Abstract
Context
Genome-wide association studies have identified more than 450 single nucleotide polymorphisms (SNPs) for type 2 diabetes (T2D).
Objective
To facilitate use of these SNPs in future genetic risk score (GRS)-based analyses, we aimed to classify the SNPs based on physiology. We also sought to validate GRS associations with insulin-related traits in deeply phenotyped Mexican Americans.
Design, Setting, and Participants
A total of 457 T2D SNPs from the literature were assigned physiologic function based on association studies and cluster analyses. All SNPs (All-GRS), beta-cell (BC-GRS), insulin resistance (IR-GRS), lipodystrophy (Lipo-GRS), and body mass index plus lipids (B + L–GRS) were evaluated for association with diabetes and indices of insulin secretion (from oral glucose tolerance test), insulin sensitivity and insulin clearance (from euglycemic clamp), and adiposity and lipid markers in 1587 Mexican Americans.
Results
Of the 457 SNPs, 52 were classified as BC, 30 as IR, 12 as Lipo, 12 as B + L, whereas physiologic function of 351 was undefined. All-GRS was strongly associated with T2D. Among nondiabetic Mexican Americans, BC-GRS was associated with reduced insulinogenic index, IR-GRS was associated with reduced insulin sensitivity, and Lipo-GRS was associated with reduced adiposity. B + L–GRS was associated with increased insulin clearance. The latter did not replicate in an independent cohort wherein insulin clearance was assessed by a different method.
Conclusions
Supporting their utility, BC-GRS, IR-GRS, and Lipo-GRS, based on SNPs discovered largely in Europeans, exhibited expected associations in Mexican Americans. The novel association of B + L–GRS with insulin clearance suggests that impaired ability to reduce insulin clearance in compensation for IR may play a role in the pathogenesis of T2D. Whether this applies to other ethnic groups remains to be determined.
Keywords: genetic risk score, diabetes, insulin resistance, beta-cell, insulin clearance, lipodystrophy
By 2018, genome-wide association studies (GWAS) and GWAS meta-analyses had identified approximately 140 single nucleotide polymorphisms (SNPs) associated with type 2 diabetes (T2D) (1). This number was increased to approximately 400 by the DIAMANTE (DIAbetes, Meta-ANalysis Trans-Ethnic) Consortium, which brought together 32 European-origin GWAS (2). Follow-up efforts to diabetes GWAS have attempted to characterize the physiology underlying these loci by examining their association with markers of insulin sensitivity or insulin secretion (3). These include early studies that specifically examined T2D (or related quantitative trait) loci for association with insulin sensitivity and insulin secretion derived from detailed phenotyping (4, 5). More recently, T2D loci have been examined in GWAS for oral glucose tolerance test (OGTT)-derived markers of glucose-stimulated insulin secretion (6), OGTT-derived markers of insulin sensitivity (7), and intravenous glucose tolerance test (IVGTT)-derived markers of first-phase insulin secretion (8). Cluster analyses applied to T2D SNP associations with multiple quantitative traits have also been used to assign physiologic function to these loci (4, 9–11). T2D SNP associations with increased fasting insulin, increased triglycerides, and reduced high-density lipoprotein cholesterol (HDL-C) have suggested a lipodystrophy-like mechanism to a subset of these SNPs, supported by the finding of a greater burden of risk alleles at these SNPs in individuals with familial partial lipodystrophy type 1 (12). Association in general cohorts of genetic risk scores (GRS) based on such SNPs with reduced body mass index (BMI), reduced peripheral fat, inability to expand peripheral fat over time, increased visceral-to-subcutaneous adipose tissue ratio, and increased hepatic fat suggest they contribute to insulin resistance (IR) and T2D by shifting fat storage from peripheral and subcutaneous depots to visceral and hepatic depots (11, 12).
Whereas chip-based high-throughput genome-wide genotyping has accelerated the pace of SNP discovery, the lab-based experimentation in cellular and animal models needed to elucidate the function of these SNPs will take more time. However, even without complete knowledge of molecular function, the genetic findings, particularly when aggregated as GRS, are useful risk factors of disease that are not affected by reverse causation. Although early analyses based on relatively few (16 to 18) SNPs examining the ability of GRS to predict T2D found little additional predictive value over clinical risk factors (13, 14), more recent GRS based on higher numbers (48 to 65) of SNPs did find that the GRS significantly increased the predictive value (15, 16). GRS are particularly promising for clinical use in type 1 diabetes, for which they may be useful in elucidating the underlying etiology of diabetes in newborns (17) or determining the cause of diabetes in adults with ambiguous clinical presentations (18).
GRS may also be used as instrument variables in Mendelian randomization studies. For example, this approach has found that diabetes may cause coronary heart disease and large-vessel stroke, but not cardioembolic stroke or pancreatic cancer (19–22). There is also much interest in instrumenting IR or beta-cell (BC) dysfunction to examine their causality for metabolic outcomes. Whereas Mendelian randomization studies looking at diabetes as the risk factor have a straightforward SNP selection, selection of SNPs to represent insulin-related endophenotypes is more challenging. Some studies used SNPs for fasting insulin or homeostatic model assessment of IR (HOMA-IR) (23), whereas others attempted to create more specific scores for IR by requiring that the fasting insulin-raising alleles also be associated with increased triglycerides and reduced HDL-C (24–26). Similarly, rather than using homeostatic model assessment of BC function (HOMA-B), efforts to create more specific scores for insulin secretion have used indices of early insulin response after oral or intravenous glucose stimulation (25). To assist the field in using T2D SNP–based GRS as risk factors or instrument variables, we first reviewed the literature to generate a comprehensive classification of all T2D SNPs currently available. To validate and assess the utility of T2D GRS in non-European–origin individuals, we then examined different classes of GRS in a deeply phenotyped Mexican American cohort. In addition to validating expected GRS associations outside the European group, we further observed a novel association of one of the GRS classes with insulin clearance.
Materials and Methods
Construction of master list of type 2 diabetes single nucleotide polymorphisms
We started with the list of 403 SNPs independently associated with T2D by the DIAMANTE consortium (2). We then compared this list with the list of 141 T2D SNPs summarized by Morris (1), because the latter is a comprehensive review of the GWAS that preceded the DIAMANTE publication. SNPs in the 2 lists were compared by chromosomal location (not by locus name) using linkage disequilibrium (LD) estimates from the European populations in the 1000 Genomes database. Only Morris SNPs that were in not in LD (r2 > 0.1) with a DIAMANTE SNP were added to the master list. This added 32 SNPs to the list. We also performed a similar procedure to add independent SNPs from large-scale GWAS reported after the Morris review (27, 28), which added 21 SNPs. We did not consider findings from recent GWAS in small sample sizes or coding variant scans. The SNP rs61736969 associated with T2D in Greenland was removed from the list because the risk allele present in Greenland is not present in other populations. The resulting master list includes 457 T2D SNPs (29).
