Abstract
Background and Purpose
Nociceptin/orphanin FQ (N/OFQ) peptide and its cognate receptor (NOP) are widely expressed in mesolimbic brain regions where they play an important role in modulating reward and motivation. Early evidence suggested that NOP receptor activation attenuates the rewarding effects of drugs of abuse, including alcohol. However, emerging data indicate that NOP receptor blockade also effectively attenuates alcohol drinking and relapse. To advance our understanding of the role of the N/OFQ‐NOP receptor system in alcohol abuse, we examined the effect of NOP receptor blockade on voluntary alcohol drinking at the neurocircuitry level.
Experimental Approach
Using male and female genetically selected alcohol‐preferring Marchigian Sardinian (msP) rats, we initially evaluated the effects of the selective NOP receptor antagonist LY2817412 (3, 10, and 30 mg·kg−1, p.o.) on alcohol consumption in a two‐bottle free‐choice paradigm. We then microinjected LY2817412 (3 and 6 μg·μl−1 per rat) in the central nucleus of the amygdala (CeA), ventral tegmental area (VTA), and nucleus accumbens (NAc).
Key Results
Peripheral LY2817412 administration dose‐dependently and selectively reduced voluntary alcohol intake in male and female msP rats. Central injections of LY2817412 markedly attenuated voluntary alcohol intake in both sexes following administration in the CeA and VTA but not in the NAc.
Conclusion and Implications
The present results revealed that the CeA and VTA are neuroanatomical substrates that mediate the effects of NOP receptor antagonism on alcohol consumption. Overall, our findings support the potential of NOP receptor antagonism as a treatment strategy to attenuate alcohol use and addiction.
Abbreviations
- 2BC
two‐bottle free‐choice test
- AP
anterior/posterior
- AUD
alcohol use disorder
- BNST
bed nucleus of the stria terminalis
- CeA
central nucleus of the amygdala
- DV
dorsal/ventral
- Kb
antagonist affinity
- Ki
inhibition constant
- ML
medial/lateral
- mPFC
medial prefrontal cortex
- msP rats
Marchigian Sardinian alcohol‐preferring rats
- N/OFQ
nociceptin/orphanin FQ
- NAc
nucleus accumbens
- NOP receptor
nociceptin/orphanin FQ receptor
- ORL‐1
opioid receptor‐like 1
- VTA
ventral tegmental area
What is already known
Targeting NOP receptors is an emerging therapeutic strategy for treating alcohol use disorder.
Systemic administration of the NOP receptor antagonist LY2940094 reduces voluntary alcohol drinking in alcohol‐preferring rats.
What does this study add
NOP receptor blockade attenuates alcohol drinking through mechanisms that involve CeA and VTA neurotransmission
What is the clinical significance
NOP receptor antagonism may represent a novel approach to the treatment of alcohol‐use disorders
1. INTRODUCTION
https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1681 (N/OFQ) peptide and its cognate https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=320, previously named opioid receptor‐like 1 (ORL‐1), are members of the endogenous opioid system (Meunier, 1997; Meunier et al., 1995; Mollereau et al., 1994; Reinscheid et al., 1995). Because of the lack of the N‐terminal tyrosine residue, N/OFQ peptide is unable to activate https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=319, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=317, or https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=318&familyId=50&familyType=GPCR receptors. In turn, classic opioid ligands do not bind NOP receptors (Bunzow et al., 1994; Meunier et al., 1995; Witkin et al., 2014). N/OFQ peptide and NOP receptors are highly expressed in various brain areas, including the central nucleus of the amygdala (CeA), bed nucleus of the stria terminalis (BNST), medial prefrontal cortex, ventral tegmental area (VTA), and lateral hypothalamus. Moderate expression is also detected in the nucleus accumbens (NAc), locus coeruleus, and dorsal raphe (Darland, Heinricher, & Grandy, 1998; Neal et al., 1999). In these areas, the N/OFQ‐NOP receptor system regulates motivation, emotion, stress reactivity, and various aspects of addiction‐like behaviours (Ciccocioppo et al., 2014; Ciccocioppo, Economidou, Fedeli, & Massi, 2003; Narendran et al., 2017; Toledo et al., 2014). Conditioned place preference studies demonstrate that NOP activation attenuates the rewarding effects of various psychoactive substances, including https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2299, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1627, and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2286 (Kotlinska et al., 2003; Kotlinska, Wichmann, Legowska, Rolka, & Silberring, 2002; Kuzmin, Sandin, Terenius, & Ogren, 2003; Zhao et al., 2003). Intracranial N/OFQ administration also reduces alcohol drinking and seeking in genetically selected Marchigian Sardinian alcohol‐preferring (msP) rats, an animal line that is selected for excessive alcohol consumption (Ciccocioppo, Panocka, Froldi, et al., 1999; Ciccocioppo, Panocka, Polidori, et al., 1999; Ciccocioppo et al., 2003; Ciccocioppo et al., 2004; Martin‐Fardon, Ciccocioppo, Massi, & Weiss, 2000). NOP receptor agonists also effectively attenuate alcohol self‐administration in heterogeneous Wistar rats with a previous history of alcohol dependence, whereas they have less or no effects in non‐dependent rats (Ciccocioppo et al., 2004; de Guglielmo, Martin‐Fardon, Teshima, Ciccocioppo, & Weiss, 2015; Economidou et al., 2008; Kuzmin, Kreek, Bakalkin, & Liljequist, 2007).
The above findings demonstrate that NOP receptor activation attenuates alcohol drinking and seeking in rats. However, these findings appear to be inconsistent with molecular data that show that (a) animals with heightened N/OFQ‐NOP expression, either innate (msP rats), or adaptive (post‐dependent Wistar rats) drink higher amounts of alcohol and are more prone to relapse (Aujla et al., 2013; Cippitelli et al., 2016; Economidou et al., 2008; Hansson et al., 2006) and (b) engineered rats with constitutive NOP receptor deletion self‐administer less alcohol compared with wild type controls (Kallupi et al., 2017). More importantly, a recent study demonstrated that the selective NOP receptor antagonist https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=9462 attenuated alcohol intake and relapse to alcohol seeking in both msP and Indiana alcohol‐preferring (P) rats (Rorick‐Kehn et al., 2016). Additionally, preliminary clinical evidence shows the efficacy of LY2940094 in decreasing heavy drinking days and increasing abstinence days in depressed alcohol users (Post et al., 2016). These studies support an alternative hypothesis that NOP receptor antagonism (rather than agonism) may be useful for the treatment of alcohol use disorder (AUD). The reason why both NOP receptor agonists and antagonists reduce alcohol drinking is unclear. Notably, however, following an acute injection, the effect of NOP agonists on alcohol drinking is low or in some cases absent, but it increases over repeated administrations and remains for several days after treatment discontinuation (Ciccocioppo et al., 2014). The effect of a NOP receptor antagonist, in contrast, appears after the first administration (Rorick‐Kehn et al., 2016). Based on these findings, we recently hypothesized that the effect of NOP agonists on alcohol drinking may depend on their ability to desensitize the receptor system, thus acting as functional antagonists (Ciccocioppo et al., 2019).
To confirm the potential of NOP antagonism to reduce alcohol drinking, we tested the effect of another recently available selective NOP receptor blocker, LY2817412, in male and female msP rats. We also sought to elucidate the neurocircuitry through which NOP receptor blockade attenuates alcohol consumption by microinjecting LY2817412 in the CeA, VTA, and NAc. These three brain areas were chosen based on their role in the regulation of motivated behaviours and their expression levels of N/OFQ‐NOP.
2. METHODS
2.1. Animals
All animal care and experimental procedures were performed in accordance with the guidelines of the European Community Council Directive for Care and Use of Laboratory Animals and European legislation (2010/63/EU). Formal approval to conduct the experiments was obtained from the Italian Ministry of Health and Organism Responsible for Animal Welfare of the University of Camerino (protocol no. 1D580.1). The animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2010) and the recommendations made by the British Journal of Pharmacology.
A total of 61 male msP rats (derived from 16 litters) and 52 female msP rats (derived from 15 litters), bred and selected at the University of Camerino (Italy), were used. The rats were between 10 and 11 weeks old (postnatal days 70–77) at the beginning of the experiments. The animals were randomly assigned and housed two per cage in standard clear plastic cages with conventional bedding until the drinking experiments began. They were maintained in a temperature (20–22°C) and humidity (45–55%) controlled and pathogen‐free vivarium under a reverse 12 hr/12 hr light/dark cycle (lights off at 8:30 a.m. and on at 8:30 p.m.). During the experiments, the animals were given ad libitum access to tap water and food pellets (4RF18, Mucedola, Settimo Milanese, Italy). To mitigate anxiety that is associated with the experimenters and familiarize the rats to the procedures, they were handled for 5 days (~3 min of handling per animal per day) before behavioural testing.
All of the experimental sessions were conducted during the dark phase of the light/dark cycle. All efforts were made to minimize the number of animals used and their discomfort. Group sizes were unequal because few subjects had to be excluded because of incorrect cannula placement in the intracranial studies. Male and female rats were randomly assigned to the treatment groups so that comparable numbers of subjects of each sex in each experiment were used for the statistical analysis. The two‐bottle free‐choice (2BC) test was conducted in parallel, with male and female rats that were housed in different rooms to avoid somatosensory cues (e.g., sight and smell) of the opposite sex from influencing the behavioural results. Rooms where male and female rats were kept were next to each other and identical. The experimenters who were responsible for data collection and analysis were blind to treatments.
