Abstract
Studies demonstrated that pneumonia can decrease vitamin A productions and vitamin A reduction/deficiency may promote asthma development. Our previous study showed that neonatal Streptococcus pneumoniae (S. pneumoniae) infection promoted asthma development. Whether neonatal S. pneumoniae pneumonia induced asthma was associated with vitamin A levels remains unclear. The aim of this study was to investigate the effects of neonatal S. pneumoniae pneumonia on vitamin A expressions, to explore the effects of vitamin A supplement after neonatal S. pneumoniae pneumonia on adulthood asthma development. Non-lethal S. pneumoniae pneumonia was established by intranasal inoculation of neonatal (1-week-old) female BALB/c mice with D39. S. pneumoniae pneumonia mice were supplemented with or without all-trans retinoic acid 24 hours after infection. Vitamin A concentrations in lung, serum and liver were measured post pneumonia until early adulthood. Four weeks after pneumonia, mice were sensitized and challenged with OVA to induce allergic airway disease (AAD). Twenty-four hours after the final challenge, the lungs and bronchoalveolar lavage fluid (BALF) were collected to assess AAD. We stated that serum vitamin A levels in neonatal S. pneumoniae pneumonia mice were lower than 0.7µmol/L from day 2–7 post infection, while pulmonary vitamin A productions were significantly lower than those in the control mice from day 7–28 post infection. Vitamin A supplement after neonatal S. pneumoniae pneumonia significantly promoted Foxp3+Treg and Th1 productions, decreased Th2 and Th17 cells expressions, alleviated airway hyperresponsiveness (AHR) and inflammatory cells infiltration during AAD. Our data suggest that neonatal S. pneumoniae pneumonia induce serum vitamin A deficiency and long-time lung vitamin A reduction, vitamin A supplement after neonatal S. pneumoniae pneumonia inhibit the progression of asthma by altering CD4+T cell subsets.
Subject terms: Asthma, Asthma
Introduction
Asthma is a heterogeneous disease characterized by airway chronic inflammation together with airway hyperresponsiveness1. Asthma is more common in childhood, and most adult asthma originate from childhood, which suggesting childhood events play an important role in the pathogenesis of asthma2–4. Childhood is a pivotal period for the immune system maturation. Specific pathogen may play a critical role in the allergic airway diseases (AAD) pathogenesis by affecting the immune system5. Epidemiological studies show that neonates colonized with S. pneumoniae, Haemophilus influenzae or Moraxella catarrhalis significantly increases the risk of asthma in the first 5 years of life6,7. S. pneumoniae is the most common bacterial pathogen of community acquired pneumonia in childhood. Our previous study suggested that neonatal S. pneumoniae infection promoted OVA-induced asthma development8. The prevention and treatment of asthma induced by S. pneumoniae pneumonia is crucial, while it remains indistinctly.
Pneumonia continues to be a serious health issue worldwide, affecting millions annually, increasing morbidity and mortality globally9–12. Pneumonia can decrease vitamin A productions in children under five years old13. Vitamin A reduction/deficiency (serum vitamin A level below 0.7 µmol/L14,15) may be associated with asthma development16,17. We have stated that the course and severity of infant wheezing was inverse correlation with vitamin A concentrations18. Whether neonatal S. pneumoniae pneumonia promoted adulthood allergic asthma was associated with vitamin A levels remains unclear. In this study, we established a neonatal non-lethal S. pneumoniae pneumonia mice model and monitored vitamin A levels in lung, serum and liver until early adulthood. We also explored the effects of vitamin A supplement after neonatal S. pneumoniae pneumonia on the development of adulthood allergic asthma. Our data indicated that neonatal S. pneumoniae pneumonia induce serum vitamin A deficiency and long-time lung vitamin A reduction, vitamin A supplement after neonatal S. pneumoniae pneumonia inhibit the progression of asthma by altering CD4+T cell subsets.
Results
Neonatal S. pneumoniae pneumonia significantly decreased lung vitamin A expressions in mice model
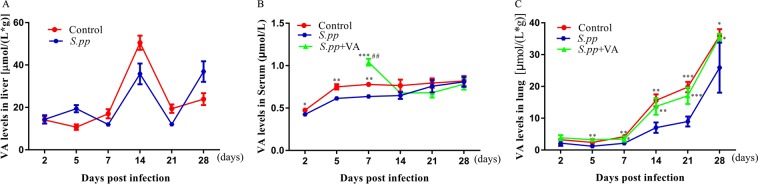
To investigate the effects of neonatal S. pneumoniae pneumonia (S.pp) on vitamin A expressions, vitamin A levels in lung, serum and liver were measured after pneumonia. We showed that vitamin A productions in liver were similar between neonatal S. pneumoniae pneumonia and mock-infection control groups (Fig. 1A). In contrast, neonatal S. pneumoniae pneumonia inhibited serum vitamin A increasing as compared with the control mice. Serum vitamin A levels in neonatal S. pneumoniae pneumonia mice were lower than 0.7 µmol/L from day 2–7 post infection (Fig. 1B). Furthermore, neonatal S. pneumoniae pneumonia mice had significantly lower lung vitamin A expressions than those in the control mice from day 7–28 post infection (Fig. 1C). These results indicated that neonatal S. pneumoniae pneumonia inhibited serum and lung vitamin A increasing, which inducing serum vitamin A deficiency and long-time lung vitamin A reduction in mice model.
Figure 1.
Vitamin A status in liver (A), serum (B) and lung (C) in BALB/C mice. *P < 0.05, **P < 0.01, ***P < 0.001 as compared with the infected (S.pp) group, n = 7 mice/group, #P < 0.05, ##P < 0.01 as compared with control group, (n = 5–7 mice/group).
