Abstract
Objective
To determine whether the adoption of laws that limit opioid prescribing or dispensing is associated with changes in the volume of opioids distributed in states.
Methods
State-level data on total prescription opioid distribution for 2015–2017 were obtained from the US Drug Enforcement Administration. We included in our analysis states that enacted an opioid prescribing law in either 2016 or 2017. We used as control states those that did not have an opioid prescribing law during the study period. To avoid confounding, we excluded from our analysis states that enacted or modified mandates to use prescription drug monitoring programs (PDMPs) during the study period. To estimate the effect of opioid prescription laws on opioid distribution, we ran ordinary least squares models with indicators for whether an opioid prescription law was in effect in a state-quarter. We included state and quarter fixed effects to control for time trends and time-invariant differences between states.
Results
With the exception of methadone and buprenorphine, the amount of opioids distributed in states fell during the study period. The adoption of opioid prescribing laws was not associated with additional decreases in opioids distributed.
Conclusions
We did not detect an association between adoption of opioid prescribing laws and opioids distributed. States may instead wish to pursue evidence-based efforts to reduce opioid-related harm, with a particular focus on treatment access and harm reduction interventions.
Keywords: Opioids, Law, Prescribing, Policy, Pain Management
Introduction
The epidemic of opioid-related harm is a continuing public health crisis. More than 70,000 drug overdose deaths occurred in the United States in 2017, of which nearly 48,000 involved an opioid [1]. Possibly because prescription opioids (POs) can be regulated more directly than the illicit opioid market, as well as widespread belief that overprescribing of opioids has been a key driver of the epidemic, states have undertaken a number of legal and policy efforts to attempt to reduce the level of POs prescribed and dispensed, including creating and mandating the use of prescription drug monitoring programs (PDMPs) [2–4].
Although the volume of POs distributed through legal channels has been falling since 2012, it remains well above historical trends [5]. To attempt to further reduce potentially inappropriate PO access and related harms and, in some cases, in response to guidelines issued by the Centers for Disease Control and Prevention (CDC) that state that “three days or less” of opioid therapy for acute pain will “often be sufficient” and that “more than seven days will rarely be needed” [6], a majority of states have passed laws that limit the amount of opioids that may be prescribed or dispensed to patients with acute pain [7]. Although there is some variation in the specifics of these laws, the majority limit initial opioid prescriptions to seven days or less. A minority of states also impose limits on the maximum dosage of opioids that can be prescribed.
The CDC recommendations and these state laws are premised largely on the fact that several characteristics of initial opioid prescriptions, including the length of the prescription and the morphine milligram equivalent (MME) prescribed, are associated with long-term opioid use in some patients [8–10]. It is therefore believed that reducing one or both of these variables may reduce the unintended continuation of prescription opioid use, potentially decreasing PO-related harm.
Concerns have been raised, however, that limitations on opioid prescribing may have negative unintended consequences [11,12]. The Food and Drug Administration has recently cautioned providers from rapidly tapering or discontinuing opioid therapy, a practice that may be associated with misperceptions of the CDC guidelines regarding opioid prescribing, as well as efforts to comply with state opioid prescribing laws [13]. There is evidence that some pain patients on chronic opioids have sought out opioids on the illicit market when they became unable to access them via their medical providers, though it is unclear how widespread this phenomenon is [14,15]. Finally, although research suggests that nonopioid therapy can be as effective as opioid therapy for many types of pain, inflexible restrictions on opioid prescribing may leave some patients’ pain inadequately treated [16–18].
All of these concerns are dependent on opioid prescribing and dispensing laws significantly affecting the distribution of prescription opioids. To date, there have been no analyses of whether these laws, most of which have been passed since 2016, have such an effect. Our research found that, contrary to the hopes of legislators and the fears of some advocates, these laws were not associated with significant changes in the volume of POs distributed in states with such laws compared with control states, suggesting that they may have a limited impact on opioid-related harm in either direction.
