Abstract
High pressure in the lower limb veins is often associated with chronic venous insufficiency (CVI) and varicose veins (VVs), making it important to search for the mechanisms and agents that control venous function. We have shown that protracted increases in venous stretch/wall tension reduce vein contraction and augment matrix metalloproteinase (MMP) −2 and −9. Also, MMP-2 and MMP-9 promote venodilation, a hallmark of VVs. Sulodexide (SDX) is a blend of glycosaminoglycans with efficient pro-fibrinolysis and anti-thrombosis activities, but its actions on vein function and the mechanisms involved are unclear. We tested the hypothesis that SDX enhances venous contractile response by decreasing MMP expression/activity in veins subjected to protracted stretch. Rat inferior vena cava (IVC) rings were treated with SDX (0.001–1mg/ml) or vehicle, equilibrated under control 0.5g-resting tension or protracted 2g-stretch for 18h, and the contractile response to 96mM-KCl and phenylephrine (Phe) in SDX-treated and non-treated veins was recorded. In IVC rings under control 0.5g-resting tension, SDX caused dose-dependent contraction, 96mM-KCl caused marked contraction (176mg/mg tissue), and Phe caused dose-dependent contraction with a maximum (56mg/mg tissue) at 10−5M. In IVC subjected to protracted 2g-stretch, 96mM-KCl-induced contraction was reduced to 112mg/mg and maximal Phe-induced contraction was decreased to 23mg/mg. In IVC subjected to protracted 2g-stretch plus SDX, 96mM-KCl-induced contraction was restored to 228mg/mg and maximal Phe-induced contraction was improved to 115mg/mg. Gelatin zymography and Western blots revealed increases in MMP-2 and MMP-9 levels/gelatinolytic activity in veins subjected to protracted 2g-stretch, and reversal to control levels in veins subjected to 2g-stretch plus SDX. Thus, SDX improves vein function and augments the contractile response in veins subjected to protracted stretch. The SDX-induced improvement of contraction and restoration of vein function appear to involve decreases in MMP-2 and MMP-9 and may contribute to the benefits of SDX in CVI and VVs.
Keywords: calcium, chronic venous disease, hyperpolarization, sulodexide, varicose veins, vena cava
INTRODUCTION
Peripheral venous disease in the lower limb could present as venous thrombosis and chronic venous disease. Venous thrombosis occurs as a result of endothelial cell damage/dysfunction, slow venous blood flow, and increased blood coagulation, and could have serious consequences including pulmonary embolism and edema.1 Lower limb chronic venous disease is a common disorder with significant socio-economic ramifications. The clinical-etiology-anatomy-pathophysiology (CEAP) classification has categorized chronic venous disease into 7 different stages termed C0–6. Stage-C0 shows no visible signs of chronic venous disease, stage-C1 shows telangiectasias and spider veins, stage-C2 is associated with varicose veins (VVs), stage-C3 shows edema, stage-C4a is characterized by eczema and skin pigmentation, stage-C4b shows atrophie blanche and lipodermatosclerosis, stage-C5 shows healed ulcer, while stage-C6 shows active ulcer. The advanced stages of chronic venous disease C4–6 are often referred to as chronic venous insufficiency (CVI).2 VVs is a common venous disorder affecting ~25 million of the United States population. Clinically, VVs is manifested as excessively bulging, distended, twisted and often painful superficial veins of the lower limb. Pathologically, VVs show vein wall dilation, incompetent venous valves and venous reflux.3
Several non-surgical methods have been adopted in order to minimize the serious complications of venous thrombosis and to curtail the socio-economic burden associated with chronic venous disease, Anti-coagulation, anti-platelet and anti-thrombosis drugs including coumarin derivatives, warfarin, low-molecular-weight heparin, non-fractionated heparin and dermatan sulfate are being used clinically as therapeutic and protective agents against venous thrombo-embolism.4, 5 Compression stockings can also be used to decrease the incidence of venous thrombo-embolism. Venotonic agents have also been used in chronic venous disease to enhance venous contractile function and improve capillary permeability. Sulodexide (SDX) is a heparinoid extracted from the pig digestive mucosa. SDX, is a finely-purified blend of electrophoresis-induced rapidly-moving heparin fraction with binding affinity to anti-thrombin-III (80%), and dermatan sulfate with binding affinity to heparin cofactor-II (20%).6–8 When administered orally, SDX has both pro-fibrinolysis and anti-thrombosis effects, with reduced risk of bleeding.9–12 Clinically, SDX has shown benefits in the treatment and prophylaxis against numerous vascular disorders. Specifically, SDX has been used in the treatment of advanced stages of CVI and venous leg ulcer,13–15 and in the prophylaxis against the recurrence of venous thrombo-embolism and post-thrombosis syndromes,7, 16, 17
Pharmacological analyses have shown that oral SDX is absorbed rapidly and progressively reaching a peak plasma level, then widely distributed in different tissues including the blood vessel wall. Although glycosoaminoglycans have strong affinity to endothelial cells in both the arterial and venous wall,18, 19 we know little about the actions of SDX on blood vessels’ functions. In a recent study, we have examined the effects of SDX on rat aortic and mesenteric arterial segments and demonstrated that it promotes arterial relaxation.20, 21 However, it is difficult to generalize these effects to the whole vascular system and to both arteries and vein. Veins are structurally and functionally different form arteries. For instance, the venous endothelium has different secretory properties when compared to the arterial endothelium. Also the tunica media in the arteries has numerous well-organized layers of vascular smooth muscle (VSM) cells, whereas the tunica media in the veins has few layers of VSM cells and is less-organized. Additionally, the extracellular matrix has different composition in the arteries and veins, and matrix metalloproteinases (MMPs) play a different role in degrading extracellular matrix proteins and promoting vascular remodeling in arteries versus veins.22, 23 Therefore, it is necessary to examine how SDX could affect venous function and alter the venous expression/activity of MMPs.
We have previously used the rat inferior vena cava (IVC) model to demonstrate that subjecting the veins to extended periods of stretch/wall tension causes decreases in vein contraction and increases in the levels/activity of MMP-2 and MMP-9. The stretch-associated decreases in vein contraction were averted when the IVC was pre-treated with MMP inhibitory agents, suggesting that MMPs could function as a linking mechanism between the high venous pressure and elevated vein wall stretch, and the decreases in vein contractile response and increases in venodilation associated with venous disease and VVs.24 In the current study we tested the hypothesis that SDX affects venous function and examined the potential underlying mechanisms. We used the rat IVC model to test whether: 1) SDX modulates the vein contractile response, 2) SDX recovers venous function and improves the vein contractile response in veins subjected to prolonged stretch/wall tension, and 3) SDX affects the expression/activity of MMPs in veins under prolonged stretch.
METHODS
Tissue Preparation
Sprague Dawley male rats 12 week of age and 225–250g of weight) were purchased from Charles River Lab. (Wilmington, MA) and maintained in the animal housing in 12h:12h light:dark cycle, at 22°C room temperature. The rats were maintained on tap water and normal rodent chow (Purina, St. Louis, MO) ad libitum. The rats were euthanized by inhalation of CO2 followed by exsanguination and rapid harvesting of vital organs. After euthanasia was confirmed by stoppage of the animal heart beats and breathing, a longitudinal incision was made in the abdomen and the IVC was quickly isolated and transferred to oxygenated physiological Krebs’ solution. Using a dissection microscope, the IVC was thoroughly dissected, the surrounding fat and connective tissue were removed, then the IVC was cut into 4 rings; each ring is approximately 3mm-wide. The IVC rings were transferred to isometric contraction set-up for measurement of venous function. Different IVC rings were used for different vein contraction experiments. The rat IVC is very delicate and as thin as tissue paper, and extensive manipulation and experimentation could compromise its contractile response. In a previous study to determine endothelial function in rat IVC, we were very careful during the IVC dissection procedure in order to avoid injury to the endothelium, and found that acetylcholine caused significant relaxation, supporting integrity of the endothelium.25 Because the present study was centered on measuring the effects of SDX on venous smooth muscle contraction, we did not test the integrity of the endothelium, but we followed the same careful dissection procedure, avoided injury to the endothelium, and did not attempt to remove the endothelium as previously described,25 so presumably the endothelium was left intact under these conditions, After recording the IVC contraction, the veins were collected for biochemical studies to measure MMPs levels/activity. All experiments were performed according to the recommendations of the National Institutes of Health, and were approved by the Brigham and Women’s Hospital IACUC (Protocol:2016N000259).
