Abstract
This study investigated the physicochemical, instrumental and bacterial parameters of tilapia fillets subjected to oxygen-scavenger packaging, alone or in combination with UV-C radiation at two doses (0.102 and 0.301 J/cm2), stored at 4 ± 1 °C for 23 days. The oxygen scavenger, both UV-C doses, and the oxygen scavenger combined with UV-C, independently of the dose, extended the shelf life in 5, 6 and 7 days, respectively, by decreasing the bacterial growth rate and the formation of degradation compounds (e.g., TVB-N and ammonia). Oxygen-scavenger packaging, alone or in combination with UV-C at 0.102 J/cm2 and 0.301 J/cm2 showed lower amounts of free amino acids (FAA; 34.39, 34.49 and 34.50 mg L-lysine/kg fish tissue, 3.63, 3.57 and 3.61 mg L- ornithine/kg fish tissue, 27.52, 27.63 and 27.67 mg L-arginine/kg fish tissue), biogenic amines (BA; 3.81, 3.87 and 3.89 mg cadaverine/kg fish tissue, 12.88, 12.91 and 12.86 mg putrescine/kg fish tissue, 2.41, 2.44 and 2.47 mg spermidine/kg fish tissue), redness (2.53, 2.55 and 2.59), yellowness (6.65, 6.69 and 6.72), lipid oxidation (1.52, 1.53 and 1.58 mg malondialdehyde/kg fish tissue) and protein oxidation (5.06, 5.11 and 5.18 nmol carbonyls/mg protein), with higher hardness (3273.41, 2652.98 and 2687.57 g) than control (air packaging; 41.97 mg L-lysine/kg fish tissue, 4.83 mg L- ornithine/kg fish tissue, 37.33 mg L-arginine/kg fish tissue, 4.82 mg cadaverine/kg fish tissue, 16.56 mg putrescine/kg fish tissue, 3.21 mg spermidine/kg fish tissue, 4.26 of redness, 8.17 of yellowness, 2.88 mg malondialdehyde/kg fish tissue, 9.44 nmol carbonyls/mg protein and 2092.58 g of hardness), respectively, on day 13 of storage when the control fillets were unfit for consumption (7 log CFU/g) (p < 0.05). However, in the same day of storage, both UV-C doses had similar values for BA (p > 0.05), higher amounts of FAA (44.28 and 44.13 mg L-lysine/kg fish tissue, 5.16 and 5.12 mg L- ornithine/kg fish tissue, 40.20 and 40.28 mg L-arginine/kg fish tissue), redness (4.86 and 5.33), yellowness (9.32 and 10.01), lipid oxidation (3.09 and 3.52 mg malondialdehyde/kg fish tissue) and protein oxidation (10.27 and 11.93 nmol carbonyls/mg protein), as well as lower hardness (1877.54 and 1767.39 g), respectively, than control fillets (p < 0.05). The combined preservation methods were the most effective in extending the shelf life and prolonging the physicochemical quality of the refrigerated tilapia fillets and the O2 scavenger proved to be a potential alternative to prevent the negative changes induced by both UV-C doses.
Subject terms: Biochemistry, Microbiology
Introduction
Fish is rich in nutrients, but is highly perishable due to rapid endogenous enzyme and bacterial activity in the postmortem period, resulting in the production of undesirable metabolites (e.g., total volatile basic nitrogen, ammonia and biogenic amines), limited shelf life and loss of quality1,2. According to the United Nations Food and Agriculture Organization3, approximately 27% of the fish catch is discarded because of loss of quality between capture and final consumption, leading to economic loss. Nile tilapia (Oreochromis niloticus) is the most important freshwater fish species contributing to the increase in global production and consumption of fish from aquaculture systems3. Tilapia is usually consumed as fillets, which contain high amounts of protein (23%) and unsaturated fatty acids (66%), making the flesh more susceptible to protein and lipid oxidation4,5. Previous studies have been suggested a relationship between lipid and protein oxidation, wherein protein oxidation is favored by secondary compounds from lipid oxidation, and the free iron from protein oxidation catalyzes the lipid oxidation, causing changes in the color and texture and accelerating deterioration4,6–9.
Vacuum packaging (VP) and modified-atmosphere packaging (MAP) are widely used for fish flesh, to minimize oxygen-induced reactions and to inhibit the growth of obligate aerobic microorganisms; however, these packaging systems require costly equipment and do not prevent O2 from penetrating through the packaging film during storage10,11. O2 scavengers or O2 absorbers, which can prevent O2 penetration, are commercially available in the form of sachets, labels, cards or films. Their mechanism of action is based mainly on iron oxidation, wherein ferrous oxide (Fe2+) is converted to ferric oxide (Fe3+), reducing O2 levels in the package to less than 0.01%10,12. O2 scavengers do not require the use of equipment and, therefore it may be an efficient and economical alternative to the use of VP and MAP. Additionally, the effectiveness of the O2 scavengers in increasing shelf life and preventing oxidative processes in fish species have been described in the literature13–15.
UV-C radiation (wavelengths of 200–280 nm) is an emerging non-thermal technology that is effective in improving the bacterial quality and extending the shelf life of fish flesh through direct action on the microbial DNA, by formation of cross-linking between thymine and cytosine, and indirect action by water radiolysis, releasing free radicals16,17. This technology has several advantages, including ease of implementation, low cost and absence of toxic residues17. Previous studies confirmed that UV-C radiation is able to reduce the bacterial growth rate during refrigerated storage of fish species1,18. However, in general, the UV-C doses needed to significantly extend the shelf life may lead to the production of reactive oxygen species (ROS), which remove a hydrogen atom from a weak C-H bond, consequently initiating a free-radical chain reaction and intensifying the oxidative processes, changes in texture and color during refrigerated storage1,19. This effect depends mainly on the type and load of microorganisms present in the food matrix and the food composition1,11,17 and UV-C is therefore not necessarily dose-dependent20. Deprivation of oxygen in the package during storage could minimize the adverse effects of UV-C radiation.
The demand for a longer shelf life while maintaining the physicochemical characteristics without the use of chemical preservatives has increased, and represents one of the main challenges for the food industry and scientific community. The number of studies on combined preservation methods has increased1,7,13,14, but there are no reports about the use of an O2 scavenger in combination with UV-C radiation to treat any food matrix. Therefore, this study investigated the effect of an O2 scavenger and two different doses of UV-C radiation (0.102 and 0.301 J/cm2), alone or in combination, on the quality attributes of Nile tilapia fillets stored at 4 ± 1 °C for 23 days.
Material and Methods
Experimental design
Twenty-five kilograms of fresh tilapia (Oreochromis niloticus) fillets packed in low-density polyethylene bags were purchased from a local fish farm in Rio de Janeiro, Brazil (22°27′46″S 042°39′10″W). Fillets (111.24 g ± 7.18 g each) were transported in ice chests to the laboratory, where they were individually packed in nylon/polyethylene bags (15 cm width, 22 cm height, 80 µm thickness) with barrier properties of 66.31 cc/m2/day for O2 transmission rate (OTR) and 4.91 gm/m2/day for water-vapor transmission rate (WVTR) according to the information from the manufacturer (Gabrilina, São Paulo, Brazil). The fillets were randomly divided into six treatments according to packaging conditions (air or oxygen scavenger) and exposure to different UV-C doses (0.102 J/cm2 or 0.301 J/cm2). The treatments were AP (air packaging), OSP (oxygen-scavenger packaging), AUV1 (air packaging + UV-C at 0.102 J/cm2), OSUV1 (oxygen-scavenger packaging + UV-C at 0.102 J/cm2), AUV3 (air packaging + UV-C at 0.301 J/cm2) and OSUV3 (oxygen-scavenger packaging + UV-C at 0.301 J/cm2). After the O2 scavenger sachets were placed and the samples were radiated with UV-C, they were stored at 4 ± 1 °C and analyzed for total aerobic mesophilic count (TAMC), total aerobic psychrotrophic count (TAPC), Enterobacteriaceae count, free amino acids, biogenic amines, total volatile basic nitrogen (TVB-N), ammonia (NH3), lipid oxidation, protein oxidation, and instrumental color and texture parameters. The packaging headspace was 47.88 ± 1.20 mL in all treatments. AP was evaluated on days 0, 1, 2, 3, 4, 5, 6, 9, 11 and 13; and OSP, AUV1, OSUV1, AUV3 and OSUV3 were evaluated on days 0, 1, 2, 3, 4, 5, 6, 9, 11, 13, 15, 17, 19, 21, and 23. The criterion for determining the days of storage was based on a predictive primary model designed by Baranyi & Roberts21, using the DMFit program version 2.0 (Institute of Food Research, Norwich, UK), until the stationary phases of the bacterial groups (TAMC, TAPC, and Enterobacteriaceae count) were reached. All experiment was carried out in duplicate (n = 2).
Oxygen scavenger system
In the OSP, OSUV1 and OSUV3 treatments, an oxygen-scavenger sachet was placed inside the package before sealing. The sachet used was the Ageless SS-50, with O2 absorption capacity of 50 mL (Mitsubishi Gas Chemical Co., Inc., Tokyo, Japan). This sachet reduces oxygen levels through spontaneous iron oxidation, converting ferrous oxide (Fe2+) to ferric oxide (Fe3+) in the presence of oxygen, resulting in an O2 concentration <0.01% according to information from the manufacturer (Mitsubishi Gas Chemical Co., Inc., Tokyo, Japan).
