Abstract
Background
Few resources exist for prospective, longitudinal analysis of the relationships between early life environment and later obesity in large diverse samples of children in the United States (US). In 2016, the National Institutes of Health launched the Environmental influences on Child Health Outcomes (ECHO) program to investigate influences of environmental exposures on child health and development. We describe demographics and overweight and obesity prevalence in ECHO, and ECHO’s potential as a resource for understanding how early life environmental factors affect obesity risk.
Methods
In this cross-sectional study of 70 extant US and Puerto Rico cohorts, 2003–2017, we examined age, race/ethnicity, and sex in children with body mass index (BMI) data, including 28,507 full-term post-birth to <2 years and 38,332 aged 2–18 years. Main outcomes included high BMI for age <2 years, and at 2–18 years overweight (BMI 85th to <95th percentile), obesity (BMI ≥ 95th percentile), and severe obesity (BMI ≥ 120% of 95th percentile).
Results
The study population had diverse race/ethnicity and maternal demographics. Each outcome was more common with increasing age and varied with race/ethnicity. High BMI prevalence (95% CI) was 4.7% (3.5, 6.0) <1 year, and 10.6% (7.4, 13.7) for 1 to <2 years; overweight prevalence increased from 13.9% (12.4, 15.9) at 2–3 years to 19.9% (11.7, 28.2) at 12 to <18 years. ECHO has the statistical power to detect relative risks for ‘high’ BMI ranging from 1.2 to 2.2 for a wide range of exposure prevalences (1–50%) within each age group.
Conclusions
ECHO is a powerful resource for understanding influences of chemical, biological, social, natural, and built environments on onset and trajectories of obesity in US children. The large sample size of ECHO cohorts adopting a standardized protocol for new data collection of varied exposures along with longitudinal assessments will allow refined analyses to identify drivers of childhood obesity.
Introduction
Over the last few decades, the obesity epidemic has grown rapidly as a public health challenge [1]. Particularly worrisome is that obesity and its associated comorbidities are rapidly increasing among children, with diagnoses at increasingly younger ages (e.g., type 2 diabetes, hypertension, dyslipidemia, obstructive sleep apnea, and mobility impairment) [2], thus pointing toward their early life, environmental origins [3]. Approximately one-third of today’s United States (US) children and adolescents are now overweight or have obesity [4], with persisting socio-economic and racial disparities [5, 6].
Research has consistently shown that excess caloric intake and limited physical activity are immediate contributors to childhood obesity [7]. Individual behaviors, however, do not fully explain the obesity epidemic [7], while lifestyle and behavioral intervention efforts to prevent or treat childhood obesity have had disappointingly limited success [8]. A growing body of evidence suggests that a wide array of likely interrelated environmental exposures (e.g., chemical, nutritional, physical, social), operating at multiple levels (e.g., individual, familial, community, societal), especially during sensitive periods of development (such as during fetal and early postnatal life), contribute to unhealthy growth trajectories and increased childhood obesity risk [9]. Thus, novel paradigms, such as complex systems [10] and developmental origins approaches [9] have been proposed to further advance discovery and translational efforts related to childhood obesity. Given the increasing availability of “big data” from electronic medical records, geographic information systems, high-throughput omics and sequencing assays, as well as advanced modeling techniques, such approaches may now be ripe for exploration in large, diverse, longitudinal studies.
In response to these challenges and opportunities, in 2016, the US National Institutes of Health launched the Environmental influences on Child Health Outcomes (ECHO) program to address existing knowledge gaps in early life predictors of child health and disease risk. ECHO will enroll and follow >50,000 diverse children (Fig. 1), and will integrate data already collected via extant parent study protocols with new data collected through an ECHO-supported data-collection protocol that includes multiple exposures, outcomes, and potentially responsible mechanisms to comprehensively investigate the influence of early life environment on child health and development. The present manuscript focuses on childhood obesity, one of ECHO’s focus areas, and describes, using already collected data: (1) the prevalence of childhood “high body mass index (BMI) for age”, overweight, and obesity in the extant ECHO cohorts; and (2) ECHO’s potential as a research resource for improving our understanding of the impact of early life environmental factors on obesity risk in US children.
Fig. 1.
Cohorts and participant recruiting sites for the Environmental influences on Child Health Outcomes (ECHO) study
Materials and methods
We pooled existing, already collected data from 84 cohorts in the ECHO program that met eligibility criteria (https://www.nih.gov/echo/pediatric-cohorts). None of the cohorts oversampled by weight status, although several selected participants at higher demographic risk for obesity. One cohort specifically oversampled for poverty and African American ethnicity in low-income, non-urban counties in Pennsylvania and in North Carolina. Other cohorts enrolled exclusively Hispanic/Latino or Puerto Rican participants (four), African American participants (two), or American Indian/Alaskan Natives from the Northern Plains (one). There were also cohorts that exclusively enrolled infants born preterm.
We excluded cohorts if they had only self-reported weight and height measurements (n = 2); no weight or height measurements (n = 6); or no measurements of height or weight since 2003 (n = 6), resulting in 70 cohorts providing data on weight and height status of 46,222 participants ages 0–18 years. We further excluded children for the following reasons: (1) missing values for sex, age, or race/ ethnicity (n = 1,835); (2) missing gestational age (GA) or weight at birth (n = 1,656); (3) GA < 22 weeks at birth and full-term births with no data on height and/or recumbent length (n = 1,271); (4) height but not recumbent length (<2 years) or with length but not height (≥2 years) (n = 165); (5) measurements performed before 2003 (n = 821); (6) parent-reported recumbent length/height/weight (n = 1,868); (7) biologically implausible values, such as (a) z-scores <−5 or >5 for BMI-for-age for children <2 years old [11], and (b) z-scores <−4 or >8 for BMI-for-age for children ≥2 years old (n = 126); [12] and (8) if a participant had >1 observation within an age category, then a random selection of 1 observation was selected, excluding anthropometric data from preterm births from after birth to 3 years (n = 877). These exclusions resulted in having 37,603 children available for this report.
