Abstract
Background
African Americans (AAs) are at a higher risk for developing type 2 diabetes compared with non-Hispanic whites (NHWs). The causal role of β-cell glucose sensitivity (β-GS) and insulin clearance in hyperinsulinemia in AA adults is unclear.
Objective
Using a cross-sectional study design, we compared β-cell function and insulin clearance in nondiabetic AAs (n = 36) and NHWs (n = 47) after a mixed meal test (MMT).
Methods
Insulin secretion rate, glucose sensitivity, rate sensitivity, and insulin sensitivity during MMT were derived from a mathematical model. Levels of insulin-degrading enzyme (IDE) and carcinoembryonic antigen-related cell adhesion molecule-1 (CEACAM1), key players in insulin clearance, were measured (by enzyme-linked immunosorbent assay) in hepatic cytosolic fractions from age-, sex-, and body mass index–matched AA and NHW cadaveric donors (n = 10).
Results
Fasting and mean postprandial plasma glucose levels were similar in both ethnic groups. AAs had significantly higher fasting and mean postprandial plasma insulin levels. However, fasting ISR, total insulin output, and insulin sensitivity during MMT were not different between the groups. β-GS and rate sensitivity were higher in AAs. Fasting and meal plasma insulin clearance were lower in AAs. Hepatic levels of IDE and CEACAM-1 were similar in AAs and NHWs. Hepatic IDE activity was significantly lower in AAs.
Conclusions
In this study, lower insulin clearance contributes to higher plasma insulin levels in AAs. Reduced insulin clearance may be explained by lower IDE activity levels in AAs. Further confirmatory studies are needed to investigate diminished insulin clearance in AAs as a result of lower IDE activity levels.
Keywords: insulin clearance, African Americans, insulin degrading enzyme, β-cell function
African Americans (AAs) are at a higher risk for the development of type 2 diabetes mellitus (T2DM) compared with non-Hispanic whites (NHWs) (1-3). Differences in diet, body composition, insulin sensitivity, and pancreatic β-cell function have been proposed to contribute to this increased risk (1, 4-6). Insulin sensitivity as measured by the intravenous glucose tolerance test (IVGTT) is reduced in nondiabetic AA adults (7, 8). Acute insulin response (AIR) following an IVGTT is frequently higher in AAs and is often suggested to be a compensatory response to lower insulin sensitivity (7, 8). However, in adolescents, for the same degree of insulin sensitivity, AAs have a higher AIR to intravenous glucose when compared with NHWs (9). Similarly, plasma insulin concentrations in the fasting state and following an oral glucose load is often higher in nondiabetic AA adults (10-14). Diminished insulin clearance may contribute partly to these observed differences in circulating insulin levels (12, 15-18). However, the role of β-cell glucose responsivity following an oral glucose load in adults is unclear.
Insulin clearance rate is an important determinant of and accounts for nearly 25% of the variability in plasma insulin concentrations (19). The liver and kidney are major sites for insulin clearance (20, 21). Following insulin binding, the insulin receptor is internalized, and receptor-bound insulin is degraded by insulin-degrading enzyme (IDE) (22). Although, cellular mechanisms regulating IDE are not clear, IDE polymorphisms are associated with higher risk for developing T2DM (23). In addition to IDE, carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1), a transmembrane protein plays an important role in hepatic insulin clearance (24). Activated insulin receptor, a tyrosine kinase, phosphorylates CEACAM1 to enhance the rate of internalization and degradation of insulin-bound insulin receptor complexes (25). Reduced insulin clearance contributes to hyperinsulinemia in AAs (12, 15-18), but the cellular basis for the lower insulin clearance is unknown. Specifically, there are no studies that have examined ethnic differences in the expression and activity of hepatic IDE and CEACAM1. The purposes of this study were to a) compare β-cell glucose sensitivity (β-GS) and insulin clearance following a meal in nondiabetic AAs and NHWs, and b) examine ethnic differences in the concentrations and activity of hepatic IDE and CEACAM1.
Methods
Study design and participants
Participants in this study were part of an ongoing cross-sectional study conducted at the Clinical Research Center, National Institutes of Health, in Bethesda, Maryland (ClinicalTrials.gov identifier: NCT00428987). The study protocol was approved by the institutional review board of the National Institute of Diabetes and Digestive and Kidney Diseases, and all procedures followed were in accordance with institutional guidelines. Written informed consent was obtained from all participants. Individuals older than 18 years with a body mass index (BMI) greater than 18.5 kg/m2 and stable weight over the last 3 months were included in the study. A subset of individuals from the original cohort who were nondiabetic (based on medical history and hemoglobin A1C < 6.5%) and not on any medications known to modulate insulin sensitivity, glucose metabolism, or weight loss were included in this study. Exclusion criteria were pregnancy, diabetes, liver disease, pulmonary disease, renal insufficiency, coronary heart disease, heart failure, peripheral vascular disease, coagulopathy, or other systemic disease. Three patients were on antihypertensives (angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and thiazide diuretics); 4 were on hypolipidemic agents (statins, n = 3 and niacin, n = 1); and 4 women were on oral contraceptives. All participants were admitted to the Metabolic Clinical Research Unit at the National Institutes of Health Clinical Center for 1 or more days.
Study procedures
Assessment of body composition.
Body weight was measured using a digital balance (Scale-Tronix 5702; Scale-Tronix). Body composition was measured by dual-energy X-ray absorptiometry with a Lunar iDXA scanner (GE Healthcare).
Measurement of β-cell function, insulin sensitivity, and glucagon-like peptide 1 following a mixed meal test.
Each participant underwent a mixed meal test (MMT) after a 12-hour overnight fast. Participants received a liquid meal with an approximate total nutrient content of 360 kcal (50% carbohydrates, 16% protein, and 34% fat). Samples for determination of plasma glucose, insulin, and C-peptide were drawn at –10, 0, 15, 30, 45, 60, 120, and 180 minutes. Insulin and C-peptide concentrations were measured in serum using the Roche Cobas 6000 analyzer (Roche Diagnostics). For analysis of glucagon-like peptide 1 (GLP-1), blood samples were collected in EDTA tubes with dipeptidyl peptidase IV (DPP-IV) and protease inhibitors, immediately centrifuged, and the plasma was stored at −80°C until analysis. In a subset of AAs (n = 22) and NHWs (n = 21) for whom plasma samples were available, intact GLP-1 concentrations were measured with Mesoscale enzyme-linked immunosorbent assay (ELISA) kits.