Measurement of vein contraction
Isolated IVC rings were placed between two tungsten wire loops, one loop was attached to a glass holder in the bottom of an organ bath and the other loop was attached to a force transducer (Grass-FT03, Astro-Med, West Warwick, RI). In order to minimize the effects of variability in tissue size on the observed vein contraction, the IVC rings were placed in different organ baths at random. Veins were equilibrated in a water-jacketed temperature controlled (37°C) organ bath containing 50ml physiological Krebs’ solution constantly infused with a gas mixture of 95%O2-5%CO2. The vein contraction to different stimuli was displayed on a polygraph (Grass-Model-7D, Astro-Med). To correct for differences in the IVC ring diameter and size, the vein contractile response was normalized to the vein segment weight and shown as mg/mg tissue. In preliminary experiments to examine the relationship between incremental resting tension and IVC contraction, high 96mM-KCl showed maximal contraction at 0.5g-resting tension.24 When the vein resting tension was increased to 1g or 2g no further increases in 96mM-KCl-induced contraction were observed in comparison with the contraction at 0.5g-resting tension. Further increases in the vein resting tension to 3g, were associated with marked decreases in 96mM-KCl-induced contractile response. Therefore, we used 0.5g-resting tension as the control vein wall tension that generates maximal IVC contractile response, and 2g-stretch as the maximal vein wall tension that could still generate maximal IVC contraction but does not cause potential damage to the veins from extended tissue stretch.24, 26, 27
To test the actions of SDX on the vein contractile function, IVC rings under control 0.5g-resting tension were first pre-contracted with the α-adrenergic receptor agonist phenylephrine (Phe) at submaximal concentration of 6×10−7M. Cumulative concentrations of SDX (0.001–1mg/ml) were then applied and the changes in vein contractile response were recorded and presented either in mg/mg tissue weight, or as % of the initial Phe pre-contraction, or as % of control 96mM-KCl-induced contraction. We selected the SDX 0.001–1mg/ml concentrations following its pharmacokinetics properties in human studies which demonstrated that when one dose of 50–100mg SDX is taken orally it is rapidly and progressively absorbed through the intestine, and reaches a peak plasma level of 0.2–1mg/L after 1–10h of its administration.6 We also selected the SDX 0.001–1mg/ml concentrations because concentrations >1mg/ml showed limitations in its solubility in Krebs’ solution, and the solution became turbid and not clear, and thus these high SDX concentrations were not used. In another set of experiments, IVC rings were challenged with accumulating Phe concentrations (10−9–10−5M), Phe concentration-response relationships were plotted, and the Phe pEC50 (-log M) were determined. The IVC rings were then pre-treated with SDX (0.5mg/ml) for 30min, then challenged again with accumulating Phe concentrations (10−9–10−5M), and the effects of SDX on Phe concentration-response relationship and the Phe pEC50 were determined.
To test the actions of SDX on stretched veins we followed a prolonged vein stretch protocol as previously described.24, 26, 27 First, IVC rings were equilibrated under control 0.5g-resting tension for 1h, a control contractile response to 96mM-KCl was elicited, then the rings were rinsed in Krebs’ solution 3×10min. After a control concentration-contraction curve to Phe (10−9–10−5M), the IVC rings were rinsed again in Krebs’ solution 3×10min. We then changed the incubation solution from Krebs to a culture medium, and the IVC rings were exposed to control 0.5g-resting tension for 18h, or high 2g-stretch for 18h, or high 2g-stretch plus SDX (0.5mg/ml) for 18h. To minimize any potential bias, IVC rings were assigned at random to any of the three experimental conditions. After 18h equilibration at the assigned resting tension and SDX treatment, the incubation solution was switched back to Krebs’ solution, and another vein contractile response to 96mM-KCl and concentration-contraction curve to Phe (10−9–10−5M) were elicited to determine if protracted stretch and SDX treatment affect vein contraction. After completing the vein contraction measurements, the IVC rings were dabbed gently between dry filter papers, weighed, and saved at −80°C for subsequent gelatin zymography and Western blot experiments.
Vein tissue homogenization
IVC rings were pulverized in a tight-fitting homogenizer (2-ml, Kontes-Glass, Vineland, NJ) and a buffer composed of 3-[N-morpholino] propane sulfonic acid (20mM), sodium dodecyl sulfate (SDS, 4%), glycerol (10%), dithiothreitol (2.3mg), ethylenediaminetetraacetic acid (EDTA, 1.2mM), bovine serum albumin (BSA, 0.02%), leupeptin (5.5μM), pepstatin (5.5μM), aprotinin (2.15μM) and 4-(2-aminoethyl)-benzenesulfonyl fluoride (20μM), The vein homogenate was subjected to centrifugation at 10,000×g for 10min and the supernatant was pipetted-out. If the supernatant showed excessive tissue debris, the vein homogenate was centrifuged again in order to obtain a clearer supernatant. Protein concentration was measured in the supernatant using a protein-assay system from Bio-Rad (Hercules, CA).
Gelatin zymography
Vein protein extracts (with no dithiothreitol) were run electrophoretically on 8% SDS-polyacrylamide gel supplemented with gelatin (0.1%, Sigma, St. Louis, MO). The gel was then placed in renaturing buffer supplemented with Triton-X-100 (2.5%, Sigma) and gently agitated at room temperature for 30min. The gel was transferred to developing buffer composed of Tris (50mM), NaCl (0.2M), CaCl2 (5mM), Brij35 (0.02%, Fisher, Pittsburgh, PA), and ZnCl2 (1μM, Sigma) at an adjusted pH 6.7, first at room temperature for 30min then at 37°C for 16h. The gel was stained with coomassie blue R-250 (0.5%, Sigma) for 30min, then destained in a solution composed of methanol:acetic acid:water at 50:10:40 ratio. Proteolytic areas representing MMP-2 and MMP-9 showed as clear bands against blue background. Actin showed as dark blue area at 43kDa against light blue background. In all experiments, equal amount (1μg) of protein from various tissue samples was used to load the gels. The clear MMP proteolytic bands were analyzed using optical densitometry and ImageJ (NIH), and the integrated gelatinolytic activity was presented as pixel intensity×mm2 relative to actin to correct for any differences in sample loading and variations among different gels.28–30
Western blot analysis
Vein protein extracts (20μg) were mixed with an equal amount of 2× Laemmli loading buffer, heated to boiling for 5min, loaded on 8% SDS-polyacrylamide gels, then size fractionated using electrophoresis. Proteins in the gels were transferred to nitrocellulose membranes using electroblotting. The membranes were placed in phosphate-buffered saline (PBS)-Tween containing 5% dry non-fat milk for 1h, then reacted with rabbit polyclonal MMP-2 antibody (SC10736, 1:1000), or MMP-9 antibody (SC10737, 1:1000) (Santa Cruz Biotech, Dallas, TX) at 4°C for 24h. Control studies were conducted using similar protocol but without the MMP antibody, and did not show any measurable immunoreactivity. In other control studies, the membranes were exposed to heat-damaged MMP antibody subjected to alternating heating at 75°C for 30sec followed by cooling at 4°C for 1min repeatedly for 10 times,31 and still no measurable MMP immunoreactivity was observed. Membranes were rinsed 3×15min with PBS-Tween then reacted with horseradish peroxidase-conjugated secondary antibody (1:1000) for 2h. Membranes were rinsed again 5×15min with PBS-Tween, and the MMP bands were identified using enhanced chemiluminescence ECL detection system (GE Biosciences, Piscataway, NJ). The immunoblots were next re-probed for house-keeping protein using β-actin antibody (1:5000). The MMP immunoreactive bands were analyzed using optical densitometry and ImageJ. The MMP pixel intensity was normalized to β-actin to correct for any differences in sample loading.28–30
Experimental Solutions and Test Drugs
Krebs’ solution was composed of 120mM NaCl, 5.9mM KCl, 25mM NaHCO3, 1.2mM NaH2PO4, 11.5mM dextrose, 2.5mM CaCl2, 1.2mM MgCl2, and infused with a gas mixture of 95%O2-5%CO2, at pH 7.4. High 96mM-KCl solution had similar composition as Krebs’ except for using KCl as substitute of NaCl. Phe stock solution (10−1M, Sigma) was made using double distilled H2O. SDX, a generous gift from Alfasigma, Bologna, Italy, was freshly prepared at appropriate concentrations in Krebs’ solution. The culture medium used for incubating the veins under extended stretch was made of Minimum Essential Medium with penicillin, amphotericin B and streptomycin supplementation (Invitrogen, Grand Island, NY). The PBS used in the Western blotting studies was composed of 137mM NaCl, 2.7mM KCl, 8mM Na2HPO4, 2mM KH2PO4, with pH adjusted to 7.4. All other chemical ingredients were reagent grade or higher.
