Abstract
Arylamine N-acetyltransferases (NATs) are xenobiotic metabolizing enzymes responsible for detoxification of many drugs and carcinogens. Two NAT proteins (NAT1 and NAT2) are expressed in humans and they both N-acetylate aromatic amine carcinogens such as 4-aminobiphenyl (ABP). Arylamines such as ABP represent a large class of chemical carcinogens. Exposure to ABP occurs in the chemical, dye and rubber industries as well as in hair dyes, paints, and cigarette smoke. NAT2 is subject to a genetic polymorphism resulting in rapid, intermediate and slow acetylator phenotypes. We investigated the role of the NAT2 genetic polymorphisms on the N-acetylation of ABP in cryopreserved human hepatocytes in which NAT2 genotype and deduced phenotype were determined. Differences in sulfamethazine (selectively N-acetylated via NAT2) and ABP (N-acetylated by both NAT1 and NAT2) N-acetylation rates among rapid, intermediate, and slow NAT2 acetylator genotypes were tested for significance by one-way analysis of variance. In vitro ABP N-acetyltransferase activities differed significantly between rapid, intermediate, and slow acetylators at 10 μM (p=0.0102) or 100 μM (p= 0.0028). N-acetylation of ABP in situ also differed significantly between human hepatocytes from rapid, intermediate, and slow acetylators at 10 μM (p= 0.0015) and 100 μM (p= 0.0216). A gene dose response relationship was exhibited as intermediate acetylators catalyzed ABP N-acetylation both in vitro and in situ at rates arithmetically between rapid and slow acetylators. In conclusion, N-acetylation of ABP is NAT2 genotype-dependent in human hepatocytes. These results suggest refinement of the exposure limit and safety for arylamine carcinogens according to NAT2 genotype.
Keywords: N-acetyltransferase 2, acetylator polymorphism, 4-aminobiphenyl, human hepatocytes
Introduction
Arylamine N-acetyltransferases are xenobiotic metabolizing enzymes which play important roles in the metabolism and detoxification of many drugs [1]. Two arylamine N-acetyltransferase genes (NAT1 and NAT2) are expressed in human liver. NAT2 is subject to genetic polymorphism segregating human populations into rapid, intermediate and slow acetylators. NAT1 and NAT2 differ in substrate specificity. Arylamine drugs such as sulfamethazine (SMZ) exhibit the NAT2 genetic polymorphism in cryopreserved human hepatocytes whereas substrates such as p-aminobenzoic acid, which is selective for NAT1, do not [2].
Arylamine carcinogens are ubiquitous in the human environment and over 10% of all known or suspected human carcinogens are either an arylamine or metabolized to one [3]. 4-Aminobiphenyl (ABP) is present in chemical, dye and rubber industries as well as in hair dyes, paints, and cigarette smoke with sufficient evidence for listing as a known human carcinogen [4]. Since aromatic amines require metabolic activation to exert their carcinogenic effects, genetic polymorphisms in carcinogen-metabolizing enzymes such as NAT2 may modify cancer risk following exposure [5]. Higher levels of ABP-DNA adducts have been reported in human breast [6] from rapid versus slow NAT2 acetylators, whereas ABP-hemoglobin adducts are higher in slow acetylators [7]. As previously reported [8] and recently reviewed [3], ABP is N-acetylated by both NAT1 and NAT2.
Cryopreserved human hepatocytes have been useful in illustrating the role of the N-acetylation polymorphism for drugs such as isoniazid and hydralazine. These substrates are highly selective for NAT2 and its genotype modifies their drug efficacy and/or toxicity [1]. The purpose of this study was to assess the role of NAT2 acetylator genotype in the N-acetylation of carcinogenic aromatic amines such as ABP in cryopreserved human hepatocytes in vitro and in situ.
