL | Severe fatigue | Sa,b (mental distress; OR = 1.15) | ‐ | ‐ | ‐ | NS (≥ 30 kg/m2; OR = 1.8) | ‐ | ‐ |
| Ho 2019c | Mix | Fatigue (continuous) | Sa (7 ‐ 12 yrs b = 0.21; 13 ‐ 18 yrs b = 0.23) | ‐ | ‐ | ‐ | ‐ | Sa (7 ‐ 12 yrs b = −0.56; 13 ‐ 18 yrs b = −0.51) | ‐ |
| Langeveld 2003 | Mix | Fatigue (continuous) | Sa (b = 0.54) | ‐ | ‐ | ‐ | ‐ | ‐ | Sa (b = 0.14) |
| Meeske 2005 | ALL | Moderate to severe fatigue | S (OR = 32.9) | Sa (OR = 6.15) | Sa (OR = 5.56) | ‐ | Sa (obesity; OR = 3.80) | ‐ | S (OR = 1.73) |
| Mulrooney 2008 | Mix | Fatigued | Sa (OR = 7.5) | ‐ | ‐ | ‐ | NSa (BMI 30+ kg/m2; OR = 1.3) | ‐ | ‐ |
| Ruccione 2013 | Mix | Fatigue (continuous) | S (r = 0.64) | ‐ | S (r = 0.42) | S (r = 0.65) | ‐ | ‐ | ‐ |
| Rueegg 2013 | Mix | Fatigued | ‐ | ‐ | ‐ | ‐ | NSa (BMI ≥ 25 kg/m2; OR = 1.44) | ‐ | ‐ |
| Verberne 2012 | CNS | Fatigue (continuous) | ‐ | S (DOES r = −0.78; SWTD r = −0.37) NS (DIMS r = −0.15; SHY r = −0.08; daytime sleepiness r = −0.30) |
‐ | ‐ | ‐ | ‐ | ‐ |
| Zeller 2014ad | ALL, NHL, HL | Persistent severe fatigue | Sa (OR = 1.3) | NSa (insomnia, no further information) | NSa (no further information) | ‐ | NS (cases mean 25.1 vs controls mean 24.6) | NSa (number of steps per day, no further information) | ‐ |
Clinical and psychological variables associated with fatigue. Presented results are from univariable analyses, unless multivariable analyses were available. In that case, we present only the results of the multivariable analyses in this table. More detailed information about the effect estimates can be found in the Characteristics of included studies tables.
ALL: acute lymphoblastoma leukaemia; ASHQ: Adolescent Sleep Habits Questionnaire; b: beta coefficient linear regression; BMI: body mass index; CNS: central nervous system tumour; CSHQ: Childrens Sleep Habits Questionnaire; DIMS: disorders maintaining sleep; DOES: disorders of excessive somnolence; HADS: Hospital Anxiety and Depression scale; HL: Hodgkins lymphoma; Mix: covering a broad range of childhood cancer diagnoses; NHL: non‐Hodgkins lymphoma; NS: non‐significant; OR: odds ratio; r: correlation coefficient; S: Significant; SHY: sleep hyperhydrosis; SWTD: sleep wake transition disorder
aresults of multivariable analyses. bmental distress was assessed with the HADS questionnaire for depression and anxiety. cHo 2019 performed separate analysis for survivors aged 7‐12 years and 13‐18 years at the time of fatigue assessment.
dZeller 2014a is a longitudinal study. Therefore, the investigated factors can be interpreted as risk factors instead of associated factors.
5. Clinical and psychological variables part 2.
| Characteristics of study | Associated factors | ||||||||
| Study ID | Tumour type | Dependent factor | Thyroid problems | Cardiac problems | Lung fibrosis | Neurocognitive impairment | Second malignancy | Chronic headaches or migraines | Seizures |
| Hamre 2013a | ALL, NHL, HL | Severe fatigue | NSa (present hypothyroidism; OR = 1.4) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Meeske 2005 | ALL | Moderate to severe fatigue | S (thyroid status; OR = 4.32) | NS (OR = 1.18) | ‐ | Sa (OR = 2.56) | NS (OR = 0.85) | S (OR = 5.32) | S (OR = 4.32) |
| Mulrooney 2008 | Mix | Fatigued | NSa (hypothyroidism; OR = 0.9) | Sa (OR = 2.9) | Sa (OR = 2.9) | ‐ | ‐ | ‐ | ‐ |
| Rueegg 2013 | Mix | Fatigued | Sa (OR = 2.12) | ‐ | ‐ | Sa (memory problems; OR = 3.74) | ‐ | ‐ | ‐ |
Clinical and psychological variables associated with fatigue. Presented results are from univariable analyses, unless multivariable analyses were available. In that case, we present only the results of the multivariable analyses in this table. More detailed information about the effect estimates can be found in the Characteristics of included studies tables.
ALL: acute lymphoblastoma leukaemia; HL: Hodgkins lymphoma; Mix: covering a broad range of childhood cancer diagnoses; NHL: non‐Hodgkins lymphoma; NS: non‐significant; OR: odds ratio; S: significant
aresults of multivariable analyses.
6. Clinical and psychological variables part 3.
| Characteristics of study | Associated factors | ||||||||
| Study ID | Tumour type | Dependent factor | Exercise‐induced symptoms | Surgical procedure following therapy | Menopausal symptoms | Gonadal failure | Growth hormone deficiency | Hepatitis C | Anaemia in past 12 months |
| Meeske 2005 | ALL | Moderate to severe fatigue | Sa (OR = 2.98) | S (OR = 2.43) | S (OR = 9.22) | S (OR = 6.45) | NS (OR = 2.26) | NS (OR = 0.94) | NS (OR = 2.16) |
Clinical and psychological variables associated with fatigue. Presented results are from univariable analyses, unless multivariable analyses were available. In that case, we present only the results of the multivariable analyses in this table. More detailed information about the effect estimates can be found in the Characteristics of included studies tables.
ALL: acute lymphoblastoma leukaemia; NS: non‐significant; S: significant; OR: odds ratio
aresults of multivariable analyses.
7. Clinical and psychological variables part 4.
| Characteristics of study | Associated factors | ||||||
| Study ID | Tumour type | Dependent factor | Hearing problems | Vision impairments | Digestive problems | Musculoskeletal/neurological problems | Psychiatric comorbidity |
| Meeske 2005 | ALL | Moderate to severe fatigue | NS (OR = 1.20) | NS (OR = 1.21) | ‐ | ‐ | ‐ |
| Puhr 2019 | CNS | Severe fatigue | ‐ | ‐ | ‐ | ‐ | NS (no further information) |
| Rueegg 2013 | Mix | Fatigued | Sa (OR = 2.85) | Sa (OR = 1.87) | Sa (OR = 3.15) | Sa (OR = 2.03) | ‐ |
Clinical and psychological variables associated with fatigue. Presented results are from univariable analyses, unless multivariable analyses were available. In that case, we present only the results of the multivariable analyses in this table. More detailed information about the effect estimates can be found in the Characteristics of included studies tables.
ALL: acute lymphoblastoma leukaemia; CNS: central nervous system tumour; Mix: covering a broad range of childhood cancer diagnoses; NS: non‐significant; S: significant; OR: odds ratio
aresults of multivariable analyses.
Multivariable analyses
Seven studies performed multivariable analyses including clinical and psychological variables (Hamre 2013a; Ho 2019; Langeveld 2003; Meeske 2005; Mulrooney 2008; Rueegg 2013; Zeller 2014a). As described above, the multivariable models of these seven studies differed considerably. Hamre 2013a investigated mental distress and thyroid dysfunction in relation to fatigue, with mental distress found to be significantly associated with fatigue. Ho 2019 performed separate analyses for survivors aged seven to 12 years and 13 to 18 years at time of fatigue assessment and found in both groups a significant effect for depression and physical activity. Langeveld 2003 found significant associations with fatigue for depression and the presence of late effects. Meeske 2005 identified exercise‐induced symptoms, neurocognitive impairment, sleep problems, pain and obesity all to be significantly associated with fatigue. Mulrooney 2008 found significant associations with fatigue for cardiac problems, lung fibrosis and depression. Non‐significant associations were found for thyroid dysfunction and BMI. Rueegg 2013 identified no significant association with fatigue for BMI, but significant associations with fatigue for hearing, memory, digestive, musculoskeletal/neurological, vision and thyroid problems. Zeller 2014a found an increased risk of severe fatigue in survivors with a higher level of depressive symptoms. Non‐significant effects were found for insomnia, pain, BMI and physical activity, expressed in the number of steps taken each day.
Discussion
Summary of main results
As the survival rates of childhood cancer are increasing, there are more childhood cancer survivors (CCS) who are at risk for developing severe fatigue as a late effect. The aim of this review was to estimate the prevalence of severe fatigue after successful treatment for childhood cancer. Secondary objectives were to describe the course of severe fatigue after completion of cancer treatment and to examine risk factors for fatigue, or factors associated with it. We include and evaluate 30 studies, of which 18 contribute to the main objective and 22 to the secondary objectives.
The prevalence of severe fatigue after treatment for childhood cancer ranges from 0% to 61.7%. There were substantial differences between studies in terms of cancer diagnosis, cancer treatment, age of participants, questionnaires used to assess fatigue and sample size. This resulted in clinical and statistical heterogeneity and prevented us from pooling the results.
Four studies reported the prevalence data in controls (range 3.1% to 10.3%), who were either siblings or population‐based controls. In all four studies, the prevalence of severe fatigue in the controls was lower compared to the prevalence in CCS, but only significantly different in two studies. These data suggest that, compared to the general population, severe fatigue might be more prevalent in CCS. However, limited information was available about the prevalence of severe fatigue in the general population and we can draw no firm conclusions from these data.
We formed clinically relevant subgroups based on cancer type and age at assessment. Prevalence of severe fatigue in survivors of haematological cancers ranged from 1.8% to 35.9%. Prevalence of severe fatigue in brain cancer survivors was presented in two studies, at 14.6% and 21.1% respectively (Brand 2016; Puhr 2019). One study presented a prevalence for bone cancer survivors of 0.0% (Lopez 2011). Studies including only children showed prevalence rates ranging from 6.7% to 12.5%, while studies predominantly in adults showed prevalence rates ranging from 4.4% to 61.7%. It was not possible to form subgroups based on cancer treatment, gender, age at cancer diagnosis or time since cancer diagnosis.
With the exception of genetic factors and tumour stage, all other predefined associated and risk factors for fatigue were reported in at least one study. Definitions of the factors under study were often at variance, and the results of the associated and risk factors were inconsistent. Despite these variations, several interesting observations could be made, although all are based on very weak evidence. Depression was consistently found to be associated with fatigue. On the other hand, age at diagnosis and education level did not appear to influence fatigue. We were unable to calculate an overall risk estimate for any of the reported risk and associated factors because we could not perform meta‐analyses. The magnitude of possible associations and risk factors therefore remains unknown in this review.
Only one study provided information on the course of severe fatigue over time (Zeller 2014a). They found that 32 of 102 participants (31.4%) reported persistent severe fatigue over a mean time interval of 2.7 years.
Overall completeness and applicability of evidence
None of the 30 included studies scored well on all items for external validity. Most of the studies (70%) did not describe cancer treatment in detail (e.g. chemotherapeutic agents and doses) and 26.6% of the studies did not mention all primary treatment modalities (i.e. surgery, chemotherapy and radiotherapy). A lack of information about cancer treatment might be problematic for interpreting and extrapolating the results, especially since treatment protocols for different types of cancer are very heterogeneous and treatment protocols have changed over the years.
The focus of this review was originally on severe fatigue that was established with a published cut‐off score or based on normative data. This was not available in all included studies, with the consequence that 40% of them did not address the main objective. However, in these studies fatigue was assessed with a variety of different methods, for example, fatigue reported on a continuous scale, fatigue defined as moderate to severe, or fatigue defined using other criteria. To be able to extract all potentially relevant information, we decided to extend the outcome for the secondary objective of risk and associated factors to fatigue, instead of limiting it to severe fatigue.
The variation between the 10 different fatigue measurements used by the included studies was a limitation. Questionnaires have different cut‐off points for severe fatigue, and several questionnaires assessed fatigue as a one‐dimensional approach, while others assessed fatigue multidimensionally. They also differ in the dimensions of fatigue that are assessed, for example cognitive, emotional and/or physical fatigue. This leads to variability in the assessment and definition of fatigue between studies and could have influenced the results.
In addition, in only one‐third of the included studies was fatigue the primary outcome of interest. Most studies focused on several other late effects or quality of life. In these studies fatigue was part of a comprehensive questionnaire, which contributed to the variation in assessment and definition of fatigue, and resulted, in general, in less extensive analyses with fatigue as an outcome.
Both the primary and secondary objectives of this review could be addressed, but with great variation between studies in several characteristics, making it impossible to combine results. Limited information was available about the course of severe fatigue and about risk and associated factors for fatigue. In addition, the prevalence rates of severe fatigue after treatment for childhood cancer were diverse and remain unknown.
In summary, external validity varied greatly between studies, and interpreting and extrapolating the results to all survivors of childhood cancer must only be done with caution.
Quality of the evidence
We noted risks of bias in all the included studies. Risk of selection bias was high in 86.7% of the studies. For 13.3% of the studies it was unclear whether the study population was representative of the original cohort of childhood cancer survivors. Selection bias could therefore not be ruled out in this review, and might result in an over‐ or underestimation of the prevalence of severe fatigue.
We found a low risk of attrition bias in this review. Twenty‐nine of 30 included studies assessed fatigue in at least 65% of the described study group, with 83.3% of the studies assessing fatigue in at least 95% of the study group.
In an ideal setting, the outcome of interest (in this case fatigue) is assessed by an independent person who is blinded to the outcome status of the participant, to limit detection bias. Fatigue is always assessed with a self‐reported questionnaire, so blinding of outcome assessors was not possible in the included studies. As an inevitable consequence, detection bias was present and limited all included studies equally.
Only one study incorporated all predefined possible confounding factors in the multivariable analysis. For 70% of the studies we could not rule out risk of confounding and the remaining studies did not perform risk factor analyses (26.7%). This could result in an over‐ or underestimation of the real effect of the risk and associated factors.
Overall the quality of the evidence of this review is very weak.
Potential biases in the review process
Potential bias in the methods is limited because we deployed a broad search strategy and studies were not excluded on the basis of language. However, we could not acquire all relevant data from the included studies. For some studies original data were no longer available or we could not contact corresponding authors. We can not rule out the possibility of selection bias based on the inclusion criterion of 'in complete remission', as not all eligible articles specifically stated that their study population was in complete remission at the time of the study. For six studies we interpreted this inclusion criterion based on the mean time since diagnosis, on the assumption that the chance of a recurrence after a mean time since diagnosis of at least five years is small (Barrera 2012; Daniel 2019; Mulrooney 2008; Reulen 2007; Rueegg 2013; Zeller 2014a). Time since diagnosis is an indicator for being in complete remission, but we can not completely exclude a recurrence or second malignancy in these studies. The lack of reporting on this issue in the literature is a limitation, and attention should be focused on a clear description of the study population in future publications.
Authors' conclusions
Implications for practice.
Due to a considerable variation in the included studies, this review provides limited evidence about the prevalence of severe fatigue after treatment for childhood cancer, and about risk and associated factors that might be involved in the development of fatigue. As a result, we can draw no robust or definitive conclusions for either objective of this review.
However, for professionals it is important to know that severe fatigue can be a late effect of treatment after childhood cancer, and screening for fatigue in follow‐up clinics might be valuable to gain more insight into fatigue within this population.
Implications for research.
This review provides a comprehensive overview of current knowledge of (severe) fatigue, and identifies the gaps in the literature about severe fatigue after treatment for childhood cancer.
For future research it would be valuable to describe a large well‐defined cohort including all childhood cancer diagnoses, to be more representative of the childhood cancer survivor population, including rarer diagnoses. Concerning the analyses, it is important to perform multivariable analysis in order to account for possible confounders, and to adequately assess risk and associated factors. Furthermore, longitudinal studies should be performed to assess the course of severe fatigue over time and to identify risk factors for severe fatigue.
There is no unambiguous construct to measure fatigue, and no clear consensus on which measurement should be used to assess fatigue after treatment for childhood cancer. It would therefore be valuable to investigate which fatigue measurements are most reliable, valid and easy to use. Consensus on the definition of severe fatigue, including different dimensions of fatigue, would contribute to a more coherent interpretation and assessment of severe fatigue after treatment for childhood cancer.
Acknowledgements
We would like to acknowledge the editorial base of Cochrane Childhood Cancer for their advice and support.
We would like to thank Prof. Maroeska Rovers for her contributions to the development of the protocol and her methodological advice during the execution of the review.
We would like to thank the study authors that kindly provided additional information about their study (Berbis 2013; Brand 2016; Calaminus 2014; Crom 1999; Daniel 2019; Hamre 2013a; Harila 2010; Kenney 2010; Puhr 2019; Reulen 2007; Ruccione 2013; Sundberg 2013; Tremolada 2018; Van Dijk 2008; Verberne 2012; Adams 2004; Berg 2009; Berg 2013; De Ruiter 2016; Korinthenberg 2011; Nies 2017; Nugent 2018; Robert 2012).
The authors are grateful to the following peer reviewer for their time and comments: Katrin Scheinemann MD, Division of Hematology/ Oncology, Department of Pediatrics, Kantonsspital Aarau, Switzerland; Division of Hematology/ Oncology, University Children’s Hospital Basel and University of Basel, Switzerland; Department of Pediatrics, McMaster Children’s Hospital and McMaster University Hamilton, Canada.
This review is supported by the Dutch Cancer Society (KUN 2014‐6985). In addition, the editorial base of Cochrane Childhood Cancer has been funded by KIKA and is located in the Prinsess Máxima Center for Pediatric Oncology, Utrecht, the Netherlands.
Appendices
Appendix 1. Search strategy for Cochrane Central Register of Controlled Trials (CENTRAL)
1. For fatigue the following text words were used:
fatigue or fatigu* or tired or tiredness or tired* or asthenia or astheni* or exhaustion or exhausted or exhaust* or loss of energy or energy loss or loss of vitality or (vital* and loss) or weary or weariness or weakness or apathy or apath* or lassitude or lethargy or letharg* or sleep or sleep deprivation or sleepiness or drowsy or drowsiness
2. For children the following text words were used:
infan* OR newborn* OR new‐born* OR perinat* OR neonat* OR baby OR baby* OR babies OR toddler* OR minors OR minors* OR boy OR boys OR boyfriend OR boyhood OR girl* OR kid OR kids OR child OR child* OR children* OR schoolchild* OR schoolchild OR school child OR school child* OR adolescen* OR juvenil* OR youth* OR teen* OR under*age* OR pubescen* OR pediatrics OR pediatric* OR paediatric* OR peadiatric* OR school OR school* OR prematur* OR preterm*
3. For childhood cancer the following text words were used:
leukemia OR leukemi* OR leukaemi* OR childhood ALL OR AML OR lymphoma OR lymphom* OR hodgkin OR hodgkin* OR T‐cell OR B‐cell OR non‐hodgkin OR sarcoma OR sarcom* OR Ewing* OR osteosarcoma OR osteosarcom* OR wilms tumor OR wilms* OR nephroblastom* OR neuroblastoma OR neuroblastom* OR rhabdomyosarcoma OR rhabdomyosarcom* OR teratoma OR teratom* OR hepatoma OR hepatom* OR hepatoblastoma OR hepatoblastom* OR PNET OR medulloblastoma OR medulloblastom* OR PNET* OR primitive neuroectodermal tumors OR retinoblastoma OR retinoblastom* OR meningioma OR meningiom* OR glioma OR gliom* OR pediatric oncology OR paediatric oncology OR childhood cancer OR childhood tumor OR childhood tumors OR brain tumor* OR brain tumour* OR brain neoplasms OR central nervous system neoplasm OR central nervous system neoplasms OR central nervous system tumor* OR central nervous system tumour* OR brain cancer* OR brain neoplasm* OR intracranial neoplasm*
4. For cancer the following text words were used:
cancer OR cancers OR cancer* OR oncology OR oncolog* OR neoplasm OR neoplasms OR neoplasm* OR carcinoma OR carcinom* OR tumor OR tumour OR tumor* OR tumour* OR tumors OR tumours OR malignan* OR malignant OR hematooncological OR hemato oncological OR hemato‐oncological OR hematologic neoplasms OR hematolo*
5. Forsurvivors the following text words were used:
Survivor OR survivors OR Long‐Term Survivors OR Long Term Survivors OR Long‐Term Survivor OR survivo* OR surviving
Final search
1 AND 2 AND (3 OR 4) AND 5
The search was performed in title, abstract or keywords.
[*= zero or more characters]
Appendix 2. Search strategy for MEDLINE/PubMed
1. For fatigue the following MeSH headings and text words were used:
fatigue[mh] OR fatigue OR fatigu* OR tired[tiab] OR tiredness[tiab] OR tired* OR asthenia[mh] OR asthenia OR astheni* OR exhaustion OR exhausted OR exhaust* OR loss of energy[tiab] OR energy loss[tiab] OR loss of vitality OR (vital* AND loss) OR weary[tiab] OR weariness[tiab] OR weakness OR apathy[mh] OR apath* OR lassitude[tiab] OR lethargy[mh] OR letharg* OR sleep OR sleep deprivation OR sleepiness[tiab] OR drowsy[tiab]OR drowsiness[tiab]
2. For children the following MeSH headings and text words were used (Leclercq 2013):
infan* OR newborn* OR new‐born* OR perinat* OR neonat* OR baby OR baby* OR babies OR toddler* OR minors OR minors* OR boy OR boys OR boyfriend OR boyhood OR girl* OR kid OR kids OR child OR child* OR children* OR schoolchild* OR schoolchild OR school child[tiab] OR school child*[tiab] OR adolescen* OR juvenil* OR youth* OR teen* OR under*age* OR pubescen* OR pediatrics[mh] OR pediatric* OR paediatric* OR peadiatric* OR school[tiab] OR school*[tiab] OR prematur* OR preterm*
3. For childhood cancer the following MeSH headings and text words were used:
leukemia OR leukemi* OR leukaemi* OR (childhood ALL) OR AML OR lymphoma OR lymphom* OR hodgkin OR hodgkin* OR T‐cell OR B‐cell OR non‐hodgkin OR sarcoma OR sarcom* OR sarcoma, Ewing's OR Ewing* OR osteosarcoma OR osteosarcom* OR wilms tumor OR wilms* OR nephroblastom* OR neuroblastoma OR neuroblastom* OR rhabdomyosarcoma OR rhabdomyosarcom* OR teratoma OR teratom* OR hepatoma OR hepatom* OR hepatoblastoma OR hepatoblastom* OR PNET OR medulloblastoma OR medulloblastom* OR PNET* OR neuroectodermal tumors, primitive OR retinoblastoma OR retinoblastom* OR meningioma OR meningiom* OR glioma OR gliom* OR pediatric oncology OR paediatric oncology OR childhood cancer OR childhood tumor OR childhood tumors OR brain tumor* OR brain tumour* OR brain neoplasms OR central nervous system neoplasm OR central nervous system neoplasms OR central nervous system tumor* OR central nervous system tumour* OR brain cancer* OR brain neoplasm* OR intracranial neoplasm* OR leukemia lymphocytic acute
4. For cancer the following MesH headings and text words were used:
cancer OR cancers OR cancer* OR oncology OR oncolog* OR neoplasm OR neoplasms OR neoplasm* OR carcinoma OR carcinom* OR tumor OR tumour OR tumor* OR tumour* OR tumors OR tumours OR malignan* OR malignant OR hematooncological OR hemato oncological OR hemato‐oncological OR hematologic neoplasms OR hematolo*
5. Forsurvivors the following MeSH headings and text words were used:
Survivor OR survivors OR Long‐Term Survivors OR Long Term Survivors OR Long‐Term Survivor OR Survivor, Long‐Term OR Survivors, Long‐Term OR survivo* OR surviving
Final search
1 AND 2 AND (3 OR 4) AND 5
[tiab = title, abstract; mh = MeSH term; *=zero or more characters]
Appendix 3. Search strategy for Embase/Ovid
1. For fatigue the following Emtree terms and text words were used:
1. fatigue/ or cancer fatigue/ or muscle fatigue/ 2. (fatigue or fatigu$ or tired or tiredness or tired$).mp. 3. exp asthenia/ or (asthenia or astheni$).mp. 4. exp exhaustion/ or (exhaustion or exhausted or exhaust$).mp. 5. (loss of energy or energy loss).mp. 6. ((loss adj2 vital$) or (loss adj2 energy)).mp. 7. exp weakness/ or (weakness or weary or weariness).mp. 8. exp apathy/ or (apathy or apath$).mp. 9. lassitude.mp. or exp lassitude/ 10. exp lethargy/ or (lethargy or letharg$).mp. 11. exp sleep/ or exp sleep deprivation/ or (sleep or sleep deprivation or sleepiness).mp. 12. exp drowsiness/ or (drowsy or drowsiness).mp. 13. or/1‐12
2. For children the following Emtree terms and text words were used:
1. infan$.mp. 2. (newborn$ or new‐born$).mp. 3. (perinat$ or neonat$).mp. 4. baby/ 5. (baby or baby$ or babies).mp. 6. toddler$.mp. 7. (minors or minors$).mp. 8. (boy or boys or boyfriend or boyhood).mp. 9. girl$.mp. 10. (kid or kids).mp. 11. child/ 12. (child or child$ or children$).mp. 13. school child/ 14. (schoolchild$ or schoolchild).mp. 15. (school child or school child$).ti,ab. 16. (adolescen$ or youth$ or teen$).mp. 17. (juvenil$ or under$age$).mp. 18. pubescen$.mp. 19. exp pediatrics/ 20. (pediatric$ or paediatric$ or peadiatric$).mp. 21. (school or school$).mp. 22. (prematur$ or preterm$).mp. 23. or/1‐22
3. For childhood cancer the following Emtree terms and text words were used:
1. (leukemia or leukemi$ or leukaemi$ or (childhood adj ALL) or acute lymphocytic leukemia).mp. 2. (AML or lymphoma or lymphom$ or hodgkin or hodgkin$ or T‐cell or B‐cell or non‐hodgkin).mp. 3. (sarcoma or sarcom$ or Ewing$ or osteosarcoma or osteosarcom$ or wilms tumor or wilms$).mp. 4. (nephroblastom$ or neuroblastoma or neuroblastom$ or rhabdomyosarcoma or rhabdomyosarcom$ or teratoma or teratom$ or hepatoma or hepatom$ or hepatoblastoma or hepatoblastom$).mp. 5. (PNET or medulloblastoma or medulloblastom$ or PNET$ or neuroectodermal tumors or primitive neuroectodermal tumor$ or retinoblastoma or retinoblastom$ or meningioma or meningiom$ or glioma or gliom$).mp. 6. (pediatric oncology or paediatric oncology).mp. 7. ((childhood adj cancer) or (childhood adj tumor) or (childhood adj tumors) or childhood malignancy or (childhood adj malignancies) or childhood neoplasm$).mp. 8. ((pediatric adj malignancy) or (pediatric adj malignancies) or (paediatric adj malignancy) or (paediatric adj malignancies)).mp. 9. ((brain adj tumor$) or (brain adj tumour$) or (brain adj neoplasms) or (brain adj cancer$) or brain neoplasm$).mp. 10. (central nervous system tumor$ or central nervous system neoplasm or central nervous system neoplasms or central nervous system tumour$).mp. 11. intracranial neoplasm$.mp. 12. LEUKEMIA/ or LYMPHOMA/ or brain tumor/ or central nervous system tumor/ or teratoma/ or sarcoma/ or osteosarcoma/ 13. nephroblastoma/ or neuroblastoma/ or rhabdomyosarcoma/ or hepatoblastoma/ or medulloblastoma/ or neuroectodermal tumor/ or retinoblastoma/ or meningioma/ or glioma/ or childhood cancer/ 14. or/1‐13
4. For cancer the following Emtree terms and text words were used:
1. (cancer or cancers or cancer$).mp. 2. (oncology or oncolog$).mp. or exp oncology/ 3. (neoplasm or neoplasms or neoplasm$).mp. or exp neoplasm/ 4. (carcinoma or carcinom$).mp. or exp carcinoma/ 5. (tumor or tumour or tumor$ or tumour$ or tumors or tumours).mp. or exp tumor/ 6. (malignan$ or malignant).mp. 7. (hematooncological or hemato oncological or hemato‐oncological or hematologic neoplasms or hematolo$).mp. or exp hematologic malignancy/
8. or/1‐7
5. Forsurvivors the following Emtree terms and text words were used:
1. (survivor or survivors or (long adj term survivor) or (long adj term survivors) or survivo$).mp. 2. survivor/ or cancer survivor/ 3. surviving.mp. 4. 1 or 2 or 3
Final search
1 AND 2 AND (3 OR 4) AND 5
[mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name; ti,ab = title, abstract; / = Emtree term; $=zero or more characters; adj=adjacent]
Appendix 4. Search strategies for conference proceedings
The pdf files of SIOP, ESLCCC, ASPHO and The International Conference on Long‐Term Complications of Treatment of Children and Adolescents for Cancer abstracts were searched for “fatigue”, "fatique", "tired", "tiredness", "exhaustion", "exhausted", "weakness", "asthenia" and "survivor". The ASCO abstracts were searched for “fatigue survivor” in the abstracts (http://meetinglibrary.asco.org/abstracts).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barrera 2012.