Methods
Data on PO distribution were obtained from the US Drug Enforcement Administration’s (DEA’s) Automation of Reports and Consolidated Orders System (ARCOS), an automated, comprehensive controlled substance reporting system. Entities that legally manufacture and distribute controlled substances are required by federal law to report transactions involving those substances to the DEA, which compiles them into a publicly available data set.
Our analysis uses quarterly 2015–2017 ARCOS data for eight opioids primarily used for pain (oxycodone, hydrocodone, morphine, codeine, hydromorphone, oxymorphone, meperidine, and fentanyl), as well as buprenorphine and methadone, which are used for both pain management and opioid use disorder treatment [19]. ARCOS data are reported in grams per 100,000 persons in each state-quarter. We converted grams of each type of opioid into morphine gram equivalents (MGE) per 100,000 persons to account for the relative potency of each opioid [20].
Data on states that enacted PO prescribing or dispensing laws by December 2017 (N = 26) were obtained from Davis et al. [7] For this analysis, we examined states that enacted such a law in either 2016 or 2017. To avoid confounding, we excluded states that enacted or modified a mandate that prescribers access the state PDMP during the study period (January 1, 2016, through December 31, 2017), as recent research has suggested that such mandates may be independently associated with decreases in opioid prescribing [21,22].
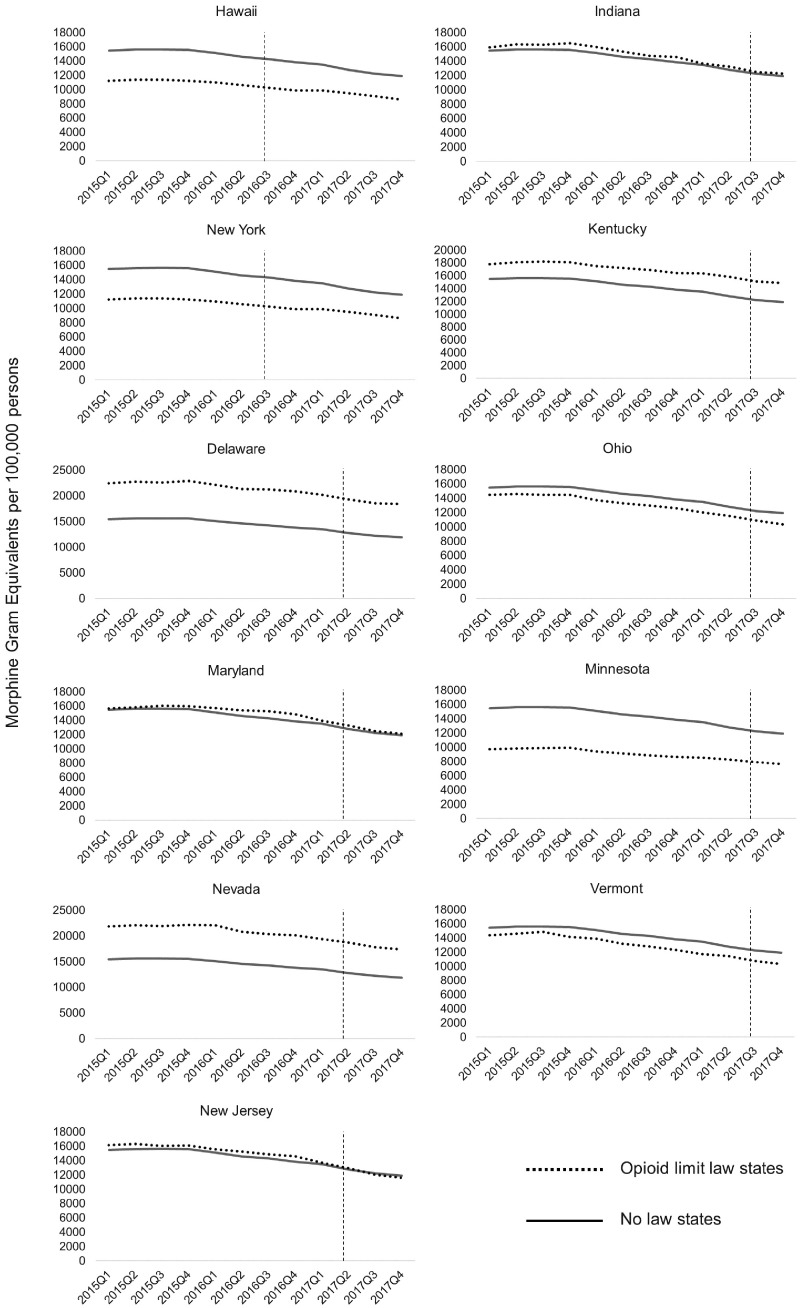
Two states adopted PO laws in the third quarter of 2016 (HI and NY), four states in the second quarter of 2017 (DE, MD, NJ, NV), and five states in the third quarter of 2017 (IN, KY, MN, OH, VT). All of these states impose limits on initial prescriptions of seven days or less, except for NV (14 days), HI (30 days), and MD, which does not have a day limit but which, like New Jersey, limits prescriptions to the “lowest effective dose” of an opioid. Additionally, Ohio limits MME to 30 per day, whereas Nevada imposes a 90-MME limit. Limits in Vermont vary from 24 MME for moderate pain to 50 MME for extreme pain. None of the other states in the sample impose MME limits.
