Abstract
The formation of premetastatic niches creates a fertile environment for the seeding of disseminated cancer cells in selected secondary organs. This is crucial for the development of metastasis in various malignancies, including breast cancer (BC). We previously reported that the loss of FBXW7 in bone marrow‐derived stromal cells promoted cancer metastasis by increasing the production of the chemokine CCL2, which attracts myeloid‐derived suppressor cells and macrophages to the premetastatic niche. Furthermore, treatment with the CCL2 inhibitor propagermanium (PG), which has been used in Japan as a therapeutic agent against chronic hepatitis B, was shown to block the enhancement of metastasis in FBXW7‐deficient mice through inhibiting the formation of premetastatic niches. Here, we describe a phase I dose‐escalation study of PG used as an antimetastatic drug for perioperative patients with primary BC. The primary end‐point was the percentage of patients who experience dose‐limiting toxicity. Twelve patients were enrolled in the study. Dose‐limiting toxicity was not observed, and the maximum dose was determined to be 90 mg/body/day. The serum concentrations of PG were nearly within the normal range in all observation days. We observed an inverse correlation between FBXW7 mRNA levels in blood and the serum concentrations of CCL2 and interleukin (IL)‐6, in agreement with our previous mouse model. Also, IL‐6 was downregulated in a PG dose‐dependent manner, as observed in mice. Thus, PG was given safely and it is expected to have antimetastatic potential in BC. This trial is registered in the UMIN Clinical Trials Registry as UMIN000022494.
Keywords: breast cancer, CCL2, FBXW7, premetastatic niche, propagermanium
A Phase I dose‐escalation study of the CCL2 inhibitor propagermanium (PG) administration was performed as an antimetastatic drug for perioperative patients with primary breast cancer (BC). This study has demonstrated a manageable safety profile and provides a basis for exploring the clinical use of PG as an antimetastatic drug for BC patients.

1. INTRODUCTION
Distant metastasis is the leading cause of mortality in cancer patients, including those with breast cancer (BC), which is one of the most common malignant tumors worldwide1 and remains a major cause of cancer death, despite advances in diagnosis and treatment.1, 2
The process of tumor metastasis involves the interaction of primary tumor cells with the environment of distant organs (the premetastatic niche). Also important is the interaction of cancer cells with their environment after arriving in a distant organ (metastatic niche).3 A premetastatic niche creates a fertile environment for the seeding of disseminated cancer cells in selected secondary organs.4
The C‐C motif chemokine 2 (CCL2, also known as monocyte chemoattractant protein‐1 [MCP‐1]) and the signaling pathway modulated by its receptor (CCR2) play a crucial role in the recruitment of monocytes to the sites of inflammation.5 Recently, the signaling pathway in the tumor microenvironment has generated increasing interest due to its association with tumor progression and metastasis, including that in BC.6, 7, 8, 9 CCL2 is overexpressed in breast cancer tissues,10 and patients with high expression of CCL2 have early recurrence or poor prognosis in breast cancer.11, 12, 13 We previously showed that increased expression of CCL2 due to downregulated expression of F‐box and WD repeat domain containing 7 (FBXW7) in bone marrow‐derived stromal host cells promoted the formation of premetastatic niches through recruitment of myelomonocytic cell‐derived suppressor cells, thereby promoting metastatic tumor growth.14 Our findings provided strong evidence that host cells play a critical role in tumor metastasis through the formation of premetastatic niches through the CCL2/CCR2 signaling pathway.15 Furthermore, we showed that treatment with a CCL2 inhibitor, propagermanium (PG), blocked the enhancement of metastasis in FBXW7‐deficient mice through inhibiting the formation of premetastatic niches.14
Propagermanium is an organic germanium compound with a chemical structure of [(O1/2)3GeCH2CH2CO2H]n. It has been used clinically in Japan since 1994 as a safe therapeutic agent against hepatitis B virus e antigen (HBe)‐positive chronic hepatitis type B. Treatment with PG decreased or eliminated HBe antigen and increased anti‐HBe Ab, resulting in seroconversion. Also, the decrease of hepatitis B virus (HBV)‐DNA polymerase activity and HBV‐DNA content in blood has been reported.16 Propagermanium targets glycosylphosphatidylinositol‐anchored proteins that are closely associated with CCR and selectively inhibit CCL2/CCR2 signaling.17
There has been growing interest in repurposing previously approved noncancer drugs as potential anticancer treatments. This approach offers the advantages of shorter development time, lower research cost, and lower drug prices.18 Propagermanium could be repositioned as an antimetastatic drug for BC patients.
