Abstract
Activation‐induced cell death (AICD) mediated by the Fas/Fas ligand (FasL) system plays a key role in regulating immune response. Although normal natural killer (NK) cells use this system for their homeostasis, malignant NK cells seem to disrupt the process. Extranodal NK/T‐cell lymphoma, nasal type (ENKL) is a rare but fatal disease, for which novel therapeutic targets need to be identified. We confirmed that ENKL‐derived NK cell lines NK‐YS and Hank1, and primary lymphoma cells expressed procaspase‐8/FADD‐like interleukin‐1β‐converting enzyme (FLICE) modulator and cellular FLICE‐inhibitory protein (c‐FLIP), along with Fas and FasL. Compared with Fas‐sensitive Jurkat cells, NK‐YS and Hank1 showed resistance to Fas‐mediated apoptosis in spite of the same expression levels of c‐FLIP and the death‐inducing signaling complex (DISC) formation. Unexpectedly, the long isoform of c‐FLIP (c‐FLIPL) was coimmunoprecipitated with Fas predominantly in both ENKL‐derived NK cell lines after Fas ligation. Indeed, c‐FLIPL was more sufficiently recruited to the DISC in both ENKL‐derived NK cell lines than in Jurkat cells after Fas ligation. Knockdown of c‐FLIPL per se enhanced autonomous cell death and restored the sensitivity to Fas in both NK‐YS and Hank1 cells. Although ENKL cells are primed for AICD, they constitutively express and efficiently utilize c‐FLIPL, which prevents their Fas‐mediated apoptosis. Our results show that c‐FLIPL could be a promising therapeutic target against ENKL.
Keywords: activation‐induced cell death; c‐FLIPL; extranodal NK/T-cell lymphoma, nasal type; Fas; FasL
Extranodal natural killer (NK)/T‐cell lymphoma, nasal type (ENKL)‐derived NK cells are primed for activation‐induced cell death (AICD) like activated normal NK cells. ENKL‐derived NK cells efficiently recruit the long isoform of c‐FLIP to the death‐inducing signaling complex and show resistance to AICD.

Abbreviations
- c‐FLIP
cellular FLICE‐inhibitory protein
- EBV
Epstein‐Barr virus
- DISC
death‐inducing signaling complex
- FADD
Fas‐associated death domain‐containing protein
- FasL
Fas ligand
- FCM
flow cytometry
- FLICE
FADD‐like interleukin‐1β‐converting enzyme
- HRS
Hodgkin‐Reed‐Sternberg
- Ms
mouse
- NF‐κB
nuclear factor‐kappa B
- NK
natural killer
- PARP
poly(ADP‐ribose) polymerase
- PFA
paraformaldehyde
- RIP
receptor‐interacting protein
- TNF
tumor necrosis factor
- TRAIL
TNF‐related apoptosis‐inducing ligand
1. INTRODUCTION
Natural killer (NK) cells are innate lymphoid cells, which act on the early defenses against tumors and virus‐infected cells.1, 2 To induce target cell death, they elicit cytotoxic protein release following calcium‐dependent granule exocytosis and produce TNF‐α, FasL, and TRAIL.1, 2 Immature NK cells likely use TRAIL‐dependent cytotoxicity rather than FasL‐ or granule release‐dependent cytotoxicity, whereas mature NK cells mainly use the latter two.3 During the immune response, the expanded activated NK cells come to express Fas and eventually undergo AICD mediated by their own secretion of FasL.4, 5 The AICD signaling involves clustering of Fas on the cell surface and recruitment of the FADD to the trimerized intracellular death domain of Fas.6 In turn, FADD recruits procaspase‐8/FLICE, leading to the formation of the DISC.6 Once the DISC is completely formed, procaspase‐8/FLICE is activated by its autolytic cleavage and forms caspase‐8, which initiates the apoptotic signaling pathway.6
Extranodal NK/T‐cell lymphoma, nasal type (ENKL) is rare in North America and Europe but frequently develops in East Asia.7 Most ENKL cells are derived from EBV‐infected NK cells.7 Although l‐asparaginase‐containing chemotherapy has significantly improved the clinical outcome, such therapeutic regimens still fail in approximately 30% of cases, resulting in a fatal course.8 Therefore, novel therapeutic targets are needed for ENKL. Lymphoma cells abundantly express FasL, which could induce surrounding tissue necrosis and vascular damage.9 Although they can also express Fas,9, 10 the alteration of the Fas/FasL system in ENKL remains to be fully elucidated.
Cellular FLICE‐inhibitory protein (c‐FLIP) is known to be a crucial modulator of procaspase‐8/FLICE.11 In particular, the long isoform of c‐FLIP (c‐FLIPL), which is structurally similar to procaspase‐8/FLICE, has the potency to interfere with caspase‐8 activation and restores sensitivity to Fas‐mediated apoptosis in several cancer cells.12, 13, 14, 15 In ENKL‐derived cell lines, cycloheximide treatment was shown to abolish expression of c‐FLIP and to increase sensitivity to several apoptotic stimuli.16 However, cycloheximide reduces expression not only of c‐FLIPL but also of the short isoform (c‐FLIPS) and RIP kinases, which are involved in caspase‐8‐mediated pathways.16, 17, 18 In addition, the protein synthesis inhibitor also induces cell death in a Fas‐independent manner.18 Although c‐FLIPL might function in ENKL, its direct evidence for Fas resistance has not yet been provided. Hence, we investigated the biological behavior of c‐FLIPL in ENKL cells.
