Abstract
Lately, cellular-based cartilage joint therapies have gradually gained more attention, which leads to next generation bioengineering approaches in the development of cell-based medicinal products for human use in cartilage repair. The greatest hurdles of chondrocyte-based cartilage bioengineering are: (i) preferring the cell source; (ii) differentiation and expansion processes; (iii) the time necessary for chondrocyte expansion pre-implantation; and (iv) fixing the chondrocyte count in accordance with the lesion surface area of the patient in question. The chondrocyte presents itself to be the focal starting material for research and development of bioengineered cartilage-based medicinal products which promise the regeneration and restoration of non-orthopedic cartilage joint defects. Even though chondrocytes seem to be the first choice, inevitable complications related to proliferation, dedifferentation and redifferentiation are probable. Detailed studies are a necessity to fully investigate detailed culturing conditions, the chondrogenic strains of well-defined phenotypes and evaluation of the methods to be used in biomaterial production. Despite a majority of the current methods which aid amelioration of joint functionality, they are insufficient in fully restoring the natural structure and composition of the joint cartilage. Hence current studies have trended towards gene therapy, mesenchymal stem cells and tissue engineering practices. There are many studies addressing the outcomes of chondrocytes in the clinical scene, and many vital biomaterials have been developed for structuring the bioengineered cartilage. This study aims to convey to the audience the practical significance of chondrocyte-based clinical applications.
Keywords: Autologous chondrocyte implantation, Cartilage bioengineering, Cartilage isolation, Chondrocyte, Chondrocyte characterization, Chondrocyte isolation
Introduction
Chondrocytes—comprising only a 10%-volume of the cartilage—are cells which assist tissue articulation. These cells also mechanically support the cartilage by protecting the extracellular matrix (ECM). They provide synthesis of glycoproteins, collagen, proteoglycans and hyalurons (Archer and Francis-West 2003). Autologous chondrocytes are the main source of chondrocyte lines. Nevertheless, the differentiation potential of mesenchymal stem cells (MSCs) provide an alternative for cell line sources of chondrocytes. By the help of growth and transcription factors, MSCs first differentiate to chondrocyte progenitor cells, then to chondroprogenitor cells, chondroblasts, and finally chondrocytes. Chondrogenesis is regulated by the Sox9 transcription factor which is expressed during the condensation of mesenchymal progenitor cells. This causes the formation of immature chondrocytes (Kozhemyakina et al. 2015). Chondrocytes mature by the continuous Sox9 expression, yielding hypertrophic chondrocytes (Cooper et al. 2013). Mature chondrocytes end up with a long mitochondria, enlarged Golgi region, and basophilic cytoplasm in the healthy cartilage (Lin et al. 2006). Articular cartilage is located on the surface of the diarthrodial joints of the knee, and contains more than 98% of extracellular matrix (ECM). The ECM contains approximately 90% of collagen type II (Sopena Juncosa et al. 2000).
Joint cartilage loss is characterized by the cleavage of collagen and proteoglycan portions of the ECM via the role of cytokines and growth factors, and the changes in biomechanics and cellular responses. This can lead to seriously debilitating degenerative pathologies such as osteoarthritis. When the activity is studied at the level of genes, matrix metalloproteinases (MMPs), disintegrin (ADAM-TS4) and a thrombospondin motif metalloproteinase (ADAM-TS5) are found responsible (Lin et al. 2006; Mobasheri et al. 2014; Waters et al. 2014). The first time that the structure, function and diseases of the joint cartilage and the problems in ameliorating the synovial joints were studied, was expressed by William Hunter in 1743: “If we were to gather all information from surgeons who lived from the time of Hippocrates until today, we would find that ulcerated cartilage is absolutely bound to lead to a severe disorder in the future and once corrupted, can never be regenerated” (Hunter 1995). In the years that followed Hunter’s evaluation, experimental and clinical studies revealed that cartilage defects are not fully recovered or partially recovered by the fibrous tissue, and that, opposed to the bone tissue, the joint cartilage is not able to self-regenerate into its original state. During nearly three centuries after 1743, the effort for finding the actual cure to such pathologies has exponentially risen and is continuously proceeding ever since.
Treatment of joint cartilage defects are yet a difficult issue due to the limited and controversial regeneration potential. Joint surgery is an area of orthopedic and traumatologic surgical interventions in which novel treatment approaches are still being studied. Recently, the focus is on forming structures that allow similarity to the native bone-cartilage interface (Deng et al. 2016). Well-established microfracture and mosaicplasty surgeries already being applied extensively, autologous chondrocyte implantation (ACI) applications are also progressing (Ochi et al. 2001; Marcacci et al. 2007; Wakitani et al. 2008; Adkisson et al. 2010; Baugé and Boumédiene 2015; Atilla et al. 2018). ACI has pioneered the development of regenerative cartilage medicine since the late 1980s: first in rabbits in 1987 and first in humans in 1994 (Brittberg et al. 1994). Afterwards, the U.S. Food and Drug Administration (FDA) approved the autologous cultured chondrocyte—Carticel®: Genzyme Biosurgery, Cambridge, MA, USA—in 1997 for the repair of cartilage defects (Zhang et al. 2016). A detailed list of other cell-based, tissue engineered autologous chondrocyte therapies which were are either currently on the market, were reluctant to reach the market or were withdrawn can be found in “Appendix 1”.
The main aim of treatment approaches in cartilage lesions is to achieve the histological, functional and biomechanical properties of the original hyaline cartilage, and thus intend retention of the healthy cartilage tissue. Even though current treatment options intended to repair cartilage degeneration are satisfactory, there is crucial need for developing an ideal protocol encompassing cells, biomaterials and the tissue engineering technology. As there is a massive amount of cells wasted during tissue engineering studies, simple and affordable isolation techniques can be an extremely supportive factor and may be able to speed up the translation of processes into the clinical setting. Isolated chondrocytes may be used as the active component of ACIs with the aim of cartilage regeneration. Such cells are regarded as precious models for maintaining the cartilage phenotype along with exploring the magnitude of cellular response resulting from changes in the nature of the cell and the cartilage tissue (Naranda et al. 2017). However, differences between isolation methods by means of unsafe neocartilage formation related to low cell counts and disqualified cells are occasionally observed (Oseni et al. 2013).
Injuries and other complications, including the cell source of chondrocyte and dedifferentiation, are the main questions to be solved (Baugé and Boumédiene 2015). For instance, the best atmosphere for MSC differentiation without a chondrocyte-to-fibroblast differentiation is indicated (Calus et al. 2010; Nukavarapuet and Amini 2011; Amini et al. 2012; Danisovic et al. 2012; Nukavarapu and Dorcemus 2013). Demonstrating which type of cells have the better ability to regenerate tissues is a debate. The cell source alone is not sufficient. The presence of growth factors is suggested for promotion of cell proliferation (Angele et al. 1999; Wang et al. 2009; Im and Lee 2010). As all of these treatments have their disadvantages besides their advantages, it is still not certain which one is the absolute treatment option in cartilage defects. This set aside, as Benya and Schaffer (1982) pinpointed in the early 1980s, the native cartilage is still difficult and, moreover, impossible to be tailored in the laboratory setting.
Joint cartilage injuries are yet an evolving field of orthopedic surgery which causes problems in joint surgery due to limited and conflicted potential recovery, and for which novel treatment approaches are studied. Even though successful methods such as microfractures, osteoarticular autograft transfers, mosaicplasty and ACI exist today, a gold standard has yet to be reported due to the limitations specific to each method. Despite a majority of the current methods which aid amelioration of joint functionality, they are insufficient in fully restoring the natural structure and composition of the joint cartilage. Hence current studies have trended towards gene therapy, MSCs and tissue engineering practices. There are many studies addressing the outcomes of chondrocytes in the clinical scene, and many vital biomaterials have been developed for structuring the bioengineered cartilage. This study aims to convey to the audience the practical significance of chondrocyte-based clinical applications.
Current treatment options for cartilage injuries
Whatever the applied method, the worldwide consensus in regeneration of the cartilage joint lesions is that a hyaline cartilage structure formation is vital (Wakitani et al. 2008). The nearest clinical results to the intent with regeneration in the presence of the hyaline cartilage is either osteochondral autograft transfer or ACI of cartilage-like tissue engineered products. Yet, the main intent has not been achieved. Hence research continues as regards exploring different cellular sources, growth factor components and genetic modification techniques (Ochi et al. 2001).
Palliative treatment methods only provide temporary and partial cartilage amelioration whereas reparative methods present certain biological materials which have physiological action potential. Due to the unique nature of the cartilage, bone and cartilage-bone interface, regenerative and restorative treatment methods manifest as the most ideal outcomes. Cartilage tissue engineering is the fastest evolving field of biomedical engineering, where chondrocytes are most frequently translated from in vitro models to the clinic (Oseni et al. 2013). Cartilage regeneration and restoration with human medicinal products is thus on the agenda as the next generation studies of cartilage tissue engineering. Table 1 shows the current status of clinical approaches to ameliorating cartilage lesions.
Table 1.