Classification of single nucleotide polymorphisms
We used key published studies that attempted to assign physiology to T2D SNPs. Several evaluated the association of T2D SNPs with physiologic measures of insulin secretion or IR from OGTT, the euglycemic-hyperinsulinemic clamp, the insulin suppression test, or the frequently sampled intravenous glucose tolerance test (FSIGT) (4–8). Other studies assessed the association of T2D SNPs with multiple cardiometabolic traits, using those associations in hierarchical cluster analyses to assign the SNPs to functional groups (4, 9–11). In the present effort, we did not include annotations made by “soft clustering,” whereby SNPs could fall into more than 1 category. One study assigned IR as the mechanism to SNPs associated with increased fasting insulin, increased triglycerides, and reduced HDL-C (12). We considered the latter method as a type of clustering analysis; however, SNPs associated with these 3 traits tended to be associated with reduced BMI and body fat percentage, which was confirmed in a cluster analysis of fasting insulin SNPs that identified an overlapping group of SNPs (11). In a few instances, a SNP was considered unclassified in the publication but had nominal evidence (P < .05), allowing functional annotation. SNPs that were associated only with a fasting-based trait (eg, fasting insulin, HOMA-B) were not classified herein based on the fasting trait alone.
The T2D SNPs evaluated in each of the studies listed previously were compared to the SNPs in the master list. SNPs on the same chromosomes were compared using LD from 1000 Genomes European populations. Classifications were assigned to list SNPs that were in LD (r2 > 0.1) with SNPs evaluated in these studies. We did not attempt to match loci by names because locus names are labels of convenience, and some loci contain multiple independent SNPs. SNPs with annotations of insulin secretion, proinsulin, insulin processing, BC differentiation, and BC proliferation were herein classified as BC SNPs. Signals assigned the early classification of “hyperglycemia” (4) were assigned here as BC, given subsequent clustering of the SNP as BC (eg, GCK, rs878521) or association with insulin secretion indices at genome-wide significance (eg, MTNR1B, rs10830963). SNPs assigned to the IR category via cluster analyses or association with physiologic measures of reduced insulin sensitivity were herein designated as IR SNPs (IR, includes the “insulin resistance plus lipids 1” cluster [9]). SNPs that were solely associated with increased fasting insulin and triglycerides and decreased HDL-C were classified as lipodystrophy SNPs (Lipo) because of their association with reduced adiposity (11, 12). Other classifications included BMI plus lipids (B + L, primary known effect on BMI or clustered as BMI and lipids [9, 10]), unclassified (U, examined in one of the studies listed above, but no function could be assigned), or never examined (NE, SNPs for which no studies to date attempted physiologic annotation).
After collecting the annotations from the literature, we applied several rules to make final physiologic assignments. Classifications made by cluster analyses or by SNP associations with a physiologic trait at the genome-wide significance level (P < 5 × 10–8) were considered robust. If a SNP was associated with a classification at nominal significance (P < .05) in multiple distinct publications, it was assigned to that classification (eg, KCNQ1, rs2237895). SNPs associated with a physiologic trait at nominal significance in only 1 study were classified as undetermined (eg, FAF1, rs58432198). Assignments made in cluster analyses took priority over conflicting assignments made by nominal associations (eg, ABO, rs505922; ANKRD55, rs465002; BCAR1, rs72802342). In cases where cluster analyses did not agree (ADCY5, rs11708067; CMIP, rs2925979) or evidence was mixed (GIPR, rs10406431 and rs2238689), the assignment from the most recent cluster analysis, which was based on the greatest number of SNP associations with metabolic and anthropometric traits, was used (10).
Selection of proxy single nucleotide polymorphisms and genetic risk score construction
The risk allele listed in the Morris or DIAMANTE publications was taken as the risk allele in our Mexican American cohort. For palindromic SNPs (A/T or G/C) for which the risk allele frequency was between 0.35 and 0.65 (total of 21 SNPs), we selected a linked (r2 > 0.8) nonpalindromic proxy SNP from the 1000 Genomes database (29). SNPs were selected for GRS construction from genome-wide genotyping data available in our study participants, all of whom were genotyped on the Illumina HumanOmniExpress Beadchip, as previously described (30), with subsequent imputation using 1000 Genomes phase 3.
GRS were constructed based on the SNPs described above, focusing on the alleles of each SNP that were associated with increased risk of T2D. Allele counts (0, 1, and 2) were used for directly genotyped SNPs, and dosage was used for imputed SNPs. We computed a weighted GRS as follows: GRS = n × [(SNP1 × effect size1) + (SNP2 × effect size2) + … + (SNPn × effect sizen)]/the sum of the effect sizes, where n is the number of SNPs, SNPi is the number or dosage of T2D-increasing alleles, and effect sizes were the natural log of the odds ratios. For approximately 90% of the SNPs, the odds ratios were obtained from the DIAMANTE GWAS (2); for the remaining SNPs not reported as main results in DIAMANTE, the odds ratios were obtained from the original reports that discovered them (1, 27, 28, 31). All-GRS was constructed using all available T2D SNPs, BC-GRS was based on the SNPs in the BC category, IR-GRS was based on the IR SNPs, B + L–GRS was based on the BMI-plus-lipids group of SNPs, and Lipo-GRS was based on the SNPs solely associated with increased fasting insulin and triglycerides and reduced HDL-C (Table 1). The BMI-plus-lipids group of SNPs was further explored by splitting the group into SNPs predominantly associated with BMI (POC5 rs2307111, TFAP2B rs3798519, NRXN3 rs17836088, FTO rs1421085, NFAT5 rs862320, MC4R rs523288) and with lipid parameters (ZMIZ1 rs703967, MAP3K11 rs1783541, BPTF rs61676547, TOMM40/APOE rs429358, HNF4A rs11696357, and PNPLA3 rs738408) and using these to generate B-GRS and L-GRS, respectively.
Table 1.
