Abstract
Interoceptive signals concerning the internal physiological state of the body influence motivational feelings and action decisions. Cardiovascular arousal may facilitate inhibition to mitigate risks of impulsive actions. Baroreceptor discharge at ventricular systole underpins afferent signalling of cardiovascular arousal. In a modified Go/NoGo task, decisions to make or withhold actions on ‘Choose’ trials were not influenced by cardiac phase, nor individual differences in heart rate variability. However, cardiac interoceptive awareness and insight predicted how frequently participants chose to act, and their speed of action: Participants with better awareness and insight tended to withhold actions and respond slower, while those with poorer awareness and insight tended to execute actions and respond faster. Moreover, self-reported trait urgency correlated negatively with intentional inhibition rates. These findings suggest that lower insight into bodily signals is linked to urges to move the body, putatively by engendering noisier sensory input into motor decision processes eliciting reactive behaviour.
Subject terms: Cardiovascular biology, Human behaviour
Introduction
Intentional inhibition is the volitional withholding of cognitive processes, which include the withholding of motor action1, thoughts2, and memories3. In ‘what-when-whether’ models of voluntary action decisions1,4, intentional inhibition encompasses the ‘whether’ category of whether to perform an action or inhibit it. This can occur in response to an external decision cue, or to an internal thought or prompt5.
Within the brain, intentional decisions to withhold actions are associated with enhanced activation within prefrontal and motor preparation areas, including the pre-supplementary motor area (preSMA), extending ventrally to mid-cingulate cortex and the rostral cingulate zone, and the dorsolateral prefrontal cortex and inferior frontal gyrus4,6–8. Interestingly, neuroimaging studies of intentional inhibition also show activation of the anterior insular cortex4,9. The insula is implicated as a substrate for motivational feelings, through the integration of interoceptive information concerning the internal state of the body, cueing homeostatic adjustments of behaviour through midline motor pathways10–12. This implies that afferent information concerning bodily physiology can influence intentional inhibition decisions, for example via ‘somatic markers’, proposed by Damasio13 to function as rapid, unconscious, cues to guide behaviour.
Beyond interoceptive cues, signals from other sensory domains, such as vision, can guide intentional action decisions. A modified version of a Go/NoGo task incorporates ‘Choose’ trials that require participants to decide whether to act or to withhold a button press. In this task, subliminal (unconscious) priming by visual cues that precede Choose trials can influence motor choices, by reducing a participant’s tendency to withhold action14. Thus sensory cues which do not enter awareness may nevertheless shape volitional motor behaviour.
In the interoceptive domain, cardiovascular arousal, signalled by activation of baroreceptors in vessel walls with each heartbeat, influences many cognitive processes, including pain, visual perception of fearful stimuli, and memory15. Arterial baroreceptors are activated as blood is ejected out of the heart. The maximal activation of aortic and carotid baroreceptors typically peaks ~300 ms after an ECG R-wave15–17. In the domain of action control, we previously observed that reactive response inhibition, as measured from performance on the stop signal task, is influenced by such heartbeat signals18. Participants showed increased response inhibition efficiency (indexed by shorter stop signal reaction times) when ‘stop’ cues were presented at the point within the cardiac cycle when the heart contracts (systole), eliciting baroreceptor afferent firing, compared to when the heart fills between beats (diastole) and baroreceptors are quiescent. These momentary interoceptive cardiac cues are also associated with the timing of voluntary movements, with onset of actions to sample information more likely to coincide with systole19,20, and the offset of self-paced action sequences with diastole21. However, it is yet to be established if such cardiac cycle effects impact upon ‘whether’ voluntary decisions to make or withhold action.
In this study, we used a modified Go/NoGo task that incorporated ‘Choose’ trials on which participants chose whether to act or to withhold a button press. The task was combined with online dynamic monitoring of participants’ electrocardiograms (ECG), to deliver the onset of each trial at either cardiac systole or diastole. Given the facilitatory effect of systole on reactive response inhibition in the stop signal task18, and the long-held view that heartbeats may broadly invoke inhibitory processes across behavioural domains22, we predicted that heartbeats would promote intentional inhibition, such that participants would choose to withhold button presses more frequently during systole than diastole.
A previous literature describes the effects of action inhibition on changes to heart rate, with cardiac deceleration occurring during the inhibition of movements23,24, including intentional inhibition on the ‘marble’ task25. Here, we focused instead on the impact that momentary cardiac cues have on intentional inhibition, dynamically monitoring participants’ ECG such that trial onsets were delivered accurately even with fluctuations in heart rate.
Individual differences exist in interoceptive sensitivity, expressed across dissociable dimensions of interoception. These include objective interoceptive accuracy (according to performance on interoceptive tasks), subjective (trait) interoceptive sensibility (measured from self-report scales), and metacognitive interoceptive awareness or insight (reflecting trial-by-trial correspondence between subjective and objective measures)26,27. In addition, trait interoceptive prediction error reflects the magnitude of a discrepancy between an individual’s objective (accuracy) and belief-based (trait) subjective (sensibility) dimensions of interoception28,29. Such individual differences in psychological dimensions of interoceptive sensitivity can affect the impact of cardiac cues on cognition30. By extension, interoceptive abilities may influence voluntary decisions to make or withhold action.
In addition to measuring participants’ interoceptive abilities, we also investigated the relationship between reactive and intentional inhibition, as measured by NoGo and Choose-NoGo trials, with autonomic reactivity, measured by heart rate variability, and with trait impulsivity, measured by self-report questionnaires. Heart rate variability (HRV), which indexes cardiac (parasympathetic) vagal tone, is positively associated with enhanced executive function and emotion regulation31. Greater HRV predicts better response inhibition on the stop signal task32, and better suppression on an intentional thought inhibition paradigm33, suggesting that HRV may similarly be associated with volitional action inhibition. Moreover, an impulsive neurocognitive endophenotype may predispose individuals to poorer inhibitory control34, whereby higher trait impulsive individuals may choose to move more often, and choose to intentionally withhold actions less often, than individuals with lower trait impulsivity.
Methods
Participants
43 right-handed participants gave written informed consent to participate. The data failed to save for one participant, and one further participant was excluded from analysis due to a very large number of NoGo commission errors (>2 standard deviations from group mean). Data are presented from the remaining 41 participants (17 male, age 18–28, mean 22 years). Participants were recruited from students at the University of Sussex who had no reported history of psychiatric or neurological disorders, and no intake of medications affecting neural or peripheral physiological function. Prospective participants reporting colour-blindness were excluded. The study was approved by the Brighton and Sussex Medical School Research Governance and Ethics Committee, and all research was performed in accordance with relevant guidelines and regulations.
Cardiac intentional inhibition task
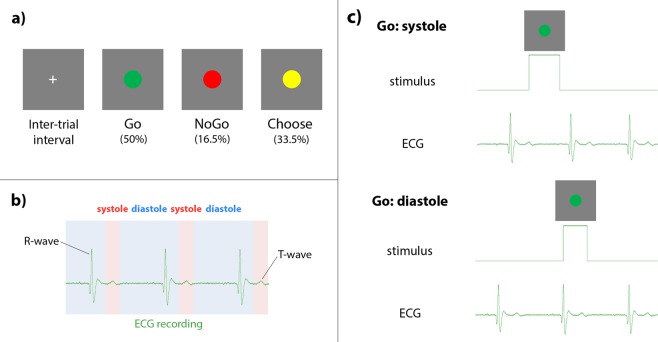
Participants performed an intentional inhibition task in which the onset of the motor cues was timed to either cardiac systole (when the heart is contracting, and baroreceptors in arterial walls are activated) or cardiac diastole (when the heart is relaxed between beats, and baroreceptors are quiescent). The task was presented using Cogent2000 (version 1.32, http://www.vislab.ucl.ac.uk/cogent_2000.php) in Matlab (R2013a, Mathworks). Task stimuli comprised green, red, and yellow circles, presented in the centre of the screen on a grey background (Fig. 1a). Green Go cues indicated a button press of the left arrow key to be made using the right index finger. Red NoGo cues indicated the participant should withhold their button press. Yellow ‘Choose’ cues indicated participants should choose whether to press the button or to withhold. There were 400 trials in total: 200 Go trials (50%), 66 NoGo (16.5%), and 134 Choose (33.5%). The higher frequency of Go trials was designed to invoke a prepotent tendency to go, as in traditional Go/NoGo tasks, so that withholding on the NoGo trials was sufficiently challenging to invoke reactive inhibitory control35–37. 50% of each trial type were delivered at cardiac systole, and 50% at diastole. Participants were instructed to respond as quickly as possible on the Go trials, to withhold button presses on NoGo trials, and to choose quickly and make a fresh decision whether to press the button or not on each Choose trial. All cues were presented for a maximum duration of 1000 ms, with the trial ending sooner if the participant pressed the button. In between trials, a white fixation cross was displayed in the centre of the screen for a duration of 1000 ms, plus the duration during which the participants’ ECG was monitored to establish the time of the next R-wave peak (see ‘ECG recording and stimulus delivery’ below). The task was divided into four blocks of 100 trials, giving participants a rest break in between each block, in order to reduce movement during task performance and ensure that ECG recording was uncontaminated by movement artifact. The task took approximately half an hour to complete. An in-house Matlab script calculated four key indices of motor behaviour: 1) reaction times on Go trials, 2) % of NoGo trials on which participants made commission errors (pressed the button instead of withholding), 3) % of Choose trials on which participants decided to act (% Choose-Go), and 4) reaction times on Choose-Go trials.
Figure 1.