Vitamin A supplement after neonatal S. pneumoniae pneumonia suppressed inflammatory cells infiltrate during AAD
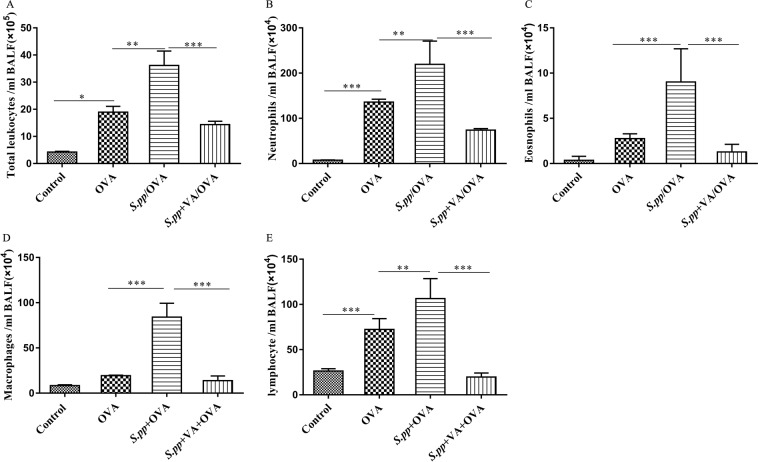
To investigate the effects of vitamin A levels after neonatal pneumonia on asthma development, mice were supplemented with or without all-trans retinoic acid after neonatal S. pneumoniae pneumonia and induced allergic airway disease (AAD) at early adulthood. Before inducing AAD, we first monitored vitamin A concentrations both in serum and in lung after vitamin A supplementation in neonatal S. pneumonia infection mice. Our results indicated vitamin A supplementation after neonatal S. pneumonia infection didn’t result in hypervitaminosis A in this study (Fig. 1B,C). As expected, the total inflammatory cells, neutrophils, eosinophils, macrophages and lymphocyte in the infected allergic (S.pp/OVA) group were significantly increased as compared with the mock-infected allergic (OVA) control mice. In contrast, the number of total inflammatory cells, neutrophils, eosinophils, macrophages and lymphocyte was dramatically reduced in the infected and vitamin A supplement allergic (S.pp+VA/OVA) group when compared to the infected allergic (S.pp/OVA) control mice (Fig. 2A–E). These results demonstrated that vitamin A supplement after neonatal S. pneumoniae pneumonia significantly alleviated inflammatory cells infiltration during AAD.
Figure 2.
Vitamin A supplement after neonatal S. pneumoniae pneumonia significantly reduced inflammatory cells infiltration during AAD. Total cells (A), neutrophils (B), eosinophils (C), macrophages (D) and lymphocyte (E) were counted from bronchoalveolar lavage fluid (BALF) collected 24 h after the final challenge. Control (mock-infected, non-allergic); OVA (mock-infected, allergic); S.pp/OVA (neonatal infected, allergic); S.pp+VA/OVA (vitamin A supplementary after neonatal infection, allergic). Data are shown as mean ± standard error from three separate experiments (n = 6–8 mice/group). *P < 0.05, **P < 0.01, ***P < 0.001.
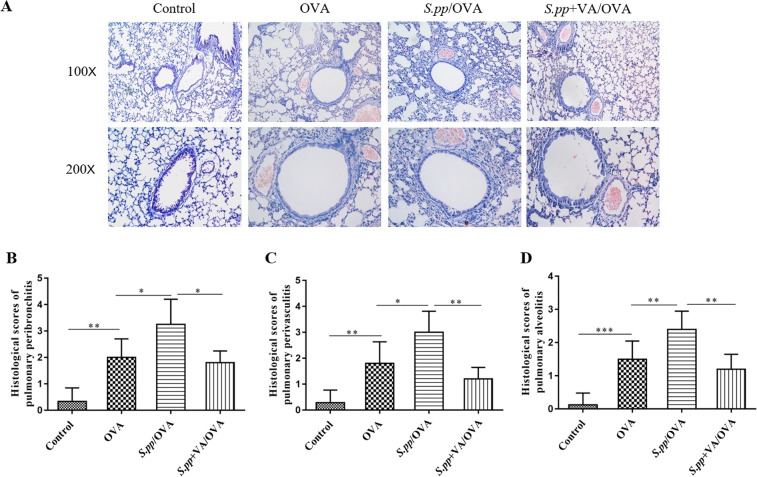
Next, we explored the effects of vitamin A supplement after neonatal S. pneumoniae pneumonia on pulmonary histopathology during AAD. Our results demonstrated that neonatal S. pneumoniae pneumonia remarkably increased the accumulation of inflammatory cells around pulmonary alveoli, bronchioles and pulmonary vascular during AAD as compared with the mock-infected control mice. Furthermore, there were fewer inflammatory cells around pulmonary alveoli, bronchioles and pulmonary vascular in S.pp+VA/OVA group as compared with S.pp/OVA control mice (Fig. 3A). The inflammation scores of pulmonary peribronchitis, perivasculitis and alveolitis in the S.pp/OVA group were significantly higher than those in the OVA group. In contrast, the inflammation scores in S.pp+VA/OVA group were remarkably lower than those in the S.pp/OVA mice (P < 0.05) (Fig. 3B–D). Taken together, these results demonstrated that vitamin A supplement after neonatal S. pneumoniae pneumonia remarkably suppressed inflammatory cells infiltration during AAD.
Figure 3.
Vitamin A supplement after neonatal S. pneumoniae pneumonia significantly reduced lung inflammation during AAD. (A) Hematoxylin and eosin (H&E) staining of lung tissue sections from mock-infected, non-allergic (Control), mock-infected, allergic (OVA), neonatal infected, allergic (S.pp/OVA) and vitamin A supplementary after neonatal infection, allergic (S.pp +VA/OVA) mice. Magnification:100X, ×200X. Histological scores of pulmonary peribronchitis (B), pulmonary perivasculitis (C) and pulmonary alveolitis (D). Data are shown as mean ± standard error from three separate experiments (n = 6–8 mice/group). *P < 0.05, **P < 0.01, ***P < 0.001.
Vitamin A supplement after neonatal S. pneumoniae pneumonia decreased AHR during AAD
Twenty-four hours after the final challenge, AHR was assessed by calculating the Penh values (i.e, enhanced respiratory pausing). The Penh value in S.pp+OVA group was significantly higher than that in the OVA group at methacholine concentrations of 12.5 mg/ml (4.58 ± 1.77 vs 2.08 ± 0.59, P < 0.001), 25 mg/ml (4.76 ± 1.43 vs 2.38 ± 0.55, P < 0.001) and 50.0 mg/ml (6.36 ± 1.53 vs 2.74 ± 0.61, P < 0.001). Interestingly, the Penh value in S.pp+VA/OVA group was remarkably lower than that in S.pp/OVA group at methacholine concentrations of 12.5 mg/ml (2.63 ± 0.94 vs 4.58 ± 1.77, P < 0.001), 25 mg/ml (3.06 ± 0.94 vs 4.76 ± 1.43, P < 0.001) and 50.0 mg/ml (2.90 ± 1.01 vs 6.36 ± 1.53, P < 0.001). Our results showed that vitamin A supplement after neonatal S. pp significantly decreased AHR during AAD (Fig. 4).
