Abstract
The use of an immunologic adjuvant to augment the immune response is essential for modern vaccines which are relatively ineffective on their own. In the past decade, researchers have been consistently reporting that skin treatment with a physical parameter, namely laser light, augments the immune response to vaccine and functions as an immunologic adjuvant. This “laser adjuvant” has numerous advantages over the conventional chemical or biological agents; it is free from cold-chain storage, hypodermic needles, biohazardous sharp waste, irreversible formulation with vaccine antigen, undesirable biodistribution in vital organs or unknown long-term toxicity. Since vaccine formulations are given to healthy populations, these characteristics render the “laser adjuvant” significant advantages for clinical use and open a new developmental path for a safe and effective vaccine. In addition, laser technology has been used in the clinic for more than three decades and is therefore technically matured and has been proved to be safe. Currently, four classes of laser adjuvant have been reported; ultra-short pulsed, non-pulsed, non-ablative fractional and ablative fractional lasers. Since each class of the laser adjuvant shows a distinct mechanism of action, a proper choice is necessary to craft an effective vaccine formulation toward a desired clinical benefit for a clinical vaccine to maximize protection. In addition, data also suggest that further improvement in the efficacy is possible when a laser adjuvant is combined with chemical or biological adjuvant(s). To realize these goals, further efforts to uncover the molecular mechanisms of action of the laser adjuvants is warranted. This review provides a summary and comments of the recent updates in the laser adjuvant technology.
Keywords: laser, skin, adjuvant, vaccine
Introduction
An immunologic adjuvant is a component that accelerates, prolongs, or enhances adaptive immune responses to an antigen toward a desired clinical benefit (1–4). Since modern vaccine formulations in a form of highly-targeted recombinant molecules are inefficient in inducing protective immunity, they require an immunologic adjuvant to achieve clinical significance (5, 6). Currently, most of clinical or experimental adjuvants are chemical or biological substances (5, 6). These conventional adjuvants are known to often induce undesirable local reactogenicity or systemic toxicity (7–10). Due to the safety concerns, only a limited number of adjuvants have been used with clinically approved vaccines (1, 11, 12).
In the past decade, studies have consistently demonstrated that laser light functions as an immunologic adjuvant. Since laser is a physical parameter, it does not remain in exposed tissues and has less chance to induce side effects in comparison to either chemical or biological adjuvants. This new and safe strategy has been logically combined with skin vaccination to maximize the efficacy of vaccines and induce protection. The skin is enriched with professional antigen presenting dendritic cells (DCs) and has been considered to be an ideal vaccination site to initiate adaptative immune responses and protection when DCs are provided with a proper cue (13). Skin-based vaccination to target antigen-presenting cells (APCs) has been consistently shown to be superior to the conventional intramuscular route (14–16) and offer dose sparing (17–19). In response to these advantages, various technologies are now in use or under development for skin delivery of vaccines (17, 20).
To date, there are four major categories of the “laser adjuvant” that have been classified based on the nature of the laser treatment: (i) ultrashort pulsed lasers, (ii) non-pulsed lasers, (iii) non-ablative fractional lasers, and (iv) ablative fractional lasers. In this review, the initial studies as well as the most recent updates on this technology are summarized and commented.
1. Ultra-short pulsed laser adjuvant
Ultra-short pulsed laser (UPL) adjuvants are laser pulses with durations in the nanosecond range or shorter that cover the exposed tissue to enhance immune responses. These ultra-short pulses are delivered at repetition rates ranging from 10 to 1,000 Hertz over several minutes. Typically, a dose chosen for this approach causes no frank tissue damage (Table 1).
Table 1.
Four classes of the laser adjuvant.
| Class | Laser type | Wavelength | Pathway Activated | Immune responses stimulated | Tested in | References |
|---|---|---|---|---|---|---|
| Ultra-short pulsed laser (UPL) | Copper vapor | 511/578 nm | Extracellular release of HSP70 to enhance Langerhans cell activation and antigen presentation | Ab against influenza and hepatitis B Augment cancer vaccines |
Mice, humans | (21–23) |
| Q switched Nd:YAG | 532 nm | Migration and activation of APCs in the skin | Ab against influenza Ab and T cell responses against OVA |
Mice | (24) | |
| Potassium titanyl phosphate (KTP) | 532 nm | n.d. | Ab and T cell responses against OVA upon intramuscular injection | Mice | (24) | |
| Q switched Nd:YAG | 532 nm | Disarray of the extracellular matrix in the dermis facilitates migration of DCs | Cytotoxic T cell response against solid tumor | Mice | (84) | |
| Q switched Nd:YAG | 532 nm | n.d. | Ab against nicotine | Mice | (25) | |
| Q switched Nd:YVO4 | 532 or 1064 nm | A selective upregulation of chemokines and cytokines in the skin leading to APCs activation and migration | Ab against influenza and OVA | Mice | (26) | |
| Q switched Nd:YAG (532) Pulsed dye laser (595) |
532 and 595 nm | Increase in vascular permeability increases translocation of sporozoite to liver | Sporozoite-specific antibody and CD8+ T cell responses | Mice | (27) | |
| Non-pulsed laser (NPL) | Semiconductor laser | 980 nm | Photothermal effect induced cancer cell death and subsequent DC and CTL activation and recruitment in tumor microenvironment | CTL response against pancreatic cancer and melanoma | Mice | (32) |
| n.d. | 808 nm | Laser along with imiquimod induced intracellular expression of DAMPs | Suppression of tumor growth of skin SCC | Mice and Humans | (35) | |
| Diode laser | 805 nm | n.d. | Synergistic effects between laser immunotherapy and immune checkpoint inhibitor on tumor growth of melanoma | Humans | (36) | |
| Diode laser | 808 nm | Laser along with imiquimod treatment induced lymphocyte infiltration into warts | Suppression of tumor growth of refractory warts | Humans | (37) | |
| Diode laser | 980 nm | Photothermal effect induced cancer cell death and subsequent DC and CTL activation and recruitment in tumor microenvironment | Induction of tumor-specific CTLs in breast cancer model |
Mice | (34) | |
| n.d. | 808 nm | Photothermal effect induced thermal lysis of the tumor cell membrane and release of tumor-specific antigens | Maturation of DCs and induction of tumor infiltration of CTLs in breast cancer model | Mice | (38) | |
| CW Nd:YVO4 | 1064 nm | Photochemical reaction in skin tissue leading to immunostimulatory microenvironment for APCs via expression of a selective chemokines | Ab and T cell responses against influenza and OVA | Mice, human (no immunogenicity study) | (26) | |
| GaAs (1061) InP (1258, 1301), CW Nd:YVO4 (1064) |
1061, 1258, 1301 nm | n.d. | Ab response against influenza | Mice | (41) | |
| CW Nd:YVO4 | 1064 nm | Selective activation of Lang+ and Lang-CD11b- skin DCs and recruitment of Ly6C+ monocytes | Ab response against influenza | Mice | (42) | |
| CW Nd:YVO4 | 1064 nm | Photochemical reaction in mast cells and keratinocytes leading to ROS generation and chemokine/cytokine expression and immunostimulatory microenvironment for skin DCs | Ab response against influenza | Mice | (43) | |
| Custom diode laser | 1064 nm | n.d. | Induction of migration response of skin DCs | Human (no immunogenicity study) | (47) | |
| Non-ablative fractional laser (NAFL) | Fractional ER:Glass (PaloVia laser, mice; Fraxel SR-1500, pigs) | 1410 nm (mice) 1540 nm (pigs) |
Sterile inflammation caused by an array of MTZs in skin recruits pDCs | Ab and T cell responses against influenza with imiquimod | Mice, pigs | (49) |
| Fractional ER:Glass (PaloVia laser, mice; Fraxel SR-1500, pigs) | 1410 nm (mice) 1540 nm (pigs) |
Activation of dsDNA sensing pathway and subsequent interferon upregulation by MTZs in skin facilitates migration of DCs | Ab and T cell responses against influenza using microneedles | Mice, pigs | (51) | |
| Fractional ER:Glass (PaloVia laser) | 1410 nm | Adjuvant effect of micro-injury in skin | Ab against OVA using hyaluronan nanocarriers | Mice, pigs (no immunogenicity study) | (53) | |
| Fractional ER:Glass (PaloVia laser) | 1410 nm | Direct activation of skin-resident DCs | TEM and TRM CD8+ T cell responses against herpes virus with imiquimod | Mice | (52) | |
| Fractional ER:Glass (PaloVia laser) | 1410 nm | ATRA stimulated upregulation of cytosolic nucleic acid sensors and their downstream factors and enhanced type I interferon expression with NAFL | Ab against influenza with ATRA | Mice | (54) | |
| Ablative fractional laser (AFL) | Fractional CO2 (UltraPulse device) | 10600 nm | Efficient delivery of vaccine to skin DCs | Ab against OVA | Mice | (61) |
| Fractional CO2 (UltraPulse device) | 10600 nm | Efficient delivery of vaccine to skin DCs | Ab against OVA and T cell responses against OVA-expressing vaccinia virus | Mice, pigs (no immunogenicity study), humans (no immunogenicity study) | (65) | |
| Fractional ER:YAG (P.L.E.A.S.E. device) | 2940 nm | The mild inflammatory milieu created in the dermis activates skin-resident DCs and induces infiltration of APCs | T cell response against melanoma using a vaccibody targeting XCR1+ DCs | Mice | (66) | |
| Fractional ER:YAG (P.L.E.A.S.E. device) | 2940 nm | The limited inflammatory response activates Langerhans cells and facilitates DC migration | Ab response to OVA and recombinant pertussis toxin using Viaskin® | Mice | (73) | |
| Fractional ER:YAG (P.L.E.A.S.E. device) | 2940 nm | The limited inflammatory response induced by laser microporation activates DC | TH1/TH17-biased Ab and T cell responses using pollen allergen-mannan neoglycoconjugates | Mice, human skin explant (no immunogenicity study) | (80) | |
| Fractional ER:YAG (P.L.E.A.S.E. device) | 2940 nm | Suppressing allergenic responses if AFL and TH1-chemical adjuvant are combined in immunotherapy for pre-sensitized mice | Suppressed allergenic IgE and T cell responses to the grass pollen allergen Phl p 5 with CpG-ODN | Mice | (77) | |
| Fractional ER:YAG (P.L.E.A.S.E. device) | 2940 nm | Efficient antigen delivery to distinct APC population in the skin | TH2-biased Ab and T cell responses to model vaccines with CpG-ODN | Mice, Pig ear skin preparation (no immunogenicity study) | (62) | |
| Fractional ER:YAG (P.L.E.A.S.E. device) | 2940 nm | n.d. | Ab responses against HBsAg is dependent on a laser parameter and adjuvant to be combined | Mice | (64) | |
| Fractional ER:YAG (P.L.E.A.S.E. device) | 2940 nm | Increasing cytokine expression for TH1/Treg induction while suppressing TH2 response. | Enhanced Tregs, suppressed IgE response against OVA in combination with chemical adjuvants | Mice | (79) | |
| Fractional ER:YAG (P.L.E.A.S.E. device) | 2940 nm | Increasing cytokine expression | The resultant immune responses were largely not affected by the presence of γδ T cells or MCs. | Mice | (81) |
Ab, antibody; APC, antigen presenting cell; ATRA, all-trans retinoic acid; CpG-ODN, CpG-oligodeoxynucleotides; CW, continuous wave; CTLs, cytotoxic T lymphocytes; DC, dendritic cell; DAMPs, damage-associated molecular patterns, ER:YAG, erbium:yttrium-aluminium-garnet; MCs, mast cells; MTZ, microthermal zone; Nd:YAG, neodymium-doped yttrium aluminum garnet; Nd:YVO4, neodymium-doped yttrium orthovanadate; n.d., Not described; KTP, potassium titanyl phosphate; SCC, squamous cell carcinoma; TEM, effector memory; TRM, tissue-resident.
