Abstract
Diagnosis of cartilage damage in early stages of arthritis is vital to impede the progression of disease. In this regard, considerable progress has been made in near-infrared fluorescence (NIRF) optical imaging technique. Arthritis can develop due to various mechanisms but one of the main contributors is the production of matrix metalloproteinases (MMPs), enzymes that can degrade components of the extracellular matrix (ECM). Especially, MMP-1 and MMP-13 have main role in RA and OA because they enhance collagen degradation in the process of arthritis. We present here a novel NIRF imaging strategy that can used to determine the activity of matrix metalloproteinases (MMPs) and cartilage damage simultaneously by detection of exposed type II collagen in cartilage tissue. In this study, retroorbital injection of mixed fluorescent dyes, MMPSense® 750 FAST (MMP750) dye and Alexa Fluor® 680 conjugated monoclonal mouse antibody immune-reactive to type II collagen (MabCII_680F), was administered in the arthritic mice. Both dyes were detected with different intensity according to degree of joint destruction in the animal. Thus, our dual fluorescence imaging method can be used to detect cartilage damage as well as MMP activity simultaneously in early stage arthritis.
Keywords: cartilage, arthritis, type II collagen, MMP, optical imaging, diagnosis, fluorescence
INTRODUCTION
The global burden of arthritis has become a serious concern in recent times [1]. In addition, arthritis not only results in damaged cartilage but also increases the risk of systemic complications [2]. Modern techniques available for the detection of arthritis such as magnetic resonance imaging (MRI) or positron emission tomography (PET), are time consuming, expensive and have limitation to detect early stage of joint destruction. Therefore, a trend towards near-infrared fluorescence (NIRF) optical imaging has emerged and has reported in detection of arthritis [3]. NIRF imaging is a non-ionizing and non-invasive technique with fast acquisition times [4, 5].
It has been reported that matrix metalloproteinases (MMPs) levels increase in sera of arthritis patients. Different forms of MMPs, such as MMP-1 and -13, have been reported to cause destruction of joint tissues through breakdown of cartilage extracellular matrix, especially type II collagen [6]. Thus, MMPs have been considered as a diagnostic marker for arthritis.
Recently, we described detection of cartilage degradation by the use of monoclonal collagen type II (MabCII) antibody [7, 8]. As type II collagen (CII) is only exposed in the damaged cartilage so the MabCII only bind to the damaged cartilage. This implies that such a technique can help in early diagnosis of arthritis.
Therefore, we hypothesized that detection of both MMP activity and exposed CII will be an efficient method to diagnose arthritic joints. In this study, we combined MMP detection probe and fluorescent labelled MabCII for the detection of cartilage damage and inflammation simultaneously in early phase of arthritis in a mouse model of arthritis.
MATERIAL AND METHODS
Monoclonal mouse IgG and anti-collagen II antibody
The generation and characterization of MabCII was done as described previously [9]. Binding and specificity of these antibodies for type II collagen purified from mouse cartilage was confirmed by immunoassay. Results indicated that antibody designated as MabCII, has strong immuno-reactivity to collagen, and henceforth used in this study. Purified monoclonal antibody was obtained from the VA Program Project Scientific Core at the VA Medical Center (Memphis, TN). The monoclonal mouse IgG2A antibody (MabCont; Clone 20102) of the same subclass (R&D Systems, Minneapolis, MN) was used as control. The antibodies were conjugated with XenoLight CF™ 680 dye (PerkinElmer, Waltham, MA) and designated as MabCII_680F and MabCont_680F respectively. MMPSense® 750 FAST Fluorescent Imaging Agent (Perkin Elmer, Waltham, MA) designated as MMP750 was used to assess MMP activity in vivo.
