Abstract
Aberrant activation of the Wnt/β‐catenin signaling pathway has been observed in a wide range of human tumors. Deregulation of the pathway is closely linked to various aspects of human carcinogenesis such as cell viability, regulation of cell cycle, epithelial‐mesenchymal transition, and maintenance of stemness. In addition, recent studies have disclosed the involvement of Wnt signaling in immune evasion of tumor cells. The accumulation of β‐catenin in the nucleus is a common feature of cancer cells carrying defects in the pathway, which leads to the continuous activation of T‐cell factor (TCF)/LEF transcription factors. Consequently, a genetic program is switched on, leading to the uncontrolled growth, prolonged survival, and acquisition of mesenchymal phenotype. As β‐catenin/TCF serves as a signaling hub for the pathway, β‐catenin/TCF‐dependent transcriptional activity is a relevant readout of the pathway. To date, a wide variety of synthetic TCF/LEF reporters has been developed, and high‐throughput screening (HTS) using these reporters has made significant contributions to the discovery of Wnt inhibitors. Indeed, HTS led to the identification of chemical probes targeting porcupine, a membrane bound O‐acyltransferase, and CREB‐binding protein, a transcriptional coactivator. This review focuses on various screening strategies for the discovery of Wnt inhibitors and their mode of action to help the creation of new concepts for assay/screening methods.
Keywords: chemical probe, high‐throughput screening, reporter assay, TCF/LEF transcription factor, Wnt/β‐catenin signaling pathway
Establishment of a well‐designed high‐throughput screening system is essential to identify Wnt inhibitors. We comprehensively review Wnt inhibitors and discuss the strategies involved in their identification.
1. INTRODUCTION
The Wnt/β‐catenin signaling pathway (also called the canonical Wnt signaling pathway) was originally recognized as an essential pathway for embryonic development and adult tissue homeostasis. Importantly, aberration of Wnt/β‐catenin signaling was later found in a wide range of cancers. Recent analysis of genetic alterations using more than 9000 tumors revealed oncogenic pathway signatures in various tumor types.1 The frequency of activation of the Wnt/β‐catenin signaling pathway varies widely, depending on the tumor type and/or subtype, and its high frequency is particularly observed in colorectal tumors. In addition to colorectal cancer, high frequencies of Wnt/β‐catenin pathway activation were observed in uterine corpus endometrial carcinoma carrying microsatellite instability (MSI) and DNA polymerase epsilon (POLE) mutation (70%), stomach and esophageal cancer carrying MSI and POLE mutation (70%) and diffuse large B‐cell lymphoma (70%).1 Somatic mutations in various components within this pathway, including APC regulator of WNT signaling pathway (APC; previous name, adenomatous polyposis coli), β‐catenin (CTNNB1), transcription factor 7 like 2 (TCF7L2), ring finger protein 43 (RNF43), R‐spondin (RSPO), and AXIN1, cause its aberrant activation. In line with previous reports,2, 3 APC mutations are observed in approximately 50% of colorectal tumors in 2 curated databases of somatic mutations in human cancer (The Catalogue of Somatic Mutations in Cancer [https://cancer.sanger.ac.uk/cosmic] and The cBioPortal for Cancer Genomics [https://www.cbioportal.org/]). A high frequency of mutations in the CTNNB1 gene has also been found in pituitary (41%), soft tissue (36%), and liver tumors (21%).
In the Wnt/β‐catenin signaling pathway, β‐catenin is suppressed by a degradation complex consisting of APC, Axin, glycogen synthase kinase‐3β, and casein kinase 1α (CK1α). However, dysfunction in any of the components of the complex or activating mutations in β‐catenin itself causes abnormal accumulation of β‐catenin in the cells. Translocated into the nucleus, β‐catenin forms a complex with members of the T‐cell factor (TCF) family of DNA‐binding proteins, and consequently leads to transcriptional activation of their target genes. These genes, so‐called “Wnt target genes”, include protooncogenes, cell cycle regulators, stem cell markers, and negative feedback regulators of the Wnt pathway.
To date, there have been a number of proof‐of‐concept studies targeting the Wnt pathway for the treatment of cancer. Using Apc shRNA transgenic mice, Dow et al4 showed that restoration of Apc could reestablish the control of crypt homeostasis in colorectal hyperproliferative polyps and cancer. The capacity for proliferation and self‐renewal of CML cells carrying activated β‐catenin was attenuated by the ectopic expression of Axin.5 β‐Catenin knockdown by RNAi significantly suppressed anchorage‐independent growth and proliferation of liver cancer cells.6 In addition, there is a growing body of evidence suggesting that Wnt/β‐catenin signaling plays an essential role in the immune system. In metastatic melanoma, activation of the Wnt/β‐catenin signaling pathway correlates with T cell exclusion.7 Consistent with this view, multiomics analysis revealed that colorectal tumors with biallelic loss of the APC gene or nuclear accumulation of β‐catenin protein were negatively correlated with tumor‐infiltrating lymphocytes.8 These reports suggested that activated Wnt signaling mediates cancer immune evasion and resistance to immunotherapies. Thus, blocking the Wnt pathway is an attractive approach to improve cancer immunotherapy. These data have prompted a search for chemical probes targeting this pathway (hereafter referred to as Wnt inhibitors). High‐throughput screening (HTS) using a wide variety of assays has made significant contributions to the discovery of Wnt inhibitors. Indeed, HTS successfully identified small molecule compounds targeting porcupine and CREB‐binding protein (CBP), and these compounds have already entered clinical trials. The establishment of a well‐designed HTS system is crucial to identify Wnt inhibitor. Here, we comprehensively review Wnt inhibitors, and discuss the strategies involved in their identification.
2. DEVELOPMENT OF REPORTER ASSAYS OF THE WNT/β‐CATENIN SIGNALING PATHWAY
β‐Catenin has been suggested to bind DNA mainly through the TCF/lymphoid enhancer‐binding factor (LEF) transcription factors in mammals9 and Drosophila.10 The first observational evidence linking TCF/LEF directly to Wnt signaling resulted from yeast 2 hybrid screening using TCF111 or β‐catenin12 as bait. Another group also reported a physical interaction between β‐catenin and LEF1.13 In addition, these studies disclosed a responsible domain for the interaction, known as a β‐catenin binding domain, in the amino terminus of the TCF/LEF protein. Deletion of the β‐catenin binding domain produces a dominant negative form (dnTCF), which can outcompete with WT TCF/LEF for binding to the target sites.14 As dnTCF7L2 abrogated the recruitment of β‐catenin to the target chromatin regions in LS174T cells, TCF/LEF factors play a major role in the recruitment of β‐catenin, at least in colorectal cancer cells.9 However, in the absence of TCF/LEF factors, β‐catenin is recruited through other transcription factors in HEK293T cells.15 As TCF/LEF transcription factors act as major end‐point mediators of this pathway, their DNA binding motif (Figure 1A) has been used as a faithful reporter for monitoring the Wnt/β‐catenin signaling activity.
