Abstract
Background
Aspirin-exacerbated respiratory disease (AERD) is the triad of asthma, nasal polyposis, and sensitivity to cyclooxygenase-1 inhibitors. Treatment options include medical management, surgical intervention, and aspirin desensitization (AsaD).
Methods
AERD patients were identified using the MarketScan Database from 2009 to 2015. Patients were included using International Classification of Diseases, 9th edition (ICD-9) codes for asthma, nasal polyposis, and drug allergy. Treatments were determined by Current Procedural Terminology (CPT) codes for drug desensitization and endonasal procedures. Geographic trends and timing of interventions between those exposed and not exposed to desensitization were explored.
Results
A total of 5628 patients met inclusion criteria for AERD, with mean age 46 years, 60% female; 395 (7%) underwent AsaD and 2171 (39%) underwent sinus surgery. Among patients who were desensitized, 229 (58%) underwent surgery, of whom 201 (88%) had surgery prior to AsaD (median [quartile 1, quartile 3]; 61 days [30, 208] prior to desensitization). For patients undergoing surgery following AsaD (n = 46), surgery was performed a median of 302 (163, 758) days after AsaD. Nineteen patients had multiple surgeries post-AsaD with median time between surgeries being 734 days (312, 1484); 261 patients were not desensitized to aspirin but did undergo multiple surgeries, with the median of the median time between surgeries being 287 days (15, 617), which is shorter than for patients post-AsaD (p< 0.001).
Conclusion
A very small percentage of AERD patients undergo AsaD. Patients who had AsaD underwent surgery approximately 2 months prior to AsaD. Patients who underwent AsaD experienced an increased time between surgeries compared to patients who did not undergo AsaD.
Keywords: asthma, chronic rhinosinusitis, sinusitis, AERD, aspirin desensitization
Aspirin-exacerbated respiratory disease (AERD) is defined as an inflammatory disease with 3 clinical features: asthma, nasal polyps, and nonsteroidal anti-inflammatory drug (NSAID) sensitivity to all COX-1 inhibitors. Approximately 1,368,000 patients are thought to have AERD within the United States, based on the estimated prevalence of asthma in this country and estimates of AERD within the asthma population.1
Several treatment options for AERD exist: medical therapy including biologic therapy, functional endoscopic sinus surgery, and aspirin desensitization (AsaD). Due to regrowth of polyps, patients often require several revision surgeries in their lifetime. AsaD is recommended after surgery to help avoid recurrence of nasal polyps.1 AsaD has been shown to be cost effective because for many patients revision surgery can then be delayed or avoided.1
Barriers to drug desensitization include the need for specific training to perform AsaD. In a recent survey study of allergists in the United States, only 62.5% of participants reported performance of AsaD for AERD patients and most performed <5 procedures per year.2 Aspirin maintenance following desensitization requires commitment from the patient to adhere to long-term aspirin intake, and side effects include gastritis and gastrointestinal (GI) or other bleeding.3 Additionally, although desensitization protocols have shortened in duration, 1 to 2 days are required for current protocols, which may be a burden to the patient.4
Multiple trials have shown that AsaD is beneficial to AERD patients with reduction in prescription use and improved quality of life.5 Yet the treatment practices for AERD are variable and have not been well studied. Therefore, despite evidence of patient tolerance and safety, resources for AsaD are not universally accessible to AERD patients.
The MarketScan database contains insurance claim information for over 20 billion patient encounters between 2009 and 2015 (https://truvenhealth.com; now IBM Watson Health, Armonk, NY). The database can be queried for patient diagnoses and procedures using International Classification of Diseases, 9th edition (ICD-9) and Current Procedural Terminology (CPT) coding. The aim of this study was to evaluate the current treatment practices for AERD in the United States using a large national insurance claims database.
