Abstract
Prior small reports have postulated a link between gastrointestinal polyposis and childhood and young adulthood cancer (CYAC) treatment (therapy-associated polyposis; TAP), but this remains a poorly understood phenomenon. The aim of this study is to describe the phenotypic spectrum of TAP in a multi-institutional cohort. TAP cases were identified from 8 high risk cancer centers. Cases were defined as patients with ≥10 gastrointestinal polyps without known causative germline alteration or hereditary CRC predisposition syndrome who had a history of prior treatment with chemotherapy and/or radiotherapy for CYAC.
34 TAP cases were included (original CYAC: 27 Hodgkin lymphoma, 3 neuroblastoma, 1 acute myeloid leukemia, 1 medulloblastoma, 1 nephroblastoma, 1 non-Hodgkin lymphoma). Gastrointestinal polyposis was first detected at a median of 27 years (interquartile range [IQR] 20-33) after CYAC treatment. 12/34 (35%) TAP cases had ≥50 colorectal polyps. 32/34 (94%) had >1 histologic polyp type. 25/34 (74%) had clinical features suggestive of ≥1 CRC predisposition syndrome (e.g., attenuated familial adenomatous polyposis (FAP), serrated polyposis syndrome, extracolonic manifestations of FAP, mismatch repair deficient CRC, or hamartomatous polyposis) including 8/34 (24%) with features of multiple such syndromes. TAP is an apparently acquired phenomenon that should be considered in patients who develop significant polyposis without known causative germline alteration but who have had prior treatment for a CYAC. TAP patients have features that may mimic various hereditary CRC syndromes, suggesting multiple concurrent biologic mechanisms, and recognition of this diagnosis may have implications for cancer risk and screening.
Keywords: radiation, adenoma, secondary cancer, MSI-H, gastrointestinal malignancy
INTRODUCTION:
Survivors of childhood and young adulthood cancers (CYAC) are at increased risk for a variety of neoplastic and non-neoplastic adverse effects years after original cancer treatment (1–3), including colorectal adenomas and colorectal cancer (CRC) (4–7). Exposure to abdominopelvic radiotherapy (RT) and/or alkylating chemotherapy has been associated with an increased risk of developing such gastrointestinal neoplasia, although the biological mechanisms remain poorly understood (4–6,8,9). Because of this increased risk, Children’s Oncology Group (COG) long term follow up guidelines were recently updated to recommend initiation of colonoscopy for CYAC survivors who received abdominopelvic RT either at age 30 or 5 years after RT, whichever occurs later, and continue every 5 years, with those without prior abdominopelvic RT recommended to begin CRC screening at age 45 and continue at 10 year intervals (10).
We previously described a phenomenon of striking gastrointestinal polyposis developing in 5 CYAC survivors in the absence of identifiable germline or familial susceptibility (11). This apparently acquired phenotype was postulated to have been induced by prior chemotherapy and/or radiation exposure, and was therefore termed therapy-associated polyposis (TAP). More recently, Dutch investigators published an additional series of 3 apparent TAP cases in CYAC survivors treated with prior radiotherapy (12). Notably, gastrointestinal polyposis is a hallmark feature of rare hereditary CRC predisposition syndromes (including familial adenomatous polyposis [FAP], attenuated FAP, MUTYH-associated polyposis, serrated polyposis syndrome [SPS], hamartomatous polyposis syndromes, and others), and all 8 TAP cases in the literature to date lacked an identifiable germline variant in the high-risk polyposis genes Adenomatous Polyposis Coli (APC) and MutY Homolog (MUTYH).
Polyposis is also a known risk factor for the development of gastrointestinal cancers, and patients with inherited polyposis syndromes may warrant earlier and more frequent cancer screening and/or more invasive interventions. While in a large registry study of the general population (unselected for history of childhood and adulthood cancer), adenomatous polyps were seen in 26% of colonoscopies among 50-64 year old and 36% among those 65 and older, and serrated polyps were detected in 9% of colonoscopies for both the 50-64 and 65 and older groups (13), the presence of multiple polyps, however, is much less frequent such that multiple professional societies recommend that patients with polyposis be referred for genetic evaluation and high risk assessment[ The recognition of a non-hereditary polyposis phenomenon (such as TAP) would thus have important implications for management of patients and their families. The primary aim of this study is therefore to further characterize the phenotypic spectrum of TAP in a multi-institutional cohort.
METHODS:
For the purposes of this analysis, we defined TAP cases as individuals who developed polyposis without known genetic predisposition, in the setting of prior exposure to chemotherapy and/or radiotherapy for a CYAC. Polyposis was defined as cumulative lifetime incidence of ≥10 gastrointestinal polyps of any type, inclusive of the entire gastrointestinal tract. We included individuals with CYAC diagnosed at age ≤30 years or individuals diagnosed with CYAC between ages 31-45 years, if their first gastrointestinal polyp were identified ≥10 years after initial CYAC treatment. Individuals known to have a personal or family diagnosis of pathogenic or likely pathogenic germline variants in any gene(s) linked to inherited CRC susceptibility were excluded. Potential TAP cases were ascertained from IRB-approved registries at 8 cancer genetics programs (Supplementary Table 1). Investigators from individual sites identified cases with suspected TAP based on the above criteria.