The primary objective of this phase 1 study was to assess the dose‐limiting toxicity (DLT) of PG as a single agent and to determine its maximum tolerated dose (MTD) in perioperative patients with primary BC. The secondary objectives were to examine the serum concentration of PG and the safety of treatment of BC patients.
2. MATERIALS AND METHODS
2.1. Patients
It is well known that surgery and other perioperative stresses can promote metastasis,19 suggesting that it is important to suppress cancer metastasis in the perioperative setting. Therefore, in this study of primary BC patients, we examined the perioperative use of PG, which has the potential to suppress cancer metastasis by inhibiting the formation of premetastatic niches.
Eligible patients had primary BC. The treatment plan consisted of curative surgery without neoadjuvant therapy. Patients were 20‐ to 70‐year‐old women with histologically confirmed invasive ductal carcinoma. They had an ECOG performance status of 0 or 1, adequate organ function, no HBV infection, and no ongoing systemic infections or other malignancies. The stages and grades of the tumors were classified according to the AJCC/UICC 7th TNM classification and stage groupings. The clinical data were obtained from medical records.
2.2. Propagermanium
Serocion Capsule 10, containing PG, was produced by Sanwa Kagaku Kenkyusho. The active pharmaceutical ingredient in each capsule was 10 mg PG. Serocion was launched in Japan in 1994 as an oral drug for chronic HBV infection. The patients took PG capsules 3 times daily in the observation period.
2.3. Study objectives
The primary objectives were to determine DLT and to estimate the MTD. The secondary objective was to evaluate the safety and pharmacokinetics of PG.
2.4. Study design and procedures
This multiinstitutional, open‐label, phase I study, a dose‐escalation trial, was carried out in compliance with the study protocol, the Declaration of Helsinki, and Japanese Good Clinical Practice guidelines. All patients provided written informed consent. Propagermanium was given from 7 days before surgery to the final day except for the day of surgery. The PG dose was escalated in a “3 + 3 design”, with cohorts of 3 patients per dose level of 30, 60, and 90 mg/body/day followed by a cohort of 3 patients receiving 90 mg/body/day (Figure 1). Standard postoperative therapy was scheduled after PG treatment. The trial was carried out at Kyushu University Beppu Hospital, National Cancer Center Hospital East, and The Cancer Institute Hospital Ariake of the Japanese Foundation for Cancer Research after the protocol was approved by each institutional review board and registered as UMIN000022494. The Trial Coordination Office for this trial was EP‐CRSU Co., Ltd. Safety was monitored by EP‐CRSU Co., Ltd.
Figure 1.
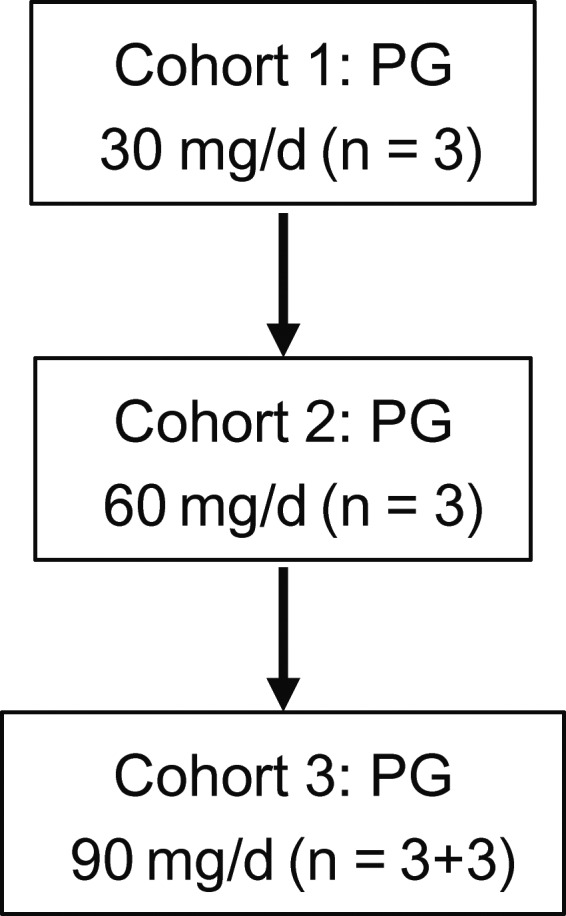
Design of a phase I dose‐escalation study of propagermanium (PG) used as an antimetastatic drug for perioperative patients with primary breast cancer
2.5. Dose‐limiting toxicity