Figure 4.
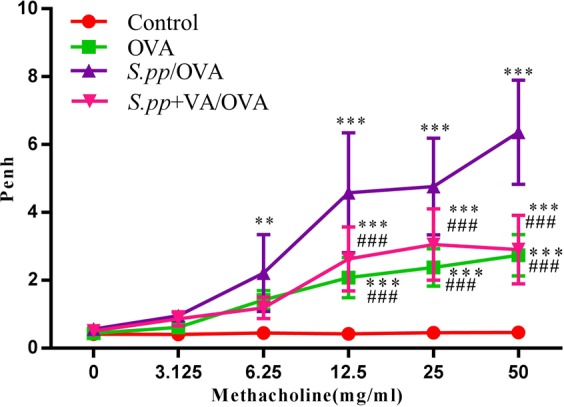
Vitamin A supplement after neonatal S. pneumoniae pneumonia alleviated AHR during AAD. Whole-body plethysmography in mock-infected, non-allergic (Control), mock-infected, allergic (OVA), neonatal infected, allergic (S.pp/OVA) and vitamin A supplement after neonatal infected, allergic (S.pp+VA/OVA) mice was conducted 24 h following challenge with methacholine (n = 6–8 mice/group). **P < 0.01, ***P < 0.001 as compared with the control group, ###P < 0.001 as compared with S.pp/OVA group.
Vitamin A supplement after neonatal S. pneumoniae pneumonia affected cytokines productions during AAD
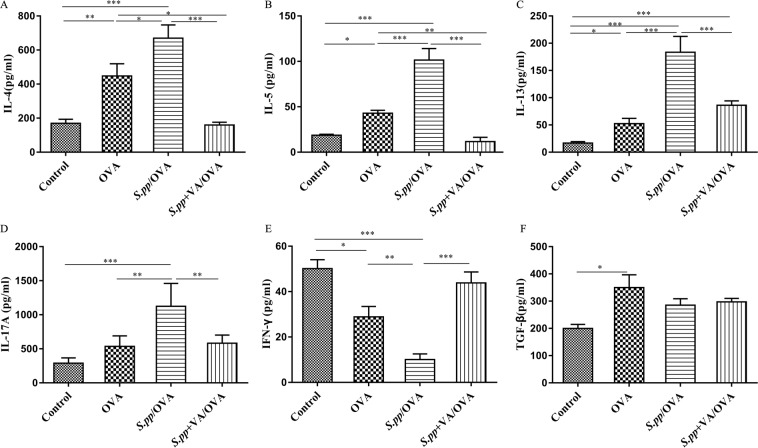
Twenty-four hours after the final challenge, BALF was obtained to detect the cytokines by ELISA. The productions of IL-4, IL-5, IL-13, IL-17A were significantly higher (P < 0.05), while IFN-γ was remarkably lower in the S.pp/OVA group compared with the OVA group (P < 0.01). In contrast, IL-4, IL-5, IL-13, IL-17A productions were significantly decreased accompanied by IFN-γ production increased dramatically in the S.pp+VA/OVA group when compared to the S.pp/OVA mice. There had no significant difference of TGF-β production among OVA, S.pp/OVA and S.pp+VA/OVA groups (Fig. 5).
Figure 5.
Concentrations of interleukin (IL)-4, IL-5, IL-13, IL-17A,interferon (IFN)-γ, and transforming growth factor (TGF)-β in the BALF of mock-infected, non-allergic (Control), mock-infected, allergic (OVA), neonatal infected, allergic (S.pp/OVA) and vitamin A supplement after neonatal infected, allergic (S.pp+VA/OVA) mice were measured by ELISA. Data are reported as mean ± standard error from three separate experiments (n = 6–8 mice/group). *P < 0.05, **P < 0.01, ***P < 0.001 as compared with the control group, #P < 0.05, ##P < 0.01, ###P < 0.001 as compared with S.pp/OVA group.
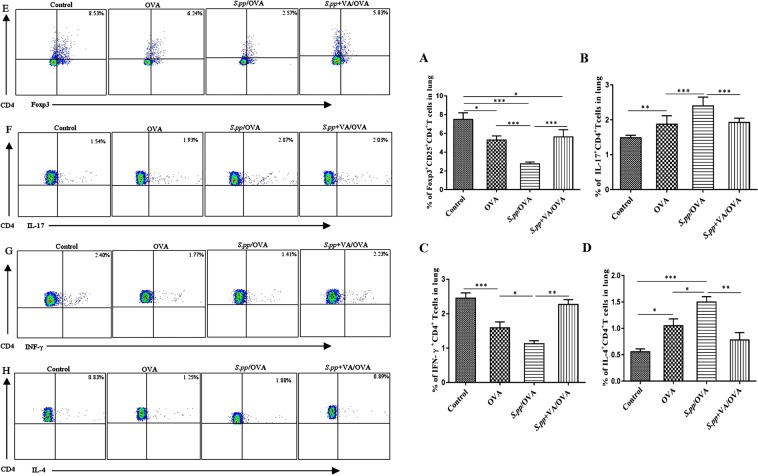
Vitamin A supplement after neonatal S. pneumoniae pneumonia altered the productions of CD4+T cells during AAD
To determine the effects of vitamin A supplement after neonatal S. pneumoniae pneumonia on the differentiation of lung CD4+T cells differentiation during AAD, flow cytometry was used to analyze the population of Foxp3+Treg, Th17, Th1and Th2 cells 24 h after the final challenge. Our data revealed that Foxp3+Treg and Th1 cells decreased significantly in S.pp/OVA group as compared with the OVA group (2.75 ± 0.72% vs 5.30 ± 1.29%, P < 0.001, 1.13 ± 0.33% vs 1.59 ± 0.39%, P < 0.05), accompanied with Th17 and Th2 cells increased remarkably(2.40 ± 0.25% vs 1.88 ± 0.24%, P < 0.001, 1.50 ± 0.17% vs 1.05 ± 0.31%, P < 0.05). Vitamin A supplement after neonatal S. pneumoniae pneumonia remarkably increased Foxp3+Treg, Th1 cells production when compared to the S.pp/OVA group mice (5.64 ± 2.11% vs 2.75 ± 0.72%, P < 0.001, 2.27 ± 0.36% vs 1.13 ± 0.33%, P < 0.01), while the number of Th17 and Th2 cells significantly decreased (1.93 ± 0.12% vs 2.40 ± 0.25%, P < 0.001, 0.78 ± 0.31% vs 1.50 ± 0.17%, P < 0.01). Thus, vitamin A supplement after neonatal S. pneumoniae pneumonia altered the productions of lung CD4+T cells during AAD (Fig. 6A–D).
