Abstract
BACKGROUND
High-dose erythropoietin has been shown to have a neuroprotective effect in preclinical models of neonatal brain injury, and phase 2 trials have suggested possible efficacy; however, the benefits and safety of this therapy in extremely preterm infants have not been established.
METHODS
In this multicenter, randomized, double-blind trial of high-dose erythropoietin, we assigned 941 infants who were born at 24 weeks 0 days to 27 weeks 6 days of gestation to receive erythropoietin or placebo within 24 hours after birth. Erythropoietin was administered intravenously at a dose of 1000 U per kilogram of body weight every 48 hours for a total of six doses, followed by a maintenance dose of 400 U per kilogram three times per week by subcutaneous injection through 32 completed weeks of postmenstrual age. Placebo was administered as intravenous saline followed by sham injections. The primary outcome was death or severe neurodevelopmental impairment at 22 to 26 months of postmenstrual age. Severe neurodevelopmental impairment was defined as severe cerebral palsy or a composite motor or composite cognitive score of less than 70 (which corresponds to 2 SD below the mean, with higher scores indicating better performance) on the Bayley Scales of Infant and Toddler Development, third edition.
RESULTS
A total of 741 infants were included in the per-protocol efficacy analysis: 376 received erythropoietin and 365 received placebo. There was no significant difference between the erythropoietin group and the placebo group in the incidence of death or severe neurodevelopmental impairment at 2 years of age (97 children [26%] vs. 94 children [26%]; relative risk, 1.03; 95% confidence interval, 0.81 to 1.32; P = 0.80). There were no significant differences between the groups in the rates of retinopathy of prematurity, intracranial hemorrhage, sepsis, necrotizing enterocolitis, bronchopulmonary dysplasia, or death or in the frequency of serious adverse events.
CONCLUSIONS
High-dose erythropoietin treatment administered to extremely preterm infants from 24 hours after birth through 32 weeks of postmenstrual age did not result in a lower risk of severe neurodevelopmental impairment or death at 2 years of age. (Funded by the National Institute of Neurological Disorders and Stroke; PENUT ClinicalTrials.gov number, NCT01378273.)
Advances in the care of infants born before 28 weeks of gestation have been associated with a dramatic improvement in survival.1 However, one or more major impairments (e.g., cerebral palsy, intellectual disability, deafness, or blindness) develop in approximately 40% of infants born before 28 weeks.2,3 In addition, long-term follow-up studies have increasingly identified a higher risk of behavioral disorders — including attention-deficit disorder,4 autism,5 and psychiatric disorders6 — among children born preterm than among those born at term. There is a critical need for neuroprotective agents that can improve outcomes in preterm infants.7,8
Erythropoietin, which is used clinically for its erythropoietic effects, is also an important trophic factor in fetal brain development.9–12 Erythropoietin has been shown to have neuroprotective effects in preclinical models of neonatal brain injury.13 In addition, results of a meta-analysis of four randomized, controlled trials involving a total of 1133 infants showed that fewer infants who received erythropoietin than those who received placebo had a score of less than 70 (which corresponds to 2 SD below the mean, with higher scores indicating better performance) on the Mental Developmental Index of the Bayley Scales of Infant and Toddler Development, third edition (Bayley-III) (odds ratio, 0.51; 95% confidence interval [CI], 0.31 to 0.81; number needed to treat to prevent a score of <70 in one child, 14).14 We performed the Preterm Erythropoietin Neuroprotection Trial (PENUT), a phase 3, randomized, placebo-controlled, double-blind trial, to assess the safety and efficacy of early high-dose erythropoietin for neuroprotection in extremely preterm infants.15
METHODS
PATIENTS, TRIAL DESIGN, AND OVERSIGHT
Infants were eligible if they were born between 24 weeks 0 days and 27 weeks 6 days of gestation. Parental consent was obtained before or after birth, as permitted by the institutional review board at each site. Exclusion criteria were known life-threatening anomalies, chromosomal anomalies, disseminated intravascular coagulopathy, twin-to-twin transfusion, a hematocrit level above 65%, hydrops fetalis, or known congenital infection.
Randomization was stratified according to recruitment site, single or multiple birth, and gestational age (24 weeks 0 days to 25 weeks 6 days or 26 weeks 0 days to 27 weeks 6 days). We used block randomization within sites with variable blocks of 4, 6, 8, and 10 infants. Infants from the same pregnancy (i.e., twins or triplets) were assigned to the same group. Randomization sequences were generated at a central data coordinating center and were provided directly to the research pharmacy with the use of a trial binder that contained the complete set of trial identification numbers and associated randomization assignments.