Data Analysis
Data from different veins were shown as means±SEM with the “n” value referring to the number of rats. For analysis of vein function, the contractile response was recorded at increasing Phe concentrations, individual Phe concentration-response relationships were plotted using non-linear regression, the data were fitted to sigmoidal curves using the least squares method, and the Phe pEC50 (−log EC50, drug concentration eliciting half-maximum response) were determined using GraphPad (Prism v.5.01, San Diego, CA). Data were first analyzed using ANOVA with different classification criteria [resting tension (veins under control 0.5g-resting tension vs veins under protracted 2g-stretch); vein treatment (pre-treated with SDX vs control non-treated control veins)]. When a difference was found, the data were analyzed using Student-Newman-Keuls post hoc test for multiple comparisons. Data were also analyzed using paired and unpaired Student t-test. P<0.05 was considered significantly different.
RESULTS
Effects of SDX on contraction in veins under control 0.5g-resting tension
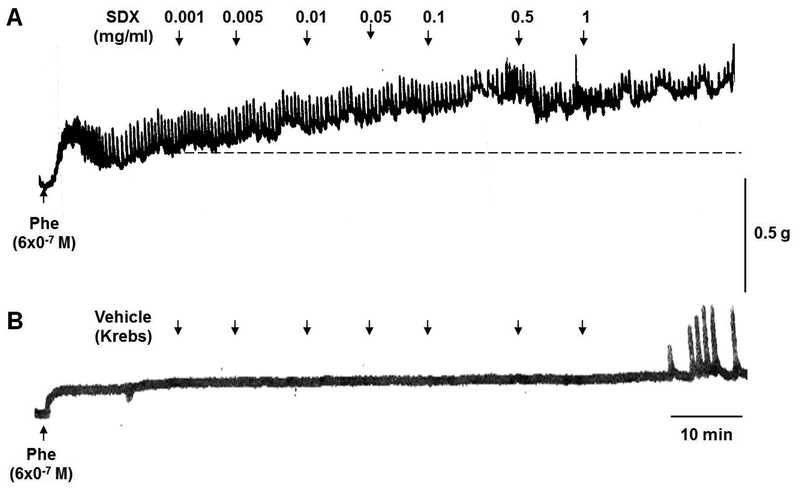
In IVC rings under control 0.5g-resting tension, Phe 6×10−7M caused a submaximal contraction. Applying SDX 0.001–1mg/ml to the veins elicited increases in contraction (Fig.1A). Application of similar volumes of the vehicle Krebs’ solution caused no measurable changes in vein contraction (Fig.1B).
Fig.1.
Effect of SDX on IVC contraction. Rat IVC rings were pre-contracted with submaximal Phe concentration (6×10−7M). When Phe precontraction reached steady-state (as indicated by dashed line), increasing concentrations of SDX (0.001–1mg/ml) were added and the increases in IVC contraction were observed (A). In control experiments, IVC rings were treated with equal volumes of the vehicle Krebs (B).
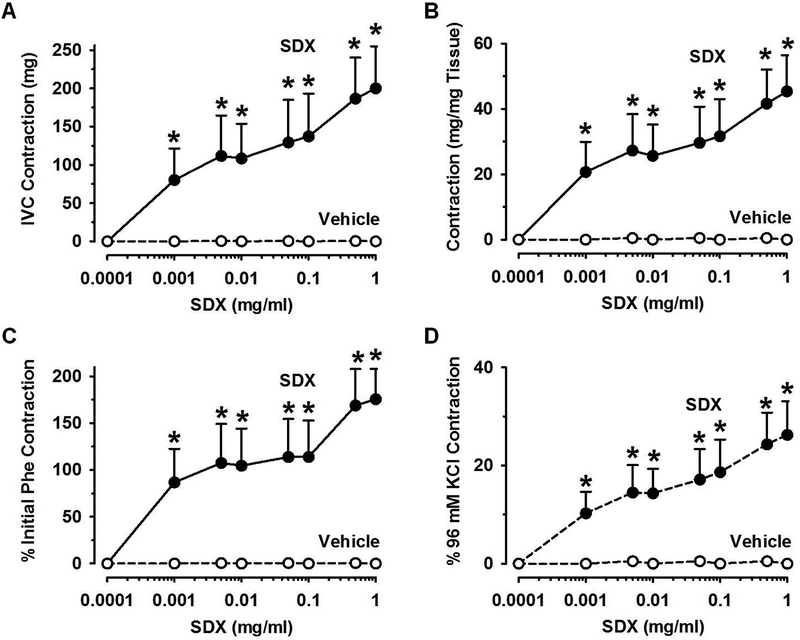
SDX-induced dose-dependent vein contraction
Cumulative data showed that accumulating concentrations of SDX (0.001–1 mg/ml) elicited dose-dependent increases in the IVC contractile response and a maximal response at 0.5mg/ml (Fig.2A). Because of the variability in the size of the IVC rings, the SDX-induced contractile response was presented as mg/mg tissue weight, and SDX still showed increases in contraction (Fig.2B). To further normalize the IVC contraction data, the SDX contraction was presented as % of the initial Phe pre-contraction (Fig.2C) or as % of control 96mM-KCl-induced contraction (Fig.2D), and SDX still showed dose-dependent vein contraction that reached a maximal effect at 0.5mg/ml (Fig.2C, 2D).
Fig.2.
SDX-induced concentration-dependent vein contraction. Rat IVC rings were pre-contracted with submaximal Phe concentration (6×10−7M). Increasing concentrations of SDX (0.001–1mg/ml) or equal volumes of the vehicle Krebs were added and the effects on IVC contraction in milligrams (mg) were measured (A). To correct for variability in the vein segment size and its responsiveness to vasoconstrictors, the SDX-induced contraction was normalized and presented in mg/mg tissue weight (B), as % of initial Phe pre-contraction (C), and as % of control 96mM-KCl contraction (D). Data represent means±SEM, n=6. * Significantly different (P<0.05) versus vehicle.
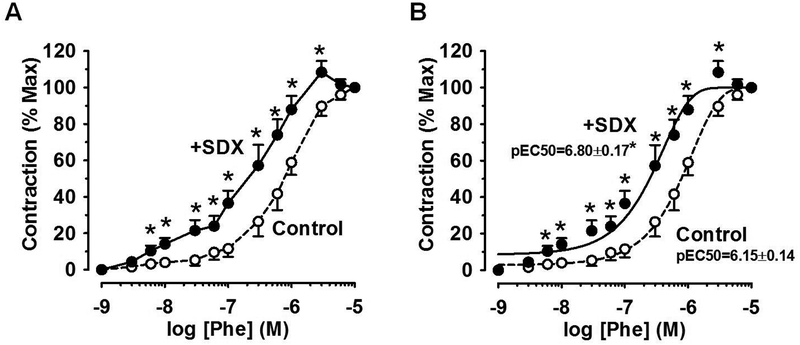
Effects of SDX on Phe-induced contractile response
In control IVC rings, Phe elicited a dose-dependent contractile response and a maximal effect at 10−5M with pEC50=6.15±0.14. Treatment of IVC rings with SDX (0.5mg/ml) caused measurable contraction on its own and in the absence of Phe (12.1±1.8 mg/mg tissue), and this response was considered the baseline prior to measuring the concentration-response curve to Phe in SDX-pretreated veins. In IVC rings pre-treated with SDX 0.5mg/ml for 30min, the veins were more responsive to lower Phe concentrations, and the Phe concentration-response relationship was located to the left, with pEC50=6.80±0.17 (Fig.3).
Fig.3.
