Abstract
Background and Objective
The aim of the present study was to evaluate the effects of synbiotic on glycemic status, lipid profile, and biomarkers of oxidative stress in type 1 diabetes mellitus (T1DM) patients.
Materials and Methods
In this double-blind clinical trial, 50 T1DM patients were randomly allocated to intervention (n = 25) and control (n = 25) groups and received either synbiotic powder (Lactobacillus sporogenes GBI-30 (probiotic), maltodextrin and fructooligosaccharide (prebiotic)) or placebo 2 g per day for 8 weeks. Fasting blood samples were collected before and after the intervention to measure fasting blood glucose (FBG), insulin concentration, hemoglobin A1c (HbA1c), lipid profile, and biomarkers of oxidative stress such as total antioxidant capacity (TAC) and hs-C-reactive protein (hs-CRP).
Results
Supplementation with synbiotic resulted in a significant decrease in the mean serum levels of HbA1c and hs-CRP (p = 0.01 and p = 0.004, respectively), and marginally significant decrease in FBG (p = 0.05) in the intervention group post- intervention. Also, the mean changes of FBG and hs-CRP were significantly lower in the intervention group compared with the control group (p = 0.03 and p = 0.005, respectively). There were no significant changes found in lipid profile in intervention group post-intervention (p≥ 0.05). The mean serum levels of insulin and TAC were significantly increased in the intervention group post-intervention (p = 0.001). There was a significant increase in the mean changes of TAC (p = 0.005) in the intervention group compared with the control group.
Conclusion
The 8-week synbiotic supplementation in T1DM patients may be effective in improvement of FBG, HbA1c, insulin, hs-CRP, and TAC.
Keywords: type 1 diabetes mellitus, synbiotic, glycemic status, lipid profile, inflammation
Introduction
Type 1 diabetes mellitus (T1DM) is an autoimmune disease characterized by the progressive destruction of beta-pancreatic cells and the insufficiency of insulin production by these cells.1 Genetic background and environmental factors are involved in the process of this disease.2 The progression of disease is slow and may start before the diagnosis of T1DM. The disease progress is mostly occurred in early childhood or adolescence,3,4 when the autoantibodies against beta cells appeared in the peripheral circulation.5 T1DM is characterized by chronic hyperglycemia along with accelerated protein glycosylation.6 The chronic hyperglycemia can induce the high levels of bioactive molecules including superoxide free radicals, tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) initiate early atherosclerosis by the formation of oxidized low-density lipoprotein (LDL), foam cells and proliferation of smooth muscle cells.7 In fact, some epidemiological studies found a positive relationship between high levels of blood sugar, lipid dysfunction and cardiovascular diseases.8
The incidence of T1DM is increased by 3–4% annually.9 About 26,000 infants, children, and adolescents in the United States suffer from the disease.10 Over 85% of patients are under 20 years old.11 The increasing prevalence of diabetes in adolescents and young adults has become a major health problem.12 Such a rapid changes and decreases in the age of affliction are not due to genetics. Therefore, it is suggested that the recent increase in diabetes may be due to changes in the living environment affecting exposure to pathogenic microbes, as well as the composition of microbial flora in the gut regulating immunity and metabolism.13 The intestinal mucus is considered as a defense barrier and breaking down of this defense barrier11,14 lead to invading bacteria and toxins to the gastrointestinal tract, organs, and tissues.15 Gut microbiomes play a key role in health.16 The main benefits of the microbiota for the host include carbohydrate fermentation and digestion, vitamin synthesis, expansion of intestinal lymphoid tissues, eliciting specific immune responses and preventing the accumulation of pathogens.17 According to the health hypothesis, the sudden changes in human intestinal microflora are probably one of the causative factors of increased incidence of autoimmune diseases.
A new version of the “Health Hypothesis” suggests that reduced exposure to environmental and/or intestinal stimuli, including germs, is responsible for an increased incidence of childhood autoimmune diseases.18 In T1DM children, variations in the intestinal microflora have been reported; for instance, in children with T1DM in Spain, a reduced butyrate-producing bacteria with anti–inflammatory impacts was observed.16
Probiotic, the Greek word, meaning “for life”19 has been shown to activate monocytes, macrophages, and dendritic cells in vitro that affect the immune system.20 In addition, prebiotics which are non-digestible carbohydrates consumed by the intestinal bacteria for fermentation,21 are used in medicine to produce short-chain fatty acids (SCFAs)22 such as inulin or fructooligosaccharides (FOS), which stimulate the growth and metabolism of probiotics in the intestine. A mixture of prebiotics and probiotics as synbiotic has beneficial effects against several diseases.23 Several studies also suggest that the use of synbiotic foods may help control metabolic profiles, inflammatory factors, and oxidative stress biomarkers. Nevertheless, such effects have been mainly observed in animal models or nondiabetic patients.24 The hypoglycemic effects of Lactobacillus and Bifidobacterium have been investigated in several human studies. Several clinical trials have suggested that probiotic and synbiotic compounds alleviate or prevent elevated blood glucose in diabetic and non-diabetic subjects.25 Previous studies have reported that the synergistic effects of synbiotic supplements on the intestine and immune system are significantly stronger than probiotics and prebiotics alone.26 Recently, few studies have also reported that synbiotic and probiotic intake can improve insulin sensitivity and reduce inflammatory factors.27 The effects of synbiotic use in T1DM have not been investigated by any study so far. The aim of the present study was to evaluate the impacts of synbiotic supplementation on glycemic control, lipid profile, and biomarkers of oxidative stress in T1DM Patients.
Materials and Methods
In this double-blind clinical trial, the patients with type 1 diabetes referred to an endocrinologist’s clinic in Ahvaz city, were selected. The inclusion criteria were as the age of 4 to 18 years old, males and females, body mass index <95% percentile (according to BMI for age chart for children under 18) and at least 1 year diagnosed with T1DM. The exclusion criteria were as: kidney disease, coronary artery disease, acute and chronic pulmonary inflammation, short bowel syndrome, allergies, lactation or pregnancy, traveling for more than 2 weeks, smoking, using dietary supplements, anti–inflammatory drugs, using any antioxidant supplements since last 3 months, using immunosuppressive drugs, antibiotics and synbiotic products, following specific diets, changing diet and weight loss.
