Abstract
The article presents a case report and literature review of hemifacial microsomia with cervical vertebral anomalies. Unilateral hypoplasia of the mandible, congenital anomalies of the external ear and cervical spine pathology identified in this case are common major signs/symptoms of Goldenhar (Goldenhar-Gorlin) syndrome. Complete fusion of bodies and spinous processes of the second and third cervical vertebrae as well as atlantooccipital assimilation and anterior cleft of the atlas were also found. All abnormalities were accidentally identified and not accompanied by clinical symptoms.
Keywords: Hemifacial microsomia, Goldenhar (Goldenhar-Gorlin) syndrome, craniofacial microsomia, oculo-auriculo-vertebral dysplasia, first and second branchial arch syndrome, lateral facial dysplasia, unilateral oto-mandibular dysostosis, facio-auriculo-vertebral sequence, oculo-auriculo-vertebral spectrum, vertebral anomalies, temporomandibular joint abnormalities, cone beam computed tomography
CASE REPORT
An 18-year-old Caucasian male was referred by the medical board of the Military Registration and Recruitment Office to the oral and maxillofacial surgeon with the chief complaint of having facial deformity since childhood. Supposedly, unilateral (left side) mandibular hypoplasia and left and right ear skin tags were noticed at birth with no other anomalies detected. The patient underwent surgery for ear malformation at 1 year of age. Signed informed consent regarding radiological images and data publication was obtained.
Cone beam computed tomography performed on Galileos GAX5 (Germany) revealed the following findings:
Facial skull asymmetry due to a relatively smaller size of the left half of the face mainly expressed in the region of the mandible (Figure 1).
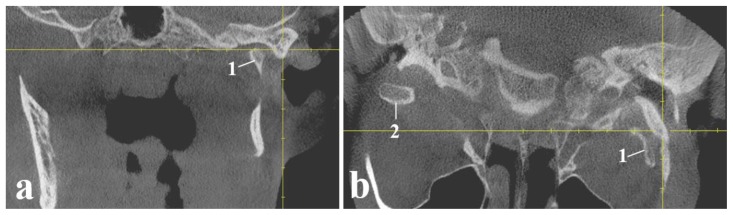
Elongated left styloid complex including the styloid process and ossified stylohyoid ligament measuring 41.38 mm (Figure 2).
Shortening of the left mandibular ramus with medial and anterior shift and the condyle size reduction. Articular tubercle was located laterally from condylar head (Figure 3).
Complete fusion of C2–C3 vertebral bodies and spinous processes; the parasagittal slit-like defect of the anterior arch of the atlas (Figure 4); atlantooccipital assimilation (fusion of the left lateral masses of the atlas with the occipital condyle (Figure 5).
Absence of visible pathology of the external, middle and inner ear.
Absence of the pneumatization of the mastoid processes on both sides.
Figure 1.
18-year-old male adolescent with hemifacial microsomia
Findings: Facial skull asymmetry due to unilateral hypoplasia of the mandible (the left side is hypoplastic).
Technique: Cone beam computed tomography - panoramic view (a) and cone beam computed tomography volumetric rendering technique image (b), 85 kV; tube current 5–7 mA; acquisition period 14 s; effective radiation time 2–6 s; voxel size 0.3 × 0.3 × 0.3 mm
Figure 2.
18-year-old male adolescent with hemifacial microsomia
Findings: Calcified left stylohyoid ligament (red arrow).
Technique: Cone beam computed tomography - 3D reconstruction (a) and CBCT sagittal slice by drawing perpendicular lines to the long axis of the stylohyoid complex (b), 85 kV; tube current 5–7 mA; acquisition period 14 s; effective radiation time 2–6 s; voxel size 0.3 × 0.3 × 0.3 mm
Figure 3.
18-year-old male adolescent with hemifacial microsomia
Findings: CBCT is showing relationships of articular surfaces of left temporomandibular joint. Yellow crosshair represents the apex of the articular tubercle located laterally from the head of mandible. 1 - the left head of the mandible; 2 - the right head of the mandible.
Technique: Cone beam computed tomography - coronal (a) and axial (b) view; 85 kV; tube current 5–7 mA; acquisition period 14 s; effective radiation time 2–6 s; voxel size 0.3 × 0.3 × 0.3 mm
Figure 4.
