Abstract
Context
Polycystic ovary syndrome (PCOS) is a common endocrine disorder and is associated with metabolic syndrome (MS). Development of MS in PCOS is likely multifactorial and may relate to poor sleep.
Objective
The objective of this research is to investigate differences in objective markers of sleep in adolescents with obesity and PCOS with and without MS. We also aimed to examine the relationships between markers of sleep with MS markers.
Design
A cross-sectional study was conducted.
Participants
Participants included adolescents with PCOS and obesity with MS (N = 30) or without MS (N = 36).
Outcome Measures
Hormone and metabolic measurements, abdominal magnetic resonance imaging for hepatic fat fraction, actigraphy to estimate sleep, and overnight polysomnography (PSG).
Results
Adolescents with obesity and PCOS who also had MS had significantly worse sleep-disordered breathing including higher apnea-hypopnea index (AHI, P = .02) and arousal index (P = .01) compared to those without MS. Actigraphy showed no differences in habitual patterns of sleep behaviors including duration, timing, or efficiency between groups. However, a greater number of poor sleep health behaviors was associated with greater number of MS components (P = .04). Higher AHI correlated with higher triglycerides (TG) (r = 0.49, P = .02), and poorer sleep efficiency correlated with higher percentage of liver fat (r = –0.40, P = .01), waist circumference (r = –0.46, P < .01) and higher TG (r = –0.34, P = .04).
Conclusions
Among girls with PCOS and obesity, sleep-disordered breathing was more prevalent in those with MS, and poor sleep behaviors were associated with metabolic dysfunction and more MS symptoms. Sleep health should be included in the assessment of adolescents with PCOS and obesity.
Keywords: obstructive sleep apnea, polycystic ovarian syndrome, insulin resistance, sleep-disordered breathing, metabolic disease, pediatrics
Polycystic ovary syndrome (PCOS) is a common endocrine disorder currently affecting 6% to 10% of reproductive-aged women, with prevalence increasing with rising rates of obesity (1, 2). Women with PCOS and obesity are at risk for metabolic syndrome (MS), composed of central adiposity, hypertension, hypertriglyceridemia, dysglycemia, insulin resistance (IR), and often hepatic steatosis, which in turn increases risk for future type 2 diabetes, nonalcoholic fatty liver disease, and cardiovascular disease (3-6). The development of MS in women with PCOS is multifactorial, with hormonal, metabolic, and environmental contributors, and emerging evidence suggests poor sleep health, including sleep duration, timing, quality, and sleep-disordered breathing (SDB), may be a risk factor.
The focus of sleep research in PCOS has predominantly focused on SDB, with evidence that adult women with PCOS have higher rates of SDB than healthy women (7, 8). Women with PCOS were found to be 30 times more likely to have polysomnography (PSG)-assessed symptoms of SDB than healthy control women (9). Further, women with PCOS and SDB have more features of MS, including higher body mass index (BMI), waist circumference, blood pressure, triglyceride (TG) levels, worse lipid profiles, and impaired glucose metabolism compared to women with PCOS but without SDB, suggesting correlation of SDB with MS in PCOS (10-13).
Reported prevalence of SDB in adolescents with PCOS is contradictory, with estimates ranging from 0% to 57% (14-17). Only one study compared PSG parameters in adolescents with a) obesity, PCOS, and MS to adolescents with b) obesity and PCOS alone, c) obesity alone, and d) healthy-weight adolescents, and found no differences among groups for apnea-hypopnea index (AHI) (18). However, additional research is needed in adolescents to fully understand SDB in the context of PCOS and MS given that effective treatments for SDB are available and may prevent development of components of MS.
In addition to SDB risk, adult women with PCOS report more clinically significant insomnia symptoms and excessive daytime sleepiness than women without PCOS (19). A large Australian cohort study found that women with PCOS reported similar sleep duration but increased difficulty sleeping and more severe sleepiness compared to women without PCOS, and that these findings were maintained after accounting for BMI and depressive symptoms (20). Healthy women with shorter self-reported sleep duration were more likely to have irregular menses and markers of IR (21). However, these studies are limited by reliance on self-reported measures of sleep, and the extant literature in adolescents is limited.
We have previously reported that adolescents with obesity and PCOS had worse actigraphy-estimated sleep, including longer sleep-onset latency and reduced sleep efficiency (SE), compared to adolescents with obesity but without PCOS (22). Additionally, morning circadian misalignment was associated with IR in adolescents with PCOS (22). Another study found that adolescent girls with PCOS treated with metformin (850 mg twice daily) demonstrated significant improvements in self-reported sleep disturbance and daytime sleepiness at 3-month follow-up, in addition to improvements in BMI and insulin sensitivity (23). However, literature on sleep health in the context of MS in adolescents is lacking.
Thus, given that no prior research has comprehensively examined sleep-disordered breathing and sleep in adolescents with PCOS and with and without MS, the purpose of this study was to investigate differences in objective markers of sleep health including duration, timing, quality, and SDB in girls with obesity and PCOS with and without MS, and examine associations between sleep health and markers of MS. We hypothesized that those with MS would have worse sleep health compared to those without MS, and that a greater number of sleep risk factors, including short sleep duration, late sleep timing, sleep variability, and poor SE, would be associated with more components of the MS.
