Abstract
Aims
As health systems around the world increasingly look to measure and improve the value of care that they provide to patients, being able to measure the outcomes that matter most to patients is vital. To support the shift towards value-based health care in atrial fibrillation (AF), the International Consortium for Health Outcomes Measurement (ICHOM) assembled an international Working Group (WG) of 30 volunteers, including health professionals and patient representatives to develop a standardized minimum set of outcomes for benchmarking care delivery in clinical settings.
Methods and results
Using an online-modified Delphi process, outcomes important to patients and health professionals were selected and categorized into (i) long-term consequences of disease outcomes, (ii) complications of treatment outcomes, and (iii) patient-reported outcomes. The WG identified demographic and clinical variables for use as case-mix risk adjusters. These included baseline demographics, comorbidities, cognitive function, date of diagnosis, disease duration, medications prescribed and AF procedures, as well as smoking, body mass index (BMI), alcohol intake, and physical activity. Where appropriate, and for ease of implementation, standardization of outcomes and case-mix variables was achieved using ICD codes. The standard set underwent an open review process in which over 80% of patients surveyed agreed with the outcomes captured by the standard set.
Conclusion
Implementation of these consensus recommendations could help institutions to monitor, compare and improve the quality and delivery of chronic AF care. Their consistent definition and collection, using ICD codes where applicable, could also broaden the implementation of more patient-centric clinical outcomes research in AF.
Keywords: Atrial fibrillation, Outcomes, Patient-reported, Value-based health care
Introduction
Atrial fibrillation (AF) is an increasingly prevalent clinical and public health problem, affecting more than 33 million people worldwide1 and predicted to affect nearly 18 million people in Europe alone by 2060.2 Atrial fibrillation is associated with adverse health outcomes, poor health-related quality of life (HRQoL), and high healthcare costs with hospitalization being the main cost driver.3 The goals of any health care provider managing patients with AF should be:
to improve HRQoL, including alleviating symptoms and consequent problems such as treatment burden and anxiety;
to reduce the risk of long-term sequelae, i.e. systemic embolism, stroke, or heart failure;
to reduce mortality; and
to deliver care in a cost-sensitive manner.
Currently, significant variation exists in AF care and treatment practices between institutions and countries.4,5 The 2017 Lancet Global Burden of Disease study estimated that non-communicable diseases account for 73% of all deaths globally,6 of which over half are attributable to four cardiovascular risk factors (hypertension, smoking, diabetes mellitus, and obesity). The largest number of deaths from non-communicable diseases was from cardiovascular diseases. Efforts to address preventable cardiovascular morbidity and mortality, therefore, should be enhanced and encouraged.6
The notion of ‘value’ in healthcare has taken on increasing importance over the last 15 years. Value may be defined within the healthcare context as patient-relevant outcomes divided by the costs per patient across the full cycle of care.7 As healthcare systems worldwide work to improve the value of care that they provide to patients, particularly in the context of growing demands on providers amid significant resource constraints, being able to measure outcomes important to patients is crucial.8 Although clinicians gather more data today than ever before, what is measured may have little relationship to the goals of healthcare that matter most to patients. The ability to define ‘value’ in healthcare is, therefore, not straightforward.
A challenge related to value in healthcare is that its measurement relies on the validity and robustness of measurements. A good source of robust clinical outcomes and their definitions is found in clinical trials and in the output of major registries. Efforts to report outcomes of routine AF care exist already. For example, there are a number of large registries reporting on thromboprophylaxis and stroke prevention,9–11 some that report on antiarrhythmic drug therapy and cardioversion,12–15 and a few on ablation.16–18 Some international societies have produced position papers and guidelines in an attempt to standardize care across their jurisdictions.19–21 Although several position papers have been published attempting to do so,22,23 there is no single internationally accepted standardized approach to report outcomes of care in AF. A fully standardized approach would include both the outcomes that are measured as well as the process of measurement and recording, e.g. using electronic health records. While survival or hospitalization outcomes are frequently recorded, patient-reported outcomes are still rarely measured and/or recorded despite increasing recognition of their importance in disease management.24,25 The lack of a standardized approach hinders routine monitoring and benchmarking of different clinical practices. To support the development of a standardized outcome set in AF for integration into routine clinical practice, the International Consortium for Health Outcomes Measurement (ICHOM) convened an international multidisciplinary Working Group (WG) of experts and patient representatives. As a not-for-profit organization, ICHOM has developed 27 standard sets of value-based outcomes for use in routine clinical practice in various medical conditions, such as coronary artery disease,26 stroke,27 and cancer (including breast,28 colorectal,29 and prostate cancer30). Over 600 organizations have implemented ICHOM sets including 15 national registries. Standard sets are reviewed and updated annually by ICHOM.
The aim of the project was to propose a standardized minimum set of outcomes for AF, including patient-reported outcomes, and case-mix factors, for comparisons across treatment modalities and institutions.
Methods
Composition of the working group
ICHOM established a geographically diverse WG covering a broad range of sub-specialties within the AF community. The WG consisted of 30 members, including clinicians, registry experts, epidemiologists, research scientists, and six patients and patient representatives from 11 countries in Europe, North America, Latin America, the Middle East, and Asia-Pacific. Twenty percent of WG members were either AF patients or represented AF patient organizations. Two of the patient representatives in the WG represented the two largest AF patient organizations globally. A project team (W.H.S., Z.D.-G., A.J.-O., and A.J.C.) guided the efforts of the WG.
Development of the atrial fibrillation standard set
The WG convened using seven teleconferences between January 2018 and March 2019, following a structured process similar to that of previous ICHOM WGs (Supplementary material online, Item S1). The development of the standard set involved several phases: defining the scope of the project; prioritizing and defining outcome domains; evaluating and selecting outcome measures that would be used to measure the outcome domains, including clinical data and patient-reported outcome measures (PROMs); and selecting and defining case-mix variables.