Effect of SDX on Phe response. Rat IVC rings were equilibrated under 0.5g-resting tension and a control concentration-contraction curve to Phe (10−9–10−5M) was elicited. The IVC rings were pre-treated with SDX (0.5mg/ml) for 30min, and another concentration-contraction curve to Phe was elicited. The response to each Phe concentration was presented as % of maximal Phe contraction (A), best fitted using least square method and the cumulative Phe pEC50 was calculated (B). Data represent means±SEM, n=8. * Significantly different (P<0.05) in SDX-treated versus non-treated veins.
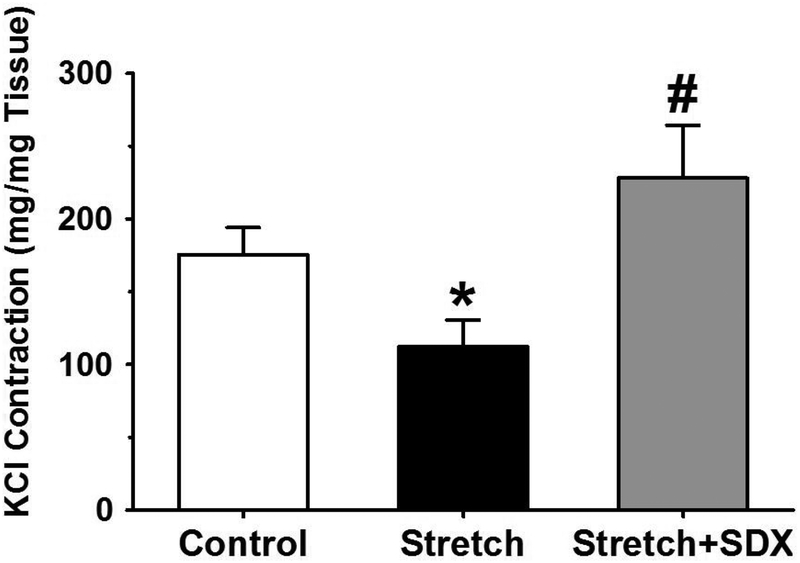
Effects of SDX on 96mM-KCl contraction in veins under protracted stretch
In IVC rings under control 0.5g-resting tension, 96mM-KCl elicited significant contractile response. The IVC contractile response to 96mM-KCl was significantly reduced in veins subjected to 2g-stretch for 18h compared with veins under control 0.5g-resting tension. In IVC rings subjected to extended 2g-stretch plus SDX 0.5mg/ml for 18h, 96mM-KCl-induced contraction was improved to levels significantly different from those in veins subjected to 2g-stretch alone, and insignificantly greater (P=0.062) than those in veins under control 0.5g-resting tension for 18h (Fig.4).
Fig.4.
Effect of SDX on 96mM-KCl contraction in stretched veins. Rat IVC rings were equilibrated under 0.5g-resting tension and a control 96mM-KCl contraction was elicited. The vein segments were washed in Krebs then subjected to extended 2g-stretch for 18h in the absence or presence of SDX (0.5mg/ml), and another 96mM-KCl was elicited. Data represent means±SEM, n=7 to 8. * Significantly reduced (P<0.05) in veins under 2g-stretch versus control veins under 0.5g-resting tension. # Significantly greater (P<0.05) in veins subjected to extended stretch plus SDX versus veins subjected to extended stretch alone.
Effects of SDX on Phe-induced contraction in veins under protracted stretch
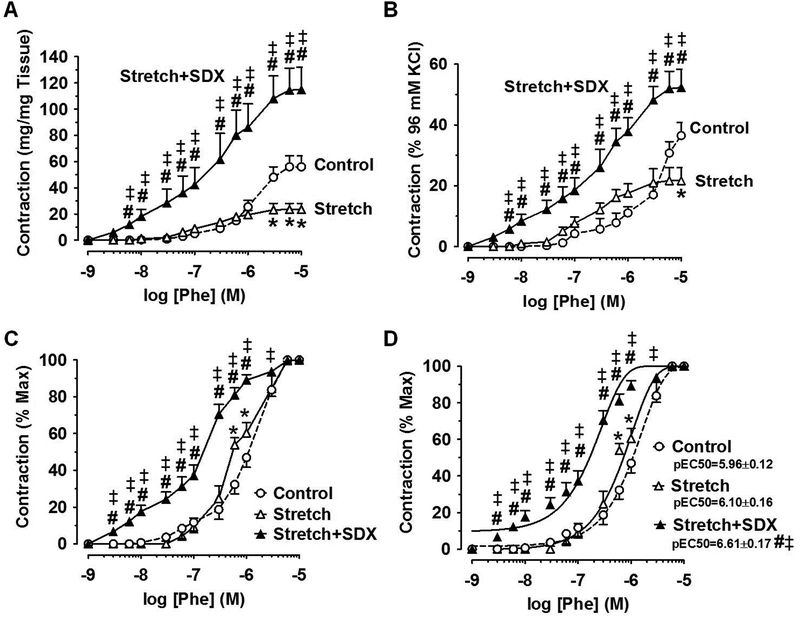
In IVC rings exposed to 2g-stretch for 18h, the magnitude of Phe contraction in mg/mg tissue was decreased compared with that in veins under control 0.5g-resting tension. In IVC rings exposed to extended 2g-stretch plus SDX (0.5mg/ml) for 18h, the Phe concentration-contraction relationship was restored and was significantly greater than that in veins under extended stretch for 18h, and was enhanced to levels even greater than those in veins under control 0.5g-resting tension (Fig.5A).
Fig.5.
Effect of SDX on Phe contraction in stretched veins. Rat IVC rings were equilibrated under 0.5g-resting tension and a control concentration-contraction curve to Phe (10−9–10−5M) was elicited. The IVC rings were washed in Krebs then subjected to extended 2g-stretch for 18h in the absence or presence of SDX (0.5mg/ml), and another concentration-contraction curve to Phe was elicited. The Phe response was presented in mg/mg tissue (A), as % of control 96mM-KCl contraction (B), as % of maximal Phe contraction (C), or best fitted using the least square method and the cumulative Phe pEC50 was calculated (D). Data represent means±SEM, n=8. * Significantly different (P<0.05) in stretched veins versus control veins under 0.5g-resting tension. # Significantly enhanced (P<0.05) in veins under stretch+SDX versus veins under stretch alone. ‡ Significantly enhanced (P<0.05) in veins under stretch+SDX versus control veins under 0.5g-resting tension.
When the Phe-induced contraction was presented as % of control 96mM-KC-induced contraction, the contractile response to different Phe concentrations was largely not different, but the maximal Phe (10−5M)-induced contraction was significantly decreased in veins subjected to extended 2g-stretch versus veins under control 0.5g-resting tension. In IVC rings under protracted 2g-stretch plus SDX 0.5mg/ml, the Phe concentration-contraction curve as % of 96mM-KCl contraction was significantly greater than that in veins under extended 2g-stretch for 18h, and enhanced to levels even greater than those in veins under control 0.5g-resting tension (Fig.5B).
When the Phe-induced contraction was measured as % of maximal Phe contraction, a slight shift to the left particularly to high concentrations of Phe (6×10−7M and 10−6M) was observed in veins under extended 2g-stretch compared with veins under control 0.5g-resting tension. However, when the individual Phe responses were fitted to a non-linear curve and the cumulative Phe pEC50 was calculated, no significant differences were observed in veins under extended 2g-stretch (pEC50=6.10±0.16) compared with veins under control 0.5g-resting tension (pEC50=5.96±0.12). In comparison, the Phe concentration-contraction curve in veins under extended 2g-stretch plus SDX 0.5mg/ml was markedly shifted to the left, and the cumulative Phe pEC50 (pEC50=6.61±0.17) was significantly different when compared to veins under extended 2g-stretch alone, and also compared to veins under control 0.5g-resting tension, suggesting enhanced vein sensitivity to Phe (Fig.5C, 5D).