Clinical data were obtained from medical record review and querying of cancer genetics registry data, including original CYAC diagnostic and treatment history; endoscopic, surgical, and pathologic findings; genetic testing results; family history of cancer and polyps; and other medical history. Quantification of polyp data were obtained from pathology reports and endoscopic records, when available, and from text descriptions included in provider notes. If number of polyps were documented as a numeric range, the lowest end of this range was used for quantification of lifetime polyp burden. If only qualitative descriptions of polyp burden were provided, “few” was coded as 3 polyps, “many” as 5 polyps, and “numerous” as 10 polyps. Tubular, villous and tubulo-villous were all categorized as “adenomas” for this study.
Each TAP case was assessed for clinical features of inherited CRC predisposition syndromes, even though none of the cases (by definition) had a known personal or familial diagnosis of any genetically defined syndrome. Patients with thyroid cancer, osteomas, hepatoblastoma, desmoid tumors, duodenal polyps, or epidermoid cysts were classified as having extracolonic features of FAP. While fundic gland polyps are part of the spectrum of FAP, these polyps typically present in large numbers (>30) in FAP (14), and we elected to consider fundic gland polyps separately given limited data available on the number of fundic gland polyps seen in our cohort. The presence of ≥10 colorectal adenomas was considered a feature of attenuated FAP. Individuals whose polyp history fulfilled the World Health Organization (WHO) 2010 SPS criterion 1 (≥5 serrated polyps proximal to the sigmoid colon, ≥2 of which were ≥1 cm) or criterion 3 (>20 serrated polyps anywhere in the colorectum) were classified as having features of SPS[ Individuals with ≥3 hamartomatous polyps of the gastrointestinal tract were classified as having features of a hamartomatous polyposis syndrome. Cases with mismatch repair deficient (MMR-D) or microsatellite instability-high (MSI-H) CRC were also considered to have features of Lynch syndrome.
RESULTS:
Thirty-four TAP patients were identified from 8 institutions (Table 1, Supplementary Table 1). Twenty-seven had previously been treated for Hodgkin lymphoma, 3 for neuroblastoma, 1 for acute myeloid lymphoma, 1 for medulloblastoma, 1 for nephroblastoma [Wilms’ tumor], and 1 for non-Hodgkin lymphoma). Subjects’ median age at the time of their original CYAC diagnosis was 18 years (interquartile range (IQR) 14-24 years). 20/34 TAP cases (59%) received known alkylating chemotherapy for their initial CYAC, 21 (62%) received abdominopelvic RT, and 12 (35%) received both alkylating chemotherapy and abdominopelvic RT (Table 1, Supplementary Table 2).
Table 1:
Clinical characteristics and original cancer history of TAP patients (n=34)
| n | (%)* | |
|---|---|---|
| Gender | ||
| Male | 21 | (62) |
| Female | 13 | (38) |
| Type of original cancer | ||
| Hodgkin lymphoma | 27 | (79) |
| Neuroblastoma | 3 | (9) |
| Acute myeloid leukemia | 1 | (3) |
| Medulloblastoma | 1 | (3) |
| Nephroblastoma | 1 | (3) |
| Non-Hodgkin lymphoma | 1 | (3) |
| Median age (years) at original cancer diagnosis (IQR) | 18(14-24) | |
| Treatment received for original cancer** | ||
| Chemotherapy (any) | 29 | (85) |
| Alkylating chemotherapy | 20 | (59) |
| Radiation (any) | 28 | (82) |
| Abdominopelvic Radiation | 21 | (62) |
| Unknown | 3 | (9) |
| Family history | ||
| FDR with CRC before age 50 | 0 | (0) |
| FDR with ≥ 10 polyps | 2 | (6) |
| SDR with CRC before age 50 | 1‡ | (3) |
| SDR with ≥ 10 polyps | 0 | (0) |
CRC: colorectal cancer, FDR: first-degree relative; IQR: interquartile range; SDR: second-degree relative
Percentages listed are of total cohort (n=34)
Categories not mutually exclusive. Please see Supplementary Table 2 for more details
Maternal grandmother with rectal cancer at age 42
Among the 34 TAP cases, gastrointestinal polyposis was first detected at a median age of 49 years (IQR 37-54) and at a median of 27 years (IQR 20-33) after initial CYAC treatment (Table 2). Patients had gastrointestinal surveillance data available from a median of 4 (IQR 2-6) colonoscopies obtained over 6 (IQR 3-9) years.