Identification of potential outcomes and case-mix variables
The project team performed a systematic literature review, following Preferred Reporting Items for systematic Reviews and Meta-Analyses (PRISMA) guidelines to identify potential outcome domains, PROMs, and case-mix variables. The search strategy is included in a supplement to this article (Supplementary material online, Item S2). This search retrieved 2121 articles. The 100 most recent PubMed-indexed articles published as of 1 March 2018 were subsequently included for review.
Atrial fibrillation registries were reviewed to extract additional outcome measures and case-mix adjustment variables. These registries were identified from a systematic review by Mazurek et al.31 and by internet searches. Some of these registries were specifically designed to assess outcomes that had previously been reported in clinical trials. Patient representatives from the WG participated as a patient advisory group in a separate breakout session in order to explore their perspectives on the importance of different outcomes. An additional literature review was performed to identify studies of patients’ perspectives on the most relevant outcome domains in AF.
Consensus process
Following each teleconference, the project team circulated an electronic survey to the WG to gather feedback on each key decision. An online three round modified Delphi process was performed for selection of outcomes, following the RAND/University of California (Los Angeles) methodology32 and based on a literature review,33 to achieve consensus on which outcomes should be included. Inclusion in the standard set required that at least 80% of the WG voted an item as ‘essential’ (score 7–9 on a 9-point Likert scale) in either voting round. Outcomes were excluded if at least 80% of the WG voted an item as ‘not recommended’ (Score 1–3). The WG voted on all inconclusive outcome domains in the final third round with 70% consensus required for the domain to be included (yes/no scale).
Selection of patient-reported outcome measures
After the outcomes were chosen for inclusion in the standard set, corresponding PROMs were identified from the literature and registry review. The original and validation studies of the instruments were examined in order to evaluate the psychometric quality, domain coverage, and feasibility of measurement and implementation. An advisory group consisting of academics and patients with particular expertise in PROMs in AF was convened in a breakout session in order to review the list of potential instruments compiled by the project team.
A similar process was used to agree on which PROM tools and case-mix variables should be recommended. The results of each vote were reviewed by the WG at the subsequent teleconference. The criteria by which outcome domains were assessed for inclusion in the set were: (i) frequency of the outcome, (ii) impact on the patient, (iii) potential for modifying the outcome, and (iv) feasibility of measuring the outcome. Variables to be used as case-mix adjusters were assessed on: (i) relevance, (ii) independence, and (iii) the measurement feasibility.
Open review
Atrial fibrillation patients, recruited via professional associations (Arrhythmia Alliance and StopAfib.org) reviewed the final draft of the standard set, and independently provided feedback using an online survey that was shared by the associations through email and social media. Respondents were asked to rate their confidence regarding several elements of the set on a 9-point Likert scale34 (1 not important, 5 somewhat important, 9 most important), with an open field for comments.
Results
Scope
The outcomes and measures included in the AF standard set were defined for a target population of adult patients, 18 years and older, diagnosed with AF; including patients with asymptomatic AF. Cardiotoxic AF (e.g. acute AF related to drugs and/or toxic substances) was excluded. The treatment approaches covered are categorized: management of cardiovascular risk factors and initiation of preventive therapy (e.g. lifestyle interventions and patient education); pharmacological management (e.g. rate control, rhythm control and anticoagulants); and non-pharmacological management (e.g. pacemaker, DC cardioversion, catheter-based atrial ablation, surgical ablation, atrioventricular node ablation, catheter-based left atrial appendage occlusion, and left atrial appendage excision/exclusion).
Outcomes
Through the literature review, registry search and patient advisory group discussions, the WG selected 18 outcome domains. The Reference Guide containing all consensus definitions is published on the ICHOM website at www.ichom, org. The outcome domains were categorized into three major groups: long-term consequences of disease, complications of treatment, and patient-reported outcomes (Table 1). The results of the Delphi process are shown in Supplementary material online, Item S3.
Table 1.
Summary of ICHOM atrial fibrillation standard set of outcomes
| Domain | Measure | Details | Timing | Data source |
|---|---|---|---|---|
| Long-term consequences of disease | Mortality (all-cause and cardiovascular) | Cardiovascular cause of deatha | Ongoing | Clinician |
| Ischaemic stroke, systemic embolism, and unclassified stroke | Occurrence of a cardiovascular eventb | |||
| Heart failure | Clinical diagnosis of heart failure and value of LVEFc | |||
| Cardiovascular hospitalization | Hospital admissiond due to an unplanned cardiovascular causee | |||
| Freedom from fast atrial arrhythmia post-treatment | Detection of fast atrial arrhythmia and mode of treatmentf and monitoringg administered | |||
| Anticoagulation management | Rationaleh for prescribing anticoagulation therapy and prescription of a treatment devicei | 1–6 months and 1 year | ||
| Cognitive functioning | Assessment of cognition | |||
| Complications of treatment | Haemorrhagic stroke | Occurrence of a cardiovascular eventb | Ongoing | |
| Life-threatening/major bleeding | Occurrence of fatal bleeding, symptomatic bleeding in a critical area or organ,j and/or fall in haemoglobink | |||
| Serious adverse events post-intervention | Occurrence of serious adverse eventsl due to an interventionm | |||
| Medication side effects | Occurrence of medication side effectn that led to discontinuing of prescribed medicationo | |||
| Patient-reported outcomes | Health-related quality of life | Measured with the SF-12 ® or the PROMIS Global Health ® | 1–6 months and 1 year | Patient |
| Physical functioning | ||||
| Emotional functioning | ||||
| Exercise tolerance | Measured with the AFEQTp or the AFSSq | |||
| Symptom severity | ||||
| Ability to work | Measured with the WPAIr | |||
| Cognitive functioning | Measured with the PROMIS Cognitive Function® |
An acute myocardial infarction; sudden cardiac death; heart failure; stroke; cardiovascular procedure; cardiovascular haemorrhage; other cardiovascular causes, e.g. peripheral arterial disease; other cause of death; unknown.
An ischaemic stroke; a systemic embolism; an unclassified stroke; none of above. Date (DD/MM/YYYY).
