Abstract
RNA interference is a biological process whereby small RNAs inhibit gene expression through neutralizing targeted mRNA molecules. This process is conserved in eukaryotes. Here, recent work regarding the mechanisms of how small RNAs move within and between organisms is examined. Small RNAs can move locally and systemically in plants through plasmodesmata and phloem, respectively. In fungi, transportation of small RNAs may also be achieved by septal pores and vesicles. Recent evidence also supports bidirectional cross‐kingdom communication of small RNAs between host plants and adapted fungal pathogens to affect the outcome of infection. We discuss several mechanisms for small RNA trafficking and describe evidence for transport through naked form, combined with RNA‐binding proteins or enclosed by vesicles.
Keywords: cross kingdom, extracellular vesicles, small RNAs, transportation
We summarize the evidence and possible mechanisms that small RNAs move within and between plants and fungi in naked form, combined with RNA‐binding proteins or enclosed in vesicles.
Abbreviations
- AGO
Argonaute
- CC
companion cell
- CW
cell wall
- CYP
cytochrome P450 lanosterol C‐14 α‐demethylase
- DCL
dicer‐like
- DM
desmotubule
- dsRNA
double‐stranded RNA
- EHMx
extrahaustorial matrix
- ER
endoplasmic reticulum
- EV
extracellular vesicle
- FCW
fungal cell wall
- FIGS
filamentous organism‐induced gene silencing
- FPM
fungal plasma membrane
- G
Golgi
- GFP
green fluorescent protein
- HIGS
host‐induced gene silencing
- miRNA
microRNA
- MP
movement protein
- MVB
multiple vesicle body
- N
nucleus
- PCM
plant plasma membrane
- PCW
plant cell wall
- PD
plasmodesmata
- piRNA
PIWI‐interacting RNA
- PM
plasma membrane
- RBP
RNA‐binding proteins
- RDRP
RNA‐dependent RNA polymerase
- RNAi
RNA interference
- RNPC
ribonucleoprotein complex
- SC
source cell
- SE
sieve tube element
- siRNA
small interfering RNA
- SP
sieve tube plate or septal pore
1. INTRODUCTION
Small RNAs were first discovered in Escherichia coli in 1984 (Mizuno et al., 1984). Subsequently, they have been found in all kingdoms of life operating as noncoding RNA with diverse functions (Wassarman et al., 2001; Saito, Kakeshita, and Nakamura, 2009; Pantaleo et al., 2010; Li et al., 2016). Most small RNAs serve as regulators of gene expression (Hammond et al., 2000; McCaffrey et al., 2002; Paul et al., 2002). In eukaryotes, small RNAs induce silencing of target genes, known as RNA interference (RNAi), at both transcriptional and post‐transcriptional levels. Here, their defining features are short length (c.20–30 nucleotides) and association with proteins of the Argonaute family, with whose help they can recognize target mRNAs and lead to their reduced expression (Ghildiyal and Zamore, 2009). Based on origin, they are typically classified as small interfering RNA (siRNA), microRNA (miRNA), and PIWI (P‐element‐induced wimpy testes)‐interacting siRNA (piRNA; Ghildiyal and Zamore, 2009).
siRNA, miRNA as well as piRNA all act to control gene expression and play important roles in many fundamental biological processes in eukaryotic organisms. They have been tied to vital processes such as cell growth, tissue differentiation, heterochromatin formation, cell proliferation, and disease resistance (Blair and Olson, 2015; Yuan et al., 2015; Tassetto et al., 2017; Czech et al., 2018; Mondal et al., 2018; Almeida et al., 2019). Research over the past few decades has led to powerful insight into the structure and function of small RNAs, which has been summarized in several reviews (Eamens et al., 2008; Ghildiyal and Zamore, 2009; Peters and Meister, 2007; Pratt and MacRae, 2009; Holoch and Moazed, 2015; Quinn and Chang, 2016; Zhang, Cozen et al., 2016; Zhang et al., 2019). The purpose of this review, however, is to highlight what is known and not known about the mechanisms of how small RNAs move within and between organisms. Indeed, small RNAs can travel both short and long distances in plants, as well as in fungi. Below, we summarize their movement in plants and fungi before considering how small RNAs move between fungi and plants.
2. SHORT‐ AND LONG‐DISTANCE MOVEMENT OF SMALL RNAS IN PLANTS AND FUNGI
In plants, small RNAs are produced to coordinate plant development, maintain genome integrity, and combat adverse environmental conditions (Buchon and Vaury, 2006; Chen, 2009; Ruiz‐Ferrer and Voinnet, 2009). The mobility of small RNAs was presumed to be a prerequisite for carrying out these functions. Evidence now shows that small RNAs can move both short and long distances in plants (Sarkies and Miska, 2014). Primary siRNA can spread 10–15 cells without producing secondary siRNA (Kim, 2005), while long‐distance small RNA movement involves amplification of silencing signals through RNA‐dependent RNA polymerases (RDRPs) that are transported primarily through the phloem (Wassenegger and Krczal, 2006). Transitivity and secondary siRNA production amplify the RNAi so silencing persists even in the absence of the initiator double‐stranded RNA (dsRNA)(Baulcombe, 2004). As early as 1928, Wingard found the upper leaves of a tobacco plant whose lower leaves had been inoculated with tobacco ringspot virus and showed strong symptoms became resistant to the same virus (Wingard, 1928). We now know that the recovery from virus disease involves small RNAs derived from the virus moving from the infection site to upper leaves and conferring small RNA‐mediated resistance in the distal tissues (Ratcliff, 1997; Baulcombe, 2004).
2.1. Cell‐to‐cell (short‐range) movement in plants
The early clear evidence for mobile small RNAs was reported using Nicotiana benthamiana plants expressing the GFP transgene. Leaf infiltration with Agrobacterium also expressing GFP resulted in a ring of GFP silencing that was consistently observed spreading over 10–15 cells beyond the agroinfiltration zone without triggering small RNA amplification. When an RNA silencing suppressor was co‐infiltrated, GFP silencing was abolished (Johansen, 2002). In addition to siRNA generated by transgenes, endogenous miRNAs have also been observed to spread from cell to cell. For example, when miR390 precursor loci were transcribed in the vascular system and pith region of Arabidopsis, mature miR390 were found only in the shoot apical meristem and young leaf primordia where their precursors were not detected (Chitwood et al., 2009). Similarly, miR165/166 precursors were transcribed mainly in the endodermis of Arabidopsis root, but mature miR166 were observed in adjacent cell layers (Carlsbecker et al., 2010). These and other examples are consistent with the cell‐to‐cell movement of miRNA (Chitwood et al., 2009; Martínez et al., 2016; Wu and Zheng, 2019) (see Figure 1a).
Figure 1.
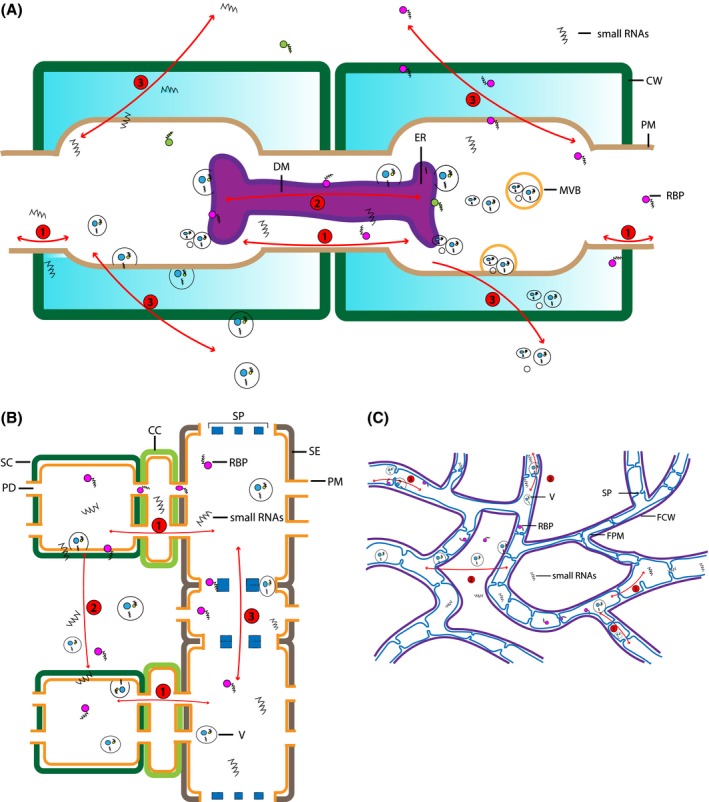
Short‐ and long‐distance transportation of small RNAs in plants and fungi. (a) Cell‐to‐cell movement in plants: 1, naked small RNAs, small RNAs bound to RNA‐binding proteins (RBP), and small RNAs enclosed in vesicles can move from cell to cell through spaces between the plant plasma membrane (PM) and desmotubule (DM); 2, small RNAs can be transported through the DM, which connects the endoplasmic reticulum (ER) of two adjacent cells; 3, small RNAs can be secreted from the PM and travel through the plant cell wall (CW) to extracellular spaces, and small RNAs can also be taken up by other cells (multiple vesicle bodies, MVB). Note: Vesicle transport through plasmodesmata by active gating is hypothetical at this time. (b) Long‐distance movement in plants: 1, naked small RNAs, small RNAs bound to RBP, and small RNAs inside vesicles can be transported from source cells (SC) to companion cells (CC) and then to sieve tube elements (SE) through plasmodesmata; 2, small RNAs can be secreted out of PM and travel through the plant cell wall (CW) to extracellular spaces and subsequently be absorbed by other cells; 3, small RNAs can be transported to distal plant cells through the sieve tube elements (sieve tube plates, SP). (c) Movement in fungi: 1, naked small RNAs, small RNAs bound to RBP, and small RNAs inside vesicles can be transported short distances cell to cell through the septal pore (SP); 2, small RNAs can be secreted out of fungal plasma membrane (FPM) and travel through the fungal cell wall (FCW) to extracellular spaces. Later, small RNAs can be absorbed by distal fungal cells and in this way small RNAs can be dispersed systemically throughout the whole fungal colony; 3, small RNAs can be transferred through the FPM. Unlike nonselective transportation through septal pores, FPM can conduct selective transportation by binding, fusion, and secretion. Note: small RNA movement in fungi needs more evidence.