Materials and Methods
Participants
The data presented includes participants from the APPLE (NCT02157974) and PLUM studies (NCT03041129), who were recruited from referral-based tertiary care obesity clinics. Inclusion criteria included age 12 to 21 years, female sex, BMI greater than or equal to 90th percentile for age and sex, and being habitually physically inactive (< 3 h/wk physical activity). Exclusion criteria included diabetes mellitus, alanine transferase greater than 80 IU/mL, blood pressure greater than 140/90 mm Hg, hemoglobin less than 9 mg/dL, serum creatinine greater than 1.5 mg/dL, smoking, medications affecting primary end points including insulin sensitivity, blood pressure, or lipids, and pregnancy or breastfeeding. Participants were excluded from data analysis if they were home-schooled or if study participation occurred over school breaks. PCOS was diagnosed using the National Institutes of Health criteria of oligomenorrhea and hyperandrogenism, with adaptation for adolescents of 1.5 or more years postmenarche (2, 24). Participants were studied in the follicular phase of the menstrual cycle, confirmed via serum progesterone. This study was approved by the University of Colorado Combined Multiple Institutional Review Board and the Children’s Hospital Colorado’s Scientific Advisory Review Committee. Participants older than 18 years provided written informed consent; participants younger than 18 years provided assent and informed consent was obtained from parents or guardians.
Procedures
Participants completed 2 study visits. The first visit included medical history, qualification laboratory tests, and a physical exam. A subsequent overnight study visit composed of dual-energy x-ray absorptiometry and magnetic resonance imaging of the abdomen on day 1, and a 76- to 78-g, 6.5-hour oral sugar tolerance test (OSTT) the next day following an overnight monitored fast. A subset of participants (N = 21) received overnight PSG. All participants were asked to avoid exercising 3 days before the second visit. In addition, all participants were provided a weight-maintenance isocaloric snack and dinner (55% carbohydrate, 30% fat, 15% protein) the night before the OSTT from the University of Colorado Anschutz Clinical and Translational Research Center metabolic kitchen.
Measures
Actigraphy.
Participants wore a Spectrum Plus actigraphy monitor (Phillips Respironics) on their nondominant wrist for 7 days before the second study visit. They completed a concurrent sleep diary reporting their sleep and wake times to allow for accurate scoring of sleep and wake episodes. Total sleep time, sleep-onset latency, SE, and bedtimes and wake times were calculated using Actiware Sleep V6 software (Phillips Respironics) with standard scoring rules and moderate sensitivity (25).
Physical activity.
All participants wore an Actigraph GT3x accelerometer on their hip for 7 days before the second visit (Actigraph Corp). After correcting for wear time, the data from the accelerometer were categorized into 6 activity levels (sedentary, light, lifestyle, moderate, vigorous, and very vigorous) according to cut points corresponding to standardized activity intensity standards (26).
Abdominal magnetic resonance imaging.
A 3 Tesla magnet (GE Healthcare) and 15M4 software was used to measure hepatic and visceral fat. As described previously, the Dixon method involving a multi–breath-hold double-gradient echo T1-weighted sequence was used to quantify hepatic fat (27-30).
Polysomnography.
A subgroup of the sample (N = 21) had their sleep architecture and respiratory patterns evaluated via PSG using pediatric criteria from the American Thoracic Society and standard sleep scoring techniques. Surface electrodes and monitoring devices (Somnoligica) measured signals from the central electroencephalogram, right and left electro-oculogram, surface electromyogram, electrocardiogram, chest and abdominal wall motion, pulse oximetry (Masimo), and end-tidal PCO2 (Novametrix). An oronasal thermistor was used to measure airflow (Pro Tech). All studies were performed by a registered PSG technologist and monitored with real-time video for motion analysis and snoring recording. Studies were interpreted by a board-certified pulmonologist.
Oral sugar tolerance test.
After a monitored overnight fast, participants consumed a 76- to 78-g glucose load with an additional 25 g of fructose. Blood samples were collected before the sugar load for fasting measures and continued for 6 hours post–sugar load to allow return to baseline. For the purpose of the present analyses, 2-hour glucose values were used as criteria for MS. Additionally, homeostatic model assessment of insulin resistance (HOMA-IR) ([fasting insulin (uU/ml) × fasting glucose (mmol/l)]/22.5) (31) and Matsuda index (10 000/√[[fasting insulin (uU/ml) × fasting glucose (mmol/l)] × [mean OSTT insulin uU/ml) × mean OSTT glucose (mmol/l)]) (32) were calculated to quantify insulin sensitivity, with higher HOMA-IR and lower Matsuda index indicative of worse insulin sensitivity.