UV-C radiation exposure
After packaging, AUV1, OSUV1, AUV3 and OSUV3 were subjected to UV-C radiation in an apparatus containing six 30-W lamps and six 55-W lamps (Osram HNS, OFR, Munich, Germany) designed by Lázaro et al.20. The samples were placed in the center of the UV-C apparatus at a distance of 14 cm from the lamps. The intensity levels were monitored with a UV radiometer (MRUR-203, Instrutherm Ltda., São Paulo, Brazil) wrapped with the same sample packaging, and the exposure times were measured every 5 s until the doses of 0.102 ± 0.001 J/cm2 for AUV1 and OSUV1, and 0.301 ± 0.001 J/cm2 for AUV3 and OSUV3 were reached. These doses were chosen due to its effectiveness in increasing shelf life while causing physicochemical changes in refrigerated tilapia fillets conforming previously reported by some authors1,4,7.
Bacterial analysis
Serial dilutions were inoculated through the pour-plate technique into Petri dishes containing a plate-count agar (PCA, Merck, Darmstadt, Germany) for TAMC and TAPC, and Violet-Red-Bile-Glucose agar (VRBG-agar, Merck, Darmstadt, Germany) for Enterobacteriaceae, using a Spiral Plater (Eddy Jet 2, IUL Instruments, USA) mode E50. TAMC, TAPC and Enterobacteriaceae were enumerated in the electronic counter (Flash & Go, IUL instruments, USA) after incubation at 37 °C for 48 h, 10 °C for 7 days, and 35 °C for 24 h, respectively22. The results were expressed as log CFU/g fish tissue.
Free amino acids analysis
L-lysine, L-ornithine and L-arginine were analyzed as described by Gatti et al.23 with modifications in the sample deproteinization step. In brief, 0.1 g of sample (tissue) was mixed with 1 mL of 1.5 M perchloric acid (v/v) to remove proteins. After 2 min at room temperature, 0.325 mL H2O and 0.5 mL potassium carbonate were added. The tubes were centrifuged at 10,000 × g for 2 min. The sample (50 μL) was mixed with 50 μL H2O and 40 μL of 2,5-dimethyl-1H-pyrrole-3,4-dicarbaldehyde (DPD) reagent solution (v/v) for 10 min. 360 μL of the mobile phase (0.05 M triethylammonium phosphate buffer) was added to the derivatized solution, which was immediately analyzed by HPLC. The HPLC device was equipped with an ACE C18 3-μm reversed-phase column (250 × 4.6 mm I.D.), a 5-μm Ascentis C18 reversed-phase guard column (20 × 4.6 mm I.D.) and an RF-10AXL photodiode array detector (SHIMADZU, Kyoto, Japan), monitoring the absorbance at 320 nm. The results were expressed as mg free amino acids/kg fish tissue.
Biogenic amines analysis
Cadaverine, putrescine and spermidine were determined according to the method of Lázaro et al.24, using an HPLC (SHIMADZU, Kyoto, Japan) equipped with a CBM-20A controller composed of an LC-20AD pump, SPD-M20A diode-array detector, CTO-20A oven and SIL-20AC autosampler. The amines were separated using a Spherisorb ODS2 C18 column (15 × 0.46 cm I.D., 5 μm particle size) for the stationary phase, and an acetonitrile:water mixture (42:58, v/v) as the mobile phase, under isocratic conditions. The biogenic amines were detected at 198 nm, and the results were expressed as mg biogenic amines/kg fish tissue.
Determination of total volatile basic nitrogen (TVB-N) and ammonia (NH3)
TVB-N was determined by Conway’s microdiffusion method25 and the results were expressed as mg TVB-N/100 g fish tissue. Ammonia was quantified by the colorimetric method, using a UV-1800 spectrophotometer (SHIMADZU, Kyoto, Japan) at 425 nm according to the protocol of Rodrigues et al.11. Results were expressed as µg NH3/g fish tissue, based on a standard curve (R2 = 0.996) constructed from seven different NH3 concentrations (1 to 15 µg NH3).
Determination of lipid and protein oxidation
Lipid oxidation was evaluated by the thiobarbituric acid-reactive substances (TBARS) assay according to the method of Yin et al.26 adapted by Joseph et al.27. The absorbance values were read at 532 nm, using a UV-1800 spectrophotometer (SHIMADZU, Kyoto, Japan), and the results were expressed as mg malonaldehyde (MDA)/kg fish tissue from a standard curve (R2 = 0.999) constructed with eight different MDA concentrations (0.5 to 400 µmol). Protein oxidation was evaluated by the carbonyl content, following the method of Oliver et al.28 with modifications29,30. The absorbance values were measured at 280 nm (protein) and 370 nm (carbonyl) by a UV-1800 spectrophotometer (SHIMADZU, Kyoto, Japan), and the results were expressed as nmol carbonyls/mg protein. Protein content was determined by a standard curve (R2 = 0.999) constructed from five different concentrations of bovine serum albumin (0.1–1.0 mg), while the carbonyl content was calculated using an absorptivity coefficient for the protein hydrazones of 21.0/mM/cm.
Instrumental color measurements
Lightness (L*), redness (a*) and yellowness (b*) values were measured with an illuminant A, 8 mm-diameter aperture, and 10° standard observer through a Minolta CM-600D portable spectrophotometer (Minolta Camera Co., Osaka, Japan). The color parameters were determined at four random locations on the surface of each fillet immediately after it was removed from the packaging31.
Instrumental texture profile
The texture-profile analysis (TPA) was measured utilizing a TA.XTplus Texture Analyser (Stable Micro Systems, Surrey, UK) equipped with a cylindrical P/36 R probe. Each fillet was cut transversely into four pieces (2 × 2 × 2 cm3), which were compressed twice to 50% of their original height with the time of 5 s between the two compression cycles, and pre-test, test speed, and post-test of 1 mm/s following conditions established by Sun et al.32. The parameters determined were hardness, chewiness, cohesiveness, springiness, and resilience.
Statistical analysis
The experiment was conducted in duplicate, using a fully randomized design (n = 2). A linear regression analysis was performed separately for each treatment to investigate the relationship between each physicochemical parameter and days of storage. The area under the curve (AUC), calculated by the trapezoidal method, was used to calculate the total amount of each physicochemical parameter produced during a time interval. To identify differences in the AUC among treatments (AP, OSP, AUV1, OSUV1, AUV3 and OSUV3), a one-way ANOVA was used. An additional post-hoc test with Tukey’s adjustment was performed. All analyses were performed with a 0.05 confidence level, using GraphPad Prism version 5.00 (GraphPad Software, San Diego, California, USA). The bacterial growth curves were obtained by the predictive primary model21 through the DMFit program version 2.0 (Institute of Food Research, Norwich, UK), and the differences among treatments regarding bacterial growth parameters (lag, log and stationary phases) were identified by one-way ANOVA with Tukey post-hoc test (p < 0.05).
Results and Discussion
Bacterial growth during storage
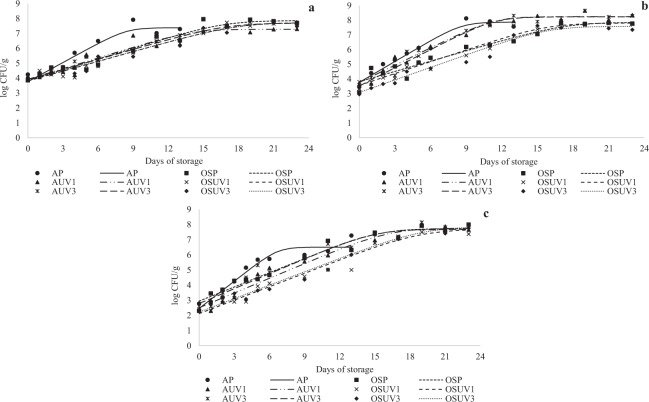
The results for TAMC, TAPC and Enterobacteriaceae are shown in Table 1 and Fig. 1a–c. The lag phase was absent in all bacterial groups. Although the number of colonies in the stationary phase of the fillets treated with the oxygen scavenger and/or UV-C radiation (0.102 or 0.301 J/cm2) was higher than in the fillets in air packaging (AP), these emerging techniques alone or in combination extended the shelf life of the tilapia fillets by decreasing (p < 0.05) the exponential growth rate (EGR) of the microorganisms (Table 1). The initial bacterial counts were 4.24 log CFU/g for TAMC, 3.45 log CFU/g for TAPC and 2.78 log CFU/g for Enterobacteriaceae. Considering the limit of 3 log CFU/g for initial counts of Enterobacteriaceae proposed by the International Commission on Microbiological Specifications for Foods33, the tilapia fillets showed good initial microbial quality. The limit of 7 log CFU/g for TAMC proposed by ICMSF33 was also used as the microbiological criterion to establish the shelf life of tilapia fillets during refrigerated storage. AP exceeded the limit of 7.0 log CFU/g for TAMC on day 9, while OSP, AUV1, AUV3, OSUV1 and OSUV3 reached this limit on storage days 14, 15, 15, 16 and 16, respectively.
Table 1.
