Abstract
Recent studies have reported that tumor‐infiltrating mast cells (TIM) play an important role in tumor regression, but the effect of TIM in gallbladder cancer (GBC) remains unclear. The present study aims to investigate the prognostic value of TIM in GBC patients and its responsiveness to gemcitabine‐based adjuvant chemotherapy (ACT). A total of 298 GBC patients from Zhongshan Hospital were recruited for this study. TIM infiltration was measured by immunohistochemical staining. Accumulation of TIM is significantly associated with prolonged overall survival in GBC patients. The benefit from gemcitabine‐based ACT was superior among patients with high infiltration of TIM with GBC. Multivariate analysis identified TIM infiltration as an independent prognostic factor for overall survival. A heatmap showed that TIM‐activated gene signatures were positively correlated with CD8+ T cells' gene signatures. Gene set enrichment analysis (GSEA) suggested that TIM was related to multiple T cell‐related processes and signaling pathways, including the interferon gamma signaling pathway and the leukocyte migration signaling pathway. It was confirmed that CD8+ T cell infiltration was positively correlated with high TIM infiltration in tissue microarray (TMA), suggesting that TIM infiltration was linked to the immune surveillance in GBC. TIM can be used as an independent prognostic factor and a predictor of therapeutic response of gemcitabine‐based ACT in GBC patients, which may mediate immune surveillance by recruiting and activating CD8+ T cells in GBC.
Keywords: adjuvant chemotherapy, gallbladder cancer, prognosis, tumor immune, tumor‐infiltrating mast cells
In gallbladder cancer patients who received chemotherapy, a part of population has been associated with better prognosis. In these patients, elevated recruitment of tumor‐infiltrating mast cells (TIM) resulted in reduced tumor burden and enhanced anti–tumor effect through recruitment and activation of CD8+ T cells. The mechanisms responsible for a tumor‐protective function of TIM and links to the known anti–tumor function of infiltrating cells have remained elusive. Based on the results reported here, we hypersized that interaction between TIM and CD8+ T cells with a pivotal role of chemokines and their receptors underlies these clinical observations.
1. INTRODUCTION
Gallbladder cancer (GBC) is the most common malignant biliary cancer, with strong capability for invasion and metastasis.1, 2 Most patients with GBC are diagnosed at advanced stage and, therefore, miss the opportunity to undergo radical surgery. Chemotherapy is important to improve the survival of patients with advanced GBC.3 Unfortunately, the effect of chemotherapy and targeted therapy for GBC remains limited.4
The recent breakthroughs in tumor immune therapy have shown promising therapeutic effects against cancer.5, 6, 7 Among the diverse components of the tumor microenvironment (TME), tumor‐infiltrating immune cells are regarded as a key factor influencing immunotherapy response.8, 9 Tumor‐infiltrating mast cells (TIM) accumulate in the TME in the early stages, and the infiltration of TIM is generally correlated with poor prognosis in multiple malignant tumors.10, 11 However, in human kidney cancer and gastric cancer, the accumulation of TIM is associated with favorable prognosis.12, 13 Although it is reported that mast cells can directly inhibit tumor progression by releasing mediators such as TNF and heparin, mast cells can also generate effective anti–tumor immunity by recruiting and activating the immune cells in the TME, including natural killer (NK) cells, CD8+ T cells and dendritic cells.10, 14, 15 Recent studies have suggested that mast cells recruit T cells at sites of inflammation by secreting CCL3, CCL5, CXCL10 and LTB4.16 In addition, mast cells can activate T cells through cell to cell interactions via expression of the B7 and OX40L.17
The infiltration of TIM in GBC has not been investigated sufficiently. In this study, GBC patients with abundant TIM infiltration have a favorable prognosis and can significantly benefit from adjuvant chemotherapy. Further bioinformatics analysis and immunohistochemistry assays confirm that TIM are positively correlated with CD8+ T cell infiltration and activation. We propose that TIM have an anti–tumor effect through recruitment and activation of CD8+ T cells in GBC.