Table 2:
Gastrointestinal polyposis and other clinical manifestations of TAP (n=34)
| n | (%)* | |
|---|---|---|
| Median time (years) from initial cancer treatment to first colorectal polyp (IQR) | 27(20-33) | |
| Median age (years) at first polyp (IQR) | 49(37-54) | |
| Median number of colonoscopies (IQR) | 4(2-6) | |
| Median number of colorectal polyps (IQR) | 32(16-52) | |
| At least 20 colorectal polyps | 23 | (68) |
| At least 50 colorectal polyps | 12 | (35) |
| Clinical features suggestive of other inherited GI cancer syndromes | 25 | (74) |
| Attenuated adenomatous polyposis (≥ 10 colorectal adenomas) | 18 | (53) |
| Serrated polyposis syndrome (WHO 2010 criteria) | 10 | (29) |
| Extracolonic FAP-related neoplasia** | 6 | (18) |
| Lynch syndrome-like (MMR-D/MSI-H colorectal cancer) | 3 | (9) |
| Hamartomatous polyposis (≥3 GI hamartomatous polyps) | 1 | (3) |
| More than one of the above | 8 | (24) |
| Presence of gastroduodenal polyps† | 7 | (30) ‡ |
| Gastric hamartoma | 2 | (9) ‡ |
| Gastric hyperplastic polyps | 2 | (9) ‡ |
| Duodenal adenoma | 1 | (4) ‡ |
| Duodenal hyperplastic/serrated polyp | 1 | (4) ‡ |
| Duodenal inflammatory polyp | 1 | (4) ‡ |
| Colorectal cancer diagnosis | 10 | (29) |
| Median age (years) at colorectal cancer diagnosis (IQR) | 46(33-57) |
CRC: colorectal cancer; FAP: familial adenomatous polyposis; GI: gastrointestinal; IQR: interquartile range; MMR-D: mismatch repair deficient; MSI-H: microsatellite instability-high; WHO: World Health Organization
Percentages listed are of 34, unless otherwise specified
Includes: thyroid cancer (n=5,15%); desmoid tumor (n=2,6%); duodenal adenoma (n=1,3%); osteoma (n=1,3%)
Does not include fundic gland polyps (n=17,50%)
Percentage out of 23 with known EGD.
Of the 21 patients who received abdominopelvic RT, 5 (24%) had polyps detected prior to the age they would have been recommended to start colonoscopic screening by COG guidelines. Of the 10 cases who did not receive abdominopelvic RT, 3 (30%) had a polyp detected < age 45 (the age that would be recommended by COG to initiate colonoscopy). Three patients had unknown radiation exposure, but all had first polyp detected after age 45. Similarly, of the 9 patients who developed CRC and had known RT exposure, 3 (33%) were diagnosed prior to the COG recommended age to start colonoscopy screening (2 with history of abdominopelvic RT, 1 without abdominopelvic RT).
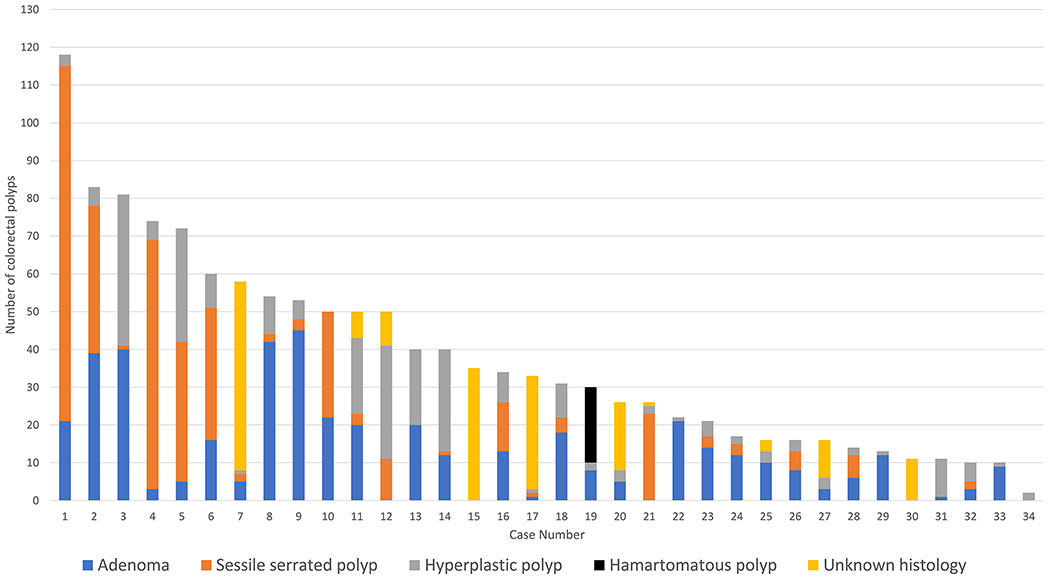
All TAP cases had colorectal polyps, with a median lifetime aggregate of 32 polyps (IQR 16-52). 23/34 TAP cases (68%) had a lifetime aggregate of ≥20 colorectal polyps, and 12 (35%) had ≥50 colorectal polyps (Figure 1). 32/34 TAP cases (94%) had more than one histologic type of colorectal polyps (the remaining two (Figure 1, cases 16 and 31) did not have polyp histology data available). Of the 23 patients known to have undergone an EGD, 7(30%) had gastric and/or duodenal polyps with one patient having an upper gastrointestinal-predominant phenotype (Figure 1, case 34). Including fundic gland polyps, 19/23 (74%) patients who underwent EGD had upper gastrointestinal polyp findings.