High BMI, overweight, and obesity classification
We classified full-term infants between 48 weeks post gestation and <2 years of age as having high BMI if their BMI was at or above the 97.7th percentile of the World Health Organization (WHO) sex-specific BMI-for-age growth standards [13]. BMI was calculated as weight in kilograms divided by length in meters squared.
We classified children and adolescents born full-term (≥37 completed weeks of gestation) between 2 and 18 years of age and those children born preterm (22 to < 37 weeks gestation) between 3 and 18 years of age as having overweight (BMI ≥ 85th and <95th percentile) or obesity (BMI ≥ 95th percentile) using Centers for Disease Control and Prevention (CDC) sex-specific BMI-for-age reference data [14, 15]. We further classified severe obesity (BMI ≥ 120% of 95th percentile), and class III severe obesity (≥140% of the 95th percentile or BMI ≥ 40 kg/m2). We explored using BMI ≥ 35 kg/m2 as a further input to defining severe obesity though this did not change results.
Maternal socio-demographic characteristics
We described the sample of children contributing anthropometric measurements as a function of maternal characteristics, obtained via data already collected by the study-specific questionnaires and/or abstracted from medical records. We combined race and Hispanic origin into five categories: non-Hispanic (NH) white, NH black, NH Asian, NH other, and Hispanic. NH other included non-Hispanic persons who identified their race as American Indian, Alaska Native, Pacific Islander, or multiple races. We categorized marital status as married or cohabitating or not; primary language spoken in the home as English, Spanish, or other; level of education as less than high school, or high school diploma/General Education Degree (GED) or higher; and health insurance coverage as none, private, Medicaid, or other (e.g., military or Medicare).
Selection of age and race categories
We selected age groupings to ensure sufficient precision of prevalence estimates (>2000 children per age group). The resulting age categories included full-term infants post birth to <1 year; or 1 to <2 years; and pre- and full-term births 2–3 (preterm ≥ 3 only), 4–5, 6–7, 8–11, and 12–18 years. If a child had >1 observation within an age group, we selected 1 observation at random to give each child equal weight in estimating prevalences. If a child had >1 observation across age categories, they were included in each respective age group.
Participants available for high BMI for age, overweight, and obesity
Detailed sample sizes for age, sex, and race/ethnicity categories are available in Table 1. The number of cohorts that contributed to analyses by age and sex groups are in Table 2.
Table 1.
Maternal socio-demographics for participants with recumbent length/height and weight for preterm birth through 18 years
| Demographics (No. participants providing maternal data) | Cohorts/sub-cohorts providing data | No. participants (%) |
|---|---|---|
| Overall | 70 | 37,603 (100) |
| Maternal marital status (n = 30,470)a | 50 | |
| Married or cohabitating | 21,096 (69) | |
| Not married or cohabitating | 6726 (22) | |
| Race (n = 34,260)b | 63 | |
| Non-Hispanic | ||
| White | 15,951 (46) | |
| Black | 4745 (14) | |
| Asian | 2188 (6) | |
| Other | 3773 (11) | |
| Hispanic | 7603 (22) | |
| Primary language spoken at home (n = 19,555)c | 45 | |
| English | 15,159 (77) | |
| Spanish | 1668 (9) | |
| Other | 472 (2) | |
| Mother’s education (n = 28,679)d | 64 | |
| <12th grade, no diploma | 4202 (12) | |
| High school graduate/GED or higher | 28,874 (80) | |
| Maternal health insurance coverage (n = 28,663)e | 41 | |
| None | 568 (2) | |
| Private plan | 17,962 (63) | |
| Medicaid | 7241 (25) | |
| Other (military, Medicare) | 1970 (7) | |
| Preterm birth (n = 3,440)f | ||
| Small for gestational age | 10.6 (8.8, 12.3) | |
| Large for gestational age | 7.9 (5.2, 10.6) |
Marital status missing: n = 2648 (9%)
Race/ethnic origin missing: n = 0 (0%)
Primary language missing: n = 2256 (12%)
Education missing: n = 3154 (9)%
Health insurance missing: n = 922 (3%)
Point estimate, 95% confidence interval; weight-for-gestational age z-scores: small for gestational age: <10th percentile; large for gestational age: >90th percentile of the Fenton sex-specific growth charts, respectively [59].
Table 2.