Figure 1.
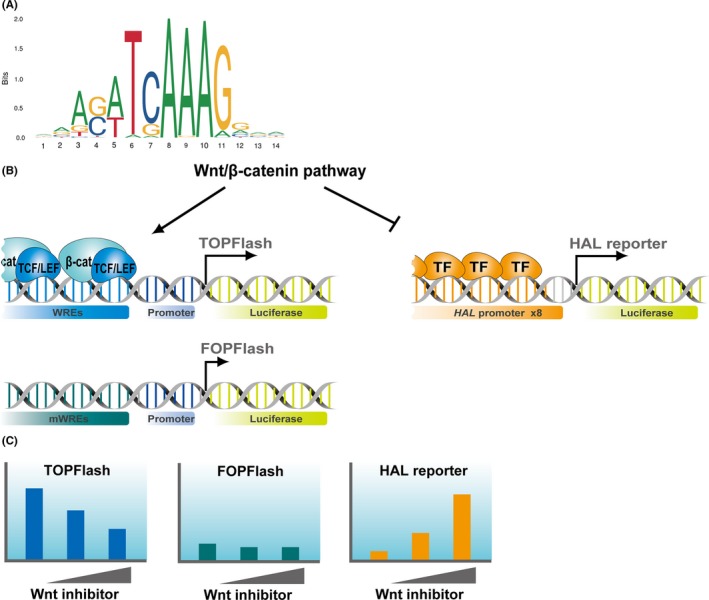
A, Position frequency matrix of the T‐cell factor (TCF) motif was obtained from the JASPAR database (http://jaspar.genereg.net). B, TOPFlash consists of tandemly repeated TCF motifs (Wnt response elements [WREs]) upstream of a minimal promoter that drives luciferase gene expression. FOPFlash has mutated motifs (mWREs) and is used to normalize the TOPFlash activity. HAL reporter was developed as a luciferase reporter driven by 8 copies of the promoter of histidine ammonia‐lyase (HAL). Transcription factor (TF) that regulates the activity of HAL promoter is under investigation. C, These reporter plasmids were designed for monitoring the activity of Wnt/β‐catenin pathway in cultured cells. When the pathway is inhibited, TOPFlash and HAL reporter activities are decreased and increased, respectively
The TCF/LEF reporter plasmid originally incorporated 7 copies of approximately 30 bp of the CD3E enhancer region upstream of a minimal thymidine kinase promoter, and the chloramphenicol acetyltransferase gene (CAT) as a reporter (pMW567). As negative control, a mutant plasmid was prepared in which the WT TCF/LEF‐binding motif AACAAAG was replaced by CCGCGGT (pMW56Sac7).16 TOPFlash, another synthetic TCF/LEF reporter plasmid containing tandemly repeated TCF motifs upstream of a minimal c‐fos promoter and the versatile luciferase gene as a reporter, and FOPFlash, the negative control plasmid, were constructed (Figure 1B).17, 18 As c‐fos is a transcriptional target of Wnt/β‐catenin signaling19 and might affect the β‐catenin‐dependent transactivation,20 thymidine kinase promoter‐driven reporter plasmids have been used for this purpose. SuperTOPFlash, a plasmid with an increased number of TCF motifs, is currently available.21 Although use of the transgenic TCF reporters to detect Wnt/β‐catenin signaling in vivo remains controversial,22 cell‐based assays with TOPFlash or SuperTOPFlash are useful strategies for monitoring the activity of the Wnt signal and the evaluation of chemicals that could affect the activity.
Intriguingly, identification of genes negatively regulated by the β‐catenin/TCF complex led to the development of a new reporter plasmid (Figure 1B).23 This plasmid contains 8 copies of the promoter region of histidine ammonia‐lyase (HAL) upstream of the luciferase gene. Unlike TOPFlash, the HAL reporter activity is inversely correlated with Wnt/β‐catenin signaling activity (Figure 1C). The combination of HAL reporter and TOPFlash plasmids could serve as an effective screening system for the discovery of new Wnt inhibitors.
3. APPLICATION OF REPORTER ASSAY FOR HTS
Reporter assays using synthetic promoters containing multiple copies of a responsive element have been applied for HTS of small molecules and natural compounds affecting transcription and/or cellular signaling pathways. Because sensitivity, specificity, robustness, technical simplicity, and cost effectiveness are required for HTS, various strategies have been devised to meet the conditions. The Z′‐factor has been widely accepted for quality control of the HTS assay.24 An acceptable assay for HTS usually requires a Z′‐value more than 0.5. Reportedly, the Z′‐factor of a luciferase assay using HEK293 cells stably expressing TCF reporter and LiCl for the activation of the reporter was as high as 0.89.25 Regarding the luciferase assay using fly cell‐optimized TCF reporter and Drosophila imaginal disc‐derived clone 8 cells, the Z′‐factor was 0.77.26 In the reciprocal assay, the Z′‐factors for TOPFlash and the HAL promoter luciferase assays were 0.69 and 0.79, respectively.23
Bioluminescent assays have been used in HTS as a major strategy, due to their high sensitivity, broad linearity, and robustness to chemicals.27 Firefly luciferase (Photinus pyralis) and Renilla luciferase (Renilla reniformis) are commonly used as reporter genes. Firefly luciferase is an enzyme of 61 kDa that catalyzes oxidation of a substrate (luciferin) in the presence of ATP and O2. This chemical reaction results in an emission of a yellow‐green light with a spectral maximum of 560 nm.27 Renilla luciferase, a 36 kDa enzyme, is often used as an internal control in the dual luciferase format. Genetically engineered luciferase genes have improved assay sensitivity by increasing intensity of the luminescent signal and enhanced response dynamics by reducing expression lifetime. NanoLuc luciferase has emerged as a potential alternative to firefly/Renilla luciferase for reporter gene assay because it showed an approximately 100‐fold greater activity than that of firefly or Renilla luciferase.28 Importantly, since NanoLuc is a relatively small protein (19 kDa) it could have less effect on nonspecific chemical binding.
4. CELL‐BASED REPORTER ASSAY FOR SCREENING COMPOUNDS IN LIBRARIES
To date, cell‐based HTS with TCF/LEF reporter plasmids have been frequently utilized for the identification of Wnt inhibitors. One of the greatest advantages in using secreted alkaline phosphate (SEAP) as a reporter is that there is no need to lyse the cells to measure its levels, because it is secreted directly into the culture medium. For example, screening of a library of 11 600 compounds using a SEAP reporter driven by Wnt response elements (WREs) identified FH535 and FH615 that suppressed Wnt/β‐catenin signaling.29 However, we might struggle with a high background in the assay because mammalian cells have endogenous alkaline phosphatase activity.