All patients had a confirmed diagnosis of T1DM (FBG ≥126 mg/dl or 2 hr glucose) 2 hpp (≥200 mg/dl or HbA1c ≥6.5%).28 The study protocol was confirmed by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (Ref No. IR.AJUMS.REC.1396.1032) and was recorded in the Iranian Registry of Clinical Trials website (IRCT20180310039020N1).
Study Design
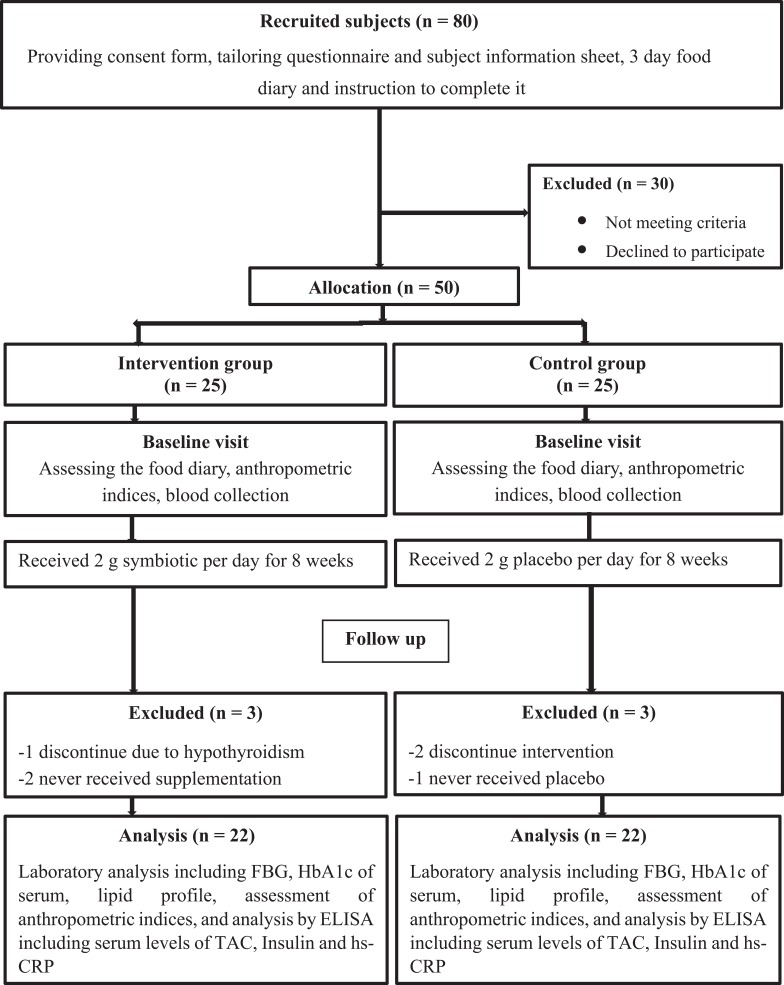
In this study, 50 families announced their child’s readiness to participate in the study, of which 44 patients completed the study (Figure 1). Patients were randomly (block design based on the combined analysis) divided into two intervention and control groups (25 subjects in each group). The subjects in the intervention group received 2 g of synbiotic powder (containing 109 CFU Lactobacillus sporogenesis GBI-30 as probiotic and maltodextrin and fructooligosaccharide as prebiotic) in one glass of water as once daily after a main meal for 8 weeks. The subjects in the control group received 2 g of starch powder with a glass of water once daily for 8 weeks. Both the supplement and placebo powder were provided by the “Parsilact Company,” Shiraz, Iran. The placebo and supplement were matched in terms of shape, color, size, and taste. The dosage of supplement was as 80% probiotic and 20% prebiotic. The patients were asked to avoid using any probiotic products during the study and parents were contacted weekly to control the usage of supplements.
Figure 1.
Flow diagram of the study.
Abbreviations: FBG, fasting blood glucose; HbA1c, hemoglobin A1c; hs-CRP, hs-C-reactive protein; TAC, total antioxidant capacity; ELISA, enzyme-linked immunosorbent assay.
Anthropometric and Nutritional Assessments
A three-day food record (including two working days and one weekend day) was completed pre and post-intervention. The dietary analysis was performed using the “Nutritionist 4” software, and the mean intakes of macronutrients and energy intake were calculated and compared with International Society for Pediatric and Adolescent Diabetes (ISPAD) guidelines.29 Also, the body weight was measured using the “Seca” scale with an accuracy of 0.5 kg and the height was measured using the tape meter with an accuracy of 0.1 cm. The BMI was calculated as weight/height2, kg/m2. According to the standard deviation scores (SDS) of weight, height and BMI calculated using the World Health Organization (WHO) data, SDS of patients were calculated.30,31 The physical activity was also evaluated by IPAQ questionnaire.32
Biochemical Measurements
A 5 mL blood sample was collected from each subject after 12 hrs of fasting and before insulin administration pre- and post-intervention. The FBG was immediately measured by the enzymatic method using laboratory kits (Pars Azmoon, Tehran, Iran) and an auto-analyzer. HbA1c was measured by enzymatic method using nycocard laboratory kits (Norway). All samples were stored in a freezer (Snijders, Germany) at −80°C until the analysis. Insulin, hs-CRP and TAC were measured by enzyme-linked immunosorbent assay (ELISA) method using kit (Tarvand Sina, Esfehan, Iran). Also, the serum levels of TG, cholesterol, and HDL-c was measured by the colorimetric method using the laboratory kits of Pars Azmoon (Tehran, Iran). The LDL-c and VLDL-c were calculated by the following formula (Friedewald formula):33
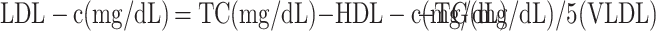 |
Statistical Analysis
Considering the FBG as the primary outcome21 and the 90% of power study and the withdrawal rate of % 15, the sample size calculated as 25 subjects in each group. All data were presented as mean ± SD for the quantitative variables or number (percentage) for the qualitative variables. The data were tested using Kolmogorov-Smirnoff-test for normal distribution with SPSS 20 software. The Chi-square test was used to compare the qualitative variables. The Independent sample t-test was used to compare quantitative variables between two groups. The Paired-sample test was also used to identify within-group differences (before and after intervention). Results with P < 0.05 were considered statistically significant.