Laboratory analyses
All laboratory analyses were performed at the Children’s Hospital Colorado Laboratory and the University of Colorado Research Core Laboratory. ELISA was used to analyze TG and total cholesterol (Hitachi 917 autoanalyzer; Boehringer Mannheim Diagnostics). The Friedewald equation was used to evaluate low-density lipoprotein cholesterol. Diabetes Control and Complications Trials–calibrated high-performance ion-exchange liquid chromatography was sourced for hemoglobin A1c (Bio-Rad Laboratories). Radioimmunoassays were used for serum adiponectin, and insulin (EMD Millipore). Immunoturbidimetric assay was used to evaluate C-reactive protein (Beckman Coulter). Chemiluminescent immunoassay was used to analyze estradiol (Beckman Coulter). Alanine transferase was measured using VITROS 5600 (Ortho Clinical Diagnostics). Electrochemiluminescence immunoassay was used for evaluating sex hormone-binding globulin (SHBG) (Esoterix). Liquid chromatography–tandem mass spectrometry was used to measure total testosterone (Esoterix). Total testosterone and SHBG values were used to determine the free androgen index.
Calculations
The following 5 MS components were used to classify participants as being in either the MS group (> 3 components) or the non–metabolic syndrome (NMS) group (< 2 components): 2-hour OSTT glucose greater than 140 mg/dL, systolic blood pressure greater than 130 mmHg, waist circumference greater than 80 cm, TG concentration greater than 150 mg/dL, and percentage hepatic fat greater than 5.5(33). Two-hour glucose was used per World Health Organization and American Association of Clinical Endocrinology guidelines because fasting dysglycemia was rare in our population. Because all participants were obese and had a high-density lipoprotein less than 50 mg/dL, we did not add either of these into the count of MS components. We included hepatic steatosis because the addition of nonalcoholic fatty liver disease to MS was associated with significant increases in mortality (34). A sleep risk score of 0 to 4 was calculated based on 4 markers of sleep health, with lower values indicating better sleep health (35). Participants received a score of either 0 (absence) or 1 (presence) for each of the following: 1) less than 7 hours of total sleep time on weeknights, 2) bedtime after midnight on weeknights, 3) more than 1-hour variability between weekday and weekend bedtimes, and 4) weekday SE less than 85%.
Statistical analyses
Descriptive statistics are presented as mean ± SD or median (25%, 75%). A normality test was run on all results, and a Mann-Whitney Rank Sum test was used if the normality test failed. An independent-sample t test was performed to compare participants with and without MS. Spearman correlation coefficient was used to examine the relationships between sleep variables and metabolic syndrome markers. The chi-square test was used to assess relationships between the number of MS symptoms and sleep risk score. A P value less than .05 denoted statistical significance. All statistical analyses were performed using SigmaStat software, version 13.1 (Systat Software, Inc).
Results
Of the 66 participants in the study, 36 were classified as NMS and 30 were classified as MS. Groups did not significantly differ in terms of demographic or physical characteristics, including age, age of menarche, acne severity, hirsutism score, BMI percentile, or sedentary time; thus these variables were not controlled for in subsequent analyses (Table 1). As expected, the MS group had significantly higher waist to hip ratio, systolic blood pressure, 2-hour OSTT glucose, fasting TG, and hepatic fat (P < .001). Whereas testosterone was similar between the 2 groups (P > .05), free androgen index and ALT were significantly higher and SHBG and adiponectin were significantly lower in the MS group (Table 2). Hemoglobin A1c was similar, but HOMA-IR, Matsuda index, fasting insulin, and 1/fasting insulin were significantly worse in the MS group (all P < .01).
Table 1.
Participant descriptive characteristics
| MS (N = 30) | NMS (N = 36) | P | |
|---|---|---|---|
| Age, y | 15.8 ± 1.8 | 16.0 ± 1.9 | .61 |
| BMI percentile | 98.2 (97.2, 99.1) | 97.7 (96.0, 99.0) | .36 |
| Race/Ethnicity | |||
| Caucasian/Hispanic/Black/Other | 10/18/1/1 | 15/10/6/5 | .24 |
| Menarche age, y | 12 (11, 13) | 11 (11, 13) | .49 |
| Ferriman Gallwey score (0-32) | 6 (3, 12) | 8 (2, 11) | .98 |
| Acne (0-3) | 1 (1, 2) | 2 (1, 2) | .08 |
| Total body fat, % from DXA | 44 ± 4 | 44 ± 5 | .93 |
| Accelerometer, % sedentary | 55 (48, 67) | 57 (49, 72) | .59 |
| MS components | |||
| Waist to hip ratio | 0.95 (0.87, 0.98) | 0.88 (0.83, 0.92) | < .001 |
| Systolic blood pressure, mm Hg | 122 ± 10 | 119 ± 7 | .10 |
| Fasting glucose, mg/dL | 91 ± 8 | 88 ± 8 | .07 |
| OSTT 2-h glucose, mg/dL | 146 ± 25 | 123 ± 24 | < .001 |
| Fasting triglycerides, mg/dL | 146 (106, 192) | 103 (76, 129) | < .001 |
| Liver fat, % | 8.8 (6.1, 13.8) | 4.1 (3.0, 5.3) | < .001 |
Data are presented as mean ± SD or median (25%, 75%). P less than .05 is shown in bold and indicates a significant difference on independent-samples t tests between the MS and NMS groups. Higher Ferriman Gallwey score indicates worse hirsutism and higher acne score indicates worse acne.
Abbreviations: BMI, body mass index; DXA, dual-energy x-ray absorptiometry; MS, metabolic syndrome; NMS, non–metabolic syndrome; OSTT, oral sugar tolerance test.
Table 2.