Left ventricular ejection fraction (LVEF).
Admission = at least one overnight stay at a hospital or acute care facility from first atrial fibrillation diagnosis.
Cardiovascular causes for admission are ones in which the principal admitting diagnosis relates to the cardiovascular system: myocardial infarction/ischaemic heart disease, heart failure, stroke/TIA, peripheral arterial disease, AF, venous thromboembolism/PE, etc.
Rate control drugs; pharmacological cardioversion; electrical cardioversion; atrial ablation; AVN/His-bundle ablation; surgical atrial ablation; pacemaker.
A 12-lead ECG; ambulatory monitoring; implantable devices; wearable devices/smartphones.
Not recommended by current guidelines. Anticoagulants are not appropriate for beneficial reasons, e.g. young patient with no underlying heart conditions; Not recommended by current guidelines; anticoagulants inappropriate for harmful reason or due to harm reasons, e.g. patients with serious bleeding events; patient refusal; medication and/or monitoring/follow-up unavailable; cognitive dysfunction; short life expectancy; high costs (including health insurance issue); other (specify).
Left atrial appendage occlusion device, closure or excision of the left atrial appendage.
Intracranial, intraspinal, intraocular, retroperitoneal, intra-articular, or pericardial, or intramuscular with compartment syndrome.
Haemoglobin >2 g/dL or transfusion of >2 units of whole blood/red cells.
In hospital death; vascular complications (postoperative haemorrhage, postoperative haemorrhage requiring transfusion, pericardial tamponade); required open heart surgery; required repeat ablation procedure; ventricular arrhythmias; respiratory complications(pneumothorax; phrenic nerve palsy; pulmonary vein stenosis; other iatrogenic respiratory complications); trauma embolic complications, stroke, TIA, systemic or pulmonary embolism; postprocedure infections; atrio-oesophageal fistula; other (specify).
Catheter-based ablation (four subcategories); surgical ablation procedure (including MAZE); hybrid catheter and surgical ablation; left atrial appendage closure/occlusion (device); left atrial appendage ligation/excision (surgical); electrical cardioversion; pacemaker implantation; pharmacological cardioversion.
Dizziness, fainting, lightheadedness, or loss of consciousness; erectile dysfunction; hair loss; memory problems, brain for or poor concentration; mental health issues such as depression or anxiety; muscle or joint pain; shortness of breath; stomach problems such as nausea, vomiting or diarrhoea; unexplained bruising or bleeding; unusual weakness or tiredness; weight loss; other (specify).
Antithrombotic (anticoagulation, antiplatelet) rhythm control; rate control; other (specify); unknowns.
Atrial Fibrillation Effect on Quality-of-Life Questionnaire.
University of Toronto Atrial Fibrillation Severity Scale.
Work Productivity and Activity Impairment Questionnaire: General Health V2.0.
Long-term consequences of disease
This outcome domain consists of the following sub-domains: mortality (all-cause and cardiovascular); ischaemic stroke, systemic embolism, unclassified stroke; heart failure; cardiovascular hospitalization; freedom from rapid and/or symptomatic atrial arrhythmia post-treatment; anticoagulation management; clinician-reported patient cognitive functioning.
Complications of treatment
Complications of AF treatment selected for inclusion were: haemorrhagic stroke, life-threatening/major bleeding, serious adverse events post-intervention, and medication side effects; all of which were highly ranked by patients during the patient advisory group discussion. Building upon the International Society on Thrombosis and Haemostasis definition for bleeding, the WG expanded the definition by specifying, ‘bleeds that require medical intervention by healthcare professional, leading to hospitalization or increase in the level of care, or prompting a face-to-face evaluation’.
Patient-reported outcomes
As the primary aim of the standard set is to measure outcomes that matter most to patients with AF, the WG recommended specific patient-reported outcomes. The following sub-domains were selected: HRQoL, emotional functioning, physical functioning, exercise tolerance, symptom severity, ability to work, and cognitive functioning.
Baseline characteristics and case-mix variables
In addition to the outcome measures, the WG selected important baseline health characteristics that are important to collect to enable comparison between providers. These baseline health characteristics include demographics, comorbidities, cognitive function, date of diagnosis, disease duration, medications prescribed, and procedure type. The WG also identified lifestyle intervention variables that may affect the management and care of patients with AF and influence their outcomes, including smoking, BMI, alcohol intake, and physical activity (Table 2).
Table 2.
Summary of ICHOM atrial fibrillation standard set case-mix variables
| Category | Measure | Details | Timing | Data source | |
|---|---|---|---|---|---|
| Case-mix variables | Demographic factors | Age | Year of birth | Baseline | Patient |
| Sex | Sex at birth | ||||
| Level of education | Highest attained educationa | ||||
| Ethnicity (optional) | Country specific | ||||
| Lifestyle interventions | Smoking status | Never/former/current | Baseline and annually | Clinician/administrative | |
| BMI | BMI (height and weight)b | ||||
| Alcohol intake | Number of standard alcoholic drinks do you drink per weekc | Patient | |||
| Physical activity | Frequency of engagement in moderate to strenuous exercised | Patient/clinician | |||
| Baseline health status | Comorbidities | Indicate whether the patient has a documented history or is currently diagnosede | Baseline at the time of diagnosis | Clinician | |
| Cognitive function | Measured with the MoCAf | Baseline | |||
| Diagnosis | Types of atrial fibrillationg | Baseline and annually | |||
| Disease duration | Diagnosis dateh | ||||
| Medications prescribed | Indicate whether the patient is currently being prescribed medication for atrial fibrillationi | ||||
| Procedure type | Eight broad categoriesj |
ISED classification (none/primary/secondary/tertiary).
BMI <18.5 underweight; 18.5–24.9 normal weight; 25–29.9 pre-obesity; and >30.0 obesity.