Collagen-Induced Arthritis (CIA)
A total of twenty-four (n=8 per group) DR1*0101 (DR1) transgenic mice were used in this study [10]. Mice were kept in a pathogen free environment with adequate food and water ad libitum. All animal experiments were performed according to approved protocols by IACUC and experimental procedures at the University of Tennessee Health Science Center. The mouse model of CIA was developed in sixteen animals (n=16) as described previously [11]. Briefly, each animal was injected intra-dermally in tail by CII (Chondrex, Redmond, WA) in Complete Freund adjuvant (CFA) (BD Biosciences, Franklin Lakes, NJ). Booster dose was given 14 days after the primary immunization with CII in Incomplete Freund adjuvant (IFA) (BD Biosciences, Franklin Lakes, NJ). Animals were examined 2-3 times per week for signs and symptoms of arthritis. The evaluation involved scoring of each animal on a scale of 0-4 for each paw, with 4 being the most severe form of arthritis [11]. Mice were injected retroorbitally with 100 μl of solution containing MMP750 and MabCon_680 or MabCII_680 in 1:1 ratio when incidence of arthritis was detected.
Dual fluorescent imaging and quantification
Mice were divided into three groups (n=8 per group) according to the presence and absence of arthritis and injection administered (Table 1). Injection was given retroorbitally into the right eye. After 24 h, the mice under anesthesia were scanned using the in vivo imaging system (IVIS® Lumina XR System, Perkin Elmer, Hopkinton, MA) with a high range filter set. The excitation and emission wavelengths used for MMPSense® 750 FAST were 745 nm and 800 nm respectively, and XenoLight 680 CF™ Dye were 675 nm and 720 nm respectively. Fluorescence in each knee joint was quantified using Living Image 4.0 software to calculate the flux radiating omni-directionally from the region of interest (ROI). Calculations are represented in graphical form as radiant efficiency (photons/s/cm2/str)/(μW/cm2). Standardized ROI of the knee fluorescence was measured by capturing the same area for each mouse. Background fluorescence was removed by subtracting the fluorescence of null or background captured area (consisting of muscle and skin tissue) from each articular reading. The ROI results were compared to arthritis score in same mouse.
Table 1.
Control and experimental groups used in vivo
| Group | Injected fluorescent dye |
|---|---|
| Normal mice | MabCII_680F1 + MMP7502 |
| Arthritic mice | MabCont_680F3 + MMP750 |
| Arthritic mice | MabCII_680F + MMP750 |
Monoclonal mouse anti-type II collagen antibody labeled with XenoLight 680 CF™ Dye
MMPSense® 750 FAST
Monoclonal mouse IgG labeled with XenoLight 680 CF™ Dye
Statistical analysis
All experiments were performed independently in triplicate. Student’s t tests were performed to determine statistical significance. A P ≤ 0.05 was considered statistically significant.
RESULTS
Detection of MabCII_680F and MMP750 by IVIS imaging
To evaluate if the fluorescence signal from MabCII_680F and MMP750 interfere with each other, the two dyes were measured by IVIS scan with different filter sets in vitro. MabCII_680F was only detected at excitation wavelength of 675 nm and emission wavelength of 720 nm. Similarly, only MM750 was detected at excitation wavelength of 745 nm and emission wavelength of 800 nm. Further, no overlapping signal was confirmed by mixing the two dyes in equal proportion and measuring with IVIS scan at respective wavelengths (Figure 1A). Each dye was detected distinctly at respective wavelength. Intensity for both 680F and 750F was significantly high at their respective filter set (Figure 1 B and C).
Figure 1.