An alternative approach is the use of fluorescent proteins as a reporter. Waaler et al30 prepared HEK293 cells stably expressing GFP reporter under the control of a synthetic TCF‐responsive promoter. They screened 37 000 compounds using the reporter cells after activation with Wnt3a‐conditioned medium and identified 77 compounds as primary hits by image analysis. Subsequent analysis identified 2 potent inhibitors, namely JW67 and JW74. Fluorescence‐based reporter gene assays are cost effective because the addition of substrate is not required for its activity. However, these assays tend to have higher backgrounds, leading to the low signal‐to‐background ratio. Details about the property of biological reporters are described elsewhere.31
5. WNT INHIBITORS DISCOVERED BY HTS
Over the past 2 decades, a wide range of HTS systems have been developed and applied for the screening of Wnt inhibitors. The inhibitors found in HTS are divided into 6 groups according to their mode of action: (i) inhibition of tankyrase (TNKS); (ii) inhibition of porcupine; (iii) activation of CK1α; (iv) inhibition of the β‐catenin‐TCF interaction; (v) inhibition of transcriptional co‐activators; and (vi) induction of β‐catenin degradation (Figure 2). Their chemical structures, assay methods, compound libraries, and four physicochemical parameters of the Lipinski's rule of five (RO5)32 are listed in Table 1. The RO5 is a rule of thumb to evaluate drug‐likeness, and defines 4 parameter ranges (molecular weight, 500 or less; calculated octanol/water partition coefficient value, 5 or less; H‐bond donors, 5 or less; and H‐bond acceptors, 10 or less).
Figure 2.
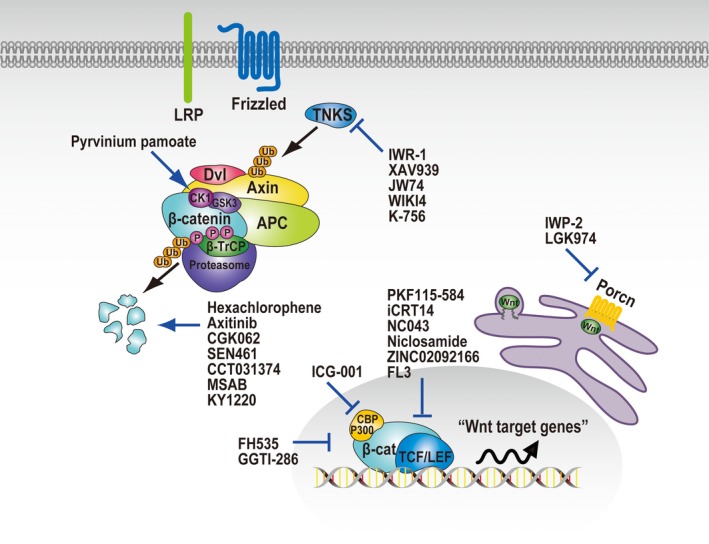
Pharmacological manipulation of the activity of Wnt/β‐catenin signaling pathway. High‐throughput screening identified chemical probes that target tankyrase (TNKS), porcupine (Porcn), casein kinase 1α (CK1α), β‐catenin‐TCF interaction, transcriptional co‐activators of TCF, and β‐catenin degradation (see Table 1 for more details and references). β‐TrCP, β‐transducin repeat containing E3 ubiquitin protein ligase; APC, APC regulator of WNT signaling pathway (adenomatous polyposis coli); CBP, CREB‐binding protein; CK1, casein kinase‐1; Dvl, Dishevelled segment polarity protein; GSK‐3, glycogen synthase kinase‐3; LEF, lymphoid enhancer binding factor; LRP, LDL receptor related protein; TCF7L2, transcription factor 7 like 2; TNKS, tankyrase
Table 1.
Inhibitors of Wnt/β‐catenin signaling discovered by high‐throughput screening (HTS)
| Chemical probe | HTS assay | Cells (condition) | Library | Mode of action | Parameters of RO5 | Structure | References |
|---|---|---|---|---|---|---|---|
| IWR‐1 | Luciferase reporter (SuperTOPFlash) | Mouse L cells (Wnt3a‐CM) | 198 080 (UTSouthwestern chemical library) | Tankyrase inhibition |
MWT = 409.45 CLog P = 2.522 H‐bond donors = 1 H‐bond acceptors = 4 |
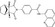
|
33 |
| XAV939 | Luciferase reporter (SuperTOPFlash) | HEK293 | N/A | Tankyrase inhibition |
MWT = 312.31 CLog P = 3.82964 H‐bond donors = 1 H‐bond acceptors = 3 |
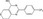
|
34 |
| JW74 | Fluorescence imaging (SuperTOP‐d1EGFP) | HEK293 (Wnt3a‐CM) | 37 000 | Tankyrase inhibition |
MWT = 456.52 CLog P = 4.05445 H‐bond donors = 0 H‐bond acceptors = 6 |
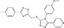
|
30 |
| WIKI4 | Luciferase reporter (BAR, TCF reporter) | A375 (Wnt3a‐CM) | 6492 (KINASet library, Chembridge) | Tankyrase inhibition |
MWT = 521.60 CLog P = 5.21344 H‐bond donors = 0 H‐bond acceptors = 6 |
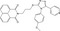
|
35 |
| K‐756 | Luciferase reporter (TCF reporter) | DLD‐1 | N/A | Tankyrase inhibition |
MWT = 433.51 CLog P = 3.4062 H‐bond donors = 1 H‐bond acceptors = 5 |
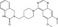
|
36 |
| IWP‐2 | Luciferase reporter (SuperTOPFlash) | Mouse L cells (Wnt3a‐CM) | 198 080 (UTSouthwestern chemical library) | Porcupine inhibition |
MWT = 466.59 CLog P = 4.94517 H‐bond donors = 1 H‐bond acceptors = 4 |
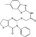
|
33 |
| LGK974 | Luciferase reporter (SuperTOPFlash) | TM3 cells (co‐cultured with L‐cell Wnt3A) | ~2 400 000 | Porcupine inhibition |
MWT = 396.45 CLog P = 1.77295 H‐bond donors = 1 H‐bond acceptors = 6 |
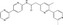
|
38 |
| Pyrvinium pamoate | Luciferase reporter (β‐catenin‐Fluc/Axin‐RLuc fusion proteins) | Xenopus egg extracts (LRP6ICD) | FDA‐approved drug library etc | Activation of CK1α |
MWT = 382.53 CLog P = 2.78575 H‐bond donors = 0 H‐bond acceptors = 0 |
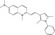
|
41 |
| ICG‐001 | Luciferase reporter (TOPFlash) | SW480 | 5000 | Inhibition of β‐catenin‐CBP interaction |
MWT = 548.64 CLog P = 6.13165 H‐bond donors = 2 H‐bond acceptors = 4 |
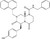
|
54 |