2.2. Intracranial surgery and infusion procedure
For intracranial surgery, the animals were anaesthetized by an intramuscular injection of a solution (100–150 μl) that contained tiletamine (58.17 mg·ml−1) and zolazepam (7.5 mg·ml−1). Guide cannulas (0.65 mm outside diameter) for the microinjections were implanted and cemented bilaterally into the CeA, VTA, and NAc. Different groups of animals were used for the three different brain areas.
The following stereotaxic coordinates, relative to bregma, were used: male rats (CeA: anterior/posterior (AP), −2.5 mm; medial/lateral (ML), ±4.3 mm; dorsal/ventral (DV), −6.5 mm; VTA: AP, −5.6 mm; ML, ±2.2 mm; DV, −7.4 mm, 12° angle; NAc: AP, +1.5 mm; ML, ±1.1 mm; DV, 5.2 mm), female rats (CeA: AP, −1.8 mm; ML, ±4.0 mm; DV, −7.4 mm; VTA: AP, −5.7 mm; ML, ±2.2 mm; DV, −7.4 mm, 10° angle; NAc: AP, +1.40 mm; ML, ±1.0 mm; DV, −5.5 mm). All of the coordinates were based on a rat brain atlas (Paxinos & Watson, 2007 ) and adjusted for the animals' body weight and sexes. Following surgery, the animals received a single subcutaneous injection of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4795 (2.5 mg·kg−1, s.c.) and were allowed to recover for 1 week in their home cages. During this recovery period, the rats were handled daily and habituated to the microinjection procedure, consisting of insertion of the injector into the guide cannulas, for at least 3 days before the behavioural tests began. The rats were bilaterally injected with LY2817412 or vehicle over a period of 60 s, approximately 15 min before they were given access to alcohol. A stainless‐steel injector, 1.5 mm longer than the guide cannula, was left in place for few seconds after the injection to allow diffusion of the solution. At the completion of the experiments, to verify cannula placements, the rats were anaesthetized with https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2505 and subsequently injected with black India ink (0.3 μl per site) in the CeA, VTA, and NAc. Immediately afterward, the rats were killed, and the brains were collected for histological analyses of cannula placements (see Supporting Information).
2.3. Effect of systemic LY2817412 administration on 10% alcohol intake in a two‐bottle free‐choice test
The two‐bottle free‐choice (2BC) paradigm (choice between water and 10% alcohol) was used to measure alcohol drinking and preference (Koob et al., 2003; Tabakoff & Hoffman, 2000). The week before the 2BC test began, msP rats were singly housed in experimental chambers for acclimation. For the next 15 days, they were given continuous access to 10% alcohol and water (24 hr·day−1) under free access conditions to achieve a stable baseline of drinking and high preference for alcohol (≥80% preference for alcohol over water during the last 3 days). Once baseline drinking was reached, we tested the effect of LY2817412 on voluntary alcohol intake. Cages (30 cm length × 30 cm width × 30 cm height) that were used for the experiments were equipped with two graduated drinking tubes (100‐ml volume) with metallic drinking spouts and food containers. Fluids were delivered through graduated drinking tubes, and consumption was measured by reading the volume that was consumed 2, 8, and 24 hr after alcohol was offered to the animals. The tubes were switched daily to avoid the development of side preference. Food intake was measured by weighing the food containers and taking into account spillage. Alcohol, water, and food intake was calculated as absolute values at each time interval (2, 8, and 24 hr) and are expressed as grams per kilogram to control for the influence of body weight differences (Becker & Lopez, 2004; Finn et al., 2007; Rimondini, Sommer, & Heilig, 2003). Drug tests were performed using a within‐subject counterbalanced Latin square design (i.e., each animal received a single injection of each treatment dose) in male msP rats (n = 11, derived from three litters) and female msP rats (n = 10, derived from three litters). At least a 3‐day interval, during which a stable baseline of alcohol drinking was re‐established, was allowed between drug tests. Before the treatments began, rats were habituated to the gavage administration procedures for three consecutive days, during which they received distilled water to familiarize them with the injection procedures. Following the acquisition of a stable baseline of alcohol consumption (≥80% preference for alcohol over the last 3 days), LY2817412 (3, 10, and 30 mg·kg−1) or its vehicle were administered orally 1 hr before the 2BC test began. Drug doses and the time of administration were chosen based on our previous experience with this compound and published data (Toledo et al., 2014). The treatments were counterbalanced across rats from different litters and across sexes. The experimenters were blind to the treatments.
2.4. Effect of systemic LY2817412 administration on standard food and water intake
Male (n = 10, derived from three litters) and female (n = 10, derived from three litters) msP rats were singly housed in experimental chambers for at least 1 week before the test began. To evaluate the effect of systemic LY2817412 administration on the caloric effects of food, water and standard chow were available ad libitum. Water and food intake was calculated as absolute values at each time interval (2, 8, and 24 hr) and are expressed as grams per kilogram to control for the influence of body weight differences (Becker & Lopez, 2004; Finn et al., 2007; Rimondini et al., 2003). Similar to the previous experiment, the rats were habituated to the administration procedures, and the treatments were counterbalanced in a Latin square design. After the acclimation period (15 days), LY2817412 (30 mg·kg−1) or its vehicle was administered orally 1 hr before the test began. The treatments were counterbalanced across rats from different litters and across sexes. The experimenters were blind to the treatments.
2.5. Effect of intracranial LY2817412 administration
Rats were single‐housed in experimental chambers for at least 1 week before the 2BC test began. To evaluate the effect of intracranial LY2817412 administration, water and food were available ad libitum, but access to alcohol was restricted to 2 hr·day−1 (8:30–10:30 a.m.). The 2BC test was restricted to 2 hr because after site‐specific administration, drugs can easily redistribute, and their concentration at the injection site can decrease rapidly over time.
Male (n = 13 for CeA, n = 17 for VTA, n = 10 for NAc) and female (n = 9 for CeA, n = 12 for VTA, n = 11 for NAc) msP rats received LY2817412 (3 and 6 μg·μl−1 per rat) or its vehicle 15 min before access to 10% alcohol. Fluid (alcohol and water) intake and food intake were recorded after 2‐hr access. The drug tests were conducted according to a within‐subject Latin square counterbalanced design. At least a 3‐day interval, during which a stable baseline of alcohol drinking was re‐established, was allowed between drug tests. Only data from rats with correct cannula placements were included in the statistical analysis (male: n = 8 for CeA [derived from four litters], n = 9 for VTA [derived from four litters], n = 8 for NAc [derived from two litters]; female: n = 7 for CeA [derived from three litters], n = 8 for VTA [derived from three litters], n = 9 for NAc [derived from three litters]). The effect of intracranial LY2817412 administration in each brain area was studied in both sexes simultaneously in the following order: CeA, VTA, and NAc. The treatments were counterbalanced across rats from different litters and across sexes. The experimenters were blind to the treatments.
2.6. In vitro NOP receptor binding
A filtration‐based https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1688 binding assay was used to determine the affinity (Ki) of LY2817412 using previously described conditions (Statnick et al., 2016). Assay incubations were performed in deep‐well 96‐well plates with [3H]‐nociceptin (final assay concentration = 0.2 nM) and 5–10 μg of membrane protein (isolated from CHO cells that expressed cloned human NOP receptors) in a final volume of 0.5 ml of HEPES buffer (20 mM, pH 7.4) that contained 5‐mM MgCl2, 1‐mM EDTA, 100‐mM NaCl, and 0.1% BSA and incubated at 25°C for 60 min. Reactions were terminated by filtration on glass fibre filtermats (GF/C Filtermat A, Perkin‐Elmer Life and Analytical Sciences, Waltham, MA, USA) that were pretreated with 0.3% polyethyleneimine. The filters were washed three times with 5 ml of ice‐cold filtermats in Tris·HCl buffer (50 mM, pH 7.4), dried, and embedded with MeltiLex scintillant (Perkin‐Elmer Life and Analytical Sciences, Waltham, MA, USA). Bound radioactivity was counted in a Microbeta Trilux device (Perkin‐Elmer Life and Analytical Sciences, Waltham, MA, USA). Specific binding was determined by displacement with 100‐nM unlabelled nociceptin. Curves were plotted as the percentage of specific inhibition relative to LY2817412 concentration. IC50 values were determined using four‐parameter nonlinear regression (XLFit version 4.0, IDBS). Ki values were calculated from IC50 values according to Cheng and Prusoff, where Ki = IC50 × (1 + D × K D–1)−1 (Cheng & Prusoff, 1973). Reported values for Ki are shown as geometric means ± SEM from n = 3 independent assays. Geometric means were calculated by the following equation: GeoMean = 10 (average [log Ki1 + log Ki2 + … log KIn]/square root of the number of replicates, n).
2.7. In vitro opioid receptor binding
To determine receptor selectivity to classic opioid receptors, the binding affinity (Ki) of LY2817412 was determined in CHO (CHO, RRID:CVCL_0213) cell membranes that expressed cloned human μ, δ, or κ receptors as previously described (Emmerson et al., 2004). Briefly, membranes (4–6 μg protein per reaction) were added to buffer that contained 50‐mM Tris–HCl, 100‐mM NaCl, 1‐μM guanosine diphosphate, 5‐mM MgCl2, and 1‐mM EDTA (pH 7.4). The reactions were initiated by the addition of 0.2‐nM [3H]‐https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1617 to yield a 500‐μl final assay volume. The reaction was incubated at 25°C for 120 min. Specific binding was determined by displacement with 10‐μM https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1639. Reactions were terminated by rapid filtration through glass fibre filters. Radioactivity was measured, and the data were analysed using procedures that were identical to those that were used for NOP receptor binding.