β-cell function parameters [(β-GS (in pmol.min−1.m−2. mM−1), rate sensitivity (pmol.m−2.mM−1), and potentiation] were derived by mathematical modeling of plasma glucose and C-peptide concentrations during the MMT as previously described (26). β-GS is the slope of the relationship between insulin secretion rate and plasma glucose concentration. Rate sensitivity is the magnitude of the β-cell insulin response to the rate of change in plasma glucose concentration. The potentiation factor ratio (PFR) modulates the relationship between glucose concentration and insulin secretion by increasing the sensitivity of the β cells to resulting plasma glucose concentration. The PFR accounts for various potentiating factors such as incretins, neurotransmitters, and secretagogues. PFR was quantified using ratios between mean values at times 160 to 180 and 0 to 20 minutes. Area under the curve (AUC) was calculated with the use of the trapezoidal method. AUC of insulin secretion during the 3-hour MMT represents the total insulin output (expressed in nmol.m−2). The AUCs for glucose and insulin concentration were divided by the accumulation time of the MMT to provide a time-averaged value (mmol/L) or pmol/L, respectively. Assuming an absence of hepatic C-peptide extraction, basal and stimulated insulin clearance was calculated from the ratio of insulin secretion AUC/insulin AUC from temporal curves that were followed for 180 minutes, allowing plasma levels to return to baseline. A correction factor [V*(Insulin180 min – Insulin0 min)/AUCInsulin)] was applied to insulin clearance to account for differences in baseline by the end of the sampling period (T = 180).
Insulin sensitivity parameters.
A model-based index of insulin sensitivity (oral glucose insulin sensitivity, OGIS) was calculated using plasma glucose and insulin concentrations during the 3-hour MMT (27). The Quantitative Insulin-sensitivity Check Index (QUICKI) was calculated as previously described (28). Insulin sensitivity index, SI, was determined from the minimal model using data from the insulin-modified FSIVGTT as previously described (version 6.02; MinMOD Millennium) (29). Briefly, after a 12-hour overnight fast, 2 intravenous catheters were placed, 1 in each arm near or in the antecubital veins. Baseline blood samples were obtained, and at time 0 minutes, dextrose (0.3 g/kg) was administered intravenously. Insulin was given as a bolus at 20 minutes (0.03 U/kg/min). Blood samples for glucose and insulin were obtained at –10, –1, 0, 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, 14, 16, 20, 22, 23, 24, 25, 27, 30, 40, 50, 60, 70, 80, 90, 100, 120, 150, and 180 minutes.
Hepatic carcinoembryonic antigen-related cell adhesion molecule-1 and insulin-degrading levels and activity.
IDE is mostly present in the cytosol (~95%) (30) and therefore we measured the levels and activity of IDE in hepatic cytosolic fractions. Protein concentrations of human hepatic cytosolic fractions (Xenotech) from age-, sex-, and BMI-matched AA and NHW cadaveric liver donors (n = 10) were measured using a BCA Protein Assay Reagent Kit (Thermo Scientific). The cause of death of liver donors was cerebral anoxia following head trauma or cerebrovascular accident (CVA). Human hepatic cytosolic fractions were from a commercial source, and additional information regarding comorbidities was unavailable. CEACAM1 levels in individual hepatic cytosolic fractions (n = 6) were measured by ELISA (RayBiotech Life). IDE levels were measured using a human IDE ELISA kit (Fivephoton Biochemicals; catalog No. IDE-ELISA [96T]). IDE activity in liver cytosolic fractions was measured for 30 minutes using the SensoLyte IDE Activity Assay Kit (AnaSpec; catalog No. AS-72231) according to the manufacturer’s instructions.
Statistical analysis
Variables with normal distributions are expressed as mean ± SD. Variables with a non-normal distribution are expressed as median (interquartile range). Comparisons between groups were assessed by the independent unpaired t test or The Wilcoxon-Mann-Whitney test. A P value less than .05 was considered statistically significant. Relationships between parameters were assessed by linear regression analyses using the Pearson correlation coefficient. Ethnic differences in indices of insulin action, β-cell function, and insulin clearance were examined using ANCOVA, with ethnicity as a fixed factor, and relevant covariates. Data were analyzed with JMP version 13.0 (SAS Institute) and GraphPad Prism 7 (GraphPad Software Inc).
Results
Participant characteristics and baseline metabolic profiles
Baseline characteristics and metabolic parameters of the study participants are depicted in Table 1. Fifty-six percent of AAs (n = 20) vs 30% of NHWs (n = 14) were obese (BMI ≥ 30 kg/m2) in this cohort (P = .02). Compared with NHWs, AAs had a significantly higher BMI, waist circumference, and fat-free mass, but a similar percentage of total body and truncal fat. There were a higher proportion of AAs with prediabetes (defined as hemoglobin A1C, 5.7%-6.4%) compared with NHWs (42 vs 13%, P = .002). The percentage of individuals with impaired fasting glucose (defined as a fasting plasma glucose of 100-125 mg/dL) was not significantly different between the groups (22 vs 19%, P = .73). Plasma triglyceride levels were lower in AAs, and there were no ethnic differences in total, low-density lipoprotein, and high-density lipoprotein cholesterol levels. Fasting plasma insulin and hemoglobin A1C levels were higher in AAs, whereas fasting glucose and C-peptide were not significantly different. AAs had a lower SI and QUICKI, but OGIS was similar between the groups. Ethnic differences in SI and QUICKI remained significantly different even after adjustment for BMI, percentage total body fat, and percentage truncal fat (data not shown). AIR was higher (~2.7-fold) in AAs (Table 1) and remained significant even after adjusting for BMI and SI (478.1 vs 235.0 μU·ml–1·min–1 [ratio of geometric mean 2.0, 95% CI 1.5-2.8]; P < .001). Results were similar when total percentage body or truncal fat was used as a covariate instead of BMI (data not shown). These results suggest that the higher AIR is above the magnitude expected from a compensatory response to reduced insulin sensitivity in AA. Serum creatinine and estimated glomerular filtration rate (eGFR) were significantly higher in AAs.
Table 1.