2. MATERIALS AND METHODS
2.1. Antibodies
We used the following primary Abs: anti‐Fas mouse (Ms) IgM 7C11 (Beckman Coulter) or CH‐11 (MBL); anti‐Fas Ms IgG1 ZB4 (Beckman Coulter); anti‐Fas Ms IgG1 UB2 (Beckman Coulter); anti‐Fas Ms IgG1 G‐9 (Santa Cruz Biotechnology); anti‐Fas rabbit Ab C‐20 (Santa Cruz Biotechnology); anti‐FasL Ms IgG1 NOK‐1 (BD Biosciences); anti‐FasL rabbit Ab Q‐20 (Santa Cruz Biotechnology); anti‐FADD Ms IgG1 FD19 (Santa Cruz Biotechnology); anti‐FADD rabbit Ab (Cell Signaling Technology); anti‐caspase‐8 Ms IgG1 1C12 (Cell Signaling Technology); anti‐caspase‐8 rabbit IgG (GeneTex); anti‐c‐FLIPL Ms IgG3 5D8 (Santa Cruz Biotechnology); anti‐c‐FLIPγ/δ rabbit Ab (Sigma‐Aldrich); anti‐c‐FLIP rabbit IgG D5J1E (Cell Signaling Technology); anti‐c‐FLIP Ms IgG1 NF6 (AdipoGen); anti‐RIP Ms IgG2a 38 (BD Bioscience); anti‐BCL2 Ms IgG1 C‐2 (Santa Cruz Biotechnology); anti‐BCL‐xL rabbit IgG E18 (Abcam); anti‐MCL1 rabbit IgG Y37 (Abcam); anti‐caspase‐3 Ms IgG1 31A1067 (Santa Cruz Biotechnology); anti‐cleaved PARP1 Ms IgG2b 194C1439 (Santa Cruz Biotechnology); and anti‐β‐actin Ms IgG2a AC74 (Sigma‐Aldrich).
2.2. Cell lines
Two EBV‐positive ENKL‐derived NK cell lines, NK‐YS and Hank1, were kindly provided by Dr Junjiro Tsuchiyama (Kawasaki Medical School, Kurashiki, Japan) and Dr Masao Seto (Kurume University, Kurume, Japan), respectively. Human T‐cell leukemia line Jurkat purchased from ATCC was used as a positive control for Fas‐mediated apoptosis, because this cell line efficiently undergoes apoptosis by Fas ligation with agonistic Ab 7C11 (Beckman Coulter) and CH‐11 (MBL). These cell lines were maintained as described previously.19, 20
2.3. Detection of Fas and FasL by FCM
We used FCM to detect Fas and FasL in each cell line. To confirm cell surface expression of Fas, 1 × 106 cells were stained with FITC‐conjugated UB2 (Beckman Coulter) for 30 minutes after blocking of Fcγ receptor on NK cell lines with human IgG (Sigma‐Aldrich). Intracytoplasmic expression of FasL was evaluated with NOK‐1 (BD Bioscience) and FITC‐conjugated goat anti‐Ms IgG (Beckman Coulter) using the PFA/saponin procedure as described previously.21
2.4. Cytokine assays
We further measured cytokine concentration in culture supernatants of Jurkat and Hank1 (3 × 106 cells/mL) in a time course (3, 6, and 12 hours) because the doubling time for each cell line was estimated to be 26 to 28 hours. Detection of FasL, TRAIL, and TNF‐α was carried out using Quantikine ELISA Kit (R&D Systems) according to the manufacturer’s instructions.
2.5. Immunohistochemistry for Fas, FasL, and c‐FLIP on clinical samples
To evaluate the expression of Fas, FasL, and c‐FLIP, immunohistochemistry was carried out on formalin‐fixed, paraffin‐embedded specimens from ENKL patients. After deparaffinization and rehydration, endogenous peroxidase was blocked with hydrogen peroxide (DAKO Japan). Heat‐induced antigen retrieval was carried out using 0.05% citraconic anhydride, pH 7.4 (Nisshin EM Co., Ltd.). Primary Abs were C‐20 (Santa Cruz Biotechnology) for Fas, Q‐20 (Santa Cruz Biotechnology) for FasL, and NF6 (Adipogen) for c‐FLIP. The Ab signals were enhanced using EnVision (DAKO Japan). Definition of positivity was made when more than 30% of the tumor cells were stained in the CD56‐positive area. The results were determined by outsourcing pathology services (Kotobiken Medical Laboratories). All specimens were obtained at the biopsy undertaken for initial diagnosis of ENKL, according to the WHO classification 20177 at Juntendo University Hospital. This study protocol was approved by the Ethics Review Board of Juntendo University.
