Abstract
Caenorhabditis elegans presents functioning, biologically relevant phenotypes and is frequently used as a bioindicator of toxicity. However, most C. elegans in vivo effect-assessment methods are laborious and time consuming. Therefore, we developed a novel method to measure the oxygen consumption rate of C. elegans as a sublethal endpoint of toxicity. This protocol was tested by exposing 50 larval stage one C. elegans individuals for 48 h (at 20 °C) to different concentrations of two toxicants i.e. benzylcetyldimethylammonium chloride (BAC-C16) and cadmium (Cd). Following exposures, the oxygen consumption rate of the C. elegans individuals were measured using the high-throughput functionality of the Seahorse XFe96 Extracellular Flux Analyzer. Dose-response curves for BAC-C16 (R2 = 0.93; P = 0.001) and Cd (R2 = 0.98; P = 0.001) were created. Furthermore, a strong, positive correlation was evidenced between C. elegans oxygen consumption rate and a commonly used, ecologically relevant endpoint of toxicity (growth inhibition) for BAC-C16 (R2 = 0.93; P = 0.0001) and Cd (R2 = 0.91; P = 0.0001). The data presented in this study show that C. elegans oxygen consumption rate can be used as a promising functional measurement of toxicity.
Subject terms: Caenorhabditis elegans, High-throughput screening, Toxicology
Introduction
Caenorhabditis elegans Maupas, 1900 has been extensively used to study the toxic effect of pollutants, drugs, and environmental samples1. One clear benefit is the exposure of an intact animal with functioning digestive, endocrine, neuromuscular, reproductive, and sensory systems2, i.e. phenotypes that are biologically relevant3. This species is also small in size, easy to culture, and can even be maintained axenically2,4. Furthermore, studies have shown that C. elegans bioassays can be used to predict mammalian development at a fraction of the cost of traditional animal testing2,5. These qualities and benefits complement C. elegans as a model organism for toxicity testing, as well as its use in high-throughput assessment protocols3, as has been developed for drugs6 and pollutants of environmental concern7.
Commonly used toxicity endpoints include feeding, fertility, growth, movement, reproduction, and survival of C. elegans1,8. Respiration of C. elegans has also been used, although infrequently, to study the effect of toxicant exposure9–11. This endpoint has been shown to serve as an effective measure of toxicity of especially metal pollution in, among others, microbes12, daphnia13 and earthworms14. Therefore, with the development of state-of-the-art, high-throughput respirometers, such as the Seahorse XFe96 Extracellular Flux Analyzer, the relevance and applicability of C. elegans oxygen consumption rate (OCR) measurements have greatly improved. This has led to the development of acute response protocols that measure C. elegans OCR before and after the injection of pre-loaded compounds, typically oligomycin, FCCP, rotenone and antimycin A. Such compounds facilitate the measurement of mitochondrial respiratory chain functionality in organisms by determining, for example, ATP production, proton leak, maximal respiration, spare respiratory capacity and non-mitochondrial respiration15–17.
However, with such short exposure periods and the lack of food, these OCR protocols have limited environmental relevance. Longer exposure periods would allow the measurement of a chronic response, which is facilitated by the short life cycle (approximately 3 days at 20 °C) of C. elegans3,18. Furthermore, by adding a food source, nematode respiration would be sustained16 and toxicant bioaccessibility increased as feeding nematodes will ingest dissolved and bacterial-bound substrates19. Therefore, the aim of this research was to develop an environmentally relevant C. elegans OCR protocol for sublethal toxicity testing by utilizing the high-throughput capabilities of the Seahorse XFe96 Extracellular Flux Analyzer.
Results and discussion
Food density
A food density bioassay was performed in order to determine the minimum amount of food (Escherichia coli OP50) required to ensure uninhibited nematode development during the present study’s experimental procedures. The relationship between food density and nematode length is illustrated in Fig. 1. Nematode length (or rather growth) was clearly inhibited by decreased food availability, a well-studied response often used to investigate the effect of dietary restrictions on C. elegans development20,21. Therefore, using a segmented regression model, the minimum density of food that allowed unrestricted nematode development was determined. This model indicated that a plateau for nematode growth was reached at a food (E. coli) OD of 0.59 (595 nm). Subsequently, an OD of 0.6 was used for the experimental bioassays.
Figure 1.
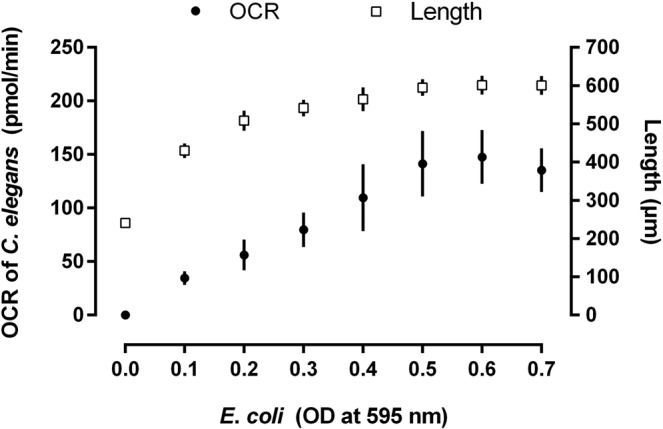
Food density bioassay. Average length and total oxygen consumption rate (OCR) of 50 larval stage one Caenorhabditis elegans nematodes are considered at different optical densities (at 595 nm) of its bacterial food source (Escherichia coli OP50) after 48 h incubation at 20 °C.
Furthermore, the relationship between C. elegans length and OCR was investigated (Fig. 1) and visualised as a non-linear exponential growth curve (Fig. 2a). In order to infer statistical meaning from this relationship, a linear regression model (Fig. 2b) was applied. The slope of the log10 transformed linear model () differed significantly (P = 0.0002) from zero with nematode length explaining 98% of the variation in OCR of C. elegans. The 95% confidence bands, as illustrated in Fig. 2b, indicated a low degree of uncertainty. Previous studies have reported on the change in OCR as a function of C. elegans larval development and/or adult aging16,22–26. However, these observations were mainly made per life stage or for L4 and adult nematodes. It should be noted that the reason for the slight decrease in OCR at a food density of 0.7 OD (at 595 nm) (Fig. 1) remains unknown, however, it is possible, although unconfirmed, that high densities of E. coli cells can impair OCR measurement. Nonetheless, the findings presented here clearly show that following incubation, C. elegans OCR correlate to its growth stage as influenced by food availability.