2.2. Long‐range movement in plants
Long‐range systemic movement was first demonstrated by Dalmay in 2000 using a phloem‐restricted virus expressing a GFP reporter gene. The virus was applied to GFP expressing plants and GFP silencing was observed for entire leaves (Dalmay et al., 2000). Later, Pant and colleagues demonstrated the long‐range movement of miRNA through micrografting Arabidopsis plants. In grafted plants with miR393 overexpressing shoots and wild‐type roots, high levels of miR393 accumulated in the roots, suggesting the long‐range movement (shoot to root) of miR393 (Pant et al., 2008). Molnar demonstrated that both exogenous and endogenous small RNAs could pass through the graft union (Molnar et al., 2010). Other studies using grafted Nicotiana tabacum as well as Arabidopsis showed small RNAs can transfer from source tissue (leaves) to meiotically active cells such as anthers and flowers (Zhang et al., 2014; see Figure 1b).
2.3. Movement of small RNAs in fungi
In contrast to plants, fungi are simple organisms and lack defined cellular transportation systems for the movement of nutrients and metabolites. Fungi may exist as unicellular forms or as extensive multicellular hyphal branched networks. A number of fungi, including Zygomycota, are usually aseptate; in contrast, other fungal divisions like Ascomycota and Basidiomycota hyphae are separated by septa, which usually have pores. Small RNAs have also been well characterized in fungi (Drinnenberg et al., 2009; Nicolas et al., 2010; Nunes et al., 2011; Mueth et al., 2015; Campo et al., 2016; Donaire and Ayllón, 2017). In 1992, small RNAs were first demonstrated to mediate gene silencing, termed quelling, in Neurospora crassa (Romano and Macino, 1992). Subsequently, similar phenomena were reported in many fungal phyla, including Ascomycetes and Basidiomycetes, as well as in fungal‐like Oomycota (Nicolás, Torres‐Martínez, and Ruiz‐Vázquez, 2003; Latijnhouwers et al., 2004; Wang et al., 2010; Nunes et al., 2011). Studies of the direct movement of small RNAs within fungal colonies and tissues are largely absent. However, transfection of protoplasts with dsRNA can lead to targeted gene silencing that is maintained for several months across a growing colony, suggestive of both amplification and movement (Caribé dos Santos et al., 2009; Saraiva et al., 2014).
3. TRANSPORTATION PATHWAYS OF SMALL RNAS IN PLANTS AND FUNGI
Conceptually, molecules, including small RNAs, can be transported between cells and tissues within an organism via two principal routes: through direct internal connections (symplast) or externally (apoplast). In either case, evidence exists that small RNAs can be transported either in naked form or encased in vesicles (Bucher et al., 2001; Cai et al., 2018a; Kehr and Buhtz, 2008; Koch et al., 2016; Vogler et al., 2008).
3.1. Transport as either a naked form or encased in vesicles
Evidence for transport of naked forms is primarily inferred from direct application of small RNAs to cells. Both plants and fungi, including fungal‐like oomycetes, have the capacity to import naked small RNAs. Whisson and colleagues described the first application of transient gene silencing by delivering in vitro synthesized dsRNA directly into protoplasts of the oomycete Phytophthora infestans to trigger silencing (Whisson et al., 2005). Similar gene silencing results were observed using the basidiomycete Moniliophthora perniciosa, which causes witches’ broom disease on cacao. In this instance, protoplast transfection with synthesized dsRNA led to targeted gene silencing for as long as 4 months after dsRNA treatment (Caribé dos Santos et al., 2009). Production of secondary siRNA may occur to amplify the silencing effect and small RNAs may move through the whole fungal colony. In Saprolegnia parasitica, dsRNA‐mediated long‐term gene silencing has also been reported (Saraiva et al., 2014). Moreover, when artificial synthesized siRNA were co‐cultured with the model filamentous fungus Aspergillus nidulans, silencing of the reporter GFP gene as well as endogenous AnrasA & B genes was induced, supporting the possibility that this may be a natural means of small RNA transport in fungi (see Figure 1c) (Kalleda et al., 2013).
Direct application of RNA molecules to plants has been shown to down‐regulate endogenous transcript levels. Sammons et al. (2011), in a patent application, showed that direct application of various nucleic acids, including dsRNA and siRNA, down‐regulated herbicide resistance (Sammons et al., 2011). Through root soaking, dsRNA targeting Mob1A and WRKY23 was delivered into Arabidopsis and rice tissue. Suppression of root growth, seed germination, and failure of bolt or flower were detected along with silencing of the targeted genes (Li et al., 2015). Besides suppression of plant endogenous genes, a number of studies have demonstrated that direct application of dsRNAs can effectively silence transgenes such as GFP or YFP in plants (Dubrovina et al., 2019).
As an alternative to the naked form, small RNAs can also be transported through a pathway involving vesicular migration from the endoplasmic reticulum (ER) to the Golgi apparatus and then loading to a complex network of vesicles. Small RNAs can be sorted to transporting vesicles fusing with the plasma membrane and then released by exocytosis (Bonifacino and Glick, 2004). Compared to plants, knowledge of vesicular transport in fungi is extensive. Such extracellular vesicles (EVs) have been discovered in many different species of fungi, such as Cryptococcus neoformans, Histoplasma capsulatum, Candida albicans, Candida parapsilosis, Sporothrix schenckii, and Saccharomyces cerevisiae (Albuquerque et al., 2008; Rodrigues et al., 2008, 2007). In addition to proteins, neutral lipids, glycans, and pigments, fungal RNA has also been found in EVs (Rodrigues et al., 2007; Oliveira et al., 2010, 2009; Vallejo et al., 2012; Garcia‐Silva et al., 2014). Different types of noncoding small RNAs have been characterized inside EVs from C. neoformans, Paracoccidiodes brasiliensis, and C. albicans as well as from S. cerevisiae (Da Silva et al., 2015).
As each cell has two endomembrane systems, one for outgoing traffic and the other for incoming traffic (Hilbi and Haas, 2012), small RNAs can be released from the cell through EVs as well as be absorbed by the recipient cell through membrane fusion. This has been demonstrated using synthetic EVs composed of siRNA inside cationic lipid/liposomes (Spagnou et al., 2004). Moreover, the trafficking of EVs by fungal cells is regulated by both cell turgor and cell wall structure (Eisenman et al., 2005; Brown et al., 2015). Thus, the fungal cell wall may play an important role in regulating the movement of small RNAs (via EVs) between fungal cells and to plant hosts (Figure 2).
Figure 2.
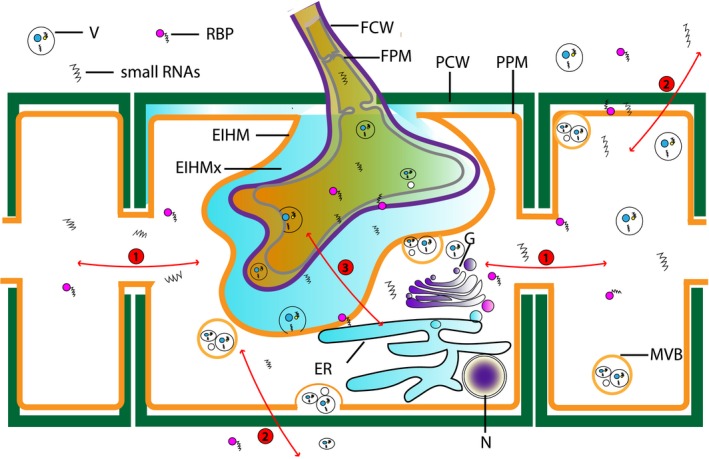
Trans‐kingdom transportation of small RNAs between plants and fungi: 1, inside plant cells, naked small RNAs, small RNAs bound with RBP, and small RNAs inside vesicles can be transported cell to cell through plasmodesmata (PM); 2, small RNAs can be secreted through the plant plasma membrane (PPM) and plant cell wall (PCW) to extracellular spaces, where they can also be taken up by other cells; 3, small RNAs can be transferred through the fungal plasma membrane (FPM)–fungal cell wall (FCW)–extra‐invasive hyphae matrix (EIHMx)‐extra‐invasive hyphae membrane (EIHM)–plant cytoplasm pathway. This transportation pathway can be bidirectional. N, nucleus; G, Golgi. Note: Vesicles transport through the plasmodesmata by active gating is a hypothesis.
3.2. Movement of small RNAs via the symplast and apoplast
In plants, for movement through the symplast, small RNAs probably move through the plasmodesmata (PD), a plasma membrane‐lined pore acting as an intercellular channel that connects the plant cytoplasm of connected cells (Figure 1a). There are several lines of evidence supporting the symplast route. Mature guard cells that are symplastically isolated from adjacent cells escape transitive GFP silencing (Voinnet et al., 1998; Vatén et al., 2011). The presence of the tobacco mosaic virus movement protein (MP) increased PD aperture size and enhanced the spread of transgene silencing (Bucher et al., 2001; Vogler et al., 2008). Several viruses transfer their RNA genome to plant cells through ER protrusions that extend through the PD (Chou et al., 2013; Pyott and Molnar, 2015).
The PD size exclusion limit is 30–50 kDa and may dictate which forms of small RNAs can move through the PD. Naked small RNAs are around 15 kDa, thus their free diffusion through the PD should not be limited (Crawford and Zambryski, 2000). As the size of plant vesicles (>10 nm) (Huang et al., 2017; Rutter and Innes, 2017) is generally larger than the diameter of PD microchannels (3–4 nm) (Ding et al., 1999; Sager and Lee, 2018), vesicles containing small RNAs may not diffuse freely through the PD. However, PD permeability can be significantly increased through dilation, active gating, and structural remodelling (Lucas and Lee, 2004). Thus, naked as well as vesicle‐enclosed small RNAs may be trafficked across the PD actively in plants.
