Abstract
Increasing evidence indicates that extracellular vesicles (EVs) play an important role in cancer cell‐to‐cell communication. The Epstein‐Barr virus (EBV)‐encoded latent membrane protein 1 (LMP1), which is closely associated with nasopharyngeal carcinoma (NPC) pathogenesis, can trigger multiple cell signaling pathways that affect cell progression. Several reports have shown that LMP1 promotes EV secretion, and LMP1 trafficking by EVs can enhances cancer progression and metastasis. However, the molecular mechanism by which LMP1 promotes EV secretion is not well understood. In the present study, we found that LMP1 promotes EV secretion by upregulated syndecan‐2 (SDC2) and synaptotagmin‐like‐4 (SYTL4) through nuclear factor (NF)‐κB signaling in NPC cells. Further study indicated that SDC2 interacted with syntenin, which promoted the formation of the EVs, and SYTL4 is associated with the release of EVs. Moreover, we found that stimulation of EV secretion by LMP1 can enhance the proliferation and invasion ability of recipient NPC cells and tumor growth in vivo. In summary, we found a new mechanism by which LMP1 upregulates SDC2 and SYTL4 through NF‐κB signaling to promote EV secretion, and further enhance cancer progression of NPC.
Keywords: extracellular vesicle, LMP1, nasopharyngeal carcinoma, SDC2, SYTL4
We found a new mechanism by which latent membrane protein 1 upregulated syndecan‐2 and synaptotagmin‐like‐4 through nuclear factor‐kB signaling to promote exosome secretion, and further enhance cancer progression of nasopharyngeal carcinoma.

1. INTRODUCTION
The tumor microenvironment (TME) is closely related to the occurrence and development of tumors. Tumor cells cannot only contact cells directly, but can also secrete soluble cytokines and extracellular vesicles (EVs) to connect with other cells in the TME.1, 2 Extracellular vesicles include vesicles present in extracellular space (both exosomes and microvesicles); exosomes are small nanosized vesicles (endosomal origin, 50‐150 nm) and microvesicles vary in their sizes (plasma membrane origin, 100‐2000 nm).3, 4 Extracellular vesicles contain large numbers of information molecules of their origin cells, and transmit these molecules to recipient cells, inducing related signaling pathways and performing a variety of important biological functions.5, 6, 7
Nasopharyngeal carcinoma (NPC) is a malignant nasopharyngeal epithelial tumor that occurs mostly in Southeast Asia and southern China.8 Epstein‐Barr virus (EBV) has been confirmed to have a significant correlation with the progression of NPC.9 The EBV‐encoded latent membrane protein 1 (LMP1) is considered to be a major oncogenic protein.10 Intracellular LMP1 activates a variety of signaling pathways, including NF‐κB, JNK/SAPK, PI3K, P38/MAPK, ERK/MAPK, JAK/STAT, and others, in a ligand‐independent manner, and further regulates some cellular proteins that contribute to NPC development by affecting cell proliferation, antiapoptosis, cell migration, angiogenesis, and therapy resistance.11, 12, 13
Several reports showed that LMP1 can be localized to the Golgi apparatus and polycystic endosomes and further encapsulated in the EV endocrine system to the extracellular environment.14 Furthermore, transmission of LMP1‐EVs to recipient cells activates PI3K/Akt and MAPK/ERK signaling pathways to promote malignant cell growth, invasion, and metastasis.15, 16 CD63 is a critical player in LMP1 exosomal trafficking and LMP1‐mediated enhancement of EV production,17, 18, 19 and it has been found that C‐terminal farnesylation of UCH‐L1 is also one of the mechanisms by which LMP1 is sorted to EVs.20 Therefore, LMP1 cannot only be loaded in EVs, but might also be involved in the regulation of EV secretion. However, the molecular mechanism by which LMP1 promotes EV secretion is not well understood.
In the present study, we found that LMP1 promotes EV secretion associated with syndecan‐2 (SDC2) and synaptotagmin‐like‐4 (SYTL4) through NF‐κB signaling in NPC cells. Further studies indicated that SDC2 is associated with the formation of EVs, and SYTL4 is associated with the release of EVs. In addition, we found that stimulation of EV secretion can enhance cancer progression of NPC.
2. MATERIALS AND METHODS
2.1. Cell culture and transfection
CNE1, HONE1, and HK1 are LMP1‐negative NPC cell lines. CNE1‐LMP1 (CM) is an LMP1‐transfected CNE1 cell. C666‐1 is an NPC cell line that carries the EBV virus and expresses LMP1.21 Cells were supplemented with 10% FBS (Invitrogen) using RPMI‐1640 (Gibco BRL). Cells were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. The LMP1‐, SDC2‐, SYTL4‐, p65‐, and control siRNAs were synthesized by Ribo Bio‐Technology, and the sequences of siRNA are described in Table S1.
2.2. Extracellular vesicle isolation
After 48 hours of cell culture with EV‐free FBS medium (SBI), EVs were isolated used Exoquick‐TC reagent (EXOTC10A‐1, SBI) according to the manufacturer’s protocol. We have submitted all relevant data of our experiments to the EV‐TRACK knowledgebase (EV‐TRACK ID: EV190100).22
2.3. Transmission electron microscopy analysis
The isolated EVs were dissolved in PBS, 10 μL of the samples were added to the copper mesh for 1 minute, 10 μL uranyl acetate was added to the copper mesh for 1 minute, and then the filter paper was sucked off and floated at room temperature for several minutes. The results were observed with a transmission electron microscope (FEI).
2.4. Nanoparticle tracking analysis
The isolated EVs were resuspended and further diluted in PBS to analyze their size distribution and concentration using the NanoSight NS300 system (Malvern Instruments) according to the manufacturer’s instructions.
2.5. Quantification of EV protein
Extracellular vesicles were lysed with IP lysis buffer (Pierce, Thermo Fisher Scientific) to obtain total protein. A BCA assay was used to measure the protein concentrations used BCA Assay reagent (Pierce Chemical) following the manufacturer’s protocol.
2.6. Animal experiments
Animal work was approved by the Animal Care Committee of Xiangya Hospital (Changsha, China) in accordance with Institutional Animal Care and Use Committee guidelines. HK1 cells (1 × 107) mixed with different concentrations of CM‐EVs (0, 100, or 200 μg) were injected in each 6‐week‐old female athymic nude mouse (BALB/C) to establish xenografts, and then accordant quantity EVs were injected every 2 days in the vicinity of the s.c. tumors. Tumor formation was examined every 3 days. At the indicated time points, animals were killed, and tissues were collected and fixed with 10% buffered formalin for immunohistochemical (IHC) analysis.