Study-wide essential data elements to be collected across the child developmental life coursea for the Environmental influences on Child Health Outcomes (ECHO) program
| Domain/Concept | Preconception | Prenatal | Perinatal | Infancy | Early childhood | Middle childhood | Adolescence |
|---|---|---|---|---|---|---|---|
| Socio-demographicsb | X | X | X | X | X | ||
| Child and family health historiesc | X | X | X | X | X | X | |
| Pregnancy-related data elements (biological mother)d | X | X | |||||
| Child physical health and functioning-relatede | X | X | X | X | X | ||
| Child neurodevelopmental health & functioningf | X | X | X | X | |||
| Child health behaviors/lifestyleg | X | X | X | X | X | ||
| Child social role performance & functioningh | X | X | X | ||||
| Child well-being life satisfactioni | X | X | X | X | |||
| Caregiver neuropsychosocialj | X | X | X | X | |||
| Built/physical environmentk | X | X | X | X | X | X | |
| Home/social environmentl | X | X | X | X | |||
| Child sleep healthm | X | X | X | X | |||
| Biospecimensn | X | X | X | X | X | X | X |
Postnatal assessments at birth (preterm 22 to <37; full-term 37–46 weeks gestation), infancy (46 weeks post-last menstrual period through 11 months + 30 days), early childhood (EC; 1–6 years) and middle childhood (MC; 5–12 years) through adolescence (adol; (11–21 years). Full protocol and measures can be accessed
Race, ethnic origin, age, education, income, insurance, biological relationship with parents, country of origin, household income, federal assistance, financial strain, household composition, primary language, lifetime addresses, early care and education, K12 education, gender identity, adolescent occupation, biological family (child, mother, father, grandparents) demographic, rearing family (child, caregiver) demographics, caregiver and partner occupation
Anthropometrics, blood pressure, caregiver health insurance status, lifetime first-degree relative family medical conditions, lifetime/updated child medical history includes major congenital anomalies, medications, hospitalizations, airways-related health outcomes, and health insurance, pubertal development scale
Diet, substance use, anthropometrics, weight gain, conditions/interventions, infections, sleep quality, health and impairment, travel, labor and delivery complications, supplements, prenatal care, food contaminants, pregnancy dating/ultrasound, social support
Airways-related health outcomes, blood pressure, body composition height, weight, waist circumference, pubertal development
Developmental milestones, general cognition, emotional and behavioral functioning, social cognition, perceived stress, temperament
Infant feeding and introduction to solids, child diet, food contaminants, physical activity, sexual behavior, substance use (adolescence)
Peer relationships, academic ability & performance, child sleep health, quality and impairment
Global health, life satisfaction, meaning and purpose
Global health, depressive symptoms, perceived stress, social support, stressful life events in adulthood, discrimination, caregiver IQ, stressful life events in childhood (<18 years)
Chemical exposures, household exposure to secondhand smoke, maternal and paternal work exposures
Caregiving quality, parenting behaviors, family relationships, child negative life events
Child sleep health, quality and impairments
Maternal (biologic mother): sample for DNA; whole blood, hair, urine, toenails; Index Child biospecimen: sample appropriate for DNA, hair, toenails, urine, whole blood/blood spot, shed teeth, whole blood/plasma/serum/red blood cells, urine, stool, hair (opioid exposure), buccal swab, skin swab
Statistical analysis
This analysis involved individual participant data meta-analysis, in which the cohorts used common statistical code to analyze their own data and the results of those analyses were combined at the ECHO Data Analysis Center using meta-analytic methods [16]. This approach standardized the statistical methods used by each contributing cohort and did not require the sharing of individual-level data.
Conventionally, meta-analysis of summary estimates of binomial outcome measures is based on a normal model (using a double arcsine transformation to satisfy the normality assumption of the model). Stratifying our population across five race/ethnicity and five age categories resulted in some cases in small numbers (<50) and did not meet this model’s assumption of an approximately normal with in study likelihood. Therefore, we chose to use an exact binomial likelihood through a Normal-Binomial (N-B) non-linear random-effects model following the recommendation of Ma et al. [17]. Let Xi denotes the number of “events” observed (children classified as obese) from a total of ni children in the ith cohort with a probability of events pi, i = 1, 2, …K. The N-B model obtains an estimate of the pooled proportion (e.g., percentage of obese children) in two stages. In the first stage, conditional on (ni, pi) provided by the K independent cohorts, the Xi is assumed to follow a binomial within-study distribution. In the second stage, the N-B model fits a generalized linear mixed effects model on a logit scale of pi with a normal distribution
where μ0 and τ2 represent mean and between-cohort variance of the transformed pi, respectively. In cases where multiple observation were available on a child, 1 observation was selected at random within each age strata to satisfy the independence assumption. The models were fit using the SAS procedure NLMIXED (SAS Institute Inc. Cary, NC) using code provided by Ma et al. [17]. The confidence interval (CI) of the pooled prevalence estimate describes uncertainty in the estimates from the different studies (the width of the CI tends toward zero as the number of studies in the meta-analysis increases). The supplemental tables provide mean + standard deviation (SD)-specific estimates and 95% CIs for the various age, sex, and race/ethnicity categories.
Results
Maternal socio-demographic characteristics
The majority of mothers were married or cohabitating with a partner (Table 1). Forty-six percent of mothers reported their race/ethnicity to be NH white, followed in decreasing order by Hispanic, NH black, NH other, and NH Asian. English was the primary language spoken in the majority of homes. Eighty percent of mothers completed a high school education or higher. Using health insurance as an indicator of socio-economic status, 25% of mothers had Medicaid insurance, with 2% reporting no insurance coverage.
High BMI for age: Post-birth to <2-year age for full-term births
There were 23,406 children contributing 28,507 observations included in the analysis to estimate high BMI for age for full-term infants. Prevalence estimates for high BMI for age were 4.7% (95% CI, 3.5, 6.0) post-birth to <1 year, and 10.6% (95% CI, 7.4, 13.7) at 1 to <2 years (Table 3). Similar prevalence estimates were observed across all sex and race/ethnic categories.
