Abstract
Chronic stress leads to sex-dependent outcomes on spatial memory by producing deficits in males, but not in females. Recently it was reported that compared to daily restraint, intermittent restraint (IR) produced more robust stress and anxiety responses in male rats. Whether IR would be sufficiently robust to impair hippocampal-dependent spatial memory in both male and female rats was investigated. IR involved mixing restraint with non-restraint days over weeks before assessing spatial memory and anxiety profile on the radial arm water maze, object placement, novel object recognition, Y-maze, open field and novelty suppressed feeding. Experiments 1 and 2 used Sprague-Dawley male rats only and determined that IR for 6hrs/d (IR6), but not 2hrs/d, impaired spatial memory and that task order was important. In experiment 3, IR6 was extended for 6wks before spatial memory testing commenced using both sexes. Unexpectedly, an extended IR6 paradigm failed to impair spatial memory in either sex, suggesting that by 6wks IR6 may have become predictable. In experiment 4, an unpredictable IR (UIR) paradigm was implemented, in which restraint duration (30 or 60-min) combined with orbital shaking, time of day, and the days off from UIR were varied. UIR impaired spatial memory in males, but not in females. Together with other reports, these findings support the interpretation that chronic stress negatively impairs hippocampal-dependent function in males, but not in females. We interpret these findings to show that females are more resilient to chronic stress than are males as it pertains to spatial ability.
Keywords: Anxiety, Cognition, Chronic stress, Depression, Sex differences, Spatial memory
1. Introduction
Major Depressive Disorder (MDD) affects more than 300 million people worldwide and is the leading cause of global disability [1,2]. Despite the wide variety of interventions, approximately a third of MDD patients fail to improve with treatment [3,4], emphasizing the need to identify novel mechanisms for potentially new therapeutic targets. Chronic stress in preclinical research is commonly used to study depressive-like symptoms [5-7] and the respective changes in cognitive function [8,9]. While no one animal model produces all symptoms of MDD, together they can offer new novel insights [10].
Chronic restraint is a common manipulation in rodents, but also carries some caveats. Restraint is relatively cost-effective, has readily available materials for construction, and is straightforward to implement. Restraint also produces fairly consistent outcomes across animals, which is not always the case for paradigms that require two animals to engage, such as with social defeat [11,12]. Some caveats include the concern that restraint stress is not ecologically relevant [13]; however, this is less of an issue when the goal is to induce neurobiological changes in certain limbic structures, such as the hippocampus, before initiating behavioral assessments. In addition, chronic restraint is a homotypic (i.e., a repeat of the same type of) stressor, leading to adrenal response habituation in which the stress steroid, corticosterone, levels in the blood become less pronounced than compared to the first restraint exposure [14-16]. Again, this is less of a concern because the muted corticosterone response aligns with adrenal dysregulation found in patients with MDD [16-21]. Consequently, restraint stress in rodents produces a subset of outcomes that align with those found in MDD, especially as they pertain to the hippocampus and stress responsivity.
A puzzling outcome following chronic daily restraint is the sex differences observed in spatial ability. In male rodents, chronic restraint stress compromises the hippocampus and impairs hippocampal-dependent spatial learning and memory [22-28]. In contrast, female rodents fail to show hippocampal-dependent memory deficits following chronic restraint [29-32] or even other chronic stressors [33-35]. Instead, female rodents almost seem to be resilient in the face of chronic stress, and may even show improved spatial ability in the Morris Water Maze, Y-maze and Radial arm water maze (RAWM), [29,31,33,36-40]. The concern is that in humans, women are nearly twice as likely as men to be diagnosed with MDD, even when accounting for willingness to seek out help [41,42]. Consequently, one aim is to identify a chronic stress paradigm that leads to cognitive deficits in female rodents.
When characterizing the behavioral phenotype in animal models, obtaining several behavior measures is helpful. For that reason, behavioral batteries using multiple tests can be advantageous in order to examine different aspects of the spatial memory domain and other cognitive abilities. This requires measuring cognition over multiple days, but the timeline of the daily restraint paradigm is restrictive because spatial memory deficits begin to improve in the days and weeks after chronic stress has ended [43-45]. Given the limited window of time to capture cognitive deficits following chronic restraint, identifying a paradigm that allows for multiple cognitive assessments during this window may be of great benefit.
Recently, Zhang and colleagues [46] found that a modified restraint model using a work-week design, produced stress and anxiety responses that were greater than that observed with chronic daily restraint for the same duration. Specifically, in male Sprague- Dawley rats, restraint for 20 minutes/day in a hemi-cylinder for 5 days, followed by two days off, and then with restraint for two more days produced robust effects on stress responses, body weight gain and anxiety levels than compared to restraint daily for the same period. This raised the question as to whether the robust nature of this interrupted restraint stress paradigm may be useful in producing more substantial effects on spatial memory in both male and female rats.
While this interrupted restraint paradigm has the potential to be a more robust stressor than daily restraint, many questions remain as it pertains to the way it is used to assess spatial memory. The goal of the current series of experiments was to use a modified version of the interrupted restraint paradigm described by Zhang et al., [46], which we termed intermittent restraint (IR), on hippocampal function in both male and female rodents. First, it was unclear whether an extended duration of three weeks (instead of nine days as used by Zhang et al., [46]) would have similar potentiating effects on impairing hippocampal function in males when compared to the daily restraint paradigm. Second, Zhang and colleagues [46] used plastic hemi-cylinders to restrain rats for just 20 min each day; however, past work from our team found that daily restraint in wire mesh for 2 hours/day for three weeks failed to impair spatial memory in males [47]. Consequently, experiment 1 included intermittent restraint for two-hours (IR2) and six-hours (IR6), as well as the traditional daily restraint paradigm for six hours/day (DR6) and compared outcomes on spatial ability in male rats. In addition, the IR may produce a more robust deficit on spatial memory and so a behavioral battery was incorporated in the event that spatial memory impairments persisted beyond the few days after restraint ended. Hence, experiments 1 and 2 used a behavioral battery in male rats, with experiment 2 testing from the least to most aversive task. Experiment 3 tested both male and female rats using an extended IR6 paradigm for a minimum of 6 weeks. Experiment 4 implemented an unpredictable intermittent restraint (UIR) paradigm in both sexes, with behavioral testing occurring on days when UIR was not used. We tested the hypothesis that IR and/or UIR would produce spatial memory deficits in both male and female rats and that these deficits would be long-lasting.
2. Materials and methods
2.1. Subjects.
Arizona State University Institutional Animal Care and Use Committee approved the procedures, which align with the Guide for the Care and Use of Laboratory Animals. Male and Female Sprague-Dawley (Charles River Laboratories, Hollister, CA, USA) rats weighing approximately 200-225 grams upon arrival were pair housed in standard laboratory cages (21-22 °C, corncob bedding). Male and female rats were housed in same-sex housing units and were tested on separate days or in different rooms. Except where noted below, animals were allowed food and water ad libitum. Animals were housed on a reverse 12:12 light cycle; lights off at 07:00. All procedures occurred during the dark phase of the light cycle.
2.2. Chronic stress procedure
Rats were chronically stressed by wire mesh restraint. Restrainers were constructed from wire mesh (19 cm diameter × 26.5 cm long for males, 16.5 cm diameter x 26.5 cm long for females, aluminum screen wire Model #3001120, Lowes) with the cut edges and ends sealed with Plasti Dip (Performix #075815116024). Once rats were placed in the restraint, the ends were secured with black binder clips (Staples Inc., Framingham, MA, USA). Animals were upgraded to larger wire-mesh restrainers as they grew (21.5 cm diameter x 29 cm long for males, 19 cm circumference x 26.5 cm long for females). Control rats (CON) were always housed in a chamber separate than the stressed rats in order to reduce the likelihood of communication through odor, sounds and sight. To maintain similar handling procedures and access to food and water across groups, CON rats were handled daily, and food restricted for the same duration as the restrained rats. Body weights were measured weekly for all groups. For experiments 1-3, restraint occurred between the hours of 09:00 and 15:00 of the dark phase of the light cycle. In experiment 4, restraint occurred between the hours of 07:00 and 21:00. One to three days following the last behavioral testing day in Experiments 1 and 4, rats were euthanized using isoflurane and rapidly decapitated. Adrenal glands, thymus and uterus were excised and weighed for a secondary measure of stressor effectiveness.
2.3. Treatment conditions
Experiment 1: Effects of three weeks IR2 and IR6 on spatial memory in male rats. One stress group was restrained for 6h/day for 21 consecutive days (daily restraint, 6-hours/d, DR6), another was restrained for 6h/day in an interrupted pattern: 5 days restrained then two days without restraint over a period of 23 days (Intermittent restraint, 6-hours/d, IR6) and the third was restrained for 2h/day over a period of 23 days (IR2). The sum total of restraint days for DR and IR was 21 and 17 days, respectively. CON rats were not restrained. Each group, n=12 for a total of 48 rats.
Experiment 2: Effects of three weeks IR6 on a behavioral battery, ordered with the least aversive task first and using male rats. Three treatment conditions were used: CON (n= 14), IR6 (n= 12) and DR6 (n=12). DR6 and IR6 restraint were described in experiment 1.
Experiment 3: Effects of an extended IR6 paradigm on spatial memory in male and female rats. IR6 was used on half the male and female rats and was performed for 6 weeks before the first spatial task was implemented. Details are listed in experiment 1. Each group (n= 12) for a total of 48 rats. One female rat died from unrelated complications during the experiment, and so her behavior was included up until that point.
Experiment 4: Effect of unpredictable intermittent restraint (UIR) on spatial memory in male and female rats. The stressor was changed to be unpredictable and robust, termed “Unpredictable Intermittent Restraint” or UIR. Restraint occurred for 30 or 60 min, while on an orbital shaker (120 rpm) and at different times of day (ranging from 7:00 to 21:00) and for different consecutive day lengths (2-6 days) before one or two days off without a stressor. There were four groups (n= 12) for a total of 48 rats.
2.4. Behavioral tests
In order to minimize potential behavioral outcomes that may arise from rats being exposed to different investigators, worn/unlaundered t-shirts were located in the testing room out of the rodent’s view when inside the apparatus [48]. Fans were placed in the room to provide white noise and to disperse odors. Four different testing rooms were used, and curtains drawn as needed to ensure that testing environments and cues differed across tasks. All behavior was recorded by cameras (GoPro Hero3) mounted on the ceiling. Unless otherwise noted, three home cages of pair-housed rats were carted from the animal colony and placed in an adjacent room until it was time for testing. An investigator retrieved the home-cage from the adjacent room and brought it to the testing room. The investigator remained behind a curtain during trials, out of sight of the rats, but could view the rats on a live video feed via a computer monitor.
2.4.1. Y-maze
The Y-maze is a task that requires hippocampal function and spatial memory to navigate [23,25]. Y-maze testing occurred over two days to accommodate the large number of rats (20-24 rats tested on each day, counterbalanced for treatment).
2.4.1.1. Y-maze apparatus.
Two Y-maze apparatuses were located in the same room and were constructed of black Plexiglas. Three identical and symmetrical arms (58.4 cm long × 20.3 cm wide x 38.1 cm height) radiated from the center. The sides were tall enough that the rats could not jump out of the maze. Outside the maze, large, explicit cues (painted shapes on the walls and furniture in the room) were located on the walls and around the room in order to encourage the use of extra-maze cues. The light intensity at the floor of the maze was 80-90 lux for the duration of testing. No explicit cues were present inside of the maze. Corncob rat bedding covered the maze floor to about 3 cm thick.
2.4.1.2. Y-maze procedure
Cage mates were tested simultaneously, in side-by-side Y-mazes. For trial 1, a rat was placed in one arm, which was then designated the “start” arm for that rat. Another arm was blocked with black Plexiglas, so the rat was able to explore the start and the other open arm, called the “other” arm. Rats were given 15 min to explore the maze and two accessible arms before they were removed, returned to their home cage and brought back to the animal colony. After each Y-maze exposure, the bedding in the maze was mixed to dissipate the odors before the next set of rats were tested. At the end of trial 1 and before the start of trial 2, the two Y-mazes were swapped so that rats would be tested in a new Y-maze, but in the exact same position as before to further reduce the likelihood that they would use intramaze cues.
Trial 2 began after a 4-hr intertrial interval (ITI). Rats were brought back to the testing room as before and now the previously blocked arm, the “novel” arm, was open to investigation. Rats started in the same arm as in trial 1 and were given 5 minutes to explore. The start, other, and novel arms were counter-balanced across groups but held constant for a given rat.