Clinical and metabolic characteristics in African Americans and non-Hispanic whites
| NHW (n = 47) | AA (n = 36) | P | |||||
|---|---|---|---|---|---|---|---|
| Age, y | 39 | ± | 13 | 39 | ± | 11 | .99 |
| Female, %, n | 68 (n = 32) | 56 (n = 20) | .24 | ||||
| BMI, kg/m2 | 26.3 (9.7) | 31.2 (12.3) | .02 | ||||
| Fat free mass, kg | 49 | ± | 11 | 57 | ± | 11 | .002 |
| Total body fat, % | 35 | ± | 11 | 38 | ± | 12 | .24 |
| Waist circumference, cm | 86.2 | ± | 16.4 | 95.8 | ± | 16.8 | .02 |
| Truncal fat, % | 35.0 | ± | 13.3 | 39.9 | ± | 12.4 | .08 |
| Systolic blood pressure, mm Hg | 119 | ± | 12 | 125 | ± | 12 | .02 |
| Diastolic blood pressure, mm Hg | 70 | ± | 8 | 72 | ± | 8 | .40 |
| Fasting plasma glucose, mmol/L | 4.92 | ± | 0.38 | 5.03 | ± | 0.39 | .49 |
| Fasting plasma insulin, pmol/L | 17.4 (49.8) | 45 (55.8) | .007 | ||||
| Fasting C-peptide, nmol/ | 0.49 (0.53) | 0.56 (0.42) | .16 | ||||
| Hemoglobin A1c, % | 5.3 (0.5) | 5.6 (0.8) | .001 | ||||
| QUICKI | 0.389 (0.104) | 0.353 (0.073) | .003 | ||||
| SI, min–1·μU·ml–1 | 5.47 (6.37) | 2.93 (2.12) | < .001 | ||||
| OGIS, mL.min−1.m−2 | 360 | ± | 41 | 347 | ± | 34 | .10 |
| AIR, μU·mL–1·min–1 | 230 (210) | 611(632) | < .001 | ||||
| Total cholesterol, mmol/L | 4.98 | ± | 0.73 | 4.67 | ± | 0.70 | .06 |
| LDL cholesterol, mmol/L | 2.89 | ± | 0.79 | 2.83 | ± | 0.64 | .43 |
| HDL cholesterol, mmol/L | 1.50 | ± | 0.43 | 1.36 | ± | 0.37 | .12 |
| Triglycerides, mmol/L | 0.95 (0.89) | 0.73 (0.53) | .03 | ||||
| Serum creatinine, µmol/L | 74.6 ± 14.7 | 82.8 ± 18.4 | .03 | ||||
| eGFR, mL.min−1.1.73 m−2 | 93 ± 22 | 103 ± 20 | .02 |
Data are presented as arithmetic mean ± SD or as median (interquartile range); n, No. of participants. P values indicate significance for comparisons between ethnic groups.
Abbreviations: AA, African American; AIR, acute insulin response; BMI, body mass index; eGFR, estimated glomerular filtration rate by the abbreviated Modification of Diet in Renal Disease equation; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NHW, non-Hispanic white; OGIS, oral glucose insulin sensitivity index; QUICKI, quantitative insulin-sensitivity check index; SI, insulin sensitivity index derived from frequently sampled intravenous glucose tolerance test.
β-cell function and insulin clearance during a mixed meal test
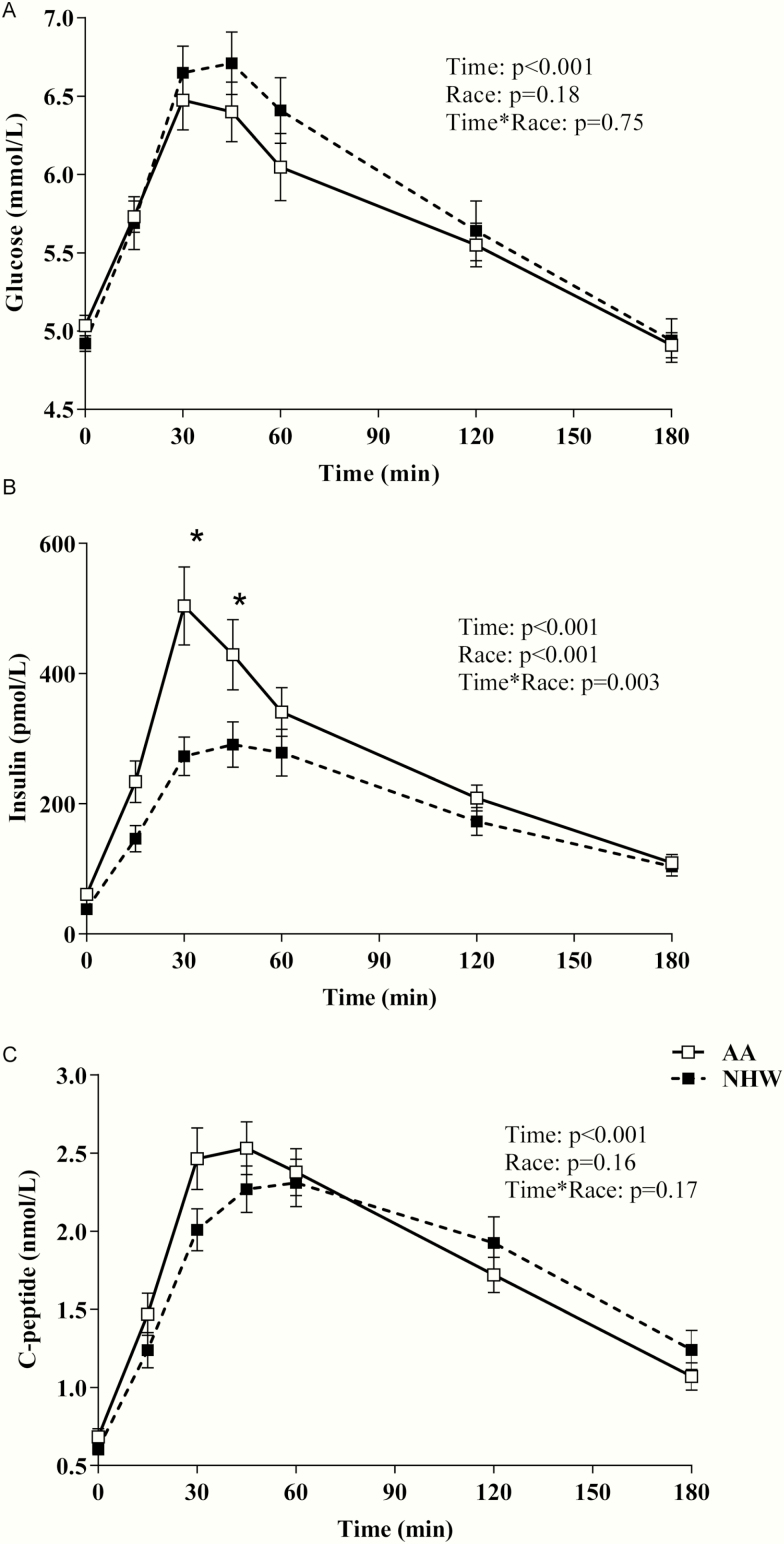
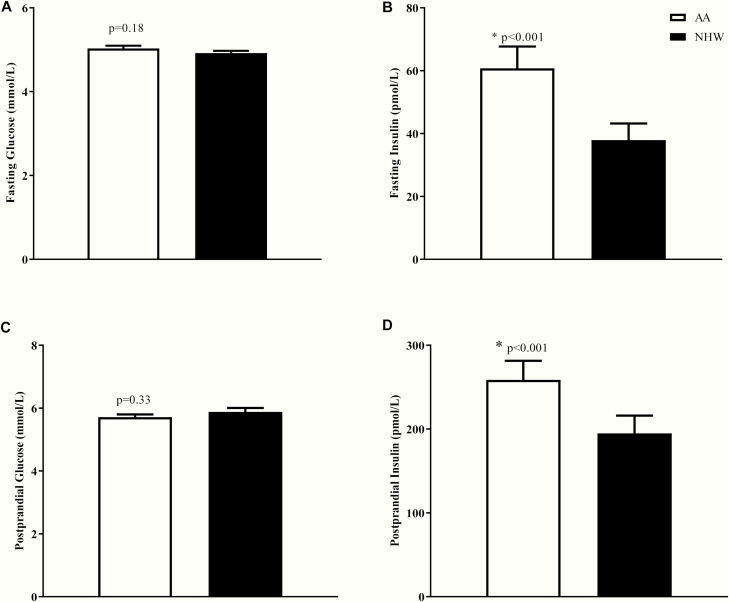
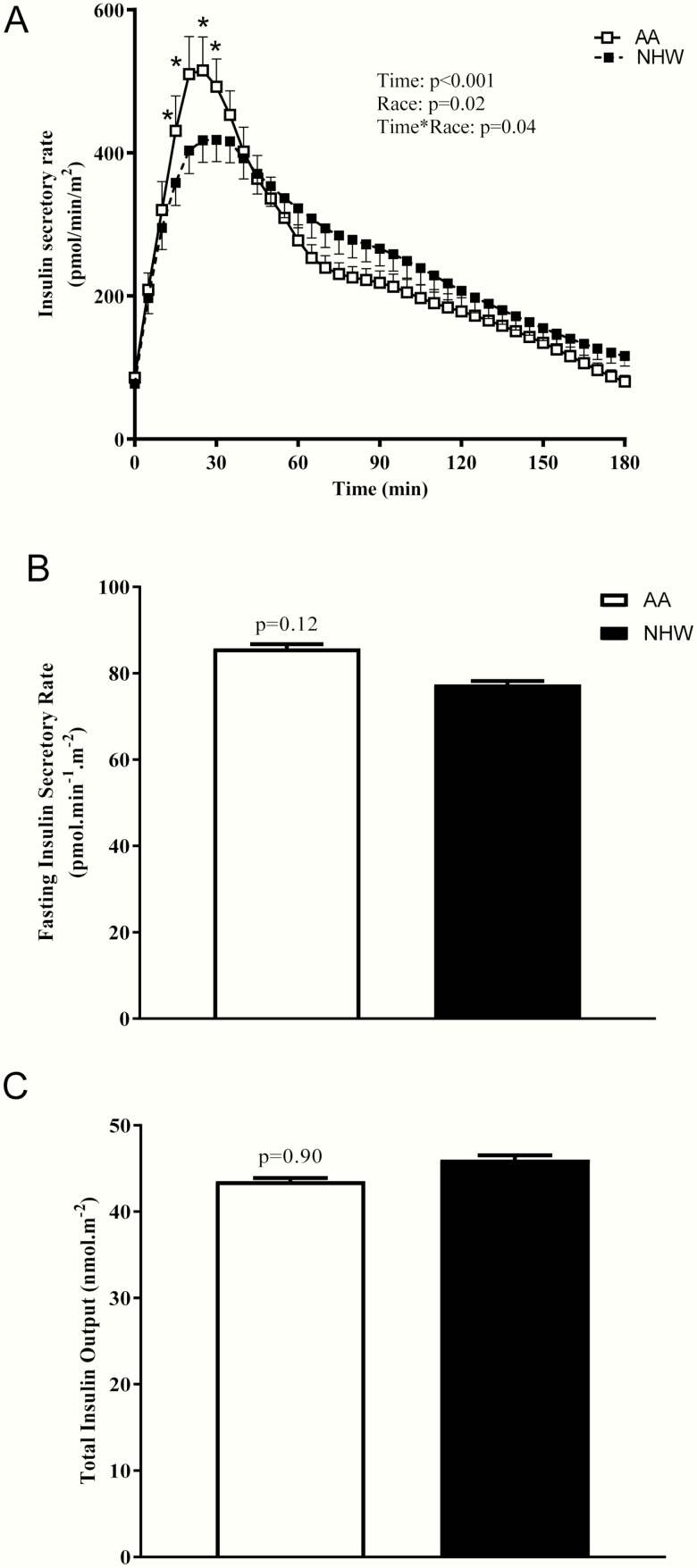