Several lines of evidence indicate that long‐range movement of small RNAs is primarily by means of the phloem (Figure 1b). In 2008, Buhtz identified a large number of small RNAs in the phloem of oilseed rape plants but not in the xylem. Analysis of phloem sap contents revealed different types of RNAs (Kehr and Buhtz, 2008), while for xylem exudates only minerals, peptides, and proteins were found (Turnbull and Lopez‐Cobollo, 2013). In 2010, Varkonyi and co‐workers also found a subset of miRNAs present in the phloem of apple (Buhtz et al., 2008; Varkonyi‐Gasic et al., 2010). In addition, Roberts showed that treatment of plants with a nontoxic concentration of cadmium to block phloem transport of specific virus movement also inhibited systemic RNA silencing (Ghoshroy et al., 1998; Ueki and Citovsky, 2001). In vascular plants, phloem is a living tissue that conveys organic compounds made during photosynthesis from source (typically leaves) to sink tissues (such as roots and buds) (Van Bel, 2003). However, in several solanaceous species as well as Arabidopsis, upward long‐distance mobile silencing has also been shown to be phloem mediated (Liang et al., 2012).
Proteins may assist in both short‐ and long‐range transportation of naked small RNAs. In plants, RNA‐binding proteins (RBPs), which are at the core of ribonucleoprotein complexes (RNPCs), are important for RNA movement (Kedde et al., 2007). Phloem Small‐RNA Binding Protein 1 (CmPSRP1) from pumpkin (Cucurbita maxima) phloem binds single‐stranded small RNAs moving from cell to cell through the PD (Yoo et al., 2004). This protein can also shuttle small RNAs through the companion cell–sieve element complex (Ham et al., 2014). Several small RBPs have been identified in the phloem of other plant species (Barnes et al., 2004; Go, 2004; Giavalisco et al., 2006). Argonaute (AGO) proteins have also been suggested to be involved in siRNA movement (Marín‐González and Suárez‐López, 2012).
For fungal cells that are linked to each other, intercellular communication may be achieved via septal pores, similar to plasmodesmata in plant cells (Bloemendal and Kück, 2013). Septal pores were first reported by Bary in 1884 (Bary, 1884). Later, in 1893, Wahrlich observed cytoplasmic flow between different fungal compartments (Wahrlich, 1893). Septa can be described as a simple plate with a central pore about 50–500 nm in diameter that allows the passage of cytoplasm and organelles like mitochondria, vacuoles, and nuclei (Gull, 1978; Esser, 1982). Moreover, microtubules have also been found to direct the transport process in filamentous fungi and the range of cargo can be expanded to include endosomes, mRNA, peroxisomes, and secretory vesicles (Egan et al., 2012). It was further demonstrated that tubules can move cargo in either direction across the septal pores as well as transport material between cells (Shepherd et al., 1993). In sum, the septal pore, a plasmodesmata‐like structure associated with ER or the desmotubule, a membranous cell wall‐spanning structure, may enable small RNAs either in naked form or enclosed in vesicles to move throughout the whole mycelial network (Zarnack and Feldbrügge, 2007; Figure 1c).
Transport via symplastic routes is probably valuable for movement within an organism and where direct cellular connections exist. For the apoplastic pathway, small RNAs are proposed to be exported by the cell through the membrane to the apoplast and are subsequently imported into a recipient cell, potentially another organism in intimate proximity. Direct evidence demonstrating the apoplastic transportation pathway for small RNAs within plants is lacking. However, the discovery of diverse small RNA species in Arabidopsis extracellular vesicles is consistent with this pathway where small RNAs could be loaded into vesicles and secreted to the apoplast (Baldrich et al., 2019). Extracellular vesicles play critical roles in fungal growth and the ability to derive nutrients from their environment (including invading potential hosts). Recently, a number of different types of noncoding small RNAs have been characterized inside EVs from fungi as described above (Garcia‐Silva et al., 2014; Peres da Silva et al., 2015; Rayner et al., 2017).
4. CROSS‐KINGDOM TRAFFICKING OF SMALL RNAS
Insight gained into the conservation of mechanisms of RNA silencing and understanding the movement of small RNAs within different organisms opens up the distinct possibility that small RNAs could be readily shared between organisms in close association to induce gene silencing. Bidirectional small RNA movement between host and parasite was first reported in 2012 between the honeybee and Varroa destructor (Garbian et al., 2012). Today the evidence suggests that cross‐kingdom RNAi can occur between diverse living systems (Roney et al., 2006; David‐Schwartz et al., 2008; Lamonte et al., 2012; Cheng et al., 2013; Garcia‐Silva et al., 2014; Weiberg et al., 2014; Quintana et al., 2015).
4.1. Endogenous small RNA transfer between plants and fungi
The role of cross‐kingdom RNAi for defining interactions between fungal pathogens and plant hosts was pioneered by Hailing Jin's group. They showed that to promote virulence, the necrotrophic fungal pathogen Botrytis cinerea produces small RNAs during infection that hijack the host plant's RNAi machinery to silence genes of Arabidopsis and tomato involved in host immunity. B. cinerea dcl1 dcl2 double mutants that could no longer produce Bc‐sRNA exhibited reduced virulence, whereas the Arabidopsis ago1 mutant that lost RNAi function regained resistance to B. cinerea. This kind of host plant gene silencing triggered by small RNAs from the fungus has been termed filamentous organism‐induced gene silencing (FIGS; Baulcombe, 2015; Weiberg et al., 2014).
The trafficking of small RNAs is bidirectional; plants can also deliver endogenous small RNAs into invading fungal pathogens. For example, miR166 and miR159 generated in cotton have been shown to be transferred to the hyphae of the wilt pathogen Verticillium dahliae during infection, where they reduced expression of genes encoding a Ca2+‐dependent cysteine protease (Clp‐1) and an isotrichodermin C‐15 hydroxylase (HiC‐15). Deletion of those two genes in the fungus inhibited microsclerotia formation or hyphae growth, respectively, and down‐regulation of Clp‐1 and HiC‐15 through small RNAs from the host plant interfered with fungal pathogenicity (Zhang et al., 2016). A growing number of recent studies suggest that both plants and fungi use cross‐kingdom RNAi strategies for their own benefit (Table 1).
Table 1.
Summary of small RNAs movement between plants and fungi
| Plant host | Fungal life style | Fungal pathogen | Target genes | Evidence | Reference |
|---|---|---|---|---|---|
| Barley | Biotrophic | Blumeria graminis | Effector gene Avra10 | Reduced fungal development | Nowara et al. (2010) |
| Barley | Biotrophic | B. graminis | 50 Blumeria effector candidates | Eight were identified contributing to infection | Pliego et al. (2013) |
| Wheat | Biotrophic | Puccinia striiformis f. sp. tritici | Calcineurin homologs Pscna1/Pscnb1 | Slower extension of fungal hyphae and reduced production of urediospores | Zhang et al. (2012) |
| Wheat | Biotrophic | P. striiformis f. sp. tritici | MAPK kinase gene PsFUZ7 | Hyphal development strongly restricted, necrosis of plant cells in resistance responses induced | Zhu et al. (2017) |
| Wheat | Biotrophic | P. striiformis f. sp. tritici | PKA catalytic subunit gene PsCPK1 | Significant reduction in the length of infection hyphae and disease phenotype | Qi et al. (2018) |
| Wheat | Biotrophic | Puccinia triticina | MAP kinase (PtMAPK1), cyclophilin (PtCYC1), and calcineurin B (PtCNB) | Disease suppression, compromising fungal growth and sporulation | Panwar et al. (2013a) |
| Wheat | Biotrophic | P. triticina | Three predicted pathogenicity genes encoding MAPK, cyclophilin, and calcineurin regulatory subunit | Suppressed disease phenotype | Panwar et al. (2013b) |
| Wheat | Biotrophic | Puccinia graminis f. sp. tritici | Haustoria‐enriched genes | Reduced fungi development | Yin et al. (2015) |
| Lettuce | Biotrophic oomycete | Bremia lactucae | Highly abundant message #34 (HAM34), cellulose synthase (CES1) | Greatly reduced growth and inhibition of sporulation | Govindarajulu et al. (2015) |
| Potato | Biotrophic oomycete | Phytophthora infestans | Three genes important in the infection, PiGPB1, PiCESA2, and PiPEC, together with PiGAPDH taking part in basic cell maintenance | Hp‐PiGBP1 targeting the G protein β‐subunit (PiGPB1) important for pathogenicity resulted in most restricted disease progress | Jahan et al. (2015) |
| Potato | Biotrophic oomycete | P. infestans | RXLR effector Avr3a gene | Imparted partial resistance to late blight disease | Sanju et al. (2015) |
| Arabidopsis, barley | Hemibiotrophic | Fusarium graminearum | Fungal cytochrome P450 lanosterol C‐14α‐demethylase (CYP51) genes | Inhibition of fungal growth | Koch et al. (2013) |
| Banana | Hemibiotrophic | Fusarium oxysporum f. sp. cubense | Velvet, Fusarium transcription factor 1 | Resisted disease at 8 months post‐inoculation | Ghag et al. (2014) |
| Arabidopsis | Hemibiotrophic | F. oxysporum | F‐box protein required for pathogenicity 1 (FRP1), F. oxysporum Wilt 2 (FOW2), plant 12‐oxophytodienoate‐10,11‐reductase gene (OPR) | Survival rates after fungal infection were higher in the transgenic lines | Hu et al. (2015) |
| Wheat | Hemibiotrophic | F. graminearum | Chitin synthase (Chs) 3b | High levels of stable, consistent resistance to both fusarium head blight and fusarium stem blight throughout the T3 to T5 generations | Cheng et al. (2015) |
| Wheat | Hemibiotrophic | F. graminearum | β‐1,3‐glucan synthase gene FcGls1 | Aberrant, swollen fungal hyphae | Chen, Kastner et al. (2016) |
| Arabidopsis, barley | Hemibiotrophic | F. graminearum | CYP51 genes | Spray‐induced gene silencing also conferred resistance against F. graminearum in unsprayed distal leaf parts | Koch et al. (2016), Wang and Jin (2017) |
| Wheat, barley | Hemibiotrophic | F. graminearum | TRI6, a transcription factor that positively regulates deoxynivalenol synthesis | Silencing of TRI6 | Hunter et al. (2018) |
| Cotton | Hemibiotrophic | Verticillium dahliae | Two V. dahliae genes encoding a Ca2+‐dependent cysteine protease (Clp‐1) and an isotrichodermin C‐15 hydroxylase (HiC‐15) | Cotton plants increased production of microRNA 166 (mir166) and mir159 that silence Clp‐1 and hic‐15 | Zhang et al. (2016) |
| Cotton | Hemibiotrophic | V. dahliae | V. dahliae hygrophobins1 (VdH1) gene | Induced silencing of the target mRNA and conferred resistance to V. dahliae infection | Zhang et al. (2016) |
| Arabidopsis, tomato | Hemibiotrophic | V. dahliae | Three previously identified virulence genes of V. dahliae (Ave1, Sge1, and NLP1) | Reduced verticillium wilt disease in two of the three targets | Song and Thomma (2018) |
| Arabidopsis, tomato | Necrotrophic | Botrytis cinerea | B. cinerea Dicer‐like protein encoding genes: Bc‐DCL1 and Bc‐DCL2 | Silenced Bc‐DCL genes and attenuated fungal pathogenicity and growth | Weiberg et al. (2014), Wang et al. (2016) |
| Arabidopsis | Necrotrophic | B. cinerea | small RNAs‐containing vesicles accumulate at the infection sites and are taken up by the fungal cells | Transferred host sRNAs induced silencing of fungal genes critical for pathogenicity | Cai et al. (2018) |
| Tall fescue | Necrotrophic | Rhizoctonia solani | Genes encoding RNA polymerase, importin beta‐1 subunit, Cohesin complex subunit Psm1, and a ubiquitin E3 ligase | Lesion size was reduced by as much as 90% | Zhou et al. (2016) |
| Tobacco | Necrotrophic | Sclerotinia sclerotiorum | Chitin synthase (Chs) | Reduction in disease severity | Andrade et al. (2016) |
| Maize | Saprotrophic | Aspergillus species | AflC gene encodes an enzyme in the Aspergillus aflatoxin biosynthetic pathway | Aflatoxin could not be detected | Thakare et al. (2017) |
4.2. HIGS: artificial small RNAs transfer from plants to fungi
Observations that naturally occurring endogenous small RNAs move between organisms led to studies that showed that artificial transgene‐derived small RNAs are also able to move between interacting organisms. This has been exploited for the development of host‐induced gene silencing (HIGS), a novel RNA‐based technology for the efficient control of fungal pathogens and other pests (see Table 1). Conceptually, HIGS involves generating small RNAs targeting a pathogen gene in the host plant, which results in the uptake of small RNAs and gene silencing in the invading pathogen. HIGS has been demonstrated in a number of diverse fungal pathosystems and provides a promising disease control alternative to chemical control (Nowara et al., 2010; Yin et al., 2011; Zhang et al., 2012; Panwar et al., 2013; Hu et al., 2015; Deising et al., 2016; Song and Thomma, 2018; Zhang et al., 2016; Zhou et al., 2016; Zhu et al., 2017; Qi et al., 2018). In addition, it also can be used as a tool to screen potentially crucial fungal genes without the need to produce knockout mutants, which is challenging in a number of pathogens (Yin et al., 2015).