Results
Forty-four subjects (22 in the intervention group and 22 in the control group as 23 females and 21 males) completed the study with the mean age of 10.36 ± 2.50. There were no adverse effects were reported during the study. Table 1 showed that there were no significant difference in terms of demographic characteristics, the standard deviation scores (SDS) of weight, height and BMI, physical activity, using insulin (data not shown), and duration of diabetes between the two intervention and control groups at baseline (p ≥ 0.05). Moreover, no significant differences were observed in dietary intakes including calories, protein intake, carbohydrates, fat, cholesterol, saturated fat, and dietary fiber between groups pre- and post-intervention (p ≥ 0.05) (Table 2). According to acceptable macronutrient distribution range recommended by the ISPAD guidelines (2018), energy contribution of macronutrients was normal. Dietary fiber intakes of patients were lower than recommended (children >2 years old, Age in years +5 = grams of fiber per day).29
Table 1.
The Characteristics of Subjects at Baseline
| Variable | Control Group (n=22) | Intervention Group (n=22) | *P-value | SDS (Z score) |
|---|---|---|---|---|
| Age (years) | 10.04 ± 2.08 | 10.36 ± 2.53 | 0.65 | |
| Gender | ||||
| Female (N) (%) | 12 (54) | 11 (50) | 1.00a | |
| Male (N) (%) | 10 (45) | 11 (50) | ||
| Weight (kg) | 34.90 ± 13.56 | 43.20 ± 17.94 | 0.09 | |
| Z-score for weight | −0.14 ± 1.75 | 0.39 ± 1.78 | 0.32 | −2,+1 |
| Height (m) | 137.65 ± 18.01 | 144.38 ± 21.48 | 0.26 | |
| Z-score for height | −0.04 ±2.45 | 0.45 ± 2.04 | 0.46 | −2,+3 |
| BMI (kg/m2) | 17.86 ± 3.45 | 19.77 ± 4.18 | 0.20 | |
| Z-score for BMI | −0.02 ±1.44 | 0.54 ±1.04 | 0.13 | −2,+1 |
| Disease duration (years) | 4.04 ± 1.36 | 4.45 ± 1.96 | 0.42 |
Notes: Values are expressed as means ± SD. P<0.05 was considered as significant. *P<0.05 was considered as significant using independent T-test between the two groups at baseline. aP<0.05 was considered as significant using Chi-square test.
Abbreviation: SDS, standard deviation scores.
Table 2.
Mean ± SD of Energy, Macronutrients Intake at Baseline and Post-Intervention
| Variable | Baseline | Post-Intervention | **P-value | ISPAD |
|---|---|---|---|---|
| Energy (kcal/d) | ||||
| Control group | 1616.86 ± 150.59 | 1617.63 ± 147.89 | 0.78 | |
| Intervention group | 1655.81 ± 150.65 | 1667.77 ± 165.20 | 0.26 | |
| *P-value | 0.39 | 0.29 | ||
| Carbohydrate (g/d) | ||||
| Control group | 203.66 ± 20.76 | 203.95 ± 20.16 | 0.61 | |
| Intervention group | 212.62,± 23.90 | 213.26 ± 24.09 | 0.14 | |
| *P-value | 0.19 | 0.17 | ||
| Carbohydrate (%) | ||||
| Control group | 50 | 50 | 0.77 | 45–50 |
| Intervention group | 51 | 51 | 0.46 | |
| *P-value | 0.59 | 0.69 | ||
| Protein (g/d) | ||||
| Control group | 62.91 ± 13.72 | 62.99 ± 13.87 | 0.62 | |
| Intervention group | 70.38 ± 17.07 | 70.27 ± 16.87 | 0.66 | |
| *P-value | 0.11 | 0.12 | ||
| Protein (%) | ||||
| Control group | 15.5 | 15.5 | 0.65 | 15–20 |
| Intervention group | 17 | 16.8 | 0.51 | |
| *P-value | 0.31 | 0.20 | ||
| Fat (g/d) | ||||
| Control group | 59.76 ± 3.18 | 59.57 ± 3.30 | 0.66 | |
| Intervention group | 60.57 ± 3.50 | 60.45 ± 3.62 | 0.68 | |
| *P-value | 0.42 | 0.40 | ||
| Fat (%) | ||||
| Control group | 33 | 33 | 0.60 | <35 |
| Intervention group | 33 | 32.5 | 0.58 | |
| *P-value | 0.12 | 0.26 | ||
| Cholesterol (mg/d) | ||||
| Control group | 191.83 ± 59.71 | 189.17 ± 54.99 | 0.14 | |
| Intervention group | 185.40 ± 56.79 | 185.50 ± 56.97 | 0.76 | |
| *P-value | 0.71 | 0.82 | ||
| Saturated fat (g/d) | ||||
| Control group | 13.91 ± 2.81 | 13.59 ± 2.47 | 0.41 | <10 |
| Intervention group | 13.88 ± 2.35 | 13.57 ± 2.69 | 0.02 | |
| *P-value | 0.96 | 0.97 | ||
| Dietary fiber (g/d) | ||||
| Control group | 9.39±2.70 | 9.08±2.41 | 0.20 | 15.04 |
| Intervention group | 9.33±2.77 | 9.04±2.43 | 0.20 | 15.36 |
| *P-value | 0.94 | 0.95 | ||
| Dietary fiber (g/1000 kcal) | ||||
| Control group | 5.81± 1.67 | 5.61±1.49 | 0.20 | |
| Intervention group | 5.78±1.75 | 5.42±1.46 | 0.10 | |
| *P-value | 0.96 | 0.66 |
Notes: Values are expressed as means ± SD. *P<0.05 was considered as significant at baseline and post-intervention using independent T-test between two groups. **P<0.05 was considered as significant using paired T-test.
