Abstract
Primary aortoenteric fistulas are rare, with the annual incidence of such fistulas estimated to be 0.007 per million. The most common predisposing conditions for primary aortoenteric fistulas are atherosclerotic abdominal aortic aneurysms or penetrating atherosclerotic ulcers. We illustrate a rare case of an inflammatory aortic aneurysm causing a primary aortic fistula, with a direct fistulous jet from the aorta to the bowel with resultant catastrophic bleeding. In contrast to atherosclerotic aneurysms, most inflammatory aneurysms are symptomatic and show dense perianeurysmal fibrosis and periaortic wall thickening. A direct jet of contrast extravasation from the aorta into a bowel loop, while rarely seen, remains the most specific sign of a primary aorta-enteric fistula. A comprehensive literature review of the clinical presentation, imaging features, and differential diagnosis of a primary aortoenteric fistula are also discussed.
Keywords: Aortoenteric fistula, primary, secondary, inflammatory abdominal aortic aneurysm, mycotic aneurysm, infectious aortitis, CT, MRI, ultrasound, radiotracer, abdomen
CASE REPORT
A 73-year-old lady with a past medical history of ischemic heart disease, diabetes mellitus, hypertension, and hyperlipidemia, presented to the gynecology clinic for vaginal discharge. She also reports having a long-standing poor appetite and weight loss of 10 kg over 6 months.
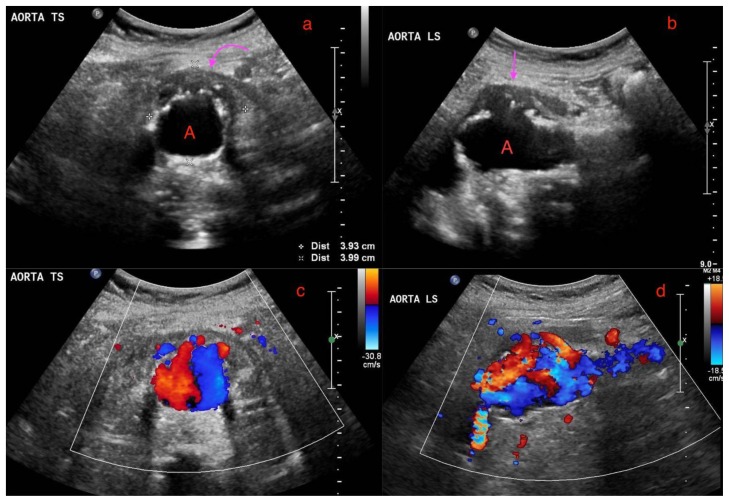
During her bedside pelvic ultrasound scan, she complained of epigastric pain. Physical examination revealed a pulsatile abdominal mass which was confirmed to be an abdominal aortic aneurysm on screening ultrasound. She was subsequently sent for a formal ultrasound of the aorta which showed a 3.9 × 4.0cm saccular abdominal aortic aneurysm with a suggestion of periaortic soft tissue (Figure 1a and 1b) and a “pseudo Yin-Yang” sign (Figure 1c and 1d). A Computed Tomography (CT) aortogram was recommended for further evaluation.
Figure 1.
A 73-year-old lady with an inflammatory aortic aneurysm on initial ultrasound abdomen 7.5 months prior to presentation with an aortoenteric fistula and catastrophic hemorrhage.
FINDINGS: 1(a) and 1(b) demonstrates a perianeurysmal cuff of hypoechoic soft tissue (curved arrow) around a fusiform abdominal aortic aneurysm (labelled A) with saccular outpouchings, measuring 3.9 × 4.0cm. 1(c) and 1(d) demonstrates turbulent flow within the abdominal aortic aneurysm with the “Pseudo Yin-Yang” sign.
TECHNIQUE: Ultrasound of the abdominal aorta, low frequency 2–5 MHz curvilinear probe, 1(a) and 1(b) grey scale images, 1(c) and 1(d) color doppler images
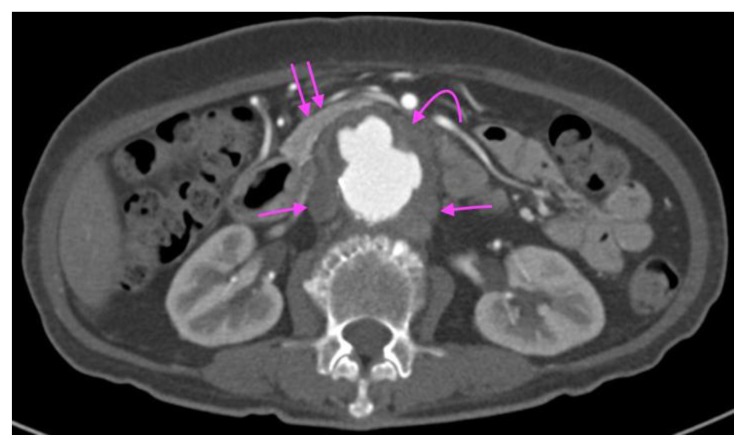
CT aortogram performed 3.5 months later showed a complex fusiform and saccular infrarenal aortic aneurysm with associated peripheral thrombi, overall measuring 4.8 × 4.7 cm over a length of 4.5 cm (Figures 2, 3, 4 and 5). The suprarenal aorta also showed tortuosity and at least two other saccular aneurysms. Of note, there was a cuff of periaortic soft tissue density with minimal adjacent fat stranding (Figures 2, 3, 4 and 5). This cuff of perianeurysmal soft tissue showed T2 intermediate-low signal and progressive enhancement into the 20 minutes delayed phase with primovist on a Magnetic Resonance Imaging (MRI) liver which was performed for the workup of an incidental liver lesion (Figures 6 and 7). There was also draping of the duodenum over the inflammatory AAA and distortion of the inferior vena cava producing a cleft like appearance (Figures 2, 3 and 6)
Figure 2.
A 73-year-old lady with an inflammatory aortic aneurysm on initial CT Aortogram 3.5 months from the initial ultrasound and 4 months prior to presentation with aortoenteric fistula and catastrophic hemorrhage.
FINDINGS: Axial CT Aortogram in the arterial phase demonstrates a perianeurysmal cuff of fibrotic soft tissue (curved arrow) around a fusiform abdominal aortic aneurysm with saccular outpouchings and peripheral thrombi. A small bowel loop is seen tethered and draped over the fibrotic soft tissue (Double arrows). Incidental note of a dual inferior vena cava, one on each side of the posterior aspect of the abdominal aorta (Single arrows).
TECHNIQUE: Axial CT Aortogram, arterial phase, 163 mAS, 100 KV, 3mm slice thickness, arterial phase, 75 ml Omnipaque 350
Figure 3.
A 73-year-old lady with an inflammatory aortic aneurysm on initial CT Aortogram 4 months prior to presentation with aortoenteric fistula and catastrophic hemorrhage.
FINDINGS: Axial CT Aortogram in the venous phase demonstrates a perianeurysmal cuff of fibrotic soft tissue (curved arrow) around a fusiform abdominal aortic aneurysm with saccular outpouchings and peripheral thrombi. A small bowel loop is seen tethered and draped over the fibrotic soft tissue (Double arrows). Incidental note of a dual inferior vena cava, one on each side of the posterior aspect of the abdominal aorta (single arrow).
TECHNIQUE: Axial CT Aortogram, venous phase, 302 mAS, 100 KV, 3mm slice thickness, venous phase, 75 ml Omnipaque 350
Figure 4.
A 73-year-old lady with an inflammatory aortic aneurysm on initial CT Aortogram 4 months prior to presentation with aortoenteric fistula and catastrophic hemorrhage.
FINDINGS: Coronal slice of CT Aortogram in the arterial phase demonstrates a perianeurysmal cuff of fibrotic soft tissue (curved arrow) around a fusiform abdominal aortic aneurysm with saccular outpouchings.
TECHNIQUE: CT Aortogram, coronal multiplanar reconstruction, 163 mAS, 100KV, 3mm slice thickness, arterial phase, 75 ml Omnipaque 350
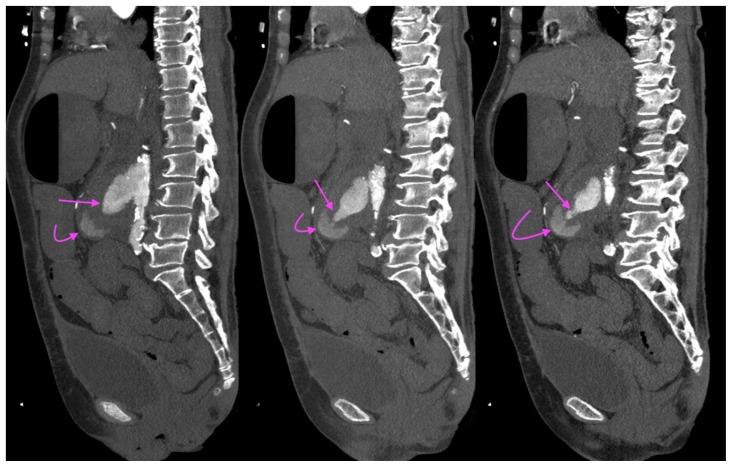
Figure 5.