| Methods | Study design: cross‐sectional study Instrument used to assess fatigue: EORTC‐QLQ‐C30 symptom scale fatigue Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: NAa Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: survivors of lower extremity bone tumours, 16 years or older at the time of study, younger than 21 at the time of diagnosis, at least 5 years from diagnosis, had a limb salvage or amputation surgery Exclusion criteria: nm |
|
| Participants | Sample characteristics: N of participants original cohort: unknown; N of participants described study group: 28; N of participants study group of interest: 28; N of participants fatigue assessed: 28 Participant characteristics: Tumour type: bone tumour n = 28, i.e. osteogenic sarcoma n = 23, Ewings sarcoma n = 5 Tumour stage: nm Age at diagnosis: mean 11.6 years (SD 3.3; range 6 ‐ 16 years) Time since diagnosis and/or end of therapy: not reported, at least 5 years since diagnosis Age at assessment: mean 25.1 years (SD 4.5) F/M: 14/14 BMI: nm Race/ethnicity: white n = 20, mixed n = 2, Asian n = 1, East Indian n = 1; Hispanic n = 1, Native American Indian n = 1, not reported n = 2 Marital status: single n = 20, married/common law n = 7, not reported n = 1 Highest completed education level: grade school n = 2, high school n = 15, college diploma/university degree n = 9, post‐graduate/professional n = 2 Employment: nm Physical activity level: EORTC physical functioning subscale mean (SD): 85.71 (19.93) Sleeping problems: EORTC symptom scale insomnia mean (SD): 20.24 (29.17) Psychosocial problems: nm Comorbidities: nm Genetic factors/mutations: nm |
|
| Interventions | N of participants surgery: 28 N of participants chemotherapy: nm N of participants radiotherapy: nm Type of surgery: limb salvage n = 19, amputation n = 9 |
|
| Outcomes | Severe fatigue: N of participants with severe fatigue: NDa Risk and associated factors: Dependent factor: fatigue (continuous)b Univariable: Significant: type of surgery (limb salvage (mean 22.81) vs amputation (mean 9.88), P = 0.033), gender (female (mean 26.19) vs male (mean 11.11), P = 0.047) Non‐significant: age at assessment (age ≤ 25 years (mean 14.07) vs age ≥ 26 years (mean 23.93), P = 0.206) |
|
| Notes | Funding sources: Grant sponsor Canadian Institutes of Health Research Declaration of interest: Nothing to declare aAuthors report fatigue on a continuous scale. Additional information on severe fatigue was requested but not available. bAnalyses were performed with fatigue score on a continuous scale as outcome (T‐test). Effect estimates were not reported in the article. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | High risk | Size of original cohort is unclear; 70 eligible participants, 28 described study group, < 90% |
| Adequate follow‐up assessment (attrition bias) | Low risk | Outcome was assessed for > 95% of the study group of interest |
| Blinded outcome assessor (detection bias) All outcomes | High risk | Outcome assessors were not blinded to the investigated determinant |
| Adjustment important confounders | High risk | No multivariable analyses |
| Well‐defined study group (reporting bias) | High risk | Only surgery is mentioned as type of treatment, no information available about chemotherapy and radiotherapy. Inclusion and exclusion criteria are described |
| Well‐defined follow‐up (reporting bias) | Unclear risk | Length of follow‐up is not reported, but at least 5 years since diagnosis (based on inclusion criteria) |
| Well‐defined outcome fatigue (reporting bias) | Low risk | Authors reported which instrument they used to assess fatigue, and how they described fatigue (continuous scale) |
| Well‐defined risk estimation | Low risk | Mean fatigue scores of both groups are presented |
Berbis 2013.
| Methods | Study design: cross‐sectional study (questionnaire survey) Instrument used to assess fatigue: SF‐36 Vitality subscale Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: score ≤ 50 Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: diagnosis of de novo AML or de novo ALL since January 1980 (not excluding secondary leukaemias), < age 18 years at the time of diagnosis, complete remission 24 months after the diagnosis for AML participants and ALL participants grafted in first complete remission or complete remission at 48 months after the diagnosis for ALL participants not grafted in first complete remission; agreement to participate in the study with parents or legal guardians authorising participation for any child < age 18 years Exclusion criteria: nm |
|
| Participants | Sample characteristics: N of participants original cohort: 1115; N of participants described study group: 943; N of participants study group of interest: 943; N of participants fatigue assessed: 431a Participant characteristics: Tumour type: ALL n = 807, AML n = 136 Tumour stage: nm Age at diagnosis: mean 6.4 years (SD 4.2) Time since diagnosis: mean 11.9 years (SD 6.4) Age at assessment: mean 18.3 years (SD 7.1); < 8 years n = 55, 8 ‐ 10 years n = 102, 11 ‐ 17 years n = 294, > 18 years n = 492 F/M: 423/520 BMI: overweight (BMI ≥ 25) n = 346 Race/ethnicity: nm Marital status: nm Highest completed education level: nm Employment: nm Physical activity level: nm Sleeping problems: nm Psychosocial problems: nm Comorbidities: at least 1 late event n = 674, height growth failure n = 375, gonadal dysfunction n = 129/706, hypothyroidism n = 75, second tumour n = 46, bone mineral deficiency n = 11/163, alopecia n = 32, cardiac side effect n = 24, cataract n = 115, severe neurological dysfunctions n = 22, diabetes n = 6, iron overload n = 91, osteonecrosis n = 24, viral transmission n = 18, metabolic syndrome n = 26/298 Genetic factors/mutations: nm |
|
| Interventions | N of participants surgeryb: 0 N of participants only chemotherapyb: 203 N of participants radiotherapy and chemotherapyb: 93 N of participants chemotherapy and SCT with or without radiotherapyb: 135 N of participants cranial irradiation: 148 N of participants testicular irradiation: 21 N of participants testicular irradiation boost: 24 N of participants total body irradiation: 186 N of participants SCT: 256 |
|
| Outcomes | Severe fatigue: N of participants with severe fatigue: 128/431 (29.70%) Risk and associated factors: Dependent factor: no analysis performed with fatigue as outcome |
|
| Notes | Funding sources: Supported by the French national cancer institute (InCa) and the regional council PACA Declaration of interest: Nothing to declare The following data were obtained from the study author: N of participants with severe fatigue a the SF‐36 questionnaire was only administered to the adult survivors (age at assessment > 18 years) b numbers are based on the survivors for whom fatigue could be assessed (n = 431) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | High risk | The described study group consisted of < 90% of the original cohort |
| Adequate follow‐up assessment (attrition bias) | High risk | Outcome was assessed for < 65% of the study group of interest |
| Blinded outcome assessor (detection bias) All outcomes | High risk | Outcome assessors were not blinded to the investigated determinant |
| Well‐defined study group (reporting bias) | Unclear risk | Type of cancer and cancer treatment are mentioned but information about specific agents and doses are not reported. Inclusion and exclusion criteria are described |
| Well‐defined follow‐up (reporting bias) | Low risk | Length of follow‐up is mentioned |
| Well‐defined outcome severe fatigue (reporting bias) All outcomes | Low risk | The authors reported which instrument they used to assess fatigue; definition of severe fatigue is based on data query |
Brand 2016.
| Methods | Study design: cross‐sectional study (part of longitudinal cohort study REACH) Instrument used to assess fatigue: PedsQL Multidimensional Fatigue Scale Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: normative mean total score for USA population: 80.49 (SD 13.33); cut‐off score for severe fatigue: total fatigue score < 53.83 Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: recruited from project REACH (≥ 2 years from cancer diagnosis, ≥ 1 year from completion of cancer therapy, English‐speaking), returned for a subsequent survivorship visit at long‐term follow‐up clinic, brain tumour survivor Exclusion criteria: ill at the time of their clinic visit, severe cognitive limitations (documented IQ < 70), sensory limitation that would interfere with their ability to complete self‐reported measures independently (i.e. blindness), missing or incomplete assessments |
|
| Participants | Sample characteristics:
N of participants original cohort: unknown; N of participants described study group: 142; N of participants study group of interest: 142; N of participants fatigue assessed: 142 Participant characteristics: Tumour type: brain tumour n = 142 Tumour stage: nm Age at diagnosis: mean 9.72 years (SD 4.87, range 4 months ‐ 22 years)a Time since diagnosis: mean 10.55 years (SD 5.57, range 2 ‐ 27) Age at assessment: mean 20.24 years (SD 4.81, range 12 ‐ 32) F/M: 76/66 BMI: nm Race/ethnicity: White n = 128, African‐American n = 4, Hispanic n = 2, other or unknown n = 8 Marital status: married or living as married n = 4, single/never married n = 79, unknown n = 59 Highest completed education level: < high school graduate n = 4, high school graduate n = 54, college graduate n = 22, graduate degree n = 3, unknown n = 59 Employment: paid full‐time work n = 15, paid part‐time work n = 29, student n = 105, receiving social benefit n = 5 Physical activity level: nm Sleeping problems: nm Psychosocial problems: nm Comorbidities: Thyroid disorder (self‐reported) n = 26 Genetic factors/mutations: nm |
|
| Interventions | N of participants surgery: 133 N of participants chemotherapy: 60 N of participants radiotherapy: 79 N of participants SCT: 4 | |
| Outcomes | Severe fatigue: N of participants with severe fatigue: 30/142 (21.1%) Risk and associated factors: No analysis performed with fatigue as outcome |
|
| Notes | Funding sources: nm Declaration of interest: The authors declare that they have no conflict of interest. The following data were obtained from the study author: N of participants with severe fatigue, cancer treatment, marital status, highest completed education level, employment and comorbidities. For employment, participants could select more than 1status. aInformation received from study author: 135 of the 142 participants (95.1%) were under 18 at diagnosis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | High risk | Size of original cohort is unclear, > 245 eligible participants, 142 described study group, < 90% |
| Adequate follow‐up assessment (attrition bias) | Low risk | Outcome was assessed for > 95% of the study group of interest |
| Blinded outcome assessor (detection bias) All outcomes | High risk | Outcome assessors were not blinded to the investigated determinant |
| Well‐defined study group (reporting bias) | Unclear risk | Type of cancer and cancer treatment are mentioned but information about specific agents and doses are not reported Inclusion and exclusion criteria are described |
| Well‐defined follow‐up (reporting bias) | Low risk | Length of follow‐up is mentioned |
| Well‐defined outcome severe fatigue (reporting bias) All outcomes | Low risk | The authors reported which instrument they used to assess fatigue; definition of severe fatigue is based on data query |
Calaminus 2014.
| Methods | Study design: cross‐sectional study Instrument used to assess fatigue: EORTC QLQ‐C30 symptom scale fatigue Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: a score ≥ 70 Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: treated for Hodgkins disease, enrolled in 1 of the following trials: German–Austrian consecutive multicentre trials DAL‐HD‐78, HD‐82, HD‐85, HD‐87, HD‐90 and the European trial GPOH‐HD‐95 Exclusion criteria: patients reporting at least 1 event related to the treatment of Hodgkins disease (including progress, relapse and secondary malignant neoplasia), < 21 years at assessment, > 41 years at assessment, incomplete information for evaluation |
|
| Participants | Sample characteristics: N of participants original cohort: unknown; N of participants described study group: 725; N of participants study group of interest: 725; N of participants fatigue assessed: 725 Participant characteristics: Tumour type: Hodgkins disease n = 725 Tumour stage: nm Age at diagnosis: mean 13.63 years (SD 3.09)a Time since diagnosis: mean 15.26 years (SD 5.89) Age at assessment: mean 28.44 years (SD 5.21) F/M: 392/333 BMI: nm Race/ethnicity: nm Marital status: married n = 311, single n = 393, divorced n = 16, data not given n = 5 Highest completed education level: low (intermediate school completion) n = 129, medium (secondary school and vocational school completion) n = 209, high (post‐secondary and/or university) n = 378, none/other n = 3, data not given n = 6 Employment: employed n = 392, unemployed n = 70, student (school) n = 2, college/vocational/university student n = 259, data not given n = 2 Physical activity level: EORTC physical functioning mean scores for males and females separately: males 94.61 (SD 11.3). females 92.69 (SD 11.1) Sleeping problems: EORTC symptom scale insomnia mean scores for males and females separately: males 15.32 (SD 25.6). females 22.96 (SD 31.4) Psychosocial problems: nm Comorbidities: nm Genetic factors/mutations: nm |
|
| Interventions | N of participants chemotherapy: 697 N of participants radiotherapy: 695 Maximum dose radiotherapy: ≤ 20 Gy n = 167, > 20 Gy ‐ ≤ 30 Gy n = 299, > 30 Gy n = 229 N of participants surgery: nm |
|
| Outcomes | Severe fatigue: N of participants with severe fatigue: 32/725 (4.41%) Risk and associated factors: Dependent factor: fatigue (continuous)b Univariable: Non‐significant: age at diagnosis, time since diagnosis |
|
| Notes | Funding sources: Funded by a project grant of the German Childhood Cancer Trust (Deutsche Kinderkrebsstiftung), grant number: DKS 2005/04. Declaration of interest: nm The following data were obtained from the study author: N of participants with severe fatigue aInformation received from study author: all participants were < 18 at diagnosis. bAnalyses were performed with fatigue score on a continuous scale as outcome (univariable regression analyses). Effect estimates and P values were not reported in the article |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | High risk | Size of original cohort is unclear, 1819 eligible participants, 725 described study group, < 90% |
| Adequate follow‐up assessment (attrition bias) | Low risk | Outcome was assessed for > 95% of the study group of interest |
| Blinded outcome assessor (detection bias) All outcomes | High risk | Outcome assessors were not blinded to the investigated determinant |
| Adjustment important confounders | High risk | No multivariable analyses |
| Well‐defined study group (reporting bias) | High risk | Type of cancer and cancer treatment are mentioned but information about surgery, specific chemotherapeutic agents and doses are not available. Inclusion and exclusion criteria are described |
| Well‐defined follow‐up (reporting bias) | Low risk | Length of follow‐up is mentioned |
| Well‐defined outcome severe fatigue (reporting bias) All outcomes | Low risk | The authors reported which instrument they used to assess fatigue; definition of severe fatigue is based on data query |
| Well‐defined outcome fatigue (reporting bias) | Low risk | Authors reported which instrument they used to assess fatigue, and how they described fatigue (continuous scale) |
| Well‐defined risk estimation | High risk | None were calculated |
Cheung 2017.
| Methods | Study design: cross‐sectional study
Instrument used to assess fatigue: PedsQL Multidimensional Fatigue Scale Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: NAa Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: active survivors who were receiving paediatric follow‐up care, at least 5 years from diagnosis, over 8 years of age, alive Exclusion criteria: previous treatment with cranial irradiation for CNS relapse or bone marrow transplantation or additional chemotherapy for a secondary cancer, pre‐existing non‐cancer‐related neurodevelopmental or genetic disorder associated with cognitive impairment or brain injury unrelated to cancer, lack of proficiency in English, not eligible for follow‐up |
|
| Participants | Sample characteristics: N of participants original cohort: 408; N of participants described study group: 70; N of participants study group of interest: 70; N of participants fatigue assessed: 70 Participant characteristics: Tumour type: ALL n = 70 Tumour stage: nm Age at diagnosis: female mean 6.8 years (SD 4.5; range 1.9 ‐ 17.7); male mean 7.0 years (SD 4.8; range 1.2 ‐ 16.5) Time since diagnosis: female mean 7.1 years (SD 1.7; range 5.1 ‐ 11.6); male mean 7.8 years (SD 2.0; range 5.1 ‐ 12.5) Age at assessment: female mean 13.9 years (SD 4.3; range 8.1 ‐ 25.4); male mean 14.8 years ( SD 5.1; range 8.5 ‐ 25.5) F/M: 35/35 BMI: female mean 22.2 kg/m2 (SD 5.0; range 14.1 ‐ 36.7); male mean 23.1 kg/m2 (SD 8.3; range 15.4 ‐ 46.4) Race: White n = 56, Black n = 12, other n = 2 Ethnicity: Hispanic n = 6, non‐Hispanic n = 64 Marital status: nm Highest completed education level: education in years, female mean 7.4 (SD 3.6; range 2 ‐ 14); male mean 7.6 (SD 3.9; range 2 ‐ 14) Employment: nm Physical activity level: nm Sleeping problems: sleep duration in hours, female mean 9.05 (SD 1.7); male mean 8.96 (SD 1.1) Psychosocial problems: nm Comorbidities: nm Genetic factors/mutations: nm |
|
| Interventions | N of participants chemotherapy: 70 N of participants radiotherapy: 0 N of participants surgery: 0 Chemotherapeutic agents, cumulative dose: Oral dexamethasone, mg/m2: female mean 1061.0 (SD 204.1; range 444.2 ‐ 1534.8); male mean 1148.0 (SD 326.2; range 412.3 ‐ 1690.1) IV Erwinia‐asparaginase, 1000 U/m2: female mean 375.2 (SD 227.8; range 146.0 ‐ 741.2); male mean 402.0 (SD 284.6; range 178.9 ‐ 871.9) IV L‐asparaginase, 1000 U/m2: female mean 215.5 (SD 147.0; range 85.9 ‐ 584.5); male mean 314.7 (SD 198.5; range 44.4 ‐ 539.9) IV Peg‐asparaginase, 1000 U/m2: female mean 7.8 (SD 3.8; range 2.5 ‐ 12.9); male mean 17.1 (SD 15.5; range 2.5 ‐ 44.7) IV cytarabine Standard dose, g/m2: female mean 1.4 (SD 1.6; range 0.3 ‐ 4.8); male mean 2.6 (SD 1.8; range 0.6 ‐ 4.9) IV cytarabine High dose, g/m2: female mean 7.4 (SD 1.6; range 3.9 ‐ 8.1); male mean 8.6 (SD 2.7; range 7.7 ‐ 19.6) IV cyclophosphamide, g/m2: female mean 1.9 (SD 1.6; range 1.0 ‐ 4.9); male mean 2.9 (SD 1.8; range 0.9 ‐ 6.2) IV daunorubicin, mg/m2: female mean 49.0 (SD 10.5; range 25.0 ‐ 91.9); male mean 48.8 (SD 9.1; range 24.6 ‐ 71.0) IV doxorubicin, mg/m2: female mean 90.4 (SD 51.2; range 58.9 ‐ 209.0); male mean 127.0 (SD58.6; range 58.4 ‐ 191.5) IV leucovorin, mg/m2: female mean 337.8 (SD 155.0; range 200.0 ‐ 655.0); male mean 379.1 (SD 269.6; range 75.0 ‐ 1645.0) IV methotrexate standard dose, g/m2: female mean 3.3 (SD 0.8; range 0.2 ‐ 5.1); male mean 5.2 (SD 4.4; range 2.7 ‐ 29.4) IV methotrexate high ‐ dose ‐ low ‐ risk arm, g/m2: female n=26, mean 11.6 (SD 1.8; range 0.9 ‐ 16.4); male n=15, mean 11.5 (SD 3.2; range 2.5 ‐ 17.1) IV methotrexate high ‐ dose ‐ standard ‐ risk arm, g/m2: female n=9, mean 20.8 (SD 4.8; range 15.6 ‐ 29.3); male n=20, mean 19.5 (SD 4.4; range 7.4 ‐ 26.3) IT methotrexate, mL: female mean 148.7 (SD 34.1; range 93.0 ‐ 276.0); male mean 180.1 (SD 52.5; range 60.0 ‐ 288.0) IV vincristine, mg/m2: female mean 59.1 (SD 11.4; range 31.1 ‐ 73.3); male mean 58.1 (SD 13.3; range 28.7 ‐ 74.6) IT chemotherapy, no of counts: female mean 13.1 (SD 3.1; range 9 ‐ 23); male mean 15.6 (SD 4.3; range 11 ‐ 24) |
|
| Outcomes | Severe fatigue: N of participants with severe fatigue: NDa Risk and associated factors: Dependent factor: fatigue (continuous)b Univariable: Non‐significant: gender (P = 0.19) |
|
| Notes | Funding sources: Supported by the National Institute of Mental Health (grant MH085849 to K.R.K.), the National Cancer Institute (grant CA195547 to M.M.H and L.L.R), and the American Lebanese Syrian Associated Charities. Support to St Jude Children’s Research Hospital was also provided by the Cancer Center Support (CORE) grant (CA21765, CharlesW. M. Roberts, Principal Investigator) Declaration of interest: Angela Panoskaltsis‐Mortari reports a family member employed by BioTechne, which owns the company from which some of the kits used for biomarker analysis were purchased (R&D Systems) aAuthors report fatigue on a continuous scale. Additional information on severe fatigue was requested and not available. bAnalysis was performed with the subscale score for general fatigue as outcome (T‐test). Effect estimate was not reported in the article. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | High risk | The described study group consisted of < 90% of the original cohort of cancer survivors |
| Adequate follow‐up assessment (attrition bias) | Low risk | Outcome was assessed for > 95% of study group of interest |
| Blinded outcome assessor (detection bias) All outcomes | High risk | Outcome assessors were not blinded to the investigated determinant |
| Adjustment important confounders | High risk | No multivariable analyses |
| Well‐defined study group (reporting bias) | Low risk | Type of cancer and cancer treatment are mentioned, including specific chemotherapeutic agents and cumulative dose. Inclusion and exclusion criteria are described. |
| Well‐defined follow‐up (reporting bias) | Low risk | Length of follow‐up is mentioned |
| Well‐defined outcome fatigue (reporting bias) | Low risk | Authors reported which instrument they used to assess fatigue, and how they described fatigue (continuous scale) |
| Well‐defined risk estimation | High risk | None were calculated |
Crom 1999.
| Methods | Study design: cross‐sectional study Instrument used to assess fatigue: EORTC QLQ‐C30 symptom scale fatigue Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: NAa Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: at least 18 years of age at assessment, survived a solid tumour for at least 15 years Exclusion criteria: occurrence of second malignancies, presence of mental retardation, lost to follow‐up |
|
| Participants | Sample characteristics: N of participants original cohort: unknown; N of participants described study group: 220; N of participants study group of interest: 220; N of participants fatigue assessed: 220 Participant characteristics: Tumour type: HL n = 67, WT n = 30, neuroblastoma n = 26, rhabdomyosarcoma n = 17, retinoblastoma n = 20, osteosarcoma n = 16, Ewings sarcoma n = 11, germ cell tumour n = 9, brain tumour n = 5, nasopharyngeal carcinoma n = 7, melanoma n = 2, other n=10 Tumour stage: nm Age at diagnosis: mean 8.91 years (SD 6.55)b Time since diagnosis: mean 22.48 years (SD 3.85) Age at assessment: mean 31.40 years (SD 7.39) F/M: 104/116 BMI: nm Race/ethnicity: white n = 182, African‐American n = 35, Hispanic n = 3 Marital status: single n = 84, living as married n = 15, married n = 99, divorced n = 22 Highest completed education level: nm Employment: nm Physical activity level: nm Sleeping problems: nm Psychosocial problems: nm Comorbidities: number of comorbid conditions: 0 n = 158, 1 n = 51, 2 n = 11 Genetic factors/mutations: nm |
|
| Interventions | N of participants surgery: nm N of participants chemotherapy: nm N of participants radiotherapy: nm The authors graded therapy as low intensity (n = 17) and high intensity therapy (n = 203) |
|
| Outcomes | Severe fatigue: N of participants with severe fatigue: NDa Risk and associated factors: Dependent factor: fatigue (continuous)c Univariable: Significant: diagnosis (HL with mantle radiation) |
|
| Notes | Funding sources: Grant from the National Cancer Institute and American Lebanese Syrian associated charities Declaration of interest: nm aInformation provided by the study author: additional data are no longer available bThe study author confirmed that at least 90% of the participants were under 18 at diagnosis cAnalysis was performed with fatigue score on a continuous scale as outcome. Effect estimates and P values were not reported in the article. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | Unclear risk | Study group was a random sample with respect to treatment intensity, but it was unclear which specific treatments were received by the study participants |
| Adequate follow‐up assessment (attrition bias) | Low risk | Outcome was assessed for > 95% of the study group of interest |
| Blinded outcome assessor (detection bias) All outcomes | High risk | Outcome assessors were not blinded to the investigated determinant |
| Adjustment important confounders | High risk | No multivariable analyses |
| Well‐defined study group (reporting bias) | High risk | Specific type of cancer treatment was not mentioned; authors classified treatment as low and high intensity. Inclusion and exclusion criteria are described |
| Well‐defined follow‐up (reporting bias) | Low risk | Length of follow‐up is mentioned |
| Well‐defined outcome fatigue (reporting bias) | Low risk | Authors reported which instrument they used to assess fatigue, and how they described fatigue (continuous scale) |
| Well‐defined risk estimation | High risk | None were calculated |
Daniel 2019.
| Methods | Study design: cross‐sectional study Instrument used to assess fatigue: FACIT ‐ Fatigue Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: score ≤ 30 Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: diagnosed before the age of 21 years, survived at least 5 years following diagnosis, over 18 years of age at time of the sleep survey Exclusion criteria: nm |
|
| Participants | Sample characteristics: N of participants original cohort: 14,355; N of participants described study group: 1933; N of participants study group of interest: 1933; N of participants fatigue assessed: 1821 Participant characteristics: Tumour type: leukaemia n = 302, CNS malignancy n = 303, HL n = 1018, soft tissue sarcoma n = 151, bone cancer n = 159 Tumour stage: nm Age at diagnosis: mean 11.6 years (SD 5.7)a Time since diagnosis: not reported, at least 5 years Age at assessment: mean 35.1 years (SD 7.6) F/M: 981/952 BMI: normal/underweight n = 871, overweight n = 597, obese n = 398b Race/ethnicity: white n = 1717, black n = 64, Hispanic n = 86, Asian n = 17, American India/Alaska native n = 16, other n = 27b Marital status: nm Highest completed education level: nm Employment: nm Physical activity level: nm Sleeping problems: Daytime sleepiness (ESS score ≥ 10): n = 358 Psychosocial problems: nm Comorbidities: nm Genetic factors/mutations: nm |
|
| Interventions | N of participants surgery: nm N of participants chemotherapy: 1140 N of participants alkylating agents: 897 N of participants anthracyclines: 481 N of participants platinum derivatives: 68 N of participants alkylating agent cyclophosphamide‐equivalent: 875 N of participants radiotherapy: 1371 N of participants cranial radiation: < 20 Gy n = 165, ≥ 20 Gy n = 306 N of participants neck radiation: < 30 Gy n = 239, ≥ 30 Gy n = 695 N of participants chest radiation: < 30 Gy n = 211, ≥ 30 Gy n = 682 N of participants abdominal radiation: < 30 Gy n = 172, ≥ 30 Gy n = 474 |
|
| Outcomes | Severe fatigue: N of participants with severe fatigue: 256/1821 (14.1%) Risk and associated factors: no analysis performed with fatigue as outcome |
|
| Notes | Funding sources: National Cancer Institute (CA55727, G.T. Armstrong, Principal Investigator) Declaration of interest: nothing to declare The following data were obtained from the study author: N of participants with severe fatigue aBased on information of the total CCSS cohort, it was possible to estimate the percentage of participants under 18 at diagnosis of the 5 included diagnostic groups (92.2%) bNumbers do not add up to total N of described study group. Unclear if this is due to missing values. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | High risk | The described study group consisted of < 90% of the original cohort |
| Adequate follow‐up assessment (attrition bias) | Low risk | Outcome was assessed for 65% ‐ 95% of the study group of interest |
| Blinded outcome assessor (detection bias) All outcomes | High risk | Outcome assessors were not blinded to the investigated determinant |
| Well‐defined study group (reporting bias) | Unclear risk | Type of cancer and cancer treatment are mentioned but information about doses of specific chemotherapeutic agents is not reported. Inclusion and exclusion criteria are described |
| Well‐defined follow‐up (reporting bias) | Unclear risk | Length of follow‐up is not reported, but at least 5 years since diagnosis (based on inclusion criteria) |
| Well‐defined outcome severe fatigue (reporting bias) All outcomes | Low risk | The authors reported which instrument they used to assess fatigue; definition of severe fatigue is based on data query |
Gordijn 2013.
| Methods | Study design: cross‐sectional study
Instrument used to assess fatigue: PedsQL Multidimensional Fatigue scale
Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: NAa Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: aged 5 ‐ 17 years, ALL treated according to ALL 9 or ALL 10 protocol Exclusion criteria: being under treatment for relapsed ALL, deficient Dutch language skills |
|
| Participants | Sample characteristics: N of participants original cohort: unknown; N of participants described study group: 62; N of participants study group of interest: 62; N of participants fatigue assessed: 62, 35 with child form Participant characteristics: Tumour type: ALL n = 62 Tumour stage: nm Age at diagnosis: nm, based on age at assessment, the maximum age at diagnosis is 17 years Time since end of therapy: median 36 months (IQR 22 ‐ 62 months) Age at assessment: mean 9.7 years (SD 3.2; range 5 ‐ 17) F/M: 31/31 BMI: nm Race/ethnicity: nm Marital status: NA Highest completed education level: nm Employment: NA Physical activity level: Physical functioning subscale of CHQ: mean 96.1 (SD 6.41) Sleeping problems: Total score of CSHQ child form: mean 31.70 (SD 4.32); CSHQ parent form: mean 44.10 (SD 7.99). Total score of ASHQ child form: mean 39.40 (SD 11.15); ASHQ parent form: mean 33.36 (SD13.16) Psychosocial problems: Total score of CDI: female mean 4.94 (SD 4.28), male mean 5.22 (SD 3.69) Comorbidities: nm Genetic factors/mutations: nm |
|
| Interventions | N of participants chemotherapy: 62 N of participants radiotherapy: 0 N of participants surgery: 0 |
|
| Outcomes | Severe fatigue: N of participants with severe fatigue: NDa Risk and associated factors: Dependent factor: fatigue (continuous)b Univariable: Significant: sleeping difficulties (CSHQ, parent form r = −0.60, P < 0.01; ASHQ, parent form r = −0.74, P < 0.01), symptoms of depression (CDI; r = −0.45, P < 0.01) Non‐significant: sleeping difficulties (CSHQ, child form r = −0.44, P > 0.05; ASHQ, child form r = −0.47, P > 0.05) |
|
| Notes | Funding sources: Dutch Cancer Society; Grant number: VU 2010‐4859. Declaration of interest: nothing to declare a Authors report fatigue on continuous scale. Additional information on severe fatigue was requested and not available. bAnalyses were performed with fatigue score on a continuous scale as outcome (Pearson correlation). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | High risk | Size of original cohort is unclear, 146 eligible participants, 62 described study group, < 90% |
| Adequate follow‐up assessment (attrition bias) | Low risk | Outcome was assessed for > 95% of the study group of interest |
| Blinded outcome assessor (detection bias) All outcomes | High risk | Outcome assessors were not blinded to the investigated determinant |
| Adjustment important confounders | High risk | Important prognostic factors and follow‐up were not taken into account |
| Well‐defined study group (reporting bias) | Unclear risk | Type of cancer and cancer treatment are mentioned but information about specific chemotherapeutic agents and doses are not reported. Inclusion and exclusion criteria are described. |
| Well‐defined follow‐up (reporting bias) | Low risk | Length of follow‐up is mentioned |
| Well‐defined outcome fatigue (reporting bias) | Low risk | Authors reported which instrument they used to assess fatigue, and how they described fatigue (continuous scale) |
| Well‐defined risk estimation | Low risk | Correlation coefficients are calculated |
Hamre 2013a.