2.6. Western blot analysis
Cells were dissolved on ice in 250 µL ice‐cold lysis buffer (CelLytic M; Sigma‐Aldrich) containing protease inhibitor (Sigma‐Aldrich) as described previously.19, 21 Equal amounts of protein (50 µg/well) were separated on a discontinuous SDS‐10% polyacrylamide gel and blotted onto a nitrocellulose membrane (Bio‐Rad). Western blot analysis was carried out using the following primary Abs: G‐9 (Santa Cruz Biotechnology) for Fas; FD19 (Santa Cruz Biotechnology) and anti‐FADD rabbit Ab (Cell Signaling Technology) for FADD; 1C12 (Cell Signaling Technology) and anti‐caspase‐8 rabbit Ab (GeneTex) for procaspase‐8/FLICE and caspase‐8; 5D8 (Santa Cruz Biotechnology) for c‐FLIPL; anti‐c‐FLIPγ/δ rabbit Ab (Sigma‐Aldrich) for c‐FLIPS; D5J1E (Cell Signaling Technology) for both forms and the cleaved c‐FLIPL; 38 (BD Bioscience) for RIP1; C‐2 (Santa Cruz Biotechnology) for BCL2; E18 (Abcam) for BCL‐xL; Y37 (Abcam) for MCL1; 31A1067 (Santa Cruz Biotechnology) for procaspase‐3 and the cleaved forms; 194C1439 (Santa Cruz Biotechnology) for cleaved PARP1; and AC74 (Sigma‐Aldrich) for β‐actin. Antibody signals were visualized using Western Blue (Promega). We quantified the expression levels by comparison with each β‐actin level using ImageJ version 1.52k (://imagej.nih.gov/ij/).
2.7. Induction of Fas‐mediated apoptosis
Each cell line was washed twice with PBS and incubated (5 × 105 cells/mL) with 2.0 μg/mL Fas agonistic 7C11 (Beckman Coulter) or CH‐11 (MBL), Fas antagonistic ZB4 (Beckman Coulter), or control Ms IgM (Beckman Coulter) for 1 hour at 37°C in fresh medium. Cell viability was assessed using the MTT assay (Promega). The viability was calculated as the percentage absorbance of formazan products, that is, (OD570 7C11 treated / OD570 control Ms IgM treated) × 100%. We also evaluated the cell death process by FITC‐conjugated annexin V‐binding and 7‐AAD rejection assays (Beckman Coulter). Apoptotic cell change was assessed on a Cell Lab Quanta SC flow cytometer (Beckman Coulter). During our experimental period, 7C11 (Beckman Coulter) became unavailable due to discontinuation. Therefore, the assessment of cleavage of procaspase‐3 and PARP1 by western blot analyses was undertaken after Fas ligation with CH‐11 (MBL).
2.8. Analysis of DISC formation
Briefly, cells were incubated with 2.0 μg/mL 7C11 (Beckman Coulter) or control Ms IgM (Beckman Coulter) for 10 minutes at 37°C. They were washed twice, harvested, and subjected to the analysis. Whole‐cell lysate (200 μg total protein) was added to 1.0 μg C‐20 (Santa Cruz Biotechnology) or 1C12 (Cell Signaling Technology) and adjusted to 150 μL of volume with lysis buffer (Sigma‐Aldrich). Rabbit or mouse serum‐saturated protein G‐Sepharose beads (20 μL) (GE Healthcare) were added to each lysate, and immunoprecipitation was carried out for 1 our while rotating at 4°C. After incubation, the beads were washed three times with ice‐cold lysis buffer and boiled for 3 minutes with 20 μL loading buffer. The supernatant was electrophoresed and analyzed by western blot analysis.
Confocal fluorescence imaging was also undertaken. After incubation with 7C11 (Beckman Coulter) or control Ms IgM (Beckman Coulter), NK‐YS, Hank1, and Jurkat cells were fixed and permeabilized using the PFA/saponin procedure and stained with primary Abs for 1 hour at room temperature. The primary Abs were as follows: C‐20 (Santa Cruz Biotechnology) for Fas; 1C12 (Cell Signaling) for procaspase‐8/FLICE and caspase‐8; and 5D8 (Santa Cruz Biotechnology) for c‐FLIPL. After staining with primary Abs, cells were washed twice with PBS containing 0.03% saponin and 2% goat serum and further incubated with Alexa 594‐conjugated goat anti‐rabbit Ab (SouthernBiotech) and Alexa 488‐conjugated goat anti‐Ms Ab (SouthernBiotech). After washing with the saponin‐containing PBS, nuclei were stained DAPI (Invitrogen). Confocal microscopic analysis with Cytospin preparations was undertaken on a Leica confocal scanning microscope (Leica Microsystems).
2.9. RNA interference for c‐FLIPL
The siRNA duplexes against c‐FLIPL (Silencer Select s444339) and control scrambled siRNA (Silencer s4390843) were synthesized by Applied Biosystems. Transfection of siRNA was carried out using Nucleofector I (Lonza) as described previously.19 NK‐YS and Hank1 cells (1 × 106 cells) were resuspended in 100 μL Solution R and T with 200 nmol/L each siRNA, respectively. After 24 hours, each treated cell line (2 × 106 cells) was subjected to western blot analysis and apoptosis assay, respectively.
