Dear Editor,
Uscanga‐Palomeque et al reported that PKHB1, a peptide analog of 4N1K with increased stability in serum, directly induced T‐leukemic cell death by engaging the CD47 receptor.1 CD47 is commonly upregulated in tumor cells, and its binding to macrophage SIRPα inhibits phagocytosis and engagement of the innate immune response.2 It is well documented that CD47 ligation with antibodies can invoke cell death in the absence of effector cells.3, 4 The potential to convert CD47 from a protumor antigen to one that is antitumor has attracted the search for CD47‐targeted therapeutics. In this vein, mapping studies of thrombospondin, a natural ligand of CD47, led to discovery of the VVM‐motif containing peptides, 4N1 and 7N3, that mediate binding to the CD47 IgV domain. To improve solubility, N/C‐terminal lysines were added to yield the peptide known as 4N1K, with the sequence KRFYVVMWKK. 5 PKHB1 has an identical sequence, but with terminal D‐lysines instead of the L‐enantiomer found in 4N1K.6
It was reported in 2001 that 4N1K mediated significant biological effects that were CD47‐independent.7, 8 In 2014, we published a study that highlighted the propensity of 4N1K to bind nonspecifically to proteins in vitro as well as to the plasma membrane, and that this phenomenon was a major contributor to the cellular effects mediated by 4N1K.9 This included the apparent “induction” of integrin activation that was presumed to occur through CD47 ligation by 4N1K. We showed that 4N1K treatment promoted binding of several antibodies to Jurkat T‐leukemic cells, including the binding of β1‐integrin specific antibodies to a Jurkat derivative that expressed no β1‐integrins.9 We obtained similar results using only secondary antibodies, leading us to posit that 4N1K mediated CD47‐independent binding to a variety of Ig domains on the cell surface. Indeed, we used cell‐free assays to show that different antibodies readily interacted with 4N1K that was immobilized on plastic. Thus, we concluded that 4N1K had significant CD47‐independent effects and, as such, any experiment using this and similar peptides should be accompanied by stringent negative controls, such as CD47 knockdown or knockout cells. Another group working on microglia biology has since corroborated our results on 4N1K.10 Given that 4N1K is known to have substantial CD47‐independent effects, it is conceivable that PKHB1 may as well.
One aspect we had not investigated previously was CD47‐mediated cell death.9 As such, we evaluated WT and CD47−/− Jurkat and MOLT4 cell lines to evaluate the ability of PKHB1 and 4N1K to induce T‐leukemic cell death. Here, we report that both PKHB1 and 4N1K induced significant cell death in a CD47‐independent manner, ascertained in two ways: (i) the forward and side scatter profile of WT and CD47−/− cells treated with PKHB1 or 4N1K was similar, presenting a population with decreased size and increased granularity, characteristic of cells undergoing cell death (Figure 1A); and (ii) there was significant annexin V binding when CD47−/− cells were treated with 4N1K or PKHB1 (Figure 1B). In contrast, and as previously reported,4 the anti‐CD47 antibody CC2C6 induced cell death in WT, but not in CD47−/−, Jurkat cells. We note with interest that MOLT4 cells were not sensitive to CC2C6‐CD47 ligation‐induced death, but more importantly, both WT and CD47−/− MOLT4 cells exhibit equal susceptibility to PKHB1 or 4N1K treatment‐induced death (Figure 1B).
Figure 1.
