Abstract
OBJECTIVES:
Severe acute pancreatitis (SAP) is still a big challenge. Accumulated data showed that overexpression of cyclooxygenase-2 (COX-2) in acute pancreatitis and experimental pancreatitis could be attenuated with COX-2 inhibitors. This study was aimed to evaluate whether the occurrence of SAP could be prevented by selective COX-2 inhibitors.
METHODS:
A total of 190 patients with predicted SAP were randomized into convention group or convention plus COX-2 inhibitors (C+COX-2-Is) group. Besides conventional treatment to all patients in 2 groups, parecoxib (40 mg/d intravenous injection for 3 days) and celecoxib (200 mg oral or tube feeding twice daily for 7 days) were sequentially administrated to the patients in the C+COX-2-Is group. The primary outcome was predefined as the occurrence of SAP. The serum levels of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) for all of the patients were measured.
RESULTS:
The occurrence of SAP in the C+COX-2-Is group was decreased 47.08% compared with the convention group, 21.05% (20/95) vs 39.78% (37/93), P = 0.005. A reduction of late local complications was also shown in the C+COX-2-Is group, 18.95% (18/93) vs 34.41% (32/95), P = 0.016. The serum levels of IL-6 and TNF-α were significantly lower in the C+COX-2-Is group than those in the convention group, P < 0.05. Parecoxib relieved abdominal pain more rapidly and decreased the consumption of meperidine. An incremental reduction of cost for 1% decrease of SAP occurrence was RMB475.
DISCUSSION:
Sequential administration of parecoxib and celecoxib in patients with predicted SAP obtained about half-reduction of SAP occurrence through decreasing serum levels of TNF-α and IL-6. This regimen presented good cost-effectiveness.
INTRODUCTION
Severe acute pancreatitis (SAP) is characterized by persistent organ failure (OF) lasting more than 48 hours (1). Although much progress has been made in the management of SAP, the miserable suffering, high mortality, and heavy financial burden on health-care resources make SAP still a big challenge. Predicted SAP is defined as a special type of acute pancreatitis (AP) at its early stage with a score of acute physiology and chronic health evaluation (APACHE) II over or equal to 8 (2–4). It has been reported that about 70%–80% predicted SAP may progress into SAP (3–5). Therefore, interception of the development from predicted SAP to SAP may be crucial to prevent the occurrence of SAP and improve its prognosis.
The progression from onset of AP to SAP is driven by the inflammatory cascade, which is initiated by toll-like receptor (TLR)-nuclear factor κB (NF κB) activation and cytokine production in acinar cells (6,7). During the early stage of AP, a variety of proinflammatory mediators, including tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-6, IL-8, and cyclooxygenase-2 (COX-2), are released into the blood circulation and amplify the inflammatory response, consequently systemic inflammatory response syndrome (SIRS) develops (8–12). Severe and persistent SIRS inevitably result in multiple organs failure (13).
Previous studies indicate that somatostatin (SST) showed significant anti-inflammatory effect on AP (14,15). The basic researches of our group reported that octreotide, an analogue of SST, could decrease the proinflammatory cytokines by suppressing the TLR4-NF κB-cytokine pathway, inhibiting the activity of intestinal mucosal mast cells, and improving B-cell mature in macaques (16–20). Our prospective randomized controlled trials have shown that octreotide may attenuate SAP of obese patients and prevent the development of SAP in patients with high risk of SAP through reverting plasma SST to a normal level and decreasing TNF-α and IL-6 (5,21). SST and octreotide have been recommended in AP guideline of the Chinese Society of Gastroenterology (22). Therefore, octreotide was used as a conventional treatment in patients with predicted SAP in this study.
In experimental studies, overexpression of COX-2 was found in rats with AP (23,24). Mice deficient in COX-2 genes showed marked attenuation in the severity of pancreatitis and pancreatitis-associated lung injury (25,26). Moreover, NF-κB activation and the expression of messenger ribose nucleic acid of TNF-α in the pancreas of rats with AP could be suppressed by COX-2 inhibitors, leading to the decreased serum levels of TNF-α, IL-1, and IL-6 (27,28). COX-2 inhibitors also attenuated the severity of pancreatitis and improved renal and respiratory function (25–27,29,30). Lornoxicam, a COX-1/COX-2 inhibitor, could reduce TLRs expression and production of proinflammatory cytokines in AP patients (31). Those data implicate that COX-2 inhibitors may effectively attenuate the inflammatory process in AP. However, up to now, there is no clinical trial of COX-2 inhibitors on AP in literature.
Parecoxib, an injective COX-2 inhibitor, is usually used to alleviate postoperative pain (32,33) for no more than 3 days because there is limited clinical experience of usage for more than 3 days according to the instruction of parecoxib. Celecoxib, an oral dosage form, has been widely used for osteoarthritis. The hypothesis of this pioneering study was that the sequential administration of these 2 dosage forms of COX-2 inhibitors may intercept the development of SAP from predicted SAP. Thus, a prospective randomized controlled trial was designed and conducted in our single center.