Laboratory measures
| MS (N = 30) | NMS (N = 36) | P | |
|---|---|---|---|
| Free testosterone, pg/mL | 9.1 (6.6, 13.3) | 7.9 (5.5, 10.5) | .12 |
| Total testosterone, ng/dL | 44 (29, 56) | 43 (33, 51) | .67 |
| Free androgen index | 9.6 (7.1, 13.7) | 6.6 (5.2, 9.3) | < .01 |
| SHBG, nmol/L | 14.6 (11.0, 20.1) | 20.0 (13.7, 28.9) | < .01 |
| Estradiol, pg/mL | 50.0 (38.8, 79.8) | 49.0 (38.3, 68.3) | .88 |
| Adiponectin, ng/mL | 5.6 (4.2, 7.1) | 7.0 (5.5, 10.6) | .01 |
| Cholesterol, mg/dL | 147 (133, 166) | 138 (123, 164) | .25 |
| HDL, mg/dL | 36.3 ± 8.8 | 36.7 ± 7.3 | .84 |
| LDL, mg/dL | 82 (66, 95) | 82 (67, 97) | .74 |
| ALT, U/L | 37 (31, 46) | 32 (27, 41) | .02 |
| AST, U/L | 41 (36, 45) | 44 (35, 50) | .99 |
| hs-CRP, mg/dL | 2.2 (0.8, 4.7) | 2.8 (1.0, 7.1) | .44 |
| Glucose metabolism | |||
| HbA1c, % | 5.5 ± 0.3 | 5.4 ± 0.4 | .24 |
| Fasting insulin, µIU/mL | 32 (23, 44) | 21 (15, 29) | < .01 |
| Fasting glucose, mg/dL | 91 ± 8 | 88 ± 8 | .07 |
| OGTT 2-h insulin, µIU/mL | 408 (191, 659) | 138 (59, 220) | < .001 |
| Mean OGTT glucose, mg/dL | 137 ± 18 | 121 ± 18 | < .001 |
| Mean OGTT insulin, µIU/mL | 295 (188, 432) | 128 (92, 192) | < .001 |
| Matsuda index | 0.94 (0.62, 1.48) | 1.89 (1.38, 2.61) | < .001 |
| HOMA-IR | 7.6 (4.8, 10.0) | 4.3 (3.2, 5.9) | < .001 |
| TG/HDL | 3.9 (2.7, 5.7) | 2.7 (2.1, 3.6) | < .01 |
| 1/Fasting insulin | 0.03 (0.02, 0.04) | 0.05 (0.03, 0.07) | < .01 |
Data are presented as mean ± SD or median (25%, 75%). P less than .05 is shown in bold and indicates a significant difference on independent samples t tests between the MS and NMS groups.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance; hs-CRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; MS, metabolic syndrome; NMS, non–metabolic syndrome; OSTT, oral sugar tolerance test; SHBG, sex hormone-binding globulin; TG, triglycerides.
Among those with PSG (Table 3, MS = 12; NMS = 9), girls with MS had significantly higher AHI (MS = 6.5 (2.9, 22.5); NMS = 0.7 (0.2, 6.7); P = .02) and arousal index (P = .01). Analyses showed that there was no significant difference in actigraphy-assessed bedtime, wake time, total sleep time, number of awakenings, sleep-onset latency, and SE between participants with and without MS (Table 4). Additionally, social jet lag, defined as a more than a 1-hour difference between weekend and weekday bedtime, was not significantly different between the 2 groups (P = .78).
Table 3.
Polysomnography
| MS (N = 12) | NMS (N = 9) | P | |
|---|---|---|---|
| AHI | 6.5 (2.9, 22.5) | 0.7 (0.2, 6.7) | .02 |
| % REM sleep | 19 (15, 23) | 17 (9, 21) | .41 |
| Arousal index | 9.9 (6.8, 16.4) | 5.9 (3.4, 7.0) | .01 |
| Oxygen desaturation index | 6.2 (1.5, 12.3) | 0.3 (0.0, 8.7) | .08 |
Data are presented as mean ± SD or median (25%, 75%). P less than .05 is shown in bold and indicates a significant difference on independent-samples t tests between the MS and NMS groups.
Abbreviations: AHI, apnea-hypopnea index; MS, metabolic syndrome; NMS, non–metabolic syndrome; REM, rapid-eye movement.
Table 4.
Actigraphy
| MS (N = 30) | NMS (N = 36) | |||
|---|---|---|---|---|
| Weekday | Weekend | Weekday | Weekend | |
| Bedtime, h | 23:18 ± 1.3 | 23:36 ± 1.4 | 23:37 ± 1.6 | 1:20 ± 1.7 |
| Wake time, h | 7:35 ± 1.6 | 8:58 ± 1.7 | 8:23 ± 1.9 | 9:06 ± 2.1 |
| TST, min | 417 ± 56 | 463 ± 102 | 432 ± 60 | 443 ± 71 |
| SOL, min | 16.8 (9.3, 22.4) | 10.0 (5.5, 24.5) | 10.1 (6.3, 19.5) | 6.5 (2.0, 12.8) |
| Sleep efficiency, % | 82 (76, 87) | 85 (80, 88) | 84 (75, 87) | 86 (82, 89) |
Data are presented as mean ± SD or median (25%, 75%). Independent-samples t tests showed no significant differences in any weekday and weekend objective sleep markers between the MS and NMS groups (all P > .05).