2.7. Statistical analysis
Data were processed by GraphPad Prism5, and the method was the mean ± SD (ie, Χ ± S). Student’s t test was used for calculating the significance between different groups, and the correlations between IHC‐determined protein expression levels were evaluated by Spearman’s correlation coefficient analysis. The statistical significance was indicated by P < .05.
Other materials and methods such as Abs and reagents, RNA sequencing analysis, quantitative RT‐PCR, western blot analysis, and coimmunoprecipitation (Co‐IP) analysis, immunofluorescence assay, ChIP, plasmid constructs and luciferase reporter assay, IHC, cell proliferation assay, and Transwell experiment are described in Appendix S1, and the primers for quantitative RT‐PCR, ChIP, and plasmid construction are provided in Table S2.
3. RESULTS
3.1. Latent membrane protein 1 promotes EV secretion in NPC cells
First, we validated the expression of LMP1 in experimental cell lines at mRNA and protein levels (Figure 1A,B). Cells were then cultured in EV‐depleted FBS medium for 48 hours, EVs were isolated from the supernatants of cells using Exo‐QuickTC reagent, and the EVs were examined by transmission electron microscopy, nanoparticle tracking analysis, and western blot analysis for EV protein markers. The data indicated the presence of 80‐100 nm vesicles (Figure 1C), the size distribution ranged from 30 to 200 nm, and the peak was approximately 100 nm. Approximately 3.0 × 102 particles/cells and 2.9 × 102 particles/cells were obtained from LMP1‐negative CNE1 and HK1 cells, respectively, and approximately 5.7 × 102 particles/cells and 5.2 × 102 particles/cells were obtained from the LMP1‐positve CM and C666‐1 cells, respectively (Figure 1D,E). Furthermore, we characterized the CD63 and heat shock protein (HSP)70 (putative markers for EVs) as well as calnexin (marker for the endoplasmic reticulum). The data, as seen in Figure 1F, also indicated that the isolated vesicles were mostly EVs according to MISEV 2018.3 Next, we detected the protein content of EVs in the supernatant of different cells using the BCA Protein Assay, as the total protein of the EVs indirectly reflects the number of EVs.23 The data showed that the number of secreted EVs by LMP1‐positive CM and C666‐1 cells was greater than the same number of LMP1‐negative CNE1 and HK1 cells (P < .01) (Figure 1G). After knockdown of LMP1 using target LMP1 siRNA in two LMP1‐positive NPC cells, the total EVs protein content was obviously reduced compared with untreated and negative control siRNA groups (Figure 1H,I). Furthermore, after overexpression of LMP1 in the LMP1‐negative NPC cell lines HONE1 and HK1, there was a distinct increase of the total EVs protein content compared with control groups (P < .01) (Figure 1J‐L). These data revealed an increase in EV secretion following LMP1 expression.
Figure 1.
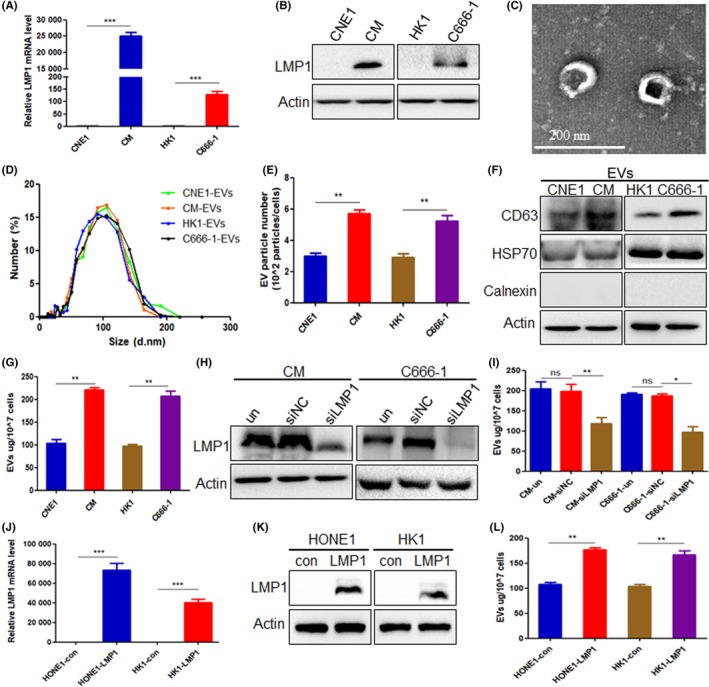
Latent membrane protein 1 (LMP1) promotes extracellular vesicle (EV) secretion in nasopharyngeal carcinoma (NPC) cells. A, Quantitative RT‐PCR was used to detect the mRNA expression of LMP1 in CNE1, CNE1‐LMP1 (CM), HK1, and C666‐1 cells. B, Western blot analysis was used to detect the protein expression of LMP1 in CNE1, CM, HK1, and C666‐1 cells. C, Representational image of isolated EVs from NPC cell CM by transmission electron microscopy analysis. D, Nanoparticle tracking analysis showing the particle size of EVs isolated from CNE1, CM, HK1, and C666‐1 cells. E, Comparison of the particle numbers of EVs isolated from CNE1, CM, HK1, and C666‐1 EVs. F, Western blot analysis was used to detect the molecular positive markers CD63, heat shock protein (HSP)70, and negative marker calnexin of EVs isolated from equal numbers of CNE1, CM, HK1, and C666‐1 cells. G, EV protein content of equal numbers of NPC cells was detected by BCA assay. H, Western blot analysis was used to detect the knockdown effect of LMP1 using siRNA. I, After transfection with siLMP1 for 48 h, the EV protein content of equal numbers of NPC cells was detected by BCA assay. J, Quantitative RT‐PCR was used to detect the mRNA expression of LMP1 in HONE1 and HK1 cells after transfection of pGV141‐LMP1‐wt vector. K, Western blot analysis was used to detect the protein expression of LMP1 in HONE1 and HK1 cells after transfection of pGV141‐LMP1‐wt vector. L, After transfection with pGV141‐LMP1‐wt vector for 48 h, the EV protein content of equal numbers of NPC cells was detected by BCA assay. *P < .05, **P < .01, ***P < .001. con, control empty plasmid; ns, not significant; siNC, negative control siRNA; un, untreated
3.2. Latent membrane protein 1 upregulates the expression of SDC2 and SYTL4
To clarify the key signaling molecules of LMP1 promoting EV secretion in NPC cells, the mRNA sequencing was carried out for CNE1 and CM cells and the expression changes of genes related to EV secretion were screened. The results showed that there were 10 genes whose expression were upregulated in CM cells rather than CNE1 cells, including CD37, RAB7B, RAB25, RAB9B, TBC1D10A, CD28, CD53, SYTL4, SDC2, and SNAP‐25 (Figure 2A). The quantitative RT‐PCR further indicated 4 increased molecules in CM cells, and SDC2 and SYTL4 were selected because of obvious differential expression (P < .001) (Figure 2B). Western blot analysis showed that the protein expression level of SDC2 and SYTL4 in LMP1‐positive CM and C666‐1 cells was higher than that in LMP1‐negative CNE1 and HK1 cells (Figure 2C). After knockdown of LMP1 expression with siRNA in CM and C666‐1 cells, it was found that the expression of SDC2 and SYTL4 was distinctly decreased (Figure 2D). Furthermore, after overexpression of LMP1 in HONE1 and HK1 cells, there was an obvious increase in the expression of SDC2 and SYTL4 compared with control groups (Figure 2E). These results indicated that LMP1 can upregulate the expression of SDC2 and SYTL4 in NPC.