METHODS
Study design and registration
This prospective single-center randomized controlled trial was designed and conducted in the Department of Gastroenterology, West China Hospital, Sichuan University, PR China. Before initiation of the trial, the study protocol was approved by China Ethics Committee of Registering Clinical Trials (Number: ChiECRCT-20140023) and registered at Chinese Clinical Trial Registry (Number: ChiCTR-TRC-14005059). The clinical investigators had the experiences of good clinical practice training, and all of them took their special roles individually in this study.
Participants and randomization
On admission, each patient diagnosed as AP in the emergency department was re-evaluated to confirm the diagnosis by the Atlanta definitions in 2012 (1). A score of APACHE II was calculated for each patient. The criteria for inclusion included (i) either gender aged 18–70 years; (ii) a confirmed diagnosis of AP; (iii) the time interval from the onset of symptoms to admission was no more than 48 h; (iv) a score of APACHE II ≥ 8; and (v) written informed consent was obtained from the patients or their legal representatives.
Exclusion criteria included (i) pregnancy and breast feeding mother; (ii) severe chronic diseases such as cardiac dysfunction, chronic obstructive pulmonary disease, renal insufficiency, cirrhosis, inflammatory bowel diseases, and malignancies; (iii) peptic ulcer; (iv) pancreatitis due to trauma; (v) drug allergy; and (vi) drug abuse and psychosis.
The participants were randomized in a 1:1 ratio into convention or convention + COX-2-inhibitors (C+COX-2-Is) group by using computer-generated randomization numbers (from SPSS version 18.0 for Windows). Two nurses who were not directly involved in medical care were assigned to allocate the eligible patients into 2 groups with the sequence number concealed in a container. Blindness of group assignments was maintained for the investigators who involved specially in clinical management and data collection, as well as the technicians who measured cytokines, until all data collection and data queries had been completed and the database was locked.
Intervention and follow-up
All enrolled patients received conventional management according to AP guidelines of International Association of Pancreatology (34) and the Chinese Society of Gastroenterology (22), including goal-directed fluid resuscitation, oxygen supply even mechanical ventilation, and nutritional support if necessary. Octreotide (Sandoz, Basel, Switzerland) was given routinely to all of patients with intravenous infusion of 50 μg/hr for 3 days (5,21,22). Meperidine 50–100 mg was injected intramuscularly if the patients complained of severe abdominal pain with the interval between 2 injections no less than 4 h. The patients started oral or nasogastric/nasojejunal tube feeding as soon as possible when abdominal pain alleviated or bowel sounds recovered. Enteral nutrition emulsion Fresubin (Fresenius Kabi SSPC, Wuxi, PR China) was feeding through oral or nasogastric tube. On day 5 after admission if the nutritional goals (25 kcal/kg/d) cannot be reached with oral or tube feeding, parenteral nutrition was supplemented by intravenous injection of fat emulsion, amino acids (17), and glucose (11%) injection Kabiven PI (Fresenius Kabi SSPC). Endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic papillotomy were performed for patients with persistently obstructive jaundice or cholangitis. Imipenem or meropenem was administered intravenously in the patients with infected necrosis or suspected infection of necrotic pancreatitis.
Besides the conventional treatment, selective COX-2 inhibitors, parecoxib and celecoxib, were given to the patients in the C+COX-2-Is group as a sequential regimen: parecoxib (Kalamazoo MI-49001; Pharmacia and Upjohn Company, Kalamazoo, MI) 40 mg per day intravenous injection for 3 days (first to third day), then celecoxib (Pfizer Pharmaceuticals LLC, Vega Baja, Puerto Rico) 200 mg oral or tube feeding twice daily for 7 days (fourth to tenth day).
All the patients were evaluated daily by the scores of APACHE-II, SIRS, and OF (1) for 8 days after the initiation of treatment. OF was evaluated according to the modified Marshall scoring system (35). The numeral rating scores of abdominal pain were assessed by the patients themselves every 4 hours during the first 3 days, ranging from 0 to 10 (0 score was painless, and 10 score was extreme pain). The serum of each patient was obtained on admission and day 4, day 8 to analyze inflammatory mediators at Department of Laboratory Medicine, West China Hospital of Sichuan University. C-reaction protein (CRP) was measured by scattering turbidimetric immunoassay with antibody against CRP from Beckman Coulter, Carlsbad. IL-6 was assessed by electrical chemiluminescent immunoassay with antibody against IL-6 from Roche, Mannheim, Germany. TNF-α was determined by microparticle chemiluminescence immunoassay with corresponding antibodies from Siemens Healthcare Diagnostics Products Limited, Gwynedd, UK. On day 8, abdominal contrast-enhanced computed tomography (CT) scans were performed and the modified CT severity index (36) was calculated. Patients were discharged when they had significantly improved and could tolerate oral diet with no fever, no abdominal pain, and almost normal blood routine and biochemical tests. During 6-month observation, all the enrolled patients were kept follow-up through outpatient interview or telephone connection with an interval of 2 weeks after discharge. On day 28–30, contrast-enhanced CT scans were performed again to detect any late local complications.