Bacterial growth parameters of tilapia (Oreochromis niloticus) fillets non- and treated with oxygen scavenger packaging (OSP) and ultraviolet radiation (UV-C) stored at 4 ± 1 °C for 23 days.
| Microorganisms¥ | Parameters# | Treatments€ | |||||
|---|---|---|---|---|---|---|---|
| AP | OSP | AUV1 | OSUV1 | AUV3 | OSUV3 | ||
| TAMC | Lag | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| EGR | 0.45 ± 0.01a | 0.24 ± 0.01b | 0.21 ± 0.01b | 0.12 ± 0.01c | 0.20 ± 0.01b | 0.13 ± 0.01c | |
| NC | 7.35 ± 0.01b | 7.86 ± 0.02a | 7.54 ± 0.34ab | 7.72 ± 0.02a | 7.70 ± 0.00ab | 7.74 ± 0.02a | |
| TAPC | Lag | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| EGR | 0.48 ± 0.01a | 0.30 ± 0.01c | 0.38 ± 0.01b | 0.24 ± 0.01d | 0.37 ± 0.01b | 0.23 ± 0.01d | |
| NC | 7.87 ± 0.14b | 7.85 ± 0.00b | 8.24 ± 0.01a | 7.80 ± 0.02c | 8.22 ± 0.02a | 7.63 ± 0.04d | |
| Enterobacteriaceae | Lag | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| EGR | 0.60 ± 0.00a | 0.30 ± 0.01b | 0.31 ± 0.01b | 0.23 ± 0.00c | 0.30 ± 0.01b | 0.24 ± 0.01c | |
| NC | 6.63 ± 0.17b | 7.76 ± 0.04a | 7.80 ± 0.06a | 7.70 ± 0.01a | 7.72 ± 0.04a | 7.84 ± 0.05a | |
Results are expressed as means ± standard deviation (n = 2). a,b,c,dDifferent letters in the same row indicate significant differences (p < 0.05) among treatments. ¥TAMC - Total aerobic mesophilic count; TAPC - Total aerobic psychrotrophic count. #Lag – lag phase (h); EGR – exponential growth rate (log CFU/g/h); NC – number of colonies in the stationary phase (log CFU/g). €AP (air packaging); OSP (oxygen scavenger packaging); AUV1 (air packaging + UV-C at 0.102 J/cm2); OSUV1 (oxygen scavenger packaging + UV-C at 0.102 J/cm2); AUV3 (air packaging + UV-C at 0.301 J/cm2); and OSUV3 (oxygen scavenger packaging + UV-C at 0.301 J/cm2).
Figure 1.
Total aerobic mesophilic count (a), Total aerobic psychrotrophic count (b), and Enterobacteriaceae count (c) in tilapia (Oreochromis niloticus) fillets non- and treated with oxygen scavenger packaging (OSP) and ultraviolet radiation (UV-C) stored at 4 ± 1 °C for 23 days. Results are expressed as the mean of log CFU (colony forming units)/g ± standard deviation (n = 2). AP (air packaging); OSP (oxygen scavenger packaging); AUV1 (air packaging + UV-C at 0.102 J/cm2); OSUV1 (oxygen scavenger + UV-C at 0.102 J/cm2); AUV3 (air packaging + UV-C at 0.301 J/cm2); and OSUV3 (oxygen scavenger packaging + UV-C at 0.301 J/cm2).
The microbiota of tropical freshwater fishes such as tilapia is composed predominantly of Gram-negative aerobic and facultative anaerobic bacteria, including bacteria from the family Enterobacteriaceae, and Gram-positive bacteria34. Molinari et al.35 and Pakingking et al.36, evaluating the microbiota of tilapia, found a wide variety of bacterial genera and species, including Pseudomonas spp., Shewanella putrefaciens, Aeromonas spp., Pasteurella pneumotropica, Photobacterium damselae, Plesiomonas shigelloides, Vibrio spp., Burkholderia cepacia, Chromobacterium violaceum, and Flavimonas oryzihabitans (Gram-negative aerobic and facultative anaerobic bacteria); Citrobacter spp., Edwardsiella spp., Enterobacter cloacae, Klebsiella oxytoca, Escherichia coli (Enterobacteriaceae); and Bacillus sp. and Staphyloccocus sp. (Gram-positive bacteria). However, along with the increase in the storage time under aerobic conditions, Pseudomonas spp. became the dominant spoilage bacteria in refrigerated fish, due to low temperature34.
In our study, the oxygen scavenger and both UV-C doses (OSP, AUV1 and AUV3) had similar effects on EGR for TAMC and Enterobacteriaceae. However, UV-C radiation (AUV1 and AUV3) showed a higher (p < 0.05) EGR for TAPC than OSP. This fact may be explained by antimicrobial effect of the UV-C16,17. Gram-negative bacteria are more sensitive to UV-C radiation due to their lack of a thick cell wall, which prevents UV-C absorption by microbial DNA37. Nevertheless, although Pseudomonas spp. are Gram-negative, they are resistant to radiation due to their ability to form a biofilm in response to UV-C induced stress, in an attempt to repair damaged DNA38,39. On the other hand, obligate aerobic bacteria such as Pseudomonas spp. are highly sensitive to low oxygen concentrations from O2 scavenger13. Our results demonstrated that the O2 scavenger delayed the EGR of Enterobacteriaceae, which are facultative anaerobic bacteria. This delay can be attributed to the sensitivity of these bacteria to carbon dioxide, which increases in the package headspace due to the relative decrease in the O2 level caused by O2 scavengers13,40.
With respect to the combined preservation methods, the oxygen scavenger plus UV-C radiation, at both doses (OSUV1 and OSUV3), was the most effective in delaying the EGR in all bacterial groups, indicating a synergistic effect between the two preservation methods. While the O2 scavenger inhibits the growth of obligate aerobic bacteria and decreases the growth rate of facultative anaerobic bacteria of family Enterobacteriaceae by removing O2 and increasing the CO2 level inside the package, UV-C radiation decreases the growth rate of the microorganisms, especially Gram-negative bacteria, through direct or indirect damage to microbial DNA13,16,17.
OSP, AUV1, OSUV1, AUV3 and OSUV3 showed more viable cells in the stationary phase than AP. This difference may be explained by sublethal injury induced by CO2 and UV-C radiation to bacterial cells, which at first grow more slowly than intact cells, and more rapidly after recovery, mainly in an environment without natural competition17,41,42.
In agreement with the present results, previous researchers demonstrated that O2 scavengers were effective in extending the shelf life of refrigerated rainbow-trout fillets14 and ground beef43 by 5 and 2 days, respectively. Mohan et al.15 found an extension of 6–7 days in the shelf life of sardines packed with an O2 scavenger. Likewise, Bottino et al.18 reported that UV-C at 0.055 and 0.160 J/cm2 extended the shelf life of tambacu (Colossoma macropomum × Piaractus mesopotamicus) fillets stored at 4 °C by 50% and 100%, respectively. Monteiro et al.1 observed that the shelf life of refrigerated tilapia fillets exposed to UV-C radiation at 0.103 J/cm2 was extended by at least 2.5-fold.
Free amino acids and biogenic amines
The levels of free amino acids (L-lysine, L-ornithine, L-arginine) and biogenic amines (cadaverine, putrescine, spermidine) increased in all treatments throughout the storage period (p < 0.05; Table 2). AUV1 and AUV3 showed higher total amounts (p < 0.05), while OSP, OSUV1 and OSUV3 had lower (p < 0.05) total amounts of free amino acids than AP throughout the storage period (Table 2). The results of free amino acids and biogenic amines in all days of storage can be found as Supplementary Table S1. The increase of free amino acids during storage is attributed to the action of endogenous and microbial proteolytic enzymes44. Our results are attributable to the resistance of Pseudomonas spp. to UV-C radiation, together with the effect of UV-C in increasing the amount of oxidized proteins, which are more susceptible to proteolysis, resulting in a high level of free amino acids17,38,39. On the other hand, oxygen scavenger is highly effective against Pseudomonas spp.13 and it is able to minimize ROS-induced oxidation45.
Table 2.
Free amino acids and biogenic amines of tilapia (Oreochromis niloticus) fillets non- and treated with oxygen scavenger packaging (OSP) and ultraviolet radiation (UV-C) stored at 4 ± 1 °C for 23 days.