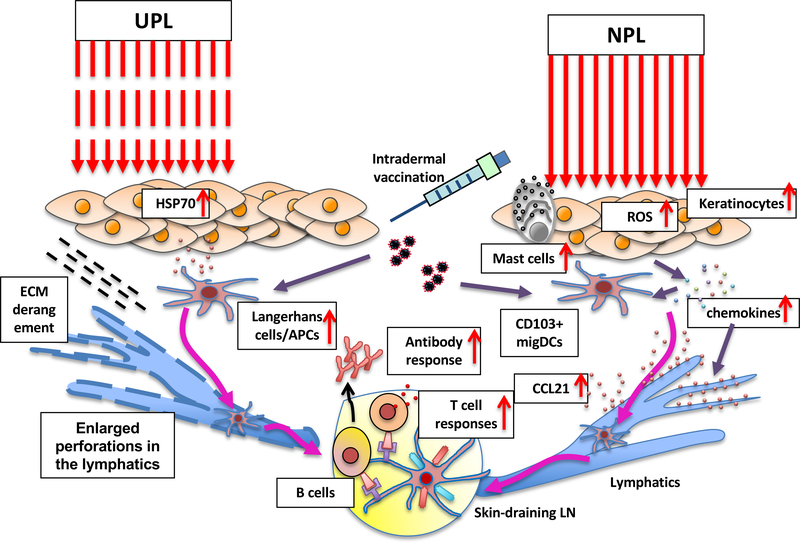
UPL adjuvant was first reported by Russian scientists using copper vapor lasers emitting nanosecond pulses of yellow 510 nm and green 578 nm light with kilohertz repetition rates at a power density between 1–6 W/cm2 applied on circular skin exposures of about 5–10 mm in diameter over 1–3 min (21–23). Onikienko et al. treated human subjects or mice with UPL followed by intradermal injection of a commercial prophylactic influenza or hepatitis B vaccine. The group found that the UPL treatment resulted in significant increases of antigen-specific antibody titers both in human and mice. The same Russian investigators also reported the potentiating effects of the UPL adjuvant on experimental therapeutic vaccines for chronic hepatitis B and cancer in human subjects (22). The group observed that the UPL treatment induced rapid release of heat shock protein 70 (HSP70) by skin fibroblasts and keratinocytes and concluded that this release enhanced immune responses via recruitment and activation of Langerhans cells (21).
Consistently, Chen et al. demonstrated that a Q-switched neodymium-doped yttrium aluminum garnet (Q-Nd:YAG) laser emitting 532 nm light at a repetition rate of 10 Hz (Spectra-Physics Inc., Mountain View, CA) possessed the adjuvant effect. The group found that the UPL treatment of mouse skin followed by intradermal vaccination significantly increased humoral immune responses to model and inactivated influenza vaccines without inducing any tissue damage or inflammation (24). Interestingly, this approach could augment humoral and antigen-specific CD4+ and CD8+ T cell responses to intramuscular immunization using a hair-like optical fiber emitting a long-pulsed potassium titanyl phosphate (KTP)/532 nm laser (Laserscope, San Jose, CA). The group demonstrated that the mechanisms of action involved increased motility and migration and enhanced antigen uptake and presentation of MHC class II-positive APCs in skin after the UPL treatment. Chen et al. consistently showed the adjuvant effect of the UPL adjuvant on an intradermal nicotine vaccine augmenting production of antibody against nicotine (25). Interestingly, the adjuvant effect of the UPL adjuvant was further augmented if it was combined with a TH1 adjuvant monophosphoryl lipid A (MPL) without inducing significant local reaction in the skin.
Chen et al. further demonstrated that this approach can be used to augment the efficacy of DC vaccine against solid tumors. UPL adjuvant treatment enhanced migration and maturation of APCs in the skin as well as therapeutic DCs following intradermal administration of the cells, ultimately resulting in expansion of cytotoxic CD8+ T cells and enhancement of anti-tumor immunity (25). In this study, the group observed enlarged perforations in the basement membrane of the lymphatics and disarray of the extracellular matrix in the dermis upon the UPL treatment, which was considered to be responsible for the enhanced migration of APCs and DCs in the skin and lymphatic vessels. The green light pulsed laser used in this study was featured with a pulse peak power over 2,500 times greater as compared to the UPL used by Russian researchers, which could have contributed to this ultrastructural change.
Our group demonstrated that pre-treatment with a Q-switched neodymium-doped yttrium orthovanadate (Q-Nd:YVO4) laser, emitting either 532 nm or 1064 nm light in 7 nanosecond pulses at a repetition rate of 10 kHz, at an average irradiances of 1 for and 5 W/cm2 for 532 and 1064 nm over 1–4 min showed the adjuvant effect on intradermal vaccination with model and influenza vaccines (26). In this study, the laser parameter for 532 nm was closer to what the Russian investigators used compared to those used by Chen et al.
Zhou et al. expanded the use of this laser parameter showing that UPL of 532 nm at 1 J/cm2 increased microvascular permeability in the exposed skin tissue and translocation of intradermally-administered radiation-attenuated sporozoites (RAS) to liver (27). Since hemoglobin and oxyhemoglobin have peak absorbance from 540 nm to several parameters of 578 nm, 532 nm Nd:YAG laser and a 595 nm pulsed dye laser (Vbeam, Candela, MA) were tested if they increased the microvascular permeability and the efficacy of the malaria vaccine. Remarkably, the UPL treatment significantly augmented sporozoite-specific antibody and CD8+ T cell responses and conferred protection against malaria challenge. Interestingly, confocal microscopic analysis revealed that many sporozoites were recruited close to blood vessels in laser-treated skin in this study, which suggests a possible involvement of a chemotactic cue released by the UPL treatment. However, the exact molecular pathways ultimately leading to the adjuvant effect with the UPL adjuvant remains largely elusive.
Collectively, these studies show that UPL adjuvants create immunostimulatory milieu for APCs in the skin and augments the immune response to vaccine administered to the exposed skin without inducing pain or tissue damage (Figure 1). Each UPL could be further tailored with other strategies including additional chemical or biological adjuvant to achieve a particular goal of vaccination of interest.
Figure 1. The mechanisms of action of the UPL and NPL adjuvants.
Ultrashort-pulsed laser (UPL) induces extracellular release of HSP70 and disarray of extracellular metrices activating Langerhans cell and facilitating migration of DCs, respectively. Non-pulsed laser (NPL) a near-infrared (NIR) range stimulates skin cells including keratinocytes and mast cells via reactive oxygen species (ROS) and induces the expression of a defined set of chemokines including CCL2 and CCL20 that ultimately induce CCL21 expression in the lymphatics and migrational changes of skin-resident migratory CD103+ and Lang−CD11b− DCs.
2. Non-pulsed laser adjuvant
Non-pulsed laser (NPL) adjuvant is featured by continuous wave laser light to stimulate immune responses. Phototherapy with continuous wave light has been used to treat skin diseases for more than 3,000 years (28). Concentrated light has been well established to be effective to treat lupus vulgaris (29), suggesting that light can modulate host-pathogen interactions. Accordingly, a concept of laser immunotherapy combining photothermal therapy and immunostimulation is proposed by Dr. Wei R. Chen in 1997 (30). A series of work demonstrated that photothermal effect primarily drives the induction of robust anti-cancer immune response (31). Typically, this approach combines laser exposures with photoabsorber indocyanine green (ICG) and immunologic adjuvant (such as glycated chitosan, GC) to induce dye-enhanced thermal interaction and immune stimulation. It has been demonstrated that this approach is mainly mediated by photothermal effect, but photochemical effect could contribute to the formation of the robust anti-cancer immunity. Zhou et al. showed that mice treated with photothermal therapy (PTT) alone had a significant smaller tumor burden than that of the control mice. A 10-min treatment with 980 nm laser at a density of 0.85 W/cm2 (510 J/cm2 in total) increased the temperature of tumor tissue, which caused cell death in mouse models of pancreatic cancer and melanoma (32). In this approach, heat generation in tissues is considered to induce immunologic cell death with release of heat shock proteins (HSPs), calreticulin (CRT), high-mobility group box 1 (HMGB1) protein and adenosine triphosphate (ATP) (33). In response to this, DCs are stimulated and produce IFN-γ and increase their expression of MHCII and CD80 and recruitment of DCs and T cells in tumor microenvironment. Consistently, a 10-min treatment with 805–808 nm light at an irradiance of 1–1.5 W/cm2 along with topical imiquimod application has been shown to induce release of damage-associated molecular patterns (DAMPs) (HSP70, HSP90 and HMGB1) suppressed tumor growth in mammary tumor (34) and skin squamous cell carcinoma in humans and mice (35), and melanoma (36) and refractory cutaneous warts in humans (37). In this approach, immunogenic cell death also provides sources of tumor neoantigens, creating an in situ autologous cancer vaccine.