Figure 1:
Colorectal polyps per TAP case, stratified by histologic type of polyp (if known).
32/34 TAP cases (94%) had prior germline genetic testing. 22/34 (65%) had multi-gene panel testing (including APC and MUTYH among other genes); none had pathogenic or likely pathogenic variants in any tested gene (Supplementary Table 2). The remaining 10/34 (29%) had only single-gene testing including APC and MUTYH although one patient had testing performed after allogenic stem cell transplant with thereby uninformative results. Only 1 TAP case had a first- or second-degree relative with CRC diagnosed prior to age 50 and 2 cases (6%) had one first-degree relative with a reported history of ≥10 colorectal polyps.
25/34 (74%) TAP cases had clinical features of an inherited gastrointestinal cancer syndrome (Table 2): 18 (53%) had a colorectal adenomatous polyp burden consistent with attenuated FAP; 10 (29%) met WHO 2010 SPS criteria; 6 (18%) had extracolonic FAP related neoplasia; 3(9%) had an MMR-D/MSI-H CRC; and 1 (3%) had hamartomatous polyposis. 8/34 (24%) had features of ≥1 syndrome. If we included the presence of fundic gland polyps as an extracolonic feature of FAP, 31/34 (94%) would have met criteria for a hereditary syndrome, and 15/34 (44%) met more criteria for ≥1 hereditary CRC predisposition syndrome.
10/34 (29%) TAP cases were diagnosed with CRC. Seven CRC cases were diagnosed on the individuals’ first ever colonoscopy, with 6/7 cases (86%) detected in patients less than age 50. The majority had early stage disease (7 had stage 0/I), with only 1 each with Stage IIa and Stage IIIa CRC (1 with stage unknown). MMR-D was identified in 3 cases of CRC, all of whom had negative germline testing for the five Lynch syndrome genes. Two MMR-D CRC cases (one with loss of MSH6 and the other with loss of MSH2/MSH6) were ultimately identified to have biallelic somatic MMR gene inactivation identified on paired tumor/germline testing). The third case had loss of MLH1/PMS2 but did not have available MLH1 promoter hypermethylation testing or paired somatic testing data.
14/34 TAP (41%) cases underwent some degree of colorectal surgical resection (Supplementary Table 3); 7 were performed as treatment for a CRC and 7 were performed for management of polyposis alone.
25/34 (74%) TAP cases had other medical history suggestive of treatment-related complications: 17/34 (50%) had non-colorectal neoplastic conditions; and 16/34 (47%) had non-neoplastic sequelae of prior treatment (Table 3).
Table 3:
Other (non-polyposis) medical comorbidities and sequelae of original anti-cancer treatment (n=34)
| n | %* | |
|---|---|---|
| Non-colorectal neoplasms** | 17 | (50) |
| Barrett’s esophagus | 5 | (15) |
| Non-melanomatous skin cancer | 5 | (15) |
| Breast cancer | 4 | (12) |
| Meningioma | 2 | (6) |
| Prostate cancer | 2 | (6) |
| Schwannoma | 2 | (6) |
| Melanoma | 1 | (3) |
| Non-small cell lung cancer | 1 | (3) |
| Pancreatic adenocarcinoma | 1 | (3) |
| Renal cell carcinoma | 1 | (3) |
| Non-neoplastic conditions | 16 | (47) |
| Cardiovascular | ||
| early onset coronary artery disease | 3 | (9) |
| cardiomyopathy | 2 | (6) |
| heart block | 2 | (6) |
| valvular heart disease | 1 | (3) |
| early cardiac disease (unknown type) | 1 | (3) |
| Endocrine | ||
| hypothyroidism | 7 | (21) |
| hypogonadism | 4 | (12) |
| Gynecologic | ||
| endometriosis/polyps | 2 | (6) |
| uterine fibroids | 2 | (6) |
| Neurologic | ||
| cataracts | 1 | (3) |
| cognitive impairment | 1 | (3) |
| Pulmonary | ||
| pulmonary fibrosis | 2 | (6) |
| Any of the above (neoplastic and/or non-neoplastic) | 25 | (74) |
Percentages listed are of total cohort (n=34)
Does not include thyroid cancers (n=5) or desmoid tumors (n=2) as these were included in Table 2 as “Extracolonic FAP-related neoplasia”
DISCUSSION
In this multi-institutional study, we present 34 patients with TAP, an apparently acquired gastrointestinal polyposis phenomenon manifesting years after chemotherapy and/or radiotherapy exposure. While there already is robust literature on the development of at least one adenoma or advanced lesion in childhood and young adulthood cancer survivors (4,6,15), the identification of frank polyposis after CYAC treatment has only been described in 8 patients in the literature to date[ Importantly, despite the absence of larger studies, we suspect that this is underrecognized, and other survivor cohorts may also include potential TAP cases even if not characterized as such. For example, in an analysis of 101 Dutch Hodgkin lymphoma survivors who underwent their first colonoscopy, 6 (6%) met WHO 2010 criteria for SPS; as they were only assessed for the presence of at least one adenoma (and only had results from the first colonoscopy), it is possible that some of these patients may have met TAP criteria (6). As the default is often to manage polyposis patients as if there were a familial syndrome present, which would lead to increased screening and/or other invasive interventions for both patients and relatives, it is therefore critical to better identify patients with TAP. In our expanded cohort, nearly all TAP patients actually had features mimicking specific hereditary CRC predisposition syndromes in spite of the apparently acquired biology, and almost half of TAP cases demonstrated manifestations of multiple such syndromes.