Figure 6.
Vitamin A supplement after neonatal S. pneumoniae pneumonia altered CD4+T cells productions during AAD. Foxp3+Treg (A), Th17 (B), Th1 (C) and Th2 (D) cells productions were measured in mock-infected, non-allergic (Control), mock-infected, allergic (OVA), neonatal infected, allergic (S.pp/OVA) and vitamin A supplement after neonatal infected, allergic (S.pp+VA/OVA) mice. The data (E-H), respectively, represent, percentages of positively stained cells of Foxp3+Treg, Th1, Th2 and Th17 within the lymphocyte gate of lung in BALB/c mice. (n = 6–8 mice/group). *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
Asthma is one of the most common chronic diseases in children19. Epidemiological studies have demonstrated the association between early-life infections and subsequent asthma development20,21. It is now well accepted that asthma is a heterogeneous disease with different clinical subtypes. Viral infections have been implicated in asthma pathogenesis as well as exacerbation22–25. Atypical pathogen infection (such as Mycoplasma pneumoniae) also appears to play an important role in the induction and exacerbation of asthma both in children and adults26,27. Recent studies suggest some bacterial infection have important role in asthma pathogenesis28,29. Clinical study stated acute episodes of wheezing in some children are closely associated with bacterial infection26 and S. pneumoniae infection may increase the risk of asthma exacerbation30. Our previous study indicated that neonatal S. pneumoniae infection promoted early adulthood allergic asthma development8. As clinical study indicated pneumonia decrease vitamin A levels significantly in children under five years old13. In this study, we monitored vitamin A levels after neonatal S. pneumoniae pneumonia and investigated the effect of vitamin A supplement post-infection on the development of allergic asthma. Our findings demonstrated that neonatal S. pneumoniae pneumonia induced serum vitamin A deficiency and long-time lung vitamin A reduction in mice model, vitamin A supplement after neonatal S. pneumoniae pneumonia inhibited airway neutrophils and eosinophils recruitment, alleviated airway inflammation and decreased AHR during AAD. Vitamin A supplement not only promoted Foxp3+Treg and Th1 cells, but also inhibited Th2 and Th17 cells productions, accompanied by IFN-γ productions increased, type II cytokines and IL-17A expressions decreased during AAD. These results indicated that vitamin A supplement after neonatal S. pneumoniae pneumonia inhibit the progression of experimental asthma by altering CD4+T cell subsets.
Studies indicated S. pneumoniae or other pathogens infection in infants and young children decreased retinol productions31–33. Here, we showed neonatal S. pneumoniae pneumonia induced serum vitamin A deficiency and long-time lung vitamin A reduction. In contrast, Katherine et al.9 stated that S. pneumoniae infection hardly affected serum and lung vitamin A levels in adult vitamin A adequate BALB/C mice, which indicating that S. pneumoniae infection at different periods of life may induce different effects on vitamin A expressions. Possible explanations for long-time lung vitamin A reduction after neonatal S. pneumoniae pneumonia include: (1) insufficient vitamin A storage in neonates; (2) increased vitamin A consumption: fast growth in neonates increases the need of vitamin A, repairing the damaged epithelial may increase vitamin A consumption13; (3) vitamin A decreasing may reduce retinol binding protein (RBP) production, leading to decreased mobilization of vitamin A from liver34; (4) ordinary food supply after neonatal pneumonia is insufficient to restore vitamin A concentrations35.
Epidemiological studies demonstrate vitamin A deficiency is common in asthmatic patients18,36–38. Growing evidence indicate that vitamin A directing immune cell differentiation and inducing allergic disease. Intestinal studies in vivo and vitro showed that sufficient retinoic acid (a kind of metabolites of vitamin A) can promote intestinal regulatory T cells productions39,40. Akiko et al.41 stated the differentiation of Foxp3+Treg from naïve CD4+T cell is decreased in vitamin A deficient mice. Animal studies suggest that sufficient vitamin A can suppress Th2 reaction and promote Foxp3+Treg and Th1 cells productions42–44. Consistent with these studies, our data demonstrated that neonatal S. pneumoniae pneumonia reduced Foxp3+Treg and Th1 productions, increased Th2 and Th17 cells expressions during AAD, which aggravated allergic inflammation and AHR. Vitamin A supplement after neonatal S. pneumoniae pneumonia inhibits the progression of experimental asthma by altering CD4+T cell subsets productions.
Studies have reported negative correlation between vitamin A levels and the risk of asthma development17,45,46. Sufficient vitamin A inhibits asthma or allergic disease by downregulating oxidative stress47, or via direct effects on the immune system44,48–50. One study demonstrated dexamethasone combined with vitamin A therapy promoted allergic asthmatic epithelium repair by down-regulating leucine zipper (GILZ) expression and activating MAPK-ERK signaling51. However, some studies suggested infants supplemented vitamin A or multivitamin increased the risk of allergic disease52,53. In addition, a study in Norwegian adults showed that daily intake of cod liver oil (rich in vitamin A) for ≥1 month significantly increased the incidence of adult-onset asthma54. One possible explanation for these inconsistencies is vitamin A supplement in those without vitamin A reduction/deficiency may lead to hypervitaminosis A55. Hypervitaminosis A has been reported to be associated with airway hyperresponsiveness and increase the risk of asthma in mice model56. So it is important to determine vitamin A levels before vitamin A supplementation both in basic and clinical studies.