| Methods | Study design: cross‐sectional study Instrument used to assess severe fatigue: Fatigue Questionnaire Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: sum score ≥ 4 for all 11 dichotomised items and duration of symptoms for ≥ 6 months Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: treatment for HL as first cancer at university hospitals in Norway or treatment at Oslo University Hospital for ALL or NHL, diagnosed between 1970 and 2000 (1970 and 2002 for ALL), age at diagnosis ≤ 18 years (≤ 16 years for ALL), survival for ≥ 5 years, age at survey > 18 years, alive at June 2007 (April 2009 for ALL)a Exclusion criteria: second cancer or pregnancy (except for comparison to control group), incomplete questionnaires |
|
| Participants | Sample characteristics: N of participants original cohort: unknown; N of participants described study group: 290; N of participants study group of interest: 290; N of participants fatigue assessed: 290 Participants characteristics: Tumour type: ALL n = 151, HL n = 92, NHL n = 47 Tumour stage: nm Age at diagnosis: median 9.5 years (range 0.3 ‐ 18.4) Time since diagnosis: median 21.1 years (range 6.9 ‐ 39.4) Age at assessment: median 29.6 years (range 18.3 ‐ 54.5) F/M: 139/140 BMI: > 30 kg/m2 n = 31 Race/ethnicity: nm Marital status: in a partnership: n = 145 Highest completed education: education ≥ 12 years: n = 175 Employment: nm Physical activity level: nm Sleeping problems: nm Psychosocial problems: mental distress: HADS total score mean 8.4 (SD 6.2) Comorbidities: impaired heart function n = 73, reduced lung function n = 70, present hypothyroidism n = 49 Genetic factors/mutations: nm |
|
| Interventions | N of participants chemotherapy: 179 N of participants radiotherapy: 13 N of participants chemotherapy + radiotherapy: 87 N of participants surgery: 0 N of participants cranial irradiation: 19; dose median range: 20 Gy (12 ‐ 54 Gy) N of participants radiotherapy mediastinum: 69; dose median range: 36 Gy (18 ‐ 44 Gy) N of participants other radiotherapy:12; dose median range: 40 Gy (13 ‐ 41 Gy) N of participants anthracyclines: 199; dose median range: 150 mg/m2 (40 ‐ 510 mg/m2) |
|
| Outcomes | Severe fatigue: N of participants with severe fatigue: 79/290 (27.2%); ALL 34/151 (22.5%), NHL 14/47 (29.8%), HL 32/92 (34.8%) Risk and associated factors: Dependent factor: severe fatigue (yes/no)b Univariable: Significant: age at survey (OR 1.04 (95% CI 1.00 to 1.07), P = 0.01), tumour type (ALL = ref, NHL OR 1.4 (95% CI 0.6 to 2.9), P = 0.4, HL OR 1.8 (95% CI 1.0 to 3.3), P = 0.05), mental distress (OR 1.1 (95% CI 1.1 to 1.2), P < 0.001)) Non‐significant: BMI ≥ 30 kg/m2 (OR 1.8 (95% CI 0.8 to 4.0), P = 0.1), education (≤ 11 years, OR 1.6 (95% CI 0.8 to 2.7), P = 0.1), marital status (not in a partnership, OR 0.7 (95% CI 0.4 to 1.3), P = 0.3), present hypothyroidism (OR 1.8 (95% CI 0.9 to 3.4), P = 0.09), gender (female, OR 0.9 (95% CI 0.5 to 1.5), P= 0.7) Multivariable: Significant: mental distress (OR 1.15 (95% CI 1.1 to 1.2), P < 0.001) Non‐significant: age at survey (OR 1.05 (95% CI 1.00 to 1.1), P = 0.1), present hypothyroidism (OR 1.4 (95% CI 0.7 to 3.0), P= 0.4), gender (female, OR 0.8 (95% CI 0.46 to 1.5), P = 0.6), tumour type (ALL = ref, NHL OR 1.5 (95% CI 0.6 to 3.4), P = 0.4, HL OR 1.7 (95% CI 0.8 to 3.5), P = 0.2) |
|
| Notes | Funding sources: Supported by “Helse Sorost HF” Declaration of interest: No competing financial interest exist Numbers of gender, BMI and interventions is based on n = 279 aStudy author confirmed that the study population had no evidence of disease at time of the study. bAnalysis for associated factors is based on n = 279, participants who were pregnant (n = 6) or had a second cancer (n = 5) were excluded from risk factor analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | High risk | Size of original cohort unclear, > 430 eligible participants, 290 described study group, < 90% |
| Adequate follow‐up assessment (attrition bias) | Low risk | Outcome was assessed for > 95% of the study group of interest |
| Blinded outcome assessor (detection bias) All outcomes | High risk | Outcome assessors were not blinded to the investigated determinant |
| Adjustment important confounders | High risk | Follow‐up was not taken into account |
| Well‐defined study group (reporting bias) | Unclear risk | Type of cancer and cancer treatment are mentioned, specific chemotherapeutic agents and doses is not reported (only for anthracyclines). Inclusion and exclusion criteria are described. |
| Well‐defined follow‐up (reporting bias) | Low risk | Length of follow‐up is mentioned |
| Well‐defined outcome severe fatigue (reporting bias) All outcomes | Low risk | The authors reported which instrument they used to assess fatigue and what they considered to be severe fatigue |
| Well‐defined outcome fatigue (reporting bias) | Low risk | Authors reported which instrument they used to assess fatigue, and how they described fatigue (severe fatigue) |
| Well‐defined risk estimation | Low risk | Prevalence rates for subgroup diagnosis are provided and odds ratio are calculated for all factors |
Harila 2010.
| Methods | Study design: cross‐sectional study (questionnaire survey) Instrument used to assess fatigue: SF‐36 Vitality subscale Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: a score ≤ 50 Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: at least 18 years old within 6 months of the invitation, diagnosed with ALL at least 10 years earlier, no evidence of leukaemia at the time of the evaluation, living in Finland Exclusion criteria: Down syndrome, severe mental retardation, severe brain injury caused by an accident, deficient Finnish language skills |
|
| Participants | Sample characteristics: N of participants original cohort: unknown; N of participants described study group: 74; N of participants study group of interest: 74; N of participants fatigue assessed: 74 Participant characteristics: Tumour type: ALL n = 74 Tumour stage: nm Age at diagnosis: mean 5 years (range 0 ‐ 15) Time since diagnosis: mean 20 years (range 10 ‐ 32) Age at assessment: mean 24 years (range 17 ‐ 37) F/M: 48/26 BMI: nm Race/ethnicity: white n = 74 Marital status: married/cohabiting n = 17, unmarried n = 57 Highest completed education level: no vocational education n = 27, vocational school n = 34, polytechnical university n = 10, university n = 3 Employment: employed n = 36, student n = 14, unemployed n = 4, not student or unemployed n = 20 Physical activity level: SF‐36 physical functioning subscale mean 94 (SD 11) Sleeping problems: nm Psychosocial problems: nm Comorbidities: according to CTCAEv3: no late effects n = 19, low‐grade effect n = 21, high grade effect n = 23a Genetic factors/mutations: nm |
|
| Interventions | N of participants chemotherapy: 28 N of participants chemotherapy + cranial irradiation: 46 Cranial irradiation doses: 18 ‐ 23 Gy n = 16, 24 ‐ 25 Gy n = 23, 30 ‐ 48 Gy n = 7 N of participants BMT: 1 N of participants surgery: 0 |
|
| Outcomes | Severe fatigue: N of participants with severe fatigue: 6/74 (8.1%) Risk and associated factors: Dependent factor: fatigue (continuous)b Univariable: Non‐significant: radiotherapy (irradiated (mean 77) versus non‐irradiated (mean 73)) |
|
| Notes | Funding sources: Grant sponsor: Nona and Kullervo VäreFoundation, Finland. Grant sponsor: Foundation for Pediatric Research, Finland
Grant sponsor: Finnish Cancer Society and Cancer Society of Northern Finland Declaration of interest: nothing to declare The following data were obtained from the study author: N of participants with severe fatigue, and ethnicity aComorbidities could only be assessed for 63 survivors who agreed to participate in an additional 2‐day examination visit at the hospital bAnalysis was performed with fatigue score on a continuous scale as outcome(T‐test). P value was not reported in the article |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | High risk | Size of original cohort is unclear, 87 eligible participants, 74 described study group, < 90% |
| Adequate follow‐up assessment (attrition bias) | Low risk | Outcome was assessed for > 95% of the study group of interest |
| Blinded outcome assessor (detection bias) All outcomes | High risk | Outcome assessors were not blinded to the investigated determinant |
| Adjustment important confounders | High risk | No multivariable analyses |
| Well‐defined study group (reporting bias) | Unclear risk | Cancer type and cancer treatment are mentioned but information about specific chemotherapeutic agents are not reported. Inclusion and exclusion criteria are described |
| Well‐defined follow‐up (reporting bias) | Low risk | Length of follow‐up is mentioned |
| Well‐defined outcome severe fatigue (reporting bias) All outcomes | Low risk | The authors reported which instrument they used to assess fatigue; definition of severe fatigue is based on data query |
| Well‐defined outcome fatigue (reporting bias) | Low risk | Authors reported which instrument they used to assess fatigue, and how they described fatigue (continuous scale) |
| Well‐defined risk estimation | Low risk | Mean fatigue scores are provided for both subgroups |
Ho 2019.
| Methods | Study design: cross‐sectional study Instrument used to assess fatigue: Fatigue scale for children and Fatigue scale for adolescents, Chinese versions Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: NAa Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: cancer survivors who had completed treatment at least 6 months, aged between 7 and 18 years, able to speak Cantonese and read Chinese Exclusion criteria: evidence of recurrence or second malignancies, cognitive and learning problems as identified from their medical records |
|
| Participants | Sample characteristics: N of participants original cohort: unknown; N of participants described study group: 400; N of participants study group of interest: 400; N of participants fatigue assessed: 400 Participant characteristics: Tumour type: leukaemia n = 158, lymphoma n = 103, brain tumour n = 68, osteosarcoma n = 35, kidney tumour n = 19, germ‐cell tumour n = 17 Tumour stage: nm Age at diagnosis: nm, based on age at assessment, the maximum age at diagnosis is 18 years Time since end of therapy: 6 ‐ 12 months n = 87, 13 ‐ 24 months n = 69, 25 ‐ 36 months n = 67, 37 ‐ 48 months n = 69, 49 ‐ 60 months n = 53, > 60 months n = 55 Age at assessment: 7 ‐ 12 years n = 200, mean 9.4 (SD 1.7); 13 ‐ 18 years n = 200, mean 15.9 (SD 1.6) F/M: 187/213 BMI: nm Race/ethnicity: nm Marital status: nm Highest completed education level: nm Physical activity level: CUHK‐PARCY mean score 4.34 (SD 2.7) Sleeping problems: nm Psychosocial problems: depression: CES‐DC mean score 37.9 (SD 8.8) Comorbidities: nm Genetic factors/mutations: nm |
|
| Interventions | N of participants chemotherapy: 179 N of participants surgery: 39 N of participants surgery + radiotherapy: 24 N of participants surgery + chemotherapy: 40 N of participants chemotherapy + radiotherapy: 27 N of participants BMT: 38 N of participants BMT + chemotherapy: 53 |
|
| Outcomes | Severe fatigue: N of participants with severe fatigue: NDa Risk and associated factors: Dependent factor: fatigue (continuous)b Survivors aged 7 ‐ 12 years Univariable: Significant: treatment received (b = −0.22, SE = 0.74, P = 0.00), time since treatment completion (b = −0.19, SE = 1.28, P = 0.01), number of depressive symptoms (b = 0.52, SE = 0.07, P = 0.00), physical activity levels (b = −0.68, SE = 0.19, P = 0.00) Non‐significant: age (b = 0.01, SE = 0.35, P = 0.95), gender (b = 0.03, SE = 2.53, P = 0.67), diagnosis (b = 0.08, SE = 0.29, P = 0.32) Multivariable: Significant: time since treatment completion (b = −0.14, SE = 1.19, P = 0.04), number of depressive symptoms (b = 0.21, SE = 0.09, P = 0.01), physical activity levels (b = −0.56, SE = 0.21, P = 0.00) Non‐significant: age (b = −0.09, SE = 0.27, P = 0.18), gender (b = −0.03, SE = 1.95, P = 0.64), diagnosis (b = −0.01, SE = 0.24, P = 0.87), treatment received (b = −0.11, SE = 0.53, P = 0.10) Survivors aged 13 ‐ 18 years Univariable: Significant: time since treatment completion (b = −0.20, SE = 1.12, P = 0.01), number of depressive symptoms (b = 0.56, SE = 0.09, P = 0.00), physical activity levels (b = −0.62, SE = 0.19, P = 0.00) Non‐significant: age (b = 0.03, SE = 0.54, P = 0.87), gender (b = 0.15, SE = 1.30, P = 0.08), diagnosis (b = −0.11, SE = 0.27, P = 0.22), treatment received (b = 0.14, SE = 0.31, P = 0.05) Multivariable: Significant: number of depressive symptoms (b = 0.23, SE = 0.08, P = 0.02), physical activity levels (b = −0.51, SE = 0.20, P = 0.00) Non‐significant: age (b = 0.01, SE = 0.44, P = 0.88), gender (b = 0.06, SE = 1.01, P = 0.42), diagnosis (b = −0.05, SE = 0.22, P = 0.46), treatment received (b = 0.08, SE = 0.10, P = 0.27), time since treatment completion (b = −0.13, SE = 1.01, P = 0.05) |
|
| Notes | Funding sources: no significant financial support for this work that could have influenced its outcome Declaration of interest: authors confirm that there are no known conflicts of interest associated with this publication aThe original cut‐off score for severe fatigue of the FS‐C and FS‐A was not used and has not been validated yet for the Chinese versions. bAnalysis was performed with fatigue score on a continuous scale as outcome (linear regression analysis) and separately for survivors aged 7 ‐ 12 years and survivors aged 13 ‐ 18 years. Age, number of depressive symptoms and physical activity levels were operationalised as continuous variable. Gender, diagnosis, treatment and time since treatment completed were operationalised as categorical variables. Diagnosis included leukaemia, lymphoma, brain tumour, osteosarcoma, kidney tumour and germ‐cell tumour. Treatment received included surgery, chemotherapy, bone marrow transplant and mixed methods; time since treatment completed included 6 – 12 months, 13 – 24 months, 25 – 36 months, 37 – 48 months, 49 – 60 months,> 60 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | High risk | Size of original cohort is unclear, 472 eligible participants, 400 described study group, < 90% |
| Adequate follow‐up assessment (attrition bias) | Low risk | Outcome was assessed for > 95% of the study group of interest |
| Blinded outcome assessor (detection bias) All outcomes | High risk | Outcome assessors were not blinded to the investigated determinant |
| Adjustment important confounders | High risk | Multivariable analyses, but comorbidities were not taken into account |
| Well‐defined study group (reporting bias) | Unclear risk | Type of cancer and cancer treatment are mentioned but information about specific chemotherapeutic agents, radiotherapy fields and doses are not reported. Inclusion and exclusion criteria are described |
| Well‐defined follow‐up (reporting bias) | Low risk | Length of follow‐up is mentioned |
| Well‐defined outcome fatigue (reporting bias) | Low risk | Authors reported which instrument they used to assess fatigue, and how they described fatigue (continuous scale) |
| Well‐defined risk estimation | Low risk | Regression coefficients of linear regression are provided |
Johannsdottir 2012.
| Methods | Study design: cross‐sectional study Instrument used to assess fatigue: Fatigue Questionnaire Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: sum score of ≥ 4 on the Fatigue Questionnaire and symptom duration of ≥ 6 months Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: a histologically‐verified diagnosis of AML, IA, or WT, treatment according to established protocols in the period from 1985 to 2001, age ≥ 1 year at time of diagnosis, age ≥ 13 years at time of assessment, complete remission at time of assessment, no secondary malignancy, no cancer treatment during the previous 3 years Exclusion criteria: Down syndrome or mental retardation |
|
| Participants | Sample characteristics: N of participants original cohort: unknown; N of participants described study group: 398 ; N of participants study group of interest: 398; N of participants fatigue assessed: 395 Participant characteristics: Tumour type: AML n = 90, WT n = 183, IA n = 125 Tumour stage: nm Age at diagnosis: range 1 ‐ 18 years, YG mean 5.5 years (SD 2.9), OG mean 8.0 years (SD 4.1) Time since diagnosis: YG mean 10 years (SD 3.2), OG mean 16 years (SD 3.7) Age at assessment: YG mean 16 years (SD 1.7), OG mean 24 (SD3.3) F/M:YG 73/78, OG 136/111 BMI: nm Race/ethnicity: nm Marital status: nm Highest completed education level: nm Physical activity level: nm Sleeping problems: nm Psychosocial problems: nm Comorbidities: nm Genetic factors/mutations: nm |
|
| Interventions | N of participants chemotherapy: 34 N of participants surgery: 94 N of participants surgery + radiotherapy: 20 N of participants surgery + chemotherapy: 104 N of participants surgery, chemotherapy and radiotherapy:73 N of participants BMT/SCT: 56 N of participants treatment unknown: 11 N of participants treatment not stated: 6 |
|
| Outcomes | Severe fatigue: N of participants with severe fatigue: 43/395 (10.9%), YG 10/149 (6.7%), OG 33/246 (13.4%) Risk and associated factorsa: Dependent factor: severe fatigue (yes/no) Univariable: Signifcant: diagnosis (ref = controls, AML OR 1.63 (95% CI 0.62 to 4.30), IA OR 2.56 (95% CI 1.30 to 5.06) P < 0.01, WT OR 2.98 (95% CI 1.61 to 5.50) P < 0.01) Non‐significant: age at assessment (OR 1.03 (95% CI 0.97 to 1.08)), gender (OR 1.58 (95% CI 0.97 to 2.55)), academic education (OR 0.69 (95% CI 0.40 to 1.20)), married/cohabiting (OR 1.32 (95% CI 0.83 to 2.11)), gainfully employed (OR 1.10 (95% CI 0.67 to 1.80)), treatment modalities, time since diagnosis Multivariable: Significant: age at assessment (OR 1.08, (95% CI 1.01 to 1.16) P < 0.05) Non‐significant: gender (female; OR 1.54 (95% CI 0.94 to 2.54), academic education (OR 0.63 (95% CI 0.36 to 1.12)), married/cohabiting (OR 1.09 (95% CI 0.64 to 1.85)), gainfully employed (OR 1.18 (95% CI 0.67 to 2.07)) |
|
| Notes | Funding sources: The study was supported by a grant from the Norwegian Cancer Society and the Nordic Cancer Union without any involvement in the conduction of study or writing of this article Declaration of interest: nm Results are presented separately for young survivors (YG; aged 13 ‐ 18 years, n = 151) and older survivors (OG; aged ≥ 19 years, n = 247) aResults are presented of the analysis with the fatigued OG (n = 33) and control group (n = 44). Effect estimates for treatment modalities and time since diagnosis were not reported. P values were not reported for non‐significant risk and associated factors in the article |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | High risk | Size of original cohort is unclear, 584 eligible participants, 398 described study group, < 90% |
| Adequate follow‐up assessment (attrition bias) | Low risk | Outcome was assessed for > 95% of the study group of interest |
| Blinded outcome assessor (detection bias) All outcomes | High risk | Outcome assessors were not blinded to the investigated determinant |
| Adjustment important confounders | High risk | Follow‐up was not taken into account |
| Well‐defined study group (reporting bias) | Unclear risk | Type of cancer and cancer treatment are mentioned but information about specific chemotherapeutic agents, radiotherapy fields and doses are not reported. Inclusion and exclusion criteria are described |
| Well‐defined follow‐up (reporting bias) | Low risk | Length of follow‐up was mentioned |
| Well‐defined outcome severe fatigue (reporting bias) All outcomes | Low risk | The authors reported which instrument they used to assess fatigue and what they considered to be severe fatigue |
| Well‐defined outcome fatigue (reporting bias) | Low risk | Authors reported which instrument they used to assess fatigue, and how they described fatigue (severe fatigue) |
| Well‐defined risk estimation | Unclear risk | Prevalence rates are provided for subgroups based on age at assessment and odds ratio are calculated, but not for all factors |
Kenney 2010.
| Methods | Study design: cross‐sectional study (questionnaire survey as part of cohort study) Instrument used to assess fatigue: Functional Assessment of Chronic Illness Therapy ‐ Fatigue Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: score < 30 Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: part of the original cohort of CCSS, have survived to age 50 years, speak English, be a US resident, have a confirmed malignant diagnosisa Exclusion criteria: nm |
|
| Participants | Sample characteristics: N of participants original cohort: 1100; N of participants described study group: 55; N of participants study group of interest: 55; N of participants fatigue assessed: 50 Participant characteristics: Tumour type: sarcoma n = 18, NHL n = 10, WT n = 10, HL n = 6, neuroblastoma n = 5, other (ALL,CNS, etc) n = 6 Tumour stage: nm Age at diagnosis: median 8.0 years (range < 1 ‐ 17) Time since diagnosis: median 48 years (range 36 ‐ 65) Age at assessment: median 56 years (range 51 ‐ 71) F/M: 32/23 BMI: nm Race/ethnicity: nm Marital status: married/widowed/living as married n = 40, divorced/separated n = 9, unknown n = 6 Highest completed education level: not a college graduate n = 25, college graduate n = 27, unknown n = 3 Employment: nm Physical activity level: nm Sleeping problems: nm Psychosocial problems: Psychological distress: BSI mean score 34.80 (SD 2.83) Comorbidities: Hypertension n = 26, liver disease n = 6, diabetes n = 5, thyroid disease n = 16, valvular heart disease n = 3, coronary artery disease n = 9, osteoporosis n = 14, renal disease n = 4, arrhythmia n = 5, congestive heart failure n = 2, cerebral vascular disease n = 2, obesity n = 14, mental illness n = 14, lung disease n = 5 Genetic factors/mutations:nm |
|
| Interventions | N of participants chemotherapy: 14 N of participants radiotherapy: 15 N of participants surgery: 4 N of participants chemotherapy + radiotherapy: 22 |
|
| Outcomes | Severe fatigue: N of participants with severe fatigue: 8/50 (16.0%) Risk and associated factors: no analysis performed with fatigue as outcome |
|
| Notes | Funding sources: Supported by the David B. Perini, Jr Quality of Life Clinic at the Dana‐Farber Cancer Institute and the Carl J. Herzog Foundation Declaration of interest: The authors made no disclosures aStudy author confirmed that the study population was in remission at time of enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | High risk | The described study group consisted of < 90% of the original cohort of cancer survivors |
| Adequate follow‐up assessment (attrition bias) | Low risk | Outcome was assessed for 65% ‐ 95% of the study group of interest |
| Blinded outcome assessor (detection bias) All outcomes | High risk | Outcome assessors were not blinded to the investigated determinant |
| Well‐defined study group (reporting bias) | Unclear risk | Type of cancer and cancer treatment are mentioned but information about specific chemotherapeutic agents, radiotherapy fields and doses are not reported. Inclusion and exclusion criteria are described |
| Well‐defined follow‐up (reporting bias) | Low risk | Length of follow‐up is mentioned |
| Well‐defined outcome severe fatigue (reporting bias) All outcomes | Low risk | The authors reported which instrument they used to assess fatigue and what they considered to be severe fatigue |
Khan 2014.
| Methods | Study design: cross‐sectional study Instrument used to assess fatigue: Brief Fatigue Inventory Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: mean score > 7a Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: treatment on institutional protocol, at least 5 years from the time of ALL diagnosis, at least 1 year from completion of all cancer therapy, English as a primary language, absence of a pre‐existing cognitive disorder preventing study evaluation Exclusion criteria: nm |
|
| Participants | Sample characteristics: N of participants original cohort: unknown; N of participants described study group: 162; N of participants study group of interest: 162; N of participants fatigue assessed: 162 Participant characteristics: Tumour type: ALL n = 162 Tumour stage: nm Age at diagnosis: median 3.9 years (range 0.4 ‐ 18.6) Time since diagnosis and/or end of therapy: median 10.2 years (range 5 ‐ 22.7) and median 7.4 years (range 1.9 ‐ 20.3) Age at assessment: median 15.7 years (range 6.9 ‐ 29.0) F/M: 72/90 BMI: nm Race/ethnicity: White n = 146 Marital status: nm Highest completed education level: nm Employment: nm Physical activity level: nm Sleeping problems: nm Psychosocial problems: nm Comorbidities: pre‐hypertension n = 15, definite hypertension n = 6, seizures n = 17, back pain n = 37 Genetic factors/mutations: nm |
|
| Interventions | N of participants chemotherapy: 162 N of participants cranial irradiation: 23 N of participants surgery: 0 N of participants SCT: 0 Received chemotherapeutic agents: triple intrathecal therapy with cytarabine, methotrexate and hydrocortisone: n = 162 |
|
| Outcomes | Severe fatigue: N of participants with severe fatigue: 3/162 (1.9%) Risk and associated factors: Dependent factor: mild to severe fatigue (yes/no)b Multivariable: Significant: relapse (OR 8.35 (95% CI 1.16 to 59.93), P = 0.03) |
|
| Notes | Funding sources: Grant CA21765 from the National cancer institute and by the Lebanese Syrian associated charities Declaration of interest: nothing to declare aNot every survivor completed the BFI, only if the screening question was positive for fatigue/energy loss bAnalysis was performed with mild to severe fatigue as outcome (mean score ≥ 1). It is unclear which other variables were included in the multivariable model |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | High risk | Size of original cohort is unclear, 432 eligible participants, 162 described study group, < 90% |
| Adequate follow‐up assessment (attrition bias) | Low risk | Outcome was assessed for > 95% of the study group of interest |
| Blinded outcome assessor (detection bias) All outcomes | High risk | Outcome assessors were not blinded to the investigated determinant |
| Adjustment important confounders | Unclear risk | Unclear which other variables were included in multivariable model |
| Well‐defined study group (reporting bias) | Unclear risk | Type of cancer and cancer treatment are mentioned but information about doses is not reported. Inclusion and exclusion criteria are described |
| Well‐defined follow‐up (reporting bias) | Low risk | Length of follow‐up is mentioned |
| Well‐defined outcome severe fatigue (reporting bias) All outcomes | Low risk | The authors reported which instrument they used to assess fatigue and what they considered to be severe fatigue |
| Well‐defined outcome fatigue (reporting bias) | Low risk | Authors reported which instrument they used to assess fatigue, and how they described fatigue (mild to severe fatigue) |
| Well‐defined risk estimation | Unclear risk | Odds ratio is provided for significant factor. Unclear which other variables were included in multivariable model. |
Langeveld 2003.