Dose‐limiting toxicity was defined as a treatment‐related grade 3 or higher adverse event (AE) observed during the DLT evaluation period in each stage (Figure 2). We previously showed in a mouse model that the enhancement of metastasis could be blocked by administration of PG at a concentration equivalent to 30 mg/day in humans.14 In addition, PG was reported to inhibit the CCL2/CCR2 pathway in a dose‐dependent manner in vitro.17 These results suggested that PG could suppress metastasis in a dose‐dependent manner. Furthermore, the previous phase I study of PG for human chronic hepatitis B determined 90 mg/body/day as the highest dose among 15, 30, 60, and 90 mg/body/day (http://www.info.pmda.go.jp/go/pack/3919007M1021_1_09/?view=frame%26style=SGML%26lang=ja). Therefore, we undertook a phase I dose‐escalation study of PG (30, 60, and 90 mg/body/day) in BC patients. The MTD was the highest dose at which no more than 1 of 3 patients experienced a DLT. Three additional patients were planned to be enrolled at the MTD or the highest planned dose (90 mg/body/day) to confirm safety.
Figure 2.
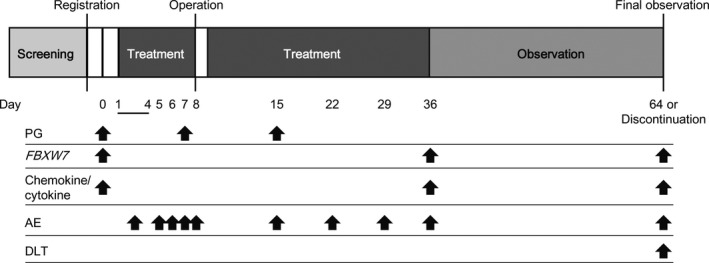
Treatment and evaluation procedure of a phase I dose‐escalation study of propagermanium (PG) used as an antimetastatic drug for perioperative patients with primary breast cancer. AE, adverse event; DLT, dose‐limiting toxicity
2.6. Safety
The safety of each treatment group was assessed by evaluating study drug exposure, AEs, all deaths, changes in laboratory determinations, and vital sign parameters. Adverse events reported from the start of PG treatment until 28 days after discontinuation of PG were collected (Figure 2). Adverse events were assessed according to the Common Terminology Criteria for Adverse Events version 4.0.
2.7. Serum concentration of PG
The serum concentration of PG was measured in blood samples (3 mL) collected on days 0 (predose), 7, and 15 (PG dose) (Figure 2). The concentrations were measured by liquid chromatography/mass spectrometry at Sumika Chemical Analysis Service (Osaka, Japan). Serum concentrations over 120, 260, and 400 ng/mL at 30, 60, and 90 mg/day, respectively, were considered abnormal based on the Cmax from Sanwa Kagaku Kenkyusho, as reported on the 2016 Japanese interview form of approved medicines by the Japanese Ministry of Health, Labour and Welfare (http://www.info.pmda.go.jp/go/pack/3919007M1021_1_09/?view=frame%26style=SGML%26lang=ja).
2.8. FBXW7 mRNA expression in blood
The expression level of FBXW7 mRNA was analyzed by quantitative RT‐PCR as described previously14 using blood samples (2 mL) collected on day 0 (predose), day 36 (PG dose), and the final postdose day (Figure 2). Reverse transcription was undertaken with random hexamers using M‐MLV reverse transcriptase (Invitrogen). Quantitative PCR was carried out with LightCycler FastStart DNA Master SYBR Green I (Roche Diagnostics). The raw data are presented as the relative quantity of target genes, normalized to GAPDH. The primer sequences for PCR were as follows: FBXW7 forward 5′‐CTCTCCCAGATAATGGCACTCTCA‐3′ and reverse 5′‐ AGAGTCATCTGACCAAGAAATAGCC‐3′; GAPDH forward 5′‐TTGGTATCGTGGAAGGACTC‐3′ and reverse 5′‐AGTAGAGGCAGGGATGATGT‐3′. The quantitative RT‐PCR was carried out at LSI Medience Corporation (Fukuoka, Japan).