We used as control states those that did not have an opioid prescribing law and did not enact or modify a mandatory PDMP access law in 2016 or 2017 (CO, DC, FL, IA, ID, KS, MI, MS, MT, ND, NE, OK, OR, SD, WA, WY). Of these states, ND and OK had PDMP mandates applicable to all prescribers during the entirety of the study period, whereas CO mandated that the PDMP be utilized on intake into methadone treatment programs and in some worker compensation cases, MS mandated PDMP usage by pain clinics and opioid treatment programs, and WA mandated PDMP usage in the worker compensation context.
We grouped these states by the quarter in which the law was effective and plotted the MGE per 100,000 persons for each drug. We also plotted the total combined MGE per 100,000 persons for all drugs excluding buprenorphine and methadone by state. We ran ordinary least squares (OLS) models with an indicator for whether an opioid prescription law was in effect in a state-quarter as well as state and quarter fixed effects. These models estimate the effect of opioid prescription laws on the MGE per 100,000 persons, controlling for time-invariant differences between states and time trends. The general form of our models was OPIOIDsq = STATEs + QUARTERq + LAWsq + εs, where STATEs referred to state-specific indicators, QUARTERq referred to quarter-specific indicators, and LAWsq referred to state-quarter indicators of whether a PO law was in effect in each state-quarter. Standard errors were clustered at the state level to account for correlation of outcomes within states over time.
Results
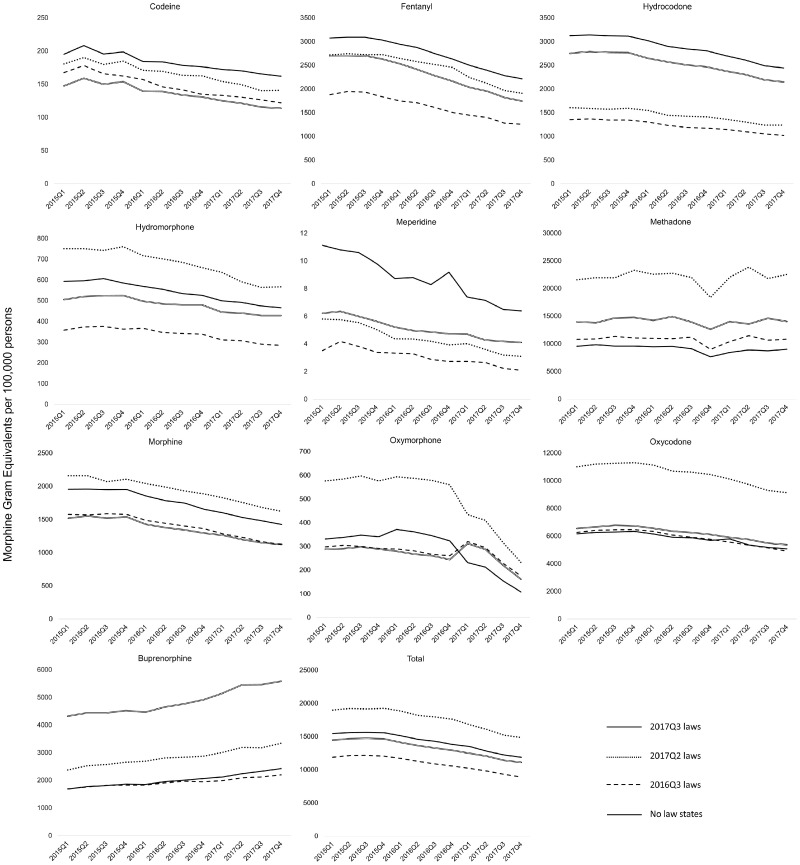
Consistent with a trend that has existed since approximately 2012, volume for all opioids other than buprenorphine fell throughout the study period, whereas methadone levels remained roughly constant. The overall average MGE for pain opioids fell by 23% in control states from the first quarter of 2015 to the last quarter of 2017, whereas buprenorphine volume increased by 44%. The overall average MGE for pain opioids similarly fell across the study period by 25% in states with PO laws effective in 2016Q3, by 22% in states with PO laws effective in 2017Q2, and by 23% in states with PO laws effective in 2017Q3.