2. MATERIALS AND METHODS
2.1. Study population
The study enrolled 289 consecutive patients with GBC who underwent surgical resection between January 2008 and October 2014 at Zhongshan Hospital, Fudan University (Shanghai, China). This study was approved by the Ethics Committee of Zhongshan Hospital, and written informed consent was obtained from all patients. The clinicopathological and baseline demographic characteristics of the patients, including age, gender, tumor differentiation, vascular invasion and TNM stage, were collected retrospectively. The tumor TNM stage assessment was undertaken by two independent pathologists from the Department of Pathology, Zhongshan Hospital, according to the 7th edition of the UICC/AJCC cancer staging manual. Overall survival (OS) was calculated from the date of surgery to the date of death or last visit.
2.2. Tissue microarray, immunohistochemistry and immunofluorescence
Tissue microarray (TMA) was established with formalin‐fixed paraffin‐embedded surgical specimens, and immunohistochemical staining was performed on TMA according to the protocols previously described18 with appropriate antibodies after control staining (anti–tryptase antibody, ab2378 diluted 1:10 000; anti–CD8, ab4055, diluted 1:400; FOXP3 ab22510, diluted 1:200; CCR2 1:100, ab203128 and CCR5, ab7346, 20 µg/mL). The negative control sections were treated equally, with primary antibody omitted. The number of TIM, CD8+ T cells and Treg cells per field were evaluated with Image Pro Plus 6.0 (Media Cybernetics). Then, two secondary antibodies, Alexa 594 goat anti–rabbit IgG and Alexa 488 goat anti–mouse IgG, were applied. Images were acquired with a Nikon Eclipse Ti‐S Microscope. Identical settings were used for each photograph. Positive staining was calculated under a high magnification field (400×). The cutoff point for the high/low infiltrated mast cells was determined via X‐tile software through a “minimum P‐value” method based on the patients' OS information.
2.3. Gene set enrichment analysis
Despite the lack of data in The Cancer Genome Atlas (TCGA) on GBC, TCGA cholangiocarcinoma data were extracted to conduct differential expression analysis to establish whether the gallbladder shares embryonic origin with bile ducts. The mRNA expression data of TCGA were downloaded from cBioPortal and were already normalized to RSEM format. A total of 36 samples were analyzed in this study. Gene set enrichment analyses (GSEA) were applied to determine the biological pathway divergences between high and low TIM. Significant differential gene expression between groups with high and low infiltration of TIM was explored using the edgeR package. The cutoff value of TIM in BLCA data was determined as median.
2.4. Statistical analyses
Statistical analysis was performed using SPSS 22.0 (IBM), Medcalc Software (version 15.2.2) and Stata SE, version 13.0. The χ2 test or the Fisher exact test was used to evaluate the correlation between clinicopathological factors and immunohistochemical variables. Continuous variables were analyzed using the t test. OS curves were plotted using the Kaplan‐Meier method, and the log‐rank test was used to analyze the difference between subgroups. Cox univariate and multivariate regression analysis was performed. All statistical analyses were two‐sided, and P < 0.05 was regarded as statistically significant.
3. RESULTS
3.1. Correlation between baseline variables including tumor‐infiltrating mast cell and gallbladder cancer patient prognosis
For insight into the role of TIM infiltration in GBC, we first performed immunohistochemical staining in 289 GBC TMA. Tryptase positive staining identified diffuse mast cells in the tumor tissues (Figure 1A). The detailed correlations between TIM infiltration and clinicopathological factors of 289 GBC patients are summarized in Table 1. Remarkably, high infiltration of TIM correlated negatively with TNM stage (Figure 1B). Multivariate Cox regression analyses further identified that TIM accumulation was significantly associated with OS (Table 2).
Figure 1.