| PKF115‐584 | ELISA (β‐catenin/ GST‐TCF4 recombinant proteins) | N/A | 7000 (natural compounds library) | Inhibition of β‐catenin‐TCF interaction |
MWT = 790.77 CLog P = 6.87757 H‐bond donors = 3 H‐bond acceptors = 11 |
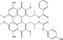
|
44 |
| iCRT14 | Luciferase reporter (TCF reporter) | Drosophila Cl8 cells (dAxin‐dsRNA) | 14 977 (ICCB‐Longwood collection, Harvard) | Inhibition of β‐catenin‐TCF interaction |
MWT = 375.45 CLog P = 5.70349 H‐bond donors = 0 H‐bond acceptors = 3 |
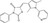
|
26 |
| NC043 | Luciferase reporter (TOPFlash) |
HEK293T (Wnt1 overexpression, Wnt3a‐CM, or LiCl) |
4000 | Indirect inhibition of β‐catenin‐TCF interaction |
MWT = 330.42 CLog P = 1.2362 H‐bond donors = 1 H‐bond acceptors = 3 |
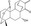
|
48 |
| Niclosamide | Luciferase reporter (S1004A4 promoter reporter) | HCT116 | 1280 (LOPAC Sigma‐Aldrich) | Inhibition of formation of β‐catenin/TCF complex |
MWT = 327.12 CLog P = 4.34465 H‐bond donors = 2 H‐bond acceptors = 2 |
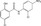
|
47 |
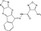
| ZINC02092166 | Fluorescence polarization and AlphaScreen assays (β‐catenin/fluorescein‐TCF4 recombinant proteins) | N/A | 2093 (Sigma‐Aldrich, Pfizer, NCI etc) | Inhibition of β‐catenin‐TCF interaction |
MWT = 349.27 CLog P = 3.91846 H‐bond donors = 2 H‐bond acceptors = 8 |
|
45 |
| LF3 | AlphaScreen assay (GST‐β‐catenin/His‐TCF4 recombinant proteins) | N/A | 16 671 (WDI compounds, ChemBioNet) | Inhibition of β‐catenin‐TCF interaction |
MWT = 416.56 CLog P = 2.582 H‐bond donors = 2 H‐bond acceptors = 4 |
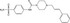
|
46 |
| Hexachlorophene | Luciferase reporter (TOPFlash) | HEK293 expressing hFz‐1 (Wnt3a‐CM) | 960 (Genesis Plus Collection, MicroSource Discovery) | β‐Catenin degradation through SIAH‐1 induction |
MWT = 406.89 CLog P = 7.02708 H‐bond donors = 2 H‐bond acceptors = 2 |

|
56 |
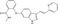
| Axitinib | Luciferase reporter (SuperTOPFlash) | HEK293FT (GSK‐3 inhibitor, 6BIO) | 460 (FDA‐approved drug library) | β‐Catenin degradation through SHPRH stabilization |
MWT = 386.47 CLog P = 3.3269 H‐bond donors = 3 H‐bond acceptors = 4 |
|
58 |
| CGK062 | Luciferase reporter (TOPFlash) | HEK293 expressing hFz‐1 (Wnt3a‐CM) | 800 | β‐Catenin degradation through PKCα activation |
MWT = 408.41 CLog P = 3.75537 H‐bond donors = 2 H‐bond acceptors = 5 |
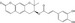
|
59 |
| SEN461 | Luciferase reporter (TCF reporter) | DBTRG.05MG | 16 000 (Siena Biotech internal compounds collection) | β‐Catenin degradation through Axin stabilization |
MWT = 486.57 CLog P = 2.0554 H‐bond donors = 0 H‐bond acceptors = 6 |
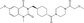
|
60 |
| CCT031374 | Luciferase reporter (TCF reporter) | HEK293 expressing Dvl2‐ER (Disheveled‐estrogen receptor fusion) | 63 040 (The Cancer Research UK Center for Cancer Therapeutics compound library) | β‐Catenin degradation + unknown mechanism |
MWT = 353.43 CLog P = 4.5174 H‐bond donors = 0 H‐bond acceptors = 2 |

|
25 |
| MSAB | Luciferase reporter (TOPFlash) | HCT116 | 22 000 (Chembridge and Broad Institute) | β‐Catenin binding and degradation |
MWT = 305.35 CLog P = 3.405 H‐bond donors = 1 H‐bond acceptors = 3 |
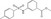
|
61 |
| KY1220 | Luciferase reporter (TOPFlash) | HEK293 (Wnt3a‐CM) | ~3599 (Chemdiv and Sigma LOPAC 1280) | Axin binding and β‐catenin degradation |
MWT = 314.32 CLog P = 1.60552 H‐bond donors = 2 H‐bond acceptors = 2 |
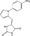
|
62 |
| FH535 | SEAP reporter (TOPFlash) | HepG2 | 11 600 (DIVERSet collection, ChemBridge) | Repression of β‐catenin recruitment |
MWT = 361.19 CLog P = 3.95088 H‐bond donors = 1 H‐bond acceptors = 2 |
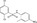
|
29 |
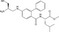
| GGTI‐286 | Eye phenotype | Zebrafish embryos (GSK‐3 inhibitor, 6BIO) | 282 (SCADS inhibitor kit) | Inhibition of nuclear accumulation of β‐catenin |
MWT = 429.58 CLog P = 3.4042 H‐bond donors = 3 H‐bond acceptors = 3 |
|
63 |
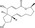
| Brefeldin A | Luciferase reporter (TOPFlash and HAL promoter reporter) | HepG2 | 361 (SCADS inhibitor kit) | Repression of β‐catenin mRNA |
MWT = 280.36 CLog P = .538999 H‐bond donors = 2 H‐bond acceptors = 3 |
|
23 |
Chemical structures were drawn using ChemDraw. The molecular weights (MWT), calculated octanol/water partition coefficient (CLog P) values, and number of hydrogen bond donors and acceptors were calculated using ChemDraw or Molecular Operating Environment (MOE).
BAR, β‐catenin‐activated reporter; CBP, CREB‐binding protein; CK1α, casein kinase‐1α; GSK‐3, glycogen synthase kinase‐3; N/A, not available; PKCα, protein kinase Cα; SCADS, Screening Committee of Anticancer Drugs; SEAP, secreted alkaline phosphate; TCF, T‐cell factor.