Figure 2.

Latent membrane protein 1 (LMP1) upregulates the expression of syndecan‐2 (SDC2) and synaptotagmin‐like‐4 (SYTL4). A, Heat map analysis of the expression differences of extracellular vesicle (EV) secretion‐related genes in CNE1 and CM cells through mRNA sequencing. B, Quantitative RT‐PCR was used to detect the expression difference of EV secretion‐related genes in CNE1 and CM cells. C, Protein expression levels of SDC2 and SYTL4 in CNE1, CM, HK1, and C666‐1 cells by western blot analysis. D, Expression of SDC2 and SYTL4 in nasopharyngeal carcinoma (NPC) cells by western blot analysis after transfection with siLMP1 for 48 h. E, Expression of SDC2 and SYTL4 in NPC cells by western blot analysis after transfection with pGV141‐LMP1‐wt vector for 48 h. **P < .01, ***P < .001 con, control empty plasmid; siNC, negative control siRNA; un, untreated
3.3. Latent membrane protein 1 promotes EV secretion through SDC2 and SYTL4
Using CD63 as a marker of multivesicular bodies (MVBs) to detect the number of intracellular MVBs by an immunofluorescence experiment as previously described.23 After overexpression of LMP1 in HONE1 and HK1 cells, there was an obvious increase in the number of MVBs compared to control groups (P < .01) (Figure 3A). To determine whether SDC2 and SYTL4 are key molecules for the EV secretion regulated by LMP1, we synthesized siRNA targeting SDC2 and SYTL4 and found that si‐SDC2‐2 and si‐SYTL4‐2 had the best inhibitory effects at both transcriptional and protein levels (Figure 3B,C). After knockdown of SDC2 or SYTL4 with targeted siRNA, the EVs in the tumor cell supernatant was isolated, and data showed that EV secretion was reduced in CM and C666‐1 cells (Figure 3D). Studies have reported that syndecan can mediate the formation of EV.24 Furthermore, as an effector molecule of RAB27A, SYTL4 mainly promotes the fusion of vesicles with cell membranes to secrete EVs out of the extracellular environment.23 Results of immunofluorescence experiments indicated that the number of MVBs in LMP1‐positive CM and C666‐1 cells was more than in LMP1‐negative CNE1 and HK1 cells. After knockdown of the expression of SDC2 in CM and C666‐1 cells, the number of MVBs was reduced (P < .05 and P < .01, respectively), indicating that SDC2 mediates the formation of EVs following LMP1 expression. Meanwhile, after knockdown of the expression of SYTL4 in CM and C666‐1 cells, the number of MVBs was obviously increased (P < .05 and P < .01, respectively), suggesting that MVBs could not be fused with the cell membrane with silencing of SYTL4, resulting in a large intracellular MVB accumulation (Figure 3E). These results indicated that SYTL4 mediates the release of EVs following LMP1 expression.
Figure 3.
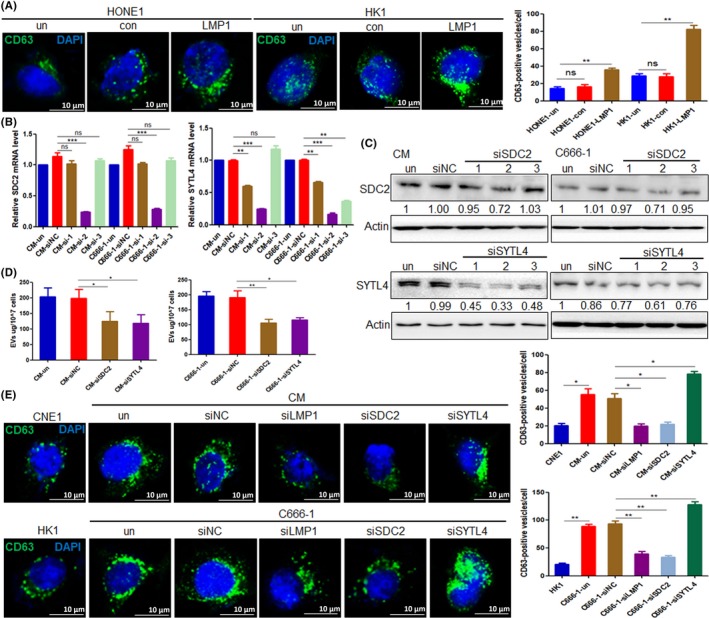
Latent membrane protein 1 (LMP1) promotes extracellular vesicle (EV) secretion through syndecan‐2 (SDC2) and synaptotagmin‐like‐4 (SYTL4). A, Quantities of multivesicular bodies (MVBs) in HONE1 and HK1 cells were detected by immunofluorescence assay, after transfection with pGV141‐LMP1‐wt vector for 48 h. B, mRNA expression levels of SDC2 and SYTL4 after transfection with SDC2 and SYTL4 siRNA by quantitative RT‐PCR analysis in CM and C666‐1 cells, respectively. C, Protein expression levels of SDC2 and SYTL4 after transfection with SDC2 and SYTL4 siRNA by western blot analysis in CM and C666‐1 cells, respectively. D, Protein content of EVs isolated from CM and C666‐1 cells was detected by BCA assay, after transfection with siSDC2‐2 and siSTYL4‐2 for 48 h. E, Quantities of MVBs in nasopharyngeal carcinoma cells were detected by immunofluorescence assay, after transfection with siSDC2‐2 and siSTYL4‐2 for 48 h. *P < .05, **P < .01, ***P < .001. con, control empty plasmid; ns, not significant; siNC, negative control siRNA; un, untreated
3.4. Latent membrane protein 1 upregulates SDC2 and SYTL4 expression through NF‐κB signaling