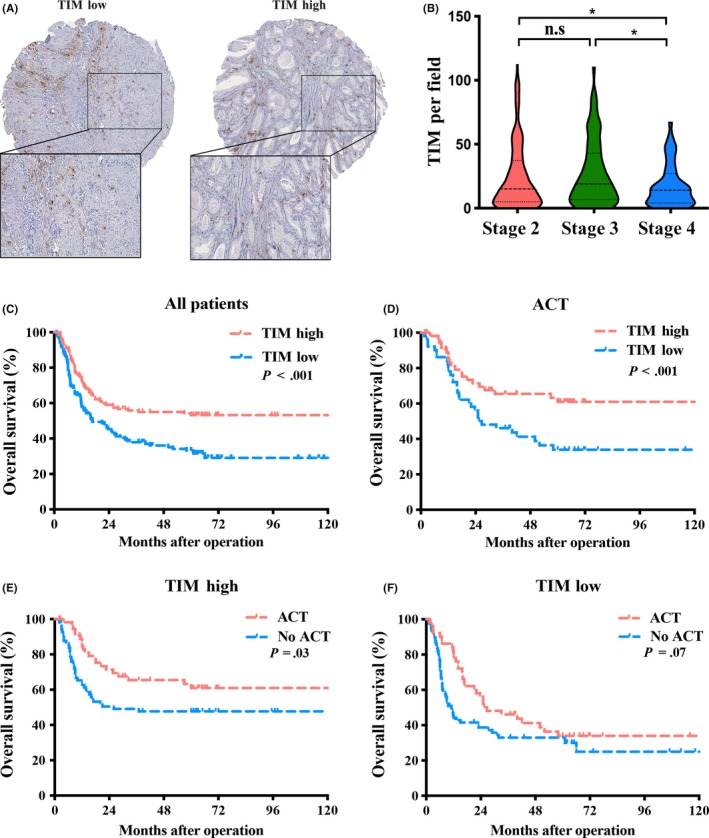
Evaluation of tumor‐infiltrating mast cells (TIM) by immunohistochemical staining in gallbladder cancer (GBC) patients. A, Representative immunohistochemistry images of TIM infiltration in different tumor tissues. B, Distribution of TIM in different TNM stages of Zhongshan cohort. C‐F, Kaplan‐Meier survival analysis of overall survival in all patients (C), patients who received adjuvant chemotherapy (ACT) (D), patients with TIM high (E) and patients with TIM low (F)
Table 1.
Correlation between mast cells infiltration and patient characteristics
| Characteristic | Patients (n = 289) | TIM infiltration | P a | ||
|---|---|---|---|---|---|
| Number | % | Low (n = 145) | High (n = 144) | ||
| Age at surgery, yearsb | |||||
| Mean ± SD | 63.09 ± 11.06 | 61.68 ± 11.19 | 64.50 ± 10.77 | 0.031 | |
| Gender | |||||
| Female | 185 | 64.0 | 92 | 93 | 0.84 |
| Male | 104 | 36.0 | 53 | 51 | |
| pT‐stage | |||||
| T2 | 171 | 59.2 | 87 | 84 | 0.81 |
| T3 | 85 | 29.4 | 40 | 45 | |
| T4 | 33 | 11.4 | 18 | 15 | |
| pN‐stage | |||||
| N0 | 226 | 78.2 | 113 | 113 | 0.837 |
| N1 | 53 | 18.3 | 26 | 27 | |
| N2 | 10 | 3.5 | 6 | 4 | |
| TNM stage | |||||
| II | 159 | 55.0 | 80 | 79 | 0.537 |
| III | 79 | 27.3 | 35 | 44 | |
| IV | 51 | 17.7 | 30 | 21 | |
| Tumor differentiation | |||||
| Well, moderate | 154 | 53.3 | 72 | 82 | 0.304 |
| Poor | 135 | 46.7 | 73 | 42 | |
| Residual tumor | |||||
| R0 | 255 | 88.2 | 131 | 124 | 0.266 |
| R1 | 34 | 11.8 | 14 | 20 | |
| Vascular invasion | |||||
| Absent | 196 | 67.8 | 101 | 95 | 0.504 |
| Present | 93 | 32.2 | 44 | 49 | |
| ACT | |||||
| Absent | 179 | 61.9 | 93 | 86 | 0.441 |
| Present | 110 | 38.1 | 52 | 58 | |
ACT, adjuvant chemotherapy; TIM, tumor‐infiltrating mast cells; SD, standard deviation.