Enrollment and initial administration of erythropoietin or placebo occurred within 24 hours after birth. Infants received erythropoietin at a dose of 1000 U per kilogram or placebo (saline) intravenously every 48 hours for a total of six doses; thereafter, infants received maintenance subcutaneous injections of erythropoietin at a dose of 400 U per kilogram of body weight or sham injections, three times per week through 32 weeks 6 days of postmenstrual age. With the exception of the staff at the data coordinating center, the site pharmacist, and the staff who administered the maintenance injections, all trial personnel were unaware of the trial-group assignments. Additional details of the protocol have been published previously15; the protocol, with the statistical analysis plan, is available with the full text of this article at NEJM.org.
The trial was approved by the institutional review board at each participating site and was registered with the Food and Drug Administration (investigational new drug application 12656).15 The first, second, and last authors vouch for the accuracy and completeness of the data and analyses and for the fidelity of the trial to the protocol.
ULTRASONOGRAPHY, IRON SUPPLEMENTATION, AND TRANSFUSIONS
After enrollment and before the administration of erythropoietin or placebo, ultrasonography of the head was performed. Sequential ultrasonographic images were obtained as part of routine clinical care on day 7, 8, or 9 and at 36 weeks of postmenstrual age.
If breast milk was unavailable, infants received a standard iron-containing formula. All infants began receiving iron supplementation when their enteral feeding volume had reached 60 ml per kilogram per day and they were at least 7 days old. Infants initially received 3 mg per kilogram per day of enteral iron. The dose was increased to 6 mg per kilogram per day when infants reached a feeding volume of 100 to 120 ml per kilogram per day.16 Serum ferritin or the ratio of zinc protoporphyrin to heme17 was assessed on days 14 and 42, and the dose of supplemental iron was adjusted accordingly. Infants who did not receive enteral feedings received parenteral iron (1.5 mg per kilogram twice a week, adjusted on the basis of the ratio of zinc protoporphyrin to heme or serum ferritin values).
Site-specific transfusion practices were allowed. The transfusion strategies used by the trial sites ranged from liberal to restrictive, which reflects the current lack of consensus regarding guidelines for transfusion strategies.18
PRIMARY OUTCOME
The primary outcome was death or severe neurodevelopmental impairment at 22 to 26 months of postmenstrual age. Severe neurodevelopmental impairment was defined as the presence of severe cerebral palsy or a Bayley-III composite motor score or composite cognitive score of less than 70. Cerebral palsy was classified as hemiplegia, diplegia, or quadriplegia, and severity was determined according to the Gross Motor Function Classification System (GMFCS) (levels range from 0 [no impairment] to 5 [most severe impairment]). Severe cerebral palsy was defined as a GMFCS level higher than 2.19–22 Personnel who performed the Bayley-III assessments and standardized neurologic examinations were certified centrally. To minimize bias, the examiners were unaware of the participants’ medical histories and the results of brain-imaging studies.
SECONDARY OUTCOMES
The key secondary outcome was death or moderate-to-severe neurodevelopmental impairment. Moderate-to-severe neurodevelopmental impairment was defined as moderate cerebral palsy (a GMFCS level of 2) or a Bayley-III composite motor score or composite cognitive score of less than 85 (which corresponds to 1 SD below the mean).15 Other prespecified secondary outcomes were adverse events and death or severe neurodevelopmental impairment assessed according to sex. A subgroup of 220 infants at selected sites underwent magnetic resonance imaging of the head at 36 weeks of postmenstrual age, and plasma biomarkers were evaluated. Additional secondary analyses involving these measures are described in the statistical analysis plan and are not reported here. Data regarding transfusions (average cumulative volume, number of transfusions, and the number of unique donors per infant) were compared between groups in post hoc analyses.
ADVERSE EVENTS
We recorded the incidence and severity of common sequelae of prematurity: bronchopulmonary dysplasia (defined by the use of supplemental oxygen at 36 weeks of postmenstrual age),23 any intracranial hemorrhage (classified according to Papile grade),24 periventricular leukomalacia,25 any culture-positive sepsis, any stage of necrotizing enterocolitis (classified according to Bell’s stage, with stages ranging from 1 to 3 and higher stages indicating greater severity of disease),26 patent ductus arteriosus, any stage of retinopathy of prematurity,27 and hemangiomas present on day 5 or at the time of the physical examination performed at discharge.
Serious adverse events were prespecified and had to be reported within 72 hours after identification. Serious adverse events that were classified as potentially related to erythropoietin on the basis of safety profiles in adults included any symptomatic thrombosis involving a major vessel that was unrelated to an infusion catheter and that resulted in anticoagulation therapy (e.g., superior vena cava syndrome), polycythemia (defined as a hematocrit level >65% [measured from a blood sample obtained from a vein] or an increase of ≥15% in hematocrit level in the absence of a preceding blood transfusion), hypertension (defined by receipt of antihypertensive therapy for more than 1 month, discharge with medication, or both), or other expected or unexpected life-threatening event. Serious adverse events potentially related to prematurity (but not necessarily to erythropoietin) included severe pulmonary hemorrhage, severe necrotizing enterocolitis (defined as Bell’s stage 2b or 3),26 severe retinopathy of prematurity resulting in laser surgery or bevacizumab therapy, severe sepsis (defined as culture-proven bacterial or fungal sepsis resulting in blood-pressure support or substantive new respiratory support), grade 3 or 4 intracranial hemorrhage, cardiac arrest that did not result in death, and death.