Our study indicated that neonatal S. pneumoniae pneumonia resulted in serum vitamin A deficiency and long-time lung vitamin A reduction. Vitamin A supplementation after neonatal S. pneumoniae pneumonia promoted Foxp3+Treg and Th1 productions, reduced Th2 and Th17 cells expressions when exposed to the allergen, which decreasing AHR and inflammatory cells infiltration, and eventually inhibit the progression of experimental asthma in adulthood. Our finding may provide a novel strategy for the prevention of allergic asthma induced by S. pneumoniae pneumonia. While further researches are needed to explore the mechanisms of neonatal S. pneumoniae pneumonia induced serum vitamin A deficiency and long-time vitamin A reduction. More studies are needed to clarify whether our results can be extrapolated to other pathogens and other animals.
Materials and Methods
Animals
Parturient BALB/C mice were purchased from Animal Resources Centre, Chongqing medical university. Pregnant mice were kept separately and monitored for births. Newborn female mice were raised in a pathogen-free environment, and housed at 24 °C under a 12 h light, 12 h dark cycle, and given a normal diet and water. All experiments performed in mice were permitted by the Institutional Animal Care and Research Advisory Committee at the Chongqing Medical University. All experimental animals were used in accordance with the guidelines issued by the Chinese Council on Animal Care.
Establishment of a neonatal non-lethal S. pneumoniae pneumonia mice model
Neonatal S. pneumoniae pneumonia (S.pp) was established based on the procedures described in our previous study8. Briefly, Streptococcus pneumoniae (D39) training onto trypicsoy broth (Pangtong, China), at 37 °C and 5% CO2 environment to cultivate 10–14 hours, then washed, and suspended in sterile phosphate buffered saline (PBS). Conscious neonatal (1-week-old) BALB/c mice were infected intranasally with 2 × 106 CFU of S.pneumonia in 5ul of PBS. Mock-infected mice were injected intranasally with 5ul of PBS.
Determination of vitamin A concentrations in tissues
Lung, serum and liver were collected from uninfected controls and neonatal S. pneumoniae pneumonia mice on 2, 5, 7, 14, 21, 28 days post infection. After grinding, the liver and lung were extracted in ethane. Thereafter the extracted samples and the untreated serum were degassed and redissolved. Total retinol concentration in lung, liver and serum was determined by high performance liquid chromatograph (HPLC, Model G1315 A, Agilent Technologies, Palo Alto) using trimethylmethoxyphenyl-retinol as an internal standard.
Establishment of a vitamin A supplement model
Retinyl palmitate (Sigma) with all-trans retinoic acid (Sigma) in the ratio of 10:1 was dissolved in rapeseed oil to config to the vitamin A used for subsequent experiments57. 24 hours after infection, mice were administrated orally with a dose of 20IU/g of vitamin A once daily for four consecutive days58 to establish the vitamin A supplement model.
Induction of Allergic airway disease (AAD)
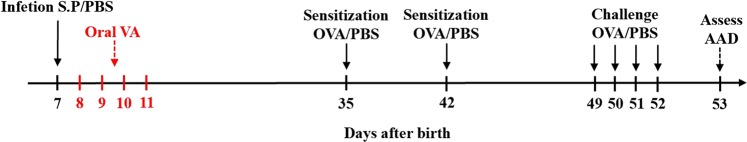
On the basis of the procedures described in our previous study8, four weeks after neonatal S. pneumoniae pneumonia (mice have grown-up into early adulthood), mice were divided into the following four groups: mock-infected non-allergic (control), mock-infected allergic (OVA), infected allergic (S.pp/OVA), infected and vitamin A supplement allergic (S.pp+VA/OVA). In order to induce AAD, mice in the OVA, S.pp /OVA, S.pp+VA/OVA groups were sensitized with intraperitoneal injections of 100 μg OVA (Sigma-Aldrich, St. Louis, MO, USA) diluted in 50% aluminum hydroxide gel (Sigma-Aldrich) for a total volume of 200 μL on days 35 and 42. Mice were exposed to 1% OVA aerosols for 30 min/d, from days 49–52. The controls were sensitized and challenged with same volume of sterile PBS at the same time. Within 24 h after the final challenge AAD was assessed (Fig. 7). Each experiment was repeated three times with a sample size of a total of four to eight mice per group.
Figure 7.
Establishment of models and schematic of study protocol. Neonatal S. pneumoniae pneumonia BALB/c mice were divided into the following groups: mock-infected, non-allergic (Control), mock-infected, allergic (OVA), neonatal infected, allergic(S.pp/OVA) and vitamin A supplement after neonatal infected, allergic (S.pp+VA/OVA). Mice were infected intranasally with S. pneumoniae or phosphate-buffered saline (PBS) on day 7 (1 week-old), and supplemented orally with vitamin A on days 8–11. Mice were sensitized by an intraperitoneal (i.p) injection of ovalbumin (OVA) or PBS on days 35 and 42, and challenged with aerosolized OVA or PBS to induce allergic airways disease (AAD) from 49 to 52 days.
Measurement of airway hyperresponsiveness (AHR)
AHR was assessed in vivo by measuring the changes in transpulmonary resistance using a mouse plethysmograph and methods previously described8,59–61. Briefly, 24 hours after the final challenge, AHR was measured in conscious, unrestrained mice by whole-body plethysmography (Emca instrument; Allmedicus, France). Each mouse was exposed to aerosolized PBS followed by increasing concentrations of aerosolized methacholine (Sigma-Aldrich, St. Louis, Mo. USA) solution (3.125, 6.25, 12.5, 25, and 50 mg/ml; Sigma) in PBS for 3 min and then rested for 2 min. The average Penh for each concentration was calculated from the continuously recorded pressure and flow data for 5 min. Penh is a dimensionless value and correlates with pulmonary airflow resistance. It represents a function of the ratio of peak expiratory flow to peak inspiratory flow and a function of the timing of expiration.