2.8. In vitro functional activity on G‐protein activation
The NOP receptor antagonist affinity (Kb) of LY2817412 was measured in CHO membranes that expressed cloned human NOP receptors with a GTPγ‐[35S] binding assay according to previously described protocols with minor modifications (DeLapp, 2004). The assays were performed in a 200‐μl volume with 20‐mM HEPES buffer that contained 100‐mM NaCl, 5‐mM MgCl2, 1‐mM EDTA, 0.1% BSA, 3‐μM guanosine diphosphate, and 0.5‐nM GTPγ[35S]. NOP receptor membrane suspension was added at a concentration of 20 μg protein per well, and receptor stimulation was achieved with 300‐nM nociceptin. Wheat germ agglutinin‐coated scintillation proximity assay (SPA) beads (Perkin‐Elmer Life and Analytical Sciences, Waltham, MA, USA) were added at 1 mg per well to detect membrane‐bound GTPγ[35S]. The plates were sealed and incubated for 2 hr at 25°C and then placed at 4°C overnight to allow the SPA beads to settle. The plates were then counted for radioactivity in a Microbeta Trilux instrument. Specific GTPγ[35S] binding was determined as the difference in counts per minute that were observed in the absence and presence of 10‐μM unlabelled GTPγS. The data were plotted as the percentage of specific bound GTPγ[35S], from which IC50 values were calculated using four‐parameter nonlinear regression routines (XLFit version 4.0, IDBS). Antagonist affinity (Kb) was estimated according to DeLapp et al. (2004) using a modification of the equation of Cheng and Prusoff (1973), where Kb = IC50 × (1 + D × EC50–1)−1. Reported values for Kb are shown as the geometric mean ± SEM from three independent experiments.
2.9. Blinding and randomization
The laboratory animals were randomly assigned to the experimental groups, and the treatments were assessed blindly. The order of treatment administration was also randomized. All of the animal samples were studied, and the analyses were performed in a blinded manner.
2.10. Data and statistical analysis
The data and statistical analysis in this study comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018). The statistical analysis was performed only for groups with a group size of n ≥ 5, and the experimental groups were designed accordingly. The declared group size represents the number of independent values, and the statistical analysis was performed using these independent values. The optimum sample sizes and animal numbers were determined by power analysis of pre‐existing data. The effects of systemic LY2817412 administration on alcohol, water, and food intake were analysed in male and female msP rats using three‐way ANOVA, with treatment and time as the within‐subject factor and sex as the between‐subject factor. The effects of intracranial LY2817412 administration in the CeA, VTA, and NAc on alcohol, water, and food intake were analysed using two‐way repeated‐measures ANOVA, with treatment as the within‐subject factors and sex as the between‐subject factor. Before performing the ANOVAs, the data were analysed to confirm a normal distribution using the Shapiro–Wilk test and to confirm the homogeneity of variance using the Levene test. All data were normally distributed. Significant main effects and interactions in the ANOVA were followed by the Newman–Keuls post hoc test only when the F value attained P < .05, and there was no significant inhomogeneity of variances. The data are presented as the mean ± SEM. For determining whether groups differ, the level of probability (P) was set at P < .05 for constituting the threshold for statistical significance. The statistical analyses were performed using Prism 7 software (GraphPad Prism, RRID:SCR_002798, La Jolla, CA, USA).
2.11. Materials
The alcohol (10%, v/v) drinking solution was prepared by diluting 95% alcohol (FL Carsetti SNC, Camerino, Italy) in tap water. The NOP receptor antagonist LY2817412 was synthetized and provided by Eli Lilly (Indianapolis, IN, USA) and dissolved in a vehicle that consisted of a 1:1 mixture of distilled water and 1‐M H3PO4, (Sigma‐Aldrich, Milan, Italy). Solutions were freshly prepared before the tests. For peripheral administration, LY2817412 (3, 10, and 30 mg·kg−1) was administered in a volume of 1 ml·kg−1 and given by gavage (p.o.) 1 hr before the tests. For intracerebral microinjections, LY2817412 (3 and 6 μg·μl−1 per rat) was suspended in 10% DMSO (Sigma‐Aldrich, Milan, Italy), 3% Tween‐80, and 87% deionized water. LY2817412 was administered bilaterally in a volume of 0.5 μl per side. Diprenorphine and naltrexone were supplied by Tocris (USA) and [3H]‐nociceptin by PerkinElmer (USA).
2.12. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander et al., 2019).
3. RESULTS
3.1. Systemic LY2817412 administration reduces voluntary alcohol drinking in male and female msP rats
As shown in Figure 1a, LY2817412 significantly reduced alcohol drinking in male and female msP rats. At 2 hr, no differences in alcohol consumption were found in male and female animals, with no effect of LY2817412 at doses of 3, 10, and 30 mg·kg−1. At 8 hr, female msP rats consumed more alcohol than male msP rats. LY2817412 at a dose of 30 mg·kg−1 significantly reduced alcohol drinking in female msP rats but not in male msP rats. At 24 hr, female msP rats drank more alcohol than male msP rats, and 30 mg·kg−1 LY2817412 reduced alcohol consumption in both male and female msP rats. As shown in Figure 1b, a significant increase in water consumption was observed in female msP rats after 30 mg·kg−1 LY2817412 administration. As shown in Figure 1c, food intake was unaffected by LY2817412. These data suggest that LY2817412 reduced alcohol drinking in both male and female msP rats.
Figure 1.
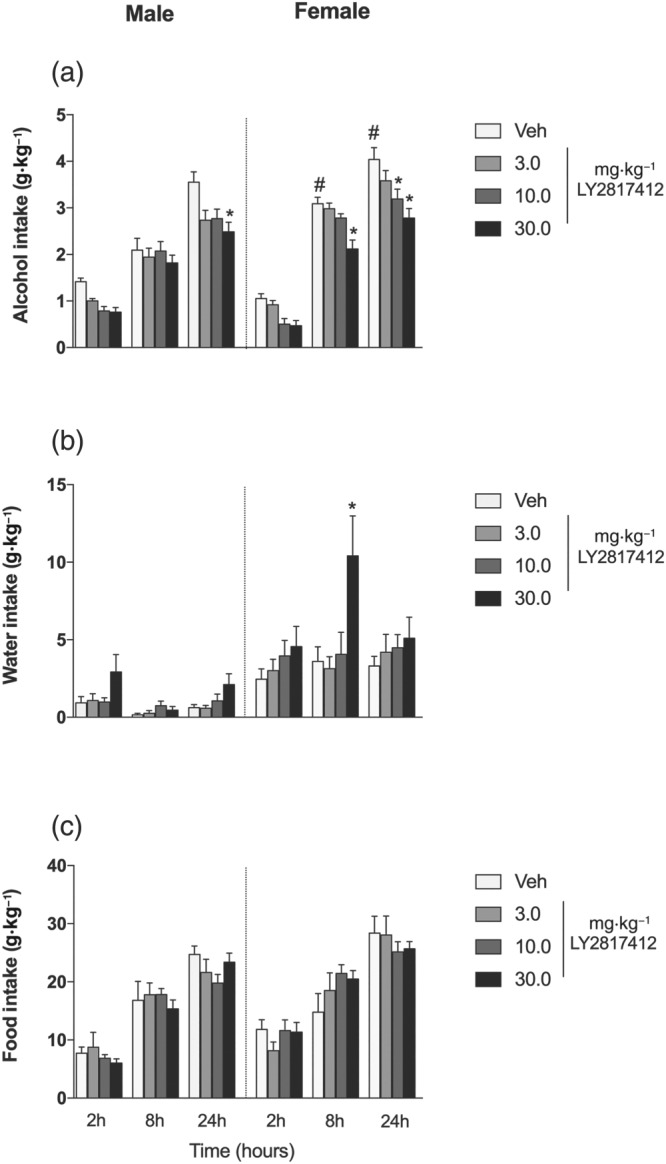
Effect of systemic LY2817412 administration on voluntary alcohol drinking in male and female msP rats. Male (n = 11) and female (n = 10) msP rats were subjected to a two‐bottle free‐choice (2BC) test. Following treatment with LY2817412 (3, 10, and 30 mg·kg−1) or vehicle, the voluntary intake of (a) alcohol, (b) water, and (c) food was recorded at 2, 8, and 24 hr in male and female msP rats. The data are expressed as mean ± SEM. * P < .05, significantly different from vehicle; # P < .05, significant difference between males and females; three‐way ANOVA followed by Newman–Keuls post hoc test)
3.2. LY2817412 administration does not modify standard food or water intake in male and female msP rats
To exclude the possibility that the effect of LY2817412 on alcohol drinking was attributable to the non‐specific inhibition of caloric intake or general ingesta, food and water intake was evaluated in the absence of alcohol availability. Male and female msP rats were singly housed, and water and standard chow pellets were available ad libitum. As shown in Figure S4A, no difference in water intake was found among sexes, with no effect of LY2817412 at a dose of 30 mg·kg−1. Similarly, no sex differences in food intake were observed. Moreover, food and water intake was unaffected by LY2817412 (Figure S4B). Altogether, these data confirm the specific inhibitory effect of LY2817412 on alcohol drinking.
