Angioimmunoblastic T-cell lymphoma (AITL) is one of the most common specific types of peripheral T-cell lymphomas (PTCL)1. The cell of origin for AITL is T follicular helper cells (TFH)2,3. AITL has an immunophenotype closely akin to that of normal TFH; CD4+, CD8−, T-cell receptor (TCR) alpha/beta, and often expressing CXCL13, CD10, BCL6, Programmed-Death-1 (PD-1), ICOS, and CD2002,4–6. Diagnosis of AITL is challenging especially in small needle core biopsy due to the presence of admixed abundant inflammatory cells. The differential diagnosis is broad, and a definitive diagnosis requires a use of ancillary testing, including large panels of immunohistochemistry and molecular genetic studies. Immunophenotyping by flow cytometry is a common study for the diagnosis as well as for monitoring minimal residual disease of hematologic malignancies7–13, but contribution of flow cytometry to diagnose AITL, while often reported, has been unclear due to lack of systemic studies with integrated morphologic assessment14. Here, we evaluated the utility of highly sensitive flow cytometry in diagnosing AITL using an antibody against PD-1 in the context of other T-cell antigen. Tissue biopsies, bone marrow biopsies, and peripheral blood samples were retrieved from pathology archives between May 2015 and December 2018 at Memorial Sloan Kettering Cancer Center. This study was performed following the declaration of Helsinki and was approved by the institutional review board. The group was composed of 81 patients with PTCL and 40 patients with no T-cell lymphomas, but with potential morphologic mimics. Specifically, 36 patients with AITL, 6 with ALK-negative anaplastic large cell lymphoma, 2 with ALK-positive anaplastic large cell lymphoma, 9 with adult T-cell leukemia/lymphoma, 11 with peripheral T-cell lymphoma, not otherwise specified, 6 with nodal involvement by mycosis fungoides, 5 with T-cell large granular lymphocytic leukemia, 6 with T-cell prolymphocytic leukemia, 4 with nodular lymphocyte-predominant Hodgkin lymphoma, 1 with T-cell/histiocyte-rich large B-cell lymphoma, 5 with diffuse large B-cell lymphoma, germinal center B-cell type, 6 with follicular lymphoma, 12 with classical Hodgkin lymphoma, and 12 with benign reactive lymph nodes. Overall we obtained 94 tissue biopsies, 59 peripheral blood specimens, and 21 bone marrow aspirate specimens (Supplementary Table 1). Expression of PD-1 (CD279) on lymphoma cells was evaluated by BD FACSCanto 10-color flow cytometry (BD Biosciences, San Jose, CA) with BV605-conjugated anti-CD279 antibody (EH12.2H7, BioLegend, San Diago, CA) along with other T-cell antigens frequently evaluated in this context. The results were analyzed with Woodlist software (Dr. B.L.Wood, University of Washington). Abnormal T-cell populations were identified by visual assessment of aberrant antigen expression. Mean fluorescence intensity (MFI) of anti-CD279 antibody was also evaluated. For selected cases, Beta Mark TCR Vbeta Repertoire Kit (Beckman-Coulter, Miami, FL) was used to confirm clonality. Flow sorting was performed in conjunction with molecular genetic analysis as a part of the study. Detailed methods are described in supplementary information.