Options: none; 1–6; 7–14; >14. Supporting definition: standard drink according to WHO is 20 g of pure alcohol that is: a can or bottle of beer (375 mL or 12 oz at 3.5% alcohol by volume) or a small glass of red wine (100 mL or 3–4 oz at 13% alcohol by volume) or a shot of whiskey or other spirit (30 mL or 1 oz at 40% alcohol by volume).
Measured by the physical activity vital sign.
Answer (yes/no): gastrointestinal/other major haemorrhage, intracranial haemorrhage; hypertension; diabetes mellitus; heart failure; ischaemic heart disease/myocardial infarction; vascular diseases, e.g. coronary disease, arterial disease; chronic obstructive pulmonary disease; chronic kidney disease; hyperthyroidism; obstructive sleep apnoea; stroke/TIA; cancer (excluding non-melanoma skin cancer).
The Montreal Cognitive Assessment.
Paroxysmal (episode of AF that terminates spontaneously or with intervention in <7 days); persistent (AF that lasts for >7 days and requires intervention in order for cardioversion to occur); long-standing persistent (episodes of AF extending >12 months); permanent (AF that will not be cardioverted or has failed cardioversion).
Answer options: diagnosed date (DD/MM/YYYY); recent (less than a year and date unknown); unknown.
Three major categories with sub-options: antithrombotic agents (two subcategories); rate control agents (four subcategories); rhythm control agents (four subcategories).
Catheter-based ablation (four subcategories); surgical ablation procedure (including MAZE); hybrid catheter and surgical ablation; left atrial appendage closure/occlusion (device); left atrial appendage ligation/excision (surgical); electrocardioversion; pharmacological cardioversion; pacemaker implantation.
Open review
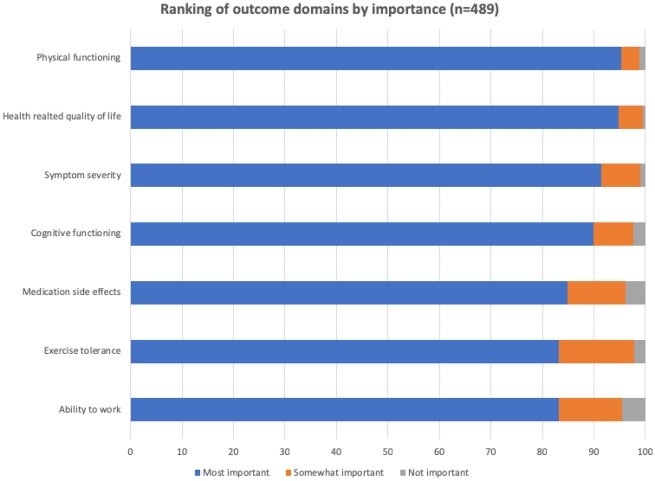
An electronic survey was distributed to AF patients via patient networks through email and social media. Four hundred and eighty-nine respondents replied electronically to the validation survey seeking feedback on the outcomes included in the standard set. The majority (87%) of respondents agreed that the standard set captures all the important outcomes. All patient-reported outcomes were rated as very important, with a range from 83% for ability to work to 95% for physical functioning (Figure 1). The additional outcomes that respondents suggested adding to the standard set included mental health impact such as depression, anxiety, feeling worried and stressed, and the health literacy domain that included aspects such as patient–clinician communication, disease understanding, treatment options knowledge, and empowerment to control disease. The WG agreed to include the emotional functioning domain in the core set but decided to exclude the health literacy domain as it covers aspects outside of the scope of the current project.
Figure 1.
Relevance of outcomes included in ICHOM AF set according to patient open review.
Discussion
In this project, an international WG comprising AF experts, patients and patient representatives, developed a consensus set of the most important outcomes and outcome measures in AF. The production of such a standard set could help institutions to monitor, compare and improve the quality and delivery of chronic AF care. The WG also defined a set of case-mix variables that must be adjusted for when comparing outcomes across institutions or regions. This is the first global effort to develop a standardized minimum set of patient-centred outcomes in AF for use in clinical practice.
The approach taken in this project goes some way to supporting the principles of value-based healthcare and a value-based outcomes framework. Value-based healthcare has the potential to benefit stakeholders across the healthcare spectrum.35 For example, patients could in future choose providers based on informed expectations of outcomes and the associated costs.36 Providers that deliver superior outcomes at competitive costs may excel, while others are forced to improve or lose market share. Equally, payers could negotiate contracts based on results and encourage innovation to achieve those results.37 The life science community could succeed by marketing its products based on value, showing improved patient outcomes relative to costs. While additional work above and beyond a consensus process would be required to reach these aspirations, nevertheless a consensus process can identify areas of importance for future academic research, e.g. PROMs in AF. Indeed, one of the recommendations of the WG was that there is a need to develop and validate further AF-specific PROMs. The WG acknowledges that other PROMs could have been selected for inclusion in the standard set, however, those ultimately included were considered to be the most appropriate tools currently available.
The ability to measure outcomes most relevant to patients in a standardized manner globally is key to unlocking the potential of value-based health care.38 By establishing a geographically and clinically diverse WG of AF patients and experts in AF, we have developed a minimum set of outcomes for AF, focused on patient-centred measures, that could be recorded in routine clinical practice. This may support the shift towards value-based health care in AF.8
Our literature review of recent randomized clinical trials in AF revealed heterogeneity in outcomes. Nevertheless, there was overlap in outcomes collected by trials, registries, and those reported by patients. This is unsurprising given that some registries are designed to assess outcomes previously reported in clinical trials. Prioritizing the outcomes that would feature in the agreed standard set posed a challenge for the WG. There was consensus that a balance must be struck between clinical outcomes and PROMs as well as a comprehensive set of outcomes and a standard set that is feasible to measure. Although there was debate over which outcomes should be included in the final set, it became clear through the Delphi process that certain outcomes were essential, e.g. all-cause mortality, cardiovascular mortality, ischaemic stroke/systemic embolism/unclassified stroke, medication side effects, haemorrhagic stroke, life-threatening/major bleeding, HRQoL, physical functioning, exercise tolerance and symptom severity.