STATISTICAL ANALYSIS
Our trial design assumed that 18% of the infants would die and that trial-group assignment would have no effect on mortality; moreover, we hypothesized that treatment with erythropoietin would result in a 25% lower rate of neurodevelopmental impairment than placebo (30% vs. 40%). We calculated that 376 infants per group (a total of 752) would be needed to give the trial more than 80% power to show this 25% difference.15 Because multiple births account for 25% of extremely preterm infants, we increased the total sample size to 846 infants, using a variance inflation factor of 1.125 to account for correlation within siblings from the same pregnancy (assuming a correlation of 0.5 and an average cluster size of 1.25). We anticipated attrition of 12.5% of the infants and therefore planned for an overall total enrollment of 940 infants.
A modified intention-to-treat approach was used for the safety analysis, which included all infants who underwent randomization and received the first dose of erythropoietin or placebo. The safety analysis compared the percentage of serious adverse events in the two groups that occurred from the time of the first dose to the time of hospital discharge. For comparisons between groups, we accounted for potential correlation within siblings from multiple gestations by using generalized estimating equations with robust standard errors to provide valid statistical inference and to fully account for the potential correlation of outcomes in siblings from the same pregnancy. We used a Wald test with a Poisson regression model for the analysis of total serious adverse events and a logistic-regression model for the analysis of individual events, with adjustment for gestational age at birth (24 weeks 0 days to 25 weeks 6 days or 26 weeks 0 days to 27 weeks 6 days) and recruitment site as fixed effects. All other noncategorical data were assessed with generalized estimating equation regression models appropriate for continuous outcomes.
We also evaluated the primary outcome of death or neurodevelopmental impairment using generalized estimating equations to account for potential correlation within siblings from the same pregnancy, with adjustment for gestational age at birth and recruitment site as a fixed effect. The primary analysis included infants with complete data and excluded data from infants known to be alive but in whom neurodevelopmental outcomes were not assessed. In sensitivity analyses, we included infants who died before the first infusion, outcomes regarding neurodevelopmental impairment in infants who were not followed up within the follow-up window, and adjudicated consensus outcomes regarding neurodevelopmental impairment in infants with missing data for the primary outcome because of a partially completed follow-up examination. For the remaining infants with missing data on neurodevelopmental impairment outcomes, we conducted multiple-imputation analyses with the multivariate imputation by chained equations (MICE) algorithm in R software28 (10 imputations) to impute missing data on neurodevelopmental impairment using variables, determined at baseline and through telephone follow-up, associated with missing primary-outcome data owing to loss to follow-up or data missing because an assessment was not possible owing to neurodevelopmental impairment in the child. Subgroups defined according to sex and gestational age at birth were prespecified; the heterogeneity of effect with respect to the primary outcome was evaluated separately for each subgroup variable with the use of a Wald test for the interaction between each term and treatment. Confidence intervals are reported for the treatment effect in each subgroup.
A two-sided significance level of 0.05 was used for the prespecified efficacy hypothesis test and for the final safety analysis that compared serious adverse events between groups. An independent data and safety monitoring board formally reviewed the accumulated serious adverse events in three interim analyses (when 25%, 50%, and 75% of infants had been enrolled). A fourth (final) analysis used significance thresholds of 0.031 for death and 0.004 for the 10 individual serious adverse events because of sequential monitoring with an O’Brien–Fleming stopping boundary. All other comparisons were considered exploratory and were not adjusted for multiple testing. Statistical analyses were performed with the use of R software, version 3.3.0.
RESULTS
PATIENTS
A total of 941 infants were enrolled from December 2013 through September 2016 at 19 sites that comprised 30 hospitals; 477 infants were assigned to the erythropoietin group and 464 to the placebo group (Fig. 1). Three infants in the placebo group and 1 infant in the erythropoietin group died before administration of the first infusion and were not included in the analyses. One infant in the placebo group was excluded because of a complication with consent. The modified intention-to-treat population for the evaluation of safety therefore included 476 infants assigned to erythropoietin and 460 assigned to placebo. The evaluation of efficacy at 2 years of age included 376 children in the erythropoietin group (79%) and 365 in the placebo group (79%).
Figure 1.