Flow cytometric analysis of all 12 reactive lymph nodes demonstrated that non-neoplastic T-cell population with bright PD-1 (CD279) uniformly co-express CXCR5 (CD185) and ICOS (CD278), consistent with TFH (Fig. 1a–f). No other physiological subset of normal T-cell showed PD-1 expression with similar intensity indicating that bright PD-1 expression is a hallmark of TFH. These findings were reproducible in neoplastic T-cells of AITL and confirmed that bright PD-1 expression is an excellent surrogate to assign TFH phenotype to T-cells (Fig. 1g–i). Subsequently, we compared the expression levels of PD-1 by flow cytometry among PTCL cases. The expression of PD-1 in abnormal T-cell population in the tissue biopsy was significantly higher in AITL than that of other PTCL (CD279-BV605 MFI; Mean ± SEM; 6990.0 ± 934.9 vs. 547.3 ± 155.5; p < 0.0001) (Fig. 1j). PD-1 MFI differentiated AITL from other PTCL with high sensitivity and specificity (AUC 0.978; Fig. 1k). In 54 patients with PTCL who had circulating lymphoma cells in peripheral blood, the significant increase in PD-1 expression was a consistent finding and it differentiated AITL from other PTCL with high sensitivity and specificity (CD279-BV605 MFI; Mean ± SEM; 3079.0 ± 508.8 vs. 341.8 ± 54.17; p < 0.0001, AUC 0.986, Fig. 1l, m). Among 28 AITL patients with a tissue biopsy, 14 patients had concomitant peripheral blood samples. Circulating AITL cells were detected in all 14 patients, including 7 patients with new diagnosis of AITL and 7 patients with relapsed/persistent AITL (median 0.52% of total white blood cells; Range 0.094–7.7%). ddPCR for selected mutations was performed on flow sorted PD-1 bright T-cell population in peripheral blood and bone marrow in 3 and 2 patients with AITL, respectively, and same genetic abnormalities seen in tissue sample were identified, confirming clonal identity between tissue and peripheral blood/bone marrow. These findings suggest that peripheral blood flow cytometry with anti-PD-1 antibody is a useful screening tool for diagnosis of AITL. Aberrant T-cells with bright PD-1 expression were not identified in bone marrow or in peripheral blood of the patients with B-cell lymphoma or reactive follicular hyperplasia.
Fig. 1. Flow cytometric findings of PD-1 (CD279), ICOS (CD278), and CXCR5 (CD185) expression in non-neoplastic T-cells and T-cell lymphoma.
Non-neoplastic T-cell population with bright PD-1 expression in reactive lymph node (green arrow) shows co-expression of ICOS and CXCR5 (a–c). Non-neoplastic T-cell population with bright PD-1 expression in lymph node with follicular lymphoma (red arrow) shows co-expression of ICOS and CXCR5 (d–f). Abnormal CD4-positive T-cell population with loss of surface CD3 identified in lymph node with AITL (g, black arrow; Case 11). This abnormal T-cell population shows bright PD-1 expression (h). This population also co-express ICOS and CXCR5 (i, CD185 plot not shown). PD-1 mean fluorescent intensity (MFI) in tissue sample. MFI in AITL is significantly higher than that of non-AITL PTCL (j). Receiver Operating Characteristic (ROC) Curve of PD-1 MFI in tissue sample shows high sensitivity and specificity observed in this assay differentiating AITL from other PTCL (p < 0.001, AUC 0.978: sensitivity 93.15% and specificity 92.86% for a cutoff of MFI 1560.64) (k). PD-1 mean fluorescent intensity (MFI) in peripheral blood sample. MFI in AITL is significantly higher than that of non-AITL PTCL (l). ROC curve of PD-1 MFI in peripheral blood sample also shows high sensitivity and specificity in differentiating AITL from other PTCL (p < 0.001, AUC 0.986: sensitivity 93.75% and specificity 95.45% for a cutoff of MFI 836.33) (m).
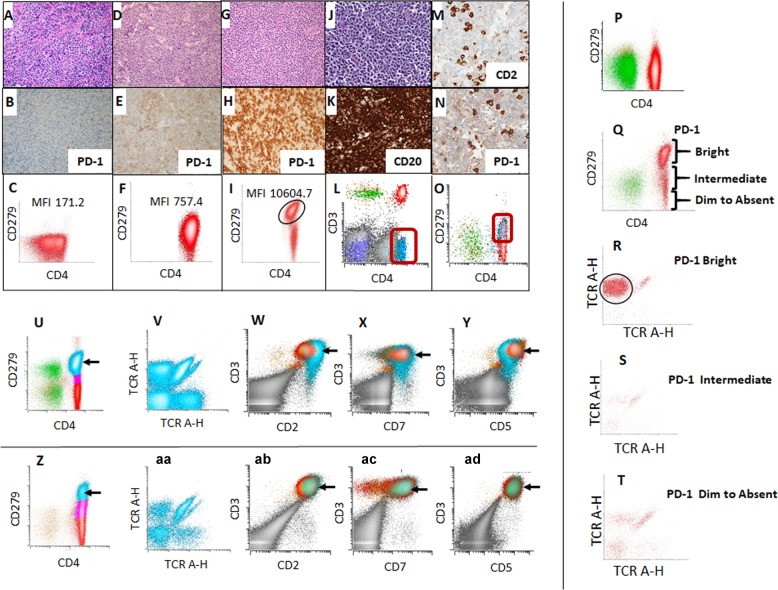
All 28 AITL cases with tissue biopsy showed CD3 and PD-1 immunoreactivity by immunohistochemistry, and PD-1 expression on abnormal T-cells by flow cytometry showed excellent correlation with immunohistochemistry (Supplementary Table 2, Fig. 2a–i). In tissue samples, we frequently encountered that other standard TFH markers were not informative by immunohistochemistry, or interpretation of immunohistochemistry was challenging. In our cohort, expression of other TFH markers was not observed by immunohistochemistry in seven cases (25% of cases; Case #3, 7, 8, 12, 15, 19, 28). Additional three cases (11% of cases; Case #21, 22, 27) presented with concomitant lymphoid