Cardiac intentional inhibition task. (a) Intentional inhibition task: following an inter-trial interval, on Go trials (50%) cues instructed participants to make a button press, on NoGo trials (16.5%) to withhold a button press, and on Choose trials (33.5%) to decide whether to execute or inhibit a button press. Stimuli enlarged for illustrative purposes. (b) Cardiac cycle in relation to ECG signal: systole (cardiac contraction) is signalled neurally by arterial baroreceptor firing. The activation of aortic and carotid baroreceptors is maximal 280 ms after the R-wave. (c) Example cardiac timing of task cue onsets. On systole trials, the onset of the task cue is 290 ms following R-wave; on diastole trials, the onset of the task cue is 10 ms prior to the R-wave.
ECG recording and stimulus delivery
The onset of task cues was synchronised to specific time points within the cardiac cycle (Fig. 1b) using ECG recording, with Cambridge Electronic Design (CED) hardware and Spike2 physiological recording software (version 7.18), to interface cardiac events with the task in Matlab. Three Ag/AgCl electrodes (3 M Healthcare, Neuss, Germany) were attached with foam tape: two on the upper left and right chest, and a ground electrode above the left hipbone. The ECG signal was sampled at 1000 Hz, amplified (1902, CED) and relayed to Spike2 recording software via an analogue-to-digital recorder (1401, CED). An interactive threshold in the Spike2 recording isolated each ECG R-wave peak. This enabled the dynamic monitoring of inter-beat intervals for three beats prior to each trial, giving a median inter-beat interval to provide a precise temporal prediction of the next R-wave peak. Task cues could thus be delivered at either cardiac systole (290 ms following R-wave peak), or cardiac diastole (10 ms prior to R-wave peak) (Fig. 1c).
Although electrical depolarisation events, evident in the QRS complex of an ECG, are critical for initiating cardiac contraction, they do not correspond in time to the neural and cognitive perception of a heartbeat, which is signalled to the central nervous system (in part) via baroreceptor activation15. Imaging and catheter studies of cardiac function suggest that arterial baroreceptor discharge in the aorta and carotid sinus peaks around 300 ms after the ECG R-wave. The pre-ejection period between the start of myocardial depolarisation (QRS ECG complex) and the triggered onset of ventricular contraction is typically 130–160 ms16,38. The following left ventricular ejection time (LVET) is ~350–500 ms, with peak systolic pressure occurring within 100 ms of LVET16,39,40. The delay between left ventricular and systolic pressure peaks is ~50 ms17, altogether suggesting the activation of aortic and carotid baroreceptors is maximal after 280 ms from the R-wave.
To determine the achieved precision of trial event timing within the cardiac cycle, we used in-house Spike2 and Matlab scripts to extract the stimulus onsets from the Spike recording, and plot their relative position to the R-wave peak, in 50 ms time bins (Fig. 2). Dynamic monitoring of the inter-beat interval throughout the task ensured accurate synchronisation of the task cues with specified cardiac events, even when participants’ heart rates may vary over time, with attention, fatigue, and potentially, in response to stimuli and to action execution or inhibition23–25.
Figure 2.
Precision of cue timing within the cardiac cycle, relative to the R-wave peak, in 50 ms time bins, for (a) Go, (b) NoGo, (c) Choose-Go, and (d) Choose-NoGo trials. Over 90% of trials were within 200 ms of the intended timing for diastole trials at 10 ms prior to the R-wave (blue), and for systole trials at 290 ms following R-wave (red). Dark blue indicates overlap of diastole and systole trial timings (minimal for all trial types).
Interoception heartbeat tracking task
Individual differences in interoceptive sensitivity were quantified using a heartbeat perception task. Participants’ heartbeats were monitored using a pulse oximeter attached to their left index finger (‘soft’ mount PureLight sensor; Nonin Medical Inc, MN, USA). Participants were instructed to “silently count the number of heartbeats you feel from the time you hear ‘start’ to when you hear ‘stop’”, on six trials of varying duration (25, 30, 35, 40, 45, 50 s), presented in a randomised order41. Following each trial, participants gave a confidence rating in their reported perceived number of heartbeats on a visual analogue scale, from ‘total guess (no heartbeat awareness)’ to ‘complete confidence (full perception of heartbeat’), scored from 0 (no heartbeat awareness) to 10 (full perception of heartbeat).
Dimensions of interoception
We summarise the interoceptive indices calculated in this study and the dimensions of interoception to which they belong in Table 1.
Table 1.
Interoceptive indices calculated in this study, the dimensions of interoception to which they belong, task employed, which accuracy equation is used to calculate the values, and whether the index represents a state or trait assessment of interoception.
| Index | ‘Standard’ accuracy | ‘Alternative’ accuracy | Insight | Awareness | Confidence | Sensibility | Trait interoceptive prediction error (TIPE) |
|---|---|---|---|---|---|---|---|
| Dimension | Accuracy | Accuracy | Metacognition / State discrepancy | Metacognition | Sensibility | Trait discrepancy | |
| Task | Heartbeat tracking | Heartbeat tracking | Heartbeat tracking & confidence | Heartbeat tracking & confidence | Confidence | Body Perception Questionnaire | Heartbeat tracking & Body Perception Questionnaire |
| Accuracy calculation method | ‘Standard’ accuracy | ‘Alternative’ accuracy | ‘Standard’ accuracy | ‘Alternative’ accuracy | ‘Alternative’ accuracy | ||
| State or trait | State | State | State | State | State | Trait | Trait |
Interoceptive accuracy
Interoceptive accuracy, reflecting objective interoceptive performance, was calculated using an in-house Matlab script according to the trial-by-trial ratio of perceived to actual heartbeats, with two equations: (1 - (nbeatsreal – nbeatsreported) / nbeatsreal) (‘standard’ accuracy), and (1 – (nbeatsreal – nbeatsreported) / (nbeatsreal + nbeatsreported) / 2) (‘alternative’ accuracy)26,27,42. For each accuracy method, the ratios for the six trials were averaged to give a mean heartbeat tracking score. The ‘standard’ accuracy calculation is appropriate only in cases where participants do not estimate more than 2x the number of real heartbeats. When participants do substantially overestimate, the ‘alternative’ accuracy calculation is more appropriate26,27. One participant was a substantial over-estimator, according to this heuristic: we therefore report ‘standard’ accuracy scores for the remaining 40 participants, and ‘alternative’ accuracy scores for the full sample of 41 participants.
Interoceptive awareness and insight
Interoceptive awareness and insight, reflecting metacognitive perception of one’s interoceptive accuracy performance, were calculated using an in-house Matlab script, according to two approaches: a discrepancy index between the participant’s accuracy and confidence scores (using ‘standard’ accuracy scores), and the Pearson correlation between interoceptive accuracy and confidence rating on each trial (using ‘alternative’ accuracy scores)26,27. To calculate the discrepancy index (insight), confidence ratings were converted from a 0 to 10 scale to a percentage (confidence rating / 10), then an absolute difference score calculated for each trial (‘standard’ accuracy – confidence), which was averaged across the six trials. Because the ‘alternative’ accuracy calculation method can generate negative values, it was possible to apply the discrepancy index to ‘standard’ accuracy scores only from the 40 participants who did not substantially overestimate their perceived heartbeats. The within-participant correlation between accuracy and confidence scores (awareness) was calculated using ‘alternative’ accuracy scores in all 41 participants.
We describe the discrepancy index as ‘interoceptive insight’ and the correlation measure as ‘interoceptive awareness’, following the original terminology of the authors who introduced these methods26,27. An insight discrepancy score of 0 indicates high interoceptive metacognition, with optimum alignment of accuracy and confidence (accuracy exactly matches confidence rating). The further the discrepancy score from 0, the poorer the interoceptive metacognition, with negative discrepancy scores indicating low performance but high confidence, and positive discrepancy scores indicating high performance but low confidence. In contrast, low (negative) awareness correlation scores indicate poor interoceptive metacognition, while high (positive) awareness correlation scores indicate better interoceptive metacognition.
In addition to the metacognitive measures of interoceptive awareness and insight, we also calculated the mean confidence rating on the heartbeat tracking task, to provide a general indication of confidence, separate to interoceptive processes.
Interoceptive sensibility
Participants completed the awareness section of the Body Perception Questionnaire43. The BPQ was used to calculate (trait) interoceptive sensibility, reflecting self-reported subjective sensitivity to bodily sensations, according to the mean score of the response to the 45 items (each scored from 1 to 5).
Trait interoceptive prediction error (TIPE)
Heartbeat tracking accuracy (‘alternative’ scores) and BPQ scores were converted to standardised z-scores (SPSS), and TIPE calculated as the discrepancy between z-scored accuracy and z-scored sensibility (z-sensibility – z-accuracy). Positive TIPE values reflect a tendency for individuals to over-estimate interoceptive ability, while negative values reflect a tendency to under-estimate28.
Heart rate variability
To calculate heart rate variability (HRV), ECG recording continued for 2.5 minutes following the intentional inhibition task, during which participants were instructed to rest quietly with eyes open. Each participant’s recording was visually inspected to confirm no movement artifacts, then thresholded to isolate each R-wave peak, and the inter-beat intervals calculated using in-house Spike2 and Matlab scripts. The inter-beat intervals were entered to the HRVAS toolbox44 in Matlab. Heart rate variability was indexed as the root mean square of the successive differences (RMSSD). The HRVAS output also provided heart rate in beats per minute (bpm), which was entered to multiple regressions as a confounding factor.
Trait impulsivity
Participants completed three questionnaires indexing facets of trait impulsivity: the Barratt Impulsivity Scale (BIS)45, giving a total and three attention, motor, and planning subscale scores; the Conners Adult ADHD Rating Scale – Short Scale (CAARS-SS)46, giving an index of ADHD symptomology; and the UPPS-P47, giving five subscale scores on negative urgency, lack of premeditation, lack of perseverance, sensation seeking, and positive urgency.