Small RNAs have been shown to transfer bidirectionally between plants and fungi; however, the mechanism(s) of how they move remains to be fully determined.
4.3. Possible pathways for small RNA cross‐kingdom movement
Based on studies of small RNA movement in plants and fungi described above, there are several pathways for cross‐kingdom small RNA transportation. Because naked small RNAs can move short and long distances in plants (Hyun et al., 2011) and can also be taken up by fungal cells (Wang, Thomas, and Jin, 2017), trafficking during plant–fungus interactions may involve naked small RNAs. For instance, when small RNAs targeting B. cinerea DCL1 and DCL2 genes were directly sprayed to Arabidopsis and tomato, treated plants gained resistance to grey mould disease, suggesting the naked exogenous small RNAs were assimilated into the pathogen and interfered with fungal virulence (Wang et al., 2016). Furthermore, spraying dsRNA of the fungal CYP3 gene on barley conferred resistance to Fusarium graminearum not only at the local sprayed area but also at distal nonsprayed areas. CYP encodes a protein required for fungal ergosterol synthesis, and silencing of this gene is lethal for fungi (Koch et al., 2016). McLoughlin and colleagues applied 59 in vitro‐synthesized dsRNAs onto the leaf surface of oilseed rape and Arabidopsis, 20 of which suppressed disease symptoms caused by Sclerotinium sclerotiorum and B. cinerea along with reduced expression levels of target genes (Mcloughlin et al., 2018). The number of examples of direct RNA molecule uptake leading to local and systemic resistance against fungal pathogens is growing (Wang et al., 2016; Song et al., 2018; Gu et al., 2019). Direct application of small RNAs has been referred to as spray‐induced gene silencing (SIGS). In most studies, however, direct uptake is limited without tissue wounding (Song et al., 2018). How these molecules are taken up and first assimilated to the plant before transfer to the pathogen remains to be determined. The process may be facilitated by RBPs in plants protecting small RNAs from degradation (McEwan et al., 2012). However, there are no similar reports regarding small RNA‐binding proteins in fungi or of receptors on the fungal cell surface to recognize small RNAs. Thus, movement via membrane‐bound vesicles may better explain small RNA communication between plants and fungi.
For both plants and fungi, vesicles containing small RNAs can be generated inside cells as well as secreted to the extracellular environment. Evidence suggests that such vesicles can also be taken up by fungi (Jiang et al., 2012; Brown et al., 2015). Recently, Arabidopsis cells have been shown to secrete extracellular vesicles to deliver plant small RNAs into the fungal pathogen B. cinerea, resulting in silencing of fungal genes critical for pathogenicity (Cai et al., 2018).
Studies of mammals such as mice also suggested that small RNAs can be transferred between different species mediated by EVs (Knip et al., 2014). Such vesicles probably enable genetic communication between phylogenetic distantly related organisms. For example, plant‐derived exosome‐like nanoparticles have been detected in the guts of mice after consuming plant material. These ingested plant‐derived exosome‐like nanoparticles contain proteins, lipids, and small RNAs (Mu et al., 2014). Direct evidence for vesicle involvement in plant–pathogen interactions has also been obtained in barley leaves under attack by powdery mildew pathogen Blumeria graminis. Light microscope‐visible vesicle‐like bodies were observed accumulating around papillae, which formed at sites where the fungal penetration was halted, suggesting such vesicles may be important for host immunity (An et al., 2006). These vesicles are known to contain antimicrobial compounds, such as phytoalexins, phenolics or reactive oxygen species (Tam et al., 2015). In addition, vesicle‐like inclusions have been shown to accumulate around penetration sites in sorghum leaves attacked by the hemibiotrophic fungus Colletotrichum graminicola (Nielsen et al., 2004). In onion, membrane‐bound electron‐dense vesicles were observed in epidermal cells in response to necrotrophic fungus Botrytis allii. Furthermore, vesicle budding and fusion of vesicle‐like structures with the fungal plasma membrane have been observed in the Arabidopsis–Golovinomyces orontii interaction. Fungal multiple vesicle bodies (MVBs) were abundant in haustoria and putative exosome vesicles were detected in the extracellular space and extrahaustorial matrix (EHMx), suggesting the existence of an exosome‐mediated secretion pathway for the interaction area between plants and fungi. These and numerous other examples support a role of vesicles in plant–fungal pathogen interactions (Chowdhury, 2016; Stewart and Mansfield, 1985). Evidence to date also suggests vesicles derived from both plants and fungi can contain small RNAs. However, further research is required to confirm whether vesicles at the fungal–host plant interface do, indeed, contain small RNAs, and that they are released into the extracellular space and are subsequently taken up by the associated partner. The movement of EVs between organisms may be highly regulated and directed rather than occur by simple diffusion (see Figure 2).
Direct evidence for the role of vesicles in cross‐kingdom communication could be obtained through the isolation of vesicles derived from HIGS transgenic plants followed by evaluation of the presence of target small RNAs inside vesicles. Such vesicles could then be co‐cultured with fungi to confirm the ability to confer RNA silencing. Using fluorescence or radioactively labelled small RNAs would facilitate monitoring of small RNA movement. Chemical inhibitors such as Brefeldin A (Nebenfuhr et al., 2002), prieurianin (Robert et al., 2008; Tõth et al., 2012), and secramine (Pelish et al., 2006) that block vesicle secretion may be valuable to confirm the function of extracellular vesicles.
The possible mechanisms of small RNA absorption remain unknown. Secretion and absorption may involve cell membrane proteins. Currently, there are limited reports regarding protein channels for small RNA movement in plants. In addition, how specific small RNAs are sorted for secretion and absorption remain enigmatic. Though much work needs to be done, studies of small RNA communication will probably provide applications for enhancing sustainable agriculture. Gene silencing of pathogen genes by HIGS or the direct application of dsRNA, for instance, is a highly promising strategy to provide resistance to plant disease.
5. CONCLUSION
The RNAi system is largely conserved among eukaryotes. It plays important biological roles, including in disease processes. Both plants and fungi can generate small RNAs and induce gene silencing. In plants small RNAs can be transported both short and long distances. For the latter, small RNAs are transferred from cell to cell through plasmodesmata and the plant phloem. Small RNAs can be transferred nakedly, bound by proteins, or packed into vesicles. In fungi small RNAs can be transferred through septa pores as well as secreted and absorbed in vesicles. EVs are present at the interface of plant–fungus interactions. However, compelling data are still needed to illuminate the underlying mechanisms by which small RNA communication occurs across plants and fungal cells. The knowledge of how small RNAs are sorted and transferred to target cells is also unclear. Answers to these questions and others related to cross‐kingdom communication will not only enrich our understanding of plant disease processes but also aid in the development of powerful new tools for disease control.
ACKNOWLEDGEMENTS
We are grateful for financial support from the USDA‐NIFA program 2013‐68004‐20378 and North Carolina State University. Special thanks are extended to members of the Dean laboratory and participants of the USDA‐NIFA project for their inspiration and support of this work.
Wang M, Dean RA. Movement of small RNAs in and between plants and fungi. Molecular Plant Pathology. 2020;21:589–601. 10.1111/mpp.12911
Funding information
We are grateful for financial support from the USDA‐NIFA program 2013‐68004‐20378 and North Carolina State University
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this study.