3.3. Microinjection of LY2817412 in the CeA and VTA but not NAc reduces voluntary alcohol drinking in male and female msP rats
No main sex differences in alcohol (Figure 2a), water (Figure 2b), or food (Figure 2c) intake were detected in msP rats that received microinjections of LY2817412 in the CeA. LY2817412 significantly reduced alcohol drinking at doses of 3 and 6 μg·μl−1 per rat, in both male and female msP rats. Water and food intake was unaffected by LY2817412 (Figure 2b,c, respectively). No main sex differences in alcohol (Figure 3a), water (Figure 3b), or food (Figure 3c) intake were detected in msP rats that received microinjection of LY2817412 in the VTA. LY2817412 significantly reduced alcohol drinking at doses of 3 and 6 μg·μl−1 per rat in both male and female msP rats. Water and food intake was unaffected by LY2817412 (Figure 3b,c, respectively). Microinjection of LY2817412 at doses of 3 and 6 μg·μl−1 per rat in the NAc did not affect alcohol (Figure 4a), water (Figure 4b), or food (Figure 4c) intake in either male or female msP rats. Overall, these data suggest that neuronal circuitries in the CeA and VTA but not NAc mediated the effects of LY2817412 on alcohol drinking in male and female msP rats.
Figure 2.
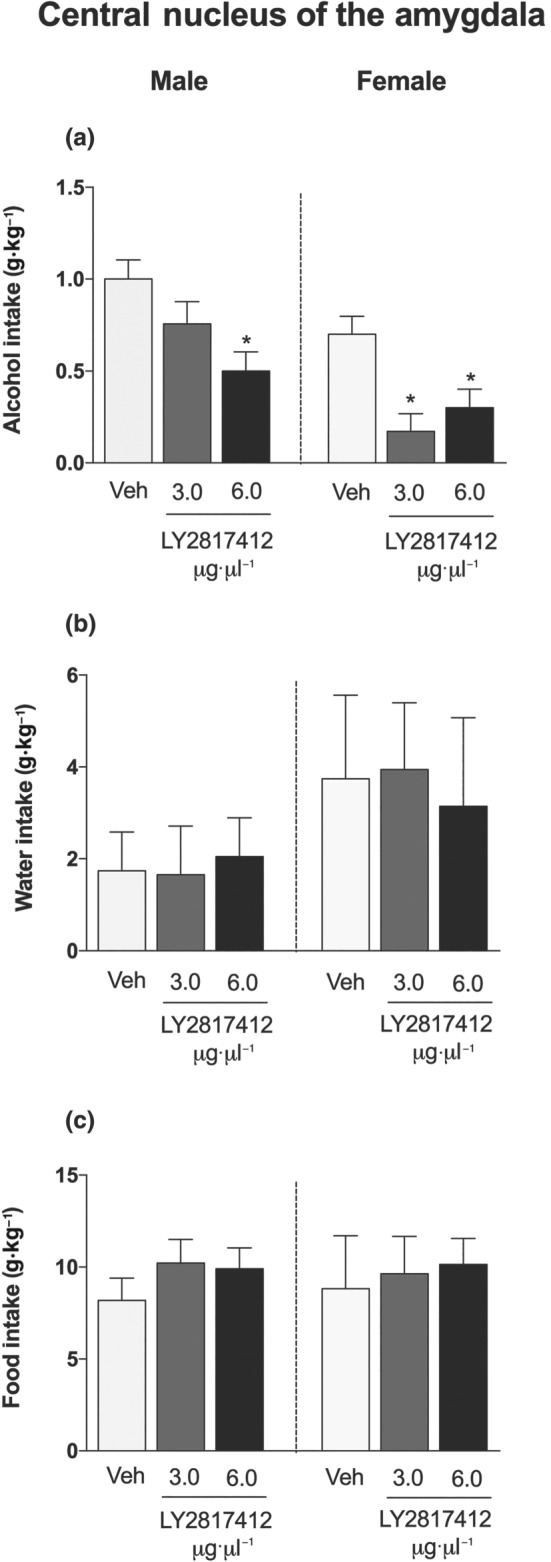
Effect of intra‐CeA microinjection of LY2817412 on voluntary alcohol drinking in male and female msP rats. Male (n = 8) and female (n = 7) msP rats were implanted with bilateral cannulas in the CeA and then subjected to a two‐bottle free‐choice test. Following treatment with LY2817412 (3 and 6 μg·μl−1 per rat) or vehicle, the voluntary intake of (a) alcohol, (b) water, and (c) food was recorded at 2 hr in male and female msP rats. The data are expressed as mean ± SEM. * P < .05, significantly different from vehicle; two‐way ANOVA followed by Newman–Keuls post hoc test
Figure 3.
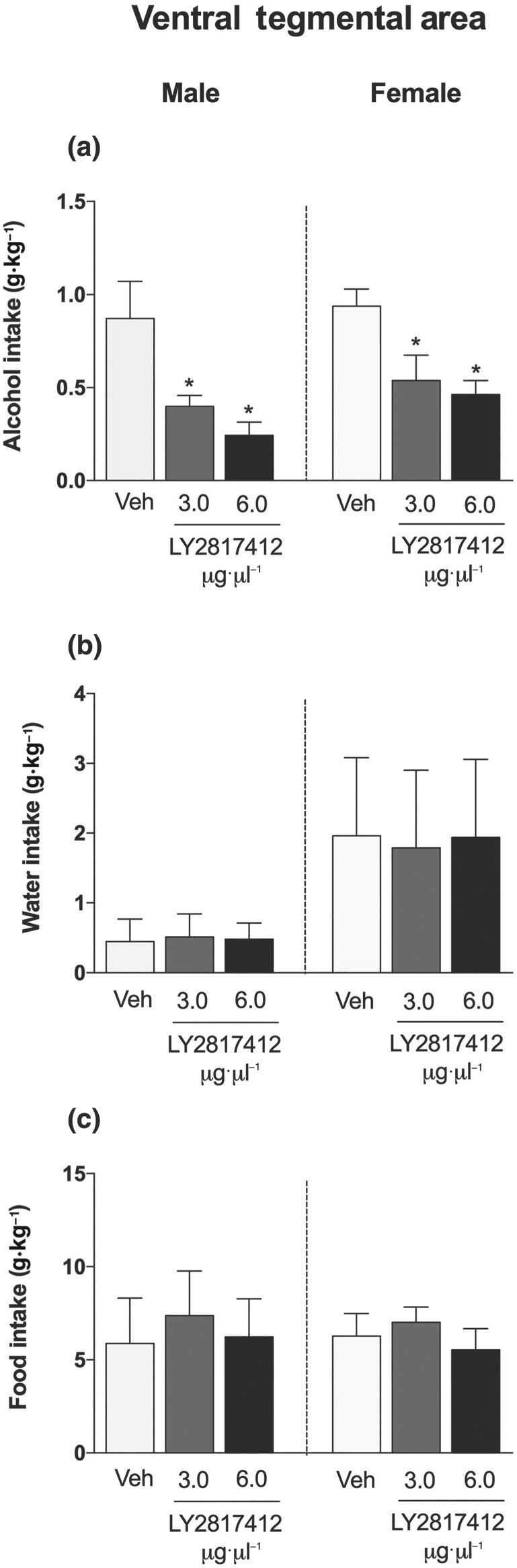
Effect of intra‐VTA microinjections of LY2817412 on voluntary alcohol drinking in male and female msP rats. Male (n = 9) and female (n = 8) msP rats were implanted with bilateral cannulas in the VTA and then subjected to a two‐bottle free‐choice test. Following treatment with LY2817412 (3 and 6 μg·μl−1 per rat) or vehicle, the voluntary intake of (a) alcohol, (b) water, and (c) food was recorded 2 hr after drinking onset in both male and female msP rats. The data are expressed as mean ± SEM. * P < .05, significantly different from vehicle; two‐way ANOVA followed by Newman–Keuls post hoc test
Figure 4.
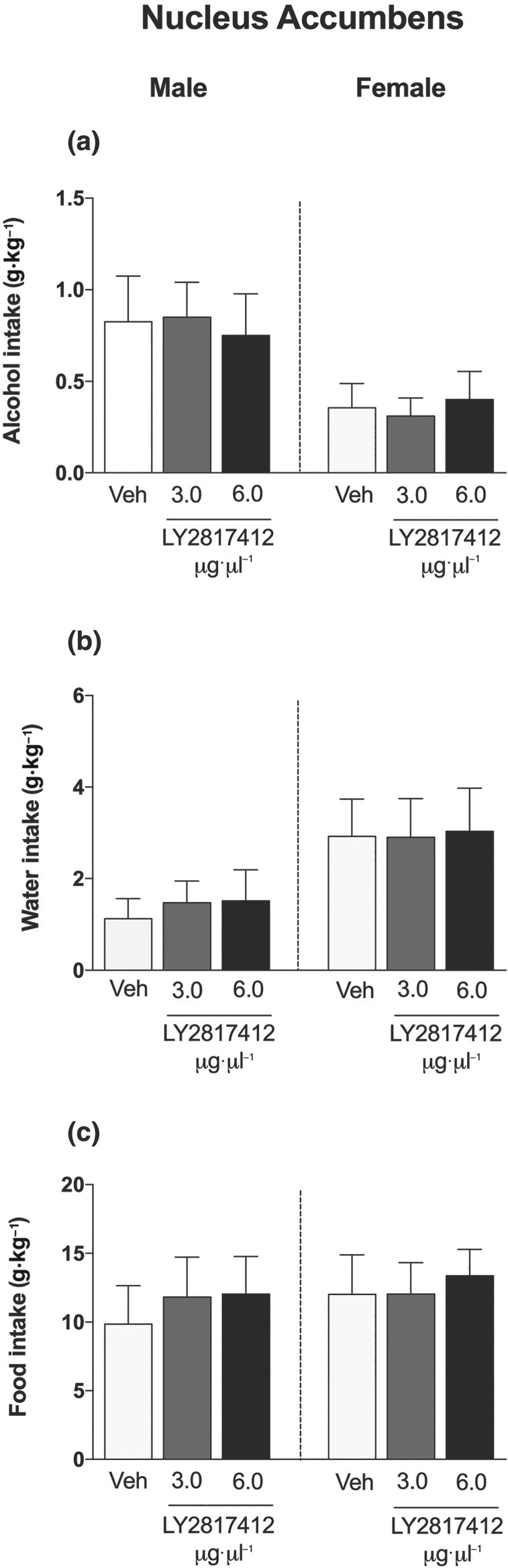
Effect of intra‐NAc microinjections of LY2817412 on voluntary alcohol drinking in male and female msP rats. Male (n = 8) and female (n = 9) msP rats were implanted with bilateral cannulas in the NAc and then subjected to a two‐bottle free‐choice test. Following treatment with LY2817412 (3 and 6 μg·μl−1 per rat) or vehicle, the voluntary intake of (a) alcohol, (b) water, and (c) food was recorded at 2 hr in both male and female msP rats. The data are expressed as mean ± SEM. No significant effects of LY2817412 were observed; two‐way ANOVA followed by Newman–Keuls post hoc test
3.4. LY2817412 is a potent and selective NOP receptor antagonist
LY2817412 had a high degree of affinity for the recombinant human NOP receptor (Ki = 0.18 nM). The antagonist potency of LY2817412 at the NOP receptor was similarly high (Kb = 0.27 nM). No residual agonist activity of LY2817412 was detected at concentrations up to 10 μM. Moreover, the selectivity of LY2817412 over the other classic opioid receptors (μ, δ, and κ receptors) was greater than 2,500‐fold (Table 1).