Adverse events were monitored carefully, and the proper managements were given as soon as possible to ensure the safety. All data were collected and recorded in the case report form.
Efficacy outcomes and endpoints
The primary outcomes were predefined as the occurrence of SAP, which is defined by the presence of persistent OF lasting more than 48 h (1). The secondary outcomes included evolvement of APACHE II and SIRS, scores of abdominal pain, the length of hospitalization, cost, and the serum levels of CRP, TNF-α, and IL-6. Late local complications include pancreatic pseudocyst and walled-off necrosis detected by contrast-enhanced CT scans after 4 weeks of onset of AP.
The endpoints include death and a complete recovery which is defined as almost normal state that the patients could tolerate oral diet with no symptoms (such as fever, abdominal pain, and vomiting), no abnormality of laboratory results (including blood/urine/stool routine examinations and blood biochemical tests), and no local complications on CT scans.
Statistical analysis
Our previous study showed that about 40% patients with predicted SAP developed into SAP (5). By using 2-sided calculations with an α of 0.05 and a power of 90% on the basis of 20% reduction of SAP, a sample size of 90 in each group was sufficient to detect the reduction caused by COX-2 inhibitors. Data were entered into a database for analysis (SPSS version 18.0 for Windows). Continuous variables were compared using the independent-sample T test or Mann-Whitney U Test. Categorical variables were compared with the χ2 test or Fisher exact test. A P value < 0.05 was considered statistically significant.
RESULTS
Participant characteristics
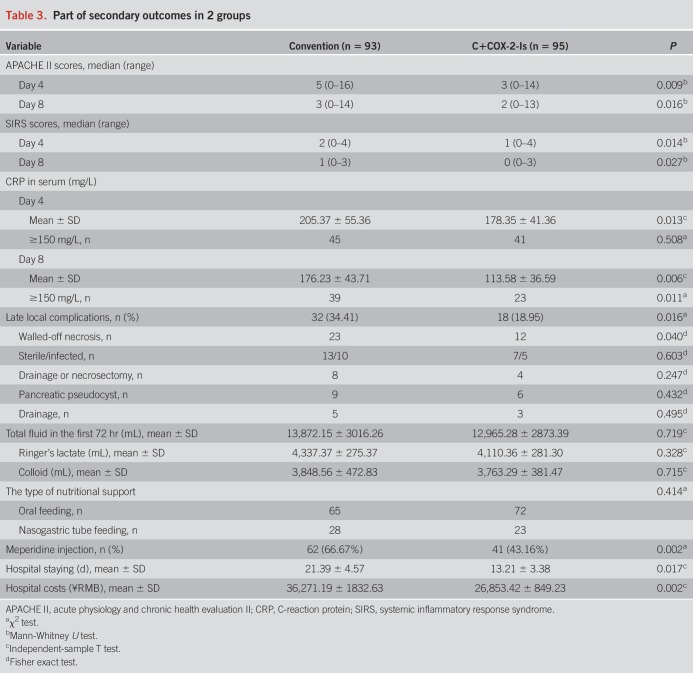
From August 2014 to July 2017, a total of 3,482 patients were diagnosed as AP in West China Hospital. Among these patients, 199 patients with predicted SAP were eligible for inclusion criteria, in which 9 patients were excluded because they met exclusion criteria. Then, 190 patients were enrolled and randomized into either convention group (n = 94) or C+ COX-2-Is group (n = 96). A 68-year-old man in the C+ COX-2-Is group discontinued the therapy because he was obviously relieved at day 5 and requested to be transferred to his local hospital. A 53-year-old woman in the convention group was lost to follow-up after discharge from hospital at day 12. Then, 188 patients (93 in the convention group and 95 in the C+ COX-2-Is group) were included into the final analysis. A patient flow chart was shown in Figure 1. The baseline data in the 2 groups were comparable, P > 0.05 (Table 1).
Figure 1.
Flow chart of recruitment and participants.
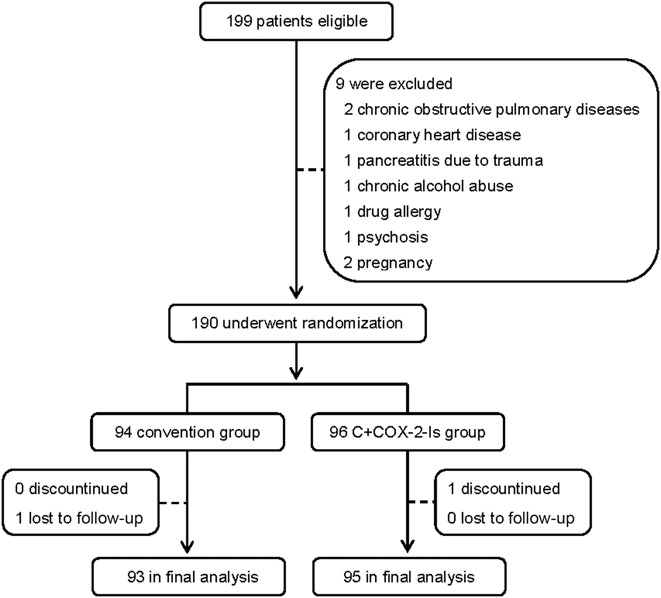
Table 1.