Figure 2.
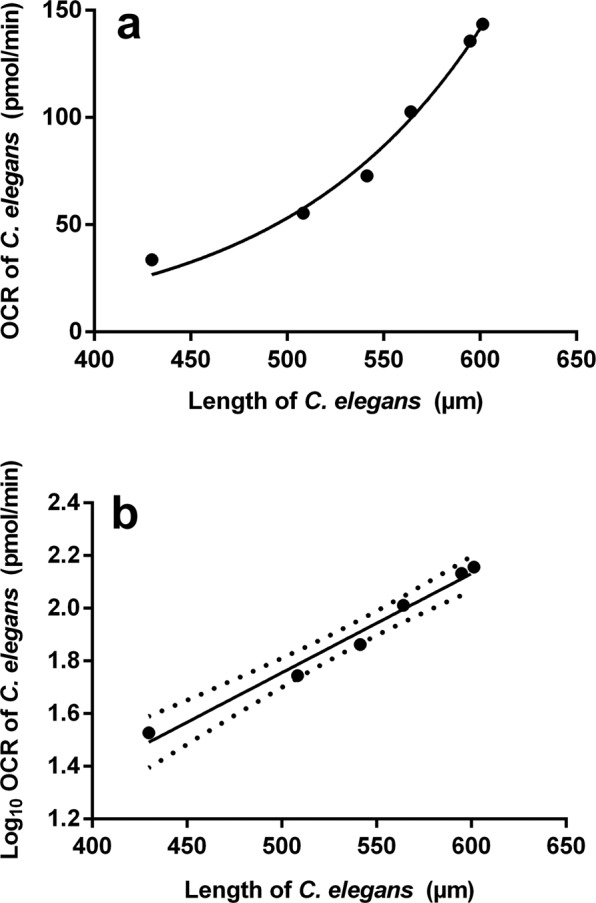
Oxygen consumption rate (OCR) of Caenorhabditis elegans. The OCR of Caenorhabditis elegans (of 50 larval stage one nematodes) is considered against the average length of such specimens as an (a) exponential (non-linear) growth curve and (b) log10 transformed linear model (Y = 0.003761X − 0.1247). The slope of the linear model differed significantly (P = 0.0002) from zero with nematode length explaining 98% of the OCR variation.
Oxygen consumption rate inhibition due to toxicant exposure
For each toxicant bioassay the percentage OCR inhibition of C. elegans per exposure concentration was measured against a negative control (M9 medium). Following, concentration-response curves for Benzylcetyldimethylammonium chloride monohydrate (BAC-C16) (R2 = 0.93; P = 0.001) (Fig. 3) and cadmium (Cd) (R2 = 0.98; P = 0.001) (Fig. 4) were created and used to derive ECx values (Table 1) at the 10, 20, and 50% inhibition levels.
Figure 3.
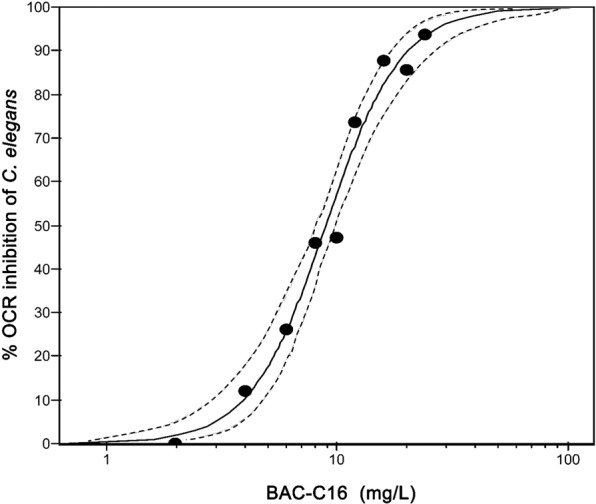
Oxygen consumption rate (OCR) inhibition following benzylcetyldimethylammonium chloride monohydrate (BAC-C16) exposure. Concentration-response curve of Caenorhabditis elegans oxygen consumption rate (OCR) inhibition following exposure to BAC-C16. The R2 value was calculated as 0.93 (P = 0.001) and 95% confidence bands are indicated as dotted lines.
Figure 4.
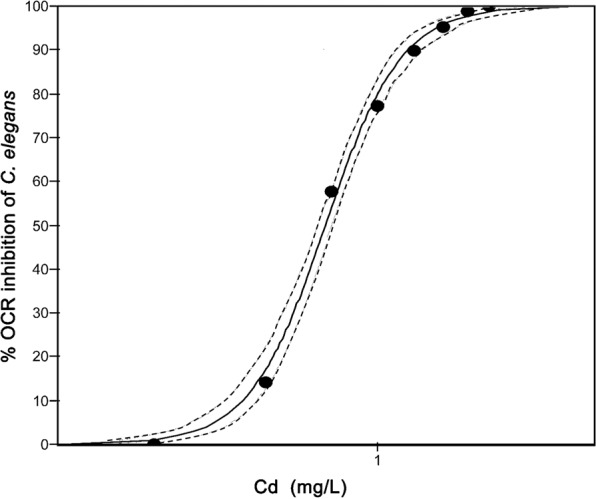
Oxygen consumption rate (OCR) inhibition following cadmium (Cd) exposure. Concentration-response curve of Caenorhabditis elegans oxygen consumption rate (OCR) inhibition following exposure to Cd. The R2 value was calculated as 0.98 (P = 0.001) and 95% confidence bands are indicated as dotted lines.
Table 1.