2.9. Serum concentrations of multiple cytokines and chemokines
Serum concentrations of multiple cytokines and chemokines, including interferon‐γ, interleukin (IL)‐1β, IL‐2, IL‐4, IL‐5, IL‐8, IL‐10, IL‐12p70, IL‐13, tumor necrosis factor‐α, granulocyte macrophage colony‐stimulating factor (GM‐CSF), IL‐6, IL‐18 and CCL2 were measured with ECL using a V‐PLEX Plus Human Biomarker Kit (MesoScale) from blood samples (2 mL) collected on day 0 (predose), day 36 (PG dose), and final day (postdose) at LSI Medience Corporation (Figure 2).
2.10. Statistical analysis
Associations between the variables were tested with the Mann‐Whitney U test, Student’s t test or Fisher’s exact test. The degree of linearity was estimated by Spearman’s rank correlation coefficient. A 2‐sided P < .05 was deemed statistically significant. Statistical analyses were undertaken using JMP Pro 13 software (SAS Institute, Cary, NC, USA) or R version 3.5.1 (Vienna, Austria; URL: http://www.R-project.org/).
3. RESULTS
3.1. Patient characteristics
A total of 12 female BC patients (3 in a 30 mg/body/day cohort, 3 in a 60 mg/body/day cohort, and 6 in a 90 mg/body/day dose‐expansion cohort) were enrolled in this study and received at least 1 dose of SK‐818. All patients were at TNM stage I, II, or III, and had undergone curative surgery. The patient characteristics are summarized in Table 1.
Table 1.
Characteristics of perioperative patients with breast cancer treated with propagermanium
| Characteristic | n = 12 |
|---|---|
| Age, years | |
| Median (range) | 63 (40‐69) |
| ECOG performance status, n | |
| 0 | 12 |
| 1 | 0 |
| Subtype, n | |
| HR+/HER2− | 11 |
| HR ± HER2+ | 0 |
| TN | 1 |
| Surgery, n | |
| Bp + SNB | 2 |
| Bt + SNB | 5 |
| Bt + Ax | 5 |
| Pathologic stage, n | |
| I/IA | 4 |
| IIA | 2 |
| IIB | 3 |
| IIIA | 2 |
| IIIB | 0 |
| IIIC | 1 |
Abbreviations: Ax, axillary lymph node dissection; Bp, partial mastectomy; Bt, total mastectomy; ER, estrogen receptor; PgR, progesterone receptor; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; SNB, sentinel lymph node biopsy; TN, triple negative.
3.2. Safety and DLTs
Seven AEs were observed in 12 patients during the PG treatment period. All AEs were grade 1/2, with the most common being back pain (16.7%) and fever (16.7%). Fever was considered a treatment‐emergent AE. The AEs for all dose levels are listed in Table 2. No DLTs were observed at any dose level of PG in BC patients. Thus, we could not determine the MTD. The maximum dose was 90 mg/body/day. In addition, the AEs for all dose levels of the patients with chronic hepatitis B are shown in Table S1, which is based on the 2016 Japanese interview form of approved medicines by the Japanese Ministry of Health, Labour and Welfare (http://www.info.pmda.go.jp/go/pack/3919007M1021_1_09/?view=frame%26style=SGML%26lang=ja). Papula was the most frequent AE (2.7%) in the patients with chronic hepatitis B. Adverse events following PG treatment might be observed more frequently in patients with BC compared to chronic hepatitis B, although no DLTs were observed in patients with either BC or chronic hepatitis B.
Table 2.
Adverse events (AE) in perioperative patients with breast cancer treated with propagermanium
| AE/CTCAE grade | 30 mg/day (n = 3) | 60 mg/day (n = 3) | 90 mg/day (n = 6) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | ≥3 | 1 | 2 | ≥3 | 1 | 2 | ≥3 | |
| Back pain | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Diarrhea | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Wound infection | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Arthritis | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Pain in extremity | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Fever | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| Pharyngitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
Abbreviation: CTCAE, Common Terminology Criteria for Adverse Events.