Statistical analyses
Data were analysed in JASP (https://jasp-stats.org/), using both frequentist tests and Bayesian equivalents, extending insights to guide interpretation of significance (p values) according to how likely the alternative hypothesis is versus the null (BF10). For t-tests, in addition to the statistic and significance value, we give the 95% confidence intervals of the mean difference. For the Bayesian analyses, in the absence of pilot data on relationships amongst intentional inhibition, cardiac cycle, and dimensions of interoception, we used the default JASP priors, which assume a medium effect size on a Cauchy distribution of 0.707 for t-tests, beta binomials of 1 for multiple regressions, and stretched beta prior widths of 1 for correlations. The.jasp file, containing the data, analysis options, and output, is available at https://osf.io/3xezy. This also contains graphical robustness checks examining evidence for H1 and H0 under different prior widths, for t-tests. We interpret Bayes Factors (BF10) according to the heuristic of 1–3 indicating anecdotal evidence in favour of H1, 3–10 moderate evidence, and >10 strong evidence48.
Firstly, to compare behaviour on the intentional inhibition task between systole and diastole trials, we used four 2-tailed repeated measures t-tests, for (1) Go reaction times, (2) % NoGo commission errors, (3) % of Choose trials on which participants chose to go (% Choose-Go), and (4) reaction times on Choose-Go trials. We report 95% confidence intervals of the mean difference. While participants’ intentional inhibition rates (% Choose-Go), and reactive inhibition in the form of the NoGo trials, were the primary measures of interest, we include reaction time analyses to provide complementary analyses of different facets of motor behaviour. The %NoGo commission error data were not normally distributed, and so for this comparison we report the results of a non-parametric Wilcoxon-test.
Secondly, to investigate how individual differences in interoception relate to motor behaviour, we generated a correlation matrix of 2-tailed Pearson correlations between all seven interoceptive indices and four motor behaviour measures (Go reaction times, % NoGo errors, % Choose-Go, Choose-Go reaction times).
Thirdly, to investigate the influence of HRV on motor behaviour, we used four linear regressions, with (1) Go reaction time, (2) % NoGo commission errors, (3) % Choose-Go, and (4) Choose-Go reaction time as the dependent variable, and RMSSD and beats per minute as the independent variables, using the ‘Enter’ method (which does not assume a stronger contribution of any one of the independent variables in the prediction of the dependent variable). Bayesian regressions compared a full inclusion model to the null model.
Fourthly, to explore how individual differences in trait impulsivity relate to motor behaviour, we generated a correlation matrix of 2-tailed Pearson correlations between the four behavioural measures on the intentional inhibition task (Go reaction times, % NoGo errors, % Choose-Go, Choose-Go reaction times), and the impulsivity questionnaire scores (BIS: total, and the attention, motor, and planning subscales; CAARS-SS ADHD index; and the five subscales of the UPPS-P).
Finally, to investigate how individual differences in interoception relate to trait impulsivity, we generated a correlation matrix of 2-tailed Pearson correlations between the seven interoceptive indices and a subset of the impulsivity questionnaire scores (BIS: total, attention, motor, planning; CAARS-SS ADHD index).
Results
Descriptive statistics (mean, standard deviation, minimum and maximum) for all experimental measures are given in Table 2.
Table 2.
Demographic details of participants, and measures of performance on the cardiac intentional inhibition task, dimensions of interoception, trait impulsivity, and heart rate variability.
| Category | Variable | Mean | Standard deviation | Minimum | Maximum |
|---|---|---|---|---|---|
| Age | 22 | 2 | 18 | 28 | |
| Cardiac intentional inhibition task | Go reaction time: systole (ms) | 483 | 59 | 352 | 623 |
| Go reaction time: diastole (ms) | 481 | 56 | 360 | 607 | |
| Go reaction time: mean (ms) | 482 | 57 | 356 | 615 | |
| % Go omissions: systole | 2 | 2 | 0 | 9 | |
| % Go omissions: diastole | 2 | 2 | 0 | 13 | |
| % Go omissions: mean | 1 | 2 | 0 | 5 | |
| % NoGo errors: systole | 4 | 5 | 0 | 18 | |
| % NoGo errors: diastole | 4 | 6 | 0 | 27 | |
| % NoGo errors: mean | 4 | 5 | 0 | 18 | |
| NoGo reaction time: systole (ms) | 408 | 72 | 299 | 546 | |
| NoGo reaction time: diastole (ms) | 397 | 62 | 289 | 522 | |
| NoGo reaction time: mean (ms) | 405 | 61 | 294 | 538 | |
| % Choose-Go: systole | 60 | 13 | 37 | 87 | |
| % Choose-Go: diastole | 61 | 11 | 40 | 84 | |
| % Choose-Go: mean | 60 | 11 | 42 | 84 | |
| Choose-Go reaction time: systole (ms) | 524 | 86 | 357 | 712 | |
| Choose-Go reaction time: diastole (ms) | 522 | 81 | 360 | 686 | |
| Choose-Go reaction time: mean (ms) | 523 | 82 | 359 | 689 | |
| Interoception | Heartbeat tracking accuracy (‘Standard’) | 0.71 | 0.18 | 0.24 | 0.97 |
| Heartbeat tracking accuracy (‘Alternative’) | 0.62 | 0.29 | −0.23 | 0.97 | |
| Heartbeat tracking insight (accuracy-confidence discrepancy) | 0.24 | 0.21 | −0.29 | 0.69 | |
| Heartbeat tracking awareness (accuracy-confidence correlation) | 0.26 | 0.48 | −0.88 | 0.98 | |
| Confidence | 4.71 | 1.92 | 0.85 | 7.87 | |
| Sensibility (BPQ) | 2.5 | 0.64 | 1.49 | 3.89 | |
| Trait interoceptive prediction error (TIPE) | <0.01 | 1.39 | −2.81 | 3.81 | |
| BIS | Attention | 18 | 4 | 11 | 26 |
| Motor | 24 | 4 | 16 | 32 | |
| Planning | 24 | 5 | 14 | 36 | |
| Total | 66 | 11 | 49 | 86 | |
| CAARS-SS | ADHD index | 13 | 4 | 4 | 21 |
| UPPS-P | Negative urgency | 29 | 7 | 16 | 42 |
| Premeditation | 23 | 4 | 14 | 30 | |
| Perseverance | 20 | 3 | 14 | 30 | |
| Sensation seeking | 38 | 7 | 21 | 51 | |
| Positive urgency | 29 | 9 | 16 | 49 | |
| Heart rate variability | RMSSD | 54 | 29 | 15 | 175 |
| Beats per minute (bpm) | 75 | 10 | 53 | 95 |
Cardiac intentional inhibition task
There was no significant effect of cardiac timing on motor behaviour in the intentional inhibition task, on any of the four task measures (Fig. 3).
Figure 3.
No significant difference between systole and diastole for (a) reaction time on Go trials, (b) % errors on NoGo trials, (c) frequency of choosing to go on Choose trials (% Choose-Go), or (d) reaction time on Choose-Go trials. Error bars represent standard error of the mean (SEM).
Go reaction time was not significantly different at systole (483 ms) to diastole (481 ms) (t(40) = 0.583, p = 0.563, mean difference = 2 ms [−4, 7], with BF10 = 0.198, suggesting moderate evidence for H0).
Participants did not make commission errors any more frequently on NoGo trials at systole (4%) than diastole (4%) (Z = 224.500, p = 0.886, mean difference = <1% [−3, 3], with BF10 = 0.171, suggesting moderate evidence for H0). However, we interpret this comparison with caution, given that on the whole, participants made very few NoGo errors, with a mean NoGo error rate of 4% – equating to an average of only 3 NoGo errors per participant throughout the experiment – while 10 participants did not make a single commission error at all. This means that paucity of NoGo error trials, leading to floor effects, could obscure any potential cardiac effect.
On the critical trial type of interest, Choose trials - when participants selected whether to act or to inhibit their button press - how frequently they chose to go (% Choose-Go) was not significantly different at systole (60%) to diastole (61%) (t(40) = −1.553, p = 0.128, mean difference = 2% [−4, 1], with BF10 = 0.510, suggesting anecdotal evidence for H0).
On Choose trials where participants elected to go, reaction times were not significantly different when the Choose cue had been presented at systole (524 ms) to diastole (522 ms) (t(40) = 0.390, p = 0.699, mean difference = 2 ms [−7, 11], with BF10 = 0.181, suggesting moderate evidence for H0).
Dimensions of interoception
A correlation matrix investigated how individual differences in interoception relate to motor behaviour (Table 3). There were no significant associations between interoception and Go reaction times or % NoGo errors. However, interoceptive awareness correlated negatively with % Choose-Go (r = −0.438, p = 0.004, BF10 = 10.214), such that the lower the interoceptive awareness, the more frequently participants decided to act on Choose trials (Fig. 4a). A weak negative correlation between heartbeat tracking confidence ratings and % Choose-Go (r = −0.273, p = 0.084, BF10 = 0.825) that did not reach statistical significance suggested this may be partly explained by participants who have lower confidence choosing to act more frequently (Fig. 4b). Interestingly, interoceptive insight did not relate to % Choose-Go, but was associated with reaction times on Choose-Go trials (r = −0.398, p = 0.011, BF10 = 4.495), such that the poorer the interoceptive insight (higher discrepancy scores), the faster the reaction time (Fig. 4c). A significant correlation between confidence ratings and Choose-Go reaction times (r = 0.354, p = 0.023, BF10 = 2.331) suggests the association with interoceptive insight may be partly explained by participants who have lower confidence responding more quickly (Fig. 4d).