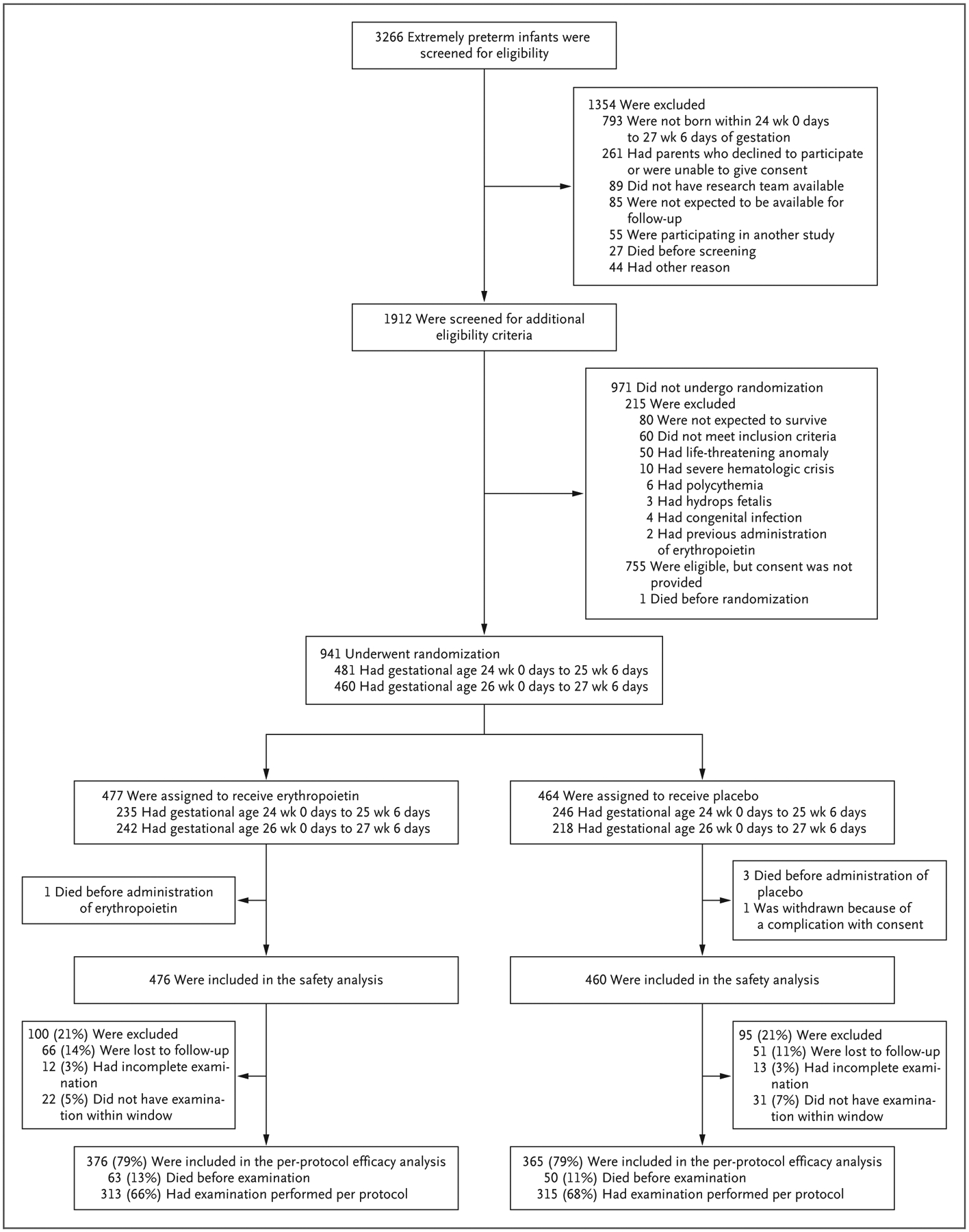
Screening, Randomization, and Follow-up.
The baseline characteristics of the mothers, infants, and pregnancies are shown in Table 1. The majority of infants received all six intravenous doses (432 of 476 infants [91%] in the erythropoietin group and 424 of 460 infants [92%] in the placebo group); 25 infants (5%) in the erythropoietin group and 26 (6%) in the placebo group died before they received all six doses. The number of infants who received all subcutaneous doses of erythropoietin or all placebo sham injections was similar in the two groups (389 infants [82%] in the erythropoietin group and 390 [85%] in the placebo group). A similar number of infants in the two groups (420 [88%] in the erythropoietin group and 412 [90%] in the placebo group) were discharged alive; the time until discharge was also similar in the two groups (median, 101 days [interquartile range, 82 to 121] and 102 days [interquartile range, 86 to 128], respectively).
Table 1.