6. TANKYRASE INHIBITORS
Using a cell‐based SuperTOPFlash assay, Chen et al33 screened a ~200 000 synthetic chemical library to identify Wnt inhibitors. Secondary tests were carried out to select specific Wnt inhibitors from the first hit compounds, such as dose‐dependent test (cytotoxicity), firefly/Gaussia luciferase assays (firefly luciferase inhibitor/exocytosis), and Notch/Hedgehog reporter assays (stem cell‐associated signal transduction pathways). This screening strategy identified 5 inhibitors of Wnt response (IWR) compounds that abrogated destruction of Axin proteins. Axin is an essential scaffold protein required for assembly of the β‐catenin destruction complex. Degradation of Axin is controlled through its poly‐ADP‐ribosylation (PARsylation) by TNKS.34 Subsequently, IWR compounds turned out to be TNKS inhibitors (Figure 3A). Soon after, Huang et al34 discovered another Axin stabilizer, XAV939 (Figure 3B), that directly binds TNKS and inhibits its PARsylation activity. An additional phenotype‐based assay using zebrafish fin corroborated the inhibitory effect of Wnt activity by IWR and XAV939. Other TNKS inhibitors such as JW74,30 WIKI4,35 and K‐75636 have also been found by cell‐based HTS.
Figure 3.
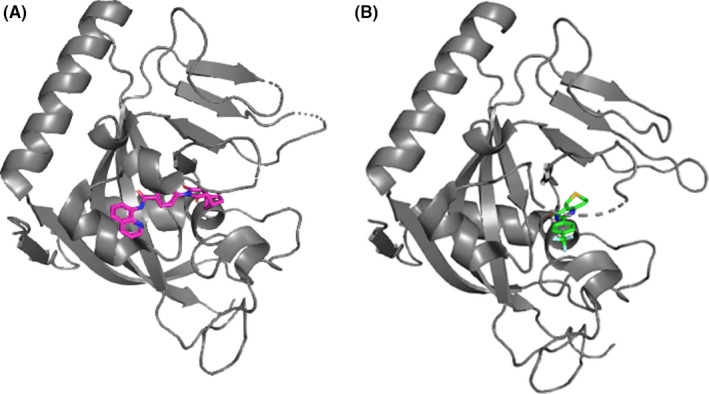
Crystal structures of tankyrase‐1 (TNKS1) in complex with 2 small molecule compounds, IWR1 (A) and XAV939 (B). Data taken from RCSB Protein Data Bank (IWR1, PDB ID:4OA7 and XAV939, PDB ID:3UH4) and visualized using PyMOL molecular graphics software (https://pymol.org/2/). TNKS1, IWR1, and XAV939 are colored by gray, magenta, and green, respectively
7. PORCUPINE INHIBITORS
One of the possible therapeutic strategies for targeting Wnt‐driven cancers is to block the production of Wnt ligands. Porcupine (PORCN), a membrane‐bound O‐acyltransferase, was found to catalyze the palmitoylation of Wnt ligands, which is an essential step in the processing of Wnt into active ligands.37 IWP‐2, one of the inhibitors of Wnt production (IWPs), directly binds to PORCN, and inhibits the function of PORCN.33 Another potent PORCN inhibitor, LGK974, was discovered by the screening of ~2 400 000 compounds using a TCF/LEF reporter assay, where Wnt3a‐secreting cells were cocultured with mouse Leydig TM3 cells expressing SuperTOPFlash for the activation of the Wnt pathway.38 An in vivo study reported that LGK974 diminished/eradicated tumors carrying RSPO fusions from the intestinal mucosa without effects on normal intestinal crypts.39 In addition, LGK974 prevented proliferation and induced differentiation of RNF43‐mutant pancreatic adenocarcinoma in xenograft models.40 Currently, a phase I clinical trial (Clinicaltrials.gov ID NCT01351103) of LGK974 for patients with malignancies of histological origin carrying genetic alterations upstream in the Wnt signaling pathway (eg, RNF43 mutation or RSPO fusion) is underway.
8. CASEIN KINASE‐1α ACTIVATOR
To explore chemical probes that inhibit the turnover of Axin and promote the degradation of β‐catenin, Thorne et al41 developed an assay system using extract of Xenopus eggs expressing β‐catenin‐firefly luciferase and Axin‐Renilla luciferase fusion proteins mixed with a soluble form of LRP6 for the activation of the Wnt signaling. This assay was designed to analyze the reciprocal stability of β‐catenin and Axin, thus Wnt inhibitors should decrease firefly luciferase (β‐catenin) and increase Renilla luciferase (Axin). As Xenopus egg extracts are transcriptionally and translationally inactive, hit compounds are expected to modulate Wnt signaling through posttranslational events. In addition, compounds that target energy metabolism (reduce both reporter activities) and general protein degradation (increase both reporter activities) would be avoided from the first hits. By the screening of an FDA‐approved drug library, they identified pyrvinium pamoate, a CK1α activator, previously used in the treatment of pinworm infection. However, this effect was not confirmed by other studies.42 Details about cytotoxic effects and molecular targets of pyrvinium pamoate are described elsewhere (http://www.oncm.org/v03p0001.htm).
9. INHIBITORS OF INTERACTION BETWEEN β‐CATENIN AND TCF
Activating mutations in β‐catenin are frequently observed in several types of cancer, such as hepatocellular carcinoma,43 where CTNNB1 mutations disrupt the phosphorylation and degradation of the β‐catenin protein. In this case, inhibition of the upstream components is unable to induce the degradation of β‐catenin. Thus, inhibition of the interaction of TCF/LEF with β‐catenin or the coactivators is a rational and straightforward approach. To identify small molecules that disrupt the interaction, Lepourcelet et al44 developed ELISA‐based HTS using β‐catenin and GST‐fused TCF7L2 recombinant proteins. Consequently, they obtained 6 hits including PKF115‐584 and CGP049090 from a library of 7000 natural compounds. Two other groups independently adopted AlphaScreen (Amplified Luminescent Proximity Homogeneous Assay), a method for detecting intramolecular binding, for the discovery of inhibitors of the β‐catenin‐TCF interaction, and discovered LF3 and ZINC02092116.45, 46
Cell‐based approaches have also discovered small molecule compounds iCRT, NC043, and niclosamide that target the β‐catenin‐TCF interaction.26, 47, 48 For example, niclosamide was identified by HTS with 1280 pharmacologically active compounds in HCT116 cells expressing luciferase reporter driven by the promoter of the S1004A4 gene, a transcriptional target of the Wnt/β‐catenin pathway.47 Gonsalves et al identified iCRT3, iCRT4, and iCRT14 by screening 14 977 compounds using fly cell‐optimized TCF reporter and Drosophila imaginal disc‐derived clone 8 cells that had silenced dAxin expression for the activation of the reporter.26 According to crystallographic analysis, the TCF7L2‐binding region on β‐catenin overlaps with the binding regions for APC and E‐cadherin49, 50; this might become a potential obstacle to develop selective Wnt inhibitor. Nevertheless, further characterization of iCRTs revealed that these compounds inhibited β‐catenin‐TCF interaction, whereas they had little or no effect on β‐catenin‐E‐cadherin or β‐catenin‐α‐catenin interaction.26 In contrast, PKF222‐815, PKF115‐584, and CGP049090 blocked the interaction of β‐catenin with TCF7L2, and unfavorably blocked its interaction with APC.44 Increasing the specificity of inhibitors targeting the interaction of β‐catenin and TCF remains a major challenge.