A 73-year-old lady with an inflammatory aortic aneurysm on initial CT Aortogram 4 months prior to presentation with aortoenteric fistula and catastrophic hemorrhage.
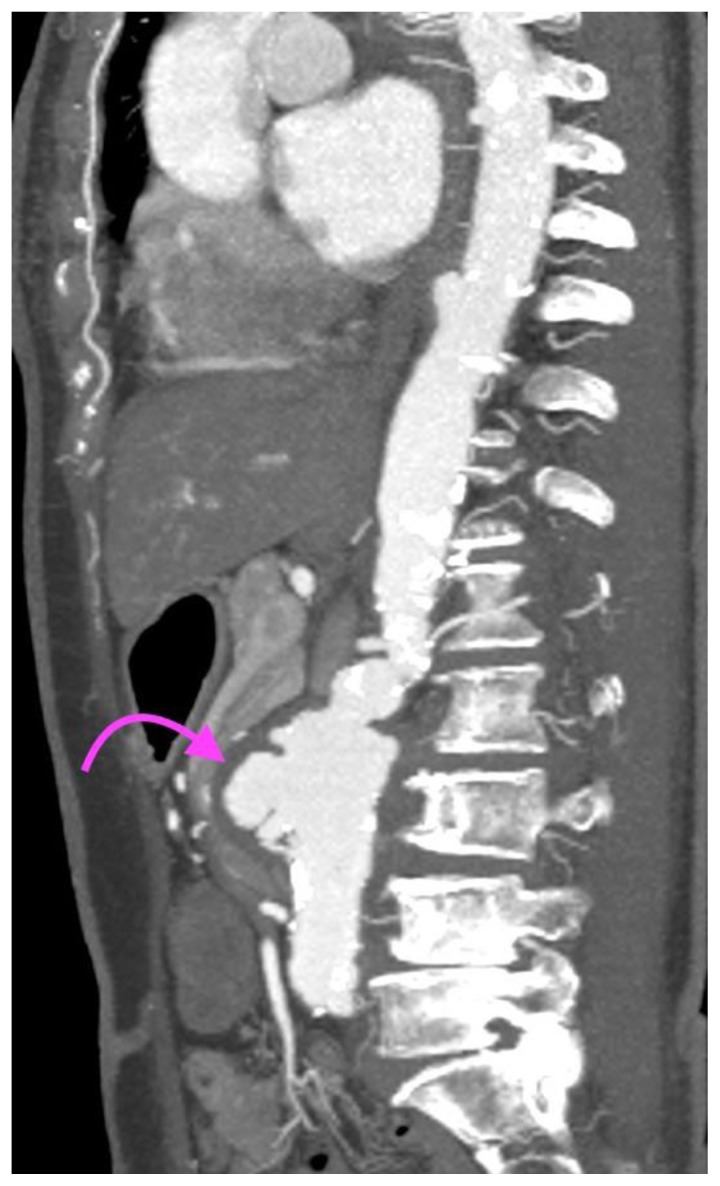
FINDINGS: Sagittal CT Aortogram in the arterial phase demonstrates a perianeurysmal cuff of fibrotic soft tissue (curved arrow) around a fusiform abdominal aortic aneurysm with saccular outpouchings.
TECHNIQUE: CT Aortogram, sagittal MIP (maximum intensity projection), 10mm slice thickness, arterial phase, 163 mAS, 100 KV, 75 ml Omnipaque 350
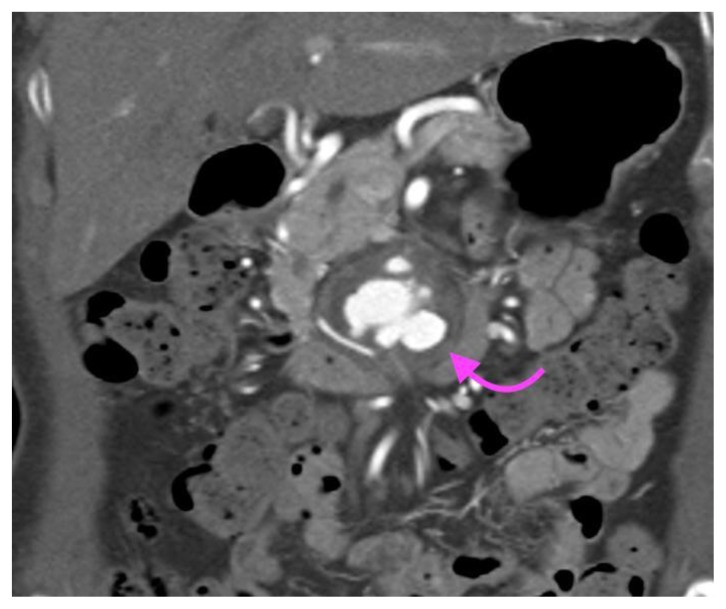
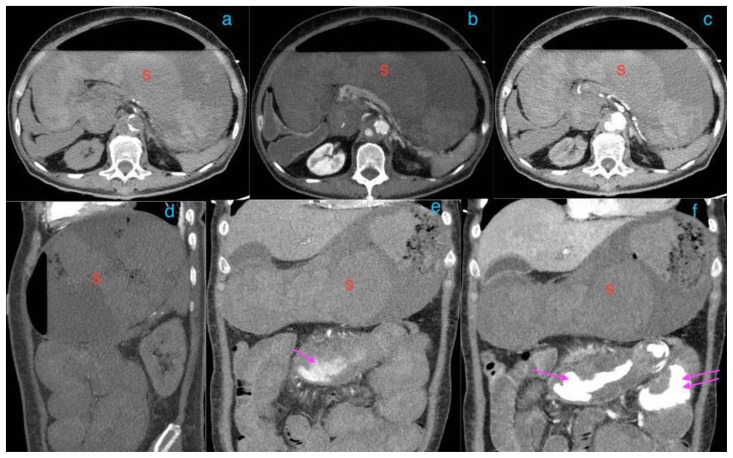
Figure 6.
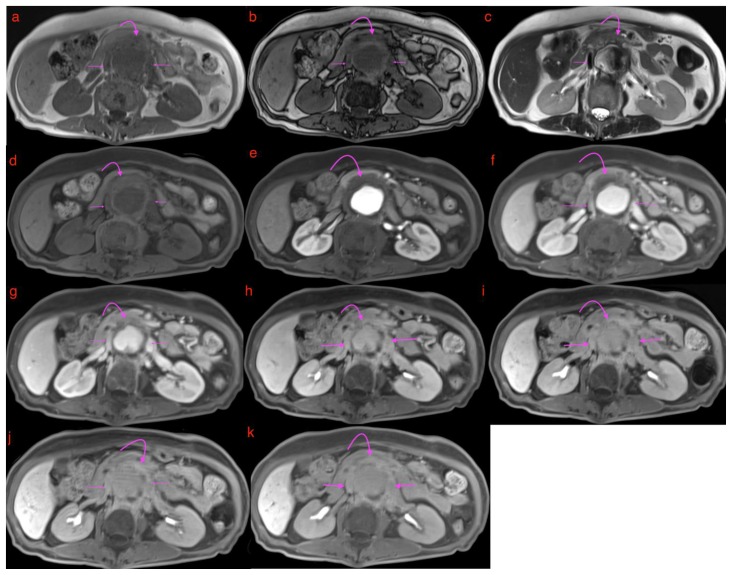
A 73-year-old lady with an inflammatory aortic aneurysm depicted on MR liver (with primovist) performed for the purpose of further characterization for an incidental liver lesion picked up on CT aortogram performed earlier.
FINDINGS: MRI liver with primovist shows aortic wall thickening and a cuff of perianeurysmal soft tissue (curved arrow) which shows intermediate T2 signal and progressive/persistent enhancement into the 20-minute phase with primovist. Double arrows point to dual inferior vena cava tethered to the inflammatory abdominal aortic aneurysm with a cleft like appearance. Note also elongation and draping of the duodenum over the inflammatory abdominal aortic aneurysm.
TECHNIQUE: MRI liver with primovist, axial, 6(a)T1 Volumetric Interpolated Breath-hold Examination(VIBE) Dixon in phase (TR/TE 4.05/2.58, 3mm slice thickness), 6(b)T1 Volumetric Interpolated Breath-hold Examination(VIBE) Dixon oppose phase (TR/TE 4.05/1.35, 3mm slice thickness), 6(c)T2 Half-fourier-acquired single-shot turbo spin echo (HASTE) (TR/TE 1400/84, 5mm slice thickness), 6(d)T1 fat saturated pre contrast( TR/TE: 3.36/1.03, 3mm slice thickness), T1 dynavibe fat saturated with contrast at 6(e)1st, measurement(arterial phase), 6(f) 2nd measurement(venous phase), 6(g) 3rd measurement(equilibrium phase), 6(h) 5 minutes delayed, 6(i) 10 minutes delayed, 6(j) 15minutes delayed, 6(k) 20 minutes delayed ( TR/TE: 3.36/1.03, 3mm slice thickness, 9ml intravenous primovist).