REFERENCES
- Albuquerque, P.C. , Nakayasu, E.S. , Rodrigues, M.L. , Frases, S. , Casadevall, A. , Zancope‐Oliveira, R.M. et al (2008) Vesicular transport in Histoplasma capsulatum: An effective mechanism for trans‐cell wall transfer of proteins and lipids in ascomycetes. Cellular Microbiology, 10, 1695–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida, M.V. , Andrade‐Navarro, M.A. and Ketting, R.F. (2019) Function and evolution of nematode RNAi pathways. Non‐Coding RNA, 5, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An, Q. , Hückelhoven, R. , Kogel, K.H. and van Bel, A.J.E. (2006) Multivesicular bodies participate in a cell wall‐associated defence response in barley leaves attacked by the pathogenic powdery mildew fungus. Cellular Microbiology, 8, 1009–1019. [DOI] [PubMed] [Google Scholar]
- Andrade, C.M. , Tinoco, M.L.P. , Rieth, A.F. , Maia, F.C.O. and Aragão, F.J.L. (2016) Host‐induced gene silencing in the necrotrophic fungal pathogen. Plant Pathology, 65, 626–632. [Google Scholar]
- Baldrich, P. , Rutter, B.D. , Zand Karimi, H. , Podicheti, R. , Meyers, B.C. and Innes, R.W. (2019) Plant extracellular vesicles contain diverse small RNA species and are enriched in 10 to 17 nucleotide “tiny” RNAs. The Plant Cell, 31, 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes, A. , Bale, J. , Constantinidou, C. , Ashton, P. , Jones, A. and Pritchard, J. (2004) Determining protein identity from sieve element sap in Ricinus communis L. by quadrupole time of flight (Q‐TOF) mass spectrometry. Journal of Experimental Botany, 55, 1473–1481. [DOI] [PubMed] [Google Scholar]
- Bary, A. (1884) Vergleichende morphologie und biologie der pilze, mycetozoen und bacterien. Leipzig: Wilhelm Engelmann. [Google Scholar]
- Baulcombe, D. (2004) RNA silencing in plants. Nature, 431, 356–363. [DOI] [PubMed] [Google Scholar]
- Baulcombe, D.C. (2015) VIGS, HIGS and FIGS: small RNA silencing in the interactions of viruses or filamentous organisms with their plant hosts. Current Opinion in Plant Biology, 26, 141–146. [DOI] [PubMed] [Google Scholar]
- Blair, C.D. and Olson, K.E. (2015) The role of RNA interference (RNAi) in arbovirus–vector interactions. Viruses, 7, 820–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemendal, S. and Kück, U. (2013) Cell‐to‐cell communication in plants, animals, and fungi: A comparative review. Naturwissenschaften, 100, 3–19. [DOI] [PubMed] [Google Scholar]
- Bonifacino, J.S. and Glick, B.S. (2004) The mechanisms of vesicle budding and fusion. Cell, 116, 153–166. [DOI] [PubMed] [Google Scholar]
- Brown, L. , Wolf, J.M. , Prados‐Rosales, R. and Casadevall, A. (2015) Through the wall: Extracellular vesicles in Gram‐positive bacteria, mycobacteria and fungi. Nature Reviews Microbiology, 13, 620–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher, G.L. , Tarina, C. , Heinlein, M. , Di Serio, F. , Meins, F. and Iglesias, V.A. (2001) Local expression of enzymatically active class I β‐1,3‐glucanase enhances symptoms of TMV infection in tobacco. The Plant Journal, 28, 361–369. [DOI] [PubMed] [Google Scholar]
- Buchon, N. and Vaury, C. (2006) RNAi: A defensive RNA‐silencing against viruses and transposable elements. Heredity (Edinb), 96, 195–202. [DOI] [PubMed] [Google Scholar]
- Buhtz, A. , Springer, F. , Chappell, L. , Baulcombe, D.C. and Kehr, J. (2008) Identification and characterization of small RNAs from the phloem of Brassica napus . The Plant Journal, 53, 739–749. [DOI] [PubMed] [Google Scholar]
- Cai, Q. , Qiao, L. , Wang, M. , He, B. , Lin, F. , Palmquist, J. et al (2018) Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science, 1129, 1126–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo, S. , Gilbert, K.B. , Carrington, J.C. and Guo, H.S. (2016) Small RNA‐based antiviral defense in the phytopathogenic fungus Colletotrichum higginsianum . PLoS Path, 12, e1005640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caribé dos Santos, A.C. , Sena, J.A.L. , Santos, S.C. , Dias, C.V. , Pirovani, C.P. , Pungartnik, C. et al (2009) dsRNA‐induced gene silencing in Moniliophthora perniciosa, the causal agent of witches’ broom disease of cacao. Fungal Genetics and Biology, 46, 825–836. [DOI] [PubMed] [Google Scholar]
- Carlsbecker, A. , Lee, J.Y. , Roberts, C.J. , Dettmer, J. , Lehesranta, S. , Zhou, J. et al (2010) Cell signalling by microRNA165/6 directs gene dose‐dependent root cell fate. Nature, 465, 316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X. (2009) Small RNAs and their roles in plant development. Annual Review of Cell and Developmental Biology, 25, 21–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, G. , Luo, R. , Hu, C. , Cao, J. and Jin, Y. (2013) Deep sequencing‐based identification of pathogen‐specific microRNAs in the plasma of rabbits infected with Schistosoma japonicum . Parasitology, 140, 1751–1761. [DOI] [PubMed] [Google Scholar]
- Cheng, W. , Song, X.S. , Li, H.P. , Cao, L.H. , Sun, K. , Qiu, X.L. et al (2015) Host‐induced gene silencing of an essential chitin synthase gene confers durable resistance to Fusarium head blight and seedling blight in wheat. Plant Biotechnology Journal, 13, 1335–1345. [DOI] [PubMed] [Google Scholar]
- Chen, W. , Kastner, C. , Nowara, D. , Oliveira‐Garcia, E. , Rutten, T. , Zhao, Y. et al (2016) Host‐induced silencing of genes protects wheat from infection. Journal of Experimental Botany, 67, 4979–4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood, D.H. , Nogueira, F.T.S. , Howell, M.D. , Montgomery, T.A. , Carrington, J.C. and Timmermans, M.C.P. (2009) Pattern formation via small RNA mobility. Genes & Development, 23, 549–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou, Y.L. , Hung, Y.J. , Tseng, Y.H. , Hsu, H.T. , Yang, J.Y. , Wung, C.H. et al (2013) The stable association of virion with the triple‐gene‐block protein 3‐based complex of bamboo mosaic virus. PLoS Pathogens, 9, 10.1371/journal.ppat.1003405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury, M.J. (2016) The role of barley cell wall polysaccharides in host plant defence mechanisms against powdery mildew. PhD thesis, Adeleide, Australia: University of Adeleide. [Google Scholar]
- Crawford, K.M. and Zambryski, P.C. (2000) Subcellular localization determines the availability of non‐target proteins to pladsmodesmatal transport. Current Biology, 10, 1032–1040. [DOI] [PubMed] [Google Scholar]
- Czech, B. , Munafò, M. , Ciabrelli, F. , Eastwood, E.L. , Fabry, M.H. , Kneuss, E. et al (2018) piRNA‐guided genome defense: from biogenesis to silencing. Annual Review of Genetics, 52, 131–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva, R.P. , Puccia, R. , Rodrigues, M.L. , Oliveira, D.L. , Joffe, L.S. , César, G.V. et al (2015) Extracellular vesicle‐mediated export of fungal RNA. Scientific Reports, 5, 10.1038/srep07763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmay, T. , Hamilton, A. , Mueller, E. and Baulcombe, D.C. (2007) Potato virus X amplicons in Arabidopsis mediate genetic and epigenetic gene silencing. The Plant Cell, 12, 369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmay, T. , Hamilton, A. , Rudd, S. , Angell, S. and Baulcombe, D.C. (2000) An RNA‐dependent RNA polymerase gene in Arabidopsis is required for posttranscriptional gene silencing mediated by a transgene but not by a virus. Cell, 101, 543–553. [DOI] [PubMed] [Google Scholar]
- David‐Schwartz, R. , Runo, S. , Townsley, B. , MacHuka, J. and Sinha, N. (2008) Long‐distance transport of mRNA via parenchyma cells and phloem across the host–parasite junction in Cuscuta . New Phytologist, 179, 1133–1141. [DOI] [PubMed] [Google Scholar]
- Ding, B. , Itaya, A. and Woo, Y.‐M. (1999) Plasmodesmata and cell‐to‐cell communication in plants. International Review of Cytology, 190, 251–316. [Google Scholar]
- Drinnenberg, I.A. , Weinberg, D.E. , Xie, K.T. , Mower, J.P. , Wolfe, K.H. , Fink, G.R. et al (2009) RNAi in budding yeast. Science, 326, 544–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaire, L. and Ayllón, M.A. (2017) Deep sequencing of mycovirus‐derived small RNAs from species. Molecular Plant Pathology, 18, 1127–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovina, A.S. , Aleynova, O.A. , Kalachev, A.V. , Suprun, A.R. , Ogneva, Z.V. and Kiselev, K.V. (2019) Induction of transgene suppression in plants via external application of synthetic dsRNA. International Journal of Molecular Sciences, 20, e1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eamens, A. , Wang, M.‐B. , Smith, N.A. and Waterhouse, P.M. (2008) RNA silencing in plants: yesterday, today, and tomorrow. Plant Physiology, 147, 456–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan, M.J. , McClintock, M.A. and Reck‐Peterson, S.L. (2012) Microtubule‐based transport in filamentous fungi. Current Opinion in Microbiology, 15, 637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman, H.C. , Nosanchuk, J.D. , Webber, J.B.W. , Emerson, R.J. , Camesano, T.A. and Casadevall, A. (2005) Microstructure of cell wall‐associated melanin in the human pathogenic fungus Cryptococcus neoformans . Biochemistry, 44, 3683–3693. [DOI] [PubMed] [Google Scholar]
- Esser, K. (1982) Cryptogams: Cyanobacteria, Algae, Fungi, Lichens. London, UK: Cambridge University Press. [Google Scholar]