When evaluating PROMs to be collected as part of the standard set, consideration was given to the appropriate mix of generic PROMs and AF-specific PROMs. Some uncertainty surrounds PROMs in AF, and the methodology to develop robust patient-reported outcomes was refined in 2013.39 While generic PROMs are more commonly used, and have been better validated than AF-specific PROMs, they measure general health and functioning that is not specific to AF, and scores are influenced by patient demographics and comorbidities.40 However, AF-specific instruments assess with high sensitivity domains exclusive and/or relevant to patients with AF, although they are, in general, less well-validated and may add to the burden of assessment for patients with other comorbid conditions.41 It is hoped that these tools could be embedded into a provider’s electronic health record in order to ease adoption of PROMs in routine clinical practice and reduce the burden on both providers and patients. It was also important to consider the psychometric and measurement qualities of the various instruments. Although few studies of the measurement properties of AF-specific instruments exist, a recent systematic review has provided useful insights into their overall utility.41 While the AF-specific instruments recommended in the standard set have been used as outcome measures in trials, including a recent large randomized trial of catheter ablation compared to medical therapy in AF,42 they have not yet been used with the intention of guiding therapy. The WG’s intention in recommending these tools is to support the adoption of AF-specific tools and to increase awareness and use of these tools to allow further validation and development.
Although the WG was able to reach consensus on the majority of outcomes to be collected, as well as instruments by which to collect the outcomes and case-mix variables to allow benchmarking and comparisons across institutions, one area which remains controversial is how best to collect data on the side effects of prescribed medication. Although some trials collect data on discontinuation of medications, there is little granularity to these data.3,44 For example, it is often not possible to discern whether patients have discontinued their medication because of a side effect of the medication or whether they have discontinued their medication because of a perceived lack of effect or a lack of knowledge of the prescribed therapy. It is also difficult to capture whether patients are taking their medicines according to the prescribed regimen or whether there are inadvertent errors with adherence. This is particularly likely to be the case with oral anticoagulants where complex dosing regimens may exist.45 The WG would support efforts to improve the evidence base behind medication side effects in AF.
It is envisaged that the AF standard set could be implemented in a comprehensive, integrated care model as has been proposed previously.46 Use of the integrated care approach in AF has been associated with reduced cardiovascular hospitalizations and all-cause mortality.47 Nurse-coordinated management could encompass not only the medical aspects of care but also the education that is vital to many of the outcomes identified.
There are, of course, limitations to our approach. The standard set methodology is reliant on the composition of the WGs. Every effort was made to ensure a balanced global representation of disease experts allowing for implementation in different healthcare contexts. Our WG members practice in 11 countries around the world representing high-, low-, and middle-income countries. Furthermore, since the standard set was developed with implementation in mind, feasibility of measuring outcomes was a key concern during the outcome selection stage and therefore not all outcomes could be collected. This meant that some outcomes could not be included, e.g. burden of AF, treatment adherence, perceived control over AF, which are nevertheless recognized by the WG as being important.
Implementation of the standard set involves several phases. The initial phase involves engaging clinical leaders to champion the adoption of the set within their institutions, assessing an institution’s starting point and identifying its goals. This cultivation phase may not be immediately straightforward, for example particularly if provider reimbursement is tied to outcomes. Before such a situation could be envisaged, risk-adjustment models would require significant analysis before meaningful algorithms could be developed which carry the confidence of providers. After the initial phase, a diagnostic phase, in which current measurement practice and infrastructure is evaluated to identify gaps preventing the collection of clinical data and PROMs. It is necessary in this phase to identify at what points data would be captured and how it would be collected, possibly using electronic health records. A patient education campaign could be undertaken at this stage in order to demonstrate, for example, the value of PROMs. This is likely to increase patient compliance with the additional questionnaires. To support data collection, a summary of ICD codes for outcomes included in the standard set that can be coded in this way was generated (Supplementary material online, Item S4). Pilot studies would then follow to test strategies for data collection. Incorporating feedback from these studies, a measurement phase identifies and adopts the best strategies to relay data back to clinical teams and current and future patients.
The WG defined time-points for outcomes measurement that align with existing clinical time points to minimize the burden on patients and healthcare professionals.
Conclusions
We have developed a consensus recommendation for a standardized minimum set of outcomes that are deemed most important to patients with AF comprising long-term consequences of disease outcomes, complications of treatment outcomes, and patient-reported outcomes. This recommendation is targeted for integration into routine clinical practice and research. Use of the standard set may enable institutions to monitor, compare, and improve the quality of their care for patients living with AF.
Supplementary Material
Acknowledgements
F.D.R.H. acknowledges his part-funding from the National Institute for Health Research (NIHR) School for Primary Care Research, the NIHR Collaboration for Leadership in Health Research and Care (CLARHC) Oxford, the NIHR Oxford Biomedical Research Centre (BRC) UHT, and the NIHR Oxford Medtech and In-Vitro Diagnostics Co-operative (MIC). B.A.S. is supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K23HL143156. D.A.C. has received congress sponsorship from Bayer in the last 5 years and has received research grants from Abbott and Biotronik. Am.B. acknowledges support from the BigData@Heart Consortium, funded by the Innovative Medicines Initiative-2 Joint Undertaking under grant agreement No. 116074. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA; it is chaired, by DE Grobbee and SD Anker, partnering with 20 academic and industry partners and ESC. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. S.G. acknowledges the financial support from MEXT/JSPS KAKENHI 17K19669, 18H01726, and 19H03661. S.G. also acknowledge grant support from the Vehicle Racing Commemorative Foundation and Nakatani Foundation for Advancement of Measuring Technologies in Biomedical Engineering. S.G. is an associated Editor for Circulation, an associate Editor for Journal of Biorheology, an associate Editor for Archives of Medical Science, section Editor for Thrombosis and Hemostasis.