Abbreviation: ISPAD, International Society for Pediatric and Adolescent Diabetes.
Glycemic Control
No significant differences were seen in FBG, insulin, and HbA1c between 2 groups at baseline (p ≥ 0.05). At the end of the study, there was a significant reduction (p = 0.01) in the mean serum levels of HbA1c (8.90±1.95 vs 8.61±1.85%, respectively, for baseline and after the intervention) in the intervention group. However, the decrease in the mean serum levels of FBG showed a marginal trend toward significant (199.72±81.10 vs 163.68±75.88 mg/dl; p = 0.05) (Table 3). Also, the mean changes of FBG were significantly lower in the intervention group compared with the control group (−36.04±81.87 vs 9.31±45.34, respectively; p = 0.03). Also, after adjusting for confounding factors, the results did not change in terms of significance; except for HbA1c after adjusting, there was a significant decrease (p = 0.03) (Table 3).
Table 3.
Glycemic Status and Lipid Profile at Baseline and Post-Intervention
| Variables | Intervention Group (n=22) | Control Group (n=22) | P-value* | P-value** | P-value*** |
|---|---|---|---|---|---|
| FBG (mg/dl) | |||||
| Baseline | 199.72±81.10 | 162.31±68.11 | 0.10 | ||
| End | 163.68±75.88 | 171.63±73.89 | 0.72 | ||
| P-value | 0.05 | 0.34 | |||
| Difference | −36.04±81.87 | 9.31±45.34 | 0.03 | 0.02 | |
| Insulin (µg/mL) | |||||
| Baseline | 6.37±6.32 | 5.97±5.02 | 0.81 | ||
| End | 10.90±8.20 | 7.57±7.12 | 0.15 | ||
| P-value | <0.001 | 0.18 | |||
| Difference | 4.52±4.53 | 1.59±5.46 | 0.06 | 0.06 | |
| HbA1c (%) | |||||
| Baseline | 8.90±1.95 | 9.60±2.23 | 0.27 | ||
| End | 8.61±1.85 | 9.08±2.59 | 0.96 | ||
| P-value | 0.01 | 0.08 | |||
| Difference | −0.28±0.52 | −0.52±1.36 | 0.44 | 0.03 | |
| LDL-c (mg/dl) | |||||
| Baseline | 79.81±13.55 | 74.45±17.06 | 0.25 | ||
| End | 81.86±15.01 | 79.31±17.90 | 0.61 | ||
| P-value | 0.14 | 0.06 | |||
| Difference | 2.04±6.34 | 4.86±11.47 | 0.31 | 0.31 | |
| HDL-c (mg/dl) | |||||
| Baseline | 54.13±9.47 | 49.13±8.02 | 0.06 | ||
| End | 56.04±9.29 | 51.22±8.24 | 0.07 | ||
| P-value | 0.26 | 0.12 | |||
| Difference | 1.90±7.73 | 2.09±6.16 | 0.93 | 0.06 | |
| VLDL-c (mg/dl) | |||||
| Baseline | 16.75±6.20 | 14.93±2.48 | 0.21 | ||
| End | 15.65±9.67 | 15.00±3.73 | 0.77 | ||
| P-value | 0.36 | 0.92 | |||
| Difference | −1.10±5.53 | 0.06±3.26 | 0.40 | 0.30 | |
| TG (mg/dl) | |||||
| Baseline | 83.77±31.03 | 74.68±12.44 | 0.21 | ||
| End | 78.27±48.39 | 75.00±18.69 | 0.77 | ||
| P-value | 0.36 | 0.92 | |||
| Difference | −5.50±27.68 | 0.31±16.30 | 0.40 | 0.40 | |
| CHOL (mg/dl) | |||||
| Baseline | 156.90±21.55 | 156.81±21.46 | 0.98 | ||
| End | 158.63±21.32 | 158.31±21.26 | 0.96 | ||
| P-value | 0.65 | 0.69 | |||
| Difference | 1.72±17.59 | 1.50±17.70 | 0.92 | 0.93 |
Notes: Values are expressed as means ± SD. P<0.05 was considered as significant using paired T-test. *P<0.05 was considered as significant using independent T-test between the two groups before and post-intervention. **P<0.05 was considered as significant changes using independent T-test between the two groups post-intervention. ***P<0.05 was considered as significant using analysis of covariance (ANCOVA) between the two groups post-intervention after adjusting for confounding factors.
Abbreviations: FBG, fasting blood glucose; HbA1c, glycosylated hemoglobin; TG, triglyceride; CHOL, total cholesterol; HDL, high-density; LDL, low-density lipoprotein cholesterol.
Lipid Profile
The results of this study showed that there were no significant difference (p ≥ 0.05) in the mean levels of lipid profile such as triglyceride (TG), total cholesterol (CHOL), high-density lipoprotein cholesterol (HDL−c), low-density lipoprotein cholesterol (LDL−c), and very low-density lipoproteins (VLDL) between two groups at baseline and after the intervention. Also, after adjusting for confounding factors, the results did not change in terms of significance (Table 3).
Biomarkers of Stress Oxidative and Inflammatory Status
According to results of Table 4, there were no significant difference (p ≥ 0.05) in the mean levels of TAC and hs-CRP between two groups at baseline. At the end of the study, synbiotic supplementation significantly reduced serum levels of hs-CRP (3054.64±3009.89 vs 1807.10±2258.92 ng/mL, respectively; p = 0.004) in the intervention group.
Table 4.