2.4.1.3. Y-maze quantification
Behavior was quantified at a later date by an investigator who was blind to the treatments and novel/other arm identities. The dependent variables measured in trial 2 were the number of entries made (entry) and time spent in each arm (dwell) during each minute. An entry was defined as the forelimbs crossing from the middle of the maze into an arm entrance. The first two minutes of exploration during testing were used for analysis because rats habituate quickly to the Y-maze [49]. The start arm was not included in the analysis because the rats were placed there at the beginning of the trial, causing an inherent bias compared to the novel and other arms. Entry and dwell data were converted into percentages for all three arms (novel, start, and other), with chance being equal to 33.3%. Discrimination performance for the novel arm compared to the other arm was calculated by subtracting the percentage of entries into the other arm from the percentage of entries into the novel arm. Dwell was calculated similarly for percentage of time spent in the arms. For simplicity, only entry data are shown in the figures. Chance for the discrimination index would be 50% and with a preference for the novel arm being a value greater than 50%.
2.4.2. Open Field (OF)
OF acclimates rats to the environment in which they are to be tested on subsequent days. Moreover, OF can serve as a measure of locomotor activity and anxiety-like behavior [50,51].
2.4.2.1. OF apparatus
The OF apparatus consisted of two side-by-side black square fields (96.5 cm x 96.5 cm) with high walls (38.1 cm height) to prevent escape and yet permitting the rats to see the extra-maze cues around the walls and room (painted geometric shapes, shelving). The light intensity at the floor of the field was 150-160 lux for the duration of OF testing and during the following testing days.
2.4.2.2. OF procedure
An investigator carted three pairs of rats from the home colony room and placed in an adjacent room until OF testing. In the OF test, rats were tested in pairs with their cage mate in an adjacent OF apparatus. Rats were allowed to explore for 10 min and then removed and returned to the animal colony. After each trial, an investigator wiped the arena with paper towels and Lime/Sea Salt Scented, Method All-Purpose cleaner.
2.4.2.3. OF quantification
Behavior was quantified at a later date by an investigator who was unaware of the treatment identities. The 10-min open field trial was quantified in two, 5-min blocks, utilizing a 4 × 4 grid. The first 5-min block was utilized for analyses because rats readily habituate to OF testing [52,53], but the full ten minutes provided rats with time to acclimate to the arena and room. Peripheral crossings were quantified as the front two paws crossing a line on the periphery of the grid. Central crossings were quantified as the front two paws crossing a center gridline. An anxiety index was calculated as used in our past work [54]:
Total locomotor activity was scored as the number of line crossings (front two paws crossing any line).
2.4.3. Novel Object Recognition (NOR) and Object Placement (OP)
NOR can assess a type of memory that does not necessarily require the hippocampus [55-57] and was used to provide minimal cognitive challenge with just 1 minute inter-trial-interval (ITI). Experiment 4 also implemented a 1-hour ITI NOR test as an added measure of cognitive ability. OP testing assesses hippocampal-mediated spatial memory in which rats use the spatial context to detect familiar objects in new locations [54,57-59]. A 1-hour ITI in OP has been shown to be effective in assessing hippocampal mediated deficits [60,61]. For this reasoning, a 1-hour ITI was utilized for OP testing in the experiments in this study.
2.4.3.1. NOR and OP apparatus
NOR and OP occurred in the same OF arena in which rats were previously acclimated and in a testing room under similar conditions. When shifting from one task to the next, the OF remained in the same location, but vinyl curtains were used to obscure/change the cues in the room.
The objects were sufficiently large so that rats could not climb or topple them and were made of ceramic, metal, or glass for easy cleaning. Each object had at least four duplicates so that they could be swapped quickly. The objects included a plastic red opaque rectangle (24 cm high x 10 cm x 8 cm), a tall, slender opaque green glass bottle (36 cm high, 9 cm wide), and a tall, slender gold rectangle attached to a heavy rectangular base (23 cm high, 6 cm wide). To ensure object stability, the objects were either filled with sand (if hollow) or secured to a heavy aluminum base. Before each trial, the objects and arena were cleaned with Method All-Purpose Cleaner. Different scented cleaners were used for OP and NOR, but the same scent was used within a session.
2.4.3.2. NOR and OP procedure
All rats were tested on the same day with pairs of cage mates being tested simultaneously in adjacent fields. During trial 1, the arena contained two identical objects. A rat was placed in a corner of the arena, away from the objects and allowed to explore for 3 min. After the trial ended, the rat was removed and returned to its home cage with its cage mate. The arena and objects were wiped clean. After the designated ITI, rats were returned to the arena in trial 2 at the same starting location as before, but with the following differences. In the NOR, both objects were replaced, but one object was identical to the object used in trial 1 and the other was exchanged for an object that was unique. These objects were placed in the same location as was used in trial 1. In the OP, the objects were replaced with new, but identical objects to those used in trial 1; however, one object was moved to a novel location and the other remained in the same location as in trial 1. In trial 2, rats were allowed to explore the objects in for 3 min. The start locations of the rats and the object locations were counter-balanced across groups but held constant for a given rat.
2.4.3.3. NOR and OP quantification
Behavior was quantified at a later date by an investigator who was unaware of the treatment identities. Exploration was defined as the rat facing the object within 3 cm and attentively interacting with the object. Rats were excluded from analyses if they failed to explore both objects in trial 1 or explored the objects for less than 10 second total in either trial 1 or 2. An object exploration index was calculated as reported elsewhere [54]:
2.4.4. Radial Arm Water Maze (RAWM) win-stay paradigm with 1 platform
RAWM testing was conducted because of its use in measuring spatial ability in rodents [45,62,63] and in assessing spatial learning and memory following chronic stress [33,45,63,64].
2.4.4.1. RAWM apparatus
Two RAWM mazes and rooms were used. The RAWM was constructed of black polypropylene, with eight symmetrical arms (27.9 cm long × 12.7 cm wide) originating from a circular center (48 cm diameter). The maze was filled with water and allowed to equilibrate to room temperature ranging from 20 to 22 °C. Black powder tempera paint was added to the water until its opacity was sufficient to conceal a black rubber platform placed in one of the eight arms. The testing rooms provided several prominent extra-maze cues including the door to the room, shelves, heat lamps, and cues made of black and white construction paper located on the walls.
2.4.4.2. RAWM procedure
Groups were counterbalanced between the two testing rooms and experimenters. Rats were tested in squads of 6-8 (e.g. two rats from each experimental group). Once a rat finished a trial, the other rats in the squad completed the given trial before the first rat in the squad began the next trial. Testing occurred over three days with 8 trials occurring on each of the first 2 days and a single retention trial occurring on the third day (17 total trials). Each trial consisted of releasing a rat into an arm (start arm) that did not contain the platform, the start arm was also never directly across from the platform arm to increase the navigational demand of the task. For each rat, the start arm location varied across trials, while the platform arm remained in the same location. Once a rat reached the hidden platform, it was permitted to remain on the platform for 15 s to visualize the room spatially before being returned to its testing cage in the same room, under a heat lamp. If a rat failed to find the platform within 3 min, the experimenter used a pole to guide the rat to the hidden platform. After each trial, any debris was skimmed and removed with a net and the water stirred to reduce the likelihood of rats using non-spatial cues.
2.4.4.3. RAWM quantification
The investigator recorded arm entrances during the behavioral testing and then arm entries were quantified at a later date. An entrance was recorded when the tip of the rat’s nose crossed a mark on the outside of the maze (about 22 cm into the arm). Reference memory errors were considered the number of first-time entries into arms that did not contain the platform within a given trial (first entries into the start arm were also quantified as reference memory errors). Working memory errors were considered the number of repeat entries into an arm that did not contain the platform within a given trial (i.e. repeat entries into an arm where a reference memory error was previously committed in the same trial).
2.4.5. Novelty Suppressed Feeding (NSF)
Anxiety-like behavior was assessed using a test to examine how readily rats will eat food in a novel environment [65,66]. Slow latency to approach the food and begin eating may be indicative of high anxiety.
2.4.5.1. NSF apparatus
NSF occurred in a novel testing room, but using the same dual-field apparatus as implemented for the OF, OP and NOR. The OF arena was brightly lit (170-180 lux) and located in a novel testing room without obvious spatial cues. Before each rat was tested, the arena was cleaned with 70% isopropyl alcohol.
2.4.5.2. NSF procedure
Twenty-four hours prior to NSF, rats were food deprived, but with unlimited access to drinking water. On the day of testing, rats were carted, two pairs at a time in their home cages, to a holding room prior to testing. Then, an investigator retrieved the cage and brought it to the NSF testing arena. In the center of the arena there was a pile of standard rodent chow. The rat was placed in one corner of the field, and the amount of time it took the rat to approach the food was recorded. If the rat did not approach the food after 8 min, the testing was terminated, and the animal was given a score of 480 sec. When completed, the animals were returned to the animal colony and placed individually in their cages for 10-minutes. In each cage was a pre-weighed piece of rat chow with water still available. The amount of food consumed during the home-cage feeding was measured to assess the motivation of the rats to eat in a familiar environment. Littermates were re-united after the end of the home-cage eating assessment and food was provided ad libitum.
2.4.6. Elevated Platform (EP) stressor
When implemented seven days following chronic stress, novel acute stressors may increase activation in corticolimbic structures in female rats [67,68]. The impact of this novel stressor was assessed on spatial ability at the end of the fourth experiment. Seven days following the last UIR day, rats that previously underwent UIR were exposed to this EP stressor and then spatial memory using the Y-maze was tested one day after the EP stressor concluded.
2.4.6.1. EP Apparatus
An elevated platform (12 cm x 12 cm, elevated 90 cm from ground) was used as a unique stressor. EP occurred in a novel testing room.
2.4.6.2. EP procedure
The UIR rats were transported in pairs in their home cages and the rat pairs were tested simultaneously in two separate rooms. Rats were placed on the top of the EP for 30 minutes. In the event that a rat fell, an investigator immediately returned it to the platform. The EP was performed seven days after the end of UIR, based upon a study that found cognitive deficits using a similar acute stressor seven days after the end of restraint in female rats [67]. One day after the EP stressor, all rats (UIR and CON), were tested in the Y-maze as described previously.
2.5. Statistical analyses
Data were analyzed using SPSS (v. 23). Analysis of variance (ANOVA) was used to analyze parametric data. Body weights were analyzed as a change in body weight from the start of the study to the end of the study, as the experimental timelines varied. Fisher’s LSD post hoc tests were used when ANOVA reached significance. In some cases, planned comparisons were performed. Parametric data were represented as means ± S.E.M. For object tests and the Y-Maze, Wilcoxon Signed Rank tests were used on nonparametric data and represented as medians and quartiles. Statistical significance was defined when p-values were equal to or less than 0.05.
3. Results
3.1. Experiment 1: Effects of three weeks IR2 and IR6 on spatial memory in male rats
Two IR paradigms using 2 and 6 hours of restraint were compared with the 6 hours of daily restraint paradigm on several spatial tasks (Fig. 1A). Rats were tested first on the RAWM because of consistent chronic stress effects from past work [33,45], and because the most robust chronic stress effects occur in RM assessment [33,45].
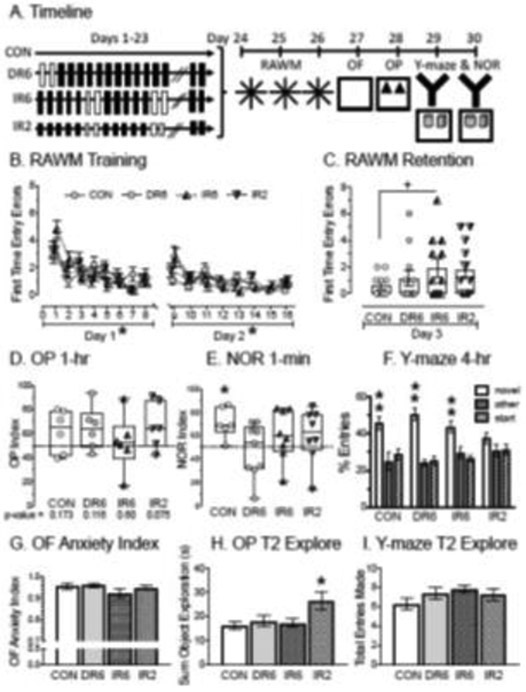
Fig. 1.