| Parameters | Treatments€ | AUC¥ | Linear regression coefficients | ||||
|---|---|---|---|---|---|---|---|
| AUC¥0–13 | AUC¥15–23 | y-intercept | slope | p-value | r-squared | ||
| L-lysine (mg lysine/kg fish tissue) | AP | 367.30 ± 1.48b | NA | 11.83 ± 0.79 | 2.49 ± 0.12 | <0.0001 | 0.962 |
| OSP | 302.40 ± 2.59c | 324.20 ± 1.52b | 11.72 ± 0.69 | 1.57 ± 0.06 | <0.0001 | 0.966 | |
| AUV1 | 402.90 ± 2.50a | 396.20 ± 1.62a | 17.76 ± 1.15 | 1.75 ± 0.09 | <0.0001 | 0.927 | |
| OSUV1 | 303.70 ± 1.30c | 323.90 ± 0.79b | 12.15 ± 0.63 | 1.54 ± 0.05 | <0.0001 | 0.971 | |
| AUV3 | 401.30 ± 1.08a | 397.60 ± 0.61a | 17.52 ± 1.13 | 1.77 ± 0.09 | <0.0001 | 0.930 | |
| OSUV3 | 304.20 ± 1.51c | 324.20 ± 0.58b | 12.16 ± 0.63 | 1.55 ± 0.05 | <0.0001 | 0.970 | |
| L-ornithine (mg ornitine/kg fish tissue) | AP | 47.04 ± 0.28b | NA | 1.89 ± 0.14 | 0.26 ± 0.02 | <0.0001 | 0.903 |
| OSP | 34.15 ± 0.32c | 33.56 ± 0.30b | 1.66 ± 0.06 | 0.14 ± 0.01 | <0.0001 | 0.962 | |
| AUV1 | 51.62 ± 0.27a | 47.90 ± 0.26a | 2.60 ± 0.12 | 0.18 ± 0.01 | <0.0001 | 0.926 | |
| OSUV1 | 34.83 ± 0.44c | 33.77 ± 0.38b | 1.81 ± 0.05 | 0.13 ± 0.00 | <0.0001 | 0.970 | |
| AUV3 | 51.58 ± 0.39a | 48.11 ± 0.34a | 2.60 ± 0.12 | 0.19 ± 0.01 | <0.0001 | 0.929 | |
| OSUV3 | 35.08 ± 0.23c | 33.82 ± 0.35b | 1.82 ± 0.05 | 0.13 ± 0.00 | <0.0001 | 0.968 | |
| L-arginine (mg arginine/kg fish tissue) | AP | 337.80 ± 4.96b | NA | 10.30 ± 1.09 | 2.34 ± 0.16 | <0.0001 | 0.922 |
| OSP | 244.10 ± 3.35c | 275.00 ± 4.50b | 9.63 ± 0.51 | 1.31 ± 0.04 | <0.0001 | 0.973 | |
| AUV1 | 373.70 ± 2.21a | 393.60 ± 2.85a | 15.04 ± 1.16 | 1.84 ± 0.09 | <0.0001 | 0.933 | |
| OSUV1 | 244.20 ± 1.65c | 274.30 ± 1.03b | 9.68 ± 0.45 | 1.31 ± 0.04 | <0.0001 | 0.979 | |
| AUV3 | 374.40 ± 2.12a | 392.40 ± 2.44a | 15.15 ± 1.18 | 1.83 ± 0.10 | <0.0001 | 0.929 | |
| OSUV3 | 244.30 ± 1.24c | 274.00 ± 1.15b | 9.75 ± 0.44 | 1.30 ± 0.04 | <0.0001 | 0.980 | |
| Cadaverine (mg cadaverine/kg fish tissue) | AP | 46.97 ± 0.54a | NA | 2.14 ± 0.09 | 0.22 ± 0.01 | <0.0001 | 0.945 |
| OSP | 36.83 ± 0.28b | 34.40 ± 0.19b | 1.98 ± 0.04 | 0.12 ± 0.00 | <0.0001 | 0.979 | |
| AUV1 | 47.19 ± 0.31a | 44.02 ± 0.18a | 2.39 ± 0.09 | 0.17 ± 0.01 | <0.0001 | 0.955 | |
| OSUV1 | 36.94 ± 0.30b | 34.28 ± 0.26b | 1.98 ± 0.05 | 0.12 ± 0.00 | <0.0001 | 0.974 | |
| AUV3 | 47.35 ± 0.32a | 44.01 ± 0.25a | 2.42 ± 0.09 | 0.17 ± 0.01 | <0.0001 | 0.955 | |
| OSUV3 | 37.13 ± 0.28b | 34.40 ± 0.26b | 1.98 ± 0.05 | 0.13 ± 0.00 | <0.0001 | 0.973 | |
| Putrescine (mg putrescine/kg fish tissue) | AP | 178.40 ± 0.37a | NA | 12.13 ± 0.10 | 0.25 ± 0.02 | <0.0001 | 0.939 |
| OSP | 155.70 ± 1.40b | 110.30 ± 1.32b | 11.15 ± 0.11 | 0.14 ± 0.01 | <0.0001 | 0.890 | |
| AUV1 | 178.60 ± 0.52a | 129.70 ± 0.53a | 12.35 ± 0.11 | 0.21 ± 0.01 | <0.0001 | 0.953 | |
| OSUV1 | 156.30 ± 0.42b | 110.80 ± 0.62b | 11.24 ± 0.11 | 0.14 ± 0.01 | <0.0001 | 0.889 | |
| AUV3 | 179.20 ± 0.70a | 129.90 ± 0.46a | 12.41 ± 0.10 | 0.20 ± 0.01 | <0.0001 | 0.958 | |
| OSUV3 | 155.90 ± 0.62b | 110.50 ± 0.27b | 11.26 ± 0.13 | 0.13 ± 0.01 | <0.0001 | 0.880 | |
| Spermidine (mg spermidine/kg fish tissue) | AP | 26.36 ± 0.44a | NA | 0.96 ± 0.04 | 0.17 ± 0.01 | <0.0001 | 0.977 |
| OSP | 20.92 ± 0.29b | 26.32 ± 0.38b | 0.81 ± 0.04 | 0.13 ± 0.00 | <0.0001 | 0.984 | |
| AUV1 | 26.58 ± 0.30a | 31.75 ± 0.39a | 1.03 ± 0.04 | 0.16 ± 0.00 | <0.0001 | 0.990 | |
| OSUV1 | 21.04 ± 0.29b | 26.26 ± 0.28b | 0.83 ± 0.04 | 0.13 ± 0.00 | <0.0001 | 0.985 | |
| AUV3 | 26.51 ± 0.28a | 31.97 ± 0.23a | 1.02 ± 0.04 | 0.16 ± 0.00 | <0.0001 | 0.988 | |
| OSUV3 | 21.09 ± 0.21b | 26.30 ± 0.19b | 0.83 ± 0.03 | 0.13 ± 0.00 | <0.0001 | 0.988 | |
Results are expressed as means ± standard deviation (n = 2). a,b,cDifferent superscripts in the same column indicate significant differences (p < 0.05) among treatments. ¥AUC – Area under curve; AUC0–13 – from day 0 to 13 among treatments AP, OSP, AUV1, OSUV1, AUV3, and OSUV3; AUC15–23 – from day 15 to 23 among treatments OSP, AUV1, OSUV1, AUV3, and OSUV3. NA – Not applicable. €AP (air packaging); OSP (oxygen scavenger packaging); AUV1 (air packaging + UV-C at 0.102 J/cm2); OSUV1 (oxygen scavenger packaging + UV-C at 0.102 J/cm2); AUV3 (air packaging + UV-C at 0.301 J/cm2); and OSUV3 (oxygen scavenger packaging + UV-C at 0.301 J/cm2).
Regarding biogenic amines, cadaverine, putrescine and spermidine are formed mainly by bacterial decarboxylation of precursor free amino acids such as L-lysine, L- ornithine and L-arginine, respectively46. Metabolization of L-arginine to L-ornithine is another pathway to formation of putrescine46, which explains the high amount of this amine in relation to others (cadaverine and spermidine). The present study found no difference (p > 0.05) in the total amounts of cadaverine, putrescine and spermidine among AP, AUV1 and AUV3; whereas OSP, OSUV1 and OSUV3 resulted in lower (p < 0.05) total amounts of these biogenic amines than the other treatments (Table 2). Although O2 OSP, AUV1 and AUV3 had similar effect in controlling the growth of Enterobacteriaceae, which is the main bacterial group associated with the formation of biogenic amines47, UV-C radiation may cause oxidative decarboxylation of amino acids by catalyzing the production of Fe3+ 48,49. On the other hand, O2 absorber has the capacity to minimize the oxidative reaction pathways by oxygen scavenging45, explaining our results for combined preservation methods (OSUV1 and OSUV3).
Currently, there is little information about the effect of O2 absorbers and UV-C radiation on the production of free amino acids and biogenic amines in fish species during refrigerated storage. Similarly to our results, an increase in the amount of free amino acids by UV-C has been previously reported in fish stored at 4 °C11,19. Likewise, Mohan et al.50 observed a delay in the formation of putrescine, cadaverine and spermidine by use of an O2 scavenger in seer fish (Scomberomorus commerson) stored under refrigeration. However, no effect on the formation of putrescine, cadaverine and spermidine by similar UV-C doses was reported in freshwater fish species during refrigerated storage11,19.
Total volatile basic nitrogen (TVB-N) and ammonia (NH3)
The initial levels of TVB-N and NH3 were 10.08 ± 0.00 mg TVB-N/100 g and 7.66 ± 0.04 µg NH3/g fish tissue. As expected, the TVB-N and ammonia levels increased (p < 0.05) in all treatments during the storage period, with the highest increases in the tilapia fillets under aerobic packaging (AP; Table 3). However, no treatment exceeded the limit of 25 mg TVB-N/100 g established by the Commission of the European Community51 until the end of storage, indicating that N-TVB was not a good indicator of bacterial spoilage and quality loss in tilapia fillets stored under refrigeration. On days 9, 14, 15, 16, 15 and 16 of refrigerated storage, when the acceptable microbial limit of 7 log CFU/g was reached, the TVB-N levels were 17.75 ± 0.89, 15.85 ± 0.07, 17.35 ± 0.81, 14.15 ± 0.27, 17.39 ± 0.10 and 14.09 ± 0.10 mg TVB-N/100 g for AP, OSP, AUV1, OSUV1, AUV3 and OSUV3, respectively (Supplementary Table S2). In freshwater fish species, TVB-N values are related mainly to the ammonia concentration, due to absence or low level of trimethylamine oxide in vivo2,52. However, there is no limit for ammonia content in freshwater fish species. In the present study, at the point when the fillets were unfit for consumption (7 log CFU/g), the ammonia levels were 10.60 ± 0.03, 10.44 ± 0.32, 11.46 ± 0.05, 9.99 ± 0.22, 11.47 ± 0.03 and 10.04 ± 0.20 µg of NH3/g of fish tissue for AP, OSP, AUV1, OSUV1, AUV3 and OSUV3, respectively (Supplementary Table S2).
Table 3.