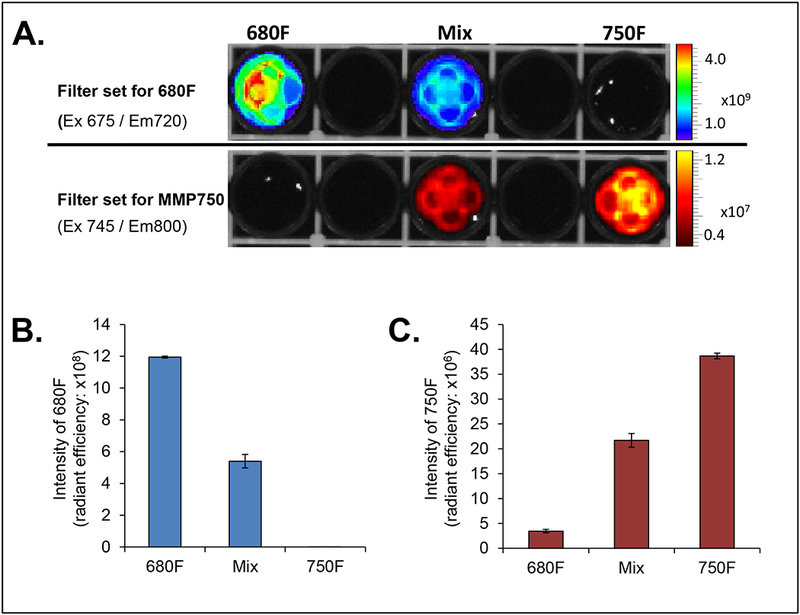
In vitro assessment of 680F and 750F. (A) Representative image for 680F and 750F measured at respective wavelengths. (B) Graphical representation for 680F. Data is expressed as mean ± SD (C) Graphical representation for 750F. Data is expressed as mean ± SD
We performed macroscopic evaluation and IVIS scan of paws from arthritic animal model to assess progression of disease. Macroscopic examination was done based on the scoring system as follows: 0 = No arthritis, 1 = Swelling/redness in 1-2 interphalangeal (IP) joints, 2 = Involvement of one larger joint or 3-4 IP joints, 3 = Swelling/redness in more than 4 joints, and 4 = Severe arthritis in entire paw. Macroscopic examination of joints clearly showed signs of arthritis in CIA mouse model (Figure 2A). The maximum score obtained for arthritis was 3.
Figure 2.
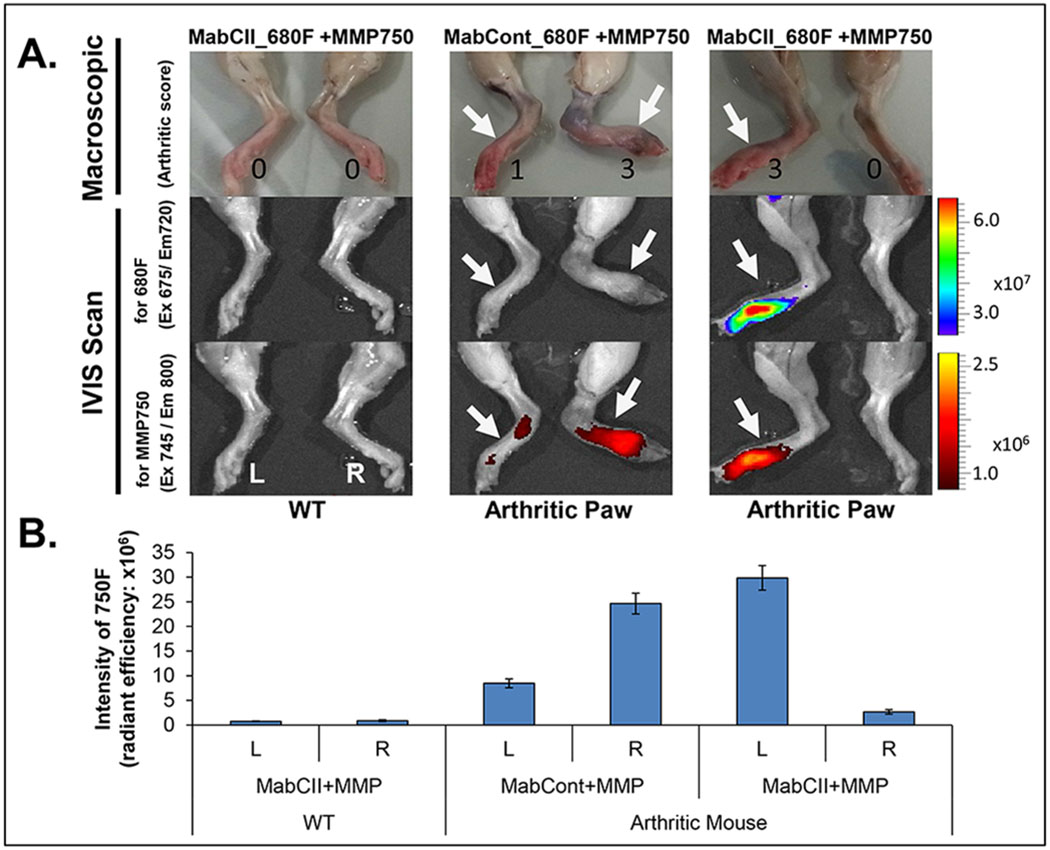
Analysis of joints in normal and arthritis mice. (A) Macroscopic and IVIS analysis. (B) Graphical representation of inflammation. MMP activity was measured as intensity of 750F. Data is expressed as mean ± SD
The IVIS scan results shown that no fluorescence was detected in joints of control group injected with a combination of MabCII_680F and MMP750 (Figure 2A). Arthritic joints injected with MabCont_680F and MMP750, only showed fluorescence for MMP750. On the other hand fluorescence was detected for both dyes in arthritic joints injected with a combination of MabCII_680F and MMP750. Distinct signals were obtained for each dye. The fluorescence signals were in accordance with arthritis score.
The degree of inflammation was further confirmed by measuring the intensity of 750F in arthritic mice (Figure 2B). Intensity of 750F was significantly higher in arthritic animals for both right and left paws as compared with normal joints. Further, the intensity of 750F was in accordance with the arthritis score.
DISCUSSION
The short acquisition time with minimal intervention makes NIRF optical imaging an ideal technique to examine the extent of joint damage in arthritis [4, 5]. However, it is essential to design such techniques that will help in early diagnosis to prevent the progression of the disease [12, 13]. Therefore, in this study we evaluated if MMP750 and MabCII_680F can be used in combination to detect MMP activity and cartilage damage simultaneously in a CIA mouse model.