Effective concentration (EC) values representing 10, 20 and 50% of Caenorhabditis elegans oxygen consumption rate (OCR) and growth inhibition.
| BAC-C16 | OCR | Growth | ||||
|---|---|---|---|---|---|---|
| EC10 | EC20 | EC50 | EC10 | EC20 | EC50 | |
| Value (mg/L) | 3.99 | 5.26 | 8.94 | 3.4 | 4.96 | 9.47 |
| Lower 95% Cl | 2.96 | 4.23 | 8.07 | 2.72 | 4.25 | 8.8 |
| Upper 95% Cl | 4.82 | 6.08 | 9.83 | 4.00 | 5.58 | 10.5 |
| Cadmium | ||||||
| Value (mg/L) | 0.44 | 0.52 | 0.73 | 0.43 | 0.56 | 0.86 |
| Lower 95% Cl | 0.39 | 0.48 | 0.69 | 0.32 | 0.45 | 0.79 |
| Upper 95% Cl | 0.47 | 0.55 | 0.76 | 0.51 | 0.63 | 0.94 |
These values were calculated with a Probit analysis using the linear maximum likelihood regression algorithm. Upper and lower 95% confidence intervals (CI) values, based on Fieller’s Theorem, are also provided.
The BAC-C16 and Cd concentrations at which 50% OCR inhibition of C. elegans occurred were calculated as 8.94 mg/L and 0.73 mg/L (Table 1), respectively. Benzylcetyldimethylammonium chloride monohydrate is routinely used as a positive control for C. elegans toxicity assays27,28, while also serving as the positive control in standardised toxicity testing29. Although C. elegans OCR inhibition following exposure to BAC-C16 has never been investigated, Schouest et al.11 used fluorescence oxygen sensing and recorded significant OCR reduction following 24 h exposure of C. elegans adults to Cd, as well as other toxicants (e.g. zinc and rotenone). The latter authors calculated an EC50 value (for OCR inhibition) following exposure to Cd of 60.85 µM (6.84 mg/L). This is substantially higher than the EC50 value reported for this study (0.73 mg/L). However, in the present study longer exposure periods of 48 h (vs 24 h) and larval stage one (vs adult) nematodes were used. It is known that longer exposure periods can lead to greater toxicity30, while larval stages are typically more sensitive to metal exposure than adults31.
Also worth considering is the sensitivity of OCR compared to reproduction, since the latter is regarded as one of the most sensitive C. elegans endpoints of toxicity19,29. Comparing our results to the findings of other studies, it seems that OCR might be slightly less sensitive than reproduction (EC50 of BAC-C16: 7.5 mg/L; EC50 of Cd: 0.21 mg/L)19,27. However, these direct comparisons should be made with caution since different exposure periods were used for deriving ECx values for OCR (48 h) and reproduction (96 h).
Growth inhibition due to toxicant exposure
Caenorhabditis elegans growth inhibition was also investigated for two reasons: (1) a strong relationship, as evidenced in the food density bioassay, existed between C. elegans OCR and length and (2) C. elegans growth inhibition is routinely used as an endpoint of toxicity and can therefore be used to evaluate the sensitivity of C. elegans OCR inhibition as an endpoint of toxicity. Growth inhibition concentration-response curves for BAC-C16 (R2 = 0.97, P = 0.001) and Cd (R2 = 0.95, P = 0.001) are illustrated on Figs. 5 and 6, respectively.
Figure 5.
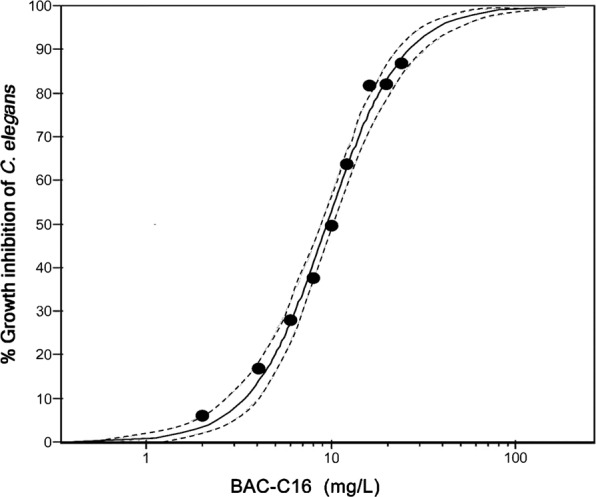
Growth inhibition following benzylcetyldimethylammonium chloride monohydrate (BAC-C16) exposure. Concentration-response curve of Caenorhabditis elegans growth inhibition following exposure to BAC-C16. The R2 value was calculated as 0.97 (P = 0.001) and 95% confidence bands are indicated as dotted lines.
Figure 6.
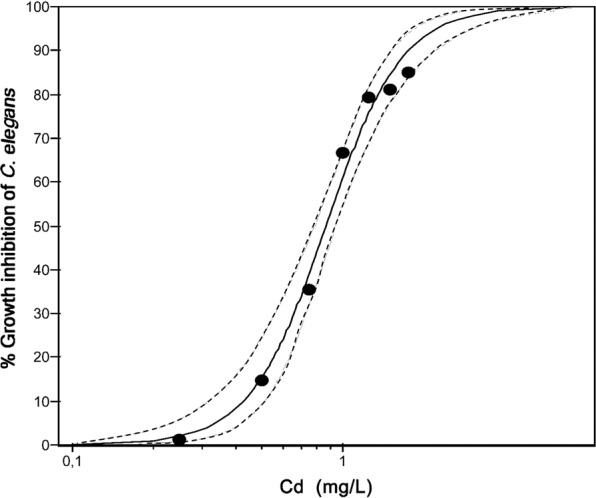
Growth inhibition following cadmium (Cd) exposure. Concentration-response curve of Caenorhabditis elegans growth inhibition following exposure to Cd. The R2 value was calculated as 0.95 (P = 0.001) and 95% confidence bands are indicated as dotted lines.
The BAC-C16 concentration at which 50% growth inhibition of C. elegans occurred was calculated as 9.47 mg/L (Table 1). Although EC values of C. elegans OCR inhibition for BAC-C16 have not previously been reported, ISO1087229 states that the EC50 value for BAC-C16 growth inhibition typically ranges between 8 and 22 mg/L. Most studies have reported EC50 values of approximately 15 mg/L27,28, however, Schertzinger et al.32 reported EC50 values for two separate tests of 9.1 and 10.8 mg/L, respectively. The latter values are therefore similar to results reported in this study.
According to Hanna et al.28 growth inhibition by BAC-C16 is substantially influenced by food density, with lower densities presenting higher inhibition rates. However, this was not viewed as a concern during the present study as the minimum required amount of food for C. elegans was used at a constant density in control and exposure wells of toxicant bioassays.