neoplasm, which obscured morphologic and immunohistochemical evaluation for AITL (Fig. 2j–o). In 10 patients (36% of cases), AITL could not be initially established by morphology and immunohistochemistry by expert review; however, flow cytometry reliably detected AITL in all these 10 challenging cases. Next-generation sequencing was performed with FFPE tissue on 14 AITL cases, including these challenging cases, and all cases had at least one mutation either in RHOA, TET2, IDH2, or DNMT3A (Supplementary Tables 3 and 4). Sorted bright PD-1 positive neoplastic T-cells in two cases of AITL with RHOA G17V mutation showed the presence of same mutation with over 0.45 allele frequency, confirming that this population represents AITL. We performed TCR Vbeta fragment analysis by flow cytometry on selected AITL cases when other phenotypic abnormalities were subtle. The abnormal PD-1 bright T-cell population showed restricted Vbeta usage indicating monoclonality (Fig. 2p–t). Monoclonality was also confirmed by molecular TCR analysis. None of the 6 patients with morphologic mimics with bright PD-1 expression which were tested for TCR Vbeta analysis showed evidence of monoclonality, and findings were also confirmed by molecular TCR analysis (Fig 2u–ad). All 28 cases of AITL showed at least one loss (negative or dim) of T-cell antigen by flow cytometry (Supplementary Table 2). Among 40 cases of morphologic mimics of AITL, T-cell antigens were preserved with exception of populations of CD4+ T cells showing reduction of CD7, commonly seen in memory T cells (Fig 2u–ad). In our study, TCR Vbeta restriction along with loss of T-cell antigen(s) in PD-1 bright T-cell population reliably discriminated morphologic mimics from AITL with 100% sensitivity and specificity. We also found that PD-1 flow cytometry is useful for evaluating staging bone marrow biopsy especially for the cases with minimal involvement or cases having other concomitant hematolymphoid malignancy (Supplementary Fig. 1).
Fig. 2. Utility of flow cytometry in diagnosing angioimmunoblastic T-cell lymphoma.
Histology, PD-1 immunohistochemistry and PD-1 flow cytometric findings in peripheral T-cell lymphoma, NOS (PTCL-NOS; a–c), adult T-cell leukemia/ lymphoma (ATLL; d–f), and angioimmunoblastic T-cell lymphoma (AITL, Case 28; g–i). PD-1 expression is not observed in PTCL-NOS both by immunohistochemistry (b) and flow cytometry (c). ATLL shows intermediate expression of PD-1 by immunohistochemistry (e) and flow cytometry (f). AITL shows bright PD-1 expression by immunohistochemistry (h) and flow cytometry (i). Lymph node involved by both AITL and diffuse large B-cell lymphoma (Case 21; Fig 2j–o). Lymph node shows atypical lymphocytic infiltrate composed of large cells (j). Immunohistochemistry for CD20 highlights sheets of large cells (k). CD2 highlights scattered T-cells (m). Scattered T-cells also express PD-1 (n). Flow cytometry identifies abnormal CD4+ T-cell population with aberrant loss of surface CD3 (l; aqua dots). This aberrant population expresses PD-1/CD279 (o; aqua dots). PD-1 expression on normal lymph node and lymph node involved by AITL. Normal lymph node does not show distinct CD4+/PD-1 bright population (p). Lymph node involved by AITL show distinct CD4+/PD-1 bright population (q, Case 17). Vbeta fragment analysis performed on sorted PD-1 bright population shows that this population is clonal (r, Case 17). Vbeta fragment analysis performed on PD-1 intermediate and dim to absent populations show that these populations are polyclonal (s, t, Case 17). Flow cytometric findings of patients with nodular lymphocyte-predominant Hodgkin lymphoma (u–y) and reactive follicular hyperplasia (Z-AD). Prominent CD4-positive T-cell population with bright PD-1 expression is seen in NLPHL (u; blue dots). This population is polyclonal by Vbeta analysis (v). PD-1 bright population shows normal T-cell immunophenotype of CD2+/CD3+/CD4+/CD5+/CD7+(w–y; blue dots). Expansion of CD4-positive T-cell population with bright PD-1 expression is present in reactive follicular hyperplasia (z; blue dots). This population is polyclonal by Vbeta analysis (aa). PD-1 bright population shows normal T-cell immunophenotype of CD2+/CD3+/CD4+/CD5+/CD7+(ab–ad; blue dots). a, d, g, j: Hematoxylin and Eosin, ×400; b, e, h, n: PD-1 immunohistochemistry, ×400; k: CD20 immunohistochemistry, ×400; m: CD2 immunohistochemistry, ×400.