Materials and Methods
Source and Processing of Cryopreserved Human Hepatocytes:
Cryopreserved human hepatocytes were received from Bioreclamation IVT, (Baltimore, MD) and stored in liquid nitrogen until use. Upon removal from liquid nitrogen, hepatocytes were thawed according to the manufacturer’s instructions as previously described [2]. To mitigate possible instability of human NAT1, supernatant aliquots were thawed only once and used immediately to carry out the enzymatic reactions.
Determination of NAT2 Genotype and Deduced Phenotype:
Genomic DNA was isolated from pelleted cells prepared from human cryopreserved hepatocyte samples as described above using the QIAamp DNA Mini Kit (QIAGEN, Valencia, CA) according to the manufacturer’s instructions. NAT2 genotypes and deduced phenotypes were determined as described previously [9]. Controls (no DNA template) were run to ensure that there was no amplification of contaminating DNA.
Measurement of ABP N-Acetyltransferase Activity in Vitro:
N-acetyltransferase assays containing hepatocyte lysate (< 2 mg of protein/ml), ABP (10 or 100 μM) and 1 mM acetyl coenzyme A were incubated at 37°C for 10 minutes. Reactions were terminated by the addition of 1/10 volume of 1 M acetic acid and the reaction tubes were centrifuged in a small biofuge at 15,000 × g for 10 minutes to precipitate protein. The amount of the acetylated products was determined following separation and quantitation by high performance liquid chromatography (HPLC) as previously described [2]. For all samples, protein concentrations were determined using the Bio-Rad protein assay kit (Bio-Rad, Richmond, CA).
Cryopreserved human hepatocytes were selected at random with rapid NAT2*4/*4 (n=8), intermediate NAT2*4/*5B (n=5), NAT2*4/*6A (n=3) and slow NAT2*5B/*6A (n=5), NAT2*5B/*5B (n=2), and NAT2*7B/*7B (n=2) acetylator genotypes.
Measurement of SMZ and ABP N-Acetylation In Situ:
Cryopreserved human hepatocytes previously identified as rapid, intermediate, or slow NAT2 acetylator genotypes were thawed as described above and transferred to 50 mL conical tubes containing 12 mL of InVitroGRO CP media. One mL of hepatocyte/media mixture was transferred to each well of 12 well Biocoat® collagen coated plates to allow cells to attach for 24 hours at 37 °C. Following culture in growth media for 24 hours, the cells were washed 3 times with 500 μL 1X PBS to remove non-attached or dead hepatocytes and replaced with media with 10 or 100 μM SMZ or ABP. Hepatocytes were incubated for up to 24 hours after which media was removed and protein precipitated by addition of 1/10 volume of 1 M acetic acid. Media was centrifuged at 15,000 × g for 10 min and the supernatant used to separate and quantitate all substrates and their acetylated products by HPLC as described previously for SMZ [10] and ABP [11].
Cell number was determined after 24 hours of incubation with each substrate. For SMZ rapid genotypes NAT2*4/*4 (n=6); intermediate genotypes NAT2*4/*5B (n=4), NAT2*4/*6A (n=2); and slow genotypes NAT2*5B/*6A (n=6), NAT2*5B/*5B (n=1), NAT2*7B/*7B (n=1), NAT2*6A/*6A (n=1). For ABP rapid genotypes NAT2*4/*4 (n=6); intermediate genotypes NAT2*4/*5B (n=5), NAT2*4/*6A (n=1); and slow genotypes NAT2*5B/*6A (n=6), NAT2*5B/*5B (n=1), NAT2*7B/*7B (n=1), NAT2*6A/*6A (n=1).
Statistical Analysis
Differences in N-acetylation rates among rapid, intermediate, and slow NAT2 acetylator genotypes were tested for significance by one-way analysis of variance followed by Tukey-Kramer post-hoc test (GraphPad Prism v5.01 Software, La Jolla, CA, USA).