The Cd concentration at which 50% growth inhibition of C. elegans occurred was calculated as 0.86 mg/L (Table 1). As with BAC-C16, Cd toxicity has been linked to food density, with increasing toxicity as food density decreases19. The latter authors attributed this to a decrease in the bioavailability of freely dissolved Cd at high bacterial densities. Traunspurger et al.33 reported a lowest observed effect concentration (LOEC; 72 h exposure) of 0.14 mg/L for the growth of C. elegans. This can be compared to the present study’s EC10 and EC20 values of 0.43 and 0.56 mg/L, respectively, following 48 h exposure. Van Kessel et al.34 on the other hand, showed a substantially higher LOEC (11.2 mg/L) for C. elegans after 48 h exposure to Cd. However, this was in the absence of food, which results in highly reduced Cd bioaccessibility. The presence of bacteria (food source) stimulates C. elegans pharyngeal pumping, which promotes the uptake of dissolved and bacterial-bound Cd19.
Comparing OCR and growth inhibition
Similar ECx values for OCR and growth inhibition of C. elegans were evidenced (Table 1). Furthermore, the correlations between OCR and growth inhibition for BAC-C16 and Cd were strong [R2 = 0.93 (P = 0.001) and R2 = 0.91 (P = 0.001), respectively] and thus support the relationship evidenced between nematode length and OCR in the food density bioassay.
Lastly, this protocol and the use of C. elegans as a test organism can be further studied by, for example, investigating the sensitivity of C. elegans organismal vs. cellular respiration15–17. Considering that for the tested toxicants, OCR was equally sensitive to growth (an accepted and routinely used endpoint of toxicity1,29) we have clearly demonstrated that OCR inhibition can be used as an alternative method that facilitate high-throughput and rapid measurements.
Quality control
A number of steps were taken to maximise the confidence in the generated results. Firstly, for accurate OCR measurements, oxygen consumption from other organisms than the test organism should be excluded. Since E. coli, serving as food source for the C. elegans, is an aerobic bacterium, the bacterial cultures had to be inactivated before use [see Stock preparation of the food source (Escherichia coli)]. Additionally, Penicillin-Streptomycin (Pen-Strep) was added to each well (see Experimental procedure) to ensure the inactivation of the E. coli food source and prevent bacterial contamination. Bacterial inactivation was checked by measuring the OCR of four extra wells with inactivated bacteria, but without nematodes (see Bioassay plate layout). In case of any oxygen consumption in these “E. coli” controls, the whole bioassay plate was discarded.
Secondly, temperature drifts occur during the operation of the Seahorse respirometer (see Temperature requirements). However, using a Seahorse respirometer, Koopman et al.16 studied the OCR of L4 and adult C. elegans and found no significant (P > 0.05) difference in OCR between 20 °C and 25 °C. Also in this study, OCR data measured with the Seahorse respirometer at 20 °C and 24.5 °C were not significantly different (P > 0.05), indicating that temperature shifts in this range have only a negligible influence on the outcome of the bioassays.
Lastly, all wells of the bioassay plate should be sufficiently oxygenated during OCR measurement in order to avoid inaccurate readings. During this study, the unprocessed oxygen level data (in mmHg) was checked (following OCR measurement) in order to confirm that anoxic conditions were never reached.
Final considerations
The results presented in this study provide support for the use of C. elegans OCR inhibition as a functional and ecologically relevant endpoint of toxicity. Although different methods and instruments can be utilized, the high-throughput capability of modern respirometers facilitate simultaneous and rapid toxicity measurement of substances at various concentrations. Also, with further advancement and optimization of respirometers (e.g. better temperature management) it is likely that preparation and operating times will be reduced and measurement accuracy increased. Lastly, the authors recommend to explore more appropriate bacterial inactivation methods (e.g. without the addition of chemicals), or even the use of anaerobic bacteria as a food source.
Methods
Cultures and reagents
Cultures of C. elegans N2 and E. coli OP50 (food source) were obtained from the Caenorhabditis Genetics Centre (https://cbs.umn.edu/cgc/home). Stock solutions of sterile M9 medium (buffer) and cholesterol were prepared, as well as cultures of C. elegans reared, following ISO1087229. Penicillin-Streptomycin (Gibco 100X) was obtained from Thermo Fisher Scientific while the remaining reagents used in this study were obtained from Sigma-Aldrich®.
Stock preparation of the food source (Escherichia coli)
An important step in the preparation of food stocks is the culturing, inactivation, and density adjustment of E. coli before the commencement of a bioassay. Escherichia coli was cultured, washed, and pelleted according to ISO1087229. The pellet was re-suspended in M9 medium after which an aliquot was diluted (1 → 10) and the optical density (OD) measured at 595 nm35 using a Pharo 300 Spectroquant (spectrophotometer). Finally, the density of the E. coli suspension was adjusted to an OD of 3 (595 nm). Thereafter, 5 mL aliquots of food stocks were transferred to 15 mL conical centrifuge tubes and heat inactivated (30 min at 65 °C) using a water bath36. The stocks were stored at −80 °C for a maximum of two weeks.