Maternal, Pregnancy, and Infant Characteristics at Enrollment.*
| Characteristic | Erythropoietin (N = 476) | Placebo (N = 460) |
|---|---|---|
| Maternal characteristics | ||
| Age — yr | 29.1±6.2 | 28.8±6.2 |
| Hispanic ethnic group — no. (%)† | 104 (22) | 96 (21) |
| Race — no. (%)† | ||
| White | 309 (65) | 302 (66) |
| Black | 131 (28) | 109 (24) |
| Unknown or not reported | 36 (8) | 49 (11) |
| Education — no. (%) | ||
| High school or less | 148 (31) | 159 (35) |
| Some college | 153 (32) | 132 (29) |
| College degree or greater | 113 (24) | 119 (26) |
| Unknown or not reported | 62 (13) | 50 (11) |
| Pregnancy characteristics | ||
| Complications of pregnancy | ||
| Maternal indications for delivery — no. (%)‡ | 76 (16) | 76 (17) |
| Risk of infection — no. (%)§ | 349 (73) | 340 (74) |
| Pregnancy-induced hypertension — no. (%) | 39 (8) | 32 (7) |
| Prenatal glucocorticoid use — no. (%) | 430 (90) | 412 (90) |
| Prenatal magnesium sulfate use — no. (%) | 374 (79) | 375 (82) |
| Delivery complications — no. (%)¶ | 79 (17) | 70 (15) |
| Cesarean delivery — no. (%) | 337 (71) | 314 (68) |
| Delayed cord clamping — no./total no. (%) | 171/346 (49) | 147/334 (44) |
| Pregnancy with multiple fetuses — no. (%) | 125 (26) | 118 (26) |
| Infant characteristics | ||
| Female sex — no. (%) | 219 (46) | 229 (50) |
| Gestational age at birth — no. (%) | ||
| 24 wk | 113 (24) | 119 (26) |
| 25 wk | 121 (25) | 124 (27) |
| 26 wk | 103 (22) | 118 (26) |
| 27 wk | 139 (29) | 99 (22) |
| Mean gestational age at birth — wk | 26.0±1.2 | 25.8±1.1 |
| Weight — g | 806.4±194.6 | 792.9±182.2 |
| Weight <10th percentile for gestational age — no. (%) | 69 (14) | 78 (17) |
| Head circumference <10th percentile — no. (%) | 80 (17) | 81 (18) |
| Apgar score at 5 min | 6.1±2.2 | 6.2±2.1 |
| Apgar score <5 at 5 min — no. (%) | 104 (22) | 85 (18) |
| Intracranial hemorrhage before first infusion — no. (%) | 100 (21) | 94 (20) |
| Median age at first infusion (interquartile range) — hr | 21.1 (15.3–23.5) | 20.0 (14.8–23.3) |
Plus–minus values are means ±SD. Percentages may not total 100 because of rounding.
Hispanic ethnic group and race were reported by the mother.
Maternal indications for delivery were defined as eclampsia, preeclampsia, or seizures.
Risk of infection was defined as pyrexia, chorioamnionitis, prolonged rupture of membranes, administration of antibiotic agents, or preterm labor.
Delivery complications were defined as the presence of one or more of the following complications during delivery: cord prolapse, true cord knot, tear or rupture of the cord, placental abruption, twin-to-twin transfusion, fetal or maternal bleeding, uterine rupture, or complications related to delivery with instruments.
OUTCOMES
Death or severe neurodevelopmental impairment occurred in 97 of 376 children (26%) in the erythropoietin group and in 94 of 365 (26%) in the placebo group (relative risk, 1.03; 95% CI, 0.81 to 1.32; P = 0.80) (Fig. 2). Death occurred in 13% in the erythropoietin group and in 11% in the placebo group (relative risk, 1.27; 95% CI, 0.91 to 1.79), and severe neurodevelopmental impairment occurred in 11% and 14%, respectively (relative risk, 0.79; 95% CI, 0.51 to 1.22). The rate of death or severe neurodevelopmental impairment was lower among children who had been born at a later gestational age, but there was no difference in treatment effect according to gestational-age group or sex. No meaningful treatment effect was observed with respect to moderate neurodevelopmental impairment or in sensitivity analyses of the primary outcome that included deaths that occurred before the first dose (4 infants), outcome assessments that were not performed within the prespecified time windows (53 infants), or adjudicated outcomes because of partially completed follow-up examinations (25 infants). An analysis that used multiple imputation for missing primary-outcome data showed results that were similar to those of the primary analysis (117 children; relative risk, 1.00; 95% CI, 0.80 to 1.25).
Figure 2. Primary and Secondary Efficacy Outcomes.

The primary outcome was death or severe neurodevelopmental impairment at 22 to 26 months of postmenstrual age. Severe neurodevelopmental impairment was defined as severe cerebral palsy or a composite motor score or composite cognitive score of less than 70 (which corresponds to 2 SD below the mean, with higher scores indicating better performance) on the Bayley Scales of Infant and Toddler Development, third edition (Bayley-III). Severe cerebral palsy was defined as a Gross Motor Function Classification System (GMFCS) level higher than 2 (levels range from 0 [no impairment] to 5 [most severe impairment]). Moderate-to-severe neurodevelopmental impairment was defined as moderate cerebral palsy (a GMFCS level of 2) or a Bayley-III composite motor score or composite cognitive score of less than 85 (which corresponds to 1 SD below the mean). Relative risks were generated with the use of generalized estimating equation models adjusted for gestational age at birth and recruitment site and accounting for clustering of siblings from the same pregnancy. The diamonds indicate that the result is a key measure of interest; the center of the diamond represents the point estimate, and the width of the diamond represents the 95% confidence interval.