3.3. Serum concentration of PG
Serum concentrations of PG on days 0 (predose), 7, and 15 (PG dose) are shown in Figure 3. The concentrations on all days except day 15 at a 30 mg dose were within the normal range reported for healthy donors on the 2016 Japanese interview form of approved medicine by the Japanese Ministry of Health, Labour and Welfare (http://www.info.pmda.go.jp/go/pack/3919007M1021_1_09/?view=frame%26style=SGML%26lang=ja).
Figure 3.
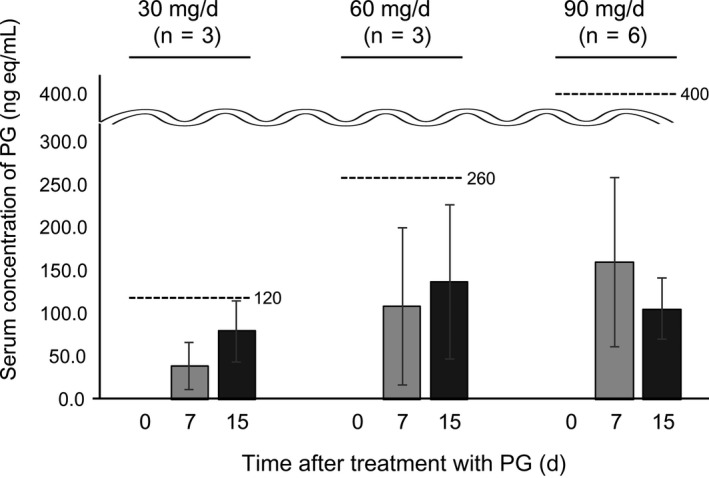
Serum concentrations of propagermanium (PG; n = 36) as an antimetastatic drug for perioperative patients with primary breast cancer. The upper limits of normal at the 30, 60, and 90 mg/day of PG were 120, 260, and 400 ng/mL, respectively
3.4. Correlation among FBXW7 mRNA levels in blood and serum concentrations of multiple cytokines and chemokines
In our previous study of FBXW7‐deficient mice, the serum concentrations of various cytokines and chemokines were examined to explore how FBXW7 affected the formation of the premetastatic niche.14 In this clinical trial, we also investigated the correlation between FBXW7 mRNA levels in the blood and serum concentrations of multiple cytokines and chemokines. The correlation diagram is shown in Figure S1. Interleukin‐1β, IL‐2, IL‐4, IL‐12p70, IL‐13, and GM‐CSF were not assessed because they were not detected. As expected, Spearman’s rank correlation coefficient showed a significant inverse correlation between FBXW7 mRNA expression and the levels of CCL2 and IL‐6 in blood (IL‐6 is induced by CCL2) (r = 0.404 and 0.356, P = .015 and .033, respectively) (Figure 4).
Figure 4.
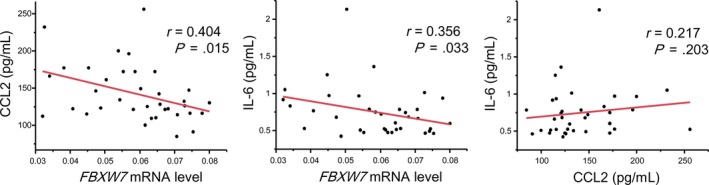
Correlation among FBXW7 mRNA levels and serum concentrations of C‐C motif chemokine 2 (CCL2) and interleukin (IL)‐6 in perioperative patients with primary breast cancer treated with propagermanium
3.5. Relationship between dose level of PG and serum concentrations of multiple cytokines and chemokines
The correlation diagram of PG and the serum concentrations of multiple cytokines and chemokines are shown in Figure S2. There was no statistically significant correlation between the dose level of PG and FBXW7 mRNA expression level or CCL2 level in blood. Of note, IL‐6 was downregulated in a PG dose‐dependent manner (Figure 5).
Figure 5.