Summary of Method of Functional Assignments
| Category | Total SNPs | Cluster Analysis | Genome-wide Significant Association | Multiple Nominal Associations | Multiple Levels of Evidence |
|---|---|---|---|---|---|
| BC | 52 | 51 | 9 | 10 | 17 |
| IR | 30 | 29 | 2 | 0 | 1 |
| Lipo | 12 | 12 | N/A | N/A | N/A |
| B + L | 12 | 12 | N/A | N/A | N/A |
Number of SNPs classified are listed. The multiple levels of evidence column indicates SNPs classified by cluster analysis that also had consistent evidence of association (either genome-wide or multiple nominal associations). Lipo and B + L classifications were made solely by cluster analyses. Lipo indicates SNPs associated with increased fasting insulin and triglycerides and reduced high-density lipoprotein cholesterol.
Abbreviations: B + L, body mass index plus lipids; BC, beta-cell; IR, insulin resistance; Lipo, lipodystrophy; N/A, not available; SNP, single nucleotide polymorphisms.
Participants
The current study was conducted in 1426 nondiabetic and 161 diabetic (fasting glucose ≥126 mg/dl or 2-hour glucose ≥200 mg/dl) individuals from 2 independent family-based Mexican American cohorts recruited from the Los Angeles area, the Mexican-American Coronary Artery Disease (MACAD, 821 participants from 223 families) and the Hypertension-Insulin Resistance (HTN-IR, 766 participants from 155 families) cohorts, described previously (32, 33). All studies were approved by Human Subjects Protection Institutional Review Boards at UCLA, the University of Southern California, LA BioMed/Harbor-UCLA, and Cedars-Sinai Medical Center. All participants gave written informed consent before participation.
Phenotyping
The 161 participants with diabetes were included only for analyses of association between GRS and the diagnosis of diabetes. Association analyses of GRS with quantitative traits were conducted only in the 1426 individuals without diabetes, who comprised 774 participants from MACAD and 652 HTN-IR participants who had undergone phenotyping with the hyperinsulinemic-euglycemic clamp as described previously (30). These participants were free of major medical illness, and none were taking glucocorticoids or other drugs that could affect glucose homeostasis when they were phenotyped. The glucose infusion rate (M value) during the last 30 minutes of steady-state glucose and insulin levels during the clamp reflects glucose uptake by all tissues of the body (mainly insulin-mediated glucose uptake in muscle) and is directly correlated with tissue insulin sensitivity. Insulin sensitivity index (M/I) is calculated as M divided by the steady-state plasma insulin level. In this study, to clearly distinguish between insulin sensitivity and insulin clearance, we relied on M as the measure for insulin sensitivity because the calculations of M/I and insulin clearance both use steady-state insulin in the denominator (34). The metabolic clearance rate of insulin (MCRI) was calculated as the insulin infusion rate divided by the insulin concentration during the steady state of the euglycemic clamp (35).
Multipoint OGTT was available in participants from MACAD. In those individuals, we calculated acute insulin secretion using the insulinogenic index at 30 minutes (IGI30) as the change in insulin (0 to 30 minutes) divided by change in glucose (0 to 30 minutes).
Fasting triglycerides and HDL-C from both cohorts were examined in this study. BMI was calculated as weight in kilograms divided by height in meters squared. Total body fat mass as assessed by whole-body dual–x-ray absorptiometry was available in MACAD participants. To obtain estimates of fat mass in both cohorts, we also calculated body adiposity index (BAI) as (hip circumference in centimeters)/(height in meters)1.5–18), which is highly correlated with percentage of body fat in Mexican Americans (36).
Statistical analysis
Log-transformed (BMI, BAI, IGI30, triglycerides, HDL-C) or square root–transformed (M value, MCRI, body fat) trait values were used to normalize the distributions for all analyses. T tests for quantitative traits and chi-square tests for categorical traits were used to compare traits between participants with and without diabetes and between cohorts.
Associations between GRS and phenotypes were evaluated using the general estimating equations (GEE1) method to adjust for familial relationships. The weighted GEE1 (37) was computed assuming an exchangeable correlation structure and using the sandwich estimator of the variance to account for familial correlation present in family data. GEE was used to derive standardized regression coefficients. Untransformed estimates were generated for significant associations to illustrate clinical effect sizes. All GRS association analyses were adjusted for age, sex, BMI, and cohort with the following exceptions. GRS associations with adiposity measures (BMI, BAI, and body fat) were not adjusted for BMI. Association with traits available only in MACAD (IGI30 and body fat) were not adjusted for cohort. As a further precaution against cohort effects, all significant association models were repeated with the inclusion of an interaction term between GRS and cohort status.
Replication effort
We sought to replicate novel association results concerning insulin clearance in an independent Mexican American cohort, the Insulin Resistance Atherosclerosis Family Study (IRAS-FS) (38). Genome-wide genotyping and phenotyping of insulin clearance using the FSIGT have been previously described (30). The same beadchip was used to genotype IRAS-FS, MACAD, and HTN-IR. SNPs comprising B + L–GRS, B-GRS, and L-GRS were extracted and used to construct the scores as outlined above in 958 participants with available data. Family-based association of these scores with MCRI in IRAS-FS was conducted using SOLAR (Sequential Oligogenic Linkage Analysis Routines) (39), with adjustment for age, sex, BMI, and study site.