Though, it was evident that MMP750 and MabCII_680F would be read at two distinct wavelengths, we still confirmed that the two dyes do not interfere with each other. We measured the fluorescent signal for each dye in vitro at their respective wavelengths and found no overlapping pattern between the two dyes. Further, intensity of each dye was significantly high as compared with either mixed solution or other dye at respective wavelength for a specific dye. This confirmed that both 680F and 750F can be detected distinctly at their respective wavelength with no overlapping signal from the other dye.
The results of in vivo study further strengthen our observations. No fluorescence signal in normal mice showed that these dyes can be used for the detection of arthritic joints without any noise. In mice, combination of MabCont_680F and MMP750 only showed fluorescence signal for MMP750. This indicates that the control antibody did not bind to the damaged cartilage. On the other hand, signal was obtained for both dyes at their respective filter set when MabCII_680F and MMP750 were used in combination. These results are in confirmation with our previous results that showed the binding of CII only to the damaged cartilage [7]. Furthermore, degree of inflammation was measured by examining the MMP activity in arthritic joints. Pronounced inflammation was confirmed by IVIS scan that also correlated with arthritis score. Combined together, we were able to determine cartilage damage and inflammation in arthritic mice using MabCII_680F and MMP750.
Thus we present here a technique that can be used to detect early stage cartilage damage in arthritic mouse. This will help in understanding the mechanisms involved in the onset of cartilage damage and monitoring the therapeutic efficacy of drugs against arthritis using animal model in a preclinical study. Translation of this technical concept into clinics may help to monitor the progression of disease in arthritic patients.