Table 1.
In vitro receptor binding profile of LY2817412 in CHO membranes that expressed cloned human opioid receptors
| Receptor subtype | Binding affinity (hKi ± SEM) | Antagonist potency (hKb ± SEM) |
|---|---|---|
| NOP receptor | 0.18 ± 0.05 nM | 0.27 ± 0.12 nM |
| κ receptor | >450 nM | na |
| μ receptor | >450 nM | na |
| δ receptor | >450 nM | na |
Note. The Ki values (n = 3 independent assays) and Kb values (n = 3 independent assays) are shown as geometric means ± SEM.
Abbreviation: na, not applicable.
4. DISCUSSION
The present results showed that systemic administration of the potent and selective NOP receptor antagonist LY2817412 significantly reduced alcohol drinking in male and female msP rats. The binding data indicated a high degree of selectivity and affinity of LY2817412 for the NOP receptor, thus excluding the possible involvement of binding other opioid receptors in the effects of LY2817412. The activity of LY2817412 was also specific to alcohol drinking, in which food and water intake was unaffected. In previous studies, we demonstrated that alcohol drinking in msP rats occurs in bouts of ~0.7–1.5 g·kg−1 (Ciccocioppo et al., 2006). At these doses, blood alcohol levels (BALs) can be as high as 45–55 mg·dl−1 (0.7 g·kg−1) or 110–135 mg·dl−1 (1.5 g·kg−1) and leads to the expression of marked conditioned place preference (Ciccocioppo et al., 2006; Ciccocioppo, Panocka, Froldi, et al., 1999; Ciccocioppo, Panocka, Polidori, et al., 1999). In the present experiment in which LY2817412 was administered systemically, the rats' alcohol intake was comparable to these earlier studies, demonstrating the pharmacological effects of alcohol. However, in the present intracranial treatment experiment, possibly because of the discomfort that is caused by the microinjection procedures, alcohol intake was lower than in the systemic treatment experiment. One may question, therefore, whether alcohol intake in these experiments was sufficient to achieve pharmacologically relevant actions of alcohol, thus raising another question of whether the effect of LY2817412 was directed towards the actions of alcohol. Despite this limitation, it is, however, worth considering that in the present study, the NOP receptor antagonist LY2817412 exerted inhibitory actions against alcohol intake when the rats consumed higher amounts of alcohol in the peripheral/systemic treatment experiment. The brain microinjection/intracranial experiment demonstrated that NOP receptor antagonism in both sexes attenuated alcohol drinking through neuroanatomical substrates that involve the CeA and VTA but not NAc. Consistent with the data from the systemic experiment, site‐specific microinjections of LY2817412 did not affect food or water intake, further confirming the pharmacological specificity of the NOP receptor antagonist. The experiments were performed using a within‐subject counterbalanced Latin square design, which may theoretically open the possibility of lingering effects of NOP receptor antagonism on alcohol drinking. However, this is unlikely because in vivo pharmacokinetic data showed that the half‐life of LY2817412 was short (t 1/2 = 12.1 ± 1.8 hr) relative to the ≥3‐day intervals between tests (Toledo et al., 2014).
These findings further support the potential usefulness of NOP receptor antagonists for attenuating alcohol intake. Recent studies showed that the administration of LY2940094, a NOP receptor antagonist with receptor affinity and selectivity that are similar to LY2817412, reduced alcohol intake in msP and Indiana P rats (Rorick‐Kehn et al., 2016). Indirect evidence of the putative therapeutic potential of NOP receptor antagonism comes from studies in genetically modified NOP receptor knockout rats. Compared with wild type controls, these engineered animals self‐administered significantly less alcohol, cocaine, and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=9082, whereas the motivation for a natural reward (i.e., https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5432) remained unchanged (Kallupi et al., 2017). These findings provide strong preclinical support for the possibility that genetically determined, and pharmacologically evoked reductions of NOP receptor activity are protective against the intake of alcohol and possibly other drugs of abuse. In a recent clinical trial with 88 patients who were diagnosed with AUD, LY2940094 treatment did not reduce the number of drinks per day (i.e., the primary clinical endpoint of the study) but significantly reduced the percentage of heavy drinking days and increased the percentage of abstinent days in 1 month compared with placebo (Post et al., 2016). These data, although inconclusive, may indicate some therapeutic potential of NOP receptor antagonism on AUD. Consistent with clinical data that showed sex‐independent effects of the NOP receptor antagonist, we found that male and female msP rats in the present study responded to both systemic and intra‐CeA and intra‐VTA administration of LY2817412.
Over the last two decades, our laboratory and other research groups have been systematically exploring the role of the N/OFQ‐NOP receptor system in alcohol and drug abuse. Historical data that were generated by us and others suggested that NOP receptor agonists are effective for treating alcohol abuse. For instance, we showed that alcohol drinking and alcohol‐induced conditioned place preference in msP rats are both reduced by the intracranial administration of N/OFQ or other peptidergic NOP receptor agonists (Ciccocioppo et al., 2004; Ciccocioppo et al., 2003; Ciccocioppo, Panocka, Froldi, et al., 1999; Ciccocioppo, Panocka, Polidori, et al., 1999; Ciccocioppo, Stopponi, et al., 2014; Economidou et al., 2006). These findings were later confirmed by studies that systemically administered low MW synthetic NOP agonists. For example, the peripheral administration of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1684 attenuated alcohol self‐administration in Wistar rats and prevented the acquisition and expression of alcohol‐induced conditioned place preference in mice (Kuzmin et al., 2003; Kuzmin et al., 2007). MT‐7716, another potent NOP receptor agonist, decreased alcohol intake in msP rats and attenuated alcohol withdrawal symptoms in alcohol‐dependent Wistar rats (Ciccocioppo, Stopponi, et al., 2014). MT‐7716 also attenuated alcohol drinking in post‐dependent Wistar rats and was less effective in nondependent controls (de Guglielmo et al., 2015). AT‐312, a novel NOP receptor agonist, was recently shown to reduce the rewarding effects of alcohol in a place conditioning paradigm (Zaveri et al., 2018). Finally, SR‐8993, another selective NOP receptor agonist, reduced anxiety‐like behaviour that was associated with alcohol withdrawal and attenuated home‐cage alcohol drinking in Wistar rats (Aziz et al., 2016). The expression of alcohol withdrawal symptoms and alcohol‐induced anxiety‐like behaviour was attenuated by central N/OFQ administration (Aujla et al., 2013; Economidou et al., 2011). A recent study reported that the activation of NOP receptors by the selective NOP receptor agonist Ro 64‐6198 in male and female adolescent Wistar rats attenuated alcohol‐induced anxiolysis and alcohol‐induced behavioural stimulation but not alcohol drinking (Miranda‐Morales & Pautassi, 2016). These findings are consistent with an earlier study that showed that N/OFQ, despite being effective in msP rats, did not reduce alcohol intake in Wistar rats (Economidou et al., 2008). The reason why NOP receptor activation is ineffective in Wistar rats but attenuates alcohol drinking in msP rats and post‐dependent rats is unclear. One possibility is that the NOP receptor system plays different roles when drinking is triggered by recreational mechanisms in non‐dependent Wistar rats or by negative reinforcement in msP rats and post‐dependent Wistar rats.
The reason why both NOP receptor agonism and antagonism are able to reduce the motivation for alcohol is still unclear, but several hypotheses may be proposed to reconcile these unexpected observations. One possibility is that the effects of NOP receptor agonists and antagonists are mediated by different neurocircuitries. However, if the present findings are placed within the context of historical data, then this possibility appears unlikely. Earlier brain microinjection studies in msP rats showed that, similar to the present findings with LY2817412, alcohol drinking was reduced by the site‐specific administration of N/OFQ in the CeA (Economidou et al., 2008). Moreover, electrophysiological studies revealed that the activation of NOP receptors in the CeA reduced the alcohol‐induced facilitation of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1067‐ and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1369‐mediated transmission and prevented the expression of anxiety‐like behaviour (Roberto & Siggins, 2006). To our knowledge, the effects of NOP receptor antagonists on CeA GABA and glutamate neurotransmission have not yet been investigated. Therefore, the direction in which CeA GABA and glutamate transmission are affected by pharmacological NOP receptor antagonism remains unknown. However, the CeA is a common brain site of action that mediates the effects of both NOP receptor agonists and antagonists, thus weakening the hypothesis that the effects of these pharmacological agents are mediated by distinct neurocircuitries.