Results and Discussion
Previous studies showed that human NAT1 and NAT2 recombinantly expressed in E. Coli were both capable of catalyzing the N-acetylation of ABP exhibiting apparent KM of 108 and 25.8 μM for recombinant human NAT1 and NAT2, respectively [8]. While the affinity of ABP for recombinant human NAT2 was slightly higher than recombinant human NAT1, the intrinsic clearance (Vmax/KM) ratio (NAT1/NAT2) was 24 suggesting the effect of the NAT2 acetylator polymorphism could be confounded by the contribution of NAT1. Previous studies reported 2-fold differences in the N-acetylation of ABP in vitro in hepatocyte samples from rapid and slow acetylators [2] suggesting the effect of the NAT2 polymorphism on ABP metabolism may be quite modest. However, these previous studies were carried out at a high concentration (1 mM) of ABP. In the current study, we selected 10 and 100 μM ABP for both the in vitro and in situ experiments as appropriate for the apparent KM reported for recombinant human NAT1 and NAT2 towards ABP. Although we only tested two ABP concentrations (10 and 100 μM) in situ, this data generated an estimated ABP KM in situ of 16.1 μM, which is much closer to the 25.8 μM reported for recombinant human NAT2 than to the 108 μM reported for recombinant human NAT1 [8]. Thus, it appears that N-acetylation of ABP is catalyzed primarily by human NAT2 with a substantial influence of the NAT2 acetylation polymorphism on the rate of N-acetylation at relatively lower concentrations of 10 and 100 μM.
In our current study, N-acetylation rates in the cryopreserved human hepatocytes from rapid, intermediate, and slow NAT2 acetylators exhibited NAT2 genotype dependent N-acetylation of ABP (Figure 1). In vitro ABP N-acetyltransferase activities differed significantly between rapid, intermediate, and slow acetylators at 10 μM (p=0.0102) or 100 μM (p= 0.0028). The highest levels