Fasting plasma glucose concentrations as well was postprandial glucose concentrations (AUC) during an MMT were not significantly different between the groups (Fig. 1). Fasting and postprandial insulin levels, but not C-peptide levels, were higher in AAs (Figs. 1 and 2). Differences in mean postprandial insulin concentrations remained significant even after adjustment for mean glucose levels during the meal. Mean insulin was higher in AAs (by ~32%) when compared with NHWs (258.6 ± 136.9 vs 194.7 ± 146.1 pmol/L, P < .001) (Fig. 2D). Basal insulin secretory rate was similar between the groups (Fig. 3B), but early insulin secretion was higher in AAs (time*race interaction, P = .04; Fig. 3A). When total insulin production during the MMT was compared, there was no significant difference between the groups (Fig. 3C). β-GS (main effect of race, P = .004, Fig. 4A) and rate sensitivity (Fig. 4B) were significantly higher in AAs. PFR (Fig. 4C) and the potentiation factor time course were similar in the 2 groups (data not shown). Intact GLP-1 levels during MMT (Fig. 4D) and GLP-1 AUC were comparable between AAs and NHWs (34.6 ± 24.7 vs 25.9 ± 10.3 pM.180 minutes, P = .51). We also compared GLP-1 AUC during the first 45 minutes of the MMT and found no significant differences between the groups (data not shown). A mixed-effects model analysis revealed that intact GLP-1 levels for both groups increased over time (P < .001); the increase appears to be the same for both groups (P = .15), and there was no significant time*race interaction (P = .64) (Fig. 4D). Insulin clearance during fasting and a meal were significantly lower in AAs when compared with NHWs (Fig. 5A and 5B). In the entire group, meal insulin clearance was positively correlated with OGIS (r = 0.44, P < .001) and SI (r = 0.63, P < .001); slopes of regressions showed no significant ethnic differences. Meal insulin clearance remained lower in AAs even after adjusting for SI and BMI when compared with NHWs (1.09 vs 1.36 L.min–1.m2, adjusted ratio of geometric means 0.80, 95% CI [0.71-0.89, P = .001]). Results were similar when total percentage body or truncal fat was used as a covariate instead of BMI (data not shown). Higher eGFR may lead to rapid insulin clearance (peripheral component). However, despite the higher eGFR, AA had a lower insulin clearance. In the entire cohort, eGFR was not related to fasting (r = –0.16, P = .13) or meal insulin clearance (r = –0.17, P = .10). There were no ethnic differences in these relationships. Finally, there was no statistically significant interaction between race and prediabetes status on insulin clearance (during fasting and during meal), rate sensitivity, SI, AIR, and mean meal insulin concentration.