Hereditary syndromes provide important biologic models for understanding pathways of colorectal carcinogenesis and the role of benign polyp precursors. In FAP, adenomatous polyps undergo malignant transformation via activation of Wnt signaling pathway (due to germline APC mutations) and the resultant chromosomal instability (16,17); the majority of sporadic colorectal tumors (due to somatic APC mutations) arise from adenomatous polyps via this same adenoma-carcinoma sequence (18–20). Early case reports of familial hyperplastic polyposis (now known as serrated polyposis syndrome) suggested the cancerous potential of serrated polyps (21–23); we know now that serrated polyps can be precursors for sporadic MSI-H CRC via activating BRAF mutations and the CpG island hypermethylation phenotype (CIMP) as part of the serrated neoplasia pathway (24,25). Conversely, Lynch syndrome-associated MSI-H CRC (by definition in the setting of germline alterations in MMR genes) have classically been thought to arise in adenomatous polyps via the microsatellite instability pathway (26–29). While these inherited CRC predisposition syndromes are typically associated with a single histologic polyp type, the polyps seen in TAP actually varied between and even within cases (Figure 1). For example, case 10 developed both 22 adenomas and 28 serrated polyps, meeting our criteria for attenuated FAP and SPS respectively, in addition to having an extracolonic manifestation of FAP (thyroid cancer). In fact, all TAP patients with available polyp histology data had more than one histologic type of polyp identified. We thus speculate that the development of multiple histologic polyp types in TAP may be driven by more than one molecular pathway, and that these appear to be co-occurring within the same individual.
The varying polyp histologies occurred in patients both with and without abdominopelvic radiation exposure, suggesting that any biological mechanism is not exclusive to radiation injury. It is well established that prior exposure to radiation and/or chemotherapy in CYAC survivors is associated with a broad range of late organ effects (1,2). We found that almost 75% of TAP cases also developed other non-polyposis medical conditions (e.g., secondary cancers, endocrinologic disorders, early-onset cardiac disease) and we therefore speculate that TAP patients might possess a systemic susceptibility to treatment-related toxicities, rather than a specific susceptibility to polyposis alone.
Clinical concern for an inherited polyposis syndrome, however, may be how TAP patients are first identified, especially given that distinctive features of inherited CRC syndromes are frequently present. The diagnosis of TAP may also therefore have significant implications for the screening and CRC risk of family members. In fact, whereas multiple professional society guidelines recommend early initiation of colonoscopy for relatives in suspected high-risk polyposis families (as early as age 10 for first-degree relatives of individuals with suspected FAP) (30–32), we suspect that a diagnosis of TAP may not be associated with risks of gastrointestinal neoplasia for relatives, given its presumed acquired nature. In fact, we did not find any early-onset CRC in first-degree relatives of TAP patients, although this result must be qualified by the limitations of a descriptive study. Thus, it remains unclear whether relatives of TAP cases require any early or enhanced screening for gastrointestinal neoplasia.
For childhood and young adulthood cancer survivors overall, however, CRC screening guidelines by the COG were revised in 2018 to recommend earlier and more frequent colonoscopy screening, especially among those treated with abdominopelvic RT. In our cohort, almost 20% had polyps first detected at an age prior to the COG recommended start time for colonoscopy screening, as were 33% of colon cancers; both patients with and without prior abdominopelvic fell outside of the COG screening guidelines. We emphasize that our data are insufficient to develop definitive screening recommendations, but we would propose that COG guidelines be expanded to include individuals who received chemotherapy (without abdominopelvic radiation), and that initiation of screening begin at age 35 or 10 years after age of chemotherapy, whichever occurs first. With these guidelines, none of the patients in this cohort would have been missed. Additionally, COG guidelines do not currently address upper gastrointestinal tract screening among CYAC survivors. Given that almost a third of TAP cases who underwent EGD screening had polyps in the stomach or duodenum, we would propose consideration of at least a baseline EGD at the age when colorectal polyposis is first identified.