18-year-old male adolescent with hemifacial microsomia
Findings: Parasagittal slit-like defect of the anterior arch on the right (red arrow).
Technique: Cone beam computed tomography - axial (a) and coronal (b) view; 85 kV; tube current 5–7 mA; acquisition period 14 s; effective radiation time 2–6 s; voxel size 0.3 × 0.3 × 0.3 mm
Figure 5.
18-year-old male adolescent with hemifacial microsomia
Findings: Complete fusion of C2–C3 vertebral bodies and spinous processes (white arrows indicate the site of the fusion of the vertebral bodies, white arrowhead shows the fusion of C2–C3 spinous processes) and atlantooccipital fusion (the red arrow).
Technique: Cone beam computed tomography - sagittal (a) and coronal (b) view; 85 kV; tube current 5–7 mA; acquisition period 14 s; effective radiation time 2–6 s; voxel size 0.3 × 0.3 × 0.3 mm
DISCUSSION
Hemifacial microsomia (HFM) is an asymmetric craniofacial malformation, variably affecting structures derived from the first and second pharyngeal arches [1, 2, 3, 4]. Many terms have been used for this malformation, thus indicating a wide spectrum of anomalies observed and emphasized by the authors from various disciplines. In addition to HFM, the malformation has been called craniofacial microsomia (some authors believe, that currently this is the most accepted term to mention this pathology), oculo-auriculo-vertebral dysplasia, first and second branchial arch syndrome, lateral facial dysplasia, unilateral oto-mandibular dysostosis, facio-auriculo-vertebral sequence and oculo-auriculo-vertebral spectrum [5].
HFM is characterized by unilateral hypoplasia of a mandible and ear, including a shortened mandibular ramus, small glenoid fossa, malformed condyle, hypoplastic coronoid process and preauricular tags [6]. As many as 55% of patients with HFM also have extracranial anomalies including central nervous system, skeletal, cardiac, lung, gastrointestinal and kidney defects [1, 7, 8]. The reported prevalence of vertebral anomalies in HFM varied from 8% to 79% and the most often reported anomalies without the specified location were hemivertebrae, block vertebrae, scoliosis/kyphoscoliosis and spina bifida [9].
Goldenhar syndrome was considered to be a variant of HFM [4]. It was first described by the Swiss ophthalmologist Maurice Goldenhar in 1952 as a syndrome by the presence of the same classic triad of eye, ear and vertebral changes.
Etiology & demographics
The estimated prevalence of HFM ranges from 1/3,500 to 1/26,550 in live births making this malformation the second most common craniofacial abnormality after cleft lip and palate [2, 3, 10, 11]. In Europe the prevalence of oculo-auriculo-vertebral spectrum defined as microtia/ear anomalies and at least one major characteristic anomaly was 3,8 per 100 000 births [11]. The major discrepancy in the literature on the incidence of HFM may be due to the lack of information on fetal deaths and terminated pregnancies, and the inclusion or exclusion of mild or extreme cases that are given a different diagnosis [12]. The documented prevalence of vertebral anomalies in HFM varied from 8% to 79% [9].
While the majority of cases are sporadic and appear to have a multifactorial etiology, cases with chromosomal abnormalities (mainly in chromosomes 5 [5p deletion], 18 [trisomy] and 22 [22q11.2 deletion]), mosaicism and families with autosomal recessive and autosomal dominant inheritance have been reported [11, 13, 14]. Despite previous efforts to reveal a genomic etiology of Goldenhar syndrome (GS), investigators have not been able to identify a causative gene [12].
Within the environmental causes of HFM there are various risk factors associated with pregnancy, such as vasoactive medications, vaginal bleeding during the second quarter, multiple gestations, the use of assisted reproductive technology by the mother and preexisting or gestational diabetes [3].
Although the precise etiology of hemifacial microsomia is unknown, disruption of the first and second branchial arches during the first 6 weeks of gestation is thought to be causative [15].