Figure 1.
Plasma levels of A, glucose, B, insulin, and C, C-peptide, in adult African Americans (AA) and non-Hispanic whites (NHW) during a mixed meal test (MMT). Data shown are mean ± SEM. P values for the effects of racial group and time and the race*time interaction were obtained with repeated-measures ANOVA with post hoc Bonferroni test.
Figure 2.
Plasma levels of A, fasting glucose, B, fasting insulin, C, mean postprandial glucose, and D, mean postprandial insulin in adult African Americans (AA) and non-Hispanic whites (NHW) during a mixed meal test (MMT). Data shown are mean ± SEM. Comparisons between groups were assessed by independent unpaired t test or the Wilcoxon-Mann-Whitney test.
Figure 3.
Model-derived A, insulin secretory rate, B, fasting insulin secretory rate, and C, total insulin output, in adult African Americans (AA) and non-Hispanic Whites (NHW) during a mixed meal test (MMT). Data shown are mean ± SEM. In panel A, P values for the effects of racial group and time and the race*time interaction were obtained with repeated-measures ANOVA with post hoc Bonferroni test. Comparisons between groups (in panels B and C) were assessed by independent unpaired t test or the Wilcoxon-Mann-Whitney test.
Figure 4.
Relationship between insulin secretory rate and A, plasma glucose levels, B, rate sensitivity, C, potentiation factor ratio, and intact D, glucagon-like peptide 1 concentrations in adult African Americans (AA) and non-Hispanic whites (NHW) during a mixed meal test (MMT). Data shown are mean ± SEM. In panel A, P values for the effects of racial group and time and the race*time interaction were obtained with repeated-measures ANOVA with post-hoc Bonferroni test. Comparisons between groups in panel B were assessed by independent unpaired t test or the Wilcoxon-Mann-Whitney test.
Figure 5.
A, Fasting insulin clearance and B, meal insulin clearance in adult African Americans (AA) and non-Hispanic whites (NHW) during a mixed meal test (MMT). C, Insulin degrading enzyme levels and activity were measured in hepatic cytosolic fractions from age-, sex-, and body mass index–matched AA and NHW cadaveric donors (n = 10). Data shown are mean ± SEM. Comparisons between groups were assessed by independent unpaired t test or the Wilcoxon-Mann-Whitney test.
Ethnic differences in hepatic carcinoembryonic antigen-related cell adhesion molecule-1 levels and insulin-degrading enzyme levels and activity
Age (AAs vs NHWs: 40.3 ± 10.8 vs 37.8 ± 6.4 y) and BMI (31.5 ± 10.9 vs 31.3 ± 6.2 kg/m2) were not significantly different between the groups (P > .05). IDE concentrations in hepatic cytosol fractions comparable between the groups (Fig. 5C). However, hepatic IDE activity in AA was 29% lower when compared with NHW (Fig. 5D). Hepatic CEACAM1 levels were not different between the 2 groups (AAs vs NHWs: 1.04 ± 0.23 vs 1.17 ± 0.48 ng/µg).
Discussion
In the present study, we found that early insulin secretion, β-GS, and rate sensitivity following a mixed meal was higher, but the cumulative insulin secretion was not different in nondiabetic AAs. Fasting and prandial insulin clearance were lower in AAs. This suggests that the higher postprandial insulin concentration is a result of reduced insulin clearance and not due to higher total insulin secretion. Consistent with this finding, we demonstrate for the first time that hepatic IDE activity, but not levels, was lower in AAs when compared with NHWs.
Ethnic differences in β-cell glucose sensitivity
Increased AIR is a characteristic trait in AAs and may be the result of lower SI, reduced insulin clearance, and enhanced β-cell function. Dynamic responsivity, a measure of the capacity of β cells to respond to the rate of increase of glucose concentration, can be assessed following an intravenous or oral glucose load and represented by Φ 1 and Φ D, respectively (31). Similarly, static responsivity, a parameter that reflects the ability of β cells to deliver new pools of insulin granules in response to continued increases in glucose concentration, can also be derived from these tests and is represented by Φ 2 and Φ S, respectively (31). Only a couple of studies have examined differences in β-cell glucose responsivity in AA and NHW women (14, 32). In healthy, normoglycemic AA adult women, first-phase glucose responsivity was significantly higher when compared with their white counterparts during an IVGTT (32). Similarly, Φ D and dynamic responsivity following a mixed meal were higher in AA women (14). However, there were no ethnic differences in static responsivity in both studies (14, 32). Higher rate sensitivity observed in our study is consistent with these studies, suggesting that β-cell function is enhanced in AAs when compared with NHWs. Whether this feature is intrinsic to the β cell in AAs is unknown and an important area of investigation. Surprisingly, there are no studies examining the racial differences in dynamic insulin secretion in human islet preparations in vitro. In one exploratory study, the effect of race was examined on the pattern of insulin secretion in isolated islet preparations using a perifusion system (33). Basal, glucose-stimulated, and 3′,5′-cyclic AMP (cAMP)-mediated insulin secretion (in the presence of cAMP phosphodiesterase, IBMX) were examined. Islet preparations from AAs had a smaller magnitude of glucose and cAMP-induced insulin secretion (fold-change over baseline) than NHW preparations. However, basal insulin secretion was higher in AAs. In addition to replicating this finding, future studies addressing the cellular basis for this difference are warranted.
Incretin hormones, GLP-1, and glucose-dependent insulinotropic polypeptide (GIP), are known to increase β-GS in humans (34, 35). Prior studies in obese adults have reported higher levels of insulin and intact GLP-1 during an OGTT in AAs (36). But, there were no ethnic differences in the levels of incretins during an MMT in AAs when compared with NHW women (14). In agreement with the latter study, we did not observe any ethnic differences in MMT-induced increases in circulating intact GLP-1 levels (Fig. 4D). This finding was consistent with a lack of race-related differences in PF, a parameter that reflects cumulative effects of changes in incretins, neuronal input to β cells, and glucose concentration (Fig. 4C).
Ethnic differences in insulin clearance
Circulating insulin concentration is a net result of insulin secretion and clearance. In our study, despite similar insulin secretory rates and total insulin secretion during a meal between the groups, AAs had higher levels of postprandial insulin. The relative hyperinsulinemia can be the result of reduced hepatic and/or peripheral insulin clearance. The findings of reduced insulin clearance during fasting and a meal in our study are consistent with other studies in AA adults (12, 14, 18, 32). Reduced insulin clearance may be a compensatory response to reduced insulin sensitivity (37). In our study, OGIS, a surrogate measure of insulin sensitivity, was comparable between the groups. Consistent with other studies, the more widely used measure of insulin sensitivity derived from FSIVGTT (8), SI was significantly lower in AAs. OGIS is insensitive to detect lower insulin sensitivity in AAs in this cohort because the main differences in insulin and glucose between the groups are in the early part of the meal (Fig. 1) as observed in other studies (14, 36). OGIS was also similar in black and white adolescents (38). QUICKI is a fasting surrogate index of insulin sensitivity based on fasting glucose and insulin levels. Reduced insulin clearance may affect reliability of these indices, especially in AAs (13). Conversely, it is possible that SI assessed by the minimal model may underestimate insulin sensitivity in AAs who manifest higher peak of insulin concentrations (AIR) compared with NHWs (8). Nevertheless, even after adjusting for the differences in SI between the groups, meal insulin clearance was significantly lower in AAs. Ethnic difference in insulin clearance, a highly heritable trait (39, 40), may explain hyperinsulinemia frequently observed in AAs.