Overweight, obesity, and severe obesity for ages 2–18 years
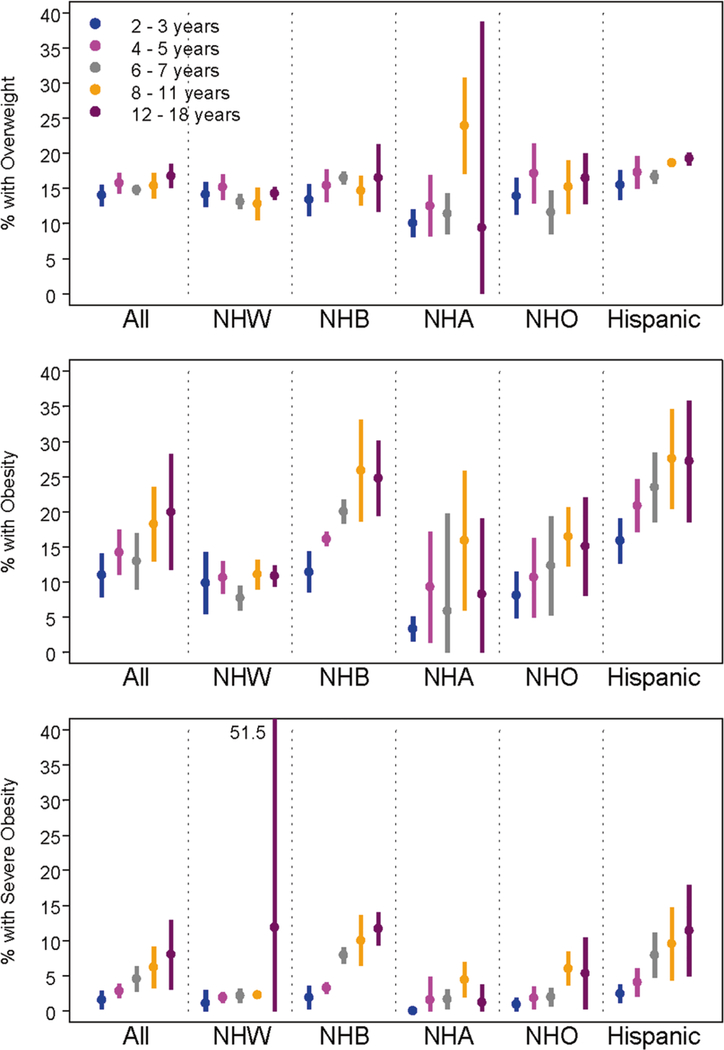
There were 20,080 children contributing 38,332 observations included in the analysis to estimate the prevalence of overweight, obesity, and severe obesity for ages 2–18 years. Overall the prevalence of overweight, obesity, and severe obesity increased with age, with point estimates of 14.0% (95% CI, 12.5, 15.5) for 2–3 years to 16.8% (95% CI, 15.0, 18.5) for 12–18 years for overweight; 11.0% (95% CI, 7.9, 14.0) for 2–3 years to 19.9% (95% CI, 11.7, 28.2) for 12–18 years for obesity; and 1.5% (95% CI, 0.2, 2.8) for 2–3 years to 8.0% (95% CI, 3.1, 12.9) for 12–18 years for severe obesity (Fig. 2). A similar pattern was seen in the prevalence of class III severe obesity with 0.2% (95% CI: 0.0, 0.4) for 2–3 years to 2.2% (95% CI: 0.9, 3.4%) for 12–18 years (Tables 4–7). However, there was considerable variability across race/ethnic groups, with NH white estimates remaining relatively stable with increasing age for all three outcomes of overweight, obesity, and severe obesity. Hispanics and NH black participants had the highest prevalences for all three outcomes, which increased with age. Obesity was more common in males than females for NH white, NH Asian, and NH other participants ages 6–18 years. In contrast, NH black females had a higher age-specific prevalence than their male counterparts (Tables 4–7).
Fig. 2.
Prevalences (%) of overweight, obesity, and severe obesity in the Environmental influences on Child Health Outcomes (ECHO) study. NH non-hispanic, W white, B black, A Asian, O other, Overweight = BMI ≥ 85th and <95th percentile; Obesity = BMI at or above the 95th percentile; Severe Obesity = BMI at or above 120% of the sex-specific 95th percentile. Each vertical bar in color represents estimated prevalence; with the point estimate as a circle and the extended vertical line representing the corresponding 95% confidence intervals obtained from meta-analysis using Normal-Binomial, non-linear, mixed-effects modeling
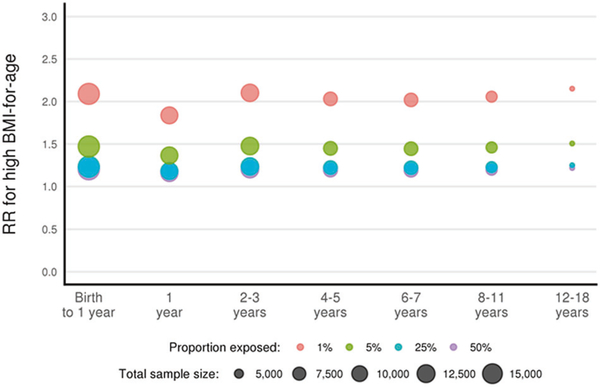
Statistical power to detect relative risks for associations of ‘high’ BMI with low to high frequency exposures in ECHO cohorts
Extant cohorts were included in the ECHO program based on their ability to provide extant high-quality data on exposures in utero through age 5 and to investigate cutting-edge scientific questions. The common ECHO-wide protocol extends and expands the follow-up of mother and child pairs from the prenatal period through age 18 years. Figure 3 provides the estimated sample sizes available for age groups and the detectable risk ratios for associations of high BMI or obesity (versus normal weight) with a range of exposures with varied prevalences (1–50%). For example, among 2–3-year-old children, given a sample size of 12,000, we expect to have ≥80% power to detect a 1.3 risk ratio for obesity vs. normal weight associated with an exposure that has a prevalence of 5% (Table 8).