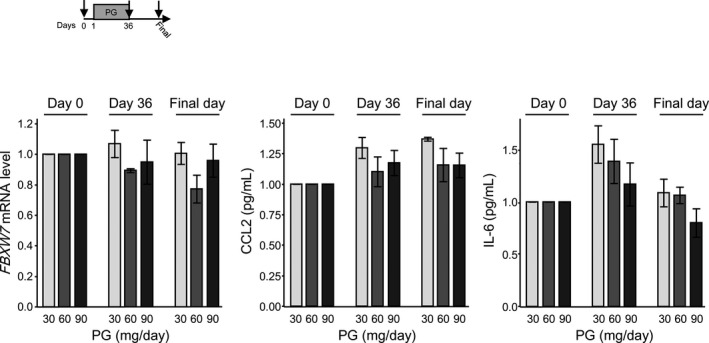
Relationship between the dose level of propagermanium (PG) and FBXW7 mRNA level and serum concentrations of C‐C motif chemokine 2 (CCL2), and interleukin (IL)‐6 in perioperative patients with primary breast cancer. PG given as 30 mg/day (n = 3), 60 mg/day (n = 3), and 90 mg/day (n = 6)
3.6. Relationship between FBXW7 mRNA expression in blood and clinicopathologic factors in primary BC
Our previous study showed that BC patients with low expression of FBXW7 in blood had poor prognoses.14 In this study, the relationship between clinicopathologic factors and FBXW7 mRNA expression in blood on day 0 was examined. As shown in Table 3, FBXW7 mRNA levels in blood were inversely associated in the group with the high malignant potential such as high nuclear grade (2, 3) and with high Ki‐67 (20% or higher) (P = .003 and .037, respectively).
Table 3.
Correlation between FBXW7 mRNA expression in blood and clinicopathological factors in breast cancer
| Factors | n | FBXW7 (average ± SD) | P value |
|---|---|---|---|
| Age (years) | |||
| <65 | 7 | 0.061 ± 0.005 | .5889 |
| ≥65 | 5 | 0.065 ± 0.006 | |
| Pathological tumor size (cm) | |||
| <2 | 7 | 0.064 ± 0.005 | .7045 |
| ≥2 | 5 | 0.061 ± 0.006 | |
| Lymph node metastasis | |||
| − | 6 | 0.068 ± 0.005 | .1824 |
| + | 6 | 0.057 ± 0.005 | |
| Nuclear grade | |||
| 1 | 6 | 0.072 ± 0.004 | .0025 |
| 2, 3 | 6 | 0.053 ± 0.004 | |
| ER | |||
| − | 1 | 0.061 ± 0.014 | .5345 |
| + | 11 | 0.062 ± 0.004 | |
| PgR | |||
| − | 4 | 0.057 ± 0.007 | .812 |
| + | 8 | 0.065 ± 0.005 | |
| Ki‐67 (%) | |||
| <20 | 9 | 0.067 ± 0.004 | .0373 |
| ≧20 | 3 | 0.049 ± 0.006 | |
Abbreviations: ER, estrogen receptor; PgR, progesterone receptor.
4. DISCUSSION
This phase I dose‐escalation study evaluated a CCL2 inhibitor, PG, in perioperative patients with primary BC. To our knowledge, this clinical trial is the first to test PG as an antimetastatic drug in the setting of BC. Treatment with PG showed low‐grade AEs and no DLTs, similar to observations made when used for patients with chronic hepatitis B. Furthermore, the blood concentrations of PG on all days were within the normal range, except day 15 at a 30 mg dose. Thus, the maximum dose of PG was determined to be 90 mg per day. These results indicate an acceptable safety profile of PG treatment for BC patients.
It is well known that the IL‐6/STAT3/vascular endothelial growth factor A axis plays a crucial role in tumor angiogenesis.20, 21 Interleukin‐6 is downstream from CCL2.20, 21, 22 In a mouse model of metastatic BC, IL‐6 was downregulated by CCL2 neutralization by anti‐CCL2 Ab, resulting in inhibition of metastasis.23 Consistent with these reports, serum IL‐6 levels were downregulated in a PG dose‐dependent manner, implying that PG might have the potential to inhibit metastasis in human BC. Furthermore, the maximum dose at 90 mg PG per day could be the most promising dose for preventing metastasis.
Interestingly, serum CCL2 levels increased after surgery and remained high until the final observation day, which was approximately 2 months after surgery, suggesting that CCL2 continued to be activated for at least 2 months after surgery, possibly a result of operative stress or inflammation,24 and might promote cancer metastasis. Given this result, it might be better to administer PG for 2 months or longer as adjuvant therapy to inhibit metastasis. Further study will be required to determine the duration of PG treatment for prevention of metastasis after surgery.