- Garbian, Y. , Maori, E. , Kalev, H. , Shafir, S. and Sela, I. (2012) Bidirectional transfer of RNAi between honey bee and Varroa destructor: Varroa gene silencing reduces Varroa population. PLoS Pathogens, 8, 10.1371/journal.ppat.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Silva, M.R. , Cura Das Neves, R.F. , Cabrera‐Cabrera, F. , Sanguinetti, J. , Medeiros, L.C. , Robello, C. et al (2014) Extracellular vesicles shed by Trypanosoma cruzi are linked to small RNA pathways, life cycle regulation, and susceptibility to infection of mammalian cells. Parasitology Research, 113, 285–304. [DOI] [PubMed] [Google Scholar]
- Ghag, S.B. , Shekhawat, U.K.S. and Ganapathi, T.R. (2014) Host‐induced post‐transcriptional hairpin RNA‐mediated gene silencing of vital fungal genes confers efficient resistance against Fusarium wilt in banana. Plant Biotechnology Journal, 12, 541–553. [DOI] [PubMed] [Google Scholar]
- Ghildiyal, M. and Zamore, P.D. (2009) Small silencing RNAs: An expanding universe. Nature Reviews Genetics, 10, 94–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoshroy, S. , Freedman, K. , Lartey, R. and Citovsky, V. (1998) Inhibition of plant viral systemic infection by non‐toxic concentrations of cadmium. The Plant Journal, 13, 591–602. [DOI] [PubMed] [Google Scholar]
- Giavalisco, P. , Kapitza, K. , Kolasa, A. , Buhtz, A. and Kehr, J. (2006) Towards the proteome of Brassica napus phloem sap. Proteomics, 6, 896–909. [DOI] [PubMed] [Google Scholar]
- Go, G. (2004) A long‐distance translocatable phloem protein from cucumber forms a ribonucleoprotein complex in vivo with hop stunt viroid RNA. Journal of Virology, 78, 10104–10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajulu, M. , Epstein, L. , Wroblewski, T. and Michelmore, R.W. (2015) Host‐induced gene silencing inhibits the biotrophic pathogen causing downy mildew of lettuce. Plant Biotechnology Journal, 13, 875–883. [DOI] [PubMed] [Google Scholar]
- Gu, K. , Song, X. , Xiao, X. , Duan, X. , Wang, J. and Duan, Y. (2019) A β-2‐tubulin dsRNA derived from Fusarium asiaticum confers plant resistance to multiple phytopathogens and reduces fungicide resistance. Pesticide Biochemistry and Physiology, 153, 36–46. [DOI] [PubMed] [Google Scholar]
- Gull, K. (1978) Form and function of septa in filamentous fungi. In: Smith J.E. and Berry D.R. (Eds.), The Filamentous Fungi, vol. 3. New York: John Wiley & Sons, Inc, pp. 78–93. [Google Scholar]
- Ham, B.K. , Li, G. , Jia, W. , Leary, J.A. and Lucas, W.J. (2014) Systemic delivery of siRNA in pumpkin by a plant PHLOEM SMALL RNA‐BINDING PROTEIN 1‐ribonucleoprotein complex. The Plant Journal, 80, 683–694. [DOI] [PubMed] [Google Scholar]
- Hammond, S.M. , Bernstein, E. , Beach, D. and Hannon, G.J. (2000) An RNA‐directed nuclease mediates post‐transcriptional gene silencing in Drosophila cells. Nature, 404, 293–296. [DOI] [PubMed] [Google Scholar]
- Hilbi, H. and Haas, A. (2012) Secretive bacterial pathogens and the secretory pathway. Traffic, 13, 1187–1197. [DOI] [PubMed] [Google Scholar]
- Holoch, D. and Moazed, D. (2015) RNA‐mediated epigenetic regulation of gene expression. Nature Reviews Genetics, 16, 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, Z. , Parekh, U. , Maruta, N. , Trusov, Y. and Botella, J.R. (2015) Down‐regulation of Fusarium oxysporum endogenous genes by host‐delivered RNA interference enhances disease resistance. Frontiers in Chemistry, 3, 10.3389/fchem.2015.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C. , Quinn, D. , Sadovsky, Y. , Suresh, S. and Hsia, K.J. (2017) Formation and size distribution of self‐assembled vesicles. Proceedings of the National Academy of Sciences of the United States of America, 114, 2910–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun, T.K. , Uddin, M.N. , Rim, Y. and Kim, J.Y. (2011) Cell‐to‐cell trafficking of RNA and RNA silencing through plasmodesmata. Protoplasma, 248, 101–116. [DOI] [PubMed] [Google Scholar]
- Jahan, S.N. , Åsman, A.K.M. , Corcoran, P. , Fogelqvist, J. , Vetukuri, R.R. and Dixelius, C. (2015) Plant‐mediated gene silencing restricts growth of the potato late blight pathogen Phytophthora infestans . Journal of Experimental Botany, 66, 2785–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, M. , Sang, X. and Hong, Z. (2012) Beyond nutrients: Food‐derived microRNAs provide cross‐kingdom regulation. BioEssays, 34, 280–284. [DOI] [PubMed] [Google Scholar]
- Johansen, L.K. (2002) Silencing on the spot. Induction and suppression of RNA silencing in the Agrobacterium‐mediated transient expression system. Plant Physiology, 126, 930–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalleda, N. , Naorem, A. and Manchikatla, R.V. (2013) Targeting fungal genes by diced siRNAs: A rapid tool to decipher gene function in Aspergillus nidulans . PLoS ONE, 8, 10.1371/journal.pone.0075443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedde, M. , Strasser, M.J. , Boldajipour, B. , Vrielink, J.A.F.O. , Slanchev, K. , le Sage, C. et al (2007) RNA‐binding protein Dnd1 inhibits MicroRNA access to target mRNA. Cell, 131, 1273–1286. [DOI] [PubMed] [Google Scholar]
- Kehr, J. and Buhtz, A. (2008) Long distance transport and movement of RNA through the phloem. Journal of Experimental Botany, 59, 85–92. [DOI] [PubMed] [Google Scholar]
- Kim, J.Y. (2005) Regulation of short‐distance transport of RNA and protein. Current Opinion in Plant Biology, 8, 45–52. [DOI] [PubMed] [Google Scholar]
- Knip, M. , Constantin, M.E. and Thordal‐Christensen, H. (2014) Trans‐kingdom cross‐talk: Small RNAs on the move. PLoS Genetics, 10, 10.1371/journal.pgen.1004602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, A. , Biedenkopf, D. , Furch, A. , Weber, L. , Rossbach, O. , Abdellatef, E. et al (2016) An RNAi‐based control of Fusarium graminearum infections through spraying of long dsRNAs involves a plant passage and is controlled by the fungal silencing machinery. PLoS Pathogens, 12, 10.1371/journal.ppat.1005901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, A. , Kumar, N. , Weber, L. , Keller, H. , Imani, J. and Kogel, K.‐H. (2013) Host‐induced gene silencing of cytochrome P450 lanosterol C14 ‐demethylase‐encoding genes confers strong resistance to Fusarium species. Proceedings of the National Academy of Sciences of the United States of America, 110, 19324–19329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamonte, G. , Philip, N. , Reardon, J. , Lacsina, J.R. , Majoros, W. , Chapman, L. et al (2012) Translocation of sickle cell erythrocyte microRNAs into Plasmodium falciparum inhibits parasite translation and contributes to malaria resistance. Cell, Host & Microbe, 12, 187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latijnhouwers, M. , Ligterink, W. , Vleeshouwers, V.G.A.A. , Van West, P. and Govers, F. (2004) A Gα subunit controls zoospore motility and virulence in the potato late blight pathogen Phytophthora infestans . Molecular Microbiology, 51, 925–936. [DOI] [PubMed] [Google Scholar]
- Li, D. , Liu, Z. , Gao, L. , Wang, L. , Gao, M. , Jiao, Z. et al (2016) Genome‐wide identification and characterization of microRNAs in developing grains of Zea mays L. PLoS ONE, 11, 10.1371/journal.pone.0153168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. , Guan, R. , Guo, H. and Miao, X. (2015) New insights into an RNAi approach for plant defence against piercing‐sucking and stem‐borer insect pests. Plant, Cell & Environment, 38, 2277–2285. [DOI] [PubMed] [Google Scholar]
- Liang, D. , White, R.G. and Waterhouse, P.M. (2012) Gene silencing in Arabidopsis spreads from the root to the shoot, through a gating barrier, by template‐dependent, nonvascular, cell‐to‐cell movement. Plant Physiology, 159, 984–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas, W.J. and Lee, J.Y. (2004) Plasmodesmata as a supracellular control network in plants. Nature Reviews Molecular Cell Biology, 5, 712–726. [DOI] [PubMed] [Google Scholar]
- Marín‐González, E. and Suárez‐López, P. (2012) “And yet it moves”: Cell‐to‐cell and long‐distance signaling by plant microRNAs. Plant Science, 196, 18–30. [DOI] [PubMed] [Google Scholar]
- Martínez, G. , Panda, K. , Köhler, C. and Slotkin, R.K. (2016) Silencing in sperm cells is directed by RNA movement from the surrounding nurse cell. Nature Plants, 2, 16030. [DOI] [PubMed] [Google Scholar]
- McCaffrey, A.P. , Meuse, L. , Pham, T.‐T.‐T. , Conklin, D.S. , Hannon, G.J. and Kay, M.A. (2002) RNA interference in adult mice. Nature, 418, 38–39. [DOI] [PubMed] [Google Scholar]
- McEwan, D.L. , Weisman, A.S. and Hunter, C.P. (2012) Uptake of extracellular double‐stranded RNA by SID‐2. Molecular Cell, 47, 746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcloughlin, A.G. , Wytinck, N. , Walker, P.L. , Girard, I.J. , Rashid, K.Y. , Kievit, T.D. et al (2018) Identification and application of exogenous dsRNA confers plant protection against Sclerotinia sclerotiorum and Botrytis cinerea . Scientific Reports, 8, 7320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno, T. , Chou, M.Y. and Inouye, M. (1984) A unique mechanism regulating gene expression: Translational inhibition by a complementary RNA transcript (micRNA). Proceedings of the National Academy of Sciences of the United States of America, 81, 1966–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar, A. , Melnyk, C.W. , Bassett, A. , Hardcastle, T.J. , Dunn, R. and Baulcombe, D.C. (2010) Small silencing RNAs in plants are mobile and direct epigenetic modification in recipient cells. Science, 328, 872–875. [DOI] [PubMed] [Google Scholar]