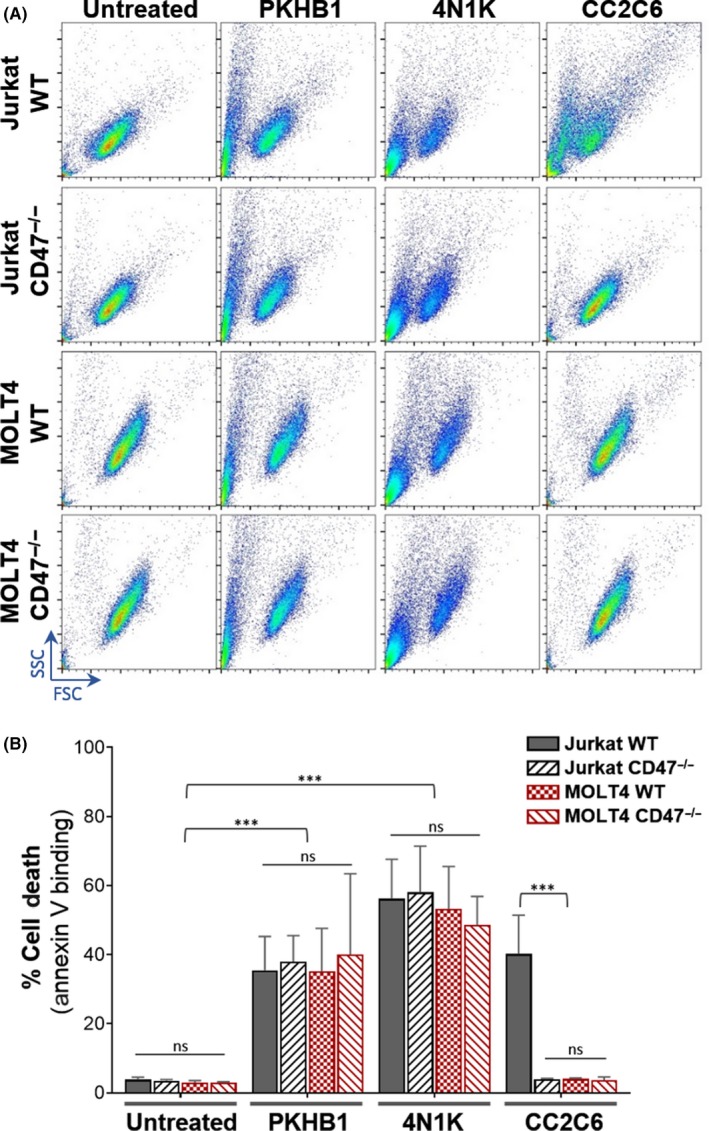
PKHB1 and 4N1K induce CD47‐independent cell death in Jurkat and MOLT4 cells. A, Cells were untreated or treated with 200 µmol/L PKHB1, 200 µmol/L 4N1K, or 125 ng/mL mAb CC2C6 for 2 h and analyzed by flow cytometry as (A) side scatter (SSC) vs forward scatter (FSC) plots or (B) percentage of annexin V‐stained cells. ***P < .001
Given that the CD47‐independent effects of PKHB1 were likely to be similar to those of 4N1K, we repeated select key experiments performed previously9 to shed light on the mechanism of PKHB1‐mediated, but CD47‐independent, effects. Wild‐type and CD47−/− cells treated with or without peptides were incubated with an anti‐CD47 antibody, B6H12, followed by a fluorophore‐conjugated secondary antibody. As shown in Figure 2A, both CD47−/− cells treated with PKHB1 or 4N1K exhibited significant labeling with B6H12. The non‐CD47‐specific labeling is directly attributed to nonspecific antibody binding to PKHB1 or 4N1K treated cells, as evident from positively labeled cells incubated with only secondary antibodies (Figure 2A). Importantly, this phenomenon is peptide dose‐dependent, as demonstrated by increased binding of CD47−/− cells with increasing concentrations of PKHB1 or 4N1K, but not of 4NGG (Figure 2B). Previously, we showed, using a simple cell‐free assay, that 4N1K immobilized on plastic effectively bound a fluorophore‐conjugated secondary antibody.9 We repeated this assay to include PKHB1, and found that, similar to 4N1K, PKHB1 also bound to a secondary antibody in a dose‐dependent manner (Figure 2C).
Figure 2.
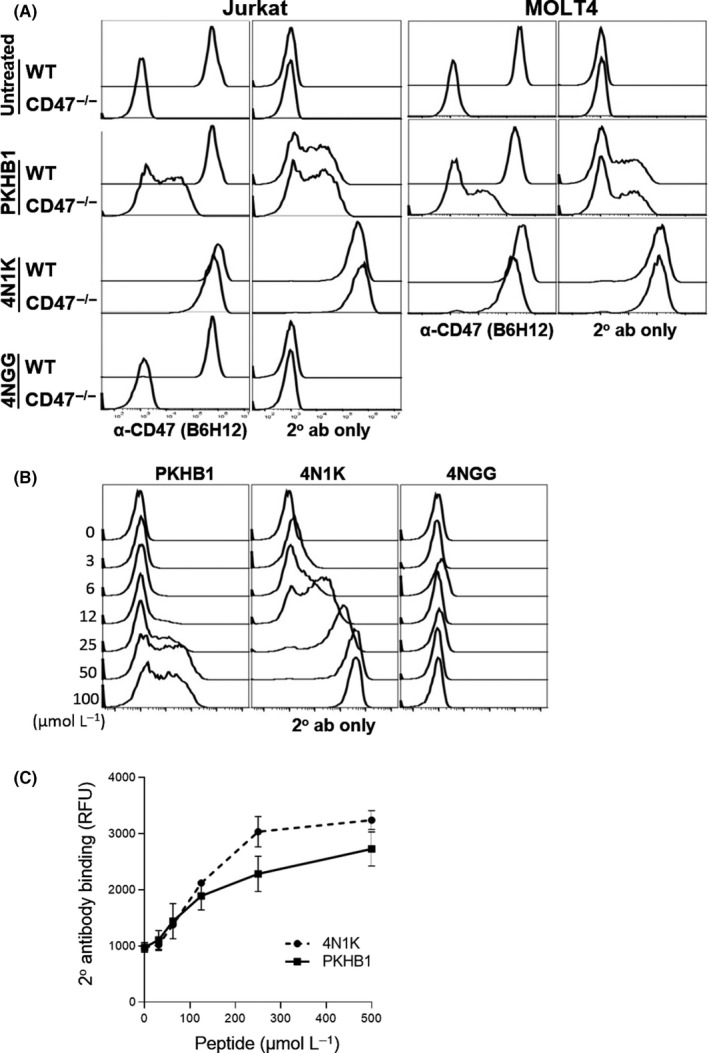
PKHB1 and 4N1K promote antibody binding to CD47−/− Jurkat and MOLT4 cells. A, Cells were untreated or treated with 200 µmol/L peptides for 30 min, and binding to B6H12 or secondary antibody (2° ab) only assessed by flow. B, CD47−/− Jurkat cells were treated with 0‐100 µmol/L peptides, and binding to secondary antibody only assessed by flow. C, Microtiter wells was coated with peptides, and binding to secondary antibody assessed by fluorometry. Detailed methods as previously reported.9 RFU, relative fluorescence unit
Our results essentially agree with those of Uscanga‐Palomeque et al1 in that PKHB1 and 4N1K are peptides that appear to induce leukemic cell death in an efficient and rapid manner, similar to what has been reported for certain CD47 antibodies.3, 4 However, the peptides clearly function in a manner that does not involve CD47 as a receptor, a definitive result based on the use of two CD47−/− leukemic cell lines as the required and critical controls. We reiterate exercising caution when interpreting 4N1K‐ or PKHB1‐induced cell phenomena; the accumulated evidence clearly indicates that the effects have no bearing on CD47 as the presumptive receptor, and calls into question the further development of these peptides and other derivatives as CD47‐targeted therapeutics.
