Abstract
As the interface between the fetal and maternal circulation, the placenta facilitates both nutrient and waste exchange for the developing fetus. Iron is essential for healthy pregnancy, and transport of iron across the placenta is required for fetal growth and development. Perturbation of this transfer can lead to adverse pregnancy outcomes. Despite its importance, our understanding of how a large amount of iron is transported across placental membranes, how this process is regulated, and which iron transporter proteins function in different placental cells remains rudimentary. Mechanistic studies in mouse models, including placenta-specific deletion or overexpression of iron-related proteins will be essential to make progress. This review summarizes our current understanding about iron transport across the syncytiotrophoblast under physiological conditions and identifies areas for further investigation.
Keywords: Iron, Pregnancy, Placenta
1.1. Iron is an essential micronutrient
Iron is an essential, multifunctional micronutrient. The ability of iron to easily transition between two oxidation states - ferrous (Fe2+) and ferric (Fe3+) - underlies its involvement in a broad range of biological processes including oxygen transport, function of the electron transport chain and DNA synthesis [1]. However, the reactive free iron also generates hydroxyl radicals (− HO•), superoxide anions (OH−) and hydroperoxyl radicals (HOO•) that oxidize and damage proteins, lipids and nucleic acids [2]. Consequently, iron levels must be tightly regulated. As there is no biological mechanism for the excretion of excess iron from the human body, regulation of total body iron occurs at the level of dietary absorption. This regulation is accomplished through the action of the master iron regulator, hepcidin, and its receptor and iron exporter, ferroportin. Under certain stresses, such as after hemorrhage or during pregnancy, iron requirements increase several fold.
1.2. Iron requirements during pregnancy
Pregnancy is a biological state that profoundly affects maternal metabolism, including iron homeostasis. Maternal iron requirements increase substantially to allow for expansion of maternal red cell mass, placental development and function, and fetal development, requiring around 1 g of iron over the duration of pregnancy (reviewed in [3]). Iron supply to the fetus is wholly dependent on the transfer across the placenta. The fetus requires approximately 270 mg iron [4] and the placenta itself around 90 mg [5]. The flux of iron through the placenta is unidirectional [6], and is greatest in the third trimester [7], with several milligrams of iron transferred to the fetus daily [5].
1.3. Iron species transported by the placenta
Nutrient transport across the placenta is carried out by the syncytiotrophoblast, the primary barrier between the maternal and fetal circulation. These cells express numerous nutrient transporters including those mediating iron transport. In this review, we will mainly focus on iron handling by the syncytiotrophoblast. Whether other cell types in the placenta (e.g. mesenchymal cells, Hofbauer cells, fibro-blasts, endothelial cells, etc.) influence iron transport to the fetus is entirely unknown.
Iron is delivered to the placenta by the maternal circulation, where iron is found complexed with transferrin, ferritin or heme. Transferrin-bound iron is thought to be the predominant iron source taken up by the placenta. Global knockout of transferrin receptor 1 (TFR1) in mice, wherein both embryos and placentas lack TFR1, results in embryonic lethality before embryonic day (E)12.5 due to severe anemia [8]. Analysis of the placenta, however, was not reported. In mice, the placenta begins developing at E3.5 and matures by E14.5 [9,10]. Interestingly, although Tfr1−/− embryos were not viable at E12.5, embryonic development and erythropoiesis were evident at E10.5 [8], suggesting that other sources of iron must have been taken up and transferred by the placenta during early development. It is entirely unknown whether and under which conditions the placenta may utilize and transport any other iron species, including ferritin, non-transferrin-bound iron or heme.
1.4. Mouse and human placental iron handling
Mouse models have been essential for defining the pathogenesis of iron disorders [11] because the key regulatory and transport mechanisms are remarkably similar to those in humans. Regarding the utility of mouse models in pregnancy studies, some important differences between humans and mice exist, including differences in the uterine shape, the number of embryos, and the site of progesterone synthesis in late gestation (placenta in humans, corpus luteum in mice [12]). Nevertheless, many anatomical and physiological features of pregnancy are similar. The placentas in both species are hemochorial, that is, maternal blood comes in direct contact with the chorion. Although the nutrient-transporting mouse labyrinth contains two syncytiotrophoblast layers (SynT-I and SynT-II) vs a single layer in humans, the syncytiotrophoblasts in the two species are functionally similar [10]. Mouse SynT-I and SynT-II have direct communication between their cytoplasms via gap-junctions, and the distribution of key iron transporters is highly similar between human and mouse syncytiotrophoblast: TFR1 is facing maternal circulation (SynT-I in mice), and ferroportin (FPN, Slc40a1) is facing the fetal circulation (SynT-II in mice) (Fig. 1). Despite the known limitations of animal models, they are essential for mechanistic studies of pregnancy, including those related to iron (patho) biology.
Fig. 1. Iron trafficking across the syncytiotrophoblast.
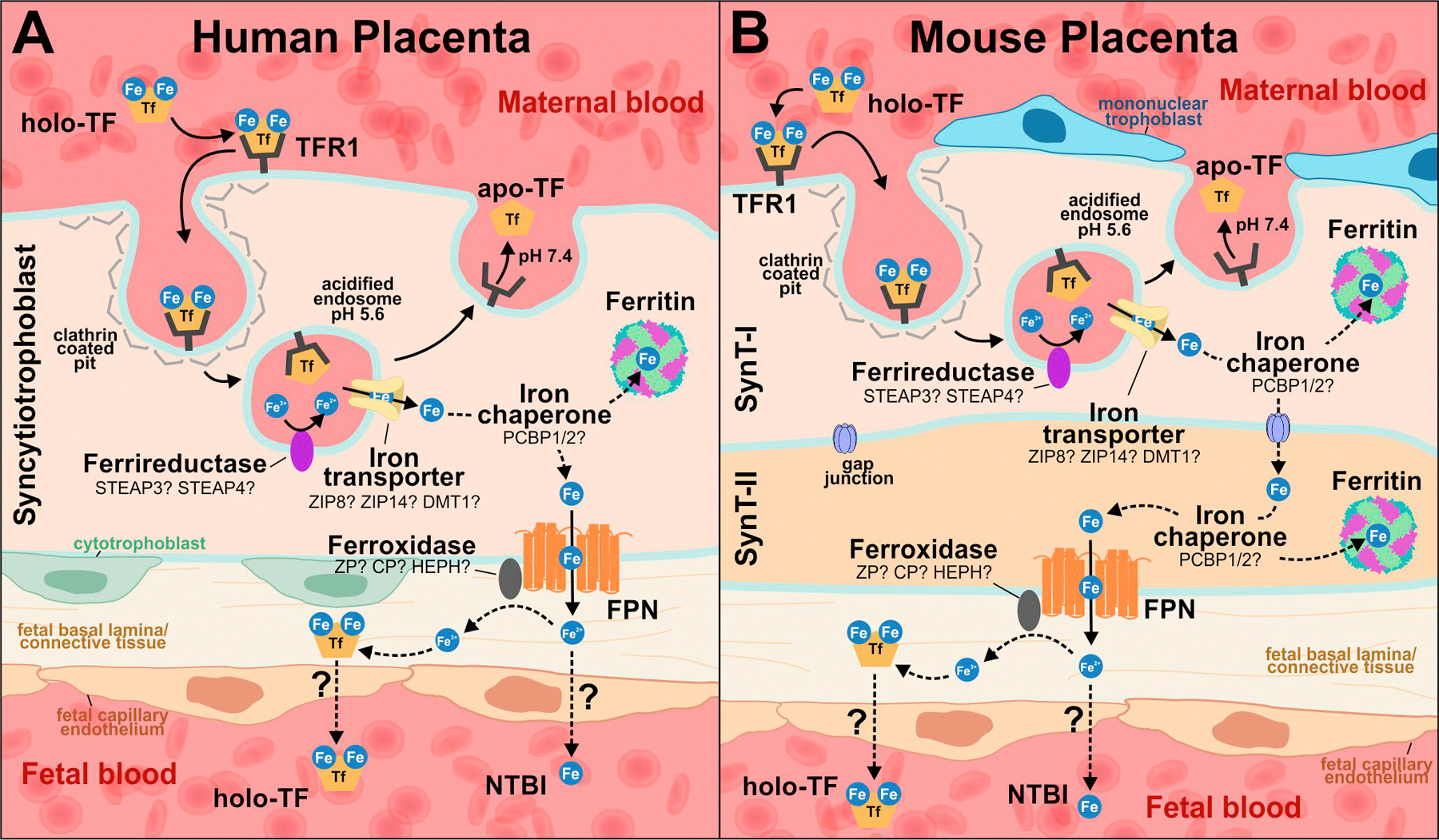