When examining unadjusted trends in drugs distributed in states that enacted PO limits, we did not find clear changes in trends around the time of law enactment (Figure 1). We similarly did not find clear changes in trends when plotting total MGE per 100,000 for pain opioids in each state that passed an opioid limitation law (Figure 2). In our OLS models, we did not find consistent effects of PO laws on the volume of drugs distributed. We found a statistically significant negative effect on codeine, but positive effects on meperidine and hydrocodone. No significant changes were found for the other opioid medications examined (Table 1).
Figure 1.
Total pain opioids distributed in states that passed prescription opioid laws. Vertical lines indicate the quarter when the law became effective for each state.
Figure 2.
Opioids distributed by type of drug and timing of opioid prescription laws.
Table 1.
Effect of opioid prescription laws on morphine gram equivalents per 100,000 persons
| Coefficient | Confidence Interval | |
|---|---|---|
| Buprenorphine | 92.58 | –156.8 to 342.0 |
| Codeine | –5.874* | –11.47 to –0.281 |
| Fentanyl | 17.79 | –108.3 to 143.9 |
| Hydrocodone | 137.8* | 7.349 to 268.2 |
| Hydromorphone | –3.993 | –46.63 to 38.64 |
| Meperidine | 1.253* | 0.218 to 2.287 |
| Methadone | 851.5 | –15.04 to 1718.0 |
| Morphine | 42.59 | –53.71 to 138.9 |
| Oxycodone | –299.1 | –605.1 to 6.941 |
| Oxymorphone | 11.57 | –82.81 to 106.0 |
| Total | –84.88 | –571.8 to 402.1 |
P < 0.05; **P < 0.01; ***P < 0.001.
In sum, we do not find compelling evidence of a significant overall association between laws limiting the prescribing or dispensing of POs and PO distribution volume. Consistent with prior research, we observed that the volume of opioids distributed was generally falling among all states during the study period, and we detected no overall effect on opioid prescribing laws modifying this trend.
Discussion
Limitations on opioid prescribing or dispensing may be attractive to legislators and health officials who are attempting to reduce PO-related harm in their states. At the same time, reasonable concerns have been raised that the relatively strict and inflexible limitations on prescription opioid access imposed by these laws may have unintended negative consequences, including an increase in untreated or undertreated pain and substitution with illicit opioid use [11,12]. This preliminary analysis suggests that laws limiting the prescribing or dispensing of opioids for acute pain were not significantly associated with changes in PO distribution.
The amount of opioids distributed in the study states was decreasing when these laws were passed, and this secular decline persisted throughout the study period, suggesting that the laws did not have additional effects beyond already-existing legal provisions and already-changing prescriber practices. This study was unable to determine why that might be the case. It is possible that providers were unaware of the laws, that most prescriptions were already within the limitations, or that the lack of clear enforcement mechanisms in most laws was insufficient to modify prescriber behavior. These laws generally target new opioid prescriptions, which may represent a falling share of opioid distribution as provider prescribing practices change. These are all promising avenues for further research.