Effects of SDX on MMP proteolytic activity
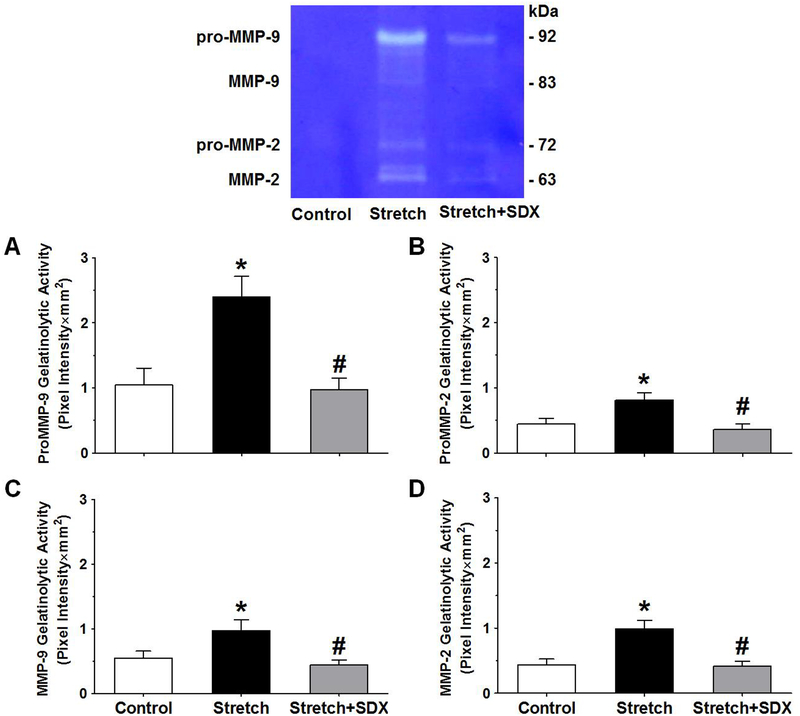
In search of the contribution of MMPs to the observed effects of SDX on vein contraction, gelatin zymography of vein tissue homogenate showed more prominent gelatinolytic bands of MMP-9 and MMP-2 in veins under extended 2g-stretch compared with veins under control 0.5g-resting tension (Fig.6). In comparison, the intensity of the MMP gelatinolytic bands was markedly decreased in veins under 2g-stretch plus SDX 0.5mg/ml versus veins subjected to extended 2g-stretch alone, and the band intensity was not significantly different compared with that in veins under control 0.5g-resting tension (Fig.6).
Fig.6.
Effect of SDX on MMP gelatinolytic activity in stretched veins. Vein tissue homogenates from rat IVC rings under 0.5g-resting tension, or extended 2g-stretch alone, or extended 2g-stretch plus SDX (0.5mg/ml) were prepared for gelatin zymography experiments. The gelatinolytic bands corresponding to pro-MMP-9 (A), pro-MMP-2 (B), MMP-9 (C) and MMP-2 (D) were analyzed using ImageJ, presented as pixel intensity×mm2, and normalized to actin to correct for loading. Bar graphs represent means±SEM, n=5. * Significantly different (P<0.05) in stretched veins versus control veins under 0.5g-resting tension. # Significantly different (P<0.05) in veins subjected to extended 2g-stretch plus SDX versus veins subjected to extended 2g-stretch alone.
Effects of SDX on MMP levels
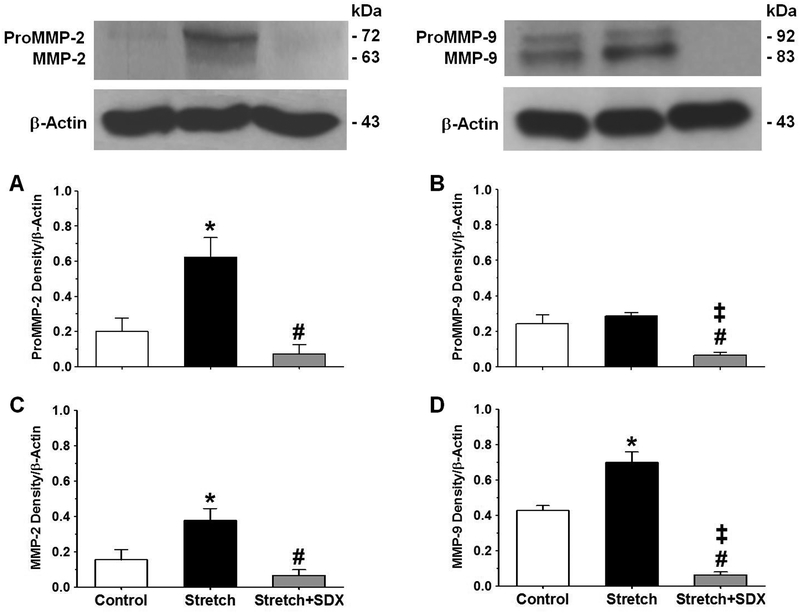
Western blot experiments showed more prominent pro-MMP-2, MMP-2, and MMP-9 in veins under extended 2g-stretch versus veins under control 0.5g-resting tension (Fig.7). The MMP-2 and MMP-9 immunoreactive bands were markedly decreased in veins under extended 2g-stretch plus SDX 0.5mg/ml versus veins under extended 2g-stretch alone. In veins under extended 2g-stretch plus SDX, the levels of pro-MMP-2 and MMP-2 were not significantly different, while the levels of pro-MMP-9 and MMP-9 were significantly less than those in veins under control 0.5g-resting tension (Fig.7).
Fig.7.
Effect of SDX on MMP levels in stretched veins. Vein tissue homogenates from rat IVC rings under 0.5g-resting tension, or extended 2g-stretch, or extended 2g-stretch plus SDX (0.5mg/ml) were prepared for Western blot analysis using antibodies to MMP-2 (1:1000) and MMP-9 (1:1000). The optical density of immunoreactive bands corresponding to pro-MMP-2 (A), pro-MMP-9 (B), MMP-2 (C) and MMP-9 (D) was measured using ImageJ and normalized to β-actin. Bar graphs represent means±SEM, n=4. * Significantly different (P<0.05) in veins under extended 2g-stretch versus control veins under 0.5g-resting tension. # Significantly different (P<0.05) in veins subjected to extended 2g-stretch plus SDX versus veins subjected to extended 2g-stretch alone. ‡ Significantly different (P<0.05) in veins subjected to extended 2g-stretch plus SDX versus control veins under 0.5g-resting tension.
DISCUSSION
In this study we found that: 1) SDX promotes venous contraction in a concentration-dependent fashion. 2) Protracted vein stretch causes reduction in vein contraction, and SDX restores the vein contractile function in veins subjected to protracted stretch. 3) Protracted vein stretch causes increases in the levels/gelatinolytic activity of MMP-2 and MMP-9, and SDX reduces MMP-2 and MMP-9 in veins under protracted stretch.
SDX is a blend of glycosaminoglycans with reported pro-fibrinolysis and anti-thrombosis actions, and efficient lipid reducing and anti-inflammation properties.9, 32–34 SDX has also been shown to be efficacious and safe in several vascular disorders,6, 7, 9, 35, 36 including both arterial diseases37–39 and chronic venous disease.15, 32, 40–43 SDX-mediated fibrinolysis is related to the release of tissue plasminogen activator, and the reduction in plasminogen activator inhibitor-1,33, 35 plasma fibrinogen, and blood viscosity.44 The SDX-mediated anti-thrombosis properties stem from its dual catalysis of thrombin inhibition by anti-thrombin-III and heparin cofactor-II, while at the same time not promoting bleeding.7 Glycosaminoglycans also show high affinity to the endothelium of both arteries and veins.18, 19 SDX reduces the gene expression of the pro-thrombosis and pro-inflammation factors von Willebrand factor, interleukin-6 and vascular cell adhesion molecule-1 in human arterial endothelial cells treated with serum from individuals with atherosclerotic peripheral artery disease.45 SDX also decreases von Willebrand factor gene expression and the release of free radical, interleukin-6 and monocyte chemoattractant protein-1 in human venous endothelial cells.46, 47 Also, SDX prevents the release of intercellular adhesion molecule-1 from venous endothelial cells treated with serum from individuals with chronic venous disease.48 Therefore, the effects of SDX on vascular cells of not only the arteries but also the veins warrant careful examination.
In a recent study, we have shown that SDX decreases contraction in rat aortic and mesenteric arterial segments.20, 21 We have also shown that SDX causes arterial relaxation by promoting endothelium-derived nitric oxide production.20, 21 In the current study, we examined the direct actions of SDX in isolated vein segments from the rat IVC. In contrast with our observations that SDX reduced contraction in the arteries, our experiments in veins under control 0.5g-resting tension showed that SDX elicited concentration-dependent increases in vein contraction. The observed contractile effects of SDX in the veins were specific because equal amounts of the vehicle Krebs’ solution caused no measurable changes in vein contraction. Also, when the contractile response to SDX was normalized relative to the vein segment weight or the control contraction to Phe or 96mM-KCl, SDX still showed significant contraction in veins under control 0.5g-resting tension. Additionally, veins under control 0.5g-resting tension and pre-treated with SDX 0.5mg/ml for 30min were more responsive to low Phe concentrations, and the Phe dose-response relationship showed leftward shift, suggesting enhanced sensitivity to Phe in SDX-treated veins.