Studies have shown that LMP1 can activate the transcription factor NF‐κB, and phosphorylated activated NF‐κB enters the cellular nucleus to regulate the expression of multiple target genes.10, 11 We validated that more p‐p65 expression occurred in LMP1‐positive CM and C666‐1 cells than in LMP1‐negative CNE1 and HK1 cells, and found that p‐p65 was decreased after knockdown of LMP1 expression in NPC cells (Figure 4A). It was also found that p‐p65 was increased after overexpression of LMP1 in HONE1 and HK1 cells (Figure 4B). Bioinformatics analysis by Genematix Software (https://www.genomatix.de/) predicted that NF‐κB binding sites would be found in the SDC2 and SYTL4 promoter regions (Figure 4C). The ChIP‐PCR analysis showed that more NF‐κB p65 bound to the promoter of SDC2 and SYTL4 in LMP1‐positive NPC cells than in LMP1‐negative cells (Figure 4D). Subsequently, the WT and mutated binding sites of the SDC2 and SYTL4 promoters were respectively cloned into luciferase reporter vectors, and luciferase reporter assays showed that the luciferase activity was obviously higher in the WT vector group than in the basic control vector group (P < .01 and P < .05, respectively). However, the mutations decreased the promoter activity (Figure 4E). These results revealed that SDC2 and STYL4 were direct targets of NF‐κB p65 in NPC cells. Furthermore, after inhibiting NF‐κB activity by curcumenol, which inhibits NF‐κB activation by suppressing the NF‐κB p65 subunit, and siRNA targeting p65, the data showed that the expression of SDC2 and SYTL4 was decreased (Figure 4F,G). Collectively, these results suggest that LMP1 can activate SDC2 and SYTL4 expression through NF‐κB signaling.
Figure 4.
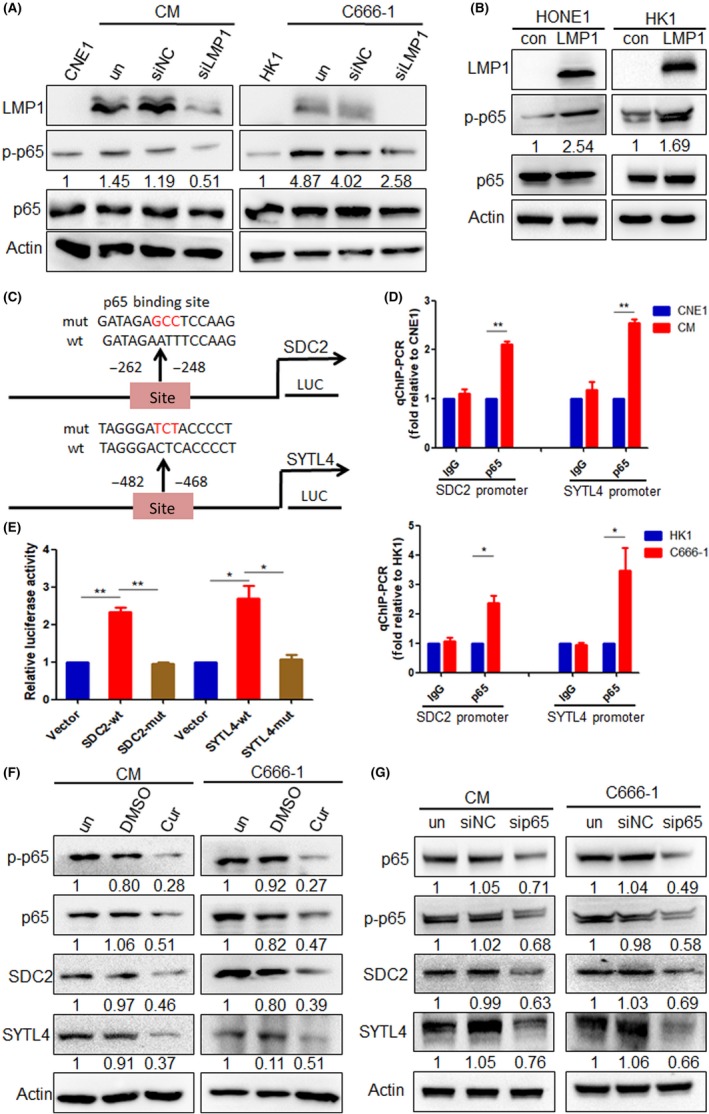
Latent membrane protein 1 (LMP1) upregulates syndecan‐2 (SDC2) and synaptotagmin‐like‐4 (SYTL4) expression through nuclear factor (NF)‐κB signaling. A, NF‐κB p65 and p‐p65 protein expression levels of nasopharyngeal carcinoma (NPC) cells was analyzed by western blotting, after transfection with siLMP1 for 48 h. B, NF‐κB p65 and p‐p65 protein expression level of HONE1 and HK1 cells after transfection with pGV141‐LMP1‐wt vector for 48 h was analyzed by western blotting. C, Wild‐type (wt) and mutant (mut) type of binding sites of NF‐κB binding to SDC2 and SYTL4 promoter. D, ChIP analysis was carried out using a p65 Ab, and the enrichment of the SDC2 and STYL4 promoter sequence was detected by quantitative RT‐PCR. E, 293T cells were cotransfected with luciferase plasmids (pGL3 vector, pGL3‐SDC2/STYL4‐wt, and pGL3‐SDC2/STYL4‐mut) and GFP‐p65, and dual‐luciferase reporter gene assays were carried out. F, Protein expression of SDC2 and SYTL4 in NPC cells, after inhibiting NF‐κB activity by 20 μmol/L curcumenol for 24 h, were detected using western blotting. G, Protein expression of SDC2 and SYTL4 in NPC cells, after transfection with siRNA targeting p65 for 48 h, were detected using western blotting. *P < .05, **P < .01. con, control empty plasmid; siNC, negative control siRNA; un, untreated
3.5. Latent membrane protein 1 promotes interaction of SDC2‐syntenin
Studies have confirmed that syndecan can interact with syntenin to promote EV formation,24, 25 suggesting that SDC2 might promote EV formation through the SDC2‐syntenin axis. We used Co‐IP experiments to determine whether LMP1 regulates the interaction between SDC2 with syntenin in HK1 and C666‐1 cells. The results indicated that LMP1 could upregulate SDC2 expression and further promoted the interaction of SDC2 with syntenin; they also showed that syntenin interacted with SDC2, but did so independently of LMP1 (Figure 5A). Furthermore, the colocalization of SDC2 and syntenin in NPC cells was detected by immunofluorescence assay. The results indicated that the fluorescence intensity of SDC2 in LMP1‐positive C666‐1 cells was higher than that in LMP1‐negative HK1 cells. In addition, SDC2 and syntenin colocalized in both cells, but the fluorescence intensity of SDC2 colocalized with syntenin was stronger in C666‐1 cells. After knockdown of LMP1 expression in C666‐1 cells, the fluorescence intensity of SDC2 was weakened, and the fluorescence intensity of colocalization of SDC2 and syntenin was also attenuated (Figure 5B). The above results suggested that LMP1 promotes EV secretion through the SDC2‐syntenin axis.