We recognize that there are other limitations to our study. First, there is an inherent ascertainment bias and the specific age and polyp cutoffs used to define TAP cases were somewhat arbitrary. Cases were identified from high risk or cancer genetics clinics by individual providers rather than systematically from a CYAC survivor registry and we are also therefore unable to infer the prevalence of TAP. Due to the descriptive nature of this study and size of the cohort, we were unable to assess predictive factors that might suggest a CYAC survivor is at risk for TAP or would benefit from earlier colonoscopic screening.
Medical records were also incomplete regarding specific childhood and young adulthood cancer treatments, pathology reports, as many TAP patients were treated at least a decade prior to our study and/or underwent colonoscopies at outside centers. We relied on historical reports and documentation in clinic notes as available. Accordingly, we were unable to determine the anatomic location of colorectal polyps or determine if polyposis was present within radiation fields (although polyposis clearly did occur in patients without any prior abdominopelvic radiation exposure.) There also was no centralized pathologic review of polyps so it is possible that histology types may have been misclassified, particularly with regard to differentiating between hyperplastic polyps and sessile serrated polyps given the known intra-observer variability (33) and changes in WHO classification in 2010 (34). We also did not specifically have data on the frequency of advanced adenomas or other high-risk features within out cohort.
Importantly, due to limited records we did not have reliable data on the indications for colonoscopies (whether obtained for COG-based screening or diagnostic and related to patients’ symptoms), so we cannot directly assess effectiveness of these guidelines. We also could not control for unknown lifestyle factors (cigarette smoking, alcohol use, obesity and/or aspirin for example) that may also impact the risk of polyp formation.
A key limitation is that the majority of cases did not have genetic testing for all genes with known possible associations to polyposis nor did we have full germline panels for all patients. We recognize that it is therefore is possible that we may have inadvertently included cases with an inherited polyposis or cancer predisposition syndrome. Additionally, while a known history of genetic predisposition was an exclusion criterion, we did include two patients without documented negative APC and MUTYH testing, as they otherwise appeared similar to TAP patients and did not have any concerning family history. The presence of somatic APC mosaicism, which has been identified in small series of patients with unexplained polyposis (35), could not be excluded as a potential cause of adenomatous polyposis in this cohort, although this would not account for the mixed histologic types we saw in 94% of patients We were also limited by the lack of molecular-based polyp or CRC-tissue testing, so we are currently only able to speculate about the biology of TAP, though we plan to investigate this in future studies.
In conclusion, this series demonstrates that TAP should be considered in patients with significant polyposis, no known pathologic germline variant and/or family history of gastrointestinal neoplasia, and a history of prior CYAC treatment. TAP appears to be an acquired phenomenon that may mimic biologically distinct forms of inherited CRC predisposition syndromes; this raises the potential for misdiagnosis, with concomitant implications for both patient- and family-specific cancer screening recommendations. The heterogenous phenotypes and varied histologic polyp types may also suggest that multiple diverse biologic pathways are involved in TAP. Further work is needed to better understand the underlying mechanisms for polyposis development, as this may in turn inform management of TAP and other treatment-related sequelae.
Supplementary Material
Acknowledgements:
Preliminary data from this manuscript were presented in abstract form at the Annual Meeting of the Collaborative Group of the Americas on Inherited Gastrointestinal Cancer (CGA-IGC) in October, 2018, and at the 2019 International Society for Gastrointestinal Hereditary Tumours (InSiGHT) biennial meeting in March, 2019.