DISCLOSURE
The authors have no conflict of interest.
Leclair and Kim contributed equally to this work.
REFERENCES
- 1. Uscanga‐Palomeque AC, Calvillo‐Rodriguez KM, Gomez‐Morales L, et al. CD47 agonist peptide PKHB1 induces immunogenic cell death in T‐cell acute lymphoblastic leukemia cells. Cancer Sci. 2019;110(1):256‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Soto‐Pantoja DR, Kaur S, Roberts DD. CD47 signaling pathways controlling cellular differentiation and responses to stress. Crit Rev Biochem Mol Biol. 2015;50(3):212‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mateo V, Lagneaux L, Bron D, et al. CD47 ligation induces caspase‐independent cell death in chronic lymphocytic leukemia. Nat Med. 1999;5(11):1277‐1284. [DOI] [PubMed] [Google Scholar]
- 4. Leclair P, Liu CC, Monajemi M, Reid GS, Sly LM, Lim CJ. CD47‐ligation induced cell death in T‐acute lymphoblastic leukemia. Cell Death Dis. 2018;9(5):544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gao AG, Frazier WA. Identification of a receptor candidate for the carboxyl‐terminal cell binding domain of thrombospondins. J Biol Chem. 1994;269(47):29650‐29657. [PubMed] [Google Scholar]
- 6. Martinez‐Torres AC, Quiney C, Attout T, et al. CD47 agonist peptides induce programmed cell death in refractory chronic lymphocytic leukemia B cells via PLCgamma1 activation: evidence from mice and humans. PLoS Medicine. 2015;12(3):e1001796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tulasne D, Judd BA, Johansen M, et al. C‐terminal peptide of thrombospondin‐1 induces platelet aggregation through the Fc receptor gamma‐chain‐associated signaling pathway and by agglutination. Blood. 2001;98(12):3346‐3352. [DOI] [PubMed] [Google Scholar]
- 8. Barazi HO, Li Z, Cashel JA, et al. Regulation of integrin function by CD47 ligands. Differential effects on alpha vbeta 3 and alpha 4beta1 integrin‐mediated adhesion. J Biol Chem. 2002;277(45):42859‐42866. [DOI] [PubMed] [Google Scholar]
- 9. Leclair P, Lim CJ. CD47‐independent effects mediated by the TSP‐derived 4N1K peptide. PLoS ONE. 2014;9(5):e98358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karki S, Nichols MR. CD47 does not mediate amyloid‐beta(1–42) protofibril‐stimulated microglial cytokine release. Biochem Biophys Res Commun. 2014;454(1):239‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]