10. INHIBITORS OF TRANSCRIPTIONAL COACTIVATORS OF β‐CATENIN
Targeting the coactivators of β‐catenin‐dependent transcription is another strategy for the suppression of aberrant Wnt signaling. Molecular studies have identified a number of coactivators that interact with β‐catenin (Wnt homepage, http://web.stanford.edu/group/nusselab/cgi-bin/wnt/protein_interactions).51, 52, 53 High‐throughput screening using TOPFlash assay in APC‐mutated SW480 colorectal cancer cells identified ICG‐001 that targets CBP, an interacting protein of β‐catenin.54 Intriguingly, treatment with ICG‐001 suppressed β‐catenin/TCF‐mediated transcription of survivin in HCT116 cells, suggesting that CBP inhibitor can block Wnt/β‐catenin signaling. In this regard, B‐cell lymphoma 9 (BCL9) and BCL9‐like (B9L) might also be targets of the nuclear coactivator. Carnosic acid that blocked the binding of β‐catenin to BCL9 resulted in the inhibition of β‐catenin‐dependent transcription in colorectal cancer cells.55
11. SMALL MOLECULES THAT PROMOTE β‐CATENIN DEGRADATION
Enzymes such as ubiquitin ligases are associated with β‐catenin degradation, and can be an attractive target of small molecule compound. Park et al screened 960 bioactive compounds using TOPFlash luciferase assay, and discovered hexachlorophene that promotes β‐catenin degradation through induction of SIAH‐1, an E3 ubiquitin ligase.56 Recently, another E3 ubiquitin ligase, SHPRH that controls β‐catenin stability, was also found to be targeted by a small molecule compound. Screening of an FDA‐approved drug library using SuperTOPFlash luciferase assay led to the discovery of axitinib, a known inhibitor of multireceptor tyrosine kinases, especially vascular endothelial growth factor receptors,57 that stabilize SHPRH protein, thereby increasing the degradation of β‐catenin.58 Another screening of 800 compounds using TOPFlash assay identified CGK062 that promotes protein kinase Cα‐mediated phosphorylation of β‐catenin at Ser33/Ser37.59 De Robertis et al60 identified SEN461 using TCF/LEF reporter with an increased number of WRE and DBTRG.05MG human glioma cells. Although SEN461 enhanced the degradation of β‐catenin through the stabilization of Axin, it showed limited effect on auto‐PARsylation and stabilization of TNKS compared with a TNKS inhibitor, XAV939, suggesting that TNKS are not the pharmacological target of SEN461. In addition, the HTS using a cell‐based TOPFlash assay identified a set of small molecules including MSAB61 and KY1220.62 It was reported that MSAB and KY1220 physically interact with the Armadillo repeat region of β‐catenin and the regulator of G‐protein signaling domain of Axin, respectively. These interactions might promote the formation of β‐catenin destruction complex.
12. SMALL MOLECULES THAT AFFECT β‐CATENIN FUNCTION OR EXPRESSION
Development of novel screening strategies has discovered different types of Wnt inhibitors. Nishiya et al63 explored compounds that suppress the chemically induced eyeless phenotype in zebrafish embryos. This phenotypic screening resulted in the discovery of GGTI‐286, and they found that GGTI‐286 reduces nuclear translocation of β‐catenin through the inhibition of geranylgeranyltransferase (GGTase). It has been reported that GGTase catalyzes the addition of the geranylgeranyl group to Rac1 protein that is required for its membrane association and biological activity.64 Rac1 activates JNK2 that in turn phosphorylates β‐catenin at Ser191 and regulates its nuclear translocation,65 suggesting that the reduced nuclear translocation of β‐catenin by GGTI‐286 might be associated with its interference with Rac1 protein. A reciprocal reporter assay using TOPFlash coupled with HAL reporter led to a decrease in the number of false positives, and identified brefeldin A (BFA), a fungal metabolite, that suppresses the expression of β‐catenin.23 Although BFA was reported to inhibit protein secretion by blocking transport from the endoplasmic reticulum to the Golgi,66 the precise mechanism(s) underlying the reduction in β‐catenin by BFA needs further investigation.
13. CONCLUSIONS
The discovery of Wnt inhibitors should provide potential benefits for cancer therapy. Although systematic unbiased screenings of the pathway have helped the identification of Wnt inhibitors, much of the underlying mechanisms remain to be elucidated. The target identification of small molecules from unbiased HTS is a challenge to be resolved.
DISCLOSURE
The authors have no conflict of interest.
ACKNOWLEDGEMENTS
We thank Drs. Tsuneo Ikenoue and Kiyoko Takane (The University of Tokyo) for helpful discussions. The research that has led up to this review was supported in part by grants from Takeda Science Foundation (KY), the Project of Translational and Clinical Research Core Centers from the Japan Agency for Medical Research and Development, AMED (YF and KY), the Japan Society for the Promotion of Science (JSPS) for the “Institutional Program for Young Researcher Overseas Visits” (KY), and the Mansfield‐PhRMA Research Scholars Program (KY). This work was supported by Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research [BINDS]) from AMED of Japan under Grand Number JP19am0101094 (KT).