Results
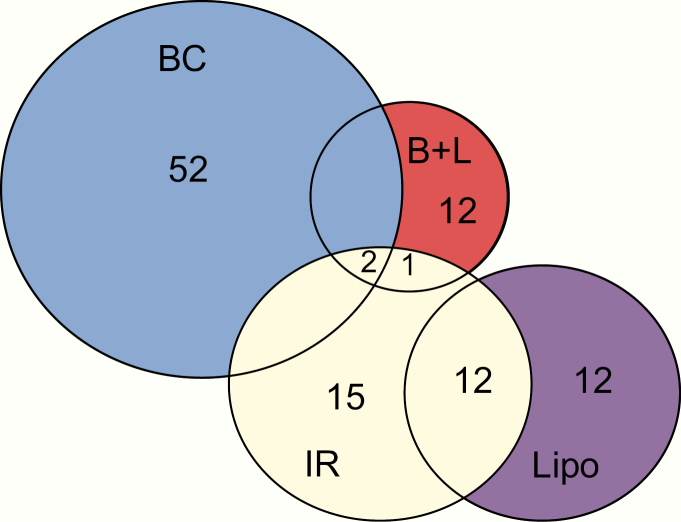
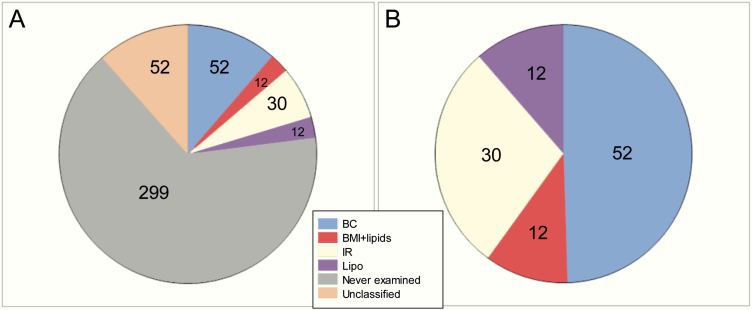
Of the 457 T2D SNPs, 52 were classified as BC, 30 as IR, 12 as Lipo, 12 as B + L, 52 as U, and 299 as NE. Cluster analysis accounted for the majority of functional annotations, whereas SNP association with physiologic measures provided the most corroborating evidence for BC SNPs (Table 1). As shown in Fig. 1, little overlap was observed between functional classes, except for IR SNPs, many of which were also associated with increased fasting insulin and triglycerides and reduced HDL-C (ie, the Lipo class). Fig. 2 displays the final distribution after application of the prioritization rules to resolve conflicting assignments. Of the 457 SNPs, 432 were available in our Mexican American data. Of the 25 SNPs that were not available, 23 were rare (minor allele frequency < 0.01), and all were from the NE category except for 2 BC SNPs (ANKH, rs146886108 and DUSP9, rs5945326). This resulted in 50 BC, 30 IR, 12 Lipo, and 12 B + L SNPs being available for GRS generation. We also generated a score based on all 432 available SNPs (All-GRS).
Figure 1.
Single nucleotide polymorphism (SNP) classification before final assignments. The Venn diagram displays the SNP classifications based on cluster analysis or association with physiologic markers of insulin secretion and insulin sensitivity before prioritization rules were applied to finalize the categorization. Colors indicate the final classifications.
Figure 2.
Distribution of final single nucleotide polymorphism (SNP) classifications. A, Distribution of all 457 SNPs. B, Distribution of the 106 SNPs that were assigned to a functional category.
The entire cohort was used to assess the association of the 5 GRS with T2D (Table 2). The individuals with diabetes were older and had lower insulin sensitivity (M value), insulin secretion (IGI30), and insulin clearance (MCRI) and greater adiposity. Those with diabetes also had lower HDL-C and greater triglycerides. All-GRS was strongly associated with T2D (Table 3). Of the subscores, BC-GRS was also associated with T2D. IR-GRS, Lipo-GRS, and B + L–GRS, though not achieving statistical significance, exhibited directionally consistent positive odds ratios for association with T2D (Table 3).
Table 2.
Clinical Characteristics of Entire Study Cohort
| Diabetes (n = 161) | No Diabetes (n = 1426) | P | |
|---|---|---|---|
| Age, y | 51.7 (19.9) | 34.0 (15.0) | < .0001 |
| Sex, female % | 60.0% | 57.8% | .52 |
| IGI30, pmol/mmol | 69.2 (85.4) | 142.7 (153.5) | .0002 |
| M, µmol·min–1·m–2 | 742.7 (351.4) | 1250.1 (821.5) | < .0001 |
| MCRI, ml·m–2·min–1 | 434.5 (123.3) | 456.6 (140.1) | .0008 |
| BMI, kg/m2 | 30.7 (7.9) | 28.1 (6.6) | < .0001 |
| BAI, % | 34.1 (10.2) | 31.7 (8.5) | < .0001 |
| Body fat, kg | 26.5 (15.6) | 23.7 (11.2) | .006 |
| HDL-C, mmol/l | 1.11 (0.41) | 1.19 (0.39) | .03 |
| Triglycerides, mmol/l | 1.66 (1.29) | 1.16 (0.96) | < .0001 |
Data are median (interquartile range) except for sex. Body fat and IGI30 were available only in Mexican-American Coronary Artery Disease cohort participants.
Abbreviations: BAI, body adiposity index; BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; IGI30, insulinogenic index at 30 minutes; M, glucose infusion rate; MCRI, metabolic clearance rate of insulin.
Table 3.
Association of GRS With Diabetes in Entire Cohort
| All-GRS | BC-GRS | IR-GRS | Lipo-GRS | B + L-GRS | |
|---|---|---|---|---|---|
| Odds ratio | 1.54 | 1.34 | 1.09 | 1.15 | 1.21 |
| 95% CI | 1.24-1.91 | 1.07-1.66 | 0.88-1.35 | 0.96-1.39 | 1.00-1.48 |
| P | 7.2 × 10–5 | .009 | .45 | .14 | .05 |
Covariates are age, sex, body mass index, and cohort.
Abbreviations: B + L, body mass index plus lipids; BC, beta-cell; GRS, genetic risk score; Lipo, lipodystrophy.
In the individuals without diabetes (Table 4), the association of the 5 GRS was assessed against 3 insulin-related traits (Table 5). The All-GRS was not associated with any of these traits. As expected, the BC-GRS was associated with reduced insulinogenic index, and the IR-GRS was associated with reduced insulin sensitivity. The BC-GRS effect per unit increment in weighted score was –13.6 pmol/mmol for IGI30; the IR-GRS effect per increment was –16.3 µmol min–1 m–2 for M. A novel association was observed between the B + L–GRS and increased insulin clearance (2.9 ml·m–2·min–1 per increment). The Lipo-GRS was associated with decreased BMI (–0.22 kg/m2 per increment) and reduced body fat (–365.4 g per increment).
Table 4.
Clinical Characteristics of Participants Without Diabetes
| HTN-IR (n = 652) | MACAD (n = 774) | P | |
|---|---|---|---|
| Age, y | 34.2 (19.4) | 34.0 (13.8) | < .0001 |
| Sex, female % | 58.0% | 57.3% | .79 |
| IGI30, pmol/mmol | N/A | 142.7 (153.5) | N/A |
| M, µmol·min–1·m–2 | 1217.3 (801.0) | 1293.9 (827.1) | .03 |
| MCRI, ml·m–2·min–1 | 442.7 (126.7) | 470.0 (146.3) | .02 |
| BMI, kg/m2 | 28.0 (6.9) | 28.2 (6.3) | .37 |
| BAI, % | 32.0 (9.0) | 31.5 (8.4) | .96 |
| Body fat, kg | N/A | 23.7 (11.3) | N/A |
| HDL-C, mmol/l | 1.19 (0.44) | 1.16 (0.39) | .009 |
| Triglycerides, mmol/l | 1.08 (0.92) | 1.22 (1.03) | .001 |
Data are median (interquartile range) except for sex. Body fat and IGI30 were available only in MACAD participants.