| Methods | Study design: cross‐sectional study Instrument used to assess fatigue: Multidimensional Fatigue Inventory Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: NAa Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: aged 16 or older, pathological confirmation of malignancy, cancer had to have been diagnosed before the participants were 19 years of age Exclusion criteria: schizophrenic, developmentally delayed, ineligible because of a current health problem causing emotional upset |
|
| Participants | Sample characteristics: N of participants original cohort: unknown; N of participants described study group: 416; N of participants study group of interest: 416; N of participants fatigue assessed: 416 Participant characteristics: Tumour type: Leukaemia/non‐Hodgkins lymphoma without CRT n = 116; Leukaemia/non‐Hodgkins lymphoma with CRT n = 87, solid tumour n =183, brain/CNS tumour n = 30 Tumour stage: nm Age at diagnosis: mean 8 years (SD 4.7; range 0 ‐ 18) Time since end of therapy: mean 15 years (SD 5.9; range 5 ‐ 33) Age at assessment: mean 24 years (SD 5.2; range 16 ‐ 49) F/M: 200/216 BMI: nm Race/ethnicity: nm Marital status: single n = 300, living together/married n = 116 Highest completed education level: lower level (less than high school) n = 278, higher level (high school or advanced degree) n = 138 Employment: employed n = unknown, student/homemaker n = unknown, unemployed n = 42 Physical activity level: nm Sleeping problems: nm Psychosocial problems: nm Comorbidities: medical limitations (graded with an adapted version of the Scale for Medical Limitations): none/mild n = 68, moderate n = 189, severe n = 159 Genetic factors/mutations: nm |
|
| Interventions | N of participants chemotherapy (with or without surgery): 197 N of participants radiotherapy (with or without surgery): 29 N of participants radiotherapy + chemotherapy (with or without surgery): 190 |
|
| Outcomes | Severe fatigue: N of participants with severe fatigue: NDa Risk and associated factors: Dependent factor: fatigue (continuous)b Multivariable: Significant: gender (female, b = 0.19, P < 0.001), employment status (ref = unemployed; student/homemaker, b = −0.12; employed, b = −0.20, P < 0.05), late effects/health problems (b = 0.14, P < 0.05), depression (b = 0.54, P < 0.001), CRT (b = −0.16, P < 0.05) Non‐significant: age at follow‐up (b = 0.01), marital status (married, b = 0.04), educational level (higher level, b = 0.03), age at diagnosis (b = 0.06), treatment (ref = CT, RT b = 0.02, RT+CT b = 0.04), years since completion of therapy (b = 0.02), diagnosis (ref = leukaemia/NHL without CRT, solid tumour b = 0.02, CNS tumour b = −0.08) |
|
| Notes | Funding sources: Supported by a grant from the Dutch Cancer Society Declaration of interest: nm aAuthors report fatigue on continuous scale. Additional information on severe fatigue was requested and not available. bAnalyses were performed with fatigue score on a continuous scale as outcome (regression analyses). P values were not reported for non‐significant risk and associated factors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | High risk | Size of original cohort is unclear, 459 eligible participants, 416 described study group, < 90% |
| Adequate follow‐up assessment (attrition bias) | Low risk | Outcome was assessed for > 95% of the study group of interest |
| Blinded outcome assessor (detection bias) All outcomes | High risk | Outcome assessors were not blinded to the investigated determinant |
| Adjustment important confounders | Low risk | Important prognostic factors and follow‐up were taken into account |
| Well‐defined study group (reporting bias) | Unclear risk | Type of cancer and cancer treatment are mentioned but information about specific chemotherapeutic agents, radiotherapy fields and doses are not reported. Inclusion and exclusion criteria are described |
| Well‐defined follow‐up (reporting bias) | Low risk | Length of follow‐up is mentioned |
| Well‐defined outcome fatigue (reporting bias) | Low risk | Authors reported which instrument they used to assess fatigue, and how they described fatigue (continuous scale) |
| Well‐defined risk estimation | Low risk | Regression coefficients of linear regression are provided |
Lopez 2011.
| Methods | Study design: cross‐sectional study Instrument used to assess fatigue: EORTC‐QLQ‐C30 symptom scale fatigue Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: score ≥ 70 Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: diagnosis of Ewing's sarcoma of the bone, < 17 years at diagnosis, long‐term survivors (≥ 5 years past diagnosis), treated between 1990 to 2004 Exclusion criteria: nm |
|
| Participants | Sample characteristics: N of participants original cohort: 21; N of participants described study group: 17; N of participants study group of interest: 17; N of participants fatigue assessed: 17 Participant characteristics: Tumour type: Ewings sarcoma n = 17 Tumour stage: stage II n = 15, stage IV n = 2 Age at diagnosis: median 10 years (range 2 ‐ 14) Time since end of therapy: median 9 years (range 6 ‐ 19) Age at assessment: median 19 years (range 11 ‐ 27) F/M: 8/9 BMI: nm Race/ethnicity: nm Marital status: nm Highest completed education level: nm Employment: nm Physical activity level: EORTC physical functioning subscale mean 93.3 (SD 21.2) Sleeping problems: EORTC symptom scale insomnia mean 7.84 (SD 25.08) Psychosocial problems: nm Comorbidities/late effects: Musculoskeletal deformity n = 12, joint range of motion decreased n = 9, growth suppression n = 5, generalised muscle weakness n = 7, localised oedema n = 1, fracture n = 1 Genetic factors/mutations: nm |
|
| Interventions | N of participants surgery + chemotherapy: 8 N of participants chemotherapy + radiotherapy: 5 N of participants surgery, chemotherapy and radiotherapy: 4 N of participants BMT/SCT: 7 |
|
| Outcomes | Severe fatigue: N of participants with severe fatigue: 0/17 (0.0%) Risk and associated factors: no analysis performed with fatigue as outcome |
|
| Notes | Funding sources: nm Declaration of interest: nm The following data were obtained from the study author: N of participants with severe fatigue |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | High risk | The described study group consisted of < 90% of the original cohort of cancer survivors |
| Adequate follow‐up assessment (attrition bias) | Low risk | Outcome was assessed for > 95% of the study group of interest |
| Blinded outcome assessor (detection bias) All outcomes | High risk | Outcome assessors were not blinded to the investigated determinant |
| Well‐defined study group (reporting bias) | Unclear risk | Type of cancer and cancer treatment are mentioned but information about specific chemotherapeutic agents, radiotherapy fields and doses are not reported. Inclusion and exclusion criteria are described |
| Well‐defined follow‐up (reporting bias) | Low risk | Length of follow‐up is mentioned |
| Well‐defined outcome severe fatigue (reporting bias) All outcomes | Low risk | The authors reported which instrument they used to assess fatigue and definition of severe fatigue is based on data query |
Meeske 2005.
| Methods | Study design: cross‐sectional study Instrument used to assess fatigue: Revised‐Piper fatigue scale Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: total score ≥ 7 Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: diagnosed with ALL at Childrens Hospital Los Angeles before age 18, between 01 January 1975 and 31 December 1995, disease‐free, off treatment for a minimum of 1 year, English‐speaking, at least 18 years old at time of the study Exclusion criteria: severe developmental delay or mental retardation |
|
| Participants | Sample characteristics: N of participants original cohort: unknown; N of participants described study group: 161; N of participants study group of interest: 161; N of participants fatigue assessed: 161 Participant characteristics: Tumour type: ALL n = 161 Tumour stage: nm Age at diagnosis: mean 7.4 (range 0 ‐ 18) Time since end of therapy: mean 13.9 years (range 4 ‐ 23) Age at assessment: 18 ‐ 19 years n = 34; 20 ‐ 24 years n = 57; 25 ‐ 29 years n = 39; 30 ‐ 41 years n = 31 F/M: 87/74 BMI: obesity n = 46 Race/ethnicity: White n = 79, Hispanic n = 63, other n = 19 Marital status: married n = 32 Highest completed education level: ≤ high school graduate n = 43, some college n = 80, college graduate n = 38 Employment: work full‐time n = 56, work part‐time n = 13, student n = 34, student and working n = 40, not student or employed n = 18 Physical activity level: nm Sleeping problems: sleep problems (self‐reported) n = 78 Psychosocial problems: depressed (CES‐D score ≥ 16) n = 50 Comorbidities/late effects: hearing loss n = 15, vision problems not corrected with glasses n = 21, neurocognitive impairment n = 62, chronic headaches or migraines n = 49, seizures n = 13, hepatitis C n = 7, anaemia in the past 12 months n = 13, cardiac problem n = 3 exercise‐induced symptoms n = 42, thyroid abnormality n = 13, pain n = 49, second malignancy n = 5, surgical procedures following therapy n = 63 growth hormone deficiency n = 24, menopausal symptoms n = 10, gonadal failure n = 7 (all self‐reported) Genetic factors/mutations: nm |
|
| Interventions | N of participants chemotherapy: 46 N of participants chemotherapy + cranial irradiation: 103 N of participants BMT: 12 N of participants surgery: 0 Received chemotherapeutic agents: anthracyclines n = 104 (dose: 75 ‐ 349 mg/m2 n = 80, ≥ 350 mg/m2 n = 24) Cranial irradiation dose: 18 Gy n = 84, ≥ 24 Gy n = 19 |
|
| Outcomes | Severe fatigue: N of participants with severe fatigue: 10/161 (6.2%) Risk and associated factors: Dependent factor: moderate to severe fatigue (yes/no)a Univariable: Significant: gender (female, OR 2.11 (95% CI 1.04 to 4.27)), employment (ref = work full‐time, work part‐time OR 1.88 (95% CI 0.53 to 6.68), student OR 1.25 (95% CI 0.48 to 3.25), student and working OR 0.64 (95% CI 0.23 to 1.76), unemployed OR 6.00 (95% CI 1.90 to 18.98)), ethnicity (ref = white, Hispanic OR 2.56 (95% CI 1.23 to 5.34), other OR 1.30 (95% CI 0.41 to 4.13)), relapse (OR 2.68 (95% CI 1.04‐6 to 96)), depression (OR 32.9 (95% CI 12.8 to 80.1)), sleep problems (OR 7.71 (95% CI 3.39 to 17.5)), obesity/BMI (OR 3.14 (95% CI 1.52 to 6.48)), thyroid status (OR 4.32 (95% CI 1.33 to 14.0)), neurocognitive impairment (OR 3.25 (95% CI 1.61 to 6.56)), chronic headaches or migraine (OR 5.32 (95% CI 2.55 to 11.1)), seizures (OR 4.32 (95% CI 1.33 to 14.0)), exercise‐induced symptoms (OR 3.41 (95% CI 1.62 to 7.15)), pain (OR 5.32 (95% CI 2.47 to 11.1)), surgical procedure following therapy (OR 2.43 (95% CI 1.22 to 4.84)), menopausal symptoms (OR 9.22 (95% CI 1.82 to 46.8)), gonadal failure (OR 6.45 (95% CI 1.21 to 34.5)), number of late effects (OR 1.73 (95% CI 1.43 to 2.09)) Non‐significant: age at assessment (ref = 18 ‐ 19 yrs, 20 ‐ 24 yrs OR 0.74 (95% CI 0.27 to 2.00), 25 ‐ 29 yrs OR 1.93 (95% CI 0.72 to 5.22), 30 ‐ 41 yrs OR 1.53 (95% CI 0.53 to 4.41)), education (ref ≤ high school grad, some college OR 0.84 (95% CI 0.38 to 1.86), college graduate OR 0.84 (95% CI 0.33 to 2.18)), marital status (married OR 0.60 (95% CI 0.24 to 1.50)), cranial irradiation (ref = 0 Gy, 18 Gy OR 1.93 (95% CI 0.89 to 4.22), ≥ 24 Gy OR 2.14 (95% CI 0.69 to 6.62)), anthracycline (ref = 0 mg/m2, 75 ‐ 349 mg/m2 OR 0.82 (95% CI 0.38 to 1.78), ≥ 350 mg/m2 OR 1.96 (95% CI 0.72 to 5.29)), BMT (OR 0.76 (95% CI 0.20 to 2.95)), years of follow‐up (ref ≤10 yrs, 11 ‐ 15 yrs OR 0.64 (95% CI 0.27 to 8.00), ≥ 16 yrs OR 0.87 (95% CI 0.35 to 2.15)), age at diagnosis (ref ≤ 3 yrs, 4 ‐ 6 yrs OR 0.62 (95% CI 0.2 to 1.93), 7 ‐ 9 yrs OR 1.26 (95% CI 0.5 to 3.2), ≥ 10 yrs OR 1.04 (95% CI 0.41 to 2.65)), cardiac problems (OR 1.18 (95% CI 0.11 to 13.3)), growth hormone deficiency (OR 2.26 (95% CI 0.93 to 5.50)), second malignancy (OR 0.58 (95% CI 0.07 to 5.33)), anaemia in the past 12 months (OR 2.16 (95% CI 0.69 to 6.82)), hepatitis C (OR 0.94 (95% CI 0.18 to 5.02)), hearing loss (OR 1.20 (95% CI 0.39 to 3.71)), vision problems not corrected with glasses (OR 1.21 (95% CI 0.45 to 3.21)) Multivariable: Significant: marital status (OR 0.11 (95% CI 0.02 to 0.5)), obesity (OR 3.80 (95% CI 1.41 to 10.3)), sleep problems (OR 6.15 (95% CI 2.33 to 16.2)), neurocognitive impairment (OR 2.56 (95% CI 1.02 to 6.38)), exercise‐induced symptoms (OR 2.98 (95% CI 1.11 to 8.02)), pain (OR 5.56 (95% CI 2.13 to 14.5)) |
|
| Notes | Funding sources: Grant from Nationa Cancer Institute and Toys R Us Children's foundation Declaration of interest: The authors indicated no potential conflicts of interest aAnalyses were performed with moderate to severe fatigue as outcome (total score ≥ 4). P values were not reported in the article |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | High risk | Size of original cohort unclear, 364 eligible participants, 161 described study group, < 90% |
| Adequate follow‐up assessment (attrition bias) | Low risk | Ouctome was assessed for > 95% of the study group of interest |
| Blinded outcome assessor (detection bias) All outcomes | High risk | Outcome assessors were not blinded to the investigated determinant |
| Adjustment important confounders | High risk | Important prognostic factors and follow‐up were not taken together into account |
| Well‐defined study group (reporting bias) | Unclear risk | Type of cancer and cancer treatment are mentioned, specific chemotherapeutic agents and doses are not reported (only for anthracyclines). Inclusion and exclusion criteria are described |
| Well‐defined follow‐up (reporting bias) | Low risk | Length of follow‐up is mentioned |
| Well‐defined outcome severe fatigue (reporting bias) All outcomes | High risk | The authors reported which instrument they used to assess fatigue, but not what they considered to be severe fatigue |
| Well‐defined outcome fatigue (reporting bias) | Low risk | Authors reported which instrument they used to assess fatigue, and how they described fatigue (moderate to severe fatigue) |
| Well‐defined risk estimation | Low risk | Odds ratios were calculated for all factors |
Mulrooney 2008.
| Methods | Study design: cross‐sectional study Instrument used to assess fatigue: FACIT ‐ Fatigue Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: NAa Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: diagnosed before the age of 21 years with ALL, a CNS malignancy, Hodgkin disease, soft tissue sarcoma, or bone malignancy, at 1 of 26 participating institutions, diagnosis between 1970 and 1986, survived at least 5 years following diagnosis, completed second follow‐up survey Exclusion criteria: nm |
|
| Participants | Sample characteristics: N of participants original cohort: unknown; N of participants described study group: 1897; N of participants study group of interest: 1897; N of participants fatigue assessed: 1897 Participant characteristics: Tumour type: leukaemia n = 298, CNS malignancy n = 299, HL n = 995, soft tissue sarcoma n = 150, bone cancer n = 155 Tumour stage: nm Age at diagnosis: 0 ‐ 4 years n = 353, 5 ‐ 9 years n = 390, 10 ‐ 14 years n = 523, 15+ n = 631b Time since diagnosis: 15 ‐ 19 years n = 495, 20 ‐ 24 years n = 646, 25 ‐ 29 years n = 493, 30+ n = 263 Age at assessment: 18 ‐ 29 years n = 452, 30 ‐ 39 years n = 879, 40 ‐ 49 years n = 532, 50+ n = 34 F/M: 964/933 BMI: nm Race/ethnicity: White n = 1682, black n = 72, Hispanic n = 86, Asian n = 25, American India/Alaska native n = 19, other n = 13 Marital status: married or living as married n=1064, not married n=816, not indicated n=7 Highest completed education level: grade school n = 8, high school n = 290, technical school n = 128, college n = 1175, postgraduate n = 281, not indicated n = 5 c Employment: working full time n = 1240, not working full time n = 657 Physical activity level: nm Sleeping problems: Sleep quality: Total score PSQI mean 6.1 (SD 0.1); Daytime sleepiness: total score ESS mean 6.2 (SD 0.1) Psychosocial problems: Depressed (BSI score ≥ 63) n = 154 Comorbidities: Congestive heart failure n = 42, lung fibrosis n = 60, BMI 30+ kg/m2 n = 392 Genetic factors/mutations: nm |
|
| Interventions | N of participants chemotherapy: 1121 N of participants radiotherapy: 1332 N of participants surgery: nm |
|
| Outcomes | Severe fatigue: N of participants with severe fatigue: NDa Risk and associated factors: Dependent factor: fatigued (yes/no) d Multivariable: Significant: gender (female, OR 1.9 (95% CI 1.5 to 2.4)), radiotherapy (OR 1.7 (95% CI 1.3 to 2.3)), congestive heart failure (OR 2.9 (95% CI 1.4 to 6.1)), lung fibrosis (OR 2.9 (95% CI 1.5 to 5.4)), depressed (OR 7.5 (95% CI 5.1 to 10.9)), marital status (not married, OR 2.7 (95% CI 2.0 to 3.6)) Non‐significant: diagnosis (ref = ALL, CNS OR 1.3 (95% CI 0.8 to 2.1), HL OR 1.2 (95% CI 0.7 to 1.8), soft tissue sarcoma OR 1.0 (95% CI 0.6 to 1.7), bone cancer OR 1.3 (95% CI 0.7 to 2.3)), age at diagnosis (ref = 15+ yrs, 10 ‐ 14 yrs OR 0.8 (95% CI 0.6 to 1.1), 5 ‐ 9 yrs OR 0.9 (95% CI 0.6 to 1.4), 0 ‐ 4 yrs OR 0.7 (95% CI 0.4 to 1.2)), chemotherapy (OR 1.0 (95% CI 0.8 to 1.4)), hypothyroidism (OR 0.9 (95% CI 0.7 to 1.3)), BMI (30+ kg/m2 OR 1.3 (95% CI 0.9 to 1.7)), employment status (not working full‐time OR 1.2 (95% CI 0.3 to 1.6)) |
|
| Notes | Funding sources: supported by grant U24 CA 55727, National Cancer Institute, Bethesda, MD, the Children’s Cancer Research Fund, Minneapolis, MN, and the American Lebanese Syrian Associated Charities (ALSAC) Declaration of interest: The other authors have indicated no financial conflicts of interest aAuthors defined fatigued as scoring below the 10th percentile of the siblings' scores (control group). Additional information on severe fatigue was requested and not available. bBased on information of the total CCS cohort, it was possible to estimate the percentage of participants under 18 at diagnosis of the five included diagnostic groups (92.2%). c Numbers do not add up to total N of described study group. Unclear what correct numbers were. dAnalyses were performed with fatigued as outcome (defined as scoring below the 10th percentile of the siblings' scores). Two multivariable analyses were conducted, one with cancer and treatment‐related variables and the other with medical conditions and sociodemographic factors. P values were not reported in the article. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | High risk | Random sample with respect to cancer diagnosis and not cancer treatment. |
| Adequate follow‐up assessment (attrition bias) | Low risk | Outcome was assessed for > 95% of the study group of interest |
| Blinded outcome assessor (detection bias) All outcomes | High risk | Outcome assessors were not blinded to the investigated determinant |
| Adjustment important confounders | High risk | Follow‐up was not taken into account |
| Well‐defined study group (reporting bias) | High risk | Only chemotherapy and radiotherapy are mentioned as type of treatment, no information available about surgery. Inclusion and exclusion criteria are described |
| Well‐defined follow‐up (reporting bias) | Low risk | Length of follow‐up is mentioned |
| Well‐defined outcome fatigue (reporting bias) | Low risk | Authors reported which instrument they used to assess fatigue, and how they described fatigue (defined fatigued as scoring below the 10th percentile of the siblings' scores) |
| Well‐defined risk estimation | Low risk | Odds ratios were calculated |
Mört 2011.
| Methods | Study design: cross‐sectional study Instrument used to assess fatigue: PedsQL Multidimensional Fatigue Scale Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: NAa Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: aged 11 ‐ 18 years at the time of the study, an extracranial cancer diagnosis at age 16 or younger, alive at the end of 2006, survived at least 4 years post‐diagnosis, free of cancer at the time of the study Exclusion criteria: nm |
|
| Participants | Sample characteristics: N of participants original cohort: unknown; N of participants described study group: 199; N of participants study group of interest: 199; N of participants fatigue assessed: 192 Participant characteristics: Tumour type: leukaemia n = 110, NHL n = 13, HL n = 5, neuroblastoma n = 15, WT n = 16, gonadal tumour n = 7, osteosarcoma n = 6, retinoblastoma n = 6, soft tissue sarcoma n = 13, other n = 8 Tumour stage: nm Age at diagnosis: mean 3.6 years (SD 2.98; range 0 ‐ 12) Time since diagnosis: mean 10 years (SD 3.25; range 4 ‐ 17 years) Age at assessment: mean 14.4 years (range 11 ‐ 18) F/M: 98/101 BMI: nm Race/ethnicity: nm Marital status: NA Highest completed education level: nm Employment: NA Physical activity level: nm Sleeping problems: nm Psychosocial problems: nm Comorbidities: nm Genetic factors/mutations: nm |
|
| Interventions | N of participants surgery: 7 N of participants chemotherapy (alone or with surgery): 115 N of participants radiotherapy (alone or with chemotherapy or surgery): 32 N of participants SCT: 26 N of participants therapy not known or not stated: 19 |
|
| Outcomes | Severe fatigue: N of participants with severe fatigue: NDa Risk and associated factors: Dependent factor: total fatigue (continuous)b Univariable: Significant: age at assessment (r = −0.18, P = 0.01), time since diagnosis (4 ‐ 10 yrs (median 84.72) vs 11 ‐ 17 yrs (median 80.56), r = −0.18, P = 0.01) Non‐significant: diagnosis (median ranges from 75.69 to 91.67) , treatment modality (median ranges from 79.17 to 83.33), age at diagnosis (0 ‐ 4 yrs (median 81.94) vs 5 ‐ 9 yrs (median 83.33) vs 10 ‐ 12 yrs (median 84.72), relapse (yes (median 86.81) vs no (median 81.94)) Multivariable: Significant: age at assessment (b = −1.87, P < 0.001), diagnosis (ref = leukaemia, sarcoma b = −14.28, P < 0.05, NHL b = −2.49, P > 0.05, neuroblastoma b = −2.3, P > 0.05, other b = −0.85, P > 0.05) Non‐significant: gender (female, b = 2.99), treatment modality (ref = surgery alone, CT b = −4.2, RT b = −8.73, SCT b = −3.17, other treatment b = −5.09) , time since diagnosis (> 10 yrs, b = −3.6) |
|
| Notes | Funding sources: supported by research grants from the Finnish Cancer Society, the Cancer Society of South‐West Finland, the Hospital District of Southwest Finland‐Foundation, and Turku University of Applied Sciences Declaration of interest: nm aAuthors report fatigue on continuous scale. Additional information on severe fatigue was requested and not available. bAnalyses were performed with total fatigue score on a continuous scale as outcome (univariable: spearman correlation; multivariable: step‐wise selection model). Correlation coefficient and P values of non‐significant variables were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | High risk | Size of original cohort is unclear, 384 eligible participants, 199 described study group, < 90% |
| Adequate follow‐up assessment (attrition bias) | Low risk | Outcome was assessed for > 95% of the study group of interest |
| Blinded outcome assessor (detection bias) All outcomes | High risk | Outcome assessors were not blinded to the investigated determinant |
| Adjustment important confounders | High risk | Comorbidities were not taken into account |
| Well‐defined study group (reporting bias) | Unclear risk | Type of cancer and cancer treatment are mentioned, but information about specific agents, radiotherapy fields and doses are not reported. Inclusion and exclusion criteria are described |
| Well‐defined follow‐up (reporting bias) | Low risk | Length of follow‐up is mentioned |
| Well‐defined outcome fatigue (reporting bias) | Low risk | Authors reported which instrument they used to assess fatigue, and how they described fatigue (continuous scale) |
| Well‐defined risk estimation | Unclear risk | Regression coefficients were calculated for all variables in multivariable analyses. Correlation coefficients of non‐significant variables in univariable analyses were not reported |
Pemberger 2005.
| Methods | Study design: cross‐sectional study (questionnaire survey) Instrument used to assess fatigue: SF‐36 Vitality subscale Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: NAa Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: at least 18 years of age, at least 5 years off therapy, no evidence of disease, diagnosed with cancer in the years 1975 ‐ 1995 Exclusion criteria: nm |
|
| Participants | Sample characteristics: N of participants original cohort: unknown; N of participants described study group: 78; N of participants study group of interest: 78; N of participants fatigue assessed: 78 Participant characteristics: Tumour type: haematological n = 44, bone and soft tissue sarcoma n = 17, CNS tumour n = 4, tumours of internal organs n = 13 Tumour stage: nm Age at diagnosis: mean 8.0 years (SD 5.0)b Time since diagnosis and/or end of therapy: mean 14.7 years (SD 4.0) and mean 13.6 years (SD 3.8) Age at assessment: mean 22.6 years (SD 3.8) F/M: 38/40 BMI: nm Race/ethnicity: nm Marital status: married n = 9, unmarried n = 66, other marital status n = 3 Highest completed education level: still attending school n = 24, university degree n = 2, college n = 13, vocational school n = 22, technical college n = 13, high school n = 4 Employment: nm Physical activity level: nm Sleeping problems: nm Psychosocial problems: nm Comorbidities/late effects: no late effect n = 26, single late effect n = 23, multiple late effects (≥ 2) n = 29; cardiovascular n = 3, constitutional n = 11, dermatology/skin n = 5, endocrine n = 11, gastrointestinal n = 1, hepatic n = 13, immunology n = 2, musculoskeletal n = 17, neurology n = 19, ocular/visual n = 1, pain n = 3, pulmonary n = 3, renal n = 4, sexual/reproductive function n = 9 Genetic factors/mutations: nm |
|
| Interventions | N of participants chemotherapy: 76 N of participants radiotherapy: 52 N of participants surgery: 50 |
|
| Outcomes | Severe fatigue N of participants with severe fatigue: NDa Risk and associated factors: Dependent factor: fatigue (continuous)c Significant: gender (female) |
|
| Notes | Funding sources: supported by the Kinder‐Krebs‐Hilfe Declaration of interest: nm aAuthors report fatigue on continuous scale. Additional information on severe fatigue was requested and not available. bThe study author confirmed that at least 90% of the participants were under 18 at diagnosis. cAnalysis was performed with fatigue score on a continuous scale as outcome. No effect estimate or P value was reported and unclear if analysis was univariable or multivariable. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | High risk | Size of original cohort is unclear, 106 eligible participants, 78 described study group, < 90% |
| Adequate follow‐up assessment (attrition bias) | Low risk | Outcome was assessed for > 95% of the study group of interest |
| Blinded outcome assessor (detection bias) All outcomes | High risk | Outcome assessors were not blinded to the investigated determinant |
| Adjustment important confounders | High risk | Important prognostic factors and follow‐up were not taken into account |
| Well‐defined study group (reporting bias) | Unclear risk | Type of cancer and cancer treatment are mentioned but information about specific chemotherapeutic agents, radiotherapy fields and doses are not reported. Inclusion and exclusion criteria are described |
| Well‐defined follow‐up (reporting bias) | Low risk | Length of follow‐up is mentioned |
| Well‐defined outcome fatigue (reporting bias) | Low risk | Authors reported which instrument they used to assess fatigue, and how they described fatigue (continuous scale) |
| Well‐defined risk estimation | High risk | None were calculated |
Puhr 2019.
| Methods | Study design: cross‐sectional study Instrument used to assess fatigue: Fatigue Questionnaire Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: sum score of ≥ 4 on the fatigue questionnaire and symptom duration of ≥ 6 months Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: treatment for paediatric brain tumour during the first 16 years of life, diagnosis during the period 1990 ‐ 2012, aged 18 ‐ 30 at the time of recruitment, completed treatment no later than 2 years prior to recruitment in the study Exclusion criteria: self‐reported severe difficulties with activities of daily life, self‐reported severe sensory and motor disabilities, pre‐tumour cognitive/neurological problems due to non‐tumour diagnosis evidenced in patient records |
|
| Participants | Sample characteristics: N of participants original cohort: 353; N of participants described study group: 114; N of participants study group of interest: 114; N of participants fatigue assessed: 110 Participant characteristics: Tumour type: brain tumour n = 114 Tumour stage: nm Age at diagnosis: mean 9.4 years (range 0.5 ‐ 17; SD 4.43) Time since end of therapy: mean 13.9 years (range 2.6 ‐ 25.1; SD 5.61) Age at assessment: mean 23.4 years (SD 3.5) F/M: 66/48 BMI: nm Race/ethnicity: nm Marital status: nm Highest completed education level: 1st to 10th grade: 0.1%, vocational studies: 16.7%, general studies: 27.2%, higher education: 55.3% Employment: nm Physical activity level: nm Sleeping problems: nm Psychosocial problems: SCL‐90‐R depression subscale mean 51.3 (SD 11.58), SCL‐90‐R anxiety subscale mean 52.4 (SD 10.05) Comorbidities: nm Genetic factors/mutations: nm |
|
| Interventions | N of participants surgery only: 74 N of participants chemotherapy only: 1 N of participants surgery + chemotherapy: 2 N of participants surgery + cranial irradiation: 5 N of participants surgery + chemotherapy + cranial irradiation: 22 N of participants no treatment: 3 N of participants treatment unknown: 7 |
|
| Outcomes | Severe fatigue: N of participants with severe fatigue: 16/110 (14.5%) Risk and associated factors: Dependent factor: severe fatigue (yes/no)a Univariable: Significant: type of treatment (surgery, CRT and chemotherapy: fatigued 26.9% vs non‐fatigued 8.3%, P = 0.017), time since treatment (fatigued mean 10.95 years vs non‐fatigued mean 14.32, P = 0.045) Not significant: gender, age at time of survey, age at diagnosis, type of brain tumour, psychiatric comorbidity |
|
| Notes | Funding sources: Supported by the Norwegian Cancer Society (6865381); the Norwegian ExtraFoundation for Health and Rehabilitation (2013/2/0234); Oslo University Hospital’s Childrens’ Foundation (36219) Declaration of interest: nm The following data were obtained from the study author: N of participants with severe fatigue aUnivariable analysis for continuous data and Chi2 test for categorical data were applied (characteristics fatigued vs non‐fatigued were compared). Effect estimates were not reported for non‐significant factors. Psychiatric comorbidity was classified according to ICD‐10 codes F01‐F99. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | High risk | The described study group consisted of < 90% of the original cohort |
| Adequate follow‐up assessment (attrition bias) | Low risk | Outcome was assessed for > 95% of the study group of interest |
| Blinded outcome assessor (detection bias) All outcomes | High risk | Outcome assessors were not blinded to the investigated determinant |
| Adjustment important confounders | High risk | No multivariable analyses |
| Well‐defined study group (reporting bias) | Unclear risk | Type of cancer and cancer treatment are mentioned but information about specific chemotherapeutic agents, radiotherapy fields and doses are not reported. Inclusion and exclusion criteria are described |
| Well‐defined follow‐up (reporting bias) | Low risk | Length of follow‐up is mentioned |
| Well‐defined outcome severe fatigue (reporting bias) All outcomes | Low risk | The authors reported which instrument they used to assess fatigue and what they considered to be severe fatigue |
| Well‐defined outcome fatigue (reporting bias) | Low risk | Authors reported which instrument they used to assess fatigue, and how they described fatigue (severe fatigue) |
| Well‐defined risk estimation | Unclear risk | Effect estimated were not reported for all factors |
Reulen 2007.