Conflict of interest: F.D.R.H. has received occasional speaker fees or congress sponsorship from Amgen, Bayer, Boehringer Ingelheim, Novartis, Novo Nordisk, Pfizer in the past 5 years. E.A. has received occasional speaker fees from Biosense Webster. Am.B. has participated in advisory boards for Boehringer-Ingelheim, Astra-Zeneca, Pfizer, Bristol-Myers-Squibb, and Novo-Nordisk. B.A.S. receives research support from Boston Scientific and Janssen; consulting to Janssen and Merit Medical; speaking for NACCME (funded by Sanofi). J.S.H. has received research grants and speaking fees from BMS/Pfizer, Servier, Medtronic, Boston Scientific and Abbott. M.F. has participated in advisory boards of Abbott, AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol Myers Squibb, Medtronic, Oberoi Consulting, Pfizer, Roche, Sanofi-Aventis and Servier. J.M.H. is supported by a Future Leader Fellowship from the Australian Heart Foundation. S.G. received independent research grant from Bristol-Myers Squibb (33999603). S.G. also received research funding from Sanofi, Pfizer, and Ono. M.V.H. has received research grants from ZONMW, Boehringer Ingelheim, Bayer Healthcare, Pfizer-BMS and Aspen and has consultancy fees from Boehringer Ingelheim, Bayer Healthcare, Pfizer-BMS and Aspen. D.L. has received investigator-initiated educational grants from Bristol-Myers Squibb and Boehringer Ingelheim; has been a speaker for Boehringer Ingelheim, Bayer, and Bristol-Myers Squibb/Pfizer; and has consulted for Bristol-Myers Squibb, Bayer, Boehringer Ingelheim, and Daiichi-Sankyo.
References
- 1. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ.. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 201 Study. Circulation 2014;129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krijthe BP, Kunst A, Benjamin EJ, Lip GY, Franco OH, Hofman A, Witteman JC, Stricker BH, Heeringa J.. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J 2013;34:2746–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wolowacz SE, Samuel M, Brennan VK, Jasso-Mosqueda JG, Van Gelder IC.. The cost of illness of atrial fibrillation: a systematic review of the recent literature. Europace 2011;13:1375–1385. [DOI] [PubMed] [Google Scholar]
- 4. Oldgren J, Healey JS, Ezekowitz M, Commerford P, Avezum A, Pais P, Zhu J, Jansky P, Sigamani A, Morillo CA, Liu L, Damasceno A, Grinvalds A, Nakamya J, Reilly PA, Keltai K, Van Gelder IC, Yusufali AH, Watanabe E, Wallentin L, Connolly SJ, Yusuf S; RE-LY Atrial Fibrillation Registry Investigators. Variations in cause and management of atrial fibrillation in a prospective registry of 15,400 emergency department patients in 46 countries: the RE-LY Atrial Fibrillation Registry. Circulation 2014;129:1568–1576. [DOI] [PubMed] [Google Scholar]
- 5. Piccini JP, Stevens SR, Lokhnygina Y, Patel MR, Halperin JL, Singer DE, Hankey GJ, Hacke W, Becker RC, Nessel CC, Mahaffey KW, Fox KA, Califf RM, Breithardt G; ROCKET AF Steering Committee & Investigators. Outcomes after cardioversion and atrial fibrillation ablation in patients treated with rivaroxaban and warfarin in the ROCKET AF trial. J Am Coll Cardiol 2013;61:1998–2006. [DOI] [PubMed] [Google Scholar]
- 6.GBD 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1859–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Porter ME. What is value in health care? NEJM 2010;363:2477–2481. [DOI] [PubMed] [Google Scholar]
- 8. Stowell C, Akerman C. Better value in health care requires focusing on outcomes. Harvard Business Review 2015. https://hbr.org/2015/09/better-value-in-health-care-requires-focusing-on-outcomes (27 December 2019).
- 9. Apenteng PN, Murray ET, Holder R, Hobbs FDR, Fitzmaurice DA; UK GARFIELD Investigators and GARFIELD Steering Committee. An international longitudinal registry of patients with atrial fibrillation at risk of stroke (GARFIELD): the UK protocol. BMC Cardiovasc Disord 2013;13:31.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huisman MV, Lip GYH, Diener HC, Dubner SJ, Halperin JL, Ma CS, Rothman KJ, Teutsch C, Zint K, Ackermann D, Clemens A, Bartels DB.. Design and rationale of global registry on long-term oral antithrombotic treatment in patients with atrial fibrillation: a global registry program on long-term oral antithrombotic treatment in patients with atrial fibrillation. Am Heart J 2014;167:329–334. [DOI] [PubMed] [Google Scholar]
- 11. Ezekowitz MD, Connolly S, Parekh A, Reilly PA, Varrone J, Wang S, Oldgren J, Themeles E, Wallentin L, Yusuf S.. Rationale and design of RE-LY: randomized evaluation of long term anticoagulant therapy, warfarin, compared with dabigatran. Am Heart J 2009;157:805–810. [DOI] [PubMed] [Google Scholar]
- 12. Crijns HJGM, Bash LD, Chazelle F, Le Heuzey J-Y, Lewalter T, Lip GYH, Maggioni AP, Martín A, Ponikowski P, Rosenqvist M, Sanders P, Scanavacca M, Bernhardt AA, Unniachan S, Phatak HM, Gitt AK.. RHYTHM-AF: design of an international registry on cardioversion of atrial fibrillation and characteristics of participating centers. BMC Cardiovasc Disord 2012;12:85.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ha ACT, Breithardt G, Camm AJ, Crijns HJ, Fitzmaurice GM, Kowey PR, Le Heuzey JY, Naditch-Brule L, Prystowsky EN, Schwartz PJ, Torp-Pedersen C, Weintraub WS, Dorian P.. Health-related quality of life in patients with atrial fibrillation treated with rhythm control versus rate control: insights from a prospective international registry (Registry on cardiac rhythm disorders assessing the control of atrial fibrillation: RECORD-AF). Circ Cardiovasc Qual Outcomes 2014;7:896–904. [DOI] [PubMed] [Google Scholar]