Effect of two different IR durations on spatial ability and anxiety profiles using male rats. A) Timeline of manipulations. Rats were restrained for 6hrs (long black-filled rectangles) or 2hrs (short black-filled rectangles). White-filled rectangles indicate days off from restraint. CON = control, DR6 = daily restraint for 6-hrs, IR6 = intermittent restraint for 6-hrs., IR2 = intermittent restraint for 2-hrs. The day after restraint ended, rats were tested on the RAWM for 3 days, followed by the OF, OP and then the Y-maze and NOR. B) First time entry errors on the RAWM during training on days 1 and 2. All groups acquired the task by decreasing first time entry errors over days. There were no group differences. C) Single retention trial on RAWM. IR6 made more first-time entry errors than did CON. D) OP Index from the second trial after a 1-hr ITI. Preference for the moved object will show values greater than 0.5 with the dashed horizontal line indicating chance levels. All groups performed at chance, with p-values listed below each group name; however, half the rats failed to explore, reducing the power of the analyses. E) NOR Index from the second trial with a 1-min ITI, which reflects minimum cognitive load. Preference for the new object will show values greater than 0.5 with the dashed horizontal line indicating chance levels. CON spent more time with the new object than the familiar object despite a low subject number (n=6). The remaining groups performed at chance levels. F) Y-maze performance showing the %Entry in each of the arms (novel, start, other) in trial 2 after a 4-hr ITI. Six to seven days after IR ended, CON, DR6 and IR6 entered (and spent more time in) the novel arm than the other arm (dwell data are not shown). IR2 performed at chance levels. Two asterisks indicate significance in both entry and dwell measures. G) OF anxiety index. All groups showed statistically similar and high anxiety profiles. H) OP total time exploring both objects during trial 2. IR2 spent more time exploring both objects in trial 2 than did CON, DR6, and IR6. There were no other group differences. I) Entries made in all three arms of the Y-maze during Trial 2. All groups made similar number of entries. *p < 0.05, +p < 0.05 with covariate, CON = control, DR6 = daily restraint for 6hrs., IR6 = intermittent restraint for 6hrs, IR2 = intermittent restraint for 2hrs. Boxes represent median and inter-quartile ranges. All other data points are mean ± S.E.M.
As expected, all groups acquired the task rapidly, as shown by decreased errors as trials progressed (Day 1, F7,301 = 13.515, p < 0.05; Day 2 F7,301 = 8.224, p < 0.05), with no significant main effect of group or interaction (Fig. 1B). Surprisingly, on Day 3 when a single retention trial was given, no significant effects were observed (Fig. 1C). However, the data showed high variability and so a subsequent analysis was performed comparing IR6 with CON, as we expected IR6 to be most impaired [47] and errors from training day 1 and 2 were used as a covariate to reduce the variance and revealed a significant effect. IR6 made more errors than did CON on the retention test (p < 0.05 for group, using T1 and T17 as the within-subjects variable).
The next tests performed used an appetitive incentive in the OP, NOR and Y-maze. Unexpectedly, many of the rats failed to sufficiently explore OP and NOR, despite acclimation to the OF under similar parameters used with success in past studies in which RAWM testing was followed by object testing [64]. In the OP, 50% of the rats failed to meet criteria and this was distributed similarly across the experimental groups (n = 6/all groups). Distributions of the OP index from individual rats were plotted (Fig. 1D). While the subject number is low and with insufficient power, it is notable that the group with an OP index distributed around chance levels is IR6. In the NOR with a 1-min ITI and minimal cognitive load, the subject number ranged from six (CON), eight (IR6, IR2) and nine (DR6). Wilcoxon paired analysis revealed that CON spent more time with the novel object than they did with the familiar object (p < 0.05, Fig. 1E). The NOR index from the other groups did not reach statistical significance, even though they had more rats than did the CON cohort (p > 0.1 for DR6, IR6, IR2). On the Y-maze, which was performed six and seven days after the end of IR, nearly all rats explored the arms in trial 2 with just one rat in each in CON and DR6 failing to leave the start arm. Wilcoxon paired tests showed that rats in most treatment conditions entered and spent more time in the novel arm compared to the other arm over the first two minutes for CON (p < 0.05), DR6 (p < 0.05), and IR6 (p < 0.05). IR2 failed to show a significant preference for the novel arm and performed at chance levels (Fig. 1F).
To determine whether anxiety or motor ability may have impacted performance, additional assessments were performed on the OF, OP and Y-maze. An anxiety index was calculated to determine whether the groups differed in anxiety profile regardless of locomotor activity. In the OF, the anxiety index was high and similar for all groups (greater than 90%, Fig. 1G). Consequently, anxiety profile was unlikely to explain differences in performance among groups. However, the groups demonstrated heightened anxiety overall, perhaps from the prior day exposures on the RAWM. This may also explain the lack of investigation for many of the rats on the OP, which requires motivation to explore. For the total time spent exploring objects during trial 2 in OP, an ANOVA revealed significant differences between groups on Trial 2 (F1,3 = 3.56, p < 0.05). LSD post-hoc tests showed that IR2 spent more time exploring both objects than the rest of the groups during OP (p < 0.05 compared to CON, DR6, IR6, Fig. 1H, note, no group differences were found on NOR for trial 2, data not shown). No other statistical differences were found. On the Y-maze, all groups entered a similar number of arms over the first two minutes, ranging from 6.3 ± 0.6 for CON to 7.8 ± 0.4 for IR6 (Fig. 1I). These OP data suggest that motor or motivation may have contributed to the IR2 group’s spatial profile on OP, but a lack of an effect on the total entries of the Y-maze suggest that motor/motivation was unlikely to contribute to spatial ability in the Y-maze. Importantly, CON and IR6 showed similar motor/motivational ability and suggests that they were similarly motivated.
In summary, patterns were observed to suggest that IR6 may have exhibited impaired spatial memory on the RAWM compared to CON, but that performance on the OP and NOR may have been obscured by high anxiety. In addition, spatial memory was displayed on the Y-maze by days 6 and 7 from the CON, DR6 and IR6, but not IR2. Given the high anxiety metric and the exploration differences on OP, the behavior for IR2 was harder to interpret than performance observed earlier on RAWM. These findings suggest that IR6 may have compromised spatial ability on the RAWM, but that this effect was not long lasting.
3.2. Experiment 2: Effects of three weeks IR6 on a behavioral battery, ordered with the least aversive task first and using male rats
The effects of the IR6 and DR6 on a behavioral battery were compared, but with the testing order starting with the least aversive task first (Y-maze) and ending with the most aversive task (RAWM, Fig. 2A).
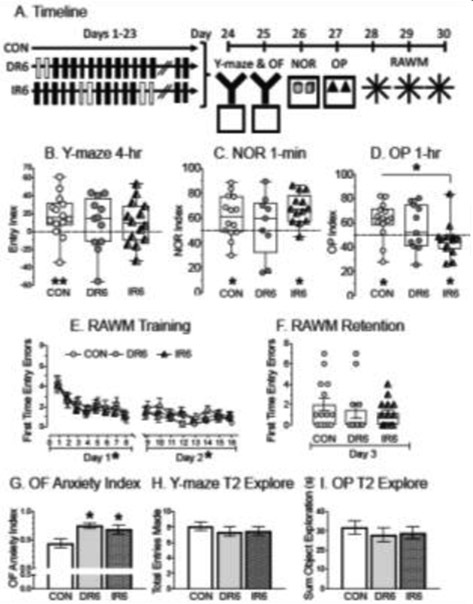
Fig. 2:
Effects of three weeks IR6 on a behavioral battery, ordered with the least aversive task first and using male rats A) Timeline of manipulations. Solid black rectangles indicate restraint day and white-filled rectangles indicate when rats were given a day off. CON = control, DR6 = daily restraint for 6hrs., IR6 = intermittent restraint for 6hrs. The day after restraint ended, rats were tested on the Y-maze and OF, followed by NOR, OP and then the RAWM (last 3 days). B) Y-maze performance showing the %Entry in each of the arms (novel, start, other) in trial 2 after a 4-hr ITI. CON entered (and spent more time in) the novel arm than the other arm, whereas DR6 and IR6 performed at chance levels. Two asterisks indicate significance for each assessment in entry and dwell (dwell data are not shown). C) NOR Index from trial 2 with a 1-min ITI when cognitive load should be minimal. Preference for the new object will show values greater than 0.5 with the dashed horizontal line indicating chance levels. CON and IR6 spent more time with the new object than the familiar object when cognitive load was minimal (1-min ITI). DR6 performed at chance levels. D) OP Index from the second trial after a 1-hr ITI. Preference for the moved object will show values greater than 0.5 with the dashed horizontal line indicating chance levels. CON preferred the object in the novel location. DR6 performed at chance while IR6 preferred the object in the familiar location. IR6 significantly differed from CON in OP index. E) First time entry errors on the RAWM during training on days 1 and 2. All groups acquired the task by decreasing first time entry errors over days. There were no group differences. F) Single retention trial on RAWM. There were no group differences. G) OF anxiety index. DR6 and IR6 showed greater anxiety profiles compared to CON. H) Entries made in all three arms of the Y-maze during Trial 2. All groups made similar number of entries. I) OP total object exploration time. All groups spent similar time exploring both objects. *p < 0.05, CON = control, DR6 = daily restraint for 6hrs., IR6 = intermittent restraint for 6hrs.
On the first two days after restraint ended, rats were tested on the Y-maze. CON rats demonstrated spatial memory, whereas DR6 and IR6 did not (Fig. 2B). For the CON rats, Wilcoxon Signed Rank Tests indicated a significantly greater number of entries in the novel arm than in the other arm (p < 0.05) as well as significantly more time spent in the novel arm (p < 0.05, data not shown). For the DR6 and IR6 rats, the Wilcoxon analyses failed to reveal a significant difference for entries made (or time spent, data not shown) in the novel and other arms.
The rats were tested in the NOR on the third day after the end of restraint. Since rats had just 1-min ITI, all groups were expected to recognize and spend more time with the novel object compared to the familiar object. A Wilcoxon Signed Rank Test showed that CON and IR6 rats explored the novel object significantly more than the familiar object (p < 0.05), an effect that was not found with the DR6 rats (Fig. 2C).
OP occurred on the fourth day after the end of restraint. Wilcoxon Signed Rank Tests were performed to determine whether each group explored the object in the novel location more than the object in the same location after a 1-hr delay (Fig. 2D). The CON rats spent significantly more time with the object in the novel location than the object in the same location (p < 0.05). DR6 rats performed at chance by exploring both objects similarly. Interestingly, IR6 rats explored the object in the same location more than the new location (p < 0.05). Additional analysis was performed to compare across groups using a 1-way ANOVA for the OP discrimination index, revealing a significant effect (F2,31 = 6.200, p < 0.05, Power = 0.860, Fig. 2D). LSD post-hoc analyses found a significant difference between the OP discrimination index for CON and IR6 rats, with CON rats having a greater OP discrimination index than did IR6 (p < 0.05, Fig. 2D).
RAWM testing began on the fifth day following the end of restraint and occurred over three days. RAWM testing has typically revealed differences in performance between chronically stress male rats and non-stressed controls [33,64]. During acquisition on days 1 and 2, all three groups made fewer first time entry errors as trials proceeded (Fig. 2E). A repeated measures ANOVA for groups across the 8 trials on day 1 showed a significant effect of trial on first time entry errors (F7,259 = 8.838, p < 0.05). By day 2, a repeated measures ANOVA for groups across the 8 trials did not show a significant effect of trial on first time entry errors to suggest that the groups reached a plateau. However, when these trials were analyzed in bins of 2 trials (e.g., a repeated measure of four bins), a significant effect of bin was observed with rats making fewest errors during the last bin compared to the first (F3,111 = 3.537, p < 0.05). There were no other significant effects on either day 1 or 2. On the third day, a one-way ANOVA for first time entry errors was not significant to reveal that rats were making similar number of first-time entry errors (Fig. 2F).
To determine whether anxiety or motor ability may have impacted performance, additional assessments were performed on the OF, OP and Y-maze. A one-way ANOVA performed on anxiety index in the OF revealed significant differences (F2,33 = 6.644, p < 0.05, Power = 0.980, Fig. 2G). LSD post-hoc analyses showed that DR6 and IR6 rats expressed a higher anxiety profile than did CON (p < 0.05). To determine whether locomotor activity or motivation to explore the Y-maze differed across groups, total entries (sum of entries into Novel, Start and Other arms over minutes 1 and 2) were analyzed using a one-way ANOVA. No significant differences were detected (Fig. 2H). The total number of entries averaged 8.1 ± 0.6 for CON, 7.5 ± 0.6 for DR6 and 7.5 ± 0.5 for IR6. Therefore, differences in spatial memory in the Y-maze were unlikely due to motivation to explore. For the OP, the total time spent exploring the objects was compared with a 1-way ANOVA and revealed no significant effects. The total time exploring objects (in seconds) averaged 31.7 ± 3.6 for CON, 28.1 ± 3.6 for DR6 and 28.8 ± 3.2 for IR6 (Fig. 2I).