Wang et al. recently showed that a personalized cancer vaccine encapsulating JQ1, a bromodomain-containing protein 4 (BRD4) inhibitor, and ICG along with tumor cells in a hydrogel matrix inhibited the tumor relapse by promoting the maturation of dendritic cells and eliciting tumor infiltration of cytotoxic T lymphocyte (CTL) upon 808 nm laser treatment (Changchun New Industries Optoelectronics Tech. Co., Ltd, Changchun, China) for 2 min at an irradiance of 2.0 W/cm2 (38). In this system, temperature increase in the treated area with 808 nm near-infrared (NIR) laser triggered on-demand release of tumor-specific antigens and cytokine expression, subsequently facilitating the activation of dendritic cells. Interestingly, since the group did not observe the comparable DC maturation with heat generation only, the immune response seems to have been caused by increased release of tumor antigens, not photothermal effect. The difference in laser parameters used in these studies may explain the difference in the effects of lasers used.
Currently, this concept has been further expanded to photoimmunotherapy which uses monoclonal antibody tagged with the fluorophore and efficiently mounts anti-cancer immunity with heat generated upon light exposure (39, 40). These results suggest that laser immunotherapy is mainly mediated by photothermal effect, but photochemical effect may contribute to the formation of strong anti-cancer immunity.
All the reports on NPL adjuvant to date use a NIR range of laser light (1061 – 1301 nm) (Table 1). A dose chosen for this approach has been reported to cause no thermal damage nor inflammation in the exposed tissue.
Our group reported for the first time the adjuvant effect of a continuous wave Nd:YVO4 laser (RMI Laser, Lafayette, CO) emitting 1064 nm light (26). We showed that a one-minute exposure at a 5 W/cm2 irradiance of the skin on a 5 mm spot to the continuous wave 1064 nm laser augmented antibody titers to intradermal model and influenza vaccines and conferred better protection in an influenza lethal challenge. The dose and irradiance used in this study were confirmed to be non-tissue damaging in mice and below the level of pain or tissue damage in human subjects. The 1064 nm laser was reported to induce the expression of selected cytokines and chemokines including CCL2 and CCL20 and functional and migrational changes in DCs in the skin in this study. This immunostimulatory milieu with the laser adjuvant led to a mixed TH1-TH2 immune response to the whole inactivated influenza vaccination. Kimizuka et al. expanded this finding showing that economical semiconductor laser diodes have adjuvant effect (41). A Gallium arsenide (GaAs) diode laser emitting 1061 nm (Axcel Photonics, Marlborough, MA) at an average irradiances of 5 W/cm2 or Indium phosphide (InP) diode lasers emitting 1258 (Innolume, Dortmund, Germany) or 1301 nm (SemiNex Co., Peabody, MA) at an average irradiances of 1–2 W/cm2 over 1 min replicated the adjuvant effect of the large diode-pumped solid-state Nd:YVO4 laser system on the intradermal influenza vaccine without inducing damage or inflammation in the exposed tissue. These low-cost handheld laser devices are 10–100 times less expensive than high-frequency, ultrashort pulsed lasers, which establish the feasibility of the laser adjuvant approach for use in the clinic. It should be noted that the representative adjuvant including alum and MF59, but no laser adjuvant induced allergenic IgE response to the vaccination. Morse et al. further demonstrated that the same 1064 nm laser adjuvant modulated migratory DCs in the skin, specifically activating the Lang+ and CD11b−Lang− subsets and recruited Ly6C+ monocytes (42). Consistently, and the adjuvant effect was dependent on DC subsets expressing Langerin and CCR2. In this study, it has been suggested that thermal effect played a minimal role as the same dose of 1064 nm laser with a pulsed wave showed limited effects on the DC subsets and immune responses. Kimizuka et al. further revealed that continuous wave 1064 nm laser induced generation of reactive oxygen species (ROS) in the exposed skin and transiently stimulated mast cells, leading to expression of a defined set of chemokines including CCL2 and CCL20 that ultimately induced CCL21 expression in the lymphatics without overt inflammation (43). Our group further demonstrated that the immunostimulatory milieu established with the laser exposure induced migrational changes of skin-resident migratory CD103+ DCs, which played a pivotal role in augmentation of the adaptive immune response to the intradermal influenza vaccination (Figure 1). High-fluence low-power laser irradiation (HF-LPLI) has been consistently reported to target cytochrome c oxidase (COX) in electron transport chain (ETC) in mitochondria and induce generation of ROS. A 10-min exposure with 633nm He–Ne laser at an irradiance 0.2 W/cm2 (the fluence of 120 J/cm2) induced ROS generation, mitochondrial permeability transition (MPT) and apoptosis in cultured human lung adenocarcinoma cells (ASTC-a-1) (44, 45). Consistently, 635 nm continuous wave semiconductor laser for 10 min at a fluence rate of 50–200mW/cm2 inhibit enzymatic activity of COX and cause respiratory chain superoxide anion (O2−) burst and tumor killing in cultured human lung and mammary cancer cell lines. It has been further shown that exposure of xenografts in vivo with 635 nm laser at an irradiance of 500 mW/cm2 for 40 min significantly suppresses tumor growth (46). Although the laser vaccine adjuvant uses a smaller dosage than the laser immunotherapy, the mode of action of laser immunotherapy targeting mitochondrial ETC to augment the immune response is similar to that of the laser vaccine adjuvant. Further study is necessary to understand specific photochemical events in response to laser-tissue interactions between a laser parameter and target tissue to evoke desirable responses for a therapeutic purpose.
Gelfand et al. performed clinical testing to determine if the effect of the NPL adjuvant was consistent between mice and humans, as the skin structure of humans is quite different from that of mice (47). The group treated human subjects with continuous wave 1064 nm laser from a modified handheld diode laser device (IPG Photonics, Oxford, MA) for 1 min on 5 mm2 areas of the lower back skin at an irradiance of 5 W/cm2. All subjects tolerated this dose of the laser with no evidence of skin damage. Immunohistochemical analysis on skin biopsy samples taken at 4 h revealed reductions in the number of CD1a+ Langerhans cells and CD11c+ dermal DCs in the dermis, indicating emigration of these cell types out of the exposed skin. Consistently, gene expression analysis revealed increases in chemokine expression including CCL17 and CCL20, which were also observed in the mouse skin exposed to continuous wave 1064 nm laser. These results confirm that the responses of the skin cells to the NPL adjuvant in mice are similar to that in humans.
In short, these studies show that NPL adjuvant is a safe and effective to augment the efficacy of vaccine. These results collectively show that the use of continuous wave NIR laser with distinct wavelength and power is an effective tool to reproducibly modulate innate programs in the skin and can be explored for the broader applications such as the treatment of immune-related skin diseases. On a note, unlike other classes of the laser adjuvants, a combination approach of the NPL adjuvant with other adjuvant has never been tested for the potential synergistical effect in the context of vaccination.
3. Non-ablative fractional laser adjuvant
Fractional lasers are small diameter laser beams to create controlled damage to the skin and generate wound-healing responses. Fractional laser devices are therefore employed in skin rejuvenation and treating skin conditions including scarring (48). The treatment typically takes seconds creating an array of vertical columns of damaged or ablated skin within the epidermis and dermis. Power is optimized to induce minimal damage outside the treated tissue and pain while maximizing the wound healing response.
Non-ablative fractional laser (NAFL) induces controlled coagulation of tissue to produce micro-sterile inflammation and self-renewable microthermal zones (MTZs) in the skin. These laser devices emit microbeam pulses in the range of 1410 to 1550 nm to coagulate a series of vertical columns of tissue. The resultant dead cells in MTZs release nucleic acid and activate nucleic acid sensing pathways including the cGAS/STING pathway to induce sterile inflammatory responses and skin tissue regeneration. Therefore, NAFL has been successfully used for skin rejuvenation.