Fig. 3.
Minimum detectable risk ratios for overweight/obesity associated with environmental exposures (80% power and 5% alpha error tolerance). High weight defined as: BMI ≥ 97.7th percentile of (age and sex-specific) WHO 2006 growth charts for birth to 2 years of age; BMI ≥ 95th percentile of (age and sex-specific) CDC growth charts
Discussion
In this analysis of >23,000 children from birth to 2 years old and >20,000 children ages 2–18 years enrolled in ECHO cohorts from across the US, we found that high BMI for age is common, increases with advancing age, and varies by race/ethnicity. Further, in ECHO pediatric cohorts the age-specific prevalences of overweight, obesity, and severe obesity combined are remarkedly similar to those reported for the National Health and Nutrition Examination Survey (NHANES; 2011–2016) [18] for 2–5 years (11% vs. 9–14%); 6–11 years (18% vs. 17–18%); and 12–18 years (20% vs. 21–25%) [4, 18–20]. Similar racial/ethnic disparities in overweight, obesity, and severe obesity for ages 2–18 exist in ECHO and NHANES, with the highest prevalence for Hispanics (16–28% vs. 15.6–26%) and NH blacks (11–26% vs. 9–22%), followed in decreasing order by NH whites (7–11% vs. 3–19%) and NH Asians (3–16% vs. 3–11%), respectively [4, 18–20]. The demographic similarities also support potential generalizability of findings from future ECHO analyses examining environmental predictors and health sequelae related to childhood obesity to the US population.
This manuscript also confirms the substantial statistical power of ECHO to answer questions regarding the environmental and preventable causes of childhood obesity even for low prevalence exposures. However, like other large national efforts, including NHANES [18, 19], the ECHO cohorts have more limited sample sizes when simultaneously stratified by race/ethnicity, age, and sex, which may result in reduced power to detect associations in some substrata, especially in the context of rare exposures.
Complex exposures across multiple levels with a standardized protocol
In addition, ECHO will improve upon previous designs by prospectively gathering a comprehensive battery of measures, biological samples, and geospatial information and prospective follow-up data (Table 2) from participants in the extant cohorts to address overarching questions put forth by the ECHO Obesity Working Group (www.nih.gov/echo), as well as ECHO-funded and outside investigators. The standardized ECHO-wide protocol will also serve as a model for the design of US cohorts in the future that can add to the statistical power and ability to identify environmental and preventable determinants of adiposity and downstream risks. For example, the ECHO-wide protocol will capture key residential neighborhood built environment domains that include: neighborhood food access (e.g., fast food, convenience stores, supermarkets); access to parks, playgrounds, and green space [21–23]; neighborhoods that support walking and cycling [21, 24]; and whether the context was urban, suburban, or rural [25]. Chemical exposures such as bisphenols [26, 27], organotins [28, 29], and toxic metals [30] will be evaluated with archived and prospectively collected specimens through the Children’s Health Exposure Analysis Resource [31]. Standardized collection of behaviors such as diet and physical activity, culture and socio-demo graphic factors, at the individual [32–37], family [38–41], community [42], and health care levels [43–45] will be collected, beginning in pregnancy. Furthermore, ECHO will incorporate linked administrative databases/sources such as crime, unemployment, neighborhood poverty, and standardized indices that characterize child vulnerability and will have the opportunity to capture the positive and negative attributes of neighborhoods in relation to obesity risk.
In recent years, the field of genomics has exploded with advances in technologies and biostatistical approaches that allow us to investigate millions of genetic variants distributed across the genome. Yet, while 15 independent loci associated with childhood BMI have been reported [46], these loci explain only 2% of the variance in obesity risk. ECHO cohorts will have specimens available to examine genetic modifications and better unravel the complexity of gene-environment interactions as contributors. ECHO can also leverage advancements in high-throughput sequencing and computing, which have heightened our appreciation for the role of microbial communities in “programming” childhood obesity risk. Finally, advances in statistical methods (including instrumental variable approaches, such as Mendelian randomization) will be used for causal inference in modelling associations of early life exposures with childhood obesity and its sequela.
What is novel about ECHO, and why is it needed?
Other longitudinal prebirth or birth cohort studies of similar size, such as the Avon Longitudinal Study of Parents and Children in the United Kingdom [47], the Promotion of Breastfeeding Intervention Trial study in Belarus [48], the Danish National Birth Cohort [49], and the Norwegian Mother and Child Cohort (MoBa) [50], are well equipped to answer questions about the environmental and developmental origins of childhood obesity. However, few are well poised to examine origins of disparities in obesity which are so prominent among US children. For example, 62% of children in the Generation R cohort are Dutch or other European, 11% are Moroccan or Dutch Antillean, and 8% each are Surinamese, Turkish and Other [51]. Findings from that sample are not generalizable to Latinos, who represent 24% of US children and have disproportionate exposure to endocrine disrupting chemicals, which have been identified as potential contributors to obesity [52]. ECHO also includes children of Asian origin, a growing segment of the US population and a population underrepresented in extant cohorts. Participants in other European cohorts are overwhelmingly or exclusively non-Hispanic white.
The utility of ECHO is also evident for studies of built environment exposures, which may be associated with race/ethnicity, and diet, physical activity and other obesogenic behaviors. Other exposures such as crime my affect stress that can affect adiposity accrual and distribution via numerous pathways, including alterations in circulating hormones and methylation of genes related to development of obesity. Few studies in the United States have the potential of ECHO to unravel the exposures, moderators and mediators that may explain the observed disparities in the US population. ECHO has assembled a large racially/ethnically diverse US cohort poised to provide much-needed answers to important questions regarding the early life determinants of childhood obesity risk, using a longitudinal, prospective design.