Figure 7.
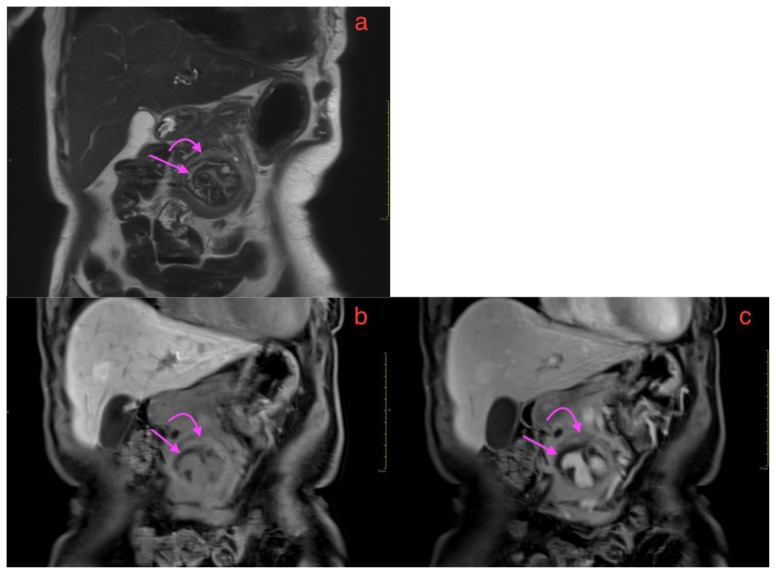
A 73-year-old lady with an inflammatory aortic aneurysm depicted on coronal MRI liver (with primovist), performed for the purpose of further characterization for an incidental liver lesion picked up on CT aortogram performed earlier.
FINDINGS: Coronal MRI liver with primovist shows a fusiform abdominal aortic aneurysm with saccular outpouchings, T2 hyperintense non enhancing peripheral thrombi(straight arrow), aortic wall thickening and a cuff of perianeurysmal soft tissue(curved arrow) which shows intermediate T2 signal and enhancement in the 7(b) 5 minutes delayed and 7(c) 20 minutes phase with primovist.
TECHNIQUE: MR liver with primovist (a) Coronal oblique T2 HASTE (TR/TE 1800/101, slice thickness 3mm), Coronal T1 dynavibe fat saturated with contrast at 7(b) 5 minutes delayed and 7(c) 20 minutes delayed (TR/TE: 3.36/1.05, slice thickness 3mm, 9ml intravenous primovist)
She was subsequently listed for elective endovascular repair of the aneurysm. At the preadmission clinic, however, she was found to have a drop-in hemoglobin level from her baseline of 9.7g/dL to 7g/dL. She was hemodynamically stable and reported having no further episodes of abdominal pain since her first ultrasound scan. She also denied symptoms related to anemia or per rectal bleeding. She was admitted on the same day for further management and was transfused with a pint of packed red cells.
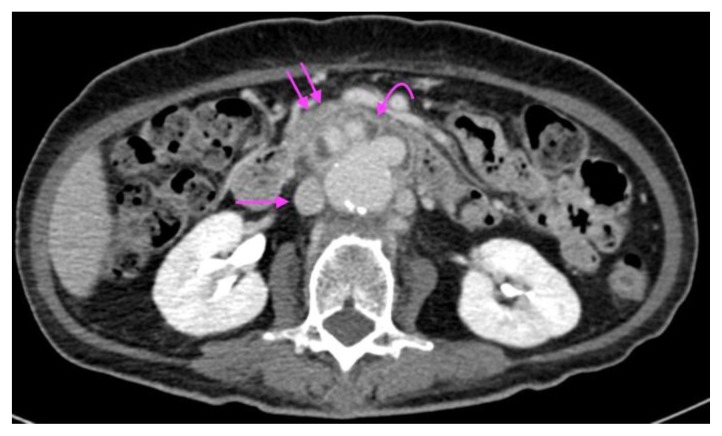
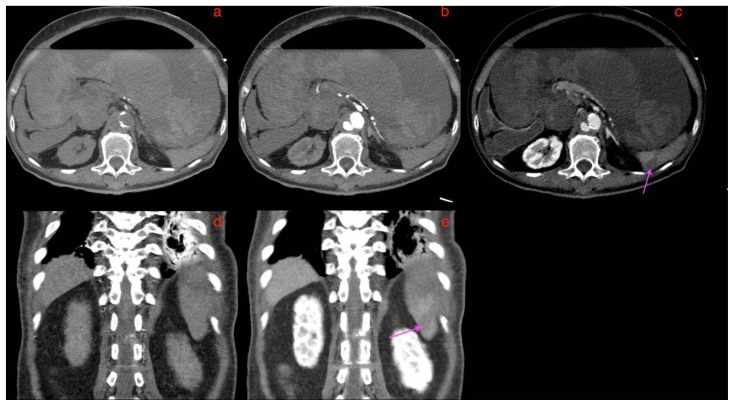
The following day, she had an episode of hematemesis and was found to be pale, diaphoretic and drowsy, with a blood pressure of 70/50 mmHg and tachycardia, compatible with hemorrhagic shock. Immediate Code Blue activation, fluid, and packed red cell transfusion, as well as Stat 80 mg of esomeprazole followed by 8mg/hr infusion, were performed. Shortly after, she had another 2 further episodes of fresh hematemesis as well as fresh melena. A stat CT mesenteric angiogram was performed, which showed an increase in the size of the fusiform infrarenal aortic aneurysm from 4.8 × 4.7cm to 5.7 × 6.7cm over a period of 4 months as well as active arterial contrast extravasation from the infra-renal aortic aneurysm into the junction of the 2nd and 3rd part of the duodenum (Figures 8, 9, 10, 11, 12, 13). Findings were compatible with an aortoenteric fistula (AEF). The gastric wall was thin and hypo-enhancing, raising concern for mural ischemia (Figure 14). New wedge shaped hypodensities in the right renal lower pole (Figure 16) and spleen (Figure17) were observed, likely representing infarcts. Hyper-enhancing adrenal glands (Figure 15b) along with a slit-like inferior vena cava (Figure 15) were compatible with the hypoperfusion complex.
Figure 8.
A 73-year-old lady with an inflammatory abdominal aortic aneurysm causing a primary aortoenteric fistula with catastrophic hemorrhage.
FINDINGS: Unenhanced axial CT mesenteric angiogram demonstrates hyperdense contents within the D3 segment of the duodenum compatible with acute blood products (curved arrow). Straight arrow points to the abdominal aortic aneurysm.
TECHNIQUE: Axial CT mesenteric angiogram, plain, 138 mAS, 100 KV, 3mm slice thickness, nil contrast
Figure 9.
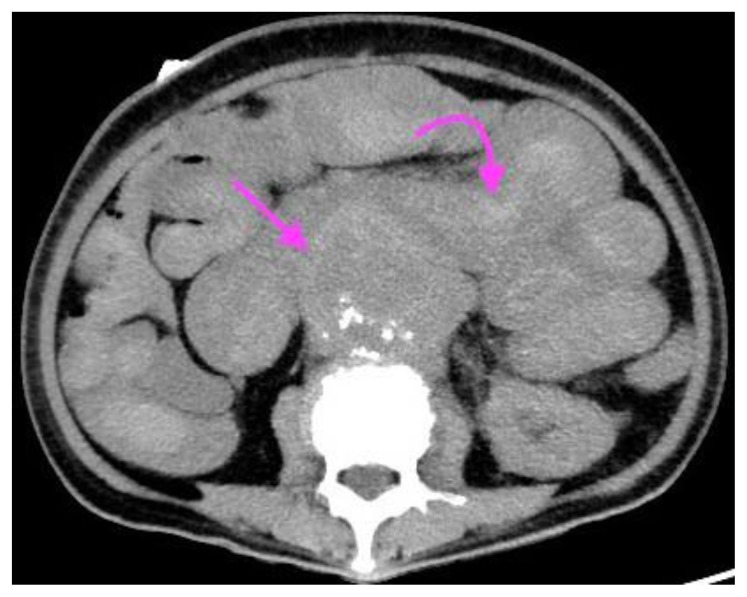
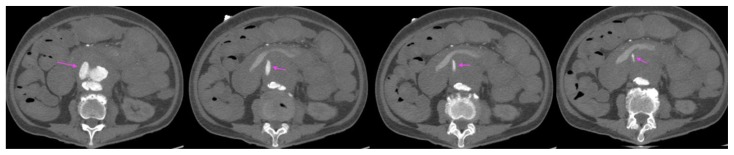
A 73-year-old lady with an inflammatory abdominal aortic aneurysm causing a primary aortoenteric fistula with catastrophic hemorrhage.
FINDINGS: axial CT mesenteric angiogram in the arterial phase demonstrates a jet of contrast extravasation from the abdominal aortic aneurysm to the D2/3 segment of the duodenum (straight arrow), with contrast filling of the duodenum.