Table 3.
Correlations (2-tailed) between performance on the intentional inhibition task, and interoceptive indices. Significant (p < 0.05) correlations highlighted in bold.
| Go RT | % NoGo error | % Choose-Go | Choose-Go RT | ‘Standard’ accuracy | ‘Alternative’ accuracy | Insight | Awareness | Confidence | Sensibility | Trait interoceptive prediction error (TIPE) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Go RT | |||||||||||
| % NoGo error |
r −0.394 p 0.011 BF10 4.516 |
||||||||||
| % Choose-Go |
r −0.213 p 0.182 BF10 0.461 |
r 0.170 p 0.287 BF10 0.336 |
|||||||||
| Choose-Go RT |
r 0.748 p < 0.001 BF10 804247.390 |
r −0.330 p 0.035 BF10 1.660 |
r −0.353 p 0.024 BF10 2.308 |
||||||||
| ‘Standard’ accuracy |
r −0.101 p 0.534 BF10 0.237 |
r 0.028 p 0.866 BF10 0.200 |
r −0.081 p 0.620 BF10 0.222 |
r −0.084 p 0.604 BF10 0.224 |
|||||||
| ‘Alternative’ accuracy |
r −0.095 p 0.553 BF10 0.231 |
r 0.039 p 0.807 BF10 0.200 |
r 0–0.107 p 0.505 BF10 0.241 |
r −0.093 p 0.563 BF10 0.229 |
r 0.991 p < 0.001 BF10 ∞ |
||||||
| Insight |
r −0.250 p 0.119 BF10 0.635 |
r 0.087 p 0.593 BF10 0.226 |
r 0.187 p 0.248 BF10 0.375 |
r −0.398 p 0.011 BF10 4.495 |
r 0.523 p < 0.001 BF10 63.674 |
r 0.539 p < 0.001 BF10 97.196 |
|||||
| Awareness |
r −0.084 p 0.602 BF10 0.222 |
r 0.211 p 0.186 BF10 0.452 |
r −0.438 p 0.004 BF10 10.214 |
r −0.011 p 0.948 BF10 0.195 |
r 0.329 p 0.038 BF10 1.574 |
r 0.326 p 0.037 BF10 1.579 |
r −0.013 p 0.934 BF10 0.198 |
||||
| Confidence |
r 0.172 p 0.281 BF10 0.341 |
r −0.069 p 0.667 BF10 0.213 |
r −0.273 p 0.084 BF10 0.825 |
r 0.354 p 0.023 BF10 2.331 |
r 0.376 p 0.017 BF10 3.120 |
r 0.343 p 0.028 BF10 1.995 |
r −0.593 p < 0.001 BF10 500.456 |
r 0.326 p 0.037 BF10 1.576 |
|||
| Sensibility |
r 0.195 p 0.222 BF10 0.400 |
r −0.027 p 0.866 BF10 0.197 |
r −0.009 p 0.957 BF10 0.195 |
r 0.079 p 0.625 BF10 0.218 |
r 0.046 p 0.780 BF10 0.205 |
r 0.035 p 0.830 BF10 0.199 |
r −0.027 p 0.866 BF10 0.200 |
r 0.123 p 0.445 BF10 0.258 |
r 0.070 p 0.664 BF10 0.213 |
||
| Trait interoceptive prediction error (TIPE) |
r 0.209 p 0.190 BF10 0.446 |
r −0.048 p 0.768 BF10 0.203 |
r 0.071 p 0.661 BF10 0.214 |
r 0.124 p 0.441 BF10 0.259 |
r −0.671 p < 0.001 BF10 10026.857 |
r −0.694 p < 0.001 BF10 39767.605 |
r −0.403 p 0.010 BF10 4.849 |
r −0.146 p 0.362 BF10 0.291 |
r −0.196 p 0.220 BF10 0.403 |
r 0.695 p < 0.001 BF10 41427.547 |
Figure 4.
Correlations of interoceptive awareness (accuracy-confidence correlation), interoceptive insight (accuracy-confidence discrepancy), and confidence with how frequently participants chose to go on Choose trials (% Choose-Go) and their reaction times. Low (negative) accuracy-confidence correlation scores indicate poor interoceptive awareness, while high (positive) accuracy-confidence correlation scores indicate better interoceptive awareness. An insight score of 0 indicates high interoceptive metacognition, with optimum alignment of accuracy and confidence. The further the discrepancy score from 0, the poorer the interoceptive metacognition, with negative discrepancy scores indicating low performance but high confidence, and positive discrepancy scores indicating high performance but low confidence.
Heart rate variability
Four multiple regressions tested for an influence of HRV (RMSSD) and heart rate (bpm) on motor behaviour. There was no significant relation between HRV, bpm, and Go reaction times (F(2,38) = 1.153, p = 0.327, with BF10 = 0.311), nor % NoGo errors (F(2,38) = 0.401, p = 0.672, with BF10 = 0.179), nor % Choose-Go (F(2,38) = 1.439, p = 0.250, with BF10 = 0.383), or Choose-Go reaction times (F(2,38) = 0.993, p = 0.380, with BF10 = 0.277).
Trait impulsivity
We generated two correlation matrices of 2-tailed Pearson correlations between 1) the four behavioural measures on the intentional inhibition task, and impulsivity questionnaire scores (Table 4), and 2) the seven interoceptive indices and a subset of the impulsivity questionnaire scores (Table 5).
Table 4.
Correlations (2-tailed) between performance on the intentional inhibition task, and impulsivity questionnaire scores. Significant (p < 0.05) correlations highlighted in bold.
| Go RT | % NoGo error | % Choose-Go | Choose-Go RT | BIS attention | BIS motor | BIS planning | BIS total | CAARS-SS ADHD index | Negative urgency | Pre-meditation | Per-severance | Sensation seeking | Positive urgency | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Go RT | ||||||||||||||
| % NoGo error |
r −0.394 p 0.011 BF10 4.516 |
|||||||||||||
| % Choose-Go |
r −0.213 p 0.182 BF10 0.461 |
r 0.170 p 0.287 BF10 0.336 |
||||||||||||
| Choose-Go RT |
r 0.748 p < 0.001 BF10 804247.390 |
r −0.330 p 0.035 BF10 1.660 |
r −0.353 p 0.024 BF10 2.308 |
|||||||||||
| BIS attention |
r −0.294 p 0.062 BF10 1.043 |
r 0.131 p 0.416 BF10 0.268 |
r 0.104 p 0.516 BF10 0.238 |
r −0.033 p 0.838 BF10 0.199 |
||||||||||
| BIS motor |
r −0.303 p 0.054 BF10 1.171 |
r 0.122 p 0.446 BF10 0.257 |
r 0.012 p 0.939 BF10 0.195 |
r 0.022 p 0.892 BF10 0.196 |
r 0.545 p < 0.001 BF10 136.533 |
|||||||||
| BIS planning |
r −0.333 p 0.034 BF10 1.720 |
r 0.161 p 0.313 BF10 0.318 |
r 0.051 p 0.750 BF10 0.204 |
r −0.154 p 0.336 BF10 0.304 |
r 0.343 p 0.028 BF10 2.003 |
r 0.544 p < 0.001 BF10 133.432 |
||||||||
| BIS total |
r −0.385 p 0.013 BF10 3.857 |
r 0.173 p 0.280 BF10 0.342 |
r 0.065 p 0.687 BF10 0.210 |
r −0.077 p 0.634 BF10 0.217 |
r 0.728 p < 0.001 BF10 238571.500 |
r 0.861 p < 0.001 BF10 1.513e + 10 |
r 0.824 p < 0.001 BF10 2.940e + 8 |
|||||||
| CAARS-SS ADHD index |
r −0.425 p 0.006 BF10 7.914 |
r 0.213 p 0.180 BF10 0.463 |
r 0.286 p 0.070 BF10 0.950 |
r −0.155 p 0.333 BF10 0.306 |
r 0.647 p < 0.001 BF10 4716.556 |
r 0.527 p < 0.001 BF10 81.839 |
r 0.587 p < 0.001 BF10 492.847 |
r 0.719 p < 0.001 BF10 148176.743 |
||||||
| Negative urgency |
r −0.078 p 0.626 BF10 0.218 |
r 0.160 p 0.317 BF10 0.316 |
r 0.362 p 0.020 BF10 2.645 |
r −0.009 p 0.953 BF10 0.195 |
r 0.490 p 0.001 BF10 32.307 |
r 0.279 p 0.077 BF10 0.878 |
r 0.320 p 0.041 BF10 1.460 |
r 0.435 p 0.004 BF10 9.679 |
r 0.600 p < 0.001 BF10 786.692 |
|||||
| Premeditation |
r 0.009 p 0.955 BF10 0.195 |
r −0.062 p 0.702 BF10 0.209 |
r −0.182 p 0.256 BF10 0.363 |
r −0.036 p 0.823 BF10 0.199 |
r 0.309 p 0.049 BF10 1.264 |
r 0.490 p 0.001 BF10 32.363 |
r 0.444 p 0.004 BF10 11.501 |
r 0.522 p < 0.001 BF10 71.666 |
r 0.341 p 0.029 BF10 1.946 |
r 0.173 p 0.281 BF10 0.341 |
||||
| Perseverance |
r −0.292 p 0.064 BF10 1.022 |
r −0.141 p 0.381 BF10 0.282 |
r −0.125 p 0.438 BF10 0.260 |
r −0.013 p 0.935 BF10 0.195 |
r 0.277 p 0.080 BF10 0.857 |
r 0.278 p 0.079 BF10 0.868 |
r 0.319 p 0.042 BF10 1.426 |
r 0.362 p 0.020 BF10 2.663 |
r 0.288 p 0.068 BF10 0.970 |
r 0.082 p 0.609 BF10 0.221 |
r 0.277 p 0.080 BF10 0.857 |
|||
| Sensation seeking |
r −0.054 p 0.736 BF10 0.206 |
r 0.063 p 0.694 BF10 0.210 |
r −0.084 p 0.603 BF10 0.222 |
r 0.073 p 0.650 BF10 0.215 |
r 0.445 p 0.004 BF10 11.901 |
r 0.555 p < 0.001 BF10 181.521 |
r 0.258 p 0.103 BF10 0.702 |
r 0.505 p < 0.001 BF10 45.800 |
r 0.314 p 0.045 BF10 1.343 |
r 0.391 p 0.011 BF10 4.262 |
r 0.478 p 0.002 BF10 24.001 |
r 0.034 p 0.835 BF10 0.199 |
||
| Positive urgency |
r −0.341 p 0.029 BF10 1.951 |
r 0.439 p 0.004 BF10 10.450 |
r 0.224 p 0.159 BF10 0.507 |
r −0.104 p 0.519 BF10 0.238 |
r 0.670 p < 0.001 BF10 12697.285 |
r 0.604 p < 0.001 BF10 890.075 |
r 0.457 p 0.003 BF10 15.039 |
r 0.696 p < 0.001 BF10 43698.378 |
r 0.678 p < 0.001 BF10 18082.328 |
r 0.674 p < 0.001 BF10 14984.850 |
r 0.214 p 0.179 BF10 0.466 |
r 0.190 p 0.235 BF10 0.384 |
r 0.580 p < 0.001 BF10 394.4 |
Table 5.