Supported by the National Institutes of Health (National Cancer Institute) K24CA113433 (S. Syngal), R01CA132829 (S. Syngal), K07CA151769 (F. Kastrinos), The Pussycat Foundation Helen Gurley Brown Presidential Initiative (C. Ukaegbu), and American Cancer Society Mentored Research Scholar Grant MRSG-13-144-01-CPHPS (J. Weiss)
Abbreviations:
- APC
Adenomatous Polyposis Coli
- COG
Children’s Oncology Group
- CRC
colorectal cancer
- CYAC
childhood and young adulthood cancer
- FAP
familial adenomatous polyposis
- IQR
interquartile range
- MMR-D
mismatch repair deficient
- MSI-H
microsatellite instability-high
- MUTYH
MutY Homolog
- RT
radiotherapy
- SPS
serrated polyposis syndrome
- TAP
therapy-associated polyposis
- WHO
World Health Organization
Footnotes
Disclosures/Conflict of Interest
LHB: no conflict of interest to report
CU: no conflict of interest to report
TGD: no conflict of interest to report
CAB: no conflict of interest to report
YC: no conflict of interest to report
AC: no conflict of interest to report
JMC: no conflict of interest to report
ESK: no conflict of interest to report
BHL: Advisory Board: Invitae, Speakers Bureau Myriad Genetics Lab
EL: no conflict of interest to report
RML: no conflict of interest to report
ML: no conflict of interest to report
ESM: no conflict of interest to report
RS: no conflict of interest to report
GI: no conflict of interest to report
FK: no conflict of interest to report
ES: no conflict of interest to report
JMW: no conflict of interest to report
MJH: no conflict of interest to report
MFK: no conflict of interest to report
ZKS: Immediate Family Member, Ophthalmology: Consulting/Advisory Role: Allergan, Adverum Biotechnologies, Alimera Sciences, Biomarin, Fortress Biotech, Genentech, Novartis, Optos, Regeneron, Regenxbio, Spark Therapeutics
SS: consultant for Myriad Genetics and Digital China Health Technologies and has rights to an inventor portion of the licensing revenue from PREMM5
MBY: no conflicts of interest to report
REFERENCES:
- 1.Reulen RC, Frobisher C, Winter DL, Kelly J, Lancashire ER, Stiller CA, et al. Long-term risks of subsequent primary neoplasms among survivors of childhood cancer. JAMA. 2011;305(22):2311–2319. [DOI] [PubMed] [Google Scholar]
- 2.Turcotte LM, Neglia JP, Reulen RC, Ronckers CM, Van Leeuwen FE, Morton LM, et al. Risk, risk factors, and surveillance of subsequent malignant neoplasms in survivors of childhood cancer: A review. J Clin Oncol. 2018;36(21):2145–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhakta N, Liu Q, Ness KK, Baassiri M, Eissa H, Yeo F, et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet. 2017;390(10112):2569–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teepen JC, Kok JL, van Leeuwen FE, Tissing WJE, Dolsma W V, van der Pal HJ, et al. Colorectal Adenomas and Cancers After Childhood Cancer Treatment: A DCOG-LATER Record Linkage Study. JNCI J Natl Cancer Inst. 2018;110(7):758–767. [DOI] [PubMed] [Google Scholar]
- 5.Henderson TO, Oeffinger KC, Whitton J, Leisenring W, Neglia J, Meadows A, et al. Secondary Gastrointestinal Cancer in Childhood Cancer Survivors. Ann Intern Med. 2012;156(11):757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rigter LS, Spaander MCW, Aleman BMP, Bisseling TM, Moons LM, Cats A, et al. High prevalence of advanced colorectal neoplasia and serrated polyposis syndrome in Hodgkin lymphoma survivors. Cancer. 2019;125(6):990–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daly PE, Samiee S, Cino M, Gryfe R, Pollett A, Ng A, et al. High prevalence of adenomatous colorectal polyps in young cancer survivors treated with abdominal radiation therapy: Results of a prospective trial. Gut. 2017. [DOI] [PubMed] [Google Scholar]
- 8.Rigter LS, Schaapveld M, Janus CPM, Krol ADG, van der Maazen RWM, Roesink J, et al. Overall and disease-specific survival of Hodgkin lymphoma survivors who subsequently developed gastrointestinal cancer. Cancer Med. 2019;8(1):190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nottage K, McFarlane J, Krasin MJ, Li C, Srivastava D, Robison LL, et al. Secondary colorectal carcinoma after childhood cancer. J Clin Oncol. 2012;30(20):2552–2558. [DOI] [PubMed] [Google Scholar]
- 10.Children’s Oncology Group. Long-Term Follow Up Guidelines. http://www.survivorshipguidelines.org/pdf/2018/COG_LTFU_Guidelines_v5.pdf. Published 2018. Accessed February 1, 2019.