| Methods | Study design: cross‐sectional study (retrospective analysis as part of cohort study) Instrument used to assess fatigue: SF‐36 Vitality subscale Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: score ≤ 50 Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: diagnosed with childhood cancer between 1940 and 1991 in Britain, survived for at least 5 yearsa Exclusion criteria: nm |
|
| Participants | Sample characteristics: N of participants original cohort: 14,540; N of participants described study group: 10,189; N of participants study group of interest: 10,189; N of participants fatigue assessed: 9920 Participant characteristics: Tumour type: leukaemia n = 2558, HL n = 736, NHL n = 533, CNS tumour n = 2188, neuroblastoma n = 392, retinoblastoma n = 676, WT n = 939, malignant bone tumour n = 393, soft tissue sarcoma n = 702, other n = 1072 Tumour stage: nm Age at diagnosis: mean 6.7 years (SD 4.4, max 15 years)b Time since diagnosis: not reported, at least 5 years Age at assessment: mean 30.4 years (SD 10.3) F/M: 4979/5210 BMI: nm Race/ethnicity: nm Marital status: nm Highest completed education level: nm Employment: nm Physical activity level: nm Sleeping problems: nm Psychosocial problems: nm Comorbidities: nm Genetic factors/mutations: nm |
|
| Interventions | N of participants chemotherapy: 166 N of participants radiotherapy: 89 N of participants surgery: 1382 N of participants surgery + radiotherapy: 1774 N of participants surgery +chemotherapy: 549 N of participants chemotherapy + radiotherapy: 1908 N of participants surgery, chemotherapy and radiotherapy: 1163 N of participants no surgery, chemotherapy or radiotherapy: 28 N of participants complete treatment info not available: 3130 |
|
| Outcomes | Severe fatigue: N of participants with severe fatigue: 3250/9920 (32.8%) Risk and associated factors: no analysis performed with fatigue as outcome |
|
| Notes | Funding sources: Grant sponsors: Cancer Research UK, Kay Kendall Leukaemia Fund; RCR is a Cancer Research UK Graduate Training Fellow Declaration of interest: nm The following data were obtained from the study author: N of participants with severe fatigue aAdditional information provide by the study author: "Information regarding remission status of the survivors is not available in the BCCSS cohort". bThe website of the BCCSS cohort states that "all neoplasms diagnosed in British residents under 15 years of age" were included. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | High risk | The described study group consisted of < 90% of the original cohort |
| Adequate follow‐up assessment (attrition bias) | Low risk | Outcome was assessed for > 95% of the study group of interest |
| Blinded outcome assessor (detection bias) All outcomes | High risk | Outcome assessors were not blinded to the investigated determinant |
| Well‐defined study group (reporting bias) | Unclear risk | Type of cancer and cancer treatment are mentioned but information about specific chemotherapeutic agents, radiotherapy fields and doses are not reported. Inclusion and exclusion criteria are described |
| Well‐defined follow‐up (reporting bias) | Unclear risk | Length of follow up is not reported, but at least 5 years since diagnosis (based on inclusion criteria) |
| Well‐defined outcome severe fatigue (reporting bias) All outcomes | Low risk | The authors reported which instrument they used to assess fatigue; definition of severe fatigue is based on data query |
Ruccione 2013.
| Methods | Study design: cross‐sectional study (subanalysis from a prospective longitudinal study) Instrument used to assess fatigue: PedsQL Multidimensional Fatigue Scale Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: NAa Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: completed cancer treatment, be disease‐free, English‐speaking, cognitively able to complete study questionnaires Exclusion criteria: incomplete information |
|
| Participants | Sample characteristics: N of participants original cohort: unknown; N of participants described study group: 94; N of participants study group of interest: 94; N of participants fatigue assessed: 94 Participant characteristics: Tumour type: leukaemia n = 36, lymphoma n = 23, CNS tumour n = 9, bone tumour n = 7, soft tissue tumour n = 19 Tumour stage: nm Age at diagnosis: 3 ‐ 12 years n = 40, 13 ‐ 15 years n = 21, ≥ 16 years n = 22b Time since end of therapy: ≤ 6 months n = 94 Age at assessment: mean 14.8 year (SD 2.74) F/M: 45/49 BMI: nm Race/ethnicity: White non‐Hispanic n = 40, Hispanic n = 44, other n = 10 Marital status: NA Highest completed education level: nm Employment: NA Physical activity level: PedsQL Physical functioning summary score mean 75.9 (SD 21.3) Sleeping problems: nm Psychosocial problems: depression: CES‐DC mean score 11 (SD 9.7) Comorbidities/late effects: pain (single item) mean 1.4 (SD 1.8); post‐traumatic stress (Post‐traumatic Stress Disorder Reaction Index) mean 17.6 (SD 15.4) Genetic factors/mutations: nm |
|
| Interventions | N of participants chemotherapy: 90 N of participants radiotherapy: 36 N of participants surgery: nm N of participants SCT: 5 |
|
| Outcomes | Severe fatigue: N of participants with severe fatigue: NDa Risk and associated factors: Dependent factor: fatigue (continuous)c Univariable: Significant: depression (r = 0.64, P < 0.0001), pain (r = 0.42, P < 0.0001), post‐traumatic stress (r = 0.65, P < 0.0001) |
|
| Notes | Funding sources: Funding was provided by CureSearch National Childhood Cancer Foundation for Adolescent and Young Adult Oncology Research through the generosity of Aflac, Inc. Dr Meeske is supported by a STOP Cancer Career Development Award Declaration of interest: The authors have no conflicts of interest to disclose aAuthors report fatigue on continuous scale. Additional information on severe fatigue was requested and not available. bInformation received from study author: all participants were under 18 at diagnosis. Numbers do not add up to total N of described study group. Unclear what correct numbers were. cAnalyses were performed with the subscale score for general fatigue as outcome (Pearson correlation) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | High risk | Size of original cohort is unknown, 119 eligible participants, 94 described study group, < 90% |
| Adequate follow‐up assessment (attrition bias) | Low risk | Outcome was assessed for > 95% of the study group of interest |
| Blinded outcome assessor (detection bias) All outcomes | High risk | Outcome assessors were not blinded to the investigated determinant |
| Adjustment important confounders | High risk | No multivariable analyses |
| Well‐defined study group (reporting bias) | High risk | Only chemotherapy and radiotherapy are mentioned as type of treatment, no information available about surgery. Inclusion and exclusion criteria are described |
| Well‐defined follow‐up (reporting bias) | Unclear risk | Length of follow‐up is not reported, but all participants had completed therapy within the past 6 months |
| Well‐defined outcome fatigue (reporting bias) | Low risk | Authors reported which instrument they used to assess fatigue, and how they described fatigue (continuous scale) |
| Well‐defined risk estimation | Low risk | Correlation coefficients are calculated |
Rueegg 2013.
| Methods | Study design: cross‐sectional study (questionnaire survey as part of Swiss childhood cancer survivor study) Instrument used to assess fatigue: SF‐36 Vitality subscale Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: NAa Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: registered in the Swiss Childhood Cancer Registry, survived for ≥ 5 years, were diagnosed < 16 years of age between 1976 and 2005, age ≥ 16 years at the time of survey Exclusion criteria: nm |
|
| Participants | Sample characteristics: N of participants original cohort: unknown; N of participants described study group: 1593; N of participants study group of interest: 1593; N of participants fatigue assessed: 1576 Participant characteristics: Tumour type: leukaemia n = 573, lymphoma n = 290, CNS tumour n = 210, neuroblastoma n = 67, retinoblastoma n = 37, renal tumour n = 107, hepatic tumour n = 10, bone tumour n = 77, soft tissue sarcoma n = 89, germ cell tumour n = 45, other tumour n = 19, langerhans cell histiocytosis n = 69 Tumour stage: nm Age at diagnosis: mean 7.6 years (SD 4.7) Time since diagnosis: mean 17.4 years (SD 6.9) Age at assessment: mean 25.1 years (SD 6.9) F/M: 746/847 BMI: Overweight (≥ 25 BMI) n = 360 Race/ethnicity: migration background; Swiss n = 1543, foreign background n = 140b Marital status: nm Highest completed education level: nm Employment: nm Physical activity level: nm Sleeping problems: nm Psychosocial problems: nm Comorbidities/late effects: self‐reported; vision impairment n = 175, hearing problem n = 158, memory problem n = 192, digestive problem n = 218, musculoskeletal/neurological problem n = 434, thyroid problem n = 151 Genetic factors/mutations: nm |
|
| Interventions | N of participants chemotherapy: 1340 N of participants surgery: 684 N 0f participants radiotherapy: 611 N of participants body and limb irradiation: 314 N of participants cranial and spinal irradiation: 297 N of participants BMT: 67 |
|
| Outcomes | Severe fatigue: N of participants with severe fatigue: NDa Risk and associated factors: Dependent factor: fatiguedc Multivariable: Significant: hearing problems (OR 2.85 (95% CI 1.84 to 4.42), P < 0.001), memory problems (OR 3.74 (95% CI 2.40 to 5.84), P < 0.001), digestive problems (OR 3.15 (95% CI 2.13 to 4.66), P < 0.001), musculoskeletal/neurological problems (OR 2.03 (95% CI 1.42 to 2.88), P < 0.001), thyroid problems (OR 2.12 (95% CI 1.31 to 3.43), P = 0.002), vision impairments (OR 1.87 (95% CI 1.15 to 3.05), P = 0.012)) Non‐significant: overweight (OR 1.44 (95% CI 0.97 to 2.15), P = 0.071) |
|
| Notes | Funding sources: Swiss Cancer League (KLS‐01605‐10‐2004, KLS‐2215‐02‐2008, KLS‐02783‐02‐2011), Cancer League Aargau, Cancer League Zurich, Swiss Bridge, and Stiftung zur Krebsbekämpfung. Gisela Michel and Claudia Kuehni were funded by the Swiss National Science Foundation (G.M.—Ambizione Fellowship grant PZ00P3_121682/1 and PZ00P3_141722; C.K.—PROSPER grant 3233‐069348) Declaration of interest: No conflict of interest stated for any of the authors aAuthors report fatigue on continuous scale. Additional information on severe fatigue was requested and not available. bNumbers do not add up to total N of described study group. Unclear what correct numbers were. cAnalyses were performed with fatigued as outcome (defined as scoring below the 10th percentile of sibling scores and described in the paper as Energy and vitality). The authors tested the effect of each chronic health problem using multivariable logistic regression models, adjusting for age, gender, and parents’ education. Due to multiple testing concerns, the authors interpreted the results cautiously, looking only at associations with P < 0.001. OR and P values were not reported in the article for the adjusting factors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | High risk | Size of original cohort is unclear, 2526 eligible participants, 1593 described study group, < 90% |
| Adequate follow‐up assessment (attrition bias) | Low risk | Outcome was assessed for > 95% of the study group of interest |
| Blinded outcome assessor (detection bias) All outcomes | High risk | Outcome assessors were not blinded to the investigated determinant |
| Adjustment important confounders | High risk | Multiviariable analyses, but treatment was not taken into account |
| Well‐defined study group (reporting bias) | Unclear risk | Type of cancer and cancer treatment are mentioned but information about specific chemotherapeutic agents and doses are not reported. Inclusion and exclusion criteria are described |
| Well‐defined follow‐up (reporting bias) | Low risk | Length of follow‐up is mentioned |
| Well‐defined outcome fatigue (reporting bias) | Low risk | Authors reported which instrument they used to assess fatigue, and how they described fatigue (defined fatigued as scoring below the 10th percentile of the siblings' scores) |
| Well‐defined risk estimation | Low risk | Odds ratios were calculated |
Sundberg 2013.
| Methods | Study design: cross‐sectional study (questionnaire survey) Instrument used to assess fatigue: SF‐36 Vitality subscale Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: score ≤ 50 Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: SCT survivor group: treatment in the paediatric SCT programme between October 1985 and June 1999, young adults > 18 years old, no history of chronic GVHD and immunosuppressive therapy Non‐SCT group: ALL diagnosis, diagnosed at age < 18 years, during period 1985 ‐ 1999, at least 18 years of age at time of the study Exclusion criteria: nm |
|
| Participants | Sample characteristics: N of participants original cohort: 415; N of participants described study group: 70; N of participants study group of interest: 70; N of participants fatigue assessed: 64 Participant characteristics: Tumour type: SCT group: ALL or lymphoblastic lymphoma n = 18, non‐SCT group: ALL n = 52 Tumour stage: nm Age at diagnosis: SCT group: median 5 years (range 1 ‐ 15), non‐SCT group: median 6.5 (range 0 ‐ 16) Time since end of therapy: SCT group: median 18 years (range 10 ‐ 22), non‐SCT group: median 14 years (range 5 ‐ 18) Age at assessment: SCT group: median 27 years (range 18 ‐ 37), non‐SCT group: median 22 years (range 19 ‐ 33) F/M: SCT group: 8/10, non‐SCT group: 27/25 BMI: nm Race/ethnicity: nm Marital status: SCT group: married/partnered n = 6, non‐SCT group: married/partnered n = 13 Highest completed education level: SCT group: junior compulsory n = 3, senior high school n = 9, postgraduate/university n = 6 non‐SCT group: junior compulsory n = 16, senior high school n = 30, postgraduate/university n = 6 Employment: SCT group: student n = 5, working n = 9, unemployed n = 2, sick leave n = 2 non‐SCT group: student n = 22, working n = 25, unemployed n = 4, sick leave n = 1 Physical activity level: SF‐36 physical functioning, SCT group: mean 90.0 (SD 13.1), non‐SCT group mean 95.3 (SD 11.2) Sleeping problems: nm Psychosocial problems: nm Comorbidities/late effects: nm Genetic factors/mutations: nm |
|
| Interventions | N of participants chemotherapy: nm N of participants radiotherapy: nm N of participants surgery: nm N of participants cranial irradiation: SCT group 7, non‐SCT group 21 N of participants SCT: SCT group 18 |
|
| Outcomes | Severe fatigue: N of participants with severe fatigue: 23/64 (35.94%) Risk and associated factors: no analysis performed with fatigue as outcome |
|
| Notes | Funding sources: Swedish Childrens’s Cancer Foundation Declaration of interest: Nothing to declare The following data were obtained from the study author: N of participants with severe fatigue |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | High risk | The described study group consisted of < 90% of the original cohort |
| Adequate follow‐up assessment (attrition bias) | Low risk | Outcome was assessed for 65% ‐ 95% of the study group of interest |
| Blinded outcome assessor (detection bias) All outcomes | High risk | Outcome assessors were not blinded to the investigated determinant |
| Well‐defined study group (reporting bias) | High risk | Type of cancer is mentioned and information on cancer treatment is limited to cranial irradiation and SCT. Inclusion and exclusion criteria are described |
| Well‐defined follow‐up (reporting bias) | Low risk | Length of follow‐up is mentioned |
| Well‐defined outcome severe fatigue (reporting bias) All outcomes | Low risk | The authors reported which instrument they used to assess fatigue and definition of severe fatigue is based on data query |
Tremolada 2018.
| Methods | Study design: cross‐sectional study Instrument used to assess fatigue: SF‐36 Vitality subscale Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: a score ≤ 50 Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: being cured for cancer by HSCT in paediatric age, at least 5 years from the end of the therapies, being currently 15 ‐ 25 years old Exclusion criteria: childhood cancer survivors treated for brain tumours, survivors with sensory deficiencies or genetic syndromes, survivors who were unable to complete the questionnaires autonomously |
|
| Participants | Sample characteristics: N of participants original cohort: unknown; N of participants described study group: 32; N of participants study group of interest: 32; N of participants fatigue assessed: 32 Participant characteristics: Tumour type: haematological tumours (leukaemias, non‐Hodgkin lymphoma) n = 21, solid tumours (Hodgkin lymphoma, solid tissue, other) n = 11 Tumour stage: nm Age at diagnosis: mean 8.1 years (SD 4.3; range 0.4 ‐ 16.1) Time since end of therapy: mean 8.5 years (SD 3.2; range 5 ‐ 16) Age at assessment: mean 19.4 years (SD 3.84; range 14.1 ‐ 25) F/M: 15/17 BMI: nm Race/ethnicity: White n = 32 Marital status: engaged n = 19, single n = 6, not reported n = 7 Highest completed education level: 0 ‐ 8 years of schooling n = 9, 9 ‐ 13 years of schooling n = 21, > 13 years of schooling n = 1, not reported n = 1 Employment: not working, student n = 19, looking for a job n = 4, part‐time n = 2, full‐time n = 7 Physical activity level: nm Sleeping problems: nm Psychosocial problems: nm Comorbidities/late effects: nm Genetic factors/mutations: nm |
|
| Interventions | N of participants chemotherapy: 32 N of participants radiotherapy: 32 N of participants surgery: 0 N of participants SCT: 32 N of participants total body irradiation: 32 |
|
| Outcomes | Severe fatigue: N of participants with severe fatigue: 8/32 (25.0%) Risk and associated factors: Dependent factor: fatigue (continuous)a Univariable: Non‐significant: gender (r = −0.150, P = 0.411), age at assessment (r = 0.081, P = 0.659), age at diagnosis (r = 0.218, P = 0.230), diagnosis type (r = −0.042, P = 0.818), time since end of therapy (r = −0.012, P = 0.950), relapse (r = −0.200, P = 0.272), years of schooling |
|
| Notes | Funding sources: this study was supported by a grant from foundation Istituo della Ricerca Pediatrica Città della Speranza. Open access sponsored by Provincia Autonoma di Bolzano, Alto Adige Declaration of interest: nm The following data were obtained from the study author: N of participants chemotherapy, radiotherapy or surgery, N of participants with severe fatigue aAnalysis was performed with fatigue score on a continuous scale as outcome (Pearson correlation). No effect estimate or P value was reported for years of schooling |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | Unclear risk | Size of original cohort is unclear |
| Adequate follow‐up assessment (attrition bias) | Low risk | Outcome was assessed for > 95% of the study group of interest |
| Blinded outcome assessor (detection bias) All outcomes | High risk | Outcome assessors were not blinded to the investigated determinant |
| Adjustment important confounders | High risk | No multivariable analyses |
| Well‐defined study group (reporting bias) | Unclear risk | Type of cancer and cancer treatment are mentioned but information about specific chemotherapeutic agents, radiotherapy fields and doses are not reported. Inclusion and exclusion criteria are described |
| Well‐defined follow‐up (reporting bias) | Low risk | Length of follow‐up is mentioned |
| Well‐defined outcome severe fatigue (reporting bias) All outcomes | Low risk | The authors reported which instrument they used to assess fatigue; definition of severe fatigue is based on data query |
| Well‐defined outcome fatigue (reporting bias) | Low risk | Authors reported which instrument they used to assess fatigue, and how they described fatigue (continuous scale) |
| Well‐defined risk estimation | Unclear risk | Correlation coefficients are calculated, but not for all factors |
Van Dijk 2008.
| Methods | Study design: cross‐sectional study Instrument used to assess fatigue: SF‐36 Vitality subscale Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: a score ≤ 50 Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: history of cancer in childhood or adolescence, finished treatment at least 5 years ago, survivor aged between 16 and 40 years, attended late effects outpatient clinic between November 2004 and December 2005 Exclusion criteria: mental retardation |
|
| Participants | Sample characteristics: N of participants original cohort: unknown; N of participants described study group: 60; N of participants study group of interest: 60; N of participants fatigue assessed: 60 Participant characteristics: Tumour type: ALL n = 27, AML n = 5, (non) Hodgkin lymphoma n = 15, solid tumour n = 11, brain tumour n = 2 Tumour stage: nm Age at diagnosis: mean 8.3 years (SD 4.5; range 1 ‐ 16) Time since diagnosis: mean 15.2 years ( SD 5.3; range 6 ‐ 28) Age at assessment: mean 24.6 years (SD 5.6; range 17 ‐ 39) F/M: 29/31 BMI: nm Race/ethnicity: White n = 60 Marital status: married or living as married n = 16, other n = 41, unknown n = 3 Highest completed education level: nm Employment: nm Physical activity level: SF‐36 physical functioning mean 87.3 (SD 20) Sleeping problems: nm Psychosocial problems: nm Comorbidities/late effects: nm Genetic factors/mutations: nm |
|
| Interventions | N of participants chemotherapy: 20 N of participants radiotherapy: 1 N of participants surgery: 2 N of participants surgery + radiotherapy: 1 N of participants surgery +chemotherapy: 8 N of participants chemotherapy + radiotherapy: 20 N of participants surgery, chemotherapy and radiotherapy: 8 |
|
| Outcomes | Severe fatigue: N of participants with severe fatigue: 37/60 (61.7%) Risk and associated factors: Dependent factor: fatigue (continuous)a Univariable: Non‐significant: age at diagnosis (< 12 years (mean 53.7) vs ≥ 12 years (mean 49.8)) |
|
| Notes | Funding sources: supported by the 'Stichting Vrouwen VU‐hulp' Declaration of interest: nm The following data were obtained from the study author: N of participants with severe fatigue aAnalysis was performed with fatigue score on a continuous scale as outcome (Mann‐Whitney U‐test). Effect estimate and P value were not reported in the article. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | High risk | Size of original cohort is unclear, 71 eligible participants, 60 described study group, < 90% |
| Adequate follow‐up assessment (attrition bias) | Low risk | Outcome was assessed for > 95% of the study group of interest |
| Blinded outcome assessor (detection bias) All outcomes | High risk | Outcome assessors were not blinded to the investigated determinant |
| Adjustment important confounders | High risk | No multivariable analyses |
| Well‐defined study group (reporting bias) | Unclear risk | Type of cancer and cancer treatment are mentioned but information about specific chemotherapeutic agents, radiotherapy fields and doses are not reported. Inclusion and exclusion criteria are described |
| Well‐defined follow‐up (reporting bias) | Low risk | Length of follow‐up is mentioned |
| Well‐defined outcome severe fatigue (reporting bias) All outcomes | Low risk | The authors reported which instrument they used to assess fatigue; definition of severe fatigue is based on data query |
| Well‐defined outcome fatigue (reporting bias) | Low risk | Authors reported which instrument they used to assess fatigue, and how they described fatigue (continuous scale) |
| Well‐defined risk estimation | Low risk | Mean fatigue scores for both groups are presented |
Verberne 2012.
| Methods | Study design: cross‐sectional study Instrument used to assess fatigue: PedsQL Multidimensional Fatigue Scale Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: normative mean total score for Dutch population of the PedsQL child form: 76.84 (SD 12.67); cut‐off score for severe fatigue: total fatigue score < 51.50 Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: between 4 and 18 years old, > 6 months after end of treatment, diagnosed after 2003 Exclusion criteria: Down syndrome |
|
| Participants | Sample characteristics: N of participants original cohort: unknown; N of participants described study group: 109; N of participants study group of interest: 109; N of participants fatigue assessed: 109, 71 with child forma Participant characteristics: Tumour type: CNS tumour n = 31, HL n = 4, NHL n = 11, ALL n = 12, WT n = 13, neuroblastoma n = 7, bone tumour n = 7, non‐CNS germ cell tumour n = 4, liver tumour n = 3, soft tissue or other tumour n = 17 Tumour stage: nm Age at diagnosis: mean 7.1 years (range 0.0 ‐ 16.9) Time since end of therapy: mean 2.4 years (range 0.5 ‐ 6.0) Age at assessment: mean 10.3 years (range 4.0 ‐ 17.9) F/M: 48/61 BMI: < 25 n = 60, ≥ 25 ‐ ≤ 30 n = 5, > 30 n = 3, unknown n = 3 Race/ethnicity: White n = 47, Hispanic n = 0, other n = 14, unknown n=10 Marital status: NA Highest completed education level: nm Employment: NA Physical activity level: nm Sleeping problems: sleep duration: 9 ‐ 11 hrs n = 62, 8 ‐ 9 hrs n = 26, 7 ‐ 8 hrs n = 13, 5 ‐ 7 hr n = 3, < 5 hr n = 1b DIMS: mean 11.7 (SD 3.8)c SWTD: mean 7.8 (SD 2.6)c DOES: mean 8.8 (SD 2.8)c SHY: mean 2.6 (SD 1.6)c Daytime sleepiness (ESS): mean 4.5 (SD 4.0)c Psychosocial problems: nm Comorbidities/late effects: nm Genetic factors/mutations: nm |
|
| Interventions | N of participants surgery: 20 N of participants surgery + chemotherapy: 34 N of participants surgery + chemotherapy + cranial irradiation: 11 N of participants surgery + chemotherapy + radiotherapy: 12 N of participants surgery + radiotherapy: 2 N of participants chemotherapy: 20 N of participants chemotherapy + radiotherapy: 7 N of participants no treatment: 3 |
|
| Outcomes | Severe fatigue: N of participants with severe fatigue: 8/71 (11.3%) Risk and associated factors: Dependent factor: fatigue (continuous)d Univariable: Significant: DOES ( r = −0.78, P < 0.001), SWTD (r = −0.37, P < 0.05) Non‐significant: DIMS (r = −0.15), SHY (r = −0.08), daytime sleepiness (r = −0.30) |
|
| Notes | Funding sources: This study was not funded Declaration of interest: The authors declare that there is no conflict of interest The following data were obtained from the study author: N of participants with severe fatigue, BMI and race/ethnicity aonly for the participants that filled in the child form of the PedsQL questionnaire (8 ‐ 18 years at assessment), fatigue was assessed based on the presented criterion for severe fatigue. bNumbers do not add up to total N of described study group. Unclear what correct numbers were. cPresented means are from CNS tumour survivors only dAnalyses were performed with fatigue score on a continuous scale as outcome (Pearson correlation). P values were not reported for non‐significant risk and associated factors in the article. Analyses included only CNS tumour survivors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | High risk | Size of original cohort is unclear, 129 eligible participants, 109 described study group, < 90% |
| Adequate follow‐up assessment (attrition bias) | Low risk | Outcome was assessed for 65% ‐ 95% of the study group of interest |
| Blinded outcome assessor (detection bias) All outcomes | High risk | The outcome assessors were not blinded to the investigated determinant |
| Adjustment important confounders | High risk | No multivariable analyses |
| Well‐defined study group (reporting bias) | Unclear risk | Type of cancer and cancer treatment are mentioned but information about specific chemotherapeutic agents and doses are not reported. Inclusion and exclusion criteria are described |
| Well‐defined follow‐up (reporting bias) | Low risk | Length of follow‐up is mentioned |
| Well‐defined outcome severe fatigue (reporting bias) All outcomes | Low risk | The authors reported which instrument they used to assess fatigue; definition of severe fatigue is based on data query |
| Well‐defined outcome fatigue (reporting bias) | Low risk | Authors reported which instrument they used to assess fatigue, and how they described fatigue (continuous scale) |
| Well‐defined risk estimation | Low risk | Correlation coefficients are calculated |
Wright 2013.
| Methods | Study design: cross‐sectional study (part of mixed method study, quantitative part is questionnaire survey) Instrument used to assess fatigue: Fatigue Scale for Adolescents Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: score ≥ 31 Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: completed treatment for cancer during childhood or adolescence, aged 13 ‐ 18 years, ability to complete written questionnaire Exclusion criteria: antecedent neurological, developmental or genetic disorder |
|
| Participants | Sample characteristics: N of participants original cohort: unknown; N of participants described study group: 48; N of participants study group of interest: 48; N of participants fatigue assessed: 48 Participant characteristics: Tumour type: leukaemia 66.6%, solid tumour 12.5%, lymphoma 18.7%, CNS tumour 2.1% Tumour stage: nm Age at diagnosis: mean 7.0 (SD 4.3; range 1.7 ‐ 14.6) Time since end of therapy: mean 6.9 years (SD 3.8; range 0.5 ‐ 13.0) Age at assessment: mean 16.0 years (SD 2.1; range 13 ‐ 18) F/M: 19/29 BMI: underweight 2.1%, healthy 74.5%, overweight 14.9%, obese 8.5% Race/ethnicity: nm Marital status: nm Highest completed education level: nm Employment: nm Physical activity level: Leisure Score Index of the GLTEQ mean 60.0 (SD 32.8) Sleeping problems: nm Psychosocial problems: nm Comorbidities/late effects: nm Genetic factors/mutations: nm |
|
| Interventions | N of participants surgery: nm N of participants chemotherapy: nm N of participants radiotherapy: nm |
|
| Outcomes | Severe fatigue: N of participants with severe fatigue: 6/48 (12.5%) Risk and associated factors: no analysis performed with fatigue as outcome |
|
| Notes | Funding sources: nm Declaration of interest: nm |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | Unclear risk | Size of original cohort is unclear |
| Adequate follow‐up assessment (attrition bias) | Low risk | Outcome was assessed for > 95% of the study group of interest |
| Blinded outcome assessor (detection bias) All outcomes | High risk | Outcome assessors were not blinded to the investigated determinant |
| Well‐defined study group (reporting bias) | High risk | Type of cancer treatment was not mentioned. Inclusion and exclusion criteria are described. |
| Well‐defined follow‐up (reporting bias) | Low risk | Length of follow‐up is mentioned |
| Well‐defined outcome severe fatigue (reporting bias) All outcomes | Low risk | The authors reported which instrument the used to assess fatigue and what they considered to be severe fatigue |
Zeller 2014a.