TECHNIQUE: Axial CT mesenteric angiogram, arterial phase,116 mAS, 100 KV, 3mm slice thickness, 75 ml of omnipaque 350
Figure 10.
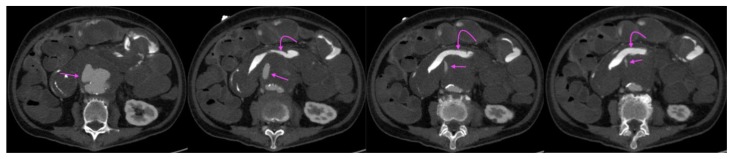
A 73-year-old lady with an inflammatory abdominal aortic aneurysm causing a primary aortoenteric fistula with catastrophic hemorrhage.
FINDINGS: axial CT mesenteric angiogram in the venous phase demonstrates a jet of contrast extravasation from the abdominal aortic aneurysm to the D2/3 segment of the duodenum (straight arrow), with contrast accumulation within the duodenum (curved arrow).
TECHNIQUE: Axial CT mesenteric angiogram, venous phase,116 mAS, 100 KV, 3mm slice thickness, 75 ml of omnipaque 350
Figure 11.
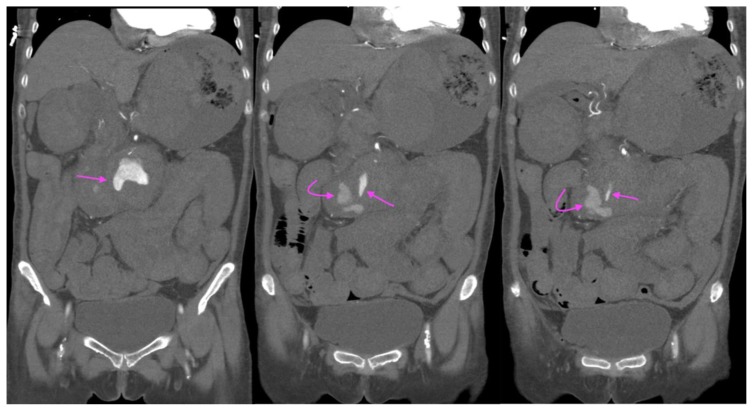
A 73-year-old lady with an inflammatory abdominal aortic aneurysm causing a primary aortoenteric fistula with catastrophic hemorrhage.
FINDINGS: CT mesenteric angiogram coronal multiplanar reconstruction in the arterial phase demonstrates a jet of contrast extravasation from the abdominal aortic aneurysm to the D2/3 segment of the duodenum (straight arrow), with contrast filling of the duodenum (curved arrow).
TECHNIQUE: CT mesenteric angiogram, coronal multiplanar reconstruction, arterial phase, 116 mAS, 100 KV, 3mm slice thickness, 75 ml of omnipaque 350
Figure 12.
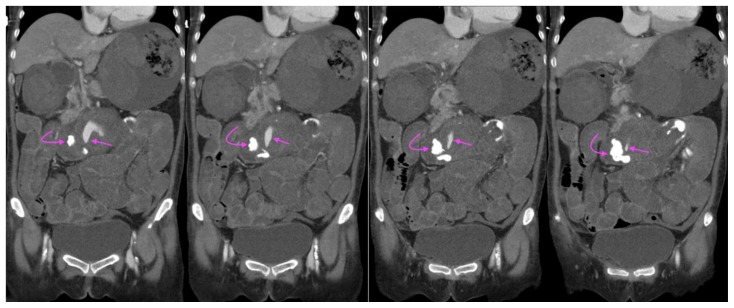
A 73-year-old lady with an inflammatory abdominal aortic aneurysm causing a primary aortoenteric fistula.
FINDINGS: CT mesenteric angiogram coronal multiplanar reconstruction in the venous phase demonstrates a jet of contrast extravasation from the abdominal aortic aneurysm to the D2/3 segment of the duodenum (straight arrow), with contrast accumulation within the duodenum (curved arrow).
TECHNIQUE: CT mesenteric angiogram, coronal multiplanar reconstruction, venous phase, 116 mAS, 100 KV, 3mm slice thickness, 75 ml of omnipaque 350
Figure 13.
A 73-year-old lady with an inflammatory abdominal aortic aneurysm causing a primary aortoenteric fistula with catastrophic hemorrhage.
FINDINGS: CT mesenteric angiogram Sagittal maximum intensity projection (MIP) in the arterial phase demonstrates a jet of contrast extravasation from the abdominal aortic aneurysm to the D2/3 segment of the duodenum (straight arrow), with contrast accumulation within the duodenum (curved arrow).
TECHNIQUE: CT mesenteric angiogram, sagittal, maximum intensity projection (MIP), arterial phase, 116 mAS, 100 KV, 3mm slice thickness, 75 ml of omnipaque 350
Figure 14.
A 73-year-old lady with an inflammatory aortic aneurysm causing a primary aortoenteric fistula with catastrophic hemorrhage.
FINDINGS: CT mesenteric angiogram in the axial 14(a)plain 14(b) arterial 14(c) venous phase and coronal multiplanar reconstruction in the 14(d) plain, 14(e) arterial, 14(d) venous phase. There is marked distension of the stomach (labelled S), with thin, poorly enhancing walls, suggestive of ischemia. Note hyperdense acute hematoma within the stomach in image 14(a) and 14(d), with contrast accumulation within the duodenum (single straight arrow) and jejunum (double straight arrows) in image 14(e) and 14(f).
TECHNIQUE: CT mesenteric angiogram in the axial 14(a)plain 14(b) arterial 14(c) venous phase and coronal multiplanar reconstruction in the 14(d) plain, 14(e) arterial 14(d) venous phase, 116 mAS, 100 KV, 3mm slice thickness, 75 ml of omnipaque 350
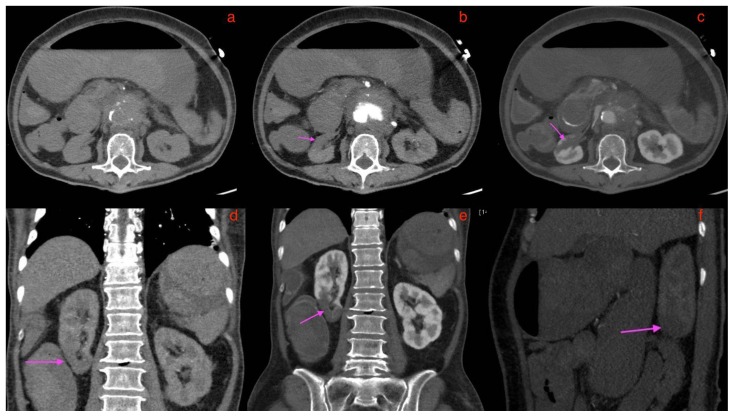
Figure 16.
A 73-year-old lady with an inflammatory abdominal aortic aneurysm causing a primary aortoenteric fistula with catastrophic hemorrhage, demonstrating right lower renal pole infarct.
FINDINGS: CT mesenteric angiogram in the axial 16(a) plain 16(b) arterial 16(c) venous phases, coronal multiplanar reconstruction in the 16(d) arterial and 16(b) venous phases, and sagittal maximum intensity projection in the 16(f)-arterial phase. Pink arrows in 16 b, c, d, e and f point to a wedge shaped right lower renal pole hypodensity representing an infarct. This is not well seen in the plain axial section of 16(a)
TECHNIQUE: CT mesenteric angiogram in the axial 16(a)plain 16(b) arterial 16(c) venous phase, coronal multiplanar reconstruction in the 16(d) arterial 16(e) venous phase, sagittal maximum intensity projection in the 16(f) arterial phase, 116 mAS, 100 KV, 3mm slice thickness, 75 ml of omnipaque 350
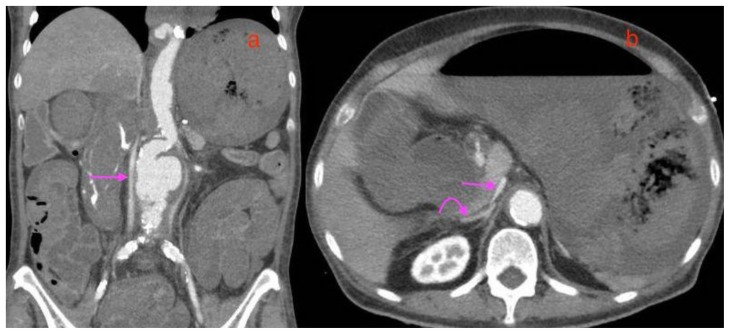
Figure 15.
A 73-year-old lady with an inflammatory abdominal aortic aneurysm causing a primary aortoenteric fistula with catastrophic hemorrhage, demonstrating the features of the CT hypoperfusion complex.