(A) Human placenta, with a single layer of syncytiotrophoblast. (B) Mouse placenta, with two syncytiotrophoblast layers (SynT-I and SynT-II). In both humans and mice, transferrin-bound iron (holo-TF) from the maternal circulation binds to transferrin receptor, TFR1, expressed on the apical membrane of placental syncytiotrophoblast (SynT-I in mice). The iron-transferrin-receptor complex is internalized via clathrin-mediated endocytosis, and ferric iron (Fe3+) is released from transferrin in acidified endosomes. The apo-TF/TFR1 complex is recycled back to the cell surface. Fe3+ in the endosome is thought to be reduced to ferrous iron (Fe2+) by a ferrireductase and exported into the cytoplasm through an endosomal iron transporter. Cytoplasmic Fe may be chaperoned, possibly by PCBP1 or 2, either to ferritin for storage or to FPN on the basal membrane (SynT-II in mice) for export toward the fetal circulation. In the mouse placenta (B), it is unknown how Fe is transported from SynT-I to SynT-II, but it likely occurs through gap junctions. The fate of iron following export through FPN is unclear; it may enter the fetal circulation as NTBI or oxidized to Fe3+ by ferroxidases and loaded onto transferrin prior to reaching the fetal circulation.
1.5. Placental trafficking of transferrin-bound iron
1.5.1. Internalization of transferrin-bound iron
Our current understanding of transferrin-bound iron transport across the placenta is depicted in Fig. 1. TFR1 is highly expressed in both mouse [13] and human [14,15] placentas. Its expression is primarily localized to the apical side of placental syncytiotrophoblasts, which faces the maternal circulation (SynT-I layer in mice) [16,17]. At physiological pH 7.4, maternal holo-transferrin (Fe3+ bound to transferrin) binds to placental TFR1; the resulting iron-transferrin-TFR1 complex is internalized via clathrin-mediated endocytosis [18]. As the pH within the endocytic vesicle decreases from pH 7.4 to below pH 5.5, Fe3+ dissociates from transferrin [19] and is reduced to ferrous iron (Fe2+) by ferrireductases. Iron-free transferrin (apo-transferrin) is then recycled to the cell surface where it dissociates from TFR1 at pH 7.4 [19], and can again bind iron atoms for the next round of iron transport. With a half-life of approximately 8 days in humans, apo-transferrin undergoes up to 100 cycles of iron delivery [20].
1.5.2. Endosomal trafficking
Following dissociation from transferrin in the acidified endosome, Fe3+ is reduced to the more soluble Fe2+ form by ferrireductases. The ferrireductases in the placenta have yet to be definitively identified. Potential ferrireductases include six-transmembrane epithelial antigen of prostate (STEAP)3 and STEAP4, which are highly expressed in the placenta and fetal liver [21,22]. Mice lacking Steap3 (nm1054 mice) have increased prenatal lethality on a C57BL/6 background, likely due to hydrocephaly [23], and those that are born are runted and anemic [23], consistent with the role of Steap3 in erythroid iron acquisition. However, the mice do not display systemic iron deficiency. Steap4 knockout mice are fully viable with no visible abnormalities [24]. These mouse models suggest functional redundancy among placental ferrireductases [22]. Duodenal cytochrome b (DCYTB; encoded by the CYBRD1 gene), a ferrireductase expressed at the brush border of duodenal enterocytes, is not highly expressed in the placenta. Knockout (Cybrd1−/−) mice are viable with no overt iron phenotype [25]. Additional ferrireductases from the cytochrome b561 family, lysosomal cytochrome b (LCYTB) and chromaffin granule cytochrome b (CGCYTB), have been detected in the placenta [26] although their roles remain unknown.
After iron is reduced, Fe2+ is transported across the endosomal membrane into the cytosol. Although the transport mechanism is still not fully understood, a number of potential candidates have been suggested, including divalent metal transporter 1 (DMT1; encoded by Slc11a2), ZRT/IRT-like protein (ZIP)14 (ZIP14; encoded by Slc39a14) and ZIP8 (encoded by Slc39a8). DMT1 is required for intestinal iron absorption and erythropoietic iron acquisition [27], and has been localized on the human placenta intracellularly and at the basal membrane [17]. However, DMT1 knockout (Slc11a2−/−) mice were born alive and, although anemic, their total body iron content normalized to body weight was normal [27], suggesting that DMT1 is dispensable for iron transport in the placenta. ZIP14 and ZIP8 are members of the ZIP (SLC39) family of metal-ion import proteins initially described as zinc transporters, but later determined to transport a number of divalent metals including iron. Both ZIP14 and ZIP8 are able to transport Fe2+ and are expressed in human [28,29] and mouse [30] placentas. However, Zip14 mRNA expression in the mouse placenta is much lower than Zip8. ZIP14 knockout (Slc39a14−/−) mice are viable [31] albeit smaller than wild-type littermate controls, a phenotype attributed to abnormal chondrocyte differentiation in growth plates rather than dysregulation of iron [32]. Therefore, knockout mouse models indicate a non-essential or redundant role of DMT1 and ZIP14 in placental iron transport.
ZIP8 is highly expressed both in mouse placentas (second only to lung [30]) and in human placentas (second only to the pancreas [29]). ZIP8 iron transport function is pH dependent with optimal function between pH 6.5 and pH 7.5 [30], suggesting it may function as an endosomal transporter. During mouse pregnancy, placental Zip8 mRNA expression increased 3-fold from E13.5 to E16.5 [33]. Knockdown of endogenous ZIP8 in the human choriocarcinoma placenta cell line, BeWo, resulted in decreased iron uptake [30] raising the possibility of an iron transporting role for ZIP8 in the placenta. Global disruption of Zip8 expression during embryonic development in the Zip8 hypomorph mouse [34] resulted in severe anemia and severely diminished embryo survival, with evidence of systemic iron deficiency [33]. Although this phenotype implies a physiologic role of ZIP8 in gestational iron metabolism, the specific role of ZIP8 in the placenta was not addressed. Zhang et al. [35] compared global Zip8−/− embryos to Meox2-Cre;-Zip8flox/flox embryos in whom Zip8 expression is preserved in the placenta and extraembryonic visceral endoderm (exVE). Notable differences between these models included: 1) greater iron accumulation in placentas from Zip8−/− compared to Meox2-Cre;Zip8flox/flox model; and 2) shorter survival of Zip8−/− embryos compared to Meox2-Cre;Zip8flox/flox (E14.5 vs E17.5). These results suggest that Zip8 is not necessary for iron import into the placenta, but is required for eventual iron delivery to the fetus, although it remains to be determined whether ZIP8 specifically facilitates endosomal iron export.