In summary, changing the task order helped with behavioral assessment as nearly all rats explored the mazes throughout the behavioral battery. In the first task using the Y-maze, both IR6 and DR6 showed impaired spatial memory at a time when the CON rats exhibited spatial memory by entering the novel arm more than they did the other arm. As testing continued in different mazes over days, IR6 and DR6 began to show the potential to demonstrate spatial ability. In the OP performed on day 4 after stress, CON showed a better OP discrimination Index than did IR6, but IR6 may have avoided the moved object. On the NOR when cognitive load was minimal with 1-min ITI, CON and IR6 preferred the novel object over the familiar one, but IR2 performed at chance. By the time they were tested on the RAWM, the last task of the session, all rats acquired it and performed similarly. Motor abilities were unlikely to explain the spatial memory differences observed in the beginning on the Y-maze and OP, and although anxiety profiles were elevated for both DR6 and IR6, the rats explored similarly and well during the low cognitive load test. We conclude that a 6-hour IR paradigm may lead to impaired hippocampal-dependent spatial ability with comparably robust deficits as found with DR6. However, these effects failed to be long-lasting, as groups performed similarly by days five and seven.
3.3. Experiment 3: Effects of an extended IR6 paradigm on spatial memory in male and female rats
The effects of an extended 6-hr IR paradigm were explored in both male and female rats by increasing the stress period from three to six weeks before the first behavioral test, as longer periods of restraint resulted in more robust spatial memory deficits on the RAWM in male rats [69]. After six weeks of IR6, rats were tested on the Y-maze and then an additional three weeks of IR6 was implement before a second Y-maze test. Thereafter, behavioral assessments on different tasks occurred weekly (Fig. 3A).
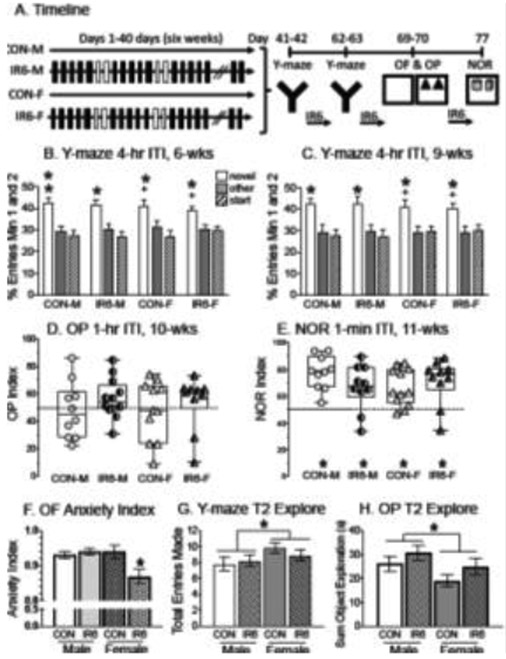
Fig. 3:
Effects of an extended IR6 paradigm on spatial memory in male and female rats. A) Timeline of manipulations. Black-filled rectangles indicate restraint was performed and white-filled rectangles indicate when rats were given a day off. After 6-weeks of restraint, rats were tested on the Y-maze and then returned to the restraint paradigm for an additional 3-weeks. Behavioral testing then occurred weekly on days without restraint, starting with restraint for 6hrs. the Y-maze, followed by OF/OP (consecutive days) and ending with NOR. B) Y-maze performance on trial 2 after 6-weeks of IR for entries in the first two minutes. CON-M entered (and spent more time in, dwell data not shown) the novel arm than the other arm. IR6-M, CON-F and IR6-F entered the novel arm more than the other arm. Two symbols indicate significance or tendency toward significance in both entry and dwell measures with entry measure listed first, followed by dwell. C) Y-maze performance on trial 2 after 9-weeks of IR for entries in the first two minutes. CON-M, IR6-M and CON-F entered the novel arm more than the other arm. IR6-F showed a tendency to enter the novel arm more than the other arm. Two symbols indicate significance or a tendency toward significance in both entry and dwell measures with entry measure listed first, followed by dwell (dwell data are not shown). D) OP performance after 10 weeks of IR. The OP Index represents data from the second trial after a 1-hr ITI. Preference for the moved object will show values greater than 0.5 with the dashed horizontal line indicating chance levels. All groups performed at chance. E) NOR performance after 11 weeks of IR. The OP Index represents data from the second trial with a 1-min ITI to reflect a low cognitive load. Preference for the new object will show values greater than 0.5 with the dashed line indicating chance levels. All groups spent more time with the new object than the familiar object to show that they would seek the novel object when cognitive demand was low. G) OF anxiety index used from the same arena that rats were given acclimation before OP and NOR. IR6-F had a significantly lower anxiety profile compared to CON-F, CON-M, and IR6-M, but this did not seem to change performance on OP or NOR. H) Assessment of the total number of entries made in all three arms of the Y-maze as a measure of motivation. Data were taken from the second Y-maze assessment after 9 weeks of IR and reflect activity during the first two minutes from trial 2. Female rats made more total entries compared to male rats, but no other group differences were found. I) The total time spent exploring both objects in OP during trial 2 after 10 weeks of IR. Male rats spent significantly more time exploring both objects compared to female rats, but this fails to explain why none of the groups showed object placement recognition. *p < 0.05, + p < 0.1, CON-M = control males, IR6-M = intermittent restraint for 6hrs. males, CON-F = control females, IR6-F = intermittent restraint for 6hrs females.
In the first Y-Maze following 6-wks of IR, all groups (CON-M, IR6-M, CON-F, IR6-F) entered (or spent more time in, data not shown) the novel arm than the other arm to reflect intact spatial ability (Fig. 3B). Wilcoxon Signed Rank Tests revealed that rats entered (and/or spent more time in, data not shown) the novel arm than the other arm (p < 0.05, CON-M, IR6-M, CON-R, IR6-F). A two-way ANOVA across treatment conditions for the entry index did not show a significant effect.
The rats were given another three weeks of IR and then tested again in the Y-maze in a different room. After 9-wks of IR, the rats still showed spatial ability (Fig. 3C). Wilcoxon Signed Rank Tests indicated a significantly greater number of entries (and/or time spent) in the novel arm than in the other arm for CON-M, IR6-M, CON-F, and IR6-F (p < 0.05). A two-way ANOVA revealed no significant effects on %entry index across groups.
After another week of IR, the rats were tested on the OP, which occurred during the 10th week of restraint. Wilcoxon Signed Rank Tests were performed to determine whether each group explored the object in the novel location more than the object in the same location. Unexpectedly, no significant differences were detected: all groups, including the controls (CON-M, IR6-M, CON-F and IR6-F), explored the objects in the novel and same location similarly. A two-way ANOVA also found no significant differences across groups for OP discrimination (Fig. 3D).
After another week of IRS, the rats were tested in the NOR during the 11th week of restraint. Since rats had just 1-min ITI with a minimum cognitive load, all groups were expected to recognize and spend more time with the novel object compared to the familiar object. Wilcoxon Signed Rank Tests showed that all groups explored the novel object significantly more than the familiar object (p < 0.05, Fig. 3E). A two-way ANOVA found no significant differences across groups in NOR discrimination.
To determine whether anxiety or motor ability may have impacted performance, additional assessments were performed on the OF, OP and Y-maze. A two-way ANOVA performed on anxiety index in the OF revealed a significant interaction of stress and sex (F1,43 = 3.827, p = 0.05, Power = 0.481, Fig. 3F). LSD post-hoc analyses showed that IR6-F rats expressed a reduced anxiety profile compared to CON-F (p < 0.05), CON-M (p < 0.05), and IR6-M (p < 0.05, Fig. 3F). To determine whether locomotor activity or motivation to explore the Y-maze differed across groups, total entries (sum of entries into novel, start and other arms over minutes 1 and 2) were analyzed using two-way ANOVAs. No significant differences were detected in the first Y-maze after 6-weeks of IR6 to suggest that the groups were similarly motivated to explore (data not shown). The total number of entries averaged 7.8 ± 0.8 for CON-M, 8.2 ± 0.7 for IR6-M, 9.8 ± 0.6 for CON-F and 8.9 ± 0.7 for IR6-F. For the Y-maze after 9-weeks of IR6, a two-way ANOVA revealed a significant effect of sex with female rats making more total entries than male rats (F1,42 = 10.205, p < 0.05, Power = 0.877, Fig. 3G) with no other significant effects. The total number of entries averaged 7.5 ± 0.8 for CON-M, 7.7 ± 1.0 for IR6-M, 10.4 ± 0.8 for CON-F and 9.9 ± 0.6 for IR6-F. In OP, the total time spent exploring the objects was compared with a two-way ANOVA and revealed a significant effect of sex with male rats spending more time exploring objects compared to female rats (F1,39 = 4.228, p < 0.05, Power = 0.518, Fig. 3H) with no other significant effects. The total time exploring objects (in seconds) averaged 48.0 ± 6.5 for CON-M, 56.7 ± 4.2 for IR6-M, 48.4 ± 6.5 for CON-F and 54.3 ± 6.1 for IR6-F.
In summary, an extended IR paradigm for six and even nine weeks failed to lead to impaired spatial memory. Both IR6-M and IR6-F showed spatial memory on the Y-maze by entering (and/or spending more time in) the novel arm than the other arm. OP behavior was less clear as all groups, including controls, failed to discriminate and spent similar amounts of time exploring both objects. On the NOR when cognitive load was minimal, all groups discriminated and preferred the novel object over the familiar one. We conclude that when the IR paradigm was extended beyond three weeks to six or nine weeks, IR failed to lead to impaired spatial memory, perhaps due to the paradigm becoming predictable.
3.4. Experiment 4: Effects of UIR on spatial ability in male and female rats.
In experiments 1 and 2, IR led to impaired spatial memory at three weeks, but experiment 3 showed that when IR was extended to six or nine weeks, spatial memory deficits were not detected to suggest that IR may have become predictable. Under predictable circumstances, chronic stress responses becomes less robust [14,70]. Consequently, IR was modified as an unpredictable intermittent restraint (UIR) and spatial memory was explored in both male and female rats. The UIR paradigm involved varying the time of day which restraint occurred, as well as the duration of restraint (either 30 min or 1 hr), with restraint repeating once a day for a period of 2 to 6 consecutive days before a 1- or 2-day restraint hiatus. Moreover, restraint occurred on an orbital shaker to increase the robustness of the restraint with a shorter duration. After a 26-day UIR period (reflecting 21 restraint days), behavioral testing began and occurred weekly on days without restraint with UIR continuing the day after behavioral testing. As a final assessment, a robust heterotypic stressor was performed at the end of UIR because it produced sex differences in set-shifting ability in a recent report [67]. However, its effect on spatial ability is unknown. A timeline of the experiment is shown in figure 4A.