| Methods | Study design: case‐control study (follow‐up study of Hamre 2013a) Instrument used to assess severe fatigue: Fatigue Questionnaire Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: sum score ≥ 4 for all 11 dichotomised items and duration of symptoms for ≥ 6 months Time points at which outcome data were collected: 2 time points, mean time between fatigue assessments 2.7 years (range 1 ‐ 4.3) Cases: n = 27; severe fatigue at both time points (persistent severe fatigue) Controls: n = 35; no severe fatigue at either time point Controls matched on diagnosis, age, gender Inclusion criteria: diagnosis between 1970 and 2002, age at diagnosis of 18 years and below (16 years and below for ALL), follow‐up time from diagnosis of at least 5 years, age at survey 18 years and above, participated in part 1 of the study (Hamre 2013a), did not change fatigue status (only for the risk factor analysis) Exclusion criteria: major somatic comorbidities, pregnancy (only for the risk factor analysis) |
|
| Participants | N of participants original cohort: unknown; N of participants described study group: 102; N of participants study group of interest: 102; N of participants fatigue assessed: 102a Tumour type: cases: lymphoma n = 14, ALL n = 13; controls: lymphoma n = 19, ALL n = 16 Tumour stage: nm Age at diagnosis: based on inclusion criteria, the maximum age at diagnosis is 18 years Time since diagnosis: cases: mean 23.0 years (SD 8.2), controls: mean 24.0 years (SD 7.9) Age at assessment: cases: mean 33.7 years (SD 6.6), controls: mean 34.4 years (SD 7.3) F/M: cases: 18/9, controls: 19/16 BMI: cases: mean 25.1 (SD 5.0), controls: mean 24.6 (SD 3.7) Race/ethnicity: nm Marital status: nm Highest completed education level: higher level of education ≥ 12 years, cases n = 11, controls n = 21 Employment: at present in paid work, cases n = 17, controls n = 26 Physical activity level: SF‐36 physical functioning scale, cases: median 80.0 (range 45 ‐ 100), controls: median 100 (range 70 ‐ 100). Number of steps per day, cases: mean 6861 (SD 2801), controls: mean 8687 (SD 2420) Sleeping problems: insomnia, cases n = 16, controls n = 7. Sleep duration in hours, cases: median 8 (range 3.5 ‐ 13), controls: median 7 (range 4 ‐ 9) Psychosocial problems: Anxiety (STAI), cases: median 49 (range 32 ‐ 70), controls: median 33 (range 23 ‐ 65) Depression (modified PHQ8), cases: median 7 (range 3 ‐ 21), controls: median 2.5 (range 0 ‐ 17) Comorbidities/late effects: Pain severity score (BPI), cases: median 10 (range 0 ‐ 28), controls: median 3.0 (range 0 ‐ 19) Genetic factors/mutations: nm |
|
| Interventions | N of participants radiotherapy: cases: n = 13, controls: n = 14 N of participants chemotherapy: nm N of participants surgery: nm Cumulative dosis anthracyclines: cases mean 166.2 mg (SD 139.9), controls mean 170.0 mg (SD 127.6) |
|
| Outcomes | Severe fatigue: N of participants with severe fatigue: at time point 1: 79/290 (27.2%); at time point 2: 41/102 (40.2%) N of participants with persistent severe fatigue: 32/102 (31.4%) Risk factors:b Dependent factor: persistent severe fatigue Univariable: Significant: depression (P < 0.001), insomnia (P = 0.002), pain severity score (P < 0.001), numbers of steps per day (P = 0.009) Non‐significant: radiotherapy (P = 0.437), time since diagnosis (P = 0.614), education (P = 0.102), employment (P = 0.246), BMI (P = 0.628) Multivariable: Significant: depression (OR 1.3 (95% CI 1.1 to 1.7), P = 0.014) Non‐significant: insomnia, pain severity score, numbers of steps per day |
|
| Notes | Funding sources: supported by the Norwegian Extra Foundation for Health and Rehabilitation Declaration of interest: The authors declare no conflict of interest a102 survivors participated in the second fatigue assessment, of which 62 were identified as case or control. bThe 62 cases and controls were included in the risk factor analyses. Univariable: Mann‐Whitney‐Wilcoxon test, independent sample t‐test or Chi2 test; multivariable: logistic regression. Only OR for depression was reported in the article |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | Unclear risk | Size of original cohort is unknown |
| Adequate follow‐up assessment (attrition bias) | Low risk | Outcome was assessed for > 95% of the study group of interest |
| Blinded outcome assessor (detection bias) All outcomes | High risk | Outcome assessors were not blinded to the investigated determinant |
| Adjustment important confounders | High risk | Follow‐up was not taken into account |
| Well‐defined study group (reporting bias) | High risk | Cancer type is mentioned and only radiotherapy is reported as cancer treatment. Inclusion and exclusion criteria are described |
| Well‐defined follow‐up (reporting bias) | Low risk | Length of follow‐up is mentioned |
| Well‐defined outcome severe fatigue (reporting bias) All outcomes | Low risk | The authors reported which instrument they used to assess fatigue and what they considered to be severe fatigue |
| Well‐defined outcome fatigue (reporting bias) | Low risk | Authors reported which instrument they used to assess fatigue, and how they described fatigue (persistent severe fatigue) |
| Well‐defined risk estimation | Unclear risk | Odds ratio is only provided for significant risk factor |
AML: acute myeloid leukaemia; ALL: acute lymphoblastoma leukaemia; ASHQ: Adolescent Sleep Habits Questionnaire; b: regression coefficient; BCCSS: British Childhood Cancer Survivor Study; BMI: body mass index; BMT: bone marrow transplantation; BPI: Brief Pain Inventory; BSI: Brief Symptom Inventory; CCS: childhood cancer survivor; CCSS: Childhood Cancer Survivors Study; CDI: Childrens Depression Inventory; CES‐D: Center for Epidemiology Studies Depression scale; CES‐DC: Center for Epidemiology Studies Depression scale for Children; CHQ: Child Health Questionnaire; CNS: central nervous system; CRT: cranial irradiation; CSHQ: Childrens Sleep Habits Questionnaire; CT: chemotherapy; CTCAEv3: CommonTerminology Criteria for Adverse Events, Version 3; CUHK‐PARCY: Chinese University of Hong Kong: physical activity rating for children and youth; DIMS: disorder maintaining sleep; DOES: disorder of excessive somnolence; EORTC‐QLQ‐C30: European Organisation for Research and Treatment of Cancer Quality of Life core questionnaire; ESS: Epworth Sleepiness Scale; F: female; FACIT: Functional Assessment of Chronic Illness Therapy; GLTEQ: Godin‐Leisure‐Time Exercise Questionnaire; GVHD: graft versus host disease; Gy: Gray; HADS: Hospital Anxiety and Depression Scale; HL: Hodgkins lymphoma; HSCT: hematopoietic stem cell transplantation; IA: infratentorial astrocytoma; IQR: interquartile range; IT: intrathecal; IV: intravenous; M: male; NA: not applicable; ND: no data; NHL: non‐Hodgkins lymphoma; nm: not mentioned; OR: odds ratio; PedsQL: Pediatric Quality of Life; PHQ: Patient Health Questionnaire; PSQI: Pittsburgh Sleep Quality Index; r: correlation coefficient; REACH: Research Evaluating After‐Cancer Health project; RT: radiotherapy; SCL‐90‐R: Symptom Checklist‐90‐Revised; SCT: stem cell transplantation; SD: standard deviation; SE: standard error; SF‐36: Short Form ‐ 36; SHY:sleep hyperhydrosis; STAI: Spielberger Trait Anxiety Inventory; SWTD: sleep wake transition disorder; WT: Wilms tumour
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adams 2004 | Not CCS; > 90% of participants were 18 years or older at the time of cancer diagnosis |
| Al‐Gamal 2016 | Study population is not in complete remission; both on and off treatment |
| Ander 2016 | Study population is not in complete remission; both on and off treatment |
| Anestin 2018 | Type of outcome measure; validated questionnaire without published cut‐off score or normative data available |
| Arpaci 2016 | Type of outcome measure; non‐validated questionnaire without published cut‐off score or normative data available |
| Arroyave 2008 | Type of outcome measure; measures barriers to exercise |
| Berg 2009 | Type of outcome measure; fatigue not measured with questionnaire |
| Berg 2013 | Type of outcome measure; non‐validated questionnaire without published cut‐off score or normative data available |
| Bower 2019 | Study design: Review paper |
| Burghardt 2019 | Type of outcome measure; validated questionnaire without published cut‐off score or normative data available |
| Carlson 2017 | Not CCS; both adult cancer survivors and childhood cancer survivors |
| Chang 2017 | Study design: Review paper |
| Clanton 2011 | Same study population as Mulrooney 2008 and Rach 2017 |
| Cox 2009 | Type of outcome measure; non‐validated questionnaire without published cut‐off score or normative data available |
| Daniel 2016 | Type of outcome measure; fatigue measured dichotomously |
| De Ruiter 2016 | Preselection on fatigue‐related factors |
| Enskar 2007 | Type of outcome measure; non‐validated questionnaire without published cut‐off score or normative data available |
| Finnegan 2009 | Type of outcome measure; validated questionnaire without published cut‐off score or normative data available |
| Fortmann 2018 | Study population is not in complete remission; both on and off treatment, and participants that had not yet received treatment |
| Geenen 2007 | Type of outcome measure; fatigue not measured with questionnaire |
| Gordijn 2012 | Same study population as Gordijn 2013 |
| Graef 2016 | Not CCS; Stem cell transplant survivors, not primarily cancer survivors |
| Hamre 2013b | Same study population as Hamre 2013a, Kanellopoulos 2013, Zeller 2014a and Zeller 2014b |
| Henderson 2018 | Type of outcome measure; validated questionnaire without published cut‐off score or normative data available |
| Ho 2015 | Same study population as Ho 2019 |
| Ho 2016 | Same study population as Ho 2019 |
| Hsiao 2017 | Type of outcome measure; fatigue measured dichotomously |
| Johannsdottir 2017 | Same study population as Hamre 2013a |
| Johnson 2018 | Not CCS; non‐malignant tumors are included (craniopharyngioma, pilocytic astrocytoma, low‐grade astrocytoma) |
| Jones 2018 | Type of outcome measure; fatigue not measured with questionnaire |
| Kanellopoulos 2013 | Same study population as Hamre 2013a, Hamre 2013b, Zeller 2014a and Zeller 2014b |
| Korinthenberg 2011 | Study population is not in complete remission; tumour status at fatigue assessment is unclear |
| Lai 2017 | Study population is not in complete remission; both on and off treatment |
| Lai 2019 | Study population is not in complete remission; both on and off treatment |
| MacArtney 2014 | Type of outcome measure; validated questionnaire without published cut‐off score or normative data available |
| Macpherson 2015 | Type of outcome measure; non‐validated questionnaire without published cut‐off score or normative data available |
| Manley 2012 | Type of outcome measure; fatigue assessed as part of the outcome 'fatigue and sleep related problems' |
| McClellan 2013 | Type of outcome measure; non‐validated questionnaire without published cut‐off score or normative data available |
| McLoone 2011 | Type of outcome measure; fatigue not measured with questionnaire |
| Mellblom 2017 | Type of outcome measure; fatigue not measured with questionnaire and as part of emotional concern |
| Nagai 2012 | Type of outcome measure, no published cut‐off score or normative data available |
| Nicklin 2019 | Study design: Review paper |
| Nies 2017 | Study population is not in complete remission; participants with persistent disease were included |
| Norum 1996 | Not CCS; Adult cancer survivors population |
| Nugent 2018 | Not CCS; additional information provided by study author: > 90% of participants were 18 years or older at the time of cancer diagnosis |
| Nwachukwu 2015 | Study population is not in complete remission |
| Pan 2017 | Study population is not in complete remission; both on and off treatment |
| Rach 2017 | Same study population as Clanton 2011 and Mulrooney 2008 |
| Raj 2018 | Type of outcome measure; fatigue not measured with questionnaire |
| Reiter‐Purtill 2003 | Type of outcome measure; validated questionnaire without published cut‐off score or normative data available |
| Ross 2018 | Type of outcome measure; measures barriers to exercise |
| Rueegg 2017 | Same study population as Rueegg 2013 |
| Sadighi 2014 | Same study population as Khan 2014 |
| Schmielau 2017 | Study design: Review paper |
| Simioni 2018 | Study design: Review paper |
| Sterkenburg 2015 | Not CCS; craniopharyngioma is not a malignant tumour |
| Van Santen 2004 | Type of outcome measure; fatigue not measured with questionnaire |
| Vannatta 1998 | Type of outcome measure; validated questionnaire without published cut‐off score or normative data available |
| Withycombe 2018 | Study population is not in complete remission; both on and off treatment |
| Wogksch 2019 | Same study population as Zhang 2018 |
| Wu 2018 | Type of outcome measure; validated questionnaire without published cut‐off score or normative data available |
| Yano 2016 | Not CCS; craniopharyngioma is not a malignant tumour |
| Yeh 2009 | Study population is not in complete remission; participants on treatment |
| Yi 2014 | Type of outcome measure; fatigue measured dichotomously |
| Zebrack 2002 | Type of outcome measure; validated questionnaire without published cut‐off score or normative data available |
| Zeller 2014b | Same study population as Hamre 2013a, Hamre 2013b, Kanellopoulos 2013, Zeller 2014a |
| Zeltzer 2009 | Study design: Review paper |
CCS: childhood cancer sufferer
Characteristics of studies awaiting assessment [ordered by study ID]
Frederick 2016.
| Methods | Study design: cross‐sectional study (part of longitudinal cohort study REACH) Instrument used to assess fatigue: Pediatric Quality of Life Multidimensional Fatigue Scale Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: NAa Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: survivors of a malignancy other than non‐melanomatous skin cancer, ≥ 2 years from cancer diagnosis, ≥ 1 year from completion of cancer therapy, willing to complete a yearly self‐reported survey of health outcomes, be able to complete forms independently in English Exclusion criteria: nm |
| Participants | Sample characteristics: N of participants original cohort: unknown; N of participants described study group: 268; N of participants study group of interest: 268; N of participants fatigue assessed: 268 Participant characteristics: Tumour type: leukaemia n = 94, HL n = 41, NHL n = 24, bone tumour n = 25, soft tissue sarcoma n = 20, neuroblastoma n = 27, Wilms tumour n = 20, other n = 17 Tumour stage: nm Age at diagnosis: median 6.4 years Time since diagnosis: mean 13.1 years (range 2 ‐ 46) Age at assessment: median 21.4 years (range 12 ‐ 49) F/M: 139/129 BMI: nm Race/ethnicity: White n = 235, African American n = 12, Hispanic n = 8, other n = 13 Marital status: nm Highest completed education level: nm Employment: nm Physical activity level: nm Sleeping problems: nm Psychosocial problems: depressed (Brief symptom inventory‐18): n = 14 Comorbidities/late effects: number of chronic health conditions: 0 n = 107, 1 ‐ 2 n = 123, 3 or more n = 36 asthma n = 55, cardiac condition n = 6, cataract n = 37, diabetes n = 6, elevated cholesterol n = 25, epilepsy (seizures) n = 6, gallstones n = 5, hepatitis n = 6, liver condition n = 7, migraines n = 26, osteoporosis n = 6, thyroid problem n = 46 Genetic factors/mutations: nm |
| Interventions | N of participants surgery: 117 N of participants chemotherapy: 239 N of participants radiotherapy: 171 N of participants BMT: 33 N of participants cranial irradiation: 84 Received chemotherapeutic agent: doxorubicin 194 |
| Outcomes | Severe fatigue: N of participants with severe fatigue: NDa Risk and associated factors: Several demographic and cancer related variables were investigated |
| Notes | Funding sources: nm Declaration of interest: The authors declare that they have no conflict of interest Unclear if this study meets the inclusion criteria 'at least 90% of the participants were under 18 at diagnosis' aAuthors defined fatigued participants as those who scored in the bottom quintile on the PedsQL MDF scale (most fatigued 20%) |
Griffith 2000.
| Methods | Study design: cross‐sectional study
Instrument used to assess fatigue: POMS fatigue subscale
Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: NAa Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: self‐indicated interest in college education by completion of the American College Testing examination between 1985 and 1996, had had a diagnosis of cancer between the ages of 12 and 17 years while living in Iowa, at least 18 years of age, be able to read English Exclusion criteria: nm |
| Participants | Sample characteristics: N of participants original cohort: unknown; N of participants described study group: 21; N of participants study group of interest: 21; N of participants fatigue assessed: 21 Participant characteristics: Tumour type: soft tumour n = 8, solid tumour n = 13, brain tumour n = 0 Tumour stage: nm Age at diagnosis: mean 16 years (SD 1.64) Time since diagnosis/end of therapy: nm Age at assessment: mean 21.5 years (SD 1.78) F/M: 18/3 BMI: nm Race/ethnicity: nm Marital status: nm Highest completed education level: nm, only which programme the participants were enrolled in Employment: nm Physical activity level: Karnofsky Performance Status Index: no problems n = 20, requires occasional assistance n = 1 Sleeping problems: nm Psychosocial problems: POMS depression mean score 7.48 (SD 7.79) Comorbidities: nm Genetic factors/mutations: nm |
| Interventions | N of participants surgery: nm N of participants chemotherapy: nm N of participants radiotherapy: nm |
| Outcomes | Severe fatigue: N of participants with severe fatigue: NDa Risk and associated factors: no analysis performed with fatigue as outcome |
| Notes | Funding sources: nm Declaration of interest: nm Unclear if this study meets the inclusion criteria 'in complete remission'. Contact details of the study author are not available. aAuthors report fatigue on continuous scale. |
Liu 2018.
| Methods | Study design: cross‐sectional study Instrument used to assess fatigue: Chinese version of Pediatric Patient‐Reported Outcomes Measurement Information System Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: T‐score ≥ 70 Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: children aged 8 ‐ 17 years, diagnosis of cancer either in treatment of in survivorshipa, able to speak and read Chinese, willing to participate in this study and have their parents' permission Exclusion criteria: diagnosed psychiatric condition or a cognitive impairment (e.g. visual) that would interfere with participation, declined or had a parent declined to participate, were receiving end‐of‐life care (life expectancy < 6 weeks) |
| Participants | Sample characteristics: N of participants original cohort: 304; N of participants described study group: 272; N of participants study group of interest: 76b; N of participants fatigue assessed: 74 Participant characteristics: Tumour type: leukaemia n = 62, lymphoma n = 7, solid tumours (brain tumours included) n = 5, others n = 2 Tumour stage: nm Age at diagnosis: nm, based on age at assessment, the maximum age at diagnosis is 17 years Time since end of therapy: nm Age at assessment: mean 12.5 years (SD 2.85; range 8 ‐ 17) F/M: nm BMI: nm Race/ethnicity: nm Marital status: nm Highest completed education level: nm Employment: nm Physical activity level: nm Sleeping problems: nm Psychosocial problems: nm Comorbidities/late effects: other health problem n = 7 Genetic factors/mutations: nm |
| Interventions | N of participants surgery: nm N of participants chemotherapy: nm N of participants radiotherapy: nm |
| Outcomes | Severe fatigue: N of participants with severe fatigue: NDc Risk and associated factors: no analysis performed with fatigue as outcome |
| Notes | Funding sources: This study was supported by the National Natural Science Foundation of China (project no. 71473262) and Intergovernmental International Cooperation Program of Science and Technology Commission of Shanghai Municipality, China (project no.18410711700) Declaration of interest: The authors have no conflicts of interest to disclose aDefinition of survivorship was requested through the corresponding author and is: who finished all the treatment and is in regular follow‐up? bDescribed study group contains both participants on treatment and in survivorship; only the data of the in survivorship group is extracted. cN of participants with severe fatigue is not reported in article. Additional information on severe fatigue was requested and not available. |
Lowe 2016.
| Methods | Study design: cross‐sectional study (part of mixed‐methods study, quantitative part is questionnaire survey) Instrument used to assess fatigue: POMS Fatigue subscale Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: NAa Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: diagnosed with cancer before age 18, being between 18 and 34 years oldb Exclusion criteria: nm |
| Participants | Sample characteristics: N of participants original cohort: unknown; N of participants described study group: 104; N of participants study group of interest: 104; N of participants fatigue assessed: 104 Participant characteristics: Tumour type: HL n = 24, NHL n = 9, Burkitts lymphoma n = 4, ALL n = 17, AML n = 3, blastoma n = 6, sarcoma n = 11, thyroid cancer n = 10, other n = 20 Tumour stage: nm Age at diagnosis: nm Time since diagnosis: mean 8.42 years (SD 5.73) Age at assessment: mean 22.13 (SD 3.18) F/M: 51/53 BMI: nm Race: White n = 82, black n = 21, other n = 1 Ethnicity: Hispanic or Latino n = 5, not Hispanic or Latino n = 99 Marital status: married/living with partner n = 19, other n = 85 Highest completed education level: nm Employment: employed n = 35, student n = 52, other n = 16c Physical activity level: ≥ 5 days of aerobic PA a week n = 52, ≥ 2 days of strength training a week n = 31 Sleeping problems: nm Psychosocial problems: POMS depression mean 4.36 (SD 5.05) Comorbidities: nm Genetic factors/mutations: nm |
| Interventions | N of participants chemotherapy: 86 N of participants surgery: 81 N of participants radiotherapy: 58 |
| Outcomes | Severe fatigue: N of participants with severe fatigue: NDa Risk and associated factors: no analysis performed with fatigue as outcome |
| Notes | Funding sources: Supported by the Georgia Cancer Coalition (PI: Berg), the National Cancer Institute (PI: Berg; 5K07CA139114), the Emory Egleston Children’s Research Center (PI: Esiashvili), and the Winship Cancer Institute Kennedy Survivorship Award (PI: Berg) Declaration of interest: The authors declare that they have no competing interests. aAuthors report fatigue on continuous scale. Additional information on severe fatigue was requested and not available. bStudy author confirmed that the study population had no evidence of disease at time of the study c Numbers do not add up to total N of described study group. Unclear what correct numbers were. |
Maunsell 2006.
| Methods | Study design: cross‐sectional study (part of Canadian Childhood Cancer Surveillance and Control Program) Instrument used to assess fatigue: Short‐Form 36 Vitality subscale Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: NAa Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: survived 5 years after diagnosis, between 1981 and 1990, primary cancer diagnosis before age 20 Exclusion criteria: nm |
| Participants | Sample characteristics: N of participants original cohort: unknown; N of participants described study group: 1334; N of participants study group of interest: 1334; N of participants fatigue assessed: 1303 Participant characteristics: Tumour type: leukaemia n = 333, lymphoma n = 312, CNS tumour n = 238, carcinoma n = 95, soft tissue cancer n = 89, bone cancer n = 78, germ cell and other gonadal cancer n = 75, kidney n = 66, other (retinoblastoma, neuroblastoma, hepatic cancer) n = 48 Tumour stage: nm Age at diagnosis: 0 ‐ 4 yrs n = 326, 5 ‐ 9 yrs n = 275, 10 ‐ 14 yrs n = 196, 15 ‐ 19 yrs n = 537 Time since diagnosis: 5 ‐ 9 yrs n = 220, 10 ‐ 14 yrs n = 684, 15 ‐ 19 yrs n = 430 Age at assessment: mean 23 years (SD 5.2; range 15 ‐ 37) F/M: 693/641 BMI: nm Race/ethnicity: ethnic background one or both parents white n = 1199, neither parent white n = 119, unknown n = 16 Marital status: nm Highest completed education level: nm Employment: nm Physical activity level: SF‐36 physical functioning mean score female 88.4, male 91.7 Sleeping problems: nm Psychosocial problems: nm Comorbidities/late effects: endocrine (diabetes, osteoporosis) 29.2%, hormonal (pituitary/growth, thyroid) 18.1%, neurological 43.9%, cardiovascular 12.7%, renal 11.5%, pulmonary 23.8% Genetic factors/mutations: nm |
| Interventions | N of participants surgery only: 224 N of participants chemotherapy only: 123 N of participants radiotherapy only: 38 N of participants chemotherapy + surgery: 216 N of participants chemotherapy + radiotherapy: 272 N of participants surgery + radiotherapy: 174 N of participants surgery, chemotherapy and radiotherapy: 263 N of participants no treatment or missing: 24 |
| Outcomes | Severe fatigue: N of participants with severe fatigue: NDa Risk and associated factors: Several demographic and cancer related variables were investigated |
| Notes | Funding sources: Supported by Health Canada Declaration of interest: The authors indicated no potential conflicts of interest Unclear if this study meets the inclusion criteria 'at least 90% of the participants were under 18 at diagnosis' aAuthors report fatigue on continuous scale. |
Meeske 2004.
| Methods | Study design: cross‐sectional study Instrument used to assess fatigue: Pediatric Quality of Life Multidimensional Fatigue Scale parent‐proxy Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: NAa Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: English‐ or Spanish‐speaking parents of children ages 2 ‐ 18 years with a diagnosis of brain tumour or ALL Exclusion criteria: families whose children were recently diagnosed (< 6 weeks ago), families in medical crisis |
| Participants | Sample characteristics: N of participants original cohort: unknown; N of participants described study group: 256; N of participants study group of interest: 103; N of participants fatigue assessed: 103b Participant characteristics: Tumour type: ALL n = 53, brain tumour n = 50 Tumour stage: nm Age at diagnosis: nm, based on age at assessment, the maximum age at diagnosis is 18 years Time since end of therapy: no treatment for < 12 months n = 52, no treatment for at least 12 months n = 51 Age at assessment: nm, max 18 years F/M: nm BMI: nm Race/ethnicity: nm Marital status: NA Highest completed education level: nm Employment: NA Physical activity level: nm Sleeping problems: nm Psychosocial problems: nm Comorbidities: nm Genetic factors/mutations: nm |
| Interventions | N of participants surgery: nm N of participants chemotherapy: nm N of participants radiotherapy: nm |
| Outcomes | Severe fatigue: N of participants with severe fatigue: NDa Risk and associated factors: no analysis performed with fatigue as outcome |
| Notes | Funding sources: Supported by the Kellerman Foundation Declaration of interest: nm NA: not applicable; ND: no data available aAuthors report fatigue on a continuous scale. Additional information on severe fatigue was requested and not available. bOnly data of the participants off treatment were extracted |
Meeske 2007.
| Methods | Study design: cross‐sectional study (retrospective questionnaire survey) Instrument used to assess fatigue: 1 item, adapted from the National Comprehensive Cancer Network clinical practice guidelines that asks the child to rate his/her fatigue over the past 4 weeks Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: NAa Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: cancer‐free, at least 5 years from diagnosis, off treatment for a minimum of 2 years, aged 8 ‐ 18 years Exclusion criteria: incomplete data, Downs syndrome |
| Participants | Sample characteristics: N of participants original cohort: unknown; N of participants described study group: 86; N of participants study group of interest: 86; N of participants fatigue assessed: 77 Participant characteristics: Tumour type: leukaemia n = 31, lymphoma n = 16, WT n = 10, brain tumour n = 12, retinoblastoma n = 4, neuroblastoma n = 3, rhabdomyosarcoma n = 5, germ cell tumour n = 3, hepatoblastoma n = 2 Tumour stage: nm Age at diagnosis: mean 4.0 years (SD 2.6), based on age at assessment, the maximum age at diagnosis is 18 years Time since end of therapy: mean 7.8 years (SD 2.5) Age at assessment: mean 13.3 years (SD 2.9) F/M: 35/51 BMI: nm Race/ethnicity: White n = 29, Hispanic n = 41, other n = 16 Marital status: NA Highest completed education level: NA Employment: NA Physical activity level: nm Sleeping problems: nm Psychosocial problems: nm Comorbidities/late effects: mild n = 36, moderate n = 27, severe n = 23 Genetic factors/mutations: nm |
| Interventions | N of participants chemotherapy: 30 N of participants surgery: 4 N of participants surgery + radiotherapy: 2 N of participants surgery + chemotherapy: 22 N of participants chemotherapy + radiotherapy: 9 N of participants surgery, chemotherapy and radiotherapy: 19 N of participants cranial irradiation: 13 |
| Outcomes | Severe fatigue: N of participants with severe fatigue: NDa Risk and associated factors: no analysis performed with fatigue as outcome |
| Notes | Funding sources: Dr. Meeske is supported by a NCI training grant Declaration of interest: nm aAuthors report moderate to severe fatigue (score ≥ 4). Additional information on severe fatigue was requested and not available. |
Robert 2012.
| Methods | Study design: cross‐sectional study Instrument used to assess fatigue: PedsQL Multidimensional Fatigue Scale Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: NAa Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: English‐ or Spanish‐speaking survivor of childhood cancer, > 2 years after treatment, participating in MD Anderson Survivorship Clinic, at least 25 years of age, completed adapted versions of the PedsQL Exclusion criteria: cognitive impairment |
| Participants | Sample characteristics: N of participants original cohort: unknown; N of participants described study group: 64; N of participants study group of interest: 64; N of participants fatigue assessed: 64 Participant characteristics: Tumour type: CNS tumour n = 9, haematological/leukaemia n = 11, lymphoma n = 11, solid tumour n = 33 Tumour stage: nm Age at diagnosis: mean 9.6 years (SD 5.3; range 1 ‐ 21)b Time since diagnosis: mean 25.2 years (SD 9.3; range 5 ‐ 43) Age at assessment: mean 34.5 years (SD7.4; range 25 ‐ 53) F/M: 38/26 BMI: nm Race/ethnicity: Asian n = 2, black n = 5, Hispanic n = 15, white n = 42 Marital status: nm Highest completed education level: nm Employment: nm Physical activity level: PedsQL physical health subscale mean 73.8 (SD 25.0) Sleeping problems: nm Psychosocial problems: nm Comorbidities: nm Genetic factors/mutations: nm |
| Interventions | N of participants surgery: nm N of participants chemotherapy: nm N of participants radiotherapy: nm |
| Outcomes | Severe fatigue: N of participants with severe fatigue: NDa Risk and associated factors: no analysis performed with fatigue as outcome |
| Notes | Funding sources: supported by the Astros’ Baseball Team Long‐Term Survivor Fund and the National Institutes of Health through MD Anderson’s Cancer Center Support Grant CA016672 Declaration of interest: Nothing to report aAuthors report fatigue on continuous scale. Additional information on severe fatigue was requested and not available. bInformation received from the study author: 62 of the 64 participants (96%) were under 18 at diagnosis. |
Veneroni 2017.