were observed in rapid acetylator, lower levels in intermediate acetylator, and the lowest levels in slow acetylator hepatocytes and the differences between rapid and slow acetylator hepatocytes were more robust at 10 μM (8-fold) and 100 μM (6-fold) than previously reported at 1 mM (2-fold). At 10 μM, the mean ABP N-acetyltransferase activity exhibited in intermediate acetylators (0.0975 nmoles/min/mg protein) was very close to the arithmetic average exhibited in rapid and slow acetylators (0.114 nmoles/min/mg). Similarly, at 100 μM, the mean ABP N-acetyltransferase activity exhibited in intermediate acetylators (0.589 nmoles/min/mg) was very close to the arithmetic average in rapid and slow acetylators (0.704 nmoles/min/mg). This confirms the co-dominant expression of N-acetyltransferase activity previously reported in rabbit [12], mouse [13], Syrian hamster [14], rat [15] and human [2] liver.
Figure 1:
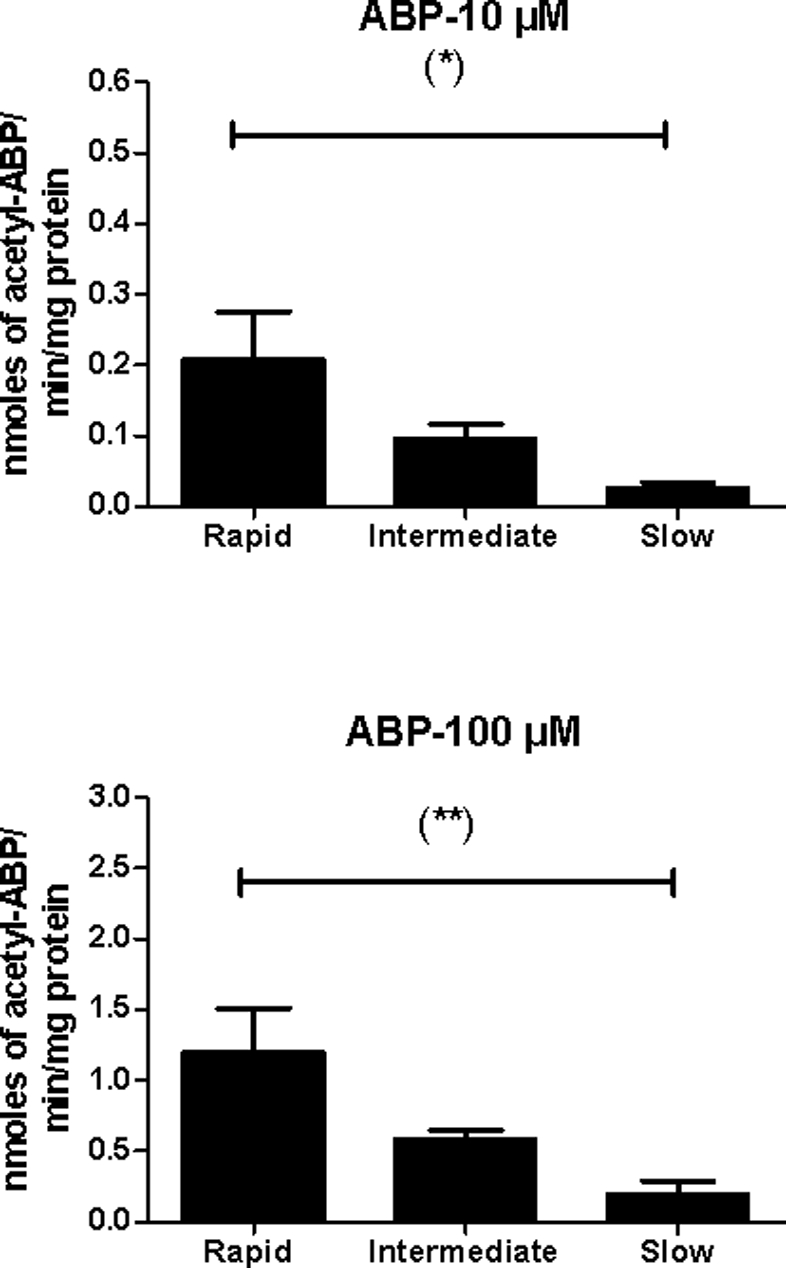
In vitro ABP N-acetyltransferase catalytic activities. Data points represent mean ± S.E.M for individual human hepatocyte samples of rapid (n=8), intermediate (n=8), and slow (n=9) NAT2 acetylator genotype. ABP N-acetyltransferase activities differed significantly between rapid, intermediate, and slow acetylators at 10 μM (p=0.0102) or 100 μM (p= 0.0028).
Previous studies reported the N-acetylation of SMZ in vitro in rapid and slow acetylator hepatocytes but these studies were conducted at higher concentrations of SMZ (300 μM) and did not include determinations in situ. We chose to also investigate the N-acetylation of a NAT2-selective substrate (SMZ) at the same low concentrations (10 and 100 μM) for which we tested ABP. In situ N-acetylation of SMZ and ABP in cryopreserved human hepatocytes was both concentration- and time-dependent (Figure 2). This is consistent with other previous reports on isoniazid [16] and hydralazine [17].