Characteristics of the patients at baseline
Primary outcomes
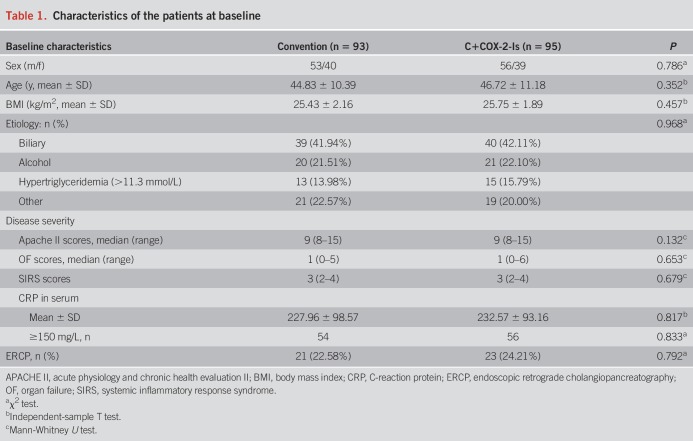
During the study, totally 57 of 188 patients (30.32%) developed to SAP. The occurrence of SAP in the C+COX-2-Is group was significantly decreased compared with the convention group (21.05% vs 39.78%, P = 0.005) (Table 2). The odds ratio (OR) for SAP of the C+COX-2-Is group to convention group was 0.404 (95% CI: 0.212–0.769). Although there was no significant difference in the occurrence of OF between 2 groups (58.06% vs 53.68%, P = 0.545), more patients underwent transient OF in the C+COX-2-Is group than the convention group (31/20 vs 17/37, P = 0.003). Accordingly, the scores of OF in the C+COX-2-Is group were significantly lower on either day 4 or day 8. Death occurred in 5 patients (2 died of OF and 3 died of infection) during 6-month observation. There was no significant difference in the mortality rate between 2 groups, 2.11% (2/95) vs 3.23% (3/93), P = 0.633 (Table 2).
Table 2.
The occurrence of SAP and organ failure in 2 groups
Secondary outcomes
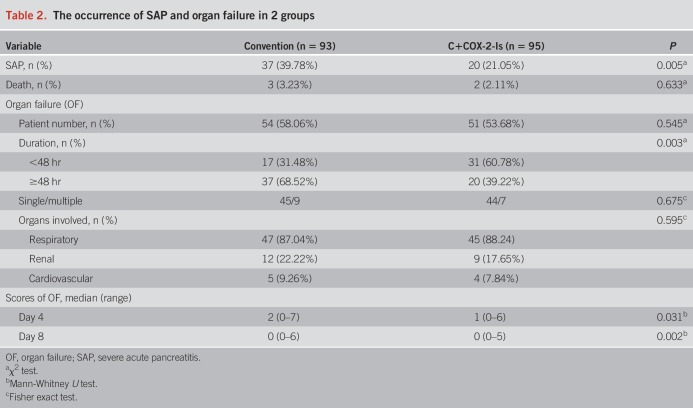
The scores of APACHE II and SIRS were significantly lower in the C+COX-2-Is group than the convention group on either day 4 or day 8 (Table 3). The late local complications occurred in 18 of 93 (18.95%) patients in the C+COX-2-Is group and 32 of 95 (34.41%) patients in the convention group (P = 0.016, Table 3). There were 12 patients with infected walled-off necrosis who had undergone catheter drainage or surgical necrosectomy. Percutaneous or endoscopic catheter drainage of pseudocysts had been performed in 8 cases (Table 3). There was no difference on the total amount of fluid in the first 72 hours and type of nutritional support between 2 groups (Table 3).
Table 3.
Part of secondary outcomes in 2 groups
On admission, the scores of abdominal pain were as high as approximately 8.0 points in both groups. Then, the scores dropped down more rapidly in the C+COX-2-Is group. The interval time from admission to significant improvement of abdominal pain (the score of 3.0) was 32 hours in the C+COX-2-Is group and 60 hours in the convention group, respectively (Figure 2). In addition, the consumption of meperidine was significantly reduced in the C+COX-2-Is group (Table 3). In addition, the length of hospital stay of the C+COX-2-Is group was significantly shorter than the convention group, P = 0.017, and the hospital costs of the C+COX-2-Is group were remarkably reduced (Table 3). An incremental reduction of cost for 1% decrease of SAP occurrence was RMB475.
Figure 2.