The most plausible hypothesis on the pathogenesis of HFM is the vascular abnormality and hemorrhage theory [6]. Between 1973 and 1975, Poswillo proposed that the pathogenetic makeup of hemifacial microsomia was based on an embryonic hematoma formation arising from the anastomosis that precedes the formation of the stapedial artery [16]. Eseonu and Vieira [16] considered the severity, variation, and heterogeneity of the symptoms of hemifacial microsomia to be a direct result of the size and amount of hematoma collection and expansion, where small hematomas cause less damage than their larger counterparts in regard to branchial arch growth.
Meckel’s cartilage plays a crucial role in development of the mandible and middle ear, and damage to Meckel’s cartilage may secondarily lead to its abnormal development, which can partially account for the skeletal defects of HFM [6]. Direct experimental evidence, based on surgical interference of mandibular development in the chick embryo confirms the latter concept [17]. Asymmetrical perturbation of Meckel’s cartilage has been shown to result in asymmetry of the mandible. This hypothesis focused mainly on the HFM-type skeletal defects and was independent of any single pathogenic factor. The perturbation of the auriculofacial cartilage model may involve either of the fundamental processes of growth and morphogenesis and can be interfered by vascular events, teratogens and genetic defects among other factors. For example, hemorrhage in the vicinity of Meckel’s cartilage is responsible for the disruption of normal chondrogenesis giving rise to malformed auditory ossicles and mandible. It was also revealed that impaired secretion of vascular endothelial growth factor is responsible for the reduced blood supply to Meckel’s cartilage ultimately generating mandibular hypoplasia [18].
The presence of an elongated styloid complex indicates abnormal morphogenesis of simultaneous first and second branchial arch derivatives. This justifies the use of the term “first and second branchial arch syndrome” as a synonym for Goldenhar syndrome.
The crucial role in craniofacial development is played by cranial neural crest cells (CNCCs). Given that most craniofacial structures involved in HFM are derivatives of CNCCs, direct disturbances of the migration, proliferation and differentiation of CNCCs due to chromosomal abnormalities or a single gene mutation were then proposed as a possible mechanism for HFM [19].
Vertebral anomalies may be a result of unilateral defects of formation and segmentation of primitive vertebrae at the mesenchymal stage of development or are thought to be due to the localized failure of vascularization of the developing cartilaginous centrum [20].
Clinical & imaging findings
Reports focused mainly on the craniofacial dysmorphic features and limited attention was paid to the vertebral abnormalities in patients with Goldenhar syndrome [20].
In the present case report radiological manifestation of hemifacial macrosomia and vertebral anomalies in cervical spine were detected using CBCT. Unilateral mandibular hypoplasia, congenital anomalies of the external ear and pathology of the cervical spine identified in this case are common major findings in Goldenhar (Goldenhar-Gorlin) syndrome. By the degree of hypoplasia of the mandibular ramus and the shape and location of the articular head they correspond to Type IIB of hemifacial microsomia according to the classification of Pruzansky-Kaban [21] shown in Table 1. Nevertheless, the patient could function with both temporomandibular joints and did not complain of any deficiency in chewing.
Table 1.
Classification of the skeletal deformity by Pruzansky modified by Kaban [21]
| Type | Anatomy of the mandible and temporomandibular joint |
|---|---|
| I | Small mandible |
| IIA | Short mandibular ramus of abnormal shape; glenoid fossa in satisfactory position |
| IIB | TMJ abnormally placed inferiorly, medially and anteriorly |
| III | Absent TMJ |
Vertebral anomalies in HFM are most common in the cervical spine, followed by the thoracic spine and ribs [9]. In our case it was possible to observe only the cervical part of the vertebral column where a number of radiographic signs were detected including complete fusion of C2–C3 vertebral bodies and spinous processes, atlantooccipital assimilation, the parasagittal slit-like defect of the anterior arch of atlas and fusion of C2 and C3 spinous processes. All these abnormalities were identified accidentally and were not accompanied by clinical symptoms.
Treatment & Prognosis
Treatment of HFM varies with age and according to systemic associations [22]. Reconstructive surgery is usually necessary in these patients. The treatment seeks to improve functionality along with optimum facial symmetry in order to increase the size of the affected mandibular side and its associated soft tissue and create a joint simulating the temporomandibular joint (TMJ) in cases where it is absent [3]. Patients with mandibular hypoplasia can be submitted to reconstructive surgery using rib bone grafts [22]. Mild cases can be potentially managed with orthodontic appliances alone [23]. Orthodontic treatment usually involves the use of a specialized type of functional appliance, hybrid appliances.