The Mean ± SD of Hs-CRP and TAC at Baseline and Post-Intervention
| Variables | Intervention Group (n=22) | Control Group (n=22) | P-value* | P-value** |
|---|---|---|---|---|
| Hs-CRP (ng/mL) | ||||
| Baseline | 3054.64±3009.89 | 2267.73±2087.29 | 0.31 | |
| End | 1807.10±2258.92 | 2293.01±1899 | 0.44 | |
| P-value | 0.004 | 0.89 | ||
| Difference | −1247.54±1793.66 | 25.28±858.14 | 0.005 | |
| TAC (mmol/lit) | ||||
| Baseline | 94.16±14.29 | 100.07±11.49 | 0.33 | |
| End | 101.12±14.35 | 92.37±4.27 | 0.002 | |
| P-value | 0.001 | 0.08 | ||
| Difference | 6.96±8.61 | −7.70±11.58 | 0.005 |
Notes: Values are expressed as means ± SD. P<0.05 was considered as significant using paired T-test. *P<0.05 was considered as significant using Independent T-test between the two groups at baseline and post-intervention. **P<0.05 was considered as significant changes using Independent T-test between the two groups post-intervention
Abbreviations: hs-CRP, Hs-C-reactive protein; TAC, total antioxidant capacity.
In addition, the mean changes of serum levels of hs-CRP were significantly lower in the intervention group compared with the control group (−1247.54±1793.66 vs 25.28±858.14 ng/mL, respectively; p = 0.005). The results of this study showed that in intervention group the mean of TAC was increased significantly (p = 0.001) post-intervention compared with baseline (94.16±14.29 to 101.12±14.35 mmol/lit, respectively). Consumption of synbiotic, compared to the control, resulted in a significant increase in the mean changes of TAC (p = 0.005) (Table 4).
Discussion
According to this study, 8-week synbiotic supplementation in T1DM patients improved FBG, HbA1c, insulin, hs-CRP, and TAC. The earlier studies investigated the impact of the synbiotic supplementation in type 2 diabetes and to our best of knowledge, the current study was the first study to investigate synbiotic supplementation among patients with T1DM.
Dietary Fibers and Microbiota
The microbiota can be altered through the intake of certain dietary ingredients including fiber and prebiotics. There are several studies showing an increase in the gut microbiota diversity and population (specifically the Clostridia class) and the reduction of the gut pH and transit time by consumption of fiber and whole grain.34,35 The transit time in the upper intestine has a key role in the regulation of satiety and appetite, glycemic control and the hormone signaling in the gut. On the other hand, the transit time in the lower intestine (colon) mainly determine the gut microbiota.36 It is indicated that the short-chain fatty acid (SCFA) production through the fermentation of non-digestible carbohydrates may impact on the appetite suppressing effects. It was found that increasing the colonic production of the SCFA propionate can significantly increase postprandial concentrations of the anorexigenic gut hormones peptide YY and glucagon-like peptide-1 (GLP-1), and sharply diminish the energy intake in humans.37 More dietary fiber intake is associated with increased diversity of the gastrointestinal microbial community.38
Diabetes and Microflora Composition
Several studies found that the permeability of butyrate is increased in the patients with DM. The butyrate is secreted by the intestinal epithelial cells and mainly supply the energy for them. Therefore, the disturbance of the butyrate secretion can be a factor impairing the tight barrier function of intestinal epithelial cells.39 Kieler et al in a study reported that the diversity of gut microbiota was lower in the diabetic cats than in the lean cats. There are growing evidence to support the presence of dysbiosis in the gut microbiota of T2DM patients. It is suggested that in general, the balance between beneficial and harmful bacteria is disturbed in the patients with DM, so that the population of beneficial bacteria decrease and that of harmful bacteria increase.40 Therefore, it may be concluded that the diabetes condition can affect the composition of microbiota.
Anti-Metabolic Effects of Synbiotic
In a double-blinded clinical trial T2DM patients consumed 200 mL/d of a synbiotic shake containing 108 UFC/mL Lactobacillus acidophilus, 108 UFC/mL Bifidobacterium bifidum and 2 g oligofructose for 30 days and the results showed that the intervention significantly reduced the levels of FBG, which was almost similar to the results of our study. Also, a significant increase was observed in HDL-c which was not concurred with the present study. Similar to the present study, no significant change was observed in other lipid profile factors.21 In another study, Ekhlasi et al, studied the effects of synbiotic and vitamin E supplementation on 60 patients with nonalcoholic fatty liver disease and found a significant decrease in FBG, insulin concentrations, TG, and TC in the synbiotic and synbiotic + vitamin E groups. Furthermore, the intake of synbiotic plus Vitamin E supplements led to a more significant decrease in LDL-C compared with control group.15 In agreement to this study, in two other clinical trials, no significant changes were observed in LDL-c, TC, HDL-c, and TG.24,41 The quantity and type of bacteria used in different studies as well as the duration of the intervention, target population, and type of disease may be factors influenced in the results of the studies.
It is indicated that synbiotic produces short-chain fatty acids, carbon disulfide, and methyl acetate, which can increase lipolytic activity.24 It has been suggested that probiotics and prebiotics might counteract the development of the metabolic syndrome through replacing the aggravating bacteria in the gut, which in turn can improve serum lipid levels and insulin resistance.26 Previous studies suggested some possible mechanisms. Synbiotics can increase the GLP-1 and GLP-2 hormones. The GLP-1 reduces blood sugar and GLP-2, a proglucagon-derived peptide, reduces intestinal permeability. GLP-1 and GLP-2 secretion can lead to weight loss, hypoglycemia, and HbA1c depletion.42 The loss of gut microbiota balance can affect on various organs such as adipose tissue, skeletal muscle, and liver, so it may affect on insulin resistance and glycemic status.
The Effects of Synbiotic on Inflammatory and Antioxidant Parameters
Systemic inflammation is common in the diabetes patients. It may lead to various complications including the cardiovascular disease in diabetes patients. There is no treatment strategy for reducing inflammation in diabetes patients has been established yet.34,43 In a study by Asgharian et al, 80 patients with nonalcoholic fatty liver received a capsule containing synbiotic for 8 weeks. There was no significant difference reported in CRP index in any of the intervention and control groups in this study.43 In agreement with the findings of the present study, after synbiotic supplementation in T2DM patients, there was a significant decrease in CRP in the intervention group compared with the control group.44 In the study carried out by Malangwara et al, LDL-c and hs-CRP were found to be significantly improved in 66 patients after 24 weeks of supplementation with synbiotic. In terms of hs-CRP as an inflammatory factor, the results were consistent with our study. But the LDL-c had the opposite effect.44 Regarding with the oxidative stress biomarkers, consuming bread-containing synbiotic for 8 weeks did not improve TAC in patients with type 2 diabetes.45 Raygan et al in a study for 12 weeks in patients with diabetic CHD investigated the effects of vitamin D and probiotics. Unlike our study, the supplementation did not influence the FBG but similar to our study, the TAC and fasting insulin were improved.46 Sonigisepp et al, observed a significant improvement in TAC after 3-week probiotic supplementation in healthy subjects.47 The results of this study were more similar to the results of our study. In the study of Bahmani et al, the effect of synbiotic supplementation in type 2 patients was investigated and unlike our study, there was no effect observed on TAC.45 There are other contradictory results have been observed in various studies. It is suggested that having a different population, using different dosages of synbiotic, duration of intervention, size effect of synbiotic, dosage of bacterial strain, and the type of prebiotic may be considered as possible reasons for different findings.