Previous studies identified abnormal T-cell population with sCD3-/CD4+immunophenotype in AITL9–13. In our cohort, ~15% of AITL show two distinct abnormal T-cell populations with and without surface CD3 expression (Case 10, 12, 17, 23). For these cases, in addition to the frankly abnormal T-cell population with loss of sCD3, an additional population with sCD3+/CD4+/PD-1 bright phenotype was revealed to be monoclonal by Vbeta fragment analysis and molecular TCR analysis (Supplementary Fig. 2). Our study demonstrated that the tumor burden could be highly underestimated when only surface CD3-negative population is considered. Co-existence of surface CD3 positive and negative populations within the same AITL is to our knowledge a novel finding that deserves further pathophysiologic investigation.
The current WHO classification recognizes other nodal lymphomas of TFH origin, including follicular T-cell lymphoma and nodal PTCL with TFH phenotype; that said this entity is rare, and the diagnosis likely has similar clinical implications to AITL5. Among our 28 cases, one case has a diagnosis of PTCL with TFH phenotype based on the morphological assessment (Case 26). Although case number is limited, our finding of bright PD-1 expression by flow cytometry was a consistent finding in PTCL with TFH phenotype.
In conclusion, flow cytometric immunophenotyping with anti-PD-1 antibody reliably detects neoplastic population in AITL including challenging and complex cases. Flow cytometric analysis reliably differentiates AITL from other PTCL and morphologic mimics with high sensitivity and specificity. Peripheral blood flow cytometry for PD-1 expression is a very useful tool in supporting or not supporting the diagnosis of AITL in corresponding tissue biopsy. Without exception, AITL appears to be a systemic (stage IV) disease at presentation when assessed with sensitive techniques, and this implies the utility of flow cytometry for peripheral blood-based screening for AITL. Measurement of PD-1 expression by flow cytometry is a powerful tool in diagnosis and monitoring of AITL.
Supplementary information
Acknowledgements
This research was funded through the National Institutes of Health, National Cancer Institute Cancer Center Support grant P30 CA008748 and P50 CA192937.
Author contributions
M.Y., A.D., and M.R. designed the study, performed, and analyzed the data, and wrote the manuscript; Q.G. analyzed data and conducted flow cytometric analysis; S.H. analyzed data and conducted molecular genetic analysis; N.O., N.L, and J.D.P. gathered and assembled data, A.J.M., and S.M.H. provided patients care, and all authors read and edited the paper.
Conflict of interest
A.J.M.: Consultancy from ADC Therapeutics, Erytech Pharma, Bristol-Myers Squibb, Cell Medica, Takeda Pharmaceuticals, Kyowa Hakko Kirin Pharma, miRagen Therapeutics Inc, Seattle Genetics. Research Funding from Bristol-Myers Squibb, Incyte, Kyowa Hakko Kirin Pharma, miRagen Therapeutics Inc, Seattle Genetics and Merck. Honoraria from Seattle Genetics. S.M.H.: Clinical research support/ Data safety monitoring board from ADC Therapeutics, AILERON, BeiGene, Celgene Corporation, Daiichi- Sankyo Co., Forty Seven, Inc., GlaxoSmithKline, Merck & Co., Inc., Miragen, Portola Pharmaceuticals, Inc., Seattle Genetics, Inc., Takeda/ Millennium, Trillium and Verastem. Scientific advisory boards, consultant or expert witness from Astex Pharmaceuticals, Celgene Corporation, Kura Oncology, Inc., Kyowa Hakko Kirin Co., Ltd., Portola Pharmaceuticals, Inc., Seattle Genetics, Inc., Takeda Pharmaceuticals North America, Inc. and Verastem. A.D.: Personal fees from Roche, Corvus Pharmaceuticals, Physicians’ Education Resource, Seattle Genetics, Peerview Institute, Oncology Specialty Group, Pharmacyclics, Celgene, Novartis, Takeda, EUSA Pharma and research grants from National Cancer Institute and Roche. M.R.: Physicians’ Education Resource: Other: Provision of services; Celgene: Other: Provision of Services; Auron Therapeutics: Equity Ownership, Other: Provision of services. Other authors declare no relevant financial interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41408-020-0301-x).