Depending on the individual’s stage of development and severity of the vertebral defect different options exist for treatment. In order to prevent severe scoliosis it is best to take action early. Non-surgical options include bracing and physical therapy [15]. Depending on the defect surgical intervention such as spinal fusion or stabilizing growing rods may be necessary [24]. Prognosis is guarded in cases with systemic involvement, but is good in otherwise uncomplicated cases without any systemic associations [22].
Differential Diagnoses
The differential diagnosis is broad and includes other conditions in which facial asymmetry is a prominent clinical feature, for instance, like Parry Romberg syndrome (PRS) and Townes-Brocks syndrome. In Goldenhar syndrome unilateral facial asymmetry is usually seen, unlike in PRS, that condition is typically congenital and nonprogressive [25]. The majority of individuals with Goldenhar syndrome do not have such clinical manifestations as upper-limb or anal malformations which are typical for Townes-Brocks syndrome [26]. Vertebral abnormalities have been not included as a precise index to differentiate between these clinical entities from Goldenhar syndrome [20].
Table 2 provides a comparison of HFM (Goldenhar syndrome) with similar disorders [12].
Table 2.
Differential diagnosis table for HFM (Goldenhar syndrome) [12]
| Goldenhar syndrome | Hemifacial microsomia | Treacher Collins syndrome | Branchio-oto-renal syndrome | |
|---|---|---|---|---|
| Incidence | 1/3,500–1/26,550 | 1/3,500–1/26,550 | 1/50,000 | 1/40,000 |
| Etiology | Multifactorial | Multifactorial | AD: loss of function mutation of TCOF1 gene on chromosome 5 | AD:
|
| Unilateral or bilateral facial deformity | Usually unilateral | Usually unilateral | Usually bilateral | Not applicable |
| Type of hearing loss | Usually conductive | Usually conductive | Usually conductive | Conductive, sensorineural, or mixed |
| Ocular anomalies | Epibulbar dermoids, upper lid colobomas, microphthalmia, anophthalmia, palpebral ptosis | Anomalies similar to GS but without epibulbar dermoids | Lower lid colobomas, absent medial lower eyelashes, downward slanting of palpebral fissures, affected vision, skeletal dysmorphism of the orbits | None |
| Auricular anomalies | Microtia, preauricular skin tags, atresia/stenosis of external auditory canal, absence of auricle, anotia, preauricular pits/sinus, malformation of middle ear and inner ear are less common | Anomalies similar to GS, and occur in 65–99% of patients | Deformed external ear, microtia or anotia, stenosis or atresia of external auditory canal, misshaped tympanic membrane | Preauricular pits (75–85% of cases), preauricular tags, lop or bat ears, microtia, atretic external auditory canal; some cases of abnormal ossicles, facial nerve, and fallopian canals; some cases of hypoplastic cochlea and absent or hypoplastic semicircular canals |
| Craniofacial anomalies | Mandibular hypoplasia, also mandibular ramus asymmetry, maxillary and malar hypoplasia, TMJ abnormalities, micrognathia, cleft palate with or without cleft lip | Anomalies similar to GS | Malar hypoplasia is most common, mandibular and maxillary hypoplasia, micrognathia, retrognathia, cleft palate | High arched or cleft palate, deep overbite |
| Other musculoskeletal anomalies | Vertebral defects, may have clubbing, polydactyly, clinodactyly, camptodactyly, or single palmar crease | No vertebral defects | Vertebral defects rare | None |
| Additional defining anomalies | Renal dysplasia in more than 2/3 of cases, branchial fistulae (usually bilaterally in lower part of the neck) | |||
| Associated anomalies | Cardiac, renal, genital, gastrointestinal, may have some cognitive disability | Anomalies similar to GS | Cardiac, renal, cryptorchidism, airway abnormalities | Aplasia or stenosis of the lacrimal ducts |
Brachio-oto-rental syndrome, mandibulofacial dysostosis (Treacher Collins syndrome), maxillofacial dysostosis, Nager acrofacial dysostosis, and axial mesodermal dysplasia complex are also included in the differential diagnosis of HFM. Unlike HFM, individuals with the conditions listed above typically have symmetric facial malformations. Additional information about the phenotypic differences between HFM and rare genetic disorders could be found in the review published by Heike et al. [27].