1.5.3. Cytoplasmic handling of iron in the syncytiotrophoblast
It is not known how iron is trafficked within the cytoplasm of the syncytiotrophoblast. By extension from other cell types, it is possible that cytosolic iron is chaperoned by poly(rC)-binding protein 2 (PCBP2) to be delivered to ferroportin [36] for export out of the syncytiotrophoblast. Alternatively, cytosolic iron may be delivered to ferritin by PCBP1 and subsequently released through the process of ferritinophagy [37] for eventual export to the fetus. Although global knockouts of PCBP1, PCBP2 and ferritin H subunit all result in embryonic lethality [38], any placenta-specific roles of these proteins are still unknown.
1.6. Ferritin, heme and non-transferrin-bound iron as a placental iron source
Apart from the transferrin-bound iron, iron is present in maternal circulation in the form of ferritin and possibly even heme. Cell-free heme and hemoglobin were detected in blood of healthy subjects [39], likely bound to their scavengers, hemopexin and haptoglobin. Whether any of these molecules represent a significant iron source for the placental transfer is entirely unknown. Although circulating ferritin tends to be iron-poor, ferritin could potentially be taken up into the placenta by TFR1 [40] or scavenger receptor class A member 5 (SCARA5) [41], the latter being expressed only at low levels in the placenta. Heme-hemopexin receptor LRP/CD91 is highly expressed on syncytiotrophoblast [42], whereas hemoglobin-haptoglobin receptor CD163 is expressed on Hofbauer cells (fetal macrophages) [43], which are not in direct contact with the maternal circulation.
Non-transferrin bound iron (NTBI) is an iron species that appears in circulation when saturation of transferrin exceeds 70 or 80%, and is usually found in subjects with iron overload or impaired erythropoiesis [44]. NTBI is not measurable in maternal circulation in healthy pregnancies, except perhaps transiently and at low levels in iron-supplemented pregnancies [45]. Thus, NTBI is unlikely to be a physiologically-relevant source of iron from the maternal circulation.
It remains to be determined to what extent any form of iron other than transferrin-bound contributes to placental iron transfer in normal or complicated pregnancies.
1.7. Export of iron to the fetal circulation
Iron is exported from the placental syncytiotrophoblasts to the fetus through the iron exporter ferroportin, located on the basal (fetal-facing) membrane of the placental syncytiotrophoblast [16,46]. In human placentas from iron-sufficient non-anemic pregnancies collected between weeks 24 and 40, placental FPN expression increased with gestation age [47]. In mice, early in pregnancy FPN was shown to be expressed in the exVE, a cell layer responsible for early nutrient transport [48], and later in the syncytiotrophoblast (likely SynT-II layer) [49]. Global knockout of FPN in mice was embryonic lethal [50]; however, when FPN expression was preserved in only the exVE and placenta, embryo development was rescued [50], confirming an essential and non-redundant role of FPN in placental iron export. Following export through FPN, the immediate fate of iron is unclear.
Exoplasmic ferroxidases may oxidize exported ferrous iron to ferric iron for loading onto fetal transferrin. FPN transport function is facilitated by the presence of ferroxidases [51], although in vitro studies have also demonstrated function in the absence of ferroxidases [52]. In order to facilitate iron export across basal membrane of the syncytiotrophoblast to the fetal circulation, the ferroxidases would need to be produced by the syncytiotrophoblast itself or by the fetus. All three known mammalian multi-copper ferroxidases, ceruloplasmin, hephaestin and zyklopen, were localized by immunohistochemistry to the placenta [53–57]. Ceruloplasmin (CP) is a soluble copper-dependent ferroxidase essential for movement of iron from hepatocytes and reticuloendothelial cells to plasma [53,58]. Fetal liver and lung Cp transcript levels increase with gestational age [59]; however, mRNA expression was not detected in term rat placentas nor the placenta cell line, BeWo [59]. Despite lack of detectable placental transcripts, in human placentas, CP protein localizes to syncytiotrophoblast and fetal capillaries [56]. However, Cp knockout mice are viable with no iron phenotype at birth [53] suggesting that CP is not essential for placental iron transport. Hephaestin (HEPH) is a Cp homolog found mutated in sex-linked anemia (sla) mice [60]. Sla mice are viable but have lower hemoglobin at birth compared to wild-type littermates [61]. This is a result of decreased intestinal iron absorption in pregnant animals rather than impaired placental iron transfer as intraperitoneal administration of radioactive iron to pregnant carrier (sla/+) animals resulted in similar radioactive iron accumulation in embryos [61]. Additionally, Heph knockout mice are viable and appear grossly normal at birth, suggesting that HEPH is not essential for placental iron transport [51]. Furthermore, mice lacking both CP and HEPH (Cp−/−Heph−/Y) are viable with no reported birth abnormalities [62], although Hep−/Y still retain some HEPH ferroxidase activity. Unlike CP, which is predominantly expressed in the liver, or HEPH, which is predominantly expressed in intestine, the third ferroxidase, Zyklopen (ZP, encoded by HEPHL1), is predominantly expressed in the placenta [55]. It localizes to the placental labyrinth, spongiotrophoblasts and yolk sac of mouse placentas and contains membrane-bound region that positions the ferroxidase domain extracellularly, the appropriate topology to interact with FPN [55]. However, mice null for Zp appear grossly normal at birth except for curly whiskers, suggestive of a copper rather than iron deficiency [63]. Despite evidence that all three ferroxidases are present in the placenta, single knockout studies suggest that these ferroxidases likely have redundant roles. Interestingly, even triple ferroxidase knockout mice were reported to be viable though anemic [63], raising the possibility that as yet unidentified ferroxidase facilitates placental iron export, or that ferroxidases are not essential for placental iron transport. Of note, detailed characterization of the triple ferroxidase mouse has not yet been published.
Once iron is exported across the basal side of the syncytiotrophoblast through ferroportin, iron still needs to cross the fetal endothelium to reach fetal circulation. This process is not understood [64,65]. Iron exported through ferroportin may be loaded onto transferrin, whose concentrations in fetal circulation gradually increases during gestation [66]. Fetal transferrin is produced by the fetal liver [67] and possibly some other organs [68]. However, NTBI is also found in fetal circulation, at least in the first trimester [69], when transferrin concentrations are relatively low and transferrin saturation in the fetal circulation is high. NTBI appears to be able to support fetal development. Hypo-transferrinemic (Tfrhpx/hpx) mice are severely deficient in serum transferrin; nevertheless, they are born alive but severely anemic [70]. Although it is possible that the residual amount of transferrin in Tfrhpx/hpx embryos is sufficient to promote organogenesis, it is also very likely that NTBI is a relevant fetal source of iron, at least when transferrin-bound iron is unavailable. This is further supported by the observation that newborns with cardiomyocyte-specific deletion of TFR1 had no obvious heart abnormalities [71], indicating that non-transferrin-related sources of iron were sufficient to support heart development in utero. More research is required to determine if NTBI delivered by the placenta into fetal circulation is a source of iron for normal fetal development and if so, in which tissues. The summary of candidate proteins involved in placental iron transport is provided in Table 1.