Synchronization of Caenorhabditis elegans
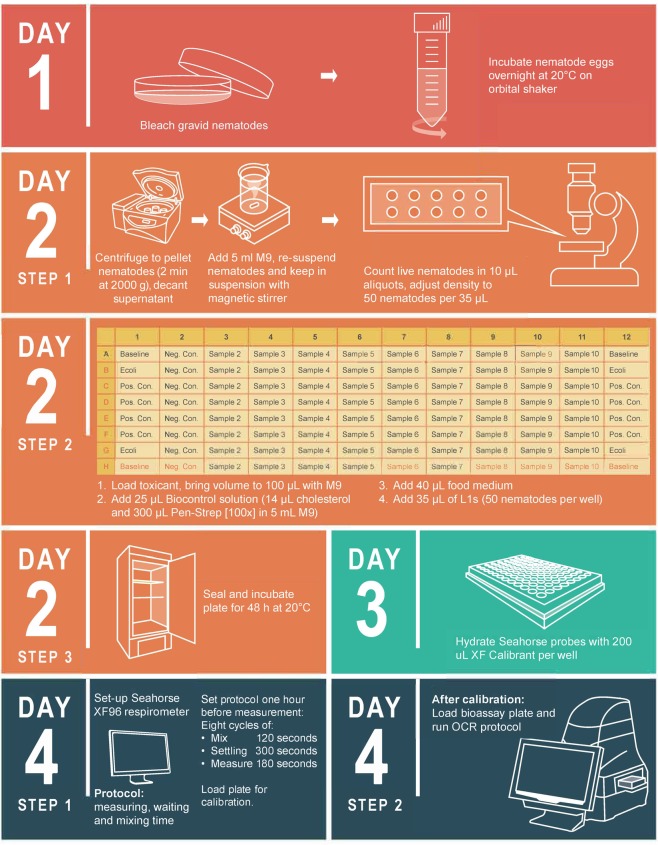
A schematic overview of the experimental procedures, as well as the appropriate timeline, is provided in Fig. 7. On day one, C. elegans eggs were extracted from culture plates using the sodium hypochlorite (bleaching) method, followed by three wash cycles to remove any residual chemicals15. Subsequently, synchronized larval stage one (L1) nematodes were obtained after overnight (12–20 h) incubation in 50 mL sterile conical centrifuge tubes at 20 °C on an orbital shaker (100 rpm)15. This procedure also kills and dissolves E. coli, which could otherwise influence the outcome of the bioassay. It should be noted that prior to bleaching, the culture plates were studied using a Nikon SMZ1000 stereo microscope (100x magnification) to confirm the presence of eggs and gravid females.
Figure 7.
Experimental setup. Schematic overview of the experimental procedure and associated timeline for culturing Caenorhabditis elegans and its bacterial food source (Escherichia coli OP 50). The timeline for preparing and incubating the bioassay plate, as well as for C. elegans oxygen consumption rate (OCR) measurement using the Seahorse XFe96 Extracellular Flux Analyzer, is also provided.
Number of nematodes
An important consideration is the number of nematodes per well to be used for OCR measurements. In order to effect a broad OCR range, 50 L1s per well were used. Koopman et al.16 reported this to be the largest number of larval stage four (L4) (expected life stage after incubation) nematodes to be used per well without inducing anoxic conditions.
Food density and nematode development bioassay
Since food availability can have a substantial impact on C. elegans development36, a food density (0.1–0.7 OD, 595 nm) bioassay (see Bioassay plate layout) was performed (as described in Experimental procedure), with 50 L1s per well. The OCR of C. elegans was measured as detailed in Seahorse respirometer setup and oxygen consumption rate measurement.
Toxicant stock solutions
In order to test the viability of OCR inhibition of C. elegans as a sublethal endpoint of toxicity, benzylcetyldimethylammonium chloride monohydrate (BAC-C16) and cadmium (Cd) were selected as toxicants. Benzylcetyldimethylammonium chloride monohydrate is routinely used as a positive control for C. elegans growth inhibition27–29, while Cd is regarded as an environmentally relevant, non-essential metal19,37,38.
The exposure solutions for BAC-C16 (made up in M9 medium) had the following concentrations: 2, 4, 6, 8, 10, 12, 16, 20, and 24 mg/L. For Cd, the exposure concentrations in M9 medium were 0.25, 0.5, 0.75, 1, 0.25, 1.5, 1.75, and 2 mg/L. For both assays (designed, performed, and measured as described in the following sections) the negative control consisted of M9 medium.
Bioassay plate layout
The bioassay was carried out in 96-well culture plates, which will from hereon be referred to as ‘bioassay plates’. The bioassay plate layout (Fig. 7) was designed to allow for the maximum number of exposure concentrations with sufficient replication. The four corners (A1, H1, A12, and H12) represented the baseline wells, which the Seahorse respirometer uses for background correction of zero oxygen consumption. Four wells (B1, G1, B12, and G12) labelled “Ecoli” were reserved for E. coli food stocks (0.6 OD, 595 nm) containing a biocontrol solution (Pen-Strep and cholesterol), brought to a final volume of 200 µL with M9 medium. These wells were included to ensure that zero E. coli oxygen consumption, which could substantially impact OCR measurements, occurred (also see Quality Control).
Wells C1 – F1 and C12 – F12 were assigned for future use as a positive control for which BAC-C16 is recommended at a concentration of 8.94 mg/L (EC50 value of OCR inhibition as determined during this study). For the positive control the oxygen consumption rate inhibition of C. elegans (compared to the negative control), should range between 20% and 80%29. Column 2 was represented by the negative control, while columns 3 to 11 were used for the measurement of nine treatments with eight replicates each.
Experimental procedure
Working in a sterile environment, the following preparation steps were executed on day two (Fig. 7):
A tube of E. coli food stock was allowed to thaw and reach room temperature.
Synchronized L1 nematodes were pelleted (2 min at 2000x g) and the supernatant discarded in order to remove residual material. Next, the pellet was re-suspended in 5 mL M9 medium using a magnetic stirrer. It should be noted that studying L1 nematodes using a Nikon Eclipse 50i light microscope (1000x magnification) after being subjected to stirring revealed no physical damage. Similarly, Van Aardt et al.39 reported that stirring speed had no effect on the OCR of Meloidogyne incognita (Kofoid & White, 1919) Chitwood, 1949 second-stage juveniles.
Suspended nematodes were transferred, in 10 µL aliquots, to a microscope slide and counted using a Nikon SMZ1000 stereo microscope (40-100x magnification). The average number of nematodes in 10 replicates were calculated per 1 µL. Thereafter, the concentration of nematodes in suspension was adjusted to 50 individuals per 35 µL.
- Stock solutions of the studied toxicants (BAC-C16 and Cd) were prepared at twice the concentration of the highest exposure concentration as stock solutions were diluted 1:2 with food, biocontrol solution (see below), and nematode suspensions (Fig. 7). By taking into account the final volume (200 µL) of each well, the required volume of toxicant stock was calculated for each exposure concentration using the following equation:
where V1 represents the volume (unit: µL) of toxicant stock, C2 the final exposure concentration (unit: mg/L), V2 the final well volume of 200 µL, and C1 the toxicant stock concentration (unit: mg/L). -
Biocontrol solution: A solution of cholesterol stock (14 µL), Pen-Strep (200 µL), and M9 medium (5 mL) was prepared. Cholesterol is necessary for the development of C. elegans40, while Pen-Strep ensured the inactivation of the E. coli food source and prevented bacterial contamination35. Pen-Strep is frequently used in the cultivation of C. elegans41,42 and was not expected to significantly influence the results. It is also important to consider that Pen-Strep were added to both control and exposure wells, which therefore renders it potential effect (if any) on the final results negligible.