Abbreviations: BAI, body adiposity index; BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; HTN-IR, Hypertension–Insulin Resistance cohort; IGI30, insulinogenic index at 30 minutes; M, glucose infusion rate; MACAD, Mexican-American Coronary Artery Disease Study; MCRI, metabolic clearance rate of insulin; N/A, not available.
Table 5.
Association of GRS With Quantitative Phenotypes in Mexican Americans Without Diabetes
| All-GRS | BC-GRS | IR-GRS | Lipo-GRS | B + L-GRS | |
|---|---|---|---|---|---|
| IGI30 | –0.031 (.44) | –0.14 (.001) | 0.039 (.43) | 0.029 (.55) | –0.063 (.19) |
| M | –0.032 (.21) | 0.038 (.16) | –0.084 (.001) | 0.016 (.54) | –0.018 (.51) |
| MCRI | 0.014 (.62) | 0.028 (.36) | –0.034 (.17) | –0.028 (.36) | 0.056 (.04) |
| BMI | 0.0032 (.91) | –0.010 (.72) | 0.0008 (.98) | –0.076 (.007) | 0.025 (.41) |
| BAI | 0.0014 (.95) | –0.011 (.65) | 0.0024 (.92) | –0.065 (.005) | 0.0069 (.78) |
| Body fat | –0.0065 (.84) | –0.0099 (.77) | 0.011 (.78) | –0.080 (.02) | –0.004 (.92) |
| HDL-C | –0.028 (.32) | –0.0048 (.85) | –0.055 (.07) | –0.015 (.59) | 0.053 (.06) |
| Triglycerides | 0.046 (.09) | 0.013 (.64) | 0.029 (.32) | 0.022 (.42) | –0.042 (.11) |
Data are β (P value). Significant associations are highlighted in bold. Body fat and IGI30 were available only in Mexican-American Coronary Artery Disease cohort participants.
Abbreviations: B + L, body mass index plus lipids; BAI, body adiposity index; BC, beta-cell; BMI, body mass index; GRS, genetic risk score, HDL-C, high-density lipoprotein cholesterol; IGI30, insulinogenic index at 30 minutes; IR, insulin resistance; Lipo, lipodystrophy; M, glucose infusion rate; MCRI, metabolic clearance rate of insulin.
To further characterize the association of B + L–GRS with insulin clearance, we created B-GRS and L-GRS based on SNPs primarily associated with BMI or with lipid fractions, respectively. The B-GRS was associated with increased insulin clearance (β = 0.073, P = .005; 5.5 ml·m–2·min–1 per increment), whereas the L-GRS showed no association (β = 0.002, P = .95), revealing that the BMI SNPs are responsible for the B + L–GRS association with insulin clearance.
Regarding significant associations of genetic risk scores with diabetes and the quantitative traits, all analyses were repeated with the addition of an interaction term between GRS and cohort status. None of these interaction terms were significantly associated with phenotype, and their inclusion in the models did not substantively alter the significance of the association of GRS with phenotype.
In IRAS-FS, associations with insulin clearance were not observed for B + L–GRS (β = –0.0003, P = .95), B-GRS (β = –0.004, P = .52), or L-GRS (β = 0.004, P = .48).
Discussion
We found that BC-GRS, IR-GRS, and Lipo-GRS, based on SNPs discovered largely in Europeans, also translated successfully to Mexican Americans (ie, exhibited expected physiologic associations). The B + L–GRS (driven by BMI SNPs) was newly found to associate with insulin clearance, consistent with a role for insulin clearance in diabetes pathogenesis. Regarding the insulin-related endophenotypes, each was associated with a single GRS. This observation indicates that these GRS may have utility in dissecting out distinct mechanisms in studies wherein SNP data are available but physiologic measurements are not. The All-GRS, though strongly associated with T2D, was not associated with endophenotypes, suggesting no single mechanism predominates in the pathophysiology of diabetes, contrary to the conventional view that BC dysfunction dominates T2D genetics (40).
Of 457 SNPs associated with T2D, the majority have not been examined for association with an insulin-related trait, mainly because the latest GWAS greatly expanded the number of T2D SNPs (2). Of the 299 NE SNPs, 265 were from the DIAMANTE consortium, and 172 of these were novel discoveries. Considering the SNPs that do have a functional assignment, the greatest number fell into the BC category, consistent with prior observations (40). However, the number of IR SNPs is relatively higher in the current survey than in the past, mainly arising from cluster analyses but also reflecting increased efforts to associate these variants with measures of insulin sensitivity (7). Another challenge to assigning insulin resistance as a function may be that surrogate measures, including those from OGTT, may not accurately represent insulin sensitivity (41). This is particularly true of fasting-based measures. Fasting insulin has often been used as a measure of insulin sensitivity in epidemiologic studies. Although it does correlate with direct measures of insulin sensitivity (eg, derived from the euglycemic hyperinsulinemic clamp), fasting insulin is also highly and independently correlated with insulin secretion, insulin clearance, and adiposity (34). Furthermore, we previously found that a dynamic measure of insulin sensitivity (SI from the FSIGT) had a genetic basis different from fasting insulin or HOMA-IR because the genetic correlation of SI with either fasting-based trait was only 0.5 (42). Therefore, unlike earlier SNP classification efforts, we did not consider association with fasting insulin or the closely related HOMA-IR as evidence that a SNP acted via reduced insulin sensitivity.
In efforts to assign physiology to T2D SNPs, genome-wide significance (P < 5 × 10–8) has not typically been required. In fact, physiologic classification has often been made even when the association between T2D SNP and insulin-related trait is nominal based on the recognition that these SNPs are robust, detailed physiologic studies cannot be performed in massive sample sizes, and measurement error in physiologic traits may obscure association signals (3). In a GWAS for insulin secretion measured by IVGTT techniques, conducted in up to 5567 individuals, this approach allowed reduced first-phase insulin secretion to be assigned as the mechanism of 21 of 76 T2D loci examined, representing new evidence for 8 loci (8). To increase the robustness of the assignments made in the present effort, we prioritized assignments for which there were multiple lines of evidence. In only 5 cases did we not make an assignment because the only evidence was nominal association with an insulin-related trait in a single study. The consistency between evidence from differing studies adds confidence to the physiologic assignments.