Table 1.
Candidate placental iron transporters.
| Protein (gene) | Function | Genotype | Disruption | Phenotype | Ref |
|---|---|---|---|---|---|
| Internalization of transferrin-bound iron | |||||
| TFR1 (TFRC) | Transferrin receptor | TFR1−/− | Global; deletion of essential sequences in exon 3–5 | Embryonic lethal at E12.5 due to severe anemia | [8] |
| TFR1flox/flox | loxP sites flanking exons 3–6 | Not yet crossed with Cre expressing mice for analysis of placental-specific iron transfer | [96] | ||
| Endosomal trafficking | |||||
| STEAP3 (STEAP3) | Ferrireductase | nm1054 mice | Global; spontaneous deletion of ~400 kb region of chromosome 1 | Significant prenatal lethality on C57BL/6 background (likely due to hydrocephaly), viable embryos runted and anemic at birth, but mice do not display systemic iron deficiency | [21,23] |
| STEAP4 (STEAP4) | Ferrireductase | Stamp2−/− | Global; targeted disruption, Deltagen tracking number ZBB519 | Viable and fertile with no visible abnormalities; iron parameters not reported for embryos or neonates | [24] |
| DCYTB (CYBRD1) | Ferrireductase | Cybrd1−/− | Global; targeted disruption, deleted exon 2 | Viable with no visible phenotype; iron parameters normal in adult mice | [25] |
| DMT1 (SLC11A2, NRAMP2) | Iron transporter | mk/mk mice | Global; missense mutation | Viable but pale at birth, microcytic anemia, defect is due to impaired erythrocyte iron uptake | [97–99] |
| Slc11a2−/− | Global; targeted disruption, deleted exons 6–8 | Viable but severely anemic; total body iron content normalized to body weight was similar to wild-type littermate controls suggesting efficient placental iron transfer | [27] | ||
| Slc11a2flox/flox | loxP sites flanking exons 6–8 | Not yet crossed with Cre expressing mice for analysis of placental specific iron transfer | [27] | ||
| ZIP8 (SLC39A8) | Iron transporter | Slc39a8neo/neo | Global; hypomorph, neomycin gene in exon 3 | Severely decreased viability, newborns exhibit stunted growth, are pale with malformations | [33] |
| Zip8−/− | Global | Embryonic lethal at E14.5, iron accumulation in the placenta | [35] | ||
| Meox2-Cre;Zip8flox/flox | Inactivation in all embryonic tissues except exVE and placenta | Embryonic lethal at E17.5, lesser iron accumulation in the placenta compared to Zip8−/− | [35] | ||
| ZIP14 (SLC39A14) | Iron transporter | Slc39a14−/− | Global; targeted disruption, deleted exons 5–8, splice variants also deleted | Viable with growth retardation, defect due to abnormal chondrocyte differentiation in growth plates; iron parameters not reported for embryos or at birth | [32] |
| Cytoplasmic handling of iron in the syncytiotrophoblast | |||||
| PCBP1 (PCBP1) | Iron chaperone | Pcbp1−/− | Global; TALEN-mediated deletions after the translation initiation codon | Embryonic lethal between E.5–8.5 | [38] |
| Pcbp1flox/flox | Generated by InGenious Targeting Laboratory | Not yet crossed with Cre expressing mice for analysis of placental-specific iron transfer | [37] | ||
| PCBP2 (PCBP2) | Iron chaperone | Pcbp2−/− | Global; targeted disruption, delete promoter and exons 1–2 | Embryonic lethal between E12.5–13.5, cardiovascular and hematopoietic abnormalities including defective erythropoiesis; iron parameters not reported for embryos | [38] |
| Pcbp2flox/flox | loxP sites flanking promoter and exons 1–2 | Not yet crossed with Cre expressing mice for analysis of placental-specific iron transfer | [38] | ||
| Export of iron from the syncytiotrophoblast | |||||
| Ferroportin (FPN1, SLC40A1) | Iron exporter | FPN−/− | Global; targeted disruption, deleted exons 6–7 | Lethal by E7.5. At E7, iron accumulated in the exVE suggesting defective iron transfer from the exVE to the embryo | [50] |
| FPNflox/floxMeox2-Cre | loxP sites flanking exons 6–7, inactivation in all embryonic tissues except exVE and placenta | Viable and appear identical to wild-type littermates; iron parameters not reported for embryos or neonates | [50] | ||
| Ceruloplasmin (CP) | Ferroxidase | Cp−/− | Global; targeted disruption, disrupt exons 17 and 18 | Viable and normal at birth; iron parameters not reported for embryos or neonates | [53] |
| Hephaestin (HEPH) | Ferroxidase | Hephflox/floxElla-Cre or Hephflox/YElla-Cre | Global; targeted disruption, deleted exon 4 | Viable and grossly normal at birth; iron parameters not reported for embryos or neonates | [51] |
| Sla mice | Global; spontaneous deletion of 582 nt (2094–2676), predicting an in-frame omission of 194 aa | Viable but have lower hemoglobin and total body iron at birth compared to wild-type littermates, defect is due to decreased intestinal iron absorption in pregnant animals rather than impaired placental iron transfer | [60,61,100] | ||
| Zyklopen (ZP, HEPHL1) | Ferroxidase | Zp−/− | Global | Viable and grossly normal except for hair abnormalities including curly whiskers (copper-related); iron parameters not reported for embryos or neonates | [63] |
1.8. Regulation of placental iron transport
Placental iron transport may be regulated by maternal, placental and fetal signals. A common misconception is that the fetus is a “perfect parasite”, able to acquire adequate iron irrespective of the mother’s iron status [72]. However, several human and macaque studies confirmed that neonatal iron stores are compromised when the mother is irondeficient or anemic [73]. Understanding of the regulation of placental iron transport should improve the prevention and treatment of fetal iron deficiency and anemia.
1.8.1. Maternal iron availability
Transfer of iron across the placenta to the fetus is dependent on the bioavailability of iron in the maternal circulation. Iron absorption during pregnancy increases with gestational age [74,75]. Additional iron needs are met through mobilization of liver and spleen iron stores [76–78]. These systemic adaptions are mediated at least in part by maternal hepcidin. Hepcidin functionally inhibits major flows of iron into the circulation from macrophages recycling senescent erythrocytes, duodenal enterocytes absorbing dietary iron, and hepatic stores [79,80]. Thus, bioavailable iron levels in plasma inversely correlate with hepcidin levels. Hepcidin expression is regulated by plasma iron concentrations, body iron stores, infection and inflammation, erythropoiesis and pregnancy (Reviewed in [81]). During pregnancy, maternal hepcidin levels decrease to nearly undetectable levels in the second and third trimesters of human pregnancy [82,83] and in the third week of pregnancy in rats [84], presumably to maximize iron bioavailability and enhance transport across the placenta. In fact, one study demonstrated a negative correlation between maternal hepcidin concentration (albeit measured at delivery) and net dietary nonheme and heme iron that was transferred to the fetus (as determined by stable iron isotopes) [85]. The mechanism of maternal hepcidin suppression is currently unknown, but maternal iron status contributes to hepcidin regulation during pregnancy, with iron deficiency resulting in a more profound hepcidin suppression [83].