The current clinical approaches to ameliorating cartilage lesions
| Non-therapeutic approaches | Therapeutic approaches |
|---|---|
|
Palliative treatment methods (open, arthroscopic interventions) Arthroscopic debridement Arthroplasty Chondroplasty |
Chondrocyte-based regenerative/restorative medicine applications Cell therapy medicinal products Manufactured from different cell sources Matrix-induced tissue engineering products Gene therapy medicinal products |
|
Reparative treatment methods Microfracture Mosaicplasty |
Although there are still surgical, non-therapeutic approaches, clinicians’ preferences have lately trended towards therapeutic approaches as chondrocyte-based next generation therapeutic approaches continue to emerge
Chondrocyte-based medicinal products in regenerative/restorative medicine
Cell therapy medicinal products manufactured from different cell sources
Chondrocytes
Autologous chondrocyte implantation (ACI)
In recent years, ACI has become a clinically approved procedure for cartilage defects, especially when marrow stimulation techniques fail to generate good clinical results (Jones and Peterson 2006). ACI is primitively performed by embedding chondrocytes isolated and cultured from healthy cartilage onto defected cartilage covered with periosteum.
Advantages of such a method is that (i) cell viability is continuous throughout the graft, (ii) there is no need for allogeneic tissue transplantation, and (iii) there is no donor site morbidity. Disadvantages are that (i) there is a need for gradual surgical intervention, open surgery and transfer in between the hospital and the laboratory, (ii) the recovery period is lengthy, and (iii) such an implementation is quite costly. Additionally, 26% of the complications is related to arthrotomy. The most frequently experienced complication is intraarticular adhesion.
ACI provides hyaline-like cartilage containing collagen type II. The long term clinical success rate is approximately 85%. Numerous techniques for the enrichment of chondrocyte cultures have been studied yet with obstacles encountered in the formation of homogenous and natural cartilage in vivo (Ishibashi et al. 2017). This treatment alternative involves a single-layered chondrocyte expansion phase which results in differentiated phenotype loss. The biggest issue is that chondrocytes tend to transform into fibroblasts during the culturing process (Gosset et al. 2008). Thus in spite of increased quality of life in patients, the newly formed cartilage is actually fibrocartilage. Ideally, a successful ACI is expected to contain active and phenotypically stable chondrocytes related to the specific physiology of the chondrocytes in vivo (Demoor et al. 2014). There is a necessity for a high viable chondrocyte cell count for a wider clinical use (Oseni et al. 2013).
With regard to sufficient cell count along with the maintenance of a viable cell population which is able to proliferate and provide matrix formation, researchers may come across some problems in the optimization of cell isolation. This way, typical cell populations yielded from the cartilage digestion product differ from one donor to the other and also according to the level of experience of the operator (Vedicherla and Buckley 2017).
Adult nasal chondrocytes
During first studies involving the chondrogenic features of cartilage, cells sourced from adult nasal cartilage were found to have a greater capacity for collagen type II and glycosaminoglycan (GAG) production than articular chondrocytes (Kafienah et al. 2002). In recent years, adult nasal chondrocytes have promised a higher proliferative potential than adult knee chondrocytes (Shafiee et al. 2016). In the first-in-human clinical trial of nasal chondrocyte-based autologous engineered product for articular cartilage defects, Mumme et al. (2016) showed that cartilage repair occurred with rich collagen and GAG production (Mumme et al. 2016). This suggests that nasal chondrocytes might majorly contribute to a high level of repair by producing a native hyaline cartilage. Adult nasal chondrocytes are also clinically superior to other cartilage sources, owing to its ability to not require interference with already compromised cartilage to enable repair.
Cartilage is not the only source from which chondrocytes can be obtained. Figure 1 consolidates the different cell sources from which chondrocytes can be yielded.
Fig. 1.
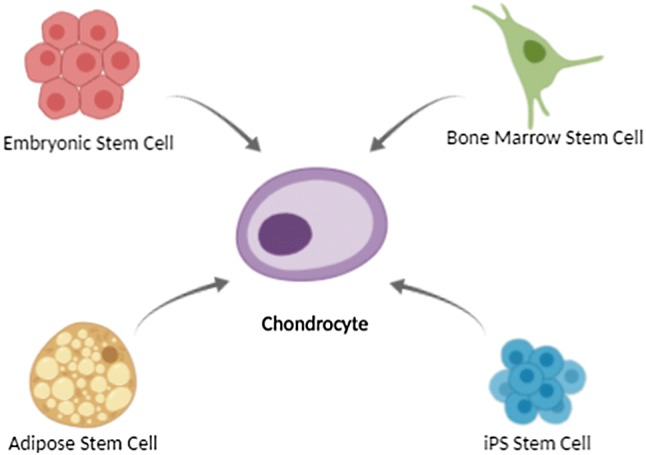
Different cell sources with the ability to differentiate into chondrocytes
MSC differentiation into chondrocytes
With their high proliferation rate, MSCs seem superior to chondrocytes. This is an advantage as MSCs substantially tend to differentiate towards chondrocytes. MSCs are also much easier to harvest. The ECM macromolecules are involved in cartilage function—i.e., fibronectin, collagen and GAGs—as well as cytokines, growth factors, chemokines, and colony stimulating factors (Somoza et al. 2014).
Synovium-derived MSCs
The synovium has proven to effectively induce chondrogenesis and yield high quality cartilage, in vitro (Jones and Peterson 2006; Pei et al. 2008) and in vivo (Pei et al. 2009). Among MSCs from all tissues, synovium-derived MSCs are more effective in their chondrogenic potential. Sakaguchi et al. (2005) explained that synovium-derived MSCs generated larger pellets and a more structured ECM consistent with toluidine blue cartilage matrix staining, concluding that these are a superior source for chondrogenesis in comparison with adipose tissue.
The ECM deposited by synovium-derived MSCs overcome chondrocyte dedifferentiation and redifferentiation (Pei and He 2012).The cartilage matrix secreted in this scenario is rich in collagen type II and aggrecan but not collagen type I or collagen-X, and is mechanically similar to articular cartilage (Zhou et al. 2014).
Adipose-derived MSCs
Adipose-derived MSCs might be suitable as they are easy to harvest, easy to obtain from fat, and stable when cultured (D’Andrea et al. 2008). In treatment of osteoarthritis, adipose tissue is safer than bone marrow due to its lower risks of complications (Koh and Choi 2012; Koh et al. 2013). The chondrogenic potency of adipose-based MSCs is comparable with that of bone marrow-derived MSCs and chondrocytes (Garcia et al. 2016). With the aid of a three-dimensional (3D) gel scaffold, adipose-derived autologous MSCs can produce hyaline-like cartilage in osteoarthritis patients (Onofrillo et al. 2018), and they also promise to function in animals (Jones et al. 2018; Song et al. 2018).
Bone marrow-derived MSCs
MSCs from bone marrow can differentiate into multiple lineages—i.e., to osteoblasts, adipocytes and chondrocytes—providing an ideal source for cartilage repair and regeneration (Dominici et al. 2006). The safety of bone-marrow derived-MSCs has been well established since the time when Watkitani et al. (1994) performed the first in-patient trial in 1988 by implanting autologous bone marrow derived-MSCs to repair articular cartilage, and studies repeated thereafter (Wakitani et al. 2011; Jo et al. 2014; Vega et al. 2015). Co-culture of chondrocytes with bone marrow derived-MSCs result in ECM production more than that of chondrocyte mono-cultures (Lai et al. 2013; de Windt et al. 2015; Owida et al. 2017).
Bone marrow derived-MSCs are one of the most preferable cellular therapeutic tissue engineering products in cartilage defects, especially in osteoarthritis (Davatchi et al. 2011; Zhang et al. 2016). MSCs first revealed their effectiveness in pain relief and are currently the focus for cartilage repair. Even though, harvesting cells is difficult and painful and may pose risks of complications. Effort continues in translational research for justifying the MSCs’ age of consent (Zhang et al. 2016). Bone marrow-derived human MSCs can differentiate into bone, fat, tendon, muscle, marrow stroma and, of course, cartilage. Despite this extensive ability, applications requiring a large number of cells can not always be afforded with MSCs. As MSCs expand, phenotypical changes and spontaneous transformation occur. MSC numbers naturally decline by age, the proliferation and differentiation capacities also decrease. Embryonic stem cells (ESCs) are a possible option to substitute for MSCs, where they lack performance (Cheng et al. 2014).
Human embryonic stem cells (hESCs) and inducable pluripotent stem cells (iPSCs)
Since its first discovery in 1998, hESCs are known to differentiate into multiple cell types (Thomson et al. 1998). In some countries, researchers have developed protocols to manipulate ESCs towards chondrogenesis (Oldershaw et al. 2010; Cheng et al. 2014). It is not a surprise to see that hESCs have poor expression of chondrogenic factors in comparison to adult chondrocytes; therefore, further studies are being performed to solve the problem (Olee et al. 2014; Cheng et al. 2018).
iPSCs are derived from fully differentiated adult cells by modifying the expression of unique transcription factors to restore their pluripotency (Takahashi and Yamanaka 2006). iPSCs were produced by reprogramming the synovial cells obtained from osteoarthritis patients and used in the generation of the chondrocytes (Kim et al. 2011) iPSC-derived chondrocyte function has been found comparable to that of juvenile chondrocytes (Lee et al. 2017).