ADVERSE EVENTS
No significant differences between groups were observed in the percentage of infants with serious adverse events or common complications of prematurity. The frequencies of severe bronchopulmonary dysplasia, medically or surgically treated patent ductus arteriosus, and all grades of intracranial hemorrhage, necrotizing enterocolitis, and retinopathy of prematurity were similar in the two groups (Fig. 3). Among infants discharged home, hemangiomas occurred in 32 of 420 (8%) in the erythropoietin group and in 26 of 412 (6%) in the placebo group. In post hoc analyses, the median number of transfusions was 2 (interquartile range, 0 to 5) in the erythropoietin group and 4 (interquartile range, 2 to 7) in the placebo group. (Additional post hoc analyses of transfusions and iron supplementation in the two groups are shown in Figs. S2 and S3 in the Supplementary Appendix, available at NEJM.org.) The use of concomitant medications was similar in the two groups (Fig. S4).
Figure 3. Adverse Events.

Relative-risk estimates were generated with the use of generalized estimating equation models adjusted for gestational age at birth and clustering of siblings from the same pregnancy. The relative risk and 95% confidence interval were not calculated for polycythemia because of the small number of events. The diamonds indicate that the result is a key measure of interest; the center of the diamond represents the point estimate, and the width of the diamond represents the 95% confidence interval. Necrotizing enterocolitis was classified according to Bell’s stage (stages range from 1 to 3, with higher stages indicating greater severity of disease). Intracranial hemorrhage was classified according to Papile grade. In the category of complications of prematurity, data for severe bronchopulmonary dysplasia are shown for the 459 infants in the erythropoietin group and the 446 infants in the placebo group who survived to 36 weeks of post-natal age, and data for retinopathy of prematurity are shown for the 424 infants in the erythropoietin group and the 421 infants in the placebo group who had an ophthalmologic examination before discharge. The total numbers of serious adverse events per patient were evaluated with the use of generalized estimating equation models appropriate for count data. The assessment of blood transfusions was a post hoc analysis.
DISCUSSION
In this multicenter, placebo-controlled, randomized trial of the use of erythropoietin in extremely preterm infants, we found no significant difference between groups in the primary outcome of death or severe neurodevelopmental impairment at 2 years of age. These results are in contrast to the conclusion of a meta-analysis of four randomized trials that showed that erythropoietin reduced the risk of a Mental Developmental Index score of less than 70 at a postmenstrual age of 18 to 22 months but showed no significant effect with respect to motor function, hearing, or vision.14 Previous studies have used different dosing regimens and different durations of treatment: 400 U per kilogram, starting within 48 hours after birth29 or within 96 hours after birth30 and administered three times a week through 35 weeks of postmenstrual age; 3000 U per kilogram, administered within 3 hours after birth, at 12 to 18 hours after birth, and at 36 to 42 hours after birth31; and 500 U per kilogram, administered every other day for 2 weeks beginning within 72 hours after birth.32 Moreover, the infants enrolled in these studies were, on average, more mature (27 weeks to 30 weeks of gestational age at birth) than the infants in our trial.31,32
The current trial is larger than the previous trials, and the infants included in this trial were born at 24 weeks 0 days to 27 weeks 6 days of gestation. On the basis of animal models of brain injury, we chose a high initial erythropoietin dose to achieve neuroprotective serum levels during the first days after birth, when physiologic vulnerability is highest.33,34 The maintenance dose was chosen on the basis of previous studies of erythropoiesis-stimulating agents, with some smaller studies showing both hematologic and neurologic benefit.29 The duration of treatment was determined on the basis of the period of oligodendrocyte vulnerability (24 weeks to 32 weeks of gestational age).35 We speculate that the contributing factors to neurologic dysfunction are heterogeneous and that the targets that are responsive to erythropoietin may be diluted by pathways not affected by erythropoietin, particularly in the most premature infants.
A limitation of this trial was the use of neurodevelopmental testing at 2 years of age, which provides less reliable information than assessments performed at older ages.36 In some children who were born preterm, evaluations performed at older ages show that neurodevelopmental outcomes are better than when the child was evaluated at a younger age; in other children, assessments performed at older ages show that neurodevelopmental outcomes have become worse. A meta-analysis of 24 studies showed that positive predictive values of evaluations, performed with the use of either Bayley-III or the Griffiths Scales of Mental Development, from 1 to 3 years of age ranged from 20 to 89%, and negative predictive values ranged from 48 to 95% when children were assessed after 5 years of age. The sensitivity of early assessment to identify cognitive deficit in school-age children was 55% (95% CI, 46 to 64), and the specificity was 84% (95% CI, 77 to 89).37 Long-term follow up of the PENUT cohort is needed to identify cognitive and physical problems that may not become apparent until later in life.
The rate of death or severe neurodevelopmental impairment (26%) was lower than the predicted rate of 40%; similarly, the observed rate of the composite outcome of death or moderate-to-severe neurodevelopmental impairment (48%) was lower than the anticipated rate of 60%. The difference between the observed and predicted rates was most likely the result of our exclusion of infants with conditions known to be associated with a higher risk of death and conditions known to have adverse effects on neurodevelopment.