We have previously used IVC segments under prolonged stretch for 18h to study the potential mechanisms underlying vein wall dilation and venous dysfunction in chronic venous disease.24, 26, 27 In accordance with our recent reports,24, 26, 27 we found that the magnitude of IVC contraction to the α-adrenergic receptor agonist Phe in mg/mg tissue was decreased in veins under extended 2g-stretch compared with veins under control 0.5g-resting tension. However, when the Phe-induced contraction was measured as % of its own maximal response, no apparent shift in the concentration-response curve was observed in stretched vs control veins. Thus while stretch alone reduced the magnitude of Phe contraction, it did not affect the receptor sensitivity to different concentrations of Phe, suggesting that stretch alone does not reduce the sensitivity of the α-adrenergic receptor to Phe, but rather the venous contraction mechanisms downstream from receptor activation. Importantly, pre-treatment of veins under extended 2g-stretch with SDX restored vein contraction to levels greater than those in veins under extended 2g-stretch alone, and improved Phe contraction to levels even greater than those in veins under control 0.5g-resting tension.
In searching for the potential mechanisms involved in the decreased vein contraction in stretched veins, we have previously demonstrated that the venous tissue levels/activity of MMP-2 and MMP-9 are augmented in veins under prolonged stretch.24, 26 We have also reported that MMP-2 and MMP-9 inhibit vein contraction in a concentration-dependent fashion, and suggested that MMP-induced inhibition of vein contraction could be involved in the excessive venodilation associated with chronic venous disease and VVs.24, 49, 50 In agreement with our previous studies,24, 26 the current study demonstrated that the decreased contractile response in veins under extended 2g-stretch was associated with increases in the levels/gelatinolytic activity of MMP-2 and MMP-9. Importantly, pre-treatment of vein segments under extended 2g-stretch with SDX caused decreases in the levels/gelatinolytic activity of MMP-2 to levels significantly less than those in veins under 2g-stretch alone, and not significantly different from those in control veins under 0.5g-resting tension. Also, pre-treatment of vein segments under extended 2g-stretch with SDX caused decreases in the levels/gelatinolytic activity of MMP-9 to levels significantly lower than those in veins under 2g-stretch alone, and even lower than those in veins under control 0.5g-resting tension. These findings are consistent with a recent report showing that SDX treatment of cultured leukemia white blood cells caused marked decreases in the pro- and complexed MMP-9 in a concentration-dependent manner.51 Also, in a recent study in chronic venous disease patients at CEAP-stage-C5, treatment with SDX for 8 weeks markedly decreased serum concentration of MMP-9.41 With regard to the vascular effects of MMPs, our recent studies on isolated rings of the rat aorta have demonstrated that MMP-2 and MMP-9 cause inhibition of Phe-stimulated45Ca2+ influx.52 We have also shown that MMP-2 inhibits Ca2+ entry through membrane channels without affecting intracellular Ca2+ release in rat IVC.49 Also, we have demonstrated that MMP-2 cause inhibition of Phe-induced vein contraction by promoting membrane polarization and enhancing the activity of K+ channels particularly the large conductance Ca2+-activated type.50 Taken together these findings conform with the possibility that the SDX-induced enhancement of vein contraction to Phe could be partly due to decreasing MMP-2 and MMP-9 and reversing their inhibitory actions on Ca2+ entry, and their promoting effects on vein hyperpolarization and venodilation. Importantly, several studies have suggested changes in the levels/activity of MMPs during the course of chronic venous disease and VVs,23, 53, 54 making MMPs an attractive target for treatment of chronic venous disease. The observed SDX-induced reduction in MMP-2 and MMP-9 and improvement in vein contractile response add to the benefits of SDX in chronic venous disease.
Other points for consideration include: 1) MMPs is a family of 28 subtypes including not only the gelatinase types such as MMP-2 and MMP-9 but also the collagenase, stromelysin, matrilysin, and membrane type MMPs,55, 56 and whether SDX affects these MMPs needs to be examined. 2) SDX could enhance vein contraction by other mechanisms. We have previously shown that the decreased vein contraction in stretched veins is partly reversed in veins pretreated with tissue inhibitor of metalloproteases (TIMPs),24 which inhibit MMP-2 and MMP-9,22, 55 and it is possible that SDX may enhance venous contraction by increasing TIMPs and promoting their inhibition of MMPs. 3) Some metalloproteases are known to breakdown big endothelin into endothelin-1, an endothelium-derived vasoconstrictor,57 and whether SDX through its effects on metalloproteases could affect the metabolism/release of endothelin-1 needs to be examined. Of note, because the present study was centered on measuring venous smooth muscle contraction, the integrity of the endothelium was not tested, and whether the presence or absence of intact endothelium would affect the SDX-induced changes in vein contraction should be examined in future experiments. 4) SDX appears to enhance venous contraction partly by enhancing the α-adrenergic receptor sensitivity to Phe. This is consistent with the current finding that in veins under control 0.5g-resting tension, the Phe concentration-contraction relationship was located to the left in SDX-treated versus non-treated veins. Also, the Phe concentration-contraction relationship was markedly moved to the left in veins under extended 2g-stretch plus SDX compared to veins subjected to extended 2g-stretch alone or control veins under 0.5g-resting tension. 5) SDX could improve IVC contraction by enhancing the contraction pathways downstream from the α-adrenergic receptor. VSM contractile response is triggered by elevation of intracellular Ca2+ concentration as a result of Ca2+ mobilization from the sarcoplasmic reticulum and Ca2+ entry through plasmalemmal channels. The VSM contractile response can be further augmented by other pathways that increase the myofilament force sensitivity to Ca2+, e.g. Rho-kinase and protein kinase-C.58, 59 It is likely that SDX could improve VSM contractile response by increasing cytosolic [Ca2+] or stimulating the Ca2+ sensitization pathways. This is consistent with the current finding that in stretched veins, SDX enhanced contraction to 96mM-KCl which causes membrane depolarization and activates plasmalemmal Ca2+ channels. Future studies should examine the effects of SDX on KCl contraction, Ca2+ entry and Ca2+ sensitization mechanisms not only in stretched veins, but also in control veins. 6) While the IVC is less susceptible to become varicose, we chose the rat IVC model because it is easily dissected and elicits reproducible response to vasoconstrictor/vasodilator stimuli in a consistent manner.50 Future experiments on rat femoral vein would add to the applicability to lower extremity chronic venous disease and VVs. 7) In the present study we used ex vivo model of extended vein wall stretch. Recent studies have assessed vein valves and MMP levels in rats with increased venous pressure produced by surgical induction of a femoral arterio-vein fistula.60–62 These rats showed increases in femoral vein pressure and reflux 6-weeks after inducing the arterio-venous fistula. Furthermore, the vein valves downstream from the arterio-vein fistula showed enlarged size, fibrosis, and augmented MMP-2 and MMP-9 3–6 weeks after inducing venous hypertension,62 making it an interesting model to test the actions of SDX in venous disease.
In conclusion, SDX promotes concentration-dependent contraction in rat IVC. Extended vein stretch causes reduction in the vein contractile response, and SDX restores the contractile properties in veins subjected to extended stretch. Extended vein stretch causes increases in MMP-2 and MMP-9, and SDX reduces the levels/activity of MMP-2 and MMP-9 in veins under extended stretch. The SDX-induced improvement in venous contractile response and reduction in MMP-2 and MMP−9 could bear benefits not only in advanced CVI and venous leg ulcer, but also in the vendilation associated with early stage chronic venous disease and VVs.
ACKNOWLEDGEMENT
This study was supported by Alfasigma S.p.A, Bologna, Italy. Dr. Khalil was in part funded by National Heart, Lung, and Blood Institute grants (HL111775 and 1R56HL147889–01). Dr. Yu was a research scholar from Dept. of Obstetrics/Gynecology, Capital Medical Univ., Beijing, China. Dr. Wang was a research scholar from Dept. of Obstetrics/Gynecology, 2nd Xiangya Hospital, Central South Univ., Hunan, China.