P < 0.05 is considered statistically significant.
The results of continuous variables are presented as mean ± SD.
Bold value is considered statistically significant.
Table 2.
Univariate and multivariate Cox regression analysis of overall survival
| Characteristic | Univariate analysis P a | Multivariate analysis | |
|---|---|---|---|
| Hazard ratio (95% CI) | P a | ||
| Age at surgery, years | 0.842 | ||
| Gender | |||
| Female | 0.591 | ||
| Male | |||
| pT‐stage | |||
| T2 | <0.001 | Reference | 0.170 |
| T3 | 1.958 (0.705‐5.439) | 0.086 | |
| T4 | 2.097 (0.702‐6.267) | 0.110 | |
| pN‐stage | |||
| N0 | 0.05 | Reference | 0.243 |
| N1 | 1.315 (0.519‐3.335) | ||
| N2 | 1.808 (0.718‐4.554) | ||
| TNM stage | |||
| II | <.001 | Reference | 0.001 |
| III | 2.472 (1.289‐4.743) | 0.06 | |
| IV | 3.956 (1.855‐8.435) | <0.001 | |
| Tumor differentiation | |||
| Well‐moderate | .043 | Reference | 0.768 |
| Poor | 1.008 (0.79‐1.490) | ||
| Residual tumor | |||
| R0 | 0.082 | ||
| R1 | |||
| Vascular invasion | |||
| Absent | 0.252 | ||
| Present | |||
| TIM infiltration | |||
| Low | <0.001 | Reference | <0.001 |
| High | 0.522 (0.373‐0.730) | ||
CI, confidence interval.
P < 0.05 is considered statistically significant.
Bold values are considered statistically significant.
Kaplan‐Meier analysis suggested that patients with high infiltration of TIM showed a prolonged OS compared to those with low infiltration of TIM (P < 0.001, Figure 1C). We further estimated the benefit of gemcitabine‐based chemotherapy according to the level of TIM in patients who received adjuvant chemotherapy. The subgroup with high infiltration of TIM could significantly benefit from adjuvant chemotherapy (ACT), unlike the subgroup with low infiltration of TIM (P < 0.001, Figure 1D‐F). Thus, the data define the potential value of the infiltration of TIM in predicting GBC OS and gemcitabine sensitivity, indicating that GBC patients with higher TIM infiltration have a better prognosis and chemosensitivity.
3.2. Accumulation of tumor‐infiltrating mast cells correlated with CD8+ T infiltration in The Cancer Genome Atlas dataset
To investigate the potential mechanism of TIM anti–tumor effects, we conducted a gene profile investigation in the TCGA cholangiocarcinoma cohort (n = 36). As depicted in Figure 2A, The gene signatures of mast cells were positively associated with activated CD8+ T cells' signatures and negatively correlated with regulatory T cells' signatures. We then performed GSEA to narrow down potential immune lineage changes related to high infiltration of TIM. Of note, TIM infiltration was positively related to multiple T cell‐related processes and signaling pathways, including the interferon gamma signaling pathway, the leukocyte migration signaling pathway, the leukocyte activation signaling pathway and the immune response signaling pathway (Figure 2B).
Figure 2.