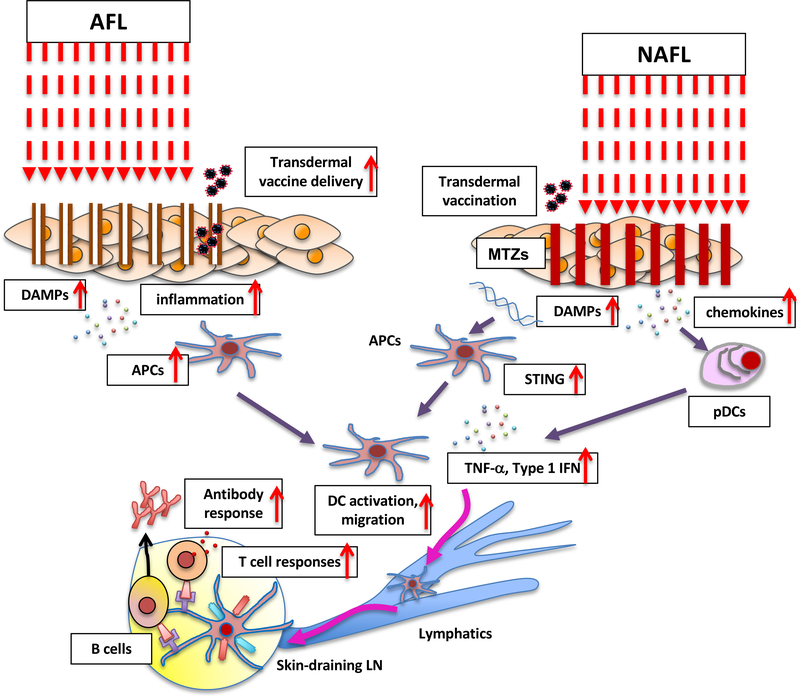
A series of studies have shown that NAFL can also function as an immunologic adjuvant for skin vaccination (Table 1). Wang et al. used a US Food and Drug Administration- (FDA-) approved NAFL devices PaloVia® Skin Renewing Laser developed by Palomar emitting a 1410 nm laser microbeam to generate an array of MTZ in the mouse skin or Fraxel SR-1500 laser by Solta Medical in the pig skin (49). The NAFL treatment followed by intradermal vaccination augmented humoral immune responses to influenza vaccine. The group further showed that topical application of imiquimod, which acts on toll-like receptor 7 (TLR7) and activates innate and adaptive immune responses, synergistically augmented humoral and T cell responses with the NAFL treatment. Topical imiquimod is FDA-approved for the treatment of anogenital warts, actinic keratosis, and superficial basal cell carcinomas (50) and a feasible choice for a combination approach. This particular combination approach in this study conferred cross-protection in a lethal challenge murine model of influenza. The mechanisms of action involve DAMPs released from MTZs, which recruit a large number of APCs to ultimately augment CD4+ and CD8+ T cell responses. In this sequence, the transient expression of a selective set of cytokines including TNF-α released from plasmacytoid DCs (pDCs) play a pivotal role in the strong adjuvant effect and T cell responses via IFN regulatory factor7 (IRF7). Wang et al. further showed that the NAFL treatment combined with microneedle vaccination augmented anti-influenza immunity while reducing local reactogenicity (51). The NAFL treatment enhanced the uptake and transportation of antigens by APCs and immune responses to vaccine packaged in the microneedle. This enhancement was mediated by dsDNA sensing STING pathway and subsequent interferon upregulation, leading to APC recruitment and migration (Figure 2). The group further demonstrated that this approach induced cross-protective immunity against influenza infection in mice and pigs.
Figure 2. The mechanism of action of the AFL and NAFL adjuvants.
AFL skin treatment induces the mild inflammatory milieu created by a narrow layer of coagulated dead tissue around each micro-channel. The efficient delivery of transdermal vaccine to skin DCs is also facilitated by the micro-channels. The NAFL treatment induces skin cell death (called microthermal treatment zones, MTZs) releasing damage-associated molecular patterns (DAMPs) including dsDNA and micro-sterile inflammation array. dsDNA is taken up by antigen presenting cells (APCs) and recognized by intracellular pattern recognition receptors (PRRs). PRRs then transduce signals to STING activating IRF3 and NF-κB. Type I interferons, proinflammatory cytokines and chemokines are produced to enhance the maturation and migration of APCs. AFL treatment also induces expression of chemokines in the skin. These chemokines recruit plasmacytoid dendritic cells (pDCs) from circulation system into the skin. The pDCs release a number of factors to enhance the maturation and migration of APCs.
Remarkably, Lopez et al. demonstrated that this approach can be used to induce protective effector memory (TEM) and tissue-resident (TRM) CD8+ T cell responses against herpes virus in the local mucosa (52). In this study, the treatment of the skin with NAFL (1410-nm PaloVia Laser) along with the local application of imiquimod cream followed by intradermal vaccination of peptide vaccine containing the immunodominant herpes simplex virus 2 (HSV-2) induced long-term memory CD8+ T cell responses and synergistically augmented protective immunity against genital herpes infection challenge. Interestingly, this study examined direct effect of NAFL on cultured DCs. An exposure of immature DCs to laser induced phenotypic maturation and promoted production of proinflammatory cytokines including IL-12 and TNF-α. The NAFL treatment was confirmed to induce accumulation of functionally matured DCs in the treated skin in vivo, which seems to be the central mechanism of action by which laser adjuvant enhances protective T-cell responses with NAFL. This is the first to demonstrate that laser-based adjuvant has potential to promote generation of TRM which play a key role in protection against viral infection.
Kim et al. combined transdermal vaccination using hyaluronan (HA) nanocarriers with NAFL to enhance the efficacy of transdermal vaccination (53). HA is a natural macromolecule with intrinsic high permeability into the skin and was used as a carrier of a model antigen for non-invasive vaccination on the skin in this study. Conjugates of ovalbumin (OVA) and HA also facilitated endocytosis via HA receptor and induced maturation of DCs in vitro, providing an additional adjuvant-like effect. The conjugates were confirmed to penetrate into the mouse and porcine skin more efficiently. Consistently, NAFL treatment using 1410-nm PaloVia Laser device significantly augmented humoral responses to OVA after non-invasive transdermal application of the conjugates.
Li et al. took a different approach to augment intradermal influenza vaccination using the NAFL adjuvant. The group combined 1410 nm NAFL treatment using 1410-nm PaloVia Laser device with topical application of all-trans retinoic acid (ATRA, a vitamin A derivative) to synergistically augment immune responses (54). ATRA is an established pleiotropic modulator of innate and adaptive immunity (55). In addition, topical application of ATRA is approved by the FDA for acne treatment (56) and represents a feasible choice for a combination strategy. In this study, ATRA stimulated upregulation of cytosolic nucleic acid sensors and their downstream factors, leading to enhancement of type I interferon expression caused by release of DAMPs upon the NAFL treatment. The combination approach significantly enhanced antibody responses against an inactivated influenza virus vaccine by 10-fold. The group also observed this approach induced cross-reactive neutralizing antibody and conferred cross-protection against infection with heterogeneous viral strains. Since ATLA alone induced a TH2-biased immune response, the combination approach was proved to be essential to induce TH1-TH2-balanced immune responses.
These results consistently demonstrate that NAFL from a clinically approved device is a feasible and effective approach to augment the efficacy of vaccine. Most of studies also show its synergistic effect with chemical adjuvants to induce robust T cell responses and protection. Further complementary development of skin vaccination technology is desired to fully take advantage of this approach.
4. Ablative fractional laser adjuvant
Ablative fractional laser (AFL) typically uses 2790, 2940 or 10600 nm laser to create micro-channel arrays (50–150 μm in diameter with a depth of less than one millimeter) in the skin (Table 1). These wavelengths show high absorption by water and cause explosive superheating of the aqueous content of the tissue column, leading to progressive ablation of the narrow columns of tissue. Each micro-channel is surrounded by a narrow (around 5 μm-thick) layer of coagulated dead tissue. AFL treatment promotes collagen formation and retraction of the dermis and epidermis to tighten the skin and is therefore used for skin resurfacing (57). In addition, AFL has been investigated for a safe method to enhance transcutaneous delivery of drugs (58, 59). Since the skin is enriched with APCs, the literature indicates that the delivery of vaccine to the skin is more effective vaccination than the conventional intramuscular or subcutaneous delivery (14–16, 19, 60). It appears that AFL not only facilitates delivery of antigens to APCs in the skin, but also acts as an adjuvant augmenting the immune response to intradermal or transdermal vaccination.
Chen et al. used AFL treatment of the skin to generate microchannel arrays in the epidermis to facilitate vaccine delivery (61). An UltraPulse® Fractional CO2 Laser emitting 10600 nm (Lumenis Inc., Yorkneam, Israel) was used to create microchannels. This approach was proved to deliver a model protein vaccine (OVA) efficiently with gauze skin patches onto the skin and enhance antigen take-up by APCs in the skin augmenting the immunogenicity of the vaccine compared to the conventional tape-stripping method. Weiss et al. used the P.L.E.A.S.E.® (Precise Laser Epidermal System) device developed by Pantec Biosolutions AG (Ruggell, Liechtenstein) to improve vaccine delivery, which is based on a 2940 nm diode-pumped, erbium:yttrium-aluminium-garnet (ER:YAG) laser (62). A model antigen application after AFL treatment enhanced antigen uptake by skin DCs and induced significantly higher antigen-specific T cell responses compared to the subcutaneous injection. However, compared to the subcutaneous injection of the same allergen, this approach rather led to the induction of a TH2-biased response. Interestingly, this response was significantly redirected toward TH1 responses by co-application of a TLR agonist CpG oligodeoxynucleotides (ODN) with their TH1-promoting immunomodulatory capacity (63). These results show that AFL treatment enhances transdermal delivery of vaccine antigen to APC populations in the skin and at the same time strongly influences on the magnitude and polarization of T cells, which could be further optimized by an addition of a chemical adjuvant.
Scheiblhofer et al. further advanced this approach to elicit high antibody titers by exploring an optimal laser parameter and adjuvant to be combined (64). The group found that antibody responses against transcutaneously administered hepatitis B surface antigen vaccine were dependent on micropore depth and peaked at a laser power density of 8.4 J/cm2, while being independent of micropore density. Having optimized the laser parameter, the group further tested combination of AFL with 5 representative adjuvants of MPL, heat labile enterotoxin (LT)-B subunit from E. coli, CpG-ODN 1826, alum, CRM197 (a nontoxic diphtheria toxin derivative) to have found that alum significantly reduced antibody titers and other adjuvants induced marginal changes only. LT-B and CpG shifted the polarization of the immune response toward TH1, suggesting that a laser parameter and choice of an adjuvant to be combined should be carefully selected depending on a specific goal of immunization.
Chen et al. used an approach to combine topical application of powder vaccine-coated array patches with AFL treatment of the skin to generate micro-channel arrays in the epidermis to adjuvant vaccine (65). The UltraPulse® Fractional CO2 Laser was used to create microchannels. This approach was proved to deliver vaccine into the skin efficiently and able to maintain the same immunogenicity as the intradermal injection of live-attenuated or chemically-adjuvanted inactivated vaccine formulation, while reducing local inflammation that causes permanent scars generally found in the intradermal administration of these vaccines. These results suggest that the limited inflammation around microchannels may be sufficient to augment the immunogenicity of the intradermal vaccine.