Patients and methods
AERD patients were identified from the MarketScan Database between January 1, 2009, and October 1, 2015. Patients meeting criteria had ICD-9 diagnosis codes consistent with all 3 components of AERD: asthma, nasal polyposis, and drug allergy; patients with ICD-9 codes for other lung diseases and immunodeficiency were excluded. This query was performed using previously published methodology.6 Because aspirin allergy is not represented by a designated ICD-9 code, drug allergy ICD-9 codes were used as surrogate codes for this analysis. CPT codes for drug desensitization and endoscopic endonasal procedures were used to determine treatments received during the study time period. Drug desensitization was used as a surrogate code for AsaD due to lack of a specific CPT code for this procedure. Geographic variability and timing of surgical intervention and drug desensitization treatments were explored. The locations of desensitization procedures performed were determined and categorized by Metropolitan Statistical Areas (MSAs), which are densely populated areas containing 1 or more related counties within the United States. Statistical analysis was performed using SAS, version 9.4 (SAS Institute, Inc., Cary, NC), with p < 0.05 used for statistical significance.
Results
Study cohort
A total of 5628 patients met criteria for study inclusion. The average age was 46 ± 11 years (mean ± standard deviation [SD]); 60% of the cohort was female (Table 1).
TABLE 1.
Demographics of cohort
| Demographic characteristic | Value |
|---|---|
| Total cohort, n | 5628 |
| Female, n (%) | 2230 (60) |
| Age (years), mean ± SD | 46 ± 11 |
| Desensitization cases, n (%) | 395 (7) |
SD = standard deviation.
Locations of drug desensitization
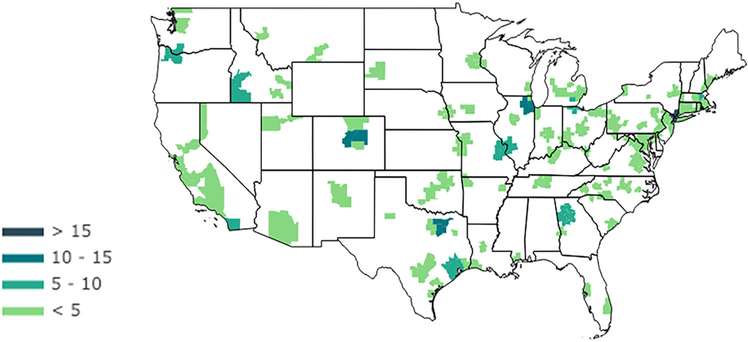
Of the included cohort, 395 (7%) underwent drug desensitization (Table 1). MSAs where drug desensitization was performed were identified with the highest number of desensitization procedures occurring in the following regions: (1) New York-White Plains-Wayne, NY-NJ; (2) Chicago-Joliet-Naperville, IL; (3) Dallas-Plano-Irving, TX; and (4) Denver-Aurora-Broomfield, CO (Fig. 1).
FIGURE 1.
Locations of desensitization cases in the United States between 2009 and 2015 in the MarketScan Database. Locations of desensitization procedures performed within the United States were categorized by MSAs. Absolute number of desensitization events is shown. MSA = metropolitan statistical area
Timing of surgical intervention
Among AERD patients, 2171 (39%) underwent sinus surgery at some point during the 6-year study time period. Among patients who were desensitized, 229 (58%) underwent at least 1 surgical intervention. Of these patients, 201 (88%) underwent surgery prior to AsaD (median [quartile 1, quartile 3] of 61 days [30, 208] prior to AsaD). Surgery was performed a median of 302 (163, 758) days after AsaD in patients who underwent surgery after AsaD (n = 46).
Time interval between surgical interventions
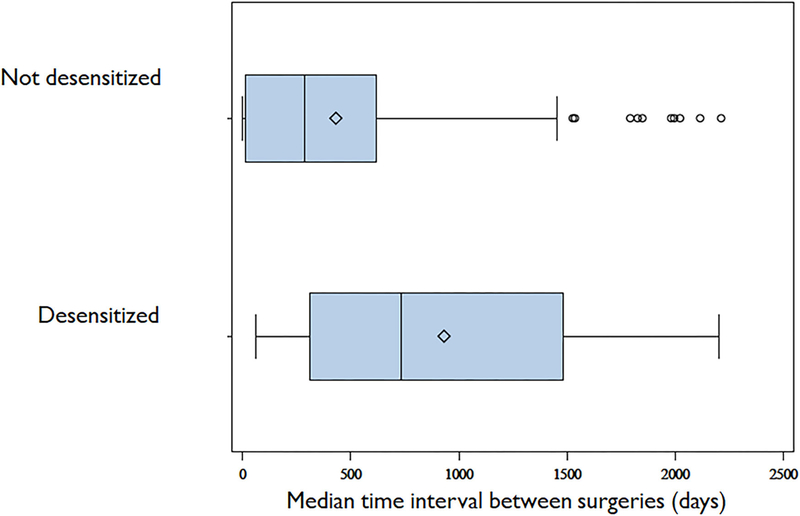
For patients who underwent AsaD and had multiple surgeries post-AsaD (n = 19), the median time between surgeries was 734 (312, 1484) days. For patients not desensitized who underwent multiple surgeries (n = 241), the median time interval between surgeries was calculated for each individual and then pooled for the group. The median for the group was reported; this median of the median-times between surgeries was 287 (15, 617) days. This time interval for non-desensitized patients was noted to be shorter than the comparable time interval for revision surgery for patients who underwent AsaD (p < 0.001). Figure 2 shows the median and interquartile range for the time interval between surgeries.