Figure 5.
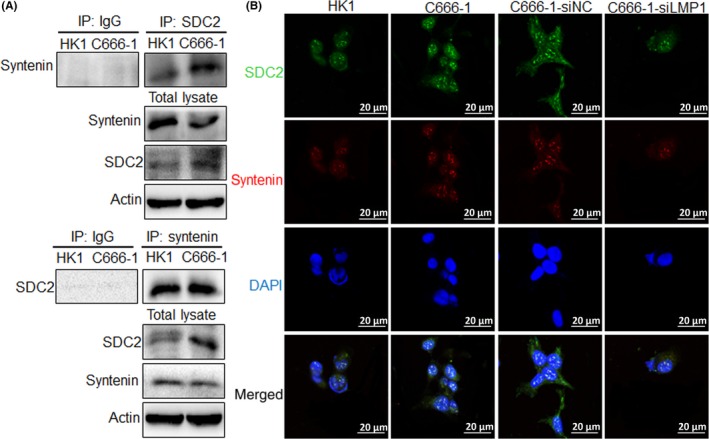
Latent membrane protein 1 (LMP1) promotes the interaction of syndecan‐2 (SDC2) and syntenin. A, Interaction between SDC2 and syntenin was detected by coimmunoprecipitation. B, Colocalization of SDC2 and syntenin in nasopharyngeal carcinoma cells was detected by immunofluorescence assay. IP, immunoprecipitant
3.6. Latent membrane protein 1 promotes EV secretion and enhances tumor cell proliferation and invasion
To investigate the effect of EV secretion on the proliferation of recipient NPC cells, HK1 cells were cocultured with conditional medium of CNE1 and CM, and HONE1 cells were cocultured with conditional medium of HK1 and C666‐1, respectively. The MTS assay results showed that the tumor cells cultured with conditional medium of LMP1‐positive NPC cells had higher proliferation ability than the cells cultured with conditional medium of LMP1‐negative NPC cells (P < .05 and P < .01, respectively) (Figure 6A). These effects could be caused by the different content of the EVs or the quantity of the EVs, the secretion of which was promoted by LMP1. Therefore, when HK1 and HONE1 were treated with EVs isolated from CM (100 μg, approximately 2.59 × 109 particles) and C666‐1 cells (100 μg, approximately 2.51 × 109 particles), respectively, at different concentrations, the results indicated that an increasing quantity (0, 100, and 200 μg) of EVs could enhance the proliferation of recipient NPC cells (P < .01) (Figure 6B). Furthermore, HK1 cells were cocultured using conditional medium of CM cells with LMP1, SDC2, and SYTL4 knockdown, respectively, and HONE1 cells were cocultured using conditional medium of C666‐1 cells with LMP1, SDC2, and SYTL4 knockdown, respectively, as well. The results showed that, compared with the control group, the proliferation decrease of HK1 and HONE1 cells were observed after being cocultured by conditional medium of LMP1‐positive cells with silencing of LMP1, SDC2, and SYTL4 (Figure 6C).
Figure 6.
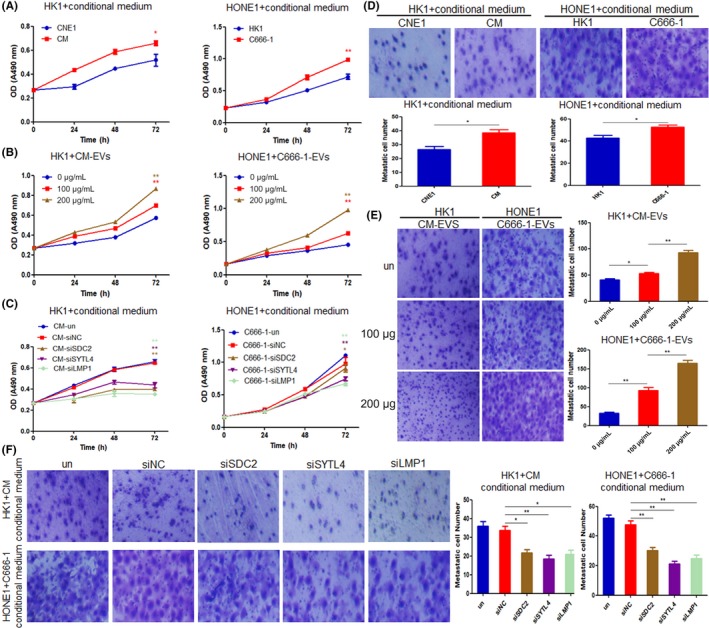
Latent membrane protein 1 (LMP1) promotes extracellular vesicle (EV) secretion and enhances tumor cell proliferation and invasion. A, Proliferation of HK1 and HONE1 cells cocultured with conditional medium of CNE1, CM, HK1, and C666‐1 cells, were detected by MTS assay. B, Proliferation of HK1 and HONE1 cells cocultured with different concentrations of EVs isolated from CM or C666‐1 cells, respectively, were detected by MTS assay. C, Proliferation of HK1 and HONE1 cells cocultured with conditional medium of CM or C666‐1 cells, which were transfected with siLMP1, siSDC2‐2, and siSTYL4‐2 for 48 h, were detected by MTS assay. D, Invasion of HK1 and HONE1 cells cocultured with conditional medium of CNE1, CM, HK1, and C666‐1 cells, were detected by Transwell experiments. E, Invasion of HK1 and HONE1 cells cocultured with different concentrations of EVs isolated from CM or C666‐1 cells, respectively, were detected by Transwell experiments. F, Invasion of HK1 and HONE1 cells cocultured with conditional medium of CM or C666‐1 cells, which were transfected with siLMP1, siSDC2‐2, and siSTYL4‐2 for 48 h, were detected by Transwell assay. *P < .05, **P < .01. Magnification, ×200
Next, we explored the effects of EV secretion on the invasion of recipient NPC cells. The Transwell assay results showed that the tumor cells cultured with conditional medium of LMP1‐positive NPC cells obtained stronger invasion ability than the cells cultured with conditional medium of LMP1‐negative NPC cells (Figure 6D). Furthermore, increasing the quantity of EVs could promote the invasion of recipient HK1 and HONE1 cells (Figure 6E). HK1 and HONE1 cells were cocultured with conditional medium of CM and C666‐1 cells with knockdown of LMP1, SDC2, and SYTL4, respectively. The results showed that, compared with the control group, the obviously decreased invasion of HK1 and HONE1 cells were observed after being cocultured with conditional medium of LMP1‐positive cells that knock down LMP1, SDC2, and SYTL4, respectively (Figure 6F). The above results indicate that LMP1 promotes EV secretion through SDC2 and STYL4 and enhances the proliferation and invasion of recipient tumor cells.