FINDINGS: 15(a) Coronal multiplanar reconstruction CT mesenteric angiogram in the arterial phase and 15 (b) axial venous phase. Single straight arrows in 15(a) and 15(b) point to the collapsed slit like inferior vena cava and curved arrow in (b) points to the hyperenhancing right adrenal gland, features of the CT hypoperfusion complex.
TECHNIQUE: CT mesenteric angiogram, 15(a) Coronal multiplanar reconstruction, arterial phase and 15(b) axial venous phase, 116 mAS, 100 KV, 3mm slice thickness, 75 ml of omnipaque 350
The patient was sent immediately for an emergency laparotomy. Intraoperative findings revealed an inflammatory abdominal aortic aneurysm complicated by an aortoenteric fistula, with large amounts of clots in the stomach and small bowel. The stomach was markedly distended and appeared ischemic, with large perforation along the lesser curve and gross peritoneal contamination. Some bleeding was also noted from the spleen. Ligation of the infrarenal aorta, axillo-bifemoral bypass, gastrectomy, splenectomy was performed. As the patient was coagulopathic and actively oozing, a decision was made for packing and temporary abdominal closure, with plans for a relook laparotomy in 24–48 hours after the correction of coagulopathy.
Shortly after, the patient suffered a cardiac arrest with pulseless electrical activity, and cardiopulmonary resuscitation was started. There was a return of spontaneous circulation after 5 minutes. Upon transfer to the surgical intensive care unit, continuous bleeding from the abdominal wound was noted. She was transfused with packed red cells, cryosupernatant plasma, cryoprecipitate, fresh frozen plasma and prothrombin complex concentrate. Her blood pressure was supported with adrenaline and noradrenaline.
The patient subsequently had an asystolic collapse the following day, with unsuccessful resuscitation.
DISCUSSION
Etiology & Demographics
Inflammatory abdominal aortic aneurysms comprise 5%–25% of all abdominal aortic aneurysms [1] and are defined by the presence of a thickened aneurysm wall, marked peri-aneurysmal and retroperitoneal fibrosis and dense adhesions to surrounding structures [2]. Inflammatory abdominal aortic aneurysms are more common in males, with a male to female ratio of 80% to 20%, [3]. The mean age of occurrence ranges from 62 to 68 years, which is 5–10 years younger than the mean age of patients with atherosclerotic aneurysms [2]. Smoking is also a known major risk factor [4,5]. The etiology of inflammatory aneurysms is poorly understood but is thought to be related to periaortic retroperitoneal fibrosis and various autoimmune diseases, including rheumatoid arthritis, systemic lupus erythematosus, giant cell arteritis and Takayasu’s arteritis [6,7].
Aortoenteric fistulas can be divided into primary and secondary forms [8].
A primary aortoenteric fistula is a direct communication between the native aorta and the adjacent bowel in a patient with no previous aortic surgery or trauma. Primary aortoenteric fistulas are rare, with the annual incidence of such fistulas estimated to be 0.007 per million [8]. The most common predisposing conditions for primary aortoenteric fistulas are atherosclerotic abdominal aortic aneurysms or penetrating atherosclerotic ulcers [9,10,11,12,13]. Less commonly encountered causes include inflammatory or infectious aortitis, diverticulitis, appendicitis, foreign bodies and gastrointestinal malignancies [9,10].
Secondary aortoenteric fistulas are more common with an annual incidence of 0.6–2% and occur as complications of aortic reconstructive surgery with or without the placement of an aortic stent-graft [8]. The cause of secondary fistulas is thought to be the result of chronic perigraft infection or prolonged pressure upon the bowel by a graft [8,9,14,15]. Secondary fistulas that result from perigraft infection may occur between 2 weeks and 10 years after surgery [8]. Patients at particular risk include those who undergo emergent surgery for a ruptured aneurysm, have postoperative complications such as bowel injury, and those with endoleaks or stent migration [9,16].
Clinical & Imaging findings
In contrast to atherosclerotic aneurysms, most inflammatory aneurysms are symptomatic. The triad of abdominal or back pain, weight loss, and an elevated erythrocyte sedimentation rate (ESR) in a patient with an AAA is highly suggestive of one with an inflammatory component [2]. A significant amount of weight loss and anorexia have been reported in up to 40% of patients with an inflammatory abdominal aortic aneurysm [2,17,18]. In approximately 20%–30% of patients, hydronephrosis or renal failure is present at the time of diagnosis because the inflammatory process involves one or both ureters [6].
Contrast-enhanced CT has been reported to have 83% sensitivity and almost 100% specificity for the diagnosis of inflammatory aneurysms [1]. Idiopathic inflammatory aneurysms differ from atherosclerotic aneurysms because of the presence of dense perianeurysmal fibrosis and a thickened aortic wall [1]. Periaortic wall thickening typically affects the anterior wall and spares the posterior wall [1,18,19]. Delayed enhancement of the soft-tissue component is usually present [1,19]. When the dense perianeurysmal fibrosis entraps adjacent structures such as the bowel(most commonly duodenum) and inferior vena cava, imaging features which could provide clues of this include elongation or draping of the bowel loops over the inflammatory abdominal aortic aneurysm, and distortion or narrowing of the inferior vena cava producing a cleft like or comma-shaped appearance [20]. On MRI, periaortic fibrotic tissue is of intermediate to low T2 signal [20].
Ultrasound features of an inflammatory abdominal aortic aneurysm would include a hypoechoic mass that represents the inflammatory process with surrounding echogenic tissue and thickening of the aortic wall [1]. Recognition of a cuff of soft tissue around the calcified margin of the aneurysm should prompt the possibility of an inflammatory component [2]. The detection of hydroureteronephrosis in the absence of an obvious pelvic cause for the obstruction should also arouse suspicion for this [2].
The classic triad of gastrointestinal bleeding, abdominal pain, and palpable mass is found in only 11% of patients with aortoenteric fistulas [7,21]. However, the clinical picture of gastrointestinal bleeding is critical to the diagnosis of aortoenteric fistula, especially in differentiating it from other entities such as perigraft infection without fistulation. In a study by Kim JY et al, 60 % of patients had “herald bleeding”, defined as an episode of minor and brisk bleeding which stops spontaneously [22]. In this study, the time interval between the initial bleeding and massive gastrointestinal hemorrhage ranged from one hour to two days, providing a window period for the opportunity to perform definitive surgical treatment for the aortoenteric fistula [22].
Aortoenteric fistulas can occur with any part of the gastrointestinal tract, the most common location is the transverse portion of the duodenum(D3), which is involved in 60% of cases [3,9,11,12]. This is due to its relatively fixed position as compared with the rest of the bowel, and the close relationship of this part of the duodenum to the aorta [3].
CT aortogram is currently the first-line imaging modality to evaluate an aortoenteric fistula bleed, with a widely variable sensitivity of 40%–90% and a specificity of 33%–100% [8]. In the appropriate clinical setting, contrast-enhanced CT is reported to have an overall specificity of 100% and a sensitivity of 50% in diagnosing an aortoenteric fistula [23].
The most specific signs of direct extravasation of contrast from the aorta into a bowel loop and breach of the aortic wall may not always be present on CT [8,9,14]. Similarly, the leakage of enteric contrast directly into the periaortic space is a highly specific sign, but rare especially since enteric contrast should not be administered during CT evaluation of acute gastrointestinal bleeding [8,9,24].
Other suggestive findings include hematoma in the retroperitoneum or within the bowel wall or lumen [8]. In the setting of gastrointestinal bleeding, pseudoaneurysm formation at site of fistula, focal thickening and tethering of a bowel loop to the aorta with loss of fat plane between them, periaortic gas in the absence of surgery or 3–4 weeks after surgery, and graft disruption or migration should raise the possibility of an aortoenteric fistula [8,9]. These secondary signs may be picked up on unenhanced CT or even ultrasound.
Tagged red blood cell scanning can be useful particularly in slow or intermittent bleeding from aortoenteric fistulas [8]. This can show an accumulation of radiotracer within the bowel lumen or retroperitoneum, and diagnostic confidence can be improved with the addition of Single-Photon Emission Computed Tomography/Computed Tomography (SPECT/CT) [8].
Though MRI may have similar specificity and sensitivity to CT, the lack of availability in the emergency setting in addition to a longer acquisition time and need for greater technical expertise often limits its use.
Treatment & Prognosis (Inflammatory Abdominal Aortic Aneurysm)
Inflammatory abdominal aortic aneurysms are generally considered to be less prone to rupture than atherosclerotic aneurysms [25,26,27]. They, however, show a tendency to enlargement and rupture that may be increased by corticosteroid therapy [17,25,28,29,30].
The goals of inflammatory abdominal aortic aneurysm treatment are the prevention or correction of aortic rupture and the correction of periaortic fibrosis and hydronephrosis. This can be accomplished by open repair or endovascular aneurysm repair. To date, there is no conclusive evidence as to whether open repair or endovascular repair is the best treatment for an inflammatory aortic aneurysm [25]. A Cochrane review in 2015 by Capoccia and Riambu showed no published randomized controlled trials, quasi randomized controlled trials or controlled clinical trials comparing open repair and elective endovascular repair for an inflammatory abdominal aortic aneurysm for immediate (30-day), intermediate (up to one-year follow-up) and long-term (more than one-year follow-up) mortality or complications rates. Compared to an atherosclerotic abdominal aortic aneurysm, open repair of an inflammatory abdominal aortic aneurysm carries increased risks of iatrogenic injury to adjacent organs and structures such as the vena cava, ureters, and bowel, in view of the presence of periaortic fibrotic adhesions that would require extensive adhesiolysis and surgical dissection [25].