CONCLUSION
We were able to detect MMP activity and damage cartilage simultaneous in the same joint by using two dyes at their respective filter sets. This is a reliable, inexpensive, time-effective and least intervention technique that can be used to detect early stage of arthritis in the same animal. We suggest further studies to determine the biosafety and time-dependent efficacy of the administered agents using this method.
Acknowledgments
This research was supported by a grant funded by VA Merit Review award from the Department of Veterans Affairs, an R21 from NIH (AR060408), a CTSI award from the UTHSC (K. Hasty), and Arthritis Foundation Fellowship Award, The William and Ella Owens Medical Research Foundation Award (H. Cho); A. Yi supported by grants from NIH (AR064723) and Arthritis Foundation (IRG 5942). The authors would like to thank Christy Patterson who is the technical director of the University of Tennessee for her assistance with this study and invaluable comments.
Footnotes
Conflict of interest
Authors declare no conflict of interest.
References
- 1.Pereira D, Ramos E, Branco J. Osteoarthritis. Acta Med Port. 2015;28:99–106. [DOI] [PubMed] [Google Scholar]
- 2.Pap T, Korb-Pap A. Cartilage damage in osteoarthritis and rheumatoid arthritis-two unequal siblings. Nat Rev Rheumatol. 2015. [DOI] [PubMed] [Google Scholar]
- 3.Krohn M, Ohrndorf S, Werner SG, Schicke B, Burmester GR, Hamm B, et al. Near-infrared Fluorescence Optical Imaging in Early Rheumatoid Arthritis: A Comparison to Magnetic Resonance Imaging and Ultrasonography. The Journal of rheumatology. 2015;42:1112–8. [DOI] [PubMed] [Google Scholar]
- 4.Wunder A, Tung CH, Muller-Ladner U, Weissleder R, Mahmood U. In vivo imaging of protease activity in arthritis: a novel approach for monitoring treatment response. Arthritis and rheumatism. 2004;50:2459–65. [DOI] [PubMed] [Google Scholar]
- 5.Chen WT, Mahmood U, Weissleder R, Tung CH. Arthritis imaging using a near-infrared fluorescence folate-targeted probe. Arthritis research & therapy. 2005;7:R310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:529–43. [DOI] [PubMed] [Google Scholar]
- 7.Cho H, Pinkhassik E, David V, Stuart JM, Hasty KA. Detection of early cartilage damage using targeted nanosomes in a post-traumatic osteoarthritis mouse model. Nanomedicine. 2015;11:939–46. [DOI] [PubMed] [Google Scholar]
- 8.Cho H, Stuart JM, Magid R, Danila DC, Hunsaker T, Pinkhassik E, et al. Theranostic immunoliposomes for osteoarthritis. Nanomedicine : nanotechnology, biology, and medicine. 2014;10:619–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terato K, Hasty KA, Reife RA, Cremer MA, Kang AH, Stuart JM. Induction of arthritis with monoclonal antibodies to collagen. J Immunol. 1992;148:2103–8. [PubMed] [Google Scholar]
- 10.Rosloniec EF, Brand DD, Myers LK, Whittington KB, Gumanovskaya M, Zaller DM, et al. An HLA-DR1 transgene confers susceptibility to collagen-induced arthritis elicited with human type II collagen. J Exp Med. 1997;185:1113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brand DD, Latham KA, Rosloniec EF. Collagen-induced arthritis. Nat Protoc. 2007;2:1269–75. [DOI] [PubMed] [Google Scholar]
- 12.Finckh A, Liang MH, van Herckenrode CM, de Pablo P. Long-term impact of early treatment on radiographic progression in rheumatoid arthritis: A meta-analysis. Arthritis Rheum. 2006;55:864–72. [DOI] [PubMed] [Google Scholar]
- 13.Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, van Zeben D, Kerstens PJ, Hazes JM, et al. Comparison of treatment strategies in early rheumatoid arthritis: a randomized trial. Ann Intern Med. 2007;146:406–15. [DOI] [PubMed] [Google Scholar]