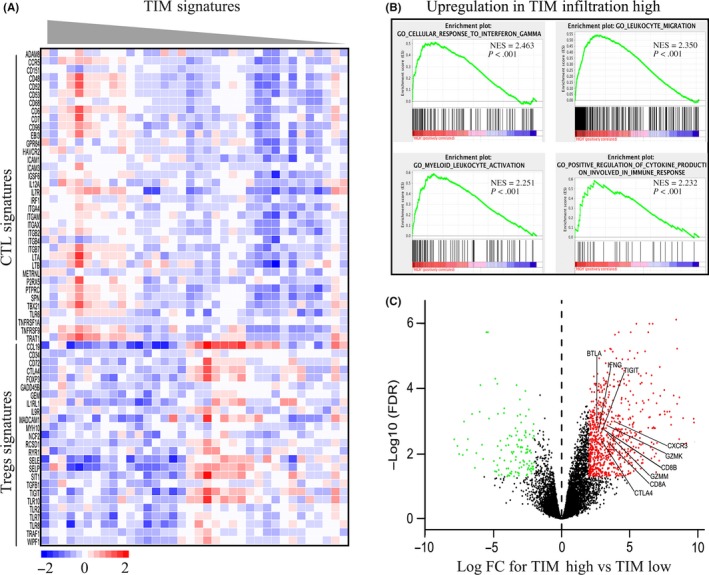
Tumor‐infiltrating mast cell (TIM) accumulation defines the immune surveillance context. A, Heatmap of CD8+ T cell signatures and Treg signatures in activated TIM gene signature tumors. B, Gene set enrichment analysis (GSEA) revealed an enrichment of the biology pathway involved in TIM high tumors. NES, normalized enrichment score. C, Volcano plot shows differential gene expression involved in CD8+ T cell reaction between TIM low and high groups. mRNA levels with a P < 0.05 and fold change ≥2 or ≤0.5 were perceived as differentially expressed
To further explore the role of TIM, differential gene expression analyses were performed. As illustrated in Figure 2C, the high TIM infiltration group were enriched with genes involved in T cell activation, such as GZMK, GZMM, CD8A, CTLA4, BTLA, IFNG and TIGIT. We also observed that Th1 chemokines receptor CXCR3, which recruits CD8+ T cells, was highly enriched in the high TIM infiltration group. These results suggested that high TIM infiltration might mediate immune surveillance through recruitment and activation of CD8+ T cells in biliary cancer TME.
3.3. CD8+ T cell infiltration positively correlates with tumor‐infiltrating mast cell presence in tissue microarray dataset
After exploration of the TCGA cholangiocarcinoma cohort, we further investigated the relationships between TIM and CD8+ T cells in GBC patients through immunohistochemistry. Similar to TCGA database results, TIM high infiltration tissue specimens were more likely to have high CD8+ T cell infiltration and potentially presented a negative relationship with regulatory T cells (Figure 3A). Significant positive correlation was also detected between the presence of TIM and CD8+ T cells (Spearman's ρ = 0.268, P < 0.001, Figure 3B). Although there was no statistical difference in the correlation coefficient between TIM and Treg (Spearman's ρ = −0.034, P = 0.5595, Figure 3C), TIM infiltration was significantly associated with the CD8+/FOXP3 ratio value (Spearman's ρ = 0.512, P < 0.001, Figure 3D). These results demonstrated that the increased CD8 T cell density is similarly present in GBC patients with high TIM infiltration.
Figure 3.
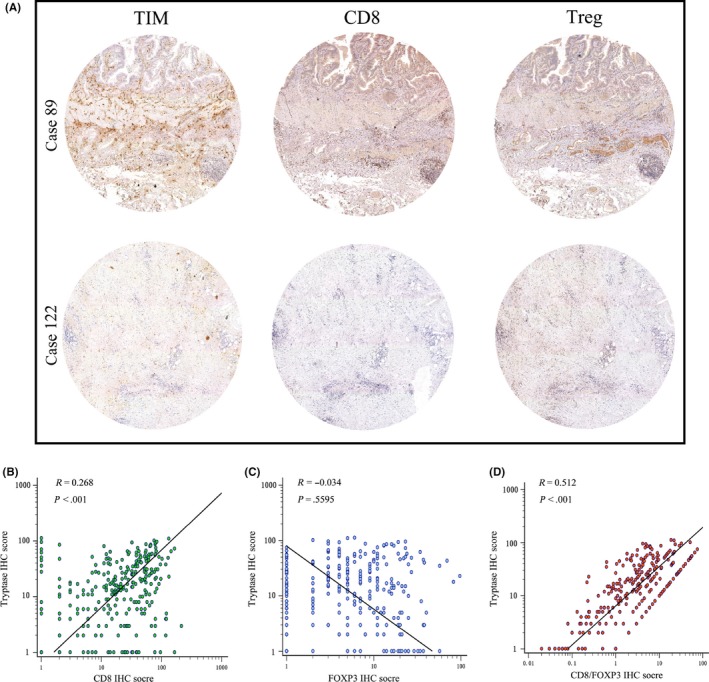
Correlation between tumor‐infiltrating mast cells (TIM) and tumor‐infiltrating lymphocytes in gallbladder cancer (GBC). A, Representative immunohistochemistry images of CD8 +T cell and Treg infiltration in corresponding low (left upper) and high (left lower) TIM infiltration patients. B‐D, Correlations assessed by Spearman analysis between TIM and tumor‐infiltrating lymphocyte immunohistochemistry score in GBC: (B) CD8+ T cells, (C) Treg and (D) CD8/Treg ratio
3.4. Combination of tumor‐infiltrating mast cells and CD8+ T cells in gallbladder cancer is associated with better prognosis of in gallbladder cancer patients