2.10. Statistical analysis
The appearance of fusion signals between Fas and c‐FLIPL was counted in each 100 cells on confocal microscopy. Differences among Jurkat, NK‐YS, and Hank1 cells were statistically evaluated. Moreover, the proportion of annexin V‐positive cells was compared after knockdown of c‐FLIPL in NK‐YS and Hank1 cells. These data were obtained from 3 separate experiments. Differences in the mean values were determined using Student’s t test using SPSS Statistics software (IBM Japan). All P values were 2‐sided, and values <.05 were considered to be significant.
3. RESULTS
3.1. ENKL cells express c‐FLIP along with Fas and FasL
Flow cytometry confirmed that NK‐YS and Hank1 cells coexpressed Fas and FasL (Figure 1A). We also detected secreted FasL but not TRAIL in supernatant of Hank1 cell culture (Figure 1B). Western blot analysis showed that they also had the components of the DISC, including Fas, FADD, procaspase‐8/FLICE, c‐FLIPL, and c‐FLIPS (Figure 1C). The expression levels of these molecules in both ENKL‐derived NK cell lines were approximately the same as those in Fas‐sensitive Jurkat cells (Figure 1C). Coexpression of Fas and FasL was also confirmed in clinical samples of ENKL (Figure 1D). Immunohistochemistry was carried out in diagnostic specimens from a total of nine cases (Table S1). All nine cases expressed FasL. Eight of them (89%) expressed Fas simultaneously. Furthermore, seven cases (78%) expressed c‐FLIP along with Fas and FasL. Although the results indicate that most ENKL cells were ready to undergo AICD, they were indeed surviving and proliferating. This situation raises the possibility that they should have mechanisms to escape AICD.
Figure 1.

Extranodal natural killer (NK)/T‐cell lymphoma, nasal type (ENKL) expresses cellular Fas‐associated death domain‐containing protein (FADD)‐like interleukin‐1β‐converting enzyme (FLICE)‐inhibitory protein (c‐FLIP) along with Fas and Fas ligand (FasL). A, Flow cytometry showing that ENKL‐derived NK cell lines, NK‐YS and Hank1, clearly expressed cell surface Fas and intracytoplasmic FasL. B, FasL, tumor necrosis factor (TNF)‐related apoptosis‐inducing ligand (TRAIL), and TNF‐α levels in culture supernatants of Hank1 and Jurkat. Each cytokine concentration was measured three times and the mean value was represented in the time‐course graph. Hank1 secretes FasL and abundant TNF‐α. C, Western blot analysis detected Fas, FADD, procaspase‐8/FLICE, and long and short forms of c‐FLIP (c‐FLIPL and c‐FLIPS, respectively) at approximately the same levels in NK‐YS, Hank1, and Jurkat cells. D, Immunohistochemistry for Fas, FasL, and c‐FLIP was carried out using diagnostic specimens of 9 cases of ENKL. Simultaneous expression of Fas, FasL, and c‐FLIP was observed in 7 of 9 examined cases (78%). Two representative cases (UPN1 and UPN2) are presented
3.2. ENKL‐derived NK cell lines show resistance to Fas‐mediated apoptosis
We next evaluated the susceptibility to Fas‐mediated apoptotic stimuli in NK‐YS and Hank1 cells. To eliminate the effects of humoral inhibitory factors, we undertook direct Fas ligation with agonistic 7C11 in both NK cell lines. The MTT assay showed that the stimulation with 7C11, but not with control Ms IgM or antagonistic ZB4, decreased the viability of each cell line (Figure 2A). Although the effect was statistically significant among the 3 lines, the viability was markedly decreased in Fas‐sensitive Jurkat cells (Figure 2A). Flow cytometry confirmed that more than 40% of the cells were positive for annexin V, whereas most NK‐YS and Hank1 cells failed to show apoptotic changes even after Fas ligation (Figure 2B,C). Although NK‐YS and Hank1 cells might have reduced their ability to proliferate after Fas ligation, they clearly showed resistance to direct Fas‐mediated apoptotic stimuli.
Figure 2.

Extranodal natural killer (NK)/T‐cell lymphoma, nasal type cells show resistance to Fas‐mediated apoptosis. A, MTT assay showed that the stimulation of Fas with agonistic 7C11 but not with control mouse (Ms) IgM or antagonistic ZB4 decreased cell viability, particularly in Fas‐sensitive Jurkat cells. Although the viability of Jurkat cells was decreased to 10% 1 h after Fas ligation with 7C11, those of NK‐YS and Hank1 stayed at approximately 50% and 70%, compared with each control, respectively. The effects were statistically significant (Jurkat, *P < .001; NK‐YS, **P < .001; Hank1, ***P < .001; Student’s t test). B, Flow cytometry detected that only Fas‐sensitive Jurkat cells increased annexin V‐positive cell fractions 1 h after Fas ligation with 7C11. C, More than 40% of Jurkat cells showed apoptotic changes, whereas most NK‐YS and Hank1 cells failed to increase apoptotic changes. Only events in Jurkat cells reached significance (*P < .001, Student's t test)
3.3. DISC formation in NK‐YS, Hank1, and Jurkat cells
To determine whether Fas‐mediated signaling is functional, we investigated DISC formation in NK‐YS and Hank1 cells. Ten minutes after Fas ligation with 7C11, FADD and the cleaved forms of caspase‐8 were coimmunoprecipitated with Fas in NK‐YS and Hank1 as well as in Jurkat cells (Figure 3A). Colocalization of these molecules was also visualized on confocal microscopy (Figure 3B). Similar to Fas‐sensitive Jurkat cells, yellow fusion signals between Fas and caspase‐8 were detected in both ENKL‐derived NK cell lines 10 minutes after treatment with 7C11 but not with control Ms IgM (Figure 3B). These findings indicate that the DISC was equally generated among the 3 lines after Fas ligation.