We found no meaningful differences between groups in any serious adverse events, including those known to occur in adults who receive long-term erythropoietin treatments (e.g., hyper-tension, thromboses, and polycythemia), or in common complications known to occur in extremely preterm infants. Specifically, in contrast to previous meta-analyses, treatment with erythropoietin did not result in a higher rate or greater severity of retinopathy of prematurity than placebo.38–40 No other safety concerns arose in this trial — a finding similar to that in the other published studies of erythropoietin for neuroprotection in neonates.14 In post hoc analyses, we found that the erythropoietin dosing regimen used in PENUT stimulated erythropoiesis, as evidenced by the lower number and lower volume of transfusions and the lower exposure to blood donors in the erythropoietin group than in the placebo group.
In summary, we did not observe that treatment with high-dose erythropoietin in extremely preterm infants resulted in a lower risk of death or in better neurodevelopmental outcomes at 2 years of age than placebo.
Supplementary Material
Acknowledgments
Supported by grants (U01NS077955 and U01NS077953) from the National Institute of Neurological Disorders and Stroke.
We thank the trial staff at all participating sites and the families who participated in the trial.
Footnotes
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Dr. Hartman reports receiving consulting fees from Best Doctors. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Matthews TJ, MacDorman MF, Thoma ME. Infant mortality statistics from the 2013 period linked birth/infant death data set. Natl Vital Stat Rep 2015; 64: 1–30. [PubMed] [Google Scholar]
- 2.Younge N, Goldstein RF, Bann CM, et al. Survival and neurodevelopmental outcomes among periviable infants. N Engl J Med 2017; 376: 617–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jarjour IT. Neurodevelopmental outcome after extreme prematurity: a review of the literature. Pediatr Neurol 2015; 52: 143–52. [DOI] [PubMed] [Google Scholar]
- 4.Franz AP, Bolat GU, Bolat H, et al. Attention-deficit/hyperactivity disorder and very preterm/very low birth weight: a meta-analysis. Pediatrics 2018; 141(1): e20171645. [DOI] [PubMed] [Google Scholar]
- 5.Joseph RM, O’Shea TM, Allred EN, et al. Prevalence and associated features of autism spectrum disorder in extremely low gestational age newborns at age 10 years. Autism Res 2017; 10: 224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Treyvaud K, Ure A, Doyle LW, et al. Psychiatric outcomes at age seven for very preterm children: rates and predictors. J Child Psychol Psychiatry 2013;54: 772–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrou S, Henderson J, Bracewell M, Hockley C, Wolke D, Marlow N. Pushing the boundaries of viability: the economic impact of extreme preterm birth. Early Hum Dev 2006; 82: 77–84. [DOI] [PubMed] [Google Scholar]
- 8.Rushing S, Ment LR. Preterm birth: a cost benefit analysis. Semin Perinatol 2004; 28: 444–50. [DOI] [PubMed] [Google Scholar]
- 9.Liu C, Shen K, Liu Z, Noguchi CT. Regulated human erythropoietin receptor expression in mouse brain. J Biol Chem 1997; 272: 32395–400. [DOI] [PubMed] [Google Scholar]
- 10.Juul SE, Anderson DK, Li Y, Christensen RD. Erythropoietin and erythropoietin receptor in the developing human central nervous system. Pediatr Res 1998; 43: 40–9. [DOI] [PubMed] [Google Scholar]
- 11.Dame C, Bartmann P, Wolber E, Fahnenstich H, Hofmann D, Fandrey J. Erythropoietin gene expression in different areas of the developing human central nervous system. Brain Res Dev Brain Res 2000; 125: 69–74. [DOI] [PubMed] [Google Scholar]
- 12.Shingo T, Sorokan ST, Shimazaki T, Weiss S. Erythropoietin regulates the in vitro and in vivo production of neuronal progenitors by mammalian forebrain neural stem cells. J Neurosci 2001; 21: 9733–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rangarajan V, Juul SE. Erythropoietin: emerging role of erythropoietin in neonatal neuroprotection. Pediatr Neurol 2014; 51: 481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer HS, Reibel NJ, Bührer C, Dame C. Prophylactic early erythropoietin for neuroprotection in preterm infants: a meta-analysis. Pediatrics 2017; 139(5): e20164317. [DOI] [PubMed] [Google Scholar]
- 15.Juul SE, Mayock DE, Comstock BA, Heagerty PJ. Neuroprotective potential of erythropoietin in neonates: design of a randomized trial. Matern Health Neonatol Perinatol 2015; 1: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franz AR, Mihatsch WA, Sander S, Kron M, Pohlandt F. Prospective randomized trial of early versus late enteral iron supplementation in infants with a birth weight of less than 1301 grams. Pediatrics 2000; 106: 700–6. [DOI] [PubMed] [Google Scholar]