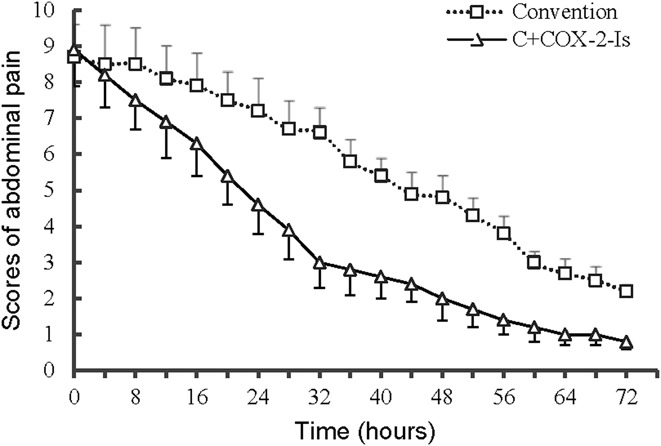
Abdominal pain scores in the C+COX-2-Is and conventional treatment groups.
On admission, serum levels of CRP of all patients in this study were increased sharply, and the levels over 150 mg/L occurred in more than half of them. After treatment (On day 4 and day 8), CRP levels decreased, more greatly in the C+COX-2-Is group than the convention group. Serum levels of TNF-α and IL-6 in all of enrolled patients were significantly higher over the upper limit of normal (TNF-α < 8.1 pg/mL, IL-6 < 7.00 pg/mL) at enrollment (day 0), and there was no difference between the 2 groups. After treatment, the levels decreased in both groups on day 4 and day 8, significantly in the C+COX-2-Is group compared with the convention group, P < 0.05 (Figure 3).
Figure 3.
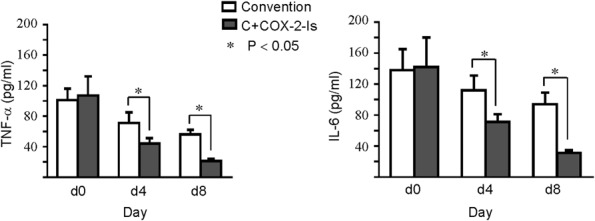
Serum levels of TNF-α and IL-6 in the C+COX-2-Is and conventional treatment groups. IL-6, interleukin-6; TNF-α, tumor necrosis factor-α.
Adverse event
No severe adverse events related to parecoxib or celecoxib were observed. Scattered red maculopapule on chest and both upper limbs was found in a 26-year-old man with calculous cholecystitis in the convention group at the second day after admission. It was considered as allergic reaction to intravenous moxifloxacin. The rash disappeared soon after intramuscular injection of 25-mg promethazine hydrochloride.
DISCUSSION
Overexpression of COX-2 in rats with AP indicated that COX-2 may participate in the inflammatory reaction during AP, and experiments further demonstrated that COX-2 inhibitors attenuated the severity of pancreas and improved the renal and respiratory function (24–27,29,30). However, there is no report of COX-2 inhibitors on the treatment of AP patients till now. This is the first study to investigate the effects of COX-2 inhibitors on AP patients. The results showed that COX-2 inhibitors significantly reduced the occurrence of SAP by 47.08% in patients with predicted SAP, and the odds ratio for SAP of the C+COX-2-Is group to convention group was 0.404. It indicated that the sequential regimen of parecoxib/celecoxib may be effective on prevention of SAP.
Persistent OF has been demonstrated as the primary determinant of outcome of AP in its early phase (37,38). In this study, initially intravenous parecoxib quickly exerted its anti-inflammatory effect and then oral celecoxib kept blocking the inflammatory reaction in the C+COX-2-Is group. The data showed great reduction in the serum levels of CRP, TNF-α, and IL-6, as well as the scores of SIRS and APACHE II on day 4 and day 8. These results were similar to the experiments of rats treated with COX-2 inhibitors (26–28,39). Subsequently, more patients in the C+COX-2-Is group manifested transient OF (transient/persistent: 31/20 vs 17/37, P = 0.003) and the incidence of persistent OF was significantly decreased (21.05% vs 39.78%, P = 0.005), although there were no difference in the incidence of OF between the C+COX-2-Is group and convention group in total (53.68% vs 58.06%, P = 0.545). It was indicated that COX-2 inhibitors may block the progression of OF through anti-inflammation effect and made more patients having transient OF. However, with regard to the prognosis, no difference in mortality rate between 2 groups was shown. It may be associated with the insufficient death cases in both groups.
Local complications, especially infected pancreatic necrosis, are believed to be the main causes of bad prognosis in late phase of SAP. In this study, COX-2 inhibitors significantly decreased late local complications, 18.95% vs 34.41%, P = 0.016. Infected pancreatic necrosis occurred in 5 cases in the C+COX-2-Is group and 10 cases in the convention group, respectively, with no difference between 2 groups (P = 0.603). In literature, AP with infected pancreatic necrosis and persistent OF was called as critical AP (CAP) (40). It was reported that CAP patients had poor prognosis, its mortality increased as high as 32% (41,42). In this study, 12 patients could be diagnosed as CAP, in which 3 cases (25%) were died. However, insufficient CAP cases in this trial could not show the benefits of COX-2 inhibitors. Further studies with enough patients may be necessary to verify its effects.