Bronchoalveolar lavage fluid and cell counting
Mice were anesthetized with 10% chloral hydrate (0.1 mL/100 g, i.p), twenty-four hours after the final challenge. Bronchoalveolar lavage fluid (BALF) was obtained through a cannulated trachea by flushing the lungs twice with 1 ml each of PBS. The two aliquots were then pooled to obtain one sample for each mouse. Erythrocytes were lysed, and the remaining cells were centrifuged at 3000 rpm for 5 min. Total cell numbers in the BALF were determined using a standard hemocytometer. Differential cell counts were classified according to the standard morphology and staining characteristics of at least 250 cells per sample. Supernatants were stored at −80 °C.
Histo-pathology of lungs
Twenty-four hours after the final challenge, to harvest the lungs, mice were euthanized by an intraperitoneal injection of a lethal dose of 10% chloral hydrate (0.3 mL/100 g, i.p). After fixing in formaldehyde for 24 hours, lungs were dissected and paraffin-embedded. The lung tissue was cut into thin slices four microns thick and then stained with hematoxylin and eosin (H&E; Sigma-Aldrich). At least five bronchi were selected from each mouse based on size (150–350 mm in diameter) for analysis. In order to reduce evaluator bias, the degree of airway inflammatory cell infiltration was scored in a single-blind fashion. Lung lesions were scored semi-quantitatively using a measurement tool as previously described62. Images were captured under a Nikon Eclipse E200 microscope connected to a Nikon Coolpix 995 camera (Nikon, Tokyo, Japan). The severity of inflammation was evaluated by assigning a value of 0 point for normal; 1 point for few cells; 2 points for a ring of inflammatory cells 1 cell layer deep; 3 points for a ring of inflammatory cells 2 to 4 cells deep; 4 points for a ring of inflammatory cells of >4 cells deep.
BALF cytokines measurements
Concentrations of IL-4, IL-5, IL-13, IL-17A interferon (IFN)-γ and TGF-β (Xin Bosheng, Shenzhen, China) in BALF were detected by commercially available enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s instructions.
Flow cytometric analysis of lung CD4+T cells
Lungs were minced and incubated 1 mL of RPMI 1640 containing 0.2% collagenase I (Sigma-Aldrich) for 15 min at 37 °C. Single cell suspension was obtained by forcing tissue through a 70 μm cell filter (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) After centrifugation, 3 ml erythrocyte lysis buffer was added to the sediment. Fifteen minutes later, the cells were then harvested and washed and divided into two aliquots. One aliquot was stained for surface-associated CD11c-FITC (Rat anti-mouse; EB Biosciences) and CD4-FITC (Rat anti-mouse, BD Biosciences), CD25-PE (Rat anti-mouse, BD Biosciences.), Foxp3-PEcy5 (Rat anti-mouse, BD Biosciences) and the other was resuspended in RPMI 1640 medium containing 10% fetal bovine serum. The resuspended cells were incubated at 37 °C and 5% CO2 for 4–6 h in 15 ml centrifuge tube in 1 mL medium containing phorbol 12-myristate 13-acetate (50 ng/mL; Sigma-Aldrich), ionomycin (500 ng/mL; Sigma-Aldrich) and GolgiPlug-containing brefeldin A (Becton, Dickinson and Company). To detect the subsets of Th1 and Th2 cells in lungs, cells were stained for intracellular IFN-γ-PerCP-Cy5.5 (Rat anti-mouse; Pharmingen), IL-17A-PE (Rat anti-mouse; Pharmingen), IL-4-APC (Rat anti-mouse; Pharmingen). Stained cells were detected by flow cytometry (FACS Canto; Becton, Dickinson and Company) and data were analyzed with CellQuest software (Becton, Dickinson and Company).
Statistical analysis
Results were analyzed using GraphPad Prism (version 5.0; GraphPad, La Jolla, CA, USA) and values are expressed as mean ± standard error. Statistical analysis was performed by either one-way analysis of variance (ANOVA) with Tukey’s post-test or two-way ANOVA with Bonferroni’s post-test. A value of P < 0.05 was considered significant.
Acknowledgements
We thank the Department of Laboratory Medicine, Key Laboratory of Diagnostic Medicine, Chongqing Medical University for providing the S.pneumoniae strain D39.We also thank the Experimental Animal Center at Chongqing Medical University for providing the BALB/c mice. This work was supported by the National Natural Science Foundation of China (81270086, 814700222), the Science and Technology Department of Chongqing (cstc2017jcyjB0160), the Science and Technology Department of Yuzhong district, Chongqing (20170120).
Author contributions
Z.X.L. conceived and designed the experiments. Q.Q.T., Y.L.T., Y.W., X.P. and Y.X.C. performed the experiments. Y.L.T., G.L.Z., X.Y.T., L.R. and Z.X.L. analyzed the data. Y.L.T., Q.Y.L. and Z.X.L. drafted or revised the manuscript. All authors approved the final manuscript and agree to be accountable for all of the work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yonglu Tian and Qinqin Tian.