| Methods | Study design: cross‐sectional study Instrument used to assess fatigue: EORTC‐QLQ‐C30 symptom scale fatigue Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: NAa Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: diagnosis with metastatic medulloblastoma, completed therapy at least 36 months before their enrolment, no evidence of relapse Exclusion criteria: already had a neurological or psychiatric disorder unrelated to their medulloblastoma |
| Participants | Sample characteristics: N of participants original cohort: unknown; N of participants described study group: 25; N of participants study group of interest: 25; N of participants fatigue assessed: 17 Participant characteristics: Tumour type: medulloblastoma n = 25 Tumour stage: nm Age at diagnosis: median 10.8 years (IQR 7 ‐ 13.9) Time since end of therapy: median 12.6 years (IQR 7.4 ‐ 14.9) Age at assessment: median 23.7 years (IQR 18.9 ‐ 27.4) F/M: 21/4 BMI: nm Race/Ethnicity: nm Marital status: nm Highest completed education level: nm Employment: nm Physical activity level: EORTC physical functioning median 80.0 (IQR 54 ‐ 87) Sleeping problems: EORTC symptom scale insomnia median 0.0 (IQR 0.0 ‐ 33.3) Psychosocial problems: nm Comorbidities/late effects: nm Genetic factors/mutations: nm |
| Interventions | N of participants surgery: nm N of participants chemotherapy: 25 N of participants radiotherapy: 25 Exact type of cancer treatment is unclear, population description states that participants received chemotherapy and hyperfractionated accelerated radiotherapy (HART) |
| Outcomes | Severe fatigue: N of participants with severe fatigue: NDa Risk and associated factors: no analysis performed with fatigue as outcome |
| Notes | Funding sources: nm Declaration of interest: On behalf of all authors, the corresponding author states that there is no conflict of interest aAuthors report fatigue on continuous scale. Additional data on severe fatigue was requested and not available. |
Wu 2019.
| Methods | Study design: cross‐sectional study Instrument used to assess fatigue: Fatigue scale for adolescent with cancer Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: unknown Time points at which outcome data were collected: Inclusion criteria: be 13 ‐ 18 years old, actively receiving cancer treatment or have already completed cancer treatment, be able to speak and read Mandarin Chinese, be able to understand the study information Exclusion criteria: nm |
| Participants | Sample characteristics: N of participants original cohort: unknown; N of participants described study group: 100; N of participants study group of interest: 62a; N of participants fatigue assessed: 62 Participant characteristics: Tumour type: leukaemia n = 36, lymphoma n = 14, solid tumours n = 12 Tumour stage: nm Age at diagnosis: nm, based on age at assessment, the maximum age at diagnosis is 18 years Time since diagnosis and/or end of therapy: nm Age at assessment: mean age 16.45 years (SD 1.90; range 13 ‐ 18) F/M: 28/34 BMI: nm Race/ethnicity: nm Marital status: nm Highest completed education level: current education: elementary and junior high school n =16, senior high school n = 37, college or university n = 9 Employment: nm Physical activity level: nm Sleeping problems: Sleep quality: Total score Pittsburgh sleep quality index mean 3.82 (SD 2.92) Psychosocial problems: nm Comorbidities: nm Genetic factors/mutations: nm |
| Interventions | Type of cancer treatment is not mentioned |
| Outcomes | Severe fatigue: N of participants with severe fatigue: NA Risk and associated factors: no analysis performed with severe fatigue as outcome |
| Notes | Funding sources: Funded by the Ministry of Science and Technology, Taiwan (grant no. MOST 103‐2314‐B‐002‐192‐MY3) Declaration of interest: The authors have no conflicts of interest to disclose Unclear if the questionnaire meets inclusion criteria 'scoring above a published cut‐off score on a validated or non‐validated fatigue questionnaire' aDescribed study group contains both participants on treatment and off treatment; only the data of the off treatment group are extracted. |
Zeltzer 1997.
| Methods | Study design: cross‐sectional study Instrument used to assess fatigue: POMS fatigue subscale Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: NAa Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: diagnosis of ALL on or after 1970, treatment on a Children's Cancer Group (CCG) protocol before age 20 years, age at least 18 years by 15 October 1990, survival for at least 2 years after diagnosis, alive, in remission, not receiving antileukaemia treatment at study entry Exclusion criteria: unable to be interviewed (Down syndrome, drug‐dependent, non‐English‐speaking, brain damage) |
| Participants | Sample characteristics: N of participants original cohort: unknown; N of participants described study group: 580; N of participants study group of interest: 580; N of participants fatigue assessed: 552 Participant characteristics: Tumour type: nm Tumour stage: nm Age at diagnosis: nm Time since diagnosis and/or end of therapy: nm Age at assessment: mean age 22.6 years (SD 3.2; range 18.02 ‐ 33.25) F/M: 287/293 BMI: nm Race/ethnicity: White n = 511, minorities n = 69 Marital status: never married n = 395, married n = 150, separated/divorced n = 35 Highest completed education level: nm Employment: unemployed n = 56, student n = 72, < 20 hours/wk n = 65, 20 ‐ 34 hours/wk n = 77, ≥ 35 hours/wk n = 282, keeping house n = 13, other n = 15 Physical activity level: nm Sleeping problems: nm Psychosocial problems: POMS depression scale mean 8.95 (SD 9.09) Comorbidities: nm Genetic factors/mutations: nm |
| Interventions | Type of cancer treatment is not mentioned |
| Outcomes | Severe fatigue: N of participants with severe fatigue: NDa Risk and associated factors: no analysis performed with severe fatigue as outcome |
| Notes | Funding sources: Supported by the Division of Cancer Treatment, National Cancer Institute, National Institutes of Health, Department of Health and
Human Services, Bethesda, MD Declaration of interest: nm Unclear if this study meets the inclusion criteria 'at least 90% of the participants were under 18 at diagnosis' Information provided by the study author: additional data are no longer available aAuthors report fatigue on continuous scale. |
Zhang 2018.
| Methods | Study design: cross‐sectional study (part of St. Jude Lifetime Cohort study) Instrument used to assess fatigue: Short Form 36 Vitality subscale Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: NAa Time points at which outcome data were collected: NA, cross‐sectional study Inclusion criteria: diagnosis of childhood malignancy treated at St. Jude Childrens's Research Hospital, survival of ≥ 10 years from diagnosis, current age ≥ 18 years Exclusion criteria: unreliable reporting for dietary intake |
| Participants | Sample characteristics: N of participants original cohort: unknown; N of participants described study group: 2480; N of participants study group of interest: 2480; N of participants fatigue assessed: 2480 Participant characteristics: Tumour type: leukaemia n = 952, lymphoma n = 488, embryonal tumours n = 324, sarcoma n = 311, CNS tumour n = 229, other n = 172 b Tumour stage: nm Age at diagnosis: mean 8.3 years (SD 5.6) Time since diagnosis: mean 24.1 years (SD 8.1) Age at assessment: mean 32.3 years (SD 8.3) F/M: 1205/1275 BMI: nm Race/ethnicity: nm Marital status: nm Highest completed education level: nm Employment: nm Physical activity level: nm Sleeping problems: nm Psychosocial problems: nm Comorbidities/late effects: nm Genetic factors/mutations: nm |
| Interventions | N of participants surgery: nm N of participants chemotherapy: nm N of participants alkylating agents: 1567 N of participants anthracyclines: 1444 N of participants antimetabolites: 1320 N of participants glucocorticoids: 1226 N of participants radiotherapy: 1487 N of participants head/neck radiotherapy only: 664 N of participants head/neck and chest radiotherapy: 158 N of participants head/neck, chest and abdomen/pelvis radiotherapy: 442 N of participants chest with or without abdomen/pelvis radiotherapy: 172 |
| Outcomes | Severe fatigue: N of participants with severe fatigue: NDa Risk and associated factors: Several demographic and health‐related variables were investigated |
| Notes | Funding sources: Supported by National Institutes of Health/National Cancer Institute grant 1R03CA199516‐01 (to Fang Fang Zhang), National Institutes of Health/National Cancer Institute grant U01CA194457 (to Melissa M. Hudson and Leslie L. Robison), and the American Lebanese Syrian Associated Charities. Declaration of interest: The authors made no disclosures Unclear if this study meets the inclusion criterion 'at least 90% of the participants were under 18 at diagnosis' aAuthors report fatigue on continuous scale. bAt different locations, different numbers are presented. The numbers that are presented here come from Table 1 of the study report. |
ALL: acute lymphoblastoma leukaemia; AML: acute myeloid leukaemia; BMI: body mass index: F: female; HL: Hodgkins lymphoma; NHL: non‐Hodgkins lymphoma; M: male; PA: physical activity; POMS: Profile of mood states; SD: standard deviation
Characteristics of ongoing studies [ordered by study ID]
Kunin‐Batson 2015.
| Trial name or title | Healthy kids after cancer: a physical activity and nutrition intervention |
| Methods | Study design: randomised controlled trial (RCT) Instrument used to assess fatigue: PedsQL multidimensional fatigue scale Validated questionnaire: yes Cut‐off score or criterion for severe fatigue: NA Time points at which outcome data were collected: 2 time points, start and 6 months after start Inclusion criteria: between 4 and < 11 years of age at the time of recruitment, previously diagnosed with ALL and currently in remission, between 1 ‐ 5 years post‐completion of chemotherapy, BMI ≥ 85 percentile for age and sex or at risk for obesity Exclusion criteria: history of cranial radiation therapy, history of bone marrow transplant, history of relapse of ALL, diagnosis of Down Syndrome, comorbidities of obesity that require immediate subspecialist referral, significant pulmonary, cardiovascular, orthopaedic, or musculoskeletal problems that would limit ability to participate in physical activity, significant psychiatric or neurologic disorders that would impair compliance with study protocol, current participation in a weight‐loss programme |
| Participants | NA |
| Interventions | NA |
| Outcomes | NA |
| Starting date | February 2015 |
| Contact information | Principal Investigator: Alicia S Kunin‐Batson, PhD University of Minnesota ‐ Clinical and Translational Science Institute |
| Notes | clinicaltrials.gov/ct2/show/nct02361047 |
Differences between protocol and review
Objectives: We removed the sentence "We will not include studies that assess the genetic basis of severe fatigue" from the review. In the final peer review of the protocol stage, we reconsidered the decision to exclude studies that assess the genetic basis of severe fatigue, and we decided to include genetic factors as a possible risk factor for fatigue.
Types of participants: For clarification on how the inclusion criterion 'in complete remission' was assessed, we added the following sentence to the Types of participants section: We interpreted 'complete remission' as participants being off treatment or having no active disease; or a recurrence or second malignancy; or had no evidence of the disease at the time of the study; or visited a long‐term follow‐up clinic; or had a mean time since diagnosis of at least five years
Primary outcome: When designing the protocol, we had intended to exclude studies which used a questionnaire without having a published cut‐off score. However, we found several relevant publications that assessed fatigue with a questionnaire without a published cut‐off score, but with available normative data from a healthy reference group, and thus provided additional data relevant to the aims of this review. These questionnaires were not designed to have a cut‐off score and would otherwise be excluded from the review. With the addition of normative data to the definition of severe fatigue, we aimed to provide a better and more complete overview of severe fatigue after treatment for childhood cancer.
If an included study performed fatigue assessment in a control group, we also extracted the prevalence of severe fatigue in that control group. This enabled us to compare the prevalence of severe fatigue of childhood cancer survivors with controls.
Secondary outcomes: In the original protocol we stated that cross‐sectional studies cannot inform us about risk factors for fatigue, but only about associations with fatigue. However, gender, ethnicity, and treatment‐related variables that are assessed in cross‐sectional studies do provide information about the risk of fatigue. These variables were measured in the past and can not be changed. We therefore corrected this sentence in the Methods section of the review.
During the data extraction phase it became clear that only four of the 30 included studies used severe fatigue (as we defined it in the Methods section) as an outcome variable in their analysis of risk and associated factors. Because other studies (n = 15) did perform analyses with fatigue as the outcome, we decided to extract data from these studies as well. These results provided information about a possible association and risk factors for fatigue. We therefore changed the outcome for the secondary objective from 'Severe fatigue' to 'Fatigue' in the description of risk and associated factors in the Methods section: Types of outcome measures.
Searching other resources: We screened all conference proceedings electronically. We added experts in the field as a source for possible eligible studies.
Assessment of risk of bias: In the protocol we stated that for case‐control studies we would slightly adapt the 'Risk of bias' criteria as described in the module of Cochrane Childhood Cancer. We only found one case‐control study, and to be able to compare risks of bias between studies we decided to assess risks of bias for this case‐control study with the modified checklist for observational studies.
'Risk of bias' domain confounding: We found no definite prognostic factors during the writing of the protocol and we also found no strong evidence for potentially important prognostic factors. To avoid confusion for the readers of this review, we slightly modified the 'Risk of bias' domain of confounding, and changed it to 'possibly important prognostic factors'.
Table 1: 'Risk of bias' assessment for observational studies: for clarification, we added short explanations of our interpretation of the 'Risk of bias' items to Table 1.
Subgroup analyses: We added a subgroup based on age at assessment to the Methods section, because several studies noted that there might be a difference in fatigue between several age groups of cancer survivors (13 to 18 years versus more than 18 years, Johannsdottir 2012; 15 to 29 years versus 30 to 70 years, Heutte 2009).
Contributions of authors
SvD: identified studies meeting inclusion criteria, searched conference proceedings and reference lists of included articles for eligible studies, performed data extraction and 'Risk of bias' assessment, analysed the data and interpreted the results, wrote and revised the manuscript.
AB: developed and wrote protocol, developed the search strategies (together with the Information Specialist of Cochrane Childhood Cancer), identified studies meeting inclusion criteria, performed data extraction and 'Risk of bias' assessment, critically reviewed the manuscript.
JL, HK, EDU: developed and wrote the protocol, developed the search strategies (together with the Information Specialist of Cochrane Childhood Cancer), act as arbiter in case of disagreement, interpreted the results and critically reviewed the manuscript.
NB: contributed to the development of the protocol and critically reviewed the manuscript.
All authors approved the final version.
Sources of support
Internal sources
No sources of support supplied
External sources
Dutch Cancer Society, Netherlands.
Declarations of interest
Review author Eline van Dulmen‐den Broeder is a co‐author of the included study Van Dijk 2008.
None of the other review authors are co‐authors of any of the included studies.
New
References
References to studies included in this review
Barrera 2012 {published data only}
- Barrera M, Teall T, Barr R, Silva M, Greenberg M. Health related quality of life in adolescent and young adult survivors of lower extremity bone tumors. Pediatric Blood & Cancer 2012;58(2):265‐73. [DOI] [PubMed] [Google Scholar]
Berbis 2013 {published and unpublished data}
- Berbis J, Michel G, Chastagner P, Sirvent N, Demeocq F, Plantaz D, et al. A French cohort of childhood leukemia survivors: impact of hematopoietic stem cell transplantation on health status and quality of life. Biology of Blood and Marrow Transplantion 2013;19(7):1065‐72. [DOI] [PubMed] [Google Scholar]
Brand 2016 {published and unpublished data}
- Brand SR, Chordas C, Liptak C, Manley P, Recklitis C. Screening for fatigue in adolescent and young adult pediatric brain tumor survivors: accuracy of a single‐item screening measure. Supportive Care in Cancer 2016;24(8):3581‐7. [DOI] [PubMed] [Google Scholar]
Calaminus 2014 {published and unpublished data}
- Calaminus G, Dorffel W, Baust K, Teske C, Riepenhausen M, Bramswig J, et al. Quality of life in long‐term survivors following treatment for Hodgkin's disease during childhood and adolescence in the German multicentre studies between 1978 and 2002. Supportive Care in Cancer 2014;22(6):1519‐29. [DOI] [PubMed] [Google Scholar]
Cheung 2017 {published data only}
- Cheung YT, Brinkman TM, Mulrooney DA, Mzayek Y, Liu W, Banerjee P, et al. Impact of sleep, fatigue, and systemic inflammation on neurocognitive and behavioral outcomes in long‐term survivors of childhood acute lymphoblastic leukemia. Cancer 2017;123(17):3410‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crom 1999 {published data only}
- Crom DB, Chathaway DK, Tolley EA, Mulhern RK, Hudson MM. Health status and health‐related quality of life in long‐term adult survivors of pediatric solid tumors. International Journal of Cancer 1999;12:25‐31. [DOI] [PubMed] [Google Scholar]
Daniel 2019 {published and unpublished data}
- Daniel LC, Wang M, Srivastava D, Schwartz L, Brinkman T, Edelstein K, et al. Sleep, emotional distress, and physical health in survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Psycho‐Oncology 2019;28(4):903‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gordijn 2013 {published data only}
- Gordijn MS, Litsenburg RR, Gemke RJ, Huisman J, Bierings MB, Hoogerbrugge PM, et al. Sleep, fatigue, depression, and quality of life in survivors of childhood acute lymphoblastic leukemia. Pediatric Blood & Cancer 2013;60(3):479‐85. [DOI] [PubMed] [Google Scholar]
Hamre 2013a {published data only}
- Hamre H, Zeller B, Kanellopoulos A, Kiserud CE, Aakhus S, Lund MB, et al. High prevalence of chronic fatigue in adult long‐term survivors of acute lymphoblastic leukemia and lymphoma during childhood and adolescence. Journal of Adolescent and Young Adult Oncology 2013;2(1):2‐9. [Google Scholar]
Harila 2010 {published and unpublished data}
- Harila MJ, Salo J, Lanning M, Vilkkumaa I, Harila‐Saari AH. High health‐related quality of life among long‐term survivors of childhood acute lymphoblastic leukemia. Pediatric Blood & Cancer 2010;55(2):331‐6. [DOI] [PubMed] [Google Scholar]
Ho 2019 {published data only}
- Ho KY, Li WH, Lam KW, Wei X, Chiu SY, Chan CG, et al. Relationships among fatigue, physical activity, depressive symptoms, and quality of life in Chinese children and adolescents surviving cancer. European Journal of Oncology Nursing 2019;38:21‐7. [DOI] [PubMed] [Google Scholar]
Johannsdottir 2012 {published data only}
- Johannsdottir IM, Hjermstad MJ, Moum T, Wesenberg F, Hjorth L, Schroder H, et al. Increased prevalence of chronic fatigue among survivors of childhood cancers: A population‐based study. Pediatric Blood & Cancer 2012;58(3):415‐20. [DOI] [PubMed] [Google Scholar]
Kenney 2010 {published data only}
- Kenney LB, Nancarrow CM, Najita J, Vrooman LM, Rothwell M, Recklitis C, et al. Health status of the oldest adult survivors of cancer during childhood. Cancer 2010;116(2):497‐505. [DOI] [PubMed] [Google Scholar]
Khan 2014 {published data only}
- Khan RB, Hudson MM, Ledet DS, Morris EB, Pui CH, Howard SC, et al. Neurologic morbidity and quality of life in survivors of childhood acute lymphoblastic leukemia: a prospective cross‐sectional study. Journal of Cancer Survivorship 2014;8(4):688‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Langeveld 2003 {published data only}
- Langeveld NE, Grootenhuis MA, Voute PA, Haan RJ, Bos C. No excess fatigue in young adult survivors of childhood cancer. European Journal of Cancer 2003;39(2):204‐14. [DOI] [PubMed] [Google Scholar]
Lopez 2011 {published and unpublished data}
- Lopez‐Guerra JL, Marquez‐Vega C, Praena‐Fernandez JM, Ramirez‐Villar GL, Ordonez R, Cabrera P, et al. Health related quality of life and late side effects of long‐term survivors of Ewing's sarcoma of bone. Journal of B.U.O.N. 2011;16(3):528‐36. [PubMed] [Google Scholar]
Meeske 2005 {published data only}
- Meeske KA, Siegel SE, Globe DR, Mack WJ, Bernstein L. Prevalence and correlates of fatigue in long‐term survivors of childhood leukemia. Journal of Clinical Oncology 2005;23(24):5501‐10. [DOI] [PubMed] [Google Scholar]
Mört 2011 {published data only}
- Mört S, Lähteenmäki PM, Matomäki J, Salmi TT, Salanterä S. Fatigue in young survivors of extracranial childhood cancer: A Finnish nationwide survey. Oncology Nursing Forum 2011;38(6):E445‐54. [DOI] [PubMed] [Google Scholar]
Mulrooney 2008 {published data only}
- Mulrooney DA, Ness KK, Neglia JP, Whitton JA, Green DM, Zeltzer LK, et al. Fatigue and sleep disturbance in adult survivors of childhood cancer: A report from the childhood cancer survivor study (CCSS). Sleep 2008;31(2):271‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pemberger 2005 {published data only}
- Pemberger S, Jagsch R, Frey E, Felder‐Puig R, Gadner H, Kryspin‐Exner I, et al. Quality of life in long‐term childhood cancer survivors and the relation of late effects and subjective well‐being. Supportive Care in Cancer 2005;13(1):49‐56. [DOI] [PubMed] [Google Scholar]
Puhr 2019 {published and unpublished data}
- Puhr A, Ruud E, Anderson V, Due‐Tonnesen BJ, Skarbo AB, Finset A, et al. Self‐reported executive dysfunction, fatigue, and psychological and emotional symptoms in physically well‐functioning long‐term survivors of pediatric brain tumor. Developmental Neuropsychology 2019;44(1):88‐103. [DOI] [PubMed] [Google Scholar]
Reulen 2007 {published and unpublished data}
- Reulen RC, Winter DL, Lancashire ER, Zeegers MP, Jenney ME, Walters SJ, et al. Health‐status of adult survivors of childhood cancer: a large‐scale population‐based study from the British Childhood Cancer Survivor Study. International Journal of Cancer 2007;121(3):633‐40. [DOI] [PubMed] [Google Scholar]
Ruccione 2013 {published data only}
- Ruccione K, Lu Y, Meeske K. Adolescents' psychosocial health‐related quality of life within 6 months after cancer treatment completion. Cancer Nursing 2013;36(5):E61‐72. [DOI] [PubMed] [Google Scholar]
Rueegg 2013 {published data only}
- Rueegg CS, Gianinazzi ME, Rischewski J, Beck Popovic M, Weid NX, Michel G, et al. Health‐related quality of life in survivors of childhood cancer: the role of chronic health problems. Journal of Cancer Survivorship 2013;7(4):511‐22. [DOI] [PubMed] [Google Scholar]
Sundberg 2013 {published and unpublished data}
- Sundberg KK, Wettergren L, Frisk P, Arvidson J. Self‐reported quality of life in long‐term survivors of childhood lymphoblastic malignancy treated with hematopoietic stem cell transplantation versus conventional therapy. Pediatric Blood & Cancer 2013;60(8):1382‐7. [DOI] [PubMed] [Google Scholar]
Tremolada 2018 {published and unpublished data}
- Tremolada M, Bonichini S, Taverna L, Basso G, Pillon M. Health‐related quality of life in AYA cancer survivors who underwent HSCT compared with healthy peers. European Journal of Cancer Care 2018;27(6):e12878. [DOI] [PubMed] [Google Scholar]
Van Dijk 2008 {published and unpublished data}
- Dijk EM, Dulmen‐den Broeder E, Kaspers GJ, Dam EW, Braam KI, Huisman J. Psychosexual functioning of childhood cancer survivors. Psycho‐oncology 2008;17(5):506‐11. [DOI] [PubMed] [Google Scholar]
Verberne 2012 {published and unpublished data}
- Verberne LM, Maurice‐Stam H, Grootenhuis MA, Santen HM, Schouten‐Van Meeteren AY. Sleep disorders in children after treatment for a CNS tumour. Journal of Sleep Research 2012;21(4):461‐9. [DOI] [PubMed] [Google Scholar]
Wright 2013 {published data only}
- Wright M, Bryans A, Gray K, Skinner L, Verhoeve A. Physical activity in adolescents following treatment for cancer: Influencing factors. Leukemia Research and Treatment 2013;2013:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zeller 2014a {published data only}
- Zeller B, Loge JH, Kanellopoulos A, Hamre H, Wyller VB, Ruud E. Chronic fatigue in long‐term survivors of childhood lymphomas and leukemia: Persistence and associated clinical factors. Journal of Pediatric Hematology/Oncology 2014;36(6):438‐44. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adams 2004 {published data only}
- Adams MJ, Lipsitz SR, Colan SD, Tarbell NJ, Treves ST, Diller L, et al. Cardiovascular status in long‐term survivors of Hodgkin's disease treated with chest radiotherapy. Journal of Clinical Oncology 2004;22(15):3139‐48. [DOI] [PubMed] [Google Scholar]
Al‐Gamal 2016 {published data only}
- Al‐Gamal E, Long T. Health‐related quality of life and its association with self‐esteem and fatigue among children diagnosed with cancer. Journal of Clinical Nursing 2016;25(21‐22):3391‐9. [DOI] [PubMed] [Google Scholar]
Ander 2016 {published data only}
- Ander M, Grönqvist H, Cernvall M, Engvall G, Hedström M, Ljungman G, et al. Development of health‐related quality of life and symptoms of anxiety and depression among persons diagnosed with cancer during adolescence: a 10‐year follow‐up study. Psycho‐oncology 2016;25(5):582‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anestin 2018 {published data only}
- Anestin AS, Lippe S, Robaey P, Bertout L, Drouin S, Krajinovic M, et al. Psychological risk in long‐term survivors of childhood acute lymphoblastic leukemia and its association with functional health status: A PETALE cohort study. Pediatric Blood & Cancer 2018;65(11):e27356. [DOI] [PubMed] [Google Scholar]
Arpaci 2016 {published data only}
- Arpaci T, Kilicarslan Toruner E. Assessment of problems and symptoms in survivors of childhood acute lymphoblastic leukaemia. European Journal of Cancer Care 2016;25(6):1034‐43. [DOI] [PubMed] [Google Scholar]
Arroyave 2008 {published data only}
- Arroyave WD, Clipp EC, Miller PE, Jones LW, Ward DS, Bonner MJ, et al. Childhood cancer survivors' perceived barriers to improving exercise and dietary behaviors. Oncology Nursing Forum 2008;35(1):121‐30. [DOI] [PubMed] [Google Scholar]
Berg 2009 {published data only}
- Berg C, Neufeld P, Harvey J, Downes A, Hayashi RJ. Late effects of childhood cancer, participation, and quality of life of adolescents. OTJR Occupation, Participation and Health 2009;29(3):116‐24. [Google Scholar]
Berg 2013 {published data only}
- Berg C, Hayashi RJ. Participation and self‐management strategies of young adult childhood cancer survivors. OTJR Occupation, Participation and Health 2013;33(1):21‐30. [Google Scholar]
Bower 2019 {published data only}
- Bower JE. The role of neuro‐immune interactions in cancer‐related fatigue: Biobehavioral risk factors and mechanisms. Pediatric Blood & Cancer 2019;125(3):353‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burghardt 2019 {published data only}
- Burghardt J, Klein E, Brähler E, Ernst M, Schneider A, Eckerle S, et al. Prevalence of mental distress among adult survivors of childhood cancer in Germany—Compared to the general population. Cancer Medicine 2019;8(4):1865‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carlson 2017 {published data only}
- Carlson LE, Zelinski EL, Speca M, Balneaves LG, Jones JM, Santa Mina D, et al. Protocol for the MATCH study (Mindfulness and Tai Chi for cancer health): A preference‐based multi‐site randomized comparative effectiveness trial (CET) of Mindfulness‐Based Cancer Recovery (MBCR) vs. Tai Chi/Qigong (TCQ) for cancer survivors. Contemporary Clinical Trials 2017;59:64‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2017 {published data only}
- Chang E, Goldsby R, Mueller S, Banerjee A. Late effects of treatment and palliative care. In: Gupta N, Banerjee A, Haas‐Kogan DA editor(s). Pediatric CNS Tumors. Cham: Springer International Publishing, 2017:365‐87. [Google Scholar]
Clanton 2011 {published data only}