Terhorst et al. tested the effect of AFL on a vaccibody, which is a homodimeric chimeric protein consisting of a XC-chemokine XCL1, a dimerization unit made of the hinge and CH3 domain of human IgG3, and moiety of antigen for the targeted delivery of vaccine antigen to target XCR1+ dermal DCs (66). The XCR1+ DC subset is increasingly recognized as being critical for formation of cytotoxic T cell and TH1 responses, long-term adaptive memory responses (67–71) and early and long-term antibody responses (72). In order to facilitate the delivery of the vaccibody, this group employed the P.L.E.A.S.E.® device. The treatment of skin generated an array of several hundred micropores of approximately 150 μm diameter over a 14 mm2 spot. Interestingly, the combination of the AFL treatment with the targeted delivery of a model cancer antigen on prophylactic or therapeutic cancer vaccine to dermal XCR1+ DCs significantly augmented antigen-specific CD8+ and CD4+ T cell responses and protection against melanoma. The mild inflammatory milieu created by dead keratinocytes upon the AFL treatment was considered to have contributed to the adjuvant effect of AFL. Hervé et al. recently advanced this approach further. The group used the P.L.E.A.S.E.® system to create laser-induced epidermis-limited perforation to augment the efficacy of epicutaneous vaccination (73) using a non-invasive and needle-free skin patch Viaskin® which has been successfully used for transcutaneous delivery of food allergen for immunotherapy (74) and genetically-inactivated recombinant pertussis toxin (75) and respiratory syncytial virus (RSV) N-nanorings (76) for vaccination. This approach induced limited skin inflammation with upregulation of IL-1β and IL-33, enhanced Langerhans cell activation and facilitated migration of antigen-positive DCs in skin to draining lymph nodes, ultimately inducing a rapid increase in antibody titers against a model vaccine and recombinant pertussis toxin.
Hessenberger et al. used this methodology to improve immunotherapy for allergy with the skin patch (77). Allergen-specific immunotherapy involves gradual administration of allergen using various routes to induce the reinstatement of immunologic tolerance toward allergens (78). Compared to the conventional subcutaneous immunotherapy (SCIT) involving subcutaneous injection of allergen using the hypodermic needle, transcutaneous application, which is called epicutaneous immunotherapy (EPIT), has been consistently found to be safe but with the modest efficacy and needs improvement (78). The transcutaneous immunization using skin patch with a recombinant glass pollen allergen Phl p 5 via laser-generated micropores led to induction of an allergenic TH2-biased response compared with the subcutaneous injection of the same allergen. Interestingly, this response was significantly suppressed by co-application of a TLR agonist CpG-ODN with the TH1-promoting immunomodulatory capacity (63) in these pre-sensitized mice but not by SCIT. Kumar et al. similarly treated mice with AFL before application of a model allergen (79). The group treated the mouse skin with P.L.E.A.S.E.® laser to generate 75 micropores per cm2 (50–75 μm in base diameter and 20–30 μm in depth). The AFL treatment with topically applied OVA using the skin patch facilitated the delivery of the allergen into the skin and take-up by APCs with no inflammation. Since a combination of CpG-ODN and 1,25-dihydroxyvitamin D3 (VD3) was found to profoundly increase TGF-β and IL-10, two primary cytokines for regulatory T cell (Treg) and subsequent immunological tolerance induction, and IL-12 and IL-18 for a TH1 response while suppressing IL-4 for a TH2 response in the skin among other tested combinations of adjuants, the group then applied OVA mixed with CpG-ODN with VD3 onto each micropore in OVA-sensitized mice. Consistently, the combination therapy significantly enhanced Tregs, suppressed IgE response and alleviated allergic airway responses in OVA-sensitized mice while the conventional SCIT failed to do so. Machado et al. used mannan-neoglycoconjugates which binds C-type lectin receptors and targets CD14+ DCs and Langerhans cells to avoid TH2-biased immune responses and induce proper modulation of the immune response upon EPIT (80). The group pre-treated the skin with the AFL prior to the transcutaneous application of a pollen allergen-mannan neoglycoconjugates to facilitate the transdermal delivery. This approach synergistically augmented TH1/TH17-biased humoral and cellular immune responses and suppressed IgE responses to the pollen allergen in mice. The suppression of IgE responses with AFL was found to be superior to intradermal injection of the allergen. Consistently, the expression of proinflammatory cytokines and chemokines was increased upon with the AFL treatment in the skin, being responsible for the synergistic effect. Interestingly, in contrast to NPL, depletion of mast cells (MCs) had no substantial effect on adaptive immune responses in the context of AFL-mediated epicutaneous immunization (81). These results show that AFL treatment could offer a strong immunomodulatory effect to skin-based immunotherapy.
Further investigation is warranted to find a tailored combination of AFL and other adjuvants to maximize the efficacy of the current and candidate vaccine and immunotherapy.
Discussion
A series of studies clearly demonstrates that all the four classes of the laser adjuvants are able to function as an immunologic adjuvant in preclinical models and clinical studies. Contrary to the conventional chemical or biological adjuvants, all the preclinical and clinical safety studies indicate that the laser adjuvant is safe and induces no significant local or systemic side effects. The laser adjuvants show a number of advantages over the conventional adjuvants. i) Laser is a physical parameter and does not persist in the tissues, reducing risk of side effects. ii) The laser adjuvant presents no requirement of irreversible formulation with vaccine nor poses risk of alteration to the vaccine antigen. iii) The laser device is free from cold-chain storage and suitable for long-term storage. iv) The laser application requires no hypodermic needles and produces no biohazardous sharp waste. Although no clinical trial has been conducted to evaluate the efficacy of the laser adjuvant to date, further efforts on rigorous preclinical safety and toxicology would open a path for the laser adjuvant to be used in the clinic.
It is challenging to directly compare the efficacy of a class of the laser adjuvant with each other because of the variability of the immunization models used in the literature. For example, co-application of imiquimod adjuvant with NAFL treatment was essential to induce T cell responses to a peptide herpes vaccine and protection in a preclinical model of herpes infection (52) and to inactivated influenza vaccine and protection in a preclinical model of influenza infection (49). On the other hand, NAFL treatment alone was sufficient to induce T cell responses and confer protection when combined with a PR8 model influenza vaccine-packaged, biodegradable microneedle array (51). UPL adjuvant alone was also sufficient to confer protection in a mouse model of influenza (21, 22), induce CD8+ T cell response to a sporozoite vaccine and protection in a malaria challenge model (27) and CD8+ T cell response to vaccinia virus-based vaccine and protection in a vaccinia challenge model (65), while the other laser adjuvants require an addition of other adjuvants to induce T cell responses. Since immunologic adjuvants receive regulatory approval only with specific vaccines, further mechanistic investigation to fully characterize the mode of action of each laser adjuvant is warranted to achieve clinical significance.
Although limited, the literature shows that each class of the laser adjuvant has the distinct molecular mechanisms of action depending on the type of laser and parameters used (Table 2). There seems to be common mechanisms across the classes, while some are very specific to a certain class. Disrupting the stratum corneum in the epidermis has been shown to induce the secretion of pro-inflammatory cytokines, chemokines and release of DAMPs, acting as an inherent adjuvant (82). In this process, damaged keratinocytes release cytokines such as IL-25, IL-33 and TSLP and subsequently activate other skin-resident immune cells (83). In this regard, the mechanisms of action involved in the NAFL adjuvant are relatively clear as they create controlled damages, induce DAMPs release and activation of dsDNA sensing STING pathway, and then evoke limited inflammation around the damaged tissue in the treated skin, ultimately stimulating APC migration and function and augmenting adaptive immune responses (51). The mechanisms of action involved in the AFL adjuvant appear to be similar to those of NAFL. Upon AFL treatment, the mild inflammatory milieu is created by dead keratinocytes surrounding columns, which is considered to stimulate the immune response in a similar way as NAFL. These fractional laser-based approaches generally lead to the induction of TH2-biased responses (62) and often need TH1 adjuvant to redirect immune responses to confer protection (52). In comparison, the molecular pathways involved in the UPL or NPL remain relatively unclear. High-frequency UPL induces HSP70 release while lower frequency UPL creates disarray of the extracellular matrix, but like other adjuvants used in licensed vaccines, both eventually activate key Langerhans cells (21) and APCs in skin (24). On the other hand, NPL adjuvant induces ROS generation and activation of innate programs including up-regulation of a selective set of chemokines, which were not seen in UPL adjuvant (24), ultimately activating migratory DCs (42, 43). In contrast to the AFP or NAFL adjuvant, no tissue damage has been detected by histological examination after the UPL or NPL treatment in the human and mouse skin (24, 26, 47). The photoreception mechanisms, exact molecular identity of photoreceptors, and subsequent signaling pathways involved in the adjuvant effect of the UPL or NPL adjuvant remain elusive. In order to direct the current and candidate vaccines with the laser adjuvant toward the desired clinical benefits with minimize side effects, it is imperative to advance basic research on the molecular mechanisms of action of the laser adjuvant.
Table 2.