When transforming growth factor beta1/bone morphogenic protein (TGF-β1/BMP) family growth factors were applied to fibroblastic/MSC-like cells derived from hESCs, hard tissue was obtained in pellet culture (Nakagawa et al. 2009). Mesenchymal progenitor cells have also been derived from hESCs through a mesodermal–epithelial transition. This is performed by applying microvascular endothelial growth media 2 (EGM2-MV), forming epithelial cell sheets (Boyd et al. 2009). Murine iPSCs subjected to micromass culture showed ability to execute good hyaline cartilage-like tissue in vitro in agarose gels (Diekman et al. 2012).
The principal step in the differentiation of embryonic stem cells (ESCs) is the formation of embryoid bodies (EBs) (Trzeciak et al. 2014). EBs from hESCs are formed within the three-dimensional porous alginate scaffolds in a rotating bioreactor system. With the help of alginate scaffolds which resemble ECM, cell seeding is efficient. Moreover, porosity can be controlled during the whole process (Shafa et al. 2012). When hESC-derived chondrocytes are cultured in hyaluronic acid (HA)-based hydrogel, long-term ability to repair rat osteochondral defects is achieved with no evidence of tumorigenicity (Toh et al. 2010; Diekman et al. 2012). Craft et al. (2013)‘s experiment involving chondrocyte populations was able to form cartilage-like tissue in vitro and support cartilage tissue phenotype within niche of immunodeficient murine models in vivo (Craft et al. 2013).
Although promising results have been reported, chondrogenic efficacy, ex vivo purification, genetic modification associated with reprogramming protocols, in vivo tissue malformations and the teratogenic potential is still being questioned (Trzeciak et al. 2014; Makris et al. 2015).
An insight to the evolutionary journey of the matrix-induced cartilage tissue engineering products
A procedure for optimization of ex vivo selection and expansion of chondrocyte research has recently been active in the field of tissue engineering. A major advantage of using autologous chondrocytes as a cell source for cartilage repair is that their application has long translated itself to clinical practice, since its first documented, promising experimental results in 1994 (Birttberg et al. 1994, 2001, 2003; Davies and Kuiper 2019). ACI has undergone three generations of development before evolving into its current state of matrix-induced cartilage tissue engineered products. The first generation involved re-implanting monolayer expanded autologous chondrocytes into the damaged region under a natural or synthetic membrane via an open joint procedure. This was clinically challenging due to complications associated with periosteal hypertrophy (Birttberg 2008; Ogura et al. 2017).
Issues with the native periosteum were rapidly resolved as ACI moved to the second generation. Here, porcine-based collagen type I and III membrane was used to replace the periosteum. Even if clinical outcomes were satisfactory, delamination, graft failure, or the inability of the regenerated tissue to integrate with the surrounding native cartilage were still present (Haddo et al. 2004; Niemeyer et al. 2008) Long-term follow-up provided evidence that the second generation of ACI alleviated pain and swelling to increase the level of knee functionality and appeared to result in a better quality of repair tissue (McCarthy et al. 2013; Niemeyer et al. 2014).
Third generation ACI consists of suspending expanded chondrocytes in a hydrated scaffold. It is commonly known as matrix-induced ACI. The third generation brings several advantages which include removing the need for a native or synthetic periosteal patch, better control of cell distribution throughout the defect, and the potential to manage more extensive osteochondral defects (Gille et al. 2016).
The use of synthetic- or xenogenic-based matrices may lead to certain safety and efficacy problems added onto evident quality issues. Therefore, more studies are needed to improve such issues in the field of xenogeneic and allogeneic chondrocytes. This attracts attention to the urgent need for products of complete human-based and autologous origin. There are a number of different approaches to autologous chondrocyte isolation and characterization (Gosset et al. 2008; Oseni et al. 2013; Shui et al. 2013; Demoor et al. 2014; Dewan et al. 2014; Ishibashi et al. 2017; Naranda et al. 2017).
Combined strategies of chondrocyte-based therapies
Biological scaffolds and biomaterials
Scaffolds have clinical superiority over a scaffold-free environment as they have several advantages such as having increased control to fill the cartilage defect, that no surgical procedures are required to obtain tissue from the patient, and that recovery time for the patient is influenced by graft stability (Caron et al. 2012). The geometry and microarchitecture of scaffolds affect factors that determine cell adhesion and migration (Oh et al. 2010). Pore size is also quite critical. It should be large enough to allow cell migration and conclusive production of the ECM (Chen et al. 2006). Pore size should also be small enough to provide a large surface area for cells to adhere.
The biomaterials should have matching mechanical properties to those of the native cartilage, properly integrate with adjacent cartilage, and adequately biodegrade. Natural biomaterials have the advantage of good cellular interaction. Ligands within, facilitate adhesion and promote the activation of various chondrogenic activation pathways. The most common natural biomaterials include collagen, Matrigel™, hyaluronic acid, chitosan, agarose and alginate (Glowacki and Mizuno 2008). The natural chondrocyte is surrounded by a hyaluronan-based pericellular matrix. Therefore, hydrogels consisting of hyaluronan are commonly used in combined models (Guilak et al. 2006). Chondrocytes can firmly attach to such hyaluronan-based matrices (Foss et al. 2013). Even if, the newly formed matrix lacks sufficient mechanical integrity (Nagaya et al. 1999). Alginate, another natural biopolimer, is derived from brown algae. It is contains l-glucuronic acid and d-manuronic acid (Hori et al. 2009). Its biocompatibility and low toxicity make alginate a commonly used biomaterial across laboratories. Autologous or allogeneic cartilage fragments have also been used. When the fragments are of allogeneic origin, it is crucial to omit the cellular component which will immediately lead to immune response once implanted in vivo. Such matrices are decellularized by various protocols (Elder et al. 2009; Tavassoli et al. 2014). They are advantageous as they support the production of collagen type II and proteoglycans. They additionally cooperate with growth factors, with the aim to minimize the hypertrophy of the newly formed tissues (Diekman et al. 2012).
Synthetic polymers provide better control of structural and mechanical features than that of natural ones. Polyglycolic acid (PGA) and polylactic acid (PLA) are common preferences (Stoop 2008). However, synthetic polymers, unfortunately, do not offer specific biological functions to the organism (Shen et al. 2014). Cell adhesion is generally facilitated by interaction with bioactive molecules.
A scoffold with the ability to maintain equilibrium between chondrocyte proliferation rate and differentiation of MSCs within a 3D matrix can be considered as an ideal support (Grigolo et al. 2002). Cell quality characterization is of equal importance (Hoch and Leach 2014; Somoza et al. 2014).
Figure 2 is a basic illustration as to how this combined strategies work in chondrocyte-based therapeutic modeling.
Fig. 2.
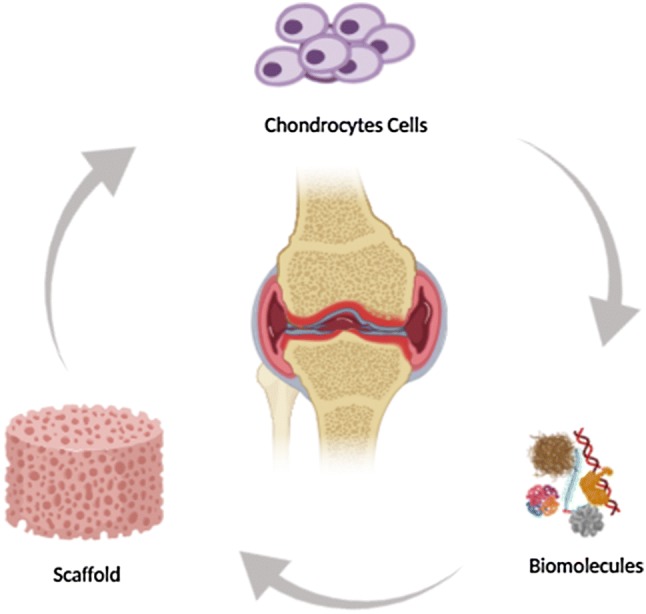
Combined strategies including biomolecules and scaffolds
Articular cartilage regeneration using the cell sheet technology
Okano et al. (1993) pioneered cell sheet technology using temperature-responsive culture dishes (Okano et al. 1993). Thereafter, it was widely used in regenerative medicine as corneal, myocardial, hepatic applications (Kushida et al. 2000; Harimoto et al. 2002; Nishida et al. 2004; Shimizu et al. 2006; Hamahashi et al. 2015). Of these, Kushida et al. (2000) reported that the multilayered, 3D tissue structures can be created without the need for scaffolds, because cell sheets have intact ECM and adhesion factors.
Artificial articular cartilage
In the attempt to treat degenerative diseases, artificial chondral matrices to serve as non-cellular 3D scaffolds. A challenge in this matter is to create a material which would recreate the microarchitecture of the respective chondral layers and also have strong mechanical resistance capacity. The best results until today have been reported with the use of hydrogels. The quality of a material accounts for similarity to soft tissues. Additionally, biocompatibility, thrombogenicity and good permeability for oxygen and other water-soluble metabolites are also questioned. They form environments which are very effective and conductive to accelerated cell growth when compared with polymeric membranes. The highly hydrated hydrogels mimic the chemical and physical conditions of the ECM, which facilitates good integration with the site of trauma as well as efficient multiplication and differentiation of cells (Arakaki et al. 2010; Martowicz and Laska 2010).