In the present study, intra‐VTA administration of LY2817412 reduced alcohol drinking. As far as we know, the effect of intra‐VTA administration of a NOP receptor agonist on alcohol drinking has not been previously investigated. However, intra‐VTA administration of N/OFQ was reported to attenuate https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=940 release in the NAc (Murphy & Maidment, 1999), whereas intracerebroventricular N/OFQ administration suppressed the morphine‐induced increase in extracellular dopamine levels in the NAc (Di Giannuario & Pieretti, 2000). We recently found that the selective blockade of NOP by LY2940094 completely blocked the ability of alcohol to evoke dopamine release in the NAc (Rorick‐Kehn et al., 2016). The effects of NOP receptor antagonists on basal mesolimbic dopamine release are mixed, in which they were shown to increase extracellular dopamine levels in some studies but decrease them in others (Koizumi, Midorikawa, Takeshima, & Murphy, 2004; Koizumi, Sakoori, Midorikawa, & Murphy, 2004; Marti et al., 2004). Overall, these findings suggest that NOP receptor agonists and antagonists, at least under some circumstances, are both able to modulate the mesolimbic dopamine system and reduce NAc dopamine release that is elicited by drugs of abuse.
An alternative hypothesis is that the exogenous administration of non‐physiological doses of NOP receptor agonists depresses N/OFQ transmission through receptor desensitization. If so, then NOP receptor agonism may result in paradoxical functional antagonism. Although highly speculative, this hypothesis is supported by data showing that NOP receptors undergo rapid desensitization that can occur within minutes after high‐dose agonist administration or after chronic agonist treatment (Toll, Bruchas, Calo', Cox, & Zaveri, 2016). Desensitization of NOP receptors depends crucially on the ligand that is used, which may explain why different agonists may sometimes produce different effects (Donica, Awwad, Thakker, & Standifer, 2013). Notably, nociceptin fragments, such as NC(1–13)NH(2) that is endowed with partial agonist activity, produce less receptor desensitization and thus are less effective against alcohol drinking (Ciccocioppo et al., 2002; Corbani, Gonindard, & Meunier, 2004; Okawa et al., 1999; Toll et al., 2016). Importantly, a previous study investigated the effect of chronic administration of the potent and selective NOP receptor agonist MT‐7716 and found that pharmacological effects progressively emerged during repeated administration with alcohol drinking, and alcohol intake remained low for several days after MT‐7716 treatment discontinuation (Ciccocioppo, Stopponi, et al., 2014). The NOP receptor desensitization hypothesis is also indirectly supported by data that demonstrated that msP rats have a higher propensity than Wistar rats to excessively consume alcohol, and they also exhibit higher N/OFQ and NOP receptor mRNA expression in numerous mesolimbic brain areas, including the CeA and VTA (Ciccocioppo, de Guglielmo, et al., 2014; Economidou et al., 2008). Furthermore, Wistar rats that were exposed to chronic intoxicating concentrations of alcohol exhibited a greater propensity to excessively consume alcohol and exhibited the up‐regulation of NOP receptor transcripts in the CeA and BNST (Aujla et al., 2013; Sommer et al., 2008). Thus, the increase in alcohol intake in genetically selected rats and in animals with a post‐dependent phenotype may be attributable to an increase in N/OFQ transmission in specific brain areas (e.g., CeA and VTA). Notably, animal models that are characterized by heightened alcohol drinking and the overexpression of NOP receptor have been shown to be more sensitive to NOP receptor agonist treatment (Cruz, Herman, Kallupi, & Roberto, 2012; de Guglielmo et al., 2015). This observation may be attributable to more pronounced receptor down‐regulation (or more pronounced consequences associated with it) when NOP receptors, which are to a large extent not functional and are rather stored at the intracellular level, become activated (Ozawa et al., 2015).
In summary, the present results indicate that pharmacological modulation of the N/OFQ‐NOP receptor system attenuates alcohol drinking through mechanisms that involve CeA and VTA neurotransmission. The inhibitory effect of LY2817412 on alcohol intake was observed in both male and female msP rats and specific to alcohol drinking, in which food and water intake were unaffected by LY2817412 treatment. The reasons why agonists and antagonists are both effective and produce similar effects on alcohol‐motivated behaviours require further investigation. However, based on the present data, NOP receptor desensitization may occur after NOP receptor agonist administration. NOP receptor blockade may thus represent one alternative and possibly a safer approach to the treatment of AUD, compared with NOP receptor agonism.
CONFLICT OF INTEREST
The authors have no conflict of interest with the subject matter or materials discussed in the manuscript.
AUTHOR CONTRIBUTIONS
A.M.B. designed the project, performed surgeries, behavioural tests, data analysis, and wrote the manuscript. Y.F. performed surgeries and behavioural tests. S.S., G.B., M.P., and F.F.C., performed behavioural tests. R.C., P.R., S.C., R.N., M.U., L.M.R.K., and F.W. conceived and designed the project, supervised the experiments, and contributed to writing the manuscript. All authors reviewed the manuscript.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for https://bpspubs.onlinelibrary.wiley.com/doi/abs/10.1111/bph.14207, and https://bpspubs.onlinelibrary.wiley.com/doi/abs/10.1111/bph.14206 and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Figure S1. Schematic representation of intra‐CeA sites of injection assessed by histological analysis.
Figure S2. Schematic representation of intra‐VTA sites of injection assessed by histological analysis.
Figure S3. Schematic representation of intra‐NAc sites of injection assessed by histological analysis.
Figure S4. Effect of systemic LY2817412 administration on standard food pellet and water consumption in male and female msP rats.
ACKNOWLEDGEMENTS
We thank Rina Righi, Agostino Marchi, and Mariangela Fiorelli for animal care and Alfredo Fiorelli for technical support. The authors also thank Federica Benvenuti and Martina Mondaini for their support with the behavioural experiments and Michael Arends for proofreading the manuscript. This work was supported by the National Institutes of Health Grant R01 AA014351 from the National Institute on Alcohol Abuse and Alcoholism.
Borruto AM, Fotio Y, Stopponi S, et al. NOP receptor antagonism reduces alcohol drinking in male and female rats through mechanisms involving the central amygdala and ventral tegmental area. Br J Pharmacol. 2020;177:1525–1537. 10.1111/bph.14915
REFERENCES
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Alistair, P. , … CGTP Collaborators (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: G protein‐coupled receptors. British Journal of Pharmacology, 176, S21–S141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aujla, H. , Cannarsa, R. , Romualdi, P. , Ciccocioppo, R. , Martin‐Fardon, R. , & Weiss, F. (2013). Modification of anxiety‐like behaviors by nociceptin/orphanin FQ (N/OFQ) and time‐dependent changes in N/OFQ‐NOP gene expression following ethanol withdrawal. Addiction Biology, 18, 467–479. 10.1111/j.1369-1600.2012.00466.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz, A. M. , Brothers, S. , Sartor, G. , Holm, L. , Heilig, M. , Wahlestedt, C. , & Thorsell, A. (2016). The nociceptin/orphanin FQ receptor agonist SR‐8993 as a candidate therapeutic for alcohol use disorders: Validation in rat models. Psychopharmacology, 233, 3553–3563. 10.1007/s00213-016-4385-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker, H. C. , & Lopez, M. F. (2004). Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcoholism, Clinical and Experimental Research, 28, 1829–1838. [DOI] [PubMed] [Google Scholar]
- Bunzow, J. R. , Saez, C. , Mortrud, M. , Bouvier, C. , Williams, J. T. , Low, M. , & Grandy, D. K. (1994). Molecular cloning and tissue distribution of a putative member of the rat opioid receptor gene family that is not a μ, δ or κ opioid receptor type. FEBS Letters, 347, 284–288. 10.1016/0014-5793(94)00561-3 [DOI] [PubMed] [Google Scholar]
- Cheng, Y. , & Prusoff, W. H. (1973). Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochemical Pharmacology, 22, 3099–3108. 10.1016/0006-2952(73)90196-2 [DOI] [PubMed] [Google Scholar]