Figure 3.
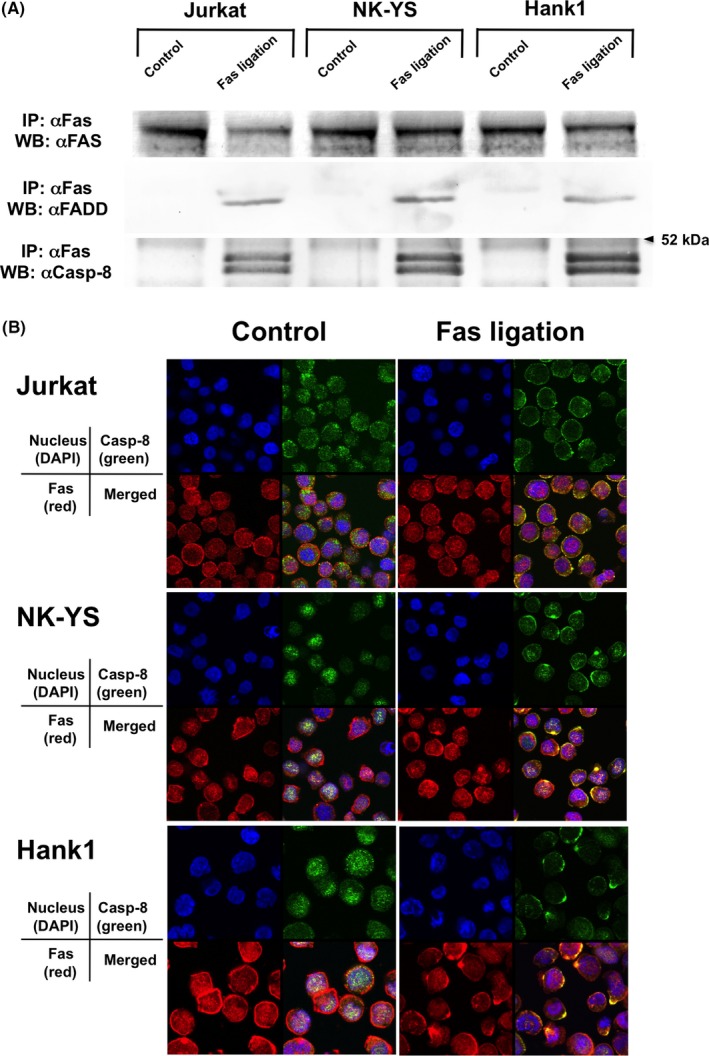
Death‐inducing signaling complex was generated equally among both extranodal natural killer (NK)/T‐cell lymphoma, nasal type cells and Fas‐sensitive Jurkat cells after Fas ligation. A, Immunoprecipitation (IP) and western blot (WB) analyses similarly detected Fas‐associated death domain‐containing protein (FADD) and the cleaved forms of caspase‐8 (Casp‐8), which were coimmunoprecipitated with Fas after treatment with 7C11 but not with control mouse (Ms) IgM in NK‐YS, Hank1, and Fas‐sensitive Jurkat cells. B, Confocal microscopy showed the fusion yellow signals of Fas (red) and procaspase‐8/FLICE (Casp‐8, green) at the cell membrane after treatment with 7C11, but not with control Ms IgM in the 3 cell lines
3.4. c‐FLIPL is efficiently recruited to DISC in ENKL cells
Although the DISC formation was similarly initiated among NK‐YS, Hank1, and Jurkat cells, a cleaved form of c‐FLIPL was coimmunoprecipitated with Fas and caspase‐8, predominantly in both ENKL‐derived NK cell lines after Fas ligation (Figure 4A). Despite the same expression levels of c‐FLIPL, the intracellular behavior of this molecule in these ENKL‐derived NK cell lines seems to be different from that in Fas‐sensitive Jurkat cells. The colocalization of Fas and c‐FLIPL were frequently detected in NK‐YS and Hank1 after Fas ligation (Figure 4B). Furthermore, yellow fusion signals between Fas and c‐FLIPL were significantly increased in both ENKL‐derived NK cell lines, compared with in Jurkat (Jurkat vs NK‐YS, *P < .001; Jurkat vs Hank1, **P < .001; Student's t test) (Figure 4C). These results suggest that efficient recruitment of c‐FLIPL to the DISC should affect the proximal Fas‐mediated signaling predominantly in NK‐YS and HANK1 cells.
Figure 4.