Physicochemical parameters of tilapia (Oreochromis niloticus) fillets non- and treated with oxygen scavenger packaging (OSP) and ultraviolet radiation (UV-C) stored at 4 ± 1 °C for 23 days.
| Parameters | Treatments€ | AUC¥ | Linear regression coefficients | ||||
|---|---|---|---|---|---|---|---|
| AUC¥0–13 | AUC¥15–23 | y-intercept | slope | p-value | r-squared | ||
| Ammonia (µg NH3/g fish tissue) | AP | 129.80 ± 0.33a | NA | 7.44 ± 0.09 | 0.40 ± 0.01 | <0.0001 | 0.981 |
| OSP | 117.90 ± 0.22c | 90.33 ± 0.22b | 7.65 ± 0.08 | 0.20 ± 0.01 | <0.0001 | 0.970 | |
| AUV1 | 122.80 ± 0.33b | 94.43 ± 0.20a | 7.88 ± 0.11 | 0.21 ± 0.01 | <0.0001 | 0.953 | |
| OSUV1 | 109.70 ± 0.19d | 84.01 ± 0.16c | 7.40 ± 0.04 | 0.16 ± 0.00 | <0.0001 | 0.991 | |
| AUV3 | 122.90 ± 0.32b | 94.47 ± 0.24a | 7.88 ± 0.12 | 0.21 ± 0.01 | <0.0001 | 0.949 | |
| OSUV3 | 109.80 ± 0.35d | 84.16 ± 0.21c | 7.40 ± 0.04 | 0.16 ± 0.00 | <0.0001 | 0.991 | |
| TVB-N (mg TVB-N/100 g fish tissue) | AP | 216.50 ± 9.09a | NA | 11.98 ± 0.60 | 0.72 ± 0.09 | <0.0001 | 0.788 |
| OSP | 178.50 ± 4.24c | 152.60 ± 3.34b | 10.60 ± 0.31 | 0.45 ± 0.02 | <0.0001 | 0.923 | |
| AUV1 | 192.00 ± 4.94b | 170.80 ± 3.04a | 11.11 ± 0.35 | 0.54 ± 0.03 | <0.0001 | 0.930 | |
| OSUV1 | 154.00 ± 7.20d | 125.60 ± 3.76c | 9.86 ± 0.20 | 0.31 ± 0.02 | <0.0001 | 0.928 | |
| AUV3 | 191.70 ± 3.31b | 171.10 ± 1.27a | 11.08 ± 0.34 | 0.54 ± 0.03 | <0.0001 | 0.932 | |
| OSUV3 | 154.20 ± 3.70d | 125.70 ± 1.73c | 9.87 ± 0.18 | 0.31 ± 0.01 | <0.0001 | 0.942 | |
| Lipid oxidation (mg malonaldehyde/kg fish tissue) | AP | 19.46 ± 0.20c | NA | 0.03 ± 0.00 | 0.22 ± 0.00 | <0.0001 | 0.963 |
| OSP | 9.84 ± 0.09d | 20.91 ± 0.12c | 0.09 ± 0.01 | 0.14 ± 0.01 | <0.0001 | 0.960 | |
| AUV1 | 23.13 ± 0.18b | 28.88 ± 0.14b | 0.49 ± 0.01 | 0.17 ± 0.01 | <0.0001 | 0.934 | |
| OSUV1 | 10.11 ± 0.11d | 21.05 ± 0.20c | 0.07 ± 0.00 | 0.14 ± 0.01 | <0.0001 | 0.960 | |
| AUV3 | 27.99 ± 0.30a | 32.70 ± 0.10a | 0.76 ± 0.01 | 0.18 ± 0.01 | <0.0001 | 0.928 | |
| OSUV3 | 10.36 ± 0.10d | 21.07 ± 0.29c | 0.04 ± 0.00 | 0.14 ± 0.01 | <0.0001 | 0.963 | |
| Protein oxidation (nmol carbonyl/mg protein) | AP | 71.08 ± 1.67c | NA | 1.68 ± 0.16 | 0.58 ± 0.02 | <0.0001 | 0.971 |
| OSP | 43.78 ± 1.36d | 48.35 ± 0.83c | 1.71 ± 0.12 | 0.23 ± 0.01 | <0.0001 | 0.951 | |
| AUV1 | 88.09 ± 1.67b | 97.15 ± 2.35b | 3.60 ± 0.26 | 0.46 ± 0.02 | <0.0001 | 0.944 | |
| OSUV1 | 44.04 ± 1.85d | 48.78 ± 0.62c | 1.74 ± 0.13 | 0.23 ± 0.01 | <0.0001 | 0.948 | |
| AUV3 | 98.12 ± 2.65a | 117.20 ± 4.80a | 3.76 ± 0.29 | 0.58 ± 0.02 | <0.0001 | 0.956 | |
| OSUV3 | 44.71 ± 2.55d | 48.69 ± 1.10c | 1.80 ± 0.14 | 0.23 ± 0.01 | <0.0001 | 0.941 | |
Results are expressed as means ± standard deviation (n = 2). a,b,c,dDifferent superscripts in the same column indicate significant differences (p < 0.05) among treatments. ¥AUC – Area under curve; AUC0-13 – from day 0 to 13 among treatments AP, OSP, AUV1, OSUV1, AUV3, and OSUV3; AUC15-23 – from day 15 to 23 among treatments OSP, AUV1, OSUV1, AUV3, and OSUV3. NA – Not applicable. €AP (air packaging); OSP (oxygen scavenger packaging); AUV1 (air packaging + UV-C at 0.102 J/cm2); OSUV1 (oxygen scavenger packaging + UV-C at 0.102 J/cm2); AUV3 (air packaging + UV-C at 0.301 J/cm2); and OSUV3 (oxygen scavenger packaging + UV-C at 0.301 J/cm2).
AP had the highest (p < 0.05) total amounts of TVB-N and ammonia produced during the storage period (AUC), followed by tilapia fillets exposed to UV-C radiation alone, at both doses (AUV1 and AUV3), OSP alone, and OSP and UV-C in combination (OSUV1 and OSUV3; Table 3). These results agree with those obtained for bacterial growth parameters (Table 1) and free amino acids (Table 2), which are important substrates for ammonia formation34. Similarly, Bottino et al.18 and Monteiro et al.1 observed that, although the initial formation of TVB-N and ammonia in freshwater fish flesh was increased by UV-C radiation, it was still delayed during the storage period as a whole, compared to their control counterparts. The effectiveness of an O2 scavenger in reducing TVB-N and ammonia levels throughout refrigerated storage of fish species was also previously reported13–15,45.
Lipid and protein oxidation
An increase in the malonaldehyde (MDA) and carbonyl levels was observed during refrigerated storage in all treatments, especially in AUV1 and AUV3 (Table 3). The increases in lipid and protein oxidation by UV-C radiation were dose-dependent. AUV3 showed the highest (p < 0.05) MDA and carbonyl levels during the storage period, followed by AUV1, AP, and treatments with the O2 scavenger (OSP, OSUV1 and OSUV3), which did not differ from each other (p > 0.05; Table 3).
A concomitant lipid and protein oxidation has been described in literature6,9 and it was also observed in our study. Lipid and protein oxidation occur mainly in the presence of reactive oxygen species (ROS)6,53. Therefore, our findings may be attributed to pro-oxidant properties of the UV-C radiation6,17,54 and capacity of the O2 scavenger in minimizing ROS-induced oxidation45. In agreement with the results of this study, an increase in the MDA and carbonyl levels by UV-C radiation was observed during refrigerated storage of sea bass fillets19 and tilapia fillets4. Some previous studies also found that an O2 scavenger retarded the lipid oxidation of rainbow trout fillets14, fresh cobia13 and sardines15 stored under refrigeration; however, only limited information is available regarding the effect of O2 scavengers on protein oxidation of fish species.
Two milligrams of MDA/kg is considered the limit above which meat is unfit for human consumption55. AP, OSP, AUV1, OSUV1, AUV3 and OSUV3 exceeded this limit on days 9, 19, 6, 19, 5 and 17, respectively (Supplementary Table S2). In spite of the importance of protein oxidation to food quality, there are no regulatory limits on carbonyl levels in meat products. In our study, when the acceptable microbial limit of 7 log CFU/g was reached, the carbonyl levels were 6.47 ± 0.06, 5.12 ± 0.00, 10.68 ± 0.00, 5.61 ± 0.40, 13.12 ± 0.22 and 5.58 ± 0.40 nmol of carbonyl/mg of protein for AP, OSP, AUV1, OSUV1, AUV3 and OSUV3, respectively (Supplementary Table S2). These results indicate the effectiveness of the O2 scavenger in retarding oxidative processes, even when oxidation-inducing treatments were used.
Instrumental color measurements
Lightness (L*), redness (a*) and yellowness (b*) increased with the increasing storage period in all treatments (p < 0.05; Table 4). Throughout the entire storage period, no difference (p < 0.05) was found for L* values between treatments. AUV3 showed the highest (p < 0.05) a* and b* values during the entire storage period, followed by AUV1, AP, and treatments containing an O2 absorber (OSP, OSUV1 and OSUV3), which did not differ from each other (p > 0.05; Table 4). As in the lipid and protein oxidation, UV-C radiation increased the a* and b* values in a dose-dependent manner. The results of instrumental color parameters in all days of storage can be observed in Supplementary Table S3.
Table 4.
Instrumental color parameters of tilapia (Oreochromis niloticus) fillets non- and treated with oxygen scavenger packaging (OSP) and ultraviolet radiation (UV-C) stored at 4 ± 1 °C for 23 days.