FIGURE 2.
Time interval between surgical interventions. Median and interquartile range for time interval between surgeries in AsaD patients and non-AsaD patients. AsaD = aspirin desensitization.
Discussion
In this study of a large insurance claims database, we identified a small percentage of AERD patients who underwent drug desensitization. Furthermore, the geographic availability of AsaD was found to be sparse throughout the United States. The lack of accessibility to AsaD is likely a contributing factor for low overall rates of AsaD in the AERD population.
Regarding timing of surgical intervention, for those patients who underwent AsaD as well as surgery during the 6-year period, surgery occurred a median of 61 days before AsaD. Although formal recommendations for timing of AsaD after surgery are not published, clinically, AsaD is often performed within 1 to 3 months after surgery, coinciding with the timeframe when the polyp burden is relatively low but after healing has taken place.7,8 For patients who underwent multiple surgeries, the time interval between surgeries was approximately 2.5 times greater in patients who had undergone AsaD, demonstrating a potential protective effect of desensitization in these patients.
Limitations of this study are similar to those for all retrospective reviews of a clinical database such as the deidentified MarketScan database. These limitations are balanced by allowing for evaluation of a large number of patients throughout the entire United States. Because we were unable to review charts for this database, selection bias for patients undergoing AsaD cannot be assessed. Similarly, increased duration of time between surgeries following AsaD may be due to a number of patient factors, including treatment preference, other than specific AsaD benefits. We are limited by ICD-9 and CPT coding as well as the subset of patients with insurance carriers available in the dataset, which has been estimated to be approximately 50% of privately insured and Medicare patients within the United States. Deidentified patient charts cannot be retrospectively reviewed and thus details such as patient symptoms and verification of AERD diagnosis are not available. Still, although patients and interventions may be underestimated in this study, we believe that this dataset is representative of the AERD population because we used a reliable algorithm for our search strategy.9 Our cohort identification strategy was designed based on a prior published model; however, our dataset is much larger in scale, which does introduce other possible confounding factors.
A major limitation of this database is the lack of aspirin/NSAID allergy and aspirin/NSAID desensitization codes. Thus, drug allergy and drug desensitization codes were used as surrogate codes to represent this diagnosis and treatment option. This limitation of available ICD-9 and CPT codes requires acknowledgement that our cohort identification and treatments identified may have inaccuracies or be incomplete. Last, the data collected in this study encompasses a 6-year time period. Although this timeframe was sufficiently long to capture multiple revision surgeries, we were not able to capture treatments including surgery and desensitization outside of this timeframe.
Future work includes an effort to better understand the benefits of AsaD in the AERD population. Further investigation of the MarketScan dataset regarding potential decrease in medication usage following AsaD is warranted. A prospective study to identify the ideal polyp burden at the time of AsaD and evaluate timing of regrowth would better delineate the appropriate timing for intervention. Sinus surgery can often be performed while patients are taking 81 mg of aspirin, and resumption of maintenance dose is acceptable in patients within 48 hours of interruption.8 Therefore, patients are able to safely undergo surgery after AsaD if needed, resume their aspirin doses following surgery, and avoid the need for a formal repeat desensitization. This benefit, along with the advantages of AsaD identified in the present study and many others,7,10 argue in favor of AsaD as the standard of care in appropriate AERD patients. Yet this treatment option remains relatively poorly accessible even in the United States. The training of future allergists and otolaryngologists should emphasize AsaD as a treatment option for refractory polyp patients. Further work to investigate the appropriate timing, indications, and aspirin maintenance dosing is warranted. As biologic therapy becomes available for use in patients with nasal polyps and asthma, the value and economic benefit of AsaD for AERD patients will be critical to making recommendations for safe, cost-effective care.