TEACHING POINT
Serious structural abnormalities of the temporomandibular joint and the cervical spine can remain compensated for a long time not forcing a patient to seek for medical care. Abnormalities of the first and second branchial arches are often combined with a congenital defect of the cervical spine and can be detected using cone beam computed tomography.
ABBREVIATIONS
- AD
Autosomal dominant
- CBCT
Cone beam computed tomography
- CFM
Craniofacial microsomia
- CNCCs
Cranial neural crest cells
- GS
Goldenhar syndrome
- HFM
Hemifacial microsomia
- TMJ
Temporomandibular joint
REFERENCES
- 1.Birgfeld CB, Heike C. Craniofacial microsomia. Semin Plast Surg. 2012 May;26(2):91–104. doi: 10.1055/s-0032-1320067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagheri SC. Clinical Review of Oral and Maxillofacial Surgery: A Case-based Approach. 2nd ed. Mosby; 2013. Syndromes of the Head and Neck; pp. 457–487. [Google Scholar]
- 3.Véliz MS, Agurto VP, Leiva VN. Hemifacial microsomia. a literature review. [Accessed April 24, 2019];Rev Fac Odontol Univ Antioq [Internet] 2016 Jan;27(2):404–424. Available at: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0121246X2016000100404&lng=en. [Google Scholar]
- 4.Resnick CM, Kaban LB, Padwa BK. Maxillofacial Surgery. 3rd ed. Churchill Livingstone; 2017. Hemifacial Microsomia: The Disorder and Its Surgical Management; pp. 870–893. [Google Scholar]
- 5.Posnick JC. Hemifacial Microsomia: Evaluation and Treatment. In: Posnick JC, editor. Orthognathic surgery: principles and practice. St Louis (MO): Elsevier; 2014. pp. 1095–1158. [Google Scholar]
- 6.Chen Q, Zhao Y, Shen G, Dai J. Etiology and Pathogenesis of Hemifacial Microsomia. J Dent Res. 2018 Nov;97(12):1297–1305. doi: 10.1177/0022034518795609. [DOI] [PubMed] [Google Scholar]
- 7.Horgan JE, Padwa BL, LaBrie RA, Mulliken JB. OMENS-Plus: analysis of craniofacial and extracraniofacial anomalies in hemifacial microsomia. Cleft Palate Craniofac J. 1995 Sep;32(5):405–12. doi: 10.1597/1545-1569_1995_032_0405_opaoca_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 8.Strömland K, Miller M, Sjögreen L, et al. Oculo-auriculo-vertebral spectrum: associated anomalies, functional deficits and possible developmental risk factors. Am J Med Genet A. 2007 Jun 15;143A(12):1317–25. doi: 10.1002/ajmg.a.31769. [DOI] [PubMed] [Google Scholar]
- 9.Renkema RW, Caron CJJM, Mathijssen IMJ, et al. Vertebral anomalies in craniofacial microsomia: a systematic review. Int J Oral Maxillofac Surg. 2017 Oct;46(10):1319–1329. doi: 10.1016/j.ijom.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 10.Hartsfield JK. Review of the etiologic heterogeneity of the oculo-auriculo-vertebral spectrum (hemifacial microsomia) Orthod Craniofac Res. 2007 Aug;10(3):121–8. doi: 10.1111/j.1601-6343.2007.00391.x. [DOI] [PubMed] [Google Scholar]
- 11.Barisic I, Odak L, Loane M, et al. Prevalence, prenatal diagnosis and clinical features of oculo-auriculo-vertebral spectrum: a registry-based study in Europe. Eur J Hum Genet. 2014 Aug;22(8):1026–33. doi: 10.1038/ejhg.2013.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meenan K, Kadakia S, Bernstein J. Revisiting the work of Maurice Goldenhar - an overview of Goldenhar syndrome. Eur J Plast Surg. 2014;37(11):575–582. [Google Scholar]