- Clanton NR, Klosky JL, Li C, Jain N, Srivastava DK, Mulrooney D, et al. Fatigue, vitality, sleep, and neurocognitive functioning in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer 2011;117(11):2559‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cox 2009 {published data only}
- Cox CL, Montgomery M, Oeffinger KC, Leisenring W, Zeltzer L, Whitton JA, et al. Promoting physical activity in childhood cancer survivors: results from the Childhood Cancer Survivor Study. Cancer 2009;115(3):642‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Daniel 2016 {published data only}
- Daniel L, Kazak AE, Li Y, Hobbie W, Ginsberg J, Butler E, et al. Relationship between sleep problems and psychological outcomes in adolescent and young adult cancer survivors and controls. Supportive Care in Cancer 2016;24(2):539‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Ruiter 2016 {published data only (unpublished sought but not used)}
- Ruiter MA, Schouten‐van Meeteren AY, Vuurden DG, Maurice‐Stam H, Gidding C, Beek LR, et al. Psychosocial profile of pediatric brain tumor survivors with neurocognitive complaints. Quality of Life Research 2016;25(2):435‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Enskar 2007 {published data only}
- Enskar K, Essen L. Prevalence of aspects of distress, coping, support and care among adolescents and young adults undergoing and being off cancer treatment. European Journal of Oncology Nursing 2007;11(5):400‐8. [DOI] [PubMed] [Google Scholar]
Finnegan 2009 {published data only}
- Finnegan L, Campbell RT, Ferrans CE, Wilbur J, Wilkie DJ, Shaver J. Symptom cluster experience profiles in adult survivors of childhood cancers. Journal of Pain and Symptom Management 2009;38(2):258‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fortmann 2018 {published data only}
- Fortmann J, Fisher A, Hough R, Gregory A, Pugh G. Sleep quality, fatigue, and quality of life among teenage and young adult cancer survivors. Journal of Adolescent and Young Adult Oncology 2018;7(4):465‐71. [DOI] [PubMed] [Google Scholar]
Geenen 2007 {published data only}
- Geenen MM, Cardous‐Ubbink MC, Kremer LC, Bos C, Pal HJ, Heinen RC, et al. Medical assessment of adverse health outcomes in long‐term survivors of childhood cancer. JAMA 2007;297(24):2705‐15. [DOI] [PubMed] [Google Scholar]
Gordijn 2012 {published data only}
- Gordijn MS, Litsenburg RR, Gemke RJ, Bierings MB, Hoogerbrugge PM, Ven PM, et al. Hypothalamic‐pituitary‐adrenal axis function in survivors of childhood acute lymphoblastic leukemia and healthy controls. Psychoneuroendocrinology 2012;37(9):1448‐56. [DOI] [PubMed] [Google Scholar]
Graef 2016 {published data only}
- Graef DM, Phipps S, Parris KR, Martin‐Elbahesh K, Huang L, Zhang H, et al. Sleepiness, fatigue, behavioral functioning, and quality of life in survivors of childhood hematopoietic stem cell transplant. Journal of Pediatric Psychology 2016;41(6):600‐9. [DOI] [PubMed] [Google Scholar]
Hamre 2013b {published data only}
- Hamre H, Zeller B, Kanellopoulos A, Ruud E, Fosså SD, Loge JH, et al. Serum cytokines and chronic fatigue in adults surviving after childhood leukemia and lymphoma. Brain, Behavior, and Immunity 2013;30:80‐7. [DOI] [PubMed] [Google Scholar]
Henderson 2018 {published data only}
- Henderson JR, Kiernan E, McNeer JL, Rodday AM, Spencer K, Henderson TO, et al. Patient‐reported health‐related quality‐of‐life assessment at the point‐of‐care with adolescents and young adults with cancer. Journal of Adolescent and Young Adult Oncology 2018;7(1):97‐102. [DOI] [PubMed] [Google Scholar]
Ho 2015 {published data only}
- Ho KY, Li WH, Lam KW, Chui SY, Chan CF. Psychometric properties of the Chinese version of the fatigue scale‐adolescent. BMC Cancer 2015;15:938. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ho 2016 {published data only}
- Ho KY, Li WH, Lam KW, Chiu SY, Chan CF. The Psychometric Properties of the Chinese Version of the Fatigue Scale for Children. Cancer Nursing 2016;39(5):341‐8. [DOI] [PubMed] [Google Scholar]
Hsiao 2017 {published data only}
- Hsiao CC, Chiou SS, Hsu HT, Lin PC, Liao YM, Wu LM. Adverse health outcomes and health concerns among survivors of various childhood cancers: perspectives from mothers. European Journal of Cancer Care 2017;e12661:1‐10. [DOI] [PubMed] [Google Scholar]
Johannsdottir 2017 {published data only}
- Johannsdottir IM, Hamre H, Fosså SD, Loge JH, Drolsum L, Lund MB, et al. Adverse health outcomes and associations with self‐reported general health in childhood lymphoma survivors. Journal of Adolescent and Young Adult Oncology 2017;6(3):470‐6. [DOI] [PubMed] [Google Scholar]
Johnson 2018 {published data only}
- Johnson A, Rice M, Turner‐Henson A, Haase JE, Azuero A. Perceived stress and the fatigue symptom cluster in childhood brain tumor survivors. Oncology Nursing Forum 2018;45(6):775‐85. [DOI] [PubMed] [Google Scholar]
Jones 2018 {published data only}
- Jones CM, Baker JN, Keesey RM, Eliason RJ, Lanctot JQ, Clegg JL, et al. Importance ratings on patient‐reported outcome items for survivorship care: comparison between pediatric cancer survivors, parents, and clinicians. Quality of life research 2018;27(7):1877‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kanellopoulos 2013 {published data only}
- Kanellopoulos A, Hamre HM, Dahl AA, Fosså SD, Ruud E. Factors associated with poor quality of life in survivors of childhood acute lymphoblastic leukemia and lymphoma. Pediatric Blood & Cancer 2013;60(5):849‐55. [DOI] [PubMed] [Google Scholar]
Korinthenberg 2011 {published data only}
- Korinthenberg R, Neuburger D, Nikkhah G, Teske C, Schnabel K, Calaminus G. Assessing quality of life in long‐term survivors after 125I brachytherapy for low‐grade glioma in childhood. Neuropediatrics 2011;42(3):110‐5. [DOI] [PubMed] [Google Scholar]
Lai 2017 {published data only}
- Lai JS, Beaumont JL, Nowinski CJ, Cella D, Hartsell WF, Han‐Chih Chang J, et al. Why computerized adaptive testing In pediatric brain tumor clinics. Journal of Pain and Symptom Management 2017;54(3):289‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lai 2019 {published data only}
- Lai JS, Kupst MJ, Beaumont JL, Manley PE, Chang JH, Hartsell WF, et al. Using the Patient‐Reported OutcomesMeasurement Information System (PROMIS) to measure symptom burden reported by patients with brain tumors. Pediatric Blood & Cancer 2019;66(3):e27526. [DOI] [PMC free article] [PubMed] [Google Scholar]
MacArtney 2014 {published data only}
- MacArtney G, Vandenkerkhof E, Harrison MB, Stacey D. Symptom experience and quality of life in pediatric brain tumor survivors: A cross‐sectional study. Journal of Pain and Symptom Management 2014;48(5):957‐67. [DOI] [PubMed] [Google Scholar]
Macpherson 2015 {published data only}
- Macpherson CF, Hooke MC, Friedman DL, Campbell K, Withycombe J, Schwartz CL, et al. Exercise and fatigue in adolescent and young adult survivors of Hodgkin lymphoma: A report from the children's oncology group. Journal of Adolescent and Young Adult Oncology 2015;4(3):137‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Manley 2012 {published data only}
- Manley PE, McKendrick K, McGillicudy M, Chi SN, Kieran MW, Cohen LE, et al. Sleep dysfunction in long term survivors of craniopharyngioma. Journal of Neuro‐Oncology 2012;108(3):543‐9. [DOI] [PubMed] [Google Scholar]
McClellan 2013 {published data only}
- McClellan W, Klemp JR, Krebill H, Ryan R, Nelson EL, Panicker J, et al. Understanding the functional late effects and informational needs of adult survivors of childhood cancer. Oncology Nursing Forum 2013;40(3):254‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
McLoone 2011 {published data only}
- McLoone JK, Wakefield CE, Butow P, Fleming C, Cohn RJ. Returning to school after adolescent cancer: A qualitative examination of Australian survivors' and their families' perspectives. Journal of Adolescent and Young Adult Oncology 2011;1(2):87‐94. [DOI] [PubMed] [Google Scholar]
Mellblom 2017 {published data only}
- Mellblom AV, Ruud E, Loge JH, Lie HC. Do negative emotions expressed during follow‐up consultations with adolescent survivors of childhood cancer reflect late effects?. Patient Education and Counseling 2017;100(11):2098‐101. [DOI] [PubMed] [Google Scholar]
Nagai 2012 {published data only}
- Nagai A, Zou N, Kubota M, Kojima C, Adachi S, Usami I, et al. Fatigue in survivors of childhood acute lymphoblastic and myeloid leukemia in Japan. Pediatrics International 2012;54(2):272‐6. [DOI] [PubMed] [Google Scholar]
Nicklin 2019 {published data only}
- Nicklin E, Velikova G. Long‐term issues and supportive care needs of adolescent and young adult childhood brain tumour survivors and their caregivers: A systematic review. Psycho‐Oncology 2019;28(3):477‐87. [DOI] [PubMed] [Google Scholar]
Nies 2017 {published data only (unpublished sought but not used)}
- Nies M, Klein Hesselink MS, Huizinga GA, Sulkers E, Brouwers AH, Burgerhof JG, et al. Long‐term quality of life in adult survivors of pediatric differentiated thyroid carcinoma. Journal of Clinical Endocrinology and Metabolism 2017;102(4):1218‐26. [DOI] [PubMed] [Google Scholar]
Norum 1996 {published data only}
- Norum J, Wist EA. Quality of life in survivors of Hodgkin's disease. Quality of Life Research 1996;5(3):367‐74. [DOI] [PubMed] [Google Scholar]
Nugent 2018 {published and unpublished data}
- Nugent BD, Bender CM, Sereika SM, Tersak JM, Rosenzweig M. Cognitive and occupational function in survivors of adolescent cancer. Journal of Adolescent and Young Adult Oncology 2018;7(1):79‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nwachukwu 2015 {published data only}
- Nwachukwu CR, Youland RS, Chioreso C, Wetjen N, NageswaraRao A, Keating G, et al. Health related quality of life (HRQOL) in long‐term survivors of pediatric low grade gliomas (LGGs). Journal of Neuro‐Oncology 2015;121(3):599‐607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pan 2017 {published data only}
- Pan HT, Wu LM, Wen SH. Quality of life and its predictors among children and adolescents with cancer. Cancer Nursing 2017;40(5):343‐51. [DOI] [PubMed] [Google Scholar]
Rach 2017 {published data only}
- Rach AM, Crabtree VM, Brinkman TM, Zeltzer L, Marchak JG, Srivastava D, et al. Predictors of fatigue and poor sleep in adult survivors of childhood Hodgkin's lymphoma: a report from the Childhood Cancer Survivor Study. Journal of Cancer Survivorship : Research and Practice 2017;11(2):256‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raj 2018 {published data only}
- Raj SP, Narad ME, Salloum R, Platt A, Thompson A, Baum KT, et al. Development of a web‐based psychosocial intervention for adolescent and young adult survivors of pediatric brain tumor. Journal of Adolescent and Young Adult Oncology 2018;7(2):187‐95. [DOI] [PubMed] [Google Scholar]
Reiter‐Purtill 2003 {published data only}
- Reiter‐Purtill J, Vannatta K, Gerhardt CA, Correll J, Noll RB. A controlled longitudinal study of the social functioning of children who completed treatment of cancer. Journal of Pediatric Hematology/Oncology 2003;25(6):467‐73. [DOI] [PubMed] [Google Scholar]
Ross 2018 {published data only}
- Ross WL, Le A, Zheng DJ, Mitchell HR, Rotatori J, Li F, et al. Physical activity barriers, preferences, and beliefs in childhood cancer patients. Supportive Care in Cancer 2018;26(7):2177‐84. [DOI] [PubMed] [Google Scholar]
Rueegg 2017 {published data only}
- Rueegg CS, Kriemler S, Zuercher SJ, Schindera C, Renner A, Hebestreit H, et al. A partially supervised physical activity program for adult and adolescent survivors of childhood cancer (SURfit): study design of a randomized controlled trial [NCT02730767]. BMC Cancer 2017;17:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sadighi 2014 {published data only}
- Sadighi ZS, Ness KK, Hudson MM, Morris EB, Ledet DS, Pui CH, et al. Headache types, related morbidity, and quality of life in survivors of childhood acute lymphoblastic leukemia: a prospective cross sectional study. European Journal of Paediatric Neurology 2014;18(6):722‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmielau 2017 {published data only}
- Schmielau J, Rick O, Reuss‐Borst M, Kalusche‐Bontemps EM, Steimann M. Rehabilitation of cancer survivors with long‐term toxicities. Oncology Research and Treatment 2017;40(12):764‐71. [DOI] [PubMed] [Google Scholar]
Simioni 2018 {published data only}
- Simioni C, Zauli G, Martelli AM, Vitale M, Ultimo S, Milani D, et al. Physical training interventions for children and teenagers affected by acute lymphoblastic leukemia and related treatment impairments. Oncotarget 2018;9(24):17199‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterkenburg 2015 {published data only (unpublished sought but not used)}
- Sterkenburg AS, Hoffmann A, Gebhardt U, Warmuth‐Metz M, Daubenbuchel AM, Muller HL. Survival, hypothalamic obesity, and neuropsychological/psychosocial status after childhood‐onset craniopharyngioma: newly reported long‐term outcomes. Neuro‐Oncology 2015;17(7):1029‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vannatta 1998 {published data only}
- Vannatta K, Gartstein MA, Short A, Noll RB. A controlled study of peer relationships of children surviving brain tumors: Teacher, peer, and self ratings. Journal of Pediatric Psychology 1998;23(5):279‐87. [DOI] [PubMed] [Google Scholar]
Van Santen 2004 {published data only}
- Santen HM, Aronson DC, Vulsma T, Tummers RF, Geenen MM, Vijlder JJ, et al. Frequent adverse events after treatment for childhood‐onset differentiated thyroid carcinoma: a single institute experience. European Journal of Cancer (Oxford, England : 1990) 2004;40(11):1743‐51. [DOI] [PubMed] [Google Scholar]
Withycombe 2018 {published data only}
- Withycombe JS, Baek MJ, Jordan DH, Thomas NJ, Hale S. Pilot study evaluating physical activity and fatigue in adolescent oncology patients and survivors during summer camp. Journal of Adolescent and Young Adult Oncology 2018;7(2):254‐7. [DOI] [PubMed] [Google Scholar]
Wogksch 2019 {published data only}
- Wogksch MD, Howell CR, Wilson CL, Partin RE, Ehrhardt MJ, Krull KR, et al. Physical fitness in survivors of childhood Hodgkin lymphoma: A report from the St. Jude Lifetime Cohort. Pediatric Blood & Cancer 2019;66(3):e27506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2018 {published data only}
- Wu WW, Lin KC, Liang SY, Jou ST. Using a patient‐centered approach to identify symptom clusters among adolescents with cancer. Cancer Nursing 2018;42(3):198‐207. [DOI] [PubMed] [Google Scholar]
Yano 2016 {published data only}
- Yano S, Kudo M, Hide T, Shinojima N, Makino K, Nakamura H, et al. Quality of life and clinical features of long‐term survivors surgically treated for pediatric craniopharyngioma. World Neurosurgery 2016;85:153‐62. [DOI] [PubMed] [Google Scholar]
Yeh 2009 {published data only}
- Yeh CH, Wang CH, Chiang YC, Lin L, Chien LC. Assessment of symptoms reported by 10‐ to 18‐year‐old cancer patients in Taiwan. Journal of Pain and Symptom Management 2009;38(5):738‐46. [DOI] [PubMed] [Google Scholar]
Yi 2014 {published data only}
- Yi J, Kim MA, Tian T. Perceived long‐term and physical health problems after cancer: adolescent and young adult survivors of childhood cancer in Korea. European Journal of Oncology Nursing 2014;18(2):145‐50. [DOI] [PubMed] [Google Scholar]
Zebrack 2002 {published data only}
- Zebrack BJ, Chesler MA. Quality of life in childhood cancer survivors. Psycho‐Oncology 2002;11(2):132‐41. [DOI] [PubMed] [Google Scholar]
Zeller 2014b {published data only}
- Zeller B, Ruud E, Havard Loge J, Kanellopoulos A, Hamre H, Godang K, et al. Chronic fatigue in adult survivors of childhood cancer: associated symptoms, neuroendocrine markers, and autonomic cardiovascular responses. Psychosomatics 2014;55(6):621‐9. [DOI] [PubMed] [Google Scholar]
Zeltzer 2009 {published data only}
- Zeltzer LK, Recklitis C, Buchbinder D, Zebrack B, Casillas J, Tsao JCI, et al. Psychological status in childhood cancer survivors: a report from the childhood cancer survivor study. Journal of Clinical Oncology 2009;27(14):2396‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Frederick 2016 {published data only}
- Frederick NN, Kenney L, Vrooman L, Recklitis CJ. Fatigue in adolescent and adult survivors of non‐CNS childhood cancer: a report from project REACH. Supportive Care in Cancer 2016;24(9):3951‐9. [DOI] [PubMed] [Google Scholar]
Griffith 2000 {published data only}
- Griffith KC, Hart LK. Characteristics of adolescent cancer survivors who pursue postsecondary education. Cancer Nursing 2000;23(6):468‐76. [DOI] [PubMed] [Google Scholar]
Liu 2018 {published and unpublished data}
- Liu Y, Yuan C, Wang J, Shen N, Shen M, Hinds PS. Chinese version of pediatric patient‐reported outcome measurement information system short form measures. Cancer Nursing 2018;Publish ahead of print:1‐9. [DOI] [PubMed] [Google Scholar]
Lowe 2016 {published data only}
- Lowe K, Escoffery C, Mertens AC, Berg CJ. Distinct health behavior and psychosocial profiles of young adult survivors of childhood cancers: a mixed methods study. Journal of Cancer Survivorship 2016;10(4):619‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maunsell 2006 {published data only}
- Maunsell E, Pogany L, Barrera M, Shaw AK, Speechley KN. Quality of life among long‐term adolescent and adult survivors of childhood cancer. Journal of Clinical Oncology 2006;24(16):2527‐35. [DOI] [PubMed] [Google Scholar]
Meeske 2004 {published data only}
- Meeske K, Katz ER, Palmer SN, Burwinkle T, Varni JW. Parent proxy‐reported health‐related quality of life and fatigue in pediatric patients diagnosed with brain tumors and acute lymphoblastic leukemia. Cancer 2004;101(9):2116‐25. [DOI] [PubMed] [Google Scholar]
Meeske 2007 {published data only}
- Meeske KA, Patel SK, Palmer SN, Nelson MB, Parow AM. Factors associated with health‐related quality of life in pediatric cancer survivors. Pediatric Blood & Cancer 2007;49(3):298‐305. [DOI] [PubMed] [Google Scholar]
Robert 2012 {published data only}
- Robert RS, Paxton RJ, Palla SL, Yang G, Askins MA, Joy SE, et al. Feasibility, reliability, and validity of the pediatric quality of life inventoryTM generic core scales, cancer module, and multidimensional fatigue scale in long‐term adult survivors of pediatric cancer. Pediatric Blood & Cancer 2012;59(4):703‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Veneroni 2017 {published data only}
- Veneroni L, Boschetti L, Barretta F, Clerici CA, Simonetti F, Schiavello E, et al. Quality of life in long‐term survivors treated for metastatic medulloblastoma with a hyperfractionated accelerated radiotherapy (HART) strategy. Childs Nervous System 2017;33(11):1969‐76. [DOI] [PubMed] [Google Scholar]
Wu 2019 {published data only}
- Wu WW, Jou ST, Liang SY, Tsai SY. The mediating role of exercise on relationships between fatigue, sleep quality, and quality of life for adolescents with cancer. Cancer Nursing 2019;42(2):E10‐9. [DOI] [PubMed] [Google Scholar]
Zeltzer 1997 {published data only}
- Zeltzer LK, Chen E, Weiss R, Guo MD, Robison LL, Meadows AT, et al. Comparison of psychologic outcome in adult survivors of childhood acute lymphoblastic leukemia versus sibling controls: a Cooperative Children's Cancer Group and National Institutes of Health Study. Journal of Clinical Oncology 1997;15(2):547‐56. [DOI] [PubMed] [Google Scholar]
Zhang 2018 {published data only}
- Zhang FF, Hudson MM, Huang I, Bhakta N, Ness KK, Brinkman TM, et al. Lifestyle factors and health‐related quality of life in adult survivors of childhood cancer: a report from the St. Jude Lifetime Cohort Study. Cancer 2018;124(19):3918‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Kunin‐Batson 2015 {published data only}
- NCT02361047. Let's play! Healthy kids after cancer: a physical activity and nutrition intervention. clinicaltrials.gov/ct2/show/NCT02361047 (first received 11 February 2015).
Additional references
Aaronson 1993
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute. 1993;85(5):365‐76. [DOI] [PubMed] [Google Scholar]
Abrahams 2016
- Abrahams HJ, Gielissen MF, Schmits IC, Verhagen CA, Rovers MM, Knoop H. Risk factors, prevalence, and course of severe fatigue after breast cancer treatment: a meta‐analysis involving 12,327 breast cancer survivors. Annals of Oncology 2016;27(6):965‐74. [DOI] [PubMed] [Google Scholar]
Abrahams 2018
- Abrahams HJ, Gielissen MF, Verhagen CA, Knoop H. The relationship of fatigue in breast cancer survivors with quality of life and factors to address in psychological interventions: A systematic review. Clinical Psychology Review 2018;63:1‐11. [DOI] [PubMed] [Google Scholar]
American Cancer Society 2014
- American Cancer Society. Cancer facts & figures 2014. www.cancer.org/research/cancer‐facts‐statistics/all‐cancer‐facts‐figures/cancer‐facts‐figures‐2014.html 2014.
Armstrong 2014
- Armstrong GT, Kawashima T, Leisenring W, Stratton K, Stovall M, Hudson MM, et al. Aging and risk of severe, disabling, life‐threatening, and fatal events in the childhood cancer survivor study. Journal of Clinical Oncology 2014;32(12):1218‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barsevick 2010
- Barsevick A, Fros M, Zwinderman A, Hall P, Halyard M. I'm so tired: biological and genetic mechanisms of cancer‐related fatigue. Quality of Life Research 2010;19(10):1419‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berger 2015
- Berger AM, Mitchell SA, Jacobsen PB, Piri WF. Screening, evaluation, and management of cancer‐related fatigue: ready for implementation to practice?. CA: A Cancer Journal for Clinicians 2015;65(3):190‐211. [DOI] [PubMed] [Google Scholar]
Bower 2006
- Bower JE, Ganz PA, Desmond KA, Bernaards C, Rowland JH, Meyerowitz BE, et al. Fatigue in long‐term breast cancer survivors. A longitudinal investigation. Cancer 2006;106(4):751‐8. [DOI] [PubMed] [Google Scholar]
Bower 2014a
- Bower JE. Cancer‐related fatigue: mechanisms, risk factors, and treatments. Nature Reviews Clinical Oncology 2014;11(10):597‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bower 2014b
- Bower JE, Bak K, Berger A, Breitbart W, Escalante CP, Ganz PA, et al. Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical Oncology clinical practice guideline adaptation. Journal of Clinical Oncology 2014;32(17):1840‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Daniels 2013
- Daniels LA, Oerlemans S, Krol AD, Poll‐Franse LV, Creutzberg CL. Persisting fatigue in Hodgkin lymphoma survivors: a systematic review. Annals of Hematology 2013;92(8):1023‐32. [DOI] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG, editor(s) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking metaanalyses. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Diller 2009
- Diller L, Chow EJ, Gurney JG, Hudson MM, Kadin‐Lottick NS, Kawashima TI, et al. Chronic disease in the Childhood Cancer Survivor Study Cohort: a review of published findings. Journal of Clinical Oncology 2009;27(14):2339‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Epitools 2018 [Computer program]
- Sergeant, ESG. Epitools epidemiological calculators. Ausvet Pty Ltd., 2018.
Gatta 2014
- Gatta G, Botta L, Rossi S, Aareleid T, Bielska‐Lasota M, Clavel J, et al. Childhood cancer survival in Europe 1999‐2007: results of EUROCARE‐5‐‐a population‐based study. Lancet Oncology 2014;15(1):35‐47. [DOI] [PubMed] [Google Scholar]
Gielissen 2007
- Gielissen MF, Schattenberg AV, Verhagen CA, Rinkes MJ, Bremmers ME, Bleijenberg G. Experience of severe fatigue in long‐term survivors of stem cell transplantation. Bone Marrow Transplantation 2007;39(10):595‐603. [DOI] [PubMed] [Google Scholar]
Grimes 2002
- Grimes DA, Schulz KF. Cohort studies: marching towards outcomes. Lancet 2002;359(9303):341‐5. [DOI] [PubMed] [Google Scholar]
Heutte 2009
- Heutte N, Flecthner HH, Mounier N, Mellink WA, Meerwaldt JH, Eghbali H, et al. Quality of life after successful treatment of early stage Hodgkins lymphoma: 10 year follow‐up of the EORTC‐GELA H8 randomised controlled trial. Lancet Oncology 2009;10(12):1160‐70. [DOI] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Hjermstad 2006
- Hjermstad MJ, Oldervoll L, Fosså SD, Holte H, Jacobsen AB, Loge JH. Quality of life in long‐term Hodgkin's disease survivors with chronic fatigue. European Journal of Cancer 2006;42(3):327‐33. [DOI] [PubMed] [Google Scholar]
Hudson 2013
- Hudson MM, Ness KK, Gurney JG, Mulrooney DA, Chemaitilly W, Krull KR, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA 2013;309(22):2371‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacobsen 2004
- Jacobsen PB. Assessment of fatigue in cancer patients. Journal of the National Cancer Institute Monographs 2004;2004(32):93‐7. [DOI] [PubMed] [Google Scholar]
Kooijmans 2019
- Kooijmans EC, Bökenkamp A, Tjahjadi NS, Tettero JM, Dulmen den Broeder E, Pal HJ, et al. Early and late adverse renal effects after potentially nephrotoxic treatment for childhood cancer. Cochrane Database of Systematic Reviews 2019, Issue 3. [DOI: 10.1002/14651858.CD008944.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kremer 2016
- Kremer LC, Jellema P, Leclercq E, Noorman JK, Dalen EC. Cochrane Childhood Cancer. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)) 2016, Issue 5:Art. No.: CHILDCA.
Leclercq 2013
- Leclercq E, Leeflang MM, Dalen EC, Kremer LC. Validation of search filters for identifying pediatric studies in PubMed. Journal of Pediatrics 2013;162(3):629‐34. [DOI] [PubMed] [Google Scholar]
Minton 2008
- Minton O, Stone P. How common is fatigue in disease‐free breast cancer survivors? A systematic review of the literature. Breast Cancer Research and Treatment 2008;112(1):5‐13. [DOI] [PubMed] [Google Scholar]
Mock 2000
- Mock V, Atkinson A, Barsevick A, Cella D, Cimprich B, Cleeland C, et al. NCCN practice guidelines for cancer‐related fatigue. Oncology 2000;14(11a):151‐61. [PubMed] [Google Scholar]
Mulder 2019
- Mulder RL, Bresters D, Hof M, Koot BG, Castellino SM, Loke YK, et al. Hepatic late adverse effects after antineoplastic treatment for childhood cancer. Cochrane Database of Systematic Reviews 2019, Issue 4. [DOI: 10.1002/14651858.CD008205.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nathan 2016
- Nathan PC, Amir E, Abdel‐Qadir H. Cardiac outcomes in survivors of pediatric and adult cancers. Canadian Journal of Cardiology 2016;32(7):871‐80. [DOI] [PubMed] [Google Scholar]
Oeffinger 2006
- Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Childhood Cancer Survivor Study. Chronic health conditions in adult survivors of childhood cancer. New England Journal of Medicine 2006;355(15):1572‐82. [DOI] [PubMed] [Google Scholar]
Prue 2006
- Prue G, Rankin J, Allen J, Gracy J, Cramp F. Cancer‐related fatigue: a critical appraisal. European Journal of Cancer 2006;42(7):846‐63. [DOI] [PubMed] [Google Scholar]
R Statistical Software 2016 [Computer program]
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2016.
Reinertsen 2010
- Reinersten KV, Varova MC, Loge JH, Edvardsen H, Wist, E, Fosså SD. Predictors and course of chronic fatigue in long‐term breast cancer survivors. Journal of Cancer Survivorship 2010;4(4):405‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Reyes‐Gibby 2008
- Reyes‐Gibby CC, Wu Z, Spitz M, Kurzrock R, Fisch M, Bruera E, et al. Molecular epidemiology, cancer‐related symptoms, and cytokines pathway. Lancet Oncology 2008;9(8):777‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ryan 2007
- Ryan JL, Carroll JK, Ryan EP, Mustian KM, Fiscella K, Morrow GR. Mechanisms of cancer‐related fatigue. Oncologist 2007;12(suppl1):22‐34. [DOI] [PubMed] [Google Scholar]
Servaes 2002
- Servaes P, Verhagen C, Bleijenberg G. Fatigue in cancer patients during and after treatment: prevalence, correlates and interventions. European Journal of Cancer 2002;38(1):27‐43. [DOI] [PubMed] [Google Scholar]
Servaes 2007
- Servaes P, Gielissen M, Verhagen S, Bleijenberg G. The course of severe fatigue in disease‐free breast cancer patients: a longitudinal study. Psycho‐oncology 2007;16(9):787‐95. [DOI] [PubMed] [Google Scholar]
Skinner 2006
- Skinner R, Wallace WH, Levitt GA, UK Children's Cancer Study Group Late Effects Group. Long‐term follow‐up of people who have survived cancer during childhood. Lancet Oncology 2006;7(6):489‐98. [DOI] [PubMed] [Google Scholar]
Spathis 2015
- Spathis A, Booth S, Grove S, Hatcher H, Kuhn I, Barclay S. Teenage and young adult cancer‐related fatigue is prevalent, distressing, and neglected: it is time to intervene. A systematic literature review and narrative synthesis. Journal of Adolescent and Young Adult Oncology 2015;4(1):3‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2017
- Sterne JA, Egger M, Moher D, Boutron I, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Von Elm 2007
- Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology 2007;18(6):800‐4. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Boonstra 2017
- Boonstra A, Dulmen‐den Broeder E, Rovers MM, Blijlevens N, Knoop H, Loonen J. Severe fatigue in childhood cancer survivors. Cochrane Database of Systematic Reviews 2017, Issue 6. [DOI: 10.1002/14651858.CD012681] [DOI] [Google Scholar]