In developed countries, most women are iron-replete. Extensive use of maternal iron supplements in this population raises the possibility that in some pregnancies, the placenta may be exposed to high iron concentrations. Whether this has an effect on placental function is unclear. Excess free iron is known to catalyze generation of free radicals and cause tissue damage in disease of iron overload. It remains to be determined whether the placenta can become iron-loaded in different pathological states or due to iron supplementation in pregnancy, and whether this iron load is sufficient to increase placental oxidative stress to such a degree as to cause placental damage. Large epidemiological studies have demonstrated a U-shaped association between the iron marker ferritin and the risk of adverse pregnancy outcomes, including preterm birth and impaired fetal growth [86]. However, association of high ferritin with adverse outcomes may not only be related to high iron, but also to inflammation or a combination of the two [87]. Any direct effects of iron excess on the placenta remain to be elucidated.
1.8.2. Fetal and placental regulation
In addition to maternal hepcidin regulating iron bioavailability in maternal circulation, fetal hepcidin could determine the rate of placental iron transfer to the fetal circulation through regulation of placental FPN. Placental FPN localizes to the basolateral side of syncytiotrophoblasts and is thus accessible only to fetal and not maternal hepcidin. Increased fetal hepcidin, either as a consequence of transgenic hepcidin overexpression or mutations in the hepcidin regulator TMPRSS6, was able to regulate placental ferroportin [88,89], resulting in severe fetal iron deficiency and even death. However, under normal physiological conditions, animal studies have demonstrated very low levels of fetal hepcidin [88,89]. This is suggestive of a minimal role for fetal hepcidin in regulating placental FPN in healthy pregnancy. In humans, cord blood hepcidin has been assessed [82,85,90]; however, because of the stress of delivery, these levels likely do not reflect fetal hepcidin concentrations. Indeed, in mice, hepcidin levels transiently increase immediately after birth, between post-natal day (P)0 and P2 [88].
In addition to the iron availability in maternal circulation, the amount of iron transferred to the fetus will depend on the expression levels of iron transport proteins in the placenta. Iron transporters appear to be regulated in response to maternal iron status, presumably mediated by the alterations in the iron concentrations in the placenta itself. Maternal iron deficiency in rats increased TFR1 and DMT1 expression in the placenta but not Fpn mRNA [91]. This is consistent with the role of cellular iron-regulatory proteins 1 and 2 (IRP1 and 2), which post-transcriptionally regulate iron uptake, storage and export proteins. Briefly, during cellular iron deficiency, IRPs bind to ‘iron response elements’ (IREs) within UTRs of iron-related genes. Binding of IRPs to 3’ IREs promotes stabilization of mRNAs involved in increasing iron uptake (i.e. TFR1), whereas binding to 5’ IREs prevents translation of mRNAs involved in iron storage and export (i.e. ferritin and FPN) (Reviewed in [92]). Both IRP1 and IRP2 activity have been detected in human placentas [47,93]. In one study, in placentas from diabetic and non-diabetic pregnancies, iron-deficient placentae had higher placental IRP1 activity and increased expression of TFR1 mRNA [93]. Another study in iron-replete non-anemic mothers found an inverse correlation between cord blood ferritin and placental IRP1 and 2, suggesting that fetal iron status may also affect placental iron regulation [47]. IRP regulation of placental FPN is less clear. Two human studies found no changes in placental FPN protein related to maternal iron deficiency and anemia [54,94,95], but improved availability of validated FPN antibodies should help address this question.
1.9. Challenges and prospects
The importance of adequate iron supply during pregnancy is well appreciated, but our understanding of placental iron transport mechanisms and their regulation is very limited. Mouse models with conditional knockout of genes in the cell of the placenta (e.g. Gcm1-Cre for SynT-II, or Cyp19-Cre or Tpbpar/Adaf-AdaP-Cre for most trophoblast subtypes) will be required to conclusively demonstrate the role of different regulators and transporters. Complementary studies in human placentas will require the availability of validated reagents. Defining specific iron species that are transported across placenta or can be utilized by the fetus, and elucidating maternal, fetal and placental regulatory mechanisms governing placental iron transport, will be essential for improved understanding of iron metabolism in healthy and complicated pregnancies, and optimal management of pregnancy.
Acknowledgments
The work was supported by the UCLA Pulmonary & Critical Care Medicine Training Grant (T32 HL072752), and the Iris Cantor-UCLA Women’s Health Center Executive Advisory Board and NCATS UCLA CTSI Grant Number UL1TR000124.
Footnotes
Disclosures
VS has no conflict of interest. EN is a consultant and stockholder of Intrinsic LifeSciences (a company developing hepcidin diagnostics) and Silarus Therapeutics (a company developing erythroferrone-targeted therapeutics).
References
- [1].Duck KA, Connor JR, Iron uptake and transport across physiological barriers, Biometals: Int. J. Role Metal. Ions Biol. Biochem. Med 29 (2016) 573–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wardman P, Candeias LP, Fenton chemistry: an introduction, Radiat. Res 145 (1996) 523–531. [PubMed] [Google Scholar]
- [3].Fisher AL, Nemeth E, Iron homeostasis during pregnancy, Am. J. Clin. Nutr 106 (2017) 1567S–1574S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Widdowson EM, Spray CM, Chemical development in utero, Arch. Dis. Child 26 (1951) 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bothwell TH, Iron requirements in pregnancy and strategies to meet them, Am. J. Clin. Nutr 72 (2000) 257S–264S. [DOI] [PubMed] [Google Scholar]
- [6].van Dijk JP, Iron metabolism and placental transfer of iron in the term rhesus monkey (Macaca mulatta): a compartmental analysis, Eur. J. Obstet. Gynecol. Reprod. Biol 7 (1977) 127–139. [DOI] [PubMed] [Google Scholar]
- [7].Glasser SR, Wright C, Heyssel RM, Transfer of iron across the placenta and fetal membranes in the rat, Am. J. Physiol 215 (1968) 205–210. [DOI] [PubMed] [Google Scholar]
- [8].Levy JE, Jin O, Fujiwara Y, Kuo F, Andrews NC, Transferrin receptor is necessary for development of erythrocytes and the nervous system, Nat. Genet 21 (1999) 396–399. [DOI] [PubMed] [Google Scholar]
- [9].Cross JC, Genetic insights into trophoblast differentiation and placental morpho-genesis, Semin. Cell Dev. Biol 11 (2000) 105–113. [DOI] [PubMed] [Google Scholar]
- [10].Rossant J, Cross JC, Placental development: lessons from mouse mutants, Nat. Rev. Genet 2 (2001) 538–548. [DOI] [PubMed] [Google Scholar]