References
- 1.Chawes, B. L. Low-grade disease activity in early life precedes childhood asthma and allergy. Dan. Med. J.63 (2016). [PubMed]
- 2.Piippo-Savolainen E, Remes S, Kannisto S, Korhonen K, Korppi M. Early predictors for adult asthma and lung function abnormalities in infants hospitalized for bronchiolitis: a prospective 18- to 20-year follow-up. Allergy Asthma Proc. 2006;27:341–349. doi: 10.2500/aap.2006.27.2912. [DOI] [PubMed] [Google Scholar]
- 3.Sears MR, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N. Engl. J. Med. 2003;349:1414–1422. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 4.von ME. Paediatric origins of adult lung disease. Thorax. 2001;56:153–157. doi: 10.1136/thorax.56.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lodge CJ, Dharmage SC. Breastfeeding and perinatal exposure, and the risk of asthma and allergies. Curr. Opin. Allergy Clin. Immunol. 2016;16:231–236. doi: 10.1097/ACI.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 6.Bisgaard H, et al. Childhood asthma after bacterial colonization of the airway in neonates. N. Engl. J. Med. 2007;357:1487–1495. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 7.Vissing NH, Chawes BL, Bisgaard H. Increased risk of pneumonia and bronchiolitis after bacterial colonization of the airways as neonates. Am. J. Respir. Crit. Care Med. 2013;188:1246–1252. doi: 10.1164/rccm.201302-0215OC. [DOI] [PubMed] [Google Scholar]
- 8.Yang B, et al. Neonatal Streptococcus pneumoniae infection may aggravate adulthood allergic airways disease in association with IL-17A. PLoS One. 2015;10:e0123010. doi: 10.1371/journal.pone.0123010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Restori KH, McDaniel KL, Wray AE, Cantorna MT, Ross AC. Streptococcus pneumoniae-induced pneumonia and Citrobacter rodentium-induced gut infection differentially alter vitamin A concentrations in the lung and liver of mice. J. Nutr. 2014;144:392–398. doi: 10.3945/jn.113.186569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pneumococcal conjugate vaccine for childhood immunization–WHO position paper. Wkly Epidemiol Rec82, 93–104 (2007). [PubMed]
- 11.Sommer A. Moving from science to public health programs: lessons from vitamin A. Am. J. Clin. Nutr. 1998;68:513S–516S. doi: 10.1093/ajcn/68.2.513S. [DOI] [PubMed] [Google Scholar]
- 12.Sommer, A. Rahmathullah, L. Underwood, B. et al. Potential interventions for the prevention of childhood pneumonia in developing countries: a meta-analysis of data from field trials to assess the impact of vitamin A supplementation on pneumonia morbidity and mortality. The Vitamin A and Pneumonia Working Group. Bull World Health Organ73, 609–619 (1995). [PMC free article] [PubMed]
- 13.Reyes H, et al. Frequency and determinants of vitamin A deficiency in children under 5 years of age with pneumonia. Arch. Med. Res. 2002;33:180–185. doi: 10.1016/S0188-4409(01)00361-7. [DOI] [PubMed] [Google Scholar]
- 14.Awasthi S, et al. Vitamin A supplementation every 6 months with retinol in 1 million pre-school children in north India: DEVTA, a cluster-randomised trial. Lancet. 2013;381:1469–1477. doi: 10.1016/S0140-6736(12)62125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.From the Centers for Disease Control and Prevention. Vitamin A deficiency among children–Federated States of Micronesia, 2000. 286, 667 (2001). [PubMed]
- 16.Julia V, Macia L, Dombrowicz D. The impact of diet on asthma and allergic diseases. Nat. Rev. Immunol. 2015;15:308–322. doi: 10.1038/nri3830. [DOI] [PubMed] [Google Scholar]
- 17.Nurmatov U, Devereux G, Sheikh A. Nutrients and foods for the primary prevention of asthma and allergy: systematic review and meta-analysis. J. Allergy Clin. Immunol. 2011;127(724–733):e1–30. doi: 10.1016/j.jaci.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Luo ZX, et al. Vitamin A deficiency and wheezing. World J. Pediatr. 2010;6:81–84. doi: 10.1007/s12519-010-0012-7. [DOI] [PubMed] [Google Scholar]
- 19.Vos T, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cabieses B, Uphoff E, Pinart M, Antó JM, Wright J. A systematic review on the development of asthma and allergic diseases in relation to international immigration: the leading role of the environment confirmed. PLoS One. 2014;9:e105347. doi: 10.1371/journal.pone.0105347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ducharme FM, Tse SM, Chauhan B. Diagnosis, management, and prognosis of preschool wheeze. Lancet. 2014;383:1593–1604. doi: 10.1016/S0140-6736(14)60615-2. [DOI] [PubMed] [Google Scholar]
- 22.Kusel MM, et al. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J. Allergy Clin. Immunol. 2007;119:1105–1110. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Webley WC, Hahn DL. Infection-mediated asthma: etiology, mechanisms and treatment options, with focus on Chlamydia pneumoniae and macrolides. Respir. Res. 2017;18:98. doi: 10.1186/s12931-017-0584-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Garawi A, et al. Influenza A facilitates sensitization to house dust mite in infant mice leading to an asthma phenotype in adulthood. Mucosal Immunol. 2011;4:682–694. doi: 10.1038/mi.2011.35. [DOI] [PubMed] [Google Scholar]
- 25.Rosas-Salazar C, et al. Nasopharyngeal Microbiome in Respiratory Syncytial Virus Resembles Profile Associated with Increased Childhood Asthma Risk. Am. J. Respir. Crit. Care Med. 2016;193:1180–1183. doi: 10.1164/rccm.201512-2350LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bisgaard H, et al. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978. doi: 10.1136/bmj.c4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Annagür A, Kendirli SG, Yilmaz M, Altintas DU, Inal A. Is there any relationship between asthma and asthma attack in children and atypical bacterial infections; Chlamydia pneumoniae, Mycoplasma pneumoniae and Helicobacter pylori. J. Trop. Pediatr. 2007;53:313–318. doi: 10.1093/tropej/fmm040. [DOI] [PubMed] [Google Scholar]
- 28.Reibman J, et al. Asthma is inversely associated with Helicobacter pylori status in an urban population. PLoS One. 2008;3:e4060. doi: 10.1371/journal.pone.0004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Essilfie AT, et al. Haemophilus influenzae infection drives IL-17-mediated neutrophilic allergic airways disease. PLoS Pathog. 2011;7:e1002244. doi: 10.1371/journal.ppat.1002244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kloepfer KM, et al. Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. J. Allergy Clin. Immunol. 2014;133(1301–1307):1307.e1–3. doi: 10.1016/j.jaci.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stephensen CB. Vitamin A, infection, and immune function. Annu. Rev. Nutr. 2001;21:167–192. doi: 10.1146/annurev.nutr.21.1.167. [DOI] [PubMed] [Google Scholar]