Free radicals can cause several complications of diabetes. There are many factors in diabetes which can increase the amount of these radicals including glucose autoxidation, leukocyte activation, etc.48 With 12 weeks of probiotic supplementation in patients undergoing hemodialysis, Soleimani et al observed a significant increase in the TAC of these patients.49 The precise mechanism behind the hypoglycemic effect and improvement of total antioxidant index by synbiotic have not been fully elucidated. It is indicated that lipopolysaccharide (LPS) derived from the outer membrane of Gram-negative bacteria increases the production of pro-inflammatory cytokines. One of the proposed mechanisms to explain how gut microbiota affects blood glucose is their effects on epithelial integrity of intestine, short-chain fatty acid production and its effect on energy homeostasis and blood glucose. Another mechanism is the conversion of primary bile acids to secondary ones by intestinal bacteria and activation of GLP1 by intestinal L cells.50 Improvement of metabolic profiles, biomarkers of inflammation and oxidative stress by probiotics may be due to their effects on increasing concentrations of GSH, scavenging superoxide and hydroxyl radicals, reduced inflammatory signaling and decreased adiposity. Therefore, the beneficial effect of probiotics on biomarkers of oxidative stress is probably due to butyrate production in the intestine, and an increase in glutamate-cysteine-ligase activity.51 In further researches can investigate the effects of synbiotic supplementation on microflora composition and other biomarkers of oxidative stress in type 1 diabetes.
Strengths and Limitation of the Study
This study was the first study to evaluate synbiotic supplementation in patients with (T1DM). The limitation of this study was its sample size.
Conclusion
It is suggested that supplementation with synbiotic for 8 weeks in patients with T1DM can improve FBG, HbA1c, insulin, hs-CRP, and TAC. So, this supplement can be used along with other diabetes control treatments.
Acknowledgments
Authors express thanks for Parsilact Company, Shiraz, Iran that provided synbiotic product for the present study. Also, we thank all the patients who participated in this study.
Funding Statement
This study was part of M.Sc thesis of MS Jamalvandi. This research project was sponsored by the Vice-Chancellor for Research Affairs of Ahvaz Jundishapur University of Medical Sciences (NRC-9635).
Abbreviations
BMI, body mass index; FBG, fasting blood glucose; HbA1c, glycated hemoglobin A1C; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein density; SD, standard deviation; TC, total cholesterol; TG, triglyceride; Hs-CRP, Hs-C-reactive protein; TAC, total antioxidant capacity.
Compliance with Ethical Standards
The design of this study was done according to the guidelines of the Helsinki Declaration and all procedures involving human patients were approved by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (Ethical Code. IR.AJUMS.REC.1396.1032). In present study, a parent or legal guardian provided written informed consent before initiating the study.
Data Sharing Statement
The datasets are not publicly available because of lack of agreement for disclosing individual raw data in public but are available from the corresponding author on reasonable request.
Disclosure
The authors have declared that there are no conflicts of interest in this work.
References
- 1.Groele L, Szajewska H, Szypowska A. Effects of Lactobacillus rhamnosus GG and bifidobacterium lactis Bb12 on beta-cell function in children with newly diagnosed type 1 diabetes: protocol of a randomised controlled trial. BMJ Open. 2017;7(10):1–7. doi: 10.1136/bmjopen-2017-017178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Javid AZ, Bazyar H, Gholinezhad H, et al. The effects of ginger supplementation on inflammatory, antioxidant, and periodontal parameters in type 2 diabetes mellitus patients with chronic periodontitis under non-surgical periodontal therapy. A double-blind, placebo-controlled trial. Diabetes Metab Syndr Obes. 2019;12:1751–1761. doi: 10.2147/DMSO.S214333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Endesfelder D, Engel M, Zu Castell W. Gut immunity and type 1 diabetes: a melange of microbes, diet, and host interactions? Curr Diab Rep. 2016;16(7):60. doi: 10.1007/s11892-016-0753-3 [DOI] [PubMed] [Google Scholar]
- 4.Kondrashova A, Hyöty H. Role of viruses and other microbes in the pathogenesis of type 1 diabetes. Int Rev Immunol. 2014;33(4):284–295. doi: 10.3109/08830185.2014.889130 [DOI] [PubMed] [Google Scholar]
- 5.Knip M, Honkanen J. Modulation of type 1 diabetes risk by the intestinal microbiome. Curr Diab Rep. 2017;17(11):105. doi: 10.1007/s11892-017-0933-9 [DOI] [PubMed] [Google Scholar]
- 6.Jaisson S, Souchon P-F, Desmons A, Salmon A-S, Delemer B, Gillery P. Early formation of serum advanced glycation end-products in children with type 1 diabetes mellitus: relationship with glycemic control. J Pediatr. 2016;172:56–62. doi: 10.1016/j.jpeds.2016.01.066 [DOI] [PubMed] [Google Scholar]
- 7.Babar G, Clements M, Dai H, Raghuveer G. Assessment of biomarkers of inflammation and premature atherosclerosis in adolescents with type-1 diabetes mellitus. J Pediatr Endocrinol Metab. 2019;32(2):109–113. doi: 10.1515/jpem-2018-0192 [DOI] [PubMed] [Google Scholar]
- 8.Cicek B, Arslan P, Kelestimur F. The effects of oligofructose and polydextrose on metabolic control parameters in type-2 diabetes. Pak J Med Sci. 2009;25(4):573–578. [Google Scholar]