List of Abbreviations:
- Ca2+
calcium
- CVI
chronic venous insufficiency
- IVC
inferior vena cava
- K+
potassium
- MMP
matrix metalloproteinase
- Phe
phenylephrine
- SDX
sulodexide
- VSM
vascular smooth muscle
- VVs
varicose veins
Footnotes
Conflict Statement
Fiorella Calanni and Paolo Mattana are employees of Alfasigma. The other authors do not have any conflict.
REFERENCES
- 1.Karino T, Motomiya M. Flow through a venous valve and its implication for thrombus formation. Thromb Res. 1984;36(3):245–257. [DOI] [PubMed] [Google Scholar]
- 2.Eklof B, Rutherford RB, Bergan JJ, Carpentier PH, Gloviczki P, Kistner RL, Meissner MH, Moneta GL, Myers K, Padberg FT, Perrin M, Ruckley CV, Smith PC, Wakefield TW. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg. 2004;40(6):1248–1252. [DOI] [PubMed] [Google Scholar]
- 3.Beebe-Dimmer JL, Pfeifer JR, Engle JS, Schottenfeld D. The epidemiology of chronic venous insufficiency and varicose veins. Ann Epidemiol. 2005;15(3):175–184. [DOI] [PubMed] [Google Scholar]
- 4.Blann AD, Khoo CW. The prevention and treatment of venous thromboembolism with LMWHs and new anticoagulants. Vascular health and risk management. 2009;5:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nenci GG. Dermatan sulphate as an antithrombotic drug. Pathophysiol Haemost Thromb. 2002;32(5–6):303–307. [DOI] [PubMed] [Google Scholar]
- 6.Lasierra-Cirujeda J, Coronel P, Aza M, Gimeno M. Use of sulodexide in patients with peripheral vascular disease. J Blood Med. 2010;1:105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lauver DA, Lucchesi BR. Sulodexide: a renewed interest in this glycosaminoglycan. Cardiovasc Drug Rev. 2006;24(3–4):214–226. [DOI] [PubMed] [Google Scholar]
- 8.Veraldi N, Guerrini M, Urso E, Risi G, Bertini S, Bensi D, Bisio A. Fine structural characterization of sulodexide. Journal of pharmaceutical and biomedical analysis. 2018;156:67–79. [DOI] [PubMed] [Google Scholar]
- 9.Coccheri S, Mannello F. Development and use of sulodexide in vascular diseases: implications for treatment. Drug Des Devel Ther. 2014;8:49–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callas DD, Hoppensteadt DA, Jeske W, Iqbal O, Bacher P, Ahsan A, Fareed J. Comparative pharmacologic profile of a glycosaminoglycan mixture, Sulodexide, and a chemically modified heparin derivative, Suleparoide. Semin Thromb Hemost. 1993;19 Suppl 1:49–57. [PubMed] [Google Scholar]
- 11.Harenberg J. Review of pharmacodynamics, pharmacokinetics, and therapeutic properties of sulodexide. Med Res Rev. 1998;18(1):1–20. [DOI] [PubMed] [Google Scholar]
- 12.Barbanti M, Guizzardi S, Calanni F, Marchi E, Babbini M. Antithrombotic and thrombolytic activity of sulodexide in rats. Int J Clin Lab Res. 1992;22(3):179–184. [DOI] [PubMed] [Google Scholar]
- 13.Scondotto G, Aloisi D, Ferrari P, Martini L. Treatment of venous leg ulcers with sulodexide. Angiology. 1999;50(11):883–889. [DOI] [PubMed] [Google Scholar]
- 14.Andreozzi GM. Role of sulodexide in the treatment of CVD. Int Angiol. 2014;33(3):255–262. [PubMed] [Google Scholar]
- 15.Coccheri S, Scondotto G, Agnelli G, Aloisi D, Palazzini E, Zamboni V. Randomised, double blind, multicentre, placebo controlled study of sulodexide in the treatment of venous leg ulcers. Thromb Haemost. 2002;87(6):947–952. [PubMed] [Google Scholar]
- 16.Andreozzi GM, Bignamini AA, Davi G, Palareti G, Matuska J, Holy M, Pawlaczyk-Gabriel K, Dzupina A, Sokurenko GY, Didenko YP, Andrei LD, Lessiani G, Visona A. Sulodexide for the Prevention of Recurrent Venous Thromboembolism: The Sulodexide in Secondary Prevention of Recurrent Deep Vein Thrombosis (SURVET) Study: A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial. Circulation. 2015;132(20):1891–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luzzi R, Belcaro G, Dugall M, Hu S, Arpaia G, Ledda A, Ippolito E, Corsi M, Ricci A, Cotellese R, Agus G, Errichi BM, Cornelli U, Cesarone MR, Hosoi M. The efficacy of sulodexide in the prevention of postthrombotic syndrome. Clin Appl Thromb Hemost. 2014;20(6):594–599. [DOI] [PubMed] [Google Scholar]
- 18.Jaques LB, Hiebert LM, Wice SM. Evidence from endothelium of gastric absorption of heparin and of dextran sulfates 8000. J Lab Clin Med. 1991;117(2):122–130. [PubMed] [Google Scholar]
- 19.Boneu B, Caranobe C, Cadroy Y, Dol F, Gabaig AM, Dupouy D, Sie P. Pharmacokinetic studies of standard unfractionated heparin, and low molecular weight heparins in the rabbit. Semin Thromb Hemost. 1988;14(1):18–27. [DOI] [PubMed] [Google Scholar]
- 20.Raffetto JD, Calanni F, Mattana P, Khalil RA. Sulodexide promotes venous contraction in rat inferior vena cava. J Vasc Surg Venous Lymphat Disord. 2017;5(1):145. [Google Scholar]
- 21.Raffetto JD, Calanni F, Mattana P, Khalil RA. Sulodexide promotes arterial relaxation via endothelium-dependent nitric oxide-mediated pathway. Biochemical pharmacology. 2019;166:347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochemical pharmacology. 2008;75(2):346–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacColl E, Khalil RA. Matrix Metalloproteinases as Regulators of Vein Structure and Function: Implications in Chronic Venous Disease. The Journal of pharmacology and experimental therapeutics. 2015;355(3):410–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raffetto JD, Qiao X, Koledova VV, Khalil RA. Prolonged increases in vein wall tension increase matrix metalloproteinases and decrease constriction in rat vena cava: Potential implications in varicose veins. J Vasc Surg. 2008;48(2):447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raffetto JD, Yu P, Reslan OM, Xia Y, Khalil RA. Endothelium-dependent nitric oxide and hyperpolarization-mediated venous relaxation pathways in rat inferior vena cava. Journal of vascular surgery. 2012;55(6):1716–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim CS, Qiao X, Reslan OM, Xia Y, Raffetto JD, Paleolog E, Davies AH, Khalil RA. Prolonged mechanical stretch is associated with upregulation of hypoxia-inducible factors and reduced contraction in rat inferior vena cava. Journal of vascular surgery. 2011;53(3):764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anwar MA, Vorkas PA, Li J, Adesina-Georgiadis KN, Reslan OM, Raffetto JD, Want EJ, Khalil RA, Holmes E, Davies AH. Prolonged Mechanical Circumferential Stretch Induces Metabolic Changes in Rat Inferior Vena Cava. Eur J Vasc Endovasc Surg. 2016;52(4):544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li W, Mata KM, Mazzuca MQ, Khalil RA. Altered matrix metalloproteinase-2 and −9 expression/activity links placental ischemia and anti-angiogenic sFlt-1 to uteroplacental and vascular remodeling and collagen deposition in hypertensive pregnancy. Biochemical pharmacology. 2014;89(3):370–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yin Z, Sada AA, Reslan OM, Narula N, Khalil RA. Increased MMPs expression and decreased contraction in the rat myometrium during pregnancy and in response to prolonged stretch and sex hormones. Am J Physiol Endocrinol Metab. 2012;303(1):E55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dang Y, Li W, Tran V, Khalil RA. EMMPRIN-mediated induction of uterine and vascular matrix metalloproteinases during pregnancy and in response to estrogen and progesterone. Biochem Pharmacol. 2013;86(6):734–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim CH, Lisman JE. A labile component of AMPA receptor-mediated synaptic transmission is dependent on microtubule motors, actin, and N-ethylmaleimide-sensitive factor. J Neurosci. 2001;21(12):4188–4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mannello F, Ligi D, Raffetto JD. Glycosaminoglycan sulodexide modulates inflammatory pathways in chronic venous disease. Int Angiol. 2014;33(3):236–242. [PubMed] [Google Scholar]