As these laws appear to have no significant impact on state-level prescription opioid distribution, it seems unlikely that they have a large effect on opioid-related harm in the short term, although in the long term they may contribute to changes in provider perception of opioid risks that inform their practice. This finding is in keeping with recent research that has found mixed effects for PDMPs on fatal and nonfatal opioid overdose, as well as a recent simulation that suggests that interventions targeting PO misuse are likely to have only a modest effect on opioid-related deaths [23,24].
This state-level analysis was unable to detect changes at the level of individual patients or medical professionals. Although the sample size in this study was limited, the estimated effects from our model were centered around zero, suggesting that the null findings result from a lack of effect rather than imprecise model estimates. Our follow-up period was similarly limited; however, we expected changes in opioid prescribing in response to laws to occur in the same quarter they became effective. As more recent data become available, we will be able to explore longer-term effects.
We were unable to examine whether the laws affected initial opioid prescribing, as several of them are intended to do. It is possible that these laws are associated with changes in specific subgroups, as has been reported with laws regulating pain clinics [25]. We also did not examine the effects of policies other than laws that may impact opioid prescribing such as changes in insurer policies, guidelines that do not have the force of law, or limits that apply only to chronic opioid prescribing [26,27]. If these policies have an effect on prescribing behavior and are present in control states, the effect of the laws studied may be obscured. Further research is indicated to determine whether changes are observed with specific types of opioid prescribing laws or individuals with certain characteristics, such as those with high-PO utilization, as well as whether changes in guidelines, policies, and insurer practices impact opioid prescribing and dispensing.
Conclusions
Limitations on opioid prescribing and dispensing have been advanced as promising measures to reduce drug-related harm, while their potential negative consequences have been viewed with concern by some. This study suggests that these laws have limited effects on statewide PO distribution, suggesting that both the hopes and fears associated with them may be overstated. Further research is indicated to determine if these laws have effects on particular prescriber or patient populations, as well as to determine how, if at all, they may be made more effective in reducing prescription opioid–related harm while not adversely impacting medically indicated use.
Funding sources: B. Piper is supported by the Fahs-Beck Fund for Research and Experimentation and the Health Resources Services Administration (D34HP31025). A. Gertner is supported by a National Research Service Award from the National Institute on Drug Abuse (F30 DA044668).
Conflicts of interest: None.
References
- 1. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G.. Drug and opioid-involved overdose deaths—United States, 2013-2017. MMWR Morb Mortal Wkly Rep 2018;67(5152):1419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parker AM, Strunk D, Fiellin DA.. State responses to the opioid crisis. J Law Med Ethics 2018;46(2):367–81. [DOI] [PubMed] [Google Scholar]
- 3. Kolodny A, Courtwright DT, Hwang CS, et al. The prescription opioid and heroin crisis: A public health approach to an epidemic of addiction. Annu Rev Public Health 2015;36(1):559–74. [DOI] [PubMed] [Google Scholar]
- 4. Davis CS, Pierce M, Dasgupta N.. Evolution and convergence of state laws governing controlled substance prescription monitoring programs, 1998-2011. Am J Public Health 2014;104(8):1389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guy GP Jr, Zhang K, Bohm MK, et al. Vital signs: Changes in opioid prescribing in the United States, 2006-2015. MMWR Morb Mortal Wkly Rep 2017;66(26):697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dowell D, Haegerich TM, Chou R.. CDC guideline for prescribing opioids for chronic pain–United States, 2016. JAMA 2016;315(15):1624–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davis CS, Lieberman AJ, Hernandez-Delgado H, Suba C.. Laws limiting the prescribing or dispensing of opioids for acute pain in the United States: A national systematic legal review. Drug Alcohol Depend 2019;194:166–72. [DOI] [PubMed] [Google Scholar]