- 9.Paun A, Yau C, Danska JS. Immune recognition and response to the intestinal microbiome in type 1 diabetes. J Autoimmun. 2016;71:10–18. doi: 10.1016/j.jaut.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 10.Blair JC, McKay A, Ridyard C, et al. Continuous subcutaneous insulin infusion versus multiple daily injection regimens in children and young people at diagnosis of type 1 diabetes: pragmatic randomised controlled trial and economic evaluation. BMJ. 2019;365:l1226. doi: 10.1136/bmj.l1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caferoğlu Z, İnanç N, Hatipoğlu N, Kurtoğlu S. Health-related quality of life and metabolic control in children and adolescents with type 1 diabetes mellitus. J Clin Res Pediatr Endocrinol. 2016;8(1):67. doi: 10.4274/jcrpe [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mottl AK, Divers J, Dabelea D, et al. The dose–response effect of insulin sensitivity on albuminuria in children according to diabetes type. Pediatr Nephrol. 2016;31(6):933–940. doi: 10.1007/s00467-015-3276-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paun A, Danska JS. Modulation of type 1 and type 2 diabetes risk by the intestinal microbiome. Pediatr Diabetes. 2016;17(7):469–477. doi: 10.1111/pedi.2016.17.issue-7 [DOI] [PubMed] [Google Scholar]
- 14.Rewers M, Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet. 2016;387(10035):2340–2348. doi: 10.1016/S0140-6736(16)30507-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ekhlasi G, Mohammadi RK, Agah S, et al. Do symbiotic and Vitamin E supplementation have favorite effects in nonalcoholic fatty liver disease? A randomized, double-blind, placebo-controlled trial. J Res Med Sci. 2016;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho J, Reimer RA, Doulla M, Huang C. Effect of prebiotic intake on gut microbiota, intestinal permeability and glycemic control in children with type 1 diabetes: study protocol for a randomized controlled trial. Trials. 2016;17(1):347. doi: 10.1186/s13063-016-1486-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Oliveira GLV, Leite AZ, Higuchi BS, Gonzaga MI, Mariano VS. Intestinal dysbiosis and probiotic applications in autoimmune diseases. Immunology. 2017;152(1):1–12. doi: 10.1111/imm.12765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Özdemir Ö. Any role for probiotics in the therapy or prevention of autoimmune diseases? Up-to-date review. J Complement Integr Med. 2013;10(1):229–250. doi: 10.1515/jcim-2012-0054 [DOI] [PubMed] [Google Scholar]
- 19.Markowiak P, Śliżewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. 2017;9(9):1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ljungberg M, Korpela R, Ilonen J, Ludvigsson J, Vaarala O. Probiotics for the prevention of beta cell autoimmunity in children at genetic risk of type 1 diabetes—the PRODIA study. Ann N Y Acad Sci. 2006;1079(1):360–364. doi: 10.1196/annals.1375.055 [DOI] [PubMed] [Google Scholar]
- 21.Moroti C, Magri LFS, de Rezende Costa M, Cavallini DC, Sivieri K. Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Dis. 2012;11(1):29. doi: 10.1186/1476-511X-11-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kavitha K, Reddy AG, Reddy KK, Kumar CS, Boobalan G, Jayakanth K. Hypoglycemic, hypolipidemic and antioxidant effects of pioglitazone, insulin and synbiotic in diabetic rats. Vet World. 2016;9(2):118. doi: 10.14202/vetworld. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajkumar H, Kumar M, Das N, Kumar SN, Challa HR, Nagpal R. Effect of probiotic Lactobacillus salivarius UBL S22 and prebiotic fructo-oligosaccharide on serum lipids, inflammatory markers, insulin sensitivity, and gut bacteria in healthy young volunteers: a randomized controlled single-blind pilot study. J Cardiovasc Pharmacol Ther. 2015;20(3):289–298. doi: 10.1177/1074248414555004 [DOI] [PubMed] [Google Scholar]
- 24.Asemi Z, Khorrami-Rad A, Alizadeh S-A, Shakeri H, Esmaillzadeh A. Effects of synbiotic food consumption on metabolic status of diabetic patients: a double-blind randomized cross-over controlled clinical trial. Clin Nutr. 2014;33(2):198–203. doi: 10.1016/j.clnu.2013.05.015 [DOI] [PubMed] [Google Scholar]
- 25.Nikbakht E, Khalesi S, Singh I, Williams LT, West NP, Colson N. Effect of probiotics and synbiotics on blood glucose: a systematic review and meta-analysis of controlled trials. Eur J Nutr. 2018;57(1):95–106. doi: 10.1007/s00394-016-1300-3 [DOI] [PubMed] [Google Scholar]
- 26.Eslamparast T, Zamani F, Hekmatdoost A, et al. Effects of synbiotic supplementation on insulin resistance in subjects with the metabolic syndrome: a randomised, double-blind, placebo-controlled pilot study. Br j Nutr. 2014;112(3):438–445. doi: 10.1017/S0007114514000919 [DOI] [PubMed] [Google Scholar]
- 27.Tajadadi-Ebrahimi M, Bahmani F, Shakeri H, et al. Effects of daily consumption of synbiotic bread on insulin metabolism and serum high-sensitivity C-reactive protein among diabetic patients: a double-blind, randomized, controlled clinical trial. Ann Nutr Metab. 2014;65(1):34–41. doi: 10.1159/000365153 [DOI] [PubMed] [Google Scholar]
- 28.Bazyar H, Gholinezhad H, Moradi L, et al. The effects of melatonin supplementation in adjunct with non-surgical periodontal therapy on periodontal status, serum melatonin and inflammatory markers in type 2 diabetes mellitus patients with chronic periodontitis: a double-blind, placebo-controlled trial. Inflammopharmacology. 2019;27(1):67–76. doi: 10.1007/s10787-018-0539-0 [DOI] [PubMed] [Google Scholar]
- 29.Smart CE, Annan F, Bruno LP, Higgins LA, Acerini CL. Nutritional management in children and adolescents with diabetes. Pediatr Diabetes. 2014;15(S20):135–153. doi: 10.1111/pedi.2014.15.issue-S20 [DOI] [PubMed] [Google Scholar]