We assessed the impact of different TIM and CD8+ T cell infiltration on patient survival. Using Kaplan–Meier analysis, TIM and CD8+ T cell double positive/high cases demonstrated markedly better OS compared with the double negative/low cases (P < 0.001, Figure 4A), followed by the CD8+ T cell high and TIM low cases (P < 0.05, Figure 4A). In patients who received chemotherapy, those with high infiltration of both TIM and CD8+ T cells had longer survival compared with those with low infiltration of both subsets (P < 0.05, Figure 4B).
Figure 4.
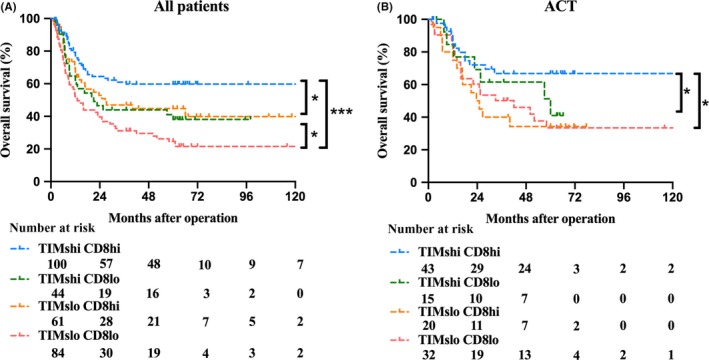
Kaplan‐Meier survival analysis of overall survival (OS) according to tumor‐infiltrating mast cells (TIM) and CD8+ T cells. A, Kaplan‐Meier survival analysis of OS in all patients, B, Patients who received adjuvant chemotherapy (ACT)
4. DISCUSSION
The role of the immune system in the control of cancer initiation and progression has been demonstrated in recent decades.19 Although most tumors evade immune surveillance at the time of presentation, the residual signs of active anticancer immune response present a favorable prognosis.9, 20 High TIM density is associated with positive prognosis in some tumors.10, 13, 21 It is reported that TIM arrest the growth and induce apoptosis of breast cancer through secretion of IL‐4.22 In the current study, we observed that TIM were positively correlated with prolonged OS. In addition, the high TIM subgroup could benefit more from ACT than the low TIM subgroup. These findings indicate that TIM could be a vital factor for predicting chemotherapeutic response, which could be valuable for selection and management of patients who receive ACT.
Considering the role that mast cells play in the developing tumor, we proposed that mast cells might present an anti–tumor effect through activating cytotoxic T cells and inducing an immune‐stimulating environment. Through bioinformatics analysis, we found that TIM was related to multiple T cell‐related processes and signaling pathways. Spearman's correlation analysis indicated that TIM infiltration was positively correlated with anti–tumor CD8+ T cells in TMA. These findings confirmed the hypothesis that TIM may play a critical role in activating and promoting CD8+ T cells to reject tumors. Several previous studies of inflammation have suggested that activated mast cells facilitate anti–infection by enhancing recruitment of NK and CD8+ T cells.23, 24 Oldford et al demonstrated that mast cells could display anti–tumor activity by recruiting CD8+ T cells by secreting CCL3 in the tumor context.16 The stromal infiltration of CD8+ T cells in the tumor context is the basis for patients who receive immunotherapy. Accordingly, TIM might serve as an important prognostic factor in identifying biliary tract cancer patients for the combination of chemotherapy and immunotherapy.