- 8. Deyo RA, Hallvik SE, Hildebran C, et al. Association between initial opioid prescribing patterns and subsequent long-term use among opioid-naive patients: A statewide retrospective cohort study. J Gen Intern Med 2017;32(1):21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jeffery MM, Hooten WM, Hess EP, et al. Opioid prescribing for opioid-naive patients in emergency departments and other settings: Characteristics of prescriptions and association with long-term use. Ann Emerg Med 2018;71(3):326–36 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shah A, Hayes CJ, Martin BC.. Characteristics of initial prescription episodes and likelihood of long-term opioid use—United States, 2006-2015. MMWR Morb Mortal Wkly Rep 2017;66(10):265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Samet JH, Kertesz SG.. Suggested paths to fixing the opioid crisis: Directions and misdirections. JAMA Netw Open 2018;1(2):e180218.. [DOI] [PubMed] [Google Scholar]
- 12. Mundkur ML, Gordon AJ, Kertesz SG.. Will strict limits on opioid prescription duration prevent addiction? Advocating for evidence-based policymaking. Subst Abuse 2017;38(3):237–8. [DOI] [PubMed] [Google Scholar]
- 13.US Food and Drug Administration. FDA identifies harm reported from sudden discontinuation of opioid pain medicines and requires label changes to guide prescribers on gradual, individualized tapering [press release]. 2019. https://www.fda.gov/drugs/drug-safety-and-availability/fda-identifies-harm-reported-sudden-discontinuation-opioid-pain-medicines-and-requires-label-changes, (accessed May 3, 2019).
- 14. Dasgupta N, Creppage K, Austin A, Ringwalt C, Sanford C, Proescholdbell SK.. Observed transition from opioid analgesic deaths toward heroin. Drug Alcohol Depend 2014;145:238–41. [DOI] [PubMed] [Google Scholar]
- 15. Compton WM, Jones CM, Baldwin GT.. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med 2016;374(2):154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Derry CJ, Derry S, Moore RA.. Single dose oral ibuprofen plus paracetamol (acetaminophen) for acute postoperative pain. Cochrane Database Syst Rev 2013;(6):CD010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gaskell H, Derry S, Moore RA, McQuay HJ.. Single dose oral oxycodone and oxycodone plus paracetamol (acetaminophen) for acute postoperative pain in adults. Cochrane Database Syst Rev 2009;(3):CD002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: The SPACE randomized clinical trial. JAMA 2018;319(9):872–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Piper BJ, Shah DT, Simoyan OM, McCall KL, Nichols SD.. Trends in medical use of opioids in the U.S., 2006-2016. Am J Prev Med 2018;54(5):652–60. [DOI] [PubMed] [Google Scholar]
- 20. Cabrera FF, Gamarra ER, Garcia TE, et al. Opioid distribution trends (2006-2017) in the US territories. PeerJ 2019;7:e6272.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Winstanley EL, Zhang Y, Mashni R. et al. Mandatory review of a prescription drug monitoring program and impact on opioid and benzodiazepine dispensing. Drug Alcohol Depend 2018;188:169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wen H, Schackman BR, Aden B, Bao Y.. States with prescription drug monitoring mandates saw a reduction in opioids prescribed to Medicaid enrollees. Health Affairs 2017;36(4):733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen Q, Larochelle MR, Weaver DT, et al. Prevention of prescription opioid misuse and projected overdose deaths in the United States. JAMA Netw Open 2019;2(2):e187621.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fink DS, Schleimer JP, Sarvet A. et al. Association between prescription drug monitoring programs and nonfatal and fatal drug overdoses: A systematic review. Ann Intern Med 2018;168(11):783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lyapustina T, Rutkow L, Chang HY. et al. Effect of a “pill mill” law on opioid prescribing and utilization: The case of Texas. Drug Alcohol Depend 2016;159: 190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heins SE, Frey KP, Alexander GC, Castillo RC.. Reducing high-dose opioid prescribing: State-level morphine equivalent daily dose Policies, 2007–2017. Pain Med 2019; (doi: 10.1093/pm/pnz038). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Al Achkar M, Grannis S, Revere D, MacKie P, Howard M, Gupta S.. The effects of state rules on opioid prescribing in Indiana. BMC Health Serv Res 2018;18(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]