- [11].Fleming RE, Feng Q, Britton RS, Knockout mouse models of iron homeostasis, Annu. Rev. Nutr 31 (2011) 117–137. [DOI] [PubMed] [Google Scholar]
- [12].Ratajczak CK, Fay JC, Muglia LJ, Preventing preterm birth: the past limitations and new potential of animal models, Dis. Models Mech 3 (2010) 407–414. [DOI] [PubMed] [Google Scholar]
- [13].Muller R, Verma IM, Adamson ED, Expression of c-onc genes: c-fos transcripts accumulate to high levels during development of mouse placenta, yolk sac and amnion, EMBO J 2 (1983) 679–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wada HG, Hass PE, Sussman HH, Transferrin receptor in human placental brush border membranes. studies on the binding of transferrin to placental membrane vesicles and the identification of a placental brush border glycoprotein with high affinity for transferrin, J. Biol. Chem 254 (1979) 12629–12635. [PubMed] [Google Scholar]
- [15].Seligman PA, Schleicher RB, Allen RH, Isolation and characterization of the transferrin receptor from human placenta, J. Biol. Chem 254 (1979) 9943–9946. [PubMed] [Google Scholar]
- [16].Bastin J, Drakesmith H, Rees M, Sargent I, Townsend A, Localisation of proteins of iron metabolism in the human placenta and liver, Br. J. Haematol 134 (2006) 532–543. [DOI] [PubMed] [Google Scholar]
- [17].Georgieff MK, Wobken JK, Welle J, Burdo JR, Connor JR, Identification and localization of divalent metal transporter-1 (DMT-1) in term human placenta, Placenta 21 (2000) 799–804. [DOI] [PubMed] [Google Scholar]
- [18].Srai SK, Bomford A, McArdle HJ, Iron transport across cell membranes: molecular understanding of duodenal and placental iron uptake, Best. Pract. Res. Clin. Haematol 15 (2002) 243–259. [DOI] [PubMed] [Google Scholar]
- [19].Dautry-Varsat A, Ciechanover A, Lodish HF, pH and the recycling of transferrin during receptor-mediated endocytosis, Proc. Natl. Acad. Sci. USA 80 (1983) 2258–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Harford JB, Rouault TA, Huebers HA, Klausner RD, Molecular Mechanisms of Iron Metabolism, W.B. Saunders Co, Philadelphia, 1994. [Google Scholar]
- [21].Ohgami RS, Campagna DR, Greer EL, Antiochos B, McDonald A, Chen J, Sharp JJ, Fujiwara Y, Barker JE, Fleming MD, Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells, Nat. Genet 37 (2005) 1264–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ohgami RS, Campagna DR, McDonald A, Fleming MD, The steap proteins are metalloreductases, Blood 108 (2006) 1388–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ohgami RS, Campagna DR, Antiochos B, Wood EB, Sharp JJ, Barker JE, Fleming MD, nm1054: a spontaneous, recessive, hypochromic, microcytic anemia mutation in the mouse, Blood 106 (2005) 3625–3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wellen KE, Fucho R, Gregor MF, Furuhashi M, Morgan C, Lindstad T, Vaillancourt E, Gorgun CZ, Saatcioglu F, Hotamisligil GS, Coordinated regulation of nutrient and inflammatory responses by STAMP2 is essential for metabolic homeostasis, Cell 129 (2007) 537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gunshin H, Starr CN, Direnzo C, Fleming MD, Jin J, Greer EL, Sellers VM, Galica SM, Andrews NC, Cybrd1 (duodenal cytochrome b) is not necessary for dietary iron absorption in mice, Blood 106 (2005) 2879–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhang DL, Su D, Berczi A, Vargas A, Asard H, An ascorbate-reducible cytochrome b561 is localized in macrophage lysosomes, Biochim. Et. Biophys. Acta 1760 (2006) 1903–1913. [DOI] [PubMed] [Google Scholar]
- [27].Gunshin H, Fujiwara Y, Custodio AO, Direnzo C, Robine S, Andrews NC, Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver, J. Clin. Investig 115 (2005) 1258–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nomura N, Nagase T, Miyajima N, Sazuka T, Tanaka A, Sato S, Seki N, Kawarabayasi Y, Ishikawa K, Tabata S, Prediction of the coding sequences of unidentified human genes. II. The coding sequences of 40 new genes (KIAA0041-KIAA0080) deduced by analysis of cDNA clones from human cell line KG-1, DNA Res. Int. J. Rapid Publ. Rep. Genes Genomes 1 (1994) 223–229. [DOI] [PubMed] [Google Scholar]
- [29].Begum NA, Kobayashi M, Moriwaki Y, Matsumoto M, Toyoshima K, Seya T, Mycobacterium bovis BCG cell wall and lipopolysaccharide induce a novel gene, BIGM103, encoding a 7-TM protein: identification of a new protein family having Zn-transporter and Zn-metalloprotease signatures, Genomics 80 (2002) 630–645. [DOI] [PubMed] [Google Scholar]
- [30].Wang CY, Jenkitkasemwong S, Duarte S, Sparkman BK, Shawki A, Mackenzie B, Knutson MD, ZIP8 is an iron and zinc transporter whose cell-surface expression is up-regulated by cellular iron loading, J. Biol. Chem 287 (2012) 34032–34043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Aydemir TB, Chang SM, Guthrie GJ, Maki AB, Ryu MS, Karabiyik A, Cousins RJ, Zinc transporter ZIP14 functions in hepatic zinc, iron and glucose homeostasis during the innate immune response (endotoxemia), PLoS One 7 (2012) e48679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hojyo S, Fukada T, Shimoda S, Ohashi W, Bin BH, Koseki H, Hirano T, The zinc transporter SLC39A14/ZIP14 controls G-protein coupled receptor-mediated signaling required for systemic growth, PLoS One 6 (2011) e18059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Galvez-Peralta M, He L, Jorge-Nebert LF, Wang B, Miller ML, Eppert BL, Afton S, Nebert DW, ZIP8 zinc transporter: indispensable role for both multiple-organ organogenesis and hematopoiesis in utero, PLoS One 7 (2012) e36055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wang B, He L, Dong H, Dalton TP, Nebert DW, Generation of a Slc39a8 hypomorph mouse: markedly decreased ZIP8 Zn(2)(+)/(HCO(3)(−))(2) transporter expression, Biochem. Biophys. Res. Commun 410 (2011) 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zhang W, Jenkitkasemwong S, Chan A, Knutson MD The metal-ion transporter Zip8 (Slc39a8) and iron transport across the placenta in: Proceedings of the 6th Meeting of the International BioIron Society American Journal of Hematology The Zijingang Campus of Zhejiang University, Hangzhou, China: 2015. [Google Scholar]