T2D SNPs have come mainly from studies in European ancestry cohorts, followed in number by discoveries in East Asians. Though individually these variants generally are not strongly associated with diabetes in Hispanic individuals, when aggregated as a GRS, they were strongly associated with diabetes in the Study of Latinos (43). Furthermore, that study, which examined 80 T2D SNPs, found a greater than expected number of nominal associations with diabetes in Hispanics, suggesting that T2D loci found in Europeans and Asians generally do translate to diabetes in Hispanics. Consistent with this notion, in the present study we found that in Mexican Americans without diabetes the BC-GRS and IR-GRS exhibited the expected associations with decreased insulin secretion (derived from OGTT) and reduced insulin sensitivity (derived from the euglycemic clamp), respectively. Furthermore, the Lipo-GRS exhibited the expected association with decreased adiposity. With increasing efforts by the DIAMANTE consortium and others focusing on T2D genetics in Hispanic cohorts, it is hoped that Hispanic-specific variants may be discovered. In the meantime, however, it appears that existing T2D SNPs can be reliably applied in Hispanic individuals.
T2D loci have not been extensively examined for association with insulin clearance. Our prior investigation of 53 loci for T2D and related quantitative traits did not find association of any of the index GWAS SNPs with insulin clearance (44). Given recent evidence that reduced insulin clearance is an early adaptive response to insulin resistance (45), potentially relieving physiologic stress on BCs (46), the uncertainty regarding whether insulin clearance is a physiologic mechanism of action of T2D loci represents a significant knowledge gap. Herein, we found that the B + L class of SNPs was uniquely associated with increased insulin clearance. This is noteworthy given that this group of SNPs was assembled in independent hierarchical clustering efforts that did not consider insulin clearance (9, 10). Though this cluster was named based on associations of constituent SNPs with BMI and/or lipids, the general function of SNPs in this cluster (apart from the FTO and MC4R obesity loci) remained vague. The variants that fell into the B + L class were found to be enriched for liver-specific enhancer and promoter states (9). The liver is the primary organ that carries out insulin clearance; up to 80% of endogenously secreted insulin is cleared by the liver (~50% at first pass) (47). Our results allow the first assignment of an insulin-related endophenotype to this class of SNPs. The fact that the B + L–GRS was associated with increased insulin clearance suggests that SNPs in this group may affect diabetes risk via relatively reduced insulin levels. In concert with genetically reduced insulin secretion from the BC SNPs, this would be expected to increase the risk of diabetes by impairing the compensatory hyperinsulinemia needed to overcome IR. Whether the B + L–GRS also affects insulin clearance in groups other than Mexican Americans remains to be determined.
We followed up this novel result by dissecting the B + L–GRS into 2 scores based on SNPs mainly associated with BMI or lipid fractions, respectively. This revealed that the association is driven by the BMI SNPs within this overall group (at the FTO, MC4R, POC5, TFAP2B, NRXN3, and NFAT5 loci) that had previously been identified in GWAS for BMI (48, 49). For these SNPs, adjusting for BMI attenuated but did not abrogate the association with T2D (2), suggesting these SNPs may pleiotropically influence diabetes risk through nonobesity mechanisms. The latter may involve insulin clearance, given our finding that B-GRS was associated with insulin clearance despite adjustment for BMI.
We also attempted to replicate the B + L–GRS and B-GRS associations with insulin clearance in an independent Mexican American cohort (IRAS-FS). Though ethnically similar to our cohort, phenotyping of insulin clearance was performed using a different technique (FSIGT). We have previously observed differences in the genetic architecture of insulin clearance between clamp- and FSIGT-derived insulin clearance, with the latter manifesting less heritability (50). In addition, the clamp assesses insulin clearance at steady state whereas the FSIGT (as implemented in IRAS-FS) assesses clearance dynamically after a single injection of insulin. Though both are measures of whole-body insulin clearance, they reflect different proportions of hepatic and extrahepatic clearance, which are independently regulated (51). These genetic and physiologic differences in clearance measures may explain the lack of replication.
We must also consider the possibility that the insulin clearance association is a chance finding because the analyses reported herein did not correct for multiple testing. Multiple testing correction is not strictly warranted when testing a GRS against the corresponding trait (eg, BC-GRS with IGI30). On the other hand, novel discoveries are more compelling when multiple testing is considered. Given that we examined 5 types of quantitative traits (insulin secretion, insulin sensitivity, insulin clearance, adiposity, lipids), an adjusted P value cutoff for significance of .01 (0.05/5) could be applied to the results in Table 5. Though the Lipo-GRS association with body fat (available only in MACAD) does not meet this P value, the association with the surrogate marker BAI (available in the entire sample) is significant. Accounting for multiple testing, the B + L–GRS association with insulin clearance is nominal, whereas the B-GRS subset association is indeed significant. Future studies will be needed to validate the novel findings regarding insulin clearance.
A strength of the current effort is that we used multiple complementary sources to annotate SNPs, including association with physiologic markers and hierarchical cluster analyses. We avoided making classifications based solely on fasting-based markers or single nominal associations. This generated a robust list that can be used in multiple future GRS investigations, including clinical prediction or Mendelian randomization. To focus on SNPs most relevant to disease, we did not classify SNPs that have been associated with quantitative traits (eg, fasting glucose, fasting insulin, 2-hour glucose, hemoglobin A1c) but not with T2D itself. In focusing on studies that examined multiple T2D SNPs, we may have missed physiologic assignments from studies interrogating single loci. For the previous reasons, the lists of SNPs for insulin secretion and IR may be incomplete. Another potential weakness is that we used LD to harmonize annotations of loci with multiple SNPs. This might have led to some SNPs being misclassified, especially in cases in which LD was modest. However, this does not appear to have had a major adverse effect, given that we were able to validate expected associations with insulin secretion, IR, and adiposity for the BC-GRS, IR-GRS, and Lipo-GRS, respectively. The appropriateness of the SNP classification is supported by the observation that a GRS based on the 352 unclassified SNPs was not associated with any of the insulin-related traits or adiposity (data not shown). As additional future GWAS are published, the list of 457 T2D SNPs will need to be updated. We plan to update the annotations periodically to maintain utility of the list. We also anticipate that the long list of SNPs never examined for physiological function will shrink as future classification efforts include these SNPs.