| Parameters | Treatments€ | AUC¥ | Linear regression coefficients | ||||
|---|---|---|---|---|---|---|---|
| AUC¥0–13 | AUC¥15–23 | y-intercept | slope | p-value | r-squared | ||
| L* | AP | 739.10 ± 27.37a | NA | 54.36 ± 0.54 | 0.37 ± 0.08 | <0.0001 | 0.366 |
| OSP | 730.30 ± 20.98a | 476.40 ± 15.34a | 54.17 ± 0.53 | 0.30 ± 0.04 | <0.0001 | 0.469 | |
| AUV1 | 726.80 ± 38.90a | 481.20 ± 21.89a | 53.44 ± 0.72 | 0.37 ± 0.06 | <0.0001 | 0.423 | |
| OSUV1 | 723.50 ± 27.49a | 477.40 ± 16.77a | 53.40 ± 0.54 | 0.35 ± 0.04 | <0.0001 | 0.534 | |
| AUV3 | 719.80 ± 29.39a | 481.00 ± 14.66a | 53.01 ± 0.52 | 0.38 ± 0.04 | <0.0001 | 0.592 | |
| OSUV3 | 720.90 ± 25.06a | 476.90 ± 14.51a | 53.17 ± 0.50 | 0.35 ± 0.04 | <0.0001 | 0.578 | |
| a* | AP | 32.12 ± 1.80c | NA | 1.27 ± 0.06 | 0.19 ± 0.01 | <0.0001 | 0.916 |
| OSP | 25.17 ± 1.11d | 28.08 ± 1.48c | 1.24 ± 0.04 | 0.12 ± 0.00 | <0.0001 | 0.954 | |
| AUV1 | 38.35 ± 1.52b | 40.59 ± 1.10b | 1.71 ± 0.07 | 0.18 ± 0.01 | <0.0001 | 0.953 | |
| OSUV1 | 24.96 ± 1.46d | 28.33 ± 1.34c | 1.21 ± 0.04 | 0.12 ± 0.00 | <0.0001 | 0.955 | |
| AUV3 | 44.27 ± 1.87a | 46.77 ± 1.65a | 2.01 ± 0.07 | 0.21 ± 0.01 | <0.0001 | 0.956 | |
| OSUV3 | 25.35 ± 1.22d | 28.68 ± 0.64c | 1.23 ± 0.03 | 0.12 ± 0.00 | <0.0001 | 0.971 | |
| b* | AP | 84.33 ± 5.62c | NA | 4.27 ± 0.13 | 0.33 ± 0.02 | <0.0001 | 0.896 |
| OSP | 73.09 ± 4.04d | 63.33 ± 3.91c | 4.24 ± 0.09 | 0.20 ± 0.01 | <0.0001 | 0.928 | |
| AUV1 | 94.82 ± 4.35b | 81.84 ± 2.25b | 5.14 ± 0.14 | 0.28 ± 0.01 | <0.0001 | 0.913 | |
| OSUV1 | 73.42 ± 3.90d | 63.75 ± 1.68c | 4.27 ± 0.07 | 0.20 ± 0.00 | <0.0001 | 0.960 | |
| AUV3 | 104.50 ± 3.67a | 92.56 ± 3.00a | 5.57 ± 0.16 | 0.33 ± 0.01 | <0.0001 | 0.915 | |
| OSUV3 | 73.77 ± 2.72d | 63.70 ± 1.99c | 4.32 ± 0.07 | 0.19 ± 0.01 | <0.0001 | 0.953 | |
Results are expressed as means ± standard deviation (n = 2). a,b,c,dDifferent superscripts in the same column indicate significant differences (p < 0.05) among treatments. ¥AUC – Area under curve; AUC0-13 – from day 0 to 13 among treatments AP, OSP, AUV1, OSUV1, AUV3, and OSUV3; AUC15-23 – from day 15 to 23 among treatments OSP, AUV1, OSUV1, AUV3, and OSUV3. NA – Not applicable. €AP (air packaging); OSP (oxygen scavenger packaging); AUV1 (air packaging + UV-C at 0.102 J/cm2); OSUV1 (oxygen scavenger packaging + UV-C at 0.102 J/cm2); AUV3 (air packaging + UV-C at 0.301 J/cm2); and OSUV3 (oxygen scavenger packaging + UV-C at 0.301 J/cm2).
The increase in lightness has been reported previously in freshwater fish species stored under refrigeration4,56, and has been associated with changes in the reflectance of the meat surface due to protein denaturation, exposing hydrophobic groups57. On the other hand, the increase in the a* and b* values in refrigerated white fish species leads to darkening, which has been related to discoloration58. It occurs due to myoglobin autoxidation, where ferrous iron (Fe2+) is oxidized to ferric iron (Fe3+), resulting in the formation and accumulation of metmyoglobin (MetMb)8. MDA can also contribute to an increase in MetMb accumulation by inactivating the metmyoglobin-reducing system and/or by interacting with myoglobin molecules through covalent bonds, which alters their primary structure, making myoglobin more susceptible to redox reactions8,9. In this study, the increase in the a* and b* values agrees with and can be explained by our results for lipid and protein oxidation, including the differences found among the treatments. Similarly, Monteiro et al.4 and Park & Ha58 observed that UV-C radiation increased a* and b* values in tilapia fillets and fresh chicken breast, respectively, over the refrigerated period. Chounou et al.43 reported that an O2 absorber was effective in preventing discoloration in ground meat stored under refrigeration.
Instrumental texture parameters
Hardness, chewiness, cohesiveness, springiness and resilience decreased (p < 0.05) during the refrigerated period in all treatments (Table 5). OSP, OSUV1 and OSUV3 showed the highest (p < 0.05) hardness and chewiness, followed by samples submitted to air packaging (AP) and UV-C radiation at both doses (AUV1 and AUV3) during the storage period (Table 5). Cohesiveness, springiness and resilience were not affected (p > 0.05) by the O2 absorber and/or UV-C radiation, regardless of the dose, during the refrigerated storage period. The results of instrumental texture parameters in all days of storage can be found as Supplementary Table S4.
Table 5.
Instrumental texture parameters of tilapia (Oreochromis niloticus) fillets non- and treated with oxygen scavenger packaging (OSP) and ultraviolet radiation (UV-C) stored at 4 ± 1 °C for 23 days.
| Parameter | Treatments€ | AUC¥ | Linear regression coefficients | ||||
|---|---|---|---|---|---|---|---|
| AUC¥0–13 | AUC¥15–23 | y-intercept | slope | p-value | r-squared | ||
| Hardness (g) | AP | 40113.00 ± 507.00b | NA | 4248.24 ± 74.65 | −177.70 ± 10.92 | <0.0001 | 0.923 |
| OSP | 47535.00 ± 591.80a | 18273.00 ± 438.40b | 4570.65 ± 53.12 | −97.70 ± 4.63 | <0.0001 | 0.906 | |
| AUV1 | 35239.00 ± 326.30c | 12302.00 ± 211.70a | 3604.38 ± 71.26 | −112.82 ± 5.82 | <0.0001 | 0.906 | |
| OSUV1 | 47462.00 ± 249.30a | 17574.00 ± 288.50b | 4078.27 ± 35.60 | −102.03 ± 2.92 | <0.0001 | 0.965 | |
| AUV3 | 35751.00 ± 301.40c | 12012.00 ± 162.40a | 3651.05 ± 66.53 | −118.42 ± 5.52 | <0.0001 | 0.911 | |
| OSUV3 | 46852.00 ± 505.20a | 17344.00 ± 194.60b | 4103.58 ± 51.52 | −106.18 ± 4.20 | <0.0001 | 0.930 | |
| Chewiness (g × mm) | AP | 8987.00 ± 104.40b | NA | 1077.30 ± 55.60 | −56.51 ± 8.52 | <0.0001 | 0.647 |
| OSP | 9724.00 ± 126.90a | 3560.00 ± 86.95b | 1149.48 ± 40.09 | −33.96 ± 3.16 | <0.0001 | 0.724 | |
| AUV1 | 8166.00 ± 65.45c | 2932.00 ± 25.87a | 874.83 ± 40.14 | −28.63 ± 3.25 | <0.0001 | 0.648 | |
| OSUV1 | 9676.00 ± 76.26a | 3540.00 ± 15.95b | 994.77 ± 39.66 | −32.39 ± 3.45 | <0.0001 | 0.710 | |
| AUV3 | 8214.00 ± 65.28c | 2906.00 ± 39.60a | 889.79 ± 43.33 | −29.62 ± 3.47 | <0.0001 | 0.629 | |
| OSUV3 | 9686.00 ± 77.45a | 3501.00 ± 26.47b | 1021.49 ± 39.39 | −33.18 ± 3.11 | <0.0001 | 0.736 | |
| Cohesiveness (ratio) | AP | 5.22 ± 0.09a | NA | 0.459 ± 0.007 | −0.009 ± 0.001 | <0.0001 | 0.627 |
| OSP | 5.09 ± 0.09a | 2.50 ± 0.04a | 0.445 ± 0.005 | −0.007 ± 0.000 | <0.0001 | 0.845 | |
| AUV1 | 5.20 ± 0.08a | 2.57 ± 0.06a | 0.449 ± 0.005 | −0.007 ± 0.000 | <0.0001 | 0.821 | |
| OSUV1 | 5.33 ± 0.07a | 2.61 ± 0.06a | 0.459 ± 0.005 | −0.007 ± 0.000 | <0.0001 | 0.855 | |
| AUV3 | 5.35 ± 0.10a | 2.60 ± 0.07a | 0.463 ± 0.006 | −0.007 ± 0.000 | <0.0001 | 0.839 | |
| OSUV3 | 5.37 ± 0.09a | 2.55 ± 0.05a | 0.464 ± 0.005 | −0.008 ± 0.000 | <0.0001 | 0.863 | |
| Springiness (ratio) | AP | 7.01 ± 0.13a | NA | 0.596 ± 0.011 | −0.008 ± 0.002 | <0.0001 | 0.440 |
| OSP | 7.10 ± 0.12a | 3.71 ± 0.08a | 0.599 ± 0.008 | −0.007 ± 0.001 | <0.0001 | 0.701 | |
| AUV1 | 7.03 ± 0.07a | 3.64 ± 0.08a | 0.593 ± 0.007 | −0.007 ± 0.001 | <0.0001 | 0.766 | |
| OSUV1 | 7.10 ± 0.13a | 3.71 ± 0.09a | 0.596 ± 0.009 | −0.007 ± 0.001 | <0.0001 | 0.662 | |
| AUV3 | 7.14 ± 0.10a | 3.71 ± 0.04a | 0.599 ± 0.007 | −0.007 ± 0.001 | <0.0001 | 0.774 | |
| OSUV3 | 7.16 ± 0.10a | 3.66 ± 0.05a | 0.601 ± 0.007 | −0.007 ± 0.001 | <0.0001 | 0.780 | |
| Resilience (ratio) | AP | 1.80 ± 0.03a | NA | 0.169 ± 0.004 | −0.005 ± 0.001 | <0.0001 | 0.692 |
| OSP | 1.80 ± 0.03a | 0.77 ± 0.02a | 0.164 ± 0.003 | −0.004 ± 0.000 | <0.0001 | 0.854 | |
| AUV1 | 1.82 ± 0.02a | 0.77 ± 0.02a | 0.167 ± 0.002 | −0.004 ± 0.000 | <0.0001 | 0.889 | |
| OSUV1 | 1.83 ± 0.03a | 0.81 ± 0.01a | 0.168 ± 0.003 | −0.004 ± 0.000 | <0.0001 | 0.864 | |
| AUV3 | 1.83 ± 0.03a | 0.83 ± 0.02a | 0.166 ± 0.002 | −0.004 ± 0.000 | <0.0001 | 0.853 | |
| OSUV3 | 1.82 ± 0.02a | 0.82 ± 0.02a | 0.165 ± 0.002 | −0.003 ± 0.000 | <0.0001 | 0.859 | |
Results are expressed as means ± standard deviation (n = 2). a,b,c,dDifferent superscripts in the same column indicate significant differences (p < 0.05) among treatments. ¥AUC – Area under curve; AUC0-13 – from day 0 to 13 among treatments AP, OSP, AUV1, OSUV1, AUV3, and OSUV3; AUC15–23 – from day 15 to 23 among treatments OSP, AUV1, OSUV1, AUV3, and OSUV3. NA – Not applicable. €AP (air packaging); OSP (oxygen scavenger packaging); AUV1 (air packaging + UV-C at 0.102 J/cm2); OSUV1 (oxygen scavenger packaging + UV-C at 0.102 J/cm2); AUV3 (air packaging + UV-C at 0.301 J/cm2); and OSUV3 (oxygen scavenger packaging + UV-C at 0.301 J/cm2).