Mechanisms of action of the laser adjuvants.
| Molecular pathways activated | Laser type | Wavelength | Immunological consequences | References |
|---|---|---|---|---|
| Extracellular release of HSP70 | UPL | 532 nm | Langerhans cell activation and antigen presentation | (21–23) |
| Disarray of the extracellular matrix in the dermis | UPL | 532 nm | facilitates migration of DCs | (84) |
| Release of DAMPs (HSP70, HSP90 and HMGB1) | NPL | 980 nm | Activation of DCs | (32) (34–37) |
| Release of tumor-specific antigens | NPL | 808 nm | Maturation of DCs | (38) |
| ROS generation | NPL | 1064 nm | Selective activation skin DCs | (43) |
| Release of DAMPs Activation of nucleic acid sensing pathways including the cGAS/STING pathway |
NAFL | 1410 – 1540 nm | Recruitment of pDCs | (49, 51, 54) |
| Direct physical effect of laser | NAFL | 1410 nm | Direct activation of skin-resident DCs | (52) |
| Physical enhancement in vaccine delivery | AFL | 2940 – 10600 nm | Enhanced antigen take-up by skin APCs | (61, 62, 65) |
| Mild inflammatory milieu created in the dermis | AFL | 2940 nm | Activation of skin-resident DCs | (66, 73, 80, 81) |
AFL, ablative fractional laser; APCs, antigen presenting cells; cGAS, cyclic GMP-AMP synthase; DAMPs, damage-associated molecular patterns, DCs, dendritic cells; HMGB1, High Mobility Group Box 1; HSP70, heat shock protein 70; NAFL, non-ablative fractional laser; NPL, non-pulsed laser; pDCs, plasmacytoid DCs; ROS, reactive oxygen species; STING, stimulator of interferon genes; UPL, ultra-short pulsed laser.
Each class of the laser adjuvants shows distinct characteristics as an immunologic adjuvant, suggesting that the judicial use of the laser adjuvant is needed to achieve the specific goals of a particular vaccine or immunotherapeutic. It appears that the laser adjutant generally leads to the induction of a TH2-biased response. As such, in order to confer protection with vaccination or reverse allergenic responses, combination strategies have been extensively tested (Table 3). Interestingly, in some cases, the use of chemical adjuvant is necessary to achieve the goals. For example, co-application of additional adjuvant with its TH1-promoting immunomodulatory capacity was essential to confer protection (49, 52) or suppress allergenic responses (62, 77), while majority of studies employ it to further augment therapeutic efficacy. Since the use of additional adjuvant would pose a significant regulatory hurdle, most of the studies focus on licensed chemical or biological adjuvants. In order to take advantages of non-invasive nature of the laser adjuvant, topical application of additional adjuvant is desirable for a combination approach. Not surprisingly, combination strategies to date employed FDA-approved topical agents including imiquimod and ATRA (Table 3).
Table 3.
Combination approaches with the laser adjuvants.
| Chemical adjuvant | FDA approval | Laser type | Wavelength | Distinct effect of combination | References |
|---|---|---|---|---|---|
| MPL | Used in a licensed vaccine as adjuvant | UPL | 532 nm | Further augmentation of ab response to nicotine | (25) |
| Imiquimod | Treatment for anogenital warts, actinic keratosis, and superficial basal cell carcinomas (topical application) | NPL | 808 nm | Synergistic suppressive effect on tumor growth | (35, 37) |
| NAFL | 1410 – 1540 nm | Synergistic augmentation of humoral and T cell responses | (49, 51) | ||
| NAFL | 1410 nm | Addition of imiquimod is necessary for the laser adjuvant to show the effect | (52) | ||
| ATRA | Acne treatment (topical application) | NAFL | 1410 nm | Further augmentation of ab response to influence vaccine | (54) |
| CpG-ODN | No | AFL | 2940 nm | Suppressing allergenic responses if AFL and the chemical adjuvant are combined | (62, 77) |
| CpG-ODN + VD3 | No | AFL | 2940 nm | Increasing cytokine expression for TH1/Treg induction while suppressing TH2 response when combined with AFL | (79) |
AFL, ablative fractional laser; ATRA, all-trans retinoic acid; CpG-ODN, CpG-oligodeoxynucleotides; MPL, monophosphoryl lipid A; NAFL, non-ablative fractional laser; NPL, non-pulsed laser; UPL, ultra-short pulsed laser; VD3, 1,25-dihydroxyvitamin D3.
With the proven safety and efficacy, AFL adjuvant has reached clinical development. A group at Medical University of Vienna sponsored by Pantec Biosolutions AG completed Phase I clinical trial of “EPIMMUN Influenza” comparing laser-assisted epidermal using the P.L.E.A.S.E.® system to intradermal administration of Sanofi Pasteur/MSD seasonal influenza vaccine INTANZA 15μg (NCT02988739, the result of the study is not available yet). The other laser adjuvants have not yet reached advanced preclinical development. It should be noted that the first demonstration of the effect of the laser adjuvant was performed in cancer patients in Russia (21–23). A recent expanded list of intradermal vaccines along with the matured medical laser industry is expected to boost initiation of the clinical translation of this technology into intradermal or transdermal vaccination. In addition, the recent studies demonstrate that the laser adjuvant could be used to augment the efficacy of immunotherapy for allergy. Exploration of broader applications of this approach to the treatment of immune-related diseases is expected to follow this path in the near future.
Acknowledgments
Funding
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number R01AI105131 (S.K.) and R21AI144103 (S.K.) and Massachusetts General Hospital Executive Committee On Research (ECOR) Interim Support Funding (S.K.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Non-standard abbreviations
- AFL
ablative fractional laser
- ATP
adenosine triphosphate
- ATRA
all-trans retinoic acid
- APC
antigen-presenting cell
- BRD4
bromodomain-containing protein 4
- CRT
calreticulin
- CpG-ODN
oligodeoxynucleotides
- COX
cytochrome c oxidase
- CTL
cytotoxic T lymphocyte
- DC
dendritic cells
- ETC
electron transport chain
- EPIT
epicutaneous immunotherapy
- GaAs
Gallium arsenide
- GC
glycated chitosan
- LT
heat labile enterotoxin
- HSP
heat shock protein
- HSV
herpes simplex virus
- HF-LPLI
high-fluence low-power laser irradiation
- HMGB1
high-mobility group box 1
- HA
hyaluronan
- InP
Indium phosphide
- ICG
indocyanine green
- MTZ
microthermal zones
- MPT
mitochondrial permeability transition
- MPL
monophosphoryl lipid A
- NIR
near-infrared
- NAFL
non-ablative fractional laser
- NPL
non-pulsed laser
- OVA
ovalbumin
- PTT
photothermal therapy
- KTP
potassium titanyl phosphate
- Q-Nd:YAG
Q-switched neodymium-doped yttrium aluminum garnet
- Q-Nd:YVO4
Q-switched neodymium-doped yttrium orthovanadate
- RAS
radiation-attenuated sporozoites
- ROS
reactive oxygen species
- RSV
respiratory syncytial virus
- SCIT
subcutaneous immunotherapy
- TLR
toll-like receptor
- UPL
ultra-short pulsed laser
- VD3
1,25-dihydroxyvitamin D3
Footnotes
Potential conflict of interest
All authors: No reported conflicts.
References
- 1.Bergmann-Leitner ES, and Leitner WW (2014) Adjuvants in the Driver’s Seat: How Magnitude, Type, Fine Specificity and Longevity of Immune Responses Are Driven by Distinct Classes of Immune Potentiators. Vaccines (Basel) 2, 252–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schijns VE, and Lavelle EC (2011) Trends in vaccine adjuvants. Expert Rev Vaccines 10, 539–550 [DOI] [PubMed] [Google Scholar]
- 3.Coffman RL, Sher A, and Seder RA (2010) Vaccine adjuvants: putting innate immunity to work. Immunity 33, 492–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Del Giudice G, Rappuoli R, and Didierlaurent AM (2018) Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin Immunol 39, 14–21 [DOI] [PubMed] [Google Scholar]
- 5.Bonam SR, Partidos CD, Halmuthur SKM, and Muller S (2017) An Overview of Novel Adjuvants Designed for Improving Vaccine Efficacy. Trends Pharmacol Sci [DOI] [PubMed] [Google Scholar]
- 6.Reed SG, Orr MT, and Fox CB (2013) Key roles of adjuvants in modern vaccines. Nat Med 19, 1597–1608 [DOI] [PubMed] [Google Scholar]
- 7.Garcon N, Segal L, Tavares F, and Van Mechelen M (2011) The safety evaluation of adjuvants during vaccine development: the AS04 experience. Vaccine 29, 4453–4459 [DOI] [PubMed] [Google Scholar]
- 8.Gupta RK, Rost BE, Relyveld E, and Siber GR (1995) Adjuvant properties of aluminum and calcium compounds. Pharmaceutical biotechnology 6, 229–248 [DOI] [PubMed] [Google Scholar]
- 9.Batista-Duharte A, Portuondo D, Carlos IZ, and Perez O (2013) An approach to local immunotoxicity induced by adjuvanted vaccines. Int Immunopharmacol 17, 526–536 [DOI] [PubMed] [Google Scholar]
- 10.Batista-Duharte A, Lindblad EB, and Oviedo-Orta E (2011) Progress in understanding adjuvant immunotoxicity mechanisms. Toxicology letters 203, 97–105 [DOI] [PubMed] [Google Scholar]