3.7. Latent membrane protein 1 promotes EV secretion and enhances tumor growth in vivo
For investigating whether CM cell‐derived EVs promote tumor growth in vivo, and whether the quantity of EVs was responsible for this effect, the animal experiments were undertaken. As shown in Figure 7A,B, the results indicated that EVs derived from CM cells promoted tumor growth compared with control groups (P < .05). Furthermore, as the quantity of EVs increased from 100 μg to 200 μg, it resulted in significant increased tumor volume (P < .01). Moreover, in the clinicopathological specimens of NPC patients, IHC staining showed that the expression of LMP1 was positively correlated with SDC2, STYL4, and CD63, respectively, and expression of SDC2 and STYL4 were also positively correlated with CD63 (P < .001) (Figure 7C). These data further indicated that LMP1 promotes EV secretion and enhances tumor growth in vivo.
Figure 7.
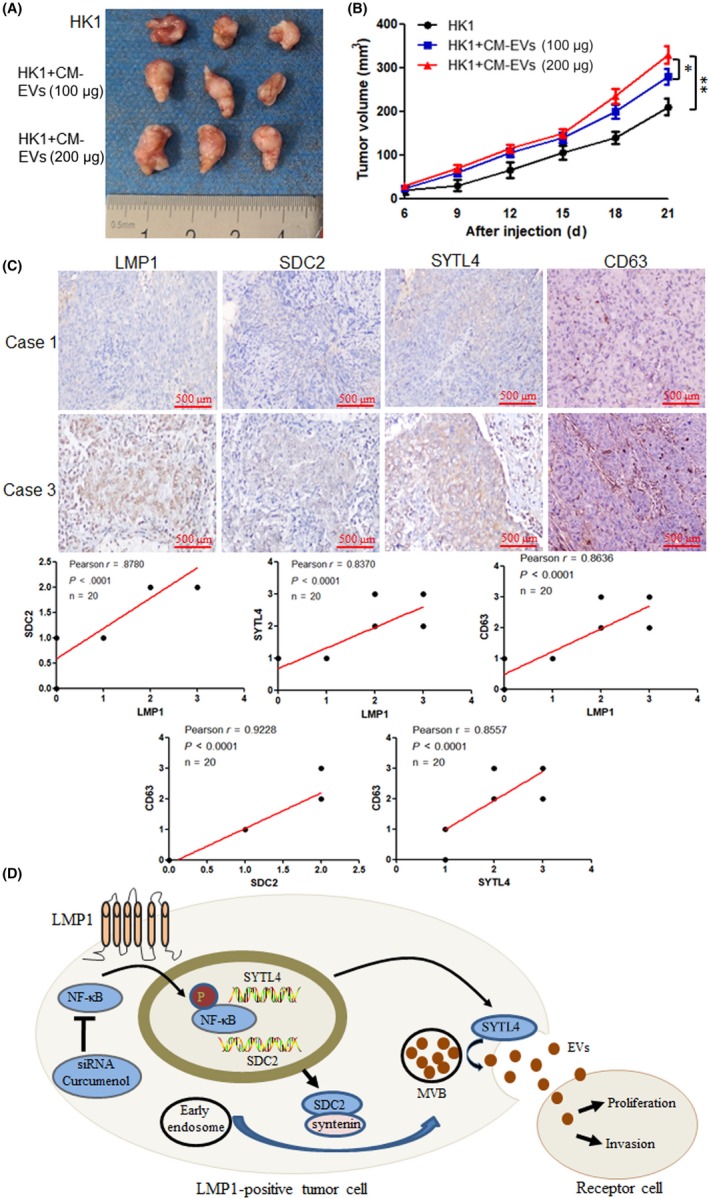
Latent membrane protein 1 (LMP1) promotes extracellular vesicle (EV) secretion and enhances tumor growth in vivo. Female BALB/c nude mice were s.c. inoculated with 1 × 107 HK1 cells. Xenografts were treated with different concentrations of CM EVs (0, 100, or 200 μg). A, At the end of the experiment, the mice were killed and the tumors were separated. Tumor mass of each group is shown in the graph. B, Tumor volume was examined every 3 d and shown in the graph. C, Immunohistochemical stain of the clinicopathologic specimens of nasopharyngeal carcinoma patients, and the correlation analysis between LMP1 and syndecan‐2 (SDC2), synaptotagmin‐like‐4 (SYTL4), and CD63, and the correlation analysis between CD63 and SDC2 and SYTL4. D, Model of LMP1 promotion of EV secretion. Stimulation of EV secretion enhances the proliferation and invasion of recipient tumor cells
4. DISCUSSION
Extracellular vesicle secretion involves 2 processes: the formation process, and the release process.26 Studies have shown that the RAB GTPase proteins are important regulators of intracellular vesicle transport, regulating vesicle trafficking at different stages, such as vesicle budding, vesicle and organelle flow, vesicle and accurate convergence, and fusion of cell membranes.27, 28 Sorting of cargo into EVs is a key step in the formation of EVs, which is regulated by a series of molecules, including endosomal sorting complex required for transport (ESCRT), 4 transmembrane protein CD63, and liposomes.29, 30 In recent years, studies have been undertaken to discover the formation of EVs in the syndecan‐dependent pathway.31, 32 The cytoplasmic domain of syndecan can be associated with the PDZ region of syntenin,33 and the N‐terminal domain of syntenin interacts directly with the accessory protein ALIX of ESCRT. Thus, syndecan forms a trimeric complex with syntenin and ALIX to regulate EV formation.34 The release process of EVs is a process in which MVBs fuse with the cell membrane to release EVs. Rab proteins also play an important role in the release of EVs, and Rab proteins need to interact with their effector molecules to execute their function. SYTL4, as an effector of RAB27A, plays an important role in the precise integration and fusion of MVBs and cell membranes.35, 36
Previous studies indicated that CD63 is a critical player in shuttling LMP1 into EVs or exosomes.17, 18, 19 However, the molecular mechanism by which LMP1 promotes EV secretion is not well understood. In the present study, we showed that LMP1 upregulates the expression of SDC2, and the interaction of SDC2 with syntenin, which promotes the formation of EVs in NPC cells. Additionally, LMP1 could regulate EV release through SYTL4. As LMP1 can activate NF‐κB, and activated NF‐κB regulates the expression of multiple target genes,37 we found that LMP1 could regulate SDC2 and SYTL4 expression through NF‐κB signaling. These results suggest that SDC2 and STYL4 are the critical players in LMP1‐mediated enhancement of EV production.