Softening during the post-mortem period is related to the activity of endogenous and microbial proteolytic enzymes, which results in protein breakdown59. The results for hardness and chewiness in this study can be explained by the results for free amino acids, MDA level, carbonyl content, and TAPC. The pro-oxidant effect of the UV-C radiation increased the amount of free amino acids, indicating a higher proteolysis rate, while ROS formation at 0.102 and 0.301 J/cm2 was mitigated by O2 absorber. Furthermore, when compared to OSP, OSUV1 and OSUV3, both UV-C doses were less effective against growth of aerobic psychrotrophic bacteria, where Pseudomonas spp. is the dominant proteolytic spoilage bacteria in freshwater fish species34. There are no studies related to instrumental texture parameters in fish species packed with an O2 scavenger. However, in agreement with our study, Monteiro et al.7 reported that a similar UV-C dose decreased the hardness and chewiness but did not affect the cohesiveness and springiness of tilapia fillets stored under refrigeration. Molina et al.19 also observed that UV‐C treatment increased collagen degradation in sea bass fillets.
Conclusion
The O2 scavenger, both UV-C doses (0.102 and 0.301 J/cm2) and combinations of these preservation methods, independently of the radiation dose, retarded the bacterial growth and the formation of TVB-N and ammonia, increasing the shelf life of refrigerated tilapia fillets by more than 50%, 60% and 70%, respectively. While UV-C doses induced adverse changes in the color, texture and oxidative processes, O2 scavenger demonstrated to be an effective and simple alternative to reduce the negative effects of UV-C radiation. Therefore, the O2 scavenger combined with UV-C radiation, regardless of the dose (0.102 or 0.301 J/cm2), was the most effective method to extend the shelf life and retard the loss of physicochemical quality of tilapia fillets stored under refrigeration.
Supplementary information
Acknowledgements
The authors are thankful for the financial support provided by the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), grant numbers E-26/202.305/2017; E-26/202.306/2017 and E-26/010.101.007/2018; and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) grant number 406777/2018-7. Furthermore, this study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. The authors also thank to Mitsubishi Gas Chemical Corporation for providing the oxygen absorbers (Ageless SS-50).
Author contributions
M.L.G.M., E.T.M. and C.A.C.J. designed the experiment. M.L.G.M., Y.S.M., V.S.C., R.V.B.P.M. and T.S.A. performed the experiments. M.L.G.M. wrote the main manuscript text. All authors revised the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-61293-8.
References
- 1.Monteiro MLG, et al. Combined effect of high hydrostatic pressure and ultraviolet radiation on quality parameters of refrigerated vacuum-packed tilapia (Oreochromis niloticus) fillets. Sci. Rep. 2018;8:e9524. doi: 10.1038/s41598-018-27861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ocaño-Higuera VM, et al. Freshness assessment of ray fish stored in ice by biochemical, chemical and physical methods. Food Chem. 2011;125:49–54. doi: 10.1016/j.foodchem.2010.08.034. [DOI] [Google Scholar]
- 3.FAO. The State of World Fisheries and Aquaculture – Meeting the Sustainable Development Goals http://www.fao.org/3/i9540en/I9540EN.pdf (2018).
- 4.Monteiro MLG, et al. Impact of UV-C light on the fatty acid profile and oxidative stability of Nile tilapia (Oreochromis niloticus) fillets. J. Food Sci. 2017;82:1028–1036. doi: 10.1111/1750-3841.13685. [DOI] [PubMed] [Google Scholar]
- 5.USDA. National Nutrient Databasehttps://ndb.nal.usda.gov/ndb (2018).
- 6.Kumar Y, Yadav DN, Ahmad T, Narsaiah K. Recent trends in the use of natural antioxidants for meat and meat products. Compr. Rev. Food Sci. Food Saf. 2015;14:796–812. doi: 10.1111/1541-4337.12156. [DOI] [Google Scholar]
- 7.Monteiro MLG, Mársico ET, Rosenthal A, Conte‐Junior CA. Synergistic effect of ultraviolet radiation and high hydrostatic pressure on texture, color, and oxidative stability of refrigerated tilapia fillets. J. Sci. Food Agric. 2019;99:4474–4481. doi: 10.1002/jsfa.9685. [DOI] [PubMed] [Google Scholar]
- 8.Suman SP, Joseph P. Myoglobin chemistry and meat color. Annu. Rev. Food Sci. Technol. 2013;4:79–99. doi: 10.1146/annurev-food-030212-182623. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z, He Z, Emara AM, Gan X, Li H. Effects of malondialdehyde as a byproduct of lipid oxidation on protein oxidation in rabbit meat. Food Chem. 2019;288:405–412. doi: 10.1016/j.foodchem.2019.02.126. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed I, et al. A comprehensive review on the application of active packaging technologies to muscle foods. Food Control. 2017;82:163–178. doi: 10.1016/j.foodcont.2017.06.009. [DOI] [Google Scholar]
- 11.Rodrigues BL, et al. Influence of vacuum and modified atmosphere packaging in combination with UV-C radiation on the shelf life of rainbow trout (Oncorhynchus mykiss) fillets. Food Control. 2016;60:596–605. doi: 10.1016/j.foodcont.2015.09.004. [DOI] [Google Scholar]
- 12.Janjarasskul T, Suppakul P. Active and intelligent packaging: The indication of quality and safety. Crit. Rev. Food Sci. Nutr. 2018;58:808–831. doi: 10.1080/10408398.2016.1225278. [DOI] [PubMed] [Google Scholar]
- 13.Remya S, Mohan CO, Venkateshwarlu G, Sivaraman GK, Ravishankar CN. Combined effect of O2 scavenger and antimicrobial film on shelf life of fresh cobia (Rachycentron canadum) fish steaks stored at 2 °C. Food Control. 2017;71:71–78. doi: 10.1016/j.foodcont.2016.05.038. [DOI] [Google Scholar]
- 14.Mexis SF, Chouliara E, Kontominas MG. Combined effect of an oxygen absorber and oregano essential oil on shelf life extension of rainbow trout fillets stored at 4 °C. Food Microbiol. 2009;26:598–605. doi: 10.1016/j.fm.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Mohan CO, Abin J, Kishore P, Panda SK, Ravishankar CN. Effect of vacuum and active packaging on the biochemical and microbial quality of indian oil sardine (Sardinella longiceps) during iced storage. J. Packag. Technol. Res. 2019;3:43–55. doi: 10.1007/s41783-018-00053-6. [DOI] [Google Scholar]
- 16.Byelashov, O. A. & Sofos, J. N. Strategies for on-line decontamination of carcasses in Safety of meat and processed meat (ed Toldrá F.) 149–182 (Springer, 2009).
- 17.Koutchma, T., Forney, L. & Moraru, C. Principles and applications of UV technology in Ultraviolet light in food technology (eds. Koutchma T., Forney L. & Moraru C) 1–32 (CRC Press, 2009).
- 18.Bottino FO, Rodrigues BL, Ribeiro JDN, Lazaro CA, Conte-Junior CA. Influence of UV-C radiation on shelf life of vacuum package tambacu (Colossoma macropomum × Piaractus mesopotamicus) fillets. J. Food Process. Preserv. 2017;41:e13003. doi: 10.1111/jfpp.13003. [DOI] [Google Scholar]
- 19.Molina B, Sáez MI, Martínez TF, Guil-Guerrero JL, Suárez MD. Effect of ultraviolet light treatment on microbial contamination, some textural and organoleptic parameters of cultured sea bass fillets (Dicentrarchus labrax) Innov. Food Sci. Emerg. Technol. 2014;26:205–213. doi: 10.1016/j.ifset.2014.07.002. [DOI] [Google Scholar]
- 20.Lázaro CA, et al. Effects of ultraviolet light on biogenic amines and other quality indicators of chicken meat during refrigerated storage. Poult. Sci. 2014;93:2304–2313. doi: 10.3382/ps.2013-03642. [DOI] [PubMed] [Google Scholar]
- 21.Baranyi J, Roberts TA. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994;23:277–294. doi: 10.1016/0168-1605(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 22.APHA. Compendium of methods for the microbiological examination of foods (4th ed.). Washington DC, USA: American Public Health Association (2001).