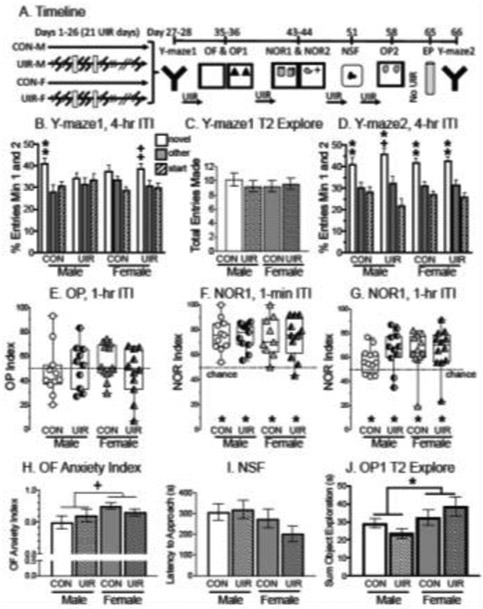
Fig. 4:
Effects of a novel UIR paradigm on spatial ability and anxiety profiles in both male and female rats. A) Timeline of manipulations. The stressor was 30-min (small zigzag) or 60-min (large zigzag) restraint with gentle shaking. White-filled rectangles indicate when rats were given a day off. After 28 days of UIR (which was 21 days of actual restraint), rats were tested on the Y-maze and then returned to the UIR paradigm as indicated. At the end of UIR, rats were given seven days before being placed on the EP for 30-min and then tested one day later on the Y-maze. B) Y-maze1 performance on trial 2 after 26 days of UIR for entries in the first two minutes after a 4-hr ITI. CON entered (and spent more time in, data not shown) the novel arm than the other arm. UIR-F tended to enter (and spend more time in, data not shown) the novel arm than the other arm. UIR-M and CON-F performed at chance levels. Two symbols indicate significance or tendency toward significance in both entry and dwell measures. C) Entries made in all three arms of the Y-maze1 during Trial 2 for the first two minutes. The groups made a similar number of total entries during trial 2 of the Y-maze to demonstrate similar motivation to explore. D) Y-maze2 performance following seven days after the end of UIR and one day after EP (post-EP) for entries made in minutes 1 and 2 during trial 2 after a 4-hr ITI. CON-M, UIR-M, CON-F and UIR-F entered (and spent more time in, data not shown) the novel arm more than the other arm. Chance is denoted by the dashed horizontal line. Two symbols indicate significance (or tendency toward significance) in both entry and dwell measures. E) OP1 performance after 36 days of UIR. The OP Index reflects time spent with the moved object compared to total time spent with both objects in the second trial after a 1-hr ITI. Preference for the moved object will show values greater than 0.5 with the dashed horizontal line indicating chance levels. All groups performed at chance. F) NOR performance after 43 days of UIR. The NOR Index reflects time spent with the novel object compared to total time with both objects in the second trial with a 1-min ITI. Preference for the new object will show values greater than 0.5 with the dashed horizontal line indicating chance levels. All groups spent more time with the new object than the familiar object to demonstrate that they were able to recognize the new object when cognitive load was minimal. G) NOR performance after 44 days of UIR, with the NOR Index from the second trial with a 1-hr ITI. All groups spent more time with the new object than the familiar object even with a longer delay than the day before. H) OF anxiety index. Female rats tended to have an elevated anxiety profile compared to male rats, but this does not explain the poor performance on OP for all groups. I) NSF latency to feed. The time it took for rats to approach the chow was similar across groups. J) The total time spent exploring both objects in OP1 during trial 2 after 36 days of UIR. Female rats spent significantly more time exploring both objects compared to male rats. There were no other group differences. *p < 0.05, +p < 0.1, CON-M = control males, UIR-M = unpredictable intermittent restraint males, CON-F = control females, UIR-F = unpredictable intermittent restraint females.
In the first spatial task, sex differences were observed in the Y-maze (Fig. 4B). In the males, Wilcoxon Signed Rank Tests indicated a significantly greater number of entries and time spent in the novel arm than in the other arm for the controls (CON-M, p < 0.05), but not in UIR-M. In the females, a tendency to enter and spend more time in the novel arm more than the other arm was found in UIR-F (p < 0.10, Fig. 4B), but not in CON-F. A two-way ANOVA did not reveal any significant effects on the %entry index (or %dwell index). Motivation to explore was unlikely to have impacted performance, as total entries on the Y-maze (sum of entries into Novel, Start and Other arms over minutes 1 and 2) were statistically similar (Fig. 4C). The total number of entries averaged 10.2 ± 0.9 for CON-M, 9.3 ± 0.8 for UIR-M, 9.3 ± 0.8 for CON-F and 9.6 ± 0.8 for UIR-F. Therefore, differences in spatial memory in the Y-maze were unlikely due to motivational differences to explore.
A second Y-maze test was performed seven days after the last UIR session concluded and one day after an acute 30-min EP stressor, as this manipulation has been shown to impair prefrontal cortex-mediated function [67]. However, no differences among groups were detected on the post-EP Y-maze from a two-way ANOVA and all groups demonstrated spatial memory (Fig. 4D). Wilcoxon Signed Rank Tests indicated that all groups significantly entered (and/or spent more time in) the novel arm than in the other arm (p < 0.05).
OP occurred twice in this experiment, during the 2nd and 5th weeks of behavioral testing. In both OP tasks (1-hr ITI), none of the groups showed a significant preference for one object over the other and explored both objects similarly (Wilcoxon Signed Rank, Fig. 4E for the first task, data not shown for the second task). Moreover, a two-way ANOVA for the OP indexes did not reveal any significant effects.
The rats were tested in two versions of the NOR (1-min and 1-hr ITI) during the 3rd week of behavioral testing. Wilcoxon Signed Rank Tests showed that all groups discriminated and explored the novel object significantly more than the familiar object under both conditions (ITI 1-min, p < 0.05, Fig. 4F; 1-hr ITI p < 0.05, Fig. 4G). A two-way ANOVA did not show any significant effects among groups for the NOR index in either task.
To determine whether anxiety may have impacted performance, the OF and NSF were used. In the OF, a two-way ANOVA performed on the anxiety index did not reveal any significant differences among groups, although there was a tendency for females to have a higher anxiety index than in males (F1,44 = 3.265, p < 0.10, Fig. 4H). In the NSF, there were no significant differences across groups in latency to approach food (Fig. 4I) and home cage feeding was statistically similar. Together, the OF and NSF data suggest that the UIR groups had similar overall anxiety profiles as the CON groups. Although females may have had a higher anxiety index, this does not explain why UIR-F may have differed from CON-F.
In OP1 and OP2, the total time spent exploring the objects in trial 2 was compared with a two-way ANOVA and revealed a significant effect of sex in OP1 (F1,40 = 5.338, p < 0.05, Power = 0.616, Fig. 4J) and OP2 (F1,41 = 16.641, p < 0.05, Power = 0.978, data not shown), with no other significant effects. The total time exploring objects (in seconds) averaged for OP1: 29.3 ± 2.5 for CON-M, 23.6 ± 2.6 for UIR-M, 32.5 ± 4.5 for CON-F and 38.9 ± 5.3 for UIR-F (Fig. 4J) and for OP2: 27.8 ± 5.1 for CON-M, 24.4 ± 3.8 for UIR-M, 37.8 ± 4.8 for CON-F and 43.5 ± 3.5 for UIR-F. While females spent more time with the objects than did males, all rats performed similarly and at chance on the OP.
In summary, the UIR paradigm was effective in leading to impaired spatial memory in male, but not female rats. In the first spatial assessment, CON-M entered the novel arm more than the other arm, while UIR-M performed at chance, which could not be explained by motor differences or anxiety profile. In contrast to males, UIR in females did not result in spatial memory deficits and may have even been beneficial, as UIR-F rats showed improved discrimination compared to CON-F, with a tendency for more novel arm exploration than the other arm in separate assessments for number of entries and time spent in the novel arm (0.1 > p >0.05). Motor abilities were unlikely to explain the spatial memory differences observed in the first Y-maze and the lack of an effect in OP. We did not find any deficits in spatial memory in the Y-maze following the EP stressor, suggesting rats had either recovered from deficits by that time point and/or the EP procedure did not interfere with spatial ability. We conclude that a EUR paradigm may lead to impaired hippocampal-dependent spatial ability with robust deficits in male rats that fail to present in female rats.
3.5. Physiological Measures
In all four experiments, IR or UIR attenuated body weight gain compared to the same-sex controls (Table 1). Follow-up post-hoc tests from a significant one-way ANOVA in experiment 1 (F3,44 = 16.3 5 7, p < 0.05), showed that DR6, IR6, IR2 gained less weight compared to CON over three weeks (p < 0.05) and that, DR6 gained the least body weight compared to IR6 and IR2 (p < 0.05). IR6 and IR2 gained similar amounts of weight. In experiment 2, follow-up from a significant one-way ANOVA (F3,44= 1153.777, p < 0.05), showed that DR6 and IR6 gained less weight than CON (p < 0.05) and that DR6 gained significantly less body weight than did IR6 (p < 0.05). In experiment 3 and 4, two-way ANOVAs were performed for stress and sex with both revealing significant effects of stress (Exp 3, F1,43 = 86.088, p < 0.05; Exp 4, F1,44 = 59.523, p < 0.05), sex (Exp 3, F1,43 = 454.343, p < 0.05; Exp 4, F1,44 = 436.739, p < 0.05), and interaction (Exp 3, F1,43 = 11.888, p < 0.05, Exp 4, F1,44 = 6.359, p < 0.05). Post-hoc tests from the interaction revealed that while the stressors attenuated body weight gain in both sexes (p < 0.05), males showed more robust effects than found with females.
Table 1:
Experiments 1 and 2 body weight change (g) ± SEM over 3 weeks of intermittent restraint.
| Experiment | CON | DR6 | IR6 | IR2 |
|---|---|---|---|---|
| 1 | 123.8 ±9.8 | 51.8 ± 6.3 | 73.9 ± 5.4 | 93.4 ± 8.0 |
| 2 | 134.0 ± 5.1 | 77.2 ±4.9 | 99.6 ±4.5 | — |
In experiment 4, thymus, adrenal and uterine weights were analyzed as an additional measure of stressor effectiveness (Table 2). A two-way ANOVA for thymus weight revealed a significant effect of stress (F1,44 = 10.982, p < 0.05) and sex (F1,44 = 27.557, p < 0.05) with no significant interaction. UIR increased thymus weight in both males and females with males showing heavier thymus. A two-way ANOVA for adrenal gland weights revealed a significant effect of sex (F1,43 = 15.090, p < 0.05), with no significant stress or interaction. Males had larger adrenals than females, as would be expected. Uterine weights in females were not statistically different.
Table 2:
Experiment 3 body weight change (g) ± SEM over 11 weeks of intermittent restraint.
| Experiment | CON-M | IR-M | CON-F | IR-F |
|---|---|---|---|---|
| 3 | 254.4 ±9.2 | 174.5 ±5.1 | 98.8 ±5.5 | 62.2 ±3.4 |
4. Discussion
The current study investigated whether an IR paradigm could be extended to study chronic restraint effects on spatial memory deficits that would be more robust and/or longer lasting than daily restraint in both male and female rats. We report that IR may be useful to investigate spatial ability in male rats within a relatively brief period, such as for three weeks of IR, but not after an extended IR duration of six or nine weeks. Moreover, when spatial memory deficits were detected in male rats, the effects of IR appeared marginally more robust than daily restraint (Fig. 2D). In addition, when spatial memory deficits were detected in male rats, the effects of IR were transient because spatial memory deficits begin to improve within a few days after restraint ended. When IR continued for an extended duration for up to six weeks, IR male and female rats failed to demonstrate spatial memory impairments, suggesting that the IR paradigm may have become predictable. A modified version of IR was subsequently designed to be unpredictable (UIR) through restraining rats at different number of consecutive days restrained (2 to 6 days), changing the time of day restrained, and mixing up the duration of restraint (30 or 60 min) when combined with gentle shaking. The outcome showed that UIR males were impaired on spatial ability, whereas UIR females were not. In summary, UIR was an effective chronic stressor when studying spatial memory impairments in males and can be useful when spatial memory is to be investigated repeatedly on the non-restraint days, and further highlights the sex differences in how chronic stress impacts spatial ability.
An important outcome of these experiments is the corroboration of sex differences in how chronic stress alters spatial memory. UIR impaired spatial memory in the Y-maze of male rats without impairing spatial memory of female rats. For the stressed females (Fig. 4B), they showed a non-significant tendency (p<0.1) to prefer the novel arm, while their same-sex controls failed to reach this p-value. The results are consistent with the findings of others documenting that males show deficits in spatial ability [23,25,27,32,33,39,40,44,45,54,71,72], but not in females. For the females more specifically, an extensive literature shows that chronic stress either fails to alter spatial memory [33,73,74] or even enhances it [31,37,75]. Chronic stress may also alter female motivation, as we found that stressed female rats delayed their exploration of the novel arm exploration in the Y-maze [29], though this did not occur in the current experiments. It is unlikely that the stressed females perceived the UIR differently than males, as physiological measures validated UIR effectiveness, consistent other reports on chronic stress in rodents [39,76-80]. Once possible explanation as to why stressed females were less vulnerable to spatial memory deficits than stressed males may be that chronic stress altered a different cognitive function in females than was investigated in the current study. For example, chronic stress impaired cognitive flexibility in females using a set-shifting task [81], which requires the prefrontal cortex [82]. Or perhaps the type of stressor could be important, as heterotypic stressors, defined as a novel stressor unique from prior stressors, impaired set-shifting in females but not males [67]. Altogether, we interpret our results in the context of how the control females performed and published reports that UIR did not impair spatial ability of female rats and corroborates the literature highlighting the sex differences in chronic stress-induced spatial memory effects.