Immediately following these preparation steps, the bioassay plate (Fig. 7) was loaded in the following sequence:
The required volume of M9 medium was added to ensure a final volume per well of 200 µL.
The calculated volume of toxicant stock per exposure concentration was loaded.
E. coli food stocks were briefly vortexed at room temperature after which 40 µL was added to all bioassay wells (excluding ‘Baseline’ wells). This ensured a final OD of 0.6 (595 nm).
Finally, 25 µL of the biocontrol solution were added to all bioassay wells (excluding ‘Ecoli’ wells).
The bioassay plate was placed on an orbital shaker (100 rpm) for 15 min in order to ensure sufficient mixture of reagents.
Lastly, 35 µL of the nematode solution was added to each control and exposure well. No nematodes were added to the ‘baseline’ or ‘Ecoli’ wells. Using a Nikon TS100 inverted microscope (40-100x magnification) each well was checked to ensure correct loading of reagents and nematodes.
The bioassay plate was sealed with parafilm and incubated for 48 h at 20 °C. This incubation period was chosen to effect high OCR readings without risk of oxygen depletion during measurement16.
Additional considerations for the execution of this protocol follows:
A final volume of 200 µL was assayed in order to simplify the calculation of the concentration and volume of solutions. However, according to the manufacturer (Agilent Technologies, Santa Clara, United States) the final well volume can range between 150 µL and 275 µL43. Therefore, the protocol can be adjusted accordingly if lower or higher well volumes are required.
Seahorse respirometer setup and oxygen consumption rate measurement
Cartridge hydration
The Seahorse respirometer makes use of optic fibre bundles, which insert into solid state sensor probes (containing polymer embedded fluorophores) and emit light to excite the fluorophores. These optic fibres then measure the change in fluorophore emission resulting from the change in oxygen concentration and thus serves as an indirect measurement of oxygen consumption. On day three (Fig. 7), the Seahorse respirometer cartridge, which houses the probes, was hydrated by adding 200 µL XF calibrant to each well followed by overnight incubation at 37 °C.
Temperature requirements
The Seahorse respirometer was designed for cell OCR measurement at 37 °C, contrary to C. elegans’ typical culture temperature range of between 16 and 25 °C18. While the instrument is capable of regulating the temperature in this range, it requires a room temperature of 4 °C, which was logistically not possible. Therefore, the room was cooled to the lowest possible temperature of 16 °C. The respirometer’s temperature was set to 24 °C and the internal heater switched off. It should be noted that the Seahorse respirometer generates heat during operation and was therefore only powered on directly before use. On day four (Fig. 7), the Seahorse cartridge was removed from the incubator two hours prior to use and left to cool and reach ambient temperature (16 °C).
Seahorse settings
Prior to OCR measurement, the following Seahorse respirometer protocol was programmed (Fig. 7):
Two min mixing: This involves the raising and lowering of the cartridge in order to replenish the oxygen levels within each well.
Five min waiting: The cartridge remains stationary in the ‘raised’ position to allow the nematodes to settle.
Three min measuring: The cartridge is lowered and a microchamber (of 3 µL) is created at the bottom of each well in which nematode oxygen consumption is measured. The decrease in oxygen is converted to a single OCR value per well.
This represents one measurement cycle, which was repeated eight times. After programming and 60 min prior to the OCR test, the Seahorse XF cartridge was inserted into the Seahorse respirometer for calibration. The calibration step is standard in every Seahorse analysis and cannot be unselected. During this calibration of the sensors, the instrument reads the coefficients of the sensor cartridge and bioassay plate to ensure accurate data acquisition. Once calibrated, the bioassay plate containing the nematodes was inserted into the Seahorse respirometer after which it underwent an equilibration period (12 min) during which temperature stability across the plate is ensured, followed by the above detailed OCR protocol. Oxygen levels during and after OCR measurement were checked to ensure that anoxic conditions were not induced. The Agilent Seahorse Wave 2.4 software package was used for exporting OCR data.
Upon completion of OCR measurement, 100 µL Bengal Rose (used here as a nematode staining agent) was added to each well and the bioassay plate heat inactivated (10 min at 80 °C)29. Bioassay plates were stored, for a maximum of 7 days, at 4 °C. Nematode length was measured and growth calculated as described in ISO1087229.
Statistical analyses
Food density bioassay
The average OCR (of the eight measurement cycles) and length of C. elegans were calculated per well and graphically illustrated, at different food densities, using GraphPad Prism 7 software package. Thereafter, the density of food required to allow maximum nematode development was calculated using a segmented regression model. Briefly, the growing phase of the curve was fitted with a quadratic model. The plateau, in turn, was fitted using a constant representing a line running parallel to the density food axis defining the maximum nematode development. The plateau point was determined under a condition of continuity and smoothness as defined in the supplementary material44. This analysis was performed using SAS/STAT software package 9.4.
The relationship between OCR and nematode length was explained by an exponential growth (non-linear) regression model. In order to further study this relationship, the dependant variable (OCR) was log10 transformed and a linear regression model fitted. The 95% confidence limits were also calculated. These graphs were created and analyses performed using GraphPad Prism 7 software package.
Toxicant concentration-response bioassays
The average nematode OCR and growth of the eight measurement cycles were calculated per exposure concentration for the BAC-C16 and Cd concentration-response bioassays. Using ToxRat Professional 3 software package, the percentage decrease per exposure concentration was calculated against the negative control. Thereafter, the Probit analysis using the linear maximum likelihood regression algorithm was performed. The Chi-squared test was used to indicate the goodness-of-fit of the regression line. Furthermore, the effective concentrations (EC10, EC20, and EC50) for OCR and growth inhibition of C. elegans were calculated, while 95% confidence limits were based on Fieller’s Theorem. Lastly, in order to study the relationship between OCR and growth inhibition of C. elegans, the data were tested for normality using the D’Agostino & Pearson omnibus normality test45. The data presented a normal distribution and therefore the Pearson correlation coefficient test was performed.