- 23.Gatti R, Gioia MG, Leoni A, Andreani A. 2,5-Dimethyl-1H-pyrrole-3,4-dicarbaldehyde as a precolumn derivatization reagent for HPLC/UV detection of amino acids. J. Pharm. Biomed. Anal. 2010;53:207–211. doi: 10.1016/j.jpba.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 24.Lázaro CA, et al. Validation of an HPLC methodology for the identification and quantification of biogenic amines in chicken meat. Food Anal. Method. 2013;6:1024–1032. doi: 10.1007/s12161-013-9565-0. [DOI] [Google Scholar]
- 25.Conway EJ, Byrne A. An absorption apparatus for the micro-determination of certain volatile substances I. The micro-determination of ammonia. J. Biochem. 1933;27:419–429. [PMC free article] [PubMed] [Google Scholar]
- 26.Yin MC, Faustman C, Riesen JW, Williams SN. α‐Tocopherol and ascorbate delay oxymyoglobin and phospholipid oxidation in vitro. J. Food Sci. 1993;58:1273–1276. doi: 10.1111/j.1365-2621.1993.tb06164.x. [DOI] [Google Scholar]
- 27.Joseph P, Suman SP, Rentfrow G, Li S, Beach CM. Proteomics of muscle-specific beef color stability. J. Agric. Food Chem. 2012;60:3196–3203. doi: 10.1021/jf204188v. [DOI] [PubMed] [Google Scholar]
- 28.Oliver CN, Ahn BW, Moerman EJ, Goldstein S, Stadtman ER. Age-related changes in oxidized proteins. J. Biol. Chem. 1987;262:5488–5491. [PubMed] [Google Scholar]
- 29.Armenteros M, Heinonen M, Ollilainen V, Toldra F, Estevez M. Analysis of protein carbonyls in meat products by using the DNPH-method, fluorescence spectroscopy and liquid chromatography–electrospray ionisation–mass spectrometry (LC–ESI–MS) Meat Sci. 2009;83:104–112. doi: 10.1016/j.meatsci.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Mercier Y, Gatellier P, Viau M, Remignon H, Renerre M. Effect of dietary fat and vitamin E on colour stability and on lipid and protein oxidation in turkey meat during storage. Meat Sci. 1998;48:301–318. doi: 10.1016/S0309-1740(97)00113-7. [DOI] [PubMed] [Google Scholar]
- 31.AMSA. Meat color measurement guidelines (2nd ed.). Champaign IL, USA: American Meat Science Association (2012).
- 32.Sun T, Hao W, Li J, Dong Z, Wu C. Preservation properties of in situ modified CaCO3–chitosan composite coatings. Food Chem. 2015;183:217–226. doi: 10.1016/j.foodchem.2015.03.036. [DOI] [PubMed] [Google Scholar]
- 33.ICMSF. Microorganisms in foods (2nd ed.). New York NY, USA: Kluwer Academic/Plenum Publishers (2001).
- 34.Gram L, Huss HH. Microbiological spoilage of fish and fish products. Int. J. Food Microbiol. 1996;33:121–137. doi: 10.1016/0168-1605(96)01134-8. [DOI] [PubMed] [Google Scholar]
- 35.Molinari LM, et al. Bacterial microflora in the gastrointestinal tract of Nile tilapia, Oreochromis niloticus, cultured in a semi-intensive system. Acta Sc. Biol. Sci. 2003;25:267–271. [Google Scholar]
- 36.Pakingking R, Palma P, Usero R. Quantitative and qualitative analyses of the bacterial microbiota of tilapia (Oreochromis niloticus) cultured in earthen ponds in the Philippines. World J. Microbiol. Biotechnol. 2015;31:265–275. doi: 10.1007/s11274-014-1758-1. [DOI] [PubMed] [Google Scholar]
- 37.Jagger, J. Physiological effects of near-ultraviolet radiation on bacteria in Photochemical and photobiological reviews (ed. Smith, K. C.) 1–75 (Springer, 1983).
- 38.Pedrós-Garrido S, et al. Efficacy of ultraviolet light (UV-C) and pulsed light (PL) for the microbiological decontamination of raw salmon (Salmo salar) and food contact surface materials. Innov. Food Sci. Emerg. Technol. 2018;50:124–131. doi: 10.1016/j.ifset.2018.10.001. [DOI] [Google Scholar]
- 39.Said MB, Daly I, Nasr H, Hassen A. Monitoring of biofilm production by Pseudomonas aeruginosa strains under different conditions of UVC irradiation and phage infection. Ann. Microbiol. 2013;63:433–442. doi: 10.1007/s13213-012-0487-7. [DOI] [Google Scholar]
- 40.López-Caballero ME, Gonçalves A, Nunes ML. Effect of CO2/O2-containing modified atmospheres on packed deepwater pink shrimp (Parapenaeus longirostris) Eur. Food Res. Technol. 2002;214:192–197. doi: 10.1007/s00217-001-0472-z. [DOI] [Google Scholar]
- 41.Izumi H, Inoue A. Viability of sublethally injured coliform bacteria on fresh-cut cabbage stored in high CO2 atmospheres following rinsing with electrolyzed water. Int. J. Food Microbiol. 2018;266:207–212. doi: 10.1016/j.ijfoodmicro.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 42.Li J, Kolling GL, Matthews KR, Chikindas ML. Cold and carbon dioxide used as multi‐hurdle preservation do not induce appearance of viable but non‐culturable Listeria monocytogenes. J. Appl. Microbiol. 2003;94:48–53. doi: 10.1046/j.1365-2672.2003.01795.x. [DOI] [PubMed] [Google Scholar]
- 43.Chounou N, et al. Shelf life extension of ground meat stored at 4 °C using chitosan and an oxygen absorber. Int. J. Food Sci. Tech. 2013;48:89–95. doi: 10.1111/j.1365-2621.2012.03162.x. [DOI] [Google Scholar]
- 44.Ordóñez JA, Hierro E, Bruna EM, Hoz L. Changes in the components of dry‐fermented sausages during ripening. Crit. Rev. Food Sci. Nutr. 1999;39:329–367. doi: 10.1080/10408699991279204. [DOI] [PubMed] [Google Scholar]
- 45.Mohan CO, Ravishankar CN, Gopal TKS, Kumar KA. Nucleotide breakdown products of seer fish (Scomberomorus commerson) steaks stored in O2 scavenger packs during chilled storage. Innov. Food Sci. Emerg. Technol. 2009;10:272–278. doi: 10.1016/j.ifset.2008.11.012. [DOI] [Google Scholar]
- 46.Silla Santos MH. Biogenic amines: their importance in foods. Int. J. Food Microbiol. 1996;29:213–231. doi: 10.1016/0168-1605(95)00032-1. [DOI] [PubMed] [Google Scholar]
- 47.Lázaro CA, et al. Biogenic amines as bacterial quality indicators in different poultry meat species. LWT – Food Sci. Technol. 2015;60:15–21. doi: 10.1016/j.lwt.2014.09.025. [DOI] [Google Scholar]
- 48.Fan SW, Srivastava AK, Dravid VP. UV-activated room-temperature gas sensing mechanism of poly crystalline ZnO. Appl. Phys. Lett. 2009;95:106–142. [Google Scholar]
- 49.Nashalian O, Yaylayan VA. Thermally induced oxidative decarboxylation of copper complexes of amino acids and formation of strecker aldehyde. J. Agric. Food Chem. 2014;62:8518–8523. doi: 10.1021/jf502751n. [DOI] [PubMed] [Google Scholar]
- 50.Mohan CO, Ravishankar CN, Gopal TKS, Kumar KA, Lalitha KV. Biogenic amines formation in seer fish (Scomberomorus commerson) steaks packed with O2 scavenger during chilled storage. Food Res. Int. 2009;42:411–416. doi: 10.1016/j.foodres.2009.01.015. [DOI] [Google Scholar]
- 51.Commission of the European Community Decision 95/149/EC of 8 March 1995 fixing the total volatile basic nitrogen (TVB-N) limit values for certain categories of fishery products and specifying the analysis methods to be used. OJEC L. 1995;097:84–87. [Google Scholar]
- 52.Baliño-Zuazo L, Barranco A. A novel liquid chromatography–mass spectrometric method for the simultaneous determination of trimethylamine, dimethylamine and methylamine in fishery products. Food Chem. 2016;196:1207–1214. doi: 10.1016/j.foodchem.2015.09.086. [DOI] [PubMed] [Google Scholar]
- 53.Stadtman ER, Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25:207–218. doi: 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- 54.Chan WH, Wu CC, Yu JS. Curcumin inhibits UV irradiation-induced oxidative stress and apoptotic biochemical changes in human epidermoid carcinoma A431 cells. J. Cell. Biochem. 2003;90:327–338. doi: 10.1002/jcb.10638. [DOI] [PubMed] [Google Scholar]
- 55.Campo MM, et al. Flavour perception of oxidation in beef. Meat Sci. 2006;72:303–311. doi: 10.1016/j.meatsci.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 56.Santos JSL, et al. Effect of the UV-C radiation on shelf life of vacuum-packed refrigerated pirarucu (Arapaima gigas) fillets. J. Aquat. Food Prod. Technol. 2018;27:48–60. doi: 10.1080/10498850.2017.1402840. [DOI] [Google Scholar]
- 57.Zhang Z, Yang Y, Tang X, Chen Y, You Y. Chemical forces and water holding capacity study of heat-induced myofibrillar protein gel as affected by high pressure. Food Chem. 2015;188:111–118. doi: 10.1016/j.foodchem.2015.04.129. [DOI] [PubMed] [Google Scholar]
- 58.Park SY, Ha SD. Ultraviolet-C radiation on the fresh chicken breast: Inactivation of major foodborne viruses and changes in physicochemical and sensory qualities of product. Food Bioprocess Tech. 2015;8:895–906. doi: 10.1007/s11947-014-1452-1. [DOI] [Google Scholar]
- 59.Rodrigues BL, et al. Instrumental texture parameters as freshness indicators in five farmed Brazilian freshwater fish species. Food Anal. Method. 2017;10:3589–3599. doi: 10.1007/s12161-017-0926-y. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.