Long isoform of cellular FLICE‐inhibitory protein (c‐FLIPL) is efficiently recruited to the death‐inducing signaling complex in extranodal natural killer (NK)/T‐cell lymphoma, nasal type (ENKL) cells after Fas ligation. A, A cleaved form of c‐FLIPL (indicated by red arrowhead) was coimmunoprecipitated with Fas predominantly in both ENKL cells after Fas ligation. Cleaved c‐FLIPL was also bound to caspase‐8 after Fas ligation predominantly in both ENKL cells. B, Confocal microscopy also confirmed the colocalization of Fas (red) and c‐FLIPL (green) predominantly at the cell membrane in both NK‐YS and Hank1, compared with Jurkat cells. C, Yellow fusion signals between Fas and c‐FLIPL were counted in each 100 cells, in triplicate. Compared with Jurkat cells, the mean values were significantly high in NK‐YS and Hank1 (Jurkat vs NK‐YS, *P < .001; Jurkat vs Hank1, **P < .001; Student’s t test). IP, immunoprecipitation; WB, western blot
3.5. c‐FLIPL plays a critical role in resistance to Fas‐mediated apoptosis in ENKL cells
We then evaluated Fas‐mediated apoptosis in the presence or absence of c‐FLIPL in both ENKL‐derived NK cell lines. RNA interference for c‐FLIPL effectively knocked down the expression of c‐FLIPL in NK‐YS and Hank1 cells 24 hours after the transfection (Figure 5A). The RNA interference did not alter the expression levels of c‐FLIPS, RIP1, or antiapoptotic molecules including BCL2, BCL‐xL, and MCL1 (Figure 5A). Although the cells transfected with control siRNA hardly showed apoptotic changes even after Fas ligation, knockdown of c‐FLIPL per se significantly increased annexin V‐positive fractions in NK‐YS and Hank1 cells (Figure 5B,C) (*P = .005 in NK‐YS and # P = .002 in Hank1; Student’s t test). Furthermore, the suppression of c‐FLIPL expression remarkably sensitized both ENKL‐derived NK cell lines to Fas stimuli. Flow cytometry detected that more than 50% of cells became positive for annexin V in NK‐YS and Hank1 cell lines (Figure 5B). This effect was statistically significant (**P < .001 in NK‐YS and ## P < .001 in Hank1; Student’s t test) (Figure 5C). Fas ligation indeed promoted the processing of procaspase‐3 in both ENKL‐derived NK cell lines (Figure 5D). Although PARP1 seems to be processed by caspase‐3 even after RNA interference in Hank1, knockdown of c‐FLIPL clearly accelerated procaspase‐3 processing and increased the cleavage of PARP1 in both ENKL‐derived NK cell lines (Figure 5D). These results indicated that c‐FLIPL plays a critical role in resistance to Fas‐mediated apoptosis in ENKL cells.
Figure 5.

Long isoform of cellular FLICE‐inhibitory protein (c‐FLIPL) plays a critical role in resistance to Fas‐mediated apoptosis in extranodal natural killer (NK)/T‐cell lymphoma, nasal type (ENKL) cells. A, RNA interference for c‐FLIPL showed effective knockdown of c‐FLIPL in NK‐YS and Hank1 cells, while the expression levels of the short isoform (c‐FLIPS), receptor‐interacting protein (RIP)1, and antiapoptotic molecules including BCL2, BCL‐xL, and MCL1 were unchanged. B, Although the cells transfected control (CNT) siRNA hardly showed apoptotic changes even after Fas ligation, knockdown of c‐FLIPL per se the increased annexin V‐positive fraction even after treatment with mouse (Ms) IgM in both ENKL cell lines. Fas ligation obviously enhanced the effect. Flow cytometry detected that more than 50% of both ENKL‐derived NK cell lines were positive for annexin V. C, These events were statistically significant (CNT siRNA‐7C11 vs c‐FLIPL siRNA‐Ms IgM, *P = .005 in NK‐YS and # P = .002 in Hank1; c‐FLIPL siRNA‐Ms IgM vs c‐FLIPL siRNA‐7C11, **P < .001 in NK‐YS and ## P < .001 in Hank1; Student’s t test). D, Although Fas ligation with CH‐11 promoted the processing of procaspase‐3, knockdown of c‐FLIPL clearly accelerated this process and increased the cleavage of poly (ADP‐ribose) polymerase (PARP)1 in NK‐YS and Hank1 cells. Only this experiment was carried out using CH‐11 for Fas ligation
4. DISCUSSION
In this study, we showed that most ENKL cells coexpressed Fas and FasL and were in a similar state to activated normal NK cells in AICD. Nevertheless, ENKL‐derived NK cell lines NK‐YS and Hank1 showed resistance to Fas‐mediated apoptosis in spite of the DISC formation. Previous studies indicated that loss‐of‐function mutations of the FAS gene are considered a major mechanism of resistance to Fas‐mediated apoptosis in ENKL.22, 23 Here we confirmed the direct evidence of c‐FLIPL for the resistance to AICD in ENKL and provided another mechanism.