- Ciccocioppo, R. , Borruto, A. M. , Domi, A. , Teshima, K. , Cannella, N. , & Weiss, F. (2019). NOP‐related mechanisms in substance use disorders. Handbook of Experimental Pharmacology, 254, 187–212. 10.1007/164_2019_209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo, R. , de Guglielmo, G. , Hansson, A. C. , Ubaldi, M. , Kallupi, M. , Cruz, M. T. , … Roberto, M. (2014). Restraint stress alters nociceptin/orphanin FQ and CRF systems in the rat central amygdala: Significance for anxiety‐like behaviors. The Journal of Neuroscience, 34, 363–372. 10.1523/JNEUROSCI.2400-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo, R. , Economidou, D. , Cippitelli, A. , Cucculelli, M. , Ubaldi, M. , Soverchia, L. , … Massi, M. (2006). Genetically selected Marchigian Sardinian alcohol‐preferring (msP) rats: An animal model to study the neurobiology of alcoholism. Addiction Biology, 11, 339–355. 10.1111/j.1369-1600.2006.00032.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo, R. , Economidou, D. , Fedeli, A. , Angeletti, S. , Weiss, F. , Heilig, M. , & Massi, M. (2004). Attenuation of ethanol self‐administration and of conditioned reinstatement of alcohol‐seeking behaviour by the antiopioid peptide nociceptin/orphanin FQ in alcohol‐preferring rats. Psychopharmacology, 172, 170–178. 10.1007/s00213-003-1645-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo, R. , Economidou, D. , Fedeli, A. , & Massi, M. (2003). The nociceptin/orphanin FQ/NOP receptor system as a target for treatment of alcohol abuse: A review of recent work in alcohol‐preferring rats. Physiology & Behavior, 79, 121–128. 10.1016/s0031-9384(03)00112-4 [DOI] [PubMed] [Google Scholar]
- Ciccocioppo, R. , Panocka, I. , Froldi, R. , Colombo, G. , Gessa, G. L. , & Massi, M. (1999). Antidepressant‐like effect of ethanol revealed in the forced swimming test in Sardinian alcohol‐preferring rats. Psychopharmacology, 144, 151–157. 10.1007/s002130050988 [DOI] [PubMed] [Google Scholar]
- Ciccocioppo, R. , Panocka, I. , Polidori, C. , Regoli, D. , & Massi, M. (1999). Effect of nociceptin on alcohol intake in alcohol‐preferring rats. Psychopharmacology, 141, 220–224. 10.1007/s002130050828 [DOI] [PubMed] [Google Scholar]
- Ciccocioppo, R. , Polidori, C. , Antonelli, L. , Salvadori, S. , Guerrini, R. , & Massi, M. (2002). Pharmacological characterization of the nociceptin receptor which mediates reduction of alcohol drinking in rats. Peptides, 23, 117–125. 10.1016/s0196-9781(01)00587-3 [DOI] [PubMed] [Google Scholar]
- Ciccocioppo, R. , Stopponi, S. , Economidou, D. , Kuriyama, M. , Kinoshita, H. , Heilig, M. , … Teshima, K. (2014). Chronic treatment with novel brain‐penetrating selective NOP receptor agonist MT‐7716 reduces alcohol drinking and seeking in the rat. Neuropsychopharmacology, 39, 2601–2610. 10.1038/npp.2014.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli, A. , Schoch, J. , Debevec, G. , Brunori, G. , Zaveri, N. T. , & Toll, L. (2016). A key role for the N/OFQ‐NOP receptor system in modulating nicotine taking in a model of nicotine and alcohol co‐administration. Scientific Reports, 6:26594 10.1038/srep26594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbani, M. , Gonindard, C. , & Meunier, J. C. (2004). Ligand‐regulated internalization of the opioid receptor‐like 1: A confocal study. Endocrinology, 145, 2876–2885. 10.1210/en.2004-0062 [DOI] [PubMed] [Google Scholar]
- Cruz, M. T. , Herman, M. A. , Kallupi, M. , & Roberto, M. (2012). Nociceptin/orphanin FQ blockade of corticotropin‐releasing factor‐induced γ‐aminobutyric acid release in central amygdala is enhanced after chronic ethanol exposure. Biological Psychiatry, 71, 666–676. 10.1016/j.biopsych.2011.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting: II. Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175, 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darland, T. , Heinricher, M. M. , & Grandy, D. K. (1998). Orphanin FQ/nociceptin: A role in pain and analgesia, but so much more. Trends in Neurosciences, 21, 215–221. 10.1016/s0166-2236(97)01204-6 [DOI] [PubMed] [Google Scholar]
- DeLapp, N. W. (2004). The antibody‐capture [35S]GTPγS scintillation proximity assay: A powerful emerging technique for analysis of GPCR pharmacology. Trends in Pharmacological Sciences, 25, 400–401. 10.1016/j.tips.2004.06.003 [DOI] [PubMed] [Google Scholar]
- Di Giannuario, A. , & Pieretti, S. (2000). Nociceptin differentially affects morphine‐induced dopamine release from the nucleus accumbens and nucleus caudate in rats. Peptides, 21, 1125–1130. 10.1016/s0196-9781(00)00250-3 [DOI] [PubMed] [Google Scholar]
- Donica, C. L. , Awwad, H. O. , Thakker, D. R. , & Standifer, K. M. (2013). Cellular mechanisms of nociceptin/orphanin FQ (N/OFQ) peptide (NOP) receptor regulation and heterologous regulation by N/OFQ. Molecular Pharmacology, 83, 907–918. 10.1124/mol.112.084632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou, D. , Cippitelli, A. , Stopponi, S. , Braconi, S. , Clementi, S. , Ubaldi, M. , … Ciccocioppo, R. (2011). Activation of brain NOP receptors attenuates acute and protracted alcohol withdrawal symptoms in the rat. Alcoholism, Clinical and Experimental Research, 35, 747–755. 10.1111/j.1530-0277.2010.01392.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou, D. , Fedeli, A. , Fardon, R. M. , Weiss, F. , Massi, M. , & Ciccocioppo, R. (2006). Effect of novel nociceptin/orphanin FQ‐NOP receptor ligands on ethanol drinking in alcohol‐preferring msP rats. Peptides, 27, 3299–3306. 10.1016/j.peptides.2006.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou, D. , Hansson, A. C. , Weiss, F. , Terasmaa, A. , Sommer, W. H. , Cippitelli, A. , … Heilig, M. (2008). Dysregulation of nociceptin/orphanin FQ activity in the amygdala is linked to excessive alcohol drinking in the rat. Biological Psychiatry, 64, 211–218. 10.1016/j.biopsych.2008.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerson, P. J. , McKinzie, J. H. , Surface, P. L. , Suter, T. M. , Mitch, C. H. , & Statnick, M. A. (2004). Na+ modulation, inverse agonism, and anorectic potency of 4‐phenylpiperidine opioid antagonists. European Journal of Pharmacology, 494, 121–130. 10.1016/j.ejphar.2004.04.050 [DOI] [PubMed] [Google Scholar]
- Finn, D. A. , Snelling, C. , Fretwell, A. M. , Tanchuck, M. A. , Underwood, L. , Cole, M. , … Roberts, A. J. (2007). Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D‐Phe‐CRF(12‐41). Alcoholism, Clinical and Experimental Research, 31, 939–949. 10.1111/j.1530-0277.2007.00379.x [DOI] [PubMed] [Google Scholar]
- de Guglielmo, G. , Martin‐Fardon, R. , Teshima, K. , Ciccocioppo, R. , & Weiss, F. (2015). MT‐7716, a potent NOP receptor agonist, preferentially reduces ethanol seeking and reinforcement in post‐dependent rats. Addiction Biology, 20, 643–651. 10.1111/adb.12157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson, A. C. , Cippitelli, A. , Sommer, W. H. , Fedeli, A. , Bjork, K. , Soverchia, L. , … Ciccocioppo, R. (2006). Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proceedings of the National Academy of Sciences of the United States of America, 103, 15236–15241. 10.1073/pnas.0604419103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS guide to pharmacology in 2018: Updates and expansion to encompass the new guide to immunopharmacology. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallupi, M. , Scuppa, G. , de Guglielmo, G. , Calo, G. , Weiss, F. , Statnick, M. A. , … Ciccocioppo, R. (2017). Genetic deletion of the nociceptin/orphanin FQ receptor in the rat confers resilience to the development of drug addiction. Neuropsychopharmacology, 42, 695–706. 10.1038/npp.2016.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. The Journal of Gene Medicine, 12, 561–563. 10.1002/jgm.1473 [DOI] [PubMed] [Google Scholar]
- Koizumi, M. , Midorikawa, N. , Takeshima, H. , & Murphy, N. P. (2004). Exogenous, but not endogenous nociceptin modulates mesolimbic dopamine release in mice. Journal of Neurochemistry, 89, 257–263. 10.1111/j.1471-4159.2003.02322.x [DOI] [PubMed] [Google Scholar]
- Koizumi, M. , Sakoori, K. , Midorikawa, N. , & Murphy, N. P. (2004). The NOP (ORL1) receptor antagonist Compound B stimulates mesolimbic dopamine release and is rewarding in mice by a non‐NOP‐receptor‐mediated mechanism. British Journal of Pharmacology, 143, 53–62. 10.1038/sj.bjp.0705906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob, G. F. , Roberts, A. J. , Kieffer, B. L. , Heyser, C. J. , Katner, S. N. , Ciccocioppo, R. , & Weiss, F. (2003). Animal models of motivation for drinking in rodents with a focus on opioid receptor neuropharmacology. Recent Developments in Alcoholism, 16, 263–281. [DOI] [PubMed] [Google Scholar]
- Kotlinska, J. , Rafalski, P. , Biala, G. , Dylag, T. , Rolka, K. , & Silberring, J. (2003). Nociceptin inhibits acquisition of amphetamine‐induced place preference and sensitization to stereotypy in rats. European Journal of Pharmacology, 474, 233–239. 10.1016/s0014-2999(03)02081-8 [DOI] [PubMed] [Google Scholar]