- Mondal, M. , Klimov, P. and Flynt, A.S. (2018) Rewired RNAi‐mediated genome surveillance in house dust mites. PLoS Genetics, 14, e 10.1371/journal.pgen.1007183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu, J. , Zhuang, X. , Wang, Q. , Jiang, H. , Deng, Z.B. , Wang, B. et al (2014) Interspecies communication between plant and mouse gut host cells through edible plant derived exosome‐like nanoparticles. Molecular Nutrition & Food Research, 58, 1561–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueth, N.A. , Ramachandran, S.R. and Hulbert, S.H. (2015) Small RNAs from the wheat stripe rust fungus (Puccinia striiformis f.sp. tritici). BMC Genomics, 16, 718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebenfuhr, A. , Ritzenthaler, C. and Robinson, D.G. (2002) Brefeldin A: Deciphering an enigmatic inhibitor of secretion. Plant Physiology, 130, 1102–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas, F.E. , Moxon, S. , de Haro, J.P. , Calo, S. , Grigoriev, I.V. , Torres‐MartÍnez, S. et al (2010) Endogenous short RNAs generated by Dicer 2 and RNA‐dependent RNA polymerase 1 regulate mRNAs in the basal fungus Mucor circinelloides . Nucleic Acids Research, 38, 5535–5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolás, F.E. , Torres‐Martínez, S. and Ruiz‐Vázquez, R.M. (2003) Two classes of small antisense RNAs in fungal RNA silencing triggered by non‐integrative transgenes. EMBO Journal, 22, 3983–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, K.A. , Gotfredsen, C.H. , Buch‐Pedersen, M.J. , Ammitzbøll, H. , Mattsson, O. , Duus, J. et al (2004) Inclusions of flavonoid 3‐deoxyanthocyanidins in Sorghum bicolor self‐organize into spherical structures. Physiological and Molecular Plant Pathology, 65, 187–196. [Google Scholar]
- Nowara, D. , Gay, A. , Lacomme, C. , Shaw, J. , Ridout, C. , Douchkov, D. et al (2010) HIGS: Host‐induced gene silencing in the obligate biotrophic fungal pathogen Blumeria graminis . The Plant Cell, 22, 3130–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes, C.C. , Gowda, M. , Sailsbery, J. , Xue, M. , Chen, F. , Brown, D.E. et al (2011) Diverse and tissue‐enriched small RNAs in the plant pathogenic fungus, Magnaporthe oryzae . BMC Genomics, 12, 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira, D.L. , Nakayasu, E.S. , Joffe, L.S. , Guimarães, A.J. , Sobreira, T.J.P. , Nosanchuk, J.D. et al (2010) Characterization of yeast extracellular vesicles: Evidence for the participation of different pathways of cellular traffic in vesicle biogenesis. PLoS ONE, 5, e 10.1371/journal.pone.0011113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira, D.L. , Nimrichter, L. , Miranda, K. , Frases, S. , Faull, K.F. , Casadevall, A. et al (2009) Cryptococcus neoformans cryoultramicrotomy and vesicle fractionation reveals an intimate association between membrane lipids and glucuronoxylomannan. Fungal Genetics and Biology, 46, 956–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant, B.D. , Buhtz, A. , Kehr, J. and Scheible, W.R. (2008) MicroRNA399 is a long‐distance signal for the regulation of plant phosphate homeostasis. The Plant Journal, 53, 731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo, V. , Szittya, G. , Moxon, S. , Miozzi, L. , Moulton, V. , Dalmay, T. et al (2010) Identification of grapevine microRNAs and their targets using high‐throughput sequencing and degradome analysis. The Plant Journal, 62, 960–976. [DOI] [PubMed] [Google Scholar]
- Panwar, V. , McCallum, B. and Bakkeren, G. (2013) Endogenous silencing of Puccinia triticina pathogenicity genes through in planta‐expressed sequences leads to the suppression of rust diseases on wheat. The Plant Journal, 73, 521–532. [DOI] [PubMed] [Google Scholar]
- Paul, C.P. , Good, P.D. , Winer, I. and Engelke, D.R. (2002) Effective expression of small interfering RNA in human cells. Nature Biotechnology, 20, 505–508. [DOI] [PubMed] [Google Scholar]
- Pelish, H.E. , Peterson, J.R. , Salvarezza, S.B. , Rodriguez‐Boulan, E. , Chen, J.L. , Stamnes, M. et al (2006) Secramine inhibits Cdc42‐dependent functions in cells and Cdc42 activation in vitro . Nature Chemical Biology, 2, 39–46. [DOI] [PubMed] [Google Scholar]
- Peres da Silva, R. , Puccia, R. , Rodrigues, M.L. , Oliveira, D.L. , Joffe, L.S. , César, G.V. et al (2015) Extracellular vesicle‐mediated export of fungal RNA. Scientific Reports, 5, 7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, L. and Meister, G. (2007) Argonaute proteins: Mediators of RNA silencing. Molecular Cell, 26, 611–623. [DOI] [PubMed] [Google Scholar]
- Pliego, C. , Nowara, D. , Bonciani, G. , Gheorghe, D.M. , Xu, R. , Surana, P. et al (2013) Host‐induced gene silencing in barley powdery mildew reveals a class of ribonuclease‐like effectors. Molecular Plant‐Microbe Interactions, 26, 633–642. [DOI] [PubMed] [Google Scholar]
- Pratt, A.J. and MacRae, I.J. (2009) The RNA‐induced silencing complex: A versatile gene‐silencing machine. Journal of Biological Chemistry, 284, 17897–17901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyott, D.E. and Molnar, A. (2015) Going mobile: Non‐cell‐autonomous small RNAs shape the genetic landscape of plants. Plant Biotechnology Journal, 13, 306–318. [DOI] [PubMed] [Google Scholar]
- Qi, T. , Zhu, X. , Tan, C. , Liu, P. , Guo, J. , Kang, Z. et al (2018) Host‐induced gene silencing of an important pathogenicity factor PsCPK1 in Puccinia striiformis f. sp. tritici enhances resistance of wheat to stripe rust. Plant Biotechnology Journal, 16, 797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn, J.J. and Chang, H.Y. (2016) Unique features of long non‐coding RNA biogenesis and function. Nature Reviews Genetics, 17, 47–62. [DOI] [PubMed] [Google Scholar]
- Quintana, J.F. , Makepeace, B.L. , Babayan, S.A. , Ivens, A. , Pfarr, K.M. , Blaxter, M. et al (2015) Extracellular Onchocerca‐derived small RNAs in host nodules and blood. Parasites and Vectors, 8, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff, F. (1997) A similarity between viral defense and gene silencing in plants. Science, 276, 1558–1560. [DOI] [PubMed] [Google Scholar]
- Rayner, S. , Bruhn, S. , Vallhov, H. , Andersson, A. and Billmyre, R.B. (2017) Identification of small RNAs in extracellular vesicles from the commensal yeast Malassezia sympodialis . Scientific Reports, e 10.1038/srep39742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert, S. , Chary, S.N. , Drakakaki, G. , Li, S. , Yang, Z. , Raikhel, N.V. et al (2008) Endosidin1 defines a compartment involved in endocytosis of the brassinosteroid receptor BRI1 and the auxin transporters PIN2 and AUX1. Proceedings of the National Academy of Sciences of the United States of America, 105, 8464–8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues, M.L. , Nimrichter, L. , Oliveira, D.L. , Frases, S. , Miranda, K. , Zaragoza, O. et al (2007) Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans‐cell wall transport. Eukaryotic Cell, 6, 48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues, M.L. , Nimrichter, L. , Oliveira, D.L. , Nosanchuk, J.D. and Casadevall, A. (2008) Vesicular trans‐cell wall transport in fungi: A mechanism for the delivery of virulence‐associated macromolecules? Lipid Insights, 2, 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano, N. and Macino, G. (1992) Quelling: transient inactivation of gene expression in Neurospora crassa by transformation with homologous sequences. Molecular Microbiology, 6, 3343–3353. [DOI] [PubMed] [Google Scholar]
- Roney, J.K. , Khatibi, P.A. and Westwood, J.H. (2006) Cross‐species translocation of mRNA from host plants into the parasitic plant dodder. Plant Physiology, 143, 1037–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz‐Ferrer, V. and Voinnet, O. (2009) Roles of plant small RNAs in biotic stress responses. Annual Review of Plant Biology, 60, 485–510. [DOI] [PubMed] [Google Scholar]
- Rutter, B.D. and Innes, R.W. (2017) Extracellular vesicles isolated from the leaf apoplast carry stress‐response proteins. Plant Physiology, 173, 728–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager, R.E. and Lee, J.‐Y. (2018) Plasmodesmata at a glance. Journal of Cell Science, 131, jcs209346. [DOI] [PubMed] [Google Scholar]
- Saito, S. , Kakeshita, H. and Nakamura, K. (2009) Novel small RNA‐encoding genes in the intergenic regions of Bacillus subtilis . Gene, 428, 2–8. [DOI] [PubMed] [Google Scholar]
- Sammons, R.D. , Ivashuta, S.I. , Liu, H. , Wang, D. , Feng, P.C.C. , Kouranov, A.Y. and Andersen, S.E. (2011) Polynucleotide Molecules for Gene Regulation in Plants. Monsanto Technology Llc (USA). [Google Scholar]
- Sanju, S. , Siddappa, S. , Thakur, A. , Shukla, P.K. , Srivastava, N. , Pattanayak, D. et al (2015) Host‐mediated gene silencing of a single effector gene from the potato pathogen Phytophthora infestans imparts partial resistance to late blight disease. Functional & Integrative Genomics, 15, 697–706. [DOI] [PubMed] [Google Scholar]
- Saraiva, M. , de Bruijn, I. , Grenville‐Briggs, L. , McLaggan, D. , Willems, A. , Bulone, V. et al (2014) Functional characterization of a tyrosinase gene from the oomycete Saprolegnia parasitica by RNAi silencing. Fungal Biology, 118, 621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkies, P. and Miska, E.A. (2014) Small RNAs break out: The molecular cell biology of mobile small RNAs. Nature Reviews Molecular Cell Biology, 15, 525–535. [DOI] [PubMed] [Google Scholar]