The new age: chondrocyte-based gene therapy medicinal products
The hypothesis as to the expression of certain genes in the lesion which might augment the repair cascade led to the idea of developing gene therapeutic approaches for cartilage regeneration, which has made notable progress within the last decade or two. The route of administration of gene delivery to articular lesions is simply intra-articular injection of complementary DNA (cDNA)-encoding medical products which focus on expressing therapeutic proteins to promote cartilage repair (Weisleder et al. 2012; Bartus et al. 2014). This has already been accomplished for both in vivo and ex vivo gene delivery (Li and Hu 2015; Bellavia et al. 2018). Different vectors to deliver membrane repair proteins, transcription factors, and growth factors to promote cellular synthesis and control paracrine cascades have been used (Kafienah et al. 2003; Evans et al. 2004; Weisleder et al. 2012).
The transforming growth factor-β1 (TGF-β1) is a chondrogenic factor that can augment chondrocyte differentiation from bone marrow-derived MSCs. In animal models of full thickness cartilage defects, the role of TGF-β1 in the repair of cartilage defects and the ability of TGF-β1 to prevent chondrocyte hypertrophy were investigated. TGF-β1 promoted chondrogenic differentiation via the canonical Smad pathway. TGF-β1 was also able to reduce chondrocyte hypertrophy evidenced by reduced expression of the cell hypertrophy marker gene, Hippo (Madry et al. 2001). So, long term expression of TGF-β1 might be useful in cartilage regeneration. Kim et al. (2018) reported the clinical efficacy of a cell and gene therapy for knee osteoarthritis in humans consisting of nontransformed and transduced chondrocytes retrovirally transduced to overexpress TGF-β1.
The insulin-like growth factor-1 (IGF-1) is well-established for its effects on cartilage repair (Madry et al. 2001, 2005; Yu et al. 2006; Shi et al. 2010). Viral and nonviral vectors have been used to transduce chondrocytes ex vivo to upregulate IGF-1 production before therapeutic cell delivery (Hellgren et al. 2000; Saxer et al. 2001; Shi et al. 2010; Madry and Cucchiarini 2013). IGF-1 expression peaks for a month’s time or more, then makes a sharp drop (Brower-Toland et al. 2001; Yu et al. 2006; Brigham 2007). The Brigham & Women’s Hospital (2007) reported that this is a remarkably short time compared with the 6–12 months ordinarily required for effective cartilage repair and is not likely to produce beneficial cartilage repair in vivo (Brigham 2007). Accordingly, there is a necessity to develop methods to prolong the expression of IGF-1 in cartilage cells.
In 2004, gene delivery to cartilage defects was studied using coagulated bone marrow aspirate (Pasher et al. 2004). Results suggested that coagulates formed from aspirated bone marrow can be useful as a means of gene delivery to cartilage. Over a decade thereafter, Venkatesan et al. (2017) analyzed the impact of mechanical stimulation on the chondrogenic processes in human bone marrow aspirates modified to overexpress Sox9 via recombinant adeno-associated virus (rAAV) vector. These findings showed the value of genetically modifying human bone marrow aspirates upon mechanical stimulation by rAAV Sox9 as a promising strategy for future treatments to improve cartilage repair by implantation in lesions, where the tissue is submitted to natural mechanical forces. A recent report of Grol and Lee (2018) stated that lately, several therapeutic gene approaches—monotherapies—have demonstrated effectiveness in preclinical models of disease, and a number of them are being assessed in clinical trials. In the same year, Bellavia et al. (2018) stated that gene therapy might represent a promising strategy for chondral and osteochondral defect repair.
To date, none of the currently authorized techniques have demonstrated the ability to produce natural-appearing and functioning hyaline cartilage in the articulations. Gene therapy might offer the possibility to deliver on this goal of cartilage repair for cartilage pathologies once bottlenecks are left behind.
Future perspectives of cartilage-based restorative and regenerative medicine applications
Lately, cellular-based cartilage joint therapies have gradually gained more attention which, at the end of the day, leads to next generation bioengineering approaches in development of cell-based medicinal products for human use in cartilage repair. The greatest hurdles of chondrocyte-based cartilage bioengineering are: (i) preferring the cell source, (ii) differentiation and expansion processes, (iii) the time necessary for chondrocyte expansion pre-implantation, and (iv) fixing the chondrocyte count in accordance with the lesion surface area of the patient in question. A number of strategies are being developed to circumvent such obstacles. For instance, a slight change in the formulation of the expansion medium and reduction of the number of passages may be helpful in controlling chondrocyte differentiation. Various chemical stimulators such as growth factors and digestion enzymes can be used for increasing the structure of the designed product. Additionally, mechanical stimulators such as cutting fluids, tension and compression can be applied for structural quality (Phull et al. 2016). All such strategies will be useful for the effective manufacture and clinical application of chondrocytes. The chondrocyte presents itself to be the focal starting material for research and development of bioengineered cartilage-based medicinal products which are promising in the regeneration and restoration of non-orthopedic cartilage joint defects.
Conclusion
To sum up, chondrocytes are unique cells which are exclusively positioned in the cartilage tissue. These cells play a crucial role in the support of the ECM. Chondrocytes are the focus of a number of medical and surgical applications. Cell- and gene-based medicinal products involving chondrocytes are substantially developed with the intent to regenerate for cartilage lesions. Even though chondrocytes seem to be the first choice, inevitable complications related to proliferation, dedifferentation and redifferentiation are probable. Detailed studies are a necessity to to fully investigate detailed culturing conditions, the chondrogenic strains of well-defined phenotypes and evaluation of the methods to be used in biomaterial production.
Appendix 1
A list of some cell-based, tissue engineered autologous chondrocyte therapies which are either on the market, were reluctant to reach the market or were withdrawn (Huang et al. 2016; Atilla et al. 2018)
| Product proprietary name | Active composition | Current regulatory status |
|---|---|---|
| Biocart II | Autologous chondrocytes (passage number unknown) | No progress after Phase II clinical studies |
| Bioseed-C | Autologous chondrocytes (passage number unknown) | Open to access only in some European countries |
| Cartipatch | Autologous chondrocytes (up to passage 3) | Phase III clinical studies ceased |
| Chondrosphere | Autologous chondrocytes (passage number unknown) | Phase III clinical studies expected to finish in 2020 |
| Hyalograft C | Autologous chondrocytes (up to passage 3) | Withdrawn from the market |
| MACI | Autologous chondrocytes (passages 1-3) | Too high price margin, manufacturing plant shut down due to reluctancy in the cover of expenditures |
| NeoCart | Autologous chondrocytes (passage number unknown) | Phase III clinical studies completed in 2017 |
| NOVOCART 3D | Autologous chondrocytes (passage 1) | Phase III clinical studies expected to finish in 2019 |
| RevaFlex | Allogeneic, juvenile chondrocytes (passage number unknown) | Phase III clinical studies expected to finish in 2019 |
| CaReS | Autologous chondrocytes (primary culture) | Delivered to Turkey, Iran and China but no access due to the high price margin |
| INSTRUCT | Autologous chondrocytes (primary culture) + bone marrow cells | Phase II clinical studies completed in 2014 |
| ChondroCelect | Autologous chondrocytes containing specific marker proteins | Withdrawn from the market upon request of the authorization holder |
| Spherox | Autologous chondrocyte spheroids | On the market |
Compliance with ethical standards
Conflict of interest
The authors have no conflicts of interest to declare. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for profit sectors. Pelin Kilic, Damla Alkaya and Cansu Grucan performed the literature search and data analysis, and all authors had the idea for the article and thereafter drafted and critically revised the work.