In conclusion, this study integrated the current collection of T2D SNPs with several major efforts that have sought to assign physiologic function to these SNPs. We have generated robust classifications of BC function, IR, Lipo, and BMI plus lipids. The latter group of SNPs may promote diabetes by impairing the body’s ability to reduce insulin clearance in response to IR. This novel finding highlights the unrecognized role of insulin clearance in diabetes, which may have implications for the development of future antidiabetic agents.
Acknowledgments
Financial Support: This work was supported in part by National Institutes of Health grants from the National Heart, Lung, and Blood Institute (R01-HL088457, R01-HL67974, P50-HL055005), the National Institute of Diabetes and Digestive and Kidney Disease (R01-DK079888, P30-DK063491), M01-RR00425 (General Clinical Research Center Grant from the National Center for Research Resources), and UL1TR001881 (University of California Los Angeles Clinical and Translational Science Institute Grant from the National Center for Advancing Translational Sciences). M.O.G. was supported by the Eris M. Field Chair in Diabetes Research.
Glossary
Abbreviations
- B + L
body mass index plus lipids
- BAI
body adiposity index
- BC
beta-cell
- BMI
body mass index
- GRS
genetic risk score
- GWAS
genome-wide association studies
- HDL-C
high-density lipoprotein cholesterol
- IGI30
insulinogenic index at 30 minutes
- IR
insulin resistance
- Lipo
lipodystrophy
- M
glucose infusion rate
- MCRI
metabolic clearance rate of insulin
- SNP
single nucleotide polymorphism
- T2D
type 2 diabetes
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Morris AP. Progress in defining the genetic contribution to type 2 diabetes susceptibility. Curr Opin Genet Dev. 2018;50:41–51. [DOI] [PubMed] [Google Scholar]
- 2. Mahajan A, Taliun D, Thurner M, et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet. 2018;50(11):1505–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Frayling TM, Hattersley AT. Physiology helps GWAS take a step closer to mechanism. Diabetes. 2014;63(6):1836–1837. [DOI] [PubMed] [Google Scholar]
- 4. Dimas AS, Lagou V, Barker A, et al. ; MAGIC Investigators Impact of type 2 diabetes susceptibility variants on quantitative glycemic traits reveals mechanistic heterogeneity. Diabetes. 2014;63(6):2158–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ingelsson E, Langenberg C, Hivert MF, et al. ; MAGIC investigators Detailed physiologic characterization reveals diverse mechanisms for novel genetic loci regulating glucose and insulin metabolism in humans. Diabetes. 2010;59(5):1266–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prokopenko I, Poon W, Mägi R, et al. A central role for GRB10 in regulation of islet function in man. PloS Genet. 2014;10(4):e1004235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walford GA, Gustafsson S, Rybin D, et al. Genome-wide association study of the modified Stumvoll insulin sensitivity index identifies BCL2 and FAM19A2 as novel insulin sensitivity loci. Diabetes. 2016;65(10):3200–3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wood AR, Jonsson A, Jackson AU, et al. ; Diabetes Research on Patient Stratification (DIRECT) A genome-wide association study of IVGTT-based measures of first-phase insulin secretion refines the underlying physiology of type 2 diabetes variants. Diabetes. 2017;66(8):2296–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scott RA, Scott LJ, Mägi R, et al. ; DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium An expanded genome-wide association study of type 2 diabetes in Europeans. Diabetes. 2017;66(11):2888–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mahajan A, Wessel J, Willems SM, et al. ; ExomeBP Consortium; MAGIC Consortium; GIANT Consortium Refining the accuracy of validated target identification through coding variant fine-mapping in type 2 diabetes. Nat Genet. 2018;50(4):559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yaghootkar H, Scott RA, White CC, et al. Genetic evidence for a normal-weight “metabolically obese” phenotype linking insulin resistance, hypertension, coronary artery disease, and type 2 diabetes. Diabetes. 2014;63(12):4369–4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lotta LA, Gulati P, Day FR, et al. ; EPIC-InterAct Consortium; Cambridge FPLD1 Consortium Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nat Genet. 2017;49(1):17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lyssenko V, Jonsson A, Almgren P, et al. Clinical risk factors, DNA variants, and the development of type 2 diabetes. N Engl J Med. 2008;359(21):2220–2232. [DOI] [PubMed] [Google Scholar]
- 14. Meigs JB, Shrader P, Sullivan LM, et al. Genotype score in addition to common risk factors for prediction of type 2 diabetes. N Engl J Med. 2008;359(21):2208–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Talmud PJ, Cooper JA, Morris RW, et al. ; UCLEB Consortium Sixty-five common genetic variants and prediction of type 2 diabetes. Diabetes. 2015;64(5):1830–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kwak SH, Choi SH, Kim K, et al. Prediction of type 2 diabetes in women with a history of gestational diabetes using a genetic risk score. Diabetologia. 2013;56(12):2556–2563. [DOI] [PubMed] [Google Scholar]
- 17. Sharp SA, Rich SS, Wood AR, et al. Development and standardization of an improved type 1 diabetes genetic risk score for use in newborn screening and incident diagnosis. Diabetes Care. 2019;42(2):200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oram RA, Patel K, Hill A, et al. A type 1 diabetes genetic risk score can aid discrimination between type 1 and type 2 diabetes in young adults. Diabetes Care. 2016;39(3):337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carreras-Torres R, Johansson M, Gaborieau V, et al. The role of obesity, type 2 diabetes, and metabolic factors in pancreatic cancer: a Mendelian randomization study. J Natl Cancer Inst. 2017;109(9):djx012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Larsson SC, Scott RA, Traylor M, et al. ; METASTROKE Collaboration and NINDS Stroke Genetics Network (SiGN) Type 2 diabetes, glucose, insulin, BMI, and ischemic stroke subtypes: Mendelian randomization study. Neurology. 2017;89(5):454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ahmad OS, Morris JA, Mujammami M, et al. A Mendelian randomization study of the effect of type-2 diabetes on coronary heart disease. Nat Commun. 2015;6:7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ross S, Gerstein HC, Eikelboom J, Anand SS, Yusuf S, Paré G. Mendelian randomization analysis supports the causal role of dysglycaemia and diabetes in the risk of coronary artery disease. Eur Heart J. 2015;36(23):1454–1462. [DOI] [PubMed] [Google Scholar]