- 11.Lee S, and Nguyen MT (2015) Recent advances of vaccine adjuvants for infectious diseases. Immune Netw 15, 51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rappuoli R, Mandl CW, Black S, and De Gregorio E (2011) Vaccines for the twenty-first century society. Nat Rev Immunol 11, 865–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nirschl CJ, and Anandasabapathy N (2016) Duality at the gate: Skin dendritic cells as mediators of vaccine immunity and tolerance. Hum Vaccin Immunother 12, 104–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fehres CM, Garcia-Vallejo JJ, Unger WW, and van Kooyk Y (2013) Skin-resident antigen-presenting cells: instruction manual for vaccine development. Front Immunol 4, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Combadiere B, and Liard C (2011) Transcutaneous and intradermal vaccination. Hum Vaccin 7, 811–827 [DOI] [PubMed] [Google Scholar]
- 16.Sticchi L, Alberti M, Alicino C, and Crovari P (2010) The intradermal vaccination: past experiences and current perspectives. J Prev Med Hyg 51, 7–14 [PubMed] [Google Scholar]
- 17.Zehrung D, Jarrahian C, and Wales A (2013) Intradermal delivery for vaccine dose sparing: overview of current issues. Vaccine 31, 3392–3395 [DOI] [PubMed] [Google Scholar]
- 18.Nicolas JF, and Guy B (2008) Intradermal, epidermal and transcutaneous vaccination: from immunology to clinical practice. Expert Rev Vaccines 7, 1201–1214 [DOI] [PubMed] [Google Scholar]
- 19.Lambert PH, and Laurent PE (2008) Intradermal vaccine delivery: will new delivery systems transform vaccine administration? Vaccine 26, 3197–3208 [DOI] [PubMed] [Google Scholar]
- 20.Kim DH, Byamba D, Wu WH, Kim TG, and Lee MG (2012) Different characteristics of reactive oxygen species production by human keratinocyte cell line cells in response to allergens and irritants. Exp Dermatol 21, 99–103 [DOI] [PubMed] [Google Scholar]
- 21.Onikienko SB, Zemlyanoy AB, Margulis BA, Guzhova IV, Varlashova MB, Gornostaev VS, Tikhonova NV, Baranov GA, and Lesnichiy VV (2007) Diagnostics and correction of the metabolic and immune disorders. Interactions of bacterial endotoxins and lipophilic xenobiotics with receptors associated with innate immunity. . Donosologiya (St. Petersburg) 1, 32–54 [Google Scholar]
- 22.Kashiwagi S, Brauns T, Gelfand J, and Poznansky MC (2014) Laser vaccine adjuvants. History, progress, and potential. Hum Vaccin Immunother 10, 1892–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kashiwagi S, Brauns T, and Poznansky MC (2016) Classification of Laser Vaccine Adjuvants. J Vaccines Vaccin 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, Kim P, Farinelli B, Doukas A, Yun SH, Gelfand JA, Anderson RR, and Wu MX (2010) A novel laser vaccine adjuvant increases the motility of antigen presenting cells. PLoS ONE 5, e13776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X, Pravetoni M, Bhayana B, Pentel PR, and Wu MX (2012) High immunogenicity of nicotine vaccines obtained by intradermal delivery with safe adjuvants. Vaccine 31, 159–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kashiwagi S, Yuan J, Forbes B, Hibert ML, Lee EL, Whicher L, Goudie C, Yang Y, Chen T, Edelblute B, Collette B, Edington L, Trussler J, Nezivar J, Leblanc P, Bronson R, Tsukada K, Suematsu M, Dover J, Brauns T, Gelfand J, and Poznansky MC (2013) Near-infrared laser adjuvant for influenza vaccine. PLoS One 8, e82899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou C, Chen X, Zhang Q, Wang J, and Wu MX (2015) Laser mimicking mosquito bites for skin delivery of malaria sporozoite vaccines. Journal of controlled release : official journal of the Controlled Release Society 204, 30–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ackroyd R, Kelty C, Brown N, and Reed M (2001) The history of photodetection and photodynamic therapy. Photochem Photobiol 74, 656–669 [DOI] [PubMed] [Google Scholar]
- 29.Grzybowski A, and Pietrzak K (2012) From patient to discoverer--Niels Ryberg Finsen (1860–1904) --the founder of phototherapy in dermatology. Clin Dermatol 30, 451–455 [DOI] [PubMed] [Google Scholar]
- 30.Chen WR, Adams RL, Carubelli R, and Nordquist RE (1997) Laser-photosensitizer assisted immunotherapy: a novel modality for cancer treatment. Cancer letters 115, 25–30 [DOI] [PubMed] [Google Scholar]
- 31.Zhou F, Nordquist RE, and Chen WR (2016) Photonics immunotherapy — A novel strategy for cancer treatment. J Innov Opt Health Sci 9, 1630001 [Google Scholar]
- 32.Zhou F, Yang J, Zhang Y, Liu M, Lang ML, Li M, and Chen WR (2018) Local Phototherapy Synergizes with Immunoadjuvant for Treatment of Pancreatic Cancer through Induced Immunogenic Tumor Vaccine. Clinical cancer research : an official journal of the American Association for Cancer Research 24, 5335–5346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kroemer G, Galluzzi L, Kepp O, and Zitvogel L (2013) Immunogenic cell death in cancer therapy. Annu Rev Immunol 31, 51–72 [DOI] [PubMed] [Google Scholar]
- 34.Zhou F, Li X, Song S, Acquaviva III JT, Wolf RF, Howard EW, and Chen WR (2013) Anti-tumor responses induced by laser irradiation and immunological stimulation using a mouse mammary tumor model. J Innov Opt Heal Sci 6, 1350039 [Google Scholar]
- 35.Luo M, Shi L, Zhang F, Zhou F, Zhang L, Wang B, Wang P, Zhang Y, Zhang H, Yang D, Zhang G, Chen WR, and Wang X (2018) Laser immunotherapy for cutaneous squamous cell carcinoma with optimal thermal effects to enhance tumour immunogenicity. Int J Hyperthermia 34, 1337–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naylor MF, Zhou F, Geister BV, Nordquist RE, Li X, and Chen WR (2017) Treatment of advanced melanoma with laser immunotherapy and ipilimumab. J Biophotonics 10, 618–622 [DOI] [PubMed] [Google Scholar]
- 37.Shi L, Luo M, Zhang F, Zhang L, Wang B, Liu P, Zhang Y, Zhang H, Yang D, Zhang G, Zhou F, Stepp H, Sroka R, Chen WR, and Wang X (2019) Photothermal therapy enhanced the effectiveness of imiquimod against refractory cutaneous warts through boosting immune responses. J Biophotonics 12, e201800149. [DOI] [PubMed] [Google Scholar]
- 38.Wang T, Wang D, Yu H, Feng B, Zhou F, Zhang H, Zhou L, Jiao S, and Li Y (2018) A cancer vaccine-mediated postoperative immunotherapy for recurrent and metastatic tumors. Nature communications 9, 1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mew D, Wat CK, Towers GH, and Levy JG (1983) Photoimmunotherapy: treatment of animal tumors with tumor-specific monoclonal antibody-hematoporphyrin conjugates. J Immunol 130, 1473–1477 [PubMed] [Google Scholar]
- 40.Mitsunaga M, Ogawa M, Kosaka N, Rosenblum LT, Choyke PL, and Kobayashi H (2011) Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat Med 17, 1685–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kimizuka Y, Callahan JJ, Huang Z, Morse K, Katagiri W, Shigeta A, Bronson R, Takeuchi S, Shimaoka Y, Chan MP, Zeng Y, Li B, Chen H, Tan RY, Dwyer C, Mulley T, Leblanc P, Goudie C, Gelfand J, Tsukada K, Brauns T, Poznansky MC, Bean D, and Kashiwagi S (2017) Semiconductor diode laser device adjuvanting intradermal vaccine. Vaccine 35, 2404–2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morse K, Kimizuka Y, Chan MPK, Shibata M, Shimaoka Y, Takeuchi S, Forbes B, Nirschl C, Li B, Zeng Y, Bronson RT, Katagiri W, Shigeta A, Sirbulescu RF, Chen H, Tan RYY, Tsukada K, Brauns T, Gelfand J, Sluder A, Locascio JJ, Poznansky MC, Anandasabapathy N, and Kashiwagi S (2017) Near-Infrared 1064 nm Laser Modulates Migratory Dendritic Cells To Augment the Immune Response to Intradermal Influenza Vaccine. J Immunol 199, 1319–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kimizuka Y, Katagiri W, Locascio JJ, Shigeta A, Sasaki Y, Shibata M, Morse K, Sirbulescu RF, Miyatake M, Reeves P, Suematsu M, Gelfand J, Brauns T, Poznansky MC, Tsukada K, and Kashiwagi S (2018) Brief Exposure of Skin to Near-Infrared Laser Modulates Mast Cell Function and Augments the Immune Response. J Immunol 201, 3587–3603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu S, Xing D, Gao X, and Chen WR (2009) High fluence low-power laser irradiation induces mitochondrial permeability transition mediated by reactive oxygen species. J Cell Physiol 218, 603–611 [DOI] [PubMed] [Google Scholar]
- 45.Wu S, Xing D, Wang F, Chen T, and Chen WR (2007) Mechanistic study of apoptosis induced by high-fluence low-power laser irradiation using fluorescence imaging techniques. J Biomed Opt 12, 064015. [DOI] [PubMed] [Google Scholar]
- 46.Wu S, Zhou F, Wei Y, Chen WR, Chen Q, and Xing D (2014) Cancer phototherapy via selective photoinactivation of respiratory chain oxidase to trigger a fatal superoxide anion burst. Antioxid Redox Signal 20, 733–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gelfand JA, Nazarian RM, Kashiwagi S, Brauns T, Martin B, Kimizuka Y, Korek S, Botvinick E, Elkins K, Thomas L, Locascio J, Parry B, Kelly KM, and Poznansky MC (2019) A pilot clinical trial of a near-infrared laser vaccine adjuvant: safety, tolerability, and cutaneous immune cell trafficking. FASEB J 33, 3074–3081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saedi N, Petelin A, and Zachary C (2011) Fractionation: a new era in laser resurfacing. Clin Plast Surg 38, 449–461, vii [DOI] [PubMed] [Google Scholar]
- 49.Wang J, Shah D, Chen X, Anderson RR, and Wu MX (2014) A micro-sterile inflammation array as an adjuvant for influenza vaccines. Nature communications 5, 4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hanna E, Abadi R, and Abbas O (2016) Imiquimod in dermatology: an overview. Int J Dermatol 55, 831–844 [DOI] [PubMed] [Google Scholar]