Abbreviations: MS, metabolic syndrome; NMS, non–metabolic syndrome; SOL, sleep-onset latency; TST, total sleep time.
Table 5 shows correlations between sleep variables with markers of MS. Lower weekday and weekend SE was associated with higher percentage of liver fat (r = –0.36, P = .02 and r = –0.40, P = .013, respectively). Lower actigraphy-measured weekday SE was associated with higher 2-hour OGTT glucose and waist to hip ratio (r = –0.29, P = .08 and r = –0.46, P = .004, respectively). Additionally, lower weekend SE correlated with higher TG (r = –0.34; P = .04). PSG AHI positively correlated with TG (r = 0.49; P = .02).
Table 5.
Correlations Between MS Markers and Sleep Health Variables
| MS Markers | |||||
|---|---|---|---|---|---|
| Sleep Health Variables (N = 66) | % Liver Fat | 2-h OSTT Glucose | SBP | WC | TG |
| AHI (N = 21) | 0.37; .08 | 0.36; .10 | 0.27; .20 | 0.12; .56 | 0.49; .02 |
| Weekday TST | –0.29; .08 | –0.02; .89 | –0.20; .23 | –0.20; .23 | –0.11; .50 |
| Weekend TST | –0.28; .08 | –0.07; .69 | –0.09; .25 | –0.19; .25 | –0.08; .62 |
| Weekday SE | –0.36; .02 | –0.29; .08 | 0.03; .85 | –0.46; < .01 | –0.19; .24 |
| Weekend SE | –0.40; .01 | –0.15; .36 | –0.02; .92 | –0.12; .48 | –0.34; .04 |
Data are presented as r value; P value. P less than .05 is shown in bold and indicates a significant Spearman correlation coefficient between the sleep health marker and MS marker.
Abbreviations: AHI, apnea-hypopnea index; MS, metabolic syndrome; NMS, non–metabolic syndrome; OSTT, oral sugar tolerance test; SE, sleep efficiency; TG triglycerides; TST, total sleep time; WC, waist circumference.
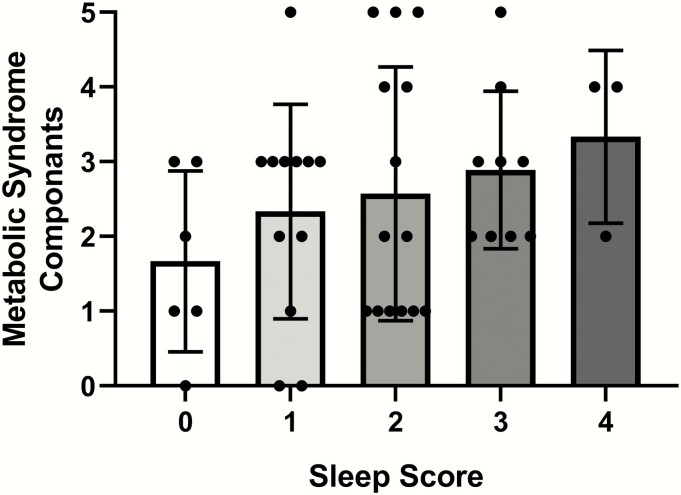
The number of MS components per participant were grouped based on her sleep risk score (Fig. 1). The proportion of MS components increased continuously as the sleep risk score increased from 0 to 4 (P = .04).
Figure 1.
Number of metabolic syndrome components (0-5, 1. percentage hepatic fat, 2. 2-hour oral sugar tolerance test glucose concentration, 3. systolic blood pressure, 4. waist circumference, and 5. triglyceride concentration) per sleep score (0-4, 1. less than 7 hours total sleep time on weeknights, 2. bedtime after midnight on weeknights, 3. more than 1-hour variability between weekday and weekend bedtimes, and 4. weekday sleep efficiency < 85%) are shown. The proportion of those with more components of metabolic syndrome was higher in those with worse sleep score. Shaded bars represent means and error bars represent SD. Symbols represent individual patient scores. The chi-square difference between the groups is P = .04.
Discussion
In this sample of adolescent girls with PCOS and obesity, SDB was more prevalent in those with MS, and higher AHI and poorer SE were associated with worse metabolic health. Moreover, a greater number of unhealthy sleep behaviors were associated with increased severity of MS.
These data are consistent with the prior literature in adult women with PCOS reporting higher rates of PSG-assessed SDB compared to healthy controls (7-9), and that women with PCOS and SDB have more MS symptoms than women with PCOS but without SDB (10-13). In addition, from a retrospective chart review, Nandalike et al (16, 17) found that adolescent girls with PCOS had a greater prevalence of SDB compared to controls, and that those with PCOS and SDB had significantly higher proportions of MS compared to those without SDB. Our study extends these findings with the addition of prospective objective sleep measurement. However, our findings contrast with a series of papers by de Sousa and colleagues that found no differences in AHI in adolescents with PCOS compared to controls with and without obesity, and no differences in adolescents with PCOS and obesity with or without the MS, though multiple group comparisons with small samples (~14) per group may have resulted in a lack of power to detect differences (15, 18).