In addition, findings from older cohorts may also not be generalizable due to the rapid replacement of many known obesogenic chemicals with others that may have similar properties. A prime example is bisphenol A, which has been increasingly replaced with forty or more other chemically similar phenols for use in polycarbonate plastics, aluminum can linings and thermal paper receipts. One replacement, bisphenol S has been found to be as estrogenic [53], toxic to embryos [54], and persistent in the environment [55]. As attention has focused on phthalates used in soft polyvinyl chloride plastics such as those used food packaging, including di-2-ethylhexylphthalate, replacement chemicals have emerged. These include 1,2-cyclohexane dicarboxylic acid, diisononyl ester; diisodecyl (DIDP) and diisononylphthalate (DINP), phthalates. DIDP and DINP have been associated with insulin resistance and elevations in blood pressure in children [56, 57].
The cohorts are different in timing of enrollment, eligibility for participation, region of the country, race/ethnicity. Examining these many interacting factors in a mosaic of participants together with a broad array of environmental factors will yield a valuable database for use by researchers nationally as well as internationally. The intention of the ECHO program is for data sharing in the broadest way possible, while protecting privacy and confidentiality. Both ECHO-funded and external investigators can propose new analytic concepts leveraging biological and environmental samples, as well as extant data to examine novel and emerging exposures, and consider long-debated questions in a more representative sample of the United States.
The ECHO program overcomes an important obstacle experienced by the National Children’s Study through the leverage of existing cohorts. This manuscript demonstrates pooling of phenotypic data is feasible. The power of the ECHO cohorts will ultimately be leveraged through harmonization of data, as proposed in the ECHO-wide protocol for extant and prospectively collected data. Extant data were collected with a wide variety of protocols and instruments, prior to ECHO’s existence, and even the prospective standardized protocol allows for some flexibility in choice of instruments and procedures to collect key domains of data.
There will still be other challenges to the ECHO program’s success in identifying environmental and preventable risks for obesity. Maximal retention of study participants is paramount and will occur through acknowledging and minimizing cumulative and within-visit burden on participants, given the planned data collection. Major challenges will require balancing multiple potentially competing scientific questions within a larger study of child health, acknowledging that not all questions will require tens of thousands of children to study. Though not all extant cohorts will have the same depth of archived biospecimen or previously collected data available, sub-sample analyses will still provide large analyses of obesogenic environmental exposures [58].
ECHO will be positioned to clarify the roles of multiple and interacting environmental and preventable risk factors for obesity in US children and to assist in setting the stage for intervention studies and policy experiments. Rigorous investigations, several already proposed within ECHO and many more to come, may address policies, urban design/planning, public health, behavioral science, commercial interests, and health care practices to curb or reverse the escalating prevalence of obesity, especially in the most disadvantaged segments of society.
Conclusion
The ECHO program has made substantial investments to develop a powerful program to study and understand how environmental influences drive the development of obesity, beginning in preconception and continuing through adolescence and young adulthood. The size and diversity of the study population, the multidimensional and systematic approach to the development of new tools and measures as part of an extensive ECHO-wide protocol, and the leveraging of cutting-edge scientific knowledge is unprecedented for the US. The research program as designed holds promise to provide insight into the mechanisms that promote overweight and obesity in US children and provide rigorous data for novel programs to lessen the burden on individuals, families, and society.
Supplementary Material
Acknowledgements
The authors wish to thank our ECHO colleagues; the medical, nursing, and program staff; as well as the children and families participating in the ECHO cohorts.
Funding Research reported in this publication was supported by the Environmental influences on Child Health Outcomes (ECHO) program, Office of The Director, National Institutes of Health, under Award Numbers U2COD023375, U24OD023382, UG3OD023271, UG3OD0 23289, UG3OD023286, UG3OD023248, UH3OD023290, P50 ES009 600, UG3OD023275 NIEHS P01ES022832, EPA RD 83544201, UG3 OD023286, 4UG3OD023287-03, K01HL141589, UG3OD023285, UG 3OD023316, UG3OD023289, UG3OD023289, UG30D023318, UH3O D023249, 1UG1HD090899-01, UG3OD023320, UG3 (UH3) OD023305.
Footnotes
Conflict of interest The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors declare that they have no conflict of interest.
Compliance with ethical standards
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Members of the Environmental influences on Child Health Outcomes are listed at the Supplementary Information “ECHO Program Collaborators”.
Supplementary information The online version of this article (https://doi.org/10.1038/s41366-019-0470-5) contains supplementary material, which is available to authorized users.