Considering the TIM anti–tumor effect via recruitment of CTL, it is appealing to investigate the mechanism behind the TIM accumulation in GBC tissues. High CXCR3 expression mast cells are observed in synovial, which appears to recruit activated T lymphocytes through secreting Th1 chemokines CXCL9 and CXCL10.25 It has been reported that expression of CCR2 and CCR5 changes during the maturation of mast cells, with high expression in mast cell progenitors that is subsequently downregulated in mature mast cells. In ACKR2–/–ApcMin/+ intestinal adenomas, mast cells with CCR2 and CCR5 overexpression modulate CD8+ T cell recruitment via the LTB4‐BLT1 axis.26 To examine whether TIM in GBC follow a similar pattern, we measured CCR2 and CCR5 protein expression via immunofluorescence assays in TMA (Figure S1). The CCR5 protein is present in the majority of TIM in GBC tissues. However, CCR2 expression is relatively rare in TIM. These results confirm that chemokine receptor CCR5 plays an important role in TIM accumulation in GBC tissues. It is suggested that in GBC tissues, CCR5 is potentially required for TIM to home to tumor tissues.
The paper has limitations in that it is a retrospective single institution study and the sample size is relatively small. In addition, the results were based on immunohistochemistry of tissue microarrays, which is a semiquantitative method and may not reflect the actual situation completely. A prospective, larger, multi‐centered randomized trial is required to validate these findings in future.
In conclusion, this study identified that TIM is associated with a favorable prognosis of GBC patients and can be used as an independent prognostic factor. Patients with high TIM infiltration tend to have improved outcomes after receiving adjuvant gemcitabine‐based ACT. TIM accumulation is correlated with more infiltration of CD8+ T cells, which may suggest an immune surveillance function of TIM in the microenvironment of GBC.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
This study was supported by grants from the National Natural Science Foundation of China (no. 81872352) and the Foundation of Shanghai Science and Technology Committee (16411952000) and the JianFeng Project of XuHui Provincial Commission of Health and Family Planning (SHXH201703), the Shanghai Medical Discipline of Key Programs for General Surgery (2017ZZ02007) and the Clinical Study of Zhongshan Hospital (2018ZSLC24).
Bo X, Wang J, Wang C, et al. High infiltration of mast cells is associated with improved response to adjuvant chemotherapy in gallbladder cancer. Cancer Sci. 2020;111:817–825. 10.1111/cas.14302
Bo, Wang and Wang contributed equally to this work
Contributor Information
Yueqi Wang, Email: yueqiwang@fudan.edu.cn.
Houbao Liu, Email: houbaoliu@aliyun.com.