Conclusion
Few AERD patients undergo AsaD, which is not available in many geographic regions of the United States. Patients who had AsaD underwent surgery approximately 2 months prior to AsaD, which is in line with clinical practice. We found an increased median time interval between surgeries in patients who underwent AsaD as compared to patients who did not undergo AsaD. This evidence supports the potential benefit of drug desensitization in the AERD cohort.
Acknowledgments
Funding sources for the study: Triological Society Research Career Development Award; NIH (National Center for Advancing Translational Sciences [NCATS]: KL2TR002381, UL1TR002378 to J.M.L.); Lyle Finley Foundation.
Footnotes
Potential conflict of interest: S.K.W.: Consultant (Stryker, NeurENT), Scientific Advisory Board (OptiNose, SinopSys Surgical, ALK-Abello). J.M.D.: Consultant (Medtronic), Research support (Spirox). K.N.C.: Advisory Board (Teva, Regeneron, Novartis). T.M.L.: Advisory Board (GlaxoSmithKline, Regeneron). No conflicts of interest reported for L.T.R., C.N., H.W., and R.M. Presented at the Annual ARS Meeting on September 13–14, 2019, in New Orleans, LA.
References
- 1.White AA, Stevenson DD. Aspirin-exacerbated respiratory disease. N Engl J Med. 2018;379:1060–1070. 10.1056/NEJMra1712125 [DOI] [PubMed] [Google Scholar]
- 2.Waldram JD, White AA. A survey of aspirin desensitization practices among allergists and fellows in training in the United States. J Allergy Clin Immunol Pract. 2016;4:1253–1255. 10.1016/j.jaip.2016.06.016 [DOI] [PubMed] [Google Scholar]
- 3.Berges-Gimeno MP, Simon RA, Stevenson DD. Longterm treatment with aspirin desensitization in asthmatic patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2003;111:180–186. 10.1067/mai.2003.7 [DOI] [PubMed] [Google Scholar]
- 4.White AA, Stevenson DD. Aspirin desensitization: faster protocols for busy patients. J Allergy Clin Immunol Pract. 2019;7:1181–1183. 10.1016/j.jaip.2018.10.019 [DOI] [PubMed] [Google Scholar]
- 5.Tajudeen BA, Schwartz JS, Bosso JV. The role of aspirin desensitization in the management of aspirin-exacerbated respiratory disease. Curr Opin Otolaryngol Head Neck Surg. 2017;25:30–34. 10.1097/MOO.0000000000000331 [DOI] [PubMed] [Google Scholar]
- 6.Roland LT, Wang H, Mehta CC, et al. Longitudinal progression of aspirin-exacerbated respiratory disease: analysis of a national insurance claims database. Int Forum Allergy Rhino. (in press). Epub 2019 Aug 23. 10.1002/alr.22412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adappa ND, Ranasinghe VJ, Trope M, et al. Outcomes after complete endoscopic sinus surgery and aspirin desensitization in aspirin-exacerbated respiratory disease. Int Forum Allergy Rhinol. 2018;8:49–53. 10.1002/alr.22036 [DOI] [PubMed] [Google Scholar]
- 8.Cook KA, Stevenson DD. Current complications and treatment of aspirin-exacerbated respiratory disease. Expert Rev Respir Med. 2016;10:1305–1316. 10.1080/17476348.2016.1258306 [DOI] [PubMed] [Google Scholar]
- 9.Cahill KN, Johns CB, Cui J, et al. Automated identification of an aspirin-exacerbated respiratory disease cohort. J Allergy Clin Immunol. 2017;139:819–825.e6. 10.1016/j.jaci.2016.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez-Jimenez JC, Moreno-Paz FJ, Teran LM,Guani-Guerra E. Aspirin exacerbated respiratory disease: current topics and trends. Respir Med. 2018;135: 62–75. 10.1016/j.rmed.2018.01.002 [DOI] [PubMed] [Google Scholar]