Figure 2:
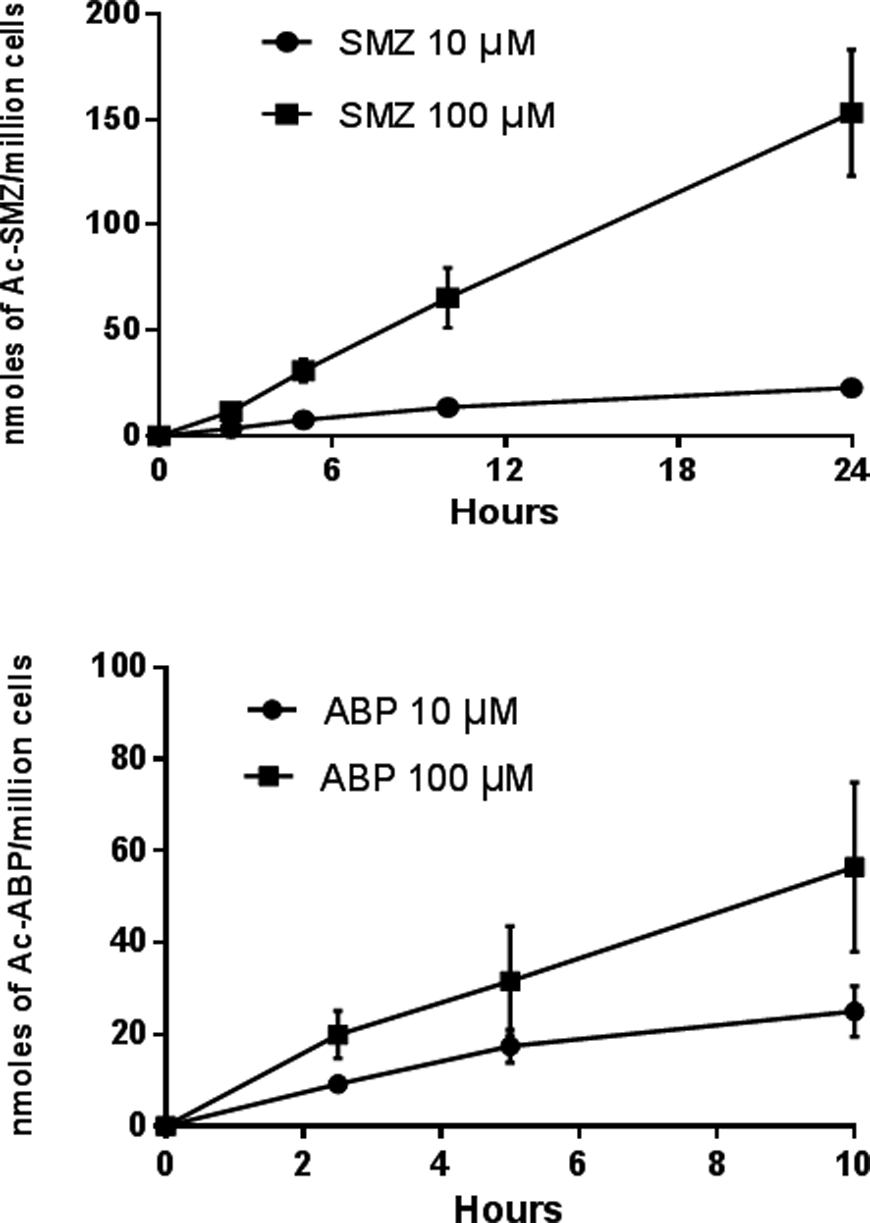
Concentration- and time-dependent N-acetylation of SMZ and ABP in cryopreserved human hepatocytes in situ. Each data point illustrates the mean ± S.E.M. in cryopreserved human hepatocytes from six individual rapid NAT2 acetylator genotypes.
The N-acetylation of SMZ and ABP was NAT2-genotype dependent with highest rates in rapid acetylators, lower rates in intermediate acetylators, and lowest rates in slow acetylators (Figure 3).