Yamaguchi K, Nagatoishi S, Tsumoto K, Furukawa Y. Discovery of chemical probes that suppress Wnt/β‐catenin signaling through high‐throughput screening. Cancer Sci. 2020;111:783–794. 10.1111/cas.14297
REFERENCES
- 1. Sanchez‐Vega F, Mina M, Armenia J, et al. Oncogenic signaling pathways in the Cancer Genome Atlas. Cell. 2018;173:321‐337 e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miyoshi Y, Nagase H, Ando H, et al. Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Hum Mol Genet. 1992;1:229‐233. [DOI] [PubMed] [Google Scholar]
- 3. Powell SM, Zilz N, Beazer‐Barclay Y, et al. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359:235‐237. [DOI] [PubMed] [Google Scholar]
- 4. Dow L, O’Rourke K, Simon J, et al. Apc restoration promotes cellular differentiation and reestablishes crypt homeostasis in colorectal cancer. Cell. 2015;161:1539‐1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jamieson CHM, Ailles LE, Dylla SJ, et al. Granulocyte‐macrophage progenitors as candidate leukemic stem cells in blast‐crisis CML. N Engl J Med. 2004;351:657‐667. [DOI] [PubMed] [Google Scholar]
- 6. Zeng G, Apte U, Cieply B, Singh S, Monga SP. siRNA‐mediated beta‐catenin knockdown in human hepatoma cells results in decreased growth and survival. Neoplasia. 2007;9:951‐959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spranger S, Bao R, Gajewski TF. Melanoma‐intrinsic beta‐catenin signalling prevents anti‐tumour immunity. Nature. 2015;523:231‐235. [DOI] [PubMed] [Google Scholar]
- 8. Grasso CS, Giannakis M, Wells DK, et al. Genetic mechanisms of immune evasion in colorectal cancer. Cancer Discov. 2018;8:730‐749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schuijers J, Mokry M, Hatzis P, Cuppen E, Clevers H. Wnt‐induced transcriptional activation is exclusively mediated by TCF/LEF. EMBO J. 2014;33:146‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Franz A, Shlyueva D, Brunner E, Stark A, Basler K. Probing the canonicity of the Wnt/Wingless signaling pathway. PLoS Genet. 2017;13:e1006700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Molenaar M, van de Wetering M, Oosterwegel M, et al. XTcf‐3 transcription factor mediates beta‐catenin‐induced axis formation in Xenopus embryos. Cell. 1996;86:391‐399. [DOI] [PubMed] [Google Scholar]
- 12. Behrens J, von Kries JP, Kuhl M, et al. Functional interaction of beta‐catenin with the transcription factor LEF‐1. Nature. 1996;382:638‐642. [DOI] [PubMed] [Google Scholar]
- 13. Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann BG, Kemler R. Nuclear localization of beta‐catenin by interaction with transcription factor LEF‐1. Mech Dev. 1996;59:3‐10. [DOI] [PubMed] [Google Scholar]
- 14. Fujita M, Furukawa Y, Tsunoda T, Tanaka T, Ogawa M, Nakamura Y. Up‐regulation of the ectodermal‐neural cortex 1 (ENC1) gene, a downstream target of the beta‐catenin/T‐cell factor complex, in colorectal carcinomas. Cancer Res. 2001;61:7722‐7726. [PubMed] [Google Scholar]
- 15. Doumpas N, Lampart F, Robinson MD, et al. TCF/LEF dependent and independent transcriptional regulation of Wnt/beta‐catenin target genes. EMBO J. 2019;38:e98873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van de Wetering M, Oosterwegel M, Dooijes D, Clevers H. Identification and cloning of TCF‐1, a T lymphocyte‐specific transcription factor containing a sequence‐specific HMG box. EMBO J. 1991;10:123‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Korinek V, Barker N, Morin PJ, et al. Constitutive transcriptional activation by a beta‐catenin‐Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784‐1787. [DOI] [PubMed] [Google Scholar]
- 18. van de Wetering M, Cavallo R, Dooijes D, et al. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell. 1997;88:789‐799. [DOI] [PubMed] [Google Scholar]
- 19. Staal FJ, Weerkamp F, Baert MR, et al. Wnt target genes identified by DNA microarrays in immature CD34+ thymocytes regulate proliferation and cell adhesion. J Immunol. 2004;172:1099‐1108. [DOI] [PubMed] [Google Scholar]
- 20. Toualbi K, Guller MC, Mauriz JL, et al. Physical and functional cooperation between AP‐1 and beta‐catenin for the regulation of TCF‐dependent genes. Oncogene. 2007;26:3492‐3502. [DOI] [PubMed] [Google Scholar]
- 21. Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol. 2003;13:680‐685. [DOI] [PubMed] [Google Scholar]
- 22. Barolo S. Transgenic Wnt/TCF pathway reporters: all you need is Lef? Oncogene. 2006;25:7505‐7511. [DOI] [PubMed] [Google Scholar]
- 23. Yamaguchi K, Zhu C, Ohsugi T, Yamaguchi Y, Ikenoue T, Furukawa Y. Bidirectional reporter assay using HAL promoter and TOPFLASH improves specificity in high‐throughput screening of Wnt inhibitors. Biotechnol Bioeng. 2017;114:2868‐2882. [DOI] [PubMed] [Google Scholar]
- 24. Zhang JH, Chung TD, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen. 1999;4:67‐73. [DOI] [PubMed] [Google Scholar]
- 25. Ewan K, Pajak B, Stubbs M, et al. A useful approach to identify novel small‐molecule inhibitors of Wnt‐dependent transcription. Cancer Res. 2010;70:5963‐5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gonsalves FC, Klein K, Carson BB, et al. An RNAi‐based chemical genetic screen identifies three small‐molecule inhibitors of the Wnt/wingless signaling pathway. Proc Natl Acad Sci USA. 2011;108:5954‐5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fan F, Wood KV. Bioluminescent assays for high‐throughput screening. Assay Drug Dev Technol. 2007;5:127‐136. [DOI] [PubMed] [Google Scholar]
- 28. Hall MP, Unch J, Binkowski BF, et al. Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS Chem Biol. 2012;7:1848‐1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Handeli S, Simon JA. A small‐molecule inhibitor of Tcf/beta‐catenin signaling down‐regulates PPARgamma and PPARdelta activities. Mol Cancer Ther. 2008;7:521‐529. [DOI] [PubMed] [Google Scholar]
- 30. Waaler J, Machon O, von Kries JP, et al. Novel synthetic antagonists of canonical Wnt signaling inhibit colorectal cancer cell growth. Cancer Res. 2011;71:197‐205. [DOI] [PubMed] [Google Scholar]
- 31. Jiang T, Xing B, Rao J. Recent developments of biological reporter technology for detecting gene expression. Biotechnol Genet Eng Rev. 2008;25:41‐75. [DOI] [PubMed] [Google Scholar]
- 32. Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3‐26. [DOI] [PubMed] [Google Scholar]
- 33. Chen B, Dodge ME, Tang W, et al. Small molecule‐mediated disruption of Wnt‐dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang S‐M, Mishina YM, Liu S, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614‐620. [DOI] [PubMed] [Google Scholar]