- 51.Wang J, Li B, and Wu MX (2015) Effective and lesion-free cutaneous influenza vaccination. Proc Natl Acad Sci U S A 112, 5005–5010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lopes PP, Todorov G, Pham TT, Nesburn AB, Bahraoui E, and BenMohamed L (2018) Laser Adjuvant-Assisted Peptide Vaccine Promotes Skin Mobilization of Dendritic Cells and Enhances Protective CD8(+) TEM and TRM Cell Responses against Herpesvirus Infection and Disease. J Virol 92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim KS, Kim H, Park Y, Kong WH, Lee SW, Kwok SJ, Hahn SK, and Yun SH (2016) Noninvasive Transdermal Vaccination Using Hyaluronan Nanocarriers and Laser Adjuvant. Adv Funct Mater 26, 2512–2522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li P, Wang J, Cao M, Deng Q, Jiang S, Wu MX, and Lu L (2018) Topical Application of a Vitamin A Derivative and Its Combination With Non-ablative Fractional Laser Potentiates Cutaneous Influenza Vaccination. Front Microbiol 9, 2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Larange A, and Cheroutre H (2016) Retinoic Acid and Retinoic Acid Receptors as Pleiotropic Modulators of the Immune System. Annu Rev Immunol 34, 369–394 [DOI] [PubMed] [Google Scholar]
- 56.Tan AU, Schlosser BJ, and Paller AS (2018) A review of diagnosis and treatment of acne in adult female patients. Int J Womens Dermatol 4, 56–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Preissig J, Hamilton K, and Markus R (2012) Current Laser Resurfacing Technologies: A Review that Delves Beneath the Surface. Semin Plast Surg 26, 109–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haedersdal M, Sakamoto FH, Farinelli WA, Doukas AG, Tam J, and Anderson RR (2010) Fractional CO(2) laser-assisted drug delivery. Lasers Surg Med 42, 113–122 [DOI] [PubMed] [Google Scholar]
- 59.Bachhav YG, Summer S, Heinrich A, Bragagna T, Bohler C, and Kalia YN (2010) Effect of controlled laser microporation on drug transport kinetics into and across the skin. Journal of controlled release : official journal of the Controlled Release Society 146, 31–36 [DOI] [PubMed] [Google Scholar]
- 60.Teunissen MB, and Zehrung D (2015) Cutaneous vaccination - Protective immunization is just a skin-deep step away. Vaccine 33, 4659–4662 [DOI] [PubMed] [Google Scholar]
- 61.Chen X, Shah D, Kositratna G, Manstein D, Anderson RR, and Wu MX (2012) Facilitation of transcutaneous drug delivery and vaccine immunization by a safe laser technology. Journal of controlled release : official journal of the Controlled Release Society 159, 43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weiss R, Hessenberger M, Kitzmuller S, Bach D, Weinberger EE, Krautgartner WD, Hauser-Kronberger C, Malissen B, Boehler C, Kalia YN, Thalhamer J, and Scheiblhofer S (2012) Transcutaneous vaccination via laser microporation. Journal of controlled release : official journal of the Controlled Release Society 162, 391–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bode C, Zhao G, Steinhagen F, Kinjo T, and Klinman DM (2011) CpG DNA as a vaccine adjuvant. Expert Rev Vaccines 10, 499–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scheiblhofer S, Strobl A, Hoepflinger V, Thalhamer T, Steiner M, Thalhamer J, and Weiss R (2017) Skin vaccination via fractional infrared laser ablation - Optimization of laser-parameters and adjuvantation. Vaccine 35, 1802–1809 [DOI] [PubMed] [Google Scholar]
- 65.Chen X, Kositratna G, Zhou C, Manstein D, and Wu MX (2014) Micro-fractional epidermal powder delivery for improved skin vaccination. Journal of controlled release : official journal of the Controlled Release Society 192, 310–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Terhorst D, Fossum E, Baranska A, Tamoutounour S, Malosse C, Garbani M, Braun R, Lechat E, Crameri R, Bogen B, Henri S, and Malissen B (2015) Laser-assisted intradermal delivery of adjuvant-free vaccines targeting XCR1+ dendritic cells induces potent antitumoral responses. J Immunol 194, 5895–5902 [DOI] [PubMed] [Google Scholar]
- 67.Kitano M, Yamazaki C, Takumi A, Ikeno T, Hemmi H, Takahashi N, Shimizu K, Fraser SE, Hoshino K, Kaisho T, and Okada T (2016) Imaging of the cross-presenting dendritic cell subsets in the skin-draining lymph node. Proc Natl Acad Sci U S A 113, 1044–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alexandre YO, Ghilas S, Sanchez C, Le Bon A, Crozat K, and Dalod M (2016) XCR1+ dendritic cells promote memory CD8+ T cell recall upon secondary infections with Listeria monocytogenes or certain viruses. J Exp Med 213, 75–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eickhoff S, Brewitz A, Gerner MY, Klauschen F, Komander K, Hemmi H, Garbi N, Kaisho T, Germain RN, and Kastenmuller W (2015) Robust Anti-viral Immunity Requires Multiple Distinct T Cell-Dendritic Cell Interactions. Cell 162, 1322–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gerner MY, Torabi-Parizi P, and Germain RN (2015) Strategically localized dendritic cells promote rapid T cell responses to lymph-borne particulate antigens. Immunity 42, 172–185 [DOI] [PubMed] [Google Scholar]
- 71.Yamazaki C, Sugiyama M, Ohta T, Hemmi H, Hamada E, Sasaki I, Fukuda Y, Yano T, Nobuoka M, Hirashima T, Iizuka A, Sato K, Tanaka T, Hoshino K, and Kaisho T (2013) Critical roles of a dendritic cell subset expressing a chemokine receptor, XCR1. J Immunol 190, 6071–6082 [DOI] [PubMed] [Google Scholar]
- 72.Gudjonsson A, Lysen A, Balan S, Sundvold-Gjerstad V, Arnold-Schrauf C, Richter L, Baekkevold ES, Dalod M, Bogen B, and Fossum E (2017) Targeting Influenza Virus Hemagglutinin to Xcr1+ Dendritic Cells in the Absence of Receptor-Mediated Endocytosis Enhances Protective Antibody Responses. J Immunol 198, 2785–2795 [DOI] [PubMed] [Google Scholar]
- 73.Herve PL, Dhelft V, Plaquet C, Rousseaux A, Bouzereau A, Gaulme L, Tilleul S, Ligouis M, Donne N, Lambert PH, Hong-Thai P, Wijagkanalan W, Sampson HA, and Mondoulet L (2019) Epidermal micro-perforation potentiates the efficacy of epicutaneous vaccination. Journal of controlled release : official journal of the Controlled Release Society 298, 12–26 [DOI] [PubMed] [Google Scholar]
- 74.Mondoulet L, Dioszeghy V, Ligouis M, Dhelft V, Dupont C, and Benhamou PH (2010) Epicutaneous immunotherapy on intact skin using a new delivery system in a murine model of allergy. Clin Exp Allergy 40, 659–667 [DOI] [PubMed] [Google Scholar]
- 75.Gavillet BM, Mondoulet L, Dhelft V, Eberhardt CS, Auderset F, Pham HT, Petre J, Lambert PH, Benhamou PH, and Siegrist CA (2015) Needle-free and adjuvant-free epicutaneous boosting of pertussis immunity: Preclinical proof of concept. Vaccine 33, 3450–3455 [DOI] [PubMed] [Google Scholar]
- 76.Herve PL, Descamps D, Deloizy C, Dhelft V, Laubreton D, Bouguyon E, Boukadiri A, Dubuquoy C, Larcher T, Benhamou PH, Eleouet JF, Bertho N, Mondoulet L, and Riffault S (2016) Non-invasive epicutaneous vaccine against Respiratory Syncytial Virus: Preclinical proof of concept. Journal of controlled release : official journal of the Controlled Release Society 243, 146–159 [DOI] [PubMed] [Google Scholar]
- 77.Hessenberger M, Weiss R, Weinberger EE, Boehler C, Thalhamer J, and Scheiblhofer S (2013) Transcutaneous delivery of CpG-adjuvanted allergen via laser-generated micropores. Vaccine 31, 3427–3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Globinska A, Boonpiyathad T, Satitsuksanoa P, Kleuskens M, van de Veen W, Sokolowska M, and Akdis M (2018) Mechanisms of allergen-specific immunotherapy: Diverse mechanisms of immune tolerance to allergens. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology 121, 306–312 [DOI] [PubMed] [Google Scholar]
- 79.Kumar MNK, Zhou C, and Wu MX (2016) Laser-facilitated epicutaneous immunotherapy to IgE-mediated allergy. Journal of controlled release : official journal of the Controlled Release Society 235, 82–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Machado Y, Duinkerken S, Hoepflinger V, Mayr M, Korotchenko E, Kurtaj A, Pablos I, Steiner M, Stoecklinger A, Lubbers J, Schmid M, Ritter U, Scheiblhofer S, Ablinger M, Wally V, Hochmann S, Raninger AM, Strunk D, van Kooyk Y, Thalhamer J, and Weiss R (2017) Synergistic effects of dendritic cell targeting and laser-microporation on enhancing epicutaneous skin vaccination efficacy. Journal of controlled release : official journal of the Controlled Release Society 266, 87–99 [DOI] [PubMed] [Google Scholar]
- 81.Joubert IA, Kovacs D, Scheiblhofer S, Winter P, Korotchenko E, Strandt H, and Weiss R (2019) Mast cells and γδ T cells are largely dispensable for adaptive immune responses after laser-mediated epicutaneous immunization. Vaccine, in press. [DOI] [PubMed] [Google Scholar]
- 82.Wood LC, Jackson SM, Elias PM, Grunfeld C, and Feingold KR (1992) Cutaneous barrier perturbation stimulates cytokine production in the epidermis of mice. J Clin Invest 90, 482–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gutowska-Owsiak D, and Ogg GS (2012) The epidermis as an adjuvant. J Invest Dermatol 132, 940–948 [DOI] [PubMed] [Google Scholar]
- 84.Chen X, Zeng Q, and Wu MX (2012) Improved efficacy of dendritic cell-based immunotherapy by cutaneous laser illumination. Clinical cancer research : an official journal of the American Association for Cancer Research 18, 2240–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]