References
- Adkisson HD, Milliman C, Zhang X, Mauch K, Maziarz RT, Streeter PR. Immune evasion by neocartilage-derived chondrocytes: implications for biologic repair of joint articular cartilage. Stem Cell Res. 2010;4(1):57–68. doi: 10.1016/j.scr.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng. 2012;40(5):363–408. doi: 10.1615/critrevbiomedeng.v40.i5.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angele P, Kujat R, Nerlich M, Yoo J, Goldberg V, Johnstone B. Engineering of osteochondral tissue with bone marrow mesenchymal progenitor cells in a derivatized hyaluronan-gelatin composite sponge. Tissue Eng. 1999;5(6):545–554. doi: 10.1089/ten.1999.5.545. [DOI] [PubMed] [Google Scholar]
- Arakaki K, Kitamura N, Fujiki H, Kurokawa T, Iwamoto M, Ueno M, Kanaya F, Osada Y, Gong JP, Yasuda K. Artificial cartilage made from a novel double-network hydrogel: in vivo effects on the normal cartilage and ex vivo evaluation of the friction property. J Biomed Mater Res A. 2010;93(3):1160–1168. doi: 10.1002/jbm.a.32613. [DOI] [PubMed] [Google Scholar]
- Archer CW, Francis-West P. The chondrocyte. Int J Biochem Cell Biol. 2003;35(4):401–404. doi: 10.1016/s1357-2725(02)00301-1. [DOI] [PubMed] [Google Scholar]
- Atilla E, Kilic P, Gurman G. Cellular therapies: day by day, all the way. Transfus Apher Sci. 2018;57(2):187–196. doi: 10.1016/j.transci.2018.04.019. [DOI] [PubMed] [Google Scholar]
- Bartus K, James ND, Didangelos A, Bosch KD, Verhaagen J, Yáñez-Muñoz RJ, Rogers JH, Schneider BL, Muir EM, Bradbury EJ. Large-scale chondroitin sulfate proteoglycan digestion with chondroitinase gene therapy leads to reduced pathology and modulates macrophage phenotype following spinal cord contusion injury. J Neurosci. 2014;34(14):4822–4836. doi: 10.1523/JNEUROSCI.4369-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugé C, Boumédiene K. Use of adult stem cells for cartilage tissue engineering: current status and future developments. Stem Cells Int. 2015 doi: 10.1155/2015/438026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellavia D, Veronesi F, Carina V, Costa V, Raimondi L, De Luca A, Alessandro R, Fini M, Giavaresi G. Gene therapy for chondral and osteochondral regeneration: is the future now? Cell Mol Life Sci. 2018;75(4):649–667. doi: 10.1007/s00018-017-2637-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30(1):215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- Boyd NL, Robbins KR, Dhara SK, West FD, Stice SL. Human embryonic stem cell-derived mesodermlike epithelium transitions to mesenchymal progenitor cells. Tissue Eng Part A. 2009;15:1897. doi: 10.1089/ten.tea.2008.0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigham & Women’s Hospital Massachusetts, US (2007) Standard of care: autologous chondrocyte implantation (ACI), pp 1–8. https://www.brighamandwomens.org/assets/BWH/patients-and-families/rehabilitation-services/pdfs/knee-aci-bwh.pdf. Accessed 09 Jan 2020
- Brittberg M. Autologous chondrocyte implantation–technique and long-term follow-up. Injury. 2008;39(Suppl 1):40–49. doi: 10.1016/j.injury.2008.01.040. [DOI] [PubMed] [Google Scholar]
- Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- Brittberg M, Tallheden T, Sjögren-Jansson B, Lindahl A, Peterson L. Autologous chondrocytes used for articular cartilage repair: an update. Clin Orthop Relat Res. 2001;391(Suppl):337–348. doi: 10.1097/00003086-200110001-00031. [DOI] [PubMed] [Google Scholar]
- Brittberg M, Peterson L, Sjögren-Jansson E, Tallheden T, Lindahl A (2003) Articular cartilage engineering with autologous chondrocyte transplantation. A review of recent developments. J Bone Jt Surg Am. 85-A(Suppl 3):109–115. 10.2106/00004623-200300003-00017 [DOI] [PubMed]
- Brower-Toland BD, Saxer RA, Goodrich LR, Mi Z, Robbins PD, Evans CH, Nixon AJ. Direct adenovirus-mediated insulin-like growth factor I gene transfer enhances transplant chondrocyte function. Hum Gene Ther. 2001;12(2):117–129. doi: 10.1089/104303401750061186. [DOI] [PubMed] [Google Scholar]
- Caron MM, Emans PJ, Coolsen MM, Voss L, Surtel DA, Cremers A, van Rhijn LW, Welting TJ. Redifferentiation of dedifferentiated human articular chondrocytes: comparison of 2D and 3D cultures. Osteoarthr Cartil. 2012;20(10):1170–1178. doi: 10.1016/j.joca.2012.06.016. [DOI] [PubMed] [Google Scholar]
- Chen FH, Rousche KT, Tuan RS. Technology insight: adult stem cells in cartilage regeneration and tissue engineering. Nat Clin Pract Rheumatol. 2006;2(7):373–382. doi: 10.1038/ncprheum0216. [DOI] [PubMed] [Google Scholar]
- Cheng A, Kapacee Z, Peng J, Lu S, Lucas RJ, Hardingham TE, Kimber SJ. Cartilage repair using human embryonic stem cell-derived chondroprogenitors. Stem Cells Transl Med. 2014;3(11):1287–1294. doi: 10.5966/sctm.2014-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A, Cain SA, Tian P, Baldwin AK, Uppanan P, Kielty CM, Kimber SJ. Recombinant extracellular matrix protein fragments support human embryonic stem cell chondrogenesis. Tissue Eng Part A. 2018;24:968–978. doi: 10.1089/ten.TEA.2017.0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus S, Aubert-Foucher E, Demoor M, Camuzeaux B, Paumier A, Piperno M, Damour O, Duterque-Coquillaud M, Galéra P, Mallein-Gerin F. Chronic exposure of bone morphogenetic protein-2 favors chondrogenic expression in human articular chondrocytes amplified in monolayer cultures. J Cell Biochem. 2010;111(6):1642–1651. doi: 10.1002/jcb.22897. [DOI] [PubMed] [Google Scholar]
- Cooper KL, Oh S, Sung Y, Dasari RR, Kirschner MW, Tabin CJ. Multiple phases of chondrocyte enlargement underlie differences in skeletal proportions. Nature. 2013;495(7441):375–378. doi: 10.1038/nature11940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft AM, Ahmed N, Rockel JS, Baht GS, Alman BA, Kandel RA, Grigoriadis AE, Keller GM. Specification of chondrocytes and cartilage tissues from embryonic stem cells. Development. 2013;140:2597–2610. doi: 10.1242/dev.087890. [DOI] [PubMed] [Google Scholar]
- D’Andrea F, De Francesco F, Ferraro GA, Desiderio V, Tirino V, De Rosa A, Papaccio G. Large-scale production of human adipose tissue from stem cells: a new tool for regenerative medicine and tissue banking. Tissue Eng Part C Methods. 2008;14(3):233–242. doi: 10.1089/ten.tec.2008.0108. [DOI] [PubMed] [Google Scholar]
- Danisovic L, Varga I, Zamborsky R, Böhmer D. The tissue engineering of articular cartilage: cells, scaffolds and stimulating factors. Exp Biol Med (Maywood). 2012;237(1):10–17. doi: 10.1258/ebm.2011.011229. [DOI] [PubMed] [Google Scholar]
- Davatchi F, Abdollahi BS, Mohyeddin M, Shahram F, Nikbin B. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int J Rheum Dis. 2011;14(2):211–215. doi: 10.1111/j.1756-185X.2011.01599.x. [DOI] [PubMed] [Google Scholar]
- Davies RL, Kuiper NJ. Regenerative medicine: a review of the evolution of autologous chondrocyte implantation (ACI) therapy. Bioengineering (Basel) 2019 doi: 10.3390/bioengineering6010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Windt TS, Saris DB, Slaper-Cortenbach IC, van Rijen MH, Gawlitta D, Creemers LB, de Weger RA, Dhert WJ, Vonk LA. Direct cell-cell contact with chondrocytes is a key mechanism in multipotent mesenchymal stromal cell-mediated chondrogenesis. Tissue Eng Part A. 2015;21(19–20):2536–2547. doi: 10.1089/ten.TEA.2014.0673. [DOI] [PubMed] [Google Scholar]
- Demoor M, Ollitrault D, Gomez-Leduc T, Bouyoucef M, Hervieu M, Lafont J, Denoix JM, Audigié F, Mallein-Gerin F, Legendre F, Galera P. Cartilage tissue engineering: molecular control of chondrocyte differentiation for proper cartilage matrix reconstruction. Biochem Biophys Acta. 2014;1840:2414–2440. doi: 10.1016/j.bbagen.2014.02.030. [DOI] [PubMed] [Google Scholar]