- 17.German K, Vu PT, Grelli KN, Denton C, Lee G, Juul SE. Zinc protoporphyrinto-heme ratio and ferritin as measures of iron sufficiency in the neonatal intensive care unit. J Pediatr 2018; 194: 47–53. [DOI] [PubMed] [Google Scholar]
- 18.Valentine SL, Bembea MM, Muszynski JA, et al. Consensus recommendations for RBC transfusion practice in critically ill children from the Pediatric Critical Care Transfusion and Anemia Expertise Initiative. Pediatr Crit Care Med 2018; 19: 884–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 1997; 39: 214–23. [DOI] [PubMed] [Google Scholar]
- 20.Palisano RJ, Cameron D, Rosenbaum PL, Walter SD, Russell D. Stability of the Gross Motor Function Classification System. Dev Med Child Neurol 2006; 48: 424–8. [DOI] [PubMed] [Google Scholar]
- 21.Palisano RJ, Rosenbaum P, Bartlett D, Livingston MH. Content validity of the expanded and revised Gross Motor Function Classification System. Dev Med Child Neurol 2008; 50: 744–50. [DOI] [PubMed] [Google Scholar]
- 22.Rosenbaum PL, Palisano RJ, Bartlett DJ, Galuppi BE, Russell DJ. Development of the Gross Motor Function Classifica tion System for cerebral palsy. Dev Med Child Neurol 2008; 50: 249–53. [DOI] [PubMed] [Google Scholar]
- 23.Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics 1988; 82: 527–32. [PubMed] [Google Scholar]
- 24.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 1978; 92: 529–34. [DOI] [PubMed] [Google Scholar]
- 25.Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res 2001; 50: 553–62. [DOI] [PubMed] [Google Scholar]
- 26.Kliegman RM, Walsh MC. Neonatal necrotizing enterocolitis: pathogenesis, classification, and spectrum of illness. Curr Probl Pediatr 1987; 17: 213–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol 2005; 123: 991–9. [DOI] [PubMed] [Google Scholar]
- 28.van Buuren S, Groothuis-Oudshoorn K. MICE: multivariate imputation by chained equations in R. J Stat Softw 2011; 45: 1–67 (https://www.jstatsoft.org/article/view/v045i03). [Google Scholar]
- 29.Ohls RK, Kamath-Rayne BD, Christensen RD, et al. Cognitive outcomes of preterm infants randomized to darbepoetin, erythropoietin, or placebo. Pediatrics 2014; 133: 1023–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohls RK, Ehrenkranz RA, Das A, et al. Neurodevelopmental outcome and growth at 18 to 22 months’ corrected age in extremely low birth weight infants treated with early erythropoietin and iron. Pediatrics 2004; 114: 1287–91. [DOI] [PubMed] [Google Scholar]
- 31.Natalucci G, Latal B, Koller B, et al. Effect of early prophylactic high-dose recombinant human erythropoietin in very preterm infants on neurodevelopmental outcome at 2 years: a randomized clinical trial. JAMA 2016; 315: 2079–85. [DOI] [PubMed] [Google Scholar]
- 32.Song J, Sun H, Xu F, et al. Recombinant human erythropoietin improves neurological outcomes in very preterm infants. Ann Neurol 2016; 80: 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kellert BA, McPherson RJ, Juul SE. A comparison of high-dose recombinant erythropoietin treatment regimens in brain-injured neonatal rats. Pediatr Res 2007; 61: 451–5. [DOI] [PubMed] [Google Scholar]
- 34.Juul SE, McPherson RJ, Bauer LA, Led-better KJ, Gleason CA, Mayock DE. A phase I/II trial of high-dose erythropoietin in extremely low birth weight infants: pharmacokinetics and safety. Pediatrics 2008; 122: 383–91. [DOI] [PubMed] [Google Scholar]
- 35.Back SA, Riddle A, McClure MM. Maturation-dependent vulnerability of perinatal white matter in premature birth. Stroke 2007; 38: Suppl: 724–30. [DOI] [PubMed] [Google Scholar]
- 36.Marlow N, Wolke D, Bracewell MA, Samara M. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med 2005; 352: 9–19. [DOI] [PubMed] [Google Scholar]
- 37.Wong HS, Santhakumaran S, Cowan FM, Modi N. Developmental assessments in preterm children: a meta-analysis. Pediatrics 2016; 138(2): e20160251. [DOI] [PubMed] [Google Scholar]
- 38.Aher SM, Ohlsson A. Early versus late erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev 2006; 3: CD004865. [DOI] [PubMed] [Google Scholar]
- 39.Ohlsson A, Aher SM. Early erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev 2014; 4: CD004863. [DOI] [PubMed] [Google Scholar]
- 40.Ohlsson A, Aher SM. Early erythropoiesis-stimulating agents in preterm or low birth weight infants. Cochrane Database Syst Rev 2017; 11: CD004863. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