After taking into account factors such as the individual patient’s comorbidities, the potential for complications, and accessibility for repair, endovascular aneurysm repair can be considered as an alternative if anatomically suitable [31]. In a recent large systematic review by Paravastu and colleagues in 2009, endovascular aneurysm repair was found to be associated with lower all-cause mortality at 1 year compared to open surgical repair (2 % in endovascular aneurysm repair versus 14 % in open surgical repair, p = 0.02) [32]. However, while endovascular aneurysm repair and open repair show comparable regression rates for periaortic fibrosis, endovascular aneurysm repair show lower regression rates for hydronephrosis compared to open repair. Furthermore, endovascular aneurysm repair may not last as well and may not be as effective in relieving inflammation, with secondary reintervention rates reported to be as high as 22% [25]. In the absence of clinically apparent complications, endovascular aneurysm repair would also require a more rigorous follow-up to evaluate for endoleak, graft migration, and evidence of device failure, underscoring the importance of patient compliance to follow-up reviews and imaging [31].
Treatment & Prognosis (Primary Aortoenteric Fistula)
Early diagnosis and prompt surgical treatment are crucial, as mortality in the absence of intervention is virtually 100% [9,22].
Two primary concerns for the treatment of an aortoenteric fistula are achieving adequate control of hemorrhage at the time of presentation and reducing the risk of late complications associated with bleeding or infection [22].
Aortoenteric fistulas can be managed via an endovascular approach or open surgery. Open surgical options include resection of the diseased segment or infected graft and creation of an extra-anatomic bypass graft (extra-anatomic reconstruction, EAR) or in situ aortic reconstruction (ISR) using a prosthetic graft, antibiotic-impregnated prosthetic graft, autogenous femoral vein graft, or cryopreserved aortic allograft [22]. Extra-anatomic bypass grafting is known to be associated with remarkably high mortality rates (up to 90%), along with a high rate of limb amputations (up to 50%) and aortic stump disruptions (up to 38%) [9].
Key factors to be taken into consideration upon deciding the appropriate treatment method include the patient’s hemodynamic state and the estimated life expectancy according to known comorbidities. If the patient is hemodynamically unstable or has a limited life expectancy, endovascular aortic repair (with aggressive infectious treatment) offers a minimally invasive treatment to close the fistula, stop the bleeding, provide time for further stabilization or as a palliative measure [16,33]. Important benefits of endovascular stenting are the avoidance of a large incision, aortic cross-clamping, interference with respiratory function, revascularization, and significant blood loss [34]. After the patient has made a full recovery, a discussion can be held with the patient regarding the necessity for a second definitive open operation to avoid late reinfection with its disastrous consequences [33].
If the patient is hemodynamically stable upon presentation and has a reasonable life expectancy, an open treatment is usually indicated [33]. The type of surgery is dependent on surgical findings- axillobifemoral bypass for periaortic purulence or in situ aortic reconstruction for no gross purulence [22]. Both involve periaortic debridement, irrigation with antiseptic solution, and coverage of the aortic stump or graft with a pedicled greater omentum.
Postoperatively, antibiotic treatment is recommended for at least 1 week following negative cultures and systemic antibiotic treatment continued for 4 – 6 weeks if cultures prove positive [21].
Differential diagnosis for Inflammatory Abdominal Aortic Aneurysms and Primary Aortoenteric Fistulas
Retroperitoneal fibrosis
At CT, retroperitoneal fibrosis manifests as a rind-like layer of fibroinflammatory soft-tissue that envelops the aorta, usually in the region of the level of the renal arteries to the iliac vessels, often progressing through the retroperitoneum to envelop the inferior vena cava and ureters with consequent vascular compression and obstructive hydroureteronephrosis [35,36]. The irregular periaortic soft tissue mass usually lies anterior and lateral to the aorta, spares the posterior aspect, and does not typically displace the aorta off the spine unlike lymphadenopathy from lymphoma [36].
Unlike aortoenteric fistula, retroperitoneal fibrosis is not associated with periaortic ectopic gas. Retroperitoneal fibrosis may be focal or diffuse, symmetric or off the midline, well defined or infiltrating. The soft-tissue plaque enhances to varying degrees depending on the stage of maturation [37]. In the early stages of the disease, avid enhancement may be present, whereas, in the late, inactive stages, little or absent enhancement may be seen [37,38].
Typical features on MRI include low signal intensity on T1 and variable signal intensity on T2 sequences depending on the degree of associated active inflammation (hypercellularity and edema). In early idiopathic retroperitoneal fibrosis, active inflammation is predominant and would demonstrate high T2 signal intensity and early contrast enhancement [38, 39]. Conversely, the late inactive stage is relatively acellular and hypovascular, with predominant fibrosis, hence usually exhibiting low T2 signal and little or no contrast enhancement [38,39].
Ultrasonography has poor overall sensitivity in the detection of retroperitoneal fibrosis with overlying gas- or fluid-filled bowel loops obscuring early subtle changes [36,40]. Typically, a well-demarcated hypoechoic or isoechoic retroperitoneal mass with irregular contours anterior to the lower lumbar spine or sacral promontory may be seen with ultrasound [36,37,41].
Increased uptake at Gallium-67 Scintigraphy and 18Fluorine-fluorodeoxyglucose Positron Emission Tomography/Computed Tomography (18F-FDG PET/CT) is particularly common during the active inflammatory stage [1]. 18F-FDG PET/CT may also reveal remote sites of disease, determine disease activity and guide immunosuppressive therapy [36].
Mycotic aneurysm
Mycotic aneurysms can be similar to inflammatory abdominal aortic aneurysms in morphologic appearance. They differ in clinical presentation of sepsis with positive microbiological culture results and may have periaortic gas or abscess or infective complications of the adjacent structures. Periaortic gas is not associated with inflammatory abdominal aortic aneurysms unless they are complicated by aortoenteric fistula.
The most common location for a mycotic aneurysm is the infrarenal aorta, followed by the descending thoracic aorta, thoracoabdominal aorta, juxta renal aorta, and ascending aorta [1,42].
Mycotic aneurysms are mostly saccular (> 90%) rather than fusiform in atherosclerotic aneurysms, and the aneurysm can be multiloculated [1,42]. On CT, concentric or eccentric periaortic inflammatory soft tissue, fat stranding, fluid or abscess may be seen. The periaortic inflammatory soft tissue may appear as a hypoattenuating concentric rim with homogeneous contrast enhancement or appear heterogeneous in attenuation with poor contrast enhancement or rim enhancement upon developing necrosis [1]. Periaortic gas may be present, though uncommon [43].
Adjacent structures may be involved, for example causing spondylodiscitis and psoas abscess formation [1]. Rapid aneurysmal expansion of the aorta over a short period of time (as short as 7 days from the onset of symptoms) on serial CT examinations should arouse the suspicion of a mycotic aneurysm [6,42,44]. Mycotic aneurysms are also prone to rupture/hemorrhage due to the friable nature of their wall.
At MRI, the aneurysm may show similar morphologic features as in CT. The wall of the aneurysm can show intense enhancement, which may be most apparent on black blood post-contrast sequences. Periaortic edema has low signal intensity on T1-weighted images and high signal intensity on T2-weighted images [43,45]. Similarly, the periaortic soft tissue mass is usually low signal intensity on T1, high signal on T2 and demonstrate uniform enhancement [43]. The inflammatory mass can develop necrosis, for which variable signal intensity, rim enhancement or poor enhancement after administration of contrast material may be seen [43].
Ultrasound is not reliable as an initial imaging modality for the diagnosis of infected abdominal aortic aneurysms. Its use is mainly limited to the peripheral arteries [43]. At gray-scale sonography, a mycotic aneurysm can appear as a hypoechoic structure with flow demonstrated on color or spectral doppler imaging, in association with perilesional hypoechoic or heterogeneous inflammatory soft tissue or hematoma. [43].
Scintigraphy with SPECT using radiotracers Gallium-67 citrate and 111-In labelled white blood cells would show increased tracer uptake within the soft tissues surrounding the aneurysm [46,47]. Mycotic aneurysms also tend to show FDG-avidity on 18F-FDG PET/CT, sometimes of 4.5 SUV max or more compared with non-infected aneurysms [44,48].
Infectious aortitis
Infectious aortitis is inflammation of the aorta caused by a bacterial, viral, or fungal infection.
Infectious aortitis and infected (mycotic) aortic aneurysm represent different points along the same disease spectrum: The aorta maintains its native caliber in infectious aortitis, but in an infected aortic aneurysm, the aorta is dilated and may rupture.