- Deng Z, Jin J, Zhao J, Xu H. Cartilage defect treatments: with or without cells? Mesenchymal stem cells or chondrocytes? Traditional or matrix-assisted? A systematic review and meta-analyses. Stem Cells Int. 2016 doi: 10.1155/2016/9201492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewan AK, Gibson MA, Elisseeff JH, Trice ME. Evolution of autologous chondrocyte repair and comparison to other cartilage repair techniques. Biomed Res Int. 2014;2014:272481. doi: 10.1155/2014/272481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekman BO, Christoforou N, Willard VP, Sun H, Sanchez-Adams J, Leong KW, Guilak F. Cartilage tissue engineering using differentiated and purified induced pluripotent stem cells. Proc Natl Acad Sci USA. 2012;109:19172. doi: 10.1073/pnas.1210422109/-/DCSupplemental. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Dj Prockop, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Elder BD, Eleswarapu SV, Athanasiou KA. Extraction techniques for the decellularization of tissue engineered articular cartilage constructs. Biomaterials. 2009;30(22):3749–3756. doi: 10.1016/j.biomaterials.2009.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CH, Gouze JN, Gouze E, Robbins PD, Ghivizzani SC. Osteoarthritis gene therapy. Gene Ther. 2004;11(4):379–389. doi: 10.1038/sj.gt.3302196. [DOI] [PubMed] [Google Scholar]
- Foss C, Merzari E, Migliaresi C, Motta A. Silk fibroin/hyaluronic acid 3D matrices for cartilage tissue engineering. Biomacromolecules. 2013;14(1):38–47. doi: 10.1021/bm301174x. [DOI] [PubMed] [Google Scholar]
- Garcia J, Mennan C, McCarthy HS, Roberts S, Richardson JB, Wright KT. Chondrogenic potency analyses of donor-matched chondrocytes and mesenchymal stem cells derived from bone marrow, infrapatellar fat pad, and subcutaneous fat. Stem Cells Int. 2016;2016:6969726. doi: 10.1155/2016/6969726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille J, Behrens P, Schulz AP, Oheim R, Kienast B. Matrix-associated autologous chondrocyte implantation: a clinical follow-up at 15 years. Cartilage. 2016;7(4):309–315. doi: 10.1177/1947603516638901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowacki J, Mizuno S. Collegen scaffolds for tissue engineering. Biopolymers. 2008;89(5):338–344. doi: 10.1002/bip.20871. [DOI] [PubMed] [Google Scholar]
- Gosset M, Berenbaum F, Thirion S, Jacques C. Primary culture and phenotyping of murine chondrocytes. Nat Protoc. 2008 doi: 10.1038/nprot.2008.95. [DOI] [PubMed] [Google Scholar]
- Grigolo B, Lisignoli G, Piacentini A, Fiorini M, Gobbi P, Mazzotti G, Duca M, Pavesio A, Facchini A. Evidence for redifferentiation of human chondrocytes grown on a hyaluronan-based biomaterial (HYAff 11): molecular, immunohistochemical and ultrastructural analysis. Biomaterials. 2002;23(4):1187–1195. doi: 10.1016/s0142-9612(01)00236-8. [DOI] [PubMed] [Google Scholar]
- Grol MW, Lee BH. Gene therapy for repair and regeneration of bone and cartilage. Curr Opin Pharmacol. 2018;40(1):59–66. doi: 10.1016/j.coph.2018.03.005. [DOI] [PubMed] [Google Scholar]
- Guilak F, Alexopoulos LG, Upton ML, Youn I, Choi JB. The pericellular matrix as a trancducer of biomechanical and biochemical signals in articular cartilage. Ann N Y Acad Sci. 2006;1068:498–512. doi: 10.1196/annals.1346.011. [DOI] [PubMed] [Google Scholar]
- Haddo O, Mahroof S, Higgs D, David L, Pringle J, Bayliss M, Cannon SR, Briggs TW. The use of chondrogide membrane in autologous chondrocyte implantation. Knee. 2004;11(1):51–55. doi: 10.1016/S0968-0160(03)00041-3. [DOI] [PubMed] [Google Scholar]
- Hamahashi K, Sato M, Yamato M, Kokubo M, Mitani G, Ito S, Nagai T, Ebihara G, Kutsuna T, Okano T, Mochida J. Studies of the humoral factors produced by layered chondrocyte sheets. J Tissue Eng Regener Med. 2015;9(1):24–30. doi: 10.1002/term.1610. [DOI] [PubMed] [Google Scholar]
- Harimoto M, Yamato M, Hirose M, Takahashi C, Isoi Y, Kikuchi A, Okano T. Novel approach for achieving double-layered cell sheets co-culture: overlaying endothelial cell sheets onto monolayer hepatocytes utilizing temperature-responsive culture dishes. J Biomed Mater Res. 2002;62(3):464–470. doi: 10.1002/jbm.10228. [DOI] [PubMed] [Google Scholar]
- Hellgren I, Drvota V, Pieper R, Enoksson S, Blomberg P, Islam KB, Sylvén C. Highly efficient cell-mediated gene transfer using non-viral vectors and FuGene6: in vitro and in vivo studies. Cell Mol Life Sci. 2000;57(8–9):1326–1333. doi: 10.1007/pl00000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch AI, Leach JK. Concise review: optimizing expansion of bone marrow mesenchymal stem/stromal cells for clinical applications. Stem Cells Transl Med. 2014;3(5):643–652. doi: 10.5966/sctm.2013-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori Y, Winans AM, Irvine DJ. Modular injectable matrices based on alginate solution/microsphere mixtures that gel in situ and co-deliver immunomodulatory factors. Acta Biomater. 2009;5(4):969–982. doi: 10.1016/j.actbio.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang BJ, Hu JC, Athanasiou KA. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials. 2016;98:1–22. doi: 10.1016/j.biomaterials.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter W. Of the structure and disease of articulating cartilages. 1743. Clin Orthop Relat Res. 1995;317:3–6. doi: 10.1098/rstl.1742.0079. [DOI] [PubMed] [Google Scholar]
- Im GI, Lee JH. Repair of osteochondral defects with adipose stem cells and a dual growth factor-releasing scaffold in rabbits. J Biomed Mater Res B Appl Biomater. 2010;92(2):552–560. doi: 10.1002/jbm.b.31552. [DOI] [PubMed] [Google Scholar]
- Ishibashi M, Hikita A, Fujihara Y, Takato T, Hoshi K. Human auricular chondrocytes with high proliferation rate show high production of cartilage matrix. Regen Ther. 2017;6:21–28. doi: 10.1016/j.reth.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo CH, Lee YG, Shin WH, Kim H, Chai JW, Jeong EC, Kim JE, Shim H, Shin JS, Shin IS, Ra JC, Oh S, Yoon KS. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32(5):1254–1266. doi: 10.1002/stem.1634. [DOI] [PubMed] [Google Scholar]
- Jones DG, Peterson L. Autologous chondrocyte implantation. J Bone Jt Surg Am. 2006;88(11):2502–2520. doi: 10.2106/00004623-200611000-00025. [DOI] [PubMed] [Google Scholar]
- Jones IA, Wilson M, Togashi R, Han B, Mircheff AK, Thomas Vangsness Jr C. A randomized, controlled study to evaluate the efficacy of intra-articular, autologous adipose tissue injections for the treatment of mild-to-moderate knee osteoarthritis compared to hyaluronic acid: a study protocol. BMC Musculoskelet Disord. 2018;19(1):383. doi: 10.1186/s12891-018-2300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafienah W, Jakob M, Démarteau O, Frazer A, Barker MD, Martin I, Hollander AP. Three-dimensional tissue engineering of hyaline cartilage: comparison of adult nasal and articular chondrocytes. Tissue Eng. 2002;8(5):817–826. doi: 10.1089/10763270260424178. [DOI] [PubMed] [Google Scholar]
- Kafienah W, Al-Fayez F, Hollander AP, Barker MD. Inhibition of cartilage degradation: a combined tissue engineering and gene therapy approach. Arthritis Rheum. 2003;48(3):709–718. doi: 10.1002/art.10842. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Son MJ, Son MY, Seol B, Kim J, Park J, Kim JH, Kim YH, Park SA, Lee CH, Lee KS, Han YM, Chang JS, Cho YS. Generation of human induced pluripotent stem cells from osteoarthritis patient-derived synovial cells. Arthritis Rheum. 2011;63(10):3010–3021. doi: 10.1002/art.30488. [DOI] [PubMed] [Google Scholar]
- Kim MK, Ha CW, In Y, Cho SD, Choi ES, Ha JK, Lee JH, Yoo JD, Bin SI, Choi CH, Kyung HS, Lee MC. A multicenter, double-blind, phase III clinical trial to evaluate the efficacy and safety of a cell and gene therapy in knee osteoarthritis patients. Hum Gene Ther Clin Dev. 2018;27(1):10. doi: 10.1089/humc.2017.249. [DOI] [PubMed] [Google Scholar]
- Koh YG, Choi YJ. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee. 2012;19(6):902–907. doi: 10.1016/j.knee.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Koh YG, Jo SB, Kwon OR, Suh DS, Lee SW, Park SH, Choi YJ. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy. 2013;29(4):748–755. doi: 10.1016/j.arthro.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Kozhemyakina E, Lassar AB, Zelzer E. A pathway to bone: signaling molecules and transcription factors involved in chondrocyte development and maturation. Development. 2015;142(5):817–883. doi: 10.1242/dev.105536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushida A, Yamato M, Konno C, Kikuchi A, Sakurai Y, Okano T. Temperature-responsive culture dishes allow nonenzymatic harvest of differentiated Madin–Darby canine kidney (MDCK) cell sheets. J Biomed Mater Res. 2000;51(2):216–223. doi: 10.1002/(sici)1097-4636(200008)51:2<216::aid-jbm10>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Lai JH, Kajiyama G, Smith RL, Maloney W, Yang F. Stem cells catalyze cartilage formation by neonatal articular chondrocytes in 3D biomimetic hydrogels. Sci Rep. 2013;3:3553. doi: 10.1038/srep03553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Smeriglio P, Chu CR, Bhutani N. Human iPSC-derived chondrocytes mimic juvenile chondrocyte function for the dual advantage of increased proliferation and resistance to IL-1β. Stem Cell Res Ther. 2017;8(1):244. doi: 10.1186/s13287-017-0696-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li KC, Hu YC. Cartilage tissue engineering: recent advances and perspectives from gene regulation/therapy. Adv Healthc Mater. 2015;4(7):948–968. doi: 10.1002/adhm.201400773. [DOI] [PubMed] [Google Scholar]