- [36].Yanatori I, Richardson DR, Imada K, Kishi F, Iron Export through the Transporter Ferroportin 1 Is Modulated by the Iron Chaperone PCBP2, J. Biol. Chem 291 (2016) 17303–17318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ryu MS, Zhang D, Protchenko O, Shakoury-Elizeh M, Philpott CC, PCBP1 and NCOA4 regulate erythroid iron storage and heme biosynthesis, J. Clin. Investig 127 (2017) 1786–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ghanem LR, Kromer A, Silverman IM, Chatterji P, Traxler E, Penzo-Mendez A, Weiss MJ, Stanger BZ, Liebhaber SA, The poly(C) binding protein Pcbp2 and its retrotransposed derivative Pcbp1 Are independently essential to mouse development, Mol. Cell. Biol 36 (2016) 304–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Oh JY, Hamm J, Xu X, Genschmer K, Zhong M, Lebensburger J, Marques MB, Kerby JD, Pittet JF, Gaggar A, Patel RP, Absorbance and redox based approaches for measuring free heme and free hemoglobin in biological matrices, Redox Biol 9 (2016) 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Li L, Fang CJ, Ryan JC, Niemi EC, Lebron JA, Bjorkman PJ, Arase H, Torti FM, Torti SV, Nakamura MC, Seaman WE, Binding and uptake of H-ferritin are mediated by human transferrin receptor-1, Proc. Natl. Acad. Sci. USA 107 (2010) 3505–3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Li JY, Paragas N, Ned RM, Qiu AD, Viltard M, Leete T, Drexler IR, Chen X, Sanna-Cherchi S, Mohammed F, Williams D, Lin CS, Schmidt-Ott KM, Andrews NC, Barasch J, Scara5 Is a ferritin receptor mediating non-transferrin iron delivery, Dev. Cell 16 (2009) 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hvidberg V, Maniecki MB, Jacobsen C, Hojrup P, Moller HJ, Moestrup SK, Identification of the receptor scavenging hemopexin-heme complexes, Blood 106 (2005) 2572–2579. [DOI] [PubMed] [Google Scholar]
- [43].Tang Z, Niven-Fairchild T, Tadesse S, Norwitz ER, Buhimschi CS, Buhimschi IA, Guller S, Glucocorticoids enhance CD163 expression in placental Hofbauer cells, Endocrinology 154 (2013) 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Brissot P, Ropert M, Le Lan C, Loreal O, Non-transferrin bound iron: a key role in iron overload and iron toxicity, Biochim. Et. Biophys. Acta 1820 (2012) 403–410. [DOI] [PubMed] [Google Scholar]
- [45].Baron J, Ben-David G, Hallak M, Changes in non-transferrin-bound iron (NTBI) in pregnant women on iron supplements, Eur. J. Obstet. Gynecol. Reprod. Biol 140 (2008) 281–282. [DOI] [PubMed] [Google Scholar]
- [46].Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt SJ, Moynihan J, Paw BH, Drejer A, Barut B, Zapata A, Law TC, Brugnara C, Lux SE, Pinkus GS, Pinkus JL, Kingsley PD, Palis J, Fleming MD, Andrews NC, Zon LI, Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter, Nature 403 (776–781) (2000). [DOI] [PubMed] [Google Scholar]
- [47].Bradley J, Leibold EA, Harris ZL, Wobken JD, Clarke S, Zumbrennen KB, Eisenstein RS, Georgieff MK, Influence of gestational age and fetal iron status on IRP activity and iron transporter protein expression in third-trimester human placenta, Am. J. Physiol. Regul. Integr. Comp. Physiol 287 (2004) R894–R901. [DOI] [PubMed] [Google Scholar]
- [48].Bielinska M, Narita N, Wilson DB, Distinct roles for visceral endoderm during embryonic mouse development, Int. J. Dev. Biol 43 (1999) 183–205. [PubMed] [Google Scholar]
- [49].Mok H, Mendoza M, Prchal JT, Balogh P, Schumacher A, Dysregulation of ferroportin 1 interferes with spleen organogenesis in polycythaemia mice, Development 131 (2004) 4871–4881. [DOI] [PubMed] [Google Scholar]
- [50].Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC, The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis, Cell Metab. 1 (2005) 191–200. [DOI] [PubMed] [Google Scholar]
- [51].Fuqua BK, Lu Y, Darshan D, Frazer DM, Wilkins SJ, Wolkow N, Bell AG, Hsu J, Yu CC, Chen H, Dunaief JL, Anderson GJ, Vulpe CD, The multicopper ferroxidase hephaestin enhances intestinal iron absorption in mice, PLoS One 9 (2014) e98792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Mitchell CJ, Shawki A, Ganz T, Nemeth E, Mackenzie B, Functional properties of human ferroportin, a cellular iron exporter reactive also with cobalt and zinc, Am. J. Physiol. Cell Physiol 306 (2014) C450–C459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Harris ZL, Durley AP, Man TK, Gitlin JD, Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux, Proc. Natl. Acad. Sci. USA 96 (1999) 10812–10817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Li YQ, Bai B, Cao XX, Yan H, Zhuang GH, Ferroportin 1 and hephaestin expression in BeWo cell line with different iron treatment, Cell Biochem. Funct 30 (2012) 249–255. [DOI] [PubMed] [Google Scholar]
- [55].Chen H, Attieh ZK, Syed BA, Kuo YM, Stevens V, Fuqua BK, Andersen HS, Naylor CE, Evans RW, Gambling L, Danzeisen R, Bacouri-Haidar M, Usta J, Vulpe CD, McArdle HJ, Identification of zyklopen, a new member of the vertebrate multicopper ferroxidase family, and characterization in rodents and human cells, J. Nutr 140 (2010) 1728–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Guller S, Buhimschi CS, Ma YY, Huang ST, Yang L, Kuczynski E, Zambrano E, Lockwood CJ, Buhimschi IA, Placental expression of ceruloplasmin in pregnancies complicated by severe preeclampsia, Lab. Investig. J. Tech. Methods Pathol 88 (2008) 1057–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Danzeisen R, Ponnambalam S, Lea RG, Page K, Gambling L, McArdle HJ, The effect of ceruloplasmin on iron release from placental (BeWo) cells; evidence for an endogenous Cu oxidase, Placenta 21 (2000) 805–812. [DOI] [PubMed] [Google Scholar]
- [58].Roeser HP, Lee GR, Nacht S, Cartwright GE, The role of ceruloplasmin in iron metabolism, J. Clin. Investig 49 (1970) 2408–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Fleming RE, Gitlin JD, Primary structure of rat ceruloplasmin and analysis of tissue-specific gene expression during development, J. Biol. Chem 265 (1990) 7701–7707. [PubMed] [Google Scholar]
- [60].Vulpe CD, Kuo YM, Murphy TL, Cowley L, Askwith C, Libina N, Gitschier J, Anderson GJ, Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse, Nat. Genet 21 (1999) 195–199. [DOI] [PubMed] [Google Scholar]
- [61].Kingston PJ, Bannerman CEM, Bannerman RM, Iron-deficiency anemia in newborn sla mice - genetic defect of placental iron transport, Br. J. Haematol 40 (1978) 265–276. [DOI] [PubMed] [Google Scholar]
- [62].Hahn P, Qian Y, Dentchev T, Chen L, Beard J, Harris ZL, Dunaief JL, Disruption of ceruloplasmin and hephaestin in mice causes retinal iron overload and retinal degeneration with features of age-related macular degeneration, Proc. Natl. Acad. Sci. USA 101 (2004) 13850–13855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Fuqua B, Lu Y, Darshan D, Frazer D, Wilkins S, Page K, Vulpe C, Anderson G, The role of multicopper ferroxidases in mammalian iron homeostasis, FASEB J. 28 (2014). [Google Scholar]
- [64].Cao C, Fleming MD, The placenta: the forgotten essential organ of iron transport, Nutr. Rev 74 (2016) 421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Elad D, Levkovitz R, Jaffa AJ, Desoye G, Hod M, Have we neglected the role of fetal endothelium in transplacental transport? Traffic 15 (2014) 122–126. [DOI] [PubMed] [Google Scholar]
- [66].Fryer AA, Jones P, Strange R, Hume R, Bell JE, Plasma protein levels in normal human fetuses: 13 to 41 weeks’ gestation, Br. J. Obstet. Gynaecol 100 (1993) 850–855. [DOI] [PubMed] [Google Scholar]
- [67].Gitlin D, Biasucci A, Development of gamma G, gamma A, gamma M, beta IC-beta IA, C 1 esterase inhibitor, ceruloplasmin, transferrin, hemopexin, haptoglobin, fibrinogen, plasminogen, alpha 1-antitrypsin, orosomucoid, beta-lipoprotein, alpha 2-macroglobulin, and prealbumin in the human conceptus, J. Clin. Investig 48 (1969) 1433–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Levin MJ, Tuil D, Uzan G, Dreyfus JC, Kahn A, Expression of the transferrin gene during development of non-hepatic tissues: high level of transferrin mRNA in fetal muscle and adult brain, Biochem. Biophys. Res. Commun 122 (1984) 212–217. [DOI] [PubMed] [Google Scholar]