Figure 3:
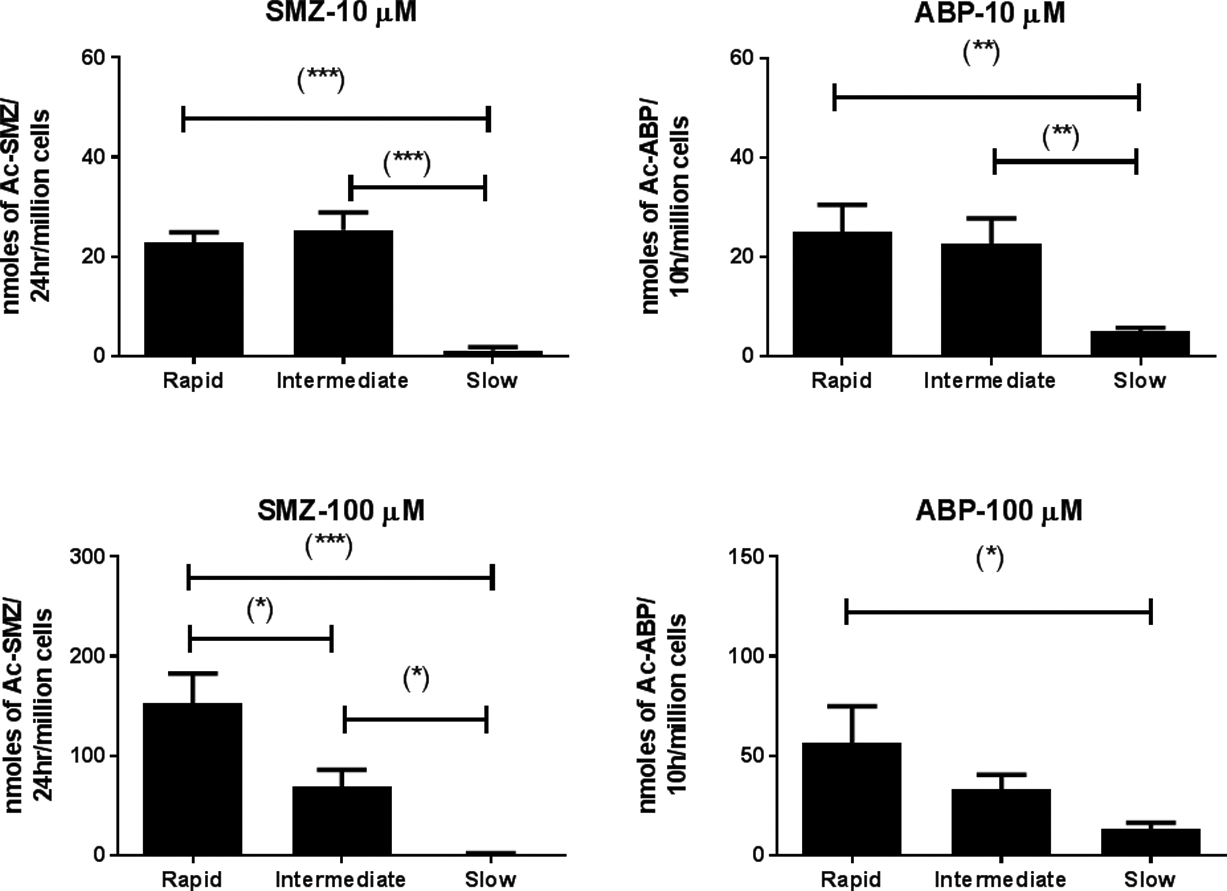
N-acetylation of SMZ (left panels) and ABP (right panels) in situ in cryopreserved human hepatocytes from rapid, intermediate, and slow NAT2 acetylator genotype. Data illustrates mean ± S.E.M. N-acetylation rates in rapid (n=6), intermediate (n=6), and slow (n=9) NAT2 acetylator genotypes. SMZ N-acetylation rates differed significantly at 10 μM and 100 μM (p< 0.0001). ABP N-acetylation rates differed significantly at 10 μM (p= 0.0015) and at 100 μM (p= 0.0216). *, p<0.05; **, p<0.01; ***, p<0.001.
The single exception to this finding was the N-acetylation of SMZ in situ at 10 μM, wherein the rapid and intermediate acetylators did not differ significantly. The apparent KM of SMZ for recombinant human NAT2 has been reported as 116 μM [8]. The observation that SMZ N-acetylation in situ was clearly NAT2 genotype-dependent at 100 μM but not at 10 μM may have resulted because the SMZ concentration of 10 μM was over 10-fold below the apparent KM for recombinant human NAT2. Trimodal distributions in N-acetylation capacity in human populations has previously been reported for SMZ following dosing regimens that provide concentrations higher than 10 μM [18,19].