References
- 1.Vose J, Armitage J, Weisenburger D. International T-Cell Lymphoma Project: International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J. Clin. Oncol. 2008;26:4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 2.de Leval L, et al. The gene expression profile of nodal peripheral T-cell lymphoma demonstrates a molecular link between angioimmunoblastic T-cell lymphoma (AITL) and follicular helper T (TFH) cells. Blood. 2007;109:4952–4963. doi: 10.1182/blood-2006-10-055145. [DOI] [PubMed] [Google Scholar]
- 3.Grogg KL, et al. Angioimmunoblastic T-cell lymphoma: a neoplasm of germinal-center T-helper cells? Blood. 2005;106:1501–1502. doi: 10.1182/blood-2005-03-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Leval L, Gisselbrecht C, Gaulard P. Advances in the understanding and management of angioimmunoblastic T-cell lymphoma. Br. J. Haematol. 2010;148:673–689. doi: 10.1111/j.1365-2141.2009.08003.x. [DOI] [PubMed] [Google Scholar]
- 5.Dogan A., et.al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (Revised 4th edn) 407–412 (IARC, Lyon, 2017).
- 6.Dorfman DM, Shahsafaei A. CD200 (OX-2 membrane glycoprotein) is expressed by follicular T helper cells and in angioimmunoblastic T-cell lymphoma. Am. J. Surg. Pathol. 2011;35:76–83. doi: 10.1097/PAS.0b013e31820065c9. [DOI] [PubMed] [Google Scholar]
- 7.David JA, Huang JZ. Diagnostic utility of flow cytometry analysis of reactive T cells in nodular lymphocyte-predominant hodgkin lymphoma. Am. J. Clin. Pathol. 2016;145:107–115. doi: 10.1093/ajcp/aqv017. [DOI] [PubMed] [Google Scholar]
- 8.de Mel S, et al. The utility of flow cytometry in differentiating NK/T cell lymphoma from indolent and reactive NK cell proliferations. Cytometry B Clin. Cytom. 2018;94:159–168. doi: 10.1002/cyto.b.21529. [DOI] [PubMed] [Google Scholar]
- 9.Serke S, et al. Circulating CD4+ T lymphocytes with intracellular but no surface CD3 antigen in five of seven patients consecutively diagnosed with angioimmunoblastic T-cell lymphoma. Cytometry. 2000;42:180–187. doi: 10.1002/1097-0320(20000615)42:3<180::AID-CYTO4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 10.Singh A, et al. Peripheral blood sCD3(-) CD4(+) T cells: a useful diagnostic tool in angioimmunoblastic T cell lymphoma. Hematol. Oncol. 2014;32:16–21. doi: 10.1002/hon.2080. [DOI] [PubMed] [Google Scholar]
- 11.Stacchini A, et al. The usefulness of flow cytometric CD10 detection in the differential diagnosis of peripheral T-cell lymphomas. Am. J. Clin. Pathol. 2007;128:854–864. doi: 10.1309/MC7QRGPTV0LRR98X. [DOI] [PubMed] [Google Scholar]
- 12.Alikhan M, et al. Peripheral T-cell lymphomas of follicular helper T-cell type frequently display an aberrant CD3(-/dim)CD4(+) population by flow cytometry: an important clue to the diagnosis of a Hodgkin lymphoma mimic. Mod. Pathol. 2016;29:1173–1182. doi: 10.1038/modpathol.2016.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loghavi S, et al. Immunophenotypic and diagnostic characterization of angioimmunoblastic T-cell lymphoma by advanced flow cytometric technology. Leuk. Lymphoma. 2016;57:2804–2812. doi: 10.3109/10428194.2016.1170827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Dongen JJ, et al. EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia. 2012;26:1908–1975. doi: 10.1038/leu.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.