- [69].Evans P, Cindrova-Davies T, Muttukrishna S, Burton GJ, Porter J, Jauniaux E, Hepcidin and iron species distribution inside the first-trimester human gestational sac, Mol. Human. Reprod 17 (2011) 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Trenor CC 3rd, Campagna DR, Sellers VM, Andrews NC, Fleming MD, The molecular defect in hypotransferrinemic mice, Blood 96 (2000) 1113–1118. [PubMed] [Google Scholar]
- [71].Xu W, Barrientos T, Mao L, Rockman HA, Sauve AA, Andrews NC, Lethal Cardiomyopathy in Mice Lacking Transferrin Receptor in the Heart, Cell Rep 13 (2015) 533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Allen LH, Anemia and iron deficiency: effects on pregnancy outcome, Am. J. Clin. Nutr 71 (2000) 1280S–1284S. [DOI] [PubMed] [Google Scholar]
- [73].Radlowski EC, Johnson RW, Perinatal iron deficiency and neurocognitive development, Front. Human. Neurosci 7 (2013) 585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Barrett JF, Whittaker PG, Williams JG, Lind T, Absorption of non-haem iron from food during normal pregnancy, Bmj 309 (1994) 79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Milman N, Iron and pregnancy–a delicate balance, Ann. Hematol 85 (2006) 559–565. [DOI] [PubMed] [Google Scholar]
- [76].Hubbard AC, Bandyopadhyay S, Wojczyk BS, Spitalnik SL, Hod EA, Prestia KA, Effect of dietary iron on fetal growth in pregnant mice, Comp. Med 63 (2013) 127–135. [PMC free article] [PubMed] [Google Scholar]
- [77].Gao G, Liu SY, Wang HJ, Zhang TW, Yu P, Duan XL, Zhao SE, Chang YZ, Effects of pregnancy and lactation on iron metabolism in rats, BioMed. Res. Int 2015 (2015) 105325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Ganz T, Nemeth E, Hepcidin and iron homeostasis, Biochim. Et. Biophys. Acta 1823 (2012) 1434–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Ganz T, Systemic iron homeostasis, Physiol. Rev 93 (2013) 1721–1741. [DOI] [PubMed] [Google Scholar]
- [80].Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J, Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization, Science 306 (2004) 2090–2093. [DOI] [PubMed] [Google Scholar]
- [81].Sangkhae V, Nemeth E, Regulation of the iron Homeostatic hormone hepcidin, Adv. Nutr 8 (2017) 126–136 (Bethesda, Md). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].van Santen S, Kroot JJ, Zijderveld G, Wiegerinck ET, Spaanderman ME, Swinkels DW, The iron regulatory hormone hepcidin is decreased in pregnancy: a prospective longitudinal study, Clin. Chem. Lab. Med. CCLM / FESCC 51 (2013) 1395–1401. [DOI] [PubMed] [Google Scholar]
- [83].Koenig MD, Tussing-Humphreys L, Day J, Cadwell B, Nemeth E, Hepcidin and iron homeostasis during pregnancy, Nutrients 6 (2014) 3062–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Millard KN, Frazer DM, Wilkins SJ, Anderson GJ, Changes in the expression of intestinal iron transport and hepatic regulatory molecules explain the enhanced iron absorption associated with pregnancy in the rat, Gut 53 (2004) 655–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Young MF, Griffin I, Pressman E, McIntyre AW, Cooper E, McNanley T, Harris ZL, Westerman M, O’Brien KO, Maternal hepcidin is associated with placental transfer of iron derived from dietary heme and nonheme sources, J. Nutr 142 (2012) 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Dewey KG, Oaks BM, U-shaped curve for risk associated with maternal hemoglobin, iron status, or iron supplementation, Am. J. Clin. Nutr 106 (2017) 1694S–1702S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Taylor CL, Brannon PM, Introduction to workshop on iron screening and supplementation in iron-replete pregnant women and young children, Am. J. Clin. Nutr 106 (2017) 1547S–1554S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Nicolas G, Bennoun M, Porteu A, Mativet S, Beaumont C, Grandchamp B, Sirito M, Sawadogo M, Kahn A, Vaulont S, Severe iron deficiency anemia in transgenic mice expressing liver hepcidin, Proc. Natl. Acad. Sci. USA 99 (2002) 4596–4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Willemetz A, Lenoir A, Deschemin JC, Lopez-Otin C, Ramsay AJ, Vaulont S, Nicolas G, Matriptase-2 is essential for hepcidin repression during fetal life and postnatal development in mice to maintain iron homeostasis, Blood 124 (2014) 441–444. [DOI] [PubMed] [Google Scholar]
- [90].Rehu M, Punnonen K, Ostland V, Heinonen S, Westerman M, Pulkki K, Sankilampi U, Maternal serum hepcidin is low at term and independent of cord blood iron status, Eur. J. Haematol 85 (2010) 345–352. [DOI] [PubMed] [Google Scholar]
- [91].Gambling L, Danzeisen R, Gair S, Lea RG, Charania Z, Solanky N, Joory KD, Srai SK, McArdle HJ, Effect of iron deficiency on placental transfer of iron and expression of iron transport proteins in vivo and in vitro, Biochem. J 356 (2001) 883–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wilkinson N, Pantopoulos K, The IRP/IRE system in vivo: insights from mouse models, Front. Pharmacol 5 (2014) 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Georgieff MK, Berry SA, Wobken JD, Leibold EA, Increased placental iron regulatory protein-1 expression in diabetic pregnancies complicated by fetal iron deficiency, Placenta 20 (1999) 87–93. [DOI] [PubMed] [Google Scholar]
- [94].Li YQ, Yan H, Bai B, Change in iron transporter expression in human term placenta with different maternal iron status, Eur. J. Obstet. Gynecol. Reprod. Biol 140 (2008) 48–54. [DOI] [PubMed] [Google Scholar]
- [95].Best CM, Pressman EK, Cao C, Cooper E, Guillet R, Yost OL, Galati J, Kent TR, O’Brien KO, Maternal iron status during pregnancy compared with neonatal iron status better predicts placental iron transporter expression in humans, FASEB J.: Off. Publ. Fed. Am. Soc. Exp. Biol 30 (2016) 3541–3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Chen AC, Donovan A, Ned-Sykes R, Andrews NC, Noncanonical role of transferrin receptor 1 is essential for intestinal homeostasis, Proc. Natl. Acad. Sci. USA 112 (2015) 11714–11719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Fleming MD, Trenor CC 3rd, Su MA, Foernzler D, Beier DR, Dietrich WF, Andrews NC, Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene, Nat. Genet 16 (1997) 383–386. [DOI] [PubMed] [Google Scholar]
- [98].Russell ES, Nash DJ, Bernstein SE, Kent EL, McFarland EC, Matthews SM, Norwood MS, Characterization and genetic studies of microcytic anemia in house mouse, Blood 35 (1970) 838–850. [PubMed] [Google Scholar]
- [99].Edwards JA, Hoke JE, Red cell iron uptake in hereditary microcytic anemia, Blood 46 (1975) 381–388. [PubMed] [Google Scholar]
- [100].Pinkerton PH, Bannerman RM, Hereditary defect in iron absorption in mice, Nature 216 (1967) 482–483. [DOI] [PubMed] [Google Scholar]