Although it has been demonstrated that the excessive inflammatory reaction is the crucial mechanism of SAP, there is a lack of effective anti-inflammatory strategy to block this miserable cascade. Classical anti-inflammatory agents, such as glucocorticoids and nonsteroidal anti-inflammatory drugs (NSAIDs), have not been recommended for AP because of limited studies and their potential adverse events. Recently, a number of clinical studies and meta-analysis reported that rectal administration of NSAIDs (indomethacin or diclofenac) significantly reduce the incidence of AP after the procedure of ERCP (43–45), and NSAIDs have been recommended to prevent post-ERCP pancreatitis by the international guidelines (46,47). It indicates that NSAIDs have good effectiveness and safety on prevention of AP after ERCP. COX-2 inhibitors (parecoxib and celecoxib) belong to NSAIDs, selectively inhibiting on COX-2 receptors, having similar anti-inflammatory effects and less adverse reaction. This study firstly investigated the effects of COX-2 inhibitors on AP patients; the results showed good effectiveness and safety on prevention of SAP in patients with predicted SAP.
Meperidine has been commonly used to relieve abdominal pain in AP patients despite of its side-effects and dependence. Enlightened from parecoxib widely used for postoperative pain these years (32,33), we hypothesized the sequential regimen of parecoxib/celecoxib in the management of AP in this study. The results showed that abdominal pain was relieved effectively and rapidly with 57% reduction of meperidine consumption. High cost of SAP consumes a lot of health-care resources throughout the world (48). This study showed that COX-2 inhibitors markedly shortened the hospital staying and reduced the cost in patients with predicted SAP. About 1% decrease of SAP occurrence with COX-2 inhibitors may save RMB475. The good cost-effective regimen would be of a great value in alleviating the heavy financial burden.
There were some limitations in this clinical trial. The lack of placebo to attain double blinding may affect the pain scoring system since it is a patient-reported outcome. Being open and conducted in a single center, there might be some data bias. The Mann-Whitney U test used in this study might cut down the power of the statistical tests. Although the results showed that COX-2 inhibitors significantly reduced the occurrence of SAP, no difference in mortality rate between 2 groups was found. It may be associated with insufficient patients, indicating the necessity of a larger sample size for further evaluation.
In conclusion, sequential administration of COX-2 inhibitors (parecoxib and celecoxib) in patients with predicted SAP obtained about half-reduction of SAP occurrence through decreasing serum levels of TNF-α and IL-6. This regimen presented good cost-effectiveness and relieved abdominal pain rapidly with less meperidine consumption.
CONFLICTS OF INTEREST
Guarantor of the article: Chengwei Tang, MD, PhD.
Specific author contributions: Z.H., X.M., and X.J.: contributed equally to this work. Z.H.: study design, screening the patients and drafting of the manuscript. X.M.: collecting data and performed analyses. X.J.: follow-up of the patients after discharge and data analysis. R.W. and L.L.: clinical management and observation. M.Z.: assigning the patients and documents management. X.W.: study design and data analysis (as a statistician). C.T.: study concept and design, critical revision of the manuscript for important intellectual content. L.H.: study concept and design, clinical management, and drafting of the manuscript.
Financial support: Natural Science Funds of China (U1702281, 81670551, and 81873584); National Key R&D Program of China (2017YFA0205404); Chinesisch-Deutsches Zentrum fṻr Wissenschaftsfὅrderung (GZ1065); and Science and Technology Department of Sichuan province (2015FZ0063). Funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ SAP is still a big challenge.
✓ The experimental pancreatitis could be attenuated with COX-2 inhibitors.
WHAT IS NEW HERE
✓ Sequential administration of parecoxib and celecoxib in patients with predicted SAP obtained about half-reduction of SAP occurrence.
✓ COX-2 inhibitors prevent SAP through decreasing serum levels of TNF-α and IL-6.
✓ This regimen presented good cost-effectiveness.
Clinical trial registry website and trial number: Chinese Clinical Trial Registry (http://www.chictr.org.cn); clinical trail number ChiCTR-TRC-14005059.