- 14. Meinertz T, Kirch W, Rosin L, Pittrow D, Willich SN, Kirchhof P, Atrium I.. Management of atrial fibrillation by primary care physicians in Germany: baseline results of the ATRIUM registry. Clin Res Cardiol 2011;100:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andrade JG, Connolly SJ, Dorian P, Green M, Humphries KH, Klein GJ, Sheldon R, Talajic M, Kerr CR.. Antiarrhythmic use from 1991 to 2007: insights from the Canadian Registry of Atrial Fibrillation (CARAF I and II). Heart Rhythm 2010;7:1171–1177. [DOI] [PubMed] [Google Scholar]
- 16. Reiffel JA, Kowey PR, Myerburg R, Naccarelli GV, Packer DL, Pratt CM, Reiter MJ, Waldo AL; AFFECTS Scientific Advisory Committee and Investigators. Practice patterns among United States cardiologists for managing adults with atrial fibrillation (from the AFFECTS Registry). Am J Cardiol 2010;105:1122–1129. [DOI] [PubMed] [Google Scholar]
- 17. Bunch TJ, May HT, Bair TL, Weiss JP, Crandall BG, Osborn JS.. Atrial fibrillation ablation patients have long-term stroke rates similar to patients without atrial fibrillation regardless of CHADS2 score. Heart Rhythm 2013;10:1272–1277. [DOI] [PubMed] [Google Scholar]
- 18. Temporelli PL, Tilz RR, Arbelo E, Dagres N, Laroche C, Crijns HJ, Blomstrom‐Lundqvist C, Kirchhof P, Lip GYH, Boriani G, Pokushalov E, Nakou E, Brugada J, Tavazzi L.. Clinical characteristics of heart failure patients undergoing atrial fibrillation ablation today in Europe. Data from the atrial fibrillation registries of the European Society of Cardiology and the European Heart Rhythm Association. Eur J Heart Fail 2019;21:690.. [DOI] [PubMed] [Google Scholar]
- 19. January CT, Wann L S, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, Heidenreich PA, Murray KT, Shea JB, Tracy CM, Yancy CW.. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Circulation 2019;140:e125–e151. [DOI] [PubMed] [Google Scholar]
- 20. Andrade JG, Verma A, Mitchell LB, Parkash R, Leblanc K, Atzema C, Healey JS, Bell A, Cairns J, Connolly S, Cox J, Dorian P, Gladstone D, McMurtry MS, Nair GM, Pilote L, Sarrazin J-F, Sharma M, Skanes A, Talajic M, Tsang T, Verma S, Wyse DG, Nattel S, Macle L.. 2018 focused update of the Canadian Cardiovascular Society guidelines for the management of atrial fibrillation. Can J Cardiol 2018;34:1371–1392. [DOI] [PubMed] [Google Scholar]
- 21. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GY, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K.. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 22. Calkins H, Gliklich RE, Leavy MB, Piccini JP, Hsu JC, Mohanty S, Lewis W, Nazarian S, Turakhia MP.. Harmonized outcome measures for use in atrial fibrillation patient registries and clinical practice: endorsed by the Heart Rhythm Society Board of Trustees. Heart Rhythm 2019;16:e3–e16. [DOI] [PubMed] [Google Scholar]
- 23. McNamara RL, Brass LM, Drozda JP Jr, Go AS, Halperin JL, Kerr CR, Lévy S, Malenka DJ, Mittal S, Pelosi F Jr, Rosenberg Y, Stryer D, Wyse DG, Radford MJ, Goff DC Jr, Grover FL, Heidenreich PA, Malenka DJ, Peterson ED, Redberg RF; American College of Cardiology; American Heart Association. ACC/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Data Standards on Atrial Fibrillation). Circulation 2004;109:3223–3243. [DOI] [PubMed] [Google Scholar]
- 24. Anker SD, Agewall S, Borggrefe M, Calvert M, Jaime Caro J, Cowie MR, Ford I, Paty JA, Riley JP, Swedberg K, Tavazzi L, Wiklund I, Kirchhof P.. The importance of patient-reported outcomes: a call for their comprehensive integration in cardiovascular clinical trials. Eur Heart J 2014;35:2001–2009. [DOI] [PubMed] [Google Scholar]
- 25. Rumsfeld JS, Alexander KP, Goff DC, Graham MM, Ho PM, Masoudi FA, Moser DK, Roger VL, Slaughter MS, Smolderen KG, Spertus JA, Sullivan MD, Treat-Jacobson D, Zerwic JJ; American Heart Association Council on Quality of Care and Outcomes Research, Council on Cardiovascular and Stroke Nursing, Council on Epidemiology and Prevention, Council on Peripheral Vascular Disease, and Stroke Council. Cardiovascular health: the importance of measuring patient-reported health status: a scientific statement from the American Heart Association. Circulation 2013;127:2233–2249. [DOI] [PubMed] [Google Scholar]
- 26. McNamara RL, Spatz ES, Kelley TA, Stowell CJ, Beltrame J, Heidenreich P, Tresserras R, Jernberg T, Chua T, Morgan L, Panigrahi B, Rosas Ruiz A, Rumsfeld JS, Sadwin L, Schoeberl M, Shahian D, Weston C, Yeh R, Lewin J.. Standardised outcome measurement for patients with coronary artery disease: consensus from the International Consortium for Health Outcomes Measurement (ICHOM). J Am Heart Assoc 2015;4:e001767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Salinas J, Sprinkhuizen S, Ackerson T, Bernhardt J, Davie C, George MG, Gething S, Kelly AG, Lindsay P, Liu L, Martins SC, Morgan L, Norrving B, Ribbers GM, Silver FL, Smith EE, Williams LS, Schwamm LH.. An international standard set of patient-centered outcome measures after stroke. Stroke 2016;47:180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ong WL, Schouwenburg MG, van Bommel ACM, Stowell C, Allison KH, Benn KE, Browne JP, Cooter RD, Delaney GP, Duhoux FP, Ganz PA, Hancock P, Jagsi R, Knaul FM, Knip AM, Koppert LB, Kuerer HM, McLaughin S, Mureau MAM, Partridge AH, Reid DP, Sheeran L, Smith TJ, Stoutjesdijk MJ, Vrancken Peeters MJTFD, Wengström Y, Yip C-H, Saunders C.. A standard set of value-based patient-centered outcomes for breast cancer: the International Consortium for Health Outcomes Measurement (ICHOM) initiative. JAMA Oncol 2017;3:677–685. [DOI] [PubMed] [Google Scholar]