Correlations (2-tailed) between interoceptive indices, and impulsivity questionnaire scores. Significant (p < 0.05) correlations highlighted in bold.
| ‘Standard’ accuracy | ‘Alternative’ accuracy | Insight | Awareness | Confidence | Sensibility | Trait interoceptive prediction error (TIPE) | BIS attention | BIS motor | BIS planning | BIS total | CAARS-SS ADHD index | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ‘Standard’ accuracy | ||||||||||||
| ‘Alternative’ accuracy |
r 0.991 p < 0.001 BF10 ∞ |
|||||||||||
| Insight |
r 0.523 p < 0.001 BF10 63.674 |
r 0.539 p < 0.001 BF10 97.196 |
||||||||||
| Awareness |
r 0.329 p 0.038 BF10 1.574 |
r 0.326 p 0.037 BF10 1.579 |
r −0.013 p 0.934 BF10 0.198 |
|||||||||
| Confidence |
r 0.376 p 0.017 BF10 3.120 |
r 0.343 p 0.028 BF10 1.995 |
r −0.593 p < 0.001 BF10 500.456 |
r 0.326 p 0.037 BF10 1.576 |
||||||||
| Sensibility |
r 0.046 p 0.780 BF10 0.205 |
r 0.035 p 0.830 BF10 0.199 |
r −0.027 p 0.866 BF10 0.200 |
r 0.123 p 0.445 BF10 0.258 |
r 0.070 p 0.664 BF10 0.213 |
|||||||
| Trait interoceptive prediction error (TIPE) |
r −0.671 p < 0.001 BF10 10026.857 |
r −0.694 p < 0.001 BF10 39767.605 |
r −0.403 p 0.010 BF10 4.849 |
r −0.146 p 0.362 BF10 0.291 |
r −0.196 p 0.220 BF10 0.403 |
r 0.695 p < 0.001 BF10 41427.547 |
||||||
| BIS attention |
r 0.216 p 0.181 BF10 0.468 |
r 0.196 p 0.220 BF10 0.402 |
r 0.067 p 0.681 BF10 0.214 |
r 0.361 p 0.021 BF10 2.598 |
r 0.132 p 0.411 BF10 0.270 |
r 0.134 p 0.402 BF10 0.273 |
r −0.044 p 0.784 BF10 0.202 |
|||||
| BIS motor |
r 0.296 p 0.064 BF10 1.037 |
r 0.290 p 0.065 BF10 1.002 |
r −0.041 p 0.804 BF10 0.203 |
r 0.135 p 0.401 BF10 0.274 |
r 0.324 p 0.039 BF10 1.524 |
r 0.125 p 0.437 BF10 0.261 |
r −0.119 p 0.460 BF10 0.253 |
r 0.545 p < 0.001 BF10 136.533 |
||||
| BIS planning |
r 0.004 p 0.978 BF10 0.197 |
r 0.006 p 0.969 BF10 0.195 |
r 0.076 p 0.642 BF10 0.219 |
r −0.064 p 0.690 BF10 0.210 |
r −0.077 p 0.632 BF10 0.217 |
r −0.142 p 0.375 BF10 0.285 |
r −0.107 p 0.506 BF10 0.241 |
r 0.343 p 0.028 BF10 2.003 |
r 0.544 p < 0.001 BF10 133.432 |
|||
| BIS total |
r 0.197 p 0.224 BF10 0.402 |
r 0.189 p 0.236 BF10 0.383 |
r 0.042 p 0.796 BF10 0.203 |
r 0.146 p 0.362 BF10 0.290 |
r 0.414 p 0.378 BF10 0.283 |
r 0.028 p 0.861 BF10 0.197 |
r −0.116 p 0.471 BF10 0.250 |
r 0.728 p < 0.001 BF10 238571.500 |
r 0.861 p < 0.001 BF10 1.513e + 10 |
r 0.824 p < 0.001 BF10 2.940e + 8 |
||
| CAARS-SS ADHD index |
r 0.032 p 0.845 BF10 0.201 |
r 0.045 p 0.779 BF10 0.202 |
r 0.111 p 0.495 BF10 0.247 |
r −0.034 p 0.834 BF10 0.199 |
r −0.084 p 0.600 BF10 0.222 |
r −0.023 p 0.886 BF10 0.196 |
r −0.023 p 0.886 BF10 0.204 |
r 0.647 p < 0.001 BF10 4716.556 |
r 0.527 p < 0.001 BF10 81.839 |
r 0.587 p < 0.001 BF10 492.847 |
r 0.719 p < 0.001 BF10 148176.743 |
Go reaction times correlated negatively with BIS planning (r = −0.333, p = 0.034, BF10 = 1.720), BIS total (r = −0.385, p = 0.013, BF10 = 3.857), CAARS-SS ADHD index (r = −0.425, p = 0.006, BF10 = 7.914), and UPPS-P positive urgency (r = −0.341, p = 0.029, BF10 = 1.951), such that the greater the impulsivity questionnaire score, the shorter the Go reaction time. NoGo errors correlated positively with UPPS-P positive urgency (r = 0.439, p = 0.004, BF10 = 10.450), such that the greater a participant’s positive urgency (reflecting a tendency to act impulsively while experiencing strong positive emotions), the more frequently they made NoGo errors. Finally, % Choose-Go correlated positively with UPPS-P negative urgency (r = 0.362, p = 0.020, BF10 = 2.645), such that the greater a participant’s negative urgency (reflecting a tendency to act impulsively while experiencing strong negative emotions), the more often they chose to act on Choose trials.
Interoceptive awareness correlated positively with BIS attention (r = 0.361, p = 0.021, BF10 = 2.598), and confidence ratings correlated positively with BIS motor (r = 0.324, p = 0.039, BF10 = 1.524), such that the greater a participant’s attentional impulsivity, the higher their interoceptive awareness, and the greater their motor impulsivity, the higher their confidence on the heartbeat tracking task.
Discussion
Physiological signals from the body influence emotion, cognition, and action13,15,49. This includes heartbeats, which can cue more efficient response inhibition18. We tested whether these effects extend to intentional inhibition, in which participants choose whether to act, or to withhold movements, with Choose cues presented at either cardiac systole or diastole. In fact, we found no conclusive evidence that choice cues presented at systole led to more frequent choices to withhold actions than choice cues presented at diastole. However, individual differences in interoceptive ability – specifically, interoceptive awareness and insight – were related to participants’ intentional inhibition rates and speed of decision. Participants with better interoceptive awareness and insight tended to choose to withhold actions and respond more slowly, while participants with poorer interoceptive awareness and insight tended to choose to execute actions and respond faster, suggesting lower (metacognitive) insight into bodily perception is connected to feelings of urges to move the body.
We also investigated whether autonomic and neurocognitive endophenotype traits, as measured by heart rate variability and self-report impulsivity questionnaires, were related to motor behaviour and interoceptive indices. We found little compelling evidence to that effect. Our primary observations were that faster Go reaction times were associated with greater impulsivity across a range of scales; NoGo error and Choose-Go rates with feelings of urgency on the UPPS-P; and higher BIS subscale trait impulsivity with greater interoceptive awareness and state sensibility (confidence), albeit with Bayes Factors that were often anecdotal.
Cardiac cues to inhibitory processes
When the heart contracts, baroreceptors in blood vessel walls signal the strength and timing of heartbeats to the central nervous system10. During states of high cardiovascular arousal, when heightened attention to salient stimuli and reprioritisation of action plans are important, increased baroreceptor signalling to brainstem and thalamic relay nuclei, then insular cortex, update the central representation of bodily physiology11,15. Fast homeostatic readjustments of behaviour are often required in scenarios of heightened autonomic arousal to adjust sensory perception and trigger aversive or avoidant reactions. On this basis, one can predict that cardiac signals would enhance inhibitory control. Indeed a broad invocation of inhibitory processes across behavioural domains at cardiac systole has been asserted historically22.