- 23. Mahendran Y, Jonsson A, Have CT, et al. Genetic evidence of a causal effect of insulin resistance on branched-chain amino acid levels. Diabetologia. 2017;60(5):873–878. [DOI] [PubMed] [Google Scholar]
- 24. Nowak C, Salihovic S, Ganna A, et al. Effect of insulin resistance on monounsaturated fatty acid levels: a multi-cohort non-targeted metabolomics and mendelian randomization study. PloS Genet. 2016;12(10):e1006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scott RA, Fall T, Pasko D, et al. ; RISC study group; EPIC-InterAct consortium Common genetic variants highlight the role of insulin resistance and body fat distribution in type 2 diabetes, independent of obesity. Diabetes. 2014;63(12):4378–4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang Q, Holmes MV, Davey Smith G, Ala-Korpela M. Genetic support for a causal role of insulin resistance on circulating branched-chain amino acids and inflammation. Diabetes Care. 2017;40(12):1779–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xue A, Wu Y, Zhu Z, et al. ; eQTLGen Consortium Genome-wide association analyses identify 143 risk variants and putative regulatory mechanisms for type 2 diabetes. Nat Commun. 2018;9(1):2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bonàs-Guarch S, Guindo-Martínez M, Miguel-Escalada I, et al. Re-analysis of public genetic data reveals a rare X-chromosomal variant associated with type 2 diabetes. Nat Commun. 2018;9(1):321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goodarzi MO, Cui J, Guo X, et al. Data from: classification of type 2 diabetes genetic variants and a novel genetic risk score association with insulin clearance. Dryad Digital Repository. 2019. Deposited 20 November 2019. https://doi.org/10.5061/dryad.s1rn8pk3t [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Palmer ND, Goodarzi MO, Langefeld CD, et al. Genetic variants associated with quantitative glucose homeostasis traits translate to type 2 diabetes in Mexican Americans: the GUARDIAN (Genetics Underlying Diabetes in Hispanics) Consortium. Diabetes. 2015;64(5):1853–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsai FJ, Yang CF, Chen CC, et al. A genome-wide association study identifies susceptibility variants for type 2 diabetes in Han Chinese. PloS Genet. 2010;6(2):e1000847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goodarzi MO, Guo X, Taylor KD, et al. Determination and use of haplotypes: ethnic comparison and association of the lipoprotein lipase gene and coronary artery disease in Mexican-Americans. Genet Med. 2003;5(4):322–327. [DOI] [PubMed] [Google Scholar]
- 33. Xiang AH, Azen SP, Raffel LJ, et al. Evidence for joint genetic control of insulin sensitivity and systolic blood pressure in Hispanic families with a hypertensive proband. Circulation. 2001;103(1):78–83. [DOI] [PubMed] [Google Scholar]
- 34. Goodarzi MO, Cui J, Chen YD, Hsueh WA, Guo X, Rotter JI. Fasting insulin reflects heterogeneous physiological processes: role of insulin clearance. Am J Physiol Endocrinol Metab. 2011;301(2):E402–E408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–E223. [DOI] [PubMed] [Google Scholar]
- 36. Bergman RN, Stefanovski D, Buchanan TA, et al. A better index of body adiposity. Obesity (Silver Spring). 2011;19(5):1083–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 38. Henkin L, Bergman RN, Bowden DW, et al. Genetic epidemiology of insulin resistance and visceral adiposity. The IRAS Family Study design and methods. Ann Epidemiol. 2003;13(4):211–217. [DOI] [PubMed] [Google Scholar]
- 39. Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62(5):1198–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Perry JR, Frayling TM. New gene variants alter type 2 diabetes risk predominantly through reduced beta-cell function. Curr Opin Clin Nutr Metab Care. 2008;11(4):371–377. [DOI] [PubMed] [Google Scholar]
- 41. Watanabe RM. The genetics of insulin resistance: where’s Waldo? Curr Diab Rep. 2010;10(6):476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bergman RN, Zaccaro DJ, Watanabe RM, et al. Minimal model-based insulin sensitivity has greater heritability and a different genetic basis than homeostasis model assessment or fasting insulin. Diabetes. 2003;52(8):2168–2174. [DOI] [PubMed] [Google Scholar]
- 43. Qi Q, Stilp AM, Sofer T, et al. ; MEta-analysis of type 2 DIabetes in African Americans (MEDIA) Consortium Genetics of type 2 diabetes in U.S. Hispanic/Latino Individuals: results from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Diabetes. 2017;66(5):1419–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Goodarzi MO, Guo X, Cui J, et al. Systematic evaluation of validated type 2 diabetes and glycaemic trait loci for association with insulin clearance. Diabetologia. 2013;56(6):1282–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jung SH, Jung CH, Reaven GM, Kim SH. Adapting to insulin resistance in obesity: role of insulin secretion and clearance. Diabetologia. 2018;61(3):681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kim SP, Ellmerer M, Kirkman EL, Bergman RN. Beta-cell “rest” accompanies reduced first-pass hepatic insulin extraction in the insulin-resistant, fat-fed canine model. Am J Physiol Endocrinol Metab. 2007;292(6):E1581–E1589. [DOI] [PubMed] [Google Scholar]
- 47. Ferrannini E, Wahren J, Faber OK, Felig P, Binder C, DeFronzo RA. Splanchnic and renal metabolism of insulin in human subjects: a dose-response study. Am J Physiol. 1983;244(6):E517–E527. [DOI] [PubMed] [Google Scholar]
- 48. Hoffmann TJ, Choquet H, Yin J, et al. A large multiethnic genome-wide association study of adult body mass index identifies novel loci. Genetics. 2018;210(2):499–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Locke AE, Kahali B, Berndt SI, et al. ; LifeLines Cohort Study; ADIPOGen Consortium; AGEN-BMI Working Group; CARDIOGRAMplusC4D Consortium; CKDGen Consortium; GLGC; ICBP; MAGIC Investigators; MuTHER Consortium; MIGen Consortium; PAGE Consortium; ReproGen Consortium; GENIE Consortium; International Endogene Consortium Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Goodarzi MO, Langefeld CD, Xiang AH, et al. Insulin sensitivity and insulin clearance are heritable and have strong genetic correlation in Mexican Americans. Obesity (Silver Spring). 2014;22(4):1157–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Polidori DC, Bergman RN, Chung ST, Sumner AE. Hepatic and extrahepatic insulin clearance are differentially regulated: results from a novel model-based analysis of intravenous glucose tolerance data. Diabetes. 2016;65(6):1556–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]