The original intent of using the IR paradigm was to determine whether the potentiated effects on stress responsiveness and anxiety reported previously [46] could be extended to spatial memory. While experiment 1 showed some evidence for three weeks of IR to lead to more robust spatial memory deficits than observed for DR (Fig. 1C), this was not always observed. In experiment 2, DR6 and IR6 males performed poorly and similarly on the Y-maze. On the OP task performed on day 4 after chronic stress ended in experiment 2, DR6 males performed at chance, but IR6 males recognized the novel arm by showing avoidance behavior, which is consistent with chronic stress facilitating neurocircuitries that favor habits rather than flexible behaviors [83]. Then for experiment 3 when IR was extended to six and even nine weeks, no spatial memory deficits were detected in either sex. The longer paradigm was used because our past study showed that that five weeks of chronic daily restraint resulted in more robust spatial memory deficits in males than compared to a three week exposure [69]. The lack of an effect with six- and nine-weeks of IR to impair spatial memory in the current study was unlikely attributed to stressor effectiveness because IR attenuated body weight gain, a reliable measure of chronic stress in rodents [84-88]. Perhaps the consistent five day exposure and two days off from restraint led to a muted stress response, a phenomenon documented to occur with repeated exposures to the same stressor [89], and as such, could have increased predictability [16] to thereby make IR less stressful [14]. While this interpretation does not explain why DR would lead to more severe spatial memory deficits when extended from three to five weeks [69], this may apply to the IR paradigm, perhaps by making it more tolerable by having predicted days off from restraint. Therefore, IR may produce potentiated stress responses and anxiety after 1-1/2 weeks, see [46], but its effects on producing robust spatial memory deficits are mixed after 3 weeks and certainly ineffective at leading to impairments after 6 weeks.
A consistent theme following chronic stress is that males show spatial memory deficits, which improve in the days after the chronic stressor ends. In one of the first studies to investigate this phenomenon, 4 weeks of chronic stress impaired spatial learning on the Morris Water Maze task, with these deficits improving after one month passed from the end of the stressor [43]. Later, our lab found that 3 weeks of chronic restraint hindered spatial memory on the RAWM, an effect that improved with the passage of time [45]. Our current experiments add to the literature and suggest that the timeframe from when spatial memory deficits improve following the end of these chronic restraint paradigms is shorter, within the range of four to seven days. These findings are consistent with the changes in hippocampal CA3 apical dendritic arbors following chronic stress: chronic stress leads to CA3 apical dendritic retraction [63,76,77,90-92], and these dendritic arbors become more complex within four to ten days after chronic stress has ended [92].
Another consideration for the relatively fast spatial memory improvement is that the rats may have benefited from the repeated behavioral assessments. For example, environmental enrichment counteracts chronic stress-induced learning and memory deficits [22,69,93,94]. Aspects of the cognitive assessments implemented in this study, such as the opportunity to explore objects and environments, could be perceived as enriching and may have similar effects as environmental enrichment in rats. For instance, CA3 synaptic density recovers rapidly after two days of water maze training, suggesting that learning may promote synaptic plasticity and counteract the chronic stress effects [71]. Another interpretation is that the rats were able to transfer information from one testing situation to another [95-97], but this likelihood was minimized by using unique testing rooms and contexts for each cognitive task. Whether spatial memory deficits improved from repeated testing or from the passage of time is unclear, but it is important to note that the phenomenon was found in all three of the restraint paradigms (IR, UIR and DR) and could not be explained by motor or motivational issues.
The UIR paradigm was introduced after concluding experiment 3 because the ability to behaviorally test both control and stress rodents on full days without disrupting the stress manipulation would be of great benefit in experiments that require multiple assessments. Moreover, the issue that spatial memory improves in as little as 5 days after the chronic stress manipulation has ended makes an intermittent stress paradigm valuable when repeated behavioral testing is desired. Experiment 4 demonstrated that UIR was effective at producing spatial memory deficits in males, an outcome that would be expected from the literature [27-29,33,44,98,99]. Consequently, UIR provides a way to obtain multiple behavioral measures without disrupting the chronic stress paradigm.
An unforeseen outcome was the failure of the majority of control rats across groups to explore the moved object more than the unmoved object during OP testing in experiments 3 (Fig. 3D) and 4 (Fig. 4E). We incorporated the OP into our behavioral battery based upon our own success (and others) with using it. Our past work using young adult Sprague-Dawley female rats revealed robust effects using OP in non-stressed rats [100] and after chronic stress [39], as have others [40,101,102]. Similar robust effects on OP were reported with male rats by us and others [44,54,64]. However, our lab recently discovered that middle-aged female rats failed to explore during object testing even with repeated exposure [103], bringing some questions about OP reliability as it pertains to females. As we reflect on the tests used in the current work, experiments 3 and 4 started with Y-maze testing, which differed from experiment 1. Testing on the Y-maze first may have had implications on willingness to explore in OP later because rats were able to seek out novelty in the Y-maze using thigmotaxis, but were unable to explore objects in the open field unless they ventured away from the wall. Additional differences that would have encouraged exploration on the Y-maze was that Y-maze testing occurred with bedding material and lower light intensity (lux = 80-90) than used for OP (lux = 150-160), which was done to enhance visualization of spatial cues and differentiate it from other tasks. In comparison with other reports that had success with OP, the OP was the only task used [37,39,44,54,104] or a different type of task preceded it [64,103], but we have not found an example in which OP followed Y-maze testing. Consequently, it is possible that the controls may have been less willing to explore or pay attention to the objects in the OP after being exposed to the Y-maze.
We also observed that the behavioral testing order impacted performance. In experiment 1, when the RAWM occurred first, followed without breaks in daily testing by the OP and NOR, half of the rats failed to explore despite being presented with an OF arena for acclimation. In experiment 2, when the Y-maze occurred first, subsequent object exploration was greatly increased, ranging from 83% to 100% participation across treatment conditions. Other reports documented order effects and one found that mice explored less in the open field and the Y-Maze when a behavioral battery preceded them, but how a behavioral battery impacted performance on the Morris Water Maze was less obvious [105]. When aversive tasks, such as the Morris Water Maze, precede comparatively less aversive tasks, such as OF, mice exhibited reduced locomotion [106,107]. Taken together, the current series of studies corroborate the literature that if multiple behavioral tasks are to be used to assess chronic stress effects on spatial ability, then perhaps testing should start from the least to the most aversive paradigm, except in the case that the Y-maze is used and have the Y-maze follow tests that require open field exploration, such as OP.
In conclusion, the results from the present set of experiments show important sex differences in how chronic stress alters spatial ability and introduce UIR as a useful paradigm to probe these effects when repeated behavioral measures are needed. We report that UIR led to spatial memory deficits in male, but not female rats. The overwhelming evidence from the current study and others suggest that chronic stress affects male and female rats differently. Chronic stress impairs hippocampal function in male rats, as evidenced by poor spatial ability, but fails to impair spatial ability in female rats. Instead, other studies suggest chronic stress may alter the vulnerability of females to cognitive arousal and related attentional tasks [108]. Future studies should continue to probe the types of respective cognitive vulnerabilities exhibited by males and females in response to chronic stress.
Table 3:
Experiment 4 body weight change (g) ± SEM over 9 weeks of unpredictable intermittent restraint.
| Experiment | CON-M | UIR-M | CON-F | UIR-F |
|---|---|---|---|---|
| 4 | 238.2 ± 8.5 | 169.8 ± 7.7 | 81.8 ±6.0 | 47.2 ±3.4 |
Table 4:
Experiment 4 post-mortem physiological measures (mg) ± SEM.
| Organ | CON-M | UIR-M | CON-F | UIR-F |
|---|---|---|---|---|
| Adrenal | 62.5 ±3.7 | 64.3 ±3.0 | 81.6 ±4.3 | 74.9 ±4.1 |
| Thymus | 419.7 ±26.1 | 325.3 ±22.0 | 286.0 ± 13.2 | 245.8 ± 17.6 |
| Uterus | — | — | 602.7 ±37.5 | 574.4 ±38.4 |
Highlights.
In males, unpredictable intermittent restraint (UIR) impaired spatial ability.
In females, spatial ability was spared after UIR.
Impaired spatial ability in males improved from 4 to 7 days after stressor ended.
UIR allows repeated behavioral testing without interrupting the stressor pattern.
Acknowledgements
The authors gratefully acknowledge the contributions of Amanda Acuna, Jinah Kim, J. Bryce Ortiz, Cindy Reynolds, Vrishti Shah, Elliot Smith, Rujuta Takalkar, Gillian Thornton, Iva Vracar and Chris Willis for their assistance with the study. The authors would also like to gratefully acknowledge the Arizona State University Department of Animal Care and Technologies for their exemplary care for the animals used in this study. This work was funded in part by Arizona State University’s College of Liberal Arts and Sciences (Conrad), Arizona State University’s Graduate College Fellowship (Peay), and the University of Texas at El Paso’s BUILDing Scholars program (National Institute of General Medical Sciences of the National Institutes of Health under linked Award Numbers RL5GM118969, TL4GM118971, and UL1GM118970, Parada).
Abbreviations
- ANOVA
analysis of variance
- CON
control
- DR6
daily restraint-6 hours
- EP
elevated platform stressor
- IR
intermittent restraint
- IR2
intermittent restraint-2 hours
- IR6
intermittent restraint-6 hours
- ITI
inter-trial interval
- MDD
major depressive disorder
- OF
open field
- OP
object placement
- NOR
novel object recognition
- NSF
novelty suppressed feeding
- RAWM
radial arm water maze
- UIR
unpredictable intermittent restraint
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].WHO, Depression and other common mental disorders: global health estimates, 2017. doi:CC BY-NC-SA 3.0 IGO.