In the domain of reactive response inhibition, we previously found that stopping efficiency, as measured by stop signal reaction times on the stop signal task, was enhanced if the stop cues were presented at cardiac systole18. Here, we extend this observation from reactive motor inhibition to examine the volitional withholding of actions, i.e. ‘intentional inhibition’5. Interestingly, this form of motor inhibition was unaffected by cardiac cycle. Participants were equally likely to choose to act, or to withhold actions, when Choose cues were presented at systole and diastole. This carries a number of interesting implications.
Firstly, it may be that momentary state signals representing cardiovascular arousal are important cues to invoke the fast, rapid, reactive motor inhibition required during the stop signal task – and during threatening, or even dangerous, scenarios in everyday life, while less important for internally generated choices to act or to withhold motor plans. These action processes may be driven by sustained goal-oriented cognitive sets rather than momentary fluctuations in arousal state. Alternatively, it is possible that physiological state cues do still cue intentional inhibition, but the artificiality of tasks that require participants to make volitional choices ‘on cue’ in an experimental context obscures our sensitivity to measure truly voluntary action. We used a modified Go/NoGo task, incorporating Choose trials on which participants chose whether to ‘Go’ or ‘NoGo’. This paradigm was applied previously to measure intentional inhibition8,14, but arguably has reduced ecological validity in relation to the intentional inhibition processes that occur in everyday life. The ‘marble’ task50, and pain avoidance task7, may better reflect such everyday intentional inhibition processes. However, these are challenging to implement in a ‘cardiac cycle study’ since trial lengths are typically longer than a typical cardiac cycle. Finally, there are substantial individual differences in feelings of urges to move, which may relate less to transient signalling of autonomic processes, and more to longer-lasting trait level phenotypes.
However, we note that the Bayes Factor comparing % Choose-Go between systole and diastole (0.510) suggested only anecdotal evidence in favour of the null hypothesis, and a larger sample size would likely enable us to more firmly conclude in favour of no impact of cardiac cycle on intentional inhibition.
By employing a modified Go/NoGo task, it was possible to simultaneously investigate the impact of cardiac cycle on reactive inhibition within the same participants. This provides a complementary investigation to our previous study employing the stop signal task18, since NoGo trials require participants to withhold a prepotent response, while stop signal trials require participants to cancel an action that has already been initiated. In contrast to our previous finding that reactive inhibition on the stop signal task is improved during systole, here we found no evidence for a similar effect on NoGo inhibition. However, we interpret this finding with caution since, here, the paucity of NoGo error trials likely led to floor effects, obscuring our ability to detect any potential cardiac influence. A more traditional NoGo task, i.e. not including the additional Choose trials, may better invoke the prepotent tendency to act and hence elicit a higher NoGo error rate to better study this form of reactive inhibition. Nevertheless, the number of NoGo error trials will inevitably remain small, and vary between participants. Thus, the stop signal task is better suited to investigations of reactive inhibition where minimum trial numbers are necessary to compare between cardiac states18.
Individual differences in interoceptive awareness and insight
Although we found no conclusive evidence for an effect of cardiac cycle, we did observe some notable individual participant differences in intentional inhibition rates – with some choosing to move as infrequently as 40% on choice trials, and others as frequently as 80% – which correlated with interoceptive awareness. In addition, Choose-Go reaction times correlated with interoceptive insight (accuracy-confidence discrepancy), such that participants with poorer insight tended to move faster.
Interoceptive awareness and insight reflect the metacognitive perception of one’s interoceptive accuracy performance, according to confidence-accuracy correspondence. This suggests that while dynamic interoceptive cues (such as individual heartbeats) may not influence intentional motor decisions, higher-order (state-based) perception of the body forms one factor associated with frequency of volitional decisions to move or to inhibit.
Interoceptive awareness reflects ‘the ability of the individual to know when they are making good or bad interoceptive decisions’26, and may contribute to control of impulsive decision-making. A lack of insight into the accuracy of one’s ‘somatic markers’ can arise from prefrontal dysfunction (as opposed to damage to the insula, which impairs instead the representation and detection of such internal bodily changes). This can lead to increased risky decisions for short-term over long-term gain51. Our finding raises the possibility that ability to reflect on interoceptive sensitivity also embeds more elemental aspects of motor control by reducing feelings of urge to move. Thus, those who are better able to explain and accommodate changes in internal state through higher awareness are less likely to choose to act.
This process may operate via accumulation-of-evidence processes that underpin motor control and the release or inhibition of actions52. Voluntary action decisions to move can be explained by accumulation of activity to a motor threshold53, and response inhibition processes can similarly be modelled by drift diffusion models of activity accumulation for release or inhibition of an action54,55. Poorer interoceptive awareness could engender noisier afferent input to motor decision processes for homeostatic adjustment of behaviour, tipping accumulators for execution of action towards threshold. Further evidence for this putative mechanism comes from the association between faster Choose-Go reaction times and poorer interoceptive insight (high accuracy-confidence discrepancies), with potentially faster accumulator processes driving rapid ramps to action execution threshold in those with noisier interoceptive insight. However, the interoceptive insight discrepancy index is not a linear metric: a score of 0 indicates high interoceptive metacognition, with optimum alignment of accuracy and confidence. The further the discrepancy score from 0, the poorer the interoceptive metacognition, with negative discrepancy scores indicating low performance but high confidence, and positive discrepancy scores indicating high performance but low confidence. In our sample, five individuals who showed poorer insight, with low accuracy but high confidence, appear to behave similarly, in terms of faster reaction times, to those who also show poorer insight, but due to high accuracy and low confidence (Fig. 4c). This raises the possibility that insight abilities follow a ‘U-shaped’ curve, although in our present dataset, we were limited in our ability to explore this by the much smaller group of participants (n = 5) who showed low accuracy and high confidence, combined with the fact that the magnitude of their discrepancy is much smaller (0 to 0.3) than those showing the opposite (up to 0.7).
We did not observe effects between decisions to act and trait-level awareness of interoceptive ability, measured using trait interoceptive prediction error (TIPE), which indexes discrepancy between an individual’s objective (accuracy) and belief-based subjective (sensibility) dimensions of interoception28,29. State-based (e.g. awareness and insight) indices versus trait-based (TIPE) indices may reflect separate constructs neurally, and cognitively56,57.
In addition, it is notable that there were weak correlations between both intentional inhibition rates and Choose-Go reaction times with confidence ratings on the heartbeat tracking task, suggesting that the associations with the metacognitive interoceptive indices may be explained in part by cognitive processes that are unspecific to interoception. However, the larger Bayes Factors for the awareness and insight correlations than for confidence ratings hints that there is still a role for metacognitive interoceptive processes specifically in driving voluntary action, beyond generic judgements of mere confidence.
Interestingly, in people with Tourette Syndrome, who experience uncomfortable bodily sensations preceding involuntary tics, sensitivity to bodily sensations predicts severity of their premonitory urges to move29,58. This is further evidence that interoceptive signals can cue motor behaviour, via signals to midline motor regions12,59.
Autonomic reactivity and trait impulsivity
We predicted that autonomic reactivity, as measured by heart rate variability, and trait impulsivity, measured by self-report questionnaires, might also shape reactive and intentional inhibition, as measured by NoGo and Choose-NoGo trials. Interestingly, despite previous associations between heart rate variability and inhibitory control31–33, we found no evidence for such a relationship within our data, including when additionally controlling for the potential confound of heart rate. The associations between autonomic reactivity and inhibitory control processes may occur more commonly under explicitly affective conditions, such as in the regulation of emotional expression31 or perhaps stopping in response to valenced stimuli32.
We did observe correlations between reactive inhibition (NoGo Errors), and intentional inhibition (% Choose-Go), with positive and negative urgency scores on the UPPS-P questionnaire. Urgency as measured by this questionnaire reflects a tendency to act impulsively while experiencing emotions (for example, “When I feel bad, I will often do things I later regret in order to make myself feel better now”). This perhaps lends weight to the conclusion that trait affective processes, which incorporate higher-order representation of the body, impact on frequency of volitional decisions to move or to inhibit. However, the Bayes Factors associated with this correlation was merely anecdotal in favour of H1. Otherwise, it is notable that other facets of trait impulsivity did not relate to intentional inhibition (or reactive): this may reflect a limitation of our (high-functioning) university student sample, in whom there are likely to be fewer individuals with very high (maladaptive) impulsivity levels than in the general population, and clinical samples may show otherwise.
We also tested whether trait impulsivity related to individual differences in interoception. Previously, we found a (non-significant) weak correlation between higher trait impulsivity on the BIS and poorer interoceptive accuracy on the heartbeat tracking task18, and that poorer performance on a two alternative forced choice synchronicity task predicted lower attentional impulsivity as measured by the BIS60. Here, having studied only the heartbeat tracking task, it was not possible to replicate Herman et al.60. Although we did not observe evidence for associations between heartbeat tracking accuracy and trait impulsivity in the present sample, there were positive correlations between BIS subscales and interoceptive awareness and confidence ratings. The anecdotal status of the Bayes Factors for these tests signifies the probably weak associations between interoceptive indices and trait impulsivity. This suggests that interoception may be a more potent driver of momentary urges to act, as measured by the intentional inhibition task, than trait-level endophenotypes; or alternatively, that such associations only become strongly evident in clinical populations.
Study limitations and future directions
The heartbeat tracking task provides a valuable heuristic approach for quantifying individual differences in bodily sensitivity41. However, the task is subject to a number of confounds which limit its interpretation61. For example, participants may count elapsed seconds and use this as a proxy for number of heartbeats. Asking participants to estimate passage of time alongside estimation of heartbeats can provide a control62,63. Nevertheless, such task confounds may arguably influence our measures of metacognitive interoceptive awareness and insight, with which we observed an effect with intentional inhibition, to a lesser degree than objective task performance measures of interoceptive accuracy. Alternatively, two alternative forced choice tasks, in which participants report whether exteroceptive stimuli are synchronous or asynchronous with their heartbeat, can be employed64, but often many participants perform at chance, and so such approaches may not comprehensively capture individual differences in physiological and interoceptive function61,63. Recently developed heartbeat matching tasks, in which participants use a slider to adjust the rate of a pulsing visual stimulus to their own heart rate, show promise for addressing these methodological drawbacks63.