References
- 1.Murray CJ, Atkinson C, Bhalla K, Birbeck G, Burstein R, Chou D, et al. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar S, Kelly AS. Review of childhood obesity: from epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clinic Proc. 2017;92:251–65. [DOI] [PubMed] [Google Scholar]
- 3.Hanson MA, Gluckman PD. Developmental origins of health and disease-global public health implications. Best Pract Res Clin Obstet Gynaecol. 2015;29:24–31. [DOI] [PubMed] [Google Scholar]
- 4.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of obesity and severe obesity in US Children, 1999–2016. Pediatrics. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan L, May AL, Wethington H, Dalenius K, Grummer-Strawn LM. Incidence of obesity among young U.S. children living in low-income families, 2008–2011. Pediatrics. 2013;132:1006–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung A, Backholer K, Wong E, Palermo C, Keating C, Peeters A. Trends in child and adolescent obesity prevalence in economically advanced countries according to socioeconomic position: a systematic review. Obes Rev. 2016;17:276–95. [DOI] [PubMed] [Google Scholar]
- 7.Trasande L, Cronk C, Durkin M, Weiss M, Schoeller DA, Gall EA, et al. Environment and obesity in the National Children’s Study. Environ Health Perspect. 2009;117:159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Connor EA, Evans CV, Burda BU, Walsh ES, Eder M, Lozano P. Screening for obesity and intervention for weight management in children and adolescents: evidence report and systematic review for the US preventive services task force. JAMA. 2017;317:2427–44. [DOI] [PubMed] [Google Scholar]
- 9.Gluckman PD, Hanson MA. Developmental origins of disease paradigm: a mechanistic and evolutionary perspective. Pediatr Res. 2004;56:311–7. [DOI] [PubMed] [Google Scholar]
- 10.Hammond RA. Complex systems modeling for obesity research.Prev Chronic Dis. 2009;6:A97. [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. A SAS program for the 2000 CDC growth charts (ages 0 to <20 years) 2019. https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm. Accessed 18 Sept 2019.
- 12.Centers for Disease Control and Prevention. A SAS program for the WHO Growth Charts (ages 0 to <2 years). 2019. https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas-who.htm. Accessed 18 Sept 2019.
- 13.Grummer-Strawn LM, Reinold C, Krebs NF. Use of World Health Organization and CDC Growth Charts for Children Aged 0–59 Months in the United States. 2010. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5909a1.htm. [PubMed]
- 14.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: Methods and Development. Vital Health Stat. 2002;1–190. [PubMed] [Google Scholar]
- 15.Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation. 2013;128:1689–712. [DOI] [PubMed] [Google Scholar]
- 16.Lesko CR, Jacobson LP, Althoff KN, Abraham AG, Gange SJ, Moore RD, et al. Collaborative, pooled and harmonized study designs for epidemiologic research: challenges and opportunities. Int J Epidemiol. 2018;47:654–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma Y, Chu H, Mazumdar M. Meta-analysis of proportions of rare events-a comparison of exact likelihood methods with robust variance estimation. Commun Stat Simul Comput. 2016;45:3036–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogden CL, Carroll MD, Kit BK, Flegal KM. PRevalence of childhood and adult obesity in the united states, 2011–2012. JAMA. 2014;311:806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK. et al. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 Through 2013–2014. JAMA. 2016;315:2292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007–2008 to 2015–2016. JAMA. 2018;319:1723–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duncan DT, Sharifi M, Melly SJ, Marshall R, Sequist TD, Rifas-Shiman SL, et al. Characteristics of walkable built environments and BMI z-scores in children: evidence from a large electronic health record database. Environ Health Perspect. 2014;122:1359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bancroft C, Joshi S, Rundle A, Hutson M, Chong C, Weiss CC, et al. Association of proximity and density of parks and objectively measured physical activity in the United States: A systematic review. Socail Sci Med. 2015;138:22–30. [DOI] [PubMed] [Google Scholar]
- 23.Kondo MC, Fluehr JM, McKeon T, Branas CC. Urban green space and its impact on human health. Int J Environ Res Public Health. 2018;15: pii: E445. 10.3390/ijerph15030445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Timperio A, Reid J, Veitch J. Playability: built and social environment features that promote physical activity within children. Curr Obes Rep. 2015;4:460–76. [DOI] [PubMed] [Google Scholar]
- 25.Kim JH, Lee C, Sohn W. Urban natural environments, obesity, and health-related quality of life among hispanic children living in inner-city neighborhoods. Int J Environ Res Public Health. 2016;13: pii: E121. 10.3390/ijerph13010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trasande L, Attina TM, Blustein J. Association between urinary bisphenol a concentration and obesity prevalence in children and adolescents. JAMA. 2012;308:1113–21. [DOI] [PubMed] [Google Scholar]
- 27.Jacobson MH, Woodward M, Bao W, Liu B, Trasande L. Urinary bisphenols and obesity prevalence among US children and adolescents. J Endocr Soc. 2019;3:1715–26. 10.1210/js.2019-00201. eCollection 2019 Sep 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janesick A, Blumberg B. Obesogens, stem cells and the developmental programming of obesity. Int J Androl; 35:437–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kotake Y Molecular mechanisms of environmental organotin toxicity in mammals. Biol Pharm Bull. 2012;35:1876–80. [DOI] [PubMed] [Google Scholar]
- 30.Padilla MA, Elobeid M, Ruden DM, Allison DB. An examination of the association of selected toxic metals with total and central obesity indices: NHANES 99–02. Int J Environ Res Public Health. 2010;7:3332–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Institute of Environmental Health Sciences. Exposure analysis resources. 2019. https://www.niehs.nih.gov/research/supported/exposure/chear/index.cfm. Accessed 18 Sept 2019.