Coexpression of Fas and FasL was also observed in HRS cells.13 Although the normal counterpart of HRS cells should undergo the cell‐death fate in germinal centers, HRS cells can survive partially through the function of c‐FLIPL.12, 13 In activated normal NK cells, the expression of c‐FLIP seems to be transiently upregulated but subsequently suppressed.24 The prolonged expression of c‐FLIPL in activated normal NK cells has been shown to have an advantage in survival during the AICD process.25 The present data and the previous findings suggest that c‐FLIPL could be a key regulator of the resistance to AICD in NK cells. Our previous observation had shown that EBV infection of NK cells conferred resistance to cell stress such as DNA damage and starvation.26 When the stress was induced, only EBV‐infected NK cells maintained the expression levels of c‐FLIPL, partially through the activation of NF‐κB.26 The presence of EBV could contribute to the prolonged expression of c‐FLIPL under conditions of proapoptotic stress in NK cells.
We further found that c‐FLIPL was more sufficiently recruited to the DISC in Fas‐resistant ENKL‐derived NK cell lines than in Fas‐sensitive Jurkat in spite of the same expression levels. After Fas ligation, the DISC formation is similarly initiated among NK‐YS, Hank1, and Jurkat cells. Nevertheless, both ENKL‐derived NK cell lines could have an advantageous system for the recruitment of c‐FLIPL into the DISC. Our study clearly showed that disparities in the intracellular trafficking, rather than the expression levels, should determine functional differences of c‐FLIPL in the inhibition of Fas‐mediated signaling. In Ms T cells, mitogenic stimulation seems to induce the posttranslational modification of procaspase‐8/FLICE and alters the intracellular localization.27 Although posttranslational modifications of c‐FLIPL remain unknown, differences in the intracellular localization might be acquired during the tumorigenic process of ENKL.
In conclusion, our findings indicate that ENKL‐derived NK cell lines are similar to activated normal NK cells in being primed for AICD. However, they constitutively express and efficiently utilize c‐FLIPL, which prevents their Fas‐mediated apoptosis (Figure 6). The specific downregulation of c‐FLIPL accelerates the autonomous cell death in ENKL cells. Therefore, c‐FLIPL could be a promising therapeutic target against ENKL.
Figure 6.

Predicted mechanisms of resistance to activation‐induced cell death (AICD) in extranodal natural killer (NK)/T‐cell lymphoma, nasal type (ENKL) cells. Although ENKL cells are similar to activated normal NK cells in being primed for AICD, they efficiently utilize the long isoform of cellular Fas‐associated death domain‐containing protein (FADD)‐like interleukin‐1β‐converting enzyme (FLICE)‐inhibitory protein (c‐FLIPL), which prevents their Fas‐mediated apoptosis, and can survive in spite of the coexpression of Fas and Fas ligand (FasL)
DISCLOSURE
All authors declare no conflict of interest.
Supporting information
ACKNOWLEDGEMENTS
We thank Dr Junjiro Tsuchiyama (Kawasaki Medical School, Kurashiki, Japan) and Dr Masao Seto (Kurume University, Kurume, Japan) for providing NK‐YS and Hank1, respectively. We also thank Ms Asami Yamada and Ms Junko Asano for technical assistance.
Masuda A, Isobe Y, Sugimoto K, Yoshimori M, Arai A, Komatsu N. Efficient recruitment of c‐FLIPL to the death‐inducing signaling complex leads to Fas resistance in natural killer‐cell lymphoma. Cancer Sci. 2020;111:807–816. 10.1111/cas.14296
Azuchi Masuda and Yasushi Isobe contributed equally to this work.
REFERENCES
- 1. Sun JC, Lanier LL. NK cell development, homeostasis and function: parallels with CD8+ T cells. Nat Rev Immunol. 2011;11:645‐657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krzewski K, Coligan JE. Human NK cell lytic granules and regulation of their exocytosis. Front Immunol. 2012;3:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zamai L, Ahmad M, Bennett IM, et al. Natural killer (NK) cell‐mediated cytotoxicity: differential use of TRAIL and Fas ligand by immature and mature primary human NK cells. J Exp Med. 1998;188:2375‐2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ida H, Robertson MJ, Voss S, et al. CD94 ligation induces apoptosis in a subset of IL‐2‐stimulated NK cells. J Immunol. 1997;159:2154‐2160. [PubMed] [Google Scholar]
- 5. Felices M, Lenvik TR, Ankarlo DEM, et al. Functional NK cell repertoires are maintained through IL‐2Rα and Fas ligand. J Immunol. 2014;192:3889‐3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lavrik IN, Krammer PH. Regulation of CD95/Fas signaling at the DISC. Cell Death Differ. 2012;19:36‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chan JKC, Quintanilla‐Martinez L, Ferry JA, et al. Extranodal NK/T‐cell lymphoma, nasal type In: Swerdlow SH, Campo E, Harris NL, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed Lyon: IARC; 2017:368‐371. [Google Scholar]