The findings that poor sleep health is associated with greater metabolic dysfunction is also in line with the extant literature. A recent systematic review and meta-analysis of adolescent male and female individuals with obesity found that obstructive sleep apnea is independently associated with metabolic impairment, above and beyond obesity risk (36). In addition, a review by Bussler and colleagues (37) reported that sleep disturbance is emerging as an associated factor with MS in youth. Specifically, we found that poorer SE and higher AHI were associated with symptoms of MS. From animal models, it has been posited that intermittent hypoxia from SDB leads to IR and hepatic metabolism dysfunction due to effects such as increased oxidative stress, inflammation, and mitochondrial dysfunction (38). Indeed, it has been demonstrated in children that oxidative stress from nocturnal hypoxia leads to progression of pediatric nonalcoholic fatty liver disease (39). Furthermore, intermittent hypoxia increases lipogenesis transcription factor HIF1α, which causes oxidative stress due to increased beta-oxidation (40). Specific investigations on the role of SE are lacking, but it may be that sleep disruption is also a risk factor for metabolic dysfunction.
In this sample, a greater number of sleep risk factors, including short sleep duration, late bedtime, sleep variability, and poor SE were associated with an increased number of MS components. However, sleep duration, timing, or quality did not differ by MS status in this sample. In our previously reported study, adolescents with obesity and PCOS had longer sleep-onset latency and lower SE assessed via actigraphy compared to adolescents with obesity but without PCOS (22). Thus, it may be that PCOS status, rather than MS status, is more salient for sleep health. Moreover, examining sleep health behaviors together as a multidimensional construct, rather than individually, may be more meaningful (35). To further elucidate the role of PCOS per se, additional work is needed to determine whether similar patterns are found in nonobese adolescents with PCOS as compared to similar-weight girls without PCOS.
Strengths of the presented data include controlling for many potential confounding variables, including physical activity, menstrual cycle, and diet before study visits, because these factors can affect sleep as well as insulin sensitivity. Furthermore, participants who were home-schooled or who participated during school breaks were excluded from analysis because sleep patterns may be different from those during the traditional academic year (41, 42). Whereas previous studies have relied on self-reported questionnaires or a single night of PSG, our study used multiple objective measures of sleep, including PSG and actigraphy. However, findings should be considered in the context of study limitations, including that our overall sample was relatively small, and only a subgroup of participants obtained PSG, which limited our sample size and statistical power. Moreover, although the sleep risk score was based on the Sleep Health Composite score created by Dong and colleagues (35), future work is needed to examine the utility of such a score in metabolic research. Future research is needed to further understand the mechanisms involved in the relationships between sleep health and metabolic dysfunction in adolescents with PCOS. Additionally, testing the impact of sleep health interventions on subsequent changes in MS symptoms would be informative, as would studies of the impact of PCOS medications on sleep health. Our participants were recruited from referral-based obesity clinics within a tertiary care center, and thus are likely not wholly representative of girls seen with uncomplicated obesity or PCOS with obesity who receive all their care from community providers. Finally, we included hepatic steatosis as one of our criteria for MS because hepatic steatosis is very common in this population (5) and based on recent analysis of National Health and Nutrition Examination Survey data that this better identifies those at risk for early mortality (34). It remains to be determined whether hepatic steatosis should be added as a formal criterion for MS.
In conclusion, in our sample of adolescent girls with PCOS and obesity, SDB was more prevalent in those with MS, and poor SE and AHI were associated with metabolic dysfunction. Additionally, a greater number of sleep health risk factors were associated with greater MS symptoms. Of concern, the most recent international PCOS guidelines (24) lack recommendations regarding sleep screening beyond SDB or for reproductive health, which is concerning given the emerging evidence that insufficient and mistimed sleep is associated with cardiometabolic morbidity in adolescents (22, 43), and the pervasiveness of chronic short and delayed sleep in adolescents (44, 45). Our data suggest the importance of comprehensive assessment of sleep health including SDB as well as duration, timing, and quality in adolescents with PCOS and obesity, given the association with MS and metabolic dysfunction.
Acknowledgments
We express our appreciation to the participants and their families, and the University of Colorado Anschutz Clinical and Translational Research Center nursing staff. We also acknowledge Carol Kline, NP, for her help with PSG interpretation.
Financial Support: This work was supported by National Institutes of Health K12HD057022; National Institute of Diabetes and Digestive and Kidney Diseases K23DK107871; Doris Duke Foundation Caregivers Fellowship (Grant 2015212); Children’s Hospital Colorado Research Institute funding (to M.C.G.); University of Colorado Denver Center for Women’s Health Research Pilot Award (to S.L.S.); National Center for Advancing Translational Sciences UL1TR002535 (to Colorado Clinical and Translational Sciences Institute).
Author Contributions: S.L.S, K.J.N., and M.C.G. designed the study; S.L.S., H.R., A.M.C., Y.G.R., A.H., and M.C.G. collected and assembled the data; S.L.S., H.R., and L.P. analyzed data and performed statistical analyses; S.L.S., H.R., K.J.N., and M.C.G. interpreted the results; S.L.S., H.R., and M.C.G. wrote the manuscript. All authors reviewed and critiqued the manuscript, contributed to revisions, and approved the final manuscript.