Several clinical trials of CCL2 inhibitors have been undertaken for therapeutic intervention in solid malignancies.8, 9 In a mouse model, Bonapace et al found that the interruption of treatment with anti‐CCL2 neutralizing Ab led to enhanced metastasis due to an influx of monocytes into the metastatic site and a rebound of IL‐6 levels.23 However, overshooting of IL‐6 serum levels was not observed following PG treatment in our study. Given this result, PG might be preferable for CCL2 inhibition compared to anti‐CCL2 neutralizing Ab in human BC.
Finally, an inverse correlation was observed between FBXW7 mRNA expression and CCL2 or IL‐6 levels in the blood. Moreover, low expression of FBXW7 mRNA in blood was associated with clinicopathologic factors showing tumor aggressiveness, including high nuclear grade and high Ki‐67 staining in BC patients. These observations support our previous clinical data that BC patients with low expression of FBXW7 in blood had poor overall survival and disease‐free survival after undergoing curative surgery.14 Furthermore, these results confirm our finding that the FBXW7/CCL2 axis is important for BC progression, and thus provides a rationale for the efficacy of PG for inhibition of metastasis of BC patients. However, the mechanisms by which FBXW7 mRNA levels are downregulated in some BC patients are unknown. The expression of FBXW7 in blood might depend on individual constitutional variability, as we previously suggested.14 Further studies will be required to clarify this mechanism.
In conclusion, this study of PG as monotherapy in BC patients has shown a manageable safety profile and provides a basis for exploring the clinical use of PG as an antimetastatic drug for BC patients. In the future, phase II clinical trials would be required to test the maximum dose (90 mg/body/day) of PG for direct assessment of its antimetastatic potential and other potential mechanisms of action.
CONFLICT OF INTEREST
Takahito Jomori is an employee of Sanwa Kagaku Kenkyusho Co., Ltd. The other authors have no conflicts of interest to declare.
Supporting information
ACKNOWLEDGMENTS
This study was supported by AMED (Translational Research Network Program) of the Japan Agency for Medical Research and Development (grant no. 16ck0106160h0002). Propagermanium was kindly provided by Sanwa Kagaku Kenkyusho Co., Ltd. (Nagoya, Japan). We thank Atsushi Kishimoto, Naomi Takayanagi, Makiko Uchiyama and other staff of the Center for Clinical and Translational Research, Kyushu University Hospital, for data collection, analysis, and interpretation, as well as helpful advice. We thank Dr Yusuke Tsuruda and Dr Tyler Lahusen for helpful comments and English proofreading. We also thank Dr Norihiro Hirata of the Academic Research and Industrial Collaboration Management Office of Kyushu University for conducting this study, and N. Mishima and T. Kawano for their excellent technical assistance. This work was supported in part by the following grants and foundations: Japan Agency for Medical Research and Development (16ck0106160h0002, 18ae0101016, 18cm0106504, 18kk0205003, and 18ck0106259); Japan Society for the Promotion of Science (JSPS) Grant‐in‐Aid for Science Research (19H03715, 19K09176, 16K09220, 19K18057, 18K08649, 18K15323, 15H05912, 15H05707 and 15H05791); JST AIP‐PRISM (JPMJCR18Y5); OITA Cancer Research Foundation; and Priority Issue on Post‐K computer (hp170227, hp160219, and hp170227).