- Kotlinska, J. , Wichmann, J. , Legowska, A. , Rolka, K. , & Silberring, J. (2002). Orphanin FQ/nociceptin but not Ro 65‐6570 inhibits the expression of cocaine‐induced conditioned place preference. Behavioural Pharmacology, 13, 229–235. [DOI] [PubMed] [Google Scholar]
- Kuzmin, A. , Kreek, M. J. , Bakalkin, G. , & Liljequist, S. (2007). The nociceptin/orphanin FQ receptor agonist Ro 64‐6198 reduces alcohol self‐administration and prevents relapse‐like alcohol drinking. Neuropsychopharmacology, 32, 902–910. 10.1038/sj.npp.1301169 [DOI] [PubMed] [Google Scholar]
- Kuzmin, A. , Sandin, J. , Terenius, L. , & Ogren, S. O. (2003). Acquisition, expression, and reinstatement of ethanol‐induced conditioned place preference in mice: Effects of opioid receptor‐like 1 receptor agonists and naloxone. The Journal of Pharmacology and Experimental Therapeutics, 304, 310–318. 10.1124/jpet.102.041350 [DOI] [PubMed] [Google Scholar]
- Marti, M. , Mela, F. , Veronesi, C. , Guerrini, R. , Salvadori, S. , Federici, M. , … Morari, M. (2004). Blockade of nociceptin/orphanin FQ receptor signaling in rat substantia nigra pars reticulata stimulates nigrostriatal dopaminergic transmission and motor behavior. The Journal of Neuroscience, 24, 6659–6666. 10.1523/JNEUROSCI.0987-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin‐Fardon, R. , Ciccocioppo, R. , Massi, M. , & Weiss, F. (2000). Nociceptin prevents stress‐induced ethanol‐ but not cocaine‐seeking behavior in rats. Neuroreport, 11, 1939–1943. 10.1097/00001756-200006260-00026 [DOI] [PubMed] [Google Scholar]
- Meunier, J. C. (1997). Nociceptin/orphanin FQ and the opioid receptor‐like ORL1 receptor. European Journal of Pharmacology, 340, 1–15. 10.1016/s0014-2999(97)01411-8 [DOI] [PubMed] [Google Scholar]
- Meunier, J. C. , Mollereau, C. , Toll, L. , Suaudeau, C. , Moisand, C. , Alvinerie, P. , … Monsarrat, B. (1995). Isolation and structure of the endogenous agonist of opioid receptor‐like ORL1 receptor. Nature, 377, 532–535. 10.1038/377532a0 [DOI] [PubMed] [Google Scholar]
- Miranda‐Morales, R. S. , & Pautassi, R. M. (2016). Pharmacological characterization of the nociceptin/orphanin FQ receptor on ethanol‐mediated motivational effects in infant and adolescent rats. Behavioural Brain Research, 298, 88–96. 10.1016/j.bbr.2015.04.016 [DOI] [PubMed] [Google Scholar]
- Mollereau, C. , Parmentier, M. , Mailleux, P. , Butour, J. L. , Moisand, C. , Chalon, P. , … Meunier, J. C. (1994). ORL1, a novel member of the opioid receptor family: Cloning, functional expression and localization. FEBS Letters, 341, 33–38. 10.1016/0014-5793(94)80235-1 [DOI] [PubMed] [Google Scholar]
- Murphy, N. P. , & Maidment, N. T. (1999). Orphanin FQ/nociceptin modulation of mesolimbic dopamine transmission determined by microdialysis. Journal of Neurochemistry, 73, 179–186. 10.1046/j.1471-4159.1999.0730179.x [DOI] [PubMed] [Google Scholar]
- Narendran, R. , Ciccocioppo, R. , Lopresti, B. , Paris, J. , Himes, M. L. , & Mason, N. S. (2017). Nociceptin receptors in alcohol use disorders: A positron emission tomography study using [11C]NOP‐1A. Biological Psychiatry, 84, 708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal, C. R. Jr. , Mansour, A. , Reinscheid, R. , Nothacker, H. P. , Civelli, O. , & Watson, S. J. Jr. (1999). Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. The Journal of Comparative Neurology, 406, 503–547. [DOI] [PubMed] [Google Scholar]
- Okawa, H. , Nicol, B. , Bigoni, R. , Hirst, R. A. , Calo', G. , Guerrini, R. , … Lambert, D. G. (1999). Comparison of the effects of [Phe1Ψ(CH2‐NH)Gly2]nociceptin(1‐13)NH2 in rat brain, rat vas deferens and CHO cells expressing recombinant human nociceptin receptors. British Journal of Pharmacology, 127, 123–130. 10.1038/sj.bjp.0702539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa, A. , Brunori, G. , Mercatelli, D. , Wu, J. , Cippitelli, A. , Zou, B. , … Toll, L. (2015). Knock‐in mice with NOP‐eGFP receptors identify receptor cellular and regional localization. The Journal of Neuroscience, 35, 11682–11693. 10.1523/JNEUROSCI.5122-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos, G. , & Watson, C. (2007). The rat brain in stereotaxic coordinates (6th edition). ISBN: 9780125476126. [Google Scholar]
- Post, A. , Smart, T. S. , Jackson, K. , Mann, J. , Mohs, R. , Rorick‐Kehn, L. , … Wong, C. J. (2016). Proof‐of‐concept study to assess the nociceptin receptor antagonist LY2940094 as a new treatment for alcohol dependence. Alcoholism, Clinical and Experimental Research, 40, 1935–1944. 10.1111/acer.13147 [DOI] [PubMed] [Google Scholar]
- Reinscheid, R. K. , Nothacker, H. P. , Bourson, A. , Ardati, A. , Henningsen, R. A. , Bunzow, J. R. , … Civelli, O. (1995). Orphanin FQ: A neuropeptide that activates an opioidlike G protein‐coupled receptor. Science, 270, 792–794. 10.1126/science.270.5237.792 [DOI] [PubMed] [Google Scholar]
- Rimondini, R. , Sommer, W. , & Heilig, M. (2003). A temporal threshold for induction of persistent alcohol preference: Behavioral evidence in a rat model of intermittent intoxication. Journal of Studies on Alcohol, 64, 445–449. 10.15288/jsa.2003.64.445 [DOI] [PubMed] [Google Scholar]
- Roberto, M. , & Siggins, G. R. (2006). Nociceptin/orphanin FQ presynaptically decreases GABAergic transmission and blocks the ethanol‐induced increase of GABA release in central amygdala. Proceedings of the National Academy of Sciences of the United States of America, 103, 9715–9720. 10.1073/pnas.0601899103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorick‐Kehn, L. M. , Ciccocioppo, R. , Wong, C. J. , Witkin, J. M. , Martinez‐Grau, M. A. , Stopponi, S. , … Statnick, M. A. (2016). A novel, orally bioavailable nociceptin receptor antagonist, LY2940094, reduces ethanol self‐administration and ethanol seeking in animal models. Alcoholism, Clinical and Experimental Research, 40, 945–954. 10.1111/acer.13052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer, W. H. , Rimondini, R. , Hansson, A. C. , Hipskind, P. A. , Gehlert, D. R. , Barr, C. S. , & Heilig, M. A. (2008). Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala Crhr1 expression following a history of dependence. Biological Psychiatry, 63, 139–145. 10.1016/j.biopsych.2007.01.010 [DOI] [PubMed] [Google Scholar]
- Statnick, M. A. , Chen, Y. , Ansonoff, M. , Witkin, J. M. , Rorick‐Kehn, L. , Suter, T. M. , … Pintar, J. E. (2016). A novel nociceptin receptor antagonist LY2940094 inhibits excessive feeding behavior in rodents: A possible mechanism for the treatment of binge eating disorder. The Journal of Pharmacology and Experimental Therapeutics, 356, 493–502. 10.1124/jpet.115.228221 [DOI] [PubMed] [Google Scholar]
- Tabakoff, B. , & Hoffman, P. L. (2000). Animal models in alcohol research. Alcohol Research & Health, 24, 77–84. [PMC free article] [PubMed] [Google Scholar]
- Toledo, M. A. , Pedregal, C. , Lafuente, C. , Diaz, N. , Martinez‐Grau, M. A. , Jimenez, A. , … Kahl, S. D. (2014). Discovery of a novel series of orally active nociceptin/orphanin FQ (NOP) receptor antagonists based on a dihydrospiro (piperidine‐4,7′‐thieno[2,3‐c]pyran) scaffold. Journal of Medicinal Chemistry, 57, 3418–3429. 10.1021/jm500117r [DOI] [PubMed] [Google Scholar]
- Toll, L. , Bruchas, M. R. , Calo', G. , Cox, B. M. , & Zaveri, N. T. (2016). Nociceptin/orphanin FQ receptor structure, signaling, ligands, functions, and interactions with opioid systems. Pharmacological Reviews, 68, 419–457. 10.1124/pr.114.009209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin, J. M. , Statnick, M. A. , Rorick‐Kehn, L. M. , Pintar, J. E. , Ansonoff, M. , Chen, Y. , … Ciccocioppo, R. (2014). The biology of nociceptin/orphanin FQ (N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence. Pharmacology & Therapeutics, 141, 283–299. 10.1016/j.pharmthera.2013.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaveri, N. T. , Marquez, P. V. , Meyer, M. E. , Polgar, W. E. , Hamid, A. , & Lutfy, K. (2018). A novel and selective nociceptin receptor (NOP) agonist (1‐(1‐((cis)‐4‐isopropylcyclohexyl)piperidin‐4‐yl)‐1H‐indol‐2‐yl)methanol (AT‐312) decreases acquisition of ethanol‐induced conditioned place preference in mice. Alcoholism, Clinical and Experimental Research, 42, 461–471. 10.1111/acer.13575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, R. J. , Woo, R. S. , Jeong, M. S. , Shin, B. S. , Kim, D. G. , & Kim, K. W. (2003). Orphanin FQ/nociceptin blocks methamphetamine place preference in rats. Neuroreport, 14, 2383–2385. 10.1097/00001756-200312190-00019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Schematic representation of intra‐CeA sites of injection assessed by histological analysis.
Figure S2. Schematic representation of intra‐VTA sites of injection assessed by histological analysis.
Figure S3. Schematic representation of intra‐NAc sites of injection assessed by histological analysis.
Figure S4. Effect of systemic LY2817412 administration on standard food pellet and water consumption in male and female msP rats.