Glossary
Abbreviations
- BMI
body mass index
- IR
insulin resistance
- MS
metabolic syndrome
- NMS
non–metabolic syndrome
- OSTT
oral sugar tolerance test
- PCOS
polycystic ovary syndrome
- PSG
polysomnography
- SDB
sleep-disordered breathing
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83(9):3078–3082. [DOI] [PubMed] [Google Scholar]
- 2. Legro RS, Arslanian SA, Ehrmann DA, et al. ; Endocrine Society . Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(12):4565–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baranova A, Tran TP, Birerdinc A, Younossi ZM. Systematic review: association of polycystic ovary syndrome with metabolic syndrome and non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2011;33(7):801–814. [DOI] [PubMed] [Google Scholar]
- 4. Ehrmann DA, Liljenquist DR, Kasza K, Azziz R, Legro RS, Ghazzi MN; PCOS/Troglitazone Study Group . Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91(1):48–53. [DOI] [PubMed] [Google Scholar]
- 5. Cree-Green M, Bergman BC, Coe GV, et al. Hepatic steatosis is common in adolescents with obesity and PCOS and relates to de novo lipogenesis but not insulin resistance. Obesity (Silver Spring). 2016;24(11):2399–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patel SS, Truong U, King M, et al. Obese adolescents with polycystic ovarian syndrome have elevated cardiovascular disease risk markers. Vasc Med. 2017;22(2):85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fogel RB, Malhotra A, Pillar G, Pittman SD, Dunaif A, White DP. Increased prevalence of obstructive sleep apnea syndrome in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2001;86(3):1175–1180. [DOI] [PubMed] [Google Scholar]
- 8. Kumarendran B, Sumilo D, O’Reilly MW, et al. Increased risk of obstructive sleep apnoea in women with polycystic ovary syndrome: a population-based cohort study. Eur J Endocrinol. 2019;180(4):265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vgontzas AN, Legro RS, Bixler EO, Grayev A, Kales A, Chrousos GP. Polycystic ovary syndrome is associated with obstructive sleep apnea and daytime sleepiness: role of insulin resistance. J Clin Endocrinol Metab. 2001;86(2):517–520. [DOI] [PubMed] [Google Scholar]
- 10. Chatterjee B, Suri J, Suri JC, Mittal P, Adhikari T. Impact of sleep-disordered breathing on metabolic dysfunctions in patients with polycystic ovary syndrome. Sleep Med. 2014;15(12):1547–1553. [DOI] [PubMed] [Google Scholar]
- 11. Lin TY, Lin PY, Su TP, et al. Risk of developing obstructive sleep apnea among women with polycystic ovarian syndrome: a nationwide longitudinal follow-up study. Sleep Med. 2017;36:165–169. [DOI] [PubMed] [Google Scholar]
- 12. Kahal H, Kyrou I, Tahrani AA, Randeva HS. Obstructive sleep apnoea and polycystic ovary syndrome: a comprehensive review of clinical interactions and underlying pathophysiology. Clin Endocrinol (Oxf). 2017;87(4):313–319. [DOI] [PubMed] [Google Scholar]
- 13. Kahal H, Kyrou I, Uthman O, et al. The association between obstructive sleep apnea and metabolic abnormalities in women with polycystic ovary syndrome: a systematic review and meta-analysis. Sleep. 2018;41(7):1–12. [DOI] [PubMed] [Google Scholar]
- 14. de Sousa G, Schlüter B, Buschatz D, et al. A comparison of polysomnographic variables between obese adolescents with polycystic ovarian syndrome and healthy, normal-weight and obese adolescents. Sleep Breath. 2010;14(1):33–38. [DOI] [PubMed] [Google Scholar]
- 15. de Sousa G, Schlüter B, Menke T, Trowitzsch E, Andler W, Reinehr T. Relationships between polysomnographic variables, parameters of glucose metabolism, and serum androgens in obese adolescents with polycystic ovarian syndrome. J Sleep Res. 2011;20(3):472–478. [DOI] [PubMed] [Google Scholar]
- 16. Nandalike K, Agarwal C, Strauss T, et al. Sleep and cardiometabolic function in obese adolescent girls with polycystic ovary syndrome. Sleep Med. 2012;13(10):1307–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nandalike K, Strauss T, Agarwal C, et al. Screening for sleep-disordered breathing and excessive daytime sleepiness in adolescent girls with polycystic ovarian syndrome. J Pediatr. 2011;159(4):591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Sousa G, Schlüter B, Menke T, Trowitzsch E, Andler W, Reinehr T. A comparison of polysomnographic variables between adolescents with polycystic ovarian syndrome with and without the metabolic syndrome. Metab Syndr Relat Disord. 2011;9(3):191–196. [DOI] [PubMed] [Google Scholar]
- 19. Franik G, Krysta K, Madej P, et al. Sleep disturbances in women with polycystic ovary syndrome. Gynecol Endocrinol. 2016;32(12):1014–1017. [DOI] [PubMed] [Google Scholar]
- 20. Mo L, Mansfield DR, Joham A, et al. Sleep disturbances in women with and without polycystic ovary syndrome in an Australian National Cohort. Clin Endocrinol (Oxf). 2019;90(4):570–578. [DOI] [PubMed] [Google Scholar]