- [2].American Psychiatric Association, DSM-V, 2013. doi: 10.1176/appi.books.9780890425596.744053. [DOI] [Google Scholar]
- [3].Souery D, Amsterdam J, De Montigny C, Lecrubier Y, Montgomery S, Lipp O, Racagni G, Zohar J, Mendlewicz J, Treatment resistant depression: Methodological overview and operational criteria, Eur. Neuropsychopharmacol 9 (1999) 83–91. doi: 10.1016/S0924-977X(98)00004-2. [DOI] [PubMed] [Google Scholar]
- [4].Keller MB, Issues in treatment-resistant depression, J. Clin. Psychiatry 66 (2005) 5–12. [PubMed] [Google Scholar]
- [5].Willner P, Animal models as simulations of depression, Trends Pharmacol. Sci (1991). doi: 10.1016/0165-6147(91)90529-2. [DOI] [PubMed] [Google Scholar]
- [6].Nestler EJ, Hyman SE, Animal models of neuropsychiatric disorders, Nat. Neurosci (2010). doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Willner P, Mitchell PJ, The validity of animal models of predisposition to depression, Behav. Pharmacol (2002). doi: 10.1097/00008877-200205000-00001. [DOI] [PubMed] [Google Scholar]
- [8].De Kloet ER, Joels M, Holsboer F, Stress and the brain: From adaptation to disease, Nat. Rev. Neurosci (2005). doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- [9].Marin MF, Lord C, Andrews J, Juster RP, Sindi S, Arsenault-Lapierre G, Fiocco AJ, Lupien SJ, Chronic stress, cognitive functioning and mental health, Neurobiol. Learn. Mem (2011). doi: 10.1016/j.nlm.2011.02.016. [DOI] [PubMed] [Google Scholar]
- [10].Lapiz-Bluhm MDS, Bondi CO, Doyen J, Rodriguez GA, Bedard-Arana T, Morilak DA, Behavioural assays to model cognitive and affective dimensions of depression and anxiety in rats, J. Neuroendocrinol 20 (2008) 1115–1137. doi: 10.1111/j.1365-2826.2008.01772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Koolhaas JM, De Boer SF, De Ruitter AJH, Meerlo P, Sgoifo A, Social stress in rats and mice, Acta Physiol. Scand. Suppl (1997). [PubMed] [Google Scholar]
- [12].Martinez M, Calvo- Torrent A, Pico- Alfonso MA, Social defeat and subordination as models of social stress in laboratory rodents: A review, Aggress. Behav (1998). doi:. [DOI] [Google Scholar]
- [13].Koolhaas JM, de Boer SF, Buwalda B, Stress and Adaptation, Curr. Dir. Psychol. Sci (2006). doi: 10.1111/j.0963-7214.2006.00417.x. [DOI] [Google Scholar]
- [14].Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flügge G, Korte SM, Meerlo P, Murison R, Olivier B, Palanza P, Richter-Levin G, Sgoifo A, Steimer T, Stiedl O, van Dijk G, Wohr M, Fuchs E, Stress revisited: A critical evaluation of the stress concept, Neurosci. Biobehav. Rev (2011). doi: 10.1016/j.neubiorev.2011.02.003. [DOI] [PubMed] [Google Scholar]
- [15].Babb JA, Masini CV, Day HEW, Campeau S, Habituation of hypothalamic-pituitary-adrenocortical axis hormones to repeated homotypic stress and subsequent heterotypic stressor exposure in male and female rats, Stress. (2014). doi: 10.3109/10253890.2014.905534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Grissom N, Bhatnagar S, Habituation to repeated stress: Get used to it, Neurobiol. Learn. Mem 92 (2009)215–224. doi: 10.1016/j.nlm.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jean Kant G, Eggleston T, Landman-Roberts L, Kenion CC, Driver GC, Meyerhoff JL, Habituation to repeated stress is stressor specific, Pharmacol. Biochem. Behav 22 (1985) 631–634. doi: 10.1016/0091-3057(85)90286-2. [DOI] [PubMed] [Google Scholar]
- [18].Pitman DL, Ottenweller JE, Natelson BH, Plasma corticosterone levels during repeated presentation of two intensities of restraint stress: Chronic stress and habituation, Physiol. Behav 43 (1988) 47–55. doi: 10.1016/0031-9384(88)90097-2. [DOI] [PubMed] [Google Scholar]
- [19].Stamp JA, Herbert J, Multiple immediate-early gene expression during physiological and endocrine adaptation to repeated stress, Neuroscience. 94 (1999) 1313–1322. doi: 10.1016/S0306-4522(99)00368-1. [DOI] [PubMed] [Google Scholar]
- [20].Marti, Armario, Influence of Regularity of Exposure to Chronic Stress on the Pattern of Habituation of Pituitary-Adrenal Hormones, Prolactin and Glucose., Stress. 1 (1997)179–189. [DOI] [PubMed] [Google Scholar]
- [21].Grissom N, Iyer V, Vining C, Bhatnagar S, The physical context of previous stress exposure modifies hypothalamic-pituitary-adrenal responses to a subsequent homotypic stress, Horm. Behav 51 (2007) 95–103. doi: 10.1016/j.yhbeh.2006.08.011. [DOI] [PubMed] [Google Scholar]
- [22].Wright RL, Conrad CD, Enriched environment prevents chronic stress-induced spatial learning and memory deficits, Behav. Brain Res 187 (2008) 41–47. doi: 10.1016/j.bbr.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Conrad CD, Galea LAM, Kuroda Y, McEwen BS, Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment, Behav. Neurosci 110(1996) 1321–1334. doi: 10.1037/0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- [24].Conrad CD, A critical review of chronic stress effects on spatial learning and memory, Prog. Neuro-Psychopharmacology Biol. Psychiatry 34 (2010) 742–755. doi: 10.1016/j.pnpbp.2009.11.003. [DOI] [PubMed] [Google Scholar]
- [25].Wright RL, Conrad CD, Chronic stress leaves novelty-seeking behavior intact while impairing spatial recognition memory in the Y-maze, Stress. 8 (2005) 151–154. doi: 10.1080/10253890500156663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Luine V, Villegas M, Martinez C, McEwen BS, Repeated stress causes reversible impairments of spatial memory performance, Brain Res. (1994). doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- [27].Kleen JK, Sitomer MT, Killeen PR, Conrad CD, Chronic stress impairs spatial memory and motivation for reward without disrupting motor ability and motivation to explore, Behav. Neurosci 120 (2006) 842–851. doi: 10.1037/0735-7044.120.4.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sunanda, Shankaranarayana Rao BS, Raju TR, Chronic restraint stress impairs acquisition and retention of spatial memory task in rats, Curr. Sci (2000). [Google Scholar]
- [29].Conrad CD, Grote KA, Hobbs RJ, Ferayorni A, Sex differences in spatial and non-spatial Y-maze performance after chronic stress, Neurobiol. Learn. Mem 79 (2003) 32–40. doi: 10.1016/S1074-7427(02)00018-7. [DOI] [PubMed] [Google Scholar]
- [30].Luine V, Gomez J, Beck K, Bowman R, Sex differences in chronic stress effects on cognition in rodents, Pharmacol. Biochem. Behav 152 (2017) 13–19. doi: 10.1016/j.pbb.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kitraki E, Kremmyda O, Youlatos D, Alexis MN, Kittas C, Gender-dependent alterations in corticosteroid receptor status and spatial performance following 21 days of restraint stress, Neuroscience. 125 (2004) 47–55. doi: 10.1016/j.neuroscience.2003.12.024. [DOI] [PubMed] [Google Scholar]
- [32].Luine V, Sex differences in chronic stress effects on memory in rats, Stress. (2002). doi: 10.1080/1025389021000010549. [DOI] [PubMed] [Google Scholar]
- [33].Ortiz JB, Taylor SB, Hoffman AN, Campbell AN, Lucas LR, Conrad CD, Sex-specific impairment and recovery of spatial learning following the end of chronic unpredictable restraint stress: Potential relevance of limbic GAD, Behav. Brain Res 282 (2015) 176–184. doi: 10.1016/j.bbr.2014.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yuen EY, Wei J, Yan Z, Estrogen in prefrontal cortex blocks stress-induced cognitive impairments in female rats, J. Steroid Biochem. Mol. Biol (2016). doi: 10.1016/j.jsbmb.2015.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wei J, Yuen EY, Liu W, Li X, Zhong P, Karatsoreos IN, McEwen BS, Yan Z, Estrogen protects against the detrimental effects of repeated stress on glutamatergic transmission and cognition, Mol. Psychiatry (2014). doi: 10.1038/mp.2013.83. [DOI] [PubMed] [Google Scholar]
- [36].Bowman RE, Zrull MC, Luine VN, Chronic restraint stress enhances radial arm maze performance in female rats, Brain Res. 904 (2001) 279–289. doi: 10.1016/S0006-8993(01)02474-X. [DOI] [PubMed] [Google Scholar]
- [37].Beck KD, Luine VN, Sex differences in behavioral and neurochemical profiles after chronic stress: Role of housing conditions, Physiol. Behav (2002). doi: 10.1016/S0031-9384(02)00670-4. [DOI] [PubMed] [Google Scholar]
- [38].McFadden LM, Paris JJ, Mitzelfelt MS, McDonough S, Frye CA, Matuszewich L, Sex-dependent effects of chronic unpredictable stress in the water maze, Physiol. Behav 102 (2011) 266–275. doi: 10.1016/j.physbeh.2010.10.022. [DOI] [PubMed] [Google Scholar]
- [39].Conrad CD, McLaughlin KJ, Huynh TN, El-Ashmawy M, Sparks M, Chronic stress and a cyclic regimen of estradiol administration separately facilitate spatial memory: Relationship with hippocampal CA1 spine density and dendritic complexity, Behav. Neurosci (2012). doi: 10.1037/a0025770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bowman RE, Beck KD, Luine VN, Chronic stress effects on memory: Sex differences in performance and monoaminergic activity, Horm. Behav (2003). doi: 10.1016/S0018-506X(02)00022-3. [DOI] [PubMed] [Google Scholar]
- [41].Heller W, Gender differences in depression: perspectives from neuropsychology., J. Affect. Disord 29 (1993) 129–143. [DOI] [PubMed] [Google Scholar]
- [42].Weissman MM, Bland R, Joyce PR, Newman S, Wells JE, Wittchen HU, Sex differences in rates of depression: cross-national perspectives., J. Affect. Disord 29 (1993) 77–84. [DOI] [PubMed] [Google Scholar]
- [43].Sousa N, V Lukoyanov N, Madeira MD, Almeida OF, Paula-Barbosa MM, Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement., Neuroscience. 97 (2000)253–266. [DOI] [PubMed] [Google Scholar]
- [44].Luine V, Villegas M, Martinez C, McEwen BS, Repeated stress causes reversible impairments of spatial memory performance, Brain Res. 639 (1994) 167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- [45].Hoffman AN, Krigbaum A, Ortiz JB, Mika A, Hutchinson KM, Bimonte-Nelson HA, Conrad CD, Recovery after chronic stress within spatial reference and working memory domains: correspondence with hippocampal morphology, Eur. J. Neurosci 34(2011) 1023–1030. doi: 10.1111/j.1460-9568.2011.07820.x. [DOI] [PubMed] [Google Scholar]
- [46].Zhang W, Hetzel A, Shah B, Atchley D, Blume SR, Padival MA, Rosenkranz JA, Greater Physiological and Behavioral Effects of Interrupted Stress Pattern Compared to Daily Restraint Stress in Rats, PLoS One. 9 (2014). doi: 10.1371/journal.pone.0102247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].McLaughlin KJ, Gomez JL, Baran SE, Conrad CD, The effects of chronic stress on hippocampal morphology and function: An evaluation of chronic restraint paradigms, Brain Res. 1161 (2007) 56–64. doi: 10.1016/j.brainres.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, Wieskopf JS, Acland EL, Dokova A, Kadoura B, Leger P, Mapplebeck JCS, McPhail M, Delaney A, Wigerblad G, Schumann AP, Quinn T, Frasnelli J, Svensson CI, Sternberg WF, Mogil JS, Olfactory exposure to males, including men, causes stress and related analgesia in rodents, Nat. Methods 11 (2014) 629–632. doi: 10.1038/nmeth.2935. [DOI] [PubMed] [Google Scholar]
- [49].Dellu F, Mayo W, Cherkaoui J, Le Moal M, Simon H, A two-trial memory task with automated recording: study in young and aged rats, Brain Res. 588 (1992) 132–139. doi: 10.1016/0006-8993(92)91352-F. [DOI] [PubMed] [Google Scholar]
- [50].Seibenhener ML, Wooten MC, Use of the Open Field Maze to Measure Locomotor and Anxiety-like Behavior in Mice, J. Vis. Exp (2015). doi: 10.3791/52434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Prut L, Belzung C, The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review, Eur. J. Pharmacol 463 (2003) 3–33. doi: 10.1016/S0014-2999(03)01272-X. [DOI] [PubMed] [Google Scholar]
- [52].Brenes JC, Padilla M, Fornaguera J, A detailed analysis of open-field habituation and behavioral and neurochemical antidepressant-like effects in postweaning enriched rats, Behav. Brain Res. 197 (2009) 125–137. doi: 10.1016/j.bbr.2008.08.014. [DOI] [PubMed] [Google Scholar]
- [53].Walsh RN, Cummins RA, The open-field test: A critical review, Psychol. Bull 83 (1976) 482–504. doi: 10.1037/0033-2909.83.3.482. [DOI] [PubMed] [Google Scholar]
- [54].Nishimura KJ, Ortiz JB, Conrad CD, Antagonizing the GABAA receptor during behavioral training improves spatial memory at different doses in control and chronically stressed rats, Neurobiol. Learn. Mem 145 (2017) 114–118. doi: 10.1016/j.nlm.2017.09.002. [DOI] [PubMed] [Google Scholar]
- [55].Balderas I, Rodriguez-Ortiz CJ, Salgado-Tonda P, Chavez-Hurtado J, McGaugh JL, Bermudez-Rattoni F, The consolidation of object and context recognition memory involve different regions of the temporal lobe, Learn. Mem 15 (2008) 618–624. doi: 10.1101/lm.1028008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Barker GRI, Warburton EC, When Is the Hippocampus Involved in Recognition Memory?, J. Neurosci 31 (2011) 10721–10731. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Mumby DG, Hippocampal Damage and Exploratory Preferences in Rats: Memory for Objects, Places, and Contexts, Learn. Mem 9 (2002) 49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ennaceur A, Neave N, Aggleton JP, Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix., Exp. Brain Res 113 (1997) 509–519. [DOI] [PubMed] [Google Scholar]