N-acetylation of ABP in situ also differed significantly between human hepatocytes from rapid, intermediate, and slow acetylators at 10 μM (p= 0.0015) and 100 μM (p= 0.0216). At 100 μM, the mean ABP N-acetylation rate in intermediate acetylators (33.1 nmoles/10 hr/million cells) was very close to the arithmetic average in rapid and slow acetylators (34.8 nmoles/10 hr/million cells). These findings are the first to be reported for an aromatic amine carcinogen that mirror those recently reported for therapeutic drugs such as isoniazid [16], hydralazine [17], and solithromycin [20]. Previous studies in genetically engineered Chinese hamster ovary cells have clearly shown the effect of NAT2 acetylation polymorphism on 4-aminobiphenyl-induced genotoxicity [21,22].
In conclusion, N-acetylation of ABP is NAT2 genotype-dependent in human hepatocytes. These results suggest refinement of the exposure limit and safety for arylamine carcinogens according to NAT2 genotype.
Acknowledgements:
We thank Dr. Raúl A. Salazar-González and Dr. Kyung U. Hong for providing review and recommendations for the manuscript. The research was partially supported by NIH grants P20-GM113226 and P42-ES023716.
References
- 1.McDonagh EM, Boukouvala S, Aklillu E, Hein DW, Altman RB, Klein TE PharmGKB summary: very important pharmacogene information for N-acetyltransferase 2. Pharmacogenet genomics 2014; 24(8): 409–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doll MA, Zang Y, Moeller T, Hein DW. Codominant expression of N-acetylation and O-acetylation activities catalyzed by N-acetyltransferase 2 in human hepatocytes. J Pharmacol Exp Ther 2010; 334(2): 540–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang S, Hanna D, Sugamori KS, Grant DM. Primary aromatic amines and cancer: Novel mechanistic insights using 4-aminobiphenyl as a model carcinogen. Pharmacol Ther 2019; 200:179–189. [DOI] [PubMed] [Google Scholar]
- 4.NTP (National Toxicology Program) 2016. Report on Carcinogens, Fourteenth Edition.; Research Triangle Park, NC: U.S. Department of Health and Human Services, Public Health Service; https://ntp.niehs.nih.gov/go/roc14. [Google Scholar]
- 5.Hein DW, Doll MA, Fretland AJ, Leff MA, Webb SJ, Xiao GH, et al. Molecular genetics and epidemiology of the NAT1 and NAT2 acetylation polymorphisms. Cancer Epidemiol Biomarkers Prev 2000; 9: 29–42. [PubMed] [Google Scholar]
- 6.Ambrosone CB, Abrams SM, Gorlewska-Roberts K, Kadlubar FF. Hair dye use, meat intake, and tobacco exposure and presence of carcinogen-DNA adducts in exfoliated breast ductal epithelial cells. Arch Biochem Biophys 2007; 464(2):169–75. [DOI] [PubMed] [Google Scholar]
- 7.Bartsch H, Caporaso N, Coda M, Kadlubar F, Malaveille C, Skipper P et al. Carcinogen hemoglobin adducts, urinary mutagenicity, and metabolic phenotype in active and passive cigarette smokers. J Natl Cancer Inst 1990; 82(23):1826–31. [DOI] [PubMed] [Google Scholar]
- 8.Hein DW, Doll MA, Rustan TD, Gray K, Feng Y, Ferguson RJ, et al. Metabolic activation and deactivation of arylamine carcinogens by recombinant human NAT1 and polymorphic NAT2 acetyltransferases. Carcinogenesis 1993; 14: 1633–1638. [DOI] [PubMed] [Google Scholar]
- 9.Doll MA and Hein DW. Comprehensive human NAT2 genotype method using single nucleotide polymorphism-specific polymerase chain reaction primers and fluorogenic probes. Anal Biochem 2001; 288(1): 106–108. [DOI] [PubMed] [Google Scholar]