- 21. Lim AJ, Huang Z, Chua SE, Kramer MS, Yong EL. Sleep duration, exercise, shift work and polycystic ovarian syndrome-related outcomes in a healthy population: a cross-sectional study. PloS One. 2016;11(11):e0167048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Simon SL, McWhirter L, Diniz Behn C, et al. Morning circadian misalignment is associated with insulin resistance in girls with obesity and polycystic ovarian syndrome. J Clin Endocrinol Metab. 2019;104(8):3525–3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. El-Sharkawy AA, Abdelmotaleb GS, Aly MK, Kabel AM. Effect of metformin on sleep disorders in adolescent girls with polycystic ovarian syndrome. J Pediatr Adolesc Gynecol. 2014;27(6):347–352. [DOI] [PubMed] [Google Scholar]
- 24. Teede HJ, Misso ML, Costello MF, et al. ; International PCOS Network . Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Clin Endocrinol (Oxf). 2018;89(3):251–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ancoli-Israel S, Martin JL, Blackwell T, et al. The SBSM guide to actigraphy monitoring: clinical and research applications. Behav Sleep Med. 2015;13(Suppl 1):S4–S38. [DOI] [PubMed] [Google Scholar]
- 26. Freedson PS, Melanson E, Sirard J. Calibration of the computer science and applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30(5):777–781. [DOI] [PubMed] [Google Scholar]
- 27. Nadeau KJ, Zeitler PS, Bauer TA, et al. Insulin resistance in adolescents with type 2 diabetes is associated with impaired exercise capacity. J Clin Endocrinol Metab. 2009;94(10):3687–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fishbein MH, Miner M, Mogren C, Chalekson J. The spectrum of fatty liver in obese children and the relationship of serum aminotransferases to severity of steatosis. J Pediatr Gastroenterol Nutr. 2003;36(1):54–61. [DOI] [PubMed] [Google Scholar]
- 29. Cree-Green M, Newcomer BR, Brown M, et al. Method for controlled mitochondrial perturbation during phosphorus MRS in children. Med Sci Sports Exerc. 2014;46(10):2030–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cree-Green M, Newcomer BR, Brown MS, et al. Delayed skeletal muscle mitochondrial ADP recovery in youth with type 1 diabetes relates to muscle insulin resistance. Diabetes. 2015;64(2):383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gungor N, Saad R, Janosky J, Arslanian S. Validation of surrogate estimates of insulin sensitivity and insulin secretion in children and adolescents. J Pediatr. 2004;144(1):47–55. [DOI] [PubMed] [Google Scholar]
- 32. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. [DOI] [PubMed] [Google Scholar]
- 33. Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5–6):231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Golabi P, Otgonsuren M, de Avila L, Sayiner M, Rafiq N, Younossi ZM. Components of metabolic syndrome increase the risk of mortality in nonalcoholic fatty liver disease (NAFLD). Medicine (Baltimore). 2018;97(13):e0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dong L, Martinez AJ, Buysse DJ, Harvey AG. A composite measure of sleep health predicts concurrent mental and physical health outcomes in adolescents prone to eveningness. Sleep Health. 2019;5(2):166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Patinkin ZW, Feinn R, Santos M. Metabolic consequences of obstructive sleep apnea in adolescents with obesity: a systematic literature review and meta-analysis. Child Obes. 2017;13(2):102–110. [DOI] [PubMed] [Google Scholar]
- 37. Bussler S, Penke M, Flemming G, et al. Novel insights in the metabolic syndrome in childhood and adolescence. Horm Res Paediatr. 2017;88(3-4):181–193. [DOI] [PubMed] [Google Scholar]
- 38. Mesarwi OA, Loomba R, Malhotra A. Obstructive sleep apnea, hypoxia, and nonalcoholic fatty liver disease. Am J Respir Crit Care Med. 2019;199(7):830–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sundaram SS, Halbower A, Pan Z, et al. Nocturnal hypoxia-induced oxidative stress promotes progression of pediatric non-alcoholic fatty liver disease. J Hepatol. 2016;65(3):560–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aron-Wisnewsky J, Clement K, Pépin JL. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism. 2016;65(8):1124–1135. [DOI] [PubMed] [Google Scholar]
- 41. Meltzer LJ, Shaheed K, Ambler D. Start later, sleep later: school start times and adolescent sleep in homeschool versus public/private school students. Behav Sleep Med. 2016;14(2):140–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hansen M, Janssen I, Schiff A, Zee PC, Dubocovich ML. The impact of school daily schedule on adolescent sleep. Pediatrics. 2005;115(6):1555–1561. [DOI] [PubMed] [Google Scholar]
- 43. Simon SL, Behn CD, Cree-Green M, et al. Too late and not enough: school year sleep duration, timing, and circadian misalignment are associated with reduced insulin sensitivity in adolescents with overweight/obesity. J Pediatr. 2019;205:257–264.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Smaldone A, Honig JC, Byrne MW. Sleepless in America: inadequate sleep and relationships to health and well-being of our nation’s children. Pediatrics. 2007;119(Suppl 1):S29–S37. [DOI] [PubMed] [Google Scholar]
- 45. Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007;8(6): 602–612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.