Ethnic differences in hepatic and peripheral insulin clearance
Insulin secreted into the portal circulation is extracted by the liver as it undergoes first pass before delivery to the systemic circulation. In the periphery, insulin is cleared by skeletal muscle and the kidney. Hepatic IDE and CEACAM1 play an important role in hepatic insulin clearance (22, 24). Insulin is secreted at an equimolar ratio with C-peptide. Because C-peptide is not extracted in the liver, the ratio of insulin secretion to insulin concentration is a measure of endogenous insulin clearance. Recently, using a mathematical model based on serum insulin and C-peptide during an insulin-modified frequently sampled IVGTT, Polidori et al were able to estimate hepatic and peripheral insulin clearance (41). This group subsequently evaluated hepatic and peripheral insulin clearance in 29 NHW and 18 AA healthy, nondiabetic women (15). AAs had a significantly lower (by ~60%) hepatic insulin clearance compared with NHWs. However, peripheral insulin clearance was not different between the groups. These results suggest that lower insulin clearance of insulin in AAs is primarily driven by the reduced extraction of insulin in the liver. We did not measure peripheral insulin clearance in this study. Nevertheless, insulin clearance was not associated with eGFR in AAs and NHWs.
The relationship between hepatic CEACAM1 levels and insulin clearance in humans has not been assessed. In a small study (n = 7), CEACAM1 levels were measured in liver samples in obese, 45- to 50-year-old men and compared with age-, sex-, and race-matched lean individuals (n = 4). Hepatic expression of CEACAM1 was lower in the obese individuals (by 50%) (42). Similarly, IDE expression was lower in patients with T2DM when compared with healthy individuals (43). This finding was supported by a liver microarray data set from the GEO Profiles database (44). Unfortunately, there was no ethnic information provided in any of these studies. To understand the underlying cellular mechanisms mediating the lower endogenous insulin clearance in AAs, we measured the hepatic levels and activity of IDE and CEACAM-1 in age-, sex-, and BMI-matched AA and NHW cadaveric liver donors. The levels of CEACAM-1 and IDE were not significantly different between the groups. However, IDE activity was lower by approximately 30% in AAs, suggesting that differences in posttranslational regulation of the catalytic activity of IDE may contribute to the observed ethnic difference. IDE activity is known to be regulated by cytoplasmic concentrations of calcium, adenosine triphosphate, oxidative stress, fatty acids, and acyl-CoA (45). Consistent with this, hepatic expression of IDE but not CEACAM-1 was lower in dogs that were fed a high-fat diet for 6 weeks (46). Causal factors mediating lower IDE activity in AAs are unknown and remain to be investigated. Nevertheless, this is the first study to report ethnic differences in IDE levels/activity.
Strengths and limitations of the study
There are many strengths of this study. First, using mathematical modeling approaches, we have rigorously examined the contributions of β-cell function and insulin clearance to hyperinsulinemia frequently observed in AA adults. Second, we used MMT, a more physiological test to elicit insulin secretion that accounts for the incretin effect in contrast to the study using intravenous glucose (15). Some, but not all studies, suggest that incretin hormones (GLP-1 and GIP) are known to modulate hepatic insulin clearance in humans (47-52). We did not observe significant ethnic differences in plasma-intact GLP-1 levels during the meal, but a larger cohort that is sufficiently powered may uncover ethnic differences in GLP-1 secretion, especially earlier in the MMT. Third, our study included men and women, unlike other studies that comprised just women (14, 15). Lastly, this is the first to study ethnic differences in IDE/CEACAM-1 levels in human hepatic tissues in AAs and NHWs. Despite these strengths, there are many limitations. We did not directly measure hepatic and peripheral insulin clearance in this study. Assessment of IDE activity in liver biopsies concomitantly performed with studies measuring insulin secretion/clearance would be ideal. Nevertheless, the risk-benefit ratio of liver biopsies or catheterization of hepatic or renal veins in healthy nondiabetic individuals would preclude such studies. It is possible that our study is underpowered to detect differences (<50%) in hepatic CEACAM-1 levels. Higher liver fat content is associated with impaired insulin clearance in nondiabetic individuals and in patients with nonalcoholic fatty liver disease (NAFLD) (53, 54). A recent study did not demonstrate significant ethnic differences in the relationship between hepatic fat and insulin clearance in black and white men (55). Furthermore, the negative relationship between hepatic fat and insulin clearance was observed in white but not in black women (14). It is generally assumed that visceral fat content is correlated with hepatic fat. In AA adults, this is not always the case as reported by Hakim and colleagues (55). We did not measure hepatic fat in our study and absence of NAFLD was elicited by medical history. Therefore, we were unable to examine relationships between insulin clearance, intrahepatic lipid content, and visceral fat.
In conclusion, in this study of nondiabetic men and women, reduced insulin clearance appears to contribute to hyperinsulinemia in AAs when compared with NHWs. To the best of our knowledge, this study is the first to explore cellular mechanisms for the reduced hepatic extraction of insulin observed in AAs. Lower hepatic IDE activity, but not differences in IDE and CEACAM-1 levels, may explicate the ethnic differences in insulin clearance. Reduced insulin clearance could be a proximal cause for hyperinsulinemia, insulin resistance, and T2DM in ethnic groups such as AAs. Future studies examining the mechanisms for lower hepatic IDE activity and higher β-GS are warranted.
Acknowledgments
Financial Support: This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), Washington, DC.
Clinical Trial Information: The ClinicalTrials.gov identifier for this study is NCT00428987.