- 10.Leff MA, Epstein PN, Doll MA, Fretland AJ, Devanaboyina US, Rustan TD et al. Prostate-specific human N-acetyltransferase 2 (NAT2) expression in the mouse. J Pharmacol Exp Ther 1999; 290(1): 182–187. [PubMed] [Google Scholar]
- 11.Fretland AJ, Doll MA, Zhu Y, Smith L, Leff MA, Hein DW. Effect of nucleotide substitutions in N-acetyltransferase-1 on N-acetylation (deactivation) and O-acetylation (activation) of arylamine carcinogens: implications for cancer predisposition. Cancer Detect Prev 2002; 26(1):10–4. [DOI] [PubMed] [Google Scholar]
- 12.Hein DW, Smolen TN, Fox RR, and Weber WW, Identification of homozygous rapid and slow acetylators of drugs and environmental carcinogens among established inbred rabbit strains. J Pharmacol Exp Ther 1982; 223:40–44. [PubMed] [Google Scholar]
- 13.Hein DW, Trinidad A, Yerokun T, Ferguson RJ, Kirlin WG, and Weber WW Genetic control of acetyl coenzyme A-dependent arylamine N-acetyltransferase, hydrazine N-acetyltransferase, and N-hydroxyarylamine O- acetyltransferase enzymes in C57BL/6J, A/J, AC57F1, and the rapid and slow acetylator A.B6 and B6.A congenic inbred mouse. Drug Metab Dispos 1988; 16:341–347. [PubMed] [Google Scholar]
- 14.Hein DW, Doll MA, Rustan TD, Gray K, Ferguson RJ, and Feng Y. Construction of Syrian hamster lines congenic at the polymorphic acetyltransferase locus (NAT2): acetylator genotype-dependent N- and O-acetylation of arylamine carcinogens. Toxicol Appl Pharmacol 1994; 124: 16–24. [DOI] [PubMed] [Google Scholar]
- 15.Hein DW, Bendaly J, Neale JR, and Doll MA. Systemic functional expression of N-acetyltransferase polymorphism in the F344 Nat2 congenic rat. Drug Metab Dispos 2008; 36: 2452–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doll MA, Salazar-González RA, Bodduluri S, Hein DW. Arylamine N-acetyltransferase 2 genotype-dependent N-acetylation of isoniazid in cryopreserved human hepatocytes. Acta Pharm Sin B 2017; 7(4): 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen CE, Doll MA, Hein DW. N-acetyltransferase 2 genotype-dependent N-acetylation of hydralazine in human hepatocytes. Drug Metab Dispos 2017; 45(12): 1276–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chapron DJ, Kramer PA and Mercik SA. Kinetic discrimination of three sulfamethazine acetylation phenotypes. Clin Pharmacol Ther 1980; 27:104–113. [DOI] [PubMed] [Google Scholar]
- 19.Chen B, Zhang WX and Cai WM. The influence of various genotypes on the metabolic activity of NAT2 in a Chinese population. Eur J Clin Pharmacol 2006; 62:355–359. [DOI] [PubMed] [Google Scholar]
- 20.Hein DW and Doll MA. Role of the N-acetylation polymorphism in solithromycin metabolism. Pharmacogenomics 2017; 18(8): 765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bendaly J, Doll MA, Millner LM, Metry KJ, Smith NB, Pierce WM Jr et al. Differences between human slow N-acetyltransferase 2 alleles in levels of 4-aminobiphenyl-induced DNA adducts and mutations. Mutat Res, 2009; 671(1–2): p. 13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baldauf KJ, Salazar-González RA, Doll MA, Pierce WM Jr, States JC, Hein DW. Role of Human N-Acetyltransferase 2 genetic polymorphism on aromatic amine carcinogen-induced DNA damage and mutagenicity in a Chinese hamster ovary cell mutation assay. Environ Mol Mutagen, 2019; September 6 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]