Oxygen consumption rate response to temperature fluctuations
In order to determine whether temperature had a significant effect on the OCR of C. elegans, the negative control data (of eight measurements) of the two toxicant bioassays were used. Temperatures during both assays ranged between 20 °C and 24.5 °C. Firstly, the bioassay data were tested for normality using the D’Agostino & Pearson omnibus normality test45. Thereafter, the significance between the measurement means were tested using a one-way analysis of variance (ANOVA) (parametric data) or Kruskal-Wallis test (non-parametric data) with Tukey’s test and Dunn’s test applied, respectively, for multiple comparisons. These analyses were performed using GraphPad Prism 7 software package.
Supplementary information
Author contributions
G.D., H.F., M.D., V.W designed the study, G.D., H.M., G.E. prepared the bioassays and performed analyses, S.H., C.R., M.Z. provided technical support with statistical analyses and interpretation of results. All authors reviewed the manuscript.
Data availability
Data will be made available by the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-61054-7.
References
- 1.Hägerbäumer A, Höss S, Heininger P, Traunspurger W. Experimental studies with nematodes in ecotoxicology: an overview. J. Nematol. 2015;47:11–27. [PMC free article] [PubMed] [Google Scholar]
- 2.Hunt PR. The C. elegans model in toxicity testing. J. Appl. Toxicol. 2017;37:50–59. doi: 10.1002/jat.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyd WA, McBride SJ, Rice JR, Snyder DW, Freedman JH. A high-throughput method for assessing chemical toxicity using a Caenorhabditis elegans reproduction assay. Toxicol. Appl. Pharmacol. 2010;245:153–159. doi: 10.1016/j.taap.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scanlan LD, et al. Counting Caenorhabditis elegans: protocol optimization and applications for population growth and toxicity studies in liquid medium. Sci. Rep. 2018;8:904. doi: 10.1038/s41598-018-19187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harlow PH, et al. The nematode Caenorhabditis elegans as a tool to predict chemical activity on mammalian development and identify mechanisms influencing toxicological outcome. Sci. Rep. 2016;6:22965. doi: 10.1038/srep22965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Reilly LP, Luke CJ, Perlmutter DH, Silverman GA, Pak SC. C. elegans in high-throughput drug discovery. Adv. Drug Del. Rev. 2014;0:247–253. doi: 10.1016/j.addr.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung S-K, et al. Multi-endpoint, high-throughput study of nanomaterial toxicity in Caenorhabditis elegans. Environ. Sci. Technol. 2015;49:2477–2485. doi: 10.1021/es5056462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Höss, S. & Williams, P. Ecotoxicity testing with nematodes in Nematodes as environmental indicators (eds Michael J. Wilson & Thomais Kakouli-Duarte) 208–224 (CABI Publishing, 2009).
- 9.Kohra S, et al. A rapid respiratory toxicity test using Caenorhabditis elegans with an oxygen electrode system. J. Health Sci. 2002;48:269–272. doi: 10.1248/jhs.48.269. [DOI] [Google Scholar]
- 10.Zuo Y-T, et al. Toxicity of 2,6-dichloro-1,4-benzoquinone and five regulated drinking water disinfection by-products for the Caenorhabditis elegans nematode. J. Hazard. Mater. 2017;321:456–463. doi: 10.1016/j.jhazmat.2016.09.038. [DOI] [PubMed] [Google Scholar]
- 11.Schouest K, Zitova A, Spillane C, Papkovsky DB. Toxicological assessment of chemicals using Caenorhabditis elegans and optical oxygen respirometry. Environ. Toxicol. Chem. 2009;28:791–799. doi: 10.1897/08-083.1. [DOI] [PubMed] [Google Scholar]
- 12.Bérard A, et al. Soil microbial respiration and PICT responses to an industrial and historic lead pollution: a field study. Environ. Sci. Pollut. R. 2016;23:4271–4281. doi: 10.1007/s11356-015-5089-z. [DOI] [PubMed] [Google Scholar]
- 13.Bownik A. Physiological endpoints in daphnid acute toxicity tests. Sci. Total Environ. 2020;700:134400. doi: 10.1016/j.scitotenv.2019.134400. [DOI] [PubMed] [Google Scholar]
- 14.Tang H, et al. Earthworm (Eisenia fetida) behavioral and respiration responses to sublethal mercury concentrations in an artificial soil substrate. Appl. Soil Ecol. 2016;104:48–53. doi: 10.1016/j.apsoil.2015.12.008. [DOI] [Google Scholar]
- 15.Luz, A. L., Smith, L. L., Rooney, J. P. & Meyer, J. N. Seahorse Xfe24 extracellular flux analyzer‐based analysis of cellular respiration in Caenorhabditis elegans. Curr. Protoc. Toxicol., 25.27.21–25.27.15 (2015). [DOI] [PMC free article] [PubMed]
- 16.Koopman M, et al. A screening-based platform for the assessment of cellular respiration in Caenorhabditis elegans. Nat. Protoc. 2016;11:1798–1816. doi: 10.1038/nprot.2016.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luz AL, et al. Mitochondrial morphology and fundamental parameters of the mitochondrial respiratory chain are altered in Caenorhabditis elegans strains deficient in mitochondrial dynamics and homeostasis processes. PloS ONE. 2015;10:e0130940. doi: 10.1371/journal.pone.0130940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porta-de-la-Riva M, Fontrodona L, Villanueva A, Cerón J. Basic Caenorhabditis elegans methods: synchronization and observation. JoVE. 2012;64:e4019. doi: 10.3791/4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Höss S, Schlottmann K, Traunspurger W. Toxicity of ingested cadmium to the nematode Caenorhabditis elegans. Environ. Sci. Technol. 2011;45:10219–10225. doi: 10.1021/es2027136. [DOI] [PubMed] [Google Scholar]