Interestingly, we did not replicate previously identified effects of cardiac cycle on reaction times65,66, whether for Go or Choose-Go trials. However, such previously reported effects have sometimes been small, and not present in both men and women65. In our recent study of reactive inhibition, Go trials during a stop signal task were no different in response time between systole and diastole18.
In both our present and our previous stop signal studies, we targeted the presentation of motor task cues according to timepoints when arterial baroreceptors – which underpin the neural and cognitive perception of a heartbeat – will be maximally active versus quiescent. Alternatively, one can study each individual participant’s ECG post-hoc to either pinpoint whether stimuli fell into particular cardiac phases19, or bin trials into histogram plots21. It may be important to bear in mind such different methodological approaches in timing task events to specific points within the cardiac cycle when comparing cardiac timing effects across studies.
Following completion of the intentional inhibition task, we recorded ECG for 2.5 minutes during which participants rested quietly with eyes open, to investigate HRV. Longer ECG recordings give more stable HRV estimates; however, a minimum of 1 minute is considered sufficient by consensus guidelines for reliable estimates of RMSSD67.
A complementary literature has examined the effect of motor acts on heart rate, leading to acceleration or deceleration during execution and inhibition of movements, respectively23,25,68. This includes both reactive inhibition23 and intentional inhibition on the ‘marble’ task25. Here, our focus was instead on the impact that cardiac cues have on motor behaviour: by dynamically monitoring the ECG signal, we ensured the timing of our stimulus delivery was accurate (Fig. 2), even taking into account momentary increases and decreases in heart rate. It is notable that reactive inhibition shows bidirectional effects between cardiac cycle and stopping18,23, while intentional inhibition effects appear to be unidirectional: with no evidence here for an effect of cardiac cycle on frequency of choosing to inhibit, but with cardiac deceleration occurring during intentional inhibition trials on the ‘marble’ task25.
To further examine how perception of the body and volitional urges to act or to withhold movements inter-relate, clinical samples of patient groups with altered interoception and motor function will be valuable. Both people with Tourette Syndrome and patients with Parkinson’s Disease show altered interoceptive processes, which predict premonitory urge and motor symptom severity29,58,69. Interestingly, however, patients with Parkinson’s Disease show equivalent intentional inhibition to controls on the ‘marble’ task70. Intentional inhibition, and its potential impact on volitional tic suppression, is yet to be fully explored in Tourette Syndrome.
Conclusions
State interoceptive cues, namely heartbeats, do not influence voluntary decisions to act or withhold movement in an intentional inhibition task. However, higher-order perception of the body, according to interoceptive awareness and insight, is associated with individual differences in intentional inhibition rates and response times. Individuals with poorer metacognitive awareness of, and insight into, their interoceptive signals tend to move more and faster, while those with better awareness and insight tend to move less, and less rapidly. This suggests that noisier afferent input to motor decision processes may engender feelings of urges to move the body, which may be altered in clinical conditions such as Tourette Syndrome.
Acknowledgements
This work was supported by a philanthropic donation from the Dr. Mortimer and Theresa Sackler Foundation to the University of Sussex and Sackler Centre for Consciousness Science.
Author contributions
C.R., S.G. and H.C. developed the study concept and design. A.A. and D.E.O.L. performed data collection. C.G.v.P. provided physiological data acquisition and analysis tools. C.R., A.A., D.E.O.L. and M.S. analysed data. C.R. and H.C. wrote the manuscript. All authors contributed to and approved the final manuscript.
Data availability
The data reported in this study, related task code and analysis scripts, and .jasp statistical analysis file are available at https://osf.io/3xezy.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brass M, Haggard P. The what, when, whether model of intentional action. Neuroscientist. 2008;14:319–325. doi: 10.1177/1073858408317417. [DOI] [PubMed] [Google Scholar]
- 2.Alderson-Day B, et al. Intentional inhibition but not source memory is related to hallucination-proneness and intrusive thoughts in a university sample. Cortex; a J. devoted study Nerv. Syst. Behav. 2019;113:267–278. doi: 10.1016/j.cortex.2018.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson MC, Hanslmayr S. Neural mechanisms of motivated forgetting. Trends Cogn. Sci. 2014;18:279–292. doi: 10.1016/j.tics.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zapparoli L, Seghezzi S, Paulesu E. The What, the When, and the Whether of Intentional Action in the Brain: A Meta-Analytical Review. Front. Hum. Neurosci. 2017;11:238. doi: 10.3389/fnhum.2017.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filevich E, Kuhn S, Haggard P. Intentional inhibition in human action: The power of ‘no’. Neurosci. Biobehav. Rev. 2012;36:1107–1118. doi: 10.1016/j.neubiorev.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Schel MA, et al. Neural correlates of intentional and stimulus-driven inhibition: a comparison. Front. Hum. Neurosci. 2014;8:27. doi: 10.3389/fnhum.2014.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lynn MT, Demanet J, Krebs RM, Van Dessel P, Brass M. Voluntary inhibition of pain avoidance behavior: an fMRI study. Brain structure Funct. 2016;221:1309–1320. doi: 10.1007/s00429-014-0972-9. [DOI] [PubMed] [Google Scholar]
- 8.Dall’Acqua T, Begliomini C, Motta R, Miotto D, Castiello U. Effects of intentionality and subliminal information in free-choices to inhibit. Neuropsychologia. 2018;109:28–38. doi: 10.1016/j.neuropsychologia.2017.11.035. [DOI] [PubMed] [Google Scholar]
- 9.Brass M, Haggard P. The hidden side of intentional action: the role of the anterior insular cortex. Brain structure Funct. 2010;214:603–610. doi: 10.1007/s00429-010-0269-6. [DOI] [PubMed] [Google Scholar]
- 10.Critchley HD, Harrison NA. Visceral influences on brain and behavior. Neuron. 2013;77:624–638. doi: 10.1016/j.neuron.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 12.Jackson SR, Parkinson A, Kim SY, Schuermann M, Eickhoff SB. On the functional anatomy of the urge-for-action. Cognit. Neurosci. 2011;2:227–243. doi: 10.1080/17588928.2011.604717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos. Trans. R. Soc. Lond. 1996;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- 14.Parkinson J, Haggard P. Subliminal priming of intentional inhibition. Cognition. 2014;130:255–265. doi: 10.1016/j.cognition.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Garfinkel SN, Critchley HD. Threat and the Body: How the Heart Supports Fear Processing. Trends Cognit. Sci. 2016;20:34–46. doi: 10.1016/j.tics.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Lewis RP, Rittogers SE, Froester WF, Boudoulas H. A critical review of the systolic time intervals. Circulation. 1977;56:146–158. doi: 10.1161/01.cir.56.2.146. [DOI] [PubMed] [Google Scholar]
- 17.Sato K, et al. Association of Time Between Left Ventricular and Aortic Systolic Pressure Peaks With Severity of Aortic Stenosis and Calcification of Aortic Valve. JAMA Cardiol. 2019;4:549–555. doi: 10.1001/jamacardio.2019.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rae CL, et al. Response inhibition on the stop signal task improves during cardiac contraction. Sci. Rep. 2018;8:9136. doi: 10.1038/s41598-018-27513-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunzendorf S, et al. Active information sampling varies across the cardiac cycle. Psychophysiol. 2019;56:e13322. doi: 10.1111/psyp.13322. [DOI] [PubMed] [Google Scholar]
- 20.Galvez-Pol, A., McConnell, R. & Kilner, J. Voluntary visual search is modulated by the cardiac cycle. BioRxiv, https://www.biorxiv.org/content/10.1101/405902v3.