- 32.Velasquez-Melendez G, Okani ET, Kiertsman B, Roncada MJ. Vitamin A status in children with pneumonia. Eur. J. Clin. Nutr. 1995;49:379–384. [PubMed] [Google Scholar]
- 33.Jiang YL, Peng DH. Serum level of vitamin A in children with pneumonia aged less than 3 years. Zhongguo Dang Dai Er Ke Za Zhi. 2016;18:980–983. doi: 10.7499/j.issn.1008-8830.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosales FJ, Ritter SJ, Zolfaghari R, Smith JE, Ross AC. Effects of acute inflammation on plasma retinol, retinol-binding protein, and its mRNA in the liver and kidneys of vitamin A-sufficient rats. J. Lipid Res. 1996;37:962–971. [PubMed] [Google Scholar]
- 35.Rice AL, et al. Maternal vitamin A or beta-carotene supplementation in lactating bangladeshi women benefits mothers and infants but does not prevent subclinical deficiency. J. Nutr. 1999;129:356–365. doi: 10.1093/jn/129.2.356. [DOI] [PubMed] [Google Scholar]
- 36.Al SAM. Serum vitamin A and beta-carotene levels in children with asthma. J. Asthma. 2009;46:699–702. doi: 10.1080/02770900903056195. [DOI] [PubMed] [Google Scholar]
- 37.Arora P, Kumar V, Batra S. Vitamin A status in children with asthma. Pediatr. Allergy Immunol. 2002;13:223–226. doi: 10.1034/j.1399-3038.2002.00010.x. [DOI] [PubMed] [Google Scholar]
- 38.Grieger JA, Wood LG, Clifton VL. Improving asthma during pregnancy with dietary antioxidants: the current evidence. Nutrients. 2013;5:3212–3234. doi: 10.3390/nu5083212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yokota A, et al. GM-CSF and IL-4 synergistically trigger dendritic cells to acquire retinoic acid-producing capacity. Int. Immunol. 2009;21:361–377. doi: 10.1093/intimm/dxp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamoto A, et al. Vitamin A deficiency Impairs Induction of Oral Tolerance in Mice. J. Nutr. Sci. Vitaminol. 2015;61:147–153. doi: 10.3177/jnsv.61.147. [DOI] [PubMed] [Google Scholar]
- 42.Brown CC, et al. Retinoic acid is essential for Th1 cell lineage stability and prevents transition to a Th17 cell program. Immun. 2015;42:499–511. doi: 10.1016/j.immuni.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cui W, et al. Vitamin A deficiency Promotes Inflammation by Induction of Type 2 Cytokines in Experimental Ovalbumin-Induced Asthma Murine Model. Inflamm. 2016;39:1798–1804. doi: 10.1007/s10753-016-0415-2. [DOI] [PubMed] [Google Scholar]
- 44.Mucida D, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Sci. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 45.Han YY, et al. Diet and asthma: vitamins and methyl donors. Lancet Respir. Med. 2013;1:813–822. doi: 10.1016/S2213-2600(13)70126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thorburn AN, Macia L, Mackay CR. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immun. 2014;40:833–842. doi: 10.1016/j.immuni.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 47.Paiva SA, Russell RM. Beta-carotene and other carotenoids as antioxidants. J. Am. Coll. Nutr. 1999;18:426–433. doi: 10.1080/07315724.1999.10718880. [DOI] [PubMed] [Google Scholar]
- 48.Mora JR, Iwata M, von AUH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat. Rev. Immunol. 2008;8:685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J. Exp. Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Niu C, et al. Vitamin A maintains the airway epithelium in a murine model of asthma by suppressing glucocorticoid-induced leucine zipper. Clin. Exp. Allergy. 2016;46:848–860. doi: 10.1111/cea.12646. [DOI] [PubMed] [Google Scholar]
- 52.Checkley W, et al. Supplementation with vitamin A early in life and subsequent risk of asthma. Eur. Respir. J. 2011;38:1310–1319. doi: 10.1183/09031936.00006911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Milner JD, Stein DM, McCarter R, Moon RY. Early infant multivitamin supplementation is associated with increased risk for food allergy and asthma. Pediatrics. 2004;114:27–32. doi: 10.1542/peds.114.1.27. [DOI] [PubMed] [Google Scholar]
- 54.Mai XM, Langhammer A, Chen Y, Camargo CA. Cod liver oil intake and incidence of asthma in Norwegian adults–the HUNT study. Thorax. 2013;68:25–30. doi: 10.1136/thoraxjnl-2012-202061. [DOI] [PubMed] [Google Scholar]
- 55.Mawson AR. Could bronchial asthma be an endogenous, pulmonary expression of retinoid intoxication. Front. Biosci. 2001;6:D973–985. doi: 10.2741/mawson. [DOI] [PubMed] [Google Scholar]
- 56.Thorne PS, Hawk C, Kaliszewski SD, Guiney PD. The noninvasive mouse ear swelling assay. I. Refinements for detecting weak contact sensitizers. Fundam. Appl. Toxicol. 1991;17:790–806. doi: 10.1016/0272-0590(91)90186-8. [DOI] [PubMed] [Google Scholar]
- 57.Ross AC, Li NQ, Wu L. The components of VARA, a nutrient-metabolite combination of vitamin A and retinoic acid, act efficiently together and separately to increase retinyl esters in the lungs of neonatal rats. J. Nutr. 2006;136:2803–2807. doi: 10.1093/jn/136.11.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.James ML, Ross AC, Bulger A, Philips JB, Ambalavanan N. Vitamin A and retinoic acid act synergistically to increase lung retinyl esters during normoxia and reduce hyperoxic lung injury in newborn mice. Pediatr. Res. 2010;67:591–597. doi: 10.1203/PDR.0b013e3181dbac3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deng Y, et al. The antiasthma effect of neonatal BCG vaccination does not depend on the Th17/Th1 but IL-17/IFN-γ balance in a BALB/c mouse asthma model. J. Clin. Immunol. 2011;31:419–429. doi: 10.1007/s10875-010-9503-5. [DOI] [PubMed] [Google Scholar]
- 60.Zang N, et al. Resveratrol-mediated gamma interferon reduction prevents airway inflammation and airway hyperresponsiveness in respiratory syncytial virus-infected immunocompromised mice. J. Virol. 2011;85:13061–13068. doi: 10.1128/JVI.05869-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang L, et al. Infant 7-valent pneumococcal conjugate vaccine immunization alters young adulthood CD4(+)T cell subsets in allergic airway disease mouse model. Vaccine. 2014;32:2079–2085. doi: 10.1016/j.vaccine.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 62.Myou S, et al. Blockade of inflammation and airway hyperresponsiveness in immune-sensitized mice by dominant-negative phosphoinositide 3-kinase-TAT. J. Exp. Med. 2003;198:1573–1582. doi: 10.1084/jem.20030298. [DOI] [PMC free article] [PubMed] [Google Scholar]