- 29. Zerillo JA, Schouwenburg MG, van Bommel ACM, Stowell C, Lippa J, Bauer D, Berger AM, Boland G, Borras JM, Buss MK, Cima R, Van Cutsem E, van Duyn EB, Finlayson SRG, Hung-Chun Cheng S, Langelotz C, Lloyd J, Lynch AC, Mamon HJ, McAllister PK, Minsky BD, Ngeow J, Abu Hassan MR, Ryan K, Shankaran V, Upton MP, Zalcberg J, van de Velde CJ, Tollenaar R.. An international collaborative standardizing a comprehensive patient-centered outcomes measurement set for colorectal cancer. JAMA Oncol 2017;3:686–694. [DOI] [PubMed] [Google Scholar]
- 30. Morgans AK, van Bommel ACM, Stowell C, Abrahm JL, Basch E, Bekelman JE, Berry DL, Bossi A, Davis ID, de Reijke TM, Denis LJ, Evans SM, Fleshner NE, George DJ, Kiefert J, Lin DW, Matthew AG, McDermott R, Payne H, Roos IA, Schrag D, Steuber T, Tombal B, van Basten JP, van der Hoeven JJ, Penson DF.. Development of a standardised set of patient-centered outcome for advanced prostate cancer: an international effort for a unified approach. Eur Urol 2015;68:891–898. [DOI] [PubMed] [Google Scholar]
- 31. Mazurek M, Huisman MV, Lip G.. Registries in atrial fibrillation: from trials to real-life clinical practice. Am J Med 2017;130:135–145. [DOI] [PubMed] [Google Scholar]
- 32. Fitch K. The RAND/UCLA Appropriateness Method User’s Manual. Santa Monica, CA: Rand; 2001. [Google Scholar]
- 33. Boulkedid R, Abdoul H, Loustau M, Sibony O, Alberti C.. Using and reporting the Delphi method for selecting healthcare quality indicators: a systematic review. PLoS One 2011;6:e20476.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Likert R. A technique for the measurement of attitudes. Arch Psychol 1932;140:1–55. [Google Scholar]
- 35. Lee VS, Kawamoto K, Hess R, Park C, Young J, Hunter C, Johnson S, Gulbransen S, Pelt CE, Horton DJ, Graves KK, Greene TH, Anzai Y, Pendleton RC.. Implementation of a value-driven outcomes program to identify high variability in clinical costs and outcomes and association with reduced cost and improved quality. JAMA 2016;316:1061–1072. [DOI] [PubMed] [Google Scholar]
- 36. Black N. Patient reported outcome measures could help transform healthcare. BMJ 2013;346.. [DOI] [PubMed] [Google Scholar]
- 37. Choudhry NK, Rosenthal MB, Milstein A.. Assessing the evidence for value-based insurance design. Health Affairs 2010;29:1988–1993. [DOI] [PubMed] [Google Scholar]
- 38. Porter ME, Larsson S, Lee TH.. Standardizing patient outcomes measurement. N Engl J Med 2016;374:504–506. [DOI] [PubMed] [Google Scholar]
- 39. Calvert M, Blazeby J, Altman DG, Revicki DA, Moher D, Brundage MD, Consort Pro Group FT.. Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA 2013;309:814–822. [DOI] [PubMed] [Google Scholar]
- 40. Scholtes VA, Terwee CB, Poolman RW.. What makes a measurement instrument valid and reliable? Injury 2011;42:236–240. [DOI] [PubMed] [Google Scholar]
- 41. Kotecha D, Ahmed A, Calvert M, Lencioni M, Terwee CB, Lane DA.. Patient-reported outcomes for quality of life assessment in atrial fibrillation: a systematic review of measurement properties. PLoS One 2016;11:e0165790.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mark DB, Anstrom KJ, Sheng S, Piccini JP, Baloch KN, Monahan KH, Daniels MR, Bahnson TD, Poole JE, Rosenberg Y, Lee KL, Packer DL.. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019;321:1275–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Laliberte F, Nelson WW, Lefebvre P, Schein JR, Rondeau-Leclaire J, Duh MS.. Impact of daily dosing frequency on adherence to chronic medications among nonvalvular atrial fibrillation patients. Adv Ther 2012;29:675–690. [DOI] [PubMed] [Google Scholar]
- 44. Pan X, Kamble S, Burns L, Kawabata H, Mardekian J, Masseria C, Gupta K, Phatak H, Lip G.. What do real world data say about safety and resource use of oral antagonists? Early analysis of newly anticoagulated non-valvular atrial fibrillation patients using either apixaban, dabigatran, rivaroxaban or warfarin. JACC 2016;67(13 suppl):894. doi:10.1016/S0735-1097(16)30895-6. [Google Scholar]
- 45. Hixson-Wallace JA, Dotson JB, Blakey SA.. Effect of regimen complexity on patient satisfaction and compliance with warfarin therapy. Clin Appl Thromb Hemost 2001;7:33–37. [DOI] [PubMed] [Google Scholar]
- 46. Lau DH, Nattel S, Kalman JM, Sanders P.. Modifiable risk factors and atrial fibrillation. Circulation 2017;136: 583–596. [DOI] [PubMed] [Google Scholar]
- 47. Gallagher C, Elliott AD, Wong CX, Rangnekar G, Middeldorp ME, Mahajan R, Lau DH, Sanders P, Hendriks JML.. Integrated care in atrial fibrillation: a systematic review and meta-analysis. Heart 2017;103:1947–1953. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.