- 21.Palser, E. R., Glass, J., Fotopoulou, A. & Kilner, J. M. Relationship between cardiac cycle and the timing of actions during action execution and observation. bioRxiv, 10.1101/585414 (preprint). [DOI] [PMC free article] [PubMed]
- 22.Lacey BC, Lacey JI. Two-way communication between the heart and the brain. Significance of time within the cardiac cycle. Am. psychologist. 1978;33:99–113. doi: 10.1037/0003-066X.33.2.99. [DOI] [PubMed] [Google Scholar]
- 23.Jennings JR, van der Molen MW, Brock K, Somsen RJ. On the synchrony of stopping motor responses and delaying heartbeats. J. Exp. Psychol. 1992;18:422–436. doi: 10.1037//0096-1523.18.2.422. [DOI] [PubMed] [Google Scholar]
- 24.van der Veen FM, van der Molen MW, Jennings JR. Selective inhibition is indexed by heart rate slowing. Psychophysiol. 2000;37:607–613. doi: 10.1111/1469-8986.3750607. [DOI] [PubMed] [Google Scholar]
- 25.Schel MA, Windhorst DA, van der Molen MW, Crone EA. Developmental change in intentional action and inhibition: a heart rate analysis. Psychophysiol. 2013;50:812–819. doi: 10.1111/psyp.12065. [DOI] [PubMed] [Google Scholar]
- 26.Garfinkel SN, Seth AK, Barrett AB, Suzuki K, Critchley HD. Knowing your own heart: distinguishing interoceptive accuracy from interoceptive awareness. Biol. Psychol. 2015;104:65–74. doi: 10.1016/j.biopsycho.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Murphy, J. et al. Testing the independence of self-reported interoceptive accuracy and attention. Q. J. Exp. Psychol., 1–19 (2019). [DOI] [PubMed]
- 28.Garfinkel SN, et al. Discrepancies between dimensions of interoception in autism: Implications for emotion and anxiety. Biol. Psychol. 2016;114:117–126. doi: 10.1016/j.biopsycho.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Rae CL, Larsson DEO, Garfinkel SN, Critchley HD. Dimensions of interoception predict premonitory urges and tic severity in Tourette syndrome. Psychiatry Res. 2019;271:469–475. doi: 10.1016/j.psychres.2018.12.036. [DOI] [PubMed] [Google Scholar]
- 30.Garfinkel SN, et al. What the heart forgets: Cardiac timing influences memory for words and is modulated by metacognition and interoceptive sensitivity. Psychophysiol. 2013;50:505–512. doi: 10.1111/psyp.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Ann. Behav. medicine: a Publ. Soc. Behav. Med. 2009;37:141–153. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- 32.Krypotos AM, Jahfari S, van Ast VA, Kindt M, Forstmann BU. Individual Differences in Heart Rate Variability Predict the Degree of Slowing during Response Inhibition and Initiation in the Presence of Emotional Stimuli. Front. Psychol. 2011;2:278. doi: 10.3389/fpsyg.2011.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gillie BL, Vasey MW, Thayer JF. Individual differences in resting heart rate variability moderate thought suppression success. Psychophysiol. 2015;52:1149–1160. doi: 10.1111/psyp.12443. [DOI] [PubMed] [Google Scholar]
- 34.Robbins TW, Gillan CM, Smith DG, de Wit S, Ersche KD. Neurocognitive endophenotypes of impulsivity and compulsivity: towards dimensional psychiatry. Trends Cogn. Sci. 2012;16:81–91. doi: 10.1016/j.tics.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 35.Chambers CD, Garavan H, Bellgrove MA. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci. Biobehav. Rev. 2009;33:631–646. doi: 10.1016/j.neubiorev.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 36.Swick D, Ashley V, Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage. 2011;56:1655–1665. doi: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- 37.Parkinson J, Garfinkel S, Critchley H, Dienes Z, Seth AK. Don’t make me angry, you wouldn’t like me when I’m angry: Volitional choices to act or inhibit are modulated by subliminal perception of emotional faces. Cognitive, Affect. Behav. Neurosci. 2017;17:252–268. doi: 10.3758/s13415-016-0477-5. [DOI] [PubMed] [Google Scholar]
- 38.Noda K, et al. Comparison of the measured pre-ejection periods and left ventricular ejection times between echocardiography and impedance cardiography for optimizing cardiac resynchronization therapy. J. Arrhythm. 2017;33:130–133. doi: 10.1016/j.joa.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kolev N, Zimpfer M. Left ventricular ejection time and end-systolic pressure revisited. Anesth. Analg. 1995;81:889–890. doi: 10.1097/00000539-199510000-00055. [DOI] [PubMed] [Google Scholar]
- 40.Feher Joseph. Quantitative Human Physiology. 2012. Chemical Foundations of Physiology I; pp. 32–42. [Google Scholar]
- 41.Schandry R. Heart beat perception and emotional experience. Psychophysiol. 1981;18:483–488. doi: 10.1111/j.1469-8986.1981.tb02486.x. [DOI] [PubMed] [Google Scholar]
- 42.Hart N, McGowan J, Minati L, Critchley HD. Emotional regulation and bodily sensation: interoceptive awareness is intact in borderline personality disorder. J. personality Disord. 2013;27:506–518. doi: 10.1521/pedi_2012_26_049. [DOI] [PubMed] [Google Scholar]
- 43.Porges, S. W. Body Perception Questionnaire. (Laboratory of Developmental Assessment, University of Maryland 1993).
- 44.Ramshur, J. T. Design, evaluation, and application of heart rate variability analysis software (HRVAS) MSc thesis, University of Memphis (2010).
- 45.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::AID-JCLP2270510607>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 46.Conners, K. C., Erhardt, D. & Sparrow, E. Conners’ adult ADHD rating scales: CAARS. (MHS, 1999).
- 47.Cyders MA, et al. Integration of impulsivity and positive mood to predict risky behavior: development and validation of a measure of positive urgency. Psychol. Assess. 2007;19:107–118. doi: 10.1037/1040-3590.19.1.107. [DOI] [PubMed] [Google Scholar]
- 48.Lee, M. D. & Wagenmakers, E.-J. Bayesian cognitive modeling: a practical course., (Cambridge University Press 2014).
- 49.Tsakiris Manos, Critchley Hugo. Interoception beyond homeostasis: affect, cognition and mental health. Philosophical Transactions of the Royal Society B: Biological Sciences. 2016;371(1708):20160002. doi: 10.1098/rstb.2016.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuhn S, Haggard P, Brass M. Intentional inhibition: how the “veto-area” exerts control. Hum. Brain Mapp. 2009;30:2834–2843. doi: 10.1002/hbm.20711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clark L, et al. Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain. 2008;131:1311–1322. doi: 10.1093/brain/awn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gold JI, Shadlen MN. The neural basis of decision making. Annu. Rev. Neurosci. 2007;30:535–574. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- 53.Schurger A, Sitt JD, Dehaene S. An accumulator model for spontaneous neural activity prior to self-initiated movement. Proc. Natl Acad. Sci. U S Am. 2012;109:E2904–2913. doi: 10.1073/pnas.1210467109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sebastian A, Forstmann BU, Matzke D. Towards a model-based cognitive neuroscience of stopping - a neuroimaging perspective. Neurosci. Biobehav. Rev. 2018;90:130–136. doi: 10.1016/j.neubiorev.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 55.Zhang J, et al. Different decision deficits impair response inhibition in progressive supranuclear palsy and Parkinson’s disease. Brain. 2016;139:161–173. doi: 10.1093/brain/awv331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quadt L, Critchley HD, Garfinkel SN. The neurobiology of interoception in health and disease. Ann. N. Y. Acad. Sci. 2018;1428:112–128. doi: 10.1111/nyas.13915. [DOI] [PubMed] [Google Scholar]
- 57.Murphy J, Catmur C, Bird G. Classifying individual differences in interoception: Implications for the measurement of interoceptive awareness. Psychonomic Bull. Rev. 2019;26:1467–1471. doi: 10.3758/s13423-019-01632-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ganos C, et al. Premonitory urge to tic in Tourette’s is associated with interoceptive awareness. Mov. disorders: Off. J. Mov. Disord. Soc. 2015;30:1198–1202. doi: 10.1002/mds.26228. [DOI] [PubMed] [Google Scholar]
- 59.Rae CL, Critchley HD, Seth AK. A Bayesian Account of the Sensory-Motor Interactions Underlying Symptoms of Tourette Syndrome. Front. psychiatry. 2019;10:29. doi: 10.3389/fpsyt.2019.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Herman AM, Rae CL, Critchley HD, Duka T. Interoceptive accuracy predicts nonplanning trait impulsivity. Psychophysiol. 2019;56:1–14. doi: 10.1111/psyp.13339. [DOI] [PubMed] [Google Scholar]
- 61.Brener Jasper, Ring Christopher. Towards a psychophysics of interoceptive processes: the measurement of heartbeat detection. Philosophical Transactions of the Royal Society B: Biological Sciences. 2016;371(1708):20160015. doi: 10.1098/rstb.2016.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ainley V, Brass M, Tsakiris M. Heartfelt imitation: high interoceptive awareness is linked to greater automatic imitation. Neuropsychologia. 2014;60:21–28. doi: 10.1016/j.neuropsychologia.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 63.Palmer, C. E., Ainley, V. L. & Tsakiris, M. Fine-tuning your heart: a novel method for measuring interoceptive accuracy. PsyArXiv, https://psyarxiv.com/qz7r9/ (preprint).
- 64.Whitehead WE, Drescher VM, Heiman P, Blackwell B. Relation of heart rate control to heartbeat perception. Biofeedback self-regulation. 1977;2:317–392. doi: 10.1007/BF00998623. [DOI] [PubMed] [Google Scholar]
- 65.Birren JE, Cardon PV, Jr., Phillips SL. Reaction time as a function of the cardiac cycle in young adults. Sci. 1963;140:195–196. doi: 10.1126/science.140.3563.195-a. [DOI] [PubMed] [Google Scholar]
- 66.Jennings JR, Wood CC. Cardiac cycle time effects on performance, phasic cardiac responses, and their intercorrelation in choice reaction time. Psychophysiol. 1977;14:297–307. doi: 10.1111/j.1469-8986.1977.tb01179.x. [DOI] [PubMed] [Google Scholar]
- 67.Laborde S, Mosley E, Thayer JF. Heart Rate Variability and Cardiac Vagal Tone in Psychophysiological Research - Recommendations for Experiment Planning, Data Analysis, and Data Reporting. Front. Psychol. 2017;8:213. doi: 10.3389/fpsyg.2017.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Borger N, van der Meere J. Motor control and state regulation in children with ADHD: a cardiac response study. Biol. Psychol. 2000;51:247–267. doi: 10.1016/S0301-0511(99)00040-X. [DOI] [PubMed] [Google Scholar]
- 69.Ricciardi L, et al. Know thyself: Exploring interoceptive sensitivity in Parkinson’s disease. J. Neurol. Sci. 2016;364:110–115. doi: 10.1016/j.jns.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 70.Ricciardi L, et al. Acting without being in control: Exploring volition in Parkinson’s disease with impulsive compulsive behaviours. Parkinsonism Relat. Disord. 2017;40:51–57. doi: 10.1016/j.parkreldis.2017.04.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data reported in this study, related task code and analysis scripts, and .jasp statistical analysis file are available at https://osf.io/3xezy.