REFERENCES
- 1. Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Valle JW, Lamarca A, Goyal L, Barriuso J, Zhu AX. New horizons for precision medicine in biliary tract cancers. Cancer Discov. 2017;7:943‐962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zeng H, Chen W, Zheng R, et al. Changing cancer survival in China during 2003–15: a pooled analysis of 17 population‐based cancer registries. Lancet Global Health. 2018;6:e555‐e567. [DOI] [PubMed] [Google Scholar]
- 4. Bizama C, Garcia P, Espinoza JA, et al. Targeting specific molecular pathways holds promise for advanced gallbladder cancer therapy. Cancer Treat Rev. 2015;41:222‐234. [DOI] [PubMed] [Google Scholar]
- 5. Wellenstein MD, de Visser KE. Cancer‐cell‐intrinsic mechanisms shaping the tumor immune landscape. Immunity. 2018;48:399‐416. [DOI] [PubMed] [Google Scholar]
- 6. Gentles AJ, Newman AM, Liu CL, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21:938‐945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fridman WH, Zitvogel L, Sautes‐Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14:717‐734. [DOI] [PubMed] [Google Scholar]
- 8. Palucka AK, Coussens LM. The basis of oncoimmunology. Cell. 2016;164:1233‐1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McGranahan N, Swanton C. Cancer evolution constrained by the immune microenvironment. Cell. 2017;170:825‐827. [DOI] [PubMed] [Google Scholar]
- 10. Dalton DK, Noelle RJ. The roles of mast cells in anticancer immunity. Cancer Immunol Immunother. 2012;61:1511‐1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ribatti D. Mast cells as therapeutic target in cancer. Eur J Pharmacol. 2016;778:152‐157. [DOI] [PubMed] [Google Scholar]
- 12. Fu H, Zhu Y, Wang Y, et al. Tumor infiltrating mast cells (TIMs) confers a marked survival advantage in nonmetastatic clear‐cell renal cell carcinoma. Ann Surg Oncol. 2017;24:1435‐1442. [DOI] [PubMed] [Google Scholar]
- 13. Lin C, Liu H, Zhang H, et al. Tryptase expression as a prognostic marker in patients with resected gastric cancer. Br J Surg. 2017;104:1037‐1044. [DOI] [PubMed] [Google Scholar]
- 14. Oldford SA, Marshall JS. Mast cells as targets for immunotherapy of solid tumors. Mol Immunol. 2015;63:113‐124. [DOI] [PubMed] [Google Scholar]
- 15. Marichal T, Tsai M, Galli SJ. Mast cells: potential positive and negative roles in tumor biology. Cancer Immunol Res. 2013;1:269‐279. [DOI] [PubMed] [Google Scholar]
- 16. Bulfone‐Paus S, Bahri R. Mast cells as regulators of T cell responses. Front Immunol. 2015;6:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stelekati E, Bahri R, D'Orlando O, et al. Mast cell‐mediated antigen presentation regulates CD8(+) T cell effector functions. Immunity. 2009;31:665‐676. [DOI] [PubMed] [Google Scholar]
- 18. Welsh TJ, Green RH, Richardson D, Waller DA, O'Byrne KJ, Bradding P. Macrophage and mast‐cell invasion of tumor cell islets confers a marked survival advantage in non‐small‐cell lung cancer. J Clin Oncol. 2005;23:8959‐8967. [DOI] [PubMed] [Google Scholar]
- 19. DeClerck YA, Pienta KJ, Woodhouse EC, Singer DS, Mohla S. The tumor microenvironment at a turning point knowledge gained over the last decade, and challenges and opportunities ahead: a white paper from the NCI TME network. Can Res. 2017;77:1051‐1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sharma P, Hu‐Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707‐723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Podlech J, Ebert S, Becker M, Reddehase MJ, Stassen M, Lemmermann NAW. Mast cells: innate attractors recruiting protective CD8 T cells to sites of cytomegalovirus infection. Med Microbiol Immunol. 2015;204:327‐334. [DOI] [PubMed] [Google Scholar]
- 22. Gooch JL, Lee AV, Yee D. Interleukin 4 inhibits growth and induces apoptosis in human breast cancer cells. Can Res. 1998;58:4199‐4205. [PubMed] [Google Scholar]
- 23. Harries MJ, Griffiths CE, Paus R. Mast cell/CD8+T cell interactions in the pathobiology of lichen planopilaris. J Invest Dermatol. 2012;132:S24‐S24. [Google Scholar]
- 24. St John AL, Rathore APS, Yap H, et al. Immune surveillance by mast cells during dengue infection promotes natural killer (NK) and NKT‐cell recruitment and viral clearance. Proc Natl Acad Sci USA. 2011;108:9190‐9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ruschpler P, Lorenz P, Eichler W, et al. High CXCR3 expression in synovial mast cells associated with CXCL9 and CXCL10 expression in inflammatory synovial tissues of patients with rheumatoid arthritis. Arthritis Res Ther. 2003;5:R241‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bodduluri SR, Mathis S, Maturu P, et al. Cell‐dependent CD8(+) T‐cell recruitment mediates immune surveillance of intestinal tumors in Apc(Min/+) mice. Cancer Immunol Res. 2018;6:332‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