- 11.Yurgelun MB, Hornick JL, Curry VK, Ukaegbu CI, Brown EK, Hiller E, et al. Therapy-Associated Polyposis as a Late Sequela of Cancer Treatment. Clin Gastroenterol Hepatol. 2014;12(6):1046–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rigter LS, Kallenberg FGJ, Bastiaansen B, van Os TAM, van Leeuwen FE, van Leerdam ME, et al. A case series of intestinal adenomatous polyposis of unidentified etiology; a late effect of irradiation? BMC Cancer. 2016;16(1):4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson JC, Butterly LF, Goodrich M, Robinson CM, Weiss JE. Differences in detection rates of adenomas and serrated polyps in screening versus surveillance colonoscopies, based on the new hampshire colonoscopy registry. Clin Gastroenterol Hepatol. 2013;11(10):1308–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bianchi LK, Burke CA, Bennett AE, Lopez R, Hasson H, Church JM. Fundic gland polyp dysplasia Is common in Familial Adenomatous Polyposis. Clin Gastroenterol Hepatol. 2008;6(2):180–185. [DOI] [PubMed] [Google Scholar]
- 15.Au S, Marquez V, Donnellan F, Salh BS, Nimmo M, Goddard KJ, et al. Colorectal Polyps in Childhood Cancer Survivors Treated with Radiation Therapy. Dig Dis Sci. 2018;63(9):2451–2455. [DOI] [PubMed] [Google Scholar]
- 16.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61(5):759–767. [DOI] [PubMed] [Google Scholar]
- 17.Kinzler KW, Vogelstein B. Lessons from Hereditary Colorectal Cancer. Cell. 1996;87(2):159–170. [DOI] [PubMed] [Google Scholar]
- 18.Powell SM, Zilz N, Beazer-Barclay Y, Bryan TM, Hamilton SR, Thibodeau SN, et al. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359(6392):235–237. [DOI] [PubMed] [Google Scholar]
- 19.Hill MJ, Morson BC, Bussey HJR. Aetiology of adenoma--carcinoma sequence in large bowel. Lancet. 1978;1(8058):245–247. [DOI] [PubMed] [Google Scholar]
- 20.Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM BJ. Genetic Alterations during Colorectal-Tumor Development. N Engl J Med. 1988;319(9):525–532. [DOI] [PubMed] [Google Scholar]
- 21.Jeevaratnam P, Cottier DS, Browett PJ, Van De Water NS, Pokos V, Jass JR. Familial giant hyperplastic polyposis predisposing to colorectal cancer: a new hereditary bowel cancer syndrome. J Pathol. 1996;179(1):20–25. [DOI] [PubMed] [Google Scholar]
- 22.Buchanan DD, Roberts A, Walsh MD, Parry S, Young JP. Lessons from Lynch syndrome: a tumor biology-based approach to familial colorectal cancer. Future Oncol. 2010;6(4):539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young J, Jass JR. The case for a genetic predisposition to serrated neoplasia in the colorectum: hypothesis and review of the literature. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1778–1784. [DOI] [PubMed] [Google Scholar]
- 24.Guarinos C, Sanchez-Fortun C, Rodriguez-Soler M, Perez-Carbonell L, Egoavil C, Juarez M, et al. Clinical subtypes and molecular characteristics of serrated polyposis syndrome. Clin Gastroenterol Hepatol. 2013;11(6):705–711; quiz e46. [DOI] [PubMed] [Google Scholar]
- 25.Yan HHN, Lai JCW, Ho SL, Leung WK, Law WL, Lee JFY, et al. RNF43 germline and somatic mutation in serrated neoplasia pathway and its association with BRAF mutation. Gut. 2017;66(9):1645–1656. [DOI] [PubMed] [Google Scholar]
- 26.Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260(5109):816–819. [DOI] [PubMed] [Google Scholar]
- 27.Kloor M, Huth C, Voigt AY, Benner A, Schirmacher P, von Knebel Doeberitz M, et al. Prevalence of mismatch repair-deficient crypt foci in Lynch syndrome: A pathological study. Lancet Oncol. 2012;13(6):598–606. [DOI] [PubMed] [Google Scholar]
- 28.Aaltonen LA, Peltomaki P, Leach FS, Sistonen P, Pylkkanen L, Mecklin JP, et al. Clues to the pathogenesis of familial colorectal cancer. Science. 1993;260(5109):812–816. [DOI] [PubMed] [Google Scholar]
- 29.Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993;363(6429):558–561. [DOI] [PubMed] [Google Scholar]
- 30.NCCN clinical practice guidelines in oncology: genetic/familial high risk assessment: colorectal. Version 2.2019. https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf. Published August 8, 2019. Accessed August 16, 2019.
- 31.Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW. ACG Clinical Guideline: Genetic Testing and Management of Hereditary Gastrointestinal Cancer Syndromes. Am J Gastroenterol. 2015;110(2):223–62;quiz 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stoffel EM, Mangu PB, Gruber SB, Hamilton SR, Kalady MF, Lau MWY, et al. Hereditary colorectal cancer syndromes: American Society of Clinical Oncology Clinical Practice Guideline endorsement of the familial risk-colorectal cancer: European Society for Medical Oncology Clinical Practice Guidelines. J Clin Oncol. 2015;33(2):209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glatz K, Pritt B, Glatz D, Hartmann A, O’Brien MJ, Blaszyk H. A multinational, internet-based assessment of observer variability in the diagnosis of serrated colorectal polyps. Am J Clin Pathol. 2007;127(6):938–945. [DOI] [PubMed] [Google Scholar]
- 34.Snover DC, Ahnen DJ, Burt RW, Odze RD. Serrated polyps of the colon and rectum and serrated polyposis In: Bosman T, Carneiro F, Hruban R, et al. WHO Classification of Tumours of the Digestive System. Lyon, France: IARC; 2010:160–165. [Google Scholar]
- 35.Ciavarella M, Miccoli S, Prossomariti A, Pippucci T, Bonora E, Buscherini F, et al. Somatic APC mosaicism and oligogenic inheritance in genetically unsolved colorectal adenomatous polyposis patients. Eur J Hum Genet. 2018;26(3):387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.