REFERENCES
- 1.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis-2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102–11. [DOI] [PubMed] [Google Scholar]
- 2.Bollen1TL, Santvoort HC, Besselink MG, et al. The Atlanta classification of acute pancreatitis revisited. Br J Surg 2008;95:6–21. [DOI] [PubMed] [Google Scholar]
- 3.Park JY, Jeon TJ, Ha TH, et al. Bedside index for severity in acute pancreatitis: Comparison with other scoring systems in predicting severity and organ failure. Hepatobiliary Pancreat Dis Int 2013;12:645–50. [DOI] [PubMed] [Google Scholar]
- 4.Cho JH, Kim TN, Chung HH, et al. Comparison of scoring systems in predicting the severity of acute pancreatitis. World J Gastroenterol 2015;21:2387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang R, Yang F, Wu H, et al. High-dose versus low-dose octreotide in the treatment of acute pancreatitis: A randomized controlled trial. Peptides 2013;40:57–64. [DOI] [PubMed] [Google Scholar]
- 6.Sah RP, Dawra RK, Saluja AK. New insights into the pathogenesis of pancreatitis. Curr Opin Gastroenterol 2013;29:523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kylänpää L, Rakonczay Z, O'Reilly DA. The clinical course of acute pancreatitis and the inflammatory mediators that drive it. Int J Inflam 2012;2012:360685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayerle J, Dummer A, Sendler M, et al. Differential roles of inflammatory cells in pancreatitis. J Gastroenterol Hepatol 2012;27(Suppl):47–51. [DOI] [PubMed] [Google Scholar]
- 9.Mayer J, Rau B, Gansauge F, et al. Inflammatory mediators in human acute pancreatitis: Clinical and pathophysiological implications. Gut 2000;47:546–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Closa D, Rosello-Catafau J, Hotter G, et al. Cyclooxygenase and lipoxygenase metabolism in sodium taurocholate induced acute hemorrhagic pancreatitis in rats. Prostaglandins 1993;45(4):315–22. [DOI] [PubMed] [Google Scholar]
- 11.Foitzik T, Hotz HG, Hotz B, et al. Selective inhibition of cyclooxygenase-2 (COX-2) reduces prostaglandin E2 production and attenuates systemic disease sequelae in experimental pancreatitis. Hepatogastroenterology 2003;50(52):1159–62. [PubMed] [Google Scholar]
- 12.Fisic E, Poropat G, Bilic-Zulle L, et al. The role of IL-6, 8, and10, sTNFr, CRP, and Pancreatic elastase in the prediction of systemic complications in patients with acute pancreatitis. Gastroenterol Res Pract 2013;2013:282645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malmstrøm ML, Hansen MB, Andersen AM, et al. Cytokines and organ failure in acute pancreatitis: Inflammatory response in acute pancreatitis. Pancreas 2012;41(2):271–7. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Wang R, Tang C. Somatostatin and octreotide on the treatment of acute pancreatitis -basic and clinical studies for three decades. Curr Pharm Des 2011;17:1594–601. [DOI] [PubMed] [Google Scholar]
- 15.Jing L, Wen-Juan Y, Huang LM, et al. Immunomodulatory therapies for acute pancreatitis. World J Gastroenterol 2014;20(45):16935–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu H, Liu L, Tan Q, et al. Somatostatin limits intestinal ischemia-reperfusion injury in macaques via suppression of TLR4-NF-kappaB cytokine pathway. J Gastrointest Surg 2009;13(5):983–93. [DOI] [PubMed] [Google Scholar]
- 17.Tang C, Lan C, Wang C, et al. Amelioration of the development of multiple organ dysfunction syndrome by somatostatin via suppression of intestinal mucosal mast cells. Shock 2005;23:470–5. [DOI] [PubMed] [Google Scholar]
- 18.Zhou C, Li J, Wang H, et al. Decreased somatostatin is related to the hypersensitivity of intestinal epithelia to LPS via upregulated TLR4-TBK1 pathway in rats chronically exposed to ethanol. Alcohol 2009;43:293–303. [DOI] [PubMed] [Google Scholar]
- 19.Liu L, Tan Q, Hu B, et al. Somatostatin inhibits the production of interferon-γ by intestinal epithelial cells during intestinal ischemia-reperfusion in macaques. Dig Dis Sci 2014;59(10):2423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu L, Tan Q, Hu B, et al. Somatostatin improved B cells mature in macaques during intestinal ischemia-reperfusion. PLoS One 2015;10(7):e0133692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang F, Wu H, Li Y, et al. Prevention of severe acute pancreatitis with octreotide in obese patients: A prospective multi-center randomized controlled trial. Pancreas 2012;41(8):1206–12. [DOI] [PubMed] [Google Scholar]
- 22.Pancreas Study Group, Chinese Society of Gastroenterology, Chinese Medical Association, Editorial Board of Chinese Journal of Pancreatology, Editorial Board of Chinese Journal of Digestion. Chinese guidelines for the management of acute pancreatitis (Shanghai, 2013). J Clin Hepatol 2013;29:656–60. [Google Scholar]
- 23.Zhou ZG, Yan WW, Chen YQ, et al. Effect of inducible cyclooxygenase expression on local microvessel blood flow in acute interstitial pancreatitis. Asian J Surg 2004;27:93–8. [DOI] [PubMed] [Google Scholar]