- 33.Crepaldi G, Rossi A, Coscetti G, Abbruzzese E, Calveri U, Calabro A. Sulodexide oral administration influences blood viscosity and fibrinolysis. Drugs Exp Clin Res. 1992;18(5):189–195. [PubMed] [Google Scholar]
- 34.Corsi C, Bocci L, Cipriani C, Gazzini A, Marrapodi E. The effectiveness of glycosaminoglycans in peripheral vascular disease therapy: a clinical and experimental trial. J Int Med Res. 1985;13(1):40–47. [DOI] [PubMed] [Google Scholar]
- 35.Mauro M, Palmieri GC, Palazzini E, Barbanti M, Calanni Rindina F, Milani MR. Pharmacodynamic effects of single and repeated doses of oral sulodexide in healthy volunteers. A placebo-controlled study with an enteric-coated formulation. Curr Med Res Opin. 1993;13(2):87–95. [DOI] [PubMed] [Google Scholar]
- 36.Andreozzi GM. Sulodexide in the treatment of chronic venous disease. Am J Cardiovasc Drugs. 2012;12(2):73–81. [DOI] [PubMed] [Google Scholar]
- 37.Coccheri S. Biological and clinical effects of sulodexide in arterial disorders and diseases. Int Angiol. 2014;33(3):263–274. [PubMed] [Google Scholar]
- 38.Coccheri S, Scondotto G, Agnelli G, Palazzini E, Zamboni V. Sulodexide in the treatment of intermittent claudication. Results of a randomized, double-blind, multicentre, placebo-controlled study. Eur Heart J. 2002;23(13):1057–1065. [DOI] [PubMed] [Google Scholar]
- 39.Gaddi A, Galetti C, Illuminati B, Nascetti S. Meta-analysis of some results of clinical trials on sulodexide therapy in peripheral occlusive arterial disease. J Int Med Res. 1996;24(5):389–406. [DOI] [PubMed] [Google Scholar]
- 40.Saviano M, Maleti O, Liguori L. Double-blind, double-dummy, randomized, multi-centre clinical assessment of the efficacy, tolerability and dose-effect relationship of sulodexide in chronic venous insufficiency. Curr Med Res Opin. 1993;13(2):96–108. [DOI] [PubMed] [Google Scholar]
- 41.Urbanek T, Zbigniew K, Begier-Krasinska B, Baum E, Breborowicz A. Sulodexide suppresses inflammation in patients with chronic venous insufficiency. Int Angiol. 2015;34(6):589–596. [PubMed] [Google Scholar]
- 42.Elleuch N, Zidi H, Bellamine Z, Hamdane A, Guerchi M, Jellazi N. Sulodexide in Patients with Chronic Venous Disease of the Lower Limbs: Clinical Efficacy and Impact on Quality of Life. Adv Ther. 2016;33(9):1536–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flota Cervera LF, Frati Munari AC, Velazquez Herrera AE, Carbajal Contreras A. Chronic venous disease treated with sulodexide: a survey among primary care physicians in Mexico. Int Angiol. 2017;36(6):558–564. [DOI] [PubMed] [Google Scholar]
- 44.Castelluccio A, Bologna E. Effect of sulodexide on blood viscosity in patients with peripheral vascular disease. Curr Med Res Opin. 1991;12(5):325–331. [DOI] [PubMed] [Google Scholar]
- 45.Sosinska P, Baum E, Mackowiak B, Maj M, Suminska-Jasinska K, Staniszewski R, Breborowicz A. Sulodexide Reduces the Proinflammatory Effect of Serum from Patients with Peripheral Artery Disease in Human Arterial Endothelial Cells. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2016;40(5):1005–1012. [DOI] [PubMed] [Google Scholar]
- 46.Lekesiz K, Naumnik B, Borysewicz-Sanczyk H, Koc-Zurawska E, Mysliwiec M. Effect of unfractionated heparin, enoxaparin and sulodexide on the relations between secretion and expression of OPG, RANKL and vWF in HUVEC. Folia Histochem Cytobiol. 2013;51(2):156–163. [DOI] [PubMed] [Google Scholar]
- 47.Ciszewicz M, Polubinska A, Antoniewicz A, Suminska-Jasinska K, Breborowicz A. Sulodexide suppresses inflammation in human endothelial cells and prevents glucose cytotoxicity. Transl Res. 2009;153(3):118–123. [DOI] [PubMed] [Google Scholar]
- 48.Urbanek T, Krasinski Z, Suminska-Jasinska K, Baum E, Borej-Nowicka G, Begier-Krasinska B, Breborowicz A. Sulodexide reduces the inflammatory reaction and senescence of endothelial cells in conditions involving chronic venous disease. Int Angiol. 2016;35(2):140–147. [PubMed] [Google Scholar]
- 49.Raffetto JD, Barros YV, Wells AK, Khalil RA. MMP-2 induced vein relaxation via inhibition of [Ca2+]e-dependent mechanisms of venous smooth muscle contraction. Role of RGD peptides. The Journal of surgical research. 2010;159(2):755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raffetto JD, Ross RL, Khalil RA. Matrix metalloproteinase 2-induced venous dilation via hyperpolarization and activation of K+ channels: relevance to varicose vein formation. Journal of vascular surgery. 2007;45(2):373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mannello F, Medda V, Ligi D, Raffetto JD. Glycosaminoglycan sulodexide inhibition of MMP-9 gelatinase secretion and activity: possible pharmacological role against collagen degradation in vascular chronic diseases. Current vascular pharmacology. 2013;11(3):354–365. [DOI] [PubMed] [Google Scholar]
- 52.Chew DK, Conte MS, Khalil RA. Matrix metalloproteinase-specific inhibition of Ca2+ entry mechanisms of vascular contraction. Journal of vascular surgery. 2004;40(5):1001–1010. [DOI] [PubMed] [Google Scholar]
- 53.Gillespie DL, Patel A, Fileta B, Chang A, Barnes S, Flagg A, Kidwell M, Villavicencio JL, Rich NM. Varicose veins possess greater quantities of MMP-1 than normal veins and demonstrate regional variation in MMP-1 and MMP-13. The Journal of surgical research. 2002;106(2):233–238. [DOI] [PubMed] [Google Scholar]
- 54.Woodside KJ, Hu M, Burke A, Murakami M, Pounds LL, Killewich LA, Daller JA, Hunter GC. Morphologic characteristics of varicose veins: possible role of metalloproteinases. Journal of vascular surgery. 2003;38(1):162–169. [DOI] [PubMed] [Google Scholar]
- 55.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circulation research. 2003;92(8):827–839. [DOI] [PubMed] [Google Scholar]
- 56.Wang X, Khalil RA. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv Pharmacol. 2018;81:241–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332(6163):411–415. [DOI] [PubMed] [Google Scholar]
- 58.Ringvold HC, Khalil RA. Protein Kinase C as Regulator of Vascular Smooth Muscle Function and Potential Target in Vascular Disorders. Advances in Pharmacology. 2017;78:203–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Z, Khalil RA. Evolving mechanisms of vascular smooth muscle contraction highlight key targets in vascular disease. Biochemical pharmacology. 2018;153:91–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takase S, Pascarella L, Bergan JJ, Schmid-Schonbein GW. Hypertension-induced venous valve remodeling. Journal of vascular surgery. 2004;39(6):1329–1334. [DOI] [PubMed] [Google Scholar]
- 61.Takase S, Pascarella L, Lerond L, Bergan JJ, Schmid-Schonbein GW. Venous hypertension, inflammation and valve remodeling. Eur J Vasc Endovasc Surg. 2004;28(5):484–493. [DOI] [PubMed] [Google Scholar]
- 62.Pascarella L, Schmid-Schonbein GW, Bergan J. An animal model of venous hypertension: the role of inflammation in venous valve failure. Journal of vascular surgery. 2005;41(2):303–311. [DOI] [PubMed] [Google Scholar]