- Shepherd, V.A. , Orlovich, D.A. and Ashford, A.E. (1993) Cell‐to‐cell transport via motile tubules in growing hyphae of a fungus. Journal of Cell Science, 105, 1173–1178. [DOI] [PubMed] [Google Scholar]
- Song, X. , Gu, K. , Duan, X. , Xiao, X. , Hou, Y. , Duan, Y. et al (2018) A myosin5 dsRNA that reduces the fungicide resistance and pathogenicity of Fusarium asiaticum . Pesticide Biochemistry and Physiology, 150, 1–9. [DOI] [PubMed] [Google Scholar]
- Song, Y. and Thomma, B.P.H.J. (2018) Host‐induced gene silencing compromises Verticillium wilt in tomato and Arabidopsis . Molecular Plant Pathology, 19, 77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnou, S. , Miller, A.D. and Keller, M. (2004) Lipidic carriers of siRNA: Differences in the formulation, cellular uptake, and delivery with plasmid DNA. Biochemistry, 43, 13348–13356. [DOI] [PubMed] [Google Scholar]
- Steward, A. and Mansfield, J.W. (1985) The composition of wall alterations and appositions (reaction material) and their role in the resistance of onion bulb scale epidermis to colonization by Botrytis allii . Plant Pathology, 34, 25–37. [Google Scholar]
- Tam, J.P. , Wang, S. , Wong, K.H. and Tan, W.L. (2015) Antimicrobial peptides from plants. Pharmaceuticals, 8, 711–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassetto, M. , Kunitomi, M. and Andino, R. (2017) Circulating immune cells mediate a systemic RNAi‐based adaptive antiviral response in Drosophila . Cell, 169, 314–325.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakare, D. , Zhang, J. , Wing, R.A. , Cotty, P.J. and Schmidt, M.A. (2017) Aflatoxin‐free transgenic maize using host‐induced gene silencing. Science Advances, 3, e1602382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tõth, R. , Gerding‐Reimers, C. , Deeks, M.J. , Menninger, S. , Gallegos, R.M. , Tonaco, I.A.N. et al (2012) Prieurianin/endosidin 1 is an actin‐stabilizing small molecule identified from a chemical genetic screen for circadian clock effectors in Arabidopsis thaliana . The Plant Journal, 71, 338–352. [DOI] [PubMed] [Google Scholar]
- Turnbull, C.G.N. and Lopez‐Cobollo, R.M. (2013) Heavy traffic in the fast lane: Long‐distance signalling by macromolecules. New Phytologist, 198, 33–51. [DOI] [PubMed] [Google Scholar]
- Ueki, S. and Citovsky, V. (2001) Inhibition of post transcriptional gene silencing by non‐toxic concentrations of cadmium. The Plant Journal, 28, 283–291. [DOI] [PubMed] [Google Scholar]
- Vallejo, M.C. , Nakayasu, E.S. , Matsuo, A.L. , Sobreira, T.J.P. , Longo, L.V.G. , Ganiko, L. et al (2012) Vesicle and vesicle‐free extracellular proteome of Paracoccidioides brasiliensis: Comparative analysis with other pathogenic fungi. Journal of Proteome Research, 11, 1676–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bel, A.J.E. (2003) The phloem, a miracle of ingenuity. Plant, Cell and Environment, 26, 125–149. [Google Scholar]
- Varkonyi‐Gasic, E. , Gould, N. , Sandanayaka, M. , Sutherland, P. and MacDiarmid, R.M. (2010) Characterisation of microRNAs from apple (Malus domestica ‘Royal Gala’) vascular tissue and phloem sap. BMC Plant Biology, 10, 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatén, A. , Dettmer, J. , Wu, S. , Stierhof, Y.D. , Miyashima, S. , Yadav, S.R. et al (2011) Callose biosynthesis regulates symplastic trafficking during root development. Developmental Cell, 21, 1144–1155. [DOI] [PubMed] [Google Scholar]
- Vogler, H. , Kwon, M.‐O. , Dang, V. , Sambade, A. , Fasler, M. , Ashby, J. et al (2008) Tobacco mosaic virus movement protein enhances the spread of RNA silencing. PLoS Pathogens, 4, e1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voinnet, O. , Vain, P. , Angell, S. and Baulcombe, D.C. (1998) Systemic spread of sequence‐specific transgene RNA degradation in plants is initiated by localized introduction of ectopic promoterless DNA. Cell, 95, 177–187. [DOI] [PubMed] [Google Scholar]
- Wahrlich, W. (1893) Zur Anatomie der Zelle bei Pilzen und Fadenalgen. Scr. Bot. Horti Univ. Imp. Petropolitanae, 4, 101–155. [Google Scholar]
- Wang, M. , Thomas, N. and Jin, H. (2017) Cross‐kingdom RNA trafficking and environmental RNAi for powerful innovative pre‐ and post‐harvest plant protection. Current Opinion in Plant Biology, 38, 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M. , Weiberg, A. , Lin, F.M. , Thomma, B.P.H.J. , Huang, H.D. and Jin, H. (2016) Bidirectional cross‐kingdom RNAi and fungal uptake of external RNAs confer plant protection. Nature Plants, 2, 10.1038/nplants.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , Hsueh, Y.P. , Li, W. , Floyd, A. , Skalsky, R. and Heitman, J. (2010) Sex‐induced silencing defends the genome of Cryptococcus neoformans via RNAi. Genes & Development, 24, 2566–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassarman, K.M. , Repoila, F. , Rosenow, C. , Storz, G. and Gottesman, S. (2001) Identification of novel small RNAs using comparative genomics and microarrays. Genes & Development, 15, 1637–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassenegger, M. and Krczal, G. (2006) Nomenclature and functions of RNA‐directed RNA polymerases. Trends in Plant Science, 11, 142–151. [DOI] [PubMed] [Google Scholar]
- Weiberg, A. , Wang, M. , Lin, F.‐M. , Zhao, H. , Zhang, Z. , Kaloshian, I. et al (2014) Fungal small RNAs suppress plant immunity by hijacking host. Science, 342, 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whisson, S.C. , Avrova, A.O. , Van West, P. and Jones, J.T. (2005) A method for double‐stranded RNA‐mediated transient gene silencing in Phytophthora infestans . Molecular Plant Pathology, 6, 153–163. [DOI] [PubMed] [Google Scholar]
- Wingard, S.A. (1928) Hosts and symptoms of ring spot, a virus disease of plants. Journal of Agricultural Research, 37, 127–153. [Google Scholar]
- Wu, W. and Zheng, B. (2019) Intercellular delivery of small RNAs in plant gametes. New Phytologist, 224, 86–90. [DOI] [PubMed] [Google Scholar]
- Yin, C. , Downey, S.I. , Klages‐Mundt, N.L. , Ramachandran, S. , Chen, X. , Szabo, L.J. et al (2015) Identification of promising host‐induced silencing targets among genes preferentially transcribed in haustoria of Puccinia . BMC Genomics, 16, 579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, C. , Jurgenson, J.E. and Hulbert, S.H. (2011) Development of a host‐induced RNAi system in the wheat stripe rust fungus Puccinia striiformis f. sp. tritici . Molecular Plant‐Microbe Interactions, 24, 554–561. [DOI] [PubMed] [Google Scholar]
- Yoo, B.‐C. , Kragler, F. , Varkonyi‐Gasic, E. , Haywood, V. , Archer‐Evans, S. , Lee, Y.M. et al (2004) A systemic small RNA signaling. The Plant Cell, 16, 1979–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, S. , Schuster, A. , Tang, C. , Yu, T. , Ortogero, N. , Bao, J. et al (2015) Sperm‐borne miRNAs and endo‐siRNAs are important for fertilization and preimplantation embryonic development. Development, 143, 635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarnack, K. and Feldbrügge, M. (2007) mRNA trafficking in fungi. Molecular Genetics and Genomics, 278, 347–359. [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Cozen, A.E. , Liu, Y. , Chen, Q. and Lowe, T.M. , (2016) Small RNA modifications: integral to function and disease. Trends in Molecular Medicine, 22, 1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Guo, J. , Voegele, R.T. , Zhang, J. , Duan, Y. , Luo, H. et al (2012) Functional characterization of calcineurin homologs PsCNA1/PsCNB1 in Puccinia striiformis f. sp. tritici using a host‐induced RNAi system. PLoS ONE, 7, e 10.1371/journal.pone.0049262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, T. , Jin, Y. , Zhao, J.H. , Gao, F. , Zhou, B.J. , Fang, Y.Y. et al (2016) Host‐induced gene silencing of the target gene in fungal cells confers effective resistance to the cotton wilt disease pathogen Verticillium dahliae . Molecular Plant, 9, 939–942. [DOI] [PubMed] [Google Scholar]
- Zhang, W. , Kollwig, G. , Stecyk, E. , Apelt, F. , Dirks, R. and Kragler, F. (2014) Graft‐transmissible movement of inverted‐repeat‐induced siRNA signals into flowers. The Plant Journal, 80, 106–121. [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Lai, T. , Zhang, P. , Zhang, X. , Yuan, C. , Jin, Z. et al (2019) Revisiting mobile RNA silencing in plants. Plant Science, 278, 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, T. , Zhao, Y.L. , Zhao, J.H. , Wang, S. , Jin, Y. , Chen, Z.Q. et al (2016) Cotton plants export microRNAs to inhibit virulence gene expression in a fungal pathogen. Nature Plants, 2, 10.1038/nplants.2016.153. [DOI] [PubMed] [Google Scholar]
- Zhou, B. , Bailey, A. , Niblett, C.L. and Qu, R. (2016) Control of brown patch (Rhizoctonia solani) in tall fescue (Festuca arundinacea Schreb.) by host induced gene silencing. Plant Cell Reports, 35, 791–802. [DOI] [PubMed] [Google Scholar]
- Zhu, X. , Qi, T. , Yang, Q. , He, F. , Tan, C. , Ma, W. et al (2017) Host‐induced gene silencing of the MAPKK gene confers stable resistance to wheat stripe rust. Plant Physiology, 175, 1853–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.