Several reports have found that LMP1 is secreted by EVs derived from LMP1‐positive cells and can promote cancer progression and metastasis. Meckes et al15 discovered LMP1 is secreted within EVs, and that LMP1‐containing EVs can activate MAPK/ERK and PI3K/Akt signaling pathways within neighboring uninfected cells. More recently, EBV has been shown to dramatically alter the protein content of EVs released from latently infected B cells with most of the significant changes correlating with LMP1 expression.15, 38, 39 Latent membrane protein 1 signaling increases EV‐mediated secretion of cellular proinvasion factors such as fibroblast growth factor‐240 and hypoxia‐inducible factor‐1.16 Previous studies have focused on LMP1 loaded in EVs, which can promote tumorigenesis in recipient cells by transporting LMP1, or identification and analysis of the change of EV content through the presence of LMP1 in cells, leading to the biological phenotype of receptor cells. However, LMP1 promotes EV secretion and its biological function has not been elucidated yet. In the present study, we found that LMP1 upregulates EV secretion, and the stimulation of EV secretion enhances cancer progression of NPC.
In addition, it was reported that intracellular LMP1 is often degraded by ubiquitination in a proteasome‐dependent manner with a half‐life of 1.5‐7 hours, which is much shorter than the cell replication cycle (20‐24 hours), therefore, LMP1 is a short‐lived protein.41, 42 Thus, the persistence and activation of LMP1 is also the premise of its biological function execution.43 It can be seen that EV secretion is not only an important strategy for LMP1 to avoid degradation, but also an important mechanism for LMP1 to participate in intercellular signal exchange, and it continues to play a role in promoting tumor growth. Overall, this study offers new insights into the complex intersection of cellular secretory and degradative mechanisms.
Finally, this study discovered that LMP1 can promote EV secretion through SDC2 and SYTL4, and the stimulation of EV secretion enhances the proliferation and invasion ability of recipient NPC cells and tumor growth in vivo (Figure 7D). This innovative discovery will provide new insights into the tumor‐promoting effect of LMP1 by intervening TME, and the regulation of EV secretion mechanisms.
DISCLOSURES
The authors have no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
This study was supported by grants from the National Natural Science Foundation of China (No. 81372182, 81672761) and the Hunan Natural Science Foundation (2018JJ2545).
Liao C, Zhou Q, Zhang Z, et al. Epstein‐Barr virus‐encoded latent membrane protein 1 promotes extracellular vesicle secretion through syndecan‐2 and synaptotagmin‐like‐4 in nasopharyngeal carcinoma cells. Cancer Sci. 2020;111:857–868. 10.1111/cas.14305
Chaoliang Liao and Qin Zhou contributed equally to this work.
REFERENCES
- 1. Hoshino A, Costa‐Silva B, Shen TL, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang L, Zhang S, Yao J, et al. Microenvironment‐induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527:100‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thery C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sundar IK, Li D, Rahman I. Small RNA‐sequence analysis of plasma‐derived extracellular vesicle miRNAs in smokers and patients with chronic obstructive pulmonary disease as circulating biomarkers. J Extracell Vesicles. 2019;8:1684816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907‐1920. [DOI] [PubMed] [Google Scholar]
- 6. Gyorgy B, Szabo TG, Pasztoi M, et al. Membrane vesicles, current state‐of‐the‐art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667‐2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang X, Sai B, Wang F, et al. Hypoxic BMSC‐derived exosomal miRNAs promote metastasis of lung cancer cells via STAT3‐induced EMT. Mol Cancer. 2019;18:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet. 2005;365:2041‐2054. [DOI] [PubMed] [Google Scholar]
- 9. Lieberman PM. Epstein‐Barr virus turns 50. Science. 2014;343:1323‐1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kieser A, Sterz KR. The Latent Membrane Protein 1 (LMP1). Curr Top Microbiol Immunol. 2015;391:119‐149. [DOI] [PubMed] [Google Scholar]
- 11. Tao Y, Shi Y, Jia J, Jiang Y, Yang L, Cao Y. Novel roles and therapeutic targets of Epstein‐Barr virus‐encoded latent membrane protein 1‐induced oncogenesis in nasopharyngeal carcinoma. Expert Rev Mol Med. 2015;17:e15. [DOI] [PubMed] [Google Scholar]
- 12. Lu J, Tang M, Li H, et al. EBV‐LMP1 suppresses the DNA damage response through DNA‐PK/AMPK signaling to promote radioresistance in nasopharyngeal carcinoma. Cancer Lett. 2016;380:191‐200. [DOI] [PubMed] [Google Scholar]
- 13. Cao Y, Yang L, Jiang W, et al. Therapeutic evaluation of Epstein‐Barr virus‐encoded latent membrane protein‐1 targeted DNAzyme for treating of nasopharyngeal carcinomas. Mol Ther. 2014;22:371‐377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Flanagan J, Middeldorp J, Sculley T. Localization of the Epstein‐Barr virus protein LMP 1 to exosomes. J Gen Virol. 2003;84:1871‐1879. [DOI] [PubMed] [Google Scholar]