Save for its normal caliber, imaging features of infectious aortitis are otherwise similar to mycotic aneurysm. This includes include irregular thickened arterial wall, periaortic fluid, stranding, soft tissue and gas [1,49]. Intramural gas may be present [1,49]. However, in some cases of infectious aortitis, the aorta may appear normal. 18F-FDG PET/CT may show FDG-avidity of the affected aortic wall segment. There are limited case reports of increased radiotracer uptake with 111 In–labeled white blood cells [50].
TEACHING POINT
Inflammatory abdominal aortic aneurysm is a rare cause for primary aortoenteric fistula and its consequences are catastrophic if not promptly managed. While rare, the presence of a contrast jet from the aorta to the duodenum is diagnostic of an aortoenteric fistula.
Figure 17.
A 73-year-old lady with an inflammatory abdominal aortic aneurysm causing a primary aortoenteric fistula with catastrophic hemorrhage, demonstrating a focal splenic infarct.
FINDINGS: CT mesenteric angiogram in the axial 17(a) plain 17(b) arterial 17(c) venous phases, coronal multiplanar reconstruction in the 17(d) arterial and 17(e) venous phases. Pink arrows in 17(c) and 17(e) point to a wedge shaped hypodensity in the spleen representing an infarct. This is not well seen in the 17(a) axial plain phase, 17(b) axial arterial phase and 17(c) coronal arterial phase.
TECHNIQUE: CT mesenteric angiogram in the axial 17(a)plain 17(b) arterial 17(c) venous phase, coronal multiplanar reconstruction in the 17(d) arterial 17(e) venous phase, 116 mAS, 100 KV, 3mm slice thickness, 75 ml of omnipaque 350
Table 1.
Summary table for Inflammatory Abdominal Aortic Aneurysm
| Etiology |
|
| Incidence |
|
| Gender ratio |
|
| Age predilection |
|
| Risk factors |
|
| Treatment |
|
| Prognosis |
|
| Findings on imaging |
|
Table 2.
Summary table for Primary Aortoenteric Fistula
| Etiology |
|
| Incidence |
|
| Gender ratio | -- |
| Age predilection | -- |
| Risk factors |
|
| Treatment |
|
| Prognosis |
|
| Findings on imaging |
|
Table 3.
Differential diagnosis table for Primary Aortoenteric Fistula (AEF) and Inflammatory Abdominal Aortic Aneurysm
| DIAGNOSIS | CT | MR | ULTRASOUND | NUCLEAR IMAGING |
|---|---|---|---|---|
| Primary AEF |
|
|
|
|
| Inflammatory abdominal aortic aneurysm |
|
|
|
|
| Retroperitoneal fibrosis |
|
|
|
|
| Mycotic aneurysm |
|
|
|
|
| Infectious aortitis |
|
|
|
|
ACKNOWLEDGEMENTS
Dr. Wan Ying Chan
ABBREVIATIONS
- AEF
Aortoenteric fistula
- EVAR
Endovascular aneurysm repair
- IAAA
Inflammatory abdominal aortic aneurysm
REFERENCES
- 1.Restrepo CS, Ocazionez D, Suri R, Vargas D. Aortitis: imaging spectrum of the infectious and inflammatory conditions of the aorta. Radiographics. 2011 Mar-Apr;31(2):435–51. doi: 10.1148/rg.312105069.. [DOI] [PubMed] [Google Scholar]
- 2.Tang T, Boyle JR, Dixon AK, Varty K. Inflammatory abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2005 Apr;29(4):353–62. doi: 10.1016/j.ejvs.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Sintler M, Howell N, Mahmood A, Vohra RK. Ruptured inflammatory aortic aneurysm with aortoenteric fistula and infected with Streptococcus pneumoniae: a review of the literature. Indian J Surg. 2008 Jun;70(3):138–41. doi: 10.1007/s12262-008-0038-4. Epub 2008 Jul 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rasmussen TE, Hallett JW., Jr Inflammatory aortic aneurysms. A clinical review with new perspectives in pathogenesis. Ann Surg. 1997 Feb;225(2):155–164. doi: 10.1097/00000658-199702000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iijima M, Azuma R, Hieda T, Makino Y. A surgical case of inflammatory abdominal aortic aneurysm that responded remarkably to preoperative steroid therapy. J Surg Case Rep. 2018 Feb 20;2018(2):rjy020. doi: 10.1093/jscr/rjy020. eCollection 2018 Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rakita D, Newatia A, Hines JJ, Siegel DN, Friedman B. Spectrum of CT findings in rupture and impending rupture of abdominal aortic aneurysms. Radiographics. 2007 Mar-Apr;27(2):497–507. doi: 10.1148/rg.272065026. [DOI] [PubMed] [Google Scholar]
- 7.Nehme F, Rowe K, Munguti C, Nassif I. A Rare Cause of Primary Aortoenteric Fistula: Streptococcus parasanguinis Aortitis. Case Rep Gastrointest Med. 2017;2017 doi: 10.1155/2017/9087308. 9087308. Epub 2017 Jan 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vu QD, Menias CO, Bhalla S, Peterson C, Wang LL, Balfe DM. Aortoenteric fistulas: CT features and potential mimics. Radiographics. 2009 Jan-Feb;29(1):197–209. doi: 10.1148/rg.291075185.. [DOI] [PubMed] [Google Scholar]
- 9.Raman SP, Kamaya A, Federle M, Fishman EK. Aortoenteric fistulas: spectrum of CT findings. Abdom Imaging. 2013 Apr;38(2):367–75. doi: 10.1007/s00261-012-9873-7. [DOI] [PubMed] [Google Scholar]
- 10.Reckless JP, McColl I, Taylor GW. Aortoenteric fistulae: an uncommon complication of abdominal aortic aneurysms. Br J Surg. 1972;59:458–460. doi: 10.1002/bjs.1800590613. [DOI] [PubMed] [Google Scholar]
- 11.Dossa CD, Pipinos II, Shepard AD, Ernst CB. Primary aortoenteric fistula. Ann Vasc Surg. 1994 Jan;8(1):113–20. doi: 10.1007/BF02133413. [DOI] [PubMed] [Google Scholar]
- 12.Tareen AH, Schroeder TV. Primary aortoenteric fistula: two new case reports and a review of 44 previously reported cases. Eur J Vasc Endovasc Surg. 1996 Jul;12(1):5–10. doi: 10.1016/s1078-5884(96)80268-1. [DOI] [PubMed] [Google Scholar]
- 13.Voorhoeve R, Moll FL, de Letter JA, Bast TJ, Wester JP, Slee PH. Primary aortoenteric fistula: report of eight new cases and review of the literature. Ann Vasc Surg. 1996 Jan;10(1):40–8. doi: 10.1007/BF02002340. [DOI] [PubMed] [Google Scholar]
- 14.Hagspiel KD, Turba UC, Bozlar U, et al. Diagnosis of aortoenteric fistulas with CT angiography. J Vasc Interv Radiol. 2007 Apr;18(4):497–504. doi: 10.1016/j.jvir.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Champion MC, Sullivan SN, Coles JC, Goldbach M, Watson WC. Aortoenteric fistula. Incidence, presentation recognition, and management. Ann Surg. 1982 Mar;195(3):314–7. doi: 10.1097/00000658-198203000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee B, Neschis DG. Late-term complication of endograft repair for aortoenteric fistula. Vasc Endovascular Surg. 2010 2010 Jul;44(5):395–8. doi: 10.1177/1538574410366761.. Epub 2010 May 18. [DOI] [PubMed] [Google Scholar]
- 17.Nitecki SS, Hallett JW, Jr, Stanson AW, Ilstrup DM, Bower TC, Cherry KJ, Jr, Gloviczki P, Pairolero PC. Inflammatory abdominal aortic aneurysms: a case-control study. J Vasc Surg. 1996;23(5):860–868. doi: 10.1016/s0741-5214(96)70249-5. [DOI] [PubMed] [Google Scholar]
- 18.Hill J, Charlesworth D. Inflammatory abdominal aortic aneurysms: a report of thirty-seven cases. Ann Vasc Surg. 1988 Oct;2(4):352–7. doi: 10.1016/S0890-5096(06)60815-7. [DOI] [PubMed] [Google Scholar]
- 19.Cullenward MJ, Scanlan KA, Pozniak MA, Acher CA. Inflammatory aortic aneurysm (periaortic fibrosis): radiologic imaging. Radiology. 1986 Apr;159(1):75–82. doi: 10.1148/radiology.159.1.3513252. [DOI] [PubMed] [Google Scholar]