- 13.Vendramini-Pittoli S, Kokitsu-Nakata NM. Oculoauriculovertebral spectrum: report of nine familial cases with evidence of autosomal dominant inheritance and review of the literature. Clin Dysmorphol. 2009 Apr;18(2):67–77. doi: 10.1097/MCD.0b013e328323a7dd. [DOI] [PubMed] [Google Scholar]
- 14.Ballesta-Martínez MJ, López-González V, Dulcet LA, et al. Autosomal dominant oculoauriculovertebral spectrum and 14q23.1 microduplication. Am J Med Genet A. 2013 Aug;161A(8):2030–5. doi: 10.1002/ajmg.a.36007. [DOI] [PubMed] [Google Scholar]
- 15.Yates DM, Sinn DP. Maxillofacial Surgery. 3rd ed. Churchill Livingstone; 2017. Classification, Diagnosis, and Etiology of Craniofacial Deformities; pp. 803–834. [Google Scholar]
- 16.Eseonu CO, Vieira AR. Revisiting the etiology of hemifacial microsomia. Univ Odontol. 2014;33(71):19–28. [Google Scholar]
- 17.Cousley RR, Wilson DJ. Hemifacial microsomia: developmental consequence of perturbation of the auriculofacialcartilage model? Am J Med Genet. 1992 Feb 15;42(4):461–6. doi: 10.1002/ajmg.1320420410. [DOI] [PubMed] [Google Scholar]
- 18.Wiszniak S, Mackenzie FE, Anderson P, Kabbara S, Ruhrberg C, Schwarz Q. Neural crest cell-derived VEGF promotes embryonic jaw extension. Proc Natl Acad Sci U S A. 2015;112(19):6086–6091. doi: 10.1073/pnas.1419368112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beleza-meireles A, Claytonsmith J, Saraiva JM, Tassabehji M. Oculoauriculo-vertebral spectrum: a review of the literature and genetic update. J Med Genet. 2014;51(10):635–45. doi: 10.1136/jmedgenet-2014-102476. [DOI] [PubMed] [Google Scholar]
- 20.Al Kaissi A, Ben Chehida F, Ganger R, Klaushofer K, Grill F. Distinctive spine abnormalities in patients with Goldenhar syndrome: tomographic assessment. Eur Spine J. 2015 Mar;24(3):594–9. doi: 10.1007/s00586-014-3204-3. [DOI] [PubMed] [Google Scholar]
- 21.Kaban LB, Moses MH, Mulliken JB. Surgical correction of hemifacial microsomia in the growing child. Plast Reconstr Surg. 1988;82:9–19. [PubMed] [Google Scholar]
- 22.Goswami M, Bhushan U, Jangra B. Goldenhar Syndrome: A Case Report with Review. Int J Clin Pediatr Dent. 2016 Jul-Sep;9(3):278–280. doi: 10.5005/jp-journals-10005-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Littlewood SJ, Mitchell L. An introduction to Orthodontics. 5th ed. Oxford University Press; 2019. p. 333. [Google Scholar]
- 24.Hedequist D, Emans J. Congenital scoliosis: a review and update. J Pediatr Orthop. 2007 Jan-Feb;27(1):106–16. doi: 10.1097/BPO.0b013e31802b4993. [DOI] [PubMed] [Google Scholar]
- 25.Madasamy R, Jayanandan M, Adhavan UR, Gopalakrishnan S, Mahendra L. Parry Romberg syndrome: A case report and discussion. J Oral Maxillofac Pathol. 2012 Sep;16(3):406–10. doi: 10.4103/0973-029X.102498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kohlhase J. Townes-Brocks Syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet] Seattle, WA: University of Washington; 2016. [Accessed May 17, 2019]. Available at: http://www.ncbi.nlm.nih.gov/books/NBK1445/ [Google Scholar]
- 27.Heike CL, Luquetti DV, Hing AV. Craniofacial microsomia overview. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews® [Internet] Seattle, WA: University of Washington; 2014. [Accessed April 12, 2019]. Available at: https://www.ncbi.nlm.nih.gov/books/NBK5199/ [PubMed] [Google Scholar]