- 35. James RG, Davidson KC, Bosch KA, et al. WIKI4, a novel inhibitor of tankyrase and Wnt/ss‐catenin signaling. PLoS ONE. 2012;7:e50457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Okada‐Iwasaki R, Takahashi Y, Watanabe Y, et al. The discovery and characterization of K‐756, a novel Wnt/beta‐catenin pathway inhibitor targeting tankyrase. Mol Cancer Ther. 2016;15:1525‐1534. [DOI] [PubMed] [Google Scholar]
- 37. Takada R, Satomi Y, Kurata T, et al. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev Cell. 2006;11:791‐801. [DOI] [PubMed] [Google Scholar]
- 38. Liu J, Pan S, Hsieh MH, et al. Targeting Wnt‐driven cancer through the inhibition of Porcupine by LGK974. Proc Natl Acad Sci USA. 2013;110:20224‐20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Han T, Schatoff EM, Murphy C, et al. R‐Spondin chromosome rearrangements drive Wnt‐dependent tumour initiation and maintenance in the intestine. Nat Commun. 2017;8:15945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jiang X, Hao H‐X, Growney JD, et al. Inactivating mutations of RNF43 confer Wnt dependency in pancreatic ductal adenocarcinoma. Proc Natl Acad Sci USA. 2013;110:12649‐12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thorne CA, Hanson AJ, Schneider J, et al. Small‐molecule inhibition of Wnt signaling through activation of casein kinase 1alpha. Nat Chem Biol. 2010;6:829‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Venerando A, Girardi C, Ruzzene M, Pinna LA. Pyrvinium pamoate does not activate protein kinase CK1, but promotes Akt/PKB down‐regulation and GSK3 activation. Biochem J. 2013;452:131‐137. [DOI] [PubMed] [Google Scholar]
- 43. de La Coste A, Romagnolo B, Billuart P, et al. Somatic mutations of the beta‐catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci USA. 1998;95:8847‐8851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lepourcelet M, Chen YN, France DS, et al. Small‐molecule antagonists of the oncogenic Tcf/beta‐catenin protein complex. Cancer Cell. 2004;5:91‐102. [DOI] [PubMed] [Google Scholar]
- 45. Catrow JL, Zhang Y, Zhang M, Ji H. Discovery of selective small‐molecule inhibitors for the beta‐catenin/T‐cell factor protein‐protein interaction through the optimization of the acyl hydrazone moiety. J Med Chem. 2015;58:4678‐4692. [DOI] [PubMed] [Google Scholar]
- 46. Fang L, Zhu Q, Neuenschwander M, et al. A small‐molecule antagonist of the beta‐catenin/TCF4 interaction blocks the self‐renewal of cancer stem cells and suppresses tumorigenesis. Cancer Res. 2016;76:891‐901. [DOI] [PubMed] [Google Scholar]
- 47. Sack U, Walther W, Scudiero D, et al. Novel effect of antihelminthic Niclosamide on S100A4‐mediated metastatic progression in colon cancer. J Natl Cancer Inst. 2011;103:1018‐1036. [DOI] [PubMed] [Google Scholar]
- 48. Wang W, Liu H, Wang S, Hao X, Li L. A diterpenoid derivative 15‐oxospiramilactone inhibits Wnt/beta‐catenin signaling and colon cancer cell tumorigenesis. Cell Res. 2011;21:730‐740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Graham TA, Weaver C, Mao F, Kimelman D, Xu W. Crystal structure of a beta‐catenin/Tcf complex. Cell. 2000;103:885‐896. [DOI] [PubMed] [Google Scholar]
- 50. Huber AH, Weis WI. The structure of the beta‐catenin/E‐cadherin complex and the molecular basis of diverse ligand recognition by beta‐catenin. Cell. 2001;105:391‐402. [DOI] [PubMed] [Google Scholar]
- 51. Hecht A, Vleminckx K, Stemmler MP, van Roy F, Kemler R. The p300/CBP acetyltransferases function as transcriptional coactivators of beta‐catenin in vertebrates. EMBO J. 2000;19:1839‐1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kramps T, Peter O, Brunner E, et al. Wnt/wingless signaling requires BCL9/legless‐mediated recruitment of pygopus to the nuclear beta‐catenin‐TCF complex. Cell. 2002;109:47‐60. [DOI] [PubMed] [Google Scholar]
- 53. Takemaru KI, Moon RT. The transcriptional coactivator CBP interacts with beta‐catenin to activate gene expression. J Cell Biol. 2000;149:249‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Emami KH, Nguyen C, Ma H, et al. A small molecule inhibitor of beta‐catenin/CREB‐binding protein transcription [corrected]. Proc Natl Acad Sci USA. 2004;101:12682‐12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. de la Roche M, Rutherford TJ, Gupta D, et al. An intrinsically labile alpha‐helix abutting the BCL9‐binding site of beta‐catenin is required for its inhibition by carnosic acid. Nat Commun. 2012;3:680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Park S, Gwak J, Cho M, et al. Hexachlorophene inhibits Wnt/beta‐catenin pathway by promoting Siah‐mediated beta‐catenin degradation. Mol Pharmacol. 2006;70:960‐966. [DOI] [PubMed] [Google Scholar]
- 57. Hu‐Lowe DD, Zou HY, Grazzini ML, et al. Nonclinical antiangiogenesis and antitumor activities of axitinib (AG‐013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin Cancer Res. 2008;14:7272‐7283. [DOI] [PubMed] [Google Scholar]
- 58. Qu Y, Gharbi N, Yuan X, et al. Axitinib blocks Wnt/beta‐catenin signaling and directs asymmetric cell division in cancer. Proc Natl Acad Sci USA. 2016;113:9339‐9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gwak J, Lee JH, Chung YH, Song GY, Oh S. Small molecule‐based promotion of PKCalpha‐mediated beta‐catenin degradation suppresses the proliferation of CRT‐positive cancer cells. PLoS ONE. 2012;7:e46697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. De Robertis A, Valensin S, Rossi M, et al. Identification and characterization of a small‐molecule inhibitor of Wnt signaling in glioblastoma cells. Mol Cancer Ther. 2013;12:1180‐1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hwang SY, Deng X, Byun S, et al. Direct targeting of beta‐catenin by a small molecule stimulates proteasomal degradation and suppresses oncogenic Wnt/beta‐catenin signaling. Cell Rep. 2016;16:28‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cha PH, Cho YH, Lee SK, et al. Small‐molecule binding of the axin RGS domain promotes beta‐catenin and Ras degradation. Nat Chem Biol. 2016;12:593‐600. [DOI] [PubMed] [Google Scholar]
- 63. Nishiya N, Oku Y, Kumagai Y, et al. A zebrafish chemical suppressor screening identifies small molecule inhibitors of the Wnt/beta‐catenin pathway. Chem Biol. 2014;21:530‐540. [DOI] [PubMed] [Google Scholar]
- 64. Joyce PL, Cox AD. Rac1 and Rac3 are targets for geranylgeranyltransferase I inhibitor‐mediated inhibition of signaling, transformation, and membrane ruffling. Cancer Res. 2003;63:7959‐7967. [PubMed] [Google Scholar]
- 65. Wu X, Tu X, Joeng KS, Hilton MJ, Williams DA, Long F. Rac1 activation controls nuclear localization of beta‐catenin during canonical Wnt signaling. Cell. 2008;133:340‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fujiwara T, Oda K, Yokota S, Takatsuki A, Ikehara Y. Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J Biol Chem. 1988;263:18545‐18552. [PubMed] [Google Scholar]