- 8. Yamaguchi M, Kwong Y‐L, Kim WS, et al. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T‐cell lymphoma, nasal type: the NK‐cell Tumor Study Group Study. J Clin Oncol. 2011;29:4410‐4416. [DOI] [PubMed] [Google Scholar]
- 9. Ohshima K, Suzumiya J, Shimazaki K, et al. Nasal T/NK cell lymphomas commonly express perforin and Fas ligand: important mediators of tissue damage. Histopathology. 1997;31:444‐450. [DOI] [PubMed] [Google Scholar]
- 10. Ng CS, Lo STH, Chan JKC. Peripheral T and putative natural killer cell lymphomas commonly coexpress CD95 and CD95 Ligand. Hum Pathol. 1999;30:48‐53. [DOI] [PubMed] [Google Scholar]
- 11. Shirley S, Micheau O. Targeting c‐FLIP in cancer. Cancer Lett. 2013;332:141‐150. [DOI] [PubMed] [Google Scholar]
- 12. Mathas S, Lietz A, Anagnostopoulos I, et al. c‐FLIP mediates resistance of Hodgkin/Reed‐Sternberg cells to death receptor‐induced apoptosis. J Exp Med. 2004;199:1041‐1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dutton A, O'Neil JD, Milner AE, et al. Expression of the cellular FLICE‐inhibitory protein (c‐FLIP) protects Hodgkin’s lymphoma cells from autonomous Fas‐mediated death. Proc Natl Acad Sci USA. 2004;101:6611‐6616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mezzanzanica D, Balladore E, Turatti F, et al. CD95‐mediated apoptosis is impaired at receptor level by cellular FLICE‐inhibitory protein (long form) in wild‐type p53 human ovarian carcinoma. Clin Cancer Res. 2004;10:5202‐5214. [DOI] [PubMed] [Google Scholar]
- 15. Wilson TR, McLaughlin KM, McEwan M, et al. c‐FLIP: a key regulator of colorectal cancer cell death. Cancer Res. 2007;67:5754‐5762. [DOI] [PubMed] [Google Scholar]
- 16. Jeon YK, Kim H, Park SO, et al. Resistance to Fas‐mediated apoptosis is restored by cycloheximide through the downregulation of cellular FLIPL in NK/T‐cell lymphoma. Lab Invest. 2005;85:874‐884. [DOI] [PubMed] [Google Scholar]
- 17. Fulda S, Meyer E, Debatin KM. Metabolic inhibitors sensitize for CD95 (APO‐1/Fas)‐induced apoptosis by down‐regulating Fas‐associated death domain‐like interleukin 1‐converting enzyme inhibitory protein expression. Cancer Res. 2000;60:3947‐3956. [PubMed] [Google Scholar]
- 18. Kadohara K, Nagumo M, Asami S, et al. Caspase‐8 mediates mitochondrial release of pro‐apoptotic proteins in a manner independent of its proteolytic activity in apoptosis induced by the protein synthesis inhibitor acetoxycycloheximide in human leukemia Jurkat cells. J Biol Chem. 2009;284:5478‐5487. [DOI] [PubMed] [Google Scholar]
- 19. Kanemitsu N, Isobe Y, Masuda A, et al. Expression of Epstein‐Barr virus‐encoded proteins in extranodal NK/T‐cell lymphoma, nasal type (ENKL): differences in biologic and clinical behaviors of LMP1‐positive and ‐negative ENKL. Clin Cancer Res. 2012;18:2164‐2172. [DOI] [PubMed] [Google Scholar]
- 20. Sugimoto K, Tamayose K, Sasaki M, Hayashi K, Oshimi K. Low‐dose doxorubicin‐induced necrosis in Jurkat cells and its acceleration and conversion to apoptosis by antioxidants. Br J Haematol. 2002;118:229‐238. [DOI] [PubMed] [Google Scholar]
- 21. Isobe Y, Sugimoto K, Yang L, et al. Epstein‐Barr virus infection of human natural killer cell lines and peripheral blood natural killer cells. Cancer Res. 2004;64:2167‐2174. [DOI] [PubMed] [Google Scholar]
- 22. Shen L, Liang ACT, Lu L, et al. Frequent deletion of Fas gene sequences encoding death and transmembrane domains in nasal natural killer/T‐cell lymphoma. Am J Pathol. 2002;161:2123‐2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takakuwa T, Dong Z, Nakatsuka S, et al. Frequent mutations of Fas gene in nasal NK/T cell lymphoma. Oncogene. 2002;21:4702‐4705. [DOI] [PubMed] [Google Scholar]
- 24. Haux J, Johnsen A‐C, Steinkjer B, Egeberg K, Sundan A, Espevik T. The role of interleukin‐2 in regulating the sensitivity of natural killer cells for Fas‐mediated apoptosis. Cancer Immunol Immunother. 1999;48:139‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mirandola P, Ponti C, Gobbi G, et al. Activated human NK and CD8+ T cells express both TNF‐related apoptosis‐inducing ligand (TRAIL) and TRAIL receptors but are resistant to TRAIL‐mediated cytotoxicity. Blood. 2004;104:2418‐2424. [DOI] [PubMed] [Google Scholar]
- 26. Isobe Y, Sugimoto K, Matsuura I, Takada K, Oshimi K. Epstein‐Barr virus renders the infected natural killer cell line, NKL resistant to doxorubicin‐induced apoptosis. Br J Cancer. 2008;99:1816‐1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. O'Reilly LA, Divisekera U, Newton K, et al. Modifications and intracellular trafficking of FADD/MORT1 and caspase‐8 after stimulation of T lymphocytes. Cell Death Differ. 2004;11:724‐736. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