- 15. Meckes DG Jr, Shair KH, Marquitz AR, Kung CP, Edwards RH, Raab‐Traub N. Human tumor virus utilizes exosomes for intercellular communication. Proc Natl Acad Sci U S A. 2010;107:20370‐20375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aga M, Bentz GL, Raffa S, et al. Exosomal HIF1alpha supports invasive potential of nasopharyngeal carcinoma‐associated LMP1‐positive exosomes. Oncogene. 2014;33:4613‐4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Verweij FJ, van Eijndhoven MA, Hopmans ES, et al. LMP1 association with CD63 in endosomes and secretion via exosomes limits constitutive NF‐kappaB activation. EMBO J. 2011;30:2115‐2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hurwitz SN, Nkosi D, Conlon MM, et al. CD63 regulates Epstein‐Barr virus LMP1 exosomal packaging, enhancement of vesicle production, and noncanonical NF‐kappaB signaling. J Virol. 2017;91:e02251‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hurwitz SN, Cheerathodi MR, Nkosi D, York SB, Meckes DG Jr. Tetraspanin CD63 bridges autophagic and endosomal processes to regulate exosomal secretion and intracellular signaling of Epstein‐Barr Virus LMP1. J Virol. 2018;92: e01969-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kobayashi E, Aga M, Kondo S, et al. C‐terminal farnesylation of UCH‐L1 plays a role in transport of Epstein‐Barr Virus primary oncoprotein LMP1 to exosomes. mSphere. 2018;3: e00030-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xiao L, Hu ZY, Dong X, et al. Targeting Epstein‐Barr virus oncoprotein LMP1‐mediated glycolysis sensitizes nasopharyngeal carcinoma to radiation therapy. Oncogene. 2014;33:4568‐4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Consortium E‐T, Van Deun J, et al. EV‐TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat Methods. 2017;14(3):228‐232. [DOI] [PubMed] [Google Scholar]
- 23. Ostrowski M, Carmo NB, Krumeich S, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19‐30; sup pp. 1‐13. [DOI] [PubMed] [Google Scholar]
- 24. Roucourt B, Meeussen S, Bao J, Zimmermann P, David G. Heparanase activates the syndecan‐syntenin‐ALIX exosome pathway. Cell Res. 2015;25:412‐428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baietti MF, Zhang Z, Mortier E, et al. Syndecan‐syntenin‐ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14:677‐685. [DOI] [PubMed] [Google Scholar]
- 26. McGough IJ, Vincent JP. Exosomes in developmental signalling. Development. 2016;143:2482‐2493. [DOI] [PubMed] [Google Scholar]
- 27. Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75:193‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kowal J, Tkach M, Thery C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116‐125. [DOI] [PubMed] [Google Scholar]
- 29. van Niel G, Charrin S, Simoes S, et al. The tetraspanin CD63 regulates ESCRT‐independent and ‐dependent endosomal sorting during melanogenesis. Dev Cell. 2011;21:708‐721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255‐289. [DOI] [PubMed] [Google Scholar]
- 31. Lambaerts K, Wilcox‐Adelman SA, Zimmermann P. The signaling mechanisms of syndecan heparan sulfate proteoglycans. Curr Opin Cell Biol. 2009;21:662‐669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Friand V, David G, Zimmermann P. Syntenin and syndecan in the biogenesis of exosomes. Biol Cell. 2015;107:331‐341. [DOI] [PubMed] [Google Scholar]
- 33. Zimmermann P, Tomatis D, Rosas M, et al. Characterization of syntenin, a syndecan‐binding PDZ protein, as a component of cell adhesion sites and microfilaments. Mol Biol Cell. 2001;12:339‐350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513‐525. [DOI] [PubMed] [Google Scholar]
- 35. Fukuda M, Kanno E, Saegusa C, Ogata Y, Kuroda TS. Slp4‐a/granuphilin‐a regulates dense‐core vesicle exocytosis in PC12 cells. J Biol Chem. 2002;277:39673‐39678. [DOI] [PubMed] [Google Scholar]
- 36. Kariya Y, Honma M, Hanamura A, et al. Rab27a and Rab27b are involved in stimulation‐dependent RANKL release from secretory lysosomes in osteoblastic cells. J Bone Miner Res. 2011;26:689‐703. [DOI] [PubMed] [Google Scholar]
- 37. Ma X, Yang L, Xiao L, et al. Down‐regulation of EBV‐LMP1 radio‐sensitizes nasal pharyngeal carcinoma cells via NF‐kappaB regulated ATM expression. PLoS ONE. 2011;6:e24647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pegtel DM, Cosmopoulos K, Thorley‐Lawson DA, et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A. 2010;107:6328‐6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu L, Zuo L, Yang J, et al. Exosomal cyclophilin A as a novel noninvasive biomarker for Epstein‐Barr virus associated nasopharyngeal carcinoma. Cancer Med. 2019;8:3142‐3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ceccarelli S, Visco V, Raffa S, Wakisaka N, Pagano JS, Torrisi MR. Epstein‐Barr virus latent membrane protein 1 promotes concentration in multivesicular bodies of fibroblast growth factor 2 and its release through exosomes. Int J Cancer. 2007;121:1494‐1506. [DOI] [PubMed] [Google Scholar]
- 41. Aviel S, Winberg G, Massucci M, Ciechanover A. Degradation of the epstein‐barr virus latent membrane protein 1 (LMP1) by the ubiquitin‐proteasome pathway. Targeting via ubiquitination of the N‐terminal residue. J Biol Chem. 2000;275:23491‐23499. [DOI] [PubMed] [Google Scholar]
- 42. Martin J, Sugden B. Transformation by the oncogenic latent membrane protein correlates with its rapid turnover, membrane localization, and cytoskeletal association. J Virol. 1991;65:3246‐3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hong SW, Kim SM, Jin DH, Kim YS, Hur DY. RPS27a enhances EBV‐encoded LMP1‐mediated proliferation and invasion by stabilizing of LMP1. Biochem Biophys Res Commun. 2017;491:303‐309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