- Lin Z, Willers C, Xu J, Zheng MH. The chondrocyte: biology and clinical application. Tissue Eng. 2006;12(7):1971–1984. doi: 10.1089/ten.2006.12.1971. [DOI] [PubMed] [Google Scholar]
- Madry H, Cucchiarini M. Advances and challenges in gene-based approaches for osteoarthritis. J Gene Med. 2013;15(10):343–355. doi: 10.1002/jgm.2741. [DOI] [PubMed] [Google Scholar]
- Madry H, Zurakowski D, Trippel SB. Overexpression of human insulin-like growth factor-I promotes new tissue formation in an ex vivo model of articular chondrocyte transplantation. Gene Ther. 2001;8(19):1443–1449. doi: 10.1038/sj.gt.3301535. [DOI] [PubMed] [Google Scholar]
- Madry H, Kaul G, Cucchiarini M, Stein U, Zurakowski D, Remberger K, Menger MD, Kohn D, Trippel SB. Enhanced repair of articular cartilage defects in vivo by transplanted chondrocytes overexpressing insulin-like growth factor 1 (IGF-1) Gene Ther. 2005;12(15):1171–1179. doi: 10.1038/sj.gt.3302515. [DOI] [PubMed] [Google Scholar]
- Makris EA, Gomoll AH, Malizos KN, Hu JC, Athanasiou KA. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 2015;11(1):21–34. doi: 10.1038/nrrheum.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcacci M, Kon E, Zaffagnini S. Arthroscopic second generation autologous chondrocyte implantation. Knee Surg Sports Traumatol Arthrosc. 2007;15:610–619. doi: 10.1007/s00167-006-0265-9. [DOI] [PubMed] [Google Scholar]
- Martowicz M, Laska J. Polymer biomaterials for skin regeneration: a review. Eng Biomater. 2010;13:2–9. [Google Scholar]
- McCarthy HS, Roberts S. A histological comparison of the repair tissue formed when using either Chondrogide(®) or periosteum during autologous chondrocyte implantation. Osteoarthr Cartil. 2013;21(12):2048–2057. doi: 10.1016/j.joca.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Mobasheri A, Kalamegam G, Musumeci G, Batt ME. Chondrocyte and mesenchymal stem cell-based therapies for cartilage repair in osteoarthritis and related orthopaedic conditions. Maturitas. 2014;78(3):188–198. doi: 10.1016/j.maturitas.2014.04.017. [DOI] [PubMed] [Google Scholar]
- Mumme M, Barbero A, Miot S, Wixmerten A, Feliciano S, Wolf F, Asnaghi AM, Baumhoer D, Bieri O, Kretzschmar M, Pagenstert G. Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: an observational first-in-human trial. Lancet. 2016;388:1985–1994. doi: 10.1016/S0140-6736(16)31658-0. [DOI] [PubMed] [Google Scholar]
- Nagaya H, Ymagata T, Ymagata S, Iyoda K, Ito H, Hasegawa Y, Iwata H. Examination of synovial fluid and serum hyaluronidase activity as a joint marker in rheumatoid arthritis and osteoarthritis patients (by zymography) Ann Rheum Dis. 1999;58(3):186–188. doi: 10.1136/ard.58.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Lee SY, Reddi AH. Induction of chondrogenesis from human embryonic stem cells without embryoid body formation by bone morphogenetic protein 7 and transforming growth factor beta1. Arthritis Rheum. 2009;60:3686. doi: 10.1002/art.27229. [DOI] [PubMed] [Google Scholar]
- Naranda J, Gradišnik L, Gorenjak M, Vogrin M, Maver U. Isolation and characterization of human articular chondrocytes from surgical waste after total knee arthroplasty (TKA) PeerJ. 2017;21(5):e3079. doi: 10.7717/peerj.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer P, Pestka JM, Kreuz PC, Erggelet C, Schmal H, Suedkamp NP, Steinwachs M. Characteristic complications after autologous chondrocyte implantation for cartilage defects of the knee joint. Am J Sports Med. 2008;36(11):2091–2099. doi: 10.1177/0363546508322131. [DOI] [PubMed] [Google Scholar]
- Niemeyer P, Porichis S, Steinwachs M, Erggelet C, Kreuz PC, Schmal H, Uhl M, Ghanem N, Südkamp NP, Salzmann G. Long-term outcomes after first-generation autologous chondrocyte implantation for cartilage defects of the knee. Am J Sports Med. 2014;42(1):150–157. doi: 10.1177/0363546513506593. [DOI] [PubMed] [Google Scholar]
- Nishida K, Yamato M, Hayashida Y, Watanabe K, Maeda N, Watanabe H, Yamamoto K, Nagai S, Kikuchi A, Tano Y, Okano T. Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsive cell culture surface. Transplantation. 2004;77(3):379–385. doi: 10.1097/01.TP.0000110320.45678.30. [DOI] [PubMed] [Google Scholar]
- Nukavarapu SP, Amini AR. Optimal scaffold design and effective progenitor cell identification for the regeneration of vascularized bone. Conf Proc IEEE Eng Med Biol Soc. 2011 doi: 10.1109/IEMBS.2011.6090684. [DOI] [PubMed] [Google Scholar]
- Nukavarapu SP, Dorcemus DL. Osteochondral tissue engineering: current strategies and challenges. Biotechnol Adv. 2013;31(5):706–721. doi: 10.1016/j.biotechadv.2012.11.004. [DOI] [PubMed] [Google Scholar]
- O’Brien FJ, Harley BA, Yannas IV, Gibson LJ. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials. 2005;26(4):433–441. doi: 10.1016/j.biomaterials.2004.02.052. [DOI] [PubMed] [Google Scholar]
- Ochi M, Uchio Y, Tobita M, Kuriwaka M. Current concepts in tissue engineering technique for repair of cartilage defect. Artif Organs. 2001;25:172–179. doi: 10.1046/j.1525-1594.2001.025003172.x. [DOI] [PubMed] [Google Scholar]
- Ogura T, Mosier BA, Bryant T, Minas T. A 20-year follow-up after first-generation autologous chondrocyte implantation. Am J Sports Med. 2017;45(12):2751–2761. doi: 10.1177/0363546517716631. [DOI] [PubMed] [Google Scholar]
- Oh SH, Kim TH, Im GI, Lee JH. Investigation of pore size effect on chondrogenic differentiation of adipose stem cells using pore size gradient scaffold. Biomacromolecules. 2010;11(8):1948–1955. doi: 10.1021/bm100199m. [DOI] [PubMed] [Google Scholar]
- Okano T, Yamada N, Sakai H, Sakurai Y. A novel recovery-system for cultured-cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide) J Biomed Mater Res. 1993;27:1243–1251. doi: 10.1002/jbm.820271005. [DOI] [PubMed] [Google Scholar]
- Oldershaw RA, Baxter MA, Lowe ET, Bates N, Grady LM, Soncin F, Brison DR, Hardingham TE, Kimber SJ. Directed differentiation of human embryonic stem cells toward chondrocytes. Nat Biotechnol. 2010;28(11):1187–1194. doi: 10.1038/nbt.1683. [DOI] [PubMed] [Google Scholar]
- Olee T, Grogan SP, Lotz MK, Colwell CW, Jr, D’Lima DD, Snyder EY. Repair of cartilage defects in arthritic tissue with differentiated human embryonic stem cells. Tissue Eng Part A. 2014;20(3–4):683–692. doi: 10.1089/ten.TEA.2012.0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onofrillo C, Duchi S, O’Connell CD, Blanchard R, O’Connor AJ, Scott M, Wallace GG, Choong PFM, Di Bella C. Biofabrication of human articular cartilage: a path towards the development of a clinical treatment. Biofabrication. 2018;10(4):045006. doi: 10.1088/1758-5090/aad8d9. [DOI] [PubMed] [Google Scholar]
- Oseni AO, Butler PE, Seifalian AM. Optimization of chondrocyte isolation and characterization for large-scale cartilage tissue engineering. J Surg Res. 2013;181(1):41–48. doi: 10.1016/j.jss.2012.05.087. [DOI] [PubMed] [Google Scholar]
- Owida HA, De Las Heras Ruiz T, Dhillon A, Yang Y, Kuiper NJ. Co-culture of chondrons and mesenchymal stromal cells reduces the loss of collagen VI and improves extracellular matrix production. Histochem Cell Biol. 2017;148(6):625–638. doi: 10.1007/s00418-017-1602-4. [DOI] [PubMed] [Google Scholar]
- Pascher A, Palmer GD, Steinert A, Oligino T, Gouze E, Gouze JN, Betz O, Spector M, Robbins PD, Evans CH, Ghivizzani SC. Gene delivery to cartilage defects using coagulated bone marrow aspirate. Gene Ther. 2004;11(2):133–141. doi: 10.1038/sj.gt.3302155. [DOI] [PubMed] [Google Scholar]