- 30.Barlow SE, Dietz WH. Obesity evaluation and treatment: expert committee recommendations. Pediatrics. 1998;102(3):e29–e29. doi: 10.1542/peds.102.3.e29 [DOI] [PubMed] [Google Scholar]
- 31.Kuczmarski RJ, Flegal KM. Criteria for definition of overweight in transition: background and recommendations for the United States. Am J Clin Nutr. 2000;72(5):1074–1081. doi: 10.1093/ajcn/72.5.1074 [DOI] [PubMed] [Google Scholar]
- 32.Bazyar H, Adibmanesh A, Javid AZ, et al. The relationship between metabolic factors and anthropometric indices with periodontal status in type 2 diabetes mellitus patients with chronic periodontitis. Obes Med. 2019;16:100138. doi: 10.1016/j.obmed.2019.100138 [DOI] [Google Scholar]
- 33.Knopfholz J, Disserol CCD, Pierin AJ, et al. Validation of the friedewald formula in patients with metabolic syndrome. Cholesterol. 2014;2014. doi: 10.1155/2014/261878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017;8(2):172–184. doi: 10.1080/19490976.2017.1290756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dominianni C, Sinha R, Goedert JJ, et al. Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLoS One. 2015;10(4):e0124599. doi: 10.1371/journal.pone.0124599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Müller M, Canfora EE, Blaak EE. Gastrointestinal transit time, glucose homeostasis and metabolic health: modulation by dietary fibers. Nutrients. 2018;10(3):275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Byrne CS, Preston T, Brignardello J, et al. The effect of L-rhamnose on intestinal transit time, short chain fatty acids and appetite regulation: a pilot human study using combined 13CO2/H2 breath tests. J Breath Res. 2018;12(4):046006. doi: 10.1088/1752-7163/aad3f1 [DOI] [PubMed] [Google Scholar]
- 38.Healey G, Murphy R, Butts C, Brough L, Whelan K, Coad J. Habitual dietary fibre intake influences gut microbiota response to an inulin-type fructan prebiotic: a randomised, double-blind, placebo-controlled, cross-over, human intervention study. Br j Nutr. 2018;119(2):176–189. doi: 10.1017/S0007114517003440 [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, Lou X. Type 2 diabetes mellitus-related environmental factors and the gut microbiota: emerging evidence and challenges. Clinics. 2020;75. doi: 10.6061/clinics/2020/e1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kieler IN, Osto M, Hugentobler L, et al. Diabetic cats have decreased gut microbial diversity and a lack of butyrate producing bacteria. Sci Rep. 2019;9(1):1–13. doi: 10.1038/s41598-019-41195-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sadat Ebrahimi Z, Nasli-Esfahani E, Nadjarzade A, Mozaffari-khosravi H. Effect of symbiotic supplementation on glycemic control, lipid profiles and microalbuminuria in patients with non-obese type 2 diabetes: a randomized, double-blind, clinical trial. J Diabetes Metab Disord. 2017;16(1):23. doi: 10.1186/s40200-017-0304-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kooshki AA, Tofighiyan T, Rakhshani MH. Effects of synbiotics on inflammatory markers in patients with type 2 diabetes mellitus. Glob J Health Sci. 2015;7(7):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Asgharian A, Askari G, Esmailzade A, Feizi A, Mohammadi V. The effect of symbiotic supplementation on liver enzymes, c-reactive protein and ultrasound findings in patients with non-alcoholic fatty liver disease: a clinical trial. Int J Prev Med. 2016;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malaguarnera M, Vacante M, Antic T, et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig Dis Sci. 2012;57(2):545–553. doi: 10.1007/s10620-011-1887-4 [DOI] [PubMed] [Google Scholar]
- 45.Bahmani F, Tajadadi-Ebrahimi M, Kolahdooz F, et al. The consumption of synbiotic bread containing lactobacillus sporogenes and inulin affects nitric oxide and malondialdehyde in patients with type 2 diabetes mellitus: randomized, double-blind, placebo-controlled trial. Journal of the American College of Nutrition 2016;35(6):506–513. [DOI] [PubMed] [Google Scholar]
- 46.Raygan F, Ostadmohammadi V, Bahmani F, Asemi Z. The effects of vitamin D and probiotic co-supplementation on mental health parameters and metabolic status in type 2 diabetic patients with coronary heart disease: a randomized, double-blind, placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2018;84:50–55. doi: 10.1016/j.pnpbp.2018.02.007 [DOI] [PubMed] [Google Scholar]
- 47.Songisepp E, Kals J, Kullisaar T, et al. Evaluation of the functional efficacy of an antioxidative probiotic in healthy volunteers. Nutrition Journal 2005;4(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruiz C, Alegria A, Barbera R, Farre R, Lagarda M. Selenium, zinc and copper in plasma of patients with type 1 diabetes mellitus in different metabolic control states. Journal of trace elements in medicine and biology 1998;12(2):91–95. [DOI] [PubMed] [Google Scholar]
- 49.Soleimani A, Mojarrad MZ, Bahmani F, et al. Probiotic supplementation in diabetic hemodialysis patients has beneficial metabolic effects. Kidney international 2017;91(2):435–442. [DOI] [PubMed] [Google Scholar]
- 50.Kassaian N, Feizi A, Aminorroaya A, Jafari P, Ebrahimi MT, Amini M. The effects of probiotics and synbiotic supplementation on glucose and insulin metabolism in adults with prediabetes: a double-blind randomized clinical trial. Acta Diabetol. 2018;55(10):1019–1028. doi: 10.1007/s00592-018-1175-2 [DOI] [PubMed] [Google Scholar]
- 51.Jamilian M, Vahedpoor Z, Dizaji SH. Effects of probiotic supplementation on metabolic status in pregnant women: a randomized, double-blind, placebo-controlled trial. Arch Iran Med. 2016;19(10):687. [PubMed] [Google Scholar]