- 24.Yan WW, Zhou ZG, Chen YD, et al. Role of COX-2 in microcirculatory disturbance in experimental pancreatitis. World J Gastroenterol 2004;10:2095–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ethridge RT, Chung DH, Slogoff M, et al. Cyclooxygenase-2 gene disruption attenuates the severity of acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology 2002;123:1311–22. [DOI] [PubMed] [Google Scholar]
- 26.Song AM, Bhagat L, Singh VP, et al. Inhibition of cyclooxygenase-2 ameliorates the severity of pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol 2002;283:G1166–74. [DOI] [PubMed] [Google Scholar]
- 27.Seo SW, Jung WS, Piao TG, et al. Selective cyclooxygenase-2 inhibitor ameliorates cholecystokinin-octapeptide-induced acute pancreatitis in rats. World J Gastroenterol 2007;13:2298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polito F, Bitto A, Irrera N, et al. Flavocoxid, a dual inhibitor of cyclooxygenase-2 and 5-lipoxygenase, reduces pancreatic damage in an experimental model of acute pancreatitis. Br J Pharmacol 2010;161:1002–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Almeida JL, Jukemura J, Coelho AM, et al. Inhibition of cyclooxygenase-2 in experimental severe acute pancreatitis. Clinics (Sao Paulo) 2006;61(4):301–6. [DOI] [PubMed] [Google Scholar]
- 30.O'Brien G, Shields CJ, Winter DC, et al. Cyclooxygenase-2 plays a central role in the genesis of pancreatitis and associated lung injury. Hepatobiliary Pancreat Dis Int 2005;4:126–9. [PubMed] [Google Scholar]
- 31.Gorsky VA, Agapov MA, Khoreva MV, et al. The effect of lornoxicam on TLR2 and TLR4 messenger RNA expression and tumor necrosis factor-α, interleukin-6, and interleukin-8 secretion in patients with systemic complications of acute pancreatitis. Pancreas 2015;44:824–30. [DOI] [PubMed] [Google Scholar]
- 32.Lloyd R, Derry S, Moore RA, et al. Intravenous or intramuscular parecoxib for acute postoperative pain in adults. Cochrane Database Syst Rev 2009;2:CD004771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei W, Zhao T, Li Y. Efficacy and safety of parecoxib sodium for acute postoperative pain: A meta-analysis. Exp Ther Med 2013;6:525–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology 2013;13(4 Suppl 2):e1–15. [DOI] [PubMed] [Google Scholar]
- 35.Marshall JC, Cook DJ, Christou NV, et al. Multiple organ dysfunction score: A reliable descriptor of a complex clinical outcome. Crit Care Med 1995;23:1638–52. [DOI] [PubMed] [Google Scholar]
- 36.Bollen TL, Singh VK, Maurer R, et al. Comparative evaluation of the modified CT severity index and CT severity index in assessing severity of acute pancreatitis. Am J Roentgenology 2011;197(2):386–92. [DOI] [PubMed] [Google Scholar]
- 37.Johnson CD, Abu-Hilal M. Persistent organ failure during the first week as a marker of fatal outcome in acute pancreatitis. Gut 2004;53:1340–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Padhan RK, Jain S, Agarwal S, et al. Primary and secondary organ failures cause mortality differentially in acute pancreatitis and should be distinguished. Pancreas 2018;47(3):302–7. [DOI] [PubMed] [Google Scholar]
- 39.Slogoff MI, Ethridge RT, Rajaraman S, et al. COX-2 inhibition results in alterations in nuclear factor (NF)-kappaB activation but not cytokine production in acute pancreatitis. J Gastrointest Surg 2004;8:511–9. [DOI] [PubMed] [Google Scholar]
- 40.Petrov MS, Shanbhag S, Chakraborty M, et al. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology 2010;139(3):813–20. [DOI] [PubMed] [Google Scholar]
- 41.Dellinger EP, Forsmark CE, Layer P, et al. Determinant-based classification of acute pancreatitis severity: An international multidisciplinary consultation. Ann Surg 2012;256:875–80. [DOI] [PubMed] [Google Scholar]
- 42.Choi JH, Kim MH, Oh D, et al. Clinical relevance of the revised Atlanta classification focusing on severity stratification system. Pancreatology 2014;5:324–9. [DOI] [PubMed] [Google Scholar]
- 43.Li X, Tao LP, Wang CH. Effectiveness of nonsteroidal anti-inflammatory drugs in prevention of post-ERCP pancreatitis: A meta-analysis. World J Gastroenterol 2014;20:12322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Puig I, Calvet X, Baylina M, et al. How and when should NSAIDs be used for preventing post-ERCP pancreatitis? A systematic review and meta-analysis. PLoS One 2014;9:e92922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sajid MS, Khawaja AH, Sayegh M, et al. Systematic review and meta-analysis on the prophylactic role of non-steroidal anti-inflammatory drugs to prevent post-endoscopic retrograde cholangiopancreatography pancreatitis. World J Gastrointest Endosc 2015;7:1341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tenner S, Baillie J, DeWitt J, et al. American college of gastroenterology guideline: Management of acute pancreatitis. Am J Gastroenterol 2013;108:1400–15. [DOI] [PubMed] [Google Scholar]
- 47.Dumonceau JM, Andriulli A, Elmunzer BJ, et al. Prophylaxis of post-ERCP pancreatitis: European society of gastrointestinal endoscopy (ESGE) guideline—updated June 2014. Endoscopy 2014;46:799–815. [DOI] [PubMed] [Google Scholar]
- 48.Fagenholz PJ, Fernández-del Castillo C, Harris NS, et al. Direct medical costs of acute pancreatitis hospitalizations in the United States. Pancreas 2007;35:302–7. [DOI] [PubMed] [Google Scholar]