- Pei M, He F. Extracellular matrix deposited by synovium-derived stem cells delays replicative senescent chondrocyte dedifferentiation and enhances redifferentiation. J Cell Physiol. 2012;227(5):2163–2174. doi: 10.1002/jcp.22950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei M, He F, Kish VL, Vunjak-Novakovic G. Engineering of functional cartilage tissue using stem cells from synovial lining: a preliminary study. Clin Orthop Relat Res. 2008;466(8):1880–1889. doi: 10.1007/s11999-008-0316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei M, He F, Boyce BM, Kish VL. Repair of full-thickness femoral condyle cartilage defects using allogeneic synovial cell-engineered tissue constructs. Osteoarthr Cartil. 2009;17(6):714–722. doi: 10.1016/j.joca.2008.11.017. [DOI] [PubMed] [Google Scholar]
- Phull AR, Eo SH, Abbas Q, Ahmed M, Kim SJ. Applications of chondrocyte-based cartilage engineering: an overview. Biomed Res Int. 2016;2016:1879837. doi: 10.1155/2016/1879837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52(8):2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- Saxer RA, Bent SJ, Brower-Toland BD, Mi Z, Robbins PD, Evans CH, Nixon AJ. Gene mediated insulin-like growth factor-I delivery to the synovium. J Orthop Res. 2001;19(5):759–767. doi: 10.1016/S0736-0266(00)00077-2. [DOI] [PubMed] [Google Scholar]
- Shafa M, Sjonnesen K, Yamashita A, Liu S, Michalak M, Kallos MS, Rancourt DE. Expansion and long-term maintenance of induced pluripotent stem cells in stirred suspension bioreactors. J Tissue Eng Regen Med. 2012;6:462–472. doi: 10.1002/term.450. [DOI] [PubMed] [Google Scholar]
- Shafiee A, Kabiri M, Langroudi L, Soleimani M, Ai J. Evaluation and comparison of the in vitro characteristics and chondrogenic capacity of four adult stem/progenitor cells for cartilage-based repair. J Biomed Mater Res A. 2016;104(3):600–610. doi: 10.1002/jbm.a.35603. [DOI] [PubMed] [Google Scholar]
- Shen Y, Fu Y, Wang J, Li G, Zhang X, Xu Y, Lin Y. Biomaterial and mesenchymal stem cells for articular cartilage reconstruction. Curr Stem Cell Res Ther. 2014;9(3):254–267. doi: 10.2174/1574888x09666140213202700. [DOI] [PubMed] [Google Scholar]
- Shi S, Mercer S, Trippel SB. Effect of transfection strategy on growth factor overexpression by articular chondrocytes. J Orthop Res. 2010;28(1):103–109. doi: 10.1002/jor.20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Sekine H, Yang J, Isoi Y, Yamato M, Kikuchi A, Kobayashi E, Okano T. Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. FASEB J. 2006;20(6):708–710. doi: 10.1096/fj.05-4715fje. [DOI] [PubMed] [Google Scholar]
- Shui W, Yin L, Luo J, Li R, Zhang W, Zhang J, Huang W, Hu N, Liang X, Deng ZL, Hu Z, Shi LL, Luu HH, Haydon RC, He TC, Ho SH (2013) Characterization of chondrocyte scaffold carriers for cell-based gene therapy in articular cartilage repair. J Biomed Mater Res A 101(12):3542–3550. https://doi.org/10.1002/jbm.a.34661 [DOI] [PMC free article] [PubMed]
- Somoza RA, Welter JF, Correa D, Caplan AI. Chondrogenic differentiation of mesenchymal stem cells: challenges and unfulfilled expectations. Tissue Eng Part B Rev. 2014;20(6):596–608. doi: 10.1089/ten.TEB.2013.0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Du H, Dai C, Zhang L, Li S, Hunter DJ, Lu L, Bao C. Human adipose-derived mesenchymal stem cells for osteoarthritis: a pilot study with long-term follow-up and repeated injections. Regen Med. 2018;13(3):295–307. doi: 10.2217/rme-2017-0152. [DOI] [PubMed] [Google Scholar]
- Sopena Juncosa JJ, Carillo Poveda JM, Rubio Zaragoza M, Redondo Garcia JI, Serra Aguado I, Soler Canet C (2000) Estructura y función del cartílago articular. Portada (Armas Frente a la Patología Articular) 1:24–26. 10.1016/S1286-935X(05)43399-7
- Stoop R. Smart biomaterials for tissue engineering of cartilage. Injury. 2008;39(Suppl 1):77–87. doi: 10.1016/j.injury.2008.01.036. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 25 126(4):663–676. 10.1016/j.cell.2006.07.024 [DOI] [PubMed]
- Tavassoli A, Mahdavi-Shahri N, Matin MM, Fereidoni M, Shahabipour F (2014) Bovine articular cartilage decellularized matrix as a scaffold for use in cartilage tissue engineering. Iran J Vet Sci Technol 4(1):1–8. 10.22067/veterinary.v4i1.17878
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Toh WS, Lee EH, Guo XM, Chan JK, Yeow CH, Choo AB, Cao T. Cartilage repair using hyaluronan hydrogel-encapsulated human embryonic stem cell-derived chondrogenic cells. Biomaterials. 2010;31:6968–6980. doi: 10.1016/j.biomaterials.2010.05.064. [DOI] [PubMed] [Google Scholar]
- Trzeciak T, Augustyniak E, Ritcher M, Kaczmarczyk J, Suchorska WM. Induced pluripotent and mesenchymal stem cells as a promising tool for articular cartilage regeneration. J Cell Sci Ther. 2014 doi: 10.4172/2157-7013.1000172. [DOI] [Google Scholar]
- Vedicherla S, Buckley CT. Rapid chondrocyte isolation for tissue engineering applications: the effect of enzyme concentration and temporal exposure on the matrix forming capacity of nasal derived chondrocytes. Biomed Res Int. 2017 doi: 10.1155/2017/2395138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega A, Martín-Ferrero MA, Del Canto F, Alberca M, García V, Munar A, Orozco L, Soler R, Fuertes JJ, Huguet M, Sánchez A, García-Sancho J. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation. 2015;99(8):1681–1690. doi: 10.1097/TP.0000000000000678. [DOI] [PubMed] [Google Scholar]
- Venkatesan JK, Frisch J, Rey-Rico A, Schmitt G, Madry H, Cucchiarini M. Impact of mechanical stimulation on the chondrogenic processes in human bone marrow aspirates modified to overexpress sox9 via rAAV vectors. J Exp Orthop. 2017;4(1):22. doi: 10.1186/s40634-017-0097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinatier C, Mrugala D, Jorgensen C, Guicheux J, Noël D. Cartilage engineering: a crucial combination of cells, biomaterials and biofactors. Trends Biotechnol. 2009;27(5):307–314. doi: 10.1016/j.tibtech.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, Goldberg VM. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Jt Surg Am. 1994;76(4):579–592. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- Wakitani S, Kawaguchi A, Tokuhara Y, Takaoka K. Present status and future direction for articular cartilage. J Bone Miner Metab. 2008;26:115–122. doi: 10.1007/s00774-007-0802-8. [DOI] [PubMed] [Google Scholar]
- Wakitani S, Okabe T, Horibe S, Mitsuoka T, Saito M, Koyama T, Nawata M, Tensho K, Kato H, Uematsu K, Kuroda R, Kurosaka M, Yoshiya S, Hattori K, Ohgushi H. Safety of autologous bone marrow-derived mesenchymal stem cell transplantation for cartilage repair in 41 patients with 45 joints followed for up to 11 years and 5 months. J Tissue Eng Regen Med. 2011;5(2):146–150. doi: 10.1002/term.299. [DOI] [PubMed] [Google Scholar]
- Wang X, Wenk E, Zhang X, Meinel L, Vunjak-Novakovic G, Kaplan DL. Growth factor gradients via microsphere delivery in biopolymer scaffolds for osteochondral tissue engineering. J Control Release. 2009;134(2):81–90. doi: 10.1016/j.jconrel.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters NP, Stoker AM, Carson WL, Pfeiffer FM, Cook JL. Biomarkers affected by impact velocity and maximum strain of cartilage during injury. J Biomech. 2014;47(12):3185–3195. doi: 10.1016/j.jbiomech.2014.06.015. [DOI] [PubMed] [Google Scholar]
- Weisleder N, Takizawa N, Lin P, Wang X, Cao C, Zhang Y, Tan T, Ferrante C, Zhu H, Chen PJ, Yan R, Sterling M, Zhao X, Hwang M, Takeshima M, Cai C, Cheng H, Takeshima H, Xiao RP, Ma J. Recombinant MG53 protein modulates therapeutic cell membrane repair in treatment of muscular dystrophy. Sci Transl Med. 2012;4(139):139ra85. doi: 10.1126/scitranslmed.3003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, Wang X, Fu YX. Enhanced local delivery with reduced systemic toxicity: delivery, delivery, and delivery. Gene Ther. 2006;13(15):1131–1132. doi: 10.1038/sj.gt.3302760. [DOI] [PubMed] [Google Scholar]
- Zhang W, Ouyang H, Dass CR. Current research on pharmacologic and regenerative therapies for osteoarthritis. Xu J Bone Res. 2016;4:15040. doi: 10.1038/boneres.2015.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Zheng H, Seol D, Yu Y, Martin JA. Gene expression profiles reveal that chondrogenic progenitor cells and synovial cells are closely related. J Orthop Res. 2014;32(8):981–988. doi: 10.1002/jor.22641. [DOI] [PMC free article] [PubMed] [Google Scholar]