- 20.Uppaluri S, Brangwynne CP. A size threshold governs Caenorhabditis elegans developmental progression. P. Roy. Soc. B - Biol. Sci. 2015;282:20151283. doi: 10.1098/rspb.2015.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen M, et al. A role for autophagy in the extension of lifespan by dietary restriction in C. elegans. PLoS Genet. 2008;4:e24. doi: 10.1371/journal.pgen.0040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Cuyper C, Vanfleteren JR. Oxygen consumption during development and aging of the nematode Caenorhabditis elegans. Comp. Biochem. Physiol. 1982;73:283–289. doi: 10.1016/0300-9629(82)90071-8. [DOI] [Google Scholar]
- 23.Braeckman BP, Houthoofd K, Vanfleteren JR. Assessing metabolic activity in aging Caenorhabditis elegans: concepts and controversies. Aging Cell. 2002;1:82–88. doi: 10.1046/j.1474-9728.2002.00021.x. [DOI] [PubMed] [Google Scholar]
- 24.Braeckman BP, Houthoofd K, De Vreese A, Vanfleteren JR. Assaying metabolic activity in ageing Caenorhabditis elegans. Mech. Ageing Dev. 2002;123:105–119. doi: 10.1016/S0047-6374(01)00331-1. [DOI] [PubMed] [Google Scholar]
- 25.Braeckman BP, et al. No reduction of energy metabolism in Clk mutants. Mech. Ageing Dev. 2002;123:1447–1456. doi: 10.1016/S0047-6374(02)00085-4. [DOI] [PubMed] [Google Scholar]
- 26.Suda H, Shouyama T, Yasuda K, Ishii N. Direct measurement of oxygen consumption rate on the nematode Caenorhabditis elegans by using an optical technique. Biochem. Biophys. Res. Commun. 2005;330:839–843. doi: 10.1016/j.bbrc.2005.03.050. [DOI] [PubMed] [Google Scholar]
- 27.Höss S, et al. Interlaboratory comparison of a standardized toxicity test using the nematode Caenorhabditis elegans (ISO 10872) Environ. Toxicol. Chem. 2012;31:1525–1535. doi: 10.1002/etc.1843. [DOI] [PubMed] [Google Scholar]
- 28.Hanna S, et al. Feasibility of using a standardized Caenorhabditis elegans toxicity test to assess nanomaterial toxicity. Environ. Sci. Nano. 2016;3:1080–1089. doi: 10.1039/C6EN00105J. [DOI] [Google Scholar]
- 29.ISO10872. Water quality — determination of the toxic effect of sediment and soil samples on growth, fertility and reproduction of Caenorhabditis elegans (Nematoda) (International Organization for Standardization, 2010).
- 30.Connell DW, Yu QJ, Verma V. Influence of exposure time on toxicity—An overview. Toxicology. 2016;355–356:49–53. doi: 10.1016/j.tox.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 31.Chen P, Martinez-Finley E, Bornhorst J, Chakraborty S, Aschner M. Metal-induced neurodegeneration in C. elegans. Frontiers in Aging. Neuroscience. 2013;5:1–11. doi: 10.3389/fnagi.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schertzinger G, Zimmermann S, Grabner D, Sures B. Assessment of sublethal endpoints after chronic exposure of the nematode Caenorhabditis elegans to palladium, platinum and rhodium. Environ. Pollut. 2017;230:31–39. doi: 10.1016/j.envpol.2017.06.040. [DOI] [PubMed] [Google Scholar]
- 33.Traunspurger W, et al. Ecotoxicological assessment of aquatic sediments with Caenorhabditis elegans (nematoda)—a method for testing liquid medium and whole‐sediment samples. Environ. Toxicol. Chem. 1997;16:245–250. [Google Scholar]
- 34.Van Kessel W, Zaalberg RB, Seinen W. Testing environmental pollutants on soil organisms: a simple assay to investigate the toxicity of environmental pollutants on soil organisms, using CdCl2 and nematodes. Ecotoxicol. Environ. Saf. 1989;18:181–190. doi: 10.1016/0147-6513(89)90079-1. [DOI] [PubMed] [Google Scholar]
- 35.Teixeira-Castro A, et al. Serotonergic signalling suppresses ataxin 3 aggregation and neurotoxicity in animal models of Machado-Joseph disease. Brain. 2015;138:3221–3237. doi: 10.1093/brain/awv262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gruber J, Ng LF, Poovathingal SK, Halliwell B. Deceptively simple but simply deceptive – Caenorhabditis elegans lifespan studies: considerations for aging and antioxidant effects. FEBS Lett. 2009;583:3377–3387. doi: 10.1016/j.febslet.2009.09.051. [DOI] [PubMed] [Google Scholar]
- 37.Järup L, Åkesson A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009;238:201–208. doi: 10.1016/j.taap.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 38.Vellinger C, et al. Comparison of arsenate and cadmium toxicity in a freshwater amphipod (Gammarus pulex) Environ. Pollut. 2012;160:66–73. doi: 10.1016/j.envpol.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Van Aardt WJ, Fourie H, Pretorius M, Louw R, Van Dyk H. The effects of stirring, population levels and a potential anti-nematodal product on the respiration of second-stage juveniles of Meloidogyne incognita measured with different technology. Nematology. 2016;18:1053–1061. doi: 10.1163/15685411-00003014. [DOI] [Google Scholar]
- 40.Kawasaki I, Jeong M-H, Yun Y-J, Shin Y-K, Shim Y-H. Cholesterol-responsive metabolic proteins are required for larval development in Caenorhabditis elegans. Mol Cells. 2013;36:410–416. doi: 10.1007/s10059-013-0170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lev I, Bril R, Liu Y, Ceré LI, Rechavi O. Inter-generational consequences for growing Caenorhabditis elegans in liquid. Philosophical Transactions of the Royal Society B: Biological Sciences. 2019;374:20180125. doi: 10.1098/rstb.2018.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stiernagle, T. Maintenance of Caenorhabditis elegans in WormBook [Fay, D (ed)]. WormBook. (WormBook, 2006).
- 43.Agilent. Agilent Seahorse XF instruments overview and selection guide (2018).
- 44.SAS. SAS/STAT User’s Guide (SAS Institute, 2013).
- 45.Ghasemi A, Zahediasl S. Normality tests for statistical analysis: a guide for non-statisticians. Int. J. Endocrinol. Metab. 2012;10:486–489. doi: 10.5812/ijem.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available by the corresponding author upon request.