Glossary
Abbreviations
- β-GS
β-cell glucose sensitivity
- AAs
African Americans
- AIR
acute insulin response
- AUC
area under the curve
- BMI
body mass index
- CEACAM1
carcinoembryonic antigen-related cell adhesion molecule 1
- GIP
glucose-dependent insulinotropic polypeptide
- IDE
insulin-degrading enzyme
- IVGTT
intravenous glucose tolerance test
- MMT
mixed meal test
- NHWs
non-Hispanic whites
- NAFLD
nonalcoholic fatty liver disease
- OGIS
oral glucose insulin sensitivity
- PFR
potentiation factor ratio
- SI
insulin sensitivity index
- T2DM
type 2 diabetes mellitus
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Bancks MP, Kershaw K, Carson AP, Gordon-Larsen P, Schreiner PJ, Carnethon MR. Association of modifiable risk factors in young adulthood with racial disparity in incident type 2 diabetes during middle adulthood. JAMA. 2017;318(24):2457–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brancati FL, Kao WH, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in African American and white adults: the Atherosclerosis Risk in Communities Study. JAMA. 2000;283(17):2253–2259. [DOI] [PubMed] [Google Scholar]
- 3. Quiñones AR, Liang J, Ye W. Differences in diabetes mellitus onset for older black, white, and Mexican Americans. Ethn Dis. 2013;23(3):310–315. [PMC free article] [PubMed] [Google Scholar]
- 4. Resnick HE, Valsania P, Halter JB, Lin X. Differential effects of BMI on diabetes risk among black and white Americans. Diabetes Care. 1998;21(11):1828–1835. [DOI] [PubMed] [Google Scholar]
- 5. Piccolo RS, Subramanian SV, Pearce N, Florez JC, McKinlay JB. Relative contributions of socioeconomic, local environmental, psychosocial, lifestyle/behavioral, biophysiological, and ancestral factors to racial/ethnic disparities in type 2 diabetes. Diabetes Care. 2016;39(7):1208–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haffner SM, D’Agostino R, Saad MF, et al. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. The Insulin Resistance Atherosclerosis Study. Diabetes. 1996;45(6):742–748. [DOI] [PubMed] [Google Scholar]
- 7. Haffner SM, Howard G, Mayer E, et al. Insulin sensitivity and acute insulin response in African-Americans, non-Hispanic whites, and Hispanics with NIDDM: the Insulin Resistance Atherosclerosis Study. Diabetes. 1997;46(1):63–69. [DOI] [PubMed] [Google Scholar]
- 8. Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Diabetes Care. 2013;36(6):1789–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hannon TS, Bacha F, Lin Y, Arslanian SA. Hyperinsulinemia in African-American adolescents compared with their American white peers despite similar insulin sensitivity: a reflection of upregulated beta-cell function? Diabetes Care. 2008;31(7):1445–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harris MI, Cowie CC, Gu K, Francis ME, Flegal K, Eberhardt MS. Higher fasting insulin but lower fasting C-peptide levels in African Americans in the US population. Diabetes Metab Res Rev. 2002;18(2):149–155. [DOI] [PubMed] [Google Scholar]
- 11. Osei K, Cottrell DA, Harris B. Differences in basal and poststimulation glucose homeostasis in nondiabetic first degree relatives of black and white patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 1992;75(1):82–86. [DOI] [PubMed] [Google Scholar]
- 12. Osei K, Schuster DP. Ethnic differences in secretion, sensitivity, and hepatic extraction of insulin in black and white Americans. Diabet Med. 1994;11(8):755–762. [DOI] [PubMed] [Google Scholar]
- 13. Pisprasert V, Ingram KH, Lopez-Davila MF, Munoz AJ, Garvey WT. Limitations in the use of indices using glucose and insulin levels to predict insulin sensitivity: impact of race and gender and superiority of the indices derived from oral glucose tolerance test in African Americans. Diabetes Care. 2013;36(4):845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chung ST, Galvan-De La Cruz M, Aldana PC, et al. Postprandial insulin response and clearance among black and white women: the Federal Women’s Study. J Clin Endocrinol Metab. 2019;104(1):181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Piccinini F, Polidori DC, Gower BA, Bergman RN. Hepatic but not extrahepatic insulin clearance is lower in African American than in European American women. Diabetes. 2017;66(10):2564–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee CC, Haffner SM, Wagenknecht LE, et al. Insulin clearance and the incidence of type 2 diabetes in Hispanics and African Americans: the IRAS Family Study. Diabetes Care. 2013;36(4):901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wagenknecht LE, Langefeld CD, Scherzinger AL, et al. Insulin sensitivity, insulin secretion, and abdominal fat: the Insulin Resistance Atherosclerosis Study (IRAS) Family Study. Diabetes. 2003;52(10):2490–2496. [DOI] [PubMed] [Google Scholar]
- 18. Osei K, Schuster DP, Owusu SK, Amoah AG. Race and ethnicity determine serum insulin and C-peptide concentrations and hepatic insulin extraction and insulin clearance: comparative studies of three populations of West African ancestry and white Americans. Metabolism. 1997;46(1):53–58. [DOI] [PubMed] [Google Scholar]
- 19. Kim MK, Reaven GM, Chen YD, Kim E, Kim SH. Hyperinsulinemia in individuals with obesity: role of insulin clearance. Obesity (Silver Spring). 2015;23(12):2430–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bojsen-Møller KN, Lundsgaard AM, Madsbad S, Kiens B, Holst JJ. Hepatic insulin clearance in regulation of systemic insulin concentrations-role of carbohydrate and energy availability. Diabetes. 2018;67(11):2129–2136. [DOI] [PubMed] [Google Scholar]
- 21. Ferrannini E, Wahren J, Faber OK, Felig P, Binder C, DeFronzo RA. Splanchnic and renal metabolism of insulin in human subjects: a dose-response study. Am J Physiol. 1983;244(6):E517–E527. [DOI] [PubMed] [Google Scholar]
- 22. Duckworth WC, Bennett RG, Hamel FG. Insulin degradation: progress and potential. Endocr Rev. 1998;19(5):608–624. [DOI] [PubMed] [Google Scholar]
- 23. Karamohamed S, Demissie S, Volcjak J, et al. ; NHLBI Framingham Heart Study Polymorphisms in the insulin-degrading enzyme gene are associated with type 2 diabetes in men from the NHLBI Framingham Heart Study. Diabetes. 2003;52(6):1562–1567. [DOI] [PubMed] [Google Scholar]
- 24. Najjar SM. Regulation of insulin action by CEACAM1. Trends Endocrinol Metab. 2002;13(6):240–245. [DOI] [PubMed] [Google Scholar]
- 25. Poy MN, Yang Y, Rezaei K, et al. CEACAM1 regulates insulin clearance in liver. Nat Genet. 2002;30(3):270–276. [DOI] [PubMed] [Google Scholar]
- 26. Mari A, Tura A, Gastaldelli A, Ferrannini E. Assessing insulin secretion by modeling in multiple-meal tests: role of potentiation. Diabetes. 2002;51(Suppl 1):S221–S226. [DOI] [PubMed] [Google Scholar]
- 27. Mari A, Pacini G, Murphy E, Ludvik B, Nolan JJ. A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care. 2001;24(3):539–548. [DOI] [PubMed] [Google Scholar]