Masuda T, Noda M, Kogawa T, et al. Phase I dose‐escalation trial to repurpose propagermanium, an oral CCL2 inhibitor, in patients with breast cancer. Cancer Sci. 2020;111:924–931. 10.1111/cas.14306
This trial is registered in the UMIN Clinical Trials Registry as UMIN000022494
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Hori M, Matsuda T, Shibata A, et al. Cancer incidence and incidence rates in Japan in 2009: a study of 32 population‐based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2015;45:884‐891. [DOI] [PubMed] [Google Scholar]
- 3. Doglioni G, Parik S, Fendt SM. Interactions in the (Pre)metastatic niche support metastasis formation. Front Oncol. 2019;9:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Akhtar M, Haider A, Rashid S, Al‐Nabet A. Paget's, "seed and soil" theory of cancer metastasis: an idea whose time has come. Adv Anat Pathol. 2019;26:69‐74. [DOI] [PubMed] [Google Scholar]
- 5. Van Coillie E, Van Damme J, Opdenakker G. The MCP/eotaxin subfamily of CC chemokines. Cytokine Growth Factor Rev. 1999;10:61‐86. [DOI] [PubMed] [Google Scholar]
- 6. Lu X, Kang Y. Chemokine (C‐C motif) ligand 2 engages CCR2+ stromal cells of monocytic origin to promote breast cancer metastasis to lung and bone. J Biol Chem. 2009;284:29087‐29096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Qian BZ, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast‐tumour metastasis. Nature. 2011;475:222‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lim SY, Yuzhalin AE, Gordon‐Weeks AN, Muschel RJ. Targeting the CCL2‐CCR2 signaling axis in cancer metastasis. Oncotarget. 2016;7:28697‐28710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yumimoto K, Sugiyama S, Mimori K, Nakayama KI. The potentials of CCL2‐CCR2 blockers including propagermanium as anticancer agents. Cancer Sci. 2019;110(7):2090‐2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ueno T, Toi M, Saji H, et al. Significance of macrophage chemoattractant protein‐1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res. 2000;6:3282‐3289. [PubMed] [Google Scholar]
- 11. Heiskala M, Leidenius M, Joensuu K, Heikkila P. High expression of CCL2 in tumor cells and abundant infiltration with CD14 positive macrophages predict early relapse in breast cancer. Virchows Arch. 2019;474:3‐12. [DOI] [PubMed] [Google Scholar]
- 12. Saji H, Koike M, Yamori T, et al. Significant correlation of monocyte chemoattractant protein‐1 expression with neovascularization and progression of breast carcinoma. Cancer. 2001;92:1085‐1091. [DOI] [PubMed] [Google Scholar]
- 13. Fujimoto H, Sangai T, Ishii G, et al. Stromal MCP‐1 in mammary tumors induces tumor‐associated macrophage infiltration and contributes to tumor progression. Int J Cancer. 2009;125:1276‐1284. [DOI] [PubMed] [Google Scholar]
- 14. Yumimoto K, Akiyoshi S, Ueo H, et al. F‐box protein FBXW7 inhibits cancer metastasis in a non‐cell‐autonomous manner. J Clin Invest. 2015;125:621‐635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yumimoto K, Nakayama KI. Fbxw7 suppresses cancer metastasis by inhibiting niche formation. Oncoimmunology. 2015;4:e1022308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hirayama C, Suzuki H, Ito M, Okumura M, Oda T. Propagermanium: a nonspecific immune modulator for chronic hepatitis B. J Gastroenterol. 2003;38:525‐532. [DOI] [PubMed] [Google Scholar]
- 17. Yokochi S, Hashimoto H, Ishiwata Y, et al. An anti‐inflammatory drug, propagermanium, may target GPI‐anchored proteins associated with an MCP‐1 receptor, CCR2. J Interferon Cytokine Res. 2001;21:389‐398. [DOI] [PubMed] [Google Scholar]
- 18. Pantziarka P, Bouche G, Meheus L, Sukhatme V, Sukhatme VP, Vikas P. The repurposing drugs in oncology (ReDO) project. Ecancermedicalscience. 2014;8:442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Neeman E, Zmora O, Ben‐Eliyahu S. A new approach to reducing postsurgical cancer recurrence: perioperative targeting of catecholamines and prostaglandins. Clin Cancer Res. 2012;18:4895‐4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Niu G, Wright KL, Huang M, et al. Constitutive Stat3 activity up‐regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000‐2008. [DOI] [PubMed] [Google Scholar]
- 21. Lei Z, Duan H, Zhao T, et al. PARK2 inhibits osteosarcoma cell growth through the JAK2/STAT3/VEGF signaling pathway. Cell Death Dis. 2018;9:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nanki T, Nagasaka K, Hayashida K, Saita Y, Miyasaka N. Chemokines regulate IL‐6 and IL‐8 production by fibroblast‐like synoviocytes from patients with rheumatoid arthritis. J Immunol. 2001;167:5381‐5385. [DOI] [PubMed] [Google Scholar]
- 23. Bonapace L, Coissieux MM, Wyckoff J, et al. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature. 2014;515:130‐133. [DOI] [PubMed] [Google Scholar]
- 24. Carson WFT, Salter‐Green SE, Scola MM, Joshi A, Gallagher KA, Kunkel SL. Enhancement of macrophage inflammatory responses by CCL2 is correlated with increased miR‐9 expression and downregulation of the ERK1/2 phosphatase Dusp6. Cell Immunol. 2017;314:63‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