- 20.Arrive L, Correas JM, Leseche G, Ghebontni L, Tubiana JM. Inflammatory aneurysms of the abdominal aorta: CT findings. AJR Am J Roentgenol. 1995 Dec;165(6):1481–4. doi: 10.2214/ajr.165.6.7484591. [DOI] [PubMed] [Google Scholar]
- 21.Saers SJ, Schlepping MR. Primary aortoenteric fistula. Br J Surg. 2005 Feb;92(2):143–52. doi: 10.1002/bjs.4928. [DOI] [PubMed] [Google Scholar]
- 22.Kim JY, Kim YW, Kim CJ, Lim HI, Kim DI, Huh S. Successful Surgical Treatment of Aortoenteric Fistula. J Korean Med Sci. 2007 Oct;22(5):846–50. doi: 10.3346/jkms.2007.22.5.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maddu KK, Telleria J, Shuaib W, Johnson JO, Khosa F. Nontraumatic acute aortic emergencies: Part 2, Pre- and postsurgical complications related to aortic aneurysm in the emergency clinical setting. AJR Am J Roentgenol. 2014 Mar;202(3):666–74. doi: 10.2214/AJR.13.11438. [DOI] [PubMed] [Google Scholar]
- 24.Peirce RM, Jenkins RH, Maceneaney P. Paraprosthetic extravasation of enteric contrast: a rare and direct sign of secondary aortoenteric fistula. AJR Am J Roentgenol. 2005;184(suppl 3):S73–S74. doi: 10.2214/ajr.184.3_supplement.01840s73. [DOI] [PubMed] [Google Scholar]
- 25.Capoccia L, Riambau V. Endovascular repair versus open repair for inflammatory abdominal aortic aneurysms. Cochrane Database Syst Rev. 2015 Apr;16(4):CD010313. doi: 10.1002/14651858.CD010313.pub2.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Downs AR, Lye CR. Inflammatory abdominal aortic aneurysm. Canadian Journal of Surgery. 1986;29(1):50–3. [PubMed] [Google Scholar]
- 27.Goldstone J, Malone JM, Moore WS. Inflammatory aneurysms of the abdominal aorta. Surgery. 1978;83(4):425–30. [PubMed] [Google Scholar]
- 28.Crawford JL, Stowe CL, Safi HJ, Hallman CH, Crawford ES. Inflammatory aneurysms of the aorta. Journal of Vascular Surgery. 1985;2(1):113–24. [PubMed] [Google Scholar]
- 29.Pennell RC, Hollier LH, Lie JT, Bernatz PE, Joyce JW, Pairolero PC, Cherry KJ, Hallett JW. Inflammatory abdominal aortic aneurysms: a thirty-year review. J Vasc Surg. 1985 Nov;2(6):859–69. [PubMed] [Google Scholar]
- 30.Sterpetti AV, Hunter WJ, Feldhaus RJ, et al. Inflammatory aneurysms of the abdominal aorta: incidence, pathologic, and etiologic considerations. Journal of Vascular Surgery. 1989;9(5):643–9. doi: 10.1067/mva.1989.vs0090643. discussion 649–50. [DOI] [PubMed] [Google Scholar]
- 31.Hart T, Milner R. Surgical Versus Endovascular Aortic Aneurysm Repair: Evidence to Guide the Optimal Approach for the Individual Patient. Curr Atheroscler Rep. 2016 Dec;18(12):76. doi: 10.1007/s11883-016-0621-2. [DOI] [PubMed] [Google Scholar]
- 32.Paravastu SC, Ghosh J, Murray D, Farquharson FG, Serracino-Inglott F, Walker MG. A systematic review of open versus endovascular repair of inflammatory abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2009 Sep;38(3):291–7. doi: 10.1016/j.ejvs.2009.05.005. Epub 2009 Jun 21. [DOI] [PubMed] [Google Scholar]
- 33.Keunen B, Houthoofd S, Daenens K, Hendriks J, Fourneau I. A Case of Primary Aortoenteric Fistula: Review of Therapeutic Challenges. Ann Vasc Surg. 2016 May;33:230.e5–230.e13. doi: 10.1016/j.avsg.2015.11.033. Epub 2016 Mar 8. [DOI] [PubMed] [Google Scholar]
- 34.Kritpracha B1, Premprabha D, Sungsiri J, Tantarattanapong W, Rookkapan S, Juntarapatin P. Endovascular therapy for infected aortic aneurysms. J Vasc Surg. 2011 Nov;54(5):1259–65. doi: 10.1016/j.jvs.2011.03.301. discussion 1265. [DOI] [PubMed] [Google Scholar]
- 35.Zhou HJ, Yan Y1, Zhou B, Lan TF, Wang XY, Li CS. Retroperitoneal Fibrosis: A Retrospective Clinical Data Analysis of 30 Patients in a 10-year Period. Chin Med J (Engl) 2015 Mar 20;128(6):804–810. doi: 10.4103/0366-6999.152648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caiafa RO, Vinuesa AS, Izquierdo RS, Brufau BP, Ayuso Colella JR, Molina CN. Retroperitoneal fibrosis: role of imaging in diagnosis and follow-up. Radiographics. 2013 Mar-Apr;33(2):535–52. doi: 10.1148/rg.332125085.. [DOI] [PubMed] [Google Scholar]
- 37.Cronin CG, Lohan DG, Blake MA, Roche C, McCarthy P, Murphy JM. Retroperitoneal fibrosis: a review of clinical features and imaging findings. AJR Am J Roentgenol. 2008;191:423–31. doi: 10.2214/AJR.07.3629. [DOI] [PubMed] [Google Scholar]
- 38.Rajiah P, Sinha R, Cuevas C, Dubinsky TJ, Bush WH, Jr, Kolokythas O. Imaging of Uncommon Retroperitoneal Masses. Radiographics. 2011 Jul-Aug;31(4):949–76. doi: 10.1148/rg.314095132.. [DOI] [PubMed] [Google Scholar]
- 39.Hartlage GR, Palios J, Barron BJ, et al. Multimodality imaging of aortitis. JACC Cardiovasc Imaging. 2014 Jun;7(6):605–19. doi: 10.1016/j.jcmg.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Amis ES., Jr Retroperitoneal fibrosis. AJR Am J Roentgenol. 1991 Aug;157(2):321–9. doi: 10.2214/ajr.157.2.1853816. [DOI] [PubMed] [Google Scholar]
- 41.Sanders RC, Duffy T, Mcloughlin MG, Walsh PC. Sonography in the diagnosis of retroperitoneal fibrosis. J Urol. 1977 Dec;118(6):944–6. doi: 10.1016/s0022-5347(17)58257-9. [DOI] [PubMed] [Google Scholar]
- 42.Macedo TA, Stanson AW, Oderich GS, Johnson CM, Panneton JM, Tie ML. Infected aortic aneurysms: imaging findings. Radiology. 2004;231:250–257. doi: 10.1148/radiol.2311021700. [DOI] [PubMed] [Google Scholar]
- 43.Lee WK, Mossop PJ, Little AF, et al. Infected (mycotic) aneurysms: spectrum of imaging appearances and management. Radiographics. 2008 Nov-Dec;28(7):1853–68. doi: 10.1148/rg.287085054.. [DOI] [PubMed] [Google Scholar]
- 44.Deipolyi AR, Czaplicki CD, Oklu R. Inflammatory and infectious aortic diseases. Cardiovasc Diagn Ther. 2018 Apr;8(Suppl 1):S61–S70. doi: 10.21037/cdt.2017.09.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sueyoshi E, Sakamoto I, Kawahara Y, Matsuoka Y, Hayashi K. Infected abdominal aortic aneurysm: early CT findings. Abdom Imaging. 1998;23(6):645–648. doi: 10.1007/s002619900422. [DOI] [PubMed] [Google Scholar]
- 46.Bedmutha AS, Singh N, Shivdasani D. Metabolic Imaging as a Novel Strategy in Evaluation of Mycotic Abdominal Aortic Aneurysm: A Case Report and Brief Clinical Review. Indian J Nucl Med. 2017 Oct-Dec;32(4):336–339. doi: 10.4103/ijnm.IJNM_81_17.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsu CC, Huang YF, Chuang YW. Detection of an infected abdominal aortic aneurysm with three-phase bone scan and Gallium-67 scan. Clin Nucl Med. 2008 Apr;33(4):305–7. doi: 10.1097/RLU.0b013e3181662f77. [DOI] [PubMed] [Google Scholar]
- 48.Murakami M, Morikage N, Samura M, Yamashita O, Suehiro K, Hamano K. Fluorine-18-fluorodeoxyglucose positron emission tomography-computed tomography for diagnosis of infected aortic aneurysms. Ann Vasc Surg. 2014 Apr;28(3):575–8. doi: 10.1016/j.avsg.2013.04.013. Epub 2013 Nov 5. [DOI] [PubMed] [Google Scholar]
- 49.Narang AT, Rathlev NK. Non-aneurysmal infectious aortitis: a case report. J Emerg Med. 2007 May;32(4):359–63. doi: 10.1016/j.jemermed.2006.07.030. Epub 2007 Mar 26. [DOI] [PubMed] [Google Scholar]
- 50.Fink AM, Miles KA, Wraight EP. Indium-111 labelled leucocyte uptake in aortitis. Clin Radiol. 1994 Dec;49(12):863–6. doi: 10.1016/s0009-9260(05)82876-4. [DOI] [PubMed] [Google Scholar]