- 28. Chen H, Sullivan G, Quon MJ. Assessing the predictive accuracy of QUICKI as a surrogate index for insulin sensitivity using a calibration model. Diabetes. 2005;54(7):1914–1925. [DOI] [PubMed] [Google Scholar]
- 29. Muniyappa R, Sachdev V, Sidenko S, et al. Postprandial endothelial function does not differ in women by race: an insulin resistance paradox? Am J Physiol Endocrinol Metab. 2012;302(2):E218–E225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Authier F, Cameron PH, Taupin V. Association of insulin-degrading enzyme with a 70 kDa cytosolic protein in hepatoma cells. Biochem J. 1996;319(Pt 1):149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cobelli C, Toffolo GM, Dalla Man C, et al. Assessment of beta-cell function in humans, simultaneously with insulin sensitivity and hepatic extraction, from intravenous and oral glucose tests. Am J Physiol Endocrinol Metab. 2007;293(1):E1–E15. [DOI] [PubMed] [Google Scholar]
- 32. Chandler-Laney PC, Phadke RP, Granger WM, et al. Age-related changes in insulin sensitivity and β-cell function among European-American and African-American women. Obesity (Silver Spring). 2011;19(3):528–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kayton NS, Poffenberger G, Henske J, et al. Human islet preparations distributed for research exhibit a variety of insulin-secretory profiles. Am J Physiol Endocrinol Metab. 2015;308(7):E592–E602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kjems LL, Holst JJ, Vølund A, Madsbad S. The influence of GLP-1 on glucose-stimulated insulin secretion: effects on beta-cell sensitivity in type 2 and nondiabetic subjects. Diabetes. 2003;52(2):380–386. [DOI] [PubMed] [Google Scholar]
- 35. Ahrén B, Holst JJ, Mari A. Characterization of GLP-1 effects on beta-cell function after meal ingestion in humans. Diabetes Care. 2003;26(10):2860–2864. [DOI] [PubMed] [Google Scholar]
- 36. Velasquez-Mieyer PA, Cowan PA, Umpierrez GE, Lustig RH, Cashion AK, Burghen GA. Racial differences in glucagon-like peptide-1 (GLP-1) concentrations and insulin dynamics during oral glucose tolerance test in obese subjects. Int J Obes Relat Metab Disord. 2003;27(11):1359–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jung SH, Jung CH, Reaven GM, Kim SH. Adapting to insulin resistance in obesity: role of insulin secretion and clearance. Diabetologia. 2018;61(3):681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Michaliszyn SF, Lee S, Bacha F, et al. Differences in β-cell function and insulin secretion in black vs. white obese adolescents: do incretin hormones play a role? Pediatr Diabetes. 2017;18(2):143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Klimentidis YC, Divers J, Casazza K, Beasley TM, Allison DB, Fernandez JR. Ancestry-informative markers on chromosomes 2, 8 and 15 are associated with insulin-related traits in a racially diverse sample of children. Hum Genomics. 2011;5(2):79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guo X, Cui J, Jones MR, et al. Insulin clearance: confirmation as a highly heritable trait, and genome-wide linkage analysis. Diabetologia. 2012;55(8):2183–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Polidori DC, Bergman RN, Chung ST, Sumner AE. Hepatic and extrahepatic insulin clearance are differentially regulated: results from a novel model-based analysis of intravenous glucose tolerance data. Diabetes. 2016;65(6):1556–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Heinrich G, Muturi HT, Rezaei K, et al. Reduced hepatic carcinoembryonic antigen-related cell adhesion molecule 1 level in obesity. Front Endocrinol (Lausanne). 2017;8:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pivovarova O, von Loeffelholz C, Ilkavets I, et al. Modulation of insulin degrading enzyme activity and liver cell proliferation. Cell Cycle. 2015;14(14):2293–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pihlajamäki J, Boes T, Kim EY, et al. Thyroid hormone-related regulation of gene expression in human fatty liver. J Clin Endocrinol Metab. 2009;94(9):3521–3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hulse RE, Ralat LA, Wei-Jen T. Structure, function, and regulation of insulin-degrading enzyme. Vitam Horm. 2009;80: 635–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kabir M, Iyer MS, Richey JM, et al. CB1R antagonist increases hepatic insulin clearance in fat-fed dogs likely via upregulation of liver adiponectin receptors. Am J Physiol Endocrinol Metab. 2015;309(8):E747–E758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim SH, Liu A, Ariel D, et al. Pancreatic beta cell function following liraglutide-augmented weight loss in individuals with prediabetes: analysis of a randomised, placebo-controlled study. Diabetologia. 2014;57(3):455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kindmark H, Pigon J, Efendic S. Glucose-dependent insulinotropic hormone potentiates the hypoglycemic effect of glibenclamide in healthy volunteers: evidence for an effect on insulin extraction. J Clin Endocrinol Metab. 2001;86(5):2015–2019. [DOI] [PubMed] [Google Scholar]
- 49. Rudovich NN, Rochlitz HJ, Pfeiffer AF. Reduced hepatic insulin extraction in response to gastric inhibitory polypeptide compensates for reduced insulin secretion in normal-weight and normal glucose tolerant first-degree relatives of type 2 diabetic patients. Diabetes. 2004;53(9):2359–2365. [DOI] [PubMed] [Google Scholar]
- 50. Shah A, Holter MM, Rimawi F, et al. Insulin clearance after oral and intravenous glucose following gastric bypass and gastric banding weight loss. Diabetes Care. 2019;42(2):311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Meier JJ, Gallwitz B, Siepmann N, et al. The reduction in hepatic insulin clearance after oral glucose is not mediated by gastric inhibitory polypeptide (GIP). Regul Pept. 2003;113(1-3):95–100. [DOI] [PubMed] [Google Scholar]
- 52. Meier JJ, Holst JJ, Schmidt WE, Nauck MA. Reduction of hepatic insulin clearance after oral glucose ingestion is not mediated by glucagon-like peptide 1 or gastric inhibitory polypeptide in humans. Am J Physiol Endocrinol Metab. 2007;293(3):E849–E856. [DOI] [PubMed] [Google Scholar]
- 53. Kotronen A, Vehkavaara S, Seppälä-Lindroos A, Bergholm R, Yki-Järvinen H. Effect of liver fat on insulin clearance. Am J Physiol Endocrinol Metab. 2007;293(6):E1709–E1715. [DOI] [PubMed] [Google Scholar]
- 54. Utzschneider KM, Kahn SE, Polidori DC. Hepatic insulin extraction in NAFLD is related to insulin resistance rather than liver fat content. J Clin Endocrinol Metab. 2019;104(5):1855–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hakim O, Bello O, Bonadonna RC, et al. Ethnic differences in intrahepatic lipid and its association with hepatic insulin sensitivity and insulin clearance between men of black and white ethnicity with early type 2 diabetes. Diabetes Obes Metab. 2019;21(9):2163–2168. [DOI] [PubMed] [Google Scholar]