- [59].Spanswick SC, Sutherland RJ, Object/context-specific memory deficits associated with loss of hippocampal granule cells after adrenalectomy in rats, Learn. Mem 17(2010)241–245. doi: 10.1101/lm.1746710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Pitsikas N, Zisopoulou S, Tarantilis PA, Kanakis CD, Polissiou MG, Sakellaridis N, Effects of the active constituents of Crocus sativus L., crocins on recognition and spatial rats’ memory., Behav. Brain Res 183 (2007) 141–146. doi: 10.1016/j.bbr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- [61].de Bruin NMWJ, Prickaerts J, van Loevezijn A, Venhorst J, de Groote L, Houba P, Reneerkens O, Akkerman S, Kruse CG, Two novel 5-HT6 receptor antagonists ameliorate scopolamine-induced memory deficits in the object recognition and object location tasks in Wistar rats, Neurobiol. Learn. Mem 96 (2011) 392–402. doi: 10.1016/j.nlm.2011.06.015. [DOI] [PubMed] [Google Scholar]
- [62].Diamond DM, Park CR, Heman KL, Rose GM, Exposing rats to a predator impairs spatial working memory in the radial arm water maze, Hippocampus. 9 (1999) 542–552. doi:. [DOI] [PubMed] [Google Scholar]
- [63].Ortiz JB, Mathewson CM, Hoffman AN, Hanavan PD, Terwilliger EF, Conrad CD, Hippocampal brain-derived neurotrophic factor mediates recovery from chronic stress-induced spatial reference memory deficits., Eur. J. Neurosci 40 (2014) 3351–3362. doi: 10.1111/ejn.12703. [DOI] [PubMed] [Google Scholar]
- [64].Ortiz JB, Anglin JM, Daas EJ, Paode PR, Nishimura K, Conrad CD, BDNF and TrkB Mediate the Improvement from Chronic Stress-induced Spatial Memory Deficits and CA3 Dendritic Retraction, Neuroscience. (2018). doi: 10.1016/j.neuroscience.2018.07.049. [DOI] [PubMed] [Google Scholar]
- [65].Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA, Adult hippocampal neurogenesis buffers stress responses and depressive behaviour, Nature. (2011). doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Gould E, Karatsoreos IN, Kane GA, McEwen BS, Kirschen GW, LaMarca EA, Fasolino M, Bocarsly ME, Obesity diminishes synaptic markers, alters microglial morphology, and impairs cognitive function, Proc. Natl. Acad. Sci (2015). doi: 10.1073/pnas.1511593112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Moench KM, Breach MR, Wellman CL, Chronic stress produces enduring sex- and region-specific alterations in novel stress-induced c-Fos expression, Neurobiol. Stress (2019). doi: 10.1016/j.ynstr.2019.100147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Moench KM, Wellman CL, Differential dendritic remodeling in prelimbic cortex of male and female rats during recovery from chronic stress, Neuroscience. (2017). doi: 10.1016/j.neuroscience.2017.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Hutchinson KM, McLaughlin KJ, Wright RL, Bryce Ortiz J, Anouti DP, Mika A, Diamond DM, Conrad CD, Environmental enrichment protects against the effects of chronic stress on cognitive and morphological measures of hippocampal integrity, Neurobiol. Learn. Mem 97 (2012) 250–260. doi: 10.1016/j.nlm.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Galea LAM, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS, Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress, Neuroscience. (1997). doi: 10.1016/S0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- [71].Sandi C, Davies HA, Cordero MI, Rodriguez JJ, Popov VI, Stewart MG, Rapid reversal of stress induced loss of synapses in CA3 of rat hippocampus following water maze training, Eur. J. Neurosci (2003). doi: 10.1046/j.1460-9568.2003.02675.x. [DOI] [PubMed] [Google Scholar]
- [72].Moosavi M, Naghdi N, Maghsoudi N, Zahedi Asl S, Insulin protects against stress-induced impairments in water maze performance, Behav. Brain Res (2007). doi: 10.1016/j.bbr.2006.10.011. [DOI] [PubMed] [Google Scholar]
- [73].Bowman RE, Ferguson D, Luine VN, Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats, Neuroscience. (2002). doi: 10.1016/S0306-4522(02)00156-2. [DOI] [PubMed] [Google Scholar]
- [74].Bowman RE, Kelly R, Chronically stressed female rats show increased anxiety but no behavioral alterations in object recognition or placement memory: A preliminary examination, Stress. (2012). doi: 10.3109/10253890.2011.645926. [DOI] [PubMed] [Google Scholar]
- [75].Bisagno V, Grillo CA, Piroli GG, Giraldo P, McEwen B, Luine VN, Chronic stress alters amphetamine effects on behavior and synaptophysin levels in female rats, in: Pharmacol. Biochem. Behav, 2004. doi: 10.1016/j.pbb.2004.04.023. [DOI] [PubMed] [Google Scholar]
- [76].Galea LA, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS, Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress., Neuroscience. 81 (1997) 689–97. doi:S0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- [77].McKittrick CR, Magarinos AM, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR, Chronic social stress reduces dendritic arbors in CA3 of hippocampus and decreases binding to serotonin transporter sites, Synapse. (2000). doi:. [DOI] [PubMed] [Google Scholar]
- [78].Conrad CD, Mauldin-Jourdain ML, Hobbs RJ, Metyrapone reveals that previous chronic stress differentially impairs hippocampal-dependent memory, Stress. (2001). doi: 10.3109/10253890109014754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Bhatnagar S, Dallman M, Neuroanatomical basis for facilitation of hypothalamic-pituitary- adrenal responses to a novel stressor after chronic stress, Neuroscience. 84 (1998) 1025–1039. doi: 10.1016/S0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- [80].McFadden LM, Paris JJ, Mitzelfelt MS, McDonough S, Frye CA, Matuszewich L, Sex-dependent effects of chronic unpredictable stress in the water maze, Physiol. Behav. (2011). doi: 10.1016/j.physbeh.2010.10.022. [DOI] [PubMed] [Google Scholar]
- [81].Grafe LA, Cornfeld A, Luz S, Valentino R, Bhatnagar S, Orexins Mediate Sex Differences in the Stress Response and in Cognitive Flexibility, Biol. Psychiatry (2017). doi: 10.1016/j.biopsych.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Birrell JM, Brown VJ, Medial frontal cortex mediates perceptual attentional set shifting in the rat., J. Neurosci (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Dias-Ferreira E, Sousa JC, Melo F, Morgado P, Mesquita AR, Cerqueira JJ, Costa RM, Sousa N, Chronic stress causes frontostriatal reorganization and affects decision-making, Science (80-, ). (2009). doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- [84].Marin MT, Cruz FC, Planeta CS, Chronic restraint or variable stresses differently affect the behavior, corticosterone secretion and body weight in rats, Physiol. Behav (2007). doi: 10.1016/j.physbeh.2006.08.021. [DOI] [PubMed] [Google Scholar]
- [85].Marti O, Martí J, Armario A, Effects of chronic stress on food intake in rats: Influence of stressor intensity and duration of daily exposure, Physiol. Behav (1994). doi: 10.1016/0031-9384(94)90055-8. [DOI] [PubMed] [Google Scholar]
- [86].Retana-Márquez S, Bonilla-Jaime H, Vázquez-Palacios G, Domínguez-Salazar E, Martínez-García R, Velázquez-Moctezuma J, Body weight gain and diurnal differences of corticosterone changes in response to acute and chronic stress in rats, Psychoneuroendocrinology. (2003). doi: 10.1016/S0306-4530(02)00017-3. [DOI] [PubMed] [Google Scholar]
- [87].Bollinger JL, Bergeon Burns CM, Wellman CL, Differential effects of stress on microglial cell activation in male and female medial prefrontal cortex, Brain. Behav. Immun (2016). doi: 10.1016/j.bbi.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Henckens MJAG, van der Marel K, van der Toorn A, Pillai AG, Fernández G, Dijkhuizen RM, Joëls M, Stress-induced alterations in large-scale functional networks of the rodent brain, Neuroimage. (2015). doi: 10.1016/j.neuroimage.2014.10.037. [DOI] [PubMed] [Google Scholar]
- [89].Viau V, Sawchenko PE, Hypophysiotropic neurons of the paraventricular nucleus respond in spatially, temporally, and phenotypically differentiated manners to acute vs. repeated restraint stress, J. Comp. Neurol (2002). doi: 10.1002/cne.10178. [DOI] [PubMed] [Google Scholar]
- [90].Conrad CD, What is the functional significance of chronic stress-induced CA3 dendritic retraction within the hippocampus?, Behav. Cogn. Neurosci. Rev (2006). doi: 10.1177/1534582306289043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Wright RL, Lightner EN, Harman JS, Meijer OC, Conrad CD, Attenuating corticosterone levels on the day of memory assessment prevents chronic stress-induced impairments in spatial memory, Eur. J. Neurosci 24 (2006) 595–605. doi: 10.1111/j.1460-9568.2006.04948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Conrad CD, LeDoux JE, Magariños AM, McEwen BS, Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy, Behav. Neurosci 113 (1999) 902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- [93].Cui M, Yang Y, Yang J, Zhang J, Han H, Ma W, Li H, Mao R, Xu L, Hao W, Cao J, Enriched environment experience overcomes the memory deficits and depressive-like behavior induced by early life stress, Neurosci. Lett (2006). doi: 10.1016/j.neulet.2006.05.048. [DOI] [PubMed] [Google Scholar]
- [94].Ilin Y, Richter-Levin G, Enriched environment experience overcomes learning deficits and depressive-like behavior induced by Juvenile stress, PLoS One. (2009). doi: 10.1371/journal.pone.0004329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Winocur G, Mills JA, Transfer between related and unrelated problems following Hippocampal lesions in rats, J. Comp. Physiol. Psychol (1970). doi: 10.1037/h0030006. [DOI] [Google Scholar]
- [96].WINOCUR G, SALZEN EA, HIPPOCAMPAL LESIONS AND TRANSFER BEHAVIOR IN THE RAT, J. Comp. Physiol. Psychol (1968). doi: 10.1037/h0025535. [DOI] [PubMed] [Google Scholar]
- [97].Winocur G, Gilbert M, The hippocampus, context, and information processing, Behav. Neural Biol (1984). doi: 10.1016/S0163-1047(84)90146-8. [DOI] [PubMed] [Google Scholar]
- [98].Riaz MS, Bohlen MO, Gunter BW, Henry Q, Stockmeier CA, Paul IA, Attenuation of social interaction-associated ultrasonic vocalizations and spatial working memory performance in rats exposed to chronic unpredictable stress, Physiol. Behav (2015). doi: 10.1016/j.physbeh.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Rahman MM, Callaghan CK, Kerskens CM, Chattaiji S, O’Mara SM, Early hippocampal volume loss as a marker of eventual memory deficits caused by repeated stress, Sci. Rep (2016). doi: 10.1038/srep29127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].McLaughlin KJ, Bimonte-Nelson H, Neisewander JL, Conrad CD, Assessment of estradiol influence on spatial tasks and hippocampal CA1 spines: Evidence that the duration of hormone deprivation after ovariectomy compromises 17β-estradiol effectiveness in altering CA1 spines, Horm. Behav (2008). doi: 10.1016/j.yhbeh.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Bisagno V, Ferguson D, Luine VN, Chronic D-amphetamine induces sexually dimorphic effects on locomotion, recognition memory, and brain monoamines, Pharmacol. Biochem. Behav (2003). doi: 10.1016/S0091-3057(03)00017-0. [DOI] [PubMed] [Google Scholar]
- [102].Frick KM, Gresack JE, Sex Differences in the Behavioral Response to Spatial and Object Novelty in Adult C57BL/6 Mice, Behav. Neurosci (2003). doi: 10.1037/0735-7044.117.6.1283. [DOI] [PubMed] [Google Scholar]
- [103].Koebele SV, Nishimura KJ, Bimonte-Nelson HA, Kemmou S, Ortiz JB, Judd JM, Conrad CD, A long-term cyclic plus tonic regimen of 17β-estradiol improves the ability to handle a high spatial working memory load in ovariectomized middle-aged female rats, Horm. Behav. 118 (2020) 104656. doi: 10.1016/J.YHBEH.2019.104656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].McCormick CM, Nixon F, Thomas C, Lowie B, Dyck J, Hippocampal cell proliferation and spatial memory performance after social instability stress in adolescence in female rats, Behav. Brain Res (2010). doi: 10.1016/j.bbr.2009.11.003. [DOI] [PubMed] [Google Scholar]
- [105].Võikar V, Vasar E, Rauvala H, Behavioral alterations induced by repeated testing in C57BL/6J and 129S2/ Sv mice: Implications for phenotyping screens, Genes, Brain Behav. 3 (2004) 27–38. doi: 10.1046/j.1601-183X.2003.0044.x. [DOI] [PubMed] [Google Scholar]
- [106].McIlwain KL, Merriweather MY, Yuva-Paylor LA, Paylor R, The use of behavioral test batteries: Effects of training history, Physiol. Behav 73 (2001) 705–717. doi: 10.1016/S0031-9384(01)00528-5. [DOI] [PubMed] [Google Scholar]
- [107].Blokland A, ten Oever S, van Gorp D, van Draanen M, Schmidt T, Nguyen E, Krugliak A, Napoletano A, Keuter S, Klinkenberg I, The use of a test battery assessing affective behavior in rats: Order effects, Behav. Brain Res 228 (2012) 16–21. doi: 10.1016/j.bbr.2011.11.042. [DOI] [PubMed] [Google Scholar]
- [108].Bangasser DA, Wiersielis KR, Khantsis S, Sex differences in the locus coeruleus-norepinephrine system and its regulation by stress, Brain Res. (2016). doi: 10.1016/j.brainres.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]