- 32.Anderson SE, Whitaker RC. Prevalence of obesity among US preschool children in different racial and ethnic groups. Arch Pediatr Adolesc Med. 2009;163:344–8. [DOI] [PubMed] [Google Scholar]
- 33.Taveras EM, Gillman MW, Kleinman KP, Rich-Edwards JW, Rifas-Shiman SL. Reducing racial/ethnic disparities in childhood obesity: the role of early life risk factors. JAMA Pediatr. 2013;167:731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ambrosini GL. Childhood dietary patterns and later obesity: a review of the evidence. Proc Nutr Soc. 2014;73:137–46. [DOI] [PubMed] [Google Scholar]
- 35.Ambrosini GL, Emmett PM, Northstone K, Howe LD, Tilling K, Jebb SA. Identification of a dietary pattern prospectively associated with increased adiposity during childhood and adolescence. Int J Obes. 2012;36:1299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marshall S, Burrows T, Collins CE. Systematic review of diet quality indices and their associations with health-related outcomes in children and adolescents. J Hum Nutr Diet. 2014;27:577–98. [DOI] [PubMed] [Google Scholar]
- 37.Mikkila V, Rasanen L, Raitakari OT, Pietinen P, Viikari J. Consistent dietary patterns identified from childhood to adulthood: the cardiovascular risk in Young Finns Study. Br J Nutr. 2005;93:923–31. [DOI] [PubMed] [Google Scholar]
- 38.Maternal Oken E. and child obesity: the causal link. Obstet Gynecol Clin North Am. 2009;36:361–77. ix–x. [DOI] [PubMed] [Google Scholar]
- 39.Hodges EA, Johnson SL, Hughes SO, Hopkinson JM, Butte NF, Fisher JO. Development of the responsiveness to child feeding cues scale. Appetite. 2013;65:210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Savage JS, Rollins BY, Kugler KC, Birch LL, Marini ME. Development of a theory-based questionnaire to assess structure and control in parent feeding (SCPF). Int J Behav Nutr Phys Act. 2017;14:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson AL. Intergenerational impact of maternal obesity and postnatal feeding practices on pediatric obesity. Nutr Rev. 2013;71 Suppl 1:S55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Showell NN, Cole KW, Johnson K, DeCamp LR, Bair-Merritt M, Thornton RLJ. Neighborhood and parental influences on diet and physical activity behaviors in young low-income pediatric patients. Clin Pediatrics. 2017;56:1235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grossman DC, Bibbins-Domingo K, Curry SJ, Barry MJ, Davidson KW, Doubeni CA, et al. Screening for obesity in children and adolescents: US preventive services task force recommendation statement. JAMA. 2017;317:2417–26. [DOI] [PubMed] [Google Scholar]
- 44.Lopez-Quintero C, Berry EM, Neumark Y. Limited English proficiency is a barrier to receipt of advice about physical activity and diet among Hispanics with chronic diseases in the United States. J Am Dietetic Assoc. 2009;109:1769–74. [DOI] [PubMed] [Google Scholar]
- 45.Thornton RLJ, Hernandez RG, Cheng TL. Putting the US Preventive services task force recommendation for childhood obesity screening in context. JAMA. 2017;317:2378–80. [DOI] [PubMed] [Google Scholar]
- 46.Felix JF, Bradfield JP, Monnereau C, van der Valk RJ, Stergia-kouli E, Chesi A, et al. Genome-wide association analysis identifies three new susceptibility loci for childhood body mass index. Hum Mol Genet. 2016;25:389–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fraser A, Macdonald-Wallis C, Tilling K, Boyd A, Golding J, Davey Smith G, et al. Cohort profile: the avon longitudinal study of parents and children: ALSPAC mothers cohort. Int J Epidemiol. 2013;42:97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patel R, Oken E, Bogdanovich N, Matush L, Sevkovskaya Z, Chalmers B, et al. Cohort profile: the promotion of breastfeeding intervention trial (PROBIT). Int J Epidemiol. 2014;43: 679–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olsen J, Melbye M, Olsen SF, Sorensen TI, Aaby P, Andersen AM, et al. The Danish National Birth Cohort-its background, structure and aim. Scand J Public Health. 2001;29:300–7. [DOI] [PubMed] [Google Scholar]
- 50.Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C. Cohort profile: the Norwegian mother and child cohort study (MoBa). Int J Epidemiol. 2006;35:1146–50. [DOI] [PubMed] [Google Scholar]
- 51.Jaddoe V, Mackenbach J, Moll H, Steegers E, Tiemeier H, Verhulst F, et al. The generation R study: design and cohort profile. Eur J Epidemiol. 2006;21:475–84. [DOI] [PubMed] [Google Scholar]
- 52.Attina TM, Malits J, Naidu M, Trasande L. Racial/ethnic disparities in disease burden and costs related to exposure to endocrine-disrupting chemicals in the United States: an exploratory analysis. J Clin Epidemiol. 2019;108:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crump D, Chiu S, Williams KL. Bisphenol S alters embryonic viability, development, gallbladder size, and mRNA expression in chicken embryos exposed via egg injection. Environ Toxicol Chem. 2016;35:1541–9. 10.1002/etc.3313. Epub 1 Apr 2016. [DOI] [PubMed] [Google Scholar]
- 54.Ma M, Crump D, Farmahin R, Kennedy SW. Comparing the effects of tetrabromobisphenol-A, bisphenol A, and their potential replacement alternatives, TBBPA-bis(2,3-dibromopropyl ether) and bisphenol S, on cell viability and messenger ribonucleic acid expression in chicken embryonic hepatocytes. Environ Toxicol Chem. 2015;34:391–401. [DOI] [PubMed] [Google Scholar]
- 55.Trasande L Further limiting bisphenol a in food uses could provide health and economic benefits. Health Aff (Millwood). 2014;33:316–23. [DOI] [PubMed] [Google Scholar]
- 56.Trasande L, Attina TM. Association of exposure to Di-2-ethylhexylphthalate replacements with increased insulin resistance in adolescents from NHANES 2009–2012. J Clin Endocrinol Metab. 2015;jc20151686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trasande L, Attina TM. Association of exposure to di-2-ethylhexylphthalate replacements with increased blood pressure in children and adolescents. Hypertension. 2015;66:301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jaddoe VW. Early-life stressors and lifecycle health. https://lifecycle-project.eu/about-lifecycle/project-summary/. (Accessed 18 Oct 2019).
- 59.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.